Abstract
Recent studies have shown the efficacy of a home test for the self-evaluation of olfactory and gustatory functions in quarantined coronavirus disease-2019 (COVID-19) patients. However, testing was often limited to COVID-19 participants, and the accuracy of home test kits was rarely compared to standardized testing. This study aims at providing proof of concept for the validation of the new Chemosensory Perception Test (CPT) developed to remotely assess orthonasal olfactory, retronasal olfactory, and gustatory functions in various populations using common North American household items. In the 2 experiments, a total of 121 participants irrespective of having olfactory and/or gustatory complaints from various causes (COVID-19, sinunasal, post-viral, idiopathic) were tested first, with one or many of the following tests: (i) a brief chemosensory questionnaire, (ii) an olfactory test—Sniffin’ Sticks Test (SST) or University of Pennsylvania Smell Identification Test (UPSIT), and/or (iii) a gustatory test—Brief Waterless Empirical Taste Test (B-WETT). We then applied the CPT which yielded 3 different subscores, namely orthonasal, retronasal, and gustatory CPT scores. The orthonasal CPT score was significantly correlated with SST (ρ = 0.837, P < 0.001) and UPSIT (ρ = 0.364, P < 0.001) scores, and exhibited an excellent accuracy to identify olfactory dysfunction (OD) as compared to SST (area under the curve [AUC]: 0.923 [95% confidence interval {CI}, 0.822–1.000], P < 0.001). The retronasal CPT score but not the gustatory CPT score allowed to distinguish between participants with or without subjective gustatory complaint (AUC: 0.818 [95% CI, 0.726–0.909], P < 0.001). The CPT has the ability to identify OD and to quantify subjective gustatory complaints.
Keywords: chemosensory testing, COVID-19, pandemic, remote testing, home test, self-assessment
Introduction
The coronavirus disease-2019 (COVID-19) is characterized by olfactory and gustatory dysfunctions (GDs) as early, specific, and lasting symptoms, with variant-specific symptom profiles (von Bartheld et al. 2020; Haehner et al. 2020; Parma et al. 2020; Gerkin et al. 2021; Saniasiaya et al. 2021; Bussière et al. 2022; Hannum et al. 2022; Ohla et al. 2022; Whitaker et al. 2022). Systematic and safe chemosensory testing is therefore important to identify and prevent the spread of COVID-19 (Pierron et al. 2020; Larremore et al. 2021). It is crucial to monitor patients’ symptoms in both COVID-19 and other health conditions that induce chemosensory dysfunctions. This is to offer prompt treatment and support and to limit the impact of these diseases on the patients’ quality of life (Rowan et al. 2019; Gunn et al. 2021; Vaira et al. 2022).
Olfactory testing with known standardized psychophysical tests remains challenging when social distancing is required for infection control or when very large cohorts are to be tested. The Sniffin’Sticks Test (SST; Hummel et al. 1997) and the University of Pennsylvania Smell Identification Test (UPSIT; Doty et al. 1984) are widely used psychophysical tests of olfactory function that require a long administration time. Shorter versions of the SST have proven helpful in screening for post-COVID-19 olfactory dysfunction (OD) (Bagnasco et al. 2021; Vandersteen et al. 2021), but they require in-person testing, a situation that may cause safety concerns, and are time consuming. The UPSIT and similar shorter tests can be administered remotely (Doty et al. 1996; Jackman and Doty 2005; Cao et al. 2022). However, since the testing booklets should be purchased and sent individually to all participants, these tests lack feasibility and financial viability when testing large cohorts. Additionally, both these tests and their derivatives are limited to assessing orthonasal olfaction. Retronasal olfaction (smell perception when odorants enter the nasal cavity from the mouth via the nasopharynx) plays a crucial role in flavor perception. It can be assessed using various methods (Özay et al. 2019), but this is rarely done in clinical or research settings, even though retronasal olfaction is directly related to flavor perception (Zang et al. 2019) and quality of life (Oleszkiewicz et al. 2019).
Gustatory testing usually includes the determination of taste thresholds for the 5 taste qualities (salty, sour, sweet, bitter, and umami) either via stimulus drop technique (3-drop test; Gudziol and Hummel 2007) or impregnated filter papers (taste strips; Mueller et al. 2003). The 3-drop test requires in-person testing and poses logistical challenges (e.g. stimulus preparation, storage) that are less compatible with safety and large-scale testing. Tastes strips such as the Waterless Empirical Taste Test (WETT; Doty et al. 2021) and its shorter self-administered version (B-WETT) are very practical, as they can be used remotely and have a good shelf life. However, they present themselves with similar feasibility and financial issues as the previously described smell tests.
In the healthcare setting, there is a need for chemosensory testing that is (i) quick and safe, (ii) cost-effective, and (iii) can be applied remotely and repeatedly on a large scale. Remote self-administered olfactory and/or gustatory tests have already been used in the context of the COVID-19 pandemic. Since the mailable commercially available test kits are relatively expensive, researchers and clinicians introduced homemade tests with common household items as an interesting alternative (Vaira et al. 2020; Konstantinidis et al. 2020; Gupta et al. 2022; Snitz et al. 2022). However, they all often lack a comparison with the validated olfactory and gustatory tests or are limited to orthonasal olfactory testing. There is, therefore, a need to develop a test that (i) can be self-administered; (ii) uses common household items; (iii) is easy to use; (iv) assesses all aspects of chemosensory processing, including ortho- and retronasal olfaction as well as gustation; and (v) is validated by comparison to existing tests.
In response to this need, our team developed the Chemosensory Perception Test (CPT), a quick and easy-to-use at-home chemosensory test enabling accessible yet accurate self-evaluation of chemosensory functions, namely orthonasal olfaction, retronasal olfaction, and gustation. Participants are asked to smell and taste odorants and tastants available in an average North American household (Gupta et al. 2022), then rate the perceived intensity on a scale from 1 to 10. The CPT is cost-free (for the experimenter), does not need material to be sent to participants, and does not require complex solutions preparation. Also, the remote and self-administrative testing avoids safety issues encountered with in-person testing. The CPT has already demonstrated its practical use in a large population (Bussière et al. 2021, 2022), but its validity remains to be demonstrated.
We conducted 2 experiments as a proof of concept for the validation of the CPT. Those experiments aimed to compare the 3 subscores of the CPT, namely orthonasal olfactory, retronasal olfactory, and gustatory scores, with (i) the participants’ subjective olfactory or gustatory complaint and (ii) standardized testing. Our main hypotheses to be tested were the following: (i) CPT scores are lower in participants complaining of smell or taste loss; (ii) CPT scores are significantly correlated with established olfactory and gustatory testing, and this with a strong correlation (i.e. ρ > 0.7); and (iii) the CPT allows for the distinction of patients with OD or GD from individuals with a normal sense of smell or taste.
