Abstract
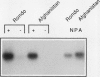
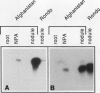
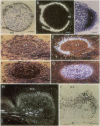
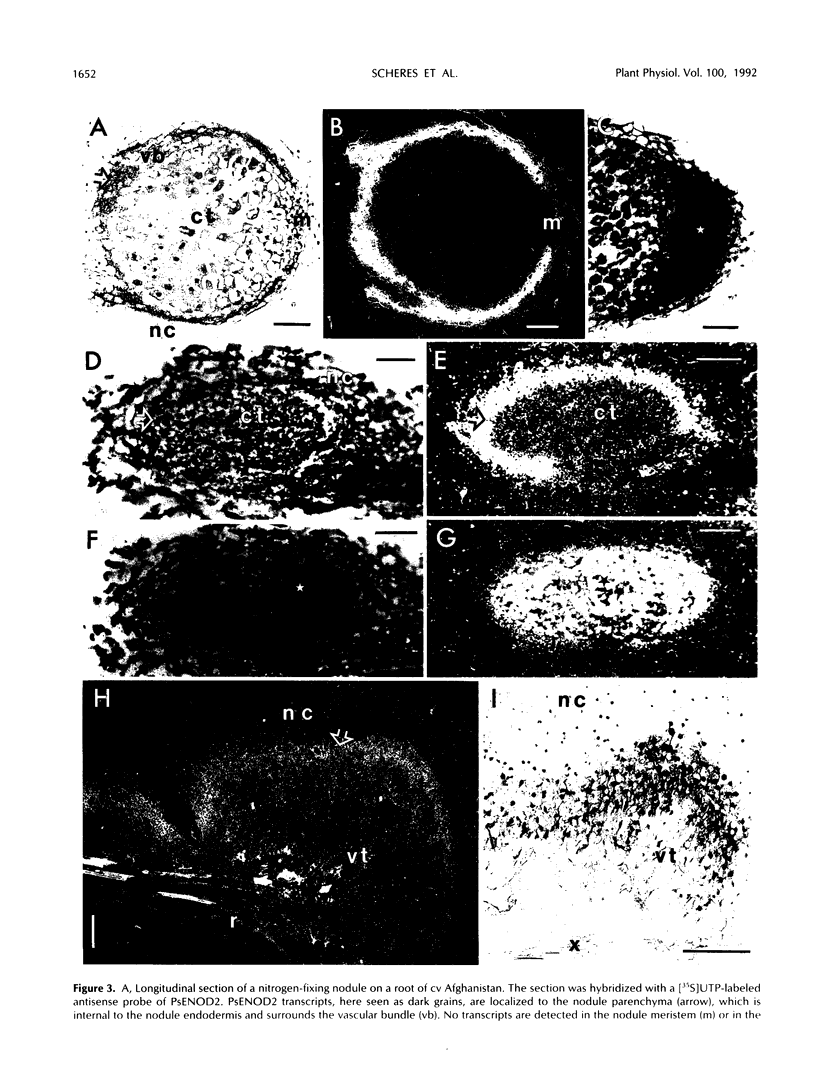
A number of early nodulin genes are expressed in specific cell types as pea (Pisum sativum) root nodules develop. The Pisum sativum early nodulin PsENOD2 is detected only in the uninfected cells of the nodule parenchyma, whereas PsENOD12 is expressed at two spatially removed sites: in root hairs and adjacent cortical cells, both of which can be invaded by Rhizobium entering through infection threads, and in derivatives of newly divided root inner cortical cells that establish the nodule primordium. We tested whether Rhizobium infection is required for triggering PsENOD12 gene expression by inducing nodule-like structures on Afghanistan pea roots with the auxin transport inhibitor N-(1-naphthyl)phthalamic acid (NPA). These nodule-like structures lack infection threads but resemble Rhizobium-induced nodules in other aspects. For one, both PsENOD2 and PsENOD12 transcripts were detected in these structures. PsENOD2 mRNA was localized by in situ hybridization to a zone equivalent to the nodule parenchyma of Rhizobium-induced nodules, whereas PsENOD12 transcripts were detected in a group of cells comparable to the nodule primordium of developing nodules. In addition, PsENOD12 mRNA was detected in uninfected root hairs 48 h after NPA treatment. These results indicate that infection is not a trigger for PsENOD12 gene expression in Afghanistan pea and rather suggest that the expression of the PsENOD2 and PsENOD12 genes is correlated with the differentiation of specific cell types in the developing nodule.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bisseling T., van den Bos R. C., van Kammen A. The effect of ammonium nitrate on the synthesis of nitrogenase and the concentration of leghemoglobin in pea root nodules induced by Rhizobium leguminosarum. Biochim Biophys Acta. 1978 Feb 13;539(1):1–11. doi: 10.1016/0304-4165(78)90115-0. [DOI] [PubMed] [Google Scholar]
- Dehio C., de Bruijn F. J. The early nodulin gene SrEnod2 from Sesbania rostrata is inducible by cytokinin. Plant J. 1992 Jan;2(1):117–128. doi: 10.1046/j.1365-313x.1992.t01-51-00999.x. [DOI] [PubMed] [Google Scholar]
- Dickstein R., Bisseling T., Reinhold V. N., Ausubel F. M. Expression of nodule-specific genes in alfalfa root nodules blocked at an early stage of development. Genes Dev. 1988 Jun;2(6):677–687. doi: 10.1101/gad.2.6.677. [DOI] [PubMed] [Google Scholar]
- Faucher C., Maillet F., Vasse J., Rosenberg C., van Brussel A. A., Truchet G., Dénarié J. Rhizobium meliloti host range nodH gene determines production of an alfalfa-specific extracellular signal. J Bacteriol. 1988 Dec;170(12):5489–5499. doi: 10.1128/jb.170.12.5489-5499.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fisher R. F., Long S. R. Rhizobium--plant signal exchange. Nature. 1992 Jun 25;357(6380):655–660. doi: 10.1038/357655a0. [DOI] [PubMed] [Google Scholar]
