Abstract
Although genomic research offering next generation sequencing (NGS) has increased the diagnoses of rare/ultra-rare disorders, populations experiencing health disparities infrequently participate in these studies. The factors underlying non-participation would most reliably be ascertained from individuals who have had the opportunity to participate, but decline. We thus enrolled parents of children and adult probands with undiagnosed disorders who had declined genomic research offering NGS with return of results with undiagnosed disorders (Decliners, n=21) and compared their data to those who participated (Participants, n=31). We assessed: 1) Practical barriers and facilitators, (2) Sociocultural factors- genomic knowledge and distrust, and 3) The value placed upon a diagnosis by those who declined participation. The primary findings were that residence in rural and medically underserved areas (MUA) and higher number of barriers were significantly associated with declining participation in the study. Exploratory analyses revealed multiple co-occurring practical barriers, greater emotional exhaustion and research hesitancy in the parents in the Decliner group compared to the Participants, with both groups identifying a similar number of facilitators. The parents in the Decliner group also had lower genomic knowledge, but distrust of clinical research was not different between the groups. Importantly, despite their non-participation, those in the Decliner group indicated an interest in obtaining a diagnosis and expressed confidence in being able to emotionally manage the ensuing results. Study findings support the concept that some families who decline participation in diagnostic genomic research may be experiencing pile-up with exhaustion of family resources - making participation in the genomic research difficult. This study highlights the complexity of the factors that underlie non-participation in clinically relevant NGS research. Thus, approaches to mitigating barriers to NGS research participation by populations experiencing health disparities need to be multi-pronged and tailored so that they can benefit from state-of -the art genomic technologies.
Keywords: Genomic Research, Genetic Counseling, disparities, exome and genome sequencing, underrepresented populations, parent
Introduction
Precision medicine, which incorporates genetic information into medical care, holds great promise in its potential to optimize an individual’s health (Manolio et al., 2019). Nowhere has this been more impactful than in the application of next-generation genomic sequencing (NGS) to undiagnosed rare/ultra-rare disorders, which collectively affect six percent of the US population, causing personal/familial distress and exerting a societal impact (Nguengang Wakap et al., 2020). Until about a decade ago, the traditional diagnostic approach of testing single genes or a small number of genes, left many of these individuals undiagnosed (Shashi et al., 2013). In recent years, the availability of NGS, which interrogates the entire exome or genome, has resulted in diagnoses in 25–50%, and an increase in new disease gene discoveries (Shickh et al., 2021). In addition to NGS being available clinically, large-scale research studies such as the NIH-Undiagnosed Diseases Network (UDN) have utilized NGS innovatively to achieve diagnoses for patients with the most intractable manifestations, improve medical care to these patients and their families and move the needle on genomic medicine and science (Schoch et al., 2021; Splinter et al., 2018). However, it has been observed since the inception of the UDN that populations experiencing health disparities, participate in low numbers (>80% of UDN applicants self-identify as White and 10% as Hispanic or Latino), with populations experiencing health disparities defined as inclusive of racial and ethnic minority groups, people with lower socioeconomic status (SES), underserved rural communities, and sexual and gender minority groups (U.S. Department of Health and Human Services, 2022). With access to state-of-the-art NGS that has direct clinical benefits to them, provided as part of the research study, it is concerning that so few affected patients and their families from populations that experience healthcare disparities participate in genomic research. Further, specific reasons for non-participation remain unclear. There is an imperative to understand the underlying factors for non-participation in NGS genomic research by these individuals so as to decrease malleable inequities that lead to low engagement.
The existing literature describes many factors influencing participation in genomic research studies by populations experiencing health disparities, with some obstacles being common to all clinical research studies. These barriers include cost, time, travel (Chou et al., 2021; Gene Hallford et al., 2020; Penon-Portmann et al., 2020), as well as factors specific to genomic research, such as doubts about the validity of genomic testing, potential negative emotional response to information learned and concern that the information would be used against a group or community, and cultural beliefs (Fisher et al., 2020; Johnson et al., 2009). Robinson et al. (2016) assessed why individuals declined genome sequencing, finding that the major reasons were practical barriers of time and study logistics, and for some fear of insurance discrimination, concerns about emotional response to the information, and concerns about privacy. However, unlike patients with undiagnosed/rare disorders, the majority of subjects who responded in this study, were healthy adults, and thus their perspective may be different from those who are experiencing a diagnostic odyssey.
The construct of genomic knowledge (Langer et al., 2017) could also influence an individual’s participation in genomic research. Previous research has reported that individuals self-identifying as African American or Hispanic/Latino, and those with lower education and SES have lower genomic knowledge and also lower uptake of genetic testing and genomic research (Canedo et al., 2019; Fisher et al., 2020; Gómez-Trillos et al., 2020; Hooker et al., 2014; Hurle et al., 2013; Krakow et al., 2017; Rini et al., 2020). Rini et al. (2020) also found that higher genomic knowledge was associated with better understanding of NGS results, but did not examine the relationship between genomic knowledge and the decision to participate in NGS genomic research.
Few data are available on the barriers and sociocultural factors specific to participation in NGS genomic research by populations experiencing health disparities. A study applying NGS in heart disease used potential participants in community-based focus groups to identify major barriers for rural and minority participants-the barriers ascertained included the use of technical language, concern of privacy, distrust of medical research, costs and concerns about what might be learned from the genomics (Skinner et al., 2015). The facilitators included the potential benefits of obtaining genomic results and altruism. The NIH Clinical Sequencing Evidence-Generating Research (CSER) consortium explored in phase I, recruitment and rates for declining to their various NGS studies and reported that 12%–64% (median 28%) of patients, declined participation with study logistics being the most common reason for declining; however, no demographics of those who declined were reported (Amendola et al., 2018). As part of the initial phase, the NCGENES study reported that 40% of patients referred to their NGS study, could not be reached, did not attend a scheduled visit, were not interested, or had poor health (Moore et al., 2017). Those identifying as African Americans were more likely to decline (44% versus 24% of those identifying as White) and after enrollment, there was greater attrition for the participants self-identifying as non-White and/or Hispanic (Moore et al., 2017). In another network-wide CSER publication, barriers identified by individuals from diverse socioeconomic backgrounds who participated in their study (including those from populations who may experience health disparities), were, in order of highest to lowest frequency: beliefs (attitudes, values, and knowledge), organizational (availability and access), financial and perception of need (distrust of healthcare system, time, avoidance), and social (language and cultural) (Gutierrez et al., 2021). Notably, these data were collected only from participants at the time of study consent or during the course of the study. CSER also strongly emphasized that patients who had limitations in access to general healthcare services, also would not have access to genomic research that recruits through the healthcare systems (Gutierrez et al 2021). The BabySeq Project, which offered NGS to both healthy and sick newborns, asked parents who declined (mostly of healthy newborns) to describe in their own words the reasons for doing so. The individual free-text responses were grouped into categories- burdensome study logistics, feeling overwhelmed postpartum, lack of interest in the genetic testing and concerns about privacy, insurability and uncertain results. Findings from this study are limited in that <30% of parents who declined provided reasons, the parents were not administered surveys or interviewed and the demographic information was incomplete (the collected data indicated the majority were White-80%). A recent study of parents of children enrolled in the UDN, targeting parents from diverse racial and ethnic backgrounds highlighted the importance of their relationships with providers, cultural norms about communication of the child’s disorder, access to resources (e.g., education, income, social connections), and language concordance as important in increasing access to the network study (Young et al., 2022). Although these studies provide a multitude of reasons for low participation in research using NGS the reasons were inferred, or ascertained from individuals either considering participation or actually participating in NGS research, and did not extract information from individuals who declined to participate.
Two genomic research studies at our institution offer diagnostic NGS- the UDN and the Duke Research Sequencing Clinic (DRSC). We reviewed the Duke Health internal referrals from medical genetics providers to both studies and found that approximately 42% of patients/parents did not participate in either study, despite initially actively agreeing to the NGS study referral and being informed that the evaluations, the NGS, and return of results were all cost-free. This was particularly perplexing since we have previously shown that despite high rates of anxiety and depression, undiagnosed patients/parents, are actively engaged in genomic research offering return of results of NGS (McConkie-Rosell et al., 2018; McConkie-Rosell et al., 2016; Spillmann et al., 2017). We suspected that there were multiple factors underlying their non-participation. Thus, we undertook the current study (Barriers study) to examine factors that contribute to the involvement or lack of involvement of families in genomic research studies. This is a novel study which identifies and surveys both decliners and participants in diagnostic NGS research studies providing return of results, clinical genetics evaluations, and genetic counseling. Using our experiences and the literature, we hypothesized that Decliners of NGS research would be more likely from a population that historically has experienced health disparities, would identify a multitude of underlying factors such as more practical barriers, fewer facilitators, demonstrate lower genomic knowledge, more distrust of clinical research, yet have a similar interest in obtaining a diagnosis, as those who participated.
Methods
Human Subjects Ethics approval was obtained from the Institutional Review Boards of the National Human Genome Research Institute (15-HG-0130), Duke University Medical Center (Pro00056651), and Duke University Medical Center (Pro00105966).
Sample Identification
Using a retrospective study design, data were collected on internal referrals from the Duke Health Genetics providers to two NGS research studies, the UDN and the DRSC, from July 2015- October 2021. The Decliners were identified from both studies and the Participants were only identified through the UDN (Duke clinical site only). We included all identified referrals in the period above and parsed them into their appropriate category those who enrolled in the UDN (Participant Group) and those who did not enroll in either the UDN or the DRSC (Decliner group)and reviewed to determine if the proband met inclusion criteria.
The UDN is funded by the NIH Common Fund, and consists of 12 clinical sites and supporting research cores and a coordinating center, with a mission to diagnose patients with mystery illnesses and move forward research for rare and undiagnosed diseases (https://undiagnosed.hms.harvard.edu/). Since most rare/ultra-rare disorders are genetic in etiology, NGS is a major component of UDN evaluations. Results of clinical assessments from medical specialists, laboratory tests, NGS [inclusive of exome sequencing (ES) and genome sequencing (GS)], are provided whether a diagnosis is established or not. The UDN processes aim to reduce patient burden, as much as possible- the twelve UDN clinical sites provide travel, hotel accommodations, and a stipend for food from research funds, to reduce the burden of geographic location or financial constraints, the individualized clinical evaluations occur within the one-week period of their stay at the clinical site and since 2020, the Duke site has been offering telehealth and hybrid telehealth/in-person evaluations to decrease the need for travel. NGS is also provided free of cost through the UDN sequencing core laboratory. At the Duke site, all clinical and laboratory evaluations are also paid for by research funds, so patients/families are accepted without any consideration of their financial status. Applicants require a referral letter from a medical provider and complete an online application with assistance provided by the UDN coordinating center if needed. The DRSC is funded by the Duke Health System where clinical genetics evaluations and NGS are provided for undiagnosed patients in this research clinic. Referrals are made directly by the health provider with participation involving one to two clinic visits. Similar to the Duke UDN site, hybrid and virtual telehealth visits are offered. All activities including the NGS are provided free of cost to the patients, with results communicated to the patients and the referring providers.
