Abstract
Liver transplant outcomes have improved over the years, and currently, the quality of life and long-term well-being of these patients needs to be improved. Improving bone health goes a long way toward achieving this objective. Poor bone health (osteopenia and osteoporosis) although prevalent, is often overlooked owing to its asymptomatic nature. It can be complicated by debilitating fracture affecting quality of life. It is recommended to assess and optimize bone health prior to liver transplant. Multiple factors contribute to poor bone health in a liver transplant recipient and it is vital to understand and ameliorate these. A careful and targeted approach with inputs from multidisciplinary team involving transplant physician, endocrinologist, occupational therapist, nutritionist, and nursing personnel may often be required. In this review, we aim to concisely discuss the various aspects related to prevalence, pathophysiology, evaluation, treatment, and follow-up of bone disease among liver transplant recipients.
Keywords: bone health, cirrhosis, liver transplant recipients, bisphosphonates, vitamin D
Over the decades, with improvement in care, post-liver transplant outcomes have improved significantly. Thus, increasingly the focus is on improving long-term well-being after transplant. One such emerging target for intervention is improving bone health of liver transplant recipient.
Poor bone health is often asymptomatic, until there is a fracture that can decrease the quality of life and lead to serious debilitation. Studies estimate a post-liver transplant prevalence varying from 3 to 35% of fractures,1,2 over a follow-up period of 1–30 years, and a much higher prevalence of its precursors—osteopenia (∼36%) and osteoporosis (∼12%).1 This suggests the importance of screening protocol, early identification, and adequate management of asymptomatic poor bone health.
In this review, we discuss the magnitude of problem, determinants of poor bone health in these patients, evaluation strategy, and medical intervention to improve bone health for liver transplant recipients.
Prevalence of poor bone health in liver disease and liver transplant recipients
Studies from India indicate a high prevalence of both osteopenia and osteoporosis in community amongst healthy men above 50 years (20% and ∼44%, respectively) and post-menopausal women (31% and 58%).3,4 Osteopenia, osteoporosis, and fracture risk are much higher among patients with liver disease.5,6 Immobilization, poor oral intake, decreased vitamin D production, and increased osteoclastic activity are factors mediating the poor bone health in cirrhosis. In fact, studies have shown a linear relation of prevalence of osteoporosis to worsening of liver disease severity.7 Certain liver diseases, for example, primary biliary cirrhosis (PBC) and primary sclerosing cirrhosis (PSC), are at an even higher risk of poor bone health and consequent fractures.8
Though in the post-transplant setting with better liver function, increasing physical activity and nutrition one would expect an improvement in bone health, the effect of immunosuppression may impede the recovery of bone loss. A prospective cohort study of liver transplant recipients estimated that almost a quarter of patients had worsening of bone mineral density, over a 10-year period post-liver transplant.9 While the data of decreased bone mineral density among children post-transplant are scarce, one Iranian study reported 36.9% prevalence of osteopenia among children one year post-liver transplant.10
Pathogenesis of bone disease in cirrhosis
Multiple factors, in addition to immobility commonly seen in advanced disease, affect bone health and increase fracture risk, in patients with cirrhosis.
Gut and Hormones
Bone health is a dynamic process that depends on multiple organs working in fine balance. The gut plays a role in the absorption of cholesterol (for vitamin D production), calcium, and phosphorus needed for bone formation. Vitamin D is formed from cholesterol as the substrate with exposure to sunlight (ultraviolet light). Vitamin D undergoes 25-hydroxylation in the liver and 1-hydroxylation in the kidney. In chronic liver disease, 25-hydroxylation of vitamin D is affected leading to functional deficiency.
While vitamin D is one of the factors affecting bone metabolism, various other hormones also play vital role namely calcitonin and estrogen, decrease bone resorption, glucocorticoids decrease bone formation, androgens increase bone formation, and parathormone (PTH) increase bone resorption.11 Cirrhosis is associated with lower testosterone among men and is shown to contribute to lower bone mineral density.12 Higher levels of serum bilirubin and low insulin-like growth factor -1 in cirrhosis are known to decrease osteoblast differentiation and proliferation.8
Receptor Activator of Nuclear Factor Kappa Beta–Receptor Activator of Nuclear Factor Kappa Beta Ligand–Osteoprotegerin Axis
Osteocytes (bone cells) are formed by osteoblasts and degraded by osteoclasts and various growth factors control this physiological process, of which the RANK (receptor activator of nuclear factor kappa beta) pathway is of therapeutic importance. RANK is activated by RANK ligand (RANKL, member of tumor necrosis factor superfamily) together they help in the differentiation of osteoclasts leading to bone resorption.11 Osteoprotegerin (OPG) produced by the osteoblast acts as a decoy receptor for RANKL and prevents the binding of RANK and RANK ligand.
Cirrhosis, especially secondary to cholestatic disorders, is known to affect the RANK–RANKL–OPG axis negatively impacting bone loss.13
Renal Dysfunction
Advanced liver disease, often is accompanied by varied severity of kidney dysfunction, which potentially negatively impacts bone health. Renal osteodystrophy is well known with decreased vitamin D hydroxylation, deranged acid–base status, and tubular defects.
Etiology of Liver Disease
Etiology of liver disease is also an important determinant of poor bone health. Primary biliary cirrhosis and primary sclerosing cholangitis are typical cholestatic liver diseases with associated decreased fat soluble vitamin absorption that leads to low vitamin D levels.14,15
The influence of alcohol on bone health is varied; however, overall evidence does not point to an alcohol driven bone weakness; however, smokers are clearly at increased risk of bone disease.16, 17, 18
Medications
Multiple drugs used in liver diseases can adversely affect bone health. Tenofovir disproxil fumarate, anti-hepatitis B nucleoside analogue, can cause poor bone health possibly secondary to renal mitochondrial tubulopathy.19 Loop diuretics, commonly used for ascites, causes calcium loss and thereby pre-dispose the individual to bone loss.20 Cholestyramine, a potent bile-acid binding agent used for cholestatic liver disease, interferes with vitamin D absorption and may affect absorption of other minerals as well.21 Steroids have multiple levels of deleterious effect on bone health.22
Pathogenesis of bone disease in post-liver transplant setting
Immediate post-liver transplant worsening of bone health is often noted, possibly secondary to poor sunlight exposure, immobilization and effect of conventional drugs, especially steroids.9 With improvement or amelioration of most factors mentioned earlier, liver transplant is expected to improve hepatic osteodystrophy over a medium-term. This improvement is partly negated by de novo risk factors, especially immunosuppressive drugs.
