Abstract
In the vascular wall, the Na,K-ATPase plays an important role in the control of arterial tone. Through cSrc signaling, it contributes to the modulation of Ca2+ sensitivity in vascular smooth muscle cells. This review focuses on the potential implication of Na,K-ATPase-dependent intracellular signaling pathways in severe vascular disorders; ischemic stroke, familial migraine, and arterial hypertension. We propose similarity in the detrimental Na,K-ATPase-dependent signaling seen in these pathological conditions. The review includes a retrospective proteomics analysis investigating temporal changes after ischemic stroke. The analysis revealed that the expression of Na,K-ATPase α isoforms is down-regulated in the days and weeks following reperfusion, while downstream Na,K-ATPase-dependent cSrc kinase is up-regulated. These results are important since previous studies have linked the Na,K-ATPase-dependent cSrc signaling to futile recanalization and vasospasm after stroke. The review also explores a link between the Na,K-ATPase and migraine with aura, as reduced expression or pharmacological inhibition of the Na,K-ATPase leads to cSrc kinase signaling up-regulation and cerebral hypoperfusion. The review discusses the role of an endogenous cardiotonic steroid-like compound, ouabain, which binds to the Na,K-ATPase and initiates the intracellular cSrc signaling, in the pathophysiology of arterial hypertension. Currently, our understanding of the precise control mechanisms governing the Na,K-ATPase/cSrc kinase regulation in the vascular wall is limited. Understanding the role of vascular Na,K-ATPase signaling is essential for developing targeted treatments for cerebrovascular disorders and hypertension, as the Na,K-ATPase is implicated in the pathogenesis of these conditions and may contribute to their comorbidity.
Keywords: blood pressure, digoxin, migraine, ouabain, sodium-potassium pump, stroke
Introduction
Distinct expression and subcellular localization of Na,K-ATPase α isoforms in the vascular wall suggest their distinct functions
The Na,K-ATPase is a vital membrane transport protein that is present in all cells of the body [1]. It plays a critical role in the control of Na+ and K+ ion distribution over the cell membrane, as it is essential for, for example, maintaining the membrane potential and the secondary active transport that is important to build up the electrochemical gradient for other ions, including Ca2+, Mg2+, Cl−, and H+ [2]. The Na,K-ATPase consists of an α- and a β-subunit and associated regulatory proteins [3,4]. The catalytic α-subunit is responsible for ion transport and enzymatic activity of the Na,K-ATPase [5], while the chaperone β-subunit is essential for subcellular localization and have modulatory action for the enzyme including the regulation of affinity for Na+ and K+ [6]. Four different isoforms of the α-subunit exist with an unequal distribution throughout the body [1]. The α1 isoform is expressed in all cells whereas the α2, α3, and α4 isoforms demonstrate cell-type specific profiles [7]. The α3 and α4 isoforms are expressed in neurons and in the testes, respectively [8,9]. The α2 isoform is mainly expressed in astrocytes and muscle tissues, including vascular smooth muscle cells [10,11].
Interactions between the Na,K-ATPase and specific lipids and proteins in the plasma membrane result in the formation of lipid–protein complexes, influencing the organization of membrane microdomains and lateral mobility of membrane components, thereby modulating cell membrane fluidity [12–15]. Changes in membrane fluidity reciprocally impact the activity of the Na,K-ATPase itself, indicating a dynamic interplay between the enzyme and the cell membrane [14,16,17]. The Na,K-ATPase is also intricately regulated by the concentrations of its substrates, i.e., Na+, K+, and intracellular ATP [12]. Catecholamines that involve numerous intracellular signaling pathways, for example, protein kinases A, G, and C, tyrosine kinases, protein phosphatases and phospholipase have also been shown to modulate the activity of the Na,K-ATPase [12,18–20]. Furthermore, the actions of insulin, aldosterone, and carbachol involve changes in the subcellular distribution of the subunits of the Na,K-ATPase [13]. In this review, we will discuss how the Na,K-ATPase may potentially have implications for diseases such as stroke, migraine, and hypertension, and propose some similarities in their background mechanisms.
Cardiotonic steroids, including ouabain, digoxin, and digitoxin that bind to the α-subunit are well-known regulators of Na,K-ATPase activity [21]. Notably, the discovery of endogenous ouabain, and some other cardiotonic steroids that are synthesized by the body and use the Na,K-ATPase as a signaling receptor, has been made [22,23]. The different cardiotonic steroids share structural similarities containing a steroid core that can be glycosylated and a five- or six-membered lactone ring [24]. These functionalities affect the binding properties of specific cardiotonic steroids [21,25] although the high-affinity binding site for the cardiotonic steroids is the same [26]. Cardiotonic steroids inhibit the Na,K-ATPase by binding to the transmembrane domain of the α-subunit from the extracellular side and by stabilization of the outward-open phosphorylated E2 state, thus antagonizing K+ binding [27]. Upon binding of cardiotonic steroids, the α isoform undergoes conformational changes that seems to be dependent on the type and number of substituents on the steroid core and the size of lactone ring of the cardiotonic steroid molecule [21]. Thus, the functional consequences of different cardiotonic steroids may vary, possibly because of different conformational changes they induce, although this remains to be validated [28]. In rodents, due to mutation in the high-affinity binding site for cardiotonic steroids, the α1 isoform exhibits a lower sensitivity to cardiotonic steroids, including ouabain, requiring concentrations approximately thousand-fold higher than those necessary to effectively inhibit the α2 and α3 isoforms [27]. Hence, functional characteristics of the α1 and α2 isoforms can be pharmacologically differentiated with cardiotonic steroids [29–31].
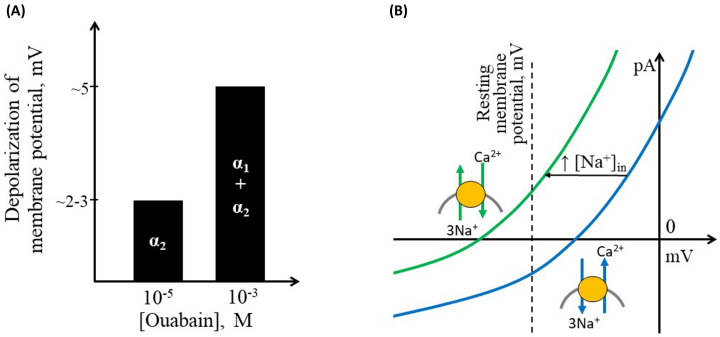
Cardiotonic steroids, particularly ouabain, have a well-described pro-contractile effect on the heart and blood vessels [11,32,33]. In cardiomyocytes and vascular smooth muscle cells, the Na,K-ATPase α2 isoform is restricted to specific areas of the plasma membrane in close proximity to the Na,Ca-exchanger (NCX) [29,34]. Both the Na,K-ATPase and the NCX are electrogenic membrane transporters. As the Na,K-ATPase pumps two K+ ions into a cell in exchange for pumping out three Na+ ions, it contributes to membrane hyperpolarization [35]. Accordingly, inhibition of the Na,K-ATPase depolarizes the membrane but the magnitude of the effect differs between the isoforms [4]. Thus, inhibition of the α2 isoform (i.e., 1–10 µM ouabain) only slightly depolarizes the smooth muscle cell membrane by approximately 2–3 mV [36,], while further elevation of ouabain to 1 mM inhibits all isoforms of the Na,K-ATPase [4] leading to further depolarization by approximately 5 mV (Figure 1A) [37]. The minor changes in membrane potential observed in the presence of ouabain below 1 mM may not be anticipated to exert a significant impact on the voltage-gated Ca2+ influx and thus arterial contraction [36,37–40].
Figure 1. Schematic presentation of key electrogenic properties of different vascular isoforms of the Na,K-ATPase and the Na,Ca-exchanger (NCX).
(A) In the rodent vasculature, ouabain in concentration of 10−5 M almost completely inhibits the Na,K-ATPase α2 isoform [4] leading to approximately 2–3 mV depolarization, while elevation of ouabain to 10−3 M further depolarizes cells with 5 mV [36,37]. (B) Current–voltage relationship for the NCX. The reversal potential of NCX in configuration 3 Na+:1 Ca2+ at a normal ion distribution over the membrane ([Na+]in 10 mM, intracellular [Ca2+]in 200 nM, [Na+]out 144 mM, [Ca2+]out 1.6 mM) is estimated to be −26 mV (blue curve). This is positive to the resting membrane potential of vascular smooth muscle cells, which is usually reported at approximately -55 mV [41,42] in peripheral arteries and −45 mV in cerebral arteries [11]. This enables extrusion of Ca2+ from the cell, i.e., forward mode of the NCX activity [43]. However, even a slight elevation of [Na+]in may shift the reversal potential of NCX to a value negative to the cell resting potential (e.g., change of [Na+]in from 10 to 15 mM moves the NCX reversal potential to −59 mV; i.e., a leftward shift of the curve indicated with green color), leading to the reverse mode of NCX transport, i.e., Ca2+ will be transported to the intracellular space [43].
The NCX, in its conventional configuration, exchanges 3 Na+ ions with 1 Ca2+ ion and has a reversal potential positive to the resting membrane potential of smooth muscle cells in the arterial wall (Figure 1B) [43]. Thus, under resting conditions, the NCX is expected to transport Ca2+ out of the cell [43]. However, a slight elevation of [Na+]in shifts the reversal potential leftwards, even below the resting membrane potential in smooth muscle cells, which is typically relatively depolarized and reported at approximately -45 – -55 mV [41,42]. This will first reduce the Ca2+ extrusion by the NCX and then, when the reversal potential is more negative than the membrane potential, change the NCX activity to a reversal mode that transports Ca2+ into the cell (Figure 1B) [43,44].
The colocalization of the Na,K-ATPase α2 isoform with the NCX may facilitate efficient transport of Ca2+ out of the cell through the NCX that utilizes the locally restricted Na+ gradient generated by the Na,K-ATPase [45] (Figure 2). Based on this isoform-specific colocalization, it has been suggested that the pro-contractile action of cardiotonic steroids is related to elevation of intracellular Ca2+ load [43,46]. The proposed mechanism stems from the inhibition of the Na,K-ATPase, which consequently prevents the active extrusion of Na+ ions and leads to secondary elevation of intracellular Ca2+ load to the sarcoplasmic reticulum by the Sarcoplasmic/Endoplasmic Reticulum Ca2+- ATPase [47]. The intracellular Ca2+, derived from the extracellular space, is normally extruded in exchange of Na+ by the NCX and through the sarcolemmal Ca2+-ATPase. However, because of combined effect of membrane depolarization and intracellular Na+ elevation, the potential for extrusion of Ca2+ by the NCX is reduced upon inhibition of the Na,K-ATPase by cardiotonic steroids. This increases the Ca2+ concentration in the cytosol that is then stored in the sarcoplasmic reticulum and contributes with greater Ca2+ release upon agonist stimulation [45,48].
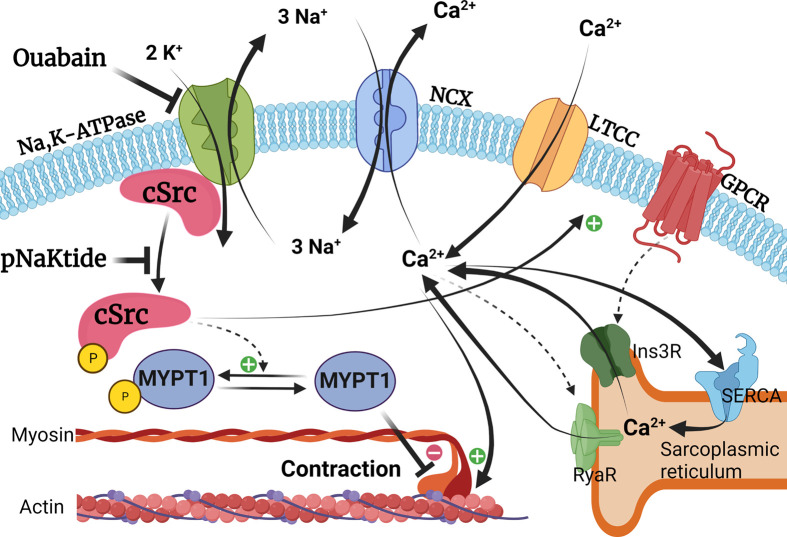
Figure 2. Signaling pathways behind potentiation of vascular smooth muscle contraction by ouabain.
The Na,K-ATPase generates the electrochemical gradient for Na+ ions, driving Ca2+ extrusion through the Na,Ca-exchanger (NCX) [45]. Upon binding of ouabain, the Na,K-ATPase pumping is impaired, reducing the NCX driving force and increasing intracellular Ca2+ concentration [43,46]. This surplus Ca2+ is then loaded into the sarcoplasmic reticulum by the sarcoendoplasmic reticulum Ca2+ ATPase (SERCA) [45,48]. Subsequently, more Ca2+ is released from the sarcoplasmic reticulum via the inositol trisphosphate receptors (Ins3R) upon G-protein coupled receptor (GPCR) stimulation and/or as a Ca2+-induced Ca2+ release through ryanodine receptors (RyaR) resulting in potentiated smooth muscle contraction [45,48]. Ouabain also induces cSrc kinase phosphorylation, ultimately increasing the sensitivity of the contractile machinery to intracellular Ca2+ [11,39]. This occurs through the phosphorylation of myosin phosphatase target subunit 1 (MYPT1), leading to the inhibition of the myosin light chain phosphatase, resulting in Ca2+ sensitization [10,11,39,49,50]. A synthetic peptide inhibitor, pNaKtide [51], inhibits cSrc kinase activation and thus, reduces smooth muscle Ca2+ sensitivity [11]. The cSrc kinase signaling may also control intracellular Ca2+ concentration through the phosphorylation of L-type calcium channels (LTCC), leading to increased Ca2+ influx [52,53].
Digoxin is a Na,K-ATPase inhibitor that is used in clinical settings to regulate cardiac frequency and potentiate the cardiac contractility [43]. However, unlike ouabain, digoxin does not have the same potentiating effect on vascular contraction, despite its similar inhibition of the ion transport [11,54]. The specific reason for these different functional effects remains to be determined but it may imply that the modulation of arterial contractility by the Na,K-ATPase cannot solely be attributed to the direct inhibition of ion pumping activity [10,55]. In discussing the importance of Na,K-ATPase for diseases, it is therefore crucial to consider its role as an intracellular signaling hub.
Na,K-ATPase-dependent cSrc signaling regulates Ca2+ sensitivity in smooth muscle cells
To elucidate the role of Na,K-ATPase for the pathologies, it is important to understand how the Na,K-ATPase signals in the cells. In this section, we will therefore summarize how signaling from the Na,K-ATPase may occur. Apart from controlling intracellular Ca2+-signaling, the Na,K-ATPase has been suggested to regulate several other intracellular signaling pathways [45,56–59] in a cation-independent manner [49, 60–65]. Two Na,K-ATPase-dependent signaling pathways has been implicated in cell proliferation, these are the EGFR/cSrc/Ras/Erk1/2 and the PI3KA1/Akt pathways [66–68] [EGFR, epidermal growth factor receptor; Erk1/2, p44/42 mitogen‐activated protein kinase; PI3KA1, phosphatidylinositide 3-kinase 1A]. Thus, activation of the PI3KA1/Akt signaling has been implicated in ouabain-induced cardiac hypertrophy [68,69]. Whether this pathway is dependent on cSrc kinase signaling remains controversial [56,66,68] and its role in the vascular wall is not yet elucidated. The Na,K-ATPase-regulated PLCγ/InsP3R signaling pathway has been suggested to modulate Ca2+ release from sarcoplasmic reticulum [45,70] [PLCγ, phospholipase Cγ; InsP3R, inositol trisphosphate receptor]. Hence, there are several signaling pathways under regulation of the Na,K-ATPase that may affect vascular tonus but their function in vascular smooth muscle cells needs to be validated. Most investigations have focused on the Na,K-ATPase-dependent pathways downstream from cSrc kinase [49, 61–65]. The Na,K-ATPase α1 isoform has been demonstrated to contain a cytosolic inhibitory cSrc-binding domain [62,71,72]. Binding of ouabain to the Na,K-ATPase [61] leads to release of the Na,K-ATPase-bound cSrc kinase and its auto-phosphorylation at Tyr-418 that activates the enzyme [36,60,63,73,74]. Importantly, the fact that cSrc is activated upon ouabain binding to the Na,K-ATPase is well-established [75,76], but the mechanism behind it remains controversial as some studies failed to demonstrate the physical interaction between the Na,K-ATPase and cSrc kinase [77,78]. Hence, it has been proposed that cSrc autophosphorylation is a result of localized increase in intracellular ATP concentration because of inhibited activity of the Na,K-ATPase [79]. Importantly, binding of digoxin to the Na,K-ATPase failed to activate cSrc kinase in rat smooth muscle cells ex vivo [80] arguing against indirect cSrc activation via the elevated ATP concentration. The reason for these different actions of the two cardiotonic steroids is unclear, although the effect of different conformational changes in the Na,K-ATPase transmembrane complex upon binding these two cardiotonic steroids has been speculated [21,39]. These different conformation changes can be a result of the variable structural and thus, binding characteristics of the different cardiotonic steroids, as discussed above.
The phosphorylation level of cSrc kinase is inversely correlated with the expression of the α1 isoform [11,72,81,51]. Whether other α isoforms than the α1 isoform are also capable of modulating cSrc kinase signaling is currently a matter of controversy [39,82]. In the studies on epithelial cells, a distinct role of the α1 isoform is suggested [83]; however, these cells lack the native expression of other Na,K-ATPase isoforms [4]. In contrast, the Na,K-ATPase-dependent cSrc signaling in the rodent vascular wall was activated by ouabain concentrations that specifically act on the α2 isoform suggesting its role in this signaling [11,36,39,49,80]. Furthermore, both the α1 and α2 isoforms were shown to interact with cSrc kinase human skeletal muscle cell culture [84]. It might, however, also be an indirect effect of the α2 isoform on cSrc activation as the Na,K-ATPase α1 isoform inhibits cSrc in its E1 conformation [62], which depends on Na+ in the cytosol [85]. Ouabain inhibition of the Na,K-ATPase α2 isoform may therefore affect the localized Na+ concentration and thus, initiate cSrc activation from the α1 isoform. Future studies need to address these possibilities.
