Abstract
Background/Aim
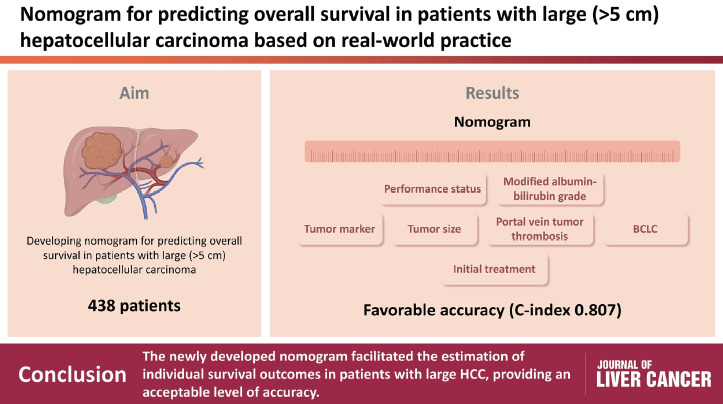
Patients with large (>5 cm) hepatocellular carcinoma (HCC) have limited treatment options, thus necessitating the identification of prognostic factors and the development of predictive tools. This study aimed to identify prognostic factors and to construct a nomogram to predict survival outcomes in patients with large HCC.
Methods
A cohort of 438 patients, who were diagnosed with large HCC at a tertiary hospital between 2015 and 2018, was analyzed. Cox proportional hazards models were used to identify key prognosticators of overall survival (OS), and an independent set of prognostic factors was used to develop a nomogram. The discrimination and calibration abilities of the nomogram were assessed and internal validation was performed using cross-validation and bootstrapping methods.
Results
During a median follow-up of 9.3 months, the median OS was 9.9 months, and the 1-year OS rate was 43.9%. Multivariable Cox regression analysis revealed that performance status, modified albumin-bilirubin grade, tumor size, extent of portal vein tumor thrombosis, and initial treatment significantly affected OS. The newly developed nomogram incorporating these variables demonstrated favorable accuracy (Harrell’s concordance index, 0.807).
Conclusions
The newly developed nomogram facilitated the estimation of individual survival outcomes in patients with large HCC, providing an acceptable level of accuracy.
Keywords: Carcinoma, hepatocellular; Prognosis; Nomograms
GRAPHICAL ABSTRACT
INTRODUCTION
Despite the identification of high-risk groups and risk factors for hepatocellular carcinoma (HCC), along with active recommendations for early and regular screening, including tumor markers and ultrasonography, patients are often diagnosed at an advanced stage with large (>5 cm) tumors with or without symptoms.1 Large tumor size can limit the implementation of curative treatment, such as radiofrequency ablation, surgical resection, or even liver transplantation, even if it is diagnosed as Barcelona Clinic Liver Cancer (BCLC) stage A without any other prognostic factors, such as portal vein tumor thrombosis (PVTT).2,3 Despite the potential for long-term survival, surgical resection in these patients is challenging due to the complexity of the required procedure(s) and their association with an elevated risk for morbidity and mortality.4-6 According to data from the Korean Nationwide Cancer Registry, 19.8% of all patients and 34.5% of patients with BCLC stage A underwent surgical resection between 2012 and 2014.7
Although various local treatments, such as transarterial chemoembolization (TACE) or radiotherapy (RT), are considered when surgery (resection or liver transplantation) is not feasible, there are limited data and little consensus regarding treatment strategies for large HCC. Patients with large HCC may present with varying tumor extent, vascular invasion, PVTT, and liver function abnormalities.8,9 As such, identifying prognostic factors is essential for predicting survival outcomes.
Recently, a nomogram, which is a graphical representation of a statistical predictive model, has been proposed as a tool for the individualized prediction and stratification of patients.10 Accordingly, the objective of this study was to identify prognostic factors in patients with large HCC (>5 cm) based on a data from a tertiary cancer center registry and to develop a nomogram incorporating these predictors.
Methods
1. Patients
From the HCC registry, 438 patients with newly diagnosed, previously untreated, large (>5 cm) HCC treated at Samsung Medical Center between 2015 and 2018, were identified.11 This single-institutional retrospective cohort study was approved by the institutional review board of Samsung Medical Center (No. 2023-06-092), and requirements for informed consent were waived because only anonymized, routinely collected data gathered during hospital visits, were used. This study is reported in accordance with the strengthening the reporting of observational studies in epidemiology guidelines (Supplementary Table 1).
2. Data collection
Data extracted included (1) patients, (2) tumors, and (3) treatment-related factors. Patient factors included age (years), sex, Eastern Cooperative Oncology Group performance status (ECOG PS), Child-Pugh class, and albuminbilirubin index (ALBI) score. Tumor-related factors included etiology, levels of tumor markers (alpha-fetoprotein [AFP] and protein induced by vitamin K absence-II [PIVKA-II]), tumor size, vessel/bile duct invasion, extent of PVTT, and tumor stage (as assessed according to the modified Union for International Cancer Control [mUICC] and BCLC).3,12,13 Treatment-related factors included details of the initial treatment.
Baseline liver function was assessed using both the Child-Pugh classification and the ALBI score. The ALBI score was calculated using the following equation.14
ALBI=(-0.085×albumin [g/L]) + (0.66×log[bilirubin] [µ mol/L]).