Steroid treatment remains pivotal as an immunosuppressant in the early post-transplant period and glucocorticoid-induced osteoporosis is well known.23 Steroids are often tapered after the first three months post-transplant, and the calcineurin inhibitors remain the backbone of life-long immunosuppression thereafter. Both cyclosporin and tacrolimus have shown to decrease bone mineral density and are associated with bone loss.24 Proton pump inhibitors routinely used along with steroid have also been associated with osteoporosis.25
Complex interactions of various risk factors, native liver disease, pre-transplant bone health, drugs and other post-transplant related effects, should prompt screening and treating of patients to prevent osteoporosis or fractures among patients with cirrhosis who undergo liver transplant.
Hungry bone syndrome is a rare condition that occurs in the post-operative state following parathyroidectomy; however, it has been described in other conditions including post-liver transplant state as well.26, 27, 28 It is characterized by persistent hypocalcemia, hypophosphatemia, and hypomagnesemia, and these electrolytes needs to be supplemented till it normalizes.29
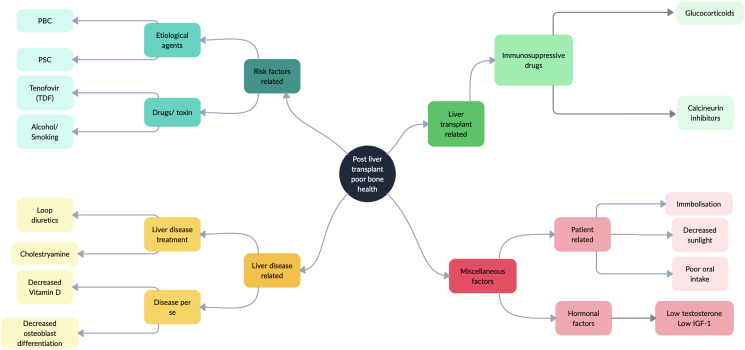
Summary of the factors predisposing to bone disease in a cirrhotic patient and liver transplant recipient is shown in Table 1 and Figure 1.
Table 1.
Risk Factors for Bone Disease in Cirrhotic and Post-transplant Patient.
| Pre-transplant risk factors for bone disease in cirrhotic patients | ||
|---|---|---|
| Modifiable risk factors | Steps to improve the risk factor | Non-modifiable risk factors |
| Vitamin D deficiency | Supplementation of Vitamin D | Immobility |
| Usage of loop diuretics | ||
| Dietary deficiencies | Screen and supplement | Cholestatic liver disease |
| Tenofovir disproxil fumarate-related tubular injury | Switch to Tenofovir alafenamide | Hypogonadism |
| Use of alcohol and nicotine smoking | Counseling and ensure abstinence | |
| Post-liver transplant risk factors for bone disease | ||
|---|---|---|
| Modifiable risk factors | Steps to improve the risk factor | Non-modifiable risk factors |
| Vitamin D deficiency | Supplementation of Vitamin D | Use of steroid and tacrolimus |
| Dietary deficiencies | Screen and supplement | |
Figure 1.
Factors causing poor bone health in a patient with cirrhosis undergoing liver transplant. IGF-1, insulin like growth factor 1; PBC, primary biliary cirrhosis; PSC, primary sclerosing cirrhosis; TDF, Tenofovir disproxil fumarate.
Evaluation for osteopenia/osteoporosis
Conventional bone health evaluation should consist of serum levels of calcium (corrected for serum albumin), vitamin D, parathormone, and bone mineral density studies.30 In cirrhosis, serum alkaline phosphatase may not be an ideal indicator as it may vary depending on nature and status of the liver disease.
Bone turn over markers (BTMs)—serum carboxy terminal telopeptide of collagen type I (s-CTX, bone resorption marker) and serum procollagen type I N-terminal pro-peptide (s-PINP, bone formation marker)—and their role in predicting the state of bone disease and response to treatment of osteoporosis have been studied and are becoming routine clinical tests in endocrine practice.31 Their role in advanced liver disease needs further defining.
