Abstract
Edibles are the only source of nutrients and energy for humans. However, ingredients of edibles have undergone many physicochemical changes during preparation and storage. Aging, hydrolysis, oxidation, and rancidity are some of the major changes that not only change the native flavor, texture, and taste of food but also destroy the nutritive value and jeopardize public health. The major reasons for the production of harmful metabolites, chemicals, and toxins are poor processing, inappropriate storage, and microbial spoilage, which are lethal to consumers. In addition, the emergence of new pollutants has intensified the need for advanced and rapid food analysis techniques to detect such toxins. The issue with the detection of toxins in food samples is the nonvolatile nature and absence of detectable chromophores; hence, normal conventional techniques need additional derivatization. Mass spectrometry (MS) offers high sensitivity, selectivity, and capability to handle complex mixtures, making it an ideal analytical technique for the identification and quantification of food toxins. Recent technological advancements, such as high-resolution MS and tandem mass spectrometry (MS/MS), have significantly improved sensitivity, enabling the detection of food toxins at ultralow levels. Moreover, the emergence of ambient ionization techniques has facilitated rapid in situ analysis of samples with lower time and resources. Despite numerous advantages, the widespread adoption of MS in routine food safety monitoring faces certain challenges such as instrument cost, complexity, data analysis, and standardization of methods. Nevertheless, the continuous advancements in MS-technology and its integration with complementary techniques hold promising prospects for revolutionizing food safety monitoring. This review discusses the application of MS in detecting various food toxins including mycotoxins, marine biotoxins, and plant-derived toxins. It also explores the implementation of untargeted approaches, such as metabolomics and proteomics, for the discovery of novel and emerging food toxins, enhancing our understanding of potential hazards in the food supply chain.
1. Introduction
Serious health hazards and outbreaks resulting from food spoilage are major concerns of food safety worldwide.1 Due to significant biological activity and poor detection by conventional testing techniques, food toxins among other contaminants constitute a serious risk to the public’s health.2,3 Food toxins are compounds that can contaminate different food products during manufacturing, processing, transit, or storage.4 According to Fletcher and Netzel, these poisons can come from a variety of sources including fungi, bacteria, algae, plants, and animals.5 Mycotoxins produced by molds, marine biotoxins from toxic algal blooms, and plant-derived toxins like alkaloids and glycoalkaloids are all well-known examples of food toxins.6,7 Mycotoxins are mainly produced by toxigenic fungal species belonging to the genera of Fusarium, Aspergillus, and Penicillium.8 These mycotoxins pose a challenge to food safety because they can contaminate food products even when good storage and processing protocols are employed for food safety. Mycotoxin contamination accounts for the major cause of food borne diseases as reported by the World Health Organization (WHO). Recent studies performed by the European Commission revealed that 80% of the samples were contaminated with at least one mycotoxin.9 Perusal of literature revealed that most of the mycotoxins are chemically and thermally stable; therefore, they can survive under storage, processing, and even cooking.10 It has been observed that crops that are stored for more than a few days become probable targets for the growth of fungi and mycotoxin formation. These mycotoxins can affect a variety of food commodities such as dried fruits, coffee, spices, nuts, cereals, oil seeds, fruits, spices, cocoa, beans, etc.11 Apart from mycotoxins, other microorganisms like bacteria, algae, and even plants also produce such metabolites that are equally toxic and lethal. Bacteria contamination is mainly attributed to poor hygiene and cleanliness and common uncleaned and poorly cooked meat and vegetable-based foods, especially fermented foods. Nem Chua fermented food prepared from pork sausage in Vietnam has the possibility for Staphylococcus aureus contamination, and New Zealand mussel (Perna canaliculus) traditional fermented food of New Zealand is usually contaminated with Clostridium botulinum.12 Likewise, aquatic and marine food like Shellfish, fish, and even water are contaminated with algal biotoxins.13 In the case of plants, some normal metabolites produced for various purposes like natural defense and stress tolerance act as toxins for other organisms. Cyanogenic glycosides in almonds, and summer fruits, furocoumarins in citrus fruits, and lectins in beans are some of the common examples of phytotoxins.14 Besides, some newly emerging chemicals include perchlorate, flame retardants, halo compounds, packaging materials, petrochemicals residues, healthcare products traces, and microplastics.15 The use of contaminated cereals, grapes, and barley used to produce wine and beer products and their consumption are the main causes of toxicological effects in human beings (Table 1).
Table 1. Various Food Toxins from Various Sources.
| Class | Toxin’s name | Source | Effect | Ref |
|---|---|---|---|---|
| Mycotoxins | Aflatoxin | Aspergillus flavus and A. parasiticus | Liver failure, cirrhosis | (16) |
| Lysergic acid (ergot alkaloids) | Claviceps purpurea | Ergotism, vasoconstriction, uterine contraction | (17) | |
| Fumonisins B1 and B2 | Fusarium verticillioides and Fusarium proliferatum | disruption of sphingolipid metabolism, leuko-encephalomalacia | (18) | |
| Ochratoxin A | Aspergillus and Penicillium | Carcinogenic, immunotoxic mutagenic, nephrotoxic, and teratogenic | (19) | |
| Patulin | Aspergillus, Byssochlamysand Penicillium | Teratogenic, carcinogenic and mutagenic | (20) | |
| Zearalenone | Fusarium graminearum, F. culmorum, F. crookwellense, F. poae, F. semitectum, and F. equiseti | Hepatotoxicity, immunotoxicity, reproductive toxicity | (21) | |
| Tentoxin | Alternaria | Genotoxic, mutagenic, and carcinogenic | (22) | |
| Bacterial toxins | Cholera toxins | Vibrio cholerae | diarrhea | (23) |
| Enterotoxins | Staphylococcus epidermidis | Toxic shock syndrome | (24) | |
| Shiga toxins | Escherichia coli | Gastrointestinal complications | (25) | |
| Botulinum toxins | Clostridium botulinum | Neurotoxic | (26) | |
| Cereulide | Bacillus cereus | Dysfunction of liver, pancreatic islet, intestines, brain, | (27) | |
| Marine biotoxins | Saxitoxin | Cyanobacteria and dinoflagellates | Neurotoxin, paralysis | (28) |
| domoic acid | Diatoms | Neurotoxin | (29) | |
| Azaspiracid | Azadiniumpoporum | Diarrheic shellfish poisoning | (30) | |
| Brevetoxin | Karenia brevis | Immunotoxicity | (31) | |
| okadaic acid | Halichondriamelanodocia and Halichondriaokadai | Diarrhea, nausea | (32) | |
| Plant-based toxins | Cyanogenic glycosides | Almonds, cassava, pome fruit, stone fruit | Tissue damage | (33) |
| Furocoumarins | Citrus fruits | Skin cancer | (34) | |
| Ptaquiloside | Bracken ferns | Carciogenic | (35) | |
| Dehydropyrrolizidine | Cyanoglossum, Senecio, Echium, Crotalaria, Heliotropium, Symphytum, Trichodesma | Carcinogenic | (36) |
The use of contaminated cereals, grapes, and barley for the production of wine and beer products is the cause of toxicological effects in human beings. Consumption of contaminated meat and milk-based products is another route for these toxins to enter the human food chain. Further, the abusive use of drugs in livestock, animal waste pollution, and the use of industrial wastewater for irrigation are also responsible factors for the entry of these contaminants into the food chain.37 Conventional methods for the detection of food toxin include immunoassays and chromatographic techniques, which, while effective, have certain limitations.38 Immunoassays, for instance, can be sensitive but may give false results if structurally related compounds are present in the testing matrix.10 Chromatographic techniques, on the other hand, require complex sample preparation and longer analysis times.39 In contrast, significant advancements in analytical techniques have revolutionized the field of food safety, and one such breakthrough is the application of mass spectrometry (MS) for the rapid and sensitive detection of food toxins. It is highly sensitive and provides selectivity and capability to handle complex mixtures, making it an ideal tool for the detection and characterization of food toxins.40,41 This led to the selection of the MS-based approach as the first choice tool among researchers, regulatory agencies, and food industries to ensure the safety and quality of the food supply chain.41−43
Recent advances and innovations in instrumentation, such as the development of high-resolution mass spectrometry (HRMS) and tandem mass spectrometry (MS/MS), have significantly improved sensitivity and selectivity, allowing for the detection of food toxins at ultralow levels.44 Additionally, several ionization methods allow rapid in situ analysis, reducing the time and resources required for analysis.45,46 Further, advancement in the field of untargeted metabolomics and several web-based repositories of metabolites allows for the detection of not only the known toxin but also the unknown variants of toxins, broadening our understanding of potential hazards in the food supply chain.47,48 The current review summarizes the overview of available detection techniques of food toxins and then further elaborates on the MS-based approaches, their benefits and drawbacks, and how they are used in various food matrices. We will also go over the difficulties in applying MS-based techniques to routine food safety monitoring as well as the potential of this technology to protect public health and global food security.
2. Conventional Methods for the Detection of Food Toxins
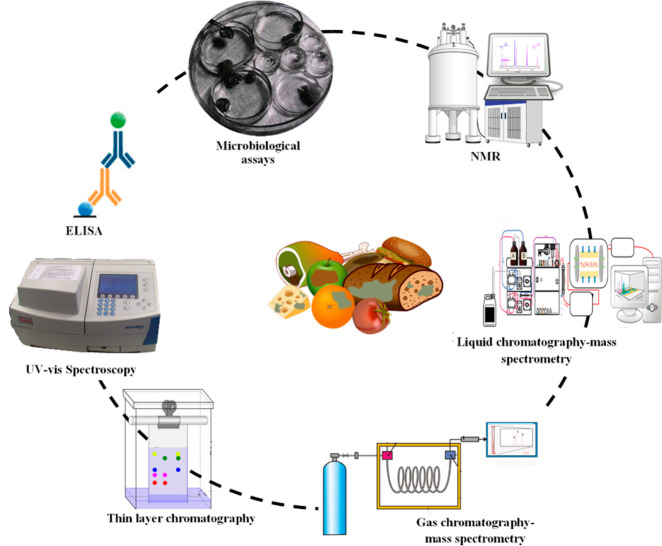
There are several specific and well-established conventional methods used on a regular basis for food safety assessment and regulation. These methods are often specific, but they may have limitations in terms of sensitivity, speed, and ability to detect a wide range of toxins (Figure 1). Some of the most common conventional methods for the detection of food toxins are mentioned below.
Figure 1.
Schematic representation of various methods used for the detection of food toxins.
2.1. Immunoassays
The most commonly used immunoassay methods in the field of food toxin detection are enzyme-linked immunosorbent assays (ELISA) and lateral flow immunoassays. These methods are very rapid and help with easy detection. Samples are allowed to interact with either labeled enzymes or antibodies, thus helping in the detection. However, compounds with similar core structures or nontoxic analogs can provide false positive results. Phycotoxins like Okadaic acid, Yessotoxin, Pectenotoxin, Azaspiracid, Cyclic imines, Palytoxin, Domoic acid, Saxitoxin, Microcystin, and Cylindrospermospsin; mycotoxins like Aflatoxin B1, Deoxynivalenol, Fumonisin B1 Zearalenone, and T-2; and bacterial toxins like Clostridium perfringens α, β, and ε toxin, Staphylococcal enterotoxins A, B, C, and E, botulinum toxins, and Escherichia coli enterotoxins49−51 are detected using immunoassay methods.
2.2. Chromatographic Techniques
Thin-layer chromatography (TLC), high-performance liquid chromatography (HPLC), and gas chromatography (GC) are commonly used to separate and quantify food toxins. Among various seafood-originated toxins, Domoic acid, paralytic shellfish toxins, and Aflatoxin B1 can be easily detected by HPLC and TLC.52−55 Among all, TLC is a simple technique in which food toxins are chromatographed on a plate layered with a thin layer of stationary phase using different mobile phase solvents, aiding the separation and visualization of the toxins. On the other hand, HPLC and GC are more advanced ways to separate toxins depending on various principles, enabling us to separate and quantify different food toxins. HPLC and GC analysis needs often include a complex protocol for sample preparation, which is one of the major limitations in the application of these techniques.
2.3. Spectroscopic-Based Techniques: UV–visible and Fluorescence Spectroscopy
Each toxin possesses a different core structure with different motifs and thus absorbs light at a particular wavelength, enabling its detection if monitored at their respective wavelength. This principle is harnessed for the detection of food toxins such as aflatoxins and can be measured with UV-fluorescence spectroscopy.56 It was suggested that if a sample is showing response at 400 and 550 nm with respect to 365 and 730 nm excitation wavelengths, it is supposed to be contaminated with aflatoxins. Singh et al.57 also reported that aflatoxin B1 and ochratoxin A have maximum absorption (λmax) at 365 and 380 nm. Both UV–visible and fluorescence spectroscopic techniques are easy to handle and cost-effective. Moreover, fluorescence spectroscopy has a high sensitivity for the detection of food toxins. Despite these advantages, there are certain toxins whose absorption and emission wavelengths may not be very selective and specific, making one of the major limitations in their detection by this technique. Other spectroscopic methods like nuclear magnetic resonance (NMR) spectroscopy are also used to elucidate the complex structure of food toxins.58 Similarly, near infrared (NIR) spectroscopy uses NIR (14000–4000 cm–1) wavelength that causes vibration of C–H, O–H, N–H, and C=O bonds in the biomolecules and can be helpful for the detection of toxins in the food. Recently, this technique was used to detect Diarrhetic shellfish toxins in the mussels.59
2.4. Biological Assays
One of the most conventional methods is the direct injection of toxic samples into live animals and monitoring of their physiological response, behavior, and mortality. This assay is commonly used for the detection of marine toxins like Diarrhetic shellfish toxins in seafood.60,61 However, these tests are time-consuming and costly and raise major ethical concerns.
2.5. Biochemical Assays
Biochemical assays do not detect food toxins by direct measurement but rather involve the measurement of toxin-induced biochemical changes such as enzymes. For example, the inhibition of phosphatase activity is used to detect the presence of diarrhetic shellfish toxins.62,63 The use of this technique is very narrow, utilizing different instruments and reagents, and cannot be applied to multiple types of toxins, thus making it less applicable for the detection of various food toxins.
2.6. Microbiological Assays
Some food toxins are produced by certain microbes. Hence, assays involving the presence of the microbe are sometimes used as a proxy to detect their presence. For instance, the detection of Bacillus cereus in the food samples can point toward the presence of enterotoxins.64,65 Although these assays are easy to operate and inexpensive, they lack sensitivity and specificity.
2.7. Sensor-Based Approaches
Sensor-based methods of toxin detection in foods are very popular since they can be used on the site. There are many toxins such as aflatoxin B1, diarrhetic shellfish toxins, and microcystins that can be detected by sensors.66 Examples may be biosensors and aptasensors. Undoubtedly, the use of sensors helps to screen samples for the possible presence of toxins. However, the sensors may vary in terms of sensitivity and specificity.
3. Mass Spectrometry-Based Detection
Mass spectrometry (MS) is a powerful analytical technique used to detect and quantify various compounds including food toxins. This method can provide highly sensitive and specific results, making it a valuable tool for food safety and quality control.67 Infectious toxins like prions and Shiga toxins can also be analyzed using mass spectrometry, where peptides are digested by proteases and then the digested proteinaceous parts are analyzed by MS.68 The pervasive contamination of food products with mycotoxins has made monitoring their levels essential. Detection of mycotoxin biomarkers in urine provides valuable and specific data for exposure assessment to these food contaminants in order to overcome the disadvantages of the indirect approach based on food analysis.69 Due to the diverse chemistry and occurrence of food toxins in feedstuffs and foods with complex matrices, the detection has become difficult. The primary source of error in the analysis results from inadequate sampling and inefficient extraction and cleaning procedures. Gas chromatography (GC)-MS is used to analyze volatile and semivolatile compounds, such as certain mycotoxins and pesticide residues, in a variety of dietary products. Before entering the mass spectrometer for ionization and detection, the compounds are vaporized and separated according to their volatility.70 The principle of detection of food toxins using GC-MS includes multiple target analyte extraction using multiresidue analytical methods like QuEChERs and adsorption extraction.71
Recently, mass spectrometry has become one of the most effective methods even for identifying specific microorganisms by using matrix-assisted laser-desorption time-of-flight (MALDI-TOF) MS, followed by recognition of MS spectra unique to that organism that create a reliable fingerprint.72 MALDI-TOF MS-based identification of bacteria is more rapid, accurate, and cost-efficient than conventional phenotypic techniques and molecular methods. Rapid and reliable identification of food-associated bacteria is of crucial significance for product quality. In contrast to genotyping methods, it can also be readily implemented in routine analysis. Due to short turnaround periods, low sample volume requirements, and low reagent costs, MALDI-TOF MS has recently emerged as a powerful tool for the identification of food toxins or toxin-producing microorganisms.73 Food toxins from various fields of seafood, fruits, vegetables, milk, dairy products, and oils can be detected using MALDI-TOF MS.74 The microbial databases with unique features relevant to each microbial species are the key components and are therefore continually building up in size with the updated information on newly discovered microbial species and their annotations.
Another powerful MS-based tool routinely used for food toxin detection is liquid chromatography (LC)-MS due to its advantages in terms of sensitivity and selectivity. LC–MS is widely used for the analysis of mycotoxins, alkaloids, marine toxins, glycoalkaloids, cyanogenic glycosides, and furocoumarins in food. The excellent sensitivity, even at low concentration levels, selectivity, and capacity to resolve coeluting compounds based on their molecular masses make LC–MS currently the most effective technique for the simultaneous detection of multiple regulated, unregulated, and emerging toxins in a single run. Commonly, the LC–MS methods for the quantitative determination of natural toxins are based on the use of a triple-quadrupole analyzer, tandem mass spectrometry, and multiple reaction monitoring (MRM) modes. The co-occurrence of natural toxins in combination with other chemical contaminants, such as pesticides, growth regulators, and veterinary drugs, as well as bioactive compounds (i.e., lignans, flavonoids, and phenolic compounds), in a wide variety of food matrices has increased the demand for analytical methods addressing the simultaneous determination of multiple analyte classes. LC–MS method used for the detection of food toxins includes alkaloids, furocoumarins, cyanogenic glycosides, marine toxins, and mycotoxins.41
Inductively coupled plasma mass spectrometry (ICP-MS) is a powerful analytical technique for the detection of elementals like heavy metals that allow multielement detection simultaneously with high speed and at very low concentrations.75 Further, for the enhanced selectivity, inductively coupled plasma mass spectrometry (ICP-MS) and tandem mass spectrometry (MS/MS) can be utilized. In the ICP-MS technique, the sample under examination is digested by employing a suitable technique such as dry ashing, acid digestion, or microwave digestion, etc. to solubilize the analytes of interest. Further, the sample is injected into an inductively coupled plasma source which ionizes the sample and detected by MS.76 Ion mobility spectrometry (IMS) is another advancement in analytical techniques that relies on ion mobility that is under the influence of velocity of ions and strength electric field.77,78 In order to take advantage, multiple MS-based approaches can be merged and operated together, which improves the efficiency of analytical techniques. Tandem mass spectrometry (TANDEM MS), also called MS/MS, is one such approach in which samples are analyzed either by multiple mass spectrometers connected to each other or with different analyzers arranged sequentially.79 A MS technique can also be coupled with immunoaffinity chromatography, and it is known as IAC-MS. This technique uses antibodies-based columns to acquire selectivity and also to isolate target analytes from the sample matrix.80 The isolated target molecules are injected into the MS component, which provides high sensitivity and identification of toxins based on mass-to-charge ratio. IAC-MS provides exceptional selectivity and can be used for the detection of various types of food toxins such as mycotoxins, pesticides, veterinary residues, and allergens, etc.81 MS-based approaches have offered diverse and advanced modules for the detection of analytes precisely. Table 2 summarizes the advantages and disadvantages of MS-based approaches under different conditions.
Table 2. Variants of Mass Spectrometry (MS) and Their Advantages and Disadvantages.
| Technique(s) | Important features | Advantages | Disadvantages | Ref |
|---|---|---|---|---|
| Accelerator mass spectrometry (AMS) | • Employed particle accelerator technology into a mass spectrometer. | • Small quantity of sample is sufficient | • High cost makes it less affordable | (82, 83) |
| • Its detection range include ion currents of more abundant stable isotopes (e.g., 12C, 13C) to very rare radionuclides (e.g., 14C) | • Need less time for estimation | • Small sample size makes it prone to contamination | ||
| Liquid chromatography mass spectrometry | • Simple and robust technique for regular analysis | • Wide linear dynamic range | • Lower accuracy | (84, 85) |
| • Able to detect nonvolatile compounds like sugar and proteins that cannot be detected in GC-MS | • Lower detection limit | • Isotopes cannot be detected | ||
| • High precision and accuracy | ||||
| Gas chromatography mass spectrometry | • Sample exposed to high temperature | • Analysis is faster and selectivity, | • Destructive method of analysis | (86−88) |
| • Used for the detection of volatile compounds from sample | • Lower detection limits | • Only thermolabile compounds can be detected | ||
| • Nonvolatile compounds like sugar can be detected after dertivatization | ||||
| High resolution mass spectrometry | • Good for the identification of unknown samples | • Highly accurate and selective measurement | • Expensive analysis | (89) |
| • Efficient for nontargeted analyses | • Able to detect mass accurately with even small change is detectable | • Data generated is hige and complex | ||
| • Not suitable for regular analysis of known samples | ||||
| Matrix-assisted laser desorption-ionization time-of-flight mass spectrometry | • Traditional method for the identification of microorganisms | • Able to differentiate between phenotypic, genotypic, and biochemical properties | • In some cases, unable to differentiate between closely related species, e.g., E. coli, and Shigella | (90) |
| • Sample is first ionized, and segregated based on mass-to-charge ratio | • Reduced analysis time | • Lack sufficient spectra in database | ||
| • Measurement is done by determining with time-of-flight | ||||
| Inductively coupled plasma mass spectrometry | • Used to measure the element level in sample | • Wide analytical range with lower detection limit | • High cost of investment and operation | (91) |
| • Sample converted to aerosol from liquid | • Need small quantity of sample | • Need experts for operation | ||
| • High throughput with multielement detection | ||||
| Surface-enhanced laser desorption/ionization time-of-flight mass spectrometry | • It is also known as SELDI-TOF-MS-based ProteinChip System | • It employes chromatographic separation techniques | • Low detection precision for individual proteins from complex | (92, 93) |
| • It is modified form of MALDI-TOF | • Less time-consuming and high through put system | • Low mass resolution | ||
| • Proteomic profiling of biological fluids | ||||
| Tandem mass spectrometry | • Two or more MS units are interconnected with quadrupoles and TOF analyzer | • Highly specific | • High operational cost | (79, 94, 95) |
| • Effective in analyzing complex mixture | • Low signal-to-noise ratio | • Limited sample through put | ||
| • Able to detect covalent modifications in proteins | ||||
| • Sensitive and reproducible |
4. Recent Advancements in Food Toxin Detection Using MS
Food toxins have become a serious concern for the society. The presence of various types of contamination and toxins like pesticides, herbicides, microbial metabolites, and plant-based toxins is highly detrimental to human health even at ppb concentrations when present in food, water, or animal feed.96 Safe food is explicitly a matter of concern and is indispensable for human health. In the current scenario, an effective and sensitive detection method becomes necessary to detect contamination of food and water with chemicals and pathogenic microbes and related products. Existing conventional methods for food analysis, based on PCR, chromatography, and spectrophotometry, have shown significant reliability and accuracy; however, the cost of analysis, time consumption, and requirement of specialized personnel impede their usage for frequent monitoring of food samples (as already summarized above). Hence, there exists an upsurging thrust for innovating rapid, accurate, robust, but inexpensive alternatives for in situ and real-time detection of contamination of food samples. The primary step involved in every methodology to identify the food toxins in the sample under examination involves the extraction of food toxins from the matrix followed by purification to remove other substances that can interfere with the analysis.97 After successful extraction of toxins, the sample is ionized into ions with ionization techniques such as electrospray ionization (ESI), chemical ionization (CI), or APCI (atmospheric pressure chemical ionization) or desorption techniques such as matrix-assisted laser desorption ionization (MALDI). These ions from the ionization chamber are accelerated followed by deflection in the magnetic field due to a difference in their masses. The beam of ions is then analyzed by the detector based on their mass-to-charge ratio. There are many types of mass analyzers available such as magnetic sector analyzers, quadrupole mass analyzers, double focusing analyzers, time-of-flight analyzers, etc. Further, MS can also distinguish between different food toxins depending upon their mass-to-charge ratio. In order to overcome the limitations associated with the conventional methods, MS can be coupled with these techniques to enhance the capabilities in complete and accurate analysis of different food toxins present in the sample.98 GC technique relies on the comparison of retention times with known standards and also lacks in the distinction of structurally similar compounds. Thus, to enhance selectivity, identification, and elucidation of the structure of various toxins present in the sample under examination, GC is coupled with the MS.99 HPLC technique requires optimization such as the selection of columns and mobile phases for each specific class of toxins. In addition to this, the sensitivity of HPLC is also low as that of MS techniques. HPLC techniques are more time-consuming than MS as they involve complex procedures for sample preparation.100 We are herein summarizing various types of food toxins and the recent advancements in their detection using MS.
