Abstract
The presence of dye in wastewater causes substantial threats to the environment, and has negative impacts not only on human health but also on the health of other organisms that are part of the ecosystem. Because of the increase in textile manufacturing, the inhabitants of the area, along with other species, are subjected to the potentially hazardous consequences of wastewater discharge from textile and industrial manufacturing. Different types of dyes emanating from textile wastewater have adverse effects on the aquatic environment. Various methods including physical, chemical, and biological strategies are applied in order to reduce the amount of dye pollution in the environment. The development of economical, ecologically acceptable, and efficient strategies for treating dye-containing wastewater is necessary. It has been shown that microbial communities have significant potential for the remediation of hazardous dyes in an environmentally friendly manner. In order to improve the efficacy of dye remediation, numerous cutting-edge strategies, including those based on nanotechnology, microbial biosorbents, bioreactor technology, microbial fuel cells, and genetic engineering, have been utilized. This article addresses the latest developments in physical, chemical, eco-friendly biological and advanced strategies for the efficient mitigation of dye pollution in the environment, along with the related challenges.
Keywords: bioremediation, dye degradation, environmental pollutants, genetic engineering, microbial fuel cells, nanotechnology, textile wastewater treatment
1. Introduction
Water is one of the most essential factors in our lives. It has been reported that approximately 71% of our planet is covered by water, consisting of 97.5% seawater and only 2.5% freshwater [1]. Industrial wastewater is very harmful for our environment and causes various adverse impacts on the ecosystem. Most wastewater is generated from the textile, cosmetics, printing, paper, and rubber industries [2]. Dyes are categorized based on their features and applications [3,4]. The textile industry generates huge amounts of highly toxic chemicals that are released at various stages of processing [5]. In addition, it is well documented that textile dyes, without proper treatment, pose serious eco-toxicological threats to living forms [6,7].
Textile dye-containing wastewater is also one of the major sources of water pollution, and it affects the environment by disturbing aquatic life, inhibiting the photosynthesis process in aquatic plants, and affects human health by causing breathing difficulties, irregular heartbeats, skin rashes, dizziness, and cancer [4,6,8]. Different types of dyes are used in the textile industry, such as sulphur dye, azo dye, acid dye, and basic dye. Most of the common dyes applied in the textile industry are called azo dyes [9,10].
Since ancient times, mankind has known about and utilized dyes [11]. In many manufacturing sectors, including the paper industry and many more, a number of techniques are effective in removing dyes [12,13,14]. It has been found that the dyes used in the textile industry have high toxicity and a potential carcinogenic nature [8,15]. The textile industry is responsible for a broad range of impacts on the environment [16]. Textile dyes can cause diseases ranging from dermatitis to central nervous system disorders [17]. Consequently, treating toxic wastewater containing different types of textile dyes is a very compelling issue currently [18,19,20]. Accommodating a single or multiple azo groups, azo dyes constitute 60–70% of known dye structures [21,22,23].
The treatment of dye-containing wastewater occurs through various methods like physical, chemical, biological, and recent advanced technologies such as using nanotechnology, microbial biosorbents, microbial films, genetic engineering, plant–microbe-mediated techniques, and others [4,14]. In recent studies, researchers have reported the use of chemical-based methods for the remediation of pollutants from wastewater [24,25,26]. Metal-based organic frameworks are also used as sensitive and selective sensors for detecting hazardous pollutants [27]. However, physical and chemical methods are not cost-effective [6].
Bioremediation is an eco-friendly and cost-effective option to remove various types of organic and inorganic toxicants from polluted environments [28]. Microbial processes using algae, bacteria, yeast, and fungi are low-cost and can be successfully utilized for dye remediation. Microorganisms are capable of breaking down the azo bonds present in dye molecules through their enzymatic activity [6,29]. Advanced treatment strategies using microorganisms can make the remediation process more effective, while appropriate environmental conditions should also be ensured for effective bioremediation [30].
Researchers have reported that non-treated wastewater has negative effects on ecosystems and raises health concerns among humans [4]. Therefore, it is important to treat dye-containing wastewater effectively with low-cost and environmentally friendly approaches to avoid the dangers of such pollutants. This review systematically addresses different technologies such as microbial mechanisms, nanotechnology, bioreactors, microbial biofilm, phytoremediation methods, challenges with these methods, and how advanced strategies can be effectively operated to clean dye-containing wastewater contributing to a greener and cleaner environment.
2. Data Collection and Bibliometric Analyses
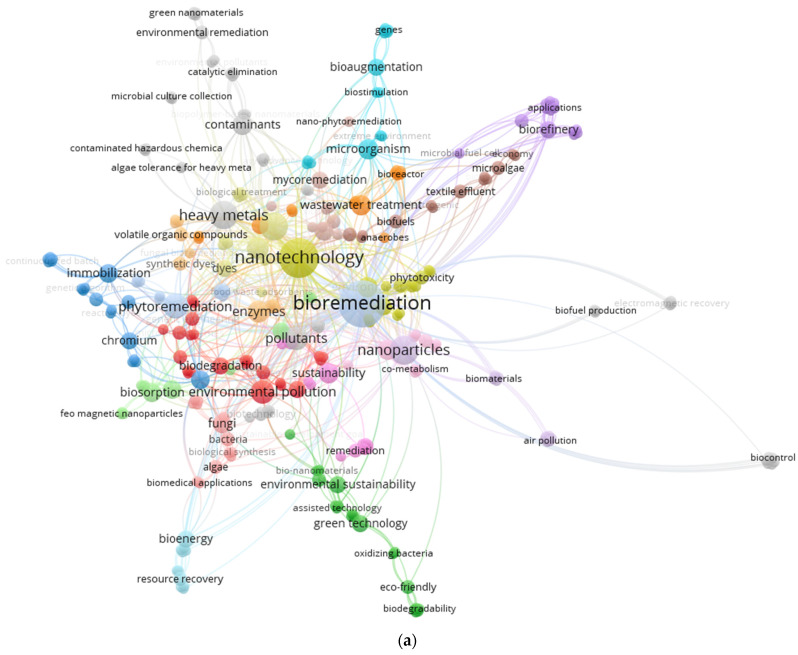
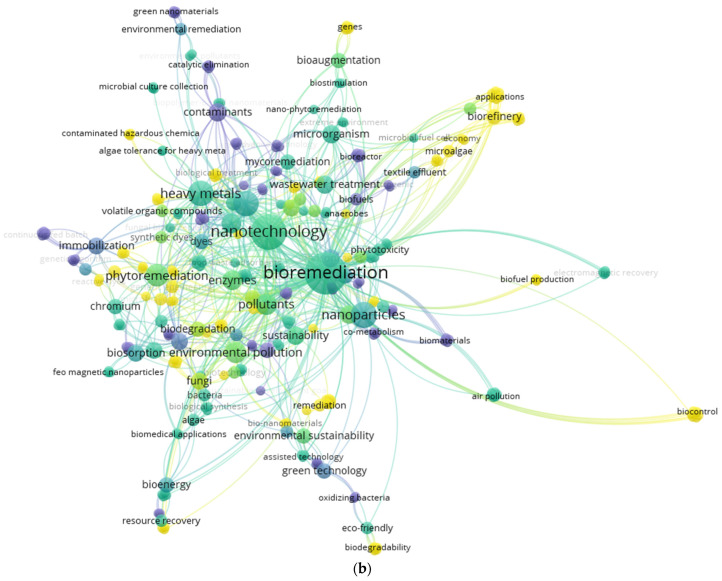
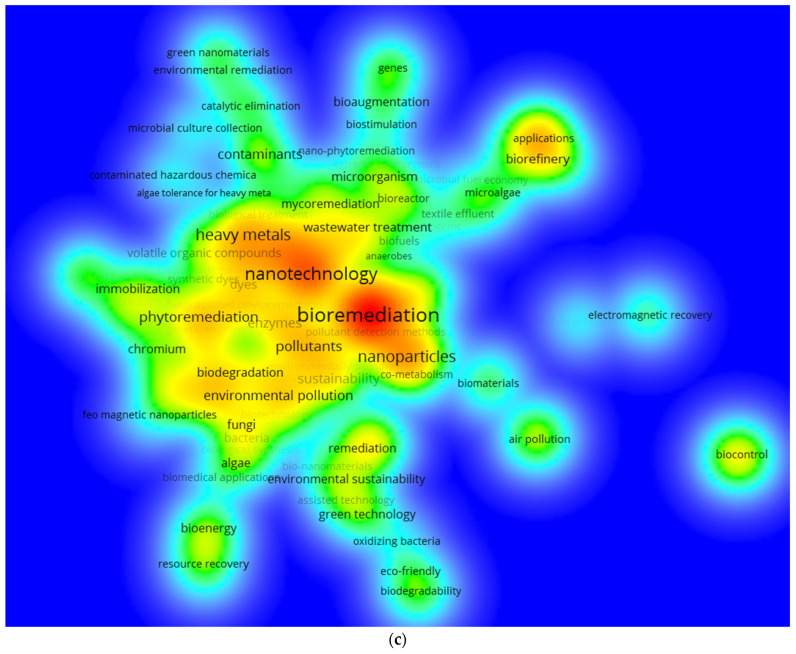
In the present study, we searched for “textile waste water treatment”, “nanotechnology”, “genetic engineering”, “bioreactors,”, “bioremediation”, “microbial fuel cells,” and “dye degradation” in the Web of Science using VOS viewer 1.6.18. The number of publications and citations relating to the selected term displayed a peak in the current year. A network visualization map indicating the co-citation of the most-cited terms is presented in Figure 1. Most recent research has paid attention to the bioremediation of textile dyes from wastewater, and the depictions reveal that bioremediation, nanotechnology, and genetic engineering are among the most studied areas in the structure of this study.
Figure 1.
(a) Network visualization of bibliometric analysis of high-frequency keyword graphs over time and clustering of keyword citation networks. (b) Overlay visualization of bibliometric analysis of high-frequency keyword graphs over time and clustering of keyword citation networks. (c) Density visualization of bibliometric analysis. Term lines, circles, and other colors in a bibliometric network represent the degree of connectedness. The label and circle of an object grow in size in proportion to its weight. The size of the label and the matching circle that represents the object change in tandem with the item’s weight. A chronology is shown using a red-to-blue color gradient, with red designating earlier articles and blue representing more recent ones. This gradient acts as a visible counter for the passage of time. Red highlights important or noteworthy content, such as prominent citations, influential writings, or often-cited sources, while the more recent works, developing fads, or sources considered foundational in a certain discipline may all be represented by the color blue. Additionally, this may identify sources with a certain theme or content.
3. Dyes Used in Textile Industry
Azo dyes are frequently used in a range of industries, including those that produce food, medicines, paper, cosmetics, textiles, leather, and other products [6,31]. In one study, Benkhaya et al. [31] discussed the classification and characteristics of dyes. They reported the chemical classification of azo dyes and their structural properties. Several researchers reported the different types of dyes that are used in the textile industry, such as acidic, basic, direct, sulphur and azo dyes, along with their applications [31,32,33,34,35,36].
4. Approaches for Dyes Remediation
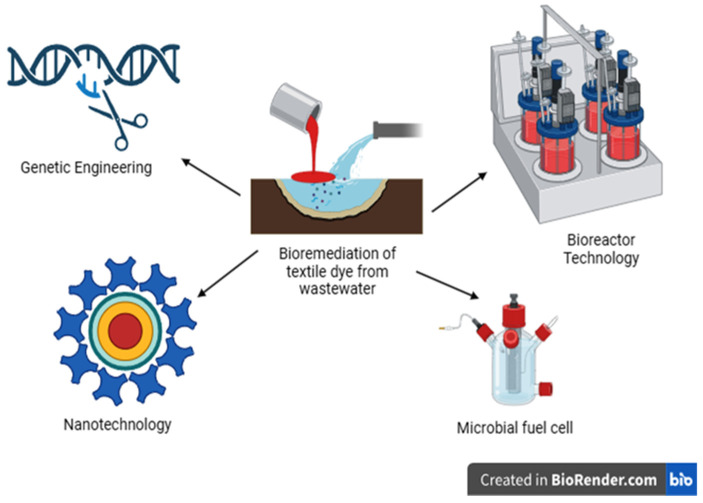
Pollutants such as dyes from the textile industry are present in wastewater. Various techniques, such as physical, chemical, and biological methods, are used for the decontamination of dyes in aquatic environments. The physical and chemical procedures used to treat textile wastewater are inadequate and not always environmentally feasible. A safe method to detoxify dyes from wastewater is bioremediation technology using microorganisms or plants. Nowadays, advanced technologies such as bioremediation, including nanotechnology, bioreactors, microbial fuel cells, genetic engineering, and others, are being applied in the treatment of dye pollution in the environment (Figure 2).
Figure 2.
Recent possible strategies for dyes remediation from aquatic environment.
Different approaches to remove various types of dye from the environment are critically described below.
4.1. Physical and Chemical Approaches
Adsorption, ion exchange, and membrane filtration are a few of the physical methods used in the remediation of environmental pollutants. Some of these physical techniques are discussed below.
