Abstract
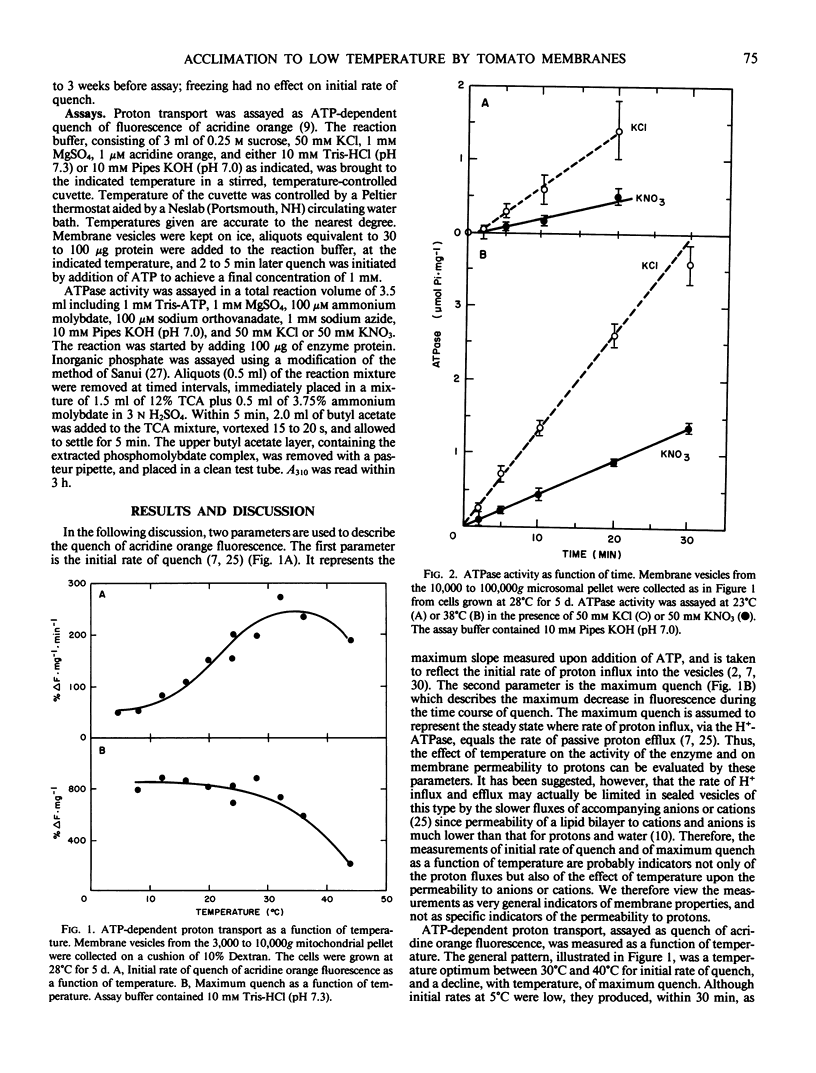
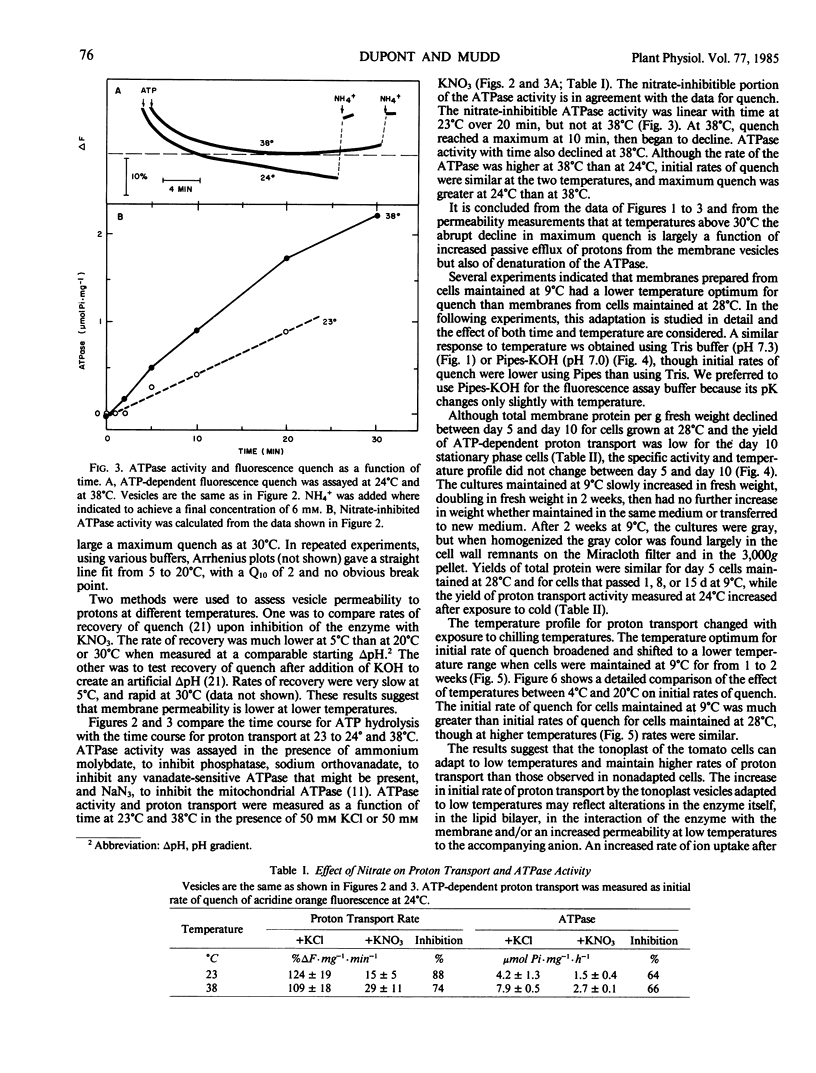
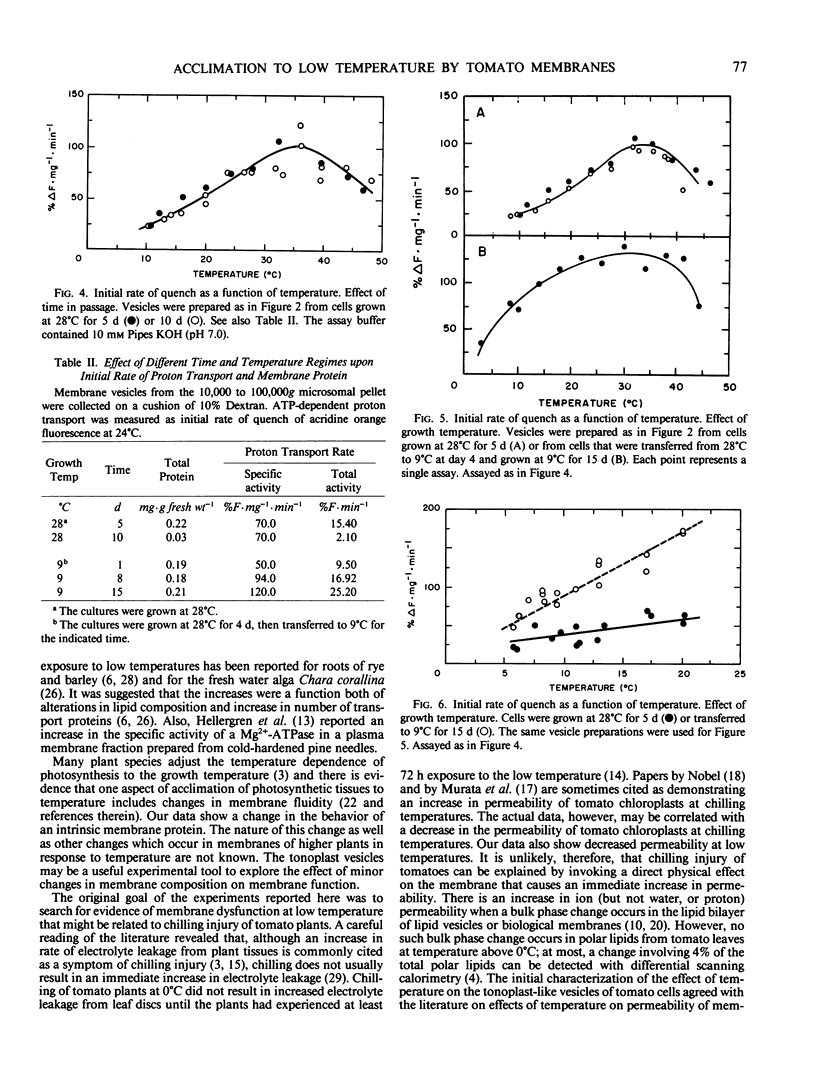
Sealed vesicles were prepared from microsomal membranes from cell suspension cultures of tomato (Lycopersicon esculentum Mill cv VF36). ATP-dependent proton transport activity by the vesicles was measured as quenching of fluorescence of acridine orange. Measurements of proton transport were correlated with the activity of a nitrate-inhibitable ATPase. The initial rate of proton influx into the vesicles was strongly temperature dependent with a Q10 of 2 and a maximum rate near 35°C. The data suggest that passive permeability did not increase at chilling temperatures but did increase rapidly with temperatures above 30°C. A comparison was made between membranes from cell cultures grown at 28°C and 9°C. The temperature optimum for proton transport broadened and shifted to a lower temperature range in membranes from cells maintained at 9°C.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett A. B., O'neill S. D., Spanswick R. M. H-ATPase Activity from Storage Tissue of Beta vulgaris: I. Identification and Characterization of an Anion-Sensitive H-ATPase. Plant Physiol. 1984 Mar;74(3):538–544. doi: 10.1104/pp.74.3.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breidenbach R. W., Waring A. J. Response to chilling of tomato seedlings and cells in suspension cultures. Plant Physiol. 1977 Aug;60(2):190–192. doi: 10.1104/pp.60.2.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont F. M., Giorgi D. L., Spanswick R. M. Characterization of a proton-translocating ATPase in microsomal vesicles from corn roots. Plant Physiol. 1982 Dec;70(6):1694–1699. doi: 10.1104/pp.70.6.1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont F. M., Staraci L. C., Chou B., Thomas B. R., Williams B. G., Mudd J. B. Effect of Chilling Temperatures upon Cell Cultures of Tomato. Plant Physiol. 1985 Jan;77(1):64–68. doi: 10.1104/pp.77.