4.1. Mycotoxins
Mycotoxins are toxic secondary metabolites produced by fungi. These toxins can accumulate in the fungal-contaminated food grains such as corn, cereals, and legumes, and upon ingestion can traverse into the food chain affecting humans and animals.101 As per the Rapid Alert System for Food and Feed of EU (RASFF) report, mycotoxins contaminate around one-quarter of global food grain production both during pre- and postharvest.102 This clearly indicates the severity of the mycotoxin problem in the food that we consume. In the literature, around 300 mycotoxins are reported, but only seven toxins are quite common in food worldwide such as aflatoxins (AF), trichothecenes (TC), zearalenone (ZEN), fumonisins (FB), ochratoxins (OTA), citrinin (CIT), and patulin (PAT).101 Fungal species belonging to the genera of Aspergillus, Penicillium, and Fusarium are predominantly toxigenic and most frequently lead to cases of mycotoxin concentration.101 AF (B1, B2, G1, and G2 produced by Aspergillus), CIT (by Penicillium, Aspergillus, and Monascus etc.), ergot alkaloids (Claviceps purpurea), FB (Fusarium sp.), ochratoxin A (produced by Penicillium and Aspergillus), PAT (Penicillium patulum), TC (Fusarium, Stachybotrys, Trichothecium, and Trichoderma sp.), and ZEN (Fusarium graminearum) are some of the well-known mycotoxins responsible for serious lethal reactions like cancer induction, kidney toxicity, immune suppression, stachybotryotoxicosis, turkey X syndrome, etc.103 Another issue with these mycotoxins is their resistance and tolerance toward thermal treatment; hence, they remain active even after heating.104
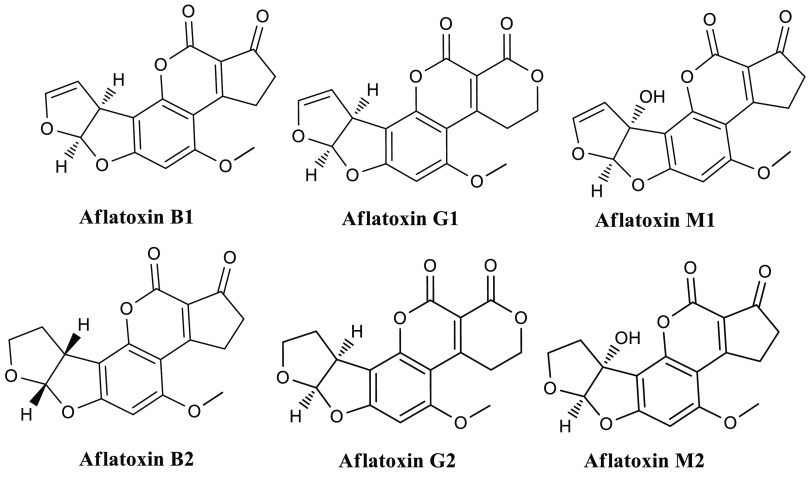
AFs are groups of potentially toxic fungal secondary metabolites reported from Aspergillus flavus, A. parasiticus, and A. nomius (Figure 2). They are produced from polyketides and commonly present in cereal crops like corn, peanuts, walnuts, wheat, etc.105 AFs can lead to chronic toxicity related to hepatic tissues, necrosis, hepatomas, periportal fibrosis, jaundice, hemorrhage, and fatty liver changes, and also exhibit teratogenicity, carcinogenicity, and immunotoxicity.105,106 They were first reported in the state of Gujrat and Rajasthan, India in 1974, which resulted in the onset of hepatitis caused by A. flavus infected staple food and maize. A. flavus, A. parasiticus, A. nomius, and sometimes Emericella spp. are the main producers of AFs.105 To date, more than 20 AFs variants have been reported, among which variant B1 is the most common and most lethal. AFs B1 and B2 are produced by A. flavus and A. parasiticus, and AF M1 is produced by A. parasiticus and can be transmitted through milk. Variants M1 and M2 are also produced by metabolism of B and B2.16 AF B1 is metabolized by the P450 monooxygenase system and generates AF 8,9-epoxide (reactive epoxide) that induces mutations and cancer by forming DNA abducts.106
Figure 2.
General structures of some of the Aflatoxin variants.
Besides Aspergillus, isolates belonging to Fusarium are also known to produce the most potent toxins, which include deoxynivalenol (DON), FBs, and ZENs (Figure 3). DON belongs to the sesquiterpenoid group of trichothecenes. It mainly contaminates corn, wheat, and barley. It is also a toxic secondary metabolite that has a negative health impact on the consumer by disturbing the intestinal barrier and has immune-stimulatory as well as immune-suppression properties at low and high doses, respectively.107 The same group also contains its acetylated derivatives named nivalenol, T-2 toxin, and HT-2 toxins.108 After ingestion, DON is absorbed and metabolized in the intestine via DON-3S, DON-GlcA, and DOM-1. In poultry birds, DON-3S and DON-15S are eliminated via bile and urine, while in swine, it is absorbed in the upper digestive system; hence, poultry birds are the least sensitive to these toxins, and swine are most sensitive to these toxins. In humans, contaminated foods like infected meat and cereals are the most common sources.107Fusarium sp. is also responsible for other mycotoxins named FBs (secreted by Fusarium verticillioides and Fusarium proliferatum). Aspergillus niger is also able to produce FBs. Such toxins are commonly reported from cereals like peanuts, maize, rye, oats, millets, and grape.109 To date, more than 15 homologous forms of fumonisin have been reported that are referred to as A, B1, B2, B3, C, P, etc. However, B1 is the most toxic form of FB.110,111 FBs are known to have carcinogenic, neurotoxic, and hepatoxic effects that cause hepatocarcinoma, defects in the neural-tube, and nephrotoxicity.109 ZEN is a nonsteroidal, estrogenic mycotoxin produced by F. acuminatum, F. crookwellense, F. culmorum, F. cerealis, F. equiseti, F. graminearum, F. oxysporum, F. sporotrichioides, F. semitectum, and F. verticillioides.112 It disrupts reproductive capacity by affecting mammalian folliculogenesis and impairs granulosa cell development and follicle steroidogenesis.113 It is thermostable114 and resistant to processing stress like milling and storage.115,116 ZEN leads to kidney damage and liver injury and causes inflammation.112
Figure 3.
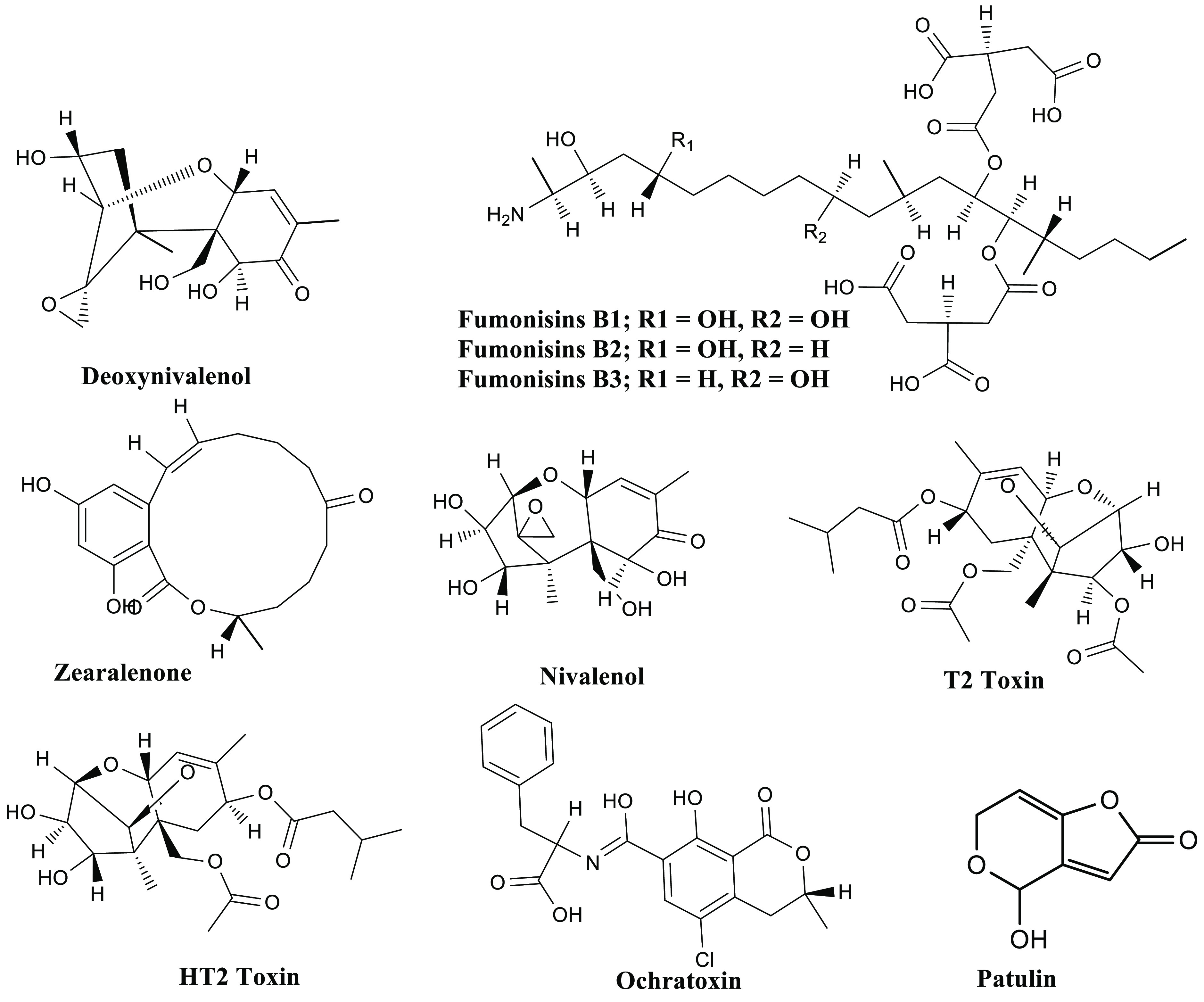
General structures of various mycotoxins.
Ergot alkaloids are toxic secondary metabolites produced by several fungi of Clavicipitaceae (Epichloë, Claviceps, Balansia, and Periglandula) and Aspergillus fumigatus. The producers mainly include Epichloë endophytes, Epichloë festucae var. lolii, Epichloë coenophialum, and Claviceps purpurea.117 They belong to compounds containing an indole group and are derived from L-tryptophan. They cause “ergotism” and their toxic effect is reflected by hyperglycemia, gastrointestinal upset, mydriasis,118 and even endocrine disruption.119 Besides these major mycotoxins, some other chemicals including OTA and PAT have also been reported from fungi. OTA is nephrotoxic and produced by a diverse range of fungi such as Aspergillus ochraceus, A. carbonarius, A. niger, and Penicillium verrucosum. It can also lead to renal tumors, Balkan endemic nephropathy, and chronic interstitial nephropathy.120 Patulin is produced by Penicillium, Aspergillus, and Byssochlamys, while alternariol (AOH) and alternariol monomethyl ether (AME) are Alternaria toxins, produced by fungi of the Alternaria genus found in fruits and related products.20
To ensure food safety with special consideration to mycotoxins, international, national, and regional agencies like the World Health Organization, Food Agriculture Organization, Codex Alimentarius Joint Expert Committee for Food Additives and Contaminants, European Food Safety Authority, GCC Standardization Organization, and Japanese Association of Mycotoxicology have determined the permission limit for mycotoxins contamination in food as well as feed.121Table 3 summarizes the permissible limit for various mycotoxins in food122−125 and animal feed126,127 as per the European Commission.
Table 3. Regulatory Permission Limits for Various Mycotoxins in Food and Feed Products As Per European Commission122−127.
| Category | Food or animal feed products | Permissible limit (μg/kg) |
|---|---|---|
| Aflatoxins B1 (AFB1) | ||
| Food | Brazil nuts, groundnuts, hazelnuts, and oilseeds for human consumption after physical treatment | 8 |
| Almonds, apricot kernels, and pistachios for human consumption after physical treatment | 12 | |
| Brazil nuts, groundnuts, hazelnuts, and oilseeds for human consumption directly (No physical treatment) | 2–5 | |
| Almonds, apricot kernels, and pistachios for human consumption directly (No physical treatment) | 8 | |
| Dairy products for consumption by infants, baby food, and processed cereal-based food | 0.1 | |
| Spices | 5 | |
| Dried fruits, and Figures for human consumption after physical treatment | 5–6 | |
| Dried fruits (except Figures) for human consumption directly (No physical treatment) | 2 | |
| Maize and rice (as ingredients) for human consumption after physical treatment | 5 | |
| Feed | Feed materials | 0.02–0.05 |
| Complete feeding stuff with the exception of | 0.05 | |
| Calves, cattle, and lambs | 0.005–0.01 | |
| Poultry | 0.02 | |
| Complementary feeding stuff | 0.005–0.05 | |
| Aflatoxins M1 (AFM1) | ||
| Food | Milk | 0.05 |
| Infants’ dairy products, baby formula and baby milk, | 0.025 | |
| Aflatoxins (AFs) total | ||
| Food | Almonds, apricot kernels, brazil nuts, groundnuts, hazelnuts, oilseeds, and pistachios for human consumption after physical treatment | 15 |
| Groundnuts, oilseeds, and processed products for human consumption directly (No physical treatment) or as ingredient | 4 | |
| Almonds, apricot kernels, brazil nuts, hazelnuts, pistachios, and for human consumption directly (No physical treatment) | 10 | |
| Spices | 10 | |
| Dried fruits, and Figures for human consumption after physical treatment | 10 | |
| Dried fruits (except Figures) for human consumption directly (No physical treatment) | 4 | |
| Maize and rice (direct or as ingredients) for human consumption after physical treatment | 10 | |
| Citrinin (CIT) | ||
| Food | Food supplements prepared from red yeast fermented rice | 2000 |
| Deoxynivalenol (DON) | ||
| Food | Unprocessed durum, maize, oats and wheat | 1750 |
| Cereals and cereal flour for direct human consumption | 750 | |
| Cereal-based processed foods and baby food | 200 | |
| Feed | Animal feed from–cereals | 8–10 |
| Complete as well as complementary feeding stuff | 0.9–5 | |
| Fumonisin (FB1+FB2) | ||
| Food | Unprocessed maize | 4000 |
| Maize for direct human consumption | 1000 | |
| Maize-based processed food for babies and young children | 200 | |
| Feed | Maze based feed | 60 |
| Complete and complementary feeding stuff | 5–50 | |
| Ochratoxins (OTA) | ||
| Food | Cereals-based unprocessed products | 3–5 |
| Cereal-based processed food and baby food, dietary foods products specially for medical purposes purposes | 0.5 | |
| Beverages based on grapes | 2 | |
| Coffee roasted/instant | 5/10 | |
| Spices | 15 | |
| Feed | Cereals-based feed materials | 0.25 |
| Complete and complementary feeding stuff for poultry | 0.1 | |
| Patulin (PAT) | ||
| Food | Fruit juices | 50 |
| Solid apple products | 25 | |
| Solid apple as well as apple juice for babies and young children | 10 | |
| Zearalenone (ZEN) | ||
| Food | Cereal products (unprocessed except maize) | 100 (350) |
| Cereals for direct human consumption | 75 | |
| Maize for direct human consumption | 100 | |
| Cereals and maize processed products for babies and young children | 20 | |
| Feed | Cereals and maze-based feed materials | 2–3 |
HPLC and GC are the common approaches for the detection and higher accuracy and sensitivity making MS an elegant and dominant analytical tool for toxicological and metabolite analysis.128 In addition, it also allows simultaneous detection of a diverse range of toxins together and aids in method standardization and implication to ensure the rapid evaluation of samples for food safety analysis. Areo et al.129 have employed UHPLC–MS/MS for the detection of AFs, ZEN, and OCT A from 100 tea samples (collected from registered shops within South Africa), prepared in acetonitrile/water/acetic acid solvent by QuEChERS extraction method. The supernatant was mixed with 900 mg of anhydrous MgSO4, 150 mg of C18, and 150 mg of primary secondary amine that separates the organic phase. The organic phase was collected and dried under a nitrogen stream and further reconstituted in methanol/water for analysis via UHPLC–MS/MS. The method selected has very high linearity (>0.99) and precision (6–29%). AFs, i.e., AFB1, AFB2, AFG2, OCT A, and ZEN, were absent in the samples, and AFG1 was present in very low amounts 1.72–5.19 μg/kg that were also below the regulated level in food as recommended by EU Commission Regulation 1881/2006.129 You et al.130 have evaluated the effect of culture medium for mycotoxin accumulation by Alternaria. Secondary metabolites were characterized by nontarget analysis with HRMS. Mycotoxins produced by Alternaria were grouped into for families: alternariol monomethyl ether (AME), alternariol (AOH), altenuene (ALU), Desmethyl dehydro altenusin (DMDA), and dehydroaltenusin (DHA) families, Altertoxin-I (ATX-I) family, tentoxin (TEN) family, and tenuazonic acid (TeA) family. Culture medium greatly influenced the type of mycotoxins produced. wiz Potato Sucrose Agar medium is suitable for AOH, AME, ALU, ALT, DHA, and DMDA, while Potato Dextrose Agar supported the accumulation of ATX-I, TEN, and TeA. Table 4 summarizes some of the research for the detection of various mycotoxins with MS-based tools.
Table 4. Detection of Mycotoxins by MS-Based Approach.
