Highlights
-
•
Insomnia is common in patients with cancer, with a higher prevalence than observed in the general population.
-
•
Insomnia is often under-recognised and inadequately treated in patients with cancer.
-
•
Brief validated screening tools are available for the evaluation of insomnia in clinical practice.
-
•
First-line therapy should be based on international guidelines recommending cognitive behavioural therapy for insomnia.
Key words: insomnia, cancer, oncology, psychiatry, psycho-oncology
Introduction
Sleep disturbance is a common problem in patients with cancer, regardless of cancer type, stage and phase of treatment.1 Sleep disorders can be identified using the criteria of the World Health Organization International Classification of Diseases 11th edition (ICD-11) (updated chapter on ‘Sleep–wake disorders’),2 the American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders fifth edition – Text Revision (DSM-5-TR)3 and the American Academy of Sleep Medicine (AASM) International Classification of Sleep Disorders (ICSD) third edition.4
The three classification systems describe a series of disorders and conditions, including sleep–wake disorders (insomnia), sleep-related breathing disorders, parasomnias, sleep-related movement disorders and circadian rhythm sleep–wake disorders (see Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2023.102047). Insomnia is by far the most frequent and clinically significant problem in patients with cancer.1,5 Sleep health is important in oncology at many different levels, including influences on the immune system, neuroendocrinological function, cognitive function, general well-being and quality of life (QoL).6 It is therefore mandatory for cancer clinicians to regularly screen their patients for insomnia. Furthermore, it is necessary to distinguish episodic from persistent insomnia, to assess the specific dimensions of insomnia and the negative consequences for patients, and to treat the disorder according to evidence-based guidelines.7
This Clinical Practice Guideline (CPG) presents an up-to-date, evidence-based approach to assessing and managing insomnia disorder in patients with cancer and cancer survivors. The authors followed the levels of evidence and grades of recommendation as detailed in the ‘Methodology’ section.
Definition, incidence and prevalence
Insomnia is defined as difficulties falling asleep and/or maintaining sleep that cause distress and have a negative impact on daily functioning. Insomnia is the most common sleep disorder, with a prevalence that is estimated to be 6%-10% in the general population and three times greater in patients with cancer and cancer survivors.1,5 Moreover, up to 95% of patients with cancer report sleep disturbances during the disease and treatment trajectory, as well as in survivorship and near the end of life.8 Variations in insomnia symptoms may be associated more with different cancer treatments than cancer site. Symptoms often peak following diagnosis, but may develop or worsen during cancer treatment.9 Insomnia frequently follows a chronic course, is strongly correlated with depressive symptoms and fatigue10 and is associated with hyperarousal,11 pain and work-related worries.12 Caregivers of patients with cancer may also experience poor quality of sleep, which in turn is associated with a reduced ability to cope with emotional problems.13 In summary, disturbed sleep remains a neglected problem in oncology, with respect to not only screening, but also providing adequate management.
Consequences
Insomnia has been associated with an increased risk of mental and physical health problems, including irritability, anxiety, depression, impaired QoL and greater disability and mortality, both in the general population and specifically in oncology.14
Decades of research into the causes of chronic insomnia have identified physiological, cognitive and emotional arousal as key factors. Hyperarousal and increased mental and physiological stress-reactivity have been hypothesised to contribute to the maintenance of insomnia by interacting with negative cognitions, dysfunctional beliefs about sleep and negative sleep-related behaviours. The ‘allostatic load’ theory suggests that sleep loss is both a precipitant of stress and a consequence of it, with evidence to suggest that insomnia-related stress may lead to persistent dysregulation of multiple biological systems15 and various adverse cancer-related outcomes. Furthermore, stressors and challenges related to cancer and its treatment can cause insomnia or worsen existing insomnia, which then exacerbates comorbid medical conditions such as pain, psychiatric conditions, daytime fatigue and somnolence, sleep-disordered breathing and napping-associated pain increase.16 Thus a deleterious feedback loop may be created which maintains or exacerbates insomnia and cancer-related comorbid conditions, including depression, anxiety, post-traumatic stress disorder symptoms, daytime fatigue and pain.
Symptoms of insomnia, depression and fatigue can maintain and amplify each other in patients with cancer via multiple mechanisms. Insomnia may lead to depression by dysregulating multiple systems involved in mood disorders. It is associated with a marked decrease in T cells and increased levels of proinflammatory markers, such as C-reactive protein, interleukin-6 and tumour necrosis factor, and may promote a state of chronic inflammation which contributes to depression. However, the role of insomnia in modulating the onset and outcome of cancer remains unclear.17
Cancer-related fatigue is a syndrome characterised by physical, mental and emotional symptoms, including generalised weakness, diminished concentration or attention, reduced motivation to engage in usual activities and emotional lability, similar to the symptoms that characterise depression.18 The aetiology of cancer-related fatigue may be best explained by a multifactorial model that considers not only the biological mechanisms but also psychological and behavioural factors, including insomnia, which has been identified as one of seven factors most commonly associated with cancer-related fatigue. Several biological mechanisms may explain the bidirectional associations among cancer-related insomnia, depression and fatigue, including cytokine and hypothalamic–pituitary–adrenal axis dysregulation.18 Treatment of insomnia is therefore recommended, not only in patients undergoing active cancer treatment, but also in long-term cancer survivors and patients undergoing palliative care.
Persistent insomnia in cancer may become a perpetuating factor in a vicious cycle of fatigue, depression and other cancer-related symptoms. These are promoted by insomnia-related dysregulations of the immune and endocrine systems, in combination with the effects of cancer treatment and the cancer itself. Insomnia can have significant consequences in patients with cancer, including an increased risk of infection, persistent symptoms after chemotherapy (ChT) and poorer recovery from depression and anxiety, leading to reduced overall well-being and QoL.19 Assessing insomnia and providing efficacious interventions can help to alleviate symptoms and late effects, as well as improving mental health, physical health and QoL in patients with cancer and cancer survivors.
Risk factors
The currently accepted risk factor model for insomnia is the diathesis–stress model, commonly known as the 3-P model, which describes predisposing, precipitating and perpetuating factors relevant to the development and maintenance of persistent insomnia. In patients with cancer, the 3-P model is useful for understanding the interaction between the general and cancer-specific factors involved in cancer-related insomnia (see Supplementary Table S2, available at https://doi.org/10.1016/j.esmoop.2023.102047).
Predisposing factors for insomnia are genetic, physiological and psychological factors that confer differential susceptibility to individuals in their sleep-related responses to stress. Such factors include advanced age, female sex, an anxiety-prone personality, tendency to ruminate and a family or personal history of insomnia and/or anxiety or depression. Circadian rhythm disruption could also contribute to the development of insomnia in these patients.
Precipitating factors include physiological, environmental and psychological stressors which may interact with predisposing factors to produce acute insomnia symptoms. Patients with cancer and cancer survivors are exposed to different types of precipitating factors for insomnia along the cancer trajectory. These include stress and distress related to the cancer diagnosis and cancer treatments, such as ChT, radiotherapy and hormone therapy. Cancer itself, as well as surgery, hospitalisation, symptoms and various treatment-related side-effects, may disrupt circadian rhythms and contribute to insomnia. In some cancers (e.g. breast cancer), antihormonal treatments may induce menopausal symptoms contributing to the development of sleep disorders.
Perpetuating factors include behavioural, cognitive and environmental factors that contribute to the maintenance and exacerbation of insomnia. Perpetuating factors common to all patients with cancer include maladaptive behaviours and beliefs that patients use to cope with sleep difficulties. Detrimental behaviours include spending extended time in bed, taking frequent and long naps, following an irregular sleep schedule and being physically inactive. Catastrophising about harms related to the inability to sleep and the daytime consequences of poor sleep may increase mental and physiological arousal that delays sleep onset and causes frequent, prolonged awakenings in patients with cancer.20
While the precipitating factors of insomnia are likely to differ between patients with cancer and the general population, most of the predisposing and perpetuating factors of primary insomnia are similar to those of insomnia in patients with cancer and in cancer survivors with comorbid insomnia. As the recommended behavioural treatments for insomnia target the perpetuating factors, the treatments that are efficacious in patients with insomnia as the primary diagnosis are also likely to be efficacious for treating insomnia in cancer settings.
Diagnosis
The diagnosis of insomnia is similar across the nosological systems, although some details differ between systems (see Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2023.102047).
In the DSM-5-TR, insomnia disorder is diagnosed as a complaint of dissatisfaction with sleep quantity or quality, associated with one or more of the three main symptoms (i.e. difficulties falling asleep, staying asleep and early awakening), with sleep difficulty occurring ≥3 nights per week, lasting ≥3 months, persisting despite adequate opportunities for sleep and causing significant impairment in daily life. Insomnia can be episodic (symptoms lasting ≥1 month but <3 months), persistent (symptoms lasting ≥3 months) or recurrent (at least two episodes within the space of 1 year). In this framework, insomnia is considered a 24-hour sleep–wake disorder, characterised by both nocturnal and diurnal symptoms.
The insomnia criteria in ICD-11 are the same as in DSM-5-TR, but ICD-11 specifies the symptoms secondary to sleep difficulties (e.g. fatigue or malaise; attention, concentration or memory impairment; mood disturbance or irritability). ICD-11 also classifies insomnia as short term (lasting <3 months) or chronic (lasting ≥3 months).
The ICSD uses the same criteria as DSM-5-TR and ICD-11, but emphasises that people who report sleep-related symptoms in the absence of daytime impairment are not regarded as having an insomnia disorder.
