Abstract
Disorders of metabolism affect multiple systems throughout the body, but may have the greatest impact on both central and peripheral nervous systems. Current available treatments and behavior changes for disorders that include diabetes mellitus (DM) and nervous system diseases are limited and cannot reverse disease burden. Greater access to healthcare and a longer lifespan has led to increased prevalence for metabolic and neurodegenerative disorders. In light of these challenges, innovative studies into the underlying disease pathways offer new treatment perspectives for Alzheimer’s Disease, Parkinson’s Disease, and Huntington’s Disease. Metabolic disorders are intimately tied to neurodegenerative diseases and can lead to debilitating outcomes, such as multi-nervous system disease, susceptibility to viral pathogens, and long-term cognitive disability. Novel strategies that can robustly address metabolic disease and neurodegenerative disorders involve a careful consideration on cellular metabolism, programmed cell death pathways, the mechanistic target of rapamycin (mTOR) and its associated pathways of mTOR Complex 1 (mTORC1), mTOR Complex 2 (mTORC2), AMP activated protein kinase (AMPK), growth factor signaling, and underlying risk factors such as the apolipoprotein E (APOE-ε4) gene. Yet, these pathways are complex and necessitate comprehensive understanding to achieve clinical outcomes that target disease susceptibility, onset, and progression.
Keywords: Alzheimer’s disease, apoptosis, autophagy, COVID-19, diabetes mellitus, erythropoietin, Huntington’s disease, mTOR, Parkinson’s disease, pyroptosis
1. The Implications for Metabolic Dysfunction
Approximately 500 million individuals have metabolic disease and diabetes mellitus (DM) across the globe [1–3]. The costs for the treatment and care for patients with DM are are significant [4, 5]. At minimum, $20,000 United States Dollars (USD) per year are required to care for each individual with DM. Individuals with DM can experience costs for care that can now exceed $760 billion USD [6]. Care and treatment and for patients with DM requires greater than 17% of the Gross Domestic Product in the US per the Centers for Medicare and Medicaid Services (CMS) [7]. On top of these costs, another seventy billion USD are consumed for individuals with DM as a result of disability and functional loss. These financial concerns also impact different age groups of the population. Almost forty-five percent of the four million annual deaths that occur in individuals with DM affect those under the age of seventy [4]. In the United States (US), thirty-five million individuals, 10% of the population, can be given a diagnosis of DM [8, 9]. These numbers do not even consider that more than 400 million individuals can be at risk for developing DM or suffer from metabolic dysfunction [5, 6, 10, 11]. It is estimated that more than 7 million individuals 18 years of age or older are currently not recognized to have DM. As an example, approximately thirty-five percent of adults in the US are considered to have prediabetes as a result of their fasting glucose and hemoglobin A1c (HbA1c) levels [12, 13].
Metabolic disease is increasing in prevalence more rapidly in low and middle income countries than in high income countries (Table 1). Approximately eighty percent of adults with DM are living in low- and middle-income countries [6]. DM prevalence has increased from nine and one-half percent during the period of 1999 to 2002 to twelve percent during the period of 2013 to 2016. Multiple factors can affect disease prevalence, such as education, co-morbidities, and socioeconomic status [13–15]. Approximately 13% of adults with less than a high school education have DM compared to 10% of individuals with a high school education and DM. If an individual has greater than a high school education, the risk decreases to seven and one-half percent. Other risk factors for leading to the progression of DM complications consist of hypertension, limited exercise, tobacco use, obesity, and elevated serum cholesterol [2, 3, 16–19]. When one considers obesity, increased body weight results in impaired glucose tolerance that that cause DM progression [20–26]. Obesity is an important factor that can raise the risk of developing DM in young individuals and can alter aging, inflammation, stem cell proliferation, oxidative stress exposure, and mitochondrial integrity [11, 27–33]. Increased weight and metabolic dysfunction also can affect underlying cellular pathways of the mechanistic target of rapamycin (mTOR) that translate to clinical disability with dementia and coronavirus disease 2019 (COVID-19) [3, 28, 34–37].
Table 1.
Highlights
|
2. Metabolic Disease and Neurodegeneration
Non-communicable diseases (NCDs) include disorders of the nervous system [15, 38]. NCDs lead to at least 70% of the annual deaths that occur each year [39, 40]. An observed increase in NCDs parallels a rise in life expectancy of the global population [41, 42]. Life expectancy is now approaching eighty years of age [43] with a one percent lowering in the age-adjusted death rate from the years 2000 through 2011 [44]. In regard to developing countries, India and China will observe an elderly population increase from five to ten percent over future years [16, 45]. Increased lifespan may be due to multiple factors that include efficient sanitation measures, greater access to healthcare, and broader public health measures that assist populations for the highest risk for disability (Table 1). Such healthcare policies can result in earlier and effective treatment for multiple chronic disorders [42, 46–55].
As a result of more effective healthcare policies combined with an increase in lifespan throughout the world, the prevalence of neurodegenerative disorders has increased [9, 29, 56–61]. Neurodegenerative disorders consist of over six hundred disease entities that result in death and disability [45, 59, 60, 62, 63]. Neurodegenerative disorders lead to disability and death in more than one billion individuals, approximately fifteen percent of the global population, and at least seven million individuals die each year from neurodegenerative disorders [64]. Interestingly, disease of the nervous system lead to treatment costs over $700 billion United States dollars (USD) in the United States (US) alone. This costs involve treatments for dementia, back pain, stroke, epilepsy, traumatic brain injury, and Parkinson’s disease (PD) (Figure 2). It is of interest to note that cognitive care is the greatest cost factor with more than $800 billion USD a year allotted for memory loss [40]. These financial considerations do not consider other expenses that are required to provide social outreach programs, adult living care, and companion care, since at least 60 million new health and social care workers will be needed [39, 40, 65].
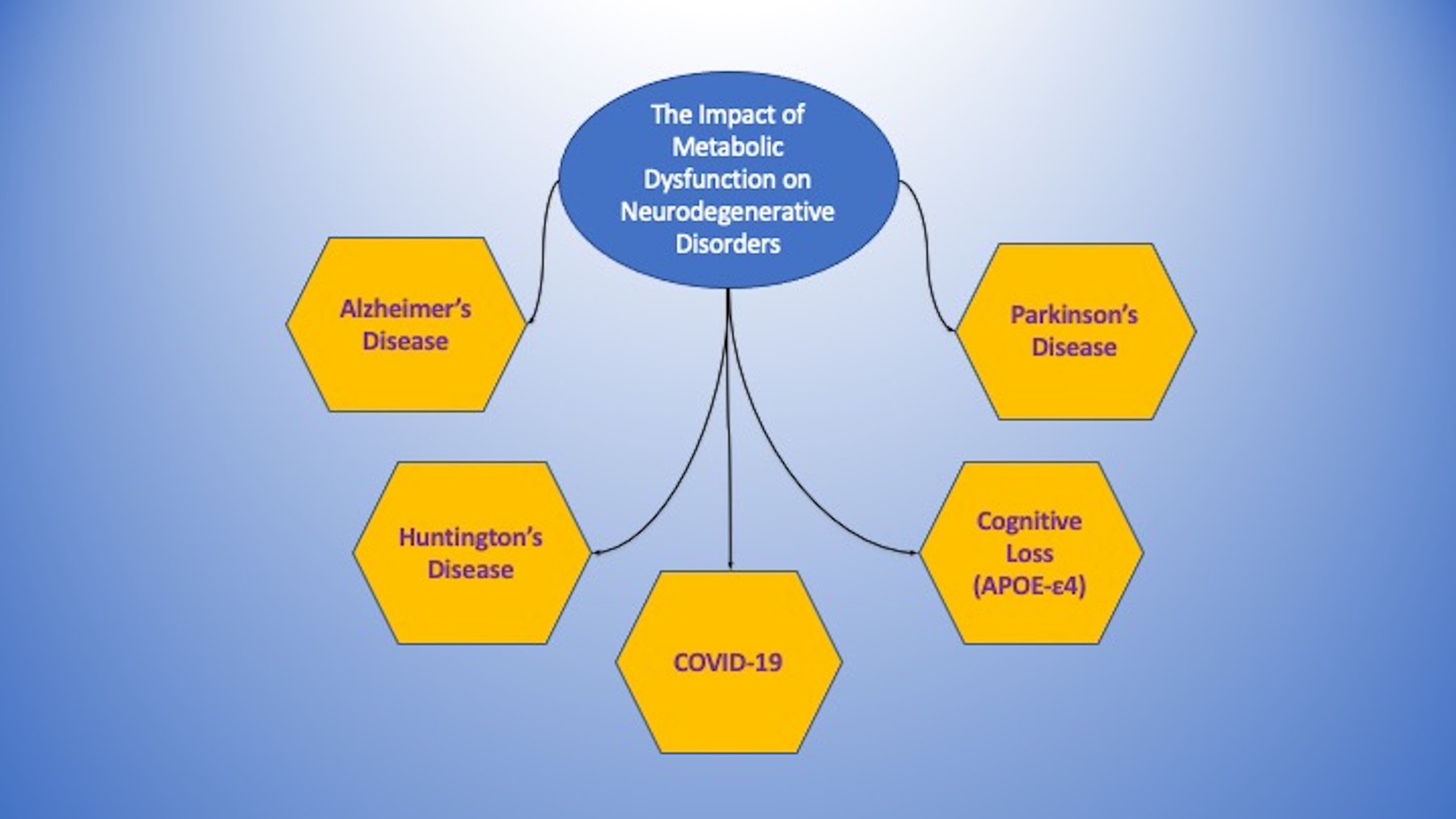
Figure 2: The Impact of Metabolic Dysfunction on Neurodegenerative Disorders.
Metabolic disorders are tightly linked to the onset and progression of neurodegenerative disorders. These include Alzheimer’s disease, Parkinson’s disease, and Huntington’s disease. In addition, other disorders, such as the apolipoprotein E (APOE-ε4) gene and coronavirus disease 2019 (COVID-19) can foster the onset and susceptibility of these neurodegenerative disorders through underlying metabolic pathways.
Early assessment of cognitive loss has become a challenge. Recognition, diagnosis, and treatment may be delayed for twelve to eighteen months following the initial onset of symptoms [4, 66, 67]. Per reports of the World Health Organization [40], dementia is present in populations throughout the globe and it is now the 7th leading cause of death [9, 67–70]. In regard to sporadic cases of Alzheimer’s disease (AD) (Figure 2), sporadic AD is expected to increase for the future and comprises almost all clinical cases [3, 48, 64, 71–73]. Sporadic AD affects at least 10% of the world’s population over the age of 65 [3, 5, 56, 72–76].
When one considers metabolic disorders such as DM, metabolic disorders affect all systems of the body and especially the central nervous system and the peripheral nervous system as well as other related systems with inflammatory and vascular [77] (Figure 1). DM can lead to both cortical and subcortical disease in the central nervous system to result in cognitive loss [3–5, 28, 78, 79]. DM can lead to insulin resistance and memory loss associated with AD [67, 80, 81]. Metabolic disorders that include DM also can affect impact stem cell proliferation [16, 32, 82–84], cytoprotective pathways [26, 30, 85], circadian rhythm pathways [3, 28, 53, 86–92], immune mediated pathways that involve microglia [93–99], and lifespan extension pathways that involve sirtuins [9, 27, 28, 100–109]. Furthermore, over 70% of diabetic individuals also have peripheral neuropathy. DM can result in peripheral nerve disorders [4, 110–112] and autonomic neuropathy [113]. DM also can lead to low-grade and acute inflammation n the immune and vascular systems [23, 33, 37, 114], endothelial dysfunction [47, 115, 116], cardiovascular disease [16, 27, 31, 117–124], and impairment of the neurovascular unit [9, 13, 26, 79, 85, 116, 125–127].
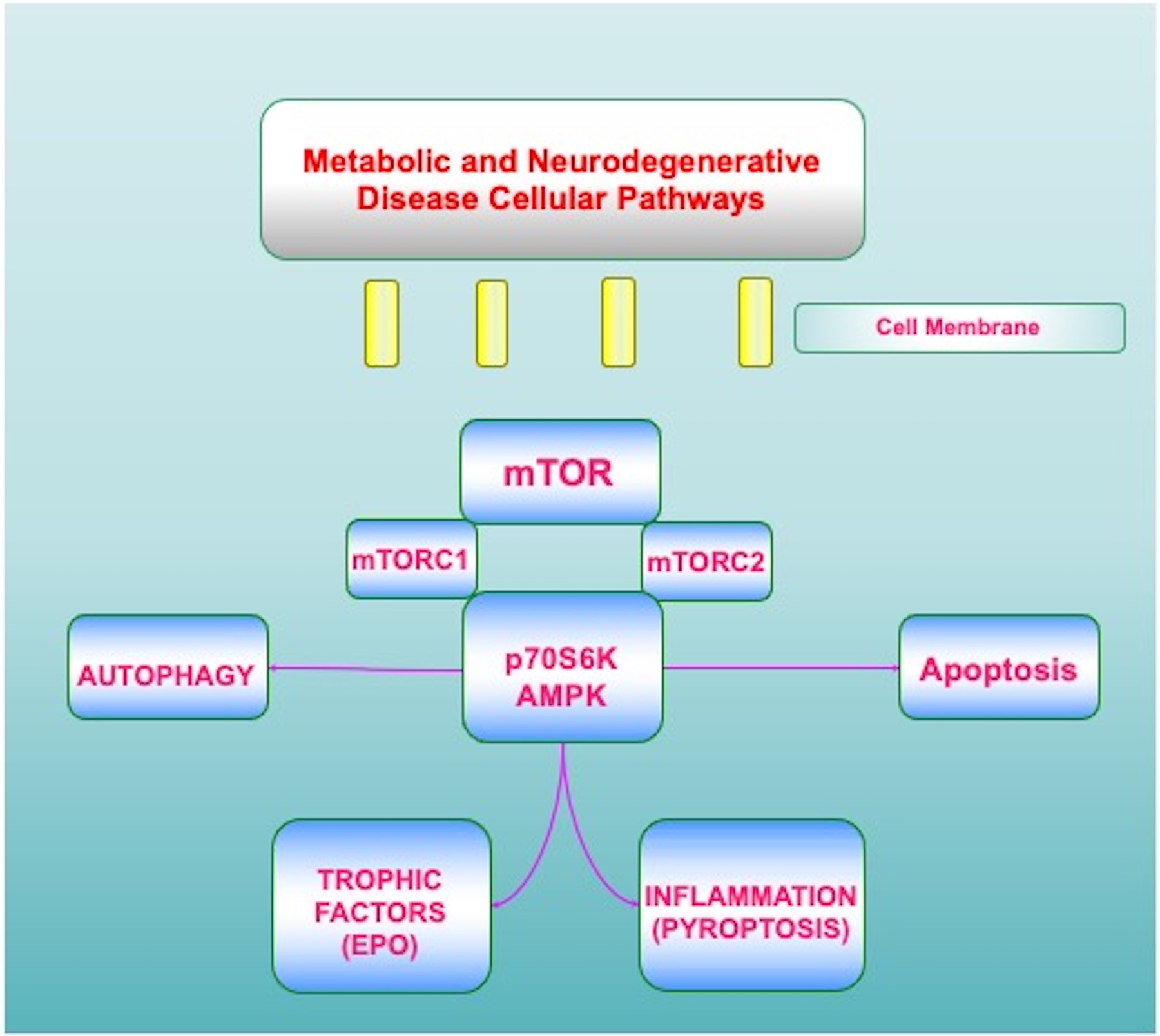
Figure 1: Metabolic and Neurodegenerative Disease Cellular Pathways.
A number of metabolic and neurodegenerative cellular pathways rely upon the mechanistic target of rapamycin (mTOR) and its associated pathways of mTOR Complex 1 (mTORC1), mTOR Complex 2 (mTORC2), AMP activated protein kinase (AMPK), and p70 ribosomal S6 kinase (p70S6K). Intimately linked to these pathways are trophic factors, such as erythropoietin (EPO), apoptosis, autophagy, and inflammation that can involve pyroptosis.
3. Novel Therapeutics for Neurodegenerative Disorders during Metabolic Disease
Metabolic disorders are intimately tied to neurodegenerative disorders. Early and targeted therapy can reduce the progression of DM and its detrimental effects on the nervous system, such as dementia onset [11, 29, 32, 52, 53, 67, 117, 128–130]. The implementation of pharmacotherapy and calorie intake monitoring can assist with the treatment of metabolic disorders and DM. However, in the attempt to limit hyperglycemic events, potential risks can ensue that can affect cellular organelles, decrease organ mass, and lead to neuronal loss through processes that involve autophagy [131, 132]. Current therapies targeted to prevent cognitive loss can have metabolic components, such as the removal of ß-amyloid (Aβ) in the brain [133] and the use of cholinesterase inhibitors [69, 75]. These may provide limited resolution of symptoms or modify disease progression over a brief or unknown period [66, 69, 134–136]. Hypertension, cardiovascular disease, low education in early life, and tobacco use also can affect cognitive decline [66, 67, 137]. Other work places attention on heightened physical activity to stabilize metabolic disease and neurodegenerative disorders linked to dementia and PD [2, 9, 19, 138, 139]. Metabolic disease that impacts the vascular system also can result in cognitive loss [41, 140–143]. With these considerations, new avenues of discovery are required for neurodegenerative disease coupled to metabolic disorders. These include targeted strategies for metabolic homeostasis, programmed cell death pathways, the mechanistic target of rapamycin (mTOR) and its associated pathways of mTOR Complex 1 (mTORC1), mTOR Complex 2 (mTORC2), AMP activated protein kinase (AMPK), growth factor signaling with erythropoietin (EPO), and critical risk factors such as the apolipoprotein E (APOE-ε4) gene (Table 1).