| Name | Sample | Method | Operating parameters | Outcome | Ref |
|---|---|---|---|---|---|
| Trichothecenes T2 | Wheat and maize | Portable mass spectrophotometer | MS parameter: heater 200 °C; sample pump 0%; mass center: m/z = 484; mass tolerance: 1; spectrum average: 10 | Limit of detection 0.2 mg/kg | (131) |
| Alternaria toxin | Fruits and vegetables | QuEChERS extraction followed by MS analysis | Triple quadrupole MS; pressure curtain gas 35 psi and collision gas 8 psi; ion spray voltage 5,500 V (positive ion mode), and −4,500 V (negative ion mode) | Detection limit 1.0–5.0 μg/kg (Extraction recoveries 73.0–120%) | (132) |
| Repeatability <12.9% | |||||
| Mycotoxins | Standard sample | UHPLC-QTrap-MS/MS | Injection volume: 20 μL; column temperature: 30 °C; flow rate: 0.2 mL min–1; mobile-phase 0.1% formic acid+ acetonitrile run time: 25 min | Limit of quantification 0.005 and 13.54 ng mL–1 (Limit of detection 0.001–9.88 ng mL–1; 0.005; Recovery 67.5–119.8%) | (133) |
| Aflatoxin B1 and M1 | Blood | HPLC–MS/MS | Allure PFPP column; positive electrospray ionization; source temp 500 °C | LOD 0.05 to 0.2 ng/mL; accuracy 92–111% | (134) |
| Alternaria toxins | Vegetable sample | UPLC–MS/MS | ACQUITY UPLC BEH C18 column; injection volume: 5 μL, column temperature: 40 °C; flow rate 0.4 mL min–1, mobile phase: 0.1% formic acid water; gradient flow 10–90% | Contaminated solanaceous vegetable: 41.1% | (135) |
| AME: 4.26%; AOH: 6.38%; altenuene: 6.38%; tentoxin: 42.6%; tenuazonic acid: 55.3% | |||||
| Aflatoxin M1 | HPLC–MS/MS | Quantitative daughter m/z: 273; qualitative daughter m/z: 259; collision energy 23 eV | Quantification limit 1.62 ppb; detection limit 0.54 ppb; 98.5% accuracy | (136) | |
| Ochratoxin A | Coffee and tea | UHPLC–MS/MS | Triple quadrupole MS; electrospray ionization, ion source temp 300 °C; flow rate 3 L/min | Sensitivity 0.30 and 0.29 ng/mL | (137) |
| Trichothecenes | Oat based products | U-HPLC–HRMS/MS | Reverse phase column; electro-ionization detector; column temperature 40 °C; ethanol+water+formic acid gradient elution | Frequency of free T2 toxin 92% | (138) |
| T2 mono glucoside 69% | |||||
| Alternaria toxins | Rice | LC–MS/MS | Separation with hyper clone column and detection with C18 column; detection with negative electrospray ionization, source temperature 300 °C | Limit of detection and quantification | (139) |
| AME: 0.03 and 0.09 μg/L; altenuene: 5.48 and 16.24 μg/L | |||||
| Alternariol monomethyl ether (AME), Alternariol (AOH), and tentoxin | Standard sample | LC-ESI-MS/MS | LC–MS/MS with triple quadrupole; separation at 25 °C with C18 column; analysis with quadrupole MS, source temperature 450 °C; ion spray voltage 5500 V | Limits of detection and quantitation: 0.7 and 3.5 ng/g recovery 80% | (140) |
4.2. Bacterial Toxins
Bacterial contamination has shown diverse causes as Shigella mainly infect via unwashed hands, while Campylobacter and Escherichia coli are usually present in raw milk, undercooked meat and poultry products, and contaminated water. In contrast, Listeria monocytogenes and Yersinia enterocolitica are found in refrigerated food.141 Bacterial toxins have been classified as endotoxins and exotoxins. Structurally, endotoxins have distinct structural regions, i.e., glycolipid is made up of disaccharide and fatty acids which are usually capric, lauric, myristic, palmitic, and stearic acids. These acids are buried within the outer cell membrane of the bacterium. The nucleus is the second important part, which is made up of a hexose- and heptose-based heteropolysaccharide. The glycolipid and nucleus are interconnected by the sugar acid 2-keto-3-deoxyoctanate. Endotoxins are lipopolysaccharides and are part of the outer membrane of Gram-negative bacteria. These are also identified as important determinants and antigenic parts of bacteria that aid in attachment with the host as well as in pathogenicity. Exotoxins are proteins in nature that are released by Gram-negative bacteria and disrupt cell division, causing lysis and tissue damage.142,143 Exotoxins are further classified into types I, II, and III based on the mechanism of action. Toxin type I can make critical changes in the host’s cells without internalizing. Superantigens secreted by Staphylococcus aureus and Streptococcus pyogenes are examples of a type I toxin. The type II group includes hemolysins, phospholipases, aerolysin, and GCAT proteins. It intrudes the host cells and creates pores to destroy the host cell’s membrane. In comparison to type I and II, type III is a diverse group in terms of activity. It has a binary structure with fractions A and B. Fraction B in the toxin facilitates the binding with receptor in the host cell, while another fraction, i.e., “A” carries enzymatic activity and is responsible for the toxin effect. Anthrax toxin (Bacillus anthracis), Cholera toxin (Vibrio cholerae), and Shiga toxin (Escherichia coli O157:H7) are some examples of exotoxins.142,144 Botulinum is 150 kDa and composed of a heavy chain of 100 kDa and a light chain of 50 kDa. Heavy chain is responsible for binding to receptors on neuron surface, while light chain cleaves proteins required for nerve signal transmission, i.e., botulinum A, C, and E cleave synaptosomal-associated protein 25, while B, D, F, and G variants act on synaptobrevin-2.72,145,146 In a similar fashion, B. anthracis produces three types of proteins or factors, e.g., lethal factor, edema factor, and protective antigen. Protective antigens split and form a fragment of 63 kDa that forms heptamers and octamers to finally find the cell surface. In addition, they also bind with lethal factors to form lethal toxin.72,147
4.2.1. Detection of Bacterial Food Toxins
Clostridium, Salmonella, Staphylococcus, and Listeria are some common pathogens causing foodborne infections in humans. Previously used methods, e.g., enzyme immunoassay (EIA), were fast and sensitive, but their accuracy was limited due to cross-reactivity reporting a high rate of false positives and misguided public health care personnel. Techniques based on MS are powerful and can be multiplexed for the detection of various protein toxins, e.g., toxins from Clostridium,148Bacillus,88 and many more with speed, sensitivity, and accuracy. Botulinum, a neurotoxin, is produced by Clostridium botulinum in seven different serotypes (A–G). Specific detection of these toxins from different strains needs high analytical sensitivity and a MS-based approach. The enzymatic activity-based approach relies upon substrate fragments generated by these toxins which can be used as targets by MALDI-TOF MS.72
In 2002, a peptide mass map of toxin variants A1 and B1 was prepared by targeting the trypsin digest of the toxin by van Baar and colleagues. The work was extended to C, D, E, and F in 2004.72 In successive generations, several advancements have shown effective approaches like endopep-MS.149 Rosen et al. have developed the endopep-MS-based method for the identification of botulinum A and E simultaneously and rapidly.150 Both A and E identify the same target SNAP 25 protein but act on different sites. 3D structures of both types of fragments were used for differential identification. Drigo et al.151 employed the same endoPep-MS approach for botulinum toxins C and D. The method has shown a sensitivity of 100% with specificity and accuracy of 96.08% and 97.47%, respectively. Integration of MS with other analytical methods like HPLC, GC, FPLC, etc. has improved bacterial toxin profiling. Toxoflavin and fervenulin are bacterial toxins produced by Berkholderia and Streptomyces hiroshimensis. These compounds are common contaminants in fermented corn flour, rice bran oil, distiller’s yeast, sweet potato starch, Tremella fuciformis Berk., and rice noodles. These compounds are sensitive to degradation in 1% ammonia solution. UHPLC-Q-TOF/MS allowed for the detection of degradation products. The modified approach led to lower down the limits of detection of toxoflavin and fervenulin to 12 μg/kg and 24 μg/kg, respectively, with recovery of 70.1–108.7%.152 The MS approach also employed natural phenomena of antigen–antibody interactions for the detection of toxins and related antigens. Salmonella typhi, Gram-negative enterobacteria, is responsible for typhoid fever and meningitis. The immunoreactive proteins of bacteria were used as targets to develop improved diagnostic tools with MS. An immunoaffinity-based proteomic approach was employed with IgG and IgM antibodies from typhoid patients. The approach aided in the identification of 28 immunoreactive proteins, out of which 14 were complementary to IgG, 4 for IgM, and the rest 10 for both, hence retained by respective charged columns. In context to antigenicity, 22 proteins have shown antigenicity and immunogenicity.153 Such an approach is helpful for rapid identification, and its reproducibility and reliability can be employed for vaccine and drug development. Peptide mass fingerprinting technique (PMF) associated with MALDI TOF/MS or ESI/MS is a top-down MS protocol where proteins are directly ionized to create a fingerprint of individual proteins and applied for detection of various microbial strains. PMF of unknown organisms is compared to those existing in PMF databases or compared to the spectrum of biomarker proteins with the proteomic spectral database, using MALDI-TOF MS. Typically a mass range m/z of 2–20 kDa is used for species-level identification where ribosomal proteins representing 60–70% of microbial cells’ dry weight and some housekeeping proteins are selected.154 Thus, by comparing with extensive commercial databases, microbial contaminants can be traced to the genus, species, or strain level, and such an identification tool is conveniently adapted in diagnostic laboratories.155 However, using biomarkers is not very common for identification since it requires prior insight into the genome sequences before creating the required databases for proteins’ molecular masses. Staphylococcus aureus delta-toxin has been detected using whole-cell (WC) MALDI-TOF/MS and LC–MS to correlate the expression of delta-toxin with the status of the agr (accessory gene regulator) status. Mass spectra of pure toxin from wild type strains and mutants for agr-rnaIII gene were compared specifically at the position of the peak for delta-toxin.156
Biosensors have taken up an important role in the accurate and fast detection of food contaminants even in very low concentrations157 using biorecognition elements, such as antibodies, enzymes, nucleic acids, phages, etc., along with electrochemical, optic, or piezo-electric devices for the detection of food contaminants. MS-based biosensors are less prevalent as compared to optical or electrochemical ones158,159 but have the potential to overcome the drawbacks of conventional models of biosensors. A multitoxin biosensor-MS was developed for the detection of multiple bacterial toxins simultaneously. Biomolecular interaction analysis-MS (BIA-MS) that used a two-step method, i.e., first bonding of toxin molecules to antibodies immobilized on a sensor chip using SPR (surface plasmon resonance) and then the bound toxin, was identified by MALDI-TOF/MS. The potential of the multiaffinity sensor chip was validated by the detection of endotoxin from Staphylococcus in mushroom and milk samples and it successfully detected multiple toxins at concentrations as low as 1 ng/mL.160
4.3. Marine Biotoxins
Marine biotoxins are natural compounds released in the marine environment by algae and phytoplankton during harmful algal blooms (Figure 4). These compounds are highly toxic for consumers and not only are related to serious illness but also lead to the death of aquatic organisms and even humans.161 Due to continuous release in the surrounding environment, these biotoxins accumulate in aquatic and marine organisms such as mollusks and fishes. Based on the chemical nature and solubility, these biotoxins are hydrophilic and lipophilic. Hydrophilic biotoxins are water-soluble and can cause amnesic shellfish poisoning, paralytic shellfish poisoning, and emerging pufferfish poisoning, while other groups of lipid-soluble biotoxins are responsible for diarrhetic shellfish poisoning and azaspiracid shellfish poisoning. There is another group of toxins with less available information that is categorized as emerging toxins and can cause unregulated ciguatera fish poisoning, cyclic imines, and neurotoxic shellfish poisoning.162−165 Paralytic shellfish poisoning (PST) is a group of more than fifty-eight related compounds, produced by Alexandrium dinoflagellates of the Atlantic and Pacific coast and Mediterranean Sea. It has a tetrahydropurine skeleton among which saxitoxin (SXT) and gonyautoxin (GNT) are common. Structurally, STX has been categorized into four subgroups named carbamate, N-sulfo-carbamoyl, decarbamoyl, and hydroxylated saxitoxins.166 The toxicity related to PST is reflected in mild as well as severe depending upon toxicity. The mild symptoms include numbness, tingling sensation around lips followed by expansion of the area, itching and prickly sensation in fingertips and toes, dizziness, headache, and nausea. Moderate and severe illness symptoms include incoherent speech, prickly sensation and stiffness in limbs, weakness, difficulty in respiration, and muscular paralysis.161
Figure 4.
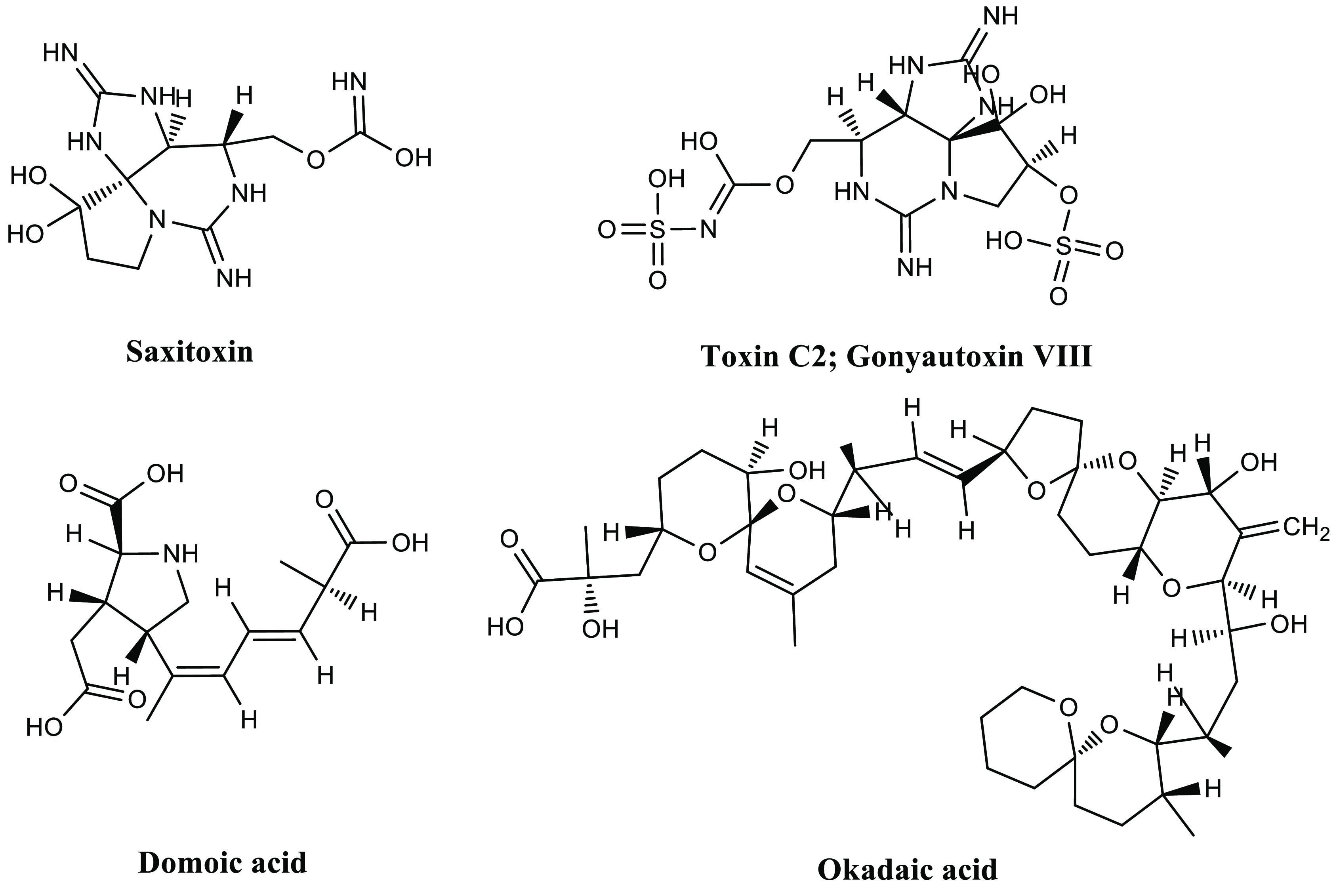
General structures of various marine biotoxins.
Amnesic shellfish poisoning is mainly caused by domoic acid (DA) and derivatives produced by marine diatoms of Pseudonitzschia. DA, cyclic tricarboxylic amino acid, can bind with glutamate receptors in the central nervous system due to structural analogy and result in excess stimulation, induced production of reactive oxygen species (ROS), and ultimately cell death.167 Consumption of DA resulted in gastrointestinal ailments including abdominal cramps, diarrhea, nausea, and vomiting. Neurological symptoms may also include confusion, disorientation, paresthesia, lethargy, short-term memory loss, and in severe toxicity cases, it may also result in coma or death.168
Diarrheic shellfish poisoning (DST) is a toxin produced by dinoflagellates of Dinophysis and Prorocentrum. It is a common type of contamination in shellfish industries due to overextended prohibitions on mussel harvesting activity.169 The responsible toxins for DST are a group of polyether compounds recognized as okadaic acid and its derivatives (dinophysistoxin); pectenotoxin; yessotoxin and its derivatives; and azaspiracid. Okadaic acid and azaspiracid consumption resulted in abdominal pain, diarrhea, nausea, and vomiting.161,170 Pectenotoxin and yessotoxin are not involved in human illness.171
Neurotoxic Shellfish Poisoning (NST) is another type of algal toxin that causes neurological as well as gastrointestinal ailments. Brevetoxins are a kind of marine biotoxin produced by Karenia brevis (Florida red tide dinoflagellate). It is a poly(ether ladder) compound (Figure 5). It causes mortality in massive fish and marine mammals. In humans, these toxins resulted in asthma-like symptoms if inhaled.172 Neurological and toxicity symptoms of NST are paralysis, seizures, paresthesia, and coma, while gastrointestinal ailments are represented by nausea, diarrhea, vomiting, cramps, and bronchoconstriction, and extreme poisoning may also lead to death.161 Ciguatera fish poisoning (CFT) is one of the most common foodborne illnesses caused by marine biotoxin of ciguatoxin.173,174 Ciguatoxins are toxic and lipid-soluble compounds found in marine organisms. Gambiertoxins, the precursor toxins, are produced by benthic dinoflagellates of Gambierdiscus genus. These toxins are accumulated in large predatory fishes like Spanish mackerels, moray eels, barracuda, and snappers.161 These compounds abnormally activate sodium ion channels and disrupt the cell membrane.175 These compounds cause abdominal pain, nausea, diarrhea, vomiting, hypertension, and bradycardia along with neurological complications.161
Figure 5.
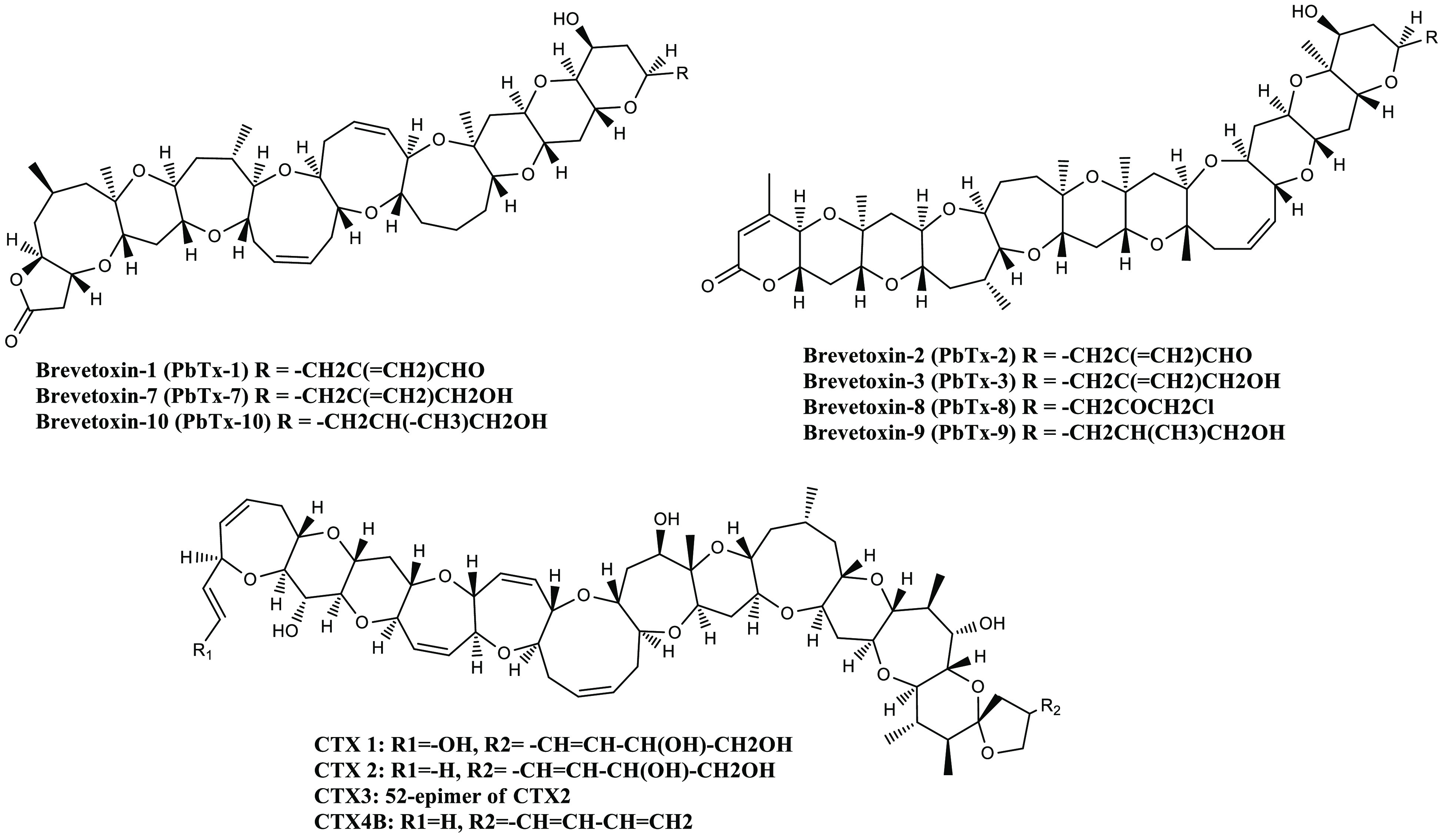
General structures of various marine biotoxin (Brevetoxin).
4.3.1. MS Analysis and Detection of Algal and Marine Biotoxins
Algal and marine biotoxins are another class of heterogeneous toxins produced by algae and cyanobacteria, Dinoflagellates and diatoms produced during algal blooms in rivers, freshwater lakes, and marine aquatic systems.176 Karunarathne et al., have found that 16,659 deaths have been reported in India between 1999 and 2018 due to poisoning.177 In the case of sea food such as shellfish, exposure and contamination to multiple toxins are possible, hence an efficient system is able to detect diverse classes of toxins at the same time effectively. Blay et al.,178 developed a method for the detection of multiple lipophilic biotoxins including azaspiracids, dinophysistoxins, and pectenotoxins as well as negative toxins via reversed-phase LC–MS within 7 min and hydrophilic toxins such as okadaic acid, dinophysistoxin-1,2, and yessotoxin from shell fish by recording scans at 2 Hz in positive and negative scans alternatively and 1 Hz in positive mode, respectively. Hydrophilic toxins including gonyautoxins domoic acid and saxitoxin were detected with mass accuracy of less than 1 ppm error and resolving power of 100,000 for the analytes (m/z 300–500). The limits of detection for lipophilic toxins were 0.041–0.10 μg/L ppm (positive ions), 1.6–5.1 μg/L (negative mode), and 3.4–14 μg/L for domoic acid and paralytic shellfish toxins.178 The biggest advantage of the method is that the analytes were detected with real time samples without any interference. Aquatic water bodies have a higher possibility of having aquatic biotoxins; hence, monitoring of water in water bodies is mandatory. Estevez et al. developed a method to seawater monitoring for marine biotoxins by hydrophilic interaction liquid chromatography coupled with HRMS. The main analytes considered for the detection were saxitoxin, decarbamoyl-saxitoxin, neosaxitoxin, gonaytoxin-2,3, and tetrodotoxin due to their adverse effects on gastrointestinal and central nervous systems in humans if taken up via seafood. Samples were processed via ultrasound-assisted solid–liquid extraction with methanol to extract toxins, followed by solid phase extraction using silica cartridges. The selected toxins are polar in nature; hence, the extraction stage is crucial for analysis, and the developed method has recoveries of 15–47% in filtrate and 26–71% in particulate fraction. Simultaneously, limit of detection was also affected with source as LOD was 0.5–5 μg/L for filtrate and 3.1–62 μg/L for particulate fraction.179
Kolrep et al.180 conducted comparative metabolite profiling to track the metabolism of okadaic acid in the liver and the role of CYP3A4 and CYP3A5 in its detoxification. It was found that LC–MS/MS can identify the metabolites distinctly from humans and rats based on the difference in +16 (+O) and +14 (+O/–H2) Da. It suggested some critical differences in the metabolism of okadaic acid in humans and rats. In continuation, it was also found that rats generated more metabolites from okadaic acid in comparison to humans in the presence of NADPH-dependent enzymes.181 The establishment of metabolic patterns and fragments might be crucial for the identification of fingerprints for toxin identification. Table 5 elaborates on the detection of bacterial and marine biotoxins from different samples.
Table 5. Detection of Bacterial and Marine Biotoxins by MS-Based Approach.
| Name | Sample | Method | Operating parameters | Outcome | Ref |
|---|---|---|---|---|---|
| Enterotoxin | Commercial | LC–HRMS | LC–MS Q-extractive mass spectrophotometer C18 reverse phase column; isocratic elution | 93 signature peptides identified for enterotoxins | (182) |
| Botulinum | Commercial | Endopep MS | Triple quadrupole mass spectrometer; Turbo Ion Spray interface; C18 column; gradient elution | Toxin detection 0.1 MLD50 and quantification 0.62 MLD50 | (183) |
| Okadaic acid | Raw and cooked food (Mussel, clam, flatfish) | Tandem mass spectrometry | C18 column with triple-quadruple mass spectrometer; ammonium format gradient elution; negative ionization mode | LOD and accuracy 0.2–5.1 μg/k | (184) |
| Dinophysistoxin | Raw and cooked food (Mussel, clam, flatfish) | Tandem mass spectrometry | C18 column with triple-quadruple mass spectrometer; ammonium format gradient elution; negative ionization mode | LOD and accuracy 0.2–5.1 μg/k | (184) |
| Anatoxins a (ATX) and Homoanatoxin-a (HAT) | Benthic-cyanobacterial-mat field samples | LC–HRMS/MS | Q Exactive HF Orbitrap MS; HESI-II electrospray ionization source; at 40 °C; resolution 60,000; collision energy 20 eV | Toxins are in conjugated form 15% ATX and 38% HAT | (185) |
| Cyanotoxins | Blue-green algae dietary supplement | Hydrophilic Interaction Liquid Chromatography-Tandem Mass Spectrometry | Electrospray ionization positive; source temperature 550 °C; ion spray voltage 5500 V; curtain gas 25 psi; collision gas 10 psi | Quantification limits 60–300 μg kg–1 | (186) |
| Paralytic shellfish toxins | Marine shellfish | Hydrophilic interaction chromatography-tandem mass spectrometry | Separation with HILIC-Z column; acetonitrile and ammonium formate-formic acid as the mobile phase; positive electrospray ionization; samples are cleaned with by ion-pair SPE using a porous graphitic carbon cartridge | Limits of detection 1.7–13.7 μg kg–1; and quantitation 5.2–41.0 μg kg–1; recoveries 76.5–95.5% | (187) |
| Tetrodotoxin | Marine shellfish | Hydrophilic interaction chromatography-tandem mass spectrometry | Separation with HILIC-Z column; acetonitrile and ammonium formate-formic acid as the mobile phase; positive electrospray ionization; samples are cleaned with by ion-pair SPE using a porous graphitic carbon cartridge | Limits of detection 1.7–13.7 μg kg–1; and quantitation 5.2–41.0 μg kg–1; recoveries 76.5–95.5% |
4.4. Phytotoxins
Phytotoxins are plant-derived compounds, including alkaloids and glycoalkaloids, that are naturally produced within plants but prove harmful if they remain in food products (Figure 6). These are secondary metabolites in plants and include cyanogenic glycosides, glucosinolates, glycoalkaloids, pyrrolizidine alkaloids, and lectins.188 Based on the site, these toxins can be classified into endotoxins and exotoxins. Endotoxins may be normal metabolites that are present in cells but become harmful if consumed in higher concentrations, and these compounds are also refereed as antinutritional factors, while exotoxins are toxic metabolites that are released from cells. Based on chemical nature, these are dehydropyrrolizidine, alkaloids, ptaquiloside, corynetoxins, and phomopsins.189 Cyanogenic glycosides (CGLs) are present in almonds, cassava, bamboo roots, sorghum, and stone fruits. These toxins are generated from proteinogenic amino acids like leucine, isoleucine, phenylalanine, tyrosine, and valine as well as nonproteinogenic amino acids like cyclopentenylglycin. CGLs are potentially toxic for humans and result in acute cyanide intoxication, high respiration rate, lower blood pressure, headache, dizziness, stomach pains, diarrhea, vomiting, and mental confusion.188,189 Furocoumarins are found in many plants including carrots, celery roots, citrus fruits, parsley, and citrus plants. These compounds are responsible for gastrointestinal ailments and phototoxicity, skin reactions under UV light.34,190 Lectins are reported from beans like kidney beans and can result in stomachache, diarrhea, and vomiting.189
Figure 6.
