Abstract
Background:
Lymphedema is common after lymphatic damage in cancer treatment, with negative impacts on function and quality of life. Evidence suggests that blood vessel microvasculature is sensitive to irradiation and trauma; however, despite knowledge regarding dedicated mural blood supply to arteries and veins (vasa vasorum), equivalent blood vessels supplying lymphatics have not been characterized. We studied collecting lymphatics for dedicated mural blood vessels in our series of 500 lymphaticovenous anastomosis procedures for lymphedema, and equivalent controls.
Methods:
Microscopic images of lymphatics from lymphedema and control patients were analyzed for lymphatic wall vascular density. Collecting lymphatics from 20 patients with lymphedema and 10 control patients were sampled for more detailed analysis (podoplanin immunostaining, light/confocal microscopy, microcomputed tomography, and transmission electron microscopy) to assess lymphatic wall ultrastructure and blood supply.
Results:
Analysis revealed elaborate, dense blood microvessel networks associating with lymphatic walls in lymphedema patients and smaller equivalent vessels in controls. These vasa vasora or “arteria lymphatica” were supplied by regular axial blood vessels, parallel to lymphatic microperforators linking dermal and collecting lymphatics. Lymphatic walls were thicker in lymphedema patients than controls, with immunohistochemistry, computed tomography, transmission electron microscopy, and confocal microscopy characterizing abnormal blood vessels (altered appearance, thickened walls, elastin loss, narrow lumina, and fewer red blood cells) on these lymphatic walls.
Conclusions:
Dedicated blood vessels on lymphatics are significantly altered in lymphedema. A better understanding of the role of these vessels may reveal mechanistic clues into lymphedema pathophysiology and technical aspects of lymphedema microsurgery, and suggest potential novel therapeutic targets.
Takeaways
Question: Do lymphatic vessels have a dedicated blood supply, and how is this altered in lymphedema?
Findings: Patients with lymphedema boast structurally abnormal lymphatics and arteria lymphatica, previously uncharacterized blood vessels associated with the lymphatic walls.
Meaning: Altered lymphatic blood supply may play a role in lymphedema.
INTRODUCTION
Lymphedema is soft-tissue swelling, which results from accumulation of interstitial lymph fluid.1 The lymphatic system is a hierarchical vascular network that provides the principal drainage route for this fluid from all body tissues, and is also integral to intestinal fat absorption and immune cell trafficking.2–5 Lymphedema may result from abnormal lymphatic development2,6; however, it more commonly occurs secondary to a range of external insults to the lymphatic network. These insults include surgery or radiotherapy to a lymph node basin for cancer treatment, other physical trauma, filarial infection, and malignant invasion.7,8
Lymphedema is notoriously difficult to manage, with conservative measures such as massage and compression still forming the mainstay of treatment.9 Surgical approaches include those pioneered by O’Brien in the 1970s, the more commonly practiced of which has been liposuction.10 Microsurgical techniques described by O’Brien include lymph node transfer11,12 and lymphaticovenous anastomosis (LVA), and aim to restore the intrinsic physiology of the lymphatic system (the thoracic duct drains into the left subclavian vein in nature3,4) and redirect lymphatic flow around the damaged lymphatic vasculature into the venous system.13,14 Before the development of these techniques, disfiguring excision of lymphedema tissues and subsequent skin grafting was performed,15–17 essentially as a palliative procedure.
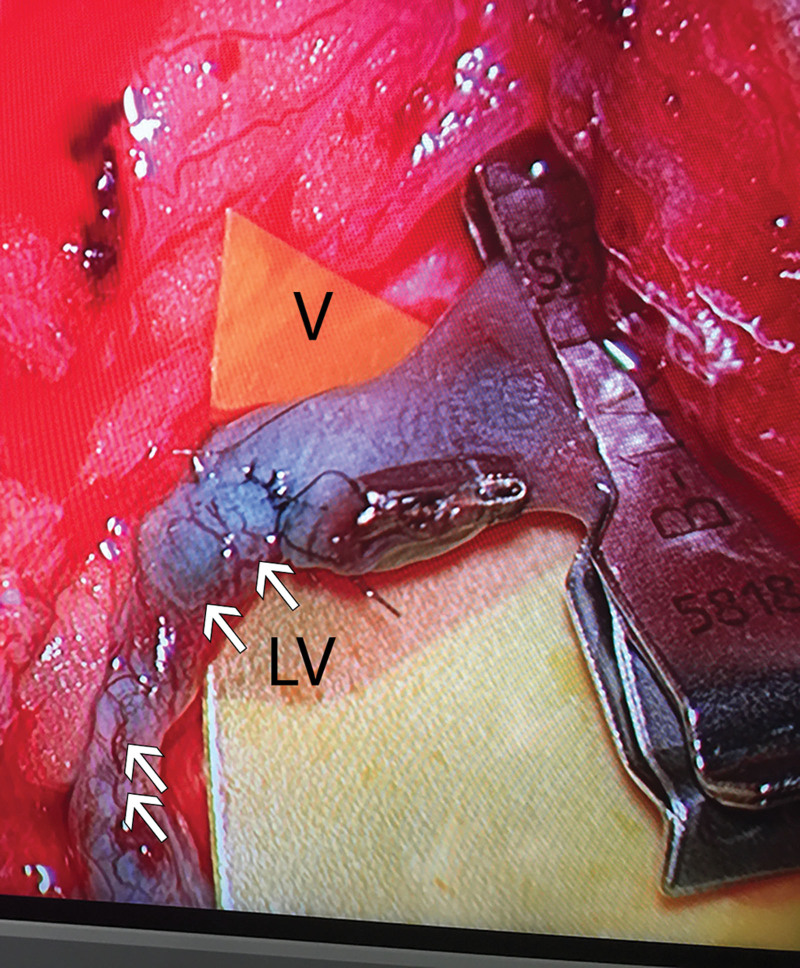
In contrast to the blood vasculature, the lymphatic system is poorly understood.18 The lymphatic system is comprised of three vessel subtypes: initial (capillary) lymphatics, precollecting lymphatics (within the dermis), and subcutaneous collecting lymphatics.3 Although thin-walled initial lymphatics are predominantly involved in absorbing fluid and protein from the interstitium, collecting lymphatics have multilayered vessel walls that are akin to those found in arteries and veins, complete with smooth muscle and valves.3,19 Scant attention has been paid to the vessels connecting the superficial to the deep lymphatics systems. During a series of more than 500 LVAs, author R.S. noted the prominence of blood vessels (BVs) related to the collecting lymphatic wall (Fig. 1). Equivalent, albeit smaller and less dense analogous vessels were seen at the same magnification in 136 vessels in patients who did not have lymphedema but were undergoing sentinel lymph node biopsy. We studied these vessels in lymphedema (n = 10) and control tissues (n = 6) using transmission electron microscopy (TEM), immunohistochemistry, and micro computed tomography (CT), to better characterize both the lymphatics and the BVs that supply them.
