Abstract
Background
Innovative restructuring of cardiac rehabilitation (CR) delivery remains critical to reduce barriers and improve access to diverse populations. Destination Cardiac Rehab is a novel virtual world technology‐based CR program delivered through the virtual world platform, Second Life, which previously demonstrated high acceptability as an extension of traditional center‐based CR. This study aims to evaluate efficacy and adherence of the virtual world–based CR program compared with center‐based CR within a community‐informed, implementation science framework.
Methods
Using a noninferiority, hybrid type 1 effectiveness‐implementation, randomized controlled trial, 150 patients with an eligible cardiovascular event will be recruited from 6 geographically diverse CR centers across the United States. Participants will be randomized 1:1 to either the 12‐week Destination Cardiac Rehab or the center‐based CR control groups. The primary efficacy outcome is a composite cardiovascular health score based on the American Heart Association Life's Essential 8 at 3 and 6 months. Adherence outcomes include CR session attendance and participation in exercise sessions. A diverse patient/caregiver/stakeholder advisory board was assembled to guide recruitment, implementation, and dissemination plans and to contextualize study findings. The institutional review board–approved randomized controlled trial will enroll and randomize patients to the intervention (or control group) in 3 consecutive waves/year over 3 years. The results will be published at data collection and analyses completion.
Conclusions
The Destination Cardiac Rehab randomized controlled trial tests an innovative and potentially scalable model to enhance CR participation and advance health equity. Our findings will inform the use of effective virtual CR programs to expand equitable access to diverse patient populations.
Registration
URL: https://www.clinicaltrials.gov; Unique identifier: NCT05897710.
Keywords: cardiac rehabilitation, cardiovascular health, health disparities, home‐based programs, social determinants of health
Subject Categories: Cardiovascular Disease, Exercise, Lifestyle, Diet and Nutrition, Secondary Prevention
Nonstandard Abbreviations and Acronyms
- AHA
American Heart Association
- CBCR
center‐based cardiac rehabilitation
- CR
cardiac rehabilitation
- CVH
cardiovascular health
- EP
exercise physiologist
- FG
focus group
- LE8
Life's Essential 8
- MACE
major adverse cardiovascular event
- NC
nurse coach
- PA
physical activity
- PCS‐AB
patient/caregiver/stakeholder advisory board
- RE‐AIM Reach
effectiveness, adoption, implementation, and maintenance
- REDCap
Research Electronic Data Capture
- VW
virtual world
- VWCR
virtual world–based cardiac rehabilitation
Despite abundant evidence demonstrating the benefits of cardiac rehabilitation (CR), including improvement in cardiovascular health, survival, functional capacity, health‐related quality of life, psychosocial factors, and reduction in major adverse cardiovascular events (MACEs), <25% of eligible patients participate. 1 , 2 , 3 , 4 Barriers to participation include scheduling conflicts, distance to CR centers, lack of transportation, and cost. 5 , 6 , 7 , 8 , 9 , 10 , 11 These social determinants of health influence access to CR, particularly among women, racial and ethnic minority groups, and patients who are socioeconomically disadvantaged. 12 , 13 , 14 Efforts to develop innovative home‐based CR programs ensued in response to a call by the American Heart Association (AHA) Presidential Advisory Board to reengineer the traditional center‐based CR (CBCR) model to mitigate these barriers and increase equitable access to an essential component of cardiovascular preventive care. 15 , 16 , 17 , 18 , 19 , 20 These efforts were amplified during the COVID‐19 pandemic, which further underscored the value of remote CR programs. 21 , 22 , 23 , 24 , 25 , 26 , 27 Unfortunately, the emergence of various novel CR programs over the past 20 years has failed to substantially improve CR participation and adherence. 15 , 28 , 29 Consequently, the goal set out by the Million Hearts CR collaborative in 2017 to increase CR participation from 20% to 70% has not been achieved, and ongoing innovation remains essential to broaden CR access. 30
Incorporating mobile and internet technologies into remote CR programs has shown promise in expanding CR uptake and adherence. 9 , 15 , 30 , 31 , 32 , 33 , 34 In addition, incorporation of nonexercise components (eg, stress management, peer support, and diet modification) has been shown to improve CR completion. 35 Thus, our study team developed a novel comprehensive CR program, Destination Cardiac Rehab, which takes place in the virtual world (VW)–based platform Second Life, an immersive computer‐based environment that mimics in‐person experiences and incorporates an important component lacking in early home‐based CR models: engagement in a social network. VW platforms have been successfully used for health education and chronic disease management. 36 , 37 , 38 , 39 Destination Cardiac Rehab capitalizes on the unique aspects of VWs and was designed to simulate the traditional CR experience while eliminating common barriers to in‐person engagement. 40 The details of the intervention and prior pilot studies are outlined below.
We will conduct a hybrid type 1 effectiveness‐implementation randomized controlled trial that aims to expand on our prior studies by directly comparing Destination Cardiac Rehab with CBCR with a particular focus on recruiting participants from populations with historically lower access to CR (ie, priority populations). 41 The objectives of this study are encompassed into 2 specific aims: (1) to evaluate the efficacy of Destination Cardiac Rehab on cardiovascular health (CVH) compared with CBCR and (2) to compare participation and adherence to Destination Cardiac Rehab versus CBCR. We hypothesize that Destination Cardiac Rehab will demonstrate noninferior efficacy and higher adherence rates compared with those of traditional CR. Study participants, CR staff, and key stakeholders will participate in postintervention focus groups (FGs) to inform future scalability efforts.
Methods
The data that will be generated from this study will be available from the corresponding author on reasonable request. Recruitment and enrollment of the first cohort are expected to begin in spring 2024. Data collection is expected to be completed in the first quarter of 2028. We aim to submit an article with the final study results in winter 2029. The study protocol has been registered (NCT05897710), and is IRB‐approved (identifier: 22‐011357).
Intervention Design and Conceptual Framework
Destination Cardiac Rehab takes place on the VW‐based platform, Second Life, where participants create and interact with peers and CR staff via avatars, designed to mimic an in‐person CR program. Group education sessions (detailed below), including lectures, tours of a virtual gym and restaurant, and peer support groups, take place within the VW. The program was designed with sound behavior change theories, including self‐determination theory, a framework used in executing successful lifestyle interventions to promote sustainable self‐management and healthy lifestyle change. 42 Self‐determination theory highlights 3 key experiences, competence, autonomy, and relatedness, that promote motivation and engagement in healthy behaviors within the context of cardiac rehabilitation. Competence involves mastering skills, supported by the Destination Cardiac Rehab education curriculum and CR staff. Autonomy entails self‐directed learning, enabled by choices in activities and learning methods in Destination Cardiac Rehab. Relatedness emphasizes social connections, central to the support groups in Destination Cardiac Rehab. In addition, the platform capitalizes on a phenomenon, known as the proteus effect, in which people tend to emulate the behaviors and attitudes of their virtual avatars. 43 On the basis of these behavioral change theories in addition to close emulation of an in‐person experience, Destination Cardiac Rehab has the potential to have similar efficacy to CBCR.
Prior Work
The results of 2 prior pilot studies evaluating Destination Cardiac Rehab have been published. Using a mixed‐methods approach, the first proof‐of‐concept study demonstrated feasibility and revealed positive participant perceptions of the VW experience. Participant feedback obtained during postintervention FGs was used to refine the program. 44 A follow‐up multisite clinical trial demonstrated excellent participant retention and attendance rates as well as high user satisfaction. 45 These positive perspectives and use were also seen among those historically underrepresented in CR (eg, women, racial and ethnic minority groups, and those with a high burden of CR access barriers). 46 Although underpowered to assess CVH outcomes, positive CVH trends in lipids, physical activity (PA), blood pressure (BP), and weight were noted. 45 Comparison of the impact of Destination Cardiac Rehab on CVH to that of CBCR is needed to establish our novel virtual world–based CR (VWCR) program as an acceptable alternative to CBCR.
Community‐Oriented Trial Design
We plan to conduct a multiphase, multicenter, 2‐arm noninferiority, hybrid type 1 effectiveness‐implementation randomized controlled trial to rigorously test the efficacy of a 12‐week VWCR intervention, Destination Cardiac Rehab (Figure 1), compared with CBCR. The study will be designed in partnership with a patient/community/stakeholder advisory board (PCS‐AB). The Consolidated Standards of Reporting Trials checklist was used when preparing the study protocol. 47 See Figure 2 for a summary of the study design and study arms.
Figure 1. Destination Cardiac Rehab logo.

Created with Adobe Illustrator.
Figure 2. Overview of study design and study arms.
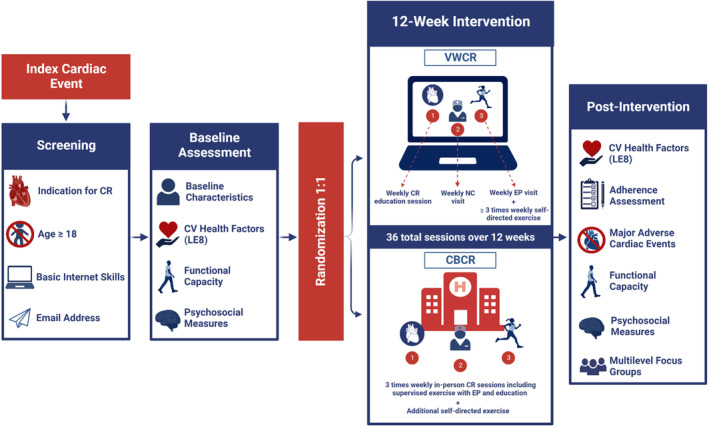
Participants will be screened for eligibility following index cardiac event and randomized 1:1 following baseline assessment. Participants will undergo a 12‐week intervention. Postintervention assessments will occur at 3 and 6 months after randomization. CBCR indicates center‐based CR; CR, cardiac rehabilitation; CV, cardiovascular; EP, exercise physiologist; LE8, Life's Essential 8; NC, nurse coach; and VWCR, virtual world–based CR. Created with BioRender.com.
