Abstract
Articular cartilage (AC) injuries often lead to cartilage degeneration and may ultimately result in osteoarthritis (OA) due to the limited self-repair ability. To date, numerous intra-articular delivery systems carrying various therapeutic agents have been developed to improve therapeutic localization and retention, optimize controlled drug release profiles and target different pathological processes. Due to the complex and multifactorial characteristics of cartilage injury pathology and heterogeneity of the cartilage structure deposited within a dense matrix, delivery systems loaded with a single therapeutic agent are hindered from reaching multiple targets in a spatiotemporal matched manner and thus fail to mimic the natural processes of biosynthesis, compromising the goal of full cartilage regeneration. Emerging evidence highlights the importance of sequential delivery strategies targeting multiple pathological processes. In this review, we first summarize the current status and progress achieved in single-drug delivery strategies for the treatment of AC diseases. Subsequently, we focus mainly on advances in multiple drug delivery applications, including sequential release formulations targeting various pathological processes, synergistic targeting of the same pathological process, the spatial distribution in multiple tissues, and heterogeneous regeneration. We hope that this review will inspire the rational design of intra-articular drug delivery systems (DDSs) in the future.
Key words: Drug delivery systems, Articular cartilage, Cartilage injury, Osteoarthritis, Cartilage regeneration, Multiple drug delivery strategy, Biomaterials, Therapeutic factors
Graphical abstract
Due to the complex and multifactorial characteristics of cartilage injury pathology and heterogeneity of the cartilage structure, drug delivery to articular cartilage is advancing from single to multiple strategy.
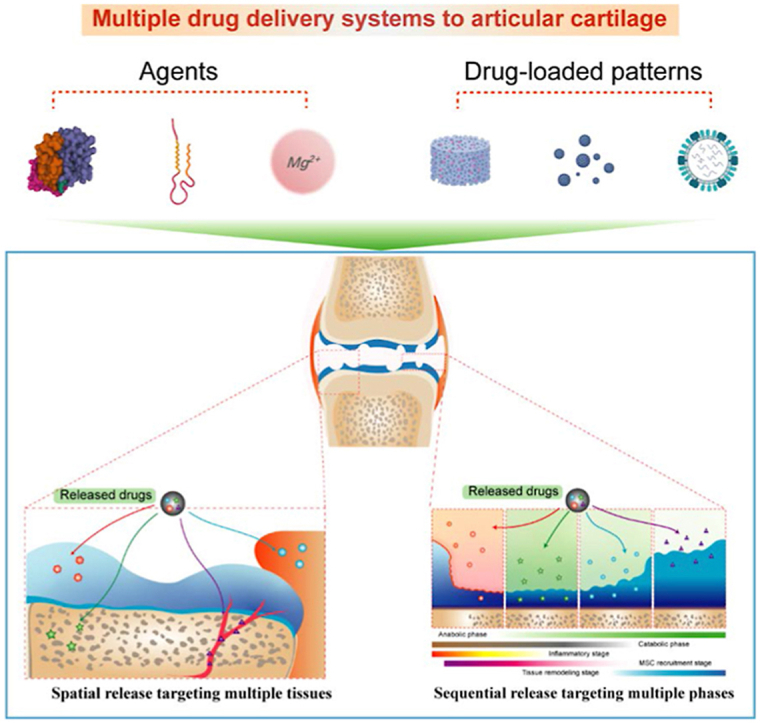
1. Introduction
Articular cartilage (AC) injuries are mainly caused by trauma and degeneration and are frequently presented in the clinic1. Due to the lack of vasculature, nerves, and lymph, cartilage exhibits relatively few self-healing properties after injury, and osteoarthritis (OA) eventually develops. Intra-articular drug delivery is a key factor in determining therapeutic outcomes by affecting cell fate and modulating the injury microenvironment. Some drugs delivered intra-articularly, such as corticosteroids, hyaluronans, glucosamine, and chondroitin, have been used in the clinic; however, the long-term clinical outcomes remain controversial2, 3, 4, 5, 6. Drug delivery is fraught with challenges, including a limited half-life, short retention time, and uncontrolled release7. To date, numerous intra-articular drug delivery systems (DDSs) in an injectable form or combined with tissue engineering have been explored to enhance delivery efficiency. The optimal strategy enables drug delivery in an appropriate spatiotemporal manner, leading to better healing effects along with fewer and less severe side effects.
Additionally, since the heterogeneity of the cartilage structure and complex physiological processes are involved in cartilage regeneration, strategies based on a single DDS have not yet been sufficiently robust to enable full cartilage regeneration. For instance, transforming growth factor β3 is considered as a classic growth factor (GF) that induces chondrogenesis. However, delivery of TGF-β3 alone is typically accompanied by chondrocyte hypertrophy and calcification8. Recently, the increasing incidence has suggested that an appropriate combination of multiple drugs may be a promising approach for more-effective cartilage regeneration9, 10, 11. Using a proper DDS to guide the release of each drug, these combinations may promote the treatment of various pathological processes, synergistically modulating the same pathological processes, targeting different tissues, and inducing heterogeneous tissue regeneration in cartilage.
This review first introduces the demand for and design of effective DDSs to increase intra-articular drug concentrations and enhance their therapeutic efficacy. In addition, since a single-factor DDS displays inadequate cartilage regenerative ability or fails to follow an effective spatiotemporal regimen, we focus mainly on emerging multiple-factor delivery strategies targeting pathological processes. Several types of delivery systems for multiple drugs are reviewed. Three multiple drug combination paradigms for multiple drug delivery are thus discussed, and currently used multiple delivery strategies are classified and summarized. Furthermore, several reasonable multiple drug combination strategies are proposed to inspire future drug design.
2. The pathophysiology of cartilage suggests the need for effective drug delivery
2.1. The structure and function of cartilage
According to the composition of its matrix, cartilage is classified into three main types: hyaline cartilage, fibrocartilage, and elastic cartilage. AC is mainly composed of hyaline cartilage localized on the surface of joints, where it acts as a cushion to decrease friction and transmit mechanical stress into subchondral bone (SB)12,13. The main components of AC are chondrocytes and the extracellular matrix (ECM). Chondrocytes, which comprise a mature and highly specialized cell type, are critical for ECM synthesis, organization, and maintenance. With a very slow turnover rate, chondrocytes account for only approximately 1%–2% of AC by volume14. The ECM, which is composed of water (68%–85% of total wet weight), collagen (60–86% of total dry weight), proteoglycans (15%–40% of total dry weight), and other glycoproteins, maintains the overall shape of the tissue, provides compression resistance, transports nutrients to chondrocytes and lubricates the articular surface15,16. The cartilage composition is mainly regulated by chondrocytes in response to extracellular chemical and mechanical changes17.
2.2. The pathophysiology of cartilage
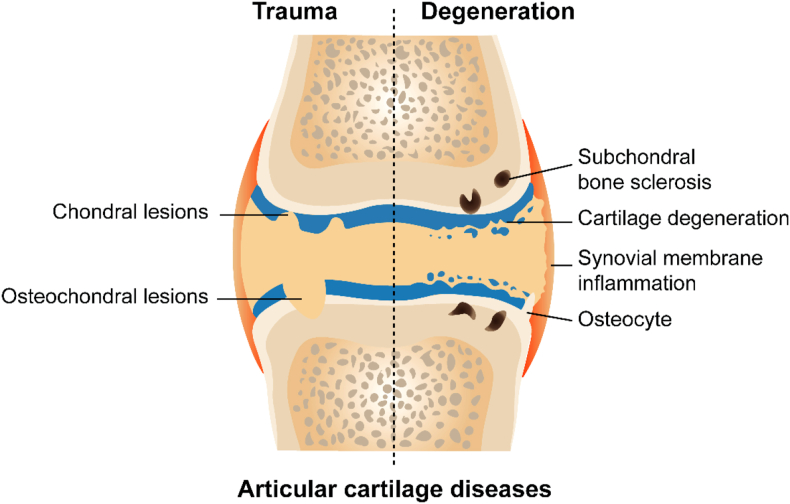
Trauma and degenerative changes are the most common causes of chondral or osteochondral lesions observed in the clinic (Fig. 1)18. After a traumatic injury, cartilage sustains a certain degree of immediate or irreversible damage, such as cell necrosis, collagen rupture, and glycosaminoglycan loss. Subsequently, acute reactions, including joint pain and swelling, inflammatory cell infiltration, inflammatory mediator secretion, ECM degradation, and apoptosis of chondrocytes adjacent to the injury, are evident within a few days19. AC degeneration is initiated with the proteolysis of aggrecan and digestion of type II collagen20, 21, 22. Chondrocytes are initially activated to proliferate, and collagen and proteoglycan production are increased to compensate23, 24, 25, 26, 27. As degradation progresses, the compensatory effect disappears, and catabolic activity is increased with a subsequent reduction in proteoglycan and type II collagen production28. The integrity of the cartilage surface is subsequently disrupted, increasing the susceptibility of the cartilage to physical forces, which may cause SB remodeling, osteophyte formation, synovial inflammation, and finally OA18,29,30.
Figure 1.
Pathological characters of cartilage injury caused by trauma and degeneration.
2.3. Inadequate clinical drug delivery efficacy
Due to the eventual onset of OA, clinicians and scientists have sought to deliver therapeutic factors, consisting of small-molecule drugs, ions, and bioactive compounds, to induce a response in host tissues or cells and thus relieve symptoms and slow disease progression31, 32, 33. Oral delivery of nonsteroidal anti-inflammatory drugs (NSAIDs) remains a common method of cartilage disease treatment because NSAIDs inhibit cyclooxygenase enzymes to reduce the synthesis of inflammation-related biological mediators (e.g., prostaglandin E2)5,6,34,35. However, some deleterious outcomes have hindered the clinical applications of these treatments. First, NSAIDs used to address cartilage injury are intended for palliative OA treatment and provide only some measure of pain and symptom relief. Second, relatively higher drug concentrations of NSAIDs in systemic tissue and relatively low drug concentrations in local tissue lead to inadequate clinical therapeutic efficacy. Moreover, the higher doses required to provide pain relief may cause significant adverse events such as renal, gastrointestinal, and cardiovascular complications36,37. Therefore, intra-articular drug delivery, including the delivery of corticosteroids, hyaluronic acid, platelet-rich plasma, and chondroitin, is another strategy to relieve symptoms because it increases local bioavailability throughout the joint and reduces the number and/or severity of adverse events5,6,34,35,38,39. However, the high viscosity, complex biological composition, and clearance ability of synovial fluid weaken drug properties, inhibit drug diffusion, and/or cause the drug concentrations within the joint to decrease rapidly. The dense networks and negatively charged molecules in cartilage ECM also block drug absorption, which leads to a short drug half-life (0.1–6 h) and retention time (6–25 days)40,41. Therefore, repeated injections to maintain therapeutic levels are necessary but cause other problems, such as joint disability, increased risk of infection, and high costs. Focusing on available disease-modifying therapy for OA, approximately 40 clinical trials are ongoing. Unfortunately, previous clinical trials have experienced a high failure rate, and to date, no disease-modifying treatment for osteoarthritis has been approved for clinical use42. Therefore, the management of cartilage injuries and OA would be substantially improved with the localized delivery and retention of these factors.
2.4. The requirements for advanced DDSs
The progression of cartilage injury may be affected by drugs that are released in a precise spatiotemporal manner. In the short term, certain treatments, including pain relief, anti-inflammatory agents, immunomodulatory agents, chondrocyte- and matrix-protective therapeutics, may limit the degree of cartilage injury and delay OA onset43,44. Additionally, in the long term, interventions such as cell supplementation, the induction of chondrogenic differentiation, and maintenance of the chondrocyte phenotype are key factors contributing to successful cartilage regeneration45. In addition to strategies targeting cartilage, treatments targeting the synovium, SB, fat pad, and meniscus effectively relieve cartilage damage46, 47, 48. This outcome indicates that advanced DDSs are required to effectively achieve the goals of these treatments. The development of an advanced DDS requires that the following criteria are met: 1. Effective agents directed to the condition are chosen. 2. The regimen extends the joint residence time and reduces off-target effects, both of which might be improved by drug modifications that make them more difficult to eliminate and more capable of targeting specific cells or damaged tissues. 3. A proper drug delivery carrier retains agent activity, protects agents from clearance, controls agent release, and delivers agents to specific cells or damaged tissues49.
3. Barriers to drug delivery in joints
A joint consists of multiple tissues, including bone, cartilage, synovium, ligament, and meniscus tissues, and each of these tissues is involved in one or more biological pathways that contribute to OA progression20. Native anatomical components in a joint provide protection, stability, and mobility but also erect multiple barriers that impede drugs from reaching their targets. The next section provides a detailed description of these barriers and state-of-the-art approaches to overcome them7.
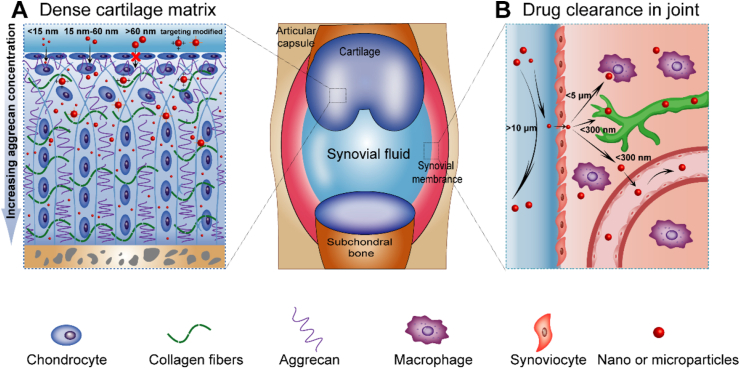
3.1. Dense cartilage matrix
Cartilage is the main target of drug delivery in joints. Agents must penetrate cartilage to a certain depth to reach chondrocytes and ECM targets in injured cartilage50. However, the dense cartilage matrix poses a substantial barrier to DDS penetration (Fig. 2A)51. Histologically, the collagen fibril network in the cartilage matrix has an approximate pore size of 60–200 nm. The sulfated glycosaminoglycan (GAG) chains, which are covalently linked to the aggrecan monomers, are spaced only 2–4 nm apart in the core monomer protein52. This matrix composition presents a substantial steric hindrance to agent penetration. Usually, agents <15 nm in size penetrate the full thickness of cartilage, while agents of approximately 15–60 nm penetrate only the superficial cartilage layer53. However, some studies have also shown that agents as large as ∼55 nm potentially penetrate the full thickness of cartilage, but others have shown that agents as small as ∼6 nm penetrate only the superficial cartilage layer54, 55, 56. These inconsistent results might have resulted from different degrees of cartilage damage and agent properties in addition to the particle size, such as charges, which may interact with negatively charged GAGs inside cartilage. Technologies have been developed to improve the penetration of delivery agents into tissue and overcome the size-exclusionary barrier. The main approaches are based on electrostatic interactions, cartilage-targeted agent-modified carriers or drugs. By designing drug–carrier conjugates with the optimal size and charge, penetration through the full thickness of cartilage and long-term retention, which are necessary for drug delivery to chondrocytes and other ECM targets, might be achieved.
Figure 2.
Barriers to drug delivery in joints. (A) Cartilage layers serve as barriers to drug penetration. The penetration depth of the drug is related to the drug size. Agents with sizes <15 nm penetrate the full thickness of cartilage, while agents with sizes of approximately 15–60 nm penetrate only the superficial cartilage layer. With targeted modification, agents may penetrate easier relative to their size. (B) Clearance of the drug within the joint cavity. The pathways of drug clearance within the articular cavity include escape via the vasculature or lymphatic system, as well as elimination via phagocytosis by immune cells.
3.2. Agent clearance from joints
The rapid clearance of therapeutic agents from a joint creates another main obstacle for intra-articular drug delivery. The pathways of drug clearance within the articular cavity include escape via the vasculature or lymphatic system, as well as elimination via phagocytosis by immune cells, including dendritic cells (DCs, Fig. 2B)57. The drug clearance speed depends mainly on the size of the therapeutic agent. Previous studies have shown that agents <300 nm escape freely from a joint cavity. Those ∼3 μm in diameter are retained in the normal synovium, whereas others escape from the inflamed synovium due to increased capillary permeability. Agents ≥10 μm are retained in a joint independent of the joint inflammatory status58. Phagocytosis of particles by immune cells in joints also contributes to drug clearance. Particles <5 μm are eliminated via phagocytosis by synovial residents and recruited macrophages and DCs59, 60, 61. New strategies for extending the joint residence time of drugs involve encapsulation into the matrix, incorporation of a neutralizing cationic coating on nanoparticles, reductions of nonspecific hydrophobic and hydrophilic interactions between particles and proteins in synovial fluid, and mimicking cells by cloaking the compound with endogenous cell membranes40,57,62,63.
