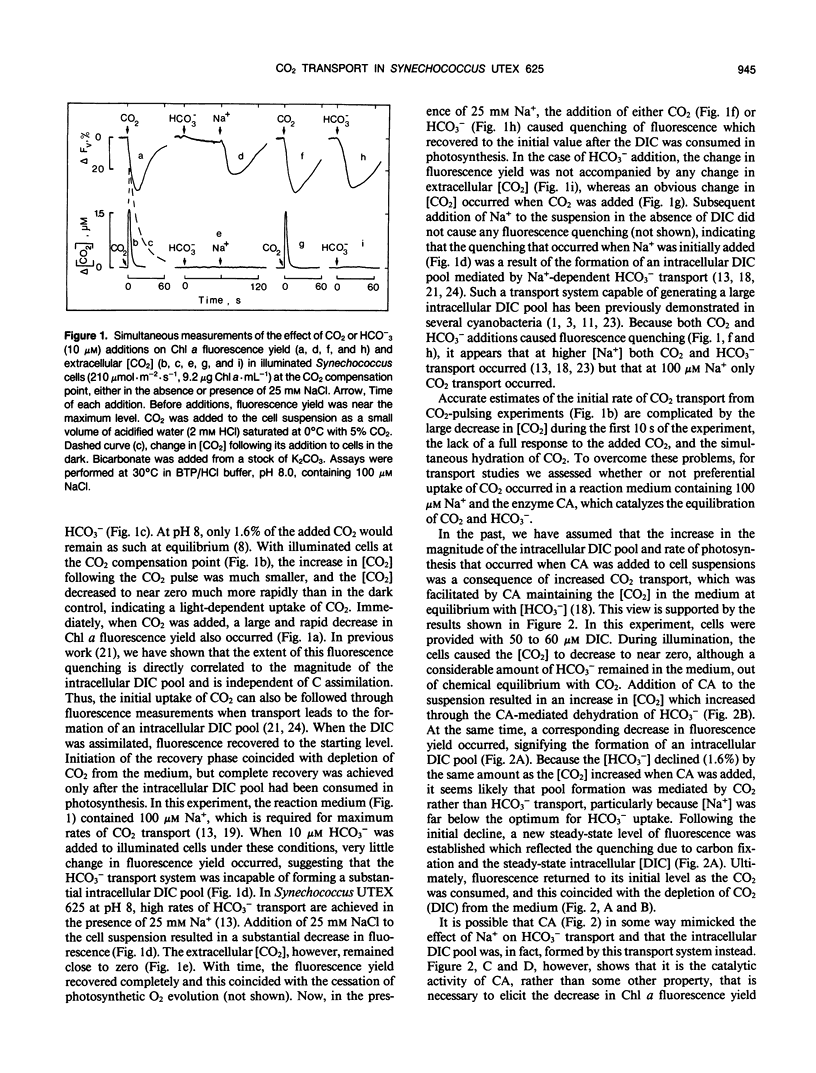
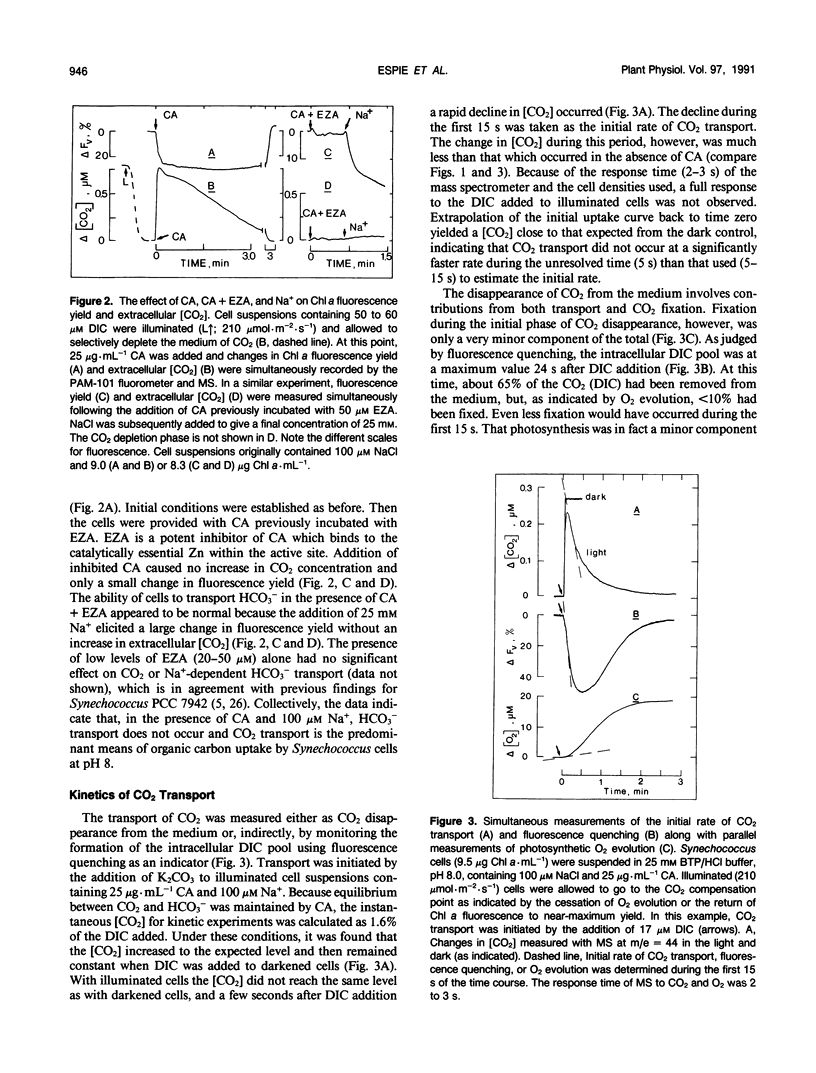
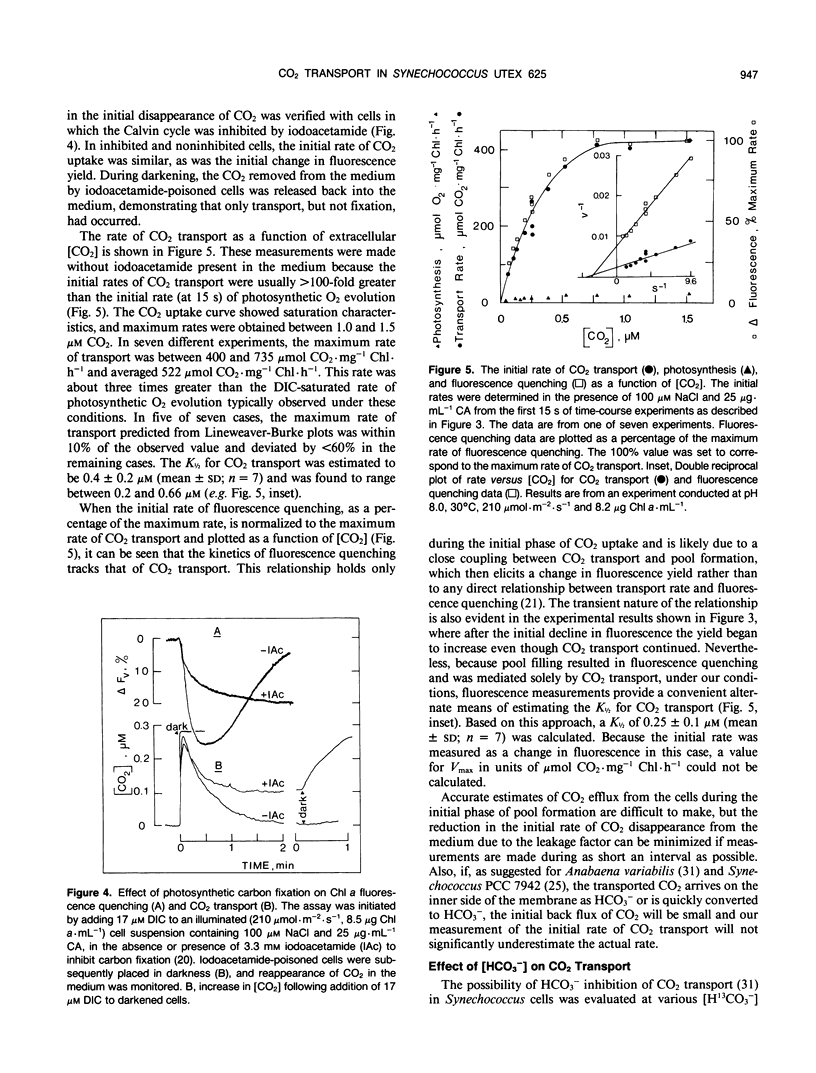
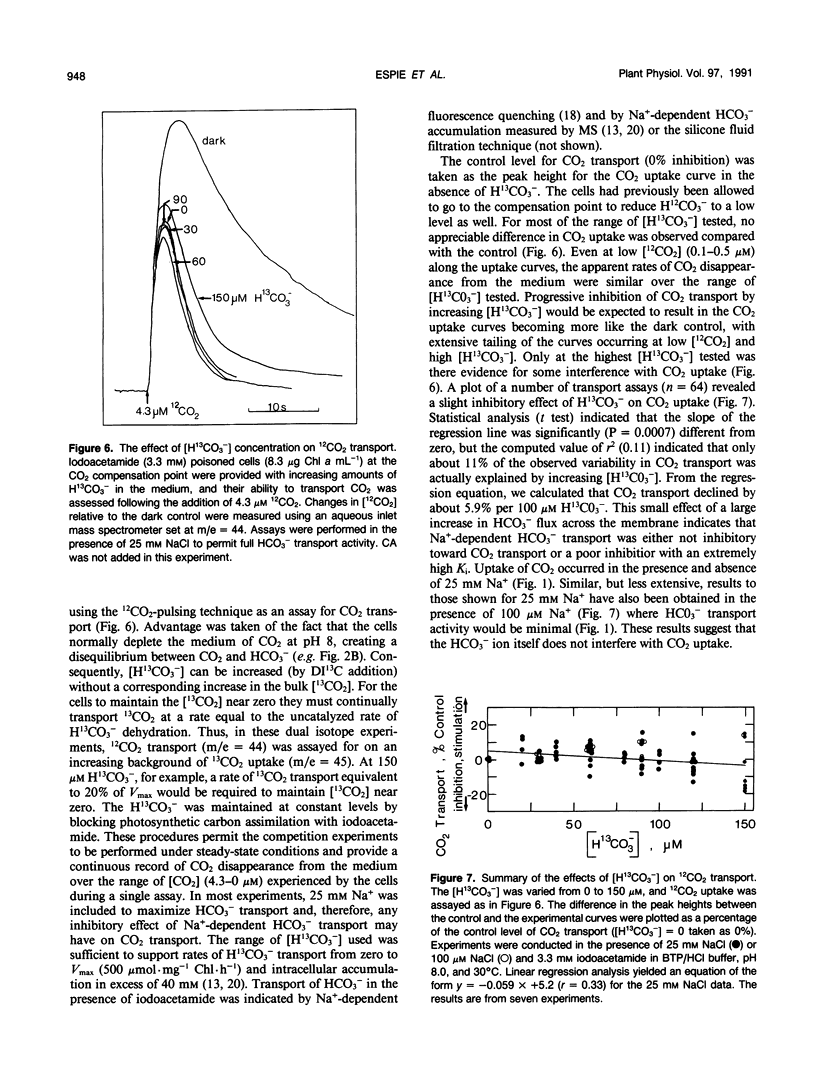
Abstract
The active transport of CO2 in Synechococcus UTEX 625 was measured by mass spectrometry under conditions that preclude HCO3− transport. The substrate concentration required to give one half the maximum rate for whole cell CO2 transport was determined to be 0.4 ± 0.2 micromolar (mean ± standard deviation; n = 7) with a range between 0.2 and 0.66 micromolar. The maximum rates of CO2 transport ranged between 400 and 735 micromoles per milligram of chlorophyll per hour with an average rate of 522 for seven experiments. This rate of transport was about three times greater than the dissolved inorganic carbon saturated rate of photosynthetic O2 evolution observed under these conditions. The initial rate of chlorophyll a fluorescence quenching was highly correlated with the initial rate of CO2 transport (correlation coefficient = 0.98) and could be used as an indirect method to detect CO2 transport and calculate the substrate concentration required to give one half the maximum rate of transport. Little, if any, inhibition of CO2 transport was caused by HCO3− or by Na+-dependent HCO3− transport. However, 12CO2 readily interfered with 13CO2 transport. CO2 transport and Na+-dependent