The frequency, severity and pattern of insomnia symptoms may vary between different cancer types and treatment regimens.
Screening and assessment
There is currently no standard tool that is routinely used to evaluate insomnia in the clinic.
The Distress Thermometer and Problem Check List (PCL), developed by the National Comprehensive Cancer Network (NCCN) within the distress management guidelines, is an internationally used screening instrument. ‘Sleep problems’ (in a yes or no format) are included in the PCL.21 The NCCN CPGs in Oncology for survivorship22 also suggest asking all cancer survivors “Are you having trouble falling asleep, staying asleep or waking up too early?” as part of a battery of survivorship-related questions. The United States National Cancer Institute’s Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events23 also includes two items on insomnia. There is no evidence, however, that this has been widely adopted in clinical practice.24 Insomnia is included in the Edmonton Symptom Assessment System, which is frequently used in routine clinical practice as a screening tool in patients with cancer due to its brevity, multidimensional domains and validation in the greatest number of languages.25
For a comprehensive evaluation of insomnia, a structured clinical interview is required (e.g. the structured clinical interview for DSM-5-TR sleep disorders).26 Addressing all significant factors that may interfere with sleep requires the evaluation of nocturnal symptoms (type, frequency and duration), daytime symptoms (degree of sleepiness and extent of daytime consequences), the presence of comorbid conditions (mental and/or medical and/or another sleep disorder) and the daytime lifestyle of the patient (e.g. use of substances, work schedule, school, social activities, light exposure, eating habits, exercise and preferred bedtime and wake-up times).27 A full clinical interview is rarely feasible in the oncology setting, but can be replaced with preconsultation assessment with one of several validated questionnaires.
The Insomnia Severity Index (ISI)28 is probably the most commonly used instrument to assess insomnia severity and has been validated in patients with cancer.29 Total scores range from 0 to 28, with higher values indicating more severe insomnia. A score of 8-14 indicates subthreshold insomnia, and scores ≥15 indicate clinical insomnia.
The Sleep Condition Indicator is an eight-item rating scale developed to screen for insomnia disorder based on DSM-5-TR criteria.30 It measures concerns about getting to sleep, remaining asleep, sleep quality (SQ), daytime personal functioning, daytime performance, duration of the sleep problem, nights per week having a sleep problem and the extent to which the patient is troubled by poor sleep. Total scores range from 0 to 32, with lower values indicating poorer sleep. A score ≤16 is considered a ‘probable insomnia disorder’.
The Pittsburgh Sleep Quality Index (PSQI)31 does not assess insomnia, but the broader concept of SQ. It is one of the most frequently used instruments in sleep research and its internal reliability and construct validity has been confirmed in oncology,32 with a study confirming the association between poor sleep and poor QoL in patients with early breast cancer.33 Global SQ scores range from 0 to 21 and a global score >5 is indicative of poor SQ. While the PSQI captures a broader spectrum of sleep than an insomnia severity scale, it may be less relevant in the daily clinical oncology setting. Further, the numerous items increase patient burden, the scoring is rather cumbersome and the index is not intended as a screening instrument for insomnia.
There is agreement among sleep researchers that it can be useful for insomnia sufferers to monitor their sleep over time on a night-by-night basis using diaries to identify maladaptive sleep patterns and track treatment effects.34 The Consensus Sleep Diary (CSD)35 was developed by an expert panel and asks patients to record their sleep, including the time they went to bed, the time they tried to fall asleep, how long it took them to fall asleep, how many times they woke up, how long these awakenings lasted, the time of their final awakening, the time they got out of bed and the perceived quality of their sleep.
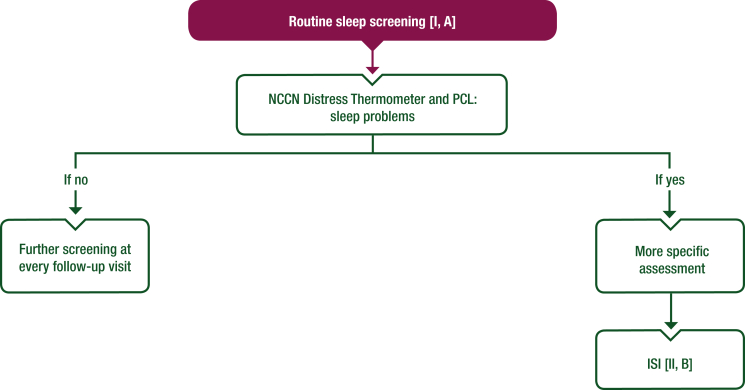
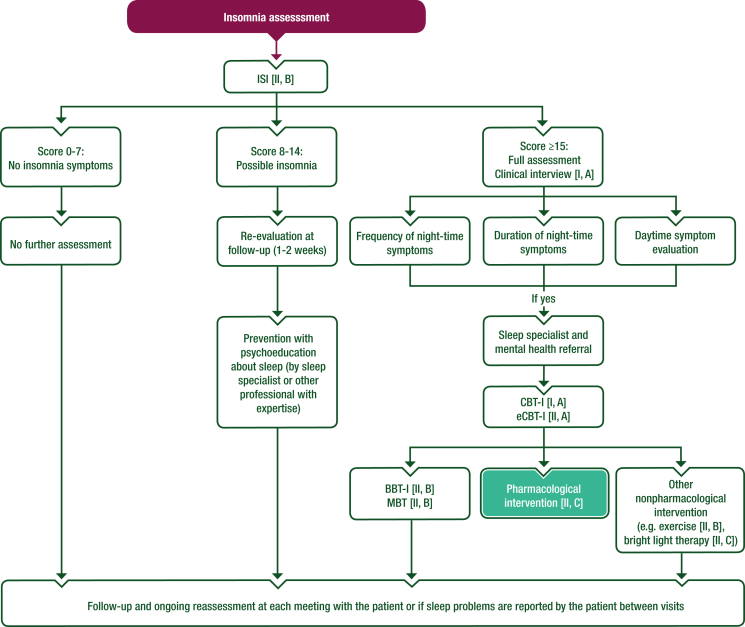
Objective measures of sleep such as polysomnography or actigraphy are not routinely required for insomnia diagnosis and evaluation but can be considered as a part of detailed exploration of sleep according to the patient’s clinical condition.16 Table 1 describes the most important tools to assess insomnia disorders in patients with cancer. Algorithms for the screening and management of insomnia are also represented in Figures 1 and 2 as adaptations of the most commonly used national guidelines.7,21,22
Table 1.
Examples of the most frequently used psychometric and nonpsychometric validated tools to assess insomnia disorders in patients with cancer
| Measures | Description |
|---|---|
| Scales | |
| ISI28 | The ISI is a self-report seven-item scale investigating sleep over the past 14 days (difficulties falling asleep, difficulties maintaining sleep, early morning awakenings, satisfaction or dissatisfaction with the current sleep pattern, how noticeable the effect on well-being is to others, how distressing the current sleep problem is and to what extent it interferes with daily functioning) Each item is scored on a four-point scale (score range 0-28) A score ≥8 indicates subthreshold insomnia and a score ≥15 indicates clinical insomnia |
| SCI30 | The SCI is an eight-item scale, comprising two quantitative items on sleep continuity (item 1: getting to sleep; item 2: remaining asleep), two qualitative items on sleep satisfaction or dissatisfaction (item 4: SQ; item 7: troubled or not), two quantitative items on severity (item 3: nights per week; item 8: duration of problem) and two qualitative items on attributed daytime consequences of poor sleep [item 5: effects on mood, energy or relationships (personal functioning); item 6: effects on concentration, productivity or ability to stay awake (daytime performance)] Each item is scored on a five-point scale (0-4), with lower scores in the 0-2 range reflecting putative DSM-5 threshold criteria for insomnia disorder Possible total score ranges from 0 to 32, with higher values indicative of better sleep. A score ≤16 is considered a ‘probable insomnia disorder’ To facilitate interpretation for clinicians and patients, total scores can be converted to a 0-10 scale by dividing the total by 3.2, where 10 represents the best possible sleep |
| PSQI31 | The self-administered PSQI measures sleep disturbance and usual sleep habits during the prior month through 19 items on a 0-3 Likert scale in seven clinically derived domains of sleep difficulties (SQ, sleep latency, sleep duration, habitual SE, sleep disturbances, use of sleeping medications and daytime dysfunction) A higher global SQ score (sum of the seven domains, score range 0-21) indicates poorer SQ A score of >5 has a diagnostic sensitivity of 89.6% and specificity of 86.5% to distinguish ‘poor’ sleepers from ‘good’ sleepers (healthy individuals) |
| Single items | |
| US NCI PRO-CTCAE23 | In the last 7 days, what was the severity of your insomnia (including difficulty falling asleep, staying asleep or waking up early) at its worst? [Score range 0 (none) to 4 (very severe)] In the last 7 days, how much did insomnia (including difficulty falling asleep, staying asleep or waking up early) interfere with your usual or daily activities? [Score range 0 (not at all) to 4 (very much)] |
| NCCN distress management in oncology21 and NCCN survivorship in oncology22 guidelines | Are you having problems falling asleep, staying asleep, waking up too early or with poor SQ? Are you experiencing excessive sleepiness (sleepiness or falling asleep in inappropriate situations or sleeping more during a 24-hour period than in the past)? Have you been told that you snore frequently or stop breathing during sleep? |
| Sleep diary | |
| CSD35 | The CSD asks patients to record (e.g. every morning for 7-10 days) the following aspects of their sleep:
|
CSD, Consensus Sleep Diary; DSM-5, Diagnostic and Statistical Manual of Mental Disorders fifth edition; ISI, Insomnia Severity Index; NCCN, National Comprehensive Cancer Network; NCI, National Cancer Institute; PRO-CTCAE, Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events; PSQI, Pittsburgh Sleep Quality Index; SCI, Sleep Condition Indicator; SE, sleep efficiency; SOL, sleep onset latency; SQ, sleep quality; TIB, time in bed; TST, total sleep time; US, United States; WASO, wakefulness after sleep onset.