4. Programmed Cell Death Pathways in Neurodegenerative and Metabolic Disease
Neuronal survival and onset of neurodegenerative disorders can be affected by programmed cell death pathways during metabolic dysfunction [144–148] (Figure 1). Programmed cell death involves a number of processes that can modulate inflammatory pathways during cellular metabolic dysfunction [4, 16, 23, 116, 124, 149]. In particular, apoptosis, autophagy, and pyroptosis can ultimately determine the fate of a cell [150–156].
Apoptosis has both an early and late phase [71]. The early phase consists of phosphatidylserine (PS) membrane asymmetry loss on the plasma membrane [157–161]. The later phase of apoptosis leads to deoxyribonucleic acid (DNA) degradation in the genome [108, 162–168]. Loss of membrane PS asymmetry can lead to microglia and inflammatory cells to identify, engulf, and remove injured cells [99, 157, 169, 170]. In the event that the activity of microglia can be blocked, PS membrane asymmetry is reversible and can permit remaining functional cells expressing membrane PS residues to be rescued [171–174]. Apoptotic cell death occurs as a result of a cascade activation of nucleases and proteases that involve caspases [50, 163, 175–180]. The destruction of cellular DNA is considered not to be reversible [134]. Modulation of apoptotic pathways can minimize cognitive loss during acute insults [34, 153, 166, 175, 181, 182]. Activation of anti-inflammatory pathways can prevent apoptotic cellular death and prevent the loss of cognition [74, 177].
Autophagy processes have been shown to recycle cytoplasmic organelles and components for eventual tissue remodeling [52, 72, 73, 150, 183–191]. Subtypes of autophagy processing include macroautophagy, microautophagy, and chaperone-mediated autophagy. Macroautophagy recycles organelles and sequesters cytoplasmic proteins and organelles into autophagosomes that combine with lysosomes for degradation and recycling [73, 192, 193]. Microautophagy refers to the process of lysosomal membranes invagination for the collection and digestion of cytoplasmic components [194]. Chaperone-mediated autophagy relies upon cytosolic chaperones to move cytoplasmic components across lysosomal membranes [195].
Autophagy-lysosome pathways have been identified during infectious process to lead to inflammatory cell injury, such as with the pathogen severe acute respiratory syndrome coronavirus (SARS-CoV-2) [35, 36, 196–200]. As a result, autophagy through inflammatory mediators can affect memory and cognition [28, 201, 202]. Autophagy activation can reduce tau and Aß neurotoxicity [203–205]. Autophagy can remove Aß levels in the brain as one possible component to limit memory loss [81, 135, 203].
Pyroptosis, another form of programmed cell death, that can oversee inflammatory pathways in the nervous system during metabolic disease [4, 13, 134, 166, 206–208]. Pyroptosis is initiated with the generation of a supramolecular complex, termed the pyroptosome or the inflammasome, that can promote caspase activation to include caspase 1, caspase 4, and caspase 5. Pyroptosis utilizes permeabilization of the plasma membrane through gasdermin protein family members. Gasdermin proteins contain both an N-terminal domain with intrinsic pore-forming properties and a C-terminal domain that can block the pore forming properties of the N-terminal domain. Disruption of the linker sequence that binds the N-terminal and the C-terminal domains allows the N-terminal domain fragment to generate pores in the plasma. Cellular membranes are then able to release pro-inflammatory cytokines such as interleukin-1 family members. Inflammatory factors, such as interleukin-1 family members, can modulate a balance in regard to assisting or hampering cell survival that involve gasdermin. Interleukin-1 family members are absent of a signal plasma membrane peptide that would permit their cellular release and therefore require gasdermin proteins to generate membrane pores [5, 209]. This opening of cell membrane pores can lead to the rupture of cell membranes, the release of cytokines, and other damage-associated molecular pattern (DAMP) molecules that includes DNA and adenosine triphosphate (ATP). DAMPs can lead to the activation of the NLR family pyrin domain containing 3 (NLRP3) inflammasome in the canonical inflammasome pathways. The noncanonical inflammasome pathway is initiated by binding of lipopolysaccharide proteins that can be found on gram-negative bacteria leading to caspase 4 and caspase 5 activation. Pyroptosis, therefore, can lead to pro-inflammatory responses that can cause cytokine storm and cell death [210]. These factors also play a role during DM and diabetic wound healing. Pro-inflammatory mediators, such as the NLRP3 inflammasome, can activate caspase 1 and cytokines, result in metabolic stress, and cause cell death and poor wound healing [207]. During reactive oxygen species (ROS) release and exposure to oxidative stress [25, 83, 211–213], pyroptosis as can have a significant role to affect cognition, AD, and DM complications that include neuronal and vascular disease [9, 25, 35, 42, 72, 181, 214, 215].
5. The Mechanistic Target of Rapamycin (mTOR)
A 289-kDa serine/threonine protein kinase, the mechanistic target of rapamycin (mTOR) is encoded by a single gene FRAP1 [3, 64, 73, 152, 186, 216, 217]. mTOR also is known as the mammalian target of rapamycin and the FK506-binding protein 12-rapamycin complex-associated protein 1 [195]. The target of rapamycin (TOR) was initially discovered in Saccharomyces cerevisiae with the genes TOR1 and TOR2 [217]. The agent rapamycin is a macrolide antibiotic in Streptomyces hygroscopicus that blocks TOR and mTOR activity [116].
mTOR is a vital component of the protein complexes mTOR Complex 1 (mTORC1) and mTOR Complex 2 (mTORC2) (Figure 1). mTORC1 contains Raptor, Deptor (DEP domain-containing mTOR interacting protein), the proline rich Akt substrate 40 kDa (PRAS40), and mammalian lethal with Sec13 protein 8, termed mLST8 (mLST8) [3, 64]. mTOR can control Raptor activity which can be blocked by rapamycin. Rapamycin may block the activity of mTORC1 by binding to immunophilin FK-506-binding protein 12 (FKBP12) that normally attaches to the FKBP12 -rapamycin-binding domain (FRB) at the carboxy (C) -terminal of mTOR and blocks the FRB domain of mTORC1 [71]. However, the mechanism of how rapamycin blocks mTORC1 activity with the interaction of the domain of FRB is unclear. One consideration may involve allosteric changes on the catalytic domain as well as the inhibition of phosphorylation of protein kinase B (Akt) and p70 ribosomal S6 kinase (p70S6K) [218]. mTORC1 appears to be more sensitive to inhibition by rapamycin than mTORC2, but chronic administration of rapamycin can inhibit mTORC2 activity as a result of the disruption of the assembly of mTORC2. Deptor, also an inhibitor, blocks mTORC1 activity by binding to the FAT domain (FKBP12 -rapamycin-associated protein (FRAP), ataxia-telangiectasia (ATM), and the transactivation/transformation domain-associated protein) of mTOR. PRAS40 blocks mTORC1 activity by limiting the association of p70 ribosomal S6 kinase (p70S6K) and the eukaryotic initiation factor 4E (eIF4E)-binding protein 1 (4EBP1) with Raptor [3, 71]. Akt is important in this pathway as a checkpoint since mTORC1 activity is increased once phosphorylation of PRAS40 occurs by Akt [50, 165, 219, 220]. This releases the binding of PRAS40 and Raptor to localize PRAS40 in the cell cytoplasm with the docking protein 14–3-3 [221–223]. mLST8 can promote the activity of mTOR [71]. This process involves the binding of p70S6K and 4EBP1 to Raptor.
Interestingly, mLST8 controls insulin signaling through the transcription factor FoxO3 [114]. It also is necessary for Akt and protein kinase C-α (PKCα) phosphorylation, and is required for Rictor to associate with mTOR [114]. mTORC1 is associated with metabolic disorders [11, 35, 224] and dementia [4, 28, 142]. mTORC1 can promote lipogenesis and fat storage [225], improve glucose homeostasis [226], and may increase pancreatic ß-cell mass [227].
mTORC2 is composed of Rictor, Deptor, the mammalian stress-activated protein kinase interacting protein (mSIN1), mLST8, and the protein observed with Rictor-1 (Protor-1) [3, 71, 106, 183, 228]. mTORC2 is involved in metabolic function [5, 11]. mTORC2 signaling is necessary for the maintenance of pancreatic β-cell proliferation and mass [229]. Absence of mTORC2 signaling results in insulin resistance, oxidative damage [230], and severe hyperglycemia [231]. mTORC2 also modulates cytoskeleton remodeling through PKCα and cell migration through the Rac guanine nucleotide exchange factors P-Rex1 and P-Rex2 and through Rho signaling. mTORC2 can foster the activity of protein kinases that includes glucocorticoid induced protein kinase 1 (SGK1), a member of the protein kinase A/protein kinase G/protein kinase C (AGC) family of protein kinases. Protor-1, a Rictor-binding subunit of mTORC2, can result in SGK1 activity [38]. mSin1 is necessary for the construction of mTORC2 and for mTORC2 to phosphorylate Akt [38]. Rictor and mSIN1 phosphorylate Akt at serine473 and foster threonine308 phosphorylation by phosphoinositide-dependent kinase 1 (PDK1) to increase cell survival.
6. The Mechanistic Target of Rapamycin (mTOR) and Programmed Cell Death
mTOR has a close association with apoptosis, autophagy and pyroptosis (Table 1). mTOR activation can block apoptotic cell death in the nervous system [232, 233]. Through mTOR activation, retinal ganglion cell regeneration can be fostered [234], microglia survival can increase during oxidative stress [235], and diabetic peripheral neuropathy can be limited[236]. Aß toxicity during mTOR activation can be blocked [34, 222, 237–239], vascular cell death is prevented [190, 240], neuroplasticity is fostered [241], mitochondria loss during oxidative stress is blocked [242], neuronal differentiation can be fostered [243], neonatal hypoxic injury is limited [244], and decreased apoptotic cell death occurs during ischemic stroke [175].
Autophagy activation can be neuroprotective under some conditions that involve mTOR blockade [5, 34, 45, 72, 73]. Cognitive loss may be limited with low calorie diets that foster autophagy and limit mTOR activity [245]. Activation of autophagy with decreased mTOR function can lead to improved memory and more robust insulin signaling that can increase Aß clearance [246]. Cognition and memory also may increase with tau clearance during autophagy activation and mTOR function that has been limited [205]. Autophagy activation during a reduction in mTOR activity can block mitochondrial loss [247], prevent dopamine cell loss [248], decrease reactive oxygen species release [249], and improve neuronal cell survival with glutamine dependent mechanisms [250]. In the setting of metabolic disorders such as DM, mTOR inhibition can increase cell survival during cerebral ischemia [251] and maintain a balance between pancreatic β-cell proliferation and cell size [229]. Other work suggests that dysregulation of autophagy can result in cognitive loss with AD, development of autism spectrum disorder [252], and the induction of DM [80]. Studies have shown that autophagy haploinsufficiency with deletion of Atg7 gene in mouse models of obesity causes elevated lipids, insulin resistance, and inflammation [253]. Autophagic protein loss of Atg7, Atg5, and LC3 can be responsible for diabetic nephropathy [254]. Autophagy offers protection by removing misfolded proteins and mitochondria that cannot function to protect β-cell function and prevent DM development [255]. Exercise in murine models has been demonstrated to increase autophagy activation and promote glucose homeostasis [256]. This may occur as a result of improved insulin sensitivity [257] and reducing microglial activity in the setting of acute glucose changes [93].
Yet, a balance between autophagy and mTOR pathways may be necessary to promote the unction of cells. mTOR activity may be required since aberrations in mTOR activity can lead to cognitive loss [71, 73, 258]. Activation of autophagy can result in ROS insults to mitochondria, lead to the death of endothelial progenitor cells, and block new blood vessel formation during elevated glucose exposure [259]. Activation of autophagy at times may result in neuronal cell death [260]. Autophagy activation also can lead to cardiac and liver tissue injury in diabetic rats during diet modification that seeks to maintain glucose homeostasis [261]. During elevated glucose levels, advanced glycation end products (AGEs), proteins that can result in complications during DM, can result in the activation of autophagy and vascular smooth muscle proliferation that can result in atherosclerosis [262] as well as cardiomyopathy [263]. Autophagy with high glucose levels can lead to endothelial progenitor cell death, mitochondrial oxidative stress [264, 265], and inhibit angiogenesis [259]. Trophic factors, such as EPO, that lead to mTOR activation while reducing autophagic processes can increase neuronal and vascular cell survival in the nervous system [162, 220, 266]. EPO can control mTOR pathways, to include PRAS40 and Akt, and result in improved neuronal survival [221, 267–269]. mTOR activation has been shown to be vital for interneuron progenitor growth in the brain during autophagy inhibition [270].
mTOR also plays a significant role in multiple metabolic pathways [3, 11, 28, 36]. Through the mTOR pathways of p70S6K and 4EBP1 can result in the secretion of insulin in pancreatic β-cells as well as promote the resistance in murine models to β-cell streptozotocin toxicity and obesity [227]. Yet, loss of p70S6K activity leads to insulin insensitivity to glucose secretion, hypo-insulinemia, glucose intolerance, and reductions in pancreatic β-cell size [271]. Activation of mTOR in patients with metabolic syndrome has been shown to be decreased and potentially account for insulin resistance with a heightened risk of vascular thrombosis [272]. Activation of mTOR pathways has been tied pancreatic β- cell protection against cholesterol-induced apoptosis [273], increased protection of neurons in models of DM [274], and decreased glucolipotoxicity [275]. mTOR inhibition can result in insulin resistance, limited β-cell function, and reduced secretion of insulin associated with DM [276]. Loss of mTOR activity can raise mortality in murine models of DM [277]. In skeletal muscle, translocation of glucose transporters to the plasma membrane are affected during blockaded of mTOR [278].
Under some conditions, memory function can be protected during mTOR activation. mTOR activity can control insulin signaling in AD experimental models and promote astrocyte survival [279], maintain glucose homeostasis [226], and reduce endothelial cell dysfunction during hyperglycemia [280]. It is believed that part of the benefits of the Mediterranean diet may be a result of mTOR. mTOR can limit Aβ toxicity in astrocytes that may be linked to the onset of AD through Akt activation that is derived from consumption of polyphenol of olives and olive oil [279].
7. Metabolic and Neurodegenerative Disease Mediated by mTOR Pathways
The AMP activated protein kinase (AMPK), a pathway of mTOR, has a central role in metabolic disease, neurodegenerative disorders, infections, and inflammation [11, 101, 106, 188, 281, 282] (Figure 1). AMPK oversees mTORC1 activity through the hamartin (tuberous sclerosis 1)/tuberin (tuberous sclerosis 2) (TSC1/TSC2) complex that inhibits mTORC1 function [101]. Modulation of the TSC1/TSC2 complex also can be overseen though phosphoinositide 3-kinase (PI 3-K), Akt, and its phosphorylation of TSC2. Extracellular signal-regulated kinases (ERKs), protein p90 ribosomal S6 kinase 1 (RSK1), and glycogen synthase kinase −3β (GSK-3β) can control TSC1/TSC2 complex activity as well. TSC2 functions as a GTPase-activating protein (GAP) that changes G protein Rheb (Rheb-GTP) into the inactive GDP-bound form (Rheb-GDP). During Rheb-GTP activation, Rheb-GTP associates with Raptor to oversee the binding of 4EBP1 to mTORC1 and increase mTORC1 activity [283]. AMPK phosphorylates TSC2 to increase GAP activity to change Rheb-GTP into the inactive Rheb-GDP and to limit mTORC1 activity [11].
AMPK is significantly involved in both cellular metabolism and programmed cell death. AMPK modulates mitochondrial homeostasis and insulin resistance [4]. During dietary restriction associated with lifespan increases[284], AMPK can change cellular metabolism to shift to oxidative metabolism that is protective [285]. AMPK can be required for resistance to senescence for mesenchymal stem cells [286] and can increase survival for endothelial progenitor cells during hyperglycemia [287, 288]. Anti-senescence cell activation may be fostered through mTOR inhibition, AMPK activation, and increased autophagic flux [289]. AMPK activation can facilitate clearance of Aß [290] and tau [205] in the brain, lead to memory improvement in models of AD and DM [291], promote pathways for healthy aging [90, 292], reduce Aß neurotoxicity [293], and reduce inflammation in neurodegenerative disorders [45, 64].
AMPK leads to activation of autophagy to oversee cellular function and cellular metabolism [3, 5, 9, 294]. AMPK activity can be necessary to enhance endothelial cell survival during elevated glucose levels [280] and increase basal autophagy activity [150, 295]. AMPK oversees apoptosis and autophagy during oxidative stress cell injury [296, 297] and coronary artery disease [298]. AMPK also functions through growth factor cell protection. EPO enhances neuronal cell function and survival through AMPK activity and increased activity of autophagy [299]. EPO employs AMPK and mTOR activities to increase cell survival under conditions of inflammation [266, 300, 301] and oxidative stress [238]. Importantly, growth factor exposure and concentration with EPO exposure can affect AMPK and mTOR activity to alleviate detrimental effects of oxidative stress [221, 302]. As a result, it appears that a precise balance of mTOR activity is needed since elevated EPO levels can lead to cell loss and limit mTOR activity [303].
