Summary
Background
Lymphedema affects one in six breast cancer survivors making it a global healthcare challenge. There is considerable debate about the efficacy of different treatments for lymphedema. We aimed to summarize the current evidence for treatments for lymphedema in breast cancer survivors.
Methods
In this overview of systematic reviews with meta-analyses (SRMAs), five databases were searched for SRMAs of randomised controlled trials (RCTs) reporting effects of medications, surgery, exercise, laser therapy, acupuncture, kinesio taping, or complex decongestive physiotherapy (CDP) for breast cancer-related lymphedema published from database inception up to March 7, 2023. Data extraction was performed for the SRMAs and RCTs, and SRMAs were appraised with AMSTAR2. Random effects meta-analyses of the RCTs provided estimates of the pooled effects sizes (Hedges’ g) for each treatment modality. This study is registered with PROSPERO, CRD42020184813.
Findings
1569 studies were identified by the search and eighteen SRMAs with 51 RCTs were included, investigating manual lymphatic drainage (MLD), compression pump, exercise, kinesio taping, laser, and acupuncture. Overall, the methodological quality of the SRMAs was low. SRMAs reached different conclusions for all treatment modalities, except for kinesio taping where the two SRMAs found no effect. The analysis of 40 RCTs with 1970 participants revealed a small effect across all interventions compared to any control (g = 0.20, p = 0.047, I2 = 0.79), corresponding to volume reductions of 119.7 ml (95% CI 135–104) and 88.0 ml (95% CI 99–77) in the intervention and control groups, respectively, and a small effect of exercise (g = 0.26, p = 0.022, I2 = 0.44). The between-group differences in volume reduction were small and did not reach statistical significance for any one treatment modality.
Interpretation
Based on the available data, there is no evidence of superiority of any one treatment on volume reduction nor any solid research refuting these treatments. Thus, definitive conclusions to inform clinical practice about the efficacy of these treatments cannot be drawn. Due to poor-quality evidence, more research is needed to untangle the efficacy of each treatment component for different stages of lymphedema.
Funding
Danish Cancer Society.
Keywords: Complex decongestive physiotherapy, Exercise, Laser therapy, Kinesio taping, Acupuncture
Research in context.
Evidence before this study
Numerous systematic reviews with meta-analysis (SRMAs) of treatment interventions for breast cancer-related arm lymphedema (BCRL) have been undertaken. Overviews of reviews synthesize evidence dispersed across multiple reviews and thus may better inform decision making in health care. We searched PubMed for overviews of systematic reviews of treatment interventions for BCRL from the inception of the database to March 27th, 2023, with the search terms “breast cancer” AND “lymphedema” AND “overview”. Two overviews were identified. Both were intervention-specific, namely for acupuncture (Zhang 2022) and laser therapy (Wang 2022) and report conflicting evidence for the benefit of these treatment modalities due to insufficient data and poor-quality evidence. To our knowledge, no overview has synthesized evidence across all treatment modalities for BCRL. We therefore performed an overview of systematic reviews with meta-analysis (SRMAs) of randomised controlled trials (RCTs) for surgery, medication, exercise, laser therapy, kinesio taping, acupuncture, and complex decongestive physiotherapy for treatment of BCRL.
Added value of this study
Across treatment modalities, the SRMAs all arrive at conflicting conclusions except for the SRMAs on kinesio taping, where both conclude that this modality does not provide benefit compared to control conditions. Our meta-analyses of the RCTs included in the SRMAs find a small effect across all interventions compared to any control (active and non-active) (Hedge’s g = 0.20; p = 0.047) and a small effect of exercise (Hedge’s g = 0.26, p = 0.022). However, the overall mean between-group difference in volume reduction was not clinically meaningful (6.1 ml, p = 0.039) and statistically non-significant for all individual treatment modalities.
Implications of all the available evidence
The available evidence does not allow for definitive conclusions regarding the efficacy of currently used treatments for reducing volume in BCRL. Health care providers should not consider the lack evidence a reason to refute these treatments to patients. Instead, it is recommended to consider cost and time effectiveness as well as closely monitor benefit with reliable, valid, and sensitive measures of improvement. Suggestions are provided of how the research community should address the shortcomings of existing evidence.
Introduction
Breast cancer-related lymphedema (BCRL) is a debilitating complication of cancer treatment, primarily from surgical removal of axillary lymph nodes and radiation of the axilla, characterized by progressive swelling typically of the arm or hand affecting more than one in six breast cancer survivors.1,2 Due to the chronic nature of BCRL, it is one of the most feared sequelae with significant personal and societal impact on those affected.3 This has increased the interest in research in preventive and treatment strategies for BCRL.
Concurrent with the development of less invasive treatments for breast cancer, the field of lymphedema management has evolved. Treatment options for patients with cancer-related lymphedema aim to reduce excess volume of the arm and symptoms of BCRL (e.g., heaviness and swelling) and consequently reduce the lymphedema severity. Complex decongestive physical therapy (CDP) uses the following modalities in isolation or combination: a) compression bandaging or garments and therapeutic exercise to reduce infiltration, remove fluid, and remodel the limb; b) manual lymphatic drainage (MLD), a specialized massage technique to encourage lymph flow; c) skin care to protect and moisturize skin to avoid cuts and infections; and d) patient education. CDP is divided into two phases, one aiming to reduce volume and symptoms especially through compression bandaging, the second using a personalized compression garment with the aim of stabilizing the condition. Other treatment modalities for BCRL include acupuncture,4 kinesio taping,5,6 laser therapy,7,8 pneumatic compression pumps,9 cardio and resistance exercise,10 and surgical approaches.11,12
There continues to be considerable debate about the efficacy of different approaches to treatment for lymphedema, particularly regarding the efficacy of MLD.13 To provide optimal treatment, health care professionals need to be able to identify the most effective evidence-based treatment options taking into account patient preferences, resources, and clinical setting.
Evidence relating to the efficacy of interventions for BCRL is often synthesized in intervention-specific systematic reviews. Overviews of reviews have the potential to improve access to evidence dispersed across multiple reviews14 and address the growing problem of information overload by providing a way to filter large bodies of complex evidence, thereby informing decision-making in health care. To date, two overviews have assessed the evidence on the effectiveness of laser therapy15 and acupuncture16 for treatment of BCRL, while no overviews have compiled evidence across treatment modalities.
The aim of this overview of systematic reviews with meta-analyses (SRMAs) was to summarize the evidence for effects of different treatment modalities for lymphedema in breast cancer survivors.
Methods
Search strategy and selection criteria
This study was conducted in accordance with the PRISMA recommendations.17 The protocol was pre-registered with PROSPERO (CRD42020184813).
A search for SRMAs of randomised controlled trials (RCTs) was conducted in the databases of PubMed, Cochrane, Embase, CINAHL, and Web of Science from the inception of the database to March 7th, 2023. Search terms included “breast cancer”, “lymphedema”, “meta-analysis”, and “systematic review”, adapted to each database (Supplementary File 1). An additional manual search was conducted in the reference lists of the identified SRMAs.
After removal of duplicates, Covidence18 was used for independent screening by two reviewers (BSR, AB). The inclusion criteria were as follows. Population: adults treated for any stage breast cancer with BCRL. Intervention: Surgical (e.g., liposuction, lymphovascular anastomosis, lymph node transfer), pharmacological (e.g., anti-inflammatory agents, immunosuppressive agents), exercise (e.g., aerobic, resistance, yoga), CDP (e.g., one or a combination of treatment modalities of CDP), laser therapy, kinesio taping, and acupuncture. Comparator: Any control group (e.g., treatment-as-usual, placebo, active control). Outcome: Any measure of lymphedema severity (e.g., volume, bioimpedance, symptoms, or function). The included SRMAs were restricted to systematic reviews with meta-analyses of RCTs.
