Abstract
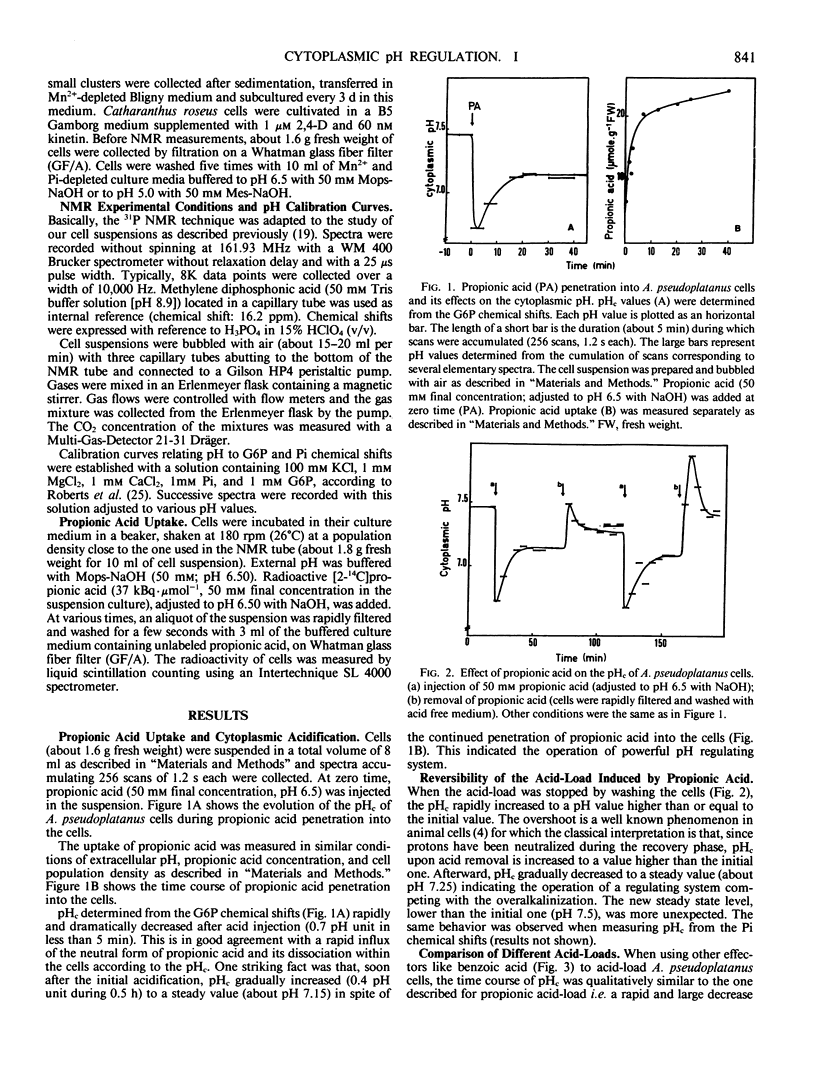
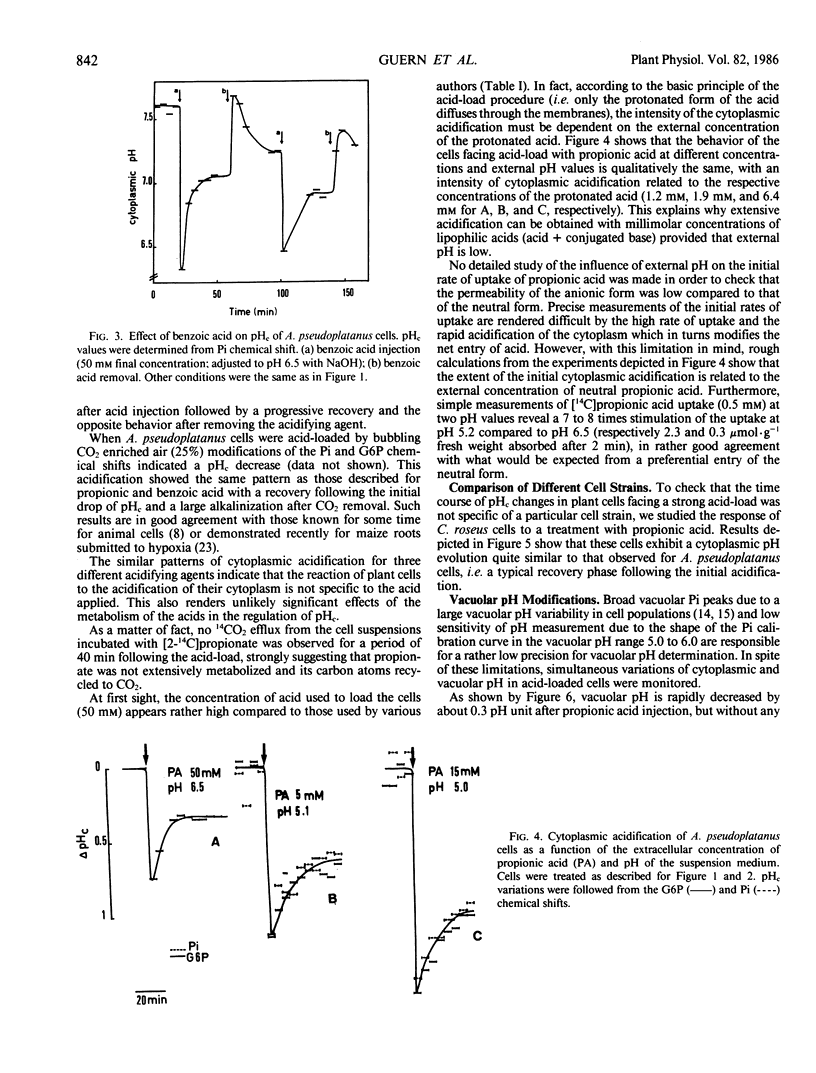
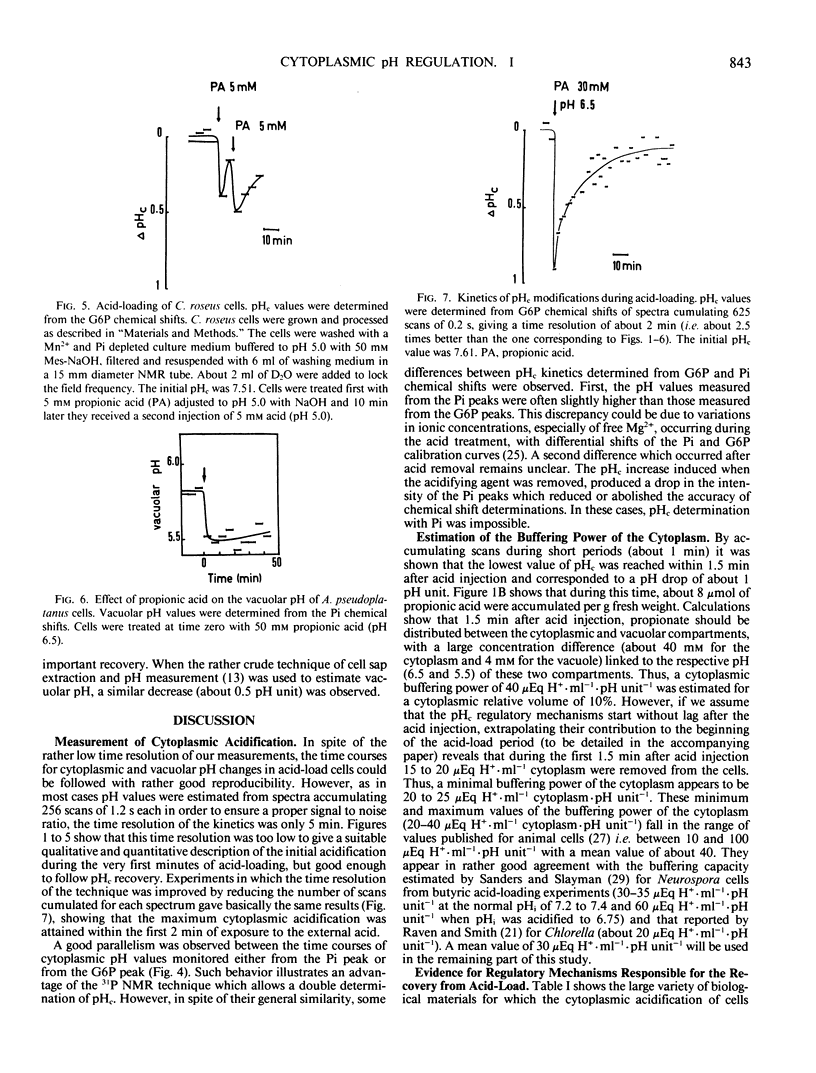
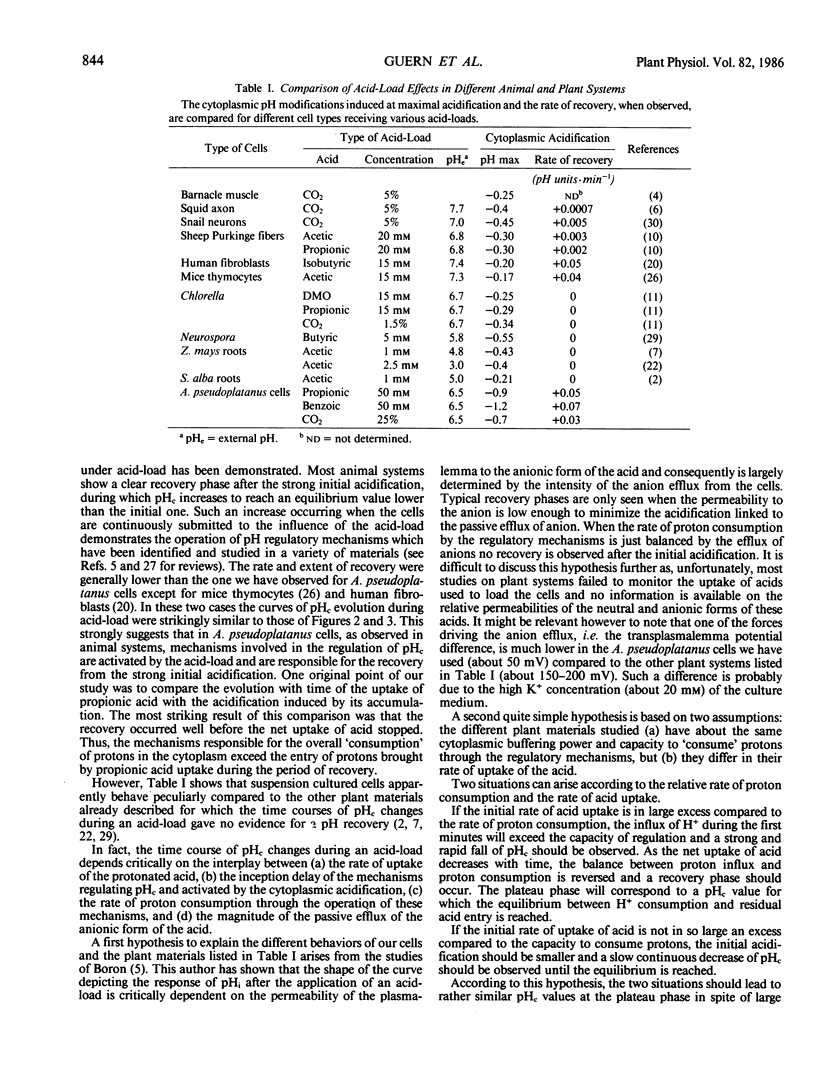
Modifications of cytoplasmic pH in Acer pseudoplatanus L. cells cultivated in suspension have been induced by acid-loads and studied by using 31P nuclear magnetic resonance spectroscopy. The initial drop of cytoplasmic pH, observed in the first minutes of exposure to weak lipophilic acids, was followed by a slow recovery to reach a plateau phase with a pH value lower than the initial one. Conversely, removal of the acid led to a sharp increase of cytoplasmic pH with in most cases an overshoot toward more alkaline values than the initial one and a subsequent decrease to more acidic values. This shows that A. pseudoplatanus cells powerfully regulate their cytoplasmic pH both on the acid side of their normal pH, during the acid-load, and on the alkaline side, after removal of acid. Similar results were obtained with different types of acid-loads, i.e. treatments with propionic or benzoic acid or bubbling with CO2-enriched air. This indicates that the occurrence of pH regulation does not depend upon the method used to acid-load the cells. The time courses of cytoplasmic pH observed for A. pseudoplatanus and also Catharanthus roseus cells are similar to those recorded for animal cells but different from those described for other plant materials for which no recovery phase was observed. This can be explained by different balances between the