Materials and methods
This study was approved by the research ethics board of Université du Québec à Trois-Rivières (CER-21-273-08-01.05).
First, we conducted a pilot experiment to establish test items of the CPT, their concentration, and the procedure (see Supplementary Material). Then, we carried out 2 experiments to compare orthonasal olfactory, retronasal olfactory, and gustatory CPT scores with (i) the SST (Experiment 1: pilot study), and (ii) subjective olfactory and gustatory complaints, the UPSIT and the B-WETT (Experiment 2).
Experiment 1: Pilot study
Data collection took place between June and August 2020.
Participants.
All participants were controls or participants with known OD recruited from previous studies (Tremblay et al. 2017; Tremblay, Mei, et al. 2020; Tremblay, Iravani, et al. 2020) and had already been tested with the SST (Hummel et al. 1997). Inclusion criteria were the following: (i) age 18 or above, (ii) French or English speaker, and (iii) previous olfactory testing with SST. Participants were excluded if they self-reported changes in taste or smell since previous testing. All participants provided verbal or written informed consent prior to participation.
We recruited a total of 36 participants (16 women, mean age 68.6; Supplementary Table 1). At the time of SST assessment (1–4 years prior to this study), 19 (53%) had been diagnosed with normosmia according to SST (9 women, age 60–78), while the 17 other participants (47%) had exhibited OD (7 women, age 57–77) from various causes (postviral, sinunasal, idiopathic). It is important to note that although this portion of the experiment took place during the initial months of the pandemic, none of the participants had suffered from COVID-19 until inclusion into the study (according to self-report).
Procedure.
Participants were contacted to be scheduled for a phone interview. Verbal consent was obtained and recorded, then the CPT was self-administered under the supervision of a research team member.
Materials.
The SST (Burghardt, Wedel, Germany) is a validated psychophysical test of orthonasal olfactory function that uses pen-like odor-dispensing devices. It measures threshold detection, odor discrimination, and odor identification that sums up to global score. We analyzed the participants’ global scores already available from previous studies. Normosmia was defined as a score above 30.5 (Rumeau et al. 2016).
The CPT is a newly developed self-administered test using common household items (Gupta et al. 2022). It assesses olfaction (orthonasal and retronasal) and gustation using stimulus intensity, as used in previous studies (Iravani et al. 2020; Vaira et al. 2020), and on the basis that this is the best single predictor to classify individuals with a normal sense of smell (Parma et al. 2021).
Prior to testing, participants were instructed to prepare a list of ingredients, namely (i) peanut butter, (ii) fruit jam or jelly, (iii) coffee of any kind, (iv) table salt, (v) household sugar, (vi) 2 glasses of lukewarm water, (vii) a measuring cup, and (viii) spoons.
During the phone interview, we assessed orthonasal function using peanut butter, fruit jam, and coffee. Participants who were intolerant/allergic to a given item or who did not have it in their possession were instructed to go directly to the next item. Participants were first asked, “Please sniff the item” and had to indicate “How strong does the item smell?” on a scale from 0 to 10 (0: not at all, 10: very strong). Then, we proceeded to the next item on the list. Once all orthonasal items were tested, we computed the orthonasal CPT score by calculating the arithmetic mean.
Next, we assessed retronasal function, by retesting the items peanut butter and jam/jelly. Participants who were intolerant/allergic to a given item or who did not have it in their possession were instructed to go directly to the next item. Participants were first instructed to “Please put a small amount of the item in your mouth” and then to quantify “How strong does the item taste?” using the same analog scale as other items (0 = “not at all,” 10 = “very strong”). Then, we proceeded to the next item. It is important to point out that although we asked participants to rate “taste” intensity, this test assesses retronasal olfactory function. Once all retronasal items were tested, we computed the retronasal CPT score by calculating the arithmetic mean.
Finally, we proceeded to the taste test. We first asked participants to prepare 2 solutions, namely (i) a salty solution by mixing 1 teaspoon of table salt in 1 cup of lukewarm water, and (ii) a sweet solution by mixing 3 teaspoons of household sugar in 1 cup of lukewarm water. We then used the same procedure for gustatory testing as for retronasal testing. We averaged the 2 scores to obtain the gustatory CPT score.
Statistical analysis.
Data were analyzed with SPSS 28.0 (IBM Corp). We assessed the correlation between orthonasal olfactory, retronasal olfactory, and gustatory CPT scores and SST scores using Pearson’s or Spearman’s rank correlation coefficients as indicated. We next compared orthonasal and retronasal CPT scores in participants with normosmia (as assessed by their SST scores) and participants with hyposmia/anosmia using t-tests. The accuracy of the orthonasal CPT score in the identification of objective OD according to SST was investigated with receiver operator characteristic curves (ROC). We derived the area under the curve (AUC) as well as sensitivity and specificity for each component, when indicated. Values of P < 0.05 were considered significant for all analyses. Results are presented as mean (SD) unless otherwise specified.
Experiment 2
The second experiment was conducted between December 2020 and August 2021.
Participants.
Participants were recruited from previously tested participants in our lab and in the public via social media, with no overlapping between Experiments 1 and 2. Inclusion criteria were the following: (i) age 18 or above, (ii) French or English speaker, (iii) with or without subjective and/or objective OD from various etiologies, including post-COVID-19 infection. All participants provided recorded verbal informed consent prior to participation.
This experiment included a total of 85 participants (63 women, mean age 45.5; Supplementary Table 2). Participants were grouped according to (i) self-reported OD, (ii) confirmed OD according to University of Pennsylvania Smell Identification Test, (iii) self-reported GD, and (iv) confirmed GD according to B-WETT (Fig. 1).
Fig. 1.
Participants flow diagram in Experiment 2. Flowchart of Experiment 2 design. Participants were grouped based on self-reported olfactory or gustatory function, objective olfactory function (University of Pennsylvania Smell Identification Test), and objective gustatory function (Brief Waterless Empirical Taste Test).
In relation to self-reported OD, 41 participants (48%) had a subjective olfactory complaint (31 women, age 25–78). In turn, according to UPSIT, 43 (51%) presented with normosmia (32 women, age 22–73) and 42 (49%) were diagnosed with objective OD (31 women, age 22–78). In participants with OD, 22 (52%) attributed their olfactory loss to COVID-19 infection and 20 (48%) presented OD from another cause (other viral infection, idiopathic or unknown).
