Abstract
These guidelines for the diagnosis and management of cholangiocarcinoma (CCA) were commissioned by the British Society of Gastroenterology liver section. The guideline writing committee included a multidisciplinary team of experts from various specialties involved in the management of CCA, as well as patient/public representatives from AMMF (the Cholangiocarcinoma Charity) and PSC Support. Quality of evidence is presented using the Appraisal of Guidelines for Research and Evaluation (AGREE II) format. The recommendations arising are to be used as guidance rather than as a strict protocol-based reference, as the management of patients with CCA is often complex and always requires individual patient-centred considerations.
Keywords: cholangiocarcinoma
Executive summary and list of recommendations
The management of CCA should be undertaken at centres with expertise across all relevant specialties, including surgery, interventional radiology, endoscopy, hepatobiliary medicine, oncology and pathology.
Recommendation 1: All patients with CCA discussed at multidisciplinary team (MDT) meetings should be classified as best as possible into either intrahepatic, perihilar or distal CCA. This should be clearly recorded in the MDT outcome discussion.
Strength of recommendation: STRONG
Quality of evidence: MODERATE
Recommendation 2: The requirement to have tissue available for molecular profiling to inform treatment decisions should be considered when immunohistochemistry is planned on lesional biopsy material.
Strength of recommendation: STRONG
Quality of evidence: HIGH
Recommendation 3: A diagnosis of combined hepatocellular-CCA should be made on morphological pathological grounds only.
Strength of recommendation: MODERATE
Quality of evidence: MODERATE
Recommendation 4: All centres managing patients with CCA should have clear established diagnostic pathways for patients presenting with jaundice/biliary obstruction, with streamlined transition to local and regional hepato-pancreato-biliary (HPB) MDT meetings.
Strength of recommendation: STRONG
Quality of evidence: HIGH
Recommendation 5: Having completed imaging, all patients should undergo a detailed review of clinical presentation, examination findings, blood investigations and imaging, ideally at a regionally coordinated hepatobiliary MDT meeting, with prompt assessment of the results and communication with the patient.
Strength of recommendation: STRONG
Quality of evidence: HIGH
Recommendation 6: Consideration should be given to possible benign causes of biliary tract stricturing/obstruction during MDT discussion, in correlation with appropriate serological investigations and clinical history, to ensure that alternative diagnoses are considered while a pathological diagnosis of CCA is secured.
Strength of recommendation: STRONG
Quality of evidence: MODERATE
Recommendation 7: Contrast enhanced multiphasic CT of the chest, abdomen and pelvis to stage the primary tumour, including assessment of local vascular relationships, should be undertaken for all types of CCA.
Strength of recommendation: STRONG
Quality of evidence: MODERATE
Recommendation 8: Contrast enhanced MRI and magnetic resonance cholangiopancreatography (MRCP) should be undertaken for perihilar and intrahepatic tumours to better delineate the extent of biliary involvement and identify any satellites/intrahepatic metastases.
Strength of recommendation: STRONG
Quality of evidence: MODERATE
Recommendation 9: For tumours involving the more distal extrahepatic duct, MRI is unlikely to add any further information over and above CT.
Strength of recommendation: MODERATE
Quality of evidence: LOW
Recommendation 10: 18 F-fluoro-deoxy-glucose positron emission tomography ( 18 FDG-PET) CT for detection of nodal and distant metastatic disease is recommended as part of staging investigations.
Strength of recommendation: STRONG
Quality of evidence: MODERATE
Recommendation 11: Ultrasound (US) or CT-guided biopsy of the primary intrahepatic tumour or metastatic lesions should be undertaken to acquire a pathological diagnosis following MDT discussion and consensus.
Strength of recommendation: STRONG
Quality of evidence: HIGH
Recommendation 12: Before undertaking any endoscopic investigations for a suspected CCA, all patients should have undergone a triple-phase CT scan of the abdomen/pelvis and chest along with dynamic MRI and MRCP if proximal biliary obstruction is suspected.
Strength of recommendation: STRONG
Quality of evidence: MODERATE
Recommendation 13: Patients with operable distal malignant tract obstruction (DMTO) should undergo a combination of endoscopic US and endoscopic retrograde cholangiopancreatography (ERCP) to try to confirm a malignant histological diagnosis before proceeding to surgery.
Strength of recommendation: MODERATE
Quality of evidence: LOW
Recommendation 14: In a suspected case of operable distal CCA, in the absence of jaundice, a standalone endoscopic ultrasound (EUS) scan should be undertaken first, to avoid the complications of ERCP, which could delay or render the patient inoperable.
Strength of recommendation: WEAK
Quality of evidence: LOW
Recommendation 15: In the presence of jaundice and DMTO, where EUS is not available, patients may only be able to have an ERCP and brush cytology in the first instance to confirm the presence of a CCA.
Strength of recommendation: MODERATE
Quality of evidence: MODERATE
Recommendation 16: At present biliary biomarkers cannot be recommended as a replacement for cytological and histological standards. However, biliary next-generation sequencing shows great promise and should be taken forward for replicative National Institute for Health Research/UK Research and Innovation (NIHR/UKRI) funded multisite studies.
Strength of recommendation: MODERATE
Quality of evidence: MODERATE
Recommendation 17: It should be realised that a cytological/histological confirmation of a malignant biliary tract obstruction (MBTO) is imperfect at present, and in cases where uncertainty remains, a decision on follow-up imaging versus surgery for a definitive diagnosis should be reached only after a full discussion between thepatient and the clinician. These guidelines acknowledge that it is acceptable to offer surgery where histological confirmation cannot exclude malignancy with absolute certainty and surgery might provide a cure and a secure diagnosis.
Strength of recommendation: STRONG
Quality of evidence: LOW
Recommendation 18: The decision to drain preoperative jaundice in distal CCA causing DMTO should be made in accordance with local hepato-pancreatico-biliary (HPB) centre guidance. In cases where rapid access to surgery can be offered, it may be appropriate to bypass biliary drainage at ERCP to avoid ERCP-related complications and postoperative sepsis.
Strength of recommendation: STRONG
Quality of evidence: HIGH
Recommendation 19: Patients with DMTO with inoperable disease from distal CCA should undergo an EUS/ERCP or standalone ERCP to confirm a pathological diagnosis and have their jaundice palliated.
Strength of recommendation: STRONG
Quality of evidence: HIGH
Recommendation 20: Patients with DMTO from distal CCA should have a fully covered self-expanding metal stent placed. Plastic stents should not be placed for long-term palliation of jaundice.
Strength of recommendation: STRONG
Quality of evidence: HIGH
Recommendation 21: Where patients cannot have a stent placed at ERCP, we recommend that EUS guided biliary drainage is undertaken rather than percutaneous transhepatic cholangiography (PTC). However, PTC can be offered if EUS bile duct drainage is not locally available.
Strength of recommendation: STRONG
Quality of evidence: HIGH
Recommendation 22: No patient with a perihilar CCA should undergo endotherapy until the case has been fully discussed at an HPB treatment centre.
Strength of recommendation: STRONG
Quality of evidence: LOW
Recommendation 23: Unilateral drainage in the future remnant lobe should be considered ahead of surgery. Bilateral/further stenting should only be considered if the level of preoperative jaundice does not improve, or there is cholangitis in residual obstructed biliary segments.
Strength of recommendation: MODERATE
Quality of evidence: MODERATE
Recommendation 24: Inoperable perihilar CCA - proximal malignant tract obstruction (PMTO) and jaundice should be considered for palliative stenting by either ERCP or PTC. Decisions about unilobar (UL) versus bilobar (BL) stenting should be predetermined by the local MDT depending on both local availability and expertise.
Strength of recommendation: STRONG
Quality of evidence: MODERATE
Recommendation 25: At present the use of adjunctive endobiliary radiofrequency ablation (RFA) and photodynamic therapy is not considered standard of care for patients with hilar and distal CCA receiving palliative care.
Strength of recommendation: STRONG
Quality of evidence: HIGH
Recommendation 26: EUS guided biliary drainage is recognised as a treatment option, but use of this technique should be planned at a MDT meeting with units adopting this approach able to show clear audit data in relation to alternative and more traditional methods of biliary drainage.
Strength of recommendation: MODERATE
Quality of evidence: MODERATE
Recommendation 27: Patients should have a clear monitoring pathway for early detection of recurrent stent blockage and on-demand endoscopic intervention.
Strength of recommendation: STRONG
Quality of evidence: LOW
Standard 28: Patients with recurrent pain after biliary stenting during their disease process should be evaluated with cross-sectional imaging. Patients with stent dysfunction should also be re-evaluated with cross-sectional imaging before any further endotherapy is undertaken.
Strength of recommendation: MODERATE
Quality of evidence: LOW
Recommendation 29: High-resolution cross-sectional imaging is essential for assessment of resectability and accurate staging.
Strength of recommendation: STRONG
Quality of evidence: HIGH
Recommendation 30: Preoperative preparation, including augmentation of the functional liver remnant (FLR) and biliary drainage, may be required to ensure safe resection.
Strength of recommendation: STRONG
Quality of evidence: HIGH
Recommendation 31: Staging laparoscopy should be used selectively.
Strength of recommendation: STRONG
Quality of evidence: MODERATE
Recommendation 32: R0 resection is the only curative treatment available.
Strength of recommendation: STRONG
Quality of evidence: MODERATE
Recommendation 33: Surgical resection of CCA should be undertaken only at high-volume centres with expertise across all relevant supporting specialties, including interventional radiology, endoscopy, hepatobiliary medicine, oncology and pathology.
Strength of recommendation: STRONG
Quality of evidence: MODERATE
Recommendation 34: Liver transplantation for selected patients with perihilar CCA (pCCA) in the presence of chronic liver disease (most commonly primary sclerosing cholangitis), less than 3 cm in size with no evidence of extrahepatic disease, results in long-term disease-free survival. This is an established indication in an increasing number of centres internationally. There is a need for evaluation of novel neoadjuvant chemoradiation strategies and assessment of long-term outcomes with national protocols and multicentre studies. Liver transplantation in the absence of background chronic liver disease remains an investigational treatment.
Strength of recommendation: MODERATE
Quality of evidence: STRONG
Recommendation 35: Neoadjuvant therapy in uncontrolled studies appears to be effective in controlling disease and selecting patients who are most likely to benefit from transplantation.
Strength of recommendation: MODERATE
Quality of evidence: LOW
Recommendation 36: Transplantation for intrahepatic CCA (iCCA) on a background of chronic liver disease precluding resection should be evaluated prospectively within a national protocol. LAG tumour size criteria to be monitored and modified to improve recruitment for evaluation.
Strength of recommendation: STRONG
Quality of evidence: MODERATE
Recommendation 37: Patients who have undergone surgical resection for CCA should be considered for 24 weeks of adjuvant chemotherapy (currently capecitabine).
Strength of recommendation: STRONG
Quality of evidence: MODERATE
Recommendation 38: The routine use of neoadjuvant chemotherapy in patients with resectable CCA is not recommended.
Strength of recommendation: STRONG
Quality of evidence: LOW
Recommendation 39: Cisplatin plus gemcitabine (CisGem) chemotherapy is recommended as the first-line treatment in patients with advanced biliary tract cancer (BTC). Immunotherapy may be added to CisGem chemotherapy, if approved and available, cognisant of the magnitude of benefit and toxicities.
Strength of recommendation: STRONG
Quality of evidence: HIGH
Recommendation 40: Combination chemotherapy is recommended in patients with adequate performance status following failure of first-line chemotherapy, particularly in the absence of a targetable molecular alteration.
Strength of recommendation: MODERATE
Quality of evidence: HIGH
Recommendation 41: CCA should be subjected to molecular profiling at the earliest opportunity, and results and treatment options should be reviewed by clinicians with appropriate expertise.
Strength of recommendation: STRONG
Quality of evidence: HIGH
Recommendation 42: Consider the use of adjuvant chemoradiotherapy for extrahepatic CCA or gallbladder cancer and a microscopically positive surgical margin resection (R1 resection) with a shared decision-making approach, considering the risk of potential harm and potential for benefit.
Strength of recommendation: MODERATE
Quality of evidence: MODERATE
Recommendation 43: Consider the delivery of stereotactic radiotherapy (SBRT) or proton beam therapy (PBT) in patients with locally advanced inoperable CCA who have received systemic therapy. Modern radiotherapy techniques should be employed to maximise radiotherapy dose and minimise toxicity.
Strength of recommendation: MODERATE
Quality of evidence: LOW
Recommendation 44a: Refer patients with symptomatic metastatic disease for consideration of palliative radiotherapy.
Strength of recommendation: MODERATE
Quality of evidence: MODERATE
Recommendation 44b: Refer for SBRT in the setting of oligometastatic disease.
Strength of recommendation: MODERATE
Quality of evidence: MODERATE
Recommendation 45: All patients with incurable CCA should have access to a palliative care assessment to fully evaluate their holistic care needs. Evidence suggests that early palliative care involvement is associated with higher health-related quality of life and lower rates of depression. Good symptom control should be delivered alongside active oncology management.
Strength of recommendation: MODERATE
Quality of evidence: MODERATE
Recommendation 46: Development and funding of clinical trials is key to fully evaluate the impact of pharmacological management of symptoms in patients with CCA and different models of care.
Strength of recommendation: MODERATE
Quality of evidence: LOW
Recommendation 47: All patients diagnosed with CCA should have access to a hepatobiliary cancer nurse specialist who can provide expertise and support to the patient and their immediate family.
Strength of recommendation: STRONG
Quality of evidence: MODERATE
Recommendation 48: All patients diagnosed with CCA should have access to a dietician.
Strength of recommendation: STRONG
Quality of evidence: MODERATE
Recommendation 49: All patients diagnosed with CCA should have timely access to high-quality information and should be diected to a dedicated CCA patient charity so that they can access support and information.
Strength of recommendation: STRONG
Quality of evidence: LOW
Recommendation 50: All patients with CCA should be enabled to access a second specialist clinical opinion if they need to seek reassurance about either their diagnosis or treatment.
Strength of recommendation: MODERATE
Quality of evidence: LOW
Scope and purpose
These guidelines have been commissioned on behalf of the British Society of Gastroenterology (BSG) liver section with the aim of updating and assisting clinicians in the diagnosis and management of patients with cholangiocarcinoma. The previous version was published in 2012.1 These guidelines do not cover gallbladder cancer or neoplasia of the ampulla of Vater/duodenum. Members of the writing committee included: gastroenterologists, hepatologists, transplant physicians, radiologists, hepatobiliary surgeons, hepatobiliary endoscopists, oncologists, histopathologists, patient representatives (from AMMF and PSC Support), and colleagues from Cholangiocarcinoma-UK (a specialist interest group within the British Association for the Study of the Liver, BASL). Where appropriate and feasible, specific clinically applicable recommendations are provided. The guidelines were reviewed and endorsed by the BSG Clinical Standards and Services Committee. We recommend this document be used in conjunction with other BSG guidelines and similar themed publications by other international bodies (such as recommendations from the European Network for the Study of Cholangiocarcinoma (ENS-CCA), the European Association for the Study of the Liver, the International Liver Cancer Association and the European Society for Medical Oncology). We also recommend revision of the guidelines in, at most, 5 years.
Evidence base
These guidelines have been produced with a systematic review of publications identified using PubMed Medline, and Cochrane database searches. Each section of the guideline was allocated at least one lead author responsible for performing a comprehensive literature search. The literature search was updated and completed in November 2022 before submission for peer review. Where possible, guidance is based on the highest levels of evidence available. Where no high-quality studies or clear evidence exist, guidance is based on the majority consensus advice of expert opinion in the literature and the writing committee. All recommendations achieved complete consensus following extensive review and discussion among the guideline development group. The grade of evidence is presented according to the international GRADE system2 3 as follows:
High-quality evidence: The authors are very confident that the estimate presented lies very close to the true value. One could interpret it as: there is very low probability of further research completely changing the presented conclusions.
Moderate-quality evidence: The authors are confident that the presented estimate lies close to the true value, but it is also possible that it might be substantially different. Hence further research might alter the conclusions completely.
Low-quality evidence: The authors are not confident of the effect estimate and the true value might be substantially different—that is, further research is likely to change the presented conclusions completely.
Very low-quality evidence: The authors do not have any confidence in the estimate and it is likely that the true value is substantially different from it. One could interpret it as: new research will most probably change the presented conclusions completely.
All members of the guideline working group were asked to complete conflicts of interest declarations. These are available as online supplemental file 1.
gutjnl-2023-330029supp001.pdf (74.7KB, pdf)
Background
CCA is a frequently lethal liver cancer arising from epithelial cells, cholangiocytes, anywhere along the biliary tree within or external to the liver.4 5 These are exceptionally desmoplastic tumours and are enmeshed in a dense network of inflammatory cells and extracellular matrix, called the tumour immune microenvironment.6 CCA are typically classified into three subtypes according to their anatomical site of origin: intrahepatic (iCCA), perihilar (pCCA) and distal (dCCA) CCA, with pCCA and dCCA collectively referred to as extrahepatic CCA (eCCA). iCCA by definition arises within the liver parenchyma, proximal to the second order bile ducts and comprises the second most common form of primary liver cancer globally, after hepatocellular carcinoma (HCC).7 pCCA is localised between the second-degree bile ducts and the insertion of the cystic duct into the common bile duct. dCCA is confined to the common bile duct below the cystic duct insertion. Historic studies report that pCCA accounts for around 50–60% of all CCA, and iCCA accounts for less than 20% of CCA.8 These CCA subtypes are heterogeneous and can vary in their respective clinical presentations, risk factors, routes to diagnosis and clinical management, as well as exhibiting distinct epidemiological, clinical, molecular and genetic characteristics.9
Patients diagnosed with CCA have a high mortality because they typically present too late for surgical resection or transplantation, the only potentially curative options. The clinical presentation of CCA typically depends on its location. pCCA and dCCA are likely to present with obstructive jaundice as well as other constitutional symptoms. iCCA, unless extending into the hilum, tends not to present with jaundice but rather with more non-specific symptoms, such as weight loss, anorexia, abdominal discomfort, nausea and malaise. iCCA can be an incidental finding in around 20% of cases4 5—for example, during surveillance for HCC, or following imaging for another reason. A diagnosis of iCCA can also occur after resection/transplant for a tumour originally deemed to have been something else, such as an HCC or a carcinoma of unknown primary. Diagnosis of anatomical subtype can be clinically and radiologically challenging with some large CCAs which extend into the perihilar or extrahepatic bile ducts, making the site of origin unclear. How to record this uncertainty at MDT meetings in a standardised and systematic way to facilitate epidemiological studies has yet to be resolved.
Epidemiology and risk factors
Recommendation 1: All patients with CCA discussed at MDT meetings should be classified as best as possible into either intrahepatic, perihilar or distal CCA. This should be clearly recorded in the MDT outcome discussion.
Strength of recommendation: STRONG
Quality of evidence: MODERATE
Consistent findings reported over the past two decades are the rising incidence and mortality rates for iCCA and declining rates for eCCA.10–13 A recent study of the National Cancer Registration Dataset reported that almost 51 000 BTC were diagnosed in England during 2001–2018.14 CCA were the most commonly diagnosed BTC (63%) followed by gallbladder (23%) and ampulla of Vater (14%). 74% of CCA were iCCA, a higher proportion compared with historic studies. Over 95% of CCA were diagnosed in patients aged 50 years or older, with the median age at diagnosis being 75. Men and women were approximately equally affected. The age -standardised incidence rate for CCA rose from 2.9 per 100 000 population in 2001–2003 to 4.6 in 2016–2018. The rise in CCA incidence was predominantly in iCCA (figure 1) with age-standardised incidence rates increasing from 2.1 to 3.4 between 2001–2003 and 2016–2018, whereas for the same time period the rise in eCCA was from 0.6 to 1.0. There was evidence of geographical variation in CCA incidence between regional Cancer Alliances in England. The age-standardised mortality rates of CCA rose from 2.6 to 4.9 between 2001–2003 and 2016–2018 in parallel with the incidence rates. The trends for eCCA and iCCA age-standardised mortality rates mirrored those of incidence, with most deaths due to iCCA. The most common route to diagnosis was the emergency route (iCCA 50.4%, eCCA 46.1%), highlighting the late presentation of this disease. Overall survival after diagnosis of CCA was less than 10%.
Figure 1.
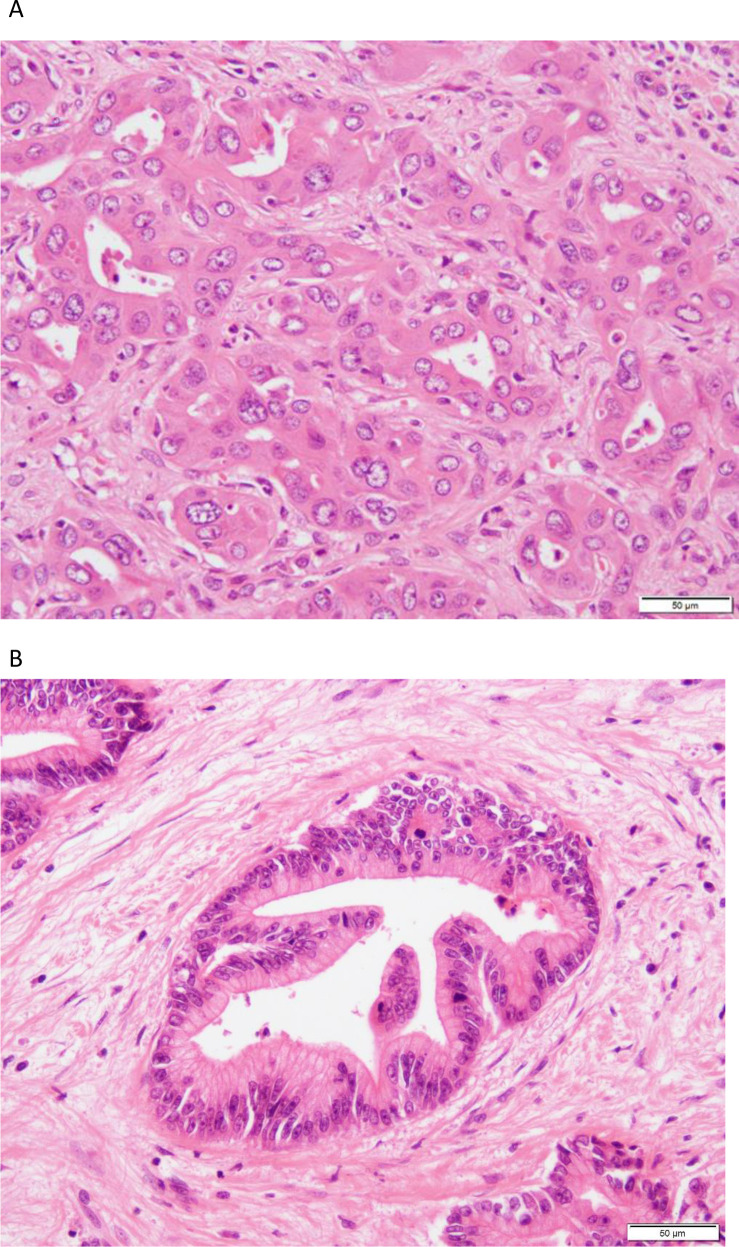
Histopathology of intrahepatic cholangiocarcinoma (iCCA). (A) Small duct iCCA shows an anastomosing tubular architecture. (B) In large duct iCCA, columnar cancer cells with intracytoplasmic mucus are arranged in a ductal structure against the background of fibrotic stroma.
An important limitation in CCA epidemiology studies is the unknown rate of pCCA specifically, as the main WHO International Classification of Diseases (ICD) coding systems have historically lacked a specific code for pCCA, which has probably been mostly miscoded to iCCA in the past.15 The lack of specific coding for pCCA is to be corrected in the latest version of ICD (2021) but this will not help with understanding the historical rates of pCCA distinct from iCCA and distal eCCA.
Aetiology and risk factors
The global variability of CCA prevalence is thought to be the result of a complex interaction between the host-specific genetic background and the geographical distribution of associated risk factors (table 1). The highest rates of CCA globally are in northeast Thailand and surrounding areas, where the main risk factor is believed to be chronic infection with liver flukes.9 With the absence of liver flukes in the Western world, the the most common known risk factor for CCA is primary sclerosing cholangitis (PSC).7 16 Of note, some risk factors are shared by both iCCA and eCCA, while others seem more specific for iCCA or eCCA.7 16 17 Most of the known major risk factors are associated with chronic inflammation of the biliary epithelium and bile stasis. However, the majority of CCA cases in the West are sporadic, without any identifiable risk factors present.
Table 1.
Risk factors for cholangiocarcinoma together with type of study (adapted from7 9 17)
| Risk factor | Type of study | OR for iCCA | OR for eCCA |
| Caroli’s disease | Population-based study | 38 | 97 |
| Primary sclerosing cholangitis | Population-based study | 22 | 41 |
| Choledochal cyst | Meta-analysis | 26.7 | 34.9 |
| Choledocholithiasis | Meta-analysis | 10.1 | 18.6 |
| Cirrhosis | Meta-analysis | 15.3 | 3.8 |
| Liver fluke (O.viverrini, C. sinensis) | Meta-analysis | OR 5 iCCA>eCCA | |
| Cholelithiasis | Meta-analysis | 3.4 | 5.9 |
| Chronic pancreatitis | Population-based study | 2.7 | 6.6 |
| Chronic hepatitis B | Meta-analysis | 4.6 | 2.1 |
| Chronic hepatitis C | Meta-analysis | 4.3 | 2 |
| Inflammatory bowel disease | Meta-analysis | 2.7 | 2.4 |
| Alcohol consumption | Meta-analysis | 3.2 | 1.8 |
| Cholecystolithiasis | Meta-analysis | 1.8 | 2.9 |
| NAFLD | Meta-analysis | 2.2 | 1.5 |
| Haemochromatosis | Population-based study | 2.1 | |
| Type 2 diabetes mellitus | Meta-analysis | 1.7 | 1.5 |
| Cigarette smoking | Meta-analysis | 1.3 | 1.7 |
| Obesity | Meta-analysis | 1.1 | 1.2 |
| Hypertension | Meta-analysis | 1.1 | 1.2 |
| Environmental toxins | |||
| Thorotrast (banned 1969) | Retrospective study | RR>300 | |
| 1,2-Dichloropropane | Retrospective study | RR 15 | |
| Asbestos | Two case–control studies | 1.1–4.8 | nil to 2.1 |
NAFLD, non-alcoholic fatty liver disease; RR, relative risk.
Polymorphisms of host genes encoding enzymes involved in xenobiotic detoxification, DNA repair, multidrug resistance, immune response and folate metabolism have also been linked to cholangiocarcinogenesis4 9 16; however, there are currently no published genome-wide association studies in CCA.
Pathology
Recommendation 2: The requirement to have tissue available for molecular profiling to inform treatment decisions should be considered when immunohistochemistry is planned on lesional biopsy material.
Strength of recommendation: STRONG
Quality of evidence: HIGH
Recommendation 3: A diagnosis of combined hepatocellular-CCA should be made on morphological pathological grounds only.
Strength of recommendation: MODERATE
Quality of evidence: MODERATE
Histological classification
In addition to the subclassification of CCA as intrahepatic and extrahepatic, WHO 5th edition furthermore classifies lesions into benign, premalignant and malignant (box 1).18
Box 1. The range of neoplastic diagnoses in the intrahepatic and extrahepatic biliary tree in accordance with WHO 5th edition.18 .
