Abstract
Polyethylene glycol (PEG)-asparaginase (pegaspargase) is a key agent in chemotherapy for acute lymphoblastic leukemia (ALL), but recipients frequently experience allergic reactions. We hypothesized that by decreasing antibody-producing CD20+ B cells, rituximab may reduce these reactions. Children and adolescents (aged 1–18 years) with newly diagnosed B-ALL treated on the St. Jude Total XVII study were randomized to induction therapy with or without rituximab on day 3 (cohort 1) or on days 6 and 24 (cohort 2). Patient clinical demographics, CD20 expression, minimal residual disease (MRD), rituximab reactions, pegaspargase allergy, anti-pegaspargase antibodies, and pancreatitis were evaluated. Thirty-five patients received rituximab and 37 did not. Among the 35 recipients, 16 (45.7%) experienced a grade 2 or higher reaction to rituximab. There were no differences between recipients and non-recipients in the incidence of pegaspargase reactions (P > 0.999), anti-pegaspargase antibodies (P = 0.327), or pancreatitis (P = 0.480). CD20 expression on day 8 was significantly lower in rituximab recipients (P < 0.001), but there were no differences in MRD levels on day 8, 15, or at the end of induction. Rituximab administration during induction in pediatric patients with B-ALL was associated with a high incidence of infusion reactions with no significant decrease in pegaspargase allergies, anti-pegaspargase antibodies, or MRD.
INTRODUCTION
Approximately 6000 cases of acute lymphoblastic leukemia (ALL) are diagnosed each year in the United States, with more than half of ALL cases presenting in patients younger than 20 years (1, 2). Asparaginase is an essential component of chemotherapy, with several FDA-approved formulations, including native L-asparaginase, pegaspargase, calaspargase pegol-mknl, and Erwinia asparaginase. Native L-asparaginase is derived from Escherichia coli, and PEG-asparaginase (pegaspargase) is the monomethoxy-polyethylene glycol succinimidyl succinate conjugate of L-asparaginase (2). Pegaspargase replaced native L-asparaginase as the first-line therapy in the United States because of its longer half-life (2.4–11.8 days vs. 17.3–19.0 hours) (3, 4) and because it caused fewer allergic reactions (2, 5, 6). In the St. Jude Total XVI study, approximately 13.5% of the patients experienced grade 2–4 allergic reactions to pegaspargase, whereas 41.2% experienced reactions to L-asparaginase in the Total XV study (5). Calaspargase pegol-mknl, which is currently approved for patients aged 21 years or younger in the United States, is also an E. coli–derived asparaginase, but it is conjugated to monomethoxy-polyethylene glycol with a succinimidyl carbonate linker, extending the half-life to approximately 16 days. Like pegaspargase, calaspargase can elicit allergic reactions (7, 8). Because anti-pegaspargase antibodies predict allergic reactions to pegaspargase (5), strategies to decrease anti-pegaspargase antibodies may reduce the incidence of allergic reactions.
Rituximab is a chimeric anti-CD20 monoclonal antibody that is used to treat B-cell malignancies and autoimmune diseases (9, 10). Adding rituximab to an adult ALL regimen during all treatment phases significantly extended event-free survival in younger adults with CD20-positive ALL and reduced allergic reactions to native L-asparaginase, possibly by targeting CD20-positive B cells and reducing anti-drug antibody production (11). We hypothesized that rituximab could also reduce anti-pegaspargase antibodies and allergic reactions to pegaspargase in children. We further hypothesized that rituximab could contribute to an improved response to remission induction therapy in children. Therefore, pediatric patients with newly diagnosed B-ALL who were treated on the St. Jude Total XVII study were randomized to receive or not to receive rituximab during induction therapy.
PATIENTS AND METHODS
Treatment
Children and adolescents (aged 1–18 years) with newly diagnosed ALL were enrolled on the Total XVII study (ClinicalTrials.gov identifier: NCT03117751). During remission induction, patients received prednisone, vincristine, daunorubicin, pegaspargase (2500 units/m2), and triple intrathecal treatment (i.e., methotrexate, hydrocortisone, and cytarabine), followed by cyclophosphamide, cytarabine, and mercaptopurine. After induction, standard-risk (SR) and high-risk (HR) patients received an additional course of vincristine, pegaspargase, cyclophosphamide, cytarabine, and mercaptopurine as early intensification therapy, whereas low-risk (LR) patients proceeded directly to consolidation.
Consolidation therapy consisted of four doses of high-dose methotrexate (HDMTX), with 2.5 g/m2 for LR and a target steady-state drug concentrations of 65 μM for SR/HR patients, administered concurrently with triple intrathecal therapy and daily mercaptopurine (12, 13). SR/HR patients received pegaspargase (1000 units/m2) after clearance of HDMTX with each course.
During continuation therapy, LR patients primarily received weekly methotrexate and daily mercaptopurine with pulses of dexamethasone and vincristine. Pegaspargase (2500 units/m2) was given during reinduction therapy in weeks 7, 9, 17, and 19. SR/HR patients received pegaspargase (2500 units/m2) every 2 weeks beginning week 1 of continuation and daily mercaptopurine with pulses of doxorubicin, vincristine, and dexamethasone for the first 20 weeks. Thereafter, they received rotating drug pairs: methotrexate plus mercaptopurine, cyclophosphamide plus cytarabine, and dexamethasone plus vincristine. The study was approved by the institutional review board of St. Jude Children’s Research Hospital. Written informed consent was obtained from all patients or from their legal guardians with assent and/or consent from the patients when appropriate.
Rituximab and pegaspargase administration
Patients with B-ALL treated between March 2017 and March 2019 were eligible to be randomized 1:1 to or not to receive rituximab (375 mg/m2) before the first dose of pegaspargase in an unblinded fashion. Randomization was stratified according to diagnostic white blood cell levels (< or ≥50 × 109/L) and CD20 expression on ALL cells (positive or negative). Rituximab was administered intravenously at 0.5 mg/kg/h for the first hour, and the rate was increased by 0.5 mg/kg/h every 30 min to a maximum of 4 mg/kg/h as tolerated. The planned infusion duration was approximately 350 min without interruptions. If an infusion reaction occurred, the infusion was interrupted and resumed at 50% of the rate achieved prior to interruption of the infusion. If the infusion was tolerated at the lower rate, the rate of infusion was increased again as described above. Rituximab was discontinued if infusion reactions did not improve with the slowest infusion.
