Abstract
Cocaine use disorder (CUD) is a significant problem worldwide, with no FDA-approved treatments. Epidemiological data indicate that only about 17 % of people that use cocaine will meet DSM criteria for CUD. Thus, the identification of biomarkers predictive of eventual cocaine use may be of great value. Two potentially useful predictors of CUD are social hierarchies in nonhuman primates and delay discounting. Both social rank and preference for a smaller, immediate reinforcer relative to a larger, delayed reinforcer have been predictive of CUD. Therefore, we wanted to determine if there was also a relationship between these two predictors of CUD. In the present study, monkeys cocaine-naive responded under a concurrent schedule of 1- vs. 3-food pellets and delivery of the 3-pellet option was delayed. The primary dependent variable was the indifference point (IP), which is the delay that results in 50 % choice for both options. In the initial determination of IP, there were no differences based on sex or social rank of the monkeys. When the delays were redetermined after ~25 baseline sessions (range 5–128 sessions), dominant females and subordinate males showed the largest increases in IP scores from the first determination to the second. Because 13 of these monkeys had prior PET scans of the kappa opioid receptor (KOR), we examined the relationship between KOR availability and IP values and found that the change in IP scores from the first to the second determination significantly negatively predicted average KOR availability in most brain regions. Future studies will examine acquisition to cocaine self-administration in these same monkeys, to determine if IP values are predictive of vulnerability to cocaine reinforcement.
Keywords: Delay discounting, Social status, Sex differences, Monkeys
1. Introduction
At present, there are no FDA-approved pharmacological treatments for cocaine use disorders (CUD). An important strategy for evaluating novel treatment interventions is the use of animal models to identify behavioral and neuronal markers associated with vulnerability and maintenance of cocaine reinforcement. CUD is a multifactorial disorder resulting from complex interactions between the pharmacological effects of a drug, biological characteristics of the individual, and environmental factors that render some individuals vulnerable (Nader et al., 2008). As such, only a subset of individuals that use cocaine develop CUDs (Anthony et al., 1994; Vsevolozhskaya and Anthony, 2016). Similarly, individual differences are observed in treatment efficacy, as well as response to exogenous challenges such as social stress and environmental enrichment (George and Koob, 2017; Koob and Volkow, 2016; Johnson et al., 2021). These variables are likely to contribute to the heterogeneity common in clinical studies, thus stressing the importance of a personalized treatment approach for CUDs, similar to other CNS disorders.
Cynomolgus monkeys living in social groups form hierarchies that are linear and transitive based primarily on outcomes of agonistic interactions (Kaplan et al., 1982; Morgan et al., 2000). As described by Morgan et al. (2000), social rank reflects a monkey’s ability to defeat other members of its social group in competitive interactions. Nonhuman primate (NHP) social groups have proven useful for studying social status-related differences in disease vulnerability and resistance, with such differences being linked to predictable variation in physiological, neurobiological and behavioral characteristics (Shively and Day, 2015; Kaplan et al., 2009; Riddick et al., 2009; Czoty et al., 2009; Morgan et al., 2000; Morgan et al., 2002; Nader et al., 2012b; Johnson et al., 2023). For example, socially subordinate monkeys are more susceptible to immune, cardiovascular and reproductive dysfunction compared to dominant monkeys (Sapolsky, 2005; Cameron, 1997; Cohen et al., 1997; Kaplan and Manuck, 2004). Of translational significance, the effects of social rank on NHP health parallel both the direct relationship between control over resources and life expectancy and the inverse relationship between socioeconomic status and susceptibility to disease in humans (Roubinov et al., 2018).
In many studies in both NHPs and humans, there are interactions between social status, receptor measures (see below) and behavior. Thus, another area of approach that has proven fruitful has been the investigation of behavioral traits or processes associated with pathological behavior, including substance use (de Wit et al., 2020; Bickel et al., 2014; Hamilton et al., 2015). One of the trait behaviors we have been investigating in our models of cocaine use involves delayed discounting (see Hamilton et al., 2011). Delayed choice responding, sometimes referred to as modeling “impulsivity” (but see Strickland and Johnson, 2021), involves subjects choosing between a smaller, more immediate reinforcers and larger, later reinforcers. Using this model in humans, it has been suggested that an “impulsive” individual may respond without consideration of alternative choices or consequences and behave inappropriately given an environmental contingency (de Wit, 2009). This phenotype is strongly associated with CUD and it has been shown to be related to stages of abstinence, relapse and treatment success (Perry and Carroll, 2008; Bickel et al., 2014; Kozak et al., 2019). Impulsive individuals initiate drug use at earlier ages, escalate to heavy use, and transition to abuse and dependence more quickly and are less likely to remain abstinent after treatment compared with non-impulsive individuals (de Wit, 2009; Dick et al., 2010; Dalley et al., 2011; Bardo et al., 2013; Vassileva and Conrod, 2019; Craft et al., 2022).
In delay discounting studies, the primary dependent variable is an indifference point (IP), which can be calculated as the delay value at which the smaller, immediate reinforcer is chosen as often as the larger, delayed reinforcer; the smaller the IP value, the more likely the individual chooses the immediate smaller reinforcer over the delayed larger one. Bickel and colleagues have shown that measures of delay discounting can serve as a behavioral marker at all stages of addiction and predicts therapeutic outcomes (Bickel et al., 2014; Craft et al., 2022).
As aforementioned, delayed choice responding is strongly associated with different stages of CUD and individuals with lower IP values have been shown to initiate drug use earlier, escalate and transition to CUD more quickly (de Wit, 2009; de Wit et al., 2020), including several animal models (Hamilton et al., 2011; Koffarnus and Woods, 2013; Huskinson et al., 2015; Huskinson et al., 2016; Freund et al., 2019). In a review on sex differences in measures of impulsivity, a limitation noted by Weafer and de Wit (2014) was the need for longitudinal studies, especially as predictors of substance abuse. The goal of the present study was to further evaluate, in socially housed monkeys, a behavioral marker, temporal discounting, associated with vulnerability, development and treatment outcome in CUD. While the present study only involved food-maintained responding, these monkeys will be studied in models of cocaine self-administration, which will provide a valuable dataset towards understanding this measure as a behavioral biomarker associated with CUD. Because social rank influences vulnerability to cocaine reinforcement, we hypothesize that the IP values for subordinate males (Morgan et al., 2002) and dominant females (Nader et al., 2012b), the two most vulnerable phenotypes to cocaine reinforcement, will be lower compared with dominant males and subordinate females, indicative of a higher degree of impulsive choice. After initially determining IP values, the delay conditions were redetermined in order to test the hypothesis that IP values represent a stable phenotype. If IP values increase (i.e., longer delays required to decrease choice for the larger reinforcer), that may be an additional biomarker associated with a subject’s ability to respond to changes in the environment.
As it relates to CUD, social rank has been shown to influence vulnerability to cocaine self-administration in male (Morgan et al., 2002) and female (Nader et al., 2012b) cynomolgus monkeys, as well as influence pharmacological and behavioral interventions (e.g., Czoty and Nader, 2012, 2015; Gould et al., 2017). NHP social rank has also been shown to influence several CNS measures associated with cocaine reinforcement, including dopamine (DA) D2/D3 receptors (D2/D3R; e. g., Grant et al., 1998; Morgan et al., 2002; Czoty et al., 2004; Nader et al., 2012b) and kappa opioid receptors (KOR; Johnson et al., 2023). Importantly, positron emission tomography (PET) studies in humans have noted similar relationships between social status and D2/D3R availability (Martinez et al., 2010; Martinez et al., 2011; Wiers et al., 2016) and KOR availability (Matuskey et al., 2019; Vijay et al., 2016). In the present study, 13 monkeys had previously received PET scans assessing KOR availability (Johnson et al., 2023). The KOR and its endogenous ligand, dynorphin, is an integral part of the brain’s stress response system; implicated in the regulation of aversive states and substance use disorders (Karkhanis et al., 2017; Estave et al., 2022; Trifilieff and Martinez, 2013; Tejeda and Bonci, 2019; Carlezon and Krystal, 2016; Martinez et al., 2019; Matuskey et al., 2019; de Laat et al., 2019; de Laat et al., 2021). Because delayed choice responding has been shown to be related to CUD, as is KOR availability, another goal of the present study was to assess the relationship between temporal discounting and measures of the KOR across various brain regions.