Fig. 1.
Operating microscopy of collecting lymphatic. Microscopic view of an LVA in a lymphedema patient, demonstrating a “meshwork” of BVs (arrows) on the collecting lymphatic (LV) wall, contrasting with the low density of BVs seen on the wall of the adjacent vein (V).
It has long been acknowledged that arterial and venous vessel walls have a designated network of blood microvasculature (the so-called vasa vasorum) supplying oxygen and nutrients to cells within the vessel wall.20–22 These microvascular networks appear altered in arterial diseases, such as atherosclerosis23,24 and vasculitis,25 and respond to arterial intimal injury by angiogenesis and expansion.24,26 Although Aglianò et al27 suggested that there may be a blood microvascular supply to the lymphatic vessels in human thigh collecting lymphatics, analysis was not performed and any significance to diseases such as lymphedema were not reported. Here, we describe our morphological and microstructural analysis of the lymphatic vasa vasorum or “arteria lymphatica” and study these vessels in lymphedema and control tissues using TEM and micro-CT, and comment on the potential role of the blood microvasculature of collecting lymphatics in lymphatic physiology and disease.
METHODS
Patients Tissue Biospecimens
Observations that lead to this study were made during routine LVA or sentinel lymph node surgery. Ethical approval (HREC-A 067/16) was obtained from the Human Research Ethics Committee of St. Vincent’s Hospital, Melbourne, Australia; and patients consented in writing to use their surplus soft-tissue biospecimens, normally removed and discarded during surgery. In LVA surgery, collecting lymphatics were identified preoperatively by magnetic resonance lymphangiography and mapped using near-infra red light excitation of intradermally injected indocyanine green dye (Diagnostic Green, Germany). Intraoperatively, collecting lymphatics were identified under operating microscopy by vessel morphology and using Patent Blue V dye (Guerbet, Asia Pacific Ltd). Biospecimens for further analysis were obtained during LVA surgery (patients with lymphedema); or in the case of control specimens, from surplus tissue from patients undergoing autologous breast reconstruction after breast cancer (no history of metastasis, radiotherapy, chemotherapy, lymph node surgery, or lymphedema), tissue reduction surgery for lymphedema, or sentinel lymph node biopsy (no metastatic disease). One-mm lengths of collecting lymphatic biospecimens were orientated using 11/0 nylon microsutures (Ethicon).
Immunohistochemistry
Immunostaining of tissue sections for light microscopy is described. (See Supplemental Digital Content 1, which shows immunostaining of tissue sections for light microscopy, http://links.lww.com/PRSGO/D28.) For confocal microscopy, specimens were fixed in 4% paraformaldehyde and the following antibodies/dilutions used: 1:20 rabbit antihuman CD31 (Thermo Fisher Scientific), 1:20 Dako mouse antihuman podoplanin (D2–40; Agilent Technologies), 1:100 rabbit antihuman von Willebrand Factor (vWF) (Millipore), 1:100 goat antirabbit Alexa Fluor 568 [Thermo Fisher (Invitrogen)], 1:100 goat antimouse Alexa Fluor 488 [Thermo Fisher (Invitrogen)]. Isotype controls: Dako mouse IgG (Agilent Technologies)
Specimens were washed in phosphate buffered saline, permeabilized in 0.5% (v/v) Triton X-100 and blocked in 10% (v/v) goat serum in 0.1% Triton X-100. Primary antibodies were diluted in 20% (v/v) goat serum for vWF, or Dako Envision Flex Diluent (Agilent Technologies) for CD31 and D240, and incubated at 4°C. Samples were washed in 0.1% Triton X-100 and secondary antibodies applied at dilutions specified in Dako Envision Flex Diluent. Following additional washing, samples were counterstained with 1 μg/mL DAPI (Molecular Probes) diluted in 0.1% Triton X-100. Antibody costaining was performed, as described, with nuclear counterstains combinations of D2–40 with either vWF or CD31. IgG isotype antibodies served as controls. Samples were prepared for imaging by glycerol immersion and imaging performed on a Leicia SP8 confocal (version 2.0.1 of LASX), using an H PlanApo CS2 20 × 0.75NA objective. Laser excitation was performed with 410–450 nm emission for DAPI, 495–550 nm for Alexa Fluor 488, and 570–650 nm for Alexa Fluor 568.