Text mining of the Duke Electronic Health Record (EPIC) was utilized to identify Participants (those who applied to the UDN and had been accepted) and Decliners (those that did not apply to the UDN after being referred by their Duke Health Provider). We reviewed the internal referrals log to the DRSC, to identify Decliners (those that had declined when contacted by the study genetic counselor or had not responded to calls or emails).
Inclusion Criteria and Recruitment for Barriers study
For the Decliner group, individuals were eligible for this study if all four criteria were met: 1) A referral to the UDN/DRSC had been made for themselves or their child by a Duke Health Genetics provider and they had actively expressed agreement to be referred, 2) Greater than 12 weeks had lapsed since the referral and no application had been received for the UDN or for the DSCR, the parents or adult proband had not responded to study staff after three attempts to contact by phone and a subsequent email/letter, 3) The proband was living (to avoid contacting families suffering from the demise of a loved one), and 4) The Proband remained undiagnosed with existing clinical testing as the purpose of the referral to UDN or DSRC was for additional diagnostic investigation. For the Participant group, parents were eligible if: 1) They or their child had been referred by a Duke Health genetics provider to the UDN after actively expressing agreement to the referral, 2) They had applied to and been accepted to the UDN, and 3) The proband was living. For both groups medical record review was specifically done focusing on the genetic clinic notes to confirm documentation of 1) discussion about the UDN/DRSC research studies (the potential benefits, limitations), 2) discussion of any clinically available options focused on diagnostic investigation (such as waiting for ES reanalysis, clinical ES or GS), 3) the patient or parents expressed agreement to be referred and 4) Proband met eligibility criteria. Both parents were eligible to participate as we were interested in learning all that we could from their experiences for both Decliner and Participant groups. Parents included biological parents and primary caregivers or guardians of children/adults who could not consent for themselves. Since most of the internal Duke referrals to both NGS studies involve children, the majority of identified eligible individuals in both groups were parents of children referred. Informed consent was provided by all individuals who agreed to participate in the study.
Measures
Demographics
The following data were collected on subjects in both groups: age, highest educational level, race, ethnicity, zip codes to identify geographic location (urban versus rural and medically underserved area (MUA) as designated by HRSA (Health Resources & Services Administration, 2022) and the Department of Agriculture (USDA ERS, 2020) [notably, many of these demographics map on to the Social Determinants of Health (SDH) as defined by the U.S. Department of Health and Human Services as including 5 domains: economic stability, education access and quality, health care access and quality, neighborhood and built environment, and social and community context (U.S. Department of Health and Human Services, 2022)]. Geographic residence was determined to be rural and MUA if the zip code mapped to one of the 76 counties in NC that meet the criteria above. We also collected information on whether the proband had undergone NGS prior to referral to the UDN or the DRSC. We note that race and ethnicity are social constructs and so should not be used as distinguishing biological or genetic variables in healthcare disparity research (Fraiman & Wojcik, 2021; Krieger, 2001; White et al., 2020) and thus we use these for descriptive purposes only and not for comparisons between the Decliners and Participants. To determine if access to services was a factor underlying participation in NGS research we examined the number of clinic visits to genetics in the Decliner and Participant groups.
Surveys and Interview
We used quantitative surveys as well as qualitative data obtained from open-ended items on the surveys for both groups and a brief interview with the Decliner group. Our measurement strategy was guided by the existing literature and our own research on psychosocial factors associated with undergoing NGS (McConkie-Rosell et al., 2018; McConkie-Rosell et al., 2016; McConkie-Rosell et al., 2019; Spillmann et al., 2017). We assessed in both Participants and Decliners: (1) Practical barriers and facilitators, and (2) Sociocultural factors, including genomic knowledge and distrust. In the Decliner group only, we ascertained the expectations of NGS, the meaning they ascribed to a diagnosis and how they would emotionally manage the information, utilizing the Genomic Empowerment Scale (GEmS) (McConkie-Rosell et al., 2019; McConkie-Rosell et al., 2022). The survey administration occurred from January 2021- February 2022 and were available to the subjects online, on paper, or through a telephone call with the study coordinator. The majority of surveys were completed online via REDCap database. For Spanish-speaking subjects, the surveys were translated into Spanish and surveys and interviews were completed with a Spanish speaking study coordinator. Participants were informed that surveys would take approximately 20–30 minutes and they would be compensated $30 for their time and instructed to think back to their reasons for either being a part of the NGS research or deciding not to participate. Parents and adult probands in both the Decliner group and the Participant group completed the following surveys:
Barriers and Facilitators Checklist.
Since there is no validated measure to assess practical/logistical barriers and facilitators to NGS, we developed a checklist after extensively reviewing the existing literature to identify the most common reasons why individuals choose to participate or decline research in general and specifically genetic/genomic research (Advani et al., 2003; Amendola et al., 2018; Burchard et al., 2015; Corbie-Smith et al., 2002; Davis et al., 2019; George et al., 2014; Gómez-Trillos et al., 2020; Hamel et al., 2016; Hughes et al., 2017; Lang et al., 2013; Oh et al., 2015). The items were developed to capture relevant SDH as well as concerns related to activities required of the genomic research, such as the ability to travel, time away from work, social support, understanding the research process, computer access, concerns about stigmatization or discrimination, and distrust of medical research. The checklist has 19 barriers and 9 facilitators, uses a Likert scale, and has two open-ended items allowing for free text responses. Preliminary reliability showed a Cronbach’s Alpha of .88 for the Barriers and .61 for the Facilitators. Higher scores are indicative of greater perceived barriers and facilitators. A total score was calculated separately for both the barriers and facilitators.
The University of North Carolina Genomic Knowledge Scale (UNC-GKS).
The UNC-GKS assesses knowledge in four domains: structure and function of genes, how they are inherited in families, their relation to health, and strengths and limitations of exome sequencing (Langer et al., 2017). The survey consists of 25 true/false items, has good reliability (Cronbach’s Alpha = .90) and assesses genomic knowledge from individuals with variable sociodemographic backgrounds. It provides a single total score of genetic health literacy, and the raw score is converted to a T-score.
Corbie-Smith Distrust Index.
This index consists of seven items, in a Likert response format assessing two types of distrust related to clinical research: interpersonal and societal distrust (Corbie-Smith et al., 2002; Durant et al., 2011). Durant defined interpersonal distrust as distrust based on personal experiences with healthcare or clinical research (e.g., belief that your doctor would not explain research fully to you if asking you to participate in a trial) and societal distrust as a general negative view of clinical research based on societal life experiences or perceptions (e.g., likelihood that you or people like you will be used as guinea pigs without permission). This index allows for the two subscale scores to be individually reported as by Durant et al. 2011.
Genome Empowerment Scale (GEmS).
For the Decliner group only, we included two scales from the GEmS, to obtain data on whether the Decliners had less interest in obtaining a diagnosis (expectation of a diagnosis from NGS and what that diagnosis could mean for them) and managing the information (confidence in their ability to emotionally manage the outcome of the genomic sequencing). Both scales have good reliability (Cronbach’s alpha .83 and.80). Total scores for each scale were compared to the previously published norms derived from UDN participants (McConkie-Rosell et al., 2019; McConkie-Rosell et al., 2022). Additionally, the two scales each have an open-ended response option that asks parents to 1) describe how “the information gained from the genetic sequencing will, in the future, improve your child’s life?” and 2) describe any worries about what they might learn about their child’s diagnosis from the sequencing.
Brief Structured Interview for Decliners
At the time of initial phone contact for the Barrier study, all Decliners were offered a brief phone interview to capture as much data as possible as a complement to the survey data. We asked all subjects in the Decliner group to reflect back on their reasons for choosing not to participate in either the UDN or the DRSC. We asked individuals in the decliner group to: 1) Inform us as to why they had decided not to participate in the UDN or DRSC, 2) What, if any, barriers made it difficult to participate, 3) If they had any recommendations for how it could be made easier to participate, including the possibility of participation through telehealth? The length of the phone interview varied, but on average lasted approximately 10–20 minutes. A structured interview format was used, conducting either in English or Spanish with responses written down by the interviewer (either the genetic counselor or Spanish speaking study coordinator) using standard interview techniques with general concepts repeated back by and confirmed by the respondent (our IRB protocol did not allow for these responses to be audio-taped).
Data Analysis
Statistical analysis of quantitative data was performed using SPSS V 28 (IBM Statistics). The demographics were compared between the groups with Student’s t-tests and chi-square analysis. Student’s t-tests were also performed for group differences on the total barriers and facilitators scores, genomic knowledge scale and, the distrust index (interpersonal and societal distrust subscales) to determine their inclusion in the logistic regressions. We did not compare groups on race/ethnicity, since a narrow focus on race and ethnicity is likely to obscure the underlying factors that may be driving the reasons for non-participation of populations experiencing health disparities in research or clinical services (Martínez-García et al., 2022; White et al., 2020). MANOVAs were computed in an exploratory manner, for group differences on the individual items in the barriers survey. The GEmS data in the Decliners were compared to previously published z-scores (McConkie-Rosell et al., 2022).
To address the primary research question of identifying significant factors that distinguished the Decliner group from the Participant group, logistic regression was used. To determine potential factors in the stepwise binary logistic regression, point biserial correlations were examined to ascertain the relationship of group membership with the total barriers scores and genomic knowledge for inclusion in the model (since these were significantly different between the groups). We also created centered interaction terms for key variables to understand the moderating effect of each on group membership. If significant, interactions would be examined by simple slopes analysis.
Qualitative data were analyzed using Atlas ti v 7.5. The interview notes from the Decliners’ brief interviews were reviewed and compared for commonality and differences to the individual items on the barriers and facilitators checklist using a directed content approach (Potter & Levine-Donnerstein, 1999). These findings were then compared to the survey responders handwritten notes on the open-ended items on the barriers and facilitators checklist. We then merged the interview notes with the parents handwritten responses on both open-ended items, so that responses from the same participant (all representing a unique probands) who wrote responses on the survey and completed the interview, were not counted twice and we were able to maximize the information from these sources. We repeated the process for the two open-ended items on the GEmS and for the participant group on the Barriers and Facilitators Checklist. These findings were compiled, themes identified, and illustrative quotes from the parents’ written responses selected.
