Abstract
Background
Bioenergetic maladaptations and axonopathy are often found in the early stages of neurodegeneration. Nicotinamide adenine dinucleotide (NAD), an essential cofactor for energy metabolism, is mainly synthesized by Nicotinamide mononucleotide adenylyl transferase 2 (NMNAT2) in CNS neurons. NMNAT2 mRNA levels are reduced in the brains of Alzheimer’s, Parkinson’s, and Huntington’s disease. Here we addressed whether NMNAT2 is required for axonal health of cortical glutamatergic neurons, whose long-projecting axons are often vulnerable in neurodegenerative conditions. We also tested if NMNAT2 maintains axonal health by ensuring axonal ATP levels for axonal transport, critical for axonal function.
Methods
We generated mouse and cultured neuron models to determine the impact of NMNAT2 loss from cortical glutamatergic neurons on axonal transport, energetic metabolism, and morphological integrity. In addition, we determined if exogenous NAD supplementation or inhibiting a NAD hydrolase, sterile alpha and TIR motif-containing protein 1 (SARM1), prevented axonal deficits caused by NMNAT2 loss. This study used a combination of techniques, including genetics, molecular biology, immunohistochemistry, biochemistry, fluorescent time-lapse imaging, live imaging with optical sensors, and anti-sense oligos.
Results
We provide in vivo evidence that NMNAT2 in glutamatergic neurons is required for axonal survival. Using in vivo and in vitro studies, we demonstrate that NMNAT2 maintains the NAD-redox potential to provide “on-board” ATP via glycolysis to vesicular cargos in distal axons. Exogenous NAD+ supplementation to NMNAT2 KO neurons restores glycolysis and resumes fast axonal transport. Finally, we demonstrate both in vitro and in vivo that reducing the activity of SARM1, an NAD degradation enzyme, can reduce axonal transport deficits and suppress axon degeneration in NMNAT2 KO neurons.
Conclusion
NMNAT2 ensures axonal health by maintaining NAD redox potential in distal axons to ensure efficient vesicular glycolysis required for fast axonal transport.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13024-023-00690-9.
Background
Glucose is the primary energy source in the brain [1, 2]. Recent human studies highlight bioenergetic maladaptations in the brain during neurodegenerative disorders of aging (NDA) [3], such as Alzheimer's disease (AD). In AD, the brain's white matter [4], which comprises long-range axonal tracts, exhibits glucose hypometabolism. Axons, the longest and the most complex subcellular compartments of neurons [5–7], are particularly vulnerable to neurodegenerative conditions [8–10]. Two key questions concerning glucose metabolism in axons during NDAs include: Does glucose hypometabolism in long-range axons cause axonopathy, an early sign of neurodegeneration [11, 12]? And what are the key steps in maintaining axonal energetics to ensure axonal function and health?
Nicotinamide adenine dinucleotide (NAD) is a cofactor essential for energy metabolism. The ratio of oxidized (NAD+) to reduced (NADH) form of NAD, named the NAD redox potential, is a pivotal driver of glycolysis, tricarboxylic acid (TCA) cycle, and oxidative phosphorylation (OXPHO) [13]. Nicotinamide mononucleotide adenylyl transferase 2 (NMNAT2) is the major NAD-synthesizing enzyme in CNS neurons [14–16], and a key axonal maintenance factor [17–20]. The mRNA levels of NMNAT2 are significantly reduced in the brains of AD, Parkinson's disease (PD), and Huntington's disease (HD) patients [21–23]. Notably, upregulating NMNAT2 and other NMNATs is protective in multiple neurodegenerative disease models [23, 24]. However, whether NMNAT2 maintains axonal health by contributing to axonal bioenergetics is not clear.
Previous studies showed that NMNAT2 is localized to Golgi-derived vesicles in axons and undergoes fast, bi-directional axonal transport in superior cervical ganglia neurons [25, 26]. Axonal transport between the cell body and axonal terminals is critical for neurotransmission, synaptic plasticity [27], and neuronal health [28]. To achieve fast and continuous movement of cargos along highly arborized axons across long distances [29–31], the bioenergetic machinery must be locally mobilized to provide ATP. It has been demonstrated that glycolytic enzymes specifically attach to fast-moving vesicular cargos to fuel fast axonal transport [32, 33]. Surprisingly, ultra-resolution electron microscopy studies find that mitochondria, the other energy powerhouse, are often small in sizes and sparsely distributed in axons [34, 35]. Moreover, in vitro evidence suggests glycolysis but not mitochondrial oxidative phosphorylation (OXPHO) supplies constant energy to fuel fast axonal transport [32]. Moving one vesicular cargo at physiological speed consumes ~ 200 ATP molecules per second [36], while each glycolytic cycle only yields 2 ATP molecules. Thus, vesicular glycolysis has to turnover rapidly to support fast axonal transport. In this study, we aimed to test the hypothesis that NMNAT2 maintains the NAD redox potential necessary to drive ATP synthesis through vesicular glycolysis for fast axonal transport.
We employed both animal (in vivo) and culture (in vitro) model systems to determine whether: (1) NMNAT2 is required for the health of long-range axons of glutamatergic neurons; (2) NMNAT2 is essential for fast axonal transport; (3) NMNAT2 ensures the proper NAD+/NADH redox potential for glycolysis on vesicular cargos; and (4) NAD supplementation rescues the axonal phenotypes caused by NMNAT2 loss. Our results reveal the importance of NMNAT2 in maintaining axonal NAD redox potential and driving the “onboard” glycolysis necessary for fast axonal transport; we also demonstrate that NMNAT2 reduction increases the vulnerability of distal axons.
Methods
Mice
The generation of NMNAT2f/f mice carrying a Cre recombinase-dependent gene trap cassette within intron 1 of NMNAT2 gene has been described [37]. Here we generated NMNAT2 conditional knockout (cKO) mice by crossing NMNAT2f/f mice with NEX-Cre mice [38] to delete NMNAT2 in cortical glutamatergic neurons after embryonic day 11.5 (E11.5). NMNAT2 cKO mice were smaller and exhibited an ataxia phenotype with wobbling movements. Thus, 5 g of DietGel 76A (Clear H2O, Westbrook, ME, USA) were given daily after animals were weaned. NEX-Cre positive NMNAT2f/+ and NMNAT2+/+ and NEX-Cre negative NMNAT2f/f littermates were used as controls for NEX-Cre positive NMNAT2f/f. The generation and genotyping of NMNAT2-Blad mice have been described previously [39]. In these mice, the NMNAT2 null mutation was generated by transposon-mediated gene-trap mutagenesis. Sarm1 KO (Snull/Snull) mice [40] were crossed with NMNAT2 cKO mice to generate cKO;Snull/ + and cKO; Snull/Snull transgenic mice. Littermate controls include NEX-Cre/ + ;NMNAT2f/+;Snull/ + , NMNAT2f/+;Snull/ + ; NEX-Cre/ + ;NMNAT2f/+;Snull/ Snull, and NMNAT2f/f;Snull/ Snull. Both male and female mice were used for all experiments. All mice were housed under standard conditions with food and water provided ad libitum and maintained on a 12 h dark/light cycle. Mice were housed and used in compliance with the NIH Guidelines for the Care and Use of Laboratory Animals and were approved by the institutional animal care and use committees at Indiana University and Max Planck Florida Institute for Neuroscience.
Genotyping
For mice, ear lysates were prepared by immersing the tissue derived from a small ear clip in digestion buffer (50 mM KCl, 10 mM Tris–HCl, 0.1% Triton X-100, 0.1 mg/ml proteinase K, pH 9.0), vortexing gently, and then incubating for 3 h at 60 °C to lyse the cells. For embryos, the same digestion buffer with 0.2 mg/ml proteinase K was used, and embryonic tail lysates were incubated at 60 °C for 15 min with 1500 rpm shaking in a thermoshaker. These lysates were then heated to 95 °C for 10 min to denature the proteinase K (Thermo Scientific, Rockford, IL, USA) and centrifuged at 16,100 g for 15 min. The supernatants were used as DNA templates for polymerase chain reactions (PCRs, EconoTaq Plus Green 2X mater mix, Lucigen, Middleton, WI, USA). For embryonic genotyping during primary culture preparation, a QIAGEN Fast Cycling PCR Kit was used. The sequences of the primers used for genotyping are listed in the reagent table. For NMNAT2f/f line: primers A and B detect WT allele; primers B and C detect NMNATf allele. For NEX-Cre line: primer Cre484 and Cre834 detect Cre positive allele. For the NMNAT2-Blad line: primers A and B detect WT allele, and primers R3 and Rf detect KO allele. For SARM1 KO line: primers WT-R and Sarm1-common detect WT allele, and primers Sarm1-common and Mut-R detect KO allele.
Scoring for hindlimb clasping motor behavior
Hindlimb clasping is a marker of motor behavioral deficits and has been observed in several neurodegenerative mouse models [41–43]. To assess clasping behavior, a mouse was lifted for 10 s by grasping its tail near its base and hindlimb responses were videotaped for scoring. The hindlimb position scoring from 0–3 was defined as previously described [44]. Score 0, the mouse splayed out both hindlimbs for most of the 10 s; Score 1, the mouse retracted one of its hindlimbs toward the abdomen for more than 5 s; Score 2, the mouse partially retracted both hindlimbs for more than 5 s; Score 3, the mouse retracted both hindlimbs close to its abdomen for more than 5 s. The behaviors were recorded and analyzed blind to genotype information.
Immunohistochemistry for brain sections
Mice were anesthetized and perfused with 4% paraformaldehyde (PFA) in PBS. Brains were harvested, post-fixed with 4% PFA in PBS overnight at 4 °C, and then rinsed with PBS. Free-floating brain sections were prepared by sectioning these fixed brains into 40-μm-thick sections in the coronal plane with a Leica VT-1000 Vibrating microtome or Sliding Microtome SM-2000R (Leica Microsystems).
For immunohistochemistry, sections were permeabilized with 0.3% Triton X-100 in PBS for 20 min at room temperature, incubated with blocking solution (3% normal goat serum prepared in PBS with 0.3% Triton X-100) for 1 h, and then incubated with primary antibodies diluted in blocking solution overnight at 4 °C. The next day, sections were washed with 0.3% Triton PBS 3 times and then incubated with the secondary antibodies diluted in blocking solution at 4 °C for 2 h. After the incubation, samples were washed with 0.3% Triton PBS 3 times. Draq5 (1:10,000 dilution, Cell Signaling) or 4´,6-diamidino-2-phenylindole (DAPI, 5 μg/ml, Invitrogen) were added during the first wash step to visualize nuclei. Dako mounting medium was used to mount the brain sections.
Microscopy, imaging and data analysis for brain sections
Bright-field images were obtained with Zeiss SteREO Discovery.V8 Microscope (Carl Zeiss Microscopy). Confocal fluorescent images were taken by a Leica TCS SPE confocal microscope (Leica DM 2500) with a 10 × objective lens (0.3 N.A.) or a 40x (0.75 N.A.) objective lens. Some confocal images were taken with a Nikon A1R laser scanning confocal (Nikon A1) with a 10x (0.5 N.A.) or a 60x (1.4 N.A.) oil objective lens. DAPI/Draq5 immunofluorescence was used to identify comparable anatomical regions across different brain sections. A minimum of three sections were imaged per mouse, and each anatomical region was imaged with both sides of the cortices, comparable across all animals. Images for particular staining were acquired with identical imaging parameters to minimize signal saturation in all experimental groups. The thickness of the corpus callosum was measured from the dorsal to ventral edges of NFM-positive axonal tracts in coronal plane brain sections. The thickness of the primary somatosensory cortex was measured from the pial surface to white matter regions using ImageJ “Straight” function. The total pixel values of APP signals and area sizes were measured by Image J to acquire APP signal densities.
NMNAT2 in situ hybridization
In situ hybridization on embryonic sections was done by the Baylor College of Medicine Advanced Technology Core Labs. The experiment was performed using a high-throughput automated platform. Cryostat sections of E14.5 embryos were placed on a standard microscope slide that was subsequently incorporated into a flow-through chamber. The chamber was then placed into a temperature-controlled rack, and the required solutions for pre-hybridization, hybridization, and signal detection reactions were added to the flow-through chamber with an automated solvent delivery system. Details can be found in a previous publication [5]. The RNA probe was generated using the Allen Brain Atlas database sequence. Images were acquired by a Leica CCD camera with a motorized stage. Multiple images were collected from the same section. Individual images were stitched together to produce a mosaic representing the entire section.
Primary cortical neuronal cultures
To culture NMNAT2 KO neurons, E15.5–16.5 embryos were harvested from NMNAT2-Blad heterozygous (HET) female mice mated to NMNAT2-Blad HET male mice. Embryos of both sexes were included for culture preparations. The cortical tissue of each embryo was dissected and then dissociated individually using the Worthington Papain Dissociation Kit (Worthington Inc.) according to the manufacturer’s protocol. Genotyping was performed before the completion of papain dissociation. Cortices from multiple NMNAT2-Blad wildtype (WT) and HET embryos were combined as control group for dissociation and plating; the cortices from multiple NMNAT2-Blad homozygous embryos were pooled together as KO group for dissociation and plating. NMNAT2f/f neuron cultures were prepared from cortical tissue dissected from E15.5–16.5 NMNAT2f/f embryos harvested from NMNAT2f/f female mice mated with NMNAT2f/f male mice. After plating, cortical neurons were maintained in Neurobasal Media (Gibco™, ThermoFisher Scientific) supplemented with 2% B-27 (Gibco™), 2 mM GlutaMAX™ (Gibco™), and 100 U/ml penicillin–streptomycin (Gibco™), incubated at 37 °C with 5% CO2 and appropriate humidity. One-third of the media was replenished every 3 days.
Immunocytochemistry and confocal imaging
About 2.5*105 cortical neurons were plated on 12 mm diameter coverslips coated with poly-D-lysine (PDL) in 24-well plates (1.3*103 cells/mm2). At indicated days in vitro, cortical neurons were first fixed by 4% PFA and 4% sucrose in PBS for 20 min, incubated for 60 min in blocking buffer (0.1% Triton X-100 and 5% Goat Serum in PBS), and then incubated with primary antibody containing blocking buffer overnight at 4 °C on a gentle rotating platform. Samples were washed with 0.1% Triton in PBS 3 times and incubated with a secondary antibody containing blocking buffer for 2 h. After the incubation, samples were washed with 0.1% Triton in PBS 3 times. Coverslips were removed to mount with ProLong™ Gold antifade mounting medium with DAPI.
Fluorescent confocal images were taken by a Leica TCS SPE confocal microscope using a 63x (1.4 N.A.) oil objective lens or by a Nikon A1R-HD25 laser scanning confocal microscope with an Apo Lambda S 60x (1.4 N.A.) oil objective lens. Z-stack images were taken with 0.5 μm Z-step size to cover the whole depth of the cultured neurons. Images were acquired with identical imaging parameters chosen to optimize the signal-to-noise ratio and avoid saturated pixels in experimental groups. To quantify APP or TUJ1, 3–5 regions were randomly selected per coverslip for imaging. Each image accounts for one data point in quantification. Two coverslips per group were imaged for each batch of culture. To sample a larger area for imaging MAP2 and βIII-tubulin staining for gross neurite area quantification, the “large image stitching” function in Nikon NIS-Elements software with 5–10% overlap was used to tile 4 adjacent fields of view together as one image.
Quantification of APP accumulation and gross neurite area in neuronal culture
ImageJ was used for quantification of the maximum intensity projected images. APP signal in somata and dendrites was manually excluded, then the default threshold method was applied to select the top 10% tail of total pixels, and the “analyze particles” function was applied to select particles of area larger than 0.9 μm2 which were identified as “accumulated APP”. The total area of “accumulated APP” was calculated in each image to evaluate the phenotype. MAP2-positive areas were identified using an auto-thresholding method and measured for each image. A MAP2 positive area was selected by the “create selection” function after thresholding, then restored and deleted from the βIII-tubulin channel. Therefore, the remaining βIII-tubulin signal solely represented axonal regions. Similarly, mean threshold was applied to measure the βIII-tubulin positive area. Live cells were distinguished from dead cells by DAPI-stained nuclei morphology, and the number of live cells was counted for each image. MAP2 and βIII-tubulin areas were normalized by dividing by the live cell number in each image. We observed a skew in the data if the cell density dramatically differed between Ctrl and KO. Therefore, only images with a live cell number between 150 to 300 within 0.31 mm2 image area (485–970 cells/mm2) were used for the analysis.
Plasmid DNA constructs
pEGFP-n1-APP was a gift from Zita Balklava and Thomas Wassmer (Addgene plasmid # 69,924; http://n2t.net/addgene:69924; RRID: Addgene_69924) [45]. pEGFP-c1-SNAP25, pEGFP-n1-SYPH, and pmCherry-n1-NMNAT2 were gifts from Michael Coleman and Jonathan Gilley [25]. pCX-EGFP was a gift from Matthew Neil Rasband [46]. pLV-mitoDsRed was a gift from Pantelis Tsoulfas (Addgene plasmid # 44,386; http://n2t.net/addgene:44386; RRID: Addgene_44386) [47]. pcDNA3.1-SoNar and pcDNA3.1-cpYFP were gifts from Yang Yi [48]. pCMV-MitoVenus was a gift from Lulu Cambronne [49]. pCAG-mCherry was a gift from Ken Mackie. pcDNA3-Syn-ATP and pcDNA3-Cyto-pHluorin were from Vidhya Rangaraju [33].
Transfection in neuronal culture
For lentiviral transduction in NMNAT2f/f neurons, a lentivirus expressing copGFP or iCre under elongation factor 1 alpha (ELF1alpha) promoter was applied at 2 multiplicity of infection (MOI) at 5 days in vitro (DIV5). For lentiviral transduction in NMNAT2 germline KO neurons, a lentivirus expressing copGFP, wt-NMNAT2, or ED(H24D)-NMNAT2 under elongation factor 1 alpha (ELF1alpha) promoter was applied at 4 multiplicity of infection (MOI) at 2 days in vitro (DIV2).
For lipofectamine transfection, about 1.6*106 cortical neurons were plated onto PDL (Poly-D-Lysine)-coated MatTek dish (P35G-1.5–20-C) (1.66*103 cells/mm2). For APP-EGFP and SNAP25-EGFP transport imaging, cortical neurons were transfected 16 to 20 h prior to the time-lapse imaging experiments using Lipofectamine 3000 (Thermo) following manufacturer’s instruction. For MitoDsRed transport imaging, cortical neurons were co-transfected with pCX-EGFP and pLV-mitoDsRed 36 to 48 h before live imaging using Lipofectamine 3000. Before adding the DNA-lipofectamine mixture, half of the conditioned culture medium was removed and saved for later. Two hours after incubation with DNA-lipofectamine mixture, one-third of the remaining medium was removed, and 2 ml of culture medium (1:1 of conditional medium and fresh medium) was added back immediately. For live imaging, all culture medium was replaced by 2–3 ml of Hibernate E low fluorescence buffer (BrainBits) supplemented with 2 mM GlutaMAX™ to maintain the ambient pH environment.
For magnetofection, about 2.56*105 cortical neurons were plated onto the center of a PDL-coated coverglass region of MatTek dish (P35G-1.5–14-C) (1.66*103 cells/mm2). For Syn-ATP imaging and Cyto-pHluorin imaging, cortical neurons were transfected 24 h before imaging using Combimag (OZ biosciences) and Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instruction. Briefly, 0.5 μg DNA was incubated with 0.5 μl lipofectamine in 50 μl transfection medium (Neurobasal media supplemented with 2 mM Glutamax without B27 or antibiotics) for 5 min, then mixed with 0.5 μl Combimag diluted in another 50 μl transfection medium for 10 min. The DNA-lipofectamine-Combimag mixture was further diluted in 125 μl of transfection medium. Then, the conditioned medium from cultured neurons was harvested, and the neurons were immediately rinsed twice in a warm transfection medium. The transfection medium used for rinsing was removed, and 150 μl of DNA-lipofectamine-Combimag mixture was added to the neurons. Neurons were placed on a magnetic plate for 20 min inside a 37 °C and 5% CO2 incubator. After 20 min, neurons were rinsed once in a warm transfection medium and replaced with the previously harvested warm, conditioned medium.
Axonal transport time-lapse video microscopy and movie analyses
Live imaging of neuronal cultures was carried out using an Olympus OSR Spinning Disk Confocal microscope (CSU-W1, Yokogawa) connected to a Hamamatsu Flash 4 V2 camera. The temperature was maintained at 37 °C by a Tokai Hit Stage Top Incubation system. For APP-EGFP and SNAP25-EGFP transport recording, movies were taken in axonal segments at least 400 μm away from the soma (defined as distal axon) and axonal segments within 200 μm of the soma (defined as proximal axon) and captured at a rate of 5 frames/s for a total recording time of 60 s with a 60 × oil objective (1.3 N.A.). For MitoDsRed transport imaging, neurons were co-transfected with EGFP, and the distance from an axonal segment of interest to soma was determined in the GFP channel under the eyepiece. Mitochondria transport videos were taken in the distal region at a 3-s time interval for a total recording time of 15 min with a 60 × oil objective (1.3 N.A.). A z-stack with 1 to 1.5 μm z-step size was taken for each time point to cover all the mitochondria within the imaged axonal segment.
Kymographs were generated using ImageJ (http://rsb.info.nih.gov/ij) with “Velocity_Measurement_Tool” macro (http://dev.mri.cnrs.fr/projects/imagej-macros/wiki/Velocity_Measurement_Tool). Velocity quantification was manually performed by following the trajectory of each particle with an angle larger than 0° relative to the time axis. Stationary and repetitive bidirectional-moving particles were excluded for velocity measurements. If an anterograde or retrograde moving particle stopped briefly during the imaging and then continued moving, the paused portion was included in the velocity calculation. Although a portion of the axon could be out of focus, resulting in a discontinuous trajectory, the discontinuous trajectory was still traced as the same if the slope and timing matched. All the traced trajectories were saved in “ROI manager” in ImageJ. Particles that remained stationary or underwent repetitive bidirectional movements for most of the recording time were defined as stationary/dynamic pause events. Each trajectory of stational/dynamic pause particles that could be visually separated was included in the number of stationary/dynamic pause events. The number of anterograde or retrograde events was counted in the same way. The stationary/dynamic pause percentage was calculated as the number of stationary/dynamic events divided by the sum of the number of anterograde, retrograde, and stationary/dynamics pause events. Anterograde and retrograde percentages were calculated similarly.
Colocalization analysis
Control neurons were plated at a density of 1.8*103 cells/mm2 on PDL-coated 12 mm diameter coverslip in 24-well culture plate (about 3.5*105 cells on 190 mm2 surface). Through transfection, pmCherry-n1-NMNAT2 was co-expressed with pEGFP-n1-APP, pEGFP-n1-SYPH, or pEGFP-c1-SNAP25 at DIV6, and samples were fixed using 4% PFA with 4% sucrose dissolved in PBS at DIV8. Immunocytochemistry was conducted to amplify the mCherry and EGFP signals after fixation. ProLong™ Gold antifade mounting medium with DAPI was used to mount the coverslips. For NMNAT2-mCherry and EGFP-tagged APP, SNAP25, or SYPH, fluorescent confocal images were taken by a Leica TCS SPE confocal microscope using a 63x (1.4 N.A.) oil objective lens with 0.17 µm xy pixel size and a system-optimized z-step size. The colocalization was further validated by structure illumination imaging using OMX-SR 3D-SIM Super-Resolution System with a 60x (1.516 N.A.) oil objective lens, following system-optimized z-step size. For NMNAT2-mCherry and MitoVenus, fluorescent confocal images were taken by a Nikon A1 laser scanning confocal microscope using a 1.4 N.A. Apo Lambda S 60 × oil objective lens with 2.88 × zoom in to achieve 0.1 µm xy pixel size and 0.15 µm z step.
JACoP (Just Another Colocalization) ImageJ plugin was used to analyze confocal images. Briefly, cell debris signal outside axon was manually deleted. “Objects based methods” was used. The threshold for both channels was manually adjusted based on the visual judgement of signal coverage. The minimum particle size was set as 0.722 µm2. The ratio of NMNAT2-mCherry puncta colocalized to other cargos or mitochondria was calculated as the number of colocalizing puncta divided by the total number of puncta in NMNAT2-mCherry channel in each image.
Mitochondria density and morphology analysis
Both control and KO neurons were plated at a density of 1.8*103 cells/mm2 on PDL-coated 12 mm diameter coverslip in 24-well culture plate (about 3.5*105 cells on 190 mm2 surface). Neurons were co-transfected with pCMV-MitoVenus and pCAG-mCherry at DIV6 and fixed using 4% PFA with 4% sucrose at DIV8. Immunocytochemistry with antibodies against GFP (Chicken) and RFP (Rabbit) was conducted to immuno-amplify the MitoVenus and mCherry signals after fixation. ProLong™ Gold antifade mounting medium with DAPI was used to mount the coverslips.
Images were taken by a Nikon A1 laser scanning confocal microscope using a 1.4 N.A. Apo Lambda S 60 × oil objective lens with 0.171 μm z-step size and 3 times zoom to cover the whole depth of the axon segment of interest. Axonal segments at least 400 μm away from the soma were identified in the RFP channel and selected for imaging. Distal axons from at least 8–10 neurons were randomly sampled per coverslip and two coverslips were imaged per group. Images were analyzed using Imaris (Oxford Instruments). RFP channel was used to generate the surface masking of the axonal region, within which the MitoVenus signal was used to create the surface representing mitochondrial morphology. To calculate mitochondria density, length of the axon segment in each image was measured. Density was calculated from mitochondria number divided by axonal length in each image. Sphericity values of mitochondrial surface from axon segments of each individual neuron were input in each column in the column table in Prism 9.0 (each column representing one individual neuron). Cumulative frequency distribution of relative percentage was generated through “column analysis” function in Prism 9.0. Statistical analysis of cumulative frequency distribution is described in “quantification and statistical analysis” section.
