Abstract
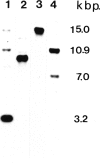
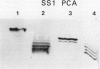
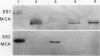
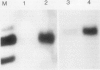
We have used antibodies directed against the two sucrose synthase (SS) isozymes, and the cDNA clones corresponding to the two nonallelic genes in maize to describe sorghum (Sorghum bicolor) SS genes and their expressions at protein and RNA levels. Western blot analyses have shown evidence of two SS isozymes, SS1 and SS2, in sorghum; these were similar, but not identical, to maize isozymes in size, charge, subunit composition, and epitope specificities against both monoclonal and polyclonal antibodies. Tissue-specific distributions of isozymes and genomic Southern hybridization data are consistent with a hypothesis that the SS1 and SS2 isozymes are encoded by two nonallelic genes, designated here as Sus1 and Sus2, respectively. Northern blot hybridizations on root RNAs showed gene-specific transcript patterns and, as in maize, the SS2-specific transcripts were slightly larger than the SS1-specific transcripts. Interestingly, no difference in the size of the SS1 and SS2 polypeptides was detected. Anaerobic induction led to significant elevations in steady-state levels of both SS1 and SS2 transcripts, but there was no detectable increase in the levels of the SS proteins. Thus, both the SS genes in sorghum were significantly regulated at the posttranscriptional level; whereas in maize, only one of the two SS genes was affected in this fashion. Another difference between maize and sorghum SS isozymes was in endosperm-specific polymerization among the SS subunits. Unlike maize endosperm where only the two SS homotetramers are seen, sorghum endosperm showed five SS isozymes attributable to a random copolymerization of SS1 and SS2 subunits, presumably due to a simultaneous expression of both genes in the endosperm cells. Physiological and molecular bases of these differences between these two crop plant species remains to be elucidated.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Chourey P. S., Nelson O. E. The enzymatic deficiency conditioned by the shrunken-1 mutations in maize. Biochem Genet. 1976 Dec;14(11-12):1041–1055. doi: 10.1007/BF00485135. [DOI] [PubMed] [Google Scholar]
- Delmer D. P. The purification and properties of sucrose synthetase from etiolated Phaseolus aureus seedlings. J Biol Chem. 1972 Jun 25;247(12):3822–3828. [PubMed] [Google Scholar]
- Echt C. S., Chourey P. S. A Comparison of Two Sucrose Synthetase Isozymes from Normal and shrunken-1 Maize. Plant Physiol. 1985 Oct;79(2):530–536. doi: 10.1104/pp.79.2.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulbert S. H., Richter T. E., Axtell J. D., Bennetzen J. L. Genetic mapping and characterization of sorghum and related crops by means of maize DNA probes. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4251–4255. doi: 10.1073/pnas.87.11.4251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Maraña C., García-Olmedo F., Carbonero P. Linked sucrose synthase genes in group-7 chromosomes in hexaploid wheat (Triticum aestivum L.). Gene. 1988 Mar 31;63(2):253–260. doi: 10.1016/0378-1119(88)90529-x. [DOI] [PubMed] [Google Scholar]
- McElfresh K. C., Chourey P. S. Anaerobiosis induces transcription but not translation of sucrose synthase in maize. Plant Physiol. 1988 Jun;87(2):542–546. doi: 10.1104/pp.87.2.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura T., Akazawa T. Enzymic mechanism of starch stynthesis in ripening rice grains. VII. Purification and enzymic properties of sucrose synthetase. Arch Biochem Biophys. 1973 Jun;156(2):644–652. doi: 10.1016/0003-9861(73)90316-0. [DOI] [PubMed] [Google Scholar]
- Springer B., Werr W., Starlinger P., Bennett D. C., Zokolica M., Freeling M. The Shrunken gene on chromosome 9 of Zea mays L is expressed in various plant tissues and encodes an anaerobic protein. Mol Gen Genet. 1986 Dec;205(3):461–468. doi: 10.1007/BF00338083. [DOI] [PubMed] [Google Scholar]
- Su J. C., Preiss J. Purification and properties of sucrose synthase from maize kernels. Plant Physiol. 1978 Mar;61(3):389–393. doi: 10.1104/pp.61.3.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taliercio E. W., Chourey P. S. Post-transcriptional control of sucrose synthase expression in anaerobic seedlings of maize. Plant Physiol. 1989 Aug;90(4):1359–1364. doi: 10.1104/pp.90.4.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]