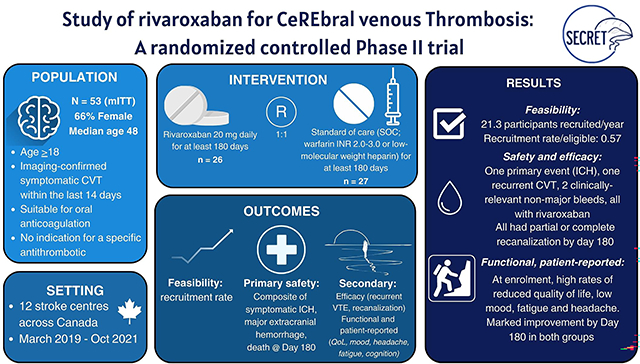
Abstract
Background:
Emerging data suggest that direct oral anticoagulants (DOACs) may be a suitable choice for anticoagulation for cerebral venous thrombosis (CVT). However, conducting high-quality trials in CVT is challenging as it is a rare disease with low rates of adverse outcomes such as major bleeding and functional dependence. To facilitate the design of future CVT trials, SECRET assessed (1) feasibility of recruitment, (2) safety of rivaroxaban compared to standard-of-care anticoagulation, and (3) patient-centered functional outcomes.
Methods:
This was a Phase II, prospective, open-label blinded-endpoint 1:1 randomized trial conducted at twelve Canadian centres. Participants were aged ≥18 years, within 14 days of a new diagnosis of symptomatic CVT and suitable for oral anticoagulation; they were randomized to receive rivaroxaban 20 mg daily, or standard-of-care anticoagulation (warfarin, target INR 2.0 – 3.0, or low molecular-weight heparin) for 180 days, with optional extension up to 365 days. Primary outcomes were annual rate of recruitment (feasibility); and a composite of symptomatic intracranial hemorrhage (sICH), major extracranial hemorrhage, or mortality at 180 days (safety). Secondary outcomes included recurrent venous thromboembolism (VTE), recanalization, clinically relevant non-major bleeding (CRNMB) and functional and patient-reported outcomes (mRS, quality of life, headache, mood, fatigue, cognition) at days 180 and 365.
Results:
Fifty-five participants were randomized. Rate of recruitment was 21.3 participants/year; 57% of eligible candidates consented. Median age was 48.0 years (interquartile range[IQR] 38.5 – 73.2); 66% were female. There was one primary event (sICH), 2 CRNMB, and one recurrent CVT by day 180, all in the rivaroxaban group. All participants in both arms had at least partial recanalization by day 180. At enrolment, both groups on average reported reduced quality of life, low mood, fatigue, and headache with impaired cognitive performance. All metrics improved markedly by day 180.
Conclusions:
Recruitment targets were reached, but many eligible participants declined randomization. There were numerically more bleeding events in patients taking rivaroxaban compared to control, but rates of bleeding and recurrent VTE were low overall and in keeping with previous studies. Participants had symptoms affecting their well-being at enrolment, but improved over time.
Registration:
Graphical Abstract
BACKGROUND AND OBJECTIVES
Cerebral venous thrombosis (CVT) affects 10–20 individuals per million annually,1 and is most common in younger women due to its increased risks with oral contraception2 and the puerperium.3 High rates of functional independence are reported, although retrospective studies report persistent headache and neuropsychiatric issues, preventing return to previous activities for many survivors.4,5
Direct oral anticoagulants (DOACs) have demonstrated efficacy and safety for treatment of pulmonary embolism and deep venous thrombosis (DVT), with noninferiority compared with vitamin K antagonists (VKA) for prevention of recurrent venous thromboembolism (VTE) and superior safety with a reduction of major or clinically relevant non-major bleeding events.6 DOACs are yet to be recommended in guidelines for the management of CVT.7,8 Small randomized trials and observational studies have shifted practice over the last several years, including the randomized RE-SPECT CVT (Safety and Efficacy of Dabigatran Etexilate vs Dose-Adjusted Warfarin in Patients With Cerebral Venous Thrombosis) trial comparing dabigatran with VKA,9 a substudy of the EINSTEIN-Jr (Oral Rivaroxaban in Children With Venous Thrombosis) pediatric VTE trial in 117 children with CVT, comparing rivaroxaban versus VKA or low-molecular weight heparin (LMWH) anticoagulation,10 and ACTION-CVT, an international retrospective observational study comparing outcomes in individuals with CVT prescribed DOAC or VKA through routine care.11 Although these studies report reassuring safety data for the use of DOACs in selected populations with CVT, high-quality data from adequately powered randomized trials are not available.
CVT is challenging to study. It is uncommon, occurring 50–100 times less frequently than DVT/PE.1,12 Trial recruitment may be additionally challenging due to CVT’s predilection for younger women. DOACs are contraindicated in pregnancy and breastfeeding, and women may be less likely to consent to participate in secondary prevention trials.13 Traditional safety and efficacy outcomes in VTE trials, including major hemorrhage and recurrent VTE, may be low in this generally young population.14 Typical functional outcome scales, such as the modified Rankin Scale (mRS), may not optimally characterize the burden of persistent symptoms, such as chronic headache and fatigue, that are common after CVT but may not compromise functional independence.
SECRET was a randomized feasibility trial with the objective of informing the design of future trials in CVT. The trial compared rivaroxaban with standard-of-care anticoagulation and aimed to (1) assess feasibility of recruitment for a clinical trial of a rare disease with a predisposition toward younger women, and (2) to generate preliminary data regarding safety of DOAC compared to standard-of-care VKA or parenteral anticoagulant therapy, and (3) functional outcomes not prospectively characterized in detail in the existing literature.
METHODS
Data sharing statement
Deidentified participant data will be available, once planned subanalyses are complete, on request following an approved proposal and with a signed data access agreement. Requests should be emailed to the corresponding author.
Study design
SECRET was a Phase II, prospective parallel arm, open-label blinded-endpoint 1:1 randomized trial comparing rivaroxaban 20 mg daily (15 mg in individuals with a creatinine clearance < 50 mL/min [Cockroft-Gault equation]) with standard-of-care anticoagulation (warfarin or LMWH) in adults presenting within 2 weeks of diagnosis of symptomatic, neuroimaging-confirmed CVT.
Participants were recruited across 12 comprehensive stroke centres in Canada. The trial was approved by the local clinical research ethics board at each site. Written informed consent was obtained from participants, or, when participants were unable to directly provide consent, from a legally authorized representative. The trial was regulated by Health Canada and adhered to ICH-GCP principles and the Declaration of Helsinki. The trial protocol is in the Data Supplement. The trial reporting was structured in keeping with the CONSORT 2010 statement and 2016 extension to randomized pilot and feasibility trials.15
Participants
Participants were aged 18 or older, were within 14 days of an imaging (computed tomography [CT] venography or magnetic resonance imaging [MR] venography)-confirmed diagnosis of CVT, and were suitable for oral anticoagulation in the judgment of the treating physician. A parenchymal brain scan (non-contrast CT or MRI) was required within 72 hours prior to randomization to assess for any baseline intracranial hemorrhage prior to initiation of study medication, but there were no specific neuroimaging-based exclusion criteria. Major exclusion criteria included known antiphospholipid antibody syndrome, an anticipated invasive procedure (e.g. thrombectomy, hemicraniectomy), being unable to swallow due to depressed level of consciousness, eCrCl < 30 mL/min, pregnancy or breastfeeding, another concurrent medical condition requiring mandatory antiplatelet or anticoagulant use, any contraindication to anticoagulation, and concomitant use of strong CYP3A4 inhibitors.
Randomization and masking
Participants were randomized in a 1:1 ratio using a fully concealed dynamic computer algorithm to receive rivaroxaban (20 mg daily) or standard-of-care anticoagulation (dose-adjusted warfarin with International Normalized Ratio [INR] 2.0–3.0, or ongoing therapeutic LMWH). Randomization was stratified by age (≤37 vs. >37 years) as older age is associated with a worse prognosis and is a predictor of adverse outcomes.16 Treatment was given for a minimum of 180 days, with optional extension up to 365 days at the discretion of the treating physician. Patients and treating clinicians were aware of treatment allocation but were fully masked to the allocation sequence; study evaluations were performed by an assessor unaware of treatment allocation.
Study procedures
Participants were screened locally to ensure that they had CVT confirmed on neuroimaging, met enrolment criteria, and had provided consent. Randomization occurred when the treating clinician considered the patient suitable for transition to oral anticoagulation based upon a parenchymal scan that had been performed ≤72 hours prior to randomization and informed consent had been obtained. Participants received their first dose of assigned treatment within 24 hours of randomization.
Interventions and outcomes
Rivaroxaban was provided by Bayer Canada and was dispensed in 20 mg tablets and taken daily with food. For patients randomized to the control arm, site investigators were provided with guidelines for antithrombotic therapy including evidence-based nomograms for INR adjustment and frequency of INR monitoring. INR monitoring was as per standard-of-care, and participants who were initiated on warfarin received bridging parenteral anticoagulation for two consecutive days after the INR was at the target of 2.0–3.0.