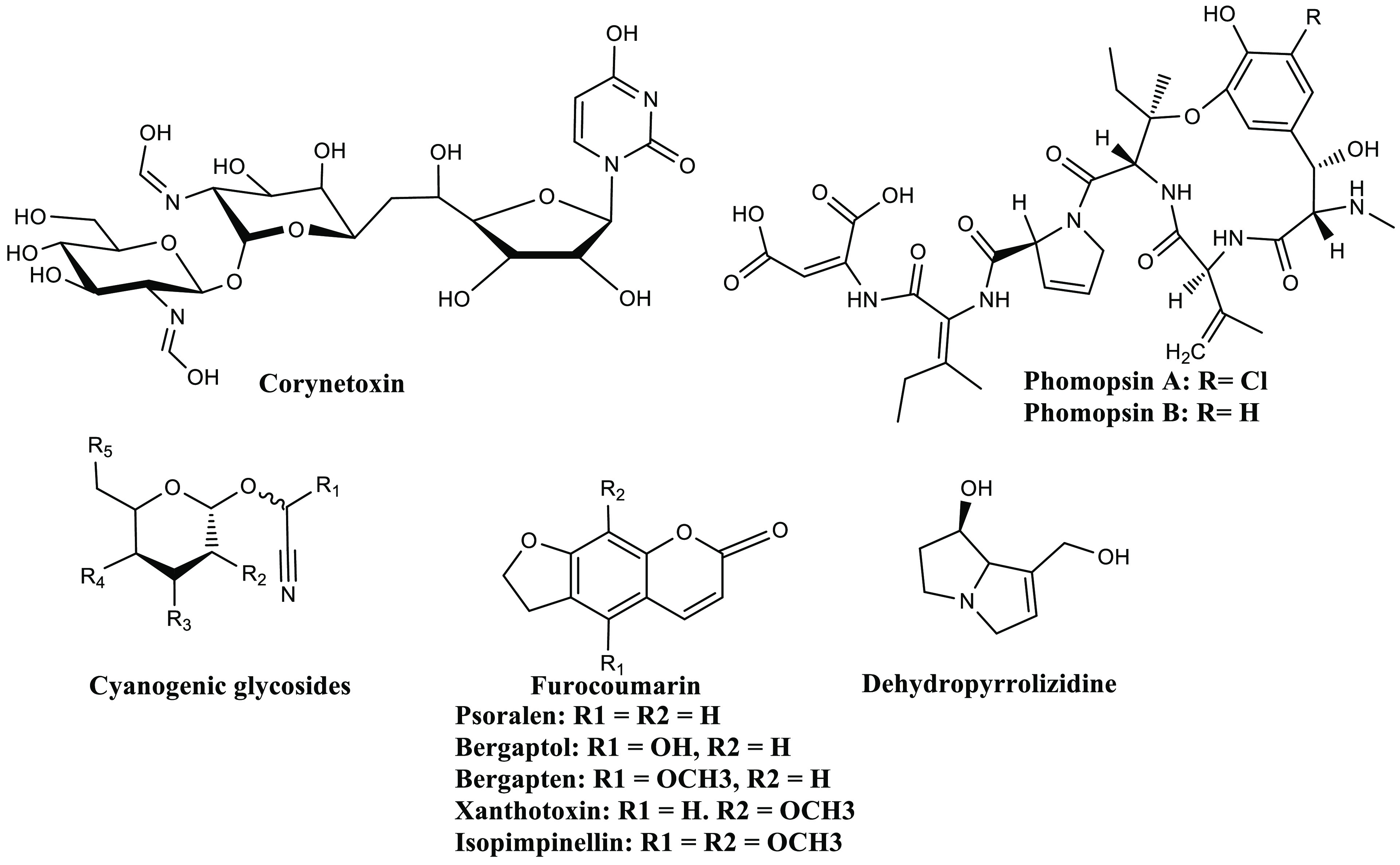
General structures of various Phytotoxins. The general scaffold for Cyanogenic glycosides, Furocoumarin and Dehydropyrrolizidine, has been depicted with providing a few examples of various compounds belonging to the class of Furocoumarin.
Phytotoxins are sometimes a part of the natural defense of plants, like Pyrrolizidine alkaloids (PAls) that are produced in Asteraceae, Boraginaceae, and Fabaceae families to defend plants against herbivores as well as insects. These toxins tend to have a common 1-hydroxymethyl pyrrolizidine core that is esterified with aliphatic acids. Besides edibles from these plants, honey is one of the common products contaminated with PAls, and hence, the extract or infusion can be used as an analyte for the detection of PAls. Ten plant samples, Anchusa officinalis, Borago officinalis, Echium italicum, Eupatorium cannabinum, Heliotropiumeuropaeum, Lithospermum officinale, Petasites hybridus, Senecio vulgaris, Symphytum officinale, and Tussilago farfara from Orto botanicodellaScuola Medica Salernitana, Salerno, Italy, were collected, and aqueous extract was prepared with salting-out assisted liquid–liquid extraction. The aqueous extracts were analyzed with UPLC–MS/MS. The analysis was able to identify 88 PAs from 282 samples with an identification limit of 0.6–30 μg kg–1 and a false negative rate <1.3% (at the concentration range of 4 μg L–1).191
For simultaneous detection of phytotoxins and microbial toxins, HRMS was employed, which applied to over 156 compounds inclusive of about 90 plant toxins (e.g., various alkaloids and aristolochic acids), about fifty-four mycotoxins, and 12 phytoestrogens (e.g., lignan, isoflavones, coumestans, etc.) in plant-protein samples, like cereals. MS library, created on fragmentation pattern obtained with both negative and positive ionization modes for each toxin, using ten different collision energies was used for analysis. A typical workflow was followed with generic QuEChERS-like sample preparation, followed by UPLC using suitable mobile phases that allowed the resolution of over 50 toxic alkaloids. The method performance was evaluated for its sensitivity at levels ranging from 1–100 μg/kg, and reproducibility. The quantitation obtained against the standard addition approach could meet SANTE/12682/2019 criteria for 132 toxins out of the tested 156 toxin samples.192 The plant toxins ricin and RCA120 were detected, differentiated, and quantified by Kalb et al.,193 via MS-based methods for the EQuATox proficiency test, in ∼9 samples. They successfully identified the samples spiked with ricin or RCA120; samples spiked with a 0.414 ng/mL concentration could not be detected. Liang et al.194 employed reversed phase LC–HRMS to detect five major phytotoxin groups including alkaloids, aromatic polyketides, flavonoids and steroids, and terpenoids at alkaline pH (>9). The developed method not only allowed the detection of 30 phytotoxins but also had forty-times higher detection sensitivity in comparison to older methods. Table 6 discusses some of the examples for phytotoxins detection using the MS-based approach.
Table 6. Phytotoxins Detection from Food Samples via MS-Based Approach.
| Name | Sample | Method | Operating parameters | Outcome | Ref |
|---|---|---|---|---|---|
| Toxoflavin and Fervenulin | Food samples and Tremella fuciformis Berk | UPLC–MS/MS | Separation column: ZORBAX SB-C18 column; oven temp: 35 °C; mobile phase: 0.1% formic acid+ methanol; flow rate 0.4 mL min–1 | Detection limits (μg/kg) Toxoflavin 12 | (152) |
| Fervenulin 24 Toxin level (mg/kg) Toxoflavin: 7.5; fervenulin: 3.2 | |||||
| Ricin | Soft drinks and serum | Surface-assisted laser desorption/ionization mass spectrometry | Pulsed Smartbeam II 2 kHz laser; wavelength 355 nm (∼3.49 eV); frequency 1000 Hz; delayed extraction time 350 ns for proteins | Limit of detection 0.5 pmol/μL | (195) |
| Pyrrolizidine Alkaloids | LC–MS/MS | Tea | UPHPLC with Quadrupole mass spectrometer; C18 Hypersil Gold column fitted; gradient elution | Total PA levels 13.4 to 286,682.2 μg/kg d.m | (196) |
| Ptaquiloside | LC–MS/MS | Bracken fern | LC–MS/MS; C18 column; gradient elution; column temperature 35 °C; electrospray positive ionization | Limits of detection 0.03 and quantification 0.09 μg/kg | (197) |
| Furanocoumarin | UPLC–MS/MS | Citrus sp. | Nexcol C18 column; column temp 40 °C; gradient elution; positive electrospray ionization | Compound recovery 94.07–114.53% | (198) |
| Amygdalin | LC–MS/MS | Kernels and Almonds | LC–MS/MS equipped with 6500 quadruple linear ion trap (QTRAP) mass spectrometer and electrospray ionization | Recovery 90–107%; limit of detection 0. Ng/g and limit of quantitation 8 and 2.5 ng/g | (199) |
| Pyrrolizidine alkaloids | quadrupole orbitrap MS | Honey | Polar C18 column; temp 40 °C; mobile phase flow rate of 400 μL min–1 | Limit of quantification 0.1–0.3 μg kg–1 | (200) |
| MS detection with positive ionization mode; scan range 250–500 m/z and 70 k (fwhm) | |||||
| Pyrrolizidine alkaloids | quadrupole orbitrap MS | Black and green tea | Polar C18 column; temp 40 °C; mobile phase flow rate of 400 μL min–1 | Limit of quantification 1–11.7 μg kg–1 | (200) |
| MS detection with positive ionization mode; scan range 250–500 m/z and 70 k (fwhm) | |||||
| Pyrrolizidine alkaloids | quadrupole orbitrap MS | Herbal infusion | Polar C18 column; temp 40 °C; mobile phase flow rate of 400 μL min–1 | Limit of quantification 0.9–2.1 μg kg–1 | (200) |
| MS detection with positive ionization mode; scan range 250–500 m/z and 70 k (fwhm) |
4.5. Emerging Toxins
In addition to conventional toxins already known and summarized above, there is a group of toxic chemicals that are continuously evolving, mainly due to rising pollution. In the last eight years, the list of emerging chemicals has increased day by day. Synthetic chemical toxins include microplastics, organophosphorus and polybrominated flame retardants, perfluoroalkyl compounds, food process and packaging, waste substances, and nanomaterials.15 Besides, heavy metals, antibiotics and drug traces and metabolic intermediates, and agricultural chemicals201 have shown bioaccumulation and have serious toxic effects if consumed, even in low concentrations. Health ailments include endocrine disruption, suppression and overexpression of the immune system, inflammation, abnormal metabolic changes, skin diseases, carcinogenesis, etc., and the toxicity relies on interaction with the cellular system and receptors.15,201,202
With the increase in pollution and intrusion of pollutants in the food web, the toxic chemicals traced in food and edibles are increasing. Some of those chemicals exhibited bioaccumulation and become silent killers, but some are potentially lethal. These emerging pollutants include pesticides, herbicides, healthcare, cosmetic chemicals, etc. Fipronil is a wide-spectrum phenylpyrazole insecticide used to control beetles, ants, cockroaches, etc. but its entry into the food chain is alarming due to its carcinogenic nature, essentiating its prohibition by the US Environmental Protection Agency (EPA). Suitable detection methods are thereby essential to identify and quantify these contaminants before they enter the food chain. The most reliable detection method includes LC–MS/MS and GC–MS, having specific sample preparation before the analyses (Table 7).203 One such preparatory method involved a modified QuEChERS sample preparation before using a triple quadrupole MS instrument coupled to ESI for detecting fipronil and its major metabolite fipronil sulfone, at concentrations of 5 μg/kg. The use of nontargeted approaches, such as SWATH-MS (sequential window acquisition of all theoretical mass spectra), enables the sequential analysis of fipronil and other such contaminants, e.g., pesticides and polyaromatic hydrocarbons. Glyphosate (insecticide) was detected in an underivatized form by innovating new extraction methods coupled with instrumentation. The QuPPe (Quick Pesticide Preparation) method was used for sample preparation204 followed by detection via sensitive MS instruments to achieve accurate quantitative results. LC–MS/MS was used in combination with the DMS (differential mobility separation) technique to terminate analytical interferences leading to improved signal by decreasing noise and, consequently, increasing accuracy and confidence in data. Using this method, LC–DMS–MS/MS was used for identification and quantification of pesticide contaminants in food samples. Triclosan is a well-known and common biocide agent against bacteria as well as fungi,205 while bisphenol analogues are used in packaging and lining.206 Morgan et al.206 employed GC–MS to monitor the levels of triclosan and five bisphenol analogues (B, F, P, S, and Z) in 776 adult solid food samples. More than 80% of the samples were contaminated with at least one target phenol. Based on the frequencies, 59% of samples were contaminated with triclosan followed by 32% bisphenol S, and 28% bisphenol Z. The maximum concentration for triclosan was 394 ng/g.
Table 7. Detection of Emerging Toxins from Food and Water Samples by MS.
| Name | Sample | Method | Operating parameters | Outcome | Ref |
|---|---|---|---|---|---|
| Cypermethrin | Baby food | liquid chromatography coupled to quadrupole Orbitrap mass spectrometry | Gradient elution; negative ionization mode; capillary temperature 300 °C and heater 305 °C | Detection concentration in baby food 10.3 μg kg–1 | (208) |
| Parabens | Surface water | UHPLC–MS/MS | LC-18 column; column temp 40 °C; gradient elution; capillary voltage −3.0 kV | Limit of detection 0.04 ng L–1 and Limit of quantification 0.82 ng L–1 | (209) |
| Bisphenol | Water | GS–MS/MS | Temperature transfer line 250 °C; ion source 230 °C and quadrupole 150 °C. solvent delay 4.5 min; electron ionization (EI) mode (70 eV) | Recovery 81.8% −96.1%; limit of detection was 0.2 ng L–1 | (210) |
| Parabens | water | GS–MS/MS | Temperature transfer line 250 °C; ion source 230 °C and quadrupole 150 °C. solvent delay 4.5 min; electron ionization (EI) mode (70 eV) | Recovery 81.8% −96.1%; limit of detection was 0.2 ng L–1 | (210) |
| Triclosan | water | GS–MS/MS | Temperature transfer line 250 °C; ion source 230 °C and quadrupole 150 °C. solvent delay 4.5 min; electron ionization (EI) mode (70 eV) | Recovery 81.8% −96.1%; limit of detection was 0.2 ng L–1 | (210) |
| Neonicotinoids | vegetables | QuEChERS-Portable MS | PDESI as ion source; ultrapure helium (≥99.999%) as carrier gas; inlet temperature 200 °C; molecular pump speed was 1375 Hz | Limit of detection 2.0 ng g–1 recovery 82.2% −109.7% | (211) |
| Carbamates | Vegetables | QuEChERS-Portable MS | PDESI as ion source; ultrapure helium (≥99.999%) as carrier gas; inlet temperature 200 °C; molecular pump speed was 1375 Hz | Limit of detection 2.0 ng g–1 recovery 82.2% −109.7% | (211) |
| Phenyl Pyrazole | Vegetables | QuEChERS-Portable MS | PDESI as ion source; ultrapure helium (≥99.999%) as carrier gas; inlet temperature 200 °C; molecular pump speed was 1375 Hz | Limit of detection 2.0 ng g–1 recovery 82.2% −109.7% | (211) |
| Pesticides | Fruits and vegetables | QuEChERS-LC–MS | Sciex QTRAP 5500 triple quadrupole MS; positive electrospray ionization; ion source temperature 550 °C | 24 pesticides detected distinctly | (212) |
| Pesticides | Milk | LC-LTQ/Orbitrap Mass Spectrometry | Separation with reverse phase C18 column; positive ionization mode; spray voltage 4 kV; auxiliary gas flow rate 10 arbitrary units; tube lens 90 V, capillary temperature 320 °C | Limit of detection 0.2–8.1 μg kg–1 and quantification 0.61–24.8 μg kg–1 | (213) |
Not only emerging toxins but also the availability of efficient and portable systems have become necessary prerequisites. Some of the recent advancements have shown the availability of portable MS systems for detection and monitoring. Maragos131 has evaluated the potential of portable MS (APCI-MS) for the detection of T-2 toxin mycotoxin in contaminated cereal grains, wheat, and maize by APCI-MS. The sample was extracted with acetonitrile+water (84:16, v/v) followed by drying and reconstitution in ammonium formate. The MS system contains a linear ion trap mass analyzer to avoid the need of an external supply of gas or air. The device and developed method were able to detect T-2 toxin above 0.2 mg/kg from soft white and hard red wheat, and yellow dent maize. The method was more efficient and hence able to lower-down the detection limit from >0.9 mg/kg. In a similar line, FB and its isoforms were detected in maize with a portable mass spectrometer. For the detection, samples were extracted with aqueous methanol followed by cleaning up in the immunoaffinity column. Ultimately, cleaned samples were successfully analyzed with the portable MS with detection limits of 0.15 (B1), 0.19 (B2/B3), and 0.28 (total FB) mg/kg maize. The method has quantification limits of 0.33, 0.59, and 0.74 mg/kg and recoveries of 93.6% to 108.6%. Wichert et al.207 have also reported such kind of advancements to detect proteinaceous toxins (912.5–66.5 kDa) from plants as well as microorganisms origin using paper spray-MS (PS-MS) with wipe samples of bench, glass, leaves, flooring, etc., and validated with biological toxin simulant for Staphylococcal enterotoxin B. Carbon sputtered porous polyethylene dominated conventional chromatography paper, carbon nanotube-coated paper, and polyethylene for paper spray. The method was able to distinguish the protein toxin simulant efficiently with a good signal-to-noise ratio.
5. Detection of Food Fraud and Food Adulteration
Food adulteration and fraud have become a common practice nowadays to gain more profit. To avoid detection by current available analytical methods, new adulteration and fraud practices are becoming advanced and sophisticated making detection one of the biggest challenges for society.214 The tracing of fraud in food products via chemical analysis has become more complex especially due to the emergence of new and unknown adulterants.74 MS has become an indistinguishable part of testing and food authentication analysis due to its potential to trace chemical compounds based on their chemical fingerprints or chemical profiles.215 Adulterants are used as ingredients and additives in food products for economic gain for the seller at the cost of the health of consumers. The use of bulking agents such as sulfated polysaccharides is very common in minced meat and is a fraudulent practice that is very difficult to detect. Kosek et al.216 have evaluated rapid evaporative MS (REIMS) to detect adulterants in sausages and burgers prepared from chopped pork and chicken meat. The technique was able to detect the adulterants efficiently with 2.5% as the threshold concentration for protein additives (carrageenan) can be detected at 1% concentration. The major advantage of the system is the quick detection of adulterants in samples, which aids in preventing any major health issue from the consumption of adulterated food products. The problem becomes more serious when consumers are infants or minors. Milk is one of the common products that is supposed to provide optimum nutrients including lipids and proteins. Piras et al.217 employed atmospheric pressure matrix-assisted laser desorption/ionization with a Q-TOF mass analyzer, which generated charged proteinaceous as well as lipids/metabolites ions to find the possible fraud with milk. The common adulteration in milk is the intermixing of milk from different sources, and the current methods were able to identify milk from camel, cow, goat, and sheep with 100% accuracy. The goat milk was analyzed for the presence of cow milk as an adulterant, and it was detected even at a lower concentration of 5% with sensitivity and specificity of 92.5% and 94.5%, respectively. The major outcome of the work is its time consumption for sample analysis, i.e., 10 s per unadulterated sample to prepare profile. The method creates differences between protein and lipid molecules in terms of the number of charged moieties as lipids are single charged, while protein moieties have multiple charges.
Integration of artificial intelligence and neural network models has further improved the demand for efficiency of MS systems. Nichani et al.218 have developed a method for the differential identification of spelt and wheat. Nontargeted LC–MS/MS along with convolutional neural network (CNN) models was developed. The employed neural network was able to learn patterns by itself and discriminated between spelt and wheat efficiently. For external validation of the model, artificial mixed spectra of spelt bread and flour, 11 untypical spelt, and six old wheat cultivars (which were not part of model training) were analyzed. The model was able to identify the nonconventional cultivars of wheat and spelt with a D value of 0.57.218
Not only food products but also active pharma formulations and ingredients are under the threat of adulteration and fraud. One such example is where MS is used to develop a model to differentiate between wild and cultivated Cordyceps sinensis harvests. Elemental analysis coupled with stable isotope ratio MS (EA-IRMS) and GasBench II coupled to isotope ratio MS (GB-IRMS) was used with orthogonal partial least-squares discriminant analysis (OPLS-DA) to identify the significant and unique markers of stable isotope ratios in both samples. Analysis identified three stable isotope markers, i.e., δ2H, δ18O, and δ15N, and their concentrations can be used to identify fresh C. sinensis samples as well as differentiating between wild and cultivated C. sinensis. δ2H values reduced sequentially in fresh samples based on respective origins. δ18O and δ15N have shown differential patterns as lower δ18O and higher δ15N represented cultivated samples, while higher δ18O and lower δ15N represented wild samples. The analyzed cultivated and wild samples have δ18O and δ15N of 18.99%, 4.80%, and 25.72%, 2.29%, respectively.219
6. Current Challenges and Future Prospects
The methods currently in practice for toxin detection are quite advanced, comprising a variety of direct and indirect analytical techniques.220 However, there are still a few challenges that make laboratory detection difficult, and toxins may sometimes go undetected.221 The following are the significant challenges for toxin analysis that are commonly encountered.
6.1. Representative Sample
Microbial toxins are rarely evenly distributed in foodstuff and edibles.222 As in the case of mycotoxins, in some regions of the victuals, called “mycotoxin pockets”, concentrations may be extremely high, whereas the rest of the material may be free from contamination. The materials are distributed more heterogeneously for products with larger particle sizes, such as nuts and figs. This necessitates representative sampling that accounts for the random distribution of the “hotspots” to provide an accurate view of the degree of contamination in the specimen. This can be performed by taking a large number of small, incremental samples from various locations distributed throughout the lot.223 The selection of incremental samples from the bulk is crucial to give all morsel particles a chance of being selected, thus reducing the statistical bias. Besides the number of samples, sample type and its processing are equally important as in some cases surface swab is sufficient, while others need extraction followed by processing like digestion of target sample as reported in the case of protein-based toxins. Kalb et al. employed endopep-MS to identify botulinum A, B, E, and F from food samples by optimizing the fingerprint peptides generated.149 Hence, detection technology must be optimized to compete with emerging challenges.
6.2. Sample Preparation
Sample preparation is a highly complex process, with various pitfalls. Every step encountered introduces a level of variability that aggregates and contributes to total variability within single analytical data. It has been generally observed that nearly 1/3 of the variability is attributed to sample preparation. On the other hand, a much smaller amount of variation is contributed by the analytical method being employed. Even with the best analytical equipment, sample preparation is critical.224,225 By adhering to the key factors of sample preparation (size reduction, sampling size, and uniformity), the root–mean–square value can be kept significantly within 5–10%, increasing the prediction result’s accuracy.
