Abstract
Sodium Glucose co-Transporter 2 (SGLT2) enables glucose and sodium reabsorption in the kidney. SGLT2-inhibitors (gliflozins, which include canagliflozin, dapagliflozin, empagliflozin, and ertugliflozin) act by increasing glycosuria, thereby reducing glycemia. These drugs are critical to reach and keep glycemic control, a crucial feature especially in patients with comorbidities, like frail individuals. A number of studies evaluated the effects of SGLT2-inhibitors in different settings beyond diabetes, revealing that they are actually pleiotropic drugs. We recently evidenced the favorable effects of SGLT2-inhibition on physical and cognitive impairment in frail older adults with diabetes and hypertension. In the present overview, we summarize the latest clinical and preclinical studies exploring the main effects of SGLT2-inhibitors on kidney and heart, emphasizing their potential beneficial actions in frailty.
Keywords: Empagliflozin, Frailty, Heart, Kidney, Metabolism, SGLT2-inhibitors, Vascular Medicine
Sodium Glucose co-Transporter 2 (SGLT2) inhibitors (also known as gliflozins) are oral antidiabetic drugs that have emerged as a cornerstone to reach and keep glycemic control1–4, particularly in older adults5–7. SGLT2 is a co-transporter that induces glucose and sodium (Na+) reabsorption in the kidney; hence, SGLT2-inhibitors act interfering with this process, reducing glycemia8, 9.
The first SGLT2 inhibitor to be approved by the Food and Drug Administration (FDA) as antihyperglycemic agent for patients with T2DM was canagliflozin in 2013, followed by dapagliflozin and empagliflozin in 2014, and ertugliflozin in 201710. The effects of SGLT2-inhibitors in type 2 diabetes mellitus (T2DM) have been evaluated by a plethora of studies, leading to groundbreaking results in cardiovascular disease and chronic kidney disease (CKD); SGLT2-inhibitors have been extensively studied beyond diabetes and are now considered pleiotropic drugs (Figure 1)11–13, having shown favorable effects in non-diabetic patients with heart failure (HF) with reduced ejection fraction (HFrEF) and preserved ejection fraction (HFpEF).
Figure 1.
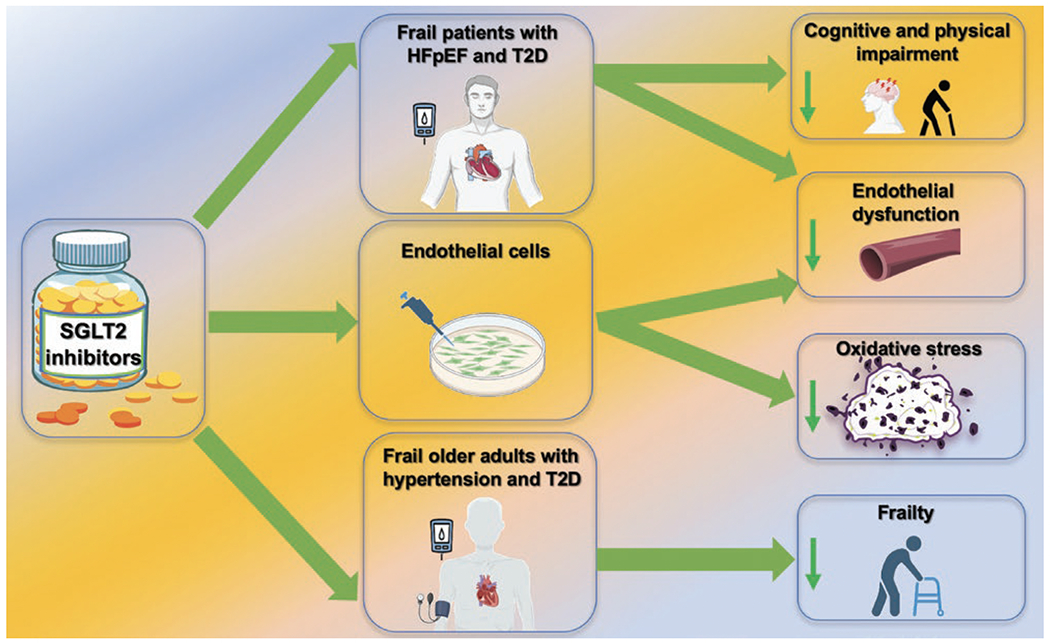
Pleiotropic actions of SGLT2-inhibitors.
Frailty and SGLT2-inhibitors
Frailty is a condition of vulnerability to stressors, which increases the risk of adverse health outcomes such as falls, disability, and hospitalization.
Our group has recently demonstrated the beneficial effects of the SGLT2 inhibitor empagliflozin on cognitive and physical impairment in frail older adults with diabetes and hypertension, highlighting how SGLT2-inhibition could attenuate mitochondrial oxidative stress in human endothelial cells14. Consistent with our findings, the efficacy and safety of SGLT2-inhibitors has been confirmed in frail elderly subjects by several investigators15–18, underscoring the large absolute benefits of treatment in these vulnerable patients, who are often needlessly denied therapy19, 20. Besides, a clinical trial known as EMPA-ELDERLY (NCT04531462), which includes 128 elderly Japanese patients with T2DM receiving Empagliflozin (10 mg) for 52-weeks has been completed in August 2022 but has yet to publish their findings21.
Clinical relevance of SGLT2-inhibitors in patients with comorbidities
Cardiovascular and renal disorders, including atherosclerotic cardiovascular disease, HF, and CKD, represent leading causes of death in patients with diabetes, and are prevailing comorbidities in frail patients22, 23. Analyses conducted by the US Diabetes Collaborative Registry found that among individuals with T2DM 94% have at least one comorbidity of which the most common are cardiovascular (32%) and renal (20%) diseases24. Likewise, over half of all patients living with HF have CKD, a decisive aspect, since the severity of renal dysfunction is associated with a graded increased risk of death25.
Pharmacology of SGLT2-inhibitors: main effects on the kidney
SGLT2 and glucose reabsorption
The kidney plays indispensable roles in the regulation of glucose level in the blood (Figure 2). It serves as the second largest producer of glucose in the organism after the liver, accounting for 20% of gluconeogenesis. No less important is glucose filtration in the renal glomeruli and further reabsorption in the proximal tubule of the nephron. Glucose freely passes through the glomerular filter and without reabsorption glucose excretion is estimated to reach 180g/day, which is roughly equal to its daily consumption. However, virtually all glucose is later reabsorbed, and a key role in this process is played by the two glucose transporters SGLT1 and SGLT2, which decrease glycemia by reducing renal glucose reabsorption and promoting glucosuria. Approximately 90% of the glucose filtered by the glomerulus is estimated to be reabsorbed via this mechanism.
Figure 2.
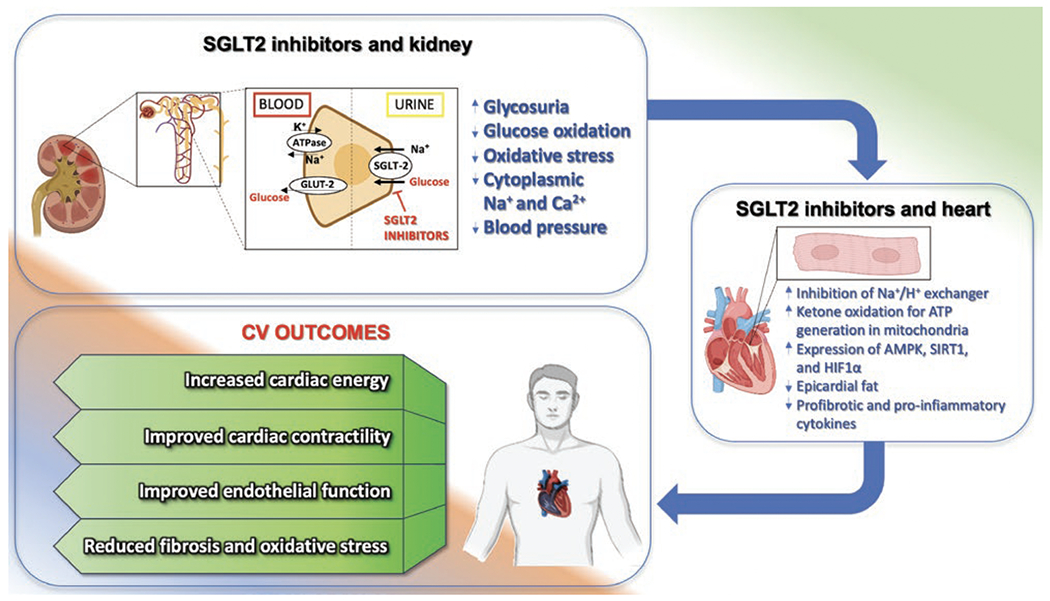
Main effects of SGLT2-inhibitors on kidney and heart.
