Abstract
Organochlorine pesticides (OCPs) are persistent environmental contaminants that act as endocrine disruptors and system toxicants. These pesticides (e.g. dichlorodiphenyltrichloroethane (DDT), dieldrin, toxaphene, among others) are ranked as some of the most concerning chemicals for human health. These pesticides (1) act as teratogens, (2) are neuroendocrine disruptors, (3) suppress the immune and reproductive systems, and (4) dysregulate lipids and metabolism. Using a computational approach, we revealed enriched endocrine-related pathways in the Comparative Toxicogenomics Database sensitive to this chemical class, and these included reproduction (gonadotropins, estradiol, androgen, steroid biosynthesis, oxytocin), thyroid hormone, and insulin. Insight from the Tox21 and ToxCast programs confirm that these agrochemicals activate estrogen receptors, androgen receptors, and retinoic acid receptors with relatively high affinity, although differences exist in their potency. We propose an adverse outcome pathway for OCPs toxicity in the fish testis as a novel contribution to further understanding of OCP-induced toxicity. Organochlorine pesticides, due to their persistence and high toxicity to aquatic and terrestrial wildlife as well as humans, remain significant agrochemicals of concern.
Keywords: pesticide, reproduction, metabolism, fish, computational toxicology, adverse outcome pathway
1. Introduction
Organochlorine pesticides (OCPs) were originally developed to remove insects and other pests from agricultural fields and thus improve crop yields. The annual loss of crops in the absence of pesticide use was significant and has been estimated roughly at one-third of the annual production (Aktar et al., 2009). OCPs were designed to interfere with and inhibit unique physiological pathways in the target organisms. In fact, the scientist who developed DDT (dichlorodiphenyltrichloroethane), Paul Hermann Müller, received the Nobel prize in 1949 for his work, as DDT also killed the mosquitos responsible for malaria and typhus (Smith, 1999). However, at the time of their release into commerce, there was little knowledge regarding the off-target effects in other organisms including invertebrates and vertebrates. As knowledge about off target effects, such as thinning of bird egg shells with exposure to DDT, became evident (Peakall, 1970), OCPs were replaced by other pesticides and removed from commerce during the 1970’s.
OCPs encompass a rather large group of agrochemicals including DDT and its metabolites, dichlorodiphenyldichloroethylene (DDE) and dichlorodiphenyldichloroethane (DDD); methoxychlor, dieldrin, toxaphene, lindane, endosulfan, among others (Figure 1). Their structures are quite distinct, with the only commonality being the presence of chlorine groups. OCPs are highly lipophilic (Kow’s > 4) and are resistant to microbial degradation and thus are persistent in the environment. Even so, many of the OCPs activate common nuclear receptors and elicit similar toxicity outcomes. For example, p,p’-DDE and methoxychlor are both weak estrogens and weak anti-androgens (Balaguer et al., 1999, Kelce et al., 1995, Sonneveld et al., 2005, Waters et al., 2001). They have been found to accumulate in fatty tissues in non-target organisms and are associated with a myriad of diseases, including cancer (Cohn et al., 2019). As a consequence of this, OCPs are at the top of the list of toxic substances compiled by the Agency for Toxic Substances and Disease Registry (ATSDR) within the United States
Figure 1.
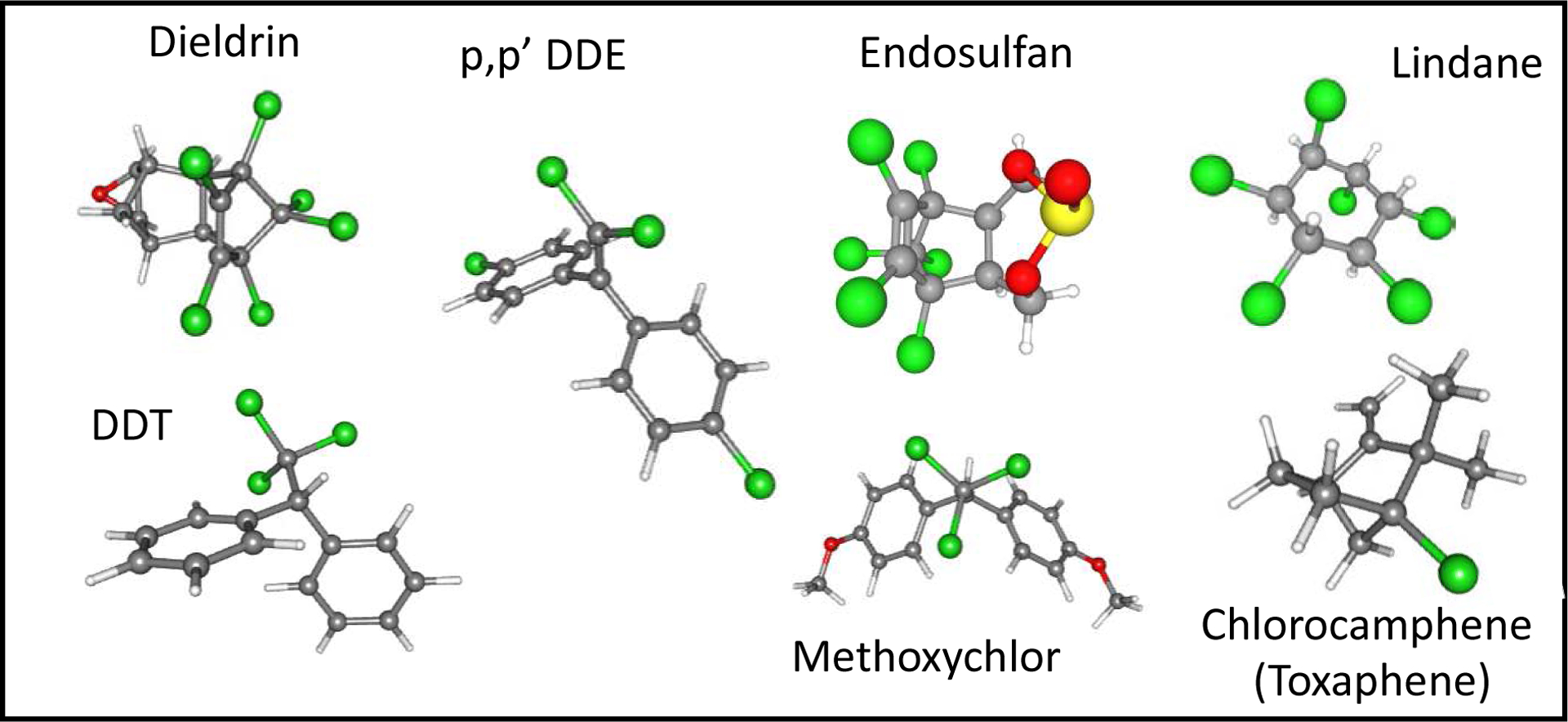
The 3D-structures of some of the major organochlorine agrochemicals. Green indicates positions of the chlorines, red indicates an oxygen, and yellow indicates the position of the sulfur group. Toxaphene is a mixture of chlorinated compounds, where chlorine can be found bonded with carbons at different positions in the molecule. Images extracted from NCBI PubChem.
Department of Health and Human Services. OCPs are frequently in the top 60 chemicals on the list, with DDT at # 13, dieldrin at #18, DDE at #21, chlordane at #22, aldrin at #25, toxaphene at #32, endosulfan at #44 and methoxychlor at #55.
Because of their persistence in the environment, it is difficult to remediate sites that were heavily contaminated with OCPs from the 1950’s to 1980’s. Some of these sites have been designated by the US EPA as Superfund Sites, which merit specific attention for cleanup. Superfund sites were established as a consequence of The Comprehensive Environmental Response, Compensation and Liability Act of 1980 (Bearden, 2012), which required the federal government to determine relative risks of contaminants in designated areas and to develop a risk mitigation strategy to protect human health. A superfund site is designated as such if there are priority contaminants above critical concentrations that are related to disease. Superfund sites are found throughout the USA and OCPs are found at many of these sites. As an example, we present a map of superfund sites that contain concerning levels of dieldrin and toxaphene (Figure 2).
