Abstract
Rationale
There is conflicting evidence whether aerobic exercise training (AET) reduces pulse wave velocity (PWV) in adults with and without long-term conditions (LTCs).
Objective
To explore whether PWV improves with AET in adults with and without LTC, to quantify the magnitude of any effect and understand the influence of the exercise prescription.
Data sources
CENTRAL, MEDLINE and EMBASE were among the databases searched.
Eligibility criteria
We included studies with a PWV measurement before and after supervised AET of at least 3 weeks duration. Exclusion criteria included resistance exercise and alternative measures of arterial stiffness.
Design
Controlled trials were included in a random effects meta-analysis to explore the effect of AET on PWV. Uncontrolled studies were included in a secondary meta-analysis and meta-regression exploring the effect of patient and programme factors on change in PWV. The relevant risk of bias tool was used for each study design.
Results
79 studies (n=3729) were included: 35 controlled studies (21 randomised control trials (RCT) (n=1240) and 12 non-RCT (n=463)) and 44 uncontrolled (n=2026). In the controlled meta- analysis, PWV was significantly reduced following AET (mean (SD) 11 (7) weeks) in adults with and without LTC (mean difference −0.63; 95% CI −0.82 to −0.44; p<0.0001). PWV was similarly reduced between adults with and without LTC (p<0.001). Age, but not specific programme factors, was inversely associated with a reduction in PWV −0.010 (−0.020 to −0.010) m/s, p<0.001.
Discussion
Short-term AET similarly reduces PWV in adults with and without LTC. Whether this effect is sustained and the clinical implications require further investigation.
Keywords: Meta-Analysis, Systematic Reviews as Topic, Risk Factors
WHAT IS ALREADY KNOWN ON THIS TOPIC
There is conflicting evidence whether aerobic exercise training (AET) reduces pulse wave velocity (PWV) in adults with and without long-term conditions (LTCs).
WHAT THIS STUDY ADDS
We found that PWV is significantly and similarly reduced following AET in adults with and without LTC.
Older age was associated with larger reductions in PWV following AET.
In the meta-analyses, different components of the exercise prescription did not affect PWV.
Whether these results are sustained in the longer term is unknown, but measures of future cardiac risk should be included as outcomes for exercise programmes in people with LTCs.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
AET should form an integral part of the management of people with a range of LTCs with the aim of reducing future cardiovascular risk.
Introduction
Arterial stiffness (AS) is a surrogate measure of cardiovascular risk that can be used to predict future cardiovascular events and all-cause mortality.1 2 Pulse wave velocity (PWV) is a non- invasive measure of the speed of a pulse wave travelling between the two sites being assessed. A rise in AS is reflected by an increase in the velocity. The measurement of PWV from the carotid to the femoral, also known as carotid-femoral PWV (cfPWV), is the non-invasive clinical measure of PWV. An increase in aortic PWV of 1 m/s corresponds to an adjusted risk increase of 14% in cardiovascular events and 15% in cardiovascular mortality and all-cause mortality over a period of 7 years.3
Cardiovascular diseases are the leading cause of death globally.4 Cardiovascular risk factors, including physical inactivity, high caloric intake and higher levels of adiposity, are shared among many long-term conditions (LTCs). Thus, identifying populations at highest risk of cardiovascular disease using surrogate markers such as AS will ensure appropriate treatment is provided. However, there is minimal evidence regarding reducing increased cardiovascular risk in adults with and without LTCs.
Strategies to reduce cardiovascular risk have been proposed with aerobic exercise training (AET) shown to reduce AS in young healthy adults.5 There are inconsistent conclusions as to whether AET improves central and peripheral PWV in adults with LTC.6–8 It is also unclear how features of the exercise prescription (frequency, intensity, type of exercise and duration of intervention and sessions) impact the change in PWV.
To date, systematic reviews suggest that AET reduces PWV in a mixed cohort of adults with and without LTCs.9–11 This approach prevents an understanding of how AET influences PWV in each separate cohort (healthy and LTC). These reviews have included studies that prescribe concurrent aerobic and resistance exercise training, and therefore, do not isolate the effects of AET on PWV. Thus, this systematic review and meta-analysis aims to extend previous work by assessing the effect of AET on PWV in each discrete adult population (eg, healthy, cardiometabolic risk factors and LTC), while also determining the magnitude of effect on a population level. In addition to this, we aimed to understand the impact of the exercise prescription and any participant factors on the influence of AET on PWV.
Methods
This review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.12 This review was not registered on PROSPERO. The protocol was prospectively developed however it was not published.
Study eligibility
The inclusion criteria for this review were studies of adults, over 18 years, undergoing a supervised AET programme for a minimum of 3 weeks, with a PWV measurement before and after the intervention. Online supplemental material 1 includes the classification of the AET programmes. The exclusion criteria included trials with resistance exercise or combined aerobic and resistance/strength exercise training and interventions with no supervision (home-based exercise).
openhrt-2023-002384supp001.pdf (431.7KB, pdf)
Literature search
The Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, EMCARE, Cumulative Index to Nursing and Allied Health Literature (CINAHL) and PeDRO databases were searched on 16 July 2021 from conception. The search terms included “pulse wave velocity”, “exercise” and “adult” (see online supplemental material 1) for the full search terms. Eligible studies were selected by two independent reviewers.
Data extraction
The following information was extracted into a preformatted spreadsheet: (1) participant demographics, (2) disease/health characteristics, (3) exercise intervention characteristics, (4) method of the assessment of PWV and (5) outcome measures.
The measure of variability of the change in PWV (SDchange) was not reported in 47 of the 79 included studies. This was calculated using the test statistic (p value) (k=13) or using a correlation coefficient specific (k=34) for this review, as recommended by the Cochrane Handbook of systematic reviews.13
The risk of bias was assessed independently by two reviewers using the relevant tool; the Revised Cochrane risk-of-bias tool for randomised trials (RoB 2) for randomised control trials (RCTs), the Risk Of Bias In Non-randomised Studies of Interventions tool for the non- RCTs and the Quality Test Tool for Observational Cohort and Cross-sectional studies for the uncontrolled studies.
Outcomes
The primary outcome was central and peripheral PWV measured by applanation tonometry, oscillometry, cardiac MRI or ultrasound. The secondary outcomes were aspects of the exercise prescription. The participant factors include age, sex at birth, body mass index, systolic blood pressure, cholesterol, Hbin A1c and exercise capacity.
Statistical analysis
The analysis was performed using the ‘metafor’ package in R (V.4.1.1). The prescribed exercise intensity was reported in different manners and thus, classified into four categories as described previously14 (online supplemental e-Table 4).
Meta-regression was performed including participant factors, programme factors and methods measuring PWV, to explore the relationships between these factors and changes in PWV following AET. The meta-regression was controlled for age, sex-at-birth and baseline cfPWV. An increase in cfPWV has been linked with an increased risk of cardiovascular events, thus, this measure was used in the meta regression. An ‘estimate’ was produced representing the change in mean difference (MD) for one-unit change in the variable of interest. All the subanalyses were run twice; once unadjusted and once adjusted for the participant factors (see online supplemental material 1).
Publication bias was evaluated using funnel plots and Egger’s regression test.15 The heterogeneity of studies was assessed using the Cochrane Q statistics; p>0.1 signifies significant heterogeneity. Additionally, an I2 test can be used to evaluate the heterogeneity of the studies; <25% shows low risk of heterogeneity, 25%–75% shows moderate risk of heterogeneity, >75% specifies high risk of heterogeneity.16 A p<0.05 was considered statistically significant.