Dual energy X-ray absorptiometry (DXA) scan is the standard method to assess patient's bone mineral density which can be compared to normal healthy gender-matched individuals (T-score). Osteopenia is defined as the patient's T-score of less than −1 SD to >−2.5 SD from that of the healthy individual and osteoporosis is defined as T-score ≤−2.5 SD compared to healthy individual.32
Fracture risk assessment tool (FRAX) score is a validated fracture risk prediction tool and can be used in cirrhotic patients as well.33 FRAX score categorizes patients as low risk (no prior hip or spine fractures, BMD T-score at the hip and spine both above −1.0, and 10-year hip fracture risk <3% and 10-year risk of major osteoporotic fractures <20%), moderate risk (no prior hip or spine fractures, a BMD T-score at the hip and spine both above −2.5, or 10-year hip fracture risk <3% or risk of major osteoporotic fractures <20%), high risk (prior spine or hip fracture, or a BMD T-score at the hip or spine of −2.5 or below, or 10-year hip fracture risk ≥3%, or risk of major osteoporotic fracture risk ≥20%) or very high risk (multiple spine fractures and a BMD T-score at the hip or spine of −2.5 or below).34
X-ray of the thoraco-lumbar spine to rule out osteoporotic fractures of the vertebrae and X-ray of the bilateral hip to identify looser zones to diagnose poor bone health related to osteomalacia are other additional tests that assist clinical care. While conventional X-rays have the risk of radiation, vertebral fracture assessment is a low radiation technique that can be done along with bone mineral density (BMD) and has good correlation with conventional radiography.35,36 Trabecular bone score is another upcoming technique that evaluates the bone microarchitecture and can independently predict fracture risk and can be done with DXA imaging.37
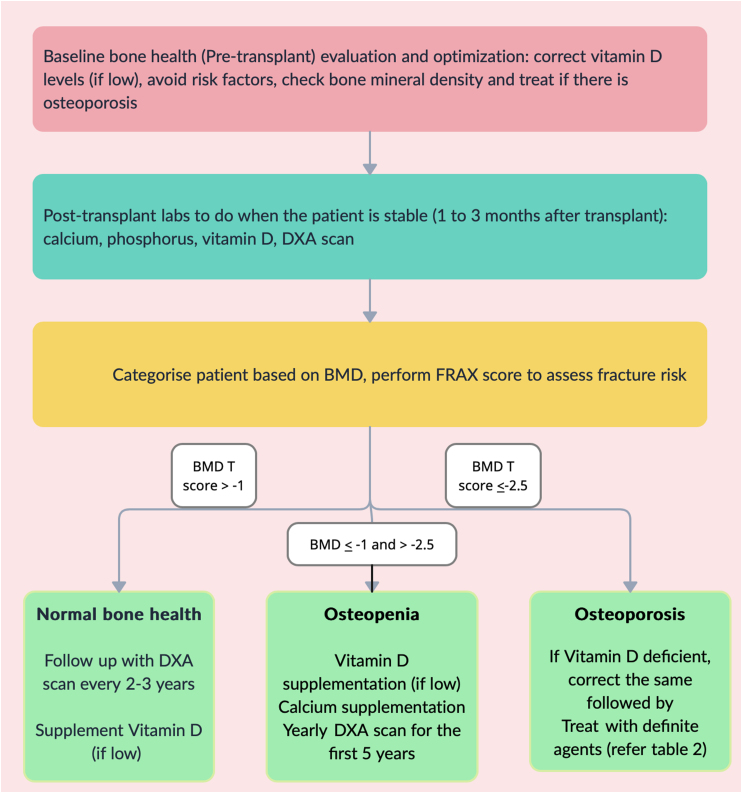
Figure 2 provides an algorithm for clinical assessment and follow-up for bone health in a post-liver transplant patient.
Figure 2.
Evaluation of bone health in a liver transplant recipient. BMD, bone mineral density; DXA, Dual energy X-ray absorptiometry.
Drug therapy in pre-transplant period
It is recommended to correct vitamin D deficiency if detected in the pre-transplant period (Figure 2). In addition, calcium supplementation is required for patients with baseline osteopenia. In patients with osteoporosis, it is advisable to initially correct vitamin D deficiency prior to treatment with specific agents (Table 2), usually with bisphosphonate (preferably single dose of intravenous Zolendronic acid).
Table 2.
Treatment Options for Liver Transplant Recipients with Osteoporosis.
| Adult dose and route of administration | Comments | ||
|---|---|---|---|
| Bisphosphonates | Alendronate | 70 mg per oral once a week for maximum 5 years | GI intolerance, hypocalcaemia, osteonecrosis of jaw (rare), atypical femur fracture (rare). Liver safe |
| Ibandronate | 150 mg per oral once a month for maximum 5 years (IV form also available) | Same as above | |
| Risedronate | 5 mg per oral daily for maximum 5 years | Same as above | |
| Zoledronic acid | 5 mg IV slow infusion over 5 min, once a year for 5 years | Infusion reactions (in addition to the above) | |
| Hormonal therapy | Teriparatide | 20 μg/day Subcutaneously daily for less than 2 years | Watch for hypercalcemia, renal impairment. Liver safe |
| Raloxifene | Not studied in post-liver transplant setting—not preferred | Vaginal bleeding, hot flushes, deep vein thrombosis, coronary artery disease. | |
| Testosterone | Not studied in post-liver transplant setting—not preferred | Causes LFT derangement Interaction with cyclosporine, tacrolimus and glucocorticoids | |
| Calcitonin | 100 IU intranasal daily or 200 IU S.C or I.M for 3–5 years | Not first choice as it is not most effective | |
| Others | Vitamin D | 50,000 IU per oral once a week for 8 weeks | Ideal to correct vitamin D deficiency for optimal response to bisphosphonate |
| Calcium | 500–1000 mg per oral/day | ||
| Denosumab | 60 mg subcutaneous once every 6 months for a period of 5 years | Liver safe, caution while using along with immunosuppression | |
GI, gastrointestinal; LFT, liver function test.
Bone health management, although, can be organized during the waiting period for cadaver transplant, should not delay transplant, especially in supra-urgent or live-donor setting.
Bone disease per se (including presence of fracture) is not a contraindication for liver transplant as generally these do not affect short-term patient outcome.38,39 However, it is common knowledge that poor bone health, especially fracture can affect the quality of life and would be ideal to optimize bone disease prior to transplant when possible.
Treatment of bone disease among patients post-liver transplant
Balanced diet rich in calcium, optimal sunlight exposure, and exercise are important to maintain bone health. Timely tapering of steroid, maintaining target levels of calcineurin inhibitors, and avoiding excess use of proton pump inhibitors are important in the post-transplant period to ensure that there is no drug-related adverse effect on bone health. Adequate counseling needs to be provided to ensure strict abstinence by patients from smoking and alcohol use.
