Abstract
Purpose of review
Warm autoimmune hemolytic anemia (wAIHA) is the most common of the immune hemolytic anemias. Although there are numerous case reports and reviews regarding this condition, some of the unusual and more recent findings have not been fully defined and may be contentious. This review will provide insight into the common specificity of the warm autoantibodies and hypothesize a novel mechanism of wAIHA, that is proposed to be linked to the controversial subject of red blood cell senescence.
Recent findings and hypotheses
It is now well established that band 3 on the red blood cell is the main target of autoantibodies in wAIHA. wAIHA targets the older red blood cells (RBCs) in about 80% of cases and, recently, it has been shown that the RBCs in these patients are aging faster than normal. It has been proposed that in these 80% of patients, that the autoantibody recognizes the senescent red blood cell antigen on band 3. It is further hypothesized that this autoantibody's production and potency has been exacerbated by hypersensitization to the RBC senescent antigen, which is processed through the adaptive immune system to create the pathogenic autoantibody. Recent publications have supported previous data that the senescent RBC antigen is exposed via a dynamic process, wherein oscillation of a band 3 internal loop flipping to the cell surface, creates a conformational neoantigen that is the RBC senescent antigen. It has also recently been shown that the cytokine profile in patients with wAIHA favors production of inflammatory cytokines/chemokines that includes interleukin-8 which can activate neutrophils to increase the oxidative stress on circulating RBCs to induce novel antigens, as has been postulated to favour exposure of the senescent RBC antigen.
Summary
This manuscript reviews new findings and hypotheses regarding wAIHA and proposes a novel mechanism active in most wAIHA patients that is due to an exacerbation of normal RBC senescence.
Keywords: aged RBCs, band 3, senescent red blood cell antigen, Type I wAIHA, warm autoimmune hemolytic anemia
INTRODUCTION
Warm autoimmune hemolytic anemia (wAIHA) is an autoimmune blood disorder whereby a patient produces an autoantibody to their own red blood cells (RBCs). It occurs at a rate of about 1 to 3 adults per 100,000 and can be idiopathic, without any known mechanism, or secondary to certain cancers, such as chronic lymphocytic leukemia, or drug administration, such as α-methyldopa or cefotetan [1,2▪▪,3▪,4]. More recently, wAIHA has been associated with use of checkpoint inhibitor therapies, such as anti-PD1 for treatment of certain cancers [5]. wAIHA is characterized by an acquired hemolytic anemia, sometimes life-threatening, a positive direct antiglobulin test (DAT) with gamma immunoglobulin (IgG), complement (C3d/g) or both [1]. The patient's eluate and serum contain antibodies to all RBCs without apparent subpopulation specificity. Historically, these autoantibodies have been associated with the Rh blood group system as they often do not react with rare Rhnull RBCs that lack Rh antigens [1]; although, there have been isolated reports of specific or mimicking antibodies to specific RBC antigens, such as D, Jka, E, Ge, Wr, that appear to resemble alloantibodies [1, see below].
Box 1.
no caption available
SPECIFICITY OF AUTOANTIBODIES
Antibodies from patients with wAIHA react serologically with all RBCs that are tested and, historically, the autoantibody(ies) have been considered to have a wide variety of target specificities [1]. Early investigators determined that many of these autoantibodies failed to react with rare RBCs that lack Rh blood group antigens, such as Rhnull or, so-called “dash-D-dash” (-D-) RBCs [1]. Thus, warm autoantibodies were originally considered reacting with Rh antigens. Over the years, however, other reports of additional specificities for warm autoantibodies began to emerge [6–8], including Jka[1,9,10], Kell [1], Gerbich [11], Wright [12], glycophorin A [1,13–15], and the D and Lansteiner-Wiener (LW) antigens [1]. As more studies were undertaken to obtain a better understanding of the specificities of warm autoantibodies, it became apparent that band 3 on RBCs was involved and possibly a main target of these autoantibodies [13–15]. Subsequent publications began to support the idea that band 3 was a major target of warm autoantibodies [16–19]. It is now generally acknowledged that the main specificity of warm autoantibodies is to components of band 3 [19]. It is important to note that glycophorin A and Rh blood group antigens are linked to band 3, and that the Wright antigen lies on band 3 [20]. Loss of Rh antigens in Rhnull and/or -D- RBCs is known to have a conformational affect on the RBC membrane, resulting in an associated anemia. Thus, previous reports of warm autoantibody specificity to Rh antigens using Rhnull RBCs may have been due to an altered band 3 so that warm autoantibodies recognizing band 3 epitopes, particularly if conformational epitopes, failed to do so, giving the impression that the autoantibodies were directed to Rh rather than band 3.
BAND 3
Band 3 (AE1/SLC4A1) is a transmembrane anion exchange protein [21,22]. It works to exchange bicarbonate and chloride and is the most highly expressed RBC membrane protein. It functions in both structural and physiological capacities [22]. Band 3 provides cell mechanical support through its physical linkage to ankyrin and the cytoskeletal network [22–25]. Band 3's interaction with multiple proteins, including Rh, and in two specific complexes contributes to cellular morphology, conservation of cellular organization, and mechanical integrity [15,22–25]. Any changes in band 3's protein-protein interactions, such as Rh, could affect the band 3 ability to adopt some conformational states, including the autoantigen recognized by warm autoantibodies. Moreover, band 3 has been postulated to be a protein that can expose a neoantigen that becomes the senescent RBC antigen [26,27]; thus, band 3 is hypothesized to be responsible for RBC senescence [26,27,28▪▪]. However, this remains controversial (see below).