The ouabain-induced potentiation of the constriction of cerebral arteries [11] and mesenteric small arteries [39] is related to the amplification of cSrc signaling. Moreover, cSrc kinase contributes to endothelin-1-induced vasoconstriction of isolated rabbit basilar arteries [49,86], and is necessary for the myogenic response of rat cerebral arteries [87]. The cSrc kinase activation is shown to lead to myosin phosphatase target subunit 1 (MYPT1) phosphorylation in vascular smooth muscle cells of cerebral arteries [11], as well as in arteries of various other tissues [10,39,49,88,50]. However, the precise signaling mechanism between cSrc and MYPT1 has not yet been fully elucidated [55] as cSrc tyrosine kinase cannot directly phosphorylate MYPT1 threonine residuals that were shown involved in this signaling [89]. MYPT1 is an enzyme, which regulates the phosphorylation of myosin light chain phosphatase and thereby the sensitivity of the contractile machinery of vascular smooth muscle cells to intracellular Ca2+ (Figure 2) [55,90]. This phenomenon is known as ‘Ca2+ sensitization’ and implies a potentiation of smooth muscle contractile state due to increased sensitivity of myofilaments to intracellular Ca2+ without any change in Ca2+ concentration [91]. It thus seems likely that ouabain consequent to binding to the Na,K-ATPase modifies cSrc signaling in smooth muscle and enhances the sensitivity of the contractile apparatus to Ca2+.
This has indeed been supported by experiments directly showning that ouabain potentiates the smooth muscle cell contraction via Ca2+ sensitization [11,39]. In accordance with the concept of sensitization of vascular smooth muscle cells to intracellular Ca2+ [91,92], ouabain-potentiated vasoconstriction is associated with minor or no change in intracellular Ca2+ [11,39,49,93]. Moreover, the source of intracellular Ca2+ and the type of contractile stimuli are not essential for ouabain-induced potentiation of contraction via the Ca2+ sensitization [11]. Thus, ouabain potentiates the contraction initiated by different agonists (e.g., noradrenaline, thromboxane, and endothelin) and K+-induced depolarization. The K+-induced depolarization and contraction depends on Ca2+ influx from the extracellular space via the voltage-gated Ca2+ channels, while the contraction upon stimulation of G-protein-coupled receptors depends on both Ca2+ influx and Ca2+ release [94]. This supports the suggested primary role of the pro-contractile ouabain-induced cSrc activation, that is Ca2+ sensitization via MYPT1 phosphorylation, although an effect of ouabain on other pathways in the vascular wall as discussed above cannot be excluded. Accordingly, it has been reported that phosphorylation by cSrc potentiates the activity of voltage-gated L-type Ca2+ channels [52,53]. Moreover, in non-muscular cells, cSrc was shown to potentiate the Ca2+ release via an activation of the inositol trisphosphate receptors in the sarcoplasmic reticulum membrane and to suppress the activity of voltage-gated K+ channels leading to further depolarization and voltage-dependent Ca2+ influx [,95] (Figure 2). Hence, there are multiple signaling pathways in which binding of ouabain to the Na,K-ATPase may potentiate the contraction of vascular smooth muscle cells but the involvement of Ca2+ sensitization seems to have prominent action (Figure 2).
Involvement of the Na,K-ATPase in ischemic stroke pathogenesis
Role of vascular dysfunction in futile recanalization
The treatment of ischemic stroke has been revolutionized by the development and implementation of endovascular thrombectomy techniques [96]. However, 54% of patients with successful macrovascular recanalization after thrombectomy are either disabled to a degree requiring external assistance or deceased 90 days after the stroke, a phenomenon known as futile recanalization [97,98]. Several mechanisms have been suggested to account for futile recanalization including vascular dysfunction [99,100]. Hence, futile recanalization has been attributed to impaired microvascular reperfusion, observed in approximately 25% of ischemic stroke patients receiving endovascular thrombectomy [101]. Preclinical studies suggest that microvascular failure after reperfusion is associated with undesirable contraction of capillary pericytes in the peri-ischemic penumbra [102] and in the ischemic core [103–106]. Although spreading depolarizations during arterial occlusion and hypoxic injury have been suggested to account for capillary pericyte contraction, the molecular mechanism behind the contraction remains largely unknown [103–108]. Importantly, constriction or vasospasm of upstream pial arteries [109] and parenchymal arterioles [100,110,111] have also been suggested to be involved in futile recanalization. Changes in arterial function following ischemic stroke have received relatively little attention compared with hemorrhagic stroke [112]. Acquiring a more comprehensive understanding of post-stroke vascular dysfunction could be of great importance in preventing infarct growth associated with secondary brain injury in ischemic stroke patients [112,113], especially given that all previous clinical trials aimed at preventing such injury have been unsuccessful [114].
Association between the Na,K-ATPase-dependent cSrc signaling and post-stroke vasospasm
Previous studies in rats indicate an increased vascular tone of rat arterioles after ischemic stroke [100,115]. This increased arteriolar tone is not associated with changed smooth muscle cell membrane potential nor changed intracellular Ca2+ concentration compared with rats undergoing sham surgery [100]. Accordingly, the excessive tonus of arteries and arterioles following stroke is suggested to be caused by elevated sensitivity of the contractile machinery to Ca2+ (Figure 2) [100,109]. Measurements of intracellular Ca2+ changes in post-stroke rat arterioles demonstrated stronger contraction at the same Ca2+ concentration in comparison with matched sham operated group suggesting the Ca2+ sensitization [100]. Further research is required to clarify if post-stroke capillary failure is also associated with increased Ca2+ sensitivity of contractile pericytes [102,103]. In the following, it will be discussed whether the Na,K-ATPase-dependent phosphorylation of cSrc kinase is important for the increased sensitivity of arteries and arterioles to intracellular Ca2+ after stroke.
The vasoconstriction of pial arteries downstream from the occlusion after reperfusion is associated with increased Na,K-ATPase-dependent phosphorylation of cSrc kinase [109]. Increased vascular tone following stroke could arise from enhanced cSrc/PLCγ/InsP3R signaling, triggering an increased release of Ca2+ from the sarcoplasmic reticulum [45,70]. However, given that the intracellular Ca2+ concentration in vascular smooth muscle cells remains similar between stroke and control rats [100], this mechanism appears unlikely. Conversely, increased Ca2+ sensitization of the contractile machinery after stroke may be mediated through activation of cSrc by phosphorylation resulting in potentiation of smooth muscle contraction [11,39,49] even at unchanged intracellular Ca2+ concentration as discussed above (Figure 2) [100]. This suggests the cSrc-associated Ca2+ sensitization as a novel treatment target to prevent post-stroke vasospasm. In fact, cSrc kinase inhibition has been studied in rodent models of experimental stroke. Pharmacological inhibition of Src family tyrosine kinases with relatively specific inhibitors, PP1 or PP2, is associated with reduced cerebral infarctions as well as improved neurological outcomes [116–119]. The inhibition of cSrc kinase decreases smooth muscle cell Ca2+ sensitivity, leading to reduced vasoconstriction of isolated mouse middle cerebral arteries [11]. Accordingly, the positive outcome resulting from PP1-induced Src family kinase inhibition following stroke is accompanied by an increase in cerebral perfusion when compared with vehicle-treated controls that also underwent stroke [116]. Since this study employed magnetic resonance imaging to quantify cerebral blood flow, the spatial resolution was insufficient to investigate the distinct contributions of different cerebrovascular beds, and other mechanisms of futile reperfusion, for example, a disrupted blood–brain barrier cannot be excluded [116–119]. Other potential reasons for the favorable stroke outcome following cSrc inhibition warrant careful consideration. Notably, the Na,K-ATPase-dependent cSrc/Ras/Erk1/2 signaling pathway has been proposed to elicit excessive generation of reactive oxygen species within the mitochondria [60,73,74], a pivotal mechanism in the pathophysiology of ischemic stroke [120]. Therefore, the improved stroke outcome linked to cSrc inhibition could potentially stem from the prevention of excessive reactive oxygen stress, thereby reducing tissue damage following stroke.
Down-regulation of the Na,K-ATPase after stroke reperfusion
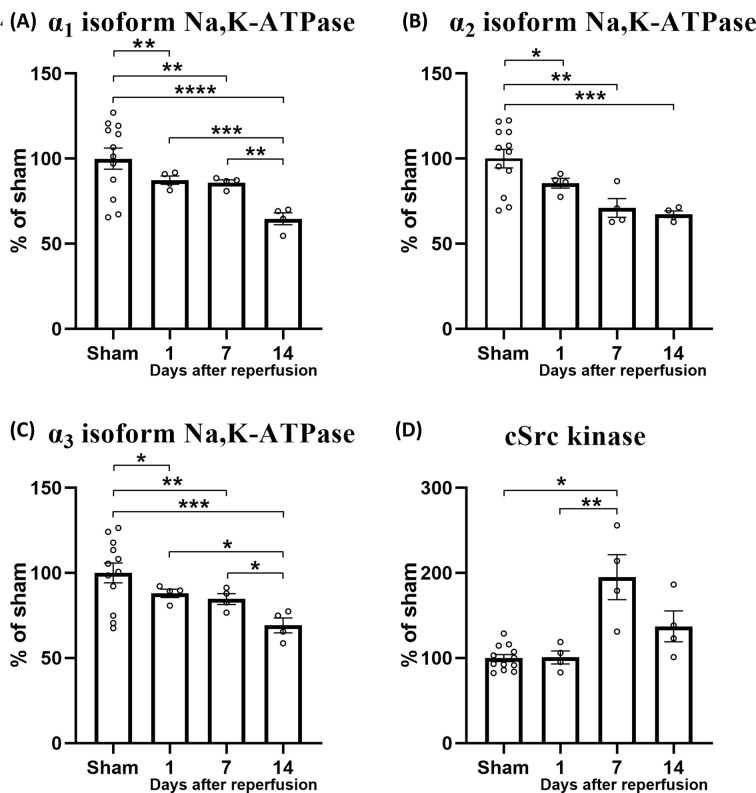
To test whether increased phosphorylation of cSrc kinase after stroke reperfusion is associated with the changes in abundancy of the Na,K-ATPase, we assessed a previously published proteomics dataset [121] (Reprinted with permission, Wen et al., 2019, J. Proteome Res., copyright © 2019 American Chemical Society). We compared the proteome of rat brains subjected to a 2-h filament-induced middle cerebral artery stroke with the proteome of matched control rats that underwent sham surgery. Samples of the rat brain were collected from the stroked hemisphere at three time points (1, 7, and 14 days) after stroke reperfusion or sham surgery to investigate the temporal changes in protein expression. Groups were compared using two-way ANOVA with Turkey’s post-hoc test. The expression of the Na,K-ATPase α1, α2, and α3 isoforms were reduced at all three time points after reperfusion compared with the sham group (Figure 3A–C). The expression of the α1 and α3 isoforms were lower at the 14 days follow-up compared with the 1- and 7-day follow-ups. Intriguingly, the expression of cSrc in the same proteomics dataset was up-regulated at the 7-day follow-up after stroke compared with the sham group (Figure 3D). Two other Src family tyrosine kinases, Lyn and Hck, were also detected by proteomics, but their expression was not affected by stroke (P>0.5). Importantly, this analysis is made on a bulk proteomics dataset [121]. A spatially restricted assessment is necessary to investigate whether cSrc up-regulation associated with the Na,K-ATPase down-regulation is localized to brain mural cells, i.e., contractile pericytes and smooth muscle cells [122].
Figure 3. Stroke led to down-regulated expression of the Na,K-ATPase α isoforms and up-regulation of cSrc kinase expression.
Proteomics analysis of brain tissue of filament-induced middle cerebral artery stroke and sham-operated rats (n=12). Brain tissue was sampled 1, 7, or 14 days after stroke (n = 4 for each follow-up). The expression of Na,K-ATPase α1 isoform was down-regulated after stroke compared with sham-operated rats (A). The expression of the α1 isoform was lower at the 14-day follow-up compared with the 1- and 7-day follow-up. The expression of the α2 (B) and α3 isoforms (C) were also reduced after stroke compared with sham-operated rats. The expression of the α3 isoform was more reduced at the 14-day follow-up compared with the 1- and 7-day follow-up (C). The expression of cSrc kinase was increased at the 7 days follow-up compared with the sham-operated group and the 1 day follow-up (D). Reprinted with permission from previously published dataset by Wen et al., J. Proteome Res. (2019) [121], Copyright © 2019 American Chemical Society. Data were normalized to the sham-operated group that was set to 100%. Groups were compared using two-way ANOVA with Turkey’s post-hoc test. *, **, ***, ****, P<0.05, 0.01, 0.001, 0.0001.
The implication of altered cSrc kinase signaling after stroke, while the expression of other Src family kinases remains unchanged, is consistent with the findings of a prior study in a mouse model of ischemic stroke [116]. In that study, it was shown that homozygous global knockout of the cSrc gene is associated with reduced infarct size, whereas homozygous knockout of another Src family kinase member, Lyn, results in a similar infarct size to that of wild-type mice [116]. These results suggest that cSrc plays a specific role in the pathology of stroke. The proteomics dataset [121] that we assessed cannot provide data on the protein phosphorylation level. Further, the dataset is not cell-type specific and the contribution of specific cell types in the brain to changed expressional levels cannot be differentiated. Nevertheless, these proteomics data strongly suggest that all three Na,K-ATPase α isoforms expressed in the brain are down-regulated after stroke. At the same time, it appears that cSrc kinase, which is normally dephosphorylated in the presence of active Na,K-ATPase in the cerebral vasculature, may become phosphorylated, which is consistent with previous observations in a mouse model of ischemic stroke.
Reduced activity of the Na,K-ATPase has been reported in various experimental models of hypoxic and ischemic pathologies [123,124]. Accordingly, hypoxia leads to reduced Na,K-ATPase expression and activity in isolated vascular smooth muscle cells of rat basilar arteries [125]. The Na,K-ATPase is one of the most ATP-consuming proteins in the body [126–128]. Hence, reduced activity or expression of the Na,K-ATPase under conditions with restricted supply of nutrients or oxygen may be an effective strategy to balance intracellular energy [129]. This fine-tuned balance may be regulated by the release of endogenous ouabain [130,131]. Hypoxia leads to increased level of oxidative stress, which has also been suggested to regulate the Na,K-ATPase through carbonylation of the α1 isoform [132,133]. Importantly, oxidative stress-mediated carbonylation of the α1 isoform increases the phosphorylation of cSrc [132,133]. Amplified cSrc signaling is associated with detrimental impact on mitochondrial respiration [60,74,93], which may further increase mitochondrial generation of reactive oxygen species and thus, a vicious circle is established [93,129,134]. In the vascular smooth muscle cells, the vicious circle resulting in impaired Na,K-ATPase and amplified cSrc kinase activity may lead to exaggerated vasoconstriction through the mechanisms discussed above. In strong support of this, pNaKtide, a specific inhibitor of the Na,K-ATPase-dependent cSrc activation [11,36,51,93,135], may break the vicious cycle. pNaKtide is a 20-amino acid peptide that mimics cSrc-binding sequence in the N-terminus of Na,K-ATPase and inhibits cSrc in an ATP-independent manner [51]. It remains, however, to be tested whether pNaKtide treatment can prevent post-stroke vasospasm and improve the outcome after stroke.
Mice with heterozygous knockout of the Na,K-ATPase α1 isoform have larger cerebral infarct size after middle cerebral artery occlusion than wild-type mice [136]. Further, reduced expression of both α1 and α2 isoforms in cerebral arteries in mice is associated with amplified cSrc kinase signaling and increased tone of the middle cerebral artery [11]. As discussed above, the role of α2 isoform in cSrc signaling is controversial [39,83]; thus, the isoform-specific contribution of the Na,K-ATPase remains to be elucidated. A further reduction in the Na,K-ATPase α isoform expression and cSrc activation following stroke, as the proteomics analysis suggested (Figure 3A–C), may lead to vasospasm. This implies that cSrc-dependent excessive vasoconstriction may contribute to the poor stroke outcome in mice with heterozygous knockout of the α1 isoform [136]. Whether the reduction in the Na,K-ATPase α2 isoform will also have similar consequences for stroke outcome remains to be studied.
The implication of the Na,K-ATPase in pathophysiology of migraine with aura
Reduced expression or pharmacological inhibition of the Na,K-ATPase increases susceptibility for spreading depressions
Migraine with aura is associated with a drop in blood flow in the affected hemisphere. This is also the case for the peri-ischemic tissue after ischemic stroke. It is generally accepted that the drop in blood flow is associated with spreading depression, i.e., cortical and peri-ischemic spreading depression in migraine aura and ischemic stroke, respectively [137]. The spreading depression is represented as a wave of depolarization propagating through the neuronal tissue across the cortex with a speed of ∼3 mm/min [102,138,139]. During spreading depression, both neuronal and glial cells undergo depolarization, resulting in a shift of the transmembrane ion balance [137]. This shift involves a substantial increase in extracellular K+ concentration [140] due to the efflux of K+ as the repolarizing current [137,141]. It is, therefore, of relevance to understand the role of the Na,K-ATPase in migraine with aura and the associated spreading depression. As the concentration of extracellular K+ rises, the cell membrane depolarizes [142]. Consequently, voltage-gated cation channels on neuronal membranes become more susceptible to opening, allowing influx of Ca2+ and Na+ ions, which further depolarizes the membrane potential [137,142].