PCS‐AB and CR Perspectives Survey
The study team principal investigator (L.C.B.) previously established a PCS‐AB from pilot and community‐based participatory research studies that informed an equity‐focused, patient‐centered Destination Cardiac Rehab program in pilot studies. 40 , 44 , 45 , 48 To further enhance the community‐oriented trial, a study‐specific PCS‐AB will be reinforced and expanded to include ≈25 diverse members from all 6 study sites (see the Study Setting section), including patients who completed CR, patients who enrolled in but did not complete CR, CR‐eligible patients who did not enroll, and caregivers of CR participants or CR‐eligible patients, as well as representatives from key stakeholder/advocacy groups (eg, American Association of Cardiology and Pulmonary Rehabilitation, AHA, payers, and information technology). The study team will partner with the PCS‐AB during the study planning and implementation phases to ensure its cultural, age, and sex appropriateness, design recruitment materials, contextualize study findings, and develop/execute a dissemination plan.
As a part of our community‐engaged process to study design, new members to the PCS‐AB will be introduced to the VWCR platform, and all members will undergo a comprehensive orientation. During the trial planning phase, the PCS‐AB will meet monthly (up to 6 virtual meetings) to provide input on study materials and feedback for the continued refinement of the VWCR platform. A subset of members will participate in a 1‐week Mock Patient Journey trial of the VWCR program and attend an additional session for program‐specific feedback. This input will be used for procedural streamlining/troubleshooting and to further enhance the platform's design, cultural appropriateness, and usability. The PCS‐AB will continue to meet throughout the trial implementation phase on a quarterly basis.
The PSC‐AB members will receive $250 for joining the advisory board in addition to $20 per virtual meeting attended. Members of the Mock Patient Journey will receive an additional $40.
Participants will also be provided with Fitbit PA monitors, Omron Evolv wireless Bluetooth BP monitors, and headsets (for optimal audio communication within the VW platform) as additional incentives. Participants who do not have access to a personal computer will be loaned a laptop for the duration of this study. In addition, patients who are eligible for CR or who have completed CR will be recruited to complete an online survey (CR Perspectives Survey) that aims to identify barriers and facilitators to CR participation and understand features of CR that are most important to patients to further inform the intervention design. The survey will be distributed nationally and across all 6 study sites.
Study Setting
The study will take place at 6 academic centers with high‐quality CBCR programs in diverse geographic regions throughout the United States, including 3 Mayo Clinic sites (Rochester, MN; Scottsdale, AZ; and Jacksonville, FL), Johns Hopkins Hospital (Baltimore, MD), University of California (Irvine, CA), and University of Mississippi Medical Center (Jackson, MS). Each site serves high volumes of diverse cardiac patients by racial and ethnic background, sex and gender, community (rural versus urban), and socioeconomic status to enhance the prospect of recruiting study participants from high‐priority populations.
Study Population/Eligibility Criteria
Eligible participants include adults (aged ≥18 years) hospitalized for a cardiac event (eg, acute coronary syndrome, stable angina or heart failure, or cardiac surgery) who have basic Internet navigation skills and an active email address. Participants will be excluded if they are high risk by the American Association of Cardiology and Pulmonary Rehabilitation risk stratification (because of safety concerns of unsupervised exercise), pregnant, or non‐English speaking, or have a visual/hearing impairment or mental disability precluding independent use of the VW platform. 49 , 50
Recruitment
Potential participants will be identified from the hospital service census and outpatient CR enrollment lists (those who have not yet completed their first CR session) by the study coordinator at each site. After confirming eligibility, each patient will be approached before dismissal and provided with a study overview and a VW demo video. Participants will provide written informed consent at the time of recruitment or at the baseline visit. Recruitment materials will be culturally and contextually tailored to high‐priority populations. We aim to recruit a diverse population that adequately represents the priority population, including ≈50% women and participants from diverse racial and ethnic backgrounds. On the basis of patient demographics of the study sites, we anticipate recruiting individuals within the following racial and ethnic distributions: 1.7% American Indian/Alaska Native individuals, 10% Asian individuals, 35% Black individuals, 10% Hispanic or Latino individuals, 3.5% Native Hawaiian or other Pacific Islander individuals, 35% White individuals, and 4.3% individuals of >1 race or ethnicity . Study coordinators will monitor sociodemographics, including age, race, and ethnicity, to provide the study team an opportunity to adjust recruitment strategies to meet the proposed goals.
All participants will receive a PA tracker (Fitbit) to accurately assess free‐living PA and heart rates and an Omron Evolv wireless Bluetooth BP monitor. Participants will receive up to $150 total by cash card at baseline and follow‐up health assessments. Participant transportation and/or parking costs to attend health assessments will be covered as needed with allocated study funding.
Randomization and Blinding
Patients will be randomly assigned to either the Destination Cardiac Rehab arm (intervention) or the CBCR arm (control) in a 1:1 ratio using a computer software‐generated list at their baseline visit using Research Electronic Data Capture (REDCap). We will use stratified permuted block randomization with sex and site as strata. Stratified randomization by site ensures balance so that within each site there are similar numbers of participants assigned to each arm. Each site as a unit represents a complex set of characteristics, including race and ethnicity of the population represented by the site, workload of site staff, or underlying practice differences. These characteristics, and hence site more broadly, may be prognostic for outcomes and may influence intervention responsiveness. Thus, stratifying randomization by site may improve statistical power compared with randomization without stratification. To ensure that staff consenting and randomizing patients are unable to predict future randomization sequence, a random sequence of block size (2, 4, and 6) will be used. Because of the nature of the intervention, neither participants nor staff will be blinded to allocation.
Study Duration/Timeline
Year 1 will encompass hiring/training study staff, obtaining Institutional Review Board (IRB) approval at all clinical sites, and finalizing the recruitment plan with the PCS‐AB. To ensure adequate and valuable interaction with study staff and participants in smaller groups within the VWCR arm, we will deliver the intervention in 3 consecutive waves/year (years 2–4). Quantitative data collection/follow‐up for study primary and secondary end points will be completed at 3 and 6 months after randomization per cohort. Participant FGs will occur at year end and will include participants from 3 waves. Staff and stakeholder FGs will occur in the first quarter of year 5. Data analysis will be completed in year 5. See Figure 3 for a summary of the study timeline.
Figure 3. Summary of study timeline.
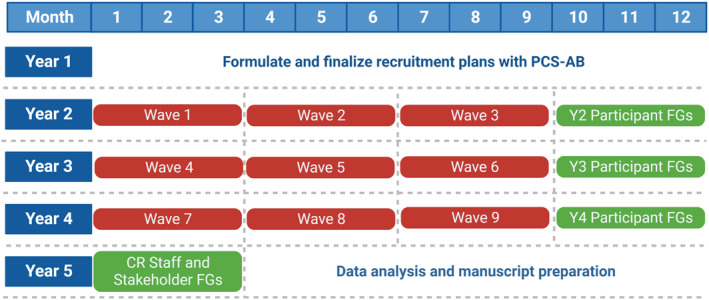
The study team will formulate and finalize recruitment plans in year (Y) 1. The intervention will occur in 3 waves per year over Y2 to Y4. Participant focus groups (FGs) will be conducted at the end of Y2 to Y4. Cardiac rehabilitation (CR) staff and stakeholder FGs will occur at the beginning of Y5. Data analysis will be complete by the end of Y5. PCS‐AB indicates patient/caregiver/stakeholder advisory board. Created with BioRender.com.
Intervention
Destination Cardiac Rehab Arm
Patients randomized to the intervention group will have access to an interactive healthy lifestyle CR community, Destination Cardiac Rehab. The intervention is delivered on a secure VW platform via an established Mayo Clinic infrastructure, Linden Lab's Second Life. To minimize access barriers and maximize equity, participants in the intervention arm will receive a loaner laptop for use during the study installed with VWCR software and/or high‐speed fifth‐generation wireless modems/service as needed. They will also receive a wireless BP monitor for home BP and heart rate self‐monitoring to share with the study team. During the baseline assessment, participants randomized to the Destination Cardiac Rehab arm will receive instructional training with the VW support staff that will provide an overview of Second Life and Destination Cardiac Rehab, create an avatar, and review the basic features and navigation of the VW platform. VW support staff will be mindful to customize training to individual digital skills/literacy needs. Technology support staff will be available during all VW sessions.
Participants will undergo an initial assessment and form an individualized treatment plan, which will be updated every 30 days during the intervention. Components of the individualized treatment plan include relevant clinical history, exercise program description, risk factor modification plan, and psychosocial assessment. Participants in the intervention arm will attend 3 synchronous virtual sessions per week over 12 weeks for a total of 36 prescribed sessions and touchpoints with CR staff and will be instructed to engage in ≥3 asynchronous self‐directed, moderate‐intensity exercise sessions per week. Participants will be encouraged to wear their PA monitor during self‐directed exercise for verification by the study team to avoid recall bias. Group education sessions will occur at a scheduled time each week in VWCR, but will be recorded for those unable to attend live. The exercise physiologist (EP) and nurse coach (NC) visits will be individualized and scheduled to meet participant scheduling needs. Exercise sessions occur asynchronously and can be completed at the convenience of participants. Figure 4 details the participant requirements by study arm.
Figure 4. Cardiac rehabilitation program participant requirements by study arm.