In the treatment of DM with metformin and biguanides, AMPK also is critical to reduce disorders such as peripheral neuropathy, demyelinating disease, and cognitive loss [28, 35, 304, 305]. Metformin blocks mTOR activity, promotes autophagy, and may function at times in an AMPK-independent manner [306]. Metformin reduces lipid peroxidation in the spinal cord and brain. These processes can be accompanied by reduced caspase activity to enhance cell survival [307]. AMPK pathways also may accelerate myelin recovery in animal models of multiple sclerosis [308]. Interestingly, metformin has recently been shown to reduce disability in patients with obesity or diabetic patients during coronavirus disease 2019 (COVID-19) [20, 309].
8. Assessing the Underlying Mechanisms for Neurodegenerative Disease Linked to Metabolic Dysfunction
There are a number of underlying mechanisms with disease of the nervous system related to cellular metabolic dysfunction. In regards to AD, the loss of cognition, the existence of metabolic disease, expression of the ε4 allele of the apolipoprotein E (APOE-ε4) gene, as well as the presence of severe acute respiratory syndrome (SARS) -CoV-2 (SARS-CoV-2) can be important clinical targets for these disorders [3, 34, 35, 57, 68, 77, 143, 194, 288, 310–317]. Individuals with the APOE-ε4 gene can have an increased risk of late-onset AD [190, 204, 318–320] (Figure 1). Apolipoprotein E (APOE) is produced in the liver. It is critical for cellular metabolism by overseeing lipid homeostasis and the transport of triglycerides, cholesterol, and phospholipids in the body [78, 321]. In the central nervous system, APOE is formed in astrocytes and facilitates through APOE receptors to transfer cholesterol to neurons [80, 190]. APOE also can help with the removal of Aβ in the brain (Table 1). It is important to recognize though that the isoform APOE-ε4 is not effective in the removal of Aβ which may result in increased risk for the onset of AD [80, 318, 322]. If two ε4 alleles are present in an individual, they can have almost 20 times the risk for acquiring AD. PS membrane exposure [323–326], part of the initial program in apoptotic cell death, can be related to Aβ aggregation. Studies suggest that some APOE isoforms can block the aggregation of Aβ through PS membrane exposure. Unfortunately, this does not occur for the isoform APOE-ε4 [327], but APOE-ε4 can increase mTOR activity [328, 329]. The effect of APOE-ε4 on mTOR and subsequently on autophagy flux has been associated with AD onset and cerebrovascular disease as a result of possible deficits in synaptic plasticity [316].
APOE-ε4 also has other effects related to infectious processes, COVID-19, and memory loss. More than twenty-two viral diseases have been identified to cause increased risk of neurodegenerative disorders, many leading to cognitive loss [330]. APOE-ε4 can foster the susceptibility of viral infection and cerebrovascular disease during COVID-19 [331] that involves the β-coronavirus family virion, SARS-CoV-2 [28, 35, 197, 305, 332, 333]. Coronaviruses are ribonucleic acid (RNA) viruses and are members of the family of Coronaviridae and the subfamily of Orthocoronavirinae [28, 334]. SARS-CoV-2 attaches to host cells, such as in the nasal epithelial region and the brain. Subsequently, the viron results in an increased response of the immune system [335]. Memory loss can follow after infection with SARS-CoV-2 [28, 35, 336]. The impairment in cognitive function can be part of a long-COVID syndrome [198]. Long-COVID, also termed as long-haul COVID, post-acute COVID-19, and chronic COVID, represents long-term effects that can occur following acute SARS-CoV-2 infection. There are a number of mechanisms that can account for long-COVID that involves metabolic pathways, oxidative stress, apoptosis, autophagy, mitochondrial dysfunction, cytokine release [56, 68, 186, 188, 337, 338]. Given the significance of APOE-ε4 in metabolism and autophagy processing, APOE-ε4 recently has been linked to the effects of long-COVID and cognitive loss [68]. Individuals with two ε4 alleles of APOE-ε4 have decreased expression of antiviral defense genes and experience heightened neuroinflammation and microvascular injury in the brain [319]. As a result, APOE-ε4 during SARS-CoV-2 infection and long COVID can lead to memory loss and cerebrovascular disease in the nervous system [19, 45, 71, 188, 339, 340].
PD is a progressive neurodegenerative disorder and is considered the second most common nervous system disease when compared to AD [71, 148, 194, 341]. PD is a movement disorder that leads to resting tremor, rigidity, and bradykinesia. It is characterized by the loss of dopaminergic neurons in the substantia nigra. More than 10 million individuals suffer from PD in the world and PD affects at least 4 percent of individuals over the age of 60 in the world. This number of individuals is expected to double by the year 2030 and presently 50,000 new cases for PD present each year in the US [45, 53, 217, 342]. In addition, at least $52 billion United States (US) dollars are spent in the US alone per year with an annual cost per patient that approaches approximately $25,000 US dollars per year.
There are a number of cellular pathways that can impact the onset and progression of PD [5, 53, 137, 342]. mTOR is one particular pathway [30, 53, 142]. In models with dopaminergic cells, activation of mTOR and p70S6K or the down-regulation of 4EBP1 can offer protection against oxidative stress [343]. Protein kinase B (Akt) pathway activation [165, 190, 193] with mTOR can block methamphetamine neurotoxicity in dopaminergic neurons [344]. PD toxic mimetics have been shown to suppress mTOR and p70S6K activity [345].
Modulation of autophagy and metabolism is another mechanism that can affect PD [134, 150, 151, 194, 346]. Autophagy can remodel cells and tissues through the recycling of cytoplasmic organelles [9, 13, 38, 347, 348]. Autophagy may protect neurons in PD through the maintenance of mitochondrial homeostasis, metabolic pathways [349–351], and even during elevated glucose levels [352]. Flavonoid metabolism in nutrients and diet may have a beneficial effect on PD [85]. However, in some cases, autophagy may be detrimental to dopamine neurons since blockade of autophagy with activation of mTOR can prevent dopaminergic neuronal injury during oxidative stress exposure [353]. Additional work addresses the role of α-synuclein toxicity in PD to support the premise that induction of autophagy degrades and eliminates α-synuclein to protect dopaminergic neurons [241, 354]. Mutation of α-synuclein and accumulation of wild-type α-synuclein in dopaminergic neurons has been tied to the onset and progression of PD [30]. Pathways of the silent mating type information regulation 2 homolog 1 (Saccharomyces cerevisiae) (SIRT1) also may be linked to autophagy in PD to foster neuroprotection [52, 71, 355]. Small non-coding ribonucleic acids (RNAs), termed microRNAs (miRNAs), may be important in PD as well [101, 120, 356–359]. MiRNAs consist of 19–25 nucleotides [19, 101, 120, 199, 360] and can modulate gene expression by silencing targeted messenger RNAs (mRNAs) translated by specific genes [67, 233, 361–363]. miRNAs can work in conjunction with mTOR, autophagy, and SIRT1 to modulate neurodegenerative processes [233, 356, 364].
In addition to AD and PD, cellular metabolic pathways, mTOR, and programmed cell death, such as autophagy, are involved in neurodegenerative disorders that involve Huntington’s disease (HD) (Figure 2) [134, 365, 366]. Metabolic pathways can be altered during HD [142, 367–371]. HD is the result of mutations leading to the polyglutamine tract expansion of CAG in the huntingtin (Htt) gene. mTOR can interact with mHtt and affect the course of HD [372]. Inhibition of mTOR activity can result in the induction of autophagy and the removal of proteins with long polyglutamine or polyalanine expansions [373]. Modulation of autophagy is important in HD to reduce mitochondrial dysfunction and improve motor function [134, 366]. Although mHtt can cellular metabolism signaling in pancreatic cells [374], mHtt can increase the activity of mTORC1 and increased mTORC1 activity can accelerate the onset of the loss of motor coordination and premature death in murine models of HD [375]. Blockade of mTORC1 alone may not be sufficient to alter autophagy or mHtt accumulation. Some studies suggest that combined inhibition of mTORC1 and mTORC2 is required for autophagy and the reduction of mHtt accumulation [376]. Studies in murine models of HD suggest that prevention of motor performance decline may be linked to decreasing the activity of p70S6K that improves muscle function instead of changes in cerebral mHtt accumulation and neuronal protection [377]. Importantly, complete elimination of mTOR activity during HD may not be beneficial requiring careful modulation for robust clinical efficacy. In HD patients and in rodent models of HD, the expression of Rhes, an mTOR activator in the striatum, is reduced [366]. Activation of mTORC1 protects against striatal atrophy, mitochondrial dysfunction, impaired dopamine signaling, and results in the induction of autophagy [366]. Furthermore, flavonoid metabolism may also protect neurons in HD[30, 85].
9. Future Considerations
Both metabolic disease and neurodegenerative disorders are significant healthcare concerns that can affect multiple disorders of the nervous system such as AD. PD, and HD (Table 1). The prevalence of DM is increasing and 700 million individuals are expected to suffer from DM by the year 2045 [6]. In addition, 7 million individuals greater than 18 years of age are undiagnosed with DM. Treatments that target tight monitoring of serum glucose, careful calorie intake assessment, and pharmaceuticals that can modulate glucose homeostasis can aide in controlling metabolic disorders. Yet, they cannot reverse the progression of metabolic dysfunction or reverse risks associated with decrease organ mass, cellular organelle injury, and neuronal cell loss through processes that involve autophagy. Of equal concern is the link of metabolic dysfunction to the onset and progression of neurodegenerative disorders that involve cognitive loss, AD, PD, and HD, all of which have limited treatment options. As a result, innovative treatment strategies are warranted and can include cellular metabolic pathways, apoptosis, autophagy, pyroptosis, mTOR, AMPK, trophic factor signaling with EPO, and the APOE-ε4 gene (Figure 1).
Investigations targeting mTOR as a basis for elucidating new treatments for metabolic dysfunction and neurodegenerative disorders can be indispensable. In conditions such as AD, activation of mTOR can reduce Aß toxicity in the nervous system. Growth factors, such as EPO, can provide cellular protection against neurodegeneration and metabolic instability through the activation of mTOR. Furthermore, mTOR components, such as mLST8, can modulate insulin signaling to maintain glucose homeostasis, mTORC1 can foster lipogenesis and fat storage, and improve glucose homeostasis, and mTORC2 signaling is required for the maintenance of pancreatic β-cell proliferation and mass. AMPK inhibition with active mTOR signaling can be required to limit Aβ toxicity, provide protection of pancreatic islet cells, and block nervous system inflammation. Yet, we also see that autophagy activation with an associated decrease in mTOR activity may be required for neuronal cell protection. Autophagy activation with blocked mTOR activity can lead to enhanced memory and improved insulin signaling that can increase Aß clearance in the nervous system. AMPK activation during mTOR inhibition can lead to memory retention, limit lipid accumulation and obesity, and result in increased cell survival. Furthermore, loss of autophagy may further the onset of cortical and memory dysfunction during metabolic disease and AD. These observations indicate the need for precision in the control of mTOR pathways to achieve a balance for optimal clinical outcome. Recent clinical studies may support this as well since immunotherapies targeted against Aß clearance for AD are successful in eliminating Aß in the brain, but the degree of clinical improvement achieved is at a much lower degree that does not correlate with the significant Aß clearance [133], suggesting that underlying cellular pathways may not be properly balanced. Similar considerations are evident for PD and HD. For example, in HD, decreased activity of p70S6K, an mTOR component, improves muscle function and neuronal protection, but in other scenarios activation of mTORC1 is necessary to prevent striatal atrophy, mitochondrial dysfunction, and impaired dopamine signaling.
The recognition that mTOR pathways, such as AMPK, form an intersection between metabolic and neurodegenerative pathways is vital in the development of future strategies for these disorders. APOE-ε4 is a prominent example of this, since APOE-ε4 oversees lipid homeostasis and the transport of triglycerides, cholesterol, and phospholipids but also is an important risk factor for developing AD. APOE-ε4 impacts mTOR signaling, increases mTOR activity, and affects autophagy flux that increases the risk for AD development. Aβ accumulation in the CNS is a result of the inability of APOE-ε4 to control apoptotic signaling with PS membrane exposure. APOE-ε4 also may promote cognitive loss, long COVID syndrome, and increase the susceptibility of viral infections and brain hemorrhages. As a central player in this scheme for new therapeutic avenues, mTOR may offer a number of innovative strategies to treat metabolic disorders tied to neurodegenerative diseases such as AD, PD, and HD given that the complexity and necessary balance for these pathways are fully comprehended.
Acknowledgments:
This research was supported by the following grants to Kenneth Maiese: American Diabetes Association, American Heart Association, NIH NIEHS, NIH NIA, NIH NINDS, and NIH ARRA.
References
- 1.Organization WH. Global Report on Diabetes. World Health Organization. 2016;Geneva:1–83. [Google Scholar]
- 2.Alves HR, Lomba GSB, Gonçalves-de-Albuquerque CF, Burth P. Irisin, Exercise, and COVID-19. Frontiers in endocrinology. 2022;13:879066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maiese K Cognitive impairment with diabetes mellitus and metabolic disease: innovative insights with the mechanistic target of rapamycin and circadian clock gene pathways. Expert Rev Clin Pharmacol. 2020;13(1):23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maiese K Novel nervous and multi-system regenerative therapeutic strategies for diabetes mellitus with mTOR. Neural regeneration research. 2016;11(3):372–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maiese K Dysregulation of metabolic flexibility: The impact of mTOR on autophagy in neurodegenerative disease. Int Rev Neurobiol. 2020;155:1–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.International Diabetes Federation. Diabetes. IDF Diabetes Atlas. 2019(9th Edition). [PubMed] [Google Scholar]
- 7.Centers for Medicare and Medicaid Services. National Health Expenditure Projections 2018–2027. wwwcmsgov. 2019.
