Abstract
Age-associated declines in aerobic capacity promote the development of various metabolic diseases. In rats selectively bred for high/low intrinsic aerobic capacity, greater aerobic capacity reduces susceptibility to metabolic disease while increasing longevity. However, little remains known how intrinsic aerobic capacity protects against metabolic disease, particularly with aging. Here, we tested the effects of aging and intrinsic aerobic capacity on systemic energy expenditure, metabolic flexibility and mitochondrial protein synthesis rates using 24-month-old low-capacity (LCR) or high-capacity runner (HCR) rats. Rats were fed low-fat diet (LFD) or high-fat diet (HFD) for eight weeks, with energy expenditure (EE) and metabolic flexibility assessed utilizing indirect calorimetry during a 48 h fast/re-feeding metabolic challenge. Deuterium oxide (D2O) labeling was used to assess mitochondrial protein fraction synthesis rates (FSR) over a 7-day period. HCR rats possessed greater EE during the metabolic challenge. Interestingly, HFD induced changes in respiratory exchange ratio (RER) in male and female rats, while HCR female rat RER was largely unaffected by diet. In addition, analysis of protein FSR in skeletal muscle, brain, and liver mitochondria showed tissue-specific adaptations between HCR and LCR rats. While brain and liver protein FSR were altered by aerobic capacity and diet, these effects were less apparent in skeletal muscle. Overall, we provide evidence that greater aerobic capacity promotes elevated EE in an aged state, while also regulating metabolic flexibility in a sex-dependent manner. Modulation of mitochondrial protein FSR by aerobic capacity is tissue-specific with aging, likely due to differential energetic requirements by each tissue.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11357-023-00985-1.
Keywords: Aging, Exercise capacity, Metabolic flexibility, Mitochondrial proteostasis
Introduction
Independent of other risk factors, aerobic capacity, commonly referred to as cardiorespiratory fitness, is the most powerful predictor of several chronic disease conditions (i.e., Type 2 diabetes, cardiovascular disease) and all-cause mortality [1]. Aerobic capacity is defined as an individual’s maximal capacity for oxygen utilization during exercise, commonly assessed by VO2 max or peak [2]. Although influenced by early life apex, reductions in aerobic capacity are well characterized with aging but this decline can be partially attenuated with exercise [3, 4]. Within healthy individuals, aerobic capacity slowly declines between the ages of 20–50 years but the rate of decline accelerates to ~ 15% per decade in individuals 50 years or older, surpassing 20% by the age of 70 [5]. However, while it is known that higher aerobic capacity can prevent the onset of age-related disease, the mechanisms that foster this protection are not well understood.
Reductions in total daily energy expenditure (EE) have been illustrated to coincide with age-related decreases in aerobic capacity [6, 7]. Total EE and energy intake are the two main contributors to energy balance [8]. With aging, it has been proposed that a decrease in aerobic capacity promotes dysregulations in energy balance through reduced EE [9], shifting the system towards a net positive energy state [10]. However, elderly individuals commonly experience weight loss even in this net positive energy state [11]. The decreases in body weight is primarily associated with the loss of lean mass (primarily skeletal muscle), which is believed to occur in relation to reduced levels of physical activity and total EE [12]. While it is unclear why this phenomenon occurs with aging, the concomitant decrease in muscle function suggests a dysregulation in systemic proteostasis (protein synthesis and degradation) [13, 14]. In particular, these alterations in proteostasis promote reductions in mitochondrial content that likely lead to the well described reductions in mitochondrial function with aging [15]. These changes are suggested to occur through altered mitochondrial protein synthesis [16, 17]. While physical activity is known to preserve mitochondrial content and function during aging [18], it remains unknown how differences in aerobic capacity, independent of physical activity, may alter mitochondrial protein synthesis and systemic EE during aging.
We have utilized rats selectively bred over several generations for high (HCR) and low endurance running capacity (LCR) to specifically examine the role of intrinsic aerobic capacity on metabolic disease susceptibility [19, 20]. Selective breeding for endurance running capacity resulted in robust divergence in intrinsic aerobic capacity despite no exposure to exercise training [19]. HCR rats are resistant to metabolic disease and have a longer lifespan than their LCR counterparts [21]. Greater mitochondrial content and higher basal energy expenditure have been reported in HCR rats compared to LCR rats [21–23]. However, the effect of aging on whole-body energy expenditure and its relationship to mitochondrial protein synthesis remains relatively unexplored. Herein, we tested the hypothesis that HCR rats would maintain higher total EE, metabolic flexibility, and mitochondrial protein fractional synthesis rates in skeletal muscle, liver, and brain than in LCR rats at ~ 2 years of age. To test this hypothesis, we utilized HCR and LCR rats fed a high fat/high sucrose diet to promote the development of metabolic disease. Here we show that in aged rats, intrinsic aerobic capacity independent of diet increases systemic energy expenditure, while also promoting greater metabolic flexibility particularly in female rats. Additionally, we found aerobic capacity modulated mitochondrial protein fractional synthesis rates in a tissue specific manner in these aged rats.
Methods
Animal strain
The HCR/LCR rat model has been extensively characterized, as previously described [20, 22, 24, 25]. HCR and LCR rats possess intrinsically diverging aerobic capacities through two-way artificial selection for running time to exhaustion on a graded exercise test [20]. Male and female rats at 21-month-old (Generation 42) were housed at ~ 25 °C (12-h light cycle) and provided ad libitum access to water and low-fat, control diet (LFD) (D12110704; 10% kcals fat, 3.5% sucrose, 3.85 kcal g−1; Research Diets, Inc.). Rats were allowed 6 weeks to acclimate to the LFD before being single-housed and randomly assigned into two dietary intervention groups (n = 6–10 per group); either maintained on LFD or transitioned to a high-fat, high-sucrose diet (HFD) (D12451; 45% kcals fat; 17% kcals sucrose, 4.73 kcals g−1). After 8 weeks of dietary intervention, fed rats were anesthetized at 24-months of age between 8 and 9 am with pentobarbital (75 mg kg−1) via intraperitoneal (i.p.) injection prior to exsanguination. Tissues were excised and immediately frozen in liquid nitrogen. Due to the age of the animals utilized, 6 male rats (2 LCR and 4 HCR) and 3 female HCR rats were unable to complete the full duration of the experimental timeline and were not included in the study. The animal protocols were approved by the Institutional Animal Care and Use Committee at the University of Kansas Medical Center.
Body composition analysis
Body composition was determined before and immediately prior to completion of the dietary intervention utilizing magnetic resonance via the EchoMRI-900 analyzer (EchoMRI, Houston, TX, USA). The difference between total body mass and fat mass was used to calculate fat free mass in all animals.
Intrahepatic lipid composition
To quantify intrahepatic lipid concentrations, ~ 30 mg of frozen liver tissue was homogenized (TissueLyser II, Qiagen) in a lipid extraction solution (1:2 vol/vol methanol:chloroform) and rotated overnight at 4 °C. Following the addition of 4 mM MgCl2, samples were centrifuged to separate inorganic and organic phases. The organic phase was removed, and the inorganic phase was let evaporate overnight. The dehydrated lipids were reconstituted following the addition of 3:2 vol:vol butanol:Triton X-114 solution. Triglyceride content was determined utilizing the commercially available kit (Sigma, F6428) and normalized to hepatic tissue weight used for analysis. A small portion of fresh hepatic tissue was fixed in 10% formalin and paraffin imbedded. Hematoxylin and eosin (H&E) and trichrome staining was performed to determine fat and collagen deposition, respectively.
Indirect calorimetry and energy metabolism
4-weeks post onset of the dietary intervention, animals were placed into the Promethion continuous monitoring system (Sable Systems International, Las Vegas, Nevada) to determine systemic energy metabolism via measurement of inspired VO2 and expired VCO2 as previously described [26]. Briefly, animals were acclimated in the indirect calorimetry cages for 3 days with ad libitum access to water and diet prior to data collection. Animals were then subjected to a metabolic challenge using a 48-h fasting/refed paradigm, composed of a continuous 24-h no access to food followed by 24-h ad libitum access to diet. Total 24-h energy expenditure (EE) was calculated during both phases of the metabolic challenge utilizing a modified form of the Weir equation (Energy expenditure (kilocalories per hour) = 60 [0.003941 × VO2 + 0.001106 × VCO2]). Resting EE was determined from the 30-min period with the lowest EE and was extrapolate to 24-h to determine total resting EE. 24-h non-resting EE was determined by the difference between total 24-h total EE and resting EE. Metabolic flexibility was assessed using hourly measures of respiratory exchange ratio (RER) during the 48-h metabolic challenge using the equation RER = VCO2/VO2 to assess substrate utilization during the initial and final hour of both the fasting and refeeding phases. Slope of RQ change over the initial 10-h period of the fasting and refeeding phases of the metabolic challenge was used to determine the rate of change in substrate utilization during these phases.
