Abstract
Background
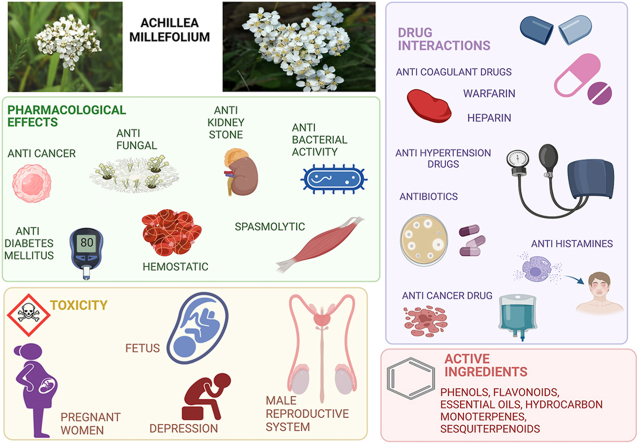
Achillea millefolium, known as Yarrow, is a medicinal plant in the Asteraceae family which is one of the oldest known botanicals used by humans and itis one of the most important medicinal plants in the pharmaceutical field. Purpose: This review discusses pharmacodynamics, pharmacokinetics, and mechanism of action of the most important component of Achillea millefolium. There are a variety of same species such as white, red and yellow yarrow and all of these species have been discussed in this manuscript. We focus on previously discovered hormonal, antibiotic, and anticancer drug interactions with Achillea millefolium that may decrease or increase the concentration of certain drugs. We categorized different interactions of this herb into minor and serious ones, such as affecting Cytochromes P450 metabolism enzyme, resulting in a concentration rise in drugs such as Erythromycin, Diazepam, and Cyclosporine.
The reason of writing a review article in this field is our enthusiasm for pharmacology of herbal ingredients and also, we want to gather other scientists’ and our knowledge in this review for future researchers who like to know more about this plant pharmacological criteria in order to make their way.
Method
Pharmacological and phytochemical-specific details of Achillea millefolium, as well as related keywords, were used to conduct a literature search across the following essential collections of electronic databases: Web of Science, Google Scholar, PubMed, and Science Direct.
Outcome
Achillea millefolium medical indications are the treatment of spasmodic gastrointestinal ulcers, inflammation, wound healing, and cancers, as well as excellent antioxidant activity. Camphene, Limonene, Apigenin and some other components show anti-inflammatory effects by cyclooxygenase inhibition, prostaglandin E2 inhibition and other mechanisms. Studies showed 90 % of its essential oil consists of monoterpenes which can be mutually beneficial with extract components.
Conclusion
A. millefolium can play a significant role as a strong antioxidant and anticancer source, positively affecting gastrointestinal inflammations.
Keywords: Achillea millefolium, Pharmacodynamics, Pharmacokinetics, Drug interactions, Toxicity, Mechanism of action
Graphical abstract
List of abbreviation
- ATP
Adenosine Triphosphate
- CAT
Catalase
- CDK
Cyclin-Dependent Kinase
- CFA
Chondrocytes and Freund's Complete Adjuvant
- CYP
Cytochrome P450 Enzymes
- DPPH
2,2-Diphenyl-1-Picrylhydrazyl
- ERK
Extracellular Signal-Regulated Kinase
- GBM
Glioblastoma
- GPx
Glutathione Peroxidase
- NLS
Nuclear Localization Signal
- NMDA
N-Methyl-d-Aspartate
- NSCLC
Non-Small Cell Lung Cancer
- MTP
Microsomal triglyceride transfer
- PARP
Poly (ADP-Ribose) Polymerase
- PERK
Phosphorylation of Extracellular Signal-Regulated Kinase
- PKG
Protein Kinases Mediated Relaxation
- ROS
Reactive Oxygen Species
- SOD
Superoxide Dismutase
1. Introduction
Herbal medicine can be a viable supplement for pharmaceutical industrial applications, where threats to human health continuously rise while synthetic drugs become less effective and include many side effects. The use of medicinal plants for the treatment of diseases is not new; in fact, humans have always attempted to treat diseases with medicinal plants []. In the investigation for innovative drugs, natural compounds, particularly plant-based medicines, have provided significant new leads [1]. One of these medicinal herbs frequently utilized in pharmaceutical fields is Achillea millefolium (A. millefolium).
A. millefolium, also known as the Yarrow or milfoili, the most well-known and widely distributed species in the Asteraceae family, has been used for medicinal purposes for more than three thousand years in both traditional and alternative medicine [2]. Essential oil and derivatives of flavonoids, notably Apigenin [3], Rutin [4], Lutein [5], and Camphor [6], are the primary phytochemical substances extracted from A. millefolium. The A. millefolium essential oil has a mixture of monoterpenes and sesquiterpenes, with the former making up around 90 % of the total [7]. Variations in essential oil content may be caused by ecotype, chemotype, phenophases, altitude, and environmental parameters like temperature, sunlight, relative humidity, and irradiance that affect plant growth. In addition, the genetic background may be relevant for altering the chemistry of secondary metabolites in plants [8]. Some of the flavonoids found in A. millefolium include Morin, Myricetin, Naringin and Naringenin [9], Millifolide A, Millifolide B, Millifolide C [10], 3-methoxytanapartholide, Seco-tanapartholide A, Seco-tanapartholide [11], Achillinin A [12], Apigenin [3], Rutin [4]. Flavonoid like aglycones, flavones and flavonol like O-glycosides are present in A. millefolium as well [3]. Generally, the flowering tops of the A. millefolium plant, which contain the essential oil, are the most efficient parts of a plant and are primarily used for the treatment of influenza [13], hemorrhage [14], dysmenorrhea [15], and diarrhea [16]. The main forms of A. millefolium extracts and oils include essential oil, infusion, alcohol extract, decoction, hydroalcoholic extract, and aqueous extract [17]. Also, the antioxidant activity and phenolic chemicals have a connection. comparatively high concentrations of the phenolic chemicals (Carvacrol and Thymol) in essential oil may be responsible for its antioxidant action [18]. It is believed that A. millefolium possesses a wide variety of pharmacological effects, such as analgesic [19], anti-inflammatory [20], antidiabetic [21], cholagogue [22], spasmolytic [23], antitumor [24], antioxidant [25], antifungal [26], antiseptic [27]. Several chemical compounds, such as essential oils, sesquiterpenes, phenolic compounds, etc., are responsible for the liver-protective actions, including essential oils, sesquiterpenes, and phenolic compounds [28]. Also, previous studies indicate that A. millefolium acts as an antiulcer agent. In a study by Sadeghi et al. (2013) ileum contractions of Wister rats were elicited by KCl or acetyl choline. The addition of the extract attenuated ileum contractions which may be attributable to flavonoids, specifically Quercetin and Apigenin [29].
The pharmacological evidence for the traditional use of A. millefolium extract for the treatment of dyspepsia was found to be provided by the extract's prokinetic effect, which was discovered by studying the effect of the aqueous extract collected from the flowering tops of A. millefolium on stomach motility [30]. Also in another study, To determine whether or not ethanol extract of A. millefolium aerial parts affected Interleukin-1 (IL-1) and iNOS gene expression in pancreatic tissue in Streptozotocin-induced diabetic rats, Zolghadri et al. (2014) conducted a study. They found that giving Streptozotocin-diabetic rats A. millefolium extract recovered their body weight, IL-1, and iNOS gene expression. Up-regulation of the protective gene iNOS and decreased production of the inflammatory cytokine IL-1 may be responsible [31]. It is reported by Jenabi et al. (2015) that A. millefolium is employed in treating disorders associated with the gastrointestinal tract, including digestive problems, dyspepsia, flatulence, abdominal pain, diarrhea, and stomachache [15]. However, other aspects of A. millefolium's toxicity, such as its biophysical impacts on male reproduction, are not fully understood. The purpose of this review is to study the efficacy, pharmacological properties, safety, toxicity, pharmacokinetics and pharmacodynamics of this plant and its extract.
The main purpose of selecting A. millefolium for the pharmacological research review article is because it is a widely used traditional medicinal plant with a range of potential pharmacological properties that have been reported in several studies. Some unique advantages of A. millefolium over other plants include its widespread availability, low toxicity, and affordability. While it is true that there are countless plants with pharmacological characteristics, A. millefolium is a plant with a long history of traditional use and has been studied extensively for its potential pharmacological benefits. Additionally, it is widely used in complementary and alternative medicine, and it has been reported to have various therapeutic properties, such as anti-inflammatory, antimicrobial, and antioxidant activities. Therefore, it is a promising candidate for further research in developing new therapeutic agents. By synthesizing the available evidence from in vitro, in vivo, and clinical studies, this review article aims to provide researchers and clinicians with a better understanding of the potential benefits and limitations of using A. millefolium as a therapeutic agent. The present review article aims to contribute to advancing research in the field of herbal medicine and provide valuable insights for clinical practice. Overall, the purpose of the review article is to provide a comprehensive overview of the mechanism of action, pharmacokinetic properties, clinical drug-drug interactions, and tolerability of A. millefolium. This review will help researchers and clinicians better understand the potential benefits and limitations of using this plant as a therapeutic agent. Although, there are many more components in A. millefolium, we discussed the pharmacodynamics and pharmacokinetics of the top components below in the table due to the percentage of presence and importance of action.
2. Research methodology
A comprehensive search was conducted using electronic databases such as PubMed, Scopus, Web of Science, and Google Scholar to identify relevant studies published in the English language from inception to September 2021. The search terms used included “Achillea millefolium,” “pharmacology,” “mechanism of action,” “pharmacokinetics,” “clinical drug interactions,” and “tolerability.” The inclusion criteria for the review article were studies that investigated the pharmacological properties of A. millefolium in vitro, in vivo, or in clinical trials. Both experimental and clinical studies that reported the effects of A. millefolium on various disease conditions were included in the review. The exclusion criteria included studies that were unrelated to the pharmacological properties of A. millefolium or were not published in English. After the initial search, the titles and abstracts of the studies were screened for relevance, and full-text articles were obtained for the selected studies. The selected studies (228 papers) were critically appraised for quality, relevance, and validity, and the data were extracted and synthesized systematically. Therefore, the methodology used for the search, inclusion and exclusion criteria, and data synthesis were aimed at ensuring the comprehensiveness, relevance, and quality of the review article.
3. Pharmacokinetics and pharmacodynamics of A. millefolium
We have prepared a table including components of A. millefolium with their details.
Table 1As mentioned, A. millefolium is indicated for some medical purposes such as inflammation, spasmodic diseases, wounds, etc. This plant contains many bioactive components with different pharmacokinetics and pharmacodynamics. In this section, these properties are discussed. There are some main components that are found in A. millefolium with the following concentrations: β-Thujone, 0.4–55.3 %; Germacrene-D, 2–20.6 %; 1,8-Cineole, 1.2–19.8 %; Isospathulenol, 0.5–36 %; Camphor, 0.6–25.5 %; Trans-nerolidol, 0.4–48.1 %; and Cubenol, 0.1–42.9 % [32]. Kaempferol, Luteolin and Apigenin are also found as main flavonoids in A. millefolium [3].
Table 1.
The most important phytochemical compound of A. millefolium.
| No | Compound name | Class of compound | Structure | Effect | Part of plant | Extract/essential oil | Half life | Mechanism of action | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Camphene | Monoterpenes | 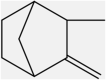 |
Anti-inflammatory, reducing triglyceride level | Leaf | Essential oil | 9.12 days | Prostaglandin e2 inhibition, cyclooxygenase (cox) inhibition. (Fig. 1), Inhibition of MTP |
[15] [33] [34] [35] [36] |
| 2 | α-pinene | Monoterpenes | 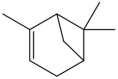 |
Antibacterial, anti inflamatory anti-tumor | Leaf | Essential oil | 1.4 h | inhibit the growth of bacteria, Decrease in inflammatory factors such as TNF-α , down-regulating Cdc25C mRNA and protein expression, |
[15] [33] [37] [38] [39] [36] |
| 3 | β-pinene | Monoterpene | 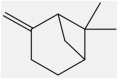 |
Antibacterial, Inhibitory effects on breast cancer and leukemia |
Leaf | Essentia oil | 4.9 h | Decreasing inflammatory factors such as TNF-α | [15] [33] [36] |
| 4 | Limonene | Monoterpene | 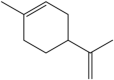 |
anti-inflammatory, antioxidant, neuroprotective | Leaf | Essential oil | 12–24 h | Reducing no production and nerve activation, thus reducing neuron cell (Fig. 2) | [40] [41] [42] [36] |
| 5 | Camphor | Terpenoid ketones |  |
Antitussive, analgesic, nasal decongestant | Leaf | Essential oil | 4–8 h | reducing TRPA1-mediated cough, Activating heat-sensitive trpv1 and trpv3 receptors | [33] [43] [44] [45] [46] [36] |
| 6 | 1,8-cineole | Monoterpene | 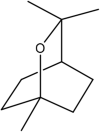 |
Anti-inflammatory Antioxidant effects |
Leaf | Essential oil | 6.7 min | Induced enzymatic and nonenzymatic cellular antioxidants and metabolic phase ii detoxifying enzymes | [33] [47] [48] [36] |
| 7 | Linalool | Monoterpenoid |  |
, decrease anxiety, sedative effects, Hepatoprotective, dermal sensitizer | Leaf | Essential oil | 3.2 h | A competitive antagonist of glutamate, and as a non-competitive antagonist of NMDA receptors in brain cortical membranes | [33] [49] [50] |
| 8 | Luteolin | Flavonoid | 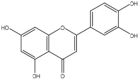 |
, anti-tumor, and anti-inflammatory | Whole Plant | Essential oil/extract | Less than 5 h | inhibited cell proliferation by inducing G0/G1 and/or G2/M cell cycle arrest, Binding to the thromboxane a2 receptor | [51] [52] [53,54] |
| 9 | Isochlorogenic acid | Polyols | 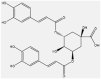 |
antioxidant and anti-inflammatory | Leaf | Extract | 227∼292 min | High restoration of glutathione levels | [55] [56] [53] [57] |
| 10 | Apigenin | Estrogenic flavonoid | 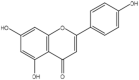 |
An antioxidant, anti-inflammatory | Whole Plant | Extract | 91.8 h | oxidant enzymes inhibition, modulation of redox signaling pathways (NF-kB, Nrf2, MAPK, and P13/Akt), Regulating a p53-bax-caspase-3 apoptotic pathway | [58] [59] [60] [53] [61] |
| 11 | Kaempferol | Flavonoid | 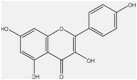 |
Anti-inflammatory, anticancer | Water extract | 2.93–3.79min | Cell cycle arrest at the G2/M phase, reducing pro-inflammatory cytokines, inducing apoptosis | [62] [63] [36] |
|
| 12 | Caffeic acid | Phenolic glycoside | 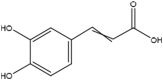 |
Hepatoprotective, anticarcinogenic, antioxidant, anti-inflammatory | Whole plant | Extract | 0.14 h | Inhibition of lipid peroxidation and shielding against ldl oxidation (Fig. 3) | [64] [65] [66] [67] [68] |
| 13 | Quercetin | f | 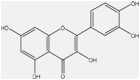 |
Anti-inflammatory, anti-bacterial | Whole plant | Extract | 3.5 h | Inhibiting lipopolysaccharide (lps)-induced tumor necrosis factor α (TNF-α) production in macrophages | [65] [69] [70] |
| 14 | Betonicine | Alkaloids |  |
Positive effect on digestive system and the blood stream | Whole plant | Extract | N.m | Not explored | [71] [72] |
*N.E: Not explored.
