Abstract
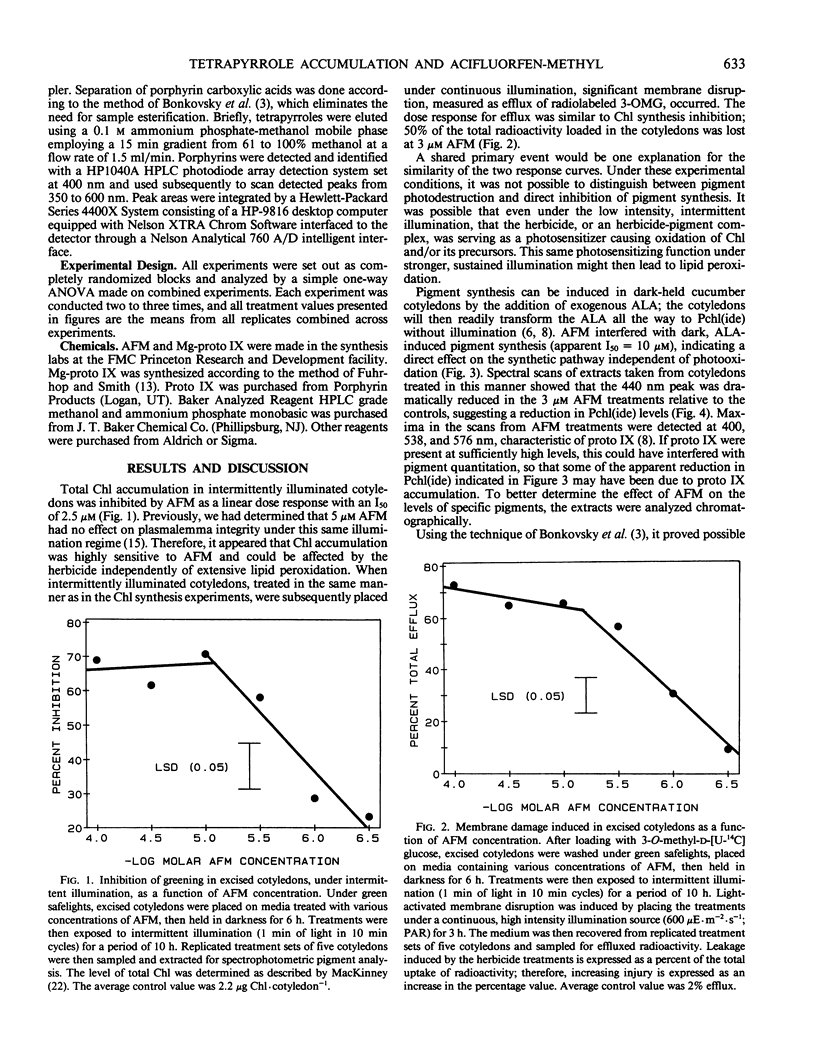
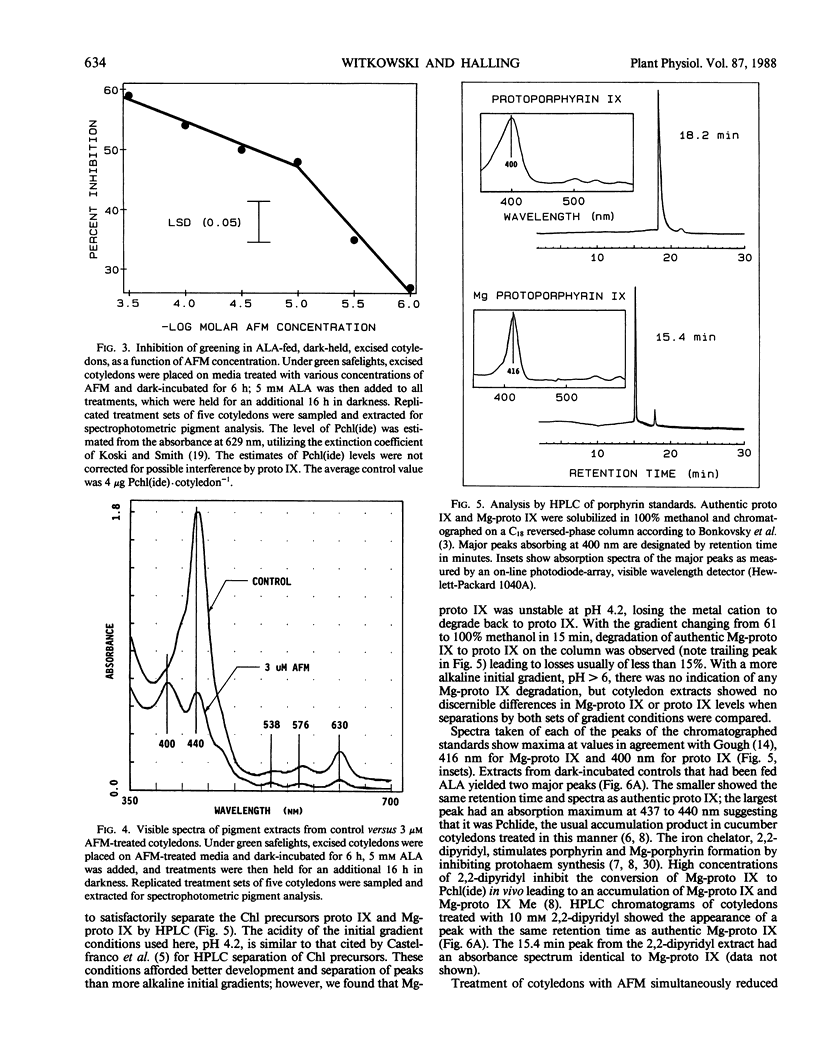
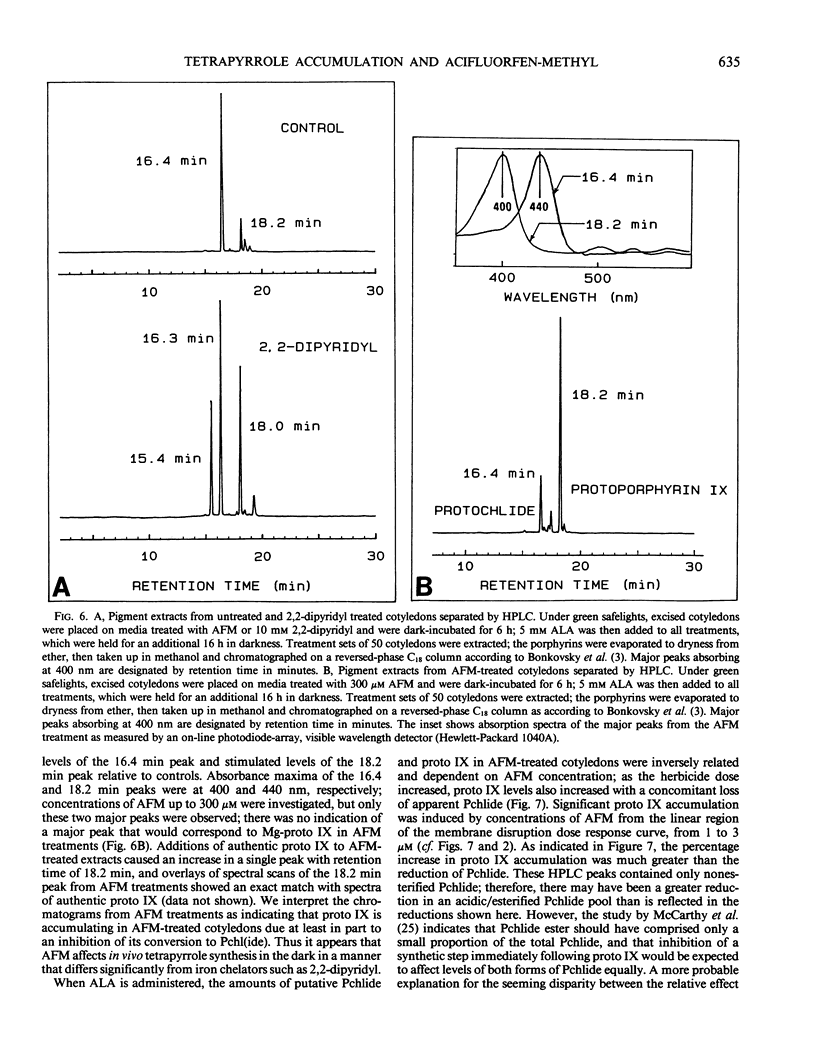
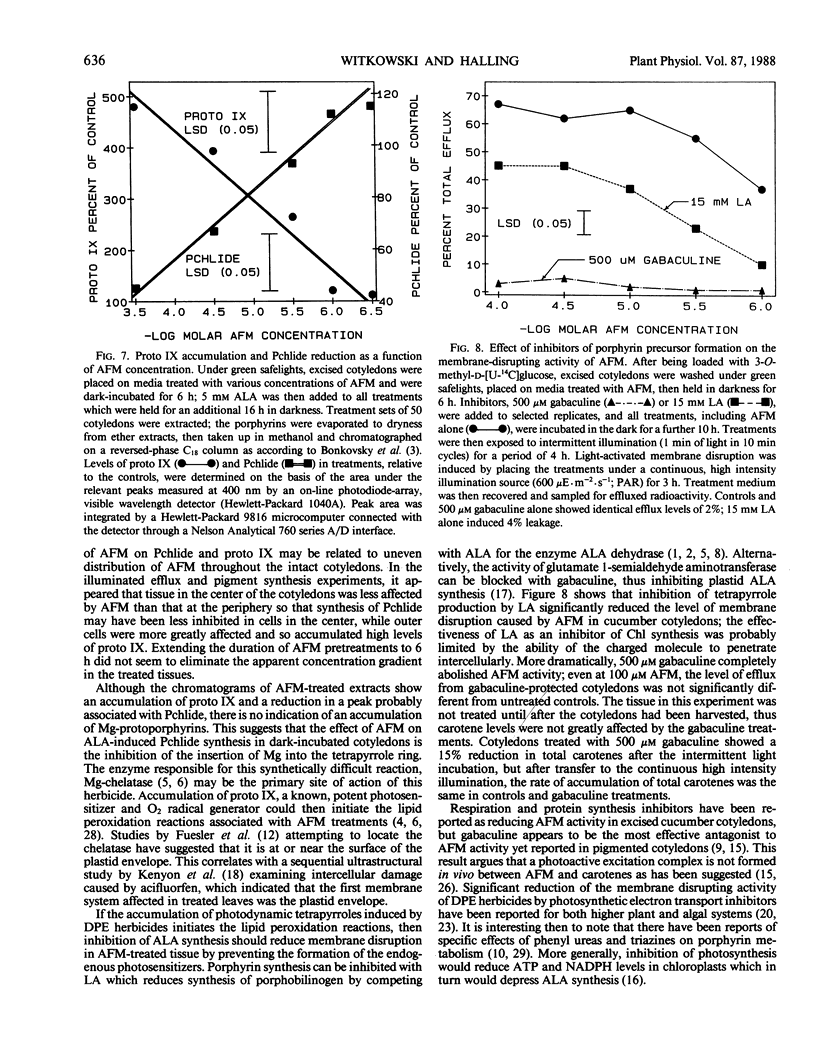
Treatment with acifluorfen-methyl (AFM), methyl 5-(2-chloro-4-[tri-fluoromethyl] phenoxy)-2-nitrobenzoate, inhibited protochlorophyllide synthesis in dark-held, δ-amino levulinic acid-fed, excised cotyledons of cucumber (Cucumis sativus L.). Protochlorophyllide and protoporphyrin IX levels in AFM-treated cotyledons were inversely related and dependent on AFM concentration; as the herbicide dose increased, protoporphyrin IX levels also increased with a concomitant loss of protochlorophyllide. Significant protoporphyrin IX accumulation was induced by concentrations of AFM from the linear region of the membrane disruption dose response curve. The pattern of precursor accumulation seen in HPLC chromatograms from extracts of AFM-treated tissue indicate that Mg insertion into the tetrapyrrole ring is inhibited, suggesting interference with Mg-chelatase. An inhibitor of δ-amino levulinic acid synthesis, gabaculine (3-amino-2,3-dihydrobenzoic acid), completely blocked the membrane disruption activity of AFM in illuminated cotyledons. Protoporphyrin IX accumulating in AFM-treated tissues may serve as the primary photosensitizer for initiating lipid peroxidation.