From March 2017 to January 2018, rituximab was given on day 3 of induction, after premedication with acetaminophen (15 mg/kg; maximum: 650 mg) and diphenhydramine (1 mg/kg; maximum: 50 mg) 30 min before rituximab infusion. Pegaspargase was given on days 4, 23, and, for SR/HR patients only, day 43 to these patients (= cohort 1). The rituximab randomization was temporarily suspended in January 2018 because of the high incidence of rituximab infusion reactions. Rituximab randomization was resumed in August 2018, with the administration times being changed to day 6 and day 24 of induction therapy to increase exposure to prednisone in an attempt to reduce infusion reactions. Patients then received premedication with acetaminophen, diphenhydramine, hydrocortisone (100 mg/m2), and ranitidine (2 mg/kg/dose; maximum: 150 mg) 30 min before rituximab infusion. Pegaspargase was given on days 7, 25, and, for SR/HR patients only, day 46 to these patients (= cohort 2).
Toxicity criteria
Adverse effects were graded according to the National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE) version 4.0. For rituximab-related and pegaspargase-related toxicities, the incidences of grade 2 or higher events were recorded. For infection and febrile neutropenia, grade 3 or higher events were used.
Minimal residual disease and CD20+ cells after rituximab administration
Bone marrow samples on day 15 and at the end of induction (around day 42) were evaluated for minimal residual disease (MRD) measured by flow cytometry as described previously (14). Peripheral blood MRD and CD20+ cells in the MRD population were examined on days 1, 3, 4, and 8 for cohort 1 and on days 1, 6, and 8 for cohort 2.
Anti-pegaspargase antibody measurement
Plasma samples were collected on days 1, 22, and 43 of induction; on day 1 of consolidation; and at weeks 7 and 17 of continuation therapy. Anti-pegaspargase antibodies were measured using an enzyme-linked immunosorbent assay (ELISA) as described previously (5). Antibody-positive cases were defined as those with an anti-pegaspargase–positive ELISA result at any timepoint after the first rituximab infusion. Samples that tested positive for anti-pegaspargase antibodies were reflexively tested for antibodies against PEG or L-asparaginase. Asparaginase activity was also assayed at the same timepoints.
Statistical analysis
The original design was based on past Total Therapy studies (5). The sample size was set to n = 400 per group and a 12% pegaspargase allergic reaction rate was assumed for the control group. With treatment group allergic reaction rates of 6% and 5%, the power of the study to detect the difference at the overall 5% significance level was expected to be 88% and 97%, respectively. The rituximab study was closed early due to unexpectedly high rate of reactions to rituximab administration. Patient demographic data (e.g., sex, age, race, initial white blood cell counts, and leukemia risk) and data on rituximab reactions, asparaginase allergies, asparaginase-associated pancreatitis, infections, febrile neutropenia, duration of each treatment phase, anti-pegaspargase antibody status, CD20 expression, and MRD were collected for analysis. Comparisons between patients who did versus did not receive rituximab during the randomization period was performed using chi-square test or Kruskal-Wallis test. P value of 0.05 or lower was considered statistically significant and no adjustment of multiple tests was applied. These data were analyzed with SAS version 9.4 and R version 4.2.1.
RESULTS
Patients
During the two study periods, 85 patients were eligible for randomization. Seventy-six patients provided consent, but two of these were not randomized because of the closure of this study objective (Fig. 1). Consequently, 37 patients were randomized to receive or not to receive rituximab in each of cohorts 1 and 2. Among the 37 patients in cohort 1, 18 were randomized to rituximab, but rituximab was not given to one of these patients because of a systemic viral infection. In cohort 2, 19 patients were randomized to rituximab, but rituximab was not given to one of these patients who was taken off treatment before day 6 because this patient had a congenital cardiac disease that made him not eligible for the protocol therapy.
Figure 1.
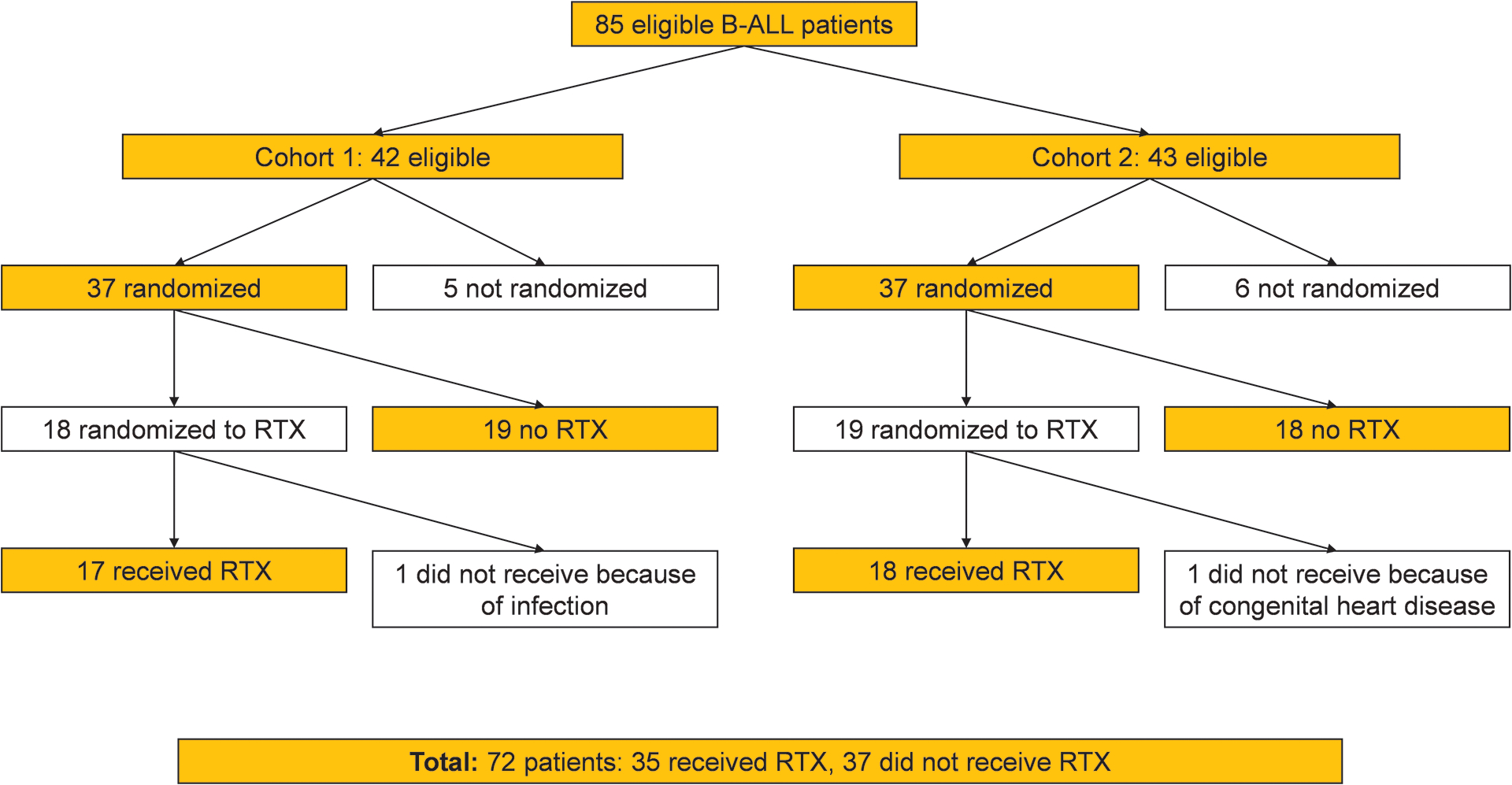
Randomization schema for this study.