Patients were categorized into four groups based on modified ALBI (mALBI) grade, as follows: grade 1, ≤-2.6; grade 2a, -2.60 to -2.27; grade 2b, -2.27 to -1.39; and grade 3, >-1.39.15,16 The extent of PVTT was classified into five grades based on the Japanese classification, as follows: (1) Vp0, no tumor thrombus in the portal vein; (2) Vp1, presence of a tumor thrombus distal to (but not in) the second order branches of the portal vein; (3) Vp2, presence of a tumor thrombus in the second-order branches of the portal vein; (4) Vp3, presence of a tumor thrombus in the first-order branches of the portal vein; and (5) Vp4, presence of a tumor thrombus in the main trunk of the portal vein and/or a portal vein branch contralateral to the primarily involved lobe.12 Information regarding the initial treatment performed in the patients is summarized in Supplementary Table 2. Eighty-eight (20.1%) patients underwent curative surgical resection (hepatectomy), while more than half (n=231 [52.7%]) underwent non-surgical local treatments, as follows: TACE (n=104 [23.7%]); TACE followed by scheduled RT (n=78 [17.8%]); RT with sorafenib (n=29 [6.6%]); and RT alone (n=20 [4.6%]).
3. Statistical analysis
The primary endpoint was overall survival (OS), calculated from the date of diagnosis to the date of death or the last follow-up visit, and analyzed using the Kaplan–Meier method. Progression-free survival (PFS) was calculated from the date of diagnosis to the date of disease progression, last follow-up visit, or death. To identify the prognostic factors related to OS and PFS, multivariable analysis was performed using the Cox proportional hazard model for statistically significant variables in the univariate analysis. The variance inflation factor was also calculated to assess multicollinearity among the factors included in the multivariable analysis. All factors exhibited a variance inflation factor <10.
The results of the coefficients introduced into the multivariable Cox proportional hazards model were used to build a new nomogram for predicting OS at 1 and 2 years. Regarding liver function, a quantitative mALBI system was selected instead of the Child-Pugh classification to eliminate multicollinearity. The final nomogram was internally validated using 1,000 bootstrap simulations. Then predictive performance of the nomogram was determined by calculating the discriminatory potential using Harrell’s concordance index (C-index) and Heagerty’s integrated area under the curve (iAUC) and by plotting calibration curves for survival probabilities at 1 and 2 years. Finally, random survival forest analysis was performed to identify the importance of the variables. In all analyses, a two-sided P<0.05 was considered statistically significant. All analyses were performed using the R package version 4.2.3 (R Foundation for Statistical Computing, Vienna, Austria).
Results
1. Patients and tumor characteristics
The baseline characteristics of the patients and tumors are summarized in Table 1. The median patient age was 57 years (interquartile range [IQR], 51-67). Underlying liver diseases included the following: hepatitis B virus infection, 293 (66.9%); non-viral infection, 121 (27.6%); hepatitis C virus infection, 17 (3.9%); and hepatitis B and C viral infections, seven (1.6%). Regarding baseline liver function, 357 (81.5%) and 244 patients (55.7 %) were classified as Child-Pugh class A and mALBI grade 1, respectively. More than one-half of patients (n=233, 53.2%) exhibited multiple lesions. The median tumor size was 15.0 cm (IQR, 9.8-15.0), and 297 patients (67.8%) exhibited tumors ≥10 cm in size. Based on the mUICC staging system, 134 patients (30.6%) had stage IVA disease, 119 (27.2%) had stage III disease, and 101 (23.1%) had stage IVB disease; BCLC stage C was the most common (n=303, 69.2%).
Table 1.
Patient and tumor characteristics
| Variable | Total (n=438) |
|---|---|
| Age, years | 57.0 (51.0-67.0) |
| ECOG PS | |
| 0 | 364 (83.1) |
| 1-2 | 66 (15.1) |
| 3-4 | 8 (1.8) |
| Gender | |
| Female | 55 (12.6) |
| Male | 383 (87.4) |
| Etiology | |
| HBV | 293 (66.9) |
| HCV | 17 (3.9) |
| HBV/HCV | 7 (1.6) |
| Non-viral | 121 (27.6) |
| Alcoholic liver disease | 40 (9.1) |
| Not specified | 81 (18.5) |
| Child-Pugh class | |
| A | 357 (81.5) |
| B | 69 (15.8) |
| C | 12 (2.7) |
| ALBI score | -2.7 (-3.0 to -2.3) |
| modified ALBI | |
| Grade 1 | 244 (55.7) |
| Grade 2a | 91 (20.8) |
| Grade 2b | 89 (20.3) |
| Grade 3 | 14 (3.2) |
| AFP, ng/mL | 882.5 (28.5-21,013.7) |
| PIVKA-II, mAU/mL | 8,530.0 (1,306.0-40,429.0) |
| Presentation | |
| Solitary | 166 (37.9) |
| Multiple | 233 (53.2) |
| Diffuse | 39 (8.9) |
| Tumor size, cm | 15.0 (9.8-15.0) |
| >5, <10 | 141 (32.2) |
| ≥10 | 297 (67.8) |
| Vascular invasion | |
| Hepatic vein | 57 (13.0) |
| Inferior vena cava | 3 (0.7) |
| Bile duct invasion | |
| Present | 22 (5.0) |
| PVTT classification | |
| Vp0 | 182 (41.6) |
| Vp1 | 17 (3.9) |
| Vp2 | 48 (11.0) |
| Vp3 | 67 (15.3) |
| Vp4 | 124 (28.3) |
| LN metastasis | |
| Present | 78 (17.8) |
| mUICC stage | |
| Stage II | 84 (19.2) |
| Stage III | 119 (27.2) |
| Stage IVA | 134 (30.6) |
| Stage IVB | 101 (23.1) |
| BCLC stage | |
| A | 80 (18.3) |
| B | 37 (8.4) |
| C | 303 (69.2) |
| D | 18 (4.1) |
Values are presented as patient number (%) or median (interquartile range).