The primary feasibility endpoint was the rate of recruitment, defined as the number of participants providing informed consent and randomized annually. The primary safety outcome was a composite of all-cause mortality, symptomatic intracranial hemorrhage (sICH), and major extracranial hemorrhage as per International Society on Thrombosis and Haemostasis (ISTH) criteria17 within 180 days of randomization. sICH was defined as a new sICH OR worsening existing intracranial hemorrhage with a ≥33% change in hematoma volume, AND either an NIH Stroke Scale (NIHSS) score increase of ≥4 points, OR a change in level of consciousness as per NIHSS item 1a, AND the clinical change was thought to be attributable to the hemorrhage. All components were adjudicated by a committee whose members were blinded to treatment allocation. Secondary endpoints at 180 and 365 days included the individual components of the primary safety endpoint, clinically relevant non-major bleeding (CRNMB) as per ISTH criteria,18 recurrent VTE (recurrent CVT, defined as involvement of a new venous segment, or reocclusion of a previously recanalized segment, DVT, pulmonary embolism, or splanchnic vein thrombosis), venous recanalization as per the de Sousa criteria,19 and functional and patient-reported outcomes including the modified Rankin Scale (mRS), EuroQoL-5 Dimensions (EQ-5D-5L), Population Health Questionnaire (PHQ-9), Headache Impact Test (HIT-6), Fatigue Assessment Scale (FAS) and Montreal Cognitive Assessment (MoCA). Proxy data was accepted for the mRS but not accepted for the patient-reported outcome measures. Neuroimaging data were reviewed at an external neuroimaging core laboratory by experienced readers unaware of treatment-group assignments. Neuroimaging was not reviewed by the core lab to determine eligibility in advance of randomization. Clinical monitoring was performed by a combination of external independent monitors and coordinating centre staff.
A schedule of assessments is summarized in the Data Supplement. Follow-up visits were at days 30, 90, 180 and 365, with a further safety follow-up visit 30 days after the day 365 follow-up. Prior to the COVID-19 pandemic, only the day 30 visit was intended to be a telephone visit; following resumption of research in the context of the COVID-19 pandemic, all follow-up visits were able to be conducted in-person or remotely.
Statistical analysis
A sample of 50 patients was chosen in keeping with realistic national recruitment targets for a rare disease, and to exclude unacceptably high rates of the primary outcome as compared with current standards of care that would preclude further studies of DOACs for the treatment of CVT.20 It was estimated that for a superiority trial powered for the primary safety outcome, 864 patients would provide 80% power to detect a 50% relative risk reduction in favour of rivaroxaban with a 10% event rate and 1864 participants for a 5% event rate.
The primary analysis population was defined as all patients, as randomized (intention-to-treat population). A modified intention-to-treat (mITT) population included those participants who consented and were confirmed to meet criteria for enrolment, and agreed to continue in the study following randomization. Participants were analyzed in their allocated treatment arm for the mITT analysis, whether or not they took the assigned treatment. The safety population was defined as all participants who took at least one dose of assigned study medication. The on-treatment cohort was defined as those participants in the mITT population who took at least 80% of their medication as treated for a minimum of the first 14 days following randomization. The per-protocol cohort was defined as those participants who took at least 80% of their medication as assigned for 180 days or longer.
Analyses of primary and secondary clinical outcomes were descriptive (counts, percentages, and 95% CI); absolute difference in proportions and 95% CIs were used to characterize differences between the rivaroxaban and standard-of-care groups. Scores for patient-reported outcomes were compared between groups using mean differences in change from baseline to day 180 or day 365, with 95% CIs. Canadian tariffs were used for the EQ-5D-5L scale.21 Missing data were not imputed.
Statistical analyses were performed using SAS 9.4 (SAS Institute, Cary NC) and SPSS V. 27.0 (IBM Corporation, Armonk NY).
RESULTS
Sites screened 219 individuals for participation between March 2019 and October 2021; 97 (44.3%) were determined to be eligible. (Figure 1, Figure S1). Reasons for ineligibility are outlined in the Data Supplement (Table S1). Recruitment rate was 1.78 participants/month (21.3/year) overall; recruitment rate as a proportion of screened patients was 0.25, and of eligible patients was 0.57. (Figures S2A and S2B)
Figure 1.
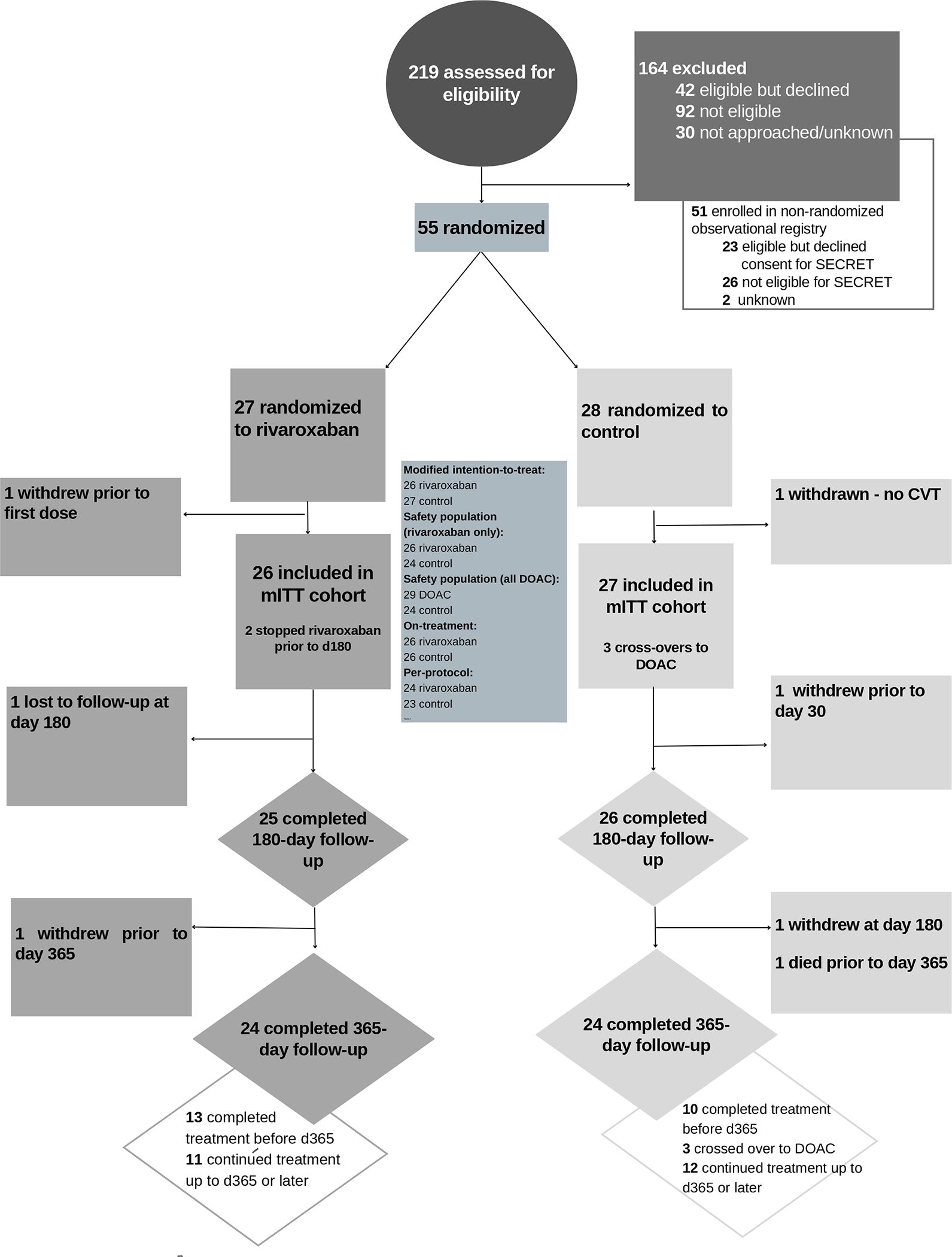
Consort diagram (color diagram is included in Data Supplement as Figure S1)
Fifty-five participants were randomized to compensate for withdrawals and anticipated losses to follow-up. Of the 55 participants who were randomized, one in the standard-of-care arm was subsequently determined not to have a diagnosis of CVT and participation in the study was terminated prior to initiation of study medication; another participant in the rivaroxaban arm withdrew immediately following randomization. Fifty-three patients were included in the mITT analysis. All 26 patients in the rivaroxaban arm and 24 patients in the standard-of-care arm took the first dose of their assigned treatment. In the mITT cohort, 96% completed 180-day follow-up and 90.6% completed 365-day follow-up. Median time on treatment in the rivaroxaban group was 352 days (interquartile range [IQR] 201.5 – 365) and 333 (IQR 184–365) days in the standard-of-care group.