The data extracted included patient characteristics, type of intervention, control, and outcome measures, the SRMAs risk of bias assessments, as well as characteristics and results of the RCTs in the SRMAs. Data extraction was performed independently by two researchers (TA, SB) using predefined extraction forms. Conflicts were solved by a third researcher (BSR or AB). Overlap of RCTs between SRMAs was explored and a quality check was done by comparing the data reported by SRMAs of the RCTs that were included in multiple SRMAs. Due to the large number of RCTs for which the SRMAs reported conflicting data, the RCTs were identified and the results from the original source extracted and used for meta-analysis. The RCT data extracted included sample sizes, age, lymphedema volume at baseline, mean lymphedema volume changes, duration of follow-up, percent mastectomies, and percent receiving axillary lymph node dissection (ALND). Summary data provided in figures only was extracted using WebPlotDigitizer (version 4.2).
AMSTAR 2 was used for critical appraisal of the included SRMAs.19 AMSTAR 2 consists of 16 items of which seven items are considered critical. The overall methodological quality is summarized as “high”, “moderate”, “low”, or “critically low”. The evaluation was performed independently by two researchers (BSR, AB) and negotiated until consensus.
Data analysis
Hedges’ g, a variation of Cohen’s d, was used as the standardized effect size (ES). It has better properties for small samples and when the sample sizes of the groups compared differ significantly.20 The conventions regarding the magnitude of Hedges’ g are the same as for Cohen’s d, i.e., 0.2, 0.5, and 0.8 correspond to small, medium, and large effect sizes.21 The ESs were pooled using a random-effects model in all analyses, with positive values indicating ESs in the hypothesized direction. If heterogeneity statistics (Q and I2)22 indicated heterogeneous ESs (I2 > 0.0), we calculated the 95% prediction interval.23 Possible sources of heterogeneity were explored by examining the ESs of subgroups of studies according to the following study characteristics: a) control type (active (e.g., home exercise program, compression garment) vs non-active (e.g., placebo)), and b) treatment modality. RCTs testing treatment modalities of MLD, compression garment, and compression pump were grouped as CDP. Continuous moderators were analysed with meta-regression, including age (years), lymphedema duration (months), lymphedema volume at baseline (ml), percent mastectomised, and percent receiving ALND. The possibility of publication bias was evaluated with funnel plots and Egger’s method.24 The analyses were conducted using Comprehensive Meta-Analysis, Version 3.25
To aid the interpretation of the results, we conducted Bayesian Model-Averaged meta-analyses26 as a supplement to the statistically significant results found in the conventional frequentist analyses (Supplementary File 8).
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. All authors had full access to all the data and have final responsibility for the decision to submit for publication.
Results
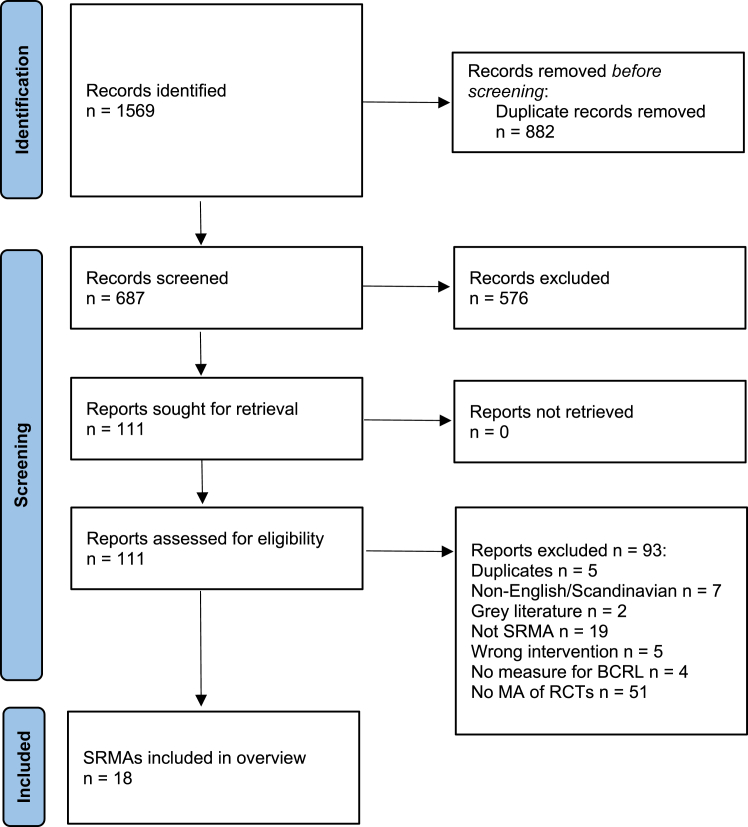
A total of 1569 articles were identified, leaving 687 articles after removal of duplicates. After screening titles and abstracts, 111 possibly relevant studies were subjected to full-text review, leaving 18 SRMAs for inclusion (Fig. 1). Supplementary File 2 provides a list of excluded SRMAs with reasons. In the 18 SRMAs, the following treatment modalities were investigated: MLD (k = 7)10,13,27, 28, 29, 30, 31; compression pump (k = 3)9,32,33; multimodal approaches (k = 1)34; exercise (k = 2)10,35; kinesio taping (k = 2)5,6; laser therapy (k = 2)7,8; and acupuncture (k = 2)4,36 (Table 1). No SRMA investigated pharmaceutical or surgical interventions.
Fig. 1.
Flow chart.
Table 1.
Characteristics of systematic reviews with meta-analyses.
| Author, year | Intervention | Aim | Inclusion | Exclusion | Funding |
|---|---|---|---|---|---|
| Shao, 201727 | MLD | To determine if addition of MLD to the standard therapy is more effective in treatment of breast cancer related lymphedema | >10% or 2 cm or 150 ml volume difference | Non-randomized studies | None |
| McNeely, 201130 | MLD | To update the evidence from RCTs concerning the benefits of conservative interventions for all cancer-related lymphedema | Lymphedema secondary to cancer | Non-randomized studies Grey literature |
Partially funded by Alberta Health Services Cancer Care |
| Huang, 201328 | MLD | To evaluate the effectiveness of MLD in the prevention and treatment of breast-cancer-related lymphedema | Axillary lymph-node dissection | Only sentinel node performed | None |
| Ezzo, 201529 | MLD | To assess the efficacy and safety of MLD in treating breast cancer related lymphedema | >2 cm or 200 ml or 10% excess volume | Use of non-manual form (i.e., electronic) of lymphatic drainage, use of different type of massage instead of MLD, use of MLD in both groups, altering more than the MLD component, prevention therapy | Manheimer and Ezzo partially funded by NCCAM (National Center for Complementary and Alternative Medicine) of the US National Institutes of Health |
| Qiao, 202331 | MLD | To evaluate the efficacy of MLD on postmastectomy BCRL | RCT, >18 years, >150 ml increase compared with preoperatively or >2 cm or 10% excess volume | <20 patients in trial, patients with serious complications, non-English literature | None |
| Liang, 202013 | MLD | To evaluate the effect of MLD on the treatment and prevention of lymphedema after breast cancer surgery | RCT, clearly defined definition of lymphedema | Grey literature | Scientific Research Foundation of Hunan Provincial Health Commission (grant no. C2017004) |
| Rogan, 201632 | Compression pump and Exercise | To evaluate the effects of compression bandages, sleeves, intermittent pneumatic compression (IPC) and active exercise on the reduction of breast cancer-related lymphedema | Mentioned one of the following keywords in the title or abstract: lymphoedema, women, mastectomy, axillary dissection, or breast cancer | Non-breast cancers, lower extremity lymphoedema, impact on fatigue only, prevention therapy | NR |
| Shao, 20149 | Compression pump | To determine if intermittent pneumatic pump manage lymphedema effectively | >10% or 2 cm excess volume | Non-randomization | None |
| Li, 202233 | Compression pump | To compare the effects of compression therapy and routine nursing in treatment of BCRL | RCT, ≥18 years, both male and female, BRCL | Repeated publications, grey literature, incorrect or incomplete data, or the evaluation index could not be obtained, non-Chinese or English publications, animal experiments | Key Project of Scientific Research in Universities in 2020, no. KJ2020A0221. |