The certainty of evidence was rated using the Grading of Recommendations, Assessment, Development and Evaluations (GRADE) approach17 using the GRADEPro online tool.
Results
Study screening
The database searches identified 8471 publications of which 79 studies were included in the quantitative analysis (figure 1). Reasons for exclusion can be found in online supplemental e-Table 1. Characteristics of included studies are shown in table 1 for controlled trials and online supplemental e-Table 2 shows the uncontrolled studies. Uncontrolled studies (USs) were defined as studies without a control group such as cohort studies. From the 79 included studies, 30 were randomised controlled trials (RCTs), 12 were non-randomised controlled trials (non-RCTs), 2 were cross-over studies and 35 were USs.
Figure 1.
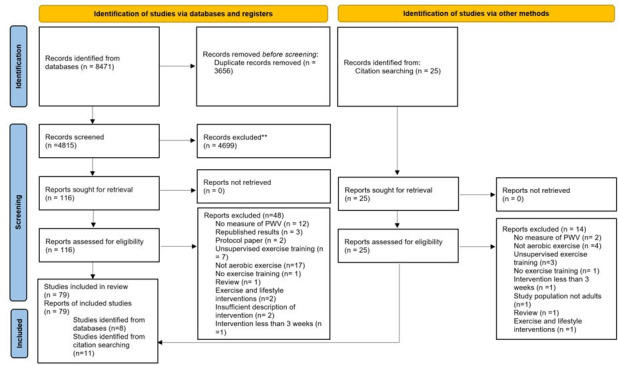
Preferred Reporting Items for Systematic Reviews and Meta-Analyses 2009 diagram of study selection. **Reasons for exclusion are detailed in online supplemental file 1.
Table 1.
Description of included randomised and non- randomised controlled trials
| Study | Country | Participant’s characteristics | Intervention group | Control group | |||||||
| N | Age (years) | Sex at birth (% male) |
BMI (kg/m2) |
Type of PWV measured | N | Age (years) | Sex at birth (% male) |
BMI (kg/m2) |
|||
| Randomised controlled trials | |||||||||||
| Adams et al,28 2017 | Canada | Testicular cancer survivors |
35 | 44 (12) | 100 | 27.2 (5.0) | cfPWV | 27 | 43 (10) | 100 | 27.9 (4.2) |
| Beck et al,23 2013 | USA | Prehypertension | 13 | 20 (1) | 69 | 28.7 (1.4) | cfPWV | 15 | 22 (1) | 67 | 27.0 (1.1) |
| Bouaziz et al,42 2019 | France | Healthy | 27 | 73 (3) | 30 | 28.7 (5.3) | cfPWV | 29 | 74 (3) | 23 | 28.8 (5.0) |
| Ciolac et al,43 2010 | Brazil | Healthy | 32 | 26 (5) | 0 | 23.9 (4.6) | cfPWV | 15 | 25 (4) | 0 | 25.3 (3.1) |
| Deiseroth et al,44 2019 | Switzerland | Healthy | 38 | 58 (5) | 82 | 33.3 (3.0) | cfPWV | 30 | 57 (6) | 37 | 33.1 (5.1) |
| Graham-Brown et al,27 2021 | UK | Haemodialysis | 51 | 56 (16) | 82 | aPWV | 50 | 59 (15) | 65 | ||
| Greenwood et al,45 2015 | UK | Kidney transplant recipients | 13 | 54 (11) | 77 | 26.6 (4.7) | cfPWV | 20 | 50 (11) | 50 | 27.3 (3.6) |
| Goldberg et al,46 2012 | Australia | Healthy | 15 | 21 (2) | 100 | 23.3 (1.9) | cfPWV | 15 | 21 (2) | 100 | 23.4 (2.3) |
| Ha et al,47 2018 | Korea | Healthy | 11 | 74 (2) | 0 | 23.9 (1.8) | cbPWV | 8 | 76 (6) | 0 | 29.1 (12.5) |
| Hannemann et al,48 2020 | Germany | Healthy | 12 | 34 (9) | 42 | 26.8 (5.6) | PWV | 12 | 37 (14) | 25 | 27.8 (6.9) |
| Hanssen et al,49 2017 | Switzerland | Episodic migraine | 25 | 36 (10) | 20 | 23.0 (6.9) | aPWV | 12 | 37 (12) | 17 | 23.4 (2.8) |
| Hasegawa et al,50 2018 | Japan | Healthy | 26 | 68 (7) | 50 | 23.5 (3.5) | cfPWV | 26 | 66 (9) | 50 | 24.7 (4.7) |
| Hasegawa et al,51 2018 | Japan | Healthy | 14 | 24 (5) | 100 | 21.9 (1.6) | aPWV | 7 | 21 (1) | 100 | 24.8 (3.4) |
| Headley et al,52 2014 | USA | Chronic kidney disease | 25 | 58 (8) | 64 | 34.9 (8.0) | aPWV | 21 | 57 (9) | 67 | 36.5 (8.9) |
| Heydari et al,7 2013 |
Australia | Healthy | 20 | 100 | 28.4 (2.4) | cfPWV | 21 | 100 | 29.0 (3.9) | ||
| Ho et al,53 2020 | Australia | Healthy | 30 | 54 (3) | 0 | 28.2 (3.5) | baPWV | 30 | 53 (3) | 0 | 27.4 (3.5) |
| Kang et al,25 2016 | Korea | Metabolic syndrome | 12 | 49 (11) | 0 | 26.7 (2.1) | baPWV | 11 | 51 (9) | 0 | 25.4 (3.1) |
| Kim et al,54 2017 | USA | Healthy | 27 | 65 (6) | 20 | 28.4 (4.0) | cfPWV | 11 | 63 (7) | 36 | 25.3 (4.6) |
| Koh et al,55 2010 | Australia | Haemodialysis | 15 | 52 (11) | 67 | 27.6 (7.2) | cfPWV | 16 | 51 (14) | 50 | 28.6 (7.3) |
| Madden et al,6 2013 | Canada | Hypertension complicated by type two diabetes and hyperlipidaemia | 25 | 69 (5) | 52 | 30.9 (5.0) | cfPWV | 27 | 70 (63) | 63 | 28.6 (4.2) |
| Madden et al,1 2009 | Canada | Hypertension complicated by type 2 diabetes and hyperlipidaemia | 17 | 72 (5) | 30.1 (4.5) | cfPWV | 17 | 71 (4) | 27.7 (4.1) | ||
| Mora-Rodriguez et al,26 2018 | Spain | Metabolic syndrome | 23 | 53 (9) | 83 | 32.8 (3.3) | cfPWV | 23 | 54 (9) | 83 | 32.9 (3.4) |
| Nualnim et al,21 2012 | USA | Prehypertension or stage 1 hypertension | 24 | 58 (10) | 29 | 29.0 (1.0) | cfPWV | 19 | 61 (9) | 21 | 31.0 (1.0) |
| Oliveira et al,56 2015 | Portugal | Acute myocardial infarction | 37 | 55 (11) | 87 | 26.5 (3.2) | cfPWV | 41 | 59 (11) | 81 | 27.1 (2.8) |
| Oudegeest-Sander et al,57 2013 | The Netherlands |