6.3. Low Concentration
Even at low concentration levels, toxins can be highly toxic.226 Different classes of toxins have different levels of toxic effects on the target. Glycoalkaloids (potato) and isoflavones (clover) have shown low toxicity, linamarin (cassava) and coniin (hemlock) are somewhat toxic, while ricin (castor beans) and cyanotoxin and saxitoxin (blue–green algae) are extremely toxic.227 Hence, the tolerable concentration range is also varied in the same proportion. The Food Safety and Standards Authority of India (FSSAI) has set limit values of 15 μg/kg in cereal products, pulses, and nuts, and 30 μg/kg in spices, whereas, for milk, the allowable range is considerably low (0.5 μg/kg).228 The values are more stringent for the US FDA and European Union, nearly 1/3 of the FSSAI-approved figures. The analysis method needs to be extremely precise and sensitive to detect such low concentrations. The low levels of toxin concentration, often not detected, are hugely responsible for reduced production efficiency and increased susceptibility to various diseases.229
6.4. Complex Matrix
Generally, the toxin matrices found in food are fairly complex and pose a major challenge for laboratories. For example, heterogeneous matrices such as spices contain numerous interfering substances, which makes it extremely difficult to detect the toxins precisely. Also, food may not be necessarily safe, even if well-known toxins are not detected during analysis. These compounds may still be present in conjugated form in masked or bound form.230 These modified toxins are derived from plants by conjugation and have their chemical structures altered, making the analysis more complex. Also, even though a large number of different toxins including mycotoxins, bacterial toxins, phytotoxins, etc. exist, only a few are characterized and regulated by law.2 In reality, several toxins may be present simultaneously, and it may be challenging to find/pinpoint specific analytes in the food particles.
6.5. Portability of Instrument
The normal trend in sample analysis is like the collection of samples from the site and transfer to the lab for further analysis. However, such a protocol seems obsolete when we need to characterize the sample rapidly. In such cases, a portable and handy instrument with easily transferable and ready-to-use techniques is required. Some of the recent research has shown the pay for portable MS-based detection systems for the food analysis and characterization of toxins like PS-MS.207 However, more advancement needed with the miniaturization of instruments with rapid detection and high accuracy is required.
6.6. Cost
A variety of factors contribute to the cost of toxin analysis. They include facilities, sophisticated analytical instruments, reagents, and logistics. Additionally, the number of tests needed for representative sampling to negate the misreporting of toxins in bulk material also contributes to the testing cost. Although effective sampling is one of the most critical factors in mycotoxin analysis, it is the costliest, and surprisingly, innovations in this area have not taken place rapidly.231 Reducing the time and cost requirements through representative sampling while increasing accuracy is the need of the hour. A balance needs to be achieved between the cost per sample and the number of runs essential to generate the most confident results that will ensure lower downtime with precise results.232
6.7. Multiple Toxins Contamination
Naturally, all the food materials are prone to be contaminated with multiple toxins due to the presence of diverse microbial contaminants simultaneously. The method should be effective and efficient to detect all of the toxins together even in minute quantity. A MS-based approach made it possible to allow the detection of diverse range of toxins. Lattanzio et al.233 have reported the detection of multiple aflatoxins including B1, B2, G1, G2, ochratoxin A, fumonisins B1 and B2, deoxynivalenol, zearalenone, T-2, and HT-2 toxins simultaneously from maize via LC–MS. In a similar line, Cheng et al.234 have also reported the detection of 15 toxic alkaloids from vegetables and meat samples using double layer pipet tip magnetic dispersive solid phase extraction method that used polyamidoamine-functionalized magnetic carbon nanotubes. Extracted samples were characterized with ultrafast liquid chromatography-tandem quadrupole mass spectrometry (UFLC–MS/MS) coupled with DPT-MSPE method. The system has shown high recovery efficiency with toxin recovery of up to 125% for meat as well as vegetable dishes. In food samples, fungal contamination is a common phenomenon; hence, it is the prime target for most of the work. Wang et al.235 and Nualkaw et al.236 have employed UPLC–MS for the detection of mycotoxins from animal feed like dairy product, poultry, and animal feed. Lee et al.184 reported the presence of okadaic acid, dinophysistoxin-1, dinophysistoxin-2, and dinophysistoxin-3 in raw as well as cooked mussels using LC–tandem mass spectrometry. The method has shown detection and quantification limit of 0.2–5.1 μg/kg with accuracy and precision of 80.5–109.8% and 0.9–20.1%, respectively. Albero et al.237 have also identified mycotoxins in aquaculture feed by LC–MS/MS. The method employed ultrasound-assisted extraction followed by LC–MS/MS, which identified 15 mycotoxins together that also included enniatins (EENB and ENNB1), beauvericin, and fumonisin B2.
In some cases, the toxins are present in modified or bounded form (masked form), which hinder its detection by conventional methods. As mentioned by Berthiller et al.,238 mycotoxins have high probability of masking due to food processing which changes its structural as well as behavior and obstruct the appearance of common characteristics of respective toxins. Masked mycotoxins have been detected in extractable conjugated and nonextractable varieties (usually to unavailability of toxins in extracted samples, bound mycotoxins are not accessible for analysis and need chemical or enzymatic treatment prior detection).238
Fiby et al.239 reported the presence of Fusarium mycotoxin like deoxynivalenol in native as well as masked form (DON-3-glucoside “D3G”, 3-acetyl-DON “3ADON”, or 15-acetyl-DON “15ADON”) in cereals. The presence was detected with a stable-isotope dilution liquid chromatography–tandem mass spectrometry-based approach that relies on labeling of toxins enzymatic byproducts with 13C. The method efficiently detected D3G (76–98%), DON (86–103%), 15ADON (68–100%), and 3ADON (63–96%).239 Zhang et al.240 employed ultrahigh-performance liquid chromatography–HRMS to detect 82 mycotoxins and categorize into 8 classes by Python program developed with “Fragmentation pattern screener (FPScreener)” and nontarget screening rules. Pascari et al.241 combined QuEChERS with liquid chromatography–triple quadrupole mass spectrometry for the detection of ZEN from oat flour. For the identification, oat and wheat flours were treated with amylolytic enzymes (α-amylase and amyloglucosidase), similar to the one used in the cereal-based baby food production process that reduced the β-zearalenol (β-ZEL) and β-ZEL-14-sulfate by 40% within 90 min and allowed the specific detection of ZEN-sulfate derivates from cereals.
7. Future Perspective
The past few decades have witnessed significant advancements in analytical techniques and technologies to detect and manage the problem of mycotoxin analysis. The global mycotoxin testing market is estimated to grow at a cumulative annual growth rate (CAGR) of 7.8%, which corresponds to a market of $1.4 billion by 2026.242 As the number indicates, the accelerated need for food safety has significantly accelerated the mycotoxin testing market. Needless to say, effective sampling, method performance, cost, and rapid detection are of paramount importance for successful mycotoxin testing. However, inexpensive and fast tests with low precision would lead to misclassification through inaccurate results and impact the business decisions of the producers. In light of this, the existing techniques must be refined/tuned for higher precision while active R&D is pursued to develop new methods that can detect multiple toxins simultaneously with high sensitivity (at regulatory levels) with minimal cost and runtime. One option could be developing miniaturized MS, which could be used in a fashion similar to the screening devices used for COVID detection at airports and train stations. The spectrometers are generally efficient and reliable and allow for rapid detection and solid sample detection through thermal absorption. One such example is mycotoxin detection in milled wheat by an MS developed by M/s BaySpec Inc.243 The portable device could detect even ppm (1.4) levels of the contaminant within less than a minute’s time.
Recent studies have highlighted that mass-sensitive microarray (MSMA)-based biosensors could be a promising tool for rapidly detecting mycotoxin material.244 The device consists of a mass-sensitive transducer based on solidly mounted resonator (SMR) technology with a specific integrated circuit. This technology allows small molecular weight toxins (up to 3 toxins simultaneously) to be rapidly detected with high sensitivity (for a single sample) within less than 10 min using mycotoxin analysis as a model example. The authors argued that with further upgrade, many more analytes (∼32) could be detected on the devices from a single sample, and with automation, the analysis was performed and printed without the requirement of any operator. The device could be an ideal system for multiplex analysis of mycotoxins; however, significant improvements must be made before it can be considered for field deployment in the analysis of real-time samples.
Biographies

Dr. Vishal Ahuja has his Ph.D. in Biotechnology from Himachal Pradesh University, Shimla, India. He has his post doc in CSIR-Indian Institute of Petroleum Dehradun India on an FTT project. Currently he is working as assistant professor of research and development (UIBT/UCRD) Chandigarh University, Punjab, India. He has published more than 30 research and review articles. His research interest is in biomass biotransformation, production of bioactive compounds, and energy products from sustainable and renewable resources. His area of work is closely aligned with current trends in biotechnology advancements in fields such as biofuels, pharmaceuticals, and environmental sustainability.

Dr. Amanpreet Singh received his Ph.D. degree from the Department of Chemistry, Indian Institute of Technology, Ropar in 2023. He earned his master’s degree in organic chemistry from the Department of Chemistry, Panjab University Chandigarh in 2017. Currently, he is an assistant professor in the Department of Chemistry, University Institute of Science, Chandigarh University, Mohali. He has published his research in well reputed international journals. His research interest lies in supramolecular organic chemistry, material chemistry, and development of carbon-based nanomaterials for sensing and catalysis. His research mainly focuses on fabrication of chemo sensors for environmental applications and sustainable development.

Dr. Debarati Paul, currently Associate Professor in Amity Institute of Biotechnology, Amity University, has completed her Ph.D. from the Institute of Microbial Technology-CSIR (Chandigarh) and post doc from EAWAG (Switzerland) and Mississippi State University (USA) for about 5 years. Her main area of research includes genomics, environmental biotechnology, antimicrobial resistance, and biodegradation. She has published over 70 papers including research articles, reviews, conference proceedings and posters, and book chapters. She has received the prestigious Indo US Science and Technology Forum award and DST travel grants, etc. She also trains students for skill development in Quality Control Chemist Microbiology, since 2019.

Dr. Diptarka Dasgupta is working as principal scientist in Material Resource Efficiency Division of CSIR-Indian Institute of Petroleum Dehradun Uttarakhand India. He has completed his Ph.D. from AcSIR- Indian Institute of Petroleum. He has expertise in microbial genomics and biotransformation of waste to industrial products. He has published more than 40 research and review articles. He is also experienced in analytical techniques for characterization of biomolecules.

Dr. Petra Urajová received her Ph.D. from University of South Bohemia, Department of Agricultural Chemistry. She has published over 25 research articles in well reputed international journals. Her research is mainly focused on chemistry of natural products, specifically algae and cyanobacteria and their secondary metabolites. Among her skills belong mainly HPLC and HPLC–HRMS techniques.

Sounak Ghosh is currently working on his final year dissertation project at Laboratory of Algal Biotechnology, Institute of Microbiology of Czech Academy of Sciences, Czech Republic. He is an ongoing integrated master’s student in biotechnology enrolled at Vellore Institute of Technology, Vellore, India. He has worked on multiple projects including Bioremediation and microbiology. His current research work is focused on genome mining of secondary metabolites, and natural product isolation from various symbiotic cyanobacteria.

Roshani Singh is currently working on her final year dissertation project at Laboratory of Algal Biotechnology, Institute of Microbiology of Czech Academy of Sciences, Czech Republic. She is an ongoing integrated master’s student in biotechnology enrolled at Vellore Institute of Technology, Vellore, India. She has worked on various projects on bioinformatics, bioremediation, and molecular biology. She is currently focused on exploration of novel cyanobacterial metabolites by direct pathway cloning.

Dr. Gobardhan Sahoo received his Ph.D. from CSIR-National Institute of Oceanography under the aegis of Academy of Scientific and Innovative Research (AcSIR), India in Science category. Further, he has pursued his postdoctoral studies at Department of Marine Microbiology, University of Haifa, Israel. Currently, he is pursing his postdoctoral study at Laboratory of Algal Biotechnology, Institute of Microbiology of Czech Academy of Sciences, Czech Republic. He has published more than 11 research papers in national and international journals. His research is mainly focused on the ecology of epibiotic diatoms and the biocommunication system in barnacles.

Dr. Daniela Ewe received her Ph.D. from Department of Biology, University of Konstanz, Germany in 2015. She published over 15 research articles in well reputed international journals. Further, she has pursued her postdoctoral studies at Laboratory of Algal Biotechnology, Institute of Microbiology of Czech Academy of Sciences, Czech Republic and CeBiTec – Center for Biotechnology, Bielefeld University, Germany. She has vast experience in the field of microalgae physiology and its metabolism.

Dr. Kumar Saurav received his Ph.D. from Vellore Institute of Technology, India followed by two postdoctoral studies at South China Sea Institute of Oceanology, Chinese Academy of Sciences and Department of Marine Microbiology, University of Haifa, Israel. Currently, he is working as junior group leader at Laboratory of Algal Biotechnology, Institute of Microbiology of Czech Academy of Sciences, Czech Republic. He has published over 50 research articles in well reputed international journals. He has vast experience in the isolation of secondary metabolites from microorganisms and their biological activity determination. He is well versed in the analytical instruments like LC–MS, HPLC, and HRMS.
Author Contributions
V.A., and K.S.: conceptualization, investigation, data analysis, original draft preparation, and reviewing. A.S., D.P., D.D., S.S., R.S., G.S., D.E., and P.U.: original draft preparation, editing, and reviewing. K.S.: supervision, data analysis, and reviewing. All authors have read and agreed to the final version of the manuscript. CRediT: Vishal Ahuja conceptualization, data curation, formal analysis, methodology, software, validation, visualization, writing-original draft, writing-review & editing; Amanpreet Singh writing-original draft; Debarati Paul writing-original draft; Diptarka Dasgupta writing-original draft; Petra Urajová software, validation, visualization, writing-review & editing; Sounak Ghosh writing-review & editing; Roshani Singh writing-review & editing; Gobardhan Sahoo formal analysis, visualization; Daniela Ewe formal analysis, validation, writing-review & editing; Kumar Saurav conceptualization, formal analysis, investigation, project administration, supervision, writing-original draft, writing-review & editing.
The authors declare no competing financial interest.
Special Issue
Published as part of Chemical Research in Toxicologyvirtual special issue “Mass Spectrometry Advances for Environmental and Human Health”.
References
- WHO Estimates of the Global Burden of Foodborne Diseases: Foodborne Disease Burden Epidemiology Reference Group 2007–2015; World Health Organization, 2015. [Google Scholar]
- Alshannaq A.; Yu J.-H. Occurrence, Toxicity, and Analysis of Major Mycotoxins in Food. Int. J. Environ. Res. Public Health 2017, 14 (6), 632. 10.3390/ijerph14060632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasheed T.; Bilal M.; Nabeel F.; Adeel M.; Iqbal H. M. N. Environmentally-Related Contaminants of High Concern: Potential Sources and Analytical Modalities for Detection, Quantification, and Treatment. Environ. Int. 2019, 122, 52–66. 10.1016/j.envint.2018.11.038. [DOI] [PubMed] [Google Scholar]
- Rather I. A.; Koh W. Y.; Paek W. K.; Lim J. The Sources of Chemical Contaminants in Food and Their Health Implications. Front Pharmacol 2017, 8, 830. 10.3389/fphar.2017.00830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher M. T.; Netzel G. Food Safety and Natural Toxins. Toxins 2020, 12 (4), 236. 10.3390/toxins12040236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dey P.; Kundu A.; Kumar A.; Gupta M.; Lee B. M.; Bhakta T.; Dash S.; Kim H. S. Analysis of Alkaloids (Indole Alkaloids, Isoquinoline Alkaloids, Tropane Alkaloids). Recent Advances in Natural Products Analysis 2020, 505–567. 10.1016/B978-0-12-816455-6.00015-9. [DOI] [Google Scholar]
- Picardo M.; Núñez O.; Farré M. Suspect and Target Screening of Natural Toxins in the Ter River Catchment Area in NE Spain and Prioritisation by Their Toxicity. Toxins 2020, 12 (12), 752. 10.3390/toxins12120752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malachová A.; Stránská M.; Václavíková M.; Elliott C. T.; Black C.; Meneely J.; Hajšlová J.; Ezekiel C. N.; Schuhmacher R.; Krska R. Advanced LC-MS-Based Methods to Study the Co-Occurrence and Metabolization of Multiple Mycotoxins in Cereals and Cereal-Based Food. Anal Bioanal Chem. 2018, 410 (3), 801–825. 10.1007/s00216-017-0750-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krska R.; de Nijs M.; McNerney O.; Pichler M.; Gilbert J.; Edwards S.; Suman M.; Magan N.; Rossi V.; van der Fels-Klerx H. j.; Bagi F.; Poschmaier B.; Sulyok M.; Berthiller F.; van Egmond H. p. Safe Food and Feed through an Integrated Toolbox for Mycotoxin Management: The MyToolBox Approach. World Mycotoxin Journal 2016, 9 (4), 487–495. 10.3920/WMJ2016.2136. [DOI] [Google Scholar]
- Turner N. W.; Subrahmanyam S.; Piletsky S. A. Analytical Methods for Determination of Mycotoxins: A Review. Anal. Chim. Acta 2009, 632 (2), 168–180. 10.1016/j.aca.2008.11.010. [DOI] [PubMed] [Google Scholar]
- Verma M.; Chaudhary M.; Singh A.; Kaur N.; Singh N. Naphthalimide-Gold-Based Nanocomposite for the Ratiometric Detection of Okadaic Acid in Shellfish. J. Mater. Chem. B 2020, 8 (36), 8405–8413. 10.1039/D0TB01195A. [DOI] [PubMed] [Google Scholar]
- Fayyaz K.; Nawaz A.; Olaimat A. N.; Akram K.; Farooq U.; Fatima M.; Siddiqui S. A.; Rana I. S.; Mahnoor M.; Shahbaz H. M. Microbial Toxins in Fermented Foods: Health Implications and Analytical Techniques for Detection. Journal of Food and Drug Analysis 2022, 30 (4), 523–537. 10.38212/2224-6614.3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abeysiriwardena N. M.; Gascoigne S. J. L.; Anandappa A. Algal Bloom Expansion Increases Cyanotoxin Risk in Food. Yale J. Biol. Med. 2018, 91 (2), 129–142. [PMC free article] [PubMed] [Google Scholar]
- Mtewa A. G.; Egbuna C.; Ngwira K. J.; Lampiao F.; Shah U.; Mtewa T. K.. Classification of Phytotoxins and Their Mechanisms of Action. In Poisonous Plants and Phytochemicals in Drug Discovery; John Wiley & Sons, Ltd, 2020; pp 19–30. 10.1002/9781119650034.ch2. [DOI] [Google Scholar]
- Campo J.; Picó Y.. 17 - Emerging Contaminants and Toxins. In Chemical Analysis of Food (Second ed.); Pico Y., Ed.; Academic Press, 2020; pp 729–758. 10.1016/B978-0-12-813266-1.00017-6. [DOI] [Google Scholar]
- Dhakal A.; Hashmi M. F.; Sbar E.. Aflatoxin Toxicity. In StatPearls; StatPearls Publishing: Treasure Island, FL, 2023. [PubMed] [Google Scholar]
- Jastrzebski M. K.; Kaczor A. A.; Wróbel T. M. Methods of Lysergic Acid Synthesis-The Key Ergot Alkaloid. Molecules 2022, 27 (21), 7322. 10.3390/molecules27217322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maragos C. M.; Barnett K.; Morgan L.; Vaughan M. M.; Sieve K. K. Measurement of Fumonisins in Maize Using a Portable Mass Spectrometer. Toxins 2022, 14 (8), 523. 10.3390/toxins14080523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L.; Wang Q.; Wang S.; Cai R.; Yuan Y.; Yue T.; Wang Z. Bio-Control on the Contamination of Ochratoxin A in Food: Current Research and Future Prospects. Curr. Res. Food Sci. 2022, 5, 1539–1549. 10.1016/j.crfs.2022.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacha S. A. S.; Li Y.; Nie J.; Xu G.; Han L.; Farooq S.. Comprehensive Review on Patulin and Alternaria Toxins in Fruit and Derived Products. Frontiers in Plant Science 2023, 14. 10.3389/fpls.2023.1139757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing S.; Liu C.; Zheng J.; Dong Z.; Guo N. Toxicity of Zearalenone and Its Nutritional Intervention by Natural Products. Food Funct. 2022, 13 (20), 10374–10400. 10.1039/D2FO01545E. [DOI] [PubMed] [Google Scholar]
- Puvača N.; Avantaggiato G.; Merkuri J.; Vuković G.; Bursić V.; Cara M. Occurrence and Determination of Alternaria Mycotoxins Alternariol, Alternariol Monomethyl Ether, and Tentoxin in Wheat Grains by QuEChERS Method. Toxins (Basel) 2022, 14 (11), 791. 10.3390/toxins14110791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charla R.; Patil P. P.; Patil V. S.; Bhandare V. V.; Karoshi V.; Balaganur V.; Joshi R. K.; Harish D. R.; Roy S.. Anti-Cholera Toxin Activity of Selected Polyphenols from Careya Arborea, Punica Granatum, and Psidium Guajava. Frontiers in Cellular and Infection Microbiology 2023, 13. 10.3389/fcimb.2023.1106293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomputius W. F.; Kilgore S. H.; Schlievert P. M. Probable Enterotoxin-Associated Toxic Shock Syndrome Caused by Staphylococcus Epidermidis. BMC Pediatr 2023, 23 (1), 108. 10.1186/s12887-023-03914-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nada H. G.; El-Tahan A. S.; El-Didamony G.; Askora A. Detection of Multidrug-Resistant Shiga Toxin-Producing Escherichia Coli in Some Food Products and Cattle Faeces in Al-Sharkia, Egypt: One Health Menace. BMC Microbiology 2023, 23 (1), 127. 10.1186/s12866-023-02873-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L.; Lv Q.; Zheng Y.; Gao S.; Huang W.; Liu P.; Kong D.; Wang Y.; Yu Y.; Jiang Y.; Jiang H.. Rapid and Sensitive Detection of Botulinum Toxin Type A in Complex Sample Matrices by AlphaLISA. Frontiers in Public Health 2022, 10. 10.3389/fpubh.2022.987517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S.; Wang Y.; Liu Y.; Jia K.; Zhang Z.; Dong Q. Cereulide and Emetic Bacillus Cereus: Characterizations, Impacts and Public Precautions. Foods 2023, 12 (4), 833. 10.3390/foods12040833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T.; Fernandes S. P. S.; Araújo J.; Li X.; Salonen L. M.; Espiña B. A Carboxyl-Functionalized Covalent Organic Polymer for the Efficient Adsorption of Saxitoxin. J. Hazard Mater. 2023, 452, 131247. 10.1016/j.jhazmat.2023.131247. [DOI] [PubMed] [Google Scholar]
