Abstract
Objective:
To evaluate the risk factors for progress of mild cognitive impairment to dementia.
Methods:
This study was based on the epidemiological survey in 2011 (No. PKJ2010-Y26) and contained 441 MCI individuals. Cognitive function was measured by the Mini-Mental Status Examination, clinical dementia rating, and montreal cognitive assessment. The association between demographic characteristics and MCI outcomes were evaluated using single-and multifactor ordered logistic regression analysis models.
Results:
Of the 441 MCI, 77 progressed to dementia (MCIp: 17.5%, 95% CI: 14.4%-21.6%), 356 remained stable (MCIs: 80.7%, 95% CI: 77.0%-88.4%), and 8 reverted to normal cognition (MCIr: 1.8%, 95% CI: 0.6%-3.0%) at follow-up in 2017. Univariate ordinal regression analysis showed that diabetes (P = .052), marriage (P = .028), worker (P = .069), and manager (P = .075) may be the risk factor for the status of MCI. Multiple ordinal regression results showed that diabetes (P = .049) and marriage (P = .04) significantly affected the cognitive function changes in the MCI patients.
Conclusion:
Nondiabetics and being married may prevent the progression from MCI to dementia.
Keywords: mild cognitive impairment, dementia, ordered logistic regression analysis, education, diabetes, past occupation
Introduction
Mild cognitive impairment (MCI) is thought to be a transitional stage between normal cognitive function and dementia among aging individuals. Compared with normal elderly people whose age and education level are matched, patients have mild cognitive decline without significant decline in functional activities of daily living. 1 Mild cognitive impairment with significant memory impairment is the initial clinical manifestation of Alzheimer’s disease (AD). 2 Bennett et al 3 and Morris et al 4 reported that more than 34% MCI individuals developed to AD over 5 years, while, 9.5 years later, the conversion was 100%. A longitudinal clinical study showed that about 80% AD were developed from MCI. 5 By the time AD was diagnosed, the cognitive decline began many years ago and accelerated during the course of the disease. 5 Substantial and irreversible neurological damage has occurred in patients with AD, and there is currently no effective treatment. Therefore, early diagnosis and prevention of AD are particularly important, and reasonable intervention in MCI may be effective for AD. 6
The number of older patients over age 60 with multiple comorbid diseases is significantly increased recently. 7 Epidemiological surveys show that the occurrence of cognitive impairment in the elderly was closely related to the metabolic diseases. 8,9 Beydoun et al conducted a meta-analysis containing 247 studies (cross-sectional and cohort studies) to analyze the modifiable factors associated with cognition and dementia. The results showed that higher homocysteine levels, lower educational attainment, and decreased physical activity were particularly strong predictors of incident AD. 10 The effect of some factors, such as occupations, economic income, hypertension, and other chronic physical diseases on the cognitive function requires further research.
The present study was based on the research conducted in 2011 (No. PKJ2010-Y26) 7 for the aim of investigation of the cognition changes in MCI, and analysis of the protective and risk factors for MCI progression to dementia by follow-up on the same community elderly in 2017.
Methods
Study Design and Ethical Considerations
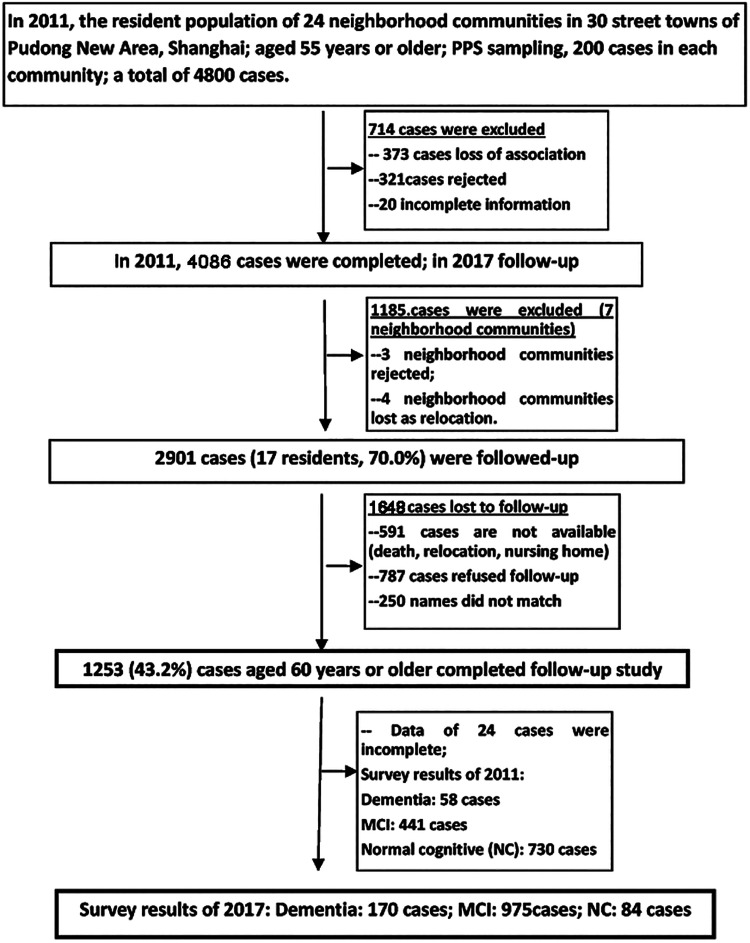
Based on the probability-proportional-to-size sampling method, community elderly involved in the cross-sectional epidemiological survey (No. PKJ2010-Y26) from July 2011 to July 2012 were involved in this study. Aged 60 years and above with audiovisual levels to complete the necessary examinations were considered as the inclusion criterions. Flow chart of follow-up process was summarized in Figure 1. Excluding the rejected and relocated communities, the remaining 17 communities participated in the follow-up survey, of which 1253 were followed up and 1648 were lost (the follow-up rate was 43.8%). The present study was approved by Ethics Committee of Shanghai Pudong New Area Mental Health Center: 2016001 and all participants signed informed consents.