Healthy | 11 | 68 (3) | 73 | 27.0 (2.6) | cfPWV | 11 | 71 (5) | 27 | 24.3 (3.3) |
| Pascoalino et al,58 2015 | Brazil | Heart transplant recipients | 31 | 45 (17) | 74 | 26.7 (5.0) | cfPWV | 9 | 45 (18) | 56 | 25.1 (7.5) |
| Sugawara et al,59 2011 |
Japan | Healthy | 11 | 59 (7) | 0 | 23.4 (1.0) | cfPWV | 11 | 59 (7) | 0 | 21.6 (0.8) |
| Oliveira e Silva et al,60 2019 | Brazil | Chronic kidney disease on haemodialysis | 15 | 50 (17) | 47 | 25.7 (3.6) | cfPWV | 15 | 58 (15) | 53 | 26.7 (4.6) |
| Yoshizawa et al,61 2009 |
Japan | Healthy | 12 | 47 (7) | 0 | 24.6 (3.8) | cfPWV | 12 | 49 (10) | 0 | 21.8 (3.5) |
| Zempo-Miyaki et al,29 2016 |
Japan | Healthy | 16 | 54 (8) | 31 | 24.4 (4.4) | cfPWV | 16 | 67 (6) | 38 | 21.1 (2.0) |
| Non-randomised controlled trials | |||||||||||
| Aghaei Bahmanbeglou et al,22 2019 | Iran | Stage 1 hypertension |
20 | 48 (5) | 100 | 28.9 (5.0) | baPWV | 10 | 47 (3) | 100 | 29.5 (5.3) |
| Donley et al,18 2014 | USA | Healthy | 11 | 41 (13) | 36 | 24.0 (3.3) | cfPWV | 10 | 40 (13) | 20 | 25.0 (3.2) |
| Donley et al,18 2014 | USA | Metabolic syndrome | 11 | 46 (13) | 27 | 38.0 (6.6) | cfPWV | 11 | 44 (10) | 45 | 34.0 (6.6) |
| Fujie et al,62 2020 | Japan | Healthy | 27 | 21 (4) | 56 | 21.1 (1.6) | cfPWV | 9 | 21 (1) | 56 | 21.8 (2.1) |
| Fujie et al,62 2020 | Japan | Healthy | 26 | 67 (7) | 39 | 23.9 (3.6) | cfPWV | 14 | 68 (6) | 43 | 21.6 (4.1) |
| Holloway et al,63 2018 |
UK | Healthy | 12 | 21 (2) | 100 | 24.0 (3.0) | cfPWV | 9 | 21(2) | 100 | 23.0 (3.0) |
| Kim et al,64 2018 | Korea | Healthy | 28 | 67 (2) | 0 | cbPWV | 12 | 66 (4) | 0 | ||
| Mamen et al,65 2020 | Norway | Healthy | 19 | 43 (11) | PWV | 37 | 38 (12) | ||||
| Shenouda et al,66 2017 |
Canada | Healthy | 19 | 28 (8) | 100 | 26.5 (5.4) | cfPWV | 6 | 26 (8) | 100 | 25.0 (7.0) |
| Soriano-Maldonado et al,67 2017 | Spain | Systemic lupus erythematosus |
26 | 43 (15) | 0 | 25.9 (3.4) | PWV | 32 | 45 (13) | 0 | 24.7 (5.6) |
| Wong et al,68 2018 | USA | Postmenopausal women with stage 2 hypertension | 20 | 59 (4) | 0 | 24.2 (3.6) | baPWV | 21 | 59 (5) | 0 | 23.8 (3.7) |
| Vivodtzev et al,69 2010 |
France | COPD | 10 | 62 (9) | 80 | 23.0 (5.0) | cbPWV | 7 | 63 (6) | 57 | 23.0 (4.0) |
| Cross-over trials | |||||||
| Study | Country | Participant’s characteristics | N | Age (years) | Sex at birth (% male) | BMI (kg/m2) | Type of PWV measured |
| Ferrier et al,24 2001 | Australia | Isolated systolic hypertension | 10 | 64 (7) | 50 | 29.1 (3.2) | cfPWV |
| Toussaint et al,70 2008 | Australia | End-stage kidney disease on haemodialysis | 9 | 69 (8) | 56 | 27.0 (4.0) | cfPWV |
| Toussaint et al,70 2008 | Australia | End-stage kidney disease on haemodialysis | 10 | 61 (16) | 40 | 24.0 (4.0) | cfPWV |
Data are expressed as mean (SD) unless specified.
aPWV, aortic PWV; baPWV, brachial-ankle PWV; BMI, body mass index; cbPWV, carotid-brachial PWV; cfPWV, carotid-femoral PWV.COPD, chronic obstructive pulmonary disease; PWV, pulse wave velocity;
Study characteristics
The total number of participants was 3076 (47% male) with a mean age of 48 (range 20–76) years. The sample sizes ranged from 6 to 71 participants and the mean duration of the intervention was 11 weeks (range: 4–52 weeks). The studies had measures of cfPWV (k=47; n=1813), aortic PWV (k=10; n=306) brachial-ankle PWV (k=13; n=363), carotid-brachial PWV (k=3; n=76), unspecified PWV (k=6; n=157) or radial PWV (k=1; n=11). Significant reductions were observed in studies that had measures of carotid-femoral, brachial ankle and carotid-brachial PWV following short-term AET (online supplemental e-Table 5). There was no difference in the method of assessing PWV (online supplemental e-Table 6).
Of the 79 studies, 25 had 2 intervention groups of which 7 were controlled studies. The studies were divided into two categories according to their population: healthy adults (k=50) and adults with LTC (k=55). Three studies possessed two intervention groups whereby one group consisted of adults with LTC and the other group included healthy adults.18–20
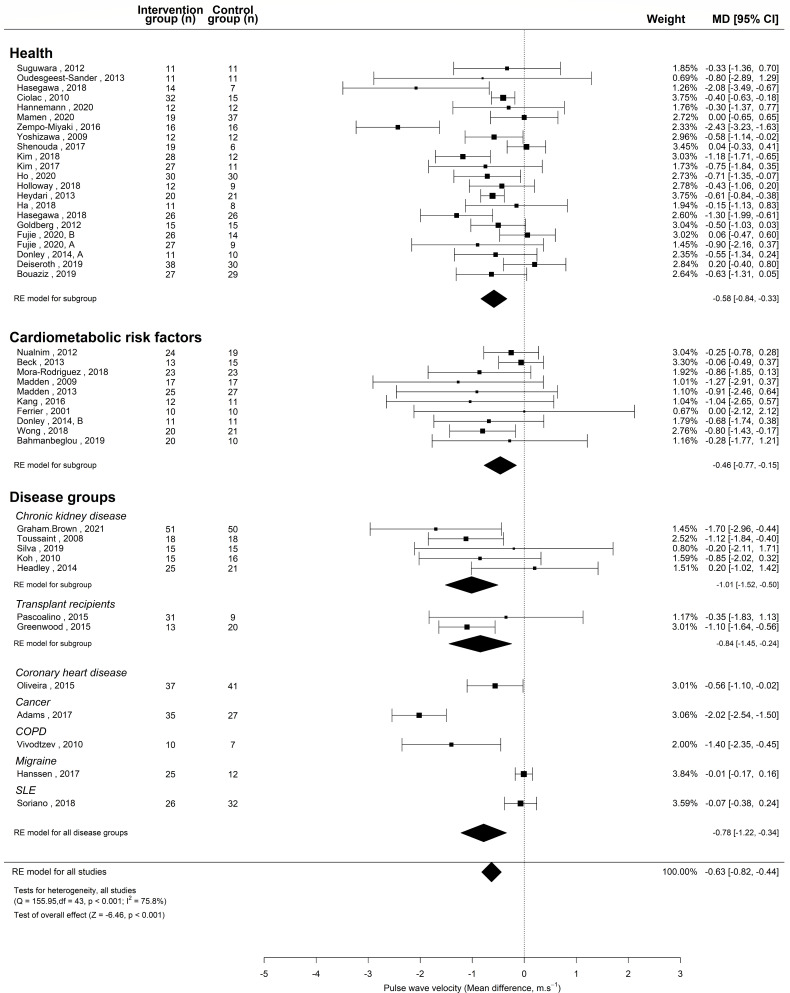
For the controlled meta-analysis, the LTCs were classified into cardiometabolic risk factors and disease groups. The cardiometabolic risk factors contained study populations of prehypertension and stage I hypertension,21–23 isolated systolic hypertension,24 metabolic syndrome18 25 26 and hypertension complicated by type II diabetes and hyperlipidaemia.1 6 The disease groups can be seen in figure 2.