SGLT2 is mainly localized at the apical membrane in the S1 and S2 segments of the proximal tubule while SGLT1 in the S3 segment, both in human and rodents. Remarkably, a higher expression of SGLT2 in females than in males has been described in rats, whereas no sex differences in this sense seem to be present in humans26, 27.
SGLT2 plays a major role in glucose reabsorption. Analysis of glomerular ultrafiltrate composition at different levels of nephron revealed that 93-97% of glucose is reabsorbed by SGLT2 and only 3% by SGLT128. However, in absence of SGLT2, SGLT1 can reabsorb a significant amount of glucose. Sglt2−/− mice develop glucosuria but maintain normal plasma glucose concentrations29. To achieve hypoglycemia in non-diabetic animals, the knockout of both transporters is required. Indeed, pharmacological inhibition of SGLT2 in euglycemic mice failed to induce hypoglycemia in wild-type but not in Sglt1−/− mice30. This partial backup of SGLT2 function by SGLT1 plays a vital role in the safety of SGLT2-inhibitors.
Glucose lowering effects of SGLT2-inhibitors can be partly attributed to the increased demand for glucose reabsorption observed in diabetes. As noted earlier, glucose passively flows through glomerular capillaries barrier, which means that hyperglycemia results in an increased amount of glucose filtered. In order to prevent glycosuria, SGLT2 expression in the kidneys is upregulated, thus enhancing glucose reabsorption31. Intriguingly, in diabetic kidneys, mainly SGLT2 expression is increased; the preferential overexpression of SGLT2 over SGLT1 is essentially explained by the fact that SGLT2 uses one Na+ ion to transport one glucose molecule, whereas SGLT1 uses two Na+ ions, making SGLT2 energetic favorable.
SGLT2 upregulation allows to prevent glycosuria in the majority of diabetic patients. However, when SGLT2 is pharmacologically inhibited in diabetes, SGLT1 is no longer capable of coping with the augmented reabsorption demand; hence, moderate glucosuria develops together with lowering glucose levels, but without significant risks of hypoglycemia31.
SGLT2 regulates glomerular filtration rate
SGLT2-inhibition exerts a number of effects on kidney function in diabetes and these effects are independent from lowering blood glucose level. First, SGLT2-inhibitors decrease Na+ reabsorption in proximal tubule. This action results in increased delivery of Na+ to the macula densa with subsequent normalization of the tubule-glomerular feedback32, 33, which is activated in diabetes due to augmented reabsorption of Na+ that is falsely sensed as a decrease in circulating blood volume. Low levels of Na+ at the macula densa level triggers adenosine-dependent relaxation of afferent arterioles and constriction of efferent arterioles, resulting in increased blood pressure in glomerular capillaries, ensuring increased filtration34. Inhibition of SGLT2 may counteract this pathological loop, also acting on the ROS-induced quenching of bioavailable nitric oxide35–38. In fact, treatment with SGLT2-inhibitors acutely decreases glomerular filtration rate (GFR), but this effect is reversible. Moreover, during the progression of diabetes, the initial increase of GFR is superseded by a decrease of GFR, as a result of CKD development. However, treatment with SGLT2-inhibitors prevents GFR decline in the longterm39.
Hyperfiltration is considered to be detrimental due to increased tensile stress applied to the capillary wall structures and heightened shear stress on the podocyte foot processes and body surface. These forces compromise the architecture of the glomerular filtration barrier, including but not limited to “stretching” the glomerular basement membrane, leading to a mismatch of the areas of glomerular basement membrane and podocyte foot processes, hypertrophy, and subsequent dysfunction of different type of cells in the glomeruli. Preclinical studies revealed that SGLT2-inhibitors are able to preserve normal architecture and function of the glomerular barrier in diabetes40.
Reduction of Na+ reabsorption by SGLT2-inhibitors is not limited to the decreased activity of the transporter per se. The inhibition of SGLT2 is coupled with a decreased activity of the Na+/H+ exchanger (NHE)41. This protein is expressed by various cells, including the apical membrane of renal epithelial cells, transporting Na+ inside and H+ outside the cell. The exact mechanism of NHE inhibition by gliflozins is not fully understood. NHE upregulation seems to be triggered by the enhancement of glycolysis and SGLT2-inhibitors diminish glucose influx. Nonetheless, microperfusion studies demonstrated the ability of SGLT2-inhibitors to inhibit NHE even in the absence of glucose42. A possible explanation for glucose-independent NHE with SGLT2-inhibitors may be the physical coupling of NHE and SGLT2 via scaffolding proteins, including PDZK1IP1/MAP1743.
SGLT2 and renal oxygen consumption
Inhibition of SGLT2 exerts dual effects on tubule oxygen consumption. The vast majority of ATP produced by oxidative phosphorylation in the renal tubular epithelium is consumed by the Na+/K+-ATPase, localized in the basal membrane, which uses ATP energy to pump Na+ out of the cell in order to allow Na+ to enter the cell via the apical membrane; in this manner, Na+ reabsorption is achieved. Inhibition of SGLT2 significantly decreases the amount of Na+ entering the cell, decreasing the energetic demand. This effect becomes even more meaningful in diabetes, when hyperperfusion augments the amount of Na+ in the ultrafiltrate44.
A marked accumulation of hypoxia-inducible factor 1 (HIF1) has been observed in the diabetic kidney, which was prevented by SGLT2-inhibition45. A direct measurement of oxygen tension in the kidney corroborated these results44. Interestingly, SGLT2-inhibitors were also capable to reduce hypoxic signaling in vitro45, a finding that may be explained by a reduced oxygen consumption by glycolysis. Yet, gliflozins evoke an increased energy demand in the lower segments of the nephron. An increased amount of glucose due to the SGLT2-inhibition upstream the nephron results in manifold augmented Na+ influx. Additionally, oxygen tension in peritubular capillaries is falling along the length of the nephron. Collectively, these processes eventually result in the activation of hypoxic signaling in distal parts of proximal tubule and in the loop of Henle46.
Effects of SGLT2-inhibition on nutrient sensing and renal mitochondria
Inhibition of SGLT2 diminishes glucose influx into the renal epithelium cells, thus mimicking the effects of caloric restriction, triggering akin protective signaling pathways. Several studies demonstrated that gliflozins can inhibit the mammalian target of rapamycin (mTOR)47, 48. Since the inhibition of SGLT2 cannot directly modify the availability of amino acids, a direct regulator of mTOR49, SGLT2-inhibitors are thought to inactivate mTOR utilizing upstream kinases, plausibly via AMP-activated protein kinase (AMPK), whose activation was demonstrated upon SGLT2-inhibition47, 50. Inhibition of mTOR is known to activate autophagy and indeed SGLT2-inhibitors have been shown to stimulate the autophagosome flux in renal epithelium47, 51.