4. Therapeutic agents for AC diseases
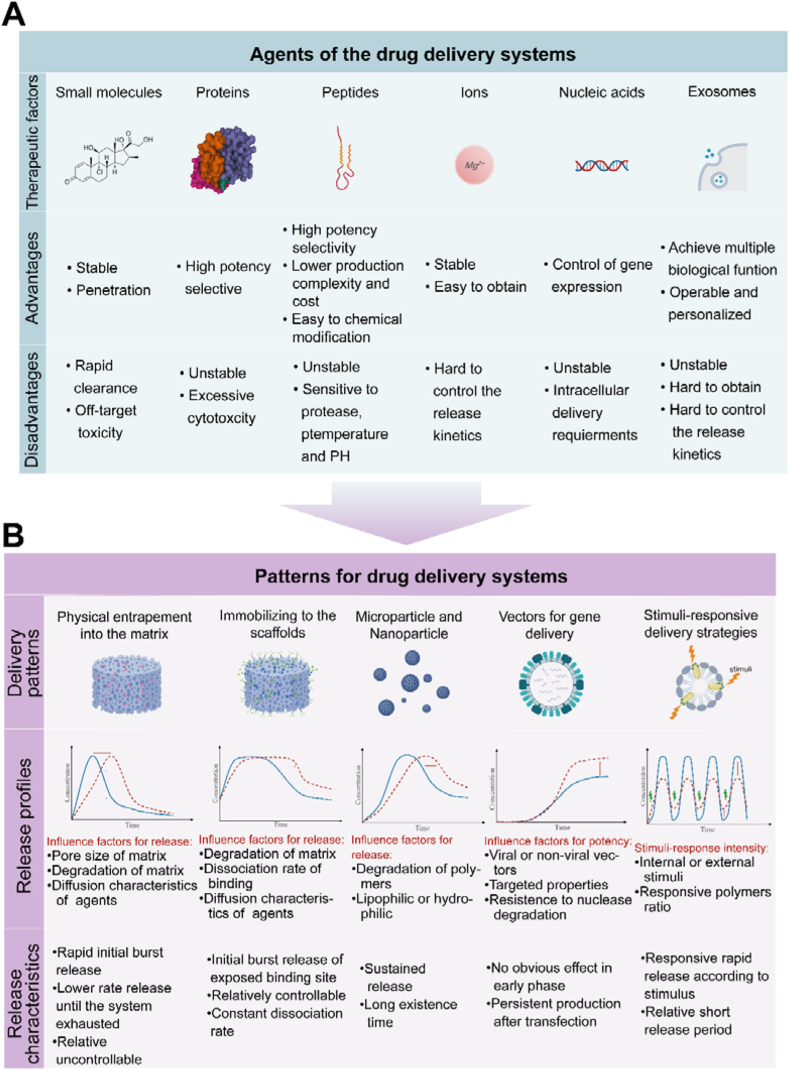
Homeostasis and repair of cartilage are regulated by various agents, such as small-molecule drugs, ions, and bioactive compounds. The different sizes, transport efficiencies, functions, and binding sites of these agents must be understood to ensure that they are loaded onto a proper DDS to maximize their effects (Fig. 3A).
Figure 3.
Characteristics of a variety of agents and delivery patterns for cartilage drug delivery system (DDS). (A) Each of the six therapeutic factors (small molecules, proteins, peptides, ions, nucleic acids and exosomes) has unique advantages and disadvantages. (B) Each of the five delivery patterns (physical entrapment into the matrix, immobilization in scaffolds, microparticles and nanoparticles, vectors for gene delivery and stimuli-responsive delivery strategies) have unique release profiles and release characteristics.
4.1. Small-molecule drugs
Small-molecule drugs (<1 kDa), such as NSAIDs, corticosteroids, and some herbs, are widely used both for systemic delivery and intra-articular delivery. The main function of most of these drugs is the suppression of symptoms and inflammation, as well as the protection of chondrocytes from oxidative stress after cartilage injury. Although small-molecule drugs penetrate the dense cartilage matrix, some challenges remain to be resolved to achieve effective intra-articular delivery. First, some drugs, such as amphotericin B, are insoluble, which limits their therapeutic utility and introduces the risk of crystal synovitis development57,64,65. Second, small-molecule drugs are cleared rapidly from lymphatic vessels and diffuse through biological fluids, which may cause off-target toxicity due to their relatively low molecular weight.
4.2. Ions
Many recent studies have shown that specific metal ions, such as Mg2+, Sr2+, Cu2+, and Zn2+, play important roles in cartilage development and maintenance66, 67, 68, 69. In conjunction with proper delivery systems, these ions induce a series of beneficial functions. For instance, Mg2+ has been shown to have osteogenic and angiogenic properties that are suitable for bone regeneration70. Moreover, a recent study indicated that Mg2+ may enhance MSC migration and chondrogenic differentiation. Sr2+ enhances MSC proliferation, promotes glycosaminoglycan secretion, and increases the chondrogenic differentiation rate71. Compared with biologics, metal ions are easier to obtain and remain stable under physiological conditions. A particle, metal oxide, or metal ion solution can be delivered by encapsulation or chelation with scaffolds. However, the kinetics of metal ion release is difficult to control, which may lead to an intolerable level of cytotoxicity.
4.3. Bioactive compounds
4.3.1. Cytokines
Cytokines constitute a broad and loose category of small proteins (∼5–25 kDa), including interleukins, interferons, members of the tumor necrosis factor superfamily, colony-stimulating factors, chemokines, and GFs, regulating innate and adaptive immunity and mediating cell proliferation, migration, and differentiation by binding to transmembrane receptors located on target cells. When a sufficient number of receptors are activated, subsequent signal transduction may trigger a series of specific cellular activities. Cytokines, including GFs and chemokines, are widely used in cartilage regeneration66,67,72. However, the release time and dose of cytokines must be precisely regulated to prevent adverse effects. For example, a study showed that compared with 10-ng BMP-2 injection treatment, 100-ng BMP-2 treatment caused adverse side effects, such as obliteration of the joint space and severe synovial reactions, in some rats 3 weeks after treatment68. High doses and prolonged delivery of GFs may lead to a loss of effectiveness over time due to the hypertrophy of differentiated chondrocytes69.
4.3.2. Functional peptides
Peptides constitute a unique class of bioactive agents that contain 2–50 amino acids and are applied to functionalize biomaterial scaffolds or nanoparticles, thus facilitating the adhesion, migration, proliferation, or chondrogenic differentiation of cells and increasing the targeting ability of agents73, 74, 75, 76, 77, 78, 79, 80, 81. By mimicking the actions of chondrogenesis-related ligands, cell-cell junction molecules and ECM components, which include a large variety of peptides, have been designed to activate the desired cell signaling pathways75, 76, 77, 78, 79, 80. Adhesive peptide sequences, such as RGD (Arg-Gly-Asp), IKVAV (Ile-Lys-Val-Ala-Val), and YIGSR (Tyr-Ile-Gly-Ser-Arg), on ECM molecules directly affect cell adhesion, morphology and the area of spread82, 83, 84, 85. Some peptides with affinity for mesenchymal stem cells (MSCs), such as PFSSTKT (PFS) and SKPPGTSS (SKP), have been combined with hydrogels to facilitate MSC homing and chondrogenic differentiation86,87. Additionally, peptides have also been used in targeted drug delivery. Certain peptides with affinity for chondrocyte or collagen II peptides, such as DWRVIIPPRPSA (CAP), WYRGRL, and collagen hybrid peptide (CHP), are immobilized with nanoparticles to deliver agents directly to chondrocytes or damaged collagen II fibers in cartilage81,88, 89, 90.
4.3.3. Nucleic acids
Nucleic acids enable the precise control of gene expression and the silencing or repair of aberrant genes, which may drive the expression of therapeutically relevant genes91, 92, 93. Gene-based therapies delivered to cells within a cartilage defect might provide sustained local production of therapeutic factors over extended periods, in contrast to the repeated intra-articular administration of recombinant GF proteins with relatively short half-lives that is currently used94. In addition to traditional gene therapy in the form of plasmid DNA (pDNA), RNA-based therapeutics such as mRNA, short interfering RNA (siRNA), and microRNA (miRNA) are promising approaches for regulating the functional activities in a cell. These RNA-based therapies prevent the risk of insertional mutagenesis that might be caused by pDNA therapy95. Additionally, some newly developed nucleic acid nanomaterials, such as aptamers (RNAs with secondary structures generated via self-pairing) and tetrahedral DNA nanostructures (TDNs), have also been used in cartilage regeneration96, 97, 98. However, the main obstacle to gene therapy is the susceptibility of nucleic acids to nucleases, which limit nucleotide half-lives and curative effects. Thus, appropriate delivery systems are crucial for nucleic acid delivery. Some advanced delivery systems for nucleic acids are summarized in Section 5.4.
4.3.4. Exosomes
Exosome comprise a class of membrane vesicles with a diameter of 50–100 nm that participate in the modulation of numerous regulatory cellular processes (e.g., proliferation, differentiation, and inflammation)99, 100, 101. Compared with MSCs, Exosome show superior regenerative effects and lower immunogenicity102. Exosome contain a variety of proteins, miRNAs, circular RNAs (circRNAs), and long noncoding RNAs (lncRNAs), all of which have been shown to promote cartilage repair in many experiments. The diversity of the molecules that they carry enables Exosome to achieve multiple biological functions, not merely one aspect of biology. Exosome generated naturally by MSCs or by preprocessing MSCs loaded onto DDSs can be used as therapeutic agents in strategies designed to meet specific needs. Advances in engineered exosome technology have made Exosome more operational and personalized because they may be specifically modified to deliver biological substances precisely and thus achieve cell-targeted effects.
5. Drug-loaded DDS patterns
This section describes the main drug-loaded patterns developed to date for the treatment of AC diseases, including physical entrapment within the matrix, immobilization on scaffolds, microparticles and nanoparticles, vectors for gene delivery, and stimuli-responsive delivery strategies (Fig. 3B). Different drug-loaded patterns lead to different drug release characters and profiles, which may adapt to specific therapeutic agents. Thus, a proper drug-loaded pattern is helpful for the enhancement of therapeutic outcomes of DDSs.
5.1. Physical entrapment within scaffold matrix
Physical entrapment within scaffold matrix is the simplest method for delivering agents. This strategy has been widely explored with hydrogels, porous matrices, 3D-printed scaffolds, and electrospinning technology loaded with agents. The agents are entrapped by mixing them with the matrix before solidification or by directly dropping them into the matrix after solidification, followed by lyophilization. In most of these systems, agents are released in a rapid initial burst within the first few hours, with the remainder released at a slower rate until the system is exhausted103. The release kinetics typically depends on the pore size and degradation rate of the matrix, indicating that the release rate is controlled by altering the matrix cross-link density or polymer stability104, 105, 106, 107, 108, 109, 110, 111. Additionally, the release rate may be altered by changing other interactions, such as the secondary binding kinetics or charges between the agent and polymer, or by incorporating hydrophobic segments to extend the release period112, 113, 114. The release profile determines that physical entrapment patterns are helpful for quickly achieving minimum effective concentration and having effective onset. Compared with other patterns, physical entrapment possesses high loading efficiency and drug preservation, while resulting in a relatively uncontrolled release, especially in the initial period, and inefficient retention during the entire treatment period.
5.2. Immobilization on scaffolds
Agents have been immobilized on a matrix through physical adsorption, chemical bonding, or secondary association. Physical adsorption is most often achieved by simple dipping procedures, which are fast, simple, cheap, and appropriate for complicated 3D surfaces115. However, dipping processes seldom lead to long-term retention of agents due to the weak interactions between the agents and scaffold, particularly in an unsuitable microenvironment. Covalent attachment leads to better-controlled release kinetics and spatiotemporal distribution but requires multistep, complicated procedures and may alter agent functionality116. For solid matrices, the immobilization of agents is typically accomplished by surface coating or through the network of a hydrogel. In these cases, the release profile of the agents is regulated by the matrix degradation rate, the polymer and agent dissociation rates, and the agent diffusion characteristics104. Immobilizing on scaffolds pattern provides a relative controlled release and thus extends retention time of therapeutic agents. However, the loading efficiency and drug bioactivity could be impaired by covalent attachment or drug modification. Therefore, drugs that are easy to modify and have stable biological activity are suitable for immobilization on scaffold pattern.
5.3. Microparticles and nanoparticles
Micro- (1–1000 μm) or nanoscale (1–1000 nm) particles are widely used as delivery agents because of their capacity for loading high concentrations of agents, protective nature, controlled release and operational properties. Particles allow minimally invasive delivery or have been combined with a macroscale system117,118. Depending on the polymer composition, particles are typically categorized into lipophilic polymeric particles, which are fabricated using synthetic polymers through the solvent extraction method, or hydrophilic particles fabricated with natural polymers through the chemical crosslinking method119. Lipophilic polymeric particles exhibit more sustainable drug release kinetics than that of hydrophilic particles. However, agent functionality may be impaired by the organic solvents used and the relatively harsh conditions for preparing lipophilic polymers. The release kinetics of these particles are regulated by modifying the particle size and polymer composition119. The drug release rate has been shown to decrease with increasing polylactic-coglycolic acid (PLGA) particle size120. When fabricating silk fibroin (SF)/polyvinyl alcohol (PVA) nanospheres, the release kinetics depended on the initial SF/PVA concentrations. As a method to overcome barriers for cartilage delivery, nanoparticles have been considered a promising delivery system due to their suitable size that penetrates the cartilage matrix. Furthermore, these nanoparticles can be decorated with numerous moieties with multiple functions, such as targeting components of cells, lubrication and multiple agent delivery.
5.4. Viral and nonviral vectors for gene delivery
In gene therapy, the choice of the gene delivery vector is crucial for effectively delivering nucleic acids to target tissue or cells. Gene delivery vectors are typically categorized as viral or nonviral. Viral vectors are generally preferred for gene therapies due to their high cell transfection efficiencies, ranging from approximately 80%–100%121. More than 70% of gene therapy clinical trials conducted to date have been based on viral vectors122. The main concerns associated with viral vectors relate to their potential immunogenicity, the risk of insertional mutagenesis that potentially results in oncogene transactivation and leukemia, and production difficulties122,123. Additionally, recombinant adeno-associated viruses (rAAV) have been investigated for applications in regenerative medicine as promising viral vectors that may overcome limitations associated with retroviruses and adenoviral vectors. Namely, rAAVs induce a reduced host immune response due to the removal of the immunogenic sequences in the viral genome and pose no risk of insertional mutagenesis, as their genetic material is maintained in episomal form, similar to nonviral pDNA-based delivery124,125. Compared to viral gene delivery, nonviral approaches to delivery offer advantages due to their temporal transfection, ease of production, safety, low immunogenicity, and ease of incorporation into 3D matrices122. Several nonviral gene vectors, such as inorganic vectors, lipid-based vectors, polymeric vectors, and protein and peptide vectors, have been developed92. A direct comparison of ex vivo liposomal and adenoviral BMP-2 delivery to bone marrow-derived mesenchymal stem cells (BMSCs) showed that although the adenovirus-infected BMSCs promoted the formation of excessive and abnormal bone in vivo, liposome-based delivery resulted in repaired tissue that was similar to the native bone in terms of thickness and shape126. These results highlight the potential of nonviral gene delivery as a physiologically suitable approach to achieve successful repair.
5.5. Stimulus-responsive delivery strategies
The aforementioned DDSs involve sustainable drug release kinetics that were determined before treatment. However, disease-related biological activities change over time, which causes sub- or supratherapeutic changes in drug concentrations at a particular time and/or in a specific space. A stimuli-responsive delivery strategy is a promising approach to release agents at target sites at the proper rate, which may minimize toxicity and enhance therapeutic efficacy. This process is summarized as either an endogenous or exogenous stimulus-responsive strategy. The release of drugs from endogenous stimulus-responsive strategies is triggered by objective pathological changes, including temperature, pH value, reactive oxygen species (ROS) levels, matrix-degrading enzyme activity, and degree of hypoxia, while the release of drugs from exogenous strategies is controlled by physicians or patients to create a stimulus, such as a magnetic field, electric field, ultrasonic field and light. However, the use of these systems might cause problems. For example, most of these systems are unable to deliver drugs over an extended period127,128. In addition, the use of certain functional materials has prompted safety concerns; for example, magnetic nanoparticles may lead to cytotoxicity129.
6. Multiple DDSs for treating AC
A series of drugs and DDSs have been developed to improve the pathological process of cartilage regeneration. Emerging therapies based on pathological mechanisms are summarized in Supporting Information. Although each therapeutic approach has shown ample potential, none seem sufficiently robust to result in full cartilage regeneration. One possible explanation for these outcomes may be related to the ability of a single DDS to attenuate only a certain category of pathological processes. For example, an anti-inflammatory drug inhibits inflammation and may further reduce a series of catabolic events; however, it does not sufficiently restore the anabolic function of the tissue to promote regeneration. Similarly, although one GF may effectively induce MSC chondrogenic differentiation, it does not control cartilage matrix remodeling to induce full hyaline cartilage regeneration. Moreover, the effect of a single drug on any single pathological process may not lead to the best outcomes. For example, the pathological processes in catabolic-related activities are closely related, and one adverse factor may hinder the therapeutic effect on another factor. For example, an inflammatory environment suppresses the induction of immunomodulation and chondrocyte protection. In addition, MMP-related matrix degradation and dysregulated immune cell activation might also lead to a sustained inflammatory response. These examples show that one drug may not be able to achieve the desired effect because of another coactive adverse factor. In addition, basic research on cartilage development has indicated that the formation of cartilage requires the initiation of a series of physiological and biochemical behaviors, and this initiation requires costimulation with multiple drugs. Because of the complexity of cartilage regeneration processes, a reasonable assumption is that single-agent delivery systems do not achieve the overall goals of tissue integration and engineering130,131. Therefore, strategies designed to explore the application of multiple DDSs in cartilage regeneration and repair are the most promising. Currently, research on matching doses and administration times and the synergy and mechanism underlying the effects of different drugs is insufficient, and therefore further exploration is still needed.
