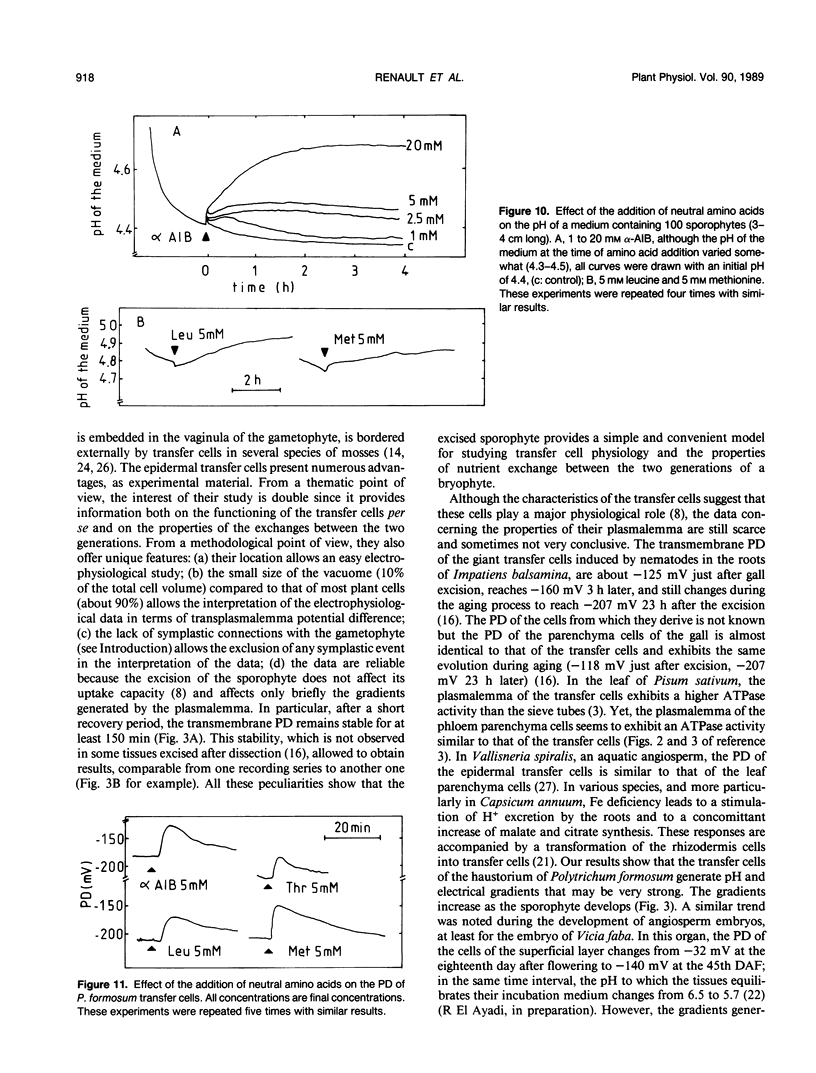
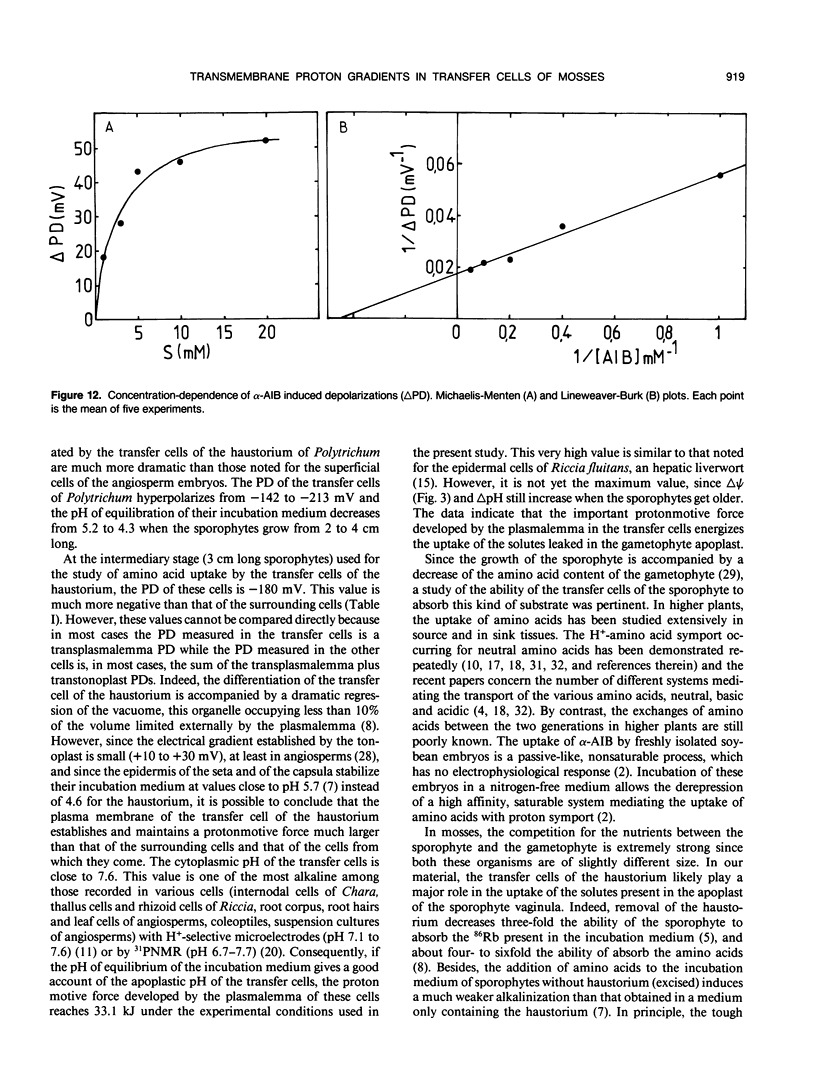
Abstract
The epidermal cells of the sporophyte haustorium of Polytrichum formosum are modified into transfer cells. These cells are located in a strategic place allowing them to control the exchanges between the two generations. Their plasmalemma creates proton gradients (Δψ and ΔpH) which increase during the development of the sporophyte. As the sporophyte grows from 2 to 4 cm long, the pH of the incubation medium of the haustoria decreases from 5.2 to 4.3, and the transmembrane potential difference (PD) hyperpolarizes form −140 to −210 millivolts. These gradients become rapidly larger than that generated by the plasmalemma of the basal cells of the sporophyte. They are used to energize the uptake of the solutes present in the apoplast of the gametophyte, particularly the amino acids. Below 20 micromolar α-aminoisobutyric acid uptake in the transfer cells is mediated by a saturable system and is optimal at acidic pH (4.0 and 4.5). It is strongly inhibited by compounds dissipating both Δψ and ΔpH (10 micromolar carbonylcyanide-m-chlorophenyl hydrazone) or only Δψ (0.1 molar KCl). The absorption of α-aminoisobutyric acid and of the other neutral amino acids tested induces an alkalinization of the medium and a depolarization of membrane potential difference which is concentration dependent. These data show that the uptake of amino acids by the transfer cells of the haustorium is a secondary translocation (proton-amino acid symport) energized by a primary translocation (proton efflux). More particularly, they show that transfer cells possess a membrane enzymic equipment particularly efficient to achieve the uptake of the solutes leaked in the apoplast from other cell types.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ammann D., Lanter F., Steiner R. A., Schulthess P., Shijo Y., Simon W. Neutral carrier based hydrogen ion selective microelectrode for extra- and intracellular studies. Anal Chem. 1981 Dec;53(14):2267–2269. doi: 10.1021/ac00237a031. [DOI] [PubMed] [Google Scholar]
