Abstract
Background
Radiation pneumonitis (RP) is the most common dose-limiting toxicity for thoracic radiation therapy. Nintedanib is used for the treatment of idiopathic pulmonary fibrosis, which shares pathophysiological pathways with the subacute phase of RP. Our goal was to investigate the efficacy and safety of nintedanib added to a prednisone taper compared to a prednisone taper alone in reducing pulmonary exacerbations in patients with grade 2 or higher (G2+) RP.
Methods
In this phase II, randomized, double-blinded, placebo-controlled trial, patients with newly diagnosed G2+ RP were randomized 1:1 to nintedanib or placebo in addition to a standard 8-week prednisone taper. The primary endpoint was freedom from pulmonary exacerbations at one year. Secondary endpoints included patient reported outcomes and pulmonary function tests. Kaplan-Meier analysis was used to estimate the probability of freedom from pulmonary exacerbations. The study was closed early due to slow accrual.
Results
34 patients were enrolled between October 2015 and February 2020. Of 30 evaluable patients, 18 were randomized to the experimental Arm A (nintedanib + prednisone taper) and 12 to control Arm B (placebo + prednisone taper). Freedom from exacerbation at one year was 72% (CI 54%–96%) in Arm A and 40% (CI 20%–82%) in Arm B (one-sided p=0.037). In Arm A there were 16 G2+ adverse events possibly or probably related to treatment compared to five in the placebo arm. There were three deaths during the study period in arm A due to cardiac failure, progressive respiratory failure, and pulmonary embolism, respectively.
Conclusions
There was an improvement in pulmonary exacerbations by the addition of nintedanib to a prednisone taper. Further investigation is warranted for the use of nintedanib for the treatment of RP.
Keywords: Radiation pneumonitis, radiation toxicity, nintedanib, thoracic radiotherapy
Introduction
Radiation pneumonitis is a common side effect of thoracic radiotherapy (RT), with rates of grade 2 or higher radiation pneumonitis near 20%.1,2 Clinical symptoms range from a minor cough to dyspnea, low grade fever, and respiratory insufficiency that typically occur within 1 to 6 months after the end of RT.3–6 The course of disease can be difficult to predict, but approximately 50% of patients experience subsequent acute pulmonary exacerbations, and the majority eventually develop radiation-induced pulmonary fibrosis.7 Severe radiation pneumonitis is associated with reduced survival, with mortality rates approaching 50%.5 The risk of radiation pneumonitis is correlated with the dose of radiation to and volume of lung tissue exposed to radiation.8,9 More recently it has also been correlated with the radiation dose to and volume of heart exposed to radiation.10,11
The pathogenesis of radiation pneumonitis is thought to be mediated through the release of multiple cytokines and growth factors, including transforming growth factor-beta (TGF-beta), vascular endothelial growth factor (VEGF), tissue necrosis factor (TNF), platelet-derived growth factor (PDGF), interleukin (IL) -6, IL-8, and NF-kappa B.12 The release of these cytokines and growth factors after radiation results in an inflammatory response, which clinically presents as subacute radiation pneumonitis. In the long term, these signaling molecules activate fibroblasts and myofibroblasts, which leads to the deposition of fibrin and permanent structural and functional changes in the lung, resulting in pulmonary fibrosis.13
The current standard treatment for radiation pneumonitis consists of an empiric course of corticosteroids over 8–10 weeks, which is associated with significant short- and long-term side effects. It is unclear whether corticosteroids prevent the late sequela of pulmonary fibrosis. Other proposed therapeutics, such as ACE inhibitors, amifostine, and pentoxifylline, have failed to show effectiveness for the prevention or treatment of radiation pneumonitis.14–16 There is a clear need for improved evidence-based management options.
Nintedanib (BIBF 1120) is an inhibitor of multiple tyrosine kinases that have been implicated in pulmonary fibrosis. It inhibits fibroblast growth factor receptor 1 and 3 (FGFR -1/3), Platelet-derived Growth Factor Receptor α and β (PDGFR α and β), and Vascular Endothelial Growth Factor Receptor 1, 2 and 3 (VEGFR 1–3). These growth factors have been investigated as possible standalone therapeutic targets for pulmonary fibrosis.17–19 In preclinical studies, treatment with nintedanib had an antifibrotic and anti-inflammatory effect in bleomycin- and silica-induced lung fibrosis in mice.20 Phase III trials in idiopathic pulmonary fibrosis (IPF) showed that nintedanib slowed progression of disease by reducing the decline in forced vital capacity (FVC) leading to its FDA approval for IPF in 2014 and EU approval in 2015.21,22Additionally, in two randomized trials, nintedanib significantly reduced the risk of having an acute exacerbation of IPF.21,23 The approval of nintedanib was expanded to chronic fibrosing interstitial lung disease (ILD) with a progressive phenotype and systemic sclerosis-associated ILD (SSc-ILD) after similar benefits in slowing the decline of FVC were observed in the INBUILD and SENCIS trials.24
Given the lack of clinically supported therapeutic treatment options for radiation pneumonitis and the overlapping pathways involved in the pathogenesis of radiation pneumonitis and IPF, we completed this phase II trial of testing nintedanib in combination with steroids for the treatment of radiation pneumonitis.
Methods
Study Design
This was a prospective, randomized, placebo controlled, double-blind, phase II multicenter trial led by Memorial Sloan Kettering Cancer Center. Participating sites included Lehigh Valley Health Network through the MSK Alliance, Brigham and Women’s hospital/Dana-Farber Cancer Institute and MD Anderson Cancer Center. The protocol was approved by the institutional review boards of the participating institutions.
Patients
Adult patients ≥18 years of age were enrolled with newly diagnosed clinical grade ≥2 radiation pneumonitis according to CTCAE version 4.0 criteria and history of thoracic malignancy or lung metastasis treated with definitive intent. Key eligibility criteria included prior thoracic radiotherapy between 4 weeks and 9 months prior to enrollment and Karnofsky performance score ≥ 70%. Key exclusion criteria included current oral steroid use ≥4 weeks prior to registration, ongoing radiotherapy or systemic therapy except pembrolizumab, nivolumab, afatinib, or hormonal therapy, or history of bleeding disorder, thrombotic events, or liver disorder. Patients were recruited by investigators and their research teams. Written consent was obtained for all patients.
Randomization and masking
Eligible patients were stratified by grade of radiation pneumonitis (grade 2 versus 3 or 4) and initiation of steroids prior to enrollment versus steroid naïve, then randomized in a 1:1 ratio to nintedanib/prednisone taper or placebo/prednisone taper using the random permuted block method in a centralized clinical research database by a clinical research coordinator. The unblinded designation was communicated to a designated pharmacist at the treatment site. Patients, investigators, and everyone involved in analyzing the study were blinded to the treatment randomization. Identical capsules of nintedanib or placebo were provided by the unblinded pharmacist based on the randomized assignment.