WARM AUTOANTIBODIES PREFERENCIALLY REACT WITH OLD RED BLOOD CELLS
In the 1980s, two different groups independently reported within one month of each other, on warm autoantibodies showing an association with the age of the RBCs [29,30]. Both groups reported that a significant proportion of the activity of warm autoantibodies from wAIHA patients reacted preferentially with older RBCs. Gray et al.[29] found that 4/5 (80%) of autoantibodies eluted from the RBCs of patients with wAIHA reacted weaker with reticulocyte-enriched compared to mature RBCs. They attributed this differential reactivity to the Rh system as they found that reticulocytes had less reactivity with anti-D than mature RBCs. Branch et al.[30] using a density gradient formulated to effectively separate reticulocytes from mature RBCs [30,31] found, in performing DATs on 24 patients with wAIHA, that 79% of patients had much less IgG on their autologous reticulocytes than on their mature, older RBCs. In 37% of patients, the DAT was negative in the reticulocyte-enriched fraction. They also showed that this differential reactivity was not due to Rh as they tested a number of different Rh antibodies on both the age-fractionated reticulocytes and older RBCs using normal healthy blood and found no differences in reactivity [31]. The autoantibodies that reacted better with reticulocyte-enriched RBCs were termed Type I and the patients as having Type I wAIHA. Those patients showing no difference or even slightly more reactivity with reticulocyte-enriched RBCs, were called Type II and patients as having Type II wAIHA. Other reports have supported these observations [1,19]. Recently, these two specificities of warm autoantibodies were confirmed in 22 patients having wAIHA using discontinuous Percoll gradients to separate reticulocyte-enriched vs. older RBCs [19]. These investigators also showed by Western blot and LC-MS that the warm autoantibodies were reacting with band 3 on the RBCs [19]. It has been suggested by these results that warm autoantibodies in the majority of patients having wAIHA may represent an exacerbation of normal RBC senescence [1,19,30].
RED BLOOD CELL SENESCENCE
Typically, RBCs live for approximately 115 to 120 days with a half-life of approximately 25 days [32,33▪▪]. These senescent RBCs are removed from circulation in the spleen or liver by macrophages; however, the exact mechanism of how these aged RBCs are removed by macrophages remains controversial [33▪▪]. Again, how RBCs die has been a subject of interest for many years and the mechanism remains a contentious topic [32,33▪▪,34▪▪,35▪▪]. In the 1980s, Marguerite Kay proposed that RBC senescence occurs due to band 3 changes in its external conformation during aging to create a neoantigen that is recognized by a naturally present autoantibody to this neoantigen [26,27,36,37]. This hypothesis has been supported by other investigators over the years [38,39]. Indeed, to date, this hypothesis as a mechanism of RBC senescence in humans has not been proven wrong; although, it has yet to be directly shown to be causative of RBC senescence, with a number of other hypotheses proposed [40]. In addition to the naturally present autoantibody to the RBC senescent antigen on band 3, other hypotheses include: (1) loss of cluster of differentiation-47 (CD47) don’t eat me signal, (2) expression of phosphatidylserine (PS) on aged RBCs and (3) loss of sialic acid on aged RBCs, or a combination of these alone or in association with an autoantibody [40].
It is generally accepted that as RBCs age, they accumulate IgG on their membrane. What is not clear as yet is why? Proponents of the autoantibody to senescent RBC antigen hypothesis would argue that this increase in IgG as RBCs age is consistent with the hypothesis, while other proposed mechanisms, such as decreased CD47 or sialic acid, or increased PS exposure, have not been clearly shown to occur. Using animal models to study RBC aging in vivo, Singer et al.[41] showed that in mice, as RBCs age they accumulate IgG. Christian et al.[42] in dogs, using biotinylated autologous RBCs to follow the RBC lifespan, found that as the RBCs reach the limits of their lifespan (126 days) they accumulate greatly increased amounts of either IgG or complement [42]. In contrast, Hudson et al.[43] in mice that were deficient in either IgG or complement, showed that in these antibody/complement deficient animals that the mouse RBCs survived normally [43]; thus, did not support a mechanism of RBC senescent that involved autoantibody and/or complement [39]. The results of Hudson et al.[43] compared to Singer et al.[41] may simply indicate redundant pathways in mice for RBC senescence and/or differences in mouse strains. Mice have many differences in hematology and biochemistry compared to humans, indicating a high rate of age-dependent RBC clearance [44,45], and may not be a good model to study human RBC senescence [34▪▪,46]. Mouse RBC half-life is much shorter than in humans, mice have high retic counts compared to human, and have some nucleated RBCs in their peripheral blood, not found in humans [44–46].