4.1.1. Adsorption
Adsorption is used for the management of industrial wastewater. Adsorption is a surface-based phenomenon in which the solid surface of the adsorbent attracts charged ions or molecules, which then adsorb onto the surface. Several types of forces are responsible for dye molecule adsorption, including hydrophobic, electrostatic interaction, hydrogen bonding, and van der Waals forces [37]. The adsorption process removes dyes from contaminated wastewater, and there is a possibility of upcycling the adsorbent for reuse in treatment [3]. The phenomenon of adsorption is dependent on the adsorbent, which contains pore-like structures that are required for the quick and systematic adsorption of dye molecules from wastewater [38,39]. Most adsorbents, such as silica gel, alumina, zeolite, and activated carbon, are commonly used for the removal of toxic dyes from contaminated wastewater [40]. Other mechanisms like complexation and ion exchange are applied in the remediation of dyes [41,42,43]. In their study, Briao et al. [44] found that ZSM-5 zeolite adsorbent is used in the treatment of dye-containing wastewater, such as basic fuchsin, crystal violet, and methylene blue, with a degradation percentage of 81.2%, 75.3%, and 86.6%, respectively. In another study, Madan et al. [45] reported 90% Congo red decolorization by ZnO, used as an adsorbent, while Harja et al. [46] reported Congo red dye remediation with the help of fly ash generated from a local powerplant. Several researchers have also reported different types of organic adsorbents and their composites for dye remediation from aqueous solutions, and some of the most interesting adsorbents are conducting polymers in their different forms, including powders and aerogels, as well as biopolymers [47,48,49].
4.1.2. Ion-Exchange Method
In the ion-exchange method, effective separation is accomplished by creating a complex bond between resin, which is a bed reactor, and a solute. Akpomie and Conardie [3] and Ahmad et al. [50] found that the process of dye removal in ion exchange mainly depends on strong interactions between charged molecules in the dye and the functional group of the resin. The cation exchangers and anion exchangers are used as resins to separate solutes with different surface charges [51]. Many researchers reported that dyes like acid orange 10 are removed by an anion-exchanger resin named Amberlite IRA-400, and the percentage of dye removed was 96.8% [52]. Another dye, acid black, was remediated at a rate of 100% by an anion exchanger that was synthesized using cellulose [53]. Disperse violet 28 dye is generally removed by cation-exchanger resin, and the dye removal percentage is 91.7 [54].
4.1.3. Membrane Filtration
Membrane filtration is one of the most important physical methods for textile dye removal from wastewater [55,56]. In this method, due to the small pore size of the membrane, molecules larger than the filter pores become trapped. Microfiltration is a membrane-based phenomenon that involves the separation of dyes in the size range of 0.1–0.10 μm [57]. In this process, the waste materials or dyes are remediated from the liquid with the help of a microporous membrane. Ultrafiltration is another membrane-based method. Collivignarelli et al. [58] reported that dye color removal capabilities are acquired from wastewater with the help of ultrafiltration. The reactive black dye solution uses an ultrafiltration ceramic membrane and decolorizes the dye at different concentrations [59]. Reverse osmosis is also a membrane filtration mechanism that is used for the treatment of industrial wastewater that contains dyes [60,61]. Several researchers have reported the application of filtration technology for the treatment of wastewater containing dyes [62,63,64].
4.1.4. Fenton Process
The two major approaches for the degradation of dyes present in textile water are Fenton and photo-Fenton [65,66]. These processes are carried out by using a Fenton reagent, namely H2O2, and a soluble iron (II) salt mixture [67,68,69]. In a study by Ledakowicz et al. [70], it was reported that the degradation of three dyes (add red 27, reactive blue 81, and add blue 62) occurred with the use of a Fenton reagent. They concluded that this reaction is very simple and fast. In another study, Chen et al. [71] used the stopped flow technique to study the degradation of methylene blue and rhodamine B with Fenton reagent. Zawadzki and Deska [72] published a review concerning the degradation of dyes by combining advanced oxidation processes with different methods, such as utilizing ozone, hydrogen peroxide, and persulfate to degrade rhodamine B. They concluded that degradation is achieved by using UV in a photo-assisted ozonation, which was the most effective method among all of the techniques tested.
4.1.5. Ozonation
Dye remediation using ozonation is another important treatment technology. Shriram and Kanmani [73] concluded in their study that H2O2, UV radiation, etc., are used in the ozonation process. They provided a detailed study about the mechanism, influencing factors, and initiators of ozonation. Venkatesh and colleagues [74] reported dye remediation by means of combined ozonation and anaerobic treatment strategies. They reported that the cost of ozonation can be reduced using an upflow anaerobic sludge blanket reactor. In another study, Cardoso et al. [75] reported nanomaterial-based catalysts for the ozonation process in the remediation of dyes. They used copper (II)-doped carbon dots as catalysts in catalytic ozonation. They also analyzed the degradation of four dyes, namely methyl orange, orange II sodium salt, reactive black, and remazol brilliant blue R (RBB-R). In a recent research work, Lanzetta et al. [76] reported using the ozonation process for color remediation from tanning wastewater. Further research is needed on the decolorization of wastewater using ozonation.
4.2. Biological Approaches
The physical and chemical techniques used in dye decolorization are costly. Traditional biological techniques are used exclusively or with chemical and physical techniques for dye decolorization. Nonetheless, advanced biological methods that are less expensive and more effective with lesser secondary sludge production are emphasized [13,28,77]. Microbial methods are effective for the bioremediation of different types of organic and inorganic pollutants [30,78,79]. On the basis of different research findings, for the decolorization of dyes, bacterial treatment serves as an efficient strategy [80]. Figure 3 presents various biological methods for the bioremediation of dyes from contaminated environments.
Figure 3.
Different strategies for the bioremediation of dyes from polluted environments.
4.2.1. Enzymatic Method
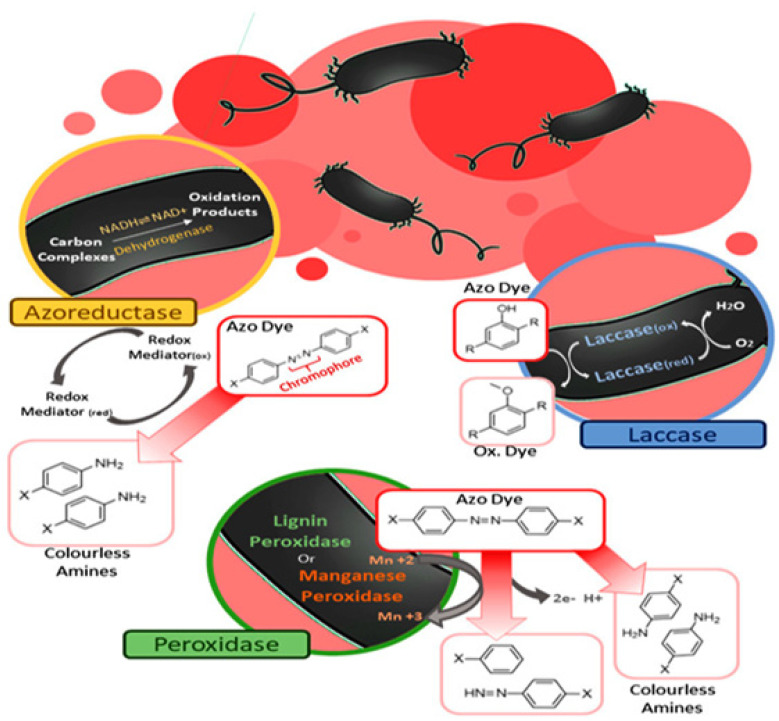
Bioremediation by enzymes is an ingenious, favorable, environmentally friendly technique [81]. The enzymatic degradation method consists of finding the attributes of microbes or genetically modified microbes, designing enzymes to metabolize the dyes, and transforming the harmful form of dyes into harmless forms or non-toxic forms [81]. Regarding azo dye degradation, a number of studies have been conducted to understand different type of enzymatic activities that help in the degradation of toxic dyes [81,82]. A class of enzyme, azoreductase, has been described by Mendes et al. [83] as carrying out the reduction reaction causing the breakdown of azo bonds (-N=N-) present in dyes, and converting the aromatic amine into colorless water. The enzyme laccase can be used in the treatment of different toxic textile dyes [84]. Lignin and Mn peroxidase have been widely studied; peroxidase enzymes have been used in the degradation of toxic textile dyes [85]. For the bioremediation of hydrocarbons and pesticides, enzymes produced by aerobic bacteria such as Alcaligene sp. and Pseudomonas sp. are also used [81]. Enzymes produced by different types of fungi, such as lignin peroxidases, azoreductases, and laccases from white rot fungi, can also take part in the biodegradation of textile dyes [86]. In a recently published review, Pinheiro et al. [87] showed the role of different enzymes in the bioremediation of dyes (Figure 4).
Figure 4.
Schematic representation of three general bacterial enzymatic degradation mechanisms of the azo chromophore group, first showing the enzymatic degradation via the action of azoreductases—yellow—in this example using NADH as an essential reducing agent for the cleavage of azo bonds, generating aromatic amines and thus discoloring the medium. Then—clockwise—we have the catalytic reaction cycle mediated by laccase—blue—with the generation of an oxidized substrate instead of potentially toxic amines, in addition to not requiring cofactors. Finally, peroxidase enzymes—green—such as lignin peroxidase and manganese peroxidase, the two enzymes most commonly used for dye degradation, illustrate some possible products according to the cleavage of their bonds, which can be symmetric or asymmetric (Figure 4 is originally printed and adapted from Pinheiro et al. [87]. Copyright: © 2023 by the authors. Licensee: MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Various researchers such as Shahid et al. [88] found that the strain MR-1/2 of multifarious Bacillus species efficiently decolorized dyes such as reactive black-5, reactive red-120, direct blue-1, and Congo red, which additionally helped in the growth of the mung bean plant by alleviating azo dye toxicity. Meanwhile, Vineh et al. [89] found 100% decolorization of most of the dyes used in the study at pH 7, 25 °C, for 60 min by using peroxidase immobilized on a functionalized reduced grapheme oxide. In another study, Navas et al. [90] reported 20–100% decolorization of dyes at pH 5–9 using laccase, which was purified and extracted from the thermophilic Thermus species. In another study, Kalsoom et al. [91] found 95% degradation of remazol turquoise blue 133G dye with peroxidase from Brassica oleracea, while Gao et al. [92] achieved 72–80% decolorization of the azo dyes reactive blue 19 and acid orange 7 at a neutral to alkaline pH at relatively high temperatures using laccase enzymes immobilized in vault nanoparticles.
4.2.2. Microbial Remediation
Several microbes, such as bacteria, fungi, yeast, algae, and actinomycetes, are utilized for treating textile dyes from aquatic environments [6,10,93,94,95,96,97].
Bacterial Remediation
Among all the groups of microbes, decolorization by bacteria is significant. From a biotechnological perspective, bacteria offer many advantages as they contain abundant degradative enzymes, consequently having the capacity to degrade dyes of a broad range [6,98,99]. The basic advantage of dealing with bacteria is their efficiency in growing quickly, and their ability to be cultured easily. The organic pollutants that are aromatic hydrocarbon-based and chlorinated can be catabolized by bacteria as their source of energy [100]. Bacteria also have the capability to oxidize textile dyes based on sulphur to H2SO4 [101].
Pure Bacterial Cultures
Using a pure bacterial culture with one type of bacteria, the biodecolorization of dyes has been reported in several studies. In one investigation, Louati et al. [102], found 100% decolorization of dyes at pH 8 by using Pseudomonas aeruginosa strain Gb30, whereas Montanez-Barragan et al. [103] observed above 90% decolorization of dyes at pH 6–11 with Halomonas species. Another researcher, Shi et al. [104], found 100% dye removal of Brilliant Crocein by using the bacteria Providencia rettgeri, while Fareed et al. [105] found 80–100% decolorization of dyes by using free and immobilized cells of Bacillus cereus at temperatures of 32 °C, 37 °C, and 45 °C. Srinivasan et al. [106] achieved 88.35–96.30% decolorization of different azo dyes, while Du et al. [107] observed the complete decolorization of malachite green and crystal violet at pH 3–10 and a temperature of 20–45 °C after 12 h of incubation under optimal environmental conditions using Serratia species, which is a new bacterial strain identified via 16S rDNA analysis. In a recent study, Tripathi et al. [10] observed 98% dye decolorization of crystal violet dye using a native multiple metal-tolerant Aeromonas caviae MT-1 isolate.
