Abstract
Objective
To assess the impact of post-covid-19 conditions among adults.
Design
Systematic review and meta-analysis of health outcomes in controlled studies.
Data sources
Two sources were searched from database inception to 20 October 2022: Cochrane covid‐19 study register (comprising Cochrane Central Register of Controlled Trials, Medline, Embase, clinicalTrials.gov, World Health Organization's International Clinical Trials Registry Platform, medRxiv) and WHO's covid-19 research database.
Eligibility criteria
Cohort studies recruiting more than 100 participants with a control group and a follow-up of at least 12 weeks were included. Adults who were documented to have SARS-CoV-2 infection based on clinical, imaging, or laboratory criteria were included.
Data extraction and synthesis
Two independent reviewers extracted data. The main outcomes included quality of life, functionality in daily activities, use of resources, recovery rates (cluster of symptoms), and the incidence of new medical diagnoses. Data were pooled using a random effects model. The risk of bias was assessed with the Joanna Briggs Institute critical appraisal tool for cohort studies.
Results
We included 63 controlled cohort studies, encompassing more than 96 million participants. Based on five studies, we found a reduction in overall quality of life between individuals with confirmed SARS-CoV-2 infection versus controls at six to 24 months follow-up, although heterogeneity was very high (mean difference in EQ-5D scale −5.28 (95% confidence interval −7.88 to 2.68; I2=93.81%). Evidence from ten studies, which could not be pooled in a meta-analysis, indicated that an increased rate of functional impairment associated with SARS-CoV-2 infection. Use of care increased compared with controls at six to 24 months follow-up at intensive care units (risk ratio 2.00 (95% confidence interval 0.69 to 5.80), five studies, I2=91.96%) and in outpatient care (1.12 (1.01 to 1.24), seven studies, I2=99.51%). Regarding persistent symptoms, individuals with documented SARS-CoV-2 infection had an increased risk of having two or more persistent symptoms at follow-up, especially those related to neurological clusters (ie, risk ratio 1.51 (95% confidence interval 1.17 to 1.93), I2=98.91%). Evidence also showed an increased incidence of a wide variety of metabolic, cardiovascular, neurological, respiratory, haematological and other incident diagnoses.
Conclusion
Evidence suggests functional impairment after SARS-CoV-2 infection, in addition to a higher use of resources and a higher incidence of widely varying medical diagnoses. These results should be interpreted with caution, considering the high heterogeneity across studies and study limitations related to outcome measurement and attrition of participants.
Systematic review registration
Open Science Framework, osf.io/drm39
Keywords: covid-19, Epidemiology, Infectious disease medicine
What is already known on this topic.
Post-acute health consequences of SARS-CoV-2 infection, widely known as long covid, include a large group of disorders
The effect of post-covid-19 conditions has been difficult to synthesise in systematic reviews
What this study adds
Evidence on the impact of post-covid-19 conditions among adults considering their quality of life, functionality in daily activities, use of resources, recovery rates, and the incidence of new medical diagnoses was synthesised
How this study might affect research, practice, or policy
High functional impairment was reported after SARS-CoV-2 infection, in addition to a higher use of resources and a higher incidence of medical diagnoses
Findings highlight the need to systematically assess the effect of SARS-CoV-2 infection on individuals and the healthcare system for improved case definition and estimates of the public health impact
Introduction
Post-acute health consequences of SARS-CoV-2 infection have been described since mid-2020 in individuals from different sociodemographic backgrounds, including people with a mild course of the disease. Guidance is available on a case definition of post-covid-19 conditions, also widely known as long covid, although this condition is considered a widely heterogeneous group of disorders.1–3 Systematic evidence synthesis is important to better understand the extent and causes of long term health consequences after SARS-CoV-2 infection and to better assess subsequent medical care needs and socioeconomic consequences.
We previously conducted an evidence map of the descriptive evidence of post-covid-19 conditions available from studies up to 5 November 2021. The results of the evidence map are available on the Robert Koch Institute website on long covid for adults, children, and adolescents4, and summarised in a scientific publication.5 At the time, 15% (83/565) of the studies included control groups that could therefore permit comparison between individuals who were infected and those who were not. Most studies considered general or organ-specific symptoms as health outcomes; a small proportion of the studies reported on the quality of life (92 (16%) of 565), ability to work (57 (10%) of 565), and rehabilitation and support conditions in everyday life (101 (18%) of 565).5
Although an increasing number of epidemiological studies have since considered one or more of these outcomes that are important to patients and also relevant to public health, synthesis of the evidence in systematic reviews has been difficult. Systematic reviews of studies on post-covid-19 in children and adolescents have been particularly hampered by the absence of a consensus case definition and the paucity of high quality data, as has been shown previously.6–10
However, even among adults, a synthesis of evidence and a critical appraisal of available results on the individual and public health consequences of post-covid-19 conditions has proven to be challenging. Along with a rapidly increasing number of studies, heterogeneity exists in primary studies concerning the selection, definition, and assessment of health outcomes as well as study designs and data sources. In this systematic review, we aimed to synthesise available evidence from controlled cohort studies on the impact of SARS-CoV-2 infection in terms of post-covid-19 conditions among adults considering their quality of life, functionality in daily activities, use of resources, recovery rates (cluster of symptoms), and the incidence of new medical diagnoses.
Methods
This review followed a predefined protocol that was prospectively registered in the Open Science Framework (https://osf.io/drm39). We followed the Joanna Briggs Institute guideline for systematic reviews of cause and risk and PRISMA 2020 guidelines for the report of the full review.11 12 World Health Organization's definition was launched after the conduct of many of the potentially included studies, therefore, we included studies during a relevant timeframe for persistent, relapsing, or new symptoms after SARS-CoV-2 infection.
Inclusion criteria
We included cohort studies that used a control group and had a follow-up of at least 12 weeks. Studies that recruited more than 100 participants were included. This criterion was used in other systematic reviews on the topic, aiming at enhancing statistical power, precision, generalisability, and overall quality of the evidence synthesis. This approach also contributes to the reliability and validity of the findings for supporting decision making in clinical and policy contexts.13
We included adults 18 years and older with documented SARS-CoV-2 infection after clinical, imaging, or laboratory criteria with an assessment of symptoms or sequelae, including those with asymptomatic or mildly symptomatic infection.
Type of outcome measures
We selected the outcome measures considering the existing core outcome sets for this condition and the patient group Long Covid Deutschland.14 15 Additionally, we incorporated an outcome related to new medical diagnoses associated with prior SARS-CoV-2 infection.16 17
We measured healh-related quality of life by including measurements of physical-mental-social functioning (SF-36 or EuroQOL or other related scales). Functioning was assessed and defined as changes in daily activities, including attendance to work and occupational activities. Use of resources was measured including the use of medical services, physical rehabilitation, nursing, social support, or other resources to restore functionality. Recovery rates were reported and defined as the absence of symptoms and return to the previous state of health prior to the illness.18 This definition includes the dynamic of symptom clusters (two or more persistent symptoms that are related to each other and occur together).19 Another measure was incident medical diagnosis, including patients having any diagnosis arising after SARS-CoV-2 infection, based on the 10th revision of the International Classification of Diseases or as defined by the study authors.20
Search methods for identification of studies
For our main database, the Cochrane covid-19 study register, our information specialist (M-IM) designed a search strategy derived from 24 publications of relevant cohort studies (published until 5 November 2021), which were identified in our evidence map.5 We conducted a text analysis of these publications using the tools PubReMiner (https://hgserver2.amc.nl/cgi-bin/miner/miner2.cgi) and Voyant (https://voyant-tools.org) and derived text word combinations that retrieved 22 (92%) of 24 relevant studies. The two studies that could not be retrieved were publications without abstracts and unspecific titles.