The role of the Na,K-ATPase in spreading depression is suggested from the finding that ouabain induces spreading depression in rat brain slices [143,144] and in vivo in mice [145]. The proposed mechanism for ouabain’s ability to induce spreading depression is through the inhibition of Na,K-ATPase ion-pumping activity [146]. Hence, ouabain may lead to an elevation of extracellular K+ concentrations resulting in membrane potential depolarization and thus initiation of the spreading depression [146,147]. Importantly, spreading depressions may be triggered by low ouabain concentrations that selectively inhibit the Na,K-ATPase α2 and α3 isoforms in rodents without any significant effect on the α1 isoform [31,148]. On strong support of this, heterozygous knockout [149] or small hairpin RNA (shRNA)-induced knockdown of the α2 isoform [145] is associated with increased susceptibility to experimental spreading depression in mice. Conversely, heterozygous knockout of the α3 isoform is associated with lower susceptibility to spreading depression whereas heterozygous knockout of the α1 isoform does not change spreading depression susceptibility [149]. The reason for this difference is unclear but possibly the different expression pattern of the isoforms leads to different extracellular K+ clearance in the brain parenchyma. The α2 isoform is expressed in astrocytes [4,7], which may be specifically important for K+ clearance [150,151]. Further, in contrast with the Na,K-ATPase α1 isoform, both α2 and α3 isoforms are not saturated at normal K+ concentrations, and therefore have a capacity to further increase their activity upon elevation of extracellular K+ [152]. These observations are of high clinical relevance since mutation in the α2 isoform is linked to familial hemiplegic migraine.
Familial hemiplegic migraine-associated mutation of the Na,K-ATPase is associated with exaggerated constriction of cerebral arteries
Familial hemiplegic migraine type 2 (FHM2; OMIM No. 602481) is a rare subtype of migraine with aura associated with mutation in the ATP1A2 gene encoding the Na,K-ATPase α2 isoform [153–155]. Some ATP1A2 mutations, including the G301R missense mutation [11,74,156–158], lead to reduced expression of the α2 isoform whereas other mutations do not change the expressional level [159] but probably affect the function or regulation of the Na,K-ATPase. In line with the α2 isoform knockout studies [145,149], mice carrying FHM2-associated mutations of the α2 isoform exhibit increased susceptibility for cortical spreading depolarization [160–162]. The α2 isoform is important for generating the driving force for extracellular space clearance by astrocytes, including reuptake of glutamate and K+ released upon neuronal excitation [150,151]. Consistent with this mechanistic view, astrocytic glutamate reuptake was shown to be reduced in two different murine models of FHM2 [157,160].
It is often overlooked in research on FHM2 [153,163] that the α2 isoform is expressed not only in glial cells but also in the endothelium and smooth muscle cells of the vascular wall [11,29,164]. As discussed above, the α2 isoform plays a key role for vascular function, and it is therefore possible that cerebrovascular abnormalities may contribute to the migraine pathophysiology of FHM2 [10,46,55,165]. Indeed, mice carrying the FHM2-associated G301R mutation, α2+/G301R mice, show abnormal neurovascular coupling [166], and excessive vasoconstriction of cerebral arteries associated with cerebral hypoperfusion [11]. Curiously, the increased vasoconstriction is associated with a smaller increase in agonist-induced intracellular Ca2+ concentration in cerebrovascular smooth muscle cells of α2+/G301R compared with wild-type mice [11]. This implies that increased cerebrovascular contractility in α2+/G301R mice cannot be explained by elevation of intracellular Ca2+ concentration in vascular smooth muscle cells of α2+/G301R mice [11]. Similar to that observed in arterioles after stroke [100], this implies that the increased vasoconstriction of cerebral arteries in α2+/G301R mice is attributed to augmented Ca2+ sensitization in smooth muscle cells that is associated with amplified Na,K-ATPase-dependent phosphorylation of cSrc kinase and MYPT1 (Figure 2) [11]. Indeed, it was shown that cSrc phosphorylation was increased in cerebral arteries of α2+/G301R mice and acute cSrc inhibition abolished the difference in vascular responses between α2+/G301R and wild-type mice [11]. Therefore, it seems likely that the cSrc pathway is important for the pathophysiology of FHM2. It is possible that other Na,K-ATPase-dependent signaling pathways affecting cell proliferation, i.e., EGFR/Src/Ras/ERK and PI3KA1/Akt pathways, may contribute to the altered cerebrovascular function in FHM2. In fact, middle cerebral arteries of α2+/G301R mice exhibit increased vascular wall thickness and media-to-lumen ratio compared with wild-type mice [11].
Migraineurs with aura exhibit interictal cerebral hypoperfusion
Whether the enhanced Ca2+ sensitivity of cerebrovascular smooth muscle cells is associated with increased vasoconstriction in other types of migraine with aura than FHM2 has yet to be studied. However, previous studies have reported cerebral hypoperfusion of migraineurs with aura in the interictal period [167–169]. Several contractile factors may provoke cerebral hypoperfusion in migraineurs with aura. For example, one study found that the plasma level of thromboxane A2, a potent vasoconstrictor, is higher in migraineurs during the interictal period than in patients with tension-type headache or healthy individuals [170]. Other studies have proposed that migraineurs with aura have elevated plasma level of another vasoconstrictor, endothelin-1, in the interictal period [171,172]. Interestingly, it has also been reported that migraineurs have an increased concentrations of endogenous ouabain in the blood in the interictal period [173], suggesting that the inhibition of the Na,K-ATPase may lead to exaggerated vasoreactivity [39,174]. Importantly, the elevation of endogenous ouabain is seen in the blood but not in the cerebrospinal fluid [173]. Due to its hydrophilic structure, ouabain cannot easily cross the blood–brain barrier [175], therefore deviding the body’s endogenous ouabain in two compartments, i.e, periphery and the brain [45]. This suggests that the action of increased endogenous ouabain in the blood of migraineurs [173] may be addressed to the cerebral vasculature from the circulation site, and not to neurons and astrocytes located behind the blood–brain barrier. However, the specific extent to which ouabain from the blood can pass the blood–brain barrier remains to be studied. The finding of elevated levels of endogenous ouabain in migraineurs [173] calls for further research to clarify the contribution of Na,K-ATPase-dependent disturbances in cerebrovascular function in migraine with aura.
The role of the Na,K-ATPase in hypertension
Endogenous cardiotonic steroid-like compounds
The first indications that the body may produce a cardiotonic steroid stems from investigations of volume-dependent hypertension [176]. Nearly half a century ago, a reduced pumping activity of the Na,K-ATPase in the vascular wall of hypertensive dogs was reported [177]. This led to the proposal of the presence of an endogenous compound similar to cardiotonic steroids [178]. Moreover, a strong correlation between the capacity of blood plasma to inhibit the Na,K-ATPase and blood pressure measured in patients with hypertension was discovered [179]. As a result, an endogenous cardiotonic steroid was isolated from human serum and was claimed to be structurally indistinguishable from ouabain [180]. As its structure is still debated, it is often termed as the endogenous ouabain-like compound [181], but it is proposed to be chemically identical to the original plant-derived alkaloid ouabain [182]. Later, other endogenous cardiotonic steroids, for example, marinobufagenin and telocinobufagin, were also found in the human body [183]. The binding site for cardiotonic steroids in the Na,K-ATPase is highly conserved through evolution [21], with only few exceptions [184]. This might suggest that ouabain is physiologically (and pathophysiologically) important and consistent with a possible role in hypertension [185].
Ouabain is synthesized in the zona glomerulosa cells of the adrenal cortex as other adrenal steroids [186,187]. Hypothalamus is also suggested as a site for production of endogenous ouabain [188,189], which may play a central neuromodulatory role leading to excitation of the central sympathoexcitatory pathways [190,191]. Two separate secretion sites and ouabain's impermeability over the blood-brain barrier [175], suggest the existence of two signaling systems for endogenous ouabain [45].
Hydroxycholesterol, pregnenalone, and progesterone have been shown to increase the secretion of endogenous ouabain, possibly acting as precursors in its biosynthetic pathway [192,193]. Interestingly, the use of the oral contraceptive, progesterone, was previously shown in migraineurs with aura to be associated with increased prevalence of ischemic stroke [194–197]. However, a direct link between the level of endogenous ouabain and ischemic stroke has not yet been investigated. The synthesis of ouabain follows a similar pathway as aldosterone [198]. The exact mechanisms and precursors directly involved in the biosynthesis of ouabain remain, however, unclear. Several factors have been suggested to increase the production of endogenous cardiotonic steroids, including blood pressure elevating hormones, drugs, and interventions such as angiotensin II, vasopressin, adrenocorticotropic hormone (ACTH), hypoxia, physical activity, and stress [130,199–201]. There are several lines of evidence to suggest that ouabain is synthesized and secreted by the adrenal cortex. First, ouabain is found at high concentrations in the adrenal cortex [180,189]. Second, bovine adrenocortical cells secrete ouabain in amounts greater than their storage capacity under in vitro conditions [202]. Third, the concentration of ouabain in adrenal veins is significantly higher than in arterial plasma [203]. Moreover, some adrenal cortex tumors are associated with excessive production and secretion of ouabain [204]. Consistently, administration of anti-ouabain antibodies leads to adrenal cortex enlargement in rats, further implicating the adrenal gland as a source of ouabain [205].
The majority of endogenous ouabain detection studies are based on blood using immunoassays, for example, enzyme-linked immunosorbent assay (ELISA) and radioimmunoassay [206]. The results of these methods are supported by high-resolution mass spectrometry and nuclear magnetic resonance spectroscopy [180]. However, the validity of endogenous ouabain detection is still questioned [181]. One study that used an ultra-performance liquid chromatography tandem mass spectrometry failed to detect the exact structure corresponding to endogenous ouabain in patients with heart failure and in healthy controls [181] while others reported endogenous ouabain in the blood of various patients [207–210]. Nevertheless, the majority of publications support the presence and functional significance of endogenous ouabain-like compound under physiological and pathological conditions, although its specific molecular identity remains to be validated [211].
Endogenous ouabain concentration is elevated in hypertensive patients
Sub-nanomolar concentrations of endogenous ouabain are detected in the blood of patients with essential hypertension [207–210]. Almost 50% of patients with uncomplicated essential hypertension have been reported to have elevated endogenous ouabain [212]. In accordance with the increased peripheral resistance and normal cardiac output in hypertension, plasma ouabain level correlates positively with elevated peripheral resistance and left ventricular hypertrophy, but not with cardiac output [208,210]. An elevated level of endogenous cardiotonic steroids are also reported in hypertensive patients with volume expansion, for example, kidney and heart failure [213,214], hyperaldosteronism [212], and preeclampsia [215].
Chronic administration of ouabain, leading to an increase of its plasma concentration to the level observed in hypertensive patients, also produces hypertension in rats [61,216,217]. This chronic elevation of blood pressure is associated with elevated peripheral vascular resistance [61,218]. Accordingly, chronic ouabain treatment induces inward arterial remodeling in the resistance vasculature [219]. Notably, the plasma level of endogenous ouabain is also elevated in several rodent models of hypertension, including reduced renal mass, Milan hypertensive rats, Dahl S rats on high-salt diet and ACTH-induced hypertension [220–223]. Intriguingly, inhibition of endogenous ouabain by systemic administration of the ouabain antagonist, rostafuroxin, or exogenous anti-ouabain antibody lowers blood pressure and even prevents hypertension in rodent hypertension models [224–226]. While anti-ouabain antibodies are thought to scavenge endogenous ouabain [225,226], rostafuroxin is a derivative of another cardiotonic steroid, digitoxigenin, and is suggested to occupy the ouabain-binding site preventing ouabain-induced cSrc activation without affecting the Na,K-ATPase transport [227]. Altogether, it suggests the therapeutic importance of putative endogenous ouabain signaling in hypertension [228].
The Na,K-ATPase may contribute to hypertension via multiple organ systems
Several mechanisms are proposed to link the Na,K-ATPase to hypertension [229]. These include genetically predisposed hyperactivation or ouabain-stimulated overexpression of the Na,K-ATPase in the renotubular epithelium [230,231]. The implication of the renal Na,K-ATPase is supported by observations of elevated endogenous ouabain in the blood of hypertensive animal models [220–223] and patients with volume expansion [213–215]. Moreover, the sympathoexcitatory activity of brain-derived endogenous ouabain is suggested to activate pathways [223] that may contribute to blood pressure elevation by neurogenic potentiation of vascular tone of peripheral resistance arteries [190,191].
Considering that the hemodynamic background of hypertension is an increase in total peripheral resistance, the role of vascular Na,K-ATPase in hypertension is not surprising. As discussed above, the pro-contractile effect of reduced abundancy or activity of the Na,K-ATPase is mainly manifested in small arteries and arterioles [55], which provide a major part of vascular resistance in the circulation [232]. The pro-contractile role of the Na,K-ATPase in these resistance arteries is well-described both in vivo [33,233–235] and in vitro [11,49,236]. Importantly, sub-micromolar and micromolar concentrations of ouabain itself do not produce significant vasoconstriction but rather potentiate agonist-induced contraction [,49] and myogenic tone [39,165,236,237] of small arteries.
The α2 isoform is important for the pro-hypertensive action of Na,K-ATPase
To date, only limited information on the hypertension-related proteomic changes in resistance arteries is available, with only one study analyzing the expressional changes in detail. This study did not identify any significant changes in the expression of Na,K-ATPase proteins in mesenteric small arteries from hypertensive rats in comparison with normotensive controls [238]. The α1 and α2 isoforms of the Na,K-ATPase are expressed in the arterial wall [40,235,239–242]. However, low plasma concentrations of ouabain are enough to induce hypertension in rodents [216,217], suggesting a primary role of the vascular α2 isoform. Accordingly, infusion of exogenous ouabain in mice that increases its plasma concentration to approximately 0.1 µM elevates blood pressure and total peripheral resistance in anesthetized mice without any significant effect on cardiac contractility and stroke volume [33]. This is further supported by ex vivo studies showing that knockout or down-regulation of the vascular α2 isoform in rodents leads to potentiation of myogenic contraction [174,235,237,243].
The contribution of the α2 isoform to vascular agonist-induced contraction is complex, as ouabain has several variables in the acute potentiating effects on the agonist-induced contraction ex vivo [39,40,244]. In most ex vivo studies, ouabain has been shown to primarily potentiate arterial sensitivity to contractile agonists [11,39,235,245–247], although this effect has not been consistently observed [40,236,242]. Thus, knockout and knockdown of the Na,K-ATPase α2 isoform potentiates the constriction of neonatal murine aorta in response to thromboxane A2 analog stimulation [164]. However, the down-regulation of the α2 isoform in adult rats suppresses the contraction induced by noradrenaline and vasopressin but potentiates pressure-induced myogenic contraction of mesenteric arteries [40]. Further, chronic treatment with low ouabain concentrations does not affect agonist-induced contraction in fourth order mesenteric arteries but potentiates the myogenic tone of these arteries [236]. In contrast, the myogenic and agonist-induced contraction of third-order mesenteric arteries are not affected by chronic ouabain treatment [219]. Altogether, this suggests that the modulatory contribution of α2 isoform Na,K-ATPase to vascular constriction depends on the vascular bed, arterial size, the contractile stimuli, and whether the effect is chronic or acute.
Ouabain affects arterial function in a vascular bed-specific manner
Different vascular beds exhibit varying responses to chronic exposure to ouabain in spite of a whole-body increase in blood pressure [242]. Thus, the aorta from chronically ouabain-treated rats is characterized by augmented expression of both α1 and α2 isoforms but reduced phenylephrine-induced contraction [242]. Mesenteric superior arteries from the same rats exhibit a similar reduction in contractility but without change in the expression of the Na,K-ATPase α isoforms [242]. In contrast, tail arteries show reduced expression of both Na,K-ATPase isoforms but no difference in the contraction in comparison with normotensive control rats [242]. These results suggest variability of the modulatory effects of ouabain in the vasculature related perhaps to different types and potency of signaling initiated by the Na,K-ATPase, for example, different distribution and subcellular localization of the α isoforms. In fact, the expression density of Na,K-ATPase isoforms varies with the arterial size even within the same vascular bed [49]. While it has been observed that ouabain has a greater effect on vasoconstriction in larger mesenteric artery branches compared with smaller ones, the underlying mechanism has yet to be fully elucidated. However, the arterial caliber-dependent vasocontractile potentiation positively correlates with the expression of the α2 isoform and the degree of ouabain-induced cSrc kinase phosphorylation [49,236]. These results imply that ouabain in large mesenteric arteries increases vascular smooth muscle cell Ca2+ sensitivity in a cSrc-dependent manner (Figure 2) [49,248]. Regardless of the underlying mechanism, the arterial caliber-dependent variability may be important for control of peripheral resistance in vivo, where smaller arteries and arterioles provide major resistance under resting conditions, while upstream arteries contribute to hemodynamic resistance during sympathetic excitation [249].
Altering the ouabain-sensitivity of the α2 isoform confers protection against hypertension
Experimental evidence for the significance of the Na,K-ATPase α2 isoform in regulating peripheral resistance and blood pressure includes experiments utilizing knock-in mutations of the α isoforms [220,235,250]. Thus, the mutation in the α2 isoform high-affinity binding site for ouabain [27] modified the normally ouabain-sensitive α2 isoform to the ouabain-resistant α2 isoform [251]. This knock-in mutation in mice does not affect the total expression level of vascular Na,K-ATPase α isoforms [250], but it prevents the ouabain-induced and ACTH-induced hypertension in mice [220,235,251]. This was further studied by introducing the mutation in Na,K-ATPase α1 isoform to increase its ouabain sensitivity [251]. When these two mutations were combined in the knock-in mice, which, in contrast with wild types, had the ouabain-sensitive α1 isoform and ouabain-insensitive α2 isoform, they remained resistant to develop hypertension following chronic ouabain treatment [251]. These findings emphasize the crucial role of the ouabain-sensitive α2 isoform in the development of hypertension [7,252].
Currently there is, however, no generally accepted molecular mechanism that explains how inhibition of the Na,K-ATPase α2 isoform leads to elevation of blood pressure [45,252]. The situation is further complicated by the fact that not all inhibitors of the Na,K-ATPase have similar effects on blood pressure. Thus, in contrast with ouabain, digoxin does not raise blood pressure and even has antihypertensive action in ouabain-dependent hypertension models [61,216]. Both ouabain and digoxin inhibit the ion pumping action of the Na,K-ATPase ex vivo in a specific manner [21,253]. This suggests that the pro-hypertensive action of ouabain is not due to disturbance in intracellular ion homeostasis as it has been validated in ex vivo studies [37,38].
