Abstract
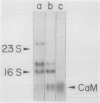
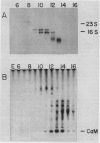
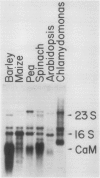
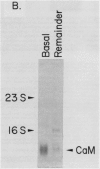
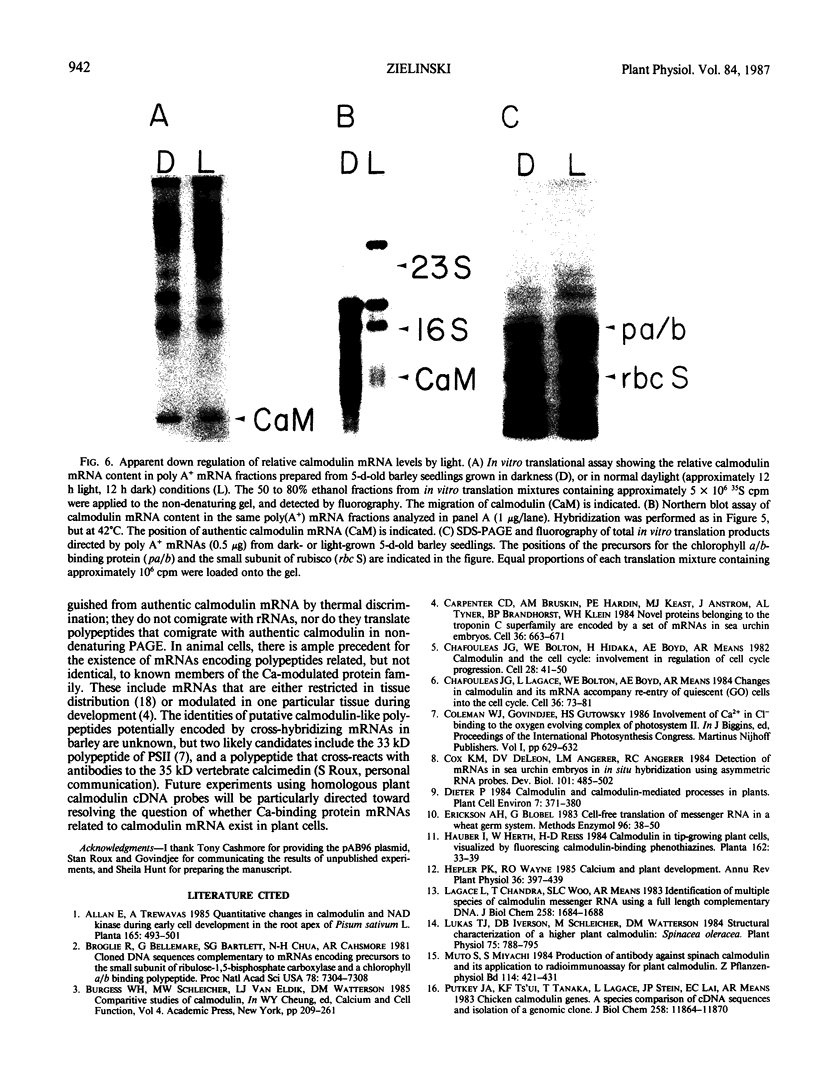
Calmodulin is encoded by a 650-nucleotide mRNA in higher plants. This messenger was identified in barley and pea by a combination of in vitro translation and blot hybridization experiments using anti-sense RNA produced from an eel calmodulin cDNA probe. In all plant tissues tested, calmodulin mRNA represents between 0.01 and 0.1% of the total translatable mRNA population. Calmodulin mRNA levels are three- to fourfold higher in the meristematic zone of the first leaf of barley. At all other stages of leaf cell differentiation, calmodulin mRNA levels are nearly identical. During light-induced development in barley leaves, the relative proportion of translatable calmodulin mRNA declines about twofold. Cytoplasmic mRNAs that may encode calmodulin-like proteins were also detected. The levels of several of these putative Ca2+-binding protein mRNAs are modulated during the course of light-induced barley leaf cell development.