Baseline characteristics were similar between groups. Of the 53 patients in the mITT cohort, 66.0% were female, all identifying as cis-gender women, with the remaining 34% who were male identifying as cis-gender men. Median age was 48.0 years (IQR 38.5 – 73.2). The median time from diagnosis to enrolment was 3.0 (IQR 2 – 6) days. (Figure 2, Figure S3) Median baseline NIHSS score was 0 (IQR 0 – 1). All but two participants received lead-in parenteral anticoagulation. (Table 1) Sinus involvement is summarized in Table S2.
Figure 2.
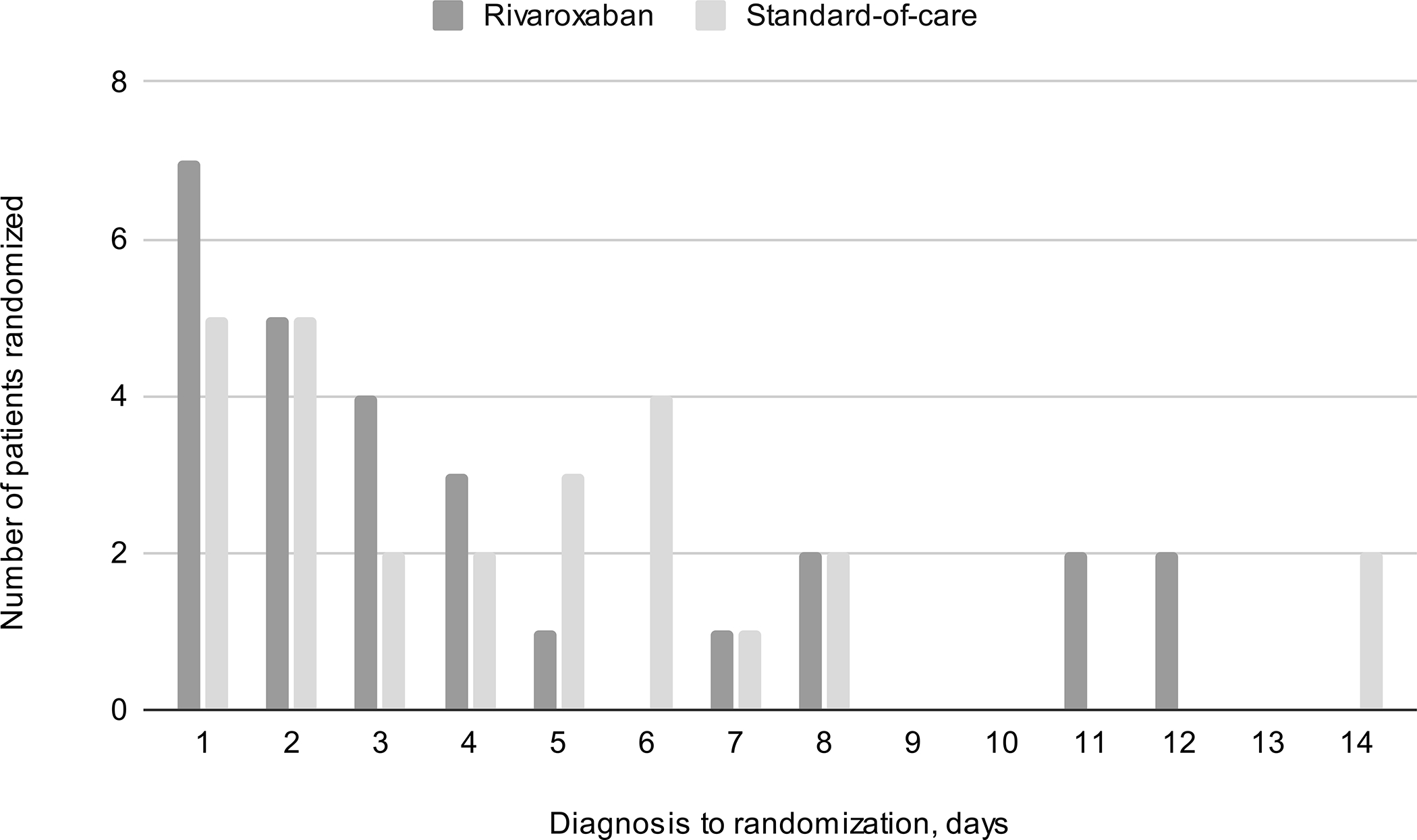
Delay from timing of diagnosis to randomization (color diagram is included in Data Supplement as Figure S3)
Table 1.
Baseline characteristics - Modified intention-to-treat cohort
| Rivaroxaban (n=26) |
Standard-of-care (n=27) |
Total (n=53) | |
|---|---|---|---|
|
| |||
| Demographics | |||
|
| |||
| Age | |||
| Median, IQR (years) | 48.5 (37.0 – 64.25) | 48.0 (39.0 – 58.0) | 48.0 (38.5 – 73.2) |
| Range (years) | 19 – 74 | 21 – 86 | 19 – 86 |
| 18–29 (N, %) | 4 (15.4) | 4 (14.8) | 8 (15.1) |
| 30–39 (N, %) | 3 (11.5) | 3 (11.1) | 6 (11.3) |
| 40–49 (N, %) | 6 (23.1) | 7 (25.9) | 13 (24.5) |
| 50–59 (N, %) | 4 (15.4) | 7 (25.9) | 11 (20.8) |
| 60–69 (N, %) | 6 (23.1) | 2 (7.4) | 8 (15.1) |
| ≥70 | 3 (11.5) | 4 (14.8) | 7 (13.2) |
|
| |||
| Female Sex (N, %) | 18 (69.2) | 17 (63.0) | 35 (66.0) |
|
| |||
| Self-identified race-ethnicity (N, %) | |||
| white | 16 (61.5) | 15 (55.5) | 31 (58.5) |
| East Asian | 5 (19.2) | 1 (3.7) | 6 (11.3) |
| South Asian | 1 (3.8) | 3 (11.1) | 4 (7.5) |
| Indigenous | 1 (3.8) | 1 (3.7) | 2 (3.8) |
| Other | 2 (7.7) | 7 (25.9) | 9 (17.0) |
| Missing | 1 (3.8) | 0 (0.0) | 1 (1.9) |
|
| |||
| Risk factors (N, %) | |||
|
| |||
| Prior VTE | 3 (12.0) | 0 (0.0) | 3 (5.8) |
|
| |||
| Active cancer | 0 (0.0) | 1 (3.7) | 1 (1.9) |
|
| |||
| Known thrombophilia | 0 (0.0) | 0 (0.0) | 0 (0.0) |
|
| |||
| Recent infection (within last month) | 6 (23.1) | 3 (11.1) | 9 (17.0) |
|
| |||
| Oral contraceptive use | 7 (26.9) | 8 (29.6) | 16 (30.2) |
| Other hormonal contraceptive | 1 (3.8) | 0 (0.0) | 1 (1.9) |
|
| |||
| Hormone replacement therapy | 0 (0.0) | 1 (3.7) | 1 (1.9) |
|
| |||
| Post-partum (6 months or less) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
|
| |||
| Surgery in the last six weeks | 2 (7.7) | 1 (3.7) | 3 (5.7) |
|
| |||
| Dehydration in last 7 days | 1 (3.8) | 3 (11.1) | 4 (7.5) |
|
| |||
| Inflammatory bowel disease | 0 (0.0) | 0 (0.0) | 0 (0.0) |
|
| |||
| Family history of VTE | 2 (7.7) | 2 (7.4) | 4 (7.5) |
|
| |||
| Presentation | |||
|
| |||
| Symptom onset to enrollment, days | |||
| Median (IQR) | 8.0 (6.75 – 14.0) | 9.0 (3 – 14.0) | 8.0 (4.5 – 14.0) |
|
| |||
| Diagnosis to enrollment, days, Median (IQR) | 4.0 (2 – 6) | 3.0 (1 – 7) | 3.0 (2 – 6) |
| Range | 1 – 14 | 1 – 12 | 1 – 14 |
| Received bridging anticoagulation prior to randomization (N, %) | 25 (96.2) | 26 (96.3) | 51 (96.2) |
| UFH | 9 (34.6) | 12 (44.4) | 21 (39.6) |
| LMWH | 12 (46.2) | 9 (34.6) | 21 (39.6) |
| UFH + LMWH | 2 (7.7) | 4 (14.8) | 6 (11.3) |
| LMWH + Oral anticoagulant | 2 (7.7) | 1 (3.7) | 3 (5.7) |
| No lead-in anticoagulation | 1 (3.8) | 1 (3.7) | 2 (3.8) |
|
| |||
| Presenting symptoms, N(%) | |||
| Headache | 23 (88.5) | 24 (88.9) | 47 (88.7) |
| Focal sensory | 5 (19.2) | 8 (28.6) | 13 (24.5) |
| Nausea/vomiting | 13 (50.0) | 14 (51.9) | 27 (50.9) |
| Visual loss/blurring | 7 (26.9) | 7 (25.9) | 14 (26.4) |
| Diplopia | 6 (23.1) | 1 (3.7) | 7 (13.2) |
| Language disturbance | 6 (23.1) | 7 (25.9) | 13 (24.5) |
| Cognitive dysfunction/encephalopathy | 10 (38.5) | 9 (33.3) | 19 (35.8) |
| Focal motor | 9 (34.6) | 8 (29.6) | 17 (32.1) |
| Seizure | 13 (50.0) | 10 (37.0) | 23 (43.4) |
| Tinnitus | 4 (15.4) | 4 (14.8) | 8 (15.1) |
|
| |||
| NIHSS Median (IQR) | 0 (0 – 1) | 0 (0 – 1) | 0 (0 – 1) |
| 0 (N, %) | 15 (57.7) | 18 (66.7) | 33 (62.3) |
| 1–4 | 7 (26.9) | 7 (25.9) | 14 (26.4) |
| 5–9 | 4 (15.4) | 2 (7.4) | 6 (11.3) |
|
| |||
| Baseline neuroimaging characteristics | |||
|
| |||
| Baseline imaging modality (N, %) | N=26 | N=27 | N=53 |
| CT/CTV | 20 (76.9) | 23 (85.2) | 43 (81.1) |
| MR/MRV | 6 (23.1) | 4 (14.8) | 11 (20.8) |
|
| |||
| Venous edema or venous infarct (N, %) |
N=25 8 (32.0) |
N=26 8 (30.1) |
N=51 16 (31.4) |
|
| |||
| Intracranial hemorrhage (N, %) | N=25 | N=26 | N=51 |
| Any | 13 (52) | 8 (30.8) | 21 (41.2) |
| HI1 | 1 (4) | 0 (0) | 1 (2.0) |
| HI2 | 1 (4) | 3 (11.5) | 4 (7.8) |
| PH1 | 5 (20) | 2 (7.7) | 7 (13.7) |
| PH2 | 2 (8) | 1 (3.8) | 3 (5.9) |
| 3c (subarachnoid) | 4 (16) | 2 (7.7) | 6 (11.8) |
No patients in the rivaroxaban group required renally-adjusted dosing of 15 mg daily. Two participants in the standard-of-care group were treated with LMWH up to day 180; the rest were transitioned to warfarin. INR data were available for 50/70 (71.4%) possible follow-ups for participants who were taking warfarin. Of those, 42/50 (84%) readings were therapeutic. There were three cross-overs within 180 days in the standard-of-care group: two participants who were initiated on apixaban following randomization, and third who switched to rivaroxaban after 162 days, short of the 180-day window (+/− 14 days). One participant was initiated on dabigatran and withdrew at day 30; pill counts could not be ascertained thus on-treatment criteria were not met and the participant was not counted as a crossover in the on-treatment analysis. One participant withdrew at day 180, and one died between days 180 and 365.