6.1. Multiple drug delivery carriers
6.1.1. Encapsulating multiple drugs into the matrix
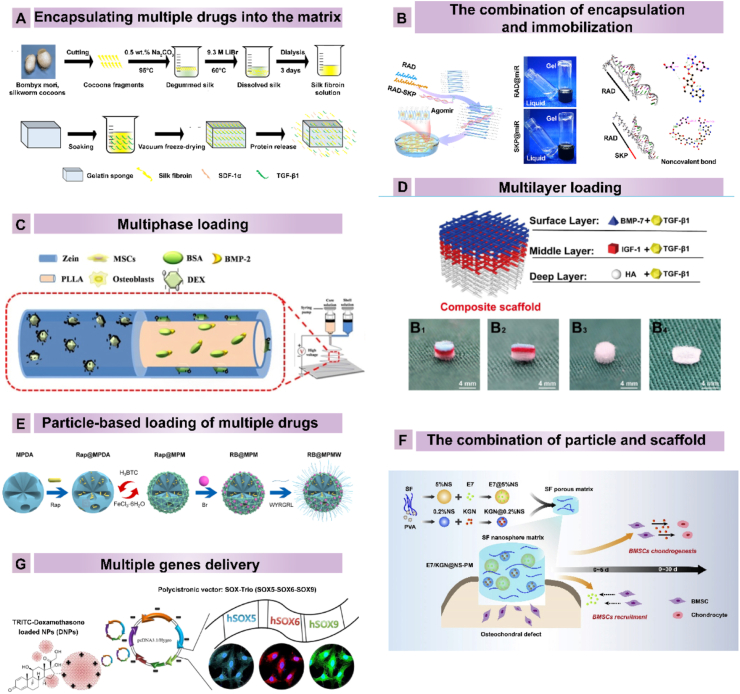
The simplest method to deliver multiple drugs is to mix them into the matrix132(Fig. 4A). This approach is widely used for the simultaneous release of multiple drugs that synergistically regulate cell fate or affect pathological states. For example, BMP-7 and TGF-β3 were loaded onto a scaffold prepared from PLGA. The scaffolds showed sustained release of both proteins over four weeks with minimal burst release133. Despite certain advantages, such as the preserved biological activity of the loaded drugs and retained scaffold properties, the disadvantages, similar to those of “physical entrapment within the matrix” mentioned above, including a rapid reduction in the drug concentration and uncontrolled loading efficiency and release properties, were even more prominent when multiple DDSs were applied because precise cooperation between different agents is needed for specific temporal and spatial release. In this system, the adjustable parameters of release kinetics are related to the dose of each drug and the overall drug release rate. This system does not enable the control of the relative release rates of drugs to ensure sequential delivery, which hinders its application to cartilage regeneration.
Figure 4.
Multiple drug delivery carriers used in cartilage DDS. (A) Construction of gelatin scaffolds encapsulated with SDF-1 and TGF-β1. Reprinted with the permission from Ref. 134. Copyright © 2019, American Chemical Society. (B) RAD and SKP peptides are immobilized to form a nanofiber hydrogel, and agomir-29b-5p is encapsulated inside the hydrogel. Reprinted with the permission from Ref. 136. Copyright © 2022, Author. (C) Construction of Zein/PLLA nanofibers loaded with BMP-2 and dexamethasone in different phases using coaxial electrospinning. Reprinted with the permission from Ref. 140. Copyright © 2018, Elsevier B.V. (D) Multilayer scaffold design loaded with BMP7, TGF-β1, and IGF-1 in different layers. Reprinted with the permission from Ref. 142. Copyright © 2022, Ivyspring International Publisher. (E) SF nanospheres with sequential release of E7/KGN from the SF porous matrix for osteochondral defect treatment. Reprinted with the permission from Ref.150. Copyright © 2020, The Authors. (F) The fabrication of MOF-coated mesoporous polydopamine loaded with Br and Rap. Reprinted with the permission from Ref.154. Copyright © 2021, The Authors. (G) SOX5, SOX6 and SOX9 were incorporated into a multicistronic plasmid and combined with the polycationic polymer polyethylenimine to induce MSC differentiation. Reprinted with the permission from Ref. 157. Copyright © 2017, American Chemical Society. Abbreviations: SDF-1α, stromal cell-derived factor 1α; TGF-β1, transforming growth factor beta 1; MSCs, mesenchymal stem cells; BSA, bovine serum albumin; BMP-2, bone morphogenetic protein 2; PLLA, poly-l-lactic acid; BMP-7, bone morphogenetic protein 7; IGF-1, insulin-like growth factor-1; HA, hydroxyapatite; Dex, dexamethasone; rap, rapamycin; Br, bilirubin; SF, silk fibroin; PVA, polyvinyl alcohol.
6.1.2. The combination of encapsulation and immobilization for drug loading
A combination of encapsulating and immobilizing different agents onto scaffolds has been developed to enable multiple drugs to act via their own tunable sustained-release profiles. In this delivery system, the release of one drug is controlled by the strength of its covalent bond with the structure, while the release of another drug is controlled by properties such as the pore size and degradation rate of the scaffold134 (Fig. 4B). Reasonable collocation can be achieved for sequential release. For example, Yang et al.135 developed a 3D bioprinted scaffold containing the aptamer HM69 and TGF-β3. The aptamer was immobilized with a decellularized cartilage extracellular matrix (DCECM) after the carboxyl groups were activated by MES, EDC, and NHS, while TGF-β3 was directly encapsulated into a DCECM/gel methacryloyl (GelMA) bioink135. The sustained release testing showed the sequential release of Aptamer and TGF-β3. Zhu et al.134 developed a stem cell-homing peptide SKPPGTSS-functionali-zed hydrogel to deliver agomir-29b-5p. Agomir-29b-5p attracts to the functional motif sequence SKPPGTSS because of the positively charged lysine. In vitro, agomirs in functionalized hydrogel were released relatively slowly to approximately 70% in 40 days. These results suggest that the combination of encapsulation and immobilization is an effective method to achieve sequential release of multiple drugs.
6.1.3. Multiphase loading
Fabricating a scaffold with multiphase release potential is another method by which to load multiple drugs and control their release. Supramolecular hydrogels and coaxial electrospinning are two methods used to generate multiphase scaffold structures. β-Cyclodextrin-based host-guest supramolecular hydrogels have been used to co-deliver both hydrophilic and hydrophobic agents. For instance, Xu et al.136 developed a stem cell-laden supramolecular hydrogel loaded with kartogenin (KGN) and β-cyclodextrin in one domain and TGF-β1 in the hydrogel network. Another class of supramolecular hydrogels has been developed based on interactions between spherical block copolymer nanoparticles and polymers137. These hydrogels have been used to encapsulate multiple drugs, with hydrophobic small molecules loaded on nanoparticles and hydrophilic molecules incorporated in the aqueous phase. Using coaxial electrospinning, two different bioinks are simultaneously injected, and core–shell nanofibers are fabricated138(Fig. 4C). Drugs are loaded into the shell layer and core layer. Generally, the drug loaded into the shell layer is released faster than the drug incorporated in the core layer, leading to the differential release behavior of the system. The release kinetics of drugs are altered by changing the fiber diameter and matrix materials of the core and shell layers139. In addition, the dose of these drugs influences drug release. A hydrophobic polymer is selected as the shell layer to prevent the need for an additional cross-linking step. However, most hydrophobic polymers, such as PCL, show poor biocompatibility. Therefore, the combination of synthetic and natural polymers (such as collagen and gelatin) is usually used for shell layer fabrication. This combination also provides flexibility that enables adjustments to the degradation behavior of the nanofibers.
6.1.4. Multilayer loading
During the development of the osteochondral unit, zone-specific GF directs MSC differentiation into different cellular phenotypes to generate osteochondral structures. Therefore, the fabrication of multilayered scaffolds with heterogeneous matrices and agents is necessary for the regeneration of defective osteochondral tissue140 (Fig. 4D). To date, several technologies have been utilized, such as multilayered lyophilization, electrospinning and 3D printing methods. The rate of agent release in each layer is adjusted by altering the molecular weight of the agents, the porous architecture of the scaffold, and the degradation of the polymers to match the parameters in native tissue development. The problem with this type of scaffold is the maintenance of fabricated agent patterns while agents are diffused between multiple layers, which may disrupt the finely tuned gradient of the original design. For instance, in one study, a BMP-2 and IGF-1 gradient distribution system was fabricated with silk sponge porous scaffolds or an aqueous alginate gel141. The level of osteochondrogenic differentiation did not follow the gradient trend of agent loading in the aqueous alginate gel because the GF was rapidly diffused. Therefore, future techniques must be developed to immobilize agents in the proper layer and maintain the heterogeneity between each layer.
6.1.5. Particle-based loading of multiple drugs
The development of nanoparticle delivery systems that combine multiple agents into a single particle is another multiple-drug-loading strategy. Nanoparticles, including liposomes, polymeric nanoparticle polymer-drug conjugates, and mesoporous nanoparticles, have been developed to deliver multiple agents. For instance, Sameer et al.142 developed a liposome-based delivery system that was fabricated through microfluidics technology. The hydrophilic agent was loaded in the aqueous phase and entrapped within the lipid bilayer, while the lipophilic agent was loaded with insolvent lipids142. Similar to liposomes, these polymeric nanoparticles delivered multiple agents through a combination of hydrophobic and hydrophilic molecules143,144. In contrast to liposomes, polymeric nanoparticles exhibit certain advantages, such as a narrow size distribution, tunable physicochemical properties, higher stability, higher lipophilic drug loading capacity, and controllable drug–release profiles145,146. In one study, fabricated thermoresponsive nanospheres with independent dual drug release profiles were used to treat OA. Specifically, KGN was covalently conjugated to an outer chitosan oligosaccharide (COS), while diclofenac (DCF) was added to the inner PPO chain of F127–COOH147. The covalently conjugated KGN was released in a sustainable manner, while the loaded DCF showed rapid burst release. Additionally, the release profile was temperature-regulated. Because of their large surface areas and pore volumes, mesoporous nanoparticles have been used to generate suitable multiple agent carriers148 (Fig. 4E). Notably, Xue et al.90 designed a dual DDS based on metal–organic framework (MOF)-functionalized mesoporous polydopamine (MPDA) nanoparticles loaded with Rap in MOF mesopores and bilirubin (Br) on the MOF shell. In addition, the collagen II-targeting peptide (WYRGRL) was conjugated onto the MOF surface. This system displayed accelerated near infrared (NIR) laser-triggered release of Rap due to the thermoresponse of polydopamine (PDA), suggesting the feasibility of multifunctional nanoparticle fabrication. Recently, mesoporous bioactive glass has attracted attention as a promising candidate carrier for delivering multiple agents due to its ion release properties when in contact with bodily fluids and capacity for therapeutic biomolecule loading149. However, the relative multiple-drug-loading application for cartilage regeneration is currently limited. Other DDSs, such as multiple agents loaded in Exos, DNA frameworks or nanogels, are prospective future carrier candidates.
6.1.6. The combination of particle and scaffold system loading
Since the degradation of microparticles or nanoparticles is altered by changing their preparation parameters, which leads to different degrees of crosslinking and different degradation properties, the release profiles from these DDSs can be controlled to a certain extent150. With a combination of scaffolds generated with hydrogel or by electrospinning or 3D printing, particles can be loaded to achieve sequential delivery based on differences between the particle-loaded agent and matrix-loaded agent90(Fig. 4F). In general, the agent loaded on particles shows a more sustained release profile than the agent loaded in the matrix. For example, the dual release of TGF-b1 and IGF-1 through gelatin-loaded microspheres incorporated in oligo(poly(ethylene glycol) fumarate) (OPF) gels was tested as a potential carrier for enhancing cartilage repair151,152. An in vitro release study showed that as much as 65.5% of the TGF-β1 loaded in the OPF gel underwent burst release within 3 days, whereas only 28.4% of the IGF loaded in the gelatin microspheres underwent burst release within 3 days. Furthermore, multiple particles were encapsulated into one matrix to codeliver multiple agents. An SF-based porous matrix containing two types of tunable drug-loaded SF nanospheres, each loaded with either the E7 peptide or KGN, was created for the dual delivery of E7 and KGN148. The loading efficiency and release kinetics of these biomolecules depended on the initial SF/PVA concentrations in the nanospheres, as well as the hydrophobicity of the loaded molecules, resulting in controllable and programmable delivery profiles. The results revealed that 5% of the nanospheres rapidly released the E7 peptide during the first 120 h, whereas the 0.2% nanospheres engaged in slow and sustained release of KGN, which was sustained for more than 30 days. These results indicated the utility of these systems and suggest that future studies should focus on the effects of the interplay between multiple agents and the release sequence151.
6.1.7. Delivery of multiple genes
Compared to the controllable release of proteins, gene therapy provides a more elegant approach for inducing cell-mediated expression of desired regulatory factors in a physiological, localized, and/or temporally controllable manner. For multiple gene delivery, microenvironment signaling during tissue development and healing may be closely imitated to achieve better regulation of the results. The transfection of a multicistronic pDNA encoding multiple genes has been applied to cartilage regeneration (Fig. 4G). In this case, SOX5, SOX6, and SOX9 were co-delivered into human MSCs using a polycistronic gene delivery system loaded with PLGA nanoparticles to maximize the efficiency of chondrogenic differentiation153, 154, 155. Combinatorial gene delivery of chondrogenic inducers, such as TGF-β1, BMP-2, and FGF-2, and hypertrophic suppressors, such as PTHrP, has been explored to suppress hypertrophic differentiation and enhance stable cartilage repair156, 157, 158, 159. In addition, by incorporating multiple nonviral vectors into materials through physical entrapment or covalent interactions, genetically activated scaffolds have been fabricated. These scaffolds are particularly flexible, enabling the delivery of multiple genes with greater temporal or spatial control than can be achieved using multicistronic pDNA. However, similar applications for cartilage regeneration are limited. In addition, the release sequence of multiple nucleic acids and timing of biomaterial-nucleic acid interactions are critical for the treatment effects and must be determined in the future.
6.2. Strategies for delivering multiple drugs
In this section, we propose three multiple drug delivery strategies for cartilage regeneration, including sequential delivery targeting various pathological processes, spatial delivery targeting various tissues or improving heterogeneous regeneration and synergistic targeting of the same pathological processes. The current status of each multiple drug delivery strategy is also systematically summarized as shown in Table S2.
6.2.1. Targeting various pathological processes
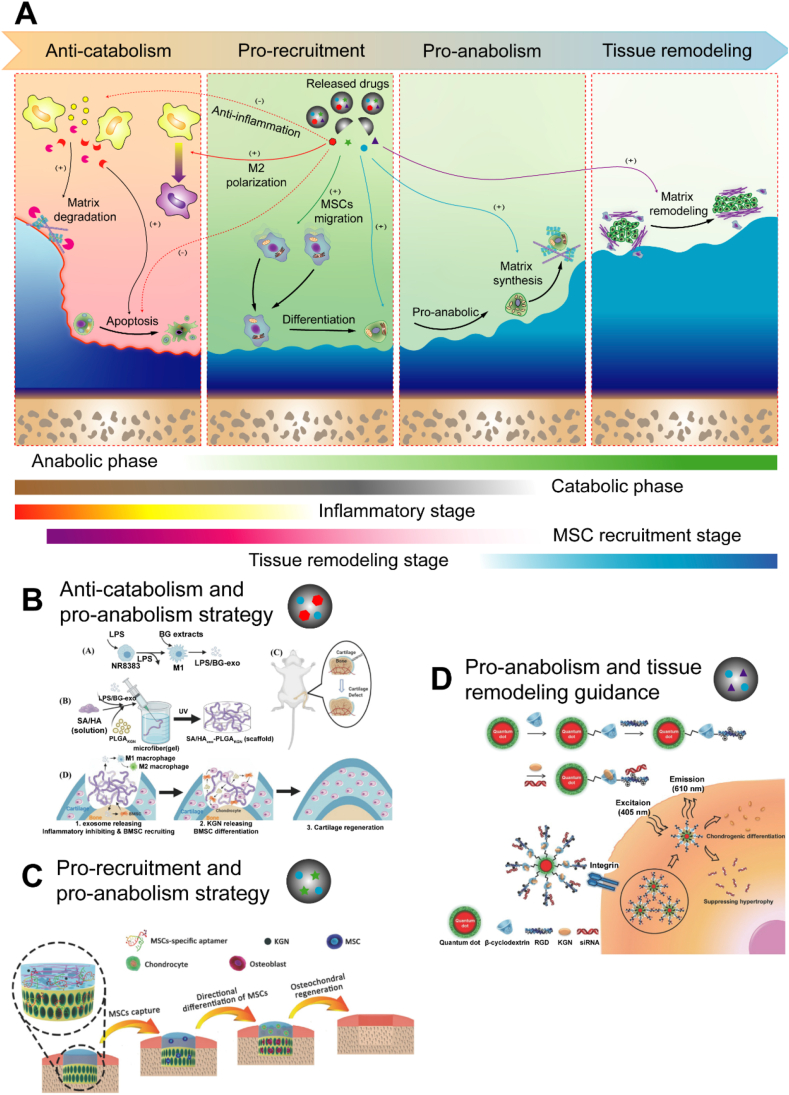
Five main phases overlap during the cartilage regeneration process: the inflammation stage, the catabolic phase, the cell recruitment stage, the anabolic phase and the tissue remodeling stage43 (Fig. 5A). The inflammatory stage and subsequent catabolic phase are evident in the first few days after injury. Then, initial anabolic activity is observed as MSCs migrate to the injury site. After 7–14 days, anabolic activities, including chondrogenic differentiation and matrix deposition, are activated and gradually dominate the process. After 4 weeks, mature neocartilage tissue forms, and cartilage, as well as SB, continues to undergo tissue remodeling, which determines the histological regeneration of the tissue types160. Multiple DDSs are needed to simulate the natural pathological process in the sequential release pattern. Because of the poor regenerative properties of cartilage, anabolic activity must be improved to establish cartilage regeneration after injury. Therefore, the inhibition of inflammation/catabolic activities, an endogenous MSC recruitment strategy, and guidance for tissue remodeling direction have been developed to function in conjunction with an anabolic activity improvement strategy to increase therapeutic effects.
Figure 5.
Targeting various pathological processes through the sequential release of multiple therapeutic factors. (A) Five main phases overlap during the cartilage regeneration process: inflammation stage, catabolic phase, cell recruitment stage, anabolic phase and tissue remodeling stage. Four therapeutic strategies were used according to pathological processes: anticatabolic, prorecruitment, pro-anabolic, and guiding tissue remodeling. (B) The combination of suppressed catabolism and increased anabolism. Reprinted with the permission from Ref. 166. Copyright © 2022 John Wiley & Sons, Inc. (C) The combination of endogenous MSC recruitment and increased anabolism. Reprinted with the permission from Ref. 175. Copyright © 2017 John Wiley & Sons, Inc. (D) The combination of increased anabolism and tissue remodeling guidance. Reprinted with the permission from Ref. 183. Copyright © 2016 John Wiley & Sons, Inc. Abbreviations: LPS, lipopolysaccharide; exo, exosome; SA/HA, sodium alginate/hyaluronic acid; PLGA, Poly(lactic-co-glycolic acid); KGN, kartogenin.