Protein fractional synthesis rate
One week prior to end of dietary intervention, an i.p. bolus of 99% deuterium oxide (D2O) was administered to enrich to 5% of total body water composition, determined based on body weight (~ 60% body weight as water). Ad libitum access to 8% D2O was provided for the remainder of the 7-day labeling period to maintain steady state of body water enrichment. Due to health concerns, 4 male rats (3 LCR and 1 HCR) and 5 HCR female rats did not receive deuterium labeling. Isotope (D2O) analysis of protein fractional synthesis rate (FSR) was performed as previously described [27]. Briefly, powdered tissue was weighed (~ 50 mg) and homogenized in isolation buffer (100 mM KCl, 40 mM Tris HCl,10 mM Tris Base, 5 mM MgCl2, 1 mM ethylenediaminetetraacetic acid (EDTA), 1 mM ATP, pH = 7.5) with protease and phosphatase inhibitors (Halt, Thermo Fisher Scientific, Waltham, MA, USA). Differential centrifugation was performed to separate mitochondrial and cytoplasmic/myofibrillar fractions. Samples were then resuspended in a second isolation buffer (100 mM KCl, 10 mM Tris HCl, 10 mM Tris Base, 1 mM MgCl2, 0.1 mM EDTA, 1.5% bovine serum albumin, pH = 7.5). Samples again underwent differential centrifugation and resuspended in 1 M NaOH. Samples were then hydrolyzed for cation exchange and derivatized alanine was analyzed using gas chromatography-mass spectrometry. Precursor enrichment was determined by D2O concentration within body water using plasma as previously described [27].
Western blotting
Liver homogenate was used to produce western-ready laemmli samples followed by SDS-PAGE to allow separation of proteins within sample. Proteins were then transferred to polyvinylidene difluoride membrane and expression of subunit proteins associated with oxidative phosphorylation was assessed using the Total OXPHOS antibody (ab110413) at a concentration of 1:2000 purchased from Abcam Inc. (Cambridge MA, USA). Quantification was analyzed using a densitometer (Bio-Rad Laboratories, Hercules, CA, USA) and normalized by total protein determined using 0.1% amido-black (Sigma-Aldrich).
Statistical analysis
Outliers were removed utilizing Grubbs method in Prism 8 (GraphPad). Three-way ANOVA were utilized to determine main effects of sex (SPSS Statistics, IBM Corp., Armonk, NY, USA). Within sex differences were assessed using a two-way ANOVA to determine main effects of strain, diet, and interactions. When a significant main effect or interaction was observed, post hoc analysis was performed using Fisher’s least significant difference (LSD) to assess specific pairwise differences. Additionally, two-way ANCOVA was used for total, non-resting, and resting EE, with total body mass used as a covariate to control for sex and strain body mass differences. A repeated measures ANOVA was used to determine changes in RER over time. Linear regression analysis was used to determine correlations between total EE and tissue mitochondrial protein fractional synthesis rates. Goodness-of-fit (r2) are presented to show linearity. For all comparisons, statistical significance was set at p < 0.05 and data are presented as means standard error mean (SEM) (GraphPad Prism 8).
Results
Body composition
Male and female rats differed significantly in final body mass, with males weighing ~ 40% more than females (Table 1, main effect of sex, p < 0.0001). Differences in body mass within male rats was driven by strain, with LCR males weighing ~ 23% more than HCR (Table 1, main effect of strain, p < 0.01). These differences in body mass were mainly attributed to disparities in fat-free mass, with LCR males possessing 19% greater fat-free mass than HCR (Table 1, main effect of strain, p < 0.01). Interestingly, while male LCR rats tended to have greater fat mass (p = 0.053), there was no effect of diet on fat mass. Similar to males, female LCR rats had greater total body mass, weighing ~ 41% more than HCR females regardless of diet (Table 1, main effect of strain, p < 0.001). LCR females possessed ~ 36% more fat mass (Table 1, main effect strain, p < 0.001) and ~ 19% fat-free mass (Table 1, main effect strain, p < 0.01), contributing to their greater total body mass. The expanded fat mass in LCR females resulted in a greater overall percent (%) fat mass compared to HCR (Table 1, main effect strain, p < 0.001). Interestingly, diet had no impact on any body composition parameter in either sex, suggesting intrinsic aerobic capacity has a more profound impact on body composition with aging than diet.
Table 1.
Animal anthropometrics following 8 week diet intervention for 24 month aged low capacity runner (LCR) and high capacituy runner (HCR) rats
| Males | Females | 2-way ANOVA | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| LCR | HCR | LCR | HCR | Within Sex Significance | ||||||
| LFD | HFD | LFD | HFD | LFD | HFD | LFD | HFD | Male | Female | |
| Final Anthropometrics | ||||||||||
| Body Mass (g) | 536.9 ± 31.1 | 596.4 ± 24.9* | 446.3 ± 27.9 | 478.4 ± 21.2 | 355.6 ± 19.9 * | 368.0 ± 22.4* | 243.3 ± 13.6 | 271.5 ± 34.5 | Strain p < 0.01 | Strain p < 0.001 |
| Fat free mass (g) | 420.4 ± 31.5* | 429.9 ± 16.0* | 350.3 ± 13.3 | 365.5 ± 16.0 | 257.9 ± 8.4 * | 262.6 ± 9.2* | 213.0 ± 7.8 | 223.8 ± 23.4 | Strain p < 0.01 | Strain p < 0.01 |
| Fat mass (g) | 116.5 ± 18.2 | 166.6 ± 23.5 | 96.0 ± 15.4 | 112.9 ± 9.2 | 97.6 ± 14.1 * | 105.4 ± 17.7* | 30.3 ± 8.0 | 47.7 ± 15.5 | Strain p = 0.053 | Strain p < 0.001 |
| Body Fat % | 21.7 ± 3.1 | 27.4 ± 3.4 | 20.8 ± 2.4 | 23.5 ± 1.3 | 26.7 ± 2.5 * | 27.5 ± 3.3* | 12.0 ± 2.6 | 16.4 ± 4.3 | Strain p < 0.01 | |
| Liver | ||||||||||
| Trigylcerides (mg/g) | 153.8 ± 42.1 # | 286.8 ± 50.9 | 262.0 ± 32.2 # | 391.3 ± 38.2 | 240.8 ± 40.8 | 213.4 ± 25.9 | 174.7 ± 32.3 | 260.3 ± 12.3 | Strain p < 0.05, Diet p < 0.01 | |
Data are report as mean ± SEM, n = 3–10 per group*p < 0.05 Main effect of Strain, #p < 0.05 Main Effect of Diet
Liver triglycerides
Intrahepatic triglyceride levels in male rats were significantly influenced by diet (Table 1, main effect diet, p < 0.01), with HFD fed LCR and HCR rats showing ~ 86% and ~ 49% more triglyceride content compared to LFD fed rats, respectively. Interestingly, male HCR rats had elevated levels of triglyceride storage in both the LFD and HFD compared to LCR within treatment group (Table 1, main effect strain, p < 0.05). Importantly, we observed no strain and diet effect on female hepatic triglyceride content, suggesting females are protected against HFD-induced liver triglyceride storage independent of intrinsic aerobic capacity. Additionally, male rats tended to have higher liver triglycerides than females (Table 1, p < 0.068). Trichrome staining of liver sections showed similar trends in hepatic lipid deposition (Supplemental Fig. 1), with minimal collagen formation present in all groups. This is supported by no transcriptional or protein content differences in various fibrosis markers (data not shown).