Intrahepatic bile ducts
Benign: bile duct adenoma, biliary adenofibroma, serous cystadenoma (microcystic adenoma).
Premalignant: mucinous cystic neoplasm (MCN), biliary intraepithelial neoplasia (BilIN), intraductal papillary neoplasm of the bile duct (IPNB).
Malignant: intrahepatic CCA, IPNB with associated invasive malignancy, MCN with associated invasive malignancy.
Extrahepatic bile ducts
Benign: none.
Premalignant: BilIN, MCN, IPNB.
Malignant: extrahepatic CCA, IPNB with associated invasive malignancy, MCN with associated invasive malignancy, neuroendocrine neoplasms.
Macroscopic features of CCA
Intrahepatic CCA has two main subtypes, large duct and small duct.18 Large duct tumours typically arise near the large central ducts and small duct tumours are found more peripherally. Small duct tumours are usually white or grey mass-forming lesions in the hepatic parenchyma. Large duct tumours typically grow along the wall of the larger ducts from which they arise. Both forms are whiter and firmer than HCCs due to their stromal component.
There are four major patterns of macroscopic growth recognised: mass-forming, periductal infiltrating, intraductal and mixed. The majority of iCCA are mass-forming tumours, with only 10% demonstrating a periductal/intraductal pattern of growth.19 Extrahepatic CCA conversely is most commonly a sclerosing, circumferential lesion or thickening of the bile duct without clear distinction from the non-lesional duct wall. Nodular or papillary types are also found.18
Histopathological features of CCA
iCCA can be subdivided into small duct and large duct. Small duct (cholangiocellular) iCCA are typically non-mucin-secreting adenocarcinomas with a ductular or tubular pattern (figure 1A). Cholangiolocarcinoma and iCCA with a ductal plate malformation pattern are considered subtypes of small duct iCCA. Large duct iCCA are typically mucin-secreting tubular adenocarcinomas resembling the perihilar and extrahepatic forms (figure 1B). Rare subtypes of eCCA can occur in large duct intrahepatic tumours. Both large and small duct forms of iCCA have a variable fibrous stroma.
Most eCCA are adenocarcinomas of pancreaticobiliary morphology with glandular structures and small groups of cells within a dense desmoplastic stroma. Other types of eCCA include: intestinal-type, foveolar-type, mucinous, signet ring, clear cell, pyloric gland, hepatoid and invasive micropapillary. Rarer types include: squamous cell carcinoma, adenosquamous carcinoma, sarcomatoid carcinoma and undifferentiated carcinoma.
Histological grade
No definitive grading system for CCA has been accepted.20 The International Collaboration on Cancer Reporting (ICCR) guidance documents a commonly used semiquantitative grading system for iCCA based on the proportion of the tumour that shows gland formation:
>95% of tumour composed of glands: well-differentiated.
50–95% of tumour composed of glands: moderately differentiated.
<50% of tumour composed of glands: poorly differentiated.
The ICCR guidance also states that the differentiation of pCCA should be classified in the same way as distal large bile duct/pancreatic adenocarcinomas, where grading is determined by the least well-differentiated component rather than the proportion of glandular elements; it should be divided into three grades and is based on gland formation, mucin production, mitotic activity and nuclear features.
Immunohistochemistry
Immunostaining is not essential for the histological diagnosis of CCA and the increasing requirement for molecular profiling of lesional tissue to determine targeted therapies should lead to a reduction in the use of tissue for purely confirmatory immunohistochemical staining. Where imaging is in keeping with CCA, particularly the absence of prior or current extrahepatic malignancy, and the morphology is that of adenocarcinoma, there is no additional diagnostic discrimination offered by immunohistochemical staining.
However, two specific diagnostic scenarios may be aided by targeted immunohistochemistry: (1) When there is a prior history of carcinoma or a possible contemporary extrahepatic primary lesion and the morphology is compatible with both CCA and a metastasis from that prior or putative extrahepatic lesion, immunohistochemistry can be used to confirm the biliary phenotype of cancer cells and differentiate them from metastasis. To aid that particular distinction, CCA is typically positive for cytokeratin (CK) 7 and CK19, and negative for CK20. Large duct iCCA, particularly in cases associated with PSC, sometimes expresses intestinal markers (eg, CK20 and CDX2).21 C-reactive protein (CRP) is a highly specific and sensitive marker for iCCA (particularly small duct type), as it is not expressed in adenocarcinomas of other organs.22 23 The site of the prior or putative extrahepatic primary lesion allows specific cell lineage–specific transcriptional factor expression to be examined; for example, TTF-1 (expressed in lung and thyroid cancers), PAX8 (renal, thyroid, ovarian and endometrial cancers) and GATA-3 (breast and urothelial cancers) are typically negative in CCA. (2) When there is no prior or contemporary extrahepatic malignancy but the histological features are equivocal in their capacity to distinguish between CCA and HCC, immunohistochemistry may be helpful. Heppar-1, arginase-1 and glypican-3 are often expressed by HCC and not by CCA, although their expression can be lost in poorly differentiated HCCs.
If subclassification of iCCA cannot be made on morphological features alone, a panel of CRP, N-cadherin and S100 calcium binding protein P (S100P) can be useful as CRP/N-cadherin and S100P are commonly expressed in small duct and large duct iCCA, respectively.23
Molecular profile
Small duct iCCA has distinct molecular features: IDH1/2 mutations (20%), BAP1 mutations (10–20%) and FGFR2 fusions (15%).24–26 In contrast, large duct iCCA and eCCA harbour alterations in KRAS (20%) and SMAD4 (10–20%).24 25 27 Mutations in TP53 are observed in either type (30%).24 26
FGFR2, NTRK and other fusions or other rearrangements can be diagnosed by RNA sequencing (preferred to immunohistochemistry) or fluorescent in situ hybridisation (FISH), and their identification prior to the use of FGFR inhibitors is essential.28 Mismatch repair protein (MMR) or microsatellite instability (MSI) tests need to be considered if clinically indicated. MMR deficiency is observed in 1–6% of CCA, and those cases often show a solid, mucinous or signet ring cell histological appearance.29–31 NTRK fusions, a category of treatment-related, pan-cancer molecular alterations, are estimated to be detectable in 1% of CCA.32 Table 2 shows the relevant codes from NHS England Genomics that can be used for sequencing. The logistics of molecular testing to guide therapeutic decisions varies within the healthcare systems of the UK’s devolved nations. For example, testing in England is provided by the NHS England Genomic Laboratory Hubs, and the available tests are listed within the test directory (https://www.england.nhs.uk/publication/national-genomic-test-directories).
Table 2.
Available assays through genomics England for cholangiocarcinoma tissue
| M220.1 | Multitarget NGS panel – structural variant | NTRK1 NTRK2 NTRK3 FGFR2 |
Structural variant detection | Panel | Patient’s clinical status means they are eligible for an NTRK inhibitor in the event an NTRK rearrangement is detected. Patient’s clinical status means they are eligible for protein kinase inhibitor therapy in the event an FGFR2 fusion is detected. |
| M220.03 | DPYD hotspot | DPYD | Small variant detection | Simple targeted mutation testing | Patient planned to receive fluoropyrimidine treatment |
| M220.5 | MSI testing | N/A | Microsatellite instability (MSI) analysis | MSI analysis | Known CCA when MMR IHC not possible/not performed, according to NICE guidelines for molecular testing to inform therapy choice. Delivery via Pathology in some regions. |
| M220.06 | Multitarget NGC panel – small variant (IDH1) | IDH1 | Small variant detection | Panel | Molecular assessment will aid diagnosis or management |
IHC, immunohistochemistry; NGS, next-generation sequencing.
Combined hepatocellular–cholangiocarcinoma
The diagnosis of combined hepatocellular–cholangiocarcinoma requires morphological confirmation of both HCC and adenocarcinoma components.33 Immunohistochemical expressions of hepatocellular markers in otherwise typical iCCA or expressions of CK7/CK19 in HCC are insufficient alone to merit designation of tumours as combined hepatocellular–cholangiocarcinoma. CK7 and CK19 are known to be expressed in 20% and 10% of HCC, respectively.34 35 Most cases of combined hepatocellular–cholangiocarcinoma harbour gene mutations that are identified in HCC (eg, TERT) even within the CCA components.36 37 Tumour, node and metastasis (TNM) staging is currently based on that of iCCA.
Premalignant neoplasms
Biliary intraepithelial neoplasia (BilIN)
Most cases of large duct CCA are thought to progress from BilIN (previous term, biliary dysplasia) via a multistep carcinogenesis.38 BilIN is diagnosed incidentally in surgically resected specimens or explanted livers. The diagnosis of BilIN in biopsy specimens should be made with caution, as BilIN is unlikely to cause biliary strictures. In contrast, premalignant lesions of small duct iCCA are unknown.
Intraductal papillary neoplasm of the bile duct (IPNB)
IPNB is characterised by an intraductal high-papillary proliferation, and is currently classified into two types.39 Type 1 IPNBs develop in intrahepatic (70%) or perihilar ducts (30%), and they are typically associated with cystic or fusiform duct dilatation and mucus overproduction.39–41 Type 2 IPNBs arise in distal (70%) or perihilar ducts (30%), and present with an intraductal solid mass and extensive dilatation of the upstream ducts. The presence of invasive malignancy is confirmed in 50% of type 1 IPNBs and >90% of type 2 IPNBs at the initial presentation.39–41 The gallbladder counterpart of IPNB is referred to as intracholecystic papillary neoplasm.42
Mucinous cystic neoplasm (MCN)
This condition was traditionally called biliary cystadenoma. MCN is defined as a cyst-forming epithelial neoplasm with ovarian-type subepithelial stroma and a lack of communication to the bile duct.43 44 Approximately 50% of MCNs develop in segment IV of the liver.44 Progression to CCA is confirmed in only 5% of surgically resected cases.43
Pathological diagnosis
Definitive histology and/or cytology are required to confirm a diagnosis of CCA. Even with successful lesional sampling, distinction of iCCA from metastatic lesions, particularly upper GI, pancreas, or extrahepatic biliary lesions, is difficult, as discussed earlier. Identification of an invasive component associated with a mucinous cystic neoplasm or intraductal papillary neoplasm on a needle biopsy can also be problematic due to its focal nature.44
Brush cytology from percutaneous or endoscopic procedures has a diagnostic sensitivity of only 30–60%,45 46 meaning negative cytology does not exclude malignancy. Combining cytology with biopsy increases the diagnostic yield.47 48 The further addition of FISH for polysomy and 9p21 detection increases sensitivity for the detection of malignancy further.48 49 However, in a meta-analysis examining patients with PSC, FISH did not increase the sensitivity to detect malignancy compared with cytology.50 In contrast, the addition of a 28-gene next-generation sequencing panel to pathological assessment of brushings or biopsies increased the sensitivity for the detection of malignancy in patients with and without PSC to over 80%.51
Reporting surgical specimens
Surgical resection specimens should be reported systematically—for example, following The Royal College of Pathologists or ICCR reporting guidance.20 Box 2 provides a summary of how the report should be structured.
Box 2. Reporting surgical specimens.
Surgical resections specimens should be reported systematically—for example, following The Royal College of Pathologists or ICCR reporting guidance.20
The final report should include:
Tumour site and number: pCCA is defined as arising above the junction of the common hepatic duct and the cystic duct up to the second-order divisions of the right and left hepatic ducts. In iCCA, the number of tumours is a prognostic factor.
Maximum tumour dimension: increasing tumour size is associated with poorer prognosis.
Histological tumour type.
Histological tumour grade.
Extent of local invasion: required for TNM classification.
Presence of vascular invasion: an important prognostic factor in iCCA and pCCA, and a component of the TNM classification.
Presence of precursor lesions.
Presence of coexistent parenchymal liver disease.
Margin and lymph node status.
Pathological staging – American Joint Committee on Cancer/Union for International Cancer Control (AJCC/UICC) TNM 8th edition.20 iCCA, pCCA and dCCA are staged using separate, specific classifications (see online supplemental figures 1–3)
Additional non-core elements should also be reported:
Tumour growth pattern: mass-forming, periductal infiltrating, intraductal or mixed.
Presence of perineural invasion: of greatest significance in perihilar tumours.
Response to neoadjuvant therapy.
Presentation
Recommendation 4: All centres managing patients with CCA should have clear established diagnostic pathways for patients presenting with jaundice/biliary obstruction, with streamlined transition to local and regional HPB MDT meetings.
Strength of recommendation: STRONG
Quality of evidence: HIGH
Recommendation 5: Having completed imaging, all patients should undergo a detailed review of clinical presentation, examination findings, blood investigations and imaging, ideally at a regionally coordinated hepatobiliary MDT meeting, with prompt assessment of and communication to the patient.
Strength of recommendation: STRONG
Quality of evidence: HIGH
Recommendation 6: Consideration should be given to possible benign causes of biliary tract stricturing/obstruction during MDT discussion, in correlation with appropriate serological investigations and clinical history, to ensure alternative diagnoses are considered while a pathological diagnosis of CCA is secured.
Strength of recommendation: STRONG
Quality of evidence: MODERATE
Most patients with a CCA will typically present through either emergency services or via referral to a secondary care centre on a 2 week-wait upper GI or jaundice related pathway.14 For patients presenting with eCCA, malignant biliary tract obstruction is a common mode of presentation. Following this, an imaging investigation followed by discussion at a local/regional hepato-pancreato-biliary MDT meeting is likely to occur, where a plan will be made on how to achieve: (1) a histological diagnosis, (2) restore bile flow for those patients with jaundice, (3) determine if the patient is an operative candidate, (4) determine what other imaging tests are required for subsequent patient management.
With regards to tissue sampling, a biopsy/cytological sample may be taken from the following sites to secure a diagnosis of CCA: a biliary stricture, periductal/intrahepatic mass lesion, lymph node metastasis, organ metastasis, pathological serosal fluid sample (pleural fluid, ascites, pericardial fluid) or peritoneal biopsy.
Malignant biliary tract obstruction can present with a wide range of symptoms that include: abnormal liver function tests, jaundice, abdominal pain, tiredness/lethargy, anorexia plus weight loss, thromboembolic disease, hypercalcaemia, paraneoplastic syndromes, abdominal masses/distant organ infiltration, malignant adenopathy, pleural disease, ascites and fever of unknown origin.1 4 5 At presentation, both distal and hilar biliary strictures essentially remain indeterminate until there is either a positive cytological or histopathological confirmation of CCA, with ultimately, over 80% of such strictures proving to be malignant.52
It is imperative that all patients presenting with possible MBTO have a detailed history taken in reference to age of presentation, country of origin, travel history, constitutional symptoms and weight loss, history of prior HPB surgery, pancreatitis, or inflammatory bowel disease, family history of inflammatory bowel disease, previous investigations to detect possible causes of indeterminate biliary strictures and history of chronic liver disease including viral hepatitis. Benign causes of a cholangiopathy/biliary stricturing should also be considered with appropriate collaborative serological testing for diseases which can mimic CCA (box 3).
Box 3. Benign diseases which can mimic cholangiocarcinoma.
Recurrent pyogenic cholangitis
Mirizzi syndrome
Stricture in primary sclerosing cholangitis
Portal hypertensive biliopathy
Heterotopic tissue
Ischaemic cholangiopathy
Inflammatory-infiltrative
Inflammatory pseudotumour
IgG4 sclerosing cholangitis
Eosinophilic cholangiopathy
Mast cell cholangiopathy
Follicular cholangitis
Xanthogranulomatous cholangitis
Sarcoidosis
A detailed family history should also be undertaken to exclude familial cancer syndromes that are associated with CCA. In a study of 267 patients, over 15% of patients had a pathogenic/likely pathogenic somatic variant in a cancer risk gene including: ATM, CHEK2, BAP1, BRCA1, MLH1, BRCA2, PALB2, TP53, APC, CDH1, MSH6, PMS2 and MUTYH.53
If a familial cancer syndrome is suspected from the patient’s genetic history, we would recommend that the patient is referred to a clinical geneticist. Things that might alert clinicians to this include: (1) three or more primary cancers in a single individual, (2) three or more cases of cancer at the same site, (3) any two of: sarcoma, breast cancer, brain tumour, leukaemia or adrenal cortical tumour, in someone under 45, (4) childhood cancer plus one close relative with cancer, (5) any individual or family with an unusual pattern of cancer—for example, rare tumours or young ages at diagnosis, (6) families with a known cancer predisposition syndrome for example, Li-Fraumeni, Lynch syndrome and Peutz Jeghers, (7) people who meet diagnostic criteria for familial genetic syndromes.
Imaging
Recommendation 7: Contrast enhanced multiphasic CT of the chest, abdomen and pelvis to stage the primary tumour, including assessment of local vascular relationships, should be undertaken for all types of CCA.
Strength of recommendation: STRONG
Quality of evidence: MODERATE
Recommendation 8: Contrast enhanced MRI and MRCP should be undertaken for perihilar and intrahepatic tumours to better delineate the extent of biliary involvement and identify any satellites/intrahepatic metastases.
Strength of recommendation: STRONG
Quality of evidence: MODERATE
Recommendation 9: For tumours involving the more distal extrahepatic duct, MRI is unlikely to add any further information over and above CT.
Strength of recommendation: MODERATE
Quality of evidence: LOW
Recommendation 10: 18 FDG-PET CT for detection of nodal and distant metastatic disease is recommended as part of staging investigations.
Strength of recommendation: STRONG
Quality of evidence: MODERATE
Recommendation 11: US or CT-guided biopsy of the primary intrahepatic tumour or metastatic lesions should be undertaken to acquire a pathological diagnosis following MDT discussion and consensus.
Strength of recommendation: STRONG
Quality of evidence: HIGH
In the diagnosis and staging of suspected CCA, the local tumour extent, vascular/biliary involvement, anatomic variations of the vessels/biliary tree and presence or absence of extrahepatic disease should be assessed. A multimodality approach is often required to combine the advantages of the various imaging techniques, which provide additive information.54–56
Imaging studies should be performed before any biliary intervention to avoid secondary inflammatory change that can mask the tumour and lead to overestimation or underestimation of its true extent.56 All imaging modalities can underestimate the longitudinal tumour extent owing to microscopic spread along the mucosal/submucosal layer of the bile duct.57
Transabdominal ultrasound (TUS)
Cholangiocarcinoma should be suspected when there is biliary ductal dilatation, particularly with a related mass and consistent clinical history. In suspected biliary obstruction, TUS is reliable for excluding gallstones but is operator-dependent and is insufficient alone for investigating suspected CCA. For detecting advanced CCA in patients with PSC, TUS offers specificity and negative predictive value of 90%, but sensitivity and positive predictive value are only 50%.58 59 TUS may miss small tumours and cannot accurately define tumour extent.58 60 The role of contrast enhanced ultrasound (CEUS) in CCA is also limited but might be helpful as an additional modality when assessing indeterminate focal liver lesions.
Contrast enhanced computed tomography (CECT)
Contrast enhanced computed tomography (CECT) should be performed in all cases of suspected CCA as the initial standard imaging modality, to include the chest, abdomen and pelvis. The main advantage is the excellent spatial resolution, providing comprehensive assessment of the primary tumour, its local vascular relationships (including any aberrant vessels) and overall resectability.56 61 62 It also allows detection of local lymphadenopathy and metastatic disease, although sensitivity is lower than that of PET.63 A meta-analysis including 448 patients from 16 studies, found data primarily related to CT, with accuracy estimates for CT evaluation of the extent of ductal tumour to be 86%; the sensitivity and specificity estimates were 89% and 92% for evaluation of portal vein involvement, 83% and 93% for hepatic artery involvement, and 61% and 88% for lymph node involvement, respectively.54
A multiphase examination of the abdomen including an unenhanced, arterial and portal venous phase is ideal. The unenhanced phase helping to differentiate high-attenuation calcified stones from enhancing tumour. Multiplanar reconstruction should be routinely used.64 Assessment of vascular involvement on CT is more difficult for the hepatic artery than the portal vein, with variable positive predictive values reported for the former ranging from 53% to 95%.56 65 66 Assessment of the extent of biliary involvement can also be difficult with CT, particularly the proximal extent of perihilar tumours.
Magnetic resonance imaging (MRI) and magnetic resonance cholangiopancreatography (MRCP)
A meta-analysis of 32 studies with 1626 patients reported a pooled sensitivity and specificity of MRI for T-stage of 0.90 and 0.84, and pooled sensitivity and specificity for N-stage of 0.64 and 0.69, respectively.67 In a recent study of 334 patients comparing CT and MRI staging of mass-forming intrahepatic CCA, MRI showed superior sensitivity for T-staging, with CT and MRI having comparable sensitivity for N-stage.68
The choice of contrast agent depends on tumour type and location. For mass forming iCCA, MRI with hepatobiliary contrast is reported to be the most accurate modality for identification of satellite lesions and intrahepatic metastases.69 70 Gadoxetic acid-enhanced MRI (Primovist in Europe/Eovist in the United States) provides better diagnostic performance and may even give prognostic information.71 On the contrary, for intraductal, periductal and perihilar tumours, particularly if there is biliary obstruction, it is recommended that extracellular contrast agents are used.57 62
Diffusion-weighted imaging should be routinely included, aiding in the characterisation of biliary and intrahepatic lesions and can detect extrahepatic disease. Using 0–100 s/mm2 and 800–1000 s/mm2 for low-b and high-b values, respectively, is optimal.72–74 For perihilar tumours, MRCP with contrast enhanced MRI helps to delineate the local extent of biliary involvement to determine resectability and for planning biliary drainage.75 76 MRCP/MRI has at least comparable results to CT and can be very helpful when appearances on CT are equivocal.54 77
18F-fluorodeoxyglucose (FDG) positron emission tomography (PET): FDG PET/FDG PET CT
A meta-analysis supports the incorporation of 18FDG-PET imaging in addition to the current standard of care imaging/diagnostic tests in CCA.63 The pooled proportion of change in management due to 18FDG-PET CT findings was 15% (95% CI 11 to 20); the majority due to disease upstaging. The results of the meta-analysis do not support the use of 18FDG-PET for diagnosis of the primary tumour in the absence of other disease sites or pathological confirmation, due to low specificity (table 3). However, 18FDG-PET is a useful tool for identification of malignant lymph nodes (N-stage), distant metastases (M-stage) and confirmation of disease relapse if diagnosis remains unclear following standard of care imaging. This is especially important when surgery or local treatments are being considered. The prognostic role of 18FDG-PET and the impact of SUV max on management require further investigation in prospective studies.
Table 3.
Recommendations for the use of 18FDG-PET for diagnosis of the primary tumour in the absence of other disease sites or pathological confirmation
| Recommended | Not recommended | |
| Tumour diagnosis/T-staging | ✓ | |
| Nodal status/ N-staging | ✓ | |
| Metastatic status/M-staging | ✓ | |
| Relapse/recurrence (R) | ✓ |
The sensitivity and specificity of 18FDG-PET for T was 91.7% (95% CI 89.8% to 93.2%) and 51.3% (95% CI 46.4% to 56.2%), respectively. For N, sensitivity was 88.4% (95% CI 82.6% to 92.8%) and specificity was 69.1% (95% CI 63.8% to 74.1%). For M, sensitivity was 85.4% (95% CI 79.5% to 90.2%) and specificity was 89.7% (95% CI 86.0% to 92.7%). For R, sensitivity was 90.1% (95% CI 84.4% to 94.3%) and specificity was 83.5% (95% CI 74.4% to 90.4%). Adapted from (10).
Interventional radiology
Biopsies
Biopsy is mandatory for confirmation of CCA diagnosis and should be performed following MDT discussion to ensure it is required and appropriate for the proposed management plan. Percutaneous liver biopsy with image guidance (mainly in the form of TUS) is suitable for intrahepatic and, where possible, pCCA if non-operable. TUS or CT guided biopsy for diagnostic purposes can also be pursued for metastatic CCA, targeting the most accessible site. There is no evidence to support and justify the routine use of CEUS in TUS-guided biopsies of focal liver lesions because of the cost and time. CEUS guidance has a role when a second biopsy is requested owing to either an insufficient initial biopsy with necrotic material or insufficient visualisation of the focal liver lesion, which could be relevant in a small percentage of cases.78
Percutaneous biliary stent drainage
In patients requiring drainage with complex hilar strictures, intent should be made for endoscopic drainage with the use of percutaneous drainage only when necessary, given not just the higher morbidity but also mortality.79
Special considerations
Assessing the background liver
As part of the imaging workup in potential surgical resection and transplant candidates, additional factors can be established to help determine resectability and postoperative risks. CT or MR liver volumetric analysis can be performed, with a small remnant increasing the risk of postoperative morbidity and mortality.80 81 Functional information from gadoxetic acid–enhanced MRI has also been reported to be useful in the prediction of future remnant liver function.82 Elastography techniques can detect and quantify any underlying hepatic fibrosis and provide prognostic information about the risk of hepatic failure,83 but must be interpreted with caution in the presence of coexisting biliary obstruction. These emerging techniques are becoming desirable in the preoperative workup of CCA but are currently not widely practised.
Diagnosing CCA in chronic liver disease
Chronic liver disease is a predisposing factor for the development of CCA (in addition to HCC), and less commonly combined HCC-CCA). Detection and characterisation of CCA in this setting has been addressed by the Liver Imaging Reporting and Data System (LI-RADS) version 2018.84 This system is now widely adopted and describes features of liver nodules in cirrhosis as an indicator of the probability of a particular nodule being HCC. It also describes features that are more suggestive of CCA-containing tumours. If CCA is suspected, biopsy is usually required to provide a definitive diagnosis as the treatment options and prognosis will differ considerably.
Endoscopy
Recommendation 12: Before undertaking any endoscopic investigations for a suspected CCA, all patients should have undergone a triple-phase CT scan of the abdomen/pelvis and chest along with dynamic MRI and MRCP if proximal biliary obstruction is suspected.
Strength of recommendation: STRONG
Quality of evidence: MODERATE
Recommendation 13: Patients with operable DMTO should undergo a combination of endoscopic US and ERCP to try to confirm a malignant histological diagnosis before proceeding to surgery.
Strength of recommendation: MODERATE
Quality of evidence: LOW
Recommendation 14: In a suspected case of operable distal CCA, in the absence of jaundice, a standalone EUS should be undertaken first, to avoid the complications of ERCP, which could delay or render the patient inoperable.
Strength of recommendation: WEAK
Quality of evidence: LOW
Recommendation 15: In the presence of jaundice and DMTO, where EUS is not available, patients may only be able to have an ERCP and brush cytology in the first instance to confirm the presence of a CCA.
Strength of recommendation: MODERATE
Quality of evidence: MODERATE
The role of endoscopy in the management of patients with CCA is essentially for three purposes: (1) to establish a tissue/cytological diagnosis, (2) to facilitate surgery and chemotherapy, (3) to palliate for jaundice and improve quality of life. In reference to endoscopic management this particularly applies to the management of dCCA and pCCA causing distal malignant tract obstruction (DMTO) and proximal malignant tract obstruction (PMTO), respectively, in both operable and inoperable settings.
Given that complications could arise from endoscopic procedures, that might affect the interpretation, sensitivity and specificity of the radiological staging, these guidelines advocate that primary diagnostic and staging imaging for DMTO and PMTO are undertaken beforehand. This will also facilitate endoscopic planning for the operator.
