Abstract
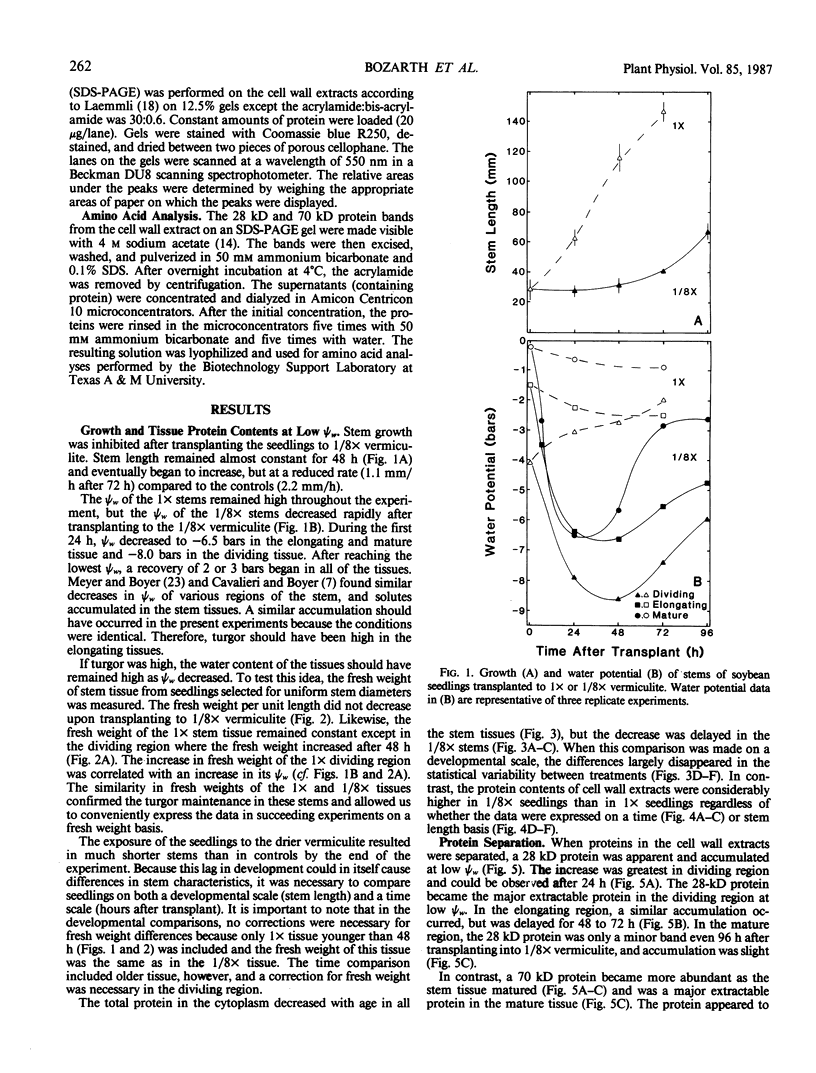
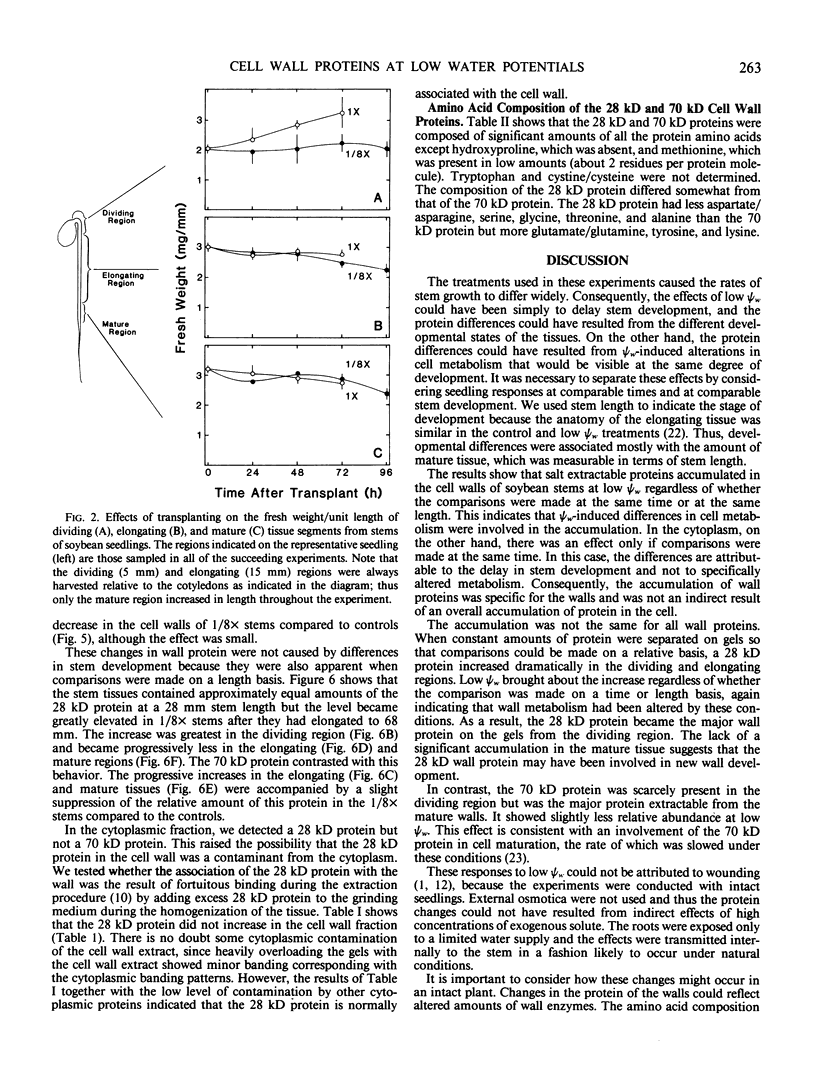
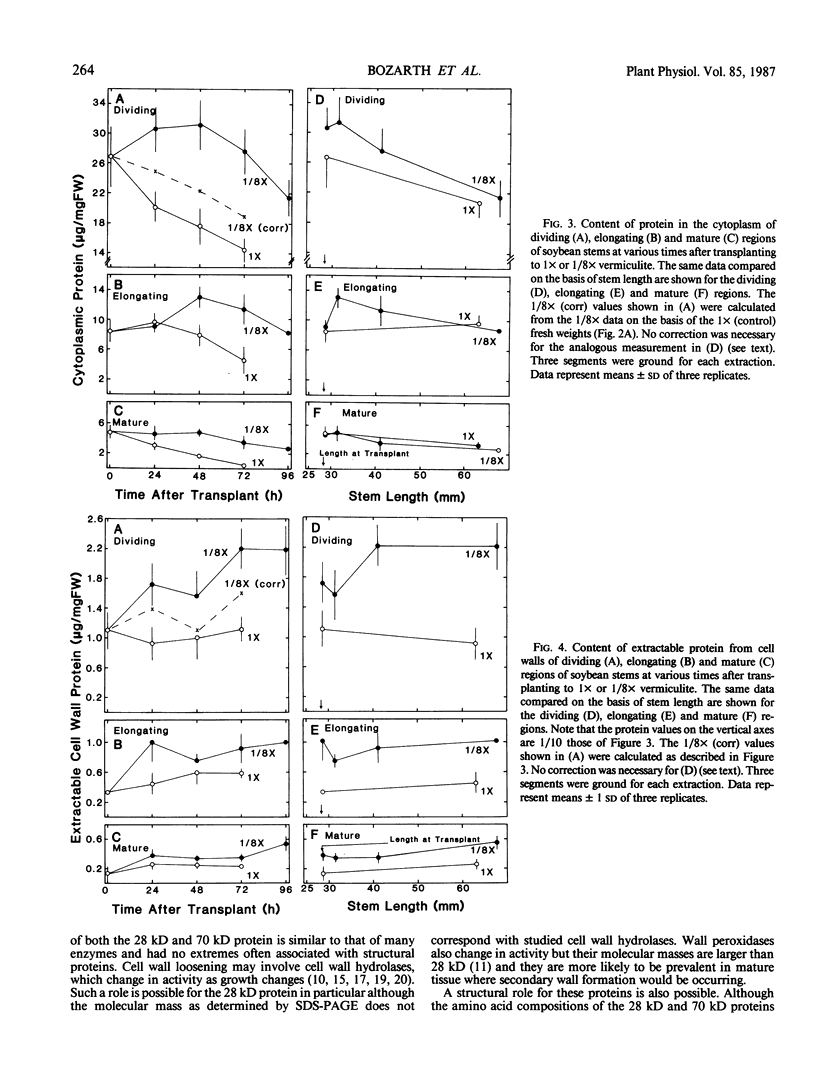
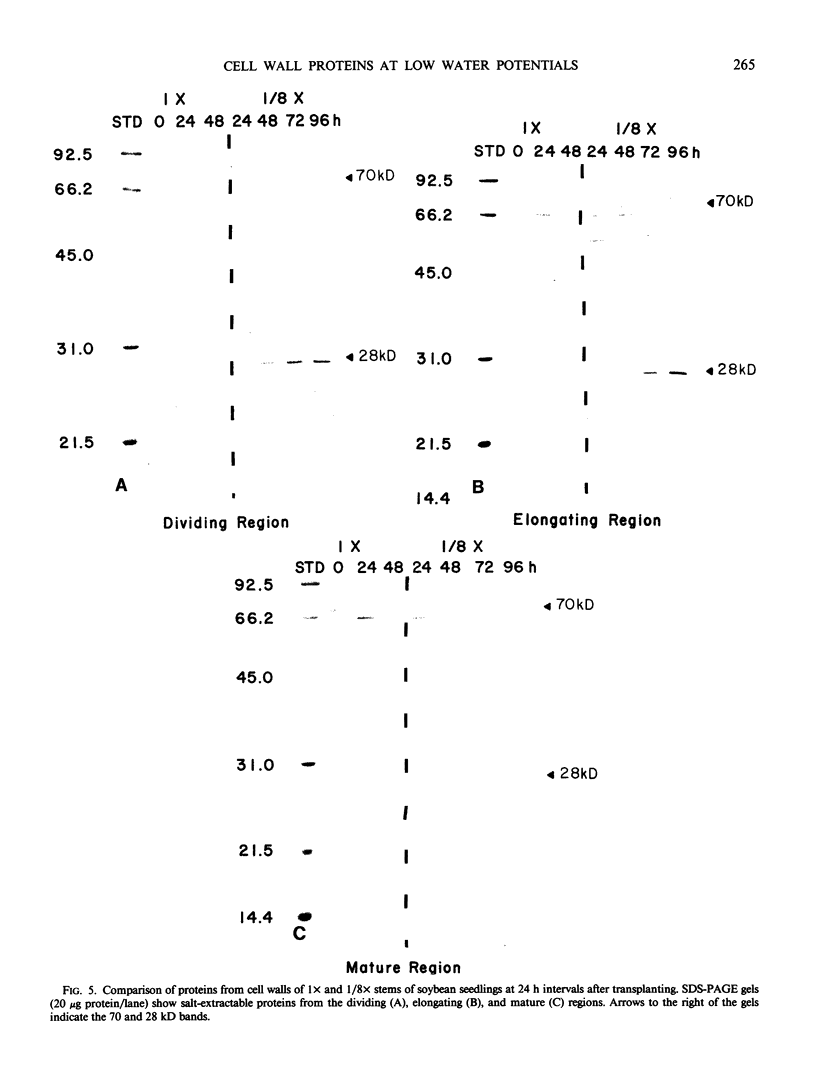
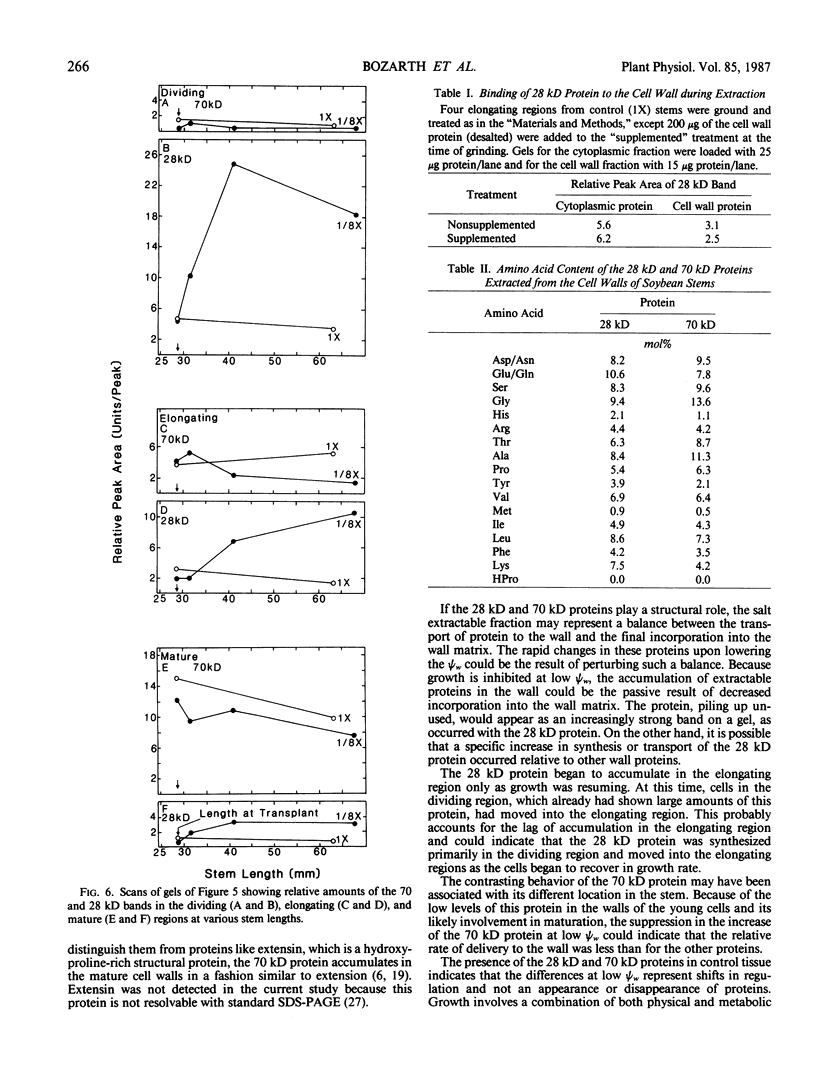
We investigated the proteins extractable from cell walls of stem tissues when plants were subjected to low water potentials (low ψw). Dark-grown soybean seedlings (Glycine max [L.] Merr.) showed decreased stem growth when the roots were exposed to vermiculite having low water content (ψw = −3 bar). After a time, growth resumed but at a reduced rate relative to the controls. The extractable protein increased in the cell walls as ψw decreased, especially a 28-kilodalton protein in the young tissue. In contrast, a 70 kilodalton protein, mainly extractable from mature cell walls, appeared to decrease slightly at low ψw. No hydroxyproline was present in either protein, which shows that neither protein is related to extensin. The level of the 28 kilodalton protein increased in the cell wall of the dividing region soon after the initial growth