In relation to self-reported GD, 35 participants (41%) complained of subjective GD (29 women, age 25–78). In turn, among the participants who completed the B-WETT (n = 84), 13 (15%) presented objective GD (7 women, age 28–78) while 71 (85%) had a normal sense of taste (55 women, age 22–72). Participants presenting with GD associated it with COVID-19 (n = 4) or other causes (post-viral, idiopathic, or unknown; n = 9).
Procedure.
Once consent was obtained, our team mailed the UPSIT and the B-WETT, as well as a list of the household items required for the CPT administration. Upon reception, participants were contacted via videoconferencing or phone by a research team member. A brief chemosensory questionnaire was completed, then self-testing was conducted under direct supervision following this order: (i) CPT, (ii) UPSIT, and (iii) B-WETT.
Materials.
1 A brief chemosensory questionnaire allows data collection about demographic characteristics and self-reported chemosensory dysfunction, prompted with the following questions: “Do you have a problem with taste?” and “Do you have a problem with smell?.”
2 We used the CPT as described above.
3 The UPSIT (Sensonics International, Haddon Heights, NJ) assesses odor identification with microencapsulated odorants presented at suprathreshold levels (Doty et al. 1984). The test was administered as described by Doty (2008). OD was defined as a score equal to or less than 33 in men and 34 in women.
4 The B-WETT (Sensonics International, Haddon Heights, NJ) is a short and self-administered version of the validated WETT (Doty et al. 2021). It assesses taste thresholds for the 5 taste qualities (sweet, sour, bitter, salty, and umami) with a series of 27 disposable taste strips of various concentrations interspersed with blank strips. This test was administered as described (Doty 2020). B-WETT scores equal to or less than 13, identified as 10th percentile in preliminary normative data, were considered representative of GD.
Statistical analysis.
Data were analyzed with SPSS 28.0 (IBM Corp, Armonk, NY). We compared (i) orthonasal olfactory, (ii) retronasal olfactory, and (iii) gustatory CPT scores in participants with subjective OD or subjective GD versus those without, by t-tests. We assessed the correlation between each CPT score and UPSIT or B-WETT raw scores using Pearson’s (for normally distributed data) or Spearman’s rank correlation coefficients (for non-normally distributed data), as indicated. Using t-tests, we compared CPT scores in participants with normosmia versus participants with hyposmia according to UPSIT, and in participants with and without GD according to B-WETT. The accuracy of each CPT score in the identification of subjective or objective dysfunction (olfactory, gustatory) was investigated and expressed with ROC and obtained the AUC. Values of P < 0.05 were considered significant for all analyses. Results are presented as mean (SD) unless otherwise specified.
Results
Experiment 1
CPT subscores were computed by averaging intensity ratings for each item in a subscore, even when one rating was missing (i.e. when a participant was allergic or did not have a test item at home). One participant did not rate peanut butter; 14 did not rate the jam; and 1 participant did not rate the salty solution.
Mean scores.
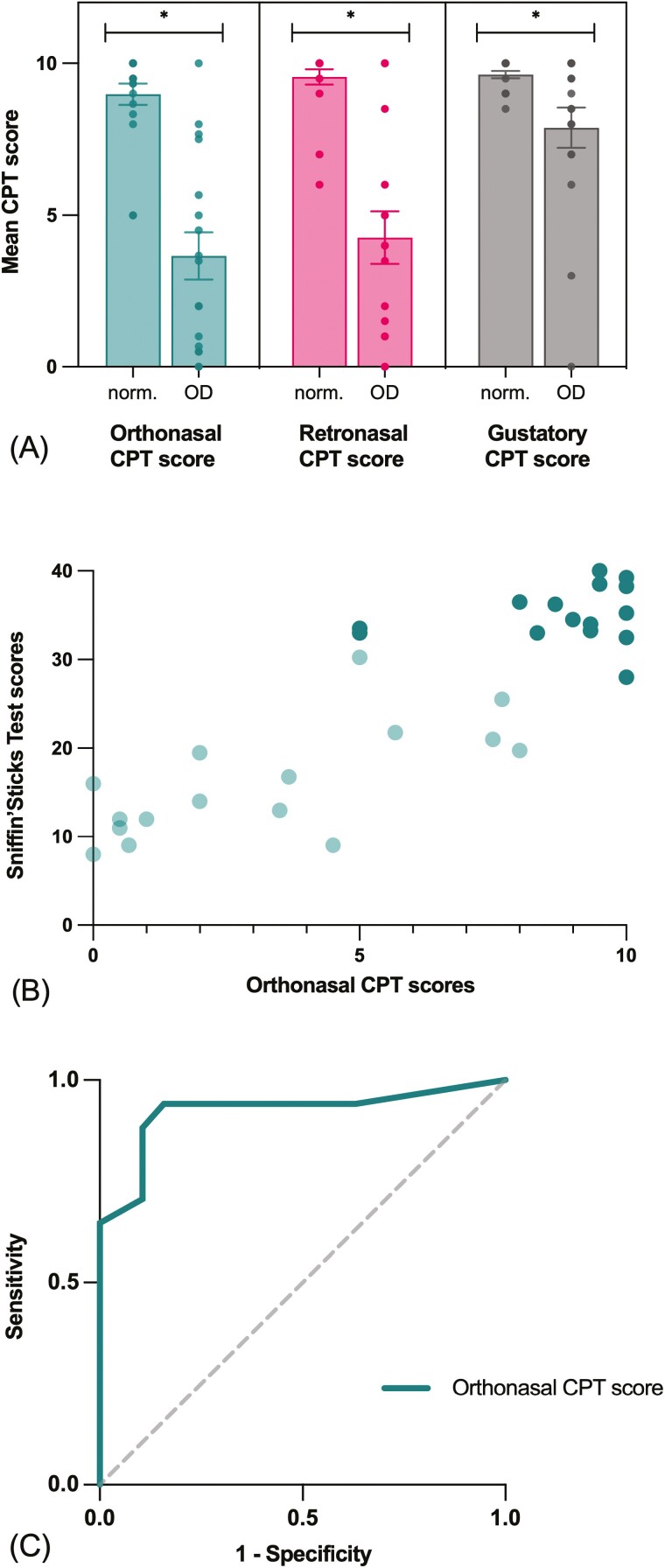
Mean SST and CPT scores are presented in Table 1. All CPT scores were significantly lower in the OD group compared to the group with normosmia (Fig. 2a; orthonasal: t(22.30) = 6.24, P < 0.001; retronasal: t(18.82) = 5.87, P < 0.001; gustatory: t(17.04) = 2.58, P = 0.019).
Table 1.