Results
Enrollment
We identified 70 probands who themselves or their parents/guardians were informed about the UDN or the DRSC by their genetics health provider and actively expressed interest and agreed to the UDN or the DRSC referral who subsequently did not apply or declined when contacted by the study team (Decliners) and 44 probands themselves or their parents/guardians who had applied to the UDN and were accepted (Participants). We were able to contact at least one parent/guardians/themselves for 52 of the 70 Decliners and for all 44 Participants. Figure 1A and 1B provides details of the response rates in both groups for the surveys and the brief interview. It is to be noted that although only 47% (n=21/52) of those in the Decliner group completed the surveys, 90% (47/52, including one adult proband and 46 parents/guardians of probands) completed the brief structured interview. Of the 21 who completed the surveys in the Decliner group, all were parents (18 declined the UDN and three the DRSC) representing 21 unique probands (Figure 1A). The Participants represented 24 unique probands (inclusive of three adult probands and 21 children) with both parents providing data for 7 probands, leading to 31 responses (Figure 1B). Twenty-nine percent (7/24) of the Participant group probands had a certain or highly likely diagnosis established by the UDN whereas none of the Decliners had a diagnosis, consistent with our inclusion criteria.
Figure 1A: Recruitment and retention of Decliners from the UDN and DRSC.
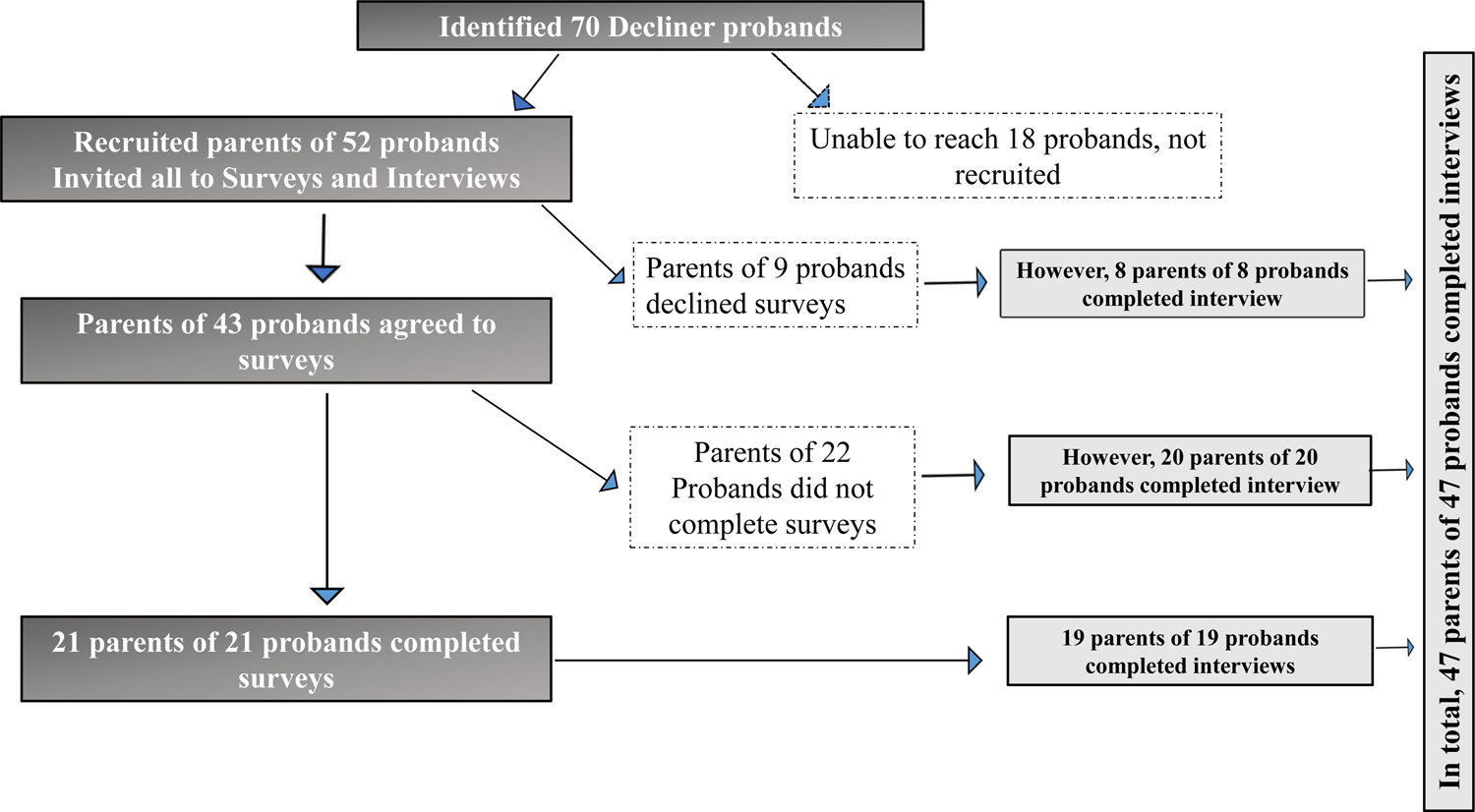
Illustration of sample identification, recruitment and study completion for the Decliner group. Recruited subjects were more willing to complete the qualitative interviews (47/52= 90%), than the quantitative surveys (21/52= 40%). There were no duplicate responses to either the surveys or the interviews in the Decliner group, with the data thus being independent.
Figure 1B: Recruitment and retention of Participants from the UDN.
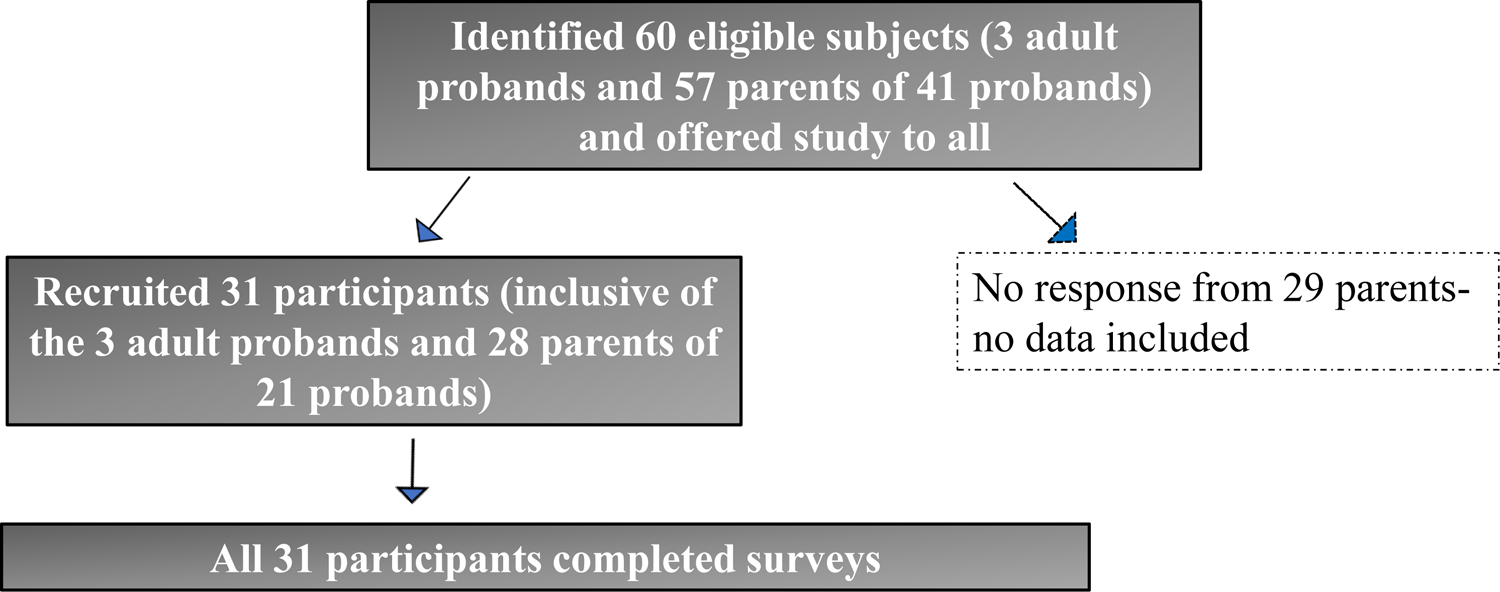
Illustration of sample identification, recruitment and study completion for Participant group. Survey completion rate was higher than in the Decliner group (24/44 probands, 54.5%). Of the 24 probands in the Participant group, seven had duplicate survey responses, due to both parents responding, with a total of 31 responders (3 adult probands who responded for themselves and 28 guardians responding for the other 21 probands). Thus, non-independence of the data was accounted for in our analyses. Interviews were not performed in the Participant group, to decrease study burden, since they had already participated in the research NGS.
Preliminary Analysis
We examined demographic characteristics between all the eligible probands in the Decliners (n =70) and Participants (n =44) groups, including those that we were unable to reach (Figure 1). Geographic location was the only significantly different demographic variable between the groups (Fisher’s exact test p = .01), with the Decliner group more likely to be from rural/MUA counties [55% (39/70)] compared to probands the Participant group [30% (13/44)]. There were no differences detected on race, ethnicity, and prior experience with non-diagnostic ES. We did not have access to educational level for the Decliners we were unable to reach so that demographic could not be assessed for the total groups. The majority of those in the Participant group who applied to the UDN did so within a few weeks of the referral (mean of 4.0 ± 6.70 weeks) (N = 44 probands). The decliner group which was defined by not applying for a minimum of 12 weeks post referral had a mean of 151.86 ± 83.87 weeks that had lapsed since referral (n = 70), but this time difference cannot be compared to the Participant group, since the Decliner group never applied to the NGS studies.
In evaluating access to genetic services across the two groups, we examined the number of visits to medical genetics clinics prior to the referrals to the UDN and DRSC: there were no significant differences between the two groups: (Decliners= mean 3.61 ±2.02 visits and Participants= mean 3.73 ± 2.20 visits prior to the referral t(112)= −.280, p= NS Twenty-six percent (18/70) of the probands in the Decliner group had also been seen at least once (mean 1.89 ± 1.20; range 1–5) in the medical genetics clinic for routine follow-up after the referral had been made to the UDN or DSRC before contact for the Barriers study.