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beale S. I., Castelfranco P. A. The Biosynthesis of delta-Aminolevulinic Acid in Higher Plants: I. Accumulation of delta-Aminolevulinic Acid in Greening Plant Tissues. Plant Physiol. 1974 Feb;53(2):291–296. doi: 10.1104/pp.53.2.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beale S. I. Studies on the Biosynthesis and Metabolism of delta-Aminolevulinic Acid in Chlorella. Plant Physiol. 1971 Sep;48(3):316–319. doi: 10.1104/pp.48.3.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonkovsky H. L., Wood S. G., Howell S. K., Sinclair P. R., Lincoln B., Healey J. F., Sinclair J. F. High-performance liquid chromatographic separation and quantitation of tetrapyrroles from biological materials. Anal Biochem. 1986 May 15;155(1):56–64. doi: 10.1016/0003-2697(86)90224-1. [DOI] [PubMed] [Google Scholar]
- Carlson R. E., Sivasothy R., Dolphin D., Bernstein M., Shivji A. An internal standard for porphyrin analysis. Anal Biochem. 1984 Aug 1;140(2):360–365. doi: 10.1016/0003-2697(84)90178-7. [DOI] [PubMed] [Google Scholar]
- Castelfranco P. A., Weinstein J. D., Schwarcz S., Pardo A. D., Wezelman B. E. The Mg insertion step in chlorophyll biosynthesis. Arch Biochem Biophys. 1979 Feb;192(2):592–598. doi: 10.1016/0003-9861(79)90130-9. [DOI] [PubMed] [Google Scholar]