Micro-CT
Tissues were fixed in 1% (w/v) glutaraldehyde, 1.5% paraformaldehyde 5 mM CaCl2 in 0.1M sodium cacodylate, then transported to the Bio21 Facility of the University of Melbourne (Parkville, Melbourne, Australia) for embedding, processing, and micro-CT imaging. Biospecimens were prepared using the glutaraldehyde/reduced osmium tetroxide/thiocarbohydrazide/OsO4 (ROTO) technique28 and embedded in Procure 812 resin, then sectioned less than 3 mm. Micro-CT scanning, including volume reconstruction, was performed (Phoenix Nanotom; GE Sensing & Inspection Technologies GmbH, Wunstorf, Germany) and xs control and Phoenix datos|x acquisition software (GE Sensing & Inspection Technologies). Micro-CT scanning was performed at 70 kV and 375 μA for varying time-periods according to the number of X-ray projections and image averaging. (See Supplemental Digital Content 2, which shows micro-CT settings, http://links.lww.com/PRSGO/D29.)
Transmission Electron Microscopy
Resin-mounted specimen blocks were trimmed to regions of interest (identified on micro-CT), and ultrathin sections cut to 70 nm for imaging [Tecnai F30 (FEI Company)] at 300 kV. Micrographs were recorded on a US1000 CCD (Gatan).
RESULTS
Lymphatic vessels were observed during LVA and sentinel lymph node surgery using patent blue V dye. Lymphatic diameter and mural thickness were significantly greater in the cohort of 507 patients with lymphedema, compared with nonlymphedema control vessels. BVs were noted on collecting lymphatic vessels; however, they were significantly higher in number and density in the walls of collecting lymphatics from patients with lymphedema compared with both veins of similar/larger caliber (Fig. 1); or collecting lymphatics viewed at identical magnification in patients with “node-negative” nonlymphedema undergoing sentinel lymph node biopsy. These lymphatic-associated BVs were designated “arteria lymphatica” or “artery of the lymphatics.” The arteria lymphatica were axially supplied by larger segmental vessels that were located between 5 and 10 mm apart. In some patients, these accompanied dilated perforating lymphatics that joined the collecting lymphatics to the layers of dermal lymphatics within the skin.
To further study these BVs that were associated with collecting lymphatics, samples of collecting lymphatics (see Methods) were obtained from 10 patients with stage 2 secondary lymphoedema29 undergoing LVA surgery, for study with immunohistochemistry using established lymphatic markers. Nonlymphedema control tissues were obtained from the inguinal region of six patients. (See table, Supplemental Digital Content 3, which shows patient demographics and biospecimens, http://links.lww.com/PRSGO/D30.) Finally, a subset of vessels was chosen for more detailed analysis using confocal microscopy, TEM, and micro-CT.
Three-dimensional Distribution of BVs Associated with Collecting Lymphatics
Serial sections stained with podoplanin and the pan-endothelial marker CD31 demonstrated multiple CD31-positive/podoplanin-negative blood microvascular structures on and within the thickened lymphatic wall of the lymphedema specimen. (See figure, Supplemental Digital Content 4, which shows light microscopy images of transverse serial sections through the lymphatic wall, http://links.lww.com/PRSGO/D31.) These structures were consistent with the arteria lymphatica of the human collecting lymphatics; however, they were less prominent in control tissues. (See figure, Supplemental Digital Content 5, which shows light microscopy images of transverse serial sections through a perforating vascular bundle from the groin of a woman without lymphedema, http://links.lww.com/PRSGO/D32.) To study the three-dimensional (3D) distribution of BVs related to the collecting lymphatic wall, lymphatic specimens from three patients with lymphedema who had undergone whole-mount staining with the lymphatic marker podoplanin and vascular endothelial marker vWF (Supplemental Digital Content 3, http://links.lww.com/PRSGO/D30; cases 1–3) were selected for further analysis.
Figure 2 provides representative confocal microscopy images of lymphatics from a patient with a 6-year history of lymphedema (Supplemental Digital Content 3, http://links.lww.com/PRSGO/D30; case 3), demonstrating a meshwork of vWF-positive/podoplainin-negative blood microvasculature on/within the lymphatic wall (Fig. 2A). The overlying network of blood microvasculature gave rise to branches that penetrated the lymphatic wall immediately overlying lymphatic endothelium (Fig. 2B), in confocal microscopy images taken from the perspective of the lymphatic lumen outwards through the lymphatic wall (Fig. 2B).
Fig. 2.
Confocal microscopy of whole immunostained lower limb lymphatic from a female patient with chronic lymphedema. External view of lymphatic wall demonstrating “meshwork” of vWF+, BVs (red), overlying the D2–40 (podoplanin)+ staining lymphatic (green) (A); with alternative angle viewed (B); and internal view from within the lymphatic lumen (C).