- Bennett A. B., Spanswick R. M. Derepression of amino Acid-h cotransport in developing soybean embryos. Plant Physiol. 1983 Jul;72(3):781–786. doi: 10.1104/pp.72.3.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Despeghel J. P., Delrot S. Energetics of Amino Acid Uptake by Vicia faba Leaf Tissues. Plant Physiol. 1983 Jan;71(1):1–6. doi: 10.1104/pp.71.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinraide T. B., Etherton B. Electrical evidence for different mechanisms of uptake for basic, neutral, and acidic amino acids in oat coleoptiles. Plant Physiol. 1980 Jun;65(6):1085–1089. doi: 10.1104/pp.65.6.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinraide T. B., Newman I. A., Etherton B. A Quantitative Simulation Model for H-Amino Acid Cotransport To Interpret the Effects of Amino Acids on Membrane Potential and Extracellular pH. Plant Physiol. 1984 Nov;76(3):806–813. doi: 10.1104/pp.76.3.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurkdjian A. C., Barbier-Brygoo H. A hydrogen ion-selective liquid-membrane microelectrode for measurement of vacuolar pH of plant cells in suspension culture. Anal Biochem. 1983 Jul 1;132(1):96–104. doi: 10.1016/0003-2697(83)90430-x. [DOI] [PubMed] [Google Scholar]
- Landsberg E. C. Function of Rhizodermal Transfer Cells in the Fe Stress Response Mechanism of Capsicum annuum L. Plant Physiol. 1986 Oct;82(2):511–517. doi: 10.1104/pp.82.2.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madore M. A., Oross J. W., Lucas W. J. Symplastic Transport in Ipomea tricolor Source Leaves : Demonstration of Functional Symplastic Connections from Mesophyll to Minor Veins by a Novel Dye-Tracer Method. Plant Physiol. 1986 Oct;82(2):432–442. doi: 10.1104/pp.82.2.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prins H. B., Harper J. R., Higinbotham N. Membrane potentials of vallisneria leaf cells and their relation to photosynthesis. Plant Physiol. 1980 Jan;65(1):1–5. doi: 10.1104/pp.65.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernooy C. D., Lin W. Amino Acid transport in protoplasts isolated from soybean leaves. Plant Physiol. 1986 May;81(1):8–11. doi: 10.1104/pp.81.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyse R. E., Komor E. Mechanism of amino Acid uptake by sugarcane suspension cells. Plant Physiol. 1984 Dec;76(4):865–870. doi: 10.1104/pp.76.4.865. [DOI] [PMC free article] [PubMed] [Google Scholar]