NAD+/NADH-Glo™ bioluminescent assay
Control or KO neurons were cultured in a 96-well plate at a density of 1.3*103 cells/mm2 (4.235*104 cells onto 32 mm2 surface). Following manufacturer’s instruction (Promega), neuronal cultures at DIV8 were washed three times with PBS and lysed in buffer containing 25 μl PBS and 25 μl 1% DTAB (Dodecyl trimethyl ammonium bromide) base buffer (100 mM Sodium Carbonate, 20 mM Sodium Bicarbonate, 10 mM Nicotinamide, 0.05% TritonX-100, pH 10–11). 30 μl of lysate were collected for BCA protein assay. 20 μl of the remaining lysate was diluted into 105 μl. 50 μl of diluted lysate was mixed with 25 μl 0.4 N HCl and incubated at 60 °C for 15 min to detect NAD+, while another 50 μl of diluted lysate (without 0.4 N HCl) was incubated at 60 °C for 15 min to detect NADH. After incubation, 25 μl of 0.5 M Tris base was added to the wells used for detecting NAD+ to neutralize the HCl; 50 μl of a solution containing 1:1 0.4 N HCl and 0.5 M Tris base was added to wells used for detecting NADH. Freshly dissolved NAD+ in PBS was used to prepare the standard curve. Luminescent signal from each sample was converted into absolute NAD+ or NADH concentration and normalized to protein concentration measured by the BCA method (Thermo Scientific™).
NAD+/NADH live imaging using the genetically encoded sensor, SoNar
Around 1.66*103 cells/mm2 cortical neurons were plated on PDL (Poly-D-Lysine)-coated MaTek dish (P35G-1.5–20-C). The NAD+/NADH sensor, SoNar, and its control cpYFP were gifts from Yi Yang’s group. On DIV6, 2.5 ug DNA of pCDNA3.1-SoNar or pCDNA3.1-cpYFP were transfected. After 48 h expression of the sensor or control (DIV8), the culture medium was replaced by Hibernate E buffer and maintained at 37 °C for imaging using a Leica TCS SP8 confocal laser scanning platform with HyD hybrid detector and an HC PL APO 40x (1.2 NA) water-immersion objective. The SoNar sensor/cpYFP was excited at 440 nm and 488 nm, and the emission at 530 ± 15 nm was detected to obtain the ratiometric measurement. Laser power intensity was maintained the same between WT/Het and KO in the same experiment. Axonal segment > 400 μm away from the soma (defined as distal axon) and axonal segment within 200 μm from the soma (defined as proximal axon) were imaged with 1 μm z-step size to cover the whole axon segment within the field of view. Maximum intensity projection was generated for further analysis. Mean grey value from 488 nm excitation (F488) and 440 nm excitation (F440) was measured in ImageJ using manually drawn regions of interest (ROIs) that circle the edges of the axons of interest. Five rectangular ROIs were randomly drawn in the background area and the average mean grey value from these five ROIs was used to subtract background. After background subtraction, the ratio was calculated as (F488axon-F488background)/ (F440axon-F440background). Data presented were normalized to control as a percentage.
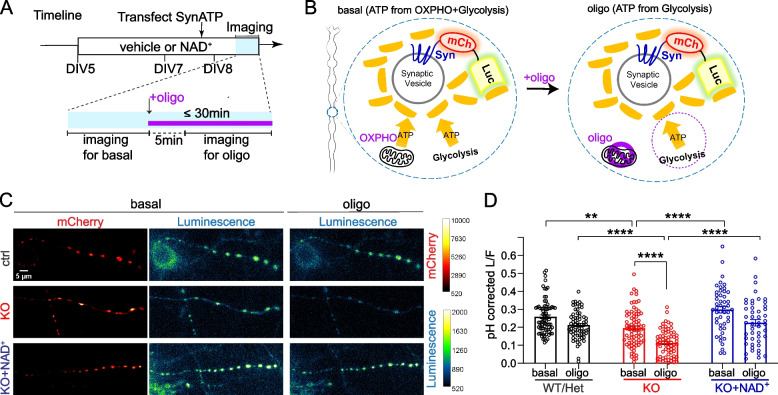
Syn-ATP live imaging
Syn-ATP imaging was performed using a custom-built inverted spinning disk confocal microscope (3i imaging systems; model CSU-W1) with two cameras: an Andor iXon Life 888 for confocal fluorescence imaging and an Andor iXon Ultra 888 electron-multiplying charge-coupled device camera for luminescence imaging. The Andor iXon Ultra 888 camera was selected for ultralow dark noise, further reduced by cooling to − 100 °C. The Andor iXon Ultra 888 camera speed used for luminescence measurements was 1 MHz with 1.00 gain and 1000 intensification. Image acquisition was controlled by Slidebook 6 software. Confocal imaging of mCherry fluorescence was performed by illuminating neurons with a 561 nm laser with 200 ms exposure and 2.3 mW laser power. To locate the varicosities of interest, mCherry fluorescence was acquired over ten frames and averaged. For luminescence measurements, luminescence photons were acquired for a period of 60 s in the presence of 2 mM D-luciferin (Promega), and the luminescence signal was measured from the same varicosities as the corresponding fluorescence image. All images were acquired through a Plan-Apochromat 63x/1.4 N.A. Oil objective, M27 with DIC III prism, using a CSU-W1 Dichroic for 561 nm excitation with Quad emitter and individual emitters for confocal fluorescence, and a 720 nm multiphoton short-pass emission filter was used for luminescence. During imaging, the temperature was maintained at 37 °C using an Okolab stage top incubator with temperature control. Distal axons at least 400 μm from the soma were selected for imaging.
Images were analyzed in ImageJ using the plugin Time series analyzer (https://imagej.nih.gov/ij/plugins/time-series.html). Regions of Interest (ROIs) of ∼1.2 μm diameter were drawn around the varicosities of interest to obtain the mean fluorescence and luminescence values from the corresponding fluorescence and luminescence images. We observed that some presynaptic varicosities in DIV8 neurons are not mature and not stable over the time course of imaging and have a heterogenous amount of synaptophysin expression, consistent with previous studies [50]. Therefore, we focused on varicosities of at least 1.2 μm diameter, stable during the 60 s luminescence acquisition. Neurons with an average mCherry fluorescence value lower than 1000 A.U. in their varicosities were excluded from the analysis, as a low fluorescence value indicates low expression of the sensor, resulting in low luminescence signal-to-noise ratio. For background subtraction of the fluorescence and luminescence values, three rectangular ROIs were drawn in the background region surrounding axons of interest. The average mean values from these background regions in the fluorescence (Fbackground) and luminescence (Lbackground) channels were used to subtract the background for each varicosity from the fluorescence (Fvaricosity) and luminescence (Lvaricosity) channels, respectively.
The average L/F value across varicosities within one neuron was used to represent that neuron's relative presynaptic ATP level, accounting for one data point in the statistical analysis.
pH measurement and pH correction of L/F measured from Syn-ATP
Cytoplasmic pHluorin (Cyto-pHluorin) was transfected in cortical neurons at DIV7, and imaging was done at DIV8 using the same equipment setting as Syn-ATP measurements. Detailed procedures are described in the previous publication [33]. In brief, neuronal culture medium was replaced by Tyrodes buffer containing (in mM) 119 NaCl, 2.5 KCl, 2 CaCl2, 2 MgCl2, 30 HEPES (buffered to pH 7.4 at 37 °C), and 25 glucose. Basal fluorescence intensity of Cyto-pHluorin was monitored for 5 min, acquired every 15 s (20 timepoints). ROIs were drawn around the axonal segment of interest and the mean fluorescence value was measured over the 20 timepoints (Faxon, frame i). Three rectangular ROIs were drawn in the background region surrounding the axon to measure the average background value across the 20 timepoints (Fbackground, frame i). Fbasal was calculated by subtracting background in each timepoint and then averaging the value across the 20 timepoints.
Then, 1 μM Oligomycin was added to the Tyrodes buffer and incubated for 5 min. Fluorescence of Cyto-pHluorin was imaged to obtain FOligo using the above calculation.
Then, Tyrodes buffer was replaced by an NH4Cl solution containing (in mM) 50 NH4Cl, 70 NaCl, 2.5 KCl, 2 CaCl2, 2 MgCl2, 30 HEPES (buffered to pH 7.4 at 37 °C), and 25 glucose. The response of Cyto-pHluorin to NH4Cl treatment was monitored for 5 min every 15 s (20 timepoints). The measured fluorescence of Cyto-pHluorin was called FMax and was background subtracted, as explained above.
Cytosolic pH of the basal and Oligomycin treatments were determined using the following modified Henderson-Hasselbalch equation:
From the known pH values at various conditions, Syn-ATP L/F value was corrected as follows:
Here, the average pH in each condition was used for correction and we did not propagate errors in pH measurements into the final error shown in L/F measurements. pH corrections of L/F values were only done for those conditions that showed a statistically significant change in pH compared to control (as in Fig. 5-S1).
Fig. 5.
NMNAT2 loss impairs glycolysis in distal axon presynaptic varicosities. A Experimental and live imaging timeline. B Schematic diagram of the Syn-ATP sensor configuration and the treatment. Syn: synaptophysin, mCh: mCherry, Luc: luciferase. C Representative Syn-ATP luminescence and fluorescence images of distal axon varicosities under basal and oligomycin-treatment conditions from DIV8 ctrl, KO, and KO neurons supplemented with NAD+ (KO + NAD+). Scale bar, 5 μm. D pH-corrected L/F (Luminescence/Fluorescence) ratio representing relative presynaptic ATP levels measured by the Syn-ATP sensor in mCherry positive varicosities of distal axons of DIV8 ctrl, KO, and KO + NAD+ neurons. Numbers (neurons imaged) and statistics: 78 ctrl, 77 KO, and 48 KO + NAD+ under basal conditions; 67 ctrl, 68 KO, and 48 KO + NAD+ under oligomycin-treated conditions. Ctrl and KO were from 5 independent experiments, and KO + NAD+ was from 2 independent experiments. Kruskal–Wallis test with Dunn's multiple comparisons test was used. All above data represents mean ± SEM, ** p = 0.004, ****p < 0.0001. In the NAD+ treated KO group, the difference between basal and oligomycin treatment conditions is marginal to significance, p = 0.0537
NAD+ supplementation in neuronal culture
NAD+ (Roche, NAD100-RO) was dissolved in PBS at a stock concentration of 100 mM, sterilized, aliquoted, and stored at -80 °C. Only less than 1-week-old stocks were used. On the starting day of supplementation, NAD+ was applied to the culture medium at a final concentration of 1 mM. In the following days before live imaging, 1/3 of culture medium was replaced with a fresh medium containing 1 mM NAD+ daily. 1 mM NAD+ was supplemented to the Hibernate E low fluorescence buffer used for live imaging. In the following days before fixation, 1/3 of culture medium was replaced with fresh medium containing 1 mM NAD+ every 3 days.
2-Deoxyglucose (2DG) treatment in neuronal culture
To acutely inhibit glycolysis while leaving mitochondrial respiration intact, 15 mM 2-DG and 10 mM methyl-pyruvate were added to the customized Hibernate E low fluorescence buffer containing 0 mM glucose (~ 262 mmol/kg osmolality). Culture medium was replaced by the 2DG Hibernate E buffer, and neurons were incubated for 30 min before imaging APP-EGFP axonal transport. For each culture dish, imaging of axonal transport was conducted within 30 min of 2DG treatment. To chronically inhibit glycolysis, 25 mM 2-DG and 10 mM methyl-pyruvate were added to the neurobasal medium containing 25 mM glucose. 2 days after treatment, neuronal culture was fixed for immunocytochemistry.
Oligomycin treatment in neuronal culture
To acutely inhibit ATP synthesized from mitochondrial respiration, 1 μM oligomycin was added to the commercial Hibernate E low fluorescence buffer containing 25 mM glucose (~ 241 mmol/kg osmolality). Culture medium was replaced by Oligo Hibernate E buffer, and neurons were incubated for 5 min before imaging APP-EGFP axonal transport, Syn-ATP, or Cyto-pHluorin. For each culture dish, live imaging was conducted within 30 min of oligomycin treatment. For APP axonal transport imaging, oligomycin (Calbiochem 495,455) was dissolved in DMSO to prepare a 1 mM stock. For Syn-ATP and Cyto-pHluorin imaging, oligomycin (Sigma-Aldrich O4876) was dissolved in 100% Ethanol to prepare a 1 mM stock.
Antisense oligonucleotide (ASO) treatment in neuronal culture
The antisense oligonucleotides (ASOs) were designed, synthesized, and preliminarily tested by Ionis Pharmaceuticals. ASOs consist of 20 chemically modified nucleotides with a central gap of 10 deoxynucleotides flanked on its 5′ and 3′ sides by five 2′-O-(2-methoxyethyl) (MOE)-modified nucleotides. Thirteen of the phosphodiester internucleotide linkages were replaced with phosphorothioate to further enhance the stability of ASOs to endogenous nucleases. The non-targeting ASO, 5’-CCTATAGGACTATCCAGGAA-3’ (ASO30), was used as a control. The ASOs targeting mouse SARM1 mRNA, 5’-GGTAAGAGCCTTAGGCACGC-3’ (ASO33) and 5’-CCACCTTTTAGTCAAGACCC-3’ (ASO47) were applied to silence SARM1 expression. Both ASOs arrived as 100 mg/ml stock. On the treatment starting day, as indicated, ASOs were diluted into 5 µM as the final concentration in neuronal culture medium. In the following days before live imaging or fixation, 1/3 of culture medium was replaced with fresh medium containing 5 µM ASOs every 3 days.
Quantitative real-time PCR
Following ASO treatment, total RNA was extracted from neuronal culture by RNeasy Mini Kit (Qiagen 74,104) and followed by on-column DNase digestion according to the manufacturer’s instructions. RNA concentration and purity was measured by NanoDrop 2000 spectrophotometer (ThermoFisher Scientific). 500 ng RNA was used as input for cDNA synthesis through iScript™ advanced cDNA synthesis kit (Bio-Rad 1,725,038) following the manufacturer’s protocol. To quantify mRNA level, quantitative real-time PCR was conducted using PrimeTime™ Gene Expression Master Mix (IDT 1055772) following the manufacturer’s instruction.
To detect SARM1 mRNA, 0.3 ul of cDNA was loaded into the reaction mix (10 ul in total) containing 450 nM of SARM1 forward primer, 450 nM of SARM1 reverse primer, and 150 nM SARM1 probe. Mouse SARM1 primer–probe set was customized through PrimeTime™ qPCR Probe Assays (IDT). SARM1 forward primer sequence is 5’ TTTGTCCTGGTGCTGTCTG 3’. SARM1 reverse primer sequence is 5’ GCCACTCAAAGCCATCAATG 3’. SARM1 probe sequence is 5’ ACAATCTCCTTGTGCACCCAGTCC 3’, with FAM reporter at 5’, internal ZEN quencher, and 3’ Iowa Black® FQ quencher. To detect GAPDH mRNA as the internal control for normalization, 0.3 ul of cDNA was loaded into the reaction mix (10 ul in total) containing 500 nM of GAPDH forward primer, 500 nM of GAPDH reverse primer, and 250 nM GAPDH probe. Mouse GAPDH primer–probe set was acquired from PrimeTime™ Predesigned qPCR Assays with same reporter and quencher (Assay ID: Mm.PT.39a.1). These assays were run in QuantStudio 7 Flex Real-Time PCR Systems (ThermoFisher Scientific). The Ct value was defined as the number of cycles required for the fluorescence to exceed the detection threshold. The ΔCt values were calculated by subtracting Ct value of GAPDH from SARM1. The ΔΔCt values were calculated by subtracting the average ΔCt of non-targeting ASO-treated control from the ΔCt of each sample. The relative fold change was calculated as 2−ΔΔCt.
Western blotting
Following ASO treatment, neuronal culture was lysed in RIPA buffer [20 mM of Tris‐base, 150 mM of NaCl, 2 mM EDTA, 1% Triton X‐100, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate (SDS), 1 × cOmplete™ protease inhibitor cocktail and phosphatase inhibitor cocktail, pH 7.4], and homogenized by motorized disposable pellet mixers for 15 s, then sonicated by VMR Branson Sonifier 250 10 times under the parameter: duty cycle 30 and output 3.
Protein concentration was measured using the BCA method (Thermo Scientific™). 10–15 ug proteins were separated on a 10% SDS–polyacrylamide gel and then transferred to a nitrocellulose membrane of 0.45 μm pore size (BioRad). To detect SARM1 protein level, the membranes were incubated overnight with the homemade primary antibody against SARM1, a gift from Dr. Yi-Ping Hsueh [51]. The next day, the membranes were incubated with a species-appropriate secondary antibody. Western blot images were acquired by LI-COR Odyssey scanner and software (LI-COR Biosciences, Lincoln, NE USA) and quantified with NIH ImageJ software.
TUJ1 fragmentation quantification
Fluorescent confocal images of TUJ1 staining were taken as described in the "immunocytochemistry and confocal imaging” section. TUJ1 fragmentation was analyzed following the degeneration index measurements method described by Kraemer et al. [52], with some modifications to their particle size and circularity indexes. TUJ1 fragmentation quantification was performed using ImageJ. The fluorescent channel corresponding to TUJ1 was isolated and converted to a binary image using the “Make Binary” function, and this image was used for all subsequent steps of the analysis. The total TUJ1 expression area was measured using the “Measure” function. Images were then processed using the “Analyze Particles” function, setting the size parameter at 0.327 µm2-817.587 µm2 (4–10,000 pixels) and circularity at 0.2–1. The area of selected particles was recorded and normalized to total area of TUJ1.
Experimental design
No statistical methods were used to predetermine the sample size. For in vivo studies, investigators were blinded to the mouse genotype during data acquisition and analysis. For in vitro manipulation, investigators were not blinded to the genotype of neuronal cultures and treatments. While quantifying APP accumulation area, TUJ1 area, MAP2 area, TUJ1 fragmentation degree, and the analysis of axonal transport, investigators were blinded to each image's genotype and treatment information. For NAD/NADH-Glo™ bioluminescent assay, investigators were not blinded, and quantification was done by plate-reader without human curation. During the acquisition and quantification of NAD+/NADH (SoNar) sensor imaging, Syn-ATP sensor imaging, and Cyto-pHluorin imaging, the investigators were not blinded to the genotype and treatment of each image. Inclusion and exclusion criteria were applied during the analysis of Syn-ATP imaging to avoid varicosities with extremely low mCherry fluorescence (an indicator of low sensor expression). For Western Blotting and qPCR, investigators were not blinded to genotype and treatment. Except for gross neurite area measurement at DIV5 & DIV14 and Cyto-pHluorin live imaging, all experiments were replicated with culture from at least 2 independent primary culture preparations, as detailed in the supplementary Excel sheets.
Quantification/statistical analysis and figure constructions
Images were analyzed using Fiji (Image J with updated plug-ins) and Imaris. NAD/NADH-Glo™ bioluminescent Assay were measured by CLARIOstar plate reader. GraphPad Prism 9.0 (GraphPad Software) was used for statistical analysis. The outliers were detected by “ROUT” method in GraphPad Prism 9.0 and excluded for statistical analysis. The normality of residuals was mostly checked by D'Agostino-Pearson normality test as recommended by the GraphPad Prism user guide. For data with normally distributed residuals, one-way ANOVA, two-way ANOVA, or unpaired student’s t-test was used. For data that did not pass the D'Agostino-Pearson test, one-way ANOVA was replaced by Kruskal–Wallis test; two-way ANOVA was replaced by Multiple Mann–Whitney test or re-formatted for Kruskal–Wallis test; unpaired student’s t-test was replaced by the Mann–Whitney test.
To analyze cumulative frequency distribution curve, the dependent variable P (the relative percentage within each bin generated by GraphPad Prism “Column analysis”) was transformed into log(P/(100-P)) so that the relationship between covariate (bin of sphericity) and the transformed dependent variable was linearized. Then, a linear mixed model with a random slope and intercept was generated by SPSS using a restricted maximum likelihood approach to compare statistical difference between control and KO. The criterion for statistical significance was set at p < 0.05 for all statistical analyses.
Figures were made with Adobe Photoshop CS6 and Illustrator CS6, and brightness/contrast, orientations, and background corrections were applied to better illustrate the staining patterns.
Results
Deleting NMNAT2 in post-mitotic cortical glutamatergic neurons results in deformed brains and age-dependent loss of long-range cortical axons
Germline deletion of NMNAT2 in mice results in premature death at birth with severe axonal outgrowth deficits and subsequent degeneration in peripheral and optic nerves [37, 39]. Using explant cultures prepared from NMNAT2 KO embryonic cortices, Gilley et al. showed NMNAT2 KO cortical axons were shorter than control axons and exhibited degenerative phenotypes [37]. However, the premature death of germline NMNAT2 KO prevented in vivo examinations of the role of NMNAT2 in cortical neurons. Using mRNA in situ hybridization, we found that nmnat2 mRNA is enriched in the embryonic cortical plate, where post-mitotic glutamatergic neurons are located (Fig. 1-S1A). Axons of cortical glutamatergic neurons often travel long distances to the contralateral hemisphere or subcortical regions via extensive and complex arbors [6]. These observations led us to hypothesize that NMNAT2 is required for axonal outgrowth and maintaining the health of cortical glutamatergic axons.
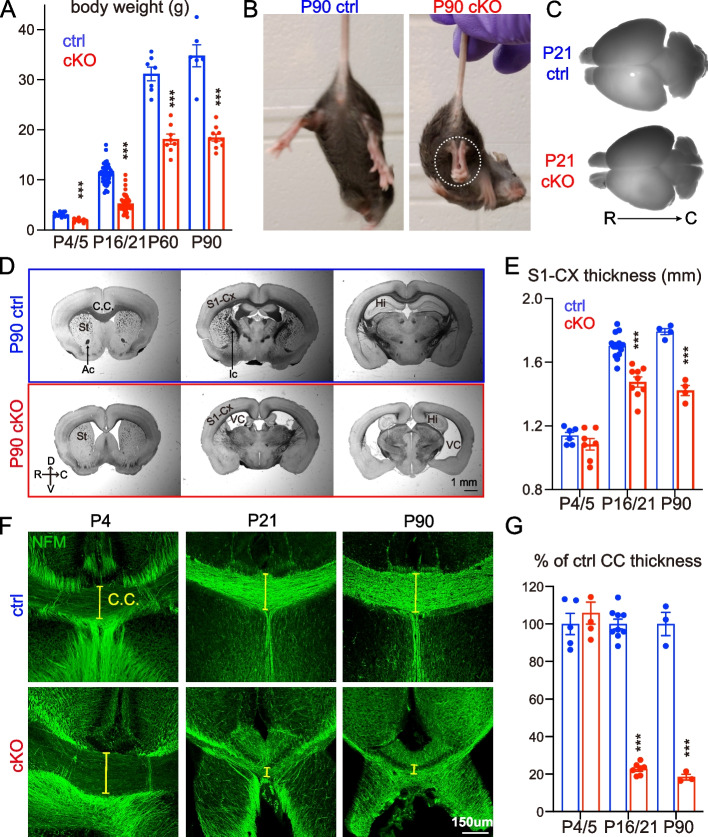
Fig. 1.
Deleting NMNAT2 in cortical glutamatergic neurons results in age-dependent axonal degeneration. A Body weight of cKO and their littermate control (ctrl) mice at P4/5, P16/21, P60, and P90. Mice numbers: P4/5, 13 ctrl and 9 cKO; P16/P21 43 ctrl and 46 cKO; P60, 7 ctrl and 8 cKO; P90, 6 ctrl and 10 cKO. B Movie screenshots showing that a P90 cKO mouse exhibits hindlimb clasping behaviors (dashed white oval), a classic motor deficit observed in many neurodegenerative models (see Sup. Movies), but not in a ctrl mouse. C, D Bright field images showing whole brains and coronal plane brain sections (rostral to caudal from left to right) from ctrl and cKO mice. In addition to the smaller brain sizes, cKO brains have enlarged ventricles and reduced cortical regions and hippocampal areas. E Quantification of the primary somatosensory (S1) cortex thickness in ctrl and cKO mice at different ages. Mice numbers: P4/5, 6 ctrl and 7 cKO; P16/P21, 14 ctrl and 9 cKO; P90, 4 ctrl and 4 cKO. F Confocal images of immunohistochemical staining of NFM (medium-size neurofilament) showing axonal tracts through the corpus callosum (CC) in ctrl and cKO brains at P4, P21, and P90. Yellow brackets mark the thickness of the CC. G Quantification of the CC thickness, normalized to its value in ctrl mice. Mice numbers: P4/5, 5 ctrl and 5 cKO; P16/P21, 9 ctrl and 7 cKO; P90, 3 ctrl and 3 cKO. Abbreviations: Ac, anterior commissure; Ic, internal capsule; CC, corpus callosum; Cx, cortex; Hi, hippocampus; St, striatum; VC, ventricle. Unpaired t-test and Mann–Whitney test were applied for the statistic result, ***p < 0.001, ****p < 0.0001
To test this hypothesis, we generated NMNAT2 conditional knockout mice (cKO) by crossing NMNAT2 conditional allele mice (NMNAT2f/f) [37] with a Nex-Cre transgenic mouse line (Fig. 1-S1B), which expresses Cre recombinase in post-mitotic glutamatergic neurons mainly in the cortex and hippocampus from embryonic day 11.5 (E11.5) [38]. Despite the restricted deletion of NMNAT2, only about 50% of cKO mice survived after birth, with no apparent lethality at E18.5 (Fig. 1-S1C, D). The surviving cKO mice weighed significantly less than their littermate controls from early postnatal ages through adulthood (Fig. 1A). NMNAT2 cKO mice exhibited evident hindlimb clasping (Fig. 1B, Fig. 1-S1F, and supplementary video), ataxia and forelimb circling phenotypes (10 out of 10 mice examined), reflecting motor behavioral deficits similar to those observed in many neurodegenerative mouse models [41–43]. cKO brains were visibly smaller than controls from postnatal ages (Fig. 1C). To evaluate gross brain morphology, we examined brain sections along rostral to caudal coronal planes with bright field microscopy. Compared to the brains of littermate controls, the brains of cKO mice displayed enlarged ventricles, smaller hippocampi, aberrant anterior commissures, a thinner corpus callosum, and a thinner cortex (Fig. 1D). We measured the thickness of the primary somatosensory cortex and found that it was significantly reduced in cKO mice compared to their littermate controls at postnatal day 16/21 (P16/21) and P90 (Fig. 1E). The degenerative brain phenotypes observed in NMNAT2 cKO brains highlight the importance of NMNAT2 in cortical neuronal health.