Figure 1.
Flow chart of investigation on cognitive function of community elderly in 2017.
Demographic Characteristics
Demographic characteristics were counted based on the questionnaires, including gender, age, height and weight, education level, marital status, occupation, economic income, health insurance, personality, the number of children, care method, family status, family history of dementia, and history of diabetes, hypertension, hyperlipidemia, and mental illness. The investigation was conducted by psychiatric medical staff with more than 2 years of clinical work experience from the district mental health center. Before the survey, investigators were trained with Mini-Mental State Examination (MMSE), clinical dementia rating (CDR), and Petersen diagnostic assessments to ensure the Kappa values ranged from 0.82 to 0.88, 7 followed by reviewing by the middle or senior psychiatrists. The survey was conducted by door-to-door interview.
Diagnosis of MCI and Dementia
According to Petersen’s diagnostic criteria, 11 participants who had the following symptoms were diagnosed as the MCI: subjective memory complaints or reported by family members for obvious memory impairment over 3 months; normal total cognitive function assessed by MMSE (illiteracy > 17 points, primary school > 20 points, others > 24 points) 12 ; CDR score of 0.5 13 ; montreal cognitive assessment (MoCA) score of ≤ 26 points 14 ; intact activities of daily living assessed by activities of daily living scale (ADL) 15 ; and no dementia. 16 Diagnostic criteria for dementia: according to MMSE, illiterate group ≤ 17 points, primary school group ≤ 20 points, middle school or above group ≤ 24 points 12 ; obvious blindness, loss of speech and difficulty in verbal expression. Diagnostic criteria for normal cognitive: MoCA > 26 points (fewer than 12 years of education, added one point) and MMSE > 24 points; MMSE score >18 points for illiteracy; MMSE score >21 points for primary school education. Hachinski ischemic score (HIS) was used for identification of the vascular dementia. 17
Statistical Analysis
Statistical analysis was performed by SPSS version 19.0 software (IBM), with P <.05 indicating statistical significance. For continuous data, the normal distribution was checked by One-Sample Kolmogorov-Smirnov Test (1 sample K-S Test) and histogram. Continuous data were expressed by mean ± standard deviation (normal distribution) or spacing values (median, quartile, extremes) for non-normal distribution. Differences between groups were analyzed by independent-samples t test (normal distribution) or nonparametric tests (Mann-Whitney U test, non-normal distribution). Qualitative data was expressed as percentages, and χ2 test was used to compare proportions between the groups. Six-year follow-up outcomes of MCI were used as the dependent variable, while, the participants’ general demographic information and physical illnesses were used as the independent variables. Then, ordered logistic regression analysis model (univariate and multiple logistic regression models) was established, and Supplemental Table 1 showed the variable assignment. The proportional odds assumption was tested by Test of Parallel Lines method. If they met the proportional odds assumption with P >.05, the ordered logistic regression analysis were performed. The factors with the P value < 0.1 in the single-factor were involved in the multifactor regression analysis which was completed by the full entry model. Odds ratios (OR) of correlations were estimated with their 95% confidence interval (CI).
Results
Demographic Characteristics of Participants and Lost to Follow-Up Population
This study was based on a survey, 6 years ago (PKJ2010-Y26), using the same tools to reinvestigate the community elderly to understand the cognition changes of MCI. Individuals who were older, had more children, not in marriage, and those with high income were prone to be lost to follow-up. While, there was no significant difference in the history of hypertension and hyperlipidemia between participants followed up and those lost to follow-up.