The Cochrane covid-19 study register is a public, continually updated database of covid-19 study references for which six primary sources are being regularly searched.21 The aim of this register is to support rapid and living evidence synthesis. An evaluation has shown its high comprehensiveness, accurate study classifications, and short publishing times.22 We, therefore, used it as our primary source and complemented it with a second database, WHO's covid-19 research database, which also comprises several primary sources. To search this database, a conceptual search strategy was developed by another information specialist (KH) and peer reviewed by M-IM. The search in this source was restricted to databases that were not included in the Cochrane covid-19 study register.
We ran searches from database inception to 20 October 2022. We searched the Cochrane covid‐19 study register, comprising: Cochrane Central Register of Controlled Trials (CENTRAL), monthly updates; Medline (PubMed), weekly updates; Embase.com, weekly updates; clinicaltrials.gov, daily updates; WHO International Clinical Trials Registry Platform (ICTRP), weekly updates; and medRxiv, weekly updates. Additionally, we searched WHO's covid-19 research database.23
We identified other potentially eligible trials or ancillary publications by inspecting the reference lists of retrieved included studies. The details of the search strategy can be accessed in the online supplemental file 1.
bmjmed-2023-000723supp001.pdf (46.6KB, pdf)
Data collection
We used EndNote for deduplication and Covidence for study selection. Two independent researchers independently scanned the abstract, title, or both, of the remaining records retrieved to determine which studies should be assessed further through Covidence. Two independent researchers investigated all potentially relevant records as full text, mapped records to studies, and classified studies as included studies, excluded studies, studies awaiting classification, or ongoing studies, following the criteria for each provided in the Cochrane Handbook for Systematic Reviews of Interventions.24 We resolved any discrepancies through consensus or recourse to a third review author (LIG or JVAF). If the resolution of a disagreement was not possible, we designated the study as "awaiting classification", and we contacted the study authors for clarification. We documented reasons for the exclusion of studies that may have reasonably been expected to be included in the review in a table of characteristics of excluded studies. We presented a PRISMA flow diagram showing the process of study selection.11 25
We developed a dedicated data abstraction form that we pilot tested. For studies that fulfilled the inclusion criteria, two independent researchers independently abstracted the following information: bibliographical details, study dates and methods, country and setting, age, gender or sex, predominant SARS-CoV-2 variant, disease severity and definition of exposition (SARS-CoV-2 infection), socioeconomic status, prognostic factors and definition, and timing of outcomes. Outcome data for continuous and dichotomous outcomes were transformed, when necessary, following the guidance from chapter 6 of the Cochrane handbook.24 We resolved any discrepancies through consensus or recourse to a third review author (LIG or JVAF). Considering that we extracted incidence and estimates for the main outcome measures used to describe and characterise post-covid-19 conditions, we assessed the risk of bias in each study using the Joanna Briggs Institute tool for cohort studies12, which has 11 questions covering different aspects related to the methodological quality of a study and the extent to which a study has addressed the possibility of bias in its design, conduct, and analysis. Each of the following questions can be answered with a yes, no, unclear, or not applicable:
Were the two groups similar and recruited from the same population?
Were the exposures measured similarly to assign people to both exposed and unexposed groups?
Was the exposure measured in a valid and reliable way?
Were confounding factors identified?
Were strategies to deal with confounding factors stated?
Were the groups/participants free of the outcome at the start of the study (or at the moment of exposure)?
Were the outcomes measured in a valid and reliable way?
Was the follow-up time reported and sufficient to be long enough for outcomes to occur?
Was follow-up complete, and if not, were the reasons to loss to follow-up described and explored?
Were strategies to address incomplete follow-up utilised?
The risk of bias is reported in the online supplemental file 2 and described narratively in the results section.
bmjmed-2023-000723supp002.pdf (759.9KB, pdf)
Data synthesis
We anticipated clinical and methodological heterogeneity in the research. When sufficient homogeneity across the study population, diagnostic approaches, and measurements of outcomes were used, we summarised data using random-effects meta-analysis of mean differences for continuous outcomes. We then adjusted risk ratios or hazard ratios following the inverse variance method according to the guidance of chapter 24 of the Cochrane Handbook.24 For outcomes for which meta-analysis was not possible, we reported these findings following the reporting guidance of synthesis without meta-analysis (SWiM).26 27 We conducted random-effects meta-analyses with due consideration of the whole distribution of effects using the Hartung-Knapp-Sidik-Jonkman method.28 Based on the input of our experts at the Robert Koch Institute, we defined a minimum set of five confounders: age, sex, race and ethnicity, comorbidities (either as a set of common or relevant conditions related to the outcome or a cumulative index of morbidity, and the number of consultations as a proxy for increased medical needs), and socioeconomic status (eg, different forms of deprivation and education). We then conducted stratified analyses based on the number of core adjustments (zero to five). If the overall estimate did not differ substantially from the group, including the most adjusted studies (eg, 20% of the point estimate), we presented the overall analysis in the main manuscript (all other analyses are available in the supplementary appendix). Otherwise, we included the estimate with the greatest adjustment (ie, the maximum number of core confounders). We tracked protocols and registers of ongoing studies and, when appropriate, generated funnel plots to assess publication bias.
We evaluated the percentage of total variation across studies due to heterogeneity by the I2 measure. We used the thresholds low (0-40%), moderate (30-60%), substantial (50-90%), and considerable (75-100%) following the Cochrane handbook.24 As we expected high heterogeneity, we aimed to explore heterogeneity by analysing our prespecified subgroups, but no cut-off point for I2 was used to decide whether to pool study data.
We had planned a series of subgroup analyses and meta-regression to explore heterogeneity. However, we had too few studies per outcome, which rendered this exploration invalid.24 We had limited studies with different characteristics defined in our protocol (ie, gender, age, and disease severity). Moreover, we could not explore the effect of comorbidities because they were mostly accounted for in the adjusted analysis of the individual studies. Furthermore, we could not incorporate settings by country income classification as only three of the included studies were conducted in upper-middle income settings. Finally, we could not conduct sensitivity analysis using WHO's case definition because the studies included the general population and did not analyse this case definition.