Figure 2.
Meta-analysis of controlled trials assessing the effect of aerobic exercise training in health and long-term condition. MD, mean difference; RE model, random effects model.
Table 2 compares the demographics of the studies conducted in adults with and without LTC. The mean cfPWV of the studies conducted in healthy populations and adults with LTC were 8.04 and 9.23 m/s, respectively. Details of the training programmes for the controlled trials are shown in online supplemental e-Table 3.
Table 2.
Study demographics between studies in health and long-term conditions
| Baseline demographics | Health (k=50; n=1666) | Long-term conditions (k=55; n=2063) |
| Age (years) | 44±6 | 52±10 |
| Male (%) | 47 | 46 |
| BMI (kg/m2) | 26.0±3.9 | 28.5±4.9 |
| cfPWV (m/s) | 8.17±2.15 | 9.23±2.48 |
| BaPWV (m/s) | 11.39±1.14 | 14.74±2.96 |
| SBP (mm Hg) | 123±13 | 132±21 |
| DBP (mm Hg) | 74±9 | 78±14 |
| VO2max (mL/kg/min) | 32.14±15.44 | 24.50±5.45 |
| Heart rate (beats/min) | 75±16 | 68±9 |
| Glucose (mg/dL) | 5.23±1.10 | 6.23±1.63 |
| Cholesterol (mg/dL) | 4.97±0.90 | 5.22±1.61 |
| HDL cholesterol (mg/dL) | 7.45±2.42 | 3.94±2.00 |
| LDL cholesterol (mg/dL) | 2.94±0.74 | 3.12±1.45 |
| Triglycerides (mg/dL) | 1.05±0.59 | 2.18±2.32 |
Data are expressed as mean±SD unless specified.
baPWV, brachial ankle pulse wave velocity; BMI, body mass index; cfPWV, carotid-femoral pulse wave velocity; DBP, diastolic blood pressure; HDL, cholesterol, high-density lipoprotein; K, number of studies in this category; LDL, cholesterol, low-density lipoprotein; PWV, pulse wave velocity; SBP, systolic blood pressure; VO2max, maximum rate of oxygen consumption.
Effect of AET on PWV
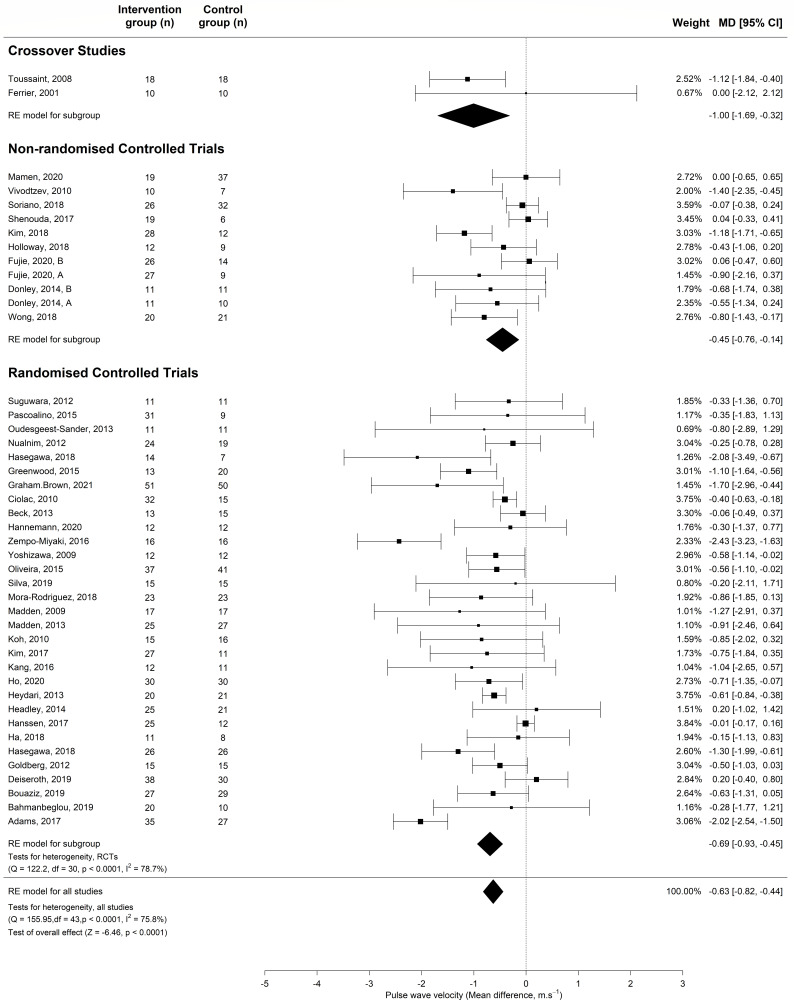
The meta-analysis of controlled studies (RCTs, non-RCTs and crossover studies) also showed a significant reduction in PWV (MD −0.63; 95% CI −0.82 to −0.44; p<0.0001) (figure 3). There was a reduction in PWV across all study designs (RCTs: MD −0.73; 95% CI −0.99 to -0.47, non-RCTs: MD −0.44; 95% CI −0.74 to -0.14, cross-over studies: MD −1.00; 95% CI −1.69 to −0.32). The larger meta-analysis combining all 79 controlled and USs showed AET significantly reduces PWV (MD −0.47; 95% CI −0.57 to −0.36; p<0.0001).
Figure 3.
Meta-analysis of controlled trials assessing the effect of aerobic exercise training vs control on PWV in adults. MD, mean difference; PWV, pulse wave velocity. RE model, random effects model
PWV was significantly reduced following AET in a meta-analysis of studies measuring PWV using carotid-femoral (MD −0.63; 95% CI −0.82 to −0.44; p<0.0001), brachial-ankle (MD −0.74; 95% CI −1.15 to −0.32; p<0.0005) and carotid-brachial (MD −0.97; 95% CI −1.62 to −0.32; p<0.005) (online supplemental e-Table 5).
Comparing adults with and without LTCs
In the meta-analysis of controlled and US, the effect of AET on PWV was not different between the studies conducted in healthy populations and those conducted in adults with LTC. Following AET, PWV was significantly reduced in adults with LTC (MD −0.86; 95% CI −1.36 to −0.37; p<0.0001) and healthy adults (MD −0.59; 95% CI −0.83 to −0.35; p<0.0001).
Subgroup and meta-regression analysis
The results of the meta-regression indicated that the population type (healthy or with LTC) did not reach statistical significance in the controlled studies (p=0.19). The meta-regression conducted in all studies also showed that the presence of LTC does not affect the impact AET has on PWV (p=0.06) with and without adjustment for age, sex at birth and baseline PWV (p=0.77).
The meta-regression for programme and subject characteristics are shown in tables 3 and 4, respectively. Age was the strongest predictor of the improvement of PWV following AET, with an inverse relationship (table 3), and remained significant after controlling for sex at birth, presence of LTC and baseline PWV (table 3). The reduction in PWV following AET was independent of all aspects of the exercise prescription and health parameters reported (table 4).
Table 3.