Mitochondrial fragmentation accompanies renal injury of different etiologies and its prevention by pharmacological inhibition or genetic ablation of fission protein dynamin-related protein 1 (DRP1) is considered nephroprotective52. Pharmacological inhibition of SGLT2 has been shown to preserve an elongated mitochondrial architecture, mostly due to the downregulation of mitochondrial DRP1 and upregulation of the fusion protein mitofusin1 (MFN1)47, 48. Another positive effect of SGLT2-inhibitors on mitochondria relates to the upregulation of Nuclear factor erythroid 2-related factor 2 (NRF2)50, a transcriptional factor that induces the expression of a variety of enzymes controlling the redox status of the cell, ultimately preventing oxidative stress53. Indeed, gliflozins were shown to reduce the generation of reactive oxygen species (ROS) in several models of kidney disease47, 50. Inhibition of SGLT2 was found to ameliorate mitochondrial fatty acid metabolism and prevent lipid accumulation in the kidney48. Finally, treating rodents with SGLT2-inhibitors diminished mitochondrial apoptosis by downregulating bcl-2-like protein 4 (BAX) and upregulating B-cell lymphoma 2 (BCL-2) expression45, 47, 51.
SGLT2-inhibitors and the Kidney: Clinical Evidence
Diabetic nephropathy, the leading cause of CKD worldwide, exacerbates the progression of atherosclerotic cardiovascular disease, systemic hypertension, and cardiac dysfunction54. In addition to demonstrating decisive findings regarding cardiovascular outcomes, the EMPA-REG OUTCOME trial was also the first major trial to demonstrate the valuable effects of an SGLT2 inhibitor on kidney function. The investigators reported a reduction of incident or worsening nephropathy, defined as the development of macroalbuminuria (urinary albumin-to-creatinine ratio >300mg/g), a two-fold increase in serum creatinine levels accompanied by an estimated GFR of 45 ml/min/1.73m2 or less, initiation of renal replacement therapy, or death resulting from renal disease. They found that patients in the experimental group were at significantly lower risk for incident or worsening nephropathy39. A meta-analysis totaling 38723 participants, revealed that SGLT2-inhibitors significantly reduce the risk of dialysis, transplantation, or death due to kidney disease55. This benefit was evident in each of the four trials and across a wide range of baseline albuminuria and GFR. Follow-up studies by the investigators of other trials—Dapagliflozin in Patients with CKD (DAPA-CKD) and Empagliflozin in Patients with CKD (EMPA-KIDNEY), which was stopped early for efficacy—confirmed that patients with and without T2DM benefit from the reno-protective effects of dapagliflozin and empagliflozin56, 57. It should be noted that these studies reported statistically similar differences in safety outcomes between groups, which had initially been a concern in several of the trials assessing cardiovascular outcomes that reported an increased incidence of lower-limb amputation, diabetic ketoacidosis, and genital mycotic infections in SGLT2-inhibitor treated groups58, 59. Although some patients with type 1 diabetes were included in some of these trials (e.g. EMPA-KIDNEY), focused analyses of these drugs in this population remain limited.
Dapagliflozin and canagliflozin have been approved by the FDA for reducing the risk of end-stage kidney disease in patients with GFR ≥25 ml/min/1.73m2. Dapagliflozin is not limited to patients with diabetic nephropathy as it is also indicated for those with CKD secondary to ischemic nephropathy, focal segmental glomerulosclerosis, IgA nephropathy, and chronic interstitial nephritis. Although SGLT2-inhibitors have shown benefits in treating IgA nephropathy and focal segmental glomerulosclerosis, we want to underscore that these drugs should not be considered a replacement for immunosuppression in cases where it is medically necessary.
The effects of dapagliflozin on kidney outcomes were similar in both diabetic and non-diabetic patients. This finding could imply that the protective effects of SGLT2-inhibitors on the kidney are mediated by mechanisms beyond systemic and/or tubulointerstitial glycemic control. Indeed, renovascular hemodynamics are thought to play a central role in the reno-protective effects of SGLT2-inhibitors. The drug class acts by reducing glomerular pressure through the above-mentioned glomerulo-tubular feedback pathways. The macula densa/adenosine-mediated reduction in filtration pressure in patients treated with SGLT2-inhibitors accounts for an early drop in GFR, reaching a nadir within the first two weeks of treatment, an event consistent across all major trials.
SGLT2-inhibitors have been proven effective not only in controlling glycemia, showing insulin-independent glucose-lowering effects (with low hypoglycemia rates), but also in protecting heart and kidneys. Patients with diabetes have an increased risk of developing cardiovascular and renal disorders, and SGLT2-inhibitors offer significant protective effects. Several large-scale clinical outcome trials have demonstrated that these agents reduce the risk of hospitalizations due to HF and CKD progression. In addition to their established cardiovascular benefits, randomized data examined by Colin Baigent and collaborators in a recent meta-analysis, support the use of SGLT2-inhibitors for modifying risk of kidney disease progression and acute kidney injury, not only in T2DM patients at high cardiovascular risk, but also in patients with CKD or HF, irrespective of diabetes status and kidney function60. While the underlying mechanisms behind these effects are not fully understood, the benefits of SGLT2-inhibitors are clear, making them a mainstay of modern treatment paradigms for patients with diabetes, HF, and—more recently—CKD.
Gliflozins increase hemoglobin and hematocrit levels
Mounting evidence suggests a link between SGLT2-inhibitors and increased hematocrit (Hct)/hemoglobin/erythropoietin levels. Indeed, in the DAPA-HF trial, anemia was more frequently corrected by dapagliflozin than placebo; similarly, dapagliflozin and empagliflozin were associated with increased Hct in the DECLARE-TIMI 58 and in the EMPA-REG OUTCOME, respectively61.
Whether this phenomenon reflects hemoconcentration due to diuretic effects, expansion of red blood cell (RBC) mass due to increased erythropoietin (EPO), a modulation of the sympathetic hyperactivity, or other mechanisms remains unclear. Notwithstanding, it may be functionally linked to a reduced CKD progression, a lower risk of heart failure hospitalization, lower mortality, and potentially advantageous effects in frail populations.
EPO is synthesized in the renal cortex by EPO-producing fibroblasts. As mentioned above, patients with diabetes have increased glucose filtration, resulting in the upregulation of SGLT1 and SGLT2 in order to increase glucose resorption capacity. However, this process is energy-consuming: the resulting relative cortical hypoxia and increased oxidative stress from higher energy demands of these transporters in the renal cortex cause the cortical fibroblasts to transform into myofibroblasts, which no longer produce EPO. By blocking these transporters, SGLT2-inhibitors reduce energy demands: henceforth, the cortical injury is reduced, the transformation reverses, and EPO production capacity is restored. Additionally, SGLT2-inhibition increases Na+ delivery to the distal portions of the nephron, which evokes the upregulation of medullary Na+ transporters in the loop of Henle and terminal nephron eventually resulting in relative medullary hypoxia, which stimulates erythropoiesis.
SGLT2-inhibitors and Cardiovascular Disease
Main clinical trials assessing the effects of SGLT2-inhibitors on cardiovascular outcomes
One of the first large placebo-controlled trials assessing the effects of an SGLT2 inhibitor specifically on cardiovascular outcomes was the Empagliflozin Cardiovascular Outcome Event Trial in T2DM Patients–Removing Excess Glucose (EMPA-REG OUTCOME) trial. It paired 4687 patients with T2DM at high risk for cardiovascular events taking 10 mg or 25 mg of empagliflozin once daily with 2333 in the placebo group. The primary endpoint was a composite of death from cardiovascular causes, nonfatal myocardial infarction, or nonfatal stroke, which was found to be significantly lower in the empagliflozin group. As adverse events, an increased rate of genital infection was observed in the empagliflozin group, but no increase in other side effects was reported62.
