Abstract
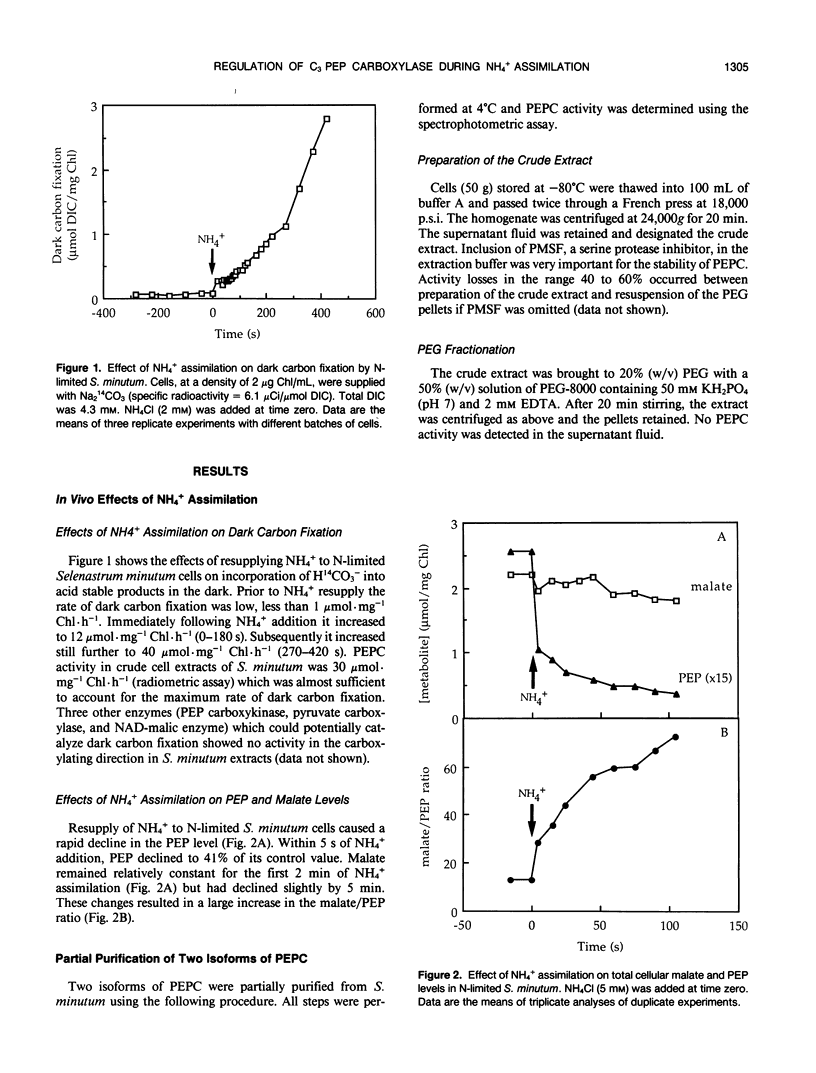
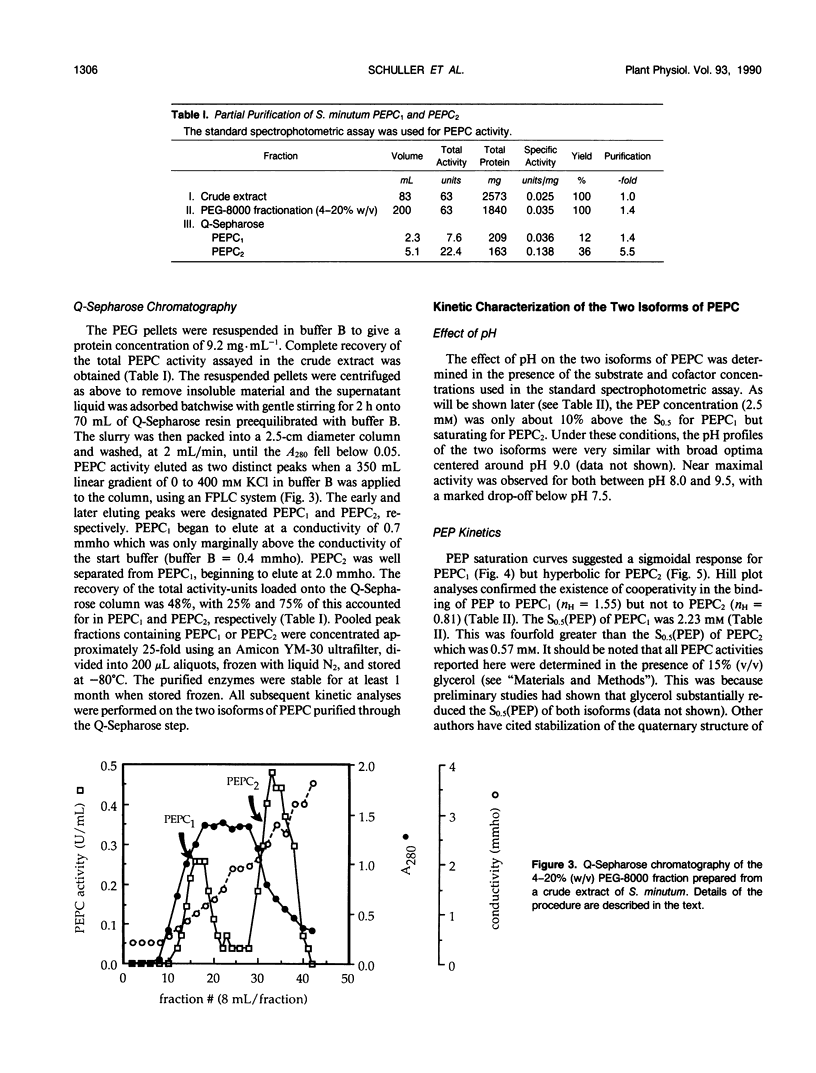
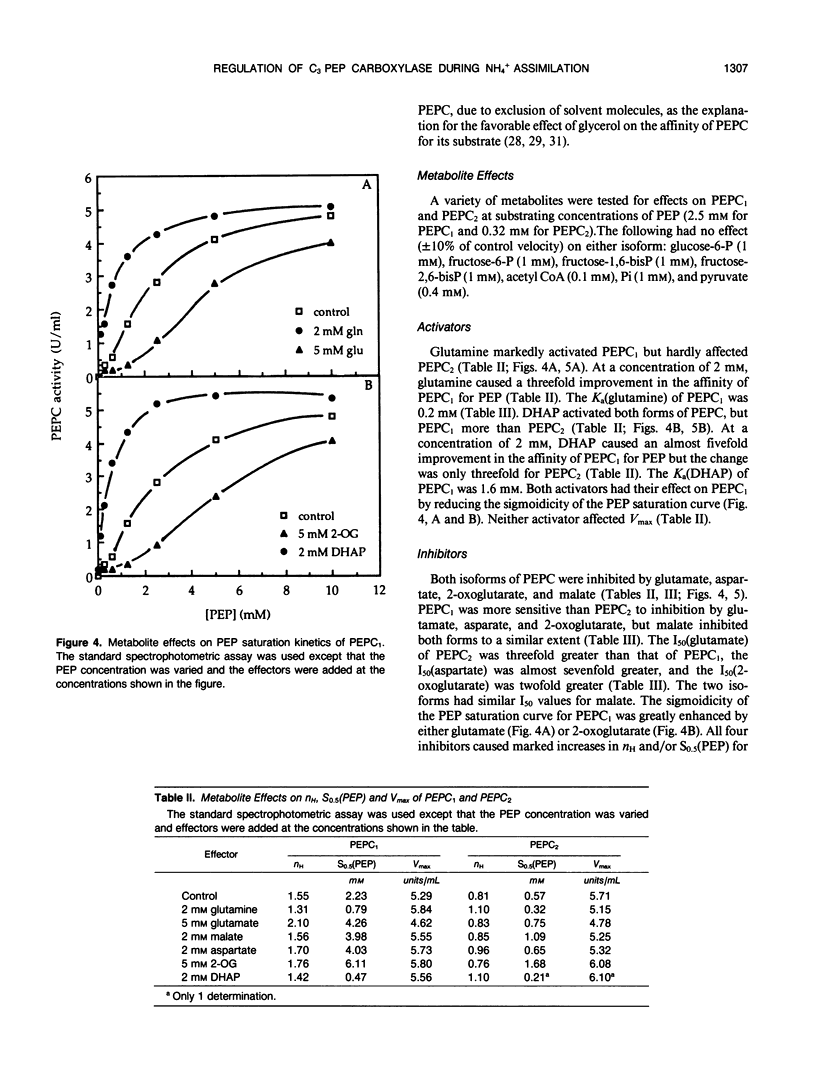
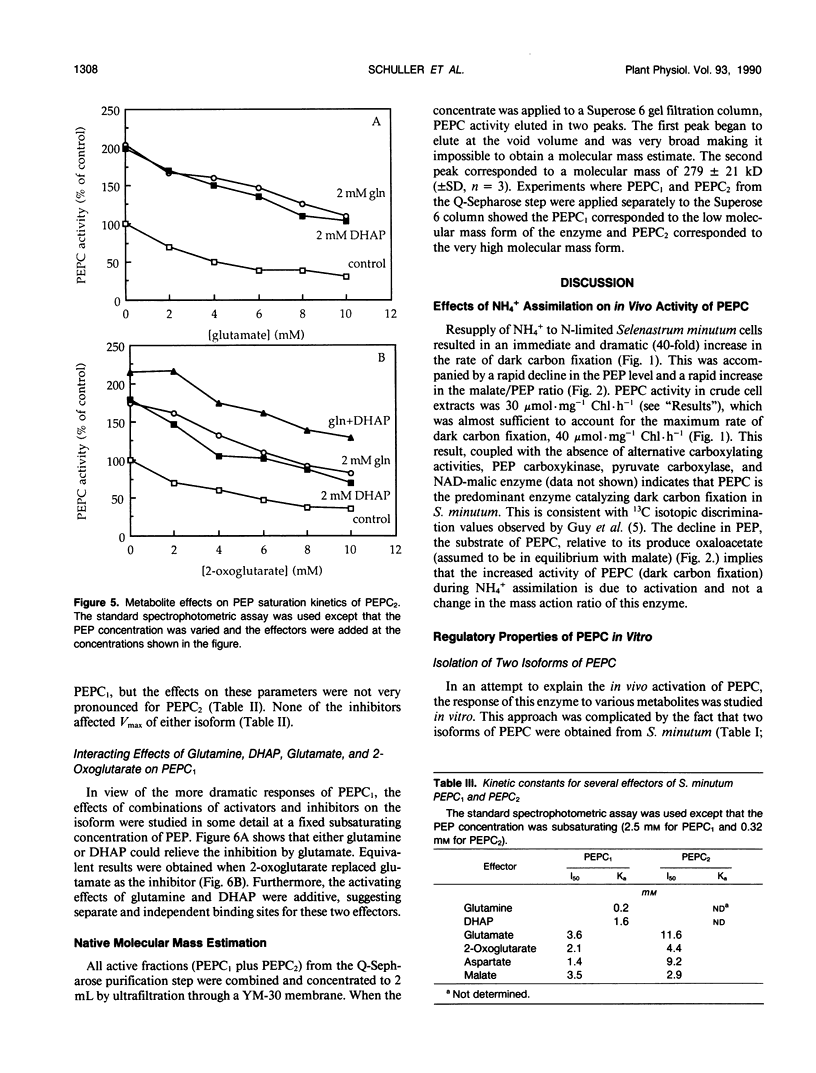
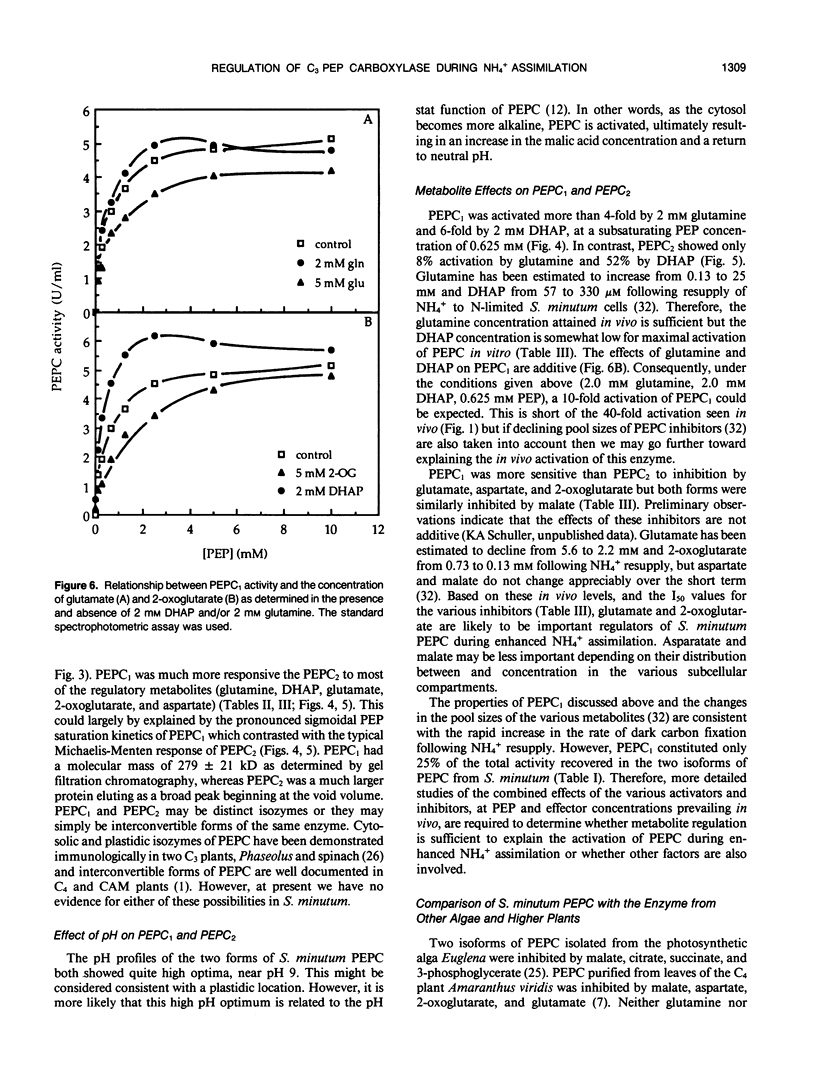
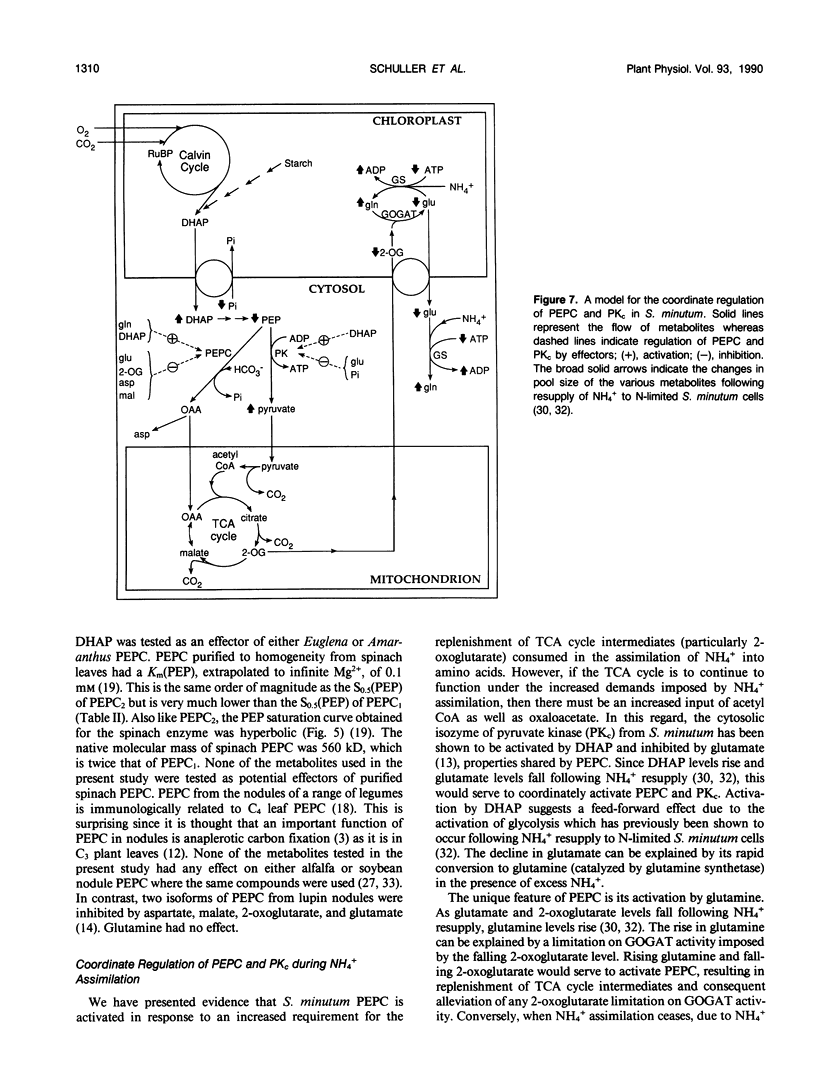
Two isoforms of phosphoenolpyruvate carboxylase (PEPC) with very different regulatory properties were partially purified from the green alga Selenastrum minutum. They were designated PEPC1 and PEPC2. PEPC1 showed sigmoidal kinetics with respect to phosphoenolpyruvate (PEP) whereas PEPC2 exhibited a typical Michaelis-Menten response. The S0.5(PEP) of PEPC1 was 2.23 millimolar. This was fourfold greater than the S0.5(PEP) of PEPC2, which was 0.57 millimolar. PEPC1 was activated more than fourfold by 2.0 millimolar glutamine and sixfold by 2.0 millimolar dihydroxyacetone phosphate (DHAP) at a subsaturating PEP concentration of 0.625 millimolar. In contrast, PEPC2 showed only 8% and 52% activation by glutamine and DHAP, respectively. The effects of glutamine and DHAP were additive. PEPC1 was more sensitive to inhibition by glutamate, 2-oxoglutarate, and aspartate than PEPC2. Both isoforms were equally inhibited by malate. All of these metabolites affected only the S0.5(PEP) not