Abstract
The aim of our research was the development of prolonged delivery systems for therapeutic agents with various properties (prevention and treatment of bone diseases, anti-neoplastic, anti-inflammatory, antioxidant) that would ensure sustained therapeutic levels of the active principle, above the minimum inhibitory concentration, without reaching toxic levels over a long period of time as alternatives to conventional routes of administration. PLGA (poly lactic-co-glycolic acid), a biodegradable and biocompatible synthetic polymer, FDA approved, with a 65:35 lactic acid (LA): glycolic acid (GA) copolymer ratio, was chosen as delivery system. Our studies have shown that in PBS it undergoes two simultaneous degradation processes, hydrolysis and autohydrolysis, degrading completely in about 40 days. The release of the active principle is determined by the diffusion from inside the polymer matrix to the outside, which occurs simultaneously with the erosion of the polymer, during 35 days
Keywords: PLGA , biocompatible , kinetic studies , release
Introduction
PLGA (poly lactic-co-glycolic acid), is a biodegradable copolymer used extensive in pharmaceutical and biomedical engineering areas as drug delivery system [1].
The synthesis of PLGA was done for the first time in the 1960s, by copolymerizing lactic acid (LA) with glycolic acid (GA).
In this way, researchers could modify the properties of the resulting polymer, observing that PLGA exhibited improved biodegradability and mechanical strength compared to pure polilactic acid (PLA), making it more suitable for various applications [2].
The unique properties of PLGA, such as its biocompatibility, made it an ideal candidate for encapsulating and releasing therapeutical agents in a controlled manner.
Using different synthesis techniques PLGA can be produced with different molecular weights, LA: GA ratios (50:50, 65:35, 75:25 and 85:15).
By increasing the amount of LA, the hydrophobicity increases which induce a slower degradation and consequently a slower release.
Also, the amount of GA influences the properties of the polymer, by increasing GA the polymer degrades faster.
The terminal group of the polymer (ester or free carboxyl group) determines hydrophilicity, molecules with free carboxyl group being more hydrophilic, absorb more water and degrade faster than those with free esterified carboxyl group [3].
This flexibility exponentially increased its applications in the biomedical field as a biodegradable scaffold for tissue engineering, nanoparticles, microparticles and even implants [4].
These delivery systems protect drugs from degradation, control their release over time and improve their therapeutic efficacy.
Furthermore, certain studies have highlighted that the shape of the nanoparticles also influences the biological properties of the polymer, more precisely, it has been proven that nanoparticles with an acicular shape have better cell membrane penetration properties than spherical ones in order to deliver the drug in the cytoplasm.
Even if the mechanism is not yet completely known, there are several theories that could explain if the penetration takes place through endocytosis or even through possible membrane disturbances [5].
All the advantages also induce certain disadvantages; in the present situation the nanoparticles with the needle shape can induce cytotoxicity, after endocytosis, even the activation of apoptosis signals [6].
Fourteen PLGA-based drug delivery systems have received FDA approval and are commercially available.
For example, sandostatin (active principle octreotide) has subcutaneous administration, 10-30mg AP/dose, every month.
Risperdal (active principle risperidone) has intramuscular administration, 12,5-37,5mg AP/dose, once at two weeks [7].
PLGA has also gained popularity in the field of regenerative medicine, with PLGA-based scaffolds for tissue engineering [8].
Ongoing research continues with new applications and enhancement of its performance in various fields (food industry, agriculture, veterinary science, etc).
Our field of expertise includes 5 types of PLGA-nanocomposite drug delivery systems (PLGA-gentamicin [9], PLGA-biphosponates [10], PLGA-doxorubicin, PLGA-polyphenols) along with other composite materials with collagen [11] and hydroxyapatite.
Materials and Methods
PLGA with LA:GA ratio of 65:35, Mw was purchased from Sigma Aldrich.
The phosphate buffer saline (PBS) solution was prepared in the laboratory using the following protocol: in 500L solution, pH 7.2 were dissolved: 4g natrium chloride salt, 0.64g disodic phosphate, 0,09g monopotasium phosphate and 0.1g sodium azide.
The pH was adjusted to 7.2 with 0.01M HCl solution.
Synthesis of simple PLGA biopolymeric nanoparticles
We used water in-oil-in-water (W1/O/W2) emulsion synthesis, a technique used to encapsulate hydrophilic compounds or aqueous droplets within oil droplets, which are further dispersed in a high aqueous medium.