- 8.Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2020. 2020;CS 314227-A:1–30. [Google Scholar]
- 9.Maiese K Nicotinamide as a Foundation for Treating Neurodegenerative Disease and Metabolic Disorders. Curr Neurovasc Res. 2021;18(1):134–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Orkaby AR, Dushkes R, Ward R, Djousse L, Buring JE, Lee IM, et al. Effect of Vitamin D3 and Omega-3 Fatty Acid Supplementation on Risk of Frailty: An Ancillary Study of a Randomized Clinical Trial. JAMA Network Open. 2022;5(9):e2231206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maiese K New Insights for nicotinamide: Metabolic disease, autophagy, and mTOR. Frontiers in bioscience (Landmark edition). 2020;25:1925–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maiese K Heightened Attention for Wnt Signaling in Diabetes Mellitus. Curr Neurovasc Res. 2020;17(3):215–7. [DOI] [PubMed] [Google Scholar]
- 13.Maiese K Prospects and Perspectives for WISP1 (CCN4) in Diabetes Mellitus. Curr Neurovasc Res. 2020;17(3):327–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nie X, Wei X, Ma H, Fan L, Chen WD. The complex role of Wnt ligands in type 2 diabetes mellitus and related complications. J Cell Mol Med. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schell M, Wardelmann K, Kleinridders A. Untangling the effect of insulin action on brain mitochondria and metabolism. J Neuroendocrinol. 2021:e12932. [DOI] [PubMed] [Google Scholar]
- 16.Maiese K SIRT1 and stem cells: In the forefront with cardiovascular disease, neurodegeneration and cancer. World J Stem Cells. 2015;7(2):235–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chong MC, Silva A, James PF, Wu SSX, Howitt J. Exercise increases the release of NAMPT in extracellular vesicles and alters NAD(+) activity in recipient cells. Aging Cell. 2022:e13647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Furtado GE, Letieri RV, Caldo-Silva A, Sardão VA, Teixeira AM, de Barros MP, et al. Sustaining efficient immune functions with regular physical exercise in the COVID-19 era and beyond. Eur J Clin Invest. 2021;51(5):e13485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maiese K Picking a bone with WISP1 (CCN4): new strategies against degenerative joint disease. J Transl Sci. 2016;1(3):83–5. [PMC free article] [PubMed] [Google Scholar]
- 20.Bramante C, Ingraham N, Murray T, Marmor S, Hoversten S, Gronski J, et al. Observational Study of Metformin and Risk of Mortality in Patients Hospitalized with Covid-19. medRxiv. 2020. [Google Scholar]
- 21.Lu M, Chen C, Lan Y, Xiao J, Li R, Huang J, et al. Capsaicin-the major bioactive ingredient of chili peppers: bio-efficacy and delivery systems. Food Funct. 2020. [DOI] [PubMed] [Google Scholar]
- 22.Maiese K Paring down obesity and metabolic disease by targeting inflammation and oxidative stress. Curr Neurovasc Res. 2015;12(2):107–8. [DOI] [PubMed] [Google Scholar]
- 23.Maiese K Erythropoietin and diabetes mellitus. World J Diabetes. 2015;6(14):1259–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Quesada I, de Paola M, Torres-Palazzolo C, Camargo A, Ferder L, Manucha W, et al. Effect of Garlic’s Active Constituents in Inflammation, Obesity and Cardiovascular Disease. Curr Hypertens Rep. 2020;22(1):6. [DOI] [PubMed] [Google Scholar]
- 25.Raut SK, Khullar M. Oxidative stress in metabolic diseases: current scenario and therapeutic relevance. Mol Cell Biochem. 2023;478(1):185–96. [DOI] [PubMed] [Google Scholar]
- 26.Yamashima T, Ota T, Mizukoshi E, Nakamura H, Yamamoto Y, Kikuchi M, et al. Intake of ω-6 Polyunsaturated Fatty Acid-Rich Vegetable Oils and Risk of Lifestyle Diseases. Adv Nutr. 2020;11(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beegum F, P VA, George KT, K PD, Begum F, Krishnadas N, et al. Sirtuins as therapeutic targets for improving delayed wound healing in diabetes. J Drug Target. 2022:1–50. [DOI] [PubMed] [Google Scholar]
- 28.Maiese K Nicotinamide: Oversight of Metabolic Dysfunction through SIRT1, mTOR, and Clock Genes. Curr Neurovasc Res. 2020;17(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fischer F, Grigolon G, Benner C, Ristow M. Evolutionarily conserved transcription factors as regulators of longevity and targets for geroprotection. Physiol Rev. 2022;102(3):1449–94. [DOI] [PubMed] [Google Scholar]
- 30.Maiese K, Chong ZZ, Wang S, Shang YC. Oxidant Stress and Signal Transduction in the Nervous System with the PI 3-K, Akt, and mTOR Cascade. International journal of molecular sciences. 2013;13(11):13830–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rotllan N, Camacho M, Tondo M, Diarte-Añazco EMG, Canyelles M, Méndez-Lara KA, et al. Therapeutic Potential of Emerging NAD+-Increasing Strategies for Cardiovascular Diseases. Antioxidants (Basel, Switzerland). 2021;10(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O’Donnell BT, Monjure TA, Al-Ghadban S, Ives CJ, L’Ecuyer MP, Rhee C, et al. Aberrant Expression of COX-2 and FOXG1 in Infrapatellar Fat Pad-Derived ASCs from Pre-Diabetic Donors. Cells. 2022;11(15). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun ZY, Yu TY, Jiang FX, Wang W. Functional maturation of immature β cells: A roadblock for stem cell therapy for type 1 diabetes. World J Stem Cells. 2021;13(3):193–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu P, Wu Z, Peng Y, Gao J, Zheng F, Tan J, et al. Neuroprotection of Triptolide against Amyloid-Beta1–42-induced toxicity via the Akt/mTOR/p70S6K-mediated Autophagy Pathway. An Acad Bras Cienc. 2022;94(2):e20210938. [DOI] [PubMed] [Google Scholar]
- 35.Maiese K The Mechanistic Target of Rapamycin (mTOR): Novel Considerations as an Antiviral Treatment. Curr Neurovasc Res. 2020;17(3):332–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pinchera B, Scotto R, Buonomo AR, Zappulo E, Stagnaro F, Gallicchio A, et al. Diabetes and COVID-19: The potential role of mTOR. Diabetes Res Clin Pract. 2022;186:109813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Swain O, Romano SK, Miryala R, Tsai J, Parikh V, Umanah GKE. SARS-CoV-2 Neuronal Invasion and Complications: Potential Mechanisms and Therapeutic Approaches. J Neurosci. 2021;41(25):5338–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maiese K Targeting the core of neurodegeneration: FoxO, mTOR, and SIRT1. Neural regeneration research. 2021;16(3):448–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.World Health Organization. Description of the global burden of NCDs, their risk factors and determinants. Global status report on noncommunicable diseases 2010. 2011(April):1–176. [Google Scholar]
- 40.World Health Organization. Global action plan on the public health response to dementia 2017–2025. 2017:1–44. [Google Scholar]
- 41.Maiese K Sirtuins: Developing Innovative Treatments for Aged-Related Memory Loss and Alzheimer’s Disease. Curr Neurovasc Res. 2018;15(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jalgaonkar MP, Parmar UM, Kulkarni YA, Oza MJ. SIRT1-FOXOs activity regulates diabetic complications. Pharmacol Res. 2022;175:106014. [DOI] [PubMed] [Google Scholar]
- 43.Maiese K Cutting through the Complexities of mTOR for the Treatment of Stroke. Curr Neurovasc Res. 2014;11(2):177–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Minino AM. Death in the United States, 2011. NCHS data brief. 2013(115):1–8. [PubMed] [Google Scholar]
- 45.Maiese K Moving to the Rhythm with Clock (Circadian) Genes, Autophagy, mTOR, and SIRT1 in Degenerative Disease and Cancer. Curr Neurovasc Res. 2017;14(3):299–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen YL, Hsieh CC, Chu PM, Chen JY, Huang YC, Chen CY. Roles of protein tyrosine phosphatases in hepatocellular carcinoma progression (Review). Oncology reports. 2023;49(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jiang W, Ding K, Yue R, Lei M. Therapeutic effects of icariin and icariside II on diabetes mellitus and its complications. Critical reviews in food science and nutrition. 2023:1–26. [DOI] [PubMed] [Google Scholar]
- 48.Li JB, Hu XY, Chen MW, Xiong CH, Zhao N, Ge YH, et al. p85S6K sustains synaptic GluA1 to ameliorate cognitive deficits in Alzheimer’s disease. Translational neurodegeneration. 2023;12(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kahmini FR, Ghaleh HD, Shahgaldi S. Sirtuins: Subtle Regulators Involved in Convoluted Mechanisms of Pregnancy. Cell Physiol Biochem. 2022;56(6):644–62. [DOI] [PubMed] [Google Scholar]
- 50.Liu D, Zhang M, Tian J, Gao M, Liu M, Fu X, et al. WNT1-inducible signalling pathway protein 1 stabilizes atherosclerotic plaques in apolipoprotein-E-deficient mice via the focal adhesion kinase/mitogen-activated extracellular signal-regulated kinase/extracellular signal-regulated kinase pathway. Journal of hypertension. 2022;40(9):1666–81. [DOI] [PubMed] [Google Scholar]
- 51.Maiese K Wnt Signaling and WISP1 (CCN4): Critical Components in Neurovascular Disease, Blood Brain Barrier Regulation, and Cerebral Hemorrhage. Curr Neurovasc Res. 2022. [DOI] [PubMed] [Google Scholar]
- 52.Maiese K Cognitive Impairment and Dementia: Gaining Insight through Circadian Clock Gene Pathways. Biomolecules. 2021;11(7):1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maiese K Neurodegeneration, memory loss, and dementia: the impact of biological clocks and circadian rhythm. Frontiers in bioscience (Landmark edition). 2021;26(9):614–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Patocka J, Kuca K, Oleksak P, Nepovimova E, Valis M, Novotny M, et al. Rapamycin: Drug Repurposing in SARS-CoV-2 Infection. Pharmaceuticals (Basel, Switzerland). 2021;14(217). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sorrells SF, Paredes MF, Zhang Z, Kang G, Pastor-Alonso O, Biagiotti S, et al. Positive Controls in Adults and Children Support That Very Few, If Any, New Neurons Are Born in the Adult Human Hippocampus. J Neurosci. 2021;41(12):2554–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Amanollahi M, Jameie M, Heidari A, Rezaei N. The Dialogue Between Neuroinflammation and Adult Neurogenesis: Mechanisms Involved and Alterations in Neurological Diseases. Mol Neurobiol. 2022. [DOI] [PubMed] [Google Scholar]
- 57.Mishra P, Davies DA, Albensi BC. The Interaction Between NF-κB and Estrogen in Alzheimer’s Disease. Mol Neurobiol. 2022. [DOI] [PubMed] [Google Scholar]
- 58.Salemi M, Mogavero MP, Lanza G, Mongioì LM, Calogero AE, Ferri R. Examples of Inverse Comorbidity between Cancer and Neurodegenerative Diseases: A Possible Role for Noncoding RNA. Cells. 2022;11(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Savu DI, Moisoi N. Mitochondria - Nucleus communication in neurodegenerative disease. Who talks first, who talks louder? Biochim Biophys Acta Bioenerg. 2022;1863(7):148588. [DOI] [PubMed] [Google Scholar]
- 60.Yalçin M, Mundorf A, Thiel F, Amatriain-Fernández S, Kalthoff IS, Beucke JC, et al. It’s About Time: The Circadian Network as Time-Keeper for Cognitive Functioning, Locomotor Activity and Mental Health. Front Physiol. 2022;13:873237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Maiese K Novel Treatment Strategies for Neurodegenerative Disease with Sirtuins. In: Sirtuin Biology in Medicine: Targeting New Avenues of Care in Development, Aging, and Disease. 2021;Academic Press, Elsevier, ISBN; 9780128224670. [Google Scholar]
- 62.Maiese K Biomarkers for Parkinson’s Disease and Neurodegenerative Disorders: A Role for Non-coding RNAs. Curr Neurovasc Res. 2022;19(2):127–30. [DOI] [PubMed] [Google Scholar]
- 63.Tang B, Zeng W, Song LL, Wang HM, Qu LQ, Lo HH, et al. Extracellular Vesicle Delivery of Neferine for the Attenuation of Neurodegenerative Disease Proteins and Motor Deficit in an Alzheimer’s Disease Mouse Model. Pharmaceuticals (Basel, Switzerland). 2022;15(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Maiese K Targeting molecules to medicine with mTOR, autophagy and neurodegenerative disorders. Br J Clin Pharmacol. 2016;82(5):1245–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.World Health Organization. Dementia: A public health priority. Geneva: World Health Organization. 2012:1–4. [Google Scholar]
- 66.Maiese K MicroRNAs for the Treatment of Dementia and Alzheimer’s Disease. Curr Neurovasc Res. 2019;16(1):1–2. [DOI] [PubMed] [Google Scholar]
- 67.Maiese K Impacting dementia and cognitive loss with innovative strategies: mechanistic target of rapamycin, clock genes, circular non-coding ribonucleic acids, and Rho/Rock. Neural regeneration research. 2019;14(5):773–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Maiese K Apolipoprotein-ε4 allele (APOE-ε4) as a Mediator of Cognitive Loss and Dementia in Long COVID-19. Curr Neurovasc Res. 2022. [DOI] [PubMed] [Google Scholar]
- 69.Ullah H, Hussain A, Asif M, Nawaz F, Rasool M. Natural products as bioactive agents in the prevention of dementia. CNS Neurol Disord Drug Targets. 2022. [DOI] [PubMed] [Google Scholar]
- 70.Zhu G, Tong Q, Ye X, Li J, Zhou L, Sun P, et al. Phototherapy for Cognitive Function in Patients With Dementia: A Systematic Review and Meta-Analysis. Frontiers in aging neuroscience. 2022;14:936489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Maiese K The mechanistic target of rapamycin (mTOR) and the silent mating-type information regulation 2 homolog 1 (SIRT1): oversight for neurodegenerative disorders. Biochem Soc Trans. 2018;46(2):351–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ding MR, Qu YJ, Hu B, An HM. Signal pathways in the treatment of Alzheimer’s disease with traditional Chinese medicine. Biomed Pharmacother. 2022;152:113208. [DOI] [PubMed] [Google Scholar]
- 73.Rapaka D, Bitra VR, Challa SR, Adiukwu PC. mTOR signaling as a molecular target for the alleviation of Alzheimer’s disease pathogenesis. Neurochem Int. 2022;155:105311. [DOI] [PubMed] [Google Scholar]
- 74.Jayaraman A, Reynolds R. Diverse pathways to neuronal necroptosis in Alzheimer’s disease. Eur J Neurosci. 2022. [DOI] [PubMed] [Google Scholar]
- 75.Mavroidi B, Kaminari A, Matiadis D, Hadjipavlou-Litina D, Pelecanou M, Tzinia A, et al. The Prophylactic and Multimodal Activity of Two Isatin Thiosemicarbazones against Alzheimer’s Disease In Vitro. Brain sciences. 2022;12(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Maiese K Addressing Alzheimer’s Disease and Cognitive Loss through Autophagy. Curr Neurovasc Res. 2020;17(4):339–41. [DOI] [PubMed] [Google Scholar]
- 77.Maiese K Cellular Metabolism: A Fundamental Component of Degeneration in the Nervous System. Biomolecules. 2023;13(5):816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hu Z, Jiao R, Wang P, Zhu Y, Zhao J, De Jager P, et al. Shared Causal Paths underlying Alzheimer’s dementia and Type 2 Diabetes. Scientific reports. 2020;10(1):4107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Min AY, Yoo JM, Sok DE, Kim MR. Mulberry Fruit Prevents Diabetes and Diabetic Dementia by Regulation of Blood Glucose through Upregulation of Antioxidative Activities and CREB/BDNF Pathway in Alloxan-Induced Diabetic Mice. Oxid Med Cell Longev. 2020;2020:1298691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Caberlotto L, Nguyen TP, Lauria M, Priami C, Rimondini R, Maioli S, et al. Cross-disease analysis of Alzheimer’s disease and type-2 Diabetes highlights the role of autophagy in the pathophysiology of two highly comorbid diseases. Scientific reports. 2019;9(1):3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Su M, Naderi K, Samson N, Youssef I, Fulop L, Bozso Z, et al. Mechanisms Associated with Type 2 Diabetes as a Risk Factor for Alzheimer-Related Pathology. Mol Neurobiol. 2019;56(8):5815–34. [DOI] [PubMed] [Google Scholar]
- 82.Jiang WJ, Peng YC, Yang KM. Cellular signaling pathways regulating beta-cell proliferation as a promising therapeutic target in the treatment of diabetes. Experimental and therapeutic medicine. 2018;16(4):3275–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Maiese K New Insights for Oxidative Stress and Diabetes Mellitus. Oxid Med Cell Longev. 2015;2015(2015:875961). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang R, Zhu Y, Qin LF, Xu ZG, Gao XR, Liu CB, et al. Comprehensive Bibliometric Analysis of Stem Cell Research in Alzheimer’s Disease from 2004 to 2022. Dement Geriatr Cogn Disord. 2023:1–27. [DOI] [PubMed] [Google Scholar]
- 85.Khan H, Tundis R, Ullah H, Aschner M, Belwal T, Mirzaei H, et al. Flavonoids targeting NRF2 in neurodegenerative disorders. Food Chem Toxicol. 2020;146:111817. [DOI] [PubMed] [Google Scholar]
- 86.Huang C, Zhang C, Cao Y, Li J, Bi F. Major roles of the circadian clock in cancer. Cancer Biol Med. 2023;20(1):1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kalam F, James DL, Li YR, Coleman MF, Kiesel VA, Cespedes Feliciano EM, et al. Intermittent fasting interventions to leverage metabolic and circadian mechanisms for cancer treatment and supportive care outcomes. J Natl Cancer Inst Monogr. 2023;2023(61):84–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mocayar Marón FJ, Ferder L, Reiter RJ, Manucha W. Daily and seasonal mitochondrial protection: Unraveling common possible mechanisms involving vitamin D and melatonin. J Steroid Biochem Mol Biol. 2020;199:105595. [DOI] [PubMed] [Google Scholar]
