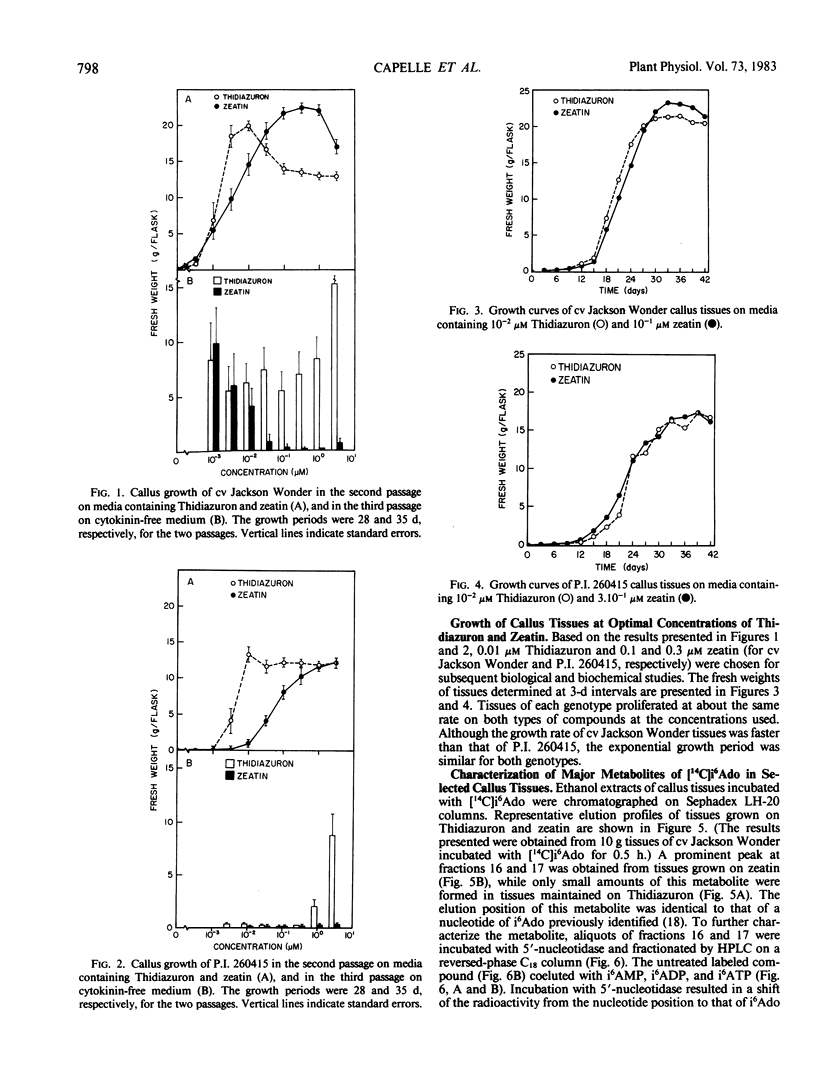
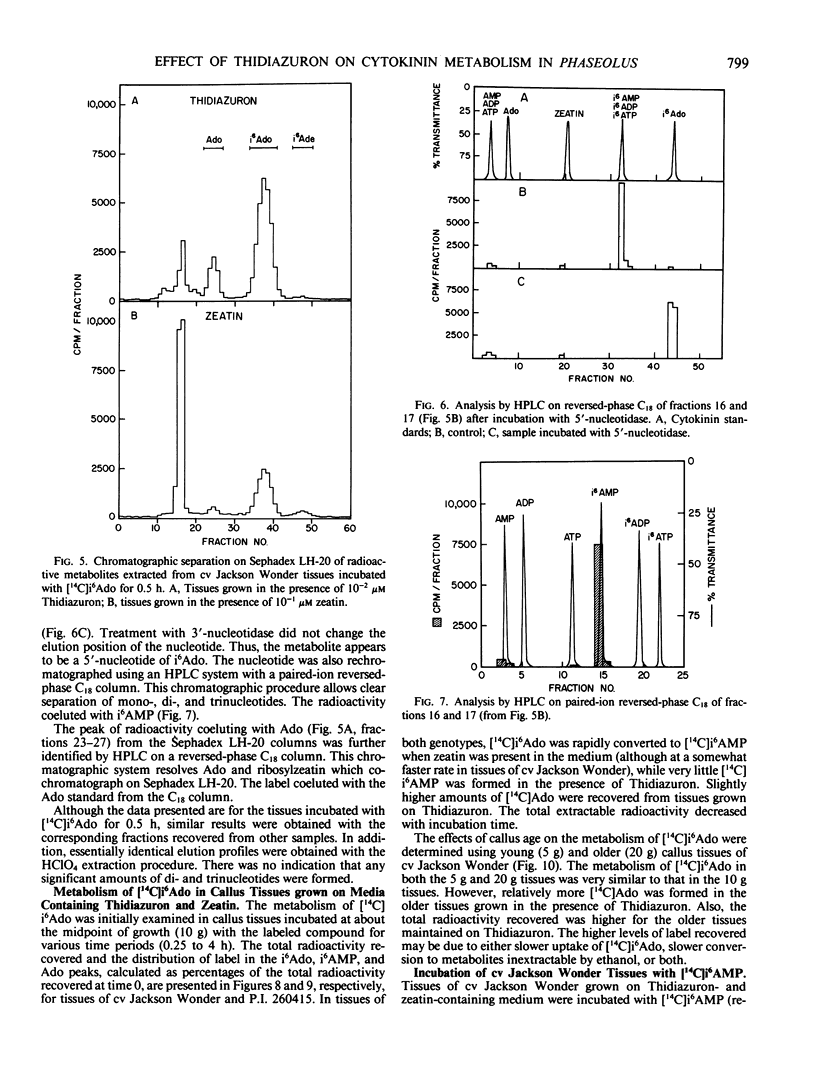
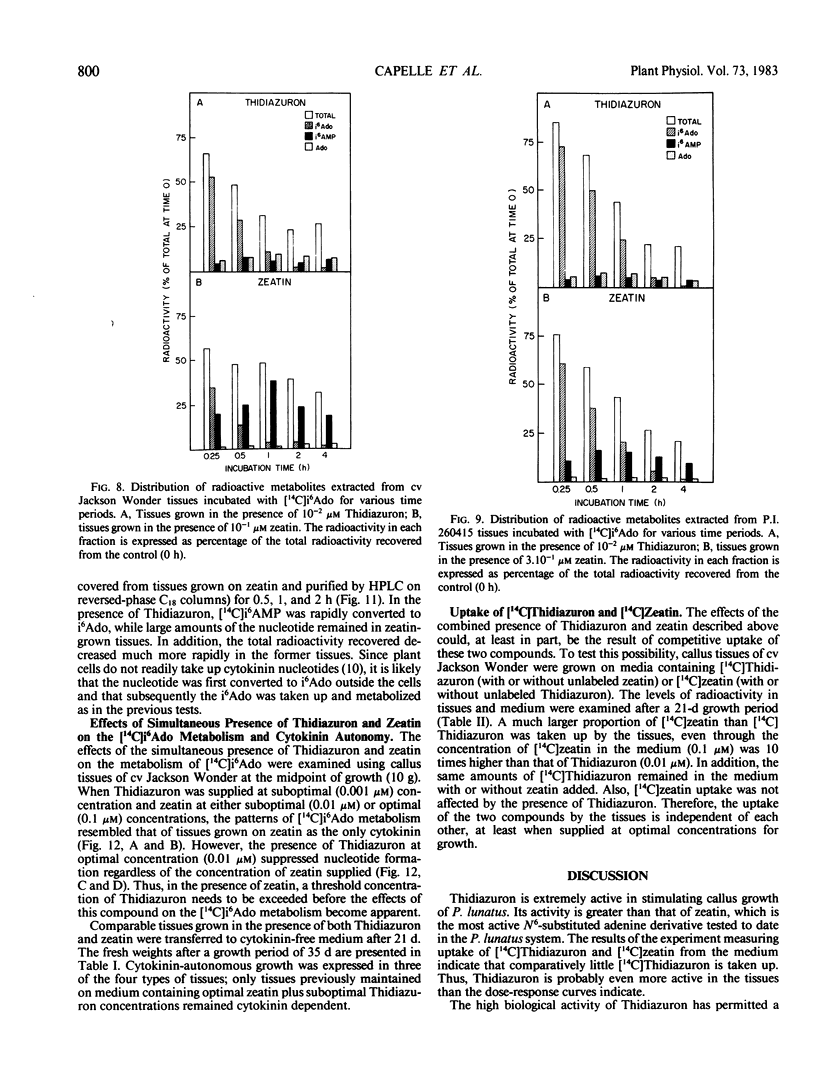
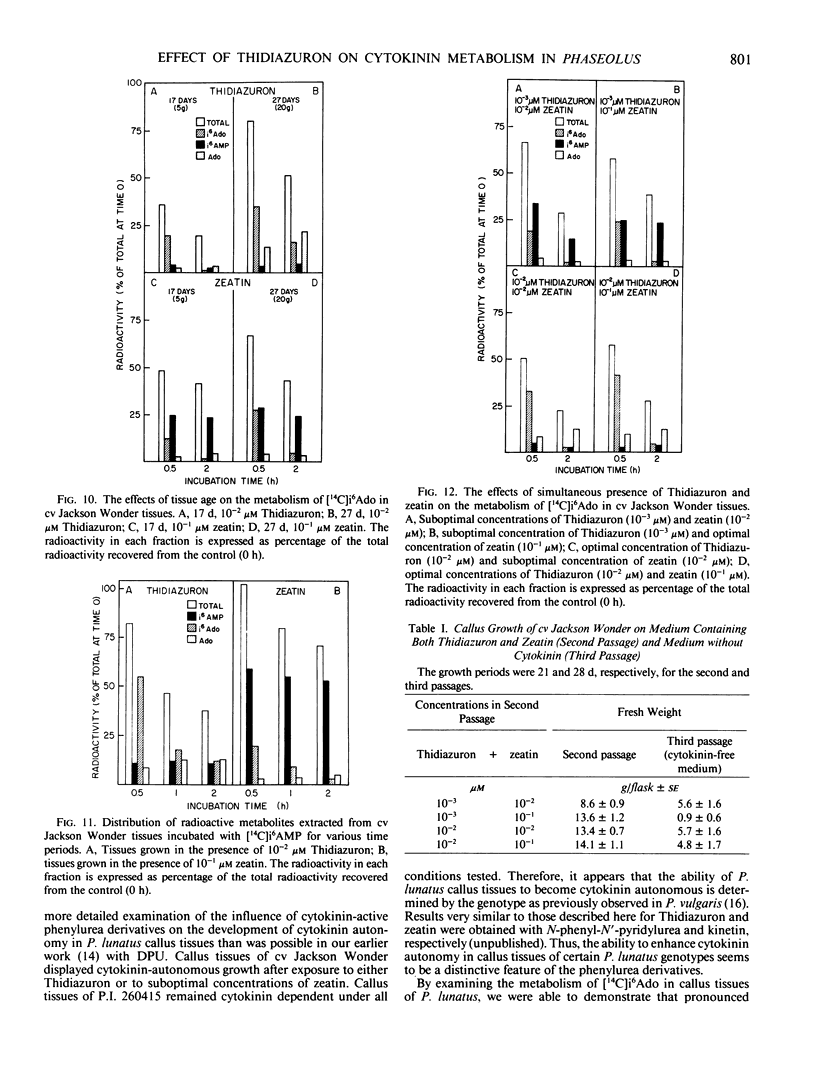
Abstract
The effects of a highly cytokinin-active urea derivative, N-phenyl-N′-1,2,3-thiadiazol-5-ylurea (Thidiazuron), and zeatin on cytokinin-autonomous growth and the metabolism of N6-(Δ2-isopentenyl)[8-14C]adenosine ([14C]i6 Ado) were examined in callus tissues of two Phaseolus lunatus genotypes, cv Jackson Wonder and P.I. 260415. Tissues of cv Jackson Wonder maintained on any concentration of Thidiazuron became cytokinin autonomous, whereas only tissues exposed to suboptimal concentrations of zeatin displayed cytokinin-autonomous growth. Tissues of P.I. 260415 remained cytokinin dependent under all these conditions. The metabolism of [14C]i6 Ado was similar for the two genotypes, but differed with the medium used. [14C]i6 Ado was rapidly converted to