Endoscopic standards for potentially operative distal CCA causing DMTO
In reality, the separation of potential causes of DMTO are not always possible following central MDT review of imaging (ie, differentiating between distal CCA, pancreatic carcinoma, ampullary cancer and periampullary cancer). In this clinical scenario the first endoscopic objective is to establish the presence of malignant histology/cytology to allow the patient to proceed to Whipple’s resection with a confirmed cancer diagnosis if operable.
For dCCA—that is, a distal malignant stricture, where the objective is to drain jaundice and acquire a pathological malignant diagnosis, a combination of linear EUS fine needle biopsy (FNB) fine needle aspiration (FNA) and ERCP-directed trans papillary brushings and stenting should be undertaken. At the time of ERCP, it may be considered that bile be sent for biliary culture to guide antibiotic treatment in the postoperative period. For suspected ampullary lesions, a side viewing duodenoscopy with surface biopsies should establish the diagnosis and be considered the first-line investigation if suspected from the primary imaging.85 The advantage of doing a combined EUS with ERCP is that it will allow complete local assessment of pathological local lymph nodes (with follow on nodal sampling),86 intrahepatic metastases and a distal bile duct associated mass (followed by a EUS FNB/FNA of the distal bile duct mass or wall of the stricture), allowing the correct cause of MBTO to be determined. At EUS samples should be placed directly into formalin, as the benefit of onsite pathology cannot be justified in terms of time and expense.87
In non-jaundiced patients with a distal biliary stricture and suspicion of MBTO, linear EUS should be undertaken, followed by review of that result by a MDT to determine if an ERCP is required to try to further establish the correct diagnosis.
For those patients proceeding to ERCP, the simplest method of tissue sampling (available at most UK sites in the presence of jaundice with suspected DMTO) is to acquire a cytological diagnosis using biliary brushings and cytological examination.88 However, this will of course mean that the bile duct is entered putting the patient at risk of both cholangitis and pancreatitis,89 which might delay surgery. The results of brushings should be classified as either: non-diagnostic, negative for malignancy, atypical, neoplastic (benign or other), suspicious for malignancy or malignant.90 Recent meta-analysis suggests that brush cytology provides, at best, the correct cytological diagnosis with a sensitivity of 45% and a specificity of closer to 99% in a series meta-analysis.91 It is recommended that the stricture is brushed more than five times with one brush to improve cellular yield and that additionally, the brush is flushed out to optimise cellular yield.92
To try to improve this low sensitivity, supplementary techniques such as FISH and digital image analysis have been suggested. The former uses a combination of molecular probes and looks for the presence of polysomy (defined as >5 cells which express two or more molecular probe markers).93 However, at present these are not routinely available in most HPB centres in the UK.
Another method of sampling the bile duct during ERCP is to obtain intraductal forceps biopsy specimens of the stricture by either wire guidance or fluoroscopic guidance. These samples are placed straight into formalin and, like brushing, offer sensitivity of around 50%, but in combination with brushings a higher sensitivity may be reached.94
Finally, in selected cases (more so for proximal bile duct strictures), direct cholangioscopy provides one additional method to make a histological diagnosis, through a combination of direct visualisation and intraductal biopsies; however, it should be noted this is more technically difficult in distal strictures than proximal ones. Most UK centres at present use the disposable through the scope Spyglass single-operator cholangioscopy.95 For visual impression, a previous meta-analysis involving eight studies and 335 patients demonstrated a sensitivity of 90% with a specificity of 80%.96 For targeted tissue biopsies, a meta-analysis of 10 studies involving 456 patients showed that the sensitivity of cholangioscopy was 60% with a specificity of 98%.97
EUS biopsy is not recommended for proximal strictures and masses that are potentially operable, owing to potential peritoneal seeding, although some studies suggest that this might not be a major concern.98 99 Confocal laser endomicroscopy, using the Miami100 and Paris101 classifications, remains a research tool.
Recommendation 16: At present biliary biomarkers cannot be recommended as a replacement for cytological and histological standards. However, biliary next-generation sequencing shows great promise and should be taken forward for replicative NIHR/UKRI funded multisite studies.
Strength of recommendation: MODERATE
Quality of evidence: MODERATE
At present, a growing number of biliary molecular markers might add to the ability to differentiate malignant from benign biliary strictures. At present none of these can be recommended as they are often based on a range of pathologies (and varying clinical stages) and are often limited to single-centre studies. Furthermore, none at present provide near 100% sensitivity or specificity, and therefore biliary molecular markers should still be considered a research tool. However, recent publications on next-generation sequencing of bile samples have shown great promise,102 but until this has been validated and standardised at national laboratories with precision, accuracy and in accordance with UK laboratory accreditation processes, this technique cannot be recommended.
In conclusion, the accuracy of cytological and histological analysis is not perfect currently. In those cases, in which clinical findings cannot completely rule out the possibility of malignancy, the decision to proceed to either surgical resection or strict observation should be discussed fully with both the patient and their family, who should have a clear voice in what approach is being taken in conjunction with the MDT consensus view.
Recommendation 17: It should be realised that the determination of a cytological/histological confirmation of MBTO is imperfect at present, and in cases where uncertainty remains, a decision on follow-up imaging versus surgery for a definitive diagnosis should be reached only after a full discussion between the patient and the clinician. These guidelines acknowledge that it is acceptable to offer surgery where histological confirmation cannot exclude malignancy with absolute certainty and surgery m provide a cure and a secure diagnosis.
Strength of recommendation: STRONG
Quality of evidence: LOW
Recommendation 18: The decision to drain preoperative jaundice in distal CCA causing DMTO should be made in accordance with local HPB guidance. In cases where rapid access to surgery can be offered, it may be appropriate to bypass biliary drainage at ERCP to avoid ERCP-related complications and postoperative sepsis.
Strength of recommendation: STRONG
Quality of evidence: HIGH
Historically it was considered that drainage of preoperative hyperbilirubinaemia improves surgical outcome for distal malignant biliary obstruction. However, studies suggest an increase in postoperative complications in those patients who have undergone preoperative biliary drainage.103 Therefore, at present, guidance has suggested that perhaps there is a threshold level of hyperbilirubinaemia at which biliary drainage should be considered, with a threshold of 250 μmol/L being the cut-off point. However, patients with intractable pruritus, cholangitis and organ dysfunction are likely to benefit from preoperative biliary drainage with lower levels of obstructive hyperbilirubinaemia. The decision therefore not to drain DMTO before surgery implies that preoperative histological confirmation might not be achieved. Ultimately, this careful balance of decisions should be made at an HPB MDT meeting prior to any planned intervention, after all radiological imaging has been obtained along with full patient discussion.
If drainage is considered, ERCP should be performed with the placement of a fully covered metal stent for DMTO; or one or more plastic stents for PMTO, if there is an expected delay in surgery more than 4 weeks. After ERCP, cholangitis, pancreatitis, cystic duct obstruction and cholecystitis are potential risks.104
Inoperable distal CCA causing DMTO
Recommendation 19: Patients with DMTO with inoperable disease from distal CCA should undergo an EUS/ERCP or standalone ERCP to confirm a pathological diagnosis and have their jaundice palliated.
Strength of recommendation: STRONG
Quality of evidence: HIGH
Recommendation 20: Patients with DMTO from distal CCA should have a fully covered self-expanding metal stent placed. Plastic stents should not be placed for long-term palliation of jaundice.
Strength of recommendation: STRONG
Quality of evidence: HIGH
Recommendation 21: Where patients cannot have a stent placed at ERCP, we recommend EUS guided biliary drainage is undertaken rather than PTC. However, PTC can be offered if EUS bile duct drainage is not locally available.
Strength of recommendation: STRONG
Quality of evidence: HIGH
In the case of inoperable dCCA causing obstructive jaundice, proceeding to a combined EUS and ERCP or ERCP alone, to make a simultaneous pathological diagnosis along with establishing biliary drainage is recommended. This is vital, because once a metal stent is placed, obtaining a pathological diagnosis can be extremely challenging in the case of dCCA. However, discussions about coexisting comorbidities and the degree of disease need to be strongly considered before endoscopic procedures are undertaken, as palliative care might be more appropriate for some patients in this clinical setting with very poor performance status.
The goals of drainage are to improve symptoms associated with biliary obstruction and the patient’s quality of life, in addition to facilitating the start of palliative chemotherapy by reducing the degree of jaundice. At present choices available for drainage include: ERCP, EUS guided drainage, PTC and surgical bypass. The application of endobiliary radiofrequency ablation (RFA) at the same time is at present not approved by the National Institute for Health and Care Excellence (NICE).105
Endoscopic stent placement is the preferred first-line intervention due to its improved morbidity and mortality compared with surgical bypass (elevated 30-day mortality – 16.3% vs 9.6%).106 At present, a choice of two stents can be considered: self‐expandable metallic stents (SEMS) and plastic stents. These guidelines endorse the use of fully covered SEMS given the lower rates of stent dysfunction (21.6% vs 46.8%), lower reintervention rates and better survival rates over plastic stents.107 108 Furthermore, for distal obstruction one would use fully covered SEMS rather than uncovered SEMS, which is supported by meta-analysis, despite the small risk of cystic duct obstruction.109
When biliary access is not achievable at ERCP, alternative options include either EUS guided biliary drainage or PTC. Although the former has a lower complication rate,110 the choice of modality will probably be driven by local availability in the UK, alongside MDT discussion.
Operable perihilar CCA causing PMTO
Recommendation 22: No patient with perihilar CCA should undergo endotherapy until the case has been fully discussed at an HPB treatment centre.
Strength of recommendation: STRONG
Quality of evidence: LOW
Recommendation 23: Unilateral drainage in the future remnant lobe should be considered ahead of surgery. Bilateral/further stenting should only be considered if the level of preoperative jaundice does not improve, or there is cholangitis in residual obstructed biliary segments.
Strength of recommendation: MODERATE
Quality of evidence: MODERATE
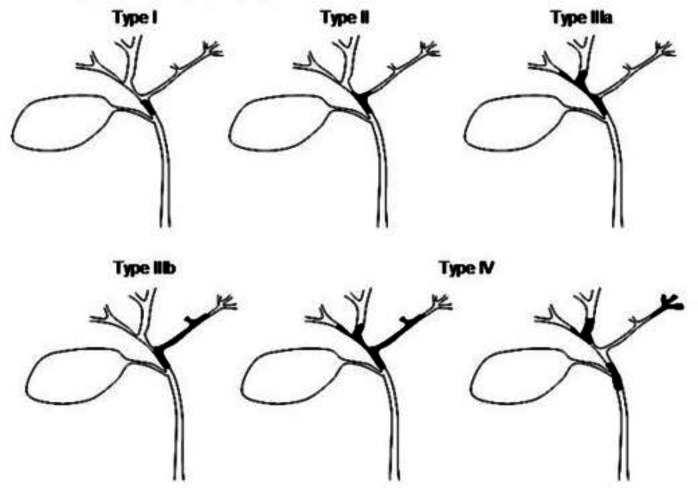
The priority in the management of pCCA is to first ensure that all imaging and clinical review has been undertaken. No patient should have biliary intervention done before the case has been fully discussed at an HPB MDT meeting where a clear plan is made of what is to be achieved and operability assessed. The standard surgical treatment for pCCA is bile duct resection combined with extended hepatectomy. In order to achieve this, planning of preoperative biliary drainage and/or portal vein embolisation aimed at improving the function of the future remnant liver function will be needed. For biliary drainage, particular attention needs to be given to the level of the PMTO, as determined by the Bismuth Corlette classification of biliary obstruction111 (figure 2).
Figure 2.
Bismuth Corlette classification of location of biliary strictures.
For tissue acquisition in operable candidates, EUS is used to confirm the presence of a ductal mass/hilar mass and to take a sample of an involved locoregional/metastatic lymph node to assist in TNM staging. The ductal/hilar mass should not be sampled as this may cause peritoneal contamination and risk causing malignant cell seeding.112
For PMTO, three different kinds of preoperative biliary drainage procedures can be considered: percutaneous transhepatic biliary drainage, endoscopic nasobiliary drainage and endoscopic biliary stenting. No clinical trials have been performed comparing these three methods to allow a definitive recommendation for jaundice resolution, or subsequent complications rate, and therefore choice will be centre specific.
On the other hand, several reports indicate the risk of portal vein injury, catheter tract recurrence and peritoneal dissemination in percutaneous transhepatic biliary drainage procedures. A recent randomised controlled trial was halted early owing to a high complication rate in the PTC arm,113 which might suggest a benefit for ERCP. Furthermore, concurrent spyglass cholangioscopy can be offered with histological sampling.
Complications of PTC and ERCP both include postprocedure related cholangitis, cholecystitis and pancreatitis. In these clinical settings repeat imaging, blood cultures/antibiotics and catheter reassessment will be required.
ERCP, spyglass cholangioscopy and PTC all offer the option of undertaking brush cytology, biliary forceps biopsy and intraductal biopsies to confirm malignant histology in perihilar CCA and exclude benign hilar stricturing.
Inoperable perihilar CCA
Recommendation 24: Inoperable perihilar CCA - proximal malignant tract obstruction (PMTO) and jaundice should be considered for palliative stenting by either ERCP or PTC. Decisions about UL versus BL stenting should be predetermined by the local MDT depending on both local availability and expertise.
Strength of recommendation: STRONG
Quality of evidence: MODERATE
It is estimated that only a third of patients will be operable at presentation with a pCCA. Given that most of these patients will be jaundiced, planning for palliative biliary drainage should be discussed at a MDT meeting. In order to achieve a meaningful reduction of hyperbilirubinaemia (to a level where chemotherapy can be offered), an uncovered SEMS can be placed during either PTC or via ERCP with selective duct cannulation. An uncovered SEMS is selected to avoid cystic duct and side-branch duct occlusion.114
At present patients can be put forward to have either unilateral (the stent is placed in either the right or left hepatic duct) or bilateral (the stent spans both the right and left hepatic duct) stents. Currently, there are no randomised controlled trials to determine the optimal approach. In a meta-analysis of 21 studies (1292 patients), Meybodi et al demonstrated equally good results with both approaches.115 However, in general the greater the volume of liver drained the greater the reduction in bilirubin.116 Furthermore, it is not advocated to drain atrophic segments in pre-drainage planning. At present these guidelines cannot favour one particular approach.
Recommendation 25: At present the use of adjunctive endobiliary RFA and photodynamic therapy is not considered standard of care for patients with hilar and distal CCA receiving palliative care.
Strength of recommendation: STRONG
Quality of evidence: HIGH
At present adjunctive biliary treatments to improve long-term stent patency and patient survival are not considered standard of care. The application of RFA via either Habib EndoHPB Bipolar Radiofrequency Catheter (Boston Scientific)117 or the ELRA (Endoluminal Radiofrequency Ablation) by Taewoong Medical in South Korea118 are designed to cause cancer necrosis while reducing damage to normal biliary mucosa. Complications of RFA include acute pancreatitis, cholangitis, cholecystitis and haemobilia. Likewise, although photodynamic therapy has been reported to increase stent patency, quality of life and survival, 10% of patients incur systemic photosensitivity.119 At present neither of these approaches has been approved by NICE.
Recommendation 26: EUS guided biliary drainage is recognised as a potential treatment option – but use of this technique should be planned at a MDT meeting with units adopting this approach able to show clear audit data in relation to alternative and more traditional methods of biliary drainage.
Strength of recommendation: MODERATE
Quality of evidence: MODERATE
EUS guided biliary drainage of the common bile duct, intrahepatic ducts and gallbladder are now established techniques in the management of patients with pCCA. ERCP fails to cannulate the obstructed system in up to 16% of the cases, although this figure varices substantially in the UK. Given the relatively close anatomical relationship between left lateral section of liver and lesser curve of gastric body, drainage of the obstructed intrahepatic ductal system can be achieved by EUS guided hepaticogastrostomy.120 In principle, this technique includes two types of procedures—rendezvous and transmural. As of 2013 the technique of EUS guided hepaticoduodenostomy for right-sided ducts has progressed with the recent publication of a case series.121 Co-temporaneous audit/governance is considered mandatory while establishing these techniques with full patient discussion and consent when undertaking this approach.
Recommendation 27: Patients should have a clear monitoring pathway for early detection of recurrent stent blockage and on-demand endoscopic intervention.
Strength of recommendation: STRONG
Quality of evidence: LOW
It is useful for patients to have serial monitoring of liver function tests to monitor for early stent blockage/cholangitis during active oncology or palliative follow-up. This may happen for a variety of reasons (table 4). Although non-evidence based, a minimum of 3-monthly repeat blood tests (full blood count, urea and electrolytes, liver function test, γ-glutamyl transferase and CRP) would seem a reasonable approach for those patients who would be suitable for treatment. Patients should have support phone numbers to call if their stent becomes blocked, and have access to a home supply of antibiotics for use if they were to develop stent cholangitis, with instructions of when to use them. It is generally recommended that repeat imaging is arranged with suspected stent blockage (recurrent bile duct blockage) to ensure that further endoscopic/EUS/PTC drainage is the correct course of action and relative anatomy determined in conjunction with the patient’s wishes and performance status.
Table 4.
Causes of recurrent biliary obstruction
| Cause | Definition |
| Tissue ingrowth/mucosal hyperplasia | Growth of cancer or hyperplastic mucosa into the lumen of SEMS |
| Tissue overgrowth | Tumour or tissue growth beyond the ends of SEMS |
| Sludge, hemobilia and food impaction | Occlusion of stent lumen by biliary sludge accumulation, clots and food impaction |
| Bile duct kinking | Obstruction at a proximal or distal end of SEMS due to an angulated bile duct |
| Stent kinking | Obstruction of stent lumen due to sharp bending of a SEMS because of an angulated bile duct or tumour growth |
SEMS, self-expandable metallic stents.
ERCP options in this situation potentially include: removing a covered stent and replacing it, sweeping the stent to clear debris, placing a plastic stent within a metal stent, or placing a metal stent within a metal stent. The most appropriate choice will be determined by the nature of the problem and the operator’s assessment of the situation.
Where expertise is available, EUS biliary drainage might be an option in the following situations: (1) failed ERCP, (2) postsurgical anatomy and (3) difficult biliary cannulation. Finally, at the time of a planned interventional procedure, the cause of the stent dysfunction should be defined for audit and research purposes.
Recommendation 28: Patients with recurrent pain after biliary stenting during their disease process should be evaluated with cross-sectional imaging. Patients with stent dysfunction should also be re-evaluated with cross-sectional imaging before any further endotherapy is undertaken.
Strength of recommendation: MODERATE
Quality of evidence: LOW
Complications other than stent blockage are also clinically important and include: pancreatitis, cholecystitis, non‐occlusion cholangitis, bleeding, ulceration, penetration, perforation and complications associated with the stent placement procedure (perforation, desaturation, aspiration pneumonia, etc).
Surgery for perihilar and intrahepatic cholangiocarcinoma
Recommendation 29: High-resolution cross-sectional imaging is essential for assessment of resectability and accurate staging.
Strength of recommendation: STRONG
Quality of evidence: HIGH
Recommendation 30: Pre-operative preparation, including augmentation of the FLR and biliary drainage, may be required to ensure safe resection.
Strength of recommendation: STRONG
Quality of evidence: HIGH
Recommendation 31: Staging laparoscopy should be used selectively.
Strength of recommendation: STRONG
Quality of evidence: MODERATE
Recommendation 32: R0 resection is the only curative treatment available.
Strength of recommendation: STRONG
Quality of evidence: MODERATE
Recommendation 33: Surgical resection of CCA should be undertaken only at high-volume centres with expertise across all relevant supporting specialties, including interventional radiology, endoscopy, hepatobiliary medicine, oncology and pathology.
Strength of recommendation: STRONG
Quality of evidence: MODERATE
Surgical resection is the only therapeutic option (other than liver transplantation for a small proportion of selected patients (see section on liver transplantation, and possibly ablation for small iCCA) that offers a potential cure for CCA.
Surgical approaches have become increasingly aggressive over the past decade with the express aim of obtaining complete tumour excision (R0) while maintaining adequate blood supply, biliary drainage and sufficient functional liver remnant (FLR) for patients to recover successfully. Distal CCA is treated with a Whipple’s resection and will not be focused on further as the surgical management of pancreatic ductal adenocarcinoma is already well described in international guidelines. For pCCA, bile duct excision, partial hepatectomy and en bloc caudate lobectomy are frequently required to achieve negative margins. For iCCA, a complete (R0) resection with an adequate liver remnant is the preferred surgical treatment. These challenging procedures are associated with significant morbidity and in-hospital mortality (up to 15%)122 and should only be undertaken in high-volume centres with the expertise required to manage such patients.123
In 2007, DeOliveira et al reported on 564 CCA resections, from a single centre. The average size of iCCA was 5.5 cm compared with 2.5 cm for pCCA and 2 cm for dCCA.124 Furthermore, despite the distinct macroscopic morphological subtypes, CCA uniformly spreads with perineural (intrahepatic 39%; perihilar and distal 75 %), lymphatic (intrahepatic 61%; perihilar 50%; distal 73 %) and vascular invasion (intrahepatic 64%; perihilar 38%; distal 73%).125 These factors often influence the surgical decision-making and outcome.
Determining resectability
This is an essential part of any surgical strategy and is reliant on high-quality imaging and accurate staging.
Staging systems and classification
iCCA staging follows the TNM staging model for epithelial tumours and lymph node metastases, and extrahepatic metastases are much more likely than HCC. Current staging classifications (including: AJCC/UICC system (8th edition) and the Okabayashi system) use variables such as tumour size (>5 cm), multifocal and bilateral tumours and vascular invasion (micro and/or macro) as prognostic determinants126 (see online supplemental figure 1). Significant changes have been made in the 8th edition of the AJCC staging of intrahepatic CCA.127 A tumour size cut-off point of 5 cm now separates the T1 category into T1a and T1b subgroups. This is because a tumour diameter >5 cm is an independent prognostic factor for overall survival and is also associated with a higher chance of microscopic vascular invasion and higher tumour grade.128–130 T2 tumours are now no longer subdivided into T2a and T2b because of the equivalent prognostic effect of vascular invasion and multifocal disease. T3 tumours are now defined as invading the visceral peritoneum (an area of controversy), while T4 are tumours involving local extrahepatic structures by direct invasion and are categorised as stage IIIB.
pCCA
Staging systems and classification
A problem with TNM staging for pCCA is that a small, badly place tumour markedly worsens prognosis, and T staging is inadequate. Current staging classifications include: the Memorial Sloan-Kettering Cancer Centre (MSKCC) system, the AJCC system (8th edition) (see online supplemental figures 2 and 3) and the modified Bismuth-Corlette (BC) classification (used to anatomically locate the tumour with reference to the bifurcation of the common hepatic duct).131 Important features common to all systems include: hepatic duct involvement (unilateral vs bilateral) and portal vein involvement (ipsilateral vs contralateral vs main). Other variables include: ipsilateral hepatic hemi-atrophy, tumour extension into second-order biliary radicles, tumour extension into surrounding adipose tissue or hepatic parenchyma, hepatic artery involvement (ipsilateral vs contralateral vs common hepatic artery), regional lymph node metastases.132
Assessment of resectability using the BC classification133 system is helpful in anatomically locating the tumour with reference to the bifurcation of the common hepatic duct (CHD), (figure 2). Tumours located distally in the biliary tree to the CHD are classified as BC type 1 tumours. Tumours located proximal to the CHD in the biliary tree are further divided into four types. The BC classification is broadly used to describe the longitudinal extension of the tumour from the CHD. Type 2 BC tumours are inclusive of the CHD, type 3a BC tumours incorporate longitudinal extension along the right hepatic main duct, and type 3b BC tumours incorporate extension along the left hepatic main duct exclusively. Type 4 BC tumour classification incorporates biductal extension and multifocal ductal disease.
While the BC system has been universally adopted as an anatomical descriptive system for tumour location, it is poor at accurately describing longitudinal extension and is commensurately poor at distinguishing between left and right duct extension.134 The BC system has been shown to have a limited usefulness in determining contraindications to surgical approach and has no evidence-based role with respect to postresection prognosis.135
The MSKCC staging system attempts to account for both longitudinal and radial extension of the primary tumour (figure 3),136–139 and is yet to be robustly validated in an external modern cohort for resectability. The majority of validation studies are concerned with determining the usefulness of the system with reference to survival.140 141
Figure 3.
Schematic Representation of the Memorial Sloan-Kettering Cancer Centre classification system.
Unresectability can result from either extensive local disease (including vascular and nodal involvement), presence of distant metastases or comorbidity of the patient. Local unresectability can be due to involvement of the portal vein and hepatic artery on the side of the future remnant liver without the possibility of a vascular reconstruction, extensive bilateral proximal infiltration of the tumour into secondary biliary radicles (segmental bile ducts) and/or massive extension of tumour into the liver parenchyma. Furthermore, extrahepatic metastases including distant lymph node metastases beyond the hepatoduodenal ligament (N2 nodes), are associated with poor survival and in most centres, are considered as unresectable disease.142
Staging laparoscopy
The usefulness of routine staging laparoscopy (SL) in stratifying patients for resection remains controversial. A meta-analysis by the Amsterdam Medical Centre (AMC) hepatobiliary group suggests that SL in modern cohorts has limited usefulness owing to the improved sensitivity of multislice CT and PET-CT in determining the presence of peritoneal and distant metastases.143 The MSKCC group advocate selected use of SL for locally advanced MSKCC stage T2 or T3 patients.139 This approach risks understaging small surgically resectable tumours that have already spread in the peritoneum beyond the surgical field. The yields of SL for peritoneal disease vary between 10% and 17% of all patients undergoing surgical assessment, precluding unnecessary laparotomy in this group.144–150 Laparoscopic ultrasound may provide additional information for determining hilar resectability, particularly with respect to defining radial extension into surrounding vascular structures; however, its usefulness has yet to be demonstrated in a large modern cohort.151 The risk of occult metastatic disease is particularly high in patients with high serum CA 19–9, major vascular invasion and suspicious lymph nodes. Exploratory laparotomy without resection should be avoided because it delays palliative systemic chemotherapy.152
Functional liver remnant (FLR)
Ensuring an adequate functional liver remnant (FLR) is essential for safe resection. This can be determined preoperatively by the use of CT volumetry, which determines the ratio of FLR to non-tumorous liver volume. The non-tumorous liver volume can be determined either by direct CT measurement or by estimation of body surface area. Ribero et al demonstrated, in a large modern cohort of 243 patients, that CT measurement underestimates the risk of hepatic insufficiency postoperatively and that body surface assessments were more accurate in determining subsequent risk.153 Augmentation of the FLR to reduce the likelihood of post-resection liver insufficiency has become a widely accepted technique.154 Augmentation typically is used in patients with right-sided intrahepatic or hilar CCA type 3A (Bismuth-Corlette staging, figure 2). FLR augmentation is typically undertaken on patients for whom the FLR post-resection would be 20% in patients with normal liver parenchyma155; 30% in post neoadjuvant chemotherapy (a rare cohort in patients with CCA); 40% in patients with established liver cirrhosis.156 The main approaches to augmentation are portal vein embolisation (PVE); portal vein ligation (PVL); and associated liver partition and portal vein ligation (ALPPS).