To distinguish whether brain dystrophy in NMNAT2 cKO is caused by axonal outgrowth deficits versus axonal maintenance failures, we examined the corpus callosum, where the long-range callosal axons of cortical glutamatergic neurons cross the midline on the way to their contralateral targets [8, 9]. To facilitate visualization of the corpus callosum, we immunostained the medium-size neurofilament (NF-M), a cytoskeleton protein enriched in axons, in P4/5, P16/21, and P90 cKO and control brains. There was no difference in corpus callosum thickness between control and cKO brains at P4/5 (Fig. 1F, G). However, a drastic reduction of corpus callosum thickness in cKO mice occurred by P16/21 and persisted until at least P90, the eldest age examined (Fig. 1F, G). Most callosal axons have already finished midline crossing at P4/5 [53, 54]. Thus, the normal corpus callosum thickness in cKO mice at P4/5 suggests that NMNAT2 deletion in glutamatergic neurons does not impair initial axonal outgrowth. Instead, the age-dependent reduction of corpus callosum thickness and degeneration-like brain morphology suggest that NMNAT2 is required to maintain the health of long-range cortical axons.
NMNAT2 loss leads to APP accumulation in axons prior to degeneration in vivo and in vitro
Based on the axon degeneration phenotype in NMNAT2 cKO brains, we hypothesized that NMNAT2 loss disrupts axonal physiology, ultimately resulting in axonal degeneration. Axonal transport plays a critical role in neuronal function and survival [28] and its deficits are thought to be a primary cause of axonopathy [55]. Amyloid Precursor Protein (APP) is rapidly and bidirectionally transported in axons [56–60] and is required for synaptogenesis and synaptic function, plasticity, etc. [61–63]. Axonal APP accumulation has been found in AD mouse models [64, 65] and traumatic brain injury [66, 67], and serves as a marker for axonal transport breakdown [68].
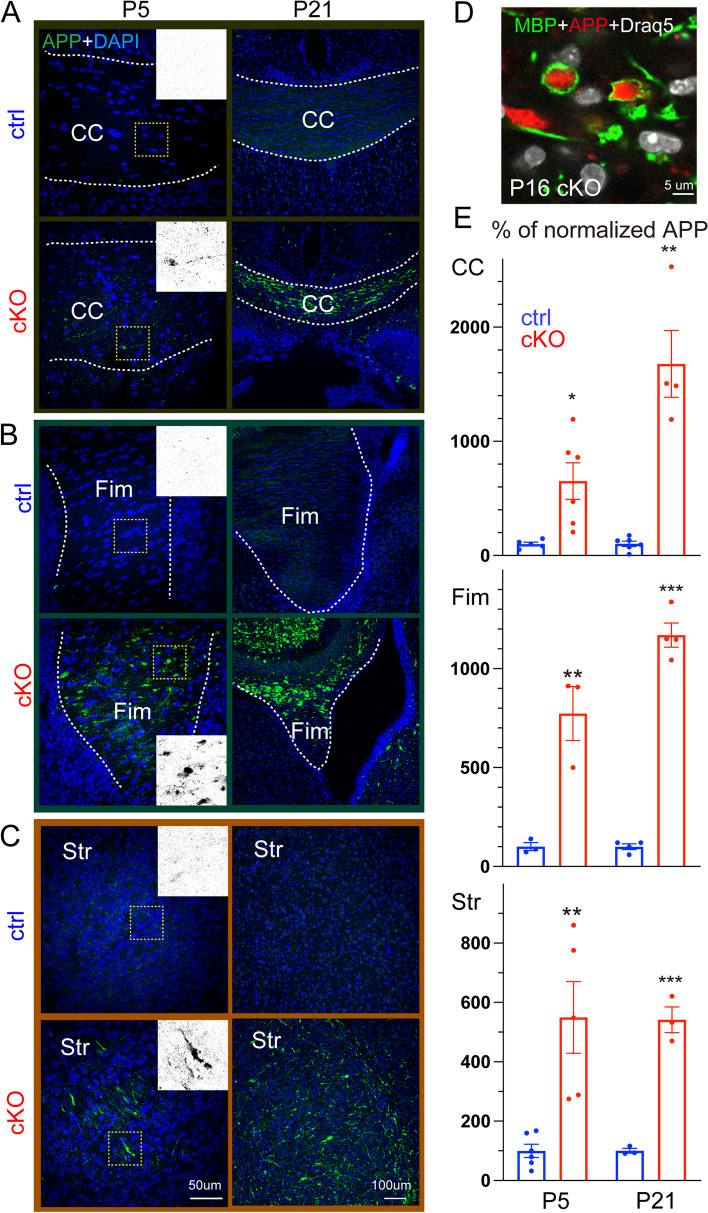
To examine whether NMNAT2 deletion results in axonal transport deficits, we immunostained APP in brains prepared from P5 and P21 cKO mice and littermate controls. At P5, most long-range cortical axons have reached their target brain regions [69, 70]. We found significant APP accumulation in the corpus callosum at P5 in cKO but not in control (Fig. 2A, E). This P5 timepoint is before the corpus callosum thickness is affected by NMNAT2 loss. In addition, we observed APP accumulation in cKO mice in brain regions where glutamatergic axons transit, including the hippocampal fimbria and striatum, despite the observation of normal axon fascicle density [71]. In contrast, little APP signal was detected in the corresponding regions of control brains (Fig. 2B, C, E). At P21, where significant axon degeneration occurs, we found a striking buildup of the APP signal in areas enriched with long-range axons. As APP accumulations are encapsulated by myelin basic protein (MBP), a marker for myelinated axons, in the corpus callosum (Fig. 2D), it suggests that the APP aggregates are present in axons.
Fig. 2.
Deleting NMNAT2 in cortical glutamatergic neurons results in Amyloid Precursor Protein (APP) accumulation (A, B, C) Representative confocal microscopy images showing APP accumulation in multiple brain regions of ctrl and cKO mice at P5 and P21. Scale bars, 50 um for all left panels, 100 um for all right panels. Dashed white lines mark the margins of corpus callosum (A) and fimbria (B). D High magnification image showing that the accumulated APP is encircled by myelin sheath labeled by myelin basic protein (MBP) staining in P16 cKO corpus callosum (observed in 3 out of 3 cKO brains). E Quantification of the APP signal in the corpus callosum, hippocampal fimbria, and striatum area of cKO mice normalized to the average signal in ctrl mice at P5 and P21. Number of mice: P5, 3–6 ctrl, and 3–6 cKO; P16/P21 3–6 ctrl and 3–4 cKO. Unpaired t-test and Mann–Whitney test were applied for the statistic result, *p < 0.05, **p < 0.01, ***p < 0.001
Interestingly, we also found analogous APP accumulations in the neurites of cortical neuron cultures prepared from NMNAT2 null (NMNAT2-Blad [39]) embryos, but not in wildtype or heterozygous (control) neurons after 8 days in vitro (DIV; Fig. 2-S1 and S2). Immunostaining showed APP accumulation in axons labeled by βIII-tubulin/TUJ1 antibody but not in MAP2-positive dendrites of KO neurons (Fig. 2-S1C, D). APP accumulation increased drastically in KO axons from DIV8 to 14 (Fig. 2-S1A, B), while the signal densities for TUJ1 and MAP2 levels were similar between KO and control neurons (Fig. 2-S2A, B). Additionally, we detected fragmented and aggregated TUJ1 signal as a sign of axon degeneration at DIV14 (Fig. 2-S1 and Fig. 8-S2C). As our cortical neuronal cultures are prepared from E15.5/E16.5 embryonic cortex, by DIV8, they likely correspond to the first postnatal week of age in vivo. Besides, we found significant axonal APP accumulation in NMNAT2-deleted cortical neurons using an alternative Cre-loxP approach (Fig. 2-S3). This in vitro recapitulation of APP accumulation and axon degeneration-like phenotype seen in vivo justifies using cultured NMNAT2 KO neurons as a cellular model to elucidate the molecular mechanisms mediating NMNAT2's function in axonal health.
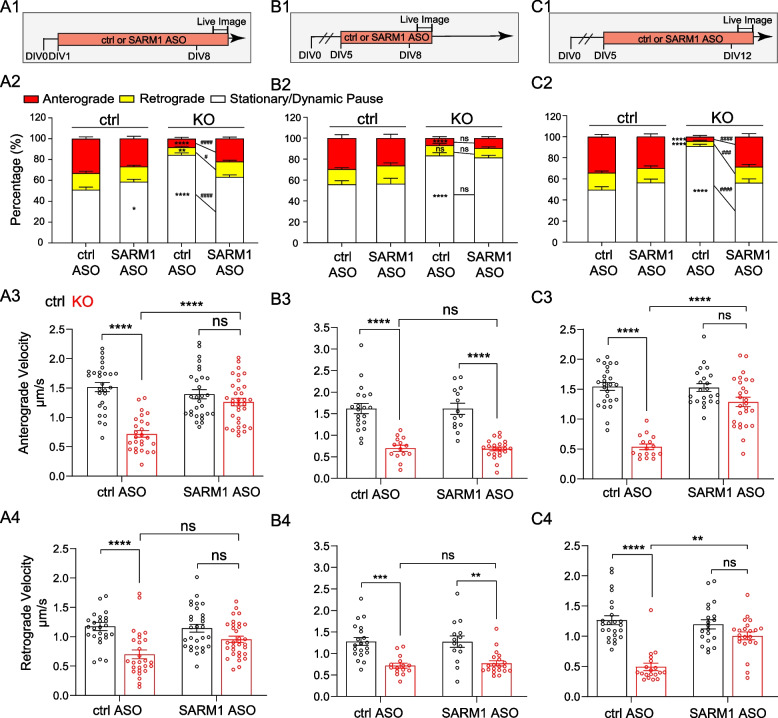
Fig. 8.
SARM1 knockdown restores APP axonal transport in NMNAT2 KO neurons (A1, B1, C1) Experimental timeline. (A2, B2, C2) Percentages of APP vesicles moving anterogradely, retrogradely, or staying stationary in distal axons of ctrl and KO neurons following the treatments indicated in A1, B1, and C1, respectively. Numbers of neurons imaged and statistics: (A2) 27 ctrl + ctrl ASO, 31 KO + ctrl ASO, 29 ctrl + SARM1 ASO, and 33 KO + SARM1 ASO, *p = 0.0286, #p = 0.018, **p = 0.0069. (B2) 20 ctrl + ctrl ASO, 22 KO + ctrl ASO, 14 ctrl + SARM1 ASO, and 26 KO + SARM1 ASO. (C2) 25 ctrl + ctrl ASO, 31 KO + ctrl ASO, 22 ctrl + SARM1 ASO, and 26 KO + SARM1 ASO, ###p = 0.0006. Significant comparison to KO + ctrl ASO group was labeled with #. (A3-4, B3-4, C3-4) Velocity of antero- and retrograde transport in distal axons of ctrl and KO neurons following the treatments indicated in (A1, B1, C1), respectively. (A3-4) 26-27 ctrl + ctrl ASO, 26-27 KO + ctrl ASO, 29 ctrl + SARM1 ASO, and 32-33 KO + SARM1 ASO. (B3-4) 20 ctrl + ctrl ASO, 14-16 KO + ctrl ASO, 14 ctrl + SARM1 ASO, and 20-22 KO + SARM1 ASO, (ctrl + SARM1 ASO vs KO + SARM1 ASO) **p = 0.0049, (ctrl + ctrl ASO vs KO + ctrl ASO) **p = 0.0022. (C3-4) 24-25 ctrl + ctrl ASO, 16-19 KO + ctrl ASO, 20-22 ctrl + SARM1 ASO, and 22-26 KO + SARM1 ASO, **p = 0.001. One-way or two-way ANOVA with Tukey's multiple comparisons test and Kruskal-Wallis test with Dunn's multiple comparisons test were used; see details in supplemented Excel. All above data represent mean ± SEM, ****p < 0.0001, ####p < 0.0001. Numbers of independent culture experiments are listed in Sup. Table
NMNAT2 is required for the transport of fast-moving vesicular cargos in distal axons
As APP accumulation is observed prior to axonal degeneration in NMNAT2 KO axons, we hypothesized that NMNAT2 is required for axonal transport. Previous work showed that NMNAT2 is localized to Golgi-derived vesicles and undergoes fast, bi-directional axonal transport in PNS axons [25, 26]. We observed a similar transport profile of NMNAT2 along axons and its colocalization (Fig. 3-S1A, B, E) and comigration with fast-moving cargos (Fig. 3-S1F, G) in cultured cortical neurons. In contrast, NMNAT2 seldom colocalizes to mitochondria in cortical neurons (Fig. 3-S1 C-E).
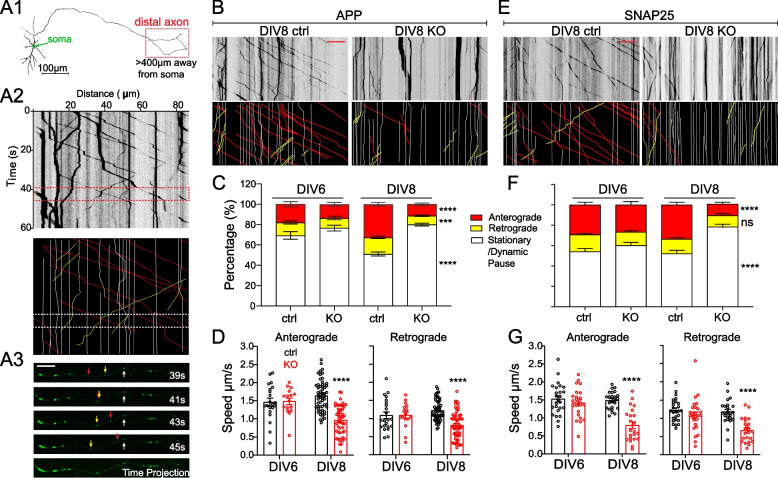
Fig. 3.
Nmnat2 is required for transporting vesicular cargos in the distal axon (A1) Diagram indicating the distal axon region (> 400 µm from the soma) where axonal transport was examined with time-lapse imaging. (A2) Example kymograph (upper) and annotations (lower) with anterograde (red lines), retrograde (yellow lines), and stationary/dynamic pause (white lines) movements indicated. (A3) Four image examples showing APP-EGFP movement along an axon segment during the times indicated in the dashed box in A2. Scale bar, 10 µm. B, E Representative kymographs for APP-EGFP and SNAP25-EGFP from DIV8 ctrl and KO distal axons. Scale bar, 20 µm. C, F Percentages of APP- or SNAP25-EGFP vesicles moving antero/retrogradely or staying stationary/dynamic pause in ctrl and KO neurons. Numbers (neurons imaged) and statistics: C DIV6, 22 ctrl, and 19 KO; DIV8, 56 ctrl, and 62 KO; compared with two-way ANOVA and Šídák's multiple comparisons for anterograde and stationary/dynamic pause categories, compared with multiple Mann–Whitney test and Holm-Šídák multiple comparisons for the retrograde category. F DIV6, 27 ctrl, 26 KO; DIV8, 24 ctrl, 27 KO; two-way ANOVA with Tukey's multiple comparisons. D, G Velocity of antero- and retrograde transport in ctrl and KO neurons from 2–3 independent experiments. Numbers (neurons imaged) and statistics: D Anterograde velocity analysis: DIV6, 21 ctrl and 16 KO; DIV8, 56 ctrl and 51 KO. Retrograde velocity analysis: DIV6: 22 ctrl, 16 KO; DIV8, 56 ctrl, 58 KO. Two-way ANOVA with Šídák's multiple comparisons. G Anterograde velocity analysis: DIV6, 25 ctrl, 24 KO; DIV8, 24 ctrl, 22 KO; two-way ANOVA with Tukey's multiple comparisons. Retrograde velocity analysis: DIV6, 25 ctrl, 26 KO; DIV8, 24 ctrl, 23 KO; multiple Mann–Whitney test with Holm-Šídák multiple comparisons. KO was compared to ctrl of the same DIV in all data sets. Data represent mean ± SEM, ****p < 0.0001. APP and SNAP25 data were collected from 3 and 2 independent experiments, respectively
To determine whether NMNAT2 is required for axonal transport, we used time-lapse imaging to quantify axonal transport of EGFP-tagged vesicular cargos, APP and SNAP25, and DsRed-tagged mitochondria in control and NMNAT2 KO neurons at DIV6 and 8 (Fig. 3 and Fig. 3-S2). APP is a component of Rab5-containing vesicles [72], while SNAP25 is a component of Piccolo-bassoon transport vesicles [73]. APP and SNAP25 undergo fast, bidirectional axonal transport [74, 75] mediated by kinesin-1 and dynein motor proteins [56, 76]. Distinct from vesicular cargos, axonal mitochondria move slowly and intermittently in both directions [77, 78], responding to the interplay between adaptor and motor proteins [79, 80]. To transfect only a few neurons with APP-EGFP, SNAP25-EGFP, or mito-DsRed expressing construct, lipofectamine transfection was conducted < 20 h before live imaging to reduce toxicity of overexpression. Such sparse labeling allowed us to identify distal (> 400 μm away from the soma) or proximal (within 200 μm of the soma) axon segments (Fig. 3-S3). Axonal transport of APP and SNAP25 was measured in distal and proximal segments (Fig. 3 and Fig. 3-S2). At DIV8, significant deficits in APP and SNAP25- transport were detected in KO distal segments (Fig. 3) but not proximal segments (Fig. 3-S2). Furthermore, we observed a substantial increase in the percentage of vesicles in the stationary and dynamic pause phases (Fig. 3C, F), a concomitant decrease in the percentage of vesicles engaging in anterograde movement, and reduced velocities in anterograde and retrograde directions (Fig. 3D, G). However, at DIV4 (data not shown) and 6 (Fig. 3C, D, F, G), APP and SNAP25 transport were unaffected in KO axons. In contrast to vesicular transport, mitochondrial distribution, morphology, and motility were unaffected in KO neurons at DIV8 (Fig. 3-S4), despite the heterogeneous mitochondrial velocities reported previously [81]. These results demonstrate that NMNAT2 is required for fast transport of vesicular cargos but not mitochondria, and that NMNAT2 loss affects distal axon transport before axon degeneration.
NMNAT2 maintains global NAD+, NADH levels and local NAD+/NADH redox potential in distal axons
NMNAT2 catalyzes NAD synthesis in the salvage pathway, the major pathway in CNS neurons for NAD biosynthesis [15]. The ratio of oxidized (NAD+) to reduced (NADH) forms of NAD establishes the NAD redox potential (NAD+/NADH) and is crucial to driving glucose metabolism [82, 83]. To determine if NMNAT2 is required for maintaining the NAD redox potential, we measured the abundance of NAD+ and NADH at DIV8, when axonal transport deficit first becomes evident in KO axons (Fig. 2-S1 C,D). Both NAD+ and NADH levels were reduced to ~ 50% of their control value in KO neurons, suggesting that NMNAT2 is a major source of NAD in cortical neurons. However, since NAD+ and NADH levels were reduced to the same extent upon NMNAT2 loss, the NAD redox potential measured from whole neurons remained unchanged (Fig. 4A).
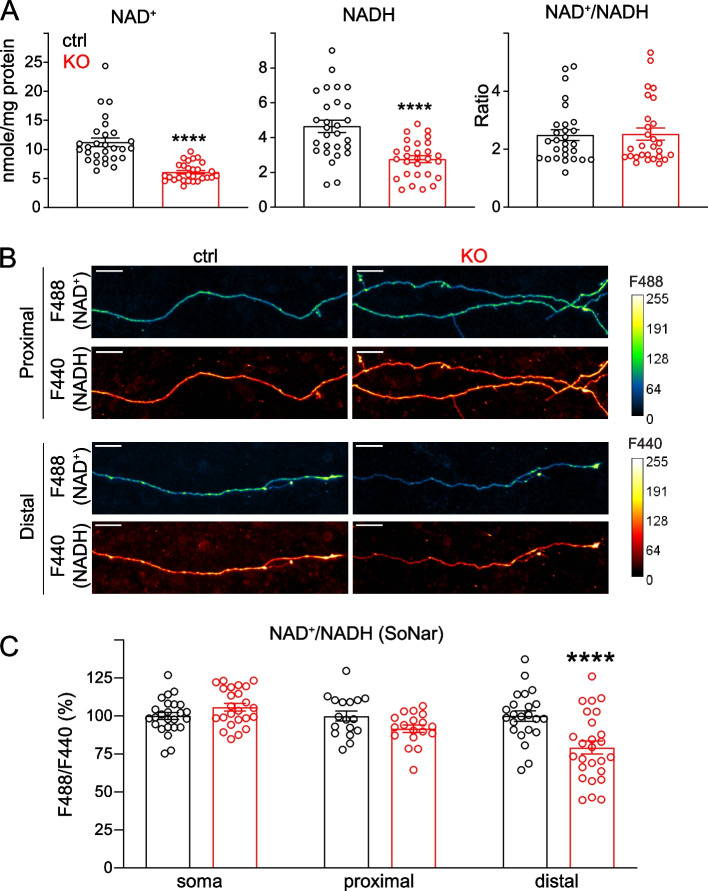
Fig. 4.
Loss of NMNAT2 reduces NAD+ levels and impairs the NAD redox potential in distal axons. A Levels of NAD+ and NADH and calculated NAD+/NADH ratios in DIV8 ctrl and KO cortical neurons as measured by the NAD+/NADH-Glo assay and normalized to protein amounts. Readings from 28 ctrl and 28 KO culture wells from 4 independent culture experiments; unpaired t-test was used for NADH while Mann–Whitney test was used for both NAD+ and NAD+/NADH ratios due to their distributions. B Representative images showing signals emitted from the SoNar (NAD+/NADH) ratiometric sensor for NAD.+ and NADH in the proximal and distal axons of DIV 8 ctrl or KO cortical neurons. Scale bar, 20 um. C NAD + /NADH ratios in soma, proximal and distal axons of DIV7 and 8 ctrl and KO cortical neurons revealed by F488/F440 ratiometric measurements of the SoNar sensor (Soma: 25 ctrl and 24 KO from 2 independent experiments; proximal axon segments: 19 ctrl and 20 KO from 3 independent experiments; distal axon segments: 26 ctrl and 30 KO from 3 independent experiments; two-way ANOVA with Tukey's multiple comparisons test). All above data represents mean ± SEM, ****p < 0.0001
One reason that NMNAT2 loss affects axonal transport in distal but not proximal axons may be that its homolog NMNAT1, which is expressed in the neuronal nucleus, is sufficient to maintain NAD redox potential in the soma and proximal axons [14, 84]. Thus, we hypothesized that NMNAT2 is only critical for maintaining NAD redox potential in distal axons, where it provides the ATP required for fast axonal transport. So, to measure NAD redox potential in sub-axonal regions, we used a genetically encoded sensor, SoNar [48], that detects cytosolic NAD redox potential with a reliable signal-to-noise ratio and high spatial resolution. Our imaging studies found that NAD redox potential was significantly reduced in distal axons but not in the soma or proximal axons of DIV8 KO neurons compared to controls (Fig. 4B, C). These data confirm the requirement of NMNAT2 in distal axons to maintain NAD redox potential. In addition, the data suggest that NMNAT2 contributes to ~ 50% of the overall NAD+ and NADH levels in cortical neurons.
NMNAT2 is critical for glycolysis on synaptic vesicles
Fast axonal transport of vesicular cargo is fueled by on-board glycolysis through vesicle-associated glycolytic enzymes that generate ATP to support motor protein movement [32, 85]. Considering the transport deficits and the reduced NAD+/NADH redox potential in the distal axons of NMNAT2 KO neurons, we hypothesized that NMNAT2 supports fast axonal transport by facilitating local glycolysis.
To measure ATP near vesicular cargos, we transfected the genetically encoded presynaptic ATP sensor (Syn-ATP) in control and NMNAT2 KO neurons and measured presynaptic ATP levels in distal axons of live neurons at DIV 8 (Fig. 5A). Syn-ATP is targeted to synaptophysin, which is one of the well-known fast-moving vesicular cargos [86]. Syn-ATP comprises an optimized luciferase to detect ATP within physiological range by luminescence and a mCherry fluorescent protein as an internal control for sensor expression level [33] (Fig. 5B). The luminescence to fluorescence ratio (L/F) measured from Syn-ATP is proportional to ATP levels near synaptic vesicles (sv-ATP) [33]. As the Syn-ATP sensor is pH sensitive [33], all the measurements in the main figure were pH-corrected (Fig. 5-S1A-C). To acquire adequate sv-ATP signal, a 60-s acquisition is required to increase the signal-to-noise ratio of the luminescence measurements as the bioluminescence reaction from the luciferase emits only up to two photons per second [33]. This acquisition criterion is not suitable for detecting ATP near fast-moving cargo. Synaptic vesicles in axonal varicosities are associated with the glycolytic machinery [33, 87], and NMNAT2 is enriched in synaptic vesicles and synaptic plasma membrane fraction [88]. Thus, we measured sv-ATP signal from the static reserve pool of synaptic vesicles located in the presynaptic varicosities [27, 89] to determine the requirement of NMNAT2 for vesicular glycolysis.