Although animal models are often used to study human conditions, this question of the mechanism of RBC senescence is complicated by immediate removal of RBCs upon the end of their lifespan, making causative conclusions difficult. What is known in humans is consistent with accumulation of autologous IgG on aged RBCs [47]. Again, it isn’t disputed that normal human RBCs accumulate IgG on their RBCs as they age, both in vitro and in vivo[1,36,47–49]. In vivo, support of a naturally present autoantibody to RBCs come from reports using normal human blood and specialized methods for the detection of IgG on the RBC membrane, such as Western immunoblotting [1,48]. Also, Franco et al.[49] using biotinylation of human RBCs found increased accumulation of autologous IgG at the end of the RBC lifespan [49]. In vitro aging of human RBCs in autologous plasma also results in accumulation of autologous IgG on the RBCs as they are stored, up to 60 days, allowing the IgG autoantibody to be eluted from the stored RBCs [35▪▪]. What is a problem with all of these studies is that there is no direct evidence that these autoantibodies are causative for the removal of the aged RBCs [34▪▪]. It is of note that the studies by Christian et al.[42] and Franco et al.[49], did not show increased PS exposure as the RBCs aged. Thus, PS exposure as a possible mechanism of RBC senescence is not supported by in vivo data and can be dismissed as a possible mechanism of RBC senescence. These investigators did not interrogate CD47 or sialic acid.
BAND 3 AND THE SENESCENT RED BLOOD CELL ANTIGEN
Studies mostly done by Marguerite Kay in the early 1980s investigating the mechanism of RBC senescence provided the first hypothesis that human RBC senescence was due to a naturally present autoantibody directed to changes in membrane components of band 3 on aging RBCs [26,27,36,37,50–55]. It was shown that the autoantibody binding was due to oxidative stress of the RBCs [56,57] Kay showed that the senescent RBC antigen had a molecular size between 55–65 kDa [37] and was comprised of two parts of the band 3 protein [53,54]. One peptide (peptide 1, aa 538–554) found on the 3rd external loop of band 3 and a second peptide (peptide 2, aa 812–830), which can access intracellular and extracellular environments [28▪▪,54–55] and, when expressed extracellularly, creates the neoantigen recognized by the autoantibody [28▪▪,55]. Furthermore, RBC aging and oxidation may favor formation of this neoantigen by influencing peptide 2's localization [28▪▪,58]. Support that both peptide 1 and peptide 2 are necessary for formation of the conformational RBC senescent antigen originally came from the work of Kay [53,54]. Recently, Badior and Casey [28▪▪] showed that peptide 2 was dynamic in its internal vs. external localization, and that it oscillates between internal and external location on the RBC membrane [28▪▪]. They hypothesized that the natural autoantibody to this conformational antigen would bind when both peptides were externally accessible and, as the peptide 2 was in a transient external state, autoantibody would bind and “hold” the conformational antigen until enough autoantibody accumulated to initiate the phagocytosis of the senescent RBC via activating Fc receptors on macrophages [28▪▪].
TYPE I WARM AUTOIMMUNE HEMOLYTIC ANEMIA ANTIBODY REACTS WITH BAND 3 RED BLOOD CELL SENESCENT ANTIGEN
It has been shown that wAIHA produces two types of warm autoantibodies, Type I that recognize older RBCs compared to reticulocyte-enriched RBCs, and Type II that recognize both younger and older RBCs equally [19,29,30]. Patients with Type I warm autoantibodies have autologous RBCs that appear to be aging faster than normal or Type II patients [19]. This hypothesis is based on the band 3 phosphorylation and discontinuous Percoll gradient patterns of Type I compared to Type II [19]. Type I patients show increased band 3 phosphorylation on lower density RBCs as well as enrichment of older (higher density) RBCs on Percoll gradients while Type II patients show little or no phosphorylation on band 3 and few to no low-density RBCs on Percoll gradients [19]. Band 3 phosphorylation is a hallmark of aged RBCs [59,60]. Investigators have suggested that these results may show that Type I wAIHA represents an exacerbation of normal RBC senescence [1,19,30,49]. Exacerbated aging of human RBCs has been previously reported in vitro due to human erythrocytes exposed to d-galactose [61▪▪]. Whether dysregulation of galactose metabolism [62▪▪] plays a role in the increased in vivo aging in Type I wAIHA requires further investigation.