In 2017, with the CANVAS Program, data from two trials, the CANVAS and the CANVAS-Renal (CANVAS-R)63, were integrated, totaling 10142 participants with T2DM and high cardiovascular risk, who were randomized to receive canagliflozin or placebo. The risk of the primary outcome, a composite of death from cardiovascular causes, nonfatal myocardial infarction, or nonfatal stroke, was higher in the placebo than in the treated group. Adverse reactions observed were genito-urinary infections, volume depletion, diuresis, and increased risk of amputation; the highest absolute risk of amputation was observed in patients with a previous history of peripheral artery disease64. Over the following decade, these findings would have been validated by several other trials drawing from populations with distinct risk factors. The most prominent examples include the Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation (CREDENCE) trial65, the Multicenter Trial to Evaluate the Effect of Dapagliflozin on the Incidence of Cardiovascular Events (DECLARE-TIMI 58)66, the Design and Baseline Characteristics of the Evaluation of Ertugliflozin Efficacy and Safety Cardiovascular Outcomes trial (VERTIS-CV)13, and the Effect of Sotagliflozin on Cardiovascular and Renal Events in Participants with Type 2 Diabetes and Moderate Renal Impairment Who Are at Cardiovascular Risk (SCORED) trial.67 A meta-analysis of some of these studies, except the SCORED, has shown that SGLT2-inhibitors significantly reduce the hazard for major adverse cardiovascular events (MACE; HR:0.90; 95%CI:0.85-0.95). Furthermore, the presence of atherosclerotic cardiovascular disease did not modify the treatment outcome on MACE68. There was a marked variability in MACE among each of the four gliflozins studied, which still needs further exploration. However, the predominant cardiovascular benefit across all these trials was noted to be a reduction in HF hospitalizations.
The cardiovascular safety profile of dapagliflozin was assessed by Wiviott and collaborators: 17,160 patients with T2DM were randomly assigned to dapagliflozin or placebo. The primary safety outcome was a composite of MACE, for which dapagliflozin resulted non-inferior to placebo (P<0.001). The primary efficacy outcomes were MACE plus a composite of cardiovascular death or hospitalization for HF; patients in dapagliflozin group had a lower rate of cardiovascular death or hospitalization for HF versus placebo group. Thus treatment with dapagliflozin resulted in a lower rate of hospitalization for HF66.
The main clinical trials substantiating the cardioprotective effects of SGLT2-inhibitors in HF are summarized in Table 1. These trials also confirmed that the main adverse effects of gliflozins were infections of the genito-urinary tract and volume depletion, without an increased risk of hypoglycemia69–71.
Table 1.
Main clinical trials assessing the efficacy and safety of SGLT2-inhibitors in HF.
| Trial name | Regimen | Number of patients | Primary outcome(s) | Results |
|---|---|---|---|---|
| CANVAS63 | Canagliflozin 100 or 300 mg | 10142 | composite of death from cardiovascular causes, nonfatal MI, or nonfatal stroke | HR:0.86; 95%CI: 0.75 to 0.97; P<0.001 for non-inferiority; P=0.02 for superiority |
| CREDENCE82 | Canagliflozin 100 mg | 4401 | composite of end-stage kidney disease, a doubling of the serum creatinine level, or death from renal or cardiovascular causes | HR:0.70; 95%CI: 0.59 to 0.82; P=0.00001 |
| DAPA-CKD57 | Dapagliflozin 10 mg | 4304 | composite of a sustained decline in the estimated GFR of at least 50%, end-stage kidney disease, or death from renal or cardiovascular causes | HR:0.61; 95%CI: 0.51 to 0.72; P<0.001 |
| DAPA-HF83 | Dapagliflozin 10 mg | 4744 | composite of cardiovascular death or episode of worsening HF | HR:0.74; 95%CI: 0.65 to 0.85; P<0.001 |
| DECLARE-TIMI 5884 | Dapagliflozin 10 mg | 17160 | composite of MACE and cardiovascular death or hospitalization for HF | HR:0.83; 95%CI: 0.73 to 0.95; P=0.005 |
| EMPA-REG OUTCOME reported62 | Empagliflozin 10 or 25 mg | 7020 | composite of death from cardiovascular causes, nonfatal MI, or nonfatal stroke | HR:0.86; 95.02%CI: 0.74 to 0.99; P=0.04 |
| EMPEROR-REDUCED85 | Empagliflozin 10 mg | 3730 | composite of adjudicated cardiovascular death or hospitalization for HF | HR:0.76; 95%CI: 0.67–0.87; P<0.0001 |
| VERTIS-CV13 | Ertugliflozin 5 or 15 mg | 8246 | composite of MACE | HR:0.97; 95.6%CI: 0.85 to 1.11; P<0.001 |
CI: Confidence Interval; GFR: Glomerular Filtration Rate; HF: Heart Failure; HR: Hazard Ratio; MACE: Major Adverse Cardiovascular Events; MI: Myocardial Infarction.
Potential mechanisms underlying the cardioprotective effects of SGLT2-inhibitors
Numerous theories exist regarding the cardiovascular benefits of SGLT2-inhibitors, which are attributed to both direct and indirect mechanisms (Figure 2). The results of the DAPA-HF trial were among the first to imply that the advantages observed in HF cannot be attributed solely to the blood glucose-lowering effects. However, the most significant impact of this innovative drug class on heart and vascular function has yet to be established. Currently, the primary pathways thought to be involved are the reduction of blood pressure (also via increased diuresis and natriuresis), weight loss (more so in patients with T2DM), decreased insulin resistance, improved cardiomyocyte Ca2+ handling, induction of autophagy and lysosomal degradation, reduced epicardial fat, suppression of adipokine and cytokine-mediated inflammation, promotion of autophagy/mitophagy, prevention of adverse cardiac remodeling, inhibition of NHE, reduction of ROS production and NLRP3-inflammasome activity, and improved cardiac mitochondrial bioenergetics—partly through an increase of circulating ketone bodies, which have been shown to play a positive adaptive role in H9, 41, 72–75.
We demonstrated that empagliflozin significantly reduces mitochondrial calcium overload through many of these pathways in human vascular endothelium; of note, ROS production triggered by high glucose in endothelial cells was ameliorated by empagliflozin, improving cell viability in response to oxidative stress14, 76. Additionally, the relationship between weight redistribution and local inflammation cannot be overlooked. In many patients with T2DM, excessive epicardial adipose tissue surrounds the aorta, coronary arteries, and ventricles, leading to the release of proinflammatory mediators (including leptin, tumor necrosis factor-α, resistin, interleukin-1β and interleukin-6) that can impair ventricular function and lead to myocardial fibrosis77–79. A 2021 meta-analysis reported that SGLT2-inhibitors markedly decrease epicardial adipose tissue in patients with T2DM (standardized mean difference 0.82; 95%CI:0.15-1.49)80. Larger studies are warranted to confirm these aspects.
The cardiovascular benefit of SGLT2-inhibitors may also be tied to their effects on renovascular hemodynamics. By increasing Na+ delivery to the macula densa, SGLT2-inhibitors increase the vasoconstriction of the afferent renal arterioles, thereby reducing hyperfiltration-mediated inflammatory pathways and renal tubule oxygen requirements78. Enhancing renal function and/or mitigating renal stress can indirectly slow the progression of HF through multiple canonical pathways, such as decreasing afferent sympathetic nervous system activation, alleviating inflammation, and further minimizing ROS production81.
Conclusions
Several major clinical trials have spotlighted the cardiac and renal protective effects of gliflozins. Nowadays, SGLT2-inhibitors are well-known not only for their efficacy in glycemic control but are also proven to decrease atherosclerotic events, hospitalizations for HF, cardiovascular mortality, and the advancement of CKD. Given the correlation between diabetes, CKD, and cardiovascular disease including HFrEF and HFpEF, these agents already play a crucial role in modern treatment paradigms.
