Abstract
Callistemon citrinus has several biological effects; it is anti-inflammatory, anti-obesogenic, antioxidant, hepatoprotection, and chemoprotective. Its bioactive compounds include terpenoids, phenolic acids, and flavonoids which have low oral bioavailability and absorption. This study aimed at developing phytosomes of C. citrinus to improve oral bioavailability and absorption. Phytosomes were formulated with soybean phosphatidylcholine and C. citrinus leaf extract using the thin layer sonication method. Phytosomes were evaluated by scanning electron microscopy (SEM), entrapment efficiency, solubility, and particle size determination. Antioxidant capacity and total phenolic, flavonoid, and terpenoid contents were also measured. The in vivo anti-obesogenic activity was evaluated. Phytosomes loaded with C. citrinus (P C.c) extract had small spherical shapes. The average particle size was 129.98 ± 18.30 nm, encapsulation efficiency 80.49 ± 0.07%, and solubility 90.00%; the stability study presented no significant changes in the average particle size at 20 °C. P C.c presented high antioxidant capacity. For the first time, ellagic acid is reported in this plant. The in vivo obesity study showed a strong anti-obesogenic activity of phytosomes with C. citrinus to reduce 40% body weight as well as morphometric and biochemical parameters.
Keywords: antioxidant capacity, bioavailability, anti-obesogenic, phosphatidylcholine
1. Introduction
Phytosomes are a delivery system of drug or plant extracts prepared with phospholipids using different types of solvents [1]. Compared with drug or plant extracts, phytosomes have a better stability profile, avoid destruction of the phytoconstituent by digestive enzymes and microbiota, increase permeability through membranes, increase bioavailability, and improve compound efficiency [2].
Oxidative stress is not only a common feature of obesity, cardiovascular, neurological, and autoimmune diseases but can also be found in aging [3]. During oxidative stress, there is an increase in reactive oxygen and nitrogen species (ROS/RNS) that are produced by endogenous and exogenous sources [4].
Antioxidants can inhibit, decrease, delay, or directly scavenge free radicals and neutralize oxidants. They act as reducing agents and metal chelators, which convert hydroperoxides into stable compounds. Transferrin, metallothionein, and ceruloplasmin are specific metal-binding proteins considered antioxidant agents and their mechanisms include binding pro-oxidant metal ions, such as iron and copper [5]. The intake of antioxidants may contribute to protecting against the damage produced by reactive oxygen species [6]. The antioxidant activity in plants is due to the phenolic, flavonoid, and terpene compounds found in them [7].
The major compounds found in plants and food are polyphenols and flavonoids. Despite that they can be found in high concentrations, they also need to be available for absorption during gastrointestinal to have beneficial effects. However, these compounds often have low bioaccessibility and bioavailability, which could be due to a number of factors that affect their absorption, stability of the compounds, and the acidic pH of the stomach and microbiota [8,9]. Gastrointestinal pH has an important role in the absorption and bioavailability of oral drugs. In the fasting state, the normal stomach pH is approximately 2.18 ± 0.18 [10]. A change in pH has an impact on the dissolution, solubility release, and stability of drugs [11]. Quin et al. [12] found that the total polyphenol and flavonoid contents of green tea infusion, at pH 1.2, decreased to 65% and 60%, respectively. In addition, the antioxidant activity was reduced as well, leading to low bioaccessibility. Polyphenols are transformed into oligomeric phenols by acidic pH in the stomach. Terpenes containing polar groups at low gastric pH allow bioaccessibility [13]. In summary, pH changes have effects on bioaccessibility that have a strong connection with bioavailability.
Callistemon citrinus (Myrtaceae) has been reported to have many biological effects, including antimicrobial, anti-inflammatory, antioxidant, hepatoprotective, and anticarcinogenic [14,15]. Recently, Ortega-Pérez et al. [16] reported that C. citrinus leaf extract has anti-obesogenic activity and reduces the oxidative stress observed in obesity. C. citrinus has many terpene compounds such as 1-8-cineole, limonene, and α-terpineol [17]. Ayala-Ruiz et al. [18] showed that the main role of these terpenes is to reduce oxidative stress generated by obesity in the animal model. The leaves and stems of C. citrinus presented phenolic and flavonoid compounds, such as eucalyptine, blumenol, gallic acid, and protocatechuic acid [19].
Despite the great biological activities of Callistemon citrinus, there are few studies about the application of nanoparticles with this plant. The biosynthesis of silver oxide nanoparticles from the aqueous leaf extract of Callistemon lanceolatus (C. citrinus) proved the in vitro antioxidant capacity and brine shrimp lethality [20]. Paosen et al. [21] reported the synthesis of silver nanoparticles from the Myrtaceae family and the characterization of their antibacterial activity. Silver nanoparticles from leaves, flowers, and seeds of C. citrinus exhibited antiplasmodial and antibacterial activity without toxicity [22]. Gold nanoparticle from the seed of C. citrinus has antibacterial activity but no antitrypanosomal activity, unlike the extract obtained by the same seed which exhibits both properties [23]. Poly (lactic-co-glycolic acid) nanoparticles loaded with C. citrinus phenolics showed anticancer activity against three breast cancer cell lines with 69% growth inhibition [24]. Recently, the use of C. citrinus silver nanoparticles from leaf aqueous extract was tested for antibacterial activity [25]. Nanotechnology is a delivery system that can be classified into two groups: inorganic as gold, silver, and copper, and organic as liposomes and polymeric nanoparticles [26]. When novel drug delivery technology is used, instead of traditional drug delivery, side effects are reduced whereas safety and efficacy are improved [27].
Obesity is a global health problem. In the Pacific Island states, 50% of the population is obese. In the United States, one-third of adults are obese [28]. In 2030, more than one billion adults and 50 million children and adolescents will be considered obese [29]. The treatment of obesity is not limited to lifestyle modifications and diets. The most common drugs used to control obesity are orlistat (pancreatic lipase inhibitor), phentermine (sympathomimetic amine), liraglutide (glucagon-like peptide 1 receptor agonist), and naltrexone-bupropion (opioid antagonist and a dopamine and noradrenaline reuptake inhibitor). However, all of them have undesired side effects [30] that can be reduced using natural products from plants as a strategy against obesity [31].
This study aimed at encapsulating Callistemon citrinus leaf extract in a phosphatidylcholine complex to enhance its bioavailability and absorption and prevent weight gain. This paper demonstrates that the phytosomes of Callistemon citrinus extract had a small size, high entrapment efficiency, and good solubility and stability. The anti-obesogenic activity was also evaluated using male Wistar rats fed with a hypercaloric diet.
2. Materials and Methods
2.1. Preparation of Callistemon citrinus Leaf Extract
Four-year-old leaves of Callistemon citrinus (Curtis) Skeels (Myrtaceae) plants were collected in the city of Morelia, Michoacán, Mexico. The plant voucher specimen EBUM23538 was identified by Professor Patricia Silva at the Biology School of Universidad Michoacana de San Nicolas de Hidalgo. The fresh leaves were macerated in a 1:10 ratio (w/v 96% ethanol) at room temperature for 5 days. Then, the extract was concentrated by a rotary evaporator at 45 °C. The yield was 20%. The extract of Callistemon citrinus was prepared according to the methodology reported by Lopez-Mejia et al. [32]. The authors concluded that the extract should be prepared with leaves of different four-year-old plants to ensure the highest concentration of its major compounds, as well as high antioxidant capacity.
2.2. Phytosome Preparation
To prepare phytosomal complex, the same concentration (200 mg/b.w.) of Callistemon citrinus and phospholipids were used. This dose has therapeutic efficacy against the inhibition of oxidative stress [15,32] and obesity amelioration [16,18].
Phytosomes were prepared using the assays reported by Baradan et al. [33] and Álvarez-Cortes [34], with slight modifications. The mixture contained 50 mL of hydration media (0.01 M phosphate buffer solution, 150 mM NaCl, pH 7.4), 1.25 g of Callistemon citrinus extract, 1.25 g of soybean phospholipids, and 0.72 g of Tween 80; 1% of ethyl acetate was added to improve solubility in the solution. The emulsion was formed using a VCX 500 ultrasonicator with an amplitude of 25% for 10 min at 10 °C. Phytosomes had a stoichiometric ratio of 1:1.
The phytosome complex was placed in an amber-colored glass bottle and stored at room temperature. Design Expert 11.0.5, an experimental design with response surface methodology of central composite design, was used to prepare phytosomes. Lecithin concentration (%w/v) and rotation speed (rpm) were selected as independent variables. Then, the effect of these variables on the vesicular size and entrapment efficiency of the phytosomes were assessed. All procedures were protected from light. Finally, to corroborate the preparation, the phytosomes were observed under optical microscopy.