If the patient had osteopenia pre-transplant, American Association for the Study of Liver Diseases suggest a yearly DXA scan for at least the first 5 years post-transplant. The interval between serial DXA scans can be 2–3 years if the patient had normal bone mineral density prior to transplant.40
Vitamin D Supplementation
Most patients do have improvement in the vitamin D levels after liver transplantation with restoration of normal liver function; however, majority of the patients still have levels that are sub-optimal, warranting Vitamin D supplementation.41,42 Therefore, it is advisable to test patients for vitamin D deficiency and treat with oral cholecalciferol (Vitamin D3) at dose of 50,000 IU (international units) once a week for 8 weeks, followed by maintenance doses.43
In osteoporosis, it is advisable to correct any vitamin D deficiency initially, to ensure an optimal response to bisphosphonate therapy.44,45
Calcium Supplementation
Recent data suggest calcium supplementation has only a weak anti-resorptive activity and may not benefit patients with osteopenia and osteoporosis. But, owing to good safety profile, the conventional practice is to supplement calcium, especially in patients on glucocorticoid therapy in the initial post-transplant period. While there are many available oral formulations of calcium, it is appropriate to ensure 500–1000 mg of elemental calcium supplementation per day in 1–2 divided doses.46
Drugs for Osteoporosis (Summarized in Table 2)
Bisphosphonate-based Therapy
Despite wide array of treatment options now available for osteoporosis, bisphosphonates still remain the backbone of therapy. Bisphosphonates are anti-resorptive agents that act by preventing hydroxyapatite (inorganic bone matrix) breakdown.47 Multiple oral agents, alendronate, ibandronate and risedronate and parenteral agents-zoledronic acid and pamidronate, are currently used.
All bisphosphonates have only minimal interactions with common transplant drugs and are safe to prescribe in post-liver transplant setting. Ibandronate and risedronate by virtue of better safety profile, are often considered as first line options in a post-transplant patient.48
Gastrointestinal side effects, hypocalcemia, infusion reactions of parenteral drugs, and musculoskeletal pains are among the common adverse effects to watch out for in patients on bisphosphonates.49 Although both oral and parenteral agents are not associated with de novo renal injury, parenteral bisphosphonates are contraindicated in patients with chronic kidney disease stage 4 and 5.50,51
PTH Analogues
Though elevated PTH levels cause increased bone resorption as in hyper-parathyroid states, teriparatide a recombinant fragment of PTH (hPTH 1–34) given subcutaneously as a low dose daily administration conversely causes bone formation.52
Teriparatide is advised for the treatment of osteoporosis for duration not more than 2 years. The side effect profile is acceptable with common side effects being nausea and vomiting, hypercalcemia, and orthostatic hypotension.
The drug has a good liver safety profile.53 It does not interact with drugs that are routinely used in a post-liver transplant setting. The usual dose is 20 mcg/day for 18–24 months.54
Denosumab
Denosumab is a fully humanized monoclonal antibody against RANK ligand that prevents bone resorption. American association of clinical endocrinology and American college of endocrinology recommend Denosumab as the first-line drug for patients with high fracture risk and who are not tolerating alternate oral agents.55 The usual dosage is 60 mg administered subcutaneously every 6 months.
As the effect of denosumab is transient and it does not have prolonged skeletal retention, the administration of denosumab should not be delayed beyond 6 months. Moreover, it should not be stopped abruptly as this can lead to a rebound increase in bone turnover and an increase in the risk of fractures. A discontinuation of denosumab warrants a transition to an oral or parenteral bisphosphonate that have prolonged skeletal retention time to curtail the abrupt and massive rebound increase in bone turnover.34
This drug is unlikely to cause liver test abnormalities.56 Since denosumab is a monoclonal antibody, literature suggest caution while using the drug while the patient is on other immunosuppressive drugs. This should not preclude usage of the drug in an appropriate post-transplant patient as the drug's efficacy and safety has been demonstrated in prior studies.57,58
The literature on the specific use of denosumab in recipients of liver transplant is limited; however, in an observational study by Brunova et al., on the use of denosumab in solid organ transplant (N = 63), it was found that denosumab was well tolerated in recipients of liver transplant (N = 14). There was significant improvement in the BMD T-scores with reduction in bone turnover.58
One of the potential side effects that may be anticipated with administration of denosumab is hypocalcaemia, which is more profound in those with concomitant renal insufficiency. It should be ensured that patients are calcium and vitamin D replete prior to denosumab therapy. Calcium levels should be monitored weekly for at least a month after initiating on denosumab therapy.
Calcitonin
Calcitonin is a hormone that is naturally produced by the C- cells of thyroid gland and functions by reducing the serum calcium level. However, the action of the hormone is not potent and may not be clinically relevant in states of vitamin D deficiency. Commercial and therapeutic calcitonin is derived from salmon.
There are studies showing the potency of nasal calcitonin in treating osteoporosis and preventing vertebral fractures; however, this is not the first choice and the use of the drug is reserved for patients who do not tolerate other agents and in the presence of severe bony pains.34,55 Among liver transplant recipients, the use of calcitonin to prevent bone loss in the first 6 months post-transplant period was not better than placebo.59 A good safety and tolerance profile and lack of drug–drug interactions allows its use in post-liver transplant setting.
Calcitonin is given as 200 IU per day intranasal dose or 100 IU per day intramuscular or subcutaneous injection for a period of 3–5 years for treatment of osteoporosis.60
Raloxifene
Raloxifene, a selective estrogen receptor modulator, has shown benefit in ameliorating osteoporosis across multiple studies in post-menopausal women.
Vaginal bleeding, hot flushes, deep vein thrombosis, and coronary artery disease are the class-related side effects that preclude a wider application of this drug. The drug is currently accepted for use only in post-menopausal women and is regarded as a weak anti-resorptive drug.55
This drug may cause liver injury and cholestatic hepatitis and exacerbation of non-alcoholic steatohepatitis has also been noted,61 and currently, there is no evidence for use of this drug in a transplant recipient with osteoporosis.
Testosterone Replacement Therapy
Cirrhosis is a state of hypogonadism and low testosterone contributes to poor bone mass is these patients. However, multiple studies exploring the role of testosterone replacement in improving bone health have yielded conflicting results.62,63
Liver-related adverse effects further deter the use of testosterone supplementation.64 Testosterone also is known to interact with cyclosporine, tacrolimus, and glucocorticoids, thereby making it difficult to use in post-liver transplant setting. Major side effects of this drug include headache, increasing hematocrit and hypertension.