Mixed Bacterial Cultures
For decolorizing diverse groups of dyes, bacteria of a single species are not efficient enough for their remediation, and this is one of the most prominent challenges for environmental biotechnologists [108]. Many researchers have worked on a consortium or mixed bacterial culture for the degradation of dyes. In a study, Ayed et al. [109] observed 90% dye decolorization at 35 °C using a bacterial consortium consisting of Sphingomonas paucimobilis, Pseudomonas putida, and Lactobacillus acidophilus. In another study, Guo et al. [110] found 93% decolorization at 40 °C and pH 10 of Methanil Yellow G dye using a consortium with Halomonas, Marinobacter, and Clostridii salibacter., Meanwhile, in another investigation, Joshi et al. [108] reported dye decolorization of 24–94% using a consortium of six bacterial strains: Pseudomonas stutzeri AK1, P. stutzeri AK2, P. stutzeri AK3, Bacillus sp. AK4, P. stutzeri AK5, and P. stutzeri AK6. Likewise, Bera et al. [111] reported 85% acid orange dye decolorization after nearly 23 h, with yeast as a supplementary source and a bacterial consortium called novel bacterial consortium SPB92 composed of four bacterial strains, i.e., Pseudomonas stutzeri (MW219251), Bacillus tequilensis (MW110471), B. flexus. (MW13 flexus and Kocuria rosea (MW 132411), whereas Neihsial et al. [112] found 85–97% degradation of dyes using a consortium including bacteria of different genera, like Acinetobacter, Comamonas, Trichococcus, Erwinia, Dysgonomonas, and Citrobacter. In another study, Barathi et al. [113] found that the bacterial consortium with three bacterial species, B. subtilis, Brevibacillus borstelensis, and B. firmus, was able to degrade dye at a better rate at lower concentrations of dye, but the ability of degradation decreased with elevated concentrations of dye. Several bacterial isolates have been reported for dye decolorization (Table 1).
Table 1.
Bacteria applied in the biodecolorization of different dyes.
| Bacteria | Dye | % Decolorization | Reference |
|---|---|---|---|
| Oerskovia paurometabola | Acid red 14 | 91% | [114] |
| Brevundimonas diminuta | Rhodamine–B | 90–95% | [115] |
|
Gerobacillus stereothermophilus
ATCC 10149 |
Remazol brilliant blue-R | 90% | [116] |
| Gerobacillus thermoleovorans | Amaranth RI Fast red E |
99% | [117] |
| Serratia spp. | Malachite green Crystal violet |
96.5% | [107] |
| Iodidimonas spp. | Cationic dyes (Malachite green, crystal violet, methylene blue) | >90% | [118] |
| Lysinibacillus sphaericus | Reactive Yellow F3R Joyfix Red RB |
96.30% 92.71% |
[106] |
Actinomycetes
Microorganisms, particularly actinomycetes, play a significant role in the decolorization of dyes. Several researchers have reported the decolorization of dyes using actinomycetes. Zhou and Zimmermann [97] observed 3–10% decolorization of dyes by Streptomyces species. On the other hand, in recent research, Dong et al. [119] observed 99% decolorization of dyes using Streptomyces sp. S27. In another study, Adenan et al. [120] observed 64–94.7% decolorization of different dyes using actinomycetes. They reported the decolorization of triphenylmethane dyes using Streptomyces bacillaris through biosorption and biodegradation mechanisms. Raja et al. [121] studied the decolorization of amido black dye by means of actinomycetes isolated from marine sediments under aerobic conditions. They found significant 88% decolorization at a 5 ppm dye concentration. Meanwhile, Blánquez et al. [122] observed decolorization of 6–70% using the actinomycetes Streptomyces ipomoeae CECT 3341. In another study, Kameche et al. [123] studied the decolorization of the azo dye Evans blue using four strains of Streptomyces isolated from soils. They observed a 97% remediation rate at an initial 50 mg/L Evans blue concentration.
Phycoremediation
Studies suggest that azo dyes are utilized as a carbon and energy source for algae, which are then degraded into aromatic amines, subsequently being converted into simple inorganic and organic compounds [124]. For the investigation of dye decolorization from textile wastewater samples, Chlorella species, Oscillatoria species, Phormidium species, and Synechocystis have been widely used [125]. Many functional groups like carboxy, carbonyl, hydroxy, phosphoryl, and amide groups are present in algal cell walls, which help in dye decolorization [126]. In another investigation, Mahajan et al. [127] reported 70–100% decolorization of methyl red dye at pH 3.5–9.5 using Chara vulgaris L. In another study by Boulkhessaim et al. [128], they reported 45–80% decolorization of dyes using Chlorella vulgaris, whereas Dellamatrice et al. [129] observed 91% dye decolorization using Cyanobacterium phormidium. In another study, Alprol et al. [130] found 75.7% and 61.11% decolorization of dyes by using Arthrospira platensis complete dry biomass and lipid-free biomass. Meanwhile, Mansour et al. [131] reported 93% decolorization of methylene blue using A. platensis. These studies indicate that algae application for effective remediation of dye pollution is a viable and eco-friendly option for a sustainable and green environment.
Yeast-Mediated Dye Decolorization
Yeast has not been as thoroughly studied and used in the decolorization of dyes as filamentous fungi and bacteria [132]. The removal of dyes using yeast was reported in the biosorption process [132]. The decolorization of azo dye through yeasts is achieved using the enzyme azoreductase present in yeast [133]. Galactomyces geotrichum MTCC1360 has the capacity to decolorize azo dyes [134]. Researchers like Guo et al. [135] reported 92% decolorization of the azo dye Acid Scarlet GR using the newly isolated salt-tolerant yeast strain Galactomyces geotrichum GG. In another study, Ali et al. [136] reported 82% decolorization of lignin-like dyes and wastewater containing textile dyes using a recently formed oleaginous yeast consortium with three yeast cultures: Yarrowia sp. SSA1642, Barnettozyma californica SSA1518, and Sterigmatomyces halophilus SSA1511.
Phytoremediation
Phytoremediation involves the plant-mediated treatment of contaminants from the environment, and it is a cost-effective solution for the clean-up of various types of pollutants [137,138]. This process operates through several mechanisms or processes to remediate contaminants. The different phytoremediation strategies, such as phytoextraction (uptake or absorption of contaminants by roots into the shoots for metabolization), phytostabilization (compounds secreted by the plant immobilize contaminants rather than degrade them), phytovolatilization (involves translocation of contaminants by roots to aerial plant parts where they volatilize into the atmosphere), and rhizofiltration (plant roots absorb the contaminants, which are then metabolized or stored), are widely used, and accepted as a cost-effective environmental restoration technology [139,140,141,142].
In phytoremediation, plants interact at the physical, chemical, biological, and microbial levels to reduce pollutant toxicity. This employs a variety of processes depending on the form and quantity of the pollutant [140]. In their investigation, Biju et al. [143] found 75 ± 0.5% and 82 ± 0.5% decolorization of a mixture of azo dyes using Salvinia species. Rane et al. [144] observed complete decolorization of sulfonated remazol red dye and effluents of the textile industry using Alternanthera philoxeroides. In another study, Imron et al. [145] found the decolorization of 80.56 ± 0.44% of methylene blue dye using duckweed (Lemna minor), whereas Baldawi et al. [146] observed 85% decolorization of methylene blue dye using the floating plant Azolla pinnata.
5. Recent Strategies for Remediation of Dyes
Nowadays, advanced methods using microbial fuel cells, nanotechnology, and others are showing promise for the treatment of dye pollution.
5.1. Genetically Engineered Microorganisms (GEMs)
Through genetically altered microbial strains, bioaugmentation has given a solution for effective remediation of pollutants from environment [147,148]. Genetic engineering is specifically applied to transformed microbes (like bacteria, fungi, and yeast) using molecular tools. GEMs can be employed for the bioremediation of environmental pollutants [148,149,150]. In their study, Bu et al. [151] performed dye decolorization experiments with mutant laccase Lacep69, D500G, and wild-type laccase. They found that the decolorization rate of D500G was better than that of wild-type laccase, with 78% decolorization for acid violet. In another study, Dixit and Garg [152] transferred the azoreductase enzyme-coding gene azoK from the bacteria Klebsiella pneumonia to Escherichia coli for successful 95% decolorization of azo dyes in less than 24 h. Meanwhile, Chang et al. [153] reported a 10% increase in the biodecolorization of azo dyes by engineering the E. coli genes coding for azoreductase present in Pseudomonas luteola.
5.2. Microbial Biosorbents
Biosorption is an energy-independent process, where sorption occurs with the help of biological molecules [154]. From water or soil, microbial biosorbents absorb or adsorb xenobiotic compounds such as carcinogens, drugs, metals, dyes, etc. [14,155]. In their investigation, Bayat et al. [156] reported the removal of 97% dye by using Chromochloris zofingiensis as a potential dye biosorbent. In another study, Sun et al. [157] reported a remediation efficiency of 92.2% at pH 2 by using the marine algae Enteromorpha prolifera for the biosorption of Direct Fast Scarlet 4BS from an aqueous solution. Silva et al. [158] observed a maximum biosorption capacity of 76.3359 mg/g by using natural biosorbents made from weeds for the biosorption of methylene blue dye. In another study, Alarcón et al. [159] reported 95% dye removal from wastewater from the textile dye industry at pH 2.6 by using orange seed powder as a suitable biosorbent. In a recently published review, Tripathi et al. [14] discussed the importance of microbial biosorbents for the bioremediation of heavy metals and dye-contaminated environments.
5.3. Bioreactors
Over the past two decades, membrane bioreactors have shown promising and efficient potential for treating contaminated wastewater [160] A researcher group, Manzoor et al. [161], reported 99% removal of dyes using a modified anaerobic forward osmosis membrane bioreactor (AnMBR) process. In another study, Jin et al. [162] also reported 99% removal of navy blue dye using modified AnMBR, while Castro et al. [163] reported 61% removal of reactive orange 16 using AnMBR treatment. In another investigation, Zhang et al. [164] found >90% dye removal of most azo dyes using an anaerobic flat-sheet ceramic membrane bioreactor. Bioreactor systems can be applied at a larger level for the abatement of dye pollution in ecosystems.
5.4. Nanoparticles Based Bioremediation
Dye and the degradation byproducts can have an adverse impact on the environment and people’s health. Traditional methods of dye remediation are less effective and more expensive. In recent years, nano-bioremediation has emerged as a viable alternative for the effective and environmentally friendly removal of dyes from the environment [165]. Numerous industries, including agriculture, energy, medicine, and the environment, all use nanotechnology [14,166,167]. The development of nanoparticles by environmentally friendly microbes is helpful for the nano-bioremediation of contaminants. Nanoparticles (NPs) have distinctive structural properties, which make them attractive for the treatment of textile wastewater. These NPs act as adsorbents for the successful removal of dyes. In their study, Balrabe et al. [165] reported that eosin yellowish displayed efficient removal (95%) at 75 ppm using Fe2O3 nanoparticles, pH 6, with a 15 min exposure time. In another study, Darwesh et al. [168] reported the F. oxysporum OSF18 strain-synthesized CuO-NPs immobilized in alginate beads, which exhibited 90% dye remediation efficiency. Functionalized nanomaterials may also be applied in dye removal. Rani and Shanker [169] reported the use of functionalized nanomaterials for the remediation of organic dyes. Further research is necessary to study the role of nanomaterials for large-scale in situ bioremediation.
5.5. Microbial Fuel Cells (MFCs)
Microbial fuel cells (MFC) are employed as oxidizing agents that oxidize organics in the anode and transfer electrons to reduce the terminal electron acceptor oxygen to the cathode electrode. The whole system setup includes electrode materials, external resistance and applied potentials, the initial level of azo dyes, and co-substrates [170]. In MFCs, microorganisms are used to generate bioelectricity [171]. MFCs can be characterized as (1) single-chambered and (2) double-standard. Double-standard chambers are separated by a proton exchange membrane. Substrates are oxidized anaerobically in the anode chamber by microbes to produce electrons. In their investigation, Jain and Zhen [172] reported a bio-electrochemical system treatment in which electrons (acceptors and donors) interact to achieve maximum dye remediation in contaminated wastewater by using the generation of electricity. In another study, Yang et al. [173] reported dye degradation with the use of a bio-electrical system. They concluded that microbes were used as oxidizing reagents to oxidize acetate at the anode. While Yadav et al. [174] reported that MFCs generate electricity during dye degradation. It was also reported that they have the highest efficiency of dye degradation, which is 96–100%. They also discussed the factors that can affect the production of electricity and the degradation of dye, which include bacteria, anodes, cathode membranes, cathode catalysts, and substrates. In another study, Yaqoob et al. [175] discussed the application of microbial fuel cells in remediation of environmental pollutants including dyes.
There are many factors that may affect the effectiveness of any of the processes used for the abatement of dye removal, as shown in Table 2.
Table 2.