6.2.1.1. The combination of inflammation/catabolic suppression and anabolic enhancement
A promising strategy for the treatment of both cartilage injury and osteoarthritis is based on the combination of inflammation/catabolic suppression and anabolic enhancement161 (Fig. 5B). It is the most reasonable strategy to induce the regeneration of damaged cartilage and inhibit cartilage deterioration. In general, drugs targeting inflammation and catabolism must be released rapidly in the short term to suppress severe inflammatory responses and catabolic activity in the early phase, while prochondrogenic differentiation and matrix deposition must be induced in the middle and late stages of injury amelioration and persist for a long period. As shown by the aforementioned KGN-/DCF-loaded nanoparticles, the rapid burst release of DCF is critical for suppressing acute inflammation, while the sustained release of KGN effectively promotes chondrogenesis. An injectable in situ formed hydrogel/microparticle system consisting of melatonin (MEL) loaded in a hydrogel and methylprednisolone (MET) covalently bound to chitosan particles has been developed to induce chondrogenesis in vitro and in vivo162. According to a previous study, MET facilitated inflammation relief for the first 3 days and enhanced chondrogenic differentiation from days 3–21163, 164, 165, 166. The drug delivery profiles suggested that 100% MET and 25% MEL were released within the first 10 days of treatment. The remaining MEL was released within 90 days. Based on the in vivo results, the combination of MET and MEL in this model enhanced hyaline cartilage formation. In another study, KGN-loaded PLGA microparticles were added to MSC aggregates to form chondrogenic microtissues. The microtissues were delivered in vivo via an injectable, anti-inflammatory hydrogel that contained GelMA loaded with curcumin. Curcumin, an anti-inflammatory factor, was used to modulate the destructive microenvironment in the cartilage defect, which was assumed to be harmful to the transplanted cells. This combination led to the effective regeneration of the osteochondral defect in a model167. In addition to multiple protein delivery systems, multiple genes inhibiting inflammation and promoting chondrogenesis have also been explored to determine the synergistic effects of their expression on cartilage repair. The intra-articular injection of IL-1RA and TGF-β1 genes complexed to lipofectamine in a rabbit OA model produced better repair outcomes than the injection of each individual gene and, surprisingly, exerted a synergistic effect, as TGF-β1 overexpression increased the endogenous expression of IL-1RA168. Thus, the combination of suppressed catabolism and increased anabolism benefits cartilage regeneration.
6.2.1.2. The combination of endogenous MSC recruitment and anabolic enhancement
When a sufficient number of endogenous stem cells accumulate in a cartilage defect, they differentiate into functional chondrocytes and deposit additional ECM, which are important for neocartilage formation169. Thus, the combination of endogenous MSC recruitment and anabolic enhancement exert a synergistic effect on neocartilage regeneration. It is suitable for the therapy of acute cartilage injury and subsequent posttraumatic osteoarthirits which needs large amount of neocartilage supplement170 (Fig. 5C). Since MSCs migrate in the early phase of MSC chondrogenic differentiation, the co-delivery of MSC recruitment agents and anabolism-enhancing agents in sequential release patterns has been investigated. Y27632 is a small-molecule drug that promotes the differentiation of MSCs in cartilage171. Similarly, a DDS containing SDF-1 and microspheres embedded with Y27632 did not require cell seeding and was directly implanted in rabbit osteochondral defects to promote cartilage regeneration through the combination of MSC recruitment and anabolic enhancement. However, in another large animal model study, the combination of SDF-1α and TGF-β3 did not promote cartilage regeneration, in contrast to the positive results obtained in other explant and small animal studies with immune-compromised rodents or rabbits172, 173, 174, 175. The presence of SDF-1α inhibited cartilage regeneration in vivo, while TGF-β3 contributed to cartilage regeneration. Inflammatory cells, including lymphocytes and macrophages175, were potentially recruited to the defect, where they may have disrupted cartilage tissue regeneration, as indicated, to some extent, in the scaffold and TGF groups. Therefore, Yang et al.135 developed multifunctional bioscaffolds with the aptamer HM69 that recognized and recruited specific endogenous MSCs to injured cartilage and promoted chondrogenic differentiation of these migratory cells stimulated with TGF-β3 to prevent a negative effect on cell migration. Following the sequential release of HM69 and TGF-β3, this system achieved better repair results than the system without the aptamer.
6.2.1.3. The combination of anabolic enhancement and tissue remodeling guidance
During the healing process, the direction of cartilage remodeling readily deviates from hyaline cartilage formation to osseous tissue or fibrous cartilage formation because of several influencing factors, such as angiogenic GFs and osteogenic factors176,177. Thus, a tissue remodeling guidance strategy is important for full cartilage regeneration178 (Fig. 5D). Bian et al.8 successfully subcutaneously implanted constructs containing TGF-β3-loaded microspheres into nude mice, resulting in superior cartilage matrix formation. However, calcification was observed 8 weeks after subcutaneous implantation. To prevent this in other experiments, the authors implanted a dual-delivery system with both TGF-β3 and PTHrP to prevent this outcome in other experiments, and they observed reduced calcification. Antihypertrophic siRNAs were utilized to silence runt-related transcription factor 2 (Runx2), which is a key osteogenic transcription factor. This controlled delivery system delivered both KGN and antihypertrophic siRNAs to enhance the chondrogenesis of human MSCs (hMSCs) and neocartilage development and to inhibit hypertrophic differentiation and related cartilage calcification178. In addition, an antiangiogenic strategy has also been used with a chondrogenic strategy to generate a more stable chondrocyte phenotype. Murphy et al.179 found that localized codelivery of BMP-2 and VEGFR1 in a hydrogel induced the differentiation of an activated skeletal stem cell population in AC. Although several attempts have been made and have generated promising results, the proper release time and sequence remain unclear for this combination strategy and must be explored in the future.
6.2.2. Synergistic targeting of the same pathological process
Multiple biologics have been used to regulate catabolic and anabolic activities under physiological conditions. Although some biological signaling pathways exhibit a cause-and-effect relationship, most exert synergistic or balanced effects to maintain a homeostatic environment in joints. Therefore, multiple drugs may induce a better regulatory effect on a pathological process, especially to achieve anti-inflammation/catabolic and pro-anabolic effects.
6.2.2.1. Synergistic inflammation/catabolic suppression
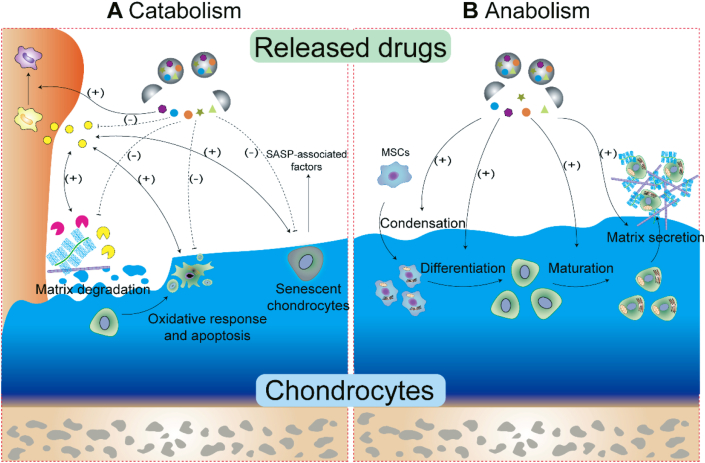
For cartilage repair in osteoarthritic joints, inflammation and a series of catabolic activities are the main obstacles to be overcome in the generation of neotissue. Since catabolic activities and related influencing factors affect several aspects of the process, such as inflammation, immunomodulation, matrix degradation, chondrocyte senescence and death, a single agent may fail to reverse the catabolic process (Fig. 6A). Therefore, the application of a combination of drugs to inhibit cartilage catabolism has become a promising strategy180, 181, 182, 183. Specifically, nitric oxide (NO) was utilized to inhibit the inflammatory response, and siRNAs targeting the Notch signaling pathway were incorporated to reduce the proliferation of inflammatory cells184. A nanoparticle was fabricated containing NO, siRNAs and thermal-responsive hemoglobin nanogenerators to precisely control NO release, and it showed remarkable inhibition of the inflammatory response and suppressed cartilage erosion in OA. In another study, a low dose of indomethacin was combined with an inhibitor of the Hedgehog/GLI pathway called GANT-61 to treat cartilage injury in mice185. This treatment obviously alleviated cartilage damage, which suggested a synergistic effect between the chondrocyte protection and anti-inflammatory effects that it produced. Since Br exhibits obvious anti-ROS and anti-apoptotic effects while responsively reducing autophagy flux, a process that maintains adaptive cellular responses and homeostasis, a dual-DDS based on a MOF decorated with MPDA, which is composed of Rap loaded into mesopores and Br loaded onto the shell of the MOF, was fabricated. The addition of Rap activated autophagy by targeting the mTOR signaling pathway186. These results indicated that the rapid release of Br from the MOF shell exhibited excellent ROS scavenging ability and antiapoptotic effects; moreover, following NIR irradiation, Rap was rapidly released from the MPDA core, further enhancing autophagy activation and chondrocyte protection. Additionally, delivery of multiple anti-inflammatory drugs not only enhanced these curative effects but also reduced the incidence of side effects. For example, combining the advantages of dexamethasone and diclofenac in the clinic, HA-loaded liposomal dexamethasone-diclofenac nanoparticles were developed for treating OA180. The results of this study suggested that this combination exerted a better effect on pain relief and inflammation and significantly reduced the incidence of side effects, such as the bleeding tendency, avascular necrosis of the humeral or femoral head and gastrointestinal irritation, when applied individually via intra-articular delivery181, 182, 183.
Figure 6.
Synergistic targeting of each catabolic and anabolic activity. (A) The crosstalk between inflammation and the immune system, matrix degradation, and chondrocyte survival is complicated. Multiple drug delivery may simultaneously target these processes to synergistically inhibit catabolic activities. (B) The chondrogenic differentiation of MSCs into mature chondrocytes involves condensation, differentiation and maturation processes. Simultaneous targeting of various processes may enhance anabolic activities and accelerate cartilage regeneration.
6.2.2.2. Synergistic effect on enhancing anabolism
Chondrogenic differentiation is important in cartilage defect disease. Cartilage development is an intricate process regulated precisely by the activation and interaction of a variety of biochemical cell signaling pathways. In summary, the chondrogenic differentiation of MSCs into mature chondrocytes involves three stages, condensation, proliferation and differentiation, and maturation, with each stage induced by a particular GF (Fig. 6B). Therefore, a single factor very likely cannot be used to recapitulate the activity of these signaling pathways during chondrogenic differentiation. Therefore, strides have been made to develop a controlled release system using multiple GFs for cartilage repair with the hope of increasing the therapeutic potency. Numerous studies have verified that different GFs, such as TGF-β1 with IGF-1, BMP with TGF-β1, and FGF2 with TGF-β3, exert synergistic effects on chondrogenesis. A classic synergistic chondrogenic differentiation enhancement strategy involves the sequential release of TGF-β1 or TGF-β3 to initially stimulate a chondrogenic synthetic response, followed by the release of IGF-1 to improve cartilage matrix production. This combination strategy increased the expression of chondrogenic markers in vitro151,152. Gugoo et al.187 showed that TGF-β1 and IGF-1 led to greater hyaline cartilage formation with well-organized cellular arrangement in vivo compared to that induced by IGF-1 delivery alone. However, other studies reported no obvious enhancement of the regenerative effects when these GFs were applied in combination in vivo, emphasizing that even when the sequence of GF release is appropriate, optimization of the dosage and release kinetics is required151,152. Ruiz et al.188 utilized five Ad-MSC chondrogenic differentiation protocols to further explore the appropriate combinatory strategy with the optimized dosage and release sequence. FGF2 stimulated early chondrogenic differentiation and maintained the chondrogenic potential by triggering a priming mechanism. The Ad-MSCs were initially exposed to 10 ng/mL FGF2 for 5 days. Then, IGF and TGF-β1 alone or in combination were added to the culture. After 4 days in monolayer culture, the cells were seeded onto scaffolds to generate a 3D culture. On day 12, the GF regimen was changed to either individual TGF-β1 or IGF-1 treatment. The cells in the scaffold were analyzed after 40 days. The expression patterns of the chondrogenic markers suggested that all cell groups underwent chondrogenesis. However, FGF2 followed by sequential IGF-1/TGF-β1 and TGF-β1 treatments resulted in the greatest increase in chondrocyte differentiation, which indicated the importance of the GF release sequence and release kinetics for chondrogenesis. In future studies, multiple factors known to initiate different aspects of anabolism, such as MSC differentiation, chondrocyte proliferation, cartilaginous ECM production or simulation of the native regenerate sequence, might be used to explore and optimize combination strategies.
6.2.3. Spatial distribution in multiple tissues and heterogeneous regeneration
6.2.3.1. Targeting multiple joint tissues
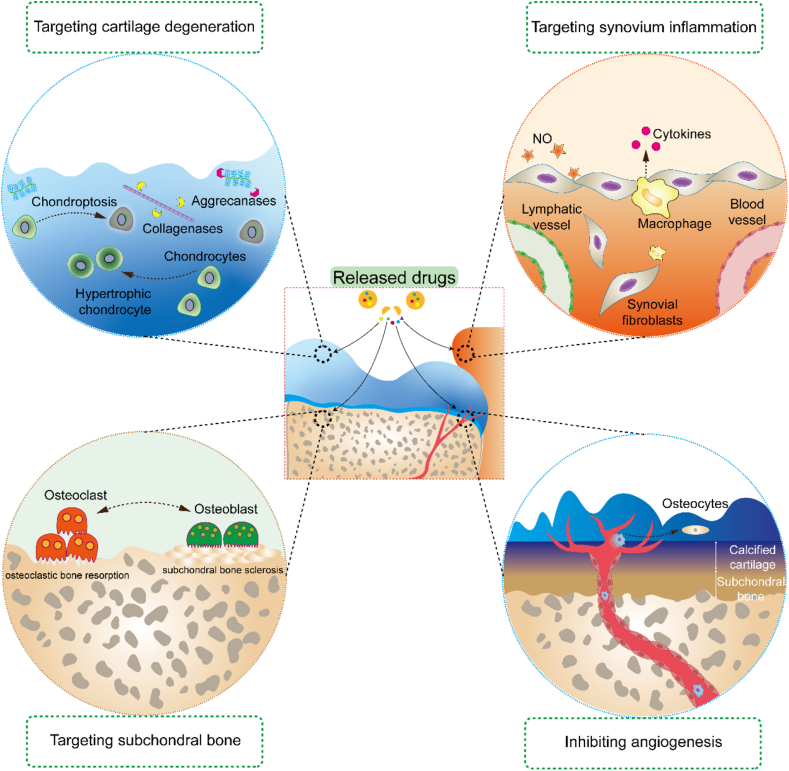
Cartilage injury is a whole-joint disease involving several tissues (e.g., cartilage, synovium, SB, and nervous system) (Fig. 7). The crosstalk between tissues is complicated; therefore, the pathological characteristics of each tissue may act together to determine the success of cartilage regeneration. Hence, in addition to treatments that induce cartilage regeneration, multiple drug delivery targets in other joint tissues may lead to synergistic effects on OA. The crosstalk between synovial tissue and cartilage plays a major role in OA pathogenesis, and therapies targeting inflammatory or senescent fibroblast-like synoviocytes prevent synovitis and suppress catabolic activities associated with AC189. Therefore, the combination of synovial catabolic suppression and increased cartilage anabolism is a potential strategy. In addition, the crosstalk between cartilage and SB is critical for osteochondral unit regeneration. A recent study indicated that the factors secreted by osteocytes may cause chondrocyte hypertrophy, cartilage degradation and mineralization190,191. In these cases, certain potential bone-targeting therapeutics, including bisphosphonates192, 193, 194, OPG (a soluble receptor antagonist of RANKL)195, an inhibitor of cathepsin-K196, and teriparatide197, have been combined in an anabolic enhancement strategy in AC. Furthermore, certain signaling pathways lead to inconsistent effects on cartilage and SB during tissue regeneration. For example, TGF-β signaling is increased in SB in an anterior cruciate ligament transection (ACLT) model and initiates pathological changes in OA, showing the opposite function to TGF-β in cartilage198. This finding suggested that an injection of a TGF-β type II receptor inhibitor prevents uncoupled bone resorption and formation while leading to some degree of proteoglycan loss in AC. Hence, multiple DDSs were applied to codeliver an SB-targeted antibody against TGF-β (1D11) in combination with a targeted cartilage anabolism-enhancing agent190,198. Similarly, therapies targeting blood vessels and nerves have been used in conjunction with cartilage anabolism-enhancing strategies. To date, certain multiple DDSs acting on multiple tissues have not been intentionally directed. Their effects may be hindered by rapid clearance from synovial fluid, barriers to cartilage matrix formation and targeting to undesired tissues. With the development of tissue-targeting delivery strategies and multiple drug delivery patterns, opportunities have been created to enhance multiple tissue-targeted treatments in joint disease through intentional drug delivery to specific drug-acting sites within a joint.
Figure 7.
Multiple drug delivery targets in various joint tissues, including cartilage, synovium, subchondral boneand blood vessels. The pathological characteristics of each tissue act together to determine the success of cartilage regeneration.