Energy expenditure and substrate utilization: Effects of 24 h fasting
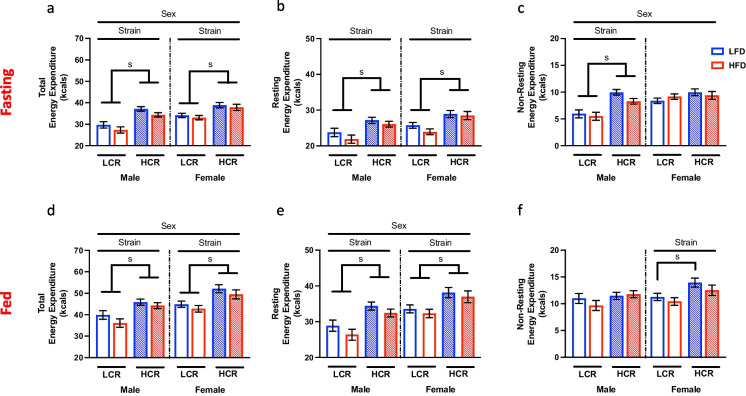
Previously, we have reported elevated EE in HCR rats when adjusted (covariate) for total body mass differences between strains [28]. Here we sought to determine if differences in EE are maintained in 2-year-old rats and the influence of HFD feeding on EE. We paired a chronic HFD with assessments of metabolic flexibility by measuring hourly response to a 24 h fast, immediately followed by a refeeding paradigm to evaluate the response to an acute metabolic stress. Analysis of total EE during the 24-h fasting period revealed sex differences in EE during the fasting period, with female rats possessing ~ 12% greater EE (Fig. 1a, main effect sex, p < 0.01,). Within male and female rats, HCR rats had elevated EE compared to LCR rats (Fig. 1a, main effect strain, p < 0.0001 and p < 0.001 respectively). Total EE can be further extrapolated into its two main components: resting EE and non-resting EE. HCR rats displayed greater resting EE than LCR rats in both male and female rats (Fig. 1b, main effect strain, p < 0.01 and p < 0.001 respectively). While there are no sex differences in resting EE (p = 0.09), non-resting EE was elevated in females compared to males (Fig. 1c, main effect sex, p < 0.05). This was in part due to LCR females having non-resting EE similar to HCR females, while this was markedly reduced in male LCR compared to male HCR (Fig. 1c, main effect strain, p < 0.0001). Therefore, EE differences in female HCR and LCR rats was driven by resting EE. However, both non-resting EE and resting EE contributed to the greater total EE seen in HCR over LCR males.
Fig. 1.
Metabolic challenge induced changes in Energy Expenditure. Daily energy expenditure (EE) was assessed during a 48-h metabolic challenge (fasting/refeed) in high-capacity (HCR) and low-capacity (LCR) runners either on a low-fat diet (LFD) or a high-fat diet (HFD). a Total EE during initial 24-h fasting period. Resting EE (b) and non-resting EE (c) were calculated from the total EE during the fast. d 24-h total EE during the refeeding period. Both resting EE (e) and non-resting EE (f) were extrapolated from the total EE during this period. Data are presented as covariate means ± SEM. n = 5–10/group. s = strain effect p < 0.05
During the 24-h refeeding period, female rats continued to maintain a ~ 9% greater total EE compared to male rats (Fig. 1d, main effect sex, p < 0.01). Mimicking the fast, HCR rats sustained expanded total EE compared to LCR in both males (Fig. 1d, main effect strain, p < 0.001) and females (Fig. 1d, main effect strain, p < 0.001). While all animals increased resting EE during the refeeding period, female rats expanded resting EE to a greater extent than males, resulting in significantly greater resting EE compared to males (Fig. 1e, main effect sex, p < 0.01) However, regardless of sex, HCR had elevated resting EE compared to LCR rats (Fig. 1e, main effect strain, Males, p < 0.001; Females, p < 0.01). Interestingly, sex differences in non-resting EE seen in the fasting period were not apparent during the refeeding period. However, female HCR expanded their non-resting EE during the refeed resulting in significantly greater non-resting EE than LCR (Fig. 1f, main effect strain, p < 0.01). Differences in non-resting EE in male LCR and HCR rats was abolished during refeeding as a result of increased non-resting EE in LCR rats.
In summary, EE in aged HCR and LCR rats appears to be largely influenced by intrinsic aerobic capacity with HCR rats sustaining higher overall EE in both females and males. In young rats, HFD has been shown to increase EE [29, 30]. However, in the present study, diet did not modulate any aspect of EE during both phases of the metabolic challenge, suggesting the aging process impairs the capacity to increase EE in response to HFD regardless of intrinsic aerobic capacity. Interestingly, aged female rats can sustain greater EE in response to acute metabolic stress compared to males. This suggests differential mechanisms of regulating EE during metabolic stress between male and female rats, independent of intrinsic aerobic capacity.
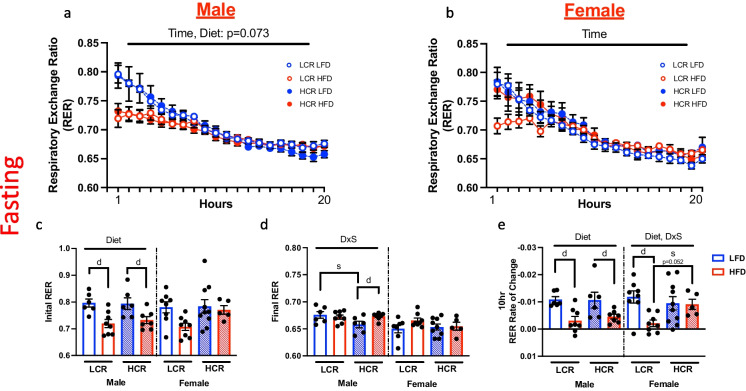
Hourly change in RER during 24-h fast
During the 24-h fasting, average hourly RER was continually monitored to assess changes in substrate utilization over the course of the fast (Fig. 2a and b). Only the first 20 h is shown graphically due to discrepancies in recording time between groups. In male rats, HFD feeding decreased RER at the start of the fast compared to LFD (Fig. 2c, main effect diet, p < 0.001), indicative of greater fat utilization for energy production (Fig. 2c). Surprisingly, this was not observed in HFD-fed female HCR, which had similar initial RER compared to LFD fed rats (Fig. 2c). Conversely, HFD did cause a lower initial RER in the HFD fed LCR females, however, there was no main effect of diet. While male LFD-fed HCR had lower final RER compared to HFD fed HCR rats (Fig. 2d, interaction diet and strain, p < 0.05), no differences were observed in the RER of LCR. All female groups had a similar final RER (Fig. 2d). To further phenotype metabolic flexibility, we calculated the rate of change in RER during the initial 10 h of the fast when the change in RER was most linear (Fig. 2e). In male rats, LFD fed rats showed a greater rate of decline in RER compared to HFD fed rats, regardless of strain (Fig. 2e, main effect of diet, p < 0.001). In contrast, while the LCR females showed a comparable response to the male (Fig. 2e, main effect diet, p < 0.01), rate of RER change was unaffected in HCR female rats by diet.
Fig. 2.
Metabolic flexibility during 24-h fasting. Hourly change in respiratory exchange ratio (RER) in HCR and LCR rats on either LFD or HFD. Hourly RER measures in male (a) and female rats (b) during the initial 20 h of fasting. c Starting RER on both male and female rats. d RER following 20 h of fasting in male and female rats. e Rate of change in RER was determined by calculating the slope between the initial RER and the RER 10 h post the initiation of the fast. Data are presented as means ± SEM. n = 5–10/group. d = diet effect p < 0.05, s = strain effect p < 0.05, Interaction = DxS p < 0.05
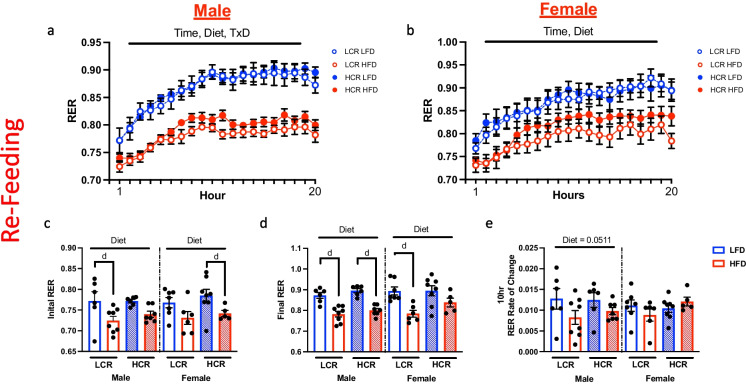
Continuous monitoring of RER was also assessed during the refeeding period (Fig. 3a and b). During the first hour of refeeding, all LFD fed rats rapidly increased RER following access to food (Fig. 3c, main effect diet, males p < 0.01; females p < 0.01) while HFD unsurprisingly promoted a lower RER (males p < 0.0001; females p < 0.001). Diet had a more significant effect on RER in the male LCR rats (p < 0.05) than HCR rats (p = 0.09). Interestingly, this was reversed in females. While strain had no influence on final RER in males, HFD influenced RER in female LCR (Fig. 3d, main effect diet, p < 0.01) to a greater extent than that observed in female HCR rats (p = 0.079). Rate of change in RER was determined during the initial 10 h of refeed (Fig. 3e). While no differences were observed within the females, HFD tended to limit the rate of change in RER during this period in males (p = 0.051). Taken together, this data suggests a unique capacity for female rats to dynamically respond to a metabolic stress. In females, this appears to be through sustained higher basal RER on HFD, allowing for more dynamic responses to metabolic stress compared to males.