The last patient follow-up was complete in October 2022. One patient taking rivaroxaban experienced a spontaneous symptomatic subdural hemorrhage (a primary clinical and safety outcome) requiring surgical evacuation after three and a half months of therapy, prior to day 180. There were no major extracranial hemorrhages. One patient in the rivaroxaban group experienced asymptomatic worsening of the hemorrhagic component of a baseline parenchymal lesion on a scan 24 hours following randomization. By day 365, one participant in the standard-of-care arm experienced a symptomatic traumatic subdural hemorrhage and simultaneous CRNMB event from scalp bleeding; the same participant later died during a separate admission to hospital unrelated to the hemorrhage. Two patients in the rivaroxaban arm and none in the standard-of-care arm experienced CRNMB prior to day 180, one related to heavy menstrual bleeding and another related to a post-operative infection after a hysterectomy. There were no additional CRNMB events reported between day 180 and day 365.
All patients in both arms experienced at least partial recanalization (De Sousa grade 1B or greater). One patient in the rivaroxaban arm who did not meet the on-treatment definition was found to have an asymptomatic new CVT event by day 180. One patient in the control arm experienced an asymptomatic extension of their pre-existing CVT between days 180 and 365. INR at the time was subtherapeutic and the participant was switched to rivaroxaban and subsequently had an ischemic stroke. This individual subsequently was found to have the Janus Kinase-2 V617F (JAK2) mutation. There were no extracranial VTE events.(Table 2, Tables S3A, S3B, S3C)
Table 2.
Clinical and radiological outcomes - modified Intention-to-treat population *
| N, % (95%CI), rivaroxaban N=26 | N, % (95% CI), standard-of-care N=27 | Difference in proportions, % (95% CI) | |
|---|---|---|---|
|
| |||
| Primary composite safety outcome, 180 days | 1 3.8 (0.1 – 19.6) |
0 0.0 (0.0 – 12.8) |
3.8 (−3.5 – 11.2) |
|
| |||
| Secondary safety outcomes - 180 days | |||
|
| |||
| All-cause mortality, 180 days | 0 | 0 | 0 |
|
| |||
| Major extracranial hemorrhage, 180 days | 0 | 0 | 0 |
|
| |||
| Symptomatic intracranial hemorrhage, 180 days | 1 3.8 (0.1 – 19.6) |
0 0.0 (0.0 – 12.8) |
3.8 (−3.5 – 11.2) |
|
| |||
| Any intracranial hemorrhage, 180 days | 2 7.7 (0.9 – 25.1) |
0 0.0 (0.0 – 12.8) |
7.7 (−2.6 – 17.9) |
|
| |||
| Clinically relevant non-major bleeding, 180 days | 2* 7.7 (0.9 – 25.1) |
0 0 (0 – 12.8) |
7.7 (−2.6 – 17.9) |
|
| |||
| Major and clinically relevant non-major bleeding, 180 days | 3 11.5 (2.4 – 30.1) |
0 0.0 (0.0 – 12.8) |
11.5 (−1.0 – 23.8) |
|
| |||
| VTE recurrence, 180 days | 1 3.8 (0.1 – 19.6) |
0 0 (0 – 12.8) |
3.8 (−3.5 – 11.2) |
|
| |||
| VTE recurrence or CVT extension, 180 days | 1 3.8 (0.1 – 19.6) |
1 3.7 (0.1 – 19) |
0.1 (−10.1 – 10.4) |
|
| |||
| Secondary safety outcomes - 365 days (cumulative) | |||
|
| |||
| Primary composite safety outcome, 365 days | 1 3.8 (0.1 – 19.6) |
1† 3.7 (0.1 – 19) |
0.1 (−10.1 – 10.4) |
|
| |||
| All-cause mortality, 365 days | 0 0 (0 – 13.2) |
1 3.7 (0.1 – 19) |
0 −3.7 (−10.8 – 3.4) |
|
| |||
| Major extracranial hemorrhage, 365 days | 0 | 0 | 0 |
|
| |||
| Symptomatic intracranial hemorrhage, 365 days | 1 3.8 (0.1 – 19.6) |
1 3.7 (0.1 – 19) |
0.1 (−10.1 – 10.4) |
|
| |||
| Any intracranial hemorrhage, 365 days | 2 7.7 (0.9 – 25.1) |
1 3.7 (0.1 – 19) |
4.0 (−8.5 – 16.5) |
|
| |||
| Clinically relevant non-major bleeding, 365 days | 2 7.7 (0.9 – 25.1) |
1 3.7 (0.1 – 19) |
4.0 (−8.5 – 16.5) |
|
| |||
| Major and clinically relevant non-major bleeding, 365 days | 3 11.5 (2.4 – 30.1) |
1** 3.7 (0.1 – 19) |
7.8 (−6.4 – 22.0) |
|
| |||
| VTE recurrence, 365 days | 1 3.8 (0.1 – 19.6) |
0 0 (0 – 12.8) |
3.8 (−3.5 – 11.2) |
|
| |||
| VTE recurrence or CVT extension, 365 days | 1 3.8 (0.1 – 19.6) |
1 3.7 (0.1 – 19) |
0.1 (−10.1 – 10.4) |
|
| |||
| Other secondary outcomes | |||
|
| |||
| Venous recanalization, De Sousa grade, 180 day scan or earlier, N(%) | N=24 | N=24 | |
| 0 (persistent occlusion of all vessels) | 0 | 0 | 0 |
| 1A (Combination of persistent occlusions and partial recanalization, without any complete recanalization) | 0 | 0 | 0 |
| 1B (Combination of persistent occlusions and complete recanalizations, with or without partial recanalizations) | 4 (16.7) | 2 (8.3) | 8.4 (−21.1 – 21.1) |
| 2A (Partial recanalization of all thrombosed vessels, without any persistent occlusion) | 0 (0) | 2 (8.3) | −8.3 (−19.4 – 2.7) |
| 2B (Combination of partial and complete recanalization, without any persistent occlusion) | 11 (45.8) | 8 (33.3) | 12.5 (−14.9 – 39.9) |
| 3 (Complete recanalization of all previously thrombosed vessels) | 9 (37.5) | 12 (50) | −12.5 (−40.3 – 15.3) |
| Any recanalization, up to 180 days, N(%) | |||
| Defined as Grade 1A or better | 24 (100) | 24 (100) | 0 |
| Defined as Grade 2A or better | 20 (83.3) | 22 (91.7) | −8.4 (−26.9 – 10.2) |
| Complete recanalization, up to 180 days N(%) | 9 (37.5) | 12 (50) | −12.5 (−40.3 – 15.3) |
|
| |||
|
Venous recanalization, De Sousa grade,
365 day scan or earlier, N(%) |
N=25 | N=25 | |
| 0 | 0 | 0 | 0 |
| 1A | 0 | 0 | 0 |
| 1B | 4 (16.0) | 2 (8.0) | 8.0 (−9.9 – 25.9) |
| 2A | 0 (0) | 2 (8.0) | −8.0 (−18.6 – 2.6) |