Abbreviations: ALL, acute lymphoblastic leukemia; RTX, rituximab
Patient characteristics are shown in Table 1. There was no significant difference in the clinical demographics between patients who did or did not receive rituximab.
Table 1.
Patient demographics
| Variables | Total (n = 72) | Rituximab (n = 35) | No Rituximab (n = 37) | P |
|---|---|---|---|---|
| Age at diagnosis [n (%)] | ||||
| 1–<10 years | 51 (70.8) | 27 (77.1) | 24 (64.9) | 0.305 |
| ≥10 years | 21 (29.2) | 8 (22.9) | 13 (35.1) | |
| Sex [n (%)] | ||||
| Male | 36 (50.0) | 18 (51.4) | 18 (48.6) | >0.999 |
| Female | 36 (50.0) | 17 (48.6) | 19 (51.4) | |
| Race [n (%)] | ||||
| White | 61 (84.7) | 28 (80.0) | 33 (89.2) | 0.275 |
| Black | 8 (11.1) | 4 (11.4) | 4 (10.8) | |
| Other | 3 (4.2) | 3 (8.6) | 0 (0) | |
| WBC count at diagnosis [n (%)] | ||||
| <50 × 109/L | 62 (86.1) | 31 (88.6) | 31 (83.8) | 0.736 |
| ≥50 × 109/L | 10 (13.9) | 4 (11.4) | 6 (16.2) | |
| CNS status at diagnosis [n (%)] | ||||
| CNS-1 | 41 (62.5) | 20 (57.1) | 21 (56.8) | 0.682 |
| CNS-2 | 22 (30.6) | 10 (28.6) | 12 (32.4) | |
| CNS-3 | 2 (2.8) | 2 (5.7) | 0 (0) | |
| TLP with blasts | 7 (9.7) | 3 (8.6) | 4 (10.8) | |
| Treatment risk [n (%)] | ||||
| Low risk | 39 (54.2) | 22 (62.9) | 17 (45.9) | 0.279 |
| Standard risk | 31 (43.1) | 12 (34.3) | 19 (51.4) | |
| High risk | 2 (2.8) | 1 (2.9) | 1 (2.7) | |
| PB MRD on day 8 (%) | ||||
| N | 71* | 35 | 36* | 0.175 |
| Mean (std) | 1.65 (5.52) | 0.97 (2.08) | 2.31 (7.47) | |
| Median (min, max) | 0.07 (0.00, 41.31) | 0.03 (0.00, 7.93) | 0.14 (0.00, 41.31) | |
| BM MRD on day 15 (%) | ||||
| N | 72 | 35 | 37 | 0.786 |
| Mean (std) | 3.22 (10.81) | 2.55 (6.94) | 3.85 (13.57) | |
| Median (min, max) | 0.05 (0.00, 80.74) | 0.13 (0.00, 27.65) | 0.05 (0.00, 80.74) | |
| BM MRD on day 42 (%) | ||||
| N | 72 | 35 | 37 | 0.972 |
| Mean (std) | 0.35 (2.99) | 0.73 (4.30) | 0.00 (0.01) | |
| Median (min, max) | 0.00 (0.00, 25.41) | 0.00 (0.00, 25.41) | 0.00 (0.00, 0.06) |
Abbreviations: n, number; WBC, white blood cell; CNS, central nervous system; TLP, traumatic tap; PB, peripheral blood; MRD, minimal residual disease; std, standard deviation; min, minimum; max, maximum; BM, bone marrow
One patient did not have PB MRD data on day 8.
Rituximab administration and adverse reactions
In cohort 1, seven of the 17 patients (41.2%) who received rituximab developed an infusion reaction to rituximab. There were five grade 2 reactions, one grade 3 reaction, and one grade 4 reaction (Table 2). In four of these seven patients, the rituximab infusion was not completed, and these patients received an estimated median 5.0% (range 0.8% to 18.6%) of the planned dose. Two patients with grade 3 or 4 reactions required epinephrine administration because of bronchospasm and hypoxia. The median rituximab infusion duration was 291 min (range: 13 min to 1093 min), while the median time for completed infusions was 348 min (range: 215 min to 1093 min).
Table 2.