We evaluated publication bias by using funnel plots representing the size of each study on the x-axis in relation to the estimated proportion on the y-axis. Bias was suspected when visible asymmetry was noted in the graph. We also performed Egger's test for asymmetry.29
All analyses were conducted with the meta suite in Stata 17.30
Results
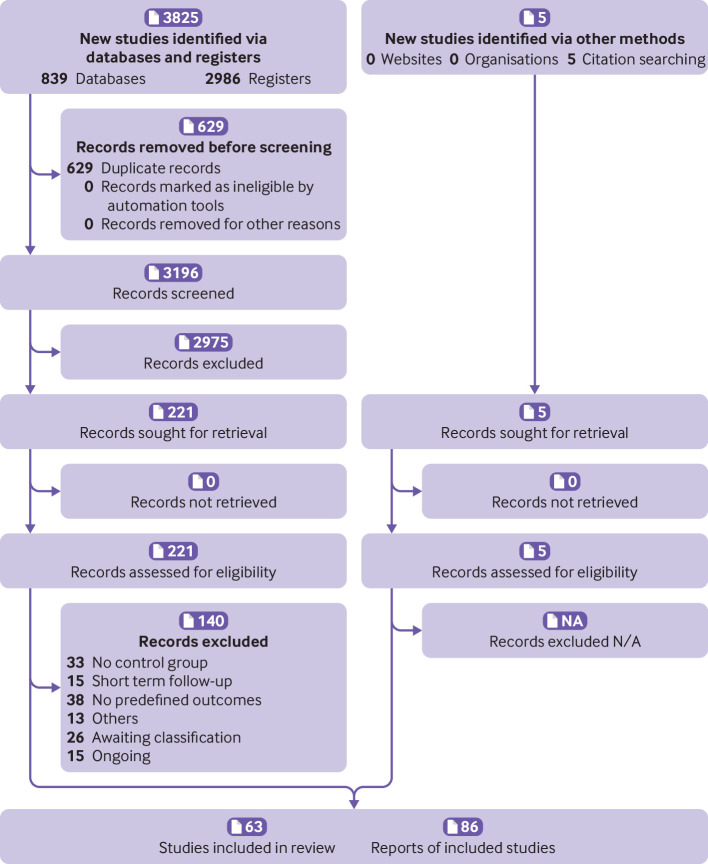
As summarised in the PRISMA flow chart (figure 1), we identified 3825 records through our database and register search. After removing duplicates, we screened 3196 records, of which 2975 were considered irrelevant after the inspection of the title and abstract. We then assessed 221 reports as full text, of which we excluded 99 reports for various reasons (the full description of included, excluded, and ongoing studies can be found in our data repository31 and a summary in table 1). We included five reports identified from other sources, primarily from the continuous surveillance of the literature on this topic or by the reference lists of included studies or other systematic reviews. We identified 15 ongoing studies and 26 studies awaiting classification (ie, we contacted authors for additional information to define eligibility, and we did not receive a response). We finally included 63 studies with 86 reports. The median follow-up is 27.6 weeks (interquartile range 19.6-48.0). Some studies had multiple secondary publications that were used as complimentary data to describe and analyse results.
Figure 1.
PRISMA flow diagram. N/A=not applicable (none excluded)
Table 1.
Characteristics of included studies by proportion (percentage), unless otherwise specified
| Characteristics | Proportion |
| Study design | |
| Publication type: | |
| Preprint | 11/63 (17) |
| Journal article | 52/63 (83) |
| English language | 63/63 (100) |
| Median sample size (interquartile range) | 72 729 participants (1106-527 856) |
| Median follow-up (interquartile range) | 27.6 weeks (19.6 to 48.0) |
| Setting | |
| Country classification (World Bank): | |
| High income | 60/63 (95) |
| Upper middle income | 3/63 (5) |
| Lower middle income | 0/63 (0) |
| Low income | 0/63 (0) |
| Year of study*: | |
| 2018 | 1/63 (2) |
| 2019 | 5/63 (8) |
| 2020 | 57/63 (90) |
| 2021 | 35/63 (56) |
| 2022 | 6/63 (10) |
| Sampling: | |
| Convenience | 60/63 (95) |
| Probabilistic | 3/63 (5) |
| Recruitment†: | |
| Community/contact tracing | 29/63 (46) |
| Outpatient | 31/63 (49) |
| Hospital | 39/63 (62) |
| Intensive care unit | 15/63 (24) |
| Data collection†: | |
| Administrative data | 34/63 (54) |
| Clinical assessment | 9/63 (14) |
| Survey (unspecified) | 10/63 (16) |
| Online survey | 6/63 (10) |
| Phone survey | 3/63 (5) |
| Population: | |
| Younger adults (19-44 years) | 49/63 (78) |
| Middle age group (45-65 years) | 56/63 (89) |
| Older adults (>65 years) | 53/63 (84) |
| Gender: | |
| Male | 62/63 (98) |
| Female | 63/63 (100) |
| Other | 2/63 (3) |
| Reported circulating variant: | |
| Not reported | 57/63 (90) |
| Alpha or wildtype | 4/63 (6) |
| Omicron/delta | 2/63 (3) |
| Severity: | |
| Mild | 28/63 (44) |
| Moderate | 23/63 (36) |
| Severe | 26/63 (41) |
| Critical | 10/63 (16) |
| Included reinfected participants | 3/63 (5) |
| Included vaccinated participants | 12/63 (19) |
| Reported socioeconomic status | 28/63 (44) |
| Prognostic factors: | |
| Symptoms/onset | 19/63 (30) |
| Severity/infection | 31/63 (49) |
| Vaccination status | 9/63 (14) |
| Gender | 48/63 (76) |
| Race/ethnicity | 18/63 (29) |
| Socioeconomic status | 17/63 (27) |
| Comorbidities | 48/63 (76) |
| Non-communicable diseases | 34/63 (54) |
| Immunosuppression | 17/63 (27) |
| Outcomes: | |
| Health related quality of life | 14/63 (22) |
| Functioning | 10/63 (16) |
| Medical and rehabilitation needs | 24/63 (38) |
| Recovery rates | 19/63 (30) |
| Incident medical diagnosis | 33/63 (52) |
*Some studies included participants during several years (including cohorts before the pandemic), leading to more than 100% overall here.
†Some studies used several sources for recruitment and data collection, leading to more than 100% overall here.
Quality assessment of included studies
Of 63 studies, 57 (90%) recruited participants from the same population for both the control and the covid-19 group. Fifty four (86%) assessed the exposure similarly (ie, diagnosis of covid-19 using polymerase chain reaction, or antigen or antibody testing), but nine studies (15%) did not specify any detail related to the test performed in the control groups. Only one study (2%) also included participants with clinically "confirmed" or "suspected" covid-19. Fifty seven (90%) studies considered potential confounding factors and 53 (84%) stated strategies to deal with them, such as adjusted logistic regression models, hierarchical linear regression analyses or propensity score matching analyses. Only 27 (43%) studies clearly stated that participants were free of the conditions considered as outcomes for this review at the start of the study, and 33 (52%) did not address the issue of possible unrecognised infections or reinfections. Regarding the outcome measures, only two studies (3%) used a non-validated survey questionnaire or unclear diagnostic criteria for the new incident diagnosis reported. Over half of the studies used large databases that relied on coding by users of healthcare records for data collection. Sixty one studies (97%) reported a follow-up time that was adequate for assessing the defined outcomes, although they may have been underpowered to detect some long term low-incidence consequences. Only eight studies (13%) provided information on loss-to-follow-up and reported high attrition rates, as they usually performed available-case analyses. However, 27 (43%) studies did not report reasons for incomplete follow-up, and 47 (75%) did not report any method to address incomplete follow-up. All but two studies (3%) used adequate analyses to calculate estimates, with the caveats mentioned in the other domains (online supplemental file 2 for the full details of quality assessment per study).