Figure 2:
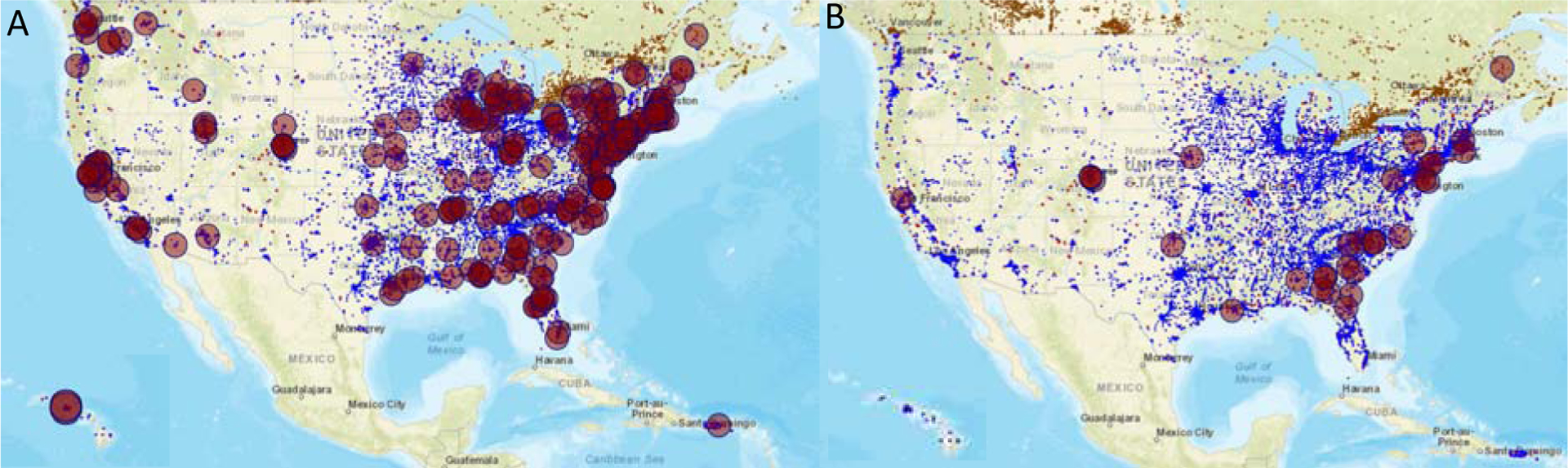
Two examples of Superfund sites (Red circles) containing organochlorine pesticides in the United States. (A) Dieldrin (B) Toxaphene. Inset to the bottom left is Hawaii. Alaska does not have any Superfund site designation for these agrochemicals. Blue indicates locations for chemical contaminants in the Toxic Release Inventory (TRI) Program, for example contaminated sites that contain cancer causing agents. Red indicates Superfund Site locations. Generated from TOXMAP (US Department of Health and Human Services)
In the past 20 years, research groups around the world have documented the health effects of exposure to OCPs in human health (for a comprehensive review, please see (Mrema et al., 2013)) and in the environment. It is clear now that OCPs function as endocrine disruptors, interfering with critical hormonal signaling events in vertebrates and invertebrates. In this review, we are limiting our discussion to OCP effects in fish, as they live in polluted environments and as vertebrates, they have conserved molecular pathways that function similarly in other vertebrates including humans. Studies indicate that many OCPs function as weak estrogens (Das and Thomas, 1999, Garcia-Reyero et al., 2018, Shanle and Xu, 2011) or anti-androgens (Kelce et al., 1997) and may also perturb other hormonal systems including those produced by the thyroid (Brown et al., 2004, Langer, 2010). They have been implicated as neuroendocrine disruptors, interfering with brain function, and several, including dieldrin, have been linked to the incidence of Parkinson’s disease. These compounds also interfere with normal metabolism (Olsvik and Softeland, 2018), mitochondrial oxidative respiration (Cowie et al., 2017, Cowie et al., 2017), and immune function (Martyniuk et al., 2016a, Martyniuk et al., 2016b). Thus, OCPs can directly or indirectly affect endocrine systems through perturbations in hormone synthesis and metabolism, and ATP production. We discuss each of these interferences in this review, starting from early development into adulthood.
2. Organochlorine pesticides as teratogens in developing fish
The endocrine system is shaped early in development, and exposure to toxicants can disrupt the developmentary trajectory of endocrine tissues. Fish embryos are used to screen chemicals for teratogenic effects, and the impacts of OCP exposure have been measured in early developmental stages of different species. Early studies reported the effects of OCPs on mortality and deformity, but more recent efforts have sought to understand how exposure to OCPs in early development affects neurotransmitter systems, which regulate hormone release, and brain development. Owens and Baer compared responses of different stages of development of the Japanese medaka exposed to DDT at concentrations ranging from 3 to 400 ng (per egg), revealing a dose dependent increase in lethality (Owens and Baer, 2000). The study showed there was little difference in survival among groups at the early stages of development, but rather toxicity was highest when the larvae began to absorb their yolk sacs (Owens and Baer, 2000). Thus, OCPs may be sequestered in lipids in developing fish and later released upon reabsorption of yolk, inducing toxicity.
An array of teratogenic effects has been reported in developing fish with OCP exposure. In a study by Ton et al., developing zebrafish larvae were exposed to the organochlorine DDT and dieldrin, and several endpoints related to neuronal development were assessed at 48 and 96 hpf (Ton et al., 2006). A teratogenicity index was calculated as the ratio of dose that causes 50% mortality (LC50) over the dose that causes 50% developmental malformations (EC50), i.e. the ratio of LC50/EC50 (Ton et al., 2006). Scoring for malformations included, heart rate, rate of circulation of blood cells, number of red blood cells, edema, hemorrhage, ventricle swelling, brain necrosis, jaw formation, caudal embryo morphology, and motility. A teratogenicity index >1 was considered teratogenic. In this study, DDT had a relative teratogenic index of 3.5 at 96 hours post fertilization, whereas dieldrin had a value of 0.1 (Ton et al., 2006). These values were compared to 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD), a highly teratogenic chemical which was given a score of 15.3.
DDT and dieldrin were also reported to slow the heart rates and induce tremors in early-stage zebrafish, as well as deplete cholinergic and dopaminergic neurons. This is relevant as acetylcholine and dopamine can stimulate or inhibit hormone releasing factors in the central nervous system (CNS). In a more recent study investigating the effects of dieldrin on early life stages, Sarty et al. (Sarty et al., 2017) exposed both chorionated and dechorionated zebrafish embryos for 48 hours to μM concentrations of dieldrin, followed by a 6-day depuration period. Zebrafish did not exhibit significant mortality until they hatched, and as expected, dechorionated fish showed a higher rate of mortality compared to fish that retained the chorion early on. Moreover, the dechorionated fish showed reduced expression of dopamine active transporter and specific dopamine receptors. Similar to the study by Ton et al., cardiac hemorrhaging, skeletal deformities, and tremors were observed with increasing concentrations of dieldrin (Sarty et al., 2017, Ton et al., 2006). It is clear from these studies that early exposure to OCPs can affect neurodevelopment, and more specifically, may impact the ontogeny of the dopaminergic system.
Other OCPs have been studied for their effects in developing fish embryos. Kapp and colleagues investigated the potential for malformations and deformity in response to different chemical structures of the pesticide toxaphene (Kapp et al., 2006). Toxaphene is a complex mixture of compounds that are created by reacting chlorine gas with camphene. Exposures in the range of 15 to 20 mg toxaphene/L induced deformities that included yolk sack edema, loss of pigmentation, and spinal malformation toxicity. The study also noted that toxaphene prevented circulation of the blood through the heart, even though there was a heartbeat; however, the mechanisms as to why this occurred remains unknown. In a follow up study, Perez-Rodrigues and colleagues exposed zebrafish embryos to toxaphene from 6 to ~126 hours post- fertilization (5 day exposure) (Perez-Rodriguez et al., 2019). Morphological and molecular endpoints were assessed, and the study revealed an increase in deformity score (encompassing pericardial edema, skeletal defects, and yolk sack edema) with higher concentrations of toxaphene. Oxidative phosphorylation rates were also assessed in ~30 hpf embryos after a 24-hour exposure to toxaphene, and it was observed that non-mitochondrial respiration was significantly decreased with 11.1 and 111 μg toxaphene/mL. While these concentrations are not environmentally relevant, it is possible that under chronic exposure paradigms effects on respiration occur at lower concentrations. Transcripts involved in oxidative stress were measured and heat shock protein 70 was induced in larvae exposed to 1.11 μg toxaphene/mL (Perez-Rodriguez et al., 2019). It was hypothesized that reduced oxidative phosphorylation was associated with the higher deformity rates in the larvae, and that the induction of heat shock protein 70 may be a protective mechanism to mitigate toxicity during development.
The mechanism of action of OCPs in embryos can be complex, affecting respiration and neural development as noted above. However, OCPs are complex molecules with the potential to act as endocrine disruptors. Toxicity assessments in zebrafish embryos and larvae to different organochlorine pesticides demonstrated that, even in early staged embryos, OCPs can regulate vitellogenin (vtg), the egg yolk precursor protein (Chow et al., 2013). In the study, OCPs such as endosulfan, methoxychlor, and hepatachlor increased vtg1 expression in either embryos or larval zebrafish. These data suggest that some OCPs act as estrogenic chemicals when fish are undergoing critical stages of sex determination. Indeed, microinjection studies with o,p’-DDT revealed complete male to female sex reversal in Japanese medaka 10 weeks after a single pulse of the chemical into the egg (Edmunds et al., 2000). Despite this understanding, there are little data in fish linking early OCP exposure to long-lasting effects later in life. There are several studies that link embryonic exposures to adult effects. For example, embryonic oxidative stress results in reproductive effects for adult zebrafish (Newman et al., 2015); exposure to azoxystrobin, an aryloxypyrimidine fungicide, delays sexual development and results in reproductive effects (Cao et al., 2019) and embryonic exposure to atrazine alters neuroendocrine function in adult zebrafish (Wirbisky et al., 2016). Clearly more research is required to link embryonic exposures to OCPs to population level effects in fish.
In summary, OCPs are teratogens and alter developmental processes of fish causing malformations. That they can act as estrogens or anti-androgens during this sensitive period, suggests that they may also alter neuroendocrine pathways during sensitive developmental endpoints that help form the brain. These endpoints are discussed further below.
3. Organochlorine pesticides as system toxicants and endocrine disruptors in adult fish
3.1. Neuroendocrine disruption
It is well documented that OCPs are readily taken up by lipid rich tissues including the brain. Studies conducted in the laboratory and investigations of fish collected from polluted sites over the years have demonstrated that the CNS is susceptible to OCP bioaccumulation. For example, a study conducted in Argentina reported that mature male silversides (Odontesthes bonariensis) collected from river watersheds showed a mean level of 5.4 ng/g wet weight of total OCPs in the male brain; whereas, in the female brain, it was reported to be four times higher at 20.6 ng/g wet weight (Barni et al., 2014). Noteworthy was that, in the pre-spawning period, the total concentration of OCPs in the male and female brain was higher than that at maturation. The exact reason for this discrepancy was not elucidated in the study but may be due to differences in biotransformation capacities of males and females throughout the year. Indeed, cycling hormones will have a pronounced effect on enzyme activity and metabolism in general. The difference between males and females could also be due to the blood brain barrier, although we are not aware of studies that have compared male and female blood brain barriers for permeability in fish. The blood brain barrier changes in mammals with age (Erdo et al., 2017) and it would be expected to do so in other vertebrates as well. Thus, sex differences can exist for deposition of OCPs in the CNS, as well as differences based on the reproductive maturity of the individual.