Ultrastructural Analysis of the Arteria Lymphatica in Lymphedema Samples
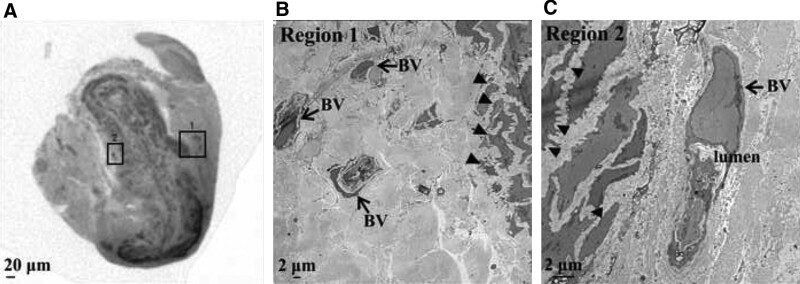
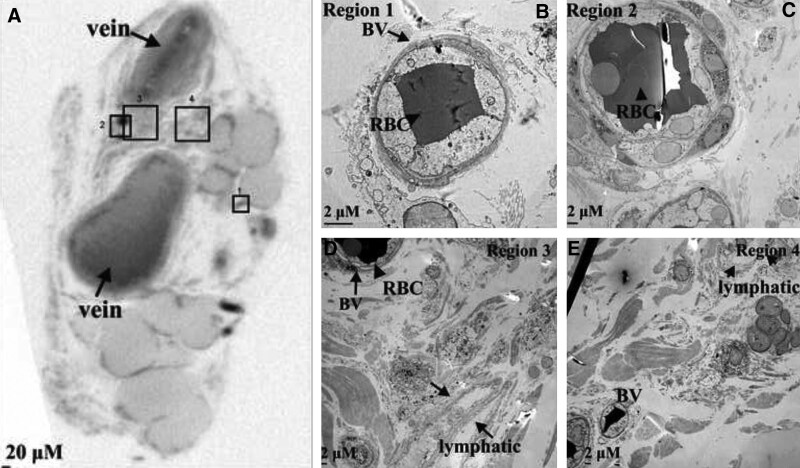
Lymphatic specimens from three patients with secondary lymphedema and controls were fixed and processed for micro-CT and TEM (Supplemental Digital Content 3, http://links.lww.com/PRSGO/D30; cases 4 and 6; control 1). Micro-CT was performed to identify blood microvasculature associated with the lymphatics, and the regions identified were further examined by TEM. In all specimens, blood microvasculature was seen on the collecting lymphatic wall; however, both the blood microvasculature and the lymphatic wall itself appeared abnormal in samples from patients with lymphedema (Figs. 3–5). These structural abnormalities were particularly evident in lower limb lymphatic specimens from the two patients with longer histories of lymphedema (5 y) (Figs. 3 and 4), compared with either patient specimens from a shorter lymphedema history (Fig. 5) or control tissues (Fig. 6).
Fig. 3.
Micro-CT and TEM images of lower limb lymphatic in lymphedema. A, Micro-CT in a transverse plane showing gross morphology of a thickened lymphatic wall with a narrowed lumen. B, TEM of region 1 showing three BVs (arrows) associated with the lymphatic wall, and the ultrastructure of the adjacent lymphatic media with abnormal, “spiky” SMCs (closed arrows). TEM of region 2 demonstrating a single BV with a narrow lumen and vascular endothelial cell nucleus (arrow). C, Abnormal SMCs (closed arrows) are seen in the adjacent lymphatic media.
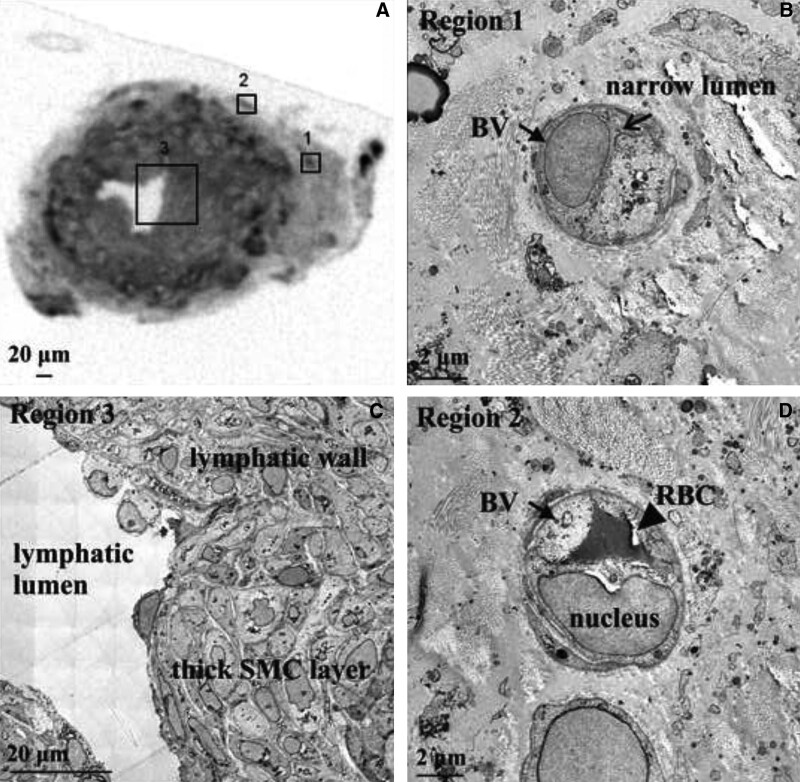
Fig. 5.
Micro-CT and TEM images of upper limb lymphatic in lymphedema. A, Micro-CT image of a transverse section through the lymphatic showing a thickened smooth muscle layer in the lymphatic wall. B, TEM demonstrating associated blood microvasculature in region 1, with a single BV with a narrow lumen (arrow and label) that is devoid of RBCs. C, TEM of associated microvasculature in region 2 showing a BV comprised of an endothelial cell (nucleus arrowed and labeled) and a RBC in the lumen (closed arrow and label). D, TEM of the lymphatic wall in region 3 showing a normal lymphatic SMC morphology and no evidence of lumen narrowing.
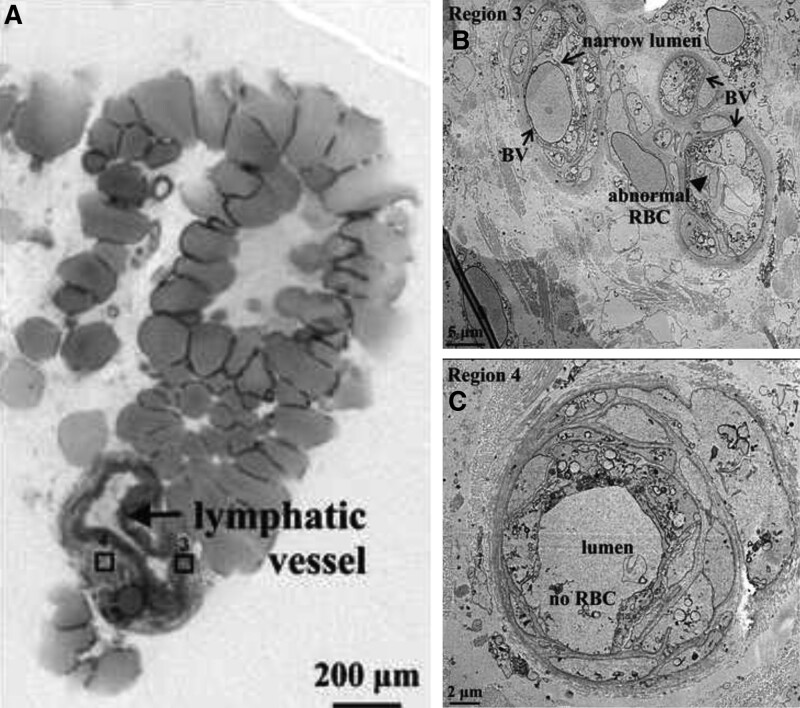
Fig. 4.