2. Methods and materials
2.1. Subjects
Experimentally naïve (N = 23) female and male cynomolgus monkeys (Macaca fascicularis), living in same-sex, stable (>18 months) social groups of 4, served as subjects. Social hierarchy has been characterized as linear and transitive, from the most dominant (no.1-ranked) to the most subordinate (no. 4-ranked) monkey, and was determined according to the outcomes of agonistic encounters. As described by Morgan et al. (2000), behaviors measured to determine social rank were categorized as “aggressive,” “submissive,” or “affiliative.” Social rank was based on the outcomes of these aggressive encounters, rather than based on the frequency or severity of these interactions. For this study, N = 14 females (7 dominant and 7 subordinate) and N = 10 males (4 dominant and 6 subordinate) were studied; of this cohort, N = 8 males (4 dominant and 4 subordinate) and N = 5 females (2 dominant and 3 subordinate) had participated in the previous PET imaging study of KOR availability (Johnson et al., 2023). Each monkey was fitted with an aluminum collar (Primate Products, Redwood City, CA) and trained to sit in a standard primate chair (Primate Products). To prevent development of obesity and cardiovascular/metabolic problems, monkeys were not fed ad libitum. They were also not maintained at a “target weight” set to be an arbitrary percentage below free-feeding weight, because the latter can change with age and other factors, and we did not plan to remove monkeys from the study for periodic redetermination of free-feeding weights. Instead, monkeys were weighed weekly and feed enough food daily (Purina Monkey Chow and fresh fruit and vegetables) to maintain a healthy body weight and appearance as determined by daily inspection and periodic veterinary examinations. Monkeys were typically fed at least 1 h after the end of behavioral sessions. Total allotment of food was based on estimated total grams needed to maintain stable body weights minus the amount of food earned during behavioral sessions. Water was available ad libitum in the home cage. For female monkeys, menstrual cycle was monitored daily by vaginal swabs and was approximately 28 days (Riddick et al., 2009). The first indication of bleeding was indicative of menses and counted as day 1 of the cycle. We considered days 2–10 the follicular phase and days 19–28 the luteal phase of the menstrual cycle. All PET studies in females occurred in the estimated follicular phase. All procedures were performed in accordance with the 2011 National Research Council Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research and were approved by the Wake Forest University Animal Care and Use Committee.
2.2. Apparatus
The apparatus for operant responding consisted of a ventilated, sound-attenuating chamber (1.5 × 0.74 × 0.76 m; Med Associates, East Fairfield, VT) designed to accommodate a primate chair. Two photo-optic switches (5 cm wide) located on one side of the chamber with a horizontal row of three stimulus lights 14 cm above each switch, and a food receptacle located between them. The food receptacle was connected with tygon tubing to a pellet dispenser (Gerbrands Corp., Arlington, MA) located on the top of the chamber for delivery of 1.0-g banana-flavored food pellets (Bio-Serv, Frenchtown, NJ).
2.3. Procedure
Monkeys were trained to respond under a concurrent fixed-ratio (FR) 30, 1- vs 3-food pellets choice paradigm using methods that have been described previously (Hamilton et al., 2011). For all monkeys, the FR value associated with both reinforcers was 30 responses with the exception of three monkeys (FR15, F-8581; FR5, F-8580 and F-8579). Under these conditions, consecutive responses completing the FR requirement on one switch resulted in delivery of a 1.0-g banana-flavored food pellet, while completing the FR requirement on the other switch resulted in the delivery of three 1.0-g banana-flavored food pellets. A response emitted on the alternate switch before an FR was completed reset the response requirement on the first switch. Monkeys were initially trained under a concurrent 1- vs. 1-pellet choice and when the conditions were changed to concurrent 1- vs. 3-pellet choice, the 3-pellet reinforcer was allocated on the switch associated with the monkey’s non-preferred side. For each monkey, responding on one side was always associated with 1 pellet reinforcer, while responding on the other delivered 3 pellets. Initially, the delay value associated with both reinforcers was 0-s. The delay value associated with 1 pellet remained at 0-s throughout the experiment, while the delay associated with the 3-pellet reinforcer varied from 0- to 200-s. Sessions began with two forced-choice trials (i.e., sampling trials). During these sampling trials, only one response switch was active with illumination of the appropriate switch light and completion of the FR resulting in the reinforcer and delay, followed by a 15-s time-out (TO), in which all lights in the chamber were extinguished. Directly after TO, the other response switch was illuminated and completion of the FR resulted in the reinforcer and delay. Once both forced-choice trials were completed, the schedule changed to a concurrent schedule with both switches active. A forced-choice was implemented during the session, if a monkey chose the same option on 5 consecutive trials, in order to limit the possibility of perseverative responding and to better assure the monkey remains in contact with the contingencies. Once the forced choice was completed, the session returned to a concurrent schedule.
After stable choice responding between 1- vs 3-food pellets (3 consecutive sessions in which >80 % choice for the 3-pellet option), delays (0- to 200-sec) were associated with the 3-pellet choice; delay values increased until percentage choice for the 3-pellet option was <20 %. As before, sessions began with two forced choices prior to the start of the concurrent schedule. During the delay, a red light above the response switch flashed on and off each second for the duration of the assigned delay. Following completion of the forced-choice trials, the schedule changed to a concurrent schedule with both response switches active. Delay values were kept constant for at least 3 consecutive sessions and until the percent choice of the larger, delayed reinforcer was deemed stable (mean percent choice ±20 % for 3 consecutive sessions). Sessions terminated after 15 concurrent choices were completed or 60 min had elapsed, whichever occurred first. For all monkeys delay values used for IP calculations were presented in ascending. After completion of the delay curve, there was a return to baseline, 1- vs. 3-pellet, with no delays associated with the 3-pellet option. Once stable, the entire delay curve was redetermined. On average, it took 24.17 (SD = 29. 41) sessions between the initial IP score determination and the beginning of the second determination with a range of 5–128 sessions.
2.4. Data analyses
The primary dependent variable was percentage of total trials in which the larger, 3-pellet option, was chosen. When delays were implemented, an IP was calculated from each monkey’s individual curve. The IP value was interpolated as the delay value that engendered 50 % choice of the larger, delayed reinforcer. Data were initially analyzed using a two-way ANOVA to look at the effect of sex and social rank on initial IP scores. Following this, data were analyzed using a mixed-effect analysis of variance (ANOVA) with IP score (initial determination and redetermined) as a within-subject factor and, sex, and social rank as between-subject factors. A significant ANOVA was followed by pairwise multiple comparisons (Holm-Sidak) post-hoc tests. Eta squared (η2) was also calculated to determine effect sizes. All statistical tests were analyzed with SPSS (IBM, Armonk, NY).
For 13 of the monkeys in this study, PET scans assessing KOR binding potentials (BP) were conducted prior to the start of this study. Those data were recently published (Johnson et al., 2023) and showed sex and social rank differences in KOR BP. To evaluate the association between PET imaging measures of KOR BP and impulsive choice in a delay discounting task, initial bivariate correlations were run between average KOR BP, initial IP scores, delta IP scores (calculated by subtracting the first and second IP score determinations), sex, and the 15 regions of interest (ROIs shown in Table 2). Given that initial IP scores were not significantly correlated with sex, delta IP scores, average KOR BP, or KOR BP in any of the ROIs (p > 0.05), no further statistics were run using initial IP scores. Instead, further analyses were run using delta IP scores. Multiple regressions were run looking at the effect of delta IP scores and sex on both average KOR availability and KOR availability in the 15 ROIs. For all statistical analyses, significance was set at p < 0.05. All statistical tests were analyzed with SPSS (IBM, Armonk, NY).
Table 2.