6.2.3.2. Improving heterogeneous regeneration
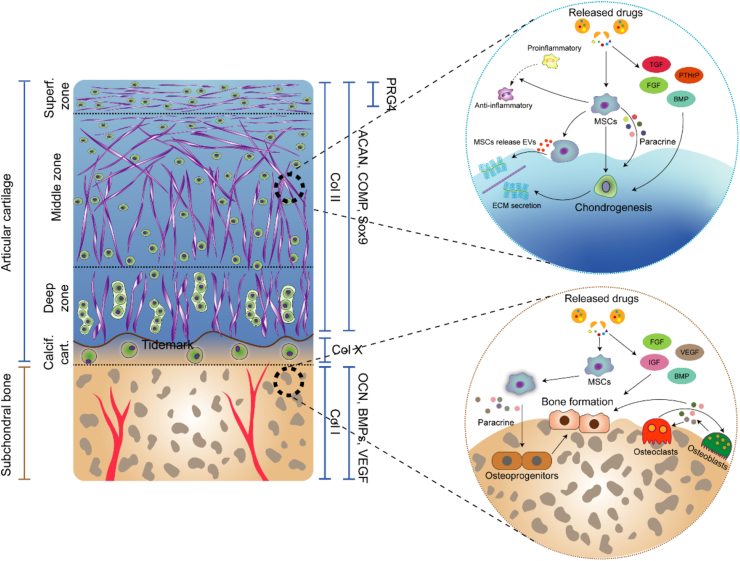
Because of the heterogeneous composition of an osteochondral unit, successful osteochondral regeneration after osteochondral defect must simulate the native heterogeneous architecture to produce biologically functional structures similar to natural tissue. Spatiotemporally controlled delivery of agents in conjunction with multiphasic scaffolds is crucial for composite osteochondral construction. The zone-specific cell phenotype is regulated by the secretion and spatial distribution of agents (Fig. 8). TGF-β and the BMP superfamily are used in classical combinations to induce chondrogenesis in AC and osteogenic differentiation of SB, respectively199, 200, 201. Previous studies have revealed that the combination of BMP-2 and TGF-β1 produced promising results in stimulating MSCs to undergo chondrogenesis and osteogenesis based on the different compositions of a scaffold202. Additionally, Wang et al.141 investigated hMSC osteogenic and chondrogenic differentiation induced with BMP-2 and IGF-1 and different carrier systems. In this study, hMSC osteogenic differentiation was most robust in a rhBMP-2/rhIGF-I silk microsphere gel system, but very little differentiation was evident in the corresponding PLGA microsphere gel system. In addition to GFs themselves, different carrier matrices that exhibit distinct GF loading and release properties are also important in determining hMSC differentiation. Various advanced techniques have been developed to incorporate multiphasic and continuous gradients into cartilage and osteochondral scaffolds. Recently, interest in the use of 3D printing to fabricate tissue engineering scaffolds has been increasing because the layer-by-layer additive patterns produced using this manufacturing technique enable the recreation of the zonal organization of native osteochondral tissue21,22. Notably, Qiao et al.203 developed a trilayered 3D printed scaffold with a spatially varying fiber configuration and biologics for layer-specific tissue induction and applied it to induce osteochondral regeneration. Specifically, the scaffold contained a superficial cartilage layer, deep cartilage layer and SB layer. With different GFs loaded in different layers (TGF-β1+BMP7 in the S layer, TGF-β1 in the D layer, and BMP2 in the B layer), a zone-specific cell phenotype was induced. As shown by the mRNA and protein expression levels, spatially stratified delivery of different GFs within the multilayered constructs significantly increased the formation of an SZP-enriched, orderly distributed matrix in the S layer, a proteoglycan-enriched, interconnected cartilaginous matrix in the D layer, and a calcium-deposited osseous matrix in the B layer. Spheroids, which are formed by spontaneous cell assembly, mimic the natural tissue-like microenvironment of cartilage or bone tissue regeneration. Moreover, Lee et al.204 developed a platform in which osteogenic and chondrogenic composite spheroids were located in a 3D-printed microchamber. The spheroids contained ECM-mimicking fibers, BMP-2 or TGF-β3 and human adipose-derived stem cells (hADSCs). More regenerated tissue filled the defect area void, and the regenerated cartilage or SB layer was stably bridged through transplanted cell–cell interactions.
Figure 8.
The zone-specific cell phenotype is regulated by the secretion and spatial distribution of agents. Spatiotemporally controllable delivery of agents in conjunction with multiphasic scaffolds is crucial for composite osteochondral construction.
7. Challenges and future prospects
Numerous multiple DDSs have been fabricated for application in cartilage regeneration. However, many problems, including an unclear understanding of the pathological and regeneration mechanisms and a lack of in vivo monitoring, have hindered the design of advanced multiple DDSs and thus must be solved.
The widely accepted theory for inducing complete regeneration of AC remains unclear. Although many studies have focused on each pathological process and some have achieved better results than nonintervention strategies, the intervention time window, concentration of each compound that should be used and the best combination strategies still require in-depth exploration. In addition, cartilage injuries have been classified into different categories based on the different pathological characteristics observed in different patients205. Personalized DDSs designed based on different injuries and patient conditions are needed. The lack of in vivo monitoring is a key area needing attention. Most of the current research on spatiotemporal drug release focuses on in vitro simulated environments, and suitable models and technologies to monitor drug release in vivo are unavailable, which contributes to the challenges to improving DDS. In addition, no measurement method is available to guide the effects of drug delivery in real time.
The future design of multiple DDSs may be improved by certain fabrication techniques and novel agent combinations, including new strategic combinations. In terms of fabrication techniques, microfluidics, microneedles, 3D printing technology, electrospinning and DNA supramolecular hydrogel generation are all promising techniques for fabricating more elaborate systems containing multiple agents206, 207, 208. In addition, particle-based technologies have also been substantially improved209. With a suitable size, operational and secondary loading properties, nanoparticles have been used to realize the precise control of agent release in a spatiotemporal manner and effectively regulate multiple pathological processes in the future. Recently, intelligent DDSs have also been explored. However, relatively few studies into intelligent multiple drug delivery have been performed in the cartilage regeneration field. The combination of multiple internal stimuli for logically assessing a disease state and the combination of internal and external stimuli for the smart release of multiple drugs according to the microenvironment in vivo and physicians’ observation ex vivo are needed. The combination of different therapeutic strategies must be explored in prospective studies. In general, in addition to the combination strategies mentioned above, a variety of possible combination strategies may benefit cartilage regeneration. Understanding the interaction in different phases is crucial for the design of new multiple drug delivery strategies in the future.
8. Gaps for clinical translation
Platelet-rich plasma (PRP), rich in various bioactive compounds, is a good example of the synergistic effect of multiple drug delivery for tissue regeneration in clinical translation. Nevertheless, other clinical applications of the multiple drug delivery strategy have so far been limited. Current preclinical studies demonstrated the potential of multiple drug delivery strategy to improve the therapeutic effects for cartilage diseases. However, there is still a long way to go between basic research and clinical development. First, the physiological, physicochemical and pharmacokinetics of multiple drug delivery systems are more complicated, which increases the difficulty of design and safety review. Second, the interaction of multiple drug delivery is unclear, and much effort is still needed in basic research to maximize therapeutic benefits and minimize the side effects between multiple drugs. Third, the desire to achieve multiple delivery strategies with precise spatiotemporal release greatly increases the development cost of clinical translation, which may discourage new technology exploration and clinical applications. Therefore, the clinical translation of multiple drug strategies needs to be simplified and developed step by step. We believe that with the development of fabrication technologies and a deep understanding of the pathological character in AC diseases, multiple drug delivery systems are expected to play an important role in clinical treatment.
9. Conclusions
The DDSs used in cartilage regeneration have been widely explored and substantially improved based on the development of new biologics, delivery carriers and therapeutic strategies. However, due to the complex and multifactorial characteristics of cartilage injury pathology, a sustained-release system loaded with a single drug fails to achieve the goal of full cartilage regeneration. Constructing a DDS that results in better curative effects by finely regulating the spatiotemporal release is a promising option. Several carrier patterns have been developed to achieve temporal and spatial control of multiple agents. Based on these advanced multiple DDSs, we proposed three combination strategies, including sequential delivery targeting various pathological processes, synergistic targeting of the same pathological processes and spatial delivery targeting various tissues or improving heterogeneous regeneration. Although numerous multiple DDSs have been proven to enhance regeneration, the rational design of these systems is more complicated than that of single drug-loaded systems. Therefore, we must obtain a profound understanding of cartilage regeneration, develop more sophisticated DDSs, precisely control the release of each agent in a spatiotemporal manner and identify new combinations of multiple drugs. We nonetheless have ample reason to believe that as more combination strategies are tested, multiple DDSs will lead to new insights into cartilage regeneration.
Acknowledgments
This work was supported by the National Key R&D Program of China (2019YFA0110600, China) and Medical Research and Development Projects (BLB20J001, China).
Footnotes
Peer review under the responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Supporting data to this article can be found online at https://doi.org/10.1016/j.apsb.2022.11.021.
Contributor Information
Quanyi Guo, Email: doctorguo_301@163.com.
Shuyun Liu, Email: clear_ann@163.com.
Author contributions
Tianyuan Zhao, Xu Li and Hao Li contributed to the conceptualization and writing of-the original draft; Haoyuan Deng and Jianwei Li contributed to the visualization; Zhen Yang, Songlin He, Shuangpeng Jiang and Xiang Sui contributed to the supervision. Quanyi Guo and Shuyun Liu contributed to the writing-review & editing.
Conflicts of interest
The authors declare no conflicts of interest.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Frank R.M., Cotter E.J., Hannon C.P., Harrast J.J., Cole B.J. Cartilage restoration surgery: incidence rates, complications, and trends as reported by the American Board of Orthopaedic Surgery Part II Candidates. Arthroscopy. 2019;35:171–178. doi: 10.1016/j.arthro.2018.08.028. [DOI] [PubMed] [Google Scholar]
- 2.Jüni P., Hari R., Rutjes A.W., Fischer R., Silletta M.G., Reichenbach S., et al. Intra-articular corticosteroid for knee osteoarthritis. Cochrane Db Syst Rev. 2015;10:Cd005328. doi: 10.1002/14651858.CD005328.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Middelkoop M., Arden N.K., Atchia I., Birrell F., Chao J., Rezende M.U., et al. The OA trial bank: meta-analysis of individual patient data from knee and hip osteoarthritis trials show that patients with severe pain exhibit greater benefit from intra-articular glucocorticoids. Osteoarthritis Cartilage. 2016;24:1143–1152. doi: 10.1016/j.joca.2016.01.983. [DOI] [PubMed] [Google Scholar]
- 4.McAlindon T.E., LaValley M.P., Harvey W.F., Price L.L., Driban J.B., Zhang M., et al. Effect of intra-articular triamcinolone vs. saline on knee cartilage volume and pain in patients with knee osteoarthritis: a randomized clinical trial. JAMA-J Am Med Assoc. 2017;317:1967–1975. doi: 10.1001/jama.2017.5283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nelson A.E., Allen K.D., Golightly Y.M., Goode A.P., Jordan J.M. A systematic review of recommendations and guidelines for the management of osteoarthritis: the chronic osteoarthritis management initiative of the U.S. bone and joint initiative. Semin Arthritis Rheum. 2014;43:701–712. doi: 10.1016/j.semarthrit.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 6.Block J.A. OA guidelines: improving care or merely codifying practice? Nat Rev Rheumatol. 2014;10:324–326. doi: 10.1038/nrrheum.2014.61. [DOI] [PubMed] [Google Scholar]
- 7.Gao J., Xia Z., Mary H.B., Joseph J., Luo J.N., Joshi N. Overcoming barriers for intra-articular delivery of disease-modifying osteoarthritis drugs. Trends Pharmacol Sci. 2022;43:171–187. doi: 10.1016/j.tips.2021.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bian L., Zhai D.Y., Tous E., Rai R., Mauck R.L., Burdick J.A. Enhanced MSC chondrogenesis following delivery of TGF-β3 from alginate microspheres within hyaluronic acid hydrogels in vitro and in vivo. Biomaterials. 2011;32:6425–6434. doi: 10.1016/j.biomaterials.2011.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Santo V.E., Gomes M.E., Mano J.F., Reis R.L. Controlled release strategies for bone, cartilage, and osteochondral engineering—part II: challenges on the evolution from single to multiple bioactive factor delivery. Tissue Eng Part B-Re. 2013;19:327–352. doi: 10.1089/ten.teb.2012.0727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen F.M., Zhang M., Wu Z.F. Toward delivery of multiple growth factors in tissue engineering. Biomaterials. 2010;31:6279–6308. doi: 10.1016/j.biomaterials.2010.04.053. [DOI] [PubMed] [Google Scholar]
- 11.Lam J., Lu S., Kasper F.K., Mikos A.G. Strategies for controlled delivery of biologics for cartilage repair. Adv Drug Deliv Rev. 2015;84:123–134. doi: 10.1016/j.addr.2014.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koo S., Andriacchi T.P. A comparison of the influence of global functional loads vs. local contact anatomy on articular cartilage thickness at the knee. J Biomech. 2007;40:2961–2966. doi: 10.1016/j.jbiomech.2007.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burstein D., Gray M., Mosher T., Dardzinski B. Measures of molecular composition and structure in osteoarthritis. Radiol Clin N Am. 2009;47:675–686. doi: 10.1016/j.rcl.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 14.Hunziker E.B., Lippuner K., Shintani N. How best to preserve and reveal the structural intricacies of cartilaginous tissue. Matrix Biol. 2014;39:33–43. doi: 10.1016/j.matbio.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 15.Chen F.H., Rousche K.T., Tuan R.S. Technology insight: adult stem cells in cartilage regeneration and tissue engineering. Nat Clin Pract Rheum. 2006;2:373–382. doi: 10.1038/ncprheum0216. [DOI] [PubMed] [Google Scholar]
- 16.Sophia Fox A.J., Bedi A., Rodeo S.A. The basic science of articular cartilage: structure, composition, and function. Sports Health. 2009;1:461–468. doi: 10.1177/1941738109350438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Archer C.W., Francis-West P. The chondrocyte. Int J Biochem Cell Biol. 2003;35:401–404. doi: 10.1016/s1357-2725(02)00301-1. [DOI] [PubMed] [Google Scholar]
- 18.Glyn-Jones S., Palmer A.J., Agricola R., Price A.J., Vincent T.L., Weinans H., et al. Osteoarthritis. Lancet. 2015;386:376–387. doi: 10.1016/S0140-6736(14)60802-3. [DOI] [PubMed] [Google Scholar]
- 19.Lotz M.K., Kraus V.B. New developments in osteoarthritis. Posttraumatic osteoarthritis: pathogenesis and pharmacological treatment options. Arthritis Res Ther. 2010;12:211. doi: 10.1186/ar3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Loeser R.F., Goldring S.R., Scanzello C.R., Goldring M.B. Osteoarthritis: a disease of the joint as an organ. Arthritis Rheum. 2012;64:1697–1707. doi: 10.1002/art.34453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Billinghurst R.C., Dahlberg L., Ionescu M., Reiner A., Bourne R., Rorabeck C., et al. Enhanced cleavage of type II collagen by collagenases in osteoarthritic articular cartilage. J Clin Invest. 1997;99:1534–1545. doi: 10.1172/JCI119316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu J.J., Lark M.W., Chun L.E., Eyre D.R. Sites of stromelysin cleavage in collagen types II, IX, X, and XI of cartilage. J Biol Chem. 1991;266:5625–5628. [PubMed] [Google Scholar]
- 23.Lippiello L., Hall D., Mankin H.J. Collagen synthesis in normal and osteoarthritic human cartilage. J Clin Invest. 1977;59:593–600. doi: 10.1172/JCI108676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ryu J., Treadwell B.V., Mankin H.J. Biochemical and metabolic abnormalities in normal and osteoarthritic human articular cartilage. Arthritis Rheum. 1984;27:49–57. doi: 10.1002/art.1780270109. [DOI] [PubMed] [Google Scholar]
