Abstract
Diabetic kidney disease (DKD) is a prevailing complication arising from diabetes mellitus. Unfortunately, there are no trustworthy and efficacious treatment modalities currently available. In recent times, compelling evidence has emerged regarding the intricate correlation between the kidney and the gut microbiota, which is considered the largest immune organ within the human physique. Various investigations have demonstrated that the perturbation of the gut microbiota and its associated metabolites potentially underlie the etiology and progression of DKD. This phenomenon may transpire through perturbation of both the innate and the adaptive immunity, leading to a burdensome allostatic load on the body and ultimately culminating in the development of DKD. Within this literature review, we aim to delve into the intricate interplay between the gut microbiota, its metabolites, and the immune system in the context of DKD. Furthermore, we strive to explore and elucidate potential chemical interventions that could hold promise for the treatment of DKD, thereby offering invaluable insights and directions for future research endeavors.
1. Introduction
Diabetic kidney disease (DKD) is one of the prevalent microvascular complications associated with diabetes mellitus (DM). A recent investigation of the epidemiology revealed that DKD is the primary cause of chronic kidney disease (CKD) in the United States [1]. Effective treatment methods to delay its progression are still lacking. Specifically, patients with early DKD exhibit mild clinical symptoms and only present with microalbuminuria, which often goes unnoticed by patients [2]. In recent years, there have been advancements in treatment options for DKD patients, such as sodium-glucose transporter 2 (SGLT2) inhibitors [3, 4]. Despite these advancements, some studies have shown that SGLT2 inhibitors are associated with an increased risk of genital infections [5] and are less effective in patients with severe renal impairment [6]. Therefore, it remains crucial to further investigate the pathogenesis and treatment of DKD. Researchers have discovered that patients with end-stage DKD often exhibit significant disruptions in their gut microbiota, immune imbalances, and allostatic loads, with the severity of gut microbiota imbalance being closely tied to the degree of renal injury. Consequently, understanding how to improve DKD by manipulating the gut microbiota to improve the allostatic load caused by immune imbalances has become a focal point of research [7]. The gut harbors a highly intricate microbial ecosystem [8]. Alterations in the composition of gut microbiota and its metabolites can profoundly impact the human immune and metabolic systems [9]. Studies have highlighted the strong connection between gut-microbial ecology and kidney function, particularly in terms of substance metabolism, immune inflammation, gut mucosa, and the composition of gut bacteria [10].
In DKD, the changes in gut microbiota can impact the immunity, ultimately resulting in the acceleration of toxic byproducts. It facilitates the transportation of bacteria and their byproducts into the systemic circulation, causing damage to tissues and organs [11]. The dysbiosis of gut microbiota and gut endotoxins exacerbate kidney injury and proteinuria in DKD patients [12–15]. Short-chain fatty acids (SCFAs) are linked to immune, oxidative stress, and inflammatory responses in DKD [16]. In DKD, dysregulated glucose and lipid metabolism, heightened immune-inflammatory response, medication, diet, and other factors further worsen the dysbiosis of gut microbiota, thereby impacting renal injury [17–21].
Considering these findings, we aim to present the article status on the connection with gut microbiota and immune dysfunction in DKD, elucidating how gut microbiota imbalance contributes to immune dysfunction in DKD mechanism and discussing relevant drug studies.
2. The Relationship between DKD and Gut Microbiota and the Immune System
2.1. Gut Microbiota and the Immune System
In healthy individuals, the gut harbors over 1,000 diverse types of microorganisms during the neonatal phase. Primarily consisting of bacteria [22], these microbial communities undergo substantial transformations in the weeks following birth [23]. Gut microbiota in infants, thereafter, is influenced by various factors like feeding habits and medications. It is worth noting that after reaching the age of 12, these microbial populations tend to stabilize progressively [24, 25].
Bacteroides, SFB (segmented filamentous bacteria), Bifidobacterium, Lactobacillus, and Bacillus proteus [26] contribute significantly to the immune system and enhancement of the physical barrier in the gut microbiota. Gut monocyte-macrophages are widespread, as they regulate immune cells in the gut and initiate nonspecific immune responses. They not only establish local immunity but also provide resistance against systemic infections [27]. Certain bacterial species, such as Clostridium, have the capacity to stimulate regulatory T cells (Tregs) in the colon and are good to the maturation of the mucosa, as well as shaping the natural killer T (NKT) cells and lymphoid structures [28]. Gut microbiota plays a crucial role in immune responses. The innate immune system involves dendritic cells (DCs), macrophages, granulocytes, and NKT cells. Of these, macrophages and DCs mainly mediate T cell-induced adaptive immune responses, particularly involving T and B cells [29–32].
2.2. Relationship between Gut Microbiota and Its Metabolite Imbalance and DKD Immune Imbalance and Allostatic Load
In the process of DKD development, the body undergoes an elevation in its allostatic load, predominantly characterized by persistent inflammation, oxidative stress, and disturbances in glucose and lipid metabolism [33]. This occurrence arises from the unbalance gut microbiota resulting in allostatic load and exacerbates the progression of DKD [34, 35].
In patients with DKD, there is a coexistence of the systemic inflammatory state and impairment of innate immune function. DKD inflammation is induced by macrophages, Toll-like receptors, NLRP3, and nuclear factor-kappa B (NF-κB) [36, 37]. Furthermore, the progression of proteinuria in DKD is promoted by an increase in T or B cells [38]. The escalation of proinflammatory factors worsens the systemic inflammatory state and facilitates the progression of DKD. Simultaneously, the compromised immune system diminishes the body's defense capability and increases susceptibility to infection [39]. Infection ranks second as the leading cause of death in end-stage renal disease [40].
Scientific research has indicated that there is a significant reduction in probiotics that can supply energy for intestinal epithelial cells and secrete SCFAs, thus improving glucose metabolism [41–43]. Among these probiotics, Bacillus proteus has demonstrated the ability to enhance the host's defense mechanisms by inducing the biosynthesis of lipopolysaccharide (LPS) and maintaining LPS levels for immune homeostasis [44, 45]. For example, exposing germ-free mice to LPS improves abnormalities in the colonic mucosa [46]. When LPS is recognized, Toll-like receptor 4 (TLR4) activates MyD88, which promotes pathogen clearance and preserves the health of intestinal epithelial cells [47]. Mice without MyD88 have demonstrated increased susceptibility to gut damage [48]. In patients with DKD, there is a positive correlation between Lactobacillus reuteri and the urinary albumin-to-creatinine ratio [49]. Additionally, the composition of the gut microbiota in DKD patients exhibits alterations in the proportion of dominant flora and their metabolites under different conditions [50]. Furthermore, in patients with early-stage DKD, an increase in Proteobacteria has been observed, causing the activation of macrophages, exacerbating the chronic low-grade inflammatory state [51]. In patients with end-stage DKD, an imbalance in the ratio of Firmicutes and Bacteroides and a low level in SCFA production have been noted, potentially disrupting glucose metabolism [52, 53].
3. The Role of Gut Microbiota in the Development of Diabetic Nephropathy by Affecting the Innate Immune System
3.1. Composition of the Gut Microbiota in the Innate Immune System
The innate immune system protects the body, and intestinal epithelial cells play a key part by secreting mucins and antimicrobial proteins (AMPs). These cells work alongside gut neutrophils to establish a physical barrier, preventing the entry of harmful pathogens [54–57]. In a healthy colon, most of the resident cells receive supplementation from monocytes through a mechanism dependent on C-C chemokine receptor type 2. This mechanism is characterized by tolerance and a lack of response to TLR stimulation. However, when there is an imbalance in gut microbiota, the normal process of monocyte-macrophage maturation is disrupted. As a result, macrophages produce tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6) when exposed to TLR stimulation, ultimately leading to kidney inflammation [58]. Peptidoglycan is the component of the innate immune system and found in the bacterial cell walls. Peptidoglycan facilitates Streptococcus pneumoniae and Staphylococcus aureus and initiates the immune response [59]. Additionally, butyrate, a type of SCFAs, has the chance to decrease NF-κB signal transduction in DCs and neutrophils. Bile acids (BAs) also contribute to immune regulation by protecting the physical barriers and reducing inflammation through their effects on cytokine bioactivity.
3.2. Dysregulation of Gut Microbiota and Its Metabolites Affects Both Innate Immunity and the Role of Persistent Inflammation in the Pathogenesis of DKD
DCs and macrophages respond to stimulation with TLR ligands [60–63] and produce interleukin-10 (IL-10) under normal conditions [62, 63]. In the context of DKD, these immune cells attract and activate humoral immunity through TLR, initiating a rapid inflammatory response [64] that ultimately leads to kidney damage. Additionally, the integrity of the gut barrier becomes compromised, leading to the accumulation of toxins. These toxins activate immune system, which lead to persistent systemic inflammation. Hence, persistent inflammation caused by intestinal barrier damage can aggravate DKD.
Research has indicated that DCs and macrophages are capable of modulating the innate immune system by exerting TLR-mediated control [65]. In a study involving patients with microalbuminuria, glomerular TLR4 expression was significantly higher in the glomeruli and tubules [66]. Another study utilizing an animal model demonstrated that enhanced TLR2 expression in renal tubules and macrophages led to a proinflammatory environment and the occurrence of microalbuminuria [67, 68].
Patients with DKD exhibit changes in the composition of their gut microbiota, such as an increase in the abundance of Escherichia-Shigella bacteria that can breach the intestinal barrier [69]. This breach allows the gut microbiota to move into other areas of the body, triggering the activation of innate immune cells and the upregulation of TLR2 and TLR4-related pathways in the kidney. These immune responses result in the production of proinflammatory cytokines, leading to immune dysfunction, heightened infection susceptibility, and kidney damage specifically in DKD patients [67, 70–72]. Interestingly, nonpathogenic Salmonella bacteria have been found to counteract renal inflammation by inhibiting the NF-κB signaling pathway [73, 74]. Several studies have demonstrated that dysregulation of metabolic endotoxin/lipopolysaccharide levels in a rat model of DKD leads to activation of the NF-κB signaling pathway, increased levels of inflammatory cytokines in both the bloodstream and kidney, and activation of the innate immune system [75]. However, experiments using TLR4 gene deficiencies in vivo have shown that TLR4 receptors have the ability to detect and activate harmful and endogenous damage signals, subsequently causing kidney injury and fibrosis in DKD patients [36, 76–78].
Furthermore, the presence of mitochondrial antiviral signaling protein (MAVS) is crucial for maintaining gut integrity and innate immunity. In one study, the gut integrity of a DKD mouse model was impaired by MAVS gene knockout, resulting in the detection of gut-derived Klebsiella oxytoca, interleukin-17 (IL-17), and kidney injury molecule-1 (KIM-1) in the circulation and kidney. The inhibition of MAVS led to gut epithelial cell inflammation and consequent renal injury [79].
3.3. Dysregulation of Gut Microbiota and Its Metabolites Affects Both Innate Immunity and the Role of Glucose and Lipid Metabolism in the Pathogenesis of DKD
In DKD, the gut microbiome is disrupted, and the balance between good and bad bacteria is altered. This imbalance then results in the disruption of carbohydrate and lipid metabolism in the body [12, 17].
The first aspect is primarily composed of the presence of advanced glycation end products and their stimulation of the expression of transforming growth factor β. This leads to increased glycation of proteins, which causes damage to renal tubular epithelial cells and local kidney damage [10]. On the other hand, the second aspect is mainly seen through the occurrence of lipid peroxidation, inhibition of extracellular matrix degradation, infiltration of monocyte-macrophages in the kidney, and the induction of glomerulosclerosis and tubulointerstitial damage.
Increased carbonylation and glycation of proteins, one of the main manifestations of allostatic load [80], disrupts the structure and function of various proteins in DKD, leading to cell dysfunction and organ damage. Proinflammatory cytokines are glycosylated proteins with immunomodulatory functions [36].
Research has shown that TLRs and NLRP3 inflammasomes can produce proinflammatory factors to mediate sterile tubulointerstitial inflammatory response. The bradykinin-releasing enzyme-bradykinin system can activate bradykinin, and its receptors can lead to renal injury. The kidneys were protected, and proteinuria was reduced upon treatment with SCFAs. This effect is related with the ability of SCFAs to inhibit glycosylated proteins [81]. Furthermore, the enhanced expression of TLR2 contributes to the upregulation of chemokine MCP1/CCL2 in both glomeruli and tubular epithelium, potentially leading to impaired renal function in individuals with diabetes [82].
3.4. Dysregulation of Gut Microbiota and Its Metabolites Affects Both Innate Immunity and the Role of Oxidative Stress in the Pathogenesis of DKD
The occurrence of DKD is related to an asymmetry in the gut microbiota and disruptions in lipid metabolism, resulting in decreased connective protein expression [83]. Consequently, it causes heightened gut permeability and translocation, ultimately impacting DKD oxidative stress pathway via the innate immune system, thereby contributing to disease progression.
As proficient stimulators of neutrophils, SCFAs elicit the proliferation and migratory response of neutrophils, generating reactive oxygen species (ROS) to safeguard beneficial bacteria. Concurrently, SCFAs impede NF-κB and foster the expansion of T cells [84, 85]. Moreover, SCFAs have the ability to fortify the physical barrier via intestinal epithelial cells, thus thwarting gut microbiota leakage [83]. Absence of GPR43 in colitis mice led to reduced colonic neutrophil counts, in contrast to the wild-type colitis mice [86].