- 89.Wang X, Xu Z, Cai Y, Zeng S, Peng B, Ren X, et al. Rheostatic Balance of Circadian Rhythm and Autophagy in Metabolism and Disease. Front Cell Dev Biol. 2020;24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Amidfar M, Garcez ML, Kim YK. The shared molecular mechanisms underlying aging of the brain, major depressive disorder, and Alzheimer’s disease: The role of circadian rhythm disturbances. Prog Neuropsychopharmacol Biol Psychiatry. 2023;123:110721. [DOI] [PubMed] [Google Scholar]
- 91.Lathe R, St Clair D. Programmed ageing: decline of stem cell renewal, immunosenescence, and Alzheimer’s disease. Biological reviews of the Cambridge Philosophical Society. 2023. [DOI] [PubMed] [Google Scholar]
- 92.Olejniczak I, Pilorz V, Oster H. Circle(s) of Life: The Circadian Clock from Birth to Death. Biology (Basel). 2023;12(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hsieh CF, Liu CK, Lee CT, Yu LE, Wang JY. Acute glucose fluctuation impacts microglial activity, leading to inflammatory activation or self-degradation. Scientific reports. 2019;9(1):840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Li F, Chong ZZ, Maiese K. Cell Life Versus Cell Longevity: The Mysteries Surrounding the NAD(+) Precursor Nicotinamide. Curr Med Chem. 2006;13(8):883–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tomita Y, Lee D, Tsubota K, Kurihara T. PPARα Agonist Oral Therapy in Diabetic Retinopathy. Biomedicines. 2020;8(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yang X, Huo F, Liu B, Liu J, Chen T, Li J, et al. Crocin Inhibits Oxidative Stress and Pro-inflammatory Response of Microglial Cells Associated with Diabetic Retinopathy Through the Activation of PI3K/Akt Signaling Pathway. J Mol Neurosci. 2017. [DOI] [PubMed] [Google Scholar]
- 97.Chong ZZ, Li F, Maiese K. The pro-survival pathways of mTOR and protein kinase B target glycogen synthase kinase-3beta and nuclear factor-kappaB to foster endogenous microglial cell protection. Int J Mol Med. 2007;19(2):263–72. [PMC free article] [PubMed] [Google Scholar]
- 98.Li F, Chong ZZ, Maiese K. Microglial integrity is maintained by erythropoietin through integration of Akt and its substrates of glycogen synthase kinase-3beta, beta-catenin, and nuclear factor-kappaB. Curr Neurovasc Res. 2006;3(3):187–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Shang YC, Chong ZZ, Hou J, Maiese K. FoxO3a governs early microglial proliferation and employs mitochondrial depolarization with caspase 3, 8, and 9 cleavage during oxidant induced apoptosis. Curr Neurovasc Res. 2009;6(4):223–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kostić M, Korićanac G, Tepavčević S, Stanišić J, Romić S, Ćulafić T, et al. Low-Intensity Exercise Affects Cardiac Fatty Acid Oxidation by Increasing the Nuclear Content of PPARα, FOXO1, and Lipin1 in Fructose-Fed Rats. Metab Syndr Relat Disord. 2023. [DOI] [PubMed] [Google Scholar]
- 101.Maiese K Harnessing the Power of SIRT1 and Non-coding RNAs in Vascular Disease. Curr Neurovasc Res. 2017;14(1):82–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Maiese K Sirtuin Biology in Cancer and Metabolic Disease: Cellular Pathways for Clinical Discovery. Academic Press, Elsevier. 2021;ISBN 9780128224670. [Google Scholar]
- 103.Maiese K Sirtuins in Metabolic Disease: Innovative Therapeutic Strategies with SIRT1, AMPK, mTOR, and Nicotinamide. In: Sirtuin Biology in Cancer and Metabolic Disease: Cellular Pathways for Clinical Discovery, ed Maiese K. 2021;Academic Press, Elsevier, ISBN 9780128141182. [Google Scholar]
- 104.Ministrini S, Puspitasari YM, Beer G, Liberale L, Montecucco F, Camici GG. Sirtuin 1 in Endothelial Dysfunction and Cardiovascular Aging. Front Physiol. 2021;12:733696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Penteado AB, Hassanie H, Gomes RA, Silva Emery FD, Goulart Trossini GH. Human sirtuin 2 inhibitors, their mechanisms and binding modes. Future Med Chem. 2023. [DOI] [PubMed] [Google Scholar]
- 106.Sadria M, Seo D, Layton AT. The mixed blessing of AMPK signaling in Cancer treatments. BMC Cancer. 2022;22(1):105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wasserfurth P, Nebl J, Rühling MR, Shammas H, Bednarczyk J, Koehler K, et al. Impact of Dietary Modifications on Plasma Sirtuins 1, 3 and 5 in Older Overweight Individuals Undergoing 12-Weeks of Circuit Training. Nutrients. 2021;13(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Watroba M, Szukiewicz D. Sirtuins at the Service of Healthy Longevity. Front Physiol. 2021;12:724506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sun C, Bai S, Liang Y, Liu D, Liao J, Chen Y, et al. The role of Sirtuin 1 and its activators in age-related lung disease. Biomed Pharmacother. 2023;162:114573. [DOI] [PubMed] [Google Scholar]
- 110.Atef MM, El-Sayed NM, Ahmed AAM, Mostafa YM. Donepezil improves neuropathy through activation of AMPK signalling pathway in streptozotocin-induced diabetic mice. Biochem Pharmacol. 2019;159:1–10. [DOI] [PubMed] [Google Scholar]
- 111.Gomes MB, Negrato CA. Alpha-lipoic acid as a pleiotropic compound with potential therapeutic use in diabetes and other chronic diseases. Diabetology & metabolic syndrome. 2014;6(1):80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Maiese K Peripheral Neuropathy: An Early Indication for Systemic Disease that Involves the Mechanistic Target of Rapamycin (mTOR). Curr Neurovasc Res. 2023. [DOI] [PubMed] [Google Scholar]
- 113.Albiero M, Poncina N, Tjwa M, Ciciliot S, Menegazzo L, Ceolotto G, et al. Diabetes causes bone marrow autonomic neuropathy and impairs stem cell mobilization via dysregulated p66Shc and Sirt1. Diabetes. 2014;63(4):1353–65. [DOI] [PubMed] [Google Scholar]
- 114.Maiese K FoxO Transcription Factors and Regenerative Pathways in Diabetes Mellitus. Curr Neurovasc Res. 2015;12(4):404–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bayaraa O, Inman CK, Thomas SA, Al Jallaf F, Alshaikh M, Idaghdour Y, et al. Hyperglycemic conditions induce rapid cell dysfunction-promoting transcriptional alterations in human aortic endothelial cells. Scientific reports. 2022;12(1):20912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Maiese K mTOR: Driving apoptosis and autophagy for neurocardiac complications of diabetes mellitus. World J Diabetes. 2015;6(2):217–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hajibabaie F, Abedpoor N, Safavi K, Taghian F. Natural remedies medicine derived from flaxseed (secoisolariciresinol diglucoside, lignans, and α-linolenic acid) improve network targeting efficiency of diabetic heart conditions based on computational chemistry techniques and pharmacophore modeling. J Food Biochem. 2022:e14480. [DOI] [PubMed] [Google Scholar]
- 118.Januszewski AS, Watson CJ, O’Neill V, McDonald K, Ledwidge M, Robson T, et al. FKBPL is associated with metabolic parameters and is a novel determinant of cardiovascular disease. Scientific reports. 2020;10(1):21655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Liu P, Liu J, Wu Y, Xi W, Wei Y, Yuan Z, et al. Zinc supplementation protects against diabetic endothelial dysfunction via GTP cyclohydrolase 1 restoration. Biochem Biophys Res Commun. 2020;521(4):1049–54. [DOI] [PubMed] [Google Scholar]
- 120.Maiese K Disease onset and aging in the world of circular RNAs. J Transl Sci. 2016;2(6):327–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Pabel S, Hamdani N, Luedde M, Sossalla S. SGLT2 Inhibitors and Their Mode of Action in Heart Failure-Has the Mystery Been Unravelled? Curr Heart Fail Rep. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zaiou M circRNAs Signature as Potential Diagnostic and Prognostic Biomarker for Diabetes Mellitus and Related Cardiovascular Complications. Cells. 2020;9(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zarneshan SN, Fakhri S, Farzaei MH, Khan H, Saso L. Astaxanthin targets PI3K/Akt signaling pathway toward potential therapeutic applications. Food Chem Toxicol. 2020;145:111714. [DOI] [PubMed] [Google Scholar]
- 124.Zhou Q, Tang S, Zhang X, Chen L. Targeting PRAS40: a novel therapeutic strategy for human diseases. J Drug Target. 2021:1–44. [DOI] [PubMed] [Google Scholar]
- 125.Chiareli RA, Carvalho GA, Marques BL, Mota LS, Oliveira-Lima OC, Gomes RM, et al. The Role of Astrocytes in the Neurorepair Process. Front Cell Dev Biol. 2021;9:665795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Engin AB, Engin A. Alzheimer’s Disease and Protein Kinases. Adv Exp Med Biol. 2021;1275:285–321. [DOI] [PubMed] [Google Scholar]
- 127.Xu T, Liu J, Li XR, Yu Y, Luo X, Zheng X, et al. The mTOR/NF-κB Pathway Mediates Neuroinflammation and Synaptic Plasticity in Diabetic Encephalopathy. Mol Neurobiol. 2021. [DOI] [PubMed] [Google Scholar]
- 128.El-Beltagy A, Saleh AMB, Attaallah A, Gahnem RA. Therapeutic role of Azadirachta indica leaves ethanolic extract against diabetic nephropathy in rats neonatally induced by streptozotocin. Ultrastruct Pathol. 2021:1–16. [DOI] [PubMed] [Google Scholar]
- 129.Kita A, Saito Y, Miura N, Miyajima M, Yamamoto S, Sato T, et al. Altered regulation of mesenchymal cell senescence in adipose tissue promotes pathological changes associated with diabetic wound healing. Commun Biol. 2022;5(1):310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Maiese K, Chong ZZ, Shang YC. OutFOXOing disease and disability: the therapeutic potential of targeting FoxO proteins. Trends Mol Med. 2008;14(5):219–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gong Q, Wang H, Yu P, Qian T, Xu X. Protective or Harmful: The Dual Roles of Autophagy in Diabetic Retinopathy. Front Med (Lausanne). 2021;8:644121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Li J, Lin FH, Zhu XM, Lv ZM. Impact of diabetic hyperglycaemia and insulin therapy on autophagy and impairment in rat epididymis. Andrologia. 2020;52(11):e13889. [DOI] [PubMed] [Google Scholar]
- 133.van Dyck CH, Swanson CJ, Aisen P, Bateman RJ, Chen C, Gee M, et al. Lecanemab in Early Alzheimer’s Disease. N Engl J Med. 2023;388(1):9–21. [DOI] [PubMed] [Google Scholar]
- 134.Maiese K FoxO Proteins in the Nervous System. Anal Cell Pathol (Amst). 2015;2015:569392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Maiese K Forkhead Transcription Factors: Formulating a FOXO Target for Cognitive Loss. Curr Neurovasc Res. 2017;14(4):415–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sharma VK, Singh TG, Singh S, Garg N, Dhiman S. Apoptotic Pathways and Alzheimer’s Disease: Probing Therapeutic Potential. Neurochem Res. 2021. [DOI] [PubMed] [Google Scholar]
- 137.Wang H, Yang F, Zhang S, Xin R, Sun Y. Genetic and environmental factors in Alzheimer’s and Parkinson’s diseases and promising therapeutic intervention via fecal microbiota transplantation. NPJ Parkinsons Dis. 2021;7(1):70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ghiasi R, Naderi R, Sheervalilou R, Alipour MR. Swimming training by affecting the pancreatic Sirtuin1 (SIRT1) and oxidative stress, improves insulin sensitivity in diabetic male rats. Hormone molecular biology and clinical investigation. 2019;40(3). [DOI] [PubMed] [Google Scholar]
- 139.Maiese K Triple play: Promoting neurovascular longevity with nicotinamide, WNT, and erythropoietin in diabetes mellitus. Biomed Pharmacother. 2008;62(4):218–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Maiese K New Directions for Dementia. Curr Neurovasc Res. 2017;14(4):305. [DOI] [PubMed] [Google Scholar]
- 141.Maiese K, Chong ZZ. Insights into oxidative stress and potential novel therapeutic targets for Alzheimer disease. Restor Neurol Neurosci. 2004;22(2):87–104. [PubMed] [Google Scholar]
- 142.Querfurth H, Lee HK. Mammalian/mechanistic target of rapamycin (mTOR) complexes in neurodegeneration. Molecular neurodegeneration. 2021;16(1):44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zhou Y, Xu J, Hou Y, Leverenz JB, Kallianpur A, Mehra R, et al. Network medicine links SARS-CoV-2/COVID-19 infection to brain microvascular injury and neuroinflammation in dementia-like cognitive impairment. Alzheimers Res Ther. 2021;13(1):110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.González-Fernández C, González P, González-Pérez F, Rodríguez F. Characterization of Ex Vivo and In Vitro Wnt Transcriptome Induced by Spinal Cord Injury in Rat Microglial Cells. Brain sciences. 2022;12(708). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Maiese K Inflammatory glial cells of the nervous system: assistants or assassins? Curr Neurovasc Res. 2005;2(3):187–8. [DOI] [PubMed] [Google Scholar]
- 146.Maiese K, Li F, Chong ZZ. Erythropoietin in the brain: can the promise to protect be fulfilled? Trends Pharmacol Sci. 2004;25(11):577–83. [DOI] [PubMed] [Google Scholar]
- 147.Jarero-Basulto J, Rivera-Cervantes M, Gasca-Martínez D, García-Sierra F, Gasca-Martínez Y, Beas-Zárate C. Current Evidence on the Protective Effects of Recombinant Human Erythropoietin and Its Molecular Variants against Pathological Hallmarks of Alzheimer’s Disease. Pharmaceuticals (Basel, Switzerland). 2020;13(424):1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kaur D, Behl T, Sehgal A, Singh S, Sharma N, Badavath VN, et al. Unravelling the potential neuroprotective facets of erythropoietin for the treatment of Alzheimer’s disease. Metab Brain Dis. 2021. [DOI] [PubMed] [Google Scholar]
- 149.Liu L, Cao Q, Gao W, Li BY, Zeng C, Xia Z, et al. Melatonin ameliorates cerebral ischemia-reperfusion injury in diabetic mice by enhancing autophagy via the SIRT1-BMAL1 pathway. Faseb j. 2021;35(12):e22040. [DOI] [PubMed] [Google Scholar]
- 150.Klionsky DJ, Abdel-Aziz AK, Abdelfatah S, Abdellatif M, Abdoli A, Abel S, et al. Guidelines for the use and interpretation of assays for monitoring autophagy (4th edition). Autophagy. 2021:1–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Maiese K, Chong ZZ, Shang YC, Wang S. Targeting disease through novel pathways of apoptosis and autophagy. Expert opinion on therapeutic targets. 2012;16(12):1203–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Gao J, Yao M, Chang D, Liu J. mTOR (Mammalian Target of Rapamycin): Hitting the Bull’s Eye for Enhancing Neurogenesis After Cerebral Ischemia? Stroke. 2022. [DOI] [PubMed] [Google Scholar]
- 153.He C, Xu Y, Sun J, Li L, Zhang JH, Wang Y. Autophagy and Apoptosis in Acute CNS injuries: from Mechanism to Treatment. Antioxid Redox Signal. 2022. [DOI] [PubMed] [Google Scholar]
- 154.Qin C, Lu Y, Bai L, Wang K. The molecular regulation of autophagy in antimicrobial immunity. J Mol Cell Biol. 2022;14(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Senousy MA, Hanafy ME, Shehata N, Rizk SM. Erythropoietin and Bacillus Calmette-Guérin Vaccination Mitigate 3-Nitropropionic Acid-Induced Huntington-like Disease in Rats by Modulating the PI3K/Akt/mTOR/P70S6K Pathway and Enhancing the Autophagy. ACS chemical neuroscience. 2022. [DOI] [PubMed] [Google Scholar]
- 156.Yan WT, Lu S, Yang YD, Ning WY, Cai Y, Hu XM, et al. Research trends, hot spots and prospects for necroptosis in the field of neuroscience. Neural regeneration research. 2021;16(8):1628–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Hou J, Chong ZZ, Shang YC, Maiese K. Early apoptotic vascular signaling is determined by Sirt1 through nuclear shuttling, forkhead trafficking, bad, and mitochondrial caspase activation. Curr Neurovasc Res. 2010;7(2):95–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Shang YC, Chong ZZ, Hou J, Maiese K. Wnt1, FoxO3a, and NF-kappaB oversee microglial integrity and activation during oxidant stress. Cell Signal. 2010;22(9):1317–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Taveira GB, Mello EO, Souza SB, Monteiro RM, Ramos AC, Carvalho AO, et al. Programmed cell death in yeast by thionin-like peptide from Capsicum annuum fruits involving activation of capases and extracelullar H(+) flux. Bioscience reports. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Almasieh M, Catrinescu MM, Binan L, Costantino S, Levin LA. Axonal Degeneration in Retinal Ganglion Cells is Associated with a Membrane Polarity-Sensitive Redox Process. J Neurosci. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Viola G, Bortolozzi R, Hamel E, Moro S, Brun P, Castagliuolo I, et al. MG-2477, a new tubulin inhibitor, induces autophagy through inhibition of the Akt/mTOR pathway and delayed apoptosis in A549 cells. Biochem Pharmacol. 2012;83(1):16–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Chong ZZ, Kang JQ, Maiese K. Erythropoietin is a novel vascular protectant through activation of Akt1 and mitochondrial modulation of cysteine proteases. Circulation. 2002;106(23):2973–9. [DOI] [PubMed] [Google Scholar]
- 163.Maiese K The bright side of reactive oxygen species: lifespan extension without cellular demise. J Transl Sci. 2016;2(3):185–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Yousafzai NA, Jin H, Ullah M, Wang X. Recent advances of SIRT1 and implications in chemotherapeutics resistance in cancer. Am J Cancer Res. 2021;11(11):5233–48. [PMC free article] [PubMed] [Google Scholar]