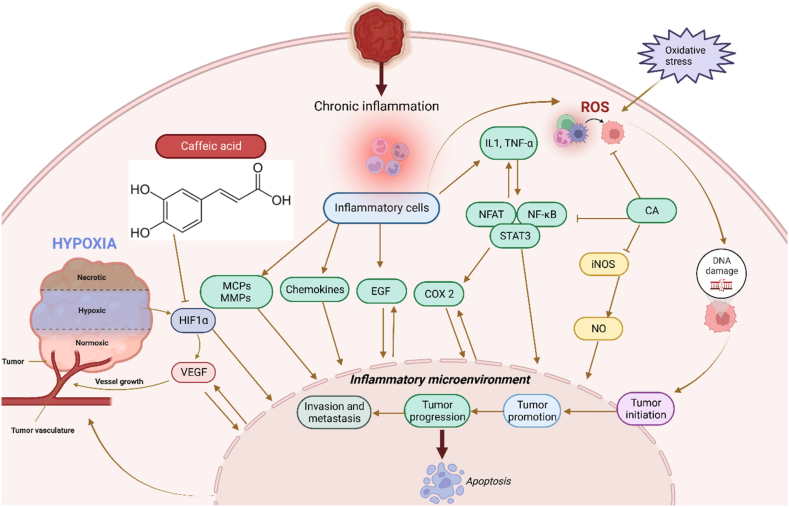
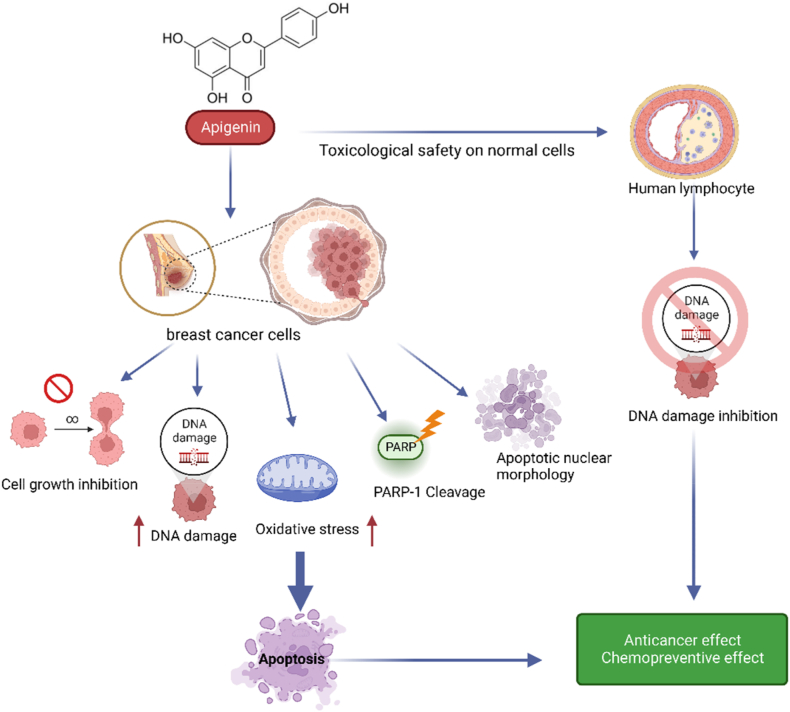
According to Table 1, caffeic acid has anti-inflammatory effects. Inflammation occurs in reaction to a stimuli, such as tissue injury. It is a physiological mechanism that may contribute to cancer growth via many intermediates. Many modulators, including NF-kB, COX-2, TNF-a, IL-6, Nrf2, iNOS, NFAT, and HIF-1α, play a role in tumor growth. caffeic acid demonstrated an inhibitory influence on NO production that also strongly inhibited the production of COX-2 and iNOS. Cancer angiogenesis can be suppressed by therapeutic drugs that target NF–B and COX-2. caffeic acid strongly inhibits ceramide-mediated NF–B action, as well as UVB-mediated COX-2 production. As a result, multiple studies have found caffeic acid to be a cell death inducer in tumor cells as well as a cancer growth inhibitor and failure in animals. In other words caffeic acid illustrated its inhibitory action on NO-making that also sturdily blocked the creation of COX -2 and iNOS which is shown in Fig. 3. Fig. 3 [73] Also, due to apoptotic effect of camphene, this plant has anti-cancer effect as shown in Fig. 1. Fig. 1 [74] and limonene has neuroprotective effects. Fig. 2 [42].
Fig. 3.
Anti-inflammatory mechanism of Caffeic acid. Many modulators involve inflammation to tumor progression, including NF-kB, COX-2, TNF-a, IL-6, Nrf2, iNOS, NFAT, and HIF-1α. Caffeic acid is an agent that target NF-κB and COX-2. This can suppress cancer angiogenesis. The role of Caffeic acid is illustrated in Fig. 3 [73].
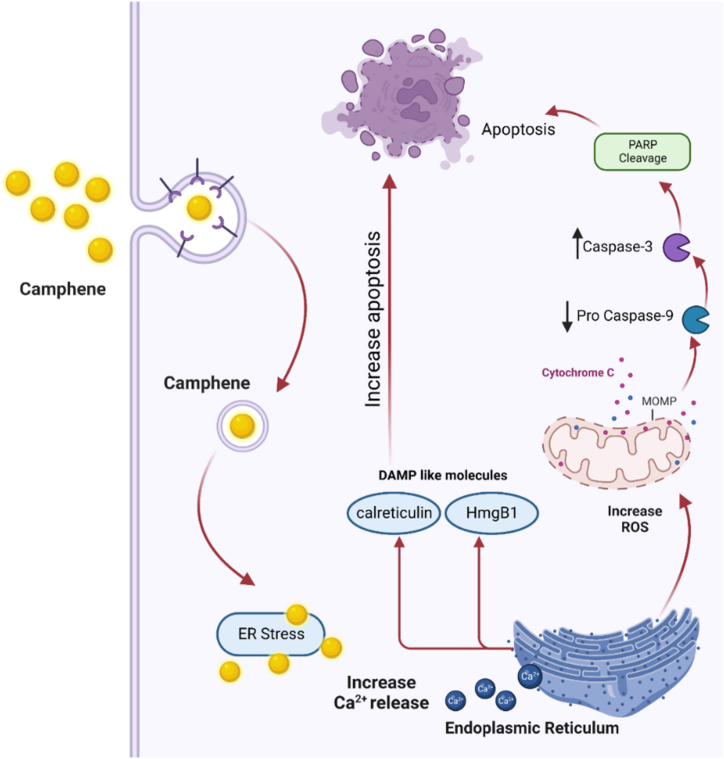
Fig. 1.
Camphene anticancer mechanism of action. Camphene has an anticancer effect through entering the cell, increasing calcium release from endoplasmic reticulum, increasing ROS (reactive oxygen species) and finally leading to apoptosis [74].
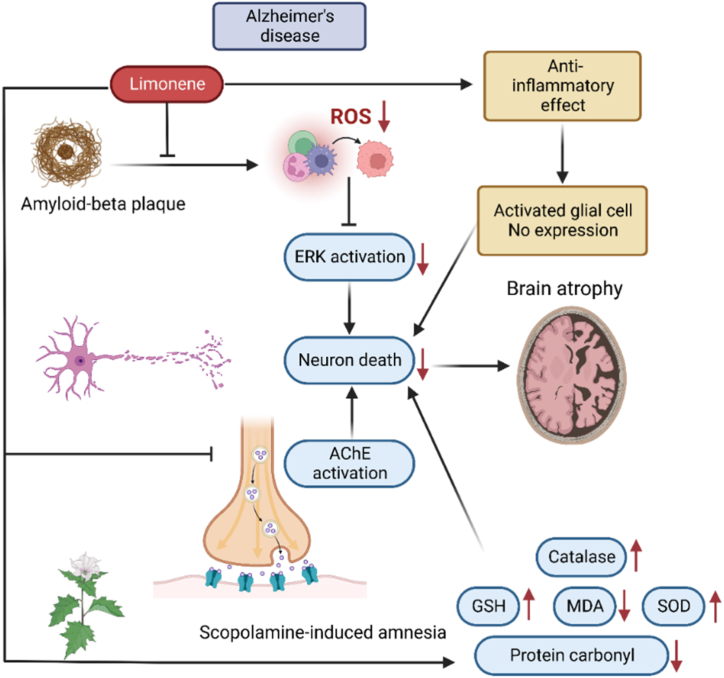
Fig. 2.
Neuroprotective mechanism of Limonene. Limonene has neuroprotective effects on brain cells by decreasing ROS, having anti-inflammatory effects, ACE activation. These effects lead to lessen neuron death and preventing brain atrophy [42].
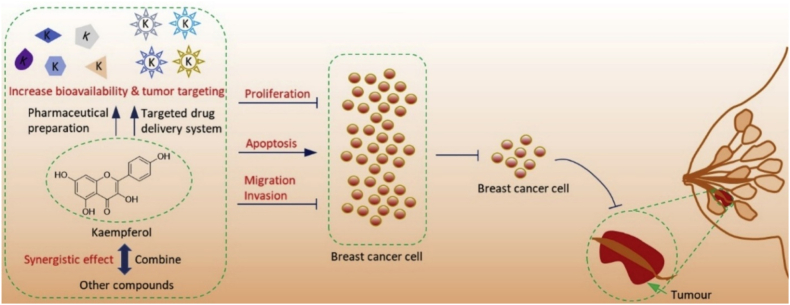
3.1. Kaempferol
Kaempferol is a typical organic component found in a wide variety of herbs. It is the most frequent flavonoid with a weak affinity for water, heated ethanol, and alkaline ether [62]. Several anti-cancer effects have been attributed to it. Kaempferol is a widely consumed flavonol. Recent research has shown that Kaempferol has promising results as a chemotherapeutic treatment for breast cancer. It has been discovered that Kaempferol primarily has anti-breast cancer effects by doing the following three things: preventing breast cancer cell growth, causing breast cancer cell death, and stopping breast cancer cell migration and invasion [75].
It is shown in Fig. 4that how Kaempferol can prevent and stop cancer [75].
Fig. 4.
The mechanism of anticancer action and potential clinical use of kaempferol in the treatment of breast cancer [75].
3.1.1. Kaempferol pharmacokinetic
Kaempferol shows a high net transfer towards the defensive side. The net absorption of this compound is high because of the less secretion of its conjugates. The rate of secretion of Kaempferol conjugates is also high which shows that considerable amounts of free Kaempferol in portal vein blood and this demonstrates the hepatic metabolism of this compound [76]. Because of Kaempferol lipophilicity, it will be absorbed in the small intestine through active transport or passive and facilitated diffusion. Metabolic transforms will give us glucuronides and sulfoconjugates formed from absorbed Kaempferol in the liver and small intestine by intestinal conjugation enzymes. Also, some bacterial microflora in the colon metabolize Kaempferol and its glycosides. These reactions lead to releasing aglycones and compounds such as 4-methylphenol, Phloroglucinol, and 4-hydroxyphenylacetic acid. There are two paths for these compounds. They can be absorbed and reach the systemic circulation or exerted in urine and feces [77].
3.1.2. Kaempferol pharmacodynamics
Kaempferol role in anti-inflammation: Kaempferol increases the number of activated macrophages and in result decreases lipopolysaccharide (LPS)-induced tumor necrosis factor-α (TNF-α), IL-1, and also interleukin 10 (IL-10) expression. In conclusion Kaempferol plays a role as an anti-inflammatory compound which increases anti-inflammatory cytokines such asIL-1, IL-10 and reduces pro-inflammatory cytokine levels [62]. Kaempferol role in suppressing inflammation in colitis: As ulcerative colitis is an inflammatory disease, Park et al. (2012) has proved that the pathogenesis of ulcerative colitis is related to an imbalance between pro-inflammatory cytokines and anti-inflammatory cytokines and Kaempferol can have a role in increasing pro-inflammatory cytokine secretion. Also, Kaempferol has many other beneficial medical effects such as preventing atherosclerosis, improving metabolic functions, anticancer efficacies, anti-inflammatory effects, etc. [62,78].
3.2. Camphor
Camphor is a natural compound which is found in the bark of the tree Cinnamomum camphora. It has demonstrated different medicinal effects since decades ago. The presence of camphor is responsible for part of A. millefolium, contraceptive [79], analeptic [80], anti-gastrointestinal disorders [81], anti-irritant [82], and anti-hypertensive properties [83]. It has demonstrated different medicinal effects since decades ago such as anti-septic, analgesic, antipruritic, rube efficient, and counter irritant [84].
3.2.1. Camphor pharmacokinetics
Camphor can be absorbed in all administration sites, including topical, inhalation, and ingestion. The peak plasma level of Camphor after ingestion is 3 h when taken alone and 1 h when it is taken with a solvent. This component can be distributed throughout the whole body with the volume of distribution 2–4 L/kg. It also passes through the placenta thus it is not recommended in pregnancy and lactation. Its protein binding in plasma is estimated at around 61 %. Finally, it undergoes metabolic procedures by the liver, which bring into hydroxycamphor metabolites by hydroxylation. This procedure is mainly done by cytochrome P450 [85]. Then, hydroxylated metabolites conjugated with glucuronic acid and excreted in the urine. The half-life of 200 mg of camphor was 167 min when ingested alone, and 93 min when ingested with a solvent (Tween 80) [86].
3.2.2. Camphor pharmacodynamics
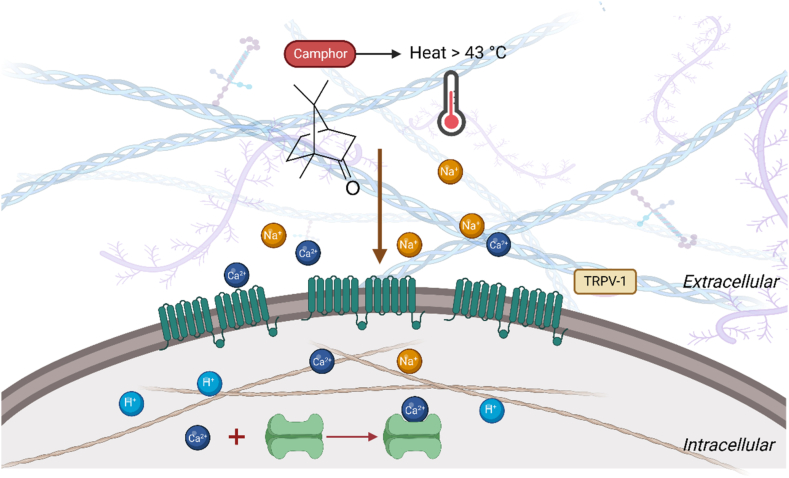
It has been shown that camphor can be effective in bronchoconstriction through being effective on histamine H1 and muscarinic M3. This can be associated with cough inhibition [84]. Also, Camphor is somehow a TRPV1 agonist, and its antipruritic activity is related to the potential of activating TRPV1 (Fig. 5) [87]. Since decades ago, Camphor has been shown to activate TRPV3 resulting in several medicinal uses such as antiseptic, analgesia, antipruritic, antispasmodic, and anti-irritant effects. Xu et al. (2005) revealed that camphor also activates the expression of TRPV1 heterologously, requiring higher concentrations than capsaicin [45]. In other investigations, camphor is considered to reduce bronchoconstriction caused by histaminergic H1 receptors and muscarinic M3 receptors. So, this function can also reduce coughs [88].
Fig. 5.
Camphor leads to desensitization through the TRPV-1 pathway. Camphor is one of the agents that has recently been shown to activates TRPV3, and also activates heterologously expressed TRPV1.
3.3. 1,8-Cineole
1,8-Cineole which is known as 11-Eucalyptol, is an aromatic component of some plants such as Salvia and Eucalyptus leave, and Arabidopsis [89]. The biological and pharmacological properties of 1,8-cineole include insecticidal and antibacterial, antiallergic and anti-inflammatory, hepatoprotective, anticancer, and gastroprotective properties. Caldas et al. (2015) proved the importance of 1,8-cineole as an ulcer healing agent and suggested that antioxidant and cytoprotective mechanisms are involved in its gastroprotective activity [90].
3.3.1. 1,8-Cineole pharmacokinetics
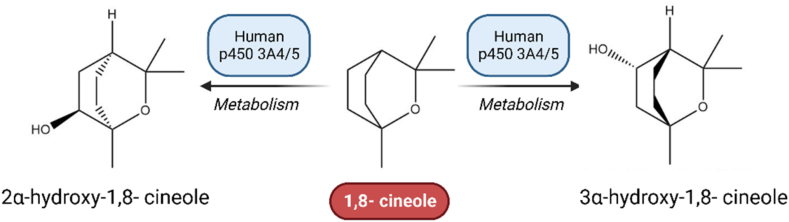
1,8-cineole is quickly absorbed and oxidized to its alcohol and carboxylic acid metabolites. So, after IV administration, the concentration of 1,8-cineole falls rapidly due to forming metabolites. Despite its quick metabolism, it has a slow elimination [91]. Cineole has a very limited bioavailability in the majority of animals following doses of 30 and 100 mg kg −1. As there is not seen any unchanged 1,8-cineole in feces, oral absorption is complete [91]. It is metabolized to hydroxycineoles: 3-hydroxy, 2-hydroxy, 9-hydroxy, and 7-hydroxy. Fig. 6 represents the metabolization of 1,8-cineole to its metabolites by cytochrome P450.
Fig. 6.
1,8-cineole is metabolized to its metabolites by cytochrome P450. Microsomal investigations have shown that P450 enzymes are involved in cineole metabolism. The predominant metabolite in liver microsomes is 2 -hydroxycineole, but 3 -hydroxycineole has also been detected in human microsomes. Both investigations discovered that CYP3A enzymes catalyzed cineole oxidation.