We also examined if there were any demographic differences between the probands in the Decliner group that we were successful in contacting (n =52) and those who we could not reach (n = 18). There were no significant group differences for the number of genetics clinic visits, geographic location (urban versus rural/MUA), and prior experience with a nondiagnostic ES. However, the Decliner probands who were unreachable for the Barriers study were more likely to be non-White (11/52; 8/18 (p<.001) and/or Hispanic (4/52; 2/18 (p = .01).
In the Participant group, there were 24 probands (three probands were adults who responded to surveys themselves and 21 probands were children or adults who could not consent for themselves, for whom parents responded). For these 24 probands we had 31 survey responses, due to duplicate parental responses from 7 families. To account for non-independence of the survey data, we performed a mixed effects generalized linear modeling with the quantitative measures, first using data from all 31 surveys, and then with the 24 unique survey responses (one for each proband, randomly selecting one parent among the duplicate responses). The results were not substantially different with the two sample sets. However, we performed logistic regression analysis only with the data from the 24 unique surveys in the participant group, since regression analysis assumes that the data are independent. Demographic data for the subset of parents and adult probands in both groups who completed the quantitative surveys are in Table 1.
Table 1:
Demographic data on parents in the Decliner and Participants groups who completed the surveys and group differences in Barriers and Facilitators, genomic knowledge and distrust in clinical research.
| Variable | Decliners who completed surveys (n=21) |
UDN Participants who completed surveys (n=31) |
|---|---|---|
| Demographics | ||
| Geographic location** | Rural= 12 Urban= 9 |
Rural= 7 Urban= 24 |
| Gender* | Female= 20 Male= 1 |
Female= 19 Male= 12 |
| Race and ethnicity | Asian = 1 Black or African American = 4 White= 15 Undefined = 1 Hispanic= 2 |
Asian = 1 Black or African American = 2 White= 24 Undefined = 4 Hispanic= 4 |
| Age in years | 40.63 (10.73) | 41.06 (7.05) |
| Education** | High School graduate or below= 6 Some post High School Education = 10 College Graduate/Post Graduate Degree = 5 |
High School graduate or below= 6 Some post High School Education = 3 College Graduate/Post Graduate Degree = 22 |
| Prior non-diagnostic Exome Sequencing | 86 % (18/21) | 87% (27/31) |
| Proband age | 11.65±7.4 years | 14.2 ±12.92 years |
| Proband Gender | Male 10 Female 10 |
Male 23 Female 8 |
| Surveys for Barriers/Facilitators and Socio-cultural Factors | ||
| Barriers*** | 56.55± 21.36 (median 55) | 34.65 ± 14.95 (median 31), t =4.3 |
| Facilitators | 54.95± 6.75 (median 56.5) | mean 55.23± 5.85 (median 57) |
| Genomic knowledge scale* | 42.94±8.66 | 49.28±9.64, t= 2.08 |
| Interpersonal Distrust | 6.86 ±3.23 | 7.23 ± 2.57 |
| Societal Distrust | 3.29 ± 2.08 | 2.68 ± 1.19 |
| Survey for interest in obtaining a diagnosis (Decliners Only) | ||
| GEmS Meaning of a Diagnosis | mean 47.90 ± 4.55 (Z =0; raw score 38–51) | |
| GEmS Emotional management | mean 35.4 ± 10.40 Z = 0; 26–41) | |
p<.05
p<.01
p<.001
Student’s t-test, Fisher-Exact test and chi-square analysis
Large effect size results are in bold.
Barriers and Facilitators, Genomic Knowledge, and Distrust in Participants versus Decliners
The findings from the Barriers and Facilitators Checklist, Genomics Knowledge Scale, and Corbie-Smith Distrust Index are in Table 1. The total mean score for barriers for parents in the Decliner group was significantly higher compared to the Participant group, t (49) = 4.31 p<.001, while the total facilitators scores did not differ between the two groups, t (49) = 0.16. On the Genomic knowledge scale, the parents in the Decliner group had significantly lower scores, indicating lower genomic knowledge, than those in the Participant group; interpersonal and societal distrust subscale scores were not significantly different between the two groups. On the GEmS, we found that parents in the Decliner group had scores on the two scales, Meaning of a Diagnosis and Emotional Management, that were within the published norms for parents prior to participating in NGS for their children in the UDN (McConkie-Rosell et al., 2022) (Table 1).
Point biserial correlations between group and the total number of barriers and genomic knowledge demonstrated that the Decliner group was associated with significantly more barriers (r= .524, p<.001) and significantly lower genomic knowledge (r= −.324, p<0.05). As a prelude to regression analysis, we examined the correlations in the entire sample, between the predictor variables that were chosen for the regression, based on the t-tests and correlations above. There was a significant negative correlation between the total barriers score and genomic knowledge (r=−.454, p<.001). Rural residence was significantly correlated with lower education (r= .396, p<.01), but not with barriers. Education and genomic knowledge were highly positively correlated (r=.758, p<.001). As we are cognizant of the effect of time on recall in both groups, we performed correlations using time interval since referral in each group, with the Barriers, Facilitators, and Genomic Knowledge. There were no significant correlations in either group and thus time elapsed since referral is not a significant factor that impacts our survey data.
Exploratory Analysis of Group Differences on Items From the Barriers and Facilitators.
We undertook an exploratory analysis of group differences on the individual items on the barriers and facilitators checklist. The overall MANOVA was statistically significant between the two groups for the individual barrier items (Pillai’s Trace = .934 F (23.01) p<.001) (Figure 2) (see Supplemental Table 1). A specific question regarding telehealth as an option instead of an in-person evaluation was added after the study was underway, and was rated significantly more often as a facilitator, by the parents in the Decliner group (n = 20) than those in the Participant group (n = 19) [(t (37) =2.46 p< .01]).
Figure 2: Many individual barriers were endorsed by the Decliner group, reflecting significant group differences in both practical considerations and cultural factors.
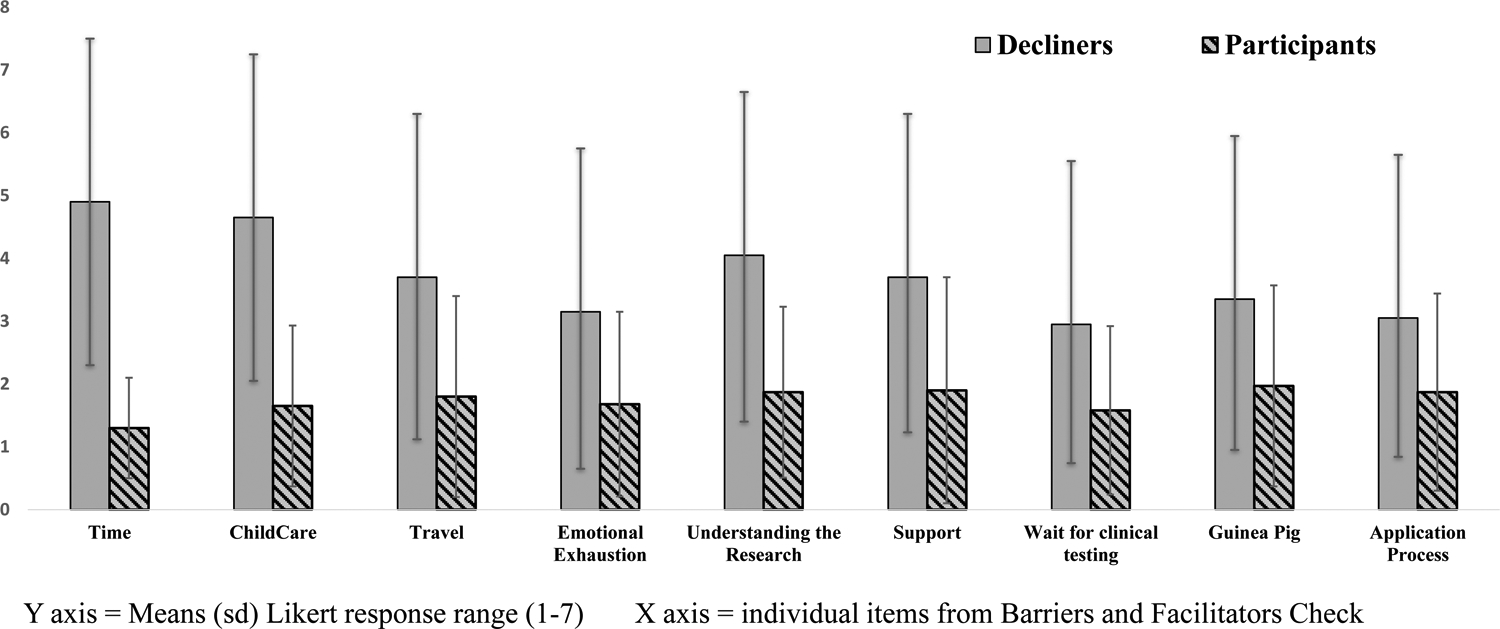
Multiple co-occurring barriers were endorsed by the Decliner group. Means (s.d.) of these barriers from the Barriers and Facilitators Checklist (Likert response options ranged from 1–7) are presented.
Predictors of Decliners and Participants Groupings
Binary logistic regression analyses with group membership as the dependent variable, and stepwise predictors chosen from our comparisons above and our hypotheses, adding in relevant interaction terms, are shown in Table 2. We found that geographic residence and the total number of barriers were significant predictors of group membership. In contrast, education and genomic knowledge were not significant predictors of group membership. This may be because of the relation between education and geographic location, with rural residents less likely to have a college or higher education (14.3% Decliners versus 77.4% Participants, X2(1) 11. 34, p<.01). Additionally, geographic location accounted for the majority of the variance with an odds ratio of 6.56 (Chen et al., 2010). Neither of the two interaction terms (barriers X genomic knowledge and Rural/Urban X genomic knowledge) were significant predictors of group membership, suggesting that genomic knowledge was not synergistic with either barriers or geographic location on the group outcomes
Table 2:
Binary logistic regression to assess variance associated with key variables in the Decliner and Participant groups: the duplicate responses in the Participant group were excluded from the regression analysis
| Step 1: Key Demographics | Step 2: Total# barriers | Step 3: Genomic knowledge | Step 4: Barriers*genomic knowledge | Step 5: Rural*genomic knowledge | ||
|---|---|---|---|---|---|---|
| Group membership (Decliners= 20 # and Participants= 24) | Rural vs Urban Odds ratio= 8.16** | 95% CI= 2.06–32.2 | Odds ratio= .91** 95% CI= .859–.969 |
Odds ratio= 1.02 95% CI= .912–1.14 |
Odds ratio= 1.00 95% CI= .994–1.01 |
Odds ratio= 1.03 95% CI= .726–1.463 |
| Education Odds ratio= 1.21 | 95% CI= .265–5.54 | |||||
p<0.05,
p<0.01,
p<0.001
CI= Confidence interval
Odds ratio: small= 1.68, medium= 3.47, and large= 6.71(Chen et al., 2010)
One participant had missing data on the surveys
Qualitative Findings From Decliners and Participants
Open-ended responses from Decliners.
