Summary
The phagocytosis of dying cells by macrophages, termed efferocytosis, is a tightly regulated process that involves the sensing, binding, engulfment, and digestion of apoptotic cells. Efferocytosis not only prevents tissue necrosis and inflammation caused by secondary necrosis of dying cells, but it also promotes pro-resolving signaling in macrophages, which is essential for tissue resolution and repair following injury or inflammation. An important factor that contributes to this pro-resolving reprogramming is the cargo that is released from apoptotic cells after their engulfment and phagolysosomal digestion by macrophages. The apoptotic cell cargo contains amino acids, nucleotides, fatty acids, and cholesterol that function as metabolites and signaling molecules to bring about this re-programming. Here, we review efferocytosis-induced changes in macrophage metabolism that mediate the pro-resolving functions of macrophages. We also discuss various strategies, challenges, and future perspectives related to drugging efferocytosis-fueled macrophage metabolism as strategy to dampen inflammation and promote resolution in chronic inflammatory diseases.
Keywords: Macrophages, Efferocytosis, Cell metabolism, Inflammation Resolution
1. INTRODUCTION
Efferocytosis, the process by which phagocytic cells engulf and clear apoptotic cells from the body, is an integral part of tissue homeostasis1. Although non-professional phagocytes such as epithelial cells and mesenchymal cells can partake in efferocytosis2,3, the majority of apoptotic cells are cleared by macrophages. In healthy tissue, efficient efferocytosis is followed by a resolution response that involves the secretion of a variety of mediators, including transforming growth factor- β (TGF-β) and interleukin-10 (IL-10), that dampen inflammation and promote tissue repair4,5. However, when apoptotic cells are not effectively cleared, they undergo secondary necrosis and release damage-associated nuclear molecular patterns (DAMPs) that elicit a pro-inflammatory response that can damage the surrounding tissue6. As such, defective efferocytosis contributes to the risk of various autoimmune and chronic inflammatory diseases7,8, including atherosclerotic cardiovascular disease, which is the leading causes of death worldwide. In advanced atherosclerosis, impaired efferocytosis leads to the formation of necrotic plaques with thin fibrous caps9–11. These so called “unstable” plaques are prone to rupture, which can trigger occlusive luminal thrombosis and thereby cause a heart attack or stroke. On the other hand, “reawakening” of the macrophage-mediated resolution response promotes regression of atherosclerosis when hypercholesterolemia is reversed12, which is associated with an increased plaque stability and a lower risk of coronary artery disease in humans13,14. Strategies that promote efferocytosis and inflammation resolution could therefore have a major therapeutic benefit in atherosclerotic cardiovascular disease as well as the plethora of other diseases associated with defective efferocytosis, such as systemic lupus erythematosus (SLE)-type autoimmune diseases, metabolic disorders, respiratory diseases, and neurodegenerative disorders7,8.
Efferocytosis is a tightly regulated process that involves three sequential steps, which has been studied mostly in macrophages, the most efficient type of efferocyte15,16. First, the macrophages need to detect apoptotic cells, which is mediated by the release of so-called “find me” signals by dying cells. Examples of these signals include CX3CL1 (fractalkine), lipid-derived molecules such as sphingosine-1-phosphate (S1P) and lysophosphatidylcholine (LPC), and the nucleotides ATP and ADP17–19. These molecules bind to their cognate receptors on the macrophage surface, which allows the macrophage to follow a chemotactic gradient of find-me signals and migrate towards the dying cell. After localizing apoptotic cells, the macrophages undergo the second step of the efferocytic process, which involves the binding of macrophages to apoptotic cells. This binding is mediated by the expression of “eat-me” signals on the surface of dying cells interacting with receptors on macrophages, with or without intermediary bridging molecules such as growth arrest-specific factor 6 (Gas6), protein S, and milk fat globule-epidermal growth factor 8 (MFG-E8)20–22. The most well-studied eat-me signal on apoptotic cells is phosphatidylserine (PtdSer). PtdSer is normally enriched on the inner leaflet of the plasma membrane, but it is translocated to the outer leaflet of the plasma membrane in a caspase-3-dependent manner in apoptotic cells23–25. Macrophages can bind to PtdSer and other molecules expressed on the surface of dying cells via a variety of receptors, including MER proto-oncogene tyrosine kinase (MERTK), the scavenger receptor CD36, and low-density lipoprotein receptor-related protein 1 (LRP1)26–28. Eat-me signals are counterbalanced by “don’t-eat-me” signals, such as CD47 that binds to macrophage signal regulatory protein alpha (SIRPα) to inhibit phagocytosis29. While the low expression of CD47 on healthy cells prevents their engulfment by macrophages, the CD47-SIRPα axis can also mediate immune evasion of tumor cells as well as defective efferocytosis of necrotic cells that have been shown to overexpress CD4730–32. The binding of apoptotic cells to macrophages sets in motion the third step of efferocytosis—apoptotic cell engulfment. Signaling through efferocytosis receptors promotes the polymerization and reorganization of the actin cytoskeleton to form a phagocytic cup, followed by internalization of the apoptotic cells33. Internalized apoptotic cells are subsequently degraded in the phagolysosome, which is followed by membrane recycling from the engulfed phagosome to restore the cell surface area and recycle efferocytosis receptors34–36. This recycling process is essential to internalize multiple apoptotic cells over a relatively short period of time, referred to as continual efferocytosis. Continual efferocytosis is particularly important in chronic inflammatory diseases such as atherosclerosis, where apoptotic cells far outnumber macrophages 34,37.
Aside from membrane recycling, there are other processes initiated by the sensing, binding, engulfment, and degradation of apoptotic cells that promote continual efferocytosis. Signaling through efferocytosis receptors as well as the release of metabolites by the degradation of apoptotic cells can reprogram macrophage metabolism to facilitate continual efferocytosis and macrophage-mediated inflammation resolution, which are essential in regaining tissue homeostasis following an inflammatory insult. These efferocytosis-induced adaptations in macrophage metabolism are the subject of this review.
2. MACROPHAGE METABOLISM IN EFFEROCYTOSIS AND RESOLUTION
2.1. Amino acid metabolism
Amino acids are not only building blocks for protein synthesis but also play central roles in the regulation of macrophage polarization and function38. Macrophages are classified as pro-inflammatory or pro-resolving based, in part, on high expression levels of inducible nitric oxide synthase (iNOS) or arginase 1 (ARG1), respectively. Both of these enzymes use the amino acid L-arginine as their substrate, but they generate different products. While iNOS in pro-inflammatory macrophages converts arginine and NADPH into nitric oxide (NO) for immune defense, ARG1 in pro-resolving macrophages generates the polyamine precursor ornithine, which is essential for continual efferocytosis. Efferocytic macrophages use arginine and ornithine derived from apoptotic cell degradation to synthesize the polyamine putrescine, which promotes Rac1 activation required for actin-mediated internalization of subsequent apoptotic cells37. As such, mice lacking myeloid ARG1 or ornithine decarboxylase (ODC), the enzyme that converts ornithine into putrescine, have defects in efferocytosis and atherosclerosis regression, whereas treatment with putrescine promoted atherosclerosis resolution37. Putrescine can be further converted into spermidine and spermine, which both accumulate in macrophages during efferocytosis37,39. Interestingly, these polyamines were shown derive from Rac1-dependent polyamine import that occurs with efferocytosis39 rather than from degraded apoptotic cells. While spermidine and spermine do not seem to enhance continual efferocytosis37, they suppress the production of pro-inflammatory IL-1β and IL-639. Spermidine, by inducing mitochondrial oxidative phosphorylation through hypusination40, may also promote pre-resolving mediator production in efferocytosing macrophages, as explained in section 2.2 below.
While spermidine and spermine were shown to suppress production of IL-1β (above), the amino acid serine has been shown to drive inflammation-induced IL-1β synthesis41,42. Multiple mechanisms may be involved in serine-dependent IL-1β production, including serine-driven synthesis of reduced glutathione to maintain cellular redox balance41, and serine-fueled generation of S-adenosylmethionine (SAM) to facilitate histone methylation and regulate inflammatory gene expression42. Although much less is known about serine in the context of efferocytosis, apoptotic cell-derived methionine has been shown to contribute to efferocytosis-induced resolution through SAM-dependent epigenetic regulation of a TGF-β resolution-repair program43. More specifically, DNA-methyltransferase-3A (DNMT3A) uses SAM to methylate Dusp4, a repressor of ERK signaling, to promote apoptotic cell-induced activation of ERK and induction of a pro-resolving Ptgs2-PGE2-TGF-β pathway in macrophages. Accordingly, bone marrow-specific deletion of DNMT3A was shown to blunt efferocytosis-induced TGF-β secretion and prevent atherosclerotic plaque resolution in mice, providing in vivo evidence for this DNMT3A-regulated TGF-β pathway. Thus, while SAM-dependent DNA methylation induces the expression of inflammatory cytokines in pro-inflammatory macrophages, it promotes the expression of resolving-repair genes in efferocytic macrophages. This suggests dynamic and context-dependent regulation of DNA methylation signatures in macrophages, potentially dependent on differential expression or activity of DNA-methyltransferases. This topic may be of particular interest to understanding how age-related loss-of-function somatic mutations in DNMT3A drive cardiovascular disease in clonal hematopoiesis44.
The essential amino acid tryptophan fuels the synthesis of kynurenine, serotonin, and indoles45. The kynurenine pathway is the most active pathway of tryptophan metabolism, and, beginning with the upstream enzyme indoleamine 2,3 dioxygenase 1 (IDO1), produces kynurenic acid, kynurenine, NAD+, and other metabolites46. A role for IDO1 in efferocytosing macrophages was first brought to light by the work of McGaha and colleagues, who found that injection of apoptotic cells into mice led to the induction of IDO1 in splenic macrophages47. When these mice were treated with an IDO1 inhibitor, they developed a T cell-mediated inflammatory response against the apoptotic cells, suggesting links between efferocytosis, IDO1, and tolerance to apoptotic cells. Using IDO1-overexpressing macrophages and the above mouse model, the group subsequently provided evidence that activation of an integrated stress response kinase called general control nonderepressible 2 (GCN2) by IDO1-mediated tryptophan depletion increased IL-10 and limited the expression of inflammatory cytokines in macrophages exposed to apoptotic cells48. However, unpublished in vitro and in vivo studies from our laboratory suggest that IDO1 mediates resolution in efferocytosing macrophages by catalyzing the conversion of tryptophan to kynurenine. Kynurenine promotes the expression of IL-10 and TGF-β and enhances continual efferocytosis by activating the macrophage aryl hydrocarbon receptor (AhR) in an autocrine/paracrine manner. These findings are consistent with other studies showing tryptophan/IDO1-derived kynurenine, in certain settings, can mediate immunosuppressive effects by activating AhR49. Although future work will be needed to sort out the different in vivo settings in which different mechanisms of IDO1 come into play, these combined studies suggest that IDO1-mediated tryptophan metabolism is an important mediator of tolerance and resolution in efferocytosing macrophages.
In addition to arginine, methionine, and tryptophan, which have well-characterized roles in the macrophage-mediated resolution response, an increasing number of amino acids are being identified as important immunometabolites. For example, it was recently shown that glutamine and spermidine are important regulators of energy metabolism in the context of efferocytosis, as highlighted in the next section.
2.2. Oxidative phosphorylation
Macrophages polarized towards a pro-resolving ARG1+ phenotype, which are more efficient efferocytes compared with pro-inflammatory macrophages37, rely mainly on oxidative phosphorylation (OXPHOS) for energy production50. Moreover, the process of efferocytosis further promotes OXPHOS in macrophages51,52, which facilitates certain aspects of the macrophage-mediated resolution program. The tricarboxylic acid (TCA cycle) oxidizes substrates from glycolysis, glutaminolysis, and fatty acid oxidation to generate NADH and FADH2 required by the electron transport chain to generate usable energy in the form of ATP through OXPHOS. ATP generated through OXPHOS has been suggested to support efferocytosis by facilitating the energy-demanding process of actin cytoskeleton reorganization required for engulfment of apoptotic cells53. Nonetheless, in efferocytic macrophages, OXPHOS may not be fully coupled to ATP production. The uncoupling protein 2 (UCP2) is upregulated in phagocytes engulfing apoptotic cells51, creating a proton leak across the inner mitochondrial membrane that depolarizes the mitochondria and uncouples OXPHOS from ATP synthesis. Macrophages from mice lacking UCP2 exhibit impaired efferocytosis in vitro as well as in vivo51, suggesting that an excessive increase in the mitochondrial membrane potential affects the ability of macrophages to effectively clear apoptotic cells, although the exact mechanism remains unknown. Aside from its effects on efferocytosis itself, OXPHOS is also involved in efferocytosis-induced expression of the anti-inflammatory, pro-resolving cytokine, IL-10. Inhibition of fatty acid oxidation by etomoxir as well as disruption of a gene encoding the Rieske iron-sulfur protein (RISP) involved in electron transport blunts efferocytosis-induced production of IL-1052. Mechanistically, OXPHOS was shown to promote activation of the NAD+-dependent enzyme sirtuin 1 (SIRT1) by elevating the NAD+/NADH ratio, which subsequently promotes transcription if the IL-10 gene. Thus, lipid metabolism and OXPHOS are not only important for efferocytosis itself but also for the inflammation resolution response that is triggered by efferocytosis.