| 2B | 12 (48.0) | 8 (32.0) | 16.0 (−10.8 – 42.8) |
| 3 | 9 (36.0) | 13 (52.0) | −16.0 (−43.2 – 11.2) |
| Any recanalization, up to 365 days, N(%) | |||
| Defined as Grade 1A or better | 25 (100) | 25 (100) | 0 |
| Defined as Grade 2A or better | 21 (84.0) | 23 (92.0) | −8.0 (−25.9 – 9.9) |
| Complete recanalization, up to 365 days, N(%) | 9 (36.0) | 13 (52.0) | −16.0 (−43.2 – 11.2) |
location of CVT is described in Supplementary Table S2; intention-to-treat, safety, on-treatment and per-protocol analyses are in Supplementary Tables S3A, S3B, S3C
Menorrhagia and vaginal bleeding secondary to infection
The same subject experienced two components of the composite outcome (symptomatic intracranial hemorrhage and death) and is counted as one composite safety event
Major and clinically relevant non-major bleeding events happened simultaneously for this subject (subdural hemorrhage and scalp bleeding) and are counted as one event for combined major bleeding and clinically relevant non-major bleeding
Functional and patient-reported outcomes are summarized in Table 3. For those participants with an mRS of ≥1 at baseline (21/26 in the rivaroxaban arm and 21/27 in the control arm), 81.0% in the rivaroxaban arm and 66.7% in the control arm had a functional improvement of ≥1 category by day 180. By day 365, 82.6% of those in the rivaroxaban arm and 84.0% in the control arm had an mRS of 0–1. Mean scores at enrolment in both arms were indicative of mild-moderate depression on the PHQ-9, substantial-severe impact of headache on the HIT-6, substantial fatigue on the FAS and impaired cognitive performance on the MoCA. On average, participants experienced improvements in all patient-reported outcomes over time between baseline and day 180 and at day 365, with no notable differences between the rivaroxaban versus control groups.
Table 3.
Patient-reported and functional outcomes*
| Outcome, mean (SD) or median (IQR) | Baseline, N completing assessment | Day 180, N | Day 365, N | |||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Rivaroxaban (N=26) | Standard-of-care (N=27) | Rivaroxaban (N=25) | Standard-of-care (N=26) | Rivaroxaban (N=24) | Standard-of-care (N=25) | |||
|
| ||||||||
| mRS, median (IQR) n(%), | n=24 2 (1 – 3) |
n=27 2 (1 – 2.5) |
n=24 1 (0 – 2) |
n=26 1 (0 – 1) |
n=23 1 (0 – 1) |
n=25 1 (0 – 1) |
||
| 0 | 2 (8.3) | 6 (21.4) | 9 (37.5) | 10 (38.5) | 10 (43.5) | 12 (48.0) | ||
| 1 | 6 (25.0) | 7 (25.9) | 8 (33.3) | 11 (42.3) | 9 (39.1) | 9 (36.0) | ||
| 2 | 9 (37.5) | 8 (29.6) | 7 (29.2) | 5 (19.2) | 3 (13.0) | 2 (8.0) | ||
| 3 | 6 (25.0) | 5 (18.5) | 0 | 0 | 1 (4.3) | 1 (4.0) | ||
| 4 | 0 (0.0) | 1 (3.7) | 0 | 0 | 0 | 0 (0.0) | ||
| 5 | 1 (4.2) | 0 (0.0) | 0 | 0 | 0 | 0 (0.0) | ||
| 6 | 0 (0.0) | 0 (0.0) | 0 | 0 | 0 | 1 (4.0) | ||
|
| ||||||||
| Rivaroxaban | Standard-of-care | Rivaroxaban | Standard-of-care |
Difference in proportions, %
(95% CI) |
Rivaroxaban | Standard-of-care | Difference in proportions, % (95% CI)> | |
|
| ||||||||
| mRS 0–1, (N, %) | 8 (33.3) | 13 (47.3) | 17 (70.8) | 21 (80.8) | −10 (−37.0 – 9.1) | 19 (82.6) | 21 (84.0) | −1.4 (−22.5 – 19.7) |
| mRS shift for baseline mRS>0 (N, %) | – | – | 17/21 (81.0) | 14/21 (66.7) | 14.3 (−12.0 – 41.0) | 17/20 (85.0) | 16/20 (80.0) | 5.0 (−18.1 – 28.5) |
|
| ||||||||
|
| ||||||||
| Rivaroxaban | Standard-of-care | Rivaroxaban | Standard-of-care | Mean difference, change from baseline to d180, rivaroxaban v control, 95% CI | Rivaroxaban | Standard-of-care | Mean difference, change from baseline to d365, rivaroxaban v control, 95% CI | |
|
| ||||||||
| EQ5D-5L, N | 22 | 27 | 22 | 25 | 0.01 (−0.08 –0.09) | 20 | 23 | −0.02 (−0.15 – 0.1) |
| Mean (SD) | 0.76 (0.16) | 0.73 (0.17) | 0.89 (0.07) | 0.89 (0.09) | 0.87 (0.10) | 0.85 (0.15) | ||
| Median (IQR) | 0.77 (0.65 – 0.89) | 0.79 (0.59 – 0.89) | 0.91 (0.87 – 0.95) | 0.93 (0.82 – 0.95) | 0.9 (0.84 – 0.95) | 0.9 (0.85 – 0.95) | ||
|
| ||||||||
| PHQ-9, N | 21 | 27 | 21 | 25 | −2.55 (−5.46 – 0.36) | 19 | 23 | −2.3 (−5.96 – 1.35) |
| Mean (SD) | 6.52 (4.05) | 9.15 (5.68) | 3.81 (4.24) | 3.80 (4.28) | 4.68 (5.16) | 4.57 (3.79) | ||
| Median (IQR) | 6.0 (4 – 9) | 9.0 (5 – 13) | 3.0 (0 – 6) | 3.0 (0 – 6) | 3.0 (0 – 8) | 4.0 (2 – 6) | ||
|
| ||||||||
| HIT-6, N | 20 | 27 | 21 | 25 | 0.9 (−5.83 – 7.63) | 19 | 23 | 4.13 (−2.81 – 11.08) |
| Mean (SD) | 59.70 (9.63) | 56.78 (11.85) | 47.00 (9.72) | 44.08 (9.53) | 43.53 (7.80) | 20.48 (7.42) | ||
| Median (IQR) | 60.5 (58 – 64) | 58.0 (49 – 67) | 48.0 (36 – 54) | 40.0 (36 – 51) | 41.0 (36 – 50) | 20.0 (15 – 23) | ||
|
| ||||||||
| FAS, N | 19 | 24 | 21 | 25 | −1.72 (−8.02 – 4.57) | 20 | 23 | 0.29 (−6.71 – 7.29) |
| Mean (SD) | 23.21 (9.16) | 25.83 (10.01) | 18.57 (7.43) | 19.72 (7.29) | 18.10 (6.66) | 20.48 (7.42) | ||
| Median (IQR) | 22.0 (16 – 32) | 24.5 (17 – 32) | 17.0 (12 – 24) | 19.0 (13 – 25) | 16.5 (36 – 49) | 20.0 (15 – 23) | ||
|
| ||||||||
| MoCA, N | 17 | 24 | 15 | 16 | 1.64 (−1.54 – 4.82) | 14 | 15 | 1.59 (−1.1 – 4.27) |
| Mean (SD) | 24.71 (3.55) | 22.88 (3.83) | 25.4 (5.12) | 26.13 (3.07) | 26.93 (1.87) | 28.36 (1.50) | ||
| Median (IQR) | 25 (23 – 28) | 24.5 (21 – 26) | 26 (25 – 28) | 27 (25 – 28) | 27 (26 – 28) | 29 (27.75 – 29.25) | ||
| <26, N(%) | 10 (58.8) | 16 (66.7) | 5 (33.3) | 5 (31.1) | 2 (13.3) | 1 (7.1) | ||
Summaries involving change in scores from baseline are based on participants with both baseline and follow-up scores.
