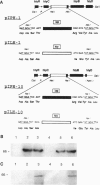
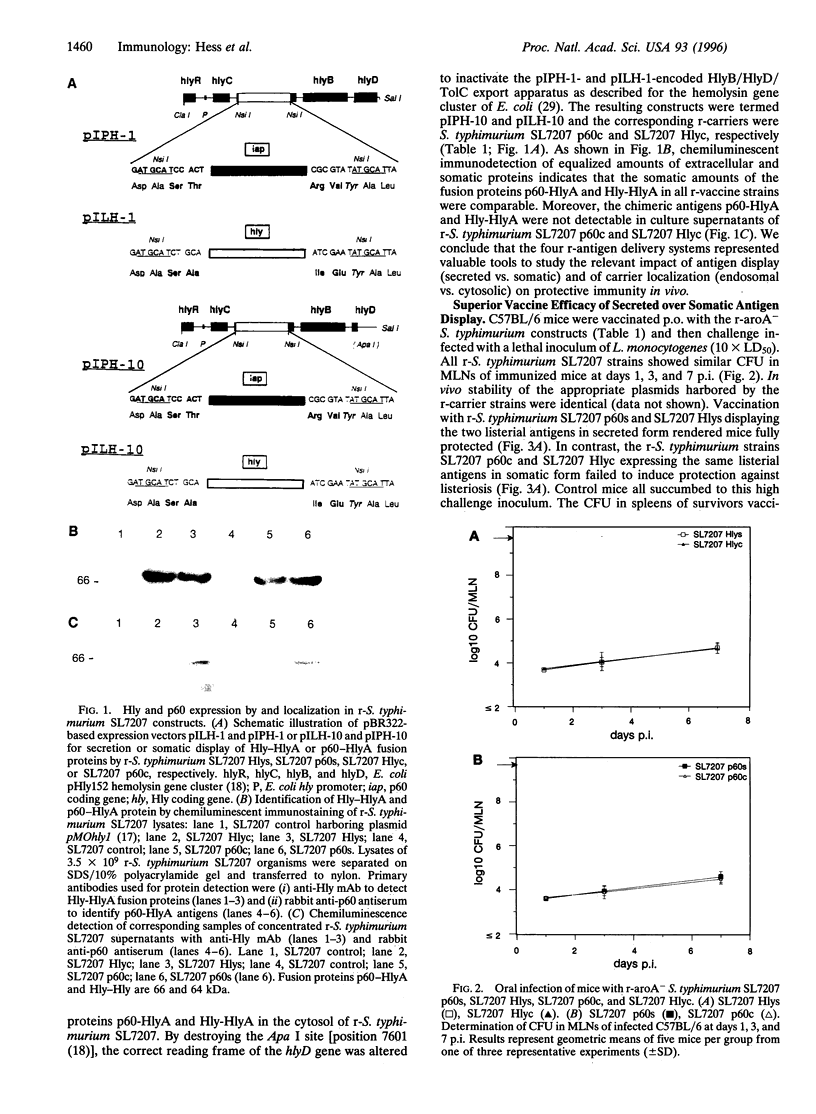
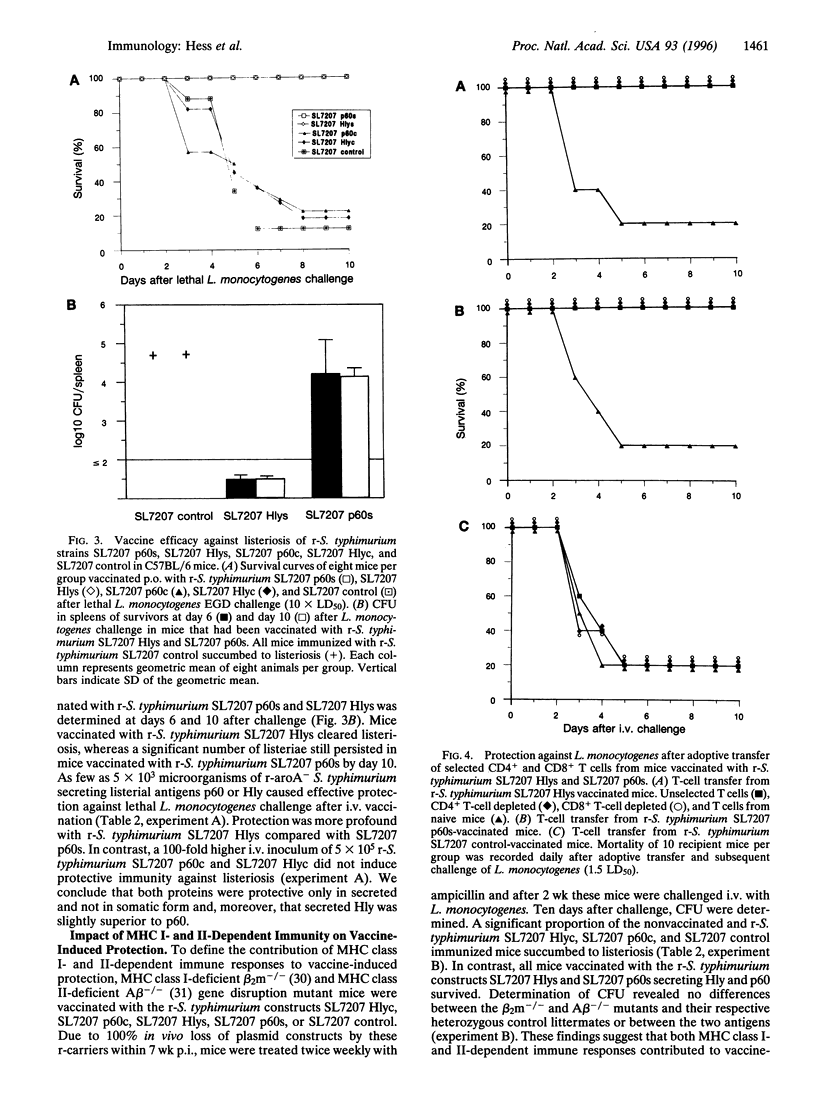
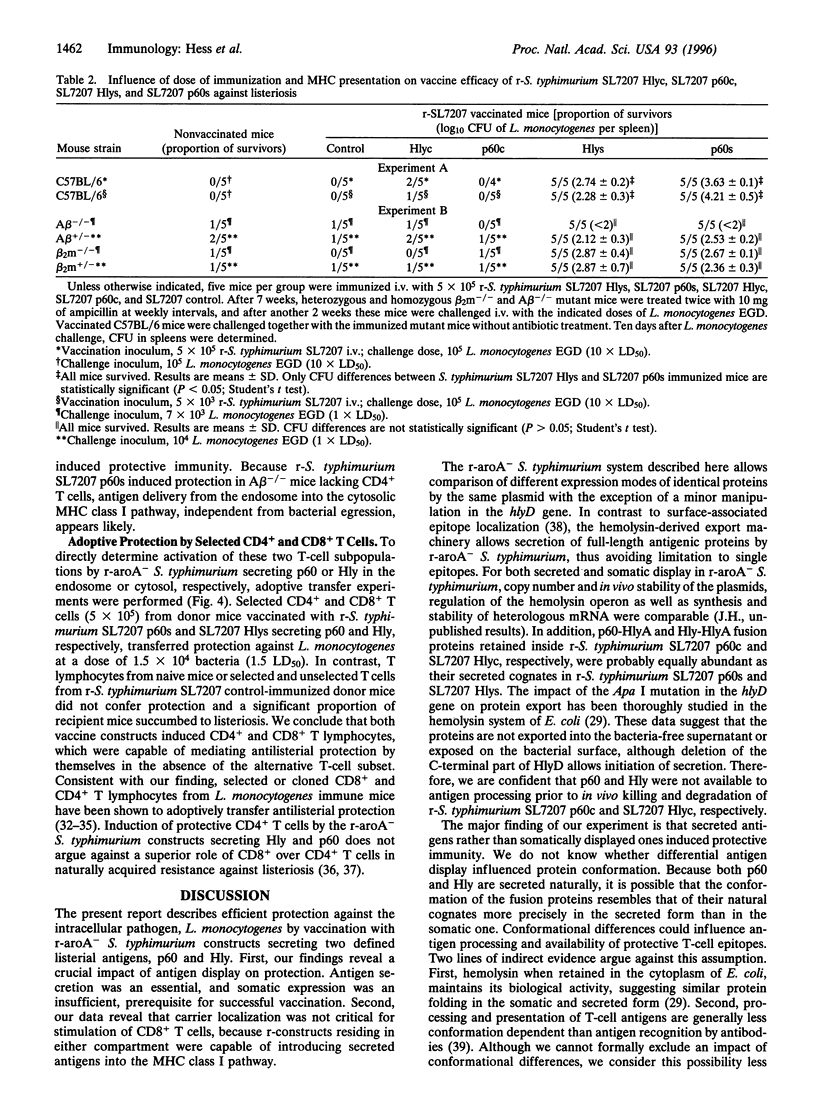
Abstract
Vaccination provides the most potent measure against infectious disease, and recombinant (r) viable vaccines expressing defined pathogen-derived antigens represent powerful candidates for future vaccination strategies. In a new approach we constructed r-aroA- Salmonella typhimurium displaying p60 or listeriolysin (Hly) antigen of Listeria monocytogenes in secreted or somatic form in the host cell. Vaccination of mice with r-aroA- S. typhimurium induced protection against the intracellular pathogen L. monocytogenes only with secreted and not with somatic antigen. Secreted Hly was slightly more potent in inducing protective immunity than secreted p60. Both r-aroA- S. typhimurium secreting p60 in the endosome and r-aroA- S. typhimurium secreting Hly in the cytosol induced protective CD4+ and CD8+ T-cells suggesting CD8+ T-cell stimulation independent from intracellular residence of r-aroA- S. typhimurium carriers. Hence, not only the type of antigen but also its display by the r-carrier within the host cell critically influences vaccine efficacy.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aggarwal A., Kumar S., Jaffe R., Hone D., Gross M., Sadoff J. Oral Salmonella: malaria circumsporozoite recombinants induce specific CD8+ cytotoxic T cells. J Exp Med. 1990 Oct 1;172(4):1083–1090. doi: 10.1084/jem.172.4.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berche P., Gaillard J. L., Sansonetti P. J. Intracellular growth of Listeria monocytogenes as a prerequisite for in vivo induction of T cell-mediated immunity. J Immunol. 1987 Apr 1;138(7):2266–2271. [PubMed] [Google Scholar]
- Blander S. J., Horwitz M. A. Vaccination with the major secretory protein of Legionella pneumophila induces cell-mediated and protective immunity in a guinea pig model of Legionnaires' disease. J Exp Med. 1989 Mar 1;169(3):691–705. doi: 10.1084/jem.169.3.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouwer H. G., Gibbins B. L., Jones S., Hinrichs D. J. Antilisterial immunity includes specificity to listeriolysin O (LLO) and non-LLO-derived determinants. Infect Immun. 