| Rangon, 202234 | Complex physical therapy | Investigate the immediate, short-term, and long-term effects of complex physical therapy and multimodal approaches on BCRL | RCT, a minimum difference of 2 cm and 10% between the upper limbs | Non-English literature | NR |
| Chen, 20198 | Laser therapy | To analyse the effectiveness and safety of laser therapy for the treatment of BCRL | RCT, breast cancer related lymphedema | Studies with inclusion of patients with medical conditions, such as current metastases, pregnancy, photosensitivity, chronic inflammatory diseases, and history of severe trauma | None |
| Smoot, 20157 | Laser therapy | To examine the effectiveness of later therapy in reducing BCRL | Breast cancer related lymphedema, LLLT alone or in combination with other treatments | Primary lymphedema, case studies | Smoot partially supported by the Building Interdisciplinary Research Careers in Women’s Health (BIRCWH) K12, Grant Number K12HD052163 NICHD/NIH, and by the National Center for Advancing Translational Sciences, National Institutes of Health, through UCSF-CTSI Grant Number KL2TR000143 |
| Lytvyn, 202010 | Exercise and MLD | To evaluate the most common conservative lymphedema treatment strategies for treating cancer-related extremity lymphedema | RCT, ≥18 years, cancer-related extremity lymphedema, published in English | Primary lymphedema, patients at risk of lymphedema | Sadeghirad received a graduate student stipend from Mitacs Canada and received funding from PIPRA AG |
| Yeung, 201835 | Exercise | To review the evidence of aquatic therapy in the management of lymphedema | Lymphedema (primary or secondary) | Non-randomization | None |
| Kasawara, 20186 | Kinesio taping | To evaluate the effects of KT on lymphedema related to breast cancer | Lymphedema after mastectomy | Case reports, congenital or primary lymphedema related to types of cancer other than breast cancer, lymphedema in the lower limbs | NR |
| Gatt, 20175 | Kinesio taping | To determine the effectiveness and safety of KT in the management of cancer related lymphedema | Cancer-related lymphedema | Primary lymphedema, non-randomized | Gatt supported by Malta Government Scholarship Scheme |
| Hou, 20194 | Acupuncture | To explore the efficacy and safety of acupuncture treatment | Breast cancer, >2 cm excess volume | Pregnant or lactating women, patients with severe damage to the heart, liver, and kidney; abnormal bone marrow function; skin ulceration of the affected limb and infectious skin diseases; severe mental disorders, and so forth | Young Scientists Fund of the National Natural Science Foundation of China (No. 81804193), the Youth Natural foundation of the Science and Technology Department of Jiangsu Province (No. BK20161083), The Leading Talents Project from Jiangsu Provincial Administration of TCM (SLJ0206) and the peak talent project of Jiangsu Province Hospital of TCM (y2018rc05) |
| Jang, 202036 | Acupuncture | To evaluate the effectiveness of acupuncture for treatment-induced symptoms in breast cancer patients | Peer-reviewed RCTs. Any stage of breast cancer, including patients with metastasis | Observational studies, cohort studies, case reports, case series, non-RCT studies, animal, and experimental studies | Traditional Korean Medicine R&D Program funded by the Ministry of Health & Welfare through the Korea Health Industry Development Institute (KHIDI) (HB16C0072) |
There was substantial overlap in RCTs across the 18 SRMAs (Supplementary File 3), which precluded statistically summarizing the effect through estimates at the levels of SRMAs, as there is no established standard to statistically take into account overlap in included RCTs.37 Specifically, 19 of the 51 RCTs were included in multiple SRMAs. Seven of the eight RCTs that assessed acupuncture were Chinese language publications, three RCTs did not report sufficient data to be included in the meta-analysis, while the full text of one RCT could not be retrieved, leaving 40 RCTs to be included in the meta-analysis. The RCTs included between seven and 90 participants in each group and were generally rated by the SRMAs as having high risk of bias. In terms of BCRL severity, the mean lymphedema duration was 33.9 months and the excess volume of the arm at baseline was 684.3 ml (min 82; max 2107) across the 21 RCTs that provided this data (Supplementary File 4).
The quality check identified incorrect data reported by eight SRMAs. This included incorrect reporting of direction of effect (i.e., inconsistency in direction of effect between figures and text13,27,31), and inconsistent data (i.e., number of participants,8,27,31 inconsistency in use of SE and SD,6 incorrect reporting of outcome data,5,29 and pooling of results with different units (i.e., ml and percentage reduction).6,7
Critical appraisal of the systematic reviews with meta-analyses
Overall, the methodological quality appraised with AMSTAR 2 was low (Supplementary File 5). Only four of the SRMAs included an explicit statement that a protocol was written prior to conduct of review (item 2), four provided a list of excluded studies (item 7), and seven investigated publication bias (item 15).
Complex decongestive physiotherapy, manual lymphatic drainage, and compression pumps
Eleven SRMAs investigated different components of CDP. Of these, seven SRMAs with a total of eleven RCTs investigated the effect of MLD in addition to standard care10,13,27, 28, 29, 30, 31 and showed mixed results on volume reduction (Table 2). Two SRMAs found significant improvements in volume reduction,27,30 while four showed no difference.10,28,29,31 For all seven SRMAs, the effects were small to moderate. One subgroup analysis found significant volume reduction for patients with mild BCRL while not for moderate or severe BCRL29 and for interventions of >20 sessions or duration of ≥2 weeks.31 Two SRMAs investigated the effect of MLD on pain and found no difference.31,34 Three SRMAs of three RCTs investigated the effect of compression pumps in addition to standard care. Two SRMAs found no difference,9,33 while one showed a small effect of additional compression pump.32 One SRMA investigated the effect of compression pumps on range of motion and found significant improvements.33 Rangon et al. included four RCTs and investigated the effect of complex physical therapy (combinations of MDL, compression, kinesio taping, and exercise) compared to control conditions consisting of other elements of CDP (typically fewer modalities).34 No difference was seen for lymphedema volume reduction or physical function, while the control condition was favored for pain reduction. No SRMA reported on the effect of compression bandages or garments only.
Table 2.
Results of systematic reviews with meta-analyses.