N6-(Δ2-isopentenyl)[8-14C]adenosine 5′-P ([14C]i6 AMP) by tissues grown on zeatin-containing medium, whereas only traces of the nucleotide were formed in tissues grown on medium with Thidiazuron. Incubation with [14C] i6 AMP of tissues grown in the presence of Thidiazuron resulted in rapid conversion to [14C]i6 Ado, while [14C]i6 AMP persisted in tissues maintained on zeatin. Thus, Thidiazuron appears to stimulate enzyme activity converting the ribonucleotide to ribonucleoside. Although the cytokininactive phenylureas and adenine derivatives differ in their effects on cytokinin autonomy as well as nucleotide formation, the two types of effects do not seem to be related.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bruce M. I., Zwar J. A. Cytokinin activity of some substituted ureas and thioureas. Proc R Soc Lond B Biol Sci. 1966 Aug 16;165(999):245–265. doi: 10.1098/rspb.1966.0067. [DOI] [PubMed] [Google Scholar]
- Burrows W. J., Leworthy D. P. Metabolism of N,N'-diphenylurea by cytokinin-dependent tobacco callus: identification of the glucoside. Biochem Biophys Res Commun. 1976 Jun 21;70(4):1109–1114. doi: 10.1016/0006-291x(76)91017-2. [DOI] [PubMed] [Google Scholar]
- Juengling E., Kammermeier H. Rapid assay of adenine nucleotides or creatine compounds in extracts of cardiac tissue by paired-ion reverse-phase high-performance liquid chromatography. Anal Biochem. 1980 Mar 1;102(2):358–361. doi: 10.1016/0003-2697(80)90167-0. [DOI] [PubMed] [Google Scholar]
- Laloue M., Terrine C., Gawer M. Cytokinins: formation of the nucleoside-5'-triphosphate in tobacco and Acer cells. FEBS Lett. 1974 Sep 15;46(1):45–50. doi: 10.1016/0014-5793(74)80331-5. [DOI] [PubMed] [Google Scholar]
- Mok M. C., Kim S. G., Armstrong D. J., Mok D. W. Induction of cytokinin autonomy by N,N'-diphenylurea in tissue cultures of Phaseolus lunatus L. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3880–3884. doi: 10.1073/pnas.76.8.3880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mok M. C., Mok D. W., Armstrong D. J., Rabakoarihanta A., Kim S. G. Cytokinin autonomy in tissue cultures of phaseolus: a genotype-specific and heritable trait. Genetics. 1980 Mar;94(3):675–686. doi: 10.1093/genetics/94.3.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mok M. C., Mok D. W., Dixon S. C., Armstrong D. J., Shaw G. Cytokinin structure-activity relationships and the metabolism of N-(delta-isopentenyl)adenosine-8-C in phaseolus callus tissues. Plant Physiol. 1982 Jul;70(1):173–178. doi: 10.1104/pp.70.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz R. Y., Skoog F. The use of dimethylsulfoxide as a solvent in the tobacco bioassay for cytokinins. Plant Physiol. 1970 Apr;45(4):537–538. doi: 10.1104/pp.45.4.537. [DOI] [PMC free article] [PubMed] [Google Scholar]