1994 Mar;62(3):1039–1045. doi: 10.1128/iai.62.3.1039-1045.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove D., Gray D., Dierich A., Kaufman J., Lemeur M., Benoist C., Mathis D. Mice lacking MHC class II molecules. Cell. 1991 Sep 6;66(5):1051–1066. doi: 10.1016/0092-8674(91)90448-8. [DOI] [PubMed] [Google Scholar]
- Daugelat S., Ladel C. H., Schoel B., Kaufmann S. H. Antigen-specific T-cell responses during primary and secondary Listeria monocytogenes infection. Infect Immun. 1994 May;62(5):1881–1888. doi: 10.1128/iai.62.5.1881-1888.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillard J. L., Berche P., Mounier J., Richard S., Sansonetti P. In vitro model of penetration and intracellular growth of Listeria monocytogenes in the human enterocyte-like cell line Caco-2. Infect Immun. 1987 Nov;55(11):2822–2829. doi: 10.1128/iai.55.11.2822-2829.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentschev I., Goebel W. Topological and functional studies on HlyB of Escherichia coli. Mol Gen Genet. 1992 Mar;232(1):40–48. doi: 10.1007/BF00299135. [DOI] [PubMed] [Google Scholar]
- Gentschev I., Sokolovic Z., Mollenkopf H. J., Hess J., Kaufmann S. H., Kuhn M., Krohne G. F., Goebel W. Salmonella strain secreting active listeriolysin changes its intracellular localization. Infect Immun. 1995 Oct;63(10):4202–4205. doi: 10.1128/iai.63.10.4202-4205.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germain R. N., Margulies D. H. The biochemistry and cell biology of antigen processing and presentation. Annu Rev Immunol. 1993;11:403–450. doi: 10.1146/annurev.iy.11.040193.002155. [DOI] [PubMed] [Google Scholar]
- Harty J. T., Bevan M. J. CD8+ T cells specific for a single nonamer epitope of Listeria monocytogenes are protective in vivo. J Exp Med. 1992 Jun 1;175(6):1531–1538. doi: 10.1084/jem.175.6.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harty J. T., Pamer E. G. CD8 T lymphocytes specific for the secreted p60 antigen protect against Listeria monocytogenes infection. J Immunol. 1995 May 1;154(9):4642–4650. [PubMed] [Google Scholar]
- Hess J., Gentschev I., Goebel W., Jarchau T. Analysis of the haemolysin secretion system by PhoA-HlyA fusion proteins. Mol Gen Genet. 1990 Nov;224(2):201–208. doi: 10.1007/BF00271553. [DOI] [PubMed] [Google Scholar]