The Outcome of MCI Patients Based on the Follow-Up Study
Among the 1229 participants with complete information, 441 individuals were diagnosed as MCI in 2011. Of the 441 MCI, 77 progressed to dementia (MCIp: 17.5%, 95% CI: 14.4%-21.6%), 356 remained stable (MCIs: 80.7%, 95% CI: 77.0%-88.4%), and 8 reverted to normal cognition (MCIr; 1.8%, 95% CI: 0.6%-3.0%) at follow-up in 2017. The basic characteristics of patients were listed in Table 1. There were no significant differences of age, gender, and body mass index in patients of MCIp, MCIs, and MCIr group (P > .05, Table 1). According to the HIS score, dementia was classified into vascular dementia in 3 cases (3.9%), AD in 59 cases (76.6%), and mixed dementia in 15 cases (19.5%).
Table 1.
The Basic Information of Patients in 3 Groups.
| Characteristics | MCIp | MCIs | MCIr | P |
|---|---|---|---|---|
| Age, n (%) | .271 | |||
| > 60 years | 67 (87.0) | 282 (79.2) | 6 (75.0) | |
| ≤ 60 years | 10 (13.0) | 74 (20.8) | 2 (25.0) | |
| Gender | .917 | |||
| Male | 23 (29.9) | 99 (27.8) | 2 (25.0) | |
| Female | 54 (70.1) | 257 (72.2) | 6 (75.0) | |
| BMI, kg/m2 | .897 | |||
| <24.0 | 8 (10.4) | 41 (11.6) | 1 (12.5) | |
| 24.0-27.9 | 29 (37.7) | 125 (35.3) | 2 (25.0) | |
| ≥28.0 | 40 (51.9) | 188 (53.1) | 5 (62.5) | |
| Family history of dementia | .611 | |||
| No | 68 (89.5) | 322 (91.0) | 8 (100.0) | |
| Yes | 8 (10.5) | 32 (9.0) | 0 (0.0) | |
| Memory decline | .913 | |||
| Yes | 62 (81.6) | 293 (82.5) | 7 (87.5) | |
| No | 14 (18.4) | 62 (17.5) | 1 (12.5) | |
| Diabetes | .135 | |||
| Yes | 12 (15.8) | 32 (9.1) | 0 (0.0) | |
| No | 64 (84.2) | 320 (90.9) | 8 (100.0) | |
| Hypertension | .809 | |||
| Yes | 38 (50.0) | 173 (48.7) | 5 (71.4) | |
| No | 38 (50.0) | 182 (51.3) | 2 (28.6) | |
| Hyperlipidemia | .620 | |||
| Yes | 3 (4.1) | 15 (4.3) | 1 (12.5) | |
| No | 70 (95.9) | 336 (95.7) | 7 (87.5) | |
| Marriage | .081 | |||
| Married | 56 (72.7) | 297 (83.4) | 7 (87.5) | |
| Else | 21 (27.3) | 59 (16.6) | 1 (12.5) | |
| Health insurance | .146 | |||
| City health care | 60 (77.9) | 258 (72.5) | 8 (100.0) | |
| Else | 17 (22.1) | 98 (27.5) | 0 (0.0) | |
| Care method | .479 | |||
| Model 6 | 54 (70.1) | 273 (76.7) | 2 (25.0) | |
| Else | 23 (29.9) | 83 (23.3) | 6 (75.0) | |
| Care frequency | .972 | |||
| Model 1 | 30 (39.0) | 146 (41.0) | 6 (75.0) | |
| Else | 47 (61.0) | 210 (59.0) | 2 (25.0) | |
| Personality | .079 | |||
| Introversive type | 15 (19.5) | 86 (24.2) | 3 (37.5) | |
| Middle type | 41 (53.2) | 149 (42.0) | 3 (37.5) | |
| Extroversive type | 21 (27.3) | 120 (33.8) | 2 (25.0) | |
| Highest education, n (%) | .083 | |||
| Primary school or below | 45 (58.4) | 195 (54.8) | 0 (0.0) | |
| Middle school or above | 32 (41.6) | 161 (45.2) | 8 (100.0) | |
| Occupation | .190 | |||
| Technical staff | 4 (5.2) | 25 (7.1) | 3 (37.5) | |
| Worker | 41 (53.2) | 178 (50.6) | 2 (25.0) | |
| Peasant | 15 (19.5) | 77 (21.9) | 0 (0.0) | |
| Service staff | 1 (1.3) | 5 (1.4) | 0 (0.0) | |
| Businessman | 1 (1.3) | 8 (2.3) | 0 (0.0) | |
| Clerk | 1 (1.3) | 8 (2.3) | 0 (0.0) | |
| Manager | 2 (2.6) | 4 (1.1) | 0 (0.0) | |
| Inconvenient classification | 12 (15.6) | 47 (13.4) | 3 (37.5) | |
| Family type | .175 | |||
| Living alone | 11 (14.3) | 31 (8.8) | 1 (12.5) | |
| Stem family | 28 (36.4) | 140 (39.5) | 5 (62.5) | |
| Core family | 38 (49.4) | 183 (51.7) | 2 (25.0) | |
| Economic income | .696 | |||
| ≥2000 Yuan/month | 22 (28.6) | 92 (25.9) | 3 (37.5) | |
| <2000 Yuan/month | 55 (71.4) | 263 (74.1) | 5 (62.5) |
Abbreviations: BMI, body mass index; MCI, mild cognitive impairment.