Meta-regression of participant characteristics
| Variable | Meta-regression ΔPWV (uncontrolled) | Meta-regression controlled for age, sex at birth, baseline PWV and presence of long-term conditions | ||||
| N= | Estimate (95% CI) | P value | N= | Estimate (95% CI) | P value | |
| Age | 100 | 0.01 (−0.02 to −0.005) | 0.0005* | 98 | 0.01 (−0.02 to −0.003) | 0.008* |
| Sex at birth | 99 | 0.001 (−0.004 to 0.002) | 0.46 | 98 | 0.003 (−0.006 to 0.0004) | 0.10 |
| BMI | 89 | 0.0003 (−0.03 to 0.03) | 0.99 | 84 | 0.02 (−0.01 to 0.06) | 0.22 |
| Systolic blood pressure | 97 | 0.01 (−0.02 to −0.0009) | 0.03* | 91 | 0.004 (−0.01 to 0.02) | 0.59 |
| Cholesterol | 51 | 0.02 (−0.10 to 0.15) | 0.74 | 46 | 0.03 (−0.18 to 0.12) | 0.73 |
| HbA1c | 21 | 0.01 (−0.03 to 0.008) | 0.26 | 18 | 0.007 (−0.25 to 0.26) | 0.96 |
| VO2 max | 65 | 0.01 (−0.006 to 0.03) | 0.23 | 61 | 0.004 (−0.03 to 0.02) | 0.78 |
*p≤0.05.
BMI, body mass index; HbA1C, Glycosylated Haemoglobin; PWV, pulse wave velocity; VO2 max, maximum rate of oxygen consumption.
Table 4.
Meta-regression of programme factors
| Variable | Meta-regression ΔPWV | Meta-regression controlled for age, sex at birth, baseline PWV and presence of long-term conditions | ||||
| No of studies | Estimate (95% CI) | P value | No of studies | Estimate (95% CI) | P value | |
| Duration programme of | 104 | 0.01 (−0.03 to 0.01) | 0.40 | 98 | 0.001 (−0.03 to 0.02) | 0.94 |
| Frequency | 103 | 0.03 (−0.20 to 0.14) | 0.73 | 97 | 0.01 (−0.18 to 0.16) | 0.94 |
| No of sessions | 103 | 0.004 (−0.01 to 0.004) | 0.34 | 97 | 0.0003 (−0.008 to 0.008) | 0.94 |
| Intensity | 104 | 0.05 (−0.19 to 0.08) | 0.44 | 98 | 0.09 (−0.22 to 0.04) | 0.19 |
| Duration of exercise session | 102 | 0.001 (−0.007 to 0.01) | 0.74 | 96 | 0.0006 (−0.009 to 0.008) | 0.88 |
| Total exercise duration | 99 | 0.0007 (−0.01 to 0.009) | 0.88 | 95 | 0.0005 (−0.01 to 0.01) | 0.93 |
PWV, pulse wave velocity.
Sensitivity analyses
In the meta-analysis of the controlled studies, three studies27–29 had a significant influence on the results potentially due to these studies showing the biggest change in PWV in the intervention group. Thus, a sensitivity analysis excluding these studies showed an MD −0.52 m/s; (95% CI −0.68 to −0.37; p<0.0001) and there was no difference between this sensitivity analysis and main meta-analysis (p=0.34). Subgroup analysis of methods of calculating SD was statistically different (p=0.02), with the greatest change in PWV using presented test statistic (p values and CIs) (MD −1.03; 95% CI −1.34 to −0.73) and the smallest change seen in studies for which SD was imputed (MD −0.44; 95% CI −0.73 to −0.15).
Bias and heterogeneity
Across the studies, the risk of bias was low except for blinding of participants and personnel, for which the risk was high in 54 studies (68%). There was no difference (p=0.45) between the effect of AET on PWV in studies with a high risk of bias (MD −0.80; 95% CI −1.26 to −0.33) and studies with low risk of bias (MD −0.60; 95% CI −0.82 to −0.37).
Egger’s regression test for publication bias was significant for the controlled and US (p=0.0014), however, there was no publication bias within the controlled studies (p=0.109). Heterogeneity was high for the meta-analysis of RCTs alone (I2=74.1%), this is also seen in the meta-analysis of all studies (I2=72.16%).
Certainty of evidence
The quality of evidence supporting the conclusion that AET reduces PWV in adults with and without LTC was assessed as very low.
Discussion
After pooling data from 79 studies, this systematic review and meta-analysis suggests short-term AET significantly reduces PWV in adults with and without LTC by a similar magnitude. The participant factors were not associated with greater improvement in PWV, however, cohorts of older age had a greater reduction in PWV following AET than younger cohorts. The impact of PWV was independent of all recorded aspects of the exercise prescription. Similarly, the technique used to measure PWV, which includes cfPWV, brachial-ankle PWV and carotid-brachial PWV, did not affect the findings and neither did the equipment used to measure PWV.
The magnitude of the change in PWV from this review is −0.63 m/s across health and disease, which is potentially associated with a risk reduction of 9.3% for cardiovascular events, 10.0% for cardiovascular and all-cause mortality over 7 years, extrapolated from existing data.3 The subanalyses showed that the presence of LTC does not impact the influence AET has on PWV. This implies that the beneficial effects of AET on vascular stiffness is not limited to healthy adults and exercise programmes incorporating AET could be of benefit for adults regardless of their health status to reduce the risk of cardiovascular events.
Eleven conditions have been collated in this review to explore the effect of AET on PWV, however, it is evident that this association has not been investigated in various conditions, including inflammatory disorders and mental health conditions associated with high levels of sedentary behaviour. Considering inflammatory markers are associated with AS and patients with primary inflammatory disorders have increased vascular stiffness,30 an AET programme could reduce the risk of cardiovascular events in adults with inflammatory conditions. AET programmes could also benefit conditions such as depression and anxiety that are associated with decreased levels of physical activity, often linked to a rise in cardiovascular risk. Rehabilitation programmes for people with chronic obstructive pulmonary disease (COPD) and chronic heart failure (CHF) usually target improvements in exercise capacity and quality of life rather than improvements in cardiovascular risk, and therefore, the effect of AET on AS has rarely been studied in these populations.
AET was chosen as the intervention as this modality confers beneficial effects on the heart and increases exercise capacity. Reduction in central and peripheral stiffness has been reported following AET in healthy populations31–35 and hypertensive adults.36 The underlying mechanisms by which AET reduces vascular stiffness is unknown, however, evidence suggests it may be via arterial remodelling, improved endothelial function and decreased sympathetic tone.31
Previous systematic reviews comparing the influences of aerobic, resistance and combined (aerobic and resistance) exercise training on a range of measures of vascular stiffness collectively report that AET significantly improves vascular stiffness whereas resistance and combined exercise had no effect in a mixed cohort of adults with and without LTC.9 11 A common hypothesis suggests the resistance exercise component of the combined exercise could limit the improvement in AS, as resistance training has been linked to increased PWV.37 Despite the influence of resistance exercise on vascular stiffness, this mode of exercise is associated with many cardiometabolic benefits including reduction of resting blood pressure and prevention and management of type 2 diabetes.38
In contrast to previous systematic reviews,9–11 this review collates studies with supervised AET rather than concurrent aerobic and resistance exercise training interventions. Despite the similar change in PWV after AET across the systematic reviews, updated studies have been added to this review alongside US enabling a clear understanding of the impact that AET has on PWV. This review also differs from the previous reviews as it explores whether any aspect of the exercise prescription or the population demographics influences the change in PWV.