Our sv-ATP measurements revealed that NMNAT2 KO neurons exhibited a modest but significant decrease in sv-ATP compared to control neurons (Fig. 5C, D). Previous studies suggests that ~ 90% of sv-ATP is maintained by local glycolysis and/or mitochondrial ATP synthesis [33, 90]. To check the degree of mitochondrial contribution to sv-ATP, we applied oligomycin, an F1-F0 ATP synthase inhibitor that blocks mitochondrial ATP production (Fig. 5A, B). We found no significant reduction in sv-ATP levels (p = 0.3621) in control distal axons upon oligomycin treatment (Fig. 5C, D), suggesting sv-ATP can be maintained by glycolysis alone in WT neurons. This result is consistent with previous studies providing evidence that glycolytic ATP production is the primary ATP source for fast-transporting cargos [32, 85]. In contrast, in KO distal axons, oligomycin treatment significantly reduced sv-ATP levels (Fig. 5C, D). These data suggest the presence of compensatory mitochondrial oxidative phosphorylation upon NMNAT2 loss to provide ATP in distal axons.
To test if the reduced sv-ATP in NMNAT2 KO axons is caused by NAD+ deficiency, neuronal cultures were supplemented with 1 mM NAD+ from DIV5 to DIV8 (Fig. 5A). Isotope labeling studies show that primary neuronal cultures can take up exogenous NAD+ [91, 92]. Here we found that NAD+ supplementation increased intracellular NAD+ levels transiently in neuronal cultures (Fig. 5-S2), and thus, we refreshed NAD+ daily. NAD+ supplementation restored sv-ATP levels in KO distal axons to control levels in both basal and oligomycin-treated conditions (Fig. 5C-D). These data suggest that NMNAT2 synthesizes the NAD+ required to drive glycolysis on synaptic vesicles.
NAD+ supplementation restores APP transport via glycolysis in the absence of NMNAT2
Next, we determined whether NAD+ supplementation can restore fast axonal transport in NMNAT2 KO distal axons. Compared to vehicle treatment, NAD+ supplementation significantly decreased the percentage of stationary/dynamic pause events, increased the percentage of anterograde and retrograde events, and restored anterograde and retrograde velocities of APP transport (Fig. 6A-C). On the other hand, NAD+ supplementation of control neurons had minimal impact on APP axonal transport (Fig. 6A-C) and NAD+ abundance (Fig. 5-S2). To further validate the requirement of NMNAT2’s NAD+ synthase activity, we overexpressed wt- or ED(H24D, enzyme dead [16])-NMNAT2 back in KO neurons through lentiviral transduction, which achieves comparable transduction rate (Fig. 6-S1B). We found ED-NMNAT2 failed to rescue APP accumulation, whereas wt-NMNAT2 reduced APP accumulation area almost to control level (Fig. 6-S1A-C). Taken together, NMNAT2’s NAD+ synthase activity is required to support APP fast axonal transport.
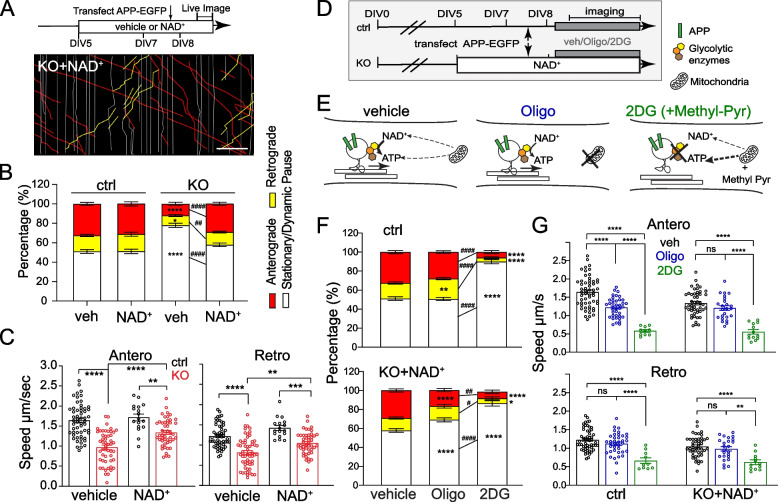
Fig. 6.
NAD+ supplementation depends on glycolysis to rescue APP transport in NMNAT2 KO neurons. A Representative kymographs from DIV8 ctrl or KO neurons supplemented with Veh (vehicle) or 1 mM NAD+ since DIV5. Scale bar, 20 µm. B Percentages of APP-EGFP vesicles moving anterogradely, retrogradely, or pausing in distal axons of DIV8 ctrl and KO neurons supplemented with Veh or NAD+. Neurons imaged and statistics: 56 ctrl + Veh, 62 KO + Veh, 17 ctrl + NAD+, and 49 KO + NAD+, *p = 0.0232, ##p = 0.0018. Significant comparison to ctrl + Veh and KO + Veh groups was labeled with* and #, respectively. C Velocity of antero- and retrograde transport of APP-EGFP in distal axons of DIV8 ctrl and KO neurons supplemented with Veh or NAD+. Numbers (neurons imaged) and statistics: 56 ctrl + Veh, 51–58 KO + Veh, 17 ctrl + NAD+, and 47–48 KO + NAD+ for anterograde velocity, (anterograde) **p = 0.0041, (retrograde) **p = 0.0013, ***p = 0.0002. E Schematic representation of how glycolysis and mitochondrial respiration are affected by Oligo or 2DG(+ Methyl-Pyr) treatment. F Percentages of APP-EGFP vesicles moving anterogradely, retrogradely, or pausing in distal axons of DIV8 ctrl and KO + NAD+ neurons in the presence of vehicle, Oligo, or 2DG. Numbers (neurons imaged) and statistics: 56 ctrl + Veh, 39 ctrl + oligo, and 20 ctrl + 2DG, **p = 0.0059; 49 KO + NAD+ + Veh, 22–25 KO + NAD+ + Oligo,and 15–18 KO + NAD+ + 2DG, *p = 0.0291, #p = 0.0252. Significant comparisons to Veh-treated and Oligo-treated groups were labeled with * and #, respectively. G Velocity of antero- and retrograde transport of APP-EGFP in distal axons of DIV8 ctrl and KO + NAD+ neurons in the presence of vehicle (black), Oligo (blue), or 2DG (green). Neurons imaged and statistics: 56 ctrl + Veh, 38–39 ctrl + oligo, 10 ctrl + 2DG, 47–48 KO + NAD+, 24 KO + NAD+ + Oligo, and 12–13 KO + NAD+ + 2DG, **p = 0.0011. All above data represent mean ± SEM, two-way ANOVA with Tukey's multiple comparisons test or Kruskal–Wallis test with Dunn's multiple comparisons test were used, see details in supplemented excel, ****p < 0.0001, ####p < 0.0001
We then tested whether glycolysis is required for NAD+ rescue of APP transport. We acutely suppressed glycolysis by transferring the neurons to a glucose-free medium containing the hexokinase inhibitor, 2-deoxyglucose (2DG), while adding methyl-pyruvate (Methyl-pyr) as an alternative substrate to support TCA cycle and OXPHO in mitochondria (Fig. 6D, E). In parallel, we tested if mitochondrial ATP production is required for NAD+ rescue of axonal transport in NMNAT2 KO neurons by blocking OXPHO in the presence of glucose (Fig. 6D, E). Glycolysis inhibition significantly impaired APP transport in control axons, as revealed by a significant increase in stationary/dynamic pause events and decrease in transport velocity (Fig. 6F, G). Very few APP-containing vesicles remain mobile in distal axons after glycolysis inhibition, and thus velocity measurements were conducted on a small number of vesicles. In contrast, OXPHO inhibition had a relatively mild impact on APP transport in control axons, including a moderate reduction in anterograde velocity and a minor but significant increase in the percentage of retrograde events. In KO axons, glycolysis inhibition abolished the rescue of APP transport provided by exogenous NAD+ and significantly reduced the number of transport events and movement velocities (Fig. 6F, G).
OXPHO inhibition also perturbed NAD+-mediated rescue of KO neurons, although not as robustly as did glycolysis inhibition, as shown by the increased percentage of stationary/dynamic pause events and decreased percentage of anterograde events (Fig. 6F). However, the transport velocities in both directions were unaffected (Fig. 6G). Additionally, we assessed APP accumulation by immunostaining and found that NAD+ supplementation from DIV8 to 14 significantly reduces APP accumulation in KO neurons. Furthermore, 48 h of glycolysis inhibition together with methyl-pyruvate supplementation abolished this rescue (Fig. 6-S2A-C). These data demonstrate that NAD+-mediated rescue of APP axonal transport in NMNAT2 KO neurons depends mainly on glycolysis with a modest contribution from mitochondrial OXPHO.
SARM1 depletion sustains APP transport and prevents axon degeneration in the absence of NMNAT2
Sterile alpha and TIR motif-containing protein 1 (SARM1) is a recently discovered NAD(P) glycol-hydrolase highly expressed in neurons [93, 94]. SARM1 senses the rise in nicotinamide mononucleotide (NMN)-to-NAD+ ratio caused by loss of NMNAT2 and responds by activating its NAD+ hydrolase domain [95, 96]. SARM1 deletion in NMNAT2 KO mice prevents perinatal lethality, preserves healthy axons and synapses in the peripheral nervous system, and maintains motor function throughout adulthood [97, 98].
We, therefore, examined if SARM1 reduction could reverse axon phenotypes in NMNAT2 cKO brains. To this end, we crossed NMNAT2 cKO mice to SARM1 KO (Snull/Snull) mice to generate NMNAT2 cKO missing one copy of SARM1 (NMNAT2 cKO; Snull/ +), which we backcrossed to SARM1 KO mice to obtain NMNAT2 cKO missing both copies of SARM1 (NMNAT2 cKO; Snull/Snull). NMNAT2 cKO; Snull/ + and NMNAT2 cKO; Snull/Snull mice survived similarly to their littermate controls, in contrast to NMNAT2 cKO mice (data not shown). Normal brain morphology and normal motor behavior were observed in NMNAT2 cKO; Snull/Snull mice (Fig. 7A, Fig. 7-S1C). In contrast to NMNAT2 cKO; Snull/Snull mice and control mice, NMNAT2 cKO; Snull/ + mice still exhibited reduced body weights and a hindlimb clasping phenotype (Fig. 7-S1B-C). Gross examination of their brains found aberrant anatomy similar to NMNAT2 cKO brains (Fig. 7A), including atrophied hippocampi, enlarged ventricles, and thinned primary somatosensory cortex (Fig. 7B) and corpus callosum (Fig. 7C, D). APP accumulation was found in the corpus callosum, fimbria, and striatum in NMNAT2 cKO; Snull/ + but not in NMNAT2 cKO; Snull/Snull brains (Fig. 7E, F ). APP accumulation was detectable at P5 in NMNAT2 cKO; Snull/ + but not NMNAT2 cKO; Snull/Snull brains, suggesting APP transport was already normalized by the complete absence of SARM1 at P5 (Fig. 7-S2). Taken together, these findings show that complete SARM1 loss prevents the impact of NMNAT2 loss in cortical axons.
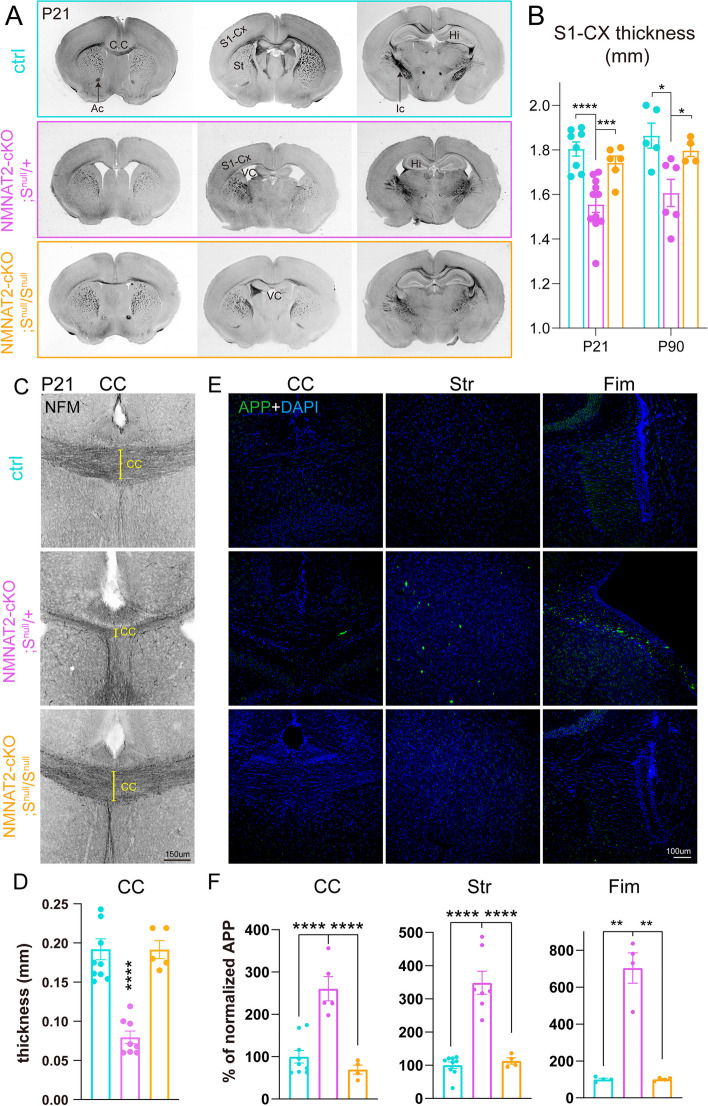
Fig. 7.
Deleting SARM1 prevents neurodegenerative phenotypes in NMNAT2 cKO brains. A Bright field images of coronal brain sections (rostral to caudal from left to right) from ctrl, NMNAT2 cKO; Snull/ + , and NMNAT2 cKO; Snull/Snull mice. Enlarged ventricle, greatly reduced corpus callosum, and internal capsule were evident in NMNAT2 cKO; Snull/ + but not in NMNAT2 cKO; Snull/Snull brains. Abbreviations: Ac, anterior commissure; Ic, internal capsule; CC, corpus callosum; S1-Cx, primary somatosensory cortex; Hi, hippocampus; St, striatum; VC, ventricle. B Thickness of the primary somatosensory cortex in P16/21 and P90 mice. Number of mice: P16/21, 8 ctrl, 11 NMNAT2 cKO; Snull/ + , 6 NMNAT2 cKO; Snull/Snull; P90, 5 ctrl, 6 NMNAT2 cKO; Snull/ + , 4 NMNAT2 cKO; Snull/Snull. ***, p = 0.0018, q = 0.0009. (Ctrl vs. NMNAT2 cKO; Snull/ +) *, p = 0.0049, q = 0.0103. (NMNAT2 cKO; Snull/ + vs. NMNAT2 cKO; Snull/Snull) *, p = 0.0341, q = 0.0358. C Representative NF-M (black) immunostaining images showing corpus callosum around the midline area in ctrl, NMNAT2 cKO; Snull/ + , and NMNAT2 cKO; Snull/Snull rostral brain section at P21. D CC thickness quantification. Number of mice: P21, 10 ctrl, 8 NMNAT2 cKO; Snull/ + , 5 NMNAT2 cKO; Snull/Snull. E Immunohistology images showing APP accumulation in the corpus callosum, fimbria, and striatum of P21 NMNAT2 cKO; Snull/ + , but not in ctrl and NMNAT2 cKO; Snull/Snull brains. F Quantification of the APP signal in the brain regions shown in (E) normalized to the value in ctrl mice. Number of mice: 7–9 ctrl, 4–5 NMNAT2 cKO; Snull/ + , 4 NMNAT2 cKO; Snull/Snull. CC, corpus callosum; Str, striatum; Fim, Fimbria. (Fim Ctrl vs. NMNAT2 cKO; Snull/ +) **, p = 0.0186, q = 0.0098; (NMNAT2 cKO; Snull/ + vs. NMNAT2 cKO; Snull/Snull) **, p = 0.0186, q = 0.0098. All above data represent mean ± SEM. One-way ANOVA with a two-stage linear step-up procedure of Benjamini, Krieger, and Yekutieli was used, ****p < 0.0001
Next, we examined whether SARM1 loss protects NMNAT2 KO neurons from axonal degeneration by preventing axonal transport deficit. We used anti-sense oligonucleotides targeting sarm1 mRNA (SARM1-ASO) to knockdown SARM1 expression at desired time points and a scrambled anti-sense oligonucleotide as the non-targeting control (ctrl-ASO). We evaluated the SARM1 knockdown efficacy of two SARM1-ASOs and one ctrl-ASO using qPCR and western blotting. ASO33, one of the two SARM1-ASOs, more efficiently decreased SARM1 protein abundance 4–7 days following ASO treatment (Fig. 8-S1). This ASO was therefore used for the rest of the experiments and referred to as SARM1-ASO. To test the impact of SARM1 knockdown on axonal transport, we added ASOs from DIV1 and DIV5 on and examined the impact at DIV8. SARM1-ASO application starting at DIV1 significantly reduced SARM1 abundance by ~ 70% and prevented APP transport deficits in NMNAT2 KO axons at DIV8 (Fig. 8A1-4). In contrast, SARM1-ASO treatment starting at DIV5 only reduced SARM1 abundance by ~ 50% and failed to rescue axonal transport (Fig. 8B1-4). Surprisingly, by DIV12, APP transport was completely rescued in the distal axons of these neurons (Fig. 8C1-4), despite the impairment at DIV8. Consistent with axonal transport analysis, APP accumulation at DIV14 was rescued by SARM1-ASO treatment starting from DIV1 or DIV5 in NMNAT2 KO neurons (Fig. 8-S2). Furthermore, the axon degeneration phenotype revealed by TUJ1 aggregates at DIV14 in ctrl-ASO-treated NMNAT2 KO axons was also reduced by SARM1-ASO treatment.
Previous studies show that activated SARM1 hydrolyzes NAD+ [93, 94]. To test if SARM1 knockdown attenuates NAD deficiency caused by NMNAT2 loss, we first measured the global levels of NAD+ and NADH at DIV8 WT and KO neurons treated with PBS (vehicle control), ctrl-ASO, or SARM1-ASO from DIV1. We found similar levels of NAD+, NADH, and NAD+/NADH ratios between SARM1-ASO treated WT and KO (Fig. 8-S3 A-C). Next, we conducted SoNar sensor imaging to examine whether SARM1 knockdown also rescues the NAD redox potential in NMNAT2 KO distal axons. Our live-cell imaging studies find that SARM1 knockdown prevents the reduction in NAD+/NADH ratios normally caused by NMNAT2 loss (Fig. 8-S3 D-E). Taken together, blocking NAD+ degradation by reducing SARM1 abundance protects axons during NMNAT2 loss in vivo and in vitro.
Discussion
NMNAT2 has been identified as an AD target (https://agora.adknowledgeportal.org/genes/nominated-targets) and an axonal maintenance factor [20]. Here we provide the first in vivo evidence that NMNAT2 is critical for the health of long-range cortical glutamatergic axons in mice. We show that NMNAT2 loss impairs glycolysis, disrupts fast axonal transport, and results in APP accumulation. NAD+ supplementation or reducing the levels of SARM1, an NAD+ hydrolase, effectively restores fast axonal transport and prevents the neurodegeneration commonly observed in NMNAT2 cKO axons both in vitro and in vivo. In summary, our present study suggests that NMNAT2 protects cortical neurons from axonal degeneration by ensuring that the energetic demands of distal axons are met. Our data also suggest glucose hypometabolism in long-range axons is likely a major cause of axonopathy during NDAs. Therefore, therapies in preventing NMNAT2 or NAD loss during aging or NDAs should maintain axonal energetics and axonal health.
NMNAT2 contributes to fast axonal transport by maintaining the local NAD redox potential for efficient vesicular glycolysis
In this study, we show that NMNAT2 is required for maintaining vesicular glycolytic activity (Fig. 5) and the fast axonal transport of APP and SNAP25-containing vesicular cargos (Fig. 3) in distal axons of cortical neurons. This role in vesicular cargo transport is consistent with earlier observations. For instance, NMNAT2 is enriched in the synaptic vesicle and membrane but not in mitochondrial fractions of cortical neurons [88]. In addition, the palmitoylation of NMNAT2 enables it to associate with the membrane of Golgi-derived, fast-moving vesicular cargos [25]. Our time-lapse imaging confirms that NMNAT2’s dynamic antero- and retrograde movement and transport velocities in axons are similar to fast-transported cargos like APP- and SNAP25-containing vesicles (Fig. 3-S1).
Glycolysis is the primary ATP source for fast axonal transport and is generated by a vesicular glycolytic complex [32, 85]. It has been estimated that one kinesin requires ~ 187.5 ATP molecules/s to move at ~ 1.5 μm/s [36], the average anterograde velocity of APP. Thus, ~ 188 NAD+ molecules are needed to drive ~ 94 glycolytic reactions per second near individual vesicular cargos to generate the ATP consumed by one kinesin. Data from our SoNar NAD+/NADH radiometric imaging studies show that NMNAT2 is essential in maintaining the NAD redox potential in distal axons. Based on in vitro biochemical studies, one NMNAT2 synthesizes ~ 0.6 NAD+ per second [88]. Knowing that lactate dehydrogenases (LDH) can catalyze the recycling of NADH back to NAD+, we hypothesize that NMNAT2 coordinates with LDH to maintain NAD redox potential for glycolytic ATP production. The necessity of NMNAT2’s NAD+ synthase activity is supported by the rescue of APP axonal transport in KO neurons by NAD+ supplementation (Fig. 6), as well as the failure of reducing APP accumulation by overexpressing enzyme inactive (H24D point mutation) NMNAT2 in KO neurons [16] (Fig. 6-S1). Taken together, our data suggest that NMNAT2’s NAD+ synthesizing activity and its proximity to the vesicular glycolytic complex ensure sufficient ATP for motor protein operation in fast axonal transport.
A glycolytic deficit likely occurs in NMNAT2 KO distal axons, reflected by the reduced sv-ATP levels when glycolysis becomes the only ATP provider upon inhibition of mitochondrial ATP production with oligomycin (Fig. 5). However, in this condition, the already reduced viability of NMNAT2 KO neurons caused by oligomycin treatment prevented us from applying 2DG subsequently to confirm glycolysis’ contribution to the remaining sv-ATP. The robust mitochondrial compensation upon glycolysis inhibition observed by Ashrafi et al. [99] and us (data not shown) also rule out the feasibility of measuring glycolysis-derived ATP by directly applying 2DG. Nevertheless, using a Seahorse glycolytic rate assay in whole neuronal cultures, we confirmed that the reserve glycolytic capacity is compromised in NMNAT2 KO neurons upon prolonged glutamate transmission (data not shown).
SARM1 is activated as the NMN-to-NAD+ ratio increases as occurs with NMNAT2 loss [95, 96]. The rescue of NMNAT2 KO phenotypes by lowering SARM1 levels raises the possibility that SARM1 activation in the absence of NMNAT2 accounts for the observed glycolysis and axonal transport deficits [97, 98, 100]. Previous studies have found that SARM1 activation triggers programmed axonal death through multifaceted mechanisms, including NAD+ degradation, reduced ATP-to-ADP ratio, release of cytokines, cyclic-ADP ribose production, etc. [101–103]. Despite the substantial SARM1 literature, it is unknown if SARM1 activation directly disrupts vesicular glycolysis and causes fast axonal transport deficits. Immunostaining data show that SARM1 puncta are widely distributed in neurites [51, 100]. Future studies should be conducted to determine if SARM1 is on transported vesicles near the glycolytic machinery and NMNAT2. Physiological concentrations of many metabolites beyond NMN, such as NADP+, nicotinic acid riboside, and free pyridines, can also modulate SARM1 activity [94]. It is plausible that glycolytic intermediate metabolites also modulate SARM1 function.
Intriguingly, before P5 in vivo and DIV8 in vitro, NMNAT2 seems to be dispensable for axonal extension and axonal transport, showing normal corpus callosum midline crossing without APP accumulation. This is different from the axonal outgrowth deficit observed in NMNAT2 germline KO mice [37]. Plausible explanations for this discrepancy include: (1) differential requirements for NMNAT2 in axonal outgrowth for different neuronal populations; (2) germline deletion of NMNAT2 disrupts the function of guidepost glia cells, critical in guiding axonal pathfinding during embryonic development [104]; and (3) the timing of NMNAT2 deletion. It remains unknown why axonal extension is insensitive to NMNAT2 loss before P5/DIV8, when glycolytic ATP supply is already needed [105]. Similarly, eliminating the NAD+ consumer, SARM1, in embryonic sensory neurons does not affect axonal branching, while its postnatal removal promotes axonal branching [106]. We speculate that other mechanisms, such as the newly discovered Wnk kinases [107], exist in the early developmental stage to ensure NAD homeostatic balance in the absence of NMNAT2, allowing a sufficient level of glycolytic flux to fuel cargo transport, cytoskeleton dynamics, and axon outgrowth. However, by DIV8 in vitro and P16/21 in vivo, NMNAT2 becomes the mandatory source of NAD+, but only in distal axonal segments. In proximal axons, NMNAT2 is dispensable for NAD redox potential maintenance and fast axonal transport. Given that the NAD+ pool between the nucleus and soma is interchangeable [49], proximal axons could potentially receive sufficient NAD+ generated by nuclear NMNAT1 due to their proximity to soma and thus maintain an adequate NAD redox potential to drive proximal axonal transport despite the absence of NMNAT2. Alternatively, OXPHO from somatic mitochondria could fuel axonal transport in proximal axons of NMNAT2 KO neurons. Electron microscopy studies of cortical layers 2/3 of the primary visual cortex show that the proximal axons within 100 μm from somata of pyramidal neurons tend to have higher mitochondrial coverage and volume than distal axons [108]. As mitochondria are smaller and less mobile in distal axons [108–111], we hypothesize that higher coverage of mitochondria in proximal axons could also compensate for the glycolysis deficits in NMNAT2 KO neurons to fuel fast axonal transport.
