Abstract
Chronic wounds, such as pressure ulcers, are a common complication of impaired peripheral circulation, such as in advanced diabetes. Factors secreted by mesenchymal stromal cells (MSCs) have been shown to enhance wound healing in vitro and in-vivo. However, there is little understanding of the impact of the chronic wound environment, namely the limited supply of nutrients and oxygen, on the ability of wound cells to respond to MSCs. In this study, we first established the effects of hypoxia (1% O2) and low serum (1% serum) concentration on the proliferation and migration of HaCaT keratinocytes. We found that hypoxia and low serum significantly slowed down these processes. Next, we found that supplementation with human MSC-concentrated conditioned media (hMSC-CM) enhanced both cell migration and proliferation in the presence of hypoxia and low serum. Furthermore, low serum and hypoxia decreased cell spreading and F-actin expression, which was reversed in the presence of hMSC-CM. Several wound healing mediators were identified in hMSC-CM, including IL-5, IL-6, IL-8, IL-9, IP-10, MCP-1, FGF-2, and VEGF. This study suggests that the concentrated secretome of MSCs can reverse the inhibitory effect of hypoxia and low serum on keratinocyte proliferation and migration. This phenomenon may contribute to the beneficial effects of hMSC-CM on wound healing in vivo.
Keywords: hMSC-CM, scratch assay, hypoxia, cytokines and growth factors, proliferation and migration, secretome
1. Introduction
The highly regulated process of wound healing is critical for restoring the barrier function of skin [1,2]. Impaired healing is often a complication of aging, diabetes, hypertension, obesity, vascular insufficiency, and/or immobilization [3]. Common characteristics of chronic wounds include prolonged inflammation, persistent infections, impaired cell proliferation, and migration. These can be attributed, in part, to the poor nutrient and oxygen supply in the wound due to impaired blood flow [4]. Keratinocytes are responsible for restoring the epidermis after injury through re-epithelialization [3], the defining parameter of successful wound closure, which is characterized by keratinocyte proliferation, migration, and ultimately differentiation [5]. In most types of chronic wounds, these processes are severely impaired [6].
Mesenchymal stromal cell (MSC)-based therapy has been shown to enhance tissue repair and regeneration in different diseases and could be a breakthrough in wound healing. MSCs secrete factors that may promote faster wound healing by decreasing inflammation and promoting cellular processes [6,7]. Studies of in vitro scratch assays with fibroblasts and keratinocytes suggest that MSC-conditioned media accelerates wound closure by enhancing cell migration [8]. Utilizing the MSC secretome circumvents some of the limitations of direct cell transplantation such as low cell engraftment and survival after implantation, tumorigenicity, and immune rejection [9]. A caveat of the in vitro scratch assay and the in vivo diabetic mouse models is that they do not mimic the hypoxic conditions found in human chronic wounds.
Therefore, in the current study, we evaluated whether the concentrated secretome of human MSC can enhance keratinocyte functions such as migration and proliferation under hypoxia and restricted serum conditions, thereby expediting wound closure under conditions mimicking the chronic wound environment.
2. Materials and methods
2.1. Human MSCs
Human bone marrow-derived MSCs (hMSCs) were acquired from the Institute of Regenerative Medicine at Texas A&M. Briefly, hMSCs (P2-3) were thawed and plated as monolayer cultures (4,000 cells/cm2) in a humidified incubator (37°C, 5% CO2). α-MEM (Gibco) containing no nucleotides, supplemented with 10% (v/v) premium FBS (Atlanta Biologicals, GA), 1% (v/v) Pen/Strep (100 U/mL penicillin + 100 mg/mL streptomycin), 2 mM L-glutamine, and 1 ng/mL bFGF (Life Technologies), was used to culture the cells. Cells were passaged at ~70% confluency and only P4 hMSCs were used to prepare hMSC-concentrated conditioned media (hMSC-CM) for subsequent experiments.
2.2. Human MSC-CM preparation
Human MSCs (P4) were seeded in α-MEM at 1.33 × 103 cells/cm2 and incubated for 5 days. On day 5, the media was removed, cells were washed once with DMEM (Gibco) supplemented with 1% FBS, and 1% Pen/Strep (low serum media). Cells were then covered with fresh low serum media. hMSC-CM was collected from ~3.4 × 103 cells/cm2 after 48 h of incubation in low serum media and filtered through a 0.2 μm pore size filter (Puradisc 25 mm, GE Healthcare) to eliminate cell debris. Then, the hMSC-CM was concentrated by centrifugation (5000 × g for 1 h, 3 kDa cut-off filter-Amicon Ultra-15, Millipore Sigma) and used as 50% v/v in all the experiments.