3.3.2. 1,8-Cineole pharmacodynamics
1,8-cineole can have an anti-inflammatory and antioxidant effect. In the cases that we have a respiratory infection or in the case of cell activity, some cytokines and ROS (reactive oxygen species) are produced. These agents will lead to hypersecretion of the mucosa and inflammation. Linalool can limit mucus hypersecretion and recurrent exacerbations as it blocks the link of ROS and cytokines with hypersecretion and inflammation [92].
3.4. Linalool
Linalool is a monoterpene alcohol with cardiovascular benefits and antihypertensive effects. It has been found in plants like A. millefolium, Ocimum canum, etc. It is a component of volatile oil, fragrance, plant metabolite, and an antimicrobial substance [93].
3.4.1. Linalool pharmacodynamics
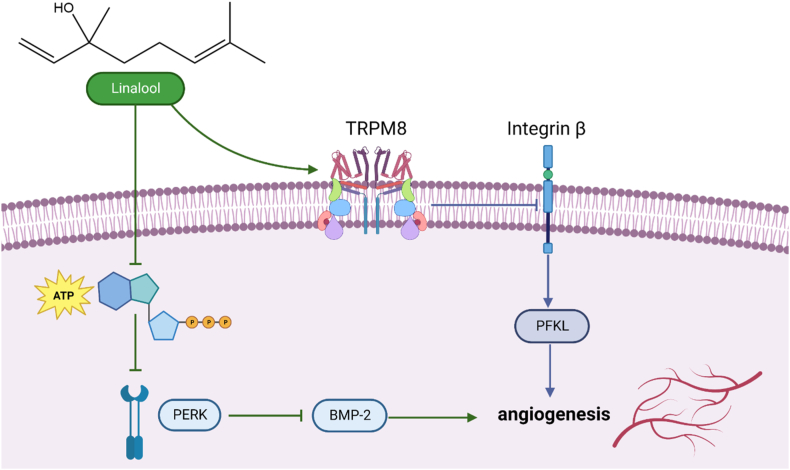
Linalool reduces morphine-induced conditioned place preference and tolerance, which can be associated with the inhibition of NMDA (N-methyl-d-aspartate) receptors, the effect on nitric oxide (NO) signaling and adenosine receptor stimulation properties [94]. Angiogenesis relates to various illnesses, such as diabetic retinopathy, cancer, endometriosis and psoriasis. As represented in Fig. 7, Linalool acts as an anti-oxidative and anti-inflammatory agent and also inhibits the angiogenic activity of endothelial cells by downregulating intracellular adenosine triphosphate (ATP) levels and activating transient receptor potential cation channel subfamily M member 8 (TRPM8) [95]. Linalool increases phosphorylation of extracellular signal-regulated kinase (PERK), decreasing intracellular ATP levels and activating the TRPM8. Linalool blocks the activity of glutamate receptors in the brain by competing with them, and it also blocks the activity of NMDA receptors in the cortex by blocking them without competing with glutamate [96].
Fig. 7.
Anticancer mechanism of action of Linalool which represent inhibition of angiogenic activity of endothelial cells by downregulating intracellular ATP levels and activating TRPM8. Linalool stimulates the transient receptor potential cation channel subfamily M (melastatin) member (TRPM) and enhances the activation of extracellular signal-regulated kinase (ERK)8. Furthermore, ATP totally reverses linalool-induced ERK phosphorylation. Furthermore, ERK activation produced by linalool inhibits the production of bone morphogenetic protein (BMP)-2. These data suggest that linalool has an anti-angiogenic impact, which is mediated via lowering intracellular ATP levels and activating TRPM8.
3.4.2. Linalool pharmacokinetics
Linalool is absorbed quickly through oral and gavage routes of administration. 10 % of administrated dose will be excreted in the urine. It's noticeable that volatile components can be absorbed and enter the blood through the lung and nasal mucosa. A study by Bickers et al. (2003) mentioned that after 72 h of administration of Linalool, 0.8 % in the skin, 1.2 % in the skeletal muscle, 0.5 % in the liver, and 0.6 % in the gut [97]. In the process of metabolization, there will be a more polar and water-soluble compound instead of Linalool to facilitate excretion. Approximately 55 % of administrated doses of excreta Linalool were found in the conjugated form with glucuronic acid via urine. 23 % of it was excreted in the form of CO2. 15 % was excreted in feces. Also, 25 % of the initial dose will be conjugated in bile, and it has enterohepatic circulation [98].
3.5. Apigenin
Apigenin is a kind of flavone that stimulates autophagy in leukemia cells it can be an anticancer function. It can have other functions, such as being an anti-neoplastic agent and a metabolite. It is found in Matricaria chamomilla, and A. millefolium [99]. Apigenin develops cell death in lung epithelium cancers, and also, besides curcumin, it has a synergic effect on the apoptosis of cells, so it can be a solution in the treatment of different types of cancers [100].
3.5.1. Apigenin pharmacodynamics
Apigenin regulates CDK inhibitors, CDK4 and CDK6 kinases, and as a result, it induces cell cycle arrest at different proliferation stages such as G1/S-phase or G2/M phase. It regulates the intrinsic apoptotic pathways, inducing the release of cytochrome C in the cytoplasm and finally leading to apoptosis (Fig. 8). Apigenin also suppresses PI3K activity by blocking the ATP-binding site of PI3K, leading to inhibiting AKT kinase activity [101].
Fig. 8.
Anticancer mechanism of Apigenin. In tumor cells, apigenin can reduce cyclin- and cycline-dependent kinases and activate inhibitor proteins, which causes cell cycle arrest. Apigenin can suppress tumor metastasis and angiogenesis in addition to inducing apoptosis by regulating anti/proapoptotic proteins. Apigenin exerts its effects through controlling gene expression and cellular signal transmission.
3.5.2. Apigenin pharmacokinetics
Apigenin is ingested in a glycosylated form. This form will be glycosylated by stomach enzymes such as β-glucosidases or microflora of the colon before systemic absorption. Free apigenin is absorbed easily or undergoes different phases of metabolism in the small intestines and liver. It can cause hydroxylated metabolites such as luteolin and others. These metabolites undergo four different options that one of them is elimination [102].
About the exertion of Apigenin, it is found that Apigenin gets out of the body through urine in rats with a difference in the percentage of ingested radioactivity recovered between male and female ones. In conclusion, it is found that less than 40 % of ingested dose will be excreted in gastric acid in 5 days [103].
4. Main safety and toxicity issues of A. millefolium
A. millefolium is one of the world's most commonly used medicinal herbs, with various applications, pharmacological effects, safety, and toxicity. The anti-inflammatory [20], spasmolytic [23], hemostatic [14], antibacterial activity against pneumonia [104], anti-kidney stones [105], anti-diarrhea [16],anti-abdominal pain [106], anticancer/tumor [24], Antiproliferative [107], analgesic effect [19], anxiolytic activity [108], skin-rejuvenating activity [109], and anthelmintic activity are linked to the safety of A. millefolium [110]. A. millefolium is also considered safe for use as a supplement [111].
Some features of A. millefolium toxicity, such as probable effects on the male reproductive system [112], are related to its extracts. The effects of an aqueous extract of A. millefolium leaves on fertility were also investigated by Dalsenter et al. (2004) in a study using Wistar rats (sperm and spermatid numbers, sperm morphology and reproductive organ weights). They were also evaluated for the level of toxicity caused by the extract. The adult male rats were given A. millefolium extract daily to be treated. With the higher dosage of A. millefolium extract, the proportion of abnormalities in sperms was shown to increase significantly. However, no other significant changes were found in any of the other reproductive endpoints studied in the male rats [113]. The findings made it abundantly clear that there would be no long-term reproductive toxicological risk associated with doses of A. millefolium that humans typically consume. However, the findings also demonstrated that the long-term use of A. millefolium extract in high doses can cause toxicity [114].
Whether prolonged exposure to A. millefolium extracts poses any health risks is unknown. Despite the plant's classification as non-poisonous and approval for use in alcoholic beverages by the Food and Drug Administration, certain harmful consequences have been reported following its use by people and in animal tests [115,116]. An investigation into the genotoxicity of the oil produced by A. millefolium was carried out by Ahmady et al. (2018). They have found that induction of mitotic non-disjunction or crossing over was correlated with the genotoxicity of the oil. As a result, the findings indicated that further research is required to determine whether or not A. millefolium essential oil can interfere with the recombinational process in mammalian cells. As a result, it is recommended that the oil be utilized with caution [117,118]. In this part, we will investigate various chemicals that play significant roles in the toxicity or safety of this plant and the extracts it produces.
4.1. Thujone
The monoterpene ketones α-Thujone (3-Thujone) and β-Thujone (3-isothujone) are naturally occurring compounds found in the Achillea species of the Asteraceae family that are frequently utilized as flavoring agents in foods and beverages [119]. Essential oil of A. millefolium, which contains Thujone as a significant constituent, has been widely used in folk medicine for the treatment of rheumatism [120], psoriasis [121], irritable bowel syndromes [122], warts, acne, and uterine carcinomas [112]. The safety of Thujon is related to these pharmacological actions. In addition to (−)-α-Thujone and (+)-β-Thujone, two more enantiomeric forms are known: (+)-α-Thujone and (−)-β-Thujone [123]. Current instruments, pharmacological, and clinical research have partially validated the toxicity and safety of Thujone. Neurotoxicity is one of Thujone's harmful consequences. also α-Thujone is more neurotoxic than β-Thujone [124]. Thujone has a totally reversible neuronal action. Thujones are GABA-gated chloride channel modulators, with α-Thujone acting around two to three times more potently than β-Thujone (GB62). However, the concentrations required in humans are unclear [125].
Additionally, Czyzewska et al. (2013) discovered that due to the different sensitivity of GABAA receptor subtypes, the action of α-Thujone may vary [126]. Also Waidyanatha et al. (2013) reported that the elimination of α-Thujone in the brain is slower than in the plasma. This effect can impact the neurotoxic effect of Thujone [127]. In addition, cannabinoid CB1 and serotonin 5-HB3 receptors were proposed as potential Thujone targets [125]. Also, Sultan et al. (2000) reported that Thujone suppressed reversibly ACh-induced currents in Xenopus oocytes with an IC50 of 24.7 M(GB65), and previous studies show that 25 mg/kg Thujone altered the behavior, mortality, organ weight, and hepatic and renal functioning of 6–8 week-old mice. Also in single-dose toxicity tests, the i. p. LD50 was 45 mg/kg body weight [128]. Chronic toxicity may result from the long-term administration of sub convulsive dosages. According to the latter authors, the optimum estimate for daily intake by humans in food or herbal preparations is around 3–7 mg/day [125,128]. Apart from neurotoxicity, thujones may be genotoxic and carcinogenic; antimutagenic and anticarcinogenic effects may also be observed [129]. These effects most likely depend on the cell type studied, genetic background, experimental setup, and concentration. According to Nikoli et al. (2011) a 20-h treatment of monkey Vero cells with 1–4 mM Thujone produces antigenotoxic effects at low concentrations but significant genotoxicity at high concentrations [130]. An in vitro study by Biswas et al. (2011) discovered that Thujone had a pro-apoptotic and cytotoxic effect on the A375 cell line [131]. In a rat model, Küpeli Akkol et al. (2015) showed that -Thujone had a favorable effect in treating polycystic ovarian syndrome [112]. According to Zhou et al. (2019) -Thujone enhances anticancer immune response. At 0.15 mol/L, this chemical increases the proliferation of CD3AK (anti-CD3 antibody-induced activated killer) cells and the cytotoxicity to colon cancer cell lines. A recent in vitro study found that β-Thujone had promising effects on glioblastoma cells, losing viability and invasive potential. Thujone reduced cancer cell proliferation and worked as a pro-apoptotic agent in a dose-dependent manner, likely via inducing oxidative stress in GBM cells (Glioblastoma) [120] (Nikoli et al., 2015). also discovered cytotoxicity (IC50: 1.0–2.8 mM) and cell growth suppression following the treatment in different human cell lines. Thujone may also act as an antigenotoxic agent and activate the DNA repair machinery when used in low dosages [129].
4.2. Camphor
Camphor (2-bomanone, 2-camphonone) is a bicyclic monoterpene widely used in industry. It is a natural substance and one of A. millefolium's most volatile essential oils (more than 20 %). The presence of camphor is responsible for part of A. millefolium contraceptive [79], analeptic [80], anti-gastrointestinal disorders [81], anti-irritant [82], and anti-hypertensive [83] properties. The presence of camphor accounts for the majority of the safety of A. millefolium in the home treatment of colds [132]. The dangers of camphor-containing products in general, and camphorated oil in particular, are mostly linked to incorrect usage [133]. Its toxic properties were first studied in the 19th century, and it is now known that the substance can be utilized medically but under specific circumstances and dosages. According to Santos et al. (2015), the fatal dose of camphor for adults is between 50 and 500 mg/kg. In addition, a dose of 2 g or more has harmful consequences, while 4 g might be fatal. The lethal dose for children is between 0.5 and 1 g, while for babies, it is 70 mg/kg [134]. Camphor is widely used, but it has high toxicity that manifests in the ovary [135], testes [135], nerves [136], liver [137], heart [138], fetus [139], pregnant women [138], the apparent toxicity to the kidneys and urinary system [140], less genotoxic [141] potential toxicity, and toxicity effects such as depression, central nervous system symptoms such as headache, dizziness, restlessness, anxiety, hallucinations, myoclonus, and hyperreflexia [140].
Camphor poisoning in children is widespread, particularly in Asia. This compound can cause poisoning through eating or skin contact [142]. The lipophilic character of camphor and its ability to permeate cell membranes must be emphasized while considering the action mechanisms. Transdermal, stomach, and inhalational absorption pathways are also possible. The liver is believed to oxidize and conjugate the monoterpene before its elimination via the kidneys [134].
4.3. Chamazulene
Chamazulene (15.84 %) is a proazulene component found in the A. millefolium essential oil which has an inhibitory effect against Staphylococcus spp [143]. It is a blue extract of A. millefolium that has antibacterial [144], anti-inflammatory [145] and antifungal effects [146] as well as antioxidant properties [147] that make it safe and the intensity of extract odor and blue color depended upon the amount of chamazulene formed by degradation of matricide during distillation. One of the important safety features of chamazulene is determined to be non-sensitizers [148]. Ding Ma et al. (2020) evaluated the protective effects of chamazulene against IL-1-induced oxidative stress. Chondrocytes and Freund's complete adjuvant (CFA) promote osteoarthritic inflammation in rats. Markers of oxidative stress, pro-inflammatory cytokines, and regulatory proteins were detected. Chamazulene significantly reverses lipid peroxidation levels, as demonstrated by the measured study. Increased the levels of superoxide dismutase (SOD), glutathione peroxidase (GPx), and catalase (CAT) enzymes against oxidative damage generated by IL-1 and CFA [149]. Chamazulene has been suggested as a safe, free radical scavenger. Its chemical nature indicates its passage through cell membranes and possible interaction with radical species. This mechanism demonstrates the safety of chamazulene [150].
4.4. p-Cymene
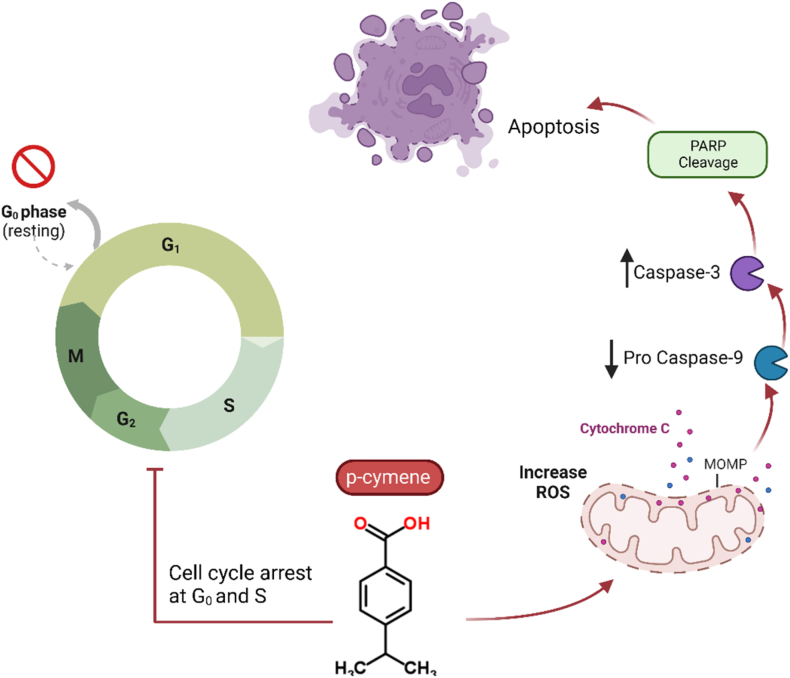
p-Cymene, also known as p-cymol or p-isopropyl toluene [1-methyl-4-(1-methyl ethyl)-benzene], is a monoterpene found in more than 100 plant species, such as A. millefolium, and utilized for medicinal purposes. The majority of A. millefolium's safety is related to p-cymene. The antioxidant [151], antifungal activity [152], anti-inflammatory [153], anti-nociceptive [154], anxiolytic [155], anticancer [156], antiparasitic activity [154], and antimicrobial properties [157] of this compound are well-known. Previous studies show that its anticancer effects are related to some mechanisms, such as the inhibition of apoptosis (Fig. 9) and cell cycle arrest, and p-cymene acts as a safe compound in anticancer properties. P-cymene exerts several anticancer effects on numerous tumor cell lines, according to several recent studies [154,158]. Zhou et al. (2013) demonstrated that p-cymene inhibits the proliferation of gastric carcinoma SGC-7901, liver carcinoma BEL-7404, and nasopharyngeal carcinoma CNE-1 cell lines with respective IC50 values of 20.7, 71.1, and 42.6 μM [159]. p-Cymene significantly inhibits 12-O-tetradecanoyl phorbol-13-acetate (TPA)-induced MMP-9 production via inhibition of ERK1/2 and p38 MAPK signaling pathways in HT1080 human fibrosarcoma cell lines, according to Li et al. (2016) [160]. p-cymene also triggered apoptosis in NSCLC (Non-Small Cell Lung Cancer) cells by activation of caspase-3/-9, activation of Poly (ADP-ribose) polymerase (PARP), and up-regulation of p53, p21, and p15 tumor suppression protein expression, which are crucial for apoptosis and cell cycle regulation [161].