ECOG PS, Eastern Cooperative Oncology Group performance status; HBV, hepatitis B virus; HCV, hepatitis C virus; ALBI, albumin-bilirubin score; AFP, alpha-fetoprotein; PIVKA-II, protein induced by vitamin K absence-II; PVTT, portal vein tumor thrombosis; LN, lymph node; mUICC, modified International Union Against Cancer; BCLC, Barcelona Clinic Liver Cancer.
2. Survival outcomes and prognostic factors
The median follow-up was 9.3 months (IQR, 3.5-31.4) for the entire cohort and 71.3 months (IQR, 61.2-83.1) for survivors. A total of 357 patients (81.5%) died during the study period. Median PFS and OS were 3.7 and 9.9 months, respectively. The 1-year PFS and OS rates were 18.9% and 43.9%, respectively (Fig. 1). The 2-year PFS and OS rates were 12.2% and 30.5%, respectively.
Figure 1.
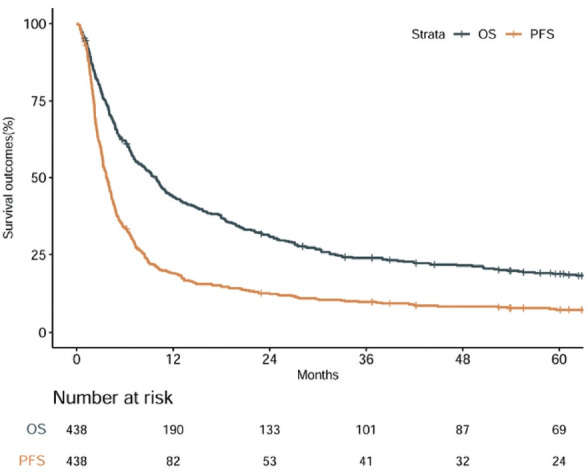
Progression-free survival (PFS) and overall survival (OS) outcomes for the entire patient cohort.
Regarding patient-related factors, ECOG PS (hazard ratio [HR], 4.10; 95% confidence interval [CI], 2.09-8.03]) and mALBI grade (grade 1 vs. grade 2a; HR, 1.49; 95% CI, 1.12-1.98 vs. grade 2b/3; HR, 2.41; 95% CI, 1.70-3.45) were associated with OS outcomes. In addition, tumor size (5-10 vs. >10 cm; HR, 1.04; 95% CI, 1.01-1.08), PVTT classification (Vp0 vs. Vp3-4; HR, 1.43; 95% CI, 1.02-2.00), and BCLC stage (A-B vs. C-D; HR, 1.65; 95% CI, 1.03-2.82) were associated with OS outcomes. Finally, multivariable analysis also demonstrated that the type of initial treatment significantly affected OS (all P<0.05) (Table 2).
Table 2.
Prognostic factors for overall survival
| Univariable analysis |
Multivariable analysis |
|||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | |
| Age, continuous (years) | 0.99 | 0.98-1.00 | 0.003 | 1.00 | 0.98-1.01 | 0.700 |
| ECOG PS, 0-1 vs. 2-4 | 9.06 | 5.33-15.41 | <0.001 | 4.10 | 2.09-8.03 | <0.001 |
| Gender, female vs. male | 1.06 | 0.76-1.46 | 0.739 | |||
| Etiology, viral vs. non-viral | 0.59 | 0.46-0.75 | <0.001 | 0.81 | 0.61-1.07 | 0.135 |
| mALBI, grade 1 vs. grade 2a | 1.94 | 1.49-2.53 | <0.001 | 1.49 | 1.12-1.98 | 0.006 |
| mALBI, grade 1 vs. grade 2b-3 | 4.95 | 3.82-6.41 | <0.001 | 2.41 | 1.70-3.45 | <0.001 |
| AFP, continuous | 1.00 | 1.00-1.00 | <0.001 | 1.00 | 1.00-1.00 | 0.001 |
| PIVKA-II, continuous | 1.00 | 1.00-1.00 | 0.708 | |||
| Child-Pugh class, A vs. B | 2.85 | 2.17-3.74 | <0.001 | 0.92 | 0.63-1.34 | 0.665 |
| Child-Pugh class, A vs. C | 31.47 | 16.34-60.6 | <0.001 | 4.57 | 2.03-10.30 | <0.001 |
| Presentation, solitary vs. multiple | 2.62 | 2.07-3.32 | <0.001 | 1.40 | 0.93-2.10 | 0.103 |
| Presentation, solitary vs. diffuse | 4.19 | 2.86-6.14 | <0.001 | 1.12 | 0.70-1.78 | 0.640 |
| Size, 5-10 vs. >10 (cm) | 1.84 | 1.46-2.32 | <0.001 | 1.04 | 1.01-1.08 | 0.043 |
| Vascular invasion, negative vs. positive | 1.35 | 1.01-1.81 | 0.04 | 0.82 | 0.62-1.05 | 0.061 |
| Biliary tract invasion, negative vs. positive | 1.24 | 0.78-1.97 | 0.365 | |||
| PVTT classification, Vp0 vs. Vp1/2 | 1.33 | 0.96-1.85 | 0.083 | 0.80 | 0.53-1.20 | 0.274 |
| PVTT classification, Vp0 vs. Vp3/4 | 3.09 | 2.44-3.91 | <0.001 | 1.43 | 1.02-2.00 | 0.036 |
| LN metastasis, negative vs. positive | 2.10 | 1.62-2.72 | <0.001 | 1.34 | 0.99-1.81 | 0.062 |
| mUICC stage, stage II vs. III | 2.24 | 1.56-3.22 | <0.001 | 0.74 | 0.44-1.29 | 0.284 |
| mUICC stage, stage II vs. IVA | 4.55 | 3.19-6.49 | <0.001 | 0.82 | 0.37-1.81 | 0.624 |
| mUICC stage, stage II vs. IVB | 5.10 | 3.54-7.37 | <0.001 | 0.72 | 0.35-1.49 | 0.378 |
| BCLC stage, A-B vs. C-D | 3.67 | 2.78-4.84 | <0.001 | 1.65 | 1.03-2.82 | 0.032 |
| Treatment, sorafenib vs. BSC | 3.53 | 2.40-5.19 | <0.001 | 1.86 | 1.13-3.13 | 0.018 |
| Treatment, sorafenib vs. surgery | 0.09 | 0.06-0.14 | <0.001 | 0.17 | 0.10-0.28 | <0.001 |
| Treatment, sorafenib vs. other local treatments | 0.45 | 0.34-0.60 | <0.001 | 0.55 | 0.40-0.76 | <0.001 |
The foreparts of the comma were set as the reference groups in the multivariable analysis.