The Ordered Logistic Regression Analysis of Outcome of MCI Patients
For single-factor ordered logistic regression, all the results for Test of Parallel Lines were P > .05. The results of single-factor ordered logistic regression analysis of outcome of MCI patients were presented in Table 2. Among the baseline characteristics, the P values of diabetes, marriage status, worker, manager were all less than 0.1. The factors with P value < 0.1 were subsequently subjected to multifactor ordered logistic regression analysis. Then, Test of Parallel Lines showed that ordered probability model met the proportional odds assumption (χ2 = 14.673, P = .108). As shown in Table 3, cognitive function changes in the MCI population were closely associated to diabetes, marriage. The possibility of stability and reversion of cognitive function in diabetic MCI patients was 0.48 (95% CI: 0.23-0.99) times higher than that of nondiabetics MCI patients (P = .047). Compared to married participants, the unmarried, widowed, divorced population had OR of 0.54 (95% CI: 0.3-0.97, P = .04) for the stability and reversion of MCI. Besides, no past occupation significantly influenced the stability and reversion of MCI progressed to dementia.
Table 2.
The Univariate Ordinal Regression Analysis of Outcome of MCI Patients.
| Factors | OR | P value |
|---|---|---|
| Age (>60 vs ≤ 60 years) | 0.59 | .110 |
| Family history of dementia (yes vs no) | 1.31 | .495 |
| Memory decline (yes vs no) | 1.10 | .753 |
| Diabetes (yes vs no) | 0.50 | .052 |
| Hypertension (yes vs no) | 1.05 | .852 |
| Hyperlipidemia (yes vs no) | 1.34 | .646 |
| Gender (male vs female) | 0.90 | .682 |
| Marriage (else vs married) | 0.53 | .028 |
| Health insurance (else vs city health care)a | 1.10 | .724 |
| Care method (else vs model 6) | 0.74 | .258 |
| Care frequency (else vs model 6) | 1.00 | .991 |
| BMI, kg/m2 | ||
| <24.0 (reference) | ||
| 24.0-27.9 | 0.88 | .609 |
| ≥28.0 | 1.07 | .866 |
| Personality | ||
| Introversive type (reference) | ||
| Middle type | 0.60 | .112 |
| Extroversive type | 0.90 | .752 |
| Highest education (middle school or above vs primary school or below) | 1.49 | .108 |
| Occupation | ||
| Technical staff (reference) | ||
| Worker | 0.35 | .069 |
| Peasant | 0.38 | .117 |
| Service staff | 0.37 | .398 |
| Businessman | 0.54 | .565 |
| Clerk | 0.54 | .565 |
| Manager | 0.16 | .075 |
| Inconvenient classification | 0.41 | .163 |
| Family typeb | ||
| Living alone (reference) | ||
| Stem family | 1.79 | .147 |
| Core family | 1.52 | .280 |
| Economic income (≥ 2000 vs < 2000 Yuan/month) | 0.94 | .831 |
Abbreviations: BMI, body mass index; OR, odds ratio.
a Health insurance includes city health care = 1; medical insurance for urban residents = 2; residents health care = 3; cooperative medical care = 4; others = 5 (including type 2/3/4/5).
b Stem family: A family contains grandparents or grandparents, parents, and third generation. Core family: A family only contains parents and unmarried children.
Table 3.
The Multiple Ordinal Regression Analysis of Outcome of MCI Patients.
| Factors | OR | P value |
|---|---|---|
| Diabetes (yes vs no) | 0.49 | .049a |
| Marriage (else vs married) | 0.54 | .040a |
| Occupation | ||
| Technical staff (reference) | ||
| Worker | 0.39 | .109 |
| Peasant | 0.41 | .149 |
| Service staff | 0.47 | .515 |
| Businessman | 0.56 | .590 |
| Clerk | 0.60 | .639 |
| Manager | 0.14 | .057 |
| Inconvenient classification | 0.43 | .193 |
Abbreviation: OR, odds ratios.