In largemouth bass (Micropterus salmoides), Dang et al. demonstrated that the brain can rapidly accumulate both p,p’ DDE and dieldrin during oral dosing experiments (Dang et al., 2016). In wild caught largemouth bass from Lake Apopka, Florida, brain burden for total Drins (dieldrin, aldrin, endrin) and DDXs (DDT, DDE, DDD) were comparable to other tissues such as the kidney and liver. It was suggested by the authors that the brain be considered a potential organ for monitoring long-term accumulation of OCPs and other lipophilic contaminants in the environment. Significant levels of OCPs in the brain have also been reported for other teleost fishes such as the female European eel (Anguilla anguilla) (Bonnineau et al., 2016). Animals collected from Belgian rivers were analyzed for an array of DDT metabolites; the concentration of p,p’-DDE, p,p’ DDD, and p,p’ DDT ranged from 4.7 to 16.2 ng/g wet weight in the brain of wild caught eels. Similarly, Wu et al. (Wu et al., 2013) collected four species of commonly consumed freshwater fish including crucian carp, grass carp, snake head fish, and silverfish from Lake Baiyangdian, a body of water on the East Coast of China in close proximity to Beijing. DDT related compounds such as o,p’ DDE, p,p’ DDT, and others were present in the brain in the low ng/g wet weight range. It is clear that OCPs accumulate in the CNS of fish over time in contaminated areas.
Once OCPs cross the blood-brain barrier, there is the potential for neurotoxicity. In developing zebrafish, Ton et al. showed that 100 μM DDT and ~20 μM dieldrin induced tremors and increased motility 96 hours post fertilization (Ton et al., 2006). The study showed that DDT at 10 and 20 μM induced apoptosis in the CNS based upon acridine orange staining. There was increased cell death, loss of catecholaminergic and dopaminergic neurons, frequent tremors and disorganized motor neurons in the caudal region of the embryo following exposure. The accumulation of these agrochemicals in the CNS is problematic, not only from the point of view of neurotoxicity, but also from the point of view of neuroendocrine disruption (Leon-Olea et al., 2014); OCPs in the brain can disrupt hormonal and neural connections between the hypothalamus, pituitary, and tissue targets leading to inappropriate endocrine signaling. This may be subtle over time compared to more overt processes such as apoptosis and neuronal cell death. For example, dieldrin can alter the expression of neurotransmitter systems in the teleost brain, such as gamma-aminobutyric acid (GABA) and dopamine signaling (Martyniuk et al., 2010, Martyniuk et al., 2012). GABA is a major stimulator of luteinizing hormone (LH) release from the pituitary while dopamine is a major inhibitor of LH release in fish (Trudeau et al., 1993). Injection of a single dose at 10 mg diledrin/kg into male and female largemouth bass altered neurotransmitter levels in the brain after 7 days in females (Martyniuk et al., 2010). GABA was significantly increased in the cerebellum and the hypothalamus of female largemouth bass but not in male bass. 3,4-dihydroxyphenylacetic acid (DOPAC) and dopamine levels were not changed in any brain region (hypothalamus, telencephalon, cerebellum) of male or female fish. A transcriptome analysis was also conducted in the female hypothalamus and a number of pathways were affected by dieldrin. These included DNA repair, inflammatory pathways, steroid signaling, base excision repair, nucleotide excision repair, and apoptosis. In a follow-up study, largemouth bass were fed 3 mg dieldrin/kg over a two-month feeding exposure (rather than chemical injection) to mimic environmentally relevant exposure scenarios. Microarray analysis in the male and female hypothalamus revealed that gene networks related to retinoic acid receptors, gonadotropin releasing hormone, and follicle-stimulating hormone were affected in females whereas in males, activin and androgen receptor signaling pathways were altered in expression (Martyniuk et al., 2013). Disruption in these hypothalamic neuroendocrine networks were hypothesized to lead to downstream effects on reproduction.
Other mechanistic studies into the neurotoxicity of OCPs have been conducted at the protein level. Proteomic investigations into the largemouth bass hypothalamus revealed that proteins responsive to dieldrin are associated with neurodegenerative diseases such as microtubule associate protein tau, myelin basic protein, ATP synthase subunit proteins, and lactate dehydrogenase (Martyniuk et al., 2010). Thus, OCPs such as dieldrin may alter cell structure in the teleostean CNS, impairing neuronal function.
There is other direct evidence for negative effects of OCPs in the CNS, Mortenson and Arukwe investigated the thyroid hormone system response in juvenile immature Atlantic salmon with waterborne exposure to 10 μg DDE/L for 5 days (Mortensen and Arukwe, 2006). The study revealed that in the brain of salmon, DDE acted to blunt the expression of thyroid receptor alpha mRNA in response to T4. DDE had no effect on thyroid receptor beta mRNA. The study revealed that, within the brain, OCPs like DDE can disrupt the expression of thyroid dependent genes.
Endosulfan, which is a broad spectrum OCP, has been shown to affect acetylcholinesterase activity in adult zebrafish (Pereira et al., 2012). The specific activity of acetylcholinesterase was decreased at a concentration of 2.4 μg endosulfan/L over 96 hours. This also corresponded to impaired swimming performance and there was a decrease in distance travelled and mean speed of the zebrafish following this treatment.
As a final example, OCPs can directly affect GnRH and gonadotropin cell populations as demonstrated in the cichlid fish Cichlasoma dimerus (Piazza et al., 2011). Larval fish exposed to 0.1 μg endosulfan/L showed decreased signal for GnRH (nucleus/cytoplasm ratio) and an increase in nuclear area of FSH beta positive cells, supporting the idea that some OCPs can affect neuroendocrine cells directly.
Taken together, an array of neurotransmitter and neuroendocrine systems appear to be impacted by OCPs in fish. This is important as wild populations have been chronically exposed to these legacy agrochemicals for many years; the long-term impact of such exposures on neuroendocrine systems, neurological function, and behavior in these animals have yet to be determined.
3.2. Reproductive Axis
OCPs are known endocrine disrupting chemicals that can bioaccumulate in reproductive organs and alter various aspects of reproductive physiology and sexual behavior (Barni et al., 2014, Feist et al., 2005, Johnson et al., 2007, Ree and Payne, 1997). For example, endosulfan did affect the behavior of cichlid fish as well as their liver and testicular morphology (Da Cuna et al., 2011).
One of the most common adverse outcomes associated with OCPs exposure is the abnormal production of sex steroid hormones. For example, testosterone has been widely associated with male courting and reproductive behavior (Mangiamele and Thompson, 2012). Both laboratory-and field-based studies have shown that OCPs can suppress the levels of testosterone in juveniles and reproductive adult fish. For example, ray-finned fish exposed to endosulfan for 30 days exhibited significantly lower levels of testosterone in testicular tissues compared to unexposed animals (Islam et al., 2017). In a field survey of immature white sturgeon, Feist et al. (Feist et al., 2005) reported a correlation between reduced levels of testosterone and high concentration of DDE and other OCPs in gonadal and liver tissues. Similarly, wild male catfish and tilapia caught in basins polluted with endosulfan, heptachlor and DDT exhibited reduced levels of testosterone in the plasma (Agbohessi et al., 2015). These findings correspond to mammalian studies that describe the anti-androgenic effects of OCPs and their impact on male sex hormones (Du et al., 2014, Fowler et al., 2007, Murono et al., 2006).
OPCs can also act as weak estrogens and alter the production of female-specific hormones and proteins. The increase of vtg in the plasma of reproductive male fish has been associated with exposure to methoxychlor, dieldrin, p,p’-DDE, o,p’-DDT and β-endosulfan (Han et al., 2011, Hemmer et al., 2001, Larkin et al., 2002, Versonnen et al., 2004). This precursor of yolk proteins is synthesized in the liver of reproductive females. Following exposure to OCPs such as dieldrin and p,p’-DDE, increased gene expression of vtg has been observed in livers of male largemouth bass and male zebrafish (Garcia-Reyero et al., 2006, Sun et al., 2016). In another study, juveniles exposed to p,p’-DDE during gonadal development expressed abnormally high levels of vtg in the plasma (Monteiro et al., 2015). These results provide further evidence of the estrogenic potency of OCPs.
Other established markers of estrogenicity such as 17β-estradiol (E2), can be perturbed by these chemicals in both males and females. However, data available suggest that the effects on E2 production may vary depending on the fish species, pesticides, and exposure duration. For example, reports of suppressed E2 levels were found in both sexes of the largemouth bass chronically exposed to dieldrin or DDE under laboratory conditions (Garcia-Reyero et al., 2006, Martyniuk et al., 2013). In contrast, wild male and female tilapia and catfish caught in an OCP-polluted river exhibited high E2 levels (Agbohessi et al., 2015). These discrepancies suggest that differences in OCP mixture composition and exposure duration can have different effects on the estrogen biosynthesis pathway. Additional studies are needed to better understand OCPs modes of actions and nonmonotonic responses in fish.
The regulation of estrogenic and androgenic pathways is tightly linked to gonadal tissue development, gamete production, fecundity and fertility. Alterations of these biological processes have been documented in OCP-contaminated organisms. In male fish, spermatogenesis appears to be sensitive to OCPs exposure. Dutta et al. (Dutta et al., 2006) reported damages to Sertoli cells and reduction in the number of Leydig cells in bluegill fish exposed to endosulfan for 2 weeks. These two types of cells play an important role in testosterone synthesis. Hence, structural and functional damages to these cells could explain the reduction in plasma testosterone levels frequently observed in OCP-exposed male fish. The decrease in circulating testosterone can in turn reduce the maturation and proliferation of sperm. This hypothesis is supported by Agbohessi et al. who measured low numbers of mature spermatozoa in the testes of wild catfish and tilapia exposed to environmental mixtures of OCPs (Agbohessi et al., 2015). However, Da Cuña et al. treated C. dimerus with low levels of endosulfan and found a build-up of spermatozoa in the gonads (Da Cuna et al., 2011). This study was conducted over 4 days and it is possible that reduced maturation and number of sperm occur over longer exposure periods. Acute exposure has led to poor quality of spermatozoa as evidenced in a study conducted by Das Neves et al. in which African catfish exposed to methoxychlor for 4 days showed increased vacuolization of the male gametes (Das Neves et al., 2018). Some laboratory studies have also reported the development of ova-testes in male fish subjected to prolonged exposure to pesticides such as p,p’ DDE and endosulfan (Han et al., 2011, Sun et al., 2016). It should be noted that the incidence and severity of testicular lesions were often dose dependent.