2.2.1. Lyophilization and Scanning Electron Microscopy (SEM)
Phytosome samples were frozen at −80 °C overnight; afterward, lyophilized in a high vacuum of 34 Pa using a lyophilizer (Labconco Plus 12; Labconco, Kansas City, MO, USA) for 8 h with a condenser at −43 °C. Lyophilized phytosomes were stored in a sealed glass ampoule at 4 °C. One drop of lyophilized sample was placed on a brass electron microscope tube and coated with copper particles for sputtering. Representative images of the samples were taken and particle diameters were calculated using scanning electron microscopy (JEOL JSM-7600F SEM) with a voltage of 20.0 KV at a working distance of 15.1 mm. Details of the morphological structure of the phytosomes were observed at up to an amplitude of 10,000× and a working distance that allowed minute observations with increasing depth of focus.
2.2.2. Particle Size
Particle size was measured with a Nano Particle Analyzer SZ-100, based on the principle of dynamic light scattering; Ludox TM silica was used as reference material [35]. Ludox TM-50 was diluted to 10% using 0.01 M KCL. A total of 10 mL of KCl/LUDOX solution was filtered through a 2.5 µm filter. The samples were placed in a plastic cuvette and analyzed at a 90° scattering angle. All the batches were analyzed in a triplicate manner and mean and SD were calculated. Table 1 shows the measurement conditions to determine the particle size.
Table 1.
Measurement conditions to determine the particle size.
| Temperature | 25 °C |
| Particle | LUDOX (1.45–0.000i) |
| Dispersion medium | Water |
| Cell | Plastic |
| Distribution type | Monodisperse narrow |
2.2.3. Stability Study
The stability analysis was assessed by storing the phytosomes at 20 ± 2 °C and 4 ± 1 °C and the particle size was measured 1, 3, 5, and 10 days after storing. Later, it was measured after three and a half months.
2.2.4. Study of Vesicular Entrapment/Encapsulation and Solubility
The entrapment efficiency of C. citrinus phytosomes was measured using UV-visible spectrophotometer [36]. A total of 1 mL of dialyzed vesicular suspension was taken and diluted with 0.1 mL of Triton X-100. The solution was centrifuged at 1350× g for 5 min and the supernatant was diluted with ethanol. The amount of drug entrapped was analyzed spectrophotometrically at a maximum of 425 nm against ethanol containing Triton X-100 as blank. Equation (1) computes the efficiency of entrapment (EE); Tdrug is the total amount of drug; Edrug is the extract entrapment in the formulation (phytosome); and Udrug is the extract not entrapped in phytosomal formulation.
| (1) |
Solubility analysis was calculated by dissolving 2 mg of each of the complexes formed (soybean phospholipid particles) and C. citrinus leaf extract in 5 mL of different solvents in small volumetric flasks. The solutions were stirred continuously for 1 h [37]. The experiments were performed in triplicate.
2.3. In Vitro Antioxidant Activity
2.3.1. DPPH Radical Assay
The 1,1-diphenyl-2-picrylhydrazyl (DPPH) assay was performed as reported by Kamarac et al. [38]. The solution reaction contained 10 μL of the sample (C. citrinus leaf extract or phytosome at 200 mg), 90 μL of methanol, and 2 mL of methanolic solution of DPPH 0.1 mM, which were mixed and incubated in the dark for 60 min at room temperature; its absorbance was measured at 517 nm. Trolox (25–800 µM) was used as standard.
2.3.2. ABTS Radical Scavenging Assay
The 2,2′-Azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) assay was performed as reported by Rufino et al. [39], with slight modifications. A total of 2.6 mM potassium persulfate solution was mixed in equimolar amounts with ABTS (ready to use, Sigma); then, the solution was stirred in the dark for 3 h at 27 °C. This working solution was diluted with ethanol to obtain an absorbance from 0.8–0.9 at 734 nm. For the tests, 1 µL of C. citrinus leaf extract (200 mg) and 1 µL of the phytosomes (200 mg) were used, and 49 µL of absolute ethanol and 950 µL of working solution were added. Subsequently, the absorbance at 734 nm was determined after 6 min of starting the reaction.
2.3.3. Ferric-Reducing Antioxidant Power (FRAP) Assay
The FRAP assay was performed as reported by Thaipong et al. [40]. Working solution contained 10 mM 2,4,6-tri [2-pyridyl-s-triazine] (TPTZ) in 40 mM HCL, 20 mM ferric chloride (FeCl3.6 H2O), and 300 mM sodium acetate buffer (pH 3.6) in a 1:1:10 ratio. A total of 0.1 mL of sample was mixed with 1.5 mL working solution and allowed to stand at room temperature for 20 min in darkness. Then, the absorbance was measured at 593 nm. Results were expressed as mean values ± one standard deviations. Trolox standards ranged from 25 to 800 µM.
2.3.4. Determination of Total Phenolic Content
The total phenolic content was determined using the reported by Pripdeevech et al. [41], with slight modifications; in brief, 0.2 mL of the sample and 1.0 mL of Folin–Ciocalteu reagent (1:9 v/v) were shaken vigorously for 5 min. Then, 1.0 mL of 7% Na2CO3 and 5.0 mL of distilled water were added. The reaction mixture was allowed to stand for 60 min at room temperature in darkness and its absorbance was measured at 765 nm. Gallic acid was used as standard (0.01–0.4 mM). Total phenolic content was expressed as mg gallic acid equivalent (mg GAE).
2.3.5. Total Flavonoid Content
The total flavonoid content was determined using the assay reported by Chang et al. [42]. In brief, 0.5 mL of the sample mixed with 1.5 mL of 95% methanol, 0.1 mL of 10% aluminum chloride, 0.1 mL of 1 M potassium acetate, and 2.8 mL of distilled water, stood for 30 min at room temperature in darkness, and the absorbance was measured at 415 nm. Water was used instead of aluminum chloride as blank. Rutin acid was used to calculate the standard curve (0.025–0.5 mg/mL).
2.3.6. Total Terpenoid Content
The total terpenoid content was determined using the methodology described by Chang and Lin [43]. A mixture containing 100 μL of sample (10 mg/mL), 150 μL of vanillin/glacial acetic acid (5% w/v), and 20 μL of sulfuric acid was incubated at 60 °C for 45 min. The mixture was left on ice for 7 min to stop reaction. Finally, 2.25 mL of glacial acetic acid was added and its absorbance was measured at 548 nm. A total of 1,8-Cineole at 1–6 mg/mL was used as standard.
2.4. GC-MS Determination
The samples were analyzed in an Agilent 7890A gas chromatography equipment (Agilent Technologies, Folsom, CA, USA) with an HP5MS30M column (5% phenyl polysilphenylene- siloxane, 30 × 0.25 × 0.25; Agilent Technologies, USA) coupled to an electronic impact ionization quadrupole mass analyzer mass spectrometer. Hewlett Packard 5975C (Hewlett Packard, Palo Alto, CA, USA, EEA). The initial temperature of the oven was 60 °C for 1 min and was increased to 280 °C at 8 °C/min. The injector temperature was 230 °C, the ionization source 230 °C, and the quadrupole temperature 150 °C. Helium was used as carrier gas at a constant flow of 1 mL/min. The mass spectrometer was operated in the EI mode at 70 eV using a range of m/z 50–500 and the voltage was −1737 V. Total ion chromatograms (TIC) were processed using the automated data processing Software MassHunter Workstation version B.06.00 (Agilent Technologies, Inc.). To identify the different compounds, the mass spectrum of each compound detected was compared to those in mass spectral databases (Wiley 275 and US National Institute of Science and Technology (NIST) V. 2.0. The quantities of compounds were calculated from a standard calibration curve using 1,8-cineole at range 1–0.2 mg/mL.
2.5. HPLC Determination
Phenolic acids were quantified by using a high-performance liquid chromatograph (HPLC, Agilent 1260 Infinity Series), equipped with a quaternary pump, auto sampler, column oven, diode array detector (DAD), and Express 90 analytical column. Å C18, 250 × 4.6, 5 µm. The column temperature was 40 °C with an injection volume of 5 µL, the flow was 0.7 mL/min. The mobile phases were A: methanol and B: 1% formic acid. The gradient elution was: 0–5 min: 2% A; 5–15 min: 2–15% A; 15–30 min: 15–25% A; 30–35 min: 25–35% A; 35–45 min: 35–55% A; 40–50 min: 55% A; 50–55 min: 55–2% A; 55–60 min: 2% A; Post time: 5 min. The DAD detector: 255, 270, 280, 310, 322, 355, 370 nm. Eleven available HPLC grade phenolic markers were considered (gallic acid, 4-hydroxybenzoic acid, chlorogenic acid, caffeic acid, vinylic acid, syringic acid, p-coumaric acid, ferulic acid, synaptic acid, ellagic acid, t-cinnamic acid, quercetin, and rutin).