FLR responsiveness to PVE has been demonstrated to be an independent preoperative predictor of postoperative liver insufficiency.157 158 PVL and ALPPS are surgical procedures used to produce FLR hypertrophy. PVL involves surgical ligation of the portal vein to ensure redirection of portal venous blood flow. ALPPS is a novel concept with a narrow evidence base and significant controversy. The procedure involves surgical splitting of the hepatic parenchyma and ligation of the right portal vein to provide complete partition and reduce the chance of collateralisation of blood supply to the FLR.159 160 Following the initial stage of vascular ligation and parenchymal microvascular isolation, a second stage right trisectionectomy is undertaken for resection of the primary tumour, following radiographically demonstrated adequate FLR hypertrophy.161 The rate of growth of the FLR is also significantly faster, with maximal growth occurring at day 9 postoperatively compared with 4–6 weeks after PVE.160 162 The short interval between the initial portal vein ligation and parenchymal transection and the peak hypertrophic stage potentially increases the likelihood of completion of the second stage of the procedure, the trisectionectomy. PVE and two-stage hepatectomy require significant periods between the initial procedure and completing the resection of the primary tumour.163 This interval produces a potential for disease progression, thereby precluding completion of resection. Laparoscopic approaches to ALPPS have also been reported demonstrating the feasibility of the procedure to be adopted as a minimally invasive approach for hypertrophy of the FLR.164–167 Although there was initial support for the use of this approach for FLR growth, data from the international ALPPS Registry has demonstrated that this technique, when used for perihilar cholangiocarcinoma is associated with excessive mortality of 48%. Hence this technique is not currently recommended in this clinical setting.168
Preoperative biliary drainage
There is clear consensus that preoperative biliary decompression is indicated in patients with cholangitis, patients undergoing preoperative antineoplastic therapy, patients with hyperbilirubinaemia-induced malnutrition, hepatic insufficiency or renal insufficiency, and patients undergoing PVE. Although some authors have advocated no preoperative biliary decompression in patients with adequate nutritional status and no cholangitis, others, especially those from centres in Asia, have advocated biliary drainage as mandatory, regardless of bilirubin level, because of the association between cholangitis and outcome.169 Decompression of the bile duct can be facilitated with either PTC or ERCP (see recommendations 23/24). In addition to the reduction of both procedural risks and need for re-intervention with ERCP, PTC catheters can provide much better delineation of the extent of the spread of endobiliary tumour within the liver for resection planning.170 171 Even though endoscopic drainage is highly successful, complex lesions may not respond adequately to endobiliary drainage and hence, particularly in patients who may be candidates for resection, the care team should not hesitate to establish durable biliary drainage with percutaneous catheters if required.172
Intraoperative surgical considerations
Resection of iCCA
A complete (R0) resection with an adequate liver remnant is the preferred surgical treatment. Most patients have a single large tumour requiring a (extended) hemi-hepatectomy. If the FLR volume is below 30% enhancement of the FLR is required (see section on FLR). ALPPS can be considered if the remnant volume remains inadequate after PVE or if intraoperatively a larger resection than expected is needed on imaging.173
About 15% of ipatients with CCA who undergo a resection present with biliary obstruction. Most of these patients will require preoperative biliary drainage, in particular in the setting of cholangitis or a small FLR (see also section on biliary drainage in the first part on pCCA). Resection without biliary drainage can be considered if the future liver remnant exceeds 50%. Resection of the biliary confluence is typically needed in patients with biliary obstruction, followed by a Roux‐en-Y hepaticojejunostomy.174 Current guidelines recommend performing a lymphadenectomy in all patients with resectable iCCA.127 152
Most patients with iCCA (75%) will require an (extended) hemi-hepatectomy with higher than 1% mortality. Mortality is higher when vascular reconstructions are needed, and for patients with cirrhosis who are operated on.175 The majority of patients will develop recurrent disease after resection of iCCA. In a large study by Hyder et al, the median recurrence-free survival of 301 patients was 20 months. Most patients developed an initial intrahepatic recurrence (61%). An initial extrahepatic recurrence was found in 21%, and 19% had a simultaneous intrahepatic and extrahepatic recurrence.176 The median overall survival after a curative resection is about 30 months with a 5-year overall survival of approximately 30%–40% based on several large series.177
Locoregional treatments
Surgical resection is the preferred treatment for resectable iCCA. Lesions of less than 3 cm located centrally in the liver may be considered for thermal ablation (such as radiofrequency or microwave ablation) especially those lesions in patients with a high surgical risk (eg, cirrhosis). The main disadvantage of thermal ablation is an increased risk of local recurrence and the inability to perform a lymphadenectomy, although nodal metastasis is unlikely in small lesions, and removing positive lymph nodes has not been shown to improve survival. In lesions too large for thermal ablation, transarterial treatments are available, such as transarterial chemoembolisation, elective internal radiation therapy with Y-90 and hepatic arterial infusion chemotherapy.178 179
pCCA
Exploratory laparotomy is undertaken following exclusion of disseminated intraperitoneal disease at SL. R0 resection of the primary tumour confers significant survival benefits compared with R1/R2 resection.180 The focus of exploratory laparotomy is to determine and confirm local resectability of the primary tumour with regards to local vascular invasion, distal biliary duct extension, and intra-abdominal nodal spread. Surgical assessment of vascular invasion includes visual inspection and palpation, with intraoperative ultrasound providing evaluation of extension of the tumour into the hilar vascular structures. Coeliac axis nodes confirmed intraoperatively on frozen section are considered to represent metastatic disease outside of the surgical field and, if detected, would constitute closure without proceeding to resection.
Distal bile duct transection occurs early in the resection to ensure adequate access to the hilar vasculature structures. During isolation and transection of the distal margin, the specimen routinely undergoes frozen section analysis. Frozen section analysis is used to determine the presence of microscopic disease at the distal resection margin. If there appears to be microscopic invasive disease threatening the resection margin then further excision can be undertaken to ensure adequate R0 resection margins. Patients undergoing re-excision of the distal margin and subsequently achieving a negative frozen section of the new margin appear to have similar survival characteristics to patients who achieved negative margins on the initial frozen section.181 Carcinoma in situ threatening or present at the resection margin does not appear to produce negative effects on survival and can essentially be considered as being equivalent to a negative frozen section result.182 Frozen section has a sensitivity of between 60% and 70%, with a significant number of false-negative and false-positive findings confirmed on subsequent full histopathological assessment.183 184 The false-negative results have been putatively linked to the use of preoperative biliary stenting producing epithelial regeneration at the site of the distal margin.185 Regeneration of the normal epithelial layer occurs in response to the friction produced by the stent at this site. Following confirmation of clear distal margins on frozen section attempted resection of the primary tumour can proceed. Some centres collect bile swabs during hilar CCA resections to guide subsequent antibiotic use.
Traditionally, concomitant resection of the caudate lobe is undertaken due to the high proportion of patients with microscopic infiltration of the caudate lobe.186 187 Sufficient resection to achieve R0 resection margins is advocated.188 189 Extended right hemi-hepatectomy, inclusive of the inferior section of segment 4 (4B) with hilar bile duct excision at the confluence, has been demonstrated to achieve good R0 resection margins for type 3a disease.190 The anatomical proximity of the portal vein to the hilar confluence has led to the development of en bloc ‘no-touch’ techniques including resection of the portal vein as necessary.191 En bloc resection has been suggested to offer improved survival, but may also be associated with increased perioperative mortality.192 193
Right-sided trisectionectomy is the preferred approach, if feasible, for resecting hilar CCA. Left-sided approaches for hilar pathology are surgically demanding and reserved solely for predominantly left-sided BC 3b tumours.194 Principally, the difficulty of the approach relates to the extrahepatic course of the respective portal vein. The right portal vein has a short extrahepatic course, which makes reconstruction of the portal vein following left-sided resection difficult.194–196 Left-sided resections for BC 3b tumours are also more likely to involve complex hepatic arterial resection and reconstruction. The right hepatic artery is potentially threatened due to its proximity to the left portal vein and its course within the hilum.197 Consequently, there is a corresponding increase in potential for postoperative liver insufficiency if the right hepatic artery is encountered during a left-sided resection.
Invasion of the portal vein is reflective of locally advanced CCA and represents T3/T4 disease. Despite portal vein invasion representing more locally advanced disease, overall survival in allcomers undergoing resection is comparable to that of patients undergoing major hepatectomy without portal vein resection.180 198 Vascular resection and reconstruction of the hepatic artery, which appears to confer limited survival benefits in highly selected patients, may be suitable.199 200
An important resection consideration is ensuring that an adequate lymphadenectomy field is achieved. Fastidious dissection of the course of the proper hepatic and common hepatic artery, in the hepatoduodenal ligament, to the level eight lymph node in the retroperitoneum is required to gain an adequate surgical field. Acquisition of lymphatic tissue is technically difficult to achieve and has substantial risk of comorbidity to the patient. Inadequate acquisition of lymphatic tissue, with fewer than five nodes resected, has a detrimental effect on overall survival due to understaging of disease.201 Acquisition of 15 lymph nodes within the resection specimen has been suggested as the optimal lymphadenectomy for accurate staging of disease and subsequent determination of prognosis.202 However, the optimal number of lymph nodes acquired within the resection specimen rarely reaches this number, with the median number of nodes acquired being between 5 and 10.203–205 The ratio of positive lymph nodes to total lymph nodes acquired has been linked to overall survival and recurrence-free survival.206 207
Postoperative prognostic factors and follow-up
A multi-institutional series of 306 resections was used to develop a prognostic nomogram, which included margin status; lymph node positivity and tumour differentiation, which predicted disease-free survival and could facilitate stratification of patients into clinical trials (figure 4).208 Subsequent to this, a large meta-analysis of in excess of 4500 resected patients with CCA has validated the above prognostic factors, as well as identifying a number of additional variables that have an impact on outcome (table 5).209
Figure 4.
Memorial Sloan-Kettering Cancer Centre postoperative prognostic nomogram. DSS, disease-specific survival.
Table 5.
Meta-analysis of prognostic factors
| Variable (default) | N | Heterogeneity | Random effects | ||
| I2 P | P value | In (HR) (95% CI) | HR (95% CI) | ||
| Tumour size (small) | 5 | 36.4% | 0.178 | 0.19 (–0.10 to 0.48) | 1.21 (0.90 to 1.62) |
| Age (young) | 10 | 0.3% | 0.435 | 0.11 (0.00 to 0.22) | 1.12 (1.00 to 1.25) |
| LN involvement (negative) | 17 | 22.9% | 0.188 | 0.62 (0.51 to 0.72) | 1.86 (1.67 to 2.05) |
| Microvascular invasion (negative) | 7 | 0.0% | 0.470 | 0.38 (0.24 to 0.52) | 1.46 (1.27 to 1.68) |
| Perineural invasion (negative) | 12 | 0.0% | 0.656 | 0.41 (0.30 to 0.51) | 1.51 (1.35 to 1.67) |
| Portal vein resection (none) | 8 | 58.7% | 0.018 | 0.31 (0.07 to 0.55) | 1.36 (1.07 to 1.73) |
| Resection margin status (negative) | 17 | 57.1% | 0.002 | 0.63 (0.47 to 0.78) | 1.88 (1.60 to 2.18) |
| Gender (male) | 9 | 29.1% | 0.187 | 0.06 (–0.09 to 0.21) | 1.06 (0.91 to 1.23) |
| Tumour status (T1–T2) | 5 | 54.9% | 0.065 | 0.43 (0.18 to 0.68) | 1.54 (1.20 to 1.97) |
| Tumour differentiation (well differentiated) | 10 | 0.0% | 0.505 | 0.43 (0.32 to 0.54) | 1.54 (1.38 to 1.72) |
LN, lymph node.
Currently there is no consensus as to frequency of clinical follow-up, imaging or the use of tumour markers following resection for CCA. This lack of consensus was demonstrated in the survey undertaken by Cholangiocarcinoma-UK of NHS units undertaking this surgery.210 However, the majority of centres undertake 6-monthly cross-sectional imaging with a CT scan of chest, abdomen and pelvis, as well as studying tumour markers for at least 2 years after resection.210
Impact of surgical activity on outcome
Correlation of surgeon activity and patient outcome is challenging to quantify as there are many confounding variables—for example, expertise of support services, which can affect patient outcomes. Idrees et al using the US National Comprehensive Cancer Network database, containing in excess of 40 000 CCA resections, showed that a hospital volume of 14 operations/year was the most sensitive and specific value associated with mortality.211 Surgery at high-volume hospitals (HR=0.92, 95% CI 0.88 to 0.97, p<0.001) was independently associated with improved overall survival.211 Another recent study determined the annual case volume for optimum outcomes for 2471 patients with resected pCCA at 471 facilities between 2010 and 2017. They reported that centres undertaking at least seven perihilar resections a year have improved 90-day mortality and improved perioperative outcomes.212
Liver transplantation for CCA
Recommendation 34: Liver transplantation for selected patients with pCCA in the presence of chronic liver disease (most commonly primary sclerosing cholangitis), less than 3 cm in size with no evidence of extrahepatic disease results in long-term disease-free survival. This is an established indication in an increasing number of centres internationally. There is a need for evaluation of novel neoadjuvant chemoradiation strategies and assessment of long-term outcomes with national protocols and multi-centre studies. Liver transplantation in the absence of background chronic liver disease remains an investigational treatment.
Strength of recommendation: MODERATE
Quality of evidence: STRONG
Recommendation 35: Neoadjuvant therapy in uncontrolled studies appears to be effective in controlling disease and selecting patients who are most likely to benefit from transplantation.
Strength of recommendation: MODERATE
Quality of evidence: LOW
Recommendation 36: Transplantation for iCCA on a background of chronic liver disease precluding resection should be evaluated prospectively within a national protocol. LAG tumour size criteria are to be monitored and modified to improve recruitment for evaluation.
Strength of recommendation: STRONG
Quality of evidence: MODERATE
Although potentially curative, unfortunately liver resection is only applicable for a minority of patients due to the extent of the cancer or the presence of background chronic liver disease at presentation. Liver transplantation is a potential treatment option for selected patients with pCCA or iCCA.
Perihilar CCA
Initial attempts at liver transplantation for pCCA produced poor outcomes, which have been attributed to selecting patients with advanced cancers, a surgical learning curve, immunosuppression management and lack of understanding of tumour biology.213–215 Liver transplantation for pCCA was initiated with publications from Nebraska216 and Mayo Clinic,217 which introduced strict selection protocols to identify a group of patients likely to benefit from liver transplantation, and these have been adopted by other centres.218–222
De Vreede et al 217 published the initial Mayo experience in 2000, with 19 patients enrolled into a neoadjuvant therapy protocol. Eleven patients underwent transplantation. Of eight with long term follow-up (median 44 months), only one patient developed recurrence. Similarly, Sudan et al published the Nebraska experience in 11 patients who underwent transplantation after neoadjuvant chemoradiotherapy, with 5 of the 11 patients alive and disease free at a median follow-up of 7.5 years.216
The majority of cases reported more recently have adopted the Mayo protocol with minor modifications.218–222 The key protocol components are: selection of patients, neoadjuvant therapy and test of time. Patients with localised cancers with a dominant stricture or less than 3 cm tumour were included. Intrahepatic and extrahepatic metastases including any nodal metastases are exclusion criteria. Controversially, histology or cytology was not considered essential for diagnosis of CCA. Elevated serum CA 19 9 of greater than 100 U/mL, ploidy of cells on bile cytology (FISH), or a dominant stricture associated with a mass lesion on imaging were considered sufficient for enrolment into the protocol. Prior attempt at transperitoneal biopsy (percutaneous or EUS guided) was another exclusion criterion, based on increased risk of tumour dissemination.
Neoadjuvant therapy involved external beam radiation therapy with concurrent chemotherapy (chemosensitisation), followed by brachytherapy whenever possible. Patients were then restaged and continued to receive systemic chemotherapy with oral capecitabine until the time of transplant. Over a period of 26 years (1993–2019), 376 patients including those with de novo CCA (148) were enrolled on this protocol at the Mayo clinic.223 Of these, 14% were excluded as they developed disease progression during neoadjuvant therapy. A further 14% of patients were excluded at staging surgery, which took place after neoadjuvant therapy because of extrahepatic metastases, and were excluded from transplant as failing the ‘test of time’ with unfavourable tumour biology.
A recent systematic review included 20 studies from 2000 until 2019, with 428 patients eligible for analysis.224 The pooled 1-, 3-, and 5-year overall survival rates following liver transplantation without neoadjuvant therapy (n=156) were 71.2% (95% CI 62.2% to 79.4%), 48.0% (95% CI 35.0% to 60.9%) and 31.6% (95% CI 23.1% to 40.7%), respectively. Patients who had neoadjuvant therapy prior to transplantation (n=272) had higher survival of 82.8% (95% CI 73.0% to 90.8%), 65.5% (95% CI 48.7% to 80.5%), and 65.1% (95% CI 55.1% to 74.5%) at 1, 3 and 5 years, respectively. Similarly, the recurrence rate was 51.7% in patients not undergoing neoadjuvant therapy compared with 24.1% for patients who did. Only 4 of the 20 studies reported pretransplant histological confirmation of adenocarcinoma or malignant/suspicious cells on cytology. Of 145 liver explants from studies not using neoadjuvant therapy, malignancy was found in 142 (97.9%), compared with no evidence of malignancy in 126 (49.4%) of 255 patients who had neoadjuvant therapy. These 126 patients are presumed to have had complete pathological response to neoadjuvant therapy, although the possibility exists that in at least some of the patients there was no malignancy at all.
Patients with background PSC had better survival than patients with de novo cancers. The most recent experience from Mayo, published in 2016, demonstrates a 5-year survival of 74% for patients with background PSC compared with 58% for de novo CCA after transplantation. Response to neoadjuvant therapy is another independent predictor of outcomes.222 Five-year disease free-survival was 93.5% in patients with complete or near-complete response to neoadjuvant therapy (<1% viable tumour in explant) compared with 51.5%, 41.6% and 27.3% for tumours with 1–10%, >10–30% and >30% viable tumour, respectively. A limitation of these data is that the prognostic assessment was histology of the explant and hence it cannot be used for case selection.
Intrahepatic CCA
Intrahepatic CCA, while rare, is increasingly diagnosed in patients with background chronic liver disease. Where feasible, liver resection remains the gold standard for treatment. The associated chronic liver disease and function, location of tumour and portal hypertension limit applicability of liver resection even in patients with small iCCA.
iCCA currently remains a contraindication for liver transplantation in most programmes worldwide.225 Recent multicentre studies showing encouraging outcomes have prompted reassessment of iCCA on a background of chronic liver disease as an indication for liver transplantation.226–230 Much of these data are retrospective, with iCCA found incidentally in the explant or being misdiagnosed as HCC radiologically. A single-centre study of 13 patients,228 reported no recurrence in four patients with well-differentiated tumours compared with 78% recurrence in those with moderately differentiated tumours, suggesting that tumour biology may be key to transplant selection. A multicentre study of eight patients from Spain with very early iCCA (defined as solitary tumours less than 2 cm in size) reported 73% 5-year survival.229 An international multicentre study again demonstrated that the number and size of tumours were key factors influencing long-term outcomes.230 Fifteen patients with very early iCCA had a 5-year 65% survival with recurrence rates comparable to those of transplant outcomes for HCC within Milan criteria. Jung et al reported the outcome of liver transplantation for 16 patients with incidental iCCA and compared their outcomes using a propensity score matched analysis with 100 patients with CCA who underwent liver resection.231 Of patients with very early iCCA there was no recurrence following liver transplantation (n=3, follow-up of 39.1±29.9 months), whereas 23% (6/26) of those undergoing resection developed recurrence. Half of the recurrences of very early iCCA were intrahepatic, and the authors argued that these patients might have potentially benefited from transplant.
A recent multicentre French study advocates a more liberal approach towards tumour size. A retrospective three-centre study compared outcomes of patients who underwent liver transplantation with incidental iCCA found at explants (n=49) with those of patients who underwent liver resection for iCCA with background chronic liver disease (n=26).232 The incidence of incidental iCCA and mixed hepatocellular–cholangiocarcinoma increased from 0.6% of transplants in 2002 to 2% by 2015. At a median follow-up of 25 months, the 1-, 3- and 5-year survival of patients who underwent liver transplantation was 90.76 and 67%, respectively, compared with 92.59 and 40% for patients who had resection. The recurrence-free survival was 75% at 5 years after transplantation compared with 36% for resection. Independent risk factors for recurrence were the size of the largest tumour and differentiation. The 1- and 5-year survival for tumours <2 cm after transplantation was 92.87 and 69% compared with 87.65 and 65% for tumours 2–5 cm in size. Combined HCC–CCA had similar outcomes to those of iCCA. Of patients who underwent liver transplantation, 55% had transarterial chemoembolisation as bridging therapy and five patients had adjuvant chemotherapy with gemcitabine and oxaliplatin.
Two studies investigated the role of neoadjuvant therapy prior to liver transplantation for large unresectable iCCA.233 234 Systemic therapy and locoregional approach with radioembolisation were used in these studies. These studies indicate potential benefit of neoadjuvant and adjuvant therapies, which needs to be investigated in larger clinical trials.
Systemic therapy
Recommendation 37: Patients who have undergone surgical resection for CCA should be considered for 24 weeks of adjuvant chemotherapy (currently capecitabine).
Strength of recommendation: STRONG
Quality of evidence: MODERATE
Recommendation 38: The routine use of neoadjuvant chemotherapy in patients with resectable CCA is not recommended.
Strength of recommendation: STRONG
Quality of evidence: LOW
Recommendation 39: CisGem chemotherapy is recommended as the first-line treatment in patients with advanced BTC. Immunotherapy may be added to CisGem chemotherapy, if approved and available, cognisant of the magnitude of benefit and toxicities.
Strength of recommendation: STRONG
Quality of evidence: HIGH
Recommendation 40: Combination chemotherapy is recommended in patients with adequate performance status following failure of first-line chemotherapy, particularly in the absence of a targetable molecular alteration.
Strength of recommendation: MODERATE
Quality of evidence: HIGH
Recommendation 41: CCA should be subjected to molecular profiling at the earliest opportunity, and results and treatment options should be reviewed by clinicians with appropriate expertise.
Strength of recommendation: STRONG
Quality of evidence: HIGH
Adjuvant treatment
The aim of adjuvant treatment is to reduce the chances of disease relapse, thereby improving survival, following potentially curative resection. The PRODIGE 12 randomised phase III trial failed to show benefit of gemcitabine and oxaliplatin chemotherapy in patients with resected biliary tract cancer (CCA or gallbladder cancer) over observation alone235; there was also no benefit from gemcitabine in a phase III study limited to patients with extrahepatic (perihilar and distal) CCA.236 The STAMP study compared gemcitabine and cisplatin with capecitabine in node-positive extrahepatic CCA. This was negative, but the sample size of 101 patients was small.237 The BILCAP phase III study, randomising patients with CCA and gallbladder cancer to receive adjuvant capecitabine versus observation failed to demonstrate an improvement in overall survival (the primary endpoint) by intention-to-treat analysis (HR=0.81; 95% CI 0.63 to 1.04; p=0.097). However, there was an improvement in survival according to the prespecified sensitivity analysis adjusted for sex, tumour grade and nodal stage (HR=0.71; 95% CI 0.55 to 0.92; p=0.010).205 This, together with the clinically meaningful numerical improvement in median survival (51.1 vs 36.4 months) favouring chemotherapy, by intention-to-treat analysis) has led to the adoption of capecitabine as standard of care and the reference for future studies.
Most recently, the results of the JCOG1202 (ASCOT) study were presented.238 In this phase III study patients with biliary tract cancer (CCA and gallbladder cancer) were randomised to receive S1 (an oral fluoropyrimidine) versus observation alone. There was an improvement in the 3-year overall survival (77.1% vs 67.6% in favour of S1; HR=0.694; 95% CI 0.514 to 0.9351; one-sided p value 0.008), although the 3-year relapse-free survival was not statistically significantly different (62.4% vs 50.9% for S1 vs surgery, respectively, HR=0.797; 95% CI 0.613 to 1.035). Further follow-up is required as there were only 40.7% of events (deaths) at the time of the analysis. Although this supports the findings of the BILCAP study, the ASCOT study did not include a Western population and therefore capecitabine is the recommended adjuvant treatment in patients following resected biliary tract cancer.
Although there have been several phase II studies evaluating systemic treatments in the neoadjuvant setting, no phase III studies are available on which to make any high-level recommendations. However, in patients with locally advanced disease who are initially deemed unresectable and who derive a good response to systemic therapy, it is recommended that treatment of the patients is rediscussed at the appropriate MDT meeting to re-evaluate potentially curative resection.
Advanced disease
It can be difficult to obtain an unequivocal histological or cytological diagnosis in CCA, particularly perihilar CCA. At least two, and if appropriate more, attempts should be made at unequivocal histological or cytological diagnosis, and the diagnosis reconsidered if persistently negative. If there is a strong clinical suspicion of malignancy despite negative unequivocal histological or cytology, systemic therapy may be considered following discussion with the MDT and the patient. This scenario should be a rare exception.
In advanced disease, patients who do not receive systemic therapy have a very short life expectancy, typically 3–4 months. Phase III studies have shown an improvement in overall survival with chemotherapy compared with supportive care alone.239 240 The UK ABC-02 study defined the combination of cisplatin and gemcitabine (CisGem) as the standard-of-care regimen in 2009 having shown an improved survival of the doublet to single-agent gemcitabine241; comparable findings were seen in the BT22 randomised phase II study.242 Use of gemcitabine alone in patients with poor performance status would be reasonable and substitution of oxaliplatin for cisplatin is reasonable in those with renal impairment.241
Intensification of chemotherapy with triplet regimens has delivered mixed results. In the randomised phase II/III PRODIGE 38 (AMEBICA) study, the modified (m)FOLFIRINOX regimen (5-FU, irinotecan and oxaliplatin) was compared with CisGem in the first-line setting.243 There was no improvement in the primary endpoint of the randomised phase II stage (6-month progression-free survival: 44.6% (90% CI 35.7% to 53.7%) with mFOLFIRINOX vs 47.3% (90% CI 38.4% to 56.3%) with CisGem), and the study did not proceed to phase III. In the KHBO1401-MITSUBA study, the triplet of CisGem plus S1 showed an improvement in overall survival versus CisGem (median 13.5 vs 12.6 months; HR=0.79; 95% CI 0.63 to 0.99; p=0.046 – pending full manuscript) in favour of the triplet with additional, although manageable, toxicity rendering it as another treatment option in Japan.244 Based on promising activity seen in a phase II study (median progression-free survival of 11.8 months, 95% CI 6.0 to 15.6; partial response rate of 45% and disease control rate of 84%; and median overall survival of 19.2 months (95% CI 13.2 to not estimable)),245 the SWOG 1815 randomised phase III study compared gemcitabine and cisplatin with or without nab-paclitaxel; however, the result was was negative.246 As such overall survival outcomes using triplet-agent chemotherapy in biliary tract cancer have been disappointing, although response rates have improved. Triplet-agent chemotherapy may be most relevant in patients where a higher response is pivotal—for example, in rendering disease potentially resectable.