Procedures
Either nintedanib or placebo was given orally at an initial dose of 150 mg two times per day for 12 weeks. A prednisone taper was given concurrently, starting at 40mg prednisone given orally each day for two weeks, followed by a dose taper of 10mg every two weeks for four weeks, followed by 10mg for one week, then 5mg for one week, for a total duration on prednisone of eight weeks. Delays in the prednisone taper or increase of the prednisone dose were permitted as clinically necessary. Office visits to evaluate safety and tolerability of the study drug and assess for acute pulmonary exacerbations were completed every three weeks for the first three months, then at four to five, six, nine, and 13 months. Patient reported outcomes were collected with the Patient Reported Outcomes version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE) surveys and the St. George’s Respiratory Questionnaire (SGRQ).
Outcomes
The primary outcome was the proportion of patients who were free from acute pulmonary exacerbations within one year of enrollment. Acute pulmonary exacerbations were defined as unexplained worsening or development of new cough, dyspnea, hypoxia, or pneumonia lasting more than 4 days with new or worse diffuse pulmonary infiltrates on CT chest without significant pneumothorax or pleural effusion, and exclusion of alternative causes such as pneumonia, congestive heart failure, pulmonary emboli, or cancer progression. Exacerbations in the first two weeks of study treatment were not counted towards the primary endpoint to allow for adequate resolution of the initial presenting symptoms. Secondary endpoints include the total number of exacerbations and results of pulmonary function tests (PFTs). Exploratory endpoints included quality of life measures by SGRQ and PRO-CTCAE. Additional endpoints to be reported in the future include radiographic changes on CT scans, and correlation of clinical presentation with potential serum biomarkers. Adverse events were monitored for any grade 4 or higher toxicities, and the sequential probability ratio test was used to define a stopping rule.
Statistical analysis
The target enrollment was 68 patients total (34 in each treatment arm) to provide 90% power to detect a decrease in freedom from pulmonary exacerbations within one year from 50% to 25% with a one-sided test, based on the decrease in acute exacerbations reported in a prior study of patients with IPF, and assuming 25% of patients would be censored during the 12 month interval due to death, cancer progression or study drop-out.25 Due to an anticipated non-negligible proportion of patients that would drop out, have cancer progression, or die during the planned one year of follow up, the primary analysis was time to event, with a one-sided test based on difference in survival outcomes at a fixed time point. All patients randomized who remained on study medication beyond the first four weeks of treatment were included in the analysis per protocol. To compare the number of exacerbations between groups, a zero-inflated Poisson model was used to model the total number of acute exacerbations in the nintedanib versus placebo groups. Wilcoxon signed-rank tests were used to compare changes in PFTs. For patient reported outcomes, Wilcoxon signed rank tests with continuity correction were used to compare results between treatment arms. The institutional Data and Safety Monitoring Committee at Memorial Sloan Kettering Cancer Center established monitoring procedures for the protocol. Data analysis was completed using R version 4.1.2. The study was registered with ClinicalTrials.gov identifier NCT02496585.
Results
Between October 16, 2015, and February 20, 2020, a total of 34 participants were enrolled. The trial was closed early due to slow accrual. After randomization, four participants withdrew from the study. Five participants discontinued treatment early but received at least 4 weeks of treatment on study and were therefore included in the analysis (Figure 1). In total, 30 participants were included in the final analysis, 18 in the nintedanib group and 12 in the placebo group, and the median follow up was 13 months (95% CI 13–15 months). Baseline characteristics of trial participants are shown in Table 1. For the course of radiotherapy preceding trial enrollment, 63% of patients received 45–66 Gy in 25–35 fractions (Table 1). The mean lung dose in the nintedanib group was 1312 cGy (SD 616) and 1016 cGy (SD 424) in the placebo arm. The mean heart dose was 1472 cGy (SD 1018) and 928 cGy (SD 660) in the nintedanib and placebo arms, respectively. Additional dose volume statistics can be found in the supplemental material. Four participants in the nintedanib arm and two in the placebo arm had grade 3 pneumonitis at enrollment (Table 1). There were no participants with grade 4 pneumonitis at baseline. Most participants (80%) started corticosteroid treatment within four weeks prior to enrollment. The median duration of prior steroid treatment among those who started prior to enrollment was one week in the nintedanib arm (range 0.2–2.5 weeks) and one week in the placebo arm (range 0.2–3.7 weeks). Four patients in the nintedanib group had been treated with durvalumab and one patient was treated with pembrolizumab prior to enrollment. One patient continued afatinib concurrently with nintedanib.
Figure 1.