Summary of rituximab reactions
| Cohort | CTCAE grade | Symptoms | Infusion duration (min) | Infusion stopped | Interventions | Received 2nd dose | Tolerated 2nd dose |
|---|---|---|---|---|---|---|---|
| Cohort 1 | 2 | Chills, vomiting | 1093 | N | Hydrocortisone, ranitidine | NA | NA |
| 2 | Urticaria, cough | 475 | N | Hydrocortisone | NA | NA | |
| 2 | Rash, itching, vomiting | 215 | N | NA | NA | ||
| 2 | Chills, tachycardia | 83 | Y | Hydrocortisone | NA | NA | |
| 2 | Tachycardia, tachypnea, hypotension, vomiting | 13 | Y | Lorazepam, normal saline bolus | NA | NA | |
| 3 | Wheezing, tachypnea, hypoxia, tachycardia, hypotension, vomiting | 102 | Y | Albuterol, epinephrine, hydrocortisone, ranitidine | NA | NA | |
| 4 | Urticaria, wheezing, hypoxia, chills, vomiting | 150 | Y | Diphenhydramine, epinephrine, hydrocortisone, ranitidine | NA | NA | |
| Cohort 2 | 2 | Urticaria | 419 | N | Diphenhydramine, ranitidine, hydrocortisone | N | NA |
| 2 | Urticaria | 483 | N | Diphenhydramine, hydrocortisone | Y | Y | |
| 2 | Urticaria | 455 | N | Diphenhydramine, ranitidine, hydrocortisone | Y | Y | |
| 2 | Urticaria | 463 | Y | Diphenhydramine, methylprednisolone, ranitidine, hydrocortisone | N | NA | |
| 2 | Urticaria | 334 | Y | Acetaminophen, diphenhydramine, hydrocortisone, ranitidine | Y | Y | |
| 2 | Chills, abdominal pain | 80 | Y | Hydrocortisone, ranitidine | N | NA | |
| 3 | Urticaria | 2179 | N | Hydrocortisone, hydroxyzine, ranitidine, acetaminophen, diphenhydramine | Y | Y | |
| 3 | Cough, dyspnea, vomiting | 141 | Y | Albuterol, diphenhydramine, methylprednisolone, hydrocortisone, ranitidine | N | NA | |
| 3 | Urticaria, facial swelling, wheezing, dyspnea | 103 | Y | Albuterol, methylprednisolone, ranitidine, hydrocortisone | N | NA |
Abbreviations: CTCAE, Common Terminology Criteria for Adverse Events; min, minutes; N, no; Y, yes; NA, not applicable
In cohort 2, nine of the 18 patients (50.0%) who received rituximab developed an infusion reaction for the first dose. There were six grade 2 reactions and three grade 3 reactions (Table 2). The rituximab infusion was discontinued in five of these nine patients, and these patients received an estimated median 22.2% (range 4.2% to 66.5%) of the planned dose. For the first dose, the median rituximab infusion duration was 396 min (range: 80 min to 2179 min), while the median time for completed infusions was 405 min (range: 282 min to 2179 min). Thirteen of the 18 patients who received rituximab in cohort 2 received a second dose. These 13 patients included four who had a reaction with the first course but completed the dose and tolerated the second infusion without infusion reactions (median infusion duration: 365 min, range: 185 min to 533 min).
Sixteen of the 35 patients (45.7%) in cohorts 1 and 2 (combined) who received rituximab experienced an infusion reaction to the treatment, and the infusion was discontinued in nine patients. Because of the high incidence of reactions to rituximab, the rituximab study was closed.
There were no significant differences in patient characteristics and treatment response between patients who did or did not develop reactions to rituximab treatment (Supplemental Table 1).
Pegaspargase reactions, anti-pegaspargase antibodies, and pancreatitis
Nine of the 72 patients in the combined cohorts (12.5%) had an allergic reaction to pegaspargase, with all such reactions occurring before or at the time of the fourth pegaspargase dose (Table 3 and Fig. 2). There was no significant difference in the frequency of pegaspargase reactions between the patients who received rituximab and those who did not receive it (P > 0.999); four of the 35 patients (11.4%) who received rituximab had a reaction to pegaspargase, as did five of the 37 patients (13.5%) who did not receive rituximab. All 4 cases of pegaspargase reactions in patients who received rituximab were observed after induction therapy, but 3 of the 5 cases of reactions in those who did not receive rituximab were observed during induction therapy. Of the four patients who received rituximab and had a reaction to pegaspargase, three had also experienced a reaction to rituximab, of whom one had the rituximab infusion discontinued.
Table 3:
Pegaspargase allergic reactions
| Cohort | Reaction to rituximab | Phase | Cumulative doses at reaction | Grade | Symptoms | Intervention | Anti-pegaspargase antibodies | |
|---|---|---|---|---|---|---|---|---|
| Received rituximab | 1 | Y | Early intensification | 3 | 3 | Periorbital edema, abdominal rash, emesis, cough, hypoxia, wheezing | Albuterol, oxygen, diphenhydramine, ranitidine, epinephrine | Positive |
| 1 | Y | Early intensification | 3 | 4 | Tachycardia, cyanosis with poor perfusion | Diphenhydramine, oxygen, normal saline boluses, epinephrine | Positive | |
| 2 | Y* | Reinduction I | 4 | 2 | Facial rash/swelling, intermittent cough | Diphenhydramine | Positive | |
| 2 | N | Early intensification | 3 | 3 | Lip swelling, emesis, tachycardia, hypotension, somnolence | Ondansetron, methylprednisolone, normal saline boluses, epinephrine | Positive | |
| No rituximab | 1 | NA | Consolidation | 4 | 2 | Emesis, urticaria on neck, arms, and chest | Diphenhydramine, hydrocortisone | Positive |
| 2 | NA | Reinduction I | 3 | 2 | Erythema/urticaria, facial edema, cough | Hydrocortisone, epinephrine | Positive | |
| 2 | NA | Induction | 2 | 2 | Emesis, eyes and lips swelling | Methylprednisolone | Positive | |
| 2 | NA | Induction | 2 | 3 | Abdominal pain and cramping, emesis, dyspnea | Diphenhydramine, albuterol, oxygen, methylprednisone, ranitidine, epinephrine | Positive | |
| 2 | NA | Induction | 1 | 3 | Nausea, throat and facial tightness | Oxygen, methylprednisolone | Positive |
Abbreviations: PEG, polyethylene glycol; Y, yes; N, no; NA, not applicable
Rituximab infusion was not completed due to the reaction.
Figure 2.