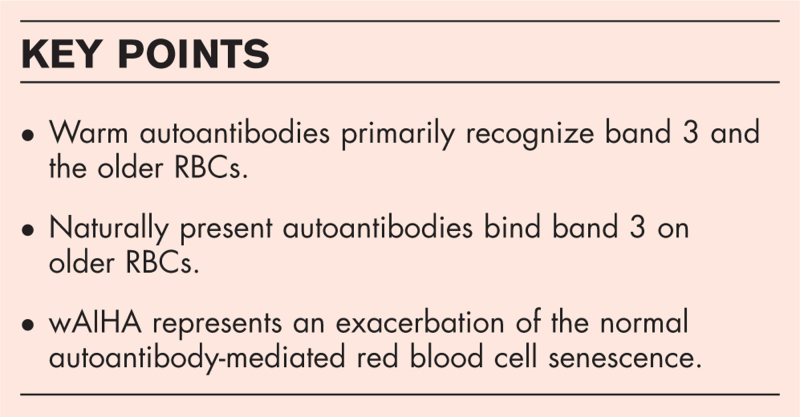
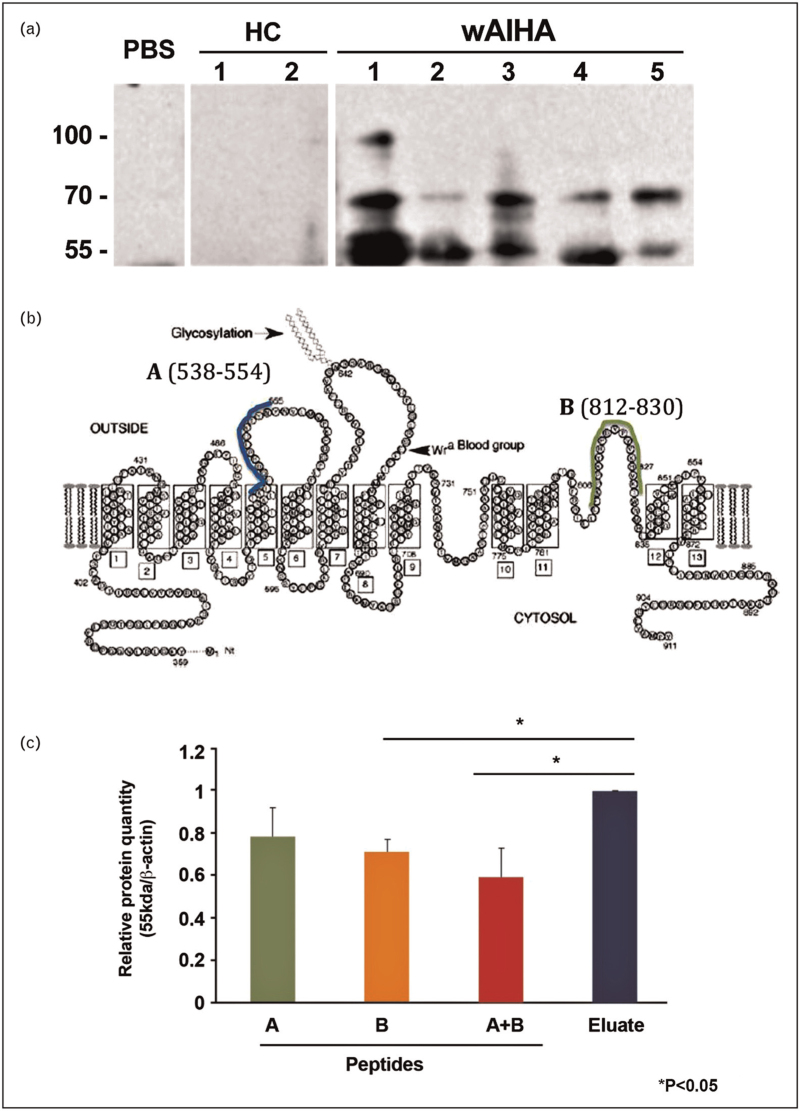
We have unpublished data that shows that eluates from Type I wAIHA patients recognize band 3 in Western immunoblotting and specifically react with a band 3 fragment between 55–65 kDa (Fig. 1A), the molecular size consistent with the RBC senescent antigen proposed by Kay [37]. We have also used synthetic peptides spanning band 3 aa 538–554 and aa 812–830 that when used together can significantly inhibit the Western blot reactivity of an eluate from a patient having Type I wAIHA (Fig. 1B, C). Investigators have suggested that the RBC senescent antigen is comprised of a conformational antigen made up of these two amino acid sequences on band 3 [28▪▪,53,54]. Also, Petz and Garratty [1] reveal in their book, “Immune Hemolytic Anemias” that they were able to inhibit warm autoantibodies that reacted preferentially with aged RBCs (Type I wAIHA) with a synthetic peptide, derived from band 3, that was provided by Dr Marguerite Kay that represented the red blood cell senescent antigen [1]. These results led them to agree with previously published work [30] that Type I wAIHA is the pathogenic result of normal RBC senescence, which would be similar to pathogenic cold agglutinin autoantibodies [1]. Whether Type I wAIHA is a pathogenic version of normal RBC senescence remains to be proven; however, with RBCs perhaps aging faster in these patients, able to present increased amounts of senescent antigen resulting in increased autoantibody response to the increased stimulation, it is possible.
FIGURE 1.
Warm autoantibodies from Type I wAIHA recognize 55–65 kDa band 3 fragment and can be inhibited by peptides proposed to form the senescent RBC antigen. (A) RBC ghosts were lysed and run on 10% sodium dodecyl sulfate polyacrylamide electrophoresis gel. Gel was transferred to nitrocellulose membrane and probed with PBS, eluates from 2 healthy controls (HC) and eluates from five patients with Type I wAIHA. (B) Schematic diagram of band 3 and the two peptides, A and B, proposed to be able to form the senescent RBC antigen (Modified from Zhu Q, Lee D W, Casey J R [69]. (C) Peptides A and B were used to inhibit the warm autoantibody used in the Western blot. Results represent the ratio of the 55 kDa band 3 result compared to beta-actin with either peptide A or B alone or in combination, compared to eluate without any inhibition (Eluate). ∗p < 0.05. HC, healthy control; PBS, phosphate buffered saline; wAIHA, warm autoimmune hemolytic anemia.