Fig. 9.
Anticancer mechanisms of p-cymene on apoptosis and cell cycle arrest. In HT1080 human fibrosarcoma cell lines, p-cymene inhibits 12-O-tetradecanoyl-phorbol-13-acetate (TPA)-induced MMP-9 production through inhibiting the ERK1/2 and p38 MAPK signaling pathways.
The antimicrobial effects appear to be used in treating communicable diseases and antimicrobial resistance [18]. This application was recently discussed in SARS-CoV-2, which showed at non-toxic concentrations, p-cymene is not genotoxic and inhibits SARS-CoV-2 and influenza H1N1 viral replication in vitro [157]. p-Cymene has been shown to reduce biofilm formation in Burkholderia xenovorans. Bacterial membrane p-Cymene accumulation and the structural changes brought on by this aromatic molecule have both been linked to this process [162]. Food product contamination by microorganisms is a major problem, especially in third-world countries. Api et al. (2021) demonstrated that essential oils and plant extracts containing diverse phytochemicals, including p-Cymene, can be utilized as a fungicide, herbicide and insecticide, for the use of monoterpenes, notably p-Cymene, in food products. This issue it proves that p-Cymene is safe to use as an antibacterial and food preservative [163].
4.5. Bornyl acetate
Bornyl acetate is an oxygenated monoterpene. One of the most important effects of A. millefolium is its anti-inflammatory properties [164], most of which are attributed to Bornyl acetate. A recent study found that Bornyl acetate reduced the production of LPS-induced pro-inflammatory cytokines like TNF-α, IL-1, and IL-6, as well as regulating T-lymphocyte subsets [165]. The antioxidant activity of bornyl acetate, which was extracted from the A. millefolium aerial part of essential oil, was examined using the DPPH assay. Bornyl acetate had the least effective free radical scavenging activity against DPPH (IC50 25 ± 0.1 μg/mL) [18]. The safety of using Bornyl acetate during pregnancy has also been confirmed because it has an anti-abortive effect via immunological balance modulation at the maternal-fetal interface [166].
4.6. Limonene
Limonene is a monocyclic monoterpene (1-methyl-4-(1-methyl ethenyl)cyclohexane). Limonene is a colorless liquid with two optical isomers, d- and l-limonene, and a racemic combination. Limonene's low toxicity to humans and lack of mutagenic, carcinogenic, or nephrotoxic potential have earned it a spot on the safe compound [167]. Limonene has been extensively investigated for its therapeutic benefits, which include anti-inflammatory [168], antidiabetic [169], anticancer [170], antioxidant [168], antinociceptive [171], antihyperalgesic [172], gastroprotective [173] and antiviral [174] properties.
In vivo research using mouse and rat models has shown that limonene affects cancer development and has allergic or sensitizing potential. Nonetheless, a comprehensive investigation of limonene's mode of action indicated that the toxicity was irrelevant to human health [175]. In terms of limonene's effect on reproduction, there were no signs of teratogenic or embryotoxic effects. Still, the compound may indirectly affect fertility by making mothers gain less weight and grow slower. Therefore, this makes their offspring much more likely to have skeletal abnormalities and delayed ossification. In the clinical management of limonene exposure, numerous adverse consequences were reported. The most prevalent is cutaneous sensitization. Ingestion of limonene has also been associated with nausea, coughing, vomiting, tongue and throat burning, ataxia, choking, fever, and tachycardia [176]. According to the harmonized classification and labelling authorized by the European Union, monoterpene is extremely harmful to aquatic life with long-lasting effects, as well as causing skin irritation and allergic skin reactions. Moreover, if limonene enters the airways after being ingested, it can be lethal [177].
4.7. Miscellaneous toxicities under investigation
A. millefolium inhibits spermatogenesis by decreasing the levels of testosterone and LH hormones in the hypophysis-gonadal hormonal axis. By making the adenylate cyclase system more sensitive, the flavons in this plant can attach to sex hormone receptors and inhibit the synthesis of sex hormones. The plant's Apigenin component inhibits phosphatidylinositol 3-kinase and aromatase and promotes the development of cell death enzymes. Takzare et al. (2011) investigated the effect of A. millefolium flower ethanol extract on spermatogenesis in mature Wistar rats. For 22 days, every other day, extract doses of 200, 400, and 800 mg/kg/day were delivered through IP injection or gavage. At a dosage of 400 mg/kg/day (IP), scattered immature cells on the basal membrane of seminiferous tubules were detected, as well as a considerable reduction in cell accumulation and vacuolization [178]. A dosage of 800 mg/kg (IP) induced thickening of the basal membrane of the seminiferous tubules, a reduction in cell accumulation in the tubules, severe disarrangement, degenerative cells, and a significant drop in sperm count. Oral dosing of 800 mg/kg/day of the extract resulted in basal membrane thickness and cell disarray. The findings indicate that A. millefolium has transitory antifertility action in adult male animals [179,180].
5. Drug-drug interactions of A. millefolium
5.1. Anti-nociceptive synergistic interaction
The effects of concomitant use of A. millefolium and Origanum vulgare L. extract encapsulated on liposomes in mice were investigated in a study. The mentioned study (Hassanzadeh-Kiabi F et al.,0.2018) reported that the extract encapsulated in liposome reduced the nociceptive behavior induced by formalin. The interaction index and iso-biologic analysis revealed that the extracts had a synergistic analgesic (anti-nociceptive) effect. Naloxone also reduced the anti-nociceptive effect of the co-administered liposome encapsulated extract [65,181].
5.2. Hypertension synergistic interaction
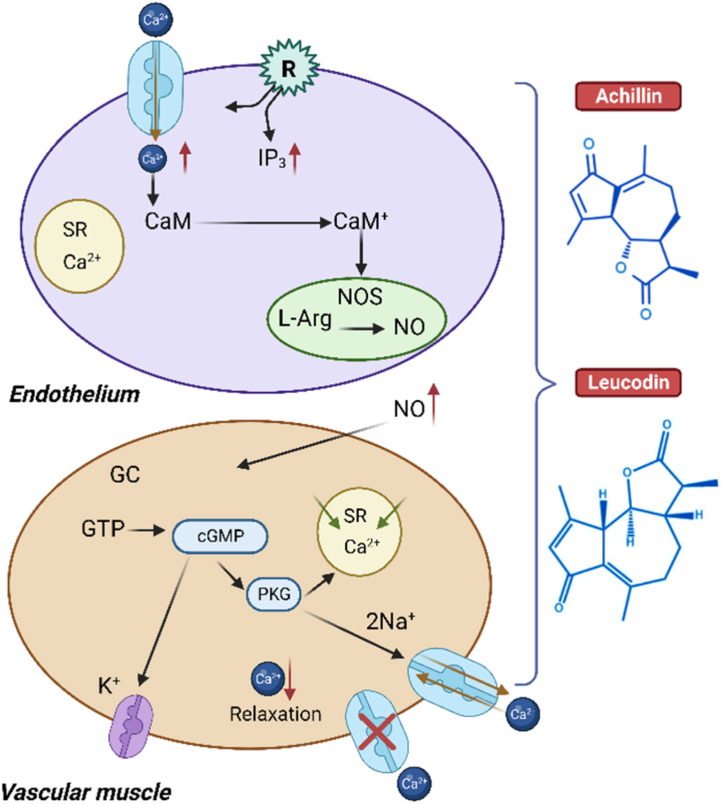
There is evidence that A. millefolium can help reduce blood pressure, and it may even enhance the effectiveness of drugs used to treat hypertension. Significant antihypertensive and vasorelaxant effects of A. millefolium can be attributed to the presence of the chemicals Leucodin and Achillin (epimeric compounds). Vasorelaxation, mediated in part by the endothelium and including NO release and cGMP increase, as well as by the blocking of membrane calcium channels, contributes significantly to the antihypertensive effect of both compounds [27].
Extract and pure chemicals like Leucodin and Achillin appear to effect independently by producing NO and blocking Ca2+ channels. No diffuses into smooth muscle cells and activates soluble guanylate cyclase (sGC). Activating sGC increases intracellular cGMP, which relaxes cells. cGMP-dependent protein kinases mediated relaxation (PKG). cGMP-PKG signaling affects cell motility, migration, and proliferation in vascular smooth muscle cells, which is crucial for angiogenesis and vascular permeability [182]. By phosphorylating the inositol trisphosphate receptor (IP3R), a protein involved in cellular relaxation, PKG-I helps reduce free cytosolic Ca2+. Myosin light chain phosphatase is partially activated by PKG-I; this decreases myosin's identification of actin and promoting relaxation. Also, cGMP-dependent ionic channels are activated, which contributes to the overall decrease in intracellular Ca2+ (through many channels) that results in cellular relaxation (Fig. 10) [27].
Fig. 10.
Mechanism of Antihypertensive and vasorelaxant effect of Leucodin and Achillin. A. millefolium hexanic extract has a significant relaxant effect by calcium channel blockade and NO release. The main bioactive compounds responsible for the relaxant action are Leucodin and Achillin.
It should be noted that a fixed combination of hawthorn and camphor has been used in the therapy of hypotension for decades, and simultaneous use of A. millefolium and hawthorn due to the existence of camphor in the A. millefolium in people with hypertension causes toxicity and is contraindicated [183]. Excessive consumption causes vomiting, nausea, anorexia, and drowsiness. A. millefolium should not be used simultaneously with antiplatelet drugs, blood pressure medications, analgesics and sedatives, and barbiturates. However, the pollen of A. millefolium is highly allergic [184,185].
5.3. Coagulating effect
Although A. millefolium has anticoagulant effects, concomitant use of immunosuppressants with anticoagulants such as Warfarin and A. millefolium due to decreased international normalized ratio may cause the plant in the environment in vivo to have a coagulating effect [186]. Consuming large amounts of A. millefolium can reduce the rate of blood clotting. Taking A. millefolium with medicines that slow down blood clotting may increase the chance of bruising and bleeding, drugs such as Aspirin, Clopidogrel, Diclofenac, Ibuprofen, Naproxen, Dalteparin, Enoxaparin [187].
5.4. Interaction with CYP
Natural compounds can also act as triggers for modifications in CYP activity. Therefore, interactions are not just confined to synthetic medications. The main enzymes for the oxidative metabolism of pharmaceuticals and other xenobiotics are members of the CYP P450 protein family, which consists of more than 50 distinct proteins. The metabolism of medicines with clinical importance seems to be most relevant for a few isoforms, including CYP1A2, CYP2C9, CYP2D6, and CYP3A4. One study looked into how chamazulene inhibits four specific human cytochromes P450 enzymes (CYP1A2, CYP2C9, CYP2D6, and CYP3A4). Then, it was discovered that CYP1A2 was active toward CYP3A4 and more sensitive than the other isoforms. The inhibition of CYP2C9 and CYP2D6 was lower. so, chamazulene in A. millefolium inhibits the activities of major human drug metabolizing enzymes and interacts with drugs that metabolize to these enzymes such as Estrogens, Estradiol, Acetaminophen, Codeine, Erythromycin, Diazepam, Cyclosporine [188,189].
Quercetin is a plant pigment (flavonoid). Quercetin increases xanthine oxidase and N-acetyltransferase activity while decreasing CYP3A4 and CYP1A2 activity in vivo. To a similar extent, quercetin inhibits the P-glycoprotein (Pgp) activity in vivo. Pgp is a drug efflux transporter that plays an important role in the intestinal and biliary transport and elimination of a wide variety of drugs and their metabolites. Quercetin may change the blood levels of all medications processed by these enzymes due to these interactions [190,191].
However, quercetin, especially in the case of “Minor severity,” reduces the effect(s) of the drug by means of pharmacodynamic antagonism. Quercetin inhibits P-glycoprotein, which is responsible for the majority of drug interactions (MDR1) carriers of efflux. As predicted, the dosage of quercetin may potentially impact drug-nutrient interactions. Consequently, a pig study revealed a fatal interaction between quercetin and digoxin, a P-glycoprotein substrate with a very limited therapeutic range; in fact, the co-administration of quercetin and digoxin (50 mg/kg and 0.02 mg/kg, respectively) led to the sudden death of two out of the three pigs within 30 min of administration [192]. Surprisingly, even though the co-administration of quercetin at a lesser dose (40 mg/kg) significantly increased the Cmax (highest concentration) of digoxin by 413 %, it had no fatal side effects [193].
In Table 2 most of the drug-plant interactions has been mentioned classified due to the drugs’ classification. Type of drug interaction and mechanism of action also has been mentioned.
Table 2.
Quercetin drug interaction severity (based on Medscape online database [194]).
| Drug classification | Example of drugs | Type of drug interaction | Mechanism |
|---|---|---|---|
| Antibiotics | Ciprofloxacin Fleroxacin Gemifloxacin Levofloxacin Ofloxacin Moxifloxacin |
Minor | Quercetin decreases effects of mentioned antibiotics by pharmacodynamic antagonism |
| Antihistamines | Fexofenadine Loratadine |
Minor | Quercetin will decrease the level or effects of these antihistamines by P-glycoprotein (MDR1) efflux transporter |
| Antidepressants | Amitriptyline Nortriptyline |
Monitor Closely | Quercetin will decrease the level or effects of these antidepressants by P-glycoprotein (MDR1) efflux transporter. Also Quercetin can also exert antidepressant effects by inhibiting the hyperactivity of the hypothalamic-pituitary-adrenal axis [195] |
| Cardiovascular | Atorvastatin Digoxin Lovastatin |
Monitor Closely | Quercetin will decrease the level or effects of these cardiovascular drugs by P-glycoprotein (MDR1) efflux transporter |
| Corticosteroids | Budesonide Cortisone Deflazacort Dexamethasone Fludrocortisone Methylprednisolone Prednisolone |
Monitor Closely | Quercetin will decrease the level or effects of Corticosteroids drugs by P-glycoprotein (MDR1) efflux transporter |
| Antigout Drugs | Colchicine | Monitor Closely | Quercetin will decrease the level or effects of Colchicine by P-glycoprotein (MDR1) efflux transporter |
| Sex Hormones | Conjugated-Estrogens Estradiol Mestranol |
Monitor Closely | Quercetin will decrease the level or effects of these drugs by P-glycoprotein (MDR1) efflux transporter |
| Immunosuppressants | Ciclosporin Sirolimus Tacrolimus |
Monitor Closely | Quercetin will decrease the level or effects of these drugs by P-glycoprotein (MDR1) efflux transporter |
| Anti-cancer | Imatinib Nilotinib Paclitaxel Vincristine Vinblastine |
Monitor Closely | Quercetin can reverse the resistance mechanisms of anticancer drugs through the inhibition of group P function and ABCB1 gene expression in many cell lines. Also it will decrease the level or effects of these drugs by P-glycoprotein (MDR1) efflux transporter |
| Antivirals | Saquinavir Indinavir Maraviroc Nelfinavir Ritonavir |
Monitor Closely | Quercetin will decrease the level or effects of these drugs by P-glycoprotein (MDR1) efflux transporter |
| Anti-cancer | Topotecan Everolimus |
Serious | Topotecan: Quercetin will increase the level or effects of topotecan by P-glycoprotein (MDR1) efflux. So, avoid concomitant use of P-glycoprotein inhibitors. The interaction with IV topotecan may be less severe. Everolimus: Quercetin will decrease the level or effects of Everolimus by P-glycoprotein (MDR1) efflux transporter |
5.5. Photosensitivity interactions
It is also mentioned in many investigations that A. millefolium interferes with photodermatitis drugs, that these drugs increase photosensitivity [196]. In other words, one of the most significant causes of airborne contact dermatitis appears to be due to A. millefolium. Although this species has been shown to have higher concentrations of ten distinct alkaloids and three polyenes, none is likely to be powerful contact sensitizers. Because they lack the crucial a-methylene group that is exocyclic to the α-lactone, as well as cyclopentenone and epoxy groups [197]. A. millefolium was reexamined, namely its flowers, which indicated the existence of five novel unsaturated guaianolides with peroxide property that had not been discovered before in Compositae species. The primary sensitizer must be considered a-peroxyachifolid [198].