Toxic metabolites build up in the systemic circulation and trigger ROS through the NADPH pathway. This leads to the activation of the NF-κB and causes inflammation, resulting in proteinuria and damage to podocytes [87]. One specific factor contributing to oxidative stress is the NLRP3 inflammasome complex, which is driven by ROS in mitochondria and mitochondrial dysfunction. The activation of the NLRP3 inflammasome complex can stimulate interleukin-18 (IL-18), thereby exacerbating kidney injury in DKD [88, 89]. Inflammasome activation has been observed in podocytes and endothelial cells, in the context of hyperglycemia, obesity, and lipotoxicity [90–92] (Figure 1).
Figure 1.
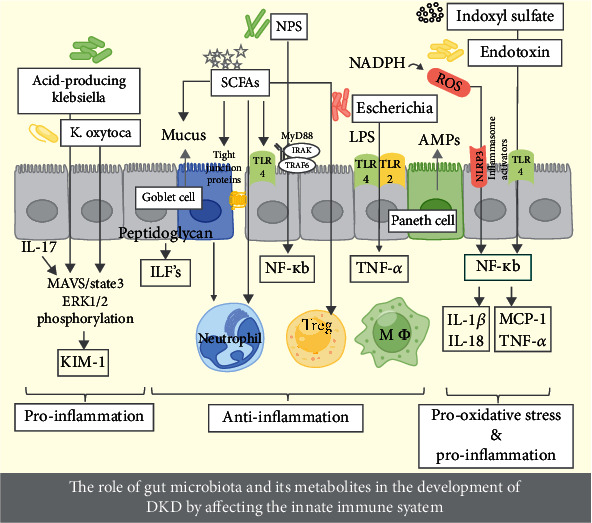
The role of gut microbiota and its metabolites in the development of DKD by affecting the innate immune system.
4. The Role of Gut Microbiota and Its Metabolites in the Development of Diabetic Nephropathy by Affecting the Adaptive Immune System
4.1. Composition of the Gut Microbiota in the Adaptive Immune System
The arrangement of gut microbiota components within the adaptive immune system primarily consists of B cells, T cells, and a majority of metabolites. The primary functions attributed to B cells involve their interaction with gut antigens [93] and the prevention of gut antigens from entering the bloodstream [94]. The equilibrium of gut immunity heavily relies on CD4+ T cells that primarily reside in the gut LP. These T cells can be classified as effector or helper CD4+ T cells, among which helper T cells can be further categorized into T-helper 1 (Th1), T-helper 17 (Th17) cells, and so forth [26]. Tregs can produce anti-inflammatory cytokines like IL-10, which aids in reducing MHC-II expression in monocyte-macrophages. Additionally, Tregs can directly impede the proliferation of proinflammatory factors and suppress the production of chemokines in DCs in an autocrine manner [95].
SCFAs inhibit inflammation through their interaction with free fatty acid receptors and GPR complex signal transduction [96–99]. Furthermore, the gut microbiota, including Lactobacillus, Clostridium, Bifidobacterium, and Enterococcus, plays a role in controlling bile acid synthesis [100] and regulating adaptive immune suppression and inflammation [101, 102]. By decomposing into indole and its derivatives, tryptophan helps maintain gut immune homeostasis by regulating adaptive immunity. The accumulation of a uremic toxin, trimethylamine n-oxide (TMAO), in the circulation is linked to renal injury [103]. Additionally, polyamines are essential in maintaining the epithelial barrier, promoting intestinal epithelial cell persistence, and inhibiting inflammation. Interestingly, the levels of phenyl sulfate (PS), a derivative of L-tyrosine in the gut, linked to the development of proteinuria in DKD.
4.2. Gut Microbiota and Its Metabolites Affect Both Adaptive Immunity and the Role of Persistent Inflammatory Response in Diabetic Nephropathy
Persistent inflammation in DKD is closely related to an imbalance in Th/Treg cells. Research indicates that the inflow rate of Tregs is associated with proteinuria, as well as glomerular and tubular damage, ultimately leading to fibrosis [104]. The gut microbiota, along with its metabolites, can directly impact adaptive immunity's T and B cells while indirectly influencing other immune cells, thereby contributing to the generation of persistent inflammatory responses [104].
The gut's Bacteroides fragilis plays a role in protecting the kidneys through its systemic induction of Th1 responses [105, 106]. DKD patients show increased concentration of interferon-g (IFN-g), which is linked to the production of interferon-γ (IFN-γ) and Th2, ultimately leading to the development of DKD [107–109]. Additionally, Bacteroides fragilis can enhance its colonization ability and increase IL-10 secretion by increasing the induction of FOXP3+ Tregs through octa capsular polysaccharide (PSA) [110–112]. This process helps regulate the biased Th/Treg levels.
Polysaccharide A of Bacteroides, which is known for its T cell-dependent properties and maintaining the gut barrier, was reduced in the population with CKD [113]. PSA restores the balance of Th1 and Th2 and inhibits renal inflammation by stimulating regulatory T cells to secrete IL-10. Additionally, Bacteroides play a role in the BAs and SCFAs, which have been shown to slow the advancement of DKD [114–116]. BAs, such as tauroursodeoxycholic acid (TUDCA), exert their effects on the immunity by increasing the bile acid receptor Gpbar1 (TGR5) and farnesoid X receptor (FXR), thereby delaying the progression of DKD [117].
Promoting Th17 cells and enhancing the defense ability of the intestinal mucosal surface, SFB has the potential to reduce infection risk and safeguard kidney health [118]. The gut serves as the entry point for Tregs, which activate to maintain a balance in effector cell activity by suppressing inflammation and promoting immune tolerance [119, 120]. Research suggests that DKD patients exhibit decreased proportions of Tregs in their peripheral blood. Furthermore, the transfer of Tregs in diabetic mice has been found to effectively ameliorate DKD [121]. Moreover, evidence indicates that the colonization of mice by SFB triggers Th cell [122]. Specifically, T cells equipped with SFB-specific antigen receptors differentiate into Th17 cells, which are responsible for the production of cytokines [123, 124].
In the gut lumen, B cells have the capability to secrete IgA (sIgA) [82, 125]. Alongside, the secretion of IgA is also facilitated by TGF-β and IL-10 [93]. The primary function of sIgA is to create a protective barrier for epithelial cells against pathogens and aid in maintaining gut immune homeostasis [94]. sIgA can control systemic adaptive T cell responses through immune rejection and maintain gut immune homeostasis against commensal bacteria [126, 127].
Gut immune responses are facilitated by gut macrophages [64, 127]. Recent research has demonstrated that mice with F4/80 gene knockout exhibit an inability to develop tolerance or reduce antigen-specific CD8+ Tregs and gut macrophages upon ingestion of soluble antigens [128]. Neutrophils play a role in decomposing bacteria and cellular fragments in the gut [129]. Gut DCs transport antigens from gut bacteria to the mesenteric lymph nodes (MLN) [130–132]. These DCs are generally less responsive under normal conditions, which helps maintain gut immune tolerance and diminish inflammation. They accomplish this by promoting the development of T cell that tolerate commensal bacteria and food antigens [60, 133]. Similarly, gut macrophages, like DCs, express low CD86, CD80, and CD40 levels [61, 134, 135]. Additionally, they increase IL-10, thereby reducing inflammation [62, 63].
Uremic toxins have a significant role in the progression of CKD [136]. The inhibition of HDAC activity by SCFAs has implications for different immune system cells [96]. Specifically, it can promote the differentiation of T cells into effector and contribute to the inflammatory response of DKD while also providing protection to the kidneys [137]. Tryptophan, another metabolite produced by gut microbiota, has various effects on gut immune cells and epithelial cells [138, 139]. It can produce inflammatory factors and hinder the development of Th17 cells [140]. The conversion of tryptophan precursors into ligands for the aryl hydrocarbon receptor (AHR) [141, 142] is facilitated by lactic acid bacteria and bifidobacteria [142]. These ligands then bind to the AHR, leading to the translocation of the receptor into the nucleus. Through this mechanism, the AHR regulates IL-22 and IL-17, resulting in functional changes and pathological alterations in diabetic mice [143–145]. Moreover, the activation of innate lymphoid cells (ILCs) and T cells, along with the production of IL-17 and IL-22, has been observed [138]. Of note is IL-22 against the colonization of pathogenic microbiota [146]. Research has shown that mice deficient in AHR or AHR ligands exhibit alterations in the composition of gut microbiota. AHR-deficient mice have lower levels of ILCs and IL-22 [147, 148]. These research indicate a relationship between AHR activity and gut microbiota metabolism in DKD [146, 149, 150]. Another investigation discovered that the production of compounds derived from indole by Lactobacillus reuteri might stimulate the conversion of CD4+ T cells within the gut epithelium into CD4+ CD8+ double-positive intraepithelial lymphocytes. This transformation is beneficial for maintaining the immune balance within the intestines [151]. The presence of polyamines can modify T cells by rising the expression of connexin and promoting the secretion of mucus, thus regulating the levels of inflammatory substances. Following the administration of PS, the foot processes vanished, podocytes suffered damage, the glomerular basement membrane thickened, and there was a rise in the mRNA levels of monocyte chemoattractant protein-1 (MCP-1) and fibronectin 1 (Fn1) in kidney tissue. In diabetic patients, the levels of inflammation and renal fibrosis were exacerbated [152].
4.3. Gut Microbiota and Its Metabolites Affect Both Adaptive Immunity and the Role of Glucose and Lipid Metabolism in the Pathogenesis of Diabetic Nephropathy
In hyperglycemia and hypercholesterolemia, DKD leads to an imbalance of gut microbiota, and it will further activate the adaptive immune system, resulting in renal inflammation [18]. Simultaneously, excessive lipid accumulation reduces SCFAs, inhibiting insulin sensitivity and causing renal injury [19].
Increased carbonylation and glycation of proteins, such as thrombin in the body, can activate receptors by activating proteases in kidney cells and recognize glycated proteins and/or glycated complement regulatory protein dysfunction through mannan-binding lectins, lead to activation of complement cascade [36], and promote renal inflammation and renal fibrosis in DKD. Meanwhile, the dysfunction of glycated albumin can cause renal injury and fibrosis by upregulating TGF-β [153].
4.4. Gut Microbiota and Its Metabolites Affect Both Adaptive Immunity and the Role of Oxidative Stress in the Pathogenesis of Diabetic Nephropathy
When renal function declines, toxic levels of TMAO [154–156] accumulate in the circulation due to the stimulation of glomerular and tubular damage by uremic toxins, promoting oxidative stress [103]. Mice were fed with TMAO or its precursor choline, resulting in renal tubular damage and renal fibrosis induction [157]. Additionally, TMAO has been shown to aggravate renal fibrosis by impacting the secretion of IL-1β and IL-18 through the inflammasome channel NLRP3 [15]. Sun et al. also discovered that 3,3-dimethyl-1-butanol prevented high-fat diet-induced renal fibrosis [158] (Figure 2).
Figure 2.
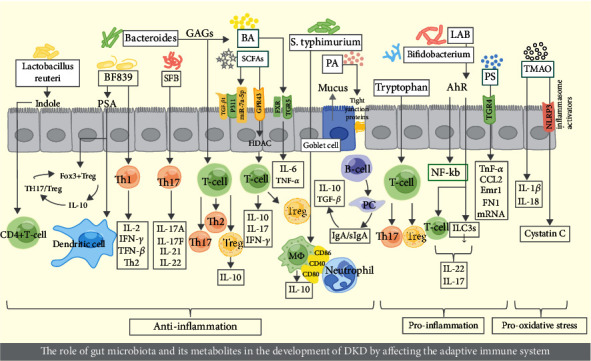
The role of gut microbiota and its metabolites in the development of DKD by affecting the adaptive immune system.
5. The Role of Gut Microecological Interventions in Retarding the Progression of DKD
DKD is impacted by disturbances in gut microbiota, which result in elevated levels of metabolites linked to gut microbial activity and compromised integrity of the gut barrier. Consequently, a sustained state of systemic inflammation arises, accompanied by weakened immune function. Hence, potential treatments for DKD are modulation of gut microbiota as well as improvement of the immune system (Figure 3).
Figure 3.
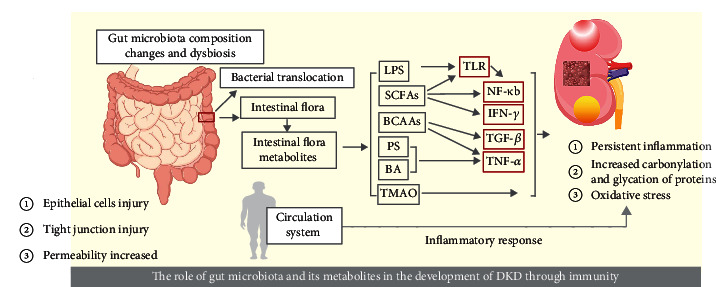
The role of gut microbiota and its metabolites in the development of DKD through immunity.