- Chereskin B. M., Castelfranco P. A. Effects of Iron and Oxygen on Chlorophyll Biosynthesis : II. OBSERVATIONS ON THE BIOSYNTHETIC PATHWAY IN ISOLATED ETIOCHLOROPLASTS. Plant Physiol. 1982 Jan;69(1):112–116. doi: 10.1104/pp.69.1.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duggan J., Gassman M. Induction of porphyrin synthesis in etiolated bean leaves by chelators of iron. Plant Physiol. 1974 Feb;53(2):206–215. doi: 10.1104/pp.53.2.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuesler T. P., Wong Y. S., Castelfranco P. A. Localization of Mg-Chelatase and Mg-Protoporphyrin IX Monomethyl Ester (Oxidative) Cyclase Activities within Isolated, Developing Cucumber Chloroplasts. Plant Physiol. 1984 Jul;75(3):662–664. doi: 10.1104/pp.75.3.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuesler T. P., Wright L. A., Castelfranco P. A. Properties of Magnesium Chelatase in Greening Etioplasts: METAL ION SPECIFICITY AND EFFECT OF SUBSTRATE CONCENTRATIONS. Plant Physiol. 1981 Feb;67(2):246–249. doi: 10.1104/pp.67.2.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gough S. Defective synthesis of porphyrins in barley plastids caused by mutation in nuclear genes. Biochim Biophys Acta. 1972 Nov 24;286(1):36–54. doi: 10.1016/0304-4165(72)90086-4. [DOI] [PubMed] [Google Scholar]