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont F. M., Zabala M. de G. Preparation of Membrane Vesicles Enriched in ATP-Dependent Proton Transport from Suspension Cultures of Tomato Cells. Plant Physiol. 1985 Jan;77(1):69–73. doi: 10.1104/pp.77.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elamrani K., Blume A. Effect of the lipid phase transition on the kinetics of H+/OH- diffusion across phosphatidic acid bilayers. Biochim Biophys Acta. 1983 Jan 5;727(1):22–30. doi: 10.1016/0005-2736(83)90364-4. [DOI] [PubMed] [Google Scholar]
- Gallagher S. R., Leonard R. T. Effect of vanadate, molybdate, and azide on membrane-associated ATPase and soluble phosphatase activities of corn roots. Plant Physiol. 1982 Nov;70(5):1335–1340. doi: 10.1104/pp.70.5.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handa S., Bressan R. A., Handa A. K., Carpita N. C., Hasegawa P. M. Solutes contributing to osmotic adjustment in cultured plant cells adapted to water stress. Plant Physiol. 1983 Nov;73(3):834–843. doi: 10.1104/pp.73.3.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King A. I., Reid M. S., Patterson B. D. Diurnal changes in the chilling sensitivity of seedlings. Plant Physiol. 1982 Jul;70(1):211–214. doi: 10.1104/pp.70.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata N., Fork D. C. Temperature dependence of the light-induced spectral shift of carotenoids in Cyanidium caldarium and higher plant leaves. Evidence for an effect of the physical phase of chloroplast membrane lipids on the permeability of the membrane to ions. Biochim Biophys Acta. 1977 Sep 14;461(3):365–378. doi: 10.1016/0005-2728(77)90226-2. [DOI] [PubMed] [Google Scholar]
- Ono T. A., Murata N. Chilling-Susceptibility of the Blue-Green Alga Anacystis nidulans: III. LIPID PHASE OF CYTOPLASMIC MEMBRANE. Plant Physiol. 1982 Jan;69(1):125–129. doi: 10.1104/pp.69.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papahadjopoulos D., Jacobson K., Nir S., Isac T. Phase transitions in phospholipid vesicles. Fluorescence polarization and permeability measurements concerning the effect of temperature and cholesterol. Biochim Biophys Acta. 1973 Jul 6;311(3):330–348. doi: 10.1016/0005-2736(73)90314-3. [DOI] [PubMed] [Google Scholar]