Previous studies indicate that BDNF axonal transport is mainly fueled by glycolysis, and unaffected by ablating mitochondrial OXPHO [32]. Our data is largely consistent with this finding on glycolysis-dependent fast axonal transport. However, upon OXPHO inhibition, we observed a mild but significant increase in the numbers of APP cargos undergoing retrograde transport, with a concomitant decrease in anterograde velocity. Using Drosophila neurodegenerative models, it has been shown that mitochondrial dysfunction stimulates a retrograde signaling response [112]. APP is partially localized on the endo-lysosomal vesicles involved in retrograde transport [113, 114]. Thus, the increase in retrograde APP cargo upon mitochondrial inhibition can reflect enhanced retrograde signaling.
Accumulation of APP, a pathological hallmark of defective axonal transport [68, 115], was found in regions enriched with long-range axons of NMNAT2 cKO brains already at P5, preceding the obvious axonal loss. No APP accumulation was observed in the cortex, where proximal or short axonal arbors are enriched. Using cultured neurons, we demonstrated that the fast axonal transport of APP and SNAP25 is severely impaired in distal axonal segments of NMNAT2 KO neurons. These in vitro observations of specific axonal transport deficits in distal axons of NMNAT2 KO cortical neurons offer a mechanistic explanation for the APP accumulation in long-range axons observed in cKO brains. Together, our study provides strong evidence that NMNAT2 is required for fast axonal transport in distal axons of cortical glutamatergic neurons.
Cumulative evidence suggests that neuronal subtypes with long and complex axonal arbors are particularly vulnerable to external insults [116, 117]. As NMNAT2 cKO brains reach the age of P16-P21, not only does APP accumulation drastically increase, but severe axon degeneration occurs, along with a reduction in cortical thickness, hippocampal atrophy, ventricle enlargement, and motor behavioral deficits, recapitulating major hallmarks of neurodegeneration. These findings support the hypothesis that defective axonal transport drives axonopathy in the pre-symptomatic phase of neurodegeneration [55, 118], which may serve as the focal basis for the gradual development and spread of secondary neuronal damage [119, 120]. However, we do not exclude a possible role of NMNAT2 in maintaining neuronal health in the dendritic and somatic compartments [121, 122], whose dysfunction could also contribute to neurodegenerative phenotypes in NMNAT2 cKO brains.
Mitochondrial OXPHO as a mediator of axonal degeneration in NMNAT2 KO cortical neurons
Our sv-ATP imaging studies show significantly reduced ATP levels in NMNAT2 KO distal axons when mitochondrial respiration is blocked (Fig. 5). By contrast, in control cortical axons, mitochondrial inhibition has a negligible impact on sv-ATP levels, potentially compensated by glycolysis-derived ATP in conjunction with the creatine kinase system [123]. These observations suggest that NMNAT2 loss results in a shift towards mitochondrial OXPHO to maintain sv-ATP levels; however, not enough to fuel fast axonal transport. The literature suggests three mechanisms that could mediate this shift in CNS neurons: (1) Ca2+ signaling-dependent regulation of mitochondrial calcium uniporters and the malate-aspartate shuttle [99, 124, 125]; (2) The O-GlcNAcylation-dependent post-translational modification of the mitochondrial proteome and mitochondrial motility [126, 127]; (3) An orchestrated gene expression that upregulates mitochondrial metabolism machineries, including OXPHO and fatty acid beta oxidation [128]. Other than the insufficiency to fuel fast axonal transport, it remains unclear why this metabolic shift fails to protect NMNAT2 KO neurons from axonal degeneration: at DIV14, NMNAT2 KO axons exhibit prominent blebbing, a degenerative-like phenotype (Fig. 8-S2B). It has been suggested that the cumulative oxidative stress and depletion of TCA cycle substrates from hyperactive mitochondrial OXPHO could be detrimental to neuronal survival in PD and AD animal models [129–131]. This observation raises the possibility that excessive mitochondrial activity, compensating for defective glycolysis in NMNAT2 KO axons, contributes to axon degeneration.
We suspect this harmful impact of excessive OXPHO in NMNAT2 KO axons is mitigated by SARM1 deletion, potentially through modulating mitochondrial metabolism and resilience by elevating mitochondrial NAD+ pool, thus rescuing NMNAT2 KO neurons. Upon mitochondrial stress or SARM1 overexpression, SARM1 localizes onto the mitochondrial outer membrane and interacts with mitophagy machinery [93, 94, 132, 133]. It has been shown that SARM1 in activated state drastically consumes NAD+ and impairs mitochondrial OXPHO [134]. In an axotomy model, the mitochondrial motility is preserved when SARM1 is genetically deleted [101]. In the Charcot-Marie-Tooth neuropathy model with mitochondrial abnormality, SARM1 deletion preserved mitochondrial morphology and motility, and protected axons and synapses from degeneration [135]. Here we find that SARM1 loss prevents several phenotypes caused by NMNAT2 loss, including the impaired fast axonal transport and axonal morphology. Currently, it is still unknown whether SARM1 removal in NMNAT2 KO axons prevents the oxidative stress caused by mitochondria hyperactivity. Further investigations into the impacts of SARM1 deletion from NMNAT2 KO axons on mitochondrial metabolic capacity, dynamics, NAD+-NADH shuttling between mitochondrial matrix and cytoplasm, and mitochondrial quality control are needed.
NMNAT2 and vesicular glycolysis as potential targets to protect white matter in neurodegenerative diseases
Compelling in vivo evidence highlights the important role of neuronal glucose uptake and neuronal glycolysis for fueling synaptic function, learning, and memory [128]. Positron emission tomography (FDG-PET) studies have shown that glucose metabolism changes during aging/NDAs in specific brain regions [136–141]. In particular, glucose hypometabolism is often detected in AD-susceptible brain regions and strongly predicts the incidence of mild-cognitive impairment (MCI) in later life [142–146]. Most AD-susceptible regions for cognitive decline identified are located in the default mode network (DMN), the topological central hub of the brain connectome [147–149]. Anatomically, these hub areas are densely inter-connected by long-range axonal tracts [150], that often show pathological changes since early-stage disease [151]. Metabolically, these connectome hubs express higher levels of glycolysis genes compared to the non-hub areas [152], and exhibit reduced aerobic glycolysis upon normal aging and AD [153, 154], which positively correlates with white matter deterioration [155]. Notably, glucose hypometabolism is observed directly in white matter tracts of AD brains [4].
We show that NMNAT2 is critical for vesicular glycolysis to fuel axonal transport in distal axons. Previously, we have demonstrated that NMNAT2 levels are significantly reduced in the prefrontal cortex of AD brains [21], one of the DMN hubs. Our biochemical assays reveal a ~ 50% reduction of NAD+ and NADH levels in NMNAT2 KO neurons (Fig. 4A), indicating that NMNAT2 is a major NAD+ and NADH provider in cortical neurons. Synthesizing these observations, we hypothesize that reduced NMNAT2 in AD brains contributes, at least in part, to the reduction in glucose metabolism observed in AD white matter.
NMNAT2 reduction has also been observed in PD and HD brains [21–23]. PD patients show hypometabolism in the premotor and parieto-occipital cortex that correlates with motor dysfunction [141], while HD patients show progressive glucose hypometabolism in the frontal lobe, temporal lobe, and striatum, accompanied by white matter volume reduction [138]. Supplementing NAD+ and its precursors remarkably ameliorates degenerative phenotypes in transgenic AD and ALS mouse models [156–158]. Similarly, increasing NMNAT2 expression broadly provides neuroprotection across mouse models of tauopathy [21, 159], familiar AD [160], and glaucoma [161]. Coincidently, upregulating glycolysis exerts neuroprotective effect in PD synucleinopathy and ALS models [162, 163]. Our study highlights the novel role of NMNAT2 in supporting glycolysis in long-range projecting axons of cortical glutamatergic neurons. Extrapolating from our findings, NMNAT2 could serve as a putative therapeutic target to boost neuronal glycolysis in order to antagonize the structural connectome breakdown occurring in many neurodegenerative disorders.
Supplementary Information
Additional file 1: Fig. 1-S1. NMNAT2 expression, cKO mice survival rate, and motor behavioral deficit (A) In situ hybridization reveals nmnat2 mRNAs (blue signal) in the E14.5 brain, mainly in the cortical plate (CP) and in the lateral ganglionic eminence (LGE) and medial ganglionic eminence (MGE). (B) Illustration of Nmnat2 conditional KO (cKO) mice generation as described previously [37]. The blue and magenta triangles indicate loxP sites. (C, D) Summary of embryonic and postnatal pups genotyping. For embryonic samples, the strategy was to breed Nex-Cre/+;NMNAT2f/+ with NMNAT2f/+ mice. The genotyping results conform to the expectations of Mendelian inheritance. The breeding strategy for postnatal pups was to cross Nex-Cre/+;NMNAT2f/+ with NMNAT2f/f mice. The genotyping result showed a smaller than expected number of mice with the Nex-Cre/+;NMNAT2f/f (cKO) genotype, suggesting a reduced survival rate for cKO mice after birth. (E) Overall numbers of postnatal male and female ctrl and cKO mice. (F) 3-5 months old NMNAT2 cKO mice exhibit evident hindlimb clasping behavior (ctrl N=3, cKO N=4 were scored), Mann-Whitney test was used, data represents mean ± SEM, *p=0.04. Abbreviations: Cre, Nex-Cre; f/f, NMNAT2f/f; IZ, intermediate zone; Str, striatum; SVZ, subventricular zone; VC, ventricle; VZ, ventricular. Fig. 2-S1. Loss of NMNAT2 triggers APP accumulation in the axon of cultured cortical neurons (A) Representative confocal image of APP immunocytochemical staining in ctrl and KO neuronal culture at DIV5, 8, 10, and 14. Scale bar 30 μm. Red arrows highlight APP accumulation. (B) Area of accumulated APP in cKO and ctrl mice normalized to the average ctrl value of the corresponding DIV. Image numbers used for analysis: DIV5: 10 ctrl, 9 KO; DIV8 & DIV10: 20 ctrl, 20 KO; DIV14: 50 ctrl, 67 KO. DIV5-10 were from 2 independent culture experiments and DIV14 was from > 3 independent experiments. Multiple Mann-Whitney test with Holm-Šídák post-hoc adjustment, KO was compared to ctrl of the same DIV, ***p=0.000202, ****p<0.0001. (C) Representative confocal images of APP, TUJ1 (βIII-tubulin), MAP2, and DAPI staining in DIV10 ctrl and KO neurons. Scale bar 20 μm. (D) Zoom-in view of axon and dendrite area highlighted by the white box in (C). Scale bar 5 μm. Fig. 2-S2. Gross neurite covering the area in vitro is not affected by Nmnat2 loss (A) Representative confocal images of MAP2, TUJ1, and DAPI staining in ctrl and KO neuronal culture at DIV10. MAP2 positive area corresponding to soma and dendrites was subtracted in TUJ1 staining images to determine area corresponding to axon regions. 100 μm scale bar in left and middle panels; 25 μm scale bar in zoom-in panels. (B) Quantification of the somatic and dendritic area labeled by MAP2 and the axonal area labeled by TUJ1 (MAP2 area subtracted) in ctrl and KO neuronal culture at DIV5, 8, 10, and 14, normalized to the average ctrl values at the same DIV. Image numbers from 1-3 independent experiments: DIV5: 10 ctrl, 10 KO; DIV8: 23 ctrl, 16 KO; DIV10: 11 ctrl, 13 KO; DIV14: 5 ctrl, 6 KO. Multiple Mann-Whitney tests with Holm-Šídák correction for comparisons were used for MAP2; two-way ANOVA with Šídák's multiple comparisons test was used for TUJ1. Data represent mean ± SEM. (C) The procedure of semi-automated quantification of accumulated APP by ImageJ. Fig. 2-S3. Loss of NMNAT2 after neurite outgrowth in vitro triggers axonal APP accumulation (A) The experimental timeline in NMNAT2f/f cortical neuronal culture. The NMNAT2 mRNA level was normalized to the LV-copGFP transduced ctrl group of the corresponding DIV and expressed as percentage. Numbers of culture wells from 1-2 independent batches of culture: DIV8: 2 copGFP, 2 iCre; DIV10 & 14: 4 copGFP, 4 iCre; (B) Representative confocal images of APP, TUJ1 and MAP2 staining in DIV10 NMNAT2f/f neurons that received lentivirus starting at DIV5, 30 μm scale bar. Zoom-in view of axon and dendrite area highlighted by magenta and orange box respectively in the upper panel, 10 μm scale bar. (C) The area of accumulated APP was normalized to the LV-copGFP transduced neurons of the corresponding DIV as percentage. Number of images acquired from 2-3 independent experiments: DIV10: 28 copGFP, 28 iCre; DIV14: 26 copGFP, 24 iCre. Two-way ANOVA with Šídák's multiple comparisons test, iCre was compared to copGFP of the same DIV, ****p<0.0001. Fig. 3-S1. NMNAT2 colocalize and co-migrate with vesicular cargos (A) Representative confocal images with maximum intensity Z stack projection showing that NMNAT2-mCherry colocalizes with APP-EGFP, SYPH-EGFP, and SNAP25-EGFP in axons of DIV8 WT cortical neurons (Scale bar, 20 μm). (B) Representative structure illumination images with maximum intensity Z stack projection showing NMNAT2-mCherry colocalizes with APP-EGFP, SYPH-EGFP, and SNAP25-EGFP in axons of DIV8 WT cortical neurons (Scale bar, 5 μm). (C) Representative confocal image shows the distribution of MitoVenus and NMNAT2-mCherry expressed in cortical axons. (Scale bar, 10 μm) (D) Representative grey value intensity plots along the axonal distance for MitoVenus and NMNAT2-mCherry or APP-GFP and NMNAT2-mCherry. (E) The ratio of NMNAT2-mCherry puncta colocalizing to APP-EGFP, SYPH-EGFP, SNAP25-EGFP, or MitoVenus quantified from confocal images. N represents the number of neurons imaged, APP (N=10), SNAP25 (N=20), SYPH (N=20), and MitoVenus (N=6). (F) Representative kymograph showing NMNAT2-mCherry comigrates with APP-EGFP in the axon of DIV8 WT neurons (Scale bar 20 μm in length, 30 s in time, 1.7 frame/second). (G) Frequency distribution of the anterograde and retrograde velocity of NMNAT2-mCherry and APP-EGFP in DIV8 distal axons. (NMNAT2 N=445, APP N=861, N represents the cargo trajectory traced in all kymographs). Fig. 3-S2. Vesicular cargo transport in the proximal axon is less affected by NMNAT2 loss at DIV8 (A) Diagram indicating the proximal axon region (< 200 µm from the soma). (B) Representative kymographs for APP-EGFP from DIV8 ctrl and KO proximal axons. Scale bar, 20 µm. (C) Distribution of APP-EGFP vesicles in anterograde motion, retrograde motion, or in a stationary/dynamic pause from 2 independent culture experiments. Numbers (neurons imaged) and statistics: 23 ctrl, 27 KO; compared with unpaired t-test. (D) Velocity of antero- and retrograde transport of APP-EGFP from 2 independent culture experiments. Numbers (neurons imaged) and statistics: anterograde, 23 ctrl, 26 KO; retrograde, 22 ctrl, 26 KO. Two-way ANOVA with Šídák's multiple comparisons. (E) Representative kymographs for SNAP25-EGFP from DIV8 ctrl and KO proximal axons. Scale bar, 20 µm. (F) Distribution of APP-EGFP vesicles in anterograde motion, retrograde motion, or in a stationary/dynamic pause from 3 independent culture experiments. Numbers (neurons imaged) and statistics: 23 ctrl, 21 KO; anterograde and retrograde categories were analyzed by Mann-Whitney test, and stationary/dynamic pause category was analyzed by unpaired t-test. (G) Velocity of antero- and retrograde transport of SNAP25-EGFP from 3 independent culture experiments. Numbers (neurons imaged) and statistics: anterograde, 23 ctrl, 21 KO; retrograde, 22 ctrl, 20 KO. Two-way ANOVA with Šídák's multiple comparisons. Data represent mean ± SEM. Fig. 3-S3. Examples of axonal morphology overview (A1, B1) Stitched confocal image of DIV8 ctrl or KO neuron expressing SNAP25-EGFP. (A2, B2) Zoom-in of the regions of interest identified as proximal and distal axons as highlighted in magenta and dark green boxes in A1, B1. (A3) Kymograph generated from the axon segment selected and highlighted in yellow in (A2, B2). Fig. 3-S4. Mitochondrial density, morphology, and mobility in distal axons are unaffected by NMNAT2 loss at DIV8 (A) Representative images of EGFP and Mito-DsRed in the distal axon of DIV8 ctrl and KO neurons. (Scale bar 10 μm). (B) Mitochondria density (# of mitochondria per 100 μm) in distal axons of DIV8 ctrl and KO neurons from 3 independent experiments. Numbers (neurons imaged) and statistics: 38 ctrl, 40 KO; unpaired t-test was used. (C) The cumulative percentage distribution of mitochondria morphology indicated by sphericity in DIV8 ctrl and KO distal axons from 3 independent experiments. Numbers (neurons imaged) and statistics: 38 ctrl, 40 KO; Log(Y/(1-Y)) transformation for the independent variable and linear mixed model with random intercept and the slope was used; see descriptions in Star Methods. (D) Representative kymographs show the mobility of MitoDsRed in distal axons of DIV8 ctrl and KO neurons. (Scale bar, 20 µm; total recording time, 15min). (E) The velocity of anterogradely and retrogradely moving MitoDsRed in distal axons of DIV8 ctrl and KO neurons from 2 independent batches of culture. (Anterograde: ctrl N=14, KO N=16, Mann-Whitney test was used; Retrograde: ctrl N=14, KO N=14, unpaired t test was used). (F) Percentage of MitoDsRed that is anterogradely moving, retrogradely moving or in stationary/dynamic pause in distal axons of DIV8 ctrl and KO neurons from 2 independent batches of culture (ctrl N=19, KO N=21). For analyzing anterograde and stationary/dynamic pause, an unpaired t-test was used; for retrograde, the Mann-Whitney test was used. N represents number of neurons imaged. All above data represents mean ± SEM. Fig. 5-S1. pH measurement and pH correction for Syn-ATP (A) Cyto-pHluorin imaging timeline and equation used for cytosolic pH determination in presynaptic varicosities. (B) pH measured by Cyto-pHluorin in various conditions. The mean value in each group is labeled above each box and whisker. Numbers (neurons imaged) and statistics: 27 ctrl, 25 KO, and 27 KO+NAD+ under basal conditions; 18 ctrl, 25 KO, and 27 KO+NAD+ under oligomycin-treated conditions. All groups were from a single experiment. Two-way ANOVA with Tukey's multiple comparisons test, **p= 0.005. (C) Equations used for pH correction of L/F measurements from Syn-ATP. (D) Raw L/F measurements in distal axon varicosities of DIV8 ctrl neurons with or without NAD+ supplementation. Numbers (neurons imaged) and statistics: 78 ctrl and 10 ctrl+NAD+ under basal conditions; 67 ctrl and 10 ctrl+NAD+ under oligomycin-treated conditions. Ctrl was from 5 experiments, and ctrl+NAD+ was from one experiment. Kruskal-Wallis test with Dunn's multiple comparisons test, *p= 0.0171, **p= 0.0018. All above data represent mean ± SEM. Fig. 5-S2. Increase of intracellular NAD+ by exogenous supplementationThe change of intracellular NAD+ abundance over time after one-time 1 mM NAD+ supplementation into the culture medium. All groups were normalized to ctrl+NAD+ 0 h time point and expressed as a percentage. Numbers (culture wells) and statistics: 13-14 ctrl and 12- 14 KO from 4 independent experiments, Kruskal-Wallis test with Dunn's multiple comparisons test was used, *p= 0.049, **p= 0.0023 Fig. 6-S1. The NAD+ synthesizing enzymatic activity of NMNAT2 is required to rescue APP accumulation in NMNAT2 KO neurons (A) Representative confocal images of APP staining of DIV12 KO neurons with or without lentiviral transduction to overexpress wt-NMNAT2 or ED(enzyme dead)-NMNAT2, scale bar 35 µm. (B) Comparable lentiviral transduction rate for wt-NMNAT2 and ED-NMNAT2 in KO neurons with 4MOI (multiplicity of infection) lentivirus. Transduction rate was calculated as the percentage of HA+ APP+ cells out of APP+ cells. N=8 per group, N represents the number of images randomly sampled, unpaired t test was used. (C) The summary for the area of accumulated APP relative to WT as percentage. N=14-18 per group from 2 independent batches of culture, N represents the number of images randomly sampled. One-way ANOVA with Tukey's multiple comparisons test was used, **p= 0.0034, ****p <0.0001. All above data represents mean ± SEM. Fig 6-S2. NAD+ supplementation depends on glycolysis to ameliorate APP accumulation in NMNAT2 KO neurons (A) Timeline of NAD+ supplementation, 2DG(+Methyl-pyr) treatment, and immunostaining. (B) Representative confocal images of APP staining of DIV14 ctrl or KO neurons under Veh (vehicle) or NAD+ supplementation, treated with Veh or 2DG. Scale bar, 30 µm. (C) Area of accumulated APP in DIV14 ctrl or KO neurons supplemented with Veh or NAD+ and treated with Veh or 2DG. All values were normalized to ctrl supplemented and treated with Veh. Numbers (images) and statistics: 18 ctrl+Veh, 20 KO+Veh, 23 ctrl+ 2DG, 12 KO+2DG, 19 ctrl+NAD+, 22 KO+NAD+, 21 ctrl+NAD++2DG, and 22 KO+NAD++2DG, Two-way ANOVA with Tukey's multiple comparisons test was used, ****p<0.0001, ####p<0.0001. Except for KO+2DG, all groups were from 2 independent experiments. All above data represent mean ± SEM. Fig 7-S1. Gender ratio, body weight, and motor behaviors in surviving NMNAT2 cKO mice lacking one or two copies of SARM1 (A) Y axis represents the percentage of postnatally alive mice of ctrl, NMNAT2 cKO; Snull/+, and NMNAT2 cKO; Snull/Snull genotypes pooled from progenies derived from various parent genotype combinations. The actual number of mice from each genotype and gender is listed on top of each bar. (B) Body weight of ctrl, NMNAT2 cKO; Snull/+, and NMNAT2 cKO; Snull/Snull mice at P16/21, and P90. Number of mice: P16/21, 61 ctrl, 34 NMNAT2 cKO; Snull/+, 24 NMNAT2 cKO; Snull/Snull; P90, 11 ctrl, 7 NMNAT2 cKO; Snull/+, 5 NMNAT2 cKO; Snull/Snull. Kruskal-Wallis test with a two-stage linear step-up procedure of Benjamini, Krieger, and Yekutieli was used for P16/21 data, ****p<0.0001. One-way ANOVA with a two-stage linear step-up procedure of Benjamini, Krieger, and Yekutieli was used for P90 data, (ctrl vs. NMNAT2 cKO; Snull/+), ***, p= 0.0004, q=0.0004; (NMNAT2 cKO; Snull/+ vs. NMNAT2 cKO; Snull/Snull), **, p= 0.0039, q= 0.002. (C) Hindlimb clasping phenotype in ctrl, NMNAT2 cKO; Snull/+, and NMNAT2 cKO; Snull/Snull mice of 3-5 months old (ctrl N=13, cKO; Snull/+ N=20, cKO; Snull/Snull N=6). Kruskal-Wallis test was used, ***p<0.0005, ****p<0.0001. All above data represents mean± SEM. Fig. 7-S2. APP accumulation in P5 brains of NMNAT2 cKO mice lacking one or two copies of SARM1 (A) Immunohistology images showing APP accumulation in the corpus callosum, fimbria, and striatum of P5 NMNAT2 cKO; Snull/+, but not in ctrl and NMNAT2 cKO; Snull/Snull brains. (B) Normalized APP signals in P5 brains. Number of mice: 4 ctrl, 4 NMNAT2 cKO; Snull/+, 3 NMNAT2 cKO; Snull/Snull. CC, corpus callosum; Str, striatum; Fim, Fimbria. For CC, one-way ANOVA with a two-stage linear step-up procedure of Benjamini, Krieger, and Yekutieli was used (Ctrl vs. NMNAT2 cKO; Snull/+) **, p=0.0032, q= 0.0021; (NMNAT2 cKO; Snull/+ vs. NMNAT2 cKO; Snull/Snull) **, p=0.004, q=0.0021. For Str, Kruskal-Wallis test with a two-stage linear step-up procedure of Benjamini, Krieger, and Yekutieli was used, (Ctrl vs. NMNAT2 cKO; Snull/+) *, p=0.0142, q=0.0298; (NMNAT2 cKO; Snull/+ vs. NMNAT2 cKO; Snull/Snull) *, p=0.0414, q= 0.0435. For Fim, one-way ANOVA with a two-stage linear step-up procedure of Benjamini, Krieger, and Yekutieli was used, ****p<0.0001. All above data represent mean ± SEM. Fig 8-S1. SARM1 knockdown validation (A) RT-qPCR quantification of sarm1 mRNA levels across ASO treatments in primary mouse cortical neurons. Cells were treated for 2 days (DIV5-7) with a control or a SARM1 ASO (33 or 47) at two different concentrations to test efficacy. Number of wells: 6 ctrl; 2 ASO33 at 1 uM or 5 uM, 2 ASO47 at 5 uM or 10 uM. (B) Representative western blotting detecting SARM1 and GAPDH (internal control) protein levels at DIV8 after ASO treatment since DIV6. Brain lysates of SARM1 WT and SARM1-null (Snull/Snull) mice were included for antibody specificity validation. (C) Quantification of SARM1 protein level across various ASO treatment duration, as detected by western blot. SARM1 protein level was normalized to GAPDH first, then normalized to its corresponding ctrl-ASO-treated group. Numbers of wells: 2 days Sarm1 ASO, n=3. 2 days Ctrl ASO, 3 days Ctrl ASO, 3 days Sarm1 ASO, 5 days Ctrl ASO, 5 days Sarm1 ASO, n=4. 8 days Ctrl ASO, 8 days Sarm1 ASO, 9 days Ctrl ASO, 9 days Sarm1 ASO, n=6. 7 days Ctrl ASO, 7 days Sarm1 ASO, n=8. 4 days Ctrl ASO, 4 days Sarm1 ASO, n=12. Statistics were conducted with the Kruskal-Wallis test and corrected for multiple comparisons by controlling the False Discovery Rate using the Two-stage step-up method of Benjamin, Krieger, and Yekutieli. All above data represent mean ± SEM. *, p<0.05, ***, p<0.001, ****, p<0.0001. Fig. 8-S2. SARM1 knockdown prevents APP accumulation and axon degeneration in NMNAT2 KO neurons in vitro (A) Representative images illustrating the effects of ctrl-ASO and SARM1-ASO on APP accumulation in WT and NMNAT2 KO primary cultured cortical neurons. (B) Quantification of APP accumulation area following ASO treatment of two timelines: DIV1 to DIV14 and DIV5 to DIV14. (C) Representative images show TUJ1 axonal staining of WT and KO cells following treatment with ctrl-ASO and SARM1-ASO. (D) Percentage of the total TUJ1 area found as aggregates following control and SARM1 ASO (ASO 33) treatments at DIV1-14 and DIV5-14. (E) Representative images of DAPI staining and quantification (F) of live cells in every sampled image. Image numbers: DIV1-14, N=6 for every group, from 2 independent experiments; DIV5-14, N=9 for every group, from 3 independent experiments. 3 images were collected from each experimental batch. Data for panel D, DIV5-14 was not normally distributed, so a Kruskal-Wallis test multiple comparisons test was performed. All other data sets were analyzed using two-way ANOVA and Tukey multiple comparisons. All above data represent mean ± SEM. ***, p<0.001, ****, p<0.0001. Fig 8-S3. SARM1 knockdown rescues global NAD deficiency and distal axonal NAD+/NADH ratio in NMNAT2 KO neurons in vitro (A-C) Levels of NAD+ (A), NADH (B), and the calculated NAD+/NADH ratios (C), in DIV8 WT and KO cortical neurons treated with PBS, ctrl-ASO (ASO30), or SARM1-ASO (ASO33) from DIV1-8. For ASO treated groups, the raw concentration is normalized to the corresponding protein amounts, and then normalized as percentages to the average value acquired from ctrl-ASO treated WT neurons. For PBS treated groups, the raw concentration is normalized to the corresponding protein amounts, and then normalized to WT average value. N represents each well of culture lysate from 2-3 independent culture experiments, WT+PBS (N=16), KO+PBS (N=16), WT+ASO30 (N=23), KO+ASO30 (N=23), WT+ASO33 (N=23), KO+ASO33 (N=23). Kruskal-Wallis test with Dunn's multiple comparisons test was used. (D) Representative images showing signals emitted from the SoNar (NAD+/NADH) ratiometric sensor for NAD+ and NADH in distal axons of DIV 8 WT or KO cortical neurons treated with ctrl-ASO or SARM1-ASO. Scale bar, 20 mm. (E) F488/F440 ratiometric measurements of the SoNar sensor, reflecting the NAD+/NADH ratios in distal axons of DIV8 WT or KO cortical neurons treated with ctrl-ASO or SARM1-ASO since DIV1. N represents the number of neurons imaged from two independent cultures, WT+ctrl-ASO (N=17), KO+ctrl-ASO (N=21), WT+SARM1-ASO (N=20), KO+SARM1-ASO (N=23). Two-way ANOVA with Tukey's multiple comparisons test was used. All data represent mean ± SEM. *p<0.05, **p<0.01, ****p<0.0001
Acknowledgements
We thank the following: Dr. Yuyu Song for helpful discussions; Light Microscopy Imaging Center and Dr. Jim Powers in Indiana University Bloomington for live cell imaging (supported by NIH1S10OD024988); Gene vector core directed by Dr. Kazu Oka at Baylor College of Medicine for lentivirus production; Dr. Yi Yang for providing SoNar sensor; Drs. Feng Guo, István Katona for sharing equipment; Dr. Ken Mackie for pCAG-mCherry plasmid and reading the manuscript; László Barna, John Reinhart, Scott Barton, Theo Knowles, Caliel D. Hines and Kelsie Godsey for technical assistance; Mr. Jason Fu for motor behavioral quantification in a blinded fashion; Dr. JangDong Seo in Indiana statistical consulting center for statistical assistance.
