Abstract
Immunotherapy is one of the fastest developing areas in the field of oncology. Many immunological treatment strategies for refractory tumors have been approved and marketed. Nevertheless, much clinical and preclinical experimental evidence has shown that the efficacy of immunotherapy in tumor treatment varies markedly among individuals. The commensal microbiome mainly colonizes the intestinal lumen in humans, is affected by a variety of factors and exhibits individual variation. Moreover, the gut is considered the largest immune organ of the body due to its influence on the immune system. In the last few decades, with the development of next-generation sequencing (NGS) techniques and in-depth research, the view that the gut microbiota intervenes in antitumor immunotherapy through the immune system has been gradually confirmed. Here, we review important studies published in recent years focusing on the influences of microbiota on immune system and the progression of malignancy. Furthermore, we discuss the mechanism by which microbiota affect tumor immunotherapy, including immune checkpoint blockade (ICB) and adoptive T-cell therapy (ACT), and strategies for modulating the microbial composition to facilitate the antitumor immune response. Finally, opportunity and some challenges are mentioned to enable a more systematic understanding of tumor treatment in the future and promote basic research and clinical application in related fields.
Keywords: Tumor immunotherapy, Microbiome, Immunity, Immune checkpoint blockade, Adoptive T-cell therapy, Probiotics, Prebiotics, Fecal microbiota transplantation
Introduction
Cancer is the second leading cause of human death. The prevention and control of cancer is still challenging. Population expansion and aging, which have been reflected in the growth rate of the number of cancer diagnoses and deaths due to cancer, inevitably challenge the process of rapid social and economic development and are important indicators of its quality. With the increasing incidence of cancer, research on its treatment has long been a popular and difficult topic in modern biology and medicine. Traditional tumor treatment methods, including chemotherapy, radiotherapy and surgical resection, are prone to drug resistance, have a high recurrence rate and greatly harm the patient’s body, which limits their prognostic effect. Therefore, new tumor treatment strategies that induce less resistance are urgently needed. Over the past few decades, a large amount of basic experimental data and clinical trial results have shown that immunotherapy has the potential to change this situation. However, these treatments have different outcomes in different patients; some patients experience lasting benefits, and others do not benefit at all, which limits their clinical application [1–4]. This situation makes the development of innovative combination therapy to overcome drug resistance and improve the response rate an important aspect of tumor therapy research.
With the progress and development of whole genome sequencing technology, a substantial amount of evidence has shown that the microbiota in the human body has an important influence on the effect of tumor therapy [5, 6]. It is estimated that a normal adult can be colonized with up to 3 × 1013 commensal microbial cells, totaling over 3000 species, with over 97% colonizing the colon and the rest distributed throughout the body [7, 8]. A variety of factors can influence the symbiotic microbial composition within an individual, such as the composition of the maternal flora, the manner in which the infant is delivered, diet, exposure to antibiotics or other drugs, lifestyle, and environmental factors [9]. Thus, in contrast to the relative uniformity of microbes among different individuals at the phylum level, the composition of gut microbes at the species level varies greatly among individuals, making it difficult for researchers to define the composition of the core healthy gut microbes in humans. This challenge suggests that it may be more appropriate to define indicators of a core healthy microbiome based on the microbial function indicated by the presence of genes involved in microbial metabolic pathways [10, 11].
Commensal microbiomes are closely related to body health, and their disorders can lead to a variety of diseases [12, 13]. Increasing attention has been given to the relationship between the gut microbiome and the occurrence and development of tumors [14–16]. In addition, with the in-depth study of tumor therapy in recent years, a large amount of data has shown that the gut microbiota has a considerable impact on the results of the treatment of various types of tumors, including lung and kidney cancers [17], melanoma [18], and colorectal cancer [19]. Most studies have confirmed that the gut microbiota affects the antitumor response by influencing the immune system. In response to these findings, several strategies have been developed to alter gut microbiome composition and ultimately prolong progression-free survival (PFS) and overall survival (OS) in patients.
Here, we review important studies published in recent years focusing on the influences of the microbiota on the maturation of the immune system. Furthermore, we emphasize the microbiota and the mechanisms underlying its effects on tumor immunotherapy, including ICB and ACT; we also highlight strategies that shape the microbial composition to facilitate the antitumor immune response to enable a more systematic understanding of tumor treatment in the future and promote basic research and clinical application in related fields.
Gut microbiome and tumor progression
One of the hallmarks of malignant tumors is gene instability and mutation [20, 21], and certain gut microbes that can induce gene mutation have an important influence on the occurrence and progression of tumors, especially in the gastrointestinal system [22, 23]. Regarding the specific mechanism of microbes affecting tumors, current research results mainly support two modes of action: direct and indirect carcinogenic effects. Some bacteria have direct carcinogenic effects and are known as carcinogenic microorganisms. For example, Helicobacter pylori can produce viral factors, including urease, which act on epithelial cells in gastric pits, leading to endoplasmic reticulum stress, autophagy, oxidative stress and other inflammatory reactions, thus promoting the pathological changes of gastric tissues, which may develop into gastric cancer [24].
Similarly, Salmonella Typhi bacteria that colonize the gallbladder can also produce a cancer-causing typhoid toxin, which causes DNA damage and cell cycle changes in gallbladder cells, leading to gallbladder cancer[25]. Enterotoxigenic Bacteroides fragilis (ETBF) has been associated with the induction of colitis and colon tumorigenesis [26]. The toxin produced by ETBF can lead to chronic inflammation in CRC. Mechanistically, ETBF can migrate from the intestinal tract and localize to the mammary gland, where it induces epithelial cell proliferation and promotes tumor growth and metastasis in a Toll-like receptor 4 (TLR4)-dependent pathway [27, 28]. Fusobacterium nucleatum adheres to colon tissues through its unique FadA adhesin, which binds to E-cadherin on the surface of colon cells and activates β-catenin signaling and Annexin A1, resulting in inflammatory and carcinogenic responses [29, 30].
In addition, carcinogenic pathogens can also induce tumorigenesis indirectly through immune cells of the tumor microenvironment. F. nucleatum can express Fap2 protein in the tumor microenvironment, which binds to the T-cell immunoglobulin and ITIM domain (TIGIT) receptor of immune cells and inhibits the cytotoxicity of natural killer (NK) cells and activation of T cells, thus producing immunosuppressive effects and promoting tumor growth and metastasis [31]. In recent impressive research reports, Pushalkar et al. induced immunogenicity reprogramming of the tumor microenvironment by reducing the microbial load in pancreatic tumors, including a reduction in myeloid-derived suppressor cells (MDSCs) and increased differentiation of M1-type macrophages, and promoted CD4+ T-cell differentiation and CD8+ T-cell activation, thus improving the effect of tumor treatment [32]. Ma and colleagues found that bile acid (BA) produced by intestinal microbial metabolism could act as a messenger to control the accumulation of chemokine-dependent NKT cells in the liver and promote liver-specific antitumor immunity [33]. Short-chain fatty acids (SCFAs) could lead to an increase in the number of Tregs in the colon, as well as the production of the anti-inflammatory cytokines interleukin-10 (IL-10) and transforming growth factor-β (TGF-β), which inhibit the development of tumors [34]. In GF- or antibiotic-treated mice, Kras mutation and p53 gene deletion cannot induce lung cancer because pulmonary symbiotic bacteria can induce the proliferation and activation of γδT cells and promote the development of inflammatory tumors through the local release of IL-17 and IL-23 [35]. Another study also found that p53 mutations induced cancer only in the presence of gallic acid, which was produced by commensal bacteria [36]. All this evidence suggests that intestinal microbes play an important role in tumor progression.
According to the current research results, most of the microbes that promote tumor progression by inducing gene instability are somewhat specific microbial species and their toxic proteins, and the disturbance of intestinal microecology or increased microbial load in the tumor microenvironment is often related to the inhibition of antitumor immunity. However, the fine regulation between intestinal microecological disorders and antitumor immune responses has not been thoroughly elucidated, and most research results lack more general trends and characteristics, which may be due to the limitation of the research scale or differences in research methods.
Microbiome-based tumor diagnosis
In view of the nonnegligible influence of the microbiome on tumor progression, identifying microbial characteristics is a valid means to diagnose the threat and progression of tumors [37]. CRC and advanced adenoma (AA) are closely related to the gut microbiome, but AA is easier to cancerate. To identify AA from CRC, metagenomic analysis was used to describe the microbiome profile and microbial single nucleotide polymorphism (SNP) characteristics [38]. Another recent study distinguished clinically relevant subtypes of precancerous colorectal polyps, such as tubular adenomas (TAs) and sessile serrated adenomas (SSAs), through microbial signatures from 971 patients [39]. Specifically, TA is associated with a decrease in microbial methanogenesis and mevalonate metabolism, while SSAs exhibit increased NAD, bile acid, and sulfate metabolic potential. This study offers humanized evidence that microbial characteristics can serve as biomarkers of the stage of tumor progression.
In addition to the gut lumen, microbiome-based tumor diagnosis can also be applied broadly. Yang et al. provided a random forest analysis approach based on the oral-gut-tumor microbiome for the early detection of hepatocellular carcinoma (HCC) [40]. The fecal microbiome is also different between cervical cancer patients and healthy controls, with Ruminococcus_2 negatively correlated with cancerous stage [41]. Moreover, with the usage of artificial intelligence, tumor diagnosis has moved to a new phase. Xu and colleagues exploited an artificial intelligence diagnosis model, called DeepMicroCancer, for a broad spectrum of cancer types [42]. Combined with random forest and transfer learning models, DeepMicroCancer covers more than twenty common types of cancer, and the accuracy that could be achieved for blood samples is satisfactory for clinical scenarios.
Impact of the gut microbiome on the immune system
The commensal microbes distributed throughout the human body maintain a continuous interaction with the host. Many researchers believe that the gut, which exhibits immunoreactions driven by a high density of microbes, is the largest immune organ in mammals [14, 43]. Mammalian immune systems have evolved to fight pathogenic microorganisms due to the interaction between the host and commensal microbes [44]. Early colonization of microbes on mucosal membranes in mammals plays an important role in the development and maturation of the immune system. The individuality and variability of commensal configuration are highest in the first 3 years, during which infants are more susceptible to pathogen infections, and such life-threatening infections rarely occur in adulthood [45].
Germ-free (GF) mice are the most commonly used animal models to study the mechanisms of gut microbes’ influence on immune system development and maturation. The immune system and lymphoid organs are severely impaired in GF mice [46, 47]; these mice exhibit a decreased number of Peyer’s patches, a thinner mucus layer, and a lack of lymphoid follicles in the lamina propria [48]. In addition, widespread defects in monocytes, macrophages and neutrophils have been found in the spleen, bone marrow and liver in GF mice. The numbers of macrophages from both embryonic and bone marrow precursors were found to be reduced in the spleen, suggesting that the gut microbiota has an important influence on the origin and development of bone marrow cells [47, 49]. Immunoregulatory Th17 cells in the lamina propria of the small intestine are absent in GF mice but are inducible upon colonization by segmented filamentous bacteria (SFB) [50]. Early B-cell maturation in the intestinal mucosa is regulated by extracellular signals of symbiotic microorganisms and affects intestinal immunoglobulin repertoires [51]. Hence, the relationship between the microbiota and host is not just characterized by parasitism that drafts nutrients from the host; instead, the symbiotic relationship matures host defenses and immunity.
The regulatory effect on local immunity
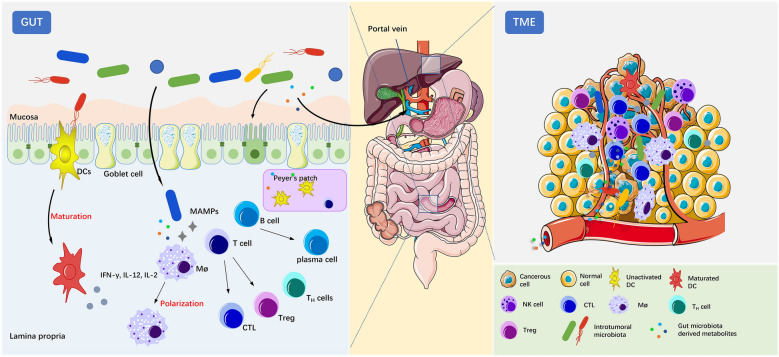
A growing number of studies have indicated that the gut microbiota may influence host immunity through multiple mechanisms, including local and systemic immunity (Fig. 1). Locally, the gut microbiota is essential for maintaining the integrity of the mucosal barrier in the intestinal lumen. Disruption of the gut microbiota can lead to a decrease in mucosal barrier function, which results in the entry of pathogenic or normal symbiotic bacteria into the bloodstream and activation of distant pattern recognition receptors (PRRs), triggering an immune response or inflammation [52, 53]. Gut microbes can activate these PRRs, such as Toll-like receptors (TLRs), to signal the immune cells in the gut-associated lymphoid tissue and mesenteric lymph nodes. Microbe-associated molecular patterns (MAMPs) in bacteria, including lipopolysaccharides (LPS) and peptidoglycan, can be presented by macrophages as antigens to Peyer’s patches, where they activate the immune response of antigen-specific B cells and promote the amplification of IgA-secreting plasma cells [54]. In addition, DCs produce tight junction (TJ) proteins between intestinal epithelial cells. At the transepithelial location, DCs can activate TJs and interact directly with bacteria and related molecules in the intestinal lumen to perceive signals [55]. Under conditions of infection, the gut microbiota can activate local phagocytes directly through PPRs to produce cytokines more efficiently [56]. The local interaction between the microbiota and host actuates defenses against most pathogens in the gut lumen and the evolution of immunity, which reemphasizes the existence of symbiotic microbes.
Fig. 1.
The gut microbiome interacts with the immune system locally in the gut and peripherally in the tumor microenvironment (TME). Within the gut, the gut microbiota plays an essential role in maintaining the mucosal barrier to protect the gut from pathogens. Microbes can interact with DCs directly and induce their maturation. Some microbiota-derived metabolites, such as SCFAs, inosine, and peptidoglycan, or invasive microbes can activate macrophages (Møs), T cells and B cells in the lamina propria or Peyer’s patches. Systemically, gut microbiota-derived metabolites can disseminate to distal sites, especially the TME, through the portal vein and interact with tumor-associated lymphocytes, including DCs, NK cells, Møs and T cells. CTL cytotoxic T lymphocyte
The regulatory effect on systemic immunity
The gut microbiota can also mediate systemic immune responses by releasing various metabolites into the circulatory system. A key example is SCFAs, which can act on G-protein-coupled receptor (GPCR) signaling pathways or affect epigenetic factors as inhibitors of histone deacetylases (HDACs). SCFAs, such as butyrate and propionate, can induce the differentiation of peripheral Tregs by epigenetic modification of Foxp3 sites [57]. It has also been reported that butyrate is able to increase interferon-γ (IFN-γ) and granzyme B (GZMB) expression in CD8+ T cells [58] and induce their transition from an effector phenotype to a memory phenotype [59]. BA is another important immunomodulator produced by microbial metabolism. Studies have shown that BA and its derivatives can control T-cell differentiation and macrophage polarization, especially inhibiting the function of Th17 cells [60], thus regulating the intestinal inflammatory response [61–63]. Generally, the abundance of metabolic genes is irregular among microbes. Thus, these varied metabolites enable the delicate regulation of the immune response by modulating the gut microbiota population.
The interplay between innate immunity and gut microbiota
Host innate immunity requires not only defense against pathogen invasion but also tolerance to nonpathogenic symbiotic microbiota, that is, maintenance of gut mucosal barrier homeostasis. Various signaling pathways in intestinal epithelial cells and intestinal immune cells play an important role in this process. First, TLRs, which are PRRs, may sense the presence of MAMPs and determine defense or tolerance. For example, polysaccharide A (PSA) from Bacteroides fragilis can act on the TIR2/1 heterodimer in cooperation with Dectin-1, thus activating downstream anti-inflammatory immune regulatory genes [64]. NOD-like receptors (NLRs) are also innate immune regulatory sensors. NOD2 inhibits inflammation of the gut by restricting commensal B. vulgatus [65].
Apart from PRRs, MyD88 and inflammasomes are also indispensable to host innate immunity for sensing symbiotic microbes and maintaining homeostasis. MyD88 is an adapter for a variety of innate immune receptors that recognize microbial signals. The absence of MyD88 in Treg cells in the small intestine leads to the expansion of Th17 cells, inactivation of the IgA immune response, and initiation of IL-17-dependent intestinal inflammation [66]. The inflammasome induces pyroptosis of infected cells in response to intense pathogen invasion, thus maintaining homeostasis. For example, the NLRP6 inflammasome regulates mucus secretion by “sentinel” goblet cells to prevent pathogenic intruders [67]. In patients with ulcerative colitis, it was found that a symbiotic microbial-induced IgG response and increased activation of FcγR signaling in the colon mucosa jointly induced NLRP3 activation in macrophages and increased production of the proinflammatory cytokine IL-1β [68]. In addition to these immune signaling pathways, innate lymphoid cells (ILCs) are a class of innate immune cells that specialize in rapidly secreting cytokines and chemokines to combat pathogen infection and promote mucosal damage repair [69]. ILCs are characterized by phenotypic diversity and functional plasticity, which are thought to be shaped by different microbial signals. Guo et al. reported that ILC3s can mediate immune surveillance, such as Citrobacter rodentium, to facilitate early colonization resistance through ID2-dependent regulation of IL-22 [70].
The interaction between adaptive immunity and gut microbiota
The interaction between the gut microbiota and adaptive immunity is important in the antitumor T-cell response. Vétizou et al. found that B. thetaiotaomicron- or B. fragilis-mediated TLR4- and IL-12-dependent Th1 responses were associated with the efficacy of cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) blockade [71]. Reconstitution of GF mice with commensal microbiota abundant in Bifidobacterium longum, Collinsella aerofaciens, and Enterococcus faecium causes an improved T-cell response and more efficient anti-PD-L1 therapy [72]. E. hirae in the gut can translocate into the secondary lymphatic organs, activating the Th17 cell response and promoting the activation of IFN-γ-producing γδT cells, thus improving the therapeutic effect of cyclophosphamide on patients with advanced lung cancer and ovarian cancer [73]. In the study of adaptive immunity induced by gut microbes, DC cells in enteric-associated lymphoid tissue or tumor-draining lymph nodes play an important role in sensing bacteria, presenting bacterial antigens, and secreting cytokines [74].
Symbiotic bacteria activate antitumor T-cell responses by molecular mimicry of tumor-associated antigens, which induces tumor antigen-specific T-cell cross-reactivity. Fluckiger et al. identified a tape measure protein (TMP)-specific H-2Kb-restricted CD8+ T lymphocyte response against a prophage found in the genome of E. hirae. In melanoma patients, tumor antigens that are cross-reactive with microbial peptides are recognized by T-cell clones [75]. Similarly, Bessell and colleagues found that T cells targeting epitope SVYRYYGL (SVY), expressed in B. breve, cross-react with a model neoantigen, SIYRYYGL (SIY), in B16-SIY. SVY-specific T cells recognized SIY-expressing melanomas in vivo and led to beneficial outcomes [76]. Moreover, human leukocyte antigen (HLA) molecules of both glioblastoma tissues and tumor cell lines present bacteria-specific peptides, which are recognized by tumor-infiltrating lymphocyte (TIL) CD4+ T-cell clones [77]. During homeostasis, Akkermansia muciniphila (AKK) can induce T follicular helper cells in Peyer’s patches to produce an IgG1 antibody-dependent immune response [78]. Moreover, metabolites produced by bacteria, typically SCFAs [74] or tryptophan derivatives [79], can also induce T-cell- or DC cell-dependent adaptive immune responses.