2.3. Keratinocyte (HaCaT) culture
HaCaT cells (P37-39) were cultured and maintained using DMEM supplemented with 10% FBS and 1% (v/v) Pen/Strep.
2.4. Cell proliferation and viability assay
HaCaT cells were plated in 24-well plates (50,000 cells/well, 3-wells/condition, 3 independent experiments) and exposed to hypoxia (H group- 5% CO2, 1% O2) or normoxia (N group- 5% CO2, 21% O2) with or without serum restriction (1% FBS group and 10% FBS group, respectively). After 72 h, cells were counted using a hemocytometer. In a parallel experiment, HaCaT cells were cultured similarly in 96-well plates (5,000 cells/well). The next day, media was replaced by media containing different conditions under hypoxia or normoxia. After 72 h, the medium was then replaced with fresh media containing 10% AlamarBlue reagent (Life Technologies, CA) according to the manufacturer's instructions. The cells were then returned to their respective incubators for another 1 h. Fluorescence (Ex/Em-571/585 nm) of the wells was read in a microplate reader (DTX 880 Multimode Detector, Beckman Coulter, CA) as relative fluorescence units (RFU) and compared between the groups to quantify cellular metabolic activity.
2.5. Scratch wound assay
HaCaT cells were grown as described above. Cells were trypsinized, counted, and then 250,000 cells/well were plated in 24-well plates and allowed to reach ~100% confluency. After 48 h, a scratch was created by passing a 200 μL sterile pipette tip over the middle of each well, and wells were washed twice (PBS) to remove cellular debris. Different treatment conditions were added in low serum fresh medium and incubated for 72 h under normoxia or hypoxia. Each well was photographed at the beginning (day 0- initial scratch time) and then every 72 h using a 10X objective (Olympus CKX41 microscope) for 9 days. The percent wound closure was quantified as follows: % wound closure = [1-(wound area at t0/wound area at t1)] × 100, where t0 is the initial time point and t1 is the time point of observation. For each treatment condition, the experiment was repeated thrice.
2.6. Cell Staining for actin cytoskeleton visualization and quantification
Actin cytoskeleton changes were assessed via filamentous actin (F-actin) staining. Briefly, cells were seeded (5,000 cells/well) in chamber slide systems (Lab-Tek, Thermo Fisher Scientific) to allow for single-cell imaging. Post-treatment, cells were fixed with 4% (w/v) paraformaldehyde (MilliporeSigma) for 20 min (room temperature, RT) and then enough PBS was added to each well to achieve 1% (w/v) paraformaldehyde. The fixed cells were stored at 4°C until staining. Fixed cells were transitioned to RT, washed (3X) with PBS, permeabilized with 0.2% Triton X-100 for 5 min (MilliporeSigma), and blocked for nonspecific binding using PBS containing 1% FBS and 0.2% Triton X-100. Following blocking, the cells were stained for 20 min with phalloidin conjugated to Alexa Fluor 488 (Molecular Probes) and Hoechst 33342 (Molecular Probes) diluted 1:100 and 1:5000 in PBS, respectively. Cells were then washed twice with PBS and maintained in 200 μL PBS for imaging. Stained cells were visualized at 40X (Zeiss LSM 780, Germany) and further analyzed using Fiji software (ImageJ, NIH). The corrected total cell fluorescence (CTCF) was calculated utilizing the area integrated intensity and mean grey value functions from Fiji and the formula CTCF= Integrated Density-(Area of selected cell × mean fluorescence of background readings).
2.7. Cytokine measurements
To identify the factors present in hMSC-CM that could affect keratinocyte behavior, a bead-based multiplex analysis (Bio-Plex Pro Human Cytokine Group I, Bio-Rad Laboratories Inc.) was used. The multiplex assay of 27 cytokines, chemokines and growth factors was performed in a Bio-Plex 200 System using samples of the low serum fresh culture media and hMSC-CM following manufacturer’s instructions.
2.8. Statistical analysis
Data are represented as the mean±standard error of the mean (SEM). Statistical analysis was done using KaleidaGraph. A p-value <0.05 is considered statistically significant.
3. Results
3.1. Effect of hypoxia and low serum on wound healing processes
Human HaCaT proliferation was inhibited by hypoxia (1% O2 versus 21% O2) and low serum concentration (1% versus 10% FBS) over 72 h. The increase in viable cell number was lower in 1% FBS as compared to 10% FBS under normoxia (p < 0.01), and hypoxia (p < 0.0001) (Fig. 1A-a). Hypoxia inhibited cell proliferation in presence of 10% FBS (p < 0.05); a similar pattern although not statistically significant was observed with 1% FBS. Using an AlamarBlue assay to estimate cell number yielded similar results (Fig. 1A-b). The RFU were decreased when lowering from 10% to 1% FBS under normoxia (p < 0.001) and hypoxia (p < 0.001). Hypoxia showed a trend although not statistically significant towards lowered RFU when compared to normoxia with 10% FBS. The effect was significant (p < 0.01) with 1% FBS.