Two randomised studies have shown an improvement in overall survival in the second-line setting; ABC-06 and NIFTY. In the phase III ABC-06 study, patients were randomised to active symptom control alone or with FOLFOX (5-FU and oxaliplatin) chemotherapy. FOLFOX-treated patients had an improved overall survival (HR=0.69, 95% CI 0.50 to 0.97, p=0.031); notably survival was greater than expected in the active symptom control alone arm (5.3 vs 4 months) highlighting the need for proactive screening, identification and treatment of disease-related complications (particularly biliary obstruction and infection).247 In the randomised phase IIb NIFTY study, Korean patients received either 5-FU monotherapy or in combination with nano-liposomal irinotecan. Combination chemotherapy was associated with an improved progression-free survival (primary endpoint, by blinded independent central review): 7.1 months, 95% CI 3.6 to 8.8 vs 1.4 months, 95% CI 1.2 to 1.5 with monotherapy (HR=0.56, 95% CI 0.39 to 0.81, p=0.0019) as well as overall survival (HR=0.68, 95% CI 0.48 to 0.98, p=0.035).248 The incremental benefit of using nano-liposomal irinotecan in preference to conventional irinotecan is unknown.
Immunotherapy
A phase III study to evaluate the benefit of adding immunotherapy (durvalumab, a programmed death-ligand 1 (PD-L1) inhibitor) to first-line chemotherapy (TOPAZ-1) has shown a reduction in risk of death by 20% (HR=0.80, 95% CI 0.66 to 0.97, p=0.021).249 The benefit is mainly seen beyond the first 6 months of treatment, with increasing divergence of the survival curves at 12 months (54.1 vs 48% alive), 18 months (35.1% vs 25.6%) and 24 months (24.9% vs 10.4%) with little difference at the median (12.8 vs 11.5 months) for durvalumab and chemotherapy, respectively. The progression-free survival and overall response rate were also statistically significantly improved, with evidence of durable responses in some patients with no new safety concerns from the new combination. No enrichment criteria have emerged to date (PD-L1 expression did not correlate with outcome) to identify patients most likely to benefit. The KEYNOTE-966 study (cisplatin and gemcitabine with either pembrolizumab or placebo) showed very similar outcomes, confirming the first-line standard of care of cisplatin, gemcitabine and immunotherapy.250
Targetable molecular alterations in biliary tract cancer
A significant proportion of biliary tract cancers have an actionable molecular alteration (table 6). Although iCCA have the largest proportion (~50%), the other sites of CCA also have up to 30%.251 Some alterations are specific to anatomical subtypes—for instance, FGFR2 and a IDH1 localised iCCA while some are present throughout the whole biliary tract, for instance BRAF. Notably, these alterations are mutually exclusive to other common drivers of malignancy, notably RAS, and consequently offer opportunities for benefit from targeted therapies. Resistance to therapies appears to be a consequence of emergent mutations within the target gene (FGFR2, IDH1), and the development of second-generation multitarget compounds is ongoing.252 253
Table 6.
Frequencies of targeted actionable alterations in biliary tract cancer
| Alteration | Frequency | Frequency (specific subtype) | Test | ESCAT score | Reference |
| IDH1 mutations* | 1–18% | 8–18% (iCCC) | NGS | IA | 1 13 14 |
| IDH2 mutations | <5% | <5% (iCCC) | NGS | IIB | 1 14 |
| FGFR2 fusions+ | <10% | 5–15% (iCCC) | RNA-seq | IIB | 6–9 15–19 |
| FGFR2 mutations | 2% | 2% (iCCC) | NGS | IIB | 11 15–21 |
| HER2 amplifications | 5–10% | 10–20% (d/pCCC, GBC) | NGS/FISH/IHC | IIIA | 10 15–19 22–24 |
| HER2 mutations | 3–5% | More frequent d/pCCC and GBC | NGS | IIIA | 10 15–19 22–24 |
| BRAF mutations | < 5% (50% V600E) | NGS | IIIA | 15–19 21 23 25 26 | |
| BRCA1/2 mutations | 3–5% | NGS | IIIA | 15–19 23 26–32 | |
| PALB2 mutations | 1% | NGS | IIIA | 1 19 30–33 | |
| KRAS G12C | <1% | NGS | IIA | 1 34 | |
| NTRK | <1% | RNA-seq | 1 35 | ||
| MSI | <1% | IHC | 1 36 | ||
| MDM2 amplification | 7% | NGS | IIB | 37 |
There is substantial heterogeneity across studies in molecular testing methodology and patient population, which limits the precision of these estimates (approved by the National Institute for Health and Care Excellence at the time of writing).
d/pCCC, distal/perhilar chiolangiocarcinoma; FISH, fluorescent in situ hybridisation ; GBC, gallbladder cancer; iCCC, intrahepatic cholangiocarcinoma; NGS, next-generation sequencing.
A primary difficulty for clinicians is the choice of profiling platform. Most commercially available platforms use hybrid DNA technologies, but these are likely to be less good at finding fusion abnormalities.254 This is currently also the case for liquid biopsies most of which still use DNA technologies and are limited by patient tumour load. A careful discussion of the options with a molecular tumour board is therefore essential.254 255
FGFR2 fusions, mutations and extracellular domain in-frame deletions are sensitive to FGF2 inhibitors.28 256–260 Several agents, such as pemigatinib and infigratinib (among others), are likely to have a similar efficacy, affording a progression-free survival in second and subsequent line therapy of 7–9 months. Molecular data from this small number of patients, supported by in vitro data, suggest that futibatinib has shown activity against emergent mutations, but this has yet to be confirmed.261 Any survival impact is currently uncertain because of the lack of randomised data to resolve the prognostic impact of FGFR2 alterations, which remain uncertain.262 Short-term toxicities are generally tolerable and manageable, although longer term emergent toxicities, such as hyperphosphataemia, may be more difficult to manage.
Ivosidenib has been shown to have progression-free survival advantage for patients with IDH1 mutations and is now adopted as standard of care in several countries, also after prior treatment with chemotherapy.263 Similar to FGFR2, resistance emerges through drug-resistant subclones. The primary co-occurring alterations are mutations within PI3KCA and may offer rational options for combination therapy as ivosidenib is very well tolerated.
HER2 has been successfully targeted in cholangiocarcinoma,264 and we await randomised studies to establish optimal sequencing and biomarkers.
Although there has been significant progress, notable challenges remain. Some biliary tract cancers, notably pCCA, are unlikely to be able to receive targeted therapy because of no apparent actionable alterations at present, but additionally the difficulty of obtaining sufficient material for profiling. Additionally, more than 50% of biliary tract cancers do not have an actionable alteration for which targeting pathways, rather than point alterations, might represent a feasible treatment option.
Finally, a number of potentially actionable alterations occur at very low frequency—for instance. BRAFV600E and IDH2 mutations.265 266 It is essential, but challenging, to incorporate these patients in clinical studies in order to provide a practice informing outcome. Targeted therapies approved by NICE are shown in table 6. Potentially beneficial therapies not precluded should be considered in the context of local approval of compassionate use programmes and clinical trials.
Radiotherapy
Recommendation 42: Consider the use of adjuvant chemoradiotherapy for extrahepatic CCA or gallbladder cancer and a microscopically positive surgical margin resection (R1 resection) with a shared decision-making approach, considering the risk of potential harm and potential for benefit.
Strength of recommendation: MODERATE
Quality of evidence: MODERATE
Recommendation 43: Consider the delivery of SBRT or PBT in patients with locally advanced inoperable CCA who have received systemic therapy. Modern radiotherapy techniques should be employed to maximise radiotherapy dose and minimise toxicity.
Strength of recommendation: MODERATE
Quality of evidence: LOW
Recommendation 44a: Refer patients with symptomatic metastatic disease for consideration of palliative radiotherapy.
Strength of recommendation: MODERATE
Quality of evidence: MODERATE
Recommendation 44b: Refer for SBRT in the setting of oligometastatic disease.
Strength of recommendation: MODERATE
Quality of evidence: MODERATE
Technological advances, including intensity-modulated/volumetric arc therapy, SBRT, image guidance and the availability of PBT, have enabled the safe and effective use of radiotherapy in the treatment of primary liver cancers. There is growing interest in the use of radiotherapy for the treatment of CCA.
Neoadjuvant
Neoadjuvant radiotherapy is currently used before attempted resection of locally advanced disease or during multimodality therapy prior to liver transplantation. Two systematic reviews on the use of chemoradiotherapy prior to resection of pCCA were of limited quality with variable treatment protocols and limited the reporting of outcomes. Data, however, demonstrate that treatment can be delivered safely in patients with unresectable disease and, in some, facilitates complete resection.267 268
Several retrospective series report outcomes following neoadjuvant radiotherapy/SBRT prior to liver transplantation for pCCA. This approach is now a standard of care in numerous high-volume liver transplant centres worldwide. The largest reported multicentre series included 287 patients with unresectable disease who received neoadjuvant radiotherapy with concurrent and/or maintenance chemotherapy. Intention-to-treat 2- and 5-year overall survival rates were encouraging at 68% and 53%, respectively.220 One prospective study showed high rates of dropout and disease progression, highlighting the importance of careful patient selection.269 A recently published meta-analysis (of mostly retrospective studies) reporting outcomes following transplantation for unresectable pCCA supports a role for neoadjuvant chemoradiotherapy in potentially improving survival outcomes.224 The ongoing French phase III TRANSPHIL trial (NCT02232932) compares this strategy with standard surgical resection.
Adjuvant
Several meta-analyses have been published on the role of adjuvant radiotherapy. One included 21 retrospective studies of more than 1400 patients with eCCA and gallbladder cancer. The 5-year overall survival rate was higher with adjuvant radiotherapy than in the non-radiotherapy group (OR=0.63, p=0.0002), with particular benefit in patients with lymph node positive disease (OR=0.15, p<0.00001) and positive surgical margins (OR=0.40, p=0.02). Local recurrence rates were reduced in those receiving radiotherapy, but no difference was demonstrated in the rate of distant metastases.270
A recent meta-analysis of retrospective studies of a variety of adjuvant therapies for iCCA showed a statistically significant benefit for the use of adjuvant chemoradiotherapy (HR=0.73, 95% CI 0.57 to 0.89), but not radiotherapy alone (HR=0.71, 95% CI 0.39 to 1.03). The use of adjuvant therapy was particularly beneficial in the setting of positive resection margin or lymph node metastases.271
A phase II feasibility study of combination adjuvant chemotherapy and chemoradiotherapy in patients with pancreaticobiliary cancers (24% biliary tract cancers) reported tolerability in the adjuvant setting, although 14.5% discontinued study therapy and 15% experienced grade 3+ toxicities with one death.272 The SWOG S0809 phase II trial enrolled patients with resected eCCA (68%) and gallbladder cancer, who were treated with adjuvant gemcitabine and capecitabine chemotherapy followed by chemoradiotherapy with oral capecitabine. Modern radiotherapy techniques were used, and a comprehensive quality assurance process employed for all cases. The 2-year overall survival rates of 67% and 60%, in R0 and R1 resection patients, respectively, were significantly higher than those expected from historical controls. Acceptable toxicity rates, with 86% patients completing all planned treatment, demonstrate tolerability of the regimen.273
Definitive radiotherapy
Definitive chemoradiotherapy and SBRT have been used in the setting of locally advanced inoperable CCA. A systematic review of 11 mixed prospective and retrospective studies of SBRT in unresectable or recurrent CCA reported 1-year local control rates of 74.7–81.8% depending on radiotherapy dose, with benefit for higher dose. Median overall survival was 13.6 months. Most common toxicity was related to luminal gastrointestinal tissues, with a late incidence of ulceration from 10% to 20%.274 A further systematic review reported pooled 1-year local control and overall survival rates of 83.4% and 58.3%, respectively. The rate of gastroduodenal complications was variable, with studies including a range of disease location and SBRT dose/fractionations.275
The phase II Fédération Francophone de Cancérologie Digestive trial randomised patients with CCA and gallbladder cancer to gemcitabine oxaliplatin chemotherapy or radiotherapy delivered concurrently with cisplatin and 5-fluorouracil. No additional chemotherapy was delivered in the chemoradiotherapy arm other than the concurrent dosing. The trial closed early owing to slow accrual, and reported chemotherapy to be at least as effective as chemoradiotherapy.276
Hong et al reported a phase II multi-institutional study investigating the use of PBT in patients with unresectable iCCA and hepatocellular carcinoma. Multifocal disease and tumours with vascular invasion were included. Given the locally advanced nature of these cancers, the 2-year local control rate of 94.1% for the CCA cohort is encouraging, and treatment was delivered with low rates of grade 3+ toxicity.277
Dose delivered correlates with outcome for SBRT and conventionally fractionated radiotherapy. Despite 20% having metastatic disease, Tao et al reported 3-year overall survival and local control rates of 73% and 78%, respectively, for patients with iCCA who received higher radiotherapy doses. Treatment was well tolerated and almost all patients received chemotherapy before radiotherapy/chemoradiotherapy.278 Brunner et al reported improved survival and disease control rates with higher SBRT dose for both iCCA and eCCA, with <5% grade 3 toxicity.279 A systematic review of the impact of treatment on quality of life shows SBRT to be well tolerated.280 The addition of SBRT to systemic chemotherapy in locally advanced biliary tract cancers is being investigated in a randomised phase II trial ABC07 (ISRCTN10639376).
Palliative radiotherapy
Palliative radiotherapy can be considered, to manage symptoms such as pain or bleeding caused by metastatic disease.281 282 A meta-analysis of retrospective and small single centre randomised controlled trials assessing intraluminal brachytherapy compared with biliary stenting alone in the management of malignant obstructive jaundice reported improvements in risk of stent occlusion and mean survival, with comparable complication rate.283 Availability of expertise in the use of intraluminal brachytherapy limits it use.
Radiotherapy for oligometastatic/recurrence disease
The phase II SABR-COMET trial has shown an improvement in overall survival and no detriment in quality of life with the addition of SBRT to standard of care in patients with 1–5 sites of oligometastatic disease from a variety of primary malignancies.284–286 While phase III trial results are awaited to confirm the survival advantage, data from a prospective UK multicentre registry show that SBRT can be delivered safely with encouraging outcomes and has led to commissioning of SBRT by NHS England.287
Palliative care
Recommendation 45: All patients with incurable CCA should have access to a palliative care assessment to fully evaluate their holistic care needs. Evidence suggests that early palliative care is associated with higher health-related quality of life and lower rates of depression. Good symptom control should be delivered alongside active oncology management.
Strength of recommendation: MODERATE
Quality of evidence: MODERATE
Recommendation 46: Development and funding of clinical trials is key to fully evaluate the impact of pharmacological management of symptoms in patients with CCA and different models of care.
Strength of recommendation: MODERATE
Quality of evidence: LOW
Despite advances in management, CCA often present late with an overall poor prognosis. Treatments are often given with palliative intent.288 Given this, consideration of excellent symptom control alongside any active oncology management is paramount, while also considering the patient’s priorities and preferences for care. Early involvement of palliative care, either in hospital or in the community, to support management in parallel to any active multidisciplinary management plan should be considered, including focused discussions of the overall goals of care.
Early palliative care is associated with higher health-related quality of life and lower rates of depression compared with standard care in patients with advanced cancers.289 However, more evidence is needed relating specifically to gastrointestinal cancer subgroups, including biliary tract cancers, but the principle should be transferable and deemed as good practice.
Patients can have symptoms associated with local or systemic consequences of their disease, alongside treatment-related symptoms.290 Depending on the stage of the disease at presentation, fatigue, jaundice, pruritus, weight loss, nausea and anorexia may be present. Relief of biliary obstruction can palliate many of the associated symptoms, but not all. The degree of biliary obstruction also does not necessarily correlate with the severity of associated symptoms.
The pathophysiology of cholestatic itch is complex and no single treatment has been identified as definitive.291 It can, however, be a hugely disabling problem for patients with an impact on quality of life. Biliary stenting is an established treatment, with evidence to support the use of metal rather than plastic stents. There is variable evidence available to support the pharmacological management of cholestatic itch.292 Treatment aims of cholestatic itch are threefold. First, to remove pruritogens from the enterohepatic circulation (eg, cholestyramine or biliary drainage), second to alter the metabolism of pruritogens in the liver and/or gut (eg, rifampicin) and third, to modify central itch signally by influencing specific receptors in the central nervous system (eg, selective serotonin reuptake inhibitors, serotonin receptor antagonists and opioid antagonists).291 The evidence underpinning all options is limited, although increasing, so for any intervention the severity of the symptom must be clearly defined, and any pharmacological intervention carefully monitored, including benefits and side effects.
The key priority of management of symptoms in patients with CCA includes detailed history taking and identification of symptoms and their severity. Identification of likely aetiology, either disease or treatment related, including specific drug-related toxicities. Pharmacological and non-pharmacological approaches to the management of all symptoms should be considered, alongside appropriate psychosocial support. A multiprofessional approach to management of all patients with CCA, whatever the stage of presentation, is key.
Patient/public perspective
Recommendation 47: All patients diagnosed with CCA should have access to a hepatobiliary cancer nurse specialist who can provide expertise and support to the patient and their immediate family.
Strength of recommendation: STRONG
Quality of evidence: MODERATE
Recommendation 48: All patients diagnosed with CCA should have access to a dietician.
Strength of recommendation: STRONG
Quality of evidence: MODERATE
Recommendation 49: All patients diagnosed with CCA should have timely access to high-quality information and should be directed to a dedicated CCA patient charity so that they can access support and information.
Strength of recommendation: STRONG
Quality of evidence: LOW
Recommendation 50: All patients with CCA should be facilitated to access a second specialist clinical opinion if they need to seek reassurance about either their diagnosis or treatment.
Strength of recommendation: MODERATE
Quality of evidence: LOW
The purpose of this guideline is to endorse the best holistic care for patients with CCA. As discussed above, patients once diagnosed with CCA should expect that their case will be discussed at an HPB treatment centre and that the MDT is represented by a surgeon gastroenterologist/hepatologist with a special interest in HPB disease, oncologist, interventional radiologist, histopathologist and HPB clinical nurse specialist.
All patients with CCA should be under a specialist team with expertise in this disease. Furthermore, patients with CCA should have the opportunity to seek a second opinion from a named consultant who specialises in this disease, for confirmation of the diagnosis, additional advice and reassurance that the management suggested is the most appropriate for them.
The patient should expect that they will be contacted and supported along their cancer journey by an HPB specialist cancer nurse. All patients with CCA should be allocated a clinical nurse specialist, knowledgeable and experienced in the care of patients with CCA, who would be responsible for them throughout their treatment, and who they could approach during this time for advice and to answer any queries they might have.293 Once the course of treatment has been decided and agreed between the consultant and the patient, the patient should have time allocated with the clinical nurse specialist to go through all aspects of the treatment they will undergo, and to share contact details.
For patients who are potentially operable they should expect to (1) receive clear instructions of the proposed surgery, (2) understand the expected length of stay, (3) the unit’s morbidity and mortality for the proposed operation, (4) the expected date of their surgery, (5) what follow-up they will receive after their operation. Liver transplantation should be considered as a potential management option for qualifying cases, under new guidance.
Patients undergoing preoperative/pretreatment endoscopic procedures or interventional radiological procedures should receive high-quality information about the procedure and the associated risks.
All patients with CCA should have access to a Dietitian/Nutritionist, and this is especially important for resected patients. These patients should be able to contact a dietician/nutritionist experienced in caring for those who have had this type of surgery, so that they can be advised if they experience symptoms and difficulties with diet and digestion following surgery. Bile acid malabsorption and small intestinal bacterial overgrowth are both relatively common for resected patients with CCA, causing distressing symptoms, and should be considered by healthcare professionals in symptomatic patients.
After potentially curative treatment, given the high recurrence risk, patients should expect regular follow-up. The European Society of Medical Oncology clinical practice guidelines, for example, recommend 3-monthly visits at their specialist centre, and during the first 2 years after surgery patients should expect to receive a clinical examination, laboratory investigation and follow-up CT scans of the thorax, abdomen and pelvis. Regular visits can be extended to 6-monthly thereafter and prolonged to yearly visits after 5 years of follow-up.294
Systemic therapy, targeted therapy, molecular profiling and clinical trials
For patients offered systemic therapy, the implications should be fully discussed with their consultant and their clinical nurse specialist. Patients should be given realistic information about the procedure and what it might mean for their quality of life and for their life expectancy, and what side effects might occur. They should know what action to take if they have worrying side effects or symptoms that concern them, and be given contact numbers for advice and help.
With a growing number of clinical studies investigating first-line treatments for those with CCA, any that are available at the time of a patient’s diagnosis should be discussed with the patient before a decision on a first-line treatment is made. Patients should be fully informed about taking part in a clinical trial before making a decision to participate.
Whenever a patient with CCA is deemed inoperable and undergoes a systemic first-line treatment, they should be further assessed by an HPB MDT for operability, either at the mid-way scan or at the end of the first line treatment. They should also have molecular profiling of their tumour.
As described earlier in the guidelines, a significant proportion of CCA have an actionable molecular alteration (table 6). With the advent of treatments targeting certain of these alterations, there are a growing number of clinical trials investigating efficacy. Some trials have reported positively and a number of treatments have now been approved in the USA and in other countries. In 2022, for the first time NICE approved a therapy for those with CCA and an FGFR2 fusion who have previously undergone a first-line therapy. Until this point, molecular profiling was available to the few who were being investigated for eligibility for clinical trials. Importantly, this NICE decision made molecular profiling available to far more patients with CCA.
Stenting is an important area in the management of many patients with CCA. This procedure, what will be involved, why it is needed and what will happen afterwards, should be explained to the patient. Stenting should be carried out only by those endoscopists and interventional radiologists who are experienced in biliary stenting. Following a stenting procedure, patients should be given information on the possibility of biliary infection following a stenting procedure, what action to take if symptoms of cholangitis occur and contact numbers for advice and help.
Symptoms associated with CCA, such as pain, itching or jaundice, will have a detrimental effect on the quality of a patient’s life. All patients should have their symptoms dealt with promptly and appropriately.288 290
Patient perspectives and support groups
Patients with CCA are reported to have lower measured physical and psychological health-related quality of life scores than controls as well as anxiety, depression and social isolation. Patients and their loved ones should be encouraged to participate in support groups,295 and be made aware of appropriate support agencies, such as:
AMMF—The Cholangiocarcinoma Charity—is the main UK support group for people with CCA and their loved ones. The AMMF website provides CCA-specific, patient-friendly details, information on new developments and clinical trials, a discussion forum and links to private discussion groups for those with CCA, plus other tools and information important to patients (https://www.ammf.org.uk).
Macmillan Cancer Support can offer general help and advice to those with cancer, especially on where to find benefits and financial support (https://www.macmillan.org.uk/).
PSC Support UK is the only UK patient organisation dedicated to improving the lives of people affected by PSC. They provide patients and families with high-quality, accessible information and support, and collaborate with healthcare providers to improve clinical care (https://www.pscsupport.org.uk).
Climate change and sustainability
The impact of climate change on CCA is unknown, but fluctuations in carcinogenic toxins excreted via the hepatobiliary system, waterborne infections and parasitic infestations could potentially be affecting disease rates around the world.289 Further data are needed to confirm these inter-relationships.
As with many aspects of healthcare, the care of patients with CCA may contribute to adverse climactic and ecological effects. Given the widespread requirement of ERCP in the management of patients with CCA, we are supportive of the concept of a more sustainable future for endoscopy as has been promoted by the “Green Endoscopy Group”,290 driven by the observation that endoscopy is the third highest source of waste in a typical hospital.
We have recommended that the management of patients with CCA occurs at centres of expertise, but offering local care where possible without compromising the need for specialist input is important to reduce travelling and greenhouse gas emissions. Further examples of good environmental practice that should be considered include reducing unnecessary tests and in-person clinic visits, increased virtual consultations, reduction of waste (for example, in packaging of medications and devices) and advance care planning. We support the principles of the Intercollegiate Green Theatre Checklist.291
Priorities for service development and research in cholangiocarcinoma
This section summarises the authors’ views on the priorities for service development and research in CCA.
Epidemiology and aetiology
Monitor future trends with the latest ICD coding system (ICD-11), which includes separate codes for pCCA.
Investigate possible causes of geographical variation.
Uncover additional risk factors and drivers for sporadic CCA and focus on developing pan-UK biobanks for CCA research.
Pathology
Improve the cytological/tissue diagnosis of biliary strictures using ancillary methods—for example, gene sequencing.
Characterise the tumour microenvironment to improve patient selection for immunotherapy and other systemic treatments, including neoadjuvant therapy. Digital image analysis with multiplex immunohistochemistry and machine learning enables the assessment of immune cell populations, cell–cell interactions and checkpoint marker expression in a time-efficient, quantitative, reproducible manner.
Elucidate resistance mechanisms to molecular targeted therapy. A second mutation in the target gene, concomitant and acquired mutations in other genes, and activation of other pathways are potential mechanisms of resistance.
Imaging
Improve detection rates of CCA in PSC and assess benefit of imaging surveillance programmes.
Determine the most effective method of assessing the function of the future liver remnant to aid surgical planning.
Endoscopy
Develop and validate next-generation sequencing of bile in the diagnosis of CCA.
Examine the best drainage option for perihilar CCA undergoing both surgery and palliation.
Surgery
Optimal preoperative stenting, PTC versus ERCP, has been examined by the DRAINAGE trial, but is there a role for metal stents.
Neoadjuvant and adjuvant studies, especially the role of targeted therapies in the adjuvant setting.
Follow-up after resection, role of CT-DNA, long term quality of life metrics.
Optimum management of oligometastatic disease.
Establishment of liver transplantation for selected patients with CCA and monitoring of outcomes.
Systemic treatment
Access to molecular profiling.
Widen access to clinical trials.
Radiotherapy
Optimum patient selection for photon or particle radiotherapy.
Identify molecular signatures/biomarkers in order to develop optimal combinations and sequencing of radiotherapy and biological agents (eg, DNA damage response pathway, immune signature).
Identify a ‘low-metastatic potential’ tumour phenotype that would benefit from the use of ablative local/radiotherapy treatments.
Palliative care
Develop evidence to confirm the benefit of early palliative care intervention in patients with CCA.
Increase evidence to inform the best models of care for integration between oncology and palliative care services.
Increase the evidence underpinning the use of various pharmacological interventions to treat specific symptoms.
Footnotes
Contributors: These guidelines were commissioned and endorsed by the British Society of Gastroenterology. A guideline development group (GDG) was set up, chaired by SAK. Individual authors wrote separate sections of the guidelines according to their specialty. Then the final document was shared for all comments and approved by the GDG. The authors listed are the members of the GDG.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Not applicable.