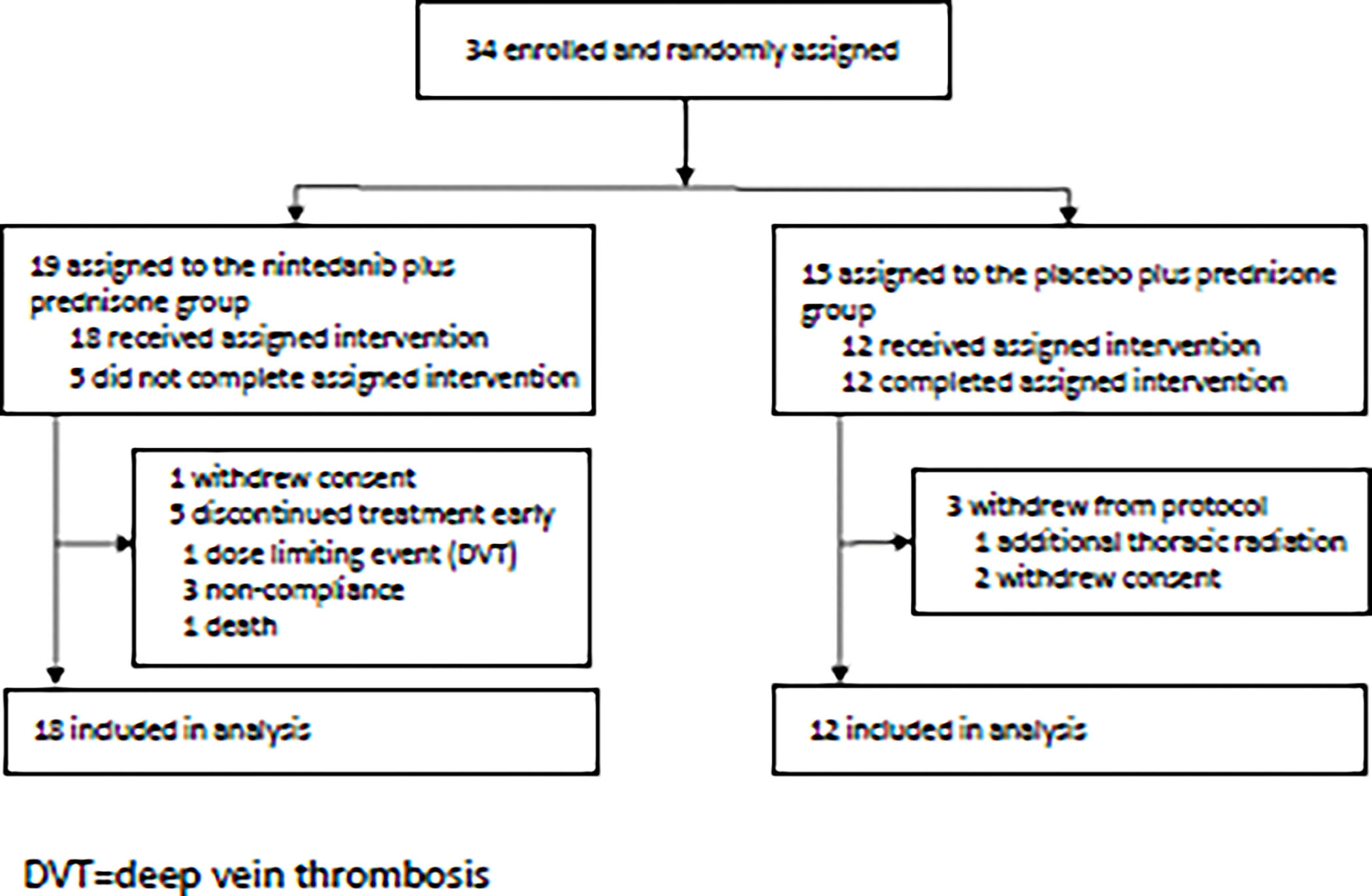
Trial Profile
Table 1.
Baseline Characteristics
| Characteristic | Overall n = 30 |
Nintedanib n = 18 |
Placebo n = 12 |
|---|---|---|---|
| Age in years | 72 (47–86) | 72 (58–80) | 70 (47–86) |
| Sex | |||
| Female | 22 (73%) | 13 (72%) | 9 (75%) |
| Male | 8 (27%) | 5 (28%) | 3 (25%) |
| Race | |||
| Asian | 3 (10%) | 1 (6%) | 2 (17%) |
| Black or African American | 1 (3%) | 0 | 1 (8%) |
| Not reported | 1 (3%) | 0 | 1 (8%) |
| White | 25 (83%) | 17 (94%) | 8 (67%) |
| Smoking Status | |||
| Former | 24 (83%) | 15 (83%) | 9 (82%) |
| Never | 5 (17%) | 3 (17%) | 2 (18%) |
| KPS | 80 (70–100) | 80 (70–90) | 80 (70–100) |
| Radiation Dose | |||
| 48–50 Gy / 4–5 fxns | 8 (27%) | 4 (22%) | 4 (33%) |
| 45–60 Gy / 10–20 fxns | 3 (10%) | 2 (11%) | 1 (8%) |
| 45–66 Gy / 25–35 fxns | 19 (63%) | 12 (67%) | 7 (58%) |
| Pneumonitis Grade | |||
| Grade 2 | 24 (80%) | 14 (78%) | 10 (83%) |
| Grade 3 | 6 (20%) | 4 (22%) | 2 (17%) |
| Prior Steroids | 24 (80%) | 13 (72%) | 11 (92%) |
| Cancer Type | |||
| NSCLC | 22 (73%) | 14 (78%) | 8 (67%) |
| Stage I | 4 (13%) | 2 (11%) | 2 (17%) |
| Stage II | 2 (7%) | 0 | 2 (17%) |
| Stage III | 12 (40%) | 8 (44%) | 4 (33%) |
| Stage IV | 3 (10%) | 3 (17%) | 0 |
| Mesothelioma | 2 (7%) | 2 (11%) | 0 |
| Thymoma | 2 (7%) | 1 (6%) | 1 (8%) |
| SCLC | 3 (10%) | 1 (6%) | 2 (17%) |
| Other | 1 (3%) | 0 | 1 (8%) |
Baseline characteristics of enrolled participants. Reported as median (range) for age and KPS, otherwise number (percent). Prior steroids refers to corticosteroid use <=4 weeks prior to enrollment. NSCLC – Non-small cell lung cancer, SCLC-small cell lung cancer. fxns- fractions
The Kaplan Meier estimate of freedom from exacerbation at one year was 72% (95% CI 54%–96%) in the nintedanib group and 40% in the placebo group (95% CI 20%–82%) (p=0.037) (Figure 2). Due to the low accrual, a one-sided log-rank test was also performed to cover the whole time up to the last event, which was not part of the prespecified analysis plan. This yielded a p-value of 0.068. Four patients were censored due to progressive or recurrent malignancy, and three patients were censored due to withdrawal from the study or non-adherence with follow up. A total of five of 18 patients in the nintedanib arm and seven of 12 patients in the placebo arm had at least one pulmonary exacerbation within one year (Figure 2b). Notably, the number of exacerbations in the nintedanib arm was lower despite a greater number of participants with grade 3 pneumonitis in that arm. The median freedom from exacerbation was 6.4 months (95% CI: 2-not reached) in the placebo arm and was not reached in the nintedanib arm. The average number of exacerbations was 0.67 in the nintedanib group and 1.1 in the placebo group. The incidence rate ratio (IRR) of having exacerbations in the nintedanib arm was 2.31 (95% CI 0.85–6.29) (p=0.1).
Figure 2. Freedom from pulmonary exacerbations.
