Visual Abstract

Abstract
During hemolysis, erythrophagocytes dispose damaged red blood cells. This prevents the extracellular release of hemoglobin, detoxifies heme, and recycles iron in a linked metabolic pathway. Complementary to this process, haptoglobin and hemopexin scavenge and shuttle the red blood cell toxins hemoglobin and heme to cellular clearance. Pathological hemolysis outpaces macrophage capacity and scavenger synthesis across a diversity of diseases. This imbalance leads to hemoglobin-driven disease progression. To meet a void in treatment options, scavenger protein-based therapeutics are in clinical development.
Introduction
An efficient clearance system comprising macrophages and scavenger proteins maintains homeostasis within the red blood cell (RBC) compartment,1 by capturing aged and damaged RBCs, shuttling heme-iron to erythropoiesis, and preventing the adverse effects of cell-free hemoglobin (Hb) and heme. Several diseases are characterized by the excessive turnover of RBCs (ie, hemolysis), either as the primary etiology in genetic hemoglobinopathies, membranopathies, and enzyme defects, or as an acquired characteristic associated with blood transfusions, infections, autoimmune diseases, and mechanical stresses.2,3 RBC lysis in hemolytic anemia may occur not only in circulating peripheral blood, the spleen, or liver, but also within closed compartments after vascular rupture and hematoma formation. Aberrant functioning of RBC membranes in all these conditions results in exposure of the body’s internal milieu to harmful erythrocyte contents, including Hb, arginase,4 urate,5 and RBC-derived microparticles.6, 7, 8 The largest body of evidence exists on the toxicities of cell-free Hb and heme, on which we focus in this review. The capacity of the RBC-Hb-heme clearance system defines the threshold at which hemolysis deviates from a physiological normal RBC turnover process into a disease amplifier. This article delineates the pathophysiological processes induced by cell-free Hb and heme. It further discusses toxicity control by macrophages, haptoglobin, and hemopexin and potential exploitation of these protective features for the development of novel therapeutics.
Pathophysiology of cell-free Hb toxicity
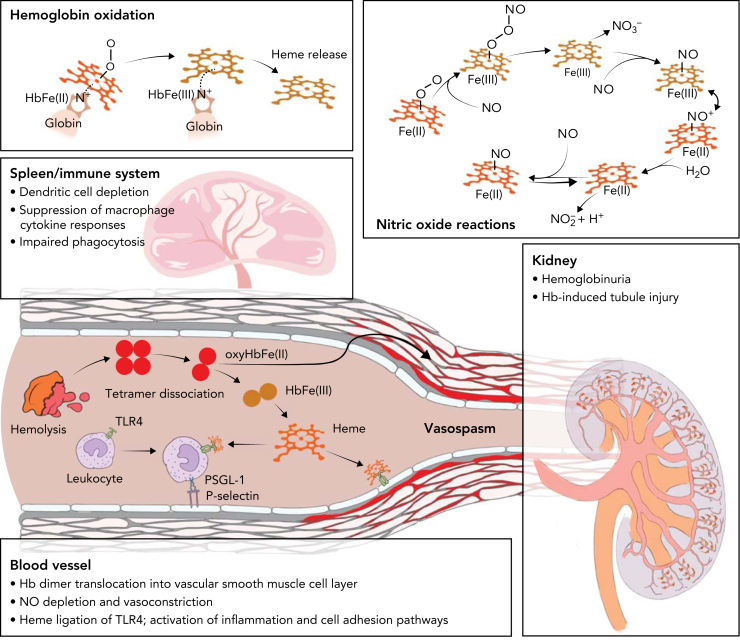
Progression of Hb-induced disease entails persistent RBC rupture, shifting of the dynamic equilibrium of Hb from tetramerization to dimerization, followed by translocation of small Hb dimers (32 kDa) across tissue barriers, such as the vascular endothelium, renal glomeruli, and the ependymal epithelium separating the ventricular cerebral spinal fluid (CSF) space from the brain parenchyma. The pathophysiological outcome of this cascade is the toxic accumulation of Hb and its by-products close to functionally critical parenchymal tissue. Ultimately, the detrimental effects of hemolysis can be traced back to Hb-catalyzed oxidative reactions and heme-ligation of cellular signaling molecules. This concept has been most extensively studied in the vasculature, kidneys, immune system, and subarachnoidal compartments.
Translocation of cell-free Hb from blood across endothelial barriers causes accumulation within interstitial spaces that surround vascular smooth muscle cells.9 Subsequent reactions between endothelium-derived nitric oxide (NO) and Hb cause vasoconstriction, aberrant hemodynamics,10,11 and chronic manifestations of endothelial dysfunction.9,12 Dioxygenation of oxy-Hb (HbFe(II)O2) initiates a sequence of NO-consumptive reactions progressing to (HbFe(III)NO+) and (HbFe(II)NO), which indicates that low quantities of interstitial Hb may cause substantial loss of autacoid-mediated vascular tone regulation.13,14
In blood, the majority of cell-free Hb remains in the reduced Fe(II)O2 state, capable of reactions with NO.15 However, in conditions with enhanced oxidative stress, Hb reactions initiated by autoxidation and endogenous peroxides can lead to higher heme iron oxidation states (Fe(III), Fe(IV), Fe(IV)=O2), redox cycling, and generation of Hb degradation and crosslinking products with cytotoxic and inflammatory activities.16, 17, 18 Furthermore, HbFe(III) oxidation destabilizes the heme-globin association,19 favoring heme partitioning into lipid compartments, such as cell membranes and lipoproteins. In these compartments, heme initiates peroxidative chain reactions that contribute to hemolysis-induced cytotoxicity and tissue injury and the generation of proinflammatory by-products.20, 21, 22, 23, 24 These reactions cumulatively leave traceable oxidative biomarker signatures in animals25 and humans,26, 27, 28 which provide information regarding the extent and duration of cell-free Hb exposure.