Figure 1.
Proposed algorithm for screening of insomnia in patients with cancer and cancer survivors. Purple: general categories or stratification; white: other aspects of management.
ISI, Insomnia Severity Index; NCCN, National Comprehensive Cancer Network; PCL, Problem Check List.
Figure 2.
Proposed algorithm for the management of insomnia in patients with cancer and cancer survivors. Purple: general categories or stratification; white: other aspects of management; turquoise: systemic therapy.
BBT-I, brief behavioural therapy for insomnia; CBT-I, cognitive behavioural therapy for insomnia; eCBT-I, digitally delivered cognitive behavioural therapy for insomnia; ISI, Insomnia Severity Index; MBT, mindfulness-based therapy.
Recommendations
-
•
All patients with cancer should be regularly screened and assessed for insomnia during all phases of their treatment (e.g. after diagnosis and prior to surgery or any other primary treatment) and during the survivorship trajectory [I, A].
-
•
Validated screening tools should be used to assess insomnia severity on a regular basis in patients who have screened positive for insomnia [II, B].
-
•
The ISI is recommended for assessment of insomnia severity in patients with cancer [II, B].
-
•
A clinical interview is needed for a detailed insomnia evaluation [I, A].
-
•
The CSD may be useful for a detailed evaluation of insomnia and treatment effects [III, C].
Management
Treatment of insomnia in patients with cancer is based on a combined approach to address both precipitating factors (e.g. hot flashes, pain, nocturia) and perpetuating factors (e.g. maladaptive sleep behaviours, dysfunctional beliefs about sleep), with nonpharmacological approaches used as first-line treatment. Pharmacological approaches to reduce precipitating factors and improve sleep have been described.7 The evidence supporting different approaches in the management of insomnia in patients with cancer and cancer survivors is summarised in Table 2 (see Supplementary Table S3, available at https://doi.org/10.1016/j.esmoop.2023.102047, for a full list of relevant studies).
Table 2.
Evidence-based recommendations regarding treatment for insomnia in patients with cancer and cancer survivorsa
| Intervention (based on studies involving different cancer types and treatment status) | LoE | GoR | Example of evidence (SRMA or RCT) |
|---|---|---|---|
| Psychological interventions | |||
| Face-to-face CBT-I | |||
|
I | A | Johnson et al. (2016)55 (SRMA) |
|
I | A | Berger et al. (2009)45 (RCT) |
|
I | A | Matthews et al. (2014)49 (RCT) |
| eCBT-I | |||
|
II | A | Zachariae et al. (2018)61 (RCT) |
|
II | A | Savard et al. (2014)50 (RCT) |
| BBT-I | |||
|
II | B | Casault et al. (2015)65 (RCT) |
|
II | B | Palesh et al. (2020)66 (RCT) |
| MBT | |||
|
II | B | Lengacher et al. (2015)72 (RCT) |
|
II | B | Zhang et al. (2017)74 (RCT) |
| Pharmacotherapy | |||
| Hypnotics [benzodiazepines (triazolam), nonbenzodiazepines (eszopiclone, zolpidem)] | |||
|
II | C | Jacobsen et al. (1994)80 (RCT) |
|
II | B | Dimsdale et al. (2011)81 (RCT) |
|
II | B | Jakobsen et al. (2022)110 (RCT) |
| Melatonin | |||
|
II | B | Hansen et al. (2014)88 (RCT) |
| Palmer et al. (2020)90 (RCT) | |||
|
II | B | Yennurajalingam et al. (2021)83 (RCT) |
| Shahrokhi et al. (2021)82 (RCT) | |||
| Other approaches | |||
| Physical exercise | |||
|
II | B | Chen et al. (2016)99 (RCT) |
|
II | B | Nguyen et al. (2021)102 (RCT) |
| Bright light therapy | |||
|
II | C | Wu et al. (2021)108 (RCT) |
|
II | C | Fox et al. (2021)107 (RCT) |
BBT-I, brief behavioural therapy for insomnia; CBT-I, cognitive behavioural therapy for insomnia; eCBT-I, digitally delivered cognitive behavioural therapy for insomnia; GoR, grade of recommendation; LoE, level of evidence; MBT, mindfulness-based therapy; RCT, randomised controlled trial; SRMA, systematic review and meta-analysis.
See Supplementary Table S3, available at https://doi.org/10.1016/j.esmoop.2023.102047, for a full list of relevant studies.
Psychological interventions
Cognitive behavioural therapy for insomnia
Various cognitive behavioural strategies to treat insomnia have been developed over the past 3-4 decades. While these approaches have been used as stand-alone therapies, they are most often offered in combination and termed cognitive behavioural therapy for insomnia (CBT-I). The robust evidence for CBT-I and the risk of adverse events (AEs) with hypnotics have led the American College of Physicians, the European Sleep Research Society and the AASM to recommend CBT-I as the standard of care for treating insomnia.36, 37, 38
The intervention strategies in CBT-I target various cognitive, emotional and physiological perpetuating factors that are known to contribute to the maintenance of insomnia. Sleep restriction therapy aims to reduce sleep onset latency (SOL) and increase sleep efficiency (SE); initially, the time spent in bed is restricted, and then it is gradually increased as SE improves. Stimulus-control therapy aims to re-establish associations of the bed and bedroom with sleepiness and sleep, thus replacing the conditioned arousal which patients with insomnia commonly experience at bedtime. Relaxation helps the patient to reduce the mental and physiological arousal that inhibits sleep.39 Cognitive therapy targets the maladaptive cognitions that interfere with falling asleep, such as rumination and sleep-related catastrophising.40 Finally, sleep hygiene education helps the patient to eliminate behaviours and lifestyle factors that make it difficult to fall asleep and maintain sleep during the night. These include drinking caffeinated beverages and alcohol late in the day, taking naps during the day and irregular bedtimes.
A number of studies have examined the effects of CBT-I on comorbid insomnia in patients with various medical and psychiatric disorders. A meta-analysis of 23 studies of patients with disorders such as chronic pain syndromes, post-traumatic stress disorder, major depressive disorder and cancer found medium to large effects of CBT-I on insomnia severity [standard mean difference (SMD) 1.22], SQ (SMD 0.88), SOL (SMD 0.75) and SE (SMD 0.93).41 The smallest effect was observed for total sleep time (TST; SMD 0.25).
At least nine randomised controlled trials (RCTs) comparing CBT-I with treatment as usual, waiting list and attention–control conditions in patients with cancer and cancer survivors have been published.42, 43, 44, 45, 46, 47, 48, 49, 50 The reported effects range from small to large for SQ (SMDs 0.19-1.16), SOL (SMDs 0.11-0.86) and SE (SMDs 0.06-1.09), and from small negative to medium positive effects for TST (SMDs −0.11 to 0.67). Several RCTs have compared CBT-I with various active treatments, including mindfulness-based stress reduction (MBSR),51 aerobic exercise,52 CBT-I without the sleep restriction component53 and a stepped-care version of CBT-I.54 CBT-I generally outperformed other active treatments.
A meta-analysis of eight RCTs evaluating CBT-I in cancer survivors revealed statistically significant medium to medium-large effects on SQ (SMD 0.76), SOL (SMD 0.58) and SE (SMD 0.53).55 The results appear robust, with effects that are similar to those reported in other meta-analyses and of a clinically relevant magnitude. It should be noted that this meta-analysis included studies comparing CBT-I with active treatment51 as well as pooled data from nonindependent samples.50 Two recent meta-analyses reported that CBT-I is effective for the management of insomnia in patients with cancer and survivors. The first, which included 16 trials with 1523 participants, indicated that CBT-I reduced insomnia severity, SOL and wake after sleep onset, and increased SE and TST.56 The second, which included 22 studies and 1461 participants, demonstrated robust efficacy of CBT-I for insomnia in cancer survivors (effect size for insomnia severity Cohen’s g = 0.78) as well as small but statistically significant improvements in fatigue, depression, anxiety symptoms and QoL after treatment.57
The available studies support robust and clinically meaningful effects of CBT-I on self-reported SQ, SOL and SE. As observed in noncancer studies, the smallest effects are observed for TST. The ISI and the PSQI were the most commonly used measures of SQ. Eight of the 17 studies monitored AEs and found none to be associated with CBT-I. It should also be noted that 17 of the 19 studies were conducted with cancer survivors, with 7 of these focusing on breast cancer survivors. It thus remains unclear whether CBT-I is effective in patients in active treatment.
Digitally delivered CBT-I
While face-to-face CBT-I is highly efficacious in treating insomnia and is desired by many patients who prefer nonpharmacological approaches,58 it has been challenging to make it available and accessible to meet population needs. The main barriers to access are the lack of trained therapists, costs, and physical and geographical constraints, the latter being particularly challenging for many patients with cancer and cancer survivors. One approach to making CBT-I more accessible is to deliver treatment online in digital formats (eCBT-I).59 A meta-analysis of 11 RCTs including 1460 participants with insomnia has shown eCBT-I to be highly efficacious, with benefits comparable with face-to-face CBT-I.60 Three RCTs evaluating eCBT-I have enrolled cancer survivors, including breast cancer survivors61, 62, 63 and the effects on self-reported SQ, SOL and SE were similar or larger than those observed with face-to-face CBT-I. AEs were only examined in one study, finding none attributable to the intervention.61 While the available evidence is of moderate quality due to the limited number of studies in patients with cancer and cancer survivors, the effects of eCBT-I on SQ are highly promising.