Therefore, the presence of microbes in the gut allows the immune system to balance the toleration of beneficial microbes and the defense against pathogens. The maintenance of this equilibrium is also influenced by the health state of the host and the stability of the microecology. Due to the close relationship between the gut microbiota and the immune system, there is clearly an important correlation between these commensal organisms and the efficiency of immune-related therapy. Several studies have found that gut microbiome disturbances could affect local and systemic antitumor immune responses [80–82]. In proportion, changing the immune response state by regulating the composition of intestinal microbes may be an effective strategy to improve the efficiency of the treatment of different tumors.
The gut microbiome in tumor immunotherapy
The rapid proliferation of tumor cells is partly believed to be caused by the failure of immune control. Tumor cells evade the surveillance of the host immune system through various mechanisms, such as downregulating target antigens or creating a TME with immunosuppressive characteristics [83, 84]. Tumor immunotherapy enhances or rebuilds the immune system to monitor, recognize and destroy tumor cells, thereby eventually prolonging the survival of patients [85, 86]. Immunotherapy has significantly prolonged survival and improved quality of life for many cancer patients in whom chemotherapy or radiotherapy regimens have failed [87, 88]. However, with the increase in the clinical application of immunotherapy, researchers have gradually found that the therapeutic effect of immunotherapy on tumor patients varies among individuals. Stool samples from clinical patients who were subjected to sequencing analysis revealed that the features of the gut microbiome and treatment effect exhibited a significant correlation (Table 1), implying that the gut microbiome has a significant impact on tumor therapy. There may be an internal mechanism that is involved in the relationship between the individual diversity of the gut microbiota and the heterogeneity of antitumor immunotherapy outcomes. However, current mechanistic research is mainly focused on the preclinical phase, and the implementation of research findings into clinical application has remained very limited.
Table 1.
Clinical study of gut microbiome effects on immunotherapy
| Tumor type | Number of included patients | Immunotherapy treatment | Favorable clinical outcomes | Unfavorable clinical outcomes | References |
|---|---|---|---|---|---|
| NSCLC | 65 | ICB: αPD-1 mAb; αPD-L1 mAb; αCTLA-4 mAb | Ruminococcus; Akkermansia; Faecalibacterium | [89] | |
| Hepatocellular carcinoma (HCC) | 11 | ICB: αCTLA-4 mAb; αPD-L1 mAb | Akkermansia | Enterobacteriaceae | [90] |
| Lung cancer | 46 | ICB: αPD-1 mAb | Fusobacterium | [91] | |
| Advanced hepatobiliary cancers | 65 | ICB: αPD-1 mAb | Lachnospiraceae bacterium-GAM79; Alistipes sp. Marseille-P5997; Ruminococcus calidus; Erysipelotichaceae bacterium-GAM147 | Veillonellaceae | [92] |
| NSCLC | 30 | ICB: αPD-1 mAb; αPD-L1 mAb | Gammaproteobacteria | [93] | |
| CRC | 39 | Chemotherapy alone (oxaliplatin or capecitabine), or combined with ACT | Bifidobacterium; Lactobacillus; Enterococcus | Dialister pneumosintes; Escherichia coli; Lactobacillus reteri; Streptococcus mutans; Enterococcus faecium; Streptococcus gordonii; Veillonella atypica; Granulicatella sp.; Trchuris trichiura | [94] |
| Advanced HCC | 8 | ICB: αPD-1 mAb (nivolumab) | Citrobacter freundii; Azospirillum sp.; Enterococcus durans; Akkermansia; | [95] | |
| Melanoma | 77 | ICB: αCTLA-4 mAb and αPD-1 mAb combined | Bacteroides intestinalis | [96] | |
| NSCLC | 88 | ICB: αPD-1 mAb | Helicobacter pylori | [97] | |
| NSCLC | 75 | ICB: αPD-1 mAb | Streptococcus | [98] | |
| NSCLC | 69 | ICB: αPD-1 mAb; αPD-L1 mAb | Phascolarctobacterium | Dialister | [99] |
| Lung cancer | 34 | ICBs | Clostridiales | Rikenellaceae | [100] |
| B-cell non-Hodgkin lymphoma | 51 | Immunochemotherapy | Dorea formicigenerans; Faecalibacterium prausnitzii | [101] | |
| Metastatic melanoma | 130 | ICB: αPD-1 mAb; αPD-L1 mAb; αCTLA-4 mAb; ICB combination | Faecalibacterium; Ruminococcaceae; Barnesiella intestinihominis | [102] | |
| NSCLC | 11 | ICB: αPD-1 mAb | Akkermansia muciniphila; Rikenellaceae; Bacteroides; Peptostreptococcaceae; Mogibacteriaceae; Clostridiaceae | [103] | |
| Metastatic renal cell carcinoma (mRCC) | 21 | ICB: αPD-1 mAb | Akkermansia muciniphila | [104] | |
| NSCLC | 17 | ICB: αPD-1 mAb | Lactobacillus; Clostridium; Syntrophococcus | Bilophila; Sutterella; Parabacteroides | [105] |
| NSCLC | 37 | ICB: αPD-1 mAb | Alistipes putredinis; Bifidobacterium longum; Prevotella copri | [106] | |
| HCC | 8 | ICB: αPD-1 mAb | Akkermansia muciniphila; Ruminococcaceae spp. | [107] | |
| Melanoma | 27 | ICB: αPD-1 mAb; αPD-L1 mAb; αCTLA-4 mAb; ICB combination | Faecalibacterium prausnitzii; Coprococcus eutactus; Prevotella stercorea; Streptococcus sanguinis; Streptococcus anginosus; Lachnospiraceae bacterium | Bacteroides ovatus; Bacteroides dorei; Bacteroides massiliensis; Ruminococcus gnavus; Blautia producta | [108] |
| Melanoma | 112 | ICB: αPD-1 mAb | Ruminococcaceae family | [18] | |
| Metastatic melanoma | 42 | ICB: αPD-1 mAb | Bifidobacterium longum; Collinsella aerofaciens; Enterococcus faecium | [72] | |
| Gastrointestinal cancer | 74 | ICB: αPD-1 mAb; αPD-L1 mAb | Prevotella; Ruminococcaceae; Lachnospiraceae | [109] | |
| Thoracic neoplasms | 42 | ICB: αPD-1 mAb | Akkermansiaceae; Enterococcaceae; Enterobacteriaceae; Carnobacteriaceae; Clostridiales Family XI bacterial families | [110] | |
| Melanoma | 94 | ICB: αPD-1 mAb | Actinobacteria phylum; Lachnospiraceae; Ruminococcaceae; Lachnospiraceae spp.; Streptococcaceae spp. | [111] | |
| NSCLC | 294 | ICB: αPD-1 mAb | Bifidobacterium; Clostridium butyricum; lactic acid bacteria | [112] | |
| NSCLC | 21 | ICB combined with chemotherapy | Bifidobacterium; Escherichia; Sarterella | [113] | |
| HCC | 35 | ICB: αPD-1 mAb | Ruminococcus | [114] | |
| Metastatic castrate resistant prostate cancer (mCRPC) | 23 | ICB combined with enzalutamide | Streptococcus salivarius | Akkermansia muciniphila; Collinsella aerofaciens | [115] |
| Melanoma | 218 | ICB: αCTLA-4 mAb and αPD-1 mAb combined | Ruminococcaceae | Bacteroidaceae | [116] |
| Advanced gastric cancer | 77 | ICB: αPD-1 mAb | Helicobacter pylori | [117] | |
| multiple myeloma (MM); acute lympholastic leukemia (ALL); non-Hodgkin lymphoma (NHL) | 78 | CAR-T | Faecalibacterium; Roseburia; Ruminococcus | [118] | |
| Nasopharyngeal carcinoma (NPC) | 57 | ICB: αPD-1 mAb | Blautia wexlera; Blautia obeum; Erysipelatoclostridium; Ruminococcaceae bacterium; Ruminococcus sp. AF46-10NS | [119] | |
| HCC | 41 | ICB: αPD-1 mAb | Lachnoclostridium; Lachnospiraceae; Veillonella | Prevotella 9 | [120] |
Immune checkpoint blockade (ICB)
Tumors, as collections of cancerous cells, can be recognized and eliminated by the immune system. However, tumor cells secrete inhibitors that recognize and bind to adaptive immune cell surface receptors and inhibit their immune response to tumor cells [121]. ICBs that have been approved by the U.S. Food and Drug Administration (FDA) target two classes of T-cell receptors (TCRs), including CTLA-4 and programmed cell death protein 1 (PD-1) and its ligand (PD-L1). Inhibitory antibodies targeting CTLA-4 [122], PD-1 [123], or PD-L1 [124] can induce antitumor effects in vivo. In addition, many antibodies and small molecule drugs targeting other immunomodulators, including LAG3, TIGIT, TIM3, CD39, CD47 and CD73, are in the process of clinical investigation.
Although ICB therapy improves outcomes for patients with many cancer types, only a portion of patients experience a stable benefit. Even among melanoma patients with the highest response rate to ICB, more than 60–70% of patients do not respond positively to anti-PD-1 antibody therapy; 20–30% of these patients eventually show tumor recurrence and progression [125, 126]. Therefore, there is an urgent need to identify new immunotherapy strategies to improve the immunotherapy response.
There is substantial clinical evidence that the baseline composition of a patient’s gut microbiome is associated with the antitumor efficacy of ICB therapy [17, 81] (Table 1). Notably, the microbes that are favorable and unfavorable to the treatment outcome in different study populations have varied greatly overall, but they may share some important metabolic pathways that allow them to be distinguished. Based on these clinical studies, stool samples from patients with different treatment outcomes were transferred to GF or antibiotic-treated tumor model mice, and the differences in ICB treatment outcomes were paralleled in these mice [18, 72, 127]. Sequencing analysis of fecal samples has often been used to reveal the signature of the bacteria in responding patients, and the signature of beneficial bacteria identified through culture isolation or directly through the use of commercial strain supplements can further confirm the role of key gut microbes in promoting ICB treatment [18, 128]. More strikingly, intestinal bacteria and fungi have opposite effects on tumor therapy, with commensal bacteria essential for an effective antitumor immune response but symbiotic fungi modulating the immunosuppressive microenvironment after treatment. The opposite effect is likely due mainly to fungi, since the size of fungal populations is significantly increased in the gut after bacterial deletion [129]. Although the mechanism by which the microbiome affects tumor therapy is not completely understood, fecal microbiota transplantation (FMT) can be regarded as a therapeutic strategy. However, the future research objective is still to use more specific and direct methods to regulate the microbiota based on a clear understanding of the mechanism of action.
To date, different studies have found that the key bacterial signatures involved in the ICB response are different, and the mechanisms of action vary as well (Fig. 2). Based on how bacteria exhibit a synergistic antitumor effect with the treatment, the mode of action can be divided as follows: (a) exopolysaccharides [71, 130] or surface proteins [131] in the structure of bacteria themselves can be used as pathogen-associated molecular patterns (PAMPs) to directly stimulate intestinal immune cells and induce innate or adaptive immune responses; (b) metabolites produced by bacteria, such as SCFAs [109, 132, 133], inosine [134], peptidoglycan [127], trimethylamine N-oxide (TMAO) [135, 136], neurotransmitters (including dopamine, norepinephrine, serotonin, or γ-aminobutyric acid) [137], ferrichrome [138], β-galactosidase [139], etc., enter the circulatory system through the portal vein to stimulate the TME, changing the immune state and reversing tumor immune tolerance and thus promoting the therapeutic effect of ICBs (Table 2). On this basis, the gut metabolomic profile was characterized in 11 non-small cell lung cancer (NSCLC) patients treated with nivolumab anti-PD-1 therapy, which showed that 2-pentanone and tridecane were significantly associated with early progression, while SCFAs, lysine and nicotinic acid were significantly associated with long-term beneficial effects [140]. Moreover, other studies have found that regulating gut microbial composition and supplementing beneficial bacteria could alleviate immune-related adverse reactions (irAEs) induced by monoclonal antibody therapy targeting CTLA-4 or PD-1 [141, 142], which reiterated the importance of the relationship between the gut microbiome and immunotherapy. Based on these findings, strategies that regulate bacterial populations in the host are reasonable and applicable.
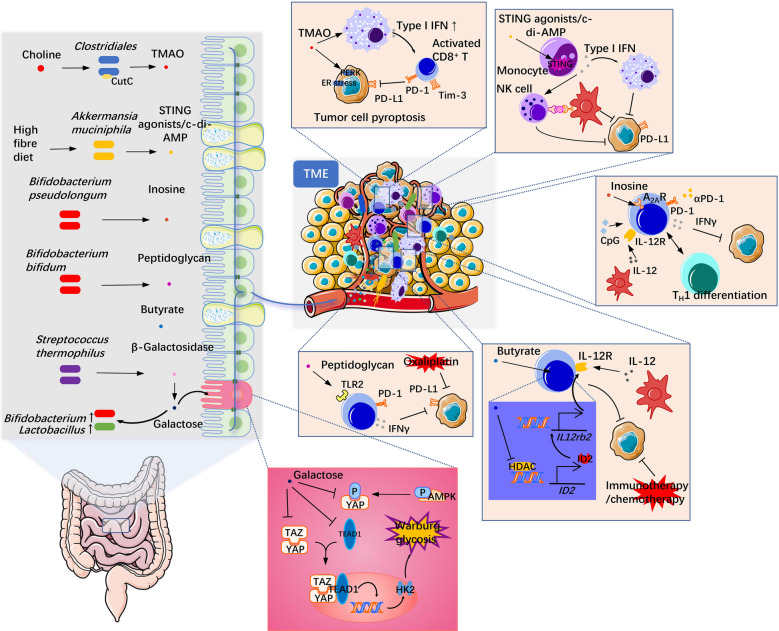
Fig. 2.
Mechanism through which some gut microbiota-derived metabolites influence antitumor therapy. These metabolites, including TMAO from Clostridiales, c-di-AMP from Akkermansia, inosine from Bifidobacterium pseudolongum, peptidoglycan from Bifidobacterium bifidum, β-galactosidase from Streptococcus thermophilus and butyrate, are representative metabolites that have been reported in the last two years. They can spread to the TME through the circulatory system or interact with mutated enterocytes directly and mediate antitumor therapy by different mechanisms and pathways. TLRs Toll-like receptors, AhR aryl hydrocarbon receptor, A2AR: adenosine 2A receptor
Table 2.
Reports on microbial metabolites that affect immunotherapy
| Tumor type | Immunotherapy treatment | Microbial metabolites | Metabolic bacterial taxa | Target | Overview of mechanism | References |
|---|---|---|---|---|---|---|
| Triple-negative breast cancer (TNBC) | αPD-1 mAb | Trimethylamine N-oxide (TMAO) | Clostridiales | PERK | TMAO induced tumor cell pyroptosis by activating ER stress kinase PERK, thus enhancing antitumor immunity mediated by CD8+ T cells | [135] |
| PDAC | αPD-1 mAb and/or αTim-3 mAb | Trimethylamine N-oxide (TMAO) | TMAO induced an immunostimulatory phenotype in macrophages, which supported effector T-cell responses in a type I IFN-dependent manner and facilitated the efficacy of αPD-1 or αTim-3 mAb | [136] | ||
| ICI-induced colitis | αCTLA-4 mAb | Indole-3-carboxaldehyde (3-IAld) | AhR | 3-IAld activates AhR/IL-22 dependent signaling pathways in the host, controls colon inflammation induced by αCTLA-4 mAb therapy, and reduces adverse reactions associated with ICB therapy by altering the composition and function of the gut microbiota | [143] | |
| Lymphoma (EL4); CRC (MC38); breast cancer (TUBO); melanoma (BRAFV600E/PTEN−/−) | αPD-1 mAb; αPD-L1 mAb | STING agonists, including c-di-AMP | Akkermansia muciniphila | STING signaling pathway | STING agonists generated by bacterial metabolism such as c-di-AMP induce intratumoral monocytes to produce type I IFN, thus promoting the polarization of tumor suppressor macrophages and antigen presentation between NK cells and DC cells, which promotes antitumor immunity | [144] |
| HNSCC | αPD-L1 mAb | Bacterial lipopeptide Pam3CSK4 | Staphylococcus aureus | TLR2 | Bacterial lipopeptide Pam3CSK4 enhances the expression of PD-L1 in multiple HNSCC cell lines and directly promotes immunosuppression | [145] |
| Hepatocellular carcinoma (HepG-2) | γδT immunotherapy combined with antibiotics | 3-Indolepropionic acid (IPA) | IPA can stimulate γδT cells to release more cytotoxic cytokines, such as granzyme B and perforin, thus improving the efficacy of immunotherapy | [146] | ||
| CRC (AOM-DSS/MC38); bladder cancer (MB49); melanoma (B16-F10) | αCTLA-4 mAb | Inosine | Bifidobacterium pseudolongum; Lactobacillus johnsonii; Olsenella species | A2A receptor | Inosine produced by the gut microbiota can translocate to the tumor microenvironment and activated T cells by adenosine A2A receptor combined with costimulation of CpG and IL-12 released by DCs for Th1 differentiation, which resulted in IFN-γ production and enhanced ICB therapy | [134] |
| CRC (MC38); Lewis lung cancer (LLC1); breast cancer (4T1) | Oxaliplatin; αPD-1 mAb | Peptidoglycan | Bifidobacterium bifidum | TLR2 | Highly levels of peptidoglycan expressed by Bifidobacterim bifidum can act on the TLR2 receptor to stimulate IFN-γ secretion and improve antitumor therapy by αPD-1 mAb or oxaliplatin | [127] |
| CRC (AOM-DSS) | αCTLA-4 mAb | Lysates | Lactobacillus acidophilus | Lysates of L. acidophilus decreased Treg and M2 cell levels and increased the proportion of memory CD8+ T cells in tumor-draining lymph nodes and mesenteric lymph nodes, thus improving αCTLA-4 mAb efficacy | [147] | |
| Sarcoma (MCA-205); RET melanoma; CRC (CT26/MC38) | αCTLA-4 mAb | Capsular polysaccharide | Bacteroides thetaiotaomicron; Bacteroides fragilis | The capsular polysaccharides of Bacteroides thetaiotaomicron and Bacteroides fragilis induce the maturation of lamina propria DCs, which combined with the Th1 immune response induced by IL-12 secretion and promoted the antitumor effect of αCTLA-4 mAb | [71] | |
| CRC (CT26) | αPD-1 mAb | Glycerophospholipid | Prevotell; Akkermansia | Changes in gut microbiota composition lead to changes in glycerophospholipid metabolism, which affect IFN-γ and IL-2 expression in the tumor microenvironment, resulting in different therapeutic effects of αPD-1 mAb | [148] | |
| CRC (MC38); lymphoma (EG7) | Oxaliplatin | Butyrate | ID2 | Butyrate could improve the expression of ID2 by inhibiting HDACs, which promoted IL12R production, and boosted the efficacy of antitumor therapy after stimulation with IL-12 from DCs | [133] |
TAM tumor associated macrophage, IL-2/12/22 interleukin-2/12/22
Given the diversity of results, increasing attention is being paid to integrating data from multiple cohorts or to further expanding the cohorts so that the optimal combination of key microbes that further improve the clinical application prospects of strategies targeting the gut microbiota for tumor immunotherapy can be identified.