Ninety percent (47/52, inclusive of the 21 who completed surveys) of the parents and one adult proband in the Decliners group provided comments on at least one of our three sources (Barriers and Facilitators Checklist, GEmS, structured interview). The majority of the reasons for non-participation were consistent with the items on the Barriers and Facilitators checklist. The most commonly endorsed reasons were lack of social support, difficulty with the application process, emotional exhaustion, lack of time, and difficulty with travel.
“I have two sons with special needs, taking one means no one is available to watch the other. I don’t have somebody to watch him. I was told that my life for a week would be at Duke Hospital, back and forth for a week and so I just couldn’t do that. And then do it twice for the other child.”
However, through this process we identified two additional barriers that were not on the checklist: 1) Other concerns taking precedence, such as health concerns for the child or for other family members and 2) Not wanting to put the child through any more tests or procedures. Approximately one fourth of parents also expressed concerns about research participation, including simply not wanting to engage with research, concerns about the uncertainty inherent in research or worry over being a guinea pig.
Facilitators identified by parents in the Decliner group were that the UDN/DRSC was perceived as the best option for a diagnosis, and the availability of telehealth would reduce the barriers of time, travel, and childcare.
I am fully aware that participation in the UDN would not guarantee a diagnosis for my daughter, but knowing that we did everything we could and that an answer could still be forthcoming would have given me some peace.
Parents in the Decliner group valued the NGS and felt that outcome of the sequencing could lead to an overall improved quality of life, improved medical management, a possible treatment, and some felt that any information that might be gained would be helpful.
“Our doctors have informed us that, barring anything shocking, my child’s course of treatment wouldn’t likely change much with a diagnosis. As a parent, the use of the word ‘likely” just isn’t good enough for me. Having answers about my child’s condition, along with possibly connecting with other families who have experienced the same thing, would feel like a very powerful, tangible outcome.”
Some parents reported worry regarding a life limiting disorder that could be uncovered, while others just wanted a diagnosis to reduce the uncertainty and negate the worry.
I’m not worried we just want answers so we can move on with getting her help or treatment at this point.
Thirty-six percent (19/52) of the parents in the Decliner group asked for assistance at the time they were contacted for the Barrier study, either for a follow up appointment in Medical Genetics (11/52) or for assistance with a UDN application (6/52) or both (2/52). The Duke clinical genetics team was informed and this assistance was provided by the genetic counselor. These requests were all from parents of children referred to the UDN.
Open-ended responses from Participants from the Barriers Survey.
Forty-eight percent (15 probands (14 parents and one adult proband) in the Participant group provided responses to the open-ended items. There were only two barriers identified: lack of social support and emotional exhaustion.
Some of my family severely discouraged me from putting my child in this program, I ultimately had to choose to ignore them which was a really hard decision.
Two of the three parents describing lack of support stated that they would not have been able to participate had it not been for the referring provider intervening.
My husband did not want to participate in UDN. I don’t know the solution to when parents do not agree about UDN. The only thing that helped was to make a genetic appointment and the geneticist explained to my husband that they wouldn’t do any tests that seem to hurt my son. And that there was potential that it could help in the future.
The responses focused mainly on facilitators: UDN being best option for a diagnosis, having good social support, costs related to participation covered and having the time needed to participate.
Discussion
Genomic research studies have had a persistent problem of disproportionately low participation by populations experiencing health disparities (Amendola et al., 2018; Fisher et al., 2020; Fraiman & Wojcik, 2021; Khoury et al., 2022; Splinter et al., 2018) with barriers to clinical research occurring at the healthcare systems level, the provider level, as well as at the patient level (Kilbourne et al., 2006). Since we found that despite offering the benefit of no-cost return of results NGS, 43% of referred patients/parents who had access through their prior clinical genetics, declined to participate, we focused on patient factors underlying non-participation. Currently, comprehensive data on the perspectives of patients/parents who decline NGS research are lacking. Our study is novel as it assesses patient perspectives who declined a clinical genomics study with a focus on return of results to patients with a clinical presentation suggestive of a genetic disorder for which clinical testing has been non-diagnostic, relative to participants, across multiple domains and factors.
We examined a number of key factors for our primary outcome of why a family would be a participant or a decliner of the two NGS research studies. Our preliminary analyses showed significant group differences, with the Decliners endorsing more barriers, having lower genomic knowledge and education level, and more often residing in a rural/MUA county. When examining specific predictors of group membership, we found that residing in a rural/MUA county and the total number of barriers were significant. While it is possible that the lower participation by rural residents is related to system based factors (e.g., less access to specialty services, clinics, and/or healthcare providers) it is unlikely to be solely due to healthcare system or provider factors, since all the parents/probands had obtained a referral to the NGS studies from their genetics providers, travel was supported for most, and there were no costs to the NGS. However, rural residence may be subsuming the effect of patient related factors such as genomic knowledge and education, with which it was highly correlated. Although it was not associated with significant variance in the regression analysis, the low genomic knowledge in the Decliner group is remarkable, since all of the responders had seen a genetics provider, and 86% had experience with a prior clinical NGS, receiving information on NGS as part of the consenting and genetic counseling. Thus, more genomic education may be required when introducing NGS research to patients/parents over and above the standard discussion of genomics that occurs in clinical practice. Parental education level was also not significantly associated with group membership, although the parents in the Participant group were more highly educated (71% had a college degree) than the parents in the Decliner group (24% with a college degree).
The CSER consortium has highlighted the importance of access to healthcare is a critical factor to participation in Genomic research (Gutierrez et al., 2021). The probands all had access to genetic services, with no difference between the total participant (n = 44) and decliner groups (n =70) regarding number of genetics clinic visits up to the point of referral to the UDN or DSRC. To further explore access, we also reviewed medical records to determine if probands in the Decliner group had been seen for interval follow-up since the referral to the Genomic research studies and found that 26% had been seen for routine care. These return visits had already occurred before being contacted for the Barriers study. It should be noted that if a proband is clinically stable and ES was non-diagnostic, the medical genetics follow-up recommendation at Duke is typically follow-up in approximately 1–2 years. Some of the probands in the Decliner group were waiting for their clinical follow up to occur. Access to care can be a barrier to being referred to genomic research studies. However, our study demonstrates that even when access to services is provided, practical barriers are operative that may reduce participation by populations experiencing healthcare disparities.
Our exploratory analyses of the individual barriers demonstrated that many co-occurred in the Decliner group--lack of time, difficulty with travel, childcare, and lack of social support, similar to the described literature (Amendola et al., 2018; Genetti et al., 2019; Gutierrez et al., 2021). While barriers of time, travel, and childcare can be addressed with telehealth, prior data report that telehealth itself can be a barrier, for some, due to difficulty with internet connectivity and computer access (Chang et al., 2021). It is important to note that the Participant group who did not endorse these as barriers at the same level as the Decliner group were also significantly less likely to endorse telehealth as a facilitator. These findings suggest that removing the barriers of time, travel, and childcare via telehealth, may increase participation, but in those who do not face those barriers, in-person evaluations may be acceptable and even preferred. A discussion about options for evaluations and sample collection should be part of the discussions with the proband and their families so that as much as possible, facilitators of participation can be tailored to the needs of the individual.
The parents in the Decliner group also expressed more research hesitancy (i.e., preferring to wait for clinical testing, concern about being treated like a guinea pig, and not understanding what could happen as part of the research), but they did not identify specific concerns with research identified by other studies, such as stigmatization or discrimination, health provider not understanding, or worry that something bad could happen as a result of the research study (Fisher et al., 2020; George et al., 2014). This may be due to the parents in the Decliner group having established close relationships with their referring genetics providers as well as frequent engagement with healthcare systems that provide care for their children’s complex medical problems.
Despite these major group differences, consistent with our hypothesis we found that the parents in the Decliner group were similar to parents who have participated in the UDN in several ways: in their desire for a diagnosis, its importance for the care and well-being of their children, and their perceived ability to emotionally manage whatever might be learned. Complementing these findings, parents in both groups reported on the facilitators survey that the UDN/DSRC were their best options to achieve a diagnosis and expressed positive perceptions of the genomic research and its outcome. The emotional exhaustion expressed as a significant barrier in the Decliner group may explain the apparent contradiction of declining NGS genomic research, while simultaneously desiring a diagnosis for their child. We and others have highlighted the emotional toll associated with searching for a diagnosis (McConkie-Rosell et al., 2018; Spillmann et al., 2017) that when compounded by the everyday caregiver toll required for a medically complex child (Caicedo, 2014; Cardinali et al., 2019) may have a negative effect on the decision to participate in genomic research, especially when co-occurring with other barriers. Remarkably, 36% of the parents in the Decliner group, at the time of contact for this study, asked for assistance with follow-up, either in the Medical Genetics Clinic or in applying to the genomic research study. These parents may have experienced emotional exhaustion or difficulty with navigation of the healthcare system. These findings and the two additional barriers on the open-ended items (i.e., other concerns taking precedent and not wanting to put the child through additional testing) suggest that some parents may need a pause in the diagnostic odyssey. We recommend that the referral to the genomic research not be offered as a one-time option, allowing for a pause in the process.
An unexpected finding in our study was that, although distrust is reported as a reason for non-participation in clinical research (Corbie-Smith et al., 2002; Durant et al., 2011), there were no group differences in societal and interpersonal distrust. Similar to what has been reported on trusting patient experiences with health care specialists (Keating et al., 2004), we surmise that because both groups had previously obtained medical care from the clinical genetics providers who referred them to the studies and since children with undiagnosed disorders have serious medical problems, they are likely to have had trusting relationships with these providers, possibly leading to less interpersonal distrust. Indeed, two parents in the Participant group commented that they would not have been able to participate in the genomic research had it not been for the clinical geneticist. The lack of societal distrust is more perplexing, but may be influenced by the recurrent interactions with societal structures such as health systems and payers for the care of their children.