References
- 1. Khan SA, Davidson BR, Goldin RD, et al. Guidelines for the diagnosis and treatment of cholangiocarcinoma: an update. Gut 2012;61:1657–69. 10.1136/gutjnl-2011-301748 [DOI] [PubMed] [Google Scholar]
- 2. Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924–6. 10.1136/bmj.39489.470347.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Balshem H, Helfand M, Schünemann HJ, et al. GRADE guidelines: 3. rating the quality of evidence. J Clin Epidemiol 2011;64:401–6. 10.1016/j.jclinepi.2010.07.015 [DOI] [PubMed] [Google Scholar]
- 4. Khan SA, Thomas HC, Davidson BR, et al. Cholangiocarcinoma. Lancet 2005;366:1303–14. 10.1016/S0140-6736(05)67530-7 [DOI] [PubMed] [Google Scholar]
- 5. Brindley PJ, Bachini M, Ilyas SI, et al. Cholangiocarcinoma. Nat Rev Dis Primers 2021;7:65. 10.1038/s41572-021-00300-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fabris L, Perugorria MJ, Mertens J, et al. The tumour microenvironment and immune milieu of cholangiocarcinoma. Liver Int 2019;39 Suppl 1:63–78. 10.1111/liv.14098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Banales JM, Marin JJG, Lamarca A, et al. Cholangiocarcinoma 2020: the next horizon in mechanisms and management. Nat Rev Gastroenterol Hepatol 2020;17:557–88. 10.1038/s41575-020-0310-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nakeeb A, Pitt HA, Sohn TA, et al. Cholangiocarcinoma. Ann Surg 1996;224:463–75. 10.1097/00000658-199610000-00005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Khan SA, Tavolari S, Brandi G. Cholangiocarcinoma: epidemiology and risk factors. Liver Int 2019;39 Suppl 1:19–31. 10.1111/liv.14095 [DOI] [PubMed] [Google Scholar]
- 10. Taylor-Robinson SD, Toledano MB, Arora S, et al. Increase in mortality rates from Intrahepatic cholangiocarcinoma in England and Wales 1968–1998. Gut 2001;48:816–20. 10.1136/gut.48.6.816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Khan SA, Taylor-Robinson SD, Toledano MB, et al. Changing International trends in mortality rates for liver, biliary and pancreatic tumours. J Hepatol 2002;37:806–13. 10.1016/s0168-8278(02)00297-0 [DOI] [PubMed] [Google Scholar]
- 12. Pericleous M, Khan SA. Epidemiology of HPB malignancy in the elderly. Eur J Surg Oncol 2021;47:503–13. 10.1016/j.ejso.2020.03.222 [DOI] [PubMed] [Google Scholar]
- 13. Vithayathil M, Khan SA. Current epidemiology of cholangiocarcinoma in Western countries. J Hepatol 2022;77:1690–8. 10.1016/j.jhep.2022.07.022 [DOI] [PubMed] [Google Scholar]
- 14. Tataru D, Khan SA, Hill R. Cholangiocarcinoma across England: a national study of temporal changes in incidence, survival and routes to diagnosis by region and socioeconomic deprivation [in press]. J Hep Reports 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Selvadurai S, Mann K, Mithra S, et al. Cholangiocarcinoma miscoding in hepatobiliary centres. Eur J Surg Oncol 2021;47:635–9. 10.1016/j.ejso.2020.09.039 [DOI] [PubMed] [Google Scholar]
- 16. Rizvi S, Khan SA, Hallemeier CL, et al. Cholangiocarcinoma-evolving concepts and therapeutic strategies. Nat Rev Clin Oncol 2018;15:95–111. 10.1038/nrclinonc.2017.157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Khan SA, Clements O, Kim JU, et al. “[reply to: 'letter regarding [risk factors for Intrahepatic and extrahepatic cholangiocarcinoma: a systematic review and meta-analysis]'” J Hepatol 2020;72:1217. 10.1016/j.jhep.2020.02.029 [DOI] [PubMed] [Google Scholar]
- 18. WHO . WHO Classification of Tumours, 5th Edition, Volume 1. Digestive System Tumours, Available: https://publications.iarc.fr/Book-And-Report-Series/Who-Classification-Of-Tumours/Digestive-System-Tumours-2019 [Accessed 14 Oct 2021].
- 19. Guglielmi A, Ruzzenente A, Campagnaro T, et al. Intrahepatic cholangiocarcinoma: prognostic factors after surgical resection. World J Surg 2009;33:1247–54. 10.1007/s00268-009-9970-0 [DOI] [PubMed] [Google Scholar]
- 20. Burt A, Alves V, Coulston A, et al. Intrahepatic cholangiocarcinoma, perihilar cholangiocarcinoma and hepatocellular carcinoma histopathology reporting guide. 2nd ed. Sydney, Australia: International Collaboration on Cancer Reporting, 2020. Available: https://www.iccr-cancer.org/datasets/published-datasets/digestive-tract/liver/ [DOI] [PubMed] [Google Scholar]
- 21. Zen Y, Quaglia A, Heaton N, et al. Two distinct pathways of carcinogenesis in primary sclerosing cholangitis. Histopathology 2011;59:1100–10. 10.1111/j.1365-2559.2011.04048.x [DOI] [PubMed] [Google Scholar]
- 22. Yeh Y-C, Lei H-J, Chen M-H, et al. C-reactive protein (CRP) is a promising diagnostic immunohistochemical marker for intrahepatic cholangiocarcinoma and is associated with better prognosis. Am J Surg Pathol 2017;41:1630–41. 10.1097/PAS.0000000000000957 [DOI] [PubMed] [Google Scholar]
- 23. Akita M, Sawada R, Komatsu M, et al. An immunostaining panel of C-reactive protein, N-Cadherin, and S100 calcium binding protein P is useful for Intrahepatic cholangiocarcinoma subtyping. Hum Pathol 2021;109:45–52. 10.1016/j.humpath.2020.12.005 [DOI] [PubMed] [Google Scholar]
- 24. Akita M, Fujikura K, Ajiki T, et al. Dichotomy in intrahepatic cholangiocarcinomas based on histologic similarities to hilar cholangiocarcinomas. Mod Pathol 2017;30:986–97. 10.1038/modpathol.2017.22 [DOI] [PubMed] [Google Scholar]
- 25. Hayashi A, Misumi K, Shibahara J, et al. Distinct clinicopathologic and genetic features of 2 histologic subtypes of intrahepatic cholangiocarcinoma. Am J Surg Pathol 2016;40:1021–30. 10.1097/PAS.0000000000000670 [DOI] [PubMed] [Google Scholar]
- 26. Nakamura H, Arai Y, Totoki Y, et al. Genomic spectra of biliary tract cancer. Nat Genet 2015;47:1003–10. 10.1038/ng.3375 [DOI] [PubMed] [Google Scholar]
- 27. Liau J-Y, Tsai J-H, Yuan R-H, et al. Morphological subclassification of intrahepatic cholangiocarcinoma: etiological, clinicopathological, and molecular features. Mod Pathol 2014;27:1163–73. 10.1038/modpathol.2013.241 [DOI] [PubMed] [Google Scholar]
- 28. Abou-Alfa GK, Sahai V, Hollebecque A, et al. Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: a multicentre, open-label, phase 2 study. Lancet Oncol 2020;21:671–84. 10.1016/S1470-2045(20)30109-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Goeppert B, Roessler S, Renner M, et al. Mismatch repair deficiency is a rare but putative therapeutically relevant finding in non-liver fluke associated cholangiocarcinoma. Br J Cancer 2019;120:109–14. 10.1038/s41416-018-0199-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ju JY, Dibbern ME, Mahadevan MS, et al. Mismatch repair protein deficiency/microsatellite instability is rare in cholangiocarcinomas and associated with distinctive morphologies. Am J Clin Pathol 2020;153:598–604. 10.1093/ajcp/aqz199 [DOI] [PubMed] [Google Scholar]
- 31. Suda R, Sakai N, Matsushita K, et al. Prediction of mismatch repair deficient biliary tract cancer: role of morphological features and host immune response detected by routine hematoxylin-eosin staining. J Hepatobiliary Pancreat Sci 2021;28:680–91. 10.1002/jhbp.988 [DOI] [PubMed] [Google Scholar]
- 32. Demols A, Rocq L, Charry M, et al. NTRK gene fusions in biliary tract cancers. J Clin Oncol 2020;38:574. 10.1200/JCO.2020.38.4_suppl.574 [DOI] [Google Scholar]
- 33. Brunt E, Aishima S, Clavien P, et al. cHCC-CCA: consensus terminology for primary liver carcinomas with both hepatocytic and cholangiocytic differentation. Hepatology 2018;68:113–26. 10.1002/hep.29789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Durnez A, Verslype C, Nevens F, et al. The clinicopathological and prognostic relevance of cytokeratin 7 and 19 expression in hepatocellular carcinoma. A possible progenitor cell origin. Histopathology 2006;49:138–51. 10.1111/j.1365-2559.2006.02468.x [DOI] [PubMed] [Google Scholar]
- 35. Akita M, Ajiki T, Fukumoto T, et al. Keratin 19-expressing hepatocellular carcinoma and small-duct type Intrahepatic cholangiocarcinoma show a similar postoperative clinical course but have distinct genetic features. Histopathology 2019;75:385–93. 10.1111/his.13884 [DOI] [PubMed] [Google Scholar]
- 36. Joseph NM, Tsokos CG, Umetsu SE, et al. Genomic profiling of combined hepatocellular-cholangiocarcinoma reveals similar genetics to hepatocellular carcinoma. J Pathol 2019;248:164–78. 10.1002/path.5243 [DOI] [PubMed] [Google Scholar]
- 37. Moeini A, Sia D, Zhang Z, et al. Mixed hepatocellular cholangiocarcinoma tumors: cholangiolocellular carcinoma is a distinct molecular entity. J Hepatol 2017;66:952–61. 10.1016/j.jhep.2017.01.010 [DOI] [PubMed] [Google Scholar]
- 38. Zen Y, Adsay NV, Bardadin K, et al. Biliary intraepithelial neoplasia: an international interobserver agreement study and proposal for diagnostic criteria. Mod Pathol 2007;20:701–9. 10.1038/modpathol.3800788 [DOI] [PubMed] [Google Scholar]
- 39. Nakanuma Y, Uesaka K, Kakuda Y, et al. Intraductal papillary neoplasm of bile duct: updated clinicopathological characteristics and molecular and genetic alterations. J Clin Med 2020;9:3991. 10.3390/jcm9123991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fujikura K, Fukumoto T, Ajiki T, et al. Comparative clinicopathological study of biliary intraductal papillary neoplasms and papillary cholangiocarcinomas. Histopathology 2016;69:950–61. 10.1111/his.13037 [DOI] [PubMed] [Google Scholar]
- 41. Komori T, Inoue D, Zen Y, et al. CT imaging comparison between Intraductal papillary neoplasms of the bile duct and papillary cholangiocarcinomas. Eur Radiol 2019;29:3132–40. 10.1007/s00330-018-5841-0 [DOI] [PubMed] [Google Scholar]
- 42. Akita M, Fujikura K, Ajiki T, et al. Intracholecystic papillary neoplasms are distinct from papillary gallbladder cancers. Am J Surg Pathol 2019;43:783–91. 10.1097/PAS.0000000000001237 [DOI] [PubMed] [Google Scholar]
- 43. Quigley B, Reid MD, Pehlivanoglu B, et al. Hepatobiliary mucinous cystic neoplasms with ovarian type stroma (so-called “hepatobiliary cystadenoma/cystadenocarcinoma. Am J Surg Pathol 2018;42:95–102. 10.1097/PAS.0000000000000963 [DOI] [PubMed] [Google Scholar]
- 44. Zen Y, Pedica F, Patcha VR, et al. Mucinous cystic neoplasms of the liver: a clinicopathological study and comparison with intraductal papillary neoplasms of the bile duct. Mod Pathol 2011;24:1079–89. 10.1038/modpathol.2011.71 [DOI] [PubMed] [Google Scholar]
- 45. Parsi MA, Deepinder F, Lopez R, et al. Factors affecting the yield of brush cytology for the diagnosis of pancreatic and biliary cancers. Pancreas 2011;40:52–4. 10.1097/MPA.0b013e3181f3aa96 [DOI] [PubMed] [Google Scholar]
- 46. Kobayashi M, Ryozawa S, Araki R, et al. Investigation of factors affecting the sensitivity of bile duct brush cytology. Intern Med 2019;58:329–35. 10.2169/internalmedicine.1551-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. de Moura DTH, Ryou M, de Moura EGH, et al. Endoscopic ultrasound-guided fine needle aspiration and endoscopic retrograde cholangiopancreatography-based tissue sampling in suspected malignant biliary strictures: a meta-analysis of same-session procedures. Clin Endosc 2020;53:417–28. 10.5946/ce.2019.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Han S, Tatman P, Mehrotra S, et al. Combination of ERCP-based modalities increases diagnostic yield for biliary strictures. Dig Dis Sci 2021;66:1276–84. 10.1007/s10620-020-06335-x [DOI] [PubMed] [Google Scholar]
- 49. Brooks C, Gausman V, Kokoy-Mondragon C, et al. Role of fluorescent in situ hybridization, cholangioscopic biopsies, and EUS-FNA in the evaluation of biliary strictures. Dig Dis Sci 2018;63:636–44. 10.1007/s10620-018-4906-x [DOI] [PubMed] [Google Scholar]
- 50. Navaneethan U, Njei B, Venkatesh PGK, et al. Fluorescence in situ hybridization for diagnosis of cholangiocarcinoma in primary sclerosing cholangitis: a systematic review and meta-analysis. Gastrointest Endosc 2014;79:943–50. 10.1016/j.gie.2013.11.001 [DOI] [PubMed] [Google Scholar]
- 51. Singhi AD, Nikiforova MN, Chennat J, et al. Integrating next-generation sequencing to endoscopic retrograde cholangiopancreatography (ERCP)-obtained biliary specimens improves the detection and management of patients with malignant bile duct strictures. Gut 2020;69:52–61. 10.1136/gutjnl-2018-317817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Singh A, Gelrud A, Agarwal B. Biliary strictures: diagnostic considerations and approach. Gastroenterol Rep (Oxf) 2015;3:22–31. 10.1093/gastro/gou072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Uson Junior PL, Kunze KL, Golafshar MA, et al. Germline cancer susceptibility gene testing in unselected patients with hepatobiliary cancers: a multi-center prospective study. Cancer Prev Res (Phila) 2022;15:121–8. 10.1158/1940-6207.CAPR-21-0189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ruys AT, van Beem BE, Engelbrecht MRW, et al. Radiological staging in patients with hilar cholangiocarcinoma: a systematic review and meta-analysis. Br J Radiol 2012;85:1255–62. 10.1259/bjr/88405305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Fábrega-Foster K, Ghasabeh MA, Pawlik TM, et al. Multimodality imaging of intrahepatic cholangiocarcinoma. Hepatobiliary Surg Nutr 2017;6:67–78. 10.21037/hbsn.2016.12.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ni Q, Wang H, Zhang Y, et al. MDCT assessment of resectability in hilar cholangiocarcinoma. Abdom Radiol (NY) 2017;42:851–60. 10.1007/s00261-016-0943-0 [DOI] [PubMed] [Google Scholar]
- 57. Joo I, Lee JM, Yoon JH. Imaging diagnosis of intrahepatic and perihilar cholangiocarcinoma: recent advances and challenges. Radiology 2018;288:7–13. 10.1148/radiol.2018171187 [DOI] [PubMed] [Google Scholar]
- 58. Kuszyk BS, Soyer P, Bluemke DA, et al. Intrahepatic cholangiocarcinoma: the role of imaging in detection and staging. Crit Rev Diagn Imaging 1997;38:59–88. [PubMed] [Google Scholar]
- 59. Charatcharoenwitthaya P, Enders FB, Halling KC, et al. Utility of serum tumor markers, imaging, and biliary cytology for detecting cholangiocarcinoma in primary sclerosing cholangitis. Hepatology 2008;48:1106–17. 10.1002/hep.22441 [DOI] [PubMed] [Google Scholar]
- 60. Ariff B, Lloyd CR, Khan S, et al. Imaging of liver cancer. World J Gastroenterol 2009;15:1289–300. 10.3748/wjg.15.1289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Aloia TA, Charnsangavej C, Faria S, et al. High-resolution computed tomography accurately predicts resectability in hilar cholangiocarcinoma. Am J Surg 2007;193:702–6. 10.1016/j.amjsurg.2006.10.024 [DOI] [PubMed] [Google Scholar]
- 62. Joo I, Lee JM. Imaging bile duct tumors: pathologic concepts, classification, and early tumor detection. Abdom Imaging 2013;38:1334–50. 10.1007/s00261-013-0027-3 [DOI] [PubMed] [Google Scholar]
- 63. Lamarca A, Barriuso J, Chander A, et al. 18F-Fluorodeoxyglucose positron emission tomography (18FDG-PET) for patients with biliary tract cancer: systematic review and meta-analysis. J Hepatol 2019;71:115–29. 10.1016/j.jhep.2019.01.038 [DOI] [PubMed] [Google Scholar]
- 64. Seo H, Lee JM, Kim IH, et al. Evaluation of the gross type and longitudinal extent of extrahepatic cholangiocarcinomas on contrast-enhanced multidetector row computed tomography. J Comput Assist Tomogr 2009;33:376–82. 10.1097/RCT.0b013e318184f3f7 [DOI] [PubMed] [Google Scholar]
- 65. Franken LC, Coelen RJS, Erdmann JI, et al. Multidetector computed tomography assessment of vascular involvement in perihilar cholangiocarcinoma. Quant Imaging Med Surg 2021;11:4514–21. 10.21037/qims-20-1303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Lee HY, Kim SH, Lee JM, et al. Preoperative assessment of resectability of hepatic hilar cholangiocarcinoma: combined CT and cholangiography with revised criteria. Radiology 2006;239:113–21. 10.1148/radiol.2383050419 [DOI] [PubMed] [Google Scholar]
- 67. Huang X, Yang J, Li J, et al. Comparison of magnetic resonance imaging and 18-fluorodeoxyglucose positron emission tomography/computed tomography in the diagnostic accuracy of staging in patients with cholangiocarcinoma. Medicine (Baltimore) 2020;99:e20932. 10.1097/MD.0000000000020932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kim Y-Y, Yeom S-K, Shin H, et al. Clinical staging of mass‐forming intrahepatic cholangiocarcinoma: computed tomography versus magnetic resonance imaging. Hepatol Commun 2021;5:2009–18. 10.1002/hep4.1774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Lee KH, Lee JM, Park JH, et al. MR imaging in patients with suspected liver metastases: value of liver-specific contrast agent gadoxetic acid. Korean J Radiol 2013;14:894–904. 10.3348/kjr.2013.14.6.894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Shimada K, Isoda H, Hirokawa Y, et al. Comparison of gadolinium-EOB-DTPA-enhanced and diffusion-weighted liver MRI for detection of small hepatic metastases. Eur Radiol 2010;20:2690–8. 10.1007/s00330-010-1842-3 [DOI] [PubMed] [Google Scholar]
- 71. Koh J, Chung YE, Nahm JH, et al. Intrahepatic mass-forming cholangiocarcinoma: prognostic value of preoperative gadoxetic acid-enhanced MRI. Eur Radiol 2016;26:407–16. 10.1007/s00330-015-3846-5 [DOI] [PubMed] [Google Scholar]
- 72. Cui X-Y, Chen H-W, Cai S, et al. Diffusion-weighted MR imaging for detection of extrahepatic cholangiocarcinoma. Eur J Radiol 2012;81:2961–5. 10.1016/j.ejrad.2011.12.040 [DOI] [PubMed] [Google Scholar]
- 73. Kaya B, Koc Z. Diffusion-weighted MRI and optimal B-value for characterization of liver lesions. Acta Radiol 2014;55:532–42. 10.1177/0284185113502017 [DOI] [PubMed] [Google Scholar]
- 74. Koc Z, Erbay G. Optimal B value in diffusion-weighted imaging for differentiation of abdominal lesions. J Magn Reson Imaging 2014;40:559–66. 10.1002/jmri.24403 [DOI] [PubMed] [Google Scholar]
- 75. Ryoo I, Lee JM, Chung YE, et al. Gadobutrol-enhanced, three-dimensional, dynamic MR imaging with MR cholangiography for the preoperative evaluation of bile duct cancer. Invest Radiol 2010;45:217–24. 10.1097/RLI.0b013e3181d2eeb1 [DOI] [PubMed] [Google Scholar]
- 76. Sun HY, Lee JM, Park HS, et al. Gadoxetic acid-enhanced MRI with MR cholangiography for the preoperative evaluation of bile duct cancer. J Magn Reson Imaging 2013;38:138–47. 10.1002/jmri.23957 [DOI] [PubMed] [Google Scholar]
- 77. Park HS, Lee JM, Choi J-Y, et al. Preoperative evaluation of bile duct cancer: MRI combined with MR cholangiopancreatography versus MDCT with direct cholangiography. AJR Am J Roentgenol 2008;190:396–405. 10.2214/AJR.07.2310 [DOI] [PubMed] [Google Scholar]
- 78. Francica G, Meloni MF, de Sio I, et al. Biopsy of liver target lesions under contrast-enhanced ultrasound guidance – a multi-center study. Ultraschall Med 2018;39:448–53. 10.1055/s-0043-122496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Bridgewater J, Webster GJ, Amin Z. How should we drain malignant obstruction at the liver hilum? Lancet Gastroenterol Hepatol 2018;3:657–8. 10.1016/S2468-1253(18)30267-X [DOI] [PubMed] [Google Scholar]
- 80. Ribero D, Zimmitti G, Aloia TA, et al. Preoperative cholangitis and future liver remnant volume determine the risk of liver failure in patients undergoing resection for hilar cholangiocarcinoma. J Am Coll Surg 2016;223:87–97. 10.1016/j.jamcollsurg.2016.01.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Lodewick TM, Arnoldussen C, Lahaye MJ, et al. Fast and accurate liver volumetry prior to hepatectomy. HPB (Oxford) 2016;18:764–72. 10.1016/j.hpb.2016.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Bi X-J, Zhang X-Q, Zhang T, et al. Quantitative assessment of liver function with hepatocyte fraction: comparison with T1 relaxation-based indices. Eur J Radiol 2021;141:109779. 10.1016/j.ejrad.2021.109779 [DOI] [PubMed] [Google Scholar]
- 83. Tapper EB, Loomba R. Noninvasive imaging biomarker assessment of liver fibrosis by elastography in NAFLD. Nat Rev Gastroenterol Hepatol 2018;15:274–82. 10.1038/nrgastro.2018.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. American College of Radiology . CT/MRI LI-RADS® V2018 core. 2018. Available: https://www.acr.org/-/media/ACR/Files/RADS/LI-RADS/LI-RADS-2018-Core.pdf?la=en [Accessed 27 2022].
- 85. Brandi G, Venturi M, Pantaleo MA, et al. Cholangiocarcinoma: current opinion on clinical practice diagnostic and therapeutic Algorithms. Digestive and Liver Disease 2016;48:231–41. 10.1016/j.dld.2015.11.017 [DOI] [PubMed] [Google Scholar]
- 86. Jo JH, Cho CM, Jun JH, et al. Same‐session endoscopic ultrasound‐guided fine needle aspiration and endoscopic retrograde cholangiopancreatography‐based tissue sampling in suspected malignant biliary obstruction: a multicenter experience. J Gastroenterol Hepatol 2019;34:799–805. 10.1111/jgh.14528 [DOI] [PubMed] [Google Scholar]
- 87. Chen Y-I, Chatterjee A, Berger R, et al. Endoscopic ultrasound (EUS)-guided fine needle biopsy alone vs. EUS-guided fine needle aspiration with rapid onsite evaluation in pancreatic lesions: a multicenter randomized trial. Endoscopy 2022;54:4–12. 10.1055/a-1375-9775 [DOI] [PubMed] [Google Scholar]
- 88. Novikov A, Kowalski TE, Loren DE. Practical management of indeterminate biliary strictures. Gastrointest Endosc Clin N Am 2019;29:205–14. 10.1016/j.giec.2018.12.003 [DOI] [PubMed] [Google Scholar]
- 89. Bailey AA, Bourke MJ, Williams SJ, et al. A prospective randomized trial of cannulation technique in ERCP: effects on technical success and post-ERCP pancreatitis. Endoscopy 2008;40:296–301. 10.1055/s-2007-995566 [DOI] [PubMed] [Google Scholar]
- 90. Pitman MarthaB, Centeno BarbaraA, Genevay M, et al. Standardized terminology and nomenclature for pancreatobiliary cytology: the Papanicolaou Society of Cytopathology guidelines. CytoJournal 2014;11:15. 10.4103/1742-6413.133343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Yoon SB, Moon S-H, Ko SW, et al. Brush cytology, forceps biopsy, or endoscopic ultrasound-guided sampling for diagnosis of bile duct cancer: a meta-analysis. Dig Dis Sci 2022;67:3284–97. 10.1007/s10620-021-07138-4 [DOI] [PubMed] [Google Scholar]
- 92. Wang J, Xia M, Jin Y, et al. More endoscopy-based brushing passes improve the detection of malignant biliary strictures: a multicenter randomized controlled trial. Am J Gastroenterol 2022;117:733–9. 10.14309/ajg.0000000000001666 [DOI] [PubMed] [Google Scholar]
- 93. Zhai J. Urovysion multi-target fluorescence in situ hybridization assay for the detection of malignant bile duct brushing specimens: a comparison with routine cytology. Acta Cytol 2018;62:295–301. 10.1159/000488636 [DOI] [PubMed] [Google Scholar]
- 94. Naitoh I, Nakazawa T, Kato A, et al. Predictive factors for positive diagnosis of malignant biliary strictures by transpapillary brush cytology and forceps biopsy. J Dig Dis 2016;17:44–51. 10.1111/1751-2980.12311 [DOI] [PubMed] [Google Scholar]
- 95. Dimas ID, Fragaki M, Vardas E, et al. Digital cholangioscopy (Spyglass) in the diagnosis of cholangiocarcinoma. Ann Gastroenterol 2017;30:253. 10.20524/aog.2016.0110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Sun X, Zhou Z, Tian J, et al. Is single-operator peroral cholangioscopy a useful tool for the diagnosis of indeterminate biliary lesion? A systematic review and meta-analysis. Gastrointest Endosc 2015;82:79–87. 10.1016/j.gie.2014.12.021 [DOI] [PubMed] [Google Scholar]
- 97. Navaneethan U, Hasan MK, Lourdusamy V, et al. Single-operator cholangioscopy and targeted biopsies in the diagnosis of indeterminate biliary strictures: a systematic review. Gastrointest Endosc 2015;82:608–14. 10.1016/j.gie.2015.04.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Heimbach JK, Sanchez W, Rosen CB, et al. Trans-peritoneal fine needle aspiration biopsy of hilar cholangiocarcinoma is associated with disease dissemination. HPB (Oxford) 2011;13:356–60. 10.1111/j.1477-2574.2011.00298.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Onoyama T, Matsumoto K, Takeda Y, et al. Endoscopic ultrasonography-guided fine needle aspiration for extrahepatic cholangiocarcinoma: a safe tissue sampling modality. J Clin Med 2019;8:417. 10.3390/jcm8040417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Meining A, Chen YK, Pleskow D, et al. Direct visualization of indeterminate pancreaticobiliary strictures with probe-based confocal laser endomicroscopy: a multicenter experience. Gastrointest Endosc 2011;74:961–8. 10.1016/j.gie.2011.05.009 [DOI] [PubMed] [Google Scholar]
- 101. Caillol F, Filoche B, Gaidhane M, et al. Refined probe-based confocal laser endomicroscopy classification for biliary strictures: the Paris classification. Dig Dis Sci 2013;58:1784–9. 10.1007/s10620-012-2533-5 [DOI] [PubMed] [Google Scholar]
- 102. Arechederra M, Rullán M, Amat I, et al. Next-generation sequencing of bile cell-free DNA for the early detection of patients with malignant biliary strictures. Gut 2022;71:1141–51. 10.1136/gutjnl-2021-325178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. van der Gaag NA, Rauws EAJ, van Eijck CHJ, et al. Preoperative biliary drainage for cancer of the head of the pancreas. N Engl J Med 2010;362:129–37. 10.1056/NEJMoa0903230 [DOI] [PubMed] [Google Scholar]
- 104. Fugazza A, Troncone E, Amato A, et al. Difficult biliary cannulation in patients with distal malignant biliary obstruction: an underestimated problem? Dig Liver Dis 2022;54:529–36. 10.1016/j.dld.2021.07.010 [DOI] [PubMed] [Google Scholar]
- 105. NICE (National Institute for Health and Care Excellence) . Endoscopic bipolar radiofrequency ablation for treating biliary obstruction caused by cancer Interventional procedures guidance [IPG614], Available: https://www.nice.org.uk/guidance/IPG614/chapter/1-Recommendations [Accessed 11 Feb 2023].