Brief behavioural therapy for insomnia
An alternative approach aimed at reducing costs and minimising patient burden is to deliver a less intensive, scaled down version of CBT-I, termed brief behavioural therapy for insomnia (BBT-I). In one study, a three-session BBT-I intervention was delivered by a nurse over the telephone.64 A similar minimal intervention consisted of six booklets and three telephone-based sessions with a psychologist.65 Another BBT-I investigated in two trials involved two 60-minute face-to-face sessions with trained staff and four 15-minute telephone calls,66,67 while a further study evaluated nurse-delivered BBT-I consisting of two face-to-face sessions, two telephone-delivered sessions and two telephone-based follow-up sessions.68 Patients in all studies were in active treatment for their cancer. Statistically significant improvements in SQ were found in four out of five studies, with effect sizes ranging from small (SMD 0.45)66 to large (SMD 1.62).68 Only one study examined AEs and found none attributable to the intervention.67 Although BBT-I appears promising, the available evidence is weak due to the small number of studies and lack of results on sleep-related outcomes other than SQ.
Mindfulness-based therapy
In recent years, there has been a growing interest in exploring the efficacy of mindfulness-based therapy (MBT), particularly MBSR, in treating insomnia. MBT involves attention training with a focus on the present moment in a nonjudgemental and self-compassionate manner.69 It is hypothesised to target mental responses, such as rumination and emotional reactivity, that contribute to maintaining sleep disturbances. A recent systematic review and meta-analysis explored the efficacy of MBT on self-reported SQ across 18 trials of participants with primary insomnia or insomnia comorbid with various illnesses.70 While there was no difference in SQ when compared with sleep-specific active control conditions such as relaxation, exercise and CBT-I (SMD −0.03), MBT was superior to nonspecific attention–control conditions, such as health education and sleep hygiene education, in improving SQ (SMD 0.33). No data were reported for other sleep outcomes.
Although several studies have explored the efficacy of MBT in treating various cancer-related symptoms, including SQ, only four studies have included effects on insomnia as a primary outcome.71, 72, 73, 74 Significant improvements in SQ were reported in three studies, with effect sizes ranging from small (SMD 0.11)72 to large (SMD 0.95).74 Overall, the evidence provided by the available studies is weak, with most outcomes limited to self-reported SQ. It remains unknown whether there are any AEs associated with MBT.
Pharmacotherapy
Some classes of drugs, particularly hypnotics, are widely used to treat insomnia. While cancer treatments are generally associated with fatigue, anticancer drugs may promote insomnia. When possible, it is therefore a priority to avoid taking drugs in the evening that promote insomnia. In addition, the physical symptoms and side-effects of cancer interventions that can also contribute to insomnia (e.g. pain, hot flashes) should be appropriately treated. Finally, careful attention to polypharmacy and possible drug–drug interactions is necessary in patients with cancer. Anticancer agents can share similar metabolic pathways with psychotropic medications, including hypnotics, especially those involving the cytochrome P450 enzyme system, through either induction or inhibition of the enzyme system (see Supplementary Table S3, available at https://doi.org/10.1016/j.esmoop.2023.102047, for references). Supplementary Table S4, available at https://doi.org/10.1016/j.esmoop.2023.102047, summarises drugs and doses used for the treatment of insomnia.
Hypnotics
Despite an increasing interest in behavioural mechanisms and nonpharmacological approaches, pharmacological agents remain the most common treatment for insomnia.75 These are primarily benzodiazepine receptor agonists, including both classical benzodiazepines (e.g. diazepam, triazolam) and the more recently developed nonbenzodiazepine ‘Z drugs’ (e.g. zopiclone, zolpidem, eszopiclone). These agents act as a sedative by targeting the gamma-aminobutyric acid (GABA) type A receptor, thereby enhancing the effects of GABA.
The use of hypnotics is common, with data from the National Health and Nutrition Examination Survey 2005-2010 estimating that >4% of the adult population in the United States had used prescription sleep medication within the past month.76 Although insomnia has been estimated to be two to three times more prevalent among patients with cancer and cancer survivors than the general population,63 their use of sleep medication may not be proportionally greater. For example, while national survey data from the United States confirmed that more cancer survivors were told by their physicians that they had trouble sleeping (34% versus 23% in the general population with no history of cancer), only 4% reported use of prescription sleep medication.77
Systematic reviews and meta-analyses have examined the efficacy and safety of sleep medication.78 Statistically significant improvements with benzodiazepines compared with placebo are generally reported for both self-reported and objectively-assessed sleep outcomes, for example, reduced SOL (−19.6 minutes and −10.0 minutes), increased TST (+52.6 minutes and +32.7 minutes) and improved SQ (SMD 0.79).79 Similar results have been reported for nonbenzodiazepines, although later reviews have concluded that the effect sizes are small and the differences between active drugs and placebo may be of questionable clinical importance.78 The AASM 2017 CPG concludes that while the benefits of short-term use of several pharmacological treatments outweigh the harms, the quality of the available evidence is either low or very low.79 Notable exceptions are trazodone, tiagabine and l-tryptophan, where harms outweigh benefits.79
Given the widespread use of sleep medication, surprisingly few RCTs have evaluated these agents in patients with cancer or cancer survivors. The authors identified only one published study comparing the effects of a benzodiazepine (triazolam) with placebo in patients with breast cancer undergoing active treatment80 and another comparing a nonbenzodiazepine drug (eszopiclone) with placebo in inpatients with haematological malignancies.81 These studies reported better sleep outcomes with triazolam or eszopiclone compared with placebo and no differences between groups in AEs. A third study compared zolpidem with melatonin82 and a fourth compared various combinations of methylphenidate, light therapy, placebo and melatonin;83 both studies reported no statistically significant differences in sleep measures, with effect sizes ranging from small (SMD 0.11) to medium (SMD 0.77). The reported beneficial effects of hypnotics across these four studies were generally limited to self-reported SQ.
While hypnotics remain the most commonly prescribed treatment for insomnia, their use should be restricted to ≤2 weeks. Long-term use is not recommended36,37 due to the risk of AEs (e.g. drowsiness and poor daytime performance), dependence and tolerance, and hypnotic-withdrawal insomnia. There are also growing concerns regarding long-term AEs, including an increased risk of infection, depression, cancer and mortality.84 Such concerns apply not only to the classical benzodiazepines but also to the more recently developed Z drugs, such as zolpidem.85 Because of the lack of published studies, little is known about AEs specifically in patients with cancer and cancer survivors, or about AEs in the different stages of cancer (e.g. adjuvant, advanced, end of life).
Melatonin
Endogenous melatonin production by the pineal gland is inhibited by daytime light cues received by the suprachiasmatic nucleus; it is released at night when no light signals are received. The primary physiological role of endogenous melatonin is to reinforce sleep propensity and to help maintain sleep during the night as the homeostatic sleep pressure is reduced. The use of exogenous melatonin has increased, particularly in the United States, due to its relatively benign side-effect profile and over-the-counter availability. In a meta-analysis of 19 studies with a total of 1683 participants, melatonin yielded an overall reduction in SOL of 7.1 minutes, an increase in TST of 8.3 minutes and a small increase in SQ (SMD 0.22).86 It is possible that the small effects were due to the low doses used in the reviewed studies (0.1-5 mg). Based on the available evidence, the AASM 2017 CPG recommends against the use of nonprescription melatonin for treating chronic insomnia due to limited effectiveness and very low quality of evidence.79
At least four trials have compared the efficacy of melatonin versus placebo for the treatment of insomnia in patients with cancer or cancer survivors, with all reporting statistically significant results in favour of melatonin.87, 88, 89, 90 The effects on SQ ranged from medium (SMD 0.47)88 to large (SMD 1.76)90 and were generally larger than those observed in patients with insomnia as their primary diagnosis. One explanation could be the use of higher doses of melatonin in the studies of patients with cancer (3, 6 and 20 mg). Another could be that sleep disturbances in patients with cancer are more likely to be associated with circadian rhythm disruption,91 which responds better to melatonin treatment than other types of sleep disturbances.92 The beneficial effects reported in these studies are mostly related to self-reported SQ, but improvements have also been demonstrated using selected actigraphy-based data.93
RCTs comparing melatonin with placebo indicate that long-term melatonin treatment only causes mild AEs, which may include dizziness, headache, nausea and sleepiness. Currently, the recommended dose for prolonged-release melatonin is 2 mg at night for a maximum of 13 weeks in patients aged >55 years. Short-term use of melatonin is generally deemed safe, even in high doses.94 While the evidence is limited, this also seems to be the case for patients with cancer.83 The low-level AE profile suggests that the benefits may outweigh the harms and that melatonin is a possible treatment for insomnia in patients with cancer when other options are limited.