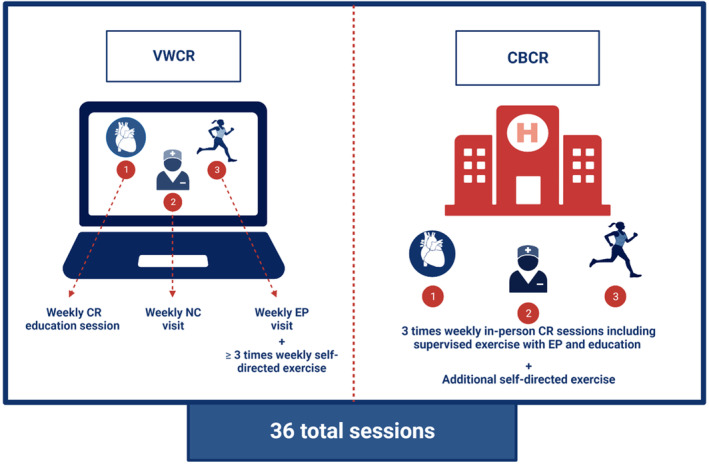
Participants in the intervention arm will participate in 3 virtual sessions per week: a group education session, nurse coach (NC) visit, and exercise physiologist (EP) visit, in addition to ≥3 self‐directed exercise sessions per week. Participants in the control arm will participate in 3 in‐person sessions per week and will also be encouraged to exercise outside of cardiac rehabilitation (CR) sessions. CBCR indicates center‐based CR; and VWCR, virtual world–based CR. Created with BioRender.com.
Education Sessions
A series of weekly, 1‐hour group sessions covering relevant CVH topics (Table 1) will occur over 12 weeks within the VWCR platform. These sessions will be led by a cardiovascular disease specialist (principal investigator and site leads) and a cardiovascular nurse educator, both trained in motivational interviewing and the Second Life application. Participants will engage via their avatar in study team–led and self‐guided virtual activities, including grocery store, home kitchen, and restaurant tours (Figure 5) with a registered dietician, as well as a variety of fitness activities.
Table 1.
Overview of Destination Cardiac Rehab Education Session Curriculum
| Session | Topic |
|---|---|
| 1 | Overview of types of heart disease |
| 2 | Managing heart disease risk factors |
| 3 | Heart medications |
| 4 | Benefits of exercise |
| 5 | Smoking cessation |
| 6 | Practical exercise tips |
| 7 | Stress management and heart disease |
| 8 | Sexuality and heart disease |
| 9 | Heart‐healthy nutrition review |
| 10 | High blood pressure |
| 11 | Fitness concepts and implementation strategies (including fitness center tour with exercise physiologist) |
| 12 | Dining out the healthy way (including interactive restaurant and grocery store tour with dietician) |
Figure 5. Images of Destination Cardiac Rehab.

A, Peer support group. B, Patient using treadmill in fitness center. C, Patient at restaurant. D, Patient practicing yoga in fitness center. Created with BioRender.com.
EP Visits
Participants will meet weekly with an EP via private virtual visits on a platform within the electronic medical record, compliant with the Health Insurance Portability and Accountability Act, to discuss and review self‐directed exercise patterns (frequency, type, intensity, and duration) and to receive a personalized exercise prescription (including cardioaerobic and resistance training). 50 , 51 Although no structured supervised exercise will occur during these sessions, participants may review exercise techniques with the EP as needed.
NC Visits
An NC will be assigned to each patient for personalized, collaborative goal setting for lifestyle change and cardiovascular risk factors. At weekly private virtual visits via the Health Insurance Portability and Accountability Act–compliant platform within the electronic medical record, the NC will review key concepts from the CR curriculum, cardiovascular symptoms, PA patterns, BPs, and medications, and assist with social determinants of health as identified by the patient (eg, local community resource referrals).
Peer Support Group Sessions and Discussion Forum
To mimic the social interaction experienced by participants in CBCR, optional weekly live peer social happy hour group sessions will be available within the VWCR platform for participants to virtually meet via their avatars to share their progress in translating healthy lifestyle changes from the VW to the physical world. A peer discussion forum will be available at all times to the participants on the Destination Cardiac Rehab platform. The peer support group is adjunctive rather than a core component of the intervention and is designed to simulate the social interactions patients may experience in CBCR.
CBCR Arm
The control group will enroll in a standard CBCR program, which consists of 36 sessions (typically 3 sessions/week) over 12 weeks at most clinical sites. Analogous to the intervention group, participants will undergo an initial session and individualized treatment plan (which will be updated every 30 days by CR clinicians), and meet with EPs and NCs regularly throughout the program for supervised exercise training with exercise prescriptions, clinical assessments, and risk factor modification (Figure 1). Participants in the CBCR group may also engage in self‐directed exercise outside of in‐person sessions and will be encouraged to wear their PA tracker for verification by the study team.
Safety Assessments and Adverse Event Reporting
Participants in the intervention arm will be instructed to self‐monitor for worrisome cardiovascular symptoms (eg, chest pain, excessive dyspnea, and lightheadedness). During each VW session (education sessions, EP visits, and NC visits), the CR staff will ask about any worrisome symptoms. If an adverse event (defined as any new unfavorable symptom/diagnosis that was either absent at baseline or worsens during the intervention) is identified, CR staff will immediately report to the site‐specific cardiovascular disease specialist for appropriate triage. Participants will be asked to discontinue self‐directed exercise until deemed safe by the cardiovascular disease specialist. Participants in both arms may discontinue CR at will or with the development of an adverse event but, with their permission, will continue interval assessments to ensure adequate intention‐to‐treat analysis. Temporary or permanent discontinuation and the reason for discontinuation will be documented by the study team.
Data Collection and Management
Health Assessments
Quantitative assessments (summarized in Table 2) in both study arms will be performed in person with the study team at baseline and 3 and 6 months after randomization. Data will be maintained on a secure site and will be deidentified for analysis.
Table 2.
Summary of Patient Assessments
| Assessment | Screening/enrollment | 3 Months after randomization | 6 Months after randomization |
|---|---|---|---|
| Individual information | |||
| Demographics (sex/gender, age, race and ethnicity, marital status, SES, and insurance status) | X | ||
| Cardiovascular disease history (indication for CR, other cardiovascular comorbidities, and current use of guideline‐based cardiovascular medication therapies) | X | ||
| General health, medical history, health status, and preventive care | X | ||
| Cardiovascular health (LE8) measures | |||
| Diet quality | X | X | X |
| PA patterns | X | X | X |
| Nicotine exposure | X | X | X |
| Sleep quality | X | X | X |
| Body mass index | X | X | X |
| Blood lipids (non‐HDL) | X | X | X |
| Blood glucose/hemoglobin A1c | X | X | X |
| BP | X | X | X |
| Cardiovascular outcomes | |||
| MACE composite | X | X | |
| Functional capacity | |||
| 6‐Min walk distance | X | X | X |
| Psychosocial measures | |||
| Quality of life | X | X | X |
| Diet/PA self‐efficacy | X | X | X |
| Diet/PA self‐regulation | X | X | X |
| Diet/PA social support | X | X | X |
BP indicates blood pressure; CR, cardiac rehabilitation; HDL, high‐density lipoprotein; LE8, Life's Essential 8; MACE, major adverse cardiovascular event; PA, physical activity; and SES, socioeconomic status.
The baseline assessment will include completion of a survey, anthropometric and laboratory data, and a functional assessment. The survey will include questions on demographics (sex/gender, age, race and ethnicity, marital status, socioeconomic status, and insurance status), cardiovascular disease history, including the indication for CR, other cardiovascular comorbidities, and current use of guideline‐based cardiovascular medication therapies (including aspirin, statin, cardioselective β‐blocker, angiotensin‐converting enzyme inhibitor/angiotensin receptor blocker/angiotensin receptor/neprilysin inhibitor, mineralocorticoid receptor antagonist, and sodium‐glucose cotransporter‐2 inhibitor, as indicated), other baseline characteristics (general health, additional medical history, health status, and preventive care), and outcome measures.
Anthropometric data will include height measurement (to nearest centimeter by stadiometer), weight measurement (with calibrated scale in kilograms), and BP (average of 3 sitting readings). Laboratory evaluation will include fasting lipid panel, hemoglobin A1c (percentage), and fasting glucose (mg/dL). Functional capacity will be assessed by measuring 6‐minute walk distance (meters). Two follow‐up assessments will occur at 3 and 6 months after randomization with similar data collected as those obtained during the baseline visit, excluding questions on demographic information and baseline characteristics.
Qualitative Assessment
Focus Groups
At 3 to 9 months after randomization, study participants will be recruited to participate in optional FGs, which will include a semistructured interview with questions probing CR satisfaction, acceptability, and facilitators/barriers to completion. An FG in year 5 will be conducted with the CR staff and key stakeholders (eg, clinicians, payers, and community organizations) to elicit feedback on the intervention and to better understand barriers/facilitators and costs projected to support or hinder sustainment/scalability using the Consolidated Framework for Implementation Research. 52
Evaluation will follow the Reach, Effectiveness, Adoption, Implementation, and Maintenance (RE‐AIM) framework to understand influences on adherence/clinical outcomes as well as cost‐effectiveness to inform the potential for future sustainability and scalability of Destination Cardiac Rehab. 53 , 54 In addition, the Clinical Sustainability Assessment tool will be used for sustainability planning. 55 Implementation outcomes or evaluation measures used to assess each RE‐AIM domain are summarized in Table 3.
Table 3.