| Author, year | Intervention | Control | K | N | Outcome | Main result |
|---|---|---|---|---|---|---|
| Manual lymphatic drainage | ||||||
| Shao, 201727 | MLD | Standard therapy (variety of compression garments, exercise, skin care, education) | 3 | 181 | Primary: volume reduction. Secondary: symptoms and arm function | Significant volume reduction by adding MLD. Mean difference 72.10 (95% CI 13.65–130.55) No subgroup analyses |
| McNeely, 201130 | MLD + compression garment or bandage + self-massage for some | Compression bandage or garment and self-massage for some | 5 | 198 | Primary: volume reduction. Secondary: QoL, function, and lymphedema symptoms (pain, tension, heaviness, discomfort) | Significant effect of addition of MLD to treatment (effect 0.37, p-value 0.02) No subgroup analyses |
| Lytvyn, 202010 | MLD, compression garment, compression pump or combination | Standard therapy | 4 | 276 | Primary: volume reduction. Secondary: fatigue, function, lymphedema symptoms, pain, QoL | Network meta-analysis. Low to very low evidence of effect of conservative treatment |
| Huang, 201328 | MLD + standard care | Compression or simple lymphatic drainage or sequential pneumatic compression or a combination | 6 | 237 | Volume reduction | No significant effect of additional MLD (mean difference 75.12; 95% CI −9.34 to 159.58) No subgroup analyses |
| Ezzo, 201529 | MLD + compression bandaging | Compression bandaging | 2 | 83 | Primary: volumetric changes in arm, hand, breast, or trunk; adverse effects. Secondary: functional measures (Range of motion, strength), subjective sensations, QoL, cost of care, any other outcome reported by the trial | No significant difference in volume reduction. Mean difference 26.21 ml (95% CI −1.04 to 53.45) Subgroup: significant percent volume reduction (MD 12.09%; 95% CI 0.15–24.04%, n = 36, p = 0.05) for mild BCRL (defined as <23% excess volume) but not for moderate/severe. No difference in relation to BCRL duration |
| Liang, 202013 | MLD | Compression bandaging or standard therapy | 8 | 338 | Volume reduction | No significant difference in volume reduction. SMD −0.09 (95% CI −0.85 to 0.67) Subgroup: significant volume reduction for those <60 years: SMD −1.77 (95% CI −2.23 to −1.31; k = 2, n = NR) and when treatment >4 weeks: SMD: −1.77 (95% CI −2.23 to −1.30; k = 4, n = NR). No difference for research region, publication year, sample size, type of surgery, ≥60 years, ≤4 weeks treatment, or the statistical analysis method |
| Qiao, 202331 | MLD | Compression bandaging or standard therapy | 8 | 457 | Primary: volume or circumference reduction. Secondary: lymphedema symptoms, anxiety, mobility, QoL | No significant difference in volume reduction. SMD 0.43 (95% CI −0.10 to 0.96) Subgroup: Significant volume reduction in favor of MLD when duration >2 weeks SMD 0.23 (95% CI 0.02–0.44; k = 5 n = 347) or ≥20 sessions SMD 0.33 (95% CI 0.03–0.58; k = 3 n = 213). No difference for pain. SMD −0.09 (95% CI −0.43 to 0.25) |
| Rangon, 202234 | Complex physical therapy | Multimodal approaches | 7 | 690a | Primary: volume reduction. Secondary: pain, physical function | Significant reduction in volume. SMD −0.18 (95% CI 0.35–0.00). No difference for pain or function |
| Compression pump | ||||||
| Rogan, 201632 | Compression pump | Standard therapy | 2 | 135 | Volume reduction | Volume reduction with additional use of pump. SMD −0.54 (95% CI −1.01 to −0.064). Other analyses include women at risk of BCRL and are thus not relevant here |
| Li, 202233 | Compression pump + CDP | CDP | 3 | 159 | Morbidity of lymphedema, volume reduction, range of motion | No difference in volume reduction. Mean difference 4.51 (95% CI −7.01 to 16.03) Significant improvement in range of motion |
| Shao, 20149 | Compression pump | MLD | 3 | 159 | Primary: percent of volume reduction. Secondary: subjective symptoms and joint mobility | No difference in adding compression pump to MLD. Mean percent difference 4.51 (95% CI −7.01 to 16.03) No subgroup analyses |
| Laser therapy | ||||||
| Chen, 20198 | Laser therapy | No treatment or conventional therapy group (including compression garments, MLD, and remedial exercises) | 6 | 239 | Primary: arm circumference, volume. Secondary: grip strength, pain scores | No difference in volume reduction. SMD 0.04 (95% CI −0.32 to 0.41). No difference in arm circumference: SMD −0.47 (95% CI −1.34 to 0.39) Subgroup: No difference in strength or pain |
| Smoot, 20157 | Laser therapy | Sham laser or no treatment or compression | 4 | 138 | Upper extremity swelling and pain | Reduction in volume with addition of laser. Pooled effect size −0.62 (95% CI −0.97 to −0.28) No difference in pain |
| Exercise | ||||||
| Yeung, 201835 | Water based exercise | Any comparison intervention, including standard care, habitual activity, wait and see, or alternative land-based exercise | 2 | 66 | Lymphedema limb volume measured by water displacement, perometer, or circumferential tape measure, tissue fluid measurement via TDC or BIS, physical function (strength, ROM), symptoms (e.g., pain, heaviness, tightness), QoL | No difference in volume reduction. SMD 0.14 (95% CI −0.37 to 0.64) No difference in function |
| Lytvyn, 202010 | Exercise (resistance, aerobic, yoga or water based) | Standard therapy | 11 | 523 | Primary: volume reduction. Secondary: fatigue, function, lymphedema symptoms, pain, quality of life | Network meta-analysis. Low to very low evidence of effect of exercise |
| Kinesio taping | ||||||
| Kasawara, 20186 | Kinesio taping + standard treatment | Standard treatment (variety of MLD, CDP, compression pump) | 6 | 199 | Lymphedema limb volume measured by perimetry or volumetry | No difference between KT and control in reduction of lymphedema volume (SMD 0.04; 95% CI −0.24 to 0.33) No subgroup analyses |
| Gatt, 20175 | Kinesio taping | Compression bandaging or compression hosiery with or without CDP (complete decongestive physiotherapy) | 4 | 159 | Primary: limb volume and/or circumference, adverse effects. Secondary: patients’ subjective experience, severity of lymphedema symptoms, QoL | No difference in volume reduction. Mean difference −413.45 ml (95% CI −896.55 to 69.64) No difference in QoL, discomfort, itching |
| Acupuncture | ||||||
| Hou, 20194 | Acupuncture | Non-acupuncture therapy, including Western medicine, functional exercise, and sham acupuncture | 5 | 374 | Total effective rate, extent of lymphedema, adverse effects | Significant improvement in total effective rate with acupuncture. OR 4.62 (95% CI 2.61–8.17) Subgroup: significant improvement in arm circumference. Mean difference 0.79 (95% CI 0.57–1.01) |
| Jang, 202036 | Manual acupuncture, ear acupuncture, and electro-acupuncture | Sham acupuncture, medicine (venlafaxine, hormone therapy), exercise, relaxation, enhanced self-care, no-treatment, and wait-list control groups | 2 | 133 | Climacteric symptoms, pain, nausea and vomiting, lymphedema (level of edema, arm circumference), neuropathic pain, cognitive impairment, and gastrointestinal symptoms | Significant reduction in arm circumference in control group. Mean difference −1.61 cm (95% CI −1.92 to −1.31) No subgroup analyses |
BIS = bioimpedance spectroscopy; CDP = complex decongestive physiotherapy; 95% CI = 95% confidence interval; K = number of RCTs; KT = kinesio taping; MLD = manual lymphatic drainage; N = total number of participants in the RCTs; QoL = quality of life; ROM = range of motion; SMD = standardized mean difference; TDC = tissue dielectric constant.
The meta-analysis by Rangon et al. includes the same RCTs multiple times and thus is the sample size (n) not the number of unique participants.
Laser therapy
Two SRMAs of seven RCTs investigated the use of laser therapy and reached different conclusions.7,8 Chen et al. found no difference in limb volume reduction, grip strength, or pain.8 Smoot et al. found volume reduction in favor of laser therapy, but no effect on pain reduction.7
Exercise
Two SRMAs of twelve RCTs investigated the effect of exercise.10,35 Yeung et al. included RCTs with aqua exercise35 and found no difference in volume or upper-body physical function. Similarly, Lytvyn et al. found little to no evidence of effect on volume from resistance exercise, aerobic plus resistance exercise, and water-based or yoga exercise.10
Kinesio taping
Two SRMAs of six RCTs investigated the effect of kinesio taping and found no difference in volume reduction5,6 and no difference in quality of life (QoL) or discomfort.5
Acupuncture
Two SRMAs of nine RCTs assessed the effect of acupuncture and reached different conclusions.4,36 Hou et al. favored acupuncture compared to western medicine (CDP and exercise).4 Jang et al. found a significantly larger volume reduction in the control group.36
Meta-analysis of the randomised controlled trials
Meta-analysis of data from the 40 RCTs was performed to explore the efficacy in reducing volume of different treatment modalities. The RCTs investigating acupuncture were not included, as all but one paper was Chinese language. A small overall effect was observed across all interventions compared to any type (active and non-active) of control condition (Hedges’s g = 0.20, I2 = 0.79, p = 0.047) (Table 3, Supplementary File 6). This result remained statistically significant when excluding three outliers in a sensitivity analysis (Hedge’s g = 0.24, I2 = 0.66, p = 0.004). With respect to individual treatment modalities, a statistically significant pooled effect was observed for exercise compared to various control conditions (Hedge’s g = 0.26, I2 = 0.44, p = 0.022). The effects of other modalities failed to reach statistical significance. It was possible to meta-analyse the effect of exercise on QoL while there were not sufficient data to conduct meta-analyses for other secondary outcomes. Compared with the control conditions, the combined effect on QoL of exercise was small and statistically non-significant (Hedges g = 0.10, I2 = 0.10, p = 0.43) (Supplementary File 7). Funnel plots and Eggers’ tests showed no indications of publication bias.