Of note, although both ouabain and digoxin are suggested to be specific ligands for the Na,K-ATPase ex vivo [21,253], it might be different in vivo. Thus, blood plasma immunoglobulin-like molecules were shown to bind ouabain and function as a delivery system for ouabain in the modulation of Na,K-ATPase in vivo [254]. Taking into account the difference in molecular charge of ouabain and digoxin [255], it might affect their binding to immunoglobulins in vivo. This needs to be tested but different effects of ouabain and digoxin on the arterial contraction ex vivo in the experiments with the saline bath solution [11] cannot be explained by immunoglobulin binding. Another concern can be made based on reports from tumor cells showing that in many cardiotonic glycosides, including ouabain and digoxin, binds to the transcriptional regulator steroid receptor co-activators, SRC1 and SRC3 modulating gene expression and tumor cell proliferation [256]. Importantly, the effects of ouabain and digoxin on SRC1 and SRC3 expression in these cells were concentration-dependent and digoxin showed significantly higher potency [256]; however, it has never been shown for other cell types than tumor cells. Further research is needed to test whether the antagonizing effect of digoxin on pro-hypertensive action of ouabain is associated with other than the Na,K-ATPase signaling ex vivo and in vivo. So although it seems well-documented that the Na,K-ATPase plays a role for the pathophysiology of hypertension, the precise mechanism for this effect is still unclear.
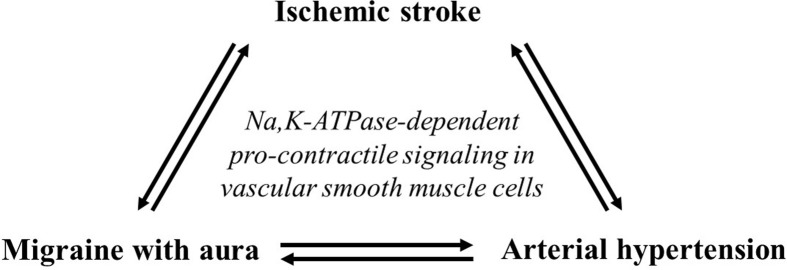
Given the similar putative mechanisms potentially involved in elevating vascular tone discussed in this review for ischemic stroke, migraine with aura, and hypertension, the comorbidity among these disorders may not be surprising. Migraine with aura significantly increases the risk of ischemic stroke [257–259], while cerebral ischemic events are also proposed to provoke migraine attacks [260,261]. Furthermore, arterial hypertension is a significant risk factor for ischemic stroke [262], and stroke patients with hypertension have a worse outcome compared with normotensive stroke patients [263]. The association between migraine with aura, stroke, and hypertension may involve increased vascular contractility linked to common Ca2+ sensitization pathways discussed in this review (Figure 4). Further research is necessary to fully comprehend these comorbidities.
Figure 4. Comorbidities between migrane with aura, ischemic stroke, and hypertension.
The hypothetical link between cerebrovascular abnormalities in ischemic stroke, migraine with aura, and hypertension, encompassing Na,K-ATPase-dependent intracellular signaling and Ca2+ sensitization in vascular smooth muscle cells.
Strengths and limitations
Ouabain is often used in laboratories to inhibit the Na,K-ATPase in tissues of various origins across different species, due to its highly conserved binding site [185,251]. The majority of the studies discussed in this review utilized genetic modifications of the Na,K-ATPase in rodents and/or ouabain to investigate Na,K-ATPase function and its physiological implications. One of the advantages of using ouabain stems from its distinct sensitivity toward the Na,K-ATPase isoforms [27,264]. Particularly, the α1 isoform in rodents requires significantly higher concentrations of ouabain for effective inhibition compared with the α2 and α3 isoforms [29–31]. This disparity allows for pharmacological differentiation and the study of functional roles of these isoforms using ouabain [27,264]. However, it is important to acknowledge the limitations associated with using ouabain to study the Na,K-ATPase. Ouabain has been shown to have effects on cellular processes such as gene expression, protein trafficking, cell adhesion, and proliferation [67,256,265–270]. These effects may be attributed to the changes in ion homeostasis or altered Na,K-ATPase-dependent intracellular signaling but may also be related to off-target binding of ouabain [256]. Unlike other cardiotonic steroids such as digoxin, ouabain is water-soluble, potentially reducing non-specific vehicle effects [271]. It is also worth noting that the concentration and exposure duration of ouabain may influence its effects and may not always reflect physiological conditions accurately.
Conclusions
The vascular Na,K-ATPase is involved in regulating arterial tone, with one mechanism being the modulation of Ca2+ sensitivity in arterial smooth muscle cells through cSrc kinase signaling. The Na,K-ATPase-dependent cSrc pathway has been implicated in vascular dysfunction after stroke, as futile recanalization and post-stroke vasospasm. Moreover, reduced expression or pharmacological inhibition of the Na,K-ATPase can lead to cSrc-dependent cerebral hypoperfusion and increased susceptibility to migraine-associated cortical spreading depression. The Ca2+ sensitization in smooth muscle modulated by the α2 isoform may play an important role in the pathophysiology of arterial hypertension, as a pro-hypertensive ouabain-mediated increase in tone of resistance arteries may be facilitated through the amplified cSrc signaling. Targeting the Na,K-ATPase-dependent cSrc signaling pathway may, therefore, provide a potential treatment strategy to prevent excessive smooth muscle cell Ca2+ sensitization in multiple vascular disorders.
Abbreviations
- ACTH
adrenocorticotropic hormone
- ELISA
enzyme-linked immunosorbent assay
- MYPT
myosin phosphatase target subunit 1
- NCX
Na,Ca-exchanger
Data Availability
This is a review article and no original data hence available. The proteomics dataset retrospectively analyzed in this review is openly accessible in the original report (Wen et al., J. Proteome Res., 2019).
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
Funding
This work was supported by the Independent Research Fund Denmark - Medical Sciences [grant number 8020-00084B] and Lundbeck Foundation [grant numbers R344-2020-952 and R412-2022-449].
CRediT Author Contribution
Christian Staehr: Conceptualization, Formal analysis, Investigation, Methodology, Writing—original draft, Project administration, Writing—review & editing. Christian Aalkjaer: Conceptualization, Supervision, Writing—original draft, Writing—review & editing. Vladimir V. Matchkov: Conceptualization, Supervision, Funding acquisition, Investigation, Methodology, Writing—original draft.
References
- 1.Clausen M.V., Hilbers F. and Poulsen H. (2017) The structure and function of the Na,K-ATPase isoforms in health and disease. Front. Physiol. 8, 1–16 10.3389/fphys.2017.00371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fedosova N.U., Habeck M. and Nissen P. (2021) Structure and function of Na,K-ATPase-the sodium-potassium pump. Compr. Physiol. 12, 2659–2679 10.1002/cphy.c200018 [DOI] [PubMed] [Google Scholar]
- 3.Morth J.P., Pedersen B.P., Toustrup-Jensen M.S., Sørensen T.L.M., Petersen J., Andersen J.P.et al. (2007) Crystal structure of the sodium–potassium pump. Nature 450, 1043–1049 10.1038/nature06419 [DOI] [PubMed] [Google Scholar]
- 4.Blanco G. and Mercer R.W. (1998) Isozymes of the Na-K-ATPase: heterogeneity in structure, diversity in function. Am. J. Physiol. 275, F633–F650 10.1152/ajprenal.1998.275.5.F633 [DOI] [PubMed] [Google Scholar]
- 5.Crambert G., Hasler U., Beggah A.T., Yu C., Modyanov N.N., Horisberger J.D.et al. (2000) Transport and pharmacological properties of nine different human Na, K-ATPase isozymes. J. Biol. Chem. 275, 1976–1986 10.1074/jbc.275.3.1976 [DOI] [PubMed] [Google Scholar]
- 6.Geering K. (2008) Functional roles of Na,K-ATPase subunits. Curr. Opin. Nephrol. Hypertens. 17, 526–532 10.1097/MNH.0b013e3283036cbf [DOI] [PubMed] [Google Scholar]
- 7.Matchkov V.V. and Krivoi I.I. (2016) Specialized functional diversity and interactions of the Na,K-ATPase. Front Physiol. 7, 179 10.3389/fphys.2016.00179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jimenez T., McDermott J.P., Sánchez G. and Blanco G. (2011) Na,K-ATPase alpha4 isoform is essential for sperm fertility. Proc. Natl. Acad. Sci. U.S.A. 108, 644–649 10.1073/pnas.1016902108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bøttger P., Tracz Z., Heuck A., Nissen P., Romero-Ramos M. and Lykke-Hartmann K. (2011) Distribution of Na/K-ATPase alpha 3 isoform, a sodium-potassium P-type pump associated with rapid-onset of dystonia parkinsonism (RDP) in the adult mouse brain. J. Comp. Neurol. 519, 376–404 10.1002/cne.22524 [DOI] [PubMed] [Google Scholar]
- 10.Staehr C., Rajanathan R. and Matchkov V.V. (2019) Involvement of the Na+,K+-ATPase isoforms in control of cerebral perfusion. Exp. Physiol. 104, 1023–1028 10.1113/EP087519 [DOI] [PubMed] [Google Scholar]
- 11.Staehr C., Hangaard L., Bouzinova E.V., Kim S., Rajanathan R., Boegh Jessen P.et al. (2018) Smooth muscle Ca(2+) sensitization causes hypercontractility of middle cerebral arteries in mice bearing the familial hemiplegic migraine type 2 associated mutation. J. Cereb. Blood Flow Metab. 39, 1570–1587 10.1177/0271678X18761712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Therien A.G. and Blostein R. (2000) Mechanisms of sodium pump regulation. Am. J. Physiol. Cell Physiol. 279, C541–C566 10.1152/ajpcell.2000.279.3.C541 [DOI] [PubMed] [Google Scholar]
- 13.Ewart H.S. and Klip A. (1995) Hormonal regulation of the Na(+)-K(+)-ATPase: mechanisms underlying rapid and sustained changes in pump activity. Am. J. Physiol.-Cell Physiol. 269, C295–C311 10.1152/ajpcell.1995.269.2.C295 [DOI] [PubMed] [Google Scholar]
- 14.Giraud F., Claret M., Bruckdorfer K.R. and Chailley B. (1981) The effects of membrane lipid order and cholesterol on the internal and external cationic sites of the Na+-K+ pump in erythrocytes. Biochim. Biophys. Acta 647, 249–258 10.1016/0005-2736(81)90253-4 [DOI] [PubMed] [Google Scholar]
- 15.Kimelberg H.K. and Papahadjopoulos D. (1974) Effects of phospholipid acyl chain fluidity, phase transitions, and cholesterol on (Na+ + K+)-stimulated adenosine triphosphatase. J. Biol. Chem. 249, 1071–1080 10.1016/S0021-9258(19)42943-8 [DOI] [PubMed] [Google Scholar]
- 16.Johannsson A., Smith G.A. and Metcalfe J.C. (1981) The effect of bilayer thickness on the activity of (Na+ + K+)-ATPase. Biochim. Biophys. Acta 641, 416–421 10.1016/0005-2736(81)90498-3 [DOI] [PubMed] [Google Scholar]
- 17.Kimelberg H.K. and Mayhew E. (1975) Increased ouabain-sensitive 86Rb+ uptake and sodium and potassium ion-activated adenosine triphosphatase activity in transformed cell lines. J. Biol. Chem. 250, 100–104 10.1016/S0021-9258(19)41986-8 [DOI] [PubMed] [Google Scholar]
- 18.Minor N.T., Sha Q., Nichols C.G. and Mercer R.W. (1998) The gamma subunit of the Na,K-ATPase induces cation channel activity. Proc. Natl. Acad. Sci. U.S.A. 95, 6521–6525 10.1073/pnas.95.11.6521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wen X.P. and Wan Q.Q. (2021) Regulatory effect of insulin on the structure, function and metabolism of Na(+)/K(+)-ATPase (Review). Exp. Ther. Med. 22, 1243 10.3892/etm.2021.10678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mohan S., Tiwari M.N., Biala Y. and Yaari Y. (2019) Regulation of neuronal Na(+)/K(+)-ATPase by specific protein kinases and protein phosphatases. J. Neurosci. 39, 5440–5451 10.1523/JNEUROSCI.0265-19.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laursen M., Gregersen J.L., Yatime L., Nissen P. and Fedosova N.U. (2015) Structures and characterization of digoxin- and bufalin-bound Na+,K+-ATPase compared with the ouabain-bound complex. Proc. Natl. Acad. Sci. U.S.A. 112, 1755–1760 10.1073/pnas.1422997112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blaustein M.P. and Hamlyn J.M. (1984) Sodium transport inhibition, cell calcium, and hypertension. The natriuretic hormone/Na+-Ca2+ exchange/hypertension hypothesis. Am. J. Med. 77, 45–59 10.1016/S0002-9343(84)80037-6 [DOI] [PubMed] [Google Scholar]
- 23.Blaustein M.P. (1996) Endogenous ouabain: Role in the pathogenesis of hypertension. Kidney Int. 49, 1748–1753 10.1038/ki.1996.260 [DOI] [PubMed] [Google Scholar]
- 24.Schönfeld W., Weiland J., Lindig C., Masnyk M., Kabat M.M., Kurek A.et al. (1985) The lead structure in cardiac glycosides is 5 beta, 14 beta-androstane-3 beta 14-diol. Naunyn Schmiedebergs Arch. Pharmacol. 329, 414–426 10.1007/BF00496377 [DOI] [PubMed] [Google Scholar]
- 25.Kanai R., Cornelius F., Ogawa H., Motoyama K., Vilsen B. and Toyoshima C. (2021) Binding of cardiotonic steroids to Na(+),K(+)-ATPase in the E2P state. Proc. Natl. Acad. Sci. U.S.A. 118, 1–12 10.1073/pnas.2020438118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laursen M., Yatime L., Nissen P. and Fedosova N.U. (2013) Crystal structure of the high-affinity Na+K+-ATPase-ouabain complex with Mg2+ bound in the cation binding site. Proc. Natl. Acad. Sci. U.S.A. 110, 10958–10963 10.1073/pnas.1222308110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lingrel J.B., Arguello J.M., Van Huysse J. and Kuntzweiler T.A. (1997) Cation and cardiac glycoside binding sites of the Na,K-ATPase. Ann. N. Y. Acad. Sci. 834, 194–206 10.1111/j.1749-6632.1997.tb52251.x [DOI] [PubMed] [Google Scholar]
- 28.Fuerstenwerth H. (2014) On the differences between ouabain and digitalis glycosides. Am. J. Ther. 21, 35–42 10.1097/MJT.0b013e318217a609 [DOI] [PubMed] [Google Scholar]
- 29.Juhaszova M. and Blaustein M.P. (1997) Distinct distribution of different Na+ pump alpha subunit isoforms in plasmalemma. Physiological implications. Ann. N.Y. Acad. Sci. 834, 524–536 10.1111/j.1749-6632.1997.tb52310.x [DOI] [PubMed] [Google Scholar]
- 30.Juhaszova M. and Blaustein M.P. (1997) Na+ pump low and high ouabain affinity alpha subunit isoforms are differently distributed in cells. Proc. Natl. Acad. Sci. U.S.A. 94, 1800–1805 10.1073/pnas.94.5.1800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Obrien W.J., Lingrel J.B. and Wallick E.T. (1994) Ouabain binding kinetics of the rat alpha two and alpha three isoforms of the sodium-potassium adenosine triphosphate. Arch. Biochem. Biophys. 310, 32–39 10.1006/abbi.1994.1136 [DOI] [PubMed] [Google Scholar]
- 32.Mueller P. (1965) Ouabain effects on cardiac contraction, action potential, and cellular potassium. Circ. Res. 17, 46–56 10.1161/01.RES.17.1.46 [DOI] [PubMed] [Google Scholar]
- 33.Rajanathan R., Pedersen T.M., Guldbrandsen H.O., Olesen L.F., Thomsen M.B., Botker H.E.et al. (2023) Augmented ouabain-induced vascular response reduces cardiac efficiency in mice with migraine-associated mutation in the Na(+), K(+)-ATPase alpha(2)-Isoform. Biomedicines 11, 1–19 10.3390/biomedicines11020344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Linde C.I., Antos L.K., Golovina V.A. and Blaustein M.P. (2012) Nanomolar ouabain increases NCX1 expression and enhances Ca2+ signaling in human arterial myocytes: a mechanism that links salt to increased vascular resistance? Am. J. Physiol.-Heart Circulatory Physiol. 303, H784–H794 10.1152/ajpheart.00399.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Glitsch H.G. (2001) Electrophysiology of the sodium-potassium-ATPase in cardiac cells. Physiol. Rev. 81, 1791–1826 10.1152/physrev.2001.81.4.1791 [DOI] [PubMed] [Google Scholar]
- 36.Hangaard L., Bouzinova E.V., Staehr C., Dam V.S., Kim S., Xie Z.et al. (2017) Na-K-ATPase regulates intercellular communication in the vascular wall via cSrc kinase-dependent connexin43 phosphorylation. Am. J. Physiol. Cell Physiol. 312, C385–C397 10.1152/ajpcell.00347.2016 [DOI] [PubMed] [Google Scholar]