Overall, our findings suggest that reasons for declining genomic sequencing are complex, and much work is needed to improve our understanding of how these complex and possibly interwoven factors may have an impact on how families make decisions about participating in diagnostic genomic research. McCubbin and Patterson’s Double ABCX model of adjustment and adaptation may offer one framework for beginning to understand these findings (McCubbin & Patterson, 1983). This model describes the interaction of factors associated with the family response and adaptation to a stressful event and predicts that families who have less resources at the start of a stressful event are more likely to experience more rapid depletion of resources. It has been extensively used and adapted to different family stress events, including well-being in parents of children with health concerns (Boettcher et al., 2021; Pozo et al., 2014). The components of this model certainly would apply to our families, experiencing the stress of a diagnostic odyssey and often medically complex children, and provide a theoretical consideration for many of our findings between the groups. The multiple co-occurring barriers, residing in a rural/MUA county, requiring travel to gain access to medical care, and the taxing the available family resources such as childcare and emotional exhaustion, support the concept that some families who decline participation in diagnostic genomic research may be experiencing pile-up with exhaustion of family resources, thus making participation in the genomic research difficult. Further studies may enable exploration of the applicability of this model to our understanding of why parents decide to participate in research or not.
Study Limitations
Although we had medium to large effect sizes for salient results, the sample size in our study was small (n=52) and thus the results should be interpreted with caution. Data collected for this study were retrospective and memories of why they made the decision to either participate or decline the genomic research may have been influenced by factors that occurred after the decision was made. We demonstrated that time-lapse from referral to contact for the study did not significantly influence the survey data, but it is possible that additional factors such as actually participating in the genomic research studies (Participant group) may have influenced survey and interview responses. Additionally, 29% of the participant probands were diagnosed through their participation in the UDN and this may also have influenced their responses, while all the Decliners remained undiagnosed. The number of parents in the Decliner group who completed the surveys was small and we were not able to audio-record responses to the Decliner brief interview, limiting the completeness of the responses, our ability to analyze the data, and assess reliability, although the genetic counselor took comprehensive notes and checked back with the respondent to ensure that notes reflected their response. We identified UDN participants and the Decliner groups through data mining of the electronic medical record by selection of records in which a specific phrase was inserted into a patient’s clinic note for referral to the UDN and this phrase may not have been used if parents declined a referral during the clinic visit and thus we may not have been able to capture data on all probands for whom these two research studies were discussed by the clinical genetics team. We found that the Decliners we were unable to reach about the Barriers study were more likely to be nonwhite or Hispanic. There may be additional underlying health disparities that we were not able to ascertain and this should be the focus of future investigation. An unexpected finding occurring during the interviews was the request for assistance in either applying to the NGS research or facilitating clinical follow up. This finding could suggest decisional instability but the study design and focus of this study did not allow us to asses this further. Importantly, parents in both groups including Decliners that we could not contact for this study, had access to clinical genetic services and thus barriers for those families not able to access this clinical service at all may be quite different and should be further explored. The majority of those enrolled were parents of children and thus we do not know if our findings are directly applicable to adult probands. We did not formally assess SDH, (U.S. Department of Health and Human Services, 2022), but many of the demographics, specific items on our barriers and facilitators survey and the sociocultural variables of genomic knowledge and distrust map onto the relevant SDH.
Conclusion
We assessed practical barriers and sociocultural factors that influence the decision to participate in genomic sequencing research in parents who declined genomic research offering NGS for their children, despite being provided access through a referral to the studies and support for travel, and being interested in obtaining a diagnosis. We found the most significant associations were rural residence and practical barriers, which are likely to reduce participation in clinical research, even if the outcome of that research is highly desired. Our findings suggest that if we are to improve participation in genomic research by patients and their families from rural and other populations experiencing healthcare disparities, barriers need to be addressed at the time of the referral and tailored to the family’s needs (e.g., offering telehealth, a further discussion of research). Further research is needed to build on these preliminary findings, to explore causal relationships, to develop and evaluate the effectiveness of interventions designed to increase the participation of populations experiencing healthcare disparity in genomic and more broadly clinical research, with the ultimate goal of improving access to state-of-the art care and improved outcomes for all.
Supplementary Material
What is known about this topic
Very little is known about the reasons why parents in search of a diagnosis for their child decline participation in genomic research providing return of results NGS.
What this paper adds to the topic
Our novel study provides insights into multiple factors that are operative to participation in genomic research in individuals who declined compared to those who do participate. We found that parents in the Decliner group more often live in rural and MUA areas and thus meet the definition of individuals who experience health disparities, have lower genomic knowledge and identify multiple barriers to NGS participation, than those in the Participant group. At the same time, we found that parents in the Decliner group valued a diagnosis and reported similar facilitators to genomic research as the Participants. These findings suggest that approaches to mitigating barriers to participation in genomic research need to be multi-pronged and should extend beyond access to services, since barriers, many of them practical, may prevent participation even if access is provided.
Acknowledgements:
We would like to thank the parents of the children evaluated by the Duke UDN clinical team and Duke Research Sequencing Clinic who participated in this study and those parents who declined participation in the genomic research and graciously gave their time to this study, and Nicole Daniels and Michelle Carreño for their assistance. Research reported in this manuscript was supported by the NIH Common Fund, through the Office of Strategic Coordination/Office of the NIH Director under Award Numbers U01HG007672 (Shashi V) and Duke Research Sequencing Clinic supported by Duke University Health System and School of Medicine. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Dr. Melanie Myers served as Action Editor on the manuscript review process and publication decision.
Members of the Undiagnosed Diseases Network (October 24, 2022)
Maria T. Acosta, Margaret Adam, David R. Adams, Justin Alvey, Laura Amendola, Ashley Andrews, Euan A. Ashley, Mahshid S. Azamian, Carlos A. Bacino, Guney Bademci, Ashok Balasubramanyam, Dustin Baldridge,,Jim Bale, Michael Bamshad, Deborah Barbouth, Pinar Bayrak-Toydemir, Anita Beck, Alan H. Beggs, Edward Behrens, Gill Bejerano, Hugo J. Bellen, Jimmy Bennet, Beverly Berg-Rood, Jonathan A. Bernstein, Gerard T. Berry, Anna Bican, Stephanie Bivona, Elizabeth Blue, John Bohnsack, Devon Bonner, Lorenzo Botto, Brenna Boyd, Lauren C. Briere, Elly Brokamp, Gabrielle Brown, Elizabeth A. Burke, Lindsay C. Burrage, Manish J. Butte, Peter Byers, William E. Byrd, John Carey, Olveen Carrasquillo, Thomas Cassini, Ta Chen, Peter Chang, Sirisak Chanprasert, Hsiao-Tuan Chao, Gary D. Clark, Terra R. Coakley, Laurel A. Cobban, Joy D. Cogan, Matthew Coggins,F. Sessions Cole, Heather A. Colley, Cynthia M. Cooper, William J. Craigen, Andrew B. Crouse, Michael Cunningham, Precilla D’Souza, Hongzheng Dai, Surendra Dasari, Joie Davis,Jyoti G. Dayal, Matthew Deardorff, Esteban C. Dell’Angelica, Katrina Dipple, Daniel Doherty, Naghmeh Dorrani, Argenia L. Doss, Emilie D. Douine, Laura Duncan, Dawn Earl, David J. Eckstein, Lisa T. Emrick, Christine M. Eng, Marni Falk, Liliana Fernandez, Elizabeth L. Fieg, Paul G. Fisher, Brent L. Fogel, Irman Forghani, William A. Gahl, Ian Glass, Bernadette Gochuico, Rena A. Godfrey, Katie Golden-Grant, Madison P. Goldrich, Alana Grajewski, Irma Gutierrez, Don Hadley, Sihoun Hahn, Rizwan Hamid, Kelly Hassey, Nichole Hayes, Frances High, Anne Hing, Fuki M. Hisama, Ingrid A. Holm, Jason Hom, Martha Horike-Pyne, Alden Huang, Yong Huang, Wendy Introne, Rosario Isasi, Kosuke Izumi, Fariha Jamal, Gail P. Jarvik, Jeffrey Jarvik, Suman Jayadev, Orpa Jean-Marie, Vaidehi Jobanputra, Lefkothea Karaviti, Jennifer Kennedy, Shamika Ketkar, Dana Kiley, Gonench Kilich, Shilpa N. Kobren, Isaac S. Kohane, Jennefer N. Kohler, Deborah Krakow, Donna M. Krasnewich, Elijah Kravets, Susan Korrick, Mary Koziura, Seema R. Lalani, Byron Lam, Christina Lam, Grace L. LaMoure, Brendan C. Lanpher, Ian R. Lanza, Kimberly LeBlanc, Brendan H. Lee, Roy Levitt, Richard A. Lewis, Pengfei Liu, Xue Zhong Liu, Nicola Longo, Sandra K. Loo, Joseph Loscalzo Richard L. Maas, Ellen F. Macnamara, Calum A. MacRae, Valerie V. Maduro, Rachel Mahoney, May Christine V. Malicdan, Laura A. Mamounas, Teri A. Manolio, Rong Mao, Kenneth Maravilla, Ronit Marom, Gabor Marth, Beth A. Martin, Martin G. Martin, Julian A. Martínez-Agosto, Shruti Marwaha, Jacob McCauley, Allyn McConkie-Rosell, Alexa T. McCray, Elisabeth McGee, Heather Mefford, J. Lawrence Merritt, Matthew Might, Ghayda Mirzaa, Eva Morava, Paolo M. Moretti, Mariko Nakano-Okuno, Stan F. Nelson, John H. Newman, Sarah K. Nicholas, Deborah Nickerson, Shirley Nieves-Rodriguez, Donna Novacic, Devin Oglesbee, James P. Orengo, Laura Pace, Stephen Pak, J. Carl Pallais, Christina GS. Palmer, Jeanette C. Papp, Neil H. Parker, John A. Phillips III, Jennifer E. Posey, Lorraine Potocki, Barbara N. Pusey, Aaron Quinlan, Wendy Raskind, Archana N. Raja, Deepak A.Rao, Anna Raper, Genecee Renteria, Chloe M. Reuter, Lynette Rives, Amy K. Robertson, Lance H. Rodan, Jill A. Rosenfeld, Natalie Rosenwasser, Francis Rossignol, Maura Ruzhnikov, Ralph Sacco, Jacinda B. Sampson, Mario Saporta, Judy Schaechter, Timothy Schedl, Kelly Schoch, C. Ron Scott, Daryl A. Scott, Vandana Shashi, Jimann Shin, Edwin K. Silverman, Janet S. Sinsheimer, Kathy Sisco, Edward C. Smith, Kevin S. Smith, Emily Solem, Lilianna Solnica-Krezel, Ben Solomon, Rebecca C. Spillmann, Joan M. Stoler, Jennifer A. Sullivan, Kathleen Sullivan, Angela Sun, Shirley Sutton, David A. Sweetser, Virginia Sybert, Holly K. Tabor, Amelia L. M. Tan, Queenie K.-G.Tan, Mustafa Tekin, Fred Telischi, Willa Thorson, Cynthia J. Tifft, Camilo Toro, Alyssa A. Tran, Brianna M. Tucker, Tiina K. Urv, Adeline Vanderver, Matt Velinder, Dave Viskochil, Tiphanie P. Vogel, Colleen E. Wahl, Melissa Walker, Stephanie Wallace, Nicole M. Walley, Jennifer Wambach, Jijun Wan, Lee-kai Wang, Michael F. Wangler, Patricia A. Ward, Daniel Wegner, Monika Weisz-Hubshman, Mark Wener, Tara Wenger, Katherine Wesseling Perry, Monte Westerfield, Matthew T. Wheeler, Jordan Whitlock, Lynne A. Wolfe, Kim Worley, Changrui Xiao, Shinya Yamamoto, John Yang, Diane B. Zastrow, Zhe Zhang, Chunli Zhao, Stephan Zuchne
Footnotes
Conflict of interest
AMR, RS, KS, JS, NW, MM, SH, VS declare that they have no conflict of interest.