6. Discussion and future perspectives
Herbal remedies continue to be an accepted complementary medical opinion throughout the world. There are more than 80 species of A. millefolium, each with its unique constituents. This review summarizes the safety, efficacy, pharmacodynamics, pharmacokinetics and herb-drug interactions of A. millefolium. A. millefolium is prescribed as a supplement for anti-inflammatory cases, liver disorders, bleeding disorders, dyspepsia and many other cases. A considerable point about this medicinal herb is that the polar portion of the extract has an antioxidant role and the oil showed antimicrobial effects. Therefore, it can have both antioxidant and antimicrobial effects. Two risk factors of kidney stone can be low volume of urine and also bacterial infections [199]. This plant increases diuresis by 25 % from initial volume without changing of urine pH in the patients with calcium type of urolithiasis. So A. milefolium has diuretic effects in addition to antimicrobial effect as mentioned [200]. So, these mechanisms can be possible for preventive and curative effects of A. millefolium [201]. Traditional and widespread beliefs hold that the herb A. millefolium is helpful for treating wounds, inflammation, and digestive issues. It may be helpful for these objectives due to its anti-inflammatory and perhaps anti-pathogenic properties, and its hemostyptic properties would independently assist ailments including skin injuries and hemorrhoids. Its less frequent usage for liver illness is consistent with its proven hepatoprotective effect. Additionally, A. millefolium is safe and well tolerated by people who are not allergic to it, according to animal research and a wealth of human experience. We have mentioned different types of interactions between A. millefolium and chemical drugs. Some of them are serious interactions like Topotecan-A. millefolium and some of them are minor ones such as antihistamines-A. millefolium. Coagulating or anticoagulating effects of A. millefolium are unreliable and can increase the risk of bleeding and bruising when taking other antiplatelet or anticoagulant medicines. As mentioned previously, there are many anticancer components in this plant. There are different mechanisms for this property, such asdifferent mechanisms for this property, for example, arresting the cell cycle at the G2/M phase in Kaempferol. Many other components have anti-inflammatory properties such as Camphene, a prostaglandin E2 inhibitor. A. millefolium by effecting on CYP450 can increase the concentration of certain drugs, which are a prostaglandin E2 inhibitor. A. millefolium by effecting on CYP450 can increase the concentration of certain drugs sensitive to hepatic enzymes. For example, increasing the concentration of Estradiol, Acetaminophen, Codein, and etc. Also, the leaf and flower extracts of A. millefolium were assumed to promote acid production in the stomach, lowering the efficiency of gastrointestinal tract medicines, but not due to CYP inhibition. The A. millefolium-CYP inhibition demonstrated here implies nonspecific activity against various human drug-metabolizing enzymes, which might result in a larger than predicted in vivo or clinical herb-drug interaction.
Also, as effective antioxidant, A. millefolium EO is suggested as a new potential source of natural additives for the food and pharmaceutical industries. Moreover, there is a link between the antioxidant activity of phenolic substances. The comparatively high concentrations of the phenolic chemicals carvacrol and thymol in EO may be responsible for its antioxidant action. However, the results showed that thymol and carvacrol are primarily responsible for the EO's antioxidant activity. On the other Nature's abundant anti-inflammatory phenol, salicylic acid (aka salicin), can be found in A. millefolium.
Also, A. millefolium has been utilized as an appetite stimulant for dyspeptic disorders and circulatory conditions such as hypertension. The active components in A. millefolium, Acillin and Leucodin were shown to lower blood pressure by relaxing blood vessels. In addition, the NO/cGMP pathway is involved in the relaxation of blood vessels. The process of the most likely by causing the endothelium to release NO and make more cGMP and by blocking calcium channels. Obviously, it is vital to evaluate the pharmacological interactions between this plant and blood pressure-lowering medications. A. millefolium hexane-ether-methanol extract exhibited antibacterial action against Staphylococcus aureus, Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, and Salmonella enteritidis. Essential oil of A. millefolium is considered effective against Streptococcus pneumoniae, Clostridium perfringens, Acetinobacter lawoffii, and Mycobacterium smegmatis. People also utilize it for its antipyretic properties and to struggle the common cold. According to the notable pharmacological effects of p-cymene of A. millefolium essential oil in the treatment of colds, and its antibacterial properties, this extract can be used to treat fever, colds, influenza and to control SARS-COVID. To influence nuclear translocation and viral proliferation, p-cymene binds to a nuclear localization signal (NLS) in the C-terminal structural domain of SARS-CoV with low micromolar affinity. P-cymene changes the NLS sites of influenza virus nucleoproteins, reducing their ability to bind with importin A. Because it works against viruses via this same mechanism, p-cymene can be used effectively in alone or in tandem with other antiviral drugs. Also, A. millefolium expands blood vessels and promotes sweating to eliminate toxins. Antibiotic properties of A. millefolium eliminate cold infections and act as a potent antimicrobial with a synergistic effect. This is why this plant is recommended for reducing cold-related coughing and sneezing.
7. Conclusion
Herb-drug interactions associated with A. millefolium were completely explained previously. Also, the pharmacokinetics and pharmacodynamics of this herb were discussed. As A. millefolium CYP the greatest interactions are for drugs with a narrow therapeutic index. No serious adverse effect was seen toward using this herb except contact dermatitis. As mentioned in this review, A. millefolium is traditionally believed to be a useful medicine for respiratory infections, digestive diseases, injuries, and inflammatory conditions. Anti-inflammatory and possibly anti-pathogenic activities can actually be beneficial in these cases. As reviewed, It has been shown to protect the liver. Activity is also compatible with its less common use. In addition, animal studies and extensive human experience have shown that yarrow is safe for those who are not allergic to it and is well tolerated. There is evidence that A. millefolium can play a significant role as a strong antioxidant source and positively affects gastrointestinal inflammations. Inflammation, cancer, dyspepsia, bacterial, viral, parasitic, helminth infections, and other pathological illnesses are only a few of the diseases and pathological conditions that the pharmacological qualities of A. millefolium suggest they be used as natural drugs in clinical settings. To determine the precise mechanism underlying, more study is required to determine the full scope of their pharmacological effects, particularly regarding their use as an antifertility agent, their ability to promote gastric motility and treat gastric ulcers, their potential to cause cytotoxic and genotoxic effects, to cardiovascular diseases, and their toxicity as a fumigant for the management. Additionally, many of the plant's pharmacological effects have neither been scientifically established nor linked to specific plant components. In addition to the uses and effects mentioned for this plant and its extract, other new studies also show new uses for it, such as the encapsulation of yarrow extract in nanoautosomal vesicles followed by encapsulation in a gel for topical delivery.
Data availability statement
Data in this study has not associated or deposited into a publicly available repository. All data in this study included in article and there's no other available data.
CRediT authorship contribution statement
Bahareh Farasati Far: Writing - review & editing, Writing - original draft, Software, Conceptualization. Golnaz Behzad: Writing - review & editing, Writing - original draft, Methodology, Investigation. Hasti Khalili: Writing - review & editing, Writing - original draft, Visualization, Software, Investigation.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
The authors would like to express their gratitude to BioRender.com for providing an invaluable platform that significantly contributed to the creation of visual elements and illustrations in this manuscript. The user-friendly interface and diverse library of scientific icons and templates offered by BioRender.com played a crucial role in enhancing the visual representation of complex concepts. The ability to seamlessly design high-quality figures greatly facilitated the communication of our research findings. We acknowledge BioRender.com for their commitment to supporting scientific communication and visualization, empowering researchers to convey their ideas with clarity and impact.
Contributor Information
Bahareh Farasati Far, Email: bahar.ferasati@gmail.com.
Golnaz Behzad, Email: golnazbehzad973@gmail.com.
Hasti Khalili, Email: hastikhalili78@gmail.com.
References
- 1.Verma R.S., Joshi N., Padalia R.C., Goswami P., Singh V.R., Chauhan A., et al. Chemical composition and allelopathic, antibacterial, antifungal and in vitro acetylcholinesterase inhibitory activities of yarrow (Achillea millefolium L.) native to India. Ind. Crop. Prod. 2017;104:144–155. [Google Scholar]
- 2.Radušiene J., Gudaityte O. Distribution of proazulenes in Achillea millefolium sl wild populations in relation to phytosociological dependence and morphological characters. Plant genetic resources. 2005;3(2):136–143. [Google Scholar]
- 3.Ayoobi F., Shamsizadeh A., Fatemi I., Vakilian A., Allahtavakoli M., Hassanshahi G., et al. Bio-effectiveness of the main flavonoids of Achillea millefolium in the pathophysiology of neurodegenerative disorders-a review. Iranian journal of basic medical sciences. 2017;20(6):604. doi: 10.22038/IJBMS.2017.8827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gorni P.H., Pacheco A.C., Lima Moro A., Silva J.F.A., Moreli R.R., de Miranda G.R., et al. Elicitation improves the leaf area, enzymatic activities, antioxidant activity and content of secondary metabolites in Achillea millefolium L. grown in the field. J. Plant Growth Regul. 2021;40(4):1652–1666. [Google Scholar]
- 5.Shrivastava A., Saxena P. Stability indicating reverse phase high performance liquid chromatography method for the estimation of capsaicin. Pharmaceutical methods. 2011;2(2):135–142. doi: 10.4103/2229-4708.84451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bimbiraitė K., Ragažinskienė O., Maruška A., Kornyšova O. Comparison of the chemical composition of four yarrow (Achilea millefolium L.) morphotypes. Biologija. 2008;54(3) [Google Scholar]
- 7.Pazouki L., Memari H.R., Kännaste A., Bichele R., Niinemets Ü. Germacrene A synthase in yarrow (Achillea millefolium) is an enzyme with mixed substrate specificity: gene cloning, functional characterization and expression analysis. Front. Plant Sci. 2015;6:111. doi: 10.3389/fpls.2015.00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zahara K., Tabassum S., Sabir S., Arshad M., Qureshi R., Amjad M.S., et al. A review of therapeutic potential of Saussurea lappa-An endangered plant from Himalaya. Asian Pac. J. Tropical Med. 2014;7:S60–S69. doi: 10.1016/S1995-7645(14)60204-2. [DOI] [PubMed] [Google Scholar]
- 9.Water F.C.O. Antioxidant activity, total phenolic and flavonoid content of water and ethanol extracts from Achillea millefolium L. Turk J Pharm Sci. 2013;10(3):385–392. [Google Scholar]
- 10.Li Y., Ni Z.-Y., Zhu M.-C., Zhang K., Wu Y.-B., Dong M., et al. Millifolides A–C. New 1, 10-Seco-guaianolides from the flowers of Achillea millefolium. Z. Naturforsch. B Chem. Sci. 2012;67(5):438–446. [Google Scholar]
- 11.Saeidnia S., Gohari A., Mokhber-Dezfuli N., Kiuchi F. A review on phytochemistry and medicinal properties of the genus Achillea. Daru: Journal of Faculty of Pharmacy, Tehran University of Medical Sciences. 2011;19(3):173. [PMC free article] [PubMed] [Google Scholar]
- 12.Li Y., Zhang M.-L., Cong B., Wang S.-M., Dong M., Sauriol F., et al. Achillinin A, a cytotoxic guaianolide from the flower of Yarrow, Achillea millefolium. Bioscience, biotechnology, and biochemistry. 2011 doi: 10.1271/bbb.110234. [DOI] [PubMed] [Google Scholar]
- 13.Akram M. Minireview on Achillea millefolium Linn. J. Membr. Biol. 2013;246(9):661–663. doi: 10.1007/s00232-013-9588-x. [DOI] [PubMed] [Google Scholar]
- 14.Vahid S., Dashti-Khavidaki S., Ahmadi F., Amini M., Surmaghi M.H.S. Effect of herbal medicine achillea millefolium on plasma nitrite and nitrate levels in patients with chronic kidney disease: a preliminary study. Iran J Kidney Dis. 2012;6(5):350. [PubMed] [Google Scholar]
- 15.Jenabi E., Fereidoony B. Effect of Achillea millefolium on relief of primary dysmenorrhea: a double-blind randomized clinical trial. J. Pediatr. Adolesc. Gynecol. 2015;28(5):402–404. doi: 10.1016/j.jpag.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 16.Liu B., Bussmann R.W., Batsatsashvili K., Kikvidze Z., Akobirshoeva A., Ghorbani A., et al. 2020. Achillea asiatica Serg. Achillea filipendulina Lam. Achillea millefolium L. Achillea setacea Waldst. & Kit. A steraceae. Ethnobotany of the Mountain Regions of Central Asia and Altai; pp. 33–43. [Google Scholar]
- 17.Başer K.H., Demirci B., Demirci F., Koçak S., Akıncı Ç., Malyer H., et al. Composition and antimicrobial activity of the essential oil of Achillea multifida. Planta Med. 2002;68(10):941–943. doi: 10.1055/s-2002-34923. [DOI] [PubMed] [Google Scholar]
- 18.Kazemi M. Phytochemical and antioxidant properties of Achillea millefolium from the eastern region of Iran. Int. J. Food Prop. 2015;18(10):2187–2192. [Google Scholar]
- 19.Sayed A., Bano H. Brinjasif (Achillea millefolium Linn): an efficacious unani medicine. Int J Herb Med. 2018;6:25–28. [Google Scholar]
- 20.Villalva M., Jaime L., Villanueva-Bermejo D., Lara B., Fornari T., Reglero G., et al. Supercritical anti-solvent fractionation for improving antioxidant and anti-inflammatory activities of an Achillea millefolium L. extract. Food Res. Int. 2019;115:128–134. doi: 10.1016/j.foodres.2018.08.027. [DOI] [PubMed] [Google Scholar]
- 21.Chávez-Silva F., Cerón-Romero L., Arias-Durán L., Navarrete-Vázquez G., Almanza-Pérez J., Román-Ramos R., et al. Antidiabetic effect of Achillea millefollium through multitarget interactions: α-glucosidases inhibition, insulin sensitization and insulin secretagogue activities. J. Ethnopharmacol. 2018;212:1–7. doi: 10.1016/j.jep.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 22.Akram M., Shah S.A., Karim M.A. Yarrow (Sultaani Buti/Barinjasif): famous aromatic and medicinal herb of Pakistan. Int J of Pharmaceuticals and Nutritional Sciences. 2018;2 [Google Scholar]