the Vmax. The regulatory properties of S. minutum PEPC in vitro are discussed in terms of (a) increased rates of dark carbon fixation (shown to be catalyzed predominantly by PEPC) and (b) changes in metabolite levels in vivo during enhanced NH4+ assimilation. Finally, a model is proposed for the regulation of PEPC in vivo in relation to its role in replenishing tricarboxylic acid cycle intermediates consumed in NH4+ assimilation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Elrifi I. R., Turpin D. H. Nitrate and Ammonium Induced Photosynthetic Suppression in N-Limited Selenastrum minutum. Plant Physiol. 1986 May;81(1):273–279. doi: 10.1104/pp.81.1.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy R. D., Vanlerberghe G. C., Turpin D. H. Significance of Phosphoenolpyruvate Carboxylase during Ammonium Assimilation: Carbon Isotope Discrimination in Photosynthesis and Respiration by the N-Limited Green Alga Selenastrum minutum. Plant Physiol. 1989 Apr;89(4):1150–1157. doi: 10.1104/pp.89.4.1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao J. A., Chollet R. Light/dark regulation of maize leaf phosphoenolpyruvate carboxylase by in vivo phosphorylation. Arch Biochem Biophys. 1988 Mar;261(2):409–417. doi: 10.1016/0003-9861(88)90357-8. [DOI] [PubMed] [Google Scholar]