- 165.Maiese K A Common Link in Neurovascular Regenerative Pathways: Protein Kinase B (Akt). Curr Neurovasc Res. 2022. [DOI] [PubMed] [Google Scholar]
- 166.Pyroptosis Maiese K., Apoptosis, and Autophagy: Critical Players of Inflammation and Cell Demise in the Nervous System. Curr Neurovasc Res. 2022. [DOI] [PubMed] [Google Scholar]
- 167.Pang Y, Qin M, Hu P, Ji K, Xiao R, Sun N, et al. Resveratrol protects retinal ganglion cells against ischemia induced damage by increasing Opa1 expression. Int J Mol Med. 2020;46(5):1707–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Tran HN, Nguyen QH, Jeong JE, Loi DL, Nam YH, Kang TH, et al. The embryonic patterning gene Dbx1 governs the survival of the auditory midbrain via Tcf7l2-Ap2δ transcriptional cascade. Cell Death Differ. 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Bailey TJ, Fossum SL, Fimbel SM, Montgomery JE, Hyde DR. The inhibitor of phagocytosis, O-phospho-L-serine, suppresses Muller glia proliferation and cone cell regeneration in the light-damaged zebrafish retina. Exp Eye Res. 2010;91(5):601–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Wei L, Sun C, Lei M, Li G, Yi L, Luo F, et al. Activation of Wnt/beta-catenin Pathway by Exogenous Wnt1 Protects SH-SY5Y Cells Against 6-Hydroxydopamine Toxicity. J Mol Neurosci. 2013;49(1):105–15. [DOI] [PubMed] [Google Scholar]
- 171.Kim S, Kang IH, Nam JB, Cho Y, Chung DY, Kim SH, et al. Ameliorating the Effect of Astragaloside IV on Learning and Memory Deficit after Chronic Cerebral Hypoperfusion in Rats. Molecules. 2015;20(2):1904–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Maiese K Novel applications of trophic factors, Wnt and WISP for neuronal repair and regeneration in metabolic disease. Neural regeneration research. 2015;10(4):518–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Xin YJ, Yuan B, Yu B, Wang YQ, Wu JJ, Zhou WH, et al. Tet1-mediated DNA demethylation regulates neuronal cell death induced by oxidative stress. Scientific reports. 2015;5:7645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Yu T, Li L, Chen T, Liu Z, Liu H, Li Z. Erythropoietin attenuates advanced glycation endproducts-induced toxicity of schwann cells in vitro. Neurochem Res. 2015;40(4):698–712. [DOI] [PubMed] [Google Scholar]
- 175.Lan T, Xu Y, Li S, Li N, Zhang S, Zhu H. Cornin protects against cerebral ischemia/reperfusion injury by preventing autophagy via the PI3K/Akt/mTOR pathway. BMC Pharmacol Toxicol. 2022;23(1):82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Liu L, Xu S, Li P, Li L. A novel adipokine WISP1 attenuates lipopolysaccharide-induced cell injury in 3T3-L1 adipocytes by regulating the PI3K/Akt pathway. Obes Res Clin Pract. 2022;16(2):122–9. [DOI] [PubMed] [Google Scholar]
- 177.Mansour RM, El Sayed NS, Ahmed MAE, El-Sahar AE. Addressing Peroxisome Proliferator-Activated Receptor-gamma in 3-Nitropropionic Acid-Induced Striatal Neurotoxicity in Rats. Mol Neurobiol. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Sabzali M, Eidi A, Khaksari M, Khastar H. Anti-inflammatory, Antioxidant, and Antiapoptotic Action of Metformin Attenuates Ethanol Neurotoxicity in the Animal Model of Fetal Alcohol Spectrum Disorders. Neurotox Res. 2022. [DOI] [PubMed] [Google Scholar]
- 179.Chong ZZ, Li F, Maiese K. Oxidative stress in the brain: Novel cellular targets that govern survival during neurodegenerative disease. Prog Neurobiol. 2005;75(3):207–46. [DOI] [PubMed] [Google Scholar]
- 180.Maiese K WISP1: Clinical Insights for a Proliferative and Restorative Member of the CCN Family. Curr Neurovasc Res. 2014;11(4):378–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Feng H, Xue M, Deng H, Cheng S, Hu Y, Zhou C. Ginsenoside and Its Therapeutic Potential for Cognitive Impairment. Biomolecules. 2022;12(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Zhuang X, Ma J, Xu G, Sun Z. SHP-1 knockdown suppresses mitochondrial biogenesis and aggravates mitochondria-dependent apoptosis induced by all trans retinal through the STING/AMPK pathways. Mol Med. 2022;28(1):125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Ali ES, Mitra K, Akter S, Ramproshad S, Mondal B, Khan IN, et al. Recent advances and limitations of mTOR inhibitors in the treatment of cancer. Cancer cell international. 2022;22(1):284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Arias C, Salazar LA. Autophagy and Polyphenols in Osteoarthritis: A Focus on Epigenetic Regulation. International journal of molecular sciences. 2021;23(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Barthels D, Prateeksha P, Nozohouri S, Villalba H, Zhang Y, Sharma S, et al. Dental Pulp-Derived Stem Cells Preserve Astrocyte Health During Induced Gliosis by Modulating Mitochondrial Activity and Functions. Cell Mol Neurobiol. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Casciano F, Zauli E, Rimondi E, Mura M, Previati M, Busin M, et al. The role of the mTOR pathway in diabetic retinopathy. Front Med (Lausanne). 2022;9:973856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Chen G, Zeng L, Yan F, Liu J, Qin M, Wang F, et al. Long-term oral administration of naringenin counteracts aging-related retinal degeneration via regulation of mitochondrial dynamics and autophagy. Frontiers in pharmacology. 2022;13:919905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Gao J, Xu H, Rong Z, Chen L. Wnt family member 1 (Wnt1) overexpression-induced M2 polarization of microglia alleviates inflammation-sensitized neonatal brain injuries. Bioengineered. 2022;13(5):12409–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Jobst M, Kiss E, Gerner C, Marko D, Del Favero G. Activation of autophagy triggers mitochondrial loss and changes acetylation profile relevant for mechanotransduction in bladder cancer cells. Arch Toxicol. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Maiese K Taking aim at Alzheimer’s disease through the mammalian target of rapamycin. Ann Med. 2014;46(8):587–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Sakai M, Yu Z, Hirayama R, Nakasato M, Kikuchi Y, Ono C, et al. Deficient Autophagy in Microglia Aggravates Repeated Social Defeat Stress-Induced Social Avoidance. Neural Plast. 2022;2022:7503553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Corti O, Blomgren K, Poletti A, Beart PM. Autophagy in neurodegeneration: New insights underpinning therapy for neurological diseases. J Neurochem. 2020;154(4):e15002. [DOI] [PubMed] [Google Scholar]
- 193.Maiese K Warming Up to New Possibilities with the Capsaicin Receptor TRPV1: mTOR, AMPK, and Erythropoietin. Curr Neurovasc Res. 2017;14(2):184–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Maiese K Driving neural regeneration through the mammalian target of rapamycin. Neural regeneration research. 2014;9(15):1413–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Maiese K Novel Stem Cell Strategies with mTOR. Molecules to Medicine with mTOR: Translating Critical Pathways into Novel Therapeutic Strategies, Academic Press, Elsevier. 2016:3–22. [Google Scholar]
- 196.He W, Gao Y, Zhou J, Shi Y, Xia D, Shen HM. Friend or Foe? Implication of the autophagy-lysosome pathway in SARS-CoV-2 infection and COVID-19. Int J Biol Sci. 2022;18(12):4690–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Maiese K Circadian Clock Genes: Targeting Innate Immunity for Antiviral Strategies Against COVID-19. Curr Neurovasc Res. 2020. [DOI] [PubMed] [Google Scholar]
- 198.Theoharides TC. Could SARS-CoV-2 Spike Protein Be Responsible for Long-COVID Syndrome? Mol Neurobiol. 2022;59(3):1850–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.You H, Zhao Q, Dong M. The Key Genes Underlying Pathophysiology Correlation Between the Acute Myocardial Infarction and COVID-19. Int J Gen Med. 2022;15:2479–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Li Q, Zhang T, Wang Y, Yang S, Luo J, Fang F, et al. Qing-Wen-Jie-Re Mixture Ameliorates Poly (I:C)-Induced Viral Pneumonia Through Regulating the Inflammatory Response and Serum Metabolism. Frontiers in pharmacology. 2022;13:891851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Maiese K Forkhead transcription factors: new considerations for alzheimer’s disease and dementia. J Transl Sci. 2016;2(4):241–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Movahedpour A, Vakili O, Khalifeh M, Mousavi P, Mahmoodzadeh A, Taheri-Anganeh M, et al. Mammalian target of rapamycin (mTOR) signaling pathway and traumatic brain injury: A novel insight into targeted therapy. Cell Biochem Funct. 2022;40(3):232–47. [DOI] [PubMed] [Google Scholar]
- 203.Cheng J, North BJ, Zhang T, Dai X, Tao K, Guo J, et al. The emerging roles of protein homeostasis-governing pathways in Alzheimer’s disease. Aging Cell. 2018;17(5):e12801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Morris G, Berk M, Maes M, Puri BK. Could Alzheimer’s Disease Originate in the Periphery and If So How So? Mol Neurobiol. 2019;56(1):406–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Zhang ZH, Wu QY, Zheng R, Chen C, Chen Y, Liu Q, et al. Selenomethionine mitigates cognitive decline by targeting both tau hyperphosphorylation and autophagic clearance in an Alzheimer’s disease mouse model. J Neurosci. 2017;37(9):2449–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Maiese K Novel Insights for Multiple Sclerosis and Demyelinating Disorders with Apoptosis, Autophagy, FoxO, and mTOR. Curr Neurovasc Res. 2021;18(2):1–4. [DOI] [PubMed] [Google Scholar]
- 207.Geng K, Ma X, Jiang Z, Huang W, Gao C, Pu Y, et al. Innate Immunity in Diabetic Wound Healing: Focus on the Mastermind Hidden in Chronic Inflammatory. Frontiers in pharmacology. 2021;12:653940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Ye M, Zhao Y, Wang Y, Xie R, Tong Y, Sauer JD, et al. NAD(H)-loaded nanoparticles for efficient sepsis therapy via modulating immune and vascular homeostasis. Nat Nanotechnol. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Wu L, Xiong X, Wu X, Ye Y, Jian Z, Zhi Z, et al. Targeting Oxidative Stress and Inflammation to Prevent Ischemia-Reperfusion Injury. Front Mol Neurosci. 2020;13:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Farahani M, Niknam Z, Mohammadi Amirabad L, Amiri-Dashatan N, Koushki M, Nemati M, et al. Molecular pathways involved in COVID-19 and potential pathway-based therapeutic targets. Biomed Pharmacother. 2021;145:112420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Ponzetti M, Rucci N, Falone S. RNA methylation and cellular response to oxidative stress-promoting anticancer agents. Cell Cycle. 2023;22(8):870–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Chong ZZ, Shang YC, Wang S, Maiese K. SIRT1: New avenues of discovery for disorders of oxidative stress. Expert opinion on therapeutic targets. 2012;16(2):167–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Xiong J, Bonney S, Gonçalves RV, Esposito D. Brassinosteroids control the inflammation, oxidative stress and cell migration through the control of mitochondrial function on skin regeneration. Life Sci. 2022;307:120887. [DOI] [PubMed] [Google Scholar]
- 214.Chong ZZ, Li F, Maiese K. Stress in the brain: novel cellular mechanisms of injury linked to Alzheimer’s disease. Brain Res Brain Res Rev. 2005;49(1):1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Chong ZZ, Maiese K. The Src homology 2 domain tyrosine phosphatases SHP-1 and SHP-2: diversified control of cell growth, inflammation, and injury. Histol Histopathol. 2007;22(11):1251–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Chen G, Li Z, Chen C, Liu J, Zhu W, She L, et al. The Molecular Landscape and Biological Alterations Induced by PRAS40-Knockout in Head and Neck Squamous Cell Carcinoma. Front Oncol. 2020;10:565669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Maiese K, Chong ZZ, Shang YC, Wang S. mTOR: on target for novel therapeutic strategies in the nervous system. Trends Mol Med. 2013;19(1):51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Xue Q, Nagy JA, Manseau EJ, Phung TL, Dvorak HF, Benjamin LE. Rapamycin inhibition of the Akt/mTOR pathway blocks select stages of VEGF-A164-driven angiogenesis, in part by blocking S6Kinase. Arterioscler Thromb Vasc Biol. 2009;29(8):1172–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Sergio CM, Rolando CA. Erythropoietin regulates signaling pathways associated with neuroprotective events. Exp Brain Res. 2022. [DOI] [PubMed] [Google Scholar]
- 220.Maiese K Regeneration in the nervous system with erythropoietin. Frontiers in bioscience (Landmark edition). 2016;21:561–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Chong ZZ, Shang YC, Wang S, Maiese K. PRAS40 Is an Integral Regulatory Component of Erythropoietin mTOR Signaling and Cytoprotection. PLoS ONE. 2012;7(9):e45456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Shang YC, Chong ZZ, Wang S, Maiese K. WNT1 Inducible Signaling Pathway Protein 1 (WISP1) Targets PRAS40 to Govern beta-Amyloid Apoptotic Injury of Microglia. Curr Neurovasc Res. 2012;9(4):239–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Wang H, Zhang Q, Wen Q, Zheng Y, Philip L, Jiang H, et al. Proline-rich Akt substrate of 40kDa (PRAS40): a novel downstream target of PI3k/Akt signaling pathway. Cell Signal. 2012;24(1):17–24. [DOI] [PubMed] [Google Scholar]
- 224.Saenwongsa W, Nithichanon A, Chittaganpitch M, Buayai K, Kewcharoenwong C, Thumrongwilainet B, et al. Metformin-induced suppression of IFN-alpha via mTORC1 signalling following seasonal vaccination is associated with impaired antibody responses in type 2 diabetes. Scientific reports. 2020;10(1):3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Chakrabarti P, English T, Shi J, Smas CM, Kandror KV. Mammalian target of rapamycin complex 1 suppresses lipolysis, stimulates lipogenesis, and promotes fat storage. Diabetes. 2010;59(4):775–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Malla R, Wang Y, Chan WK, Tiwari AK, Faridi JS. Genetic ablation of PRAS40 improves glucose homeostasis via linking the AKT and mTOR pathways. Biochem Pharmacol. 2015. [DOI] [PubMed] [Google Scholar]
- 227.Hamada S, Hara K, Hamada T, Yasuda H, Moriyama H, Nakayama R, et al. Upregulation of the mammalian target of rapamycin complex 1 pathway by Ras homolog enriched in brain in pancreatic beta-cells leads to increased beta-cell mass and prevention of hyperglycemia. Diabetes. 2009;58(6):1321–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Katsianou MA, Papavassiliou KA, Gargalionis AN, Agrogiannis G, Korkolopoulou P, Panagopoulos D, et al. Polycystin-1 regulates cell proliferation and migration through AKT/mTORC2 pathway in a human craniosynostosis cell model. J Cell Mol Med. 2022;26(8):2428–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Gu Y, Lindner J, Kumar A, Yuan W, Magnuson MA. Rictor/mTORC2 is essential for maintaining a balance between beta-cell proliferation and cell size. Diabetes. 2011;60(3):827–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Wang RH, Kim HS, Xiao C, Xu X, Gavrilova O, Deng CX. Hepatic Sirt1 deficiency in mice impairs mTorc2/Akt signaling and results in hyperglycemia, oxidative damage, and insulin resistance. J Clin Invest. 2011;121(11):4477–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Treins C, Alliouachene S, Hassouna R, Xie Y, Birnbaum MJ, Pende M. The combined deletion of S6K1 and Akt2 deteriorates glycaemic control in high fat diet. Mol Cell Biol. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Chong ZZ, Shang YC, Wang S, Maiese K. Shedding new light on neurodegenerative diseases through the mammalian target of rapamycin. Prog Neurobiol. 2012;99(2):128–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Maiese K Novel Treatment Strategies for the Nervous System: Circadian Clock Genes, Non-coding RNAs, and Forkhead Transcription Factors. Curr Neurovasc Res. 2018;15(1):81–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Teotia P, Van Hook MJ, Fischer D, Ahmad I. Human retinal ganglion cell axon regeneration by recapitulating developmental mechanisms: effects of recruitment of the mTOR pathway. Development. 2019;146(13). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Shang YC, Chong ZZ, Wang S, Maiese K. Erythropoietin and Wnt1 Govern Pathways of mTOR, Apaf-1, and XIAP in Inflammatory Microglia. Curr Neurovasc Res. 2011;8(4):270–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Dong J, Li H, Bai Y, Wu C. Muscone ameliorates diabetic peripheral neuropathy through activating AKT/mTOR signalling pathway. J Pharm Pharmacol. 2019;71(11):1706–13. [DOI] [PubMed] [Google Scholar]