Mean SST and CPT scores according to olfactory function in Experiment 1.
| Normosmia (n = 19) | Olfactory dysfunction (n = 17) | P value | |
|---|---|---|---|
| SST | 36.53 (3.18) | 16.85 (6.81) | |
| Orthonasal CPT score | 8.98 (1.52) | 3.66 (3.21) | <0.001 |
| Retronasal CPT score | 9.55 (1.12) | 4.26 (3.56) | <0.001 |
| Gustatory CPT score | 9.63 (.52) | 7.88 (2.75) | 0.019 |
Fig. 2.
CPT subscores compared with SST in Experiment 1. (A) Mean orthonasal, retronasal and gustatory CPT scores in participants with normosmia (norm.) or OD according to SST scores. Data presented as jittered dots (visibility: 0.8). Significant difference between groups (P < 0.05) is represented with *. (B) Correlation between SST and orthonasal CPT scores (ρ = 0.84, P < 0.001). Darker points indicate participants with normosmia (SST TDI > 30.5); lighter points indicate participants with olfactory dysfunction (SST TSI < 30.5). (C) Orthonasal CPT score accuracy in detecting olfactory dysfunction according to SST scores (AUC: 0.923 [95% CI, 0.822–1.000], P < 0.001).
Correlation between scores.
All CPT scores significantly correlated with SST scores. The association was strong between SST scores and orthonasal (ρ = 0.84, P < 0.001) and retronasal CPT (ρ = 0.73, P < 0.001) scores (Fig. 2b). Accordingly, orthonasal CPT scores were also strongly associated with retronasal CPT scores (ρ = 0.82, P < 0.001).
Accuracy of the CPT to classify OD.
The ability of the orthonasal CPT score to correctly identify OD (SST score) was excellent (AUC: 0.92 [95% CI, 0.82–1.00], P < 0.001; Fig. 2c). Based on the difference in mean orthonasal CPT scores between normosmia versus OD groups and ROC analyses, a cut-off score of 8 was established. With this cut-off score, the orthonasal CPT score reaches a sensitivity of 0.94 and a specificity of 0.84 according to SST’s classification of OD. Of course, results such as cut-off scores obtained in a pilot study with 36 participants are of limited clinical value. Therefore, this cut-off score should be interpreted with caution.
Experiment 2
CPT subscores were computed using the same method as in Experiment 1. Out of 85 participants, 7 did not rate peanut butter; 1 did not rate the salty solution; and 3 did not rate the sweet solution.
Mean scores.
Following a brief chemosensory questionnaire to document self-reported olfactory and/or GD, we tested all participants (n = 85) with the CPT, the UPSIT, and the WETT. However, one participant could not complete the WETT testing due to nausea. Mean UPSIT and CPT scores are presented in Table 2 (olfactory function) and Table 3 (gustatory function).
Table 2.
Mean UPSIT and CPT scores according to olfactory function in Experiment 2.
| Subjective olfactory function | Objective olfactory function | |||||
|---|---|---|---|---|---|---|
| Subjective normosmia (n = 44) | Subjective olfactory dysfunction (n = 41) | P value | Normosmia (n = 43) | Olfactory dysfunction (n = 42) | P value | |
| UPSIT | 35.93 (1.32) | 28.9 (6.57) | ||||
| Orthonasal CPT score | 8.33 (1.55) | 5.61 (2.49) | <0.001 | 7.78 (1.78) | 6.24 (2.82) | 0.004 |
| Retronasal CPT score | 8.23 (1.70) | 5.43 (3.02) | <0.001 | 7.13 (2.50) | 6.63 (3.07) | 0.411 |
| Gustatory CPT score | 7.94 (1.59) | 7.26 (2.59) | 0.154 | 7.67 (1.89) | 7.55 (2.41) | 0.811 |
Table 3.
Mean B-WETT and CPT scores according to gustatory function in Experiment 2.
| Subjective gustatory function (n = 85) | Objective gustatory function (n = 84) | |||||
|---|---|---|---|---|---|---|
| Subjective normogueusia (n = 50) | Subjective gustatory dysfunction (n = 35) | P value | Normogueusia (n = 71) | Gustatory dysfunction (n = 13) | P value | |
| B-WETT | 19.21 (3.36) | 10.85 (2.73) | ||||
| Gustatory CPT score | 7.98 (1.64) | 7.09 (2.65) | 0.081 | 7.64 (1.92) | 7.33 (3.26) | 0.740 |
| Retronasal CPT score | 8.22 (1.78) | 4.96 (2.88) | <0.001 | 7.17 (2.44) | 5.06 (3.86) | 0.078 |
| Orthonasal CPT score | 8.03 (1.79) | 5.57 (2.58) | <0.001 | 7.29 (2.23) | 5.31 (3.00) | 0.039 |
Mean scores according to subjective olfactory function.
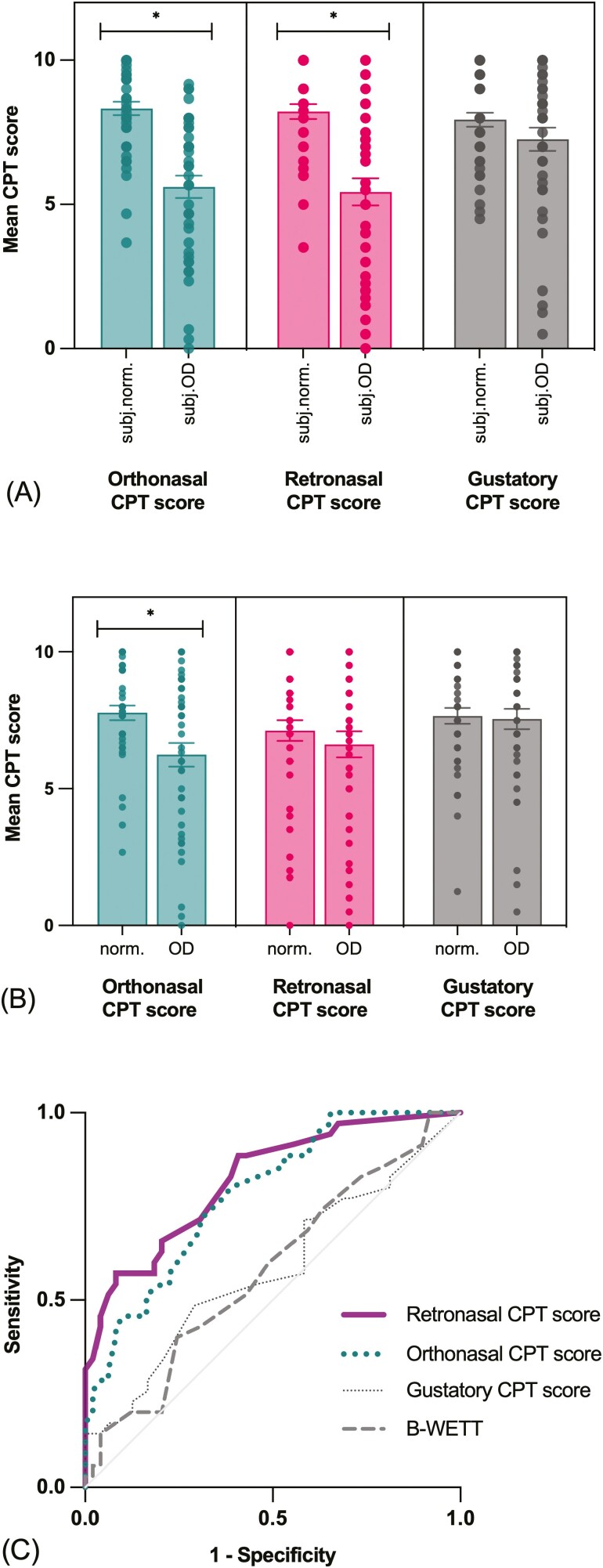
Mean orthonasal CPT score (t(65.98) = 6.00, P < 0.001) and mean retronasal CPT score (t(62.07) = 5.21, P < 0.001) were significantly lower in participants with subjective olfactory complaint compared to participants with no complaint. Mean gustatory CPT scores did not differ significantly according to subjective olfactory complaint (Fig. 3a).