DISCUSSION
Among individuals with a recent symptomatic diagnosis of CVT, feasibility of recruitment was demonstrated, although the rarity of the disease, compounded by suboptimal consent rates and low rates of serious events, underscore that broad participation from dozens of sites internationally would be essential for an appropriately powered study of anticoagulation in lower-risk individuals. Approximately half of patients screened met eligibility criteria, but few were ineligible due to contraindications to DOACs - four were pregnant, one was breastfeeding and five had antiphospholipid antibody syndrome. Of those who were eligible, 53% provided informed consent. This is consistent with previous work examining rates of consent for secondary stroke prevention studies of antithrombotic agents13 Further consultation with patients is needed to understand how to improve engagement of CVT participants in randomized therapeutic trials.
Acknowledging the study’s small sample size, there were numerically more bleeding events with rivaroxaban 20 mg at 180 days compared to the control arm, but rates did not exceed what has been reported in previous CVT studies comparing DOAC with warfarin.9–11 Rates of bleeding in the standard-of-care arm were lower than expected. Consistent with the evolving literature, there appear to be no major safety concerns for use of DOACs that would preclude their further study in CVT. At present, however, a larger phase III trial comparing DOACs to warfarin for CVT seems impractical due to a growing preference for DOACs in clinical practice. Over time, real-life treatment patterns have shifted in favour of DOACs over warfarin, both due to social distancing requirements during the pandemic, which made INR monitoring more logistically challenging,22 and with emerging evidence providing reassuring safety data.23,24,11 Still, a study comparing other antithrombotic strategies, such as a comparison between DOACs or with novel antithrombotic agents, may potentially be feasible.11
SECRET is distinct from the RE-SPECT CVT and EINSTEIN-Jr trials in that lead-in parenteral anticoagulation was not required; the other trials required a minimum of 5 days of therapy prior to initiating DOAC.9,10 Although only two participants were randomized prior to starting any anticoagulation, 62.2% of participants were randomized within 1–4 days, and 41.5% within 2 days of diagnosis. No significant safety concerns were identified related to early initiation of DOAC therapy, including no early sICH and no early symptomatic extension of CVT. Unlike treatment of acute DVT/pulmonary embolism, where initial dosing of rivaroxaban is 15 mg bid for 3 weeks followed by 20 mg daily, participants in SECRET received initial dosing of 20 mg daily. There were also no safety concerns related to this starting dose in CVT patients. The lower dosing regimen was selected to reduce risk of early worsening of intracranial hemorrhage. However, the EINSTEIN-DVT (Oral rivaroxaban for symptomatic venous thromboembolism) dose-finding study found that efficacy and safety of rivaroxaban 20 mg daily was comparable to standard therapy for acute DVT. Further, pharmacokinetic modelling shows substantial overlap in the Pk curves for the 15 mg bid and 20 mg daily dosing regimens.25,26
SECRET assessed patient-reported outcomes. Despite three-quarters of patients having a mRS in keeping with functional independence at baseline (0 – 2), there were demonstrable impairments for all metrics, which on average improved over the course of follow-up. In this younger cohort, half scored below the cutoff for cognitive impairment (<26) on the MoCA at the time of their enrolment.27 Unfortunately, there were high rates of missing cognitive data on follow-up. Participants often declined to repeat cognitive testing specifically, despite options for remote administration. Determining a strategy to assess cognitive trajectories over time in this population remains a priority. Patient-reported outcomes are an important element in considering prognosis of CVT. In considering efficient outcomes for future CVT trials, an approach combining clinical and patient-reported outcomes, such as a hierarchical composite “win ratio” design, may help to maximize information gained from smaller trials in a rare disease.28
This study has limitations. First, the study was underpowered to test superiority or non-inferiority of one antithrombotic treatment strategy over another. Still, it provides additional randomized trial data about the safety of DOACs for treatment of CVT, even when initiated early. The number of bleeding events was numerically higher in the rivaroxaban group and, although rates were not out of keeping with previous studies examining DOACs for CVT and there were no major safety concerns identified with either treatment strategy, it is possible that a larger trial may have revealed significant differences in event rates between groups. Further, early routine follow-up imaging after initiation of therapy was not performed, so we cannot preclude asymptomatic intracranial bleeding being underreported in either group. The open-label design of the study may have also led to biases towards reporting bleeding events in the experimental arm. Second, the grant-funded nature of the study precluded central INR monitoring for participants taking warfarin. However, INR monitoring was thus reflective of clinical practice, providing generalizability, and 84% of available readings were therapeutic. Estimates of best- and worst-case-scenario estimates of times in therapeutic range are in the Data Supplement (Table S4). Further, low rates of recurrent VTE and bleeding events in the control arm do not raise concerns that INRs may have been outside of the therapeutic range more often than would be expected in routine practice. Third, it is possible that not all relevant outcomes related to bleeding were considered. Heavy menstrual bleeding may have been under-ascertained and underreported.29,30 Fourth, some follow-up patient-reported outcome data is missing and we cannot exclude responder bias in interpreting results. Still, this information is important in designing feasible strategies to characterize the impact of CVT on survivors while minimizing assessment times and in-person follow-up. Fifth, COVID testing, although recommended during the pandemic, was performed in keeping with local protocols, thus rates of COVID-related CVT cannot be reliably reported. Individuals with CVT due to vaccine-associated thrombocytopenia with thrombosis were enrolled in a separate international registry and were not randomized into the trial.31 Finally, the population of CVT patients enrolled into this trial do not represent the spectrum of disease severity or pathology. In addition to excluding women who are pregnant or breastfeeding or those with antiphospholipid antibody syndrome, those with more severe presentations with depressed level of consciousness were not included, and individuals at higher risk for bleeding, such as those with head trauma or CNS infection, or recurrent VTE, such as active malignancy, were underrepresented. The role of DOAC therapy and timing of initiation in those individuals remains an area of persistent uncertainty.
CONCLUSIONS
Engagement of multiple sites across Canada led to the effective recruitment of clinical trial participants for a rare cause of stroke, although almost half of eligible participants declined to be randomized. There were numerically more bleeding events at 180 days in patients taking rivaroxaban as compared to standard-of-care, but rates of bleeding events and recurrent VTE were low overall and in keeping with previous studies of DOAC use for CVT. Despite high rates of functional independence at baseline, initial rates of impairment related to cognition, mood, and fatigue were high, with reduced quality of life. All metrics markedly improved over time.
Supplementary Material
Registration and role of funders
The trial was registered at clinicaltrials.gov (NCT03178864). Bayer provided rivaroxaban in-kind but had no role in the conduct of the study or the analysis. The study was funded by the Canadian Institutes of Health Research, the Canadian Partnership for Stroke Recovery, the Canadian Stroke Consortium, the Heart and Stroke Foundation of Canada, Michael Smith Health Research BC, and the Vancouver Coastal Health Research Institute, and design was supported by the Clinical Trials Methodology Course (NIH-NINDS R25NS088248).