Authors’ contributions
Conceptualization: SY, HCL., VR, JMT, JYH. Experimentation/Investigation: SY, ZXN, JL, AE Data acquisition: SY, ZXN, JL, AE Data curation: SY, VR, ZXN, AE Methodology: VR, JYH, PJN, KL, JG, MPC Writing: SY, ZXN, AE, VR, HCL Funding acquisition: HCL Resource: HCL, VR Supervision: HCL, VR.
Funding
This work was supported by National Institutes of Health grant NINDS NS086794 (H.C.L), NIGMS R35GM119557 (J.M.T.), Max Planck Society (V.R.), Indiana Clinical and Translational Sciences Institute funded by National Institutes of Health UL1TR002529 (Indiana University Research Community).
Availability of data and materials
All data are available in the main text or the supplementary materials. Material will be provided upon request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
All authors declare they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Sen Yang and Zhen-Xian Niou contributed equally to this work.
References
- 1.Harris JJ, Attwell D. The energetics of CNS white matter. J Neurosci. 2012;32:356–371. doi: 10.1523/JNEUROSCI.3430-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yellen G. Fueling thought: Management of glycolysis and oxidative phosphorylation in neuronal metabolism. J Cell Biol. 2018;217:2235–2246. doi: 10.1083/jcb.201803152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shokhirev MN, Johnson AA. An integrative machine-learning meta-analysis of high-throughput omics data identifies age-specific hallmarks of Alzheimer's disease. Ageing Res Rev. 2022;81:101721. doi: 10.1016/j.arr.2022.101721. [DOI] [PubMed] [Google Scholar]
- 4.Roy M, Rheault F, Croteau E, Castellano CA, Fortier M, St-Pierre V, Houde JC, Turcotte EE, Bocti C, Fulop T, et al. Fascicle- and Glucose-Specific Deterioration in White Matter Energy Supply in Alzheimer's Disease. J Alzheimers Dis. 2020;76:863–881. doi: 10.3233/JAD-200213. [DOI] [PubMed] [Google Scholar]
- 5.Wang M, Liu K, Pan J, Li J, Sun P, Zhang Y, Li L, Guo W, Xin Q, Zhao Z, et al. Brain-wide projection reconstruction of single functionally defined neurons. Nat Commun. 2022;13:1531. doi: 10.1038/s41467-022-29229-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Winnubst J, Bas E, Ferreira TA, Wu Z, Economo MN, Edson P, Arthur BJ, Bruns C, Rokicki K, Schauder D, et al. Reconstruction of 1,000 Projection Neurons Reveals New Cell Types and Organization of Long-Range Connectivity in the Mouse Brain. Cell. 2019;179(268–281):e213. doi: 10.1016/j.cell.2019.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peng H, Xie P, Liu L, Kuang X, Wang Y, Qu L, Gong H, Jiang S, Li A, Ruan Z, et al. Morphological diversity of single neurons in molecularly defined cell types. Nature. 2021;598:174–181. doi: 10.1038/s41586-021-03941-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coleman MP. The challenges of axon survival: introduction to the special issue on axonal degeneration. Exp Neurol. 2013;246:1–5. doi: 10.1016/j.expneurol.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 9.Zhang J, Long B, Li A, Sun Q, Tian J, Luo T, Ding Z, Gong H, Li X. Whole-Brain Three-Dimensional Profiling Reveals Brain Region Specific Axon Vulnerability in 5xFAD Mouse Model. Front Neuroanat. 2020;14:608177. doi: 10.3389/fnana.2020.608177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stokin GB, Lillo C, Falzone TL, Brusch RG, Rockenstein E, Mount SL, Raman R, Davies P, Masliah E, Williams DS. Axonopathy and transport deficits early in the pathogenesis of Alzheimer's disease. Science. 2005;307:1282–1288. doi: 10.1126/science.1105681. [DOI] [PubMed] [Google Scholar]
- 11.Fischer LR, Culver DG, Tennant P, Davis AA, Wang M, Castellano-Sanchez A, Khan J, Polak MA, Glass JD. Amyotrophic lateral sclerosis is a distal axonopathy: evidence in mice and man. Exp Neurol. 2004;185:232–240. doi: 10.1016/j.expneurol.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 12.Xiao AW, He J, Wang Q, Luo Y, Sun Y, Zhou YP, Guan Y, Lucassen PJ, Dai JP. The origin and development of plaques and phosphorylated tau are associated with axonopathy in Alzheimer's disease. Neurosci Bull. 2011;27:287–299. doi: 10.1007/s12264-011-1736-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones DP, Sies H. The Redox Code. Antioxid Redox Signal. 2015;23:734–746. doi: 10.1089/ars.2015.6247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berger F, Lau C, Dahlmann M, Ziegler M. Subcellular compartmentation and differential catalytic properties of the three human nicotinamide mononucleotide adenylyltransferase isoforms. J Biol Chem. 2005;280:36334–36341. doi: 10.1074/jbc.M508660200. [DOI] [PubMed] [Google Scholar]
- 15.Raffaelli N, Sorci L, Amici A, Emanuelli M, Mazzola F, Magni G. Identification of a novel human nicotinamide mononucleotide adenylyltransferase. Biochem Biophys Res Commun. 2002;297:835–840. doi: 10.1016/S0006-291X(02)02285-4. [DOI] [PubMed] [Google Scholar]
- 16.Yan T, Feng Y, Zheng J, Ge X, Zhang Y, Wu D, Zhao J, Zhai Q. Nmnat2 delays axon degeneration in superior cervical ganglia dependent on its NAD synthesis activity. Neurochem Int. 2010;56:101–106. doi: 10.1016/j.neuint.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 17.Coleman MP, Hoke A. Programmed axon degeneration: from mouse to mechanism to medicine. Nat Rev Neurosci. 2020;21:183–196. doi: 10.1038/s41583-020-0269-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gerdts J, Summers DW, Milbrandt J, DiAntonio A. Axon Self-Destruction: New Links among SARM1, MAPKs, and NAD+ Metabolism. Neuron. 2016;89:449–460. doi: 10.1016/j.neuron.2015.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Simon DJ, Watkins TA. Therapeutic opportunities and pitfalls in the treatment of axon degeneration. Curr Opin Neurol. 2018;31:693–701. doi: 10.1097/WCO.0000000000000621. [DOI] [PubMed] [Google Scholar]
- 20.Gilley J, Coleman MP. Endogenous Nmnat2 is an essential survival factor for maintenance of healthy axons. PLoS Biol. 2010;8:e1000300. doi: 10.1371/journal.pbio.1000300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ali YO, Allen HM, Yu L, Li-Kroeger D, Bakhshizadehmahmoudi D, Hatcher A, McCabe C, Xu J, Bjorklund N, Taglialatela G, et al. NMNAT2:HSP90 Complex Mediates Proteostasis in Proteinopathies. PLoS Biol. 2016;14:e1002472. doi: 10.1371/journal.pbio.1002472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bennett JP, Keeney PM. RNA-Sequencing Reveals Similarities and Differences in Gene Expression in Vulnerable Brain Tissues of Alzheimer's and Parkinson's Diseases. J Alzheimers Dis Rep. 2018;2:129–137. doi: 10.3233/ADR-180072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ali YO, Li-Kroeger D, Bellen HJ, Zhai RG, Lu HC. NMNATs, evolutionarily conserved neuronal maintenance factors. Trends Neurosci. 2013;36:632–640. doi: 10.1016/j.tins.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brazill JM, Li C, Zhu Y, Zhai RG. NMNAT: It's an NAD(+) synthase... It's a chaperone... It's a neuroprotector. Curr Opin Genet Dev. 2017;44:156–162. doi: 10.1016/j.gde.2017.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Milde S, Gilley J, Coleman MP. Subcellular localization determines the stability and axon protective capacity of axon survival factor Nmnat2. PLoS Biol. 2013;11:e1001539. doi: 10.1371/journal.pbio.1001539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hung CO, Coleman MP. KIF1A mediates axonal transport of BACE1 and identification of independently moving cargoes in living SCG neurons. Traffic. 2016;17:1155–1167. doi: 10.1111/tra.12428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guedes-Dias P, Holzbaur ELF: Axonal transport: Driving synaptic function. Science 2019, 366. [DOI] [PMC free article] [PubMed]
- 28.Sleigh JN, Rossor AM, Fellows AD, Tosolini AP, Schiavo G. Axonal transport and neurological disease. Nat Rev Neurol. 2019;15:691–703. doi: 10.1038/s41582-019-0257-2. [DOI] [PubMed] [Google Scholar]
- 29.Pacelli C, Giguere N, Bourque MJ, Levesque M, Slack RS, Trudeau LE. Elevated Mitochondrial Bioenergetics and Axonal Arborization Size Are Key Contributors to the Vulnerability of Dopamine Neurons. Curr Biol. 2015;25:2349–2360. doi: 10.1016/j.cub.2015.07.050. [DOI] [PubMed] [Google Scholar]
- 30.Gallo G. The bioenergetics of neuronal morphogenesis and regeneration: Frontiers beyond the mitochondrion. Dev Neurobiol. 2020;80:263–276. doi: 10.1002/dneu.22776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chamberlain KA, Sheng ZH. Mechanisms for the maintenance and regulation of axonal energy supply. J Neurosci Res. 2019;97:897–913. doi: 10.1002/jnr.24411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zala D, Hinckelmann MV, Yu H. Lyra da Cunha MM, Liot G, Cordelieres FP, Marco S, Saudou F: Vesicular glycolysis provides on-board energy for fast axonal transport. Cell. 2013;152:479–491. doi: 10.1016/j.cell.2012.12.029. [DOI] [PubMed] [Google Scholar]
- 33.Rangaraju V, Calloway N, Ryan TA. Activity-driven local ATP synthesis is required for synaptic function. Cell. 2014;156:825–835. doi: 10.1016/j.cell.2013.12.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Faitg J, Lacefield C, Davey T, White K, Laws R, Kosmidis S, Reeve AK, Kandel ER, Vincent AE, Picard M. 3D neuronal mitochondrial morphology in axons, dendrites, and somata of the aging mouse hippocampus. Cell Rep. 2021;36:109509. doi: 10.1016/j.celrep.2021.109509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Santuy A, Turegano-Lopez M, Rodriguez JR, Alonso-Nanclares L, DeFelipe J, Merchan-Perez A. A Quantitative Study on the Distribution of Mitochondria in the Neuropil of the Juvenile Rat Somatosensory Cortex. Cereb Cortex. 2018;28:3673–3684. doi: 10.1093/cercor/bhy159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schnitzer MJ, Block SM. Kinesin hydrolyses one ATP per 8-nm step. Nature. 1997;388:386–390. doi: 10.1038/41111. [DOI] [PubMed] [Google Scholar]
- 37.Gilley J, Adalbert R, Yu G, Coleman MP. Rescue of peripheral and CNS axon defects in mice lacking NMNAT2. J Neurosci. 2013;33:13410–13424. doi: 10.1523/JNEUROSCI.1534-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goebbels S, Bormuth I, Bode U, Hermanson O, Schwab MH, Nave KA. Genetic targeting of principal neurons in neocortex and hippocampus of NEX-Cre mice. Genesis. 2006;44:611–621. doi: 10.1002/dvg.20256. [DOI] [PubMed] [Google Scholar]
- 39.Hicks AN, Lorenzetti D, Gilley J, Lu B, Andersson KE, Miligan C, Overbeek PA, Oppenheim R, Bishop CE. Nicotinamide mononucleotide adenylyltransferase 2 (Nmnat2) regulates axon integrity in the mouse embryo. PLoS ONE. 2012;7:e47869. doi: 10.1371/journal.pone.0047869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim Y, Zhou P, Qian L, Chuang JZ, Lee J, Li C, Iadecola C, Nathan C, Ding A. MyD88-5 links mitochondria, microtubules, and JNK3 in neurons and regulates neuronal survival. J Exp Med. 2007;204:2063–2074. doi: 10.1084/jem.20070868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chou AH, Yeh TH, Ouyang P, Chen YL, Chen SY, Wang HL. Polyglutamine-expanded ataxin-3 causes cerebellar dysfunction of SCA3 transgenic mice by inducing transcriptional dysregulation. Neurobiol Dis. 2008;31:89–101. doi: 10.1016/j.nbd.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 42.Lieu CA, Chinta SJ, Rane A, Andersen JK. Age-related behavioral phenotype of an astrocytic monoamine oxidase-B transgenic mouse model of Parkinson's disease. PLoS ONE. 2013;8:e54200. doi: 10.1371/journal.pone.0054200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang X, Zhang Q, Bao R, Zhang N, Wang Y, Polo-Parada L, Tarim A, Alemifar A, Han X, Wilkins HM, et al. Deletion of Nampt in Projection Neurons of Adult Mice Leads to Motor Dysfunction, Neurodegeneration, and Death. Cell Rep. 2017;20:2184–2200. doi: 10.1016/j.celrep.2017.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guyenet SJ, Furrer SA, Damian VM, Baughan TD, La Spada AR, Garden GA: A simple composite phenotype scoring system for evaluating mouse models of cerebellar ataxia. J Vis Exp 2010. [DOI] [PMC free article] [PubMed]
- 45.Currinn H, Guscott B, Balklava Z, Rothnie A, Wassmer T. APP controls the formation of PI(3,5)P(2) vesicles through its binding of the PIKfyve complex. Cell Mol Life Sci. 2016;73:393–408. doi: 10.1007/s00018-015-1993-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Galiano MR, Jha S, Ho TS, Zhang C, Ogawa Y, Chang KJ, Stankewich MC, Mohler PJ, Rasband MN. A distal axonal cytoskeleton forms an intra-axonal boundary that controls axon initial segment assembly. Cell. 2012;149:1125–1139. doi: 10.1016/j.cell.2012.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kitay BM, McCormack R, Wang Y, Tsoulfas P, Zhai RG. Mislocalization of neuronal mitochondria reveals regulation of Wallerian degeneration and NMNAT/WLD(S)-mediated axon protection independent of axonal mitochondria. Hum Mol Genet. 2013;22:1601–1614. doi: 10.1093/hmg/ddt009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhao Y, Hu Q, Cheng F, Su N, Wang A, Zou Y, Hu H, Chen X, Zhou HM, Huang X, et al. SoNar, a Highly Responsive NAD+/NADH Sensor, Allows High-Throughput Metabolic Screening of Anti-tumor Agents. Cell Metab. 2015;21:777–789. doi: 10.1016/j.cmet.2015.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cambronne XA, Stewart ML, Kim D, Jones-Brunette AM, Morgan RK, Farrens DL, Cohen MS, Goodman RH. Biosensor reveals multiple sources for mitochondrial NAD(+) Science. 2016;352:1474–1477. doi: 10.1126/science.aad5168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fletcher TL, Cameron P, De Camilli P, Banker G. The distribution of synapsin I and synaptophysin in hippocampal neurons developing in culture. J Neurosci. 1991;11:1617–1626. doi: 10.1523/JNEUROSCI.11-06-01617.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen CY, Lin CW, Chang CY, Jiang ST, Hsueh YP. Sarm1, a negative regulator of innate immunity, interacts with syndecan-2 and regulates neuronal morphology. J Cell Biol. 2011;193:769–784. doi: 10.1083/jcb.201008050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Clements RT, Fuller LE, Kraemer KR, Radomski SA, Hunter-Chang S, Hall WC, Kalantar AA, Kraemer BR: Quantification of Neurite Degeneration with Enhanced Accuracy and Efficiency in an In Vitro Model of Parkinson's Disease. eNeuro 2022, 9. [DOI] [PMC free article] [PubMed]
- 53.Wang CL, Zhang L, Zhou Y, Zhou J, Yang XJ, Duan SM, Xiong ZQ, Ding YQ. Activity-dependent development of callosal projections in the somatosensory cortex. J Neurosci. 2007;27:11334–11342. doi: 10.1523/JNEUROSCI.3380-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fame RM, MacDonald JL, Macklis JD. Development, specification, and diversity of callosal projection neurons. Trends Neurosci. 2011;34:41–50. doi: 10.1016/j.tins.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stokin GB, Lillo C, Falzone TL, Brusch RG, Rockenstein E, Mount SL, Raman R, Davies P, Masliah E, Williams DS, Goldstein LS. Axonopathy and transport deficits early in the pathogenesis of Alzheimer's disease. Science. 2005;307:1282–1288. doi: 10.1126/science.1105681. [DOI] [PubMed] [Google Scholar]
- 56.Fu MM, Holzbaur EL. JIP1 regulates the directionality of APP axonal transport by coordinating kinesin and dynein motors. J Cell Biol. 2013;202:495–508. doi: 10.1083/jcb.201302078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gunawardena S, Goldstein LS. Disruption of axonal transport and neuronal viability by amyloid precursor protein mutations in Drosophila. Neuron. 2001;32:389–401. doi: 10.1016/S0896-6273(01)00496-2. [DOI] [PubMed] [Google Scholar]
- 58.Kamal A, Stokin GB, Yang Z, Xia CH, Goldstein LS. Axonal transport of amyloid precursor protein is mediated by direct binding to the kinesin light chain subunit of kinesin-I. Neuron. 2000;28:449–459. doi: 10.1016/S0896-6273(00)00124-0. [DOI] [PubMed] [Google Scholar]
- 59.Koo EH, Sisodia SS, Archer DR, Martin LJ, Weidemann A, Beyreuther K, Fischer P, Masters CL, Price DL. Precursor of amyloid protein in Alzheimer disease undergoes fast anterograde axonal transport. Proc Natl Acad Sci U S A. 1990;87:1561–1565. doi: 10.1073/pnas.87.4.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lazarov O, Morfini GA, Lee EB, Farah MH, Szodorai A, DeBoer SR, Koliatsos VE, Kins S, Lee VM, Wong PC, et al. Axonal transport, amyloid precursor protein, kinesin-1, and the processing apparatus: revisited. J Neurosci. 2005;25:2386–2395. doi: 10.1523/JNEUROSCI.3089-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang P, Yang G, Mosier DR, Chang P, Zaidi T, Gong YD, Zhao NM, Dominguez B, Lee KF, Gan WB, Zheng H. Defective neuromuscular synapses in mice lacking amyloid precursor protein (APP) and APP-Like protein 2. J Neurosci. 2005;25:1219–1225. doi: 10.1523/JNEUROSCI.4660-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Klevanski M, Herrmann U, Weyer SW, Fol R, Cartier N, Wolfer DP, Caldwell JH, Korte M, Muller UC. The APP Intracellular Domain Is Required for Normal Synaptic Morphology, Synaptic Plasticity, and Hippocampus-Dependent Behavior. J Neurosci. 2015;35:16018–16033. doi: 10.1523/JNEUROSCI.2009-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Muller UC, Deller T, Korte M. Not just amyloid: physiological functions of the amyloid precursor protein family. Nat Rev Neurosci. 2017;18:281–298. doi: 10.1038/nrn.2017.29. [DOI] [PubMed] [Google Scholar]
- 64.Wirths O, Weis J, Kayed R, Saido TC, Bayer TA. Age-dependent axonal degeneration in an Alzheimer mouse model. Neurobiol Aging. 2007;28:1689–1699. doi: 10.1016/j.neurobiolaging.2006.07.021. [DOI] [PubMed] [Google Scholar]
- 65.Wirths O, Weis J, Szczygielski J, Multhaup G, Bayer TA. Axonopathy in an APP/PS1 transgenic mouse model of Alzheimer's disease. Acta Neuropathol. 2006;111:312–319. doi: 10.1007/s00401-006-0041-4. [DOI] [PubMed] [Google Scholar]
- 66.Stone JR, Singleton RH, Povlishock JT. Antibodies to the C-terminus of the beta-amyloid precursor protein (APP): a site specific marker for the detection of traumatic axonal injury. Brain Res. 2000;871:288–302. doi: 10.1016/S0006-8993(00)02485-9. [DOI] [PubMed] [Google Scholar]
- 67.Hortobagyi T, Wise S, Hunt N, Cary N, Djurovic V, Fegan-Earl A, Shorrock K, Rouse D, Al-Sarraj S. Traumatic axonal damage in the brain can be detected using beta-APP immunohistochemistry within 35 min after head injury to human adults. Neuropathol Appl Neurobiol. 2007;33:226–237. doi: 10.1111/j.1365-2990.2006.00794.x. [DOI] [PubMed] [Google Scholar]
- 68.Ruhling S, Kramer F, Schmutz S, Amor S, Jiangshan Z, Schmitz C, Kipp M, Hochstrasser T. Visualization of the Breakdown of the Axonal Transport Machinery: a Comparative Ultrastructural and Immunohistochemical Approach. Mol Neurobiol. 2019;56:3984–3998. doi: 10.1007/s12035-018-1353-9. [DOI] [PubMed] [Google Scholar]
- 69.Linke R, Frotscher M. Development of the rat septohippocampal projection: tracing with DiI and electron microscopy of identified growth cones. J Comp Neurol. 1993;332:69–88. doi: 10.1002/cne.903320106. [DOI] [PubMed] [Google Scholar]
- 70.Sheth AN, McKee ML, Bhide PG. The sequence of formation and development of corticostriate connections in mice. Dev Neurosci. 1998;20:98–112. doi: 10.1159/000017306. [DOI] [PubMed] [Google Scholar]
- 71.Niou Z, Yang S, Sri A, Rodriquez H, Gilley J, Coleman MP, Lu H-C: NMNAT2 in cortical glutamatergic neurons exerts both cell and non-cell autonomous influences to shape cortical development and to maintain neuronal health. bioRxiv 2022:2022.2002.2005.479195.