Funding:
The Santulli’s Lab is currently supported in part by the National Institutes of Health (NIH): National Heart, Lung, and Blood Institute (NHLBI: R01-HL164772, R01-HL159062, R01-HL146691, T32-HL144456), National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK: R01-DK123259, R01-DK033823), National Center for Advancing Translational Sciences (NCATS: UL1-TR002556-06, UM1-TR004400) to G.S., by the Diabetes Action Research and Education Foundation (to G.S.), and by the Monique Weill-Caulier and Irma T. Hirschl Trusts (to G.S.). F.V. is supported in part by a postdoctoral fellowship of the American Heart Association (AHA-22POST915561); S.S.J. is supported in part by a postdoctoral fellowship of the American Heart Association (AHA-21POST836407); U.K. is supported in part by a postdoctoral fellowship of the American Heart Association (AHA-23POST1026190).
Abbreviations
- CKD
Chronic kidney disease
- HF
Heart failure
- SGLT2
Sodium-glucose co-transporter 2
- T2DM
Type 2 diabetes mellitus
Footnotes
Disclosure: None.
References
- 1.ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, Collins BS, Hilliard ME, Isaacs D, Johnson EL, et al. ; on behalf of the American Diabetes Association. Pharmacologic approaches to glycemic treatment: standards of care in diabetes-2023. Diabetes Care. 2023;46:S140–S157. doi: 10.2337/dc23-S009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yuan S, Song C, He J, Zhang R, Bian X, Song W, Dou K. Trends in cardiovascular risk factors control among US adults by glycemic statuses, 2007-2018. Eur J Prev Cardiol. 2023; in press. doi: 10.1093/eurjpc/zwad080 [DOI] [PubMed] [Google Scholar]
- 3.Salmen T, Serbanoiu LI, Bica IC, Serafinceanu C, Muzurović E, Janez A, Busnatu S, Banach M, Rizvi AA, Rizzo M, et al. A critical view over the newest antidiabetic molecules in light of efficacy-a systematic review and metaanalysis. Int J Mol Sci. 2023;24:9760. doi: 10.3390/ijms24119760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Samson SL, Vellanki P, Blonde L, Christofides EA, Galindo RJ, Hirsch IB, Isaacs SD, Izuora KE, Low Wang CC, Twining CL, et al. American Association of Clinical Endocrinology Consensus statement: comprehensive type 2 diabetes management algorithm - 2023 update. Endocr Pract. 2023;29:305–340. doi: 10.1016/j.eprac.2023.02.001 [DOI] [PubMed] [Google Scholar]
- 5.ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, Collins BS, Hilliard ME, Isaacs D, Johnson EL, et al. ; on behalf of the American Diabetes Association. Older adults: standards of care in diabetes-2023. Diabetes Care. 2023;46:S216–S229. doi: 10.2337/dc23-S013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lunati ME, Cimino V, Gandolfi A, Trevisan M, Montefusco L, Pastore I, Pace C, Betella N, Favacchio G, Bulgheroni M, et al. SGLT2-inhibitors are effective and safe in the elderly: the SOLD study. Pharmacol Res. 2022;183:106396. doi: 10.1016/j.phrs.2022.106396 [DOI] [PubMed] [Google Scholar]
- 7.Hias J, Hellemans L, Walgraeve K, Tournoy J, Van der Linden L. SGLT2 inhibitors in older adults with heart failure with preserved ejection fraction. Drugs Aging. 2022;39:185–190. doi: 10.1007/s40266-022-00920-7 [DOI] [PubMed] [Google Scholar]
- 8.Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, Januzzi J, Verma S, Tsutsui H, Brueckmann M, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. NEJM. 2020;383:1413–1424. doi: 10.1056/NEJMoa2022190 [DOI] [PubMed] [Google Scholar]
- 9.Varzideh F, Kansakar U, Santulli G. SGLT2 inhibitors in cardiovascular medicine. Eur Heart J Cardiovasc Pharmacother. 2021;7:e67–e68. doi: 10.1093/ehjcvp/pvab039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Teo YN, Ting AZH, Teo YH, Chong EY, Tan JTA, Syn NL, Chia AZQ, Ong HT, Cheong AJY, Li TY, et al. Effects of sodium/glucose cotransporter 2 (SGLT2) inhibitors and combined SGLT1/2 inhibitors on cardiovascular, metabolic, renal, and safety outcomes in patients with diabetes: a network meta-analysis of 111 randomized controlled trials. Am J Cardiovasc Drugs. 2022;22:299–323. doi: 10.1007/s40256-022-00528-7 [DOI] [PubMed] [Google Scholar]
- 11.Solomon SD, McMurray JJV, Claggett B, de Boer RA, DeMets D, Hernandez AF, Inzucchi SE, Kosiborod MN, Lam CSP, Martinez F, et al. Dapagliflozin in heart failure with mildly reduced or preserved ejection fraction. NEJM. 2022;387:1089–1098. doi: 10.1056/NEJMoa2206286 [DOI] [PubMed] [Google Scholar]
- 12.Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Böhm M, Brunner-La Rocca HP, Choi DJ, Chopra V, Chuquiure-Valenzuela E, et al. Empagliflozin in heart failure with a preserved ejection fraction. NEJM. 2021;385:1451–1461. doi: 10.1056/NEJMoa2107038 [DOI] [PubMed] [Google Scholar]
- 13.Cannon CP, Pratley R, Dagogo-Jack S, Mancuso J, Huyck S, Masiukiewicz U, Charbonnel B, Frederich R, Gallo S, Cosentino F, et al. Cardiovascular outcomes with ertugliflozin in type 2 diabetes. NEJM. 2020;383:1425–1435. doi: 10.1056/NEJMoa2004967 [DOI] [PubMed] [Google Scholar]
- 14.Mone P, Varzideh F, Jankauskas SS, Pansini A, Lombardi A, Frullone S, Santulli G. SGLT2 inhibition via empagliflozin improves endothelial function and reduces mitochondrial oxidative stress: insights from frail hypertensive and diabetic patients. Hypertension. 2022;79:1633–1643. doi: 10.1161/HYPERTENSIONAHA.122.19586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abdelhafiz AH, Sinclair AJ. Cardio-renal protection in older people with diabetes with frailty and medical comorbidities - A focus on the new hypoglycaemic therapy. J Diabetes Complications. 2020;34:107639. doi: 10.1016/j.jdiacomp.2020.107639 [DOI] [PubMed] [Google Scholar]
- 16.Sasaki T. Sarcopenia, frailty circle and treatment with sodium-glucose cotransporter 2 inhibitors. J Diabetes Investig. 2019;10:193–195. doi: 10.1111/jdi.12966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sinclair AJ, Pennells D, Abdelhafiz AH. Hypoglycaemic therapy in frail older people with type 2 diabetes mellitus-a choice determined by metabolic phenotype. Aging Clin Exp Res. 2022;34:1949–1967. doi: 10.1007/s40520-022-02142-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Villarreal D, Ramírez H, Sierra V, Amarís JS, Lopez-Salazar AM, González-Robledo G. Sodium-glucose cotransporter 2 inhibitors in frail patients with heart failure: clinical experience of a heart failure unit. Drugs Aging. 2023;40:293–299. doi: 10.1007/s40266-022-01004-2 [DOI] [PubMed] [Google Scholar]
- 19.Butt JH, Dewan P, Merkely B, Belohlávek J, Drożdż J, Kitakaze M, Inzucchi SE, Kosiborod MN, Martinez FA, Tereshchenko S, et al. Efficacy and safety of dapagliflozin according to frailty in heart failure with reduced ejection fraction: a post hoc analysis of the DAPA-HF trial. Ann Intern Med. 2022;175:820–830. doi: 10.7326/M21-4776 [DOI] [PubMed] [Google Scholar]