HR, hazard ratio; CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance status; mALBI, modified albumin-bilirubin score; AFP, alpha-fetoprotein; PIVKA-II, protein induced by vitamin K absence-II; PVTT, portal vein tumor thrombosis; LN, lymph node; mUICC, modified International Union Against Cancer; BCLC, Barcelona Clinic Liver Cancer; BSC, best supportive care.
Multivariable analyses revealed that patient-related factors, including ECOG PS (HR, 3.70; 95% CI, 1.99-6.84) and mALBI grade 3-4 (HR, 1.68; 95% CI, 1.18-2.41) and tumorrelated factors of AFP (HR, 1.00; 95% CI, 1.00-1.00), PIVKA-II (HR, 1.00; 95% CI, 1.00-1.00), Vp3-4 classification (HR, 1.42; 95% CI, 1.01-1.99), and lymph node metastasis (HR, 1.41; 95% CI, 1.05-1.90) significantly contributed to inferior PFS outcomes (Supplementary Table 3).
3. Nomogram
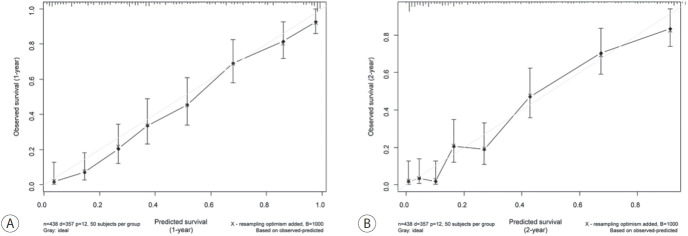
Considering the multiple factors related to OS outcomes, a nomogram for predicting the 1- and 2-year OS outcomes was developed (Fig. 2). In the random survival forest analysis, initial treatment was the most important prognostic factor, followed by BCLC stage and mALBI grade (Table 3). Tumor size and etiology were less influential than other factors. The C-index and the iAUC of the nomogram were 0.807 (95% CI, 0.749-0.855) and 0.880 (95% CI, 0.865-0.911), respectively (Table 4). The internal calibration plot for predicting the 1- and 2-year survival probability to validate the nomogram confirmed that the predicted survival rates correlated well with the actual survival rates at 1 and 2 years (Fig. 3). Harrell’s C-indices for 1- and 2-year OS were 0.808 and 0.798, respectively. The iAUC for 1- and 2-year OS were 0.873 and 0.885, respectively.
Figure 2.
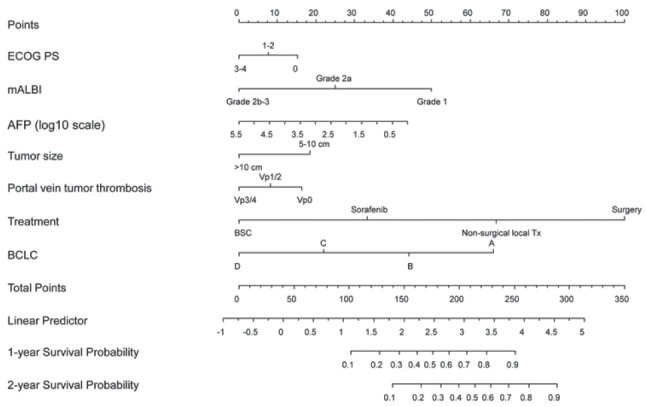
Nomogram for predicting 1-, and 2-year survival rates in patients with large hepatocellular carcinoma. The nomogram summed the points identified on the scale for each variable. The total points projected on the bottom scale indicate the probabilities of survival. ECOG PS, Eastern Cooperative Oncology Group performance status; mALBI, modified albumin-bilirubin index; AFP, alpha fetoprotein; BSC, best supportive care; Tx, treatment; BCLC, Barcelona Clinic Liver Cancer.
Table 3.
Random survival forests analysis on the development set of the nomogram
| Characteristic | Importance | Relative importance |
|---|---|---|
| Initial treatment | 0.2027 | 1.0000 |
| BCLC stage | 0.1552 | 0.7654 |
| mALBI | 0.1180 | 0.5821 |
| PVTT classification | 0.0412 | 0.2034 |
| AFP value | 0.0401 | 0.1976 |
| ECOG PS | 0.0366 | 0.1806 |
| Tumor size | 0.0150 | 0.0740 |
BCLC, Barcelona Clinic Liver Cancer; mALBI, modified albuminbilirubin score; PVTT, portal vein tumor thrombosis; ECOG PS, Eastern Cooperative Oncology Group performance status; AFP, alphafetoprotein.