- 37.Aalkjaer C. and Mulvany M.J. (1985) Effect of ouabain on tone, membrane potential and sodium efflux compared with [3H]ouabain binding in rat resistance vessels. J. Physiol. 362, 215–231 10.1113/jphysiol.1985.sp015672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mulvany M.J., Aalkjaer C. and Petersen T.T. (1984) Intracellular sodium, membrane potential, and contractility of rat mesenteric small arteries. Circ. Res. 54, 740–749 10.1161/01.RES.54.6.740 [DOI] [PubMed] [Google Scholar]
- 39.Bouzinova E.V., Hangaard L., Staehr C., Mazur A., Ferreira A., Chibalin A.et al. (2018) The α2 isoform Na,K-ATPase modulates contraction of rat mesenteric small artery via cSrc–dependent Ca2+ sensitization. Acta Physiol. (Oxf.) 224,11–15, e13059 10.1111/apha.13059 [DOI] [PubMed] [Google Scholar]
- 40.Matchkov V.V., Moeller-Nielsen N., Dam V.S., Nourian Z., Bodtkjer D.M. and Aalkjaer C. (2012) The alpha2 isoform of the Na,K-pump is important for intercellular communication, agonist-induced contraction and EDHF-like response in rat mesenteric arteries. Am. J. Physiol. 303, H36–H46 [DOI] [PubMed] [Google Scholar]
- 41.Matchkov V.V., Rahman A., Peng H., Nilsson H. and Aalkjaer C. (2004) Junctional and nonjunctional effects of heptanol and glycyrrhetinic acid derivates in rat mesenteric small arteries. Br. J. Pharmacol. 142, 961–972 10.1038/sj.bjp.0705870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Matchkov V.V., Rahman A., Bakker L.M., Griffith T.M., Nilsson H. and Aalkjaer C. (2006) Analysis of effects of connexin-mimetic peptides in rat mesenteric small arteries. Am. J. Physiol. Heart Circ. Physiol. 291, H357–H367 10.1152/ajpheart.00681.2005 [DOI] [PubMed] [Google Scholar]
- 43.Blaustein M.P. and Lederer W.J. (1999) Sodium/calcium exchange: its physiological implications. Physiol. Rev. 79, 763–854 10.1152/physrev.1999.79.3.763 [DOI] [PubMed] [Google Scholar]
- 44.Blaustein M.P., Zhang J., Chen L., Song H., Raina H., Kinsey S.P.et al. (2009) The pump, the exchanger, and endogenous ouabain: signaling mechanisms that link salt retention to hypertension. Hypertension 53, 291–298 10.1161/HYPERTENSIONAHA.108.119974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Blaustein M.P. and Hamlyn J.M. (2020) Ouabain, endogenous ouabain and ouabain-like factors: The Na(+) pump/ouabain receptor, its linkage to NCX, and its myriad functions. Cell Calcium 86, 102159 10.1016/j.ceca.2020.102159 [DOI] [PubMed] [Google Scholar]
- 46.Blaustein M.P., Chen L., Hamlyn J.M., Leenen F.H., Lingrel J.B., Wier W.G.et al. (2016) Pivotal role of α2 Na(+) pumps and their high affinity ouabain binding site in cardiovascular health and disease. J. Physiol. 594, 6079–6103 10.1113/JP272419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hirota S., Pertens E. and Janssen L.J. (2007) The reverse mode of the Na(+)/Ca(2+) exchanger provides a source of Ca(2+) for store refilling following agonist-induced Ca(2+) mobilization. Am. J. Physiol. Lung Cell. Mol. Physiol. 292, L438–L447 10.1152/ajplung.00222.2006 [DOI] [PubMed] [Google Scholar]
- 48.Shattock M.J., Ottolia M., Bers D.M., Blaustein M.P., Boguslavskyi A., Bossuyt J.et al. (2015) Na+/Ca2+ exchange and Na+/K+-ATPase in the heart. J. Physiol. 593, 1361–1382 10.1113/jphysiol.2014.282319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang L., Aalkjaer C. and Matchkov V.V. (2018) The Na,K-ATPase-dependent Src kinase signaling changes with mesenteric artery diameter. Int. J. Mol. Sci. 19, 1–18 10.3390/ijms19092489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Matsumoto T., Kobayashi T., Ishida K., Taguchi K. and Kamata K. (2010) Enhancement of mesenteric artery contraction to 5-HT depends on Rho kinase and Src kinase pathways in the ob/ob mouse model of type 2 diabetes. Br. J. Pharmacol. 160, 1092–1104 10.1111/j.1476-5381.2010.00753.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li Z., Cai T., Tian J., Xie J.X., Zhao X., Liu L.et al. (2009) NaKtide, a Na/K-ATPase-derived peptide Src inhibitor, antagonizes ouabain-activated signal transduction in cultured cells. J. Biol. Chem. 284, 21066–21076 10.1074/jbc.M109.013821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wijetunge S., Lymn J.S. and Hughes A.D. (2000) Effects of protein tyrosine kinase inhibitors on voltage-operated calcium channel currents in vascular smooth muscle cells and pp60(c-src) kinase activity. Br. J. Pharmacol. 129, 1347–1354 10.1038/sj.bjp.0703186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gui P., Wu X., Ling S., Stotz S.C., Winkfein R.J., Wilson E.et al. (2006) Integrin receptor activation triggers converging regulation of Cav1.2 calcium channels by c-Src and protein kinase A pathways. J. Biol. Chem. 281, 14015–14025 10.1074/jbc.M600433200 [DOI] [PubMed] [Google Scholar]
- 54.Song H., Karashima E., Hamlyn J.M. and Blaustein M.P. (2014) Ouabain-digoxin antagonism in rat arteries and neurones. J. Physiol. 592, 941–969 10.1113/jphysiol.2013.266866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang L., Staehr C., Zeng F., Bouzinova E.V. and Matchkov V.V. (2019) The Na,K-ATPase in vascular smooth muscle cells. Curr. Top. Membr. 83, 151–175 10.1016/bs.ctm.2019.01.007 [DOI] [PubMed] [Google Scholar]
- 56.Wu J., Akkuratov E.E., Bai Y., Gaskill C.M., Askari A. and Liu L. (2013) Cell signaling associated with Na+/K+-ATPase: activation of phosphatidylinositide 3-kinase IA/Akt by ouabain is independent of Src. Biochemistry 52, 9059–9067 10.1021/bi4011804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Aperia A. (2007) New roles for an old enzyme: Na,K-ATPase emerges as an interesting drug target. J. Intern. Med. 261, 44–52 10.1111/j.1365-2796.2006.01745.x [DOI] [PubMed] [Google Scholar]
- 58.Pratt R.D., Brickman C.R., Cottrill C.L., Shapiro J.I. and Liu J. (2018) The Na/K-ATPase signaling: from specific ligands to general reactive oxygen species. Int. J. Mol. Sci. 19, 2600 10.3390/ijms19092600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cui X. and Xie Z. (2017) Protein interaction and Na/K-ATPase-mediated signal transduction. Molecules 22, 1–20 10.3390/molecules22060990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu J., Tian J., Haas M., Shapiro J.I., Askari A. and Xie Z. (2000) Ouabain interaction with cardiac Na+/K+-ATPase initiates signal cascades independent of changes in intracellular Na+ and Ca2+ concentrations. J. Biol. Chem. 275, 27838–27844 10.1074/jbc.M002950200 [DOI] [PubMed] [Google Scholar]
- 61.Zulian A., Linde C.I., Pulina M.V., Baryshnikov S.G., Papparella I., Hamlyn J.M.et al. (2013) Activation of c-SRC underlies the differential effects of ouabain and digoxin on Ca(2+) signaling in arterial smooth muscle cells. Am. J. Physiol. Cell Physiol. 304, C324–C333 10.1152/ajpcell.00337.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ye Q., Lai F., Banerjee M., Duan Q., Li Z., Si S.et al. (2013) Expression of mutant α1 Na/K-ATPase defective in conformational transition attenuates Src-mediated signal transduction. J. Biol. Chem. 288, 5803–5814 10.1074/jbc.M112.442608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Haas M., Askari A. and Xie Z. (2000) Involvement of Src and epidermal growth factor receptor in the signal-transducing function of Na+/K+-ATPase*. J. Biol. Chem. 275, 27832–27837 10.1074/jbc.M002951200 [DOI] [PubMed] [Google Scholar]
- 64.Rajanathan R., Riera C.V.I., Pedersen T.M., Staehr C., Bouzinova E.V., Nyengaard J.R.et al. (2023) Hypercontractile cardiac phenotype in mice with migraine-associated mutation in the Na(+),K(+)-ATPase α(2)-Isoform. Cells 12, 1–21 10.3390/cells12081108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu J., Lilly M.N. and Shapiro J.I. (2018) Targeting Na/K-ATPase signaling: a new approach to control oxidative stress. Curr. Pharm. Des. 24, 359–364 10.2174/1381612824666180110101052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wu J., Akkuratov E.E., Bai Y., Gaskill C.M., Askari A. and Liu L. (2013) Cell signaling associated with Na+/K+-ATPase: activation of phosphatidylinositide 3-kinase IA/Akt by ouabain is independent of Src. Biochemistry 52, 9059–9067 10.1021/bi4011804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xie Z. and Askari A. (2002) Na(+)/K(+)-ATPase as a signal transducer. Eur. J. Biochem. 269, 2434–2439 10.1046/j.1432-1033.2002.02910.x [DOI] [PubMed] [Google Scholar]
- 68.Liu L., Zhao X., Pierre S.V. and Askari A. (2007) Association of PI3K-Akt signaling pathway with digitalis-induced hypertrophy of cardiac myocytes. Am. J. Physiol. Cell Physiol. 293, C1489–C1497 10.1152/ajpcell.00158.2007 [DOI] [PubMed] [Google Scholar]
- 69.Bai Y., Morgan E.E., Giovannucci D.R., Pierre S.V., Philipson K.D., Askari A.et al. (2013) Different roles of the cardiac Na+/Ca2+-exchanger in ouabain-induced inotropy, cell signaling, and hypertrophy. Am. J. Physiol. Heart Circ. Physiol. 304, H427–H435 10.1152/ajpheart.00462.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tian J. and Xie Z.-j. (2008) The Na-K-ATPase and calcium-signaling microdomains. Physiology 23, 205–211 10.1152/physiol.00008.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Banerjee M., Duan Q. and Xie Z. (2015) SH2 ligand-like effects of second cytosolic domain of Na/K-ATPase α1 subunit on Src kinase. PLoS ONE 10, e0142119 10.1371/journal.pone.0142119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liang M., Cai T., Tian J., Qu W. and Xie Z.J. (2006) Functional characterization of Src-interacting Na/K-ATPase using RNA interference assay. J. Biol. Chem. 281, 19709–19719 10.1074/jbc.M512240200 [DOI] [PubMed] [Google Scholar]
- 73.Xie Z. (2003) Molecular mechanisms of Na/K-ATPase-mediated signal transduction. Ann. N. Y. Acad. Sci. 986, 497–503 10.1111/j.1749-6632.2003.tb07234.x [DOI] [PubMed] [Google Scholar]
- 74.Staehr C., Rohde P.D., Krarup N.T., Ringgaard S., Laustsen C., Johnsen J.et al. (2022) Migraine-associated mutation in the Na,K-ATPase leads to disturbances in cardiac metabolism and reduced cardiac function. J. Am. Heart Assoc. 11, e021814 10.1161/JAHA.121.021814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li Z. and Xie Z. (2009) The Na/K-ATPase/Src complex and cardiotonic steroid-activated protein kinase cascades. Pflugers Arch. 457, 635–644 10.1007/s00424-008-0470-0 [DOI] [PubMed] [Google Scholar]
- 76.Aperia A., Akkuratov E.E., Fontana J.M. and Brismar H. (2016) Na+-K+-ATPase, a new class of plasma membrane receptors. Am. J. Physiol. Cell Physiol. 310, C491–C495 10.1152/ajpcell.00359.2015 [DOI] [PubMed] [Google Scholar]
- 77.Gable M.E., Abdallah S.L., Najjar S.M., Liu L. and Askari A. (2014) Digitalis-induced cell signaling by the sodium pump: on the relation of Src to Na+/K+-ATPase. Biochem. Biophys. Res. Commun. 446, 1151–1154 10.1016/j.bbrc.2014.03.071 [DOI] [PubMed] [Google Scholar]
- 78.Yosef E., Katz A., Peleg Y., Mehlman T. and Karlish S.J. (2016) Do Src kinase and caveolin interact directly with Na,K-ATPase? J. Biol. Chem. 291, 11736–11750 10.1074/jbc.M116.721084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Weigand K.M., Swarts H.G., Fedosova N.U., Russel F.G. and Koenderink J.B. (2012) Na,K-ATPase activity modulates Src activation: a role for ATP/ADP ratio. Biochim. Biophys. Acta 1818, 1269–1273 10.1016/j.bbamem.2012.01.015 [DOI] [PubMed] [Google Scholar]
- 80.Rognant S., Kravtsova V.V., Bouzinova E.V., Melnikova E.V., Krivoi I.I., Pierre S.V.et al. (2022) The microtubule network enables Src kinase interaction with the Na,K-ATPase to generate Ca(2+) flashes in smooth muscle cells. Front Physiol. 13, 1007340 10.3389/fphys.2022.1007340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chen Y., Cai T., Wang H., Li Z., Loreaux E., Lingrel J.B.et al. (2009) Regulation of intracellular cholesterol distribution by Na/K-ATPase. J. Biol. Chem. 284, 14881–14890 10.1074/jbc.M109.003574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Xie J., Ye Q., Cui X., Madan N., Yi Q., Pierre S.V.et al. (2015) Expression of Rat Na/K-ATPase alpha2 enables ion pumping but not ouabain-induced signaling in alpha1-deficient porcine renal epithelial cells. Am. J. Physiol. 309, C373–C382 10.1152/ajpcell.00103.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lai F., Madan N., Ye Q., Duan Q., Li Z., Wang S.et al. (2013) Identification of a mutant α1 Na/K-ATPase that pumps but is defective in signal transduction. J. Biol. Chem. 288, 13295–13304 10.1074/jbc.M113.467381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kotova O., Al-Khalili L., Talia S., Hooke C., Fedorova O.V., Bagrov A.Y.et al. (2006) Cardiotonic steroids stimulate glycogen synthesis in human skeletal muscle cells via a Src- and ERK1/2-dependent mechanism. J. Biol. Chem. 281, 20085–20094 10.1074/jbc.M601577200 [DOI] [PubMed] [Google Scholar]
- 85.Jorgensen P.L., Hakansson K.O. and Karlish S.J. (2003) Structure and mechanism of Na,K-ATPase: functional sites and their interactions. Annu. Rev. Physiol. 65, 817–849 10.1146/annurev.physiol.65.092101.142558 [DOI] [PubMed] [Google Scholar]
- 86.Zubkov A., Miao L. and Zhang J. (2004) Signal transduction of ET-1 in contraction of cerebral arteries. J. Cardiovasc. Pharmacol. 44, S24–S26 10.1097/01.fjc.0000166221.73217.f7 [DOI] [PubMed] [Google Scholar]
- 87.Gonzales A.L., Yang Y., Sullivan M.N., Sanders L., Dabertrand F., Hill-Eubanks D.C.et al. (2014) A PLCγ1-dependent, force-sensitive signaling network in the myogenic constriction of cerebral arteries. Sci. Signal. 7, ra49 10.1126/scisignal.2004732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lu R., Alioua A., Kumar Y., Kundu P., Eghbali M., Weisstaub N.V.et al. (2008) c-Src tyrosine kinase, a critical component for 5-HT2A receptor-mediated contraction in rat aorta. J. Physiol. 586, 3855–3869 10.1113/jphysiol.2008.153593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Feng J., Ito M., Ichikawa K., Isaka N., Nishikawa M., Hartshorne D.J.et al. (1999) Inhibitory phosphorylation site for Rho-associated kinase on smooth muscle myosin phosphatase. J. Biol. Chem. 274, 37385–37390 10.1074/jbc.274.52.37385 [DOI] [PubMed] [Google Scholar]
- 90.Qiao Y.N., He W.Q., Chen C.P., Zhang C.H., Zhao W., Wang P.et al. (2014) Myosin phosphatase target subunit 1 (MYPT1) regulates the contraction and relaxation of vascular smooth muscle and maintains blood pressure. J. Biol. Chem. 289, 22512–22523 10.1074/jbc.M113.525444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Somlyo A.P. and Somlyo A.V. (2003) Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol. Rev. 83, 1325–1358 10.1152/physrev.00023.2003 [DOI] [PubMed] [Google Scholar]
- 92.Johnson R.P., El-Yazbi A.F., Takeya K., Walsh E.J., Walsh M.P. and Cole W.C. (2009) Ca2+ sensitization via phosphorylation of myosin phosphatase targeting subunit at threonine-855 by Rho kinase contributes to the arterial myogenic response. J. Physiol. 587, 2537–2553 10.1113/jphysiol.2008.168252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kutz L.C., Cui X., Xie J.X., Mukherji S.T., Terrell K.C., Huang M.et al. (2021) The Na/K-ATPase α1/Src interaction regulates metabolic reserve and Western diet intolerance. Acta Physiol. (Oxf.) 232, e13652 10.1111/apha.13652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Brozovich F.V., Nicholson C.J., Degen C.V., Gao Y.Z., Aggarwal M. and Morgan K.G. (2016) Mechanisms of Vascular smooth muscle contraction and the basis for pharmacologic treatment of smooth muscle disorders. Pharmacol. Rev. 68, 476–532 10.1124/pr.115.010652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wijetunge S., Lymn J.S. and Hughes A.D. (2000) Effects of protein tyrosine kinase inhibitors on voltage-operated calcium channel currents in vascular smooth muscle cells and pp60c-src kinase activity. Br. J. Pharmacol. 129, 1347–1354 10.1038/sj.bjp.0703186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ansari J., Triay R., Kandregula S., Adeeb N., Cuellar H. and Sharma P. (2022) Endovascular intervention in acute ischemic stroke: history and evolution. Biomedicines 10, 1–11 10.3390/biomedicines10020418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hussein H.M., Georgiadis A.L., Vazquez G., Miley J.T., Memon M.Z., Mohammad Y.M.et al. (2010) Occurrence and predictors of futile recanalization following endovascular treatment among patients with acute ischemic stroke: a multicenter study. AJNR Am. J. Neuroradiol. 31, 454–458 10.3174/ajnr.A2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Goyal M., Menon B.K., van Zwam W.H., Dippel D.W., Mitchell P.J., Demchuk A.M.et al. (2016) Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet 387, 1723–1731 10.1016/S0140-6736(16)00163-X [DOI] [PubMed] [Google Scholar]