The quality of evidence in this review was graded very low with the main reason being the inclusion of observational studies (non-RCTs) alongside the RCTs. The high heterogeneity may also contribute to the low grading.
Strengths and limitations
The main strength of this systematic review was the focus on AET in isolation to remove the possible confounding of resistance training, and the inclusion of US to provide a larger dataset for meta-regression, allowing exploration of the impact of patient and programme factors on change in PWV.
The main limitation of this study is that it explores the effect of an exercise intervention on PWV when it is unknown whether the reduction in cardiovascular events associated with AET is mediated by AS or an alternative mechanism.
The mean duration of the AET programmes in the included studies was 11 weeks. Although a reduction in PWV was observed, the long-term benefits of AET on PWV, cardiovascular health and the effect of detraining on PWV remain unknown. One trial reported that PWV returned to baseline levels after 1 month of detraining.39
Poor reporting of exercise interventions, specifically training intensity, potentially reduced the power of the meta-regression to detect an effect for patient and programme factors. There was a greater change in PWV observed in studies where SD was estimated using presented test statistics compared with using correlation coefficient. A potential explanation is that studies reporting test statistics often show significant results demonstrating precise results with greater effects.
The included studies possessed small sample sizes (number of participants range: 6–71), indicating the studies could be predominantly underpowered. High risk of bias was present in study designs other than RCTs due to a lack of participant blinding, potentially leading to performance bias, however, this is very difficult to avoid in studies of exercise training. The presence of publication bias could indicate successful trials are more likely to be published, supported by the three positive studies that had a large influence on the change in PWV. The number of studies in individual diseases was small, suggesting the need for further exploration in these diseases.
Narrowing the focus of this review to AET restricts the application of these results to interventions that combine AET with other factors (eg, resistance training or dietary modification). In turn, this excludes populations with LTC, such as COPD and CHF, for whom rehabilitation programmes lead to beneficial effects on symptoms often include resistance and AET.40 41 Pharmacological interventions, includinganti-hypertensive and lipid-lowering medications, can influence PWV but were not taken into consideration in this review.
Finally, confining the measure of AS to PWV could limit the generalisability of the results. Although combining different measures of AS such as PWV, carotid-intima thickness and flow‐mediated vasodilation would illustrate the overall picture of AS, these parameters cannot be compared as each measure assesses a different aspect of vascular stiffness.
Conclusion
PWV is reduced following short-term AET in both adults with and without LTC, and the overall magnitude of the change is −0.63 m/s, which is of prognostic importance. There was no difference between the effect of AET on PWV between healthy populations and populations with LTC, however, cohorts with older mean age showed greater improvement in PWV following AET. The exercise prescription did not influence the impact of AET on PWV. However, the overall quality of data was graded very low, with high heterogeneity and inclusion of observational studies. Despite the paucity of studies in specific LTC, AET should form an integral part of the management of people with a range of LTCs with the aim of reducing future cardiovascular risk.
Footnotes
Twitter: @majda_bakali
Contributors: MB was involved in the conception, design, methodology, writing and revising of the manuscript and is responsible for the overall content as the guarantor. RAE, MG-B, GPM, TY, and MCS contributed to the protocol. MB and PD devised the search strategy and PD performed the searches. MB, GMH, TCJW, AVJ and RAE screened the studies. MB and AVJ extracted the data from the eligible studies. MB, AVJ and ED assessed the quality of the studies. TCJW performed the statistical analysis. RAE supervised and was involved in writing the original draft. All authors contributed to the article and approved the submitted version.
Funding: MB is funded by a National Institute for Health Research (NIHR) Biomedical Research Council (BRC)/Leicester Precision Medicine Institute (LPMI) Studentship. RAE is funded by NIHR Clinician Scientist Fellowship CS-2016-16-020.
Disclaimer: The views expressed are those of the authors and no necessarily those of the National Health Service, the NIHR, or the department of health.
Competing interests: None declared.
Provenance and peer review: Not commissioned; internally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available on reasonable request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Not applicable.
References
- 1.Madden KM, Lockhart C, Cuff D, et al. Short-term aerobic exercise reduces arterial stiffness in older adults with type 2 diabetes, hypertension and hypercholesterolemia. Diabetes 2009;32:1531–5. 10.2337/dc09-0149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benetos A. Pulse pressure and arterial stiffness in type 1 diabetic patients. J Hypertens 2003;21:2005–7. 10.1097/00004872-200311000-00005 [DOI] [PubMed] [Google Scholar]
- 3.Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol 2010;55:1318–27. 10.1016/j.jacc.2009.10.061 [DOI] [PubMed] [Google Scholar]
- 4.Organisation, W.H . Cardiovascular diseases. 2022. Available: https://www.who.int/health-topics/cardiovascular-diseases#tab=tab_2
- 5.Currie KD, Thomas SG, Goodman JM. Effects of short-term endurance exercise training on vascular function in young males. Eur J Appl Physiol 2009;107:211–8. 10.1007/s00421-009-1116-4 [DOI] [PubMed] [Google Scholar]
- 6.Madden KM, Lockhart C, Cuff D, et al. Aerobic training-induced improvements in arterial stiffness are not sustained in older adults with multiple cardiovascular risk factors. J Hum Hypertens 2013;27:335–9. 10.1038/jhh.2012.38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heydari M, Boutcher YN, Boutcher SH. High-intensity intermittent exercise and cardiovascular and autonomic function. Clin Auton Res 2013;23:57–65. 10.1007/s10286-012-0179-1 [DOI] [PubMed] [Google Scholar]
- 8.Ciolac EG, Bocchi EA, Bortolotto LA, et al. Effects of high-intensity aerobic interval training versus moderate exercise on hemodynamic, metabolic and neuro-humoral abnormalities of young normotensive women at high familial risk for hypertension. Hypertens Res 2010;33:836–43. 10.1038/hr.2010.72 [DOI] [PubMed] [Google Scholar]
- 9.Ashor AW, Lara J, Siervo M, et al. Effects of exercise modalities on arterial stiffness and wave reflection: a systematic review and meta-analysis of randomized controlled trials. PLoS One 2014;9:e110034. 10.1371/journal.pone.0110034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang C, Wang J, Deng S, et al. The effects of aerobic endurance exercise on pulse wave velocity and intima media thickness in adults: a systematic review and meta-analysis. Scandinavian Med Sci Sports 2016;26:478–87. 10.1111/sms.12495 [DOI] [PubMed] [Google Scholar]