Fig. 3.
CPT subscores compared with subjective complaint, UPSIT and B-WETT in Experiment 2. Mean orthonasal, retronasal, and gustatory CPT scores in participants with (A) subjective normosmia (subj.norm.) or olfactory dysfunction (subj.OD) and (B) normosmia (norm.) or OD according to UPSIT scores. Data presented as jittered dots (visibility: 0.8). Significant difference between groups (P < 0.05) is represented with *. (C) CPT and B-WETT scores’ accuracy in identifying subjective gustatory complaint. Retronasal and orthonasal CPT scores analysis reached significance and presented, respectively, with good (AUC: 0.82 [95% CI, 0.73–0.91], P < 0.001) and fair (AUC: 0.78 [95% CI, 0.69–0.88], P < 0.001) accuracy.
Mean scores according to objective olfactory function.
Participants with objective OD according to UPSIT scores had significantly lower orthonasal CPT scores than participants with normosmia (t(68.86) = 2.996, P = 0.004). The group difference was not significant for the retronasal and gustatory CPT scores (Fig. 3b).
Mean scores according to subjective gustatory function.
Mean gustatory CPT scores were not different between participants with or without subjective GD (t(52.46) = 1.78, P = 0.081). However, participants complaining of GD presented lower orthonasal (t(56.37) = 4.88, P < 0.001) and retronasal (t(51.97) = 5.95, P < 0.001) CPT scores as compared to participants with no gustatory complaint.
Mean scores according to objective gustatory function.
Mean gustatory CPT scores did not differ between groups of participants with or without objective GD according to WETT’s 10th percentile (t(13.58) = 0.34, P = 0.740). However, participants with GD had significantly lower orthonasal CPT score (t(14.52) = 2.27, P = 0.04). There was no difference in mean retronasal CPT scores between groups.
Correlation between scores.
Orthonasal (ρ = 0.36, P < 0.001) but not retronasal CPT scores (ρ = 0.17, P = 0.125) were significantly correlated with UPSIT scores. When considering only participants with OD (n = 42), orthonasal CPT scores (ρ = 0.692, P < 0.001), and retronasal CPT scores (ρ = 0.577, P < 0.001) were significantly associated with UPSIT scores.
We did not observe any correlation between B-WETT scores and gustatory (ρ = −0.56, P < 0.617) or retronasal CPT scores (ρ = 0.205, P < 0.062). Rather, there was a weak correlation between B-WETT scores and orthonasal CPT scores (ρ = 0.28, P = 0.011).
Orthonasal CPT scores were strongly associated with retronasal CPT scores (ρ = 0.769, P < 0.001) and less with gustatory CPT scores (ρ = 0.398, P < 0.001), while retronasal CPT scores were moderately correlated with gustatory CPT scores (ρ = 0.428, P < 0.001).
Accuracy to classify subjective OD.
The ability of the orthonasal CPT scores to correctly identify participants’ olfactory status according to their initial olfactory complaint (subjective OD) was good (AUC: 0.83 [95% CI, 0.75–0.91], P < 0.001), while the UPSIT raw scores resulted in a fair accuracy (AUC: 0.77 [95% CI, 0.67–0.87], P < 0.001). The retronasal CPT scores could identify participants’ olfactory status according to subjective OD with fair accuracy (AUC: 0.77 [95% CI, 0.67–0.87], P < 0.001). ROC analyses of the gustatory CPT score did not reach significance.
Accuracy to classify objective OD.
CPT’s ability to classify OD according to objective OD (UPSIT) in all participants was poor (AUC:.65 [95% CI, 0.53–0.77], P = 0.018). However, when grouping participants with normosmia with participants with mild hyposmia (n = 74), the accuracy improved (AUC: 0.80 [95% CI, 0.64–0.97], P = 0.001). Based on the difference in mean orthonasal CPT scores between participants with normosmia/mild hyposmia versus participants with moderate/severe hyposmia or anosmia, a cut-off score of 8 reaches a sensitivity of 0.91 and a specificity of 0.38. ROC analyses of retronasal and gustatory CPT scores did not reach significance.
Accuracy to classify subjective GD.
The gustatory CPT scores and the B-WETT raw scores’ ROC analyses did not allow the identification of participants with gustatory complaint (subjective GD). The retronasal CPT scores demonstrated the best ability to classify subjective GD, with good accuracy (AUC: 0.82 [95% CI, 0.73–0.91], P < 0.001), while the orthonasal CPT scores had a fair accuracy (AUC: 0.78 [95% CI, 0.69–0.88], P < .001; Fig. 3c).
Accuracy to classify objective GD.
The orthonasal CPT scores were the only CPT scores that allowed the classification of GD according to B-WETT’s normative values (10th percentile); however, it did so with a poor accuracy (AUC: 0.65 [95% CI, 0.52–0.78], P = 0.030). ROC analyses of retronasal and gustatory CPT scores did not reach significance.