- 23.Dalili A., Ebrahimnia Milani S., Kamali N., Mohammadi S., Pakbaz M., Jamalnia S., et al. Beneficial effects of Achillea millefolium on skin injuries; a literature review. J. Essent. Oil Res. 2022:1–11. [Google Scholar]
- 24.Mouhid L., Gómez de Cedrón M., Vargas T., García-Carrascosa E., Herranz N., García-Risco M., et al. Identification of antitumoral agents against human pancreatic cancer cells from Asteraceae and Lamiaceae plant extracts. BMC Compl. Alternative Med. 2018;18(1):1–11. doi: 10.1186/s12906-018-2322-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.El-Kalamouni C., Venskutonis P.R., Zebib B., Merah O., Raynaud C., Talou T. Antioxidant and antimicrobial activities of the essential oil of Achillea millefolium L. grown in France. Medicines. 2017;4(2):30. doi: 10.3390/medicines4020030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aydın S., Sevindik E. Achillea millefolium L. subsp. millefolium essential oil's antifungal effect. European Journal of Biological Research. 2018;8(3):153–156. [Google Scholar]
- 27.Arias-Durán L., Estrada-Soto S., Hernández-Morales M., Chávez-Silva F., Navarrete-Vázquez G., León-Rivera I., et al. Tracheal relaxation through calcium channel blockade of Achillea millefolium hexanic extract and its main bioactive compounds. J. Ethnopharmacol. 2020;253 doi: 10.1016/j.jep.2020.112643. [DOI] [PubMed] [Google Scholar]
- 28.Al-Ezzy R.M., Al Anee R.S., Kathum O.A. Hepatoprotective effects of Achillea millefolium methanolic extract on carbon tetrachloride induced hepatotoxicity on albino male mice. International Journal of Advanced Research in Biological Sciences. 2017;4(8):98–109. [Google Scholar]
- 29.Sedighi M., Nasri H., Rafieian-kopaei M., Mortazaei S. Reversal effect of Achillea millefolium extract on ileum contractions. Journal of HerbMed Pharmacology. 2013;2(1):5–8. [Google Scholar]
- 30.Borrelli F., Romano B., Fasolino I., Tagliatatela‐Scafati O., Aprea G., Capasso R., et al. Prokinetic effect of a standardized yarrow (Achillea millefolium) extract and its constituent choline: studies in the mouse and human stomach. Neuro Gastroenterol. Motil. 2012;24(2):164-e90. doi: 10.1111/j.1365-2982.2011.01827.x. [DOI] [PubMed] [Google Scholar]
- 31.Zolghadri Y., Fazeli M., Kooshki M., Shomali T., Karimaghayee N., Dehghani M. Achillea millefolium L. hydro-alcoholic extract protects pancreatic cells by down regulating IL-1β and iNOS gene expression in diabetic rats. International journal of molecular and cellular medicine. 2014;3(4):255. [PMC free article] [PubMed] [Google Scholar]
- 32.Farajpour M., Ebrahimi M., Baghizadeh A., Aalifar M. Phytochemical and yield variation among Iranian Achillea millefolium accessions. Hortscience. 2017;52(6):827–830. [Google Scholar]
- 33.Belsito D.V., Klaassen C.D., Liebler D.C., Hill R.A. 2013. Amended Safety Assessment of Achillea Millefolium-Derived Ingredients as Used in Cosmetics. [Google Scholar]
- 34.Vallianou I., Hadzopoulou-Cladaras M. Camphene, a plant derived monoterpene, exerts its hypolipidemic action by affecting SREBP-1 and MTP expression. PLoS One. 2016;11(1) doi: 10.1371/journal.pone.0147117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hachlafi N.E., Aanniz T., Menyiy N.E., Baaboua A.E., Omari N.E., Balahbib A., et al. In vitro and in vivo biological investigations of camphene and its mechanism insights: a review. Food Rev. Int. 2021:1–28. [Google Scholar]
- 36.Becker L.C., Bergfeld W.F., Belsito D.V., Hill R.A., Klaassen C.D., Liebler D.C., et al. Safety assessment of Achillea millefolium as used in cosmetics. Int. J. Toxicol. 2016;35(3_suppl):5S–15S. doi: 10.1177/1091581816677717. [DOI] [PubMed] [Google Scholar]
- 37.Bakthavatsalam N. Academic Press; San Diego: 2016. Chapter 19-semiochemicals. Ecofriendly Pest Management for Food Security. [Google Scholar]
- 38.Salehi B., Upadhyay S., Erdogan Orhan I., Kumar Jugran A., Ld Jayaweera S., Dias D A., et al. Therapeutic potential of α-and β-pinene: a miracle gift of nature. Biomolecules. 2019;9(11):738. doi: 10.3390/biom9110738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Russo E.B. Taming THC: potential cannabis synergy and phytocannabinoid‐terpenoid entourage effects. Br. J. Pharmacol. 2011;163(7):1344–1364. doi: 10.1111/j.1476-5381.2011.01238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vieira A.J., Beserra F.P., Souza M., Totti B., Rozza A. Limonene: aroma of innovation in health and disease. Chem. Biol. Interact. 2018;283:97–106. doi: 10.1016/j.cbi.2018.02.007. [DOI] [PubMed] [Google Scholar]
- 41.Gupta A., Jeyakumar E., Lawrence R. Strategic approach of multifaceted antibacterial mechanism of limonene traced in Escherichia coli. Sci. Rep. 2021;11(1):1–15. doi: 10.1038/s41598-021-92843-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eddin L.B., Jha N.K., Meeran M.N., Kesari K.K., Beiram R., Ojha S. Neuroprotective potential of limonene and limonene containing natural products. Molecules. 2021;26(15):4535. doi: 10.3390/molecules26154535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Laude E.A., Morice A.H., Grattan T.J. The antitussive effects of menthol, camphor and cineole in conscious Guinea-pigs. Pulm. Pharmacol. 1994;7(3):179–184. doi: 10.1006/pulp.1994.1021. [DOI] [PubMed] [Google Scholar]
- 44.Zheng X-b, Zhang Y-l, Li Q., Liu Y-g, Wang X-d, Yang B-l, et al. Effects of 1, 8-cineole on neuropathic pain mediated by P2X2 receptor in the spinal cord dorsal horn. Sci. Rep. 2019;9(1):1–8. doi: 10.1038/s41598-019-44282-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.del Ecuador H. In: Camphor activates and strongly desensitizes the transient receptor potential vanilloid subtype 1 channel in a vanilloid-independent mechanism. Xu H., Blair N.T., Clapham D.E., editors. J Neurosci; 1985. Plantas medicinales. Quito: editorial ediciones Libri mundi; p. 379. 2005;25(39):8924-8937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Martin D., Valdez J., Boren J., Mayersohn M. Dermal absorption of camphor, menthol, and methyl salicylate in humans. J. Clin. Pharmacol. 2004;44(10):1151–1157. doi: 10.1177/0091270004268409. [DOI] [PubMed] [Google Scholar]
- 47.Juergens U. Anti-inflammatory properties of the monoterpene 1.8-cineole: current evidence for co-medication in inflammatory airway diseases. Drug Res. 2014;64(12):638–646. doi: 10.1055/s-0034-1372609. [DOI] [PubMed] [Google Scholar]
- 48.Jäger W., Nasel B., Nasel C., Binder R., Stimpfl T., Vycudilik W., et al. Pharmacokinetic studies of the fragrance compound 1,8-cineol in humans during inhalation. Chem. Senses. 1996;21(4):477–480. doi: 10.1093/chemse/21.4.477. [DOI] [PubMed] [Google Scholar]
- 49.Altınok-Yipel F., Tekeli İ.O., Şy Özsoy, Güvenç M., Kaya A., Yipel M. Hepatoprotective activity of linalool in rats against liver injury induced by carbon tetrachloride. Int. J. Vitam. Nutr. Res. 2020;90(3–4):302–308. doi: 10.1024/0300-9831/a000581. [DOI] [PubMed] [Google Scholar]
- 50.Elisabetsky E. In: Iwu M.M., Wootton J.C., editors. vol. 1. Elsevier; 2002. Chapter 11 - traditional medicines and the new paradigm of psychotropic drug action; pp. 133–144. (Advances in Phytomedicine). [Google Scholar]
- 51.Jiang D., Li D., Wu W. Inhibitory effects and mechanisms of luteolin on proliferation and migration of vascular smooth muscle cells. Nutrients. 2013;5(5):1648–1659. doi: 10.3390/nu5051648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sarawek S., Derendorf H., Butterweck V. Pharmacokinetics of luteolin and metabolites in rats. Nat. Prod. Commun. 2008;3(12) 1934578X0800301218. [Google Scholar]
- 53.Dall'Acqua S., Bolego C., Cignarella A., Gaion R.M., Innocenti G. Vasoprotective activity of standardized Achillea millefolium extract. Phytomedicine. 2011;18(12):1031–1036. doi: 10.1016/j.phymed.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 54.Luo Y., Shang P., Li D. Luteolin: a flavonoid that has multiple cardio-protective effects and its molecular mechanisms. Front. Pharmacol. 2017;8:692. doi: 10.3389/fphar.2017.00692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim S.S., Park R.Y., Jeon H.J., Kwon Y.S., Chun W. Neuroprotective effects of 3, 5‐dicaffeoylquinic acid on hydrogen peroxide‐induced cell death in SH‐SY5Y cells. Phytother Res.: An International Journal Devoted to Pharmacological and Toxicological Evaluation of Natural Product Derivatives. 2005;19(3):243–245. doi: 10.1002/ptr.1652. [DOI] [PubMed] [Google Scholar]
- 56.Chen Y.-L., Hwang T.-L., Yu H.-P., Fang J.-Y., Chong K.Y., Chang Y.-W., et al. Ilex kaushue and its bioactive component 3, 5-dicaffeoylquinic acid protected mice from lipopolysaccharide-induced acute lung injury. Sci. Rep. 2016;6(1):1–12. doi: 10.1038/srep34243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Radušienė J., Karpavičienė B., Raudone L., Vilkickyte G., Çırak C., Seyis F., et al. Trends in phenolic profiles of Achillea millefolium from different geographical gradients. Plants. 2023;12(4):746. doi: 10.3390/plants12040746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Salehi B., Venditti A., Sharifi-Rad M., Kręgiel D., Sharifi-Rad J., Durazzo A., et al. The therapeutic potential of apigenin. Int. J. Mol. Sci. 2019;20(6):1305. doi: 10.3390/ijms20061305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sadraei H., Asghari G., Khanabadi M., Minaiyan M. Anti-inflammatory effect of apigenin and hydroalcoholic extract of Dracocephalum kotschyi on acetic acid-induced colitis in rats. Res Pharm Sci. 2017;12(4):322. doi: 10.4103/1735-5362.212050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sarkar C., Mondal M., Torequl Islam M., Martorell M., Docea A.O., Maroyi A., et al. Potential therapeutic options for COVID-19: current status, challenges, and future perspectives. Front. Pharmacol. 2020;11 doi: 10.3389/fphar.2020.572870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kashyap P., Shikha D., Thakur M., Aneja A. Functionality of apigenin as a potent antioxidant with emphasis on bioavailability, metabolism, action mechanism and in vitro and in vivo studies: a review. J. Food Biochem. 2022;46(4) doi: 10.1111/jfbc.13950. [DOI] [PubMed] [Google Scholar]
- 62.Ren J., Lu Y., Qian Y., Chen B., Wu T., Ji G. Recent progress regarding kaempferol for the treatment of various diseases. Exp. Ther. Med. 2019;18(4):2759–2776. doi: 10.3892/etm.2019.7886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zabela V., Sampath C., Oufir M., Moradi-Afrapoli F., Butterweck V., Hamburger M. Pharmacokinetics of dietary kaempferol and its metabolite 4-hydroxyphenylacetic acid in rats. Fitoterapia. 2016;115:189–197. doi: 10.1016/j.fitote.2016.10.008. [DOI] [PubMed] [Google Scholar]
- 64.Yassa N., Saeidnia S., Pirouzi R., Akbaripour M., Shafiee A. Three phenolic glycosides and immunological properties of Achillea millefolium from Iran, population of Golestan. Daru. 2007;15(1):49–52. [Google Scholar]
- 65.Pires J.M., Mendes F.R., Negri G., Duarte‐Almeida J.M., Carlini E.A. Antinociceptive peripheral effect of Achillea millefolium L. and Artemisia vulgaris L.: both plants known popularly by brand names of analgesic drugs. Phytother Res.: An International Journal Devoted to Pharmacological and Toxicological Evaluation of Natural Product Derivatives. 2009;23(2):212–219. doi: 10.1002/ptr.2589. [DOI] [PubMed] [Google Scholar]
- 66.Espindola K., Ferreira R., Narvaez L.M., Silva A.G.B., Vieira A.P.O., Monteiro M.C. Chemical and pharmacological aspects of caffeic acid and its activity in hepatocarcinoma. Front. Oncol. 2019;9:541. doi: 10.3389/fonc.2019.00541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Khan F.A., Maalik A., Murtaza G. Inhibitory mechanism against oxidative stress of caffeic acid. J. Food Drug Anal. 2016;24(4):695–702. doi: 10.1016/j.jfda.2016.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Islam M., Shehzadi N., Salman M., Zahid F., Khan H.M., Amjad S., et al. Pharmacokinetics of caffeic acid from methanol seed extract of Syzygium cumini L in rats. Trop. J. Pharmaceut. Res. 2016;15(2):363–369. [Google Scholar]
- 69.Quercetin I., Immunity Y. Li, Yao J., Han C., J Yang M.T. Chaudhry, S Wang et al Nutrients. 2016;8(3):167. doi: 10.3390/nu8030167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Novo Belchor M., Hessel Gaeta H., Fabri Bittencourt Rodrigues C., Ramos da Cruz Costa C., de Oliveira Toyama D., Domingues Passero L.F., et al. Evaluation of rhamnetin as an inhibitor of the pharmacological effect of secretory phospholipase A2. Molecules. 2017;22(9):1441. doi: 10.3390/molecules22091441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ijaz F., Nawaz H., Hanif M.A., Ferreira P.M.P. Elsevier; 2020. Yarrow. Medicinal Plants of South Asia; pp. 685–697. [Google Scholar]
- 72.Van Wyk B.-E., Wink M. CABI; 2018. Medicinal plants of the world. [Google Scholar]
- 73.Alam M., Ahmed S., Elasbali A.M., Adnan M., Alam S., Hassan M.I., et al. Therapeutic implications of caffeic acid in cancer and neurological diseases. Front. Oncol. 2022;12 doi: 10.3389/fonc.2022.860508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kim M.-H., Lee S.-M., An K.-W., Lee M.-J., Park D.-H. Usage of natural volatile organic compounds as biological modulators of disease. Int. J. Mol. Sci. 2021;22(17):9421. doi: 10.3390/ijms22179421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang X., Yang Y., An Y., Fang G. The mechanism of anticancer action and potential clinical use of kaempferol in the treatment of breast cancer. Biomed. Pharmacother. 2019;117 doi: 10.1016/j.biopha.2019.109086. [DOI] [PubMed] [Google Scholar]
- 76.Barve A., Chen C., Hebbar V., Desiderio J., Saw C.L.L., Kong A.N. Metabolism, oral bioavailability and pharmacokinetics of chemopreventive kaempferol in rats. Biopharm Drug Dispos. 2009;30(7):356–365. doi: 10.1002/bdd.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Imran M., Salehi B., Sharifi-Rad J., Aslam Gondal T., Saeed F., Imran A., et al. Kaempferol: a key emphasis to its anticancer potential. Molecules. 2019;24(12):2277. doi: 10.3390/molecules24122277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Park M.-Y., Ji G.E., Sung M.-K. Dietary kaempferol suppresses inflammation of dextran sulfate sodium-induced colitis in mice. Dig. Dis. Sci. 2012;57(2):355–363. doi: 10.1007/s10620-011-1883-8. [DOI] [PubMed] [Google Scholar]
- 79.Sikka S.C., Bartolome A.R. Perfumery, essential oils, and household chemicals affecting reproductive and sexual health. Bioenvironmental Issues Affecting Men's Reproductive and Sexual Health: Elsevier. 2018:557–569. [Google Scholar]
- 80.Gupta J.K. Safety and toxicological profile of contemporary analeptics: prodigious focus on doxapram and almitrine. Res. J. Pharm. Technol. 2021;14(2):1104–1108. [Google Scholar]
- 81.Sedaghat A., Torshizi M.K. Immune responses, intestinal microbiota, performance and blood characteristics of Japanese quail fed on diets containing camphor. Animal. 2017;11(12):2139–2146. doi: 10.1017/S1751731117001148. [DOI] [PubMed] [Google Scholar]
- 82.Kerscher M., Buntrock H. CRC Press; 2017. Treatments for sensitive skin. Sensitive Skin Syndrome; pp. 202–211. [Google Scholar]