Quality of life
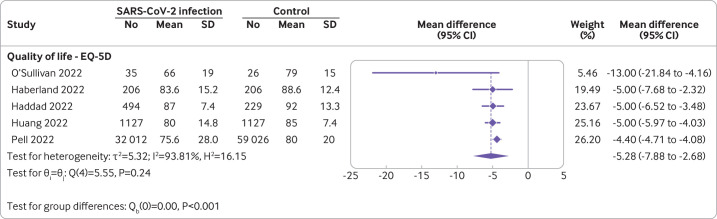
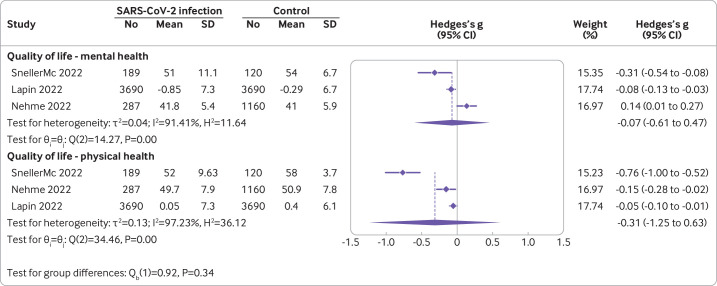
Fifteen studies with 122 503 participants reported this outcome. Only eight (53%) of these could be pooled in a meta-analysis. The remaining seven studies reported limited data or different scales for the quality of life domain. For example, some studies did not report measures of dispersion, 95% confidence intervals, or exact P values that enable conversions for meta-analysis. Other studies disaggregated the outcome into multiple subdomains or converted scales in a dichotomous fashion. The meta-analysis of the five studies reporting the EQ-5D indicated a small reduction in overall quality of life between individuals with confirmed SARS-CoV-2 infection versus controls at six to 24 months follow-up, although heterogeneity was very high (mean difference in EQ-5D scale −5.28 (95% confidence interval −7.88 to 2.68); I2=93.81%; figure 2).32–36 One small study with 113 predominantly male (86%), working age (mean 39 (standard deviation 9)) participants provided outlier results in this meta-analysis indicating lower quality of life in individuals with documented symptomatic infection versus non-infected individuals.37 Nonetheless, when pooling studies with different scales assessing subdomains of quality of life, we found little to no difference, for example, for mental health (Hedges's g −0.07 (95% confidence interval −0.61 to 0.47)) and for physical health (−0.31 (−1.25 to 0.63), figure 3).38–40 Other studies using validated scales that could not be incorporated into the pooled estimate due to missing data (eg, reporting only P values) reported a similar direction of results to the overall estimate of the meta-analysis.41 42 Too few studies were available to be able to conduct subgroup analysis or meta-regression for further explorations.
Figure 2.
Quality of life in covid-19 cases versus controls of studies reporting EQ-5D.32–36 Symptomatic and asymptomatic groups of Pell and colleagues36 were merged for the analysis. A negative mean difference value indicates lower quality of life in the covid-19 group. The dotted line indicates the pooled estimate. CI=confidence interval; SD=standard deviation
Figure 3.
Quality of life in covid-19 cases versus controls of studies reporting mental and physical health.38–40 A negative g value indicates lower quality of life in the covid-19 group. The dotted lines indicates the pooled estimates. CI=confidence interval; SD=standard deviation
Functioning
Ten studies with 103 981 participants reported a functioning outcome. Heterogeneous reporting of the outcomes (dichotomous and continuous scales using different definitions of functioning) precluded meta-analyses, so we present the findings narratively in table 2.
Table 2.
Summary of the results related to functioning
| Study, year | Follow-up | Main findings |
| Ballouz et al, 202261 | 12 months | More problems with usual activities (OR 2.04 (95% CI 1.31 to 3.21), self-care (1.85 (0.6 to 5.9), and mobility (1.56 (0.98 to 2.47)) compared with the control group |
| Buonsenso et al, 202262 | 6-9 months | No disaggregated data for adults |
| Cheung et al, 202263 | 306 days | Return to normal activities:
|
| Cortez Zamora et al, 202243 | 104 days | Mean Barthel independence score (range 0-100, higher score, higher independence): 65 in participants with documented infection v 80 in the control group Similar ambulation scores in both groups, assessed with the functional ambulation classification test |
| Haberland et al, 202234 | 200 days | Reduced post-covid-19 functional status v controls: 30.6% v 14.6% (OR 2.6 (95% CI 1.6 to 4.2)) |
| Huang et al, 202135 | 2 years | Reduced overall functionality v controls (multiple scales) |
| Nehme et al, 202239 | 12 months | Non-functional impairment at 12 months:
Severe impairment:
|
| Ollila et al, 202264 | 4 months | Participants with no disability (mRS modified Rankin scale)
|
| Platteel et al, 202242 | 12 months | No difference in SF-36 subscales for functioning in those with positive and negative serology for SARS-CoV-2 |
| Pell et al, 202236 | 12 months | Compared with controls:
|
CI=confidence interval; OR=odds ratio; RR=risk ratio.
Use of resources
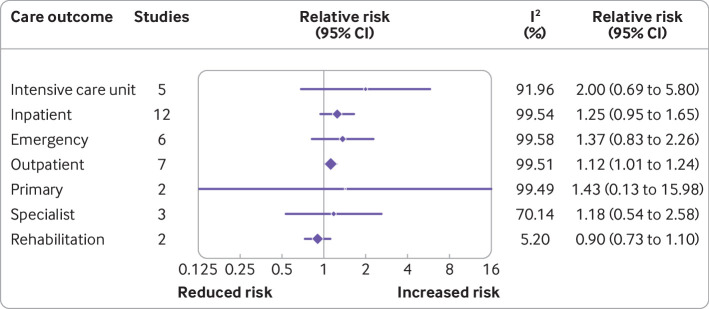
Twenty two studies with 25 169 789 participants reported on the use of resources. Some of these studies reported the incidence of hospital admissions and outpatient care following SARS-CoV-2 infection compared with controls. These studies found increased use of intensive care unit care (risk ratio 2.00 (95% confidence interval 0.69 to 5.80), five studies, I2=91.96%) and outpatient care (1.12 (1.01 to 1.24), seven studies, I2=99.51%) compared with controls at six to 24 months follow-up (figure 4). The median rate of use of resources among individuals with previous SARS-CoV-2 infection and controls who had no infection can contextualise these results. For instance, the median rate of intensive care admissions unit was nine per 10 000 individuals in the control group compared with 17 per 10 000 in the group with previous SARS-CoV-2 infection. By contrast, the rate of hospital admissions, emergency care, and specialised care were similar among both groups, although one outlier study indicated a marked increase in emergency care visits in people with prior SARS-CoV-2 infection among elderly adults living in long-term care facilities compared with controls.43 The pooled results had high heterogeneity, however, we could not explore this further through meta-regression because too few studies were available per outcome (see full details of this analysis including the relative and absolute differences in online supplemental appendix 3).
Figure 4.
Use of resources. CI=confidence interval
bmjmed-2023-000723supp003.pdf (2.7MB, pdf)
There were also only a few studies that reported on incident use of medication. Following SARS-CoV-2 infection, psychiatric drugs (ie, antidepressants and benzodiazepines and Z drugs), antihyperglycaemic drugs, bronchodilators, and medication for neuropathic pain increased at six to 12 months follow-up compared with controls (online supplemental appendix 3). This mirrors some of the findings related to incident medical diagnosis, because findings may be related to increased incidence of psychiatric disorders, diabetes mellitus, and lung disorders (see later).