P < .05.
Discussion
The present study mainly focused on the 441 MCI and analyzed their progression to dementia, reversion to normal cognition, and remained stable during 6 years of follow-up. There were 17.5% MCI community elderly progressed to dementia and 80.7% remained stable. Diabetes and marriage status as factors increased the risk of MCI progressing to dementia.
A meta-analysis conducted by our team showed that the probability of community elderly MCI progression to dementia was 34% (95% CI: 26%-42%), which was lower than clinic-based outcomes. 18 Gao et al followed up 208 MCI (among of the 437 participants older than 55 years) in Singapore and found that 4% MCI progressed to dementia and 44% MCI reversed to normal cognition during the 6-year follow-up. 19 Besides, Pandya et al reported that 35% of the 1208 participants meeting MCI criteria progressed to dementia at 2 years. 20 While, in this study, the MCI progression ratio was 17.5%. The different operational diagnostic criteria, assessment process, regional difference, and participant backgrounds might explain the wide possibility of MCI progression to dementia. Verlinden et al investigated trajectories of cognition and daily functioning in preclinical dementia, during 18 years of follow-up, revealing that dementia cases first reported memory complaints 16 years before diagnosis, followed by decline in MMSE and ADL. 21 Therefore, the age of memory complaints also affected the length of time for MCI progression to dementia.
In this study, diabetes was found to be a risk factor significantly affecting the status MCI (progression, reversion or stability). According to Degen et al, diabetes type II might lead to deficits in cognitive flexibility and visuospatial thinking, indicated that diabetes type II can be considered to be a frequent comorbid condition which can aggravate the course of MCI. 22 In our study, the risk of diabetes MCI progression to dementia was 3 times higher than those without diabetes. There was plenty of evidence to support the results. Neuropathologic studies have revealed cerebral atrophy and subclinical brain infarction evidence in diabetes patients without dementia. 23 Presumably, small-vessel disease and high levels of glycated hemoglobin which were common symptoms of chronic hyperglycemia increased the oxidative stress as well as the accumulation of advanced glycation end products, then led to the alterations in synaptic plasticity and damage of the central nervous cells. 24 -26 According to Ji and Cheng, fasting blood glucose and glycated hemoglobin levels were inversely associated with cognitive function scores, meaning that the higher the blood glucose level, the more severe the cognitive dysfunction. 27 This finding was similar to those of previous studies conducted in Beijing, 28,29 which indicated that fasting blood glucose and insulin resistance (Homeostasis model assessment for insulin resistance [HOMA]-IR, β = 1.313, P = .01) were independent influencing factors of cognitive impairment (MMSE assessment) in elderly type 2 diabetic patients. Diabetes, impaired glucose tolerance, and metabolic syndrome increased the risk of MCI progression. 30,31 High fasting blood glucose level increased the risk of dementia even in nondiabetes individuals. 32
Besides, our data also showed that the marriage status was a risk factor for the outcomes of MCI. The risk of depression, delusions, elation, and disinhibition was less in married MCI participants. 33 The declined cognitive ability in MCI patients affects the marital relationship. Conversely, the changes in marital relationship may increase the caregiving burden and depression in MCI patients. 34 Being married is reported to be negatively related with the decline of social activities. 35 The declined engagement in social activities is closely associated with the progression from mild to severe cognitive impairment in MCI patients. Therefore, we concluded that being married may decline the risk of the progression from MCI to dementia. Moreover, past occupation as a manager may be another risk factor for MCI progressed to dementia. Keohane and Balfe found that complex work which required higher mental stimulation may be protective for cognitive function. It was possible due to the continuously use of the brain increased the cognitive reserve in the technical staff. 36 The more you use your brains, the slower the cognitive function declines, and this advantage became more apparent after age 65. 37 Garibotto et al reported that education and occupation might be proxies for reserve in aMCI converters and AD Fluorodeoxyglucose positron emission tomography (FDG-PET) evidence. 38 High education or high occupational attainment (such as mid-level civil servant or management, head of a small business, academician, or specialist in a subordinate position, or senior academic position) reduced the severity and delayed the clinical expression of AD pathology. 38 The different levels of education/occupation showed a different modulatory effect on the relationship between brain metabolism and cognitive functions, which was in accordance with neuropathologic evidence. 39 Working in low-skilled occupations has been repeatedly identified as a risk factor for dementia. 40 The regression analysis also revealed that working as a farm laborer was associated with a greater risk of developing MCI. 41 Highly qualified/skilled occupation such as being a professional musician is related to increased gray matter volumes in particular brain areas. 42 The previous studies showed that compared to other occupations, the higher the complexity of the previous occupation (such as occupations with high requirements for reasoning, calculation, language, or vocational training ability), the lower the risk of dementia. In our study, univariate ordinal regression analysis showed that worker and manager might be the risk factors for the outcomes of MCI patients with P value <.01. However, the multiple ordinal regression analysis showed no past occupation significantly affected the outcomes of MCI patients. However, the possibility of manager as the risk factor for influencing the outcomes of MCI was 14% (0.02-1.06, P = .057). Our data show that the occupation of manager may be the risk factor influencing the progression, stability, and reverse of MCI, which should be validated in further studies.