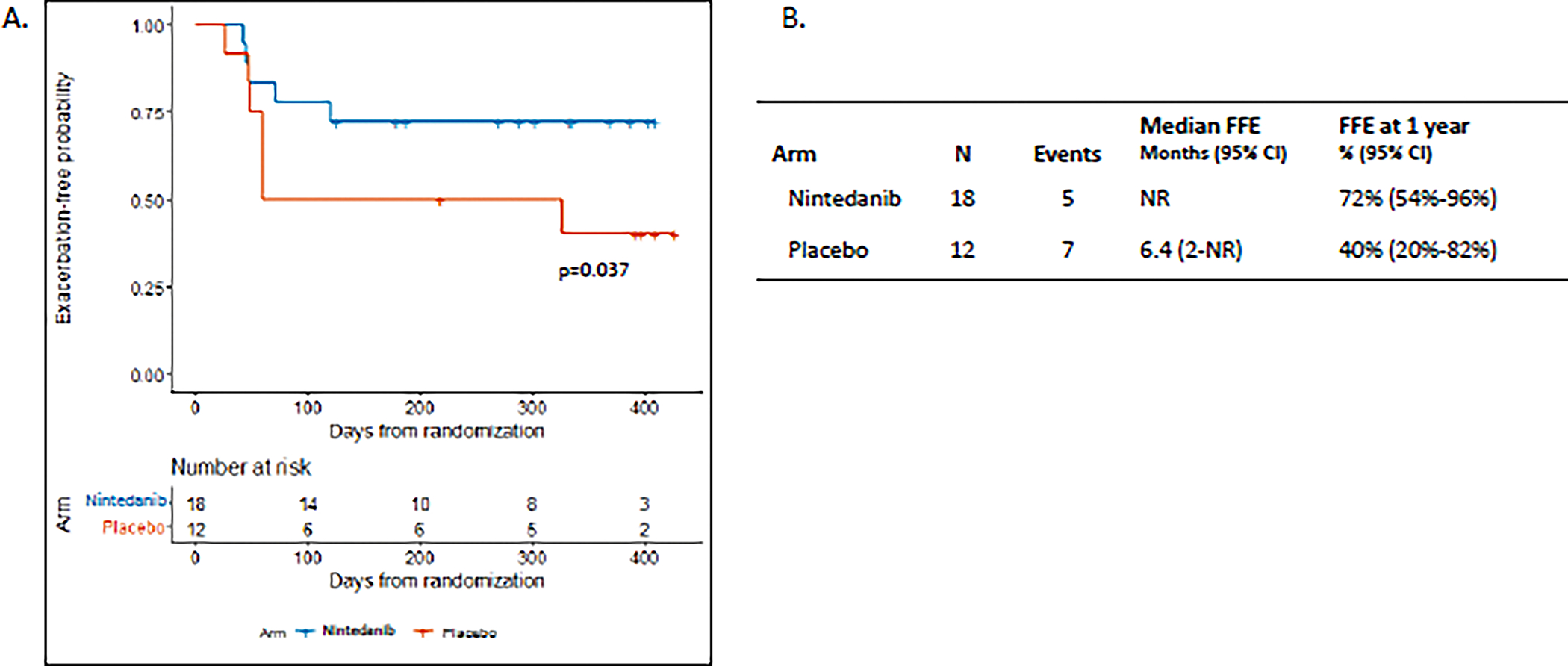
A. Kaplan-Meier plot of time to first acute pulmonary exacerbation beginning 2 weeks after the start of treatment with nintedanib + prednisone or placebo + prednisone. In the prespecified one-sided Z-test for significance at one year p=0.037. B. Kaplan-Meier estimate of median freedom from exacerbation (FFE) and estimated freedom from pulmonary exacerbation at 1 year. NR=not reached
Proportional hazards regression models were used to investigate univariable associations with exacerbation-free survival as shown in Figure 3. There were no statistically significant associations with an increased hazard of pulmonary exacerbation among age, KPS, smoking status, baseline FEV1, or number of weeks of prior steroid use. The confidence interval for smoking status was large as only 5 patients were never smokers.
Figure 3. Univariable associations with exacerbation-free survival.
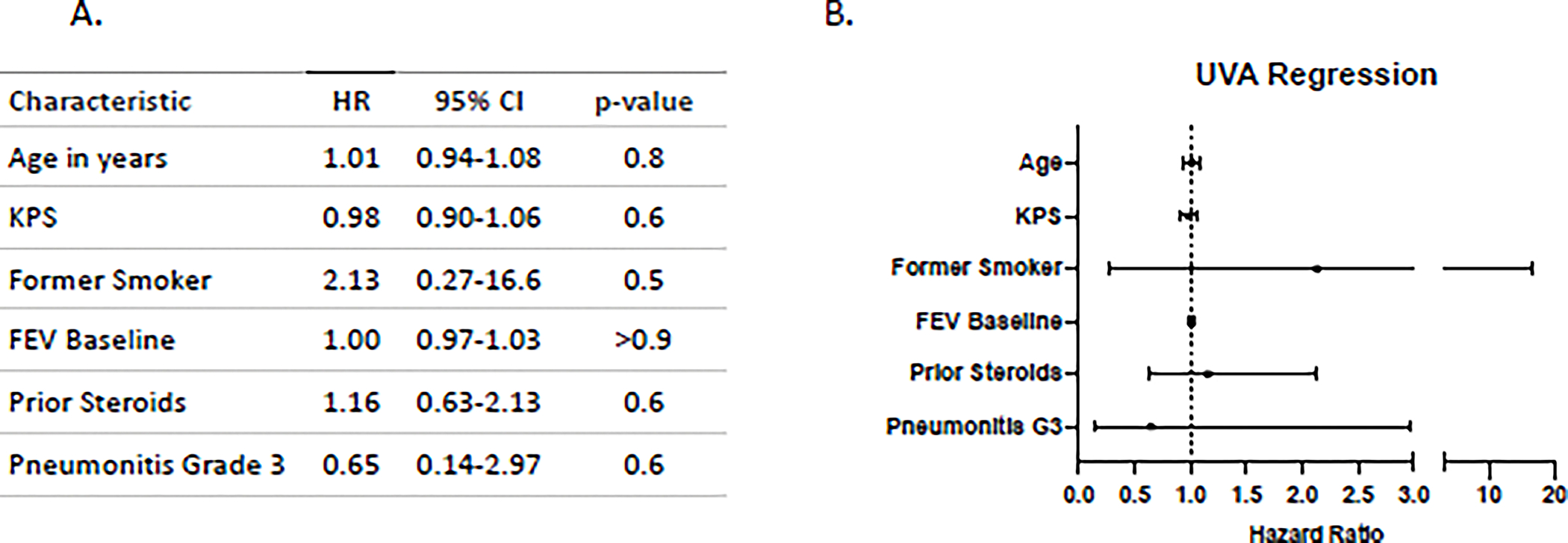
Cox Proportional hazards regression analysis of freedom from pulmonary exacerbations. Age in years, KPS, smoking status (former smoker versus never smoker), baseline FEV, and number of weeks of steroid treatment prior to enrollment did not have a statistically significant association with the hazard of pulmonary exacerbation. Hazard ratios (HR) are plotted with with 95% confidence intervals (CI) in panel B.
No unexpected safety signals occurred during the study. There were no grade 2 or higher events definitely attributed to the study intervention. Grade 2 or higher adverse events that were possibly or probably caused by the study drug as determined by the treating physician are shown in Table 2. The most frequent adverse events were diarrhea and dyspnea, both in the nintedanib group. The total number of all G2+ pulmonary-related adverse events, including those not related to the study intervention, was 21 in the nintedanib arm and 23 in the placebo arm. A table of all adverse events can be found in the supplemental material.