Table 4.
Performance of the model in the internal dataset
| Period | Harrell's C-index | Heagerty's iAUC |
|---|---|---|
| All time | 0.807 (0.749-0.855) | 0.880 (0.865-0.911) |
| 1-year | 0.808 (0.750-0.841) | 0.873 (0.862-0.900) |
| 2-year | 0.798 (0.741-0.835) | 0.885 (0.870-0.912) |
Values are presented as number (95% confidence interval).
C-index, concordance index; iAUC, integrated area under the curve.
Figure 3.
Calibration plots of the developed nomogram for internal validation. (A) One-year survival probability, (B) 2-year survival probability.
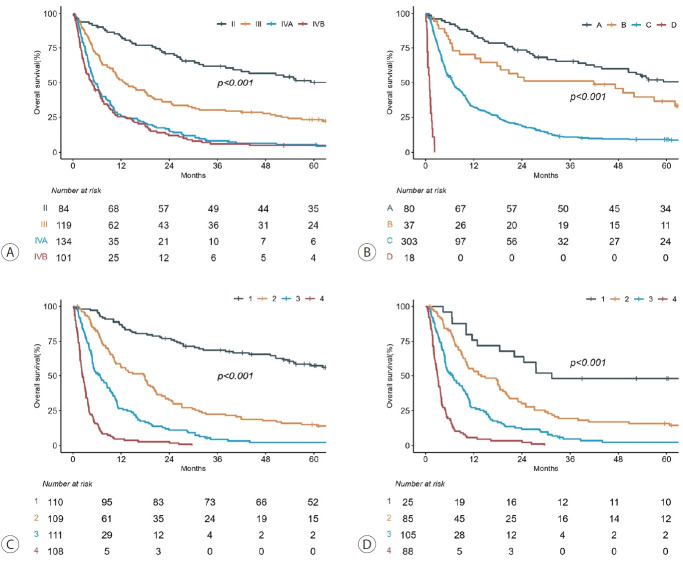
4. Survival outcomes according to the mUICC and BCLC systems
According to the mUICC system, patients with stages IVA and IVB disease exhibited similar OS outcomes (Fig. 4A), whereas significant differences in OS were observed based on the BCLC system (Fig. 4B). However, 303 patients had BCLC stage C disease. To identify differences in OS based on the new nomogram, the patients were divided into quartiles according to total points. A significant difference in OS among these classes was identified compared to the classes categorized based on mUICC (Fig. 4C). Additionally, a significant difference was observed in the OS of the 303 patients with BCLC stage C when categorizing patients based on the new nomogram (Fig. 4D). A significant difference in PFS was observed when patients were stratified according to the new nomogram (Supplementary Fig. 1).
Figure 4.
Kaplan–Meier survival curves stratified according to the (A) modified Union for International Cancer Control (mUICC) system, (B) Barcelona Clinic Liver Cancer (BCLC) system, (C) the new nomogram for all patients, and (D) the new nomogram for BCLC stage C patients.
Discussion
In this study, we developed a nomogram for predicting OS in patients with large HCC (>5 cm), which yielded accurate predictions based on internal validation. Although several studies have identified prognostic factors in this patient population, estimating individual survival outcomes remains difficult. This nomogram can help physicians predict survival probability based on patient- and tumor-specific covariates for individualized treatment decision making.
In the current analysis, we identified that an initial treatment modality favoring surgical resection was an independent prognostic factor and the most relevant factor affecting OS. Although there are no absolute size contraindications for surgical resection, large tumors are generally considered to be unresectable. Recently, several reports have described the benefits of surgical resection in this population with a tumor size exceeding the Milan criteria.17,18 Even in tumors ≥10 cm, liver resection yielded better OS outcomes than TACE, even after adjusting for clinical factors.19-22 Factors involved that may have influenced these retrospective results by overestimating the benefits of surgical resection.
In addition, non-surgical local treatment was associated with better OS outcomes than sorafenib alone in the developed nomogram. Peng et al.23 reported that the combination of TACE and radiofrequency ablation with sorafenib yielded better OS outcomes (median OS, 12 vs. 8 months; P=0.001) than sorafenib alone for recurrent HCC >5 cm. Although a phase III trial failed to improve OS outcomes after sorafenib with TACE compared to sorafenib alone in advanced HCC, combination therapy significantly improved PFS, tumor response rate, and time to progression.24,25 Regarding RT, a recent phase II trial demonstrated favorable median OS outcomes of 24.6 months after concurrent chemoradiotherapy with 5-fluorouracil and leucovorin followed by sorafenib. The median tumor size among the 47 patients was 8.4 cm (IQR, 6.5-12.0).26 Additionally, a recent randomized trial reported that the addition of RT to TACE was associated with better PFS (3 months, 86.7% vs. 34.3%), longer time to progression (31 vs. 12 weeks), and OS (median, 55 vs. 43 weeks) than sorafenib alone in patients with macroscopic vascular invasion.27 Several meta-analyses have shown that concurrent RT with sorafenib has improved OS compared to sorafenib monotherapy.28,29 Although we could not analyze the difference among non-surgical treatments due to the limited number of patients, Li et al.28 reported that RT may be the best choice for combination therapy compared with TACE or hepatic artery infusion chemotherapy with sorafenib. In summary, we suggest that surgical resection is the preferred initial treatment whenever possible. However, for patients who are ineligible for surgical resection, other local treatments (e.g., TACE and RT) should be considered in combination with systemic therapy rather than with systemic therapy alone.