5.1. Dietary Interventions
Dietary interventions are the most effective influences among the exogenous factors affecting gut microbiota [159, 160]. According to existing studies, it is evident that the progress and onset of DKD can be altered through sensible dietary interventions due to the strong correlation between diabetes development and nutritional habits [161].
5.1.1. Dietary Fiber (DF)
DF primarily consists of nonstarch polysaccharides [162]. The inclusion of dietary fiber decelerates the renal dysfunctions through the release of SCFAs and the reduction of inflammation in DKD. Previous research [12] demonstrated that when resistant starch and high fiber diets supplemented with guar gum and cellulose were administered to diabetic mice, there was an expansion observed in SCFA-producing Prevotella and Bifidobacterium genera, resulting in elevated SCFA levels and a decrease in pathological Bifidobacterium and CD68+ cells. Moreover, mice in the RS group exhibited a decrease in urinary albumin/creatinine, albuminuria, and mRNA expression of inflammatory cytokines and fibrosis-related genes. Dietary fiber has a protective and ameliorative role in DKD by modulating gut microbiota's production of SCFAs and regulating crucial pathways associated with innate immunity, inflammation, and macrophage recruitment.
5.1.2. High Linoleic Acid (LA) Diet
LA is a typical polyunsaturated fatty acid (PUFA) in plant-derived oils, mainly derived from vegetable oils and nuts in the diet [163]. A diet high in linoleic acid is typical of the Mediterranean diet, and previous studies have suggested that in patients with DKD, adopting the Mediterranean diet could potentially present a lifestyle approach that effectively postpones kidney deterioration [164, 165]. A study conducted on 366 patients as part of a clinical trial revealed that an enhanced intake of polyunsaturated fatty acids had a notable association with a decreased preponderance of DKD [166]. Animal experiments also supported these findings, wherein rats induced with T2DM through STZ and NA were administered a polyunsaturated fatty acid-rich diet. The diet effectively reversed the rise in the ratio of Bacteroides to Firmicutes within gut tract and also decreased IL-6, IL-1β, TNF-α, and IL-17A [167]. This suggests that a diet rich in linoleic acid can potentially regulate intestinal flora, leading to a delay in the progression of DKD and proposing it as a significant dietary intervention for DKD patients.
5.1.3. Cereals
Cereals, which are extensively cultivated food crops across various regions globally, have demonstrated to alleviate inflammatory components [168]. It has been highlighted in a research investigation that cereals could potentially safeguard against high-intensity inflammation and morphological deviations in DKD [169]. This protection mechanism is achieved through the facilitation of SCFA release and the restoration of gut microbiota, consequently restraining the excessive expression of MCP-1 and TNF-α.
5.1.4. Astaxanthin (AST)
AST is a naturally occurring ketocarotenoid present in many microalgae. It exhibited numerous activities both in laboratory settings and in living organisms [170]. Studies have indicated that when mice with diabetes were exposed to a high-fat diet, either with 01.0% (AST) or 02.12% (AST) for a period of one week, the addition of AST effectively delayed the progression of kidney damage by inhibiting LPS, TMAO, and IL-1 and by modulating the NF-κB signaling pathway, in comparison to the DKD group. Furthermore, including AST as part of the diet leads to gut microbiota composition changes. This is supported by the decrease in bacteria like Coriobacteriaceae UCG-002 and the increase in probiotics Ruminococcaceae [171]. AST supplements might lead to ameliorate kidney injury associated with DKD by promoting a healthier intestinal flora and subsequently influencing immune factors.
5.2. Probiotics, Prebiotics, and Synbiotics (PPS)
Synbiotics include probiotics, live microorganisms beneficial to the host in specific quantities, including Lactobacillus and Bifidobacterium, and prebiotics, substrates selectively used by host microorganisms [172, 173].
Studies have shown that synbiotics slow the progression of kidney damage in DKD patients by correcting gut microbiota balance, modulating the host immune response, reducing proinflammatory factors, and improving inflammation [10, 174]. Dai et al. [175] showed that moutan cortex polysaccharide ameliorates (MC-Pa) increased Lactobacillus, increasing SCFAs and improving serum IL-6. It alleviated the structural abnormalities of tubules in DKD rats.
5.3. Metabolic Regulation
5.3.1. SCFAs
The effect of SCFAs on DKD has been studied, and it has been found that SCFAs can help regulate inflammation in DKD [176]. Researchers have shown that SCFA supplementation can alleviate renal inflammation in DKD by acting on the immune response process [177]. In particular, butyrate, a type of SCFA, has been found to have immunomodulatory effects [37]. It can also modulate the intestinal barrier and regulate insulin sensitivity. In a study, exogenous butyrate was found to significantly reduce NF-κB and MCP-1 and IL-1β, which are markers of inflammation, through a GPR43-β-arrestin-2 mechanism. This study also showed that butyrate restored inflammatory injury in the kidneys [178–180]. Another study applied sodium-butyrate to DKD mice and indicated that it reduced TGF-β, Fn, IL-6, and MCP-1, thus improving DKD by inhibiting renal inflammation [181, 182]. Furthermore, research has shown that butyrate supplementation can decrease kidney fibrosis by mediating the TGF-β1 pathway [183]. Overall, these findings suggest that exogenous SCFAs, particularly butyrate, have the potential to prevent and treat DKD by inhibiting renal inflammation and fibrosis gene expression.
5.3.2. BAs
Gut microbiota in the gut convert primary BAs into secondary BAs [184, 185]. This conversion process was demonstrated in a study, where tauroursodeoxycholic acid (TUDCA) had a beneficial effect on db/db and STZ-induced DKD mice. Specifically, TUDCA attenuated the expression of IL-6, TNF-α, and collagen 1 α 2. The mechanism of action involved targeting FXR or TGR5 pathways, leading to the improvement of glomerular and tubular injury [13]. Importantly, TGR5 has been found to decrease renal inflammation by inhibiting the NF-κB signaling [117]. This suggests that exogenous BAs could be therapeutic agents in DKD.
5.3.3. Branched-Chain Amino Acids (BCAAs)
BCAAs are composed of leucine, valine, and isoleucine [186]. It has been demonstrated that BCAAs possess the ability to hinder the expression of TGF-β in order to mitigate the damage caused by diabetic kidney injury [14]. A study recently found that administering high quantities of BCAAs prevented kidney weight in mice with DKD, additionally, moderate amounts of BCAAs led to a reduction in TGF-β mRNA and mitigated oxidative stress by activating the TGF-β pathway, thereby lessening the severity of DKD kidney injury. Furthermore, providing oral supplementation of BCAAs to individuals may also enhance their appetite and overall nutritional status [187].
5.3.4. TMAO
TMAO is a group of metabolites that form when trimethylamine (TMA) is oxidized through the metabolism of gut microbiota [188]. According to research, TMAO promotes inflammation in the kidneys, ultimately leading to interstitial dysfunction [189]. A study found that the intake of TMAO at 0.2% (w/v) resulted in higher kidney index and urinary protein in DKD rats induced by 35 mg/kg STZ compared to the DKD group without TMAO intake. Furthermore, the TMAO group also exhibited a significant increment IL-18 and IL-1β, which further intensified kidney inflammation [15]. Therefore, it can be inferred that elevated levels of TMAO may exacerbate DKD.
5.4. Fecal Microbial Transplantation (FMT)
FMT alters the gut microbial composition of patients with DKD by applying fecal solutions from healthy donors [190]. It has been shown [191] that FMT can slow down the process of kidney injury in DKD by rebuilding abnormal gut microbial ecology. It was shown that FMT leads to increase in Odoribacter spp. In the black and tan brachyury ob/ob mouse model of DKD, there is a decrease in urine albumin-creatinine ratio (UACR) and TNF-α and an improvement in the tendency of insulin resistance [192]. Cai et al. used resveratrol-treated db/db mice as FMT donors. They showed a reduction in the proportion of thick-walled Enterobacteria and Ferribacterium as well as an increase Proteobacteria and abundance of Odoribacter spp. and Bacteroides, as well as urinary albumin excretion rates (UAER), serum creatinine, and kidney inflammatory factor level reduction, suggesting that the reduction of inflammation is a key mechanism by which FMT protects renal function in DKD [193].
5.5. Natural Drugs
Extensive research findings indicate that the utilization of natural medications exhibits a positive impact on the management of DKD by restraining inflammation and oxidative stress. In addition, these drugs help modulate the gut microbiota and related metabolic processes [194, 195].
5.5.1. Chinese Herbal Monomers and Their Extracts
(1) Bupleurum Polysaccharides (BP). Bupleurum, a perennial herb from Umbelliferae, yields a polysaccharide known as BP. According to studies, BPs may act by interrupting high mobility group box-1 protein-TLR4 [196]. This disease suppresses renal inflammation and fibrosis processes [197]. In STZ-induced DKD mice, BP has the potential to reverse the reduction in Bacteroidetes abundance and the increase in Proteobacteria and Ferribacterium abundance. In addition, studies indicate that BP can lower TLR1 levels. In doing so, it reduces the inflammatory response, repairs the intestinal barrier to reduce LPS content, and ultimately addresses glomerular hypertrophy and glomerular hyperplasia. Moreover, BP also shows promise in reducing urinary albumin in diabetic mice [198]. Given these findings, BP holds the potential to enhance DKD by modulating the gut microbiota in the kidney.
(2) Cordyceps cicadae Polysaccharides (CCP). CCP, the primary active compound found in the parasitic medicinal fungus Cordyceps sinensis, has garnered attention for its therapeutic potential in the treatment of diabetes [199]. Multiple studies have underscored CCP treatment's ability to demonstrate hypoglycemic properties, thereby reducing tissue damage typically associated with diabetes. Furthermore, CCP administration has exhibited notable changes in the ratio of Firmicutes/Bacteroidetes, including increased copiousness of Odoribacter, Bacteroides, Alloprevotella, Mucispirillum, and Parabacteroides while the populations of Lactobacillus and Helicobacter have shown significant decrease [200]. In rats with streptozotocin-induced DKD, CCP supplementation has proven effective in mitigating renal fibrosis by upgrading the maturation of Bacteroidetes and Lactobacillus communities while reducing the presence of LPS-producing bacteria. These effects are attributed to CCP's regulatory impact on gut microbiota dysbiosis by inhibiting TLR4. In turn, this regulation leads to lowered serum engrossment of TNF-α, resulting in significant improvements in 24-hour urine volume and Scr levels. The positive impact of CCP on renal inflammation in DKD rats is further evidenced by the amelioration of collagen fiber accumulation in the glomerular mesangial area, lipid accumulation in kidney tissue samples, and the thickening and widening of the kidney tubular basement membrane [35].
(3) Cornus. Cornus, a deciduous perennial tree or shrub, exhibits antifibrotic effects in DKD by inhibiting TGF-β and hypoxia-inducible factor-1 (HIF-1) signaling pathways, as determined through network pharmacological analysis [201, 202]. The efficacy of Cornus in reducing glomeruli nodular sclerosis and kidney interstitial edema in STZ-induced DKD rats, along with its ability to decrease TGF-β and enhance abundance of gut lactobacilli to elevate SCFA content, suggests that it can be used to treat DKD by restoring gut microbiota's abundance, increasing SCFA levels, and diminishing inflammatory infiltration [203].
(4) Ginsenoside Compound K (CK). Ginsenoside, the primary extract derived from ginseng, which is a perennial plant belonging to the Wujia family, is a steroidal compound [204]. One of the major metabolites of ginseng is known as ginsenoside compound K. Research conducted in the past has illustrated the favorable impacts of CK on DKD, effectively alleviating glomerular injuries [205]. Moreover, it has been found that CK supplementation of a diet containing 0.03% dosage significantly reduces proteinuria, glomerular dilatation, glomerulosclerosis, and inflammatory infiltration. This reduction is achieved by diminishing the level of Bacteroides and increasing Lactobacillus levels. Furthermore, TGF-β1 expression in the kidney is reversed, effectively inhibiting NF-κB and subsequently decreasing IL-6 and IL-1β levels. Lastly, decreased serum imidazole propionate (IMP) will guide the downregulation of protein expression induced by IMP [206].
(5) Magnesium Lithospermate B (MLB). MLB is a constituent found in water extracts of Salvia miltiorrhiza, a perennial herb belonging to the Sage genus in the Labiatae family [207]. Experimental investigations indicate that MLB can mitigate kidney injury caused by STZ-induced DKD in mice by inflecting the composition of gut microbiota derived from it [208]. The oral administration of MLB effectively suppressed the unfreezed of inflammatory cells caused by BA and resulted in a decrease in urinary albumin levels over a 24-hour period in rats with STZ-induced DKD, thus slowing down the progression of kidney injury. Moreover, MLB intervention significantly reduced the abundance of Shigella and Aspergillus species, as well as the level of BAs in the feces of the rats [209].