2.6. Anti-Obesity Evaluation of Phytosomes
2.6.1. In Vivo Study
Animals
Two-month-old male Wistar rats (180–200 g) were obtained from the laboratory animals of the Chemical-Biological Research Institute of UMSNH. All the animals were housed in plastic cages in the following conditions: 12 h light–dark cycle, relative humidity of 60–70%, and a temperature of (23–24 °C). They had ad libitum access to food and water. The animals were kept in the bioterium of the Chemical-Biological Research Institute of UMSNH. All protocols were approved and conducted in accordance with the guide for the care and use of laboratory animals by the Mexican Official Standard (NOM-062-ZOO-1999) and the Ethics Committee of the Universidad Michoacán de San Nicolás de Hidalgo.
2.6.2. Obesity Induction
A high-fat diet (HFD) containing 45.4% normal chow (Rodent diet brand Purina rat chow), 14.8% lard, 14.8% vegetable fat, and 25% fructose was daily prepared as reported in [16]. Fifty-four male Wistar rats were randomly divided into 9 (n = 6) groups to be fed. Group 1 (chow diet), Group 2 (chow diet plus vehicle), Group 3 (chow diet plus C. citrinus extract 200 mg/kg), Group 4 (HFD), Group 5 (HFD plus C. citrinus extract 200 mg/kg), Group 6 (HFD plus phytosomes loaded with C. citrinus (P C.c) 50 mg/kg), Group 7 (HFD plus P C.c 100 mg/kg), Group 8 (HFD plus P C.c 200 mg/kg) and Group 9 (HFD plus orlistat 5 mg/kg). Treatments were administered by oral gavage once daily at 9.00 a.m. in the home cage for 15 weeks. The animal’s age at the end of the treatment was 23 weeks. All blood samples were collected after 12–13 h of fasting by cardiac puncture. After blood collection, the animals were anesthetized with pentobarbital sodium injection (150 mg/kg), and all tissues were taken, washed, and stored at −80 °C for subsequent analysis.
2.6.3. Measurement of Morphometric and Biochemical Parameters
Rats were weighed weekly. The percentage of weight gain, adiposity index, and Lee index were calculated as reported by Ortega-Pérez et al. [16]. Plasma glucose, triacylglycerol, and cholesterol were measured using enzymatic colorimetric kits SPINREACT® following the manufacturer’s protocols.
2.7. Statistical Analysis
One-way ANOVA is a parametric method that can be used to determine if two or more groups of data are statistically different. Parametric tests make assumptions about the population distribution of the sample and in nonparametric tests the distribution of a population is unknown. This study selected a parametric test because it is more likely to detect significant differences with these methodologies than the use of nonparametric methods. The test results were expressed as mean ± standard error (SEM) or standard deviation (SD). Data were analyzed using GraphPad Prism (version 8.0) by one-way analysis of variance (ANOVA). To determine statistical differences (a, b, c) of nano-phytosomes, and morphometric and biochemical parameters between groups, Tukey’s multiple comparison test was conducted. * p ≤ 0.05 is a statistically significant result. Tukey’s honestly significant difference (HSD) test, is a post hoc test used in ANOVA to compare all possible pairs of means. When conducting ANOVA and finding a significant difference among group means, a post hoc test like Tukey’s is needed to determine whether the specific group means significantly differed from each other.
3. Results and Discussion
3.1. Morphology and Particle-Size Analysis
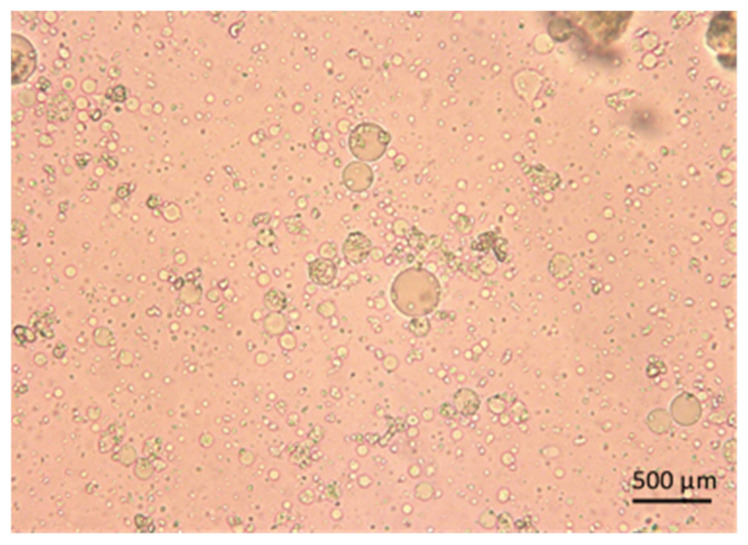
Phytosomes are a strategy used to improve the solubility and bioavailability of herbal extracts [44]. Particle size and phospholipid composition are important factors to obtain these parameters. This study used soybean phosphatidylcholine because this lipid is a main component of membranes and also provides choline, a substrate of choline acetyltransferase to produce the acetylcholine neurotransmitter. Xie et al. [45] reported that using soybean phosphatidylcholine to prepare curcumin-loaded phytosome presented small particle size, high-surface charge, stability, and drug-loading capacity. Figure 1 shows small spherical shapes of phytosomes loaded with Callistemon citrinus under an optical microscope at 40×.
Figure 1.
Optical microscope images at a 40× scale of Callistemon citrinus phytosomes.
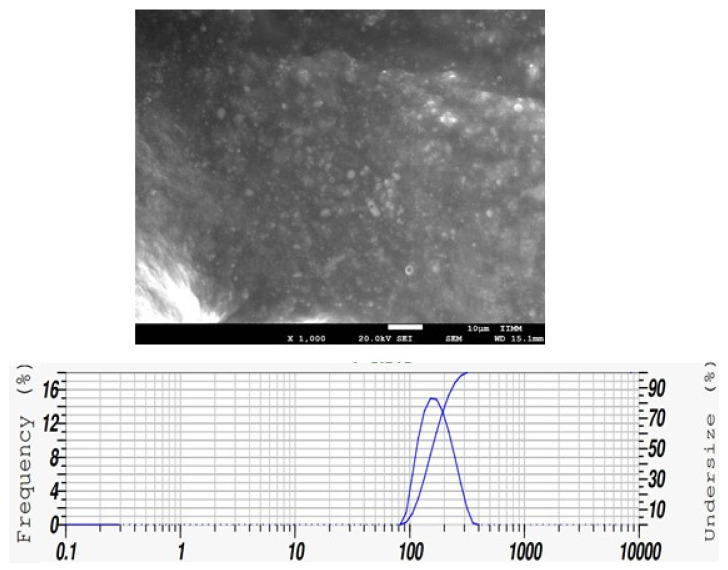
Scanning electron microscope (SEM) was used to evaluate the size and surface morphology. Figure 2 shows the SEM image confirming that phytosomes have a highly spherical structure. The average particle size of the Callistemon citrinus phytosome was 129.98 nm ± 18.30 nm in the emulsion.
Figure 2.
Scanning electron microscope images at scales of 1000×. Particle size was obtained through Nano Particle Analyzer SZ-100 of phytosomes loaded with Callistemon citrinus (200 mg/kg).
3.2. Study of Vesicular Entrapment/Encapsulation
The percentage drug entrapment was determined by extracting phytosomes with centrifugation and the supernatant was measured by UV-visible spectroscopy. Table 2 shows the drug entrapment. The results showed that the entrapment efficiency (EE) was about 80.49%. In this way, the encapsulation efficiency of phytosomes is represented by the concentration of unbound C. citrinus leaf extract (200 mg/kg); this indicates that the leaf extract and soybean phospholipids react to form the complex with a high degree of entrapment of the leaf extract.
Table 2.
Entrapment efficiency of the Callistemon citrinus phytosomes.
| Parameter | Abs |
|---|---|
| Tdrug | 0.189 ± 0.01 |
| Udrug | 0.045 ± 0.07 |
| EE | 80.49 ± 0.07% |
(Tdrug) is the total amount of drug, (EE) is the efficiency of entrapment, and (Udrug) is the extract not entrapped in phytosomal formulation. The data are expressed with the mean (n = 4) and standard deviation (±SD).
3.3. Study of Stability and Solubility
Table 3 shows the stability of the C. citrinus phytosome. During the storage period at 20 ± 2 °C, no significant changes in average particle size were observed for the phytosomes. However, low temperatures caused an increase in the particle size up to two folds. Our results indicate that a phytosome loaded with C. citrinus remained stable for three and a half months. This result is similar to a phytosome loaded with Cuscuta reflexa [46].
Table 3.