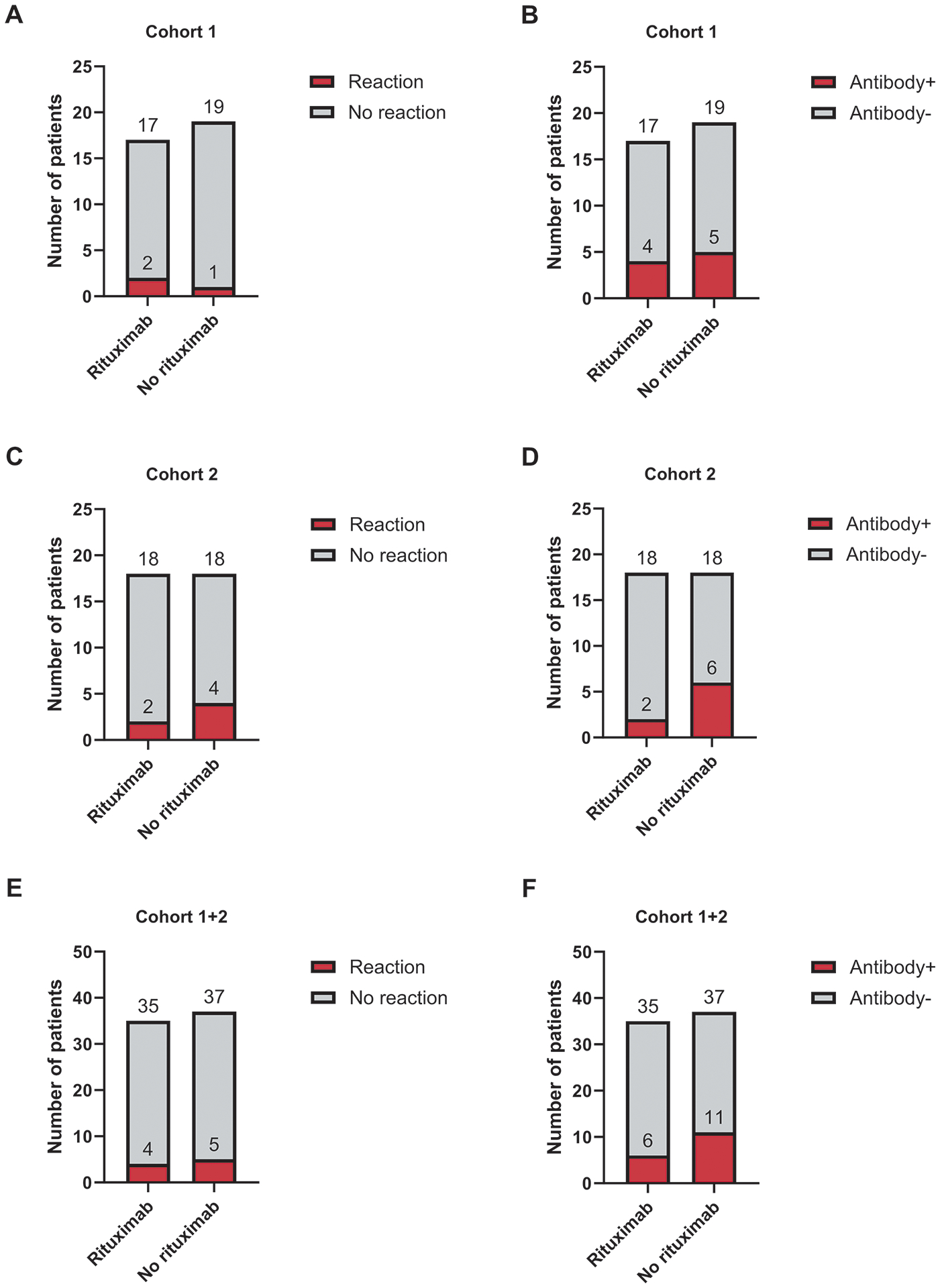
Number of patients with pegaspargase reactions (A, C, and E) and anti-pegaspargase antibodies (B, C, and F) based on rituximab randomization.
Seventeen of the 72 patients (23.6%) tested positive for anti-pegaspargase antibodies at any point in therapy (Fig. 2). Although only 17.1% of patients who received rituximab tested positive for anti-pegaspargase antibodies, compared with 29.7% of patients who did not receive rituximab, this difference was not statistically significant (P = 0.327). All 17 patients positive for anti-pegaspargase antibodies were positive for anti-PEG antibodies, and 5 patients were also positive for anti-asparaginase antibodies. Eight patients had anti-pegaspargase antibodies without reaction to pegaspargase, consistent with our previous findings on rates of pegaspargase allergy (5). Low asparaginase activity without pegaspargase reactions in the presence of positive anti-pegaspargase antibodies (i.e., silent inactivation) occurred in only 3 patients, all in the non-rituximab group.
Nine of the 72 patients (12.5%) developed pancreatitis that was attributed to pegaspargase; eight patients had grade 3 reaction and one grade 4. There was no significant difference in the incidence of pancreatitis between the patients who received rituximab (three of 35 patients, 8.6%) and those who did not (six of 37 patients, 16.2%) (P = 0.480).
Incidences of infection and febrile neutropenia and duration of each treatment phase
We analyzed grade 3 or higher infection in both overall and in the induction period. There were 31 infections in patients who received rituximab and 35 in those who did not (P = 0.42), of which 10 and 9, respectively, occurred during the induction therapy. There was no significant difference in the incidences of febrile neutropenia between patients who received rituximab (22 patients) and those who did not (18 patients) (P = 0.25), of which 5 and 7, respectively, occurred during the induction phase. When duration of each treatment phase was evaluated, there were no significant differences in the duration of induction, early intensification, consolidation, continuation weeks 1–6, reinduction 1 (weeks 7–9), continuation weeks 10–16, or reinduction 2 (weeks 17–19) between the 2 groups (Supplemental Table 2).
MRD response and CD20 expression
The MRD response was evaluated in peripheral blood at day 8 (71 patients) and in bone marrow at day 15 and at the end of induction (72 patients) (Table 1). There was no significant difference between the MRD values of patients who received rituximab and those of patients who did not receive it. MRD values were not significantly different between patients who did or did not develop reactions to rituximab (Supplemental Table 1).
We also evaluated the change in blast percentages and CD20 expression on blasts before and after rituximab administration (Fig. 3). There were no significant differences in blast percentages between patients who received rituximab and those who did not. In cohort 1, there was a significant decrease in CD20+ cells on days 4 and 8 in patients who received rituximab, as compared with those who did not (P < 0.001 for both). Similarly, in cohort 2, CD20 expression was significantly decreased on day 8 in patients who received rituximab, as compared with those who did not (P = 0.003). In the combined cohort, although CD20 expression on day 1 did not differ between patients who received rituximab and those who did not, CD20 expression on day 8 was significantly lower in the former group (P < 0.001). There were increases in CD20 expression after the initiation of chemotherapy but before rituximab therapy in patients who were randomized to the rituximab arm (on day 3 for cohort 1 and on day 6 for cohort 2) and in those who were randomized to the non-rituximab arm (on days 3, 4, and 8 for cohort 1 and on days 6 and 8 for cohort 2) (Fig. 3).