The important role of OXPHOS in the context of efferocytosis raises the question of how this metabolic pathway is regulated by macrophages. Apoptotic cell stimulation of primary macrophages promotes expression of genes involved in fatty acid oxidation, the electron transport chain, and OXPHOS, indicating transcriptional regulation of lipid utilization52. This may be in part due to the polyamine spermidine, whose levels are increased in macrophages upon efferocytosis37. Spermidine enables hypusination, a polyamine-dependent post-translational modification, of eukaryotic translation initiation factor 5A (eIF5A), which can subsequently promote the expression of mitochondrial proteins that promote TCA flux and OXPHOS40. Furthermore, the gene encoding glutaminase 1 (GLS1), an enzyme that breaks down glutamine into the TCA cycle intermediate α-ketoglutarate, was recently shown to be upregulated in macrophages by apoptotic cell ingestion53. By generating more substrate to fuel OXPHOS, glutaminolysis was found to meet the demand for high-energy actin polymerization required for efferocytosis. Whether the glutamine in this pathway is taken up from the extracellular environment or is derived from the apoptotic cells that are engulfed and degraded by macrophages is remains unknown. Besides fatty acids and glutamine, glucose can fuel the TCA cycle by generating acetyl-CoA. We elaborate on the role of glucose in efferocytosis and macrophage-mediated resolution in the following section.
2.3. Aerobic glycolysis and lactate
In contrast to OXPHOS, glycolysis is generally associated with a pro-inflammatory macrophage phenotype. Inflammatory molecules and cytokines such as bacteria-derived lipopolysaccharides (LPS) and interferon-gamma (IFN-γ), which are employed in vitro to promote pro-inflammatory macrophage polarization, upregulate the transcription factor hypoxia-inducible factor 1-alpha (HIF1α)54. HIF1α shifts the cell towards a more glycolytic phenotype by upregulating key enzymes and proteins in glycolysis, including hexokinases, pyruvate kinases, and the glucose transporter GLUT155. At the same time, several key TCA cycle enzymes are downregulated in inflammatory macrophages, resulting in a broken TCA cycle and accumulation of the TCA cycle intermediate succinate56,57. Succinate further stimulates glycolysis by promoting HIF1α stability, which drives the production of the inflammatory cytokine IL-1β and supports the inflammatory macrophage phenotype58,59.
Recent work has challenged the idea that glycolysis is linked exclusively to inflammatory macrophage metabolism by showing that glycolysis is increased in efferocytic macrophages60. Nonetheless, the mechanisms involved in glycolysis in inflammatory vs. efferocytic macrophages are distinct. The transition to glycolysis in inflammatory macrophages is irreversible and depends on genetic reprogramming and rewiring of the TCA cycle61,62. In contrast, the increased glycolysis in efferocytic macrophages is transient and depends on rapid, Akt-mediated post-translational mechanisms that increase cell-surface localization of the glucose transporter GLUT1, via phosphorylation of the GLUT1 adaptor thioredoxin-interacting protein (TXNIP), and activation of the glycolytic enzyme 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase 2 (PFBF2)63. These processes could theoretically enable glucose to be shunted into the TCA cycle via acetyl-CoA to generate more available energy required for apoptotic cell engulfment. However, efferocytosis was rather shown to promote the breakdown of glucose into lactate60, which serves several purposes. First, intracellular lactate promotes calcium-dependent recycling of the efferocytosis receptors MerTK and LRP1 required for continual efferocytosis63. Second, lactate released from the cell can act as a paracrine mediator of inflammation resolution by skewing neighboring macrophages towards an anti-inflammatory and pro-resolving phenotype60. Third, lactate availability drives lysine lactylation, a newly identified posttranslational modification of histones and non-histone proteins64. While the role of lactylation of non-histone proteins in macrophages remains to be elucidated, histone lactylation has been associated with a more pro-resolving macrophage phenotype, specifically, increased expression of Arg1, suggesting lactate-dependent epigenetic regulation of inflammation resolution64. Lactate has also been shown to enhance histone acetylation to promote polarization to a more pro-resolving phenotype in the context of tumor-induced immunosuppression65. While these epigenetic changes may be more sustained, glycolysis in efferocytic macrophages is relatively short-lived (< 24 hours). Thus, efferocytosis induces glycolysis in a rapid manner to produce a burst of lactate for pro-resolving processes, followed by a transition to OXPHOS to maintain the macrophages’ resolving-repair phenotype. Lactate itself can be converted to pyruvate to feed into the TCA cycle and OXPHOS, which may be the mechanism by which this metabolic switching occurs in efferocytic macrophages. Indeed, lactate has been identified as a key input to fuel the TCA cycle in many tissues under physiological conditions66. Furthermore, a buildup of intracellular lactate effected by silencing the lactate exporter, MCT1, can blunt oxidative phosphorylation in macrophages60,67, suggesting that macrophages require the uptake of extracellular lactate to shuttle it into OXPHOS.
In addition to lactate affecting macrophage function by feeding into the TCA cycle, lactate can signal via cell surface receptors such as the G-protein coupled receptors GPR81 and GPR13268. Both receptors have been identified previously to respond to lactate by dampening inflammation and promoting phenotypic switching of macrophages to a resolving-repair phenotype68. Given that GPR81 and GPR132 are cell surface receptors that respond to extracellular lactate, lactate secreted by efferocytic macrophages may spread pro-resolution signaling to neighboring, non-efferocytic macrophages and other cell types. Consistent with this idea, genetic targeting of the lactate exporter MCT1 in macrophages has been shown to impair tissue repair in the context of peripheral nerve injury67. Moreover, treating naïve macrophages with conditioned media collected from efferocytic macrophages increased mRNA expression of pro-resolving mediators such as Tgfb and Il10, which was blocked when the efferocytic macrophages could not export lactate60. Interestingly, GPR132 is downregulated upon LPS stimulation, which may function to sustain inflammation and blunt lactate signaling69. In a study under review from our group, we have also identified that lactate is critical to sustaining efferocytosis-induced macrophage proliferation (EIMP) through the lactate receptor GPR132. EIMP, which is discussed in section 2.5 below, is a process that increases the pool of macrophages competent in continual efferocytosis to boost the resolution-efferocytosis cycle. The central driver of EIMP is upregulation of Myc protein, and lactate was found to stabilize Myc through GPR132-mediated activation of an AMPK-NAD+-SIRT1 pathway. Both AMPK and SIRT1 have been implicated previously in macrophage polarization towards a pro-resolving phenotype52,70,71 and play a role in promoting efferocytosis72,73. As discussed above, NAD+-SIRT1 signaling was found to enhance efferocytosis-induced IL-10 expression in efferocytosing macrophages52.
Efferocytosis is a non-inflammatory form of phagocytosis despite the uptake of large quantities of potentially pro-inflammatory molecules released directly from the apoptotic cell or from the phagolysosomal degradation of engulfed apoptotic cells7,74,75. Furthermore, molecules released from apoptotic cells or derived from apoptotic cell degradation in macrophages and then secreted could cause paracrine effects in neighboring macrophages75. For example, ATP, a “find-me signal”17, is released through pannexin channels by apoptotic cells, and, conversely, high levels of extracellular ATP can trigger a strong inflammatory response76. Additional pathways are activated during efferocytosis to promote a tolerogenic response. One such pathway involves the upregulation of the chloride transporter SLC12A2, which was reported to prevent pro-inflammatory sensing of apoptotic cells74. Another key mechanism of immunosuppression in macrophages may involve lactate production, as Slc16a1, which encodes the lactate exporter MCT1, is upregulated during efferocytosis60, and, as noted above, lactate can play important roles in local immunosuppression, including in tumor-mediated immunosuppression77.
2.4. The pentose phosphate pathway
The pentose phosphate pathway (PPP) branches from glucose-6-phosphate in the glycolytic pathway, and has both an oxidative and a non-oxidative phase78. The oxidative phase of the PPP generates NADPH, which can be used by NADPH oxidases to generate reactive oxygen species (ROS) required for the defense against pathogens. In addition, this phase can be used to generate ROS-scavenging antioxidants, such as reduced glutathione, to protect the cell from oxidative-stress induced damage. The non-oxidative phase of the PPP produces ribose-6-phosphate, which is used for the synthesis of nucleotides required for cell proliferation. Pro-inflammatory macrophages exhibit increased oxidative PPP activity56, in line with the important role they play in host defense. Non-oxidative PPP activity is suppressed in pro-inflammatory macrophages as a result of downregulation of the sedoheptulose kinase CARKL, which bridges glycolysis and non-oxidative PPP by shifting glucose-3-phosphate towards sedoheptulose-7-phosphate79. In contrast, pro-resolving macrophages express higher levels of CARKL, thereby enhancing the non-oxidative branch of the PPP while decreasing overall PPP flux79. The role of the PPP in efferocytosis is controversial. While the oxidative phase of PPP was shown to support efferocytosis by generating NADPH in the setting of physiological hypoxia80, another study found that efferocytosis decreases PPP flux under normoxic conditions as a result of reduced expression of PPP-related genes. In this latter setting, restoration of PPP activity in efferocytic macrophages blunted engulfment of apoptotic cells and stimulated pro-inflammatory cytokine production81. Consistent with these findings, a third study, that showed that PPP agonism reduced efferocytosis in vitro and aggravated autoimmune disease in a mouse model of SLE82.
These findings suggest that PPP flux could be an important regulator of continual efferocytosis or the inflammatory response, depending on oxygen availability within the tissue microenvironment. Although the underlying mechanisms are largely unknown, a decreased PPP flux could be coupled to an increased glycolytic flux in efferocytic macrophages, thereby promoting lactate-mediated continual efferocytosis and resolution. However, blunted oxidative phosphorylation under hypoxic conditions may deplete intracellular NADPH, resulting in a compensatory increase in PPP as well as glycolysis to maintain NADPH levels and redox homeostasis. Whether flux through the PPP is indeed coupled to glycolysis in efferocytic macrophages, and whether this could (in part) underly the association between PPP activity and efferocytosis, remains to be investigated. Moreover, further research is needed to elucidate how oxygen availability regulates macrophage metabolism in the context of efferocytosis and inflammation resolution, particularly given that tissue hypoxia is a hallmark of chronic inflammatory diseases.