Micro-CT and TEM images of leg lymphatic in lymphedema. A, Micro-CT section through a lymphatic showing mural structure (arrow) of 4–6 SMC layers, connective tissue and associated blood vessels. B, TEM of region 3 demonstrating an abnormal BV adjacent to the lymphatic wall, with a narrow lumen (arrow) containing a permeabilized RBC (closed arrow and label). C, TEM of region 4, showing an associated BV devoid of RBCs.
Fig. 6.
Micro-CT and TEM images of control tissues from the lower limb. A, Micro-CT image of a transverse section demonstrating two veins (arrows and labels), and thin-walled lymphatics with associated blood microvasculature (regions 1–4). B, TEM imaging showing the features of the normal BV in region 1, with a rounded morphology, no luminal constriction, and circulating RBC (closed arrow). C, TEM of a normal BV in region 2 containing multiple RBCs (closed arrow). TEM of region 3 and 4 demonstrating a thin-walled lymphatic with only 1–2 SMC layers and a normal lumen (arrows) (D) and two small BVs (E).
Case 4 demonstrated two regions on micro-CT that contained blood microvasculature within the adventitia or connective tissue of the collecting lymphatic (Fig. 3; regions 1 and 2). In keeping with clinical intraoperative findings, the collecting lymphatic walls were thickened and the lumena narrowed (wall 70 µm, lumen 0 µm). In the first of these regions, examined further by TEM, the smooth muscle cells (SMCs) of the collecting lymphatic media demonstrated “spiky” morphology (Fig. 3; region 1). Three BVs on the lymphatic walls were also grossly abnormal, with a compressed vessel wall, an irregular lumen, and few observable circulating red blood cells (RBCs) (Fig. 3; region 1). The same abnormality was seen on TEM of region 2, with “spiky” lymphatic SMCs and associated expansion of the lymphatic extracellular matrix (Fig. 3; region 2). In this region, BVs in the lymphatic adventitia were also seen to contain “spiky” SMCs and lack mural elastin fibers. Additionally, the BV lumena were severely narrowed and did not contain RBCs (Fig. 3; region 2).
Case 5 demonstrated similar features in the lymphatic vessels of the lower limb of a patient with lymphedema (wall 36 µm and lumen 60 µm). Two regions were identified on micro-CT containing blood microvasculature within the adventitia and surrounding connective tissue of the collecting lymphatic (Fig. 4; regions 3 and 4). In one region, three BVs were identified, all with abnormal morphology on TEM. The lumen of these BVs was narrowed, and abnormal permeabilized RBCs were seen (Fig. 4; region 3).
In the lymphatic specimen from case 6 with a shorter history of lymphedema, micro-CT imaging also demonstrated lymphatic wall thickening (wall 103 µm and lumen 43 µm) (Fig. 5). Examination of the lymphatic wall by TEM demonstrated more normal, rounded SMC morphology (Fig. 5; region 3). Two regions containing blood microvasculature were identified on micro-CT within the adventitia and connective tissue of the collecting lymphatic wall (Fig. 5; regions 1 and 2). This specimen contained BVs with a narrow lumen and no circulating RBCs.
In a nonlymphedema control specimen, two thin-walled lymphatics and attendant blood microvasculature were seen in adjacent connective and adipose tissue (Fig. 6). Four regions identified on micro-CT were examined further by TEM (Fig. 6; regions 1–4). In contrast to the lymphedema specimens, this lymphatic was thinner-walled (one to two SMC layers) and boasted a patent lumen (wall 13 µm and lumen 13 µm) (Fig. 6; region 3). Normal blood microvasculature was seen adjacent to these vessels, with patent lumena containing RBCs (Fig. 6; regions 1–4). The density of these BVs and their association with the lymphatic wall did not resemble the blood microvasculature in lymphedema specimens (Figs. 3–5).