Evaluation Using RE‐AIM Framework
| RE‐AIM element | Data source(s) | Evaluation measures |
|---|---|---|
| Reach | ||
| Representativeness: participant | Enrollment data | Number/proportions by demographic subgroups |
| Representativeness: setting | Site characteristics | Size, location, staff, and demographic of patients served |
| Penetration/refusion reasons | Screening lists | Number/proportions not participating for each reason overall and by subgroups |
| Effectiveness | ||
| Primary outcome: adherence | Participant follow‐up data |
Number of sessions attended relative to number prescribed* Goal ≥70% of sessions: VWCR: 25 of 36 virtual sessions (education, EP, and NC sessions); CBCR: 25 of 36 in‐person sessions |
| Secondary outcome: adherence |
Number of sessions attended relative to sessions prescribed Number of self‐directed moderate‐intensity exercise sessions completed per week |
|
| Secondary outcome: retention | Percentage of participants completing baseline and 3‐ and 6‐mo and follow‐up clinical assessments | |
| Secondary outcome: clinical | 3‐mo CVH (LE8 score) outcome, MACEs | |
| Secondary outcome: cost‐effectiveness | 3‐ and 6‐mo estimated per‐participant direct and indirect health care costs for both VWCR and CBCR | |
| Adoption | ||
| Acceptability/satisfaction | Focus groups |
Participant satisfaction with VWCR intervention or CBCR CR staff (EP, NC) satisfaction with VWCR intervention |
| Barriers/facilitators to adoption |
Focus groups Implementation checklists Study team notes |
Site‐specific impediments to program execution; catalyzing factors supporting program uptake by implementers |
| Implementation | ||
| Fidelity |
Focus groups Implementation checklists Protocol variations Participant follow‐up data VW platform data Study team notes |
Fidelity to intervention (VWCR) and control (CBCR) groups will be measured as follows: Curriculum: number of sessions/participant, session type (group/individual), independent use (unscheduled visits), and completion of experiential learning activities (eg, fitness center, restaurant), EP and NC: number of virtual (or in‐person) visit sessions/participant, mode of delivery (telephone/video, in‐person), session duration, session type (group/individual), what was delivered (eg, exercise, health behavior counseling, or SDOH review), and quality of interventionist delivery (enthusiasm, confidence, and communication style) |
| Maintenance | ||
| Sustainability and scalability | Stakeholder focus groups | Projected facilitators/barriers to sustainability/scaling from stakeholders (clinicians, payers, and advocacy groups) as follows: review of direct and indirect health care costs, personnel costs (salaries), intervention materials, and facility‐level overhead costs |
CBCR indicates center‐based CR; CR, cardiac rehabilitation; CVH, cardiovascular health; EP, exercise physiologist; LE8, Life's Essential 8; MACE, major adverse cardiovascular event; NC, nurse coach; RE‐AIM, Reach, Effectiveness, Adoption, Implementation, and Maintenance; SDOH, social determinants of health; VW, virtual world; and VWCR, VW–based CR.
Number of VWCR sessions/participant, session type (group/individual), independent use (unscheduled visits), and areas visited (eg, fitness center, restaurant).
Primary Outcome Measures
Cardiovascular Health
The AHA defined 8 essential components of CVH, coined Life's Essential 8 (LE8), that when optimized are strongly associated with reduced MACEs, cardiovascular disease mortality, and all‐cause mortality. 56 , 57 , 58 , 59 Positive trends in the AHA's prior iteration of LE8, Life's Simple 7, which included 7 of 8 LE8 components, have been noted in patients attending CR 56 On the basis of this robust evidence, LE8 was selected as the primary outcome measure as it is a more comprehensive measure of CVH and has a strong correlation with the aforementioned hard end points often used to evaluate CR programs. The components include both health behaviors (diet, PA, nicotine exposure, and sleep health) and health factors (body mass index, blood lipids, blood glucose levels, and BP). The aforementioned survey completed at baseline and follow‐up assessments will include validated questionnaires, Mediterranean Eating Patterns for Americans, 60 the International Physical Activity Questionnaire, 61 National Health and Nutrition Examination Survey Smoking‐Cigarette Use Questionnaire, 62 and the Pittsburg Sleep Quality Index 63 to evaluate diet quality, PA patterns, nicotine exposure, and sleep quality, respectively. Body mass index will be calculated by the height and weight measured at the baseline and follow‐up visits and reported as kg/m2. Additional health factor metrics obtained at baseline and after intervention will include non–high‐density lipoprotein cholesterol levels (mg/dL), hemoglobin A1c (percentage), or fasting blood glucose (mg/dL), and an average of 3 sitting BP readings (mm Hg). An overall LE8 score will be generated by calculating an unweighted average score on a scale of 0 to 100 (Table 4). Average LE8 scores will be categorized as low (0–49), moderate (49–78), or high (80–100). The LE8 components will be measured at baseline and 3 and 6 months after randomization.
Table 4.
LE8 Composite Scoring Guide
| Metric | Score | |
|---|---|---|
| Diet quality | Points | Quantiles of adherence |
| 100 | ≥95th Percentile | |
| 80 | 75th–94th Percentile | |
| 50 | 50th–74th Percentile | |
| 25 | 25th–49th Percentile | |
| 0 | 1st–24th Percentile | |
| PA | Points | Min/wk |
| 100 | ≥150 | |
| 90 | 120–149 | |
| 80 | 90–119 | |
| 60 | 60–89 | |
| 40 | 30–59 | |
| 20 | 1–29 | |
| 0 | 0 | |
| Nicotine exposure | Points | Status |
| 100 | Never smoker | |
| 75 | Former smoker, quit ≥5 y ago | |
| 50 | Former smoker, quit 1–5 y ago | |
| 25 | Former smoker, quit <1 y ago | |
| 0 |
Current smoker Subtract 20 points for living with active indoor smoker in home |
|
| Sleep health | Points | Hours of sleep/night |
| 100 | 7 to <9 | |
| 90 | 9 to <10 | |
| 70 | 6 to <7 | |
| 40 | 5 to <6 or ≥10 | |
| 20 | 4 to <5 | |
| 0 | <4 | |
| BMI | Points | BMI, kg/m2 |
| 100 | <25 | |
| 70 | 25.0–29.9 | |
| 30 | 30.0–34.9 | |
| 15 | 35.0–39.9 | |
| 0 | ≥40.0 | |
| Blood lipids | Points | Non‐HDL cholesterol, mg/dL |
| 100 | <130 | |
| 60 | 130–159 | |
| 40 | 160–189 | |
| 20 | 190–219 | |
| 0 |
≥220 If on lipid‐lowering therapy, subtract 20 points |
|
| Blood glucose | Points | HbA1c, %; or FBG, mg/dL |
| 100 | No history of diabetes and FBG <100 or <5.7 | |
| 60 | No history of diabetes and FBG 100–125 or 5.7–6.4 | |
| 40 | Diabetes with HbA1c 6.4–7.0 | |
| 30 | Diabetes with HbA1c 7.0–7.9 | |
| 20 | Diabetes with HbA1c 8.0–8.9 | |
| 10 | Diabetes with HbA1c 9.0–9.9 | |
| 0 | Diabetes with HbA1c ≥10.0 | |
| BP | Points | BP, mm Hg |
| 100 | <120/80 to 120–129/<80 | |
| 75 | 130–139 or 80–89 | |
| 50 | 140–159 or 90–99 | |
| 25 | ≥160 or ≥100 | |
| 0 | Subtract 20 points if on BP treatment | |
| LE8 composite score total points/8 | ||
BMI indicates body mass index; BP, blood pressure; FBG, fasting blood glucose; HbA1c, hemoglobin A1c; HDL, high‐density lipoprotein; LE8, Life's Essential 8; and PA, physical activity.
Participation and Adherence
Studies have demonstrated a dose‐response association between CR adherence and reduction in MACEs, highlighting the importance of participant attendance and the basis for which we chose to include participation and adherence as a primary outcome measure. 64 , 65 Optimal adherence will be defined as completion of ≥70% of prescribed weekly virtual or in‐person sessions (≥25 of 36 sessions) in the Destination Cardiac Rehab and CBCR arms based on the goal set out by the Million Hearts CR Collaborative and empirical evidence from a recent meta‐analysis. 30 , 64 Attendance to the Destination Cardiac Rehab education sessions as well as EP and NC virtual visits will be monitored by the VW web administrator, EPs, and NCs, respectively. Participant adherence in the control group will be measured by number of prescribed in‐person sessions attended, as documented by CR staff.
Secondary Outcome Measures
Cardiovascular Outcomes
Cardiovascular outcomes will be measured using a MACE composite end point, including the following: (1) cardiovascular‐related hospital readmissions or (2) all‐cause or cardiovascular‐related mortality at 3 and 6 months after randomization. Data on reasons for hospital admissions will be collected through extraction of clinical documentation in the electronic medical record, and deaths will be determined from the electronic medical record and caregiver report.
Exercise Sessions
Participants in both arms will report number of self‐directed exercise sessions completed per week, which will be confirmed by data from the provided PA tracker (Fitbit) with a goal of adherence to ≥3 self‐directed and/or in‐person (control group only) moderate‐intensity exercise sessions per week in accordance with CR quality measures outlined by the American College of Cardiology/AHA and prior works demonstrating improved outcomes in patients who engage in ≥36 sessions of combined in‐person and self‐directed exercise over the course of CR. 5 , 64 , 65
Functional Capacity
The 6‐minute walk test is a commonly used metric to assess functional exercise capacity. 66 A prior meta‐analysis revealed that distance walked over 6 minutes improves in patients who have completed CR. 66 The change in 6‐minute walk test distance (measured in meters) from baseline to 3 and 6 months after randomization will be compared between the intervention and control arms.
Psychosocial Measures
Psychosocial measures, including health‐related quality of life, 67 healthy diet/regular PA self‐efficacy, 68 , 69 self‐regulation, 70 , 71 and social support,72 will be measured using validated questionnaires at 3 and 6 months after randomization.