The present study has several limitations. First, in order to save time and economic cost, this study used a noninvasive MMSE and MoCA screening scale with high reliability and validity, rather than high-cost detection methods such as magnetic resonance and genetic testing to reflect the cognitive changes. Second, in our previous study, the mean MMSE score of MCI (2011, n = 441, ≥55 years old) was 26.58 ± 2.48, which was higher than that of other investigators (2008, n = 2809 cases, ≥60 years old) with 24.37 ± 4.071. 43 This may be one of the reasons for the low MCIp rate in this study. While, this study specifically investigated the relevant factors of the MCI outcomes among the older population in Shanghai. We hope that our findings would be of guiding significance for preventing MCI progression to dementia.
Conclusion
Of the 441 MCI participants, about 17.5% progressed to dementia and 80.7% remained stable during the 6-year follow-up. The diabetes and marriage status were the risk factors influencing the outcomes of MCIs. Diabetes and nonmarital status significantly increased the cognition function impairment of MCI patients.
Supplemental Material
Supplemental Material, Highlights for Study of the Risk and Preventive Factors for Progress of Mild Cognitive Impairment to Dementia by Chengping Hu, Ling Wang, Yi Guo, Zhicheng Cao, Ying Lu and Hongyun Qin in American Journal of Alzheimer's Disease & Other Dementias
Supplementary_Table_1 for Study of the Risk and Preventive Factors for Progress of Mild Cognitive Impairment to Dementia by Chengping Hu, Ling Wang, Yi Guo, Zhicheng Cao, Ying Lu and Hongyun Qin in American Journal of Alzheimer's Disease & Other Dementias
Footnotes
Authors’ Note: The present study was approved by Ethics Committee of Shanghai Pudong New Area Mental Health Center: 2016001. C.H. contributed to conception and design of the research, obtaining funding, and drafting the manuscript; Y.L. contribute to acquisition of data; Z.C. contributed to analysis and interpretation of data; Y.G. contributed to statistical analysis; H.Q. contributed to drafting the manuscript and revision of manuscript for important intellectual content. All authors read and approved the final manuscript.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study was supported by Shanghai Pudong Municipal Commission (Number PWZxk2017-17 and PWYgy2018-10); Shanghai Municipal Commission (Number 201640298 and PKJ2016-Y29).
ORCID iD: Hongyun Qin  https://orcid.org/0000-0001-8751-0908
https://orcid.org/0000-0001-8751-0908
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Luis CA, Loewenstein DA, Acevedo A, Barker WW, Duara R. Mild cognitive impairment: directions for future research. Neurology. 2003;61(4):438–444. [DOI] [PubMed] [Google Scholar]
- 2. Guo LH, Alexopoulos P, Eisele T, Wagenpfeil S, Kurz A, Perneczky R. The National Institute on Aging-Alzheimer’s Association research criteria for mild cognitive impairment due to Alzheimer’s disease: predicting the outcome. Eur Arch Psychiatry Clin Neurosci. 2013;263(4):325–333. [DOI] [PubMed] [Google Scholar]
- 3. Bennett DA, Wilson RS, Schneider JA. et al. Natural history of mild cognitive impairment in older persons. Neurology. 2002;59(2):198–205. [DOI] [PubMed] [Google Scholar]
- 4. Morris JC, Storandt M, Miller JP. et al. Mild cognitive impairment represents early-stage Alzheimer disease. Arch Neurol. 2001;58(3):397–405. [DOI] [PubMed] [Google Scholar]
- 5. Wilson RS, Segawa E, Boyle PA, Anagnos SE, Hizel LP, Bennett DA. The natural history of cognitive decline in Alzheimer’s disease. Psychol Aging. 2012;27(4):1008–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Allan CL, Behrman S, Ebmeier KP, Valkanova V. Diagnosing early cognitive decline—When, how and for whom? Maturitas. 2016;96:103. [DOI] [PubMed] [Google Scholar]
- 7. Qin HY, Chen DH, Zheng-Wan QU. Investigation of mild cognitive impairment and its risk factors among 55 years old and above residents in Shanghai. J Clin Psychiatry. 2014;24(3):155–158. [Google Scholar]