Liver functional reserve is important for determining the initial treatment option and treatment outcomes in patients with HCC. Serum albumin and bilirubin levels are reliable markers of decline in liver function. The ALBI score is one of the most useful markers for estimating liver function.14,15,30 Fang et al.31 found that large HCCs with ALBI grade 1 exhibited comparable outcomes to those with BCLC stage A disease, and large HCCs with ALBI grade 2 or 3 exhibited similar outcomes to those with BCLC stage B disease. Therefore, the ALBI grade appears to help stratify this population. Furthermore, ALBI grade 2-3 was related to inferior OS outcomes in 143 patients with HCC ≥10 cm.22 Considering the relative importance of mALBI score, liver function assessed by mALBI has an acceptable discriminative potential to predict the prognosis of patients, even among those with large HCC.
In random survival forest analysis, PVTT classification was another important factor affecting OS outcomes. Although various treatment options have been considered for PVTT, the presence of PVTT was associated with dismal outcomes.32-34 The presence of PVTT could promote intrahepatic tumor progression, treatment failure, and deterioration of liver function.35-37 Mähringer-Kunz et al.38 reported that the extent of PVTT and OS were significantly associated. The median OS for Vp4 was 4.8 months compared to 14.6 months for Vp1. Moreover, Li et al.39 demonstrated that main trunk involvement in PVTT was related to OS outcomes in patients with main PVTT treated with TACE. Given the suboptimal outcomes for PVTT, it is essential to explore combination treatment strategies to improve survival outcomes.40
The present study had several limitations, the first of which were the inherent limitations of its single-center, retrospective design. For example, selection bias in the treatment modality could significantly affect OS outcomes (Supplementary Table 4). Because treatment modality is mainly decided based on tumor stage in clinical practice, it should not be selected based on this nomogram until further validation is performed. However, using the total points from the current nomogram (Supplementary Fig. 2) enabled discrimination between patients in each treatment modality. However, because most patients in the surgery group were allocated to the first quartile of the developed nomogram, additional validation with an external dataset is needed to predict outcomes in the surgery group. Second, the current nomogram should be validated externally in other centers, where the selection criteria for initial treatment modalities may vary substantially. However, the strength of our nomogram is the relatively large sample size and real-world data. In addition, we observed acceptable results in internal validation with bootstrapping. Finally, the nomogram did not include subsequent treatments after recurrence, which could have affected OS outcomes. However, our nomogram results, based on initial diagnosis and treatment, could be informative and helpful to both patients and physicians. Incorporating patient and treatment factors with tumor factors, such as BCLC stage, PVTT classification, AFP value, and tumor size, demonstrated an improved prediction of OS compared with the traditional mUICC and BCLC systems. Further studies, however, are required to validate the use of this nomogram in clinical practice.
In conclusion, we identified several prognostic factors related to survival outcomes in patients with large HCC. In addition, the nomogram, based on patient and tumor characteristics, demonstrated acceptable accuracy in this patient population. Continued efforts to refine and validate nomogram prediction tools are necessary to assist physicians in appraising the anticipated course of the disease and aid in decision-making for individualized treatment.
Acknowledgments
None.
Footnotes
Conflict of Interest
The authors have no conflicts of interest to disclose.
Ethics Statement
This single-institutional retrospective cohort study was approved by the institutional review board of Samsung Medical Center (No. 2023-06-092), and requirements for informed consent were waived because only anonymized, routinely collected data gathered during hospital visits, were used.
Funding Statement
This study was supported by the Korean Liver Cancer Association Research Award (2021).
Data Availability
The datasets generated and analyzed during the current study are not publicly available due to institutional data protection law and confidentiality of patient data but are available from the corresponding author on reasonable request in person.
Author Contribution
Conceptualization: JIY, HCP
Data curation: JIY, HCP, UYH, HYL, MJG, YHP
Formal analysis: NK
Investigation: JIY, HCP, UYH, HYL, MJG, YHP
Methodology: NK, JIY
Project administration: JIY, HCP
Resources: JIY, HCP
Software: NK
Supervision: JIY, HCP
Validation: NK, JIY, HCP
Visualization: NK
Writing-original draft: NK, JIY
Writing-review & editing: all authors
Approval of final manuscript: all authors
Supplementary Material
Supplementary data can be found with this article online https://doi.org/10.17998/jlc.2023.08.10.