Testosterone replacement therapy may be considered in hypogonadal men with osteopenia.
Interactions Between Osteoporosis Medications and Post-transplant Immunosuppressants
There are concerns with the use of oral bisphosphates along with tacrolimus or cyclosporine as it may interfere with the absorption of the calcineurin inhibitors. Options of treatment with intravenous bisphosphonates or adjusting the timing of oral bisphosphonates will prevent this problem.
Current evidence does not suggest any major interaction of denosumab or calcitonin with the common immunosuppressive medications.58,65 Teriparatide, on the other hand, has only limited data on its interaction with immunosuppressive medications and is not widely used in this setting.
Testosterone as a therapy for osteoporosis is less preferred as it is known to increase the blood levels of both tacrolimus and cyclosporine by decreasing their metabolism. Similarly, raloxifene, owning to its side effect profile, is better avoided in post-liver transplant setting.
Stepwise Management of Osteoporosis—choice of Drugs
Currently, there is no literature or guideline pertaining to the choice of drug in a cirrhotic or post-liver transplant patient. We suggest the stepwise approach below based on the recommendations by the Indian Society for Bone and Mineral Research position statement which could be safely followed in a patient with cirrhosis and in a post-liver transplant setting as well.66 Please refer to the above discussion on FRAX score for determination of fracture risk and refer Table 2 for specific drug dosage.
When There is Osteoporosis and High Risk of Fracture
Oral daily or weekly bisphosphonates are the first choice (alternatively yearly intravenous bisphosphonates can also be given). Generally, bisphosphonates are given up to 5 years.
Denosumab (6 monthly for up to 5 years) is the preferred second-line agent especially if patients have contraindications or are intolerant to bisphosphonate. Teriparatide can also be considered as an option if the T-score <−3.
When There is Osteoporosis and Low-moderate Risk of Fracture
Oral bisphosphonates are preferred in this group owing to their safety profile. However, intravenous bisphosphonates (2nd line) and denosumab (3rd line) are options in the presence of contraindication or reaction to oral bisphosphonates.
When There is Osteoporosis-related Vertebral Fracture
Though teriparatide is considered as good first-line anabolic agent in this scenario and is recommended subcutaneously daily for up to 2 years, due to lack of evidence in post-transplant setting, we suggest injectable bisphosphonates (Zoledronic acid) therapy yearly for 5 years.
When There is Osteoporosis-related Hip Fracture
Injectable Zoledronic acid is the apt first-line of choice and needs to be given yearly for 5 years.
Denosumab is a good second choice for this patient. Data on the use of teriparatide are limited in this setting.
Monitoring While on Treatment
Among parameters used for monitoring of patients while on treatment for osteoporosis BMD and BTM (bone turn over markers) are popular. BMD done yearly after starting of therapy to diagnose patients with non-response and to implement alternate treatment is recommended. Among patients on anti-resorptive treatment, decrease in BTM is expected and has shown to correlate with decrease in fracture risk.67
Avascular necrosis of bone
Avascular necrosis (AVN), although not related to bone health, is a common occurrence among patients with long-term iatrogenic glucocorticoid usage. AVN is also reported in pre-transplant patients with cirrhosis secondary to PBC and PSC; however, there is a sharp increase in the post-transplant incidence of the same.68
Common sites of AVN include head of femur and humerus, talus, and knee. As in the initial stage, the problem can be missed on plain X-ray, if a patient complaints of hip pain, there should be a high index of suspicion and magnetic resonance imaging should be considered.69
The pathophysiology of AVN is complex and has various postulates, but glucocorticoid association is certain; therefore, once AVN is suspected in patient, the use of steroid immunosuppression needs to be limited and other agents should be accordingly boosted.70 It is also advisable to limit the use of long-term glucocorticoids in liver transplant recipients.
Prompt intervention by orthopedic surgeons directed toward optimal definitive local treatment along with physiotherapy is often required in these patients.
Bone disease is a common occurrence in patients with liver disease and in recipients of liver transplantation. However, due to the silent nature of the disease, a high degree of suspicion and regular screening is needed to diagnose osteopenia and osteoporosis. Osteopenia can be treated with sunlight exposure, exercise, and calcium and Vitamin D supplementation, whereas osteoporosis treatment must include addition of bisphosphonate therapy as the first-line agent, with denosumab and teriparatide being the alternatives.
Credit authorship contribution statement
Santhosh E Kumar: Concept and design, Literature review, Preparation of manuscript, Review and Final approval. Kripa Elizabeth Cherian: Literature review, Critical writing/intellectual content, Review and Final approval. Thomas Paul: Critical writing/intellectual content, Review and Final approval. Ashish Goel: Concept and design, Literature review, Preparation of manuscript, Critical writing/intellectual content, Review and Final approval.
Conflicts of interest
The authors have none to declare.
Acknowledgments
Nil.
Funding
There was no financial grant received for conducting this review.