- 11.Montero D, Roche E, Martinez-Rodriguez A. The impact of aerobic exercise training on arterial stiffness in pre- and hypertensive subjects: a systematic review and meta-analysis. Int J Cardiol 2014;173:361–8. 10.1016/j.ijcard.2014.03.072 [DOI] [PubMed] [Google Scholar]
- 12.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta- analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol 2009;62:e1–34. 10.1016/j.jclinepi.2009.06.006 [DOI] [PubMed] [Google Scholar]
- 13.Higgins JPT, Thomas J, Chandler J, et al. Cochrane Handbook for systematic reviews of interventions. Version 6.3. Cochrane; 2022. [Google Scholar]
- 14.Ward TJC, Plumptre CD, Dolmage TE, et al. Change in V.O2Peak in response to aerobic exercise training and the relationship with exercise prescription in people with COPD: a systematic review and meta-analysis. Chest 2020;158:131–44. 10.1016/j.chest.2020.01.053 [DOI] [PubMed] [Google Scholar]
- 15.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Higgins JPT, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924–6. 10.1136/bmj.39489.470347.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Donley DA, Fournier SB, Reger BL, et al. Aerobic exercise training reduces arterial stiffness in metabolic syndrome. J Appl Physiol (1985) 2014;116:1396–404. 10.1152/japplphysiol.00151.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fantin F, Rossi A, Morgante S, et al. Supervised walking groups to increase physical activity in elderly women with and without hypertension: effect on pulse wave velocity. Hypertens Res 2012;35:988–93. 10.1038/hr.2012.85 [DOI] [PubMed] [Google Scholar]
- 20.Gelinas JC, Lewis NC, Harper MI, et al. Aerobic exercise training does not alter vascular structure and function in chronic obstructive pulmonary disease. Exp Physiol 2017;102:1548–60. 10.1113/EP086379 [DOI] [PubMed] [Google Scholar]
- 21.Nualnim N, Barnes JN, Tarumi T, et al. Comparison of central artery elasticity in swimmers, runners, and the sedentary. Am J Cardiol 2011;107:783–7. 10.1016/j.amjcard.2010.10.062 [DOI] [PubMed] [Google Scholar]
- 22.Aghaei Bahmanbeglou N, Ebrahim K, Maleki M, et al. Short-duration high-intensity interval exercise training is more effective than long duration for blood pressure and arterial stiffness but not for inflammatory markers and lipid profiles in patients with stage 1 hypertension. J Cardiopulm Rehabil Prev 2019;39:50–5. 10.1097/HCR.0000000000000377 [DOI] [PubMed] [Google Scholar]
- 23.Beck DT, Martin JS, Casey DP, et al. Exercise training reduces peripheral arterial stiffness and myocardial oxygen demand in young prehypertensive subjects. Am J Hypertens 2013;26:1093–102. 10.1093/ajh/hpt080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferrier KE, Waddell TK, Gatzka CD, et al. Aerobic exercise training does not modify large-artery compliance in isolated systolic hypertension. Hypertension 2001;38:222–6. 10.1161/01.hyp.38.2.222 [DOI] [PubMed] [Google Scholar]
- 25.Kang SJ, Kim EH, Ko KJ. Effects of aerobic exercise on the resting heart rate, physical fitness, and arterial stiffness of female patients with metabolic syndrome. J Phys Ther Sci 2016;28:1764–8. 10.1589/jpts.28.1764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mora‐Rodriguez R, Ramirez‐Jimenez M, Fernandez‐Elias VE, et al. Effects of aerobic interval training on arterial stiffness and microvascular function in patients with metabolic syndrome. J of Clinical Hypertension 2018;20:11–8. 10.1111/jch.13130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Graham-Brown MPM, March DS, Young R, et al. A randomized controlled trial to investigate the effects of Intra- dialytic cycling on left ventricular mass. Kidney International 2021;99:1478–86. 10.1016/j.kint.2021.02.027 [DOI] [PubMed] [Google Scholar]
- 28.Adams SC, DeLorey DS, Davenport MH, et al. Effects of high-intensity aerobic interval training on cardiovascular disease risk in testicular cancer survivors: a phase 2 randomized controlled trial. Cancer 2017;123:4057–65. 10.1002/cncr.30859 [DOI] [PubMed] [Google Scholar]
- 29.Zempo-Miyaki A, Fujie S, Sato K, et al. Elevated Pentraxin 3 level at the early stage of exercise training is associated with reduction of arterial stiffness in middle-aged and older adults. J Hum Hypertens 2016;30:521–6. 10.1038/jhh.2015.105 [DOI] [PubMed] [Google Scholar]
- 30.Jain S, Khera R, Corrales-Medina VF, et al. Inflammation and arterial stiffness in humans. Atherosclerosis 2014;237:381–90. 10.1016/j.atherosclerosis.2014.09.011 [DOI] [PubMed] [Google Scholar]
- 31.Gralla MH, McDonald SM, Breneman C, et al. Associations of objectively measured vigorous physical activity with body composition, cardiorespiratory fitness, and cardiometabolic health in youth: a review. Am J Lifestyle Med 2019;13:61–97. 10.1177/1559827615624417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee CD, Blair SN, Jackson AS. Cardiorespiratory fitness, body composition, and all- cause and cardiovascular disease mortality in men. Am J Clin Nutr 1999;69:373–80. 10.1093/ajcn/69.3.373 [DOI] [PubMed] [Google Scholar]
- 33.Pereira MS. Physical Activity and Cardiorespiratory Fitness. Dordrecht: Springer Netherlands, 2007. [Google Scholar]
- 34.Nocon M, Hiemann T, Müller-Riemenschneider F, et al. Association of physical activity with all-cause and cardiovascular mortality: a systematic review and meta-analysis. Eur J Cardiovasc Prev Rehabil 2008;15:239–46. 10.1097/HJR.0b013e3282f55e09 [DOI] [PubMed] [Google Scholar]
- 35.Hu FB, Willett WC, Li T, et al. Adiposity as compared with physical activity in predicting mortality among women. N Engl J Med 2004;351:2694–703. 10.1056/NEJMoa042135 [DOI] [PubMed] [Google Scholar]
- 36.Lopes S, Afreixo V, Teixeira M, et al. Exercise training reduces arterial stiffness in adults with hypertension: a systematic review and meta-analysis. J Hypertens (Los Angel) 2021;39:214–22. 10.1097/HJH.0000000000002619 [DOI] [PubMed] [Google Scholar]
- 37.Szekely LA, Oelberg DA, Wright C, et al. Preoperative predictors of operative morbidity and mortality in COPD patients undergoing bilateral lung volume reduction surgery. Chest 1997;111:550–8. 10.1378/chest.111.3.550 [DOI] [PubMed] [Google Scholar]
- 38.Westcott WL. Resistance training is medicine: effects of strength training on health. Curr Sports Med Rep 2012;11:209–16. 10.1249/JSR.0b013e31825dabb8 [DOI] [PubMed] [Google Scholar]
- 39.Mustata S, Chan C, Lai V, et al. Impact of an exercise program on arterial stiffness and insulin resistance in hemodialysis patients. J Am Soc Nephrol 2004;15:2713–8. 10.1097/01.ASN.0000140256.21892.89 [DOI] [PubMed] [Google Scholar]
- 40.Fu T-C, Huang S-C, Hsu C-C, et al. Cardiac rehabilitation in patients with heart failure. Acta Cardiol Sin 2014;30:353–9. [PMC free article] [PubMed] [Google Scholar]
- 41.Flynn KE, Piña IL, Whellan DJ, et al. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA 2009;301:1451–9. 10.1001/jama.2009.457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bouaziz W, Lang P-O, Schmitt E, et al. Effects of a short-term interval aerobic training program with recovery bouts on vascular function in sedentary aged 70 or over: a randomized controlled trial. Arch Gerontol Geriatr 2019;82:217–25. 10.1016/j.archger.2019.02.017 [DOI] [PubMed] [Google Scholar]
- 43.Ciolac EG, Bocchi EA, Bortolotto LA, et al. Effects of high intensity interval training versus moderate exercise on hemodynamic, metabolic, and neuro-humoral abnormalities of young normotensive women at high familial risk for hypertension. Hypertens Res 2010;33:836–43. 10.1038/hr.2010.72 [DOI] [PubMed] [Google Scholar]