- Hess J., Gentschev I., Szalay G., Ladel C., Bubert A., Goebel W., Kaufmann S. H. Listeria monocytogenes p60 supports host cell invasion by and in vivo survival of attenuated Salmonella typhimurium. Infect Immun. 1995 May;63(5):2047–2053. doi: 10.1128/iai.63.5.2047-2053.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoiseth S. K., Stocker B. A. Aromatic-dependent Salmonella typhimurium are non-virulent and effective as live vaccines. Nature. 1981 May 21;291(5812):238–239. doi: 10.1038/291238a0. [DOI] [PubMed] [Google Scholar]
- Horwitz M. A., Lee B. W., Dillon B. J., Harth G. Protective immunity against tuberculosis induced by vaccination with major extracellular proteins of Mycobacterium tuberculosis. Proc Natl Acad Sci U S A. 1995 Feb 28;92(5):1530–1534. doi: 10.1073/pnas.92.5.1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann S. H., Hahn H. Biological functions of t cell lines with specificity for the intracellular bacterium Listeria monocytogenes in vitro and in vivo. J Exp Med. 1982 Jun 1;155(6):1754–1765. doi: 10.1084/jem.155.6.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann S. H., Hug E., Väth U., Müller I. Effective protection against Listeria monocytogenes and delayed-type hypersensitivity to listerial antigens depend on cooperation between specific L3T4+ and Lyt 2+ T cells. Infect Immun. 1985 Apr;48(1):263–266. doi: 10.1128/iai.48.1.263-266.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann S. H., Rodewald H. R., Hug E., De Libero G. Cloned Listeria monocytogenes specific non-MHC-restricted Lyt-2+ T cells with cytolytic and protective activity. J Immunol. 1988 May 1;140(9):3173–3179. [PubMed] [Google Scholar]
- Kovacsovics-Bankowski M., Rock K. L. A phagosome-to-cytosol pathway for exogenous antigens presented on MHC class I molecules. Science. 1995 Jan 13;267(5195):243–246. doi: 10.1126/science.7809629. [DOI] [PubMed] [Google Scholar]