Discussion
Here, we present a proof of concept for the validation of the CPT, a newly developed, self-administered chemosensory test using common household items. Our main results are the following: (i) participants with objective OD have significantly lower CPT scores; (ii) the orthonasal CPT scores have the potential to classify individuals with regards to their subjective olfactory complaint; with regards to objective OD, its ability seems to be limited to classify individuals with moderate to severe OD versus those with normosmia or mild hyposmia; and (iii) the retronasal CPT scores have the potential ability to classify individuals with subjective gustatory complaint.
We show that the orthonasal CPT scores, obtained by averaging intensity ratings after smelling peanut butter, fruit jam, and coffee, allowed to distinguish between individuals with or without OD with good accuracy. Specifically, participants with OD exhibited lower orthonasal CPT scores. Further, the orthonasal CPT scores were significantly correlated with both the SST scores and the UPSIT scores. Finally, the orthonasal CPT scores had good accuracy in classifying OD. When comparing both experiments’ results, it is evident that the orthonasal CPT scores’ accuracy in identifying OD was more robust in Experiment 1. In fact, using a cut-off score of 8 for the orthonasal CPT scores allowed for excellent accuracy in classifying OD in Experiment 1 and good accuracy in classifying into normosmia/mild hyposmia versus moderate/severe hyposmia in Experiment 2. Differences in the design between the SST and the UPSIT may explain those results. The UPSIT is an identification test in which stimuli are presented at a suprathreshold level (Doty et al. 1984). In contrast, the SST score is a composite score of 3 olfactory components: threshold, discrimination, and identification (Hummel et al. 1997). This suggests that the CPT is more sensitive to global olfactory status, including subtle intensity alterations that do not impair smell identification abilities. Our results also demonstrate that the orthonasal CPT score is better in identifying participants with severe or moderate hyposmia from participants with mild hyposmia or with normosmia when compared with the UPSIT. Moreover, Experiment 2 had younger participants with milder forms of OD. Therefore, the poor accuracy of the CPT in this experiment could be due to less extreme score differences between both groups. The next step would be to study test–retest reliability to see if the CPT can monitor OD evolution over time, particularly in these milder forms.
In this study, we also used CPT subscores to objectify olfactory and gustatory subjective complaints. Indeed, both orthonasal and retronasal CPT scores allowed to distinguish between participants with or without olfactory complaint. While there is no surprise regarding the orthonasal CPT scores’ ability to objectify olfactory complaints, results regarding the retronasal CPT scores are of interest. To obtain the retronasal olfactory CPT scores, we asked participants to taste and then rate the perceived taste intensity of peanut butter and fruit jam. Therefore, they were not aware that we were assessing retronasal olfaction (rather than gustation). In fact, the retronasal CPT score classifies participants with or without gustatory complaint with good accuracy—better than the B-WETT. This suggests that subjective GD is very often related to flavor perception and therefore driven by retronasal olfaction (Zang et al. 2019). In other words, we show that many individuals who think they have a gustatory problem suffer in fact from OD, which is in line with earlier reports (Hunt et al. 2019; Hintschich et al. 2020; Hintschich et al. 2022).
In opposition to our initial hypothesis, we did not find any significant association between gustatory CPT scores and B-WETT scores in Experiment 2. The gustatory CPT scores are, therefore, unreliable as a taste test when compared to B-WETT. Different parameters in the test solutions could explain inconsistent results from one participant to another. Participants may have (i) used sugar or salt of different strengths and (ii) used water with different temperatures that could impact the dissolution of tastants or sensitivity. It is also possible that the suggested concentration in our protocol was not optimal for testing purposes. We further noted that a very low proportion of participants in Experiment 2 presented with an objective GD (n = 13; 15%). Future studies should improve taste solutions’ parameters and include more participants with actual GD according to B-WETT.
Limitations of our study include, for Experiment 1, asynchronous testing of participants already informed of a measured OD and, in Experiment 2, a limited number of participants with severe forms of OD. Age differences between both experiments also render comparisons and conclusions more difficult. Limits regarding the newly developed CPT include (i) an expected variability in the household items available (e.g. different kinds of jam), (ii) testing of only 2 out of 5 taste qualities (salty and sweet), and (iii) testing not blinded as we ask for intensity rating of known odorants and tastants.
The CPT was designed during the pandemic, to address issues regarding the accessibility, cost, and safe use of olfactory and gustatory established tests. It is easily self-administered under remote supervision, and it helps to quantify chemosensory complaints using intensity ratings from common household items. Moreover, the CPT is not limited to orthonasal olfactory function. Assessing both orthonasal and retronasal olfaction, as well as gustatory function can help appreciate their respective contribution to subjective olfactory and taste dysfunctions. We do not expect the CPT to replace established and more detailed olfactory and gustatory tests. However, the CPT can be helpful to screen remotely large cohorts of participants (e.g. to estimate OD prevalence in distant locations such as Northern Canada or Australia outback) and/or when in-person testing is unsafe.
Conclusion
The CPT is a new self-administered test that can be used remotely using common household items. Results from 2 experiments support the use of orthonasal and retronasal olfactory CPT scores to objectify OD and to quantify olfactory and gustatory complaints. It is a simple and inexpensive method to obtain a quantitative appreciation of subjective chemosensory complaint from various etiologies and may allow to easily monitor patients’ perspectives and experience of disease.
Supplementary Material
Acknowledgments
We would like to thank Émilie Aubry-Lafontaine, Audrey Fortin, and Cécilia Tremblay for their help during testing. We would also like to thank the participants.
Contributor Information
Cindy Levesque-Boissonneault, Department of Anatomy, Université du Québec à Trois-Rivières, Trois-Rivières, QC, Canada; Department of Speech and Language Pathology, Université du Québec à Trois-Rivières, Trois-Rivières, QC, Canada.
Nicholas Bussière, Department of Anatomy, Université du Québec à Trois-Rivières, Trois-Rivières, QC, Canada; Faculty of medicine, Université de Montreal, Montreal, QC, Canada.
Frédérique Roy-Côté, Research Center of the Sacré-Cœur hospital, CIUSSS Nord-de-l’Île-de-Montréal, Montréal, QC, Canada.
Frank Cloutier, Department of Anatomy, Université du Québec à Trois-Rivières, Trois-Rivières, QC, Canada.
Marie-Ève Caty, Department of Speech and Language Pathology, Université du Québec à Trois-Rivières, Trois-Rivières, QC, Canada.
Johannes Frasnelli, Department of Anatomy, Université du Québec à Trois-Rivières, Trois-Rivières, QC, Canada; Research Center of the Sacré-Cœur hospital, CIUSSS Nord-de-l’Île-de-Montréal, Montréal, QC, Canada; Department of Clinical Neuroscience, Karolinska Institute, Stockholm, Sweden.