W1: 4mL aqueous solution with 0.5% PVA (polyvinyl alcohol) as an emulsifier;
O: 120mg PLGA solubilized in 4mL DCM (dichloromethane);
W2: 80mL water with 0.5% PVA (polyvinyl alcohol) as an emulsifier;
Synthesis of biopolymeric PLGA nanoparticles functionalized with active principle (PA)
W1: 4mL aqueous solution with 50mg PA (gallic acid) dissolved in 0.5% PVA (polyvinyl alcohol) as an emulsifier;
O: 120mg PLGA solubilized in 4mL DCM (dichloromethane);
W2: 80mL water with 0.5% PVA (polyvinyl alcohol) as an emulsifier;
The samples were washed and centrifuged at 10,000rpm for 10 minutes, freezed overnight at-180C then subjected to a lyophilization process using Alpha 1-2LSCbasic from Martin Christ, (freezing at-55°C and vacuum at 0.002mbar for 12h, and heating under vacuum for 2h to 35°C) to obtain porous composite materials.
The composite was poured in molds to obtain discs with equal diameters (Figure 1).
Figure 1.

PLGA composites after lyophilization process (discs with equal diameters
Kinetic degradation studies
To carry out the kinetic degradation studies, one disc with the initially weighed mass was placed in screw-cap vials containing 20mL PBS and then kept at 37°C in an oven.
The methodology for carrying out the degradation studies was taken from Vey et al., with minor changes.
Potentiometric determinations
The pH was recorded using a Consort multi-parameter analyzer multimeter, using a glass electrode, after careful calibration of the device with three buffer solutions, at pH 4.0, 7.0 and 10.
Mass loss and water content
The samples were weighed before introduction into PBS (mi).
At certain time intervals, the samples were removed from the environment, left for 30 minutes to elapse and reweighed (mw).
After completing the experiment, the samples were removed from the environment, dried in an oven at 100°C for 72 hours (md).
The mass loss and water content were calculated with:

Determination of the AP release profile
The AP release profile from PLGA was observed using:
- order 0 kinetics: Qt = kt (I)
- first order kinetics (II):
Qt = Q0e-k1t (II)
- Higuchi model (III) [12]:
Qt = k_H √t (III)
Korsmeyer-Peppas model (IV):

where: Q-cumulative amount of drug release;
t - time;
kH - Higuchi constant;
Mt - amount of AP release at time t;
M - total amount of the AP.
The amount of AP (gallic acid) release was determined by the method Folin-Ciocîlteu described by Borneo et al [13], using a calibration curve.
Results
Kinetic degradation studies
PLGA samples immersed in PBS as degradation medium acquired a spongy, swollen appearance.
The water content increased continuously, through a diffusion phenomenon that trapped it inside the matrix [14] (Figure 2).
Figure 2.
Water content of the polymer film as function of time.
In Figure 3 the PLGA mass loss in time is presented.
Figure 3.

Mass loss (percentage) as function of time
In the first 10 days of the experiment, the decrease is small, about 10%, although the water diffused inside the PLGA reaches 71%.
The volume of the sample doubles due to the retained water.
After 25 days of the start of the experiment, the mass lost is 87.5% (Figure 3).
Because PLGA immersed in PBS became very soft, it was impossible to calculate the water content after 15 days.
PLGA degradation is heterogeneous, similar to observations found by other authors.
Figure 4 shows the decrease in pH concomitant with the degradation in time of the polymer.
Figure 4.
The decrease in the pH as a function of degradation time
The process is rapid, with a drop in pH to 6.2 in the first 14 days.
From the moment the samples are immersed in PBS, the carboxyl groups on the outer surface are ionized, which release H+ ions.
Between 14 and 16 days, a sudden change in pH occurs, from 6.2 to 3.89.
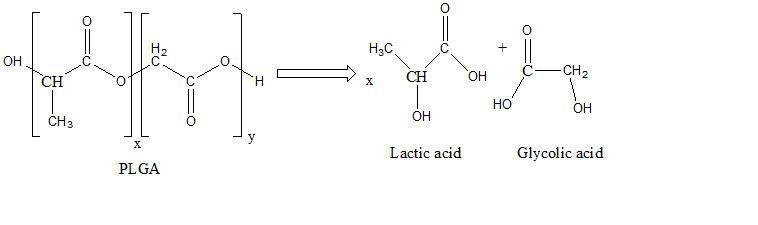
The decrease is then slow, similar to other studies, releasing lactic and glycolic acid (Figure 5).
Figure 5.

Release of lactic acid and glycolic acid in PBS.AP release profile
According to the obtained results, there is a mixed release kinetics of the AP: a burst release of AP bound to the surface of the polymer, in the first 7-10 days (60%, Figure 6) and a slower rate of release through the process of PA diffusion and erosion.