Adoptive T-cell therapy (ACT)
The therapeutic effect of ICB is dependent on the presence of preexisting tumor-specific immune cells [149], so its clearance effect is limited in certain tumors with low immunogenicity. Due to this phenomenon, artificial supplementation of tumor-specific immune cells may have a better therapeutic effect. ACT uses autoimmune T cells such as TILs or cytotoxic T lymphocytes (CTLs) to combat cancer. In 1985, transfusions of autologous mature lymphocytes were first reported to produce effective cancer regression [150]. The ACT treatment process typically consists of three steps: (1) isolating and extracting T cells from patient tumor tissues or peripheral blood vessels; (2) culturing and enriching for lymphocytes in vitro; and (3) reinjecting the amplified specific T cells into the patient [151, 152]. With the development of basic biology and immunology, research on the characteristics of immune cells has become increasingly comprehensive. Many studies have shown that T cells produce more specific TCRs or chimeric antigen receptors (CARs) in vitro through gene modification, which could produce a stronger antitumor immune response after infusion into patients [153–155]. Unlike conventional TCRs, CARs recognize antigens independent of major histocompatibility complex (MHC) antigen presentation, avoiding the restriction of MHC molecules and solving the problem of tumor immune escape caused by the inhibition of MHC molecule expression [156, 157]. Recently, ACT, especially CAR-T-cell therapy, has shown excellent efficacy in patients with hematological malignancies and metastatic melanoma [158, 159]. This rapid development of cell therapy brings more hope and possibilities for a cure to patients, but the rate of response to cell therapy also needs to be further improved.
Given the widely accepted role of the gut microbiome in ICB therapy, its impact on CAR-T-cell efficacy was supported by indirect evidence [19, 160, 161] and confirmed in a retrospective cohort study [162]. The study (n = 228) found that antibiotic use during the first 4 weeks of treatment was associated with poorer survival and increased neurotoxicity in B-cell lymphoma and leukemia patients receiving CD19-targeted CAR-T therapy. 16S rRNA and metagenomic sequencing of feces showed that Ruminococcus, Bacteroides and Faecalibacterium were related to the efficacy of CD19 CAR-T treatment. Bacterial metabolic pathways, such as peptidoglycan synthesis and pentose phosphate metabolism, may be biomarkers of CD19 CAR-T-cell efficacy. Moreover, a clinical trial revealed that the irAEs produced in response to CAR-T, especially cytokine release syndrome, are also modulated by the gut microbiome in patients with hematologic malignancies, and similarly, Faecalibacterium and Ruminococcus are enriched in patients who achieved complete remission [118]. The dominant bacteria and detailed mechanism need further confirmation and exploration. Future application of this mechanism would uniquely enable the stimulation or activation of therapeutic T cells by specific metabolites produced by microbes in vitro before their transplantation into patients.
The relationship between the gut microbiome and ACT response in solid tumors has not yet been reported. However, gut microbes can stimulate tumor cells to secrete a variety of chemokines in patients with colorectal cancer, thus recruiting more T cells into the TME and improving the prognosis of patients. These results revealed the role of the gut commensal microbiota in controlling the extent of tumor invasion by immune cells [19]. In another analysis of plasma samples from colorectal cancer patients treated with ACT in combination with chemotherapy, the abundance of Bifidobacterium, Lactobacillus and Enterococcus in the blood of responders was higher than that in nonresponders [94]. Therefore, using the blood microbiome to predict the effect of tumor immunotherapy may be a more convenient and effective method. In animal models, there were significant differences in the effectiveness of ACT against tumors in mice with similar genes but different origins. Fecal microbiota sequencing showed significant differences in the fecal microbiota composition of mice with different origins, and vancomycin exposure could enhance the therapeutic effect of ACT. This effect was demonstrated by increasing systemic CD8α+ DC and IL-12 levels and was associated with more efficient expansion of adoptive antitumor T cells in mice [160]. This study confirmed that it is possible to improve the therapeutic effect of ACT by modulating the gut microbiome composition and promoting the tumor immune response.
Currently, studies on the impact of gut microbes on CAR-T therapy are still in the stage of discovery and validation, but due to the considerable role of the gut microbiome in antitumor immunity, it is believed that there will be increasing evidence to support the effects of this relationship and the specific mechanisms involved.
Association between the gut microbiome and immunotherapy-related toxicity
Although immunotherapy has achieved remarkable results in the clinical application of tumor patients, there are still a considerable number of cases with toxic reactions, especially irAEs.
In the ICB therapy setting, irAEs correlate with the type of ICB used, such as anti-CTLA4 therapy, which tends to induce colitis and pituitary inflammation, and anti-PD-1 therapy, which tends to induce thyroid dysfunction and pneumonia. The patient baseline gut microbiome has been shown to correlate with the risk of irAEs. In a study of 77 patients with advanced melanoma treated with a combination of CTLA-4 and PD-1 blocking, analysis of blood, tumor, and gut microbes showed that irAEs were not associated with the α diversity of the microbiome but were associated with the baseline abundance of specific bacterial taxa, including Bacteroides intestinalis and Intestinibacter bartlettii. The abundance of Bacteroides was positively correlated with irAEs occurrence and IL-1β levels in the intestinal mucosa, and this conclusion was verified in mouse models [96]. In another study of patients with metastatic melanoma treated with a CTLA-4 and PD-1 blocking combination, Bacteroides dorei and B. vulgatus were found to be associated with irAEs in a reversed pattern [163]. In a study of 26 patients with metastatic melanoma treated with anti-CTLA-4 monotherapy, the author reported that the baseline abundance of Faecalibacterium and other Firmicutes were associated with both treatment efficiency and irAEs, and Bacteroidetes were associated with lower treatment response and lower irAEs [164].
The differences between the two studies may be due to differences in treatment methods or may be the result of functional redundancy between different microbes. In addition, there may be geography-related differences in the microbial communities associated with ICB efficacy and irAEs. Simpson et al. compared fecal microbes of 103 patients with metastatic melanoma from Australia and the Netherlands treated with neoadjuvant ICBs and found that the Ruminococcaceae family taxa and AKK were associated with lower treatment effectiveness and more severe irAEs.
In addition, C-reactive protein in peripheral blood can be used as a biomarker for severe irAEs [116]. In the context of immune agonist antibody (IAA) therapy, such as anti-CD40 and anti-CD137, the presence of gut microbiota is correlated with therapeutic toxic reactions such as cytokine release syndrome (CRS), liver damage and colitis. The incidence of toxic reactions was significantly reduced in GF mice or mice treated with antibiotics, while there was no significant effect on the therapeutic efficiency [165]. Although this study did not investigate the function of specific bacterial taxa in depth, the comparison of IAA with ICBs with different effects has implications for intestinal microbial immunotherapy of tumors.
CAR-mediated toxicities are still a problem in CAR-T therapy, including CRS or immune effector cell-associated neurotoxicity. Smith and colleagues reported that antibiotic use was associated with increased neurotoxicity in B-cell lymphoma and leukemia patients treated with CD19-targeted CAR-T therapy [162]. These results not only confirmed the relationship between the intestinal microbiota and the effect and side effects of tumor immunotherapy but also provided a strategic basis for improving the effect of immunotherapy by regulating the intestinal microbiota in different ways.
Modulation of the gut microbiome to facilitate tumor immunotherapy
The diversity and composition of the gut microbiota are associated with the efficacy of various cancer treatment strategies. In addition to demonstrating the correlation between the two, most studies in this area have demonstrated that the gut microbiota is a therapeutic target, and its modulation is a tool to improve the prospects of clinical application [166]. Large-scale population studies have shown that the diversity of commensal microbial communities in different populations or individuals is largely shaped by environmental factors [167]. With a focus on achieving high clinical compliance or enabling function in daily life, several strategies have been studied to regulate the composition of the gut microbiome to promote immune-related antitumor therapy. The next section reviews the literature related to these studies published in recent years.
Fecal microbiota transplantation (FMT)
Despite the controversy over its mechanism and safety, FMT has been widely used in the treatment of recurrent Clostridium difficile infection (CDI) in the past decade and has achieved good results [168], which also laid the foundation for the therapeutic application of FMT in the treatment of other diseases. Clinical studies on antitumor immunotherapy using the FMT strategy are in the initial stage, and many results have been achieved mainly in animal models [169].
FMT is performed on the premise that the degree of host response to immunotherapy can be transmitted between different individuals through fecal components. Numerous studies have found that GF mice transplanted with stool from patients who had a clinically significant response to ICB treatment are more likely to develop an antitumor immune response to ICB treatment than control mice transplanted with stool from nonresponders. Specifically, tumor progression is slowed, and overall survival is significantly prolonged [18, 72, 170]. These results suggest that the therapeutic outcome of ICB may be influenced by regulating the gut microbiota of tumor patients. More recently, Baruch et al. [128] and Davar et al. [171] verified the efficacy of FMT in anti-PD-1 immunotherapy in patients with metastatic melanoma for the first time in a clinical trial. Two separate studies each observed evidence of clinical benefit in some patients who received FMT treatment. Based on the benefits of FMT in anti-PD-1 therapy in a mouse model [72], Baruch et al. designed a phase I clinical trial (NCT03353402) in which stool donors, including two melanoma patients who received anti-PD-1 antibody therapy and achieved complete remission, were recruited to evaluate the safety and feasibility of FMT combined with anti-PD-1 antibody immunotherapy in 10 patients with refractory metastatic melanoma. Clinical response results were observed in three patients, including two partial responses and one complete response. Moreover, FMT treatment was associated with favorable changes in immune cell infiltration and gene expression profiles in the intestinal lamina propria and TME. In the clinical trial (NCT03341143) conducted by Davar et al., 6 of the 15 patients enrolled in the trial exhibited clinical benefits, including rapid and lasting changes in their gut microbiome and increased abundance of some previously reported gut microbial taxa associated with clinical response, such as Bifidobacterium. longum, Collinsella aerofaciens, and E. faecium [18, 72]. In the TME, increased activation of CD8+ T cells and a decreased proportion of myeloid cells expressing IL-8 were observed. In addition, patients treated with FMT had different proteomic and metabolomic characteristics. These two studies also became the first clinical proof-of-concept studies showing that FMT overcomes ICB resistance [172], which greatly expanded the prospects of the clinical application of FMT in antitumor immunotherapy strategies. In addition, there are many other ongoing or completed clinical trials [15, 173–176], which provide strong evidence for the safety and efficacy of FMT regulation of the gut microbiome in the treatment of tumors.
Nevertheless, the compatibility of the donor feces and the recipient intestinal microenvironment during FMT remains to be further ascertained. In addition, there are many nontarget components of donor feces that are transplanted into the recipient’s body, which have unknown effects. Thus, FMT remains a temporary, outcome-oriented solution until the mechanism of action and criteria for use are fully understood. More in-depth mechanistic exploration and the identification of more refined treatment strategies are the main directions of future research.
Probiotics
The concept of probiotics was first proposed by Metchnikoff [177] and is defined by the Food and Agriculture Organization of the United Nations as “living microorganisms beneficial to the host when injected in sufficient quantities” [178]. However, probiotics that are considered dietary supplements do not need to go through strict review by drug regulatory authorities before being marketed [179], which has resulted in the absence of formulation standards and quality control, exaggerated efficacy and lack of scientific experimental data regarding probiotics [180]. Therefore, while probiotics have been recognized academically for their health benefits, they are still misunderstood by many people. We need to continue to explore the effect and mechanism of probiotics using advanced science and revise the general understanding of probiotic therapy.
Due to the impact of probiotic supplementation on the composition of the gut microbiota, it is rational that the application of probiotics could enhance the host immune response [181, 182], as well as the efficiency of antitumor therapy (Table 3). Lee Se-Hoon et al. found that B. bifidum was significantly enriched among the fecal microbes in NSCLC treatment responders in a study of patients treated with different methods, and it was found in an animal model that supplementation with B. bif_K57 combined with oxaliplatin or anti-PD-1 antibody significantly enhanced the antitumor effect. Mechanistic studies showed that the probiotic strain B. bif_K57 could significantly enhance the immune response in the TME and increase the activation of CD4+ and CD8+ T cells, as well as the secretion of the cytokines IFN-γ and IL-2. These effects may be achieved through the ability of the B. bif_K57 strain to synthesize peptidoglycan [127]. Mager Lukas et al. found that inosine produced by Bifidobacterium pseudolongum activates the T-cell-specific A2AR signaling pathway and stimulates strong antitumor immunity in the tumor and spleen, thereby promoting the efficacy of anti-CTLA-4 antibodies in mouse colon cancer [134]. In addition to supplementation with Bifidobacterium, preclinical studies have found that supplementation with other probiotics, such as Lactobacillus [138, 147, 183] or Streptococcus thermophilus [139], could significantly improve tumor immunotherapy. In addition, AKK is another species of probiotic that has been discovered and widely accepted in recent years with the development of microbial sequencing technology [184, 185]. AKK, which can induce an adaptive immune response in follicular helper T cells (TFH) in Peyer’s patches to maintain intestinal homeostasis, has been reported [78]. Model mice orally supplemented with AKK combined with anti-PD-1 antibody [17] or cisplatin [186] showed apparently improved therapeutic outcomes. Shi et al.’s study revealed that the outer membrane protein of AKK, Amuc, could recruit more tumor-specific cytotoxic T cells in the TME by activating TLR2 signaling and reducing the levels of immunosuppressed Tregs, thus producing a considerable antitumor effect when IL-2 was combined as an adjuvant [131]. These results suggest that beneficial probiotics for health have good prospects for use in research on antitumor effects. In addition to the use of single probiotic strains, it has been suggested that a mixture of multiple strains may be needed to influence the complex microecosystem of the gut. Tanoue et al. isolated 11 strains from the feces of volunteers and mixed them, which could significantly induce the accumulation of IFNγ+ CD8+ T cells in the intestinal tract of mice, and synergistic ICB produced a significant therapeutic effect in a mouse tumor model; the induction effect was optimal only when all 11 strains were present [187]. These results implied that the interaction of the gut microbiome with the immune system and tumor may involve more complex systemic processes.
Table 3.
Studies on improving the effect of antitumor immunotherapy by probiotics
| Tumor type | Immunotherapy treatment | Species/strain | Overview of mechanism | References |
|---|---|---|---|---|
| CRC (AOM-DSS/MC38); bladder cancer (MB49); melanoma (B16-F10) | αCTLA-4 mAb | Bifidobacterium pseudomonas | Inosine produced by the gut microbiota can translocate to the tumor microenvironment and activated T cells by adenosine A2A receptor combined with costimulation of CpG and IL-12 released by DCs for Th1 differentiation, which results in IFN-γ production and enhanced ICB therapy | [134] |
| Sarcoma (MCA205); RET melanoma; Lewis lung cancer (LLC) | αPD-1 mAb; αCTLA-4 mAb combined with αPD-1 mAb | Akkermansia muciniphila | Oral supplementation with Akkermansia muciniphila restored the efficacy of ICB in an il-12-dependent manner by increasing the recruitment of CCR9+ CXCR3+ CD4+ T lymphocytes into tumor beds | [17] |
| CRC (MC38); Lewis lung cancer (LLC1); breast cancer (4T1) | Oxaliplatin; αPD-1 mAb | Bifidobacterium bifidum | Peptidoglycan expressed at high levels in Bifidobacterim bifidum can act as TLR2 receptor to stimulate IFN-γ secretion and improve antitumor therapy by αPD-1 mAb or oxaliplatin | [127] |
| CRC (MC38); melanoma (BrafV600E Pten−/−) | αCTLA-4 mAb; αPD-1 mAb | A mixture of 11 strains (11-mix) | An 11-strain mix induces the accumulation of IFN-γ+ CD8+ T cells through the effects of CD103+ DCs in the colonic lamina propria in an MHC Ia dependent manner, thereby activating the antitumor immune response | [187] |
| Colon cancer | αCTLA-4 mAb | Lactobacillus acidophilus | Lysates of Lactobacillus acidophilus reduced the number of Treg and M2 cells in tumor-draining lymph nodes and mesenteric lymph nodes, increased the ratio of memory CD8+ T cells, and promoted an antitumor immune response | [147] |
| Melanoma (B16.SIY/B16.F10) bladder cancer (MB49) | αPD-1 mAb | Bifidobacterium breve and Bifidobacterium longum mixtures | Bifidobacterium-derived signals enhanced the effector function of tumor-specific CD8+ T cells and promoted an antitumor immune response by modulating DC activation | [190] |
| Sarcoma (MCA205); RET melanoma; metastatic melanoma; CRC (MC38/CT26) | αCTLA-4 mAb | Bacteroides fragilis | The capsular polysaccharides of Bacteroides thetaiotaomicron and Bacteroides fragilis induce the maturation of lamina propria DCs, combined with the Th1 immune response induced by IL-12 secretion, and promotes the antitumor effect of αCTLA-4 mAb | [71] |
| Lewis lung cancer (LLC) | Cis-platinum | Akkermansia muciniphila | AKK combined with cis-platinum could increase the levels of IFN-γ, IL-6 and TNF-α in peripheral blood and the spleen in mice, inhibit the expression of CD4+ CD25+ Foxp3+ Treg cells, and promote an antitumor immune response | [186] |
| CRC (CT26) | αPD-1 mAb | Akkermansia muciniphila | The glycerophospholipid generation produced by AKK bacteria affects the expression of IFN-γ and IL-2 in tumor microenvironment, resulting in different therapeutic effects of αPD-1 mAb | [148] |
| Melanoma (B16-F10); CRC (CT26) | Systemic IL-2 therapy | Akkermansia muciniphila | Amuc (AKK outer membrane protein) activates antitumor immunity through the TLR2 signaling pathway | [131] |
| CRC (AOM/DSS, MC38/CT26); Lewis lung cancer (LLC); melanoma (B16-F10) | With or without αPD-1 mAb | Clostridiales (Ruminococcaceae, Lachnospiraceae): Roseburia intestinalis; Eubacterium hallii; Faecalibacterium prausnitzii; Anaerostipes caccae | Clostridiales promote antitumor immune response in a CD8+ T dependent manner | [191] |
| CRC (MC38) | αPD-1 mAb | Lactobacillus paracasei sh2020 | Colonization of Lactobacillus paracasei sh2020 induces increased CXCL10 expression in tumors, which in turn promotes recruitment of CD8+ T cells and promotes an antitumor immune response | [192] |
| Melanoma (B16-F10); sarcoma (MCA205); CRC(MC38) | αPD-1 mAb; αCTLA-4 mAb | Enterococcus faecium; Enterococcus faecalis | Enterococcus with unique NlpC/P60 peptidoglycan hydrolase activity can produce peptides that activate NOD2 activity and modulate the efficacy of ICB therapy in vivo, promoting antitumor immunity | [193] |
| CRC (CT26) | αPD-1 mAb | Lactobacillus rhamnosus | Lactobacillus rhamnosus could effectively restore the gut microbiota depleted by antibiotics, significantly increase the relative abundance of beneficial bacteria, and promote the therapeutic effect of αPD-1 mAb | [194] |
| CRC (CT26) | Neoantigen cancer vaccine | Bifidobacterium (B. bifidum, B. longum, B. lactis and B. breve) | Bifidobacterium could affect the mechanism of tumor growth, change the composition of the gut microbiota, increase the abundance of antitumor Muribaculaceae, reduce the levels of tumor-promoting Lachnospiraceae, and promote the antitumor effect of a neoantigen cancer vaccine | [195] |
| CRC (MC38); sarcoma (MCA205); lung cancer (TC-1) | Cyclophosphamide; αPD-1 mAb | Enterococcus hirae | The tape measure protein (TMP) of the probacteriophage found in the genome of Enterococcus hirae phage contains an epitope that can bind MHC-I. After treatment with cyclophosphamide or αPD-1 mAb, mice carrying Enterococcus hirae developed TMP-specific H-2Kb restrictive CD8+ T-cell responses that ultimately promoted antitumor immunotherapy | [75] |
| Melanoma (B16); CRC (CT26) | αTIM-3 mAb | Enterococcus hirae; Lactobacillus johnsonii | Probiotic administration restored the antitumor activity of αTIM-3 mAb that was impaired by antibiotics usage | [183] |
| CRC (MC38); lymphoma (EG7) | αCD-47 mAb | Mixture of Bifidobacterium species (B. bifidum, B. longum, B. brevis, B. lactis) | Systemic administration of Bifidobacterium leads to its accumulation in tumors, which can effectively stimulate STING signal transduction, increase the cross-initiation of DCs after αCD-47 mAb treatment, and promote an antitumor immune response | [196] |
| Breast cancer (4T1); liver cancer (H22) | TGF-β blockade | Escherichia coli strain Nissle 1917 (EcN) | EcN colonization could effectively promote tumor-specific effector T-cell infiltration and DC activation after TGF-β blockade, resulting in a stronger antitumor effect | [197] |
| Melanoma (B16-F10) | αPD-1 mAb | Lactobacillus kefiranofaciens ZW18 (ZW18) | ZW18 activated the immunity, promoted tumor CD8+ T-cell infiltration, and significantly increased the abundance of Akkermansia, the Prevotellaceae_NK3B31_group and Muribaculum | [198] |
| CRC (MC38/CT26/HCT116); breast cancer (4T1) | αPD-1 mAb; oxaliplatin | Lactococcus lactis GEN3013 | L. lactis GEN3013 augmented cytotoxic immune cell populations, including CD4+ T cells, CD8+ effector T cells, and NK cells, in the tumor microenvironment | [199] |
The considerable effect of oral probiotics on the antitumor immune response in mice provides a basis for the clinical study of probiotics in the tumor population. In a recent phase I trial (NCT03829111), 29 patients with mRCC were treated for the first time with nivolumab in combination with ipilimumab, and some were supplemented with CBM588, which contains Clostridium butyricum. Probiotic CBM588 significantly prolonged PFS in patients with mRCC without additional toxicity and improved response rates to combined ICB therapy [188]. Moreover, the administration of CBM588 to regulate gut microbiota improved the efficacy of ICB treatment in NSCLC cancer patients receiving PPIs [189]. Another analysis of 77 patients with advanced melanoma revealed a positive correlation between the toxicity of anti-PD-1 and anti-CTLA-4 antibodies and the presence of Bacteroides intestinalis in the feces of the patients, suggesting that the bacteria could be used as a target for reducing the side effects of ICB treatment [96]. At present, clinical studies on the use of probiotics in the treatment of cancer have been very rare, and the results of only individual clinical trials are not sufficient for cross-validation.