Table 3.
Pooled effect sizes of randomized controlled trials of the effect of treatment on lymphedema volume.
| Sample sizea |
Heterogeneityb |
Mean diff (ml) | Combined effect sizec |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| K | N | Q | p | I2 | Tau2 | Hedge’s gd | 95% CI | p | 95% PIe | ||
| Overall effect (all interventions vs. all comparisons) | 40 | 1970 | 184.1 | <0.001 | 0.79 | 0.31 | −6.1 | 0.20 | 0.00–0.40 | 0.047 | −0.95 to 1.35 |
| Overall effect (w. Sensitivity analysis)f | 37 | 1857 | 106.4 | <0.001 | 0.66 | 0.16 | −4.5 | 0.24 | 0.08–0.40 | 0.004 | −0.59 to 1.07 |
| Overall effect vs. active control | 30 | 1573 | 165.6 | <0.001 | 0.83 | 0.38 | −10.5 | 0.20 | −0.05 to 0.44 | 0.12 | −1.09 to 1.49 |
| Overall effect vs. non-active control | 10 | 397 | 18.3 | 0.032 | 0.51 | 0.11 | −3.8 | 0.23 | −0.06 to 0.52 | 0.12 | −0.61 to 1.07 |
| Exercise vs. all comparisons | 13 | 684 | 21.4 | 0.022 | 0.44 | 0.06 | −7.2 | 0.26 | 0.04–0.47 | 0.022 | −0.33 to 0.85 |
| Kinesio taping vs. all comparisons | 4 | 146 | 15.9 | <0.001 | 0.81 | 0.50 | +453.4 | −0.46 | −1.23 to 0.31 | 0.24 | −3.94 to 3.02 |
| Laser vs. all comparisons | 4 | 179 | 11.8 | 0.008 | 0.75 | 0.28 | −5.4 | 0.50 | −0.11 to 1.12 | 0.11 | −2.15 to 3.15 |
| CDP (Complex Decongestive physiotherapy) (vs. all) | 13 | 685 | 102.3 | <0.001 | 0.88 | 0.62 | −19.6 | 0.31 | −0.15 to 0.77 | 0.18 | −1.49 to 2.12 |
| MLDg + compression vs. compression | 6 | 336 | 30.1 | <0.001 | 0.83 | 0.38 | +5.7 | −0.20 | −0.75 to 0.35 | 0.48 | −2.08 to 1.68 |
| MLD + compression + pump vs. MLD + compression | 3 | 159 | 12.5 | <0.001 | 0.84 | 0.56 | −4.6 | 0.36 | −0.57 to 1.29 | 0.44 | −10.90 to 11.62 |
P-values of <0.05 are marked with bold.
K indicates number of published studies.
Q statistic: p values <0.1 taken to suggest heterogeneity; I2 statistic: 0% (no heterogeneity), 25% (low heterogeneity), 50% (moderate heterogeneity), 75% (high heterogeneity).
Effect size = Hedge’s g. Standardized mean difference, adjusting for small sample bias. A positive value indicates an effect size in the hypothesized direction, e.g., larger reduction in volume or a relative smaller increase in volume in the intervention group. To ensure independency, if a study reported results for more than one measure, the effect sizes were combined (mean), ensuring that only one effect size per study was used in the calculation.
If analyses (Eggers test: p < 0.05) had indicated the possibility of publication bias, missing studies would have been imputed and an adjusted effect size rate calculated with a random effects model. However, for the effects with a sufficient number of studies (K ≥ 10), there were no robust indications of publication bias.
PI = 95% prediction interval: The interval in which 95% of future observations will fall, given the observed data, calculated for heterogeneous ESs (I2 > 0).
Sensitivity analysis(Hedges’ g): Omitting three studies (Andersen et al., 2000, Smykla et al. 2013, and Sitzia et al., 2002) with effect sizes 2 standard deviations away from the pooled effect size (0.22 ± 1.57: <−1.35 OR >1.79). Sensitivity analyses (mean diff. ml): Omitting one study (Smykla et al., 2013).
MLD = manual lymphatic drainage.
The moderator analyses suggested that the effects on volume reduction were: a) smaller in patients with ALND for all interventions; b) larger for CDP in older patients; and c) larger for CDP in patients with longer lymphedema duration (Supplementary File 8).
The supplementary Bayesian meta-analyses confirmed the weak evidence for the efficacy of lymphedema treatments (Supplementary File 9). The results indicated only anecdotal evidence for an overall effect of treatment (k = 40), with an effect different from zero being only 1.85 times more likely than the null hypothesis. Likewise, the effect of exercise being different from zero was only 2.28 times more likely than the null hypothesis (k = 13). Similarly, while the analysis of CDP favored the null hypothesis, it was only 2.31 times more likely than the alternative hypothesis.
Across all interventions, the volume reduction was 119.7 ml (95% CI 135.0–104.5) for the intervention groups and 88.0 ml (95% CI 99.0–77.1) for the control groups (Table 4). While the overall mean between-group difference in volume reduction was small (6.1 ml) but statistically significant (p = 0.039), the mean between-group differences in volume reduction were small and non-significant for all individual treatment modalities.
Table 4.
Volume reduction and between-group difference of randomized controlled trials.
| Sample sizea |
Mean volume reduction (ml)b |
||||
|---|---|---|---|---|---|
| Kc | N | Intervention (95% CI) | Control (95% CI) | Differenced (95% CI) | |
| Overall effect (all interventions vs. all comparisons) | 38 | 1800 | −119.7 (−135.0 to −104.5) | −88.0 (−99.0 to −77.1) | −6.1 (−11.9 to −0.4) |
| Overall effect (w. Sensitivity analysis)e | 35 | 1689 | −80.2 (−94.4 to −66.1) | −69.5 (−79.8 to −59.2) | −4.5 (−9.2 to +0.2) |
| Overall effect vs. active control | 29 | 1432 | −199.8 (−229.1 to −170.5) | −143.7 (−162.9 to −124.5) | −10.5 (−20.5 to −0.57) |
| Overall effect vs. non-active control | 9 | 368 | +7.2 (−14.0 to 28.4) | −13.4 (−36.3 to +9.4) | −3.8 (−9.2 to +1.7) |
| Exercise vs. all comparisons | 11 | 514 | −48.1 (−78.2 to −18.1) | −36.7 (−61.9 to 11.5) | −7.2 (−18.1 to +3.7) |
| Kinesio taping vs. all comparisons | 4 | 146 | −448.5 (−843.6 to −53.5) | −1525.2 (−2415.2 to −635.2) | +453.4 (−113.8 to +1020.5) |
| Laser vs. all comparisons | 4 | 179 | +22.9 (+8.9 to +37.0) | +17.8 (+8.2 to +27.4) | +5.4 (+1.5 to +9.2) |
| CDP vs. all comparisons | 12 | 640 | −232.5 (−278.1 to −186.8) | −184.6 (−219.3 to −149.9) | −19.6 (−41.7 to +2.6) |
| MLDf + compression vs. compression | 6 | 336 | −228.4 (−288.7 to −168.1) | −241.7 (−318.5 to −165.0) | +5.7 (−70.9 to +82.3) |
| MLD + compression + pump vs. MLD + compression | 3 | 78 | −30.4 (−51.5 to −9.2) | −23.9 (−55.5 to +7.6) | −4.6 (−16.2 to +7.1) |
K indicates number of published studies in the analysis; N indicates total number of participants in the study.
Negative values indicate volume reduction, positive values indicate volume increase.
K may vary between mean volume reductions for the intervention (max K = 34), control (max K = 34), and difference (max K = 38), due to missing data.
Negative values indicate volume difference in favor of intervention; positive values indicate volume difference in favor of control.
Sensitivity analysis (Hedges’ g): Omitting three studies (Kim 2010, Smykla et al. 2013, and Sitzia et al., 2002) with volume reductions 2 standard deviations away from the pooled effect size (±841.6 or ±1787.6).
MLD = manual lymphatic drainage.