Fig. 3.
24-h refeeding induced changes in RER. Metabolic flexibility based on change in RER during 24-h refeeding. Hourly change in RER during refeeding in male (a) and female rats (b). c RER following the initial hour of ad libitum access to diet. d RER during the 20.th hour of refeeding. e Slope between initial RER and at 10 h post access to food was calculated to assess rate of change in RER during the initial phase of refeeding. Data are presented as means ± SEM. n = 5–10/group. d = diet effect p < 0.05
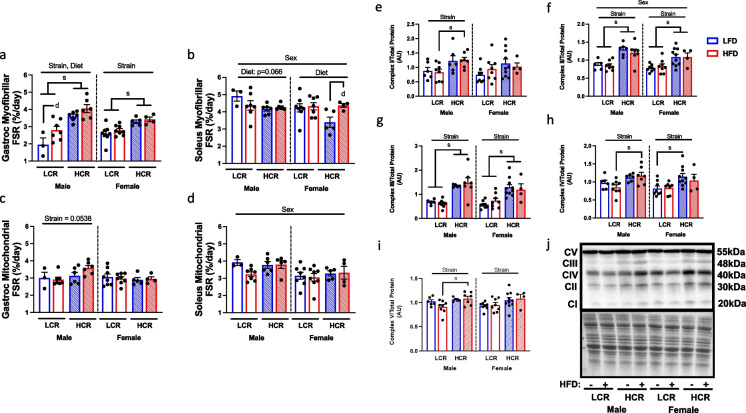
Skeletal muscle protein synthesis rate
To determine how differences in intrinsic aerobic capacity and EE modulate protein synthesis in aged HCR and LCR rats, we employed D2O labeling techniques to compute the rate of protein synthesis in skeletal muscle. We first analyzed myofibrillar protein fractional synthesis rate in both gastrocnemius (mixed red and white fibers) and soleus muscle (primarily red fibers). Within the gastrocnemius, HCR rats had elevated myofibrillar protein FSR compared to LCR rats (Fig. 4a, main effect strain, males p < 0.0001; females p < 0.001). While diet did not impact FSR in females, HFD increased myofibrillar protein FSR in males (Fig. 4a, main effect diet, p < 0.05). Intriguingly, strain had no effect on protein FSR in the more oxidative soleus muscle (Fig. 4b). Instead, myofibrillar protein FSR was driven by sex (Fig. 4b, main effect sex, p < 0.05), with female rats having lower protein FSR, largely due to decreased protein FSR in LFD fed HCR rats (main effect diet, p < 0.05). However, HFD promoted greater protein FSR in HCR rats comparable to the rates present in LCR rats. This differential regulation of myofibrillar protein FSR between gastrocnemius and soleus suggests possible influence of fiber type, degree of chronic activity, and/or motor unit activity which may influence the impact of intrinsic aerobic capacity on protein FSR.
Fig. 4.
Fractional synthesis rate, and mitochondrial protein expression in skeletal muscle. Deuterated water (D2O) was used to assess protein fractional synthesis rate (FSR) of myofibrillar and mitochondrial proteins within skeletal muscle followed by expressional analysis of mitochondrial oxidative phosphorylation proteins. Myofibrillar protein FSR was determined in gastrocnemius (a) and soleus muscle (b). Mitochondrial fractions were isolated and D2O labeling was used to determine protein FSR in gastrocnemius (c) and soleus (d) muscle. e-j Western blot analysis was performed to determine the expression of all five complexes involved in mitochondrial oxidative phosphorylation (OXPHOS) within the gastrocnemius (h). Representative images of OXPHOS proteins and amido black staining for loading control. Data are presented as means ± SEM. n = 3–10/group. Main effect d = diet effect p < 0.05, s = strain effect p < 0.05
Mitochondrial protein synthesis rate and expression
Higher mitochondrial content and enzymatic and respiratory function in skeletal muscle, liver, and brain of HCR vs. LCR rats has been identified as one of the principle diverging factors resulting from selective breeding for the two strains [25, 31]. Here we examined if mitochondrial protein synthesis in various metabolic tissues was different between aged HCR and LCR rats. In contrast to myofibrillar protein FSR in gastrocnemius, mitochondrial protein FSR in females was not different between LCR and HCR (Fig. 4c). However, male HCR tended to have greater mitochondrial protein FSR compared to male LCR (p = 0.053) in gastrocnemius muscle. In soleus muscle, there was no difference between HCR and LCR rats in mitochondrial protein FSR, however, there was a sex effect with male rats showing elevated mitochondrial protein synthesis compared to females (Fig. 4d, main effect sex, p < 0.01).
While we did not observe large strain or diet-mediated difference in bulk mitochondrial protein FSR, we next sought to assess whether expression of subunit proteins for all five complexes associated with oxidative phosphorylation (C1 subunit NDUFB8, CII subunit SDHB, CIII subunit UQCRC2, CIV subunit MTCO1, CV subunit ATP5A) was different between strains due to the known differences in oxidative capacity between HCR and LCR rats. Assessment of these protein in skeletal muscle was only performed in gastrocnemius muscle due to the soleus muscle tissue being entirely used for the fractional synthesis analysis. Intriguingly, we found that HCR rats possessed elevated expression in all five complex proteins, regardless of sex or diet (Fig. 4 d-i, p < 0.05). Within the males, strain effect was pronounced between the HCR and LCR rats on HFD for complex I, IV and V. Meanwhile, strain effect in females was greater between the LFD fed HCR and LCR in complex I, II, IV. Complex II (SDHB) was the only complex to show sexual dimorphism in expression (Fig. 4f, main effect sex, p < 0.05), with reduced expression in females. Together, this suggests that while the rate of global mitochondrial protein synthesis in skeletal muscle is largely unaltered by intrinsic aerobic capacity, HCR rats possess elevated OXPHOS protein expression, likely attributing to increased mitochondrial oxidative capacity.
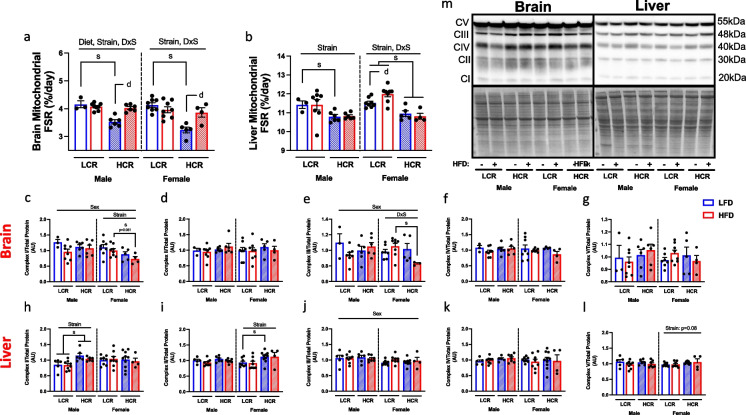
Further assessment of mitochondrial protein synthesis and expression was performed in brain and liver tissues. Unlike skeletal muscle, mitochondrial protein FSR was strongly associated with strain for both tissues. In the brain, both males and females HCR rats had lower mitochondrial protein FSR on LFD (Fig. 5a, main effects strain, male p < 0.01; female p < 0.001), however, HFD promoted greater protein FSR, similar to that observed in LCR. Similar to brain, liver mitochondrial protein FSR was reduced in both male and female HCR (Fig. 5b, main effect strain, male p < 0.05; Female p < 0.0001). While no effect of diet was observed in male rats, HFD altered protein FSR in a strain dependent manner in the females, with HFD increasing mitochondrial protein FSR in LCR rats (Fig. 5b, diet and strain interaction, p < 0.05).
Fig. 5.