Recovery rates (cluster of symptoms)
Fifteen studies with 62 729 673 participants reported on the dynamic of clusters of symptoms following SARS-CoV-2 infection compared with controls. We identified a higher incidence of clusters of two or more symptoms at three to 12 months follow-up (risk ratio 1.78 (95% confidence interval 1.09 to 2.88), I2=99.71%). However, when looking at individual clusters of two or more symptoms by system (eg, gastrointestinal, urological, cardiovascular, etc), we were unable to identify an increased incidence in the group of infected individuals versus controls, except for neurological symptoms (1.51 (1.17 to 1.93), I2=98.91%)). This analysis does not consider symptoms associated with new medical diagnoses (online supplemental appendix 4).
bmjmed-2023-000723supp004.pdf (2.9MB, pdf)
Incident medical diagnosis
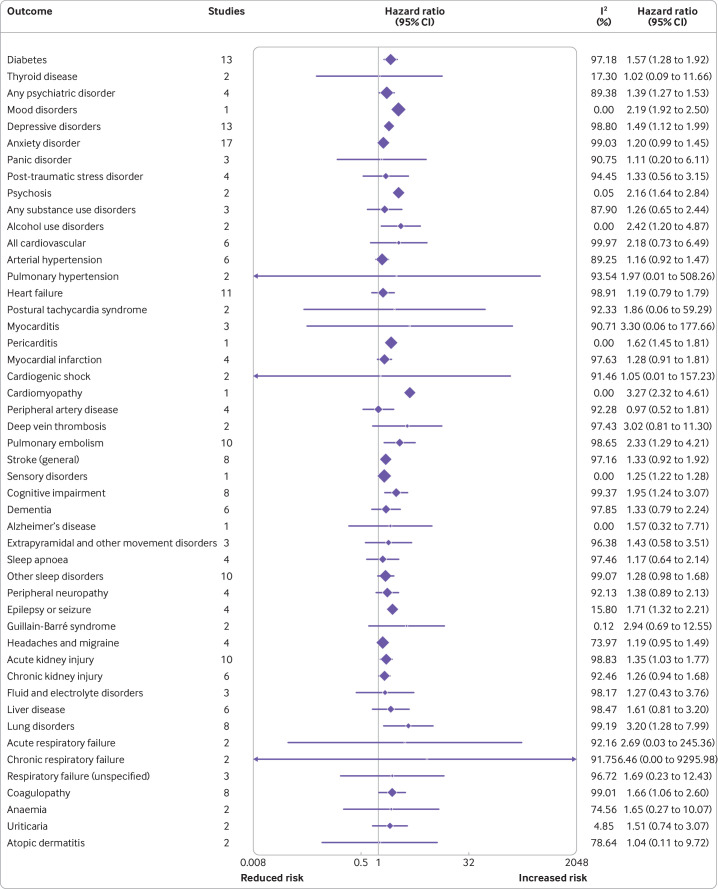
Thirty seven studies with 92 682 258 participants reported incident medical diagnosis. A summary of the meta-analyses for all reported diagnoses can be found in figure 5. People with documented SARS-CoV-2 infection had an increased incidence of diabetes mellitus, psychiatric disorders (primarily depressive, anxiety, and alcohol use disorder), cardiovascular disease (eg, myocarditis, cardiomyopathy, and postural tachycardia syndrome), deep vein thrombosis, pulmonary embolism, ischaemic stroke, haemorrhagic stroke, cognitive impairment, Alzheimer's disease, peripheral neuropathy, epilepsy or seizures, headaches or migraine, sleep disorders, acute and chronic kidney injury, lung disorders (eg, acute and chronic respiratory failure), coagulopathy, anaemia, and urticaria, among other disorders. These results can be contextualised by the median rate of the diagnosis incidence in the groups who did or did not have an SARS-CoV-2 infection. For instance, incidence of any psychiatric disorder was 761 per 10 000 in the group that did not have an infection compared with 1058 per 10 000 in the group of individuals who had infections. By contrast, the incidence of myocarditis was less than one per 10 000 in the non-infected group, corresponding to approximately two per 10 000 among those previously infected. Finally, some results could not be pooled because of heterogeneity in the definition of the outcome or multiple studies reporting on the same variant of the outcome. We highlight arrhythmias, for which we found several studies reporting a higher incidence in adults with previous SARS-CoV-2 infection compared with non-infected adults (online supplemental appendix 5).
Figure 5.
Incident medical diagnosis. CI=confidence interval
bmjmed-2023-000723supp005.pdf (20.1MB, pdf)
We had too few studies per outcome to explore heterogeneity through meta-regression, which would have rendered our findings unpowered and invalid.
Publication bias
We were able to draw funnel plots for a few comparisons, and they were mostly uninformative concerning the suspicion of publication bias. In the case of incident diabetes mellitus, the funnel plot was asymmetrical but the result from Egger's test was not significant. Nonetheless, we cannot confirm nor rule out publication bias.
Discussion
Our review synthesises a large body of evidence from controlled studies on post-covid-19 conditions. We considered health outcomes likely to impact individuals and society as a whole. These health outcomes included those reported by patients such as quality of life, recovery, and functional impairments, and outcomes reflecting the use of the healthcare system, such as incident medical diagnoses and healthcare services use. We found a small reduction in the overall health related quality of life between adults exposed to SARS-CoV-2 infection compared with adults who had no infection. Furthermore, we found evidence of an increased rate of disability in conducting daily life activities in association with prior SARS-CoV-2 infection. We also found increased attendance to outpatient visits and post-acute admission to intensive care unit and, in one study, a higher attendance to the emergency department. In terms of persistent symptoms, we found that individuals with a documented SARS-CoV-2 infection had an increased risk of having two or more symptoms at follow-up, especially those related to neurological clusters. We also found evidence of an increased incidence of metabolic, cardiovascular, neurological, and haematological diagnosis among individuals with documented SARS-CoV-2 infection compared with individuals with no infections. These results should be interpreted with caution, considering the high heterogeneity in our analysis and the study limitations, primarily related to outcome measurement (detection bias) and missing outcome data (attrition bias).