- 25.Sandy J.D., Adams M.E., Billingham M.E., Plaas A., Muir H. In vivo and in vitro stimulation of chondrocyte biosynthetic activity in early experimental osteoarthritis. Arthritis Rheum. 1984;27:388–397. doi: 10.1002/art.1780270405. [DOI] [PubMed] [Google Scholar]
- 26.Aigner T., Stöss H., Weseloh G., Zeiler G., von der Mark K. Activation of collagen type II expression in osteoarthritic and rheumatoid cartilage. Virchows Arch B. 1992;62:337–345. doi: 10.1007/BF02899701. [DOI] [PubMed] [Google Scholar]
- 27.Goldring M.B., Marcu K.B. Cartilage homeostasis in health and rheumatic diseases. Arthritis Res Ther. 2009;11:224. doi: 10.1186/ar2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mankin H.J., Dorfman H., Lippiello L., Zarins A.J.J. Biochemical and metabolic abnormalities in articular cartilage from osteo-arthritic human hips: II. correlation of morphology with biochemical and metabolic data. J Bone Joint Surg Am. 1971;53:523–537. [PubMed] [Google Scholar]
- 29.Hunter D.J., Bierma-Zeinstra S. Osteoarthritis. Lancet. 2019;393:1745–1759. doi: 10.1016/S0140-6736(19)30417-9. [DOI] [PubMed] [Google Scholar]
- 30.Zhang Y., Wang F., Tan H., Chen G., Guo L., Yang L. Analysis of the mineral composition of the human calcified cartilage zone. Int J Med Sci. 2012;9:353–360. doi: 10.7150/ijms.4276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ohba S., Hojo H., Chung U.I. Bioactive factors for tissue regeneration: state of the art. Muscles Ligaments Tendons J. 2012;2:193–203. [PMC free article] [PubMed] [Google Scholar]
- 32.Fortier L.A., Barker J.U., Strauss E.J., McCarrel T.M., Cole B.J. The role of growth factors in cartilage repair. Clin Orthop Relat Res. 2011;469:2706–2715. doi: 10.1007/s11999-011-1857-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Censi R., Dubbini A., Matricardi P. Bioactive hydrogel scaffolds—advances in cartilage regeneration through controlled drug delivery. Curr Pharm Des. 2015;21:1545–1555. doi: 10.2174/1381612821666150115150712. [DOI] [PubMed] [Google Scholar]
- 34.McAlindon T.E., Bannuru R.R., Sullivan M.C., Arden N.K., Berenbaum F., Bierma-Zeinstra S.M., et al. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthritis Cartilage. 2014;22:363–388. doi: 10.1016/j.joca.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 35.da Costa B.R., Reichenbach S., Keller N., Nartey L., Wandel S., Jüni P., et al. Effectiveness of non-steroidal anti-inflammatory drugs for the treatment of pain in knee and hip osteoarthritis: a network meta-analysis. Lancet. 2017;390:e21–e33. doi: 10.1016/S0140-6736(17)31744-0. [DOI] [PubMed] [Google Scholar]
- 36.Gallelli L., Galasso O., Falcone D., Southworth S., Greco M., Ventura V., et al. The effects of nonsteroidal anti-inflammatory drugs on clinical outcomes, synovial fluid cytokine concentration and signal transduction pathways in knee osteoarthritis. A randomized open label trial. Osteoarthritis Cartilage. 2013;21:1400–1408. doi: 10.1016/j.joca.2013.06.026. [DOI] [PubMed] [Google Scholar]
- 37.Martel-Pelletier J., Barr A.J., Cicuttini F.M., Conaghan P.G., Cooper C., Goldring M.B., et al. Osteoarthritis. Nat Rev Dis Primers. 2016;2 doi: 10.1038/nrdp.2016.72. [DOI] [PubMed] [Google Scholar]
- 38.Jones I.A., Togashi R., Wilson M.L., Heckmann N., Vangsness C.T., Jr. Intra-articular treatment options for knee osteoarthritis. Nat Rev Rheumatol. 2019;15:77–90. doi: 10.1038/s41584-018-0123-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhao K., Liu Y.S., Nie L.Y., Qian L.N., Nie N.F., Leptihn S., et al. The influence of sample size and gender composition on the meta-analysis conclusion of platelet-rich plasma treatment for osteoarthritis. J Orthop Transl. 2020;22:34–42. doi: 10.1016/j.jot.2019.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gerwin N., Hops C., Lucke A. Intraarticular drug delivery in osteoarthritis. Adv Drug Deliv Rev. 2006;58:226–242. doi: 10.1016/j.addr.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 41.Larsen C., Ostergaard J., Larsen S.W., Jensen H., Jacobsen S., Lindegaard C., et al. Intra-articular depot formulation principles: role in the management of postoperative pain and arthritic disorders. J Pharm Sci. 2008;97:4622–4654. doi: 10.1002/jps.21346. [DOI] [PubMed] [Google Scholar]
- 42.Latourte A., Kloppenburg M., Richette P. Emerging pharmaceutical therapies for osteoarthritis. Nat Rev Rheumatol. 2020;16:673–688. doi: 10.1038/s41584-020-00518-6. [DOI] [PubMed] [Google Scholar]
- 43.Anderson D.D., Chubinskaya S., Guilak F., Martin J.A., Oegema T.R., Olson S.A., et al. Post-traumatic osteoarthritis: improved understanding and opportunities for early intervention. J Orthop Res. 2011;29:802–809. doi: 10.1002/jor.21359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang Y., Pizzute T., Pei M. Anti-inflammatory strategies in cartilage repair. Tissue Eng Part B-Re. 2014;20:655–668. doi: 10.1089/ten.teb.2014.0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bhattacharjee M., Coburn J., Centola M., Murab S., Barbero A., Kaplan D.L., et al. Tissue engineering strategies to study cartilage development, degeneration and regeneration. Adv Drug Deliv Rev. 2015;84:107–122. doi: 10.1016/j.addr.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 46.Lieberthal J., Sambamurthy N., Scanzello C.R. Inflammation in joint injury and post-traumatic osteoarthritis. Osteoarthritis Cartilage. 2015;23:1825–1834. doi: 10.1016/j.joca.2015.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Greif D.N., Kouroupis D., Murdock C.J., Griswold A.J., Kaplan L.D., Best T.M., et al. Infrapatellar fat pad/synovium complex in early-stage knee osteoarthritis: potential new target and source of therapeutic mesenchymal stem/stromal cells. Front Bioeng Biotech. 2020;8:860. doi: 10.3389/fbioe.2020.00860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Belluzzi E., Stocco E., Pozzuoli A., Granzotto M., Porzionato A., Vettor R., et al. Contribution of infrapatellar fat pad and synovial membrane to knee osteoarthritis pain. BioMed Res Int. 2019;2019 doi: 10.1155/2019/6390182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vargason A.M., Anselmo A.C., Mitragotri S. The evolution of commercial drug delivery technologies. Nat Biomed Eng. 2021;5:951–967. doi: 10.1038/s41551-021-00698-w. [DOI] [PubMed] [Google Scholar]
- 50.Evans C.H. Drug delivery to chondrocytes. Osteoarthritis Cartilage. 2016;24:1–3. doi: 10.1016/j.joca.2015.08.012. [DOI] [PubMed] [Google Scholar]
- 51.Bajpayee A.G., Grodzinsky A.J. Cartilage-targeting drug delivery: can electrostatic interactions help? Nat Rev Rheumatol. 2017;13(3):183–193. doi: 10.1038/nrrheum.2016.210. [DOI] [PubMed] [Google Scholar]
- 52.Ng L., Grodzinsky A.J., Patwari P., Sandy J., Plaas A., Ortiz C. Individual cartilage aggrecan macromolecules and their constituent glycosaminoglycans visualized via atomic force microscopy. J Struct Biol. 2003;143:242–257. doi: 10.1016/j.jsb.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 53.Rothenfluh D.A., Bermudez H., O'Neil C.P., Hubbell J.A. Biofunctional polymer nanoparticles for intra-articular targeting and retention in cartilage. Nat Mater. 2008;7:248–254. doi: 10.1038/nmat2116. [DOI] [PubMed] [Google Scholar]
- 54.Brown S., Pistiner J., Adjei I.M., Sharma B. Nanoparticle properties for delivery to cartilage: the implications of disease state, synovial fluid, and off-target uptake. Mol Pharm. 2019;16:469–479. doi: 10.1021/acs.molpharmaceut.7b00484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bajpayee A.G., Wong C.R., Bawendi M.G., Frank E.H., Grodzinsky A.J. Avidin as a model for charge driven transport into cartilage and drug delivery for treating early stage post-traumatic osteoarthritis. Biomaterials. 2014;35:538–549. doi: 10.1016/j.biomaterials.2013.09.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Foy B.D., Blake J. Diffusion of paramagnetically labeled proteins in cartilage: enhancement of the 1-D NMR imaging technique. J Magn Reson. 2001;148:126–134. doi: 10.1006/jmre.2000.2216. [DOI] [PubMed] [Google Scholar]
- 57.Maudens P., Jordan O., Allémann E. Recent advances in intra-articular drug delivery systems for osteoarthritis therapy. Drug Discov Today. 2018;23:1761–1775. doi: 10.1016/j.drudis.2018.05.023. [DOI] [PubMed] [Google Scholar]
- 58.Pradal J., Maudens P., Gabay C., Seemayer C.A., Jordan O., Allémann E. Effect of particle size on the biodistribution of nano- and microparticles following intra-articular injection in mice. Int J Pharm. 2016;498:119–129. doi: 10.1016/j.ijpharm.2015.12.015. [DOI] [PubMed] [Google Scholar]
- 59.Horisawa E., Kubota K., Tuboi I., Sato K., Yamamoto H., Takeuchi H., et al. Size-dependency of DL-lactide/glycolide copolymer particulates for intra-articular delivery system on phagocytosis in rat synovium. Pharm Res (NY) 2002;19:132–139. doi: 10.1023/a:1014260513728. [DOI] [PubMed] [Google Scholar]
- 60.Champion J.A., Walker A., Mitragotri S. Role of particle size in phagocytosis of polymeric microspheres. Pharm Res (NY) 2008;25:1815–1821. doi: 10.1007/s11095-008-9562-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Edwards S.H., Cake M.A., Spoelstra G., Read R.A. Biodistribution and clearance of intra-articular liposomes in a large animal model using a radiographic marker. J Liposome Res. 2007;17:249–261. doi: 10.1080/08982100701557129. [DOI] [PubMed] [Google Scholar]
- 62.Mancipe Castro L.M., García A.J., Guldberg R.E. Biomaterial strategies for improved intra-articular drug delivery. J Biomed Mater Res A. 2021;109:426–436. doi: 10.1002/jbm.a.37074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shi Y., Lu A., Wang X., Belhadj Z., Wang J., Zhang Q. A review of existing strategies for designing long-acting parenteral formulations: focus on underlying mechanisms, and future perspectives. Acta Pharm Sin B. 2021;11:2396–2415. doi: 10.1016/j.apsb.2021.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Savjani K.T., Gajjar A.K., Savjani J.K. Drug solubility: importance and enhancement techniques. ISRN Pharm. 2012;2012 doi: 10.5402/2012/195727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kalepu S., Nekkanti V. Insoluble drug delivery strategies: review of recent advances and business prospects. Acta Pharm Sin B. 2015;5:442–453. doi: 10.1016/j.apsb.2015.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhou N., Li Q., Lin X., Hu N., Liao J.Y., Lin L.B., et al. BMP2 induces chondrogenic differentiation, osteogenic differentiation and endochondral ossification in stem cells. Cell Tissue Res. 2016;366:101–111. doi: 10.1007/s00441-016-2403-0. [DOI] [PubMed] [Google Scholar]
- 67.Sheykhhasan M., Qomi R.T., Ghiasi M. Fibrin scaffolds designing in order to human adipose-derived mesenchymal stem cells differentiation to chondrocytes in the presence of TGF-β3. Int J Stem Cell. 2015;8:219–227. doi: 10.15283/ijsc.2015.8.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yeh T.T., Wu S.S., Lee C.H., Wen Z.H., Lee H.S., Yang Z., et al. The short-term therapeutic effect of recombinant human bone morphogenetic protein-2 on collagenase-induced lumbar facet joint osteoarthritis in rats. Osteoarthr Cartil. 2007;15:1357–1366. doi: 10.1016/j.joca.2007.04.019. [DOI] [PubMed] [Google Scholar]
- 69.Zhong L., Huang X., Karperien M., Post J.N. The Regulatory role of signaling crosstalk in hypertrophy of MSCs and human articular chondrocytes. Int J Mol Sci. 2015;16:19225–19247. doi: 10.3390/ijms160819225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Qiao W., Wong K.H.M., Shen J., Wang W., Wu J., Li J., et al. TRPM7 kinase-mediated immunomodulation in macrophage plays a central role in magnesium ion-induced bone regeneration. Nat Commun. 2021;12:2885. doi: 10.1038/s41467-021-23005-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cai Z., Li Y., Song W., He Y., Li H., Liu X. Anti-inflammatory and prochondrogenic in situ-formed injectable hydrogel crosslinked by strontium-doped bioglass for cartilage regeneration. ACS Appl Mater Inter. 2021;13:59772–59786. doi: 10.1021/acsami.1c20565. [DOI] [PubMed] [Google Scholar]
- 72.Cicione C., Muiños-López E., Hermida-Gómez T., Fuentes-Boquete I., Díaz-Prado S., Blanco F.J. Alternative protocols to induce chondrogenic differentiation: transforming growth factor-β superfamily. Cell Tissue Bank. 2015;16:195–207. doi: 10.1007/s10561-014-9472-7. [DOI] [PubMed] [Google Scholar]
- 73.Sreejalekshmi K.G., Nair P.D. Biomimeticity in tissue engineering scaffolds through synthetic peptide modifications-altering chemistry for enhanced biological response. J Biomed Mater Res A. 2011;96:477–491. doi: 10.1002/jbm.a.32980. [DOI] [PubMed] [Google Scholar]
- 74.Hastar N., Arslan E., Guler M.O., Tekinay A.B. Peptide-based materials for cartilage tissue regeneration. Adv Exp Med Biol. 2017;1030:155–166. doi: 10.1007/978-3-319-66095-0_7. [DOI] [PubMed] [Google Scholar]
- 75.Lin X., Zamora P.O., Albright S., Glass J.D., Peña L.A. Multidomain synthetic peptide B2A2 synergistically enhances BMP-2 in vitro. J Bone Miner Res. 2005;20:693–703. doi: 10.1359/JBMR.041104. [DOI] [PubMed] [Google Scholar]
- 76.Bragdon B., Thinakaran S., Moseychuk O., King D., Young K., Litchfield D.W., et al. Casein kinase 2 beta-subunit is a regulator of bone morphogenetic protein 2 signaling. Biophys J. 2010;99:897–904. doi: 10.1016/j.bpj.2010.04.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Moore N.M., Lin N.J., Gallant N.D., Becker M.L. Synergistic enhancement of human bone marrow stromal cell proliferation and osteogenic differentiation on BMP-2-derived and RGD peptide concentration gradients. Acta Biomater. 2011;7:2091–2100. doi: 10.1016/j.actbio.2011.01.019. [DOI] [PubMed] [Google Scholar]
- 78.Li R., Xu J., Wong D.S.H., Li J., Zhao P., Bian L. Self-assembled N-cadherin mimetic peptide hydrogels promote the chondrogenesis of mesenchymal stem cells through inhibition of canonical Wnt/ββ-catenin signaling. Biomater. 2017;145:33–43. doi: 10.1016/j.biomaterials.2017.08.031. [DOI] [PubMed] [Google Scholar]
- 79.Chang J.C., Hsu S.H., Chen D.C. The promotion of chondrogenesis in adipose-derived adult stem cells by an RGD-chimeric protein in 3D alginate culture. Biomater. 2009;30:6265–6275. doi: 10.1016/j.biomaterials.2009.07.064. [DOI] [PubMed] [Google Scholar]
- 80.Liu S.Q., Tian Q., Hedrick J.L., Po Hui J.H., Ee P.L., Yang Y.Y. Biomimetic hydrogels for chondrogenic differentiation of human mesenchymal stem cells to neocartilage. Biomater. 2010;31:7298–7307. doi: 10.1016/j.biomaterials.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 81.Hu Q., Chen Q., Yan X., Ding B., Chen D., Cheng L. Chondrocyte affinity peptide modified PAMAM conjugate as a nanoplatform for targeting and retention in cartilage. Nanomedicine. 2018;13:749–767. doi: 10.2217/nnm-2017-0335. [DOI] [PubMed] [Google Scholar]
- 82.Hern D.L., Hubbell J.A. Incorporation of adhesion peptides into nonadhesive hydrogels useful for tissue resurfacing. J Biomed Mater Res. 1998;39:266–276. doi: 10.1002/(sici)1097-4636(199802)39:2<266::aid-jbm14>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 83.Shin H., Jo S., Mikos A.G. Biomimetic materials for tissue engineering. Biomater. 2003;24:4353–4364. doi: 10.1016/s0142-9612(03)00339-9. [DOI] [PubMed] [Google Scholar]
- 84.Fittkau M.H., Zilla P., Bezuidenhout D., Lutolf M.P., Human P., Hubbell J.A., et al. The selective modulation of endothelial cell mobility on RGD peptide containing surfaces by YIGSR peptides. Biomaterials. 2005;26:167–174. doi: 10.1016/j.biomaterials.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 85.VandeVondele S., Vörös J., Hubbell J.A. RGD-grafted poly-L-lysine-graft-(polyethylene glycol) copolymers block non-specific protein adsorption while promoting cell adhesion. Biotechnol Bioeng. 2003;82:784–790. doi: 10.1002/bit.10625. [DOI] [PubMed] [Google Scholar]
- 86.Huang B., Li P., Chen M., Peng L., Luo X., Tian G., et al. Hydrogel composite scaffolds achieve recruitment and chondrogenesis in cartilage tissue engineering applications. J Nanobiotechnol. 2022;20:25. doi: 10.1186/s12951-021-01230-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sun X., Yin H., Wang Y., Lu J., Shen X., Lu C., et al. In Situ articular cartilage regeneration through endogenous reparative cell homing using a functional bone marrow-specific scaffolding system. ACS Appl Mater Inter. 2018;10:38715–38728. doi: 10.1021/acsami.8b11687. [DOI] [PubMed] [Google Scholar]