Assessment of liver and brain mitochondrial protein synthesis and expression. Protein fractional synthesis rate and mitochondrial OXPHOS protein expression was determined in liver and brain isolated mitochondria fractions. a Protein FSR in isolated brain mitochondria. b Isolated liver mitochondrial protein FSR. c-g Protein expression of brain mitochondria OXPHOS proteins (complex I-V) using western blotting. h–l Western blot analysis of liver isolated mitochondrial OXPHOS protein expression (complex I-V). m Representative western blot images of liver and brain OXPHOS proteins and total protein staining. Data are presented as means ± SEM. n = 3–10/group. Main effect d = diet effect p < 0.05, s = strain effect p < 0.05
Mitochondrial oxidative phosphorylation subunit proteins were then quantified in both brain and liver whole homogenates. Interestingly, unlike the gastrocnemius, strain had minimal influence on complex expression (Fig. 5c-g). Male rats did possess greater complex I expression compared to female rats (Fig. 5c, main effect sex, p < 0.05). This was largely due to lower complex I expression in female HCR rats (Fig. 5c, main effect strain, p < 0.05). While there were no main effects of strain on complex III expression, HCR tended to have reduced complex III expression driven by HFD (Fig. 5e, p = 0.08). Within the liver, strain differences in complex expression were sex dependent, with HCR males possessed higher expression of complex I compared to LCR (Fig. 5h, main effect strain, p < 0.001), while HCR females had greater complex II protein expression compared to LCR (Fig. 5i, main effect strain, p < 0.05). Additionally, while females had lower protein expression of complex III (Fig. 5j, main effect sex, p < 0.05), no sex differences in complex IV and V expression were observed (Fig. 5k-l). Overall, this suggests tissue specific alterations to mitochondrial protein synthesis suggestive of a differential role of mitochondrial biogenesis and turnover rate with aging and intrinsic aerobic capacity.
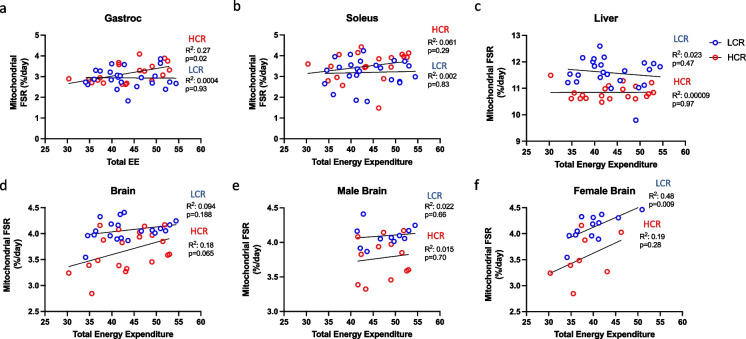
Due to observed strain differences between HCR and LCR rats in both energy expenditure and mitochondrial protein FSR, we next sought to determine if any correlations existed between these two factors to suggest co-regulation. Correlations were run within HCR and within LCR strains. While no correlations were observed within the LCR rats, we did observe a significantly positive correlation in mitochondrial FSR in the gastrocnemius of HCR rats (R2 = 0.27, p < 0.05, Fig. 6a). However, total EE did not correlate with liver, brain, or soleus mitochondrial protein FSR in either LCR or HCR rats (Fig. 6b-d). To assess sex-specific correlation, we independently assessed female and male rats between the two strains. While total EE did not correlate with brain mitochondrial FSR in male HCR (Fig. 6e, R2 = 0.015, p = 0.70) or LCR rats (R2 = 0.02, p = 0.66), female LCR rats displayed a significant correlation between brain mitochondrial FSR and total EE (Fig. 6f, R2 = 0.48, p = 0.009). This correlation was not observed in female HCR rats (R2 = 0.19, p = 0.28). No correlations between strain, total EE and mitochondrial FSR were found in any other tissue within either male or female rats (Supplemental Fig. 2).
Fig. 6.
Correlations between energy expenditure and mitochondrial fractional synthesis rates. Assessment of tissue-specific correlations in mitochondrial proteins FSR and energy expenditure between HCR and LCR rats. Correlations were assessed between HCR and LCR rats in (a) Gastrocnemius, (b) soleus, (c) liver and (d) brain tissues. Within the brain, correlations were assessed within sexes to determine differential regulation of mitochondrial FSR between (e) HCR and LCR males and (f) HCR and LCR female rats. Data are plotted based on total energy expenditure (without covariate of body mass) and tissue mitochondrial protein FSR. Significance was set to p < 0.05 with the associated R2 value to show strength of correlation between strain
Discussion
Despite aging being associated with the onset of most chronic diseases, the mechanisms that account for this increased susceptibility remain largely unknown. Likewise, determining factors that influence and even counteract the process of aging has been a highly sought-after target of scientific inquiry. Epidemiological data clearly shows that differences in aerobic capacity are one of the most powerful physiological factors influencing mortality and risk for disease during aging [32]. In this study, we sought to determine if divergence in intrinsic aerobic capacity in aging rats impacts EE, metabolic flexibility, and mitochondrial protein synthesis rates in key metabolic tissues. We leveraged rats with intrinsically high (HCR) vs. low aerobic capacity (LCR) that have previously been shown to have a significant difference in lifespan [33]. Our results show that intrinsic aerobic capacity directly modulates both resting and non-resting EE, and to a lesser extent metabolic flexibility in aged rats. Importantly, these differences in EE correspond with tissue-specific regulation of mitochondrial protein synthesis rates and oxidative phosphorylation protein content, serving as possible sources for the discrepancies in EE between HCR and LCR rats. Regardless of aerobic capacity or diet, sex appears to be a critical factor in regulating these measures during the process of aging. Overall, the data shows that elevated aerobic capacity may foster protections against age-related disease and mortality by sustaining elevations in EE regardless of dietary intervention, as well as directly modulating tissue-specific mitochondrial protein synthesis.
High vs. low aerobic capacity changes susceptibility to an array of chronic diseases, particularly cardiovascular disease, type 2 diabetes, and hypertension [34]. In addition, aerobic capacity is a powerful predictor of longevity [35]. Daily physical activity levels and exercise status, behaviors known to improve aerobic capacity, have been repeatedly shown to improve overall health status, increasing life expectancy in both men and women by approximately 4 years [36]. Utilization of models with intrinsic differences in aerobic capacity serve as powerful tools to understand how aerobic capacity modulates mortality independent of exercise. The HCR/LCR rat model shows differences in aerobic capacity between strains, with HCR rats possessing elevated untrained VO2 max compared to LCR throughout their lifespan [33]. The higher aerobic capacity in HCR rats compared to LCR rats promotes protection against HFD-induced weight gain and development of metabolic disease that may afford HCR rats an extended lifespan [21, 22, 37]. However, these studies have predominately focused on using relatively young rats, and thus have not investigated how aging may affect the response to HFD in aged HCR/LCR rats.
In the current study, aged LCR rats weighed significantly more than HCR rats regardless of diet, suggesting that even in an aged state, aerobic capacity may regulate body composition. Interestingly, we did observe increased hepatic triglyceride storage in HCR male rat. This contradicts previous studies which have shown that short-term and chronic HFD feeding is capable of rapidly increasing fat mass in young (25–30-week-old) LCR rats to a far greater extent than HCR [28, 38]. However, these studies were performed in a fasted state, which acutely drives lipolysis and lipid uptake within the liver. Other studies conducted in a fed-state have reported expanded triglyceride content in HCR rats [22], suggestive of an athlete’s paradox-like state within the HCR (higher mitochondrial content paired with higher lipid storage like that reported in skeletal muscle of athletes) [39]. It is believed that exercise-induced alterations in lipid metabolism can increased lipid content, particularly polyunsaturated lipid, in skeletal muscle and liver independent of adverse metabolic effects, such as insulin resistance development and may explain the expanded storage seen within male HCR rats [39, 40]. This increase in intrahepatic lipid storage may be due to expanded fatty acid oxidation corresponding to elevated mitochondrial oxidative capacity and content previously described in HCR rats [22, 23, 41]. Females appear to be protected against both diet-induced and strain mediated increases in lipid storage.
Beyond aerobic capacity, systemic energy expenditure (EE), particularly basal metabolic rate, is known to decrease with aging [6, 7]. Age-induced alterations in EE appear to be prominently driven by changes in body composition, primarily loss of lean body mass [12, 42]. Daily EE is a critical factor in preserving metabolic flexibility with age, particularly in women [43]. Here, we show that aged HCR rats maintain elevated EE during the aging process compared to LCR counterparts. In particular, HCR displayed higher EE in both sexes, during a two-phase metabolic challenge (24 h fast/refeed) compared to LCR and this was not influenced by diet. While female rats show reduced total EE compared to males, correcting for differences in body weight (covariate) revealed that female HCR also have higher total EE than LCR. Interestingly, increased EE in HCR rats did not appear to influence metabolic flexibility during either part of the metabolic challenge regardless of diet, a response that counteracts previous findings of wide differences in younger rats [44]. However, the RER of female rats was less influenced by diet compared to male rats regardless of strain, particularly during the 24-h fasting period. We previously reported greater metabolic flexibility in response to a HFD in mice with higher EE vs. lower EE (caused by differences in housing temperatures) leading us to posit that EE may also influence metabolic flexibility in older rats [26]. Moreover, we had previously found profound differences in metabolic flexibility between younger HCR and LCR rats measured by indirect calorimetry, and various other metabolic outcomes including metabolic tracers and hyperinsulinemic-euglycemic clamp conditions [44]. However, the current results provide evidence that differences in EE and aerobic capacity may modulate metabolic flexibility in an aged state, particularly within females. Taken together, divergence in aerobic capacity maintains a divide for total EE during aging that is not influenced by diet.