Table 2.
Adverse Events
| Nintedanib n =18 |
Placebo n=12 |
|
|---|---|---|
| Grade 2+ AEs | 16 | 5 |
| Systemic | ||
| Fatigue | 1 (6%) | 1 (8%) |
| Anorexia | 1 (6%) | - |
| Respiratory | ||
| Cough | - | 1 (8%) |
| Dyspnea | 3 (17%) | - |
| Hypoxia | 1 (6%) | - |
| Lung Infection | 2 (11%) | - |
| Pleural effusion | 1 (6%) | - |
| Gastrointestinal | ||
| Diarrhea | 3 (17%) | - |
| Nausea | - | 1 (8%) |
| Cardiovascular/Hematologic | ||
| Pericardial effusion | 1 (6%) | - |
| Hypertension | 1 (6%) | - |
| Platelet count decreased | 1 (6%) | - |
| Lymphocyte count decreased | - | 1 (8%) |
| Thromboembolic event | 1 (6%) | - |
| Skin | ||
| Rash | - | 1 (8%) |
Grade 2 or higher adverse events that were possibly or probably attributed to the study intervention. Reported as number of patients with at least one occurrence of listed event (percent). No grade 2 events were definitely attributed to intervention. Some patients had multiple adverse events. Two deaths occurred in nintedanib group, unlikely related to study. A full list of all AEs can be found in the supplemental material.
There were six participants in the nintedanib arm who had a combined total of 11 serious adverse events. These events included thrombocytopenia, lung infection, pericardial effusion, dyspnea, and venous thrombosis. One participant in the nintedanib group discontinued treatment early due to an uncomplicated grade 2 venous thrombosis. Another patient in the nintedanib group taking concurrent afatinib had a dose reduction due to grade 3 diarrhea and subsequently discontinued treatment. Two patients self-discontinued the study drug but completed full protocol follow-up. In total, five patients in the nintedanib arm did not complete the full 12 weeks of treatment, and two of these patients had a pulmonary exacerbation.
Three patients died during the course of the study in the nintedanib arm. One patient died of cardiac arrest associated with hypoxia, pleural effusion, and possible progression of disease. This patient had a history of COPD and pleural mesothelioma and had undergone coronary artery stent placement for coronary artery disease two months prior to enrollment. The second death was due to respiratory failure caused by progressive chemotherapy and radiation induced pneumonitis, and the third was due to bilateral pulmonary embolism six months after enrollment. On subsequent review by the treating physicians and study PI, these deaths were considered unlikely to be related to the study drug.
Patient reported outcomes including pulmonary-related PRO-CTCAE scores and SGRQ scores are shown in Figure 4. To reduce the number of statistical tests given the incomplete enrollment and missing values for some patients, Wilcoxon tests were limited to evaluating the change from baseline to the 3-month time point between treatment arms instead of the prespecified analysis of change from baseline to all other time points. The 3-month time point corresponds with the completion of nintedanib/placebo treatment. There were no statistically significant differences in end of treatment versus baseline changes in cough severity, wheezing, shortness of breath, fatigue, diarrhea, or SGRQ scores between treatment arms. A table of all results and p-values can be found in the supplemental material. Comparing the plotted median scores between treatment arms shows a trend toward benefit in the nintedanib arm for cough severity, wheezing severity, and potentially a more rapid improvement in the SGRQ impacts score relative to the placebo arm. Zero-inflated Poisson models showed no statistically significant changes in the incidence rate ratios of pulmonary exacerbations with shortness of breath severity (IRR 1.13, 95% CI 0.69–1.86, p=0.63) or cough severity (IRR 1.06, 95% CI 0.75–1.49), p=0.75) at 12 weeks. There were no trends in any of these patient-reported outcomes suggesting worse patient reported outcomes in the nintedanib arm.
Figure 4. Patient reported outcomes.
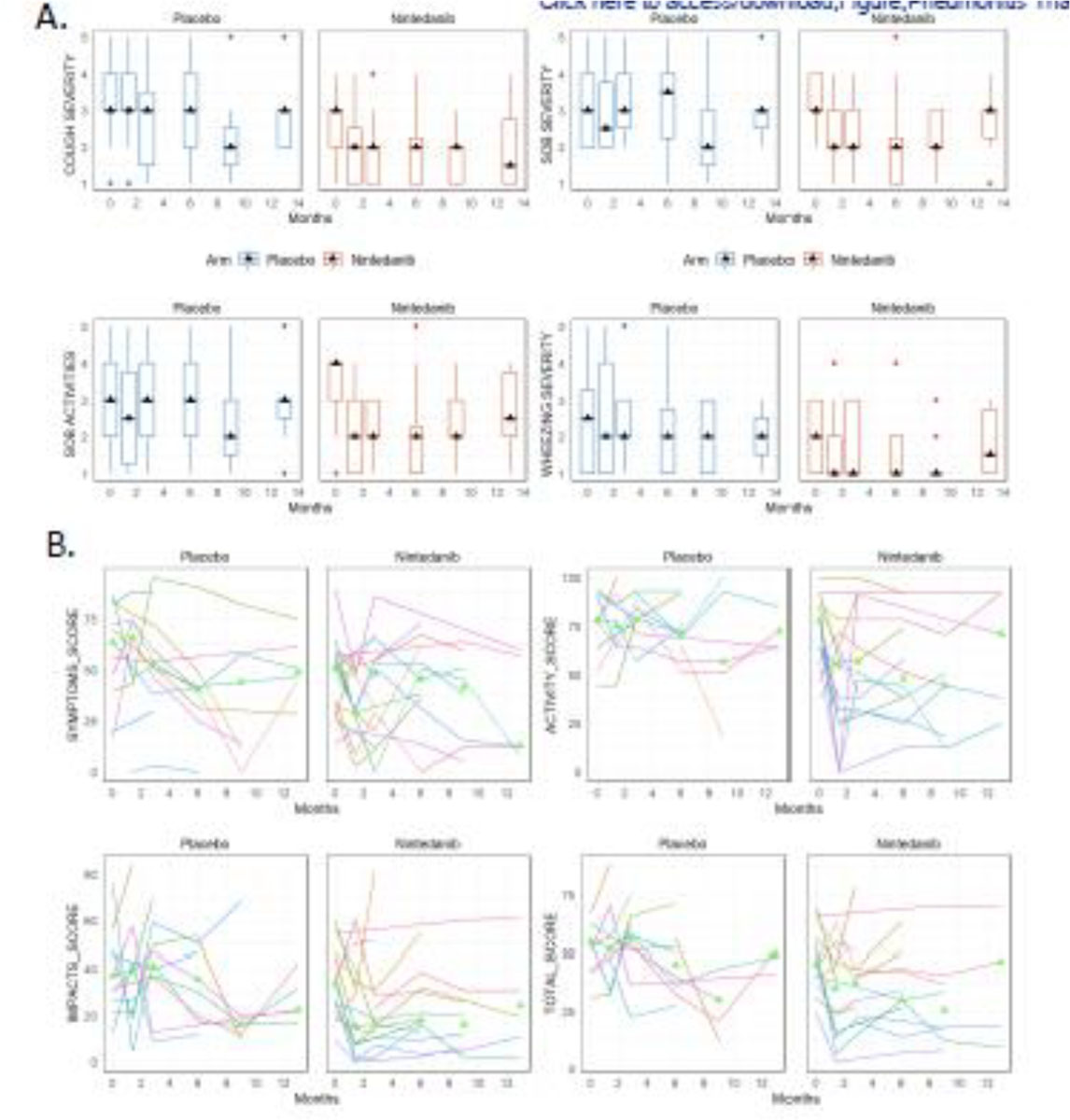
A. PRO-CTCAE scores are plotted from the following domains: cough severity, shortness of breath (SOB) severity, SOB with daily activities, and wheezing severity scores in the placebo and nintedanib arms. Triangles represent the median values at each time point. B. SGRQ spaghetti plots of total score as well as symptoms, activity, and impacts sub scores. Each line represents a single patient with triangles denoting the median scores at each time point.