Discussion
This is the first overview evaluating the efficacy of different treatment modalities for BCRL. Six modalities, namely MLD, compression pump, exercise, kinesio taping, laser therapy, and acupuncture were identified with between two and seven SRMAs assessing each modality. Overall, the methodological quality of the SRMAs was low, the risk of bias of the included primary studies were high, and they reached different conclusions for most treatment modalities, with the exception of kinesio taping where the two SRMAs were unable to demonstrate an effect. Further, our meta-analysis of the RCTs included in the SRMAs revealed no clear benefit of any of the treatment modalities, regardless of whether they were compared to active or non-active control conditions. This conclusion was further supported by the results of the Bayesian meta-analyses indicating only small differences between the probabilities of the null and alternative hypotheses and thus neither clear evidence to support nor refute the efficacy of these treatments.
The top research priorities within lymphedema management identified by patients and clinicians include identification of the most efficacious treatments.38 Given the inconclusive evidence regarding the superiority of treatments, even when compared to non-active control conditions, this remains the principal research priority. In line with our findings, two recent overviews of acupuncture16 and laser therapy15 reported poor quality of evidence and inability to demonstrate superiority of these treatments in reducing volume for BCRL. Other research priorities are to test the efficacy of MLD.38 Based on the available evidence, there appears to be no volume reduction of MLD (mean difference +5.7 ml, n = 336), compression pump (−4.6 ml, n = 78), or exercise (−7.2 ml, n = 514), which is in line with recently published work.39, 40, 41, 42
There are several challenges when evaluating the efficacy of the treatment modalities, particularly the components of CDP, due to substantial overlap in treatments being delivered in groups. In addition, heterogeneity in treatment regimens (i.e., level of compression, dose of intervention), and populations (i.e., BCRL severity) may contribute to the inconsistent conclusions in the SRMAs and high heterogeneity in our meta-analyses. Due to the relatively few and small studies, their high risk of bias, many different criteria for BCRL based on inter-arm differences and treatment combinations, it is difficult to disentangle the efficacy of the different treatment approaches. As such, individual patient data meta-analyses are warranted to investigate the efficacy of each treatment component and its moderators.43 Particularly the heterogeneity in diagnostic criteria for lymphedema is a major limitation to research and reaching consensus regarding standardization of lymphedema diagnosis should be a top research priority. An ongoing systematic review aims to establish criteria for early/sub-clinical lymphedema and chronic lymphedema based on psychometric properties of measurement tools.44
Of note, none of the SRMAs tested the efficacy of compression garments alone. This is unfortunate as: a) compression garments are widely used; b) lymphedema therapists have empirical evidence for the benefit of these, particularly for the early stages of lymphedema; and c) compression bandages and garments are recommended by international guidelines.45,46 Recent RCTs address the efficacy of compression alone in reducing volume or preventing progression of BCRL.47,48 Bundred et al. found that compression garments (compression class (ccl) 2) worn for 12 months did not prevent the increase of volume after 24 months compared to no compression among 143 women with mild BCRL (defined as 4–9% arm volume increase from pre-surgery).47 In contrast, Blom et al. found volume reductions but no difference in symptoms among women with mild BCRL (defined as skin thickness and either 5–8% volume or local tissue water increase compared to the unaffected arm) who wore daily compression garments (circular, ccl 1) for six months vs no garment.48 Of note, 43% of participants in the no compression group did not experience volume progression even without treatment.48 These participants may have had transient swelling or may have been misclassified with BCRL due to the criteria used by Blom et al. which rely on differences between arms and not within-arm increase from pre-surgery. Adherence to compression was not reported by Bundred et al., while 93% of participants in the study by Blom et al. reported to wear compression the whole day. The limited evidence of efficacy of compression garments on volume reduction or prevention of progression in volume warrants more research. As such, a SRMA is needed to investigate the efficacy of compression including level (ccl 1–4), type (circular or flat knit), timing (start of intervention), time (daytime, nighttime) and duration of compression interventions.
Our quality check revealed inconsistent reporting of the RCTs across the SRMAs. For this reason, we extracted data from all the RCTs, which revealed incorrect reporting by almost half of the SRMAs. While some of these issues may not have impacted the conclusions of a SRMA (e.g., incorrect registration of sample size), other issues may directly have impacted the result (e.g., pooling of different types of outcome measures). In contrast to the conclusions of some SRMAs, we only found a significant effect of one treatment modality, namely exercise. This underscores the absolute importance of a rigorous approach to conducting SRMAs.
Observing no benefit of any of the treatment modalities compared to active control conditions raises the question whether all modalities are equally beneficial or inefficacious. Across modalities, the mean volume reduction was 119 ml in intervention groups and 88 ml in control groups, with even smaller reductions in non-active controls (13 ml). It is important to note that clinically important differences in arm volume may be defined differently by the patient, the lymphedema therapist, and the health care system. Lymphedema therapists will typically aim for “maximum” reduction until sufficient stabilization of the condition to order a compression garment, the patient may want a “sufficient” reduction for the swelling to be invisible or have minimum impact on everyday activities, and the health care system will aim for “minimum” to reduce costs of hospitalization due to infection. Given the progressive nature of lymphedema where the condition is expected to worsen if left untreated,49 the treatment modalities may be considered to be equally beneficial. Importantly, as volume reductions are also seen in the control groups, it is unclear whether the reductions found in the intervention groups can be attributed to the intervention rather than spontaneous remission. Certainly, more work is needed by the research community to develop evidence-based treatments, including surgical interventions,12 for BCRL which affects hundreds of thousands of women worldwide. Thus, strategies to prevent BCRL are even more important. Recent and ongoing trials contribute to evidence for preventing BCRL using prospective surveillance and early management50, 51, 52 or prophylactic use of compression sleeves53 for those at high risk.
This is the first comprehensive overview that features the highest level of evidence (e.g., SRMAs of RCTs) across all treatment modalities for BCRL. Second, we used well-defined criteria for study selection and appraisal of the included SRMAs. Third, we examined not only the different interventions but also different control interventions and consulted with clinical experts when making decisions about classifying and pooling interventions. Fourth, as a consequence of our comprehensive quality check, we identified several inconsistencies and extracted data from all RCTs included in the SRMAs, and conducted a state-of-the-art meta-analysis of these data, including not only classical frequentist approaches but also supplementary Bayesian methods, which—given the available data—allow for the evaluation of the relative probability of the alternative and null hypotheses.
A limitation of the overview approach is that conclusions are based on data included in the SRMAs, leaving out possible RCTs not included in SRMAs. To address this, we performed a search in five databases for RCTs and identified 22 newly published trials. Of these, six report significant volume reductions and the remaining have statistically non-significant findings (Supplementary File 10). There were not sufficient data to conduct meta-analyses on this material. Further, most RCTs in the SRMAs were small (48 of 51 with <100 participants), and while most RCTs had high risk of bias (29 of 40), our analysis could not assess the impact of risk of bias for each treatment modality. All RCTs used inter-arm differences to define BCRL which bares the risk of misclassification due to arm asymmetry.54 However, while intra-arm differences based on changes from pre-surgery are ideal to define BCRL, such pre-surgery measurements are not available to most women with breast cancer. Thus, lymphedema therapists use inter-arm differences along with symptoms and clinical examination to quantify BCRL severity. Ideally, futures studies should use relative changes from pre-surgery as inclusion criteria to minimize risk of misclassification. Finally, the considerable variation of interventions and control conditions used in the SRMAs limited the possibility to disentangle the effects of each treatment component which may wash out potential effects and hinder firm conclusions regarding specific treatment components that are (in)efficacious.
Based on the currently available data, there is no clear evidence of superiority of any one treatment on volume reduction nor any solid research refuting these treatments. Thus, definitive conclusions to inform clinical practice about the efficacy of these treatments cannot be drawn. Generally, research in this area is weak and more work is needed to untangle the efficacy of each treatment component for different stages of BCRL. Health care providers should not consider the lack of evidence a reason to refute these treatments to patients. Instead, it is recommended to closely monitor benefit with reliable, valid, and sensitive measures of improvement.
Contributors
Conceptualization: BSR, AB, PC, RZ, CJ.
Methodology: BSR, AB, RZ, AvH, MJ, SB, TG.