2.5. Nucleotide metabolism
Nucleotide metabolism supports RNA synthesis and DNA replication required for cell growth and proliferation. Although local proliferation of inflammatory macrophages exacerbates chronic inflammatory diseases such as atherosclerosis83, proliferation of pro-resolving macrophages can be beneficial by expanding the pool of pro-resolving, pro-efferocytic macrophages. In the setting of type 2 immunity and would healing, the anti-inflammatory cytokine interleukin-4 (IL-4) has been shown to promote the proliferation of tissue-resident macrophages to facilitate tissue repair84–86. This proliferation response was not directly linked to efferocytosis, but it was blunted in mice in which the PtdSer-dependent receptors MerTK and Axl were genetically ablated84. This role of efferocytosis was addressed in a subsequent study showing that efferocytosis selectively induces the proliferation of pro-resolving macrophages and that this process, termed efferocytosis-induced macrophage proliferation (EIMP), facilitates inflammation resolution and atherosclerosis regression in vivo87. This pathway begins with the release into the macrophages of nucleotides derived from the phagolysosomal hydrolysis of apoptotic cell-DNA by DNase2a. The major function of the nucleotides is to trigger a DNA-dependent protein kinase (DNA-PK)-mTORC2 pathway that, together with MerTK signaling via ERK, drives expression of Myc mRNA and protein to stimulate cell cycling. Secondarily, the macrophages recycle the apoptotic cell-derived nucleotides to support DNA synthesis in this setting. As mentioned in section 2.3 above, another key component of EIMP related to Myc is that lactate production through efferocytosis-induced glycolysis is necessary to stabilize Myc protein expression and sustain EIMP. Finally, studies in the field of cancer have shown that Myc, which is a proto-oncogene, promotes expression of the gene encoding ODC88,89. Thus, it is possible that apoptotic cell-induced Myc, by promoting ODC-mediated putrescine synthesis (above), may also enhance the pro-resolving properties of macrophages undergoing EIMP. Consistent with this idea, EIMP macrophages are better at continual efferocytosis than naïve macrophages87, which may be attributable to increased putrescine production via ODC37. Thus, not unlike macrophage glycolysis as discussed above, macrophage proliferation has distinct mechanistic underpinnings in the setting of efferocytosis, which opens up therapeutic avenues to specifically target these processes to promote the resolution in chronic inflammatory diseases.
2.6. Cholesterol metabolism
Cholesterol plays a crucial role in regulating cell function and is an important structural component of the cell membrane. However, cellular cholesterol homeostasis must be tightly regulated to prevent free cholesterol-induced cytotoxicity90. Accordingly, the substantial increase in cellular cholesterol that occurs when macrophages engulf apoptotic cells is coupled to protective mechanisms to prevent cholesterol-induced cell death91. Key among these mechanisms is the activation of survival pathways involving NF-κB and PI-3 kinase/Akt, which protect phagocytes from apoptosis when free cholesterol levels become elevated91. Moreover, the increase in free cholesterol itself is limited in efferocytosing macrophages by several mechanisms. For example, free cholesterol is esterified to fatty acids by acyl-CoA cholesterol acyltransferase (ACAT), followed by storage of the resulting cholesterol esters within intracellular neutral lipid droplets. In addition, cholesterol is excreted from the cell via ATP-binding cassette transporter A1 (ABCA1)- and ABCG1-mediated cholesterol efflux. Transcription of ABCA1 and ABCG1 is induced by the nuclear hormone receptor LXR, which was shown to be upregulated in macrophages undergoing efferocytosis92. Aside from protecting the macrophages from free cholesterol overload, the LXR pathway induces expression of the efferocytosis receptor MerTK and dampens expression of pro-inflammatory mediators including IL-1β, suggesting a more tolerogenic macrophage phenotype92. Indeed, mice lacking LXR were shown to develop autoimmune characteristics, while administration of an LXR agonist ameliorated SLE-like autoimmune disease92. Other targets of LXR include the sterol-regulatory element-binding proteins (SREBPs), which are transcription factors that regulate the expression of genes involved in the biosynthesis of fatty acids and cholesterol. On the one hand, inflammatory signaling through toll-like receptors such as TLR4 has been shown to promote SREBP1 expression, resulting in lipid synthesis, production of inflammatory cytokines, and downregulation of the efferocytosis receptor LRP1 in human macrophages93. However, SREBP1 has also been shown to contribute to the resolution of pro-inflammatory TLR4 signaling through the synthesis of anti-inflammatory fatty acids94. LXR and SREBPs function in a coordinated manner, and transcription of LXR target genes depends on co-activation by SREBP, which facilitates context-specific regulation of lipid metabolism94. The complexity of macrophage LXR-SREBP signaling and its role in efferocytosis and resolution require further investigation.
2.7. Metabolism of fatty acids into specialized pro-resolving mediators
An important component of inflammation resolution is the conversion of polyunsaturated fatty acids into various classes of specialized pro-resolving mediators (SPMs), including lipoxins, resolvins, protectins, and maresins, that together restrain inflammation and promote tissue repair to restore homeostasis. Lipoxygenase (LOX) enzymes catalyze the formation of lipoxins from the omega-6 fatty acid arachidonic acid (AA), while they use omega-3 fatty acids such as eicosapentanoic acid (EPA) and docosahexaenoic acid (DHA) to generate resolvins, protectins, and maresins. Efferocytosis has been reported to promote the production of these SPMs by macrophages in two ways: by increasing the availability of SPM precursors (e.g., AA, EPA, and DHA) through degradation of the metabolic content of apoptotic cells and by stimulating enzymatic conversion of these precursors into mature lipid mediators7. In particular, activation of MerTK by the binding of apoptotic cells promotes excursion of the enzyme 5-LOX from the nucleus into the cytoplasm95,96. This brings 5-LOX in close proximity with cytoplasmic 12/15-LOX, which together can promote the conversion of AA into the lipoxin LXA4 and DHA into the resolvin RvD1. Moreover, efferocytosis promotes the expression of 12/15-LOX, which may further enhance SPM biosynthesis97. Conversely, LXA4 and RvD1 promote efferocytosis, suggesting a positive feedback loop between efferocytosis and SPM production98,99. Loss of 12/15-LOX activity prevents clearance of apoptotic cells by tissue-resident macrophages and promotes the development of a lupus-like autoimmune disease in mice100, consistent with a role for LOX-dependent SPM biosynthesis for efferocytosis-mediated resolution.
In summary, as schematized in Figure 1, efferocytosis causes an array of metabolic adaptations in macrophages that facilitate tissue resolution, the continual clearance of apoptotic cells, and the protection of macrophages from the stresses of substantial apoptotic cell-cargo load.
FIGURE 1.
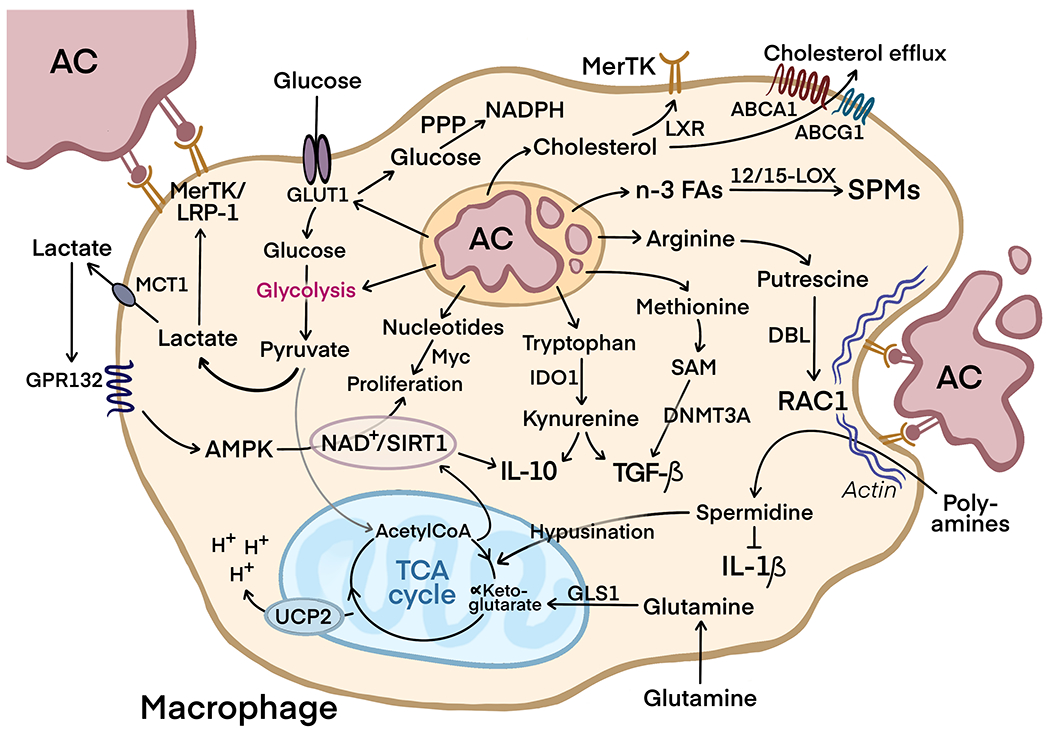
The process of efferocytosis and the release of metabolites by the degradation of apoptotic cells (AC) modulates metabolic signaling in macrophages to facilitate inflammation resolution, continual efferocytosis, and protection from excess AC-cargo load.
3. TARGETING MACROPHAGE METABOLISM IN CHRONIC INFLAMMATORY DISEASES
3.1. The therapeutic potential of modulating macrophage immunometabolism
Various anti-inflammatory therapies have been developed for the treatment of chronic inflammatory diseases, including nonsteroidal anti-inflammatory drugs (NSAIDs), glucocorticoids, and disease-modifying drugs101. NSAIDs and glucocorticoids are used mostly to relieve symptoms of these diseases, whereas disease-modifying drugs target specific pathogenic processes to prevent tissue damage and limit disease progression. A key example of a disease-modifying drug is anti-TNFα antibody, which is used for the treatment of inflammatory rheumatoid arthritis (RA) and other inflammatory joint diseases. Although this therapy has proven to be very effective in reducing pro-inflammatory cytokines and halting progressive destruction of joints102,103 and is also used as part of the treatment for ulcerative colitis and Crohn’s disease104, its use in other inflammatory conditions is limited. A more broadly applicable anti-inflammatory drug is colchicine, which is indicated for the treatment of RA and pericarditis and has recently been shown to reduce the rate of recurrent cardiovascular events105. Cardioprotective actions were also found for canakinumab, a monoclonal antibody against IL-1β, which reduces the risk of atherosclerotic cardiovascular disease in patients with previous myocardial infarction106. However, these anti-inflammatory therapies are associated with adverse events related to impaired host defense, notably infection and sepsis. A promising strategy to limit chronic inflammation while minimizing loss of essential host defense mechanisms is through boosting inflammation resolution101. Resolution-repair processes are regulated by pro-resolving type macrophages that possess distinct and dynamic metabolic signatures. This review has extensively discussed metabolic pathways that are upregulated in pro-resolving macrophages, particularly following efferocytosis. This section will focus on the clinical landscape and therapeutic potential of targeting these metabolic pathways.
3.2. Targeting metabolic signaling pathways associated with pro-resolving macrophage function
The ARG1- and ODC-mediated synthesis of the polyamines putrescine, spermidine and spermine promote pro-resolving pathways in macrophages. Although supplementation of putrescine in the drinking water of mice was shown to restore inflammation resolution and features of plaque stability in atherosclerosis37, dietary intake of putrescine has not been associated with a decreased cardiovascular risk in humans107. In contrast, high intake of spermidine in humans is associated with a reduced risk of cardiovascular disease and a lower incidence of death after myocardial infarction107. The cardioprotective role of spermidine has been attributed to its antihypertensive effects, but it is unknown whether inflammation resolution is also involved. While dietary supplementation of polyamines could be beneficial to some extent, therapeutic efficacy could be enhanced by directly targeting ARG1/ODC in macrophages to promote their pro-resolving phenotype and to boost localized release of polyamines within the inflamed tissue. This could be particularly useful in chronic inflammatory diseases, which are dominated by the presence of pro-inflammatory macrophages that express relatively low levels of ARG1108. Promoting the ARG1-dependent conversion of arginine into pro-resolving polyamines instead of the iNOS-dependent conversion of arginine into NO could drive these macrophages towards a more pro-resolving phenotype and thereby contribute to inflammation resolution. Indeed, high macrophage ARG1 expression in rabbits confers resistance to atherosclerosis109, providing proof of concept that promoting ARG1 expression in macrophages could reduce the risk of chronic inflammatory disorders.
Aside from IL-4, which is widely used to promote the pro-resolving ARG1 macrophage phenotype in vitro, signaling through the LXR is known to promote ARG1 expression110. This is accompanied by an increase in macrophage efferocytosis receptors and a dampened inflammatory response (see section 2.6), suggesting that LXR agonists can be employed to promote ARG1-mediated inflammation resolution. In this regard, a recent study found that phytanic acid, a microbiota-associated metabolite, restores efferocytosis in female lupus-prone mice by activating PPARγ/LXR signaling111. However, systemic LXR activation promotes hepatic steatosis and hypertriglyceridemia by acting on the liver112, implying the need for targeted drug delivery. Macrophage-specific drug targeting could be achieved by using targeted nanoparticles, which can deliver a broad range of therapeutic agents to inflamed tissues113. This strategy has been employed to enhance delivery of the LXR agonist GW3965 to atherosclerotic plaque macrophages, which showed superior efficacy in mice as compared to untargeted treatment without adverse effects on the liver114. Thus, by increasing therapeutic efficacy while reducing adverse effects, nanoparticle-mediated drug delivery holds great therapeutic potential.