Other approaches
Exercise
Exercise is believed to influence sleep physiology by reducing autonomic arousal and improving thermoregulation, metabolism, endocrine function and mood.95 A systematic review of three meta-analyses reported statistically significant improvements in overall SQ in 14 primary studies of exercise interventions in various populations (SMD 0.52).96 SOL was also improved (SMD 0.58), but there was no significant effect on TST (SMD 0.10) or SE (SMD 0.35). While these findings are promising, evidence for the efficacy of exercise as a stand-alone treatment for insomnia in patients with cancer is limited.97
A meta-analysis of studies that examined the effect of exercise on QoL (including sleep) in patients with cancer reported statistically significant small improvements in self-reported SQ (SMD 0.26) and SOL (SMD 0.27).98 There were no significant improvements in TST (SMD −0.15) or SE (SMD 0.16). The authors identified four studies that focused specifically on the effect of exercise on sleep in patients with cancer. Two studies investigated the effect of walking in patients with lung cancer99 and patients in active treatment for mixed cancers.100 The two remaining studies examined the efficacy of moderate exercise programmes in breast cancer survivors on objectively (actigraphy)-assessed sleep parameters.101,102 The effects on SQ ranged from medium (SMD 0.75) to large (SMD 0.90). By contrast, none of the effects on objectively assessed sleep parameters reached statistical significance. AEs were only monitored in one study;99 none were deemed related to the intervention. Taken together, the effects of exercise in the limited number of studies were similar to those observed in noncancer samples, with medium to large effects for SQ and small, nonsignificant effects for other sleep parameters. The patients evaluated in these studies varied in terms of cancer type and treatment status, and the exercise interventions ranged from walking to aerobic exercise. While exercise is generally viewed as beneficial and safe for patients with cancer undergoing active treatment and cancer survivors,103,104 the available evidence to support treatment of insomnia in these individuals is weak to moderate.
Bright light therapy
Circadian rhythms exert a strong influence on how tired individuals feel, how quickly they fall asleep and when they wake up. Light is a powerful ‘zeitgeber’, which regulates sleep–wake patterns by stimulating the suprachiasmatic nucleus in the hypothalamus, inhibiting the secretion of melatonin and alerting the ascending arousal system.105 It seems reasonable, therefore, to assume that light therapy (e.g. bright light exposure in the morning and restriction of light in the evening) could be useful for treating insomnia associated with disrupted circadian rhythms. In a systematic review and meta-analysis of trials examining the effects of light therapy on sleep problems, the pooled results of 15 studies revealed small to medium statistically significant improvements in circadian rhythm sleep disorders as well as various sleep outcomes in patients with insomnia, including SOL (SMD 0.29), TST (SMD 0.44) and SQ (SMD 0.77).106 The authors identified only two small pilot studies testing the efficacy of light therapy in patients with cancer and cancer survivors experiencing insomnia with effects on sleep as the primary outcome. One trial examined the effects of 4 weeks of light therapy on sleep outcomes in ovarian and endometrial cancer survivors.107 The other study evaluated the effects of 3 weeks of light therapy in women undergoing ChT for locoregional breast cancer.108 The effects were generally small and were not statistically significant. In conclusion, the available research is very limited and there is currently insufficient evidence to support the use of light therapy for insomnia in patients with cancer and cancer survivors.
Recommendations
-
•
CBT-I is recommended as the standard of care for treating insomnia in cancer survivors [I, A].
-
•
eCBT-I should be offered to cancer survivors with insomnia when face-to-face CBT-I is not available due to cost, lack of trained therapists, or physical or geographical constraints [II, A].
-
•
BBT-I, which reduces the patient burden of the intervention, should be considered for patients in active treatment for cancer who are experiencing insomnia [II, B].
-
•
MBT should be offered to cancer survivors with insomnia when CBT-I is not available or when MBT is preferable to survivors [II, B].
-
•
Hypnotics are an option for treating insomnia in patients with cancer and cancer survivors; however, the limited evidence for efficacy does not outweigh the side-effects and risks of AEs associated with long-term use [II, C]. This recommendation does not preclude short-term use (≤2 weeks).
-
•
Melatonin should be considered in patients with cancer and cancer survivors when circadian rhythm disruption is believed to be an important factor in maintaining the insomnia and when suitable nonpharmacological options are limited [II, B]. Doses used in studies of patients with cancer were 2-20 mg. There are currently no recommendations regarding dose.
-
•
While currently available evidence is moderate, the general benefits of physical exercise suggest that exercise tailored to the patient’s abilities and needs should be offered as a stand-alone or supplementary intervention for insomnia in patients with cancer and cancer survivors [II, B].
-
•
There is currently insufficient evidence to support the use of bright light therapy in treating insomnia in patients with cancer and cancer survivors [II, C].
Challenges and suggestions for future research
While the available evidence confirms that CBT-I should be the first-line treatment for insomnia in cancer survivors, further research is needed. The majority of studies conducted thus far have investigated breast cancer survivors and there is a need for studies evaluating CBT-I in survivors of a broader range of cancers, although there is no reason that this approach should not be as efficacious in survivors of other cancers. Other barriers include cost and the availability of trained therapists to meet the needs of patients and survivors. While the available studies on eCBT-I are very promising, more studies are needed to determine the most cost-effective approach to treating insomnia in patients with cancer and cancer survivors, including head-to-head trials comparing face-to-face CBT-I with eCBT-I and trials of stepped-care approaches.
Although CBT-I has generally been reported to be highly efficacious, there is a need to identify which specific populations benefit most, and for whom other supplementary approaches could be of value. The relative efficacy of CBT-I and melatonin could depend on whether the main mechanism driving the sleep disturbance is a disrupted circadian rhythm or maladaptive sleep-related cognitions and behaviours. In addition, further research is necessary to determine whether BBT-I and MBT are efficacious alternatives to CBT-I, and which patients are most likely to benefit from each of these approaches. Finally, longitudinal studies examining when and how insomnia develops and is maintained during the cancer treatment and survivorship trajectory are needed to identify relevant preventive measures and treatments for insomnia at the various phases of treatment and follow-up.
Methodology
This CPG was developed in accordance with the European Society for Medical Oncology (ESMO) standard operating procedures for CPG development (http://www.esmo.org/Guidelines/ESMO-Guidelines-Methodology). The relevant literature has been selected by the expert authors. Levels of evidence and grades of recommendation have been applied using the system shown in Supplementary Table S5, available at https://doi.org/10.1016/j.esmoop.2023.102047.109 Statements without grading were considered justified standard clinical practice by the authors. For future updates to this CPG, including eUpdates and Living Guidelines, please see the ESMO Guidelines website: https://www.esmo.org/guidelines/guidelines-by-topic/supportive-and-palliative-care/insomnia-in-adult-cancer-patients.
Acknowledgements
Manuscript editing support was provided by Claire Bramley (ESMO Guidelines staff) and Angela Corstorphine and Sian-Marie Lucas of Kstorfin Medical Communications Ltd (KMC); this support was funded by ESMO.
Funding
No external funding has been received for the preparation of this guideline. Production costs have been covered by ESMO from central funds.
Disclosure
LG reports personal fees as an advisory board member for Angelini and Fidia, as an invited speaker for Eisai and Med Point and as a consultant for the Istituto a Carattere Scientifico IRCSIRST Meldola (FC); royalties from Minerva Medica, Oxford University Press, Springer and Wiley; nonremunerated roles as a member of the board of directors of the World Psychiatric Association (WPA), chair of the WPA Section on Psycho-Oncology and Palliative Care and co-chair of the WPA section on Psychiatry, Medicine and Primary Care. RZ reports personal fees as an advisory board member for Janssen and an invited speaker for Eli Lilly, MSD, Novo Nordisk and Pfizer; stocks/shares in Novo Nordisk and an institutional research grant from LEO Pharma. MBR reports personal fees for writing engagements and royalties from American Psychiatric Publishing, Inc., Cambridge University Press, Springer and Wiley; nonremunerated roles as co-chair of the WPA Section on Psycho-Oncology and Palliative Care, chair of the National Comprehensive Cancer Network (NCCN) distress guidelines and member of the NCCN fatigue guidelines. MLW reports nonremunerated roles as a member of the research grants panel for Marie Curie Cancer Care, co-chair of the Living With and Beyond Cancer group of the National Cancer Research Institute and chair of the PhD awards committee of Tenovus Cancer Charity. DK reports personal fees as a consultant for Reset Pharmaceutical and royalties from Oxford University Press and Routledge; institutional fees as an invited speaker for Nippo Pharmaceutical. CIR reports personal fees as an invited speaker from Angelini, Kyowa Kirin, Molteni and Mundipharma. RC, LP, RC-R, GR, DM and DS have declared no conflicts of interest.
Supplementary data
References
- 1.Al Maqbali M., Al Sinani M., Alsayed A., et al. Prevalence of sleep disturbance in patients with cancer: a systematic review and meta-analysis. Clin Nurs Res. 2022;31(6):1107–1123. doi: 10.1177/10547738221092146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization International Classification of Diseases 11th revision. https://icd.who.int/en Available at. Published 2019.
- 3.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition- Text Revision (DSM-5-TR). 5th ed. Washington, DC: American Psychiatric Association Publishing; 2022.