- 88.Pi Y., Zhang X., Shi J., Zhu J., Chen W., Zhang C., et al. Targeted delivery of non-viral vectors to cartilage in vivo using a chondrocyte-homing peptide identified by phage display. Biomaterials. 2011;32:6324–6332. doi: 10.1016/j.biomaterials.2011.05.017. [DOI] [PubMed] [Google Scholar]
- 89.Pi Y., Zhang X., Shao Z., Zhao F., Hu X., Ao Y. Intra-articular delivery of anti-Hif-2α siRNA by chondrocyte-homing nanoparticles to prevent cartilage degeneration in arthritic mice. Gene Ther. 2015;22:439–448. doi: 10.1038/gt.2015.16. [DOI] [PubMed] [Google Scholar]
- 90.Xue S., Zhou X., Sang W., Wang C., Lu H., Xu Y., et al. Cartilage-targeting peptide-modified dual-drug delivery nanoplatform with NIR laser response for osteoarthritis therapy. Bioact Mater. 2021;6:2372–2389. doi: 10.1016/j.bioactmat.2021.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Raftery R.M., Walsh D.P., Blokpoel Ferreras L., Mencía Castaño I., Chen G., LeMoine M., et al. Highly versatile cell-penetrating peptide loaded scaffold for efficient and localised gene delivery to multiple cell types: from development to application in tissue engineering. Biomaterials. 2019;216 doi: 10.1016/j.biomaterials.2019.119277. [DOI] [PubMed] [Google Scholar]
- 92.Gonzalez-Fernandez T., Kelly D.J., O'Brien F.J. Controlled non-viral gene delivery in cartilage and bone repair: current strategies and future directions. Adv Ther. 2018;1 [Google Scholar]
- 93.Opalinska J.B., Gewirtz A.M. Nucleic-acid therapeutics: basic principles and recent applications. Nat Rev Drug Discov. 2002;1:503–514. doi: 10.1038/nrd837. [DOI] [PubMed] [Google Scholar]
- 94.Lohmander L.S., Hellot S., Dreher D., Krantz E.F., Kruger D.S., Guermazi A., et al. Intraarticular sprifermin (recombinant human fibroblast growth factor 18) in knee osteoarthritis: a randomized, double-blind, placebo-controlled trial. Arthritis Rheumatol. 2014;66:1820–1831. doi: 10.1002/art.38614. [DOI] [PubMed] [Google Scholar]
- 95.Kelly D.C., Raftery R.M., Curtin C.M., O'Driscoll C.M., O'Brien F.J. Scaffold-based delivery of nucleic acid therapeutics for enhanced bone and cartilage repair. J Orthop Res. 2019;37:1671–1680. doi: 10.1002/jor.24321. [DOI] [PubMed] [Google Scholar]
- 96.Fu L., Li P., Zhu J., Liao Z., Gao C., Li H., et al. Tetrahedral framework nucleic acids promote the biological functions and related mechanism of synovium-derived mesenchymal stem cells and show improved articular cartilage regeneration activity in situ. Bioact Mater. 2022;9:411–427. doi: 10.1016/j.bioactmat.2021.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Li P., Fu L., Liao Z., Peng Y., Ning C., Gao C., et al. Chitosan hydrogel/3D-printed poly(ε-caprolactone) hybrid scaffold containing synovial mesenchymal stem cells for cartilage regeneration based on tetrahedral framework nucleic acid recruitment. Biomaterials. 2021;278 doi: 10.1016/j.biomaterials.2021.121131. [DOI] [PubMed] [Google Scholar]
- 98.Hu X., Wang Y., Tan Y., Wang J., Liu H., Wang Y., et al. A Difunctional regeneration scaffold for knee repair based on aptamer-directed cell recruitment. Adv Mater. 2017;29:1605235. doi: 10.1002/adma.201605235. [DOI] [PubMed] [Google Scholar]
- 99.Dostert G., Mesure B., Menu P., Velot É. How do mesenchymal stem cells influence or are influenced by microenvironment through extracellular vesicles communication? Front Cell Dev Biol. 2017;5:6. doi: 10.3389/fcell.2017.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tsiapalis D., O'Driscoll L. Mesenchymal stem cell derived extracellular vesicles for tissue engineering and regenerative medicine applications. Cells. 2020;9:991. doi: 10.3390/cells9040991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Théry C., Witwer K.W., Aikawa E., Alcaraz M.J., Anderson J.D., Andriantsitohaina R., et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicl. 2018;7 doi: 10.1080/20013078.2018.1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Velot É., Madry H., Venkatesan J.K., Bianchi A., Cucchiarini M. Is Extracellular vesicle-based therapy the next answer for cartilage regeneration? Front Bio Eng Biotechnol. 2021;9 doi: 10.3389/fbioe.2021.645039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Leach J.B., Schmidt C.E. Characterization of protein release from photocrosslinkable hyaluronic acid-polyethylene glycol hydrogel tissue engineering scaffolds. Biomater. 2005;26:125–135. doi: 10.1016/j.biomaterials.2004.02.018. [DOI] [PubMed] [Google Scholar]
- 104.Tayalia P., Mooney D.J. Controlled growth factor delivery for tissue engineering. Adv Mater. 2009;21:3269–3285. doi: 10.1002/adma.200900241. [DOI] [PubMed] [Google Scholar]
- 105.Cadée J.A., de Groot C.J., Jiskoot W., den Otter W., Hennink W.E. Release of recombinant human interleukin-2 from dextran-based hydrogels. J Control Release. 2002;78:1–13. doi: 10.1016/s0168-3659(01)00483-7. [DOI] [PubMed] [Google Scholar]
- 106.Bhattarai N., Ramay H.R., Gunn J., Matsen F.A., Zhang M. PEG-grafted chitosan as an injectable thermosensitive hydrogel for sustained protein release. J Control Release. 2005;103:609–624. doi: 10.1016/j.jconrel.2004.12.019. [DOI] [PubMed] [Google Scholar]
- 107.Tabata Y., Miyao M., Ozeki M., Ikada Y. Controlled release of vascular endothelial growth factor by use of collagen hydrogels. J Biomater Sci Polym Ed. 2000;11:915–930. doi: 10.1163/156856200744101. [DOI] [PubMed] [Google Scholar]
- 108.Zan J., Chen H., Jiang G., Lin Y., Ding F. Preparation and properties of crosslinked chitosan thermosensitive hydrogel for injectable drug delivery systems. J Appl Polym Sci. 2006;101:1892–1898. JJoaps. [Google Scholar]
- 109.Mellott M.B., Searcy K., Pishko M.V. Release of protein from highly cross-linked hydrogels of poly (ethylene glycol) diacrylate fabricated by UV polymerization. Biomaterials. 2001;22:929–941. doi: 10.1016/s0142-9612(00)00258-1. [DOI] [PubMed] [Google Scholar]
- 110.Temenoff J.S., Athanasiou K.A., Lebaron R.G., Mikos A.G. Effect of poly (ethylene glycol) molecular weight on tensile and swelling properties of oligo (poly (ethylene glycol) fumarate) hydrogels for cartilage tissue engineering. J Biomed Mater Res. 2002;59:429–437. doi: 10.1002/jbm.1259. [DOI] [PubMed] [Google Scholar]
- 111.Qiu B., Stefanos S., Ma J., Lalloo A., Perry B.A., Leibowitz M.J., et al. A hydrogel prepared by in situ cross-linking of a thiol-containing poly (ethylene glycol)-based copolymer: a new biomaterial for protein drug delivery. Biomaterials. 2003;24:11–18. doi: 10.1016/s0142-9612(02)00227-2. [DOI] [PubMed] [Google Scholar]
- 112.Silva E., Mooney D.J. Spatiotemporal control of vascular endothelial growth factor delivery from injectable hydrogels enhances angiogenesis. J Thromb Haemost. 2007;5:590–598. doi: 10.1111/j.1538-7836.2007.02386.x. [DOI] [PubMed] [Google Scholar]
- 113.Kushibiki T., Tomoshige R., Fukunaka Y., Kakemi M., Tabata Y. In vivo release and gene expression of plasmid DNA by hydrogels of gelatin with different cationization extents. J Control Release. 2003;90:207–216. doi: 10.1016/s0168-3659(03)00197-4. [DOI] [PubMed] [Google Scholar]
- 114.Leonard M., De Boisseson M.R., Hubert P., Dalencon F., Dellacherie E. Hydrophobically modified alginate hydrogels as protein carriers with specific controlled release properties. J Control Release. 2004;98:395–405. doi: 10.1016/j.jconrel.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 115.Nijhuis A.W., van den Beucken J.J., Jansen J.A., Leeuwenburgh S.C. In vitro response to alkaline phosphatase coatings immobilized onto titanium implants using electrospray deposition or polydopamine-assisted deposition. J Biomed Mater Res A. 2014;102:1102–1109. doi: 10.1002/jbm.a.34776. [DOI] [PubMed] [Google Scholar]
- 116.Lee H., Rho J., Messersmith P.B. Facile conjugation of biomolecules onto surfaces via mussel adhesive protein inspired coatings. Adv Mater. 2009;21:431–434. doi: 10.1002/adma.200801222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Nof M., Shea L.D. Drug-releasing scaffolds fabricated from drug-loaded microspheres. J Biomed Mater Res. 2002;59:349–356. doi: 10.1002/jbm.1251. [DOI] [PubMed] [Google Scholar]
- 118.Ennett A.B., Kaigler D., Mooney D.J. Temporally regulated delivery of VEGF in vitro and in vivo. J Biomed Mater Res A. 2006;79:176–184. doi: 10.1002/jbm.a.30771. [DOI] [PubMed] [Google Scholar]
- 119.Panyam J., Labhasetwar V. Biodegradable nanoparticles for drug and gene delivery to cells and tissue. Adv Drug Deliver Rev. 2003;55:329–347. doi: 10.1016/s0169-409x(02)00228-4. [DOI] [PubMed] [Google Scholar]
- 120.Berkland C., King M., Cox A., Kim K., Pack D.W. Precise control of PLG microsphere size provides enhanced control of drug release rate. J Control Release. 2002;82:137–147. doi: 10.1016/s0168-3659(02)00136-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Saraf A., Mikos A.G. Gene delivery strategies for cartilage tissue engineering. Adv Drug Deliv Rev. 2006;58:592–603. doi: 10.1016/j.addr.2006.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yin H., Kanasty R.L., Eltoukhy A.A., Vegas A.J., Dorkin J.R., Anderson D.G. Non-viral vectors for gene-based therapy. Nat Rev Genet. 2014;15:541–555. doi: 10.1038/nrg3763. [DOI] [PubMed] [Google Scholar]
- 123.Williams D.A., Thrasher A.J. Concise review: lessons learned from clinical trials of gene therapy in monogenic immunodeficiency diseases. Stem Cells Transl Med. 2014;3:636–642. doi: 10.5966/sctm.2013-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Venkatesan J.K., Ekici M., Madry H., Schmitt G., Kohn D., Cucchiarini M. SOX9 gene transfer via safe, stable, replication-defective recombinant adeno-associated virus vectors as a novel, powerful tool to enhance the chondrogenic potential of human mesenchymal stem cells. Stem Cell Res Ther. 2012;3:22. doi: 10.1186/scrt113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Frisch J., Orth P., Rey-Rico A., Venkatesan J.K., Schmitt G., Madry H., et al. Peripheral blood aspirates overexpressing IGF-I via rAAV gene transfer undergo enhanced chondrogenic differentiation processes. J Cell Mol Med. 2017;21:2748–2758. doi: 10.1111/jcmm.13190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Park J., Ries J., Gelse K., Kloss F., von der Mark K., Wiltfang J., et al. Bone regeneration in critical size defects by cell-mediated BMP-2 gene transfer: a comparison of adenoviral vectors and liposomes. Gene Ther. 2003;10:1089–1098. doi: 10.1038/sj.gt.3301960. [DOI] [PubMed] [Google Scholar]
- 127.Aguilar L.M.C., Silva S.M., Moulton S.E. Growth factor delivery: Defining the next generation platforms for tissue engineering. J Control Release. 2019;306:40–58. doi: 10.1016/j.jconrel.2019.05.028. [DOI] [PubMed] [Google Scholar]
- 128.Hoare T.R., Kohane D.S. Hydrogels in drug delivery: progress and challenges. Polymer. 2008;49:1993–2007. Jp. [Google Scholar]
- 129.Feng Q., Liu Y., Huang J., Chen K., Huang J., Xiao K. Uptake, distribution, clearance, and toxicity of iron oxide nanoparticles with different sizes and coatings. Sci Rep. 2018;8:1–13. doi: 10.1038/s41598-018-19628-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Guldberg R.E. Spatiotemporal delivery strategies for promoting musculoskeletal tissue regeneration. J Bone Miner Res. 2009;24:1507–1511. doi: 10.1359/jbmr.090801. [DOI] [PubMed] [Google Scholar]
- 131.Anitua E., Sánchez M., Orive G., Andia I. Delivering growth factors for therapeutics. Trends Pharmacol Sci. 2008;29:37–41. doi: 10.1016/j.tips.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 132.Chen Y., Wu T., Huang S., Suen C.W., Cheng X., Li J., et al. Sustained release SDF-1α/TGF-β1-loaded silk fibroin-porous gelatin scaffold promotes cartilage repair. ACS Appl Mater Inter. 2019;11:14608–14618. doi: 10.1021/acsami.9b01532. [DOI] [PubMed] [Google Scholar]
- 133.Crecente-Campo J., Borrajo E., Vidal A., Garcia-Fuentes M. New scaffolds encapsulating TGF-β3/BMP-7 combinations driving strong chondrogenic differentiation. Eur J Pharm Biopharm. 2017;114:69–78. doi: 10.1016/j.ejpb.2016.12.021. [DOI] [PubMed] [Google Scholar]
- 134.Zhu J., Yang S., Qi Y., Gong Z., Zhang H., Liang K., et al. Stem cell–homing hydrogel-based miR-29b-5p delivery promotes cartilage regeneration by suppressing senescence in an osteoarthritis rat model. Sci Adv. 2022;8 doi: 10.1126/sciadv.abk0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Yang Z., Zhao T., Gao C., Cao F., Li H., Liao Z., et al. 3D-Bioprinted difunctional scaffold for in situ cartilage regeneration based on aptamer-directed cell recruitment and growth factor-enhanced cell chondrogenesis. ACS Appl Mater Inter. 2021;13:23369–23383. doi: 10.1021/acsami.1c01844. [DOI] [PubMed] [Google Scholar]
- 136.Xu J., Feng Q., Lin S., Yuan W., Li R., Li J., et al. Injectable stem cell-laden supramolecular hydrogels enhance in situ osteochondral regeneration via the sustained co-delivery of hydrophilic and hydrophobic chondrogenic molecules. Biomaterials. 2019;210:51–61. doi: 10.1016/j.biomaterials.2019.04.031. [DOI] [PubMed] [Google Scholar]
- 137.Appel E.A., Tibbitt M.W., Webber M.J., Mattix B.A., Veiseh O., Langer R. Self-assembled hydrogels utilizing polymer–nanoparticle interactions. Nat Commun. 2015;6:1–9. doi: 10.1038/ncomms7295. JNc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Li R., Ma Y., Zhang Y., Zhang M., Sun D. Potential of rhBMP-2 and dexamethasone-loaded Zein/PLLA scaffolds for enhanced in vitro osteogenesis of mesenchymal stem cells. Colloid Surface B. 2018;169:384–394. doi: 10.1016/j.colsurfb.2018.05.039. [DOI] [PubMed] [Google Scholar]
- 139.He P., Li Y., Huang Z., Guo Z.Z., Luo B., Zhou C.R., et al. A multifunctional coaxial fiber membrane loaded with dual drugs for guided tissue regeneration. J Biomater Appl. 2020;34:1041–1051. doi: 10.1177/0885328219894001. [DOI] [PubMed] [Google Scholar]
- 140.Han Y., Lian M., Sun B., Jia B., Wu Q., Qiao Z., et al. Preparation of high precision multilayer scaffolds based on melt electro-writing to repair cartilage injury. Theranostics. 2020;10:10214–10230. doi: 10.7150/thno.47909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wang X., Wenk E., Zhang X., Meinel L., Vunjak-Novakovic G., Kaplan D.L. Growth factor gradients via microsphere delivery in biopolymer scaffolds for osteochondral tissue engineering. J Control Release. 2009;134:81–90. doi: 10.1016/j.jconrel.2008.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Joshi S., Hussain M.T., Roces C.B., Anderluzzi G., Kastner E., Salmaso S., et al. Microfluidics based manufacture of liposomes simultaneously entrapping hydrophilic and lipophilic drugs. Int J Pharm. 2016;514:160–168. doi: 10.1016/j.ijpharm.2016.09.027. [DOI] [PubMed] [Google Scholar]
- 143.Croy S.R., Kwon G.S. Polymeric micelles for drug delivery. Curr Pharm Des. 2006;12:4669–4684. doi: 10.2174/138161206779026245. [DOI] [PubMed] [Google Scholar]
- 144.Miyata K., Christie R.J., Kataoka K.J.R., Polymers F. Polymeric micelles for nano-scale drug delivery. React Funct Polym. 2011;71:227–234. [Google Scholar]
- 145.Rapoport N. Physical stimuli-responsive polymeric micelles for anti-cancer drug delivery. Prog Polym Sci. 2007;32:962–990. [Google Scholar]
- 146.Kim S., Shi Y., Kim J.Y., Park K., Cheng J.X. Overcoming the barriers in micellar drug delivery: loading efficiency, in vivo stability, and micelle-cell interaction. Expert Opin Drug Deliv. 2010;7:49–62. doi: 10.1517/17425240903380446. [DOI] [PubMed] [Google Scholar]
- 147.Kang M.L., Kim J.E., Im G.I. Thermoresponsive nanospheres with independent dual drug release profiles for the treatment of osteoarthritis. Acta Biomater. 2016;39:65–78. doi: 10.1016/j.actbio.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 148.Zhang W., Ling C., Zhang A., Liu H., Jiang Y., Li X., et al. An all-silk-derived functional nanosphere matrix for sequential biomolecule delivery and in situ osteochondral regeneration. Bioact Mater. 2020;5:832–843. doi: 10.1016/j.bioactmat.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zhu H., Zheng K., Boccaccini A.R. Multi-functional silica-based mesoporous materials for simultaneous delivery of biologically active ions and therapeutic biomolecules. Acta Biomater. 2021;129:1–17. doi: 10.1016/j.actbio.2021.05.007. [DOI] [PubMed] [Google Scholar]