- 237.Shang YC, Chong ZZ, Wang S, Maiese K. Prevention of beta-amyloid degeneration of microglia by erythropoietin depends on Wnt1, the PI 3-K/mTOR pathway, Bad, and Bcl-xL. Aging (Albany NY). 2012;4(3):187–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Shang YC, Chong ZZ, Wang S, Maiese K. Tuberous sclerosis protein 2 (TSC2) modulates CCN4 cytoprotection during apoptotic amyloid toxicity in microglia. Curr Neurovasc Res. 2013;10(1):29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Wang Y, Wang YX, Liu T, Law PY, Loh HH, Qiu Y, et al. mu-Opioid receptor attenuates Abeta oligomers-induced neurotoxicity through mTOR signaling. CNS Neurosci Ther. 2015;21(1):8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Park JA, Lee CH. Temporal changes in mammalian target of rapamycin (mTOR) and phosphorylated-mTOR expressions in the hippocampal CA1 region of rat with vascular dementia. Journal of veterinary science. 2017;18(1):11–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Farmer K, Abd-Elrahman KS, Derksen A, Rowe EM, Thompson AM, Rudyk CA, et al. mGluR5 Allosteric Modulation Promotes Neurorecovery in a 6-OHDA-Toxicant Model of Parkinson’s Disease. Mol Neurobiol. 2020;57(3):1418–31. [DOI] [PubMed] [Google Scholar]
- 242.Dai C, Tang S, Biao X, Xiao X, Chen C, Li J. Colistin induced peripheral neurotoxicity involves mitochondrial dysfunction and oxidative stress in mice. Mol Biol Rep. 2019;46(2). [DOI] [PubMed] [Google Scholar]
- 243.Huang D, Shen S, Cai M, Jin L, Lu J, Xu K, et al. Role of mTOR complex in IGF-1 induced neural differentiation of DPSCs. Journal of molecular histology. 2019. [DOI] [PubMed] [Google Scholar]
- 244.Xi JS, Wang YF, Long XX, Ma Y. Mangiferin Potentiates Neuroprotection by Isoflurane in Neonatal Hypoxic Brain Injury by Reducing Oxidative Stress and Activation of Phosphatidylinositol-3-Kinase/Akt/Mammalian Target of Rapamycin (PI3K/Akt/mTOR) Signaling. Med Sci Monit. 2018;24:7459–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Dong W, Wang R, Ma LN, Xu BL, Zhang JS, Zhao ZW, et al. Influence of age-related learning and memory capacity of mice: different effects of a high and low caloric diet. Aging Clin Exp Res. 2016;28(2):303–11. [DOI] [PubMed] [Google Scholar]
- 246.Han K, Jia N, Zhong Y, Shang X. S14G-humanin alleviates insulin resistance and increases autophagy in neurons of APP/PS1 transgenic mouse. J Cell Biochem. 2017. [DOI] [PubMed] [Google Scholar]
- 247.Dai C, Ciccotosto GD, Cappai R, Wang Y, Tang S, Hoyer D, et al. Rapamycin confers neuroprotection against colistin-induced oxidative stress, mitochondria dysfunction and apoptosis through the activation of autophagy and mTOR/Akt/CREB signaling pathways. ACS chemical neuroscience. 2017;9(4):824–37. [DOI] [PubMed] [Google Scholar]
- 248.Park A, Koh HC. NF-kappaB/mTOR-mediated autophagy can regulate diquat-induced apoptosis. Arch Toxicol. 2019. [DOI] [PubMed] [Google Scholar]
- 249.Javdan N, Ayatollahi SA, Choudhary MI, Al-Hasani S, Kobarfard F, Athar A, et al. Capsaicin protects against testicular torsion injury through mTOR-dependent mechanism. Theriogenology. 2018;113:247–52. [DOI] [PubMed] [Google Scholar]
- 250.Zhao Y, Wang Q, Wang Y, Li J, Lu G, Liu Z. Glutamine protects against oxidative stress injury through inhibiting the activation of PI3K/Akt signaling pathway in parkinsonian cell model. Environmental health and preventive medicine. 2019;24(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Liu P, Yang X, Hei C, Meli Y, Niu J, Sun T, et al. Rapamycin Reduced Ischemic Brain Damage in Diabetic Animals Is Associated with Suppressions of mTOR and ERK1/2 Signaling. Int J Biol Sci. 2016;12(8):1032–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Thomas SD, Jha NK, Ojha S, Sadek B. mTOR Signaling Disruption and Its Association with the Development of Autism Spectrum Disorder. Molecules. 2023;28(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Lim YM, Lim H, Hur KY, Quan W, Lee HY, Cheon H, et al. Systemic autophagy insufficiency compromises adaptation to metabolic stress and facilitates progression from obesity to diabetes. Nature communications. 2014;5:4934. [DOI] [PubMed] [Google Scholar]
- 254.Ma L, Fu R, Duan Z, Lu J, Gao J, Tian L, et al. Sirt1 is essential for resveratrol enhancement of hypoxia-induced autophagy in the type 2 diabetic nephropathy rat. Pathology, research and practice. 2016. [DOI] [PubMed] [Google Scholar]
- 255.Liu Z, Stanojevic V, Brindamour LJ, Habener JF. GLP1-derived nonapeptide GLP1(28–36)amide protects pancreatic beta-cells from glucolipotoxicity. J Endocrinol. 2012;213(2):143–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.He C, Bassik MC, Moresi V, Sun K, Wei Y, Zou Z, et al. Exercise-induced BCL2-regulated autophagy is required for muscle glucose homeostasis. Nature. 2012;481(7382):511–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Liu Y, Palanivel R, Rai E, Park M, Gabor TV, Scheid MP, et al. Adiponectin stimulates autophagy and reduces oxidative stress to enhance insulin sensitivity during high fat diet feeding in mice. Diabetes. 2014;64(1):36–48. [DOI] [PubMed] [Google Scholar]
- 258.Radulovic J, Gabbay V. PFC mTOR signaling as a biological signature for cognitive deficits in bipolar disorder without psychosis. Cell Rep Med. 2021;2(5):100282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Kim KA, Shin YJ, Akram M, Kim ES, Choi KW, Suh H, et al. High glucose condition induces autophagy in endothelial progenitor cells contributing to angiogenic impairment. Biol Pharm Bull. 2014;37(7):1248–52. [DOI] [PubMed] [Google Scholar]
- 260.Saleem S, Biswas SC. Tribbles Pseudokinase 3 Induces Both Apoptosis and Autophagy in Amyloid-beta-induced Neuronal Death. J Biol Chem. 2017;292(7):2571–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Lee JH, Lee JH, Jin M, Han SD, Chon GR, Kim IH, et al. Diet control to achieve euglycemia induces significant loss of heart and liver weight via increased autophagy compared with ad libitum diet in diabetic rats. Exp Mol Med. 2014;46:e111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Hu P, Lai D, Lu P, Gao J, He H. ERK and Akt signaling pathways are involved in advanced glycation end product-induced autophagy in rat vascular smooth muscle cells. Int J Mol Med. 2012;29(4):613–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Lee Y, Hong Y, Lee SR, Chang KT. Autophagy contributes to retardation of cardiac growth in diabetic rats. Lab Anim Res. 2012;28(2):99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Martino L, Masini M, Novelli M, Beffy P, Bugliani M, Marselli L, et al. Palmitate activates autophagy in INS-1E beta-cells and in isolated rat and human pancreatic islets. PLoS ONE. 2012;7(5):e36188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Guo T, Chen M, Liu J, Wei Z, Yuan J, Wu W, et al. Neuropilin-1 promotes mitochondrial structural repair and functional recovery in rats with cerebral ischemia. Journal of translational medicine. 2023;21(1):297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Maiese K, Li F, Chong ZZ. New avenues of exploration for erythropoietin. Jama. 2005;293(1):90–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Lee HJ, Koh SH, Song KM, Seol IJ, Park HK. The Akt/mTOR/p70S6K Pathway Is Involved in the Neuroprotective Effect of Erythropoietin on Hypoxic/Ischemic Brain Injury in a Neonatal Rat Model. Neonatology. 2016;110(2):93–100. [DOI] [PubMed] [Google Scholar]
- 268.Maiese K, Chong ZZ, Shang YC, Wang S. Erythropoietin: new directions for the nervous system. International journal of molecular sciences. 2012;13(9):11102–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Wang GB, Ni YL, Zhou XP, Zhang WF. The AKT/mTOR pathway mediates neuronal protective effects of erythropoietin in sepsis. Mol Cell Biochem. 2014;385(1–2):125–32. [DOI] [PubMed] [Google Scholar]
- 270.Ka M, Smith AL, Kim WY. MTOR controls genesis and autophagy of GABAergic interneurons during brain development. Autophagy. 2017:0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Pende M, Kozma SC, Jaquet M, Oorschot V, Burcelin R, Le Marchand-Brustel Y, et al. Hypoinsulinaemia, glucose intolerance and diminished beta-cell size in S6K1-deficient mice. Nature. 2000;408(6815):994–7. [DOI] [PubMed] [Google Scholar]
- 272.Pasini E, Flati V, Paiardi S, Rizzoni D, Porteri E, Aquilani R, et al. Intracellular molecular effects of insulin resistance in patients with metabolic syndrome. Cardiovasc Diabetol. 2010;9:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Zhou J, Wu J, Zheng F, Jin M, Li H. Glucagon-like peptide-1 analog-mediated protection against cholesterol-induced apoptosis via mammalian target of rapamycin activation in pancreatic betaTC-6 cells -1mTORbetaTC-6. Journal of diabetes. 2015;7(2):231–9. [DOI] [PubMed] [Google Scholar]
- 274.Liu YW, Zhang L, Li Y, Cheng YQ, Zhu X, Zhang F, et al. Activation of mTOR signaling mediates the increased expression of AChE in high glucose condition: in vitro and in vivo evidences. Mol Neurobiol. 2015;53(7). [DOI] [PubMed] [Google Scholar]
- 275.Miao XY, Gu ZY, Liu P, Hu Y, Li L, Gong YP, et al. The human glucagon-like peptide-1 analogue liraglutide regulates pancreatic beta-cell proliferation and apoptosis via an AMPK/mTOR/P70S6K signaling pathway. Peptides. 2013;39:71–9. [DOI] [PubMed] [Google Scholar]
- 276.Fraenkel M, Ketzinel-Gilad M, Ariav Y, Pappo O, Karaca M, Castel J, et al. mTOR inhibition by rapamycin prevents beta-cell adaptation to hyperglycemia and exacerbates the metabolic state in type 2 diabetes. Diabetes. 2008;57(4):945–57. [DOI] [PubMed] [Google Scholar]
- 277.Sataranatarajan K, Ikeno Y, Bokov A, Feliers D, Yalamanchili H, Lee HJ, et al. Rapamycin Increases Mortality in db/db Mice, a Mouse Model of Type 2 Diabetes. J Gerontol A Biol Sci Med Sci. 2016;71(7):850–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Deblon N, Bourgoin L, Veyrat-Durebex C, Peyrou M, Vinciguerra M, Caillon A, et al. Chronic mTOR inhibition by rapamycin induces muscle insulin resistance despite weight loss in rats. Br J Pharmacol. 2012;165(7):2325–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Crespo MC, Tome-Carneiro J, Pintado C, Davalos A, Visioli F, Burgos-Ramos E. Hydroxytyrosol restores proper insulin signaling in an astrocytic model of Alzheimer’s disease. BioFactors (Oxford, England). 2017;43(4). [DOI] [PubMed] [Google Scholar]
- 280.Pal PB, Sonowal H, Shukla K, Srivastava SK, Ramana KV. Aldose reductase regulates hyperglycemia-induced HUVEC death via SIRT1/AMPK-alpha1/mTOR pathway. Journal of molecular endocrinology. 2019;63(1):11–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Hua K, Li T, He Y, Guan A, Chen L, Gao Y, et al. Resistin secreted by porcine alveolar macrophages leads to endothelial cell dysfunction during Haemophilus parasuis infection. Virulence. 2023:2171636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Guimera AM, Clark P, Wordsworth J, Anugula S, Rasmussen LJ, Shanley DP. Systems modelling predicts chronic inflammation and genomic instability prevent effective mitochondrial regulation during biological ageing. Exp Gerontol. 2022:111889. [DOI] [PubMed] [Google Scholar]
- 283.Sato T, Nakashima A, Guo L, Tamanoi F. Specific activation of mTORC1 by Rheb G-protein in vitro involves enhanced recruitment of its substrate protein. J Biol Chem. 2009;284(19):12783–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Balan V, Miller GS, Kaplun L, Balan K, Chong ZZ, Li F, et al. Life span extension and neuronal cell protection by Drosophila nicotinamidase. J Biol Chem. 2008;283(41):27810–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Moroz N, Carmona JJ, Anderson E, Hart AC, Sinclair DA, Blackwell TK. Dietary restriction involves NAD -dependent mechanisms and a shift toward oxidative metabolism. Aging Cell. 2014;13(6):1075–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Xia W, Zhang F, Xie C, Jiang M, Hou M. Macrophage migration inhibitory factor confers resistance to senescence through CD74-dependent AMPK-FOXO3a signaling in mesenchymal stem cells. Stem cell research & therapy. 2015;6(1):82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Chiu SC, Chao CY, Chiang EI, Syu JN, Rodriguez RL, Tang FY. N-3 polyunsaturated fatty acids alleviate high glucose-mediated dysfunction of endothelial progenitor cells and prevent ischemic injuries both in vitro and in vivo. The Journal of nutritional biochemistry. 2017;42:172–81. [DOI] [PubMed] [Google Scholar]
- 288.Maiese K The Implications of Telomere Length: Advanced Aging, Cell Senescence, MRI Phenotypes, Stem Cells and Alzheimer’s Disease. Curr Neurovasc Res. 2023. [DOI] [PubMed] [Google Scholar]
- 289.Zhang H, Yang X, Pang X, Zhao Z, Yu H, Zhou H. Genistein protects against ox-LDL-induced senescence through enhancing SIRT1/LKB1/AMPK-mediated autophagy flux in HUVECs. Mol Cell Biochem. 2019;455((1–2)):127–34. [DOI] [PubMed] [Google Scholar]
- 290.Zhao H, Wang ZC, Wang KF, Chen XY. Abeta peptide secretion is reduced by Radix Polygalaeinduced autophagy via activation of the AMPK/mTOR pathway. Molecular medicine reports. 2015;12(2):2771–6. [DOI] [PubMed] [Google Scholar]
- 291.Du LL, Chai DM, Zhao LN, Li XH, Zhang FC, Zhang HB, et al. AMPK Activation Ameliorates Alzheimer’s Disease-Like Pathology and Spatial Memory Impairment in a Streptozotocin-Induced Alzheimer’s Disease Model in Rats. J Alzheimers Dis. 2015;43(3):775–84. [DOI] [PubMed] [Google Scholar]
- 292.Yu M, Zhang H, Wang B, Zhang Y, Zheng X, Shao B, et al. Key Signaling Pathways in Aging and Potential Interventions for Healthy Aging. Cells. 2021;10(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Lin CL, Huang WN, Li HH, Huang CN, Hsieh S, Lai C, et al. Hydrogen-rich water attenuates amyloid beta-induced cytotoxicity through upregulation of Sirt1-FoxO3a by stimulation of AMP-activated protein kinase in SK-N-MC cells. Chem Biol Interact. 2015;240:12–21. [DOI] [PubMed] [Google Scholar]
- 294.Yang J, Suo H, Song J. Protective role of mitoquinone against impaired mitochondrial homeostasis in metabolic syndrome. Critical reviews in food science and nutrition. 2020;20:1–19. [DOI] [PubMed] [Google Scholar]
- 295.Maiese K The Many Facets of Cell Injury: Angiogenesis to Autophagy. Curr Neurovasc Res. 2012;9(2):1–2. [DOI] [PubMed] [Google Scholar]
- 296.Shokri Afra H, Zangooei M, Meshkani R, Ghahremani MH, Ilbeigi D, Khedri A, et al. Hesperetin is a potent bioactivator that activates SIRT1-AMPK signaling pathway in HepG2 cells. Journal of physiology and biochemistry. 2019;75(2):125–33. [DOI] [PubMed] [Google Scholar]
- 297.Zhao D, Sun X, Lv S, Sun M, Guo H, Zhai Y, et al. Salidroside attenuates oxidized lowdensity lipoproteininduced endothelial cell injury via promotion of the AMPK/SIRT1 pathway. Int J Mol Med. 2019;43(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Dong Y, Chen H, Gao J, Liu Y, Li J, Wang J. Molecular machinery and interplay of apoptosis and autophagy in coronary heart disease. J Mol Cell Cardiol. 2019;136:27–41. [DOI] [PubMed] [Google Scholar]
- 299.Jang W, Kim HJ, Li H, Jo KD, Lee MK, Yang HO. The Neuroprotective Effect of Erythropoietin on Rotenone-Induced Neurotoxicity in SH-SY5Y Cells Through the Induction of Autophagy. Mol Neurobiol. 2015;53(6):3812–21. [DOI] [PubMed] [Google Scholar]
- 300.Tsai CF, Kuo YH, Yeh WL, Wu CY, Lin HY, Lai SW, et al. Regulatory Effects of Caffeic Acid Phenethyl Ester on Neuroinflammation in Microglial Cells. International journal of molecular sciences. 2015;16(3):5572–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Maiese K Erythropoietin and mTOR: A “One-Two Punch” for Aging-Related Disorders Accompanied by Enhanced Life Expectancy. Curr Neurovasc Res. 2016;13(4):329–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Wang L, Di L, Noguchi CT. AMPK is involved in mediation of erythropoietin influence on metabolic activity and reactive oxygen species production in white adipocytes. Int J Biochem Cell Biol. 2014;54:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Andreucci M, Fuiano G, Presta P, Lucisano G, Leone F, Fuiano L, et al. Downregulation of cell survival signalling pathways and increased cell damage in hydrogen peroxide-treated human renal proximal tubular cells by alpha-erythropoietin. Cell Prolif. 2009;42(4):554–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Nejabati HR, Samadi N, Shahnazi V, Mihanfar A, Fattahi A, Latifi Z, et al. Nicotinamide and its metabolite N1-Methylnicotinamide alleviate endocrine and metabolic abnormalities in adipose and ovarian tissues in rat model of Polycystic Ovary Syndrome. Chem Biol Interact. 2020;324:109093. [DOI] [PubMed] [Google Scholar]
- 305.Maiese K The Oversight of Circadian Clock Genes for the Detection, Prevention, and Treatment of COVID-19 Infection. Curr Neurovasc Res. 2021;18(5):471–3. [DOI] [PubMed] [Google Scholar]
- 306.Kalender A, Selvaraj A, Kim SY, Gulati P, Brule S, Viollet B, et al. Metformin, independent of AMPK, inhibits mTORC1 in a rag GTPase-dependent manner. Cell Metab. 2010;11(5):390–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Oda SS. Metformin Protects against Experimental Acrylamide Neuropathy in Rats. Drug development research. 2017;78(7):349–59. [DOI] [PubMed] [Google Scholar]