While most of the research on OPC-induced reproductive toxicity has been conducted in male fish, there is some evidence that OCPs may have similar effects on fish ovarian tissues. For example, female African catfish exposed to dieldrin for 30 days led to a delay in gonad maturation, and elevated the number of immature oocytes (Lamai et al., 1999). Other researchers have reported follicular atresia, disorganization of ovarian tissues and reduced number of viable eggs laid (Agbohessi et al., 2015, Gormley and Teather, 2003, Ree and Payne, 1997). The impact of OPCs on egg quality is not surprising. These lipophilic pesticides are known to accumulate in the gonads and may be transferred to the embryos, thus affecting their development. This mechanism may also explain some of the teratogenic effects of OCPs on early life stages that were discussed above in section 2.
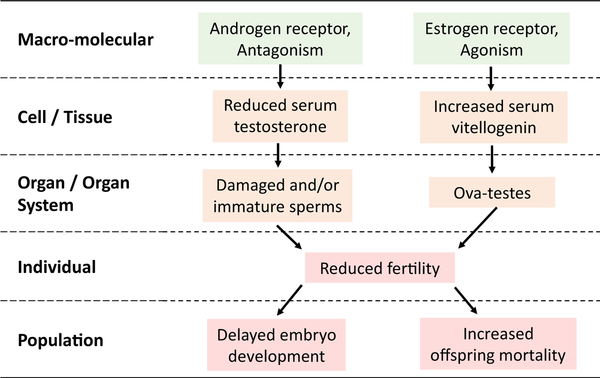
Based on the data discussed in this review, we propose an adverse outcome pathway to describe the impact of OCPs on male reproduction (Figure 3). The available data on molecular and apical endpoints suggest that prolonged exposure to environmental levels of OCPs (in low to mid ng/L range) is likely to disrupt sex hormone production, including a reduction of testosterone. At the same time there is an increase in vitellogenin, suggesting agonism of the the estrogen receptor. These changes lead to impaired sperm cell maturation and release, and reduced fecundity. Current experimental data are less complete for female reproduction compared to males, making it difficult to construct a similar AOP for females; thus we focus only on males.
Figure 3.
Proposed adverse outcome pathway based on the disruption of the androgen and estrogen receptor activities in fish testes by organochlorine pesticides.
3.3. Immune Dysfunction
There are a few studies with species of high ecological and economic relevance, such as alligators (Rooney et al., 2003) and largemouth bass (Martyniuk et al., 2016b) that show a connection between OCP exposure and immune dysfunction. A 4-month mesocosm exposure of largemouth bass to OCP mixtures present in the muck farms of central Florida north of Lake Apopka had significant effects on the immune system, in addition to the expected alterations in the reproductive axis (Martyniuk et al., 2016b). In this study, transcriptomics analysis via microarray indicated significant changes in transcripts involved in inflammatory response, platelet function, complement activation, macrophage activation and response, lymphocyte proliferation, eosinophil chemotaxis, T-cell tolerance, agglutination and hemagglutination, all of which relate to the immune system. Subsequent laboratory-based dietary exposures using food pellets spiked with p,p’DDE and methoxychlor also led to similar findings (Martyniuk et al., 2016a). Fish treated with OPC-contaminated diet for 3 months exhibited significant alterations of transcripts involved in immune function including acute phase reaction, platelet function and inhibition and these transcript changes were related to immune system diseases such as Lupus Nephritis.
Several studies have documented the decrease of leukocytes in the head kidney of fish treated with OCPs and decrease of apoptosis of granulocytes (Cuesta et al., 2008, Milston et al., 2003, Misumi et al., 2005). Increased plasma cortisol suppresses immune function in fish (Ainsworthet al., 1991, Pickering and Pottinger, 1989, Thomas et al., 1987) by depressing circulating lymphocytes and decreasing lymphocyte proliferation (Narnaware and Baker, 1996, Weyts et al., 1997). Other effects reported include decreased phagocytosis and lymphocyte mitogenesis, as well as increased apoptosis of B cells (Pulsford et al., 1994, Weyts et al., 1998, Weyts et al., 1997). Other studies suggest that cortisol synthesis in response to a stressor may be depressed by exposure to OCPs (Benguira et al., 2002).
Potential targets of endocrine disruption in the hypothalamus-pituitary-adrenal (HPA) axis includes adrenocorticotropic hormone (ACTH), which acts on sensitive tissues to release cortisol and corticosterone (Bonga, 1997). Although there is sparse information on mechanisms by which OCPs alter the immune response, o,p-DDD and p,p’-DDE suppressed the response to ACTH by interrenal tissues in the cichlid fish, Sarotherodon aureus (Ilan and Yaron, 1980), interfering with cortisol secretion. This in turn could interfered with glucocorticoid signaling since cortisol in a primary agonist of the glucocorticoid receptor. While zebrafish have only one glucocorticoid receptor (Alsop and Vijayan, 2008), other fish possess two instead of one (Meyer and Van de Peer, 2005, Prunet et al., 2006), each featuring alternative splice variants (Schaaf et al., 2008) making generalizations across fish species difficult. The glucocorticoid receptor(s) regulate genes involved with the immune system, as well as glucose metabolism, stress response, blood pressure and osmoregulation (Aluru and Vijayan, 2009, Burnstein and Cidlowski, 1989, Veillette et al., 2007, Weyts et al., 1999).
It should be noted that immunotoxicity of OCPs is well documented in mammalian models and this aligns with what we and others have reported in fish. For example, exposure to three different OCPs, chlordecone, methoxychlor and o,p’-DDT were shown to accelerate the development of systemic lupus erythematosus in ovariectomized mice (Sobel et al., 2005). The mice used in the study were female (NZB X NZW), a hybrid strain of New Zealand mice bred specifically for their susceptibility to lupus erythematosus. OCPs were administered via implantable pellets for a period of 8 weeks. The mice developed renal disease that was confirmed to be immune complex glomerulonephritis, a hallmark for lupus. The disease acceleration was most striking for chlordecone, in this study, making the link of OCP exposure of farmworkers and lupus, more tractable.
It is evident that more mechanistic research is needed to elucidate the effects of OCPs on immune system in fish and other aquatic organisms. Evidence collected to date suggests that fish respond to OCPs in ways that are similar to higher vertebrates including reduced leukocytes and lymphocytes, and lupus like symptoms. More in-depth studies are needed using aquatic organisms to identify the molecular initiating and key events involved in immune suppression and autoimmune disorders that are typically associated with OCPs exposure in mammals.
3.4. Lipids and metabolism
The transcriptomics studies conducted with largemouth bass in the field and the laboratory have indicated major alterations in lipid biosynthesis, lipid transport and lipid metabolism pathways (Martyniuk et al., 2016a, Martyniuk et al., 2016b). While lipids are mainly known for their role in membrane composition and as a reservoir for energy, there are many other roles for lipids as well in which they function as signaling molecules made on site and which are important for reproduction and immune function. Thus, many of the adverse effects noted above can also be a result of disrupted lipid metabolism. Lipids include a wide array of molecules (in the thousands) that classify based on their structures into various groups with phospholipids making an important mechanistic group. The advent of lipidomics as another OMIC method has enabled researchers to study changes in lipid moieties in relation to physiological competence of organisms (Drier et al., in review). That OCP exposure can change transcripts involved in lipid metabolism suggested that this cellular compartment is also important to consider.
Recent published studies suggest the immune system is linked to specific cellular lipid profiles (Koberlin et al., 2015), with key changes in ceramides and sphingolipids playing central roles in the cellular effects that occur after the recognition of viruses and bacteria through the toll-like receptors. Other lipids such as diacylglycerols and phospholipids can be converted into eicosanoids, which are also involved in inflammation, immune suppression and CNS function (Balazy, 2004). Lysophospholipids have also been defined as having important biological action in a number of conditions including immune function, pain and cancer (Wepy et al., 2019). More research is needed to identify how lipid metabolism fits into both immune and reproductive dysfunction for fish. This has not been studied adequately in fish to date.
Many agrochemicals affect metabolic processes, altering key processes such as glycolysis, oxidative phosphorylation, fatty acid utilization, and other biochemical pathways. Organochlorine pesticides are also reported to affect metabolic capacity and oxidative respiration in a diverse range of fish species. Endosulfan for example, alters the activity of malate dehydrogenase (MDH) in the skeletal muscle of freshwater catfish Clarias batrachus (Mishra and Shukla, 2003). The authors proposed that a decrease in the activity of MDH suggests a decline in the efficiency of aerobic energy metabolism, which can affect skeletal muscle function. A loss of activity of MDH was also reported in the liver of channel catfish exposed to levels of 2.5 nM - 7.4 nM endosulfan (1 to 3 ug endosulfan/L, respectively) for seven days (Mishra and Shukla, 1997). In another experiment, freshwater catfish exposed to 0.06 mg endosulfan/L for 21 days resulted in decreased activity of enzymes associated with glycolysis (Tripathi and Verma, 2004). More specifically, endosulfan decreased the activity of citrate synthase (CS) and glucose 6-phosphate dehydrogenase (G6-PDH) in the brain, liver and skeletal muscle as well as brain lactate dehydrogenase (LDH).