Cell-free Hb from high-intensity hemolysis is primarily eliminated from blood by renal clearance. Therefore, the kidney is the most heavily exposed organ during intravascular hemolysis. Postfiltration, the progressive acidification of the urine accelerates Hb-oxidation, globin structural destabilization, and heme release.22 This explains why most observations related to oxidation-driven Hb toxicity have been reported in the context of hemoglobinuria-induced kidney injury.29, 30, 31 In addition, because of direct exposure to RBC toxins, vascular pathophysiology is a hallmark of intermittent and chronic intravascular hemolysis. In genetic hemolytic anemias that demonstrate intravascular hemolysis, a common feature of disease progression is pulmonary hypertension caused by NO depletion and hemolysis-induced thrombosis.32, 33, 34, 35
Heme has also been identified as a driver of hemolysis-induced immune deregulation and as a disruptor of functional cell structures, including the proteasome36 and the cytoskeleton.37,38 The binding of heme to toll-like receptor 4 (TLR4) induces inflammatory NF-kB signaling in sickle cell anemia (SCA) murine models.39,40 The resulting exposure of P-selectin, which is a key mediator of multicellular adhesive interactions in SCA, suggests a plausible mechanistic framework for the initiation and propagation of vaso-occlusive crisis (VOC) and acute chest syndrome.39, 40, 41, 42 Conversely, the various detrimental effects of hemolysis on immune cells lead to hyposplenism, a state of acquired immunodeficiency that enhances the susceptibility to infections in patients with SCA and other hemolytic anemia. These immunosuppressive effects of heme progress via Hmox1,43 and activation of the transcription factor NRF2,44,45 which represses inflammatory cytokine responses of macrophages.44,45 Furthermore, activated heme-Nrf2 signaling in myeloid progenitors causes dysregulation of myeloid differentiation trajectories.46 This subsequently results in depletion of dendritic cells and reduces antigen presentation capacity and CD4 T-cell priming in murine SCA. Heme-induced distortion of the actin-cytoskeletal dynamics impairs phagocytosis and leukocyte migration and enhances susceptibility to gram-negative sepsis in mice.37 A summary of cell-free Hb driven toxicity pathways is shown in Figure 1.
Figure 1.
Toxicity pathways driven by hemolysis. From a biochemical perspective, hemolysis drives toxicity by Hb oxidation, which generates free heme, and by a sequence of consumptive NO reactions (top panels). The bottom panels symbolize the main targets of hemolysis-driven disease in the immune system, blood vessels, and kidneys. After intravascular hemolysis, cell-free tetrameric Hb dissociates into dimers. Hb dimers can translocate across tissue barriers, leading to toxic exposures of functionally critical compartments, such as vascular smooth muscle cells of arteries or renal glomeruli, leading to vasoconstriction and renal injury. Another toxicity pathway progresses via Hb oxidation, free heme generation, and heme-ligation of TLR4 on endothelial cells and leukocytes, leading to adhesion pathway activation. Finally, Hb and heme exposure of the immune system causes phagocyte dysfunction, suppression of inflammation, and impaired antigen presentation.
Hierarchical defense by erythrophagocytes, haptoglobin, and hemopexin
Protection against hemolytic stress is primarily achieved by metabolism of hemolytic toxins via engulfment and controlled phagolysosomal degradation of RBCs by specialized macrophages with high antioxidant and iron-recycling capacity (erythrophagocytes).44,47,48 Under physiological conditions, heme-mediated activation of the transcription factor SPI-C drives splenic differentiation of monocyte-derived red pulp macrophages.49,50 These homeostatic erythrophagocytes recognize aged and damaged RBCs and are largely responsible for the physiological turnover of erythrocyte metabolites. Enhanced capacity for clearance of damaged RBCs in hemolytic anemia requires recruitment of circulating monocytes to the liver, where CSF1, heme-activated NRF2, and HMOX1 regulate their differentiation into on-demand erythrophagocytes.44,51, 52, 53 A similar transcription factor network that functions via NRF2 and ATF1 promotes the differentiation of tissue-localized erythrophagocytes within the hematoma microenvironment.54,55 Heme signaling profoundly impairs the antigen presentation capacity and innate immune functions of erythrophagocytes. This process is accompanied by the downregulation of major histocompatibility complex class 2 expression and suppressed NF-κB activation subsequent to TLR4 or CD40 ligation.44,46,56 Although this immunoregulatory function of heme can cause immunodeficiency in hemolytic anemia, it likely evolved as a protective mechanism against harmful inflammation and development of autoimmunity during wound healing.
On-demand erythrophagocytes can sustain multiples of physiological RBC turnover and thereby avert cell-free Hb toxicity.51 However, saturation of the total erythrophagocytic capacity in stressed compartments, or hemolytic mechanisms evading recognition by erythrophagocytes, can lead to RBC degradation and release of cell-free Hb into extracellular spaces. This defines a physiological need for a different defense system targeting cell-free Hb and heme by the high-affinity scavenger proteins haptoglobin and hemopexin.
Haptoglobin binds dimers of HbFe(II)O2 and HbFe(III) in a stable protein complex.57 It exists in humans as a polymorphic protein with genetically determined dimeric or multimeric assemblies of Hb-binding subunits (haptoglobin β chains).58 Even the smallest possible configuration of an Hb-haptoglobin complex has a molecular weight of >100 kDa, which far exceeds size restrictions of translocation pathways that permit extravasation of cell-free Hb in the kidney and vasculature.30,59,60 This simple “size-exclusion mechanism” of haptoglobin is highly effective at protecting the kidney and vascular NO homeostasis against disruption by cell-free Hb.9 Furthermore, haptoglobin binding stabilizes the structural integrity of bound Hb dimers and enforces the closed R-state configuration of the heme pocket, thus preventing the release of heme, even under conditions when the heme iron is oxidized.16,21,57,61 The potent detoxification capacity of haptoglobin against all established modes of Hb toxicity is attributable to these unique structural features, in conjunction with the complex’s capacity to stabilize globin-based ferryl-radicals that are generated in reactions of Hb-haptoglobin complexes with endogenous peroxides.62,63 However, the destructive lysosomal clearance of the complexes via the macrophage CD163 scavenger receptor pathway results in the consumption, and often complete depletion of plasma haptoglobin in patients with hemolytic anemia.64 Therefore, despite ongoing synthesis of haptoglobin by the liver, the overall protective capacity of the haptoglobin scavenger system is limited in conditions with a high rate of cell-free Hb generation.26,64
Heme lies downstream in the hemolytic toxin release cascade. Spontaneous or oxidant-driven formation of ferric Hb (Fe(III)) weakens the noncovalent bond between globin and heme, thus enabling the transfer of heme to lipophilic compartments, which is followed by radical chain reactions, oxidative injury, inflammation, and immune dysfunction.20,39,65 Hemopexin irreversibly captures this highly reactive heme in a biochemically inert hexacoordinated complex that is cleared by endocytosis after binding to CD91 on macrophages and hepatocytes.66 Because only a small fraction of cell-free Hb is oxidized in most hemolytic conditions, the selectivity of hemopexin for oxidation-generated free heme explains the gradual depletion of hemopexin compared with that of haptoglobin during development of hemolytic anemia.21,26,67 A summary of protective mechanisms is shown in Figure 2.