- Panlilio J. M.; Hammar K. M.; Aluru N.; Hahn M. E. Developmental Exposure to Domoic Acid Targets Reticulospinal Neurons and Leads to Aberrant Myelination in the Spinal Cord. Sci. Rep 2023, 13 (1), 2587. 10.1038/s41598-023-28166-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tebben J.; Zurhelle C.; Tubaro A.; Samdal I. A.; Krock B.; Kilcoyne J.; Sosa S.; Trainer V. L.; Deeds J. R.; Tillmann U. Structure and Toxicity of AZA-59, an Azaspiracid Shellfish Poisoning Toxin Produced by Azadinium Poporum (Dinophyceae). Harmful Algae 2023, 124, 102388. 10.1016/j.hal.2023.102388. [DOI] [PubMed] [Google Scholar]
- Brammer-Robbins E.; Costa K. A.; Bowden J. A.; Martyniuk C. J.; Larkin I. V.; Denslow N. D. Putative High-Level Toxicity Pathways Based on Evidence of Brevetoxin Immunotoxicity in Marine Fauna. Aquat Toxicol 2022, 252, 106298. 10.1016/j.aquatox.2022.106298. [DOI] [PubMed] [Google Scholar]
- Qin Y.; Li J.; Kuang J.; Shen S.; Jiang J.; Zhang Z.; Zhao C.; Zhou X.; Huang B.; Han B.. Sensitive Time-Resolved Fluoroimmunoassay for the Quantitative Detection of Okadaic Acid. Frontiers in Marine Science 2022, 9. 10.3389/fmars.2022.961751 [DOI] [Google Scholar]
- Bolarinwa I. F.; Oke M. O.; Olaniyan S. A.; Ajala A. S.; Bolarinwa I. F.; Oke M. O.; Olaniyan S. A.; Ajala A. S.. A Review of Cyanogenic Glycosides in Edible Plants. In Toxicology - New Aspects to This Scientific Conundrum; IntechOpen, 2016. 10.5772/64886. [DOI] [Google Scholar]
- Melough M. M.; Lee S. G.; Cho E.; Kim K.; Provatas A. A.; Perkins C.; Park M. K.; Qureshi A.; Chun O. K. Identification and Quantitation of Furocoumarins in Popularly Consumed Foods in the U.S. Using QuEChERS Extraction Coupled with UPLC-MS/MS Analysis. J. Agric. Food Chem. 2017, 65 (24), 5049–5055. 10.1021/acs.jafc.7b01279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J. S.; Clauson-Kaas F.; Lindqvist D. N.; Rasmussen L. H.; Strobel B. W.; Hansen H. C. B. Does the Natural Carcinogen Ptaquiloside Degrade Readily in Groundwater?. Environmental Sciences Europe 2021, 33 (1), 24. 10.1186/s12302-021-00468-0. [DOI] [Google Scholar]
- Stegelmeier B. L.; Colegate S. M.; Brown A. W. Dehydropyrrolizidine Alkaloid Toxicity, Cytotoxicity, and Carcinogenicity. Toxins (Basel) 2016, 8 (12), 356. 10.3390/toxins8120356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girardi C.; Odore R. Pharmacological Treatments and Risks for the Food Chain. Vet Res. Commun. 2008, 32, S11–18. 10.1007/s11259-008-9083-5. [DOI] [PubMed] [Google Scholar]
- Kralj Cigic I.; Prosen H. An Overview of Conventional and Emerging Analytical Methods for the Determination of Mycotoxins. Int. J. Mol. Sci. 2009, 10 (1), 62–115. 10.3390/ijms10010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kole P. L.; Venkatesh G.; Kotecha J.; Sheshala R. Recent Advances in Sample Preparation Techniques for Effective Bioanalytical Methods. Biomed Chromatogr 2011, 25 (1–2), 199–217. 10.1002/bmc.1560. [DOI] [PubMed] [Google Scholar]
- Qiu N.; Sun D.; Zhou S.; Li J.; Zhao Y.; Wu Y. Rapid and Sensitive UHPLC-MS/MS Methods for Dietary Sample Analysis of 43 Mycotoxins in China Total Diet Study. Journal of Advanced Research 2022, 39, 15–47. 10.1016/j.jare.2021.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Girolamo A.; Lippolis V.; Pascale M. Overview of Recent Liquid Chromatography Mass Spectrometry-Based Methods for Natural Toxins Detection in Food Products. Toxins (Basel) 2022, 14 (5), 328. 10.3390/toxins14050328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Bargen C.; Brockmeyer J.; Humpf H.-U. Meat Authentication: A New HPLC-MS/MS Based Method for the Fast and Sensitive Detection of Horse and Pork in Highly Processed Food. J. Agric. Food Chem. 2014, 62 (39), 9428–9435. 10.1021/jf503468t. [DOI] [PubMed] [Google Scholar]
- Liu Z.; Zhang M.; Chen P.; Harnly J. M.; Sun J. Mass Spectrometry-Based Nontargeted and Targeted Analytical Approaches in Fingerprinting and Metabolomics of Food and Agricultural Research. J. Agric. Food Chem. 2022, 70 (36), 11138–11153. 10.1021/acs.jafc.2c01878. [DOI] [PubMed] [Google Scholar]
- Monbaliu S.; Van Poucke C.; Detavernier C.; Dumoulin F.; Van De Velde M.; Schoeters E.; Van Dyck S.; Averkieva O.; Van Peteghem C.; De Saeger S. Occurrence of Mycotoxins in Feed as Analyzed by a Multi-Mycotoxin LC-MS/MS Method. J. Agric. Food Chem. 2010, 58 (1), 66–71. 10.1021/jf903859z. [DOI] [PubMed] [Google Scholar]
- Kerian K. S.; Jarmusch A. K.; Cooks R. G. Touch Spray Mass Spectrometry for In Situ Analysis of Complex Samples. Analyst 2014, 139 (11), 2714–2720. 10.1039/C4AN00548A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feider C. L.; Krieger A.; DeHoog R. J.; Eberlin L. S. Ambient Ionization Mass Spectrometry: Recent Developments and Applications. Anal. Chem. 2019, 91 (7), 4266–4290. 10.1021/acs.analchem.9b00807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández-Mesa M.; Le Bizec B.; Dervilly G. Metabolomics in Chemical Risk Analysis - A Review. Anal. Chim. Acta 2021, 1154, 338298. 10.1016/j.aca.2021.338298. [DOI] [PubMed] [Google Scholar]
- Selamat J.; Rozani N. A. A.; Murugesu S. Application of the Metabolomics Approach in Food Authentication. Molecules 2021, 26 (24), 7565. 10.3390/molecules26247565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilariño N.; Louzao M. C.; Fraga M.; Rodríguez L. P.; Botana L. M. Innovative Detection Methods for Aquatic Algal Toxins and Their Presence in the Food Chain. Anal Bioanal Chem. 2013, 405 (24), 7719–7732. 10.1007/s00216-013-7108-6. [DOI] [PubMed] [Google Scholar]
- Law J. W.-F.; Ab Mutalib N.-S.; Chan K.-G.; Lee L.-H. Rapid Methods for the Detection of Foodborne Bacterial Pathogens: Principles, Applications, Advantages and Limitations. Front Microbiol 2015, 5, 770. 10.3389/fmicb.2014.00770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liew W.-P.-P.; Sabran M.-R. Recent Advances in Immunoassay-Based Mycotoxin Analysis and Toxicogenomic Technologies. J. Food Drug Anal 2022, 30 (4), 549–561. 10.38212/2224-6614.3430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quilliam M. A.; Thomas K.; Wright J. L. Analysis of Domoic Acid in Shellfish by Thin-Layer Chromatography. Nat. Toxins 1998, 6 (3–4), 147–152. . [DOI] [PubMed] [Google Scholar]
- Rodríguez I.; Vieytes M. R.; Alfonso A. Analytical Challenges for Regulated Marine Toxins. Detection Methods. Current Opinion in Food Science 2017, 18, 29–36. 10.1016/j.cofs.2017.10.008. [DOI] [Google Scholar]
- Jangampalli Adi P.; Matcha B. Analysis of Aflatoxin B1 in Contaminated Feed, Media, and Serum Samples of Cyprinus Carpio L. by High-Performance Liquid Chromatography. Food Quality and Safety 2018, 2 (4), 199–204. 10.1093/fqsafe/fyy013. [DOI] [Google Scholar]
- Siracusa M.; Bacchiocchi S.; Dubbini A.; Campacci D.; Tavoloni T.; Stramenga A.; Ciriaci M.; Dall’Ara S.; Piersanti A. A High Throughput Screening HPLC-FLD Method for Paralytic Shellfish Toxins (PSTs) Enabling Effective Official Control. Molecules 2022, 27 (15), 4702. 10.3390/molecules27154702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandraleka T.; Abinayaa S.; Rathour A.; Mahalakshmi A.; Heema C. Detection of Aflatoxin in Food Products Using UV Fluorescence Spectroscopy. IJITEE 2020, 9 (5), 1068–1071. 10.35940/ijitee.E2272.039520. [DOI] [Google Scholar]
- Singh A. K.; Lakshmi G. B. V. S.; Dhiman T. K.; Kaushik A.; Solanki P. R.. Bio-Active Free Direct Optical Sensing of Aflatoxin B1 and Ochratoxin A Using a Manganese Oxide Nano-System. Frontiers in Nanotechnology 2021, 2. 10.3389/fnano.2020.621681 [DOI] [Google Scholar]
- Ray S.Sensory Properties of Foods and Their Measurement Methods. In Techniques to Measure Food Safety and Quality: Microbial, Chemical, and Sensory; Khan M. S., Shafiur Rahman M., Eds.; Springer International Publishing: Cham, 2021; pp 345–381. 10.1007/978-3-030-68636-9_15. [DOI] [Google Scholar]
- Liu Y.; Qiao F.; Xu L.; Wang R.; Jiang W.; Xu Z.. Fast Detection of Diarrhetic Shellfish Poisoning Toxins in Mussels Using NIR Spectroscopy and Improved Twin Support Vector Machines. Frontiers in Marine Science 2022, 9. 10.3389/fmars.2022.907378 [DOI] [Google Scholar]
- Baden D. G.; Melinek R.; Sechet V.; Trainer V. L.; Schultz D. R.; Rein K. S.; Tomas C. R.; Delgado J.; Hale L. Modified Immunoassays for Polyether Toxins: Implications of Biological Matrixes, Metabolic States, and Epitope Recognition. Journal of AOAC INTERNATIONAL 1995, 78 (2), 499–507. 10.1093/jaoac/78.2.499. [DOI] [PubMed] [Google Scholar]
- Yasumoto T.; Murata M.; Oshima Y.; Sano M.; Matsumoto G. K.; Clardy J. Diarrhetic Shellfish Toxins. Tetrahedron 1985, 41 (6), 1019–1025. 10.1016/S0040-4020(01)96469-5. [DOI] [Google Scholar]
- Tubaro A.; Florio C.; Luxich E.; Sosa S.; Della Loggia R.; Yasumoto T. A Protein Phosphatase 2A Inhibition Assay for a Fast and Sensitive Assessment of Okadaic Acid Contamination in Mussels. Toxicon 1996, 34 (7), 743–752. 10.1016/0041-0101(96)00027-X. [DOI] [PubMed] [Google Scholar]
- Eberhart B.-T. L.; Moore L. K.; Harrington N.; Adams N. G.; Borchert J.; Trainer V. L. Screening Tests for the Rapid Detection of Diarrhetic Shellfish Toxins in Washington State. Mar Drugs 2013, 11 (10), 3718–3734. 10.3390/md11103718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H. U.; Goepfert J. M. Enumeration and Identification of Bacillus Cereus in Foods. I. 24-h Presumptive Test Medium. Appl. Microbiol. 1971, 22 (4), 581–587. 10.1128/am.22.4.581-587.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund T.; Granum P. E. Comparison of Biological Effect of the Two Different Enterotoxin Complexes Isolated from Three Different Strains of Bacillus Cereus. Microbiology (Reading) 1997, 143 (10), 3329–3336. 10.1099/00221287-143-10-3329. [DOI] [PubMed] [Google Scholar]
- Hasan M. R.; Pulingam T.; Appaturi J. N.; Zifruddin A. N.; Teh S. J.; Lim T. W.; Ibrahim F.; Leo B. F.; Thong K. L. Carbon Nanotube-Based Aptasensor for Sensitive Electrochemical Detection of Whole-Cell Salmonella. Anal. Biochem. 2018, 554, 34–43. 10.1016/j.ab.2018.06.001. [DOI] [PubMed] [Google Scholar]
- Tevell Åberg A.; Björnstad K.; Hedeland M. Mass Spectrometric Detection of Protein-Based Toxins. Biosecurity and Bioterrorism: Biodefense Strategy, Practice, and Science 2013, 11 (S1), S215–S226. 10.1089/bsp.2012.0072. [DOI] [PubMed] [Google Scholar]
- Silva C. J. Food Forensics: Using Mass Spectrometry To Detect Foodborne Protein Contaminants, as Exemplified by Shiga Toxin Variants and Prion Strains. J. Agric. Food Chem. 2018, 66 (32), 8435–8450. 10.1021/acs.jafc.8b01517. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Carrasco Y.; Moltó J. C.; Mañes J.; Berrada H. Development of a GC-MS/MS Strategy to Determine 15 Mycotoxins and Metabolites in Human Urine. Talanta 2014, 128, 125–131. 10.1016/j.talanta.2014.04.072. [DOI] [PubMed] [Google Scholar]
- Jadhav S. R.; Shah R. M.; Karpe A. V.; Morrison P. D.; Kouremenos K.; Beale D. J.; Palombo E. A.. Detection of Foodborne Pathogens Using Proteomics and Metabolomics-Based Approaches. Frontiers in Microbiology 2018, 9. 10.3389/fmicb.2018.03132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M.-L.; Gao Y.; Wang X.; Han X. X.; Zhao B. Comprehensive Strategy for Sample Preparation for the Analysis of Food Contaminants and Residues by GC-MS/MS: A Review of Recent Research Trends. Foods 2021, 10 (10), 2473. 10.3390/foods10102473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalb S. R.; Boyer A. E.; Barr J. R. Mass Spectrometric Detection of Bacterial Protein Toxins and Their Enzymatic Activity. Toxins 2015, 7 (9), 3497–3511. 10.3390/toxins7093497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlovic M.; Huber I.; Konrad R.; Busch U. Application of MALDI-TOF MS for the Identification of Food Borne Bacteria. Open Microbiol J. 2013, 7, 135–141. 10.2174/1874285801307010135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambonin C. MALDI-TOF Mass Spectrometry Applications for Food Fraud Detection. Applied Sciences 2021, 11 (8), 3374. 10.3390/app11083374. [DOI] [Google Scholar]
- Xing L. Determination of Toxic Elements in Food by ICP-MS Using AOAC Method 2015.01. Spectroscopy 2022, 37 (10), 7–12. 10.56530/spectroscopy.zv7982s5. [DOI] [Google Scholar]
- Ammann A. A. Inductively Coupled Plasma Mass Spectrometry (ICP MS): A Versatile Tool. Journal of Mass Spectrometry 2007, 42 (4), 419–427. 10.1002/jms.1206. [DOI] [PubMed] [Google Scholar]
- te Brinke E.; Arrizabalaga-Larrañaga A.; Blokland M. H. Insights of Ion Mobility Spectrometry and Its Application on Food Safety and Authenticity: A Review. Anal. Chim. Acta 2022, 1222, 340039. 10.1016/j.aca.2022.340039. [DOI] [PubMed] [Google Scholar]
- Hernández-Mesa M.; Ropartz D.; García-Campaña A. M.; Rogniaux H.; Dervilly-Pinel G.; Le Bizec B. Ion Mobility Spectrometry in Food Analysis: Principles, Current Applications and Future Trends. Molecules 2019, 24 (15), 2706. 10.3390/molecules24152706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büyükköroglu G.; Dora D. D.; Özdemir F.; Hızel C.. Chapter 15 - Techniques for Protein Analysis. In Omics Technologies and Bio-Engineering; Barh D.; Azevedo V., Eds.; Academic Press, 2018; pp 317–351. 10.1016/B978-0-12-804659-3.00015-4. [DOI] [Google Scholar]
- Domínguez I.; Frenich A. G.; Romero-González R. Mass Spectrometry Approaches to Ensure Food Safety. Analytical Methods 2020, 12 (9), 1148–1162. 10.1039/C9AY02681A. [DOI] [Google Scholar]
- Mazarakioti E. C.; Zotos A.; Thomatou A. A.; Kontogeorgos A.; Patakas A.; Ladavos A. Inductively Coupled Plasma-Mass Spectrometry (ICP-MS), a Useful Tool in Authenticity of Agricultural Products’ and Foods’ Origin. Foods 2022, 11 (22), 3705. 10.3390/foods11223705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jull A. J. T.; Burr G. S.. 5.20 - Accelerator Mass Spectrometry. In Treatise on Geochemistry (Second ed.); Holland H. D., Turekian K. K., Eds.; Elsevier: Oxford, 2014; pp 375–383. 10.1016/B978-0-08-095975-7.01429-7. [DOI] [Google Scholar]
- Wacker J. F.; Eiden G. C.; Lehn S. A.; Koppenaal D. W.. Accelerator Mass Spectrometry (AMS). In Encyclopedia of Spectroscopy and Spectrometry (Third ed.); Lindon J. C., Tranter G. E., Koppenaal D. W., Eds.; Academic Press: Oxford, 2017; pp 15–17. 10.1016/B978-0-12-803224-4.00043-1. [DOI] [Google Scholar]
- Metz T. O.; Zhang Q.; Page J. S.; Shen Y.; Callister S. J.; Jacobs J. M.; Smith R. D. The Future of Liquid Chromatography-Mass Spectrometry (LC-MS) in Metabolic Profiling and Metabolomic Studies for Biomarker Discovery. Biomark Med. 2007, 1 (1), 159–185. 10.2217/17520363.1.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves T. O.; D’Almeida C. T. S.; Scherf K. A.; Ferreira M. S. L.. Modern Approaches in the Identification and Quantification of Immunogenic Peptides in Cereals by LC-MS/MS. Frontiers in Plant Science 2019, 10. 10.3389/fpls.2019.01470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amirav A.; Fialkov A.; Margolin Eren K.; Neumark B.; Elkabets O.; Tsizin S.; Gordin A.; Alon T. Gas Chromatography-Mass Spectrometry (GC-MS) with Cold Electron Ionization (EI): Bridging the Gap Between GC-MS and LC-MS. 2020, 18 (4), 5–15. [Google Scholar]
- Gilbert J. R.; McCaskill D.; Fishman V. N.; Brzak K.; Markham D.; Bartels M. J.; Winniford B.; Bano Mohsin S.; Godbey J.; Akinbo O.; Lewer P.. Chapter 17 - Industrial Applications of High-Resolution GC/MS. In Comprehensive Analytical Chemistry; Ferrer I., Thurman E. M., Eds.; Advanced Techniques in Gas Chromatography-Mass Spectrometry (GC-MS-MS and GC-TOF-MS) for Environmental Chemistry; Elsevier, 2013; Vol. 61, pp 403–429. 10.1016/B978-0-444-62623-3.00017-4. [DOI] [Google Scholar]
- Vivekanandan-Giri A.; Byun J.; Pennathur S.. Chapter Five - Quantitative Analysis of Amino Acid Oxidation Markers by Tandem Mass Spectrometry. In Methods in Enzymology; Conn P. M., Ed.; The Unfolded Protein Response and Cellular Stress, Part C; Academic Press, 2011; Vol. 491, pp 73–89. 10.1016/B978-0-12-385928-0.00005-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geer Wallace M. A.; McCord J. P.. Chapter 16 - High-Resolution Mass Spectrometry. In Breathborne Biomarkers and the Human Volatilome (Second ed.); Beauchamp J., Davis C., Pleil J., Eds.; Elsevier: Boston, 2020; pp 253–270. 10.1016/B978-0-12-819967-1.00016-5. [DOI] [Google Scholar]
- Rychert J. Benefits and Limitations of MALDI-TOF Mass Spectrometry for the Identification of Microorganisms. Journal of Infectiology and Epidemiology 2019, 2 (4), 1. 10.29245/2689-9981/2019/4.1142. [DOI] [Google Scholar]
- Wilschefski S. C.; Baxter M. R. Inductively Coupled Plasma Mass Spectrometry: Introduction to Analytical Aspects. Clin Biochem Rev. 2019, 40 (3), 115–133. 10.33176/AACB-19-00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorderwülbecke S.; Cleverley S.; Weinberger S. R.; Wiesner A. Protein Quantification by the SELDI-TOF-MS-Based ProteinChip® System. Nat. Methods 2005, 2 (5), 393–395. 10.1038/nmeth0505-393. [DOI] [Google Scholar]
- Poon T. C. Opportunities and Limitations of SELDI-TOF-MS in Biomedical Research: Practical Advices. Expert Review of Proteomics 2007, 4 (1), 51–65. 10.1586/14789450.4.1.51. [DOI] [PubMed] [Google Scholar]
- Neagu A.-N.; Jayathirtha M.; Baxter E.; Donnelly M.; Petre B. A.; Darie C. C. Applications of Tandem Mass Spectrometry (MS/MS) in Protein Analysis for Biomedical Research. Molecules 2022, 27 (8), 2411. 10.3390/molecules27082411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal R. D. Tandem Mass Spectroscopy in Diagnosis and Clinical Research. Indian J. Clin Biochem 2015, 30 (2), 121–123. 10.1007/s12291-015-0498-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andjelković U.; Šrajer Gajdošik M.; Gašo-Sokač D.; Martinović T.; Josić D. Foodomics and Food Safety: Where We Are. Food Technol. Biotechnol 2017, 55 (3), 290–307. 10.17113/ftb.55.03.17.5044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu P.; Wu X.; Huang Q.; Yu Q.; et al. Mass Spectrometry-Based Multimodal Approaches for the Identification and Quantification Analysis of Microplastics in Food Matrix. Front. Nutr. 2023, 10 (April), 1–7. 10.3389/fnut.2023.1163823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanjer M. C. Mass Spectrometry in Multi-Mycotoxin and Fungal Spore Analysis 2011, 90. 10.1533/9780857090973.1.90. [DOI] [Google Scholar]
- Yang S.; Zhu X.; Wang J.; Jin X.; Liu Y.; Qian F.; Zhang S.; Chen J. Combustion of Hazardous Biological Waste Derived from the Fermentation of Antibiotics Using TG-FTIR and Py-GC/MS Techniques. Bioresour. Technol. 2015, 193 (x), 156–163. 10.1016/j.biortech.2015.06.083. [DOI] [PubMed] [Google Scholar]
- Zhou Q.; Xing A.; Zhao K. Simultaneous Determination of Nickel, Cobalt and Mercury Ions in Water Samples by Solid Phase Extraction Using Multiwalled Carbon Nanotubes as Adsorbent after Chelating with Sodium Diethyldithiocarbamate Prior to High Performance Liquid Chromatography. Journal of Chromatography A 2014, 1360, 76–81. 10.1016/j.chroma.2014.07.084. [DOI] [PubMed] [Google Scholar]
- El-Sayed R. A.; Jebur A. B.; Kang W.; El-Demerdash F. M. An Overview on the Major Mycotoxins in Food Products: Characteristics, Toxicity, and Analysis. Journal of Future Foods 2022, 2 (2), 91–102. 10.1016/j.jfutfo.2022.03.002. [DOI] [Google Scholar]
- Yu S.; Jia B.; Li K.; Zhou H.; Lai W.; Tang Y.; Yan Z.; Sun W.; Liu N.; Yu D.; Wu A. Pre-Warning of Abiotic Factors in Maize Required for Potential Contamination of Fusarium Mycotoxins via Response Surface Analysis. Food Control 2021, 121, 107570. 10.1016/j.foodcont.2020.107570. [DOI] [Google Scholar]
- Bennett J. W.; Klich M. Mycotoxins. Clin Microbiol Rev. 2003, 16 (3), 497–516. 10.1128/CMR.16.3.497-516.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadi Shad Z.; Venkitasamy C.. Mycotoxins as Food and Feed Contaminant: Effect on Health and Economy and Their Management. In Fungal Resources for Sustainable Economy: Current Status and Future Perspectives; Singh I., Rajpal V. R., Navi S. S., Eds.; Springer Nature: Singapore, 2023; pp 531–563. 10.1007/978-981-19-9103-5_20. [DOI] [Google Scholar]
- Kumar P.; Mahato D. K.; Kamle M.; Mohanta T. K.; Kang S. G.. Aflatoxins: A Global Concern for Food Safety, Human Health and Their Management. Frontiers in Microbiology 2017, 7. 10.3389/fmicb.2016.02170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirmenstein M. A.; Mangipudy R.. Aflatoxin. In Encyclopedia of Toxicology (Third ed.); Wexler P., Ed.; Academic Press: Oxford, 2014; pp 104–106. 10.1016/B978-0-12-386454-3.00224-4. [DOI] [Google Scholar]
- Pinto A. C. S. M.; De Pierri C. R.; Evangelista A. G.; Gomes A. S. d. L. P. B.; Luciano F. B. Deoxynivalenol: Toxicology, Degradation by Bacteria, and Phylogenetic Analysis. Toxins 2022, 14 (2), 90. 10.3390/toxins14020090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cope R. B.Chapter 75 - Trichothecenes. In Veterinary Toxicology (Third ed.); Gupta R. C., Ed.; Academic Press, 2018; pp 1043–1053. 10.1016/B978-0-12-811410-0.00075-1. [DOI] [Google Scholar]
- Kamle M.; Mahato D. K.; Devi S.; Lee K. E.; Kang S. G.; Kumar P. Fumonisins: Impact on Agriculture, Food, and Human Health and Their Management Strategies. Toxins (Basel) 2019, 11 (6), 328. 10.3390/toxins11060328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun M. S.; Wink M. Exposure, Occurrence, and Chemistry of Fumonisins and Their Cryptic Derivatives. Comprehensive Reviews in Food Science and Food Safety 2018, 17 (3), 769–791. 10.1111/1541-4337.12334. [DOI] [PubMed] [Google Scholar]
- Damiani T.; Righetti L.; Suman M.; Galaverna G.; Dall’Asta C. Analytical Issue Related to Fumonisins: A Matter of Sample Comminution?. Food Control 2019, 95, 1–5. 10.1016/j.foodcont.2018.07.029. [DOI] [Google Scholar]
- Ropejko K.; Twaruzek M. Zearalenone and Its Metabolites—General Overview, Occurrence, and Toxicity. Toxins (Basel) 2021, 13 (1), 35. 10.3390/toxins13010035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G.-L.; Feng Y.-L.; Song J.-L.; Zhou X.-S.. Zearalenone: A Mycotoxin With Different Toxic Effect in Domestic and Laboratory Animals’ Granulosa Cells. Frontiers in Genetics 2018, 9. 10.3389/fgene.2018.00667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Salah-Abbès J.; Belgacem H.; Ezzdini K.; Abdel-Wahhab M. A.; Abbès S. Zearalenone Nephrotoxicity: DNA Fragmentation, Apoptotic Gene Expression and Oxidative Stress Protected by Lactobacillus Plantarum MON03. Toxicon 2020, 175, 28–35. 10.1016/j.toxicon.2019.12.004. [DOI] [PubMed] [Google Scholar]
- Gromadzka K.; Waskiewicz A.; Chelkowski J.; Golinski P. Zearalenone and Its Metabolites: Occurrence, Detection, Toxicity and Guidelines. World Mycotoxin Journal 2008, 1 (2), 209–220. 10.3920/WMJ2008.x015. [DOI] [Google Scholar]
- Mally A.; Solfrizzo M.; Degen G. H. Biomonitoring of the Mycotoxin Zearalenone: Current State-of-the Art and Application to Human Exposure Assessment. Arch. Toxicol. 2016, 90 (6), 1281–1292. 10.1007/s00204-016-1704-0. [DOI] [PubMed] [Google Scholar]
- Reddy P.; Hemsworth J.; Guthridge K. M.; Vinh A.; Vassiliadis S.; Ezernieks V.; Spangenberg G. C.; Rochfort S. J. Ergot Alkaloid Mycotoxins: Physiological Effects, Metabolism and Distribution of the Residual Toxin in Mice. Sci. Rep 2020, 10 (1), 9714. 10.1038/s41598-020-66358-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiff P. L. Ergot and Its Alkaloids. Am. J. Pharm. Educ 2006, 70 (5), 98. 10.5688/aj700598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans T. J.Chapter 67 - Endocrine Disruptors. In Reproductive and Developmental Toxicology; Gupta R. C., Ed.; Academic Press: San Diego, 2011; pp 873–891. 10.1016/B978-0-12-382032-7.10067-0. [DOI] [Google Scholar]
- Bui-Klimke T. R.; Wu F. Ochratoxin A and Human Health Risk: A Review of the Evidence. Crit Rev. Food Sci. Nutr 2015, 55 (13), 1860–1869. 10.1080/10408398.2012.724480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Jaal B.; Salama S.; Al-Qasmi N.; Jaganjac M. Mycotoxin Contamination of Food and Feed in the Gulf Cooperation Council Countries and Its Detection. Toxicon 2019, 171, 43–50. 10.1016/j.toxicon.2019.10.003. [DOI] [PubMed] [Google Scholar]
- European Union Commission. Commission Regulation (EC) No 401/2006 of 23 February 2006 Laying down the Methods of Sampling and Analysis for the Official Control of the Levels of Mycotoxins in Foodstuffs; EU, 2006; Vol. 401/2006, p 12. https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=CONSLEG:2006R0401:20100313:EN:PDF.