Another advantage of using nanoparticles is that they markedly improve the chemical stability of the loaded therapeutics, which is important for the delivery of small interfering RNAs (siRNAs) and microRNAs that are sensitive to degradation by serum nucleases. Nanoparticle-mediated delivery of siRNA targeting Ca2+/calmodulin-dependent protein kinase γ (CaMKIIγ), an inhibitor of LXR signaling, restores MerTK-dependent efferocytosis in atherosclerotic lesional macrophages and promotes plaque stabilization115,116. MerTK-mediated efferocytosis may also be increased by palmitoylethanolamide (PEA), a lipid mediator that is generated by the enzyme N-acetylphosphatidylethanolamine-hydrolyzing phospholipase D (NAPE-PLD). PEA was shown to act via the orphan receptor GPR55 to promote the expression of MerTK117, whereas oleoylethanolamide (OEA), another product of NAPE-PLD, was found to drive the polarization of macrophages to a pro-resolving phenotype in an AMPK-PPARα-dependent manner118. Although the disadvantageous pharmacokinetic profile of PEA and OEA limit their clinical use, activation of NAPE-PLD has been suggested as a therapeutic strategy to treat cardiometabolic diseases, leading to the development of small molecule activators of NAPE-PLD that are effective in raising PEA and OEA levels and promoting efferocytosis in vitro119. Future studies that employ targeted delivery of NAPE-PLD activators are required to evaluate whether this strategy is effective in promoting efferocytosis and inflammation resolution in vivo.
A therapeutic approach that is garnering increased interest is to target pathogenic microRNAs (miRs), e.g., by using anti-miRNA nucleotides or other strategies to neutralize disease-causing miRs packaged in nanoparticles120. In this context, it may be possible to promote pro-resolving efferocytosis-induced glycolysis by targeting miR-646, which inhibits PFKFB2, the enzyme that mediates the pro-resolving process of efferocytosis-induced glycolysis121 (section 2.3). A circular RNA termed circFLNA was shown to act as a sponge of miR-646, thereby promoting PFKFB2 expression. Although PFKFB2 is expressed in various cell types and tissues, macrophage-specific delivery of circFLNA using nanoparticles may promote efferocytosis and inflammation resolution while limiting risks and side effects of treatment. As illustrated below, another strategy uses nanoparticles to deliver siRNAs, and this could be employed to silence genes that inhibit efferocytosis and resolution.
Most nanoparticles are constructed from a lipid-polyethylene glycol (PEG) layer conjugated to peptides as targeting agents, such as a collagen IV-targeting peptide ligand for delivery to collagen-rich atherosclerotic lesions114 or S2P that recognizes the macrophage receptor stabilin-287,115,116. However, these nanoparticles may be taken up by multiple cell types or by macrophages not in the inflamed tissue of interest. To overcome this limitation, an approach was developed to specifically target synovial inflammatory macrophages in RA122. Nanoparticles were designed to consist of a drug-based core with an oxidative stress-responsive PtdSer-coated protein corona and an outer shell of low molecular weight heparin. Heparin binds to P-selectin on activated endothelium, which localizes the nanoparticles to the inflamed tissue, i.e., the joint synovium in the case of RA. ROS within the inflamed microenvironment promotes shedding of the outer shell and exposes the PtdSer corona. Macrophages then bind to PtdSer and engulf the drug-based core of the nanoparticle in an efferocytosis-like manner. This strategy was shown to be effective in delivering siRNA against Irf5, a regulator of the inflammatory macrophage phenotype, to synovial macrophages and thereby reduce inflammation and restore joint function in a mouse model of RA122. In theory, this approach could be used to target tissue-resident macrophages in other chronic inflammatory diseases, including atherosclerotic lesional macrophages, to enable site- and cell-type specific delivery of drugs that promote pro-resolving macrophage metabolism while minimizing off-target effects.
3.3. Supplementation of pro-resolving mediators to promote macrophage-mediated inflammation resolution
Besides targeting proteins and enzymes within metabolic pathways to promote the resolution program, their precursors and end-products can be administered directly to dampen inflammation and promote tissue repair. An imbalance between SPMs and pro-inflammatory leukotrienes have been found in human atherosclerotic plaques123, airway fluid of cystic fibrosis patients124, and in blood of individuals with asthma and inflammatory periodontal disease125,126. These findings not only show that SPMs may be valuable diagnostic biomarkers127, but also suggest that raising SPM levels could be beneficial for the treatment of a variety of inflammatory diseases128. In animal models, exogenously administered resolvins have been shown to attenuate vascular injury and inflammation-induced atherogenesis129,130. Moreover, targeted nanoparticles containing Ac2-26, a peptide that acts on the same receptor as RvD1, protects against advanced atherosclerosis in mice131, similar to IL-10-containing nanoparticles that were also shown to promote inflammation resolution and efferocytosis in atherosclerosis132. In addition, injection of SPMs into inflamed human skin promotes active resolution in the short-term133, however, long-term studies with SPMs in humans with chronic inflammatory disease are lacking. These studies may be hampered by unfavorable pharmacokinetics of SPMs, such as their limited biological half-life, making it difficult to effectively raise SPM levels at the inflamed tissue site. However, synthetic analogues of SPMs with improved metabolic stability may provide therapeutic advantages. Another approach to promote local SPM generation is by administering PUFAs as precursors, which can be converted into SPMs within the target tissue. Indeed, omega-3 fatty acid supplementation is associated with increased SPM production in humans134–139, and low levels of SPMs in patients with coronary artery disease could be completely restored by a pharmaceutical formulation consisting of a mixture of the omega-3 fatty acids EPA and DHA140. Most importantly, an omega-3 fatty acid-rich marine oil supplement not only increased SPM levels in individuals with peripheral artery disease, but also increased the phagocytic activity of peripheral blood monocytes and reduced their inflammatory phenotype141. Clinical trials are currently underway to test whether restoration of SPMs by various formulations of omega-3 fatty acids can also improve clinical outcomes in patients with chronic inflammatory diseases. Of note, such a therapy depends on the activity of 12/15-LOX, and it may be less effective in individuals with, for example, a reduced 12/15-LOX expression as a result of genetics142–144 or a defect in efferocytosis, which might occur in settings of chronic inflammation11.
Lactate has many of the pro-resolving properties of SPMs, and thus boosting lactate production or administering exogenous lactate may be used as therapeutic strategies for chronic inflammatory diseases. Such strategies could also mimic the pro-resolving effects observed by our lab resulting from efferocytosis-induced lactate production to sustain the efferocytosis-resolution cycle by boosting continual efferocytosis and expanding the pool of pro-resolving macrophages. Some studies have begun to investigate such possibilities using animal models, ex vivo studies, or small-scale human trials. Direct injection of lactate into the circulation was found to be an effective method of delivery given the rapid transport of lactate through the circulation. This is a particularly effective method of lactate delivery to the brain, given that lactate readily crosses the blood-brain-barrier. In mice, both intracerebroventricular and intravenous injection of lactate were found to have a neuroprotective effect in ischemia-reperfusion injury of the brain145, which is likely explained by the anti-inflammatory and anti-oxidant properties of lactate69,146. Furthermore, injecting lactate is an effective method to activate SIRT1 in the hippocampus to boost the expression of brain-derived neurotrophic factor (BDNF), which is critical for memory and learning and has been associated with neurodegenerative diseases such as Alzheimer’s and Parkinson’s disease147,148. SIRT1 is also an important player in promoting efferocytosis73, as well as efferocytosis-associated macrophage proliferation and IL-10 production52, providing further evidence for lactate as a therapeutic tool to boost the efferocytosis-resolution cycle in vivo. One promising study found pro-resolving and tissue repair effects of lactate injection in dampening myocardial inflammation following acute myocardial infarction in humans149. Ischemic postconditioning, together with an injection of lactated Ringer’s (sodium lactate) solution with each bout of brief reperfusion in myocardial infarction patients, resulted in dampened inflammation, reduced occurrence of arrhythmias, and attenuated tissue damage following full reperfusion by stenting149. Although the authors attribute these beneficial effects to a lactate-dependent prevention of hypercontraction, lactate could also contribute to tissue repair by promoting macrophage efferocytosis and dampening inflammation. The anti-inflammatory properties of lactate have also been observed in type 1 diabetes patients, where short infusions of lactate reduced LPS-stimulated secretion of pro-inflammatory cytokines by peripheral blood mononuclear cells ex vivo150.
Given that efferocytosis itself can trigger the acute production of pro-resolving lactate through glycolysis and also leads to the intracellular accumulation of other pro-resolving, pro-efferocytic metabolites derived from apoptotic cell degradation, such as nucleotides and amino acids37,43,60,63, potential therapies to boost efferocytosis itself are important to consider151. Direct injection of apoptotic cells was found to have clinically beneficial effects in acutely reducing inflammation, e.g., by dampening cytokine storm during sepsis and attenuating arthritis-related inflammation152,153. The therapeutic potential of using apoptotic cells to stimulate efferocytosis is further illustrated by a recent study on mesenchymal stromal cell (MSC) infusion therapy, which showed that apoptosis of MSCs and subsequent efferocytosis contributes to the efficacy of this treatment154. However, strategies that involve administration of apoptotic cells are not as effective when macrophage efferocytosis is impaired, which is known to occur not only in chronic inflammatory diseases but also in aging7,155. Upregulation of the “don’t-eat-me” signals CD24 and CD47 have been suggested to contribute to the accumulation of pathogenic senescent cells in aged tissues156. Similarly, hepatocytes dying from necroptosis upregulate CD47 in inflammation-mediated liver disease, or nonalcoholic steatohepatitis (NASH). This pathological process prevents macrophage-mediated clearance of necroptotic hepatocytes, which exacerbates NASH progression31. Blocking “don’t-eat-me” signaling has therefore been suggested as a therapeutic approach to boost efferocytosis for the treatment of chronic inflammatory diseases. In mice, neutralizing antibodies that target CD47 were shown to boost efferocytosis and attenuate disease progression for both atherosclerosis and NASH31,157. A small retrospective study in patients with atherosclerotic disease showed attenuated vascular inflammation after treatment with anti-CD47 antibody158. Finally, an elegant approach involving the fusion of efferocytic receptors to a specific signaling domain within the cytoplasmic adaptor protein ELMO1 resulted in a chimeric receptor with an improved capacity for efferocytosis159. Introduction of this chimeric receptor genetically via either transgenesis or viral delivery in mice resulted in increased efferocytosis and attenuated inflammation and tissue injury in multiple pathologies, including colitis and drug-induced hepatotoxicity159. Importantly, introduction of the chimeric receptor also increased expression of glycolytic genes in phagocytes, suggesting increased production of pro-resolving lactate to boost the efferocytosis-resolution cycle159.
Aside from direct injection of lactate or boosting efferocytosis, lactate levels can be acutely raised through exercise. Exercise is well-known to generate lactate, promote anti-inflammatory signaling, and enhance tissue repair160–162. This exercise-induced resolution-repair program repairs skeletal muscle that becomes injured through exercise163. The process is initiated by the recruitment of pro-inflammatory Ly6chi monocytes by the chemokine CCL2. These monocytes develop into pro-inflammatory macrophages in an initial inflammatory phase before a local conversion to pro-resolving macrophages during the repair phase164. Moreover, efferocytosis is known to be critical for the proper repair of exercise-induced muscle damage165. Given that both inflammation and efferocytosis drive an increase in lactate production60,63, lactate may aid in the conversion of inflammatory macrophages to resolving-type macrophages. Consistent with this idea, lactate supplementation has been observed to promote tissue repair in mouse models of muscle damage. For example, oral lactate supplementation in mice with cardiotoxin-induced muscle damage stimulates skeletal muscle regeneration166, and implantation of a Matrigel plug containing lactate promotes the expression of pro-resolving macrophage markers in muscle and stimulates muscle perfusion, vascularization, and regeneration in an ischemia model of hind leg muscle injury167. Lactate has thus become recognized as a major myokine and exerkine (exercise-induced secreted signaling molecule) that promotes the systemic and local beneficial metabolic and anti-inflammatory effects of exercise168. Aside from the direct pro-resolving and tissue repair roles of lactate, a novel exercise-induced circulating metabolite N-lactoyl-phenylalanine (Lac-Phe), derived from lactate and phenylalanine, has been identified in mice and humans to suppress food intake, promote glucose balance, and prevent obesity169. We therefore think it would be interesting to examine whether Lac-Phe or other novel lactate-derived metabolites could also have pro-resolving and pro-efferocytosis effects in tissue repair and disease.