Effect of the temperature on the stability of Callistemon citrinus phytosomes at 1, 3, 5, and 10 days and 3.5 months.
| Temperature | ||
|---|---|---|
| Days | 20 ± 2 °C | 4 ± 1 °C |
| 1 | 193.62 ± 27.33 a | 285.07 ± 14.04 ab |
| 3 | 218.06 ± 59.55 a | 412.80 ± 248.22 abc |
| 5 | 256.50 ± 29.00 a | 454.23 ± 175.28 abc |
| 10 | 279.64 ± 61.21 a | 570.70 ± 132.73 bc |
| 106 | 283.82 ± 51.87 ab | 623.23 ± 142.18 c |
Values are the particle size (nm) expressed as mean ± SD (ANOVA followed by Tukey, statistically different values (a, b, c) between groups (p ≤ 0.05, n = 6)).
The low lipid solubility of some compounds may be the reason for their weak absorption [47]. Thus, the solubility is an important parameter to study. Table 4 shows that Callistemon citrinus phytosomes were completely soluble in four solvents and partially soluble in one of them. Assuming 20% for the former and 10% for the latter, Callistemon citrinus phytosomes had a 90% of solubility. It follows that C. citrinus extract, without and with tween 80, shows 80% of solubility and finally soybean liposomes. The formation of phytosomes with plant extract is based on hydrogen-bonding interaction, which increases the bioavailability and stability of the compounds [48]. Consequently, phytosomes have better lipophilicity and hydrophilicity than bioactive compounds.
Table 4.
Solubility profile of Callistemon citrinus leaf extract, Callistemon citrinus phytosomes, and soybean phospholipids.
| Solvent | C. citrinus Extract (200 mg/kg) | C. citrinus Extract (200 mg/kg) + Tween 80 | C. citrinus Phytosomes (200 mg/kg) | Soybean Liposomes + Tween 80 | Soybean Liposomes-Tween 80 |
|---|---|---|---|---|---|
| Distilled water | Partially | Partially | Soluble | Soluble | Micellar shape |
| Methanol | Soluble | Soluble | Partially | Unsolvable | Soluble |
| Dichloromethane | Soluble | Partially | Soluble | Soluble | Soluble |
| Chloroform | Soluble | Soluble | Soluble | Soluble | Partially |
| Hexane | Partially | Soluble | Soluble | Soluble | Soluble |
5 mL of each solvent was added. The solutions were placed under continuous stirring for 1 h; n = 4.
3.4. In Vitro Antioxidant Activity of Callistemon citrinus Phytosomes
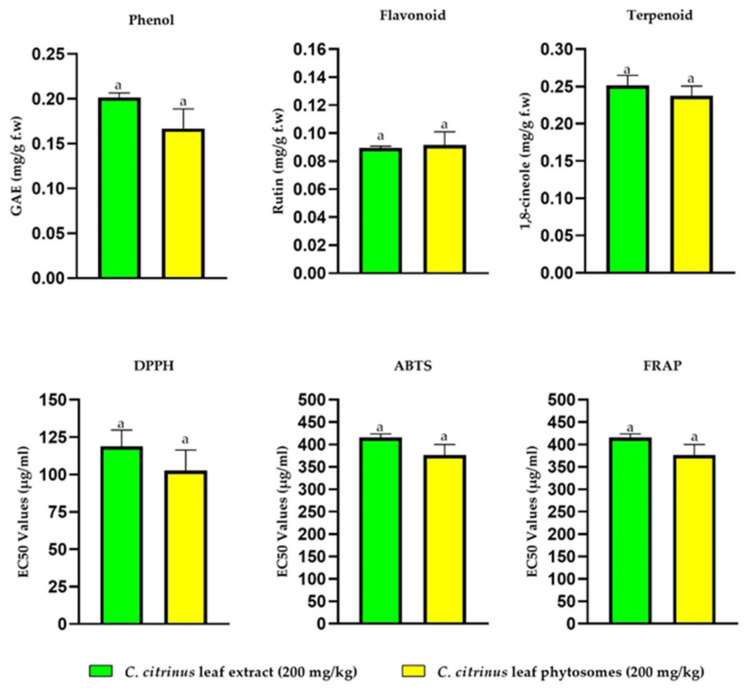
DPPH, ABTS, and FRAP are methodologies commonly used to evaluate the antioxidant capacity of plant extracts. DPPH and ABTS are based on the hydrogen or electron-donating capacity and FRAP on the capacity of reducing ferric to ferrous [49]. Ortega-Perez et al. [16] reported the strong antioxidant capacity and the total phenol, flavonoid, and terpene compounds of Callistemon citrinus leaf extract. Figure 3 shows that both C. citrinus extract and C. citrinus phytosomes exhibited significant inhibitory activity against the DPPH and ABTS radicals and a high ability to reduce ferric to ferrous. Many reports have demonstrated the correlation between total phenolic and flavonoid content and their antioxidant activities [50]. This study also found this correlation, suggesting that the compounds produced the antioxidant effect in C. citrinus, acting as hydrogen donators, singlet oxygen quenchers, and reducing agents [51].
Figure 3.
Determination of antioxidant capacity and total phenol, flavonoid, and terpenoid contents of Callistemon citrinus leaf extract and phytosomes. Data are expressed with the mean ± standard error (ANOVA followed by Tukey, n = 6). The same letter (a) meaning that there is no statistical differences.
Figure 3 shows that there are no significant differences between the bioactive compounds and the antioxidant capacity in the Callistemon citrinus extract and the phytosomes of C. citrinus. This result shows that during the process of creating phytosomes, the bioactive compounds and antioxidant capacity were retained. However, the encapsulation did not significantly improve the activity. This result agrees with Saonere et al. [52], which found antioxidant capacity in a phytophospholipid complex of Glycerrhiza glabra.
Phytosomes containing extracts of mulberry and ginger used against the metabolic syndrome improved the antioxidant system and decreased inflammatory cytokines such as IL-6 and TNF-α [53]. The use of phytosome curcumin against paracetamol-induced liver toxicity in mice showed an increase in enzymatic antioxidant activities and the reduction in lipoperoxidation products [54]. Deleanu et al. [55] reported that phytosomes with the extract of ginger rhizomes and rosehips increase the bioavailability, antioxidant, and anti-inflammatory properties in LPS-induced systemic inflammation in mice. These results suggest that the use of phytosomes can improve enzymatic antioxidant properties and reduce inflammation during oxidative stress [33].
3.5. Gas Chromatography and Mass Spectrometry Analysis
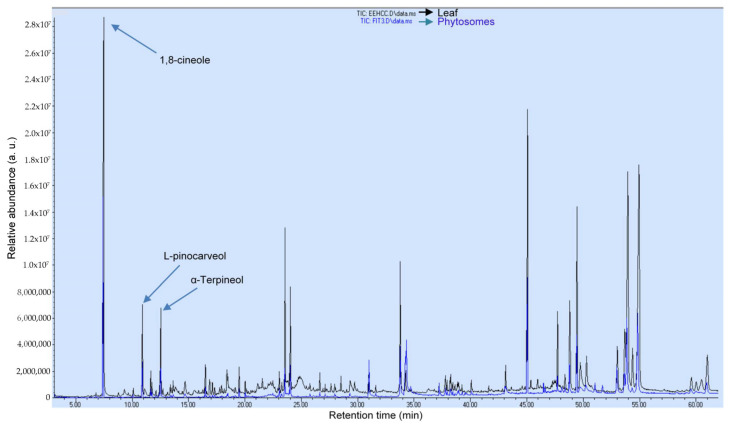
Figure 4 shows the chromatogram of C. citrinus extract and C. citrinus phytosome analyzed by GC/MS to evaluate the terpenes profile. Terpenes were quantified according to GC/MS. Table 5 shows that 1,8-cineole and α-terpineol were the main compounds of the extract and phytosome. These two monoterpenes have been reported to have hepatoprotective, antiviral, antimicrobial, antioxidant, and anticarcinogenic effects [32,56], suggesting that C. citrinus may constitute an alternative pharmacological tool to treat oxidative stress in some diseases.
Figure 4.
Comparison of terpenoid abundance (arbitrary units) in GC/MS total ion chromatogram of Callistemon citrinus leaf (black) and C. citrinus phytosomes (blue).
Table 5.