Linear regressions and significance levels between ΔIP and [11C]EKAP BP, including sex.
| Region of interest | Standardized B | p |
|---|---|---|
| Dorsal PFC | −0.68 | 0.01 |
| Medial PFC | −0.57 | 0.02 |
| Orbito FC | −0.66 | 0.02 |
| Anterior Cing Cortex | −0.60 | 0.04 |
| Caudate | −0.73 | 0.008 |
| Putamen | −0.77 | 0.005 |
| Ventral Striatum | −0.56 | 0.05 |
| Amygdala | −0.37 | 0.23 |
| Globus Pallidus | −0.52 | 0.09 |
| Insula | −0.66 | 0.03 |
| Claustrum | −0.69 | 0.01 |
| Cingulate Cortex | −0.63 | 0.03 |
| Hippocampus | −0.63 | 0.03 |
| Thalamus | −0.51 | 0.08 |
| Temporal Cortex | −0.56 | 0.06 |
| Mean ROI | −0.74 | 0.01 |
Bold values represent statistically significant correlations.
Abbreviations: PFC, prefrontal cortex; Orbito FC, orbital frontal cortex; Anterior Cing Cortex, anterior cingulate cortex; ROI, region of interest.
3. Results
Under the concurrent 1- vs. 3-pellet schedule of reinforcement, when the delay for the 3-pellet option was 0-s, monkeys chose the larger magnitude food reinforcer on nearly 100 % of the trials (see Figs. 1–4). In general, monkeys completed all 15 trials each session and there was no significant difference between the number of trials completed at a 0-s delay vs the largest delay tested in each monkey (t(23) = 1.00, p = 0.324). Increases in delay value resulted in time-dependent reductions in the percent of trials in which the larger reinforcer was chosen, real-locating choice to the smaller, immediate reinforcer. IPs were calculated based on each monkey’s individual curve, as the delay value (seconds) that engendered 50 % choice of the larger, delayed reinforcer and the smaller, immediate reinforcer (Figs. 1–4, dashed line).
Fig. 1.
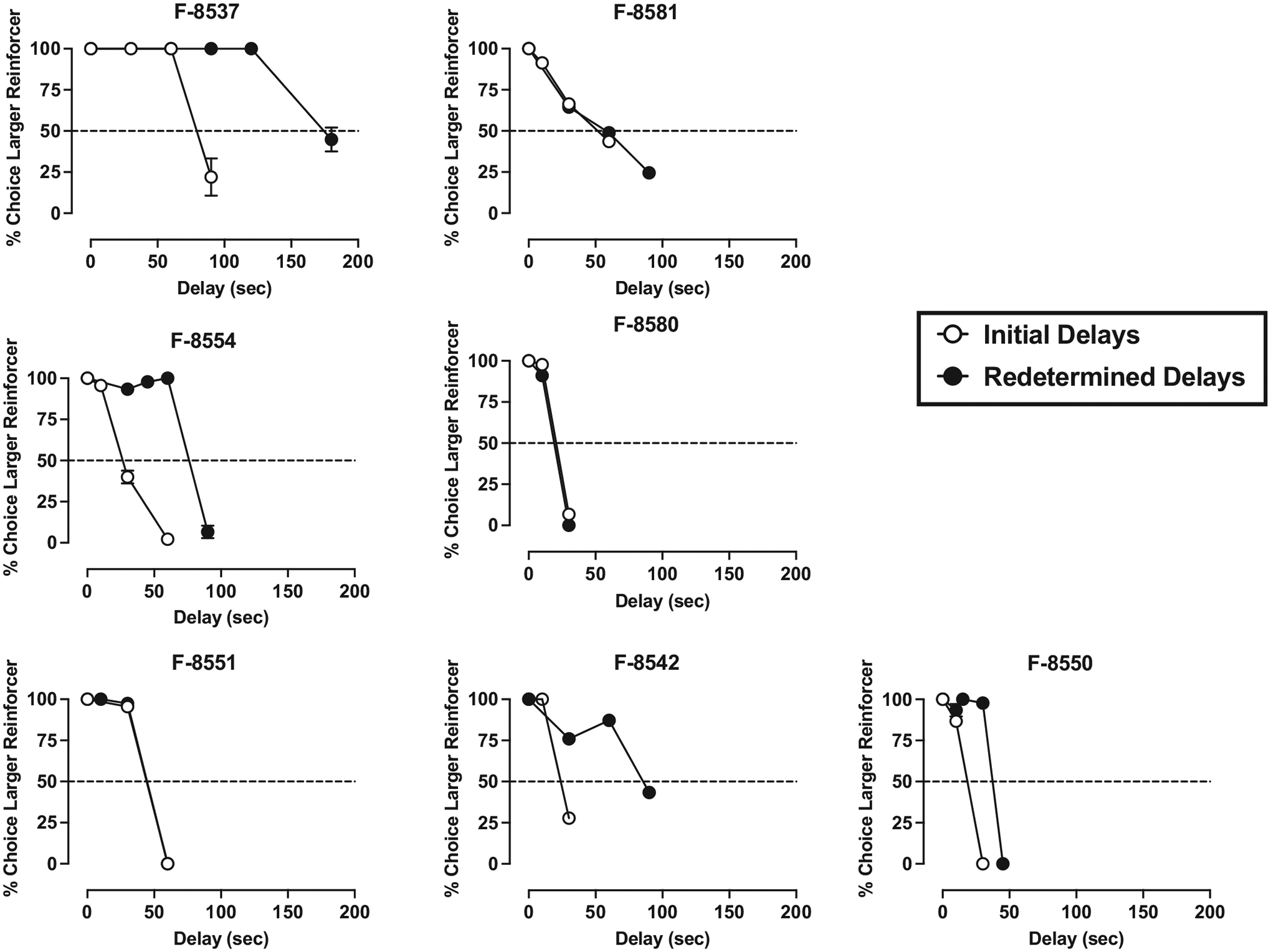
Initial (open symbols) and redetermined (closed symbols) delay discounting curves in dominant female monkeys. Ordinate: percentage of trials in which the larger, delayed reinforcer was chosen over the smaller, immediate reinforcer. Abscissae: delay value (sec) associated with the larger food reinforcer. The delay value at which the curve intersects with the dashed line (50 % choice of larger reinforcer) represents the indifference point. Each point is the mean ± SD of the last 3 days at each delay. For all monkeys, the FR value was 30, except for F-8580 (FR 5) and F-8581 (FR 15).
Fig. 4.