Figure 2.
Protective mechanisms against hemolysis-driven disease. Protection mechanisms against Hb and heme toxicity adopt a hierarchical organization to match a diversity of hemolytic scenario. Macrophage recruitment to the liver augments erythrophagocytic capacity (level 1). Haptoglobin captures cell-free Hb dimers in an irreversible complex, stabilizing the heme-pocket structure and preventing tissue-barrier translocation in blood vessels and kidneys by its size-exclusion mechanism (level 2). Ultimately, hemopexin selectively captures free heme released from HbFe(III), blocking oxidative reactions, TLR4 ligation, and heme partitioning into lipophilic compartments (level 3).
Therapeutic potential of haptoglobin and hemopexin
Targeting specific diseases and medical interventions that feature extensive hemolysis offers unique therapeutic niches for haptoglobin and hemopexin. Here, we summarize the potential indications that are most described in the literature to date.
Hemolysis is a primary feature of SCA.68 Sickle erythrocytes are highly unstable and are excessively consumed by erythrophagocytosis in the liver and spleen or by intravascular hemolysis, which is estimated to account for one-third of RBC destruction.69 The release of Hb and arginase diminishes NO bioavailability, promoting vasculopathy and recurrent VOC.12,68 Autoxidation of HbFe(II)O2 to HbFe(III) enhances heme transfer to lipids,26,70, 71, 72 neutrophils, macrophages, and the vascular endothelium,39 triggering inflammation73 and cell adhesion pathways74 that are important contributors to VOC. Murine models of SCA provide proof of concept that haptoglobin and hemopexin prevent, and in part reverse, Hb- and heme-induced vascular stasis.75,76 Because haptoglobin consumption is very rapid during brisk hemolysis characteristic of human SCA, haptoglobin replacement therapy may be challenging.26,67 Consequently, hemopexin dosing is currently under investigation in a multicenter phase 1 clinical safety, tolerability, and pharmacokinetic trial being conducted in patients with SCA (https://clinicaltrials.gov/ct2/show/NCT04285827). Dose finding and pivotal efficacy studies with clinical endpoints will reveal if there is a role for hemopexin as a prevention or treatment for VOC.
RBC damage and hemolysis are common sequelae of extracorporeal circulation. The generation of high plasma cell-free Hb correlates with the incidence of acute kidney injury and adverse outcome after on-pump cardiac surgery,77, 78, 79 and in critically ill patients on extracorporeal membrane oxygenation.80 The assessment of Hb clearance rates,80,81 endogenous plasma haptoglobin concentrations,80 exogenously administered haptoglobin,82 and in-line Hb capture systems83 suggests considerable therapeutic potential. Against this hypothesis, another small study found that acute kidney injury in cardiopulmonary bypass patients was not associated with the level of plasma Hb.84 Consequently, rigorous efforts based on large prospective clinical studies to determine patient populations that could benefit most from scavenger protein-based therapeutics are essential to establish measurable endpoints for effectiveness.
RBC storage for transfusion is associated with donor-dependent changes that may compromise the quality of individual blood bags.85, 86, 87 After transfusion, this may lead to significant erythrophagocytosis, hemolysis-induced and NO-mediated adverse vascular effects, including posttransfusion vasoconstriction in animals6 and impaired blood flow in humans.88 Furthermore, renal effects, and iron accumulation in animals89, 90, 91 and humans,92 are critical observations following transfusion. Hb toxin scavenger proteins may thus be useful in clinical scenarios involving massive transfusion (>10 units of packed RBCs in 24 hours).93 The preclinical proof of concept for haptoglobin and hemopexin administration in poor-quality, high-volume RBC transfusion has been assessed in several species, and the findings suggest potentially substantive effectiveness.29,91,94, 95, 96 However, similar to clinical device-associated hemolysis, clinical situations in transfusion medicine that would benefit from treatment need to be identified based on patient risk for poor outcome, owing to Hb and heme exposure.
Prevention and treatment of secondary brain injury in patients with aneurysmal subarachnoid hemorrhage (aSAH) represent another opportunity for scavenger protein-based therapeutics outside the classical hemolysis field. In patients with aSAH, the decomposition of the blood clot generates cell-free Hb in the CSF and concurrence of cerebral vasospasm, ischemia, and secondary brain injury with high CSF-Hb has been described in approximately one-third of patients with aSAH.13,97 Preclinical evidence demonstrated that CSF-Hb causes cerebral vasospasm and potentially toxic neuronal exposures.13,97,98 Beneficial downstream effects of intracerebroventricular haptoglobin administration have been modeled in sheep and mice.13,98
Conclusions
The entire body of work, beginning from the studies conducted by Jayle et al,99 Smithies et al,100,101 and Bunn et al102,103 to the first protein-ligand complex crystal structures of haptoglobin-Hb57 and hemopexin-heme,104 has facilitated an in-depth proof of concept for refinement of indications for the use of scavenger proteins that can be tested in randomized controlled trials. An extensive summary of the potential therapeutic roles of haptoglobin and hemopexin was published in this journal in 2013.1 Since then, a substantial volume of research evidence has reinforced the concepts of Hb-driven disease mechanisms and encouraged therapeutic development. The European Commission and Food and Drug Administration designated hemopexin as an orphan drug (CSL889, CSL Behring), for potential use in SCA in 2020, and haptoglobin as an orphan drug (CSL888, CSL Behring), for treatment of subarachnoid hemorrhage in 2021. Clinical trials are currently in initial phases to advance plasma-derived hemopexin into dose-finding and pivotal efficacy trials. In addition, a large multicenter clinical study that aims to define targetable concentrations of cell-free Hb in CSF of patients with aSAH (www.clinicaltrials.gov/ct2/show/NCT04998370) is underway. Although current treatment strategies involving haptoglobin and hemopexin use human plasma-derived proteins, recombinant platforms are being exploited to conceptualize next-generation scavengers. These proteins will be structurally homogenous and contain specific functional subunits to prolong plasma half-life, provide dual Hb and heme-binding specificities, and enhance bioavailability in specific compartments.60,105,106 These approaches will improve drug formulation, delivery, and tissue distribution.