Human Studies and Informed Consent
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The data gathered for this study was approved by the Institutional Review Boards of the National Human Genome Research Institute (15-HG0130), Duke University Medical Center (Pro00056651), and Duke University Medical Center (Pro00105966). Data was collected with written informed consent and/or under a waiver of consent as approved by the NIH and Duke IRBs.
Data Availability Statement
No restrictions. The datasets generated and/or analyzed during the current study are not publicly available due to patient confidentiality but can be redacted and made available from the corresponding author upon reasonable request.
References:
- Advani AS, Atkeson B, Brown CL, Peterson BL, Fish L, Johnson JL, Gockerman JP, & Gautier M (2003, Mar 15). Barriers to the participation of African-American patients with cancer in clinical trials: a pilot study. Cancer, 97(6), 1499–1506. 10.1002/cncr.11213 [DOI] [PubMed] [Google Scholar]
- Amendola LM, Robinson JO, Hart R, Biswas S, Lee K, Bernhardt BA, East K, Gilmore MJ, Kauffman TL, Lewis KL, Roche M, Scollon S, Wynn J, & Blout C (2018, Sep). Why Patients Decline Genomic Sequencing Studies: Experiences from the CSER Consortium. J Genet Couns, 27(5), 1220–1227. 10.1007/s10897-018-0243-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boettcher J, Zapf H, Fuerboeter M, Nazarian R, Reinshagen K, Wiegand-Grefe S, & Boettcher M (2021, Sep 9). Perceived mental health in parents of children with rare congenital surgical diseases: a double ABCX model considering gender. Orphanet J Rare Dis, 16(1), 384. 10.1186/s13023-021-01998-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burchard EG, Oh SS, Foreman MG, & Celedón JC (2015, Mar 1). Moving toward true inclusion of racial/ethnic minorities in federally funded studies. A key step for achieving respiratory health equality in the United States. Am J Respir Crit Care Med, 191(5), 514–521. 10.1164/rccm.201410-1944PP [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caicedo C (2014, Nov-Dec). Families with special needs children: family health, functioning, and care burden. J Am Psychiatr Nurses Assoc, 20(6), 398–407. 10.1177/1078390314561326 [DOI] [PubMed] [Google Scholar]
- Canedo JR, Miller ST, Myers HF, & Sanderson M (2019, Jun). Racial and ethnic differences in knowledge and attitudes about genetic testing in the US: Systematic review. J Genet Couns, 28(3), 587–601. 10.1002/jgc4.1078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinali P, Migliorini L, & Rania N (2019). The Caregiving Experiences of Fathers and Mothers of Children With Rare Diseases in Italy: Challenges and Social Support Perceptions. Front Psychol, 10, 1780. 10.3389/fpsyg.2019.01780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Cohen P, & Chen S (2010, March/31). How Big is a Big Odds Ratio? Interpreting the Magnitudes of Odds Ratios in Epidemiological Studies. Communications in Statistics - Simulation and Computation, 39, 860–864. 10.1080/03610911003650383 [DOI] [Google Scholar]
- Chou AF, Duncan AR, Hallford G, Kelley DM, & Dean LW (2021, Jul). Barriers and strategies to integrate medical genetics and primary care in underserved populations: a scoping review. J Community Genet, 12(3), 291–309. 10.1007/s12687-021-00508-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbie-Smith G, Thomas SB, & St George DM (2002, Nov 25). Distrust, race, and research. Arch Intern Med, 162(21), 2458–2463. 10.1001/archinte.162.21.2458 [DOI] [PubMed] [Google Scholar]
- Davis TC, Arnold CL, Mills G, & Miele L (2019). A Qualitative Study Exploring Barriers and Facilitators of Enrolling Underrepresented Populations in Clinical Trials and Biobanking. Front Cell Dev Biol, 7, 74. 10.3389/fcell.2019.00074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durant RW, Legedza AT, Marcantonio ER, Freeman MB, & Landon BE (2011, Feb). Different types of distrust in clinical research among whites and African Americans. J Natl Med Assoc, 103(2), 123–130. 10.1016/s0027-9684(15)30261-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher ER, Pratt R, Esch R, Kocher M, Wilson K, Lee W, & Zierhut HA (2020, Feb). The role of race and ethnicity in views toward and participation in genetic studies and precision medicine research in the United States: A systematic review of qualitative and quantitative studies. Mol Genet Genomic Med, 8(2), e1099. 10.1002/mgg3.1099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraiman YS, & Wojcik MH (2021, Jan). The influence of social determinants of health on the genetic diagnostic odyssey: who remains undiagnosed, why, and to what effect? Pediatr Res, 89(2), 295–300. 10.1038/s41390-020-01151-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gene Hallford H, Coffman MA, Obregon-Tito AJ, Morales AH, & Williamson Dean L (2020, Jun). Access barriers to genetic services for Spanish-speaking families in states with rapidly growing migrant populations. J Genet Couns, 29(3), 365–380. 10.1002/jgc4.1195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genetti CA, Schwartz TS, Robinson JO, VanNoy GE, Petersen D, Pereira S, Fayer S, Peoples HA, Agrawal PB, Betting WN, Holm IA, McGuire AL, Waisbren SE, Yu TW, Green RC, Beggs AH, & Parad RB (2019, Mar). Parental interest in genomic sequencing of newborns: enrollment experience from the BabySeq Project. Genet Med, 21(3), 622–630. 10.1038/s41436-018-0105-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- George S, Duran N, & Norris K (2014, Feb). A systematic review of barriers and facilitators to minority research participation among African Americans, Latinos, Asian Americans, and Pacific Islanders. Am J Public Health, 104(2), e16–31. 10.2105/ajph.2013.301706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Trillos S, Sheppard VB, Graves KD, Song M, Anderson L, Ostrove N, Lopez K, Campos C, Gonzalez N, & Hurtado-de-Mendoza A (2020, Aug). Latinas’ knowledge of and experiences with genetic cancer risk assessment: Barriers and facilitators. J Genet Couns, 29(4), 505–517. 10.1002/jgc4.1201 [DOI] [PubMed] [Google Scholar]
- Gutierrez AM, Robinson JO, Outram SM, Smith HS, Kraft SA, Donohue KE, Biesecker BB, Brothers KB, Chen F, Hailu B, Hindorff LA, Hoban H, Hsu RL, Knight SJ, Koenig BA, Lewis KL, Lich KH, O’Daniel JM, Okuyama S, Tomlinson GE, Waltz M, Wilfond BS, Ackerman SL, & Majumder MA (2021). Examining access to care in clinical genomic research and medicine: Experiences from the CSER Consortium. J Clin Transl Sci, 5(1), e193. 10.1017/cts.2021.855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamel LM, Penner LA, Albrecht TL, Heath E, Gwede CK, & Eggly S (2016, Oct). Barriers to Clinical Trial Enrollment in Racial and Ethnic Minority Patients With Cancer. Cancer Control, 23(4), 327–337. 10.1177/107327481602300404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Health Resources & Services Administration. (2022). Medically Underserved Area and Medically Underserved Population designations. data.HRSA.gov | Health Resources & Services Administration. https://data.hrsa.gov/tools/shortage-area/mua-find [Google Scholar]
- Hooker GW, Peay H, Erby L, Bayless T, Biesecker BB, & Roter DL (2014, May). Genetic literacy and patient perceptions of IBD testing utility and disease control: a randomized vignette study of genetic testing. Inflamm Bowel Dis, 20(5), 901–908. 10.1097/mib.0000000000000021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes TB, Varma VR, Pettigrew C, & Albert MS (2017, Apr 1). African Americans and Clinical Research: Evidence Concerning Barriers and Facilitators to Participation and Recruitment Recommendations. Gerontologist, 57(2), 348–358. 10.1093/geront/gnv118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurle B, Citrin T, Jenkins JF, Kaphingst KA, Lamb N, Roseman JE, & Bonham VL (2013, Aug). What does it mean to be genomically literate?: National Human Genome Research Institute Meeting Report. Genet Med, 15(8), 658–663. 10.1038/gim.2013.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson VA, Edwards KA, Sherman SL, Stephens LD, Williams W, Adair A, & Deer-Smith MH (2009, Fall). Decisions to participate in fragile X and other genomics-related research: Native American and African American voices. J Cult Divers, 16(3), 127–135. [PubMed] [Google Scholar]
- Keating NL, Gandhi TK, Orav EJ, Bates DW, & Ayanian JZ (2004, May 10). Patient characteristics and experiences associated with trust in specialist physicians. Arch Intern Med, 164(9), 1015–1020. 10.1001/archinte.164.9.1015 [DOI] [PubMed] [Google Scholar]