initial rate of proton influx brought in by the acids, and the capacity of proton consumption by the regulatory mechanisms. The existence of the recovery phase offers a unique possibility to study the regulation of the cytoplasmic pH of plant cells, as it has been done in animal systems.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bligny R. Growth of Suspension-cultured Acer pseudoplatanus L. Cells in Automatic Culture Units of Large Volume. Plant Physiol. 1977 Mar;59(3):502–505. doi: 10.1104/pp.59.3.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boron W. F., De Weer P. Intracellular pH transients in squid giant axons caused by CO2, NH3, and metabolic inhibitors. J Gen Physiol. 1976 Jan;67(1):91–112. doi: 10.1085/jgp.67.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boron W. F. Intracellular pH transients in giant barnacle muscle fibers. Am J Physiol. 1977 Sep;233(3):C61–C73. doi: 10.1152/ajpcell.1977.233.3.C61. [DOI] [PubMed] [Google Scholar]
- Boron W. F. Transport of H+ and of ionic weak acids and bases. J Membr Biol. 1983;72(1-2):1–16. doi: 10.1007/BF01870311. [DOI] [PubMed] [Google Scholar]
- CALDWELL P. C. Studies on the internal pH of large muscle and nerve fibres. J Physiol. 1958 Jun 18;142(1):22–62. doi: 10.1113/jphysiol.1958.sp005998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies D. D. Control of and by pH. Symp Soc Exp Biol. 1973;27:513–529. [PubMed] [Google Scholar]