Fig. 1. Effect of hypoxia and low serum concentration on wound healing processes.
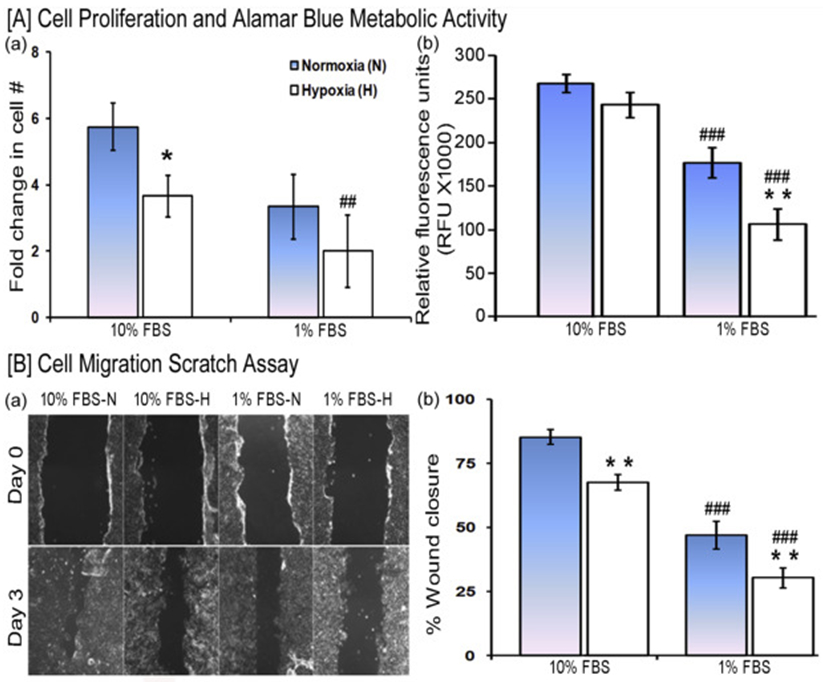
[A] HaCaT cell proliferation was evaluated after 3 days of culture in a medium supplemented with either 10% FBS or 1% FBS under normoxia and hypoxia. (a) Viable cell counts were relative to seeded cell number. (b) Metabolic activity estimated by the reduction of AlamarBlue reagent (n=9/each group). [B] Cell migration scratch assay. (a) Representative images of the scratch wound initially and after 3 days (10X). (b) Quantification of images comparing the fraction of the initial cell-free area in the scratch that has restored cell coverage. (n=6/each group). Statistical significance determined by ANOVA followed by post hoc Fisher's LSD test. * indicates a comparison between normoxia (N) and hypoxia (H) and # indicates a comparison between 10% FBS versus 1% FBS. */#P < 0.05, ##/**P < 0.01, and ###/***P < 0.001.
HaCaT cell migration, as determined in a scratch wound closure assay over 72 h (Fig. 1B-a,b), was inhibited by hypoxia (10% FBS-H versus 10% FBS-N; 1% FBS-H versus 1% FBS-N, p < 0.01), as well as low serum (1% FBS-N versus 10% FBS-N, p<0.001; 1% FBS-H versus 10% FBS-H, p < 0.001). Taken together, these results show that hypoxia (1% O2) and low serum (1% v/v) both decrease the proliferation and migration of HaCaT cells.
3.2. Effect of hMSC-CM on keratinocyte wound closure
We tested whether hMSC-CM could reverse low serum and/or hypoxia-mediated inhibition of wound closure over 9 days. During this period, cells migrated fastest in the normoxia hMSC-CM group, and thereby had the highest percent wound closure, followed by the normoxia control, hypoxia hMSC-CM, and hypoxia control group (p<0.001). The wound closure curve over 9 days showed that hypoxia slowed migration (Fig. 2AB, ANOVA p<0.001) compared to normoxia. The addition of hMSC-CM to the medium accelerated wound closure under both normoxia (p<0.05 on day 3 and p<0.0001 on days 6 and 9) and hypoxia (p<0.05 on day 9). There was a trend – although not statistically significant – of enhanced cell migration in normoxia with hMSC-CM compared to hypoxia with hMSC-CM. HaCaT cells exposed to hypoxia had lower cell attachment, as previously described in proliferation studies (Fig. 1A-a), and occupied a smaller area due to decreased spreading (high magnification insert images at day 9, Fig. 2A).