Non-standard abbreviations and acronyms:
*Acronym appears <5 times
- CVT
Cerebral venous thrombosis
- DOACs
Direct oral anticoagulants
- VTE
Venous thromboembolism
- DVT
deep venous thrombosis
- VKA
Vitamin K antagonists
- *RESPECT
CVT trial (Safety and Efficacy of Dabigatran Etexilate vs Dose-Adjusted Warfarin in Patients With Cerebral Venous Thrombosis)
- *EINSTEIN
Jr trial (Oral Rivaroxaban in Children With Venous Thrombosis)
- *ACTION
CVT study (Rivaroxaban Compared to Warfarin for Treatment of Cerebral Venous Thrombosis)
- LMWH
Low molecular-weight heparin
- mRS
Modified Rankin Scale
- CT
computed tomography
- MRI
Magnetic resonance imaging
- NIHSS
NIH Stroke Scale Score
- EuroQoL-5
Dimensions (EQ-5D-5L)
- PHQ-9
Population Health Questionnaire
- HIT-6
Headache Impact Test
- FAS
Fatigue Assessment Scale
- MoCA
Montreal Cognitive Assessment
- mITT
modified intention-to-treat
APPENDIX:
The SECRET Investigators
University of British Columbia Coordinating Centre: Thalia Field (PI), Michael Hill (co-PI, Calgary), Vanessa Dizonno (Study manager), Karina Villaluna Murray (lead monitor)
Cognitive assessments and coordination: Andrea Jones, Steven Mancini, Lauren Matsubara, Zoe O’Neill, Sarah Park, Namali Ratnaweera, Michelle Yuan
Statistical Centre (Centre for Health Outcomes and Evaluation Sciences, University of British Columbia)): Hubert Wong, Monica Norena
Neuroimaging Imaging Adjudication Core and Management (Calgary Imaging Processing and Assessment Centre, University of Calgary)): Marina Saluzzi, Ashley Dueck, Mohammed Almekhlafi, Fouzi Bala, Ibrahim Alhabli
Data management (Clinical Research Unit, University of Calgary): K. Ingrid McKibben, Qiao Zhang
Clinical events adjudication committee: Oscar Benavente (chair), Stephen Van Gaal, Deepa Suryanarayan
Data Safety and Monitoring Committee: Ken Butcher (chair), Cheryl Bushnell, Diana Aguiar de Sousa, Hubert Wong
Trial steering committee: Thalia Field, Michael Hill, Vanessa Dizonno, Oscar Benavente, Andrew Demchuk, Dar Dowlatshahi, Sylvain Lanthier, Agnes Lee, Jennifer Mandzia, Deepa Suryanarayan, Jeffrey Weitz, Hubert Wong
Vancouver General Hospital - Vancouver, British Columbia (15 participants): PI - Dr Stephen Van Gaal (April 2021 -), Dr Laura Wilson (Mar 2019 - April 2021)
Primary study coordination: Princess King-Azote
Other investigators: Oscar Benavente, Mar Lloret, Hsien Lee Lau, Genoveva Maclean, Sharanpal Mann, Colleen Murphy, Jonathan Smith, Philip Teal, Ming Yin (Dominic) Tse, Samuel Yip
Royal Columbian Hospital - New Westminster, British Columbia: PI - George Medvedev MD
Primary study coordination: VIshaya Naidoo
Other Investigators: Jaskiran Brar, Shuo Chen, Myles Horton,
Foothills Hospital - Calgary, Alberta (12): PI - Dr Michael Hill
Primary study coordination: Supriya Save
Other investigators: Shorog Althubait, Chrysi Bogiatzi, Matthew Boyko, Surbhi Chatuverdi, Shelagh Coutts, Aravind Ganesh, Kimia Ghavami, Emme Harrison, Sherry Hu, Anitha Jambula, Raed Joundi, Carol Kenney, Gary Klein, Katie Lin, Salman Mansoor, Martha Marko, Karla Ryckborst, Kayla Sage, Nishita Singh, Ming Yin (Dominic) Tse, Ankur Wadhwa, Sanchea Wasiliw
Royal University Hospital - Saskatoon, SK (5): PI - Dr Brett Graham
Study coordination: Shrijal Bhavsar, Kala Bold, Sharleen Maley, Lilian Urroz, Kaitlyn Foster, Emily Junk, Scott Corley, Aaron Gardner, Sharleen Maley, Jennifer McMullen, Ruth Whelan, Whitney Duff, Cassandra Tyson
Other investigators: Regan Cooley, Gary Hunter. Fergall Magee, Sanchea Wasyliw
London Health Sciences Centre - London ON (5): PI - Dr Luciano Sposato
Primary study coordination: Meribeth-Ann (Beth) Beauchamp, Lindsay Lambourn
Other investigators: Diana Ayan, Aleksander Khaw, Lauren Mai, Maria Bres Bullrich, Sebastian Fridman, Jennifer Mandzia
Ottawa Hospital Research Institute - Ottawa ON (4): PI - Dr Dylan Blacquiere
Primary study coordination: Brian Dewar, Zeinab Daham
Other investigators: Dar Dowlatshahi, Robert Fahad, Michel Shamy, Grant Stotts
Sunnybrook Health Sciences Centre - Toronto ON (4): PI - Dr Mark Boulos
Primary study coordination: Idris Fatadawala, Kaitlyn Lopes, Alisia Southwell
Other Investigators: Vinaya Bhandari, Tess Fitzpatrick, David Gladstone, Julia Hopyan, Maneesha Kamra, Houman Khosravani, Anne-Marie Liddy, Zhongyu Liu, Najla Popel, Keith Sivakumar, Richard Swartz, Amy Yu
University of Alberta Hospital - Edmonton, AB (3): PI - Dr Brian Buck
Primary study coordination: Paige Fairall
Other investigators: Farhat Ahmed, Juline Jabs, Leah White, Lori Piquette, Rekha Shepherd, Noman Ishaque
Centre Hospitalier de l’Université de Montréal - Montreal, Quebec (3): PI - Dr Céline Odier
Primary study coordination: Nandy-Shelwine Simon; Marlene Lapierre
Other investigators: Olena Bereznyakova, Casey Boudreau Caporuscio, Francois Cauchon, Valerie Côté, Nicole Denault, Yan Desciantre, Lyne Gauthier, Laura Gioia, Grégory Jaquin, Nadia Jadil, Sothun Lim, Alexandra Poppe, Caludia Rodriguez, Marie-Christine Rodriguez, Christian Stapf
Hamilton Health Sciences Centre - Hamilton, Ontario (2): PI - Dr Kanjana Perera
Primary study coordination: Cheyenne Vandervelde
Other investigators: Kuan Huei (Kelvin) Ng, Wes Oczkowski, Diane Lourenco, Cathay Moreau. Amber Jolie, Vinai Bhagraith, Aristeidis Katsanos, Danielle de Sa Boasquevisque, Kanchana Ratnayake
Kelowna General Hospital - Kelowna, British Columbia (1): PI - Dr Aleksander Tkach
Primary study coordination: Marie McLelland
Other investigators: Coleen Adderly, Elsadig Elamin, Camille Galloway, Michelle Smith, Tess Topor
Toronto Western Hospital - Toronto, Ontario (1): PI - Dr Aleksandra Pikula
Study coordination: Shobha Singh, William To
Other investigators: Farhana Akthar, Leanne Casaubon, Anne Cayley, Lisa Crelling, , Martin Del Campo, Mingyang Gao, Cresti Hanna, Muhammad Khalid, Nga Pham, Joanna Schaafsma, Frank Silver, TIm Stewart, Patricia Tiopanas, Rely Wigner, Janice Williams
Footnotes
Disclosure statement
Dr. Boulos reports compensation from Eisai, Jazz Pharmaceuticals, and Paladin Laboratories for consultant services, and a gift from Braebon Medical Corporation. Dr. Demchuk reports compensation from Boehringer Ingleheim, HLS Pharmaceuticals, Hoffmann-Laroche, Metronic NovaSignal, and Servier for consultant services, other from AstraZeneca, Data Safety Monitoring Board participation with Philips, and stock and a patent for stroke imaging software with Circle NVI. Dr. Dowlatshahi reports compensation from AstraZeneca for consultant services. Dr. Field reports compensation from AstraZeneca, HLS Therapeutics, Servier, Bristol Myers Squibb Pfizer, and Roche for consultant services, from the Canadian Medical Protective Association as an Expert Witness, and is on the board of DESTINE Health. Dr. Hill reports compensation for Brainsgate Ltd for consultant services, grants from the Canadian Institutes of Health Research, Medtronic, Microvention Inc, NoNO Inc, and Boehringer Ingleheim, and endpoint review committee work for Merck. Dr. Lanthier reports compensation from Bayer, Bristol Myers Squibb Pfizer, and Servier for consultant services. Dr. Lee reports compensation from Bayer and Bristol Myers Squibb for consultant services. Dr. Sposato reports compensation from Bayer, Boehringer Ingleheim, Daiichi Sanko Ltd and Pfizer for consultant services. Dr. Weitz reports compensation from Alnylam Pharmaceuticals, Anthos, Bayer, Boehringer Ingleheim, Bristol myers Squibb, Daiichi Sanko Lts, Ionis Pharmaceuticals, Janssen Global Services LLC, Merck Company Foundation, Novartis, Pfizer and Portola Pharmaceuticals for consult services. The other authors report no conflicts.