- Gutknecht J., Tosteson D. C. Diffusion of weak acids across lipid bilayer membranes: effects of chemical reactions in the unstirred layers. Science. 1973 Dec 21;182(4118):1258–1261. doi: 10.1126/science.182.4118.1258. [DOI] [PubMed] [Google Scholar]
- Kurkdjian A., Guern J. Vacuolar pH Measurement in Higher Plant Cells : I. EVALUATION OF THE METHYLAMINE METHOD. Plant Physiol. 1981 May;67(5):953–957. doi: 10.1104/pp.67.5.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leguay J. J., Guern J. Quantitative Effects of 2,4-Dichlorophenoxyacetic Acid on Growth of Suspension-cultured Acer pseudoplatanus Cells. Plant Physiol. 1975 Sep;56(3):356–359. doi: 10.1104/pp.56.3.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin J. B., Bligny R., Rebeille F., Douce R., Leguay J. J., Mathieu Y., Guern J. A P Nuclear Magnetic Resonance Study of Intracellular pH of Plant Cells Cultivated in Liquid Medium. Plant Physiol. 1982 Oct;70(4):1156–1161. doi: 10.1104/pp.70.4.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moolenaar W. H., Tertoolen L. G., de Laat S. W. The regulation of cytoplasmic pH in human fibroblasts. J Biol Chem. 1984 Jun 25;259(12):7563–7569. [PubMed] [Google Scholar]
- Roberts J. K., Callis J., Jardetzky O., Walbot V., Freeling M. Cytoplasmic acidosis as a determinant of flooding intolerance in plants. Proc Natl Acad Sci U S A. 1984 Oct;81(19):6029–6033. doi: 10.1073/pnas.81.19.6029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J. K., Callis J., Wemmer D., Walbot V., Jardetzky O. Mechanisms of cytoplasmic pH regulation in hypoxic maize root tips and its role in survival under hypoxia. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3379–3383. doi: 10.1073/pnas.81.11.3379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J. K., Wade-Jardetzky N., Jardetzky O. Intracellular pH measurements by 31P nuclear magnetic resonance. Influence of factors other than pH on 31P chemical shifts. Biochemistry. 1981 Sep 15;20(19):5389–5394. doi: 10.1021/bi00522a006. [DOI] [PubMed] [Google Scholar]
- Rogers J., Hesketh T. R., Smith G. A., Metcalfe J. C. Intracellular pH of stimulated thymocytes measured with a new fluorescent indicator. J Biol Chem. 1983 May 25;258(10):5994–5997. [PubMed] [Google Scholar]
- Roos A., Boron W. F. Intracellular pH. Physiol Rev. 1981 Apr;61(2):296–434. doi: 10.1152/physrev.1981.61.2.296. [DOI] [PubMed] [Google Scholar]
- Sanders D., Hansen U. P., Slayman C. L. Role of the plasma membrane proton pump in pH regulation in non-animal cells. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5903–5907. doi: 10.1073/pnas.78.9.5903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders D., Slayman C. L. Control of intracellular pH. Predominant role of oxidative metabolism, not proton transport, in the eukaryotic microorganism Neurospora. J Gen Physiol. 1982 Sep;80(3):377–402. doi: 10.1085/jgp.80.3.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas R. C. Intracellular pH of snail neurones measured with a new pH-sensitive glass mirco-electrode. J Physiol. 1974 Apr;238(1):159–180. doi: 10.1113/jphysiol.1974.sp010516. [DOI] [PMC free article] [PubMed] [Google Scholar]