- 44.Deiseroth A, Streese L, Köchli S, et al. Exercise and arterial stiffness in the elderly: a combined cross-sectional and randomized controlled trial (EXAMIN AGE). Front Physiol 2019;10:1119. 10.3389/fphys.2019.01119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Greenwood SA, Koufaki P, Mercer TH, et al. Aerobic or resistance training and pulse wave velocity in kidney transplant recipients: a 12-week pilot randomized controlled trial (the exercise in renal transplant [exert] trial). Am J Kidney Dis 2015;66:689–98. 10.1053/j.ajkd.2015.06.016 [DOI] [PubMed] [Google Scholar]
- 46.Goldberg MJ, Boutcher SH, Boutcher YN. The effect of 4 weeks of aerobic exercise on vascular and Baroreflex function of young men with a family history of hypertension. J Hum Hypertens 2012;26:644–9. 10.1038/jhh.2011.95 [DOI] [PubMed] [Google Scholar]
- 47.Ha M-S, Kim J-H, Kim Y-S, et al. Effects of Aquarobic exercise and Burdock intake on serum blood lipids and vascular elasticity in Korean elderly women. Exp Gerontol 2018;101:63–8. 10.1016/j.exger.2017.11.005 [DOI] [PubMed] [Google Scholar]
- 48.Hannemann J, Laing A, Glismann K, et al. Timed physical exercise does not influence circadian rhythms and glucose tolerance in rotating night shift workers: the Eurhythdia study. Diab Vasc Dis Res 2020;17:1479164120950616. 10.1177/1479164120950616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hanssen H, Minghetti A, Magon S, et al. Superior effects of high-intensity interval training vs. moderate continuous training on arterial stiffness in episodic migraine: a randomized controlled trial. Front Physiol 2017;8:1086. 10.3389/fphys.2017.01086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hasegawa N, Fujie S, Horii N, et al. Aerobic exercise training-induced changes in serum C1Q/TNF-related protein levels are associated with reduced arterial stiffness in middle-aged and older adults. Am J Physiol Regul Integr Comp Physiol 2018;314:R94–101. 10.1152/ajpregu.00212.2017 [DOI] [PubMed] [Google Scholar]
- 51.Hasegawa N, Fujie S, Horii N, et al. Effects of different exercise modes on arterial stiffness and nitric oxide synthesis. Med Sci Sports Exerc 2018;50:1177–85. 10.1249/MSS.0000000000001567 [DOI] [PubMed] [Google Scholar]
- 52.Headley S, Germain M, Wood R, et al. Short-term aerobic exercise and vascular function in CKD stage 3: a randomized controlled trial. Am J Kidney Dis 2014;64:222–9. 10.1053/j.ajkd.2014.02.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ho TY, Redmayne GP, Tran A, et al. The effect of interval sprinting exercise on vascular function and aerobic fitness of postmenopausal women. Scand J Med Sci Sports 2020;30:312–21. 10.1111/sms.13574 [DOI] [PubMed] [Google Scholar]
- 54.Kim H-K, Hwang C-L, Yoo J-K, et al. All-extremity exercise training improves arterial stiffness in older adults. Med Sci Sports Exerc 2017;49:1404–11. 10.1249/MSS.0000000000001229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Koh KP, Fassett RG, Sharman JE, et al. Effect of Intradialytic versus home-based aerobic exercise training on physical function and vascular parameters in hemodialysis patients: a randomized pilot study. Am J Kidney Dis 2010;55:88–99. 10.1053/j.ajkd.2009.09.025 [DOI] [PubMed] [Google Scholar]
- 56.Oliveira NL, Ribeiro F, Silva G, et al. Effect of exercise-based cardiac rehabilitation on arterial stiffness and inflammatory and endothelial dysfunction biomarkers: a randomized controlled trial of myocardial infarction patients. Atherosclerosis 2015;239:150–7. 10.1016/j.atherosclerosis.2014.12.057 [DOI] [PubMed] [Google Scholar]
- 57.Oudegeest-Sander MH, Olde Rikkert MGM, Smits P, et al. The effect of an advanced glycation end-product crosslink breaker and exercise training on vascular function in older individuals: a randomized factorial design trial. Exp Gerontol 2013;48:1509–17. 10.1016/j.exger.2013.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pascoalino LN, Ciolac EG, Tavares AC, et al. Exercise training improves ambulatory blood pressure but not arterial stiffness in heart transplant recipients. J Heart Lung Transplant 2015;34:693–700. 10.1016/j.healun.2014.11.013 [DOI] [PubMed] [Google Scholar]
- 59.Sugawara J, Akazawa N, Miyaki A, et al. Effect of endurance exercise training and Curcumin intake on central arterial hemodynamics in postmenopausal women: pilot study. Am J Hypertens 2012;25:651–6. 10.1038/ajh.2012.24 [DOI] [PubMed] [Google Scholar]
- 60.Oliveira e Silva VR, Stringuetta Belik F, Hueb JC, et al. Aerobic exercise training and nontraditional cardiovascular risk factors in hemodialysis patients: results from a prospective randomized trial. Cardiorenal Med 2019;9:391–9. 10.1159/000501589 [DOI] [PubMed] [Google Scholar]
- 61.Yoshizawa M, Maeda S, Miyaki A, et al. Effect of 12 weeks of moderate-intensity resistance training on arterial stiffness: a randomised controlled trial in women aged 32-59 years. Br J Sports Med 2009;43:615–8. 10.1136/bjsm.2008.052126 [DOI] [PubMed] [Google Scholar]
- 62.Fujie S, Hasegawa N, Sanada K, et al. Increased serum Salusin-Α by aerobic exercise training correlates with improvements in arterial stiffness in middle-aged and older adults. aging (Albany NY). Aging (Albany NY) 2020;12:1201–12. 10.18632/aging.102678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Holloway K, Roche D, Angell P. Evaluating the progressive cardiovascular health benefits of short-term high-intensity interval training. Eur J Appl Physiol 2018;118:2259–68. 10.1007/s00421-018-3952-6 [DOI] [PubMed] [Google Scholar]
- 64.Kim J-H, Jung Y-S, Kim J-W, et al. Effects of aquatic and land-based exercises on Amyloid beta, heat shock protein 27, and pulse wave velocity in elderly women. Exp Gerontol 2018;108:62–8. 10.1016/j.exger.2018.03.024 [DOI] [PubMed] [Google Scholar]
- 65.Mamen A, Øvstebø R, Sirnes PA, et al. High-intensity training reduces CVD risk factors among rotating shift workers: an eight-week intervention in industry. IJERPH 2020;17:3943. 10.3390/ijerph17113943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shenouda N, Gillen JB, Gibala MJ, et al. Changes in brachial artery endothelial function and resting diameter with moderate-intensity continuous but not sprint interval training in sedentary men. J Appl Physiol (1985) 2017;123:773–80. 10.1152/japplphysiol.00058.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Soriano-Maldonado A, Morillas-de-Laguno P, Sabio JM, et al. Effects of 12-week aerobic exercise on arterial stiffness, inflammation, and cardiorespiratory fitness in women with systemic LUPUS erythematosus: non-randomized controlled trial. J Clin Med 2018;7:477. 10.3390/jcm7120477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wong A, Figueroa A, Son W-M, et al. The effects of stair climbing on arterial stiffness, blood pressure, and leg strength in postmenopausal women with stage 2 hypertension. Menopause 2018;25:731–7. 10.1097/GME.0000000000001072 [DOI] [PubMed] [Google Scholar]
- 69.Vivodtzev I, Minet C, Wuyam B, et al. Significant improvement in arterial stiffness after endurance training in patients with COPD. Chest 2010;137:585–92. 10.1378/chest.09-1437 [DOI] [PubMed] [Google Scholar]
- 70.Toussaint ND, Polkinghorne KR, Kerr PG. Impact of Intradialytic exercise on arterial compliance and B-type natriuretic peptide levels in hemodialysis patients. Hemodial Int 2008;12:254–63. 10.1111/j.1542-4758.2008.00262.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
openhrt-2023-002384supp001.pdf (431.7KB, pdf)
Data Availability Statement
Data are available on reasonable request.