CYOTKINE PROFILE IN WARM AUTOIMMUNE HEMOLYTIC ANEMIA
A recent publication has examined the cytokine/chemokine profile in 54 patients having wAIHA [63▪▪]. These investigators confirmed previous reports of increases in production of TNFα and IL-10. Of note was their novel findings of two cytokine/chemokines increased in their study cohort. Both interleukin-8 (IL-8/CXCL8) and interferon gamma-inducible protein (IP10/CXCL10) were significantly increased. Both, along with TNFα, are biomarkers of inflammation. What is of interest in relationship to this review is that IL-8/CXCL8 can recruit and activate neutrophils. Activated neutrophils can produce reactive oxygen species (ROS), such as O2-, H2O2 or OH-, which could result in RBC oxidative stress and increased RBC aging [64▪▪,65▪▪,66,67]. Excessive ROS production may affect membrane lipids, integrins and cytoplasmic proteins in various circulating cells, including RBCs [66,67]. These effects are particularly critical for RBCs, which may become dysfunctional. First, excess ROS can cause oxidation of polyunsaturated fatty acids in the RBC membrane, bringing about a profound modification of the membrane lipids’ lateral and transversal distribution and organization at the nanoscale level [63▪▪,64▪▪,65▪▪,66]. This may contribute to band 3 oxidative stress resulting in increased display of senescent RBC antigen and help to explain the increased aging of RBCs in wAIHA. Indeed, Iuchi et al.[67] have reported an increase in autoimmune hemolytic anemia in NZB mice under conditions of oxidative stress, and suggested that oxidation-mediated RBC autoantibody production is a direct result of ROS-mediated oxidative stress. This observation is consistent with the hypothesis that human RBCs under oxidative stress, which results in increased aging and presentation of conformational senescent RBC neoantigen, results in faster aging of the RBCs and development of Type I wAIHA.
CONCLUSION
The published findings implicating a naturally occurring autoantibody directed to a conformational antigen on band 3 that is responsible for red blood cell senescence is substantial and compelling. However, for the most part it is circumstantial and not as yet shown to be causative. It is a conundrum that is difficult to figure out how to overcome. If autoantibody is responsible for removal, one can conceive of circulating RBCs accumulating the autoantibody recognizing band 3-associated senescent antigen, reaching a threshold of opsonization, and being removed from the circulation [28▪▪]. There is no dispute that either in vitro or in vivo aged RBCs accumulate IgG autoantibody, while evidence so far shows that PS is not increased as RBCs age and no evidence for CD47 or sialic acid involvement. This leaves the hypothesis that RBC senescence is most likely due to autoantibody recognizing a neoantigen on the aged RBCs. However, in vitro density gradients would not provide aged RBCs that present the maximum senescent antigen as those RBCs would have already been removed after reaching a threshold of antibody sensitization as previously described [28▪▪]. In vivo models are required to settle this controversy. Biotinylation of human RBCs has proven that the oldest RBCs have the most IgG detectable on the cells but do not provide proof that removal is antibody-mediated via Fc receptors on macrophages. Perhaps using biotinylation of dog RBCs as previously reported [42], that have a similar lifespan, 116–125 days, as human RBCs could be isolated and used in in vitro phagocytosis assays to determine if monocyte-macrophages can recognize these opsonized cells and phagocytose them. This testing could also be done in humans with autologous enriched for the oldest IgG-opsonized RBCs. Type I wAIHA may represent a highly potent version of the normal autoantibody to senescent RBC antigen but this is currently speculation. It is known that Type I wAIHA autoantibody results in phagocytosis, both in vitro and in vivo[68]. Perhaps if Type I warm autoantibodies could be used to isolate and purify the antigen recognized and this be shown to be similar to peptides 1 and 2 reported by Kay [54] and Badior and Casey [28▪▪], this would be helpful. Until more studies can be done in humans, the controversy over how RBCs die will continue.
Acknowledgements
Thanks to Katherine Badior and Marguerite Kay for review of the manuscript and critical discussion.
Financial support and sponsorship
None.
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1.Petz LD, Garratty G. Immune hemolytic anemias. 2nd edPhiladelphia, PA: Churchill Livingstone; 2004. [Google Scholar]
- 2▪▪.Barcellini W, Fattizzo B. How I treat warm autoimmune hemolytic anemia. Blood 2021; 137:1283–1294. [DOI] [PubMed] [Google Scholar]; A current review of warm autoimmune hemolytic anemia and the various treatment modalities.
- 3▪.National Organization for Rare Disorders. Warm Autoimmune Hemolytic Anemia. Accessed November 3, 2021. https://rarediseases.org/rarediseases/warm-autoimmune-hemolytic-anemia/. [Google Scholar]; This site contains the most up to date information on wAIHA incidence.