Terpene contents in Callistemon citrinus leaf extract and C. citrinus phytosomes (GC/MS).
| RT | RIlit | RIcalc | Ref. RIlit | Match Factor |
Prob (%) |
Compounds | Extract | Phytosomes |
|---|---|---|---|---|---|---|---|---|
| 7.47 | 1041 | 1059 | Silva et al. [57] | 972 | 93.8% | 1,8-Cineole | 0.613 ± 0.05 | 0.224 ± 0.04 |
| 10.91 | 1143 | 1131 | Radulovic et al. [58] | 950 | 67.2% | L-Pinocarveol | 0.097 ± 0.007 | 0.030 ± 0.005 |
| 11.65 | 1140 | 1114 | Muselli et al. [59] | 883 | 68.6% | Pinocarvone | 0.016 ± 0.003 | nd |
| 11.76 | 1170 | 1166 | Al-Omar [60] | 923 | 63.9% | Borneol | 0.0081 ± 0.001 | nd |
| 12.54 | 1172 | 1143 | Boti et al. [61] | 952 | 74.5% | α-Terpineol | 0.0894 ± 0.04 | 0.0233 ± 0.003 |
| 23.04 | 1567 | 1530 | Babushok et al. [62] | 929 | 55.5% | Globulol | 0.011 ± 0.002 | 0.0012 ± 0.001 |
| 33.78 | 2099 | 2045 | Babushok et al. [62] | 894 | 81.2% | Phytol | 0.1714 ± 0.03 | 0.0637 ± 0.01 |
| 45.04 | 2847 | 2914 | Zhao et al. [63] | 963 | 50.4% | Squalene | 0.1041 ± 0.01 | 0.0044 ± 0.001 |
| 53.66 | - | 2886 | - | 886 | 59.9% | Unknown 1 | 0.0957 ± 0.02 | 0.0364 ± 0.006 |
| 54.94 | - | 2848 | - | 941 | 86.5% | Unknow 2 | 0.8505 ± 0.05 | 0.2187 ± 0.03 |
RT retention time (min). RIlit retention index (iu) reported in the literature for 5% phenyl polysilphenylene-siloxane GC column. RIcalc retention index obtained through the modulated chromatogram. Ref. RIlit retention index bibliography found in the literature for 5% phenyl polysilphenylene-siloxane GC column. Extract (mg/mL), phytosomes (mg/mL). nd = Not detected. Non-polar retention index (n-alkane scale). The values are the mean ± SD (n = 3). Fragment ions (m/z) of unknown 1: 218 (100), 203, 219, 69, 95, 426 [M+], 411.4. Unknown 2: 189 (100, 95,207,93,135, 426 [M+], 411.4.
Table 5 shows the calculated retention indices and a comparison with retention indices found on the NIST home page (www.nistwebbook.com (accessed on 17 March 2023)). A total of 80% of the compounds were identified and only two of them could not be fully identified despite having a Match Factor above 800 (Good Match). According to the NIST library, Match Factor scores > 900 mean an Excellent Match, whereas Match Factors scores in the range of 800–900 are a Good Match. All identified compounds have Factor scores above 800.
3.6. High-Performance Liquid Chromatography Analysis
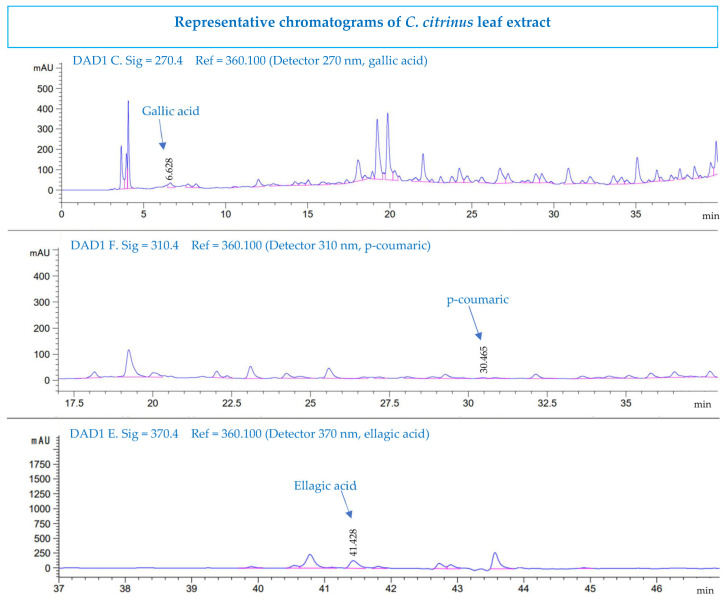
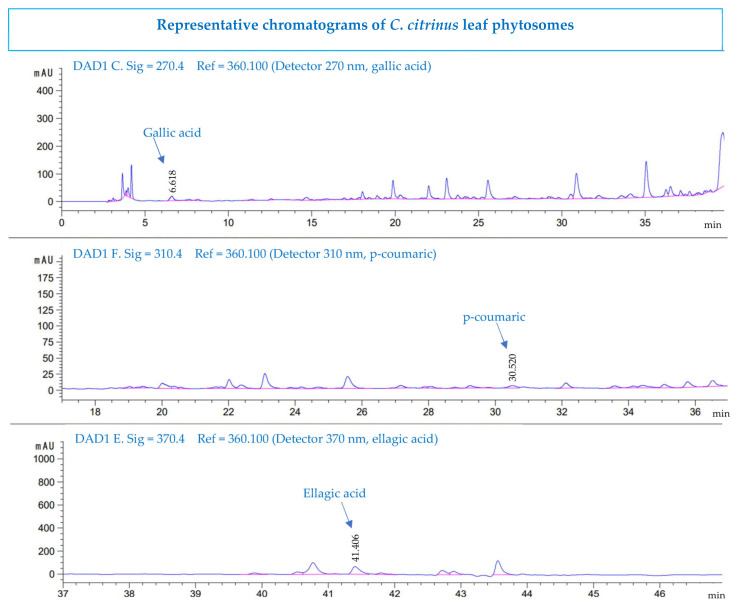
The phenolic and flavonoid compounds were identified according to their retention time in HPLC. Ellagic acid was found for the first time in C. citrinus. Figure 5 and Figure 6 show that gallic acid, p-coumaric acid, and ellagic acid are the compounds identified in the Callistemon citrinus extract and phytosome. These phenolic acids have been reported to have anticancer, antiviral, antioxidant, and anti-inflammatory activities [64]. Table 6 shows that the concentration of gallic acid, p-coumaric acid, and ellagic acid was very similar in the C. citrinus extract and in the phytosome.
Figure 5.
HPLC chromatograms of the Callistemon citrinus leaf extract.
Figure 6.
HPLC chromatograms of Callistemon citrinus leaf phytosome.
Table 6.
HPLC analysis profile in Callistemon citrinus leaf extract and phytosomes.
| Compounds | Extract (µg/mL) | Phytosomes (µg/mL) |
|---|---|---|
| gallic acid | 6.94 ± 0.06 | 5.93 ± 0.0 |
| 4-hydroxybenzoic acid | nd | nd |
| chlorogenic acid | nd | nd |
| caffeic acid | nd | nd |
| Vanillic acid | nd | nd |
| Syringic acid | nd | nd |
| p-coumaric acid | 0.47 ± 0.05 | 0.65 ± 0.07 |
| ferulic acid | nd | nd |
| synaptic acid | nd | nd |
| ellagic acid | 74.3 ± 1.3 | 67.3 ± 1.4 |
| t-cinnamic acid | nd | nd |
| quercetin | nd | nd |
| rutin | nd | nd |
nd = Not detected. Data expressed as mean ± SD (n = 3).
Until now, silver, gold, and poly (lactic-co-glycolic acid) nanoparticles loaded with Callistemon citrinus [22,23,24] have been reported. Nanoparticles have some characteristics that could affect their toxicity as nature, size, mobility, stability, surface aggregation, and storing time [65]. Metal oxide nanoparticles reduced the enzymatic activity of microorganisms [66]. However, the use of phytosomes as a delivery method of natural products has advantages. Phytosomes have an amphiphilic characteristic that allows the extract compounds to interact with the hydrophilic and hydrophobic parts, increasing the therapeutic effect.
3.7. Effect of Phytosomes on Morphometric and Biochemical Parameters
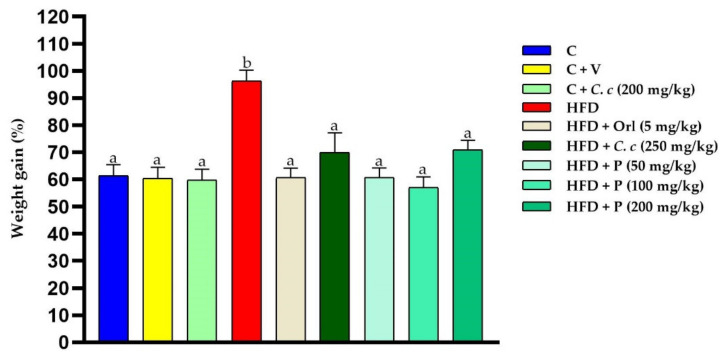
The rats fed with HFD showed increased body weight compared to the other groups. Conversely, the administration of phytosomes loaded with C. citrinus to animals fed with HFD showed significantly reduced body weight as compared to obese rats (Figure 7). These results agree with Ortega et al. [16]. A previous study showed that C. citrinus extract inhibited lipase activity in a dose-dependent manner [16]. Regarding the phytosomal dosage (50, 100, and 200 mg/kg) and C. citrinus extract (250 mg/kg), this study showed similar effects in all of them to reduce weight. This study suggests that phytosomes loaded with C. citrinus extract have stronger anti-obesogenic activity than C. citrinus extract itself; this result is probably due to the high bioavailability, which improves the solubility, allowing to reduce the dose. The Lee and adiposity indices in the HFD group were higher than those in other groups. Also, glucose and triacylglycerol levels increased in the HFD group, contrary to the rest of the groups (Table 7).