- Job D., Cochet C., Dhien A., Chambaz E. M. A rapid method for screening inhibitor effects: determination of I50 and its standard deviation. Anal Biochem. 1978 Jan;84(1):68–77. doi: 10.1016/0003-2697(78)90484-0. [DOI] [PubMed] [Google Scholar]
- Kanazawa T., Kirk M. R., Bassham J. A. Regulatory effects of ammonia on carbon metabolism in photosynthesizing Chlorella pyrenoidosa. Biochim Biophys Acta. 1970 Jun 30;205(3):401–408. doi: 10.1016/0005-2728(70)90106-4. [DOI] [PubMed] [Google Scholar]
- Larsen P. O., Cornwell K. L., Gee S. L., Bassham J. A. Amino Acid Synthesis in Photosynthesizing Spinach Cells : EFFECTS OF AMMONIA ON POOL SIZES AND RATES OF LABELING FROM CO(2). Plant Physiol. 1981 Aug;68(2):292–299. doi: 10.1104/pp.68.2.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin M., Turpin D. H., Plaxton W. C. Pyruvate kinase isozymes from the green alga, Selenastrum minutum. II. Kinetic and regulatory properties. Arch Biochem Biophys. 1989 Feb 15;269(1):228–238. doi: 10.1016/0003-9861(89)90104-5. [DOI] [PubMed] [Google Scholar]