4. CURRENT LIMITATIONS AND FUTURE DIRECTIONS
Key gaps in our understanding of macrophage metabolism will need to be addressed to optimize the refinement and implementation of the aforementioned therapeutic strategies for the prevention and treatment of chronic inflammatory diseases in humans. For example, our knowledge on macrophage phenotypes and their metabolic profile in human tissue is relatively limited. The majority of studies on macrophage metabolism have been performed in murine peritoneal and bone marrow-derived macrophages. While these cell models have given us valuable insight into macrophage biology, they do not always share the same properties as human tissue-resident macrophages. In fact, even peritoneal and bone marrow-derived macrophages from mice, while from the same species, have distinct metabolic phenotypes. Peritoneal macrophages are often collected under elicited conditions that promote their metabolic activity and increase their dependency on glucose as an energy source170,171, whereas bone marrow-derived macrophages are differentiated ex vivo and show more distinct metabolic and functional phenotypes depending on the method of differentiation. The strong dependency of elicited peritoneal macrophages on glycolysis in the resting state may explain why efferocytosis results in a higher increase in oxidative phosphorylation compared to glycolysis in these cells52. Bone marrow-derived macrophages that undergo efferocytosis also upregulate all pathways of energy metabolism, but shift transiently towards glycolysis soon after engulfment of apoptotic cells63. Nevertheless, the current paradigm of pro-resolving macrophages expressing high levels of ARG1 and pro-inflammatory macrophages expressing high levels of iNOS holds true for both macrophage populations. This paradigm is challenged by human macrophages, which express very little or even undetectable levels of iNOS and ARG1 in vitro172,173. Although this has created a debate on whether these enzymes are relevant for the metabolism and function of human macrophages174, their expression has been detected in human tissue-resident macrophages in vivo175–178. Whether ARG1 expression is induced in tissue-resident human macrophages in settings that require (continual) efferocytosis and inflammation resolution, as occurs in tissue-resident murine macrophages37, remains to be investigated. Moreover, apoptotic cells release ornithine to human macrophages after efferocytosis, which can feed the pro-resolving putrescine pathway (section 2.1) independently of ARG137. Metabolic differences between mouse and human macrophages are further exemplified by studies utilizing LPS and IL-4 to promote a pro-inflammatory and pro-resolving macrophage phenotype, respectively. Activation of mouse bone marrow-derived macrophages by LPS promotes a shift in energy metabolism from oxidative phosphorylation to glycolysis, whereas human peripheral blood monocyte-derived macrophages fail to undergo metabolic reprogramming upon LPS stimulation179. Furthermore, stimulation towards a pro-resolving macrophage phenotype by IL-4 depends on oxidative phosphorylation in murine macrophages180,181 but not in human macrophages182. These findings collectively argue for better models for studying human macrophage biology, i.e., to better understand human-specific aspects of pro-resolving macrophage metabolism and to refine therapeutic strategies that have a better chance of become clinically useful.
Another factor that may complicate the implementation of macrophage-targeted therapeutics is that macrophage metabolism is highly context-dependent. The metabolic signature and functional response of macrophages is plastic and adapts to the tissue microenvironment183,184, which is difficult to mimic in an in vitro setting. A large variability in oxidative metabolism of macrophages within different tissues has already been observed under homeostatic conditions185, and this may be further increased in the context of disease. For example, in the chronic inflammatory setting of atherosclerosis, plaque macrophages are exposed to pro-inflammatory cytokines, apoptotic cells and their releasate, and high levels of (oxidized) lipids and cholesterol, which can all affect their functional phenotype186. As such, both peritoneal macrophages and bone marrow-derived macrophages were found to be phenotypically distinct from macrophages in atherosclerotic lesions of mice187. When comparing gene expression in macrophages isolated from these lesions, researchers found a gradual decrease in pro-resolving macrophage markers with increasing disease severity187, in line with the impaired efferocytosis and resolution that occurs as atherosclerosis progresses9–11. A contributing factor may be hypoxia, since chronically inflamed tissues are often poorly oxygenated which promotes hypoxia-driven inflammatory signaling through HIF1α. Mice with hematopoietic HIF1α-deficiency show less pro-inflammatory macrophages and reduced atherosclerosis188, while reversal of hypoxia in atherosclerotic plaques of mice through carbon oxygenation enhances macrophage efferocytosis and prevents plaque progression189. The majority of in vitro studies on macrophages are performed under normoxic conditions, which is another aspect that may limit translation to an in vivo inflammatory setting characterized by a low oxygen availability.
Most chronic inflammatory diseases would benefit from a therapeutic strategy that promotes tissue resolution by shifting the balance from inflammatory to pro-resolving macrophages. In cancer, however, the situation is more complex. A persistent inflammatory stimulus can drive oncogenesis by inducing DNA damage, stimulating proliferation, and conferring resistance to apoptosis190. However, established tumors need to evade detection by the immune system in order to grow and metastasize. Many types of human tumors have developed ways of suppressing the immune system to enhance their survival, which is a major hurdle in the development of anti-cancer therapies. As mentioned in section 2.3, an important mechanism of tumor-mediated immunosuppression involves the glycolytic end-product lactate. Lactate released by cancer cells drives the pro-resolving phenotype of tumor-associated macrophages. Macrophages that undergo efferocytosis promote a permissive tumor microenvironment through the production of anti-inflammatory cytokines and SPMs. Other contributors to tumor-mediated immunosuppression include PtdSer and PtdSer-receptors such as MerTK191, major histocompatibility complex (MHC) class I192, CD24193, CD47-SIRP1 interaction194, and PD-1-PD-L1 signaling195, which are currently being investigated as therapeutic targets to increase the tumoricidal activity of the immune system. This situation raises the intriguing questions as to whether pro-efferocytic/pro-resolving therapy for chronic inflammatory diseases may run the risk of promoting nascent tumor growth and, conversely, if tumor growth can be prevented by blocking the immunosuppressive pathways of efferocytic macrophages, but at the risk of triggering of a systemic inflammatory response. Perhaps highly targeted therapy can overcome these potential problems, i.e., by promoting efferocytosis in specific areas of inflammation, as has been demonstrated preclinically for atherosclerosis116,131, and by using tumor-targeted therapy for blocking efferocytosis-induced immunosuppression151.
5. CONCLUDING REMARKS
Extensive work has shown that efferocytosis-induced signaling, including signaling triggered by the cargo of engulfed and degraded apoptotic cells, changes metabolic pathways within the efferocytosing macrophage to promote pro-resolving functions. These studies have provided insight into potentially new therapeutic strategies to promote inflammation resolution in chronic inflammatory diseases, or, conversely, to prevent tumor-mediated immunosuppression in cancer. Examples of pathways discussed in this review that may be amenable to therapeutic manipulation include ARG1/ODC-mediated polyamine metabolism, lactate signaling, and SPM biosynthesis. Although therapeutic translation of pre-clinical findings is hindered by the lack of good models that reflect human macrophage biology in vivo, there are a few hints that some of these strategies may hold promise in a clinical setting, such as anti-CD47 antibody to block “don’t-eat-me” signaling and lactate supplementation to promote inflammation resolution. However, much more extensive clinical testing, supported by robust pre-clinical studies, will be needed to fully evaluate the potential disease-modifying benefits and safety profile of drugging efferocytosis-fueled macrophage metabolism.
ACKNOWLEDGEMENTS
This work was supported by an American Heart Association Postdoctoral Fellowship (900337 to M.S.), the Niels Stensen Fellowship (to M.S.) and NIH/NHLBI grants R35-HL145228, P01-HL087123, and R01-HL159012 (to I.T.). The authors acknowledge the previous postdoctoral fellows in the Tabas laboratory whose work was cited in this review, notably, Drs. Arif Yurdagul, Jr., Brennan Gerlach, and Patrick Ampomah.
Footnotes
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
REFERENCES
- 1.Arandjelovic S, Ravichandran KS. Phagocytosis of apoptotic cells in homeostasis. Nat Immunol. 2015;16(9):907–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wood W, Turmaine M, Weber R, et al. Mesenchymal cells engulf and clear apoptotic footplate cells in macrophageless PU.1 null mouse embryos. Development. 2000;127(24):5245–5252. [DOI] [PubMed] [Google Scholar]
- 3.Juncadella IJ, Kadl A, Sharma AK, et al. Apoptotic cell clearance by bronchial epithelial cells critically influences airway inflammation. Nature. 2013;493(7433):547–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bäck M, Yurdagul A Jr., Tabas I, Öörni K, Kovanen PT. Inflammation and its resolution in atherosclerosis: mediators and therapeutic opportunities. Nat Rev Cardiol. 2019;16(7):389–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dalli J, Serhan CN. Pro-resolving mediators in regulating and conferring macrophage function. Front Immunol. 2017;8:1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Szondy Z, Garabuczi E, Joós G, Tsay GJ, Sarang Z. Impaired clearance of apoptotic cells in chronic inflammatory diseases: therapeutic implications. Front Immunol. 2014;5:354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doran AC, Yurdagul A, Tabas I. Efferocytosis in health and disease. Nat Rev Immunol. 2020;20(4):254–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morioka S, Maueröder C, Ravichandran KS. Living on the edge: efferocytosis at the interface of homeostasis and pathology. Immunity. 2019;50(5):1149–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Linton MF, Babaev VR, Huang J, Linton EF, Tao H, Yancey PG. Macrophage apoptosis and efferocytosis in the pathogenesis of atherosclerosis. Circ J. 2016;80(11):2259–2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kojima Y, Weissman IL, Leeper NJ. The role of efferocytosis in atherosclerosis. Circulation. 2017;135(5):476–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yurdagul A Jr., Doran AC, Cai B, Fredman G, Tabas IA. Mechanisms and consequences of defective efferocytosis in atherosclerosis. Front Cardiovasc Med. 2017;4:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kasikara C, Doran AC, Cai B, Tabas I. The role of non-resolving inflammation in atherosclerosis. J Clin Invest. 2018;128(7):2713–2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nicholls SJ, Puri R, Anderson T, et al. Effect of evolocumab on progression of coronary disease in statin-treated patients: the GLAGOV randomized clinical trial. JAMA. 2016;316(22):2373–2384. [DOI] [PubMed] [Google Scholar]
- 14.Nissen SE, Nicholls SJ, Sipahi I, et al. Effect of very high-intensity statin therapy on regression of coronary atherosclerosis: the ASTEROID trial. JAMA. 2006;295(13):1556–1565. [DOI] [PubMed] [Google Scholar]
- 15.Hochreiter-Hufford A, Ravichandran KS. Clearing the dead: apoptotic cell sensing, recognition, engulfment, and digestion. Cold Spring Harb Perspect Biol. 2013;5(1):a008748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elliott MR, Ravichandran KS. The dynamics of apoptotic cell clearance. Dev Cell. 2016;38(2):147–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elliott MR, Chekeni FB, Trampont PC, et al. Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature. 2009;461(7261):282–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Truman LA, Ford CA, Pasikowska M, et al. CX3CL1/fractalkine is released from apoptotic lymphocytes to stimulate macrophage chemotaxis. Blood. 2008;112(13):5026–5036. [DOI] [PubMed] [Google Scholar]
- 19.Luo B, Gan W, Liu Z, et al. Erythropoeitin signaling in macrophages promotes dying cell clearance and immune tolerance. Immunity. 2016;44(2):287–302. [DOI] [PubMed] [Google Scholar]