Different factors responsible for the advanced bioremediation of dyes.
| S. No. | Factors | Advanced Oxidation Processes |
Biological Treatment |
Phytoremediation | Nanotechnology | Biotechnological Approaches |
|---|---|---|---|---|---|---|
| 1. | Mechanism | Utilizes chemical oxidation to break down dyes and contaminants through highly reactive species (e.g., hydroxyl radicals). | Microorganisms, enzymes, and natural processes degrade dyes biologically. | Involves the use of plants to uptake, metabolize, or sequester dyes from the environment. | Utilizes nanomaterials or nanoparticles to adsorb, degrade, or facilitate the removal of dyes. | Relies on genetically modified or engineered microorganisms for enhanced dye degradation. |
| 2. | Speed of Treatment |
Generally rapid and efficient in dye degradation. | Biodegradation rates can vary and may be slower than chemical oxidation. | Treatment rates can be relatively slow, influenced by plant growth and environmental conditions. | Offers fast and efficient removal through high surface area and reactivity. | Can be engineered for rapid and targeted dye degradation. |
| 3. | Selectivity | May not exhibit high selectivity and can degrade a wide range of dyes. | Microbes may exhibit selectivity towards specific dyes, affecting treatment effectiveness. | Plant species selection influences specificity, with variations in the range of dyes targeted. | Can be designed for selectivity through nanomaterial selection and modification. | Selectivity can be engineered by designing microorganisms with specific dye-degrading enzymes. |
| 4. | Environmental Impact | May generate secondary byproducts and require careful management. | Generally environmentally friendly, with lower chemical use and reduced environmental impact. | Environmentally friendly, as it relies on natural processes and plant uptake. | Impact depends on nanomaterials used, with potential environmental concerns. | Environmental impact may vary depending on genetic modifications, but aims for minimal harm. |
| 5. | Energy Requirements |
Requires energy for chemical processes, potentially energy-intensive. | Typically energy-efficient, relying on microbial metabolism or natural processes. | Minimal energy requirements, as it mainly depends on plant growth. | Energy-efficient, but energy may be required for nanoparticle synthesis. | Energy-efficient, but genetic engineering may involve energy-intensive processes. |
| 6. | Scale-Up Complexity |
Scaling up AOPs can be complex and may require advanced infrastructure. | Scaling biological treatment systems is generally feasible but can be size-dependent. | Scalability can be challenging for large-scale phytoremediation projects due to space and time requirements. | Nanotechnology can be scaled up relatively easily but requires careful engineering. | Scalability depends on the cultivation and maintenance of engineered microorganisms, which can be challenging. |
| 7. | Cost Effectiveness |
Initial setup costs can be high due to equipment and chemical requirements. | Generally cost-effective in the long run due to lower operating costs and sustainability. | Cost-effectiveness depends on factors like plant species, maintenance, and project size. | Costs may vary depending on the nanomaterials used and their availability. | Costs can be higher due to research and development, genetic modification, and monitoring. |
| 8. | Regulatory Considerations | May face regulatory scrutiny due to chemical usage and potential byproduct generation. | Typically meets regulatory compliance easily, especially for non-genetically modified organisms. | Regulatory approval depends on plant species used and potential ecological impacts. | Regulatory concerns related to nanoparticle release and toxicity may apply. | Requires regulatory approval for genetically modified organisms and potential ecological impacts. |
Table 3 presents the comparison of conventional versus advanced approaches and the advantages of recent strategies, respectively.
Table 3.
Conventional vs. advanced dye bioremediation aspects.
| S. No. | Aspects | Conventional Techniques | Advanced Techniques |
|---|---|---|---|
| 1 | Treatment Principle | Relies mainly on natural processes and microbial action. | Integrates innovative approaches and advanced materials. |
| 2 | Effectiveness | May be limited in the removal of complex and recalcitrant dyes. | Generally, more effective in breaking down a wide range of dyes. |
| 3 | Speed of Treatment | Biodegradation rates can be slow. | Often faster due to enhanced microbial activity and optimized conditions. |
| 4 | Microbial Strains | Utilizes naturally occurring microorganisms. |
May involve the use of genetically modified or engineered microorganisms. |
| 5 | Nutrient Requirements | Requires standard nutrients and conditions for microbial growth. | May require tailored nutrient supplementation for specific dye degradation. |
| 6 | pH and Temperature Control | Typically relies on ambient conditions. | Requires precise control of pH and temperature for optimal performance. |
| 7 | Dye Specificity | Some microbes may exhibit selectivity towards certain dyes. | Can target a broader range of dyes through microbial diversity or modifications. |
| 8 | Toxic Byproducts | May produce secondary pollutants or byproducts. | Tends to generate fewer toxic byproducts due to targeted degradation. |
| 9 | Resilience to Shock Loads | Vulnerable to shock loads and fluctuations in dye concentrations. | Better equipped to handle variations in dye concentrations. |
| 10 | Scale-up Challenges | Scaling up conventional bioremediation processes can be challenging. | Advanced techniques may offer more scalability and adaptability. |
| 11 | Sustainability | Moderately sustainable, but environmental impacts may vary. | Aims for increased sustainability through optimized processes. |
| 12 | Costs | Typically lower initial costs but may require longer treatment times. | May have higher initial setup costs but can be more cost-effective in the long term. |
| 13 | Regulatory Compliance | Conventional methods may require fewer regulatory approvals. | Advanced techniques may face additional regulatory scrutiny due to genetic modifications or novel materials. |
| 14 | Treatment Principle | Relies mainly on natural processes and microbial action. | Integrates innovative approaches and advanced materials. |
| 15 | Effectiveness | May be limited in the removal of complex and recalcitrant dyes. | Generally, more effective in breaking down a wide range of dyes. |
| 16 | Speed of Treatment | Biodegradation rates can be slow. | Often faster due to enhanced microbial activity and optimized conditions. |
| 17 | Selectivity | Limited selectivity and may not effectively target specific dyes | Can be engineered for selectivity targeting specific dye pollutants |
6. Challenges and Future Perspectives
Dye-containing wastewater is a major concern for the environment and human health. The most commonly used methods for color removal are chemical, physical, and biological treatments. The physicochemical methods are proven to be efficient, but they are costly. Thus, dye removal using biological methods is somewhat promising as a viable alternative to physicochemical methods. Nowadays, researchers are paying increased attention to advanced strategies of dye remediation. Microbial fuel cells have emerged as an essential and advantageous tool because they produce electricity for the remediation of pollutants and have a higher removal potential. Other approaches include nanotechnology, enzyme-mediated techniques, microbial remediation, phycoremediation, mycoremediation, and yeast-mediated remediation of dyes. The success rate and effectiveness of these methods are major challenges. The integration of these advanced methods may represent a complete solution for the abatement of dye pollution. Future studies should explore pure culture- as well as mixed culture-mediated decolorization processes in which microbial strains should be improved by utilizing genetic engineering. Further advanced research is required to study dye degradation pathways, the genetics of dye decolorizing microorganisms, and applications of recent dye treatment strategies. There is also a need to explore low-cost environmentally friendly treatment strategies for a sustainable environment.
7. Conclusions
Dye pollution is one of the major environmental problems and has negative effects on ecosystem health. As sustainable development is crucial globally, it is essential to develop a zero-waste approach, which is cost-effective too. There is a need to develop strategies for further dye remediation that are cooperative with the industries that are releasing dyes. Biological techniques can be integrated with advanced strategies to form a multifaceted, integrated, and cost-effective approach for improved dye remediation from wastewater. An appropriate environmentally friendly, simple and cost-effective strategy is needed for the efficient treatment of dye pollution. A biological approach using different microorganisms, nanotechnology, microbial fuel cells, and microbial biosorbents has great potential to remediate dyes for a cleaner and greener environment. The technical and economic viability of dye degradation methods should be critically studied. Future research is required to develop cost-effective and environmentally friendly novel biotechnological and innovative approaches for a cleaner and greener ecosystem.
Acknowledgments
Authors acknowledge their parent institutions.
Author Contributions
Conceptualization, writing—original draft preparation, reviewing and edit ing, M.T.; writing—original draft preparation, S.S., S.P., J.K., A.M., S.B. and D.G.; writing—review and editing, R.S., P.S., P.K.S., A.K.S. and N.P.; visualization, A.M. and D.G.; supervision, N.P. and M.T.; funding acquisition, M.T. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This review article received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Petersen L., Heynen M., Pellicciotti F. Freshwater resources; past, present, future. Int. Encycl. Geogr. People Earth Environ. Technol. 2016:1–11. doi: 10.1002/9781118786352.wbieg0712.pub2. [DOI] [Google Scholar]
- 2.Younis S.A., Serp P.S., Nassar H.N. Photocatalytic and biocidal activities of ZnTiO2 oxynitrite heterojunction with MOF-5 and G-C3N4: A case study for textile wastewater treatment under direct sunlight. J. Hazard. Mater. 2021;410:124562. doi: 10.1016/j.jhazmat.2020.124562. [DOI] [PubMed] [Google Scholar]
- 3.Akpomie K.G., Conradie J. Advances in application of cotton-based adsorbents for heavy metalstrapping, surface modifications and future perspectives. Ecotoxicol. Environ. Saf. 2020;201:110825. doi: 10.1016/j.ecoenv.2020.110825. [DOI] [PubMed] [Google Scholar]
- 4.Al-Tohamy R., Ali S.S., Li F., Okasha K.M., Mahmoud Y.A.G., Elsamahy T., Jiao H., Fu Y., Sun J. A critical review on the treatment of dye-containing wastewater: Ecotoxicological and health concerns of textile dyes and possible remediation approaches for environmental safety. Ecotoxicol. Environ. Saf. 2022;231:113160. doi: 10.1016/j.ecoenv.2021.113160. [DOI] [PubMed] [Google Scholar]
- 5.Kishor R., Purchase D., Saratale G.D., Saratale R.G., Ferreira L.F.R., Bilal M., Chandra R., Bharagava R.N. Ecotoxicological and health concerns of persistent coloring pollutants of textile industry wastewater and treatment approaches for environmental safety. Environ. Chem. Eng. 2021;9:105012. [Google Scholar]
- 6.Garg S.K., Tripathi M. Microbial strategies for discoloration and detoxification of azo dyes from textile effluents. Res. J. Microb. 2017;12:1–19. [Google Scholar]