- 8. Carnevale D, Mascio G, D’Andrea I. et al. Hypertension induces brain β-amyloid accumulation, cognitive impairment and memory deterioration through activation of rage in brain vasculature. Hypertension. 2012;60(1):188–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Roberts RO, Knopman DS, Geda YE. et al. Association of diabetes with amnestic and nonamnestic mild cognitive impairment. Alzheimers Dementia. 2014;10(1):18–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Beydoun MA, Beydoun HA, Gamaldo AA, Teel A, Zonderman AB, Wang Y. Epidemiologic studies of modifiable factors associated with cognition and dementia: systematic review and meta-analysis. BMC Public Health. 2014;14(1):643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Petersen RC, Morris JC. Mild cognitive impairment as a clinical entity and treatment target. Arch Neurol. 2005;62(7):1160. [DOI] [PubMed] [Google Scholar]
- 12. Mingyue G, Yan M, Kuang WH, Qiu PY. Factors and validity analysis of Mini-mental State Examination in Chinese elderly people. J Peking Univ (Health Sciences). 2015;47(3):443–449. [PubMed] [Google Scholar]
- 13. Woolf C, Slavin MJ, Draper B. et al. Can the clinical dementia rating scale identify mild cognitive impairment and predict cognitive and functional decline? Dement Geriatr Cogn Disord. 2016;41(5-6):292–302. [DOI] [PubMed] [Google Scholar]
- 14. Chen J, Ye ZR, Yuan MQ, Fang Y. Application and progress of Montreal Cognitive Assessment Scale in screening for mild cognitive impairment. Chin J Psychiatry. 2017;50(5):292–302. [Google Scholar]
- 15. Perneczky R, Pohl C, Sorg C. et al. Complex activities of daily living in mild cognitive impairment: conceptual and diagnostic issues. Age Ageing. 2006;35(3):240–245. [DOI] [PubMed] [Google Scholar]
- 16. Dubois B, Feldman HH, Jacova C. et al. Research criteria for the diagnosis of Alzheimer’s disease: revising the NINCDS-ADRDA criteria. Lancet Neurol. 2007;6(8):734–746. [DOI] [PubMed] [Google Scholar]
- 17. Shankle WR, Oveisgharan S, Hachinski V. Optimizing the Hachinski ischemic scale. Arch Neurol. 2012;6(4):S350–S351. [DOI] [PubMed] [Google Scholar]
- 18. Hu C, Yu D, Sun X, Zhang M, Wang L, Qin H. The prevalence and progression of mild cognitive impairment among clinic and community populations: a systematic review and meta-analysis. Int Psychogeriatr. 2017;29(10):1595. [DOI] [PubMed] [Google Scholar]
- 19. Gao Q, Gwee X, Feng L. Mild cognitive impairment reversion and progression: rates and predictors in community-living older persons in the Singapore longitudinal ageing studies cohort. Dement Geriatr Cogn Disord Extra. 2018;8(2):226–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pandya SY, Lacritz LH, Weiner MF, Deschner M, Woon FL. Predictors of reversion from mild cognitive impairment to normal cognition. Dement Geriatr Cogn Disord. 2015;43(3-4):204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Verlinden VJ, van der Geest JN, de Bruijn RFAG, Hofman A, Koudstaal PJ, Ikram MA. Trajectories of decline in cognition and daily functioning in preclinical dementia. Alzheimers Dement. 2016;12(2):144–153. [DOI] [PubMed] [Google Scholar]
- 22. Degen C, Toro P, Schönknecht P, Sattler C, Schröder J. Diabetes mellitus type II and cognitive capacity in healthy aging, mild cognitive impairment and Alzheimer’s disease. Psychiatry Res. 2016;240:42–46. [DOI] [PubMed] [Google Scholar]
- 23. Biessels GJ, Strachan MWJ, Visseren FLJ, Kappelle LJ, Whitmer RA. Dementia and cognitive decline in type 2 diabetes and prediabetic stages: towards targeted interventions. Lancet Diabetes Endocrinol. 2014;2(3):246–255. [DOI] [PubMed] [Google Scholar]
- 24. Hanyu H. Diabetes mellitus and dementia [in Japanese]. Brain Nerve. 2014;66(2):129–134. [PubMed] [Google Scholar]
- 25. Strachan MWJ. R D Lawrence Lecture 2010. The brain as a target organ in type 2 diabetes: exploring the links with cognitive impairment and dementia. Diabet Med. 2011;28(2):141–147. [DOI] [PubMed] [Google Scholar]
- 26. Strachan MWJ, Reynolds RM, Marioni RE, Price JF. Cognitive function, dementia and type 2 diabetes mellitus in the elderly. Nat Rev Endocrinol. 2011;7(2):108–114. [DOI] [PubMed] [Google Scholar]