(6) Resveratrol. Resveratrol is a class of polyphenolic compounds of distyrene, which are widely found in various Chinese herbs, such as Polygonum cuspidatum and mulberry, and is a natural therapeutic products for the treatment of T2DM [210]. Previous network pharmacological studies have depicted that resveratrol is an efficacious drug in DKD [211]. Research has demonstrated that administering resveratrol orally in mice can rectify the Firmicutes/Bacteroides ratio and diminish levels of inflammatory factors, serum creatinine, blood urea nitrogen, and urinary 24-hour microalbuminuria in db/db mice. [193]
5.5.2. Chinese Herbal Compound
(1) Qing-Re-Xiao-Zheng Formula (QRXZF). QRXZF is a traditional Chinese medicine (TCM) prescription. Gao et al. showed that QRXZF (2 g/ml) reversed the increases in UACR and improved thylakoid matrix expansion and tubulointerstitial injury in STZ-induced DKD mice, which may be related to the fact that QRXZF reversed the increase in Desulfovibrionaceae and Desulfovibrio in DKD mice, reduced gut-derived LPS in the blood, and inhibited inflammatory signaling pathway [212]. In their study, Shen et al. demonstrated the potential of Salvia miltiorrhiza and Astragalus membranaceus in enhancing DKD. The predominant bacteria involved in glycolipid metabolism were identified as Lactobacillus murinus and Akkermansia muciniphila [213].
(2) Shenyan Kangfu Tablet (SYKFT). SYKFT comprises a combination of thirteen Chinese herbal medicines, namely, Panax quinquefolius, Panax ginseng, Rehmannia glutinosa, Eucommia ulmoides, Dioscorea oppositifolia, Salvia miltiorrhiza, Leonurus artemisia, Smilax glabra, Oldenlandia diffusa, Glycine max, Imperata cylindrica, Alisma plantago-aquatica, and Platycodon grandiflorus. Chen et al.'s research indicates that SYKFT led to a reduction in TNF-α levels in the kidneys, along with an improvement in gut microbiota. Specifically, SYKFT increased the presence of Firmicutes while decreasing Bacteroidetes, resulting in the alleviation of kidney insufficiency and kidney inflammation in db/db model DKD mice [214]. CK, a component of Panax ginseng, reshaped the microbiota exhibiting potential in combating inflammation associated with DKD [206].
(3) Tangshen Formula (TSF). The TSF was obtained from a combination of 7 different herbs. The ratio of these herbs used in the extraction was 10 : 5 : 4 : 3.4 : 3 : 2 : 1 (W/W). Previous studies by Zhao et al. have designated that TSF can increase the presence of bifidobacteria, while also stamping the release of intestinal-derived inflammatory substances (IS) and LPS. In addition, TSF was found to suppress the TLR4/c-Jun N-terminal kinase (JNK) and NF-κB signaling pathways in the kidneys. This suppression resulted in a decrease in microalbuminuria and serum creatinine levels, as well as the inhibition of moderate expansion of the kidney thylakoid matrix, luminal dilatation, and tubular interstitium of rats with DKD induced by STZ and uninephrectomy [215]. Furthermore, a purified preparation of anthraquinone-glycosides derived from rhubarb, which are monomeric compounds found in rhubarb, has been shown to reduce inflammation in individuals with DM [216] (Table 1).
Table 1.
Experimental study of natural drugs on gut microbiota in DKD.
| Ingredient | Source | Moulding method | Effect on gut microbiota | Action mechanism | Related literature |
|---|---|---|---|---|---|
| Bupleurum polysaccharides (BP) | Bupleurum | STZ (1 mg/kg) | Reverse the decrease of Bacteroides abundance and the increase of Proteobacteria and Ferribacterium abundance. | Inhibit TLR1 levels, reduce TNF-α and IL-6 levels in the kidney, and improve intestinal barrier. | [189] |
| Cordyceps cicadae polysaccharides (CCP) | Cordyceps sinensis | STZ (0.1 mg/kg) | Increase the abundance of Lactobacillus and Bacteroidetes, decrease Proteobacteria and Deferribacteres. | Block TLR4/NF-κB and TGF-β1 signaling pathways, decrease the concentrations of serum TNF-α, IL-1β, and IL-6. | [35] |
| Cornus | Cornus | STZ (35 mg/kg) | Increase the abundance of gut lactobacilli and increase SCFA content. | Reduce inflammatory infiltration. | [190] |
| Ginsenoside compound K | Ginseng | db/db mice | Decrease the level of Bacteroides and increase the level of Lactobacillus. | Reverse the upregulation of TGF-β1 expression, inhibit NF-κB, decrease the expression of IL-6 and IL-1β, and downregulate the expression of the IMP-induced TLR4 signaling pathway. | [191] |
| Magnesium lithospermate B (MLB) | Salvia miltiorrhiza | STZ (40 mg/kg) | Reduce the abundance of Shigella and Aspergillus species. | Regulate BA metabolism, restore intestinal barrier integrity, and inhibit inflammatory cell release. | [192] |
| Resveratrol | Polygonum cuspidatum, mulberry | db/db mice | Restore the proportion of Firmicutes/Bacteroides. | Reduce the kidney mRNA levels of TNF-α, IFN-γ, IL-6, and IL-1β. | [217] |
6. Summary and Prospects
The correlation between DKD and the microbiota residing in the gastrointestinal tract, along with its metabolic byproducts and their interaction with the innate and adaptive immune systems, has progressively been elucidated. This is closely related to the allostatic load and immune imbalance caused by gut microbiota translocation and dysregulation between gut microbiota and DKD. However, current research still needs to clarify the role of certain bacterial strains such as Bacteroidetes, Firmicutes, Fusobacteriota, and Actinobacteriota, especially the role of the abundance of Bacteroides and Firmicutes in DKD inflammation and intestinal barrier permeability. In addition, natural drugs have significant advantages in treating DKD, and existing studies lack the immune mechanism between herbal compounds and gut microbiota, which will become a future research direction for DKD prevention and treatment.
Acknowledgments
This study was supported by the National Natural Youth Science Foundation of China (No. 82004272), National Natural Science Foundation of China (Grant No. 82174342), and Chinese Medicine Inheritance and Innovation Talent Project-Leading Talent Support Program of National Traditional Chinese Medicine (Grant No. 2018 and No. 12).
Contributor Information
Yao Xian Wang, Email: wyx3203@sina.com.
Wei Jing Liu, Email: liuweijing-1977@hotmail.com.
Additional Points
Highlight. The connection between the allostatic load of DKD and gut microecology is robust, involving immunity. By influencing the immune system, the regulation of gut microbiota has the potential to enhance DKD renal inflammation, fibrotic damage, and proteinuria levels. In the treatment of DKD, targeted interventions utilizing herbal monomers and combinations, alongside interactions involving the host immune system and gut microbiota, play a crucial role.
Conflicts of Interest
The scientists confirm that the investigation occurred devoid of any business or monetary affiliations that may potentially be interpreted as a clash of interests. Yi Zhen Han, Hui Juan Zheng, Bo Xuan Du, Yi Zhang, Xing Yu Zhu, Jing Li, Yao Xian Wang, and Wei Jing Liu hereby affirm that they possess no conflicting interests.
Authors' Contributions
The topic was conceived by LW, WY, and HY. Literature inquiry was carried out by DB, ZH, HY, and ZY. Data was collected and the manuscript was drafted by DB, HY, LJ, ZH, and ZY. Manuscript review was conducted by HY and ZH. LJ and ZY took charge of all revisions and the review of the revised manuscript. This article is the joint work of all authors, and the version provided has been approved by them. Yi Zhen Han, Hui Juan Zheng, and Bo Xuan Du contributed equally to this work.
References
- 1.Afkarian M., Zelnick L. R., Hall Y. N., et al. Clinical manifestations of kidney disease among US adults with diabetes, 1988-2014. Journal of the American Medical Association . 2016;316(6):602–610. doi: 10.1001/jama.2016.10924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chan J. C., Zhang Y., Ning G. Diabetes in China: a societal solution for a personal challenge. The Lancet Diabetes & Endocrinology . 2014;2:969–1048. doi: 10.1016/S2213-8587(14)70144-5. [DOI] [PubMed] [Google Scholar]
- 3.Sawaf H., Thomas G., Taliercio J. J., Nakhoul G., Vachharajani T. J., Mehdi A. Therapeutic advances in diabetic nephropathy. Journal of Clinical Medicine . 2022;11(2):p. 378. doi: 10.3390/jcm11020378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pillai A., Fulmali D. A narrative review of new treatment options for diabetic nephropathy. Cureus. . 2023;15(1, article e33235) doi: 10.7759/cureus.33235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dave C. V., Schneeweiss S., Patorno E. Comparative risk of genital infections associated with sodium-glucose co-transporter-2 inhibitors. Diabetes, Obesity and Metabolism . 2019;21:434–438. doi: 10.1111/dom.13531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kohan D. E., Fioretto P., Tang W., List J. F. Long-term study of patients with type 2 diabetes and moderate renal impairment shows that dapagliflozin reduces weight and blood pressure but does not improve glycemic control. Kidney International . 2014;85(4):962–971. doi: 10.1038/ki.2013.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang L., Long J., Jiang W., et al. Trends in chronic kidney disease in China. The New England Journal of Medicine . 2016;375(9):905–906. doi: 10.1056/NEJMc1602469. [DOI] [PubMed] [Google Scholar]
- 8.Kim M., Huda M. N., Bennett B. J. Sequence meets function-microbiota and cardiovascular disease. Cardiovascular Research . 2022;118(2):399–412. doi: 10.1093/cvr/cvab030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ron S., Shai F., Ron M. Revised estimates for the number of human and bacteria cells in the body. PLoS Biology . 2016;14(8, article e1002533) doi: 10.1371/journal.pbio.1002533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meijers B. K., Evenepoel P. The gut-kidney axis: indoxyl sulfate, p-cresyl sulfate and CKD progression. Nephrology, Dialysis, Transplantation . 2011;26(3):759–761. doi: 10.1093/ndt/gfq818. [DOI] [PubMed] [Google Scholar]
- 11.Sato J., Kanazawa A., Ikeda F., et al. Gut dysbiosis and detection of "live gut bacteria" in blood of Japanese patients with type 2 diabetes. Diabetes Care . 2014;3(8):2343–2393. doi: 10.2337/dc13-2817. [DOI] [PubMed] [Google Scholar]
- 12.Li Y. J., Chen X., Kwan T. K., et al. Dietary fiber protects against diabetic nephropathy through short-chain fatty acid-mediated activation of G protein-coupled receptors GPR43 and GPR109A. Journal of the American Society of Nephrology . 2020;31(6):1267–1281. doi: 10.1681/ASN.2019101029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marquardt A., Ghosh S., Kohli S., et al. Farnesoid X receptor agonism protects against diabetic tubulopathy: potential add-on therapy for diabetic nephropathy. Journal of the American Society of Nephrology: JASN . 2017;28(11):3182–3189. doi: 10.1681/ASN.2016101123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang X., Liu D., He Y., Lou K., Zheng D., Han W. Branched chain amino acids protects rat mesangial cells from high glucose by modulating TGF-β1 and BMP-7. Diabetes, Metabolic Syndrome and Obesity . 2019;12:2433–2440. doi: 10.2147/DMSO.S221642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fang Q., Zheng B., Liu N., et al. Trimethylamine N-oxide exacerbates renal inflammation and fibrosis in rats with diabetic kidney disease. Frontiers in Physiology . 2021;12, article 682482 doi: 10.3389/fphys.2021.682482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fang Q., Liu N., Zheng B., et al. Roles of gut microbial metabolites in diabetic kidney disease. Frontiers in Endocrinology . 2021;12, article 636175 doi: 10.3389/fendo.2021.636175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reidy K., Kang H. M., Hostetter T., Susztak K. Molecular mechanisms of diabetic kidney disease. The Journal of Clinical Investigation . 2014;124:2333–2373. doi: 10.1172/JCI72271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bonner R., Albajrami O., Hudspeth J. Ashish Upadhyay Diabetic kidney disease. Primary Care: Clinics in Office Practice. . 2020;47(4):645–659. doi: 10.1016/j.pop.2020.08.004. [DOI] [PubMed] [Google Scholar]
- 19.Lin Y. C., Chang Y. H., Yang S. Y., Wu K. D., Chu T. S. Update of pathophysiology and management of diabetic kidney disease. Journal of the Formosan Medical Association. . 2018;117(8):662–675. doi: 10.1016/j.jfma.2018.02.007. [DOI] [PubMed] [Google Scholar]
- 20.Jha J. C., Banal C., Chow B. S. M., Cooper M. E., Jandeleit-Dahm K. Diabetes and kidney disease: role of oxidative stress. Antioxidants & Redox Signaling . 2016;25(12):657–684. doi: 10.1089/ars.2016.6664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kato M., Natarajan R. Epigenetics and epigenomics in diabetic kidney disease and metabolic memory. Nature Reviews. Nephrology . 2019;15(6):327–345. doi: 10.1038/s41581-019-0135-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singh A., Mittal M. Neonatal microbiome-a brief review. The Journal of Maternal-Fetal & Neonatal Medicine . 2020;33:3841–3848. doi: 10.1080/14767058.2019.1583738. [DOI] [PubMed] [Google Scholar]