Funding
This work was supported by grants from Canadian Institutes of Health Research [PJT 173514; JF] and Natural Sciences and Engineering Research Council of Canada [RGPIN-2015-04597; JF]. CLB, NB and JF had full access to all study data and took responsibility for the data’s integrity and data analysis’ accuracy.
Conflict of interest
None declared.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
- Bagnasco D, Passalacqua G, Braido F, Tagliabue E, Cosini F, Filauro M, Ioppi A, Carobbio A, Mocellin D, Riccio AM, et al. Quick olfactory Sniffin’ Sticks test (Q-Sticks) for the detection of smell disorders in COVID-19 patients. World Allergy Organ J. 2021:14(1):100497–100498. 10.1016/j.waojou.2020.100497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussière N, Mei J, Levesque-Boissonneault C, Blais M, Carazo S, Gros-Louis F, De Serres G, Dupré N, Frasnelli J.. Chemosensory dysfunctions induced by COVID- 19 can persist up to 7 months: a study of over 700 healthcare workers. Chem Senses 2021:46:1–9. 10.1093/chemse/bjab038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussière N, Mei J, Levesque-Boissonneault C, Blais M, Carazo S, Gros-Louis F, Laforce R Jr, De Serres G, Dupre N, Frasnelli J.. persisting chemosensory impairments in 366 healthcare workers following COVID-19: an 11-month follow-up. Chem Senses 2022:47:1–11. 10.1093/chemse/bjac010. [DOI] [PubMed] [Google Scholar]
- Cao AC, Nimmo ZM, Mirza N, Cohen NA, Brody RM, Doty RL.. Objective screening for olfactory and gustatory dysfunction during the COVID‐19 pandemic: a prospective study in healthcare workers using self‐administered testing. World J Otorhinolaryngol Head Neck Surg. 2022:8(3):249–256. 10.1016/j.wjorl.2021.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doty RL. The Smell Identification Test: Administration Manual. Sensonics 2008.
- Doty, RL. Sensonics’ Self-Administered Waterless Empirical Taste Test (SA-WETT). Sensonics. 2020. [Google Scholar]
- Doty RL, Marcus A, William Lee W.. Development of the 12-Item Cross-Cultural Smell Identification Test (CC-SIT). Laryngoscope 1996:106(3):353–356. 10.1097/00005537-199603000-00021. [DOI] [PubMed] [Google Scholar]
- Doty RL, Shaman P, Kimmelman CP, Dann MS.. University of pennsylvania smell identification test: a rapid quantitative olfactory function test for the clinic. Laryngoscope 1984:94(2):176–178. 10.1288/00005537-198402000-00004. [DOI] [PubMed] [Google Scholar]
- Doty RL, Wylie C, Potter M.. Validation of the Waterless Empirical Taste Test (WETT®). Behav Res Methods 2021:53(2):864–873. 10.3758/s13428-020-01463-8. [DOI] [PubMed] [Google Scholar]
- Gerkin RC, Ohla K, Veldhuizen MG, Joseph PV, Kelly CE, Bakke AJ, Steele KE, et al. Recent smell loss is the best predictor of COVID-19 among individuals with recent respiratory symptoms. Chem Senses 2021:46:1–12. 10.1093/chemse/bjaa081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudziol H, Hummel T.. Normative values for the assessment of gustatory function using liquid tastants. Acta Otolaryngol. 2007:127(6):658–661. 10.1080/00016480600951491. [DOI] [PubMed] [Google Scholar]
- Gunn L, Gilbert J, Nenclares P, Soliman H, Newbold K, Bhide S, Wong KH, Harrington K, Nutting C.. Taste dysfunction following radiotherapy to the head and neck: a systematic review. Radiother Oncol. 2021:157:130–140. 10.1016/j.radonc.2021.01.021. [DOI] [PubMed] [Google Scholar]
- Gupta S, Kallogjeri D, Farrell NF, Lee JJ, Smith HJ, Khan AM, Piccirillo JF.. Development and validation of a novel at-home smell assessment. JAMA Otolaryngol–Head Neck Surg. 2022:148(3):252–258. 10.1001/jamaoto.2021.3994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haehner A, Draf J, Dräger S, de With K, Hummel T.. Predictive value of sudden olfactory loss in the diagnosis of COVID-19. ORL 2020:82(4):175–180. 10.1159/000509143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannum ME, Koch RJ, Ramirez VA, Marks SS, Toskala AK, Herriman RD, Lin C, Joseph PV, Reed DR.. Taste loss as a distinct symptom of COVID-19: a systematic review and meta-analysis. Chem Senses. 2022:47:1–17. 10.1093/chemse/bjac001. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Hintschich CA, Niv MY, Hummel T.. The taste of the pandemic—contemporary review on the current state of research on gustation in coronavirus disease 2019 (COVID-19). Int Forum Allergy Rhinol. 2022:12(2):210–216. 10.1002/alr.22902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hintschich CA.Wenzel JJ, Hummel T, Hankir MK, Kühnel T, Vielsmeier V, Bohr C.. 2020. Psychophysical tests reveal impaired olfaction but preserved gustation in COVID-19 patients. Int Forum Allergy Rhinol. 1105-1107:10(97). 10.1002/alr.22655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummel T, Sekinger B, Wolf SR, Pauli E, Kobal G.. ‘Sniffin’ sticks’: olfactory performance assessed by the combined testing of odor identification, odor discrimination and olfactory threshold. Chem Senses. 1997:22(1):39–52. 10.1093/chemse/22.1.39. [DOI] [PubMed] [Google Scholar]
- Hunt JD, Reiter ER, Costanzo RM.. Etiology of subjective taste loss. Int Forum Allergy Rhinol. 2019:9(4):409–412. 10.1002/alr.22263. [DOI] [PubMed] [Google Scholar]
- Iravani B, Arshamian A, Ravia A, Mishor E, Snitz K, Shushan S, Roth Y, Perl O, Honigstein D, Weissgross R, et al. Relationship between odor intensity estimates and COVID-19 prevalence prediction in a Swedish population. Chem Senses. 2020:45(6):449–456. 10.1093/chemse/bjaa034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackman AH, Doty RL.. Utility of a three-item smell identification test in detecting olfactory dysfunction. Laryngoscope 2005:115(12):2209–2212. 10.1097/01.mlg.0000183194.17484.bb. [DOI] [PubMed] [Google Scholar]