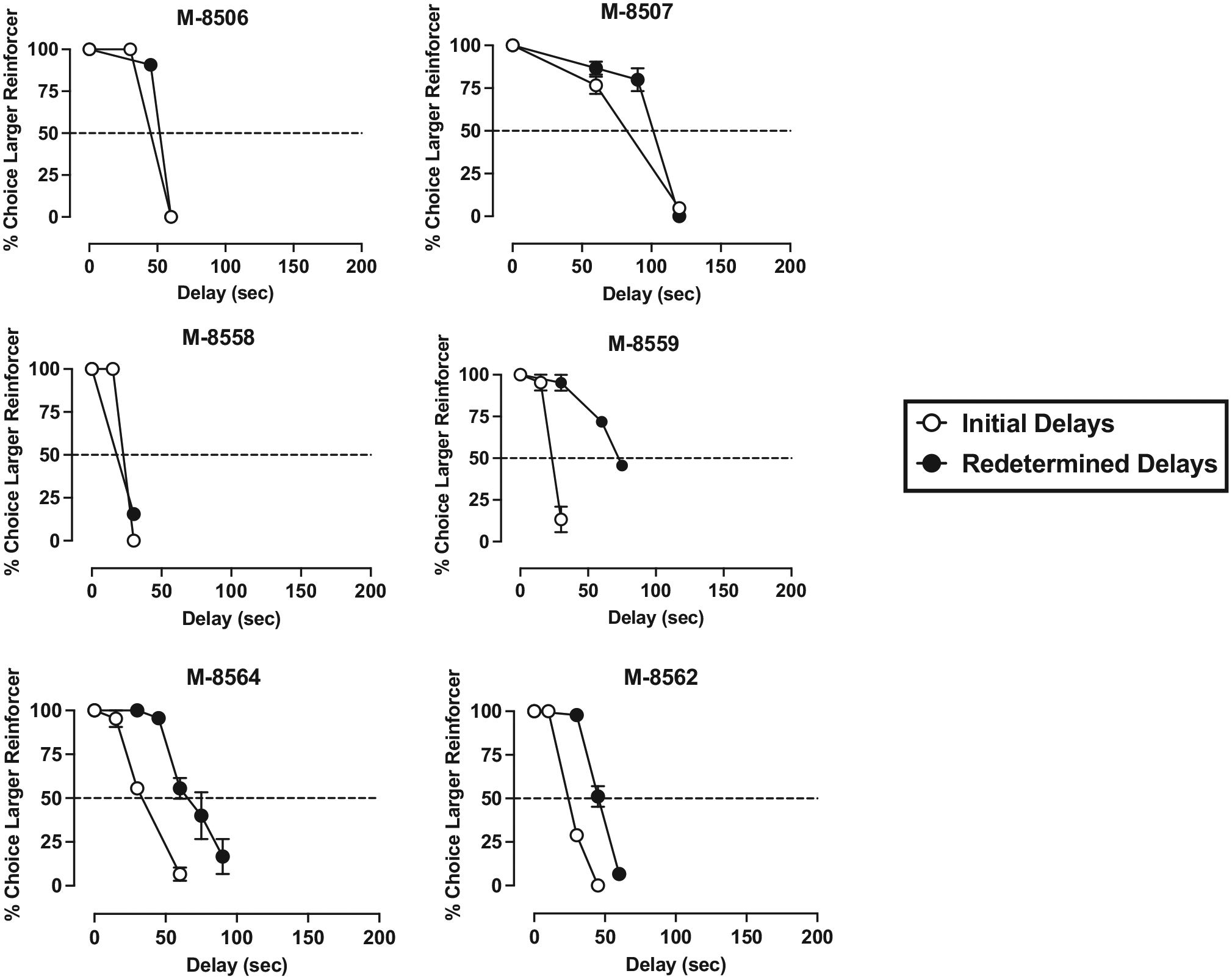
Initial (open symbols) and redetermined (closed symbols) delay discounting curves in subordinate male monkeys. All other information is as described in Fig. 1.
Initial data analyses revealed that there was no effect of sex or social rank on initial IP values and no interaction (p > 0.05). Following determination of the initial IP value, all monkeys returned to baseline-appropriate responding (~100 % choice larger magnitude of food reinforcement) when the delay was removed. Redetermination of the delay-discounting curve resulted in rightward shifts in most monkeys, and subsequent increase in IP values (Figs. 1–4). The mixed-effects ANOVA revealed that there was a significant within-subjects main effect of initial- vs. redetermined-IP score (F(1,20) = 17.05, p < 0.001, η2 = 0.460), where the redetermined IP value (64.13 ± 8.18) was significantly larger than the initial IP value (42.54 ± 5.28; Table 1). Although there were no significant interactions in the mixed-effect ANOVA, further post-hoc analyses were conducted to explore whether sex or social rank may have influenced the interaction between the initial and redetermined IP scores. This was because visual inspection of IP values at the two different timepoints (Table 1) suggested that there may be more nuanced differences in the magnitude of the shift based on sex and social rank. This exploratory analysis (Fig. 5) revealed that the dominant females experienced a significant increase in IP values from the first determination to the second (F(1,20) = 11.44, p = 0.003, η2 = 0.364), and that the subordinate males also experienced a significant increase in IP values from the first to the second determination (F(1,20) = 4.42, p = 0.048, η2 = 0.181). However, the subordinate females (p = 0.071, η2 = 0.154) and dominant males (p = 0.241, η2 = 0.068) did not experience a significant shift in IP values across the first and second determinations.
Table 1.
Calculated IP scores from the initial and redetermined delay discounting curves and resulting ΔIP values (mean ± SEM) in socially housed female and male monkeys.
| Sex | Social Status | Indifference point(s) | ||
|---|---|---|---|---|
| Delay to larger food delivery | ||||
| IP | re-IP | ΔIP | ||
| Male | Dominant | 39.35 ± 12.91 | 54.43 ± 20.02 | 15.075 ± 12.80 |
| Subordinate | 38.43 ± 10.54 | 59.85 ± 16.34 | 21.42 ± 10.45 | |
| Female | Dominant | 37.65 ± 9.51 | 69.54 ± 14.74 | 31.90 ± 9.43 |
| Subordinate | 54.73 ± 9.52 | 72.71 ± 14.75 | 17.98 ± 9.34 | |
IP represents first determination.
re-IP represents the second determination.
ΔIP is the difference between IP and re-IP.
Fig. 5.
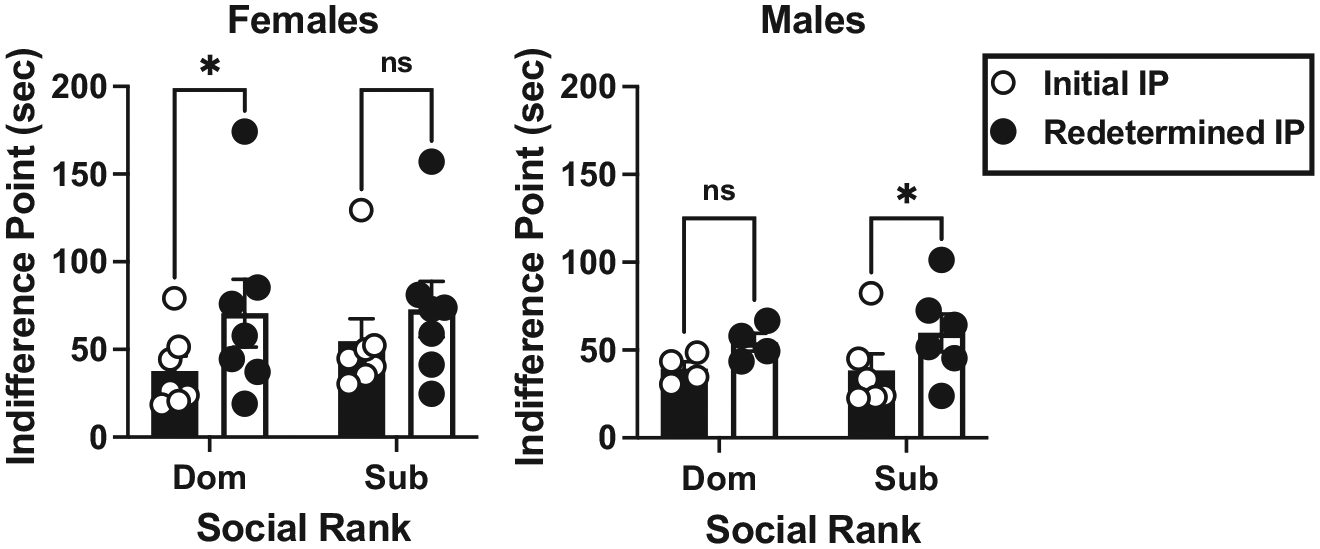
Relationship between social rank and initial (open symbols) and redetermined (closed symbols) IP scores in dominant (Dom) and subordinate (Sub) female (left, N = 13) and male (right, N = 10) cynomolgus monkeys. Each bar represents the mean (±SEM). * p < 0.05.