- 83.Sumithra M. Government Siddha Medical College; Chennai: 2017. Preclinical Evaluation of the Possible Mechanisms of Anti-hypertensive, Diuretic, Anti-oxidant Activities of Munthirikai Chooranam in Rodents. [Google Scholar]
- 84.Zuccarini P., Soldani G. Camphor: benefits and risks of a widely used natural product. Acta Biol. Szeged. 2009;53(2):77–82. [Google Scholar]
- 85.Mathew T. Essential oil-related brain disorders: an unexplored under-recognized conundrum. Neuro-Systemic Applications in Learning: Springer. 2021:243–260. [Google Scholar]
- 86.Zuccarini P., Soldani G. Camphor: benefits and risks of a widely used natural product. Acta Biol. Szeged. 2009;53:77–82. [Google Scholar]
- 87.Mihara S., Shibamoto T. The role of flavor and fragrance chemicals in TRPA1 (transient receptor potential cation channel, member A1) activity associated with allergies. Allergy Asthma Clin. Immunol. 2015;11(1):1–12. doi: 10.1186/s13223-015-0074-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Audrit K.J., Delventhal L., Aydin Ö., Nassenstein C. The nervous system of airways and its remodeling in inflammatory lung diseases. Cell Tissue Res. 2017;367(3):571–590. doi: 10.1007/s00441-016-2559-7. [DOI] [PubMed] [Google Scholar]
- 89.Christianson D.W. Structural and chemical biology of terpenoid cyclases. Chem Rev. 2017;117(17):11570–11648. doi: 10.1021/acs.chemrev.7b00287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rocha Caldas G.F., Oliveira ARdS., Araújo A.V., Lafayette S.S.L., Albuquerque G.S., Silva-Neto JdC, et al. Gastroprotective mechanisms of the monoterpene 1, 8-cineole (eucalyptol) PLoS One. 2015;10(8) doi: 10.1371/journal.pone.0134558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.McLean S., Boyle R., Brandon S., Davies N., Sorensen J. Pharmacokinetics of 1, 8-cineole, a dietary toxin, in the brushtail possum (Trichosurus vulpecula): significance for feeding. Xenobiotica. 2007;37(9):903–922. doi: 10.1080/00498250701570277. [DOI] [PubMed] [Google Scholar]
- 92.Juergens L.J., Worth H., Juergens U.R. New perspectives for mucolytic, anti-inflammatory and adjunctive therapy with 1, 8-cineole in COPD and asthma: review on the new therapeutic approach. Adv. Ther. 2020;37(5):1737–1753. doi: 10.1007/s12325-020-01279-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mohammadi T., Pirani A., Moazzeni H., Vaezi J. Achillea eriophora DC.: an ethnobotanical, pharmacological and phytochemical review. Ethnobot. Res. Appl. 2021;21(3) [Google Scholar]
- 94.Pourtaqi N., Imenshahidi M., Razavi B.M., Hosseinzadeh H. Effect of linalool on the acquisition and reinstatement of morphine-induced conditioned place preference in mice. Avicenna Journal of Phytomedicine. 2017;7(3):242. [PMC free article] [PubMed] [Google Scholar]
- 95.de Sousa D.P., de Sousa Oliveira F., de Almeida R.N. Evaluation of the central activity of hydroxydihydrocarvone. Biol. Pharm. Bull. 2006;29(4):811–812. doi: 10.1248/bpb.29.811. [DOI] [PubMed] [Google Scholar]
- 96.Becker V., Hui X., Nalbach L., Ampofo E., Lipp P., Menger M.D., et al. Linalool inhibits the angiogenic activity of endothelial cells by downregulating intracellular ATP levels and activating TRPM8. Angiogenesis. 2021;24(3):613–630. doi: 10.1007/s10456-021-09772-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bickers D., Calow P., Greim H., Hanifin J., Rogers A., Saurat J., et al. A toxicologic and dermatologic assessment of linalool and related esters when used as fragrance ingredients. Food Chem. Toxicol. 2003;41(7):919–942. doi: 10.1016/s0278-6915(03)00016-4. [DOI] [PubMed] [Google Scholar]
- 98.Kerns A.N. The Ohio State University; 2017. Elucidating the absorption and metabolism of linalool to understand its potential health benefits. [Google Scholar]
- 99.Shankar E., Goel A., Gupta K., Gupta S. Plant flavone apigenin: an emerging anticancer agent. Current pharmacology reports. 2017;3(6):423–446. doi: 10.1007/s40495-017-0113-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Johnston G.A.R., Chebib M., Duke R.K., Fernandez S.P., Hanrahan J.R., Hinton T., et al. In: Encyclopedia of Neuroscience. Squire L.R., editor. Academic Press; Oxford: 2009. Herbal products and GABA receptors; pp. 1095–1101. [Google Scholar]
- 101.Yan X., Qi M., Li P., Zhan Y., Shao H. Apigenin in cancer therapy: anti-cancer effects and mechanisms of action. Cell Biosci. 2017;7(1):1–16. doi: 10.1186/s13578-017-0179-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.DeRango-Adem E.F., Blay J. Does oral apigenin have real potential for a therapeutic effect in the context of human gastrointestinal and other cancers? Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.681477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gradolatto A., Canivenc-Lavier M.-C., Basly J.-P., Siess M.-H., Teyssier C. Metabolism of apigenin by rat liver phase I and phase II enzymes and by isolated perfused rat liver. Drug Metab Disposition. 2004;32(1):58–65. doi: 10.1124/dmd.32.1.58. [DOI] [PubMed] [Google Scholar]
- 104.Adil M., Dastagir G., Ambrin A., Bakht J. Phytochemical screening and antimicrobial activity of medicinally important achillea millefolium l and chaerophyllum villosum wall EXDC. Pakistan J. Bot. 2020;52(3):971–974. [Google Scholar]
- 105.al-Kulya H. EVALUATION OF IN-vitro activity of aerial part of BARANJASIF (Achillea millefolium Linn.) extracts. Indian Journal of Unani Medicine Vol XIV. 2021;(1):46–55. [Google Scholar]
- 106.Jaffal S.M., Abbas M.A. Antinociceptive action of Achillea biebersteinii methanolic flower extract is mediated by interaction with cholinergic receptor in mouse pain models. Inflammopharmacology. 2019;27(5):961–968. doi: 10.1007/s10787-018-0524-7. [DOI] [PubMed] [Google Scholar]
- 107.Shahani S., Hamzekanlu N., Zakeri N., Hosseinimehr S.J. Synergistic effect of Achillea millefolium L. combined with bleomycin on prostate cancer cell. Research in Molecular Medicine. 2015;3(1):12–17. [Google Scholar]
- 108.Tiwari P., Patel A.K., Dubey P., Gupta N., Bapna R., Pyati A.R., et al. Evaluation of anti-anxiety activity of Achillea millefolium Linn. International Journal of Pharmacy & Life Sciences. 2021;12(1) [Google Scholar]
- 109.Strzępek-Gomółka M., Gaweł-Bęben K., Kukula-Koch W. Achillea species as sources of active phytochemicals for dermatological and cosmetic applications. Oxid. Med. Cell. Longev. 2021:2021. doi: 10.1155/2021/6643827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Buza V., Cătană L., Andrei S., Ștefănuț L., Ș Răileanu, Matei M., et al. In vitro anthelmintic activity assessment of six medicinal plant aqueous extracts against donkey strongyles. J. Helminthol. 2020;94 doi: 10.1017/S0022149X20000310. [DOI] [PubMed] [Google Scholar]
- 111.Georgieva L., Gadjalova A., Mihaylova D., Pavlov A. Achillea millefolium L.-phytochemical profile and in vitro antioxidant activity. Int. Food Res. J. 2015;22(4) [Google Scholar]
- 112.Akkol E.K., İlhan M., Demirel M.A., Keleş H., Tümen I., Süntar İ. Thuja occidentalis L. and its active compound, α-thujone: promising effects in the treatment of polycystic ovary syndrome without inducing osteoporosis. J. Ethnopharmacol. 2015;168:25–30. doi: 10.1016/j.jep.2015.03.029. [DOI] [PubMed] [Google Scholar]
- 113.Dalsenter P.R., Cavalcanti A.M., Andrade A.J., Araújo S.L., Marques M.C. Reproductive evaluation of aqueous crude extract of Achillea millefolium L.(Asteraceae) in Wistar rats. Reprod. Toxicol. 2004;18(6):819–823. doi: 10.1016/j.reprotox.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 114.Khosh A.H., Hasanzadeh S., Jalali A.S., editors. Faculty of Veterinary Medicine. Urmia University; Urmia, Iran: 2017. Ameliorative effects of Achillea millefolium inflorescences alcoholic extract on nicotine-induced reproductive toxicity in male rat: apoptotic and biochemical evidences. Veterinary Research Forum. [PMC free article] [PubMed] [Google Scholar]
- 115.Guédon D., Abbe P., Lamaison J.L. Leaf and flower head flavonoids of Achillea millefolium L. subspecies. Biochem. Systemat. Ecol. 1993;21(5):607–611. [Google Scholar]
- 116.Bashir S., Noor A., Zargar M.I., Siddiqui N.A. Ethnopharmacology, phytochemistry, and biological activities of: a comprehensive review. Edible Plants in Health and Diseases. 2022:457–481. [Google Scholar]
- 117.Ahmadi Z., Saber M., Bagheri M., Mahdavinia G.R. Achillea millefolium essential oil and chitosan nanocapsules with enhanced activity against Tetranychus urticae. J. Pest. Sci. 2018;91(2):837–848. [Google Scholar]
- 118.Sant'Anna JRd, Franco CCdS., Miyamoto C.T., Cunico M.M., Miguel O.G., Côcco L.C., et al. Genotoxicity of Achillea millefolium essential oil in diploid cells of Aspergillus nidulans. Phytother Res.: An International Journal Devoted to Pharmacological and Toxicological Evaluation of Natural Product Derivatives. 2009;23(2):231–235. doi: 10.1002/ptr.2596. [DOI] [PubMed] [Google Scholar]
- 119.Lachenmeier D.W., Uebelacker M. Risk assessment of thujone in foods and medicines containing sage and wormwood–evidence for a need of regulatory changes? Regul. Toxicol. Pharmacol. 2010;58(3):437–443. doi: 10.1016/j.yrtph.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 120.Pudełek M., Catapano J., Kochanowski P., Mrowiec K., Janik-Olchawa N., Czyż J., et al. Therapeutic potential of monoterpene α-thujone, the main compound of Thuja occidentalis L. essential oil, against malignant glioblastoma multiforme cells in vitro. Fitoterapia. 2019;134:172–181. doi: 10.1016/j.fitote.2019.02.020. [DOI] [PubMed] [Google Scholar]
- 121.Srivastava P., Kumar P., Singh D., Singh V. Biological properties of thuja orientalis Linn. Adv. Life Sci. 2012;2(2):17–20. [Google Scholar]
- 122.Nawas T., Mitri S., Jaafar M. 2018. Inhibition of gram negative bacterial growth and biofilm formation by alpha thujone. [Google Scholar]
- 123.Tampe J., Parra L., Huaiquil K., Mutis A., Quiroz A. Repellent effect and metabolite volatile profile of the essential oil of Achillea millefolium against Aegorhinus nodipennis (Hope)(Coleoptera: Curculionidae) Neotrop. Entomol. 2015;44(3):279–285. doi: 10.1007/s13744-015-0278-5. [DOI] [PubMed] [Google Scholar]
- 124.Abass K., Reponen P., Mattila S., Pelkonen O. Metabolism of α-thujone in human hepatic preparations in vitro. Xenobiotica. 2011;41(2):101–111. doi: 10.3109/00498254.2010.528066. [DOI] [PubMed] [Google Scholar]
- 125.Pelkonen O., Abass K., Wiesner J. Thujone and thujone-containing herbal medicinal and botanical products: toxicological assessment. Regulatory Toxicology and pharmacology. 2013;65(1):100–107. doi: 10.1016/j.yrtph.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 126.Czyzewska M.M., Mozrzymas J.W. Monoterpene α-thujone exerts a differential inhibitory action on GABAA receptors implicated in phasic and tonic GABAergic inhibition. Eur. J. Pharmacol. 2013;702(1–3):38–43. doi: 10.1016/j.ejphar.2013.01.032. [DOI] [PubMed] [Google Scholar]
- 127.Waidyanatha S., Johnson J.D., Hong S.P., Robinson V.G., Gibbs S., Graves S.W., et al. Toxicokinetics of α-thujone following intravenous and gavage administration of α-thujone or α-and β-thujone mixture in male and female F344/N rats and B6C3F1 mice. Toxicol. Appl. Pharmacol. 2013;271(2):216–228. doi: 10.1016/j.taap.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 128.Höld K.M., Sirisoma N.S., Ikeda T., Narahashi T., Casida J.E. α-Thujone (the active component of absinthe): γ-aminobutyric acid type A receptor modulation and metabolic detoxification. Proc. Natl. Acad. Sci. USA. 2000;97(8):3826–3831. doi: 10.1073/pnas.070042397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Nikolić B., Vasilijević B., Mitić-Ćulafić D., Vuković-Gačić B., Knežević-Vukćević J. Comparative study of genotoxic, antigenotoxic and cytotoxic activities of monoterpenes camphor, eucalyptol and thujone in bacteria and mammalian cells. Chem. Biol. Interact. 2015;242:263–271. doi: 10.1016/j.cbi.2015.10.012. [DOI] [PubMed] [Google Scholar]
- 130.Nikolić B., Mitić-Ćulafić D., Vuković-Gačić B., Knežević-Vukčević J. Modulation of genotoxicity and DNA repair by plant monoterpenes camphor, eucalyptol and thujone in Escherichia coli and mammalian cells. Food Chem. Toxicol. 2011;49(9):2035–2045. doi: 10.1016/j.fct.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 131.Biswas R., Mandal S.K., Dutta S., Bhattacharyya S.S., Boujedaini N., Khuda-Bukhsh A.R. vol. 2011. Evid Based Complement Alternat Med; 2011. (Thujone-rich fraction of Thuja occidentalis demonstrates major anti-cancer potentials: evidences from in vitro studies on A375 cells). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Freitas M.M., Cavalcante P.M., Duarte-Filho L.A., Macedo C.A., Brito M.C., Menezes P.M., et al. Investigation of the relaxing effect of a camphor nanoemulsion on rat isolated trachea. Chem. Biol. Interact. 2021;348 doi: 10.1016/j.cbi.2021.109656. [DOI] [PubMed] [Google Scholar]
- 133.Ahmadi-Dastgerdi A., Ezzatpanah H., Asgary S., Dokhani S., Rahimi E. Phytochemical, antioxidant and antimicrobial activity of the essential oil from flowers and leaves of Achillea millefolium subsp. millefolium. Journal of Essential Oil Bearing Plants. 2017;20(2):395–409. [Google Scholar]
- 134.Santos C.D., Cabot J.C. Persistent effects after camphor ingestion: a case report and literature review. The Journal of Emergency Medicine. 2015;48(3):298–304. doi: 10.1016/j.jemermed.2014.05.015. [DOI] [PubMed] [Google Scholar]
- 135.Al-Muswie R.T., Alomer D.K., Al-Fartosi K.G. Toxopathological effects of camphor on some organs of female rats. Iraq Medical Journal. 2017;1(4) [Google Scholar]
- 136.Manorenj S., Inturi S., Pancheti N. Camphor poisoning presenting as acute diffuse demyelination of brain. Int J Community Med Public Health. 2016;3:2686–2688. [Google Scholar]
- 137.Du Z.-C., Xia Z.-S., Zhang M.-Z., Wei Y.-T., Malhotra N., Saputra F., et al. Sub-lethal camphor exposure triggers oxidative stress, cardiotoxicity, and cardiac physiology alterations in zebrafish embryos. Cardiovasc. Toxicol. 2021;21(11):901–913. doi: 10.1007/s12012-021-09682-x. [DOI] [PubMed] [Google Scholar]
- 138.Song J.-K., Du L.-D., Qiang G.-F., Du G.-H., Camphor . Springer; 2018. Natural Small Molecule Drugs from Plants; pp. 205–208. [Google Scholar]
- 139.Dosoky N.S., Setzer W.N. Maternal reproductive toxicity of some essential oils and their constituents. Int. J. Mol. Sci. 2021;22(5):2380. doi: 10.3390/ijms22052380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kumar S., Kavitha T., Angurana S.K. Kerosene, camphor, and naphthalene poisoning in children. Indian J. Crit. Care Med.: Peer-reviewed, Official Publication of Indian Society of Critical Care Medicine. 2019;23(Suppl 4):S278. doi: 10.5005/jp-journals-10071-23316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Aigbe F., Ajasa A.A., Omowumi B.T., Akpan U.-U.U., Adeyemi O.O. Cytotoxic and genotoxic activities of the aqueous extract of refined camphor. Nigerian Journal of Pharmaceutical and Applied Science Research. 2020;9(4):45–53. [Google Scholar]
- 142.Khine H., Weiss D., Graber N., Hoffman R.S., Esteban-Cruciani N., Avner J.R. A cluster of children with seizures caused by camphor poisoning. Pediatrics. 2009;123(5):1269–1272. doi: 10.1542/peds.2008-2097. [DOI] [PubMed] [Google Scholar]