In the fathead minnow, p,p’-DDT (ranging from 0.5 to 2 ug p,p’-DDT/L) affected ATPase activity in the brain of fish and there was a decline of 43% in oligomycin-sensitive mitochondrial magnesium 2+ ATPase (Desaiah et al., 1975). Inhibition of brain ATPase by DDT was noted at 118 and 266 days in fish chronically exposed to DDT in water and food in the ppb range (ug/L). Negative effects of OCPs on energy production in the central nervous system are supported by transcriptome studies in zebrafish, which point to the mitochondria as a significant target for organochlorine pesticides such as dieldrin (Cowie et al., 2017). Taken together, reduced ATP capacity and impaired metabolism are expected to impact endocrine function and overall health of fish.
4. Biomarkers of exposure: Focus on hormone signaling pathways
4.1. Comparative toxicogenomics database
Bioinformatics can uncover novel molecular signaling pathways related to organochlorine pesticide susceptibility. To determine if there were novel and conserved pathways affected by select OCPs, we retrieved publicly available data from the Comparative Toxicogenomics Database (Davis et al., 2019) (downloaded 2019-06-24). Pesticides queried included DDT, p,p’ DDE, dieldrin, methoxychlor, and toxaphene and pathway data were compiled (Supplemental Data 1). Using such an approach is useful for identifying new endocrine disruptors (Basili et al., 2018) and can unite biomarker molecules by shared factors and functional classification. We identified a number of enriched endocrine-related pathways (P<0.001) in the CTD that are affected by organochlorine pesticides at the gene level (Supplemental Table S1). These included signaling pathways related to reproduction (gonadotropins, estradiol, androgen, steroid biosynthesis, oxytocin), thyroid hormone, and insulin. These pathways were significantly enriched for all five OCPs, and clearly suggests that reproductive processes are sensitive to OCP exposure along the hypothalamic-pituitary-gonadal axis. Noteworthy is that thyroid hormone signaling was identified; however, unlike reproduction, there is less known about OCP disruption in thyroid system. Transcripts involved in estrogens signaling included estrogen receptors, heat shock proteins, and MAP kinases to name but a few. Hormones such as estrogens and progestins are intimately related to immune function, and OCPs may have affects in immune system at the gene level via estrogenic and androgenic signaling. This will be an exciting avenue of research in the future, the role of agrichemicals in modulating immune function through hormone signaling.
4.2. Insight from the Tox21 and ToxCast Screening Program
Additional evidence for organochlorine pesticides as endocrine disruptors comes from computational efforts and the Tox21 and ToxCast chemical screening programs. Using the EPA CompTox Chemicals Dashboard (version 3.0.8, released May 10th, 2019), we further elucidated potential mechanisms for select OCPs, including dieldrin, DDT, methoxychlor, and toxaphene. These high-throughput cell-based reporter assays are used to determine the potential of a chemical for endocrine disruption, as well as cytotoxicity, aryl hydrocarbon receptor activation, mitochondrial dysfunction, and oxidative stress among other modes of action. A number of organochlorine pesticides have been tested in these cell-based screening assays. Based upon the ToxCast and Chemical Activity Summaries, the data indicate that these agrochemicals activate or inhibit estrogen and androgen receptor activity (Table 1), and OCPs activate or inhibit nuclear receptors below the AC50 for cytotoxicity (Figure 4).
Table 1.
ToxCast and Chemical Activity Summary for OCPs. Organochlorine pesticides depicted in the table have significant estrogenic and anti-androgenic effects.
| Chemical | MODEL | RECEPTOR | AGONIST | ANTAGONIST | BINDING |
|---|---|---|---|---|---|
| Dieldrin | ToxCast Pathway Model (AUC) | Androgen | 0.00 | 4.15e-2 | - |
| ToxCast Pathway Model (AUC) | Estrogen | 1.81e-2 | 0.00 | - | |
| COMPARA (Consensus) | Androgen | Inactive | Active | Inactive | |
| CERAPP Potency Level (From Literature) | Estrogen | - | Inactive (Inactive) | Active (NaN) | |
| CERAPP Potency Level (Consensus) | Estrogen | Inactive (Inactive) | Inactive (Inactive) | Inactive (Inactive) | |
| DDT | ToxCast Pathway Model (AUC) | Androgen | 0.00 | 6.42e-2 | - |
| ToxCast Pathway Model (AUC) | Estrogen | 0.190 | 0.00 | - | |
| COMPARA (Consensus) | Androgen | Inactive | Active | Active | |
| CERAPP Potency Level (From Literature) | Estrogen | Active (Weak) | - | Active (Weak) | |
| CERAPP Potency Level (Consensus) | Estrogen | Active (VeryWeak) | Active (Moderate) | Active (VeryWeak) | |
| Methoxychlor | ToxCast Pathway Model (AUC) | Androgen | 0.00 | 4.29e-2 | - |
| ToxCast Pathway Model (AUC) | Estrogen | 0.254 | 0.00 | - | |
| COMPARA (Consensus) | Androgen | Inactive | Active | Active | |
| CERAPP Potency Level (From Literature) | Estrogen | Active (Weak) | - | Active (Weak) | |
| CERAPP Potency Level (Consensus) | Estrogen | Active (VeryWeak) | Active (Weak) | Active (VeryWeak) |
Figure 4.
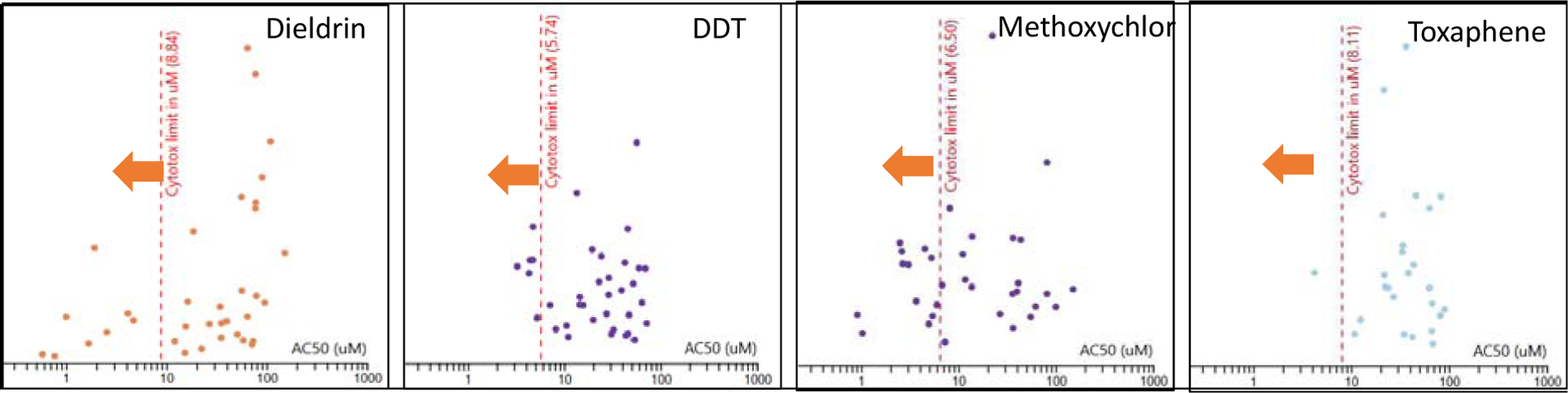
ToxCast and Chemical Activity Summary for selected organochlorine pesticides. Depicted are the ‘active” calls for assays designed to test nuclear receptor activity. All assays on the left side of the dotted line indicated by the orange arrow are active at concentrations below observed cytotoxicity. EPA CompTox Chemicals Dashboard (version 3.0.8, released May 10th, 2019).
Currently, little is known about the impact of organochlorine pesticides on retinoic acid signaling cascades in teleost fishes, which is important for thyroid hormone signaling and gene expression activation/inhibition. This is a knowledge gap that should be addressed more completely as thyroid hormone is critical for normal development and metabolism. In fact, exposure to organochlorine pesticides during early fish development can lead to adverse effects that involve metabolism and neuroendocrine signaling. For example, zebrafish exposed to toxaphene during development exhibit reduced oxygen consumption rates for non-mitochondrial respiration, a process that can lead to energy deficits with the embryo (Perez-Rodriguez et al., 2019). The study also reported developmental defects that included pericardial edema and skeletal deformities, hypothesized to be linked changes in oxygen consumption rate. Further studies should examine the effect to the organochlorine pesticides on developing eggs; this is relevant as OCPs settle into sediment, bind organic matter, and remain bound for many years, resistant to degradation. Sediment in many cases offers the ideal substrate for fish embryonic development and can lead to aberrant development trajectories in sensitive fish species. This has been observed with dieldrin exposure and zebrafish (Sarty et al., 2017).
5. Conclusions
Organochlorine pesticides remain problematic in contaminated environments and discussion on how to best remove the chemicals or reduce exposure to these chemicals is vigorously debated. Research by many over several years has revealed that these pesticides (1) affect early development of fish as teratogenic compounds, (2) act as neuroendocrine disruptors, (3) suppress male and female reproductive systems, (4) dysregulate immune functions and lipid biosynthesis, and (5) alter metabolic function. At the molecular level, these chemicals disrupt signaling pathways related to reproduction (gonadotropins, estradiol, androgen, steroid biosynthesis, oxytocin), thyroid hormone, and insulin. Weight of evidence from laboratory and field experiments, as well as insights from the Tox21 and ToxCast Screening Program indicate that these agrochemicals activate and antagonize estrogen receptors, androgen receptors, and retinoic acid receptors with relatively high affinity, although there are differences in potency of activation among organochlorine pesticides. Despite being banned, it is important to remain diligent on assessing risks for OCP exposure as these chemicals are long-lasting, persistent, and bioaccumulate to toxic levels. Future studies should evaluate multi-generational effects of such exposure, to understand how these chemicals impact molecular pathways and organismal health in the long term.