- 4.Kalfa TA. Warm antibody autoimmune hemolytic anemia. Hematol Am Soc Hematol Educ Program 2016; 1:690–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doley GEPB, Munker R. Autoimmune hemolytic anemia associated with the use of immune checkpoint inhibitors for cancer: 68 cases from the Food and Drug Administration database and review. Eur J Haematol 2019; 102:157–162. [DOI] [PubMed] [Google Scholar]
- 6.Barker RN, Casswell KM, Reid ME, et al. Identification of autoantigens in autoimmune haemolytic anaemia by a nonradioisotope immunoprecipitation method. Br J Haematol 1992; 82:126–132. [DOI] [PubMed] [Google Scholar]
- 7.Vos GH, Petz L, Fudenberg HH. Specificity of acquired haemolytic anaemia autoantibodies and their serological characteristics. Br J Haematol 1970; 19:57–66. [DOI] [PubMed] [Google Scholar]
- 8.Weiner W, Vos GH. Serology of acquired hemolytic anemias. Blood 1963; 22:606–613. [PubMed] [Google Scholar]
- 9.Ciaffoni S, Ferro I, Potenza R, Campo G. Evans’ syndrome: a case of autoimmune thrombocytopenia and autoimmune haemolytic anemia caused by anti-Jka. Haematologica 1987; 72:245–247. [PubMed] [Google Scholar]
- 10.Guastafierro S, Sessa F, Cuomo C, Tirelli A. Delayed type transfusion reaction due to anti-S in patient with anti-Jka autoantibody and multiple alloantibodies. Ann Hematol 2004; 83:307–308. [DOI] [PubMed] [Google Scholar]
- 11.Shulman IA, Vengelen-Tyler V, Thompson JC, et al. Autoanti-Ge associated with severe autoimmune hemolytic anemia. Vox Sang 1990; 59:232–234. [DOI] [PubMed] [Google Scholar]
- 12.Issitt PD, Pavone BG, Goldfinger D, et al. Anti-Wrb, and other autoantibodies responsible for positive direct antiglobulin tests in 150 individuals. Br J Haematol 1976; 34:5–18. [DOI] [PubMed] [Google Scholar]
- 13.Garratty G, Arndt P, Domen R, et al. Severe autoimmune hemolytic anemia associated with IgM warm autoantibodies directed against determinants on or associated with glycophorin A. Vox Sang 1997; 72:124–130. [DOI] [PubMed] [Google Scholar]
- 14.Victoria EJ, Pierce SW, Branks MJ, et al. IgG red blood cell autoantibodies in autoimmune hemolytic anemia bind to epitopes on red blood cell membrane band 3 glycoprotein. J Lab Clin Med 1990; 115:74–99. [PubMed] [Google Scholar]
- 15.Leddy JP, Wilkinson SL, Kissel GE, et al. Erythrocyte membrane proteins reactive with IgG (warm-reacting) antired blood cell autoantibodies: II. Antibodies coprecipitating band 3 and glycophorin A. Blood 1994; 84:650–656. [PubMed] [Google Scholar]
- 16.Janvier D, Lam Y, Elakredar L, et al. A major target of warm immunoglobulin G autoantibodies: the third external loop of band 3. Transfusion 2013; 53:1948–1955. [DOI] [PubMed] [Google Scholar]
- 17.Salama A, Janvier D, Mayer B, et al. Lethal autoimmune hemagglutination due to an immunoglobulin A autoagglutinin with band 3 specificity. Transfusion 2014; 54:1988–1995. [DOI] [PubMed] [Google Scholar]
- 18.Chadebech P, Loustau V, Janvier D, et al. Clinical severity in adult warm autoimmune hemolytic anemia and its relationship to antibody specificity. Haematologica 2017; 103:e35–e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bloch EM, Branch HA, Sakac D, et al. Differential red blood cell age fractionation and Band 3 phosphorylation distinguish two different subtypes of warm autoimmune hemolytic anemia. Transfusion 2020; 60:1856–1866. [DOI] [PubMed] [Google Scholar]
- 20.Bruce LJ, Ring SM, Anstee DJ, et al. Changes in the blood group Wright antigens are associated with a mutation at amino acid 658 in human erythrocyte band 3: a site of interaction between band 3 and glycophorin A under certain conditions. Blood 1995; 85:541–547. [PubMed] [Google Scholar]
- 21.Reithmeier RA, Casey JR, Kalli AC, et al. Band 3, the human red cell chloride/bicarbonate anion exchanger (AE1, SLC4A1), in a structural context. Biochem Biophys Acta 2016; 1858:1507–1532. [DOI] [PubMed] [Google Scholar]
- 22.Tanner MJ. The structure and function of band 3 (AE1): recent developments. Mol Membr Biol 1997; 14:155–165. [DOI] [PubMed] [Google Scholar]
- 23.Low PS, Willardson BM, Mohandas N, et al. Contribution of the band 3-ankyrin interaction to erythrocyte membrane mechanical stability. Blood 1991; 77:1581–1586. [PubMed] [Google Scholar]
- 24.Bruce L, Beckmann R, Ribeiro M, et al. A band 3-based macrocomplex of integral and peripheral proteins in the RBC membrane. Blood 2003; 101:4180–4188. [DOI] [PubMed] [Google Scholar]
- 25.Salomao M, Zhang X, Yang Y, et al. Protein 4.1R-dependent multiprotein complex: new insights into the structural organization of the red blood cell membrane. Proc Natl Acad Sci USA 2008; 105:8026–8031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kay MM. Aging of cell membrane molecules leads to appearance of an aging antigen and removal of senescent cells. Gerontology 1985; 31:215–235. [DOI] [PubMed] [Google Scholar]
- 27.Kay M. Immunoregulation of cellular life span. Ann N Y Acad Sci 2005; 1057:85–111. [DOI] [PubMed] [Google Scholar]
- 28▪▪.Badior KE, Casey JR. Large conformational dynamics in band 3 protein: significance for erythrocyte senescence signalling. Biochim Biophys Acta Biomembr 2021; 1863:183678. [DOI] [PubMed] [Google Scholar]; Paper describing the mechanism of formation of the senescent RBC antigen from band 3 and how naturally expressed autoantibody interacts with this antigen.
