Abstract
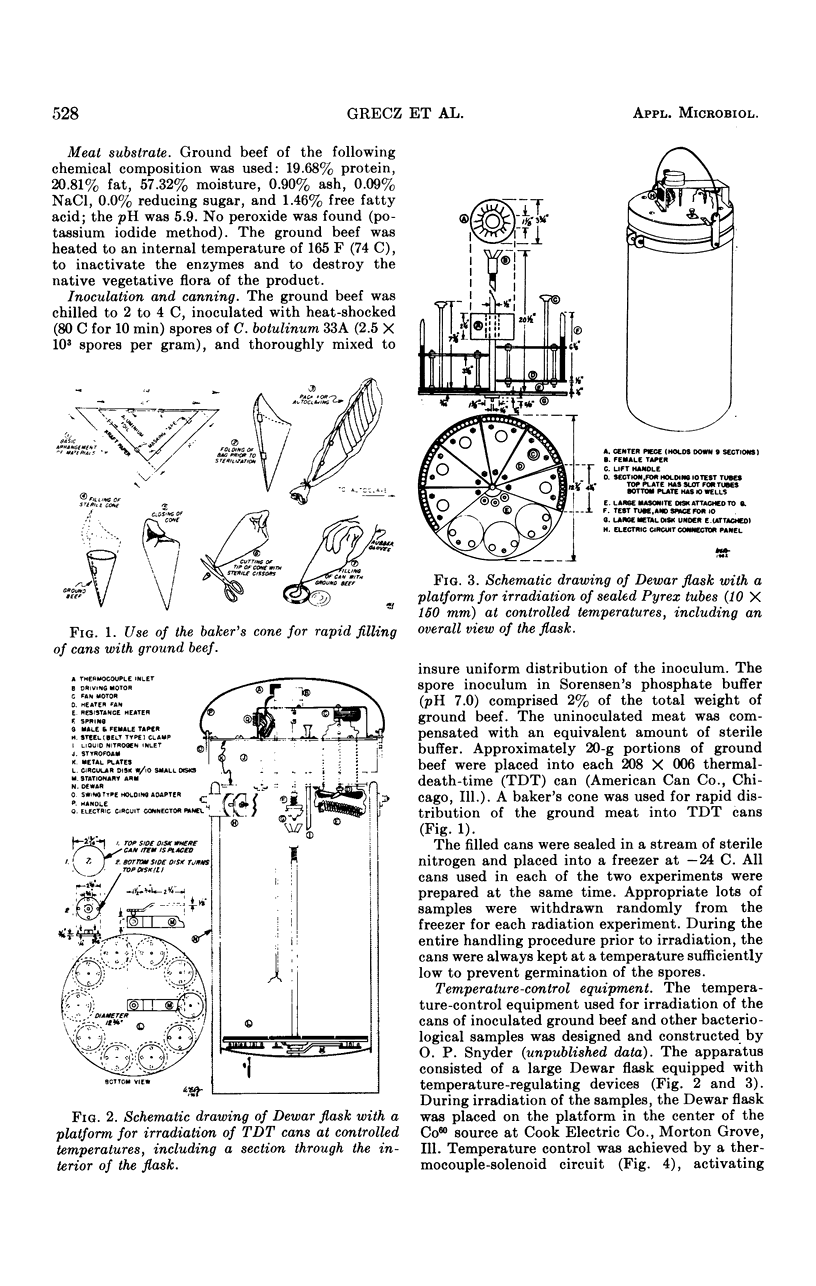
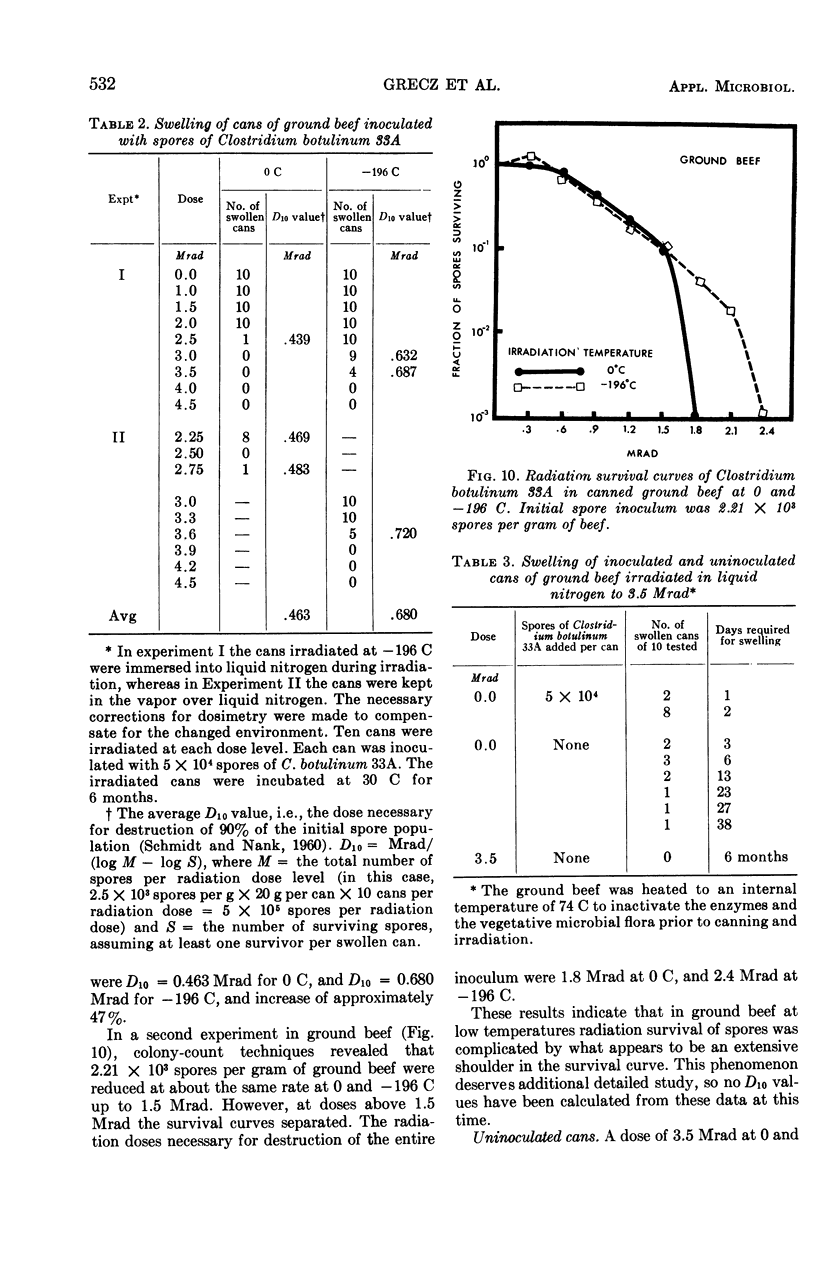
An apparatus consisting of a Dewar flask and a relay system controlling the flow of liquid nitrogen permitted the irradiation of samples in tin cans or Pyrex tubes at temperatures ranging from 0 ± 1.5 C to -194 ± 2 C. An inoculated pack comprising 320 cans of ground beef containing 5 × 104 spores of Clostridium botulinum 33A per can (10 cans per radiation dose) was irradiated with Co60 at 0 and -196 C. Incubation was carried out at 30 C for 6 months. Approximately 0.9 Mrad more radiation was required to inactivate the spores at -196 C than at 0 C. Cans irradiated at -196 C showed partial spoilage at 3.6 Mrad and no spoilage at 3.9 Mrad; the corresponding spoilage-no spoilage doses at 0 C were 2.7 and 3.0, respectively. The majority of positive cans swelled in 2 to 14 days; occasional swelling occurred as late as 20 days. At progressively higher doses, swelling was delayed proportionally to the radiation dose received. The remaining nonswollen cans had no toxin after 6 months of storage, although occasional cans contained very low numbers of viable spores comprising on the average 0.1% of the original spore inoculum. The D10 values in phosphate buffer were 0.290 Mrad for 0 C and 0.396 Mrad for -196 C; in ground beef, the corresponding D10 values were 0.463 Mrad and 0.680 Mrad, respectively. These D10 values indicate that the lethal effect of γ rays decreased at -196 C as compared with 0 C by 13.5% in phosphate buffer, and by 47% in ground beef.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANELLIS A., KOCH R. B. Comparative resistance of strains of Clostridium botulinum to gamma rays. Appl Microbiol. 