Figure 3.
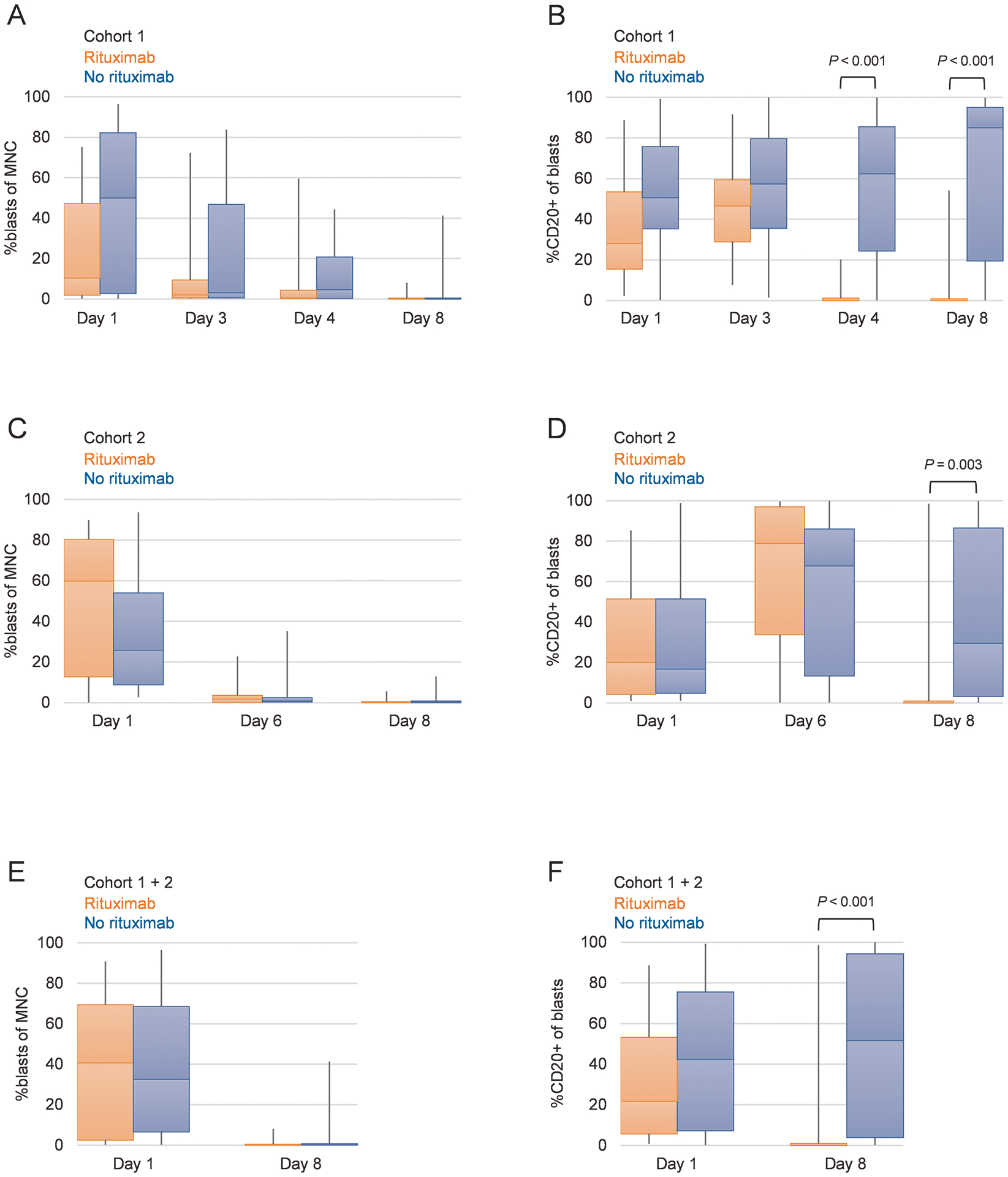
Changes in blast percentage in peripheral blood mononuclear cells and in CD20 positivity in leukemia blasts.
Abbreviations: MNC, mononuclear cells
DISCUSSION
Asparaginase remains a cornerstone of treatment of ALL (15, 16). Until the recent introduction of calaspargase, pegaspargase was the first-line asparaginase formulation in the United States, and it continues to be used globally. Although the incidence of allergic reactions to pegaspargase is lower than that of reactions to native L-asparaginase, reactions to pegaspargase can be more severe and occur in 10–15% of pediatric patients (5, 17, 18). Interruption of asparaginase therapy has been associated with inferior event-free survival in some (19, 20) but not all studies (21). In addition to causing allergic reactions, anti-pegaspargase antibodies can accelerate drug clearance of both asparaginase and other chemotherapy such as dexamethasone, neutralize asparaginase activity, and diminish drug exposure, necessitating replacement with the more expensive and less convenient Erwinia asparaginase (5, 22). Therefore, it is imperative to identify strategies to mitigate the production of anti-drug antibodies and to reduce reactions to ensure adequate anti-leukemic drug exposure and maintain the high cure rate of childhood ALL.
Rituximab targets the CD20 surface marker that is present on pre-B cells through mature B cells and induces B-cell death by three mechanisms: complement-mediated cytotoxicity, antibody-dependent cellular cytotoxicity, and direct lysis (23). Rituximab administration rapidly reduces peripheral malignant cells in patients with B-chronic lymphocytic leukemia (24) and significantly improves outcomes of CD20+ adult B-ALL (11). Because rituximab targets both leukemic and normal CD20+ cells, we hypothesized that it could decrease the incidence of allergic reactions to asparaginase by decreasing antibody production from CD20+ B cells and improve the control of CD20+ B-cell ALL.