- 20.Hanayama R, Tanaka M, Miwa K, Shinohara A, Iwamatsu A, Nagata S. Identification of a factor that links apoptotic cells to phagocytes. Nature. 2002;417(6885):182–187. [DOI] [PubMed] [Google Scholar]
- 21.Ishimoto Y, Ohashi K, Mizuno K, Nakano T. Promotion of the uptake of PS liposomes and apoptotic cells by a product of growth arrest-specific gene, gas6. J Biol Chem. 2000;127(3):411–417. [DOI] [PubMed] [Google Scholar]
- 22.Anderson HA, Maylock CA, Williams JA, Paweletz CP, Shu H, Shacter E. Serum-derived protein S binds to phosphatidylserine and stimulates the phagocytosis of apoptotic cells. Nat Immunol. 2003;4(1):87–91. [DOI] [PubMed] [Google Scholar]
- 23.Suzuki J, Denning DP, Imanishi E, Horvitz HR, Nagata S. Xk-related protein 8 and CED-8 promote phosphatidylserine exposure in apoptotic cells. Science. 2013;341(6144):403–406. [DOI] [PubMed] [Google Scholar]
- 24.Suzuki J, Imanishi E, Nagata S. Xkr8 phospholipid scrambling complex in apoptotic phosphatidylserine exposure. Proc Natl Acad Sci U S A. 2016;113(34):9509–9514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li MO, Sarkisian MR, Mehal WZ, Rakic P, Flavell RA. Phosphatidylserine receptor is required for clearance of apoptotic cells. Science. 2003;302(5650):1560–1563. [DOI] [PubMed] [Google Scholar]
- 26.Gardai SJ, McPhillips KA, Frasch SC, et al. Cell-surface calreticulin initiates clearance of viable or apoptotic cells through trans-activation of LRP on the phagocyte. Cell. 2005;123(2):321–334. [DOI] [PubMed] [Google Scholar]
- 27.Savill J, Hogg N, Ren Y, Haslett C. Thrombospondin cooperates with CD36 and the vitronectin receptor in macrophage recognition of neutrophils undergoing apoptosis. J Clin Invest. 1992;90(4):1513–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scott RS, McMahon EJ, Pop SM, et al. Phagocytosis and clearance of apoptotic cells is mediated by MER. Nature. 2001;411(6834):207–211. [DOI] [PubMed] [Google Scholar]
- 29.Kelley SM, Ravichandran KS. Putting the brakes on phagocytosis: “don’t-eat-me” signaling in physiology and disease. EMBO Rep. 2021;22(6):e52564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gerlach BD, Marinello M, Heinz J, et al. Resolvin D1 promotes the targeting and clearance of necroptotic cells. Cell Death Differ. 2020;27(2):525–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shi H, Wang X, Li F, et al. CD47-SIRPα axis blockade in NASH promotes necroptotic hepatocyte clearance by liver macrophages and decreases hepatic fibrosis. Sci Transl Med. 2022;14(672):eabp8309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chao MP, Weissman IL, Majeti R. The CD47-SIRPα pathway in cancer immune evasion and potential therapeutic implications. Curr Opin Immunol. 2012;24(2):225–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Castellano F, Montcourrier P, Chavrier P. Membrane recruitment of Rac1 triggers phagocytosis. J Cell Sci. 2000;113 (Pt 17):2955–2961. [DOI] [PubMed] [Google Scholar]
- 34.Wang Y, Subramanian M, Yurdagul A Jr., et al. Mitochondrial fission promotes the continued clearance of apoptotic cells by macrophages. Cell. 2017;171(2):331–345.e322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yin C, Argintaru D, Heit B. Rab17 mediates intermixing of phagocytosed apoptotic cells with recycling endosomes. Small GTPases. 2019;10(3):218–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aderem A How to eat something bigger than your head. Cell. 2002;110(1):5–8. [DOI] [PubMed] [Google Scholar]
- 37.Yurdagul A Jr., Subramanian M, Wang X, et al. Macrophage metabolism of apoptotic cell-derived arginine promotes continual efferocytosis and resolution of injury. Cell Metab. 2020;31(3):518–533.e510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kelly B, Pearce EL. Amino assets: how amino acids support immunity. Cell Metab. 2020;32(2):154–175. [DOI] [PubMed] [Google Scholar]
- 39.McCubbrey AL, McManus SA, McClendon JD, et al. Polyamine import and accumulation causes immunomodulation in macrophages engulfing apoptotic cells. Cell Rep. 2022;38(2):110222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Puleston DJ, Buck MD, Klein Geltink RI, et al. Polyamines and eIF5A hypusination modulate mitochondrial respiration and macrophage activation. Cell Metab. 2019;30(2):352–363.e358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rodriguez AE, Ducker GS, Billingham LK, et al. Serine metabolism supports macrophage IL-1β production. Cell Metab. 2019;29(4):1003–1011.e1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yu W, Wang Z, Zhang K, et al. One-carbon metabolism supports s-adenosylmethionine and histone methylation to drive inflammatory macrophages. Mol Cell. 2019;75(6):1147–1160.e1145. [DOI] [PubMed] [Google Scholar]
- 43.Ampomah PB, Cai B, Sukka SR, et al. Macrophages use apoptotic cell-derived methionine and DNMT3A during efferocytosis to promote tissue resolution. Nat Metab. 2022;4(4):444–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jaiswal S, Natarajan P, Silver AJ, et al. Clonal hematopoiesis and risk of atherosclerotic cardiovascular disease. N Engl J Med. 2017;377(2):111–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yao K, Fang J, Yin YL, Feng ZM, Tang ZR, Wu G. Tryptophan metabolism in animals: important roles in nutrition and health. Front Biosci (Schol Ed). 2011;3(1):286–297. [DOI] [PubMed] [Google Scholar]
- 46.Badawy AA. Kynurenine pathway of tryptophan metabolism: regulatory and functional aspects. Int J Tryptophan Res. 2017;10:1178646917691938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ravishankar B, Liu H, Shinde R, et al. Tolerance to apoptotic cells is regulated by indoleamine 2,3-dioxygenase. Proc Natl Acad Sci U S A. 2012;109(10):3909–3914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ravishankar B, Liu H, Shinde R, et al. The amino acid sensor GCN2 inhibits inflammatory responses to apoptotic cells promoting tolerance and suppressing systemic autoimmunity. Proc Natl Acad Sci U S A. 2015;112(34):10774–10779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Salminen A Role of indoleamine 2,3-dioxygenase 1 (IDO1) and kynurenine pathway in the regulation of the aging process. Ageing Res Rev. 2022;75:101573. [DOI] [PubMed] [Google Scholar]
- 50.Viola A, Munari F, Sánchez-Rodríguez R, Scolaro T, Castegna A. The metabolic signature of macrophage responses. Front Immunol. 2019;10:1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Park D, Han CZ, Elliott MR, et al. Continued clearance of apoptotic cells critically depends on the phagocyte Ucp2 protein. Nature. 2011;477(7363):220–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang S, Weinberg S, DeBerge M, et al. Efferocytosis fuels requirements of fatty acid oxidation and the electron transport chain to polarize macrophages for tissue repair. Cell Metab. 2019;29(2):443–456.e445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Merlin J, Ivanov S, Dumont A, et al. Non-canonical glutamine transamination sustains efferocytosis by coupling redox buffering to oxidative phosphorylation. Nat Metab. 2021;3(10):1313–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li C, Wang Y, Li Y, et al. HIF1α-dependent glycolysis promotes macrophage functional activities in protecting against bacterial and fungal infection. Sci Rep. 2018;8(1):3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang T, Liu H, Lian G, Zhang SY, Wang X, Jiang C. HIF1α-induced glycolysis metabolism Is essential to the activation of inflammatory macrophages. Mediators Inflamm. 2017;2017:9029327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jha AK, Huang SC, Sergushichev A, et al. Network integration of parallel metabolic and transcriptional data reveals metabolic modules that regulate macrophage polarization. Immunity. 2015;42(3):419–430. [DOI] [PubMed] [Google Scholar]
- 57.Murphy MP, O’Neill LAJ. Krebs cycle reimagined: the emerging roles of succinate and itaconate as signal transducers. Cell. 2018;174(4):780–784. [DOI] [PubMed] [Google Scholar]
- 58.Tannahill GM, Curtis AM, Adamik J, et al. Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature. 2013;496(7444):238–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mills EL, Kelly B, Logan A, et al. Succinate dehydrogenase supports metabolic repurposing of mitochondria to drive inflammatory macrophages. Cell. 2016;167(2):457–470.e413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Morioka S, Perry JSA, Raymond MH, et al. Efferocytosis induces a novel SLC program to promote glucose uptake and lactate release. Nature. 2018;563(7733):714–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Van den Bossche J, Baardman J, Otto NA, et al. Mitochondrial dysfunction prevents repolarization of inflammatory macrophages. Cell Rep. 2016;17(3):684–696. [DOI] [PubMed] [Google Scholar]
- 62.Van den Bossche J, O’Neill LA, Menon D. Macrophage immunometabolism: where are we (going)? Trends Immunol. 2017;38(6):395–406. [DOI] [PubMed] [Google Scholar]
- 63.Schilperoort M, Ngai D, Katerelos M, Power D, Tabas I. PFKFB2-mediated glycolysis promotes lactate-driven continual efferocytosis by macrophages. Nat Metab. Accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang D, Tang Z, Huang H, et al. Metabolic regulation of gene expression by histone lactylation. Nature. 2019;574(7779):575–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Noe JT, Rendon BE, Geller AE, et al. Lactate supports a metabolic-epigenetic link in macrophage polarization. Sci Adv. 2021;7(46):eabi8602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hui S, Ghergurovich JM, Morscher RJ, et al. Glucose feeds the TCA cycle via circulating lactate. Nature. 2017;551(7678):115–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jha MK, Passero JV, Rawat A, et al. Macrophage monocarboxylate transporter 1 promotes peripheral nerve regeneration after injury in mice. J Clin Invest. 2021;131(21). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Manoharan I, Prasad PD, Thangaraju M, Manicassamy S. Lactate-dependent regulation of immune responses by dendritic cells and macrophages. Front Immunol. 2021;12:691134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cai H, Wang X, Zhang Z, et al. Moderate l-lactate administration suppresses adipose tissue macrophage M1 polarization to alleviate obesity-associated insulin resistance. J Biol Chem. 2022;298(4):101768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sag D, Carling D, Stout RD, Suttles J. Adenosine 5’-monophosphate-activated protein kinase promotes macrophage polarization to an anti-inflammatory functional phenotype. J Immunol. 2008;181(12):8633–8641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Park SY, Lee SW, Lee SY, et al. SIRT1/adenosine monophosphate-activated protein kinase α signaling enhances macrophage polarization to an anti-inflammatory phenotype in rheumatoid arthritis. Front Immunol. 2017;8:1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jiang S, Park DW, Stigler WS, et al. Mitochondria and AMP-activated protein kinase-dependent mechanism of efferocytosis. J Biol Chem. 2013;288(36):26013–26026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.McCubbrey AL, Nelson JD, Stolberg VR, et al. MicroRNA-34a negatively regulates efferocytosis by tissue macrophages in part via SIRT1. J Immunol. 2016;196(3):1366–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Perry JSA, Morioka S, Medina CB, et al. Interpreting an apoptotic corpse as anti-inflammatory involves a chloride sensing pathway. Nat Cell Biol. 2019;21(12):1532–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Medina CB, Mehrotra P, Arandjelovic S, et al. Metabolites released from apoptotic cells act as tissue messengers. Nature. 2020;580(7801):130–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cauwels A, Rogge E, Vandendriessche B, Shiva S, Brouckaert P. Extracellular ATP drives systemic inflammation, tissue damage and mortality. Cell Death Dis. 2014;5(3):e1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Colegio OR, Chu NQ, Szabo AL, et al. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature. 2014;513(7519):559–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stincone A, Prigione A, Cramer T, et al. The return of metabolism: biochemistry and physiology of the pentose phosphate pathway. Biol Rev Camb Philos Soc. 2015;90(3):927–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Haschemi A, Kosma P, Gille L, et al. The sedoheptulose kinase CARKL directs macrophage polarization through control of glucose metabolism. Cell Metab. 2012;15(6):813–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang Y-T, Trzeciak AJ, Rojas WS, et al. Metabolic adaptation supports enhanced macrophage efferocytosis in limited-oxygen environments. Cell Metab. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.He D, Mao Q, Jia J, et al. Pentose phosphate pathway regulates tolerogenic apoptotic cell clearance and immune tolerance. Front Immunol. 2021;12:797091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mao Q, Luo B, Mei J, et al. Macrophage Dicer promotes tolerogenic apoptotic cell clearance and immune tolerance by inhibiting pentose phosphate pathway activity. Cell Mol Immunol. 2021;18(7):1841–1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Robbins CS, Hilgendorf I, Weber GF, et al. Local proliferation dominates lesional macrophage accumulation in atherosclerosis. Nat Med. 2013;19(9):1166–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bosurgi L, Cao YG, Cabeza-Cabrerizo M, et al. Macrophage function in tissue repair and remodeling requires IL-4 or IL-13 with apoptotic cells. Science. 2017;356(6342):1072–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jarjour NN, Schwarzkopf EA, Bradstreet TR, et al. Bhlhe40 mediates tissue-specific control of macrophage proliferation in homeostasis and type 2 immunity. Nat Immunol. 2019;20(6):687–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jenkins SJ, Ruckerl D, Cook PC, et al. Local macrophage proliferation, rather than recruitment from the blood, is a signature of TH2 inflammation. Science. 2011;332(6035):1284–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gerlach BD, Ampomah PB, Yurdagul A Jr., et al. Efferocytosis induces macrophage proliferation to help resolve tissue injury. Cell Metab. 2021;33(12):2445–2463.e2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bello-Fernandez C, Packham G, Cleveland JL. The ornithine decarboxylase gene is a transcriptional target of c-Myc. Proc Natl Acad Sci U S A. 1993;90(16):7804–7808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ben-Yosef T, Yanuka O, Halle D, Benvenisty N. Involvement of Myc targets in c-myc and N-myc induced human tumors. Oncogene. 1998;17(2):165–171. [DOI] [PubMed] [Google Scholar]