Full text
PDF
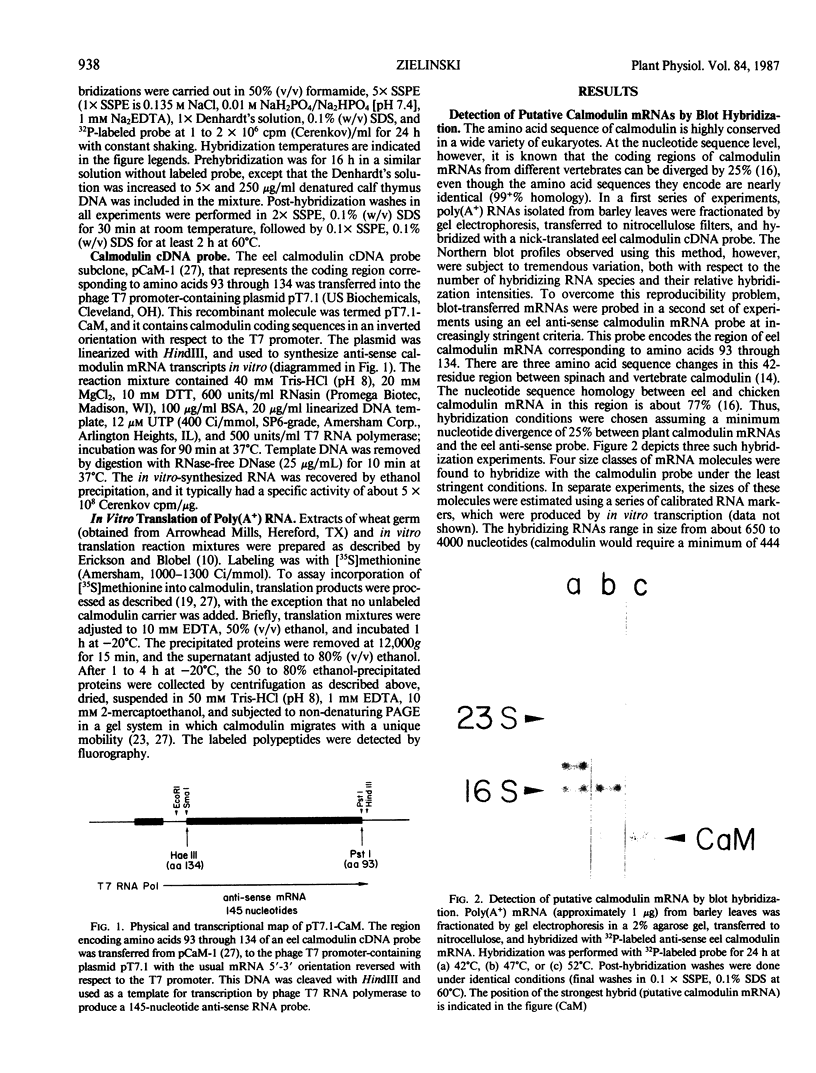
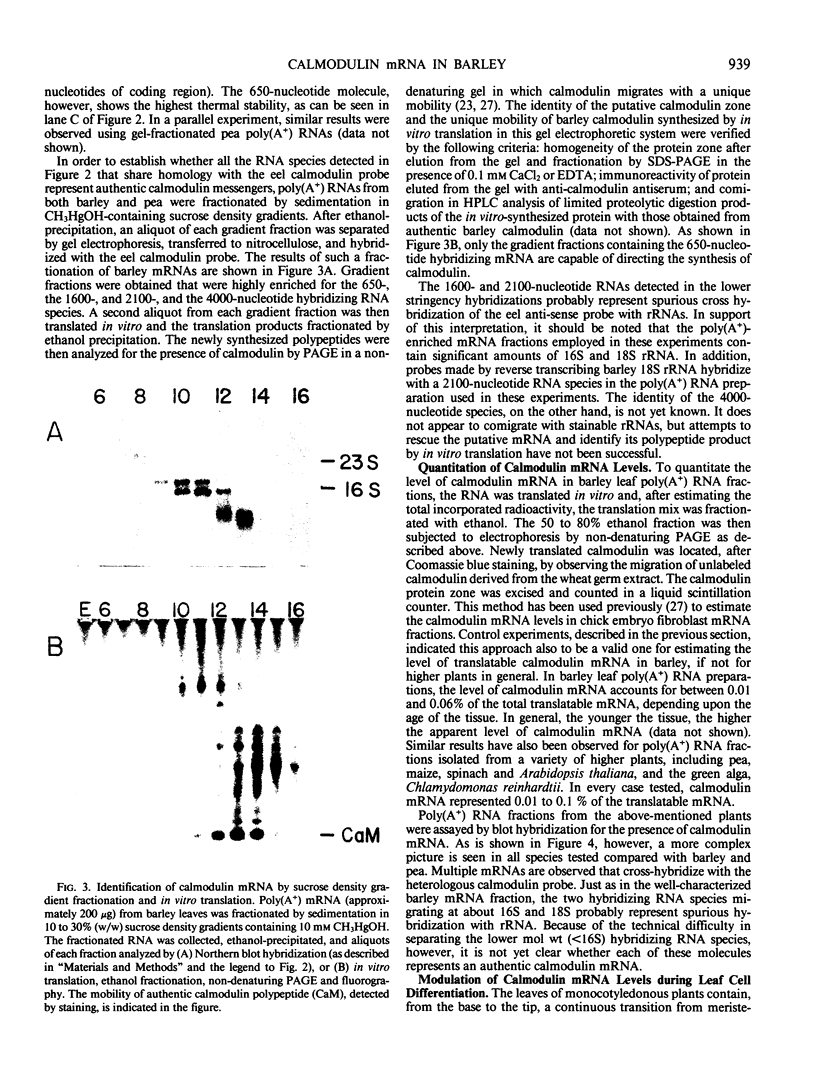

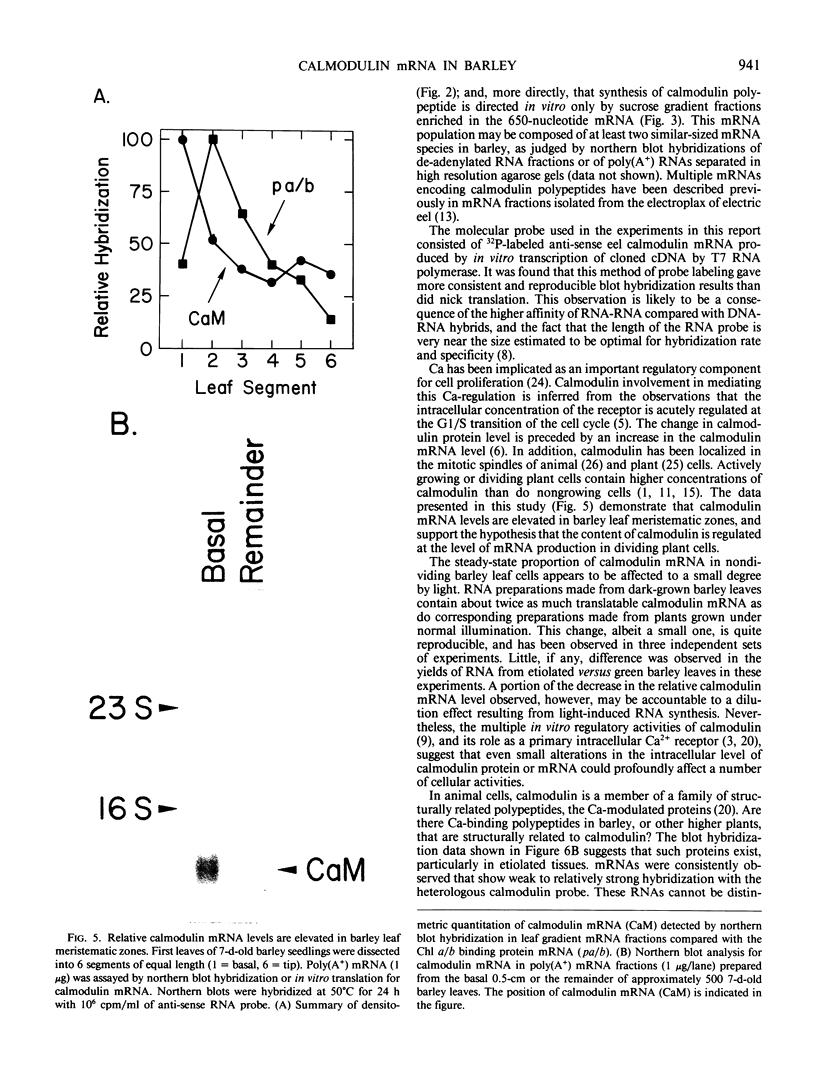

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Broglie R., Bellemare G., Bartlett S. G., Chua N. H., Cashmore A. R. Cloned DNA sequences complementary to mRNAs encoding precursors to the small subunit of ribulose-1,5-bisphosphate carboxylase and a chlorophyll a/b binding polypeptide. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7304–7308. doi: 10.1073/pnas.78.12.7304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter C. D., Bruskin A. M., Hardin P. E., Keast M. J., Anstrom J., Tyner A. L., Brandhorst B. P., Klein W. H. Novel proteins belonging to the troponin C superfamily are encoded by a set of mRNAs in sea urchin embryos. Cell. 1984 Mar;36(3):663–671. doi: 10.1016/0092-8674(84)90346-5. [DOI] [PubMed] [Google Scholar]
- Chafouleas J. G., Bolton W. E., Hidaka H., Boyd A. E., 3rd, Means A. R. Calmodulin and the cell cycle: involvement in regulation of cell-cycle progression. Cell. 1982 Jan;28(1):41–50. doi: 10.1016/0092-8674(82)90373-7. [DOI] [PubMed] [Google Scholar]
- Chafouleas J. G., Lagacé L., Bolton W. E., Boyd A. E., 3rd, Means A. R. Changes in calmodulin and its mRNA accompany reentry of quiescent (G0) cells into the cell cycle. Cell. 1984 Jan;36(1):73–81. doi: 10.1016/0092-8674(84)90075-8. [DOI] [PubMed] [Google Scholar]
- Cox K. H., DeLeon D. V., Angerer L. M., Angerer R. C. Detection of mrnas in sea urchin embryos by in situ hybridization using asymmetric RNA probes. Dev Biol. 1984 Feb;101(2):485–502. doi: 10.1016/0012-1606(84)90162-3. [DOI] [PubMed] [Google Scholar]