- 23.Tremaroli V., Bäckhed F. Functional interactions between the gut microbiota and host metabolism. Nature . 2012;489(7415):242–249. doi: 10.1038/nature11552. [DOI] [PubMed] [Google Scholar]
- 24.de Vos W. M., Tilg H., Van Hul M. Cani PD Gut microbiome and health: mechanistic insights. Gut . 2022;71:1020–1032. doi: 10.1136/gutjnl-2021-326789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patterson E., Ryan P. M., Cryan J. F., et al. Gut microbiota, obesity and diabetes. Postgraduate Medical Journal . 2016;92(1087):286–300. doi: 10.1136/postgradmedj-2015-133285. [DOI] [PubMed] [Google Scholar]
- 26.Wu H. J., Wu E. The role of gut microbiota in immune homeostasis and autoimmunity. Gut Microbes . 2012;3:4–14. doi: 10.4161/gmic.19320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sanidad K. Z., Amir M., Ananthanarayanan A., et al. Maternal gut microbiome-induced IgG regulates neonatal gut microbiome and immunity. Science Immunology . 2022;7(72, article eabh3816) doi: 10.1126/sciimmunol.abh3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nagano Y., Itoh K., Honda K. The induction of Treg cells by gut-indigenous Clostridium. Current Opinion in Immunology . 2012;24:392–399. doi: 10.1016/j.coi.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 29.Steinman R. Dendritic cells: understanding immunogenicity. European Journal of Immunology . 2007;37(Supplement 1):S53–S60. doi: 10.1002/eji.200737400. [DOI] [PubMed] [Google Scholar]
- 30.Steinman R. M. Decisions about dendritic cells: past, present, and future. Annual Review of Immunology . 2012;30(1):1–22. doi: 10.1146/annurev-immunol-100311-102839. [DOI] [PubMed] [Google Scholar]
- 31.Platt A. M., Mowat A. M. Mucosal macrophages and the regulation of immune responses in the intestine. Immunology Letters . 2018;119:22–31. doi: 10.1016/j.imlet.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 32.Hadis U., Wahl B., Schulz O., et al. Intestinal tolerance requires gut homing and expansion of FoxP3+ regulatory T cells in the lamina propria. Immunity . 2011;34(2):237–246. doi: 10.1016/j.immuni.2011.01.016. [DOI] [PubMed] [Google Scholar]
- 33.Guidi J., Lucente M., Sonino N., et al. Allostatic load and its impact on health: a systematic review. Psychotherapy and Psychosomatics . 2020;90:11–27. doi: 10.1159/000510696. [DOI] [PubMed] [Google Scholar]
- 34.Zhang Z., Li Q., Liu F., Wang D. Lycoperoside H protects against diabetic nephropathy via alteration of gut microbiota and inflammation. Journal of Biochemical and Molecular Toxicology . 2022;36(12, article e23216) doi: 10.1002/jbt.23216. Epub 2022 Sep 26. [DOI] [PubMed] [Google Scholar]
- 35.Yang J., Dong H., Wang Y., et al. Cordyceps cicadae polysaccharides ameliorated renal interstitial fibrosis in diabetic nephropathy rats by repressing inflammation and modulating gut microbiota dysbiosis. International Journal of Biological Macromolecules . 2020;163:442–456. doi: 10.1016/j.ijbiomac.2020.06.153. [DOI] [PubMed] [Google Scholar]
- 36.Tang S. C. W., Yiu W. H. Innate immunity in diabetic kidney disease. Nature Reviews Nephrology . 2020;16:206–222. doi: 10.1038/s41581-019-0234-4. [DOI] [PubMed] [Google Scholar]
- 37.Pérez-Morales R. E., Del Pino M. D., Valdivielso J. M., Ortiz A., Mora-Fernández C., Navarro-González J. F. Inflammation in diabetic kidney disease. Nephron . 2019;143(1):12–16. doi: 10.1159/000493278. [DOI] [PubMed] [Google Scholar]
- 38.Kong L., Andrikopoulos S., MacIsaac R. J., et al. Ekinci EI Role of the adaptive immune system in diabetic kidney disease. Journal of Diabetes Investigation . 2022;13:213–226. doi: 10.1111/jdi.13725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Robinson T. W., Freedman B. I. Glycated albumin and blood sugar control in advanced chronic kidney disease. Nephrology, Dialysis, Transplantation . 2018;33(7):1087–1090. doi: 10.1093/ndt/gfy059. [DOI] [PubMed] [Google Scholar]
- 40.Dalrymple L. S., Johansen K. L., Chertow G. M., Cheng S. C., Grimes B., Gold E. B. Kaysen GA Infection-related hospitalizations in older patients with ESRD. American Journal of Kidney Diseases . 2010;56:522–552. doi: 10.1053/j.ajkd.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Candela M., Biagi E., Soverini M., et al. Pianesi M Modulation of gut microbiota dysbioses in type 2 diabetic patients by macrobiotic Ma-Pi 2 diet. British Journal of Nutrition . 2016;116:80–93. doi: 10.1017/S0007114516001045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ma Q., Li Y., Wang J., et al. Investigation of gut microbiome changes in type 1 diabetic mellitus rats based on high-throughput sequencing. Biomedicine & Pharmacotherapy . 2020;124, article 109873 doi: 10.1016/j.biopha.2020.109873. [DOI] [PubMed] [Google Scholar]
- 43.Song Y., Wu M. S., Tao G., Lu M. W., Lin J. Huang JQ Feruloylated oligosaccharides and ferulic acid alter gut microbiome to alleviate diabetic syndrome. Food Research International . 2020;137, article 109410 doi: 10.1016/j.foodres.2020.109410. [DOI] [PubMed] [Google Scholar]
- 44.Koh A., Molinaro A., Ståhlman M., et al. Bergh PO Microbially produced imidazole propionate impairs insulin signaling through mTORC1. Cell . 2018;175:947–961. doi: 10.1016/j.cell.2018.09.055. [DOI] [PubMed] [Google Scholar]
- 45.Alexander C., Rietschel E. T. Bacterial lipopolysaccharides and innate immunity. Journal of Endotoxin Research . 2001;7(3):167–202. doi: 10.1179/096805101101532675. [DOI] [PubMed] [Google Scholar]
- 46.Petersson J., Schreiber O., Hansson G. C., et al. Importance and regulation of the colonic mucus barrier in a mouse model of colitis. American Journal of Physiology-Gastrointestinal and Liver Physiology . 2011;300(2):G327–G333. doi: 10.1152/ajpgi.00422.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gibson D. L., Ma C., Bergstrom K. S., Huang J. T., Man C., Vallance B. A. MyD88 signalling plays a critical role in host defence by controlling pathogen burden and promoting epithelial cell homeostasis during Citrobacter rodentium-induced colitis. Cellular Microbiology . 2008;10(3):618–631. doi: 10.1111/j.1462-5822.2007.01071.x. [DOI] [PubMed] [Google Scholar]
- 48.Rakoff-nahoum S., Paglino J., Eslami-varzaneh F., et al. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell . 2004;118(2):229–241. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 49.Hirayama A., Nakashima E., Sugimoto M., et al. Metabolic profiling reveals new serum biomarkers for differentiating diabetic nephropathy. Analytical and Bioanalytical Chemistry . 2012;404(10):3101–3109. doi: 10.1007/s00216-012-6412-x. Epub 2012 Sep 29. [DOI] [PubMed] [Google Scholar]
- 50.Devaraj S., Hemarajata P., Versalovic J. The human gut m-icrobiome and body metabolism: implications for obesity and diabetes. Clinical Chemistry . 2013;59:617–645. doi: 10.1373/clinchem.2012.187617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.He X., Sun J., Liu C., et al. Z Wang Compositional alterations of gut microbiota in patients with diabetic kidney disease and type 2 diabetes mellitus. Diabetes, Metabolic Syndrome and Obesity . 2022;15:755–765. doi: 10.2147/DMSO.S347805.eCollection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen R., Zhu D., Yang R., et al. K Hou Gut microbiota diversity in middle-aged and elderly patients with end-stage diabetic kidney disease. Annals of Translational Medicine . 2022;10:p. 750. doi: 10.21037/atm-22-2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li L., Lili R., Hui Q., et al. Furong L Combination of GLP-1 and sodium butyrate promote differentiation of pancreatic progenitor cells into insulin-producing cells. Tissue Cell . 2008;40:437–445. doi: 10.1016/j.tice.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 54.Peterson L. W., Artis D. Intestinal epithelial cells: regulators of barrier function and immune homeostasis. Immunology . 2014;14(3):141–153. doi: 10.1038/nri3608. [DOI] [PubMed] [Google Scholar]
- 55.Kim Y. S., Ho S. B. Intestinal goblet cells and mucins in health and disease: recent insights and progress. Current Gastroenterology Reports . 2010;12(5):319–330. doi: 10.1007/s11894-010-0131-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Meyer-Hoffert U., Hornef M. W., Henriques-Normark B., et al. Secreted enteric antimicrobial activity localises to the mucus surface layer. Gut . 2008;57(6):764–771. doi: 10.1136/gut.2007.141481. [DOI] [PubMed] [Google Scholar]
- 57.Hokari R., Miura S. Neutrophil elastase in colitis: more than a marker of disease activity? Journal of Gastroenterology . 2006;41(4):395–396. doi: 10.1007/s00535-006-1808-z. [DOI] [PubMed] [Google Scholar]
- 58.Yang J., Lim S. Y., Ko Y. S., et al. Intestinal barrier disruption and dysregulated mucosal immunity contribute to kidney fibrosis in chronic kidney disease. Nephrology, Dialysis, Transplantation . 2019;34(3):419–428. doi: 10.1093/ndt/gfy172. [DOI] [PubMed] [Google Scholar]
- 59.Clarke T. B., Davis K. M., Lysenko E. S., Zhou A. Y., Yu Y. JN Weiser Recognition of peptidoglycan from the microbiota by nod1 enhances systemic innate immunity. Nature Medicine . 2010;16(2):228–231. doi: 10.1038/nm.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chirdo F. G., Millington O. R., Beacock-Sharp H., Mowat A. . M. I. Immunomodulatory dendritic cells in intestinal lamina propria. European Journal of Immunology . 2005;35(6):1831–1840. doi: 10.1002/eji.200425882. [DOI] [PubMed] [Google Scholar]
- 61.Smith P. D., Smythies L. E., Shen R., Greenwell-Wild T., Gliozzi M., Wahl S. M. Intestinal macrophages and response to microbial encroachment. Mucosal Immunology . 2011;4:31–42. doi: 10.1038/mi.2010.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Denning T. L., Wang Y. C., Patel S. R., Williams I. R., Pulendran B. Lamina propria macrophages and dendritic cells differentially induce regulatory and interleukin 17-producing T cell responses. Nature Immunology . 2007;8(10):1086–1094. doi: 10.1038/ni1511. [DOI] [PubMed] [Google Scholar]
- 63.Platt A. M., Bain C. C., Bordon Y. DP Sester, AMI Mowat An independent subset of TLR expressing CCR2-dependent macrophages promotes colonic inflammation. The Journal of Immunology . 2010;184:6843–6854. doi: 10.4049/jimmunol.0903987. [DOI] [PubMed] [Google Scholar]
- 64.Chow F., Ozols E., Nikolic-Paterson D. J., Atkins R. C., Tesch G. H. Macrophages in mouse type 2 diabetic nephropathy: correlation with diabetic state and progressive renal injury. Kidney International . 2004;65(1):116–128. doi: 10.1111/j.1523-1755.2004.00367.x. [DOI] [PubMed] [Google Scholar]
- 65.Rizzello V., Bonaccorsi I., Dongarra M. L., Fink L. N., Ferlazzo G. Role of natural killer and dendritic cell crosstalk in immunomodulation by commensal bacteria probiotics. BioMed Research International . 2011;2011:10. doi: 10.1155/2011/473097.473097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Verzola D., Cappuccino L., D'Amato E., et al. Enhanced glomerular Toll-like receptor 4 expression and signaling inpatients with type 2 diabetic nephropathy and microalbuminuria. Kidney International . 2014;86:1229–1272. doi: 10.1038/ki.2014.116. [DOI] [PubMed] [Google Scholar]
- 67.Mudaliar H., Pollock C., Komala M. G., Chadban S., Wu H., Panchapakesan U. The role of Toll-like receptor proteins (TLR) 2 and 4 in mediating inflammation in proximal tubules. American Journal of Physiology-Renal Physiology . 2013;305(2):F143–F154. doi: 10.1152/ajprenal.00398.2012. [DOI] [PubMed] [Google Scholar]
- 68.Devaraj S., Tobias P., Kasinath B. S. R Ramsamooj, A Afify, I Jialal Knockout of toll-like receptor-2 attenuates both the proinflammatory state of diabetes and incipient diabetic nephropathy. Arteriosclerosis, Thrombosis, and Vascular Biology . 2011;31:1796–1804. doi: 10.1161/ATVBAHA.111.228924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Croxen M. A., Law R. J., Scholz R., Keeney K. M., Wlodarska M., Finlay B. B. Recent advances in understanding enteric pathogenic Escherichia coli. Clinical Microbiology Reviews . 2013;26(4):822–880. doi: 10.1128/CMR.00022-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lin M., Yiu W. H., Wu H. J., et al. SCW Tang Toll-like receptor 4 promotes tubular inflammation in diabetic nephropathy. Journal of the American Society of Nephrology: JASN . 2012;23:86–102. doi: 10.1681/ASN.2010111210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kaur H., Chien A., Jialal I. Hyperglycemia induces Toll like receptor 4 expression and activity in mouse mesangial cells: relevance to diabetic nephropathy. American Journal of Physiology-Renal Physiology . 2012;303:F1145–F1150. doi: 10.1152/ajprenal.00319.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Donaldson G. P., Lee S. M., Mazmanian S. K. Gut biogeography of the bacterial microbiota. Nature Reviews Microbiology . 2016;14:20–32. doi: 10.1038/nrmicro3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Collier-Hyams L. S., Sloane V., Batten B. C., Neish A. S. Cutting edge: bacterial modulation of epithelial signaling via changes in neddylation of cullin-1. Journal of Immunology . 2005;175(7):4194–4198. doi: 10.4049/jimmunol.175.7.4194. [DOI] [PubMed] [Google Scholar]