Rituximab has been used successfully with chemotherapy to treat adult ALL and was well tolerated (11, 25). To our knowledge, this is the first randomized trial and report of rituximab administration in pediatric B-ALL. However, the incidence and degree of rituximab infusion reactions were unexpectedly high. Such reactions were observed in 45.7% of the patients in the combined cohort and the median duration to complete rituximab infusions was between 5 to 6 hours which required close monitoring and intervention in the event of an infusion reaction. Therefore, the rituximab study was closed early. A high leukemia burden and insufficient premedication can contribute to reactions to rituximab treatment due to tumor lysis, mast cell degranulation, and cytokine release syndrome with elevated tumor necrosis factor-α and interleukin-6 (26). For cohort 2, we delayed rituximab administration until day 6 of induction therapy to allow more time for the leukemia burden to be reduced and to facilitate further immunosuppression. We added further premedication in the form of hydrocortisone and an H2-receptor antagonist, in addition to diphenhydramine and acetaminophen, and we titrated the infusion of rituximab to decrease cytokine release. However, the incidence of rituximab reactions did not change, despite the peripheral blast percentages being lower on day 6 than on day 3. Exposure to 5 days of prednisone and a dose of vincristine and daunorubicin was still inadequate to prevent rituximab infusion reactions in pediatric patients. The incidence and severity of hypersensitivity reactions is reported to be generally low when rituximab is used to treat mature B-cell malignancies such as Burkitt lymphoma (27). This is possibly because rituximab is given after the first course of immunosuppressive chemotherapy (one dose each of cyclophosphamide and vincristine and 7 days of prednisone). Accordingly, there were no reactions in cohort 2 to the second dose of rituximab on day 24 of induction, although five patients with reactions to the first dose did not receive a second dose. Additional factors that may have contributed to the high incidence of rituximab reactions observed in our trial but not in prior adult ALL trials are intrinsic immunologic differences between children and adults, differences in leukemia and cytokine burden, and institutional differences in the monitoring and management of infusion reactions. However, we were not able to definitively identify a cause of the unexpectedly high reactions to rituximab.
Importantly, although the number of patients in our study was small, there was no significant decrease in pegaspargase allergy with rituximab use. This contrasts with the findings of a study of rituximab in adult B-ALL, in which survival outcomes were improved and allergic reactions to native E. coli L-asparaginase were reduced (11). This divergence may reflect the recent finding that the mechanisms of pegaspargase and L-asparaginase allergies are fundamentally different (5): the PEG moiety, not L-asparaginase, is the major antigen that caused hypersensitivity reaction in patients treated with pegaspargase. Prior patient exposure to PEG-containing products, such as laxatives (Miralax®), eye drops, tablet coatings, topicals, and food, could have caused sensitization to PEG before ALL diagnosis and treatment, and the subsequent administration of pegaspargase could have re-induced and reactivated the PEG allergy. Typically, symptomatic reactions are early events, occurring at the time of the third or fourth dose of pegaspargase (5, 17). Interestingly, reactions to pegaspargase generally occurred earlier (i.e., induction) in the non-rituximab group compared to the rituximab group (Table 3), possibly suggesting that rituximab delayed the onset of reactions.
There was CD20 upregulation on ALL blasts towards day 8 after the initiation of chemotherapy. This may confirm the finding of CD20 upregulation due to prednisone treatment during induction therapy for pediatric B-ALL (28). We expected rituximab to eradicate CD20+ normal B cells and B-ALL cells. After rituximab administration, CD20+ cells significantly decreased in patients who received rituximab, as compared to those who did not, but there was no difference in MRD between the groups as was reported for the adult UKALL14 study (25). Given the multimodal induction therapy, other chemotherapy agents may have already effectively reduced the leukemia burden and lowered the MRD, and any contribution that rituximab may have made was not discernible. A limitation of this study is that event free survival (EFS) data were not available at time of analysis but given how strongly end of induction MRD predicts EFS (29), we do not expect any differences in EFS between groups.
In conclusion, rituximab administration during the early phase of induction therapy in pediatric patients with B-ALL was associated with a high incidence of infusion reactions but was not associated with significant decreases in pegaspargase allergies, anti-pegaspargase antibodies, or MRD levels. Further investigation of the mechanisms of pegaspargase allergy are needed so that alternative allergy prevention strategies can be identified and developed in future studies.
Supplementary Material
Acknowledgements
The authors thank Keith A. Laycock, PhD, ELS, for scientific editing of the manuscript. This work was supported by National Institutes of Health (NIH) grants P30CA021765, R01CA142665, and R35GM141947; by the American Lebanese Syrian Associated Charities (ALSAC); and by Sevier Pharmaceuticals, LLC. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Competing Interests:
This work was funded partially by Servier Pharmaceuticals, LLC. The company had no role in the study design, the collection or analysis of data, or the decision to publish.