- Eldik L. J., Grossman A. R., Iverson D. B., Watterson D. M. Isolation and characterization of calmodulin from spinach leaves and in vitro translation mixtures. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1912–1916. doi: 10.1073/pnas.77.4.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson A. H., Blobel G. Cell-free translation of messenger RNA in a wheat germ system. Methods Enzymol. 1983;96:38–50. doi: 10.1016/s0076-6879(83)96007-x. [DOI] [PubMed] [Google Scholar]
- Lagacé L., Chandra T., Woo S. L., Means A. R. Identification of multiple species of calmodulin messenger RNA using a full length complementary DNA. J Biol Chem. 1983 Feb 10;258(3):1684–1688. [PubMed] [Google Scholar]
- Lukas T. J., Iverson D. B., Schleicher M., Watterson D. M. Structural characterization of a higher plant calmodulin : spinacia oleracea. Plant Physiol. 1984 Jul;75(3):788–795. doi: 10.1104/pp.75.3.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putkey J. A., Ts'ui K. F., Tanaka T., Lagacé L., Stein J. P., Lai E. C., Means A. R. Chicken calmodulin genes. A species comparison of cDNA sequences and isolation of a genomic clone. J Biol Chem. 1983 Oct 10;258(19):11864–11870. [PubMed] [Google Scholar]
- Schleicher M., Lukas T. J., Watterson D. M. Further Characterization of Calmodulin from the Monocotyledon Barley (Hordeum vulgare). Plant Physiol. 1983 Nov;73(3):666–670. doi: 10.1104/pp.73.3.