- Franssen H. J., Nap J. P., Gloudemans T., Stiekema W., Van Dam H., Govers F., Louwerse J., Van Kammen A., Bisseling T. Characterization of cDNA for nodulin-75 of soybean: A gene product involved in early stages of root nodule development. Proc Natl Acad Sci U S A. 1987 Jul;84(13):4495–4499. doi: 10.1073/pnas.84.13.4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch A. M., Bhuvaneswari T. V., Torrey J. G., Bisseling T. Early nodulin genes are induced in alfalfa root outgrowths elicited by auxin transport inhibitors. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1244–1248. doi: 10.1073/pnas.86.4.1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs M., Gilbert S. F. Basal localization of the presumptive auxin transport carrier in pea stem cells. Science. 1983 Jun 17;220(4603):1297–1300. doi: 10.1126/science.220.4603.1297. [DOI] [PubMed] [Google Scholar]
- Klein S., Hirsch A. M., Smith C. A., Signer E. R. Interaction of nod and exo Rhizobium meliloti in alfalfa nodulation. Mol Plant Microbe Interact. 1988 Feb;1(2):94–100. doi: 10.1094/mpmi-1-094. [DOI] [PubMed] [Google Scholar]
- Lerouge P., Roche P., Faucher C., Maillet F., Truchet G., Promé J. C., Dénarié J. Symbiotic host-specificity of Rhizobium meliloti is determined by a sulphated and acylated glucosamine oligosaccharide signal. Nature. 1990 Apr 19;344(6268):781–784. doi: 10.1038/344781a0. [DOI] [PubMed] [Google Scholar]
- Nap J. P., Bisseling T. Developmental biology of a plant-prokaryote symbiosis: the legume root nodule. Science. 1990 Nov 16;250(4983):948–954. doi: 10.1126/science.250.4983.948. [DOI] [PubMed] [Google Scholar]
- Norris J. H., Macol L. A., Hirsch A. M. Nodulin gene expression in effective alfalfa nodules and in nodules arrested at three different stages of development. Plant Physiol. 1988 Oct;88(2):321–328. doi: 10.1104/pp.88.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheres B., Van De Wiel C., Zalensky A., Horvath B., Spaink H., Van Eck H., Zwartkruis F., Wolters A. M., Gloudemans T., Van Kammen A. The ENOD12 gene product is involved in the infection process during the pea-Rhizobium interaction. Cell. 1990 Jan 26;60(2):281–294. doi: 10.1016/0092-8674(90)90743-x. [DOI] [PubMed] [Google Scholar]
- Schultze M., Quiclet-Sire B., Kondorosi E., Virelizer H., Glushka J. N., Endre G., Géro S. D., Kondorosi A. Rhizobium meliloti produces a family of sulfated lipooligosaccharides exhibiting different degrees of plant host specificity. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):192–196. doi: 10.1073/pnas.89.1.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaink H. P., Sheeley D. M., van Brussel A. A., Glushka J., York W. S., Tak T., Geiger O., Kennedy E. P., Reinhold V. N., Lugtenberg B. J. A novel highly unsaturated fatty acid moiety of lipo-oligosaccharide signals determines host specificity of Rhizobium. Nature. 1991 Nov 14;354(6349):125–130. doi: 10.1038/354125a0. [DOI] [PubMed] [Google Scholar]
- Van De Wiel C., Norris J. H., Bochenek B., Dickstein R., Bisseling T., Hirsch A. M. Nodulin Gene Expression and ENOD2 Localization in Effective, Nitrogen-Fixing and Ineffective, Bacteria-Free Nodules of Alfalfa. Plant Cell. 1990 Oct;2(10):1009–1017. doi: 10.1105/tpc.2.10.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]