- 29.Gray LS, Kleeman JE, Masouredis SP. Differential binding of IgG anti-D and IgG autoantibodies to reticulocytes and red blood cells. Br J Haematol 1983; 55:335–345. [DOI] [PubMed] [Google Scholar]
- 30.Branch DR, Shulman IA, Sy Siok Hian AL, Petz LD. Two distinct categories of warm autoantibody reactivity with age-fractionated red cells. Blood 1984; 63:177–180. [PubMed] [Google Scholar]
- 31.Branch DR, Sy Siok Hian AL, Carlson F, et al. Erythrocyte age-fractionation using a Percoll-Renografin density gradient: application to autologous red cell antigen determinations in recently transfused patients. Am J Clin Pathol 1983; 80:453–458. [DOI] [PubMed] [Google Scholar]
- 32.Arias CF, Arias CF. How do red blood cells know when to die? R Soc Open Sci 2017; 4:160850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33▪▪.Thiagarajan P, Parker CJ, Prchal JT. How do red blood cells die? Front Physiol 2021; 12:655393. [DOI] [PMC free article] [PubMed] [Google Scholar]; Describes various proposed mechanisms of red blood cell senescence.
- 34▪▪.Zimring JC, Spitalnik SL, Hudson KE. The senescent antigen hypothesis of RBC evanescence: 50 years of correlation without causation. Transfusion 2023; 62:2414–2415. [DOI] [PubMed] [Google Scholar]; Evaluation of the published data on RBC senescence and the pitfalls of this research.
- 35▪▪.Badior KE, Bloch EM, Branch DR. A naturally present autoantibody to senescent red blood cells? Transfusion 2022; 62:1311–1312. [DOI] [PubMed] [Google Scholar]; Results showing autoantibody from normal healthy blood donors is an IgG/IgM antibody that reacts with band 3.
- 36.Kay MM. Isolation of the phagocytosis-inducing IgG-binding antigen on senescent somatic cells. Nature 1981; 289:491–494. [DOI] [PubMed] [Google Scholar]
- 37.Kay MM. The IgG autoantibody binding determinant appearing on senescent membranes residues on a 62000 MW peptide. Acta Biol Med Ger 1981; 40:385–391. [PubMed] [Google Scholar]
- 38.Lutz HU, Stringaro-Wipf G. Senescent red cell-bound IgG is attached to band 3 protein. Biomed Biochim Acta 1983; 42:S117–S121. [PubMed] [Google Scholar]
- 39.Lutz HU, Bussolino F, Flepp R, et al. Naturally occurring antiband-3 antibodies and complement together mediate phagocytosis of oxidatively stressed human erythrocytes. Proc Natl Acad Sci U S A 1987; 84:7368–7372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lutz HU, Bogdanova A. Mechanisms taqging senescent red blood cells for clearance in healthy humans. Front Physiol 2013; 4:387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Singer JA, Jennings LK, Jackson CW, et al. Erythrocyte homeostasis: antibody-mediated recognition of the senescent state by macrophages. Proc Natl Acad Sci USA 1986; 83:5498–5501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Christian JA, Rebar AH, Boon GD, Low PS. Senescence of canine biotinylated erythrocytes: increased autologous immunoglobulin binding occurs on erythrocytes aged in vivo for 104 to 110 days. Blood 1993; 82:3469–3473. [PubMed] [Google Scholar]
- 43.Hudson KE, de Wolski K, Kapp LM, et al. Antibodies to senescent antigen and C3 are not required for normal red blood cell lifespan in a murine model. Front Immunol 2017; 8:1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brown IW, Jr, Eadie GS. An analytical study of in vivo survival of limited populations of animal red blood cells tagged with radioiron. J Gen Physiol 1953; 36:327–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Burwell EL, Brickley BA, Finch CA. Erythrocyte life span in small animals: comparison of two methods employing radioiron. Am J Physiol 1953; 172:718–724. [DOI] [PubMed] [Google Scholar]
- 46.Siegel A, Walton RM. Hematology and biochemistry of small mammals. Ferrets, Rabbits Rodents 2020; 569–582. doi: 10.1016/B978-0-323-48435-0.00039-3. [Google Scholar]
- 47.Kannan R, Yuan J, Low PS. Isolation and partial characterization of antibody- and globin-enriched complexes from membranes of dense human erythrocytes. Biochem J 1991; 278:57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bloch EM, Sakac D, Branch HA, et al. Western immunoblotting as a new tool for investigating direct antiglobulin test-negative autoimmune hemolytic anemias. Transfusion 2015; 66:1529–1537. [DOI] [PubMed] [Google Scholar]