- 106. Dumonceau J-M, Tringali A, Papanikolaou IS, et al. Endoscopic biliary stenting: indications, choice of stents, and results: European Society of Gastrointestinal Endoscopy (ESGE) clinical guideline – updated October 2017. Endoscopy 2018;50:910–30. 10.1055/a-0659-9864 [DOI] [PubMed] [Google Scholar]
- 107. Pu LZCT. Endoscopic stenting for inoperable malignant biliary obstruction: a systematic review and meta-analysis. World J Gastroenterol 2015;21:13374. 10.3748/wjg.v21.i47.13374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Sawas T, Al Halabi S, Parsi MA, et al. Self-expandable metal stents versus plastic stents for malignant biliary obstruction: a meta-analysis. Gastrointest Endosc 2015;82:256–67. 10.1016/j.gie.2015.03.1980 [DOI] [PubMed] [Google Scholar]
- 109. Li J, Li T, Sun P, et al. Covered versus uncovered self-expandable metal stents for managing malignant distal biliary obstruction: a meta-analysis. PLoS ONE 2016;11:e0149066. 10.1371/journal.pone.0149066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Fugazza A, Fabbri C, Di Mitri R, et al. EUS-guided choledochoduodenostomy for malignant distal biliary obstruction after failed ERCP: a retrospective nationwide analysis. Gastrointest Endosc 2022;95:896–904. 10.1016/j.gie.2021.12.032 [DOI] [PubMed] [Google Scholar]
- 111. Banales JM, Cardinale V, Carpino G, et al. Cholangiocarcinoma: current knowledge and future perspectives consensus statement from the European Network for the Study of Cholangiocarcinoma (ENS-CCA). Nat Rev Gastroenterol Hepatol 2016;13:261–80. 10.1038/nrgastro.2016.51 [DOI] [PubMed] [Google Scholar]
- 112. Rauws EAJ, Kloek JJ, Gouma DJ, et al. Staging of cholangiocarcinoma: the role of endoscopy. HPB (Oxford) 2008;10:110–2. 10.1080/13651820801992591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Elmunzer BJ, Smith ZL, Tarnasky P, et al. An unsuccessful randomized trial of percutaneous vs endoscopic drainage of suspected malignant hilar obstruction. Clin Gastroenterol Hepatol 2021;19:1282–4. 10.1016/j.cgh.2020.05.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Moy BT, Birk JW. An update to hepatobiliary stents. J Clin Transl Hepatol 2015;3:67–77. 10.14218/JCTH.2015.00040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Aghaie Meybodi M, Shakoor D, Nanavati J, et al. Unilateral versus bilateral endoscopic stenting in patients with unresectable malignant hilar obstruction: a systematic review and meta-analysis. Endosc Int Open 2020;8:E281–90. 10.1055/a-1067-4326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Ba Y, Yue P, Leung JW, et al. Percutaneous transhepatic biliary drainage may be the preferred preoperative drainage method in hilar cholangiocarcinoma. Endosc Int Open 2020;8:E203–10. 10.1055/a-0990-9114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Larghi A, Rimbaș M, Tringali A, et al. Endoscopic radiofrequency biliary ablation treatment: a comprehensive review. Dig Endosc 2019;31:245–55. 10.1111/den.13298 [DOI] [PubMed] [Google Scholar]
- 118. So H, Oh CH, Song TJ, et al. Feasibility and safety of endoluminal radiofrequency ablation as a rescue treatment for bilateral metal stent obstruction due to tumor ingrowth in the hilum: a pilot study. J Clin Med 2021;10:952. 10.3390/jcm10050952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Kahaleh M, Mishra R, Shami VM, et al. Unresectable cholangiocarcinoma: comparison of survival in biliary stenting alone versus stenting with photodynamic therapy. Clin Gastroenterol Hepatol 2008;6:290–7. 10.1016/j.cgh.2007.12.004 [DOI] [PubMed] [Google Scholar]
- 120. Jagielski M, Zieliński M, Piątkowski J, et al. Outcomes and limitations of endoscopic ultrasound-guided hepaticogastrostomy in malignant biliary obstruction. BMC Gastroenterol 2021;21:202. 10.1186/s12876-021-01798-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Ma KW, So H, Cho DH, et al. Durability and outcome of endoscopic ultrasound‐guided hepaticoduodenostomy using a fully covered metal stent for segregated right intrahepatic duct dilatation. J Gastroenterol Hepatol 2020;35:1753–60. 10.1111/jgh.15089 [DOI] [PubMed] [Google Scholar]
- 122. Olthof PB, Miyasaka M, Koerkamp BG, et al. A comparison of treatment and outcomes of perihilar cholangiocarcinoma between Eastern and Western centers. HPB (Oxford) 2019;21:345–51. 10.1016/j.hpb.2018.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. AUGIS . AUGIS: provision of services. 2016. Available: https://www.augis.org/wp-content/uploads/2016/06/Provision-of-Services-June-2016.pdf [Accessed 28 Dec 2022].
- 124. DeOliveira ML, Cunningham SC, Cameron JL, et al. Cholangiocarcinoma: thirty-one-year experience with 564 patients at a single institution. Ann Surg 2007;245:755–62. 10.1097/01.sla.0000251366.62632.d3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Esposito I, Schirmacher P. Pathological aspects of cholangiocarcinoma. HPB (Oxford) 2008;10:83–6. 10.1080/13651820801992609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Cho CS. Prognostication systems as applied to primary and metastatic hepatic malignancies. Surg Oncol Clin N Am 2015;24:41–56. 10.1016/j.soc.2014.09.010 [DOI] [PubMed] [Google Scholar]
- 127. Lee AJ, Chun YS. Intrahepatic cholangiocarcinoma: the AJCC/UICC 8th edition updates. Chin Clin Oncol 2018;7:52. 10.21037/cco.2018.07.03 [DOI] [PubMed] [Google Scholar]
- 128. Ali SM, Clark CJ, Mounajjed T, et al. Model to predict survival after surgical resection of Intrahepatic cholangiocarcinoma: the Mayo Clinic experience. HPB (Oxford) 2015;17:244–50. 10.1111/hpb.12333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Doussot A, Gonen M, Wiggers JK, et al. Recurrence patterns and disease-free survival after resection of intrahepatic cholangiocarcinoma: preoperative and postoperative prognostic models. J Am Coll Surg 2016;223:493–505. 10.1016/j.jamcollsurg.2016.05.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Spolverato G, Ejaz A, Kim Y, et al. Tumor size predicts vascular invasion and histologic grade among patients undergoing resection of intrahepatic cholangiocarcinoma. J Gastrointest Surg 2014;18:1284–91. 10.1007/s11605-014-2533-1 [DOI] [PubMed] [Google Scholar]
- 131. Bismuth H, Corlette MB. Intrahepatic cholangioenteric anastomosis in carcinoma of the hilus of the liver. Surg Gynecol Obstet 1975;140:170–8. [PubMed] [Google Scholar]
- 132. Vilgrain V. Staging cholangiocarcinoma by imaging studies. HPB (Oxford) 2008;10:106–9. 10.1080/13651820801992617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Bismuth H, Nakache R, Diamond T. Management strategies in resection for hilar cholangiocarcinoma. Ann Surg 1992;215:31–8. 10.1097/00000658-199201000-00005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Paul A, Kaiser GM, Molmenti EP, et al. Klatskin tumors and the accuracy of the Bismuth-Corlette classification. Am Surg 2011;77:1695–9. 10.1177/000313481107701246 [DOI] [PubMed] [Google Scholar]
- 135. Ruys AT, Busch OR, Rauws EA, et al. Prognostic impact of preoperative imaging parameters on resectability of hilar cholangiocarcinoma. HPB Surg 2013;2013:657309. 10.1155/2013/657309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Jarnagin WR, Fong Y, DeMatteo RP, et al. Staging, resectability, and outcome in 225 patients with hilar cholangiocarcinoma. Ann Surg 2001;234:507–17; 10.1097/00000658-200110000-00010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Matsuo K, Rocha FG, Ito K, et al. The Blumgart preoperative staging system for hilar cholangiocarcinoma: analysis of resectability and outcomes in 380 patients. J Am Coll Surg 2012;215:343–55. 10.1016/j.jamcollsurg.2012.05.025 [DOI] [PubMed] [Google Scholar]
- 138. Rocha FG, Matsuo K, Blumgart LH, et al. Hilar cholangiocarcinoma: the Memorial Sloan‐Kettering Cancer Center experience. J Hepatobiliary Pancreat Sci 2010;17:490–6. 10.1007/s00534-009-0205-4 [DOI] [PubMed] [Google Scholar]
- 139. Lidsky ME, Jarnagin WR. Surgical management of hilar cholangiocarcinoma at Memorial Sloan Kettering Cancer Center. Ann Gastroenterol Surg 2018;2:304–12. 10.1002/ags3.12181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Zaydfudim VM, Clark CJ, Kendrick ML, et al. Correlation of staging systems to survival in patients with resected hilar cholangiocarcinoma. Am J Surg 2013;206:159–65. 10.1016/j.amjsurg.2012.11.020 [DOI] [PubMed] [Google Scholar]
- 141. Ding G, Yang Y, Cao L, et al. A modified Jarnagin-Blumgart classification better predicts survival for resectable hilar cholangiocarcinoma. World J Surg Oncol 2015;13:99. 10.1186/s12957-015-0526-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Rassam F, Roos E, van Lienden KP, et al. Modern work-up and extended resection in perihilar cholangiocarcinoma: the AMC experience. Langenbecks Arch Surg 2018;403:289–307. 10.1007/s00423-018-1649-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Coelen RJS, Ruys AT, Besselink MGH, et al. Diagnostic accuracy of staging laparoscopy for detecting metastasized or locally advanced perihilar cholangiocarcinoma: a systematic review and meta-analysis. Surg Endosc 2016;30:4163–73. 10.1007/s00464-016-4788-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Ruys AT, Busch OR, Gouma DJ, et al. Staging laparoscopy for hilar cholangiocarcinoma: is it still worthwhile Ann Surg Oncol 2011;18:2647–53. 10.1245/s10434-011-1576-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Barlow AD, Garcea G, Berry DP, et al. Staging laparoscopy for hilar cholangiocarcinoma in 100 patients. Langenbecks Arch Surg 2013;398:983–8. 10.1007/s00423-013-1104-3 [DOI] [PubMed] [Google Scholar]
- 146. Corvera CU, Weber SM, Jarnagin WR. Role of laparoscopy in the evaluation of biliary tract cancer. Surg Oncol Clin N Am 2002;11:877–91. 10.1016/s1055-3207(02)00033-9 [DOI] [PubMed] [Google Scholar]
- 147. D’Angelica M, Fong Y, Weber S, et al. The role of staging laparoscopy in hepatobiliary malignancy: prospective analysis of 401 cases. Ann Surg Oncol 2003;10:183–9. 10.1245/aso.2003.03.091 [DOI] [PubMed] [Google Scholar]
- 148. Davidson JT, Jin LX, Krasnick B, et al. Staging laparoscopy among three subtypes of extra-hepatic biliary malignancy: a 15-year experience from 10 institutions. J Surg Oncol 2019;119:288–94. 10.1002/jso.25323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Weber SM, DeMatteo RP, Fong Y, et al. Staging laparoscopy in patients with extrahepatic biliary carcinoma. Annals of Surgery 2002;235:392–9. 10.1097/00000658-200203000-00011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Rotellar F, Pardo F. Laparoscopic staging in hilar cholangiocarcinoma: is it still justified. World J Gastroenterol 2013;5:127–31. 10.4251/wjgo.v5.i7.127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Tilleman E, Castro SMM, Busch ORC. Diagnostic laparoscopy and laparoscopic ultrasound for staging of patients with malignant proximal bile duct obstruction. J Gastrointest Surg 2002;6:426–31. 10.1016/S1091-255X(02)00005-7 [DOI] [PubMed] [Google Scholar]
- 152. Weber SM, Ribero D, O’Reilly EM, et al. Intrahepatic cholangiocarcinoma: expert consensus statement. HPB (Oxford) 2015;17:669–80. 10.1111/hpb.12441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Ribero D, Amisano M, Bertuzzo F, et al. Measured versus estimated total liver volume to preoperatively assess the adequacy of the future liver remnant: which method should we use? Ann Surg 2013;258:801–6. 10.1097/SLA.0000000000000213 [DOI] [PubMed] [Google Scholar]
- 154. Kishi Y, Abdalla EK, Chun YS, et al. Three hundred and one consecutive extended right hepatectomies: evaluation of outcome based on systematic liver volumetry. Ann Surg 2009;250:540–8. 10.1097/SLA.0b013e3181b674df [DOI] [PubMed] [Google Scholar]
- 155. Dixon E, Abdalla E, Schwarz RE, et al. AHPBA/SSO/SSAT sponsored consensus conference on multidisciplinary treatment of hepatocellular carcinoma. HPB (Oxford) 2010;12:287–8. 10.1111/j.1477-2574.2010.00184.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. de Meijer VE, Kalish BT, Puder M, et al. Systematic review and meta-analysis of steatosis as a risk factor in major hepatic resection. Br J Surg 2010;97:1331–9. 10.1002/bjs.7194 [DOI] [PubMed] [Google Scholar]
- 157. Madoff DC, Abdalla EK, Vauthey JN. Portal vein embolization in preparation for major hepatic resection: evolution of a new standard of care. J Vasc Interv Radiol 2005;16:779–90. 10.1097/01.RVI.0000159543.28222.73 [DOI] [PubMed] [Google Scholar]
- 158. Pandanaboyana S, Bell R, Hidalgo E, et al. A systematic review and meta-analysis of portal vein ligation versus portal vein embolization for elective liver resection. Surgery 2015;157:690–8. 10.1016/j.surg.2014.12.009 [DOI] [PubMed] [Google Scholar]
- 159. Li J, Ewald F, Gulati A, et al. Associating liver partition and portal vein ligation for staged hepatectomy: from technical evolution to oncological benefit. World J Gastrointest Surg 2016;8:124–33. 10.4240/wjgs.v8.i2.124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Li D, Madoff DC. Portal vein embolization for induction of selective hepatic hypertrophy prior to major hepatectomy: rationale, techniques, outcomes and future directions. Cancer Biol Med 2016;13:426–42. 10.20892/j.issn.2095-3941.2016.0083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Schnitzbauer AA, Lang SA, Goessmann H, et al. Right portal vein ligation combined with in situ splitting induces rapid left lateral liver lobe hypertrophy enabling 2-staged extended right hepatic resection in small-for-size settings. Ann Surg 2012;255:405–14. 10.1097/SLA.0b013e31824856f5 [DOI] [PubMed] [Google Scholar]
- 162. Cai Y-L, Song P-P, Tang W, et al. An updated systematic review of the evolution of ALPPS and evaluation of its advantages and disadvantages in accordance with current evidence. Medicine (Baltimore) 2016;95:e3941. 10.1097/MD.0000000000003941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Moris D, Ronnekleiv-Kelly S, Kostakis ID, et al. Operative results and oncologic outcomes of associating liver partition and portal vein ligation for staged hepatectomy (ALPPS) versus two-stage hepatectomy (TSH) in patients with unresectable colorectal liver metastases: a systematic review and meta-analysis. World J Surg 2018;42:806–15. 10.1007/s00268-017-4181-6 [DOI] [PubMed] [Google Scholar]
- 164. Ferko A, Vojtko M, Adámik M, et al. Totally laparoscopic ALPPS: bilobar procedure with preservation of the S3 portobiliary triad. Ann Surg Oncol 2019;26:291. 10.1245/s10434-018-6930-7 [DOI] [PubMed] [Google Scholar]
- 165. Machado MAC, Makdissi FF, Surjan RC, et al. Transition from open to laparoscopic ALPPS for patients with very small FLR: the initial experience. HPB (Oxford) 2017;19:59–66. 10.1016/j.hpb.2016.10.004 [DOI] [PubMed] [Google Scholar]
- 166. Machado M-A, Surjan R-C, Bassères T, et al. Totally laparoscopic ALPPS for multiple and bilobar colorectal metastases (with video). J Visc Surg 2017;154:131–2. 10.1016/j.jviscsurg.2016.11.004 [DOI] [PubMed] [Google Scholar]
- 167. Machado MA, Surjan R, Basseres T, et al. Total laparoscopic reversal ALPPS. Ann Surg Oncol 2017;24:1048–9. 10.1245/s10434-016-5620-6 [DOI] [PubMed] [Google Scholar]
- 168. Olthof PB, Coelen RJS, Wiggers JK, et al. High mortality after ALPPS for perihilar cholangiocarcinoma: case-control analysis including the first series from the International ALPPS Registry. HPB (Oxford) 2017;19:381–7. 10.1016/j.hpb.2016.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Nimura Y. Preoperative biliary drainage before resection for cholangiocarcinoma (Pro). HPB (Oxford) 2008;10:130–3. 10.1080/13651820801992666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. van Delden OM, Laméris JS. Percutaneous drainage and stenting for palliation of malignant bile duct obstruction. Eur Radiol 2008;18:448–56. 10.1007/s00330-007-0796-6 [DOI] [PubMed] [Google Scholar]
- 171. Walter T, Ho CS, Horgan AM, et al. Endoscopic or percutaneous biliary drainage for Klatskin tumors J Vasc Interv Radiol 2013;24:113–21. 10.1016/j.jvir.2012.09.019 [DOI] [PubMed] [Google Scholar]
- 172. Kawakami H, Kuwatani M, Onodera M, et al. Endoscopic nasobiliary drainage is the most suitable preoperative biliary drainage method in the management of patients with hilar cholangiocarcinoma. J Gastroenterol 2011;46:242–8. 10.1007/s00535-010-0298-1 [DOI] [PubMed] [Google Scholar]
- 173. Lang H, de Santibañes E, Schlitt HJ, et al. 10th anniversary of ALPPS-lessons learned and quo vadis. Ann Surg 2019;269:114–9. 10.1097/SLA.0000000000002797 [DOI] [PubMed] [Google Scholar]
- 174. Cillo U, Fondevila C, Donadon M, et al. Surgery for cholangiocarcinoma. Liver Int 2019;39 Suppl 1:143–55. 10.1111/liv.14089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. de Jong MC, Nathan H, Sotiropoulos GC, et al. Intrahepatic cholangiocarcinoma: an international multi-institutional analysis of prognostic factors and lymph node assessment. J Clin Oncol 2011;29:3140–5. 10.1200/JCO.2011.35.6519 [DOI] [PubMed] [Google Scholar]
- 176. Hyder O, Hatzaras I, Sotiropoulos GC, et al. Recurrence after operative management of intrahepatic cholangiocarcinoma. Surgery 2013;153:811–8. 10.1016/j.surg.2012.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Groot Koerkamp B, Fong Y. Outcomes in biliary malignancy. J Surg Oncol 2014;110:585–91. 10.1002/jso.23762 [DOI] [PubMed] [Google Scholar]
- 178. Kuhlmann JB, Euringer W, Spangenberg HC, et al. Treatment of unresectable cholangiocarcinoma: conventional transarterial chemoembolization compared with drug eluting bead-transarterial chemoembolization and systemic chemotherapy. Eur J Gastroenterol Hepatol 2012;24:437–43. 10.1097/MEG.0b013e3283502241 [DOI] [PubMed] [Google Scholar]
- 179. Konstantinidis IT, Groot Koerkamp B, Do RKG, et al. Unresectable intrahepatic cholangiocarcinoma: systemic plus hepatic arterial infusion chemotherapy is associated with longer survival in comparison with systemic chemotherapy alone. Cancer 2016;122:758–65. 10.1002/cncr.29824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. de Jong MC, Marques H, Clary BM, et al. The impact of portal vein resection on outcomes for hilar cholangiocarcinoma: a multi-institutional analysis of 305 cases. Cancer 2012;118:4737–47. 10.1002/cncr.27492 [DOI] [PubMed] [Google Scholar]
- 181. Ribero D, Amisano M, Lo Tesoriere R, et al. Additional resection of an intraoperative margin-positive proximal bile duct improves survival in patients with hilar cholangiocarcinoma. Ann Surg 2011;254:776–81; 10.1097/SLA.0b013e3182368f85 [DOI] [PubMed] [Google Scholar]
- 182. Wakai T, Shirai Y, Moroda T, et al. Impact of ductal resection margin status on long-term survival in patients undergoing resection for extrahepatic cholangiocarcinoma. Cancer 2005;103:1210–6. 10.1002/cncr.20906 [DOI] [PubMed] [Google Scholar]
- 183. Endo I, House MG, Klimstra DS, et al. Clinical significance of intraoperative bile duct margin assessment for hilar cholangiocarcinoma. Ann Surg Oncol 2008;15:2104–12. 10.1245/s10434-008-0003-2 [DOI] [PubMed] [Google Scholar]
- 184. Tsukahara T, Ebata T, Shimoyama Y, et al. Residual carcinoma in situ at the ductal stump has a negative survival effect: an analysis of early-stage cholangiocarcinomas. Ann Surg 2017;266:126–32. 10.1097/SLA.0000000000001944 [DOI] [PubMed] [Google Scholar]
- 185. Shiraki T, Kuroda H, Takada A, et al. Intraoperative frozen section diagnosis of bile duct margin for extrahepatic cholangiocarcinoma. World J Gastroenterol 2018;24:1332–42. 10.3748/wjg.v24.i12.1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Nimura Y, Hayakawa N, Kamiya J, et al. Hepatic segmentectomy with caudate lobe resection for bile duct carcinoma of the hepatic hilus. World J Surg 1990;14:535–43; 10.1007/BF01658686 [DOI] [PubMed] [Google Scholar]
- 187. Yamamoto H, Hayakawa N, Komatsu S, et al. Right hepatic lobectomy and subsegmental resection of the left caudate lobe for gallbladder carcinoma involving the hepatic hilus: preservation of the ventral portion of the left caudate lobe. J Hepatobiliary Pancreat Surg 1998;5:207–11. 10.1007/s005340050035 [DOI] [PubMed] [Google Scholar]
- 188. Nagino M, Kamiya J, Arai T, et al. Anatomic’ right hepatic trisectionectomy (extended right hepatectomy) with caudate lobectomy for hilar cholangiocarcinoma. Ann Surg 2006;243:28–32. 10.1097/01.sla.0000193604.72436.63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Bhutiani N, Scoggins CR, McMasters KM, et al. The impact of caudate lobe resection on margin status and outcomes in patients with hilar cholangiocarcinoma: a multi-institutional analysis from the US extrahepatic biliary malignancy consortium. Surgery 2018;163:726–31. 10.1016/j.surg.2017.10.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Kawasaki S, Imamura H, Kobayashi A, et al. Results of surgical resection for patients with hilar bile duct cancer: application of extended hepatectomy after biliary drainage and hemihepatic portal vein embolization. Ann Surg 2003;238:84–92. 10.1097/01.SLA.0000074984.83031.02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Tamoto E, Hirano S, Tsuchikawa T, et al. Portal vein resection using the no-touch technique with a hepatectomy for hilar cholangiocarcinoma. HPB (Oxford) 2014;16:56–61. 10.1111/hpb.12067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Jonas S, Krenzien F, Atanasov G, et al. Hilar en bloc resection for hilar cholangiocarcinoma in patients with limited liver capacities-preserving parts of liver segment 4. Eur Surg 2018;50:22–9. 10.1007/s10353-017-0507-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Rui J-A, Wang S-B, Chen S-G, et al. Right trisectionectomy for primary liver cancer. World J Gastroenterol 2003;9:706–9. 10.3748/wjg.v9.i4.706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Shimizu H, Kimura F, Yoshidome H, et al. Aggressive surgical resection for hilar cholangiocarcinoma of the left-side predominance: radicality and safety of left-sided hepatectomy. Ann Surg 2010;251:281–6. 10.1097/SLA.0b013e3181be0085 [DOI] [PubMed] [Google Scholar]
- 195. Hosokawa I, Shimizu H, Yoshidome H, et al. Surgical strategy for hilar cholangiocarcinoma of the left-side predominance: current role of left trisectionectomy. Ann Surg 2014;259:1178–85. 10.1097/SLA.0000000000000584 [DOI] [PubMed] [Google Scholar]
- 196. Shimizu H, Sawada S, Kimura F, et al. Clinical significance of biliary vascular anatomy of the right liver for hilar cholangiocarcinoma applied to left hemihepatectomy. Ann Surg 2009;249:435–9. 10.1097/SLA.0b013e31819a6c10 [DOI] [PubMed] [Google Scholar]
- 197. Govil S, Bharatan A, Rammohan A, et al. Liver resection for perihilar cholangiocarcinoma - why left is sometimes right. HPB (Oxford) 2016;18:575–9. 10.1016/j.hpb.2016.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Schimizzi GV, Jin LX, Davidson JT, et al. Outcomes after vascular resection during curative-intent resection for hilar cholangiocarcinoma: a multi-institution study from the US extrahepatic biliary malignancy consortium. HPB (Oxford) 2018;20:332–9. 10.1016/j.hpb.2017.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Matsuyama R, Mori R, Ota Y, et al. Significance of vascular resection and reconstruction in surgery for hilar cholangiocarcinoma: with special reference to hepatic arterial resection and reconstruction. Ann Surg Oncol 2016;23:475–84. 10.1245/s10434-016-5381-2 [DOI] [PubMed] [Google Scholar]
- 200. Chen K-J, Yang F-C, Qin Y-S, et al. Assessment of clinical outcomes of advanced hilar cholangiocarcinoma. Hepatobiliary Pancreat Dis Int 2018;17:155–62. 10.1016/j.hbpd.2018.03.003 [DOI] [PubMed] [Google Scholar]
- 201. Giuliante F, Ardito F, Guglielmi A, et al. Association of lymph node status with survival in patients after liver resection for hilar cholangiocarcinoma in an Italian multicenter analysis. JAMA Surg 2016;151:916–22. 10.1001/jamasurg.2016.1769 [DOI] [PubMed] [Google Scholar]
- 202. Kambakamba P, Linecker M, Slankamenac K, et al. Lymph node dissection in resectable perihilar cholangiocarcinoma: a systematic review. Am J Surg 2015;210:694–701. 10.1016/j.amjsurg.2015.05.015 [DOI] [PubMed] [Google Scholar]
- 203. Ecker BL, Vining CC, Roses RE, et al. Identification of patients for adjuvant therapy after resection of carcinoma of the extrahepatic bile ducts: a propensity score-matched analysis. Ann Surg Oncol 2017;24:3926–33. 10.1245/s10434-017-6095-9 [DOI] [PubMed] [Google Scholar]