For the 13 monkeys that had also received PET scans with the KOR radiotracer [11C]EKAP, statistical analyses investigating the relationship between average KOR BP and delta IP scores determined that there was a significant negative correlation between the two variables, (r(13) = −0.681, p = 0.010). Furthermore, delta IP scores and KOR BP were significantly negatively correlated in the orbitofrontal cortex (p = 0.032), dorsal prefrontal cortex (p = 0.049), caudate nucleus (p = 0.004), putamen (p = 0.004), ventral striatum (p = 0.026), insula (p = 0.027), claustrum (p = 0.018), cingulate cortex (p = 0.021), temporal cortex (p = 0.032), and the thalamus (p = 0.045). Multiple regressions revealed that while delta-IP scores were a significant negative predictor of average EKAP BP, sex was not (t(12) = 0.891, p = 0.394). In the orbitofrontal cortex, the dorsal prefrontal cortex, anterior cingulate cortex, caudate, putamen, ventral striatum, insula, claustrum, cingulate cortex, and hippocampus change in IP was a significant negative predictor (all p < 0.05) of KOR BP, while sex was not (all p > 0.05). However, in the medial prefrontal cortex, sex (where male was the reference variable) was a positive predictor of KOR BP (t(12) = 3.27, p = 0.008) while delta IP was a significant negative predictor of KOR BP (t(12) = −2.74, p = 0.021). In the amygdala, globus pallidus, temporal cortex, and thalamus neither sex nor delta IP were significant predictors of KOR BP (p > 0.05) (Table 2).
4. Discussion
The goals of the present study in drug-naïve female and male monkeys, were to further evaluate a) a behavioral marker of impulsive choice that has been associated with vulnerability, development and treatment outcome in CUD and b) to assess the relationship between impulsive choice and brain KOR availability across various ROIs. The main finds of the study were: (1) there were no sex/social rank differences in IP (impulsive choice) following the first determination; (2) redetermination of the delay discounting curve resulted in rightward shifts and significant increase in IP from initial values (less impulsive choice); (3) this effect was likely primarily driven by dominant females and subordinate males, suggesting that delta IP was influenced by sex and social rank; (4) and delta IP significantly predicted brain KOR availability across 11/15 ROIs, such that lower delta IP was associated with higher KOR availability.
The initial determination of delay discounting curves, did not result in sex or social rank differences, suggesting that under these conditions delayed choice responding is not a behavioral phenotype associated with social hierarchy. In a review by Weafer and de Wit (2014), they noted that most human-subject studies examining sex differences in impulsive choice, found women to be more impulsive than men; this included hypothetical money rewards, actual money rewards and real candy. Consistent with this observation, they also noted that most preclinical studies report greater delay discounting in female animals compared with males. In a study conducted in adult male and female rhesus monkeys, we did not observe any sex differences in control monkeys (Hamilton et al., 2011). However, male monkeys that were prenatally exposed to cocaine had significantly lower IP values than age-matched control monkeys (Hamilton et al., 2011), suggesting an interaction of prenatal cocaine exposure on measures of delayed choice, which is consistent with the premise of Weafer and de Wit (2014).
In the present study, when the delay curves were redetermined, IP values increased in most monkeys, but were still not significantly different based on sex or social rank. However, results of an exploratory analyses revealed that changes in IP values from the first to second determination may be influenced by sex and social rank such that the subordinate males and dominant females showed the greatest increases in IP values (i.e., reductions in delayed choice) when compared to dominant males and subordinate females. These findings were unexpected given that we hypothesized that, based on other studies, the animals deemed more vulnerable to cocaine reinforcement (i.e., subordinate males and dominant females) would have lower IP scores since several measures associated with this behavioral phenotype have been related to a greater vulnerability to cocaine self-administration (Molander et al., 2011; Economidou et al., 2009). Moreover, we predicted that there would be no shifts in IP scores from the first determination to the second since studies in humans suggest that levels of impulsivity remain relatively stable over time (Rung et al., 2019; Odum, 2011). Despite this, our study has demonstrated that delayed choice appears to be influenced by other variables (e.g., experience) rather than an inherent characteristic of the individual given that IP values did not remain stable over time. Although exploratory in nature, our study does suggest that change in delayed choice behavior may also be influenced by sex and social rank.
This study also demonstrated that change in IP value significantly negatively predicted KOR availability in several different brain regions when including sex in the analysis. This suggests that the larger the shift in IP value, the lower KOR availability is in brain regions including the orbitofrontal cortex, the dorsal prefrontal cortex, anterior cingulate cortex, caudate, putamen, ventral striatum, insula, claustrum, cingulate cortex, and hippocampus. While sex was not predictive of KOR BP in any of the regions listed above, it was a significant predictor of KOR availability the medial prefrontal cortex. Future studies should be conducted to elucidate why sex and change in IP values only predict KOR availability in specific brain regions. However, previous PET studies in nonhuman primates have shown that the lowest KOR binding potentials across all ROIs were observed in dominant females and subordinate males (Johnson et al., 2023), the two most vulnerable phenotypes to cocaine reinforcement (Morgan et al., 2002; Nader et al., 2012b). Furthermore, lower KOR availability was hypothesized to occur due to high basal dynorphin levels which are indicative of chronic social stress (Johnson et al., 2023). As a result, animals with perceived higher levels of social stress (i.e., dominant females and subordinate males) who, in turn, have lower KOR binding potentials, appear to have the greatest rightward shifts in IP values across time.
While numerous studies have aimed to assess the pre-existing characteristics that may underlie an individual’s likelihood of being dominant or subordinate, prior to this study, delay discounting was not yet assessed as a behavioral phenotype related to social rank and sex. The major finding of the present study was that while baseline IP scores did not appear to be a behavioral biomarker of social rank, changes in IP values appears to be correlated with social rank in female and male monkeys. Future research will examine the significance of delta IP and whether it may provide an index of adaptability to environmental changes (Johnson et al., 2023).
We also noted in these same monkeys that the delta IP was negatively correlated with KOR availability, perhaps indicating some relationship to stress responses. An animal’s ability to respond to changes in the environment is often adaptive given that adjusting behavior based on the environment can improve the chances of survival. For example, in macaques, adaptability to the environment can help decrease the chances of being wounded in a fight with a dominant monkey (Pomerantz and Baker, 2017). Although this type of behavioral adjustment can be adaptive, it can also result in numerous maladaptive outcomes associated with chronic social stress after becoming a subordinate monkey (Nader et al., 2012a; Cameron, 1997; Cohen et al., 1997; Kaplan and Manuck, 1999). Similarly, although adaptability to the environment may result in less impulsive choice during the second IP determination, it may also increase vulnerability to cocaine self-administration. As it relates to delay discounting, there is ample experimental manipulations that can reduce discounting and delay choice responding (reviewed by: Rung et al., 2019; Smith et al., 2019; Koffarnus and Woods, 2013; Rung and Madden, 2018). There is a growing body of literature in rodents on delay exposure as an environmental manipulation that reduces delay choice responding, increases preference for the large delayed reinforcer (Stein et al., 2013; Fox et al., 2019; Stein et al., 2015). The present study had no other training than the initial food-maintained responding under an FR schedule of reinforcement. A future hypothesis to test is that dominant females and subordinate males may experience greater changes in IP values and be more vulnerable to cocaine reinforcement due to higher rates of adaptability to the environment. How this relates to the hypothesized increases in dynorphin, resulting in the lower KOR availability, will require additional research.
There are some limitations to the present study. First, we are hypothesizing that delayed choice responding under concurrent food vs. food contingencies is a behavioral phenotype associated with vulnerability to cocaine self-administration, but we have not yet given these monkeys access to cocaine. As a result, a firmer understanding of how delayed choice responding in these monkeys predicts sensitivity to cocaine reinforcement will require additional testing. A second limitation is that we only redetermined the IP values twice. It is possible that additional changes in delta IP would occur with continued testing and perhaps the social rank and sex differences would become less evident. As it relates to social rank, we did not have a control group of monkeys (individually housed or pair housed) in which IP values were redetermined. Such a control group would have impacted our understanding of how social variables influence delayed choice responding. Finally, it should be noted that this study only had four dominant males in the group, which was less than subordinate males, dominant and subordinate females. It is possible with a larger sample size, significant effects of shifts in IP values in dominant males would have been observed.
Fig. 2.