- 99.Deng G., Chu Y.H., Xiao J., Shang K., Zhou L.Q., Qin C.et al. (2023) Risk Factors, Pathophysiologic Mechanisms, and Potential Treatment Strategies of Futile Recanalization after Endovascular Therapy in Acute Ischemic Stroke. Aging Dis.[Online ahead of print] 1–17 10.14336/AD.2023.0321-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cipolla M.J., Chan S.L., Sweet J., Tavares M.J., Gokina N. and Brayden J.E. (2014) Postischemic reperfusion causes smooth muscle calcium sensitization and vasoconstriction of parenchymal arterioles. Stroke 45, 2425–2430 10.1161/STROKEAHA.114.005888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ng F.C., Churilov L., Yassi N., Kleinig T.J., Thijs V., Wu T.et al. (2022) Prevalence and significance of impaired microvascular tissue reperfusion despite macrovascular angiographic reperfusion (no-reflow). Neurology 98, e790–e801 10.1212/WNL.0000000000013210 [DOI] [PubMed] [Google Scholar]
- 102.Staehr C., Giblin J.T., Gutiérrez-Jiménez E., Guldbrandsen H., Tang J., Sandow S.L.et al. (2023) Neurovascular uncoupling is linked to microcirculatory dysfunction in regions outside the ischemic core following ischemic stroke. J. Am. Heart Assoc. 12(11) e029527 10.1161/JAHA.123.029527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yemisci M., Gursoy-Ozdemir Y., Vural A., Can A., Topalkara K. and Dalkara T. (2009) Pericyte contraction induced by oxidative-nitrative stress impairs capillary reflow despite successful opening of an occluded cerebral artery. Nat. Med. 15, 1031–1037 10.1038/nm.2022 [DOI] [PubMed] [Google Scholar]
- 104.Hall C.N., Reynell C., Gesslein B., Hamilton N.B., Mishra A., Sutherland B.A.et al. (2014) Capillary pericytes regulate cerebral blood flow in health and disease. Nature 508, 55–60 10.1038/nature13165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dalkara T. (2019) Pericytes. Stroke 50, 2985–2991 10.1161/STROKEAHA.118.023590 [DOI] [PubMed] [Google Scholar]
- 106.Korte N., Ilkan Z., Pearson C.L., Pfeiffer T., Singhal P., Rock J.R.et al. (2022) The Ca2+-gated channel TMEM16A amplifies capillary pericyte contraction and reduces cerebral blood flow after ischemia. J. Clin. Invest. 132, 1–17 10.1172/JCI154118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Khennouf L., Gesslein B., Brazhe A., Octeau J.C., Kutuzov N., Khakh B.S.et al. (2018) Active role of capillary pericytes during stimulation-induced activity and spreading depolarization. Brain 141, 2032–2046 10.1093/brain/awy143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Staehr C., Giblin J.T., Gutiérrez-Jiménez E., Guldbrandsen H., Tang J., Sandow S.L.et al. (2022) Microcirculatory dysfunction associates with neurovascular uncoupling in peri-ischemic brain regions after ischemic stroke. bioRxiv 1–212022.08.26.505245 [Google Scholar]
- 109.Guldbrandsen H.O., Staehr C., Iversen N.K., Postnov D.D. and Matchkov V.V. (2021) Does Src kinase mediated vasoconstriction impair penumbral reperfusion? Stroke 52, e250–e258 10.1161/STROKEAHA.120.032737 [DOI] [PubMed] [Google Scholar]
- 110.Shih A.Y., Friedman B., Drew P.J., Tsai P.S., Lyden P.D. and Kleinfeld D. (2009) Active dilation of penetrating arterioles restores red blood cell flux to penumbral neocortex after focal stroke. J. Cereb. Blood Flow Metab. 29, 738–751 10.1038/jcbfm.2008.166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cipolla M.J., Sweet J.G., Gokina N.I., White S.L. and Nelson M.T. (2013) Mechanisms of enhanced basal tone of brain parenchymal arterioles during early postischemic reperfusion: role of ET-1-induced peroxynitrite generation. J. Cereb. Blood Flow Metab. 33, 1486–1492 10.1038/jcbfm.2013.99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Palomares S.M. and Cipolla M.J. (2011) Vascular protection following cerebral ischemia and reperfusion. J. Neurol. Neurophysiol. 2011, 1–25 10.4172/2155-9562.S1-004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bala F., Ospel J., Mulpur B., Kim B.J., Yoo J., Menon B.K.et al. (2021) Infarct growth despite successful endovascular reperfusion in acute ischemic stroke: a meta-analysis. AJNR Am. J. Neuroradiol. 42, 1472–1478 10.3174/ajnr.A7177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Beez T., Steiger H.-J. and Etminan N. (2017) Pharmacological targeting of secondary brain damage following ischemic or hemorrhagic stroke, traumatic brain injury, and bacterial meningitis - a systematic review and meta-analysis. BMC Neurology 17, 209 10.1186/s12883-017-0994-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cipolla M.J., Sweet J.G. and Gardner-Morse I. (2011) Effect of circulating factors on cerebral artery function during hyperglycemic stroke. FASEB J. 25, 1024.6–1024.6 10.1096/fasebj.25.1_supplement.1024.6 [DOI] [Google Scholar]
- 116.Paul R., Zhang Z.G., Eliceiri B.P., Jiang Q., Boccia A.D., Zhang R.L.et al. (2001) Src deficiency or blockade of Src activity in mice provides cerebral protection following stroke. Nat. Med. 7, 222–227 10.1038/84675 [DOI] [PubMed] [Google Scholar]
- 117.Takenaga Y., Takagi N., Murotomi K., Tanonaka K. and Takeo S. (2009) Inhibition of Src activity decreases tyrosine phosphorylation of occludin in brain capillaries and attenuates increase in permeability of the blood-brain barrier after transient focal cerebral ischemia. J. Cereb. Blood Flow Metab. 29, 1099–1108 10.1038/jcbfm.2009.30 [DOI] [PubMed] [Google Scholar]
- 118.Qiu H., Qian T., Wu T., Gao T., Xing Q. and Wang L. (2021) Src family kinases inhibition ameliorates hypoxic-ischemic brain injury in immature rats. Front. Cell. Neurosci. 15, 1–11 10.3389/fncel.2021.746130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lennmyr F., Ericsson A., Gerwins P., Akterin S., Ahlström H. and Terént A. (2004) Src family kinase-inhibitor PP2 reduces focal ischemic brain injury. Acta Neurol. Scand. 110, 175–179 10.1111/j.1600-0404.2004.00306.x [DOI] [PubMed] [Google Scholar]
- 120.Rodrigo R., Fernández-Gajardo R., Gutiérrez R., Matamala J.M., Carrasco R., Miranda-Merchak A.et al. (2013) Oxidative stress and pathophysiology of ischemic stroke: novel therapeutic opportunities. CNS Neurol. Disord. Drug Targets 12, 698–714 10.2174/1871527311312050015 [DOI] [PubMed] [Google Scholar]
- 121.Wen M., Jin Y., Zhang H., Sun X., Kuai Y. and Tan W. (2019) Proteomic analysis of rat cerebral cortex in the subacute to long-term phases of focal cerebral ischemia-reperfusion injury. J. Proteome Res. 18, 3099–3118 10.1021/acs.jproteome.9b00220 [DOI] [PubMed] [Google Scholar]
- 122.Lin A., Peiris N.J., Dhaliwal H., Hakim M., Li W., Ganesh S.et al. (2021) Mural cells: potential therapeutic targets to bridge cardiovascular disease and neurodegeneration. Cells 10, 1–24 10.3390/cells10030593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Comellas A.P., Dada L.A., Lecuona E., Pesce L.M., Chandel N.S., Quesada N.et al. (2006) Hypoxia-mediated degradation of Na,K-ATPase via mitochondrial reactive oxygen species and the ubiquitin-conjugating system. Circ. Res. 98, 1314–1322 10.1161/01.RES.0000222418.99976.1d [DOI] [PubMed] [Google Scholar]
- 124.Magnani N.D., Dada L.A., Queisser M.A., Brazee P.L., Welch L.C., Anekalla K.R.et al. (2017) HIF and HOIL-1L-mediated PKCζ degradation stabilizes plasma membrane Na,K-ATPase to protect against hypoxia-induced lung injury. Proc. Natl. Acad. Sci. U.S.A. 114, E10178–E10186 10.1073/pnas.1713563114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Shen H., Liang P., Qiu S., Zhang B., Wang Y. and Lv P. (2016) The role of Na+, K+-ATPase in the hypoxic vasoconstriction in isolated rat basilar artery. Vasc. Pharmacol. 81, 53–60 10.1016/j.vph.2016.02.004 [DOI] [PubMed] [Google Scholar]
- 126.Clausen T., Van Hardeveld C. and Everts M.E. (1991) Significance of cation transport in control of energy metabolism and thermogenesis. Physiol. Rev. 71, 733–774 10.1152/physrev.1991.71.3.733 [DOI] [PubMed] [Google Scholar]
- 127.Dienel G.A. (2019) Brain Glucose Metabolism: Integration of Energetics with Function. Physiol. Rev. 99, 949–1045 10.1152/physrev.00062.2017 [DOI] [PubMed] [Google Scholar]
- 128.Rolfe D.F. and Brown G.C. (1997) Cellular energy utilization and molecular origin of standard metabolic rate in mammals. Physiol. Rev. 77, 731–758 10.1152/physrev.1997.77.3.731 [DOI] [PubMed] [Google Scholar]
- 129.Staehr C. and Matchkov V.V. (2021) Demand creates its own supply: The Na/K-ATPase controls metabolic reserve and flexibility. Acta Physiologica 232, e13673 10.1111/apha.13673 [DOI] [PubMed] [Google Scholar]
- 130.De Angelis C. and Haupert G.T. Jr (1998) Hypoxia triggers release of an endogenous inhibitor of Na(+)-K(+)-ATPase from midbrain and adrenal. Am. J. Physiol. 274, F182–F188 10.1152/ajprenal.1998.274.1.F182 [DOI] [PubMed] [Google Scholar]
- 131.Zhu M., Sun H., Cao L., Wu Z., Leng B. and Bian J. (2022) Role of Na(+)/K(+)-ATPase in ischemic stroke: in-depth perspectives from physiology to pharmacology. J. Mol. Med. (Berl.) 100, 395–410 10.1007/s00109-021-02143-6 [DOI] [PubMed] [Google Scholar]
- 132.Yan Y., Shapiro A.P., Haller S., Katragadda V., Liu L., Tian J.et al. (2013) Involvement of reactive oxygen species in a feed-forward mechanism of Na/K-ATPase-mediated signaling transduction. J. Biol. Chem. 288, 34249–34258 10.1074/jbc.M113.461020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Liu C.C., Fry N.A., Hamilton E.J., Chia K.K., Garcia A., Karimi Galougahi K.et al. (2013) Redox-dependent regulation of the Na(+)-K(+) pump: new twists to an old target for treatment of heart failure. J. Mol. Cell Cardiol. 61, 94–101 10.1016/j.yjmcc.2013.05.013 [DOI] [PubMed] [Google Scholar]
- 134.Bartlett D.E., Miller R.B., Thiesfeldt S., Lakhani H.V., Shapiro J.I. and Sodhi K. (2018) The role of Na/K-ATPase signaling in oxidative stress related to aging: implications in obesity and cardiovascular disease. Int. J. Mol. Sci. 191–13 10.3390/ijms19072139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Liu J., Tian J., Chaudhry M., Maxwell K., Yan Y., Wang X.et al. (2016) Attenuation of Na/K-ATPase mediated oxidant amplification with pNaKtide ameliorates experimental uremic cardiomyopathy. Sci. Rep. 6, 34592 10.1038/srep34592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Shi M., Cao L., Cao X., Zhu M., Zhang X., Wu Z.et al. (2018) DR-region of Na+/K+ ATPase is a target to treat excitotoxicity and stroke. Cell Death Dis. 10, 6 10.1038/s41419-018-1230-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ayata C. and Lauritzen M. (2015) Spreading depression, spreading depolarizations, and the cerebral vasculature. Physiol. Rev. 95, 953–993 10.1152/physrev.00027.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Leao A.A.P. (1944) Spreading depression of activity in the cerebral corteX. J. Neurophysiol. 7, 359–390 10.1152/jn.1944.7.6.359 [DOI] [PubMed] [Google Scholar]
- 139.Hadjikhani N., Sanchez del Rio M., Wu O., Schwartz D., Bakker D., Fischl B.et al. (2001) Mechanisms of migraine aura revealed by functional MRI in human visual cortex. Proc. Natl. Acad. Sci. 98, 4687–4692 10.1073/pnas.071582498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Vyskocil F., Kritz N. and Bures J. (1972) Potassium-selective microelectrodes used for measuring the extracellular brain potassium during spreading depression and anoxic depolarization in rats. Brain Res. 39, 255–259 10.1016/0006-8993(72)90802-5 [DOI] [PubMed] [Google Scholar]
- 141.Hansen A.J. and Zeuthen T. (1981) Extracellular ion concentrations during spreading depression and ischemia in the rat brain cortex. Acta Physiol. Scand. 113, 437–445 10.1111/j.1748-1716.1981.tb06920.x [DOI] [PubMed] [Google Scholar]
- 142.Somjen G.G. (2001) Mechanisms of spreading depression and hypoxic spreading depression-like depolarization. Physiol. Rev. 81, 1065–1096 10.1152/physrev.2001.81.3.1065 [DOI] [PubMed] [Google Scholar]
- 143.Balestrino M., Young J. and Aitken P. (1999) Block of (Na+,K+)ATPase with ouabain induces spreading depression-like depolarization in hippocampal slices. Brain Res. 838, 37–44 10.1016/S0006-8993(99)01674-1 [DOI] [PubMed] [Google Scholar]
- 144.Menna G., Tong C.K. and Chesler M. (2000) Extracellular pH changes and accompanying cation shifts during ouabain-induced spreading depression. J. Neurophysiol. 83, 1338–1345 10.1152/jn.2000.83.3.1338 [DOI] [PubMed] [Google Scholar]
- 145.Hanalioglu S., Taskiran-Sag A., Karatas H., Donmez-Demir B., Yilmaz-Ozcan S., Eren-Kocak E.et al. (2022) Cortical spreading depression can be triggered by sensory stimulation in primed wild type mouse brain: a mechanistic insight to migraine aura generation. J. Headache Pain 23, 107 10.1186/s10194-022-01474-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Cozzolino O., Marchese M., Trovato F., Pracucci E., Ratto G.M., Buzzi M.G.et al. (2018) Understanding spreading depression from headache to sudden unexpected death. Front. Neurol. 9, 19 10.3389/fneur.2018.00019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Erdener Ş.E., Kaya Z. and Dalkara T. (2021) Parenchymal neuroinflammatory signaling and dural neurogenic inflammation in migraine. J. Headache Pain 22, 138 10.1186/s10194-021-01353-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Vilsen B. (1999) Mutant Phe788 –> Leu of the Na+,K+-ATPase is inhibited by micromolar concentrations of potassium and exhibits high Na+-ATPase activity at low sodium concentrations. Biochemistry 38, 11389–11400 10.1021/bi990951t [DOI] [PubMed] [Google Scholar]
- 149.Reiffurth C., Alam M., Zahedi-Khorasani M., Major S. and Dreier J.P. (2020) Na+/K+-ATPase α isoform deficiency results in distinct spreading depolarization phenotypes. J. Cerebral Blood Flow Metab. 40, 622–638 10.1177/0271678X19833757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Benarroch E.E. (2010) Glutamate transporters. Diversity Funct. Involv. Neurol. Dis. 74, 259–264 [DOI] [PubMed] [Google Scholar]
- 151.Rose E.M., Koo J.C.P., Antflick J.E., Ahmed S.M., Angers S. and Hampson D.R. (2009) Glutamate transporter coupling to Na,K-ATPase. J. Neurosci. 29, 8143–8155 10.1523/JNEUROSCI.1081-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Larsen B.R., Assentoft M., Cotrina M.L., Hua S.Z., Nedergaard M., Kaila K.et al. (2014) Contributions of the Na+/K+-ATPase, NKCC1, and Kir4.1 to hippocampal K+ clearance and volume responses. Glia 62, 608–622 10.1002/glia.22629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Isaksen T.J. and Lykke-Hartmann K. (2016) Insights into the pathology of the α2-Na(+)/K(+)-ATPase in neurological disorders; lessons from animal models. Front Physiol. 7, 161 10.3389/fphys.2016.00161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Friedrich T., Tavraz N.N. and Junghans C. (2016) ATP1A2 mutations in migraine: seeing through the facets of an ion pump onto the neurobiology of disease. Front Physiol. 7, 239 10.3389/fphys.2016.00239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.De Fusco M., Marconi R., Silvestri L., Atorino L., Rampoldi L., Morgante L.et al. (2003) Haploinsufficiency of ATP1A2 encoding the Na+/K+ pump alpha2 subunit associated with familial hemiplegic migraine type 2. Nat. Genet. 33, 192–196 10.1038/ng1081 [DOI] [PubMed] [Google Scholar]