References
- 1.Galun D, Basaric D, Zuvela M, Bulajic P, Bogdanovic A, Bidzic N, et al. Hepatocellular carcinoma: from clinical practice to evidence-based treatment protocols. World J Hepatol. 2015;7:2274–2291. doi: 10.4254/wjh.v7.i20.2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245–1255. doi: 10.1016/S0140-6736(11)61347-0. [DOI] [PubMed] [Google Scholar]
- 3.Reig M, Forner A, Rimola J, Ferrer-Fàbrega J, Burrel M, GarciaCriado Á, et al. BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol. 2022;76:681–693. doi: 10.1016/j.jhep.2021.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Poon RT, Fan ST, Wong J. Selection criteria for hepatic resection in patients with large hepatocellular carcinoma larger than 10 cm in diameter. J Am Coll Surg. 2002;194:592–602. doi: 10.1016/s1072-7515(02)01163-8. [DOI] [PubMed] [Google Scholar]
- 5.Chen XP, Qiu FZ, Wu ZD, Zhang BX. Hepatectomy for huge hepatocellular carcinoma in 634 cases. World J Gastroenterol. 2006;12:4652–4655. doi: 10.3748/wjg.v12.i29.4652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nagasue N, Kohno H, Chang YC, Taniura H, Yamanoi A, Uchida M, et al. Liver resection for hepatocellular carcinoma. Results of 229 consecutive patients during 11 years. Ann Surg. 1993;217:375–384. doi: 10.1097/00000658-199304000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chon YE, Lee HA, Yoon JS, Park JY, Kim BH, Lee IJ, et al. Hepatocellular carcinoma in Korea between 2012 and 2014: an analysis of data from the Korean Nationwide Cancer Registry. J Liver Cancer. 2020;20:135–147. doi: 10.17998/jlc.20.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu G, Wu J, Wang B, Zhu X, Shi X, Ding Y. Importance of tumor size at diagnosis as a prognostic factor for hepatocellular carcinoma survival: a population-based study. Cancer Manag Res. 2018;10:4401–4410. doi: 10.2147/CMAR.S177663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hong SK, Lee KW, Lee S, Hong SY, Suh S, Han ES, et al. Impact of tumor size on hepatectomy outcomes in hepatocellular carcinoma: a nationwide propensity score matching analysis. Ann Surg Treat Res. 2022;102:193–204. doi: 10.4174/astr.2022.102.4.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iasonos A, Schrag D, Raj GV, Panageas KS. How to build and interpret a nomogram for cancer prognosis. J Clin Oncol. 2008;26:1364–1370. doi: 10.1200/JCO.2007.12.9791. [DOI] [PubMed] [Google Scholar]
- 11.Sinn DH, Choi GS, Park HC, Kim JM, Kim H, Song KD, et al. Multidisciplinary approach is associated with improved survival of hepatocellular carcinoma patients. PLoS One. 2019;14:e0210730. doi: 10.1371/journal.pone.0210730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kudo M, Kitano M, Sakurai T, Nishida N. General rules for the clinical and pathological study of primary liver cancer, nationwide follow-up survey and clinical practice guidelines: the outstanding achievements of the Liver Cancer Study Group of Japan. Dig Dis. 2015;33:765–770. doi: 10.1159/000439101. [DOI] [PubMed] [Google Scholar]
- 13.Korean Liver Cancer Association (KLCA) National Cancer Center (NCC) Korea 2022 KLCA-NCC Korea practice guidelines for the management of hepatocellular carcinoma. Clin Mol Hepatol. 2022;28:583–705. doi: 10.3350/cmh.2022.0294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson PJ, Berhane S, Kagebayashi C, Satomura S, Teng M, Reeves HL, et al. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J Clin Oncol. 2015;33:550–558. doi: 10.1200/JCO.2014.57.9151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hiraoka A, Michitaka K, Kumada T, Izumi N, Kadoya M, Kokudo N, et al. Validation and potential of albumin-bilirubin grade and prognostication in a nationwide survey of 46,681 hepatocellular carcinoma patients in Japan: the need for a more detailed evaluation of hepatic function. Liver Cancer. 2017;6:325–336. doi: 10.1159/000479984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kudo M. Newly developed modified albi grade shows better prognostic and predictive value for hepatocellular carcinoma. Liver Cancer. 2021;11:1–8. doi: 10.1159/000521374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamashita Y, Taketomi A, Shirabe K, Aishima S, Tsuijita E, Morita K, et al. Outcomes of hepatic resection for huge hepatocellular carcinoma (≥ 10 cm in diameter) J Surg Oncol. 2011;104:292–298. doi: 10.1002/jso.21931. [DOI] [PubMed] [Google Scholar]
- 18.Lim C, Compagnon P, Sebagh M, Salloum C, Calderaro J, Luciani A, et al. Hepatectomy for hepatocellular carcinoma larger than 10 cm: preoperative risk stratification to prevent futile surgery. HPB (Oxford) 2015;17:611–623. doi: 10.1111/hpb.12416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bogdanovic A, Bulajic P, Masulovic D, Bidzic N, Zivanovic M, Galun D. Liver resection versus transarterial chemoembolization for huge hepatocellular carcinoma: a propensity score matched analysis. Sci Rep. 2021;11:4493. doi: 10.1038/s41598-021-83868-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Min YW, Lee JH, Gwak GY, Paik YH, Lee JH, Rhee PL, et al. Long-term survival after surgical resection for huge hepatocellular carcinoma: comparison with transarterial chemoembolization after propensity score matching. J Gastroenterol Hepatol. 2014;29:1043–1048. doi: 10.1111/jgh.12504. [DOI] [PubMed] [Google Scholar]