- 4.American Academy of Sleep Medicine . 3rd ed. American Academy of Sleep Medicine; Darien, IL: 2014. International Classification of Sleep Disorders. [Google Scholar]
- 5.Büttner-Teleagă A., Kim Y.T., Osel T., et al. Sleep disorders in cancer-a systematic review. Int J Environ Res Public Health. 2021;18(21) doi: 10.3390/ijerph182111696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Besedovsky L., Lange T., Haack M. The sleep-immune crosstalk in health and disease. Physiol Rev. 2019;99(3):1325–1380. doi: 10.1152/physrev.00010.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Howell D., Oliver T.K., Keller-Olaman S., et al. A Pan-Canadian practice guideline: prevention, screening, assessment, and treatment of sleep disturbances in adults with cancer. Support Care Cancer. 2013;21(10):2695–2706. doi: 10.1007/s00520-013-1823-6. [DOI] [PubMed] [Google Scholar]
- 8.Nzwalo I., Aboim M.A., Joaquim N., et al. Systematic review of the prevalence, predictors, and treatment of insomnia in palliative care. Am J Hosp Palliat Care. 2020;37(11):957–969. doi: 10.1177/1049909120907021. [DOI] [PubMed] [Google Scholar]
- 9.Divani A., Heidari M.E., Ghavampour N., et al. Effect of cancer treatment on sleep quality in cancer patients: a systematic review and meta-analysis of Pittsburgh Sleep Quality Index. Support Care Cancer. 2022;30(6):4687–4697. doi: 10.1007/s00520-021-06767-9. [DOI] [PubMed] [Google Scholar]
- 10.Haque R., Hsu J.W., Avila C., et al. Insomnia and susceptibility to depressive symptoms and fatigue in diverse breast cancer survivors. J Womens Health (Larchmt) 2021;30(11):1604–1615. doi: 10.1089/jwh.2019.8135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Riley K.E., Garland S.N., Mao J.J., et al. Hyperarousal and insomnia in survivors of cancer. Int J Behav Med. 2021;28(6):683–691. doi: 10.1007/s12529-021-09962-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Strik H., Cassel W., Teepker M., et al. Why do our cancer patients sleep so badly? Sleep disorders in cancer patients: a frequent symptom with multiple causes. Oncol Res Treat. 2021;44(9):469–475. doi: 10.1159/000518108. [DOI] [PubMed] [Google Scholar]
- 13.Chen Q., Terhorst L., Lowery-Allison A., et al. Sleep problems in advanced cancer patients and their caregivers: who is disturbing whom? J Behav Med. 2020;43(4):614–622. doi: 10.1007/s10865-019-00088-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walker W.H., 2nd, Borniger J.C. Molecular mechanisms of cancer-induced sleep disruption. Int J Mol Sci. 2019;20(11):2780. doi: 10.3390/ijms20112780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Christensen D.S., Zachariae R., Amidi A., et al. Sleep and allostatic load: a systematic review and meta-analysis. Sleep Med Rev. 2022;64 doi: 10.1016/j.smrv.2022.101650. [DOI] [PubMed] [Google Scholar]
- 16.Palagini L., Miniati M., Massa L., et al. Insomnia and circadian sleep disorders in ovarian cancer: evaluation and management of underestimated modifiable factors potentially contributing to morbidity. J Sleep Res. 2022;31(3) doi: 10.1111/jsr.13510. [DOI] [PubMed] [Google Scholar]
- 17.Ge L., Guyatt G., Tian J., et al. Insomnia and risk of mortality from all-cause, cardiovascular disease, and cancer: systematic review and meta-analysis of prospective cohort studies. Sleep Med Rev. 2019;48 doi: 10.1016/j.smrv.2019.101215. [DOI] [PubMed] [Google Scholar]
- 18.Fabi A., Bhargava R., Fatigoni S., et al. Cancer-related fatigue: ESMO Clinical Practice Guidelines for diagnosis and treatment. Ann Oncol. 2020;31(6):713–723. doi: 10.1016/j.annonc.2020.02.016. [DOI] [PubMed] [Google Scholar]
- 19.Mogavero M.P., DelRosso L.M., Fanfulla F., et al. Sleep disorders and cancer: state of the art and future perspectives. Sleep Med Rev. 2021;56 doi: 10.1016/j.smrv.2020.101409. [DOI] [PubMed] [Google Scholar]
- 20.Savard J., Ivers H., Villa J., et al. Natural course of insomnia comorbid with cancer: an 18-month longitudinal study. J Clin Oncol. 2011;29(26):3580–3586. doi: 10.1200/JCO.2010.33.2247. [DOI] [PubMed] [Google Scholar]
- 21.National Comprehensive Cancer Network NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Distress Management. Version 2.2023. https://www.nccn.org/professionals/physician_gls/pdf/distress.pdf Available at. Published 2022.
- 22.National Comprehensive Cancer Network NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Survivorship. Version 1.2022. https://www.nccn.org/professionals/physician_gls/pdf/survivorship.pdf Available at. Published 2022.
- 23.Dueck A.C., Mendoza T.R., Mitchell S.A., et al. Validity and reliability of the US National Cancer Institute’s patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE) JAMA Oncol. 2015;1(8):1051–1059. doi: 10.1001/jamaoncol.2015.2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou E.S., Partridge A.H., Syrjala K.L., et al. Evaluation and treatment of insomnia in adult cancer survivorship programs. J Cancer Surviv. 2017;11(1):74–79. doi: 10.1007/s11764-016-0564-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ripamonti C., Leporati R., De Feo G., et al. Italian version of the Edmonton Symptom Assessment System (ESAS)-Total Care (TC): development and psychometric validation in patients undergoing cancer treatment or follow-up. Support Care Cancer. 2022;30(3):1923–1933. doi: 10.1007/s00520-021-06594-y. [DOI] [PubMed] [Google Scholar]
- 26.Taylor D.J., Wilkerson A.K., Pruiksma K.E., et al. Reliability of the structured clinical interview for DSM-5 sleep disorders module. J Clin Sleep Med. 2018;14(3):459–464. doi: 10.5664/jcsm.7000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Palagini L., Manni R., Aguglia E., et al. Expert opinions and consensus recommendations for the evaluation and management of insomnia in clinical practice: joint statements of five Italian scientific societies. Front Psychiatry. 2020;11:558. doi: 10.3389/fpsyt.2020.00558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bastien C.H., Vallières A., Morin C.M. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2(4):297–307. doi: 10.1016/s1389-9457(00)00065-4. [DOI] [PubMed] [Google Scholar]
- 29.Savard M.H., Savard J., Simard S., et al. Empirical validation of the Insomnia Severity Index in cancer patients. Psychooncology. 2005;14(6):429–441. doi: 10.1002/pon.860. [DOI] [PubMed] [Google Scholar]
- 30.Espie C.A., Kyle S.D., Hames P., et al. The Sleep Condition Indicator: a clinical screening tool to evaluate insomnia disorder. BMJ Open. 2014;4(3) doi: 10.1136/bmjopen-2013-004183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Buysse D.J., Reynolds C.F., 3rd, Monk T.H., et al. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 32.Beck S.L., Schwartz A.L., Towsley G., et al. Psychometric evaluation of the Pittsburgh Sleep Quality Index in cancer patients. J Pain Symptom Manage. 2004;27(2):140–148. doi: 10.1016/j.jpainsymman.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 33.Edmed S.L., Huda M.M., Smith S.S., et al. Sleep and health-related quality of life in women following a cancer diagnosis: results from the Women’s Wellness after Cancer Program in Australia. Support Care Cancer. 2022;30(12):10243–10253. doi: 10.1007/s00520-022-07429-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Buysse D.J., Ancoli-Israel S., Edinger J.D., et al. Recommendations for a standard research assessment of insomnia. Sleep. 2006;29(9):1155–1173. doi: 10.1093/sleep/29.9.1155. [DOI] [PubMed] [Google Scholar]
- 35.Carney C.E., Buysse D.J., Ancoli-Israel S., et al. The Consensus Sleep Diary: standardizing prospective sleep self-monitoring. Sleep. 2012;35(2):287–302. doi: 10.5665/sleep.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qaseem A., Kansagara D., Forciea M.A., et al. Management of chronic insomnia disorder in adults: a Clinical Practice Guideline from the American College of Physicians. Ann Intern Med. 2016;165(2):125–133. doi: 10.7326/M15-2175. [DOI] [PubMed] [Google Scholar]
- 37.Riemann D., Baglioni C., Bassetti C., et al. European guideline for the diagnosis and treatment of insomnia. J Sleep Res. 2017;26(6):675–700. doi: 10.1111/jsr.12594. [DOI] [PubMed] [Google Scholar]
- 38.Edinger J.D., Arnedt J.T., Bertisch S.M., et al. Behavioral and psychological treatments for chronic insomnia disorder in adults: an American Academy of Sleep Medicine Clinical Practice Guideline. J Clin Sleep Med. 2021;17(2):255–262. doi: 10.5664/jcsm.8986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bootzin R.R., Epstein D.R. Understanding and treating insomnia. Annu Rev Clin Psychol. 2011;7:435–458. doi: 10.1146/annurev.clinpsy.3.022806.091516. [DOI] [PubMed] [Google Scholar]
- 40.Belanger L., Savard J., Morin C.M. Clinical management of insomnia using cognitive therapy. Behav Sleep Med. 2006;4(3):179–198. doi: 10.1207/s15402010bsm0403_4. [DOI] [PubMed] [Google Scholar]
- 41.Geiger-Brown J.M., Rogers V.E., Liu W., et al. Cognitive behavioral therapy in persons with comorbid insomnia: a meta-analysis. Sleep Med Rev. 2015;23:54–67. doi: 10.1016/j.smrv.2014.11.007. [DOI] [PubMed] [Google Scholar]
- 42.Savard J., Simard S., Ivers H., et al. Randomized study on the efficacy of cognitive-behavioral therapy for insomnia secondary to breast cancer, part I: sleep and psychological effects. J Clin Oncol. 2005;23(25):6083–6096. doi: 10.1200/JCO.2005.09.548. [DOI] [PubMed] [Google Scholar]