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein J. P., Munjaal R. P., Lagace L., Lai E. C., O'Malley B. W., Means A. R. Tissue-specific expression of a chicken calmodulin pseudogene lacking intervening sequences. Proc Natl Acad Sci U S A. 1983 Nov;80(21):6485–6489. doi: 10.1073/pnas.80.21.6485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Eldik L. J., Zendegui J. G., Marshak D. R., Watterson D. M. Calcium-binding proteins and the molecular basis of calcium action. Int Rev Cytol. 1982;77:1–61. doi: 10.1016/s0074-7696(08)62463-8. [DOI] [PubMed] [Google Scholar]
- Watterson D. M., Van Eldik L. J., Smith R. E., Vanaman T. C. Calcium-dependent regulatory protein of cyclic nucleotide metabolism in normal and transformed chicken embryo fibroblasts. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2711–2715. doi: 10.1073/pnas.73.8.2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield J. F., Boynton A. L., MacManus J. P., Sikorska M., Tsang B. K. The regulation of cell proliferation by calcium and cyclic AMP. Mol Cell Biochem. 1979 Nov 1;27(3):155–179. doi: 10.1007/BF00215364. [DOI] [PubMed] [Google Scholar]
- Willingham M. C., Wehland J., Klee C. B., Richert N. D., Rutherford A. V., Pastan I. H. Ultrastructural immunocytochemical localization of calmodulin in cultured cells. J Histochem Cytochem. 1983 Apr;31(4):445–461. doi: 10.1177/31.4.6338107. [DOI] [PubMed] [Google Scholar]
- Zendegui J. G., Zielinski R. E., Watterson D. M., Van Eldik L. J. Biosynthesis of calmodulin in normal and virus-transformed chicken embryo fibroblasts. Mol Cell Biol. 1984 May;4(5):883–889. doi: 10.1128/mcb.4.5.883. [DOI] [PMC free article] [PubMed] [Google Scholar]