Figure 7.
Final body weight percentage in control rats and the different experimental treatments during the 15 weeks. Values are presented as mean ± standard error (ANOVA followed by Tukey, n = 6). Statistically different values (a, b) between groups.
Table 7.
Effect of C. citrinus on morphometric parameters, obesity markers, and biochemical determinations in rats.
| Measurements | Control | Control + Vehicle | Control + C. citrinus Extract (200 mg/kg) | Hypercaloric-fat Diet (HFD) | HFD + Orlistat (5 mg/kg) | HFD + C. citrinus Extract (250 mg/kg) | HFD + Phytosomes (50 mg/kg) | HFD + Phytosomes (100 mg/kg) | HFD + Phytosomes (200 mg/kg) |
|---|---|---|---|---|---|---|---|---|---|
| Morphometric parameters | |||||||||
| Abdominal circumference (cm) | 20.50 ± 0.45 a | 20.50 ± 45 a | 21.00 ± 0.45 a | 25.50 ± 0.45 b | 22.25 ± 0.45 a | 20.33 ± 1.36 a | 21.0 ± 0.52 a | 21.20 ± 20 a | 21.50 ± 0.45 a |
| Nose-to-anus length (cm) | 25.25 ± 0.60 a | 24.37 ± 0.60 a | 24.60 ± 0.54 a | 24.41 ± 0.91 a | 23.66 ± 0.91 a | 24.41 ± 0.91 a | 23.80 ± 0.54 a | 24.12 ± 0.60 a | 23.50 ± 0.60 a |
| Nose-to-tail length (cm) | 46.40 ± 0.42 a | 46.40 ± 0.42 a | 46.87 ± 0.47 a | 45.66 ± 0.38 a | 45.71 ± 0.47 a | 44.66 ± 1.63 a | 45.87 ± 0.47 a | 45.75 ± 0.47 a | 45.12 ± 0.47 a |
| Markers of obesity | |||||||||
| BMI (kg/m2) | 0.67 ± 0.03 b | 0.72 ± 0.03 ab | 0.70 ± 0.03 ab | 0.88 ± 0.04 a | 0.72 ± 0.04 ab | 0.69 ± 0.09 b | 0.66 ± 0.04 b | 0.68 ± 0.04 b | 0.76 ± 0.04 ab |
| Adiposity index | 2.78 ± 0.55 c | 2.77 ± 0.55 c | 2.52 ± 0.62 c | 9.43 ± 0.62 a | 5.56 ± 0.71 bc | 6.18 ± 0.39 b | 5.98 ± 0.71 b | 4.82 ± 0.71 bc | 4.02 ± 0.62 bc |
| Lee index | 0.30 ± 0.01 | 0.30 ± 0.02 | 0.30 ± 0.01 | 0.33 ± 0.01 a | 0.30 ± 0.01 | 0.30 ± 0.01 | 0.29 ± 0.01 | 0.30 ± 0.01 | 0.31 ± 0.01 |
| Biochemical parameters | |||||||||
| Triacylglycerol (mg/dL) | 90.66 ± 11.64 c | 103.66 ± 11.64 c | 109.66 ± 11.64 c | 202.66 ± 11.64 a | 90.66 ± 11.64 c | 136.33 ± 66.96 b | 103.50 ± 11.64 c | 105.33 ± 11.64 c | 118.66 ± 11.64 b |
| Blood glucose (mg/dL) | 93.99 ± 8.24 a | 101.37 ± 8.24 a | 95.59 ± 8.24 a | 111.11 ± 8.24 b | 100.24 ± 8.24 a | 97.00 ± 4.24 a | 104.18 ± 8.24 a | 92.24 ± 8.24 a | 96.65 ± 8.24 a |
| Total cholesterol (mg/dL) | 161.33 ± 2.69 a | 160.33 ± 2.69 a | 162.00 ± 2.69 a | 159.66 ± 2.69 a | 156.66 ± 2.69 a | 162.00 ± 2.69 a | 155.66 ± 2.69 a | 161.00 ± 2.69 a | 159.30 ± 2.69 a |
Values expressed as mean ± SEM (n = 6, ANOVA followed by Tukey test, statistically different values (a, b, c) between groups; p ≤ 0.05).
Callistemon citrinus phytosomal formulation improved oral bioavailability. Even the administration of low doses reduced the morphometrical and biochemical parameters in the treated animals.
4. Conclusions
The Callistemon citrinus phytosomal formulation improved oral bioavailability, retained the major compounds, and was stable for three and a half months when stored at 20 °C. Phytosomes of C. citrinus, even in low doses, reduced morphometrical and biochemical parameters in Wistar rats fed with a high-fat diet. The results also revealed that the supplementation of phytosomes of Callistemon citrinus reduced excessive weight in the animals.
Acknowledgments
The authors thank the Coordinación de la Investigación Científica de la Universidad Michoacana de San Nicolas de Hidalgo in Mexico for the partial support of the study. We also thank Eduardo Segundo-Jara and Mathieu Tétreault for the careful review of the manuscript.
Author Contributions
Conceptualization, formal analysis, investigation, and writing—original draft, L.G.O.-P.; performed experiments L.A.A.-R., O.R.M.-R. and J.S.P.-S.; collected and analyzed all the data, A.A.-M.; Funding acquisition and review, D.G.-H.; analyzed all the data, supervision, writing—review and editing, P.R.-C. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The animal study protocol was approval by the Institutional Bioethics and Biosecurity Committee in the Instituto de Investigaciones Químico Biológicas, Universidad Michoacana de San Nicolás de Hidalgo, approval date: 1 September 2021; Protocol ID IIQB-CIBE-06-2021.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data availability under request.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Sandhiya V., Ubaidulla U. A review on herbal drug loaded into pharmaceutical carrier techniques and its evaluation process. Future J. Pharm. Sci. 2020;6:51. doi: 10.1186/s43094-020-00050-0. [DOI] [Google Scholar]
- 2.Permana A.D., Utami R.N., Courtenay A.J., Manggau M.A., Donnelly R.F., Rahman L. Phytosomal nanocarriers as platforms for improved delivery of natural antioxidant and photoprotective compounds in propolis: An approach for enhanced both dissolution behaviour in biorelevant media and skin retention profiles. J. Photochem. Photobiol. B Biol. 2020;205:111846. doi: 10.1016/j.jphotobiol.2020.111846. [DOI] [PubMed] [Google Scholar]
- 3.Simioni C., Zauli G., Martelli A.M., Vitale M., Sacchetti G., Gonelli A., Neri L.M. Oxidative stress: Role of physical exercise and antioxidant neutraceutical in adulthood and aging. Oncotarget. 2018;9:17181–17198. doi: 10.18632/oncotarget.24729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martemucci G., Costagliola C., Mariano M., D’andrea L., Napolitano P., D’Alessandro A.G. Free radical properties, source and targets, antioxidant consumption and health. Oxygen. 2022;2:48–78. doi: 10.3390/oxygen2020006. [DOI] [Google Scholar]
- 5.Warraich U.M., Hussain F., Kayani H.U.R. Aging—Oxidative stress, antioxidant and computational modeling. Heliyon. 2020;6:e04107. doi: 10.1016/j.heliyon.2020.e04107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ali S.S., Ahsan H., Zia M.K., Siddiqui T., Khan F.H. Understanding oxidant and antioxidants: Classical team with new players. J. Food Biochem. 2020;44:e13145. doi: 10.1111/jfbc.13145. [DOI] [PubMed] [Google Scholar]
- 7.Lee M.Y., Lin W.C., Yu B., Lee T.T. Antioxidant capacity of phytochemicals and their potential effects on oxidative status in animals- A review. Asian-Australas. J. Anim. Sci. 2017;30:299–308. doi: 10.5713/ajas.16.0438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rein M.J., Renouf M., Cruz-Hernandez C., Actis-Goretta L., Thakkar S.K., Pinto M.S. Bioavailability if bioactive food compounds: A challenging journey to bioefficacy. Br. J. Clin. Pharmacol. 2012;75:588–602. doi: 10.1111/j.1365-2125.2012.04425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rahman H.S., Othman H.H., Hammadi N.I., Yeap S.K., Amin K.M., Samad N.A., Alitheen N.B. Novel drug delivery systems for loading of natural plant extracts and their biomedical applications. Int. J. Nanomed. 2020;15:2439–2483. doi: 10.2147/IJN.S227805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alqahtani M.S., Kazi M., Alsenaidy M.A., Ahmad M.Z. Advances in oral drug delivery. Front. Pharmacol. 2021;12:618411. doi: 10.3389/fphar.2021.618411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abuhelwa A.Y., Williams D.B., Upton R.N., Foster D.J.R. Food, gastrointestinal pH, and models of oral drug absorption. Eur. J. Pharma. Biopharm. 2017;112:234–248. doi: 10.1016/j.ejpb.2016.11.034. [DOI] [PubMed] [Google Scholar]