Power and Sample Size Calculations
To establish noninferiority of the primary efficacy outcome (comparison of LE8 scores at 3 months between arms), we will use a 5‐point noninferiority margin based on prior studies suggesting that a 5‐point increase in LE8 is associated with an additional 1‐year life expectancy free of disease for a 50‐year‐old individual. 45 , 57 This noninferiority margin is sufficient to conclude that Destination Cardiac Rehab is not meaningfully worse than CBCR. Assuming there is no difference between intervention arms under the alternative hypothesis, a total sample size of 110 participants (55/arm) with complete 3‐month follow‐up provides 90% power to conclude noninferiority for the LE8 outcome at 3 months based on a noninferiority margin of 5 points, assuming a residual SD of 8 points and 1‐sided test with α level 0.025. Prior population‐based studies have observed an SD of up to 12 points, but we anticipate the populations eligible for CR are more homogeneous. Furthermore, the statistical analysis will adjust for prognostic prerandomization variables, including baseline LE8 (ANCOVA model). An SD of 10 in this population would result in a residual SD of 8 if the correlation between baseline LE8 and 3‐month LE8 is moderately strong (𝜌=0.6). Assuming ≈25% dropout, we will enroll and randomize 150 participants. If the residual SD is larger than anticipated, we retain 74% power for a residual SD of 10 and nearly 60% power for a residual SD of 12.
As described previously, a participant is adherent if attending ≥70% of prescribed weekly virtual or in‐person sessions. We hypothesize that the rate of adherence (percentage of participants who are adherent) in Destination Cardiac Rehab will be noninferior to the rate of adherence in CBCR. A 25% absolute difference in the adherence rate is considered noninferior. On the basis of prior Destination Cardiac Rehab adherence data, 40 we anticipate higher adherence than observed in prior studies. Assuming a 50% adherence rate among CBCR, a 2‐sample test of proportions among 150 total participants (75 per arm) provides 86% power to conclude noninferiority with 1‐sided α level 0.025. If CBCR adherence percentage is 80%, the design provides 97% power.
Statistical Analysis
Quantitative Data Analysis
Cardiovascular Heath
The primary outcome for aim 1 is comparison of the LE8 score at 3‐month follow‐up between randomized arms. A noninferiority hypothesis will be tested, with the null hypothesis (H0) stating that δ (calculated as μ_VW‐μ_CB) ≤−5 versus the alternative hypothesis (HA) stating that δ>−5 where μ_VW and μ_CB are mean LE8 at 3 months in Destination Cardiac Rehab and CBCR groups, respectively. δ rejects H0 in favor of noninferiority of Destination Cardiac Rehab if the lower bound of the 2‐sided 95% CI on δ is above −5 points. δ will be estimated from a linear mixed effects model, which will control for baseline LE8, sex, and random effect of site. If noninferiority is established, a superiority hypothesis will be tested using a 2‐sided α level 0.05. We will assess moderators of the treatment effect by assessing interactions between baseline visit data and treatment. As the primary goal is to assess a strategy of assignment to Destination Cardiac Rehab or CBCR, the analysis of CVH outcomes will use intention‐to‐treat principles with participants analyzed according to randomized arm independent of participation and adherence.
Participation and Adherence
Adherence to Destination Cardiac Rehab (versus CBCR) will be assessed by comparing the proportion of participants in each arm attending ≥70% of sessions prescribed in each group using a Cochran‐Mantel‐Haenszel test stratified by site, estimating the risk difference (adherence probabilities). Alternatively, continuous percentage of sessions completed will be compared between arms using a Wilcoxon rank‐sum test for superiority. Quantitative data will be summarized by descriptive statistics with t tests and generalized linear models. To inform generalizability to high‐priority populations and to evaluate the possibility of bias attributable to attrition, sociodemographic and contextual factors (eg, sex/gender, age, race and ethnicity, socioeconomic status, and geographic location) of adherent/nonadherent will be compared. If there are any discovered differences, corresponding variables will be adjusted for in subsequent analyses. Mediation of intervention effects with respect to each aim 1 end point (LE8 score, MACEs) will be assessed by participant adherence level using multivariable regression and interaction analyses.
Secondary Outcomes
The MACE composite outcome will be compared using Kaplan‐Meier analysis and shared frailty Cox proportional hazards models, adjusting for sex and site using Firth correction. We will evaluate the 6‐minute walk test distance using linear and generalized linear mixed effects models. Superiority hypothesis testing will occur as noninferiority boundaries are not prespecified for these outcomes. Estimates will be reported with 95% CIs and P values.
Qualitative Data Analysis
Using rapid assessment analysis techniques, the FG moderator will provide a summary analysis of the discussion following each FG. 73 , 74 Sessions will be recorded and transcribed, and transcripts will be independently reviewed by 2 study team members to validate the summary analysis. Data from the semistructured FG interviews will be organized and aligned with research questions in a matrix framework. The study team will systematically examine and code each cell in the matrix to identify emergent themes within and across sites and by participant characteristics. 75 A third team member will assist with discrepancy resolution to ensure consensus. Content analysis will be facilitated by QSR NVivo software, version 10 (Doncaster, Victoria, Australia). We will use triangulation as cross‐verification of our results with comparison across multiple data sources (eg, survey, adherence, and FG data). 50 , 76 , 77
Missing Data
To account for missing data, sensitivity analyses will be conducted under various mechanisms. First, study coordinators will attempt to ascertain and document participant reasons for dropout. Multiple imputation under missing at random assumptions will impute plausible values based on observed data for participants in which dropout reason is unknown. For participants who provide dropout reason, sensitivity analyses may also be conducted under missing not at random assumptions. In addition, pattern‐mixture models will be used to assess sensitivity of results based on location‐shift attributable to the not at random mechanism. Missing data for MACE outcomes are expected to be rare as MACEs may be ascertained through review of electronic medical records. Adherence measures will not be missing as dropout reflects nonadherence.
Ethical Considerations
The protocol, informed consent form(s), and all participant materials will be submitted to the IRB for review and approval at all sites (Mayo Clinic will serve as the IRB of record). Approval of the protocol and consent form must be obtained before participant enrollment. Any addendum to the protocol will require review and approval by the IRB. All patient visits and data collection will occur at the clinical sites, ensuring participant confidentiality according to Health Insurance Portability and Accountability Act. Data will be collected in REDCap. Study staff will be trained to ensure consistency in data collection. Any data will be identified only by a participant identification number to maintain confidentiality. The principal investigator (L.C.B.) will have full access to all the data in the study and takes responsibility for their integrity and data analysis.
A Data Safety Monitoring Board composed of independent CR experts will be established to oversee and ensure the safety and integrity of data collection. The Data Safety Monitoring Board will operate independently of the research team. The Data Safety Monitoring Board will periodically review and assess the safety data throughout the study, including monitoring adverse events or potential risks to participation. The Data Safety Monitoring Board will also evaluate interim data analyses and provide recommendations to ensure participant safety.
Discussion
With the persistent disparity in CR participation, especially among medically underserved populations, ongoing equity‐focused innovation in CR delivery models remains critical. 6 , 9 , 10 , 12 , 29 , 32 , 33 To our knowledge, this is the first study to rigorously test the efficacy and participation in a VWCR program compared with CBCR. Our intervention meets all of the CR quality metrics outlined by the AHA and closely matches the traditional CBCR experience that has established efficacy in improving important CVH outcomes. 5 , 51 Furthermore, the intervention was designed to closely and virtually mimic an in‐person experience. Both the intervention and control groups will be prescribed 36 exercise sessions over 12 weeks and engage in 36 touch points with CR staff. As such, we predict that Destination Cardiac Rehab will demonstrate comparable effects on CVH outcomes to that of CBCR. The efficacy outcome measures chosen include the clinically relevant quality performance measures outlined by the AHA in addition to many other important outcome measures commonly used to evaluate CR efficacy. 5
Our community‐oriented clinical trial has several strengths and equity‐promoting aspects. The integration of a social network fosters a sense of group accountability and equitable inclusion, important elements to behavioral change that are often missing in home‐based CR, possibly accounting for its suboptimal engagement. 9 , 37 , 78 , 79 Furthermore, our study team capitalized on the unique features of VW platforms in addition to the various benefits of home‐based CR to create a patient‐centric intervention that has previously demonstrated excellent adherence, with 71% of patients attending ≥75% of sessions. 44 , 45 On the basis of our prior feasibility study, we expect at least comparable, if not superior, participation in the Destination Cardiac Rehab group compared with CBCR. We also evaluate both short‐ and longer‐term effects (at 3 and 6 months after randomization) of the intervention on key CR outcomes, CVH (clinical and behavioral factors), MACEs, and functional capacity. In addition, this intervention has the potential to serve as an alternative CR delivery model as it was designed with and will have ongoing input from diverse community members with a goal to reach populations with the most CR barriers. Finally, our rigorous mixed‐methods approach and further evaluation of our program using the RE‐AIM framework will inform future implementation, dissemination, and scalability to reach all CR‐eligible patients.