Taken together, these data indicate the potential advantages of probiotics for cancer treatment. However, it is still necessary to improve the quality control of commercial probiotics to confirm their antitumor effect and optimize the strategy for colonization by probiotics. In addition, the specific mechanism of antitumor immunity induced by probiotics needs to be further elucidated.
Diet and prebiotics
The microbes colonizing the gut can decompose and metabolize physical components that cannot be digested by the host, and the released nutrients can be absorbed by the body [200, 201]. Conversely, diet also affects the gut microbiome and metabolome [202, 203] (Table 4). This interaction has laid a theoretical foundation for the regulation of the bacterial consortium through specific metabolic pathways by dietary formulas.
Table 4.
Studies on improving the effect of antitumor immunotherapy by prebiotics
| Tumor type | Immunotherapy treatment | Prebiotic | Overview of mechanism | References |
|---|---|---|---|---|
| CRC (CT26); breast cancer (4T1); melanoma (B16-F10) | αCTLA-4 mAb; αPD-1 mAb | Exopolysaccharide (EPS-R1) | Dietary intake of EPS-R1 induces aggregation of CCR6+ CD8+ T cells in Peyer’s patches and enhances the therapeutic effect of ICBs in tumors in a CCL20-dependent manner | [130] |
| CRC (MC38) | αPD-1 mAb | Pectin | Pectin changes the composition of the gut microbiota by enriching for butyrate-producing bacteria and promotes butyrate production, which could promote T-cell infiltration and enhance the efficacy of αPD-1 mAb | [132] |
| Lewis lung cancer (LLC); melanoma (B16F10) | αPD-1 mAb | Ginseng polysaccharide (GPs) | GPs increased the antitumor response to αPD-1 mAb by increasing levels of the microbial metabolites valeric acid and decreasing L-kynurenine levels, as well as the ratio of Kyn/Trp, which contributed to the suppression of Treg cells and induction of Teff cells after combination treatment | [213] |
| CRC (MC38/CT26); melanoma (B16F10) | αPD-1 mAb | Inulin gel | Oral administration of inulin gel increased the abundance of SCFA-producing microbes and SCFAs, resulting in enhanced memory responses in IFN-γ+ CD8+ T cells and the formation of stem-like T-cell factor-1+ PD-1+ CD8+ T cells in the tumor microenvironment | [214] |
| Sarcoma (MCA-205); medullary breast cancer (E0771) | αPD-1 mAb | The berry of Myrciaria dubia | Castalagin, an active compound in polyphenol rich Myrciaria dubia berries, can enrich for Ruminococcaceae, increase the ratio of CD8+/Foxp3+ CD4+ T cells in the TME, and promote the biosynthesis of taurine binding bile acids, which translated into antitumor activity and a stronger anti-PD-1 response | [215] |
| CRC (MC38) | αPD-L1 mAb | Ultrafine Jujube powder | Ultrafine Jujube Powder consumption resulted in increased abundance of Clostridium (including Ruminococcaceae and Lachnospiraceae), increased SCFA production, and increased infiltration of cytotoxic CD8+ T cells in the tumor microenvironment | [191] |
| Melanoma (B16F10); breast cancer (4T1) | αPD-L1 mAb; Doxycycline | Smectite | Smectite loaded in lactic acid bacteria biofilms inhibits tumor growth and activates DCs through the TLR2 pathway, enhancing the efficacy of chemotherapy or immunotherapy | [217] |
| RET melanoma; renal cell carcinoma in situ (RENCA-luc); lung cancer in situ (TC-1_luc) | αPD-L1 mAb; αCTLA-4 mAb | Ketogenic diet; 3-hydroxybutyric acid | 3-Hydroxybutyric acid inhibited the expression of PD-L1 in ICB-associated myeloid cells, promoted the amplification of CXCR3+ T cells, and induced changes in the composition of gut microbiota, such as Eisenbergiella massiliensis, thus promoting the antitumor effect of ICB | [218] |
| Advanced NSCLC | αPD-1 mAb; αPD-L1 mAb | Fluorine-18 fluorodeoxyglucose (18F-FDG) | Physiological uptake of 18F-FDG by the colon before initiation of ICB was associated with better clinical outcomes and higher gut microbiome diversity in patients with advanced NSCLC | [219] |
| Solid tumor | αPD-1 mAb | SCFAs | High concentrations of SCFAs in feces were associated with longer PFS | [220] |
| CRC (MC38) | αPD-L1 mAb | Bilberry Anthocyanin Combo | Bilberry Anthocyanin Combo restored the species diversity of intestinal microbiota, increased the concentration and proportion of butyric acid in feces, enhanced the infiltration of CD8+ T cells in tumors, and promoted the effect of αPD-L1 mAb | [221] |
| CRC (CT26) | αPD-1 mAb | Gegen Qinlian decoction | The combined treatment of Gegen Qinlian decoction and αPD-1 mAb antibody can effectively inhibit the growth of CT26 tumor cells, change the composition of gut microbe, and promote the metabolism of glycerophospholipids and sphingolipids | [222] |
| Prostate cancer (RP-B6Myc); kidney cancer (RENCA) | αPD-1 mAb | Restricted protein diet | Dietary protein restriction alters the activity of tumor-associated macrophages and enhances their antitumor ability | [223] |
| melanoma (B16F10) | αPD-1 mAb | Diosgenin | The combination of αPD-1 mAb and diosgenin can induce enhanced response of tumor necrosis and apoptosis | [224] |
| CRC (MC38) | αPD-1 mAb combined with αCTLA-4 mAb | A standardized extract of cultured Lentinula Edodes Mycelia, AHCC® | AHCC® increased the species abundance of Ruminococcaceae, altered the composition of the gut microbiome, and promoted the antitumor effect of anti-PD-1 combined with anti-CTLA-4 immunotherapy by increasing the expression of GZMB and Ki-67 in tumor-infiltrating CD8+ T cells | [225] |
Geographically, different populations exhibit different dietary habits, which in turn act on the gut microbiota. Accordingly, a prospective trial profiled baseline gut microbiota signatures and dietary patterns among 103 patients from Australia and the Netherlands treated with ICBs for melanoma and performed an integrated analysis with data from 115 patients with melanoma treated with ICBs in the United States [116]. High dietary fiber intake in individuals from areas such as Australia and the United States may lead to greater clinical benefit for those with Bacteroidaceae-dominated microbiomes than in individuals from the Netherlands, who already have fiber-influenced microbiomes. Therefore, diet customization, especially involving dietary fiber, represents a potential strategy for improving tumor therapy. Dietary fiber components are metabolized to produce SCFAs [204, 205], which have been widely reported in the treatment of metabolic diseases [206, 207] and intestinal inflammation [200, 204]. In the last decade, the impact of SCFAs on tumor immunotherapy has also been reported (Table 4). He et al. found that butyrate could promote the therapeutic effect of the immunogenicity drugs oxaliplatin and anti-PD-1 antibody by directly activating antitumor CD8+ T cells [133]. However, another study found that a higher level of SCFAs in the peripheral blood may weaken the antitumor immune response induced by anti-CTLA-4 antibodies [208], suggesting that the effect of the metabolites is different in tissues. Therefore, the use of dietary fiber metabolites to regulate antitumor immunity requires more refined research.
Prebiotics are more specific than diet and comprise specific chemicals, which mainly include oligosaccharides and polysaccharides, that promote the growth of specific microbes [209, 210]. Prebiotic usage to promote antitumor immunotherapy has attracted increasing attention in recent years [211, 212]. Huang et al. found that oral administration of ginseng polysaccharide in mice could promote the antitumor effect of ICB, and its internal mechanism was that the supplemented prebiotic could improve the TME and systemic CD8+ T-cell function by reshaping gut microbial composition and tryptophan metabolism and inhibiting the effect of Treg cells, leading to the enhancement of the antitumor effect of the anti-PD-1 antibody [213]. In another study using an engineering approach to regulate beneficial microbes in the gut, Han Kai et al. prepared inulin gel that could be orally administered and released site-specifically in the colon to regulate microbial composition in situ, promote SCFA metabolism, induce a systemic T-cell memory response, and enhance the antitumor activity of anti-PD-1 antibody [214]. Furthermore, a recent study showed that a natural polyphenol from the berry of Myrciaria dubia could reverse ICB resistance by altering the intestinal microbiome composition [215]. These extracted or modified, one-component natural products can achieve a finer regulation of gut microbes than dietary supplements to a certain extent and offer more possibilities for tumor therapy by targeting the gut microbial structure.
Based on preclinical mouse models, a recently published clinical observational study of 128 melanoma patients treated with ICB found that 37 patients who met the 20 g/day dietary fiber intake threshold had significantly longer PFS than those who did not. Interestingly, the anti-PD-1 antibody showed better therapeutic efficacy in patients with adequate dietary fiber intake and no probiotic supplementation. To investigate the causal relationship between diet and treatment outcome, the authors established a mouse tumor model and found that a lower-fiber diet or supplementation with probiotics consisting mainly of B. longum and Lactobacillus rhamnosus GG weakened the antitumor effect of the anti-PD-1 antibody [216]. This result, particularly the contrary effect of probiotics, reemphasized the high complexity and specificity of targeting gut microbes to modulate the therapeutic effects of tumor therapy. Fortunately, gut microbes do therefore have potential as targets for antitumor immunotherapy, but the characteristics and mechanisms of action of gut microbes may be more complex than expected.
Antibiotics and other drugs
The purpose of using antibiotics for tumor patients is to prevent infections by various pathogenic microorganisms, which would inevitably change the composition of the gut microbiota. Therefore, a mass of preclinical studies and clinical cohort retrospective studies have reported that the use of antibiotics weakens the efficacy of tumor immunotherapy, especially ICB therapy (Table 5) [226]. Routy et al. evaluated the effect of antibiotics against PD-1 antibody therapy in 249 patients with advanced NSCLC, RCC, or urothelial carcinoma and found that PFS and OS were significantly shorter in patients who received antibiotics than in those who did not [17]. Chalabi et al. retrospectively analyzed two previous clinical trials investigating patients with metastatic NSCLC (NCT01903993; NCT02008227) and found that both antibiotics and another microbiome altering proton pump inhibitors (PPIs) affected atezolizumab treatment, shortening PFS and OS [227]. These results indicate that the gut microbiome plays an essential role in ICB treatment. Another piece of evidence supporting this idea is that the therapeutic effect of ICB may be affected by the spectrum of antibiotic action. Specifically, patients receiving broad-spectrum antibiotics have a shorter survival than those receiving narrow-spectrum antibiotics [228]. Interestingly, the effect of antibiotics on tumor immunotherapy is also related to the period of patient exposure to antibiotics; antibiotics may have beneficial effects before or 30 days after ICB treatment [229]. These studies showed that antibiotic usage during the ICB treatment period could weaken the tumor treatment effect, but antibiotics are still irreplaceable in preventing infection and postoperative complications after tumor surgical treatment [230, 231]. Hence, the design of an antibiotic treatment plan according to the actual situation of patients, such as an approach determined based on sequencing analysis of the gut microbiome before antibiotic treatment and customized antibiotic formulation, may reduce the disturbances mediated by broad-spectrum antibiotics in the gut microbiota and improve the therapeutic effect.
Table 5.
Studies on the inhibitory effect of antibiotics on immunotherapy
| Tumor type | Immunotherapy treatment | Number of included patients | Antibiotics | References |
|---|---|---|---|---|
| Melanoma; NSCLC; blood cancer; renal cancer; etc. | αPD-1/PD-L1 mAb, αCTLA-4 mAb, or combination treatment | 635 | β-Lactam, sulfa, quinolones, macrolides aminoglycosides, tetracycline | [236] |
| NSCLC; kidney cancer; bladder cancer; head and neck; melanoma | αPD-1/PD-L1 mAb, αCTLA-4 mAb, or combination treatment | 102 | β-Lactam; fluoroquinolone; cephalosporin; macrolides; sulfonamides | [237] |
| NSCLC; melanoma; upper airway and digestive tract carcinoma; renal cell carcinoma | αPD-1 mAb | 212 | β-Lactam; fluoroquinolone; tetracycline class | [238] |
| NSCLC | αPD-1 mAb; αPD-L1 mAb | 531 | β-Lactam | [239] |
| NSCLC; RCC; melanoma; lung cancer; etc. | αPD-1/PD-L1 mAb, αCTLA-4 mAb, or combination treatment | 5565 | β-Lactam; quinolone; vancomycin; daptomycin; linezoldone; meropenem; azithromycin; furantoin; tetracycline; penicillin; etc. | [240] |
| NSCLC; melanoma; etc. | αPD-1 mAb; αPD-L1 mAb | 196 | β-Lactam; quinolone; macrolides; sulfa; tetracycline; aminoglycosides; etc. | [241] |
| NSCLC; melanoma; RCC; etc. | αPD-1/PD-L1 mAb, αCTLA-4 mAb, or combination treatment | 12,492 | β-Lactam; fluoroquinolone; penicillins; carbapenems; metronidazole; macrolides; linezolid; vancomycin; ciprofloxacin; meropenem; tetracyclines; levofloxacin | [242] |
| Metastatic urothelial carcinoma (mUC) | αPD-1 mAb | 67 | Penicillins; cephalosporins; carbapenems; quinolone | [243] |
| Muscle-invasive bladder cancer | αPD-1 mAb | 149 | Fluoroquinolones | [244] |
| RCC | αPD-1 mAb | 93 | quinolone; amoxicillin | [245] |
| Advanced melanoma | αCTLA-4 mAb | 1585 | Systemic antibiotic | [246] |
| NSCLC; melanoma; RCC; others | αCTLA-4 mAb; αPD-L1 mAb; αPD-1 mAb | 217 | Concomitant systemic antibiotics | [247] |
| Urothelial carcinoma | αPD-L1 mAb | 1360 | Penicillins; quinolone; cephalosporins; glycopeptide; carbapenems; sulfa; macrolides; aminoglycosides; etc. | [248] |
| Acute myeloid leukemia (AML); NSCLC; RCC | ICI; chemotherapy | 338 | Piperacillin; clindamycin; metronidazole. meropenem; vancomycin; furantoin; rifampin. rifaximin; tobramycin | [249] |
| RCC | αPD-1 mAb | 69 | β-Lactam; quinolone | [170] |
| Melanoma | αPD-1 mAb; αCTLA-4 mAb; combined | 568 | Cephalosporins; penicillins; fluoroquinolone | [250] |
| Bladder cancer; head and neck carcinoma; melanoma; NSCLC; RCC; sarcoma; others | αPD-1/PD-L1 mAb, αCTLA-4 mAb, or combination treatment | 690 | β-Lactam | [251] |
| NSCLC; melanoma; urothelial carcinoma; RCC; lung cancer; sarcoma | αPD-1/PD-L1 mAb, αCTLA-4 mAb, or combination treatment | 2740 | Antibiotics | [252] |
| NSCLC | αPD-1 mAb; | 234 | β-Lactam; cephalosporins; quinolone | [253] |
| Melanoma | αPD-1 mAb; αCTLA-4 mAb; combined with chemotherapy | 74 | β-Lactam | [254] |
| NSCLC | αPD-1 mAb; αCTLA-4 mAb | 109 | β-Lactam; fluoroquinolone; etc. | [255] |
| NSCLC; RCC | Anti-PD-L1 | 360 | β-Lactam; quinolone; sulfa | [81] |
| mRCC | αPD-1/PD-L1 mAb; mTOR inhibitors; IFN-α; VEGF-TT | 4290 | β-Lactam; fluoroquinolone; macrolides; tetracyclines; etc. | [256] |
Nevertheless, there does not always seem to be a positive correlation between bacterial burden and treatment outcomes. In a retrospective review of 57 patients with MSI-H/dMMR mCRC receiving anti-PD-1 mAb, there was no association of lower response rates or survival in those patients exposed to antibiotics [232]. Gut microbes can relocate into surrounding tissues and influence tumor progression. Broad-spectrum antibiotic depletion of the gut microbiome prevented invasive PDAC and enhanced the antitumor immune response [32]. These results showed that the role of the gut microbiome in tumor therapy was contrary to previous results, suggesting that an excessive intestinal microbe load or a high concentration of microorganisms in tumors may have adverse effects on tumor therapy. Etiologically, the studies that observed negative effects of the microbiome on therapy outcomes have involved studies of pancreatic cancer [233, 234], and one possible explanation is that the pancreas and gut are anatomically connected through the pancreatic duct [235]. The continuity of the two may lead to a higher microbial load in the pancreas than in other tissues, which is related to the occurrence and immunosuppression of pancreatic cancer. These results suggest that the different benefits of the gut microbiome on different tumor types may be related to the distribution of the microbiome in different organs.
Future prospects: opportunity and challenge coexist
With the gradual increase in attention towards the important role of the gut microbiome in human health, research on the formation, development, diagnosis and treatment of tumors is steadily advancing to a new stage. Due to the widespread application of NGS, metabolomics, proteomics and other multiomics techniques, researchers have gradually gained a clearer understanding of the colonization, transfer and recolonization of microbes in various tissues and organs of the body and identified the close relationship of function and causation between health and disease states and the colonization of microbes in tissues, including those in the TME. Finally, the concept of the “polymorphic microbiome” has been regarded as a new hallmark of cancer [21].