References
- 1.A systematic review and meta-analysis on the incidence of osteoporosis and fractures after liver transplant. https://onlinelibrary.wiley.com/doi/10.1111/tri.13863 [cited 2022 Oct 27]; Available from: [DOI] [PubMed]
- 2.Premaor M.O., Das T.K., Debiram I., et al. Fracture incidence after liver transplantation: results of a 10-year audit. QJM. 2011 Jul;104:599–606. doi: 10.1093/qjmed/hcr025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khinda R., Valecha S., Kumar N., et al. Prevalence and predictors of osteoporosis and osteopenia in postmenopausal women of Punjab, India. IJERPH. 2022 Mar 4;19:2999. doi: 10.3390/ijerph19052999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shetty S., Kapoor N., Naik D., et al. Osteoporosis in healthy South Indian males and the influence of life style factors and vitamin D status on bone mineral density. J Osteoporos. 2014 Nov 11;2014 doi: 10.1155/2014/723238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee H.J., Lee D.C., Kim C.O. Association between 10-year fracture probability and nonalcoholic fatty liver disease with or without sarcopenia in Korean men: a nationwide population-based cross-sectional study. Front Endocrinol. 2021;12 doi: 10.3389/fendo.2021.599339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goubraim R., Kabbaj N., Salihoun M., Chaoui Z., Nya M., Amrani N. Metabolic bone disease in viral cirrhosis: a prospective study. Int Sch Res Notices. 2013 Apr 21;2013 doi: 10.1155/2013/276563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Poddar P., Khairnar H.B. Assessment of bone disease in cirrhosis of liver. J Clin Exp Hepatol. 2017 Jul 1;7:S92. [Google Scholar]
- 8.Gatta A., Verardo A., Di Pascoli M., Giannini S., Bolognesi M. Hepatic osteodystrophy. Clin Cases Miner Bone Metab. 2014;11:185–191. [PMC free article] [PubMed] [Google Scholar]
- 9.Li X.Y., Lew C.C.H., Kek P.C. Bone mineral density following liver transplantation: a 10-year trend analysis. Arch Osteoporos. 2021 Nov 12;16:169. doi: 10.1007/s11657-021-01037-x. [DOI] [PubMed] [Google Scholar]
- 10.Dehghani S.M., Amirhakimi A., Shahramian I., Bazi A., Hashemi M., Ilkhanipour H. The frequency and risk factors of low bone mineral density one year after liver transplantation in children: a study in Shiraz Organ Transplant Center. Biomed Res Ther. 2019 Jun 8;6:3207–3212. [Google Scholar]
- 11.Kini U., Nandeesh B.N. In: Radionuclide and Hybrid Bone Imaging [Internet] Fogelman I., Gnanasegaran G., van der Wall H., editors. Springer; Berlin, Heidelberg: 2012. Physiology of bone formation, remodeling, and metabolism; pp. 29–57. [cited 2022 Dec 7] [DOI] [Google Scholar]
- 12.Sinclair M., Grossmann M., Gow P.J., Angus P.W. Testosterone in men with advanced liver disease: abnormalities and implications. J Gastroenterol Hepatol. 2015 Feb;30:244–251. doi: 10.1111/jgh.12695. [DOI] [PubMed] [Google Scholar]
- 13.Moschen A.R., Kaser A., Stadlmann S., et al. The RANKL/OPG system and bone mineral density in patients with chronic liver disease. J Hepatol. 2005 Dec;43:973–983. doi: 10.1016/j.jhep.2005.05.034. [DOI] [PubMed] [Google Scholar]
- 14.Angulo P., Grandison G.A., Fong D.G., et al. Bone disease in patients with primary sclerosing cholangitis. Gastroenterology. 2011 Jan;140:180–188. doi: 10.1053/j.gastro.2010.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Menon K.V., Angulo P., Weston S., Dickson E.R., Lindor K.D. Bone disease in primary biliary cirrhosis: independent indicators and rate of progression. J Hepatol. 2001 Sep;35:316–323. doi: 10.1016/s0168-8278(01)00144-1. [DOI] [PubMed] [Google Scholar]
- 16.Johnson J.T., Hussain M.A., Cherian K.E., Kapoor N., Paul T.V. Chronic alcohol consumption and its impact on bone and metabolic health – a narrative review. Indian J Endocrinol Metab. 2022;26:206–212. doi: 10.4103/ijem.ijem_26_22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sampson H.W. Alcohol's harmful effects on bone. Alcohol Health Res World. 1998;22:190–194. [PMC free article] [PubMed] [Google Scholar]
- 18.Xu B., Anderson D.B., Park E.S., Chen L., Lee J.H. The influence of smoking and alcohol on bone healing: systematic review and meta-analysis of non-pathological fractures. eClinicalMedicine. 2021 Dec 1;42 doi: 10.1016/j.eclinm.2021.101179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sajith K.G., Kapoor N., Shetty S., et al. Bone health and impact of Tenofovir treatment in men with hepatitis-B related chronic liver disease. J Clin Exp Hepatol. 2018 Mar;8:23–27. doi: 10.1016/j.jceh.2017.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lim L.S., Fink H.A., Blackwell T., Taylor B.C., Ensrud K.E. Loop diuretic use and rates of hip bone loss and risk of falls and fractures in older women: loop diuretics and bone loss, falls, and fractures. J Am Geriatr Soc. 2009 May;57:855–862. doi: 10.1111/j.1532-5415.2009.02195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thompson W.G., Thompson G.R. Effect of cholestyramine on the absorption of vitamin D3 and calcium. Gut. 1969 Sep 1;10:717–722. doi: 10.1136/gut.10.9.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmidt T., Schmidt C., Strahl A., et al. A system to determine risk of osteoporosis in patients with autoimmune hepatitis. Clin Gastroenterol Hepatol. 2020 Jan;18:226–233.e3. doi: 10.1016/j.cgh.2019.05.043. [DOI] [PubMed] [Google Scholar]
- 23.Cherian K.E., Kapoor N., Paul T.V. Glucocorticoid-induced osteoporosis. Indian J Endocrinol Metab. 2017;21:652–654. doi: 10.4103/ijem.IJEM_187_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kanda J., Izumo N., Furukawa M., et al. Effects of the calcineurin inhibitors cyclosporine and tacrolimus on bone metabolism in rats. Biomed Res. 2018;39:131–139. doi: 10.2220/biomedres.39.131. [DOI] [PubMed] [Google Scholar]