HCO3− transport are separate, independent processes and the high affinity CO2 transporter is not only responsible for the initial transport of CO2 into the cell but also for scavenging any CO2 that may leak from the cell during ongoing photosynthesis.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Badger M. R., Andrews T. J. Photosynthesis and Inorganic Carbon Usage by the Marine Cyanobacterium, Synechococcus sp. Plant Physiol. 1982 Aug;70(2):517–523. doi: 10.1104/pp.70.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badger M. R., Bassett M., Comins H. N. A Model for HCO(3) Accumulation and Photosynthesis in the Cyanobacterium Synechococcus sp: Theoretical Predictions and Experimental Observations. Plant Physiol. 1985 Feb;77(2):465–471. doi: 10.1104/pp.77.2.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badger M. R., Price G. D. Carbon Oxysulfide Is an Inhibitor of Both CO(2) and HCO(3) Uptake in the Cyanobacterium Synechococcus PCC7942. Plant Physiol. 1990 Sep;94(1):35–39. doi: 10.1104/pp.94.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espie G. S., Canvin D. T. Evidence for Na-Independent HCO(3) Uptake by the Cyanobacterium Synechococcus leopoliensis. Plant Physiol. 1987 May;84(1):125–130. doi: 10.1104/pp.84.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espie G. S., Miller A. G., Birch D. G., Canvin D. T. Simultaneous Transport of CO(2) and HCO(3) by the Cyanobacterium Synechococcus UTEX 625. Plant Physiol. 1988 Jul;87(3):551–554. doi: 10.1104/pp.87.3.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espie G. S., Miller A. G., Canvin D. T. Characterization of the na-requirement in cyanobacterial photosynthesis. Plant Physiol. 1988 Nov;88(3):757–763. doi: 10.1104/pp.88.3.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espie G. S., Miller A. G., Canvin D. T. Selective and Reversible Inhibition of Active CO(2) Transport by Hydrogen Sulfide in a Cyanobacterium. Plant Physiol. 1989 Sep;91(1):387–394. doi: 10.1104/pp.91.1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayo W. P., Elrifi I. R., Turpin D. H. The Relationship between Ribulose Bisphosphate Concentration, Dissolved Inorganic Carbon (DIC) Transport and DIC-Limited Photosynthesis in the Cyanobacterium Synechococcus leopoliensis Grown at Different Concentrations of Inorganic Carbon. Plant Physiol. 1989 Jun;90(2):720–727. doi: 10.1104/pp.90.2.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayo W. P., Williams T. G., Birch D. G., Turpin D. H. Photosynthetic Adaptation by Synechococcus leopoliensis in Response to Exogenous Dissolved Inorganic Carbon. Plant Physiol. 