Limitations
Our study does have potential limitations that may limit generalizability and scalability of the intervention. Notably, the VWCR platform is not supported by mobile devices (eg, smartphones, tablets), which may limit generalizability. In addition, participants with no access to a home computer and/or Internet will be provided with a laptop and/or high‐speed wireless modems/service. Although this provides an advantage in recruiting a diverse patient population, it may limit future implementation to priority populations. However, the cost benefits of provision of relatively inexpensive devices and Internet access outweigh the exuberant costs associated with cardiovascular‐related hospitalizations and morbidity faced by patients unable to attend CBCR. 80 , 81 This highlights the importance of ongoing efforts to address the digital divide. 82 , 83 Participants will be recruited from academic medical centers, which may limit generalizability to medically underserved populations. In addition, because of constraints of the intervention platform at this time, this study could not accommodate non–English‐speaking individuals, a population that is highly underrepresented in CR programs. 84 Scalability plans will include the addition of other languages to serve non–English‐speaking patients. Finally, insurance reimbursement continues to hamper implementation of home‐based and virtual CR platforms. The US Congress recently chose to terminate Medicare coverage of virtual CR programs beyond the COVID‐19 public health emergency, which could significantly restrict implementation of Destination Cardiac Rehab. 85 Fortunately, the Sustainable Cardiopulmonary Rehabilitation Services in the Home Act was recently proposed in Congress to address this important issue, emphasizing the importance of building the evidence base and ongoing advocacy for alternative virtual CR platforms that expand access to a vital component of cardiovascular care. 86
Conclusions
Our study is the first study to evaluate the efficacy of a VWCR program compared with the standard of care. If Destination Cardiac Rehab demonstrates noninferior outcomes compared with CBCR, our VWCR program could serve as an alternative method of CR delivery, potentially expanding access to critical populations with high barriers to traditional CR participation.
Sources of Funding
This study is supported by the Bristol Myers Squibb Foundation and the American Heart Association Second Century Implementation Science Award. Dr Brewer was also supported by the American Heart Association–Amos Medical Faculty Development Program (grant 19AMFDP35040005), the Robert A. Winn Career Development Award (Bristol Myers Squibb Foundation), the National Institutes of Health (NIH)/National Institute on Minority Health and Health Disparities (grant P50MD017342), the Clinical and Translational Science Awards (grant UL1 TR000135) from the National Center for Advancing Translational Sciences (NCATS) to Mayo Clinic, and the Centers for Disease Control and Prevention (CDC; grant CDC‐ DP18‐1817) during the implementation of this work. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NCATS, NIH, or CDC. The funding bodies had no role in study design; in the collection, analysis, and interpretation of data; writing of the manuscript; and in the decision to submit the manuscript for publication.
Disclosures
None.
Acknowledgments
We are grateful to the members of the patient/caregiver/stakeholder advisory board for their contributions to the intervention and study design. The authors would also like to show gratitude to the cardiac rehabilitation center staff at all study sites for informing the trial design and protocol.
This article was sent to Saket Girotra, MD, SM, Associate Editor, for review by expert referees, editorial decision, and final disposition.
For Sources of Funding and Disclosures, see page 14.
References
- 1. Suaya JA, Shepard DS, Normand SL, Ades PA, Prottas J, Stason WB. Use of cardiac rehabilitation by Medicare beneficiaries after myocardial infarction or coronary bypass surgery. Circulation. 2007;116:1653–1662. doi: 10.1161/circulationaha.107.701466 [DOI] [PubMed] [Google Scholar]
- 2. Ritchey MD, Maresh S, McNeely J, Shaffer T, Jackson SL, Keteyian SJ, Brawner CA, Whooley MA, Chang T, Stolp H, et al. Tracking cardiac rehabilitation participation and completion among Medicare beneficiaries to inform the efforts of a national initiative. Circ Cardiovasc Qual Outcomes. 2020;13:e005902. doi: 10.1161/circoutcomes.119.005902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Goel K, Lennon RJ, Tilbury RT, Squires RW, Thomas RJ. Impact of cardiac rehabilitation on mortality and cardiovascular events after percutaneous coronary intervention in the community. Circulation. 2011;123:2344–2352. doi: 10.1161/circulationaha.110.983536 [DOI] [PubMed] [Google Scholar]
- 4. Dibben G, Faulkner J, Oldridge N, Rees K, Thompson DR, Zwisler AD, Taylor RS. Exercise‐based cardiac rehabilitation for coronary heart disease. Cochrane Database Syst Rev. 2021;11:Cd001800. doi: 10.1002/14651858.CD001800.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Thomas RJ, Balady G, Banka G, Beckie TM, Chiu J, Gokak S, Ho PM, Keteyian SJ, King M, Lui K, et al. 2018 ACC/AHA clinical performance and quality measures for cardiac rehabilitation: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures. J Am Coll Cardiol. 2018;71:1814–1837. doi: 10.1016/j.jacc.2018.01.004 [DOI] [PubMed] [Google Scholar]
- 6. Bakhshayeh S, Sarbaz M, Kimiafar K, Vakilian F, Eslami S. Barriers to participation in center‐based cardiac rehabilitation programs and patients' attitude toward home‐based cardiac rehabilitation programs. Physiother Theory Pract. 2021;37:158–168. doi: 10.1080/09593985.2019.1620388 [DOI] [PubMed] [Google Scholar]
- 7. Foster EJ, Munoz SA, Crabtree D, Leslie SJ, Gorely T. Barriers and facilitators to participating in cardiac rehabilitation and physical activity in a remote and rural population; a cross‐sectional survey. Cardiol J. 2019;28:697–706. doi: 10.5603/CJ.a2019.0091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Supervia M, Medina‐Inojosa JR, Yeung C, Lopez‐Jimenez F, Squires RW, Perez‐Terzic CM, Brewer LC, Leth SE, Thomas RJ. Cardiac rehabilitation for women: a systematic review of barriers and solutions. Mayo Clin Proc. 2017;92:565–577. doi: 10.1016/j.mayocp.2017.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chindhy S, Taub PR, Lavie CJ, Shen J. Current challenges in cardiac rehabilitation: strategies to overcome social factors and attendance barriers. Expert Rev Cardiovasc Ther. 2020;18:777–789. doi: 10.1080/14779072.2020.1816464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Castellanos LR, Viramontes O, Bains NK, Zepeda IA. Disparities in cardiac rehabilitation among individuals from racial and ethnic groups and rural communities‐a systematic review. J Racial Ethn Health Disparities. 2019;6:1–11. doi: 10.1007/s40615-018-0478-x [DOI] [PubMed] [Google Scholar]
- 11. Schopfer DW, Nicosia FM, Ottoboni L, Whooley MA. Patient perspectives on declining to participate in home‐based cardiac rehabilitation: a mixed‐methods study. J Cardiopulm Rehabil Prev. 2020;40:335–340. doi: 10.1097/hcr.0000000000000493 [DOI] [PubMed] [Google Scholar]
- 12. Mathews L, Brewer LC. A review of disparities in cardiac rehabilitation: evidence, drivers, and solutions. J Cardiopulm Rehabil Prev. 2021;41:375–382. doi: 10.1097/hcr.0000000000000659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Khadanga S, Gaalema DE, Savage P, Ades PA. Underutilization of cardiac rehabilitation in women: barriers and solutions. J Cardiopulm Rehabil Prev. 2021;41:207–213. doi: 10.1097/hcr.0000000000000629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Beatty AL, Beckie TM, Dodson J, Goldstein CM, Hughes JW, Kraus WE, Martin SS, Olson TP, Pack QR, Stolp H, et al. A new era in cardiac rehabilitation delivery: research gaps, questions, strategies, and priorities. Circulation. 2023;147:254–266. doi: 10.1161/CIRCULATIONAHA.122.061046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Thomas RJ, Beatty AL, Beckie TM, Brewer LC, Brown TM, Forman DE, Franklin BA, Keteyian SJ, Kitzman DW, Regensteiner JG, et al. Home‐based cardiac rehabilitation: a scientific statement from the American Association of Cardiovascular and Pulmonary Rehabilitation, the American Heart Association, and the American College of Cardiology. Circulation. 2019;140(1):e69–e89. doi: 10.1161/CIR.0000000000000663 [DOI] [PubMed] [Google Scholar]
- 16. Taylor RS, Dalal H, Jolly K, Zawada A, Dean SG, Cowie A, Norton RJ. Home‐based versus centre‐based cardiac rehabilitation. Cochrane Database Syst Rev. 2015;(6):Cd007130. doi: 10.1002/14651858.CD007130.pub3 [DOI] [PubMed] [Google Scholar]
- 17. Shanmugasegaram S, Oh P, Reid RD, McCumber T, Grace SL. A comparison of barriers to use of home‐ versus site‐based cardiac rehabilitation. J Cardiopulm Rehabil Prev. 2013;33:297–302. doi: 10.1097/HCR.0b013e31829b6e81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Balady GJ, Ades PA, Bittner VA, Franklin BA, Gordon NF, Thomas RJ, Tomaselli GF, Yancy CW. Referral, enrollment, and delivery of cardiac rehabilitation/secondary prevention programs at clinical centers and beyond: a presidential advisory from the American Heart Association. Circulation. 2011;124:2951–2960. doi: 10.1161/CIR.0b013e31823b21e2 [DOI] [PubMed] [Google Scholar]