- 74.Kelly D., Campbell J. I., King T. P., et al. Commensal anaerobic gut bacteria attenuate inflammation by regulating nuclear-cytoplasmic shuttling of PPAR-γ and RelA. Nature Immunology . 2004;5(1):104–112. doi: 10.1038/ni1018. [DOI] [PubMed] [Google Scholar]
- 75.Zhao T., Zhang H., Yin X., et al. Tangshen formula modulates gut microbiota and reduces gut-derived toxins in diabetic nephropathy rats. Biomedicine & Pharmacotherapy . 2020;129, article 110325 doi: 10.1016/j.biopha.2020.110325. [DOI] [PubMed] [Google Scholar]
- 76.Jialal I., Major A. M., Devaraj S. Global Toll-like receptor 4 knockout results in decreased renal inflammation, fibrosis and podocytopathy. Journal of Diabetes and its Complications . 2014;28:755–816. doi: 10.1016/j.jdiacomp.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 77.Kuwabara T., Mori K., Mukoyama M., et al. Exacerbation of diabetic nephropathy by hyperlipidaemia is mediated by Toll-like receptor 4 in mice. Diabetologia . 2012;55(8):2256–2266. doi: 10.1007/s00125-012-2578-1. [DOI] [PubMed] [Google Scholar]
- 78.Cha J. J., Hyun Y. Y., Lee M. H., et al. Renal protective effects of toll-like receptor 4 signaling blockade in type 2 diabetic mice. Endocrinology . 2013;154(6):2144–2155. doi: 10.1210/en.2012-2080. [DOI] [PubMed] [Google Scholar]
- 79.Linh H. T., Iwata Y., Senda Y., et al. Intestinal bacterial translocation contributes to diabetic kidney disease. Journal of the American Society of Nephrology . 2022;33:1105–1119. doi: 10.1681/ASN.2021060843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li G., Liu L., Hu H., et al. Age-related carbonyl stress and erythrocyte membrane protein carbonylation. Clinical Hemorheology and Microcirculation . 2010;46(4):305–311. doi: 10.3233/CH-2010-1355. [DOI] [PubMed] [Google Scholar]
- 81.Yu R. C., Bo H., Villani V. PJ Spencer, P Fu The inhibitory effect of rapamycin on toll like receptor 4 and interleukin 17 in the early stage of rat diabetic nephropathy. Kidney and Blood Pressure Research . 2016;41:55–69. doi: 10.1159/000368547. [DOI] [PubMed] [Google Scholar]
- 82.de Melo T. R., de Souza K. S. C., Ururahy M. A. G., et al. Toll-like receptor inflammatory cascade and the development of diabetic kidney disease in children and adolescents with type 1 diabetes. Journal of Paediatrics and Child Health . 2022;58(6):996–1000. doi: 10.1111/jpc.15884. [DOI] [PubMed] [Google Scholar]
- 83.Chi M., Ma K., Wang J., et al. C Liu The immunomodulatory effect of the gut microbiota in kidney disease. Journal of Immunology Research . 2021;2021:15. doi: 10.1155/2021/5516035.5516035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Brown E. M., Kenny D. J., Xavier R. J. Gut microbiota regulation of t cells during inflammation and autoimmunity. Annual Review of Immunology . 2019;37(1):599–624. doi: 10.1146/annurev-immunol-042718-041841. [DOI] [PubMed] [Google Scholar]
- 85.Rooks M. G., Garrett W. S. Gut microbiota, metabolites and host immunity. Nature Reviews. Immunology . 2016;16(6):341–352. doi: 10.1038/nri.2016.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fachi J. L., Sécca C., Rodrigues P. B., et al. Acetate coordinates neutrophil and ILC3 responses against C. difficile through FFAR2. Journal of Experimental Medicine . 2020;217, article 20190489 doi: 10.1084/jem.2019048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kikuchi K., Saigusa D., Kanemitsu Y., et al. Gut microbiome-derived phenyl sulfate contributes to albuminuria in diabetic kidney disease. Nature Communications . 2019;10(1):p. 1835. doi: 10.1038/s41467-019-09735-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Swanson K. V., Deng M., Ting J. P. The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nature Reviews. Immunology . 2019;19(8):477–489. doi: 10.1038/s41577-019-0165-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Galvan D. L., Green N. H., Danesh F. R. The hallmarks of mitochondrial dysfunction in chronic kidney disease. Kidney International . 2017;92(5):1051–1057. doi: 10.1016/j.kint.2017.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fang L., Xie D., Wu X., Cao H., Su W., Yang J. Involvement of endoplasmic reticulum stress in albuminuria induced inflammasome activation in renal proximal tubular cells. PLoS One . 2013;8, article e72344 doi: 10.1371/journal.pone.0072344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Vilaysane A., Chun J., Seamone M. E., et al. The NLRP3 inflammasome promotes renal inflammation and contributes to CKD. Journal of the American Society of Nephrology . 2010;21(10):1732–1744. doi: 10.1681/ASN.2010020143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rampanelli E., Orso E., Ochodnicky P., et al. Metabolic injury-induced NLRP3 inflammasome activation dampens phospholipid degradation. Scientific Reports . 2017;7(1):p. 2861. doi: 10.1038/s41598-017-01994-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mantis N. J., Forbes S. J. Secretory IgA: arresting microbial pathogens at epithelial borders. Immunological Investigations . 2010;39(4-5):383–406. doi: 10.3109/08820131003622635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pabst O. New concepts in the generation and functions of IgA. Nature Reviews Immunology . 2012;12:821–853. doi: 10.1038/nri3322. [DOI] [PubMed] [Google Scholar]
- 95.Kn C. iL-10: the master regulator of immunity to infection. Journal of Immunology . 2008;180(9):5771–5777. doi: 10.4049/jimmunol.180.9.5771. [DOI] [PubMed] [Google Scholar]
- 96.Yuille S., Reichardt N., Panda S., Dunbar H., Mulder I. E. Human gut bacteria as potent class I histone deacetylase inhibitors in vitro through production of butyric acid and valeric acid. PLoS ONE . 2018;13(7, article e0201073) doi: 10.1371/journal.pone.0201073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Licciardi P. V., Ververis K., Karagiannis T. C. Histone deacetylase inhibition and dietary short-chain fatty acids. ISRN Allergy . 2011;2011:8. doi: 10.5402/2011/869647.869647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Baxter N. T., Schmidt A. W., Venkataraman A., Kim K. S., Waldron C., Schmidt T. M. Dynamics of human gut microbiota and short-chain fatty acids in response to dietary interventions with three fermentable fibers. mBio . 2019;10(1, article e02566) doi: 10.1128/mBio.02566-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nogal A., Valdes A. M., Menni C. The role of short-chain fatty acids in the interplay between gut microbiota and diet in cardio-metabolic health. Gut Microbes . 2021;13(1, article 1897212) doi: 10.1080/19490976.2021.1897212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tian Y., Gui W., Koo I., et al. The microbiome modulating activity of bile acids. Gut Microbes . 2020;11(4):979–996. doi: 10.1080/19490976.2020.1732268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.McGlone E. R., Bloom S. R. Bile acids and the metabolic syndrome. Annals of Clinical Biochemistry . 2019;56:326–337. doi: 10.1177/0004563218817798. [DOI] [PubMed] [Google Scholar]
- 102.Shao J. W., Ge T. T., Chen S. Z., et al. Role of bile acids in liver diseases mediated by the gut microbiome. World Journal of Gastroenterology . 2021;27(22):3010–3021. doi: 10.3748/wjg.v27.i22.3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Evenepoe P., Poesen R., Meijers B. The gut-kidney axis. Pediatric Nephrology . 2017;32(11):2005–2014. doi: 10.1007/s00467-016-3527-x. [DOI] [PubMed] [Google Scholar]
- 104.Chow F. Y., Nikolic-Paterson D. J., Ma F. Y., Ozols E., Rollins B. J., Tesch G. H. Monocyte chemoattractant protein-1-induced tissue inflammation is critical for the development of renal injury but not type 2 diabetes in obese db/db mice. Diabetologia . 2007;50(2):471–480. doi: 10.1007/s00125-006-0497-8. [DOI] [PubMed] [Google Scholar]
- 105.Mazmanian S. K., Round J. L., Kasper D. L. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature . 2008;453(7195):620–625. doi: 10.1038/nature07008. [DOI] [PubMed] [Google Scholar]
- 106.Mazmanian S. K., Liu C. H., Tzianabos A. O., Kasper D. L. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell . 2005;122(1):107–118. doi: 10.1016/j.cell.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 107.Mezzano S., Aros C., Droguett A., et al. NF-kappaB activation and overexpression of regulated genes in human diabetic nephropathy. Nephrology Dialysis Transplantation . 2004;19(10):2505–2512. doi: 10.1093/ndt/gfh207. [DOI] [PubMed] [Google Scholar]
- 108.Imani F., Horii Y., Suthanthiran M., et al. Advanced glycosylation endproduct-specific receptors on human and rat T-lymphocytes mediate synthesis of interferon gamma: role in tissue remodeling. The Journal of Experimental Medicine . 1993;178(6):2165–2172. doi: 10.1084/jem.178.6.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhang C., Xiao C., Wang P., et al. The alteration of Th1/Th2/Th17/Treg paradigm in patients with type 2 diabetes mellitus: relationship with diabetic nephropathy. Human Immunology . 2014;75(4):289–296. doi: 10.1016/j.humimm.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 110.Telesford K. M., Yan W., Ochoa-Reparaz J., et al. A commensal symbiotic factor derived from Bacteroides fragilis promotes human CD39(+)Foxp3(+) T cells and Treg function. Gut Microbes . 2015;6:234–276. doi: 10.1080/19490976.2015.1056973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Round J. L., Lee S. M., Li J., et al. The Toll-like receptor 2 pathway establishes Colonization by a commensal of the Human Microbiota. Science . 2011;332(6032):974–977. doi: 10.1126/science.1206095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Round J. L., Mazmanian S. K. Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proceedings of the National Academy of Sciences . 2010;107(27):12204–12213. doi: 10.1073/pnas.0909122107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wang X. F., Yang S. T., Li S. H., et al. Aberrant gut microbiota alters host metabolome and impacts renal failure in humans and rodents. Gut . 2020;69(12):2131–2142. doi: 10.1136/gutjnl-2019-319766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Dupraz L., Magniez A., Rolhion N., et al. Gut microbiota-derived short-chain fatty acids regulate IL-17 production by mouse and human intestinal Γδ T cells. Cell Reports . 2021;36(1, article 109332) doi: 10.1016/j.celrep.2021.109332. [DOI] [PubMed] [Google Scholar]
- 115.Luu M., Visekruna A. Short-chain fatty acids: bacterial messengers modulating the immunometabolism of T cells. European Journal of Immunology . 2019;49:842–848. doi: 10.1002/eji.201848009. [DOI] [PubMed] [Google Scholar]
- 116.Du Y., Yang Y. T., Tang G., Jia J. S., Zhu N., Yuan W. J. Butyrate alleviates diabetic kidney disease by mediating the mi R-7a-5p/P311/TGF-β1 pathway. The FASEB Journal . 2020;34:10462–10475. doi: 10.1096/fj.202000431R. [DOI] [PubMed] [Google Scholar]
- 117.Xiao H., Sun X., Liu R., et al. Gentiopicroside activates the bile acid receptor Gpbar1 (TGR5) to repress NF-kappaB pathway and ameliorate diabetic nephropathy. Pharmacological Research . 2020;151, article 104559 doi: 10.1016/j.phrs.2019.104559. [DOI] [PubMed] [Google Scholar]
- 118.Ivanov I. I., Atarashi K., Manel N., et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell . 2009;139:485–583. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sakaguchi S., Wing K., Onishi Y., Prieto-Martin P., Yamaguchi T. Regulatory T cells: how do they suppress immune responses? International Immunology . 2009;21:1105–1116. doi: 10.1093/intimm/dxp095. [DOI] [PubMed] [Google Scholar]
- 120.Chen W. J., Jin W. W., Hardegen N., et al. Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-β induction of transcription factor Foxp3. The Journal of Experimental Medicine . 2003;198(12):1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Xu J., Su H. L., Wang J. H., Zhang C. H. Role of CD4+CD25+Foxp3+ regulatory T cells in type 2 diabetic nephropathy. Nan Fang Yi Ke Da Xue Xue Bao . 2009;29(1):137–139. [PubMed] [Google Scholar]