- 90.Tabas I Consequences of cellular cholesterol accumulation: basic concepts and physiological implications. J Clin Invest. 2002;110(7):905–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cui D, Thorp E, Li Y, et al. Pivotal advance: macrophages become resistant to cholesterol-induced death after phagocytosis of apoptotic cells. J Leukoc Biol. 2007;82(5):1040–1050. [DOI] [PubMed] [Google Scholar]
- 92.N AG, Bensinger SJ, Hong C, et al. Apoptotic cells promote their own clearance and immune tolerance through activation of the nuclear receptor LXR. Immunity. 2009;31(2):245–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Costales P, Castellano J, Revuelta-López E, et al. Lipopolysaccharide downregulates CD91/low-density lipoprotein receptor-related protein 1 expression through SREBP-1 overexpression in human macrophages. Atherosclerosis. 2013;227(1):79–88. [DOI] [PubMed] [Google Scholar]
- 94.Oishi Y, Spann NJ, Link VM, et al. SREBP1 contributes to resolution of pro-inflammatory TLR4 signaling by reprogramming fatty acid metabolism. Cell Metab. 2017;25(2):412–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cai B, Thorp EB, Doran AC, et al. MerTK cleavage limits proresolving mediator biosynthesis and exacerbates tissue inflammation. Proc Natl Acad Sci U S A. 2016;113(23):6526–6531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cai B, Kasikara C, Doran AC, Ramakrishnan R, Birge RB, Tabas I. MerTK signaling in macrophages promotes the synthesis of inflammation resolution mediators by suppressing CaMKII activity. Sci Signal. 2018;11(549). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Schif-Zuck S, Gross N, Assi S, Rostoker R, Serhan CN, Ariel A. Saturated-efferocytosis generates pro-resolving CD11b low macrophages: modulation by resolvins and glucocorticoids. Eur J Immunol. 2011;41(2):366–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dalli J, Serhan C. Macrophage proresolving mediators - the when and where. Microbiol Spectr. 2016;4(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dalli J, Serhan CN. Specific lipid mediator signatures of human phagocytes: microparticles stimulate macrophage efferocytosis and pro-resolving mediators. Blood. 2012;120(15):e60–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Uderhardt S, Herrmann M, Oskolkova Olga V, et al. 12/15-lipoxygenase orchestrates the clearance of apoptotic cells and maintains immunologic tolerance. Immunity. 2012;36(5):834–846. [DOI] [PubMed] [Google Scholar]
- 101.Tabas I, Glass CK. Anti-inflammatory therapy in chronic disease: challenges and opportunities. Science. 2013;339(6116):166–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Charles P, Elliott MJ, Davis D, et al. Regulation of cytokines, cytokine inhibitors, and acute-phase proteins following anti-TNF-alpha therapy in rheumatoid arthritis. J Immunol. 1999;163(3):1521–1528. [PubMed] [Google Scholar]
- 103.Elliott MJ, Maini RN, Feldmann M, et al. Repeated therapy with monoclonal antibody to tumour necrosis factor alpha (cA2) in patients with rheumatoid arthritis. Lancet. 1994;344(8930):1125–1127. [DOI] [PubMed] [Google Scholar]
- 104.Adegbola SO, Sahnan K, Warusavitarne J, Hart A, Tozer P. Anti-TNF therapy in Crohn’s disease. Int J Mol Sci. 2018;19(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tardif J-C, Kouz S, Waters DD, et al. Efficacy and Safety of Low-Dose Colchicine after Myocardial Infarction. New England Journal of Medicine. 2019;381(26):2497–2505. [DOI] [PubMed] [Google Scholar]
- 106.Ridker PM, Everett BM, Thuren T, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377(12):1119–1131. [DOI] [PubMed] [Google Scholar]
- 107.Eisenberg T, Abdellatif M, Schroeder S, et al. Cardioprotection and lifespan extension by the natural polyamine spermidine. Nat Med. 2016;22(12):1428–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol. 2011;11(11):723–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Teupser D, Burkhardt R, Wilfert W, Haffner I, Nebendahl K, Thiery J. Identification of macrophage arginase I as a new candidate gene of atherosclerosis resistance. Arterioscler Thromb Vasc Biol. 2006;26(2):365–371. [DOI] [PubMed] [Google Scholar]
- 110.Pourcet B, Feig JE, Vengrenyuk Y, et al. LXRα regulates macrophage arginase 1 through PU.1 and interferon regulatory factor 8. Circ Res. 2011;109(5):492–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Harder JW, Ma J, Alard P, et al. Male microbiota-associated metabolite restores macrophage efferocytosis in female lupus-prone mice via activation of PPARγ/LXR signaling pathways. J Leukoc Biol. 2023;113(1):41–57. [DOI] [PubMed] [Google Scholar]
- 112.Fessler MB. The challenges and promise of targeting the Liver X Receptors for treatment of inflammatory disease. Pharmacol Ther. 2018;181:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chen W, Schilperoort M, Cao Y, Shi J, Tabas I, Tao W. Macrophage-targeted nanomedicine for the diagnosis and treatment of atherosclerosis. Nat Rev Cardiol. 2022;19(4):228–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yu M, Amengual J, Menon A, et al. Targeted nanotherapeutics encapsulating liver X receptor agonist GW3965 enhance antiatherogenic effects without adverse effects on hepatic lipid metabolism in Ldlr(−/−) mice. Adv Healthc Mater. 2017;6(20). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Doran AC, Ozcan L, Cai B, et al. CAMKIIγ suppresses an efferocytosis pathway in macrophages and promotes atherosclerotic plaque necrosis. J Clin Invest. 2017;127(11):4075–4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Tao W, Yurdagul A Jr., Kong N, et al. siRNA nanoparticles targeting CaMKIIγ in lesional macrophages improve atherosclerotic plaque stability in mice. Sci Transl Med. 2020;12(553). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rinne P, Guillamat-Prats R, Rami M, et al. Palmitoylethanolamide promotes a proresolving macrophage phenotype and attenuates atherosclerotic plaque formation. Arterioscler Thromb Vasc Biol. 2018;38(11):2562–2575. [DOI] [PubMed] [Google Scholar]
- 118.Chen Z, Zhuo R, Zhao Y, et al. Oleoylethanolamide stabilizes atherosclerotic plaque through regulating macrophage polarization via AMPK-PPARα pathway. Biochem Biophys Res Commun. 2020;524(2):308–316. [DOI] [PubMed] [Google Scholar]
- 119.Zarrow JE, Alli-Oluwafuyi AM, Youwakim CM, et al. Small molecule activation of NAPE-PLD enhances efferocytosis by macrophages. BioRxiv. 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lee SWL, Paoletti C, Campisi M, et al. MicroRNA delivery through nanoparticles. J Control Release. 2019;313:80–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Qu J, Yang J, Chen M, Wei R, Tian J. CircFLNA acts as a sponge of miR-646 to facilitate the proliferation, metastasis, glycolysis, and apoptosis inhibition of gastric cancer by targeting PFKFB2. Cancer Manag Res. 2020;12:8093–8103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhang S, Liu Y, Jing W, et al. Remodeling articular immune homeostasis with an efferocytosis-informed nanoimitator mitigates rheumatoid arthritis in mice. Nat Commun. 2023;14(1):817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Fredman G, Hellmann J, Proto JD, et al. An imbalance between specialized pro-resolving lipid mediators and pro-inflammatory leukotrienes promotes instability of atherosclerotic plaques. Nat Commun. 2016;7(1):12859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Karp CL, Flick LM, Park KW, et al. Defective lipoxin-mediated anti-inflammatory activity in the cystic fibrosis airway. Nat Immunol. 2004;5(4):388–392. [DOI] [PubMed] [Google Scholar]
- 125.Levy BD, Bonnans C, Silverman ES, Palmer LJ, Marigowda G, Israel E. Diminished lipoxin biosynthesis in severe asthma. Am J Respir Crit Care Med. 2005;172(7):824–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Fredman G, Oh SF, Ayilavarapu S, Hasturk H, Serhan CN, Van Dyke TE. Impaired phagocytosis in localized aggressive periodontitis: rescue by Resolvin E1. PLoS One. 2011;6(9):e24422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Dalli J, Gomez EA, Jouvene CC. Utility of the specialized pro-resolving mediators as diagnostic and prognostic biomarkers in disease. Biomolecules. 2022;12(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Basil MC, Levy BD. Specialized pro-resolving mediators: endogenous regulators of infection and inflammation. Nat Rev Immunol. 2016;16(1):51–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hasturk H, Abdallah R, Kantarci A, et al. Resolvin E1 (RvE1) Attenuates Atherosclerotic Plaque Formation in Diet and Inflammation-Induced Atherogenesis. Arterioscler Thromb Vasc Biol. 2015;35(5):1123–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Miyahara T, Runge S, Chatterjee A, et al. D-series resolvin attenuates vascular smooth muscle cell activation and neointimal hyperplasia following vascular injury. FASEB J. 2013;27(6):2220–2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Fredman G, Kamaly N, Spolitu S, et al. Targeted nanoparticles containing the proresolving peptide Ac2-26 protect against advanced atherosclerosis in hypercholesterolemic mice. Sci Transl Med. 2015;7(275):275ra220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kamaly N, Fredman G, Fojas JJ, et al. Targeted interleukin-10 nanotherapeutics developed with a microfluidic chip enhance resolution of inflammation in advanced atherosclerosis. ACS Nano. 2016;10(5):5280–5292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Motwani MP, Colas RA, George MJ, et al. Pro-resolving mediators promote resolution in a human skin model of UV-killed Escherichia coli-driven acute inflammation. JCI Insight. 2018;3(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Norris PC, Skulas-Ray AC, Riley I, et al. Identification of specialized pro-resolving mediator clusters from healthy adults after intravenous low-dose endotoxin and omega-3 supplementation: a methodological validation. Sci Rep. 2018;8(1):18050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Gao Y, Su J, Zhang Y, et al. Dietary DHA amplifies LXA(4) circuits in tissues and lymph node PMN and is protective in immune-driven dry eye disease. Mucosal Immunol. 2018;11(6):1674–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Polus A, Zapala B, Razny U, et al. Omega-3 fatty acid supplementation influences the whole blood transcriptome in women with obesity, associated with pro-resolving lipid mediator production. Biochim Biophys Acta. 2016;1861(11):1746–1755. [DOI] [PubMed] [Google Scholar]
- 137.Souza PR, Marques RM, Gomez EA, et al. Enriched marine oil supplements increase peripheral blood specialized pro-resolving mediators concentrations and reprogram host immune responses: a randomized double-blind placebo-controlled study. Circ Res. 2020;126(1):75–90. [DOI] [PubMed] [Google Scholar]
- 138.Mas E, Croft KD, Zahra P, Barden A, Mori TA. Resolvins D1, D2, and other mediators of self-limited resolution of inflammation in human blood following n-3 fatty acid supplementation. Clin Chem. 2012;58(10):1476–1484. [DOI] [PubMed] [Google Scholar]