Fig. 2. Effect of hMSC-CM on cell migration and proliferation under hypoxia in presence of low serum.

Representative images of a scratch on confluent HaCaT cultures as a function of time (A). All cultures were in 1% FBS. N = normoxia. H = hypoxia. CM = hMSC-CM. Quantified wound images (n= 9/group) showing the fraction (%) of wound closure (B). Insets of magnified images represent the shapes of HaCaT cells. Statistical significance determined by ANOVA and post hoc Tukey test. * indicates the comparison between N and H, ^ indicates a comparison between N versus N+CM, and $ indicates a comparison between H and H+CM groups. HaCaT cell proliferation was evaluated after 3 days of culture (C). * compares between either 10% FBS or CM versus 1% FBS under both normoxia and hypoxia while & compares between 10% FBS versus CM under hypoxia only. */^/$/& P < 0.05 and ***/^^^ P <0.001.
3.3. Effect of hMSC-CM on keratinocyte proliferation
Low serum and hypoxia each inhibited cell proliferation (p<0.0001) (Fig. 2C). Addition of 50% v/v hMSC-CM to 1% FBS supplemented cultures restored cell proliferation to the level seen in the corresponding DMEM+10% FBS condition under both conditions (p<0.001).
3.4. Effect of hypoxia and hMSC-CM on HaCaT actin cytoskeleton
HaCaT cells appeared to adopt a more rounded morphology under hypoxia compared to normoxia in prior migration studies as shown in Fig. 2A (inset). This correlated with a more punctuated F-actin appearance in hypoxia cultures while normoxia cultures exhibited smooth filamentous actin (Fig. 3A-D). Furthermore, the F-actin appearance of hypoxic cultures exposed to hMSC-CM was similar to normoxia cultures. Quantification of these images and that of additional replicates confirmed that hypoxia decreased F-actin staining density (p<0.001). hMSC-CM treatment increased F-actin density in both normoxia (p<0.001) and hypoxia (p<0.05). Overall, under hypoxia, we found lower cell spreading in general, as cells occupied a smaller area showing a rounded morphology while presenting fragmented or punctuated F-actin filaments.
Fig. 3. Effect of hMSC-CM on HaCaT architecture and morphology.
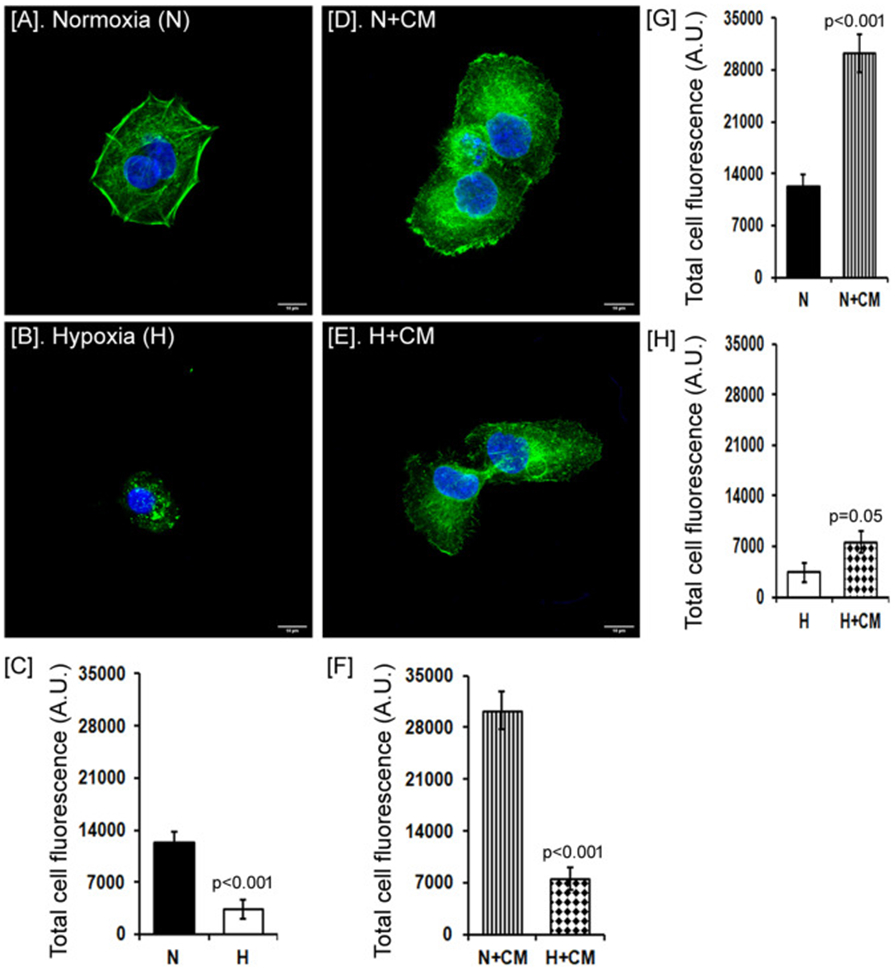
Representative images of HaCaT cells stained for F-actin (green) with nuclear counterstain (blue) under normoxia or hypoxia, and in presence of hMSC-CM (CM, A, B, D, E). Scale bar = 10 μm. Quantification of F-actin fluorescence/field of view compared between different groups as shown (C, F, G, H). The difference between the groups was analyzed by the Student t-test.