PFT results are provided in the supplemental material. The baseline median percent of predicted values for diffusion capacity for carbon monoxide (DLCO), forced expiratory volume in one second (FEV1) and forced vital capacity (FVC) in all participants were 55%, 80%, and 77%, respectively. Though the study was not powered to detect differences in PFTs, we completed prespecified non-parametric Wilcoxon sign-rank tests for changes relative to baseline values. There were no statistically significant changes in DLCO, FEV1, or FVC at any time point. The individual p-values for each test are provided in the supplemental material.
Discussion
This study showed a statistically significant improvement in freedom from exacerbations at one year with the addition of nintedanib to a standard prednisone taper. The success of nintedanib in improving outcomes from interstitial lung disease with progressive fibrosis in the INBUILD and SENICIS trials suggests that shared mechanisms of fibrosis from different etiologies can effectively be targeted with this drug.24,26 Though the primary outcome in those studies was rate of decline in FVC, secondary analysis of the INPULSIS trials in IPF showed that nintedanib significantly reduced the risk of a first acute exacerbation reported as a serious adverse event.27 Other recent studies have begun to explore the use of nintedanib in radiation pneumonitis and radiation-induced fibrosis. Preclinical studies in mice have shown that nintedanib reduces late radiation-induced fibrosis.28 Notably, in a recently published randomized trial of nintedanib for prophylaxis against radiation pneumonitis, there were more clinically significant radiation pneumonitis events in the placebo group compared to the nintedanib group.29 However, that trial was closed early due to poor accrual and the observed difference was not statistically significant.29
Diarrhea and dyspnea were the most common adverse events in the nintedanib arm of this study. This is consistent with other trial data showing diarrhea was a frequent side effects of nintedanib, with 65.9% patients experiencing this toxicity in a meta-analysis of randomized trials.30 Since one patient in the nintedanib arm who was also taking afatinib discontinued treatment due to diarrhea, this drug combination should be viewed with caution going forward as afatinib may have exacerbated this toxicity. Nausea, another common adverse event, was observed only at grade 1 in the nintedanib arm of this trial. Liver enzyme elevation has also been observed in patients taking nintedanib.30 This occurred in 3 patients in the nintedanib arm of this study but was not higher than grade 1. The total number of G2+ pulmonary-related adverse events was lower in the nintedanib arm, despite there being fewer patients in the placebo arm. Though nintedanib was generally well tolerated in trials for its use in idiopathic pulmonary fibrosis, further monitoring for safety in this patient population is warranted if investigation is pursued for its application for radiation pneumonitis, especially if used in combination with other TKIs. Nintedanib may increase the risk of bleeding since it is an inhibitor of angiogenesis, though in a pooled analysis of clinical trials of patients with IPF there was no increase in the rate of bleeding with nintedanib compared to placebo.31 In the study reported by Dy et al., one patient with squamous cell lung cancer treated with nintedanib experienced a grade 5 hemorrhagic event.29 In our study, four patients treated with nintedanib had squamous cell histology and there were no bleeding adverse events.
The primary limitation of this study is the small number of patients enrolled, with only 50% of the target enrollment prior to closing early. Additionally, five patients in the nintedanib group did not complete the full planned 12 weeks of treatment. Another limitation is that the Kaplan-Meier estimates at the fixed time point for comparison are from a time point beyond the final exacerbation time; most exacerbations occurred within 150 days of randomization. However, despite the low enrollment and incomplete treatments in 5 patients, there was still notable improvement in freedom from exacerbations in the nintedanib arm when analyzed at one year as planned per protocol. Additional enrollment would have improved the reliability of these results, therefore we recommend a larger confirmatory study prior to adopting the use of nintedanib for radiation pneumonitis in general clinical practice.