Age-associated declines in aerobic capacity increase susceptibility to various diseases, particularly metabolic diseases [5, 6]. The difference in EE between aged HCR and LCR rats in this study suggests a prominent role of total EE in modulating declines in aerobic capacity, which is further supported by evidence in humans that increasing physical activity or performing regular exercise can partially alleviate the progressive reduction in aerobic capacity that occurs across lifespan [45]. Additionally, recent studies suggest that approximately 50% of overall fitness is attributed to genetic factors [46]. Therefore, energy metabolism differences between untrained 24-month-old HCR/LCR rats can provide powerful insight into how genetic factors may influence EE. Previous studies assessing EE in young HCR and LCR rats have reported similar differences in EE as reported here [6, 47]. These studies highlight differences in thermogenesis that in part drive the different EE between HCR and LCR rats. Previous studies utilizing caloric restriction revealed that HCR rats show enhanced modulation of thermogenesis through direct regulation of non-resting EE [47]. Additionally, female HCR rats possess heightened non-resting EE when matched for lean mass to LCR rats by maintaining greater expression of mitochondrial uncouplers (UCP2 and UCP3) that promote a 1 °C higher skeletal muscle temperature [48]. Overexpression of uncoupling proteins has been shown to decrease risk weight gain and metabolic disease [49–51]. This increased expression of mitochondrial uncouplers appears to be sustained with aging [33], signifying the uncoupled-like state of HCR rats present in both young and aged HCR rats may sustain the elevated EE. This evidence suggests a central role of mitochondrial protein regulation in maintaining EE with aging.
Both total EE and metabolic flexibility are largely governed by the energetic demand of metabolically active tissues, primarily skeletal muscle, liver, brain and adipose tissues [52]. In order to maintain systemic energy homeostasis, mitochondria generate ATP through oxidative phosphorylation to meet tissue-specific and systemic energetic needs. Aerobic capacity and physical activity status are known to increase markers of mitochondrial content and oxidative metabolism in humans [53, 54], and in rodent models [23]. Moreover, we and others have previously found that adult HCR rats have greater mitochondrial number, fat oxidation, and respiratory capacity in skeletal muscle, adipose, and liver compared to LCR rats [21]. However, mitochondrial proteostasis has been shown to become altered with aging [55], resulting in reduced mitochondrial respiratory capacity and elevated reactive oxygen species production [56, 57]. Although, this continues to be debated. Using novel D2O labeling techniques, we assessed the mitochondria of various metabolic tissues by determining protein FSR and OXPHOS protein expression. Both mitochondrial protein FSR and expression of OXPHOS proteins showed dynamic tissue and sex-specific regulation in aged HCR and LCR rats. These disparities in the regulation of mitochondrial protein FSR and OXPHOS protein expression across metabolic tissues may represent differential effects of aging on tissue function, energetic requirements, and protein turnover [58].
Declining rates of mitochondrial protein synthesis are known to occur with aging in skeletal muscle, coinciding with reductions in overall mitochondrial function [17]. Exercise is able to moderate the effects of aging by promoting greater translational rates and protein content overtime [59], likely regulated by PGC1-α [60]. Within the HCR and LCR rat model strains, differences in the regulation of mitochondrial protein synthesis have been reported in multiple tissues [51, 61, 62]. Here, we show tissue-specific regulation of mitochondrial protein FSR in HCR and LCR rats. Within skeletal muscle, we observed minimal effect of aerobic capacity on FSR. Regardless of mitochondrial FSR, both male and female HCR rats displayed elevated expression of OXPHOS proteins, suggesting that HCR rats retain greater mitochondrial content and possibly function with aging and therefore proteostasis is better maintained with aging. Surprisingly, HCR rats displayed not only reduced liver mitochondrial protein FSR, but comparable expression of liver OXPHOS proteins to LCR rats (except for complex I in males and complex II in females). While mitochondrial respiration was not assessed in this study, previous studies have shown robust differences in liver mitochondrial respiratory capacity between HCR and LCR rats [23, 28], suggestive that these strain differences would remain with aging. This may explain the increased FSR for the LCR rats, which sustain an increased synthesis to compensate for age-induced deficits in respiratory capacity while the HCR rats remain protected. While other studies have reported aged HCR rats possessing greater levels of proteins regulating mitochondrial biogenesis within the hippocampus [63], we observed lower mitochondrial protein FSR within the whole brain of HCR rats. While HFD-feeding has been shown to increase expression of markers for mitochondrial biogenesis in the brain of LCR rats, here we show that HCR rats increased mitochondrial protein FSR in response to HFD to similar levels exhibited in LCR rats [64]. This HFD-induced increase in FSR within the HCR may serve as a mechanism of protection against diet-induced mitochondrial dysfunction that can occur with aging [65], likely occurring in coordination with increased levels of mitophagy or selective-degradation of damaged/low-functioning mitochondria. The LCR rats not increasing mitochondrial protein FSR could be due to decreased mitophagic flux that is known to occur with aging, which may contribute to cognitive declines reported in these animals [66].
Previous studies suggest that HFD feeding can alter protein homeostasis in metabolic tissues including influencing protein breakdown. However, we did not quantify protein degradation in the current report. Our study employed D2O labeling in the last week of the 8-weeks of high fat feeding period. It is unlikely that mitochondrial protein concentration changed during this last week of diet as the mitochondrial protein content had likely reached a new steady state. By definition, the steady state means that protein breakdown equals protein synthesis. If that was not true, protein concentrations would increase or decrease. However, we do not have mitochondrial protein concentrations at week 7 and week 8 to absolutely confirm the steady state. This issue remains a challenge to the field, where protein content markers can differ depending on which one is utilized. Another difficulty is obtaining samples in the same animal at different time points to determine if changes in protein concentrations occurred. Therefore, one can assume that the breakdown rates are equal to synthesis from week 7 to week 8. It is possible that changes in autophagy and mitophagy in metabolic tissues were induced during the initial transition to the HFD, but we did not perform D2O labeling during this period.
Several limitations exist within this study, largely due to the limited lifespan of the rats. During the course of the study, 6 male rats (2 LCR and 4 HCR) and 3 female HCR rats were euthanized early on in the intervention, with an additional set of 4 male rats (3 LCR and 1 HCR) and 5 HCR female rats experiencing declines in health several days before the completion of the study, resulting in the inability to capture final body composition or protein FSR within these animals. This limited comparisons between sexes and severely reduced statistical power. Additionally, this promotes a survival effect in which these outcome measures are biased to the animals that maintained health through the course of the study. While we did attempt to determine mitochondrial protein FSR in white adipose tissue, we were unable to attain this data due to the characteristics of adipose that hamper the extraction of mitochondrial proteins. Additionally, we were unable to attain measures of mitochondrial function beyond mitochondrial protein FSR or OXPHOS expression, reducing the ability to determine how these factors and aerobic capacity/EE contribute to metabolic disease with aging.
In summary, we demonstrated an association between intrinsic aerobic capacity and total EE in 24-month aged rats, likely contributing to lower body fat in HCR rats. We found almost no differences between HCR and LCR rats for metabolic flexibility due to fasting, re-feeding, or HFD. Additionally, regulation of mitochondrial protein fractional synthesis and expression of OXPHOS proteins showed tissue-specific regulation by aerobic capacity, diet, and sex. These data provide new insight into the role of divergence in aerobic capacity on EE and mitochondrial homeostasis in metabolic tissues providing physiological and biochemical context to the powerful role of aerobic capacity on setting a divide for mortality and risk of chronic disease.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplemental Fig. 1. Representative images of hepatic triglyceride storage. Representative images of Hematoxylin and eosin staining on liver sections in male and female HCR rats fed either a HFD or LFD (TIFF 163854 KB)
Supplemental Fig. 2. Skeletal muscle and liver correlations between energy expenditure and mitochondrial protein fractional synthesis rates. Correlations between total EE and mitochondrial protein FSR in (a) male and (b) female gastrocnemius muscle, soleus (c, d) and liver (e, f) (TIFF 4004 KB)
Acknowledgements
Edziu Franczak was involved in the investigation, statistical analysis, visualization, and writing of the original draft. Adrianna Maurer was involved in the investigation, methodology, and writing-review and editing. Vivien Csikos Drummond, Emily Wells, Madi Wenger, Frederick F. Peelor III, Abby Crosswhite, and Benjamin F. Miller were involved in the investigation, statistical analysis, visualization, and writing-review and editing, Colin S. McCoin and Benjamin A. Kugler were involved in the investigation and writing-review and editing. Steven L. Britton and Lauren G. Koch were involved in the development of rat models and writing-review. John P. Thyfault was involved in the conceptualization, methodology, formal analysis, investigation, funding acquisition, and writing-review and editing.