- 7.Parmar S., Daki S., Bhattacharya S., Shrivastav A. Development in Wastewater Treatment Research and Processes. Elsevier; Amsterdam, The Netherlands: 2022. Microorganism: An ecofriendly tool for waste management and environmental safety; pp. 175–193. [Google Scholar]
- 8.Sharma K., Sharma P., Dhiman S.K., Chadha P., Saini H.S. Biochemical, genotoxic, histological and ultrastructural effects on liver and gills of fresh water fish Channa punctatus exposed to textile industry intermediate 2 ABS. Chemosphere. 2022;287:132103. doi: 10.1016/j.chemosphere.2021.132103. [DOI] [PubMed] [Google Scholar]
- 9.Xu M., Guo J., Suh G. Biodegradation of textile azo dye by Shewanella decolorationis S12 under micro aerophillic conditions. Appl. Microbiol. Biotechnol. 2007;76:719–726. doi: 10.1007/s00253-007-1032-7. [DOI] [PubMed] [Google Scholar]
- 10.Tripathi M., Pathak N., Chaudhary V.K., Singh P., Singh P.K., Thirumalesh B.V., Bala S., Maurya A.K., Patel N., Yadav B.K. Microbial decolorization of crystal violet dye by a native multimetal tolerant Aeromonas caviae MT-1 isolate from dye contaminated soil: Optimization and phototoxicity study. Toxicol. Int. 2023;30:83–93. doi: 10.18311/ti/2023/v30i1/31254. [DOI] [Google Scholar]
- 11.Vankar P.S. Handbook on Natural Dyes for Industrial Applications: Extraction of Dyestuff from Flowers, Leaves, Vegetables. Niir Project Consultancy Services; Delhi, India: 2016. [Google Scholar]
- 12.Elgarahy A.M., Elwakeel K.Z., Mohammad S.H., Elshoubaky G.A. A critical review of biosorption of dyes, heavy metals and metalloids from wastewater as an efficient and green process. Clean. Eng. Technol. 2021;4:100209. [Google Scholar]
- 13.Tripathi M., Kumar S., Singh D.N., Pandey R., Pathak N., Fatima H. Bioremediation of dye contaminated soil. In: Parray J.A., Hashem A., Mahmoud A.E., editors. Soil Bioremediation: An Approach towards Sustainable Technology. John Wiley and Sons; Hoboken, NJ, USA: 2021. pp. 115–142. [Google Scholar]
- 14.Tripathi M., Singh P., Singh R., Bala S., Pathak N., Singh S., Chauhan R.S., Singh P.K. Microbial biosorbent for remediation of dyes and heavy metal pollution: A green strategy for sustainable environment. Front. Microbiol. 2023;14:1168954. doi: 10.3389/fmicb.2023.1168954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sharma B., Dangi A.K., Shukla P. Contemporary enzyme based technologies for bioremediation: A review. J. Environ. Manag. 2018;210:10–22. doi: 10.1016/j.jenvman.2017.12.075. [DOI] [PubMed] [Google Scholar]
- 16.Muthu S.S. Sustainability in the Textile Industry. Springer; Berlin/Heidelberg, Germany: 2017. pp. 1–8. [Google Scholar]
- 17.Khan S., Malik A. Toxicity evaluation of textile effluents and role of native soil bacterium in biodegradation of a textile dye. Environ. Sci. Pollut. Res.-Int. 2018;25:4446–4458. doi: 10.1007/s11356-017-0783-7. [DOI] [PubMed] [Google Scholar]
- 18.Seo Y.H., Park D., Oh Y.K., Yoon S., Han J.I. Harvesting of microalgae cell using oxidised dye wastewater. Bioresour. Technol. 2015;192:802–806. doi: 10.1016/j.biortech.2015.05.074. [DOI] [PubMed] [Google Scholar]
- 19.Goswami M., Chaturvedi P., Sonwani R.K. Application of arjuna (Terminalia arjuna) seed biochor in hybrid treatment system for the bioremediation of congo red dye. Bioresour. Technol. 2020;307:123203. doi: 10.1016/j.biortech.2020.123203. [DOI] [PubMed] [Google Scholar]
- 20.Kiran S., Ashraf A., Rahmat M. Green synthesis of magnesium oxide nanoparticles using leaves of Iresine herbstii for remediation of reactive brown 9 dye. Glob. NEST J. 2022;24:291–296. [Google Scholar]
- 21.Sathishkumar K., Al Salhi M.S., Sanganyado E., Devanesan S., Arulprakash A., Rajasekar A. Sequential electrochemical oxidation and bio treatment of the azo dye congo red and textile effluent. J. Photochem. Photobiol. B. 2019;200:111655. doi: 10.1016/j.jphotobiol.2019.111655. [DOI] [PubMed] [Google Scholar]
- 22.Shetty K., Krishnakumar G. Algal and cyanobacterial biomass as potential dye biodecolorizing material: A review. Biotechnol. Lett. 2020;42:2467–2488. doi: 10.1007/s10529-020-03005-w. [DOI] [PubMed] [Google Scholar]
- 23.Prabhakar Y., Gupta A., Kaushik A. Using indigenous bacteria isolate Nesterenkonia lacusekhoensis for removal of azo dyes: A low cost friendly approach for bioremediation of textile wastewaters. Environ. Dev. Sustain. 2022;24:5344–5367. doi: 10.1007/s10668-021-01661-0. [DOI] [Google Scholar]
- 24.Zhao J., Dang Z., Muddassir M., Raza S., Zhong A., Wang X., Jin J. A new Cd(II)-based coordination polymer for efficient photocatalytic removal of organic dyes. Molecules. 2023;28:6848. doi: 10.3390/molecules28196848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zheng M., Chen J., Zhang L., Cheng Y., Lu C., Liu Y., Singh A., Trivedi M., Kumar A., Liu J. Metal organic frameworks as efficient adsorbents for drugs from wastewater. Mater. Today Commun. 2022;31:103514. doi: 10.1016/j.mtcomm.2022.103514. [DOI] [Google Scholar]
- 26.Zhong Y., Chen C., Liu S., Lu C., Liu D., Pan Y., Sakiyama H., Muddassir M., Liu J. A new magnetic adsorbent of eggshell-zeolitic imidazolate framework for highly efficient removal of norfloxacin. Dalton Trans. 2021;50:18016–18026. doi: 10.1039/D1DT03020E. [DOI] [PubMed] [Google Scholar]
- 27.Zhou S., Lu L., Liu D., Wang J., Sakiyama H., Muddassir M., Nezamzadeh-Ejhieh A., Liu J. Series of highly stable Cd(ii)-based MOFs as sensitive and selective sensors for detection of nitrofuran antibiotic. CrystEngComm. 2021;23:8043–8052. doi: 10.1039/D1CE01264A. [DOI] [Google Scholar]
- 28.Bala S., Garg D., Thirumalesh B.V., Sharma M., Sridhar K., Inbaraj B.S., Tripathi M. Recent strategies for bioremediation of emerging pollutants; a review for a green and sustainable environment. Toxics. 2022;10:484. doi: 10.3390/toxics10080484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sharma P., Quanungo K. Challenges in effluents treatment containing dye. Adv. Res. Text. Eng. 2022;7:1075. [Google Scholar]
- 30.Tripathi M., Singh D.N. Bioremediation: Challenges and Advancements. Bentham Science Publishers; Singapore: 2022. [Google Scholar]
- 31.Benkhaya S., M’rabet S., El Harfi A. Classifications, properties, recent synthesis and applications of azo dyes. Heliyon. 2020;6:e03271. doi: 10.1016/j.heliyon.2020.e03271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Benkhaya S., El Harfi S., El Harfi A. Classifications, properties and applications of textile dyes: A review. Appl. J. Environ. Eng. Sci. 2017;3:311–320. [Google Scholar]
- 33.Dhodapkar R., Rao N.N., Pande S.P., Kaul S.N. Removal of basic dyes from aqueous medium using a novel polymer. Jalshakti Bioresour. Technol. 2006;97:877–885. doi: 10.1016/j.biortech.2005.04.033. [DOI] [PubMed] [Google Scholar]
- 34.Yavuz O., Aydin A.H. Removal of direct dyes from aqueous solution using various adsorbent. Pol. J. Environ. Stud. 2006;15:155–161. [Google Scholar]
- 35.Bozic M., Kokol V. Ecological alternatives to the reduction and oxidation processes in dyeing with vat and sulfur dyes. Dyes Pigment. 2008;76:299–309. doi: 10.1016/j.dyepig.2006.05.041. [DOI] [Google Scholar]
- 36.Holkar C.R., Jadhav A.J., Pinjari D.V., Mahamuni N.M., Pandit A.B. A critical review on textile wastewater treatment: Possible approaches. J. Environ. Manag. 2016;182:351–366. doi: 10.1016/j.jenvman.2016.07.090. [DOI] [PubMed] [Google Scholar]
- 37.Mudhoo A., Ramasamy D.L., Bhatnagar A.A., Usman M., Sillanpaa M. An analysis of the versatility and effectiveness of composts for sequestering heavy metal ions, dyes and xenobiotics from soils and aqueous milieus. Ecotoxicol. Environ. Saf. 2020;197:110587. doi: 10.1016/j.ecoenv.2020.110587. [DOI] [PubMed] [Google Scholar]
- 38.Samsami S., Mohamadizaniani M., Sarrafzadeh M.H., Rene E.R., Firoozbahr M. Recent advances in the treatment of dye containing wastewater from textile industries: Overview and perspectives. Process Saf. Environ. Prot. 2020;143:138–163. doi: 10.1016/j.psep.2020.05.034. [DOI] [Google Scholar]
- 39.Abu-Nada A., Abdala A., McKay G. Removal of phenols and dyes from aqueous solutions using graphene and graphene composite adsorption; a review. J. Environ. Chem. Eng. 2021;9:105858. doi: 10.1016/j.jece.2021.105858. [DOI] [Google Scholar]
- 40.Jadhav A.C., Jadhav N.C. Suitable Technologies for Textile Wastewater Treatments. Woodhead Publishing; Sawston, UK: 2021. Treatment of textile wastewater using adsorption and adsorbents; pp. 235–373. [Google Scholar]
- 41.Hisada M., Tomizawa Y., Kaware Y. Removal kinetics of cationic azo-dye from aqueous solution by gamma- glutamic acid biosorbent contribution of adsorption and complexation/precipitation to basic orange 2 removal. J. Environ. Chem. Eng. 2019;7:103157. doi: 10.1016/j.jece.2019.103157. [DOI] [Google Scholar]
- 42.Kubra K.T., Sulman M.S., Haisan M.N. Enhanced toxic dye removal from wastewater using biodegradable polymeric natural adsorbent. J. Mol. Liquids. 2021;328:115468. doi: 10.1016/j.molliq.2021.115468. [DOI] [Google Scholar]
- 43.Saifi A., Joseph J.P., Singh A.P., Pal A., Kumar K. Complexation of an azo dye by cyclodextrins: A potential strategy for water purification. ACS Omega. 2021;6:4776–4782. doi: 10.1021/acsomega.0c05684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Briao G.V., Jahn S.L., Foletto E.L., Dotto G.L. Highly efficient and reusable mesoporous zeolite synthesized from a biopolymer for cationic dyes adsorption. Colloids Surf. A Physicochem. Eng. Asp. 2018;556:43–50. doi: 10.1016/j.colsurfa.2018.08.019. [DOI] [Google Scholar]
- 45.Madan S., Shaw R., Tiwari S., Tiwari S.K. Adsorption dynamics of congo red dye removal using ZnO2 functionalized high silica zeolite particles. J. Appl. Surf. Sci. 2019;187:907–917. doi: 10.1016/j.apsusc.2019.04.273. [DOI] [Google Scholar]
- 46.Harja M., Buema G., Bucur D. Recent advances in removal of Congo Red dye by adsorption using an industrial waste. Sci. Rep. 2022;12:6087. doi: 10.1038/s41598-022-10093-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Minisy I.M., Salahuddin N.A., Ayad M.M. Chitosan/polyaniline hybrid for the removal of cationic and anionic dyes from aqueous solutions. J. Appl. Polym. Sci. 2019;136:47056. doi: 10.1002/app.47056. [DOI] [Google Scholar]
- 48.Bober P., Minisy I.M., Acharya U., Pfleger J., Babayan V., Kazantseva N., Hodan J., Stejskal J. Conducting polymer composite aerogel with magnetic properties for organic dye removal. Synth. Met. 2020;260:116266. doi: 10.1016/j.synthmet.2019.116266. [DOI] [Google Scholar]
- 49.Ayad M.M., Amer W.A., Zaghlol S., Minisy I.M., Bober P., Stejskal J. Polypyrrole-coated cotton textile as adsorbent of methylene blue dye. Chem. Pap. 2018;72:1605–1618. doi: 10.1007/s11696-018-0442-6. [DOI] [Google Scholar]
- 50.Ahmad A., Setapar M.S.H., Chuong C.S., Khatoon A., Wani W.A., Kumar R., Rafatullah M. Recent advances in new generation dye removal technologies; novel search for approaches to reprocess wastewater. RSC Adv. 2015;5:30801–30818. doi: 10.1039/C4RA16959J. [DOI] [Google Scholar]
- 51.Xu T. Ion exchange membranes: State of this development and perspective. Memb. Sci. 2005;263:1–29. doi: 10.1016/j.memsci.2005.05.002. [DOI] [Google Scholar]
- 52.Marin N.M., Pascu L.F., Demha A., Nita-Lazar M., Badea L., Aboul-Enein H.Y. Removal of the acid orange 10 by an ion-exchange and microbiological methods. Int. J. Environ. Sci. Technol. 2019;16:6357–6366. doi: 10.1007/s13762-018-2164-2. [DOI] [Google Scholar]
- 53.Waly A.I., Khedr M.A., Ali H.M., Riad B.Y., Ahmed I.M. Synthesis and characterization of ion- exchange based on waste cotton for dye removal from wastewater. Egypt. J. Chem. 2019;62:451–468. [Google Scholar]
- 54.Bayramoglu G., Kunduzcu G., Arica M.Y. Prepartion and characterization of strong cation exchange ter polymer resin as effective adsorbent for removal of dispersed dyes. Polym. Eng. Sci. 2020;60:192–201. doi: 10.1002/pen.25272. [DOI] [Google Scholar]