- 143.Daniel P.S., Lourenço E.L.B., Sete da Cruz R.M., de Souza Goncalves C.H., Marques Das Almas L.R., Hoscheid J., et al. Composition and antimicrobial activity of essential oil of yarrow ('Achillea millefolium'L.) Aust. J. Crop. Sci. 2020;14(3):545–550. [Google Scholar]
- 144.Altunkaya A., Yıldırım B., Ekici K., Ö Terzioğlu. 2018. Determining essential oil composition, antibacterial and antioxidant activity of water wormwood extracts. [Google Scholar]
- 145.Wang X., Dong K., Ma Y., Jin Q., Yin S., Wang S. Hepatoprotective effects of chamazulene against alcohol-induced liver damage by alleviation of oxidative stress in rat models. Open Life Sci. 2020;15(1):251–258. doi: 10.1515/biol-2020-0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Mahdavi B., Ghorat F., Nasrollahzadeh M.S., Hosseyni-Tabar M., Rezaei-Seresht H. Chemical composition, antioxidant, antibacterial, cytotoxicity, and hemolyses activity of essential oils from flower of Matricaria chamomilla var. chamomilla. Anti-Infective Agents. 2020;18(3):224–232. [Google Scholar]
- 147.Boris T., Baričevič D., Batič F. Essential oil content, chamazulene content and antioxidative properties of Achillea millefolium agg. extracts from Slovenia. Acta Agric. Slov. 2021;117(2):1–10. [Google Scholar]
- 148.Wasilewski T., Seweryn A., Krajewski M. Improvement in the safety of use of hand dishwashing liquids through the addition of hydrophobic plant extracts. J. Surfactants Deterg. 2016;19(6):1315–1326. doi: 10.1007/s11743-016-1868-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ma D., He J., He D. Chamazulene reverses osteoarthritic inflammation through regulation of matrix metalloproteinases (MMPs) and NF-kβ pathway in in-vitro and in-vivo models. Biosci., Biotechnol., Biochem. 2020;84(2):402–410. doi: 10.1080/09168451.2019.1682511. [DOI] [PubMed] [Google Scholar]
- 150.Querio G., Antoniotti S., Foglietta F., Bertea C.M., Canaparo R., Gallo M.P., et al. Chamazulene attenuates ROS levels in bovine aortic endothelial cells exposed to high glucose concentrations and hydrogen peroxide. Front. Physiol. 2018;9:246. doi: 10.3389/fphys.2018.00246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.de Oliveira T.M., de Carvalho R.B.F., da Costa I.H.F., de Oliveira G.A.L., de Souza A.A., de Lima S.G., et al. Evaluation of p-cymene, a natural antioxidant. Pharmaceut. Biol. 2015;53(3):423–428. doi: 10.3109/13880209.2014.923003. [DOI] [PubMed] [Google Scholar]
- 152.Pinto L., Bonifacio M.A., De Giglio E., Cometa S., Logrieco A.F., Baruzzi F. Unravelling the antifungal effect of red thyme oil (Thymus vulgaris L.) compounds in vapor phase. Molecules. 2020;25(20):4761. doi: 10.3390/molecules25204761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.de Santana M.F., Guimarães A.G., Chaves D.O., Silva J.C., Bonjardim L.R., Lucca Júnior Wd, et al. The anti-hyperalgesic and anti-inflammatory profiles of p-cymene: evidence for the involvement of opioid system and cytokines. Pharmaceut. Biol. 2015;53(11):1583–1590. doi: 10.3109/13880209.2014.993040. [DOI] [PubMed] [Google Scholar]
- 154.Balahbib A., El Omari N., Hachlafi N.E., Lakhdar F., El Menyiy N., Salhi N., et al. Health beneficial and pharmacological properties of p-cymene. Food Chem. Toxicol. 2021;153 doi: 10.1016/j.fct.2021.112259. [DOI] [PubMed] [Google Scholar]
- 155.Dougnon G., Ito M. Role of Ascaridole and p-cymene in the sleep-promoting effects of Dysphania ambrosioides essential oil via the GABAergic system in a ddY mouse inhalation model. J. Nat. Prod. 2020;84(1):91–100. doi: 10.1021/acs.jnatprod.0c01137. [DOI] [PubMed] [Google Scholar]
- 156.Rohini G., Haribabu J., Aneesrahman K., Bhuvanesh N.S., Ramaiah K., Karvembu R., et al. Half-sandwich Ru (II)(η6-p-cymene) complexes bearing N-dibenzosuberenyl appended thiourea for catalytic transfer hydrogenation and in vitro anticancer activity. Polyhedron. 2018;152:147–154. [Google Scholar]
- 157.Marchese A., Arciola C.R., Barbieri R., Silva A.S., Nabavi S.F., Tsetegho Sokeng A.J., et al. Update on monoterpenes as antimicrobial agents: a particular focus on p-cymene. Materials. 2017;10(8):947. doi: 10.3390/ma10080947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Montani M., Pazmay G.V.B., Hysi A., Lupidi G., Pettinari R., Gambini V., et al. The water soluble ruthenium (II) organometallic compound [Ru (p-cymene)(bis (3, 5 dimethylpyrazol-1-yl) methane) Cl] Cl suppresses triple negative breast cancer growth by inhibiting tumor infiltration of regulatory T cells. Pharmacol. Res. 2016;107:282–290. doi: 10.1016/j.phrs.2016.03.032. [DOI] [PubMed] [Google Scholar]
- 159.Zhou Q., Li P., Lu R., Qian Q., Lei X., Xiao Q., et al. Synthesis, X‐ray diffraction study, and cytotoxicity of a cationic p‐cymene ruthenium chloro complex containing a chelating Semicarbazone Ligand. Z. Anorg. Allg. Chem. 2013;639(6):943–946. [Google Scholar]
- 160.Li J., Liu C., Sato T. Novel antitumor invasive actions of p-Cymene by decreasing MMP-9/TIMP-1 expression ratio in human fibrosarcoma HT-1080 cells. Biol. Pharm. Bull. 2016;39(8):1247–1253. doi: 10.1248/bpb.b15-00827. [DOI] [PubMed] [Google Scholar]
- 161.Bhatti M.Z., Ali A., Duong H.-Q., Chen J., Rahman F.-U. Anticancer activity and mechanism of bis-pyrimidine based dimetallic Ru (II)(η6-p-cymene) complex in human non-small cell lung cancer via p53-dependent pathway. J. Inorg. Biochem. 2019;194:52–64. doi: 10.1016/j.jinorgbio.2019.01.019. [DOI] [PubMed] [Google Scholar]
- 162.Agullo L., Romero-Silva M.J., Domenech M., Seeger M. p-Cymene promotes its catabolism through the p-cymene and the p-cumate pathways, activates a stress response and reduces the biofilm formation in Burkholderia xenovorans LB400. PLoS One. 2017;12(1) doi: 10.1371/journal.pone.0169544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Api A., Belsito D., Biserta S., Botelho D., Bruze M., Burton G., Jr., et al. RIFM fragrance ingredient safety assessment, p-cymene, CAS Registry Number 99-87-6. Food Chem. Toxicol. 2021;149(Suppl 1) doi: 10.1016/j.fct.2021.112051. [DOI] [PubMed] [Google Scholar]
- 164.Noor N., Alyami B.A., Alqarni A.O., Alqahtani Y.S., Mahnashi M.H., Akram M., et al. Appraisal of hypotensive effect of natural monoterpene: bornyl acetate and in-silico molecular docking analysis. Pak. J. Pharm. Sci. 2021;34 [PubMed] [Google Scholar]
- 165.Yang H., Zhao R., Chen H., Jia P., Bao L., Tang H. Bornyl acetate has an anti‐inflammatory effect in human chondrocytes via induction of IL‐11. IUBMB Life. 2014;66(12):854–859. doi: 10.1002/iub.1338. [DOI] [PubMed] [Google Scholar]
- 166.Wang X., Ma A., Shi W., Geng M., Zhong X., Zhao Y. Quercetin and bornyl acetate regulate t-lymphocyte subsets and inf-γ/il-4 ratio in utero in pregnant mice. Evid Based Complement Alternat Med. 2011;2011 doi: 10.1155/2011/745262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ravichandran C., Badgujar P.C., Gundev P., Upadhyay A. Review of toxicological assessment of d-limonene, a food and cosmetics additive. Food Chem. Toxicol. 2018;120:668–680. doi: 10.1016/j.fct.2018.07.052. [DOI] [PubMed] [Google Scholar]
- 168.Santana H.S., de Carvalho F.O., Silva E.R., Santos N.G., Shanmugam S., Santos D.N., et al. Anti-inflammatory activity of limonene in the prevention and control of injuries in the respiratory system: a systematic review. Curr. Pharmaceut. Des. 2020;26(18):2182–2191. doi: 10.2174/1381612826666200320130443. [DOI] [PubMed] [Google Scholar]
- 169.Bacanlı M., Anlar H.G., Aydın S., Çal T., Arı N., Üü Bucurgat, et al. D-limonene ameliorates diabetes and its complications in streptozotocin-induced diabetic rats. Food Chem. Toxicol. 2017;110:434–442. doi: 10.1016/j.fct.2017.09.020. [DOI] [PubMed] [Google Scholar]
- 170.Mukhtar Y.M., Adu-Frimpong M., Xu X., Yu J. Biochemical significance of limonene and its metabolites: future prospects for designing and developing highly potent anticancer drugs. Biosci. Rep. 2018;38(6) doi: 10.1042/BSR20181253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.de Almeida A.A.C., Silva R.O., Nicolau L.A.D., de Brito T.V., de Sousa D.P., Barbosa ALdR., et al. Physio-pharmacological investigations about the anti-inflammatory and antinociceptive efficacy of (+)-limonene epoxide. Inflammation. 2017;40(2):511–522. doi: 10.1007/s10753-016-0496-y. [DOI] [PubMed] [Google Scholar]
- 172.Araujo-Filho H.G., Pereira E.W., Rezende M.M., Menezes P.P., Araujo A.A., Barreto R.S., et al. D-limonene exhibits superior antihyperalgesic effects in a β-cyclodextrin-complexed form in chronic musculoskeletal pain reducing Fos protein expression on spinal cord in mice. Neuroscience. 2017;358:158–169. doi: 10.1016/j.neuroscience.2017.06.037. [DOI] [PubMed] [Google Scholar]
- 173.de Souza M.C., Vieira A.J., Beserra F.P., Pellizzon C.H., Nóbrega R.H., Rozza A.L. Gastroprotective effect of limonene in rats: influence on oxidative stress, inflammation and gene expression. Phytomedicine. 2019;53:37–42. doi: 10.1016/j.phymed.2018.09.027. [DOI] [PubMed] [Google Scholar]
- 174.Gupta A., Jeyakumar E., Lawrence R. Journey of limonene as an antimicrobial agent. J. Pure Appl. Microbiol. 2021;15(3):1094–1110. [Google Scholar]
- 175.Rolseth V., Djurhuus R., Svardal A.M. Additive toxicity of limonene and 50% oxygen and the role of glutathione in detoxification in human lung cells. Toxicology. 2002;170(1–2):75–88. doi: 10.1016/s0300-483x(01)00537-6. [DOI] [PubMed] [Google Scholar]
- 176.Wojtunik-Kulesza K.A. Toxicity of selected monoterpenes and essential oils rich in these compounds. Molecules. 2022;27(5):1716. doi: 10.3390/molecules27051716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Substance Infocard. Citral. Available online: https://echa.europa.eu/pl/substance-information/-/substanceinfo/100.023.994 [Internet]. (accessed on 22 February 2022).
- 178.Takzare N., Hosseini M.-J., Hamideh Mortazavi S., Safaie S., Moradi R. The effect of Achillea millefolium extract on spermatogenesis of male Wistar rats. Hum. Exp. Toxicol. 2011;30(4):328–334. doi: 10.1177/0960327110372401. [DOI] [PubMed] [Google Scholar]
- 179.Dhandapani S., Subramanian V.R., Rajagopal S., Namasivayam N. Hypolipidemic effect of Cuminum cyminum L. on alloxan-induced diabetic rats. Pharmacol. Res. 2002;46(3):251–255. doi: 10.1016/s1043-6618(02)00131-7. [DOI] [PubMed] [Google Scholar]
- 180.Wang I.-K., Lin-Shiau S.-Y., Lin J.-K. Induction of apoptosis by apigenin and related flavonoids through cytochrome c release and activation of caspase-9 and caspase-3 in leukaemia HL-60 cells. Eur. J. Cancer. 1999;35(10):1517–1525. [PubMed] [Google Scholar]
- 181.Hassanzadeh-Kiabi F., Negahdari B. Antinociceptive synergistic interaction between Achillea millefolium and Origanum vulgare L. extract encapsulated in liposome in rat. Artificial cells, nanomedicine, and biotechnology. 2018;46(5):994–1000. doi: 10.1080/21691401.2017.1354303. [DOI] [PubMed] [Google Scholar]
- 182.Tsai E.J., Kass D.A. Cyclic GMP signaling in cardiovascular pathophysiology and therapeutics. Pharmacol. Ther. 2009;122(3):216–238. doi: 10.1016/j.pharmthera.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Csupor D., Viczián R., Lantos T., Kiss T., Hegyi P., Tenk J., et al. The combination of hawthorn extract and camphor significantly increases blood pressure: a meta-analysis and systematic review. Phytomedicine. 2019;63 doi: 10.1016/j.phymed.2019.152984. [DOI] [PubMed] [Google Scholar]
- 184.Sharififar F., Pournourmohammadi S., Arabnejad M. 2009. Immunomodulatory activity of aqueous extract of Achillea wilhelmsii C. Koch in mice. [PubMed] [Google Scholar]
- 185.Ali S.I., Gopalakrishnan B., Venkatesalu V. Pharmacognosy, phytochemistry and pharmacological properties of Achillea millefolium L.: a review. Phytother Res. 2017;31(8):1140–1161. doi: 10.1002/ptr.5840. [DOI] [PubMed] [Google Scholar]
- 186.Khalighi Sigaroodi F., Jarvandi S., Taghizadeh M. Interactions of herbs with conventional drugs (Section 2) Journal of Medicinal Plants. 2003;2(8):1–16. [Google Scholar]
- 187.Jankel C.A., McMillan J.A., Martin B.C. Effect of drug interactions on outcomes of patients receiving warfarin or theophylline. Am. J. Health Syst. Pharm. 1994;51(5):661–666. [PubMed] [Google Scholar]
- 188.Wiseman A. Elimination of major side effects due to ROS of therapeuticals through biotransformation control of the 57 cytochromes P450 isoenzymes. Med. Hypotheses. 2005;64(4):802–805. doi: 10.1016/j.mehy.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 189.Ameer B., Weintraub R.A. Drug interactions with grapefruit juice. Clin. Pharmacokinet. 1997;33(2):103–121. doi: 10.2165/00003088-199733020-00003. [DOI] [PubMed] [Google Scholar]
- 190.Choi J.-S., Piao Y.-J., Kang K.W. Effects of quercetin on the bioavailability of doxorubicin in rats: role of CYP3A4 and P-gp inhibition by quercetin. Arch Pharm. Res. (Seoul) 2011;34(4):607–613. doi: 10.1007/s12272-011-0411-x. [DOI] [PubMed] [Google Scholar]
- 191.Pal D., Mitra A.K. MDR-and CYP3A4-mediated drug–herbal interactions. Life Sci. 2006;78(18):2131–2145. doi: 10.1016/j.lfs.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 192.http://reference.medscape.com/drug/quercetin-344495#3 [Internet]. (Accessed September 16, 2015).
- 193.Wang Y.-H., Chao P.-D.L., Hsiu S.-L., Wen K.-C., Hou Y.-C. Lethal quercetin-digoxin interaction in pigs. Life Sci. 2004;74(10):1191–1197. doi: 10.1016/j.lfs.2003.06.044. [DOI] [PubMed] [Google Scholar]
- 194..
- 195.Chen S., Tang Y., Gao Y., Nie K., Wang H., Su H., et al. Antidepressant potential of quercetin and its glycoside derivatives: a comprehensive review and update. Front. Pharmacol. 2022;13 doi: 10.3389/fphar.2022.865376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Rouhi-Boroujeni H., Gharipour M., Mohammadizadeh F., Ahmadi S., Rafieian-Kopaei M. Systematic review on safety and drug interaction of herbal therapy in hyperlipidemia: a guide for internist. Acta Biomed. 2015;86(2):130–136. [PubMed] [Google Scholar]
- 197.Hausbn B., Bheuer J., Weglewski J., Rucker G. α‐Peroxyachifolid and other new sensitizing sesquiterpene lactones from yarrow (Achillea millefolium L., Compositae) Contact Dermatitis. 1991;24(4):274–280. doi: 10.1111/j.1600-0536.1991.tb01722.x. [DOI] [PubMed] [Google Scholar]
- 198.Saeidnia S., Gohari A., Mokhber-Dezfuli N., Kiuchi F. A review on phytochemistry and medicinal properties of the genus Achillea. Daru. 2011;19(3):173–186. [PMC free article] [PubMed] [Google Scholar]
- 199.Kramer G., Klingler H.C., Steiner G.E. Role of bacteria in the development of kidney stones. Curr. Opin. Urol. 2000;10(1):35–38. doi: 10.1097/00042307-200001000-00009. [DOI] [PubMed] [Google Scholar]
- 200.Gaybullaev A., Kariev S. Phytotherapy of calcium urolithiasis with extracts of medicinal plants: changes of diuresis, urine pH and crystalluria. Appl. Technol. Innovat. 2012;7(2) [Google Scholar]
- 201.Bafrani H.H., Parsa Y., Yadollah-Damavandi S., Jangholi E., Ashkani-Esfahani S., Gharehbeglou M. Biochemical and pathological study of hydroalcoholic extract of Achillea millefolium l. on ethylene glycol-induced nephrolithiasis in laboratory rats. N. Am. J. Med. Sci. 2014;6(12):638. doi: 10.4103/1947-2714.147981. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data in this study has not associated or deposited into a publicly available repository. All data in this study included in article and there's no other available data.