- 20.Pollack R, Cahn A. SGLT2 inhibitors and safety in older patients. Heart Fail Clin. 2022;18:635–643. doi: 10.1016/j.hfc.2022.03.002 [DOI] [PubMed] [Google Scholar]
- 21.Yabe D, Shiki K, Suzaki K, Meinicke T, Kotobuki Y, Nishida K, Clark D, Yasui A, Seino Y. Rationale and design of the EMPA-ELDERLY trial: a randomised, double-blind, placebo-controlled, 52-week clinical trial of the efficacy and safety of the sodium-glucose cotransporter-2 inhibitor empagliflozin in elderly Japanese patients with type 2 diabetes. BMJ Open. 2021;11:e045844. doi: 10.1136/bmjopen-2020-045844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raghavan S, Vassy JL, Ho Y-L, Song RJ, Gagnon DR, Cho K, Wilson PWF, Phillips LS. Diabetes mellitus-related all-cause and cardiovascular mortality in a national cohort of adults. J Am Heart Assoc. 2019;8:e011295. doi: 10.1161/JAHA.118.011295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jankauskas SS, Kansakar U, Varzideh F, Wilson S, Mone P, Lombardi A, Gambardella J, Santulli G. Heart failure in diabetes. Metab Clin Exp. 2021;125:154910. doi: 10.1016/j.metabol.2021.154910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arnold SV, Kosiborod M, Wang J, Fenici P, Gannedahl G, LoCasale RJ. Burden of cardio-renal-metabolic conditions in adults with type 2 diabetes within the Diabetes Collaborative Registry. Diabetes Obes Metab. 2018;20:2000–2003. doi: 10.1111/dom.13303 [DOI] [PubMed] [Google Scholar]
- 25.Damman K, Valente MAE, Voors AA, O'Connor CM, van Veldhuisen DJ, Hillege HL. Renal impairment, worsening renal function, and outcome in patients with heart failure: an updated meta-analysis. Eur Heart J. 2014;35:455–469. doi: 10.1093/eurheartj/eht386 [DOI] [PubMed] [Google Scholar]
- 26.Vrhovac I, Balen Eror D, Klessen D, Burger C, Breljak D, Kraus O, Radović N, Jadrijević S, Aleksic I, Walles T, et al. Localizations of Na(+)-D-glucose cotransporters SGLT1 and SGLT2 in human kidney and of SGLT1 in human small intestine, liver, lung, and heart. Pflugers Arch. 2015;467:1881–1898. doi: 10.1007/s00424-014-1619-7 [DOI] [PubMed] [Google Scholar]
- 27.Sabolic I, Vrhovac I, Eror DB, Gerasimova M, Rose M, Breljak D, Ljubojevic M, Brzica H, Sebastiani A, Thal SC, et al. Expression of Na+-D-glucose cotransporter SGLT2 in rodents is kidney-specific and exhibits sex and species differences. Am J Physiol Cell Physiol. 2012;302:C1174–C1188. doi: 10.1152/ajpcell.00450.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gorboulev V, Schürmann A, Vallon V, Kipp H, Jaschke A, Klessen D, Friedrich A, Scherneck S, Rieg T, Cunard R, et al. Na(+)-D-glucose cotransporter SGLT1 is pivotal for intestinal glucose absorption and glucose-dependent incretin secretion. Diabetes. 2012;61:187–196. doi: 10.2337/db11-1029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vallon V, Platt KA, Cunard R, Schroth J, Whaley J, Thomson SC, Koepsell H, Rieg T. SGLT2 mediates glucose reabsorption in the early proximal tubule. J Am Soc Nephrol. 2011;22:104–112. doi: 10.1681/ASN.2010030246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rieg T, Masuda T, Gerasimova M, Mayoux E, Platt K, Powell DR, Thomson SC, Koepsell H, Vallon V. Increase in SGLT1-mediated transport explains renal glucose reabsorption during genetic and pharmacological SGLT2 inhibition in euglycemia. Am J Physiol Renal Physiol. 2014;306:F188–F193. doi: 10.1152/ajprenal.00518.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Umino H, Hasegawa K, Minakuchi H, Muraoka H, Kawaguchi T, Kanda T, Tokuyama H, Wakino S, Itoh H. High basolateral glucose increases sodium-glucose cotransporter 2 and reduces Sirtuin-1 in renal tubules through glucose transporter-2 detection. Sci Rep. 2018;8:6791. doi: 10.1038/s41598-018-25054-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vallon V, Richter K, Blantz RC, Thomson S, Osswald H. Glomerular hyperfiltration in experimental diabetes mellitus: potential role of tubular reabsorption. J Am Soc Nephrol. 1999;10:2569–2576. doi: 10.1681/ASN.V10122569 [DOI] [PubMed] [Google Scholar]
- 33.Thomson SC, Rieg T, Miracle C, Mansoury H, Whaley J, Vallon V, Singh P. Acute and chronic effects of SGLT2 blockade on glomerular and tubular function in the early diabetic rat. Am J Physiol Regul Integr Comp Physiol. 2012;302:R75–R83. doi: 10.1152/ajpregu.00357.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kidokoro K, Cherney DZI, Bozovic A, Nagasu H, Satoh M, Kanda E, Sasaki T, Kashihara N. Evaluation of glomerular hemodynamic function by empagliflozin in diabetic mice using in vivo imaging. Circulation. 2019;140:303–315. doi: 10.1161/CIRCULATIONAHA.118.037418 [DOI] [PubMed] [Google Scholar]
- 35.Song P, Huang W, Onishi A, Patel R, Kim YC, van Ginkel C, Fu Y, Freeman B, Koepsell H, Thomson S, et al. Knockout of Na(+)-glucose cotransporter SGLT1 mitigates diabetes-induced upregulation of nitric oxide synthase NOS1 in the macula densa and glomerular hyperfiltration. Am J Physiol Renal Physiol. 2019;317:F207–F217. doi: 10.1152/ajprenal.00120.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vallon V, Gerasimova M, Rose MA, Masuda T, Satriano J, Mayoux E, Koepsell H, Thomson SC, Rieg T. SGLT2 inhibitor empagliflozin reduces renal growth and albuminuria in proportion to hyperglycemia and prevents glomerular hyperfiltration in diabetic Akita mice. Am J Physiol Renal Physiol. 2014;306:F194–F204. doi: 10.1152/ajprenal.00520.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paolocci N, Biondi R, Bettini M, Lee CI, Berlowitz CO, Rossi R, Xia Y, Ambrosio G, L’Abbate A, Kass DA, et al. Oxygen radical-mediated reduction in basal and agonist-evoked NO release in isolated rat heart. J Mol Cell Cardiol. 2001;33:671–679. doi: 10.1006/jmcc.2000.1334 [DOI] [PubMed] [Google Scholar]
- 38.Fujita H, Otomo H, Takahashi Y, Yamada Y. Dual inhibition of SGLT2 and DPP-4 promotes natriuresis and improves glomerular hemodynamic abnormalities in KK/Ta-Ins2(Akita) mice with progressive diabetic kidney disease. Biochem Biophys Res Commun. 2022;635:84–91. doi: 10.1016/j.bbrc.2022.10.034 [DOI] [PubMed] [Google Scholar]
- 39.Wanner C, Inzucchi SE, Lachin JM, Fitchett D, von Eynatten M, Mattheus M, Johansen OE, Woerle HJ, Broedl UC, Zinman B; EMPA-REG OUTCOME Investigators. Empagliflozin and progression of kidney disease in type 2 diabetes. NEJM. 2016;375:323–334. doi: 10.1056/NEJMoa1515920 [DOI] [PubMed] [Google Scholar]
- 40.Locatelli M, Zoja C, Conti S, Cerullo D, Corna D, Rottoli D, Zanchi C, Tomasoni S, Remuzzi G, Benigni A. Empagliflozin protects glomerular endothelial cell architecture in experimental diabetes through the VEGF-A/caveolin-1/PV-1 signaling pathway. J Pathol. 2022;256:468–479. doi: 10.1002/path.5862 [DOI] [PubMed] [Google Scholar]