Data Availability Statement:
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References:
- 1.Hunger SP, Mullighan CG. Acute Lymphoblastic Leukemia in Children. N Engl J Med. 2015;373(16):1541–52. [DOI] [PubMed] [Google Scholar]
- 2.Inaba H, Greaves M, Mullighan CG. Acute lymphoblastic leukaemia. Lancet. 2013;381(9881):1943–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hijiya N, van der Sluis IM. Asparaginase-associated toxicity in children with acute lymphoblastic leukemia. Leuk Lymphoma. 2016;57(4):748–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sassen SD, Mathot RA, Pieters R, Kloos RQ, de Haas V, Kaspers GJ, et al. Population pharmacokinetics of intravenous Erwinia asparaginase in pediatric acute lymphoblastic leukemia patients. Haematologica. 2017;102(3):552–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu Y, Smith CA, Panetta JC, Yang W, Thompson LE, Counts JP, et al. Antibodies Predict Pegaspargase Allergic Reactions and Failure of Rechallenge. J Clin Oncol. 2019;37(23):2051–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Woods D, Winchester K, Towerman A, Gettinger K, Carey C, Timmermann K, et al. From the Children’s Oncology Group: Evidence-Based Recommendations for PEG-Asparaginase Nurse Monitoring, Hypersensitivity Reaction Management, and Patient/Family Education. J Pediatr Oncol Nurs. 2017;34(6):387–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Angiolillo AL, Schore RJ, Devidas M, Borowitz MJ, Carroll AJ, Gastier-Foster JM, et al. Pharmacokinetic and pharmacodynamic properties of calaspargase pegol Escherichia coli L-asparaginase in the treatment of patients with acute lymphoblastic leukemia: results from Children’s Oncology Group Study AALL07P4. J Clin Oncol. 2014;32(34):3874–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vrooman LM, Blonquist TM, Stevenson KE, Supko JG, Hunt SK, Cronholm SM, et al. Efficacy and Toxicity of Pegaspargase and Calaspargase Pegol in Childhood Acute Lymphoblastic Leukemia: Results of DFCI 11–001. J Clin Oncol. 2021;39(31):3496–505. [DOI] [PubMed] [Google Scholar]
- 9.Ribrag V, Koscielny S, Bosq J, Leguay T, Casasnovas O, Fornecker LM, et al. Rituximab and dose-dense chemotherapy for adults with Burkitt’s lymphoma: a randomised, controlled, open-label, phase 3 trial. Lancet. 2016;387(10036):2402–11. [DOI] [PubMed] [Google Scholar]
- 10.Gottenberg JE, Guillevin L, Lambotte O, Combe B, Allanore Y, Cantagrel A, et al. Tolerance and short term efficacy of rituximab in 43 patients with systemic autoimmune diseases. Ann Rheum Dis. 2005;64(6):913–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maury S, Chevret S, Thomas X, Heim D, Leguay T, Huguet F, et al. Rituximab in B-Lineage Adult Acute Lymphoblastic Leukemia. N Engl J Med. 2016;375(11):1044–53. [DOI] [PubMed] [Google Scholar]
- 12.Jeha S, Pei D, Choi J, Cheng C, Sandlund JT, Coustan-Smith E, et al. Improved CNS Control of Childhood Acute Lymphoblastic Leukemia Without Cranial Irradiation: St Jude Total Therapy Study 16. J Clin Oncol. 2019;37(35):3377–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pauley JL, Panetta JC, Crews KR, Pei D, Cheng C, McCormick J, et al. Between-course targeting of methotrexate exposure using pharmacokinetically guided dosage adjustments. Cancer Chemother Pharmacol. 2013;72(2):369–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pui CH, Pei D, Coustan-Smith E, Jeha S, Cheng C, Bowman WP, et al. Clinical utility of sequential minimal residual disease measurements in the context of risk-based therapy in childhood acute lymphoblastic leukaemia: a prospective study. Lancet Oncol. 2015;16(4):465–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gupta S, Maude SL, O’Brien MM, Rau RE, McNeer JL. How the COG is Approaching the High-Risk Patient with ALL: Incorporation of Immunotherapy into Frontline Treatment. Clin Lymphoma Myeloma Leuk. 2020;20 Suppl 1:S8–S11. [DOI] [PubMed] [Google Scholar]
- 16.Inaba H, Pui CH. Advances in the Diagnosis and Treatment of Pediatric Acute Lymphoblastic Leukemia. J Clin Med. 2021;10(9):1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Henriksen LT, Harila-Saari A, Ruud E, Abrahamsson J, Pruunsild K, Vaitkeviciene G, et al. PEG-asparaginase allergy in children with acute lymphoblastic leukemia in the NOPHO ALL2008 protocol. Pediatr Blood Cancer. 2015;62(3):427–33. [DOI] [PubMed] [Google Scholar]
- 18.Burke MJ, Devidas M, Maloney K, Angiolillo A, Schore R, Dunsmore K, et al. Severe pegaspargase hypersensitivity reaction rates (grade >/=3) with intravenous infusion vs. intramuscular injection: analysis of 54,280 doses administered to 16,534 patients on children’s oncology group (COG) clinical trials. Leuk Lymphoma. 2018;59(7):1624–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Silverman LB, Stevenson KE, O’Brien JE, Asselin BL, Barr RD, Clavell L, et al. Long-term results of Dana-Farber Cancer Institute ALL Consortium protocols for children with newly diagnosed acute lymphoblastic leukemia (1985–2000). Leukemia. 2010;24(2):320–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gupta S, Wang C, Raetz EA, Schore R, Salzer WL, Larsen EC, et al. Impact of Asparaginase Discontinuation on Outcome in Childhood Acute Lymphoblastic Leukemia: A Report From the Children’s Oncology Group. J Clin Oncol. 2020;38(17):1897–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karol SE, Pei D, Smith CA, Liu Y, Yang W, Kornegay NM, et al. Comprehensive analysis of dose intensity of acute lymphoblastic leukemia chemotherapy. Haematologica. 2022;107(2):371–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kawedia JD, Liu C, Pei D, Cheng C, Fernandez CA, Howard SC, et al. Dexamethasone exposure and asparaginase antibodies affect relapse risk in acute lymphoblastic leukemia. Blood. 2012;119(7):1658–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taylor RP, Lindorfer MA. Drug insight: the mechanism of action of rituximab in autoimmune disease--the immune complex decoy hypothesis. Nat Clin Pract Rheumatol. 2007;3(2):86–95. [DOI] [PubMed] [Google Scholar]
- 24.Huhn D, von Schilling C, Wilhelm M, Ho AD, Hallek M, Kuse R, et al. Rituximab therapy of patients with B-cell chronic lymphocytic leukemia. Blood. 2001;98(5):1326–31. [DOI] [PubMed] [Google Scholar]
- 25.Marks DI, Kirkwood AA, Rowntree CJ, Aguiar M, Bailey KE, Beaton B, et al. Addition of four doses of rituximab to standard induction chemotherapy in adult patients with precursor B-cell acute lymphoblastic leukaemia (UKALL14): a phase 3, multicentre, randomised controlled trial. Lancet Haematol. 2022;9(4):e262–e75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wong JT, Long A. Rituximab Hypersensitivity: Evaluation, Desensitization, and Potential Mechanisms. J Allergy Clin Immunol Pract. 2017;5(6):1564–71. [DOI] [PubMed] [Google Scholar]
- 27.Minard-Colin V, Auperin A, Pillon M, Burke GAA, Barkauskas DA, Wheatley K, et al. Rituximab for High-Risk, Mature B-Cell Non-Hodgkin’s Lymphoma in Children. N Engl J Med. 2020;382(23):2207–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dworzak MN, Schumich A, Printz D, Potschger U, Husak Z, Attarbaschi A, et al. CD20 up-regulation in pediatric B-cell precursor acute lymphoblastic leukemia during induction treatment: setting the stage for anti-CD20 directed immunotherapy. Blood. 2008;112(10):3982–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Campana D, Pui CH. Minimal residual disease-guided therapy in childhood acute lymphoblastic leukemia. Blood. 2017;129(14):1913–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.