Supplementary Material
Acknowledgements:
This research was funded by a National Institutes of Health Pathway to Independence Award granted to C.J.M. (K99 ES016767) and by the Superfund Basic Research Program from the National Institute of Environmental Health Sciences to N.D. and D.B. (RO1 ES015449).
Abbreviations:
- ACTH
Adrenocorticotropic hormone
- ATSDR
Agency for Toxic Substances and Disease Registry
- CNS
Central nervous system
- CS
Citrate synthase
- DDD
Dichlorodiphenyldichloroethane
- DDE
Dichlorodiphenyldichloroethylene
- DDT
Dichlorodiphenyltrichloroethane
- DDXs
Combination of DDT, DDE, DDD
- DOPAC
3,4-dihydroxyphenylacetic acid
- Drins
Combination of dieldrin, aldrin, endrin
- E2
17β-estradiol
- G6-PDH
Glucose 6-phosphate dehydrogenase
- GABA
Gamma-aminobutyric acid
- HPI
Hypothalamus-pituitary-interrenal
- LDH
Lactate dehydrogenase
- LH
Luteinizing hormone
- MDH
Malate dehydrogenase
- OCP
Organochlorine pesticides
- StAR
Steroidogenic acute regulatory protein
- TCDD
2,3,7,8-Tetrachlorodibenzo-p-dioxin
- Vtg
Vitellogenin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of interest: none
REFERENCES
- Agbohessi PT, Imorou Toko I, Ouedraogo A, Jauniaux T, Mandiki SN and Kestemont P, 2015. Assessment of the health status of wild fish inhabiting a cotton basin heavily impacted by pesticides in Benin (West Africa), Sci Total Environ. 506–507, 567–84. [DOI] [PubMed] [Google Scholar]
- Ainsworth AJ, Dexiang C, Waterstrat PR and Greenway T, 1991. Effect of temperature on the immune system of channel catfish (Ictalurus punctatus)--I. Leucocyte distribution and phagocyte function in the anterior kidney at 10 degrees C, Comp Biochem Physiol A Comp Physiol. 100, 907–12. [DOI] [PubMed] [Google Scholar]
- Aktar MW, Sengupta D and Chowdhury A, 2009. Impact of pesticides use in agriculture: their benefits and hazards, Interdiscip Toxicol. 2, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsop D and Vijayan MM, 2008. Development of the corticosteroid stress axis and receptor expression in zebrafish, Am J Physiol Regul Integr Comp Physiol. 294, R711–9. [DOI] [PubMed] [Google Scholar]
- Aluru N and Vijayan MM, 2009. Stress transcriptomics in fish: A role for genomic cortisol signaling, General and Comparative Endocrinology. 164, 142–150. [DOI] [PubMed] [Google Scholar]
- Balaguer P, Francois F, Comunale F, Fenet H, Boussioux AM, Pons M, Nicolas JC and Casellas C, 1999. Reporter cell lines to study the estrogenic effects of xenoestrogens, Sci Total Environ. 233, 47–56. [DOI] [PubMed] [Google Scholar]
- Balazy M, 2004. Eicosanomics: targeted lipidomics of eicosanoids in biological systems, Prostaglandins Other Lipid Mediat. 73, 173–80. [DOI] [PubMed] [Google Scholar]
- Barni MF, Gonzalez M and Miglioranza KS, 2014. Assessment of persistent organic pollutants accumulation and lipid peroxidation in two reproductive stages of wild silverside (Odontesthes bonariensis), Ecotoxicol Environ Saf. 99, 45–53. [DOI] [PubMed] [Google Scholar]
- Basili D, Zhang JL, Herbert J, Kroll K, Denslow ND, Martyniuk CJ, Falciani F and Antczak P, 2018. In Silico Computational Transcriptomics Reveals Novel Endocrine Disruptors in Largemouth Bass (Micropterus salmoides), Environ Sci Technol. 52, 7553–7565. [DOI] [PubMed] [Google Scholar]
- Bearden DM, 2012. Comprehensive Environmental Response, Compensation, and Liability Act: A Summary of Superfund Cleanup Authorities and Related Provisions of the Act, report, June 14, 2012; Washington D.C.. (https://digital.library.unt.edu/ark:/67531/metadc93822/:. accessed August 18, 2019), University of North Texas Libraries, Digital Library, https://digital.library.unt.edu; crediting UNT Libraries Government Documents Department. [Google Scholar]
- Benguira S, Leblond VS, Weber JP and Hontela A, 2002. Loss of capacity to elevate plasma cortisol in rainbow trout (Oncorhynchus mykiss) treated with a single injection of o,p’-dichlorodiphenyldichloroethane, Environ Toxicol Chem. 21, 1753–6. [PubMed] [Google Scholar]
- Bonga SEW, 1997. The stress response in fish, Physiological Reviews. 77, 591–625. [DOI] [PubMed] [Google Scholar]
- Bonnineau C, Scaion D, Lemaire B, Belpaire C, Thome JP, Thonon M, Leermaker M, Gao Y, Debier C, Silvestre F, Kestemont P and Rees JF, 2016. Accumulation of neurotoxic organochlorines and trace elements in brain of female European eel (Anguilla anguilla), Environ Toxicol Pharmacol. 45, 346–55. [DOI] [PubMed] [Google Scholar]
- Brown SB, Adams BA, Cyr DG and Eales JG, 2004. Contaminant effects on the teleost fish thyroid, Environ Toxicol Chem. 23, 1680–701. [DOI] [PubMed] [Google Scholar]
- Burnstein KL and Cidlowski JA, 1989. Regulation of Gene-Expression by Glucocorticoids, Annual Review of Physiology. 51, 683–699. [DOI] [PubMed] [Google Scholar]
- Cao F, Martyniuk CJ, Wu P, Zhao F, Pang S, Wang C and Qiu L, 2019. Long-Term Exposure to Environmental Concentrations of Azoxystrobin Delays Sexual Development and Alters Reproduction in Zebrafish (Danio rerio), Environ Sci Technol. 53, 1672–1679. [DOI] [PubMed] [Google Scholar]
- Chow WS, Chan WK and Chan KM, 2013. Toxicity assessment and vitellogenin expression in zebrafish (Danio rerio) embryos and larvae acutely exposed to bisphenol A, endosulfan, heptachlor, methoxychlor and tetrabromobisphenol A, J Appl Toxicol. 33, 670–8. [DOI] [PubMed] [Google Scholar]
- Cohn BA, Cirillo PM and Terry MB, 2019. DDT and Breast Cancer: Prospective Study of Induction Time and Susceptibility Windows, J Natl Cancer Inst. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowie AM, Sarty KI, Mercer A, Koh J, Kidd KA and Martyniuk CJ, 2017. Molecular networks related to the immune system and mitochondria are targets for the pesticide dieldrin in the zebrafish (Danio rerio) central nervous system, J Proteomics. 157, 71–82. [DOI] [PubMed] [Google Scholar]
- Cowie AM, Sarty KI, Mercer A, Koh J, Kidd KA and Martyniuk CJ, 2017. The pesticide dieldrin disrupts proteins related to oxidative respiration and mitochondrial stress in the central nervous system, Data Brief. 11, 628–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuesta A, Meseguer J and Angeles Esteban M, 2008. Effects of the organochlorines p,p’-DDE and lindane on gilthead seabream leucocyte immune parameters and gene expression, Fish Shellfish Immunol. 25, 682–8. [DOI] [PubMed] [Google Scholar]
- Da Cuna RH, Rey Vazquez G, Piol MN, Guerrero NV, Maggese MC and Lo Nostro FL, 2011. Assessment of the acute toxicity of the organochlorine pesticide endosulfan in Cichlasoma dimerus (Teleostei, Perciformes), Ecotoxicol Environ Saf. 74, 1065–73. [DOI] [PubMed] [Google Scholar]
- Dang VD, Kroll KJ, Supowit SD, Halden RU and Denslow ND, 2016. Tissue distribution of organochlorine pesticides in largemouth bass (Micropterus salmoides) from laboratory exposure and a contaminated lake, Environ Pollut. 216, 877–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das Neves J, Barnhoorn IEJ and Wagenaar GM, 2018. The effects of environmentally relevant concentrations of aldrin and methoxychlor on the testes and sperm of male Clarias gariepinus (Burchell, 1822) after short-term exposure, Fish Physiol Biochem. 44, 1421–1434. [DOI] [PubMed] [Google Scholar]
- Das S and Thomas P, 1999. Pesticides interfere with the nongenomic action of a progestogen on meiotic maturation by binding to its plasma membrane receptor on fish oocytes, Endocrinology. 140, 1953–6. [DOI] [PubMed] [Google Scholar]
- Davis AP, Grondin CJ, Johnson RJ, Sciaky D, McMorran R, Wiegers J, Wiegers TC and Mattingly CJ, 2019. The Comparative Toxicogenomics Database: update 2019, Nucleic Acids Res. 47, D948–D954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desaiah D, Cutkomp LK, Koch RB and Jarvinen A, 1975. DDT: effect of continuous exposure on ATPase activity in fish, Pimephales promelas, Arch Environ Contam Toxicol. 3, 132–41. [DOI] [PubMed] [Google Scholar]
- Du X, Zhang H, Liu Y, Yu W, Huang C and Li X, 2014. Perinatal exposure to low-dose methoxychlor impairs testicular development in C57BL/6 mice, PLoS One. 9, e103016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta HM, Misquitta D and Khan S, 2006. The effects of endosulfan on the testes of bluegill fish, Lepomis macrochirus: a histopathological study, Arch Environ Contam Toxicol. 51, 149–56. [DOI] [PubMed] [Google Scholar]