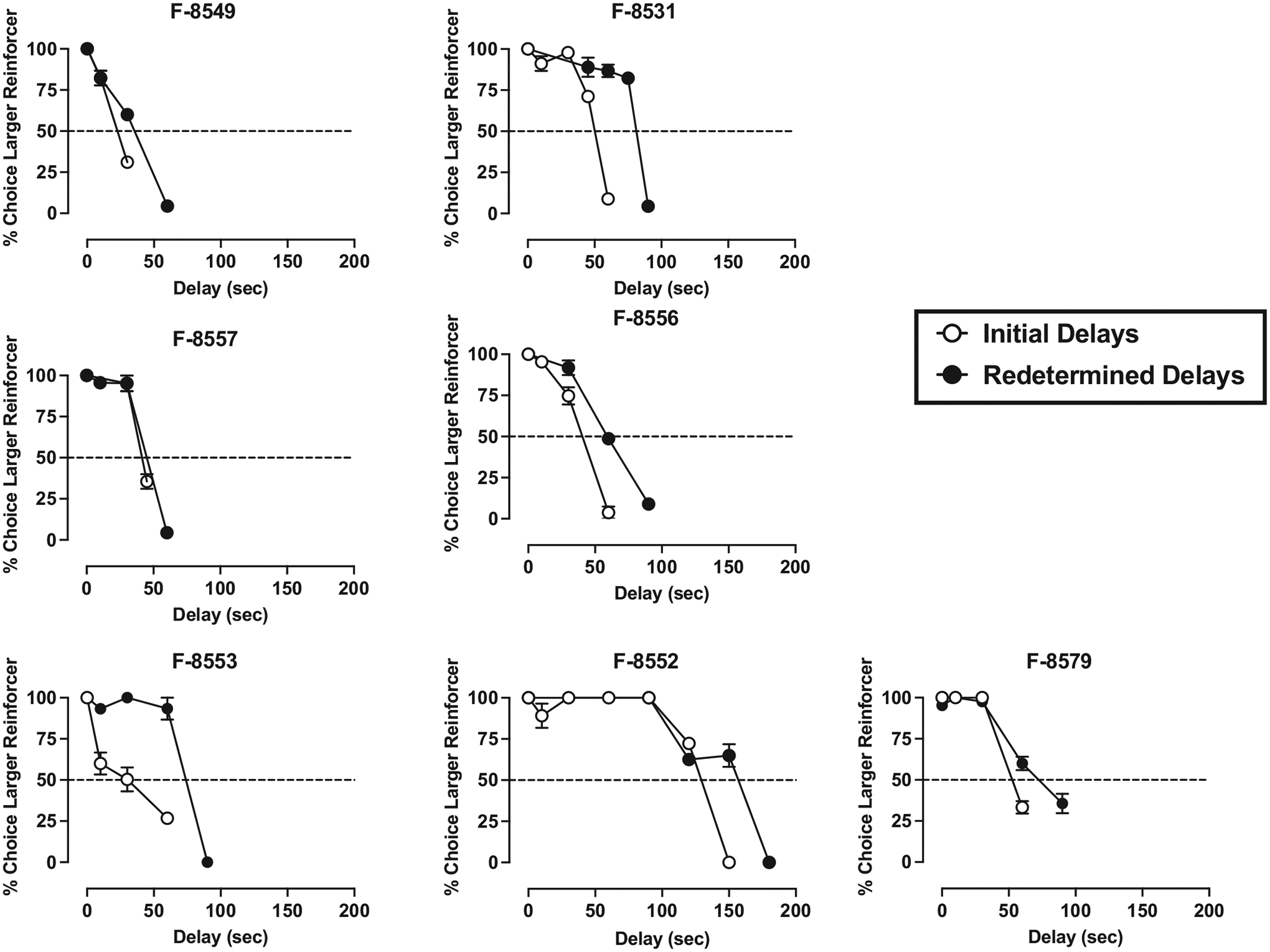
Initial (open symbols) and redetermined (closed symbols) delay discounting curves in subordinate female monkeys. All other information is as described in Fig. 1. For all monkeys, the FR value was 30, except for F-8579 (FR 5).
Fig. 3.
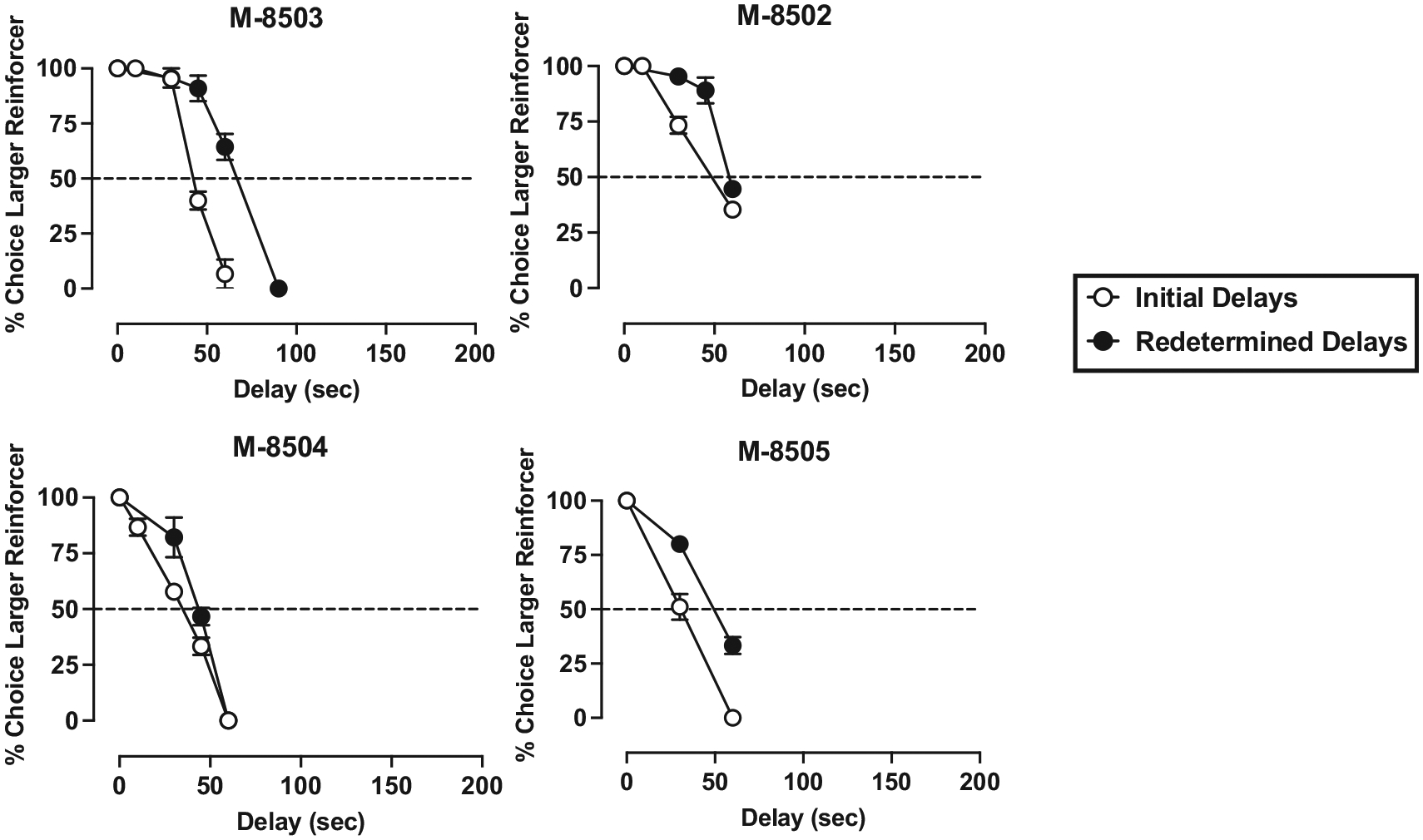
Initial (open symbols) and redetermined (closed symbols) delay discounting curves in dominant male monkeys. All other information is as described in Fig. 1.
Acknowledgment
We would like to thank Michael Coller and Jillian H. Odom for excellent technical assistance. This research was supported by R01 DA017763 (MAN) and F31 DA053776 (BNJ).
Data availability
Data will be made available on request.