Conflict-of-interest disclosure: D.J.S. is inventor of patents related to the use of haptoglobin. F.V. and D.J.S. received research grants from CSL Behring. P.W.B. declares no competing financial interests.
Acknowledgments
The authors thank Rok Humar for designing the artwork.
This work was supported by Swiss National Science Foundation grants 197823 (D.J.S.) and 201202 (F.V.), Innosuisse grant 36361.1 IP-LS (D.J.S.), National Institutes of Health, National Heart, Lung, and Blood Institute grants R01HL162120, R01HL159862, R01HL158076, and R01HL161004 (P.W.B.).
Authorship
Contribution: F.V., P.W.B., and D.J.S. conceptualized, wrote, and edited the manuscript.
Footnotes
∗F.V., P.W.B., and D.J.S. contributed equally to this article.
References
- 1.Schaer DJ, Buehler PW, Alayash AI, Belcher JD, Vercellotti GM. Hemolysis and free hemoglobin revisited: exploring hemoglobin and hemin scavengers as a novel class of therapeutic proteins. Blood. 2013;121(8):1276–1284. doi: 10.1182/blood-2012-11-451229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schaer DJ, Buehler PW. Cell-free hemoglobin and its scavenger proteins: new disease models leading the way to targeted therapies. Cold Spring Harb Perspect Med. 2013;3(6):a013433. doi: 10.1101/cshperspect.a013433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gladwin MT, Kanias T, Kim-Shapiro DB. Hemolysis and cell-free hemoglobin drive an intrinsic mechanism for human disease. J Clin Invest. 2012;122(4):1205–1208. doi: 10.1172/JCI62972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morris CR, Kato GJ, Poljakovic M, et al. Dysregulated arginine metabolism, hemolysis-associated pulmonary hypertension, and mortality in sickle cell disease. JAMA. 2005;294(1):81–90. doi: 10.1001/jama.294.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reynolds MD. Gout and hyperuricemia associated with sickle-cell anemia. Semin Arthritis Rheum. 1983;12(4):404–413. doi: 10.1016/0049-0172(83)90020-3. [DOI] [PubMed] [Google Scholar]
- 6.Donadee C, Raat NJH, Kanias T, et al. Nitric oxide scavenging by red blood cell microparticles and cell-free hemoglobin as a mechanism for the red cell storage lesion. Circulation. 2011;124(4):465–476. doi: 10.1161/CIRCULATIONAHA.110.008698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Camus SM, De Moraes JA, Bonnin P, et al. Circulating cell membrane microparticles transfer heme to endothelial cells and trigger vasoocclusions in sickle cell disease. Blood. 2015;125(24):3805–3814. doi: 10.1182/blood-2014-07-589283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garnier Y, Ferdinand S, Garnier M, et al. Plasma microparticles of sickle patients during crisis or taking hydroxyurea modify endothelium inflammatory properties. Blood. 2020;136(2):247–256. doi: 10.1182/blood.2020004853. [DOI] [PubMed] [Google Scholar]
- 9.Schaer CA, Deuel JW, Schildknecht D, et al. Haptoglobin preserves vascular nitric oxide signaling during hemolysis. Am J Respir Crit Care Med. 2016;193(10):1111–1122. doi: 10.1164/rccm.201510-2058OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eich RF, Li T, Lemon DD, et al. Mechanism of NO-induced oxidation of myoglobin and hemoglobin. Biochemistry. 1996;35(22):6976–6983. doi: 10.1021/bi960442g. [DOI] [PubMed] [Google Scholar]
- 11.Doherty DH, Doyle MP, Curry SR, et al. Rate of reaction with nitric oxide determines the hypertensive effect of cell-free hemoglobin. Nat Biotechnol. 1998;16(7):672–676. doi: 10.1038/nbt0798-672. [DOI] [PubMed] [Google Scholar]
- 12.Reiter CD, Wang X, Tanus-Santos JE, et al. Cell-free hemoglobin limits nitric oxide bioavailability in sickle-cell disease. Nat Med. 2002;8(12):1383–1389. doi: 10.1038/nm1202-799. [DOI] [PubMed] [Google Scholar]
- 13.Hugelshofer M, Buzzi RM, Schaer CA, et al. Haptoglobin administration into the subarachnoid space prevents hemoglobin-induced cerebral vasospasm. J Clin Invest. 2019;129(12):5219–5235. doi: 10.1172/JCI130630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herold S, Exner M, Nauser T. Kinetic and mechanistic studies of the NO∗-mediated oxidation of oxymyoglobin and oxyhemoglobin. Biochemistry. 2001;40(11):3385–3395. doi: 10.1021/bi002407m. [DOI] [PubMed] [Google Scholar]
- 15.Wang X, Tanus-Santos JE, Reiter CD, et al. Biological activity of nitric oxide in the plasmatic compartment. Proc Natl Acad Sci USA. 2004;101(31):11477–11482. doi: 10.1073/pnas.0402201101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schaer CA, Deuel JW, Bittermann AG, et al. Mechanisms of haptoglobin protection against hemoglobin peroxidation triggered endothelial damage. Cell Death Differ. 2013;20(11):1569–1579. doi: 10.1038/cdd.2013.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Posta N, Csősz É, Oros M, et al. Hemoglobin oxidation generates globin-derived peptides in atherosclerotic lesions and intraventricular hemorrhage of the brain, provoking endothelial dysfunction. Lab Invest. 2020;100(7):986–1002. doi: 10.1038/s41374-020-0403-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Potor L, Hendrik Z, Patsalos A, et al. Oxidation of hemoglobin drives a proatherogenic polarization of macrophages in human atherosclerosis. Antioxid Redox Signal. 2021;35(12):917–950. doi: 10.1089/ars.2020.8234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bunn HF, Jandl JH. Exchange of heme among hemoglobins and between hemoglobin and albumin. J Biol Chem. 1968;243(3):465–475. [PubMed] [Google Scholar]