Nevertheless, some substantial problems exist that prevent gut microbes from acting as more potent weapons against a variety of diseases, including tumors. First, due to the limitation of sample allocation, the collected sample information cannot truly reflect the microbial composition of the gut in space [257]. It is still to be discussed that stool samples represent the bacterial colonization in the niche of the gut-intestinal tract, and it is even more difficult to extract microbiota from tumors or other focal sites. In addition, there are also many obstacles to monitoring the temporal changes in the gut microbiome during disease progression or treatment because the gut microbiota is easily affected by diet, environment, host age, gender, lifestyle and other factors, which reflect the collective changes in multiple factors. Therefore, it is difficult to determine the causal relationship between the gut microbiome and diseases. The second is the functional diversity of the gut microbiota. For example, taxonomically, bacteria of the same species differ greatly in function. In addition, the same bacteria may show different characteristics due to environmental changes or the composition of other bacteria, and different bacteria may share similar metabolic pathways with similar functions [47, 193]. Hence, the interactions between bacteria should not be ignored. Finally, there is a diversity of data and differences in analysis techniques. Although there is a wealth of multiomics data, including human samples, indicating the microbiome associated with disease and treatment outcomes, reproducibility of results across research projects has been difficult to achieve (Table 1) [258]. This may be due to, on the one hand, the differences that researchers collect or preserve samples for research objects; on the other hand, the generation of many multiomics data makes the computational inference of data a challenge, accompanied by redundant data analysis methods. The solution to this problem may require standard analytical manual in the future, including machine learning and artificial intelligence, as well as better data and resource-availability mechanisms.
Conclusion
The existing research results have provided substantial evidence for the conclusion that the commensal microbiome impacts the efficiency of antitumor immunotherapy, and promoting the effect of antitumor immunotherapy by modulating the composition of gut microbiota has been shown to work. However, due to the variation in individual microbiomes and limitations of complex multiomics analysis, there is a lack of systematic research on the factors of the microbiome that are involved in antitumor therapy, and mutual authentication among study conclusions cannot be obtained. Moreover, precise mechanistic research on the impact of the microbiome on antitumor immunity is still scarce. As a result, the manipulation of the commensal microbiota by modern medical techniques for the treatment of diseases, including tumors, is still far from clinical application.
Nonetheless, it is optimistic that considerable resources are being devoted to research that links commensal microbiota and host health and disease. Preclinical and clinical studies have demonstrated that regulation of the gut microbiota may improve the efficacy of antitumor therapy from multiple perspectives and at multiple levels. In addition to knowledge regarding the individual differences in the commensal microbiome and the diversity of influencing factors, individual multiomics analysis combined with precision medicine, instead of broad FMT or probiotics, will inevitably become the future direction of the development of tumor immunotherapy or treatment for other diseases affected by the microbiome.
Abbreviations
- PFS
Progression-free survival
- OS
Overall survival
- NGS
Next-generation sequencing
- ICB
Immune checkpoint blockade
- ACT
Adoptive T-cell therapy
- GF
Germ-free
- PRRs
Pattern recognition receptors
- TLRs
Toll-like receptors
- MAMPs
Microbe-associated molecular patterns
- LPS
Lipopolysaccharides
- DCs
Dendritic cells
- TJ
Tight junction
- TME
Tumor microenvironment
- SCFAs
Short-chain fatty acids
- Møs
Macrophages
- NK cell
Natural killer cell
- GPCR
G-protein-coupled receptor
- HDACs
Histone deacetylases
- IFN-γ
Interferon-γ
- GZMB
Granzyme B
- BA
Bile acid
- TCRs
T-cell receptors
- CTLA-4
Cytotoxic T-lymphocyte-associated antigen 4
- PD-1
Programmed cell death protein 1
- PD-L1
Programmed cell death protein 1 ligand
- irAEs
Immune-related adverse reactions
- FMT
Fecal microbiota transplantation
- PAMPs
Pathogen-associated molecular patterns
- NSCLC
Non-small cell lung cancer
- TMAO
Trimethylamine N-oxide
- AhR
Aryl hydrocarbon receptor
- A2AR
Adenosine 2A receptor
- TILs
Tumor-infiltrating lymphocytes
- CTLs
Cytotoxic T lymphocytes
- CARs
Chimeric antigen receptors
- MHC
Major histocompatibility complex
- AKK
Akkermansia muciniphila
- PPIs
Proton pump inhibitors
- PDAC
Pancreatic ductal adenocarcinoma
- BDSCs
Myeloid-derived suppressor cells
- CLRs
C-type lectin receptors
Author contributions
All authors have read and approved the article. WJ and GC organized, wrote, and revised the manuscript. JC and GX revised the manuscript. GC, KL, XL, LK and XQ summarized the literature.
Funding
This work was supported by the Shandong Provincial Laboratory Project [SYS202202]; the National Natural Science Foundation of China [82272819, 81972888]; the Research Project of Jinan Microecological Biomedicine Shandong Laboratory [JNL-202219B, JNL-202204A, JNL-2023017D]; the Primary Research & Development Plan of Jiangsu Province [BE2022840]; and the Open Project of Chinese Materia Medica First-Class Discipline of Nanjing University of Chinese Medicine [No. 2020YLXK007].
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Chunping Jiang, Email: chunpingjiang@nju.edu.cn.
Junhua Wu, Email: wujunhua@nju.edu.cn.
References
- 1.Bagchi S, Yuan R, Engleman EG. Immune checkpoint inhibitors for the treatment of cancer: clinical impact and mechanisms of response and resistance. Annu Rev Pathol. 2021;16:223–249. doi: 10.1146/annurev-pathol-042020-042741. [DOI] [PubMed] [Google Scholar]
- 2.Galluzzi L, Chan TA, Kroemer G, Wolchok JD, Lopez-Soto A. The hallmarks of successful anticancer immunotherapy. Sci Transl Med. 2018;10(459):eaat7807. doi: 10.1126/scitranslmed.aat7807. [DOI] [PubMed] [Google Scholar]
- 3.Hegde PS, Chen DS. Top 10 challenges in cancer immunotherapy. Immunity. 2020;52(1):17–35. doi: 10.1016/j.immuni.2019.12.011. [DOI] [PubMed] [Google Scholar]
- 4.Sharma P, Hu-Lieskovan S, Wargo JA, Ribas A. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell. 2017;168(4):707–723. doi: 10.1016/j.cell.2017.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheng WY, Wu CY, Yu J. The role of gut microbiota in cancer treatment: friend or foe? Gut. 2020;69(10):1867–1876. doi: 10.1136/gutjnl-2020-321153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cullin N, Azevedo Antunes C, Straussman R, Stein-Thoeringer CK, Elinav E. Microbiome and cancer. Cancer Cell. 2021;39(10):1317–1341. doi: 10.1016/j.ccell.2021.08.006. [DOI] [PubMed] [Google Scholar]
- 7.Sender R, Fuchs S, Milo R. Revised estimates for the number of human and bacteria cells in the body. PLoS Biol. 2016;14(8):e1002533. doi: 10.1371/journal.pbio.1002533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Almeida A, Mitchell AL, Boland M, Forster SC, Gloor GB, Tarkowska A, et al. A new genomic blueprint of the human gut microbiota. Nature. 2019;568(7753):499–504. doi: 10.1038/s41586-019-0965-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tanaka M, Nakayama J. Development of the gut microbiota in infancy and its impact on health in later life. Allergol Int. 2017;66(4):515–522. doi: 10.1016/j.alit.2017.07.010. [DOI] [PubMed] [Google Scholar]
- 10.Human Microbiome Project C Structure, function and diversity of the healthy human microbiome. Nature. 2012;486(7402):207–214. doi: 10.1038/nature11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Integrative HMPRNC The integrative human microbiome project: dynamic analysis of microbiome-host omics profiles during periods of human health and disease. Cell Host Microbe. 2014;16(3):276–289. doi: 10.1016/j.chom.2014.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lynch SV, Pedersen O. The human intestinal microbiome in health and disease. N Engl J Med. 2016;375(24):2369–2379. doi: 10.1056/NEJMra1600266. [DOI] [PubMed] [Google Scholar]
- 13.Lin K, Zhu L, Yang L. Gut and obesity/metabolic disease: focus on microbiota metabolites. MedComm. 2022;3(3):e171. doi: 10.1002/mco2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sepich-Poore GD, Zitvogel L, Straussman R, Hasty J, Wargo JA, Knight R. The microbiome and human cancer. Science. 2021;371(6536):eabc4552. doi: 10.1126/science.abc4552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Helmink BA, Khan MAW, Hermann A, Gopalakrishnan V, Wargo JA. The microbiome, cancer, and cancer therapy. Nat Med. 2019;25(3):377–388. doi: 10.1038/s41591-019-0377-7. [DOI] [PubMed] [Google Scholar]
- 16.Woelk CH, Snyder A. Modulating gut microbiota to treat cancer. Science. 2021;371(6529):573–574. doi: 10.1126/science.abg2904. [DOI] [PubMed] [Google Scholar]
- 17.Routy B, Le Chatelier E, Derosa L, Duong CPM, Alou MT, Daillere R, et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science. 2018;359(6371):91–97. doi: 10.1126/science.aan3706. [DOI] [PubMed] [Google Scholar]
- 18.Gopalakrishnan V, Spencer CN, Nezi L, Reuben A, Andrews MC, Karpinets TV, et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science. 2018;359(6371):97–103. doi: 10.1126/science.aan4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cremonesi E, Governa V, Garzon JFG, Mele V, Amicarella F, Muraro MG, et al. Gut microbiota modulate T cell trafficking into human colorectal cancer. Gut. 2018;67(11):1984–1994. doi: 10.1136/gutjnl-2016-313498. [DOI] [PubMed] [Google Scholar]
- 20.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 21.Hanahan D. Hallmarks of cancer: new dimensions. Cancer Discov. 2022;12(1):31–46. doi: 10.1158/2159-8290.CD-21-1059. [DOI] [PubMed] [Google Scholar]
- 22.Scott AJ, Alexander JL, Merrifield CA, Cunningham D, Jobin C, Brown R, et al. International Cancer Microbiome Consortium consensus statement on the role of the human microbiome in carcinogenesis. Gut. 2019;68(9):1624–1632. doi: 10.1136/gutjnl-2019-318556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Song M, Chan AT. Environmental factors, gut microbiota, and colorectal cancer prevention. Clin Gastroenterol Hepatol. 2019;17(2):275–289. doi: 10.1016/j.cgh.2018.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Diaz P, Valenzuela Valderrama M, Bravo J, Quest AFG. Helicobacter pylori and gastric cancer: adaptive cellular mechanisms involved in disease progression. Front Microbiol. 2018;9:5. doi: 10.3389/fmicb.2018.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Di Domenico EG, Cavallo I, Pontone M, Toma L, Ensoli F. Biofilm producing Salmonella typhi: chronic colonization and development of gallbladder cancer. Int J Mol Sci. 2017;18(9):1887. doi: 10.3390/ijms18091887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boleij A, Hechenbleikner EM, Goodwin AC, Badani R, Stein EM, Lazarev MG, et al. The Bacteroides fragilis toxin gene is prevalent in the colon mucosa of colorectal cancer patients. Clin Infect Dis. 2015;60(2):208–215. doi: 10.1093/cid/ciu787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parida S, Wu S, Siddharth S, Wang G, Muniraj N, Nagalingam A, et al. A procarcinogenic colon microbe promotes breast tumorigenesis and metastatic progression and concomitantly activates notch and beta-catenin axes. Cancer Discov. 2021;11(5):1138–1157. doi: 10.1158/2159-8290.CD-20-0537. [DOI] [PubMed] [Google Scholar]
- 28.Liu QQ, Li CM, Fu LN, Wang HL, Tan J, Wang YQ, et al. Enterotoxigenic Bacteroides fragilis induces the stemness in colorectal cancer via upregulating histone demethylase JMJD2B. Gut Microbes. 2020;12(1):1788900. doi: 10.1080/19490976.2020.1788900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rubinstein MR, Wang X, Liu W, Hao Y, Cai G, Han YW. Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating E-cadherin/beta-catenin signaling via its FadA adhesin. Cell Host Microbe. 2013;14(2):195–206. doi: 10.1016/j.chom.2013.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rubinstein MR, Baik JE, Lagana SM, Han RP, Raab WJ, Sahoo D, et al. Fusobacterium nucleatum promotes colorectal cancer by inducing Wnt/beta-catenin modulator Annexin A1. EMBO Rep. 2019;20(4):e47638. doi: 10.15252/embr.201847638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gur C, Ibrahim Y, Isaacson B, Yamin R, Abed J, Gamliel M, et al. Binding of the Fap2 protein of Fusobacterium nucleatum to human inhibitory receptor TIGIT protects tumors from immune cell attack. Immunity. 2015;42(2):344–355. doi: 10.1016/j.immuni.2015.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pushalkar S, Hundeyin M, Daley D, Zambirinis CP, Kurz E, Mishra A, et al. The pancreatic cancer microbiome promotes oncogenesis by induction of innate and adaptive immune suppression. Cancer Discov. 2018;8(4):403–416. doi: 10.1158/2159-8290.CD-17-1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ma C, Han M, Heinrich B, Fu Q, Zhang Q, Sandhu M, et al. Gut microbiome-mediated bile acid metabolism regulates liver cancer via NKT cells. Science. 2018;360(6391):eaan5931. doi: 10.1126/science.aan5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Louis P, Hold GL, Flint HJ. The gut microbiota, bacterial metabolites and colorectal cancer. Nat Rev Microbiol. 2014;12(10):661–672. doi: 10.1038/nrmicro3344. [DOI] [PubMed] [Google Scholar]
- 35.Jin C, Lagoudas GK, Zhao C, Bullman S, Bhutkar A, Hu B, et al. Commensal microbiota promote lung cancer development via gammadelta T cells. Cell. 2019;176(5):998–1013.e16. doi: 10.1016/j.cell.2018.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kadosh E, Snir-Alkalay I, Venkatachalam A, May S, Lasry A, Elyada E, et al. The gut microbiome switches mutant p53 from tumour-suppressive to oncogenic. Nature. 2020;586(7827):133–138. doi: 10.1038/s41586-020-2541-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Song M, Chan AT, Sun J. Influence of the gut microbiome, diet, and environment on risk of colorectal cancer. Gastroenterology. 2020;158(2):322–340. doi: 10.1053/j.gastro.2019.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Han S, Zhuang J, Pan Y, Wu W, Ding K. Different characteristics in gut microbiome between advanced adenoma patients and colorectal cancer patients by metagenomic analysis. Microbiol Spectr. 2022;10(6):e0159322. doi: 10.1128/spectrum.01593-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee JWJ, Plichta DR, Asher S, Delsignore M, Jeong T, McGoldrick J, et al. Association of distinct microbial signatures with premalignant colorectal adenomas. Cell Host Microbe. 2023;31(5):827–838.e3. doi: 10.1016/j.chom.2023.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang J, He Q, Lu F, Chen K, Ni Z, Wang H, et al. A distinct microbiota signature precedes the clinical diagnosis of hepatocellular carcinoma. Gut Microbes. 2023;15(1):2201159. doi: 10.1080/19490976.2023.2201159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chang L, Qiu L, Lei N, Zhou J, Guo R, Gao F, et al. Characterization of fecal microbiota in cervical cancer patients associated with tumor stage and prognosis. Front Cell Infect Microbiol. 2023;13:1145950. doi: 10.3389/fcimb.2023.1145950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu W, Wang T, Wang N, Zhang H, Zha Y, Ji L, et al. Artificial intelligence-enabled microbiome-based diagnosis models for a broad spectrum of cancer types. Brief Bioinform. 2023;24(3):bbad178. doi: 10.1093/bib/bbad178. [DOI] [PubMed] [Google Scholar]
- 43.Chassaing B, Kumar M, Baker MT, Singh V, Vijay-Kumar M. Mammalian gut immunity. Biomed J. 2014;37(5):246–258. doi: 10.4103/2319-4170.130922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McQuade JL, Daniel CR, Helmink BA, Wargo JA. Modulating the microbiome to improve therapeutic response in cancer. Lancet Oncol. 2019;20(2):e77–e91. doi: 10.1016/S1470-2045(18)30952-5. [DOI] [PubMed] [Google Scholar]
- 45.Zheng D, Liwinski T, Elinav E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020;30(6):492–506. doi: 10.1038/s41422-020-0332-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jimenez-Saiz R, Anipindi VC, Galipeau H, Ellenbogen Y, Chaudhary R, Koenig JF, et al. Microbial regulation of enteric eosinophils and its impact on tissue remodeling and Th2 immunity. Front Immunol. 2020;11:155. doi: 10.3389/fimmu.2020.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roviello G, Iannone LF, Bersanelli M, Mini E, Catalano M. The gut microbiome and efficacy of cancer immunotherapy. Pharmacol Ther. 2022;231:107973. doi: 10.1016/j.pharmthera.2021.107973. [DOI] [PubMed] [Google Scholar]
- 48.Smith K, McCoy KD, Macpherson AJ. Use of axenic animals in studying the adaptation of mammals to their commensal intestinal microbiota. Semin Immunol. 2007;19(2):59–69. doi: 10.1016/j.smim.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 49.Marquant Q, Laubreton D, Drajac C, Mathieu E, Bouguyon E, Noordine ML, et al. The microbiota plays a critical role in the reactivity of lung immune components to innate ligands. FASEB J. 2021;35(4):e21348. doi: 10.1096/fj.202002338R. [DOI] [PubMed] [Google Scholar]
- 50.Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139(3):485–498. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wesemann DR, Portuguese AJ, Meyers RM, Gallagher MP, Cluff-Jones K, Magee JM, et al. Microbial colonization influences early B-lineage development in the gut lamina propria. Nature. 2013;501(7465):112–115. doi: 10.1038/nature12496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Honda K, Littman DR. The microbiota in adaptive immune homeostasis and disease. Nature. 2016;535(7610):75–84. doi: 10.1038/nature18848. [DOI] [PubMed] [Google Scholar]
- 53.Levy M, Kolodziejczyk AA, Thaiss CA, Elinav E. Dysbiosis and the immune system. Nat Rev Immunol. 2017;17(4):219–232. doi: 10.1038/nri.2017.7. [DOI] [PubMed] [Google Scholar]
- 54.Mowat AM, Agace WW. Regional specialization within the intestinal immune system. Nat Rev Immunol. 2014;14(10):667–685. doi: 10.1038/nri3738. [DOI] [PubMed] [Google Scholar]
- 55.Rescigno M, Urbano M, Valzasina B, Francolini M, Rotta G, Bonasio R, et al. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat Immunol. 2001;2(4):361–367. doi: 10.1038/86373. [DOI] [PubMed] [Google Scholar]