- Konstantinidis I, Delides A, Tsakiropoulou E, Maragoudakis P, Sapounas S, Tsiodras S.. Short-Term follow-up of self-isolated COVID-19 patients with smell and taste dysfunction in Greece: two phenotypes of recovery. ORL 2020:82(6):295–303. 10.1159/000511436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larremore DB, Toomre D, Parker R.. Modeling the effectiveness of olfactory testing to limit SARS-CoV-2 transmission. Nat Commun. 2021:12:1–9. 10.1038/s41467-021-23315-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller C, Kallert S, Renner B, Stiassny K, Temmel AFP, Hummel T, Kobal G.. Quantitative assessment of gustatory function in a clinical context using impregnated ‘taste strips’. Rhinology. 2003:41(1):2–6. [PubMed] [Google Scholar]
- Ohla K, Veldhuizen MG, Green T, Hannum ME, Bakke AJ, Moein ST, Tognetti A, Postma EM, Pellegrino R, Hwang DLD, et al. A follow-up on quantitative and qualitative olfactory dysfunction and other symptoms in patients recovering from COVID-19 smell loss. Rhinology. 2022:60(6):207–217. 10.4193/rhin21.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oleszkiewicz A, Park D, Resler K, Draf J, Schulze A, Zang Y, Hähner A, Hummel T.. Quality of life in patients with olfactory loss is better predicted by flavor identification than by orthonasal olfactory function. Chem Senses. 2019:44(6):371–377. 10.1093/chemse/bjz027. [DOI] [PubMed] [Google Scholar]
- Özay H, Çakır A, Ecevit MC.. Retronasal olfaction test methods: a systematic review. Balkan Medical J. 2019:36:49–59. 10.4274/balkanmedj.2018.0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parma V, Hannum ME, O’Leary M, Pellegrino R, Rawson NE, Reed DR, Dalton PH.. SCENTinel 1.0: development of a rapid test to screen for smell loss. Chem Senses. 2021:46:1–11. 10.1093/chemse/bjab012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parma V, Ohla K, Veldhuizen MG, Niv MY, Kelly CE, Bakke AJ, Cooper KW, Bouysset C, Pirastu N, Dibattista M, et al. ; GCCR Group Author. More than smell—COVID-19 is associated with severe impairment of smell, taste, and chemesthesis. Chem Senses. 2020:45(7):609–622. 10.1093/chemse/bjaa041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierron D, Pereda-Loth V, Mantel M, Moranges M, Bignon E, Alva O, Kabous J, Heiske M, Pacalon J, David R, et al. Smell and taste changes are early indicators of the COVID-19 pandemic and political decision effectiveness. Nat Commun. 2020:11:1–8. 10.1038/s41467-020-18963-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowan NR, Soler ZM, Storck KA, Othieno F, Ganjaei KG, Smith TL, Schlosser RJ.. Impaired eating-related quality of life in chronic rhinosinusitis: eating-related QOL in CRS. Int Forum Allergy Rhinolo. 2019:9(3):240–247. 10.1002/alr.22242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumeau C, Nguyen DT, Jankowski R.. How to assess olfactory performance with the Sniffin’ Sticks Test ®. Eur Ann Otorhinolaryngol Head Neck Dis. 2016:133(3):203–206. 10.1016/j.anorl.2015.08.004. [DOI] [PubMed] [Google Scholar]
- Saniasiaya J, Islam MA, Abdullah B.. Prevalence and characteristics of taste disorders in cases of COVID-19: a meta-analysis of 29,349 patients. Otolaryngol–Head Neck Surg. 2021:165(1):33–42. 10.1177/0194599820981018. [DOI] [PubMed] [Google Scholar]
- Snitz K, Honigstein D, Weissgross R, Ravia A, Mishor E, Perl O, Karagach S, Medhanie A, Harel N, Shushan S, et al. An olfactory self-test effectively screens for COVID-19. Commun Med. 2022:2:1–12. 10.1038/s43856-022-00095-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay C, Iravani B, Lafontaine EA, Steffener J, Fischmeister FP, Lundström JN, Frasnelli J.. Parkinson’s disease affects functional connectivity within the olfactory-trigeminal network. J Parkinson’s Dis. 2020:10(4):1587–1600. 10.3233/JPD-202062. [DOI] [PubMed] [Google Scholar]
- Tremblay C, Martel PD, Frasnelli J.. Trigeminal system in Parkinson’s disease: a potential avenue to detect Parkinson-specific olfactory dysfunction. Parkinsonism Relat Disord. 2017:44:85–90. 10.1016/j.parkreldis.2017.09.010. [DOI] [PubMed] [Google Scholar]
- Tremblay C, Mei J, Frasnelli J.. Olfactory bulb surroundings can help to distinguish Parkinson’s disease from non-parkinsonian olfactory dysfunction. NeuroImage Clin. 2020:28:102457–102411. 10.1016/j.nicl.2020.102457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaira LA, Gessa C, Deiana G, Salzano G, Maglitto F, Lechien JR, Saussez S, Piombino P, Biglio A, Biglioli F, et al. The effects of persistent olfactory and gustatory dysfunctions on quality of life in long-COVID-19 patients. Life 2022:12:141–113. 10.3390/life12020141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaira LA, Salzano G, Petrocelli M, Deiana G, Salzano FA, De Riu G.. Validation of a self‐administered olfactory and gustatory test for the remotely evaluation of COVID‐19 patients in home quarantine. Head Neck 2020:42(7):1570–1576. 10.1002/hed.26228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandersteen C, Payne M, Dumas L-E, Plonka A, D’Andréa G, Chirio D, Demonchy E, Risso K, Robert P, Fernandez X, et al. What about using Sniffin’ Sticks 12 items test to screen post-COVID-19 olfactory disorders? Eur Arch Otorhinolaryngol. 2021:279:3477–3484. 10.1007/s00405-021-07148-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Bartheld CS, Hagen MM, Butowt R.. Prevalence of chemosensory dysfunction in COVID-19 patients: a systematic review and meta-analysis reveals significant ethnic differences. ACS Chem Neurosci. 2020:11(19):2944–2961. 10.1021/acschemneuro.0c00460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker M, Elliott J, Bodinier B, Barclay W, Ward H, Cooke G, Donnelly CA, Chadeau-Hyam M, Elliott P.. Variant-specific symptoms of COVID-19 in a study of 1,542,510 adults in England. Nat Commun. 2022:13:1–10. 10.1038/s41467-022-34244-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zang Y, Han P, Burghardt S, Knaapila A, Schriever V, Hummel T.. Influence of olfactory dysfunction on the perception of food. Eur Arch Otorhinolaryngol. 2019:276(10):2811–2817. 10.1007/s00405-019-05558-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.