1962 Jul;10:326–330. doi: 10.1128/am.10.4.326-330.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BELLAMY W. D., LAWTON E. J. Studies on factors affecting the sensitivity of bacteria to high velocity electrons. Ann N Y Acad Sci. 1955 Feb 3;59(4):595–603. doi: 10.1111/j.1749-6632.1955.tb45972.x. [DOI] [PubMed] [Google Scholar]
- Brasch A., Huber W. Reduction of Undesirable By-Effects in Products Treated by Radiation. Science. 1948 Nov 12;108(2811):536–537. doi: 10.1126/science.108.2811.536. [DOI] [PubMed] [Google Scholar]
- HOLLAENDER A., STAPLETON G. E. Fundamental aspects of radiation protection from a microbiological point of view. Physiol Rev. 1953 Jan;33(1):77–84. doi: 10.1152/physrev.1953.33.1.77. [DOI] [PubMed] [Google Scholar]
- MOOS W. S. Variation of irradiation effects on microorganisms in relation to physical changes of their environment. J Bacteriol. 1952 May;63(5):688–690. doi: 10.1128/jb.63.5.688-690.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PEPPER R. E., BUFFA N. T., CHANDLER V. L. Relative resistances of micro-organisms to cathode rays. III. Bacterial spores. Appl Microbiol. 1956 May;4(3):149–152. doi: 10.1128/am.4.3.149-152.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PROCTOR B. E., GOLDBLITH S. A., OBERLE E. M., MILLER W. C., Jr Radiosensitivity of Bacillus subtilis under different environmental conditions. Radiat Res. 1955 Nov;3(3):295–303. [PubMed] [Google Scholar]
- STAPLETON G. E., EDINGTON C. W. Temperature dependence of bacterial inactivation by X-rays. Radiat Res. 1956 Jul;5(1):39–45. [PubMed] [Google Scholar]
- WOOD T. H., TAYLOR A. L. Dependence of x-ray sensitivity of yeast on phase state and anoxia. Radiat Res. 1957 Jun;6(6):611–625. [PubMed] [Google Scholar]