Funding
This project was supported in part by National Institutes of Health R01DK121497 (JPT) and R01DK121497-03A1 Supplement (JPT), and the National Institute of General Medical Sciences S10OD028598 (JPT). The HCR/LCR rat models were funded by National Institutes of Health Office of Research Infrastructure Programs Grant P40OD-021331 and 3P40OD021331-06S1 (LGK and SLB).
Declarations
Competing interest
The authors declare no competing interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Myers J, et al. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med. 2002;346(11):793–801. doi: 10.1056/NEJMoa011858. [DOI] [PubMed] [Google Scholar]
- 2.Hawkins MN, et al. Maximal oxygen uptake as a parametric measure of cardiorespiratory capacity. Med Sci Sports Exerc. 2007;39(1):103–107. doi: 10.1249/01.mss.0000241641.75101.64. [DOI] [PubMed] [Google Scholar]
- 3.Rogers MA, et al. Decline in VO2max with aging in master athletes and sedentary men. J Appl Physiol. 1990;68(5):2195–2199. doi: 10.1152/jappl.1990.68.5.2195. [DOI] [PubMed] [Google Scholar]
- 4.Betik AC, Hepple RT. Determinants of VO2 max decline with aging: an integrated perspective. Appl Physiol Nutr Metab. 2008;33(1):130–140. doi: 10.1139/H07-174. [DOI] [PubMed] [Google Scholar]
- 5.Fleg JL, et al. Accelerated longitudinal decline of aerobic capacity in healthy older adults. Circulation. 2005;112(5):674–682. doi: 10.1161/CIRCULATIONAHA.105.545459. [DOI] [PubMed] [Google Scholar]
- 6.Pontzer H, et al. Daily energy expenditure through the human life course. Science. 2021;373(6556):808–812. doi: 10.1126/science.abe5017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Manini TM. Energy expenditure and aging. Ageing Res Rev. 2010;9(1):1–11. doi: 10.1016/j.arr.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hill JO, Wyatt HR, Peters JC. The importance of energy balance. Eur Endocrinol. 2013;9(2):111–115. doi: 10.17925/EE.2013.09.02.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yasukata J, et al. Relationship between measured aerobic capacity and total energy expenditure obtained by the doubly labeled water method in community-dwelling, healthy adults aged 81–94 years. Geriatrics (Basel) 2022;7(2):48. doi: 10.3390/geriatrics7020048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roberts SB, Dallal GE. Effects of age on energy balance. Am J Clin Nutr. 1998;68(4):975S–979S. doi: 10.1093/ajcn/68.4.975S. [DOI] [PubMed] [Google Scholar]
- 11.Amdanee N, et al. Age-associated changes of resting energy expenditure, body composition and fat distribution in Chinese Han males. Physiol Rep. 2018;6(23):e13940. doi: 10.14814/phy2.13940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bosy-Westphal A, et al. The Age-related decline in resting energy expenditure in humans is due to the loss of fat-free mass and to alterations in its metabolically active components. J Nutr. 2003;133(7):2356–2362. doi: 10.1093/jn/133.7.2356. [DOI] [PubMed] [Google Scholar]
- 13.Goodpaster BH, et al. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci. 2006;61(10):1059–1064. doi: 10.1093/gerona/61.10.1059. [DOI] [PubMed] [Google Scholar]
- 14.Paez HG, Pitzer CR, Alway SE. Age-related dysfunction in proteostasis and cellular quality control in the development of sarcopenia. Cells. 2023;12(2):249. doi: 10.3390/cells12020249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Short KR, et al. Decline in skeletal muscle mitochondrial function with aging in humans. Proc Natl Acad Sci U S A. 2005;102(15):5618-23. [DOI] [PMC free article] [PubMed]
- 16.Markaki M, Tavernarakis N. Mitochondrial turnover and homeostasis in ageing and neurodegeneration. FEBS Lett. 2020;594(15):2370–2379. doi: 10.1002/1873-3468.13802. [DOI] [PubMed] [Google Scholar]
- 17.Rooyackers OE, et al. Effect of age on in vivo rates of mitochondrial protein synthesis in human skeletal muscle. Proc Natl Acad Sci U S A. 1996;93(26):15364-9 [DOI] [PMC free article] [PubMed]
- 18.Menshikova EV, et al. Effects of exercise on mitochondrial content and function in aging human skeletal muscle. J Gerontol A Biol Sci Med Sci. 2006;61(6):534–540. doi: 10.1093/gerona/61.6.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wisloff U, et al. Cardiovascular risk factors emerge after artificial selection for low aerobic capacity. Science. 2005;307(5708):418–420. doi: 10.1126/science.1108177. [DOI] [PubMed] [Google Scholar]
- 20.Koch LG, Britton SL. Artificial selection for intrinsic aerobic endurance running capacity in rats. Physiol Genomics. 2001;5(1):45–52. doi: 10.1152/physiolgenomics.2001.5.1.45. [DOI] [PubMed] [Google Scholar]
- 21.Thyfault JP, Morris EM. Intrinsic (genetic) aerobic fitness impacts susceptibility for metabolic disease. Exerc Sport Sci Rev. 2017;45(1):7–15. doi: 10.1249/JES.0000000000000087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morris EM, et al. Aerobic capacity mediates susceptibility for the transition from steatosis to steatohepatitis. J Physiol. 2017;595(14):4909–4926. doi: 10.1113/JP274281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morris EM, et al. Aerobic capacity and hepatic mitochondrial lipid oxidation alters susceptibility for chronic high-fat diet-induced hepatic steatosis. Am J Physiol Endocrinol Metab. 2016;311(4):E749–e760. doi: 10.1152/ajpendo.00178.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Britton SL, Koch LG. Animal genetic models for complex traits of physical capacity. Exerc Sport Sci Rev. 2001;29(1):7–14. doi: 10.1097/00003677-200101000-00003. [DOI] [PubMed] [Google Scholar]
- 25.Thyfault JP, et al. Rats selectively bred for low aerobic capacity have reduced hepatic mitochondrial oxidative capacity and susceptibility to hepatic steatosis and injury. J Physiol. 2009;587(Pt 8):1805–1816. doi: 10.1113/jphysiol.2009.169060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morris EM, et al. Difference in housing temperature-induced energy expenditure elicits sex-specific diet-induced metabolic adaptations in mice. Obesity (Silver Spring) 2020;28(10):1922–1931. doi: 10.1002/oby.22925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller BF, et al. Enhanced skeletal muscle regrowth and remodelling in massaged and contralateral non-massaged hindlimb. J Physiol. 2018;596(1):83–103. doi: 10.1113/JP275089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morris EM, et al. Intrinsic aerobic capacity impacts susceptibility to acute high-fat diet-induced hepatic steatosis. Am J Physiol-Endocrinol Metab. 2014;307(4):E355–E364. doi: 10.1152/ajpendo.00093.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Corbett SW, Stern JS, Keesey RE. Energy expenditure in rats with diet-induced obesity. Am J Clin Nutr. 1986;44(2):173–180. doi: 10.1093/ajcn/44.2.173. [DOI] [PubMed] [Google Scholar]
- 30.Jackman MR, MacLean PS, Bessesen DH. Energy expenditure in obesity-prone and obesity-resistant rats before and after the introduction of a high-fat diet. Am J Physiol Regul Integr Comp Physiol. 2010;299(4):R1097–R1105. doi: 10.1152/ajpregu.00549.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seifert EL, et al. Intrinsic aerobic capacity correlates with greater inherent mitochondrial oxidative and H2O2 emission capacities without major shifts in myosin heavy chain isoform. J Appl Physiol (1985) 2012;113(10):1624–34. doi: 10.1152/japplphysiol.01475.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bouchard C, Blair SN, Katzmarzyk PT. Less sitting, more physical activity, or higher fitness? Mayo Clin Proc. 2015;90(11):1533–1540. doi: 10.1016/j.mayocp.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 33.Koch LG, et al. Intrinsic aerobic capacity sets a divide for aging and longevity. Circ Res. 2011;109(10):1162–1172. doi: 10.1161/CIRCRESAHA.111.253807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Church TS, et al. Cardiorespiratory fitness and body mass index as predictors of cardiovascular disease mortality among men with diabetes. Arch Intern Med. 2005;165(18):2114–2120. doi: 10.1001/archinte.165.18.2114. [DOI] [PubMed] [Google Scholar]