3.5. Human hMSC-CM secretome
We identified potential soluble factors released in hMSC-CM that could enhance HaCaT proliferation and migration under hypoxia. Detectable levels of interleukin (IL-) 6, IL-7, IL-8, IL-9, monocyte chemotactic protein-1 (MCP-1), interferon gamma-induced protein (IP-)10, vascular endothelial growth factor (VEGF), and fibroblast growth factor 2 (FGF-2) were found in the hMSC-CM (Fig. 4AB). The level of these factors was 5-10-fold higher in hMSC-CM (concentrated) compared to hMSC-conditioned media (non-concentrated).
Fig. 4. Multiplex analysis of secreted factrs in hMSC-CM.

Detectable levels of different factors (IL-5, IL-6, IL-8, IL-9, IP-10, MCP-1, VEGF, FGF-2, RANTES, etc.) were quantified in hMSC-CM and hMSC-conditioned media (1% FBS, A and B).
4. Discussion
A common denominator to chronic skin wounds is locally impaired vascular perfusion causing nutrient depletion and hypoxia, both of which impair cell proliferation and migration [10-11]. MSC-based therapies have the potential to accelerate wound closure via mechanisms mediated by their secretome [12]. While keratinocytes respond to MSC-derived factors under ideal cell culture conditions, we asked whether or not MSCs could do the same in an environment that better simulates chronic wounds, namely under serum restriction and low oxygen tension. Thus, we examined the effects of the MSC secretome, prepared as concentrated hMSC-CM, on the viability, proliferation, migration and cytoskeletal arrangement of keratinocytes under hypoxia and low serum conditions. We found that hypoxia and low serum significantly inhibited cell proliferation and migration, and also caused cell rounding and disruption of the cytoskeleton. These effects were largely reversed by the addition of hMSC-CM to the cell culture media.
Acute hypoxia after an injury is known to stimulate angiogenesis, via the secretion of cytokines and other promoters of wound healing, leading to cell proliferation and migration [11,13-15]. During the re-epithelialization stage, early hypoxia in the wound margin serves as a stimulus for the initiation of keratinocyte motility and migration by activating hypoxia-inducible pathways (e.g. HIF/ARNT [16] and mTORC1/AMPK [14]). However, we found that HaCaT cell proliferation, metabolic activity, and wound closure dynamics were negatively affected under hypoxia and/or decreased serum supplementation (Fig. 1). This highlights that proper nutrition and oxygenation are important for normal wound healing. In the clinical setting, hyperbaric oxygen therapy has been used to overcome the effects of tissue hypoxia [17]; however, the availability of this treatment is limited and evidence of its efficacy is inconsistent [15,17].
Low serum cultures were performed to better mimic the low nutrient supply in chronic wounds; furthermore, FBS is rich in chemokines and growth factors, which can confound the effect of MSCs on cell proliferation and migration. Studies have shown that stem cell therapies can improve the wound healing process by decreasing inflammation and increasing angiogenesis, extracellular matrix remodeling, re-epithelialization and wound closure [9]. Addition of hMSC-CM treatment in a scratch wound assay significantly expedited wound closure in normoxia and hypoxia low serum conditions, reaching ~90% and ~50% wound closure after 9 days for the normoxia and hypoxia groups, respectively (Fig. 2AB). This presents a stark contrast with the corresponding hypoxic groups which only reached ~20% wound closure after 9 days. In addition, hMSC-CM treatment enhanced keratinocyte proliferation in low serum normoxia and hypoxia groups bringing it to comparable levels to keratinocytes cultured in fully supplemented media (10% FBS, Fig. 2C). While a prior study showed that media conditioned with adipose-derived MSCs increased keratinocyte migration and proliferation [12], this study was conducted in normoxia while the data herein show that hMSC-CM can restore these cellular functions under prolonged hypoxia and serum depletion.