Analysis: RZ.
Accessed and verified the underlying data: BSR, AB, RZ.
Writing (original draft preparation): BSR, AB.
Writing (review and editing): BSR, AB, RZ, AvH, MJ, SB, TG, PC, RZ, CJ.
Funding acquisition: PC, CJ.
Data sharing statement
The data extracted from the SRMAs and RCTs that is used in this overview along with the statistical codes used for meta-analyses will be made available upon request to corresponding author.
Declaration of interests
AvH reports lecture fees from Pfizer. All other authors declare no competing interests.
Acknowledgements
This study was funded by the Danish Cancer Society (R192-A11590-17-S59 and R192-A11611-17-S59). We are grateful to Dr. Kira Bloomquist and Jill Binkley for providing their clinical perspectives on this work and thorough review of the manuscript prior to submission.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2023.102397.
Appendix A. Supplementary data
References
- 1.DiSipio T., Rye S., Newman B., Hayes S. Incidence of unilateral arm lymphoedema after breast cancer: a systematic review and meta-analysis. Lancet Oncol. 2013;14(6):500–515. doi: 10.1016/S1470-2045(13)70076-7. [DOI] [PubMed] [Google Scholar]
- 2.Shen A., Lu Q., Fu X., et al. Risk factors of unilateral breast cancer-related lymphedema: an updated systematic review and meta-analysis of 84 cohort studies. Support Care Cancer. 2022;31(1):18. doi: 10.1007/s00520-022-07508-2. [DOI] [PubMed] [Google Scholar]
- 3.Fu M.R., Ridner S.H., Hu S.H., Stewart B.R., Cormier J.N., Armer J.M. Psychosocial impact of lymphedema: a systematic review of literature from 2004 to 2011. Psycho Oncol. 2013;22(7):1466–1484. doi: 10.1002/pon.3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hou W., Pei L., Song Y., et al. Acupuncture therapy for breast cancer-related lymphedema: a systematic review and meta-analysis. J Obstet Gynaecol Res. 2019;45(12):2307–2317. doi: 10.1111/jog.14122. [DOI] [PubMed] [Google Scholar]
- 5.Gatt M., Willis S., Leuschner S. A meta-analysis of the effectiveness and safety of kinesiology taping in the management of cancer-related lymphoedema. Eur J Cancer Care. 2017;26(5) doi: 10.1111/ecc.12510. [DOI] [PubMed] [Google Scholar]
- 6.Kasawara K.T., Mapa J.M.R., Ferreira V., et al. Effects of kinesio taping on breast cancer-related lymphedema: a meta-analysis in clinical trials. Physiother Theory Pract. 2018;34(5):337–345. doi: 10.1080/09593985.2017.1419522. [DOI] [PubMed] [Google Scholar]
- 7.Smoot B., Chiavola-Larson L., Lee J., Manibusan H., Allen D.D. Effect of low-level laser therapy on pain and swelling in women with breast cancer-related lymphedema: a systematic review and meta-analysis. J Cancer Surviv. 2015;9(2):287–304. doi: 10.1007/s11764-014-0411-1. [DOI] [PubMed] [Google Scholar]
- 8.Chen H.Y., Tsai H.H., Tam K.W., Huang T.W. Effects of photobiomodualtion therapy on breast cancer-related lymphoedema: a systematic review and meta-analysis of randomised controlled trials. Complement Ther Med. 2019;47 doi: 10.1016/j.ctim.2019.102200. [DOI] [PubMed] [Google Scholar]
- 9.Shao Y., Qi K., Zhou Q.H., Zhong D.S. Intermittent pneumatic compression pump for breast cancer-related lymphedema: a systematic review and meta-analysis of randomized controlled trials. Oncol Res Treat. 2014;37(4):170–174. doi: 10.1159/000360786. [DOI] [PubMed] [Google Scholar]
- 10.Lytvyn L., Zeraatkar D., Anbari A.B., et al. Conservative intervention strategies for adult cancer-related lymphedema: a systematic review and network meta-analysis. Oncol Nurs Forum. 2020;47(5):E171–E189. doi: 10.1188/20.ONF.E171-E189. [DOI] [PubMed] [Google Scholar]
- 11.Cormier J.N., Rourke L., Crosby M., Chang D., Armer J. The surgical treatment of lymphedema: a systematic review of the contemporary literature (2004–2010) Ann Surg Oncol. 2012;19(2):642–651. doi: 10.1245/s10434-011-2017-4. [DOI] [PubMed] [Google Scholar]
- 12.Winters H., Tielemans H.J.P., Paulus V., Hummelink S., Slater N.J., Ulrich D.J.O. A systematic review and meta-analysis of vascularized lymph node transfer for breast cancer-related lymphedema. J Vasc Surg Venous Lymphat Disord. 2022;10(3):786–795.e1. doi: 10.1016/j.jvsv.2021.08.023. [DOI] [PubMed] [Google Scholar]
- 13.Liang M., Chen Q., Peng K., et al. Manual lymphatic drainage for lymphedema in patients after breast cancer surgery: a systematic review and meta-analysis of randomized controlled trials. Medicine. 2020;99(49) doi: 10.1097/MD.0000000000023192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hunt H., Pollock A., Campbell P., Estcourt L., Brunton G. An introduction to overviews of reviews: planning a relevant research question and objective for an overview. Syst Rev. 2018;7(1):39. doi: 10.1186/s13643-018-0695-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Y., Ge Y., Xing W., et al. The effectiveness and safety of low-level laser therapy on breast cancer-related lymphedema: an overview and update of systematic reviews. Lasers Med Sci. 2022;37(3):1389–1413. doi: 10.1007/s10103-021-03446-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang X.W., Hou W.B., Pu F.L., et al. Acupuncture for cancer-related conditions: an overview of systematic reviews. Phytomedicine. 2022;106 doi: 10.1016/j.phymed.2022.154430. [DOI] [PubMed] [Google Scholar]
- 17.Page M.J., McKenzie J.E., Bossuyt P.M., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. J Clin Epidemiol. 2021;134:178–189. doi: 10.1016/j.jclinepi.2021.03.001. [DOI] [PubMed] [Google Scholar]
- 18.Veritas_Health_Innovation_Ltd . Veritas Health Innovation; Melbourne, Australia: 2021. Covidence systematic review software.www.covidence.org Available from: [Google Scholar]
- 19.Shea B.J., Reeves B.C., Wells G., et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358 doi: 10.1136/bmj.j4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hedges L., Olkin I. Academic Press; New York: 1985. Statistical methods for meta-analysis. [Google Scholar]
- 21.Cohen J. Lawrence Erlbaum Associates; Hillsdale, NJ: 1988. Statistical power analysis for the behavioral sciences. [Google Scholar]
- 22.Higgins J.P.T., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.IntHout J., Ioannidis J.P.A., Rovers M.M., Goeman J.J. Plea for routinely presenting prediction intervals in meta-analysis. BMJ Open. 2016;6(7) doi: 10.1136/bmjopen-2015-010247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Egger M., Davey Smith G., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Borenstein M., Hedges L., Higgins J., Rothstein H. Biostat; Englewood, NJ: 2013. Comprehensive meta-analysis. [Google Scholar]
- 26.Gronau Q.F., Van Erp S., Heck D.W., Cesario J., Jonas K.J., Wagenmakers E.J. A Bayesian model-averaged meta-analysis of the power pose effect with informed and default priors: the case of felt power. Compr Results Soc Psychol. 2017;2:123–138. [Google Scholar]
- 27.Shao Y., Zhong D.S. Manual lymphatic drainage for breast cancer-related lymphoedema. Eur J Cancer Care. 2017;26(5) doi: 10.1111/ecc.12517. [DOI] [PubMed] [Google Scholar]