- 122.Gaboriau-Routhiau V., Rakotobe S., Lecuyer E., et al. The key role of segmented filamentous bacteria in the coordinated maturation of gut helper T cell responses. Immunity . 2009;31:677–766. doi: 10.1016/j.immuni.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 123.Atarashi K., Tanoue T., Ando M., et al. Th17 cell induction by adhesion of microbes to intestinal epithelial cells. Cell . 2015;163(2):367–380. doi: 10.1016/j.cell.2015.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yang Y., Torchinsky M. B., Gobert M., et al. Focused specificity of intestinal Th17 cells towards commensal bacterial antigens. Nature . 2014;510(7503):152–156. doi: 10.1038/nature13279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Cerutti A. The regulation of IgA class switching. Nature Reviews. Immunology . 2008;8(6):421–434. doi: 10.1038/nri2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Peterson D. A., McNulty N. P., Guruge J. L., Gordon J. I. IgA response to symbiotic bacteria as a mediator of gut homeostasis. Cell Host & Microbe . 2007;2(5):328–339. doi: 10.1016/j.chom.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 127.Cong Y., Feng T., Fujihashi K., Schoeb T. R. CO Elson A dominant, coordinated T regulatory cell-IgA response to the intestinal microbiota. Proceedings of the National Academy of Sciences . 2009;106:19256–19317. doi: 10.1073/pnas.0812681106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lin H. H., Faunce D. E., Stacey M., et al. The macrophage F4/80 receptor is required for the induction of antigen-specific efferent regulatory T cells in peripheral tolerance. Journal of Experimental Medicine . 2005;201(10):1615–1625. doi: 10.1084/jem.20042307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Nguyen D., Ping F., Mu W., Hill P., Atkins R. C., Chadban S. J. Macrophage accumulation in human progressive diabetic nephropathy. Nephrology (Carlton) . 2006;11(3):226–231. doi: 10.1111/j.1440-1797.2006.00576.x. [DOI] [PubMed] [Google Scholar]
- 130.Voedisch S., Koenecke C., David S., et al. Mesenteric lymph nodes confine dendritic cell-mediated dissemination of Salmonella enterica serovar Typhimurium and limit systemic disease in mice. Infection and Immunity . 2009;77(8):3170–3180. doi: 10.1128/IAI.00272-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Macpherson A. J., Uhr T. Induction of protective IgA by intestinal dendritic cells carrying commensal bacteria. Science . 2004;303(5664):1662–1665. doi: 10.1126/science.1091334. [DOI] [PubMed] [Google Scholar]
- 132.Worbs T., Bode U., Yan S., et al. Oral tolerance originates in the intestinal immune system and relies on antigen carriage by dendritic cells. Journal of Experimental Medicine . 2006;203(3):519–527. doi: 10.1084/jem.20052016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Coombes J. L., Maloy K. J. Control of intestinal homeostasis by regulatory T cells and dendritic cells. Seminars in Immunology . 2007;19:116–142. doi: 10.1016/j.smim.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 134.Rogler G., Hausmann M., Vogl D., et al. Isolation and phenotypic characterization of colonic macrophages. Clinical and Experimental Immunology . 2001;112(2):205–215. doi: 10.1046/j.1365-2249.1998.00557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Uematsu S., Jang M. H., Chevrier N., et al. Detection of pathogenic intestinal bacteria by Toll-like receptor 5 on intestinal CD11c+ lamina propria cells. Nature Immunology . 2006;7(8):868–874. doi: 10.1038/ni1362. [DOI] [PubMed] [Google Scholar]
- 136.Gonzalez-Gonzalez C., Gibson T., Jauregi P. Novel probiotic-fermented milk with angiotensin I-converting enzyme inhibitory peptides produced by Bifidobacterium bifidum MF20/5. International Journal of Food Microbiology . 2013;167:131–138. doi: 10.1016/j.ijfoodmicro.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 137.Rysz J., Franczyk B., Ławiński J., Olszewski R., Ciałkowska-Rysz A., Gluba-Brzózka A. The impact of CKD on uremic toxins and gut microbiota. Toxins . 2021;13(4):p. 252. doi: 10.3390/toxins13040252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Gryp T., De Paepe K., Vanholder R., et al. Gut microbiota generation of protein-bound uremic toxins and related metabolites is not altered at different stages of chronic kidney disease. Kidney . 2020;97(6):1230–1242. doi: 10.1016/j.kint.2020.01.028. [DOI] [PubMed] [Google Scholar]
- 139.Lee J. H., Lee J. Indole as an intercellular signal in microbial communities. FEMS Microbiology Reviews . 2010;34(4):426–444. doi: 10.1111/j.1574-6976.2009.00204.x. [DOI] [PubMed] [Google Scholar]
- 140.Singh N. P., Singh U. P., Rouse M., et al. Dietary indoles suppress delayed-type hypersensitivity by inducing a switch from proinflammatory Th17 cells to anti-inflammatory regulatory t cells through regulation of microRNA. The Journal of Immunology . 2016;196:1108–1130. doi: 10.4049/jimmunol.1501727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Agus A., Planchais J., Sokol H. Gut microbiota regulation of tryptophan metabolism in health and disease. Cell Host & Microbe . 2018;23(6):716–724. doi: 10.1016/j.chom.2018.05.003. [DOI] [PubMed] [Google Scholar]
- 142.Yano J. M., Yu K., Donaldson G. P., et al. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell . 2015;161(2):264–276. doi: 10.1016/j.cell.2015.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lamas B., Natividad J. M., Sokol H. Aryl hydrocarbon receptor and intestinal immunity. Mucosal Immunology . 2018;11:1024–1038. doi: 10.1038/s41385-018-0019-2. [DOI] [PubMed] [Google Scholar]
- 144.Koper J. E. B., Loonen L. M. P., Wells J. M., Troise A. D., Capuano E., Fogliano V. Polyphenols and tryptophan metabolites activate the aryl hydrocarbon receptor in an in vitro model of colonic fermentation. Molecular Nutrition & Food Research . 2019;63, article e1800722 doi: 10.1002/mnfr.201800722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lee W. J., Liu S. H., Chiang C. K., et al. Aryl hydrocarbon receptor deficiency attenuates oxidative stress-related mesangial cell activation and macrophage infiltration and extracellular matrix accumulation in diabetic nephropathy. Antioxidants & Redox Signaling . 2016;24:217–231. doi: 10.1089/ars.2015.6310. [DOI] [PubMed] [Google Scholar]
- 146.Zelante T., Iannitti R. G., Cunha C., et al. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity . 2013;39(2):372–457. doi: 10.1016/j.immuni.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 147.Kiss E. A., Vonarbourg C., Kopfmann S., et al. Natural aryl hydrocarbon receptor ligands control organogenesis of intestinal lymphoid follicles. Science . 2011;334:1561–1566. doi: 10.1126/science.1214914. [DOI] [PubMed] [Google Scholar]
- 148.Schiering C., Wincent E., Metidji A., et al. Feedback control of AHR signalling regulates intestinal immunity. Nature . 2017;542(7640):242–245. doi: 10.1038/nature21080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Yang W., Yu T., Huang X., et al. Intestinal microbiota-derived short-chain fatty acids regulation of immune cell IL-22 production and gut immunity. Nature communications . 2020;11(1):p. 4457. doi: 10.1038/s41467-020-18262-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Liu J. R., Miao H., Deng D. Q., Vaziri N. D., Li P., Zhao Y. Y. Gut microbiota-derived tryptophan metabolism mediates renal fibrosis by aryl hydrocarbon receptor signaling activation. Cellular and Molecular Life Sciences . 2021;78:909–922. doi: 10.1007/s00018-020-03645-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Cervantes-Barragan L., Chai J. N., Tianero M. D., et al. Lactobacillus reuteriinduces gut intraepithelial CD4+CD8αα+T cells. Science . 2017;357(6353):806–810. doi: 10.1126/science.aah5825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Tuleta I., Frangogiannis N. G. Diabetic fibrosis. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease . 2021;1867(4):p. 166044. doi: 10.1016/j.bbadis.2020.166044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Verzola D., Milanesi S., Viazzi F., et al. Enhanced myostatin expression and signalling promote tubulointerstitial inflammation in diabetic nephropathy. Scientific Reports . 2020;10(1):p. 6343. doi: 10.1038/s41598-020-62875-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Liabeuf S., Cheddani L., Massy Z. A. Uremic toxins and clinical outcomes: the impact of kidney transplantation. Toxins . 2018;10(6):p. 229. doi: 10.3390/toxins10060229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Missailidis C., Hällqvist J., Qureshi A. R., et al. Serum trimethylamine-N-oxide is strongly related to renal function and predicts outcome in chronic kidney disease. PLoS One . 2016;11(1, article e0141738) doi: 10.1371/journal.pone.0141738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Xu K. Y., Xia G. H., Lu J. Q., et al. Impaired renal function and dysbiosis of gut microbiota contribute to increased trimethylamine-N-oxide in chronic kidney disease patients. Scientific Reports . 2017;7(1):p. 1445. doi: 10.1038/s41598-017-01387-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Tang W. H. W., Wang Z., Kennedy D. J., et al. Gut microbiota-dependent trimethylamine N-oxide (TMAO) pathway contributes to both development of renal insufficiency and mortality risk in chronic kidney disease. Circulation Research . 2015;116(3):448–455. doi: 10.1161/CIRCRESAHA.116.305360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Sun G., Yin Z., Liu N., et al. Gut microbial metabolite TMAO contributes to renal dysfunction in a mouse model of dietinduced obesity. Biochemical and Biophysical Research Communications . 2017;493(2):964–970. doi: 10.1016/j.bbrc.2017.09.108. [DOI] [PubMed] [Google Scholar]
- 159.Kolodziejczyk A. A., Zheng D., Elinav E. Diet-microbiota interactions and personalized nutrition. Nature Reviews Microbiology . 2019;17:742–753. doi: 10.1038/s41579-019-0256-8. [DOI] [PubMed] [Google Scholar]
- 160.Doshi S. M., Friedman A. N. Diagnosis and management of type 2 diabetic kidney disease. Clinical Journal of the American Society of Nephrology . 2017;12:1366–1373. doi: 10.2215/CJN.11111016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Liu F., Shan S., Li H., Li Z. Treatment of peroxidase derived from foxtail millet bran attenuates atherosclerosis by inhibition of CD36 and STAT3 in vitro and in vivo. Journal of Agricultural and Food Chemistry . 2020;68(5):1276–1285. doi: 10.1021/acs.jafc.9b06963. [DOI] [PubMed] [Google Scholar]
- 162.Hill C., Guarner F., Reid G., et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Gastroenterology & Hepatology . 2014;11(8):506–514. doi: 10.1038/nrgastro.2014.66. [DOI] [PubMed] [Google Scholar]
- 163.Wang Q., Wang X. The effects of a low linoleic acid/α-linolenic acid ratio on lipid metabolism and endogenous fatty acid distribution in obese mice. International Journal of Molecular Sciences . 2023;24(15, article 12117) doi: 10.3390/ijms241512117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Castro-Barquero S., Larroya M., Crispi F., et al. Diet quality and nutrient density in pregnant women according to adherence to Mediterranean diet. Frontiers in Public Health . 2023;11, article 1144942 doi: 10.3389/fpubh.2023.1144942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Alkhatib L., Velez Diaz L. A., Varma S., et al. Lifestyle modifications and nutritional and therapeutic interventions in delaying the progression of chronic kidney disease: a review. Cureus . 2023;15(2, article e34572) doi: 10.7759/cureus.34572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Dos Santos A. L. T., Duarte C. K., Santos M., et al. Low linolenic and linoleic acid consumption are associated with chronic kidney disease in patients with type 2 diabetes. PLoS One . 2018;13(8, article e0195249) doi: 10.1371/journal.pone.0195249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Zhu L., Sha L., Li K., et al. Dietary flaxseed oil rich in omega-3 suppresses severity of type 2 diabetes mellitus via anti-inflammation and modulating gut microbiota in rats. Lipids in Health and Disease . 2020;19(1):p. 20. doi: 10.1186/s12944-019-1167-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Stephen A. M., Champ M. M. J., Cloran S. J., et al. Dietary fibre in Europe: current state of knowledge on definitions, sources, recommendations, intakes and relationships to health. Nutrition Research Reviews . 2017;30:149–190. doi: 10.1017/S095442241700004X. [DOI] [PubMed] [Google Scholar]
- 169.Liu X., Qiu B., Liu W., et al. The preventive effects of fermented and germinated foxtail millet whole grain on kidney damage in a diabetic mouse model. Frontiers in Nutrition . 2022;9, article 940404 doi: 10.3389/fnut.2022.940404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Ng Q. X., De Deyn M. L. Z. Q., Loke W., Foo N. X., Chan H. W., Yeo W. S. Effects of astaxanthin supplementation on skin health: a systematic review of clinical studies. Journal of Dietary Supplements . 2021;18(2):169–182. doi: 10.1080/19390211.2020.1739187. [DOI] [PubMed] [Google Scholar]