- European Union Commission. COMMISSION REGULATION (EC) No 1881/2006 of 19 December 2006 Setting Maximum Levels for Certain Contaminants in Foodstuffs; EU, 2006; Vol. 1881/2006. https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2006:364:0005:0024:EN:PDF.
- European Union Commission. Commission Regulation (EC) No 1881/2006 of 19 December 2006 Setting Maximum Levels for Certain Contaminants in Foodstuffs (Text with EEA Relevance); EU, 2006; Vol. 364. http://data.europa.eu/eli/reg/2006/1881/oj/eng (accessed 05-28-2023).
- European Union Commission. 2013/165/EU: Commission Recommendation of 27 March 2013 on the Presence of T-2 and HT-2 Toxin in Cereals and Cereal Products Text with EEA Relevance; EU, 2013; Vol. OJ L 91, (3.4.2013), , pp 12–15. https://eur-lex.europa.eu/eli/reco/2013/165/oj#PP3Contents (accessed 05-28-2023).
- European Union Commission. Commission Recommendation of 17 August 2006 on the Presence of Deoxynivalenol, Zearalenone, Ochratoxin A, T-2 and HT-2 and Fumonisins in Products Intended for Animal Feeding (Text with EEA Relevance) (2006/576/EC); EU, 2016. http://data.europa.eu/eli/reco/2006/576/2016-08-02/eng (accessed 05-28-2023).
- European Union Commission. Directive 2002/32/EC of the European Parliament and of the Council of 7 May 2002 on Undesirable Substances in Animal Feed; EU, 2019. http://data.europa.eu/eli/dir/2002/32/2019-11-28/eng (accessed 05-28-2023).
- Mbughuni M. M.; Jannetto P. J.; Langman L. J. Mass Spectrometry Applications for Toxicology. EJIFCC 2016, 27 (4), 272–287. [PMC free article] [PubMed] [Google Scholar]
- Areo O. M.; Abafe O. A.; Gbashi S.; Njobeh P. B. Detection of Multi-Mycotoxins in Rooibos and Other Consumed Teas in South Africa by a Modified QuEChERS Method and Ultra-High Performance Liquid Chromatography Tandem Mass Spectrometry. Food Control 2023, 143, 109255. 10.1016/j.foodcont.2022.109255. [DOI] [Google Scholar]
- You Y.; Hu Q.; Liu N.; Xu C.; Lu S.; Xu T.; Mao X. Metabolite Analysis of Alternaria Mycotoxins by LC-MS/MS and Multiple Tools. Molecules 2023, 28 (7), 3258. 10.3390/molecules28073258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maragos C. M. Detection of T-2 Toxin in Wheat and Maize with a Portable Mass Spectrometer. Toxins (Basel) 2023, 15 (3), 222. 10.3390/toxins15030222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji X.; Deng T.; Xiao Y.; Jin C.; Lyu W.; Wang W.; Tang B.; Wu Z.; Yang H. Evaluation of Alternaria Toxins in Fruits, Vegetables and Their Derivatives Marketed in China Using a QuEChERS Method Coupled with Ultra-High Performance Liquid Chromatography-Tandem Mass Spectrometry: Analytical Methods and Occurrence. Food Control 2023, 147, 109563. 10.1016/j.foodcont.2022.109563. [DOI] [Google Scholar]
- Leite M.; Freitas A.; Barbosa J.; Ramos F. Mycotoxins in Raw Bovine Milk: UHPLC-QTrap-MS/MS Method as a Biosafety Control Tool. Toxins 2023, 15 (3), 173. 10.3390/toxins15030173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M.; Liu X.; Yang S.; Chen Z.; Di B.; Liu W.; Yan H. HPLC-MS/MS Method for the Simultaneous Determination of Aflatoxins in Blood: Toxicokinetics of Aflatoxin B1 and Aflatoxin M1 in Rats. Journal of Analytical Science and Technology 2022, 13 (1), 27. 10.1186/s40543-022-00336-3. [DOI] [Google Scholar]
- Tang H.; Han W.; Fei S.; Li Y.; Huang J.; Dong M.; Wang L.; Wang W.; Zhang Y. Development of Acid Hydrolysis-Based UPLC-MS/MS Method for Determination of Alternaria Toxins and Its Application in the Occurrence Assessment in Solanaceous Vegetables and Their Products. Toxins 2023, 15 (3), 201. 10.3390/toxins15030201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai C.; Liu Y.; Tang X.; Zhang W.; Zhang Q.; Li P. Development of a Toxin-Free Competitive Immunoassay for Aflatoxin M1 Based on a Nanobody as Surrogate Calibrator. LWT 2023, 182, 114829. 10.1016/j.lwt.2023.114829. [DOI] [Google Scholar]
- Prakasham K.; Gurrani S.; Shiea J.-T.; Wu M.-T.; Wu C.-F.; Ku Y.-J.; Tsai T.-Y.; Hua H.-T.; Lin Y.-J.; Huang P.-C.; Andaluri G.; Ponnusamy V. K. Rapid Identification and Analysis of Ochratoxin-A in Food and Agricultural Soil Samples Using a Novel Semi-Automated In-Syringe Based Fast Mycotoxin Extraction (FaMEx) Technique Coupled with UHPLC-MS/MS. Molecules 2023, 28 (3), 1442. 10.3390/molecules28031442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prusova N.; Behner A.; Dzuman Z.; Hajslova J.; Stranska M. Conjugated Type A Trichothecenes in Oat-Based Products: Occurrence Data and Estimation of the Related Risk. Food Control 2023, 143, 109281. 10.1016/j.foodcont.2022.109281. [DOI] [Google Scholar]
- Scheibenzuber S.; Dick F.; Asam S.; Rychlik M. Analysis of 13 Alternaria Mycotoxins Including Modified Forms in Beer. Mycotoxin Res. 2021, 37 (2), 149–159. 10.1007/s12550-021-00424-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Carrasco Y.; Mañes J.; Berrada H.; Juan C. Development and Validation of a LC-ESI-MS/MS Method for the Determination of Alternaria Toxins Alternariol, Alternariol Methyl-Ether and Tentoxin in Tomato and Tomato-Based Products. Toxins (Basel) 2016, 8 (11), 328. 10.3390/toxins8110328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielecki J. Emerging Food Pathogens and Bacterial Toxins. Acta Microbiol Pol 2003, 52, 17–22. [PubMed] [Google Scholar]
- Hernández-Cortez C.; Palma-Martínez I.; UrielGonzalez-Avila L.; Guerrero-Mandujano A.; Castro-Escarpulli R. C. S. G.; Hernández-Cortez C.; Palma-Martínez I.; UrielGonzalez-Avila L.; Guerrero-Mandujano A.; Castro-Escarpulli R. C. S. G.. Food Poisoning Caused by Bacteria (Food Toxins). In Poisoning - From Specific Toxic Agents to Novel Rapid and Simplified Techniques for Analysis; IntechOpen, 2017. 10.5772/intechopen.69953. [DOI] [Google Scholar]
- Romero Hurtado S.; Iregui C. El Lipopolisacárido. Revista de Medicina Veterinaria 2010, 1 (19), 37–45. 10.19052/mv.783. [DOI] [Google Scholar]
- Ramachandran G. Gram-Positive and Gram-Negative Bacterial Toxins in Sepsis. Virulence 2014, 5 (1), 213–218. 10.4161/viru.27024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foran P.; Lawrence G. W.; Shone C. C.; Foster K. A.; Dolly J. O. Botulinum Neurotoxin C1 Cleaves Both Syntaxin and SNAP-25 in Intact and Permeabilized Chromaffin Cells: Correlation with Its Blockade of Catecholamine Release. Biochemistry 1996, 35 (8), 2630–2636. 10.1021/bi9519009. [DOI] [PubMed] [Google Scholar]
- Schiavo G. G.; Benfenati F.; Poulain B.; Rossetto O.; de Laureto P. P.; DasGupta B. R.; Montecucco C. Tetanus and Botulinum-B Neurotoxins Block Neurotransmitter Release by Proteolytic Cleavage of Synaptobrevin. Nature 1992, 359 (6398), 832–835. 10.1038/359832a0. [DOI] [PubMed] [Google Scholar]
- Kuo S.-R.; Willingham M. C.; Bour S. H.; Andreas E. A.; Park S. K.; Jackson C.; Duesbery N. S.; Leppla S. H.; Tang W.-J.; Frankel A. E. Anthrax Toxin-Induced Shock in Rats Is Associated with Pulmonary Edema and Hemorrhage. Microbial Pathogenesis 2008, 44 (6), 467–472. 10.1016/j.micpath.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Duracova M.; Klimentova J.; Myslivcova Fucikova A.; Zidkova L.; Sheshko V.; Rehulkova H.; Dresler J.; Krocova Z. Targeted Mass Spectrometry Analysis of Clostridium Perfringens Toxins. Toxins (Basel) 2019, 11 (3), 177. 10.3390/toxins11030177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalb S. R.; Krilich J. C.; Dykes J. K.; Lúquez C.; Maslanka S. E.; Barr J. R. Detection of Botulinum Toxins A, B, E, and F in Foods by Endopep-MS. J. Agric. Food Chem. 2015, 63 (4), 1133–1141. 10.1021/jf505482b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen O.; Feldberg L.; Yamin T. S.; Dor E.; Barnea A.; Weissberg A.; Zichel R. Development of a Multiplex Endopep-MS Assay for Simultaneous Detection of Botulinum Toxins A, B and E. Sci. Rep 2017, 7 (1), 14859. 10.1038/s41598-017-14911-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drigo I.; Tonon E.; Pascoletti S.; Anniballi F.; Kalb S. R.; Bano L. Detection of Active BoNT/C and D by EndoPep-MS Using MALDI Biotyper Instrument and Comparison with the Mouse Test Bioassay. Toxins 2021, 13 (1), 10. 10.3390/toxins13010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H.; Hu L.; Chang X.; Hu Y.; Zhang Y.; Zhou P.; Cui X. Determination of Bacterial Toxin Toxoflavin and Fervenulin in Food and Identification of Their Degradation Products. Food Chem. 2023, 399, 134010. 10.1016/j.foodchem.2022.134010. [DOI] [PubMed] [Google Scholar]
- Safi A. U. R.; Mansour Salih M.; Rahman H.; Khattak B.; El Askary A.; Hussain Khalifa E.; Qasim M. Immunoaffinity-Based Mass Spectrometric Characterization of Immunoreactive Proteins of Salmonella Typhi. Saudi Journal of Biological Sciences 2023, 30 (1), 103502. 10.1016/j.sjbs.2022.103502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray P. R. What Is New in Clinical Microbiology—Microbial Identification by MALDI-TOF Mass Spectrometry. J. Mol. Diagn 2012, 14 (5), 419–423. 10.1016/j.jmoldx.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagerquist C. K.; Garbus B. R.; Miller W. G.; Williams K. E.; Yee E.; Bates A. H.; Boyle S.; Harden L. A.; Cooley M. B.; Mandrell R. E. Rapid Identification of Protein Biomarkers of Escherichia Coli O157:H7 by Matrix-Assisted Laser Desorption Ionization-Time-of-Flight-Time-of-Flight Mass Spectrometry and Top-down Proteomics. Anal. Chem. 2010, 82 (7), 2717–2725. 10.1021/ac902455d. [DOI] [PubMed] [Google Scholar]
- Gagnaire J.; Dauwalder O.; Boisset S.; Khau D.; Freydiere A.-M.; Ader F.; Bes M.; Lina G.; Tristan A.; Reverdy M.-E.; Marchand A.; Geissmann T.; Benito Y.; Durand G.; Charrier J.-P.; Etienne J.; Welker M.; Van Belkum A.; Vandenesch F. Detection of Staphylococcus Aureus Delta-Toxin Production by Whole-Cell MALDI-TOF Mass Spectrometry. PLoS One 2012, 7 (7), e40660 10.1371/journal.pone.0040660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakur M. S.; Ragavan K. V. Biosensors in Food Processing. J. Food Sci. Technol. 2013, 50 (4), 625–641. 10.1007/s13197-012-0783-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bragazzi N. L.; Amicizia D.; Panatto D.; Tramalloni D.; Valle I.; Gasparini R. Quartz-Crystal Microbalance (QCM) for Public Health: An Overview of Its Applications. Adv. Protein Chem. Struct Biol. 2015, 101, 149–211. 10.1016/bs.apcsb.2015.08.002. [DOI] [PubMed] [Google Scholar]
- Lamanna J.; Scott E. Y.; Edwards H. S.; Chamberlain M. D.; Dryden M. D. M.; Peng J.; Mair B.; Lee A.; Chan C.; Sklavounos A. A.; Heffernan A.; Abbas F.; Lam C.; Olson M. E.; Moffat J.; Wheeler A. R. Digital Microfluidic Isolation of Single Cells for -Omics. Nat. Commun. 2020, 11 (1), 5632. 10.1038/s41467-020-19394-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedelkov D.; Rasooly A.; Nelson R. W. Multitoxin Biosensor-Mass Spectrometry Analysis: A New Approach for Rapid, Real-Time, Sensitive Analysis of Staphylococcal Toxins in Food. Int. J. Food Microbiol. 2000, 60 (1), 1–13. 10.1016/S0168-1605(00)00328-7. [DOI] [PubMed] [Google Scholar]
- Visciano P.; Schirone M.; Berti M.; Milandri A.; Tofalo R.; Suzzi G.. Marine Biotoxins: Occurrence, Toxicity, Regulatory Limits and Reference Methods. Frontiers in Microbiology 2016, 7. 10.3389/fmicb.2016.01051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rambla-Alegre M.; Miles C. O.; de la Iglesia P.; Fernandez-Tejedor M.; Jacobs S.; Sioen I.; Verbeke W.; Samdal I. A.; Sandvik M.; Barbosa V.; Tediosi A.; Madorran E.; Granby K.; Kotterman M.; Calis T.; Diogene J. Occurrence of Cyclic Imines in European Commercial Seafood and Consumers Risk Assessment. Environmental Research 2018, 161, 392–398. 10.1016/j.envres.2017.11.028. [DOI] [PubMed] [Google Scholar]
- García-Altares M.; Casanova A.; Bane V.; Diogène J.; Furey A.; De la Iglesia P. Confirmation of Pinnatoxins and Spirolides in Shellfish and Passive Samplers from Catalonia (Spain) by Liquid Chromatography Coupled with Triple Quadrupole and High-Resolution Hybrid Tandem Mass Spectrometry. Marine Drugs 2014, 12 (6), 3706–3732. 10.3390/md12063706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otero P.; Pérez S.; Alfonso A.; Vale C.; Rodríguez P.; Gouveia N. N.; Gouveia N.; Delgado J.; Vale P.; Hirama M.; Ishihara Y.; Molgó J.; Botana L. M. First Toxin Profile of Ciguateric Fish in Madeira Arquipelago (Europe). Anal. Chem. 2010, 82 (14), 6032–6039. 10.1021/ac100516q. [DOI] [PubMed] [Google Scholar]
- Estevez P.; Castro D.; Pequeño-Valtierra A.; Giraldez J.; Gago-Martinez A. Emerging Marine Biotoxins in Seafood from European Coasts: Incidence and Analytical Challenges. Foods 2019, 8 (5), 149. 10.3390/foods8050149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrell S.; Gunnarsson T.; Gunnarsson K.; Clarke D.; Turner A. D. First Detection of Paralytic Shellfish Poisoning (PSP) Toxins in Icelandic Mussels (Mytilus Edulis): Links to Causative Phytoplankton Species. Food Control 2013, 31 (2), 295–301. 10.1016/j.foodcont.2012.10.002. [DOI] [Google Scholar]
- SCHWARZ M.; JANDOVÁ K.; STRUK I.; MAREŠOVÁ D.; POKORNÝ J.; RILJAK V. Low Dose Domoic Acid Influences Spontaneous Behavior in Adult Rats. Physiological Research 2014, 63, 369–376. 10.33549/physiolres.932636. [DOI] [PubMed] [Google Scholar]
- Mafra L. L.; Bricelj V. M.; Fennel K. Domoic Acid Uptake and Elimination Kinetics in Oysters and Mussels in Relation to Body Size and Anatomical Distribution of Toxin. Aquatic Toxicology 2010, 100 (1), 17–29. 10.1016/j.aquatox.2010.07.002. [DOI] [PubMed] [Google Scholar]
- Nielsen L. T.; Hansen P. J.; Krock B.; Vismann B. Accumulation, Transformation and Breakdown of DSP Toxins from the Toxic Dinoflagellate Dinophysis Acuta in Blue Mussels, Mytilus Edulis. Toxicon 2016, 117, 84–93. 10.1016/j.toxicon.2016.03.021. [DOI] [PubMed] [Google Scholar]
- Louzao M. C.; Vilariño N.; Vale C.; Costas C.; Cao A.; Raposo-Garcia S.; Vieytes M. R.; Botana L. M. Current Trends and New Challenges in Marine Phycotoxins. Mar Drugs 2022, 20 (3), 198. 10.3390/md20030198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li A.; Ma J.; Cao J.; McCarron P. Toxins in Mussels (Mytilus Galloprovincialis) Associated with Diarrhetic Shellfish Poisoning Episodes in China. Toxicon 2012, 60 (3), 420–425. 10.1016/j.toxicon.2012.04.339. [DOI] [PubMed] [Google Scholar]
- Sun P.; Leeson C.; Zhi X.; Leng F.; Pierce R. H.; Henry M. S.; Rein K. S. Characterization of an Epoxide Hydrolase from the Florida Red Tide Dinoflagellate, Karenia Brevis. Phytochemistry 2016, 122, 11–21. 10.1016/j.phytochem.2015.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traylor J.; Singhal M.. Ciguatera Toxicity. In StatPearls; StatPearls Publishing: Treasure Island (FL), 2023. [PubMed] [Google Scholar]
- Pottier I.; Lewis R. J.; Vernoux J.-P. Ciguatera Fish Poisoning in the Caribbean Sea and Atlantic Ocean: Reconciling the Multiplicity of Ciguatoxins and Analytical Chemistry Approach for Public Health Safety. Toxins 2023, 15 (7), 453. 10.3390/toxins15070453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidalgo J.; Liberona J. L.; Molgó J.; Jaimovich E. Pacific Ciguatoxin-1b Effect over Na+ and K+ Currents, Inositol 1,4,5-Triphosphate Content and Intracellular Ca2+ Signals in Cultured Rat Myotubes. Br. J. Pharmacol. 2002, 137 (7), 1055–1062. 10.1038/sj.bjp.0704980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayakumar G.; Venkatesan S. A.; Kannan V. A.; Perumal S.. Chapter8 - Detection of Food Toxins, Pathogens, and Microorganisms Using Nanotechnology-Based Sensors. In Nanotechnology Applications for Food Safety and Quality Monitoring; Sharma A., Vijayakumar P. S., Prabhakar E. P. K., Kumar R., Eds.; Academic Press, 2023; pp 155–170. 10.1016/B978-0-323-85791-8.00022-7. [DOI] [Google Scholar]
- Karunarathne A.; Bhalla A.; Sethi A.; Perera U.; Eddleston M. Importance of Pesticides for Lethal Poisoning in India during 1999 to 2018: A Systematic Review. BMC Public Health 2021, 21, 1441. 10.1186/s12889-021-11156-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blay P.; Hui J. P. M.; Chang J.; Melanson J. E. Screening for Multiple Classes of Marine Biotoxins by Liquid Chromatography-High-Resolution Mass Spectrometry. Anal Bioanal Chem. 2011, 400 (2), 577–585. 10.1007/s00216-011-4772-2. [DOI] [PubMed] [Google Scholar]
- Bosch-Orea C.; Sanchís J.; Farré M. Analysis of Highly Polar Marine Biotoxins in Seawater by Hydrophilic Interaction Liquid Chromatography Coupled to High Resolution Mass Spectrometry. MethodsX 2021, 8, 101370. 10.1016/j.mex.2021.101370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolrep F.; Hessel S.; These A.; Ehlers A.; Rein K.; Lampen A. Differences in Metabolism of the Marine Biotoxin Okadaic Acid by Human and Rat Cytochrome P450 Monooxygenases. Arch. Toxicol. 2016, 90 (8), 2025–2036. 10.1007/s00204-015-1591-9. [DOI] [PubMed] [Google Scholar]
- Kolrep F.; Rein K.; Lampen A.; Hessel-Pras S. Metabolism of Okadaic Acid by NADPH-Dependent Enzymes Present in Human or Rat Liver S9 Fractions Results in Different Toxic Effects. Toxicology in Vitro 2017, 42, 161–170. 10.1016/j.tiv.2017.04.009. [DOI] [PubMed] [Google Scholar]
- Lefebvre D.; Blanco-Valle K.; Hennekinne J.-A.; Simon S.; Fenaille F.; Becher F.; Nia Y. Multiplex Detection of 24 Staphylococcal Enterotoxins in Culture Supernatant Using Liquid Chromatography Coupled to High-Resolution Mass Spectrometry. Toxins (Basel) 2022, 14 (4), 249. 10.3390/toxins14040249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr J. R.; Moura H.; Boyer A. E.; Woolfitt A. R.; Kalb S. R.; Pavlopoulos A.; McWilliams L. G.; Schmidt J. G.; Martinez R. A.; Ashley D. L. Botulinum Neurotoxin Detection and Differentiation by Mass Spectrometry. Emerg Infect Dis 2005, 11 (10), 1578–1583. 10.3201/eid1110.041279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. Y.; Woo S. Y.; Tian F.; Park J. B.; Choi K.-S.; Chun H. S. Simultaneous Determination of Okadaic Acid, Dinophysistoxin-1, Dinophysistoxin-2, and Dinophysistoxin-3 Using Liquid Chromatography-Tandem Mass Spectrometry in Raw and Cooked Food Matrices. Food Control 2022, 139, 109068. 10.1016/j.foodcont.2022.109068. [DOI] [Google Scholar]