Hypoxia not only alters cellular gene expression and metabolic activity, but it also prompts cytoskeleton alterations [18]. Vogler et al. observed changes in architecture, adhesion, and migration in hypoxic fibroblasts characterized by cell spreading (higher area and volume), a higher number of focal adhesions, loss of cell polarization, increased actin filament rearrangement, and decrease in cell migration and wound closure [19]. Faulknor et al. observed that MSC-secreted factors promoted fibroblast-mediated contraction of collagen gels, which was also impaired under hypoxia, due to decreased α-smooth muscle actin expression [7]. In the current studies, keratinocytes exposed to hypoxia had lower cell spreading, exhibited a rounded morphology, and presented fragmented or punctuated F-actin filaments. However, treatment with hMSC-CM prompted F-actin rearrangement to yield a higher density of filaments under both normoxia and hypoxia.
Several studies have highlighted the role of multiple hMSC-secreted factors in promoting faster wound healing [8,12,20-22]. We found that 19 factors were detected in hMSC-CM at high (Fig. 4A) or moderate-low (Fig. 4B) concentrations. Many of them have pleiotropic effects, for example, FGF-2 has antifibrotic, antiapoptotic, and angiogenic functions [22] which has been shown to accelerate wound healing and skin regeneration in diabetic ulcers [12,23]. During the inflammatory phase, IL-5 [24], IL-8 [25], MCP-1, IP-10 [26], and RANTES [27] promote inflammatory and immune cell migration into the wound site to aid in removing damaged cells and pathogens. These molecules eventually assist in the transition to other stages of wound healing in conjunction with cytokines like IL-4 [28]. IL-4 is considered an anti-inflammatory cytokine due to its role in promoting the transition from inflammatory macrophages to wound healing macrophages [29] and supporting collagen synthesis [30]. Keratinocyte proliferation and migration is known to be stimulated by IL-6 [31], IL-8 [32], IL-7 [33], IP-10 [30], MCP-1 [32], eotaxin [34], and FGF-2 [35,31]. Deficient production of these factors significantly delays wound healing as evidenced by in vivo studies studying the role of IL-6 [36] and MCP-1 [37]. hMSC-CM was also rich in VEGF, consistent with other reports [38], and in vitro and in vivo studies have shown that disabling the VEGF receptor on keratinocytes decreases keratinocyte proliferation and delays re-epithelialization [39]. Our in vitro results consistently showed that all these prominent factors can promote migration (Fig. 2AB) and proliferation (Fig. 2C) of HaCaT cells.
We cannot exclude that other MSC-secreted factors with wound healing properties were not identified here due to limitations in the analyzed secretory panel. Among those are transforming growth factor (TGF-) β, epidermal growth factor (EGF), hepatocyte growth factor (HGF), collagens, fibronectin, insulin-like growth factor binding protein 7, and secreted protein acidic and rich in cysteine (SPARC) [8,12,40-41]. TGFβ1 itself has many roles in wound healing such as wound contracture, re-epithelialization, scar formation [41], and increased keratinocyte migration [40]. Our current study suggests that the hMSC-CM contains several factors previously reported to enhance wound healing of both acute and chronic wounds. Data reported herein show that the beneficial effects of hMSC-CM persist even in conditions of hypoxia and restricted serum.
Supplementary Material
Highlights:
Keratinocyte viability, proliferation, and migration are decreased by hypoxia and low serum.
Keratinocyte cytoskeleton architecture (F-actin) becomes disorganized under hypoxia and low serum.
Human MSC-CM improves keratinocyte morphology and wound healing response under hypoxia and low serum.
The human MSC secretome contains several wound healing factors.