- 20.Nagy E, Eaton JW, Jeney V, et al. Red cells, hemoglobin, heme, iron, and atherogenesis. Arterioscler Thromb Vasc Biol. 2010;30(7):1347–1353. doi: 10.1161/ATVBAHA.110.206433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deuel JW, Vallelian F, Schaer CA, Puglia M, Buehler PW, Schaer DJ. Different target specificities of haptoglobin and hemopexin define a sequential protection system against vascular hemoglobin toxicity. Free Radic Biol Med. 2015;89:931–943. doi: 10.1016/j.freeradbiomed.2015.09.016. [DOI] [PubMed] [Google Scholar]
- 22.Deuel JW, Schaer CA, Boretti FS, et al. Hemoglobinuria-related acute kidney injury is driven by intrarenal oxidative reactions triggering a heme toxicity response. Cell Death Dis. 2016;7(1) doi: 10.1038/cddis.2015.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Balla J, Jacob HS, Balla G, Nath K, Eaton JW, Vercellotti GM. Endothelial-cell heme uptake from heme proteins: induction of sensitization and desensitization to oxidant damage. Proc Natl Acad Sci USA. 1993;90(20):9285–9289. doi: 10.1073/pnas.90.20.9285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Balla G, Vercellotti GM, Muller-Eberhard U, Eaton J, Jacob HS. Exposure of endothelial cells to free heme potentiates damage mediated by granulocytes and toxic oxygen species. Lab Invest. 1991;64(5):648–655. [PubMed] [Google Scholar]
- 25.Buehler PW, D’Agnillo F. Toxicological consequences of extracellular hemoglobin: biochemical and physiological perspectives. Antioxid Redox Signal. 2010;12(2):275–291. doi: 10.1089/ars.2009.2799. [DOI] [PubMed] [Google Scholar]
- 26.Yalamanoglu A, Deuel JW, Hunt RC, et al. Depletion of haptoglobin and hemopexin promote hemoglobin-mediated lipoprotein oxidation in sickle cell disease. Am J Physiol Lung Cell Mol Physiol. 2018;315(5):L765–L774. doi: 10.1152/ajplung.00269.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Redinus K, Baek JH, Yalamanoglu A, et al. An Hb-mediated circulating macrophage contributing to pulmonary vascular remodeling in sickle cell disease. JCI Insight. 2019;4(15) doi: 10.1172/jci.insight.127860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Svistunenko DA, Patel RP, Voloshchenko SV, Wilson MT. The globin-based free radical of ferryl hemoglobin is detected in normal human blood. J Biol Chem. 1997;272(11):7114–7121. doi: 10.1074/jbc.272.11.7114. [DOI] [PubMed] [Google Scholar]
- 29.Baek JH, Yalamanoglu A, Gao Y, et al. Iron accelerates hemoglobin oxidation increasing mortality in vascular diseased guinea pigs following transfusion of stored blood. JCI Insight. 2017;2(9) doi: 10.1172/jci.insight.93577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boretti FS, Buehler PW, D’Agnillo F, et al. Sequestration of extracellular hemoglobin within a haptoglobin complex decreases its hypertensive and oxidative effects in dogs and guinea pigs. J Clin Invest. 2009;119(8):2271–2280. doi: 10.1172/JCI39115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bissinger R, Nemkov T, D’Alessandro A, et al. Proteinuric chronic kidney disease is associated with altered red blood cell lifespan, deformability and metabolism. Kidney Int. 2021;100(6):1227–1239. doi: 10.1016/j.kint.2021.08.024. [DOI] [PubMed] [Google Scholar]
- 32.Minneci PC, Deans KJ, Zhi H, et al. Hemolysis-associated endothelial dysfunction mediated by accelerated NO inactivation by decompartmentalized oxyhemoglobin. J Clin Invest. 2005;115(12):3409–3417. doi: 10.1172/JCI25040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rother RP, Bell L, Hillmen P, Gladwin MT. The clinical sequelae of intravascular hemolysis and extracellular plasma hemoglobin: a novel mechanism of human disease. JAMA. 2005;293(13):1653–1662. doi: 10.1001/jama.293.13.1653. [DOI] [PubMed] [Google Scholar]
- 34.Brzoska T, Vats R, Bennewitz MF, et al. Intravascular hemolysis triggers ADP-mediated generation of platelet-rich thrombi in precapillary pulmonary arterioles. JCI Insight. 2020;5(14) doi: 10.1172/jci.insight.139437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vats R, Brzoska T, Bennewitz MF, et al. Platelet extracellular vesicles drive inflammasome-IL-1β-dependent lung injury in sickle cell disease. Am J Respir Crit Care Med. 2020;201(1):33–46. doi: 10.1164/rccm.201807-1370OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vallelian F, Deuel JW, Opitz L, et al. Proteasome inhibition and oxidative reactions disrupt cellular homeostasis during heme stress. Cell Death Differ. 2015;22(4):597–611. doi: 10.1038/cdd.2014.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martins R, Maier J, Gorki A.-D., et al. Heme drives hemolysis-induced susceptibility to infection via disruption of phagocyte functions. Nat Immunol. 2016;17(12):1361–1372. doi: 10.1038/ni.3590. [DOI] [PubMed] [Google Scholar]
- 38.James J, Srivastava A, Varghese MV, et al. Heme induces rapid endothelial barrier dysfunction via the MKK3/p38MAPK axis. Blood. 2020;136(6):749–754. doi: 10.1182/blood.2019003986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Belcher JD, Chen C, Nguyen J, et al. Heme triggers TLR4 signaling leading to endothelial cell activation and vaso-occlusion in murine sickle cell disease. Blood. 2014;123(3):377–390. doi: 10.1182/blood-2013-04-495887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ghosh S, Adisa OA, Chappa P, et al. Extracellular hemin crisis triggers acute chest syndrome in sickle mice. J Clin Invest. 2013;123(11):4809–4820. doi: 10.1172/JCI64578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sundd P, Gladwin MT, Novelli EM. Pathophysiology of sickle cell disease. Annu Rev Pathol. 