- 204. Mizuno T, Ebata T, Yokoyama Y, et al. Adjuvant gemcitabine monotherapy for resectable perihilar cholangiocarcinoma with lymph node involvement: a propensity score matching analysis. Surg Today 2017;47:182–92. 10.1007/s00595-016-1354-0 [DOI] [PubMed] [Google Scholar]
- 205. Primrose JN, Fox RP, Palmer DH, et al. Capecitabine compared with observation in resected biliary tract cancer (BILCAP): a randomised, controlled, multicentre, phase 3 study. Lancet Oncol 2019;20:663–73. 10.1016/S1470-2045(18)30915-X [DOI] [PubMed] [Google Scholar]
- 206. Oshiro Y, Sasaki R, Kobayashi A, et al. Prognostic relevance of the lymph node ratio in surgical patients with extrahepatic cholangiocarcinoma. Eur J Surg Oncol 2011;37:60–4. 10.1016/j.ejso.2010.10.011 [DOI] [PubMed] [Google Scholar]
- 207. Guglielmi A, Ruzzenente A, Bertuzzo F, et al. Assessment of nodal status for perihilar cholangiocarcinoma location, number, or ratio of involved nodes. Hepatobiliary Surg Nutr 2013;2:281–3. 10.3978/j.issn.2304-3881.2013.08.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Groot Koerkamp B, Wiggers JK, Gonen M, et al. Survival after resection of perihilar cholangiocarcinoma-development and external validation of a prognostic nomogram. Ann Oncol 2016;27:753. 10.1093/annonc/mdw063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Bird NTE, McKenna A, Dodd J, et al. Meta-analysis of prognostic factors for overall survival in patients with resected hilar cholangiocarcinoma. Br J Surg 2018;105:1408–16. 10.1002/bjs.10921 [DOI] [PubMed] [Google Scholar]
- 210. Malik H. Ensuring quality in centres. Cholangiocarcinoma-UK Conference 2022; 2022. Available: https://www.basl.org.uk/index.cfm/content/page/cid/28 [Google Scholar]
- 211. Idrees JJ, Merath K, Gani F, et al. Trends in centralization of surgical care and compliance with National Cancer Center Network guidelines for resected cholangiocarcinoma. HPB (Oxford) 2019;21:981–9. 10.1016/j.hpb.2018.11.013 [DOI] [PubMed] [Google Scholar]
- 212. Elshami M, Hue JJ, Ahmed FA, et al. Defining facility volume threshold for optimization of short- and long-term outcomes in patients undergoing resection of perihilar cholangiocarcinoma. J Gastrointest Surg 2023;27:730–40. 10.1007/s11605-022-05465-z [DOI] [PubMed] [Google Scholar]
- 213. Stieber AC, Marino IR, Iwatsuki S, et al. Cholangiocarcinoma in sclerosing cholangitis. The role of liver transplantation. Int Surg 1989;74:1–3. [PMC free article] [PubMed] [Google Scholar]
- 214. Meyer CG, Penn I, James L. Liver transplantation for cholangiocarcinoma: results in 207 patients1. Transplantation 2000;69:1633–7. 10.1097/00007890-200004270-00019 [DOI] [PubMed] [Google Scholar]
- 215. Hidalgo E, Asthana S, Nishio H, et al. Surgery for hilar cholangiocarcinoma: the Leeds experience. Eur J Surg Oncol 2008;34:787–94. 10.1016/j.ejso.2007.10.005 [DOI] [PubMed] [Google Scholar]
- 216. Sudan D, DeRoover A, Chinnakotla S, et al. Radiochemotherapy and transplantation allow long-term survival for nonresectable hilar cholangiocarcinoma. Am J Transplant 2002;2:774–9. 10.1034/j.1600-6143.2002.20812.x [DOI] [PubMed] [Google Scholar]
- 217. De Vreede I, Steers JL, Burch PA, et al. Prolonged disease-free survival after orthotopic liver transplantation plus adjuvant chemoirradiation for cholangiocarcinoma. Liver Transpl 2000;6:309–16. 10.1053/lv.2000.6143 [DOI] [PubMed] [Google Scholar]
- 218. Rea DJ, Heimbach JK, Rosen CB, et al. Liver transplantation with neoadjuvant chemoradiation is more effective than resection for hilar cholangiocarcinoma. Ann Surg 2005;242:451–8; 10.1097/01.sla.0000179678.13285.fa [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Rosen CB, Darwish Murad S, Heimbach JK, et al. Neoadjuvant therapy and liver transplantation for hilar cholangiocarcinoma: is pretreatment pathological confirmation of diagnosis necessary J Am Coll Surg 2012;215:31–8; 10.1016/j.jamcollsurg.2012.03.014 [DOI] [PubMed] [Google Scholar]
- 220. Darwish Murad S, Kim WR, Harnois DM, et al. Efficacy of neoadjuvant chemoradiation, followed by liver transplantation, for perihilar cholangiocarcinoma at 12 US centers. Gastroenterology 2012;143:88–98. 10.1053/j.gastro.2012.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Mantel HTJ, Westerkamp AC, Adam R, et al. Strict selection alone of patients undergoing liver transplantation for hilar cholangiocarcinoma is associated with improved survival. PLoS One 2016;11:e0156127. 10.1371/journal.pone.0156127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Lehrke HD, Heimbach JK, Wu T-T, et al. Prognostic significance of the histologic response of perihilar cholangiocarcinoma to preoperative neoadjuvant chemoradiation in liver explants. Am J Surg Pathol 2016;40:510–8. 10.1097/PAS.0000000000000588 [DOI] [PubMed] [Google Scholar]
- 223. Azad AI, Rosen CB, Taner T, et al. Selected patients with unresectable perihilar cholangiocarcinoma (pCCA) derive long-term benefit from liver transplantation. Cancers (Basel) 2020;12:3157. 10.3390/cancers12113157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Cambridge WA, Fairfield C, Powell JJ, et al. Meta-analysis and meta-regression of survival after liver transplantation for unresectable perihilar cholangiocarcinoma Ann Surg 2021;274:e921–2. 10.1097/SLA.0000000000005190 [DOI] [PubMed] [Google Scholar]
- 225. Croome KP, Rosen CB, Heimbach JK, et al. Is liver transplantation appropriate for patients with potentially resectable de novo hilar cholangiocarcinoma? J Am Coll Surg 2015;221:130–9. 10.1016/j.jamcollsurg.2015.01.064 [DOI] [PubMed] [Google Scholar]
- 226. Ethun CG, Lopez-Aguiar AG, Anderson DJ, et al. Transplantation versus resection for hilar cholangiocarcinoma. Ann Surg 2018;267:797–805. 10.1097/SLA.0000000000002574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Robles R, Figueras J, Turrión VS, et al. Spanish experience in liver transplantation for hilar and peripheral cholangiocarcinoma. Ann Surg 2004;239:265–71. 10.1097/01.sla.0000108702.45715.81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Takahashi K, Obeid J, Burmeister CS, et al. Intrahepatic cholangiocarcinoma in the liver explant after liver transplantation: histological differentiation and prognosis. Ann Transplant 2016;21:208–15. 10.12659/aot.895936 [DOI] [PubMed] [Google Scholar]
- 229. Sapisochin G, Rodríguez de Lope C, Gastaca M, et al. “Very early” intrahepatic cholangiocarcinoma in cirrhotic patients: should liver transplantation be reconsidered in these patients? Am J Transplant 2014;14:660–7. 10.1111/ajt.12591 [DOI] [PubMed] [Google Scholar]
- 230. Sapisochin G, Facciuto M, Rubbia-Brandt L, et al. Liver transplantation for "very early" intrahepatic cholangiocarcinoma: international retrospective study supporting a prospective assessment. Hepatology 2016;64:1178–88. 10.1002/hep.28744 [DOI] [PubMed] [Google Scholar]
- 231. Jung D-H, Hwang S, Song G-W, et al. Clinicopathological features and prognosis of intrahepatic cholangiocarcinoma after liver transplantation and resection. Ann Transplant 2017;22:42–52. 10.12659/aot.901504 [DOI] [PubMed] [Google Scholar]
- 232. De Martin E, Rayar M, Golse N, et al. Analysis of liver resection versus liver transplantation on outcome of small intrahepatic cholangiocarcinoma and combined hepatocellular‐cholangiocarcinoma in the setting of cirrhosis. Liver Transpl 2020;26:785–98. 10.1002/lt.25737 [DOI] [PubMed] [Google Scholar]
- 233. Rayar M, Levi Sandri GB, Houssel-Debry P, et al. Multimodal therapy including yttrium-90 radioembolization as a bridging therapy to liver transplantation for a huge and locally advanced intrahepatic cholangiocarcinoma. J Gastrointestin Liver Dis 2016;25:401–4. 10.15403/jgld.2014.1121.253.y90 [DOI] [PubMed] [Google Scholar]
- 234. Lunsford KE, Javle M, Heyne K, et al. Liver transplantation for locally advanced intrahepatic cholangiocarcinoma treated with neoadjuvant therapy: a prospective case-series. Lancet Gastroenterol Hepatol 2018;3:337–48. 10.1016/S2468-1253(18)30045-1 [DOI] [PubMed] [Google Scholar]
- 235. Edeline J, Benabdelghani M, Bertaut A, et al. Gemcitabine and oxaliplatin chemotherapy or surveillance in resected biliary tract cancer (PRODIGE 12-ACCORD 18-UNICANCER GI): a randomized phase III study. J Clin Oncol 2019;37:658–67. 10.1200/JCO.18.00050 [DOI] [PubMed] [Google Scholar]
- 236. Ebata T, Hirano S, Konishi M, et al. Randomized clinical trial of adjuvant gemcitabine chemotherapy versus observation in resected bile duct cancer. Br J Surg 2018;105:192–202. 10.1002/bjs.10776 [DOI] [PubMed] [Google Scholar]
- 237. Jeong H, Kim K, Jeong JH, et al. Adjuvant gemcitabine plus cisplatin versus capecitabine in node-positive extrahepatic cholangiocarcinoma: the STAMP randomized trial. Hepatology 2023;77:1540–9. 10.1097/HEP.0000000000000046 [DOI] [PubMed] [Google Scholar]
- 238. Nakachi K, Ikeda M, Konishi M, et al. Adjuvant S-1 compared with observation in resected biliary tract cancer (JCOG1202, ASCOT): a multicentre, open-label, randomised, controlled, phase 3 trial. Lancet 2023;401:195–203. 10.1016/S0140-6736(22)02038-4 [DOI] [PubMed] [Google Scholar]
- 239. Glimelius B, Hoffman K, Sjödén PO, et al. Chemotherapy improves survival and quality of life in advanced pancreatic and biliary cancer. Ann Oncol 1996;7:593–600. 10.1093/oxfordjournals.annonc.a010676 [DOI] [PubMed] [Google Scholar]
- 240. Sharma A, Dwary AD, Mohanti BK, et al. Best supportive care compared with chemotherapy for unresectable gall bladder cancer: a randomized controlled study. J Clin Oncol 2010;28:4581–6. 10.1200/JCO.2010.29.3605 [DOI] [PubMed] [Google Scholar]
- 241. Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med 2010;362:1273–81. 10.1056/NEJMoa0908721 [DOI] [PubMed] [Google Scholar]
- 242. Okusaka T, Nakachi K, Fukutomi A, et al. Gemcitabine alone or in combination with cisplatin in patients with biliary tract cancer: a comparative multicentre study in Japan. Br J Cancer 2010;103:469–74. 10.1038/sj.bjc.6605779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Phelip J Marc, Desrame J, Edeline J, et al. Modified FOLFIRINOX versus CISGEM chemotherapy for patients with advanced biliary tract cancer (PRODIGE 38 AMEBICA): a randomized phase II study. J Clin Oncol 2022;40:262–71. 10.1200/JCO.21.00679 [DOI] [PubMed] [Google Scholar]
- 244. Sakai D, Kanai M, Kobayashi S, et al. Randomized phase III study of gemcitabine, cisplatin plus S-1 (GCS) versus gemcitabine, cisplatin (GC) for advanced biliary tract cancer (KHBO1401-MITSUBA). Ann Oncol 2018;29:viii205. 10.1093/annonc/mdy282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Shroff RT, Javle MM, Xiao L, et al. Gemcitabine, cisplatin, and NAB-paclitaxel for the treatment of advanced biliary tract cancers. JAMA Oncol 2019;5:824–30. 10.1001/jamaoncol.2019.0270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Shroff RT, Guthrie KA, Scott AJ, et al. SWOG 1815: A phase III randomized trial of gemcitabine, cisplatin, and nab-paclitaxel versus gemcitabine and cisplatin in newly diagnosed, advanced biliary tract cancers. J Clin Oncol 2023;41:LBA490. [Google Scholar]
- 247. Lamarca A, Palmer DH, Wasan HS, et al. Second-line FOLFOX chemotherapy versus active symptom control for advanced biliary tract cancer (ABC-06): a phase 3, open-label, randomised, controlled trial. Lancet Oncol 2021;22:690–701. 10.1016/S1470-2045(21)00027-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Yoo C, Kim K-P, Jeong JH, et al. Liposomal irinotecan plus fluorouracil and leucovorin versus fluorouracil and leucovorin for metastatic biliary tract cancer after progression on gemcitabine plus cisplatin (NIFTY): a multicentre, open-label, randomised, phase 2B study. Lancet Oncol 2021;22:1560–72. 10.1016/S1470-2045(21)00486-1 [DOI] [PubMed] [Google Scholar]
- 249. Oh D-Y, Ruth He A, Qin S, et al. Durvalumab plus gemcitabine and cisplatin in advanced biliary tract cancer. NEJM Evidence 2022;1. 10.1056/EVIDoa2200015 [DOI] [PubMed] [Google Scholar]
- 250. Kelley RK, Ueno M, Yoo C, et al. Pembrolizumab in combination with gemcitabine and cisplatin compared with gemcitabine and cisplatin alone for patients with advanced biliary tract cancer (KEYNOTE-966): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2023;401:1853–65. 10.1016/S0140-6736(23)00727-4 [DOI] [PubMed] [Google Scholar]
- 251. Jusakul A, Cutcutache I, Yong CH, et al. Whole-genome and epigenomic landscapes of etiologically distinct subtypes of cholangiocarcinoma. Cancer Discov 2017;7:1116–35. 10.1158/2159-8290.CD-17-0368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Goyal L, Saha SK, Liu LY, et al. Polyclonal secondary FGFR2 mutations drive acquired resistance to FGFR inhibition in patients with FGFR2 fusion–positive cholangiocarcinoma. Cancer Discov 2017;7:252–63. 10.1158/2159-8290.CD-16-1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Lowery MA, Burris HA, Janku F, et al. Safety and activity of Ivosidenib in patients with IDH1-mutant advanced cholangiocarcinoma: a phase 1 study. Lancet Gastroenterol Hepatol 2019;4:711–20. 10.1016/S2468-1253(19)30189-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254. Bekaii-Saab TS, Bridgewater J, Normanno N. Practical considerations in screening for genetic alterations in cholangiocarcinoma. Ann Oncol 2021;32:1111–26. 10.1016/j.annonc.2021.04.012 [DOI] [PubMed] [Google Scholar]
- 255. Ross JS, Sokol E, Vergilio J-A, et al. Primary versus metastatic intrahepatic cholangiocarcinoma: a comparative comprehensive genomic profiling (CGP) study. J Clin Oncol 2020;38:578. 10.1200/JCO.2020.38.4_suppl.578 [DOI] [Google Scholar]
- 256. Goyal L, Meric-Bernstam F, Hollebecque A, et al. Primary results of phase 2 FOENIX-Cca2: the irreversible FGFR1-4 inhibitor futibatinib in intrahepatic cholangiocarcinoma (iCCA) with FGFR2 fusions/rearrangements. Cancer Res 2021;81:CT010. 10.1158/1538-7445.AM2021-CT010 [DOI] [Google Scholar]
- 257. Javle MM, Roychowdhury S, Kelley RK, et al. Final results from a phase II study of Infigratinib (BGJ398), an FGFR-selective tyrosine kinase inhibitor, in patients with previously treated advanced cholangiocarcinoma harboring an FGFR2 gene fusion or rearrangement. J Clin Oncol 2021;39:265. 10.1200/JCO.2021.39.3_suppl.265 33503392 [DOI] [Google Scholar]
- 258. Mazzaferro V, El-Rayes BF, Droz Dit Busset M, et al. Derazantinib (ARQ 087) in advanced or inoperable FGFR2 gene fusion-positive intrahepatic cholangiocarcinoma. Br J Cancer 2019;120:165–71. 10.1038/s41416-018-0334-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. Meric-Bernstam F, Hanna DL, El-Khoueiry AB, et al. Zanidatamab (ZW25) in Her2-positive biliary tract cancers (BTCS): results from a phase I study. J Clin Oncol 2021;39:299. 10.1200/JCO.2021.39.3_suppl.299 [DOI] [Google Scholar]
- 260. Cleary JM, Raghavan S, Wu Q, et al. FGFR2 extracellular domain in-frame deletions are therapeutically targetable genomic alterations that function as oncogenic drivers in cholangiocarcinoma. Cancer Discov 2021;11:2488–505. 10.1158/2159-8290.CD-20-1669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261. Meric-Bernstam F, Bahleda R, Hierro C, et al. Futibatinib, an irreversible FGFR1–4 inhibitor, in patients with advanced solid tumors harboring FGF / FGFR aberrations: a phase I dose-expansion study. Cancer Discov 2022;12:402–15. 10.1158/2159-8290.CD-21-0697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262. Abou-Alfa GK, Bibeau K, Schultz N, et al. Effect of FGFR2 alterations on survival in patients receiving systemic chemotherapy for intrahepatic cholangiocarcinoma. J Clin Oncol 2021;39:303. 10.1200/JCO.2021.39.3_suppl.303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263. Abou-Alfa GK, Macarulla T, Javle MM, et al. Ivosidenib in IDH1-mutant, chemotherapy-refractory cholangiocarcinoma (ClarIDHy): a multicentre, randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol 2020;21:796–807. 10.1016/S1470-2045(20)30157-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264. Harding JJ, Fan J, Oh D-Y, et al. Zanidatamab for Her2-amplified, unresectable, locally advanced or metastatic biliary tract cancer (HERIZON-BTC-01): a multicentre, single-arm, phase 2B study. Lancet Oncol 2023;24:772–82. 10.1016/S1470-2045(23)00242-5 [DOI] [PubMed] [Google Scholar]
- 265. Boscoe AN, Rolland C, Kelley RK. Frequency and prognostic significance of Isocitrate dehydrogenase 1 mutations in cholangiocarcinoma: a systematic literature review. J Gastrointest Oncol 2019;10:751–65. 10.21037/jgo.2019.03.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266. Subbiah V, Lassen U, Élez E, et al. Dabrafenib plus trametinib in patients with BRAFV600E-mutated biliary tract cancer (ROAR): a phase 2, open-label, single-arm, multicentre basket trial. Lancet Oncol 2020;21:1234–43. 10.1016/S1470-2045(20)30321-1 [DOI] [PubMed] [Google Scholar]
- 267. Grendar J, Grendarova P, Sinha R, et al. Neoadjuvant therapy for downstaging of locally advanced hilar cholangiocarcinoma: a systematic review. HPB (Oxford) 2014;16:297–303. 10.1111/hpb.12150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268. Baltatzis M, Jegatheeswaran S, Siriwardena AK. Neoadjuvant chemoradiotherapy before resection of perihilar cholangiocarcinoma: a systematic review. Hepatobiliary Pancreat Dis Int 2020;19:103–8. 10.1016/j.hbpd.2020.02.007 [DOI] [PubMed] [Google Scholar]
- 269. Loveday BPT, Knox JJ, Dawson LA, et al. Neoadjuvant hyperfractionated chemoradiation and liver transplantation for unresectable perihilar cholangiocarcinoma in Canada. J Surg Oncol 2018;117:213–9. 10.1002/jso.24833 [DOI] [PubMed] [Google Scholar]
- 270. Ren B, Guo Q, Yang Y, et al. A meta-analysis of the efficacy of postoperative adjuvant radiotherapy versus no radiotherapy for extrahepatic cholangiocarcinoma and gallbladder carcinoma. Radiat Oncol 2020;15:15. 10.1186/s13014-020-1459-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271. Ke Q, Lin N, Deng M, et al. The effect of adjuvant therapy for patients with intrahepatic cholangiocarcinoma after surgical resection: a systematic review and meta-analysis. PLoS ONE 2020;15:e0229292. 10.1371/journal.pone.0229292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272. Cho M, Wang‐Gillam A, Myerson R, et al. A phase II study of adjuvant gemcitabine plus docetaxel followed by concurrent chemoradation in resected pancreaticobiliary carcinoma. HPB (Oxford) 2015;17:587–93. 10.1111/hpb.12413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273. Ben-Josef E, Guthrie KA, El-Khoueiry AB, et al. SWOG S0809: a phase II intergroup trial of adjuvant capecitabine and gemcitabine followed by radiotherapy and concurrent capecitabine in extrahepatic cholangiocarcinoma and gallbladder carcinoma. J Clin Oncol 2015;33:2617–22. 10.1200/JCO.2014.60.2219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274. Lee J, Yoon WS, Koom WS, et al. Efficacy of stereotactic body radiotherapy for unresectable or recurrent cholangiocarcinoma: a meta-analysis and systematic review. Strahlenther Onkol 2019;195:93–102. 10.1007/s00066-018-1367-2 [DOI] [PubMed] [Google Scholar]
- 275. Frakulli R, Buwenge M, Macchia G, et al. Stereotactic body radiation therapy in cholangiocarcinoma: a systematic review. Br J Radiol 2019;92:20180688. 10.1259/bjr.20180688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276. Phelip J-M, Vendrely V, Rostain F, et al. Gemcitabine plus cisplatin versus chemoradiotherapy in locally advanced biliary tract cancer: Fédération Francophone de Cancérologie Digestive 9902 phase II randomised study. Eur J Cancer 2014;50:2975–82. 10.1016/j.ejca.2014.08.013 [DOI] [PubMed] [Google Scholar]
- 277. Hong TS, Wo JY, Yeap BY, et al. Multi-institutional phase II study of high-dose hypofractionated proton beam therapy in patients with localized, unresectable hepatocellular carcinoma and intrahepatic cholangiocarcinoma. J Clin Oncol 2016;34:460–8. 10.1200/JCO.2015.64.2710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278. Tao R, Krishnan S, Bhosale PR, et al. Ablative radiotherapy doses lead to a substantial prolongation of survival in patients with inoperable intrahepatic cholangiocarcinoma: a retrospective dose response analysis. J Clin Oncol 2016;34:219–26. 10.1200/JCO.2015.61.3778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279. Brunner TB, Blanck O, Lewitzki V, et al. Stereotactic body radiotherapy dose and its impact on local control and overall survival of patients for locally advanced Intrahepatic and extrahepatic cholangiocarcinoma. Radiother Oncol 2019;132:42–7. 10.1016/j.radonc.2018.11.015 [DOI] [PubMed] [Google Scholar]
- 280. Mutsaers A, Greenspoon J, Walker-Dilks C, et al. Systematic review of patient reported quality of life following stereotactic ablative radiotherapy for primary and metastatic liver cancer. Radiat Oncol 2017;12:110. 10.1186/s13014-017-0818-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281. Lutz S, Berk L, Chang E, et al. Palliative radiotherapy for bone metastases: an ASTRO evidence-based guideline. Int J Radiat Oncol Biol Phys 2011;79:965–76. 10.1016/j.ijrobp.2010.11.026 [DOI] [PubMed] [Google Scholar]
- 282. Sapienza LG, Ning MS, Jhingran A, et al. Short-course palliative radiation therapy leads to excellent bleeding control: a single centre retrospective study. Clin Transl Radiat Oncol 2019;14:40–6. 10.1016/j.ctro.2018.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283. Xu X, Li J, Wu J, et al. A systematic review and meta-analysis of intraluminal brachytherapy versus stent alone in the treatment of malignant obstructive jaundice. Cardiovasc Intervent Radiol 2018;41:206–17. 10.1007/s00270-017-1827-6 [DOI] [PubMed] [Google Scholar]
- 284. Palma DA, Olson R, Harrow S, et al. Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (SABR-COMET): a randomised, phase 2, open-label trial. Lancet 2019;393:2051–8. 10.1016/S0140-6736(18)32487-5 [DOI] [PubMed] [Google Scholar]
- 285. Palma DA, Olson R, Harrow S, et al. Stereotactic ablative radiotherapy for the comprehensive treatment of oligometastatic cancers: long-term results of the SABR-COMET phase II randomized trial. J Clin Oncol 2020;38:2830–8. 10.1200/JCO.20.00818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286. Olson R, Senan S, Harrow S, et al. Quality of life outcomes after stereotactic ablative radiation therapy (SABR) versus standard of care treatments in the oligometastatic setting: a secondary analysis of the SABR-COMET randomized trial. Int J Radiat Oncol Biol Phys 2019;105:943–7. 10.1016/j.ijrobp.2019.08.041 [DOI] [PubMed] [Google Scholar]
- 287. Chalkidou A, Macmillan T, Grzeda MT, et al. Stereotactic ablative body radiotherapy in patients with oligometastatic cancers: a prospective, registry-based, single-arm, observational, evaluation study. Lancet Oncol 2021;22:98–106. 10.1016/S1470-2045(20)30537-4 [DOI] [PubMed] [Google Scholar]
- 288. Shariff MIF, Khan SA, Westaby D. The palliation of cholangiocarcinoma. Curr Opin Support Palliat Care 2013;7:168–74. 10.1097/SPC.0b013e32835f1e2f [DOI] [PubMed] [Google Scholar]
- 289. Haun MW, Estel S, Rücker G, et al. Early palliative care for adults with advanced cancer. Cochrane Database Syst Rev 2017;6:CD011129. 10.1002/14651858.CD011129.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290. Hunter LA, Soares HP. Quality of life and symptom management in advanced biliary tract cancers. Cancers 2021;13:5074. 10.3390/cancers13205074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291. Murray-Brown FL. Naltrexone for cholestatic itch: a systematic review. BMJ Support Palliat Care 2021;11:217–25. 10.1136/bmjspcare-2020-002801 [DOI] [PubMed] [Google Scholar]
- 292. Siemens W, Xander C, Meerpohl JJ, et al. Pharmacological interventions for pruritus in adult palliative care patients. Cochrane Database Syst Rev 2016;11:CD008320. 10.1002/14651858.CD008320.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293. Alessy SA, Davies E, Rawlinson J, et al. Clinical nurse specialists and survival in patients with cancer: the UK National cancer experience survey. BMJ Support Palliat Care 2022. 10.1136/bmjspcare-2021-003445 [Epub ahead of print 21 Apr 2022]. [DOI] [PubMed] [Google Scholar]
- 294. Valle JW, Borbath I, Khan SA, et al. Biliary cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2016;27:v28–37. 10.1093/annonc/mdw324 [DOI] [PubMed] [Google Scholar]
- 295. Graf J, Stengel A. Psychological burden and psycho-oncological interventions for patients with hepatobiliary cancers–a systematic review. Front Psychol 2021;12. 10.3389/fpsyg.2021.662777 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
gutjnl-2023-330029supp001.pdf (74.7KB, pdf)