- 28.Huang T.W., Tseng S.H., Lin C.C., et al. Effects of manual lymphatic drainage on breast cancer-related lymphedema: a systematic review and meta-analysis of randomized controlled trials. World J Surg Oncol. 2013;11:15. doi: 10.1186/1477-7819-11-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ezzo J., Manheimer E., McNeely M.L., et al. Manual lymphatic drainage for lymphedema following breast cancer treatment. Cochrane Database Syst Rev. 2015;(5) doi: 10.1002/14651858.CD003475.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McNeely M.L., Peddle C.J., Yurick J.L., Dayes I.S., Mackey J.R. Conservative and dietary interventions for cancer-related lymphedema: a systematic review and meta-analysis. Cancer. 2011;117(6):1136–1148. doi: 10.1002/cncr.25513. [DOI] [PubMed] [Google Scholar]
- 31.Qiao J., Yang L.N., Kong Y.H., Huang X., Li Y., Bai D.Q. Effect of manual lymphatic drainage on breast cancer-related postmastectomy lymphedema: a meta-analysis of randomized controlled trials. Cancer Nurs. 2023;46(2):159–166. doi: 10.1097/NCC.0000000000001061. https://pubmed.ncbi.nlm.nih.gov/35324506/ [cited 2023 Mar 9]; Available from: [DOI] [PubMed] [Google Scholar]
- 32.Rogan S., Taeymans J., Luginbuehl H., Aebi M., Mahnig S., Gebruers N. Therapy modalities to reduce lymphoedema in female breast cancer patients: a systematic review and meta-analysis. Breast Cancer Res Treat. 2016;159(1):1–14. doi: 10.1007/s10549-016-3919-4. [DOI] [PubMed] [Google Scholar]
- 33.Li J.X., Gao J., Song J.Y., et al. Compression therapy for the patients with breast cancer: a meta-analysis of randomized controlled trials. Cancer Nurs. 2022;45(4):E736–E745. doi: 10.1097/NCC.0000000000001005. [DOI] [PubMed] [Google Scholar]
- 34.Rangon F.B., da Silva J., Dibai-Filho A.V., Guirro R.R.J., Guirro E.C.O. Effects of complex physical therapy and multimodal approaches on lymphedema secondary to breast cancer: a systematic review and meta-analysis of randomized controlled trials. Arch Phys Med Rehabil. 2022;103(2):353–363. doi: 10.1016/j.apmr.2021.06.027. [DOI] [PubMed] [Google Scholar]
- 35.Yeung W., Semciw A.I. Aquatic therapy for people with lymphedema: a systematic review and meta-analysis. Lymphat Res Biol. 2018;16(1):9–19. doi: 10.1089/lrb.2016.0056. [DOI] [PubMed] [Google Scholar]
- 36.Jang S., Ko Y., Sasaki Y., et al. Acupuncture as an adjuvant therapy for management of treatment-related symptoms in breast cancer patients: systematic review and meta-analysis (PRISMA-compliant) Medicine. 2020;99(50) doi: 10.1097/MD.0000000000021820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lunny C., Pieper D., Thabet P., Kanji S. Managing overlap of primary study results across systematic reviews: practical considerations for authors of overviews of reviews. BMC Med Res Methodol. 2021;21(1):140. doi: 10.1186/s12874-021-01269-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Underwood E., Woods M., Riches K., Keeley V., Wallace A., Freeman J. Lymphedema research prioritization partnership: a collaborative approach to setting research priorities for lymphedema management. Lymphat Res Biol. 2019;17(3):356–361. doi: 10.1089/lrb.2018.0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.De Vrieze T., Gebruers N., Nevelsteen I., et al. Manual lymphatic drainage with or without fluoroscopy guidance did not substantially improve the effect of decongestive lymphatic therapy in people with breast cancer-related lymphoedema (EFforT-BCRL trial): a multicentre randomised trial. J Physiother. 2022;68(2):110–122. doi: 10.1016/j.jphys.2022.03.010. [DOI] [PubMed] [Google Scholar]
- 40.De Vrieze T., Gebruers N., Nevelsteen I., et al. Does manual lymphatic drainage add value in reducing arm volume in patients with breast cancer-related lymphedema? Phys Ther. 2022 doi: 10.1093/ptj/pzac137. [DOI] [PubMed] [Google Scholar]
- 41.Marotta N., Lippi L., Ammendolia V., et al. Efficacy of kinesio taping on upper limb volume reduction in patients with breast cancer-related lymphedema: a systematic review of randomized controlled trials. Eur J Phys Rehabil Med. 2023;59(2):237–247. doi: 10.23736/S1973-9087.23.07752-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hayes S.C., Singh B., Reul-Hirche H., et al. The effect of exercise for the prevention and treatment of cancer-related lymphedema: a systematic review with meta-analysis. Med Sci Sports Exerc. 2022;54(8):1389–1399. doi: 10.1249/MSS.0000000000002918. [DOI] [PubMed] [Google Scholar]
- 43.Stewart L.A., Tierney J.F. To IPD or not to IPD? Advantages and disadvantages of systematic reviews using individual patient data. Eval Health Prof. 2002;25(1):76–97. doi: 10.1177/0163278702025001006. [DOI] [PubMed] [Google Scholar]
- 44.Bloomquist K., Christensen J., Larsen A., Jensen S., Rafn B.S. Towards consensus - measurement methods for evaluating peripheral secondary lymphedema: a systematic review of measurement properties and applied cut-offs. PROSPERO protocol. https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=432603 Available at:
- 45.National Lymphedema Network Position statement of the national lymphedema network: the diagnosis and treatment of lymphedema. 2011. http://www.lymphnet.org/pdfDocs/nlntreatment.pdf; Available from:
- 46.Davies C., Levenhagen K., Ryans K., Perdomo M., Gilchrist L. Interventions for breast cancer–related lymphedema: clinical practice guideline from the academy of oncologic physical therapy of APTA. Phys Ther. 2020;100(7):1163–1179. doi: 10.1093/ptj/pzaa087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bundred N.J., Barrett E., Todd C., et al. Prevention of lymphoedema after axillary clearance by external compression sleeves PLACE randomised trial results. Effects of high BMI. Cancer Med. 2022;12:5506–5516. doi: 10.1002/cam4.5378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Blom K.Y., Johansson K.I., Nilsson-Wikmar L.B., Brogårdh C.B. Early intervention with compression garments prevents progression in mild breast cancer-related arm lymphedema: a randomized controlled trial. Acta Oncol. 2022;61(7):897–905. doi: 10.1080/0284186X.2022.2081932. [DOI] [PubMed] [Google Scholar]
- 49.Grada A.A., Phillips T.J. Lymphedema: pathophysiology and clinical manifestations. J Am Acad Dermatol. 2017;77(6):1009–1020. doi: 10.1016/j.jaad.2017.03.022. [DOI] [PubMed] [Google Scholar]
- 50.Ridner S.H., Dietrich M.S., Boyages J., et al. A comparison of bioimpedance spectroscopy or tape measure triggered compression intervention in chronic breast cancer lymphedema prevention. Lymphat Res Biol. 2022;20(6):618–628. doi: 10.1089/lrb.2021.0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rafn B.S., Christensen J., Larsen A., Bloomquist K. Prospective surveillance for breast cancer-related arm lymphedema: a systematic review and meta-analysis. J Clin Oncol. 2022;40(9):1009–1026. doi: 10.1200/JCO.21.01681. [DOI] [PubMed] [Google Scholar]
- 52.Skjødt Rafn B., Jensen S., Bjerre E.D., et al. Prospective surveillance for breast cancer-related lymphedema (PROTECT) Acta Oncol. 2023;62(7):808–813. doi: 10.1080/0284186X.2023.2197125. [DOI] [PubMed] [Google Scholar]
- 53.Paramanandam V.S., Dylke E., Clark G.M., et al. Prophylactic use of compression sleeves reduces the incidence of arm swelling in women at high risk of breast cancer–related lymphedema: a randomized controlled trial. J Clin Orthod. 2022;40(18):2004–2012. doi: 10.1200/JCO.21.02567. [DOI] [PubMed] [Google Scholar]
- 54.Sun F., Skolny M.N., Swaroop M.N., et al. The need for preoperative baseline arm measurement to accurately quantify breast cancer-related lymphedema. Breast Cancer Res Treat. 2016;157(2):229–240. doi: 10.1007/s10549-016-3821-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.