Figure 6.
Release of AP from the PLGA biopolymeric nanocomposite material (in the top square Higuchi Equation and in the in the bottom square Rigter-Peppas equation).
The erosive process creates pathways for PA to diffuse outward.
From the graphs above, it can be seen that the first segment of the release curve is of burst release type, the kinetics corresponding to this segment is of Type 1.
Then follows a segment with 0 order kinetics [15].
In order to be able to confirm that the materials synthesized by us have a mixed release, both by diffusion and by erosion, we represented the release using Higuchi and Korsmeyer-Peppas models.
The regression coefficients confirm the presence of both release mechanisms [16].
Discussions
PLGA is a synthetic polymer used intensively as a “smart” drug delivery/dispensing system.
The possibility of the encapsulated AP controlled release while reducing the frequency of administration of therapeutic doses of the drug makes it extremely attractive for biomedical applications.
Other applications follow targeted release to specific cells, tissues or organs, the PLGA surface can be conjugated with different types of ligands.
One of the researchers' new approaches is to use PLGA as a responsive delivery system. Thus, PLGA composites that can encapsulate antineoplastics respond to changes in pH, so that the degradation occurs faster at acidic pH, as it was found to be specific to the tumor environment [17, 18].
Another extremely used approach is represented by the possibility of obtaining a combined therapy, by encapsulating several drugs and releasing them simultaneously with synergistic effects.
Our studies followed conjugation of PLGA by functionalizing the surface with PDA and binding some bisphosphonates to it in order to obtain systems with local release at the bone [10].
In other studies, we have included in the PLGA sphere antibiotics such as gentamicin whose toxic effects (ototoxicity and nephrotoxicity) present with conventional administration are reduced to zero by this type of local release [9].
We also encapsulated a series of polyphenols (gallic acid, quercitin, chlorogenic acid) in PLGA in order to increase their bioavailability.
For each of these situations, we studied the degradation of the composites and the release of the active principle in order to observe similarities and differences.
A constant PA release rate is most commonly desired, which in analogy to chemical kinetics, corresponding to zero-order kinetics.
According to the method of their preparation, they are classified into physical systems, when the AP is physically incorporated into a polymer matrix (our case regardless of the encapsulated AP) and chemical systems, when the PA is linked by chemical bonds to the polymer (it is not our case).
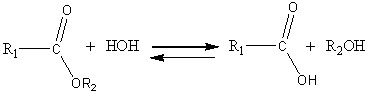
PLGA concomitantly undergoes two degradation processes in PBS.
A hydrolysis process, as shown in Figure 7A, but also a process of autohydrolysis 7B.
Figure 7.

A) Hydrolytic degradation of PLGA, B) Autocatalytic degradation of PLGA
The hydrolysis reaction takes place at the ester group in the polymer structure.
Once initiated, the hydrolysis process, predominant in the first days, can also be catalyzed by the released acids.
Due to these released acids, the pH of the medium is expected to drop to lower values [19].
When AP is released from the PLGA structure, three main synergistic mechanisms can occur: "burst" release (osmosis); diffusion release process through the polymer pores; erosion release (Hydrolytic degradation of PLGA to LA and GA).
Among the three mechanisms, the most common is diffusion. In the case of AP release through the osmosis mechanism, that water enters into the sphere of the biopolymer, it does not increase its volume, and an osmotic force is created [20].
This mechanism does not correspond to the one presented by PLGA, observing that it absorbed a very high volume of water.
In the case of our composite, with the increase in the amount of water inside the compound, the resulting pressure is compensated by the rearrangement of the polymer chains, causing it to swell [21].
Erosion occurs as a mechanism due to the degradation of the polymer over time.
Erosion creates cracks that increase the diffusion process.
The degradation of polylactic-co-glycolic acid is a combination of diffusion and erosion.
The degradation processes of the polymer and the release of the active principle are influenced by factors such as the LA:GA ratio (the LA:GA ratio of 65:35 was chosen for a slower release than in the case of LA:GA=50:50, but faster than when using PLGA with LA:GA=75:25) [22], molecular mass of PLGA [23], solubility of the active principle.
For a water-soluble PA, release from the composite biopolymer material is facilitated.
The percentage of encapsulated drug could influence the release process because the free space left after the initial, quantitatively significant release can lead to the formation of pores, helping to further increase the amount of released drug.
Our previous studies showed that the release of PA is slowed down if there is also hydroxyapatite in the composite material compared to single encapsulated PA in PLGA.