- 56.Ichinohe T, Pang IK, Kumamoto Y, Peaper DR, Ho JH, Murray TS, et al. Microbiota regulates immune defense against respiratory tract influenza A virus infection. Proc Natl Acad Sci USA. 2011;108(13):5354–5359. doi: 10.1073/pnas.1019378108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Arpaia N, Campbell C, Fan X, Dikiy S, van der Veeken J, deRoos P, et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. 2013;504(7480):451–455. doi: 10.1038/nature12726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Luu M, Weigand K, Wedi F, Breidenbend C, Leister H, Pautz S, et al. Regulation of the effector function of CD8(+) T cells by gut microbiota-derived metabolite butyrate. Sci Rep. 2018;8(1):14430. doi: 10.1038/s41598-018-32860-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bachem A, Makhlouf C, Binger KJ, de Souza DP, Tull D, Hochheiser K, et al. Microbiota-derived short-chain fatty acids promote the memory potential of antigen-activated CD8(+) T cells. Immunity. 2019;51(2):285–297.e5. doi: 10.1016/j.immuni.2019.06.002. [DOI] [PubMed] [Google Scholar]
- 60.Paik D, Yao L, Zhang Y, Bae S, D'Agostino GD, Zhang M, et al. Human gut bacteria produce TauEta17-modulating bile acid metabolites. Nature. 2022;603(7903):907–912. doi: 10.1038/s41586-022-04480-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li W, Hang S, Fang Y, Bae S, Zhang Y, Zhang M, et al. A bacterial bile acid metabolite modulates Treg activity through the nuclear hormone receptor NR4A1. Cell Host Microbe. 2021;29(9):1366–1377.e9. doi: 10.1016/j.chom.2021.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hang S, Paik D, Yao L, Kim E, Trinath J, Lu J, et al. Bile acid metabolites control TH17 and Treg cell differentiation. Nature. 2019;576(7785):143–148. doi: 10.1038/s41586-019-1785-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Campbell C, McKenney PT, Konstantinovsky D, Isaeva OI, Schizas M, Verter J, et al. Bacterial metabolism of bile acids promotes generation of peripheral regulatory T cells. Nature. 2020;581(7809):475–479. doi: 10.1038/s41586-020-2193-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Erturk-Hasdemir D, Oh SF, Okan NA, Stefanetti G, Gazzaniga FS, Seeberger PH, et al. Symbionts exploit complex signaling to educate the immune system. Proc Natl Acad Sci USA. 2019;116(52):26157–26166. doi: 10.1073/pnas.1915978116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ramanan D, Tang MS, Bowcutt R, Loke P, Cadwell K. Bacterial sensor Nod2 prevents inflammation of the small intestine by restricting the expansion of the commensal Bacteroides vulgatus. Immunity. 2014;41(2):311–324. doi: 10.1016/j.immuni.2014.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang S, Charbonnier LM, Noval Rivas M, Georgiev P, Li N, Gerber G, et al. MyD88 adaptor-dependent microbial sensing by regulatory T cells promotes mucosal tolerance and enforces commensalism. Immunity. 2015;43(2):289–303. doi: 10.1016/j.immuni.2015.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Birchenough GM, Nystrom EE, Johansson ME, Hansson GC. A sentinel goblet cell guards the colonic crypt by triggering Nlrp6-dependent Muc2 secretion. Science. 2016;352(6293):1535–1542. doi: 10.1126/science.aaf7419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Castro-Dopico T, Dennison TW, Ferdinand JR, Mathews RJ, Fleming A, Clift D, et al. Anti-commensal IgG drives intestinal inflammation and Type 17 immunity in ulcerative colitis. Immunity. 2019;50(4):1099–1114.e10. doi: 10.1016/j.immuni.2019.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Constantinides MG, McDonald BD, Verhoef PA, Bendelac A. A committed precursor to innate lymphoid cells. Nature. 2014;508(7496):397–401. doi: 10.1038/nature13047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Guo X, Liang Y, Zhang Y, Lasorella A, Kee BL, Fu YX. Innate lymphoid cells control early colonization resistance against intestinal pathogens through ID2-dependent regulation of the microbiota. Immunity. 2015;42(4):731–743. doi: 10.1016/j.immuni.2015.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vetizou M, Pitt JM, Daillere R, Lepage P, Waldschmitt N, Flament C, et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science. 2015;350(6264):1079–1084. doi: 10.1126/science.aad1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Matson V, Fessler J, Bao R, Chongsuwat T, Zha Y, Alegre ML, et al. The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science. 2018;359(6371):104–108. doi: 10.1126/science.aao3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Daillere R, Vetizou M, Waldschmitt N, Yamazaki T, Isnard C, Poirier-Colame V, et al. Enterococcus hirae and Barnesiella intestinihominis facilitate cyclophosphamide-induced therapeutic immunomodulatory effects. Immunity. 2016;45(4):931–943. doi: 10.1016/j.immuni.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 74.Uribe-Herranz M, Rafail S, Beghi S, Gil-de-Gomez L, Verginadis I, Bittinger K, et al. Gut microbiota modulate dendritic cell antigen presentation and radiotherapy-induced antitumor immune response. J Clin Invest. 2020;130(1):466–479. doi: 10.1172/JCI124332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fluckiger A, Daillere R, Sassi M, Sixt BS, Liu P, Loos F, et al. Cross-reactivity between tumor MHC class I-restricted antigens and an enterococcal bacteriophage. Science. 2020;369(6506):936–942. doi: 10.1126/science.aax0701. [DOI] [PubMed] [Google Scholar]
- 76.Bessell CA, Isser A, Havel JJ, Lee S, Bell DR, Hickey JW, et al. Commensal bacteria stimulate antitumor responses via T cell cross-reactivity. JCI Insight. 2020;5(8):e135597. doi: 10.1172/jci.insight.135597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Naghavian R, Faigle W, Oldrati P, Wang J, Toussaint NC, Qiu Y, et al. Microbial peptides activate tumour-infiltrating lymphocytes in glioblastoma. Nature. 2023;617(7962):807–817. doi: 10.1038/s41586-023-06081-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ansaldo E, Slayden LC, Ching KL, Koch MA, Wolf NK, Plichta DR, et al. Akkermansia muciniphila induces intestinal adaptive immune responses during homeostasis. Science. 2019;364(6446):1179–1184. doi: 10.1126/science.aaw7479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Guo H, Chou WC, Lai Y, Liang K, Tam JW, Brickey WJ, et al. Multi-omics analyses of radiation survivors identify radioprotective microbes and metabolites. Science. 2020;370(6516):eaay9097. doi: 10.1126/science.aay9097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang Q, Ma C, Duan Y, Heinrich B, Rosato U, Diggs LP, et al. Gut microbiome directs hepatocytes to recruit MDSCs and promote cholangiocarcinoma. Cancer Discov. 2021;11(5):1248–1267. doi: 10.1158/2159-8290.CD-20-0304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Derosa L, Hellmann MD, Spaziano M, Halpenny D, Fidelle M, Rizvi H, et al. Negative association of antibiotics on clinical activity of immune checkpoint inhibitors in patients with advanced renal cell and non-small-cell lung cancer. Ann Oncol. 2018;29(6):1437–1444. doi: 10.1093/annonc/mdy103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gopalakrishnan V, Helmink BA, Spencer CN, Reuben A, Wargo JA. The influence of the gut microbiome on cancer, immunity, and cancer immunotherapy. Cancer Cell. 2018;33(4):570–580. doi: 10.1016/j.ccell.2018.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3(11):991–998. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- 84.Igney FH, Krammer PH. Immune escape of tumors: apoptosis resistance and tumor counterattack. J Leukoc Biol. 2002;71(6):907–920. doi: 10.1189/jlb.71.6.907. [DOI] [PubMed] [Google Scholar]
- 85.Zhang Y, Zhang Z. The history and advances in cancer immunotherapy: understanding the characteristics of tumor-infiltrating immune cells and their therapeutic implications. Cell Mol Immunol. 2020;17(8):807–821. doi: 10.1038/s41423-020-0488-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lizée G, Overwijk WW, Radvanyi L, Gao J, Sharma P, Hwu P. Harnessing the power of the immune system to target cancer. Annu Rev Med. 2013;64:71–90. doi: 10.1146/annurev-med-112311-083918. [DOI] [PubMed] [Google Scholar]
- 87.Morad G, Helmink BA, Sharma P, Wargo JA. Hallmarks of response, resistance, and toxicity to immune checkpoint blockade. Cell. 2021;184(21):5309–5337. doi: 10.1016/j.cell.2021.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Stadtmauer EA, Fraietta JA, Davis MM, Cohen AD, Weber KL, Lancaster E, et al. CRISPR-engineered T cells in patients with refractory cancer. Science. 2020;367(6481):eaba7365. doi: 10.1126/science.aba7365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Newsome RC, Gharaibeh RZ, Pierce CM, da Silva WV, Paul S, Hogue SR, et al. Interaction of bacterial genera associated with therapeutic response to immune checkpoint PD-1 blockade in a United States cohort. Genome Med. 2022;14(1):35. doi: 10.1186/s13073-022-01037-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ponziani FR, De Luca A, Picca A, Marzetti E, Petito V, Del Chierico F, et al. Gut dysbiosis and fecal calprotectin predict response to immune checkpoint inhibitors in patients with hepatocellular carcinoma. Hepatol Commun. 2022;6(6):1492–1501. doi: 10.1002/hep4.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chu S, Cheng Z, Yin Z, Xu J, Wu F, Jin Y, et al. Airway Fusobacterium is associated with poor response to immunotherapy in lung cancer. Onco Targets Ther. 2022;15:201–213. doi: 10.2147/OTT.S348382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mao J, Wang D, Long J, Yang X, Lin J, Song Y, et al. Gut microbiome is associated with the clinical response to anti-PD-1 based immunotherapy in hepatobiliary cancers. J Immunother Cancer. 2021;9(12):e003334. doi: 10.1136/jitc-2021-003334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Boesch M, Baty F, Albrich WC, Flatz L, Rodriguez R, Rothschild SI, et al. Local tumor microbial signatures and response to checkpoint blockade in non-small cell lung cancer. Oncoimmunology. 2021;10(1):1988403. doi: 10.1080/2162402X.2021.1988403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yang D, Wang X, Zhou X, Zhao J, Yang H, Wang S, et al. Blood microbiota diversity determines response of advanced colorectal cancer to chemotherapy combined with adoptive T cell immunotherapy. Oncoimmunology. 2021;10(1):1976953. doi: 10.1080/2162402X.2021.1976953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chung MW, Kim MJ, Won EJ, Lee YJ, Yun YW, Cho SB, et al. Gut microbiome composition can predict the response to nivolumab in advanced hepatocellular carcinoma patients. World J Gastroenterol. 2021;27(42):7340–7349. doi: 10.3748/wjg.v27.i42.7340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Andrews MC, Duong CPM, Gopalakrishnan V, Iebba V, Chen WS, Derosa L, et al. Gut microbiota signatures are associated with toxicity to combined CTLA-4 and PD-1 blockade. Nat Med. 2021;27(8):1432–1441. doi: 10.1038/s41591-021-01406-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Oster P, Vaillant L, Riva E, McMillan B, Begka C, Truntzer C, et al. Helicobacter pylori infection has a detrimental impact on the efficacy of cancer immunotherapies. Gut. 2022;71(3):457–466. doi: 10.1136/gutjnl-2020-323392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhang C, Wang J, Sun Z, Cao Y, Mu Z, Ji X. Commensal microbiota contributes to predicting the response to immune checkpoint inhibitors in non-small-cell lung cancer patients. Cancer Sci. 2021;112(8):3005–3017. doi: 10.1111/cas.14979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhang F, Ferrero M, Dong N, D'Auria G, Reyes-Prieto M, Herreros-Pomares A, et al. Analysis of the gut microbiota: an emerging source of biomarkers for immune checkpoint blockade therapy in non-small cell lung cancer. Cancers. 2021;13(11):2514. doi: 10.3390/cancers13112514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chau J, Yadav M, Liu B, Furqan M, Dai Q, Shahi S, et al. Prospective correlation between the patient microbiome with response to and development of immune-mediated adverse effects to immunotherapy in lung cancer. BMC Cancer. 2021;21(1):808. doi: 10.1186/s12885-021-08530-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Diefenbach CS, Peters BA, Li H, Raphael B, Moskovits T, Hymes K, et al. Microbial dysbiosis is associated with aggressive histology and adverse clinical outcome in B-cell non-Hodgkin lymphoma. Blood Adv. 2021;5(5):1194–1198. doi: 10.1182/bloodadvances.2020003129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Limeta A, Ji B, Levin M, Gatto F, Nielsen J. Meta-analysis of the gut microbiota in predicting response to cancer immunotherapy in metastatic melanoma. JCI Insight. 2020;5(23):e140940. doi: 10.1172/jci.insight.140940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Vernocchi P, Gili T, Conte F, Del Chierico F, Conta G, Miccheli A, et al. Network analysis of gut microbiome and metabolome to discover microbiota-linked biomarkers in patients affected by non-small cell lung cancer. Int J Mol Sci. 2020;21(22):8730. doi: 10.3390/ijms21228730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Salgia NJ, Bergerot PG, Maia MC, Dizman N, Hsu J, Gillece JD, et al. Stool microbiome profiling of patients with metastatic renal cell carcinoma receiving anti-PD-1 immune checkpoint inhibitors. Eur Urol. 2020;78(4):498–502. doi: 10.1016/j.eururo.2020.07.011. [DOI] [PubMed] [Google Scholar]
- 105.Katayama Y, Yamada T, Shimamoto T, Iwasaku M, Kaneko Y, Uchino J, et al. The role of the gut microbiome on the efficacy of immune checkpoint inhibitors in Japanese responder patients with advanced non-small cell lung cancer. Transl Lung Cancer Res. 2019;8(6):847–853. doi: 10.21037/tlcr.2019.10.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Jin Y, Dong H, Xia L, Yang Y, Zhu Y, Shen Y, et al. The diversity of gut microbiome is associated with favorable responses to anti-programmed death 1 immunotherapy in Chinese patients with NSCLC. J Thorac Oncol. 2019;14(8):1378–1389. doi: 10.1016/j.jtho.2019.04.007. [DOI] [PubMed] [Google Scholar]
- 107.Zheng Y, Wang T, Tu X, Huang Y, Zhang H, Tan D, et al. Gut microbiome affects the response to anti-PD-1 immunotherapy in patients with hepatocellular carcinoma. J Immunother Cancer. 2019;7(1):193. doi: 10.1186/s40425-019-0650-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Peters BA, Wilson M, Moran U, Pavlick A, Izsak A, Wechter T, et al. Relating the gut metagenome and metatranscriptome to immunotherapy responses in melanoma patients. Genome Med. 2019;11(1):61. doi: 10.1186/s13073-019-0672-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Peng Z, Cheng S, Kou Y, Wang Z, Jin R, Hu H, et al. The gut microbiome is associated with clinical response to anti-PD-1/PD-L1 immunotherapy in gastrointestinal cancer. Cancer Immunol Res. 2020;8(10):1251–1261. doi: 10.1158/2326-6066.CIR-19-1014. [DOI] [PubMed] [Google Scholar]
- 110.Yin H, Yang L, Peng G, Yang K, Mi Y, Hu X, et al. The commensal consortium of the gut microbiome is associated with favorable responses to anti-programmed death protein 1 (PD-1) therapy in thoracic neoplasms. Cancer Biol Med. 2021;18(4):1040. doi: 10.20892/j.issn.2095-3941.2020.0450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.McCulloch JA, Davar D, Rodrigues RR, Badger JH, Fang JR, Cole AM, et al. Intestinal microbiota signatures of clinical response and immune-related adverse events in melanoma patients treated with anti-PD-1. Nat Med. 2022;28(3):545–556. doi: 10.1038/s41591-022-01698-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Takada K, Shimokawa M, Takamori S, Shimamatsu S, Hirai F, Tagawa T, et al. Clinical impact of probiotics on the efficacy of anti-PD-1 monotherapy in patients with nonsmall cell lung cancer: a multicenter retrospective survival analysis study with inverse probability of treatment weighting. Int J Cancer. 2021;149(2):473–482. doi: 10.1002/ijc.33557. [DOI] [PubMed] [Google Scholar]
- 113.Zhao H, Li D, Liu J, Zhou X, Han J, Wang L, et al. Bifidobacterium breve predicts the efficacy of anti-PD-1 immunotherapy combined with chemotherapy in Chinese NSCLC patients. Cancer Med. 2022;12(5):6325–6336. doi: 10.1002/cam4.5312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wu H, Zheng X, Pan T, Yang X, Chen X, Zhang B, et al. Dynamic microbiome and metabolome analyses reveal the interaction between gut microbiota and anti-PD-1 based immunotherapy in hepatocellular carcinoma. Int J Cancer. 2022;151(8):1321–1334. doi: 10.1002/ijc.34118. [DOI] [PubMed] [Google Scholar]
- 115.Peiffer LB, White JR, Jones CB, Slottke RE, Ernst SE, Moran AE, et al. Composition of gastrointestinal microbiota in association with treatment response in individuals with metastatic castrate resistant prostate cancer progressing on enzalutamide and initiating treatment with anti-PD-1 (pembrolizumab) Neoplasia. 2022;32:100822. doi: 10.1016/j.neo.2022.100822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Simpson RC, Shanahan ER, Batten M, Reijers ILM, Read M, Silva IP, et al. Diet-driven microbial ecology underpins associations between cancer immunotherapy outcomes and the gut microbiome. Nat Med. 2022;28(11):2344–2352. doi: 10.1038/s41591-022-01965-2. [DOI] [PubMed] [Google Scholar]
- 117.Che H, Xiong Q, Ma J, Chen S, Wu H, Xu H, et al. Association of Helicobacter pylori infection with survival outcomes in advanced gastric cancer patients treated with immune checkpoint inhibitors. BMC Cancer. 2022;22(1):904. doi: 10.1186/s12885-022-10004-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hu Y, Li J, Ni F, Yang Z, Gui X, Bao Z, et al. CAR-T cell therapy-related cytokine release syndrome and therapeutic response is modulated by the gut microbiome in hematologic malignancies. Nat Commun. 2022;13(1):5313. doi: 10.1038/s41467-022-32960-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Xu L, Ma Y, Fang C, Peng Z, Gao F, Moll JM, et al. Genomic and microbial factors affect the prognosis of anti-pd-1 immunotherapy in nasopharyngeal carcinoma. Front Oncol. 2022;12:953884. doi: 10.3389/fonc.2022.953884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lee PC, Wu CJ, Hung YW, Lee CJ, Chi CT, Lee IC, et al. Gut microbiota and metabolites associate with outcomes of immune checkpoint inhibitor-treated unresectable hepatocellular carcinoma. J Immunother Cancer. 2022;10(6):e004779. doi: 10.1136/jitc-2022-004779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ramsay AG. Immune checkpoint blockade immunotherapy to activate anti-tumour T-cell immunity. Br J Haematol. 2013;162(3):313–325. doi: 10.1111/bjh.12380. [DOI] [PubMed] [Google Scholar]
- 122.Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271(5256):1734–1736. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- 123.Curiel TJ, Wei S, Dong H, Alvarez X, Cheng P, Mottram P, et al. Blockade of B7–H1 improves myeloid dendritic cell-mediated antitumor immunity. Nat Med. 2003;9(5):562–567. doi: 10.1038/nm863. [DOI] [PubMed] [Google Scholar]
- 124.Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, Minato N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci USA. 2002;99(19):12293–12297. doi: 10.1073/pnas.192461099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372(21):2018–2028. doi: 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