REFERENCES
- 1.Zhou LW, Yu AYX, Ngo L, Hill MD, Field TS. Incidence of Cerebral Venous Thrombosis: A Population-Based Study, Systematic Review, and Meta-Analysis. Stroke. 2023;54:169–177. [DOI] [PubMed] [Google Scholar]
- 2.Amoozegar F, Ronksley PE, Sauve R, Menon BK. Hormonal contraceptives and cerebral venous thrombosis risk: a systematic review and meta-analysis. Front. Neurol. 2015;6:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Swartz RH, Cayley ML, Foley N, Ladhani NNN, Leffert L, Bushnell C, McClure JA, Lindsay MP. The incidence of pregnancy-related stroke: A systematic review and meta-analysis. Int. J. Stroke. 2017;12:687–697. [DOI] [PubMed] [Google Scholar]
- 4.Koopman K, Uyttenboogaart M, Vroomen PC, van der Meer J, De Keyser J, Luijckx G-J. Long-term sequelae after cerebral venous thrombosis in functionally independent patients. J. Stroke Cerebrovasc. Dis. 2009;18:198–202. [DOI] [PubMed] [Google Scholar]
- 5.Hiltunen S, Putaala J, Haapaniemi E, Tatlisumak T. Long-term outcome after cerebral venous thrombosis: analysis of functional and vocational outcome, residual symptoms, and adverse events in 161 patients. J. Neurol. 2016;263:477–484. [DOI] [PubMed] [Google Scholar]
- 6.van der Hulle T, Kooiman J, den Exter PL, Dekkers OM, Klok FA, Huisman MV. Effectiveness and safety of novel oral anticoagulants as compared with vitamin K antagonists in the treatment of acute symptomatic venous thromboembolism: a systematic review and meta-analysis. J. Thromb. Haemost. 2014;12:320–328. [DOI] [PubMed] [Google Scholar]
- 7.Saposnik G, Barinagarrementeria F, Brown RD Jr, Bushnell CD, Cucchiara B, Cushman M, deVeber G, Ferro JM, Tsai FY, American Heart Association Stroke Council and the Council on Epidemiology and Prevention. Diagnosis and management of cerebral venous thrombosis: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42:1158–1192. [DOI] [PubMed] [Google Scholar]
- 8.Ferro JM, Bousser M-G, Canhão P, Coutinho JM, Crassard I, Dentali F, di Minno M, Maino A, Martinelli I, Masuhr F, et al. European Stroke Organization guideline for the diagnosis and treatment of cerebral venous thrombosis - endorsed by the European Academy of Neurology. Eur. J. Neurol. 2017;24:1203–1213. [DOI] [PubMed] [Google Scholar]
- 9.Ferro JM, Coutinho JM, Dentali F, Kobayashi A, Alasheev A, Canhão P, Karpov D, Nagel S, Posthuma L, Roriz JM, et al. Safety and Efficacy of Dabigatran Etexilate vs Dose-Adjusted Warfarin in Patients With Cerebral Venous Thrombosis: A Randomized Clinical Trial. JAMA Neurol. 2019;76:1457–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Connor P, Sánchez van Kammen M, Lensing AWA, Chalmers E, Kállay K, Hege K, Simioni P, Biss T, Bajolle F, Bonnet D, et al. Safety and efficacy of rivaroxaban in pediatric cerebral venous thrombosis (EINSTEIN-Jr CVT). Blood Adv. 2020;4:6250–6258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yaghi S, Shu L, Bakradze E, Salehi Omran S, Giles JA, Amar JY, Henninger N, Elnazeir M, Liberman AL, Moncrieffe K, et al. Direct Oral Anticoagulants Versus Warfarin in the Treatment of Cerebral Venous Thrombosis (ACTION-CVT): A Multicenter International Study. Stroke. 2022;53:728–738. [DOI] [PubMed] [Google Scholar]
- 12.Payne JG, Tagalakis V, Wu C, Lazo-Langner A, CanVECTOR Network. Current estimates of the incidence of acute venous thromboembolic disease in Canada: A meta-analysis. Thromb. Res. 2021;197:8–12. [DOI] [PubMed] [Google Scholar]
- 13.O’Neill ZR, Deptuck HM, Quong L, Maclean G, Villaluna K, King-Azote P, Sharma M, Butcher K, Hart RG, Field TS. Who says “no” to participating in stroke clinical trials and why: an observational study from the Vancouver Stroke Program. Trials. 2019;20:313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paola Palazzo, Pierre Agius, Pierre Ingrand, Jonathan Ciron, Matthias Lamy, Aline Berthomet, Paul Cantagrel, Jean-Philippe Neau. Venous Thrombotic Recurrence After Cerebral Venous Thrombosis. Stroke. 2017;48:321–326. [DOI] [PubMed] [Google Scholar]
- 15.Eldridge SM, Chan CL, Campbell MJ, Bond CM, Hopewell S, Thabane L, Lancaster GA, PAFS consensus group. CONSORT 2010 statement: extension to randomised pilot and feasibility trials. BMJ. 2016;355:i5239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferro JM, Canhão P, Stam J, Bousser M-G, Barinagarrementeria F, ISCVT Investigators. Prognosis of cerebral vein and dural sinus thrombosis: results of the International Study on Cerebral Vein and Dural Sinus Thrombosis (ISCVT). Stroke. 2004;35:664–670. [DOI] [PubMed] [Google Scholar]
- 17.Schulman S, Angerås U, Bergqvist D, Eriksson B, Lassen MR, Fisher W, Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in surgical patients. J. Thromb. Haemost. 2010;8:202–204. [DOI] [PubMed] [Google Scholar]
- 18.Kaatz S, Ahmad D, Spyropoulos AC, Schulman S, Subcommittee on Control of Anticoagulation. Definition of clinically relevant non-major bleeding in studies of anticoagulants in atrial fibrillation and venous thromboembolic disease in non-surgical patients: communication from the SSC of the ISTH. J. Thromb. Haemost. 2015;13:2119–2126. [DOI] [PubMed] [Google Scholar]
- 19.Aguiar de Sousa D, Lucas Neto L, Arauz A, Sousa AL, Gabriel D, Correia M, Gil-Gouveia R, Penas S, Carvalho Dias M, Correia MA, et al. Early Recanalization in Patients With Cerebral Venous Thrombosis Treated With Anticoagulation. Stroke. 2020;51:1174–1181. [DOI] [PubMed] [Google Scholar]
- 20.Leon AC, Davis LL, Kraemer HC. The role and interpretation of pilot studies in clinical research. J. Psychiatr. Res. 2011;45:626–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xie F, Pullenayegum E, Gaebel K, Bansback N, Bryan S, Ohinmaa A, Poissant L, Johnson JA, Canadian EQ-5D-5L Valuation Study Group. A Time Trade-off-derived Value Set of the EQ-5D-5L for Canada. Med. Care. 2016;54:98–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brochu D, St-Arnaud A, Marchand L-É, Voisine P, Méthot J. Impact of COVID-19 on the Prescribing Pattern of Oral Anticoagulants for Atrial Fibrillation After Cardiac Surgery. J. Cardiovasc. Pharmacol. Ther. 2022;27:10742484221128124. [DOI] [PubMed] [Google Scholar]
- 23.Bose G, Graveline J, Yogendrakumar V, Shorr R, Fergusson DA, Le Gal G, Coutinho J, Mendonça M, Viana-Baptista M, Nagel S, et al. Direct oral anticoagulants in treatment of cerebral venous thrombosis: a systematic review. BMJ Open. 2021;11:e040212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yaghi S, Saldanha IJ, Misquith C, Zaidat B, Shah A, Joudi K, Persaud B, Abdul Khalek F, Shu L, de Havenon A, et al. Direct Oral Anticoagulants Versus Vitamin K Antagonists in Cerebral Venous Thrombosis: A Systematic Review and Meta-Analysis. Stroke. 2022;53:3014–3024. [DOI] [PubMed] [Google Scholar]
- 25.Mueck W, Lensing AWA, Agnelli G, Decousus H, Prandoni P, Misselwitz F. Rivaroxaban: population pharmacokinetic analyses in patients treated for acute deep-vein thrombosis and exposure simulations in patients with atrial fibrillation treated for stroke prevention. Clin. Pharmacokinet. 2011;50:675–686. [DOI] [PubMed] [Google Scholar]
- 26.Buller HR, Lensing AWA, Prins MH, Agnelli G, Cohen A, Gallus AS, Misselwitz F, Raskob G, Schellong S, Segers A, et al. A dose-ranging study evaluating once-daily oral administration of the factor Xa inhibitor rivaroxaban in the treatment of patients with acute symptomatic deep vein thrombosis: the Einstein-DVT Dose-Ranging Study. Blood. 2008;112:2242–2247. [DOI] [PubMed] [Google Scholar]
- 27.Pike NA, Poulsen MK, Woo MA. Validity of the Montreal Cognitive Assessment Screener in Adolescents and Young Adults With and Without Congenital Heart Disease. Nurs. Res. 2017;66:222–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pocock SJ, Ariti CA, Collier TJ, Wang D. The win ratio: a new approach to the analysis of composite endpoints in clinical trials based on clinical priorities. Eur. Heart J. 2012;33:176–182. [DOI] [PubMed] [Google Scholar]
- 29.Weyand AC, James PD. Sexism in the management of bleeding disorders. Res Pract Thromb Haemost. 2021;5:51–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boonyawat K, Lensing AWA, Prins MH, Beyer-Westendorf J, Prandoni P, Martinelli I, Middeldorp S, Pap AF, Weitz JI, Crowther M. Heavy menstrual bleeding in women on anticoagulant treatment for venous thromboembolism: Comparison of high- and low-dose rivaroxaban with aspirin. Res Pract Thromb Haemost. 2021;5:308–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sánchez van Kammen M, Aguiar de Sousa D, Poli S, Cordonnier C, Heldner MR, van de Munckhof A, Krzywicka K, van Haaps T, Ciccone A, Middeldorp S, et al. Characteristics and Outcomes of Patients With Cerebral Venous Sinus Thrombosis in SARS-CoV-2 Vaccine-Induced Immune Thrombotic Thrombocytopenia. JAMA Neurol. 2021;78:1314–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaatz S. Determinants and measures of quality in oral anticoagulation therapy. J. Thromb. Thrombolysis. 2008;25:61–66. [DOI] [PubMed] [Google Scholar]
- 33.Schmitt L, Speckman J, Ansell J. Quality assessment of anticoagulation dose management: comparative evaluation of measures of time-in-therapeutic range. J. Thromb. Thrombolysis. 2003;15:213–216. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