- 139.Al-Shaer AE, Regan J, Buddenbaum N, et al. Enriched marine oil supplement increases specific plasma specialized pro-resolving mediators in adults with obesity. J Nutr. 2022;152(7):1783–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Elajami TK, Colas RA, Dalli J, Chiang N, Serhan CN, Welty FK. Specialized proresolving lipid mediators in patients with coronary artery disease and their potential for clot remodeling. FASEB J. 2016;30(8):2792–2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Schaller MS, Chen M, Colas RA, et al. Treatment with a marine oil supplement alters lipid mediators and leukocyte phenotype in healthy patients and those with peripheral artery disease. J Am Heart Assoc. 2020;9(15):e016113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Assimes TL, Knowles JW, Priest JR, et al. A near null variant of 12/15-LOX encoded by a novel SNP in ALOX15 and the risk of coronary artery disease. Atherosclerosis. 2008;198(1):136–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.McCaskie PA, Beilby JP, Hung J, et al. 15-Lipoxygenase gene variants are associated with carotid plaque but not carotid intima-media thickness. Hum Genet. 2008;123(5):445–453. [DOI] [PubMed] [Google Scholar]
- 144.Zhang K, Wang YY, Liu QJ, et al. Two single nucleotide polymorphisms in ALOX15 are associated with risk of coronary artery disease in a Chinese Han population. Heart Vessels. 2010;25(5):368–373. [DOI] [PubMed] [Google Scholar]
- 145.Berthet C, Castillo X, Magistretti PJ, Hirt L. New evidence of neuroprotection by lactate after transient focal cerebral ischaemia: extended benefit after intracerebroventricular injection and efficacy of intravenous administration. Cerebrovasc Dis. 2012;34(5–6):329–335. [DOI] [PubMed] [Google Scholar]
- 146.Tauffenberger A, Fiumelli H, Almustafa S, Magistretti PJ. Lactate and pyruvate promote oxidative stress resistance through hormetic ROS signaling. Cell Death Dis. 2019;10(9):653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Miranda M, Morici JF, Zanoni MB, Bekinschtein P. Brain-derived neurotrophic factor: a key molecule for memory in the healthy and the pathological brain. Front Cell Neurosci. 2019;13:363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.El Hayek L, Khalifeh M, Zibara V, et al. Lactate mediates the effects of exercise on learning and memory through SIRT1-dependent activation of hippocampal brain-derived neurotrophic factor (BDNF). J Neurosci. 2019;39(13):2369–2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Koyama T Lactated Ringer’s solution for preventing myocardial reperfusion injury. Int J Cardiol Heart Vasc. 2017;15:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ratter JM, Rooijackers HMM, Hooiveld GJ, et al. In vitro and in vivo effects of lactate on metabolism and cytokine production of human primary PBMCs and monocytes. Front Immunol. 2018;9:2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Mehrotra P, Ravichandran KS. Drugging the efferocytosis process: concepts and opportunities. Nat Rev Drug Discov. 2022;21(8):601–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Karbian N, Abutbul A, El-Amore R, et al. Apoptotic cell therapy for cytokine storm associated with acute severe sepsis. Cell Death Dis. 2020;11(7):535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Bonnefoy F, Daoui A, Valmary-Degano S, Toussirot E, Saas P, Perruche S. Apoptotic cell infusion treats ongoing collagen-induced arthritis, even in the presence of methotrexate, and is synergic with anti-TNF therapy. Arthritis Res Ther. 2016;18(1):184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Pang SHM, D’Rozario J, Mendonca S, et al. Mesenchymal stromal cell apoptosis is required for their therapeutic function. Nature Communications. 2021;12(1):6495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.De Maeyer RPH, van de Merwe RC, Louie R, et al. Blocking elevated p38 MAPK restores efferocytosis and inflammatory resolution in the elderly. Nat Immunol. 2020;21(6):615–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Schloesser D, Lindenthal L, Sauer J, et al. Senescent cells suppress macrophage-mediated corpse removal via upregulation of the CD47-QPCT/L axis. J Cell Biol. 2022;222(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kojima Y, Volkmer JP, McKenna K, et al. CD47-blocking antibodies restore phagocytosis and prevent atherosclerosis. Nature. 2016;536(7614):86–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Jarr KU, Nakamoto R, Doan BH, et al. Effect of CD47 blockade on vascular inflammation. N Engl J Med. 2021;384(4):382–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Morioka S, Kajioka D, Yamaoka Y, et al. Chimeric efferocytic receptors improve apoptotic cell clearance and alleviate inflammation. Cell. 2022;185(26):4887–4903.e4817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Chen J, Zhou R, Feng Y, Cheng L. Molecular mechanisms of exercise contributing to tissue regeneration. Signal Transduct Target Ther. 2022;7(1):383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Gleeson M, Bishop NC, Stensel DJ, Lindley MR, Mastana SS, Nimmo MA. The anti-inflammatory effects of exercise: mechanisms and implications for the prevention and treatment of disease. Nat Rev Immunol. 2011;11(9):607–615. [DOI] [PubMed] [Google Scholar]
- 162.Xue X, Liu B, Hu J, Bian X, Lou S. The potential mechanisms of lactate in mediating exercise-enhanced cognitive function: a dual role as an energy supply substrate and a signaling molecule. Nutr Metab (Lond). 2022;19(1):52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Owens DJ, Twist C, Cobley JN, Howatson G, Close GL. Exercise-induced muscle damage: What is it, what causes it and what are the nutritional solutions? Eur J Sport Sci. 2019;19(1):71–85. [DOI] [PubMed] [Google Scholar]
- 164.Panci G, Chazaud B. Inflammation during post-injury skeletal muscle regeneration. Semin Cell Dev Biol. 2021;119:32–38. [DOI] [PubMed] [Google Scholar]
- 165.Juban G, Chazaud B. Efferocytosis during skeletal muscle regeneration. Cells. 2021;10(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ohno Y, Ando K, Ito T, et al. Lactate stimulates a potential for hypertrophy and regeneration of mouse skeletal muscle. Nutrients. 2019;11(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Zhang J, Muri J, Fitzgerald G, et al. Endothelial lactate controls muscle regeneration from ischemia by inducing M2-like macrophage polarization. Cell Metab. 2020;31(6):1136–1153.e1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Brooks GA, Osmond AD, Arevalo JA, et al. Lactate as a myokine and exerkine: drivers and signals of physiology and metabolism. J Appl Physiol. 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Li VL, He Y, Contrepois K, et al. An exercise-inducible metabolite that suppresses feeding and obesity. Nature. 2022;606(7915):785–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Pavlou S, Wang L, Xu H, Chen M. Higher phagocytic activity of thioglycollate-elicited peritoneal macrophages is related to metabolic status of the cells. J Inflamm. 2017;14(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Davies LC, Rice CM, Palmieri EM, Taylor PR, Kuhns DB, McVicar DW. Peritoneal tissue-resident macrophages are metabolically poised to engage microbes using tissue-niche fuels. Nat Commun. 2017;8(1):2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Munder M, Mollinedo F, Calafat J, et al. Arginase I is constitutively expressed in human granulocytes and participates in fungicidal activity. Blood. 2005;105(6):2549–2556. [DOI] [PubMed] [Google Scholar]
- 173.Schneemann M, Schoedon G, Hofer S, Blau N, Guerrero L, Schaffner A. Nitric oxide synthase is not a constituent of the antimicrobial armature of human mononuclear phagocytes. J Infect Dis. 1993;167(6):1358–1363. [DOI] [PubMed] [Google Scholar]
- 174.Thomas AC, Mattila JT. “Of mice and men”: arginine metabolism in macrophages. Front Immunol. 2014;5:479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Cromheeke KM, Kockx MM, De Meyer GR, et al. Inducible nitric oxide synthase colocalizes with signs of lipid oxidation/peroxidation in human atherosclerotic plaques. Cardiovasc Res. 1999;43(3):744–754. [DOI] [PubMed] [Google Scholar]
- 176.Nicholson S, Bonecini-Almeida Mda G, Lapa e Silva JR, et al. Inducible nitric oxide synthase in pulmonary alveolar macrophages from patients with tuberculosis. J Exp Med. 1996;183(5):2293–2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Mattila JT, Ojo OO, Kepka-Lenhart D, et al. Microenvironments in tuberculous granulomas are delineated by distinct populations of macrophage subsets and expression of nitric oxide synthase and arginase isoforms. J Immunol. 2013;191(2):773–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Luoma JS, Strålin P, Marklund SL, Hiltunen TP, Särkioja T, Ylä-Herttuala S. Expression of extracellular SOD and iNOS in macrophages and smooth muscle cells in human and rabbit atherosclerotic lesions: colocalization with epitopes characteristic of oxidized LDL and peroxynitrite-modified proteins. Arterioscler Thromb Vasc Biol. 1998;18(2):157–167. [DOI] [PubMed] [Google Scholar]
- 179.Vijayan V, Pradhan P, Braud L, et al. Human and murine macrophages exhibit differential metabolic responses to lipopolysaccharide - A divergent role for glycolysis. Redox Biol. 2019;22:101147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Vats D, Mukundan L, Odegaard JI, et al. Oxidative metabolism and PGC-1beta attenuate macrophage-mediated inflammation. Cell Metab. 2006;4(1):13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Huang SC-C, Everts B, Ivanova Y, et al. Cell-intrinsic lysosomal lipolysis is essential for alternative activation of macrophages. Nat Immunol. 2014;15(9):846–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Namgaladze D, Brüne B. Fatty acid oxidation is dispensable for human macrophage IL-4-induced polarization. Biochim Biophys Acta. 2014;1841(9):1329–1335. [DOI] [PubMed] [Google Scholar]
- 183.Gosselin D, Link VM, Romanoski CE, et al. Environment drives selection and function of enhancers controlling tissue-specific macrophage identities. Cell. 2014;159(6):1327–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Lavin Y, Winter D, Blecher-Gonen R, et al. Tissue-resident macrophage enhancer landscapes are shaped by the local microenvironment. Cell. 2014;159(6):1312–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Wculek SK, Heras-Murillo I, Mastrangelo A, et al. Oxidative phosphorylation selectively orchestrates tissue macrophage homeostasis. Immunity. 2023. [DOI] [PubMed] [Google Scholar]
- 186.Moore KJ, Sheedy FJ, Fisher EA. Macrophages in atherosclerosis: a dynamic balance. Nat Rev Immunol. 2013;13(10):709–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Bisgaard LS, Mogensen CK, Rosendahl A, et al. Bone marrow-derived and peritoneal macrophages have different inflammatory response to oxLDL and M1/M2 marker expression - implications for atherosclerosis research. Sci Rep. 2016;6:35234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Aarup A, Pedersen TX, Junker N, et al. Hypoxia-inducible factor-1α expression in macrophages promotes development of atherosclerosis. Arterioscler Thromb Vasc Biol. 2016;36(9):1782–1790. [DOI] [PubMed] [Google Scholar]
- 189.Marsch E, Theelen TL, Demandt JA, et al. Reversal of hypoxia in murine atherosclerosis prevents necrotic core expansion by enhancing efferocytosis. Arterioscler Thromb Vasc Biol. 2014;34(12):2545–2553. [DOI] [PubMed] [Google Scholar]
- 190.Shacter E, Weitzman SA. Chronic inflammation and cancer. Oncology (Williston Park). 2002;16(2):217–226, 229; discussion 230-212. [PubMed] [Google Scholar]
- 191.Birge RB, Boeltz S, Kumar S, et al. Phosphatidylserine is a global immunosuppressive signal in efferocytosis, infectious disease, and cancer. Cell Death Differ. 2016;23(6):962–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Barkal AA, Weiskopf K, Kao KS, et al. Engagement of MHC class I by the inhibitory receptor LILRB1 suppresses macrophages and is a target of cancer immunotherapy. Nat Immunol. 2018;19(1):76–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Barkal AA, Brewer RE, Markovic M, et al. CD24 signalling through macrophage Siglec-10 is a target for cancer immunotherapy. Nature. 2019;572(7769):392–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Majeti R, Chao MP, Alizadeh AA, et al. CD47 is an adverse prognostic factor and therapeutic antibody target on human acute myeloid leukemia stem cells. Cell. 2009;138(2):286–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Gordon SR, Maute RL, Dulken BW, et al. PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity. Nature. 2017;545(7655):495–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.