Acknowledgments
This work was partially supported by the New Jersey Commission on Spinal Cord Injury Research (CSCR15IRG010). Wilai Kosol was supported by the Louis Stokes Alliances for Minority Participation, the ARESTY Research Center at Rutgers University, and a Norman and Ruth Feller Rosenberg Fellowship. Ileana Marrero-Berrios was supported by training grants from NIGMS (T32GM008339) and the U. S. Department of Education GAANN (P200A150131). We thank Paulina Krzyszczyk and Rick Cohen from the Department of Biomedical Engineering at Rutgers University for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Han G, Ceilley R, Chronic wound healing: A review of current management and treatments, Adv. Ther, 34 (2017) 599–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Velnar T, Bailey T, Smrkolj V, The wound healing process: An overview of the cellular and molecular mechanisms, J. Int. Med. Res, 37 (2009) 1528–1542. [DOI] [PubMed] [Google Scholar]
- 3.Anderson K, Hamm RL, Factors that impair wound healing, J. Am. Coll. Clin. Wound Spec, 4 (2014) 84–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frykberg RG, Banks J, Challenges in the treatment of chronic wounds, Adv. Wound Care (New Rochelle), 4 (2015) 560–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eckert RL, Structure, function, and differentiation of the keratinocyte. Physiol Rev., 69 (1989) 1316–1346. [DOI] [PubMed] [Google Scholar]
- 6.Pastar I, Stojadinovic O, Yin NC, Ramirez H, Nusbaum AG, Sawaya A, B Patel S, Khalid L, Isseroff ER, Tomic-Canic M, Epithelialization in wound healing: A comprehensive review, Adv. Wound Care (New Rochelle), 3 (2014) 445–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Faulknor RA, Olekson MA, Nativ NI, Ghodbane M, Gray AJ, Berthiaume F, Mesenchymal stromal cells reverse hypoxia-mediated suppression of alpha-smooth muscle actin expression in human dermal fibroblasts, Biochem. Biophys. Res. Commun, 458 (2015) 8–13. [DOI] [PubMed] [Google Scholar]
- 8.Walter MN, Wright KT, Fuller HR, Macneil S, Johnson WE, Mesenchymal stem cell-conditioned medium accelerates skin wound healing: An in vitro study of fibroblast and keratinocyte scratch assays, Exp. Cell Res, 316 (2010) 1271–1281. [DOI] [PubMed] [Google Scholar]
- 9.Kucharzewski M, Rojczyk E, Wilemska-Kucharzewska K, Wilk R, Hudecki J, Los MJ, Novel trends in application of stem cells in skin wound healing, Eur. J. Pharmacol, 843 (2019) 307–315. [DOI] [PubMed] [Google Scholar]
- 10.Guo S, Dipietro LA, Factors affecting wound healing. J. Dent. Res, 89 (2010) 219–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Semenza GL, Vascular responses to hypoxia and ischemia, Arterioscler. Thromb. Vasc. Biol, 30 (2010) 648–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park SR, Kim JW, Jun HS, Roh JY, Lee HY, Hong IS, Stem cell secretome and its effect on cellular mechanisms relevant to wound healing, Mol. Ther, 26 (2018) 606–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sano H, Ichioka S, Sekiya N, Influence of oxygen on wound healing dynamics: Assessment in a novel wound mouse model under a variable oxygen environment, PLOS ONE, 7 (2012) e50212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yan T, Zhang J, Tang D, Zhang X, Jiang X, Zhao L, Zhang Q, Zhang D, Huang Y, Hypoxia regulates mtorc1-mediated keratinocyte motility and migration via the ampk pathway, PLoS One, 12 (2017) e0169155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bishop A, Role of oxygen in wound healing, J, Wound Care, 17 (2008) 399–402. [DOI] [PubMed] [Google Scholar]
- 16.Weir L, Robertson D, Leigh IM, Vass JK, Panteleyev AA, Hypoxia-mediated control of hif/arnt machinery in epidermal keratinocytes, Biochim. Biophys. Acta, 1 (2011) 60–72. [DOI] [PubMed] [Google Scholar]
- 17.Rodriguez PG, Felix FN, Woodley DT, Shim EK, The role of oxygen in wound healing: A review of the literature, Dermatol. Surg, 34 (2008) 1159–1169. [DOI] [PubMed] [Google Scholar]
- 18.Zieseniss A, Hypoxia and the modulation of the actin cytoskeleton - emerging interrelations, Hypoxia, 2 (2014) 11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vogler M, Vogel S, Krull S, Farhat K, Leisering P, Lutz S, Wuertz CM, Katschinski DM, Zieseniss A, Hypoxia modulates fibroblastic architecture, adhesion and migration: A role for hif-1 α in cofilin regulation and cytoplasmic actin distribution, PLoS One, 8 (2013) e69128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Otero-Viñas M, Falanga V, Mesenchymal stem cells in chronic wounds: The spectrum from basic to advanced therapy, Adv. wound care, 5 (2016) 149–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Mayo T, Conget P, Becerra-Bayona S, Sossa CL, Galvis V, Arango-Rodriguez ML, The role of bone marrow mesenchymal stromal cell derivatives in skin wound healing in diabetic mice, PLoS One, 12 (2017) e0177533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Collawn SS, Patel S, Adipose-derived stem cells, their secretome, and wound healing, J. Cell Sci. Ther, 5 (2014) 165. [Google Scholar]