- 27. Ji S, Ceng H. Cognitive function status and its influencing factors in elderly patients with diabetes in community. People’s Liberat Army J. 2016;33(7):28–31. [Google Scholar]
- 28. Han R, Li G, Qian YY, Wang SJ, Zhang YX, Ma LN. Relationship between insulin resistance and cognitive function in elderly patients with metabolic syndrome. J Diff Dis. 2017;16(5):473–476. [Google Scholar]
- 29. Wang J. et al. Relationship between cognitive function and insulin resistance in elderly patients with type 2 diabetes mellitus. Neurol Dis Mental Health. 2015;15(5):3. [Google Scholar]
- 30. Cooper C, Sommerlad A, Lyketsos CG, Livingston G. Modifiable predictors of dementia in mild cognitive impairment: a systematic review and meta-analysis. Am J Psychiatry. 2015;172(4):323. [DOI] [PubMed] [Google Scholar]
- 31. Luchsinger JA, Reitz C, Patel B. et al. Relation of diabetes to mild cognitive impairment. Alzheimer’s Dementia. 2007;2(3):S411–S412. [DOI] [PubMed] [Google Scholar]
- 32. Mortimer JA, Borenstein AR, Ding D. et al. High normal fasting blood glucose is associated with dementia in Chinese elderly. Alzheimers Dementia. 2010;6(6):440–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Apostolova LG, Di LJ, Duffy EL. et al. Risk factors for behavioral abnormalities in mild cognitive impairment and mild Alzheimer’s disease. Dement Geriatr Cogn Disord. 2014;37(5-6):315–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Garand L, Dew MA, Urda B, Lingler JH, Dekosky ST, Reynolds CF. Marital quality in the context of mild cognitive impairment. West J Nurs Res. 2007;29(8):976–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hughes TF, Flatt JD, Fu B, Chang CCH, Ganguli M. Engagement in social activities and progression from mild to severe cognitive impairment: the MYHAT study. Int Psychogeriatr. 2013;25(4):587–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Keohane K, Balfe M. The Nun study and Alzheimer’s disease: Quality of vocation as a potential protective factor? Dementia. 2017;18(5):1651–1662. [DOI] [PubMed] [Google Scholar]
- 37. Liang X, Chen Z, Dong X. et al. Mental work demands and late-life cognitive impairment: results from the Shanghai Aging Study. J Aging Health. 2018;31(5):883–898. [DOI] [PubMed] [Google Scholar]
- 38. Garibotto V, Borroni B, Kalbe E. et al. Education and occupation as proxies for reserve in aMCI converters and AD: FDG-PET evidence. Neurology. 2008;71(17):1342–1349. [DOI] [PubMed] [Google Scholar]
- 39. Bennett DA, Wilson RS, Schneider JA. et al. Education modifies the relation of AD pathology to level of cognitive function in older persons. Neurology. 2003;60(12):1909. [DOI] [PubMed] [Google Scholar]
- 40. Marcia S, Almeida OP, Menezes PR. The role of literacy, occupation and income in dementia prevention: the São Paulo Ageing & Health Study (SPAH). Int Psychogeriatr. 2010;22(8):1209–1215. [DOI] [PubMed] [Google Scholar]
- 41. Jia J, Zhou A, Wei C. et al. The prevalence of mild cognitive impairment and its etiological sub types in elderly Chinese. Alzheimers Dementia. 2014;10(4):439–447. [DOI] [PubMed] [Google Scholar]
- 42. Christian G, Gottfried S. Brain structures differ between musicians and non-musicians. J Neurosci. 2003;13(6):1168–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yao YH, Jiang GX, Xu RF, Tang HD, Chen SD, Cheng Q. Survey on cognitive function and analysis of associated factors among elders in Shanghai suburb. J Shanghai Jiaotong Univ (Medical Edition). 2009;29(3):283–287. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, Highlights for Study of the Risk and Preventive Factors for Progress of Mild Cognitive Impairment to Dementia by Chengping Hu, Ling Wang, Yi Guo, Zhicheng Cao, Ying Lu and Hongyun Qin in American Journal of Alzheimer's Disease & Other Dementias
Supplementary_Table_1 for Study of the Risk and Preventive Factors for Progress of Mild Cognitive Impairment to Dementia by Chengping Hu, Ling Wang, Yi Guo, Zhicheng Cao, Ying Lu and Hongyun Qin in American Journal of Alzheimer's Disease & Other Dementias