Since the approved indications of nintedanib were broadened after it was found to be effective for chronic fibrosing ILDs of different etiologies, it would be of interest to determine whether the progression of radiation pneumonitis to pulmonary fibrosis can similarly be improved by this drug.22 However, a larger study would be needed to address this question due to the variability in PFT measurements and large range of baseline values. For example, to achieve 90% power to detect a difference of 100 mL of annual FVC decline in IPF with nintedanib in the INPULSIS-1 and INPULSIS-2 trials, 1066 patients were recruited.21 Though there was a trend towards improvement in some of the patient-reported outcomes in this trial, it did not reach significance. A larger study would be more likely to link these outcomes, as analysis from the health-related quality of life (HRQoL) outcomes from the INPULSIS trials showed that acute exacerbations and reduced pulmonary function tests were associated with declining HRQoL scores.32 The improvement in freedom from pulmonary exacerbations suggests that the benefit of nintedanib may result from improvement in the acute and subacute inflammation driven by the signaling pathways that nintedanib inhibits. This finding is consistent with the increased time to first acute exacerbation seen with nintedanib for IPF in the INPULSIS-2 trial.21 Therefore, another avenue for future work would be to determine whether nintedanib decreases the severity of pulmonary exacerbations after radiation treatment, which may be the case for nintedanib in IPF.27
The increased use of immunotherapy in patients who receive thoracic radiotherapy likely plays a role in the development of radiation pneumonitis. Adjuvant durvalumab has been adopted for patients with NSCLC after an overall survival benefit was shown in the PACIFIC trial, and this may increase the rate of radiation pneumonitis compared to chemoradiotherapy alone.33,34 This makes investigating the treatment of radiation pneumonitis all the more important, since developing this complication can delay or disrupt the use of adjuvant immunotherapy. Another factor that may contribute to an increase in cases of radiation pneumonitis in modern practice is the adoption of high dose radiotherapy and multiple courses of treatment for managing oligometastatic disease. The optimal timing, sequencing, and duration of nintedanib and steroids for the treatment of radiation pneumonitis has not been established. Since nintedanib targets the pathways involved in the transition from acute inflammation to chronic pulmonary fibrosis, we don’t expect the timing of nintedanib relative to steroid initiation to be critical, as steroids are likely effective at addressing the acute inflammatory process. Though the study investigating prophylaxis by Dy et al. did not sufficiently accrue, testing the use of nintedanib for the prophylaxis of radiation pneumonitis in patients at high risk, including those receiving concurrent immunotherapy, would also be of interest.29
This was the first prospective, randomized, placebo-controlled trial for the treatment of radiation pneumonitis and will serve as a benchmark for future trials. Though a corticosteroid taper is the standard of care for treating radiation pneumonitis, the optimal dose and duration have not previously been prospectively evaluated, and this study showed that acute exacerbations are common despite corticosteroid treatment. Further investigation of nintedanib in the treatment of radiation pneumonitis is warranted given the observed benefit in reducing pulmonary exacerbations at one year and promising results of nintedanib in other subacute and chronic respiratory illnesses. Evaluation of biomarker correlates and radiographic fibrosis will also be reported in the future.
Supplementary Material
Role of the funding source
The authors meet criteria for authorship as recommended by the International Committee of Medical Journal Editors (ICMJE). This was an independent, investigator-initiated study supported by Boehringer Ingelheim Pharmaceuticals, Inc. (BIPI). BIPI had no role in the design, analysis, or interpretation of the results in this study; BIPI was given the opportunity to review the manuscript for medical and scientific accuracy as it relates to BIPI substances, as well as intellectual property considerations.
Funding
This work was supported by Boehringer Ingelheim Pharmaceuticals, Inc. (BIPI); Fiona and Stanley Druckenmiller Center for Lung Cancer Research; and NIH/NCI Cancer Center Support Grant P30 CA008748.
Abbreviations
- RP
radiation pneumonitis
- G2+
grade 2 or higher
- RT
radiotherapy
- ILD
interstitial lung disease
- SSc-ILD
systemic sclerosis-associated interstitial lung disease
- IPF
idiopathic pulmonary fibrosis
- FVC
Forced vital capacity
- PRO-CTCAE
Patient Reported Outcomes version of the Common Terminology Criteria for Adverse Events
- SGRQ
St. George’s Respiratory Questionnaire
- PFT
pulmonary function test
- DLCO
diffusion capacity for carbon monoxide (DLCO)
- FEV1
forced expiratory volume in one second
- FVC
forced vital capacity
Footnotes
Declaration of interests
AR previously consulted for Boehringer Ingelheim. MZ participated in a separate investigator-initiated trial of nintedanib in mesothelioma with Boehringer Ingelheim funding. JEC has served as consultant to AstraZeneca, Bristol Myers Squibb, Genentech, Merck, Flame Biosciences, Novartis, Regeneron-Sanofi, Guardant Health, Janssen, and received research funding to institution from AstraZeneca, Bristol Myers Squibb, Genentech, Merck, and Novartis. CMR has consulted regarding oncology drug development with AbbVie, Amgen, AstraZeneca, Epizyme, Genentech/Roche, Ipsen, Jazz, Lilly, and Syros, and serves on the scientific advisory boards of Bridge Medicines, Earli, and Harpoon Therapeutics. DRJ has consulted for AstraZeneca and serves on the clinical trial steering committee for Merck. RM has served on advisory board for AstraZeneca. All other authors declare no competing interest.
Clinical Trial Registration
NIH ClinicalTrials.gov Identifier: NCT02452463
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data Availability Statement
Research data are stored in an institutional repository and will be shared upon request to the corresponding author.
References
- 1.Yorke ED, Jackson A, Rosenzweig KE, Braban L, Leibel SA, Ling CC. Correlation of dosimetric factors and radiation pneumonitis for non-small-cell lung cancer patients in a recently completed dose escalation study. Int J Radiat Oncol Biol Phys 2005; 63(3): 672–82. [DOI] [PubMed] [Google Scholar]
- 2.Yorke ED, Jackson A, Rosenzweig KE, et al. Dose-volume factors contributing to the incidence of radiation pneumonitis in non-small-cell lung cancer patients treated with three-dimensional conformal radiation therapy. Int J Radiat Oncol Biol Phys 2002; 54(2): 329–39. [DOI] [PubMed] [Google Scholar]
- 3.Movsas B, Raffin TA, Epstein AH, Link CJ Jr. Pulmonary radiation injury. Chest 1997; 111(4): 1061–76. [DOI] [PubMed] [Google Scholar]
- 4.Mehta V Radiation pneumonitis and pulmonary fibrosis in non-small-cell lung cancer: pulmonary function, prediction, and prevention. Int J Radiat Oncol Biol Phys 2005; 63(1): 5–24. [DOI] [PubMed] [Google Scholar]