- Beach D. G.; Zamlynny L.; MacArthur M.; Miles C. O. Liquid Chromatography-High-Resolution Tandem Mass Spectrometry of Anatoxins, Including New Conjugates and Reduction Products. Anal Bioanal Chem. 2023, 415, 5281. 10.1007/s00216-023-04836-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aparicio-Muriana M. d. M.; Lara F. J.; Olmo-Iruela M. D.; Garcia-Campana A. M. Determination of Multiclass Cyanotoxins in Blue-Green Algae (BGA) Dietary Supplements Using Hydrophilic Interaction Liquid Chromatography-Tandem Mass Spectrometry. Toxins 2023, 15 (2), 127. 10.3390/toxins15020127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P.; Lu R.; Zhu J.; Ke Q.; Wang D.; Wang Y.; Zhang C.; Lu X.; Zhao M.; Wang Z.; Pan L.; Zhang M.; Ying J.; Li S. Simultaneous Determination of 13 Paralytic Shellfish Toxins and Tetrodotoxin in Marine Shellfish by Porous Graphitic Carbon Solid-Phase Extraction and Hydrophilic Interaction Chromatography-Tandem Mass Spectrometry. Food Additives & Contaminants: Part A 2023, 40 (1), 147–158. 10.1080/19440049.2022.2143576. [DOI] [PubMed] [Google Scholar]
- Urugo M. M.; Tringo T. T. Naturally Occurring Plant Food Toxicants and the Role of Food Processing Methods in Their Detoxification. Int. J. Food Sci. 2023, 2023, 9947841. 10.1155/2023/9947841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colegate S. M.; Gardner D. R.; Lee S. T.. Plant-Associated Natural Food Toxins. In Handbook of Food Chemistry; Cheung P. C. K., Mehta B. M., Eds.; Springer Berlin Heidelberg: Berlin, Heidelberg, 2015; pp 753–783. 10.1007/978-3-642-36605-5_9. [DOI] [Google Scholar]
- Arigò A.; Rigano F.; Russo M.; Trovato E.; Dugo P.; Mondello L. Dietary Intake of Coumarins and Furocoumarins through Citrus Beverages: A Detailed Estimation by a HPLC-MS/MS Method Combined with the Linear Retention Index System. Foods 2021, 10 (7), 1533. 10.3390/foods10071533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzo S.; Celano R.; Piccinelli A. L.; Serio S.; Russo M.; Rastrelli L. An Analytical Platform for the Screening and Identification of Pyrrolizidine Alkaloids in Food Matrices with High Risk of Contamination. Food Chem. 2023, 406, 135058. 10.1016/j.foodchem.2022.135058. [DOI] [PubMed] [Google Scholar]
- Bessaire T.; Ernest M.; Christinat N.; Carrères B.; Panchaud A.; Badoud F. High Resolution Mass Spectrometry Workflow for the Analysis of Food Contaminants: Application to Plant Toxins, Mycotoxins and Phytoestrogens in Plant-Based Ingredients. Food Addit Contam Part A Chem. Anal Control Expo Risk Assess 2021, 38 (6), 978–996. 10.1080/19440049.2021.1902575. [DOI] [PubMed] [Google Scholar]
- Kalb S. R.; Schieltz D. M.; Becher F.; Astot C.; Fredriksson S.-Å.; Barr J. R. Recommended Mass Spectrometry-Based Strategies to Identify Ricin-Containing Samples. Toxins (Basel) 2015, 7 (12), 4881–4894. 10.3390/toxins7124854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X.; Christensen J. H.; Nielsen N. J. Enhancing the Power of Liquid Chromatography-Mass Spectrometry for Chemical Fingerprinting of Phytotoxins in the Environment. Journal of Chromatography A 2021, 1642, 462027. 10.1016/j.chroma.2021.462027. [DOI] [PubMed] [Google Scholar]
- Hosu I. S.; Sobaszek M.; Ficek M.; Bogdanowicz R.; Coffinier Y. Boron-Doped Carbon Nanowalls for Fast and Direct Detection of Cytochrome C and Ricin by Matrix-Free Laser Desorption/Ionization Mass Spectrometry. Talanta 2023, 252, 123778. 10.1016/j.talanta.2022.123778. [DOI] [PubMed] [Google Scholar]
- Chen L.; Zhang Q.; Yi Z.; Chen Y.; Xiao W.; Su D.; Shi W. Risk Assessment of (Herbal) Teas Containing Pyrrolizidine Alkaloids (PAs) Based on Margin of Exposure Approach and Relative Potency (REP) Factors. Foods 2022, 11 (19), 2946. 10.3390/foods11192946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H.; Cho Y.; Lee J.; Lee K. M.; Kim H. J.; Lee J.; Bahn Y.-S.; Son J. Evaluation and Monitoring of the Natural Toxin Ptaquiloside in Bracken Fern, Meat, and Dairy Products. Toxins 2023, 15 (3), 231. 10.3390/toxins15030231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosoky N. S.; Satyal P.; Setzer W. N. Authentication of Citrus Spp. Cold-Pressed Essential Oils by Their Oxygenated Heterocyclic Components. Molecules 2022, 27 (19), 6277. 10.3390/molecules27196277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makovi C. M.; Parker C. H.; Zhang K. Determination of Amygdalin in Apricot Kernels and Almonds Using LC-MS/MS. J. AOAC Int. 2023, 106 (2), 457–463. 10.1093/jaoacint/qsac154. [DOI] [PubMed] [Google Scholar]
- Rizzo S.; Celano R.; Piccinelli A. L.; Russo M.; Rastrelli L. Target Screening Method for the Quantitative Determination of 118 Pyrrolizidine Alkaloids in Food Supplements, Herbal Infusions, Honey and Teas by Liquid Chromatography Coupled to Quadrupole Orbitrap Mass Spectrometry. Food Chem. 2023, 423, 136306. 10.1016/j.foodchem.2023.136306. [DOI] [PubMed] [Google Scholar]
- Gruber-Dorninger C.; Novak B.; Nagl V.; Berthiller F. Emerging Mycotoxins: Beyond Traditionally Determined Food Contaminants. J. Agric. Food Chem. 2017, 65 (33), 7052–7070. 10.1021/acs.jafc.6b03413. [DOI] [PubMed] [Google Scholar]
- Thompson L. A.; Darwish W. S. Environmental Chemical Contaminants in Food: Review of a Global Problem. J. Toxicol 2019, 2019, 2345283. 10.1155/2019/2345283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage A.; Taylor P.; Stahl-Zeng J. Novel Methods Using Mass Spectrometry for Food Safety—From Contamination to Nutrition. Column 2018, 14 (5), 13–19. [Google Scholar]
- Vass A.; Robles-Molina J.; Pérez-Ortega P.; Gilbert-López B.; Dernovics M.; Molina-Díaz A.; García-Reyes J. F. Study of Different HILIC, Mixed-Mode, and Other Aqueous Normal-Phase Approaches for the Liquid Chromatography/Mass Spectrometry-Based Determination of Challenging Polar Pesticides. Anal Bioanal Chem. 2016, 408 (18), 4857–4869. 10.1007/s00216-016-9589-6. [DOI] [PubMed] [Google Scholar]
- Gopalakrishnan S.; Ghosh R.; Renganathan T.; Pushpavanam S. Sensitive and Selective Determination of Triclosan Using Visual Spectroscopy. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 2021, 254, 119623. 10.1016/j.saa.2021.119623. [DOI] [PubMed] [Google Scholar]
- Morgan M. K.; Clifton M. S. Exposure to Triclosan and Bisphenol Analogues B, F, P, S and Z in Repeated Duplicate-Diet Solid Food Samples of Adults. Toxics 2021, 9 (3), 47. 10.3390/toxics9030047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichert W. R. A.; Dhummakupt E. S.; Zhang C.; Mach P. M.; Bernhards R. C.; Glaros T.; Manicke N. E. Detection of Protein Toxin Simulants from Contaminated Surfaces by Paper Spray Mass Spectrometry. J. Am. Soc. Mass Spectrom. 2019, 30 (8), 1406–1415. 10.1007/s13361-019-02141-6. [DOI] [PubMed] [Google Scholar]
- Prata R.; López-Ruiz R.; Petrarca M. H.; Teixeira Godoy H.; Garrido Frenich A.; Romero-González R. Targeted and Non-Targeted Analysis of Pesticides and Aflatoxins in Baby Foods by Liquid Chromatography Coupled to Quadrupole Orbitrap Mass Spectrometry. Food Control 2022, 139, 109072. 10.1016/j.foodcont.2022.109072. [DOI] [Google Scholar]
- Thuy Q. C.; Phuong P. T.; Thien T. L. T.; Minh B. Q. Determination of Seven Parabens in Surface Water Samples by UHPLC-MS/MS and Solid-Phase Extraction. Vietnam Journal of Chemistry 2022, 60 (6), 738–743. 10.1002/vjch.202200016. [DOI] [Google Scholar]
- Bian L.; Li S.; Ge X.; Wang M.; Li K.; Wang X. Determination of Bisphenols, Triclosan, and Parabens in Bottled Water by Solid-Phase Microextraction Combined with Gas Chromatography-Tandem Mass Spectrometry and Assessment of the Associated Health Risk. Journal of Food Composition and Analysis 2023, 123, 105548. 10.1016/j.jfca.2023.105548. [DOI] [Google Scholar]
- Mou B.; Zuo C.; Chen L.; Xie H.; Zhang W.; Wang Q.; Wen L.; Gan N. On-Site Simultaneous Determination of Neonicotinoids, Carbamates, and Phenyl Pyrazole Insecticides in Vegetables by QuEChERS Extraction on Nitrogen and Sulfur Co-Doped Carbon Dots and Portable Mass Spectrometry. Journal of Chromatography A 2023, 1689, 463744. 10.1016/j.chroma.2022.463744. [DOI] [PubMed] [Google Scholar]
- Zhang S.; He Z.; Zeng M.; Chen J. Impact of Matrix Species and Mass Spectrometry on Matrix Effects in Multi-Residue Pesticide Analysis Based on QuEChERS-LC-MS. Foods 2023, 12 (6), 1226. 10.3390/foods12061226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koloka O.; Boti V.; Albanis T.; Konstantinou I. Accurate Determination of Pesticide Residues in Milk by Sonication-QuEChERS Extraction and LC-LTQ/Orbitrap Mass Spectrometry. Separations 2023, 10 (3), 146. 10.3390/separations10030146. [DOI] [Google Scholar]
- Stoyke M.; Becker R.; Brockmeyer J.; Jira W.; Popping B.; Uhlig S.; Wittke S. German Government Official Methods Board Points the Way Forward: Launch of a New Working Group for Mass Spectrometry for Protein Analysis to Detect Food Fraud and Food Allergens. Journal of AOAC INTERNATIONAL 2019, 102 (5), 1280–1285. 10.1093/jaoac/102.5.1280. [DOI] [PubMed] [Google Scholar]
- Dou X.; Zhang L.; Yang R.; Wang X.; Yu L.; Yue X.; Ma F.; Mao J.; Wang X.; Zhang W.; Li P. Mass Spectrometry in Food Authentication and Origin Traceability. Mass Spectrom Rev. 2023, 42, e21779 10.1002/mas.21779. [DOI] [PubMed] [Google Scholar]
- Kosek V.; Uttl L.; Jíru M.; Black C.; Chevallier O.; Tomaniová M.; Elliott C. T.; Hajšlová J. Ambient Mass Spectrometry Based on REIMS for the Rapid Detection of Adulteration of Minced Meats by the Use of a Range of Additives. Food Control 2019, 104, 50–56. 10.1016/j.foodcont.2018.10.029. [DOI] [Google Scholar]
- Piras C.; Hale O. J.; Reynolds C. K.; Jones A. K.; Taylor N.; Morris M.; Cramer R. Speciation and Milk Adulteration Analysis by Rapid Ambient Liquid MALDI Mass Spectrometry Profiling Using Machine Learning. Sci. Rep 2021, 11 (1), 3305. 10.1038/s41598-021-82846-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichani K.; Uhlig S.; Colson B.; Hettwer K.; Simon K.; Bönick J.; Uhlig C.; Kemmlein S.; Stoyke M.; Gowik P.; Huschek G.; Rawel H. M. Development of Non-Targeted Mass Spectrometry Method for Distinguishing Spelt and Wheat. Foods 2023, 12 (1), 141. 10.3390/foods12010141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding B.; Tao Y.; Xie J.; Zeng G.; Huang H. Traceability Evaluation of Wild and Cultivated Cordyceps Sinensis by Elemental Analysis and GasBench II Coupled to Stable Isotope Ratio Mass Spectrometry. Food Anal. Methods 2023, 16 (3), 515–524. 10.1007/s12161-022-02433-w. [DOI] [Google Scholar]
- Agriopoulou S.; Stamatelopoulou E.; Varzakas T. Advances in Analysis and Detection of Major Mycotoxins in Foods. Foods 2020, 9 (4), 518. 10.3390/foods9040518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liew W.-P.-P.; Mohd-Redzwan S.. Mycotoxin: Its Impact on Gut Health and Microbiota. Frontiers in Cellular and Infection Microbiology 2018, 8. 10.3389/fcimb.2018.00060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner P. C.; Snyder J. A. Development and Limitations of Exposure Biomarkers to Dietary Contaminants Mycotoxins. Toxins (Basel) 2021, 13 (5), 314. 10.3390/toxins13050314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly R.; Elliott C.; Zhang G.; Baker B.; Meneely J. Understanding Current Methods for Sampling of Aflatoxins in Corn and to Generate a Best Practice Framework. Toxins 2022, 14 (12), 819. 10.3390/toxins14120819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheerlinck E.; Dhaenens M.; Van Soom A.; Peelman L.; De Sutter P.; Van Steendam K.; Deforce D. Minimizing Technical Variation during Sample Preparation Prior to Label-Free Quantitative Mass Spectrometry. Anal. Biochem. 2015, 490, 14–19. 10.1016/j.ab.2015.08.018. [DOI] [PubMed] [Google Scholar]
- Piehowski P. D.; Petyuk V. A.; Orton D. J.; Xie F.; Ramirez-Restrepo M.; Engel A.; Lieberman A. P.; Albin R. L.; Camp D. G.; Smith R. D.; Myers A. J.; et al. Sources of Technical Variability in Quantitative LC-MS Proteomics: Human Brain Tissue Sample Analysis. J. Proteome Res. 2013, 12 (5), 2128–2137. 10.1021/pr301146m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loi M.; Logrieco A. F.; Pusztahelyi T.; Leiter É.; Hornok L.; Pócsi I.. Advanced Mycotoxin Control and Decontamination Techniques in View of an Increased Aflatoxin Risk in Europe Due to Climate Change. Frontiers in Microbiology 2023, 13. 10.3389/fmicb.2022.1085891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen H. C. B.; Hilscherova K.; Bucheli T. D. Natural Toxins: Environmental Contaminants Calling for Attention. Environmental Sciences Europe 2021, 33 (1), 112. 10.1186/s12302-021-00543-6. [DOI] [Google Scholar]
- FSSAI . MANUAL OF METHODS OF ANALYSIS OF FOODS-Mycotoxins; Food Safety and Standards Authority of India: India, 2016; p 64. https://fssai.gov.in/upload/advisories/2018/02/5a93ebeeda71cManual_Mycotoxins_25_05_2016(1).pdf.
- Cinar A.; Onbası E.; Cinar A.; Onbası E.. Mycotoxins: The Hidden Danger in Foods. In Mycotoxins and Food Safety; IntechOpen, 2019. 10.5772/intechopen.89001. [DOI] [Google Scholar]
- Iqbal S. Z. Mycotoxins in Food, Recent Development in Food Analysis and Future Challenges; a Review. Current Opinion in Food Science 2021, 42, 237–247. 10.1016/j.cofs.2021.07.003. [DOI] [Google Scholar]
- Tittlemier S. a.; Cramer B.; Dall’Asta C.; Iha M. h.; Lattanzio V. m. t.; Maragos C.; Solfrizzo M.; Stranska M.; Stroka J.; Sumarah M. Developments in Mycotoxin Analysis: An Update for 2018–19. World Mycotoxin Journal 2020, 13 (1), 3–24. 10.3920/WMJ2019.2535. [DOI] [Google Scholar]
- Renaud J. B.; Miller J. D.; Sumarah M. W. Mycotoxin Testing Paradigm: Challenges and Opportunities for the Future. J. AOAC Int. 2019, 102 (6), 1681–1688. 10.5740/jaoacint.19-0046. [DOI] [PubMed] [Google Scholar]
- Lattanzio V. M. T.; Solfrizzo M.; Powers S.; Visconti A. Simultaneous Determination of Aflatoxins, Ochratoxin A and Fusarium Toxins in Maize by Liquid Chromatography/Tandem Mass Spectrometry after Multitoxin Immunoaffinity Cleanup. Rapid Commun. Mass Spectrom. 2007, 21 (20), 3253–3261. 10.1002/rcm.3210. [DOI] [PubMed] [Google Scholar]
- Cheng H.-L.; Wang F.-L.; Zhao Y.-G.; Zhang Y.; Jin M.-C.; Zhu Y. Simultaneous Determination of Fifteen Toxic Alkaloids in Meat Dishes and Vegetable Dishes Using Double Layer Pipette Tip Magnetic Dispersive Solid Phase Extraction Followed by UFLC-MS/MS. Anal. Methods 2018, 10 (10), 1151–1162. 10.1039/C7AY02608K. [DOI] [Google Scholar]
- Wang R.; Guo L.; Wang P.; Su X.; Song Z.; Lin G.; Zhu R. [Simultaneous determination of 37 mycotoxins in grain and animal feed by impurity adsorption purification coupled with ultra-performance liquid chromatography-tandem mass spectrometry]. Se Pu 2020, 38 (7), 817–825. 10.3724/SP.J.1123.2019.12013. [DOI] [PubMed] [Google Scholar]
- Nualkaw K.; Poapolathep S.; Zhang Z.; Zhang Q.; Giorgi M.; Li P.; Logrieco A. F.; Poapolathep A. Simultaneous Determination of Multiple Mycotoxins in Swine, Poultry and Dairy Feeds Using Ultra High Performance Liquid Chromatography-Tandem Mass Spectrometry. Toxins 2020, 12 (4), 253. 10.3390/toxins12040253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albero B.; Fernández-Cruz M. L.; Pérez R. A. Simultaneous Determination of 15 Mycotoxins in Aquaculture Feed by Liquid Chromatography-Tandem Mass Spectrometry. Toxins 2022, 14 (5), 316. 10.3390/toxins14050316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthiller F.; Crews C.; Dall’Asta C.; Saeger S. D.; Haesaert G.; Karlovsky P.; Oswald I. P.; Seefelder W.; Speijers G.; Stroka J. Masked Mycotoxins: A Review. Mol. Nutr Food Res. 2013, 57 (1), 165–186. 10.1002/mnfr.201100764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiby I.; Sopel M. M.; Michlmayr H.; Adam G.; Berthiller F. Development and Validation of an LC-MS/MS Based Method for the Determination of Deoxynivalenol and Its Modified Forms in Maize. Toxins 2021, 13 (9), 600. 10.3390/toxins13090600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y.; Chen T.; Chen D.; Liang W.; Lu X.; Zhao C.; Xu G. Suspect and Nontarget Screening of Mycotoxins and Their Modified Forms in Wheat Products Based on Ultrahigh-Performance Liquid Chromatography-High Resolution Mass Spectrometry. Journal of Chromatography A 2023, 1708, 464370. 10.1016/j.chroma.2023.464370. [DOI] [PubMed] [Google Scholar]
- Pascari X.; Weigel S.; Marin S.; Sanchis V.; Maul R. Detection and Quantification of Zearalenone and Its Modified Forms in Enzymatically Treated Oat and Wheat Flour. J. Food Sci. Technol. 2023, 60 (4), 1367–1375. 10.1007/s13197-023-05683-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EMR . Global Mycotoxin Testing Market Report and Forecast 2023–2028; EMR, 2022. https://www.expertmarketresearch.com/reports/mycotoxin-testing-market (accessed 07-03-2023).
- BaySpec Inc . ContinuityTM Portable Mass Spectrometer; BaySpec, 2023. https://www.bayspec.com/products/high-sensitivity-portable-mass-spectrometer/ (accessed 07-03-2023).
- Nolan P.; Auer S.; Spehar A.; Oplatowska-Stachowiak M.; Campbell K. Evaluation of Mass Sensitive Micro-Array Biosensors for Their Feasibility in Multiplex Detection of Low Molecular Weight Toxins Using Mycotoxins as Model Compounds. Talanta 2021, 222, 121521. 10.1016/j.talanta.2020.121521. [DOI] [PubMed] [Google Scholar]