inhibition, and it appeared in the elongating tissue at about the time growth resumed. The correlation between growth and these protein changes suggests that the two events could be related.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boyer J. S. Isopiestic technique: measurement of accurate leaf water potentials. Science. 1966 Dec 16;154(3755):1459–1460. doi: 10.1126/science.154.3755.1459. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brysk M. M., Chrispeels M. J. Isolation and partial characterization of a hydroxyproline-rich cell wall glycoprotein and its cytoplasmic precursor. Biochim Biophys Acta. 1972 Feb 29;257(2):421–432. doi: 10.1016/0005-2795(72)90295-4. [DOI] [PubMed] [Google Scholar]
- Cavalieri A. J., Boyer J. S. Water potentials induced by growth in soybean hypocotyls. Plant Physiol. 1982 Feb;69(2):492–496. doi: 10.1104/pp.69.2.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J. B., Varner J. E. Insolubilization of hydroxyproline-rich cell wall glycoprotein in aerated carrot root slices. Biochem Biophys Res Commun. 1983 Apr 15;112(1):161–167. doi: 10.1016/0006-291x(83)91811-9. [DOI] [PubMed] [Google Scholar]
- Heikkila J. J., Papp J. E., Schultz G. A., Bewley J. D. Induction of heat shock protein messenger RNA in maize mesocotyls by water stress, abscisic Acid, and wounding. Plant Physiol. 1984 Sep;76(1):270–274. doi: 10.1104/pp.76.1.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmerhorst E., Stokes G. B. Microcentrifuge desalting: a rapid, quantitative method for desalting small amounts of protein. Anal Biochem. 1980 May 1;104(1):130–135. doi: 10.1016/0003-2697(80)90287-0. [DOI] [PubMed] [Google Scholar]
- Higgins R. C., Dahmus M. E. Rapid visualization of protein bands in preparative SDS-polyacrylamide gels. Anal Biochem. 1979 Mar;93(2):257–260. doi: 10.1016/s0003-2697(79)80148-7. [DOI] [PubMed] [Google Scholar]
- Labavitch J. M., Ray P. M. Relationship between Promotion of Xyloglucan Metabolism and Induction of Elongation by Indoleacetic Acid. Plant Physiol. 1974 Oct;54(4):499–502. doi: 10.1104/pp.54.4.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Molz F. J. Growth-induced Water Potentials in Plant Cells and Tissues. Plant Physiol. 1978 Sep;62(3):423–429. doi: 10.1104/pp.62.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadava D., Chrispeels M. J. Hydroxyproline-rich cell wall protein (extensin): role in the cessation of elongation in excised pea epicotyls. Dev Biol. 1973 Jan;30(1):49–55. doi: 10.1016/0012-1606(73)90047-x. [DOI] [PubMed] [Google Scholar]
- Stuart D. A., Varner J. E. Purification and Characterization of a Salt-extractable Hydroxyproline-rich Glycoprotein from Aerated Carrot Discs. Plant Physiol. 1980 Nov;66(5):787–792. doi: 10.1104/pp.66.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twente J. W., Twente J. A. Regulation of hibernating periods by temperature. Proc Natl Acad Sci U S A. 1965 Oct;54(4):1044–1051. [PMC free article] [PubMed] [Google Scholar]
- Van Volkenburgh E., Boyer J. S. Inhibitory effects of water deficit on maize leaf elongation. Plant Physiol. 1985 Jan;77(1):190–194. doi: 10.1104/pp.77.1.190. [DOI] [PMC free article] [PubMed] [Google Scholar]