- 12.Quin W., Ketnawa S., Ogawa Y. Effect of digestive enzyme and pH on variation of bioavailability of green tea during simulated in vitro gastrointestinal digestion. Food Sci. Hum. Wellness. 2022;11:669–675. doi: 10.1016/j.fshw.2021.12.024. [DOI] [Google Scholar]
- 13.Stevanovic Z.D., Sieniawska E., Glowniak K., Obradovoc N., Pajic-Lijakovic I. Natural macromolecules as carriers for essential oils: From extraction to biomedical application. Front. Bioeng. Biotechnol. 2020;8:563. doi: 10.3389/fbioe.2020.00563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sowndhararajan K., Deepa P., Kim S. A review of the chemical composition and biological activities of Callistemon lanceolatus (Sm.) Sweet. J. Appl. Pharm. Sci. 2021;11:65–73. doi: 10.7324/JAPS.2021.1101204. [DOI] [Google Scholar]
- 15.López-Mejía A., Ortega-Pérez L.G., Godínez-Hernández D., Nateras-Marin B., Meléndez-Herrera E., Rios-Chavez P. Chemopreventive effect of Callistemon citrinus (Curtis) Skeels against colon cancer induced by 1, 2-dimethylhydrazine in rats. J. Cancer Res. Clin. Oncol. 2019;145:1417–1426. doi: 10.1007/s00432-019-02905-3. [DOI] [PubMed] [Google Scholar]
- 16.Ortega-Pérez L.G., Piñón-Simental J.S., Magaña-Rodríguez O.R., López-Mejía A., Ayala-Ruiz L.A., García-Calderón A.J., Godínez-Hernández D., Rios-Chavez P. Evaluation of the toxicology, anti-lipase, and antioxidant effects of Callistemon citrinus in rats fed with a high fat-fructose diet. Pharm. Biol. 2022;60:1384–1393. doi: 10.1080/13880209.2022.2099907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Petronilho S., Rocha S.M., Ramírez-Chávez E., Molina-Torres J., Rios-Chavez P. Assessment of the terpenic profile of Callistemon citrinus (Curtis) Skeels from Mexico. Ind. Crops Prod. 2013;46:369–379. doi: 10.1016/j.indcrop.2013.02.012. [DOI] [Google Scholar]
- 18.Ayala-Ruiz L.A., Ortega-Pérez L.G., Piñón-Simental J.S., Magaña-Rodríguez O.R., Meléndez-Herrera E., Rios-Chavez P. Role of the major terpenes of Callistemon citrinus against the oxidative stress during a hypercaloric diet in rats. Biomed. Pharmacother. 2022;153:113505. doi: 10.1016/j.biopha.2022.113505. [DOI] [PubMed] [Google Scholar]
- 19.Khanh P.N., Duc H.V., Huong T.T., Ha V.T., Van D.T., Son N.T., Kim Y.H., Viet D.Q., Cuong N.M. Phenolic compounds from Callistemon citrinus leaves and stem. J. Sci. Technol. 2016;54:190–197. doi: 10.15625/0866-708X/54/2/6469. [DOI] [Google Scholar]
- 20.Ravichandran S., Paluri V., Kumar G., Loganathan K., Venkata R.K. A novel approach for the biosynthesis of silver oxide nanoparticles using aqueous leaf extract of Callistemon lanceolatus (Myrtaceae) and their therapeutic potential. J. Exp. Nanosci. 2016;11:445–458. doi: 10.1080/17458080.2015.1077534. [DOI] [Google Scholar]
- 21.Paosen S., Saising J., Wira Septama A., Piyawan Voravuthikunchai S. Green synthesis of silver nanoparticles using plants from Myrtaceae family and characterization of their antibacterial activity. Mater. Lett. 2017;209:201–209. doi: 10.1016/j.matlet.2017.07.102. [DOI] [Google Scholar]
- 22.Rotimi L., Ojemaye M.O., Okoh O.O., Okoh A.I. Silver nanoparticles mediated by Callistemon citrinus extracts and their antimalaria, antitrypanosomal and antibacterial efficacy. J. Mol. Liq. 2019;273:615–625. [Google Scholar]
- 23.Rotimi L., Ojemaye M.O., Okoh O.O., Sadimennko A., Okoh A.I. Synthesis, characterization, antimalarial, antitrypanocidal and antimicrobialproperties of gold nanoparticle. Green Chem. Lett. Rev. 2019;12:61–68. doi: 10.1080/17518253.2019.1569730. [DOI] [Google Scholar]
- 24.Ahmed R., Tariq M., Ahmad I.S., Fouly H., Abbas F.I., Hasan A., Kushad M. Poly (lactic-co-glycolic acid) nanoparticles loaded with Callistemon citrinus phenolics exhibited anticancer properties against three breast cancer cell lines. J. Food Qual. 2019;2019:2638481. doi: 10.1155/2019/2638481. [DOI] [Google Scholar]
- 25.Gharibvand Z.S., Behbahani B.A., Noshad M., Jooyandeh H. Green synthesis of silver nanoparticles using Callistemon citrinus leaf extract and evaluation of its antibacterial activity. Iran. Food Sci. Technol. Res. J. 2022;18:151–163. [Google Scholar]
- 26.Alharbi W.S., Almughem F.A., Almehmady A.M., Jarallah S.S., Alsharif W.K., Alzahrani N.M., Alshehri A.A. Phytosomes as an emerging nanotechnology platform for the topical delivery of bioactive phytochemicals. Pharmaceutics. 2021;13:1475. doi: 10.3390/pharmaceutics13091475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Adiki S.K., Sangeetha S., Kamireddy S., Katakam P., Obilineni I. Phytosomes: A novel phytoconstituent delivery approach to improve the efficacy of obesity treatment. Curr. Nutr. Food Sci. 2023;19:229–237. doi: 10.2174/1573401318666220901125859. [DOI] [Google Scholar]
- 28.Chakkhtoura M., Haber R., Ghezzawi M., Rhayem C., Tcheroyan R., Mantzoros C.S. Pharmacotherapy of obesity: An update on the available medications and drugs under investigation. Lancet. 2023;58:1–29. doi: 10.1016/j.eclinm.2023.101882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tham K.W., Lim A.Y.L., Baur L.A. The global agenda on obesity: What does this mean for Singapore. Singapore Med. J. 2023;64:182–187. doi: 10.4103/singaporemedj.SMJ-2023-018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee P.C., Lim C.H., Asokkumar R., Chua M.W.J. Current treatment landscape for obesity in Singapore. Singapore Med. J. 2023;64:172–181. doi: 10.4103/singaporemedj.SMJ-2022-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gehan F., Raoof A. Anti-obesity potential of natural products. Egypt. J. Chem. 2022;65:329–358. [Google Scholar]
- 32.López-Mejía A., Ortega-Pérez L.G., Magaña-Rodríguez O.R., Ayala-Ruiz L.A., Piñon-Simental J.S., Godínez-Hernández D., Rios-Chavez P. Protective effect of Callistemon citrinus on oxidative stress in rats with 1,2-dimethylhydrazine-induced colon cancer. Biomed. Pharmacother. 2021;142:112070. doi: 10.1016/j.biopha.2021.112070. [DOI] [PubMed] [Google Scholar]
- 33.Baradaran S., Hajizadeh Moghaddam A., Khanjani Jelodar S., Moradi-Kor N. Protective effects of curcumin and its nano-phytosome on carrageenan-induced inflammation in mice model: Behavioral and biochemical responses. J. Inflamm. Res. 2020;13:45–51. doi: 10.2147/JIR.S232462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alvarez Cortes O. Master’s Thesis. Facultad de Químico Farmacobiología, Universidad Michoacana de San Nicolás de Hidalgo; Morelia, Mexico: 2020. Elaboración de una Biopelícula Comestible Incorporando Nanocápsulas de Aceite Esencial y Extractos Polifenólicos de Aloysia citriodora con Actividad Antiinflamatoria Evaluado en un Modelo Biológico. [Google Scholar]
- 35.Horiba Colloidal Silica as a Particle Size and Charge Reference Material. 2009. [(accessed on 22 January 2023)]. Available online: https://www.horiba.com/fileadmin/uploads/Scientific/Documents/PSA/TN158.pdf.