- Kuhn M., Goebel W. Identification of an extracellular protein of Listeria monocytogenes possibly involved in intracellular uptake by mammalian cells. Infect Immun. 1989 Jan;57(1):55–61. doi: 10.1128/iai.57.1.55-61.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladel C. H., Flesch I. E., Arnoldi J., Kaufmann S. H. Studies with MHC-deficient knock-out mice reveal impact of both MHC I- and MHC II-dependent T cell responses on Listeria monocytogenes infection. J Immunol. 1994 Oct 1;153(7):3116–3122. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mielke M. E., Niedobitek G., Stein H., Hahn H. Acquired resistance to Listeria monocytogenes is mediated by Lyt-2+ T cells independently of the influx of monocytes into granulomatous lesions. J Exp Med. 1989 Aug 1;170(2):589–594. doi: 10.1084/jem.170.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nato F., Reich K., Lhopital S., Rouyre S., Geoffroy C., Mazie J. C., Cossart P. Production and characterization of neutralizing and nonneutralizing monoclonal antibodies against listeriolysin O. Infect Immun. 1991 Dec;59(12):4641–4646. doi: 10.1128/iai.59.12.4641-4646.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oropeza-Wekerle R. L., Speth W., Imhof B., Gentschev I., Goebel W. Translocation and compartmentalization of Escherichia coli hemolysin (HlyA). J Bacteriol. 1990 Jul;172(7):3711–3717. doi: 10.1128/jb.172.7.3711-3717.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pamer E. G. Direct sequence identification and kinetic analysis of an MHC class I-restricted Listeria monocytogenes CTL epitope. J Immunol. 1994 Jan 15;152(2):686–694. [PubMed] [Google Scholar]
- Pamer E. G., Harty J. T., Bevan M. J. Precise prediction of a dominant class I MHC-restricted epitope of Listeria monocytogenes. Nature. 1991 Oct 31;353(6347):852–855. doi: 10.1038/353852a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer J. D., Wick M. J., Roberts R. L., Findlay K., Normark S. J., Harding C. V. Phagocytic processing of bacterial antigens for class I MHC presentation to T cells. Nature. 1993 Jan 28;361(6410):359–362. doi: 10.1038/361359a0. [DOI] [PubMed] [Google Scholar]
- Rakhmilevich A. L. Evidence for a significant role of CD4+ T cells in adoptive immunity to Listeria monocytogenes in the liver. Immunology. 1994 Jun;82(2):249–254. [PMC free article] [PubMed] [Google Scholar]
- Schoel B., Welzel M., Kaufmann S. H. Quantification of protein in dilute and complex samples: modification of the bicinchoninic acid assay. J Biochem Biophys Methods. 1995 Jun;30(2-3):199–206. doi: 10.1016/0165-022x(95)00009-g. [DOI] [PubMed] [Google Scholar]