- Edmunds JS, McCarthy RA and Ramsdell JS, 2000. Permanent and functional male-to-female sex reversal in d-rR strain medaka (Oryzias latipes) following egg microinjection of o,p’-DDT, Environ Health Perspect. 108, 219–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdo F, Denes L and de Lange E, 2017. Age-associated physiological and pathological changes at the blood-brain barrier: A review, J Cereb Blood Flow Metab. 37, 4–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feist GW, Webb MA, Gundersen DT, Foster EP, Schreck CB, Maule AG and Fitzpatrick MS, 2005. Evidence of detrimental effects of environmental contaminants on growth and reproductive physiology of white sturgeon in impounded areas of the Columbia River, Environ Health Perspect. 113, 1675–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler PA, Abramovich DR, Haites NE, Cash P, Groome NP, Al-Qahtani A, Murray TJ and Lea RG, 2007. Human fetal testis Leydig cell disruption by exposure to the pesticide dieldrin at low concentrations, Hum Reprod. 22, 2919–27. [DOI] [PubMed] [Google Scholar]
- Garcia-Reyero N, Barber DS, Gross TS, Johnson KG, Sepulveda MS, Szabo NJ and Denslow ND, 2006. Dietary exposure of largemouth bass to OCPs changes expression of genes important for reproduction, Aquat Toxicol. 78, 358–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Reyero N, Jayasinghe BS, Kroll KJ, Sabo-Attwood T and Denslow ND, 2018. Estrogen signaling through both membrane and nuclear receptors in the liver of fathead minnow, Gen Comp Endocrinol. 257, 50–66. [DOI] [PubMed] [Google Scholar]
- Gormley KL and Teather KL, 2003. Developmental, behavioral, and reproductive effects experienced by Japanese medaka (Oryzias latipes) in response to short-term exposure to endosulfan, Ecotoxicol Environ Saf. 54, 330–8. [DOI] [PubMed] [Google Scholar]
- Han Z, Jiao S, Kong D, Shan Z and Zhang X, 2011. Effects of beta-endosulfan on the growth and reproduction of zebrafish (Danio rerio), Environ Toxicol Chem. 30, 2525–31. [DOI] [PubMed] [Google Scholar]
- Hemmer MJ, Hemmer BL, Bowman CJ, Kroll KJ, Folmar LC, Marcovich D, Hoglund MD and Denslow ND, 2001. Effects of p-nonylphenol, methoxychlor, and endosulfan on vitellogenin induction and expression in sheepshead minnow (Cyprinodon variegatus), Environ Toxicol Chem. 20, 336–43. [DOI] [PubMed] [Google Scholar]
- Ilan Z and Yaron Z, 1980. Suppression by Organochlorines of the Response to Adrenocorticotropin of the Interrenal Tissue in Sarotherodon-Aureus (Teleostei), Journal of Endocrinology. 87, 185–193. [DOI] [PubMed] [Google Scholar]
- Islam FU, Jalali S, Shafqat MN and Shah STA, 2017. Endosulfan is toxic to the reproductive health of male freshwater fish, Cyprinion watsoni, Naturwissenschaften. 104, 104. [DOI] [PubMed] [Google Scholar]
- Johnson KG, Muller JK, Price B, Ware A, Sepulveda MS, Borgert CJ and Gross TS, 2007. Influence of seasonality and exposure on the accumulation and reproductive effects of p,p’-dichlorodiphenyldichloroethane and dieldrin in largemouth bass, Environ Toxicol Chem. 26, 927–34. [DOI] [PubMed] [Google Scholar]
- Kapp T, Kammann U, Vobach M and Vetter W, 2006. Synthesis of low and high chlorinated toxaphene and comparison of their toxicity by zebrafish (Danio rerio) embryo test, Environ Toxicol Chem. 25, 2884–9. [DOI] [PubMed] [Google Scholar]
- Kelce WR, Lambright CR, Gray LE Jr. and Roberts KP, 1997. Vinclozolin and p,p’-DDE alter androgen-dependent gene expression: in vivo confirmation of an androgen receptor-mediated mechanism, Toxicol Appl Pharmacol. 142, 192–200. [DOI] [PubMed] [Google Scholar]
- Kelce WR, Stone CR, Laws SC, Gray LE, Kemppainen JA and Wilson EM, 1995. Persistent DDT metabolite p,p’-DDE is a potent androgen receptor antagonist, Nature. 375, 581–5. [DOI] [PubMed] [Google Scholar]
- Koberlin MS, Snijder B, Heinz LX, Baumann CL, Fauster A, Vladimer GI, Gavin AC and Superti-Furga G, 2015. A Conserved Circular Network of Coregulated Lipids Modulates Innate Immune Responses, Cell. 162, 170–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamai SL, Warner GF and Walker CH, 1999. Effects of dieldrin on life stages of the African catfish, Clarias gariepinus (Burchell), Ecotoxicol Environ Saf. 42, 22–9. [DOI] [PubMed] [Google Scholar]
- Langer P, 2010. The impacts of organochlorines and other persistent pollutants on thyroid and metabolic health, Front Neuroendocrinol. 31, 497–518. [DOI] [PubMed] [Google Scholar]
- Larkin P, Sabo-Attwood T, Kelso J and Denslow ND, 2002. Gene expression analysis of largemouth bass exposed to estradiol, nonylphenol, and p,p’-DDE, Comp Biochem Physiol B Biochem Mol Biol. 133, 543–57. [DOI] [PubMed] [Google Scholar]
- Leon-Olea M, Martyniuk CJ, Orlando EF, Ottinger MA, Rosenfeld C, Wolstenholme J and Trudeau VL, 2014. Current concepts in neuroendocrine disruption, Gen Comp Endocrinol. 203, 158–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangiamele LA and Thompson RR, 2012. Testosterone rapidly increases ejaculate volume and sperm density in competitively breeding goldfish through an estrogenic membrane receptor mechanism, Horm Behav. 62, 107–12. [DOI] [PubMed] [Google Scholar]
- Martyniuk CJ, Doperalski NJ, Feswick A, Prucha MS, Kroll KJ, Barber DS and Denslow ND, 2016a. Transcriptional networks associated with the immune system are disrupted by organochlorine pesticides in largemouth bass (Micropterus salmoides) ovary, Aquat Toxicol. 177, 405–16. [DOI] [PubMed] [Google Scholar]
- Martyniuk CJ, Doperalski NJ, Kroll KJ, Barber DS and Denslow ND, 2013. Sexually dimorphic transcriptomic responses in the teleostean hypothalamus: a case study with the organochlorine pesticide dieldrin, Neurotoxicology. 34, 105–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martyniuk CJ, Doperalski NJ, Prucha MS, Zhang JL, Kroll KJ, Conrow R, Barber DS and Denslow ND, 2016b. High contaminant loads in Lake Apopka’s riparian wetland disrupt gene networks involved in reproduction and immune function in largemouth bass, Comp Biochem Physiol Part D Genomics Proteomics. 19, 140–150. [DOI] [PubMed] [Google Scholar]
- Martyniuk CJ, Feswick A, Spade DJ, Kroll KJ, Barber DS and Denslow ND, 2010. Effects of acute dieldrin exposure on neurotransmitters and global gene transcription in largemouth bass (Micropterus salmoides) hypothalamus, Neurotoxicology. 31, 356–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martyniuk CJ, Kroll KJ, Doperalski NJ, Barber DS and Denslow ND, 2010. Genomic and proteomic responses to environmentally relevant exposures to dieldrin: indicators of neurodegeneration?, Toxicol Sci. 117, 190–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martyniuk CJ, Popesku JT, Chown B, Denslow ND and Trudeau VL, 2012. Quantitative proteomics in teleost fish: insights and challenges for neuroendocrine and neurotoxicology research, Gen Comp Endocrinol. 176, 314–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A and Van de Peer Y, 2005. From 2R to 3R: evidence for a fish-specific genome duplication (FSGD), Bioessays. 27, 937–945. [DOI] [PubMed] [Google Scholar]
- Milston RH, Fitzpatrick MS, Vella AT, Clements S, Gundersen D, Feist G, Crippen TL, Leong J and Schreck CB, 2003. Short-term exposure of Chinook salmon (Oncoryhnchus tshawytscha) to o,p-DDE or DMSO during early life-history stages causes long-term humoral immunosuppression, Environ Health Perspect. 111, 1601–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra R and Shukla SP, 1997. Impact of endosulfan on cytoplasmic and mitochondrial liver malate dehydrogenase from the freshwater catfish (Clarias batrachus), Comp Biochem Physiol C Pharmacol Toxicol Endocrinol. 117, 7–18. [DOI] [PubMed] [Google Scholar]
- Mishra R and Shukla SP, 2003. Endosulfan effects on muscle malate dehydrogenase of the freshwater catfish Clarias batrachus, Ecotoxicol Environ Saf. 56, 425–33. [DOI] [PubMed] [Google Scholar]
- Misumi I, Vella AT, Leong JA, Nakanishi T and Schreck CB, 2005. p,p’-DDE depresses the immune competence of chinook salmon (Oncorhynchus tshawytscha) leukocytes, Fish Shellfish Immunol. 19, 97–114. [DOI] [PubMed] [Google Scholar]
- Monteiro MS, Pavlaki M, Faustino A, Rema A, Franchi M, Gediel L, Loureiro S, Domingues I, Rendon von Osten J and Mortagua Velho Maia Soares A, 2015. Endocrine disruption effects of p,p’-DDE on juvenile zebrafish, J Appl Toxicol. 35, 253–60. [DOI] [PubMed] [Google Scholar]