- 171.Ha M., Yang Y., Wu M., Gong T., Chen Z., Yu L. Astaxanthin could regulate the gut-kidney axis to mitigate kidney injury in high-fat diet/streptozotocin-induced diabetic mice. International Journal for Vitamin and Nutrition Research . 2023;2023:p. 22. doi: 10.1024/0300-9831/a000786. [DOI] [PubMed] [Google Scholar]
- 172.Gibson G. R., Hutkins R., Sanders M. E., et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nature reviews Gastroenterology & Hepatology . 2017;14(8):491–502. doi: 10.1038/nrgastro.2017.75. [DOI] [PubMed] [Google Scholar]
- 173.Koppe L., Mafra D., Fouque D. Probiotics and chronic kidney disease. Kidney International . 2015;88(5):958–1024. doi: 10.1038/ki.2015.255. [DOI] [PubMed] [Google Scholar]
- 174.Du L., Li Q., Yi H., Kuang T., Tang Y., Fan G. Gut microbiota-derived metabolites as key actors in type 2 diabetes mellitus. Biomedicine & Pharmacotherapy . 2022;149, article 112839 doi: 10.1016/j.biopha.2022.112839. [DOI] [PubMed] [Google Scholar]
- 175.Dai Y., Quan J., Xiong L., Luo Y., Yi B. Probiotics improve renal function, glucose, lipids, inflammation and oxidative stress in diabetic kidney disease: a systematic review and meta-analysis. Renal Failure . 2022;44(1):862–880. doi: 10.1080/0886022X.2022.2079522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Zhang M., Yang L., Zhu M., et al. Moutan cortex polysaccharide ameliorates diabetic kidney disease via modulating gut microbiota dynamically in rats. International Journal of Biological Macromolecules . 2022;206:849–860. doi: 10.1016/j.ijbiomac.2022.03.077. [DOI] [PubMed] [Google Scholar]
- 177.Bergman E. N. Energy contributions of volatile fatty acids from the gastrointestinal tract in various species. Physiological Reviews . 1990;70(2):567–590. doi: 10.1152/physrev.1990.70.2.567. [DOI] [PubMed] [Google Scholar]
- 178.Chang P. V., Hao L., Offermanns S., Medzhitov R. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proceedings of the National Academy of Sciences of the United States of America . 2014;111(6):2247–2252. doi: 10.1073/pnas.1322269111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Gonzalez A., Krieg R., Massey H. D., et al. Sodium butyrate ameliorates insulin resistance and renal failure in CKD rats by modulating intestinal permeability and mucin expression. Nephrology, Dialysis, Transplantation . 2019;34(5):783–794. doi: 10.1093/ndt/gfy238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Gao Z., Yin J., Zhang J., et al. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes . 2009;58:1509–1526. doi: 10.2337/db08-1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Huang W., Man Y., Gao C., et al. Short-chain fatty acids ameliorate diabetic nephropathy via GPR43-mediated inhibition of oxidative stress and NF-κB signaling. Oxidative Medicine and Cellular Longevity . 2020;2020:21. doi: 10.1155/2020/4074832.4074832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Zhou T., Huiwen X., Cheng X., et al. Wei Huang Sodium butyrate attenuates diabetic kidney disease partially via histone butyrylation modification. Mediators of Inflammation . 2022;2022:16. doi: 10.1155/2022/7643322.7643322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Tang M. J., Wang Y. K., Lin H. H. Butyrate and TGF-β downregulate Na,K-ATPase expression in cultured proximal tubule cells. Biochemical and Biophysical Research Communications . 1995;215(1):57–66. doi: 10.1006/bbrc.1995.2433. [DOI] [PubMed] [Google Scholar]
- 184.Ridlon J. M., Kang D. J., Hylemon P. B. Bile salt biotransformations by human intestinal bacteria. Journal of Lipid Research . 2006;47:241–300. doi: 10.1194/jlr.R500013-JLR200. [DOI] [PubMed] [Google Scholar]
- 185.Winston J. A., Theriot C. M. Diversification of host bile acids by members of the gut microbiota. Gut Microbes . 2020;11:158–171. doi: 10.1080/19490976.2019.1674124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.White P. J., Newgard C. B. Branched-chain amino acids in disease. Science . 2019;363(6427):582–583. doi: 10.1126/science.aav0558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Cano N. J., Fouque D., Leverve X. M. Application of branched-chain amino acids in human pathological states: renal failure. The Journal of Nutrition . 2006;136(1):299S–307S. doi: 10.1093/jn/136.1.299S. [DOI] [PubMed] [Google Scholar]
- 188.Janeiro M. H., Ramírez M. J., Milagro F. I., Martínez J., Solas M. Implication of trimethylamine N-oxide (TMAO) in disease: potential biomarker or new therapeutic target. Nutrients . 2018;10(10):p. 1398. doi: 10.3390/nu10101398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Sun G., Yin Z., Liu N., et al. Gut microbial metabolite TMAO contributes to renal dysfunction in a mouse model of diet-induced obesity. Biochemical and Biophysical Research Communications . 2017;493(2):964–970. doi: 10.1016/j.bbrc.2017.09.108. [DOI] [PubMed] [Google Scholar]
- 190.Napolitano M., Covasa M. Microbiota transplant in the treatment of obesity and diabetes: current and future perspectives. Frontiers in Microbiology . 2020;11, article 590370 doi: 10.3389/fmicb.2020.590370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Luo L., Luo J., Cai Y., et al. Inulin-type fructans change the gut microbiota and prevent the development of diabetic nephropathy. Pharmacological Research . 2022;183, article 106367 doi: 10.1016/j.phrs.2022.106367. [DOI] [PubMed] [Google Scholar]
- 192.Bastos R. M. C., Simplício-Filho A., Sávio-Silva C., et al. Fecal microbiota transplant in a pre-clinical model of type 2 diabetes mellitus, obesity and diabetic kidney disease. International Journal of Molecular Sciences . 2022;23(7):p. 3842. doi: 10.3390/ijms23073842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Cai T. T., Ye X. L., Li R. R., et al. Resveratrol modulates the gut microbiota and inflammation to protect against diabetic nephropathy in mice. Frontiers in Pharmacology . 2020;11:p. 1249. doi: 10.3389/fphar.2020.01249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Chen D. Q., Hu H. H., Wang Y. N., Feng Y. L., Cao G., Zhao Y. Y. Natural products for the prevention and treatment of kidney disease. Phytomedicine . 2018;50:50–60. doi: 10.1016/j.phymed.2018.09.182. [DOI] [PubMed] [Google Scholar]
- 195.Chen D. Q., Wu J., Li P. Therapeutic mechanism and clinical application of Chinese herbal medicine against diabetic kidney disease. Frontiers in Pharmacology . 2022;13, article 1055296 doi: 10.3389/fphar.2022.1055296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Yu X., Miao Z., Zhang L., Zhu L., Sheng H. Extraction, purification, structure characteristics, biological activities and pharmaceutical application of Bupleuri radix polysaccharide: a review. International Journal of Biological Macromolecules . 2023;237, article 124146 doi: 10.1016/j.ijbiomac.2023.124146. [DOI] [PubMed] [Google Scholar]
- 197.Liu Z. Z., Weng H. B., Zhang L. J., et al. Bupleurum polysaccharides ameliorated renal injury in diabetic mice associated with suppression of HMGB1-TLR4 signaling. Chinese Journal of Natural Medicines . 2019;17(9):641–649. doi: 10.1016/S1875-5364(19)30078-0. [DOI] [PubMed] [Google Scholar]
- 198.Feng Y., Weng H., Ling L., et al. Modulating the gut microbiota and inflammation is involved in the effect of Bupleurum polysaccharides against diabetic nephropathy in mice. International Journal of Biological Macromolecules . 2019;132:1001–1011. doi: 10.1016/j.ijbiomac.2019.03.242. [DOI] [PubMed] [Google Scholar]
- 199.Wang Y., Zeng T., Li H., Wang Y., Wang J., Yuan H. Structural characterization and hypoglycemic function of polysaccharides from Cordyceps cicadae. Molecules . 2023;28(2):p. 526. doi: 10.3390/molecules28020526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Wang Y., Ni Z., Li J., et al. Cordyceps cicadae polysaccharides alleviate hyperglycemia by regulating gut microbiota and its mmetabolites in high-fat diet/streptozocin-induced diabetic mice. Frontiers in Nutrition . 2023;10:p. 1203430. doi: 10.3389/fnut.2023.1203430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Huang J., Zhang Y., Dong L., et al. Ethnopharmacology, phytochemistry, and pharmacology of Cornus officinalis Sieb. et Zucc. Journal of Ethnopharmacology . 2018;213:280–301. doi: 10.1016/j.jep.2017.11.010. [DOI] [PubMed] [Google Scholar]
- 202.Zhang Y., Jin D., Zhou R., et al. Mechanism of Cornus Officinalis in treating diabetic kidney disease based on network pharmacology. Evidence-based Complementary and Alternative Medicine . 2022;2022:14. doi: 10.1155/2022/1799106.1799106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Ju C. G., Zhu L., Wang W., Gao H., Xu Y. B., Jia T. Z. Cornus officinalis prior and post-processing: regulatory effects on intestinal flora of diabetic nephropathy rats. Frontiers in Pharmacology . 2022;13:p. 1039711. doi: 10.3389/fphar.2022.1039711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Karunasagara S., Hong G. L., Park S. R., et al. Korean red ginseng attenuates hyperglycemia-induced renal inflammation and fibrosis via accelerated autophagy and protects against diabetic kidney disease. Journal of Ethnopharmacology . 2020;254:p. 112693. doi: 10.1016/j.jep.2020.112693. [DOI] [PubMed] [Google Scholar]
- 205.He J. Y., Hong Q., Chen B. X., et al. Ginsenoside Rb1 alleviates diabetic kidney podocyte injury by inhibiting aldose reductase activity. Acta Pharmacologica Sinica . 2022;43(2):342–353. doi: 10.1038/s41401-021-00788-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Chen Q., Ren D., Liu L., et al. Ginsenoside compound K ameliorates development of diabetic kidney disease through inhibiting TLR4 activation induced by microbially produced imidazole propionate. International Journal of Molecular Sciences . 2022;23(21):p. 12863. doi: 10.3390/ijms232112863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Li L. Biologically active components from traditional Chinese medicines. Pure and Applied Chemistry . 1998;70(3):547–554. doi: 10.1351/pac199870030547. [DOI] [Google Scholar]
- 208.Zhu N., Duan H., Feng Y., et al. Magnesium lithospermate B ameliorates diabetic nephropathy by suppressing the uremic toxin formation mediated by gut microbiota. European Journal of Pharmacology . 2023;953:p. 175812. doi: 10.1016/j.ejphar.2023.175812. [DOI] [PubMed] [Google Scholar]
- 209.Zhao J., Zhang Q. L., Shen J. H., Wang K., Liu J. Magnesium lithospermate B improves the gut microbiome and bile acid metabolic profiles in a mouse model of diabetic nephropathy. Acta Pharmacologica Sinica . 2019;40(4):507–513. doi: 10.1038/s41401-018-0029-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Huang D. D., Shi G., Jiang Y., Yao C., Zhu C. A review on the potential of resveratrol in prevention and therapy of diabetes and diabetic complications. Biomedicine & Pharmacotherapy . 2020;125:p. 109767. doi: 10.1016/j.biopha.2019.109767. [DOI] [PubMed] [Google Scholar]
- 211.Chen S., Li B., Chen L., Jiang H. Uncovering the mechanism of resveratrol in the treatment of diabetic kidney disease based on network pharmacology, molecular docking, and experimental validation. Journal of Translational Medicine . 2023;21(1):p. 380. doi: 10.1186/s12967-023-04233-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Gao Y., Yang R., Guo L., et al. Qing-Re-Xiao-Zheng formula modulates gut microbiota and inhibits inflammation in mice with diabetic kidney disease. Frontiers in Medicine . 2021;8, article 719950 doi: 10.3389/fmed.2021.719950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Shen Z., Cui T., Liu Y., Wu S., Han C., Li J. Astragalus membranaceus and Salvia miltiorrhiza ameliorate diabetic kidney disease via the "gut-kidney axis". Phytomedicine . 2023;121, article 155129 doi: 10.1016/j.phymed.2023.155129. [DOI] [PubMed] [Google Scholar]
- 214.Chen Q., Ren D., Wu J., et al. Shenyan Kangfu tablet alleviates diabetic kidney disease through attenuating inflammation and modulating the gut microbiota. Journal of Natural Medicines . 2021;75(1):84–98. doi: 10.1007/s11418-020-01452-3. [DOI] [PubMed] [Google Scholar]
- 215.Jiang Y., Yang L., Yang X., et al. The imidazopyridine derivative X22 prevents diabetic kidney dysfunction through inactivating NF-κB signaling. Biochemical and Biophysical Research Communications . 2020;525(4):877–882. doi: 10.1016/j.bbrc.2020.03.016. [DOI] [PubMed] [Google Scholar]
- 216.Cui H. X., Zhang L. S., Luo Y., Yuan K., Huang Z. Y., Guo Y. A purified anthraquinone-glycoside preparation from rhubarb ameliorates type 2 diabetes mellitus by modulating the gut microbiota and reducing inflammation. Frontiers in Microbiology . 2019;10:p. 1423. doi: 10.3389/fmicb.2019.01423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Mi N., Zhang X. J., Ding Y., et al. Branched-chain amino acids attenuate early kidney injury in diabetic rats. Biochemical and Biophysical Research Communications . 2015;466(2):240–246. doi: 10.1016/j.bbrc.2015.09.017. [DOI] [PubMed] [Google Scholar]