- 126.Ott PA, Bang YJ, Piha-Paul SA, Razak ARA, Bennouna J, Soria JC, et al. T-Cell-inflamed gene-expression profile, programmed death ligand 1 expression, and tumor mutational burden predict efficacy in patients treated with pembrolizumab across 20 cancers: KEYNOTE-028. J Clin Oncol. 2019;37(4):318–327. doi: 10.1200/JCO.2018.78.2276. [DOI] [PubMed] [Google Scholar]
- 127.Lee SH, Cho SY, Yoon Y, Park C, Sohn J, Jeong JJ, et al. Bifidobacterium bifidum strains synergize with immune checkpoint inhibitors to reduce tumour burden in mice. Nat Microbiol. 2021;6(3):277–288. doi: 10.1038/s41564-020-00831-6. [DOI] [PubMed] [Google Scholar]
- 128.Baruch EN, Youngster I, Ben-Betzalel G, Ortenberg R, Lahat A, Katz L, et al. Fecal microbiota transplant promotes response in immunotherapy-refractory melanoma patients. Science. 2021;371(6529):602–609. doi: 10.1126/science.abb5920. [DOI] [PubMed] [Google Scholar]
- 129.Drummond RA, Desai JV, Ricotta EE, Swamydas M, Deming C, Conlan S, et al. Long-term antibiotic exposure promotes mortality after systemic fungal infection by driving lymphocyte dysfunction and systemic escape of commensal bacteria. Cell Host Microbe. 2022;30(7):1020–33.e6. doi: 10.1016/j.chom.2022.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kawanabe-Matsuda H, Takeda K, Nakamura M, Makino S, Karasaki T, Kakimi K, et al. Dietary lactobacillus-derived exopolysaccharide enhances immune-checkpoint blockade therapy. Cancer Discov. 2022;12(5):1336–1355. doi: 10.1158/2159-8290.CD-21-0929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Shi L, Sheng J, Chen G, Zhu P, Shi C, Li B, et al. Combining IL-2-based immunotherapy with commensal probiotics produces enhanced antitumor immune response and tumor clearance. J Immunother Cancer. 2020;8(2):e000973. doi: 10.1136/jitc-2020-000973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhang SL, Mao YQ, Zhang ZY, Li ZM, Kong CY, Chen HL, et al. Pectin supplement significantly enhanced the anti-PD-1 efficacy in tumor-bearing mice humanized with gut microbiota from patients with colorectal cancer. Theranostics. 2021;11(9):4155–4170. doi: 10.7150/thno.54476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.He Y, Fu L, Li Y, Wang W, Gong M, Zhang J, et al. Gut microbial metabolites facilitate anticancer therapy efficacy by modulating cytotoxic CD8(+) T cell immunity. Cell Metab. 2021;33(5):988–1000.e7. doi: 10.1016/j.cmet.2021.03.002. [DOI] [PubMed] [Google Scholar]
- 134.Mager LF, Burkhard R, Pett N, Cooke NCA, Brown K, Ramay H, et al. Microbiome-derived inosine modulates response to checkpoint inhibitor immunotherapy. Science. 2020;369(6510):1481–1489. doi: 10.1126/science.abc3421. [DOI] [PubMed] [Google Scholar]
- 135.Wang H, Rong X, Zhao G, Zhou Y, Xiao Y, Ma D, et al. The microbial metabolite trimethylamine N-oxide promotes antitumor immunity in triple-negative breast cancer. Cell Metab. 2022;34(4):581–594.e8. doi: 10.1016/j.cmet.2022.02.010. [DOI] [PubMed] [Google Scholar]
- 136.Mirji G, Worth A, Bhat SA, El Sayed M, Kannan T, Goldman AR, et al. The microbiome-derived metabolite TMAO drives immune activation and boosts responses to immune checkpoint blockade in pancreatic cancer. Sci Immunol. 2022;7(75):eabn0704. doi: 10.1126/sciimmunol.abn0704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Leung EL, Huang J, Zhang J, Zhang J, Wang M, Zhu Y, et al. Novel anticancer strategy by targeting the gut microbial neurotransmitter signaling to overcome immunotherapy resistance. Antioxid Redox Signal. 2022;38(4):298–315. doi: 10.1089/ars.2021.0243. [DOI] [PubMed] [Google Scholar]
- 138.Konishi H, Fujiya M, Tanaka H, Ueno N, Moriichi K, Sasajima J, et al. Probiotic-derived ferrichrome inhibits colon cancer progression via JNK-mediated apoptosis. Nat Commun. 2016;7:12365. doi: 10.1038/ncomms12365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Li Q, Hu W, Liu WX, Zhao LY, Huang D, Liu XD, et al. Streptococcus thermophilus inhibits colorectal tumorigenesis through secreting beta-galactosidase. Gastroenterology. 2021;160(4):1179–1193.e14. doi: 10.1053/j.gastro.2020.09.003. [DOI] [PubMed] [Google Scholar]
- 140.Botticelli A, Vernocchi P, Marini F, Quagliariello A, Cerbelli B, Reddel S, et al. Gut metabolomics profiling of non-small cell lung cancer (NSCLC) patients under immunotherapy treatment. J Transl Med. 2020;18(1):49. doi: 10.1186/s12967-020-02231-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wang F, Yin Q, Chen L, Davis MM. Bifidobacterium can mitigate intestinal immunopathology in the context of CTLA-4 blockade. Proc Natl Acad Sci USA. 2018;115(1):157–161. doi: 10.1073/pnas.1712901115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sun S, Luo L, Liang W, Yin Q, Guo J, Rush AM, et al. Bifidobacterium alters the gut microbiota and modulates the functional metabolism of T regulatory cells in the context of immune checkpoint blockade. Proc Natl Acad Sci USA. 2020;117(44):27509–27515. doi: 10.1073/pnas.1921223117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Renga G, Nunzi E, Pariano M, Puccetti M, Bellet MM, Pieraccini G, et al. Optimizing therapeutic outcomes of immune checkpoint blockade by a microbial tryptophan metabolite. J Immunother Cancer. 2022;10(3):e003725. doi: 10.1136/jitc-2021-003725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Lam KC, Araya RE, Huang A, Chen Q, Di Modica M, Rodrigues RR, et al. Microbiota triggers STING-type I IFN-dependent monocyte reprogramming of the tumor microenvironment. Cell. 2021;184(21):5338–5356.e21. doi: 10.1016/j.cell.2021.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Mann JE, Ludwig ML, Kulkarni A, Scheftz EB, Murray IR, Zhai J, et al. Microbe-mediated activation of toll-like receptor 2 drives PDL1 expression in HNSCC. Cancers. 2021;13(19):4782. doi: 10.3390/cancers13194782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Han J, Zhang S, Xu Y, Pang Y, Zhang X, Hu Y, et al. Beneficial effect of antibiotics and microbial metabolites on expanded Vdelta2Vgamma9 T cells in hepatocellular carcinoma immunotherapy. Front Immunol. 2020;11:1380. doi: 10.3389/fimmu.2020.01380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Zhuo Q, Yu B, Zhou J, Zhang J, Zhang R, Xie J, et al. Lysates of Lactobacillus acidophilus combined with CTLA-4-blocking antibodies enhance antitumor immunity in a mouse colon cancer model. Sci Rep. 2019;9(1):20128. doi: 10.1038/s41598-019-56661-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Xu X, Lv J, Guo F, Li J, Jia Y, Jiang D, et al. Gut microbiome influences the efficacy of PD-1 antibody immunotherapy on MSS-type colorectal cancer via metabolic pathway. Front Microbiol. 2020;11:814. doi: 10.3389/fmicb.2020.00814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Yi M, Jiao D, Xu H, Liu Q, Zhao W, Han X, et al. Biomarkers for predicting efficacy of PD-1/PD-L1 inhibitors. Mol Cancer. 2018;17(1):129. doi: 10.1186/s12943-018-0864-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Rosenberg SA, Lotze MT, Muul LM, Leitman S, Chang AE, Ettinghausen SE, et al. Observations on the systemic administration of autologous lymphokine-activated killer cells and recombinant interleukin-2 to patients with metastatic cancer. N Engl J Med. 1985;313(23):1485–1492. doi: 10.1056/NEJM198512053132327. [DOI] [PubMed] [Google Scholar]
- 151.Christofi T, Baritaki S, Falzone L, Libra M, Zaravinos A. Current perspectives in cancer immunotherapy. Cancers. 2019;11(10):1472. doi: 10.3390/cancers11101472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lau HCH, Sung JJ, Yu J. Gut microbiota: impacts on gastrointestinal cancer immunotherapy. Gut Microbes. 2021;13(1):1–21. doi: 10.1080/19490976.2020.1869504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Tran E, Turcotte S, Gros A, Robbins PF, Lu YC, Dudley ME, et al. Cancer immunotherapy based on mutation-specific CD4+ T cells in a patient with epithelial cancer. Science. 2014;344(6184):641–645. doi: 10.1126/science.1251102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Dudley ME, Rosenberg SA. Adoptive-cell-transfer therapy for the treatment of patients with cancer. Nat Rev Cancer. 2003;3(9):666–675. doi: 10.1038/nrc1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zhu Y, Qian Y, Li Z, Li Y, Li B. Neoantigen-reactive T cell: an emerging role in adoptive cellular immunotherapy. MedComm. 2021;2(2):207–220. doi: 10.1002/mco2.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.June CH, O'Connor RS, Kawalekar OU, Ghassemi S, Milone MC. CAR T cell immunotherapy for human cancer. Science. 2018;359(6382):1361–1365. doi: 10.1126/science.aar6711. [DOI] [PubMed] [Google Scholar]
- 157.Zhang R, Zhang Z, Liu Z, Wei D, Wu X, Bian H, et al. Adoptive cell transfer therapy for hepatocellular carcinoma. Front Med. 2019;13(1):3–11. doi: 10.1007/s11684-019-0684-x. [DOI] [PubMed] [Google Scholar]
- 158.Wang H, Kaur G, Sankin AI, Chen F, Guan F, Zang X. Immune checkpoint blockade and CAR-T cell therapy in hematologic malignancies. J Hematol Oncol. 2019;12(1):59. doi: 10.1186/s13045-019-0746-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Holstein SA, Lunning MA. CAR T-cell therapy in hematologic malignancies: a voyage in progress. Clin Pharmacol Ther. 2020;107(1):112–122. doi: 10.1002/cpt.1674. [DOI] [PubMed] [Google Scholar]
- 160.Uribe-Herranz M, Bittinger K, Rafail S, Guedan S, Pierini S, Tanes C, et al. Gut microbiota modulates adoptive cell therapy via CD8alpha dendritic cells and IL-12. JCI Insight. 2018;3(4):e94952. doi: 10.1172/jci.insight.94952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Blumenberg V, Schubert ML, Zamir E, Schmidt S, Rohrbach R, Waldhoff P, et al. Antibiotic therapy and low gut microbiome diversity is associated with decreased response and high toxicity in BCP-ALL and DLBCL patients after treatment with CD19. CAR T-cells. Blood. 2020;136:33–34. doi: 10.1182/blood-2020-141210. [DOI] [Google Scholar]
- 162.Smith M, Dai A, Ghilardi G, Amelsberg KV, Devlin SM, Pajarillo R, et al. Gut microbiome correlates of response and toxicity following anti-CD19 CAR T cell therapy. Nat Med. 2022;28(4):713–723. doi: 10.1038/s41591-022-01702-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Usyk M, Pandey A, Hayes RB, Moran U, Pavlick A, Osman I, et al. Bacteroides vulgatus and Bacteroides dorei predict immune-related adverse events in immune checkpoint blockade treatment of metastatic melanoma. Genome Med. 2021;13(1):160. doi: 10.1186/s13073-021-00974-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Chaput N, Lepage P, Coutzac C, Soularue E, Le Roux K, Monot C, et al. Baseline gut microbiota predicts clinical response and colitis in metastatic melanoma patients treated with ipilimumab. Ann Oncol. 2017;28(6):1368–1379. doi: 10.1093/annonc/mdx108. [DOI] [PubMed] [Google Scholar]
- 165.Blake SJ, James J, Ryan FJ, Caparros-Martin J, Eden GL, Tee YC, et al. The immunotoxicity, but not anti-tumor efficacy, of anti-CD40 and anti-CD137 immunotherapies is dependent on the gut microbiota. Cell Rep Med. 2021;2(12):100464. doi: 10.1016/j.xcrm.2021.100464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Matson V, Chervin CS, Gajewski TF. Cancer and the microbiome-influence of the commensal microbiota on cancer, immune responses, and immunotherapy. Gastroenterology. 2021;160(2):600–613. doi: 10.1053/j.gastro.2020.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Rothschild D, Weissbrod O, Barkan E, Kurilshikov A, Korem T, Zeevi D, et al. Environment dominates over host genetics in shaping human gut microbiota. Nature. 2018;555(7695):210–215. doi: 10.1038/nature25973. [DOI] [PubMed] [Google Scholar]
- 168.Gough E, Shaikh H, Manges AR. Systematic review of intestinal microbiota transplantation (fecal bacteriotherapy) for recurrent Clostridium difficile infection. Clin Infect Dis. 2011;53(10):994–1002. doi: 10.1093/cid/cir632. [DOI] [PubMed] [Google Scholar]
- 169.Huang J, Zheng X, Kang W, Hao H, Mao Y, Zhang H, et al. Metagenomic and metabolomic analyses reveal synergistic effects of fecal microbiota transplantation and anti-PD-1 therapy on treating colorectal cancer. Front Immunol. 2022;13:874922. doi: 10.3389/fimmu.2022.874922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Derosa L, Routy B, Fidelle M, Iebba V, Alla L, Pasolli E, et al. Gut bacteria composition drives primary resistance to cancer immunotherapy in renal cell carcinoma patients. Eur Urol. 2020;78(2):195–206. doi: 10.1016/j.eururo.2020.04.044. [DOI] [PubMed] [Google Scholar]
- 171.Davar D, Dzutsev AK, McCulloch JA, Rodrigues RR, Chauvin JM, Morrison RM, et al. Fecal microbiota transplant overcomes resistance to anti-PD-1 therapy in melanoma patients. Science. 2021;371(6529):595–602. doi: 10.1126/science.abf3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Elkrief A, Routy B. First clinical proof-of-concept that FMT can overcome resistance to ICIs. Nat Rev Clin Oncol. 2021;18(6):325–326. doi: 10.1038/s41571-021-00502-3. [DOI] [PubMed] [Google Scholar]
- 173.Ianiro G, Rossi E, Thomas AM, Schinzari G, Masucci L, Quaranta G, et al. Faecal microbiota transplantation for the treatment of diarrhoea induced by tyrosine-kinase inhibitors in patients with metastatic renal cell carcinoma. Nat Commun. 2020;11(1):4333. doi: 10.1038/s41467-020-18127-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Kakihana K, Fujioka Y, Suda W, Najima Y, Kuwata G, Sasajima S, et al. Fecal microbiota transplantation for patients with steroid-resistant acute graft-versus-host disease of the gut. Blood. 2016;128(16):2083–2088. doi: 10.1182/blood-2016-05-717652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Bajaj JS, Salzman N, Acharya C, Takei H, Kakiyama G, Fagan A, et al. Microbial functional change is linked with clinical outcomes after capsular fecal transplant in cirrhosis. JCI Insight. 2019;4(24):e133410. doi: 10.1172/jci.insight.133410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Hartstra AV, Schuppel V, Imangaliyev S, Schrantee A, Prodan A, Collard D, et al. Infusion of donor feces affects the gut-brain axis in humans with metabolic syndrome. Mol Metab. 2020;42:101076. doi: 10.1016/j.molmet.2020.101076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Podolsky SH. Metchnikoff and the microbiome. Lancet. 2012;380(9856):1810–1811. doi: 10.1016/S0140-6736(12)62018-2. [DOI] [PubMed] [Google Scholar]
- 178.Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11(8):506–514. doi: 10.1038/nrgastro.2014.66. [DOI] [PubMed] [Google Scholar]
- 179.Venugopalan V, Shriner KA, Wong-Beringer A. Regulatory oversight and safety of probiotic use. Emerg Infect Dis. 2010;16(11):1661–1665. doi: 10.3201/eid1611.100574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Kristensen NB, Bryrup T, Allin KH, Nielsen T, Hansen TH, Pedersen O. Alterations in fecal microbiota composition by probiotic supplementation in healthy adults: a systematic review of randomized controlled trials. Genome Med. 2016;8(1):52. doi: 10.1186/s13073-016-0300-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Das M. Probiotics for chemoradiotherapy-induced oral mucositis. Lancet Oncol. 2019;20(1):E14. doi: 10.1016/S1470-2045(18)30919-7. [DOI] [PubMed] [Google Scholar]
- 182.Jiang C, Wang H, Xia C, Dong Q, Chen E, Qiu Y, et al. A randomized, double-blind, placebo-controlled trial of probiotics to reduce the severity of oral mucositis induced by chemoradiotherapy for patients with nasopharyngeal carcinoma. Cancer. 2019;125(7):1081–1090. doi: 10.1002/cncr.31907. [DOI] [PubMed] [Google Scholar]
- 183.Lee B, Lee J, Woo MY, Lee MJ, Shin HJ, Kim K, et al. Modulation of the gut microbiota alters the tumour-suppressive efficacy of Tim-3 pathway blockade in a bacterial species- and host factor-dependent manner. Microorganisms. 2020;8(9):1395. doi: 10.3390/microorganisms8091395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Corb Aron RA, Abid A, Vesa CM, Nechifor AC, Behl T, Ghitea TC, et al. Recognizing the benefits of pre-/probiotics in metabolic syndrome and type 2 diabetes mellitus considering the influence of Akkermansia muciniphila as a key gut bacterium. Microorganisms. 2021;9(3):618. doi: 10.3390/microorganisms9030618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Lordan C, Thapa D, Ross RP, Cotter PD. Potential for enriching next-generation health-promoting gut bacteria through prebiotics and other dietary components. Gut microbes. 2020;11(1):1–20. doi: 10.1080/19490976.2019.1613124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Chen Z, Qian X, Chen S, Fu X, Ma G, Zhang A. Akkermansia muciniphila enhances the antitumor effect of cisplatin in Lewis lung cancer mice. J Immunol Res. 2020;2020:2969287. doi: 10.1155/2020/2969287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Tanoue T, Morita S, Plichta DR, Skelly AN, Suda W, Sugiura Y, et al. A defined commensal consortium elicits CD8 T cells and anti-cancer immunity. Nature. 2019;565(7741):600–605. doi: 10.1038/s41586-019-0878-z. [DOI] [PubMed] [Google Scholar]
- 188.Dizman N, Meza L, Bergerot P, Alcantara M, Dorff T, Lyou Y, et al. Nivolumab plus ipilimumab with or without live bacterial supplementation in metastatic renal cell carcinoma: a randomized phase 1 trial. Nat Med. 2022;28(4):704–712. doi: 10.1038/s41591-022-01694-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Tomita Y, Goto Y, Sakata S, Imamura K, Minemura A, Oka K, et al. Clostridium butyricum therapy restores the decreased efficacy of immune checkpoint blockade in lung cancer patients receiving proton pump inhibitors. Oncoimmunology. 2022;11(1):2081010. doi: 10.1080/2162402X.2022.2081010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Sivan A, Corrales L, Hubert N, Williams JB, Aquino-Michaels K, Earley ZM, et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science. 2015;350(6264):1084–1089. doi: 10.1126/science.aac4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Montalban-Arques A, Katkeviciute E, Busenhart P, Bircher A, Wirbel J, Zeller G, et al. Commensal Clostridiales strains mediate effective anti-cancer immune response against solid tumors. Cell Host Microbe. 2021;29(10):1573–1588.e7. doi: 10.1016/j.chom.2021.08.001. [DOI] [PubMed] [Google Scholar]