- 19. Zhong W, Fu C, Xu L, Sun X, Wang S, He C, Wei Q. Effects of home‐based cardiac telerehabilitation programs in patients undergoing percutaneous coronary intervention: a systematic review and meta‐analysis. BMC Cardiovasc Disord. 2023;23:101. doi: 10.1186/s12872-023-03120-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Anderson L, Sharp GA, Norton RJ, Dalal H, Dean SG, Jolly K, Cowie A, Zawada A, Taylor RS. Home‐based versus centre‐based cardiac rehabilitation. Cochrane Database Syst Rev. 2017;6:Cd007130. doi: 10.1002/14651858.CD007130.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vigorito C, Faggiano P, Mureddu GF. COVID‐19 pandemic: what consequences for cardiac rehabilitation? Monaldi Arch Chest Dis. 2020;90:205–206. doi: 10.4081/monaldi.2020.1315 [DOI] [PubMed] [Google Scholar]
- 22. Epstein E, Patel N, Maysent K, Taub PR. Cardiac rehab in the COVID era and beyond: mHealth and other novel opportunities. Curr Cardiol Rep. 2021;23:42. doi: 10.1007/s11886-021-01482-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mureddu GF, Giallauria F, Venturini E, Fattirolli F, Ambrosetti M. Cardiac rehabilitation and secondary prevention programs during the COVID‐19 pandemic: what's "COVID on"? Article in Italian. G Ital Cardiol (Rome). 2020;21:527–528. doi: 10.1714/3386.33640 [DOI] [PubMed] [Google Scholar]
- 24. Bokolo AJ. Use of telemedicine and virtual care for remote treatment in response to COVID‐19 pandemic. J Med Syst. 2020;44:132. doi: 10.1007/s10916-020-01596-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fersia O, Bryant S, Nicholson R, McMeeken K, Brown C, Donaldson B, Jardine A, Grierson V, Whalen V, Mackay A. The impact of the COVID‐19 pandemic on cardiology services. Open Heart. 2020;7:e001359. doi: 10.1136/openhrt-2020-001359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shaw J, Brewer L, Veinot T. Health equity and virtual care: a narrative review of recommendations arising from the COVID‐19 pandemic. JMIR Form Res. 2021;5:e23233. doi: 10.2196/23233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Stefanakis M, Batalik L, Papathanasiou J, Dipla L, Antoniou V, Pepera G. Exercise‐based cardiac rehabilitation programs in the era of COVID‐19: a critical review. Rev Cardiovasc Med. 2021;22:1143–1155. doi: 10.31083/j.rcm2204123 [DOI] [PubMed] [Google Scholar]
- 28. de Araújo S, Pio C, Chaves GS, Davies P, Taylor RS, Grace SL. Interventions to promote patient utilisation of cardiac rehabilitation. Cochrane Database Syst Rev. 2019;2:Cd007131. doi: 10.1002/14651858.CD007131.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Krysler A, Dirk K, Foshaug R, Hartling L, Khoury M. Safety, feasibility and effectiveness of home‐based TELEMEDICINE cardiac rehabilitation programs: a systematic review. J Am Coll Cardiol 2022;79:1435. doi: 10.1016/S0735-1097(22)02426-3 [DOI] [Google Scholar]
- 30. Ades PA, Keteyian SJ, Wright JS, Hamm LF, Lui K, Newlin K, Shepard DS, Thomas RJ. Increasing cardiac rehabilitation participation from 20% to 70%: a road map from the Million Hearts Cardiac Rehabilitation Collaborative. Mayo Clin Proc. 2017;92:234–242. doi: 10.1016/j.mayocp.2016.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Widmer RJ, Collins NM, Collins CS, West CP, Lerman LO, Lerman A. Digital health interventions for the prevention of cardiovascular disease: a systematic review and meta‐analysis. Mayo Clin Proc. 2015;90:469–480. doi: 10.1016/j.mayocp.2014.12.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Thomas R, Scales R, Fernandes R. Alternative models to facilitate and improve delivery of cardiac rehabilitation/secondary prevention. In: Rippe JM, ed. Lifestyle Medicine. CRC Press; 2019:833–837. doi: 10.1201/9781315201108-71 [DOI] [Google Scholar]
- 33. Lee KCS, Breznen B, Ukhova A, Koehler F, Martin SS. Virtual healthcare solutions for cardiac rehabilitation: a literature review. Eur Heart J Digit Health. 2023;4:99–111. doi: 10.1093/ehjdh/ztad005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yu T, Xu H, Sui X, Zhang X, Pang Y, Yu T, Lian X, Zeng T, Wu Y, Leng X, et al. Effectiveness of eHealth interventions on moderate‐to‐vigorous intensity physical activity among patients in cardiac rehabilitation: systematic review and meta‐analysis. J Med Internet Res. 2023;25:e42845. doi: 10.2196/42845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hussain Jafri SH, Ngamdu KS, Price D, Baloch ZQ, Cohn J, Wilcox M, Freeman AM, Ornish D, Wu WC. Intensive cardiac rehabilitation attenuates the gender gap in cardiac rehabilitation participation. Curr Probl Cardiol. 2023;48:101668. doi: 10.1016/j.cpcardiol.2023.101668 [DOI] [PubMed] [Google Scholar]
- 36. Ghanbarzadeh R, Ghapanchi AH, Blumenstein M, Talaei‐Khoei A. A decade of research on the use of three‐dimensional virtual worlds in health care: a systematic literature review. J Med Internet Res. 2014;16:e47. doi: 10.2196/jmir.3097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Johnston JD, Massey AP, Devaneaux CA. Innovation in weight loss programs: a 3‐dimensional virtual‐world approach. J Med Internet Res. 2012;14:e120. doi: 10.2196/jmir.2254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Thompson A, Elahi F, Realpe A, Birchwood M, Taylor D, Vlaev I, Leahy F, Bucci S. A feasibility and acceptability trial of social cognitive therapy in early psychosis delivered through a virtual world: the VEEP study. Front Psych. 2020;11:219. doi: 10.3389/fpsyt.2020.00219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Watson AJ, Grant RW, Bello H, Hoch DB. Brave new worlds: how virtual environments can augment traditional care in the management of diabetes. J Diabetes Sci Technol. 2008;2:697–702. doi: 10.1177/193229680800200422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Brewer LC, Kaihoi B, Zarling KK, Squires RW, Thomas R, Kopecky S. The use of virtual world‐based cardiac rehabilitation to encourage healthy lifestyle choices among cardiac patients: intervention development and pilot study protocol. JMIR Res Protoc. 2015;4:e39. doi: 10.2196/resprot.4285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Curran GM, Bauer M, Mittman B, Pyne JM, Stetler C. Effectiveness‐implementation hybrid designs: combining elements of clinical effectiveness and implementation research to enhance public health impact. Med Care. 2012;50:217–226. doi: 10.1097/MLR.0b013e3182408812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Teixeira PJ, Carraça EV, Markland D, Silva MN, Ryan RM. Exercise, physical activity, and self‐determination theory: a systematic review. Int J Behav Nutr Phys Act. 2012;9:78. doi: 10.1186/1479-5868-9-78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yee N, Bailenson J. The proteus effect: the effect of transformed self‐representation on behavior. Hum Commun Res. 2007;33(3):271–290. doi: 10.1111/j.1468-2958.2007.00299.x [DOI] [Google Scholar]
- 44. Brewer LC, Kaihoi B, Schaepe K, Zarling K, Squires RW, Thomas RJ, Kopecky S. Patient‐perceived acceptability of a virtual world‐based cardiac rehabilitation program. Digit Health. 2017;3:2055207617705548. doi: 10.1177/2055207617705548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Brewer LC, Abraham H, Kaihoi B, Leth S, Egginton J, Slusser J, Scott C, Penheiter S, Albertie M, Squires R, et al. A community‐informed virtual world‐based cardiac rehabilitation program as an extension of center‐based cardiac rehabilitation: mixed methods analysis of a multicenter pilot study. J Cardiopulm Rehabil Prev. 2023;43:22–30. doi: 10.1097/HCR.0000000000000705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Content VG, Abraham HM, Kaihoi BH, Olson TP, Brewer LC. Novel virtual world‐based cardiac rehabilitation program to broaden access to underserved populations: a patient perspective. JACC Case Rep. 2022;4:911–914. doi: 10.1016/j.jaccas.2022.05.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. J Pharmacol Pharmacother. 2010;1:100–107. doi: 10.4103/0976-500x.72352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Manjunath C, Ifelayo O, Jones C, Washington M, Shanedling S, Williams J, Patten CA, Cooper LA, Brewer LC. Addressing cardiovascular health disparities in Minnesota: establishment of a community steering committee by FAITH! (Fostering African‐American Improvement in Total Health). Int J Environ Res Public Health. 2019;16:4144. doi: 10.3390/ijerph16214144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. American Association of Cardiovascular and Pulmonary Rehabilitation (AACVPR) . Risk Stratification Algorithm for Risk of Event. 2012. Accessed November 7, 2023. https://registry.dev.aacvpr.org/Documents/AACVPR%20Risk%20Stratification%20Algorithm_June2012.pdf
- 50. Keteyian SJ, Ades PA, Beatty AL, Gavic‐Ott A, Hines S, Lui K, Schopfer DW, Thomas RJ, Sperling LS. A review of the design and implementation of a hybrid cardiac rehabilitation program: an expanding opportunity for optimizing cardiovascular care. J Cardiopulm Rehabil Prev. 2022;42:1–9. doi: 10.1097/hcr.0000000000000634 [DOI] [PubMed] [Google Scholar]
- 51. Keteyian SJ, Grimshaw C, Brawner CA, Kerrigan DJ, Reasons L, Berry R, Peterson EL, Ehrman JK. A comparison of exercise intensity in hybrid versus standard phase two cardiac rehabilitation. J Cardiopulm Rehabil Prev. 2021;41:19–22. doi: 10.1097/hcr.0000000000000569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Damschroder LJ, Aron DC, Keith RE, Kirsh SR, Alexander JA, Lowery JC. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci. 2009;4:50. doi: 10.1186/1748-5908-4-50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Holtrop JS, Rabin BA, Glasgow RE. Qualitative approaches to use of the RE‐AIM framework: rationale and methods. BMC Health Serv Res. 2018;18:177. doi: 10.1186/s12913-018-2938-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kessler RS, Purcell EP, Glasgow RE, Klesges LM, Benkeser RM, Peek CJ. What does it mean to "employ" the RE‐AIM model? Eval Health Prof. 2013;36:44–66. doi: 10.1177/0163278712446066 [DOI] [PubMed] [Google Scholar]