- 156.Spadaro M., Ursu S., Lehmann-Horn F., Veneziano L., Antonini G., Giunti P.et al. (2004) A G301R Na+/K+ -ATPase mutation causes familial hemiplegic migraine type 2 with cerebellar signs. Neurogenetics 5, 177–185 10.1007/s10048-004-0183-2 [DOI] [PubMed] [Google Scholar]
- 157.Bottger P., Glerup S., Gesslein B., Illarionova N.B., Isaksen T.J., Heuck A.et al. (2016) Glutamate-system defects behind psychiatric manifestations in a familial hemiplegic migraine type 2 disease-mutation mouse model. Sci. Rep. 6, 22047 10.1038/srep22047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Tavraz N.N., Dürr K.L., Koenderink J.B., Freilinger T., Bamberg E., Dichgans M.et al. (2009) Impaired plasma membrane targeting or protein stability by certain ATP1A2 mutations identified in sporadic or familial hemiplegic migraine. Channels (Austin) 3, 82–87 10.4161/chan.3.2.8085 [DOI] [PubMed] [Google Scholar]
- 159.Bottger P., Doganli C. and Lykke-Hartmann K. (2012) Migraine- and dystonia-related disease-mutations of Na+/K+-ATPases: relevance of behavioral studies in mice to disease symptoms and neurological manifestations in humans. Neurosci. Biobehav. Rev. 36, 855–871 10.1016/j.neubiorev.2011.10.005 [DOI] [PubMed] [Google Scholar]
- 160.Leo L., Gherardini L., Barone V., De Fusco M., Pietrobon D., Pizzorusso T.et al. (2011) Increased susceptibility to cortical spreading depression in the mouse model of familial hemiplegic migraine type 2. PLos Genet. 7, e1002129 10.1371/journal.pgen.1002129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Capuani C., Melone M., Tottene A., Bragina L., Crivellaro G., Santello M.et al. (2016) Defective glutamate and K+ clearance by cortical astrocytes in familial hemiplegic migraine type 2. EMBO Mol. Med. 8, 967–986 10.15252/emmm.201505944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kros L., Lykke-Hartmann K. and Khodakhah K. (2018) Increased susceptibility to cortical spreading depression and epileptiform activity in a mouse model for FHM2. Sci. Rep. 8, 16959 10.1038/s41598-018-35285-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Sutherland H.G. and Griffiths L.R. (2017) Genetics of migraine: insights into the molecular basis of migraine disorders. Headache 57, 537–569 10.1111/head.13053 [DOI] [PubMed] [Google Scholar]
- 164.Shelly D.A., He S., Moseley A., Weber C., Stegemeyer M., Lynch R.M.et al. (2004) Na(+) pump alpha 2-isoform specifically couples to contractility in vascular smooth muscle: evidence from gene-targeted neonatal mice. Am. J. Physiol. Cell Physiol. 286, C813–C820 10.1152/ajpcell.00389.2003 [DOI] [PubMed] [Google Scholar]
- 165.Chen L., Song H., Wang Y., Lee J.C., Kotlikoff M.I., Pritchard T.J.et al. (2015) Arterial alpha2-Na+ pump expression influences blood pressure: lessons from novel, genetically engineered smooth muscle-specific alpha2 mice. Am. J. Physiol. 309, H958–H968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Staehr C., Rajanathan R., Postnov D.D., Hangaard L., Bouzinova E.V., Lykke-Hartmann K.et al. (2019) Abnormal neurovascular coupling as a cause of excess cerebral vasodilation in familial migraine. Cardiovasc. Res. 116, 2009–2020 10.1093/cvr/cvz306 [DOI] [PubMed] [Google Scholar]
- 167.Facco E., Munari M., Baratto F., Behr A.U., Dal Palù A., Cesaro S.et al. (1996) Regional cerebral blood flow (rCBF) in migraine during the interictal period: different rCBF patterns in patients with and without aura. Cephalalgia 16, 161–168 10.1046/j.1468-2982.1996.1603161.x [DOI] [PubMed] [Google Scholar]
- 168.Calandre E.P., Bembibre J., Arnedo M.L. and Becerra D. (2002) Cognitive disturbances and regional cerebral blood flow abnormalities in migraine patients: their relationship with the clinical manifestations of the illness. Cephalalgia 22, 291–302 10.1046/j.1468-2982.2002.00370.x [DOI] [PubMed] [Google Scholar]
- 169.Nowaczewska M., Straburzyński M., Waliszewska-Prosół M., Meder G., Janiak-Kiszka J. and Kaźmierczak W. (2022) Cerebral blood flow and other predictors of responsiveness to erenumab and fremanezumab in migraine-a real-life study. Front Neurol. 13, 895476 10.3389/fneur.2022.895476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kitano A., Shimomura T., Takeshima T. and Takahashi K. (1994) Increased 11-dehydrothromboxane B2 in migraine: platelet hyperfunction in patients with migraine during headache-free period. Headache 34, 515–518 10.1111/j.1526-4610.1994.hed3409515.x [DOI] [PubMed] [Google Scholar]
- 171.Gallai V., Sarchielli P., Firenze C., Trequattrini A., Paciaroni M., Usai F.et al. (1994) Endothelin 1 in migraine and tension-type headache. Acta Neurol. Scand. 89, 47–55 10.1111/j.1600-0404.1994.tb01632.x [DOI] [PubMed] [Google Scholar]
- 172.Kallela M., Farkkila M., Saijonmaa O. and Fyhrquist F. (1998) Endothelin in migraine patients. Cephalalgia 18, 329–332 10.1046/j.1468-2982.1998.1806329.x [DOI] [PubMed] [Google Scholar]
- 173.Gross N.B., Abad N., Lichtstein D., Taron S., Aparicio L., Fonteh A.N.et al. (2019) Endogenous Na+, K+-ATPase inhibitors and CSF [Na+] contribute to migraine formation. PLoS ONE 14, e0218041 10.1371/journal.pone.0218041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Iwamoto T., Kita S., Zhang J., Blaustein M.P., Arai Y., Yoshida S.et al. (2004) Salt-sensitive hypertension is triggered by Ca2+ entry via Na+/Ca2+ exchanger type-1 in vascular smooth muscle. Nat. Med. 10, 1193–1199 10.1038/nm1118 [DOI] [PubMed] [Google Scholar]
- 175.Sugimoto H., Hirabayashi H., Kimura Y., Furuta A., Amano N. and Moriwaki T. (2011) Quantitative investigation of the impact of P-glycoprotein inhibition on drug transport across blood-brain barrier in rats. Drug Metab. Dispos. 39, 8–14 10.1124/dmd.110.035774 [DOI] [PubMed] [Google Scholar]
- 176.Paczula A., Wiecek A. and Piecha G. (2016) The role of endogenous cardiotonic steroids in pathogenesis of cardiovascular and renal complications of arterial hypertension. Postepy. Hig. Med. Dosw. (Online) 70, 243–250 10.5604/17322693.1197486 [DOI] [PubMed] [Google Scholar]
- 177.Overbeck H.W., Pamnani M.B., Akera T., Brody T.M. and Haddy F.J. (1976) Depressed function of a ouabain-sensitive sodium-potassium pump in blood vessels from renal hypertensive dogs. Circ. Res. 38, 48–52 10.1161/01.RES.38.6.48 [DOI] [PubMed] [Google Scholar]
- 178.Haddy F.J. and Overbeck H.W. (1976) The role of humoral agents in volume expanded hypertension. Life Sci. 19, 935–947 10.1016/0024-3205(76)90284-8 [DOI] [PubMed] [Google Scholar]
- 179.Hamlyn J.M., Ringel R., Schaeffer J., Levinson P.D., Hamilton B.P., Kowarski A.A.et al. (1982) A circulating inhibitor of (Na+ + K+)ATPase associated with essential hypertension. Nature 300, 650–652 10.1038/300650a0 [DOI] [PubMed] [Google Scholar]
- 180.Hamlyn J.M., Blaustein M.P., Bova S., DuCharme D.W., Harris D.W., Mandel F.et al. (1991) Identification and characterization of a ouabain-like compound from human plasma. Proc. Natl. Acad. Sci. U.S.A. 88, 6259–6263 10.1073/pnas.88.14.6259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Baecher S., Kroiss M., Fassnacht M. and Vogeser M. (2014) No endogenous ouabain is detectable in human plasma by ultra-sensitive UPLC-MS/MS. Clin. Chim. Acta 431, 87–92 10.1016/j.cca.2014.01.038 [DOI] [PubMed] [Google Scholar]
- 182.Kawamura A., Guo J., Itagaki Y., Bell C., Wang Y., Haupert G.T. Jr.et al. (1999) On the structure of endogenous ouabain. Proc. Natl. Acad. Sci. U.S.A. 96, 6654–6659 10.1073/pnas.96.12.6654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Komiyama Y., Dong X.H., Nishimura N., Masaki H., Yoshika M., Masuda M.et al. (2005) A novel endogenous digitalis, telocinobufagin, exhibits elevated plasma levels in patients with terminal renal failure. Clin. Biochem. 38, 36–45 10.1016/j.clinbiochem.2004.08.005 [DOI] [PubMed] [Google Scholar]
- 184.Dostanic-Larson I., Lorenz J.N., Van Huysse J.W., Neumann J.C., Moseley A.E. and Lingrel J.B. (2006) Physiological role of the alpha1- and alpha2-isoforms of the Na+-K+-ATPase and biological significance of their cardiac glycoside binding site. Am. J. Physiol. Regul. Integr. Comp. Physiol. 290, R524–R528 10.1152/ajpregu.00838.2005 [DOI] [PubMed] [Google Scholar]
- 185.Lingrel J.B. (2010) The physiological significance of the cardiotonic steroid/ouabain-binding site of the Na,K-ATPase. Annu. Rev. Physiol. 72, 395–412 10.1146/annurev-physiol-021909-135725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Laredo J., Hamilton B.P. and Hamlyn J.M. (1995) Secretion of endogenous ouabain from bovine adrenocortical cells: role of the zona glomerulosa and zona fasciculata. Biochem. Biophys. Res. Commun. 212, 487–493 10.1006/bbrc.1995.1996 [DOI] [PubMed] [Google Scholar]
- 187.Dmitrieva R.I., Bagrov A.Y., Lalli E., Sassone-Corsi P., Stocco D.M. and Doris P.A. (2000) Mammalian bufadienolide is synthesized from cholesterol in the adrenal cortex by a pathway that Is independent of cholesterol side-chain cleavage. Hypertension 36, 442–448 10.1161/01.HYP.36.3.442 [DOI] [PubMed] [Google Scholar]
- 188.Murrell J.R., Randall J.D., Rosoff J., Zhao J.L., Jensen R.V., Gullans S.R.et al. (2005) Endogenous ouabain: upregulation of steroidogenic genes in hypertensive hypothalamus but not adrenal. Circulation 112, 1301–1308 10.1161/CIRCULATIONAHA.105.554071 [DOI] [PubMed] [Google Scholar]
- 189.Li S., Eim C., Kirch U., Lang R.E. and Schoner W. (1998) Bovine adrenals and hypothalamus are a major source of proscillaridin A- and ouabain-immunoreactivities. Life Sci. 62, 1023–1033 10.1016/S0024-3205(98)00023-X [DOI] [PubMed] [Google Scholar]
- 190.Blaustein M.P., Leenen F.H., Chen L., Golovina V.A., Hamlyn J.M., Pallone T.L.et al. (2012) How NaCl raises blood pressure: a new paradigm for the pathogenesis of salt-dependent hypertension. Am. J. Physiol. 302, H1031–H1049 10.1152/ajpheart.00899.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Leenen F.H. (2010) The central role of the brain aldosterone-“ouabain” pathway in salt-sensitive hypertension. Biochim. Biophys. Acta 1802, 1132–1139 10.1016/j.bbadis.2010.03.004 [DOI] [PubMed] [Google Scholar]
- 192.Hamlyn J.M., Lu Z.R., Manunta P., Ludens J.H., Kimura K., Shah J.R.et al. (1998) Observations on the nature, biosynthesis, secretion and significance of endogenous ouabain. Clin. Exp. Hypertens. 20, 523–533 10.3109/10641969809053230 [DOI] [PubMed] [Google Scholar]
- 193.Lichtstein D., Steinitz M., Gati I., Samuelov S., Deutsch J. and Orly J. (1998) Biosynthesis of digitalis-like compounds in rat adrenal cells: hydroxycholesterol as possible precursor. Life Sci. 62, 2109–2126 10.1016/S0024-3205(98)00186-6 [DOI] [PubMed] [Google Scholar]
- 194.Schürks M., Rist P.M., Bigal M.E., Buring J.E., Lipton R.B. and Kurth T. (2009) Migraine and cardiovascular disease: systematic review and meta-analysis. BMJ 339, b3914 10.1136/bmj.b3914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Sheikh H.U., Pavlovic J., Loder E. and Burch R. (2018) Risk of stroke associated with use of estrogen containing contraceptives in women with migraine: a systematic review. Headache 58, 5–21 10.1111/head.13229 [DOI] [PubMed] [Google Scholar]
- 196.Gillum L.A., Mamidipudi S.K. and Johnston S.C. (2000) Ischemic stroke risk with oral contraceptives: A meta-analysis. JAMA 284, 72–78 10.1001/jama.284.1.72 [DOI] [PubMed] [Google Scholar]
- 197.Calhoun A.H. and Batur P. (2017) Combined hormonal contraceptives and migraine: An update on the evidence. Cleve. Clin. J. Med. 84, 631–638 10.3949/ccjm.84a.16033 [DOI] [PubMed] [Google Scholar]
- 198.Hamlyn J.M., Laredo J., Shah J.R., Lu Z.R. and Hamilton B.P. (2003) 11-hydroxylation in the biosynthesis of endogenous ouabain: multiple implications. Ann. N. Y. Acad. Sci. 986, 685–693 10.1111/j.1749-6632.2003.tb07283.x [DOI] [PubMed] [Google Scholar]
- 199.Bauer N., Muller-Ehmsen J., Kramer U., Hambarchian N., Zobel C., Schwinger R.H.et al. (2005) Ouabain-like compound changes rapidly on physical exercise in humans and dogs: effects of beta-blockade and angiotensin-converting enzyme inhibition. Hypertension 45, 1024–1028 10.1161/01.HYP.0000165024.47728.f7 [DOI] [PubMed] [Google Scholar]
- 200.Weidemann H., Salomon N., Avnit-Sagi T., Weidenfeld J., Rosen H. and Lichtstein D. (2004) Diverse effects of stress and additional adrenocorticotropic hormone on digitalis-like compounds in normal and nude mice. J. Neuroendocrinol. 16, 458–463 10.1111/j.1365-2826.2004.01181.x [DOI] [PubMed] [Google Scholar]
- 201.Laredo J., Shah J.R., Lu Z.R., Hamilton B.P. and Hamlyn J.M. (1997) Angiotensin II stimulates secretion of endogenous ouabain from bovine adrenocortical cells via angiotensin type 2 receptors. Hypertension 29, 401–407 10.1161/01.HYP.29.1.401 [DOI] [PubMed] [Google Scholar]
- 202.Laredo J., Hamilton B.P. and Hamlyn J.M. (1994) Ouabain is secreted by bovine adrenocortical cells. Endocrinology 135, 794–797 10.1210/endo.135.2.8033829 [DOI] [PubMed] [Google Scholar]
- 203.Boulanger B.R., Lilly M.P., Hamlyn J.M., Laredo J., Shurtleff D. and Gann D.S. (1993) Ouabain is secreted by the adrenal gland in awake dogs. Am. J. Physiol. 264, E413–E419 10.1152/ajpendo.1993.264.3.E413 [DOI] [PubMed] [Google Scholar]
- 204.Komiyama Y., Nishimura N., Munakata M., Okuda K., Nishino N., Kosaka C.et al. (1999) Increases in plasma ouabainlike immunoreactivity during surgical extirpation of pheochromocytoma. Hypertens. Res. 22, 135–139 10.1291/hypres.22.135 [DOI] [PubMed] [Google Scholar]
- 205.Nesher M., Dvela M., Igbokwe V.U., Rosen H. and Lichtstein D. (2009) Physiological roles of endogenous ouabain in normal rats. Am. J. Physiol. 297, H2026–H2034 10.1152/ajpheart.00734.2009 [DOI] [PubMed] [Google Scholar]
- 206.Blaustein M.P. (2015) Letter to the Editor concerning Baecher et al. (Clin Chim Acta 2014;431:87-92). Clin. Chim. Acta 448, 248–249 10.1016/j.cca.2015.07.011 [DOI] [PubMed] [Google Scholar]
- 207.Tentori S., Messaggio E., Brioni E., Casamassima N., Simonini M., Zagato L.et al. (2016) Endogenous ouabain and aldosterone are coelevated in the circulation of patients with essential hypertension. J. Hypertens. 34, 2074–2080 10.1097/HJH.0000000000001042 [DOI] [PubMed] [Google Scholar]
- 208.Pierdomenico S.D., Bucci A., Manunta P., Rivera R., Ferrandi M., Hamlyn J.M.et al. (2001) Endogenous ouabain and hemodynamic and left ventricular geometric patterns in essential hypertension. Am. J. Hypertens. 14, 44–50 10.1016/S0895-7061(00)01225-5 [DOI] [PubMed] [Google Scholar]
- 209.Manunta P., Messaggio E., Ballabeni C., Sciarrone M.T., Lanzani C., Ferrandi M.et al. (2001) Plasma ouabain-like factor during acute and chronic changes in sodium balance in essential hypertension. Hypertension 38, 198–203 10.1161/01.HYP.38.2.198 [DOI] [PubMed] [Google Scholar]
- 210.Manunta P., Stella P., Rivera R., Ciurlino D., Cusi D., Ferrandi M.et al. (1999) Left ventricular mass, stroke volume, and ouabain-like factor in essential hypertension. Hypertension 34, 450–456 10.1161/01.HYP.34.3.450 [DOI] [PubMed] [Google Scholar]
- 211.Hamlyn J.M. and Blaustein M.P. (2016) Endogenous ouabain: recent advances and controversies. Hypertension 68, 526–532 10.1161/HYPERTENSIONAHA.116.06599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Rossi G., Manunta P., Hamlyn J.M., Pavan E., De T.R., Semplicini A.et al. (1995) Immunoreactive endogenous ouabain in primary aldosteronism and essential hypertension: relationship with plasma renin, aldosterone and blood pressure levels. J. Hypertens. 13, 1181–1191 10.1097/00004872-199510000-00013 [DOI] [PubMed] [Google Scholar]
- 213.Gottlieb S.S., Rogowski A.C., Weinberg M., Krichten C.M., Hamilton B.P. and Hamlyn J.M. (1992) Elevated concentrations of endogenous ouabain in patients with congestive heart failure. Circulation 86, 420–425 10.1161/01.CIR.86.2.420 [DOI] [PubMed] [Google Scholar]