1986 Apr;80(4):1038–1040. doi: 10.1104/pp.80.4.1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. G., Espie G. S., Canvin D. T. Chlorophyll a Fluorescence Yield as a Monitor of Both Active CO(2) and HCO(3) Transport by the Cyanobacterium Synechococcus UTEX 625. Plant Physiol. 1988 Mar;86(3):655–658. doi: 10.1104/pp.86.3.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. G., Espie G. S., Canvin D. T. Use of Carbon Oxysulfide, a Structural Analog of CO(2), to Study Active CO(2) Transport in the Cyanobacterium Synechococcus UTEX 625. Plant Physiol. 1989 Jul;90(3):1221–1231. doi: 10.1104/pp.90.3.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price G. D., Badger M. R. Ethoxyzolamide Inhibition of CO(2) Uptake in the Cyanobacterium Synechococcus PCC7942 without Apparent Inhibition of Internal Carbonic Anhydrase Activity. Plant Physiol. 1989 Jan;89(1):37–43. doi: 10.1104/pp.89.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price G. D., Badger M. R. Ethoxyzolamide Inhibition of CO(2)-Dependent Photosynthesis in the Cyanobacterium Synechococcus PCC7942. Plant Physiol. 1989 Jan;89(1):44–50. doi: 10.1104/pp.89.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volokita M., Zenvirth D., Kaplan A., Reinhold L. Nature of the Inorganic Carbon Species Actively Taken Up by the Cyanobacterium Anabaena variabilis. Plant Physiol. 1984 Nov;76(3):599–602. doi: 10.1104/pp.76.3.599. [DOI] [PMC free article] [PubMed] [Google Scholar]