- 150.Chen F.M., Zhao Y.M., Wu H., Deng Z.H., Wang Q.T., Zhou W., et al. Enhancement of periodontal tissue regeneration by locally controlled delivery of insulin-like growth factor-I from dextran-co-gelatin microspheres. J Control Release. 2006;114:209–222. doi: 10.1016/j.jconrel.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 151.Holland T.A., Tabata Y., Mikos A.G. Dual growth factor delivery from degradable oligo(poly(ethylene glycol) fumarate) hydrogel scaffolds for cartilage tissue engineering. J Control Release. 2005;101:111–125. doi: 10.1016/j.jconrel.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 152.Holland T.A., Bodde E.W., Cuijpers V.M., Baggett L.S., Tabata Y., Mikos A.G., et al. Degradable hydrogel scaffolds for in vivo delivery of single and dual growth factors in cartilage repair. Osteoarthr Cartil. 2007;15:187–197. doi: 10.1016/j.joca.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 153.Park J.S., Yi S.W., Kim H.J., Kim S.M., Kim J.H., Park K.-H., et al. Construction of PLGA nanoparticles coated with polycistronic SOX5, SOX6, and SOX9 genes for chondrogenesis of human mesenchymal stem cells. JAAM. 2017;9:1361–1372. doi: 10.1021/acsami.6b15354. [DOI] [PubMed] [Google Scholar]
- 154.Lefebvre V., Li P., de Crombrugghe B. A new long form of Sox5 (L-Sox5), Sox6 and Sox9 are coexpressed in chondrogenesis and cooperatively activate the type II collagen gene. EMBO J. 1998;17:5718–5733. doi: 10.1093/emboj/17.19.5718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Im G.I., Kim H.J. Electroporation-mediated gene transfer of SOX trio to enhance chondrogenesis in adipose stem cells. Osteoarthr Cartil. 2011;19:449–457. doi: 10.1016/j.joca.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 156.Goomer R., Deftos L., Terkeltaub R., Maris T., Lee M., Harwood F., et al. High-efficiency non-viral transfection of primary chondrocytes and perichondrial cells for ex-vivo gene therapy to repair articular cartilage defects. Osteoarthr Cartilage. 2001;9:248–256. doi: 10.1053/joca.2000.0382. [DOI] [PubMed] [Google Scholar]
- 157.Cha B.H., Kim J.H., Kang S.W., Do H.J., Jang J.W., Choi Y.R., et al. Cartilage tissue formation from dedifferentiated chondrocytes by codelivery of BMP-2 and SOX-9 genes encoding bicistronic vector. Cell Transplant. 2013;22:1519–1528. doi: 10.3727/096368912X647261. [DOI] [PubMed] [Google Scholar]
- 158.Liao J., Hu N., Zhou N., Lin L., Zhao C., Yi S., et al. Sox9 potentiates BMP2-induced chondrogenic differentiation and inhibits BMP2-induced osteogenic differentiation. PLoS One. 2014;9 doi: 10.1371/journal.pone.0089025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Cucchiarini M., Terwilliger E.F., Kohn D., Madry H. Remodelling of human osteoarthritic cartilage by FGF-2, alone or combined with Sox9 via rAAV gene transfer. J Cell Mol Med. 2009;13:2476–2488. doi: 10.1111/j.1582-4934.2008.00474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Maldonado M., Nam J. The role of changes in extracellular matrix of cartilage in the presence of inflammation on the pathology of osteoarthritis. Biomed Res Int. 2013;2013 doi: 10.1155/2013/284873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ma Z., Song W., He D., Zhang X., He Y., Li H. Smart μ-fiber hydrogels with macro-porous structure for sequentially promoting multiple phases of articular cartilage regeneration. Adv Funct Mater. 2022 JAFM. [Google Scholar]
- 162.Naghizadeh Z., Karkhaneh A., Nokhbatolfoghahaei H., Farzad-Mohajeri S., Rezai-Rad M., Dehghan M.M., et al. Cartilage regeneration with dual-drug-releasing injectable hydrogel/microparticle system: in vitro and in vivo study. J Cell Physiol. 2021;236:2194–2204. doi: 10.1002/jcp.30006. [DOI] [PubMed] [Google Scholar]
- 163.Barton K.I., Heard B.J., Sevick J.L., Martin C.R., Shekarforoush S.M.M., Chung M., et al. Posttraumatic osteoarthritis development and progression in an ovine model of partial anterior cruciate ligament transection and effect of repeated intra-articular methylprednisolone acetate injections on early disease. Am J Sports Med. 2018;46:1596–1605. doi: 10.1177/0363546518765098. [DOI] [PubMed] [Google Scholar]
- 164.Tobar-Grande B., Godoy R., Bustos P., von Plessing C., Fattal E., Tsapis N., et al. Development of biodegradable methylprednisolone microparticles for treatment of articular pathology using a spray-drying technique. Int J Nanomed. 2013;8:2065–2076. doi: 10.2147/IJN.S39327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Pei M., He F., Wei L., Rawson A. Melatonin enhances cartilage matrix synthesis by porcine articular chondrocytes. J Pineal Res. 2009;46:181–187. doi: 10.1111/j.1600-079X.2008.00646.x. [DOI] [PubMed] [Google Scholar]
- 166.Gao B., Gao W., Wu Z., Zhou T., Qiu X., Wang X., et al. Melatonin rescued interleukin 1β-impaired chondrogenesis of human mesenchymal stem cells. Stem Cell Res Ther. 2018;9:162. doi: 10.1186/s13287-018-0892-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Asgari N., Bagheri F., Eslaminejad M.B., Ghanian M.H., Sayahpour F.A., Ghafari A.M. Dual functional construct containing kartogenin releasing microtissues and curcumin for cartilage regeneration. Stem Cell Res Ther. 2020;11:289. doi: 10.1186/s13287-020-01797-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Zhang P., Zhong Z.H., Yu H.T., Liu B. Exogenous expression of IL-1Ra and TGF-β1 promotes in vivo repair in experimental rabbit osteoarthritis. Scand J Rheumatol. 2015;44:404–411. doi: 10.3109/03009742.2015.1009942. [DOI] [PubMed] [Google Scholar]
- 169.Yang Z., Li H., Yuan Z., Fu L., Jiang S., Gao C., et al. Endogenous cell recruitment strategy for articular cartilage regeneration. Acta Biomater. 2020;114:31–52. doi: 10.1016/j.actbio.2020.07.008. [DOI] [PubMed] [Google Scholar]
- 170.Hu X., Wang Y., Tan Y., Wang J., Liu H., Wang Y., et al. A difunctional regeneration scaffold for knee repair based on aptamer-directed cell recruitment. Adv Mater. 2017;29 doi: 10.1002/adma.201605235. [DOI] [PubMed] [Google Scholar]
- 171.Hung K.C., Tseng C.S., Dai L.G., Hsu S.H. Water-based polyurethane 3D printed scaffolds with controlled release function for customized cartilage tissue engineering. Biomater. 2016;83:156–168. doi: 10.1016/j.biomaterials.2016.01.019. [DOI] [PubMed] [Google Scholar]
- 172.Yu Y., Brouillette M.J., Seol D., Zheng H., Buckwalter J.A., Martin J.A. Use of recombinant human stromal cell-derived factor 1α-loaded fibrin/hyaluronic acid hydrogel networks to achieve functional repair of full-thickness bovine articular cartilage via homing of chondrogenic progenitor cells. Arthritis Rheumatol. 2015;67:1274–1285. doi: 10.1002/art.39049. [DOI] [PubMed] [Google Scholar]
- 173.Zhang F., Leong W., Su K., Fang Y., Wang D.A. A transduced living hyaline cartilage graft releasing transgenic stromal cell-derived factor-1 inducing endogenous stem cell homing in vivo. Tissue Eng Part A. 2013;19:1091–1099. doi: 10.1089/ten.tea.2012.0441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Sukegawa A., Iwasaki N., Kasahara Y., Onodera T., Igarashi T., Minami A. Repair of rabbit osteochondral defects by an acellular technique with an ultrapurified alginate gel containing stromal cell-derived factor-1. Tissue Eng Part A. 2012;18:934–945. doi: 10.1089/ten.TEA.2011.0380. [DOI] [PubMed] [Google Scholar]
- 175.Lee C., Liu Q.H., Tomkowicz B., Yi Y., Freedman B.D., Collman R.G. Macrophage activation through CCR5- and CXCR4-mediated gp120-elicited signaling pathways. J Leukoc Biol. 2003;74:676–682. doi: 10.1189/jlb.0503206. [DOI] [PubMed] [Google Scholar]
- 176.Richter W. Mesenchymal stem cells and cartilage in situ regeneration. J Intern Med. 2009;266:390–405. doi: 10.1111/j.1365-2796.2009.02153.x. [DOI] [PubMed] [Google Scholar]
- 177.Zhang W., Chen J., Zhang S., Ouyang H.W. Inhibitory function of parathyroid hormone-related protein on chondrocyte hypertrophy: the implication for articular cartilage repair. Arthritis Res Ther. 2012;14:221. doi: 10.1186/ar4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Xu J., Li J., Lin S., Wu T., Huang H., Zhang K., et al. Nanocarrier-mediated codelivery of small molecular drugs and siRNA to enhance chondrogenic differentiation and suppress hypertrophy of human mesenchymal stem cells. Adv Funct Mater. 2016;26:2463–2472. [Google Scholar]
- 179.Murphy M.P., Koepke L.S., Lopez M.T., Tong X., Ambrosi T.H., Gulati G.S., et al. Articular cartilage regeneration by activated skeletal stem cells. Nat Med. 2020;26:1583–1592. doi: 10.1038/s41591-020-1013-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Chang M.C., Chiang P.F., Kuo Y.J., Peng C.L., Chen K.Y., Chiang Y.C. Hyaluronan-loaded liposomal dexamethasone–diclofenac nanoparticles for local osteoarthritis treatment. Int J Mol Sci. 2021;22:665. doi: 10.3390/ijms22020665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Meng J., Li L.J.M. The efficiency and safety of dexamethasone for pain control in total joint arthroplasty: a meta-analysis of randomized controlled trials. Medicine. 2017;96 doi: 10.1097/MD.0000000000007126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Polderman J., Farhang-Razi V., Van Dieren S., Kranke P., DeVries J., Hollmann M., et al. Adverse side-effects of dexamethasone in surgical patients—an abridged cochrane systematic review. Anaesthesia. 2019;74:929–939. doi: 10.1111/anae.14610. [DOI] [PubMed] [Google Scholar]
- 183.Tieppo Francio V., Davani S., Towery C., Brown T.L. Oral versus topical diclofenac sodium in the treatment of osteoarthritis. J Pain Palliat Care. 2017;31:113–120. doi: 10.1080/15360288.2017.1301616. [DOI] [PubMed] [Google Scholar]
- 184.Chen X., Liu Y., Wen Y., Yu Q., Liu J., Zhao Y., et al. A photothermal-triggered nitric oxide nanogenerator combined with siRNA for precise therapy of osteoarthritis by suppressing macrophage inflammation. Nanoscale. 2019;11:6693–6709. doi: 10.1039/c8nr10013f. [DOI] [PubMed] [Google Scholar]
- 185.Liu Q., Wu Z., Hu D., Zhang L., Wang L., Liu G. Low dose of indomethacin and Hedgehog signaling inhibitor administration synergistically attenuates cartilage damage in osteoarthritis by controlling chondrocytes pyroptosis. Gene. 2019;712 doi: 10.1016/j.gene.2019.143959. [DOI] [PubMed] [Google Scholar]
- 186.Duarte J.H. Osteoarthritis: autophagy prevents age-related OA. Nat Rev Rheumatol. 2015;11:683. doi: 10.1038/nrrheum.2015.145. [DOI] [PubMed] [Google Scholar]
- 187.Gugjoo M.B., Abdelbaset-Ismail A., Aithal H.P., Kinjavdekar P., Pawde A.M., Kumar G.S., et al. Mesenchymal stem cells with IGF-1 and TGF-β1 in laminin gel for osteochondral defects in rabbits. Biomed Pharmacother. 2017;93:1165–1174. doi: 10.1016/j.biopha.2017.07.032. [DOI] [PubMed] [Google Scholar]
- 188.Garcia-Ruiz A., Sanchez-Dominguez C.N., Moncada-Saucedo N.K., Perez-Silos V., Lara-Arias J., Marino-Martinez I.A., et al. Sequential growth factor exposure of human Ad-MSCs improves chondrogenic differentiation in an osteochondral biphasic implant. Exp Ther Med. 2021;22:1282. doi: 10.3892/etm.2021.10717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Chen X., Zhang L., Shao X., Gong W., Shi T., Dong J., et al. Specific clearance of senescent synoviocytes suppresses the development of osteoarthritis based on aptamer-functionalized targeted drug delivery system. Adv Funct Mater. 2022 [Google Scholar]
- 190.Sanchez C., Deberg M.A., Piccardi N., Msika P., Reginster J.Y., Henrotin Y.E. Osteoblasts from the sclerotic subchondral bone downregulate aggrecan but upregulate metalloproteinases expression by chondrocytes. This effect is mimicked by interleukin-6, -1beta and oncostatin M pre-treated non-sclerotic osteoblasts. Osteoarthr Cartil. 2005;13:979–987. doi: 10.1016/j.joca.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 191.Sanchez C., Deberg M.A., Piccardi N., Msika P., Reginster J.Y., Henrotin Y.E. Subchondral bone osteoblasts induce phenotypic changes in human osteoarthritic chondrocytes. Osteoarthr Cartil. 2005;13:988–997. doi: 10.1016/j.joca.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 192.Davis A.J., Smith T.O., Hing C.B., Sofat N. Are bisphosphonates effective in the treatment of osteoarthritis pain? a meta-analysis and systematic review. PLoS One. 2013;8 doi: 10.1371/journal.pone.0072714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Saag K.G. Bisphosphonates for osteoarthritis prevention: "Holy Grail" or not? Ann Rheum Dis. 2008;67:1358–1359. doi: 10.1136/ard.2008.089912. [DOI] [PubMed] [Google Scholar]
- 194.Bingham C.O., 3rd, Buckland-Wright J.C., Garnero P., Cohen S.B., Dougados M., Adami S., et al. Risedronate decreases biochemical markers of cartilage degradation but does not decrease symptoms or slow radiographic progression in patients with medial compartment osteoarthritis of the knee: results of the two-year multinational knee osteoarthritis structural arthritis study. Arthritis Rheum. 2006;54:3494–3507. doi: 10.1002/art.22160. [DOI] [PubMed] [Google Scholar]
- 195.Kadri A., Ea H.K., Bazille C., Hannouche D., Lioté F., Cohen-Solal M.E. Osteoprotegerin inhibits cartilage degradation through an effect on trabecular bone in murine experimental osteoarthritis. Arthritis Rheum. 2008;58:2379–2386. doi: 10.1002/art.23638. [DOI] [PubMed] [Google Scholar]
- 196.Hayami T., Zhuo Y., Wesolowski G.A., Pickarski M., Duong L.T. Inhibition of cathepsin K reduces cartilage degeneration in the anterior cruciate ligament transection rabbit and murine models of osteoarthritis. Bone. 2012;50:1250–1259. doi: 10.1016/j.bone.2012.03.025. [DOI] [PubMed] [Google Scholar]
- 197.Bellido M., Lugo L., Roman-Blas J.A., Castañeda S., Calvo E., Largo R., et al. Improving subchondral bone integrity reduces progression of cartilage damage in experimental osteoarthritis preceded by osteoporosis. Osteoarthr Cartil. 2011;19:1228–1236. doi: 10.1016/j.joca.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 198.Zhen G., Wen C., Jia X., Li Y., Crane J.L., Mears S.C., et al. Inhibition of TGF-β signaling in mesenchymal stem cells of subchondral bone attenuates osteoarthritis. Nat Med. 2013;19:704–712. doi: 10.1038/nm.3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Zhen G., Cao X. Targeting TGFβ signaling in subchondral bone and articular cartilage homeostasis. Trends Pharmacol Sci. 2014;35:227–236. doi: 10.1016/j.tips.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.McDermott A.M., Herberg S., Mason D.E., Collins J.M., Pearson H.B., Dawahare J.H., et al. Recapitulating bone development through engineered mesenchymal condensations and mechanical cues for tissue regeneration. Sci Transl Med. 2019;11 doi: 10.1126/scitranslmed.aav7756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Khalafi A., Schmid T.M., Neu C., Reddi A.H. Increased accumulation of superficial zone protein (SZP) in articular cartilage in response to bone morphogenetic protein-7 and growth factors. J Orthop Res. 2007;25:293–303. doi: 10.1002/jor.20329. [DOI] [PubMed] [Google Scholar]
- 202.Dorcemus D.L., Kim H.S., Nukavarapu S.P. Gradient scaffold with spatial growth factor profile for osteochondral interface engineering. Biomed Mater. 2021;16 doi: 10.1088/1748-605X/abd1ba. [DOI] [PubMed] [Google Scholar]
- 203.Qiao Z., Lian M., Han Y., Sun B., Zhang X., Jiang W., et al. Bioinspired stratified electrowritten fiber-reinforced hydrogel constructs with layer-specific induction capacity for functional osteochondral regeneration. Biomaterials. 2021;266 doi: 10.1016/j.biomaterials.2020.120385. [DOI] [PubMed] [Google Scholar]
- 204.Lee J., Lee S., Huh S.J., Kang B.-J., Shin H. Directed regeneration of osteochondral tissue by hierarchical assembly of spatially organized composite spheroids. Adv Sci. 2022;9 doi: 10.1002/advs.202103525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Wang Z., Le H., Wang Y., Liu H., Li Z., Yang X., et al. Instructive cartilage regeneration modalities with advanced therapeutic implantations under abnormal conditions. Bioact Mater. 2021;11:317–338. doi: 10.1016/j.bioactmat.2021.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Luo Z., Che J., Sun L., Yang L., Zu Y., Wang H., et al. Microfluidic electrospray photo-crosslinkable κ-Carrageenan microparticles for wound healing. Eng Regener. 2021;2:257–262. [Google Scholar]
- 207.Yu Y., Wang Q., Wang C., Shang L. Living materials for regenerative medicine. Eng Regener. 2021;2:96–104. [Google Scholar]
- 208.Yu K., Yu X., Cao S., Wang Y., Zhai Y., Yang F., et al. Layered dissolving microneedles as a need-based delivery system to simultaneously alleviate skin and joint lesions in psoriatic arthritis. Acta Pharm Sin B. 2021;11:505–519. doi: 10.1016/j.apsb.2020.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Cai L., Xu D., Chen H., Wang L., Zhao Y. Designing bioactive micro-/nanomotors for engineered regeneration. Eng Regener. 2021;2:109–115. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.