- 72.Ikin AF, Annaert WG, Takei K, De Camilli P, Jahn R, Greengard P, Buxbaum JD. Alzheimer amyloid protein precursor is localized in nerve terminal preparations to Rab5-containing vesicular organelles distinct from those implicated in the synaptic vesicle pathway. J Biol Chem. 1996;271:31783–31786. doi: 10.1074/jbc.271.50.31783. [DOI] [PubMed] [Google Scholar]
- 73.Shapira M, Zhai RG, Dresbach T, Bresler T, Torres VI, Gundelfinger ED, Ziv NE, Garner CC. Unitary assembly of presynaptic active zones from Piccolo-Bassoon transport vesicles. Neuron. 2003;38:237–252. doi: 10.1016/S0896-6273(03)00207-1. [DOI] [PubMed] [Google Scholar]
- 74.Chiba K, Araseki M, Nozawa K, Furukori K, Araki Y, Matsushima T, Nakaya T, Hata S, Saito Y, Uchida S, et al. Quantitative analysis of APP axonal transport in neurons: role of JIP1 in enhanced APP anterograde transport. Mol Biol Cell. 2014;25:3569–3580. doi: 10.1091/mbc.e14-06-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shiff G, Morel N. Rapid anterograde axonal transport of the syntaxin-SNAP 25-VAMP complex. J Neurochem. 1997;68:1663–1667. doi: 10.1046/j.1471-4159.1997.68041663.x. [DOI] [PubMed] [Google Scholar]
- 76.Cai Q, Pan PY, Sheng ZH. Syntabulin-kinesin-1 family member 5B-mediated axonal transport contributes to activity-dependent presynaptic assembly. J Neurosci. 2007;27:7284–7296. doi: 10.1523/JNEUROSCI.0731-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Forman DS: Axonal transport of mitochondria. Axonal Transport 1987:155–163.
- 78.Forman DS, Lynch KJ, Smith RS. Organelle dynamics in lobster axons: anterograde, retrograde and stationary mitochondria. Brain Res. 1987;412:96–106. doi: 10.1016/0006-8993(87)91443-0. [DOI] [PubMed] [Google Scholar]
- 79.Boldogh IR, Pon LA. Mitochondria on the move. Trends Cell Biol. 2007;17:502–510. doi: 10.1016/j.tcb.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 80.Melkov A, Abdu U. Regulation of long-distance transport of mitochondria along microtubules. Cell Mol Life Sci. 2018;75:163–176. doi: 10.1007/s00018-017-2590-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Plucinska G, Misgeld T. Imaging of neuronal mitochondria in situ. Curr Opin Neurobiol. 2016;39:152–163. doi: 10.1016/j.conb.2016.06.006. [DOI] [PubMed] [Google Scholar]
- 82.Lautrup S, Sinclair DA, Mattson MP, Fang EF. NAD(+) in Brain Aging and Neurodegenerative Disorders. Cell Metab. 2019;30:630–655. doi: 10.1016/j.cmet.2019.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sasaki Y. Metabolic aspects of neuronal degeneration: From a NAD(+) point of view. Neurosci Res. 2019;139:9–20. doi: 10.1016/j.neures.2018.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Brown EE, Scandura MJ, Pierce EA. Expression of NMNAT1 in the photoreceptors is sufficient to prevent NMNAT1-associated retinal degeneration. Mol Ther Methods Clin Dev. 2023;29:319–28. 10.1016/j.omtm.2023.04.003, https://pubmed.ncbi.nlm.nih.gov/37214313/. [DOI] [PMC free article] [PubMed]
- 85.Hinckelmann MV, Virlogeux A, Niehage C, Poujol C, Choquet D, Hoflack B, Zala D, Saudou F. Self-propelling vesicles define glycolysis as the minimal energy machinery for neuronal transport. Nat Commun. 2016;7:13233. doi: 10.1038/ncomms13233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tang Y, Scott D, Das U, Gitler D, Ganguly A, Roy S. Fast vesicle transport is required for the slow axonal transport of synapsin. J Neurosci. 2013;33:15362–15375. doi: 10.1523/JNEUROSCI.1148-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ikemoto A, Bole DG, Ueda T. Glycolysis and glutamate accumulation into synaptic vesicles. Role of glyceraldehyde phosphate dehydrogenase and 3-phosphoglycerate kinase. J Biol Chem. 2003;278:5929–5940. doi: 10.1074/jbc.M211617200. [DOI] [PubMed] [Google Scholar]
- 88.Mayer PR, Huang N, Dewey CM, Dries DR, Zhang H, Yu G. Expression, localization, and biochemical characterization of nicotinamide mononucleotide adenylyltransferase 2. J Biol Chem. 2010;285:40387–40396. doi: 10.1074/jbc.M110.178913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Guedes-Dias P, Nirschl JJ, Abreu N, Tokito MK, Janke C, Magiera MM, Holzbaur ELF. Kinesin-3 Responds to Local Microtubule Dynamics to Target Synaptic Cargo Delivery to the Presynapse. Curr Biol. 2019;29(268–282):e268. doi: 10.1016/j.cub.2018.11.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cunnane SC, Trushina E, Morland C, Prigione A, Casadesus G, Andrews ZB, Beal MF, Bergersen LH, Brinton RD, de la Monte S, et al. Brain energy rescue: an emerging therapeutic concept for neurodegenerative disorders of ageing. Nat Rev Drug Discov. 2020;19:609–633. doi: 10.1038/s41573-020-0072-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Alano CC, Garnier P, Ying W, Higashi Y, Kauppinen TM, Swanson RA. NAD+ depletion is necessary and sufficient for poly(ADP-ribose) polymerase-1-mediated neuronal death. J Neurosci. 2010;30:2967–2978. doi: 10.1523/JNEUROSCI.5552-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang S, Xing Z, Vosler PS, Yin H, Li W, Zhang F, Signore AP, Stetler RA, Gao Y, Chen J. Cellular NAD replenishment confers marked neuroprotection against ischemic cell death: role of enhanced DNA repair. Stroke. 2008;39:2587–2595. doi: 10.1161/STROKEAHA.107.509158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Essuman K, Summers DW, Sasaki Y, Mao X, DiAntonio A, Milbrandt J. The SARM1 Toll/Interleukin-1 Receptor Domain Possesses Intrinsic NAD(+) Cleavage Activity that Promotes Pathological Axonal Degeneration. Neuron. 2017;93(1334–1343):e1335. doi: 10.1016/j.neuron.2017.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Angeletti C, Amici A, Gilley J, Loreto A, Trapanotto AG, Antoniou C, Merlini E, Coleman MP, Orsomando G. SARM1 is a multi-functional NAD(P)ase with prominent base exchange activity, all regulated by multiple physiologically relevant NAD metabolites. iScience. 2022;25:103812. doi: 10.1016/j.isci.2022.103812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Figley MD, Gu W, Nanson JD, Shi Y, Sasaki Y, Cunnea K, Malde AK, Jia X, Luo Z, Saikot FK, et al. SARM1 is a metabolic sensor activated by an increased NMN/NAD(+) ratio to trigger axon degeneration. Neuron. 2021;109(1118–1136):e1111. doi: 10.1016/j.neuron.2021.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Di Stefano M, Nascimento-Ferreira I, Orsomando G, Mori V, Gilley J, Brown R, Janeckova L, Vargas ME, Worrell LA, Loreto A, et al. A rise in NAD precursor nicotinamide mononucleotide (NMN) after injury promotes axon degeneration. Cell Death Differ. 2015;22:731–742. doi: 10.1038/cdd.2014.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gilley J, Orsomando G, Nascimento-Ferreira I, Coleman MP. Absence of SARM1 rescues development and survival of NMNAT2-deficient axons. Cell Rep. 2015;10:1974–1981. doi: 10.1016/j.celrep.2015.02.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gilley J, Ribchester RR, Coleman MP. Sarm1 Deletion, but Not WldS, Confers Lifelong Rescue in a Mouse Model of Severe Axonopathy. Cell Rep. 2017;21:10–16. doi: 10.1016/j.celrep.2017.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ashrafi G, de Juan-Sanz J, Farrell RJ, Ryan TA. Molecular Tuning of the Axonal Mitochondrial Ca(2+) Uniporter Ensures Metabolic Flexibility of Neurotransmission. Neuron. 2020;105(678–687):e675. doi: 10.1016/j.neuron.2019.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Osterloh JM, Yang J, Rooney TM, Fox AN, Adalbert R, Powell EH, Sheehan AE, Avery MA, Hackett R, Logan MA, et al. dSarm/Sarm1 is required for activation of an injury-induced axon death pathway. Science. 2012;337:481–484. doi: 10.1126/science.1223899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ko KW, Devault L, Sasaki Y, Milbrandt J, DiAntonio A: Live imaging reveals the cellular events downstream of SARM1 activation. Elife 2021, 10. [DOI] [PMC free article] [PubMed]
- 102.Waller TJ, Collins CA. Multifaceted roles of SARM1 in axon degeneration and signaling. Front Cell Neurosci. 2022;16:958900. doi: 10.3389/fncel.2022.958900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Li Y, Pazyra-Murphy MF, Avizonis D, de Sa Tavares Russo M, Tang S, Chen CY, Hsueh YP, Bergholz JS, Jiang T, Zhao JJ, et al: Sarm1 activation produces cADPR to increase intra-axonal Ca++ and promote axon degeneration in PIPN. J Cell Biol 2022, 221. [DOI] [PMC free article] [PubMed]
- 104.Bellon A, Mann F. Keeping up with advances in axon guidance. Curr Opin Neurobiol. 2018;53:183–191. doi: 10.1016/j.conb.2018.09.004. [DOI] [PubMed] [Google Scholar]
- 105.Ketschek A, Sainath R, Holland S, Gallo G. The Axonal Glycolytic Pathway Contributes to Sensory Axon Extension and Growth Cone Dynamics. J Neurosci. 2021;41:6637–6651. doi: 10.1523/JNEUROSCI.0321-21.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ketschek A, Holland SM, Gallo G. SARM1 Suppresses Axon Branching Through Attenuation of Axonal Cytoskeletal Dynamics. Front Mol Neurosci. 2022;15:726962. doi: 10.3389/fnmol.2022.726962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Izadifar A, Courchet J, Virga DM, Verreet T, Hamilton S, Ayaz D, Misbaer A, Vandenbogaerde S, Monteiro L, Petrovic M, et al. Axon morphogenesis and maintenance require an evolutionary conserved safeguard function of Wnk kinases antagonizing Sarm and Axed. Neuron. 2021;109(2864–2883):e2868. doi: 10.1016/j.neuron.2021.07.006. [DOI] [PubMed] [Google Scholar]
- 108.Turner NL, Macrina T, Bae JA, Yang R, Wilson AM, Schneider-Mizell C, Lee K, Lu R, Wu J, Bodor AL, et al: Multiscale and multimodal reconstruction of cortical structure and function. bioRxiv 2020:2020.2010.2014.338681.
- 109.Lewis TL, Jr, Turi GF, Kwon SK, Losonczy A, Polleux F. Progressive Decrease of Mitochondrial Motility during Maturation of Cortical Axons In Vitro and In Vivo. Curr Biol. 2016;26:2602–2608. doi: 10.1016/j.cub.2016.07.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Moutaux E, Christaller W, Scaramuzzino C, Genoux A, Charlot B, Cazorla M, Saudou F. Neuronal network maturation differently affects secretory vesicles and mitochondria transport in axons. Sci Rep. 2018;8:13429. doi: 10.1038/s41598-018-31759-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhou B, Yu P, Lin MY, Sun T, Chen Y, Sheng ZH. Facilitation of axon regeneration by enhancing mitochondrial transport and rescuing energy deficits. J Cell Biol. 2016;214:103–119. doi: 10.1083/jcb.201605101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cagin U, Duncan OF, Gatt AP, Dionne MS, Sweeney ST, Bateman JM. Mitochondrial retrograde signaling regulates neuronal function. Proc Natl Acad Sci U S A. 2015;112:E6000–6009. doi: 10.1073/pnas.1505036112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ibanez CF. Message in a bottle: long-range retrograde signaling in the nervous system. Trends Cell Biol. 2007;17:519–528. doi: 10.1016/j.tcb.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 114.Das U, Scott DA, Ganguly A, Koo EH, Tang Y, Roy S. Activity-induced convergence of APP and BACE-1 in acidic microdomains via an endocytosis-dependent pathway. Neuron. 2013;79:447–460. doi: 10.1016/j.neuron.2013.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chidlow G, Ebneter A, Wood JP, Casson RJ. The optic nerve head is the site of axonal transport disruption, axonal cytoskeleton damage and putative axonal regeneration failure in a rat model of glaucoma. Acta Neuropathol. 2011;121:737–751. doi: 10.1007/s00401-011-0807-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Burke S, Trudeau LE: Axonal Domain Structure as a Putative Identifier of Neuron-Specific Vulnerability to Oxidative Stress in Cultured Neurons. eNeuro 2022, 9. [DOI] [PMC free article] [PubMed]
- 117.Giguere N, Delignat-Lavaud B, Herborg F, Voisin A, Li Y, Jacquemet V, Anand-Srivastava M, Gether U, Giros B, Trudeau LE. Increased vulnerability of nigral dopamine neurons after expansion of their axonal arborization size through D2 dopamine receptor conditional knockout. PLoS Genet. 2019;15:e1008352. doi: 10.1371/journal.pgen.1008352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Guo W, Stoklund Dittlau K, Van Den Bosch L. Axonal transport defects and neurodegeneration: Molecular mechanisms and therapeutic implications. Semin Cell Dev Biol. 2020;99:133–150. doi: 10.1016/j.semcdb.2019.07.010. [DOI] [PubMed] [Google Scholar]
- 119.Baleriola J, Walker CA, Jean YY, Crary JF, Troy CM, Nagy PL, Hengst U. Axonally synthesized ATF4 transmits a neurodegenerative signal across brain regions. Cell. 2014;158:1159–1172. doi: 10.1016/j.cell.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lamberts JT, Hildebrandt EN, Brundin P. Spreading of alpha-synuclein in the face of axonal transport deficits in Parkinson's disease: a speculative synthesis. Neurobiol Dis. 2015;77:276–283. doi: 10.1016/j.nbd.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 121.Wen Y, Parrish JZ, He R, Zhai RG, Kim MD. Nmnat exerts neuroprotective effects in dendrites and axons. Mol Cell Neurosci. 2011;48:1–8. doi: 10.1016/j.mcn.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ji H, Sapar ML, Sarkar A, Wang B, Han C: Phagocytosis and self-destruction break down dendrites of Drosophila sensory neurons at distinct steps of Wallerian degeneration. Proc Natl Acad Sci U S A 2022, 119. [DOI] [PMC free article] [PubMed]
- 123.Wallimann T, Tokarska-Schlattner M, Schlattner U. The creatine kinase system and pleiotropic effects of creatine. Amino Acids. 2011;40:1271–1296. doi: 10.1007/s00726-011-0877-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zampese E, Wokosin DL, Gonzalez-Rodriguez P, Guzman JN, Tkatch T, Kondapalli J, Surmeier WC, D'Alessandro KB, De Stefani D, Rizzuto R, et al. Ca(2+) channels couple spiking to mitochondrial metabolism in substantia nigra dopaminergic neurons. Sci Adv. 2022;8:eabp8701. doi: 10.1126/sciadv.abp8701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Perez-Liebana I, Juaristi I, Gonzalez-Sanchez P, Gonzalez-Moreno L, Rial E, Podunavac M, Zakarian A, Molgo J, Vallejo-Illarramendi A, Mosqueira-Martin L, et al. A Ca(2+)-Dependent Mechanism Boosting Glycolysis and OXPHOS by Activating Aralar-Malate-Aspartate Shuttle, upon Neuronal Stimulation. J Neurosci. 2022;42:3879–3895. doi: 10.1523/JNEUROSCI.1463-21.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yu SB, Sanchez RG, Papich ZD, Whisenant TC, Ghassemian M, Koberstein JN, Stewart ML, Pekkurnaz G: Neuronal activity-driven O-GlcNAcylation promotes mitochondrial plasticity. bioRxiv 2023:2023.2001.2011.523512.
- 127.Pekkurnaz G, Trinidad JC, Wang X, Kong D, Schwarz TL. Glucose regulates mitochondrial motility via Milton modification by O-GlcNAc transferase. Cell. 2014;158:54–68. doi: 10.1016/j.cell.2014.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Li H, Guglielmetti C, Sei YJ, Zilberter M, Le Page LM, Shields L, Yang J, Nguyen K, Tiret B, Gao X, et al. Neurons require glucose uptake and glycolysis in vivo. Cell Rep. 2023;42:112335. doi: 10.1016/j.celrep.2023.112335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Mor DE, Sohrabi S, Kaletsky R, Keyes W, Tartici A, Kalia V, Miller GW, Murphy CT. Metformin rescues Parkinson's disease phenotypes caused by hyperactive mitochondria. Proc Natl Acad Sci U S A. 2020;117:26438–26447. doi: 10.1073/pnas.2009838117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Soman SK, Bazala M, Keatinge M, Bandmann O, Kuznicki J: Restriction of mitochondrial calcium overload by mcu inactivation renders a neuroprotective effect in zebrafish models of Parkinson's disease. Biol Open 2019, 8. [DOI] [PMC free article] [PubMed]
- 131.Calvo-Rodriguez M, Hou SS, Snyder AC, Kharitonova EK, Russ AN, Das S, Fan Z, Muzikansky A, Garcia-Alloza M, Serrano-Pozo A, et al. Increased mitochondrial calcium levels associated with neuronal death in a mouse model of Alzheimer's disease. Nat Commun. 2020;11:2146. doi: 10.1038/s41467-020-16074-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Murata H, Sakaguchi M, Kataoka K, Huh NH. SARM1 and TRAF6 bind to and stabilize PINK1 on depolarized mitochondria. Mol Biol Cell. 2013;24:2772–2784. doi: 10.1091/mbc.e13-01-0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Gerdts J, Summers DW, Sasaki Y, DiAntonio A, Milbrandt J. Sarm1-mediated axon degeneration requires both SAM and TIR interactions. J Neurosci. 2013;33:13569–13580. doi: 10.1523/JNEUROSCI.1197-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Murata H, Khine CC, Nishikawa A, Yamamoto KI, Kinoshita R, Sakaguchi M. c-Jun N-terminal kinase (JNK)-mediated phosphorylation of SARM1 regulates NAD(+) cleavage activity to inhibit mitochondrial respiration. J Biol Chem. 2018;293:18933–18943. doi: 10.1074/jbc.RA118.004578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sato-Yamada Y, Strickland A, Sasaki Y, Bloom J, DiAntonio A, Milbrandt J: A SARM1-mitochondrial feedback loop drives neuropathogenesis in a Charcot-Marie-Tooth disease type 2A rat model. J Clin Invest 2022, 132. [DOI] [PMC free article] [PubMed]
- 136.Reiman EM, Caselli RJ, Yun LS, Chen K, Bandy D, Minoshima S, Thibodeau SN, Osborne D. Preclinical evidence of Alzheimer's disease in persons homozygous for the ε4 allele for apolipoprotein E. J New Engl J Med. 1996;334:752–758. doi: 10.1056/NEJM199603213341202. [DOI] [PubMed] [Google Scholar]
- 137.Ye F, Funk Q, Rockers E, Shulman JM, Masdeu JC, Pascual B. Alzheimer's Disease Neuroimaging I: In Alzheimer-prone brain regions, metabolism and risk-gene expression are strongly correlated. Brain Commun. 2022;4:fcac216. doi: 10.1093/braincomms/fcac216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ciarmiello A, Cannella M, Lastoria S, Simonelli M, Frati L, Rubinsztein DC, Squitieri F. Brain white-matter volume loss and glucose hypometabolism precede the clinical symptoms of Huntington's disease. J Nucl Med. 2006;47:215–222. [PubMed] [Google Scholar]
- 139.Diehl-Schmid J, Licata A, Goldhardt O, Forstl H, Yakushew I, Otto M, Anderl-Straub S, Beer A, Ludolph AC, Landwehrmeyer GB, et al. FDG-PET underscores the key role of the thalamus in frontotemporal lobar degeneration caused by C9ORF72 mutations. Transl Psychiatry. 2019;9:54. doi: 10.1038/s41398-019-0381-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Cistaro A, Valentini MC, Chio A, Nobili F, Calvo A, Moglia C, Montuschi A, Morbelli S, Salmaso D, Fania P, et al. Brain hypermetabolism in amyotrophic lateral sclerosis: a FDG PET study in ALS of spinal and bulbar onset. Eur J Nucl Med Mol Imaging. 2012;39:251–259. doi: 10.1007/s00259-011-1979-6. [DOI] [PubMed] [Google Scholar]
- 141.Matthews DC, Lerman H, Lukic A, Andrews RD, Mirelman A, Wernick MN, Giladi N, Strother SC, Evans KC, Cedarbaum JM, Even-Sapir E. FDG PET Parkinson's disease-related pattern as a biomarker for clinical trials in early stage disease. Neuroimage Clin. 2018;20:572–579. doi: 10.1016/j.nicl.2018.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kalpouzos G, Chetelat G, Baron JC, Landeau B, Mevel K, Godeau C, Barre L, Constans JM, Viader F, Eustache F, Desgranges B. Voxel-based mapping of brain gray matter volume and glucose metabolism profiles in normal aging. Neurobiol Aging. 2009;30:112–124. doi: 10.1016/j.neurobiolaging.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 143.Oh H, Madison C, Baker S, Rabinovici G, Jagust W. Dynamic relationships between age, amyloid-beta deposition, and glucose metabolism link to the regional vulnerability to Alzheimer's disease. Brain. 2016;139:2275–2289. doi: 10.1093/brain/aww108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Krell-Roesch J, Syrjanen JA, Vassilaki M, Lowe VJ, Vemuri P, Mielke MM, Machulda MM, Stokin GB, Christianson TJ, Kremers WK, et al. Brain Regional Glucose Metabolism, Neuropsychiatric Symptoms, and the Risk of Incident Mild Cognitive Impairment: The Mayo Clinic Study of Aging. Am J Geriatr Psychiatry. 2021;29:179–191. doi: 10.1016/j.jagp.2020.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Baran TM, Lin FV. Alzheimer's Disease Neuroimaging I: Amyloid and FDG PET of Successful Cognitive Aging: Global and Cingulate-Specific Differences. J Alzheimers Dis. 2018;66:307–318. doi: 10.3233/JAD-180360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kelley CM, Ginsberg SD, Liang WS, Counts SE, Mufson EJ. Posterior cingulate cortex reveals an expression profile of resilience in cognitively intact elders. Brain Commun. 2022;4:fcac162. doi: 10.1093/braincomms/fcac162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Leech R, Sharp DJ. The role of the posterior cingulate cortex in cognition and disease. Brain. 2014;137:12–32. doi: 10.1093/brain/awt162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Crossley NA, Mechelli A, Scott J, Carletti F, Fox PT, McGuire P, Bullmore ET. The hubs of the human connectome are generally implicated in the anatomy of brain disorders. Brain. 2014;137:2382–2395. doi: 10.1093/brain/awu132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Yu M, Sporns O, Saykin AJ. The human connectome in Alzheimer disease - relationship to biomarkers and genetics. Nat Rev Neurol. 2021;17:545–563. doi: 10.1038/s41582-021-00529-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Hagmann P, Cammoun L, Gigandet X, Meuli R, Honey CJ, Wedeen VJ, Sporns O. Mapping the structural core of human cerebral cortex. PLoS Biol. 2008;6:e159. doi: 10.1371/journal.pbio.0060159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Mito R, Raffelt D, Dhollander T, Vaughan DN, Tournier JD, Salvado O, Brodtmann A, Rowe CC, Villemagne VL, Connelly A. Fibre-specific white matter reductions in Alzheimer's disease and mild cognitive impairment. Brain. 2018;141:888–902. doi: 10.1093/brain/awx355. [DOI] [PubMed] [Google Scholar]
- 152.Xu Z, Xia M, Wang X, Liao X, Zhao T, He Y. Meta-connectomic analysis maps consistent, reproducible, and transcriptionally relevant functional connectome hubs in the human brain. Commun Biol. 2022;5:1056. doi: 10.1038/s42003-022-04028-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Goyal MS, Vlassenko AG, Blazey TM, Su Y, Couture LE, Durbin TJ, Bateman RJ, Benzinger TL, Morris JC, Raichle ME. Loss of Brain Aerobic Glycolysis in Normal Human Aging. Cell Metab. 2017;26(353–360):e353. doi: 10.1016/j.cmet.2017.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Vlassenko AG, Gordon BA, Goyal MS, Su Y, Blazey TM, Durbin TJ, Couture LE, Christensen JJ, Jafri H, Morris JC, et al. Aerobic glycolysis and tau deposition in preclinical Alzheimer's disease. Neurobiol Aging. 2018;67:95–98. doi: 10.1016/j.neurobiolaging.2018.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Goyal MS, Blazey T, Metcalf NV, McAvoy MP, Strain J, Rahmani M, Durbin TJ, Xiong C, Benzinger TL-S, Morris JC, et al: Brain aerobic glycolysis and resilience in Alzheimer disease. bioRxiv 2022:2022.2006.2021.497006. [DOI] [PMC free article] [PubMed]