- 25.Zhou B., Huang Y., Li H., Sun W., Liu J. Proton-pump inhibitors and risk of fractures: an update meta-analysis. Osteoporos Int. 2016 Jan 1;27:339–347. doi: 10.1007/s00198-015-3365-x. [DOI] [PubMed] [Google Scholar]
- 26.Honda M., Shimata K., Sambommatsu Y., et al. Hungry bone syndrome after living donor liver transplant for biliary atresia. Exp Clin Transplant. 2021 Apr;19:386–389. doi: 10.6002/ect.2020.0413. [DOI] [PubMed] [Google Scholar]
- 27.Cartwright C., Anastasopoulou C. StatPearls [Internet] StatPearls Publishing; Treasure Island (FL): 2023. Hungry bone syndrome.http://www.ncbi.nlm.nih.gov/books/NBK549880/ [cited 2023 Mar 26]. Available from: [Google Scholar]
- 28.Nowack R., Wachtler P. Hypophosphatemia and hungry bone syndrome in a dialysis patient with secondary hyperparathyroidism treated with cinacalcet--proposal for an improved monitoring. Clin Lab. 2006;52:583–587. [PubMed] [Google Scholar]
- 29.Witteveen J.E., van Thiel S., Romijn J.A., Hamdy N.a.T. Hungry bone syndrome: still a challenge in the post-operative management of primary hyperparathyroidism: a systematic review of the literature. Eur J Endocrinol. 2013 Mar;168:R45–R53. doi: 10.1530/EJE-12-0528. [DOI] [PubMed] [Google Scholar]
- 30.Lee J., Vasikaran S. Current recommendations for laboratory testing and use of bone turnover markers in management of osteoporosis. Ann Lab Med. 2012 Mar;32:105–112. doi: 10.3343/alm.2012.32.2.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shetty S., Kapoor N., Bondu J.D., Thomas N., Paul T.V. Bone turnover markers: emerging tool in the management of osteoporosis. Indian J Endocrinol Metab. 2016;20:846–852. doi: 10.4103/2230-8210.192914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kanis J.A., Melton L.J., III, Christiansen C., Johnston C.C., Khaltaev N. The diagnosis of osteoporosis. J Bone Miner Res. 1994;9:1137–1141. doi: 10.1002/jbmr.5650090802. [DOI] [PubMed] [Google Scholar]
- 33.De A., Ray D., Lamoria S., Sharma V., Khurana T.R. Hepatic osteodystrophy and fracture risk prediction using FRAX tool in Indian patients with cirrhosis. JGH Open. 2020;4:945–949. doi: 10.1002/jgh3.12369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eastell R., Rosen C.J., Black D.M., Cheung A.M., Murad M.H., Shoback D. Pharmacological management of osteoporosis in postmenopausal women: an Endocrine Society∗ clinical practice guideline. J Clin Endocrinol Metab. 2019 May 1;104:1595–1622. doi: 10.1210/jc.2019-00221. [DOI] [PubMed] [Google Scholar]
- 35.Vertebral fracture assessment in supine position: comparison by using conventional semiquantitative radiography and visual radiography. Radiology. 2009 Jun;251:822–828. doi: 10.1148/radiol.2513080887. [DOI] [PubMed] [Google Scholar]
- 36.Drampalos E., Nikolopoulos K., Baltas C., et al. Vertebral fracture assessment: current research status and application in patients with kyphoplasty. World J Orthop. 2015 Oct 18;6:680–687. doi: 10.5312/wjo.v6.i9.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rajan R., Cherian K.E., Kapoor N., Paul T.V. Trabecular bone score—an emerging tool in the management of osteoporosis. Indian J Endocrinol Metab. 2020;24:237–243. doi: 10.4103/ijem.IJEM_147_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mehta N., Nundy S., Varma V. Indications and contraindications for liver transplantation. Int J Hepatol. 2011;2011 doi: 10.4061/2011/121862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.BC Clinical Guidelines for Liver Transplantation Jan 2023.pdf. http://www.transplant.bc.ca/Documents/Health%20Professionals/Clinical%20guidelines/BC%20Clinical%20Guidelines%20for%20Liver%20Transplantation%20Jan%202023.pdf [Internet]. [cited 2023 Apr 13]. Available from:
- 40.Lucey M.R., Terrault N., Ojo L., et al. Long-term management of the successful adult liver transplant: 2012 practice guideline by the American Association for the Study of Liver Diseases and the American Society of Transplantation. Liver Transplant. 2013;19:3–26. doi: 10.1002/lt.23566. [DOI] [PubMed] [Google Scholar]
- 41.Stein E.M., Cohen A., Freeby M., et al. Severe vitamin D deficiency among heart and liver transplant recipients. Clin Transplant. 2009;23:861–865. doi: 10.1111/j.1399-0012.2009.00989.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wisniewska H., Bander M., Bander D., et al. Vitamin D status before and after liver transplantation. https://www.clinmedjournals.org/articles/ijtrm/international-journal-of-transplantation-research-and-medicine-ijtrm-5-040.php?jid=ijtrm [cited 2022 Nov 28]; Available from:
- 43.Holick M.F., Binkley N.C., Bischoff-Ferrari H.A., et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011 Jul 1;96:1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 44.The 25(OH)D level needed to maintain a favorable bisphosphonate response is ≥33 ng/ml - PMC [Internet] https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3893033/ [cited 2022 Dec 15]. Available from:
- 45.Lems W.F., Geusens P. Are bisphosphonates effective and safe in patients with low serum vitamin D levels? Int J Clin Rheumatol. 2009 Apr;4:119–121. [Google Scholar]
- 46.Sunyecz J.A. The use of calcium and vitamin D in the management of osteoporosis. Ther Clin Risk Manag. 2008 Aug;4:827–836. doi: 10.2147/tcrm.s3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Drake M.T., Clarke B.L., Khosla S. Bisphosphonates: mechanism of action and role in clinical practice. Mayo Clin Proc. 2008 Sep;83:1032–1045. doi: 10.4065/83.9.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet] National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda (MD): 2012. Bisphosphonates.http://www.ncbi.nlm.nih.gov/books/NBK548566/ [cited 2022 Nov 28]. Available from: [Google Scholar]