- Pike C. S. Membrane Lipid Physical Properties in Annuals Grown under Contrasting Thermal Regimes. Plant Physiol. 1982 Dec;70(6):1764–1766. doi: 10.1104/pp.70.6.1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole R. J., Briskin D. P., Krátký Z., Johnstone R. M. Density gradient localization of plasma membrane and tonoplast from storage tissue of growing and dormant red beet : characterization of proton-transport and ATPase in tonoplast vesicles. Plant Physiol. 1984 Mar;74(3):549–556. doi: 10.1104/pp.74.3.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reenstra W. W., Warnock D. G., Yee V. J., Forte J. G. Proton gradients in renal cortex brush-border membrane vesicles. Demonstration of a rheogenic proton flux with acridine orange. J Biol Chem. 1981 Nov 25;256(22):11663–11666. [PubMed] [Google Scholar]
- Sanders D. Physiological Control of Chloride Transport in Chara corallina: I. EFFECTS OF LOW TEMPERATURE, CELL TURGOR PRESSURE, AND ANIONS. Plant Physiol. 1981 Jun;67(6):1113–1118. doi: 10.1104/pp.67.6.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanui H. Measurement of inorganic orthophosphate in biological materials: extraction properties of butyl acetate. Anal Biochem. 1974 Aug;60(2):489–504. doi: 10.1016/0003-2697(74)90259-0. [DOI] [PubMed] [Google Scholar]
- Siddiqi M. Y., Memon A. R., Glass A. D. Regulation of k influx in barley : effects of low temperature. Plant Physiol. 1984 Mar;74(3):730–734. doi: 10.1104/pp.74.3.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolosin J. M., Forte J. G. Kinetic properties of the KCl transport at the secreting apical membrane of the oxyntic cell. J Membr Biol. 1983;71(3):195–207. doi: 10.1007/BF01875461. [DOI] [PubMed] [Google Scholar]