- 21.Zhu SL, Zhong JH, Ke Y, Ma L, You XM, Li LQ. Efficacy of hepatic resection vs transarterial chemoembolization for solitary huge hepatocellular carcinoma. World J Gastroenterol. 2015;21:9630–9637. doi: 10.3748/wjg.v21.i32.9630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wei CY, Chen PC, Chau GY, Lee RC, Chen PH, Huo TI, et al. Comparison of prognosis between surgical resection and transarterial chemoembolization for patients with solitary huge hepatocellular carcinoma. Ann Transl Med. 2020;8:238. doi: 10.21037/atm.2019.12.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peng Z, Chen S, Wei M, Lin M, Jiang C, Mei J, et al. Advanced recurrent hepatocellular carcinoma: treatment with sorafenib alone or in combination with transarterial chemoembolization and radiofrequency ablation. Radiology. 2018;287:705–714. doi: 10.1148/radiol.2018171541. [DOI] [PubMed] [Google Scholar]
- 24.Kudo M, Imanaka K, Chida N, Nakachi K, Tak WY, Takayama T, et al. Phase III study of sorafenib after transarterial chemoembolisation in Japanese and Korean patients with unresectable hepatocellular carcinoma. Eur J Cancer. 2011;47:2117–2127. doi: 10.1016/j.ejca.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 25.Park JW, Kim YJ, Kim DY, Bae SH, Paik SW, Lee YJ, et al. Sorafenib with or without concurrent transarterial chemoembolization in patients with advanced hepatocellular carcinoma: the phase III STAH trial. J Hepatol. 2019;70:684–691. doi: 10.1016/j.jhep.2018.11.029. [DOI] [PubMed] [Google Scholar]
- 26.Kim BK, Kim DY, Byun HK, Choi HJ, Beom SH, Lee HW, et al. Efficacy and safety of liver-directed concurrent chemoradiotherapy and sequential sorafenib for advanced hepatocellular carcinoma: a prospective phase 2 trial. Int J Radiat Oncol Biol Phys. 2020;107:106–115. doi: 10.1016/j.ijrobp.2020.01.027. [DOI] [PubMed] [Google Scholar]
- 27.Yoon SM, Ryoo BY, Lee SJ, Kim JH, Shin JH, An JH, et al. Efficacy and safety of transarterial chemoembolization plus external beam radiotherapy vs sorafenib in hepatocellular carcinoma with macroscopic vascular invasion: a randomized clinical trial. JAMA Oncol. 2018;4:661–669. doi: 10.1001/jamaoncol.2017.5847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li H, Wu Z, Chen J, Su K, Guo L, Xu K, et al. External radiotherapy combined with sorafenib has better efficacy in unresectable hepatocellular carcinoma: a systematic review and meta-analysis. Clin Exp Med. 2022 Dec 10; doi: 10.1007/s10238-022-00972-4. doi: 10.1007/s10238-022-00972-4. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang M, Yuan JQ, Bai M, Han GH. Transarterial chemoembolization combined with sorafenib for unresectable hepatocellular carcinoma: a systematic review and meta-analysis. Mol Biol Rep. 2014;41:6575–6582. doi: 10.1007/s11033-014-3541-7. [DOI] [PubMed] [Google Scholar]
- 30.Hiraoka A, Kumada T, Kudo M, Hirooka M, Tsuji K, Itobayashi E, et al. Albumin-Bilirubin (ALBI) grade as part of the evidencebased clinical practice guideline for HCC of the Japan Society of Hepatology: a comparison with the liver damage and Child-Pugh classifications. Liver Cancer. 2017;6:204–215. doi: 10.1159/000452846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fang KC, Kao WY, Su CW, Chen PC, Lee PC, Huang YH, et al. The prognosis of single large hepatocellular carcinoma was distinct from Barcelona Clinic Liver Cancer stage A or B: the role of albumin-bilirubin grade. Liver Cancer. 2018;7:335–358. doi: 10.1159/000487407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee DS, Seong J. Radiotherapeutic options for hepatocellular carcinoma with portal vein tumor thrombosis. Liver Cancer. 2014;3:18–30. doi: 10.1159/000343855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Choe WH. Is multidisciplinary treatment effective for hepatocellular carcinoma with portal vein tumor thrombus? J Liver Cancer. 2022;22:1–3. doi: 10.17998/jlc.2022.03.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang F, Lu CD, Zhang XP, Chen ZH, Zhong CQ, Hu YR, et al. The impact of portal vein tumor thrombus on long-term survival after liver resection for primary hepatic malignancy. HPB (Oxford) 2020;22:1025–1033. doi: 10.1016/j.hpb.2019.10.2439. [DOI] [PubMed] [Google Scholar]
- 35.Fan J, Wu ZQ, Tang ZY, Zhou J, Qiu SJ, Ma ZC, et al. Multimodality treatment in hepatocellular carcinoma patients with tumor thrombi in portal vein. World J Gastroenterol. 2001;7:28–32. doi: 10.3748/wjg.v7.i1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yoon SM, Lim YS, Won HJ, Kim JH, Kim KM, Lee HC, et al. Radiotherapy plus transarterial chemoembolization for hepatocellular carcinoma invading the portal vein: long-term patient outcomes. Int J Radiat Oncol Biol Phys. 2012;82:2004–2011. doi: 10.1016/j.ijrobp.2011.03.019. [DOI] [PubMed] [Google Scholar]
- 37.Choi Y, Kim JW, Cha H, Han KH, Seong J. Overall response of both intrahepatic tumor and portal vein tumor thrombosis is a good prognostic factor for hepatocellular carcinoma patients receiving concurrent chemoradiotherapy. J Radiat Res. 2014;55:113–120. doi: 10.1093/jrr/rrt082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mähringer-Kunz A, Steinle V, Düber C, Weinmann A, Koch S, Schmidtmann I, et al. Extent of portal vein tumour thrombosis in patients with hepatocellular carcinoma: the more, the worse? Liver Int. 2019;39:324–331. doi: 10.1111/liv.13988. [DOI] [PubMed] [Google Scholar]
- 39.Li JH, Yin X, Fan WS, Zhang L, Chen RX, Chen Y, et al. Development of a prognostic scoring system for hepatocellular carcinoma patients with main portal vein tumor thrombus undergoing conventional transarterial chemoembolization: an analysis of 173 patients. Front Oncol. 2021;11:671171. doi: 10.3389/fonc.2021.671171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Han K, Kim JH, Ko GY, Gwon DI, Sung KB. Treatment of hepatocellular carcinoma with portal venous tumor thrombosis: a comprehensive review. World J Gastroenterol. 2016;22:407–416. doi: 10.3748/wjg.v22.i1.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.