2019;14(1):263–292. doi: 10.1146/annurev-pathmechdis-012418-012838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ghosh S, Flage B, Weidert F, Ofori-Acquah SF. P-selectin plays a role in haem-induced acute lung injury in sickle mice. Br J Haematol. 2019;186(2):329–333. doi: 10.1111/bjh.15807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cunnington AJ, de Souza JB, Walther M, Riley EM. Malaria impairs resistance to Salmonella through heme- and heme oxygenase-dependent dysfunctional granulocyte mobilization. Nat Med. 2011;18(1):120–127. doi: 10.1038/nm.2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pfefferlé M, Ingoglia G, Schaer CA, et al. Hemolysis transforms liver macrophages into antiinflammatory erythrophagocytes. J Clin Invest. 2020;130(10):5576–5590. doi: 10.1172/JCI137282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Olonisakin TF, Suber TL, Gonzalez-Ferrer S, et al. Stressed erythrophagocytosis induces immunosuppression during sepsis through heme-mediated STAT1 dysregulation. J Clin Invest. 2021;131(1) doi: 10.1172/JCI137468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vallelian F, Buzzi RM, Pfefferlé M, et al. Cell Death Differ; 2022. Heme-stress activated NRF2 skews fate trajectories of bone marrow cells from dendritic cells towards red pulp-like macrophages in hemolytic anemia [published online ahead of print 14 January 2022] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Knutson MD, Vafa MR, Haile DJ, Wessling-Resnick M. Iron loading and erythrophagocytosis increase ferroportin 1 (FPN1) expression in J774 macrophages. Blood. 2003;102(12):4191–4197. doi: 10.1182/blood-2003-04-1250. [DOI] [PubMed] [Google Scholar]
- 48.Klei TRL, Meinderts SM, van den Berg TK, van Bruggen R. From the cradle to the grave: The role of macrophages in erythropoiesis and erythrophagocytosis. Front Immunol. 2017;8:73. doi: 10.3389/fimmu.2017.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Haldar M, Kohyama M, So A.Y.-L., et al. Heme-mediated SPI-C induction promotes monocyte differentiation into iron-recycling macrophages. Cell. 2014;156(6):1223–1234. doi: 10.1016/j.cell.2014.01.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kohyama M, Ise W, Edelson BT, et al. Role for Spi-C in the development of red pulp macrophages and splenic iron homeostasis. Nature. 2009;457(7227):318–321. doi: 10.1038/nature07472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Theurl I, Hilgendorf I, Nairz M, et al. On-demand erythrocyte disposal and iron recycling requires transient macrophages in the liver. Nat Med. 2016;22(8):945–951. doi: 10.1038/nm.4146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bories GFP, Yeudall S, Serbulea V, Fox TE, Isakson BE, Leitinger N. Macrophage metabolic adaptation to heme detoxification involves CO-dependent activation of the pentose phosphate pathway. Blood. 2020;136(13):1535–1548. doi: 10.1182/blood.2020004964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim KS, Zhang D.-L., Kovtunovych G, et al. Infused wild-type macrophages reside and self-renew in the liver to rescue the hemolysis and anemia of Hmox1-deficient mice. Blood Adv. 2018;2(20):2732–2743. doi: 10.1182/bloodadvances.2018019737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Boyle JJ, Johns M, Kampfer T, et al. Activating transcription factor 1 directs Mhem atheroprotective macrophages through coordinated iron handling and foam cell protection. Circ Res. 2012;110(1):20–33. doi: 10.1161/CIRCRESAHA.111.247577. [DOI] [PubMed] [Google Scholar]
- 55.Seneviratne A, Han Y, Wong E, et al. Hematoma resolution in vivo is directed by activating transcription factor 1. Circ Res. 2020;127(7):928–944. doi: 10.1161/CIRCRESAHA.119.315528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pfefferlé M, Ingoglia G, Schaer CA, et al. Acute hemolysis and heme suppress anti-CD40 antibody-induced necro-inflammatory liver disease. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.680855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Andersen CBF, Torvund-Jensen M, Nielsen MJ, et al. Structure of the haptoglobin-haemoglobin complex. Nature. 2012;489(7416):456–459. doi: 10.1038/nature11369. [DOI] [PubMed] [Google Scholar]
- 58.Andersen CBF, Stødkilde K, Saederup KL, et al. Haptoglobin. Antioxid Redox Signal. 2017;26(14):814–831. doi: 10.1089/ars.2016.6793. [DOI] [PubMed] [Google Scholar]
- 59.Lipiski M, Deuel JW, Baek JH, Engelsberger WR, Buehler PW, Schaer DJ. Human Hp1-1 and Hp2-2 phenotype-specific haptoglobin therapeutics are both effective in vitro and in guinea pigs to attenuate hemoglobin toxicity. Antioxid Redox Signal. 2013;19(14):1619–1633. doi: 10.1089/ars.2012.5089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Buzzi RM, Owczarek CM, Akeret K, et al. Modular platform for the development of recombinant hemoglobin scavenger biotherapeutics. Mol Pharm. 2021;18(8):3158–3170. doi: 10.1021/acs.molpharmaceut.1c00433. [DOI] [PubMed] [Google Scholar]
- 61.Buehler PW, Abraham B, Vallelian F, et al. Haptoglobin preserves the CD163 hemoglobin scavenger pathway by shielding hemoglobin from peroxidative modification. Blood. 2009;113(11):2578–2586. doi: 10.1182/blood-2008-08-174466. [DOI] [PubMed] [Google Scholar]
- 62.Cooper CE, Schaer DJ, Buehler PW, et al. Haptoglobin binding stabilizes hemoglobin ferryl iron and the globin radical on tyrosine β145. Antioxid Redox Signal. 2013;18(17):2264–2273. doi: 10.1089/ars.2012.4547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vallelian F, Garcia-Rubio I, Puglia M, et al. Spin trapping combined with quantitative mass spectrometry defines free radical redistribution within the oxidized hemoglobin:haptoglobin complex. Free Radic Biol Med. 2015;85:259–268. doi: 10.1016/j.freeradbiomed.2015.04.023. [DOI] [PubMed] [Google Scholar]