- McNaughton G. A., Fewson C. A., Wilkins M. B., Nimmo H. G. Purification, oligomerization state and malate sensitivity of maize leaf phosphoenolpyruvate carboxylase. Biochem J. 1989 Jul 15;261(2):349–355. doi: 10.1042/bj2610349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melzer E., O'leary M. H. Anapleurotic CO(2) Fixation by Phosphoenolpyruvate Carboxylase in C(3) Plants. Plant Physiol. 1987 May;84(1):58–60. doi: 10.1104/pp.84.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S. S., Boylan K. L., Vance C. P. Alfalfa Root Nodule Carbon Dioxide Fixation : III. Immunological Studies of Nodule Phosphoenolpyruvate Carboxylase. Plant Physiol. 1987 Jun;84(2):501–508. doi: 10.1104/pp.84.2.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miziorko H. M., Nowak T., Mildvan A. S. Spinach leaf phosphoenolpyruvate carboxylase: purification, properties, and kinetic studies. Arch Biochem Biophys. 1974 Jul;163(1):378–389. doi: 10.1016/0003-9861(74)90489-5. [DOI] [PubMed] [Google Scholar]
- Nimmo G. A., Nimmo H. G., Hamilton I. D., Fewson C. A., Wilkins M. B. Purification of the phosphorylated night form and dephosphorylated day form of phosphoenolpyruvate carboxylase from Bryophyllum fedtschenkoi. Biochem J. 1986 Oct 1;239(1):213–220. doi: 10.1042/bj2390213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson J. B., Evans H. J. Phosphoenolpyruvate