One of the strengths of our review is the focus on controlled studies, which aims to assess the relative and absolute difference between individuals following SARS-CoV-2 infection and controls. Earlier reviews included less than ten controlled studies44 45; however, a recent systematic review included data from 194 studies, of which only 22 had a control group.46 This previous systematic review and meta-analysis indicated an increased prevalence of one or more symptoms following infection but did not assess the estimates in relation to a control group. The authors had difficulty explaining the high heterogeneity with meta-regression, which was also evident in our analysis, most likely as a result of the low power of this analysis to detect study level explanatory variables. Another recent review following the Joanna Briggs Institute method for prevalence studies found a high prevalence of mental, neurological, and respiratory problems following SARS-CoV-2 infection, with extremely high heterogeneity, but no analysis was done from data of controlled studies.47
Our review supports previous findings related to the higher risk of incident diagnosis in people who had a SARS-CoV infection compared with no infection. For example, two previous systematic reviews on the risk of diabetes included eight and nine studies each, finding a risk ratio for diabetes of 1.66 and 1.62, respectively.48 49 Our review included a larger analysis with 13 studies and found a similar relative risk of 1.65. Moreover, in our supplementary appendices, we provide absolute estimates of risk differences based on the median incidence across the control groups. Other reviews focusing on a narrower body of evidence found similar results to ours in terms of cardiovascular, neurological, and mental health diagnoses 50–52
Our review provides additional information related to important outcomes. We found no previous systematic reviews focusing on the use of resources, including outpatient, emergency, and inpatient services, nor the incidence use of new medications. We found one systematic review that reported a decreased quality of life by summarising the evidence of 24 studies of unclear study design.53 The descriptive statistics provided by this review indicate an impairment following infection but do not provide an estimate of the relative difference to controls. In our review, we found a small reduction in the quality of life of the overall population of individuals who had infections versus the control groups. This result should be interpreted with caution because we are referring to the mean quality of life in the population with documented prior SARS-CoV-2 infection. Identification of subgroups of individuals with a particularly higher burden of disease or symptoms following SARS-CoV-2 infection was beyond the scope of this study. However, while the disease mechanisms and predisposing factors remain to be elucidated, deep phenotyping of patients with particularly severe long term health impairments has shown that a subset of these patients fulfil criteria of myalgic encephalomyelitis or chronic fatigue syndrome (known as ME or CFS) and have a particularly poor chance for long term recovery.54 55
The synthesis of some of the results posed challenges and needs to be interpreted in the context of a heterogeneous body of evidence. We included studies of individuals with various clinical presentations from asymptomatic or mildly symptomatic to severe disease. As the results were not usually stratified by severity and we had too few studies per comparison, we could not explore subgroup analysis on how this would be differentially represented in the effect measures. Heterogeneity was high across all comparisons and was a common feature also described in previous systematic reviews. The sources of heterogeneity can include patient populations, study design, assignment of exposures, and outcome measures. As an example, one outcome that was particularly challenging to analyse was recovery, which included the persistence or improvement of symptoms across time. This outcome was seldom reported in controlled studies, which mostly focused on the burden of individual symptoms. Per protocol, we defined our outcome of interest as a cluster of symptoms, considering that many previous reviews had reported on the incidence of individual symptoms. However, clustering was highly variable. A recent review focused on 76 uncontrolled studies identified multiple methods of clustering in primary studies.56 Their analysis of the persistence of a cluster of symptoms was also dominated by substantial heterogeneity (I2=77-100%). Finally, the exploration of heterogeneity was limited due to the presence of too few studies per comparison, which would have resulted in underpowered and invalid subgroup analysis and meta-regression. Therefore, the interpretation of differential effects of SARS-CoV-2 infection should be interpreted with caution. For instance, a 2022 review reported a lower incidence of long covid with historical variants versus omicron; however, no formal statistical testing was done to support this statement.57
Our search strategy was empirically derived and had a high sensitivity to retrieve reports on post-covid-19 conditions in general, but might have been less sensitive for studies assessing a narrower scope of complications or outcomes than those of interest for this review. Moreover, smaller studies and poorly described ones might not have been picked up by the search. However, the sources we searched are comprehensive and were used to produce many Cochrane reviews and other reviews by members of our team.6 58–60 As such, missing relevant larger and controlled studies until the date of our search was unlikely. Additionally, data extraction resulted in challenges due to the poor reporting of included studies. In some cases, we had to infer study design (longitudinal for studies with at least two timepoints for assessment), the severity of infection of the included study population, and reported outcomes. Considering the scarcity of study registration of most of our included studies, assessing the validity of reported results is challenging. Finally, we intended to explore heterogeneity using meta-regression but we had too few studies per comparison.
Conclusions
In this review, we evaluated the evidence from 63 controlled cohort studies following more than 96 million participants for at least three months. We found a small reduction in the overall quality of life between adults with SAS-CoV-2 infection compared with non-infected adults. Furthermore, we found evidence of an increased rate of functional impairment in association with SARS-CoV-2 infection. We also found increased attendance to outpatient visits and post-acute hospital admission. In terms of persistent symptoms, we found that individuals with documented SARS-CoV-2 infection have an increased risk of having two or more symptoms at follow-up, especially those related to neurological clusters. Finally, we found evidence of an increased incidence of metabolic, cardiovascular, respiratory, neurological, and haematological diagnoses, among others. These results should be interpreted with caution, considering the high heterogeneity across studies and study limitations related to outcome measurement and attrition of participants. Given high SARS-COV-2 antigen contact rates in the population due to either vaccination or infection, including the possibility of reinfection as well as the additional effect of virus variants, adequately controlled studies will become more difficult. As such, enhancement of healthcare research and systematic identification and follow up of subgroups of patients who experience long term health consequences of SARS-CoV-2 infection are needed, according to ongoing health and functional impairments, course of acute SARS-CoV-2 infection, and pre-existing health conditions. Better case definitions based on harmonised assessment instruments and diagnostic algorithms will help to identify subgroups of people with long covid who are in need of different levels of treatment, healthcare, and social care. The results from these studies will contribute to an improved understanding of the underlying mechanisms, support the design of future clinical trials, and improve adequate delivery of services for people affected by the sequelae of SARS-CoV-2. From knowledge of the direct and indirect sequelae caused by the pandemic on population health, use of consensus agreed outcome criteria and instruments for monitoring the burden of symptoms, specific health conditions, and health related quality of life is needed at the population level as a reference.
Acknowledgments
We thank the following researchers who collaborated in the process of study selection and data extraction: Agostina Risso, Sabrina Rico, Nadia Sgarbossa, Diego Ivaldi, Lionel Trivisonno and Gisela Oltra.
Footnotes
Twitter: @juan_francomd
Contributors: Conceptualisation of the study JVAF, LIG, CSN, and RM. MIM and KH were responsible for the search methods and data curation. JVAF and LIG conducted the formal analyses and wrote the original draft. All authors contributed to writing, review and editing the manuscript. All authors approved this version of the manuscript. JVAF is the guarantor of the review. Transparency: The lead author (the guarantor) affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding: Research grant by Robert Koch Institut, Germany (Project code: 5053859-2.1.2)
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/disclosure-of-interest/ and declare: no support from any organisation for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Patient and public involvement: The patient group Long Covid Deutschland was involved during the development of the protocol of this review and provided feedback on the selection of the core outcomes. The results will be shared with this patient group. Moreover, the study results will be disseminated via the Robert Koch Institute's official channels of communication (https://www.rki.de/DE/Content/InfAZ/N/Neuartiges_Coronavirus/Long-COVID/Inhalt-gesamt.html) and social media.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available in a public, open access repository. The data that support the findings of this study are openly available on the OSF platform at https://osf.io/b7dwy/. Please contact the corresponding author if further requests are needed.
Ethics approval
Not applicable.
References
- 1. Koczulla AR, Ankermann T, Behrends U, et al. S1 guideline post-COVID/long-COVID. Pneumologie 2021;75:869–900. 10.1055/a-1551-9734 [DOI] [PubMed] [Google Scholar]
- 2. Shah W, Hillman T, Playford ED, et al. Managing the long term effects of COVID-19: summary of NICE, SIGN, and RCGP rapid guideline. BMJ 2021;372:136. 10.1136/bmj.n136 [DOI] [PubMed] [Google Scholar]
- 3. A clinical case definition of post COVID-19 condition by A Delphi consensus, 6 October . 2021. Available: https://www.who.int/publications/i/item/WHO-2019-nCoV-Post_COVID-19_condition-Clinical_case_definition-2021.1 [Accessed 1 Sep 2022]. [DOI] [PMC free article] [PubMed]
- 4. RKI - . Coronavirus SARS-CoV-2 - Scoping Review und Evidence Maps zu Long COVID, Available: https://www.rki.de/DE/Content/InfAZ/N/Neuartiges_Coronavirus/Long-COVID/Scoping-Review.html [Accessed 1 Sep 2022].