A possible explanation of this phenomenon would be the fact that hydroxyapatite is not soluble in the PBS medium and where it is present in the polymer sphere it no longer creates the same diffusion pathways for the AP located further inside the PLGA [24].
In our studies, even if the 2 mechanisms of drug release remained unchanged, they varied in percentage, erosion being predominant in some cases and diffusion in other cases.
For example, smaller particles have a larger surface area to volume ratio and lead to a faster release of the active principle.
On the other hand, the use of polyvinyl alcohol as an emulsifying agent can affect water penetration inside the matrix and thus degradation process may be disturbed.
Another extremely important factor that can affect the release kinetics is the pH of the environment.
We performed the studies in SBF to simulate as faithfully as possible the pH of the blood, but for example at acidic pH (specific to tumors) the degradation can be accelerated.
Last but not least, the hydrophobic or hydrophilic nature of the encapsulated drug must be taken into account.
Hydrophilic drugs are easier to dissolve in polar substances (most of the ones encapsulated by us).
An interesting observation of our studies is that the amount of drug encapsulated has less influence on the release profile, all having a release in a larger amount at the beginning (the first days of the experiments), followed by a prolonged release up to about a month.
Conclusions
PLGA-delivery systems can be considered sustained release systems made through a method of economic synthesis, resulting in biocompatible and biodegradable materials that contact with the body or the environment lead to the release of PA over time according to a kinetic profile 0 and 1.
The two acids copolymers (lactic and glycolic) released by the degradation of PLGA are easily eliminated from the body through the Krebs cycle.
Conflict of interests
None to declare.
Source of funding
This work was supported by the grant POCU/993/6/13/153178, ”Performanță în cercetare” - "Research performance" co-financed by the European Social Fund within the Sectorial Operational Program Human Capital 2014-2020.
References
- 1.Perez-Herrero E, Fernandez-Medarde A. Advanced targeted therapies in cancer: drug nanocarriers, the future of chemotherapy. Eur J Pharm Biopharm. 2015;93:52–79. doi: 10.1016/j.ejpb.2015.03.018. [DOI] [PubMed] [Google Scholar]
- 2.Alsaab HO, Alharbi FD, Alhibs AS, Alanazi NB, Alshehri BY, Saleh MA, Alshehri FS, Algarni MA, Almugaiteeb T, Uddin MN, Alzhrani RM. PLGA-Based Nanomedicine: History of Advancement and Development in Clinical Applications of Multiple Diseases. Pharmaceutics. 2022;14(12):2728–2728. doi: 10.3390/pharmaceutics14122728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hua Y, Su Y, Zhang H, Liu N, Wang Z, Gao X, Gao J, Zheng A. Poly (lactic-co-glycolic acid) microsphere production based on quality by design: a review. Drug Deliv. 2021;28(1):1342–1355. doi: 10.1080/10717544.2021.1943056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sharma S, Parmar A, Kori S, Sandhir R. PLGA-based nanoparticles: A new paradigm in biomedical applications. TrAC Trends Anal Chem. 2016;80:30–40. [Google Scholar]
- 5.Kolhar P, Doshi N, Mitragotri S. Polymer nanoneedle-mediated intracellular drug delivery. Small. 2011;7(14):2094–2100. doi: 10.1002/smll.201100497. [DOI] [PubMed] [Google Scholar]
- 6.Zhang B, Sai Lung, Zhao S, Chu Z, Chrzanowski W, Li Q. Shape dependent cytotoxicity of PLGA-PEG nanoparticles on human cells. Sci Rep. 2017;7(1):7315–7315. doi: 10.1038/s41598-017-07588-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang Y, Qin B, Xia G, Choi SH. FDA's Poly (Lactic-Co-Glycolic Acid) Research Program and Regulatory Outcomes. AAPS J. 2021;23(4):92–92. doi: 10.1208/s12248-021-00611-y. [DOI] [PubMed] [Google Scholar]