- 41.Onishi A, Fu Y, Patel R, Darshi M, Crespo-Masip M, Huang W, Song P, Freeman B, Kim YC, Soleimani M, et al. A role for tubular Na(+)/H(+) exchanger NHE3 in the natriuretic effect of the SGLT2 inhibitor empagliflozin. Am J Physiol Renal Physiol. 2020;319:F712–F728. doi: 10.1152/ajprenal.00264.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pessoa TD, Campos LCG, Carraro-Lacroix L, Girardi ACC, Malnic G. Functional role of glucose metabolism, osmotic stress, and sodium-glucose cotransporter isoform-mediated transport on Na+/H+ exchanger isoform 3 activity in the renal proximal tubule. J Am Soc Nephrol. 2014;25:2028–2039. doi: 10.1681/ASN.2013060588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Coady MJ, El Tarazi A, Santer R, Bissonnette P, Sasseville LJ, Calado J, Lussier Y, Dumayne C, Bichet DG, Lapointe J-Y. MAP17 is a necessary activator of renal Na+/glucose cotransporter SGLT2. J Am Soc Nephrol. 2017;28:85–93. doi: 10.1681/ASN.2015111282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.O’Neill J, Fasching A, Pihl L, Patinha D, Franzén S, Palm F. Acute SGLT inhibition normalizes O2 tension in the renal cortex but causes hypoxia in the renal medulla in anaesthetized control and diabetic rats. Am J Physiol Renal Physiol. 2015;309:F227–F234. doi: 10.1152/ajprenal.00689.2014 [DOI] [PubMed] [Google Scholar]
- 45.Huang X, Guo X, Yan G, Zhang Y, Yao Y, Qiao Y, Wang D, Chen G, Zhang W, Tang C, et al. Dapagliflozin attenuates contrast-induced acute kidney injury by regulating the HIF-1alpha/HE4/NF-kappaB pathway. J Cardiovasc Pharmacol. 2022;79:904–913. doi: 10.1097/FJC.0000000000001268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Layton AT, Vallon V. SGLT2 inhibition in a kidney with reduced nephron number: modeling and analysis of solute transport and metabolism. Am J Physiol Renal Physiol. 2018;314:F969–F984. doi: 10.1152/ajprenal.00551.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee YH, Kim SH, Kang JM, Heo JH, Kim D-J, Park SH, Sung MJ, Kim J, Oh J, Yang DH, et al. Empagliflozin attenuates diabetic tubulopathy by improving mitochondrial fragmentation and autophagy. Am J Physiol Renal Physiol. 2019;317:F767–F780. doi: 10.1152/ajprenal.00565.2018 [DOI] [PubMed] [Google Scholar]
- 48.Ke Q, Shi C, Lv Y, Wang L, Luo J, Jiang L, Yang J, Zhou Y. SGLT2 inhibitor counteracts NLRP3 inflammasome via tubular metabolite itaconate in fibrosis kidney. FASEB J. 2022;36:e22078. doi: 10.1096/fj.202100909RR [DOI] [PubMed] [Google Scholar]
- 49.Al-Bari MA, Xu P. Molecular regulation of autophagy machinery by mTORdependent and -independent pathways. Ann N Y Acad Sci. 2020;1467:3–20. doi: 10.1111/nyas.14305 [DOI] [PubMed] [Google Scholar]
- 50.Chi P-J, Lee C-J, Hsieh Y-J, Lu C-W, Hsu B-G. Dapagliflozin ameliorates lipopolysaccharide related acute kidney injury in mice with streptozotocin-induced diabetes mellitus. Int J Med Sci. 2022;19:729–739. doi: 10.7150/ijms.69031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ala M, Khoshdel MRF, Dehpour AR. Empagliflozin enhances autophagy, mitochondrial biogenesis, and antioxidant defense and ameliorates renal ischemia/reperfusion in nondiabetic rats. Oxid Med Cell Longev. 2022;2022:1197061. doi: 10.1155/2022/1197061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Perry HM, Huang L, Wilson RJ, Bajwa A, Sesaki H, Yan Z, Rosin DL, Kashatus DF, Okusa MD. Dynamin-related protein 1 deficiency promotes recovery from AKI. J Am Soc Nephrol. 2018;29:194–206. doi: 10.1681/ASN.2017060659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen QM, Maltagliati AJ. Nrf2 at the heart of oxidative stress and cardiac protection. Physiol Genomics. 2018;50:77–97. doi: 10.1152/physiolgenomics.00041.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yamaguchi S, Hamano T, Oka T, Doi Y, Kajimoto S, Sakaguchi Y, Suzuki A, Isaka Y. Low-grade proteinuria and atherosclerotic cardiovascular disease: a transition study of patients with diabetic kidney disease. PLoS One. 2022;17:e0264568. doi: 10.1371/journal.pone.0264568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Neuen BL, Young T, Heerspink HJL, Neal B, Perkovic V, Billot L, Mahaffey KW, Charytan DM, Wheeler DC, Arnott C, et al. SGLT2 inhibitors for the prevention of kidney failure in patients with type 2 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2019;7:845–854. doi: 10.1016/S2213-8587(19)30256-6 [DOI] [PubMed] [Google Scholar]
- 56.Heerspink HJL, Stefánsson BV, Correa-Rotter R, Chertow GM, Greene T, Hou FF, Mann JFE, McMurray JJV, Lindberg M, Rossing P, et al. Dapagliflozin in patients with chronic kidney disease. NEJM. 2020;383:1436–1446. doi: 10.1056/NEJMoa2024816 [DOI] [PubMed] [Google Scholar]
- 57.Herrington WG, Staplin N, Wanner C, Green JB, Hauske SJ, Emberson JR, Preiss D, Judge P, Mayne KJ, Ng SYA, et al. Empagliflozin in patients with chronic kidney disease. NEJM. 2023;388:117–127. doi: 10.1056/NEJMoa2204233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Matthews DR, Li Q, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Desai M, Hiatt WR, Nehler M, Fabbrini E, et al. Effects of canagliflozin on amputation risk in type 2 diabetes: the CANVAS Program. Diabetologia. 2019;62:926–938. doi: 10.1007/s00125-019-4839-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yau K, Dharia A, Alrowiyti I, Cherney DZI. Prescribing SGLT2 inhibitors in patients with CKD: expanding indications and practical considerations. Kidney Int Rep. 2022;7:1463–1476. doi: 10.1016/j.ekir.2022.04.094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nuffield Department of Population Health Renal Studies. Impact of diabetes on the effects of sodium glucose co-transporter-2 inhibitors on kidney outcomes: collaborative meta-analysis of large placebo-controlled trials. Lancet. 2022;400:1788–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kolkailah AA, Wiviott SD, Raz I, Murphy SA, Mosenzon O, Bhatt DL, Leiter LA, Wilding JPH, Gause-Nilsson I, Sabatine MS, et al. Effect of dapagliflozin on hematocrit in patients with type 2 diabetes at high cardiovascular risk: observations from DECLARE-TIMI 58. Diabetes Care. 2022;45:e27–e29. doi: 10.2337/dc21-1668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. NEJM. 2015;373:2117–2128. doi: 10.1056/NEJMoa1504720 [DOI] [PubMed] [Google Scholar]
- 63.Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, Shaw W, Law G, Desai M, Matthews DR; CANVAS Program Collaborative Group. Canagliflozin and cardiovascular and renal events in type 2 diabetes. NEJM. 2017;377:644–657. doi: 10.1056/NEJMoa1611925 [DOI] [PubMed] [Google Scholar]