- 49.Franco RS, Puchulu-Campanella ME, Barber LA, et al. Changes in the properties of normal human red blood cells during in vivo aging. Am J Hematol 2013; 88:44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kay MM, Bosman GJ, Johnson GJ, et al. Band-3 polymers and aggregates, and hemoglobin precipitates in red cell aging. Blood Cells 1988; 14:275–295. [PubMed] [Google Scholar]
- 51.Kay MM. Generation of senescent cell antigen on old cells initiates IgG binding to a neoantigen. Cell Mol Biol 1993; 39:131–153. [PubMed] [Google Scholar]
- 52.Kay M. Immunoregulation of cellular life span. Ann NY Acad Sci 2005; 1057:85–111. [DOI] [PubMed] [Google Scholar]
- 53.Kay MM, Lin FB. Molecular mapping of the active site of an aging antigen: senescent cell antigen requires lysine(s) for antigenicity and is located on an anion-binding segment of band 3 membrane transport protein. Gerontology 1990; 36:293–305. [DOI] [PubMed] [Google Scholar]
- 54.Kay MM, Marchalonis JJ. Molecular mapping of the active site of an aging antigen. Adv Exp Med Biol 1991; 307:303–316. [DOI] [PubMed] [Google Scholar]
- 55.Kay MM. Band 3 in aging and neurological disease. Ann N Y Acad Sci 1991; 621:179–204. [DOI] [PubMed] [Google Scholar]
- 56.Ando K, Kikugawa K, Beppu M. Involvement of sialylated poly-N-acetyllactosaminyl sugar chains of band 3 glycoprotein on senescent erythrocytes in antiband 3 autoantibody binding. J Biol Chem 1994; 269:19394–19398. [PubMed] [Google Scholar]
- 57.Beppu M, Ando K, Kikugawa K. Poly-N-acetyllactosaminyl saccharide chains of band 3 as determinants for antiband 3 autoantibody binding to senescent and oxidized erythrocytes. Cell Mol Biol (Noisy-le-grand) 1996; 42:1007–1024. [PubMed] [Google Scholar]
- 58.Badior KE, Casey JR. Molecular mechanism for the red blood cell senescence clock. IUBMB Life 2018; 70:32–40. [DOI] [PubMed] [Google Scholar]
- 59.Pantaleo A, Ferru E, Giribaldi G, et al. Oxidized and poorly glycosylated band 3 is selectively phosphorylated by Syk kinase to form large membrane clusters in normal and G6PD-deficient red blood cells. Biochem J 2009; 418:359–367. [DOI] [PubMed] [Google Scholar]
- 60.Ferru E, Giger K, Pantaleo A, et al. Regulation of membrane-cytoskeletal interactions by tyrosine phosphorylation of erythrocyte band 3. Blood 2011; 117:5998–6006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61▪▪.Remigante A, Spinelli S, Trichilo V, et al. d-galactose induced early aging in human erythrocytes: role of band 3 protein. J Cell Physiol 2022; 237:1586–1596. [DOI] [PMC free article] [PubMed] [Google Scholar]; Reports a possible mechanism for increased aging of human RBCs.
- 62▪▪.Conte F, van Buuringen N, Voermans NC, Lefeber DJ. Galactose in human metabolism, glycosylation and congenital metabolic diseases: time for a closer look. Biochim Biophys Acta 2021; 8:129898. [DOI] [PubMed] [Google Scholar]; Discusses possible sequalae of galactose metabolism in humans.
- 63▪▪.Branch DR, Leger RM, Sakac D, et al. The chemokines IP-10/CXCL10 and IL-8/CXCL8 are potential novel biomarkers of warm autoimmune hemolytic anemia. Blood Adv 2023; 7:2166–2170. [DOI] [PMC free article] [PubMed] [Google Scholar]; Most comprehensive report of cytokines/chemokines found in patients with wAIHA.
- 64▪▪.Remigante A, Morabito R, Marino A. Band 3 protein function and oxidative stress in erythrocytes. J Cell Physiol 2021; 236:6225–6234. [DOI] [PubMed] [Google Scholar]; Interesting data on what oxidative stress does to band 3.
- 65▪▪.Remigante A, Spinelli S, Basile N, et al. Oxidation stress a a mechanism of aging in human erythrocytes: protective effect of quercetin. In J Mol Sci 2022; 23:7781. [DOI] [PMC free article] [PubMed] [Google Scholar]; Another report on the affects of oxidative stress on RBC aging.
- 66.Laforge M, Elbim C, Frere C, et al. Tissue damage from neutrophil-induced oxidative stress in COVID-19. Nat Rev Immunol 2020; 20:515–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Iuchi Y, Kibe N, Tsuinoda S, et al. Implication of oxidative stress as a cause of autoimmune hemolytic anemia in NZB mice. Free Radi Biol Med 2010; 48:935–944. [DOI] [PubMed] [Google Scholar]
- 68.Gallaher MT, Branch DR, Mison A, Petz LD. Evaluation of reticuloendothelial function in autoimmune hemolytic anemia using an in vitro assay of monocyte-macrophage interaction with erythrocytes. Exp Hematol 1983; 11:82–89. [PubMed] [Google Scholar]
- 69.Zhu Q, Lee DW, Casey JR. Novel topology in C-terminal region of the human plasma membrane anion exchanger, AE1. J Biol Chem 2003; 278:3112–3120. [DOI] [PubMed] [Google Scholar]