- 64.Kristiansen M, Graversen JH, Jacobsen C, et al. Identification of the haemoglobin scavenger receptor. Nature. 2001;409(6817):198–201. doi: 10.1038/35051594. [DOI] [PubMed] [Google Scholar]
- 65.Vinchi F, De Franceschi L, Ghigo A, et al. Hemopexin therapy improves cardiovascular function by preventing heme-induced endothelial toxicity in mouse models of hemolytic diseases. Circulation. 2013;127(12):1317–1329. doi: 10.1161/CIRCULATIONAHA.112.130179. [DOI] [PubMed] [Google Scholar]
- 66.Hvidberg V, Maniecki MB, Jacobsen C, Højrup P, Møller HJ, Moestrup SK. Identification of the receptor scavenging hemopexin-heme complexes. Blood. 2005;106(7):2572–2579. doi: 10.1182/blood-2005-03-1185. [DOI] [PubMed] [Google Scholar]
- 67.Muller-Eberhard U, Javid J, Liem HH, Hanstein A, Hanna M. Plasma concentrations of hemopexin, haptoglobin and heme in patients with various hemolytic diseases. Blood. 1968;32(5):811–815. [PubMed] [Google Scholar]
- 68.Kato GJ, Steinberg MH, Gladwin MT. Intravascular hemolysis and the pathophysiology of sickle cell disease. J Clin Invest. 2017;127(3):750–760. doi: 10.1172/JCI89741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kato GJ, Piel FB, Reid CD, et al. Sickle cell disease. Nat Rev Dis Primers. 2018;4:18010. doi: 10.1038/nrdp.2018.10. [DOI] [PubMed] [Google Scholar]
- 70.Miller YI, Felikman Y, Shaklai N. The involvement of low-density lipoprotein in hemin transport potentiates peroxidative damage. Biochim Biophys Acta. 1995;1272(2):119–127. doi: 10.1016/0925-4439(95)00075-f. [DOI] [PubMed] [Google Scholar]
- 71.Miller YI, Altamentova SM, Shaklai N. Oxidation of low-density lipoprotein by hemoglobin stems from a heme-initiated globin radical: antioxidant role of haptoglobin. Biochemistry. 1997;36(40):12189–12198. doi: 10.1021/bi970258a. [DOI] [PubMed] [Google Scholar]
- 72.Miller YI, Shaklai N. Kinetics of hemin distribution in plasma reveals its role in lipoprotein oxidation. Biochim Biophys Acta. 1999;1454(2):153–164. doi: 10.1016/s0925-4439(99)00027-7. [DOI] [PubMed] [Google Scholar]
- 73.Figueiredo RT, Fernandez PL, Mourao-Sa DS, et al. Characterization of heme as activator of Toll-like receptor 4. J Biol Chem. 2007;282(28):20221–20229. doi: 10.1074/jbc.M610737200. [DOI] [PubMed] [Google Scholar]
- 74.Chang J, Patton JT, Sarkar A, Ernst B, Magnani JL, Frenette PS. GMI-1070, a novel pan-selectin antagonist, reverses acute vascular occlusions in sickle cell mice. Blood. 2010;116(10):1779–1786. doi: 10.1182/blood-2009-12-260513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Belcher JD, Chen C, Nguyen J, et al. Haptoglobin and hemopexin inhibit vaso-occlusion and inflammation in murine sickle cell disease: role of heme oxygenase-1 induction. PLoS One. 2018;13(4) doi: 10.1371/journal.pone.0196455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gentinetta T, Belcher JD, Brügger-Verdon V, et al. Plasma-derived hemopexin as a candidate therapeutic agent for acute vaso-occlusion in sickle cell disease: preclinical evidence. J Clin Med Res. 2022;11(3):630. doi: 10.3390/jcm11030630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hu J, Rezoagli E, Zadek F, Bittner EA, Lei C, Berra L. Free hemoglobin ratio as a novel biomarker of acute kidney injury after on-pump cardiac surgery: secondary analysis of a randomized controlled trial. Anesth Analg. 2021;132(6):1548–1558. doi: 10.1213/ANE.0000000000005381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Alshaikh HN, Katz NM, Gani F, et al. Financial impact of acute kidney injury after cardiac operations in the United States. Ann Thorac Surg. 2018;105(2):469–475. doi: 10.1016/j.athoracsur.2017.10.053. [DOI] [PubMed] [Google Scholar]
- 79.Mamikonian LS, Mamo LB, Smith PB, Koo J, Lodge AJ, Turi JL. Cardiopulmonary bypass is associated with hemolysis and acute kidney injury in neonates, infants, and children. Pediatr Crit Care Med. 2014;15(3):e111–e119. doi: 10.1097/PCC.0000000000000047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Graw JA, Hildebrandt P, Krannich A, et al. The role of cell-free hemoglobin and haptoglobin in acute kidney injury in critically ill adults with ARDS and therapy with VV ECMO. Crit Care. 2022;26(1):50. doi: 10.1186/s13054-022-03894-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Saylor DM, Buehler PW, Brown RP, Malinauskas RA. Predicting plasma free hemoglobin levels in patients due to medical device-related hemolysis. ASAIO J. 2019;65(3):207–218. doi: 10.1097/MAT.0000000000000801. [DOI] [PubMed] [Google Scholar]
- 82.Tanaka K, Kanamori Y, Sato T, et al. Administration of haptoglobin during cardiopulmonary bypass surgery. ASAIO Trans. 1991;37(3):M482–M483. [PubMed] [Google Scholar]
- 83.Gleason TG, Argenziano M, Bavaria JE, et al. Hemoadsorption to reduce plasma-free hemoglobin during cardiac surgery: results of REFRESH I Pilot Study. Semin Thorac Cardiovasc Surg. 2019;31(4):783–793. doi: 10.1053/j.semtcvs.2019.05.006. [DOI] [PubMed] [Google Scholar]
- 84.Wetz AJ, Richardt EM, Schotola H, Bauer M, Bräuer A. Haptoglobin and free haemoglobin during cardiac surgery-is there a link to acute kidney injury? Anaesth Intensive Care. 2017;45(1):58–66. doi: 10.1177/0310057X1704500109. [DOI] [PubMed] [Google Scholar]