- 64.Mahaffey KW, Neal B, Perkovic V, de Zeeuw D, Fulcher G, Erondu N, Shaw W, Fabbrini E, Sun T, Li Q, et al. ; CANVAS Program Collaborative Group. Canagliflozin for primary and secondary prevention of cardiovascular events: results from the CANVAS program (Canagliflozin Cardiovascular Assessment Study). Circulation. 2018;137:323–334. doi: 10.1161/CIRCULATIONAHA.117.032038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, Edwards R, Agarwal R, Bakris G, Bull S, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. NEJM. 2019;380:2295–2306. doi: 10.1056/NEJMoa1811744 [DOI] [PubMed] [Google Scholar]
- 66.Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Silverman MG, Zelniker TA, Kuder JF, Murphy SA, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. NEJM. 2019;380:347–357. doi: 10.1056/NEJMoa1812389 [DOI] [PubMed] [Google Scholar]
- 67.Bhatt DL, Szarek M, Pitt B, Cannon CP, Leiter LA, McGuire DK, Lewis JB, Riddle MC, Inzucchi SE, Kosiborod MN, et al. Sotagliflozin in patients with diabetes and chronic kidney disease. NEJM. 2020;384:129–139. doi: 10.1056/NEJMoa2030186 [DOI] [PubMed] [Google Scholar]
- 68.McGuire DK, Shih WJ, Cosentino F, Charbonnel B, Cherney DZI, Dagogo-Jack S, Pratley R, Greenberg M, Wang S, Huyck S, et al. Association of SGLT2 inhibitors with cardiovascular and kidney outcomes in patients with type 2 diabetes: a meta-analysis. JAMA Cardiol. 2021;6:148–158. doi: 10.1001/jamacardio.2020.4511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sarraju A, Li JW, Cannon CP, Chang TI, Agarwal R, Bakris G, Charytan DM, de Zeeuw D, Greene T, Heerspink HJL, et al. Effects of canagliflozin on cardiovascular, renal, and safety outcomes in participants with type 2 diabetes and chronic kidney disease according to history of heart failure: results from the CREDENCE trial. Am Heart J. 2021;233:141–148. doi: 10.1016/j.ahj.2020.12.008 [DOI] [PubMed] [Google Scholar]
- 70.McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Bělohlávek J, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. NEJM. 2019;381:1995–2008. doi: 10.1056/NEJMoa1911303 [DOI] [PubMed] [Google Scholar]
- 71.Zelniker TA, Bonaca MP, Furtado RHM, Mosenzon O, Kuder JF, Murphy SA, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, et al. Effect of dapagliflozin on atrial fibrillation in patients with type 2 diabetes mellitus: insights from the DECLARE-TIMI 58 trial. Circulation. 2020;141:1227–1234. doi: 10.1161/CIRCULATIONAHA.119.044183 [DOI] [PubMed] [Google Scholar]
- 72.Packer M, Anker SD, Butler J, Filippatos G, Ferreira JP, Pocock SJ, Carson P, Anand I, Doehner W, Haass M, et al. Effect of empagliflozin on the clinical stability of patients with heart failure and a reduced ejection fraction: the EMPEROR-Reduced trial. Circulation. 2021;143:326–336. doi: 10.1161/CIRCULATIONAHA.120.051783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Furtado RHM, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet. 2019;393:31–39. doi: 10.1016/S0140-6736(18)32590-X [DOI] [PubMed] [Google Scholar]
- 74.Requena-Ibanez JA, Santos-Gallego CG, Rodriguez-Cordero A, Vargas-Delgado AP, Mancini D, Sartori S, Atallah-Lajam F, Giannarelli C, Macaluso F, Lala A, et al. Mechanistic insights of empagliflozin in nondiabetic patients with HFrEF: from the EMPA-TROPISM study. JACC Heart Fail. 2021;9:578–589. doi: 10.1016/j.jchf.2021.04.014 [DOI] [PubMed] [Google Scholar]
- 75.Anker SD, Butler J, Filippatos G, Khan MS, Marx N, Lam CSP, Schnaidt S, Ofstad AP, Brueckmann M, Jamal W, et al. Effect of empagliflozin on cardiovascular and renal outcomes in patients with heart failure by baseline diabetes status: results from the EMPEROR-Reduced trial. Circulation. 2021;143:337–349. doi: 10.1161/CIRCULATIONAHA.120.051824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kalyani RR. Glucose-lowering drugs to reduce cardiovascular risk in type 2 diabetes. NEJM. 2021;384:1248–1260. doi: 10.1056/NEJMcp2000280 [DOI] [PubMed] [Google Scholar]
- 77.Mazidi M, Rezaie P, Gao H-K, Kengne AP. Effect of sodium-glucose cotransport-2 inhibitors on blood pressure in people with type 2 diabetes mellitus: a systematic review and meta-analysis of 43 randomized control trials with 22 528 patients. J Am Heart Assoc. 2017;6:e004007. doi: 10.1161/JAHA.116.004007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Al Jobori H, Daniele G, Adams J, Cersosimo E, Triplitt C, DeFronzo RA, Abdul-Ghani M. Determinants of the increase in ketone concentration during SGLT2 inhibition in NGT, IFG and T2DM patients. Diabetes Obes Metab. 2017;19:809–813. doi: 10.1111/dom.12881 [DOI] [PubMed] [Google Scholar]
- 79.Ferrannini E, Baldi S, Frascerra S, Astiarraga B, Heise T, Bizzotto R, Mari A, Pieber TR, Muscelli E. Shift to fatty substrate utilization in response to sodium–glucose cotransporter 2 inhibition in subjects without diabetes and patients with type 2 diabetes. Diabetes. 2016;65:1190–1195. doi: 10.2337/db15-1356 [DOI] [PubMed] [Google Scholar]
- 80.Mone P, Lombardi A, Kansakar U, Varzideh F, Jankauskas SS, Pansini A, Marzocco S, De Gennaro S, Famiglietti M, Macina G, et al. Empagliflozin improves the MicroRNA signature of endothelial dysfunction in patients with heart failure with preserved ejection fraction and diabetes. J Pharmacol Exp Ther. 2023;384:116–122. doi: 10.1124/jpet.121.001251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Christensen RH, Hansen CS, von Scholten BJ, Jensen MT, Pedersen BK, Schnohr P, Vilsbøll T, Rossing P, Jørgensen PG. Epicardial and pericardial adipose tissues are associated with reduced diastolic and systolic function in type 2 diabetes. Diabetes Obes Metab. 2019;21:2006–2011. doi: 10.1111/dom.13758 [DOI] [PubMed] [Google Scholar]
- 82.Thomson SC, Vallon V. Renal effects of sodium-glucose co-transporter inhibitors. Am J Cardiol. 2019;124(Suppl 1):S28–S35. doi: 10.1016/j.amjcard.2019.10.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gruzdeva OV, Akbasheva OE, Dyleva YA, Antonova LV, Matveeva VG, Uchasova EG, Fanaskova EV, Karetnikova VN, Ivanov SV, Barbarash OL. Adipokine and cytokine profiles of epicardial and subcutaneous adipose tissue in patients with coronary heart disease. Bull Exp Biol Med. 2017;163:608–611. doi: 10.1007/s10517-017-3860-5 [DOI] [PubMed] [Google Scholar]
- 84.Masson W, Lavalle-Cobo A, Nogueira JP. Effect of SGLT2-Inhibitors on epicardial adipose tissue: a meta-analysis. Cells. 2021;10:2150. doi: 10.3390/cells10082150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lopaschuk GD, Verma S. Mechanisms of cardiovascular benefits of sodium glucose co-transporter 2 (SGLT2) inhibitors: a state-of-the-art review. JACC Basic Transl Sci. 2020;5:632–644. doi: 10.1016/j.jacbts.2020.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]