- Mortensen AS and Arukwe A, 2006. The persistent DDT metabolite, 1,1-dichloro-2,2-bis(p-chlorophenyl)ethylene, alters thyroid hormone-dependent genes, hepatic cytochrome P4503A, and pregnane X receptor gene expressions in Atlantic salmon (Salmo salar) Parr, Environ Toxicol Chem. 25, 1607–15. [DOI] [PubMed] [Google Scholar]
- Mrema EJ, Rubino FM, Brambilla G, Moretto A, Tsatsakis AM and Colosio C, 2013. Persistent organochlorinated pesticides and mechanisms of their toxicity, Toxicology. 307, 74–88. [DOI] [PubMed] [Google Scholar]
- Murono EP, Derk RC and Akgul Y, 2006. In vivo exposure of young adult male rats to methoxychlor reduces serum testosterone levels and ex vivo Leydig cell testosterone formation and cholesterol side-chain cleavage activity, Reprod Toxicol. 21, 148–53. [DOI] [PubMed] [Google Scholar]
- Narnaware YK and Baker BI, 1996. Evidence that cortisol may protect against the immediate effects of stress on circulating leukocytes in the trout, Gen Comp Endocrinol. 103, 359–66. [DOI] [PubMed] [Google Scholar]
- Newman TAC, Carleton CR, Leeke B, Hampton MB and Horsfield JA, 2015. Embryonic oxidative stress results in reproductive impairment for adult zebrafish, Redox Biol. 6, 648–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsvik PA and Softeland L, 2018. Metabolic effects of p,p’-DDE on Atlantic salmon hepatocytes, J Appl Toxicol. 38, 489–503. [DOI] [PubMed] [Google Scholar]
- Owens KD and Baer KN, 2000. Modifications of the topical Japanese medaka (Oryzias latipes) embryo larval assay for assessing developmental toxicity of pentachlorophenol and p, p’-dichlorodiphenyltrichloroethane, Ecotoxicol Environ Saf. 47, 87–95. [DOI] [PubMed] [Google Scholar]
- Peakall DB, 1970. p,p’-DDT: effect on calcium metabolism and concentration of estradiol in the blood, Science. 168, 592–4. [DOI] [PubMed] [Google Scholar]
- Pereira VM, Bortolotto JW, Kist LW, Azevedo MB, Fritsch RS, Oliveira Rda L, Pereira TC, Bonan CD, Vianna MR and Bogo MR, 2012. Endosulfan exposure inhibits brain AChE activity and impairs swimming performance in adult zebrafish (Danio rerio), Neurotoxicology. 33, 469–75. [DOI] [PubMed] [Google Scholar]
- Perez-Rodriguez V, Nan W, Cova A, Schmidt J, Denslow ND and Martyniuk CJ, 2019. The organochlorine pesticide toxaphene reduces non-mitochondrial respiration and induces heat shock protein 70 expression and in early-stage zebrafish (Danio rerio), Comp. Biochem, Physiol C. Comp Biochem Physiol C Toxicol Pharmacol., in revision. [DOI] [PubMed]
- Piazza YG, Pandolfi M and Lo Nostro FL, 2011. Effect of the organochlorine pesticide endosulfan on GnRH and gonadotrope cell populations in fish larvae, Arch Environ Contam Toxicol. 61, 300–10. [DOI] [PubMed] [Google Scholar]
- Pickering AD and Pottinger TG, 1989. Stress responses and disease resistance in salmonid fish: Effects of chronic elevation of plasma cortisol, Fish Physiol Biochem. 7, 253–8. [DOI] [PubMed] [Google Scholar]
- Prunet P, Sturm A and Milla S, 2006. Multiple corticosteroid receptors in fish: From old ideas to new concepts, General and Comparative Endocrinology. 147, 17–23. [DOI] [PubMed] [Google Scholar]
- Pulsford AL, Lemairegony S, Tomlinson M, Collingwood N and Glynn PJ, 1994. Effects of Acute Stress on the Immune-System of the Dab, Limanda-Limanda, Comparative Biochemistry and Physiology C-Pharmacology Toxicology & Endocrinology. 109, 129–139. [Google Scholar]
- Ree GE and Payne JF, 1997. Effect of toxaphene on reproduction of fish, Chemosphere. 34, 855–67. [DOI] [PubMed] [Google Scholar]
- Rooney AA, Bermudez DS and Guillette LJ Jr., 2003. Altered histology of the thymus and spleen in contaminant-exposed juvenile American alligators, J Morphol. 256, 349–59. [DOI] [PubMed] [Google Scholar]
- Sarty KI, Cowie A and Martyniuk CJ, 2017. The legacy pesticide dieldrin acts as a teratogen and alters the expression of dopamine transporter and dopamine receptor 2a in zebrafish (Danio rerio) embryos, Comparative Biochemistry and Physiology C-Toxicology & Pharmacology. 194, 37–47. [DOI] [PubMed] [Google Scholar]
- Schaaf MJM, Champagne D, van Laanen IHC, van Wijk DCWA, Meijer AH, Meijer OC, Spaink HP and Richardson MK, 2008. Discovery of a functional glucocorticoid receptor beta-isoform in zebrafish, Endocrinology. 149, 1591–1599. [DOI] [PubMed] [Google Scholar]
- Shanle EK and Xu W, 2011. Endocrine disrupting chemicals targeting estrogen receptor signaling: identification and mechanisms of action, Chem Res Toxicol. 24, 6–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D, 1999. Worldwide trends in DDT levels in human breast milk, Int J Epidemiol. 28, 179–88. [DOI] [PubMed] [Google Scholar]
- Sobel ES, Gianini J, Butfiloski EJ, Croker BP, Schiffenbauer J and Roberts SM, 2005. Acceleration of autoimmunity by organochlorine pesticides in (NZB x NZW)F1 mice, Environ Health Perspect. 113, 323–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonneveld E, Jansen HJ, Riteco JA, Brouwer A and van der Burg B, 2005. Development of androgen- and estrogen-responsive bioassays, members of a panel of human cell line-based highly selective steroid-responsive bioassays, Toxicol Sci. 83, 136–48. [DOI] [PubMed] [Google Scholar]
- Sun J, Wang C, Peng H, Zheng G, Zhang S and Hu J, 2016. p,p’-DDE Induces Gonadal Intersex in Japanese Medaka (Oryzias latipes) at Environmentally Relevant Concentrations: Comparison with o,p’-DDT, Environ Sci Technol. 50, 462–9. [DOI] [PubMed] [Google Scholar]
- Thomas PT, Ratajczak HV, Eisenberg WC, Furedi-Machacek M, Ketels KV and Barbera PW, 1987. Evaluation of host resistance and immunity in mice exposed to the carbamate pesticide aldicarb, Fundam Appl Toxicol. 9, 82–9. [DOI] [PubMed] [Google Scholar]
- Ton C, Lin Y and Willett C, 2006. Zebrafish as a model for developmental neurotoxicity testing, Birth Defects Res A Clin Mol Teratol. 76, 553–67. [DOI] [PubMed] [Google Scholar]
- Tripathi G and Verma P, 2004. Endosulfan-mediated biochemical changes in the freshwater fish Clarias batrachus, Biomed Environ Sci. 17, 47–56. [PubMed] [Google Scholar]
- Trudeau VL, Sloley BD and Peter RE, 1993. GABA stimulation of gonadotropin-II release in goldfish: involvement of GABAA receptors, dopamine, and sex steroids, Am J Physiol. 265, R348–55. [DOI] [PubMed] [Google Scholar]
- Veillette PA, Merino M, Marcacclo ND, Garcia MM and Specker JL, 2007. Cortisol is necessary for seawater tolerance in larvae of a marine teleost the summer flounder, General and Comparative Endocrinology. 151, 116–121. [DOI] [PubMed] [Google Scholar]
- Versonnen BJ, Roose P, Monteyne EM and Janssen CR, 2004. Estrogenic and toxic effects of methoxychlor on zebrafish (Danio rerio), Environ Toxicol Chem. 23, 2194–201. [DOI] [PubMed] [Google Scholar]
- Waters KM, Safe S and Gaido KW, 2001. Differential gene expression in response to methoxychlor and estradiol through ERalpha, ERbeta, and AR in reproductive tissues of female mice, Toxicol Sci. 63, 47–56. [DOI] [PubMed] [Google Scholar]
- Wepy JA, Galligan JJ, Kingsley PJ, Xu S, Goodman MC, Tallman KA, Rouzer CA and Marnett LJ, 2019. Lysophospholipases cooperate to mediate lipid homeostasis and lysophospholipid signaling, J Lipid Res. 60, 360–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weyts FA, Flik G, Rombout JH and Verburg-van Kemenade BM, 1998. Cortisol induces apoptosis in activated B cells, not in other lymphoid cells of the common carp, Cyprinus carpio L, Dev Comp Immunol. 22, 551–62. [DOI] [PubMed] [Google Scholar]
- Weyts FA, Verburg-van Kemenade BM, Flik G, Lambert JG and Wendelaar Bonga SE, 1997. Conservation of apoptosis as an immune regulatory mechanism: effects of cortisol and cortisone on carp lymphocytes, Brain Behav Immun. 11, 95–105. [DOI] [PubMed] [Google Scholar]
- Weyts FAA, Cohen N, Flik G and Verburg-van Kemenade BML, 1999. Interactions between the immune system and the hypothalamo-pituitary-interrenal axis in fish, Fish & Shellfish Immunology. 9, 1–20. [Google Scholar]
- Wirbisky SE, Sepulveda MS, Weber GJ, Jannasch AS, Horzmann KA and Freeman JL, 2016. Embryonic Atrazine Exposure Elicits Alterations in Genes Associated with Neuroendocrine Function in Adult Male Zebrafish, Toxicol Sci. 153, 149–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu WJ, Qin N, Zhu Y, He QS, Ouyang HL, He W, Liu WX and Xu FL, 2013. The residual levels and health risks of hexachlorocyclohexanes (HCHs) and dichloro-diphenyl-trichloroethanes (DDTs) in the fish from Lake Baiyangdian, North China, Environ Sci Pollut Res Int. 20, 5950–62. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.