- 192.Zhang SL, Han B, Mao YQ, Zhang ZY, Li ZM, Kong CY, et al. Lacticaseibacillus paracasei sh2020 induced antitumor immunity and synergized with anti-programmed cell death 1 to reduce tumor burden in mice. Gut Microbes. 2022;14(1):2046246. doi: 10.1080/19490976.2022.2046246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Griffin ME, Espinosa J, Becker JL, Luo JD, Carroll TS, Jha JK, et al. Enterococcus peptidoglycan remodeling promotes checkpoint inhibitor cancer immunotherapy. Science. 2021;373(6558):1040–1046. doi: 10.1126/science.abc9113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Gao G, Ma T, Zhang T, Jin H, Li Y, Kwok LY, et al. Adjunctive probiotic Lactobacillus rhamnosus probio-M9 administration enhances the effect of anti-PD-1 antitumor therapy via restoring antibiotic-disrupted gut microbiota. Front Immunol. 2021;12:772532. doi: 10.3389/fimmu.2021.772532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Tomasi M, Dalsass M, Beghini F, Zanella I, Caproni E, Fantappie L, et al. Commensal Bifidobacterium strains enhance the efficacy of neo-epitope based cancer vaccines. Vaccines. 2021;9(11):1356. doi: 10.3390/vaccines9111356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Shi Y, Zheng W, Yang K, Harris KG, Ni K, Xue L, et al. Intratumoral accumulation of gut microbiota facilitates CD47-based immunotherapy via STING signaling. J Exp Med. 2020;217(5):e20192282. doi: 10.1084/jem.20192282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Shi L, Sheng J, Wang M, Luo H, Zhu J, Zhang B, et al. Combination therapy of TGF-beta blockade and commensal-derived probiotics provides enhanced antitumor immune response and tumor suppression. Theranostics. 2019;9(14):4115–4129. doi: 10.7150/thno.35131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Zhao J, Wang Y, Wang J, Lv M, Zhou C, Jia L, et al. Lactobacillus kefiranofaciens ZW18 from Kefir enhances the anti-tumor effect of anti-programmed cell death 1 (PD-1) immunotherapy by modulating the gut microbiota. Food Funct. 2022;13(19):10023–10033. doi: 10.1039/D2FO01747D. [DOI] [PubMed] [Google Scholar]
- 199.Kim S, Kim Y, Lee S, Kim Y, Jeon B, Kim H, et al. Live biotherapeutic Lactococcus lactis GEN3013 enhances antitumor efficacy of cancer treatment via modulation of cancer progression and immune system. Cancers. 2022;14(17):4083. doi: 10.3390/cancers14174083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Valdes AM, Walter J, Segal E, Spector TD. Role of the gut microbiota in nutrition and health. BMJ. 2018;361:k2179. doi: 10.1136/bmj.k2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Rackaityte E, Lynch SV. Rules of engagement in the gut microbiome. Nat Med. 2018;24(11):1642–1644. doi: 10.1038/s41591-018-0242-0. [DOI] [PubMed] [Google Scholar]
- 202.Carmody RN, Gerber GK, Luevano JM, Jr, Gatti DM, Somes L, Svenson KL, et al. Diet dominates host genotype in shaping the murine gut microbiota. Cell Host Microbe. 2015;17(1):72–84. doi: 10.1016/j.chom.2014.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Le Chatelier E, Nielsen T, Qin J, Prifti E, Hildebrand F, Falony G, et al. Richness of human gut microbiome correlates with metabolic markers. Nature. 2013;500(7464):541–546. doi: 10.1038/nature12506. [DOI] [PubMed] [Google Scholar]
- 204.Koh A, De Vadder F, Kovatcheva-Datchary P, Backhed F. From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell. 2016;165(6):1332–1345. doi: 10.1016/j.cell.2016.05.041. [DOI] [PubMed] [Google Scholar]
- 205.Morrison DJ, Preston T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut microbes. 2016;7(3):189–200. doi: 10.1080/19490976.2015.1134082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Kaczmarczyk MM, Miller MJ, Freund GG. The health benefits of dietary fiber: beyond the usual suspects of type 2 diabetes mellitus, cardiovascular disease and colon cancer. Metabolism. 2012;61(8):1058–1066. doi: 10.1016/j.metabol.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Rios-Covian D, Ruas-Madiedo P, Margolles A, Gueimonde M, de Los Reyes-Gavilan CG, Salazar N. Intestinal short chain fatty acids and their link with diet and human health. Front Microbiol. 2016;7:185. doi: 10.3389/fmicb.2016.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Coutzac C, Jouniaux JM, Paci A, Schmidt J, Mallardo D, Seck A, et al. Systemic short chain fatty acids limit antitumor effect of CTLA-4 blockade in hosts with cancer. Nat Commun. 2020;11(1):2168. doi: 10.1038/s41467-020-16079-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Sanders ME, Merenstein DJ, Reid G, Gibson GR, Rastall RA. Probiotics and prebiotics in intestinal health and disease: from biology to the clinic. Nat Rev Gastroenterol Hepatol. 2019;16(10):605–616. doi: 10.1038/s41575-019-0173-3. [DOI] [PubMed] [Google Scholar]
- 210.Gibson GR, Hutkins R, Sanders ME, Prescott SL, Reimer RA, Salminen SJ, et al. Expert consensus document: the International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol. 2017;14(8):491–502. doi: 10.1038/nrgastro.2017.75. [DOI] [PubMed] [Google Scholar]
- 211.Panebianco C, Andriulli A, Pazienza V. Pharmacomicrobiomics: exploiting the drug-microbiota interactions in anticancer therapies. Microbiome. 2018;6(1):92. doi: 10.1186/s40168-018-0483-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Zheng DW, Li RQ, An JX, Xie TQ, Han ZY, Xu R, et al. Prebiotics-encapsulated probiotic spores regulate gut microbiota and suppress colon cancer. Adv Mater. 2020;32(45):e2004529. doi: 10.1002/adma.202004529. [DOI] [PubMed] [Google Scholar]
- 213.Huang J, Liu D, Wang Y, Liu L, Li J, Yuan J, et al. Ginseng polysaccharides alter the gut microbiota and kynurenine/tryptophan ratio, potentiating the antitumour effect of antiprogrammed cell death 1/programmed cell death ligand 1 (anti-PD-1/PD-L1) immunotherapy. Gut. 2022;71(4):734–745. doi: 10.1136/gutjnl-2020-321031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Han K, Nam J, Xu J, Sun X, Huang X, Animasahun O, et al. Generation of systemic antitumour immunity via the in situ modulation of the gut microbiome by an orally administered inulin gel. Nat Biomed Eng. 2021;5(11):1377–1388. doi: 10.1038/s41551-021-00749-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Messaoudene M, Pidgeon R, Richard C, Ponce M, Diop K, Benlaifaoui M, et al. A natural polyphenol exerts antitumor activity and circumvents anti-PD-1 resistance through effects on the gut microbiota. Cancer Discov. 2022;12(4):1070–1087. doi: 10.1158/2159-8290.CD-21-0808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Spencer CN, McQuade JL, Gopalakrishnan V, McCulloch JA, Vetizou M, Cogdill AP, et al. Dietary fiber and probiotics influence the gut microbiome and melanoma immunotherapy response. Science. 2021;374(6575):1632–1640. doi: 10.1126/science.aaz7015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Han C, Song J, Hu J, Fu H, Feng Y, Mu R, et al. Smectite promotes probiotic biofilm formation in the gut for cancer immunotherapy. Cell Rep. 2021;34(6):108706. doi: 10.1016/j.celrep.2021.108706. [DOI] [PubMed] [Google Scholar]
- 218.Ferrere G, Tidjani Alou M, Liu P, Goubet AG, Fidelle M, Kepp O, et al. Ketogenic diet and ketone bodies enhance the anticancer effects of PD-1 blockade. JCI Insight. 2021;6(2):e145207. doi: 10.1172/jci.insight.145207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Cvetkovic L, Regis C, Richard C, Derosa L, Leblond A, Malo J, et al. Physiologic colonic uptake of (18)F-FDG on PET/CT is associated with clinical response and gut microbiome composition in patients with advanced non-small cell lung cancer treated with immune checkpoint inhibitors. Eur J Nucl Med Mol Imaging. 2021;48(5):1550–1559. doi: 10.1007/s00259-020-05081-6. [DOI] [PubMed] [Google Scholar]
- 220.Nomura M, Nagatomo R, Doi K, Shimizu J, Baba K, Saito T, et al. Association of short-chain fatty acids in the gut microbiome with clinical response to treatment with nivolumab or pembrolizumab in patients with solid cancer tumors. JAMA Netw Open. 2020;3(4):e202895. doi: 10.1001/jamanetworkopen.2020.2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Liu X, Wang L, Jing N, Jiang G, Liu Z. Biostimulating gut microbiome with bilberry anthocyanin combo to enhance anti-PD-L1 efficiency against murine colon cancer. Microorganisms. 2020;8(2):175. doi: 10.3390/microorganisms8020175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Lv J, Jia Y, Li J, Kuai W, Li Y, Guo F, et al. Gegen Qinlian decoction enhances the effect of PD-1 blockade in colorectal cancer with microsatellite stability by remodelling the gut microbiota and the tumour microenvironment. Cell Death Dis. 2019;10(6):415. doi: 10.1038/s41419-019-1638-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Orillion A, Damayanti NP, Shen L, Adelaiye-Ogala R, Affronti H, Elbanna M, et al. Dietary protein restriction reprograms tumor-associated macrophages and enhances immunotherapy. Clin Cancer Res. 2018;24(24):6383–6395. doi: 10.1158/1078-0432.CCR-18-0980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Dong M, Meng Z, Kuerban K, Qi F, Liu J, Wei Y, et al. Diosgenin promotes antitumor immunity and PD-1 antibody efficacy against melanoma by regulating intestinal microbiota. Cell Death Dis. 2018;9(10):1039. doi: 10.1038/s41419-018-1099-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Park HJ, Boo S, Park I, Shin MS, Takahashi T, Takanari J, et al. AHCC((R)), a standardized extract of cultured Lentinula edodes mycelia, promotes the anti-tumor effect of dual immune checkpoint blockade effect in murine colon cancer. Front Immunol. 2022;13:875872. doi: 10.3389/fimmu.2022.875872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Morrell S, Kohonen-Corish MRJ, Ward RL, Sorrell TC, Roder D, Currow DC. Antibiotic exposure within six months before systemic therapy was associated with lower cancer survival. J Clin Epidemiol. 2022;147:122–131. doi: 10.1016/j.jclinepi.2022.04.003. [DOI] [PubMed] [Google Scholar]
- 227.Chalabi M, Cardona A, Nagarkar DR, Dhawahir Scala A, Gandara DR, Rittmeyer A, et al. Efficacy of chemotherapy and atezolizumab in patients with non-small-cell lung cancer receiving antibiotics and proton pump inhibitors: pooled post hoc analyses of the OAK and POPLAR trials. Ann Oncol. 2020;31(4):525–531. doi: 10.1016/j.annonc.2020.01.006. [DOI] [PubMed] [Google Scholar]
- 228.Ahmed J, Kumar A, Parikh K, Anwar A, Knoll BM, Puccio C, et al. Use of broad-spectrum antibiotics impacts outcome in patients treated with immune checkpoint inhibitors. Oncoimmunology. 2018;7(11):e1507670. doi: 10.1080/2162402X.2018.1507670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Fessas P, Naeem M, Pinter M, Marron TU, Szafron D, Balcar L, et al. Early antibiotic exposure is not detrimental to therapeutic effect from immunotherapy in hepatocellular carcinoma. Liver Cancer. 2021;10(6):583–592. doi: 10.1159/000519108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Woodfield JC, Clifford K, Schmidt B, Turner GA, Amer MA, McCall JL. Strategies for antibiotic administration for bowel preparation among patients undergoing elective colorectal surgery: a network meta-analysis. JAMA Surg. 2022;157(1):34–41. doi: 10.1001/jamasurg.2021.5251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Khorasani S, Dossa F, McKechnie T, Englesakis M, Brar MS, de Buck van Overstraeten A. Association between preoperative oral antibiotics and the incidence of postoperative Clostridium difficile infection in adults undergoing elective colorectal resection: a systematic review and meta-analysis. Dis Colon Rectum. 2020;63(4):545–561. doi: 10.1097/DCR.0000000000001619. [DOI] [PubMed] [Google Scholar]
- 232.Serpas Higbie V, Rogers J, Hwang H, Qiao W, Xiao L, Dasari A, et al. Antibiotic exposure does not impact immune checkpoint blockade response in MSI-H/dMMR metastatic colorectal cancer: a single-center experience. Oncologist. 2022;27(11):952–957. doi: 10.1093/oncolo/oyac162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Sethi V, Vitiello GA, Saxena D, Miller G, Dudeja V. The role of the microbiome in immunologic development and its implication for pancreatic cancer immunotherapy. Gastroenterology. 2019;156(7):2097–2115.e2. doi: 10.1053/j.gastro.2018.12.045. [DOI] [PubMed] [Google Scholar]
- 234.Vitiello GA, Cohen DJ, Miller G. Harnessing the microbiome for pancreatic cancer immunotherapy. Trends Cancer. 2019;5(11):670–676. doi: 10.1016/j.trecan.2019.10.005. [DOI] [PubMed] [Google Scholar]
- 235.Edlund H. Developmental biology of the pancreas. Diabetes. 2001;50(Suppl 1):S5–9. doi: 10.2337/diabetes.50.2007.S5. [DOI] [PubMed] [Google Scholar]
- 236.Kostine M, Mauric E, Tison A, Barnetche T, Barre A, Nikolski M, et al. Baseline co-medications may alter the anti-tumoural effect of checkpoint inhibitors as well as the risk of immune-related adverse events. Eur J Cancer. 2021;157:474–484. doi: 10.1016/j.ejca.2021.08.036. [DOI] [PubMed] [Google Scholar]
- 237.Iglesias-Santamaria A. Impact of antibiotic use and other concomitant medications on the efficacy of immune checkpoint inhibitors in patients with advanced cancer. Clin Transl Oncol. 2020;22(9):1481–1490. doi: 10.1007/s12094-019-02282-w. [DOI] [PubMed] [Google Scholar]
- 238.Giordan Q, Salleron J, Vallance C, Moriana C, Clement-Duchene C. Impact of antibiotics and proton pump inhibitors on efficacy and tolerance of anti-PD-1 immune checkpoint inhibitors. Front Immunol. 2021;12:716317. doi: 10.3389/fimmu.2021.716317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Ochi N, Ichihara E, Takigawa N, Harada D, Inoue K, Shibayama T, et al. The effects of antibiotics on the efficacy of immune checkpoint inhibitors in patients with non-small-cell lung cancer differ based on PD-L1 expression. Eur J Cancer. 2021;149:73–81. doi: 10.1016/j.ejca.2021.02.040. [DOI] [PubMed] [Google Scholar]
- 240.Yang M, Wang Y, Yuan M, Tao M, Kong C, Li H, et al. Antibiotic administration shortly before or after immunotherapy initiation is correlated with poor prognosis in solid cancer patients: an up-to-date systematic review and meta-analysis. Int Immunopharmacol. 2020;88:106876. doi: 10.1016/j.intimp.2020.106876. [DOI] [PubMed] [Google Scholar]
- 241.Pinato DJ, Howlett S, Ottaviani D, Urus H, Patel A, Mineo T, et al. Association of prior antibiotic treatment with survival and response to immune checkpoint inhibitor therapy in patients with cancer. JAMA Oncol. 2019;5(12):1774–1778. doi: 10.1001/jamaoncol.2019.2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Wu Q, Liu J, Wu S, Xie X. The impact of antibiotics on efficacy of immune checkpoint inhibitors in malignancies: a study based on 44 cohorts. Int Immunopharmacol. 2021;92:107303. doi: 10.1016/j.intimp.2020.107303. [DOI] [PubMed] [Google Scholar]
- 243.Ishiyama Y, Kondo T, Nemoto Y, Kobari Y, Ishihara H, Tachibana H, et al. Antibiotic use and survival of patients receiving pembrolizumab for chemotherapy-resistant metastatic urothelial carcinoma. Urol Oncol. 2021;39(12):834.e21–834.e28. doi: 10.1016/j.urolonc.2021.05.033. [DOI] [PubMed] [Google Scholar]
- 244.Pederzoli F, Bandini M, Raggi D, Marandino L, Basile G, Alfano M, et al. Is there a detrimental effect of antibiotic therapy in patients with muscle-invasive bladder cancer treated with neoadjuvant pembrolizumab? Eur Urol. 2021;80(3):319–322. doi: 10.1016/j.eururo.2021.05.018. [DOI] [PubMed] [Google Scholar]
- 245.Guven DC, Acar R, Yekeduz E, Bilgetekin I, Baytemur NK, Erol C, et al. The association between antibiotic use and survival in renal cell carcinoma patients treated with immunotherapy: a multi-center study. Curr Probl Cancer. 2021;45(6):100760. doi: 10.1016/j.currproblcancer.2021.100760. [DOI] [PubMed] [Google Scholar]
- 246.Cren PY, Bertrand N, Le Deley MC, Genin M, Mortier L, Odou P, et al. Is the survival of patients treated with ipilimumab affected by antibiotics? An analysis of 1585 patients from the French National hospital discharge summary database (PMSI) Oncoimmunology. 2020;9(1):1846914. doi: 10.1080/2162402X.2020.1846914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Buti S, Bersanelli M, Perrone F, Tiseo M, Tucci M, Adamo V, et al. Effect of concomitant medications with immune-modulatory properties on the outcomes of patients with advanced cancer treated with immune checkpoint inhibitors: development and validation of a novel prognostic index. Eur J Cancer. 2021;142:18–28. doi: 10.1016/j.ejca.2020.09.033. [DOI] [PubMed] [Google Scholar]
- 248.Hopkins AM, Kichenadasse G, Karapetis CS, Rowland A, Sorich MJ. Concomitant antibiotic use and survival in urothelial carcinoma treated with atezolizumab. Eur Urol. 2020;78(4):540–543. doi: 10.1016/j.eururo.2020.06.061. [DOI] [PubMed] [Google Scholar]
- 249.Kulkarni AA, Ebadi M, Zhang S, Meybodi MA, Ali AM, DeFor T, et al. Comparative analysis of antibiotic exposure association with clinical outcomes of chemotherapy versus immunotherapy across three tumour types. ESMO Open. 2020;5(5):e000803. doi: 10.1136/esmoopen-2020-000803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Mohiuddin JJ, Chu B, Facciabene A, Poirier K, Wang X, Doucette A, et al. Association of antibiotic exposure with survival and toxicity in patients with melanoma receiving immunotherapy. J Natl Cancer Inst. 2021;113(2):162–170. doi: 10.1093/jnci/djaa057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Spakowicz D, Hoyd R, Muniak M, Husain M, Bassett JS, Wang L, et al. Inferring the role of the microbiome on survival in patients treated with immune checkpoint inhibitors: causal modeling, timing, and classes of concomitant medications. BMC Cancer. 2020;20(1):383. doi: 10.1186/s12885-020-06882-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Huang XZ, Gao P, Song YX, Xu Y, Sun JX, Chen XW, et al. Antibiotic use and the efficacy of immune checkpoint inhibitors in cancer patients: a pooled analysis of 2740 cancer patients. Oncoimmunology. 2019;8(12):e1665973. doi: 10.1080/2162402X.2019.1665973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Kim H, Lee JE, Hong SH, Lee MA, Kang JH, Kim IH. The effect of antibiotics on the clinical outcomes of patients with solid cancers undergoing immune checkpoint inhibitor treatment: a retrospective study. BMC Cancer. 2019;19(1):1100. doi: 10.1186/s12885-019-6267-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Elkrief A, El Raichani L, Richard C, Messaoudene M, Belkaid W, Malo J, et al. Antibiotics are associated with decreased progression-free survival of advanced melanoma patients treated with immune checkpoint inhibitors. Oncoimmunology. 2019;8(4):e1568812. doi: 10.1080/2162402X.2019.1568812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Zhao S, Gao G, Li W, Li X, Zhao C, Jiang T, et al. Antibiotics are associated with attenuated efficacy of anti-PD-1/PD-L1 therapies in Chinese patients with advanced non-small cell lung cancer. Lung Cancer. 2019;130:10–17. doi: 10.1016/j.lungcan.2019.01.017. [DOI] [PubMed] [Google Scholar]
- 256.Lalani AA, Xie W, Braun DA, Kaymakcalan M, Bosse D, Steinharter JA, et al. Effect of antibiotic use on outcomes with systemic therapies in metastatic renal cell carcinoma. Eur Urol Oncol. 2020;3(3):372–381. doi: 10.1016/j.euo.2019.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Zmora N, Zilberman-Schapira G, Suez J, Mor U, Dori-Bachash M, Bashiardes S, et al. Personalized gut mucosal colonization resistance to empiric probiotics is associated with unique host and microbiome features. Cell. 2018;174(6):1388–1405.e21. doi: 10.1016/j.cell.2018.08.041. [DOI] [PubMed] [Google Scholar]
- 258.Knight R, Vrbanac A, Taylor BC, Aksenov A, Callewaert C, Debelius J, et al. Best practices for analysing microbiomes. Nat Rev Microbiol. 2018;16(7):410–422. doi: 10.1038/s41579-018-0029-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.