- 35.Mandsager K, et al. Association of cardiorespiratory fitness with long-term mortality among adults undergoing exercise treadmill testing. JAMA Netw Open. 2018;1(6):e183605–e183605. doi: 10.1001/jamanetworkopen.2018.3605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reimers CD, Knapp G, Reimers AK. Does physical activity increase life expectancy? A review of the literature. J Aging Res. 2012;2012:243958. doi: 10.1155/2012/243958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morris EM, et al. Intrinsic high aerobic capacity in male rats protects against diet-induced insulin resistance. Endocrinology. 2019;160(5):1179–1192. doi: 10.1210/en.2019-00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Naples SP, et al. Skeletal muscle mitochondrial and metabolic responses to a high-fat diet in female rats bred for high and low aerobic capacity. Appl Physiol Nutr Metab. 2010;35(2):151–162. doi: 10.1139/H09-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goodpaster BH, et al. Skeletal muscle lipid content and insulin resistance: Evidence for a paradox in endurance-trained athletes. J Clin Endocrinol Metab. 2001;86(12):5755–5761. doi: 10.1210/jcem.86.12.8075. [DOI] [PubMed] [Google Scholar]
- 40.Haus JM, et al. Improved hepatic lipid composition following short-term exercise in nonalcoholic fatty liver disease. J Clin Endocrinol Metab. 2013;98(7):E1181–E1188. doi: 10.1210/jc.2013-1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aon MA, et al. Mitochondrial health is enhanced in rats with higher vs. lower intrinsic exercise capacity and extended lifespan. NPJ Aging Mech Dis. 2021;7(1):1. doi: 10.1038/s41514-020-00054-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Henry CJK. Mechanisms of changes in basal metabolism during ageing. Eur J Clin Nutr. 2000;54(3):S77–S91. doi: 10.1038/sj.ejcn.1601029. [DOI] [PubMed] [Google Scholar]
- 43.Monferrer-Marín J, et al. Impact of ageing on female metabolic flexibility: A cross-sectional pilot study in over-60 active women. Sports Med - Open. 2022;8(1):97. doi: 10.1186/s40798-022-00487-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Morris EM, et al. Reduced hepatic mitochondrial respiration following acute high-fat diet is prevented by PGC-1α overexpression. Am J Physiol Gastrointest Liver Physiol. 2013;305(11):G868–G880. doi: 10.1152/ajpgi.00179.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jackson AS, et al. Role of lifestyle and aging on the longitudinal change in cardiorespiratory fitness. Arch Intern Med. 2009;169(19):1781–1787. doi: 10.1001/archinternmed.2009.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bouchard C, Rankinen T, Timmons JA. Genomics and genetics in the biology of adaptation to exercise. Compr Physiol. 2011;1(3):1603–1648. doi: 10.1002/cphy.c100059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mukherjee SD, et al. Aerobic capacity modulates adaptive thermogenesis: Contribution of non-resting energy expenditure. Physiol Behav. 2020;225:113048. doi: 10.1016/j.physbeh.2020.113048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gavini CK, et al. Leanness and heightened nonresting energy expenditure: role of skeletal muscle activity thermogenesis. Am J Physiol Endocrinol Metab. 2014;306(6):E635–E647. doi: 10.1152/ajpendo.00555.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Son C, et al. Reduction of diet-induced obesity in transgenic mice overexpressing uncoupling protein 3 in skeletal muscle. Diabetologia. 2004;47(1):47–54. doi: 10.1007/s00125-003-1272-8. [DOI] [PubMed] [Google Scholar]
- 50.Fink BD, et al. Mitochondrial proton leak in obesity-resistant and obesity-prone mice. Am J Physiol Regul Integr Comp Physiol. 2007;293(5):R1773–R1780. doi: 10.1152/ajpregu.00478.2007. [DOI] [PubMed] [Google Scholar]
- 51.Matthew Morris E, et al. Increased aerobic capacity reduces susceptibility to acute high-fat diet-induced weight gain. Obesity (Silver Spring) 2016;24(9):1929–1937. doi: 10.1002/oby.21564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Smith RL, et al. Metabolic flexibility as an adaptation to energy resources and requirements in health and disease. Endocr Rev. 2018;39(4):489–517. doi: 10.1210/er.2017-00211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Coen PM, et al. Skeletal muscle mitochondrial energetics are associated with maximal aerobic capacity and walking speed in older adults. J Gerontol: Ser A. 2012;68(4):447–455. doi: 10.1093/gerona/gls196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lagerwaard B, et al. In vivo assessment of muscle mitochondrial function in healthy, young males in relation to parameters of aerobic fitness. Eur J Appl Physiol. 2019;119(8):1799–1808. doi: 10.1007/s00421-019-04169-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moehle EA, Shen K, Dillin A. Mitochondrial proteostasis in the context of cellular and organismal health and aging. J Biol Chem. 2019;294(14):5396–5407. doi: 10.1074/jbc.TM117.000893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Grevendonk L, et al. Impact of aging and exercise on skeletal muscle mitochondrial capacity, energy metabolism, and physical function. Nat Commun. 2021;12(1):4773. doi: 10.1038/s41467-021-24956-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lima T, et al. Pleiotropic effects of mitochondria in aging. Nature Aging. 2022;2(3):199–213. doi: 10.1038/s43587-022-00191-2. [DOI] [PubMed] [Google Scholar]
- 58.Keele GR, Zhang JG, Szpyt J, Korstanje R, Gygi SP, Churchill GA, Schweppe DK. Global and tissuespecific aging effects on murine proteomes. Cell Rep. 2023;42:112715. doi: 10.1016/j.celrep.2023.112715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Robinson MM, et al. Enhanced protein translation underlies improved metabolic and physical adaptations to different exercise training modes in young and old humans. Cell Metab. 2017;25(3):581–592. doi: 10.1016/j.cmet.2017.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Baar K, et al. Adaptations of skeletal muscle to exercise: rapid increase in the transcriptional coactivator PGC-1. Faseb j. 2002;16(14):1879–1886. doi: 10.1096/fj.02-0367com. [DOI] [PubMed] [Google Scholar]
- 61.Vieira-Potter VJ, et al. Female rats selectively bred for high intrinsic aerobic fitness are protected from ovariectomy-associated metabolic dysfunction. Am J Physiol Regul Integr Comp Physiol. 2015;308(6):R530–R542. doi: 10.1152/ajpregu.00401.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stephenson EJ, et al. Exercise training enhances white adipose tissue metabolism in rats selectively bred for low- or high-endurance running capacity. Am J Physiol Endocrinol Metab. 2013;305(3):E429–E438. doi: 10.1152/ajpendo.00544.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Choi J, et al. Brain diabetic neurodegeneration segregates with low intrinsic aerobic capacity. Ann Clin Transl Neurol. 2014;1(8):589–604. doi: 10.1002/acn3.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gan L, et al. Region-specific differences in bioenergetic proteins and protein response to acute high fat diet in brains of low and high capacity runner rats. Neurosci Lett. 2018;674:49–53. doi: 10.1016/j.neulet.2018.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kaliszewska A, et al. The interaction of diet and mitochondrial dysfunction in aging and cognition. Int J Mol Sci. 2021;22(7):3574. doi: 10.3390/ijms22073574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wikgren J, et al. Selective breeding for endurance running capacity affects cognitive but not motor learning in rats. Physiol Behav. 2012;106(2):95–100. doi: 10.1016/j.physbeh.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Fig. 1. Representative images of hepatic triglyceride storage. Representative images of Hematoxylin and eosin staining on liver sections in male and female HCR rats fed either a HFD or LFD (TIFF 163854 KB)
Supplemental Fig. 2. Skeletal muscle and liver correlations between energy expenditure and mitochondrial protein fractional synthesis rates. Correlations between total EE and mitochondrial protein FSR in (a) male and (b) female gastrocnemius muscle, soleus (c, d) and liver (e, f) (TIFF 4004 KB)