References
- Anthony JC, Warner LA, Kessler RC, 1994. Comparative epidemiology of dependence on tobacco, alcohol, controlled substances, and inhalants: basic findings from the National Comorbidity Survey. Exp. Clin. Psychopharmacol 2, 244–268. [Google Scholar]
- Bardo MT, Neisewander JL, Kelly TH, 2013. Individual differences and social influences on the neurobehavioral pharmacology of abused drugs. Pharmacol. Rev 65, 255–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Koffarnus MN, Moody L, Wilson AG, 2014. The behavioral- and neuroeconomic process of temporal discounting: a candidate behavioral marker of addiction. Neuropharmacology 76 (Pt B), 518–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron JL, 1997. Stress and behaviorally induced reproductive dysfunction in primates. Semin. Reprod. Endocrinol 15, 37–45. [DOI] [PubMed] [Google Scholar]
- Carlezon WA Jr., Krystal AD, 2016. Kappa-opioid antagonists for psychiatric disorders: from bench to clinical trials. Depress. Anxiety 33, 895–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Line S, Manuck SB, Rabin BS, Heise ER, Kaplan JR, 1997. Chronic social stress, social status, and susceptibility to upper respiratory infections in nonhuman primates. Psychosom. Med 59, 213–221. [DOI] [PubMed] [Google Scholar]
- Craft WH, Tegge AN, Athamneh LN, Tomlinson DC, Freitas-Lemos R, Bickel WK, 2022. The phenotype of recovery VII: delay discounting mediates the relationship between time in recovery and recovery progress. J. Subst. Abus. Treat 136, 108665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czoty PW, Nader MA, 2012. Individual differences in the effects of environmental stimuli on cocaine choice in socially housed male cynomolgus monkeys. Psychopharmacology 224, 69–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czoty PW, Nader MA, 2015. Effects of oral and intravenous administration of buspirone on food-cocaine choice in socially housed male cynomolgus monkeys. Neuropsychopharmacology 40, 1072–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czoty PW, Morgan D, Shannon EE, Gage HD, Nader MA, 2004. Characterization of dopamine D1 and D2 receptor function in socially housed cynomolgus monkeys self-administering cocaine. Psychopharmacology 174, 381–388. [DOI] [PubMed] [Google Scholar]
- Czoty PW, Gould RW, Nader MA, 2009. Relationship between social rank and cortisol and testosterone concentrations in male cynomolgus monkeys (Macaca fascicularis). J. Neuroendocrinol 21, 68–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley JW, Everitt BJ, Robbins TW, 2011. Impulsivity, compulsivity, and top-down cognitive control. Neuron 69, 680–694. [DOI] [PubMed] [Google Scholar]
- de Laat B, Goldberg A, Shi J, Tetrault JM, Nabulsi N, Zheng MQ, Najafzadeh S, Gao H, Kapinos M, Ropchan J, O’Malley SS, Huang Y, Morris ED, Krishnan-Sarin S, 2019. The kappa opioid receptor is associated with naltrexone-induced reduction of drinking and craving. Biol. Psychiatry 86, 864–871. [DOI] [PubMed] [Google Scholar]
- de Laat B, Nabulsi N, Huang Y, O’Malley SS, Froehlich JC, Morris ED, Krishnan-Sarin S, 2021. Occupancy of the kappa opioid receptor by naltrexone predicts reduction in drinking and craving. Mol. Psychiatry 26, 5053–5060. [DOI] [PubMed] [Google Scholar]
- de Wit H, 2009. Impulsivity as a determinant and consequence of drug use: a review of underlying processes. Addict. Biol 14, 22–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit H, Jentsch JD, SpringerLink, 2020. Recent Advances in Research on Impulsivity and Impulsive Behaviors Springer International Publishing AG Springer International Publishing : Imprint: Springer, Cham. [Google Scholar]
- Dick DM, Smith G, Olausson P, Mitchell SH, Leeman RF, O’Malley SS, Sher K, 2010. Understanding the construct of impulsivity and its relationship to alcohol use disorders. Addict. Biol 15, 217–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Economidou D, Pelloux Y, Robbins TW, Dalley JW, Everitt BJ, 2009. High impulsivity predicts relapse to cocaine-seeking after punishment-induced abstinence. Biol. Psychiatry 65, 851–856. [DOI] [PubMed] [Google Scholar]
- Estave PM, Spodnick MB, Karkhanis AN, 2022. KOR control over addiction processing: an exploration of the mesolimbic dopamine pathway. Handb. Exp. Pharmacol 271, 351–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox AE, Visser EJ, Nicholson AM, 2019. Interventions aimed at changing impulsive choice in rats: effects of immediate and relatively long delay to reward training. Behav. Process 158, 126–136. [DOI] [PubMed] [Google Scholar]
- Freund N, Jordan CJ, Lukkes JL, Norman KJ, Andersen SL, 2019. Juvenile exposure to methylphenidate and guanfacine in rats: effects on early delay discounting and later cocaine-taking behavior. Psychopharmacology 236, 685–698. [DOI] [PubMed] [Google Scholar]
- George O, Koob GF, 2017. Individual differences in the neuropsychopathology of addiction. Dialogues Clin. Neurosci 19, 217–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould RW, Czoty PW, Porrino LJ, Nader MA, 2017. Social status in monkeys: effects of social confrontation on brain function and cocaine self-administration. Neuropsychopharmacology 42, 1093–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant KA, Shively CA, Nader MA, Ehrenkaufer RL, Line SW, Morton TE, Gage HD, Mach RH, 1998. Effect of social status on striatal dopamine D2 receptor binding characteristics in cynomolgus monkeys assessed with positron emission tomography. Synapse 29, 80–83. [DOI] [PubMed] [Google Scholar]
- Hamilton LR, Czoty PW, Nader MA, 2011. Behavioral characterization of adult male and female rhesus monkeys exposed to cocaine throughout gestation. Psychopharmacology 213, 799–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton KR, Mitchell MR, Wing VC, Balodis IM, Bickel WK, Fillmore M, Lane SD, Lejuez CW, Littlefield AK, Luijten M, Mathias CW, Mitchell SH, Napier TC, Reynolds B, Schutz CG, Setlow B, Sher KJ, Swann AC, Tedford SE, White MJ, Winstanley CA, Yi R, Potenza MN, Moeller FG, 2015. Choice impulsivity: definitions, measurement issues, and clinical implications. Personal Disord 6, 182–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huskinson SL, Woolverton WL, Green L, Myerson J, Freeman KB, 2015. Delay discounting of food by rhesus monkeys: cocaine and food choice in isomorphic and allomorphic situations. Exp. Clin. Psychopharmacol 23, 184–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huskinson SL, Myerson J, Green L, Rowlett JK, Woolverton WL, Freeman KB, 2016. Shallow discounting of delayed cocaine by male rhesus monkeys when immediate food is the choice alternative. Exp. Clin. Psychopharmacol 24, 456–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BN, Norman C, Minkiewicz M, MA N, 2021. Behavioral studies in nonhuman primates: focus on models of substance use disorders. In: Encyclopedia of Behavioural Neuroscience, 2, pp. 1–12. [Google Scholar]
- Johnson BN, Kumar A, Su Y, Singh S, Sai KKS, Nader SH, Li S, Reboussin BA, Huang Y, Deep G, Nader MA, 2023. PET imaging of kappa opioid receptors and receptor expression quantified in neuron-derived extracellular vesicles in socially housed female and male cynomolgus macaques. Neuropsychopharmacology 48, 410–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan JR, Manuck SB, 1999. Status, stress, and atherosclerosis: the role of environment and individual behavior. Ann. N. Y. Acad. Sci 896, 145–161. [DOI] [PubMed] [Google Scholar]
- Kaplan JR, Manuck SB, 2004. Ovarian dysfunction, stress, and disease: a primate continuum. ILAR J 45, 89–115. [DOI] [PubMed] [Google Scholar]
- Kaplan JR, Manuck SB, Clarkson TB, Lusso FM, Taub DM, 1982. Social status, environment, and atherosclerosis in cynomolgus monkeys. Arteriosclerosis 2, 359–368. [DOI] [PubMed] [Google Scholar]
- Kaplan JR, Chen H, Manuck SB, 2009. The relationship between social status and atherosclerosis in male and female monkeys as revealed by meta-analysis. Am. J. Primatol 71, 732–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karkhanis A, Holleran KM, Jones SR, 2017. Dynorphin/Kappa opioid receptor signaling in preclinical models of alcohol, drug, and food addiction. Int. Rev. Neurobiol 136, 53–88. [DOI] [PubMed] [Google Scholar]