- 55.Zheng Y., Yao G., Cheng Q., Yu S., Liu M., Gao C. Positively charged thin–film composite hollow fibre nanofiltration membrane for the removal of cationic dyes through submerged filtration. Desalination. 2013;328:42–50. doi: 10.1016/j.desal.2013.08.009. [DOI] [Google Scholar]
- 56.Rambabu K., Bharath G., Monash P., Velu S., Banat F., Naushad M., Arthanareeswaran G., Show P.L. Effective treatment of dye polluted wastewater using nanoporous CaCl2 modified polyethersulfone membrane. Process Saf. Environ. Prot. 2019;124:266–278. doi: 10.1016/j.psep.2019.02.015. [DOI] [Google Scholar]
- 57.Cherayan M. Ultrafiltration and Microfiltration Handbook. CRC Press; Boca Raton, FL, USA: 1998. [Google Scholar]
- 58.Collivignarelli M.C., Abba A., Carnovale M., Damiani S. Treatments for color removal from wastewater: State of the art. J. Environ. Manag. 2019;236:727–745. doi: 10.1016/j.jenvman.2018.11.094. [DOI] [PubMed] [Google Scholar]
- 59.Alventosa-deLara E., Barredo-Damas S., Alcaina-Miranda M.I., Iborasa-Ciar M.I. Ultrafiltration technology with a ceramic membrane for reactive dye removal; optimization of membrane performance. J. Hazard. Mater. 2012;209–210:492–500. doi: 10.1016/j.jhazmat.2012.01.065. [DOI] [PubMed] [Google Scholar]
- 60.Wang A.J., Wang H.C., Cheng H.Y., Liang B., Liu W.Z., Han J.L., Zhang B., Wang S.S. Electrochemistry stimulated environmental bioremediation development of applicable modular electrode and system scale up. Environ. Sci. Ecotechnol. 2020;3:100050. doi: 10.1016/j.ese.2020.100050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang X., Xia J., Ding S., Zhang S., Li M., Shang Z., Lu J., Ding J. Removing organic matters from reverse osmosis concentrate using advanced oxidation biological activated carbon process combined with Fe3+/humus–reducing bacteria. Ecotoxicol. Environ. Saf. 2020;203:110945. doi: 10.1016/j.ecoenv.2020.110945. [DOI] [PubMed] [Google Scholar]
- 62.Lu Z., Hu F., Li H., Zhang X., Yu S., Liu M., Gao C. Composite nano-filtration membrane with asymmetric selective separation layer for enhanced separation efficiency to anionic dye aqueous solution. J. Hazard. Mater. 2019;368:436–443. doi: 10.1016/j.jhazmat.2019.01.086. [DOI] [PubMed] [Google Scholar]
- 63.Shao H., Qi Y., Liang S., Qin S., Yu J. Polypropylene composite hollow fibre ultrafiltration membranes with an acrylic hydrogel surface by in-situ ultrasonic wave assisted polymerization for dye removal. J. Appl. Polym. Sci. 2019;136:47099. doi: 10.1002/app.47099. [DOI] [Google Scholar]
- 64.Fradj A.B., Boubakri A., Hafiane A., Hamouda S.B. Removal of azoic dyes from aqueous solutions by chitosan enhanced ultrafilteration. J. Result Chem. 2020;2:100017. doi: 10.1016/j.rechem.2019.100017. [DOI] [Google Scholar]
- 65.Xiang H., Ren G., Yang X., Xu D., Zhang Z., Wang X. A low cost solvent free method to synthesize alpha Fe2O3 nanoparticles with applications to degrade methyl orange in photofenton system. Ecotoxicol. Environ. Saf. 2020;200:110744. doi: 10.1016/j.ecoenv.2020.110744. [DOI] [PubMed] [Google Scholar]
- 66.Zhong J., Yang B., Feng Y., Chen Y., Wang L.-G., You W.-D., Ying G.-G. Enhanced Photo–Fenton Removal Efficiency with Core-Shell Magnetic Resin Catalyst for Textile Dyeing Wastewater Treatment. Water. 2021;13:968. doi: 10.3390/w13070968. [DOI] [Google Scholar]
- 67.Saratale R.G., Sivapathan S., Saratale G.D., Banu J.R., Kim D.S. Hydroxamic acid mediated heterogenous fenton like catalyst for efficient removal of acid red 88, textile wastewater and their phytotoxicity studies. Ecotoxicol. Environ. Saf. 2019;167:385–395. doi: 10.1016/j.ecoenv.2018.10.042. [DOI] [PubMed] [Google Scholar]
- 68.Parsons S. Advanced Oxidation Processes for Water Wastewater Treatment. IWA Publishing; London, UK: 2004. [Google Scholar]
- 69.Gogate P.R., Pandit A.B. A review of imperative technologies for wastewater treatment 1: Oxidation technologies at ambient conditions. J. Adv. Environ. Resour. 2004;8:501–551. doi: 10.1016/S1093-0191(03)00032-7. [DOI] [Google Scholar]
- 70.Ledakowicz S., Maciejewska R., Gebicka L., Perkowski J. Kinetics of decolourization by Fenton’s reagent. J. Ozone Sci. Eng. 2000;22:195–205. doi: 10.1080/01919510008547220. [DOI] [Google Scholar]
- 71.Chen Y., Cheng Y., Guan X., Liu Y., Nie J., Li C. A rapid fenton treatment of bio-treated dyeing and finishing wastewater at second scale intervals; kinetics by stopped-flow technique and application in a full scale plan. Sci. Rep. 2019;9:9689. doi: 10.1038/s41598-019-45948-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zawadzki P., Deska M. Degradation efficiency and kinetics analysis of an advance oxidation process, utilizing ozone, hydrogen peroxide and persulfate to degrade the dye rhodamine. Catalysts. 2021;11:974. doi: 10.3390/catal11080974. [DOI] [Google Scholar]
- 73.Shriram B., Kanmani S. Ozonation of Textile Dyeing Wastewater: A Review. [(accessed on 16 November 2023)];2014 2014-15:3. Available online: https://www.researchgate.net/profile/ShriramBalasubramanyan/publication/281776011_Ozonation_of_Textile_Dyeing_Wastewater_-_A_Review/links/55f7f43e08ae07629dcded7a/Ozonation-of-Textile-Dyeing-Wastewater-A-Review.pdf. [Google Scholar]
- 74.Venkatesh S., Venkatesh K., Quaff A.R. Dye decomposition by combined ozonation and anaerobic treatment: Cost effective technology. J. Appl. Res. Technol. 2017;15:340–345. doi: 10.1016/j.jart.2017.02.006. [DOI] [Google Scholar]
- 75.Cardoso R.M.F., Cardoso I.M.F., da Silva P.L., Joaguim C.G., da Silva E. Copper (2) doped carbon dots as catalyst for ozone degradation of textile dyes. Nanomaterials. 2022;12:1211. doi: 10.3390/nano12071211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lanzetta A., Papirio S., Oliva A., Cesaro A., Pucci L., Capasso E.M., Esposito G., Pirozzi F. Ozonation processes for color Removal from Urban and Leather Tanning Wastewater. Water. 2023;15:2362. doi: 10.3390/w15132362. [DOI] [Google Scholar]
- 77.Chen K.C., Wu J.Y., Liou D.J., Hwang S.C.J. Decolorization of the textile dye by newly isolated bacterial strains. J. Biotechnol. 2003;101:57–68. doi: 10.1016/S0168-1656(02)00303-6. [DOI] [PubMed] [Google Scholar]
- 78.Patel H., Yadav V.K., Yadav K.K., Choudhary N., Kalasariya H., Alam M.M., Gacem A., Amanullah M., Ibrahium H.A., Park J.W., et al. A review: A recent and systematic approach towards microbial biodegradation of dyes from textile industries. Water. 2022;14:3163. doi: 10.3390/w14193163. [DOI] [Google Scholar]
- 79.Das S., Cherwoo L., Singh R. Decoding dye degradation: Microbial remediation of textile industry effluents. Biotechnol. Notes. 2023;4:64–76. doi: 10.1016/j.biotno.2023.10.001. [DOI] [Google Scholar]
- 80.Pourbabaee A.A., Malakzadeh F., Sarbolouki M.N., Najafi F. Aerobic decolorization and detoxification of disperse dye in textile effluent by a new isolate of Bacillus spp. Biotechnol. Bioeng. 2006;93:631–635. doi: 10.1002/bit.20732. [DOI] [PubMed] [Google Scholar]
- 81.Bhandari S., Poudel D.K., Marahatha R., Dawadi S., Khadaya K., Phuyal S. Microbial enzymes used in bioremediation. J. Chem. 2021;2021:8849512. doi: 10.1155/2021/8849512. [DOI] [Google Scholar]
- 82.Pham V.H.T., Kim J., Chang S., Bang D. Investigating bio-inspired degradation of toxic dyes using potential multi-enzyme producing extremophiles. Microorganisms. 2023;11:1273. doi: 10.3390/microorganisms11051273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mendes S., Robalo M.P., Martins L.O. Microbial Degradation of Synthetic Dyes in Wastewater. Springer International Publishing; Berlin/Heidelberg, Germany: 2015. Bacterial enzymes and multi-enzymatic systems for cleaning-up dyes from the environment; pp. 27–55. [Google Scholar]
- 84.Chandra R. Environmental Waste Management. CRC Press; Boca Raton, FL, USA: 2016. [Google Scholar]
- 85.Hussain Q. Peroxidase mediated decolourization and remediation of wastewater containing industrial dyes: A review. Rev. Environ. Sci. Biotechnol. 2010;9:117–140. doi: 10.1007/s11157-009-9184-9. [DOI] [Google Scholar]
- 86.Lellis B., Polonio C.Z.F., Pamphile J.A., Polonio J.C. Effects of textile dyes on health and the environment and bioremediation potential of living organisms. Biotechnol. Res. Innov. 2019;3:275–290. doi: 10.1016/j.biori.2019.09.001. [DOI] [Google Scholar]
- 87.Pinheiro L.R.S., Gradíssimo D.G., Xavier L.P., Santos A.V. Degradation of azo dyes: Bacterial potential for bioremediation. Sustainability. 2022;14:1510. doi: 10.3390/su14031510. [DOI] [Google Scholar]
- 88.Shahid M., Mahmood F., Hussain S., Shahzad T., Haider M.Z., Noman M., Mushtaq A., Fatima Q., Ahmed T., Mustafa G. Enzymatic detoxification of azo dyes by a multifarious Bacillus sp. strain MR-1/2-bearing plant growth-promoting characteristics. 3 Biotech. 2018;8:425. doi: 10.1007/s13205-018-1442-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Vineh M.B., Saboury A.A., Poostchi A.A., Ghasemi A. Biodegradation of phenol and dyes with horseradish peroxidase covalently immobilized on functionalized RGO-SiO2 nano composite. Int. J. Biol. Macromol. 2020;164:4403–4414. doi: 10.1016/j.ijbiomac.2020.09.045. [DOI] [PubMed] [Google Scholar]
- 90.Navas L.E., Carballo R., Levin L., Berretta M.F. Fast decolorization of azo dyes in alkaline solutions by a thermostable metal-tolerant bacterial laccase and proposed degradation pathways. Extremophiles. 2020;24:705–719. doi: 10.1007/s00792-020-01186-w. [DOI] [PubMed] [Google Scholar]
- 91.Kalsoom U., Bhatti H.N., Aftab K., Amin F., Jesioncowski T., Bilal M. Biocatalytic potential of Brassica oleracea L. var. botrytis leaves peroxidase for efficient degradation of textile dyes in aqueous medium. J. Bioprocess. Biosyst. Eng. 2023;46:453–465. doi: 10.1007/s00449-022-02820-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gao Y., Wang M., Shah K., Kalra S.S., Rome L.H., Mahendra S. Decolourization and detoxification of synthetic dye compounds by laccase immobilized in vault nanoparticles. Bioresour. Technol. 2022;351:127040. doi: 10.1016/j.biortech.2022.127040. [DOI] [PubMed] [Google Scholar]
- 93.Zimmermann T., Kulla H.G., Leisinger T. Properties of purified orange II azoreductase, the enzyme initiating azo dye degradation by Pseudomonas KF46. Eur. J. Biochem. 1982;129:197–203. doi: 10.1111/j.1432-1033.1982.tb07040.x. [DOI] [PubMed] [Google Scholar]
- 94.Georgiou D., Hatiras J., Aivasidis A. Microbial immobilization in a two-stage fixed-bed-reactor pilot plant for on-site anaerobic decolorization of textile wastewater. J. Enzym. Microb. Technol. 2005;37:597–605. doi: 10.1016/j.enzmictec.2005.03.019. [DOI] [Google Scholar]
- 95.Jadhav S.B., Phugare S.S., Patil P.S., Jadhav J.P. Biochemical degradation pathway of textile dye removal red and subsequent toxicological evaluation by cytotoxicity, genotoxicity and oxidative stress studies. Int. Biodeterior. Biodegrad. 2011;65:733–743. doi: 10.1016/j.ibiod.2011.04.003. [DOI] [Google Scholar]
- 96.Phugare S.S., Kalyani D.C., Surwase S.N., Jadhav J.P. Ecofriendly degradation, decolorization and detoxification of textile effluent by a developed bacterial consortium. Ecotoxicol. Environ. Saf. 2011;74:1288–1296. doi: 10.1016/j.ecoenv.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 97.Zhou W., Zimmermann W. Decolorization of industrial effluents containing reactive dyes by actinomycetes. FEMS Microbiol. Lett. 1993;107:157–161. doi: 10.1111/j.1574-6968.1993.tb06023.x. [DOI] [PubMed] [Google Scholar]
- 98.Saratale R.G., Saratale G.D., Cheng J.S., Govindwar S.P. Bacterial decolorization and degradation of azo dyes. A review. J. Taiwan Inst. Chem. Eng. 2011;42:138–157. doi: 10.1016/j.jtice.2010.06.006. [DOI] [Google Scholar]
- 99.Khan R., Bhawan P., Fulekar M.H. Microbiol decolourization and degradation of synthetic dyes: A review. Rev. Environ. Sci. Bio/Technol. 2013;12:75–97. doi: 10.1007/s11157-012-9287-6. [DOI] [Google Scholar]