carboxylase from soybean nodule cytosol. Evidence for isoenzymes and kinetics of the most active component. Biochim Biophys Acta. 1979 Apr 12;567(2):445–452. doi: 10.1016/0005-2744(79)90130-x. [DOI] [PubMed] [Google Scholar]
- Podestá F. E., Andreo C. S. Maize leaf phosphoenolpyruvate carboxylase : oligomeric state and activity in the presence of glycerol. Plant Physiol. 1989 Jun;90(2):427–433. doi: 10.1104/pp.90.2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandström G., Tärnvik A., Wolf-Watz H., Löfgren S. Antigen from Francisella tularensis: nonidentity between determinants participating in cell-mediated and humoral reactions. Infect Immun. 1984 Jul;45(1):101–106. doi: 10.1128/iai.45.1.101-106.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. G., Vanlerberghe G. C., Stitt M., Turpin D. H. Short-Term Metabolite Changes during Transient Ammonium Assimilation by the N-Limited Green Alga Selenastrum minutum. Plant Physiol. 1989 Oct;91(2):749–755. doi: 10.1104/pp.91.2.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turpin D. H., Botha F. C., Smith R. G., Feil R., Horsey A. K., Vanlerberghe G. C. Regulation of Carbon Partitioning to Respiration during Dark Ammonium Assimilation by the Green Alga Selenastrum minutum. Plant Physiol. 