- 5.Wang JY, Chen KY, Wang JT, et al. Outcome and prognostic factors for patients with non-small-cell lung cancer and severe radiation pneumonitis. Int J Radiat Oncol Biol Phys 2002; 54(3): 735–41. [DOI] [PubMed] [Google Scholar]
- 6.Carver JR, Shapiro CL, Ng A, et al. American Society of Clinical Oncology clinical evidence review on the ongoing care of adult cancer survivors: cardiac and pulmonary late effects. J Clin Oncol 2007; 25(25): 3991–4008. [DOI] [PubMed] [Google Scholar]
- 7.Williams JP, Johnston CJ, Finkelstein JN. Treatment for radiation-induced pulmonary late effects: spoiled for choice or looking in the wrong direction? Curr Drug Targets 2010; 11(11): 1386–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marks LB, Bentzen SM, Deasy JO, et al. Radiation dose-volume effects in the lung. Int J Radiat Oncol Biol Phys 2010; 76(3 Suppl): S70–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao J, Yorke ED, Li L, et al. Simple Factors Associated With Radiation-Induced Lung Toxicity After Stereotactic Body Radiation Therapy of the Thorax: A Pooled Analysis of 88 Studies. Int J Radiat Oncol Biol Phys 2016; 95(5): 1357–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yorke ED, Jackson A, Kuo LC, et al. Heart Dosimetry is Correlated With Risk of Radiation Pneumonitis After Lung-Sparing Hemithoracic Pleural Intensity Modulated Radiation Therapy for Malignant Pleural Mesothelioma. Int J Radiat Oncol Biol Phys 2017; 99(1): 61–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chun SG, Hu C, Choy H, et al. Impact of Intensity-Modulated Radiation Therapy Technique for Locally Advanced Non-Small-Cell Lung Cancer: A Secondary Analysis of the NRG Oncology RTOG 0617 Randomized Clinical Trial. J Clin Oncol 2017; 35(1): 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kong FM, Ten Haken R, Eisbruch A, Lawrence TS. Non-small cell lung cancer therapy-related pulmonary toxicity: an update on radiation pneumonitis and fibrosis. Semin Oncol 2005; 32(2 Suppl 3): S42–54. [DOI] [PubMed] [Google Scholar]
- 13.Kasmann L, Dietrich A, Staab-Weijnitz CA, et al. Radiation-induced lung toxicity - cellular and molecular mechanisms of pathogenesis, management, and literature review. Radiat Oncol 2020; 15(1): 214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang LW, Fu XL, Clough R, et al. Can angiotensin-converting enzyme inhibitors protect against symptomatic radiation pneumonitis? Radiat Res 2000; 153(4): 405–10. [DOI] [PubMed] [Google Scholar]
- 15.Ozturk B, Egehan I, Atavci S, Kitapci M. Pentoxifylline in prevention of radiation-induced lung toxicity in patients with breast and lung cancer: a double-blind randomized trial. Int J Radiat Oncol Biol Phys 2004; 58(1): 213–9. [DOI] [PubMed] [Google Scholar]
- 16.Werner-Wasik M, Scott C, Movsas B, et al. Amifostine as mucosal protectant in patients with locally advanced non-small cell lung cancer (NSCLC) receiving intensive chemotherapy and thoracic radiotherapy (RT): results of the radiation therapy oncology group (RTOG) 98–01 study. International Journal of Radiation Oncology, Biology, Physics 2003; 57(2): S216. [Google Scholar]
- 17.Abdollahi A, Li M, Ping G, et al. Inhibition of platelet-derived growth factor signaling attenuates pulmonary fibrosis. J Exp Med 2005; 201(6): 925–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu C, Wang F, Jin C, et al. Role of fibroblast growth factor type 1 and 2 in carbon tetrachloride-induced hepatic injury and fibrogenesis. Am J Pathol 2003; 163(4): 1653–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hamada N, Kuwano K, Yamada M, et al. Anti-vascular endothelial growth factor gene therapy attenuates lung injury and fibrosis in mice. J Immunol 2005; 175(2): 1224–31. [DOI] [PubMed] [Google Scholar]
- 20.Wollin L, Maillet I, Quesniaux V, Holweg A, Ryffel B. Antifibrotic and anti-inflammatory activity of the tyrosine kinase inhibitor nintedanib in experimental models of lung fibrosis. J Pharmacol Exp Ther 2014; 349(2): 209–20. [DOI] [PubMed] [Google Scholar]
- 21.Richeldi L, du Bois RM, Raghu G, et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med 2014; 370(22): 2071–82. [DOI] [PubMed] [Google Scholar]
- 22.Lamb YN. Nintedanib: A Review in Fibrotic Interstitial Lung Diseases. Drugs 2021; 81(5): 575–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Richeldi L, Kreuter M, Selman M, et al. Long-term treatment of patients with idiopathic pulmonary fibrosis with nintedanib: results from the TOMORROW trial and its open-label extension. Thorax 2018; 73(6): 581–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Flaherty KR, Wells AU, Cottin V, et al. Nintedanib in Progressive Fibrosing Interstitial Lung Diseases. N Engl J Med 2019; 381(18): 1718–27. [DOI] [PubMed] [Google Scholar]
- 25.Richeldi L, Costabel U, Selman M, et al. Efficacy of a tyrosine kinase inhibitor in idiopathic pulmonary fibrosis. N Engl J Med 2011; 365(12): 1079–87. [DOI] [PubMed] [Google Scholar]
- 26.Distler O, Highland KB, Gahlemann M, et al. Nintedanib for Systemic Sclerosis-Associated Interstitial Lung Disease. N Engl J Med 2019; 380(26): 2518–28. [DOI] [PubMed] [Google Scholar]
- 27.Kreuter M, Koegler H, Trampisch M, Geier S, Richeldi L. Differing severities of acute exacerbations of idiopathic pulmonary fibrosis (IPF): insights from the INPULSIS(R) trials. Respir Res 2019; 20(1): 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Ruysscher D, Granton PV, Lieuwes NG, et al. Nintedanib reduces radiation-induced microscopic lung fibrosis but this cannot be monitored by CT imaging: A preclinical study with a high precision image-guided irradiator. Radiother Oncol 2017; 124(3): 482–7. [DOI] [PubMed] [Google Scholar]
- 29.Dy GK, Prasad D, Kumar P, Attwood K, Adjei AA. A Phase 2 Randomized, Double-Blind, Placebo-Controlled Study Evaluating Nintedanib Versus Placebo as Prophylaxis Against Radiation Pneumonitis in Patients With Unresectable NSCLC Undergoing Chemoradiation Therapy. J Thorac Oncol 2021; 16(3): e19–e20. [DOI] [PubMed] [Google Scholar]
- 30.Chen CH, Lin HC, Wang YH, Wang CY, Lin YS, Lai CC. The safety of nintedanib for the treatment of interstitial lung disease: A systematic review and meta-analysis of randomized controlled trials. PLoS One 2021; 16(5): e0251636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lancaster L, Crestani B, Hernandez P, et al. Safety and survival data in patients with idiopathic pulmonary fibrosis treated with nintedanib: pooled data from six clinical trials. BMJ Open Respir Res 2019; 6(1): e000397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kreuter M, Wuyts WA, Wijsenbeek M, et al. Health-related quality of life and symptoms in patients with IPF treated with nintedanib: analyses of patient-reported outcomes from the INPULSIS(R) trials. Respir Res 2020; 21(1): 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Faivre-Finn C, Vicente D, Kurata T, et al. Four-Year Survival With Durvalumab After Chemoradiotherapy in Stage III NSCLC-an Update From the PACIFIC Trial. J Thorac Oncol 2021; 16(5): 860–7. [DOI] [PubMed] [Google Scholar]
- 34.Shaverdian N, Thor M, Shepherd AF, et al. Radiation pneumonitis in lung cancer patients treated with chemoradiation plus durvalumab. Cancer Med 2020; 9(13): 4622–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Research data are stored in an institutional repository and will be shared upon request to the corresponding author.