- 214.Gonick H.C., Ding Y., Vaziri N.D., Bagrov A.Y. and Fedorova O.V. (1998) Simultaneous measurement of marinobufagenin, ouabain, and hypertension-associated protein in various disease states. Clin. Exp. Hypertens. 20, 617–627 10.3109/10641969809053240 [DOI] [PubMed] [Google Scholar]
- 215.Lopatin D.A., Ailamazian E.K., Dmitrieva R.I., Shpen V.M., Fedorova O.V., Doris P.A.et al. (1999) Circulating bufodienolide and cardenolide sodium pump inhibitors in preeclampsia. J. Hypertens. 17, 1179–1187 10.1097/00004872-199917080-00018 [DOI] [PubMed] [Google Scholar]
- 216.Manunta P., Hamilton J., Rogowski A.C., Hamilton B.P. and Hamlyn J.M. (2000) Chronic hypertension induced by ouabain but not digoxin in the rat: antihypertensive effect of digoxin and digitoxin. Hypertens. Res. 23, S77–S85 10.1291/hypres.23.Supplement_S77 [DOI] [PubMed] [Google Scholar]
- 217.Yuan C.M., Manunta P., Hamlyn J.M., Chen S., Bohen E., Yeun J.et al. (1993) Long-term ouabain administration produces hypertension in rats. Hypertension 22, 178–187 10.1161/01.HYP.22.2.178 [DOI] [PubMed] [Google Scholar]
- 218.Xavier F.E., Rossoni L.V., Alonso M.J., Balfagón G., Vassallo D.V. and Salaices M. (2004) Ouabain-induced hypertension alters the participation of endothelial factors in alpha-adrenergic responses differently in rat resistance and conductance mesenteric arteries. Br. J. Pharmacol. 143, 215–225 10.1038/sj.bjp.0705919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Briones A.M., Xavier F.E., Arribas S.M., Gonzalez M.C., Rossoni L.V., Alonso M.J.et al. (2006) Alterations in structure and mechanics of resistance arteries from ouabain-induced hypertensive rats. Am. J. Physiol. 291, H193–H201 10.1152/ajpheart.00802.2005 [DOI] [PubMed] [Google Scholar]
- 220.Lorenz J.N., Loreaux E.L., Dostanic-Larson I., Lasko V., Schnetzer J.R., Paul R.J.et al. (2008) ACTH-induced hypertension is dependent on the ouabain-binding site of the alpha2-Na+-K+-ATPase subunit. Am. J. Physiol. Heart Circ. Physiol. 295, H273–H280 10.1152/ajpheart.00183.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Yamada K., Goto A., Nagoshi H., Hui C., Yagi N., Sasabe M.et al. (1994) Participation of ouabainlike compound in reduced renal mass-saline hypertension. Hypertension 23, I110–I113 10.1161/01.HYP.23.1_Suppl.I110 [DOI] [PubMed] [Google Scholar]
- 222.Ferrandi M., Minotti E., Salardi S., Florio M., Bianchi G. and Ferrari P. (1992) Ouabainlike factor in Milan hypertensive rats. Am. J. Physiol. 263, F739–F748 10.1152/ajprenal.1992.263.4.F739 [DOI] [PubMed] [Google Scholar]
- 223.Huang B.S. and Leenen F.H. (1998) Both brain angiotensin II and “ouabain” contribute to sympathoexcitation and hypertension in Dahl S rats on high salt intake. Hypertension 32, 1028–1033 10.1161/01.HYP.32.6.1028 [DOI] [PubMed] [Google Scholar]
- 224.Ferrari P. (2010) Rostafuroxin: an ouabain-inhibitor counteracting specific forms of hypertension. Biochim. Biophys. Acta 1802, 1254–1258 10.1016/j.bbadis.2010.01.009 [DOI] [PubMed] [Google Scholar]
- 225.Kaide J., Ura N., Torii T., Nakagawa M., Takada T. and Shimamoto K. (1999) Effects of digoxin-specific antibody Fab fragment (Digibind) on blood pressure and renal water-sodium metabolism in 5/6 reduced renal mass hypertensive rats. Am. J. Hypertens. 12, 611–619 10.1016/S0895-7061(99)00029-1 [DOI] [PubMed] [Google Scholar]
- 226.Krep H., Price D.A., Soszynski P., Tao Q.F., Graves S.W. and Hollenberg N.K. (1995) Volume sensitive hypertension and the digoxin-like factor. Reversal by a Fab directed against digoxin in DOCA-salt hypertensive rats. Am. J. Hypertens. 8, 921–927 10.1016/0895-7061(95)00181-N [DOI] [PubMed] [Google Scholar]
- 227.Ferrandi M., Molinari I., Torielli L., Padoani G., Salardi S., Rastaldi M.P.et al. (2010) Adducin- and ouabain-related gene variants predict the antihypertensive activity of rostafuroxin, part 1: experimental studies. Sci. Transl. Med. 2, 59ra86 10.1126/scitranslmed.3001815 [DOI] [PubMed] [Google Scholar]
- 228.Blaustein M.P., Gottlieb S.S., Hamlyn J.M. and Leenen F.H.H. (2022) Whither digitalis? What we can still learn from cardiotonic steroids about heart failure and hypertension Am. J. Physiol. Heart Circ. Physiol. 323, H1281–H1295 10.1152/ajpheart.00362.2022 [DOI] [PubMed] [Google Scholar]
- 229.Leenen F.H.H., Wang H.W. and Hamlyn J.M. (2020) Sodium pumps, ouabain and aldosterone in the brain: a neuromodulatory pathway underlying salt-sensitive hypertension and heart failure. Cell Calcium 86, 102151 10.1016/j.ceca.2019.102151 [DOI] [PubMed] [Google Scholar]
- 230.Ferrandi M., Molinari I., Barassi P., Minotti E., Bianchi G. and Ferrari P. (2004) Organ hypertrophic signaling within caveolae membrane subdomains triggered by ouabain and antagonized by PST 2238. J. Biol. Chem. 279, 33306–33314 10.1074/jbc.M402187200 [DOI] [PubMed] [Google Scholar]
- 231.Efendiev R., Chen Z., Krmar R.T., Uhles S., Katz A.I., Pedemonte C.H.et al. (2005) The 14-3-3 protein translates the NA+,K+-ATPase {alpha|1-subunit phosphorylation signal into binding and activation of phosphoinositide 3-kinase during endocytosis. J. Biol. Chem. 280, 16272–16277 10.1074/jbc.M500486200 [DOI] [PubMed] [Google Scholar]
- 232.Mulvany M.J. and Aalkjaer C. (1990) Structure and function of small arteries. Physiol. Rev. 70, 921–961 10.1152/physrev.1990.70.4.921 [DOI] [PubMed] [Google Scholar]
- 233.Pulina M.V., Zulian A., Berra-Romani R., Beskina O., Mazzocco-Spezzia A., Baryshnikov S.G.et al. (2010) Upregulation of Na+ and Ca2+ transporters in arterial smooth muscle from ouabain-induced hypertensive rats. Am. J. Physiol. 298, H263–H274 10.1152/ajpheart.00784.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Pulgar V.M., Jeffers A.B., Rashad H.M., Diz D.I. and Aileru A.A. (2013) Increased constrictor tone induced by ouabain treatment in rats. J. Cardiovasc. Pharmacol. 62, 174–183 10.1097/FJC.0b013e3182955d33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Dostanic I., Paul R.J., Lorenz J.N., Theriault S., Van Huysse J.W. and Lingrel J.B. (2005) The alpha2-isoform of Na-K-ATPase mediates ouabain-induced hypertension in mice and increased vascular contractility in vitro. Am. J. Physiol. 288, H477–H485 [DOI] [PubMed] [Google Scholar]
- 236.Zhang J., Hamlyn J.M., Karashima E., Raina H., Mauban J.R., Izuka M.et al. (2009) Low-dose ouabain constricts small arteries from ouabain-hypertensive rats: implications for sustained elevation of vascular resistance. Am. J. Physiol. Heart Circ. Physiol. 297, H1140–H1150 10.1152/ajpheart.00436.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Zhang J., Lee M.Y., Cavalli M., Chen L., Berra-Romani R., Balke C.W.et al. (2005) Sodium pump alpha2 subunits control myogenic tone and blood pressure in mice. J. Physiol. 569, 243–256 10.1113/jphysiol.2005.091801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Bastrup J.A., Aalkjaer C. and Jepps T.A. (2022) Identification of novel proteins and mechanistic pathways associated with early-onset hypertension by deep proteomic mapping of resistance arteries. J. Biol. Chem. 298, 101512 10.1016/j.jbc.2021.101512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Giachini F.R., Carneiro F.S., Lima V.V., Carneiro Z.N., Brands M.W., Webb R.C.et al. (2009) A key role for Na+/K+-ATPase in the endothelium-dependent oscillatory activity of mouse small mesenteric arteries. Braz. J. Med. Biol. Res. 42, 1058–1067 10.1590/S0100-879X2009005000033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Matchkov V.V., Gustafsson H., Rahman A., Boedtkjer D.M., Gorintin S., Hansen A.K.et al. (2007) Interaction between Na+/K+-pump and Na+/Ca2+-exchanger modulates intercellular communication. Circ. Res. 100, 1026–1035 10.1161/01.RES.0000262659.09293.56 [DOI] [PubMed] [Google Scholar]
- 241.Pritchard T.J., Bowman P.S., Jefferson A., Tosun M., Lynch R.M. and Paul R.J. (2010) Na(+)-K(+)-ATPase and Ca(2+) clearance proteins in smooth muscle: a functional unit. Am. J. Physiol. 299, H548–H556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Rossoni L.V., Salaices M., Marin J., Vassallo D.V. and Alonso M.J. (2002) Alterations in phenylephrine-induced contractions and the vascular expression of Na+,K+-ATPase in ouabain-induced hypertension. Br. J. Pharmacol. 135, 771–781 10.1038/sj.bjp.0704501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Miriel V.A., Mauban J.R., Blaustein M.P. and Wier W.G. (1999) Local and cellular Ca2+ transients in smooth muscle of pressurized rat resistance arteries during myogenic and agonist stimulation. J. Physiol. 518, 815–824 10.1111/j.1469-7793.1999.0815p.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Toda N. (1980) Mechanisms of ouabain-induced arterial muscle contraction. Am. J. Physiol. 239, H199–H205 10.1152/ajpheart.1980.239.2.H199 [DOI] [PubMed] [Google Scholar]
- 245.Vassallo D.V., Songu-Mize E., Rossoni L.V. and Amaral S.M. (1997) Effects of ouabain on vascular reactivity. Braz. J. Med. Biol. Res. 30, 545–552 10.1590/S0100-879X1997000400016 [DOI] [PubMed] [Google Scholar]
- 246.Raina H., Zhang Q., Rhee A.Y., Pallone T.L. and Wier W.G. (2010) Sympathetic nerves and the endothelium influence the vasoconstrictor effect of low concentrations of ouabain in pressurized small arteries. Am. J. Physiol. 298, H2093–H2101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Weiss D.N., Podberesky D.J., Heidrich J. and Blaustein M.P. (1993) Nanomolar ouabain augments caffeine-evoked contractions in rat arteries. Am. J. Physiol. 265, C1443–C1448 10.1152/ajpcell.1993.265.5.C1443 [DOI] [PubMed] [Google Scholar]
- 248.Zhang L., Aalkjaer C. and Matchkov V.V. (2018) The Na,K-ATPase-dependent Src kinase signaling changes with mesenteric artery diameter. Int. J. Mol. Sci. 19, 2489 10.3390/ijms19092489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Matchkov V.V. and Aalkjaer C. (2017) Reply from Vladimir V. Matchkov and Christian Aalkjaer. J. Physiol. 595, 6785–6787 10.1113/JP275145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Oshiro N., Dostanic-Larson I., Neumann J.C. and Lingrel J.B. (2010) The ouabain-binding site of the α2 isoform of Na,K-ATPase plays a role in blood pressure regulation during pregnancy. Am. J. Hypertens. 23, 1279–1285 10.1038/ajh.2010.195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Dostanic-Larson I., Van Huysse J.W., Lorenz J.N. and Lingrel J.B. (2005) The highly conserved cardiac glycoside binding site of Na,K-ATPase plays a role in blood pressure regulation. Proc. Natl. Acad. Sci. U.S.A. 102, 15845–15850 10.1073/pnas.0507358102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Blaustein M.P. and Hamlyn J.M. (2010) Signaling mechanisms that link salt retention to hypertension: endogenous ouabain, the Na(+) pump, the Na(+)/Ca(2+) exchanger and TRPC proteins. Biochim. Biophys. Acta 1802, 1219–1229 10.1016/j.bbadis.2010.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Gable M.E., Ellis L., Fedorova O.V., Bagrov A.Y. and Askari A. (2017) Comparison of digitalis sensitivities of Na(+)/K(+)-ATPases from human and pig kidneys. ACS Omega 2, 3610–3615 10.1021/acsomega.7b00591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Parhami-Seren B., Haberly R., Margolies M.N. and Haupert G.T. Jr (2002) Ouabain-binding protein(s) from human plasma. Hypertension 40, 220–228 10.1161/01.HYP.0000027134.14160.1D [DOI] [PubMed] [Google Scholar]
- 255.Soto-Blanco B. (2021) Cardiac Glycosides. In Encyclopedia of Molecular Pharmacology(Offermanns S. and Rosenthal W., eds), pp. 410–414, Springer International Publishing, Cham, editors. [Google Scholar]
- 256.Wang Y., Lonard D.M., Yu Y., Chow D.C., Palzkill T.G., Wang J.et al. (2014) Bufalin is a potent small-molecule inhibitor of the steroid receptor coactivators SRC-3 and SRC-1. Cancer Res. 74, 1506–1517 10.1158/0008-5472.CAN-13-2939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Øie L.R., Kurth T., Gulati S. and Dodick D.W. (2020) Migraine and risk of stroke. J. Neurol. Neurosurg. Psychiatry 91, 593–604 10.1136/jnnp-2018-318254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Harriott A.M. and Barrett K.M. (2015) Dissecting the association between migraine and stroke. Curr. Neurol. Neurosci. Rep. 15, 5 10.1007/s11910-015-0530-8 [DOI] [PubMed] [Google Scholar]
- 259.Etminan M., Takkouche B., Isorna F.C. and Samii A. (2005) Risk of ischaemic stroke in people with migraine: systematic review and meta-analysis of observational studies. BMJ 330, 63 10.1136/bmj.38302.504063.8F [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Leira R., Dávalos A., Aneiros A., Serena J., Pumar J.M. and Castillo J. (2002) Headache as a surrogate marker of the molecular mechanisms implicated in progressing stroke. Cephalalgia 22, 303–308 10.1046/j.1468-2982.2002.00357.x [DOI] [PubMed] [Google Scholar]
- 261.Oliveira F.A.A., Dourado-Filho M.G. and Rocha-Filho P.A.S. (2023) Acute headache attributed to ischemic stroke: assessment of its characteristics and associated factors. Arq. Neuropsiquiatr. 81, 225–232 10.1055/s-0043-1763487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Kannel W.B., Wolf P.A., Verter J. and McNamara P.M. (1996) Epidemiologic assessment of the role of blood pressure in stroke: the Framingham Study. 1970. JAMA 276, 1269–1278 10.1001/jama.1996.03540150071040 [DOI] [PubMed] [Google Scholar]
- 263.Leonardi-Bee J., Bath P.M., Phillips S.J. and Sandercock P.A. (2002) Blood pressure and clinical outcomes in the International Stroke Trial. Stroke 33, 1315–1320 10.1161/01.STR.0000014509.11540.66 [DOI] [PubMed] [Google Scholar]
- 264.Willis J.S. and Cuveellory J. (1983) Ouabain sensitivity: diversity and disparities. In Current Topics in Membranes and Transportvol. 19, (Bronner F. and Kleinzeller A., eds), pp. 277–280, Academic Press; 10.1016/S0070-2161(08)60580-8 [DOI] [Google Scholar]
- 265.Aperia A. (2007) New roles for an old enzyme: Na,K-ATPase emerges as an interesting drug target. J. Intern. Med. 261, 44–52 10.1111/j.1365-2796.2006.01745.x [DOI] [PubMed] [Google Scholar]
- 266.Liu J. and Xie Z.J. (2010) The sodium pump and cardiotonic steroids-induced signal transduction protein kinases and calcium-signaling microdomain in regulation of transporter trafficking. Biochim. Biophys. Acta 1802, 1237–1245 10.1016/j.bbadis.2010.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Riganti C., Campia I., Kopecka J., Gazzano E., Doublier S., Aldieri E.et al. (2011) Pleiotropic effects of cardioactive glycosides. Curr. Med. Chem. 18, 872–885 10.2174/092986711794927685 [DOI] [PubMed] [Google Scholar]
- 268.Orlov S.N. and Hamet P. (2015) Salt and gene expression: evidence for [Na+]i/[K+]i-mediated signaling pathways. Pflugers Arch. 467, 489–498 10.1007/s00424-014-1650-8 [DOI] [PubMed] [Google Scholar]
- 269.Lopina O.D., Tverskoi A.M., Klimanova E.A., Sidorenko S.V. and Orlov S.N. (2020) Ouabain-induced cell death and survival. Role of α1-Na,K-ATPase-mediated signaling and [Na(+)](i)/[K(+)](i)-dependent gene expression. Front Physiol. 11, 1060 10.3389/fphys.2020.01060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Ren Y.P., Huang R.W. and Lü Z.R. (2006) Ouabain at pathological concentrations might induce damage in human vascular endothelial cells. Acta Pharmacol. Sin. 27, 165–172 10.1111/j.1745-7254.2006.00244.x [DOI] [PubMed] [Google Scholar]
- 271.Manunta P., Ferrandi M. and Bianchi G. (2004) Ouabain. In Encyclopedia of Endocrine Diseases(Martini L., ed.), pp. 447–450, Elsevier, New York: 10.1016/B0-12-475570-4/00957-4 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This is a review article and no original data hence available. The proteomics dataset retrospectively analyzed in this review is openly accessible in the original report (Wen et al., J. Proteome Res., 2019).