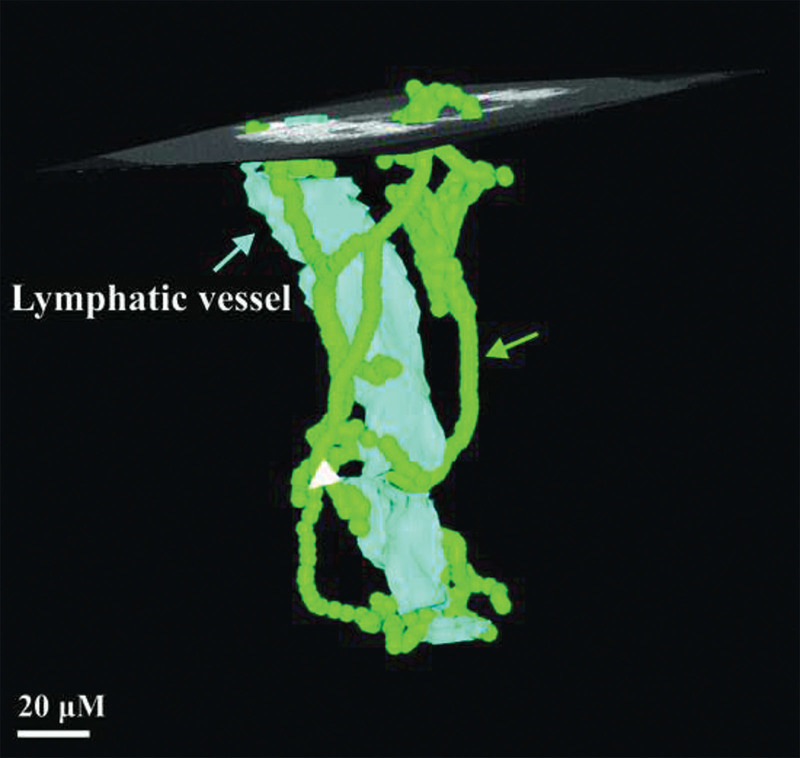
The spatial relationship between the collecting lymphatic and the network of associated blood microvasculature in chronic lymphedema (case 5) was seen on micro-CT 3D reconstruction (Fig. 7).
Fig. 7.
Three-dimensional reconstructed micro-CT image of lower limb lymphatic in Figure 4. Transverse section in Figure 4 is indicated by the superimposed orthoslice. Lymphatic endothelium is depicted in light blue, showing the outline of the lymphatic lumen (arrow and label). The course of the BVs associated with the lymphatic wall (arrow and label) are shown green.
DISCUSSION
Lymphedema affects 24%–49% of patients with breast cancer undergoing mastectomy,9 radiotherapy, and/or therapeutic lymph node surgery.7,8,30–32 Lymphedema significantly impairs quality of life30–32; however, the pathophysiology of lymphatic injury and dysfunction, particularly in the context of radiotherapy, is poorly understood.33,34 Collecting lymphatics in human limbs have a diameter greater than 200 μm and a structured vessel wall with distinct intima, media, and adventitial layers.3,19 Therefore, the cells comprising the collecting lymphatic wall require a dedicated blood supply, akin to vasa vasorum of arteries and veins.27 Although BVs can derive diffusional metabolic supply from the blood within, lymphatics do not contain blood. Therefore, larger caliber lymphatics are even more dependent on blood supply from their vasa vasorum35; meaning that injury to BVs that supply lymphatics risks causing ischemic injury to the lymphatic (as seen in nerves).
To address this question, we identified the blood microvasculature (“arteria lymphatica”) associated with collecting lymphatics in more than 500 lymphedematous human limbs, which were sparse or small in more than 130 equivalent nonlymphedema controls; and characterized their vascular morphology and microstructure using immunostaining and confocal microscopy, micro-CT, and TEM. On gross observation, the arteria lymphatica were more prominent on larger collecting lymphatics, an observation that attests to the increasing metabolic demands of a larger vessel leading to a secondary hypertrophy of these otherwise very fine-caliber vessels. Furthermore, the vessels were supplied axially via segmental BVs that may enable surgeons to retain this supply and therefore enhanced metabolism at the point at which these vessels are detached for connection into a vein, during an LVA surgery. This effectively creates a lymphatic vessel pedicled flap and suggests superior vascularity and therefore durability of anastomoses, as is seen in cardiac surgery in the case of an internal mammary artery graft (which remains attached at one end and should therefore be referred to as a flap), and a graft harvested from either the saphenous vein or radial artery.
Staining using key lymphatic and vascular markers confirmed an intricate network of mural BVs in collecting lymphatic walls, and key differences between lymphedematous and control lymphatics. To further investigate this newly described network, micro-CT and TEM were performed, demonstrating thickened lymphatic media, abnormal SMC morphology, and reduced extracellular matrix volume—features that were present regardless of lymphedema duration, but which worsened with time since lymphedema onset. Such a correlation was also seen in associated lymphatic blood microvasculature, with mural thickening and luminal narrowing, but reduction of circulating RBCs in all lymphedema specimens. In contrast, the thinner-walled control lymphatics demonstrated sparser arteria lymphatica that were normal in terms of mural SMCs, microstructure and morphology. We clearly observed fundamental differences between structural integrity of the arteria lymphatica in lymphatic vessels from lymphedematous versus control tissues in serial sections (Supplemental Digital Content 4, http://links.lww.com/PRSGO/D31 and Supplemental Digital Content 5, http://links.lww.com/PRSGO/D32).
Previous work suggested that the gut blood microvascular endothelium was the most sensitive to irradiation injury and that damage to these vessels was the primary event in radiation-induced gastrointestinal injury.36 Intestinal lymphatic endothelial cells were more resistant to radiation-induced apoptosis, compared with blood endothelial counterparts.36,37 We have previously demonstrated that radiotherapy results in transcriptional changes affecting the functionality of fibroblasts and other soft-tissue cells, potentially influencing lymphangiogenesis.38 Recently, we described a specific molecular impairment in lymphatic repair, relating to deficits in vascular endothelial growth factor receptor-3–mediated lymphangiogenic signaling in radiation injury to lymphatics.39 Injury to the lymphatic wall blood microvasculature may result in localized ischemia, important to the pathophysiology of lymphedema in lymphatics that depend on this blood supply. This injury may contribute to progressive lymphatic injury or prohibit lymphatic recovery, which may in turn lead to the lymphatic dysfunction that manifests as lymphedema.
Alternatively, arteria lymphatica may result from angiogenesis in response to lymphatic injury in an attempt to salvage lymphatic vessel integrity.24,26 Overall, the arteria lymphatica are likely to play an important role in the physiological response to lymphatic disease. Therefore, protecting these microvessels from injury may be critical in reducing the impact of cancer therapies on collecting lymphatics. Promoting angiogenesis to enhance the blood supply of lymphatics may present a novel therapeutic avenue by which to augment lymphatic function or enhance impaired lymphatics that cause lymphedema. These findings indicate that secondary lymphedema is more complex than a simple mechanical injury that “obliterates” lymphatics.