- 23.Chen S, Shi J, Zhang M, Chen Y, Wang X, Zhang L, Tian Z, Yan Y, Li Q, Zhong W, Xing M, Mesenchymal stem cell-laden anti-inflammatory hydrogel enhances diabetic wound healing, Sci. Rep, 5 (2015) 18104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wen T, Rothenberg ME, The regulatory function of eosinophils, Microbiol. Spectr, 4 (2016) MCHD-0020-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rennekampff HO, Hansbrough JF, Kiessig V, Dore C, Sticherling M, Schroder JM, Bioactive interleukin-8 is expressed in wounds and enhances wound healing, J. Surg. Res, 93 (2000) 41–54. [DOI] [PubMed] [Google Scholar]
- 26.Engelhardt E, Toksoy A, Goebeler M, Debus S, Bröcker EB, Gillitzer R, Chemokines IL-8, GROalpha, MCP-1, IP-10, and Mig are sequentially and differentially expressed during phase-specific infiltration of leukocyte subsets in human wound healing, Am. J. Pathol, 153 (1998) 1849–1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ridiandries A, Tan JTM, Bursill CA, The role of chemokines in wound healing, Int. J. Mol. Sci, 19 (2018) 3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Salmon-Ehr V, Ramont L, Godeau G, Birembaut P, Guenounou M, Bernard P, Maquart FX, Implication of interleukin-4 in wound healing, Lab. Invest, 80 (2000) 1337–1343. [DOI] [PubMed] [Google Scholar]
- 29.Woodward EA, Prêle CM, Nicholson SE, Kolesnik TB, Hart PH, The anti-inflammatory effects of interleukin-4 are not mediated by suppressor of cytokine signalling-1 (socs1), Immunology, 131 (2010) 118–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Behm B, Babilas P, Landthaler M, Schreml S, Cytokines, chemokines and growth factors in wound healing, J. Eur. Acad. Dermatol. Venereol, 26 (2012) 812–820. [DOI] [PubMed] [Google Scholar]
- 31.Hebda PA, Klingbeil CK, Abraham JA, Fiddes JC, Basic fibroblast growth factor stimulation of epidermal wound healing in pigs, J. Invest. Dermat, 95 (1990) 626–631. [DOI] [PubMed] [Google Scholar]
- 32.Raja K Sivamani, Garcia MS, Isseroff RR, Wound re-epithelialization: Modulating keratinocyte migration in wound healing, Front. Biosci, 12 (2007) 2849–2868. [DOI] [PubMed] [Google Scholar]
- 33.Bartlett A, Sanders AJ, Ruge F, Harding KG, Jiang WG, Potential implications of interleukin-7 in chronic wound healing, Exp. Ther. Med, 12 (2016) 33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tüzün Y, Antonov M, Dolar N, Wolf R, Keratinocyte cytokine and chemokine receptors. Dermatologic Clinics, 25 (2007) 467–476. [DOI] [PubMed] [Google Scholar]
- 35.Sogabe Y, Abe M, Yokoyama Y, Ishikawa O, Basic fibroblast growth factor stimulates human keratinocyte motility by rac activation, Wound Repair Regen., 14 (2006) 457–462. [DOI] [PubMed] [Google Scholar]
- 36.Lin ZQ, Kondo T, Ishida Y, Takayasu T, Mukaida N, Essential involvement of il-6 in the skin wound-healing process as evidenced by delayed wound healing in il-6-deficient mice, J. Leukoc. Biol, 73 (2003) 713–721. [DOI] [PubMed] [Google Scholar]
- 37.Low QE, Drugea IA, Duffner LA, Quinn DG, Cook DN, Rollins BJ, Kovacs EJ, Dipietro LA, Wound healing in mip-1alpha(−/−) and mcp-1(−/−) mice. Am. J. Pathol, 159 (2001) 457–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bao P, Kodra A, Tomic-Canic M, Golinko MS, Ehrlich HP, Brem H, The role of vascular endothelial growth factor in wound healing, J. Surg. Res 153 (2009) 347–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wilgus TA, Matthies AM, Radek KA, Dovi JV, Burns AL, Shankar R, Dipietro LA, Novel function for vascular endothelial growth factor receptor-1 on epidermal keratinocytes, Am. J. Pathol 167 (2005) 1257–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang XJ, Han G, Owens P, Siddiqui Y, Li AG, Role of tgfβ-mediated inflammation in cutaneous wound healing. J. Investig. Dermatol. Symp. Proc 11 (2006) 112–117. [DOI] [PubMed] [Google Scholar]
- 41.Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic-Canic M, Growth factors and cytokines in wound healing, Wound Repair Regen., 16 (2008) 585–601. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