- Koffarnus MN, Woods JH, 2013. Individual differences in discount rate are associated with demand for self-administered cocaine, but not sucrose. Addict. Biol 18, 8–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND, 2016. Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry 3, 760–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak K, Lucatch AM, Lowe DJE, Balodis IM, MacKillop J, George TP, 2019. The neurobiology of impulsivity and substance use disorders: implications for treatment. Ann. N. Y. Acad. Sci 1451, 71–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez D, Orlowska D, Narendran R, Slifstein M, Liu F, Kumar D, Broft A, Van Heertum R, Kleber HD, 2010. Dopamine type 2/3 receptor availability in the striatum and social status in human volunteers. Biol. Psychiatry 67, 275–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez D, Carpenter KM, Liu F, Slifstein M, Broft A, Friedman AC, Kumar D, Van Heertum R, Kleber HD, Nunes E, 2011. Imaging dopamine transmission in cocaine dependence: link between neurochemistry and response to treatment. Am. J. Psychiatry 168, 634–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez D, Slifstein M, Matuskey D, Nabulsi N, Zheng MQ, Lin SF, Ropchan J, Urban N, Grassetti A, Chang D, Salling M, Foltin R, Carson RE, Huang Y, 2019. Kappa-opioid receptors, dynorphin, and cocaine addiction: a positron emission tomography study. Neuropsychopharmacology 44, 1720–1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matuskey D, Dias M, Naganawa M, Pittman B, Henry S, Li S, Gao H, Ropchan J, Nabulsi N, Carson RE, Huang Y, 2019. Social status and demographic effects of the kappa opioid receptor: a PET imaging study with a novel agonist radiotracer in healthy volunteers. Neuropsychopharmacology 44, 1714–1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molander AC, Mar A, Norbury A, Steventon S, Moreno M, Caprioli D, Theobald DE, Belin D, Everitt BJ, Robbins TW, Dalley JW, 2011. High impulsivity predicting vulnerability to cocaine addiction in rats: some relationship with novelty preference but not novelty reactivity, anxiety or stress. Psychopharmacology 215, 721–731. [DOI] [PubMed] [Google Scholar]
- Morgan D, Grant KA, Prioleau OA, Nader SH, Kaplan JR, Nader MA, 2000. Predictors of social status in cynomolgus monkeys (Macaca fascicularis) after group formation. Am. J. Primatol 52, 115–131. [DOI] [PubMed] [Google Scholar]
- Morgan D, Grant KA, Gage HD, Mach RH, Kaplan JR, Prioleau O, Nader SH, Buchheimer N, Ehrenkaufer RL, Nader MA, 2002. Social dominance in monkeys: dopamine D2 receptors and cocaine self-administration. Nat. Neurosci 5, 169–174. [DOI] [PubMed] [Google Scholar]
- Nader MA, Czoty PW, Gould RW, Riddick NV, 2008. Review. Positron emission tomography imaging studies of dopamine receptors in primate models of addiction. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci 363, 3223–3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nader MA, Czoty PW, Nader SH, Morgan D, 2012a. Nonhuman primate models of social behavior and cocaine abuse. Psychopharmacology 224, 57–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nader MA, Nader SH, Czoty PW, Riddick NV, Gage HD, Gould RW, Blaylock BL, Kaplan JR, Garg PK, Davies HM, Morton D, Garg S, Reboussin BA, 2012b. Social dominance in female monkeys: dopamine receptor function and cocaine reinforcement. Biol. Psychiatry 72, 414–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odum AL, 2011. Delay discounting: trait variable? Behav. Process 87, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry JL, Carroll ME, 2008. The role of impulsive behavior in drug abuse. Psychopharmacology 200, 1–26. [DOI] [PubMed] [Google Scholar]
- Pomerantz O, Baker KC, 2017. Higher levels of submissive behaviors at the onset of the pairing process of rhesus macaques (Macaca mulatta) are associated with lower risk of wounding following introduction. Am. J. Primatol 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddick NV, Czoty PW, Gage HD, Kaplan JR, Nader SH, Icenhower M, Pierre PJ, Bennett A, Garg PK, Garg S, Nader MA, 2009. Behavioral and neurobiological characteristics influencing social hierarchy formation in female cynomolgus monkeys. Neuroscience 158, 1257–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roubinov DS, Hagan MJ, Boyce WT, Adler NE, Bush NR, 2018. Family socioeconomic status, cortisol, and physical health in early childhood: the role of advantageous neighborhood characteristics. Psychosom. Med 80, 492–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rung JM, Madden GJ, 2018. Experimental reductions of delay discounting and impulsive choice: a systematic review and meta-analysis. J. Exp. Psychol. Gen 147, 1349–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rung JM, Peck S, Hinnenkamp J, Preston E, Madden GJ, 2019. Changing delay discounting and impulsive choice: implications for addictions, prevention, and human health. Perspect. Behav. Sci 42, 397–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM, 2005. The influence of social hierarchy on primate health. Science 308, 648–652. [DOI] [PubMed] [Google Scholar]
- Shively CA, Day SM, 2015. Social inequalities in health in nonhuman primates. Neurobiol. Stress 1, 156–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith T, Panfil K, Bailey C, Kirkpatrick K, 2019. Cognitive and behavioral training interventions to promote self-control. J. Exp. Psychol. Anim. Learn. Cogn 45, 259–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein JS, Johnson PS, Renda CR, Smits RR, Liston KJ, Shahan TA, Madden GJ, 2013. Early and prolonged exposure to reward delay: effects on impulsive choice and alcohol self-administration in male rats. Exp. Clin. Psychopharmacol 21, 172–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein JS, Renda CR, Hinnenkamp JE, Madden GJ, 2015. Impulsive choice, alcohol consumption, and pre-exposure to delayed rewards: II. Potential mechanisms. J. Exp. Anal. Behav 103, 33–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strickland JC, Johnson MW, 2021. Rejecting impulsivity as a psychological construct: a theoretical, empirical, and sociocultural argument. Psychol. Rev 128, 336–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tejeda HA, Bonci A, 2019. Dynorphin/kappa-opioid receptor control of dopamine dynamics: implications for negative affective states and psychiatric disorders. Brain Res 1713, 91–101. [DOI] [PubMed] [Google Scholar]
- Trifilieff P, Martinez D, 2013. Kappa-opioid receptor signaling in the striatum as a potential modulator of dopamine transmission in cocaine dependence. Front Psychiatry 4, 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassileva J, Conrod PJ, 2019. Impulsivities and addictions: a multidimensional integrative framework informing assessment and interventions for substance use disorders. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci 374, 20180137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijay A, Wang S, Worhunsky P, Zheng MQ, Nabulsi N, Ropchan J, Krishnan-Sarin S, Huang Y, Morris ED, 2016. PET imaging reveals sex differences in kappa opioid receptor availability in humans, in vivo. Am. J. Nucl. Med. Mol. Imaging 6, 205–214. [PMC free article] [PubMed] [Google Scholar]
- Vsevolozhskaya OA, Anthony JC, 2016. Transitioning from first drug use to dependence onset: illustration of a multiparametric approach for comparative epidemiology. Neuropsychopharmacology 41, 869–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weafer J, de Wit H, 2014. Sex differences in impulsive action and impulsive choice. Addict. Behav 39, 1573–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiers CE, Shokri-Kojori E, Cabrera E, Cunningham S, Wong C, Tomasi D, Wang GJ, Volkow ND, 2016. Socioeconomic status is associated with striatal dopamine D2/D3 receptors in healthy volunteers but not in cocaine abusers. Neurosci. Lett 617, 27–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.