- 43.Epstein D.R., Dirksen S.R. Randomized trial of a cognitive-behavioral intervention for insomnia in breast cancer survivors. Oncol Nurs Forum. 2007;34(5):E51–E59. doi: 10.1188/07.ONF.E51-E59. [DOI] [PubMed] [Google Scholar]
- 44.Espie C.A., Fleming L., Cassidy J., et al. Randomized controlled clinical effectiveness trial of cognitive behavior therapy compared with treatment as usual for persistent insomnia in patients with cancer. J Clin Oncol. 2008;26(28):4651–4658. doi: 10.1200/JCO.2007.13.9006. [DOI] [PubMed] [Google Scholar]
- 45.Berger A.M., Kuhn B.R., Farr L.A., et al. Behavioral therapy intervention trial to improve sleep quality and cancer-related fatigue. Psychooncology. 2009;18(6):634–646. doi: 10.1002/pon.1438. [DOI] [PubMed] [Google Scholar]
- 46.Fiorentino L., McQuaid J.R., Liu L., et al. Individual cognitive behavioral therapy for insomnia in breast cancer survivors: a randomized controlled crossover pilot study. Nat Sci Sleep. 2010;2:1–8. doi: 10.2147/NSS.S8004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roscoe J.A., Garland S.N., Heckler C.E., et al. Randomized placebo-controlled trial of cognitive behavioral therapy and armodafinil for insomnia after cancer treatment. J Clin Oncol. 2015;33(2):165–171. doi: 10.1200/JCO.2014.57.6769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Padron A., McCrae C.S., Robinson M.E., et al. Impacts of cognitive behavioral therapy for insomnia and pain on sleep in women with gynecologic malignancies: a randomized controlled trial. Behav Sleep Med. 2022;20(4):460–476. doi: 10.1080/15402002.2021.1932500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Matthews E.E., Berger A.M., Schmiege S.J., et al. Cognitive behavioral therapy for insomnia outcomes in women after primary breast cancer treatment: a randomized, controlled trial. Oncol Nurs Forum. 2014;41(3):241–253. doi: 10.1188/14.ONF.41-03AP. [DOI] [PubMed] [Google Scholar]
- 50.Savard J., Ivers H., Savard M.H., et al. Is a video-based cognitive behavioral therapy for insomnia as efficacious as a professionally administered treatment in breast cancer? Results of a randomized controlled trial. Sleep. 2014;37(8):1305–1314. doi: 10.5665/sleep.3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Garland S.N., Carlson L.E., Stephens A.J., et al. Mindfulness-based stress reduction compared with cognitive behavioral therapy for the treatment of insomnia comorbid with cancer: a randomized, partially blinded, noninferiority trial. J Clin Oncol. 2014;32(5):449–457. doi: 10.1200/JCO.2012.47.7265. [DOI] [PubMed] [Google Scholar]
- 52.Mercier J., Ivers H., Savard J. A non-inferiority randomized controlled trial comparing a home-based aerobic exercise program to a self-administered cognitive-behavioral therapy for insomnia in cancer patients. Sleep. 2018;41(10) doi: 10.1093/sleep/zsy149. [DOI] [PubMed] [Google Scholar]
- 53.Barton D.L., Atherton P.J., Satele D.V., et al. A randomized phase II trial evaluating two non-pharmacologic interventions in cancer survivors for the treatment of sleep-wake disturbances: NCCTG N07C4 (Alliance) Support Care Cancer. 2020;28(12):6085–6094. doi: 10.1007/s00520-020-05461-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Savard J., Ivers H., Savard M.H., et al. Efficacy of a stepped care approach to deliver cognitive-behavioral therapy for insomnia in cancer patients: a noninferiority randomized controlled trial. Sleep. 2021;44(11):zsab166. doi: 10.1093/sleep/zsab166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Johnson J.A., Rash J.A., Campbell T.S., et al. A systematic review and meta-analysis of randomized controlled trials of cognitive behavior therapy for insomnia (CBT-I) in cancer survivors. Sleep Med Rev. 2016;27:20–28. doi: 10.1016/j.smrv.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 56.Gao Y., Liu M., Yao L., et al. Cognitive behavior therapy for insomnia in cancer patients: a systematic review and network meta-analysis. J Evid Based Med. 2022;15(3):216–229. doi: 10.1111/jebm.12485. [DOI] [PubMed] [Google Scholar]
- 57.Squires L.R., Rash J.A., Fawcett J., et al. Systematic review and meta-analysis of cognitive-behavioural therapy for insomnia on subjective and actigraphy-measured sleep and comorbid symptoms in cancer survivors. Sleep Med Rev. 2022;63 doi: 10.1016/j.smrv.2022.101615. [DOI] [PubMed] [Google Scholar]
- 58.Vincent N., Lionberg C. Treatment preference and patient satisfaction in chronic insomnia. Sleep. 2001;24(4):411–417. doi: 10.1093/sleep/24.4.411. [DOI] [PubMed] [Google Scholar]
- 59.Ritterband L.M., Andersson G., Christensen H.M., et al. Directions for the International Society for Research on Internet Interventions (ISRII) J Med Internet Res. 2006;8(3):e23. doi: 10.2196/jmir.8.3.e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zachariae R., Lyby M.S., Ritterband L.M., et al. Efficacy of internet-delivered cognitive-behavioral therapy for insomnia - a systematic review and meta-analysis of randomized controlled trials. Sleep Med Rev. 2016;30:1–10. doi: 10.1016/j.smrv.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 61.Zachariae R., Amidi A., Damholdt M.F., et al. Internet-delivered cognitive-behavioral therapy for insomnia in breast cancer survivors: a randomized controlled trial. J Natl Cancer Inst. 2018;110(8):880–887. doi: 10.1093/jnci/djx293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ritterband L.M., Bailey E.T., Thorndike F.P., et al. Initial evaluation of an internet intervention to improve the sleep of cancer survivors with insomnia. Psychooncology. 2012;21(7):695–705. doi: 10.1002/pon.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Howell D., Oliver T.K., Keller-Olaman S., et al. Sleep disturbance in adults with cancer: a systematic review of evidence for best practices in assessment and management for clinical practice. Ann Oncol. 2014;25(4):791–800. doi: 10.1093/annonc/mdt506. [DOI] [PubMed] [Google Scholar]
- 64.Barsevick A., Beck S.L., Dudley W.N., et al. Efficacy of an intervention for fatigue and sleep disturbance during cancer chemotherapy. J Pain Symptom Manage. 2010;40(2):200–216. doi: 10.1016/j.jpainsymman.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Casault L., Savard J., Ivers H., et al. A randomized-controlled trial of an early minimal cognitive-behavioural therapy for insomnia comorbid with cancer. Behav Res Ther. 2015;67:45–54. doi: 10.1016/j.brat.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 66.Palesh O., Solomon N., Hofmeister E., et al. A novel approach to management of sleep-associated problems in patients with breast cancer (MOSAIC) during chemotherapy: a pilot study. Sleep. 2020;43(10):zsaa070. doi: 10.1093/sleep/zsaa070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Palesh O., Scheiber C., Kesler S., et al. Feasibility and acceptability of brief behavioral therapy for cancer-related insomnia: effects on insomnia and circadian rhythm during chemotherapy: a phase II randomised multicentre controlled trial. Br J Cancer. 2018;119(3):274–281. doi: 10.1038/s41416-018-0154-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dean G.E., Weiss C., Jungquist C.R., et al. Nurse-delivered brief behavioral treatment for insomnia in lung cancer survivors: a pilot RCT. Behav Sleep Med. 2020;18(6):774–786. doi: 10.1080/15402002.2019.1685523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kabat-Zinn J. Mindfulness-based interventions in context: past, present, and future. Clin Psychol Sci Pract. 2003;10(2):144–156. [Google Scholar]
- 70.Rusch H.L., Rosario M., Levison L.M., et al. The effect of mindfulness meditation on sleep quality: a systematic review and meta-analysis of randomized controlled trials. Ann N Y Acad Sci. 2019;1445(1):5–16. doi: 10.1111/nyas.13996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nakamura Y., Lipschitz D.L., Kuhn R., et al. Investigating efficacy of two brief mind-body intervention programs for managing sleep disturbance in cancer survivors: a pilot randomized controlled trial. J Cancer Surviv. 2013;7(2):165–182. doi: 10.1007/s11764-012-0252-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lengacher C.A., Reich R.R., Paterson C.L., et al. The effects of mindfulness-based stress reduction on objective and subjective sleep parameters in women with breast cancer: a randomized controlled trial. Psychooncology. 2015;24(4):424–432. doi: 10.1002/pon.3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhao Y., Liu J.E., Lewis F.M., et al. Effects of mindfulness-based cognitive therapy on breast cancer survivors with insomnia: a randomised controlled trial. Eur J Cancer Care (Engl) 2020;29(5) doi: 10.1111/ecc.13259. [DOI] [PubMed] [Google Scholar]
- 74.Zhang R., Yin J., Zhou Y. Effects of mindfulness-based psychological care on mood and sleep of leukemia patients in chemotherapy. Int J Nurs Sci. 2017;4(4):357–361. doi: 10.1016/j.ijnss.2017.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Madari S., Golebiowski R., Mansukhani M.P., et al. Pharmacological management of insomnia. Neurotherapeutics. 2021;18(1):44–52. doi: 10.1007/s13311-021-01010-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chong Y., Fryer C.D., Gu Q. Prescription sleep aid use among adults: United States, 2005-2010. NCHS Data Brief. 2013;(127):1–8. [PubMed] [Google Scholar]