- 100.Yang H.Y., Jia R.B., Chen B., Li L. Degradation of recalcitrant aliphatic and aromatic hydrocarbons by a dioxin- degrader Rhodococcus sp. strain p52. Environ. Sci. Pollut. Res. 2014;21:11086–11093. doi: 10.1007/s11356-014-3027-0. [DOI] [PubMed] [Google Scholar]
- 101.Nguyen T.A., Fu C.C., Juang R.S. Biosorption and biodegradation of a sulphur dye in high-strength dyeing wastewater by Acidithiobacillus thiooxidans. J. Environ. Manag. 2016;182:265–271. doi: 10.1016/j.jenvman.2016.07.083. [DOI] [PubMed] [Google Scholar]
- 102.Louati I., Ellaumi-Mseddi J., Cheikhrouhou W., Hadrich B., Nasri M., Aifa S., Woodword S., Mechichi T. Simultaneous cleanup of reactive black 5 and cadmium by a desert soil bacterium. J. Ecotoxicol. Environ. Saf. 2020;190:110103. doi: 10.1016/j.ecoenv.2019.110103. [DOI] [PubMed] [Google Scholar]
- 103.Montanez-Barragan B., Sanz-Martin J.L., Gutierrez-Macias P., Barragan-Huerta B.E. Azo-dyes decolourization under high alkalinity and salinity conditions by Halomonas sp. in batch and packed bed reactor. J. Extrem. 2020;20:239–247. doi: 10.1007/s00792-019-01149-w. [DOI] [PubMed] [Google Scholar]
- 104.Shi Y., Yang Z., Xing L., Zhou J., Ren J., Ming L., Hun Z., Li X., Zhang D. Ethanol as an efficient cosubstrate for biodegradation of azo dyes by Providencia rettgeri, mechanistic analysis based on kinetics, pathways and genomics. Bioresour. Technol. 2021;31:124117. doi: 10.1016/j.biortech.2020.124117. [DOI] [PubMed] [Google Scholar]
- 105.Fareed A., Zaffar H., Bilal M., Hussain J., Jackson C., Naqvi T.A. Decolourization of azo-dyes by a novel aerobic bacterial strain Bacillus cereus strain ROC. PLoS ONE. 2022;17:e0269559. doi: 10.1371/journal.pone.0269559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Srinivasan S., Bankole P.O., Sadasivam S.K. Biodecolorization and degradation of textile azo dyes using Lysinibacilllus sphaericus MTCC9523. Front. Environ. Sci. 2022;10:990855. doi: 10.3389/fenvs.2022.990855. [DOI] [Google Scholar]
- 107.Du L., Wu H., Li G., Wei Y., Wang F., Xu L., Dong X. Efficient degradation and decolorization of triphenyl methane dyes by Serratia sp. WKD under extreme environmental conditions and the mechanism. Int. Biodeterior. Biodegrad. 2023;179:105565 [Google Scholar]
- 108.Joshi A., Hinsu A., Kothari R. Evaluating the efficacy of bacterial consortium for decolourization of diazo dye mixture. J. Arch. Microbiol. 2022;204:515. doi: 10.1007/s00203-022-03108-0. [DOI] [PubMed] [Google Scholar]
- 109.Ayed L., Ladhari N., Achour S., Chaieb K. Decolorization of reactive yellow 174 dye in real textile wastewater by active consortium; experimental factorial design for bioremediation process optimization. J. Text. Inst. 2020;112:1449–1459. doi: 10.1080/00405000.2020.1824416. [DOI] [Google Scholar]
- 110.Guo G., Hao J., Tian F., Liu C., Ding K., Xu J., Zhou W., Guan Z. Decolourization and detoxification of azo dyes by halo-alkaliphilic bacterium consortium: Systematic investigations of performance, pathway and metagenome. J. Ecotoxicol. Environ. Saf. 2020;204:111073. doi: 10.1016/j.ecoenv.2020.111073. [DOI] [PubMed] [Google Scholar]
- 111.Bera S.P., Shah M.P., Godhaniya M. Microbial remediation of textile dye acid orange by a novel bacterial consortium SPB 92. Front. Environ. Sci. 2022;10:930616. doi: 10.3389/fenvs.2022.930616. [DOI] [Google Scholar]
- 112.Neihsial R., Singha N.A., Singh A.K. Taxonomic diversity and predictive metabolic functions of a heavy metal tolerant multiple azo dye degrading bacterial consortium from textile effluents. Int. Biodeterior. Biodegrad. 2022;171:105421. doi: 10.1016/j.ibiod.2022.105421. [DOI] [Google Scholar]
- 113.Barathi S., Aruljothi K.N., Karthik C., Padikasan I.A., Ashokkumar V. Biofilm mediated decolorization and degradation of reactive red 170 dye by the bacterial consortium isolated from the dyeing industry wastewater sediments. Chemosphere. 2022;286:131914. doi: 10.1016/j.chemosphere.2021.131914. [DOI] [PubMed] [Google Scholar]
- 114.Franca R.D.G., Viera A., Corvalho G., Oehmen A., Pinheiro H.M., Barreto Crespo M.T., Lourence N.D. Oerskovia paurometabola can efficiently decolourize azo dyes Acid Red 14 and remove its recalcitrant metabolite. J. Ecotoxicol. Environ. Saf. 2020;191:110007. doi: 10.1016/j.ecoenv.2019.110007. [DOI] [PubMed] [Google Scholar]
- 115.Saravanan S., Carolina C F., Kumar P.S., Chitra B., Rangasamy G. Biodegradation of textile dye rhodamine-B by Brevundimonas diminuta and screening of their breakdown metabolites. Chemosphere. 2022;308:136–266. doi: 10.1016/j.chemosphere.2022.136266. [DOI] [PubMed] [Google Scholar]
- 116.Gianolini J.E., Britas C.N., Mulreedy C.B., Trelles J.A. Hyperstabilization of a thermophile bacterial laccase and it’s application for industrial dyes degradation. 3 Biotech. 2020;10:208. doi: 10.1007/s13205-020-02277-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rajashekarappa K.K., Mahadevan G.M., Neelagund S.E., Madhuri Sathynarayana M., Vijaya D., Mulla S.I. Decolorization of amaranth RI and fast red E azo dyes by thermophilic Geobacillus thermoleovorans KNG 112. J. Chem. Technol Biotech. 2022;97:482–489. doi: 10.1002/jctb.6834. [DOI] [Google Scholar]
- 118.Ebihara K., Yoshikawa J., Horiguchi H., Amochi S. Decolorization of cationic dyes under alkaline conditions by Iodidimonas sp. Q-1, multicopper oxidase. J. Biosci. Bioeng. 2022;133:323–328. doi: 10.1016/j.jbiosc.2022.01.007. [DOI] [PubMed] [Google Scholar]
- 119.Dong H., Guo T., Zhang W., Ying H., Wang P., Wang Y., Chen Y. Biochemical characterization of a novel azo-reductase from Streptomyces sp. application in eco-friendly decolorization of azo-dyes wastewater. J. Int. J. Biol. Macromol. 2019;140:1037–1046. doi: 10.1016/j.ijbiomac.2019.08.196. [DOI] [PubMed] [Google Scholar]
- 120.Adenan N.H., Lim Y.Y., Ting A.S.Y. Removal of triphenylmethane dyes by Streptomyces bacillaris: A study on decolorization, enzymatic reaction and toxicity of treated dye solution. J. Environ. Manag. 2022;318:115520. doi: 10.1016/j.jenvman.2022.115520. [DOI] [PubMed] [Google Scholar]
- 121.Raja M.M.M., Raja A., Salique S.M., Gajalakshmi P. Studies on effect of marine actinomycetes on amido black (azo dye) decolorization. J. Chem. Pharm. Res. 2016;8:640–644. [Google Scholar]
- 122.Blánquez A., Rodríguez J., Brissos V., Mendes S., Martins L.O., Ball A.S., Arias M.E., Hernández M. Decolorization and detoxification of textile dyes using a versatile Streptomyces laccase-natural mediator system. Saudi J. Biol. Sci. 2019;26:913–920. doi: 10.1016/j.sjbs.2018.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kameche K., Amrani S., Mouzaoui S., Aït-Amar H. Biodegradation of diazo dye Evans blue by four strains of Streptomyces isolated from soils of Algeria. Biocatal. Agric. Biotechnol. 2022;46:102529. doi: 10.1016/j.bcab.2022.102529. [DOI] [Google Scholar]
- 124.Jamee R., Siddique R. Biodegradation of synthetic dyes of textile effluent by micro-organisms: An environmentally and economically sustainable approach. Eur. J. Microb. Immunol. 2019;9:114–118. doi: 10.1556/1886.2019.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gangola S., Bhatt P., Chaudhary P., Khati P., Kumar N., Sharma A. Microbial Biotechnology in Environmental Monitoring and Cleanup. IGI Global; Hershey, PA, USA: 2018. Bioremediation of industrial waste using microbial diversity; pp. 1–27. [Google Scholar]
- 126.Mahalakshmi S., Lakshmi D., Menaga U. Biodegradation of different concentration of dye (congo red dye) by using green and blue-green algae. Int. Environ. Res. 2015;9:735–744. [Google Scholar]
- 127.Mahajan P., Kaushal J. Phytoremediation of azo dye methyl red by microalgal Chara vulgaris L.: Kinetics and equilibrium studies. Environ. Sci. Pollut. Res. 2020;27:26406–26418. doi: 10.1007/s11356-020-08977-w. [DOI] [PubMed] [Google Scholar]
- 128.Boulkhessaim S., Gacem A., Khan S.H., Amari A., Yadav V.K., Harharah H.N., Elkhaleefa A.M., Yadav K.K., Rather S., Ahn H.J., et al. Emerging trends in the remediation of persistent organic pollutants using nanomaterials and related processes: A review. Nanomaterials. 2022;12:2148. doi: 10.3390/nano12132148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Dellamatrice P.M., Silva-Stenico M.E., de Moraes L.A.B., Fiore M.F., Monteiro R.T.S. Degradation of textile dyes by cyanobacteria. Braz. J. Microbiol. 2017;48:25–31. doi: 10.1016/j.bjm.2016.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Alprol A.E., Heneash A.M.M., Ashour M., Abulnaza K.M., Alhashmialmeer D., Mansour A.T., Sharawi Z.Z., Abu-Saled M.A., Abomohra A.E.F. Potential applications of Arthrospira platensis lipid free biomass in bioremediation of organic dye from industrial textile effluents and its influence on marine rotifer (Brachionus plicatilis) Materials. 2021;16:4446. doi: 10.3390/ma14164446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Mansour A.T., Alprol A.E., Abualnaja K.M., El-Beltagi H.S., Ramadan K.M.A., Ashour M. The using of nano-particles of microalgae in remediation of toxic dye from industrial wastewater: Kinetic and isotherm studies. Materials. 2022;15:3922. doi: 10.3390/ma15113922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ngo A.C.R., Tischler D. Microbial Degradation of azo dyes: Approaches and prospects for a hazard-free conversion by microorganisms. Int. J. Environ. Res. Public Health. 2022;19:4740. doi: 10.3390/ijerph19084740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Jafari N., Soudib M.R., Kasra-Kermanshahi R. Biodegradation perspectives of azo dyes by yeasts. Microbiology. 2014;83:184–197. doi: 10.1134/S0026261714050130. [DOI] [Google Scholar]
- 134.Jadhav S.U., Kalme S.D., Govindwar S.P. Biodegradation of Methyl red by Galactomyces geotrichum MTCC 1360. Int. Biodet. Biodeg. 2008;62:135–142. doi: 10.1016/j.ibiod.2007.12.010. [DOI] [Google Scholar]
- 135.Guo G., Tian F., Zhao Y., Tang M., Liu W., Liu C., Xue S., Kong W., Sun Y., Wang S. Aerobic decolorization and detoxification of acid scarlet GR by a newly isolated salt-tolerant yeast strain Galactomyces geotrichum GG. Int. Biodeterior. Biodegrad. 2019;145:104818. doi: 10.1016/j.ibiod.2019.104818. [DOI] [Google Scholar]
- 136.Ali S.S., Al-Tohamy R., Koupra E., EL-Naggar A.H., Kornaros M., Sun J. Valorizing lignin-like dyes and textile dyeing wastewater by a newly constructed lipid producing and lignin modifying olraginous yeast consortioum valued for biodiesel and bioremediation. J. Hazard. Mater. 2021;403:123575. doi: 10.1016/j.jhazmat.2020.123575. [DOI] [PubMed] [Google Scholar]
- 137.Salt D.E., Smith R.D., Raskin I. Phytoremediation. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1998;49:643–668. doi: 10.1146/annurev.arplant.49.1.643. [DOI] [PubMed] [Google Scholar]
- 138.Greipsson S. Phytoremediation. Nat. Educ. Knowl. 2011;3:7. [Google Scholar]
- 139.Viana D.G., Filho F.B.E., Pires F.R., Soares M.B., Ferreira A.D., Bonomo R., Martins L.F. In situ barium phytoremediation in flooded soil using Typha domingensis under different planting densities. Ecotoxicol. Environ. Saf. 2021;210:111890. doi: 10.1016/j.ecoenv.2021.111890. [DOI] [PubMed] [Google Scholar]
- 140.Wei Z., Van Le Q., Peng W., Yang Y., Yang H., Gu H., Lam S.S., Sonne C. A review on phytoremediation of contaminants in air, water and soil. J. Hazard. Mater. 2021;403:123658. doi: 10.1016/j.jhazmat.2020.123658. [DOI] [PubMed] [Google Scholar]
- 141.Kafle A., Timilsina A., Gautam A., Adhikari K., Bhattarai A., Aryal N. Phytoremediation: Mechanisms, plant selection and enhancement by natural and synthetic agents. Environ. Adv. 2022;8:100203. doi: 10.1016/j.envadv.2022.100203. [DOI] [Google Scholar]