Our study serves as an anatomical description of the presence of the arteria lymphatica, with a view to stimulating future work examining the pathophysiology further. We acknowledge that the sample size is small—a reflection of the enormous cost of imaging techniques such as micro-CT and TEM, and the challenges and ethical considerations in obtaining sufficient human lymphatic material without causing further damage to the system or jeopardizing the feasibility of the LVA surgery itself. Regardless we have observed the arteria lymphatica under the operating microscope in a clinical series of more than 500 LVAs.
Further detailed molecular and mechanistic information regarding the arteria lymphatica will enhance our understanding of the etiology of secondary lymphedema and help explain additive adverse effects of radiotherapy to surgery performed to treat cancer. Such knowledge will increase our appreciation of the pathophysiology of the lymphatic system broadly, influence surgical approaches to lymphedema, and provide novel therapeutic targets for lymphatic disease.
CONCLUSIONS
We describe a novel subcategory of vascular supply to the lymphatics. The presence of arteria lymphatica supports the concept that lymphatics are not inert pipes, rather dynamic vascularized vessels with watersheds, potentially impaired by damage to their blood supply. The findings shed new light on the pathophysiology of lymphedema, suggesting either an ischemic etiology or exacerbation effect to the lymphedema, a secondary decompensation in the lymphedema lymphatics or a failure of the arteria lymphatica to compensate for this hypoxia adequately. The arteria lymphatica may also offer a further therapeutic target in our quest to enhance lymphatic function in lymphoedematous limbs occurring either after cancer treatment or as a primary genetic/developmental dysregulation, and suggest that a less aggressive stripping of the lymphatics may be beneficial to longer term survival of the lymphatics and lymphatic anastomoses in lymphatic surgery. Finally, these findings pave the way for free microsurgical transfer of lymphangions into injured lymphatic areas.
DISCLOSURES
Dr. Bendon received funding from the University of Melbourne and the Royal College of Surgeons of England. The other authors have no financial interest to declare. The work was funded by grants from The Stafford Fox and Wicking Trusts, Australia.
ACKNOWLEDGMENTS
The authors acknowledge the support from the Trace Analysis for Chemical, Earth and Environmental Sciences (TrACEES) platform from the Melbourne Collaborative Research Infrastructure Program at the University of Melbourne and thank Dr. Jay Black (School of Earth Sciences) for operating the micro-CT scanner and processing data. The authors thank Ms. Janna Taylor for assistance in preparing the figures and Mr. Jason Palmer for technical support in sectioning tissue blocks, immunohistochemical staining.
Supplementary Material
Footnotes
Published online 24 January 2024.
Disclosure statements are at the end of this article, following the correspondence information.
Related Digital Media are available in the full-text version of the article on www.PRSGlobalOpen.com.
REFERENCES
- 1.Mortimer PS. The pathophysiology of lymphedema. Cancer. 1998;83:2798–2802. [DOI] [PubMed] [Google Scholar]
- 2.Oliver G, Detmar M. The rediscovery of the lymphatic system: old and new insights into the development and biological function of the lymphatic vasculature. Genes Dev. 2002;16:773–783. [DOI] [PubMed] [Google Scholar]
- 3.Shayan R, Achen MG, Stacker SA. Lymphatic vessels in cancer metastasis: bridging the gaps. Carcinogenesis. 2006;27:1729–1738. [DOI] [PubMed] [Google Scholar]
- 4.Lord RS. The white veins: conceptual difficulties in the history of the lymphatics. Med Hist. 1968;12:174–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnson LA, Jackson DG. Control of dendritic cell trafficking in lymphatics by chemokines. Angiogenesis. 2014;17:335–345. [DOI] [PubMed] [Google Scholar]
- 6.Spiegel R, Ghalamkarpour A, Daniel-Spiegel E, et al. Wide clinical spectrum in a family with hereditary lymphedema type I due to a novel missense mutation in VEGFR3. J Hum Genet. 2006;51:846–850. [DOI] [PubMed] [Google Scholar]
- 7.Deo SV, Ray S, Rath GK, et al. Prevalence and risk factors for development of lymphedema following breast cancer treatment. Indian J Cancer. 2004;41:8–12. [PubMed] [Google Scholar]
- 8.Ugur S, Arici C, Yaprak M, et al. Risk factors of breast cancer-related lymphedema. Lymphat Res Biol. 2013;11:72–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Warren AG, Brorson H, Borud LJ, et al. Lymphedema: a comprehensive review. Ann Plast Surg. 2007;59:464–472. [DOI] [PubMed] [Google Scholar]
- 10.O’Brien BM, Khazanchi RK, Kumar PA, et al. Liposuction in the treatment of lymphoedema; a preliminary report. Br J Plast Surg. 1989;42:530–533. [DOI] [PubMed] [Google Scholar]
- 11.Chen HC, O’Brien BM, Rogers IW, et al. Lymph node transfer for the treatment of obstructive lymphedema in the canine model. Br J Plast Surg. 1990;43:578–586. [DOI] [PubMed] [Google Scholar]
- 12.Becker C, Assouad J, Riquet M, et al. Postmastectomy lymphedema: long-term results following microsurgical lymph node transplantation. Ann Surg. 2006;243:313–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O’Brien BM, Mellow CG, Khazanchi RK, et al. Long-term results after microlymphaticovenous anastomoses for the treatment of obstructive lymphedema. Plast Reconstr Surg. 1990;85:562–572. [DOI] [PubMed] [Google Scholar]