1990 May;93(1):166–175. doi: 10.1104/pp.93.1.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance C. P., Stade S. Alfalfa Root Nodule Carbon Dioxide Fixation : II. Partial Purification and Characterization of Root Nodule Phosphoenolpyruvate Carboxylase. Plant Physiol. 1984 May;75(1):261–264. doi: 10.1104/pp.75.1.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanlerberghe G. C., Horsey A. K., Weger H. G., Turpin D. H. Anaerobic Carbon Metabolism by the Tricarboxylic Acid Cycle : Evidence for Partial Oxidative and Reductive Pathways during Dark Ammonium Assimilation. Plant Physiol. 1989 Dec;91(4):1551–1557. doi: 10.1104/pp.91.4.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M. X., Wedding R. T. Regulation of Phosphoenolpyruvate Carboxylase from Crassula argentea: Further Evidence on the Dimer-Tetramer Interconversion. Plant Physiol. 1987 Aug;84(4):1080–1083. doi: 10.1104/pp.84.4.1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M. X., Wedding R. T. Regulation of phosphoenolpyruvate carboxylase from Crassula by interconversion of oligomeric forms. Arch Biochem Biophys. 1985 Aug 1;240(2):655–662. doi: 10.1016/0003-9861(85)90073-6. [DOI] [PubMed] [Google Scholar]