- 85.Yoshida T, Prudent M, D’alessandro A. Red blood cell storage lesion: causes and potential clinical consequences. Blood Transfus. 2019;17(1):27–52. doi: 10.2450/2019.0217-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.D’Alessandro A, Culp-Hill R, Reisz JA, et al. Recipient Epidemiology and Donor Evaluation Study-III (REDS-III). Heterogeneity of blood processing and storage additives in different centers impacts stored red blood cell metabolism as much as storage time: lessons from REDS-III-Omics. Transfusion. 2019;59(1):89–100. doi: 10.1111/trf.14979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nemkov T, Stefanoni D, Bordbar A, et al. Blood donor exposome and impact of common drugs on red blood cell metabolism. JCI Insight. 2021;6(3) doi: 10.1172/jci.insight.146175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Risbano MG, Kanias T, Triulzi D, et al. Effects of aged stored autologous red blood cells on human endothelial function. Am J Respir Crit Care Med. 2015;192(10):1223–1233. doi: 10.1164/rccm.201501-0145OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hod EA, Zhang N, Sokol SA, et al. Transfusion of red blood cells after prolonged storage produces harmful effects that are mediated by iron and inflammation. Blood. 2010;115(21):4284–4292. doi: 10.1182/blood-2009-10-245001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.de Wolski K, Fu X, Dumont LJ, et al. Metabolic pathways that correlate with post-transfusion circulation of stored murine red blood cells. Haematologica. 2016;101(5):578–586. doi: 10.3324/haematol.2015.139139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang D, Cortés-Puch I, Sun J, et al. Transfusion of older stored blood worsens outcomes in canines depending on the presence and severity of pneumonia. Transfusion. 2014;54(7):1712–1724. doi: 10.1111/trf.12607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hod EA, Brittenham GM, Billote GB, et al. Transfusion of human volunteers with older, stored red blood cells produces extravascular hemolysis and circulating non-transferrin-bound iron. Blood. 2011;118(25):6675–6682. doi: 10.1182/blood-2011-08-371849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Buehler PW, Karnaukhova E. When might transferrin, hemopexin or haptoglobin administration be of benefit following the transfusion of red blood cells? Curr Opin Hematol. 2018;25(6):452–458. doi: 10.1097/MOH.0000000000000458. [DOI] [PubMed] [Google Scholar]
- 94.Baek JH, D’Agnillo F, Vallelian F, et al. Hemoglobin-driven pathophysiology is an in vivo consequence of the red blood cell storage lesion that can be attenuated in guinea pigs by haptoglobin therapy. J Clin Invest. 2012;122(4):1444–1458. doi: 10.1172/JCI59770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Graw JA, Mayeur C, Rosales I, et al. Haptoglobin or hemopexin therapy prevents acute adverse effects of resuscitation after prolonged storage of red cells. Circulation. 2016;134(13):945–960. doi: 10.1161/CIRCULATIONAHA.115.019955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wagener BM, Hu PJ, Oh J.-Y., et al. Role of heme in lung bacterial infection after trauma hemorrhage and stored red blood cell transfusion: a preclinical experimental study [published correction appears in PLoS Med. 2019;16(11):e1002991] PLoS Med. 2018;15(3) doi: 10.1371/journal.pmed.1002522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Akeret K, Buzzi RM, Schaer CA, et al. Cerebrospinal fluid hemoglobin drives subarachnoid hemorrhage-related secondary brain injury. J Cereb Blood Flow Metab. 2021;41(11):3000–3015. doi: 10.1177/0271678X211020629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Garland P, Morton MJ, Haskins W, et al. Haemoglobin causes neuronal damage in vivo which is preventable by haptoglobin. Brain Commun. 2020;2(1):fcz053. doi: 10.1093/braincomms/fcz053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jayle MF, Ishak S, Gillard P. Action of haptoglobin on the peroxidase catalysis of hemoglobin; new theory on the constitution of enzymes [in Italian] ull Soc Chim Biol (Paris) 1946;28:63–80. [PubMed] [Google Scholar]
- 100.Connell GE, Dixon GH, Smithies O. Subdivision of the three common haptoglobin types based on ‘hidden’ diffrences. Nature. 1962;193(4814):505–506. doi: 10.1038/193505a0. [DOI] [PubMed] [Google Scholar]
- 101.Smithies O, Connell GE, Dixon GH. Inheritance of haptoglobin subtypes. Am J Hum Genet. 1962;14:14–21. [PMC free article] [PubMed] [Google Scholar]
- 102.Bunn HF, Esham WT, Bull RW. The renal handling of hemoglobin. I. Glomerular filtration. J Exp Med. 1969;129(5):909–923. doi: 10.1084/jem.129.5.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bunn HF, Jandl JH. The renal handling of hemoglobin. II. Catabolism. J Exp Med. 1969;129(5):925–934. doi: 10.1084/jem.129.5.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Paoli M, Anderson BF, Baker HM, Morgan WT, Smith A, Baker EN. Crystal structure of hemopexin reveals a novel high-affinity heme site formed between two β-propeller domains. Nat Struct Biol. 1999;6(10):926–931. doi: 10.1038/13294. [DOI] [PubMed] [Google Scholar]
- 105.Schaer CA, Owczarek C, Deuel JW, et al. Phenotype-specific recombinant haptoglobin polymers co-expressed with C1r-like protein as optimized hemoglobin-binding therapeutics. BMC Biotechnol. 2018;18(1):15. doi: 10.1186/s12896-018-0424-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Karnaukhova E, Owczarek C, Schmidt P, Schaer DJ, Buehler PW. Human plasma and recombinant hemopexins: heme binding revisited. Int J Mol Sci. 2021;22(3):1199. doi: 10.3390/ijms22031199. [DOI] [PMC free article] [PubMed] [Google Scholar]