- 36.Jain P.K., Kharya M., Gajbhiye A. Pharmacological evaluation of mangiferin herbosomes for antioxidant and hepatoprotection potential against ethanol induced hepatic damage. Drug Dev. Ind. Pharm. 2013;39:1840–1850. doi: 10.3109/03639045.2012.738685. [DOI] [PubMed] [Google Scholar]
- 37.Jain P.K., Khurana N., Pounikar Y., Gajbhiye A., Kharya M.D. Enhancement of absorption and hepatoprotective potential through soya-phosphatidylcholine-andrographolide vesicular system. J. Liposome Res. 2013;23:110–118. doi: 10.3109/08982104.2012.753456. [DOI] [PubMed] [Google Scholar]
- 38.Karamac M., Kosiñska A., Pegg R.B. Comparison of radical-scavenging activities for selected phenolic acids. Pol. J. Food Nutr. Sci. 2005;14:165–170. [Google Scholar]
- 39.Rufino M.S.M., Alves R.E., de Brito E.S., Perez-Jimenez J., Saura-Calixto F., Mancini-Filho J. Bioactive compounds and antioxidant capacities of 18 non-traditional tropical fruits from Brazil. Food Chem. 2010;121:996–1002. doi: 10.1016/j.foodchem.2010.01.037. [DOI] [Google Scholar]
- 40.Thaipong K., Boonprakob U., Crosby K., Cisneros-Zevallos L., Byrne D.H. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Comp. Anal. 2006;19:669–675. doi: 10.1016/j.jfca.2006.01.003. [DOI] [Google Scholar]
- 41.Pripdeevech P., Chumpolsri W., Suttiarporn P., Wongpornchai S. The chemical composition and antioxidant activities of basil from Thailand using retention indices and comprehensive two-dimensional gas chromatography. J. Serb. Chem. Soc. 2010;75:1503–1513. doi: 10.2298/JSC100203125P. [DOI] [Google Scholar]
- 42.Chang C.C., Yang M.H., Wen H.M., Chern J.C. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J. Food Drug Anal. 2002;10:178–182. [Google Scholar]
- 43.Chang C.L., Lin C.S. Phytochemical composition, antioxidant activity, and neuroprotective effect of Terminalia chebula Retzius extracts. Evid.-Based Complement. Altern. Med. 2012;2012:125247. doi: 10.1155/2012/125247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Suryawanshi J.A.S. Phytosome: An emerging trend in herbal drug treatment. J. Genet. Genom. 2011;3:109–114. [Google Scholar]
- 45.Xie J., Li Y., Song L., Pan Z., Ye S., Hou Z. Design of a novel curcumin-soybean phosphatidylcholine complex-based targeted drug delivery system. Drug Deliv. 2017;24:707–719. doi: 10.1080/10717544.2017.1303855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alshahrani S.M. Optimization and characterization of Cuscuta reflexa extract loaded phytosomes by the box-behnken design to improve the oral bioavailability. J. Oleo Sci. 2022;71:671–683. doi: 10.5650/jos.ess21318. [DOI] [PubMed] [Google Scholar]
- 47.Barani M., Sangiovanni E., Angarano M., Rajizadeh M.A., Mehrabani M., Piazza S., Gangadharappa H.V., Pardakhty A., Mehrbani M., Dell’Agli M., et al. Phytosomes as innovative delivery systems for phytochemicals: A comprehensive review of literate. Int. J. Nanomed. 2021;16:6983–7022. doi: 10.2147/IJN.S318416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Surendra T., Patel D.K., Baro L., Nair S.K. A review on phytosomes, their characterization, advancement & potential for transdermal application. J. Drug Deliv. Ther. 2013;3:147–152. [Google Scholar]
- 49.Boligon A.A., Machadi M.M., Athayde M.L. Technical evaluation of antioxidant activity. Med. Chem. 2014;4:517–522. doi: 10.4172/2161-0444.1000188. [DOI] [Google Scholar]
- 50.Khan R.A., Khan M.R., Sahreen S., Ahmed M. Evaluation of phenolic contents and antioxidant activity of various solvent extracts of Sonchus asper (L.) Hill. Chem. Cent. J. 2012;6:12. doi: 10.1186/1752-153X-6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shan B., Cai Y.Z., Sun M., Corke H. Antioxidant capacity of 26 spice extracts and characterization of their phenolic constituents. J. Agric. Food Chem. 2005;53:7749–7759. doi: 10.1021/jf051513y. [DOI] [PubMed] [Google Scholar]
- 52.Saonere J.A., Channawar M.A., Kochar N.I., Mohale D., Chandewar A.V. Preparation and evaluation of phytophospholipid complex of phenolic fractions of G. glabra for antioxidant and antimicrobial activity. Acta Sci. Pharm. Sci. 2023;7:37–44. [Google Scholar]
- 53.Palachai N., Wattanathorn J., Muchimapura S., Thukham-Mee W. Antimetabolic syndrome effect of phytosome containing the combined extracts of mulberry and ginger in an animal model of metabolic syndrome. Oxidative Med. Cell. Longev. 2019;2019:5972572. doi: 10.1155/2019/5972575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tung B.H., Hai N.T., Son P.K. Hepatoprotective effect of phytosome curcumin against paracetamol-induced liver toxicity in mice. Braz. J. Pharm. Sci. 2017;53:e16136. doi: 10.1590/s2175-97902017000116136. [DOI] [Google Scholar]
- 55.Deleanu M., Toma L., Sanda G.M., Barbălată T., Niculescu L.S., Sima A.V., Deleanu C., Săcărescy L., Suciu A., Alexandru G., et al. Formulation of phytosomes with extracts of ginger rhizomes and rosehips with improved bioavailability, antioxidant and anti-inflammatory effects in vivo. Pharmaceutics. 2023;15:1066. doi: 10.3390/pharmaceutics15041066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wojtunik-Kulesza K.A., Kasprzak K., Oniszczuk T., Oniszczuk A. Natural monoterpenes: Much more than only a scent. Chem. Biodivers. 2019;16:e1900434. doi: 10.1002/cbdv.201900434. [DOI] [PubMed] [Google Scholar]
- 57.Silva J.C., Barbosa L.C.A., Demuner J.A., Montanari R.M., Pinheiro A.L., Dias I., Andrade N.J. Chemical composition and bacterial activities from the essential oils of Myrtaceae species planted in Brazil. Química Nova. 2010;33:104–108. doi: 10.1590/S0100-40422010000100019. [DOI] [Google Scholar]
- 58.Radulovic N., Lazarevic J., Ristic N., Palic R. Chemotaxonomic significance of the volatiles in the genus Stachys (Lamiaceae): Essential oil composition of four Balkan Stachys species. Biochem. Syst. Ecol. 2007;35:196–208. doi: 10.1016/j.bse.2006.10.010. [DOI] [Google Scholar]
- 59.Muselli A., Rossi P.-G., Desjobert J.-M., Bernardini A.-F., Berti L., Costa J. Chemical composition and antibacterial activity of Otanthus maritimus (L.) Hoffmanns. & Link essential oils from Corsica. Flavour Fragr. J. 2007;22:217–223. [Google Scholar]
- 60.Al-Omar M.S. Phytochemical analysis, antioxidant, and anti-microbial activities of Suaeda vermiculata n-hexane extract in comparison to the plant’s hydrodistilled volatile oil. Pharmacogn. J. 2021;13:853–859. doi: 10.5530/pj.2021.13.109. [DOI] [Google Scholar]
- 61.Boti J.B., Koukoua G., N’Guessan T.Y., Casanova J. Chemical variability of Conyza sumatrensis and Microglossa pyrifolia from Côte d’Ivoire. Flavour Fragr. J. 2007;22:27–31. doi: 10.1002/ffj.1743. [DOI] [Google Scholar]
- 62.Babushok V.-I., Linstrom P.J., Zenkevich I.G. Retention indices for frequently reported compounds of plant essential oils. J. Phys. Chem. Ref. Data. 2011;40:043101. doi: 10.1063/1.3653552. [DOI] [Google Scholar]
- 63.Zhao C.X., Li X.N., Liang Y.Z., Fang H.Z., Huang L.F., Guo F.Q. Comparative analysis of chemical components of essential oils from different samples of Rhododendron with the help of chemometrics methods. Chemom. Intell. Lab. Syst. 2006;82:218–222. doi: 10.1016/j.chemolab.2005.08.008. [DOI] [Google Scholar]
- 64.Rahman M.M., Rahaman M.S., Islam M.R., Rahman F., Mithi F.M., Alqahtani T., Almikhlafi M.A., Alghamdi S.Q., Alruwaili A.S., Hossain M.S., et al. Role of phenolic compounds in human disease: Current knowledge and future prospects. Molecules. 2022;27:233. doi: 10.3390/molecules27010233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dikshit P.K., Kumar J., Das A.K., Sadhu S., Sharma S., Singh S., Gupta P.K., Kim B.S. Green synthesis of metallic nanoparticles: Applications and limitations. Catalysts. 2021;11:902. doi: 10.3390/catal11080902. [DOI] [Google Scholar]
- 66.Das S.R., Kundu C.N. Promising opportunities and potential risk of nanoparticle on the society. IET Nanobiotechnol. 2020;14:253–260. doi: 10.1049/iet-nbt.2019.0303. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data availability under request.