- Halling B. P., Peters G. R. Influence of chloroplast development on the activation of the diphenyl ether herbicide acifluorfen-methyl. Plant Physiol. 1987 Aug;84(4):1114–1120. doi: 10.1104/pp.84.4.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy S. A., Mattheis J. R., Rebeiz C. A. Chloroplast biogenesis: biosynthesis of protochlorophyll(ide) via acidic and fully esterified biosynthetic branches in higher plants. Biochemistry. 1982 Jan 19;21(2):242–247. doi: 10.1021/bi00531a007. [DOI] [PubMed] [Google Scholar]
- Orr G. L., Hess F. D. Mechanism of Action of the Diphenyl Ether Herbicide Acifluorfen-Methyl in Excised Cucumber (Cucumis sativus L.) Cotyledons : LIGHT ACTIVATION AND THE SUBSEQUENT FORMATION OF LIPOPHILIC FREE RADICALS. Plant Physiol. 1982 Feb;69(2):502–507. doi: 10.1104/pp.69.2.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo A. D., Chereskin B. M., Castelfranco P. A., Franceschi V. R., Wezelman B. E. ATP requirement for mg chelatase in developing chloroplasts. Plant Physiol. 1980 May;65(5):956–960. doi: 10.1104/pp.65.5.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlcek L. M., Gassman M. L. Reversal of alpha,alpha'-Dipyridyl-induced Porphyrin Synthesis in Etiolated and Greening Red Kidney Bean Leaves. Plant Physiol. 1979 Sep;64(3):393–397. doi: 10.1104/pp.64.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]