- 77.Slade A.N., Waters M.R., Serrano N.A. Long-term sleep disturbance and prescription sleep aid use among cancer survivors in the United States. Support Care Cancer. 2020;28(2):551–560. doi: 10.1007/s00520-019-04849-3. [DOI] [PubMed] [Google Scholar]
- 78.Xiang T., Cai Y., Hong Z., et al. Efficacy and safety of Zolpidem in the treatment of insomnia disorder for one month: a meta-analysis of a randomized controlled trial. Sleep Med. 2021;87:250–256. doi: 10.1016/j.sleep.2021.09.005. [DOI] [PubMed] [Google Scholar]
- 79.Sateia M.J., Buysse D.J., Krystal A.D., et al. Clinical Practice Guideline for the pharmacologic treatment of chronic insomnia in adults: an American Academy of Sleep Medicine Clinical Practice Guideline. J Clin Sleep Med. 2017;13(2):307–349. doi: 10.5664/jcsm.6470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jacobsen P.B., Massie M.J., Kinne D.W., et al. Hypnotic efficacy and safety of triazolam administered during the postoperative period. Gen Hosp Psychiatry. 1994;16(6):419–425. doi: 10.1016/0163-8343(94)90118-x. [DOI] [PubMed] [Google Scholar]
- 81.Dimsdale J.E., Ball E.D., Carrier E., et al. Effect of eszopiclone on sleep, fatigue, and pain in patients with mucositis associated with hematologic malignancies. Support Care Cancer. 2011;19(12):2015–2020. doi: 10.1007/s00520-010-1052-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shahrokhi M., Ghaeli P., Arya P., et al. Comparing the effects of melatonin and zolpidem on sleep quality, depression, and anxiety in patients with colorectal cancer undergoing chemotherapy. Basic Clin Neurosci. 2021;12(1):105–114. doi: 10.32598/bcn.12.1.1650.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yennurajalingam S., Carmack C., Balachandran D., et al. Sleep disturbance in patients with cancer: a feasibility study of multimodal therapy. BMJ Support Palliat Care. 2021;11(2):170–179. doi: 10.1136/bmjspcare-2019-001877. [DOI] [PubMed] [Google Scholar]
- 84.Kripke D.F. Hypnotic drug risks of mortality, infection, depression, and cancer: but lack of benefit. F1000Res. 2016;5:918. doi: 10.12688/f1000research.8729.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Inagaki T., Miyaoka T., Tsuji S., et al. Adverse reactions to zolpidem: case reports and a review of the literature. Prim Care Companion J Clin Psychiatry. 2010;12(6) doi: 10.4088/PCC.09r00849bro. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ferracioli-Oda E., Qawasmi A., Bloch M.H. Meta-analysis: melatonin for the treatment of primary sleep disorders. PLoS One. 2013;8(5) doi: 10.1371/journal.pone.0063773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chen W.Y., Giobbie-Hurder A., Gantman K., et al. A randomized, placebo-controlled trial of melatonin on breast cancer survivors: impact on sleep, mood, and hot flashes. Breast Cancer Res Treat. 2014;145(2):381–388. doi: 10.1007/s10549-014-2944-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hansen M.V., Madsen M.T., Andersen L.T., et al. Effect of melatonin on cognitive function and sleep in relation to breast cancer surgery: a randomized, double-blind, placebo-controlled trial. Int J Breast Cancer. 2014;2014 doi: 10.1155/2014/416531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kurdi M.S., Muthukalai S.P. The efficacy of oral melatonin in improving sleep in cancer patients with insomnia: a randomized double-blind placebo-controlled study. Indian J Palliat Care. 2016;22(3):295–300. doi: 10.4103/0973-1075.185039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Palmer A.C.S., Zortea M., Souza A., et al. Clinical impact of melatonin on breast cancer patients undergoing chemotherapy; effects on cognition, sleep and depressive symptoms: a randomized, double-blind, placebo-controlled trial. PLoS One. 2020;15(4) doi: 10.1371/journal.pone.0231379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ancoli-Israel S., Liu L., Natarajan L., et al. Reductions in sleep quality and circadian activity rhythmicity predict longitudinal changes in objective and subjective cognitive functioning in women treated for breast cancer. Support Care Cancer. 2022;30(4):3187–3200. doi: 10.1007/s00520-021-06743-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Buscemi N., Vandermeer B., Hooton N., et al. The efficacy and safety of exogenous melatonin for primary sleep disorders. A meta-analysis. J Gen Intern Med. 2005;20(12):1151–1158. doi: 10.1111/j.1525-1497.2005.0243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Madsen M.T., Hansen M.V., Andersen L.T., et al. Effect of melatonin on sleep in the perioperative period after breast cancer surgery: a randomized, double-blind, placebo-controlled trial. J Clin Sleep Med. 2016;12(2):225–233. doi: 10.5664/jcsm.5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Andersen L.P., Gögenur I., Rosenberg J., et al. The safety of melatonin in humans. Clin Drug Investig. 2016;36(3):169–175. doi: 10.1007/s40261-015-0368-5. [DOI] [PubMed] [Google Scholar]
- 95.Uchida S., Shioda K., Morita Y., et al. Exercise effects on sleep physiology. Front Neurol. 2012;3:48. doi: 10.3389/fneur.2012.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kelley G.A., Kelley K.S. Exercise and sleep: a systematic review of previous meta-analyses. J Evid Based Med. 2017;10(1):26–36. doi: 10.1111/jebm.12236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Takemura N., Cheung D.S.T., Smith R., et al. Effectiveness of aerobic exercise and mind-body exercise in cancer patients with poor sleep quality: a systematic review and meta-analysis of randomized controlled trials. Sleep Med Rev. 2020;53 doi: 10.1016/j.smrv.2020.101334. [DOI] [PubMed] [Google Scholar]
- 98.Fang Y.Y., Hung C.T., Chan J.C., et al. Meta-analysis: exercise intervention for sleep problems in cancer patients. Eur J Cancer Care (Engl) 2019;28(5) doi: 10.1111/ecc.13131. [DOI] [PubMed] [Google Scholar]
- 99.Chen H.M., Tsai C.M., Wu Y.C., et al. Effect of walking on circadian rhythms and sleep quality of patients with lung cancer: a randomised controlled trial. Br J Cancer. 2016;115(11):1304–1312. doi: 10.1038/bjc.2016.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tang M.F., Liou T.H., Lin C.C. Improving sleep quality for cancer patients: benefits of a home-based exercise intervention. Support Care Cancer. 2010;18(10):1329–1339. doi: 10.1007/s00520-009-0757-5. [DOI] [PubMed] [Google Scholar]
- 101.Roveda E., Vitale J.A., Bruno E., et al. Protective effect of aerobic physical activity on sleep behavior in breast cancer survivors. Integr Cancer Ther. 2017;16(1):21–31. doi: 10.1177/1534735416651719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nguyen N.H., Vallance J.K., Buman M.P., et al. Effects of a wearable technology-based physical activity intervention on sleep quality in breast cancer survivors: the ACTIVATE Trial. J Cancer Surviv. 2021;15(2):273–280. doi: 10.1007/s11764-020-00930-7. [DOI] [PubMed] [Google Scholar]
- 103.Mishra S.I., Scherer R.W., Snyder C., et al. Exercise interventions on health-related quality of life for people with cancer during active treatment. Cochrane Database Syst Rev. 2012;2012(8):CD008465. doi: 10.1002/14651858.CD008465.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mishra S.I., Scherer R.W., Geigle P.M., et al. Exercise interventions on health-related quality of life for cancer survivors. Cochrane Database Syst Rev. 2012;2012(8):CD007566. doi: 10.1002/14651858.CD007566.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Blume C., Garbazza C., Spitschan M. Effects of light on human circadian rhythms, sleep and mood. Somnologie (Berl) 2019;23(3):147–156. doi: 10.1007/s11818-019-00215-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.van Maanen A., Meijer A.M., van der Heijden K.B., et al. The effects of light therapy on sleep problems: a systematic review and meta-analysis. Sleep Med Rev. 2016;29:52–62. doi: 10.1016/j.smrv.2015.08.009. [DOI] [PubMed] [Google Scholar]
- 107.Fox R.S., Baik S.H., McGinty H., et al. Feasibility and preliminary efficacy of a bright light intervention in ovarian and endometrial cancer survivors. Int J Behav Med. 2021;28(1):83–95. doi: 10.1007/s12529-020-09861-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wu H.S., Davis J.E., Chen L. Bright light shows promise in improving sleep, depression, and quality of life in women with breast cancer during chemotherapy: findings of a pilot study. Chronobiol Int. 2021;38(5):694–704. doi: 10.1080/07420528.2021.1871914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dykewicz C.A. Summary of the guidelines for preventing opportunistic infections among hematopoietic stem cell transplant recipients. Clin Infect Dis. 2001;33(2):139–144. doi: 10.1086/321805. [adapted from: Gross PA, Barrett TL, Dellinger EP, et al. Purpose of quality standards for infectious diseases. Clin Infect Dis. 1994;18(3):421] [DOI] [PubMed] [Google Scholar]
- 110.Jakobsen G., Sjue K., Paulsen Ø., et al. Zopiclone versus placebo for short-term treatment of insomnia in patients with advanced cancer – a double-blind, randomized placebo-controlled clinical multicenter phase IV trial. Support Care Cancer. 2022;31(1):60. doi: 10.1007/s00520-022-07537-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.