- 156.Hou Y, Wei Y, Lautrup S, Yang B, Wang Y, Cordonnier S, Mattson MP, Croteau DL, Bohr VA: NAD(+) supplementation reduces neuroinflammation and cell senescence in a transgenic mouse model of Alzheimer's disease via cGAS-STING. Proc Natl Acad Sci U S A 2021, 118. [DOI] [PMC free article] [PubMed]
- 157.Harlan BA, Killoy KM, Pehar M, Liu L, Auwerx J, Vargas MR. Evaluation of the NAD(+) biosynthetic pathway in ALS patients and effect of modulating NAD(+) levels in hSOD1-linked ALS mouse models. Exp Neurol. 2020;327:113219. doi: 10.1016/j.expneurol.2020.113219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Gautam M, Gunay A, Chandel NS, Ozdinler PH. Mitochondrial dysregulation occurs early in ALS motor cortex with TDP-43 pathology and suggests maintaining NAD(+) balance as a therapeutic strategy. Sci Rep. 2022;12:4287. doi: 10.1038/s41598-022-08068-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ljungberg MC, Ali YO, Zhu J, Wu CS, Oka K, Zhai RG, Lu HC. CREB-activity and nmnat2 transcription are down-regulated prior to neurodegeneration, while NMNAT2 over-expression is neuroprotective, in a mouse model of human tauopathy. Hum Mol Genet. 2012;21:251–267. doi: 10.1093/hmg/ddr492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Cheng XS, Shi FX, Zhao KP, Lin W, Li XY, Zhang J, Bu YY, Zhu R, Li XH, Duan DX, et al. Nmnat2 attenuates amyloidogenesis and up-regulates ADAM10 in AMPK activity-dependent manner. Aging (Albany NY) 2021;13:23620–23636. doi: 10.18632/aging.203634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Fang F, Zhuang P, Feng X, Liu P, Liu D, Huang H, Li L, Chen W, Liu L, Sun Y, et al. NMNAT2 is downregulated in glaucomatous RGCs, and RGC-specific gene therapy rescues neurodegeneration and visual function. Mol Ther. 2022;30:1421–1431. doi: 10.1016/j.ymthe.2022.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Ren MYY, Heng KHY, Ng LY, Chong CY, Ng YT, Gorur-Shandilya S, Lee RMQ, Lim KL, Zhang J, Koh TW. MED13 and glycolysis are conserved modifiers of α-synuclein-associated neurodegeneration. Cell Report. 2022;41(12):111852. doi: 10.1016/j.celrep.2022.111852. [DOI] [PubMed] [Google Scholar]
- 163.Manzo E, Lorenzini I, Barrameda D, O'Conner AG, Barrows JM, Starr A, Kovalik T, Rabichow BE, Lehmkuhl EM, Shreiner DD, et al: Glycolysis upregulation is neuroprotective as a compensatory mechanism in ALS. Elife 2019, 8. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Fig. 1-S1. NMNAT2 expression, cKO mice survival rate, and motor behavioral deficit (A) In situ hybridization reveals nmnat2 mRNAs (blue signal) in the E14.5 brain, mainly in the cortical plate (CP) and in the lateral ganglionic eminence (LGE) and medial ganglionic eminence (MGE). (B) Illustration of Nmnat2 conditional KO (cKO) mice generation as described previously [37]. The blue and magenta triangles indicate loxP sites. (C, D) Summary of embryonic and postnatal pups genotyping. For embryonic samples, the strategy was to breed Nex-Cre/+;NMNAT2f/+ with NMNAT2f/+ mice. The genotyping results conform to the expectations of Mendelian inheritance. The breeding strategy for postnatal pups was to cross Nex-Cre/+;NMNAT2f/+ with NMNAT2f/f mice. The genotyping result showed a smaller than expected number of mice with the Nex-Cre/+;NMNAT2f/f (cKO) genotype, suggesting a reduced survival rate for cKO mice after birth. (E) Overall numbers of postnatal male and female ctrl and cKO mice. (F) 3-5 months old NMNAT2 cKO mice exhibit evident hindlimb clasping behavior (ctrl N=3, cKO N=4 were scored), Mann-Whitney test was used, data represents mean ± SEM, *p=0.04. Abbreviations: Cre, Nex-Cre; f/f, NMNAT2f/f; IZ, intermediate zone; Str, striatum; SVZ, subventricular zone; VC, ventricle; VZ, ventricular. Fig. 2-S1. Loss of NMNAT2 triggers APP accumulation in the axon of cultured cortical neurons (A) Representative confocal image of APP immunocytochemical staining in ctrl and KO neuronal culture at DIV5, 8, 10, and 14. Scale bar 30 μm. Red arrows highlight APP accumulation. (B) Area of accumulated APP in cKO and ctrl mice normalized to the average ctrl value of the corresponding DIV. Image numbers used for analysis: DIV5: 10 ctrl, 9 KO; DIV8 & DIV10: 20 ctrl, 20 KO; DIV14: 50 ctrl, 67 KO. DIV5-10 were from 2 independent culture experiments and DIV14 was from > 3 independent experiments. Multiple Mann-Whitney test with Holm-Šídák post-hoc adjustment, KO was compared to ctrl of the same DIV, ***p=0.000202, ****p<0.0001. (C) Representative confocal images of APP, TUJ1 (βIII-tubulin), MAP2, and DAPI staining in DIV10 ctrl and KO neurons. Scale bar 20 μm. (D) Zoom-in view of axon and dendrite area highlighted by the white box in (C). Scale bar 5 μm. Fig. 2-S2. Gross neurite covering the area in vitro is not affected by Nmnat2 loss (A) Representative confocal images of MAP2, TUJ1, and DAPI staining in ctrl and KO neuronal culture at DIV10. MAP2 positive area corresponding to soma and dendrites was subtracted in TUJ1 staining images to determine area corresponding to axon regions. 100 μm scale bar in left and middle panels; 25 μm scale bar in zoom-in panels. (B) Quantification of the somatic and dendritic area labeled by MAP2 and the axonal area labeled by TUJ1 (MAP2 area subtracted) in ctrl and KO neuronal culture at DIV5, 8, 10, and 14, normalized to the average ctrl values at the same DIV. Image numbers from 1-3 independent experiments: DIV5: 10 ctrl, 10 KO; DIV8: 23 ctrl, 16 KO; DIV10: 11 ctrl, 13 KO; DIV14: 5 ctrl, 6 KO. Multiple Mann-Whitney tests with Holm-Šídák correction for comparisons were used for MAP2; two-way ANOVA with Šídák's multiple comparisons test was used for TUJ1. Data represent mean ± SEM. (C) The procedure of semi-automated quantification of accumulated APP by ImageJ. Fig. 2-S3. Loss of NMNAT2 after neurite outgrowth in vitro triggers axonal APP accumulation (A) The experimental timeline in NMNAT2f/f cortical neuronal culture. The NMNAT2 mRNA level was normalized to the LV-copGFP transduced ctrl group of the corresponding DIV and expressed as percentage. Numbers of culture wells from 1-2 independent batches of culture: DIV8: 2 copGFP, 2 iCre; DIV10 & 14: 4 copGFP, 4 iCre; (B) Representative confocal images of APP, TUJ1 and MAP2 staining in DIV10 NMNAT2f/f neurons that received lentivirus starting at DIV5, 30 μm scale bar. Zoom-in view of axon and dendrite area highlighted by magenta and orange box respectively in the upper panel, 10 μm scale bar. (C) The area of accumulated APP was normalized to the LV-copGFP transduced neurons of the corresponding DIV as percentage. Number of images acquired from 2-3 independent experiments: DIV10: 28 copGFP, 28 iCre; DIV14: 26 copGFP, 24 iCre. Two-way ANOVA with Šídák's multiple comparisons test, iCre was compared to copGFP of the same DIV, ****p<0.0001. Fig. 3-S1. NMNAT2 colocalize and co-migrate with vesicular cargos (A) Representative confocal images with maximum intensity Z stack projection showing that NMNAT2-mCherry colocalizes with APP-EGFP, SYPH-EGFP, and SNAP25-EGFP in axons of DIV8 WT cortical neurons (Scale bar, 20 μm). (B) Representative structure illumination images with maximum intensity Z stack projection showing NMNAT2-mCherry colocalizes with APP-EGFP, SYPH-EGFP, and SNAP25-EGFP in axons of DIV8 WT cortical neurons (Scale bar, 5 μm). (C) Representative confocal image shows the distribution of MitoVenus and NMNAT2-mCherry expressed in cortical axons. (Scale bar, 10 μm) (D) Representative grey value intensity plots along the axonal distance for MitoVenus and NMNAT2-mCherry or APP-GFP and NMNAT2-mCherry. (E) The ratio of NMNAT2-mCherry puncta colocalizing to APP-EGFP, SYPH-EGFP, SNAP25-EGFP, or MitoVenus quantified from confocal images. N represents the number of neurons imaged, APP (N=10), SNAP25 (N=20), SYPH (N=20), and MitoVenus (N=6). (F) Representative kymograph showing NMNAT2-mCherry comigrates with APP-EGFP in the axon of DIV8 WT neurons (Scale bar 20 μm in length, 30 s in time, 1.7 frame/second). (G) Frequency distribution of the anterograde and retrograde velocity of NMNAT2-mCherry and APP-EGFP in DIV8 distal axons. (NMNAT2 N=445, APP N=861, N represents the cargo trajectory traced in all kymographs). Fig. 3-S2. Vesicular cargo transport in the proximal axon is less affected by NMNAT2 loss at DIV8 (A) Diagram indicating the proximal axon region (< 200 µm from the soma). (B) Representative kymographs for APP-EGFP from DIV8 ctrl and KO proximal axons. Scale bar, 20 µm. (C) Distribution of APP-EGFP vesicles in anterograde motion, retrograde motion, or in a stationary/dynamic pause from 2 independent culture experiments. Numbers (neurons imaged) and statistics: 23 ctrl, 27 KO; compared with unpaired t-test. (D) Velocity of antero- and retrograde transport of APP-EGFP from 2 independent culture experiments. Numbers (neurons imaged) and statistics: anterograde, 23 ctrl, 26 KO; retrograde, 22 ctrl, 26 KO. Two-way ANOVA with Šídák's multiple comparisons. (E) Representative kymographs for SNAP25-EGFP from DIV8 ctrl and KO proximal axons. Scale bar, 20 µm. (F) Distribution of APP-EGFP vesicles in anterograde motion, retrograde motion, or in a stationary/dynamic pause from 3 independent culture experiments. Numbers (neurons imaged) and statistics: 23 ctrl, 21 KO; anterograde and retrograde categories were analyzed by Mann-Whitney test, and stationary/dynamic pause category was analyzed by unpaired t-test. (G) Velocity of antero- and retrograde transport of SNAP25-EGFP from 3 independent culture experiments. Numbers (neurons imaged) and statistics: anterograde, 23 ctrl, 21 KO; retrograde, 22 ctrl, 20 KO. Two-way ANOVA with Šídák's multiple comparisons. Data represent mean ± SEM. Fig. 3-S3. Examples of axonal morphology overview (A1, B1) Stitched confocal image of DIV8 ctrl or KO neuron expressing SNAP25-EGFP. (A2, B2) Zoom-in of the regions of interest identified as proximal and distal axons as highlighted in magenta and dark green boxes in A1, B1. (A3) Kymograph generated from the axon segment selected and highlighted in yellow in (A2, B2). Fig. 3-S4. Mitochondrial density, morphology, and mobility in distal axons are unaffected by NMNAT2 loss at DIV8 (A) Representative images of EGFP and Mito-DsRed in the distal axon of DIV8 ctrl and KO neurons. (Scale bar 10 μm). (B) Mitochondria density (# of mitochondria per 100 μm) in distal axons of DIV8 ctrl and KO neurons from 3 independent experiments. Numbers (neurons imaged) and statistics: 38 ctrl, 40 KO; unpaired t-test was used. (C) The cumulative percentage distribution of mitochondria morphology indicated by sphericity in DIV8 ctrl and KO distal axons from 3 independent experiments. Numbers (neurons imaged) and statistics: 38 ctrl, 40 KO; Log(Y/(1-Y)) transformation for the independent variable and linear mixed model with random intercept and the slope was used; see descriptions in Star Methods. (D) Representative kymographs show the mobility of MitoDsRed in distal axons of DIV8 ctrl and KO neurons. (Scale bar, 20 µm; total recording time, 15min). (E) The velocity of anterogradely and retrogradely moving MitoDsRed in distal axons of DIV8 ctrl and KO neurons from 2 independent batches of culture. (Anterograde: ctrl N=14, KO N=16, Mann-Whitney test was used; Retrograde: ctrl N=14, KO N=14, unpaired t test was used). (F) Percentage of MitoDsRed that is anterogradely moving, retrogradely moving or in stationary/dynamic pause in distal axons of DIV8 ctrl and KO neurons from 2 independent batches of culture (ctrl N=19, KO N=21). For analyzing anterograde and stationary/dynamic pause, an unpaired t-test was used; for retrograde, the Mann-Whitney test was used. N represents number of neurons imaged. All above data represents mean ± SEM. Fig. 5-S1. pH measurement and pH correction for Syn-ATP (A) Cyto-pHluorin imaging timeline and equation used for cytosolic pH determination in presynaptic varicosities. (B) pH measured by Cyto-pHluorin in various conditions. The mean value in each group is labeled above each box and whisker. Numbers (neurons imaged) and statistics: 27 ctrl, 25 KO, and 27 KO+NAD+ under basal conditions; 18 ctrl, 25 KO, and 27 KO+NAD+ under oligomycin-treated conditions. All groups were from a single experiment. Two-way ANOVA with Tukey's multiple comparisons test, **p= 0.005. (C) Equations used for pH correction of L/F measurements from Syn-ATP. (D) Raw L/F measurements in distal axon varicosities of DIV8 ctrl neurons with or without NAD+ supplementation. Numbers (neurons imaged) and statistics: 78 ctrl and 10 ctrl+NAD+ under basal conditions; 67 ctrl and 10 ctrl+NAD+ under oligomycin-treated conditions. Ctrl was from 5 experiments, and ctrl+NAD+ was from one experiment. Kruskal-Wallis test with Dunn's multiple comparisons test, *p= 0.0171, **p= 0.0018. All above data represent mean ± SEM. Fig. 5-S2. Increase of intracellular NAD+ by exogenous supplementationThe change of intracellular NAD+ abundance over time after one-time 1 mM NAD+ supplementation into the culture medium. All groups were normalized to ctrl+NAD+ 0 h time point and expressed as a percentage. Numbers (culture wells) and statistics: 13-14 ctrl and 12- 14 KO from 4 independent experiments, Kruskal-Wallis test with Dunn's multiple comparisons test was used, *p= 0.049, **p= 0.0023 Fig. 6-S1. The NAD+ synthesizing enzymatic activity of NMNAT2 is required to rescue APP accumulation in NMNAT2 KO neurons (A) Representative confocal images of APP staining of DIV12 KO neurons with or without lentiviral transduction to overexpress wt-NMNAT2 or ED(enzyme dead)-NMNAT2, scale bar 35 µm. (B) Comparable lentiviral transduction rate for wt-NMNAT2 and ED-NMNAT2 in KO neurons with 4MOI (multiplicity of infection) lentivirus. Transduction rate was calculated as the percentage of HA+ APP+ cells out of APP+ cells. N=8 per group, N represents the number of images randomly sampled, unpaired t test was used. (C) The summary for the area of accumulated APP relative to WT as percentage. N=14-18 per group from 2 independent batches of culture, N represents the number of images randomly sampled. One-way ANOVA with Tukey's multiple comparisons test was used, **p= 0.0034, ****p <0.0001. All above data represents mean ± SEM. Fig 6-S2. NAD+ supplementation depends on glycolysis to ameliorate APP accumulation in NMNAT2 KO neurons (A) Timeline of NAD+ supplementation, 2DG(+Methyl-pyr) treatment, and immunostaining. (B) Representative confocal images of APP staining of DIV14 ctrl or KO neurons under Veh (vehicle) or NAD+ supplementation, treated with Veh or 2DG. Scale bar, 30 µm. (C) Area of accumulated APP in DIV14 ctrl or KO neurons supplemented with Veh or NAD+ and treated with Veh or 2DG. All values were normalized to ctrl supplemented and treated with Veh. Numbers (images) and statistics: 18 ctrl+Veh, 20 KO+Veh, 23 ctrl+ 2DG, 12 KO+2DG, 19 ctrl+NAD+, 22 KO+NAD+, 21 ctrl+NAD++2DG, and 22 KO+NAD++2DG, Two-way ANOVA with Tukey's multiple comparisons test was used, ****p<0.0001, ####p<0.0001. Except for KO+2DG, all groups were from 2 independent experiments. All above data represent mean ± SEM. Fig 7-S1. Gender ratio, body weight, and motor behaviors in surviving NMNAT2 cKO mice lacking one or two copies of SARM1 (A) Y axis represents the percentage of postnatally alive mice of ctrl, NMNAT2 cKO; Snull/+, and NMNAT2 cKO; Snull/Snull genotypes pooled from progenies derived from various parent genotype combinations. The actual number of mice from each genotype and gender is listed on top of each bar. (B) Body weight of ctrl, NMNAT2 cKO; Snull/+, and NMNAT2 cKO; Snull/Snull mice at P16/21, and P90. Number of mice: P16/21, 61 ctrl, 34 NMNAT2 cKO; Snull/+, 24 NMNAT2 cKO; Snull/Snull; P90, 11 ctrl, 7 NMNAT2 cKO; Snull/+, 5 NMNAT2 cKO; Snull/Snull. Kruskal-Wallis test with a two-stage linear step-up procedure of Benjamini, Krieger, and Yekutieli was used for P16/21 data, ****p<0.0001. One-way ANOVA with a two-stage linear step-up procedure of Benjamini, Krieger, and Yekutieli was used for P90 data, (ctrl vs. NMNAT2 cKO; Snull/+), ***, p= 0.0004, q=0.0004; (NMNAT2 cKO; Snull/+ vs. NMNAT2 cKO; Snull/Snull), **, p= 0.0039, q= 0.002. (C) Hindlimb clasping phenotype in ctrl, NMNAT2 cKO; Snull/+, and NMNAT2 cKO; Snull/Snull mice of 3-5 months old (ctrl N=13, cKO; Snull/+ N=20, cKO; Snull/Snull N=6). Kruskal-Wallis test was used, ***p<0.0005, ****p<0.0001. All above data represents mean± SEM. Fig. 7-S2. APP accumulation in P5 brains of NMNAT2 cKO mice lacking one or two copies of SARM1 (A) Immunohistology images showing APP accumulation in the corpus callosum, fimbria, and striatum of P5 NMNAT2 cKO; Snull/+, but not in ctrl and NMNAT2 cKO; Snull/Snull brains. (B) Normalized APP signals in P5 brains. Number of mice: 4 ctrl, 4 NMNAT2 cKO; Snull/+, 3 NMNAT2 cKO; Snull/Snull. CC, corpus callosum; Str, striatum; Fim, Fimbria. For CC, one-way ANOVA with a two-stage linear step-up procedure of Benjamini, Krieger, and Yekutieli was used (Ctrl vs. NMNAT2 cKO; Snull/+) **, p=0.0032, q= 0.0021; (NMNAT2 cKO; Snull/+ vs. NMNAT2 cKO; Snull/Snull) **, p=0.004, q=0.0021. For Str, Kruskal-Wallis test with a two-stage linear step-up procedure of Benjamini, Krieger, and Yekutieli was used, (Ctrl vs. NMNAT2 cKO; Snull/+) *, p=0.0142, q=0.0298; (NMNAT2 cKO; Snull/+ vs. NMNAT2 cKO; Snull/Snull) *, p=0.0414, q= 0.0435. For Fim, one-way ANOVA with a two-stage linear step-up procedure of Benjamini, Krieger, and Yekutieli was used, ****p<0.0001. All above data represent mean ± SEM. Fig 8-S1. SARM1 knockdown validation (A) RT-qPCR quantification of sarm1 mRNA levels across ASO treatments in primary mouse cortical neurons. Cells were treated for 2 days (DIV5-7) with a control or a SARM1 ASO (33 or 47) at two different concentrations to test efficacy. Number of wells: 6 ctrl; 2 ASO33 at 1 uM or 5 uM, 2 ASO47 at 5 uM or 10 uM. (B) Representative western blotting detecting SARM1 and GAPDH (internal control) protein levels at DIV8 after ASO treatment since DIV6. Brain lysates of SARM1 WT and SARM1-null (Snull/Snull) mice were included for antibody specificity validation. (C) Quantification of SARM1 protein level across various ASO treatment duration, as detected by western blot. SARM1 protein level was normalized to GAPDH first, then normalized to its corresponding ctrl-ASO-treated group. Numbers of wells: 2 days Sarm1 ASO, n=3. 2 days Ctrl ASO, 3 days Ctrl ASO, 3 days Sarm1 ASO, 5 days Ctrl ASO, 5 days Sarm1 ASO, n=4. 8 days Ctrl ASO, 8 days Sarm1 ASO, 9 days Ctrl ASO, 9 days Sarm1 ASO, n=6. 7 days Ctrl ASO, 7 days Sarm1 ASO, n=8. 4 days Ctrl ASO, 4 days Sarm1 ASO, n=12. Statistics were conducted with the Kruskal-Wallis test and corrected for multiple comparisons by controlling the False Discovery Rate using the Two-stage step-up method of Benjamin, Krieger, and Yekutieli. All above data represent mean ± SEM. *, p<0.05, ***, p<0.001, ****, p<0.0001. Fig. 8-S2. SARM1 knockdown prevents APP accumulation and axon degeneration in NMNAT2 KO neurons in vitro (A) Representative images illustrating the effects of ctrl-ASO and SARM1-ASO on APP accumulation in WT and NMNAT2 KO primary cultured cortical neurons. (B) Quantification of APP accumulation area following ASO treatment of two timelines: DIV1 to DIV14 and DIV5 to DIV14. (C) Representative images show TUJ1 axonal staining of WT and KO cells following treatment with ctrl-ASO and SARM1-ASO. (D) Percentage of the total TUJ1 area found as aggregates following control and SARM1 ASO (ASO 33) treatments at DIV1-14 and DIV5-14. (E) Representative images of DAPI staining and quantification (F) of live cells in every sampled image. Image numbers: DIV1-14, N=6 for every group, from 2 independent experiments; DIV5-14, N=9 for every group, from 3 independent experiments. 3 images were collected from each experimental batch. Data for panel D, DIV5-14 was not normally distributed, so a Kruskal-Wallis test multiple comparisons test was performed. All other data sets were analyzed using two-way ANOVA and Tukey multiple comparisons. All above data represent mean ± SEM. ***, p<0.001, ****, p<0.0001. Fig 8-S3. SARM1 knockdown rescues global NAD deficiency and distal axonal NAD+/NADH ratio in NMNAT2 KO neurons in vitro (A-C) Levels of NAD+ (A), NADH (B), and the calculated NAD+/NADH ratios (C), in DIV8 WT and KO cortical neurons treated with PBS, ctrl-ASO (ASO30), or SARM1-ASO (ASO33) from DIV1-8. For ASO treated groups, the raw concentration is normalized to the corresponding protein amounts, and then normalized as percentages to the average value acquired from ctrl-ASO treated WT neurons. For PBS treated groups, the raw concentration is normalized to the corresponding protein amounts, and then normalized to WT average value. N represents each well of culture lysate from 2-3 independent culture experiments, WT+PBS (N=16), KO+PBS (N=16), WT+ASO30 (N=23), KO+ASO30 (N=23), WT+ASO33 (N=23), KO+ASO33 (N=23). Kruskal-Wallis test with Dunn's multiple comparisons test was used. (D) Representative images showing signals emitted from the SoNar (NAD+/NADH) ratiometric sensor for NAD+ and NADH in distal axons of DIV 8 WT or KO cortical neurons treated with ctrl-ASO or SARM1-ASO. Scale bar, 20 mm. (E) F488/F440 ratiometric measurements of the SoNar sensor, reflecting the NAD+/NADH ratios in distal axons of DIV8 WT or KO cortical neurons treated with ctrl-ASO or SARM1-ASO since DIV1. N represents the number of neurons imaged from two independent cultures, WT+ctrl-ASO (N=17), KO+ctrl-ASO (N=21), WT+SARM1-ASO (N=20), KO+SARM1-ASO (N=23). Two-way ANOVA with Tukey's multiple comparisons test was used. All data represent mean ± SEM. *p<0.05, **p<0.01, ****p<0.0001
Data Availability Statement
All data are available in the main text or the supplementary materials. Material will be provided upon request.