- 55. Malone S, Prewitt K, Hackett R, Lin JC, McKay V, Walsh‐Bailey C, Luke DA. The clinical sustainability assessment tool: measuring organizational capacity to promote sustainability in healthcare. Implement Sci Commun. 2021;2:77. doi: 10.1186/s43058-021-00181-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lloyd‐Jones DM, Allen NB, Anderson CAM, Black T, Brewer LC, Foraker RE, Grandner MA, Lavretsky H, Perak AM, Sharma G, et al. Life's Essential 8: updating and enhancing the American Heart Association's construct of cardiovascular health: a presidential advisory from the American Heart Association. Circulation. 2022;146:e18–e43. doi: 10.1161/cir.0000000000001078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wang X, Ma H, Li X, Heianza Y, Manson JE, Franco OH, Qi L. Association of cardiovascular health with life expectancy free of cardiovascular disease, diabetes, cancer, and dementia in UK adults. JAMA Intern Med. 2023;183:340–349. doi: 10.1001/jamainternmed.2023.0015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Suarez AC, Brewer L, Abraham HM, Olson TP. Cardiovascular health in cardiac rehabilitation: applying the American Heart Association Life's Simple 7 in a center‐based cohort. Circulation. 2022;146:A13203. Abstract. doi: 10.1161/circ.146.suppl_1.13203 [DOI] [Google Scholar]
- 59. Lloyd‐Jones DM, Ning H, Labarthe D, Brewer L, Sharma G, Rosamond W, Foraker RE, Black T, Grandner MA, Allen NB, et al. Status of cardiovascular health in US adults and children using the American Heart Association's new "Life's essential 8" metrics: prevalence estimates from the National Health and nutrition examination survey (NHANES), 2013 through 2018. Circulation. 2022;146:822–835. doi: 10.1161/CIRCULATIONAHA.122.060911 [DOI] [PubMed] [Google Scholar]
- 60. Cerwinske LA, Rasmussen HE, Lipson S, Volgman AS, Tangney CC. Evaluation of a dietary screener: the Mediterranean Eating Pattern for Americans tool. J Hum Nutr Diet. 2017;30:596–603. doi: 10.1111/jhn.12451 [DOI] [PubMed] [Google Scholar]
- 61. Kim Y, Park I, Kang M. Convergent validity of the international physical activity questionnaire (IPAQ): meta‐analysis. Public Health Nutr. 2013;16:440–452. doi: 10.1017/s1368980012002996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. National Health and Nutrition Examination Survey . Centers for Disease Control and Prevention. September 2017. Accessed November 7, 2023. https://wwwn.cdc.gov/Nchs/Nhanes/2015‐2016/SMQRTU_I.htm
- 63. Manzar MD, BaHammam AS, Hameed UA, Spence DW, Pandi‐Perumal SR, Moscovitch A, Streiner DL. Dimensionality of the Pittsburgh Sleep Quality Index: a systematic review. Health Qual Life Outcomes. 2018;16:89. doi: 10.1186/s12955-018-0915-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Medina‐Inojosa JR, Grace SL, Supervia M, Stokin G, Bonikowske AR, Thomas R, Lopez‐Jimenez F. Dose of cardiac rehabilitation to reduce mortality and morbidity: a population‐based study. J Am Heart Assoc. 2021;10:e021356. doi: 10.1161/jaha.120.021356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Hammill BG, Curtis LH, Schulman KA, Whellan DJ. Relationship between cardiac rehabilitation and long‐term risks of death and myocardial infarction among elderly Medicare beneficiaries. Circulation. 2010;121:63–70. doi: 10.1161/circulationaha.109.876383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Bellet RN, Adams L, Morris NR. The 6‐minute walk test in outpatient cardiac rehabilitation: validity, reliability and responsiveness–a systematic review. Physiotherapy. 2012;98:277–286. doi: 10.1016/j.physio.2011.11.003 [DOI] [PubMed] [Google Scholar]
- 67. Jenkinson C, Layte R, Jenkinson D, Lawrence K, Petersen S, Paice C, Stradling J. A shorter form health survey: can the SF‐12 replicate results from the SF‐36 in longitudinal studies? J Public Health Med. 1997;19:179–186. doi: 10.1093/oxfordjournals.pubmed.a024606 [DOI] [PubMed] [Google Scholar]
- 68. Norman GJ, Carlson JA, Sallis JF, Wagner N, Calfas KJ, Patrick K. Reliability and validity of brief psychosocial measures related to dietary behaviors. Int J Behav Nutr Phys Act. 2010;7:56. doi: 10.1186/1479-5868-7-56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Carlson JA, Sallis JF, Wagner N, Calfas KJ, Patrick K, Groesz LM, Norman GJ. Brief physical activity‐related psychosocial measures: reliability and construct validity. J Phys Act Health. 2012;9:1178–1186. doi: 10.1123/jpah.9.8.1178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Anderson ES, Wojcik JR, Winett RA, Williams DM. Social‐cognitive determinants of physical activity: the influence of social support, self‐efficacy, outcome expectations, and self‐regulation among participants in a church‐based health promotion study. Health Psychol. 2006;25:510–520. doi: 10.1037/0278-6133.25.4.510 [DOI] [PubMed] [Google Scholar]
- 71. Anderson ES, Winett RA, Wojcik JR, Williams DM. Social cognitive mediators of change in a group randomized nutrition and physical activity intervention: social support, self‐efficacy, outcome expectations and self‐regulation in the guide‐to‐health trial. J Health Psychol. 2010;15:21–32. doi: 10.1177/1359105309342297 [DOI] [PubMed] [Google Scholar]
- 72. Sallis JF, Grossman RM, Pinski RB, Patterson TL, Nader PR. The development of scales to measure social support for diet and exercise behaviors. Prev Med. 1987;16:825–836. doi: 10.1016/0091-7435(87)90022-3 [DOI] [PubMed] [Google Scholar]
- 73. Kreuger RA. Focus Groups: A Practical Guide for Applied Research. 4th ed. SAGE Publications Ltd; 2009. [Google Scholar]
- 74. Beebe J. Rapid Assessment Process. Roman & Littlefield Publishers, Inc; 2001. [Google Scholar]
- 75. Hamilton AB, Finley EP. Qualitative methods in implementation research: an introduction. Psychiatry Res. 2019;280:112516. doi: 10.1016/j.psychres.2019.112516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Ozemek C, Babu AS, Arena R, Bond S, Network H‐P. Strategies to achieving the national 70% cardiac rehabilitation enrollment rate. J Cardiopulm Rehabil Prev. 2021;41:E14–E15. doi: 10.1097/HCR.0000000000000647 [DOI] [PubMed] [Google Scholar]
- 77. Ganeshan S, Jackson H, Grandis DJ, Janke D, Murray ML, Valle V, Beatty AL. Clinical outcomes and qualitative perceptions of in‐person, hybrid, and virtual cardiac rehabilitation. J Cardiopulm Rehabil Prev. 2022;42:338–346. doi: 10.1097/hcr.0000000000000688 [DOI] [PubMed] [Google Scholar]
- 78. Gold BC, Burke S, Pintauro S, Buzzell P, Harvey‐Berino J. Weight loss on the web: a pilot study comparing a structured behavioral intervention to a commercial program. Obesity (Silver Spring). 2007;15:155–164. doi: 10.1038/oby.2007.520 [DOI] [PubMed] [Google Scholar]
- 79. Freak‐Poli R, Hu J, Phyo AZZ, Barker SF. Social isolation and social support influence health service utilisation and survival after a cardiovascular disease event: a systematic review. Int J Environ Res Public Health. 2023;20:20. doi: 10.3390/ijerph20064853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Grant A, Tyner W, DeBoer L. Research & Policy INsights. Estimation of the Net Benefits of Indiana Statewide Adoption of Rural Broadband. 2018. Accessed November 7, 2023. https://pcrd.purdue.edu/wp‐content/uploads/2018/12/006‐RPINsights‐Indiana‐Broadband‐Study.pdf
- 81. Grant A, Tyner W. Research & Policy INsights: Benefit‐Cost Analysis for Implementation of Rural Broadband in the Tipmont Cooperative in Indiana. In: Purdue Center for Regional Development: Purdue University. 2018. Accessed November 7, 2023. https://pcrd.purdue.edu/wp‐content/uploads/2018/12/005‐RPINsights‐Tipmont‐Broadband.pdf
- 82. Fortuna KL, Kadakia A, Cosco TD, Rotondi A, Nicholson J, Mois G, Myers AL, Hamilton J, Brewer LC, Collins‐Pisano C, et al. Guidelines to establish an equitable mobile health ecosystem. Psychiatr Serv. 2023;74:393–400. doi: 10.1176/appi.ps.202200011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Van Iterson EH, Laffin LJ, Cho L. National, regional, and urban‐rural patterns in fixed‐terrestrial broadband internet access and cardiac rehabilitation utilization in the United States. Am J Prev Cardiol. 2023;13:100454. doi: 10.1016/j.ajpc.2022.100454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Vanzella LM, Oh P, Pakosh M, Ghisi GLM. Barriers to cardiac rehabilitation in ethnic minority groups: a scoping review. J Immigr Minor Health. 2021;23:824–839. doi: 10.1007/s10903-021-01147-1 [DOI] [PubMed] [Google Scholar]
- 85. An act making consolidated appropriations for the fiscal year ending September 30, 2023, and for providing emergency assistance for the situation in Ukraine, and for other purposes [Internet]. H.R. 2617. Section 4113. Advancing Telehealth Beyond COVID‐19. 2023. Accessed November 7, 2023. https://www.govinfo.gov/app/details/BILLS‐117hr2617enr/
- 86. Sustainable Cardiopulmonary Rehabilitation Services in the Home Act. Title XVIII. U.S.C. 1395x. Section 1861. 2023. Accessed November 7, 2023. https://www.sinema.senate.gov/wp‐content/uploads/2023/10/Sustainable‐Cardiopulmonary‐Rehabilitation‐Services‐in‐the‐Home‐Act.pdf