- 142.Alsafran M., Usman K., Ahmed B., Rizwan M., Saleem M.H., Al Jabri H. Understanding the phytoremediation mechanisms of potentially toxic elements: A proteomic overview of recent advances. Front. Plant Sci. 2022;13:881242. doi: 10.3389/fpls.2022.881242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Biju L.M., Gayathri K.V., Kumar P.S., Kavitha R., Rajagopal R., Ranasamy G. Application of Salvinia species in remediation of reactive mix azo-dyes and Cr- its pathway eleucidation. Environ. Res. 2023;216:114635. doi: 10.1016/j.envres.2022.114635. [DOI] [PubMed] [Google Scholar]
- 144.Rane N.R., Chandanshive V.V., Watharkar A.D., Khandare R.V., Patil T.S., Pawar P.K., Govindwar S.P. Phytoremediation of sulfonated remazol red dye and textile effluents by Alternanthera philoxeroides: An anatomical, enzymatic and pilot scale study. Water Res. 2015;83:271–281. doi: 10.1016/j.watres.2015.06.046. [DOI] [PubMed] [Google Scholar]
- 145.Imron M.F., Kurniawan S.B., Soegianto A., Wahyudianto F.E. Phytoremediation of methylene blue using duckweed (Lemna minor) Heliyon. 2019;5:e02206. doi: 10.1016/j.heliyon.2019.e02206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Baldawi I.A.A., Abdullah S.R.S., Anuar N., Hasan H.A. Phytotransformation of methylene blue from water using aquatic plant (Azolla pinnata) Environ. Technol. Innov. 2018;11:15–22. doi: 10.1016/j.eti.2018.03.009. [DOI] [Google Scholar]
- 147.Mishra B., Varjani S., Pradhan I., Ekambaram N., Teixeira J.A., Ngo H.H., Guo W. Insights into inter-disciplinary approaches for bioremediation of organic pollutants: Innovations, challenges and perspectives. Proc. Natl. Acad. Sci. India B Biol. Sci. 2020;90:951–958. doi: 10.1007/s40011-020-01187-x. [DOI] [Google Scholar]
- 148.Liu L., Bilal M., Duan X., Iqbal H.M. Mitigation of environmental pollution by genetically engineered bacteria- current challenges and future perspectives. Sci. Total Environ. 2019;667:444–454. doi: 10.1016/j.scitotenv.2019.02.390. [DOI] [PubMed] [Google Scholar]
- 149.Jafari M., Danesh Y.R., Goltapeh E.M., Varma A. Fungi as Bioremediators. Springer; Berlin/Heidelberg, Germany: 2013. Bioremediation and genetically modified organisms; pp. 433–451. [Google Scholar]
- 150.Sanghvi G., Thanki A., Pandey S., Singh N.K. Bioremediation of Pollutants. Elsevier; Amsterdam, The Netherlands: 2020. Engineered bacteria for bioremediation; pp. 359–374. [Google Scholar]
- 151.Bu T., Yang R., Zheng Y., Cai Y., Tang Z., Li C., Wu Q., Chen H. Improving decolorization of dyes by laccase from Bacillus licheniformis by random and site directed mutagenesis. PeerJ. 2020;8:e10267. doi: 10.7717/peerj.10267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Dixit S., Garg S. Biodegradation of environmentally hazardous azo-dyes and aromatic amines using Klebsiella pneumoniae. J. Environ. Eng. 2018;144:04018035. doi: 10.1061/(ASCE)EE.1943-7870.0001353. [DOI] [Google Scholar]
- 153.Chang J.S., Kuo T.S., Chao V.P., Ho J.Y., Lin P.J. Azo dye decolorization with a mutant E. coli strain. Biotechnol. Lett. 2000;22:807–812. doi: 10.1023/A:1005624707777. [DOI] [Google Scholar]
- 154.Fomina M., Gadd G.M. Biosorption current perspectives can concept, definition and applications. Bioresour. Technol. 2014;160:3–14. doi: 10.1016/j.biortech.2013.12.102. [DOI] [PubMed] [Google Scholar]
- 155.Kour D., Kaur T., Devi R., Yadav A., Singh M., Joshi D. Beneficial mirobiomes for bioremediation of diverse contaminated environments for environmental sustainability: Present status and future challenges. Environ. Sci. Pollut. Res. 2021;28:24917–24939. doi: 10.1007/s11356-021-13252-7. [DOI] [PubMed] [Google Scholar]
- 156.Bayat M., Salehi E., Mahdieh M. Chromochloris zofingiensis microalgae as a potential dye absorbent; adsorption thermo-kinetic, isothermal and process optimization. Algal Res. 2023;71:103043. doi: 10.1016/j.algal.2023.103043. [DOI] [Google Scholar]
- 157.Sun W., Sun W., Wang Y. Biosorption of direct fast scarlet 4BS from aqueous solution using the green tide causing marine algae Enteromorpha prolifera. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019;223:117347. doi: 10.1016/j.saa.2019.117347. [DOI] [PubMed] [Google Scholar]
- 158.Silva F., Nascimento L., Brito M., Silva K., Paschoal W., Jr., Fujiyama R. Biosorption of methylene blue dye using natural biosorbents made from weeds. Materials. 2019;12:2486. doi: 10.3390/ma12152486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Flores Alarcon M.A.D., Pacheco C.R., Bustos K.G., Meza K.T., Teran H.F., Tanaka D.A.P., Andrade G.I.C., Teran H.R. Efficient dye removal from real textile wastewater using orange seed powder as suitable bioadsorbent and membrane technology. Water. 2022;14:4104. doi: 10.3390/w14244104. [DOI] [Google Scholar]
- 160.Elmoutez S., Abushaban A., Necibi M.C., Sillanpaa M., Liu J., Dhiba D., Chehbouni A., Taky M. Design and operational aspects of anaerobic bioreactor for efficient wastewater treatment and biogas production. J. Environ. Chall. 2023;10:100671. doi: 10.1016/j.envc.2022.100671. [DOI] [Google Scholar]
- 161.Manzoor K., Khan S.J., Yasmeen M., Jamal Y., Arshad M. Assessment of anaerobic membrane distillation bioreactor hybrid system at mesophilic and thermophilic temperature treating textile wastewater. J. Water Process Eng. 2022;46:102603. doi: 10.1016/j.jwpe.2022.102603. [DOI] [Google Scholar]
- 162.Jin Y., Wang D., Zhang W. Newly designed hydrolysis acidification flat ceramic membrane bioreactor for treating high-strength dyeing wastewater. Int. J. Environ. Res. Public Health. 2019;16:777. doi: 10.3390/ijerph16050777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Castro F.D., Bassin J.P., Alves P.L.M., Sant’Anna G.L., Dezotti M. Reactive orange 16 dye degradation in anaerobic and aerobic MBBR coupled with ozonation; addressing pathways and performance. Int. J. Environ. Sci. Technol. 2021;18:1991–2010. doi: 10.1007/s13762-020-02983-8. [DOI] [Google Scholar]
- 164.Zhang W., Liu F., Wang D., Jin Y. Impact of reactor configuration on treatment performance and microbial diversity in treating high- strength dyeing wastewater. anaerobic flat- sheet ceramic membrane bioreactor versus upflow anaerobic sludge blanket reactor. Bioresour. Technol. 2018;269:269–275. doi: 10.1016/j.biortech.2018.08.126. [DOI] [PubMed] [Google Scholar]
- 165.Balrabe Y.B., Oumarou I.N.M., Korony S.A., Adjama I., Baraze I.R.A. Photooxidation of organic dye by Fe2O3 nanoparticles; catalyst, electron acceptor and polyurethane membrane effects. J. Nanotechnol. 2023;2023:1292762. [Google Scholar]
- 166.Garg D., Sridhar K., Stephen Inbaraj B., Chawla P., Tripathi M., Sharma M. Nano-biofertilizer formulations for agriculture: A systematic review on recent advances and prospective applications. Bioengineering. 2023;10:1010. doi: 10.3390/bioengineering10091010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Bala S., Sharma M., Dashora K., Siddiqui S., Diwan D., Tripathi M. Nanomaterials based sustainable bioenergy production systems: Current trends and future prospects. Nanofabrication. 2022;7:314–324. doi: 10.37819/nanofab.007.253. [DOI] [Google Scholar]
- 168.Darwesh O.M., Li H., Matter I.A. Nano-bioremediation of textile industry wastewater using immobilized CuO-NPs myco-synthesized by a novel Cu-resistant Fusarium oxysporum OSF18. Environ. Sci. Pollut. Res. 2023;30:16694–16706. doi: 10.1007/s11356-022-23360-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Rani M., Shanker U. Removal of organic dyes by functionalized nanomaterials. In: Shanker U., Hussain C.M., Rani M., editors. Handbook of Green and Sustainable Nanotechnology. Springer; Cham, Switzerland: 2023. pp. 1267–1298. [DOI] [Google Scholar]
- 170.Sun L., Mo Y., Zhang L. A mini review on bio-electrochemical systems for the treatment of azo-dye wastewater: State-of-the-art and future prospects. Chemosphere. 2022;294:133801. doi: 10.1016/j.chemosphere.2022.133801. [DOI] [PubMed] [Google Scholar]
- 171.Das A. Dye degradation using microbial fuel cell: A critical review. Int. J. Res. Appl. Sci. Eng. Technol. 2022;10:1745–1754. doi: 10.22214/ijraset.2022.47714. [DOI] [Google Scholar]
- 172.Jain A., Zhen H. Cathode-enhanced wastewater treatment in bio electro chemical system. Clean Water. 2018;1:23. doi: 10.1038/s41545-018-0022-x. [DOI] [Google Scholar]
- 173.Yang M., Korneel R., Reni A., Rozendal R.A., Yuan Z., Keller J. Decolorization of azo dyes in bioelecrochemical systems. Environ. Sci. Technol. 2009;43:5137–5143. doi: 10.1021/es900057f. [DOI] [PubMed] [Google Scholar]
- 174.Yadav A., Kumar P., Rawat D., Gara S., Mukherjee P., Farooqi F., Roy A., Sundaram S., Sharma R.S., Mishra V. Microbial fuel cells for mineralization and decolorization of azo dyes; recent advances in design and materials. Sci. Total Environ. 2022;826:154038. doi: 10.1016/j.scitotenv.2022.154038. [DOI] [PubMed] [Google Scholar]
- 175.Yaqoob A.A., Khatoon A., Mohd Setapar S.H., Umar K., Parveen T., Mohamad Ibrahim M.N., Ahmad A., Rafatullah M. Outlook on the role of microbial fuel cells in remediation of environmental pollutants with electricity generation. Catalysts. 2020;10:819. doi: 10.3390/catal10080819. [DOI] [Google Scholar]
- 176.Srivastava A., Rani R.M., Patle D.S., Kumar S. Emerging bioremediation technologies for the treatment of textile wastewater containing synthetic dyes: A comprehensive review. J. Chem. Technol. Biotechnol. 2022;97:26–41. doi: 10.1002/jctb.6891. [DOI] [Google Scholar]
- 177.Singh S., Kumar V., Datta S., Dhanjal D.S., Sharma K., Samuel J., Singh J. Current advancement and future prospect of biosorbents for bioremediation. Sci. Total Environ. 2020;709:135895. doi: 10.1016/j.scitotenv.2019.135895. [DOI] [PubMed] [Google Scholar]
- 178.Chatterjee S., Kumari S., Rath S., Das S. Microbial Biodegradation and Bioremediation. Elsevier; Amsterdam, The Netherlands: 2022. Prospects and scope of microbial bioremediation for the restoration of the contaminated sites; pp. 3–31. [Google Scholar]
- 179.Shah M., Ahmed S. Green Nanoremediation: Sustainable Management of Environmental Pollution. Springer International Publishing; Cham, Switzerland: 2023. Conventional strategies of bioremediation versus green nanoremediation; pp. 333–356. [Google Scholar]
- 180.Reddy S., Osborne J. An insight on the advancements of biological technologies in the bioremediation of textile effluents. Urban Water J. 2022;19:468–480. doi: 10.1080/1573062X.2022.2030369. [DOI] [Google Scholar]
- 181.Yogalakshmi K.N., Das A., Rani G., Jaswal V., Randhawa J.S. Nano-bioremediation: A new age technology for the treatment of dyes in textile effluents. Bioremediat. Ind. Waste Environ. Saf. Ind. Waste Its Manag. 2020;1:313–347. [Google Scholar]
- 182.Ihsanullah I., Jamal A., Ilyas M., Zubair M., Khan G., Atieh M.A. Bioremediation of dyes: Current status and prospects. J. Water Process Eng. 2020;38:101680. doi: 10.1016/j.jwpe.2020.101680. [DOI] [Google Scholar]
- 183.Sudarshan S., Harikrishnan S., RathiBhuvaneswari G., Alamelu V., Aanand S., Rajasekar A., Govarthanan M. Impact of textile dyes on human health and bioremediation of textile industry effluent using microorganisms: Current status and future prospects. J. Appl. Microbiol. 2023;134:lxac064. doi: 10.1093/jambio/lxac064. [DOI] [PubMed] [Google Scholar]
- 184.Nachiyar C.V., Rakshi A.D., Sandhya S., Jebasta N.B.D., Nellore J. Developments in treatment technologies of dye-containing effluent: A review. Stud. Chem. Case Environ. Eng. 2023;7:100339. doi: 10.1016/j.cscee.2023.100339. [DOI] [Google Scholar]
- 185.Nishshanka G.K.S.H., Thevarajah B., Nimarshana P.H.V., Prajapati S.K., Ariyadasa T.U. Real-time integration of microalgae-based bioremediation in conventional wastewater treatment plants: Current status and prospects. J. Water Process Eng. 2023;56:104248. doi: 10.1016/j.jwpe.2023.104248. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.