- Schülein R., Gentschev I., Mollenkopf H. J., Goebel W. A topological model for the haemolysin translocator protein HlyD. Mol Gen Genet. 1992 Jul;234(1):155–163. doi: 10.1007/BF00272357. [DOI] [PubMed] [Google Scholar]
- Stover C. K., de la Cruz V. F., Fuerst T. R., Burlein J. E., Benson L. A., Bennett L. T., Bansal G. P., Young J. F., Lee M. H., Hatfull G. F. New use of BCG for recombinant vaccines. Nature. 1991 Jun 6;351(6326):456–460. doi: 10.1038/351456a0. [DOI] [PubMed] [Google Scholar]
- Szalay G., Hess J., Kaufmann S. H. Presentation of Listeria monocytogenes antigens by major histocompatibility complex class I molecules to CD8 cytotoxic T lymphocytes independent of listeriolysin secretion and virulence. Eur J Immunol. 1994 Jul;24(7):1471–1477. doi: 10.1002/eji.1830240703. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma N. K., Ziegler H. K., Wilson M., Khan M., Safley S., Stocker B. A., Schoolnik G. K. Delivery of class I and class II MHC-restricted T-cell epitopes of listeriolysin of Listeria monocytogenes by attenuated Salmonella. Vaccine. 1995 Feb;13(2):142–150. doi: 10.1016/0264-410x(95)93127-u. [DOI] [PubMed] [Google Scholar]
- Wagner W., Vogel M., Goebel W. Transport of hemolysin across the outer membrane of Escherichia coli requires two functions. J Bacteriol. 1983 Apr;154(1):200–210. doi: 10.1128/jb.154.1.200-210.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wandersman C., Delepelaire P. TolC, an Escherichia coli outer membrane protein required for hemolysin secretion. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4776–4780. doi: 10.1073/pnas.87.12.4776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessel D., Flügge U. I. A method for the quantitative recovery of protein in dilute solution in the presence of detergents and lipids. Anal Biochem. 1984 Apr;138(1):141–143. doi: 10.1016/0003-2697(84)90782-6. [DOI] [PubMed] [Google Scholar]
- Zijlstra M., Bix M., Simister N. E., Loring J. M., Raulet D. H., Jaenisch R. Beta 2-microglobulin deficient mice lack CD4-8+ cytolytic T cells. Nature. 1990 Apr 19;344(6268):742–746. doi: 10.1038/344742a0. [DOI] [PubMed] [Google Scholar]