- 5. Franco JVA, Garegnani LI, Oltra GV, et al. Long-term health symptoms and sequelae following SARS-Cov-2 infection: an evidence map. Int J Environ Res Public Health 2022;19. 10.3390/ijerph19169915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Franco JVA, Garegnani LI, Oltra GV, et al. Short and long-term wellbeing of children following SARS-Cov-2 infection: A systematic review. Int J Environ Res Public Health 2022;19. 10.3390/ijerph192114392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zheng Y-B, Zeng N, Yuan K, et al. Prevalence and risk factor for long COVID in children and adolescents: A meta-analysis and systematic review. J Infect Public Health 2023;16:660–72. 10.1016/j.jiph.2023.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Behnood SA, Shafran R, Bennett SD, et al. Persistent symptoms following SARS-Cov-2 infection amongst children and young people: A meta-analysis of controlled and uncontrolled studies. J Infect 2022;84:158–70. 10.1016/j.jinf.2021.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lopez-Leon S, Wegman-Ostrosky T, Ayuzo Del Valle NC, et al. Long-COVID in children and adolescents: a systematic review and meta-analyses. Sci Rep 2022;12. 10.1038/s41598-022-13495-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pellegrino R, Chiappini E, Licari A, et al. Prevalence and clinical presentation of long COVID in children: a systematic review. Eur J Pediatr 2022;181:3995–4009. 10.1007/s00431-022-04600-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Moola S, Munn Z, Tufanaru C, et al. Chapter 7: systematic reviews of etiology and risk. In: JBI Manual for Evidence Synthesis JBI 2020. 10.46658/JBIMES-20-01 [DOI] [Google Scholar]
- 13. Pellicori P, Doolub G, Wong CM, et al. COVID-19 and its cardiovascular effects: a systematic review of prevalence studies. Cochrane Database Syst Rev 2021;3. 10.1002/14651858.CD013879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Munblit D, Nicholson T, Akrami A, et al. Core outcome set for research and clinical practice in post COVID-19 condition (long Covid): an international Delphi consensus study ‘PC-Cos. SSRN Journal 2022. 10.2139/ssrn.4017375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Long COVID Deutschland, Available: https://longcoviddeutschland.org/ [Accessed 22 Oct 2021].
- 16. Taquet M, Geddes JR, Husain M, et al. 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: a retrospective cohort study using electronic health records. The Lancet Psychiatry 2021;8:416–27. 10.1016/S2215-0366(21)00084-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Xie Y, Al-Aly Z. Risks and burdens of incident diabetes in long COVID: a cohort study. Lancet Diabetes Endocrinol 2022;10:311–21. 10.1016/S2213-8587(22)00044-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tong A, Baumgart A, Evangelidis N, et al. Core outcome measures for trials in people with Coronavirus disease 2019: respiratory failure, Multiorgan failure, shortness of breath, and recovery. Crit Care Med 2021;49:503–16. 10.1097/CCM.0000000000004817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Goldhaber NH, Kohn JN, Ogan WS, et al. Deep dive into the long haul: analysis of symptom clusters and risk factors for post-acute sequelae of COVID-19 to inform clinical care. Int J Environ Res Public Health 2022;19. 10.3390/ijerph192416841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. World Health Organization . ICD-10: International statistical classification of diseases and related health problems: tenth revision. World Health Organization 2004. Available: https://apps.who.int/iris/handle/10665/42980 [Accessed 27 Sep 2022]. [Google Scholar]
- 21. Cochrane COVID-19 study register. Cochrane, Available: https://covid-19.cochrane.org
- 22. Metzendorf M-I, Featherstone RM. Evaluation of the comprehensiveness, accuracy and currency of the Cochrane COVID-19 study register for supporting rapid evidence synthesis production. Res Synth Methods 2021;12:607–17. 10.1002/jrsm.1501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. WHO's COVID-19 research database. n.d. Available: https://search.bvsalud.org/global-literature-on-novel-coronavirus-2019-ncov
- 24. Higgins JPT, Thomas J, Chandler J, et al. Welch VA, editor. Cochrane Handbook for Systematic Reviews of Interventions Version 2023;6:4. Available: www.training.cochrane.org/handbook [Google Scholar]
- 25. Haddaway NR, Page MJ, Pritchard CC, et al. Prisma2020: an R package and shiny App for producing PRISMA 2020-compliant flow diagrams, with Interactivity for Optimised Digital transparency and open synthesis. Campbell Syst Rev 2022;18. 10.1002/cl2.1230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Campbell M, McKenzie JE, Sowden A, et al. Synthesis without meta-analysis (swim) in systematic reviews: reporting guideline. BMJ 2020;368. 10.1136/bmj.l6890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schuller-Martínez B, Meza N, Pérez-Bracchiglione J, et al. Graficando El Cuerpo de la Evidencia: lo Esencial para Comprender El Enfoque de Los Mapas de Brecha de Evidencia. Medwave 2021;21. 10.5867/medwave.2021.03.8164 [DOI] [PubMed] [Google Scholar]
- 28. IntHout J, Ioannidis JPA, Borm GF. The Hartung-Knapp-Sidik-Jonkman method for random effects meta-analysis is straightforward and considerably Outperforms the standard Dersimonian-Laird method. BMC Med Res Methodol 2014;14:25. 10.1186/1471-2288-14-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hunter JP, Saratzis A, Sutton AJ, et al. In meta-analyses of proportion studies, funnel plots were found to be an inaccurate method of assessing publication bias. J Clin Epidemiol 2014;67:897–903. 10.1016/j.jclinepi.2014.03.003 [DOI] [PubMed] [Google Scholar]
- 30. StataCorp LLC, College Station TX. STATA 17. Stata Statistical Software, 2021. [Google Scholar]
- 31. Franco JVA, Oltra G, Metzendorf M-I, et al. Longcov_521 - RKI [dataset]. Published Online First October 23, 2021. 10.17605/OSF.IO/B7DWY [DOI] [Google Scholar]
- 32. O’Sullivan O, Holdsworth DA, Ladlow P, et al. Cardiopulmonary, Functional, Cognitive and Mental Health Outcomes Post-COVID-19, Across the Range of Severity of Acute Illness, in a Physically Active, Working-Age Population. Sports Medicine - Open 2023;9:7.:7. 10.1186/s40798-023-00552-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Haddad A, Janda A, Renk H, et al. Long COVID symptoms in exposed and infected children, adolescents and their parents one year after SARS-CoV-2 infection: A prospective observational cohort study. EBioMedicine 2022;84:104245.:104245. 10.1016/j.ebiom.2022.104245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Haberland E, Haberland J, Richter S, et al. Seven months after mild COVID-19: A single-centre controlled follow-up study in the District of Constance (Fsc19-KN). Int J Clin Pract 2022;2022. 10.1155/2022/8373697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Huang L, Li X, Gu X, et al. Health outcomes in people 2 years after surviving Hospitalisation with COVID-19: a longitudinal cohort study. Lancet Respir Med 2022;10:863–76. 10.1016/S2213-2600(22)00126-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pell J, Hastie C, Lowe D, et al. Long-COVID in scotland study: a nationwide, population cohort study. In Review [Preprint] 2022. 10.21203/rs.3.rs-1656915/v1 [DOI]
- 37. O’Sullivan O, Holdsworth DA, Ladlow P, et al. Cardiopulmonary, functional, cognitive and mental health outcomes post COVID, across the range of severity of acute illness, in a physically active working age population. In Review [Preprint] 2022. 10.21203/rs.3.rs-1306776/v1 [DOI] [PMC free article] [PubMed]