- 308.Sanadgol N, Barati M, Houshmand F, Hassani S, Clarner T, Shahlaei M, et al. Metformin accelerates myelin recovery and ameliorates behavioral deficits in the animal model of multiple sclerosis via adjustment of AMPK/Nrf2/mTOR signaling and maintenance of endogenous oligodendrogenesis during brain self-repairing period. Pharmacol Rep. 2020;72(3):641–58. [DOI] [PubMed] [Google Scholar]
- 309.Ong AN, Tan CC, Cañete MT, Lim BA, Robles J. Association Between Metformin Use and Mortality among Patients with Type 2 Diabetes Mellitus Hospitalized for COVID-19 Infection. J ASEAN Fed Endocr Soc. 2021;36(2):133–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Shiravandi A, Yari F, Tofigh N, Kazemi Ashtiani M, Shahpasand K, Ghanian MH, et al. Earlier Detection of Alzheimer’s Disease Based on a Novel Biomarker cis P-tau by a Label-Free Electrochemical Immunosensor. Biosensors (Basel). 2022;12(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Agarwal D, Kumari R, Ilyas A, Tyagi S, Kumar R, Poddar NK. Crosstalk between epigenetics and mTOR as a gateway to new insights in pathophysiology and treatment of Alzheimer’s disease. International journal of biological macromolecules. 2021;192:895–903. [DOI] [PubMed] [Google Scholar]
- 312.Eshraghi M, Ahmadi M, Afshar S, Lorzadeh S, Adlimoghaddam A, Rezvani Jalal N, et al. Enhancing autophagy in Alzheimer’s disease through drug repositioning. Pharmacol Ther. 2022;237:108171. [DOI] [PubMed] [Google Scholar]
- 313.Li X, Li K, Chu F, Huang J, Yang Z. Graphene oxide enhances β-amyloid clearance by inducing autophagy of microglia and neurons. Chem Biol Interact. 2020;325:109126. [DOI] [PubMed] [Google Scholar]
- 314.Maiese K, Holloway HH, Larson DM, Soncrant TT. Effect of acute and chronic arecoline treatment on cerebral metabolism and blood flow in the conscious rat. Brain Res. 1994;641(1):65–75. [DOI] [PubMed] [Google Scholar]
- 315.Naseri A, Baghernezhad K, Seyedi-Sahebari S, Alhoseini SA, Gholipour-Khalili E, Zafarani F, et al. The association of apolipoprotein E (ApoE) genotype and cognitive outcomes in multiple sclerosis; a systematic review and meta-analysis. Mult Scler Relat Disord. 2022;65:104011. [DOI] [PubMed] [Google Scholar]
- 316.Pontifex MG, Martinsen A, Saleh RNM, Harden G, Tejera N, Müller M, et al. APOE4 genotype exacerbates the impact of menopause on cognition and synaptic plasticity in APOE-TR mice. Faseb j. 2021;35(5):e21583. [DOI] [PubMed] [Google Scholar]
- 317.Roccaro I, Smirni D. Fiat Lux: The Light Became Therapy. An Overview on the Bright Light Therapy in Alzheimer’s Disease Sleep Disorders. J Alzheimers Dis. 2020;77(1):113–25. [DOI] [PubMed] [Google Scholar]
- 318.Margrett JA, Schofield T, Martin P, Poon LW, Masaki K, Donlon TA, et al. Novel Functional, Health, and Genetic Determinants of Cognitive Terminal Decline: Kuakini Honolulu Heart Program/Honolulu-Asia Aging Study. J Gerontol A Biol Sci Med Sci. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Safdari Lord J, Soltani Rezaiezadeh J, Yekaninejad MS, Izadi P. The association of APOE genotype with COVID-19 disease severity. Scientific reports. 2022;12(1):13483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Maiese K Late Onset Alzheimer’s Disease: Novel Clinical Prospects for the Future. Curr Neurovasc Res. 2017;14(2):89. [DOI] [PubMed] [Google Scholar]
- 321.Maiese K, Chong ZZ, Hou J, Shang YC. New strategies for Alzheimer’s disease and cognitive impairment. Oxid Med Cell Longev. 2009;2(5):279–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Cacabelos R, Carril JC, Cacabelos N, Kazantsev AG, Vostrov AV, Corzo L, et al. Sirtuins in Alzheimer’s Disease: SIRT2-Related GenoPhenotypes and Implications for PharmacoEpiGenetics. International journal of molecular sciences. 2019;20(5):1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Chong ZZ, Shang YC, Hou J, Maiese K. Wnt1 neuroprotection translates into improved neurological function during oxidant stress and cerebral ischemia through AKT1 and mitochondrial apoptotic pathways. Oxid Med Cell Longev. 2010;3(2):153–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Maiese K, Chong ZZ. Nicotinamide: necessary nutrient emerges as a novel cytoprotectant for the brain. Trends Pharmacol Sci. 2003;24(5):228–32. [DOI] [PubMed] [Google Scholar]
- 325.Maiese K, Chong ZZ, Hou J, Shang YC. The vitamin nicotinamide: translating nutrition into clinical care. Molecules. 2009;14(9):3446–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Maiese K, Vincent AM. Membrane asymmetry and DNA degradation: functionally distinct determinants of neuronal programmed cell death. J Neurosci Res. 2000;59(4):568–80. [DOI] [PubMed] [Google Scholar]
- 327.Lee G, Pollard HB, Arispe N. Annexin 5 and apolipoprotein E2 protect against Alzheimer’s amyloid-beta-peptide cytotoxicity by competitive inhibition at a common phosphatidylserine interaction site. Peptides. 2002;23(7):1249–63. [DOI] [PubMed] [Google Scholar]
- 328.Li W, Su D, Zhai Q, Chi H, She X, Gao X, et al. Proteomes analysis reveals the involvement of autophagy in AD-like neuropathology induced by noise exposure and ApoE4. Environ Res. 2019;176:108537. [DOI] [PubMed] [Google Scholar]
- 329.Ojo JO, Reed JM, Crynen G, Vallabhaneni P, Evans J, Shackleton B, et al. APOE genotype dependent molecular abnormalities in the cerebrovasculature of Alzheimer’s disease and age-matched non-demented brains. Molecular brain. 2021;14(1):110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Levine KS, Leonard HL, Blauwendraat C, Iwaki H, Johnson N, Bandres-Ciga S, et al. Virus exposure and neurodegenerative disease risk across national biobanks. Neuron. 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Kurki SN, Kantonen J, Kaivola K, Hokkanen L, Mäyränpää MI, Puttonen H, et al. APOE ε4 associates with increased risk of severe COVID-19, cerebral microhaemorrhages and post-COVID mental fatigue: a Finnish biobank, autopsy and clinical study. Acta neuropathologica communications. 2021;9(1):199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Al-Kuraishy HM, Al-Buhadily AK, Al-Gareeb AI, Alorabi M, Hadi Al-Harcan NA, El-Bouseary MM, et al. Citicoline and COVID-19: vis-à-vis conjectured. Naunyn Schmiedebergs Arch Pharmacol. 2022:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Lally MA, Tsoukas P, Halladay CW, O’Neill E, Gravenstein S, Rudolph JL. Metformin is Associated with Decreased 30-Day Mortality Among Nursing Home Residents Infected with SARS-CoV2. J Am Med Dir Assoc. 2021;22(1):193–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Benotmane I, Perrin P, Vargas GG, Bassand X, Keller N, Lavaux T, et al. Biomarkers of Cytokine Release Syndrome Predict Disease Severity and Mortality From COVID-19 in Kidney Transplant Recipients. Transplantation. 2021;105(1):158–69. [DOI] [PubMed] [Google Scholar]
- 335.Abu-Eid R, Ward FJ. Targeting the PI3K/Akt/mTOR pathway: A therapeutic strategy in COVID-19 patients. Immunol Lett. 2021;240:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Jansen van Vuren E, Steyn SF, Brink CB, Möller M, Viljoen FP, Harvey BH. The neuropsychiatric manifestations of COVID-19: Interactions with psychiatric illness and pharmacological treatment. Biomed Pharmacother. 2021;135:111200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Hardeland R Redox Biology of Melatonin: Discriminating between Circadian and Non-circadian Functions. Antioxid Redox Signal. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Zuo J, Zhang Z, Luo M, Zhou L, Nice EC, Zhang W, et al. Redox signaling at the crossroads of human health and disease. MedComm (2020). 2022;3(2):e127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Maiese K, Li F, Chong ZZ, Shang YC. The Wnt signaling pathway: Aging gracefully as a protectionist? Pharmacol Ther. 2008;118(1):58–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Tang Y, Chen Y, Liu R, Li W, Hua B, Bao Y. Wnt Signaling Pathways: A Role in Pain Processing. Neuromolecular Med. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Maiese K The Challenges for Drug Development: Cytokines, Genes, and Stem Cells. Curr Neurovasc Res. 2012;9(4):231–2. [DOI] [PubMed] [Google Scholar]
- 342.Vallée A, Vallée JN, Lecarpentier Y. Parkinson’s Disease: Potential Actions of Lithium by Targeting the WNT/β-Catenin Pathway, Oxidative Stress, Inflammation and Glutamatergic Pathway. Cells. 2021;10(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Zhou Q, Liu C, Liu W, Zhang H, Zhang R, Liu J, et al. Rotenone induction of hydrogen peroxide inhibits mTOR-mediated S6K1 and 4E-BP1/eIF4E pathways, leading to neuronal apoptosis. Toxicol Sci. 2015;143(1):81–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Wu J, Zhu D, Zhang J, Li G, Liu Z, Sun J. Lithium protects against methamphetamine-induced neurotoxicity in PC12 cells via Akt/GSK3beta/mTOR pathway. Biochem Biophys Res Commun. 2015;465(3):368–73. [DOI] [PubMed] [Google Scholar]
- 345.Xu Y, Liu C, Chen S, Ye Y, Guo M, Ren Q, et al. Activation of AMPK and inactivation of Akt result in suppression of mTOR-mediated S6K1 and 4E-BP1 pathways leading to neuronal cell death in in vitro models of Parkinson’s disease. Cell Signal. 2014;26(8):1680–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Bonam SR, Tranchant C, Muller S. Autophagy-Lysosomal Pathway as Potential Therapeutic Target in Parkinson’s Disease. Cells. 2021;10(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Holling T, Bhavani GS, von Elsner L, Shah H, Kausthubham N, Bhattacharyya SS, et al. A homozygous hypomorphic BNIP1 variant causes an increase in autophagosomes and reduced autophagic flux and results in a spondylo-epiphyseal dysplasia. Hum Mutat. 2022. [DOI] [PubMed] [Google Scholar]
- 348.McCoin CS, Franczak E, Deng F, Pei D, Ding WX, Thyfault JP. Acute exercise rapidly activates hepatic mitophagic flux. J Appl Physiol (1985). 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Jeong JK, Moon MH, Bae BC, Lee YJ, Seol JW, Kang HS, et al. Autophagy induced by resveratrol prevents human prion protein-mediated neurotoxicity. Neurosci Res. 2012;73(2):99–105. [DOI] [PubMed] [Google Scholar]
- 350.Williams AC, Hill LJ, Ramsden DB. Nicotinamide, NAD(P)(H), and Methyl-Group Homeostasis Evolved and Became a Determinant of Ageing Diseases: Hypotheses and Lessons from Pellagra. Current gerontology and geriatrics research. 2012;2012:302875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Fan X, Zhao Z, Wang D, Xiao J. Glycogen synthase kinase-3 as a key regulator of cognitive function. Acta biochimica et biophysica Sinica. 2020;52(3). [DOI] [PubMed] [Google Scholar]
- 352.Das F, Dey N, Venkatesan B, Kasinath BS, Ghosh-Choudhury N, Choudhury GG. High glucose upregulation of early-onset Parkinson’s disease protein DJ-1 integrates the PRAS40/TORC1 axis to mesangial cell hypertrophy. Cell Signal. 2011;23(8):1311–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Choi KC, Kim SH, Ha JY, Kim ST, Son JH. A novel mTOR activating protein protects dopamine neurons against oxidative stress by repressing autophagy related cell death. J Neurochem. 2010;112(2):366–76. [DOI] [PubMed] [Google Scholar]
- 354.Lei Q, Wu T, Wu J, Hu X, Guan Y, Wang Y, et al. Roles of α-synuclein in gastrointestinal microbiome dysbiosis-related Parkinson’s disease progression (Review). Molecular medicine reports. 2021;24(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Sayed NH, Fathy N, Kortam MA, Rabie MA, Mohamed AF, Kamel AS. Vildagliptin Attenuates Huntington’s Disease through Activation of GLP-1 Receptor/PI3K/Akt/BDNF Pathway in 3-Nitropropionic Acid Rat Model. Neurotherapeutics : the journal of the American Society for Experimental NeuroTherapeutics. 2020;17(1):252–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Maiese K MicroRNAs and SIRT1: A Strategy for Stem Cell Renewal and Clinical Development? J Transl Sci. 2015;1(3):55–7. [PMC free article] [PubMed] [Google Scholar]
- 357.Shi X, Yan C, Liu B, Yang C, Nie X, Wang X, et al. miR-381 Regulates Neural Stem Cell Proliferation and Differentiation via Regulating Hes1 Expression. PLoS One. 2015;10(10):e0138973. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 358.Wen Z, Zhang J, Tang P, Tu N, Wang K, Wu G. Overexpression of miR185 inhibits autophagy and apoptosis of dopaminergic neurons by regulating the AMPK/mTOR signaling pathway in Parkinson’s disease. Molecular medicine reports. 2018;17(1):131–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Maiese K A Sweeping Role for MicroRNAs in Neuronal Disease, Vascular Disorders, and as Prognostic Indicators. Curr Neurovasc Res. 2018;15(1). [DOI] [PubMed] [Google Scholar]
- 360.Guo PW, Huang HT, Ma J, Zuo Y, Huang D, He LL, et al. Circular RNA-0007059 protects cell viability and reduces inflammation in a nephritis cell model by inhibiting microRNA-1278/SHP-1/STAT3 signaling. Mol Med. 2021;27(1):113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.He Z, Zhao Y, Zhu Y, Wang W, Liu X, Lu F. Interfering TUG1 Attenuates Cerebrovascular Endothelial Apoptosis and Inflammatory injury After Cerebral Ischemia/Reperfusion via TUG1/miR-410/FOXO3 ceRNA Axis. Neurotox Res. 2021. [DOI] [PubMed] [Google Scholar]
- 362.Zhang Z, Zhang HJ. Glycometabolic rearrangements-aerobic glycolysis in pancreatic ductal adenocarcinoma (PDAC): Roles, regulatory networks, and therapeutic potential. Expert opinion on therapeutic targets. 2021. [DOI] [PubMed] [Google Scholar]
- 363.Ren L Circular RNA PIP5K1A act as microRNA-552–3p sponge to regulates inflammation, oxidative damage in glucolipotoxicity-induced pancreatic INS-1 β-cells via Janus kinase 1. Bioengineered. 2022;13(3):5724–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Lu Y, Tan L, Wang X. Circular HDAC9/microRNA-138/Sirtuin-1 Pathway Mediates Synaptic and Amyloid Precursor Protein Processing Deficits in Alzheimer’s Disease. Neurosci Bull. 2019;35(5):877–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Hyrskyluoto A, Reijonen S, Kivinen J, Lindholm D, Korhonen L. GADD34 mediates cytoprotective autophagy in mutant huntingtin expressing cells via the mTOR pathway. Exp Cell Res. 2012;318(1):33–42. [DOI] [PubMed] [Google Scholar]
- 366.Lee JH, Tecedor L, Chen YH, Monteys AM, Sowada MJ, Thompson LM, et al. Reinstating aberrant mTORC1 activity in Huntington’s disease mice improves disease phenotypes. Neuron. 2015;85(2):303–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Oli V, Gupta R, Kumar P. FOXO and related transcription factors binding elements in the regulation of neurodegenerative disorders. J Chem Neuroanat. 2021;116:102012. [DOI] [PubMed] [Google Scholar]
- 368.Czubowicz K, Jesko H, Wencel P, Lukiw WJ, Strosznajder RP. The Role of Ceramide and Sphingosine-1-Phosphate in Alzheimer’s Disease and Other Neurodegenerative Disorders. Mol Neurobiol. 2019;56(8):5436–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Dhakal S, Kushairi N, Phan CW, Adhikari B, Sabaratnam V, Macreadie I. Dietary Polyphenols: A Multifactorial Strategy to Target Alzheimer’s Disease. International journal of molecular sciences. 2019;20(20). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Naia L, Rosenstock TR, Oliveira AM, Oliveira-Sousa SI, Caldeira GL, Carmo C, et al. Comparative Mitochondrial-Based Protective Effects of Resveratrol and Nicotinamide in Huntington’s Disease Models. Mol Neurobiol. 2017;54(7):5385–99. [DOI] [PubMed] [Google Scholar]
- 371.Pradhan SS, Rao KR, Manjunath M, Saiswaroop R, Patnana DP, Phalguna KS, et al. Vitamin B(6,) B(12) and folate modulate deregulated pathways and protein aggregation in yeast model of Huntington disease. 3 Biotech. 2023;13(3):96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Tourette C, Li B, Bell R, O’Hare S, Kaltenbach LS, Mooney SD, et al. A Large-scale Huntingtin Protein Interaction Network Implicates Rho GTPase Signaling Pathways in Huntington’s Disease. J Biol Chem. 2014;289(10):6709–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Berger Z, Ravikumar B, Menzies FM, Oroz LG, Underwood BR, Pangalos MN, et al. Rapamycin alleviates toxicity of different aggregate-prone proteins. Hum Mol Genet. 2006;15(3):433–42. [DOI] [PubMed] [Google Scholar]
- 374.Li L, Sun Y, Zhang Y, Wang W, Ye C. Mutant Huntingtin Impairs Pancreatic β-cells by Recruiting IRS-2 and Disturbing the PI3K/AKT/FoxO1 Signaling Pathway in Huntington’s Disease. J Mol Neurosci. 2021. [DOI] [PubMed] [Google Scholar]
- 375.Pryor WM, Biagioli M, Shahani N, Swarnkar S, Huang WC, Page DT, et al. Huntingtin promotes mTORC1 signaling in the pathogenesis of Huntington’s disease. Science signaling. 2014;7(349):ra103. [DOI] [PubMed] [Google Scholar]
- 376.Roscic A, Baldo B, Crochemore C, Marcellin D, Paganetti P. Induction of autophagy with catalytic mTOR inhibitors reduces huntingtin aggregates in a neuronal cell model. J Neurochem. 2011;119(2):398–407. [DOI] [PubMed] [Google Scholar]
- 377.Fox JH, Connor T, Chopra V, Dorsey K, Kama JA, Bleckmann D, et al. The mTOR kinase inhibitor Everolimus decreases S6 kinase phosphorylation but fails to reduce mutant huntingtin levels in brain and is not neuroprotective in the R6/2 mouse model of Huntington’s disease. Molecular neurodegeneration. 2010;5:26. [DOI] [PMC free article] [PubMed] [Google Scholar]