- 14.Mihara M, Murai N, Hayashi Y, et al. Using indocyanine green fluorescent lymphography and lymphatic-venous anastomosis for cancer-related lymphedema. Ann Vasc Surg. 2012;26:278.e1–278.e6. [DOI] [PubMed] [Google Scholar]
- 15.Charles RH. The surgical treament of elephantiasis. Ind Med Gaz. 1901;36:84–99. [PMC free article] [PubMed] [Google Scholar]
- 16.Sistrunk WE. Contribution to plastic surgery: removal of scars by stages; an open operation for extensive laceration of the anal sphincter; the Kondoleon operation for elephantiasis. Ann Surg. 1927;85:185–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Farina R. Elephantiasis of the lower limbs; treatment by dermo-fibro-lipectomy followed by free skin grafting. Plast Reconstr Surg (1946). 1951;8:430–442. [DOI] [PubMed] [Google Scholar]
- 18.Stacker SA, Williams SP, Karnezis T, et al. Lymphangiogenesis and lymphatic vessel remodelling in cancer. Nat Rev Cancer. 2014;14:159–172. [DOI] [PubMed] [Google Scholar]
- 19.Scavelli C, Weber E, Aglianò M, et al. Lymphatics at the crossroads of angiogenesis and lymphangiogenesis. J Anat. 2004;204:433–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lowenberg RI, Schumacker HB. Experimental studies in vascular repair; morphologic observations of normal vasa vasorum. Yale J Biol Med. 1948;20:395–401. [PMC free article] [PubMed] [Google Scholar]
- 21.Wolinsky H, Glagov S. Nature of species differences in the medial distribution of aortic vasa vasorum in mammals. Circ Res. 1967;20:409–421. [DOI] [PubMed] [Google Scholar]
- 22.Scotland RS, Vallance PJ, Ahluwalia A. Endogenous factors involved in regulation of tone of arterial vasa vasorum: implications for conduit vessel physiology. Cardiovasc Res. 2000;46:403–411. [DOI] [PubMed] [Google Scholar]
- 23.Mollmark JI, Park AJ, Wang TZ, et al. Fibroblast growth factor-2 is required for vasa vasorum plexus stability in hypercholesterolaemic mice. Atheroscler Thromb Vasc Biol. 2012;32:2644–2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herrmann J, Lerman LO, Rodriguez-Porcel M, et al. Coronary vasa vasorum neovascularization precedes epicardial endothelial dysfunction in experimental hypercholesterolaemia. Cardiovasc Res. 2001;51:762–766. [DOI] [PubMed] [Google Scholar]
- 25.Hamaoka-Okamoto A, Suzuki C, Yahata T, et al. The involvement of the vasa vasorum in the development of vasculitis in animal model of Kawasaki disease. Pediatr Rheumatol Online J. 2014;12:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mulligan-Kehoe MJ, Simons M. Vasa vasorum in normal and diseased arteries. Circulation. 2014;129:2557–2566. [DOI] [PubMed] [Google Scholar]
- 27.Aglianò M, Sacchi G, Weber E, et al. Vasa vasorum of superficial collecting lymphatics of human thigh. Lymphology. 1997;30:116–121. [PubMed] [Google Scholar]
- 28.Hanssen E, Dekiwadia C, Riglar DT, et al. Electron tomography of Plasmodium falciparum merozoites reveals core cellular events that underpin erythrocyte invasion. Cell Microbiol. 2013;15:1457–1472. [DOI] [PubMed] [Google Scholar]
- 29.International Society of Lymphology. The diagnosis and treatment of peripheral lymphedema: 2020 Consensus Document of the International Society of Lymphology. Lymphology. 2020;53:3–19. [PubMed] [Google Scholar]
- 30.Gärtner R, Jensen MB, Kronborg L, et al. Self-reported arm-lymphedema and functional impairment after breast cancer treatment—a nationwide study of prevalence and associated factors. Breast. 2010;19:506–515. [DOI] [PubMed] [Google Scholar]
- 31.Beaulac SM, McNair LA, Scott TE, et al. Lymphedema and quality of life in survivors of early-stage breast cancer. Arch Surg. 2002;137:1253–1257. [DOI] [PubMed] [Google Scholar]
- 32.McLaughlin SA, Wright MJ, Morris KT, et al. Prevalence of lymphedema in women with breast cancer 5 years after sentinel lymph node biopsy or axillary dissection: objective measurements. J Clin Oncol. 2008;26:5213–5219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Avraham T, Yan A, Zampell JC, et al. Radiation therapy causes loss of dermal lymphatic vessels and interferes with lymphatic function by TGF-beta1-mediated tissue fibrosis. Am J Physiol Cell Physiol. 2010;299:C589–C605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mehrara BJ, Greene AK. Lymphedema and obesity: is there a link? Plast Reconstr Surg. 2014;134:154e–160e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heistad DD, Armstrong ML, Amundsen S. Blood flow through vasa vasorum in arteries and veins: effects of luminal PO2. Am J Physiol. 1986;250:H434–H442. [DOI] [PubMed] [Google Scholar]
- 36.Paris F, Fuks Z, Kang A, et al. Endothelial apoptosis as the primary lesion initiating intestinal radiation damage in mice. Science. 2001;293:293–297. [DOI] [PubMed] [Google Scholar]
- 37.Sung HK, Morisada T, Cho CH, et al. Intestinal and peri-tumoral lymphatic endothelial cells are resistant to radiation-induced apoptosis. Biochem Biophys Res Commun. 2006;345:545–551. [DOI] [PubMed] [Google Scholar]
- 38.Shukla L, Lee S, Du M, et al. A transcriptomic dataset evaluating the effect of radiotherapy injury on cells of skin and soft tissue. Data in Brief. 2022;41:107828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pillay V, Shukla L, Herle P, et al. Radiation therapy attenuates lymphatic vessel repair by reducing VEGFR-3 signalling. Front Pharmacol. 2023;14:1152314. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.