- Khoury MJ, Bowen S, Dotson WD, Drzymalla E, Green RF, Goldstein R, Kolor K, Liburd LC, Sperling LS, & Bunnell R (2022, Aug). Health equity in the implementation of genomics and precision medicine: A public health imperative. Genet Med, 24(8), 1630–1639. 10.1016/j.gim.2022.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilbourne AM, Switzer G, Hyman K, Crowley-Matoka M, & Fine MJ (2006, Dec). Advancing health disparities research within the health care system: a conceptual framework. Am J Public Health, 96(12), 2113–2121. 10.2105/ajph.2005.077628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakow M, Ratcliff CL, Hesse BW, & Greenberg-Worisek AJ (2017). Assessing Genetic Literacy Awareness and Knowledge Gaps in the US Population: Results from the Health Information National Trends Survey. Public Health Genomics, 20(6), 343–348. 10.1159/000489117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger N (2001, Oct). A glossary for social epidemiology. J Epidemiol Community Health, 55(10), 693–700. 10.1136/jech.55.10.693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang R, Kelkar VA, Byrd JR, Edwards CL, Pericak-Vance M, & Byrd GS (2013, Mar-Apr). African American participation in health-related research studies: indicators for effective recruitment. J Public Health Manag Pract, 19(2), 110–118. 10.1097/PHH.0b013e31825717ef [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer MM, Roche MI, Brewer NT, Berg JS, Khan CM, Leos C, Moore E, Brown M, & Rini C (2017, Jan-Jun). Development and Validation of a Genomic Knowledge Scale to Advance Informed Decision Making Research in Genomic Sequencing. MDM Policy Pract, 2(1), 1–13. 10.1177/2381468317692582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manolio TA, Rowley R, Williams MS, Roden D, Ginsburg GS, Bult C, Chisholm RL, Deverka PA, McLeod HL, Mensah GA, Relling MV, Rodriguez LL, Tamburro C, & Green ED (2019, Aug 10). Opportunities, resources, and techniques for implementing genomics in clinical care. Lancet, 394(10197), 511–520. 10.1016/s0140-6736(19)31140-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-García M, Villegas Camacho JM, & Hernández-Lemus E (2022). Connections and Biases in Health Equity and Culture Research: A Semantic Network Analysis. Front Public Health, 10, 834172. 10.3389/fpubh.2022.834172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConkie-Rosell A, Hooper SR, Pena LDM, Schoch K, Spillmann RC, Jiang YH, Cope H, Palmer C, & Shashi V (2018, Jan 2). Psychosocial Profiles of Parents of Children with Undiagnosed Diseases: Managing Well or Just Managing? J Genet Couns. 10.1007/s10897-017-0193-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConkie-Rosell A, Pena LD, Schoch K, Spillmann R, Sullivan J, Hooper SR, Jiang YH, Mathey-Andrews N, Goldstein DB, & Shashi V (2016, Oct). Not the End of the Odyssey: Parental Perceptions of Whole Exome Sequencing (WES) in Pediatric Undiagnosed Disorders. J Genet Couns, 25(5), 1019–1031. 10.1007/s10897-016-9933-1 [DOI] [PubMed] [Google Scholar]
- McConkie-Rosell A, Schoch K, Sullivan J, Cope H, Spillmann R, Palmer CGS, Pena L, Jiang YH, Daniels N, Walley N, Tan KG, Hooper SR, & Shashi V (2019, Dec). The genome empowerment scale: An assessment of parental empowerment in families with undiagnosed disease. Clin Genet, 96(6), 521–531. 10.1111/cge.13635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConkie-Rosell A, Schoch K, Sullivan J, Spillmann RC, Cope H, Tan QK, Palmer CGS, Hooper SR, & Shashi V (2022, Feb). Clinical application of a scale to assess genomic healthcare empowerment (GEmS): Process and illustrative case examples. J Genet Couns, 31(1), 59–70. 10.1002/jgc4.1451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCubbin HI, & Patterson JM (1983, 1983/04/27). The Family Stress Process. Marriage & Family Review, 6(1–2), 7–37. 10.1300/J002v06n01_02 [DOI] [Google Scholar]
- Moore EG, Roche M, Rini C, Corty EW, Girnary Z, O’Daniel JM, Lin FC, Corbie-Smith G, Evans JP, Henderson GE, & Berg JS (2017). Examining the Cascade of Participant Attrition in a Genomic Medicine Research Study: Barriers and Facilitators to Achieving Diversity. Public Health Genomics, 20(6), 332–342. 10.1159/000490519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguengang Wakap S, Lambert DM, Olry A, Rodwell C, Gueydan C, Lanneau V, Murphy D, Le Cam Y, & Rath A (2020, Feb). Estimating cumulative point prevalence of rare diseases: analysis of the Orphanet database. Eur J Hum Genet, 28(2), 165–173. 10.1038/s41431-019-0508-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh SS, Galanter J, Thakur N, Pino-Yanes M, Barcelo NE, White MJ, de Bruin DM, Greenblatt RM, Bibbins-Domingo K, Wu AH, Borrell LN, Gunter C, Powe NR, & Burchard EG (2015, Dec). Diversity in Clinical and Biomedical Research: A Promise Yet to Be Fulfilled. PLoS Med, 12(12), e1001918. 10.1371/journal.pmed.1001918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penon-Portmann M, Chang J, Cheng M, & Shieh JT (2020, Jan). Genetics workforce: distribution of genetics services and challenges to health care in California. Genet Med, 22(1), 227–231. 10.1038/s41436-019-0628-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter WJ, & Levine-Donnerstein D (1999). Rethinking validity and reliability in content analysis. Journal of Applied Communication Research, 27, 258–284. [Google Scholar]
- Pozo P, Sarriá E, & Brioso A (2014, May). Family quality of life and psychological well-being in parents of children with autism spectrum disorders: a double ABCX model. J Intellect Disabil Res, 58(5), 442–458. 10.1111/jir.12042 [DOI] [PubMed] [Google Scholar]
- Rini C, Henderson GE, Evans JP, Berg JS, Foreman AKM, Griesemer I, Waltz M, O’Daniel JM, & Roche MI (2020, Jan). Genomic knowledge in the context of diagnostic exome sequencing: changes over time, persistent subgroup differences, and associations with psychological sequencing outcomes. Genet Med, 22(1), 60–68. 10.1038/s41436-019-0600-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson JO, Carroll TM, Feuerman LZ, Perry DL, Hoffman-Andrews L, Walsh RC, Christensen KD, Green RC, & McGuire AL (2016, Feb). Participants and Study Decliners’ Perspectives About the Risks of Participating in a Clinical Trial of Whole Genome Sequencing. J Empir Res Hum Res Ethics, 11(1), 21–30. 10.1177/1556264615624078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoch K, Esteves C, Bican A, Spillmann R, Cope H, McConkie-Rosell A, Walley N, Fernandez L, Kohler JN, Bonner D, Reuter C, Stong N, Mulvihill JJ, Novacic D, Wolfe L, Abdelbaki A, Toro C, Tifft C, Malicdan M, Gahl W, Liu P, Newman J, Goldstein DB, Hom J, Sampson J, Wheeler MT, Cogan J, Bernstein JA, Adams DR, McCray AT, & Shashi V (2021, Feb). Clinical sites of the Undiagnosed Diseases Network: unique contributions to genomic medicine and science. Genet Med, 23(2), 259–271. 10.1038/s41436-020-00984-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shashi V, McConkie-Rosell A, Rosell B, Schoch K, Vellore K, McDonald M, Jiang Y-H, Xie P, Need A, & Goldstein DG (2013). The utility of the traditional medical genetics diagnostic evaluation in the context of next-generation sequencing for undiagnosed genetic disorders [Original Research Article]. Genet Med. 10.1038/gim.2013.99 [DOI] [PubMed] [Google Scholar]
- Shickh S, Mighton C, Uleryk E, Pechlivanoglou P, & Bombard Y (2021, Oct). The clinical utility of exome and genome sequencing across clinical indications: a systematic review. Hum Genet, 140(10), 1403–1416. 10.1007/s00439-021-02331-x [DOI] [PubMed] [Google Scholar]
- Skinner HG, Calancie L, Vu MB, Garcia B, DeMarco M, Patterson C, Ammerman A, & Schisler JC (2015). Using community-based participatory research principles to develop more understandable recruitment and informed consent documents in genomic research. PLoS One, 10(5), e0125466. 10.1371/journal.pone.0125466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spillmann RC, McConkie-Rosell A, Pena L, Jiang YH, Schoch K, Walley N, Sanders C, Sullivan J, Hooper SR, & Shashi V (2017, Apr 17). A window into living with an undiagnosed disease: illness narratives from the Undiagnosed Diseases Network. Orphanet J Rare Dis, 12(1), 71. 10.1186/s13023-017-0623-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Splinter K, Adams DR, Bacino CA, Bellen HJ, Bernstein JA, Cheatle-Jarvela AM, Eng CM, Esteves C, Gahl WA, Hamid R, Jacob HJ, Kikani B, Koeller DM, Kohane IS, Lee BH, Loscalzo J, Luo X, McCray AT, Metz TO, Mulvihill JJ, Nelson SF, Palmer CGS, Phillips JA 3rd, Pick L, Postlethwait JH, Reuter C, Shashi V, Sweetser DA, Tifft CJ, Walley NM, Wangler MF, Westerfield M, Wheeler MT, Wise AL, Worthey EA, Yamamoto S, & Ashley EA (2018, Nov 29). Effect of Genetic Diagnosis on Patients with Previously Undiagnosed Disease. N Engl J Med, 379(22), 2131–2139. 10.1056/NEJMoa1714458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services. (2022). Healthy People 2030. U.S. Department of Health and Human Services, Office of Disease Prevention and Health Promotion. Retrieved October 22, 2022 from https://health.gov/healthypeople/priority-areas/social-determinants-health [Google Scholar]
- USDA ERS. (2020). Rural-Urban Continuum Codes 2020. Department of Agriculture; Retrieved October 26 2022 from https://www.ers.usda.gov/data-products/rural-urban-continuum-codes.aspx [Google Scholar]
- White K, Lawrence JA, Tchangalova N, Huang SJ, & Cummings JL (2020, Feb 10). Socially-assigned race and health: a scoping review with global implications for population health equity. Int J Equity Health, 19(1), 25. 10.1186/s12939-020-1137-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JL, Halley MC, Anguiano B, Fernandez L, Bernstein JA, Wheeler MT, & Tabor HK (2022). Beyond race: Recruitment of diverse participants in clinical genomics research for rare disease. Front Genet, 13, 949422. 10.3389/fgene.2022.949422 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No restrictions. The datasets generated and/or analyzed during the current study are not publicly available due to patient confidentiality but can be redacted and made available from the corresponding author upon reasonable request.