- 49.Kennel K.A., Drake M.T. Adverse effects of bisphosphonates: implications for osteoporosis management. Mayo Clin Proc. 2009 Jul;84:632–638. doi: 10.1016/S0025-6196(11)60752-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Perazella M.A., Markowitz G.S. Bisphosphonate nephrotoxicity. Kidney Int. 2008 Dec 1;74:1385–1393. doi: 10.1038/ki.2008.356. [DOI] [PubMed] [Google Scholar]
- 51.Miller P.D., Jamal S.A., Evenepoel P., Eastell R., Boonen S. Renal safety in patients treated with bisphosphonates for osteoporosis: a review. J Bone Miner Res. 2013 Oct;28:2049–2059. doi: 10.1002/jbmr.2058. [DOI] [PubMed] [Google Scholar]
- 52.Vall H., Parmar M. StatPearls [Internet] StatPearls Publishing; Treasure Island (FL): 2022. Teriparatide.http://www.ncbi.nlm.nih.gov/books/NBK559248/ [cited 2022 Nov 28]. Available from: [Google Scholar]
- 53.LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet] National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda (MD): 2012. Teriparatide.http://www.ncbi.nlm.nih.gov/books/NBK548722/ [cited 2022 Nov 28]. Available from: [Google Scholar]
- 54.Bonsity, Forteo, parathyroid hormone (teriparatide) dosing, indications, interactions, adverse effects, and more [Internet] https://reference.medscape.com/drug/bonsity-forteo-teriparatide-342831#0 [cited 2022 Nov 28]. Available from:
- 55.Tu K.N., Lie J.D., Wan C.K.V., et al. Osteoporosis: a review of treatment options. P T. 2018 Feb;43:92–104. [PMC free article] [PubMed] [Google Scholar]
- 56.LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet] National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda (MD): 2012. Denosumab.http://www.ncbi.nlm.nih.gov/books/NBK548424/ [cited 2022 Dec 7]. Available from: [Google Scholar]
- 57.Alfieri C., Binda V., Malvica S., et al. Bone effect and safety of one-year denosumab therapy in a cohort of renal transplanted patients: an observational monocentric study. J Clin Med. 2021 May 6;10:1989. doi: 10.3390/jcm10091989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brunova J., Kratochvilova S., Stepankova J. Osteoporosis therapy with denosumab in organ transplant recipients. Front Endocrinol. 2018 Apr 17;9:162. doi: 10.3389/fendo.2018.00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hay J.E., Malinchoc M., Dickson E.R. A controlled trial of calcitonin therapy for the prevention of post-liver transplantation atraumatic fractures in patients with primary biliary cirrhosis and primary sclerosing cholangitis. J Hepatol. 2001 Feb 1;34:292–298. doi: 10.1016/s0168-8278(00)00093-3. [DOI] [PubMed] [Google Scholar]
- 60.Chesnut C.H., Silverman S., Andriano K., et al. A randomized trial of nasal spray salmon calcitonin in postmenopausal women with established osteoporosis: the prevent recurrence of osteoporotic fractures study. PROOF Study Group. Am J Med. 2000 Sep;109:267–276. doi: 10.1016/s0002-9343(00)00490-3. [DOI] [PubMed] [Google Scholar]
- 61.LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet] National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda (MD): 2012. Selective estrogen receptor modulators.http://www.ncbi.nlm.nih.gov/books/NBK548475/ [cited 2022 Dec 7]. Available from: [Google Scholar]
- 62.Zhang Z., Kang D., Li H. The effects of testosterone on bone health in males with testosterone deficiency: a systematic review and meta-analysis. BMC Endocr Disord. 2020 Mar 7;20:33. doi: 10.1186/s12902-020-0509-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Isidori A.M., Giannetta E., Greco E.A., et al. Effects of testosterone on body composition, bone metabolism and serum lipid profile in middle-aged men: a meta-analysis. Clin Endocrinol. 2005 Sep;63:280–293. doi: 10.1111/j.1365-2265.2005.02339.x. [DOI] [PubMed] [Google Scholar]
- 64.LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet] National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda (MD): 2012. Androgenic steroids.http://www.ncbi.nlm.nih.gov/books/NBK548931/ [cited 2022 Dec 15]. Available from: [Google Scholar]
- 65.Bonani M., Frey D., Brockmann J., et al. Effect of twice-yearly denosumab on prevention of bone mineral density loss in de novo kidney transplant recipients: a randomized controlled trial. Am J Transplant. 2016;16:1882–1891. doi: 10.1111/ajt.13692. [DOI] [PubMed] [Google Scholar]
- 66.Bhadada S.K., Chadha M., Sriram U., et al. The Indian Society for Bone and Mineral Research (ISBMR) position statement for the diagnosis and treatment of osteoporosis in adults. Arch Osteoporos. 2021 Jun 26;16:102. doi: 10.1007/s11657-021-00954-1. [DOI] [PubMed] [Google Scholar]
- 67.Bruyère O., Reginster J.Y. Monitoring of osteoporosis therapy. Best Pract Res Clin Endocrinol Metabol. 2014 Dec 1;28:835–841. doi: 10.1016/j.beem.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 68.Guichelaar M.M.J., Schmoll J., Malinchoc M., Hay J.E. Fractures and avascular necrosis before and after orthotopic liver transplantation: long-term follow-up and predictive factors. Hepatology. 2007;46:1198–1207. doi: 10.1002/hep.21805. [DOI] [PubMed] [Google Scholar]
- 69.Hiralal, Udiya A.K., Thakral A., et al. Avascular necrosis of hip (AVN) in post renal transplant recipient: case report & review of literature. Indian J Transplant. 2014 Jan 1;8:32–35. [Google Scholar]
- 70.Lafforgue P. Pathophysiology and natural history of avascular necrosis of bone. Joint Bone Spine. 2006 Oct 1;73:500–507. doi: 10.1016/j.jbspin.2006.01.025. [DOI] [PubMed] [Google Scholar]