- 38. Lapin B, Katzan IL. Health-Related Quality of Life Mildly Affected Following COVID-19: a Retrospective Pre-post Cohort Study with a Propensity Score-Matched Control Group. J Gen Intern Med 2022;37:862–9. 10.1007/s11606-021-07340-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nehme M, Braillard O, Chappuis F, et al. One-year persistent symptoms and functional impairment in SARS-Cov-2 positive and negative individuals. J Intern Med 2022;292:103–15. 10.1111/joim.13482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sneller MC, Liang CJ, Marques AR, et al. A Longitudinal Study of COVID-19 Sequelae and Immunity: Baseline Findings. Ann Intern Med 2022;175:969–79.:M21-4905. 10.7326/M21-4905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sandmann FG, Tessier E, Lacy J, et al. Long-term health-related quality of life in non-hospitalized Coronavirus disease 2019 (COVID-19) cases with confirmed severe acute respiratory syndrome Coronavirus 2 (SARS-Cov-2) infection in England: longitudinal analysis and cross-sectional comparison with controls. Clin Infect Dis 2022;75:e962–73. 10.1093/cid/ciac151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Platteel TN, Koelmans JC, Cianci D, et al. Long-term prognosis of adults with moderate-severe SARS-cov-2 lower respiratory tract infection managed in primary care: prospective cohort study. Infectious Diseases (except HIV/AIDS) [Preprint] 2022. 10.1101/2022.06.07.22276108 [DOI]
- 43. Cortés Zamora EB, Mas Romero M, Tabernero Sahuquillo MT, et al. Psychological and functional impact of COVID-19 in long-term care facilities: the COVID-A study. Am J Geriatr Psychiatry 2022;30:431–43. 10.1016/j.jagp.2022.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Greer N, Bart B, Billington CJ, et al. COVID-19 Postacute care major organ damage: a systematic review. BMJ Open 2022;12:e061245. 10.1136/bmjopen-2022-061245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Michelen M, Manoharan L, Elkheir N, et al. Characterising long COVID: a living systematic review. BMJ Glob Health 2021;6:e005427. 10.1136/bmjgh-2021-005427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. O’Mahoney LL, Routen A, Gillies C, et al. The prevalence and long-term health effects of long Covid among hospitalised and non-hospitalised populations: A systematic review and meta-analysis. EClinicalMedicine 2023;55. 10.1016/j.eclinm.2022.101762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mudgal SK, Gaur R, Rulaniya S, et al. Pooled prevalence of long COVID-19 symptoms at 12 months and above follow-up period: A systematic review and meta-analysis. Cureus 2023;15:e36325. 10.7759/cureus.36325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zhang T, Mei Q, Zhang Z, et al. Risk for newly diagnosed diabetes after COVID-19: a systematic review and meta-analysis. BMC Med 2022;20:444. 10.1186/s12916-022-02656-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ssentongo P, Zhang Y, Witmer L, et al. Association of COVID-19 with diabetes: a systematic review and meta-analysis. Sci Rep 2022;12. 10.1038/s41598-022-24185-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Shrestha AB, Mehta A, Pokharel P, et al. Long COVID syndrome and cardiovascular manifestations: A systematic review and meta-analysis. Diagnostics (Basel) 2023;13:491. 10.3390/diagnostics13030491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Mahdizade Ari M, Mohamadi MH, Shadab Mehr N, et al. Neurological manifestations in patients with COVID-19: A systematic review and meta-analysis. J Clin Lab Anal 2022;36:e24403. 10.1002/jcla.24403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Leung CMC, Ho MK, Bharwani AA, et al. Mental disorders following COVID-19 and other epidemics: a systematic review and meta-analysis. Transl Psychiatry 2022;12:205. 10.1038/s41398-022-01946-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Nandasena HMRKG, Pathirathna ML, Atapattu AMMP, et al. Quality of life of COVID 19 patients after discharge: systematic review. PLoS One 2022;17:e0263941. 10.1371/journal.pone.0263941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Legler AF, Meyer-Arndt L, Mödl L, et al. Symptom persistence and biomarkers in post-COVID-19/chronic fatigue syndrome – results from a prospective observational cohort. Neurology [Preprint] 2023. 10.1101/2023.04.15.23288582 [DOI] [PMC free article] [PubMed]
- 55. Kedor C, Freitag H, Meyer-Arndt L, et al. A prospective observational study of post-COVID-19 chronic fatigue syndrome following the first pandemic wave in Germany and biomarkers associated with symptom severity. Nat Commun 2022;13. 10.1038/s41467-022-33784-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kuodi P, Gorelik Y, Gausi B, et al. Characterisation of post-COVID syndromes by symptom cluster and time period up to 12 months post-infection: A systematic review and meta-analysis. International Journal of Infectious Diseases 2023;134:1–7. 10.1016/j.ijid.2023.05.003 [DOI] [PubMed] [Google Scholar]
- 57. Fernández-de-Las-Peñas C, Notarte KI, Peligro PJ, et al. Long-COVID symptoms in individuals infected with different SARS-Cov-2 variants of concern: A systematic review of the literature. Viruses 2022;14. 10.3390/v14122629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ansems K, Grundeis F, Dahms K, et al. Remdesivir for the treatment of COVID-19. Cochrane Database Syst Rev 2021;8. 10.1002/14651858.CD014962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Popp M, Stegemann M, Riemer M, et al. Antibiotics for the treatment of COVID-19. Cochrane Database Syst Rev 2021;10. 10.1002/14651858.CD015025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Mikolajewska A, Fischer A-L, Piechotta V, et al. Colchicine for the treatment of COVID-19. Cochrane Database Syst Rev 2021;10. 10.1002/14651858.CD015045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ballouz T, Menges D, Anagnostopoulos A, et al. Natural course of post COVID-19 condition and implications for trial design and outcome selection: A population-based longitudinal cohort study. Epidemiology [Preprint] 2022. 10.1101/2022.06.22.22276746 [DOI]
- 62. Buonsenso D, Munblit D, Pazukhina E, et al. Post-COVID condition in adults and children living in the same household in Italy: A prospective cohort study using the ISARIC global follow-up protocol. Front Pediatr 2022;10. 10.3389/fped.2022.834875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Cheung J, Nordmeier K, Kelland S, et al. Symptom persistence and recovery among COVID-19 survivors during a limited outbreak in Canterbury, New Zealand: a prospective cohort study. Intern Med J 2023;53:37–45. 10.1111/imj.15930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ollila H, Pihlaja R, Koskinen S, et al. Long-term cognitive functioning is impaired in ICU-treated COVID-19 patients: a comprehensive controlled neuropsychological study. Crit Care 2022;26:223. 10.1186/s13054-022-04092-z [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjmed-2023-000723supp001.pdf (46.6KB, pdf)
bmjmed-2023-000723supp002.pdf (759.9KB, pdf)
bmjmed-2023-000723supp003.pdf (2.7MB, pdf)
bmjmed-2023-000723supp004.pdf (2.9MB, pdf)
bmjmed-2023-000723supp005.pdf (20.1MB, pdf)
Data Availability Statement
Data are available in a public, open access repository. The data that support the findings of this study are openly available on the OSF platform at https://osf.io/b7dwy/. Please contact the corresponding author if further requests are needed.