- 8.Zhao D, Zhu T, Li J, Cui L, Zhang Z, Zhuang X, Ding J. Poly (lactic-co-glycolic acid)-based composite bone-substitute materials. Bioact Mater. 2020;6(2):346–360. doi: 10.1016/j.bioactmat.2020.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Turcu-Știolică A, Ciocîlteu MV, Podgoreanu P, Neacșu I, Ionescu (Filip), Nicolicescu C, Neamțu J, Amzoiu E, Amzoiu E, Manda CV. PLGA-Gentamicin and PLGA-hydroxyapatite-gentamicin microspheres for medical applications. Pharm Chem J. 2022;56(5):645–653. [Google Scholar]
- 10.Postelnicu RA, Ciocîlteu MV, Neacșu IA, Nicolicescu C, Costachi A, Amzoiu M, Neamțu J, Pisoschi CG, Mocanu AG, Rău G, Amzoiu E. PLGA-bisphosphonates conjugated nanoparticles: synthesis and morphological characterization. Farmacia. 2023;71(1):83–90. [Google Scholar]
- 11.Filip Ionescu, Mocanu AG, Neacșu IA, Ciocîlteu MV, Rău G, Neamțu J. Biocompatibility studies on a collagen-hydroxyapatite biomaterial. Curr Health Sci J. 2022;48(2):217–225. doi: 10.12865/CHSJ.48.02.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Higuchi T. Mechanism of sustained action medication theoretical analysis of rate of release of solid drugs dispersed in solid matrices. J Pharm Sci. 1963;52:1145–1149. doi: 10.1002/jps.2600521210. [DOI] [PubMed] [Google Scholar]
- 13.Borneo R, León AE, Aguirre A, Ribotta P, Cantero JJ. Antioxidant capacity of medicinal plants from the Province of Córdoba (Argentina) and their in vitro testing in a model food system. Food Chem. 2009;112(3):664–670. [Google Scholar]
- 14.Engelberg I, Kohn J. Physico-mechanical properties of degradable polymers used in medical applications: a comparative study. Biomaterials. 1991;12(3):292–304. doi: 10.1016/0142-9612(91)90037-b. [DOI] [PubMed] [Google Scholar]
- 15.Miao Y, Cui H, Dong Z, Ouyang Y, Li Y, Huang Q, Wang Z. Structural Evolution of Polyglycolide and Poly(glycolide-co-lactide) Fibers during In Vitro Degradation with Different Heat-Setting Temperatures. ACS Omega. 2021;6(43):29254–29266. doi: 10.1021/acsomega.1c04974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang HT, Palmer H, Linhardt RJ, Flanagan DR, Schmitt E. Degradation of poly(ester) microspheres. Biomaterials. 1990;11(9):679–685. doi: 10.1016/0142-9612(90)90026-m. [DOI] [PubMed] [Google Scholar]
- 17.Shen X, Li T, Xie X, Feng Y, Chen Z, Yang H, Wu C, Deng S, Liu Y. PLGA-based drug delivery systems for remotely triggered cancer therapeutic and diagnostic applications. Front Bioeng Biotechnol. 2020;8:381–381. doi: 10.3389/fbioe.2020.00381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Song Y, He L, Wang Y, Wu Q, Huang W. Molecularly targeted therapy and immunotherapy for hormone receptor‑positive/human epidermal growth factor receptor 2‑negative advanced breast cancer (Review) Oncol Rep. 2020;44(1):3–13. doi: 10.3892/or.2020.7589. [DOI] [PubMed] [Google Scholar]
- 19.Kim JM, Seo KS, Jeong YK, Hai BL, Kim YS, Khang G. Co-effect of aqueous solubility of drugs and glycolide monomer on in vitro release rates from poly (D,L-lactide-co-glycolide) discs and polymer degradation. J Biomater Sci Polym Ed. 2005;16(8):991–1007. doi: 10.1163/1568562054414676. [DOI] [PubMed] [Google Scholar]
- 20.Jeon O, Kang SW, Lim HW, Hyung Chung, Kim BS. Long-term and zero-order release of basic fibroblast growth factor from heparin-conjugated poly(L-lactide-co-glycolide) nanospheres and fibrin gel. Biomaterials. 2006;27(8):1598–15607. doi: 10.1016/j.biomaterials.2005.08.030. [DOI] [PubMed] [Google Scholar]
- 21.Proikakis CS, Tarantili PA, Andreopoulos AG. The role of polymer/drug interactions on the sustained release from poly (dl-lactic acid) tablets. Eur. Polym. J. 2006;42:3269–3276. [Google Scholar]
- 22.Shah S, Cha Y, Pitt CG. Poly (glycolic acid-co-dl-lactic acid): diffusion or degradation-controlled drug delivery. J Control Release. 1992;18(3):261–270. [Google Scholar]
- 23.Andhariya JV, Jog R, Shen J, Choi S, Wang Y, Zou Y. Burgess DJ. In vitro-in vivo correlation of parenteral PLGA microspheres: Effect of variable burst release. J Control Release. 2019;314:25–37. doi: 10.1016/j.jconrel.2019.10.014. [DOI] [PubMed] [Google Scholar]
- 24.Ciocîlteu MV, Nicolaescu OE, Mocanu AG, Nicolicescu C, Rău G, Neamțu J, Amzoiu E, Amzoiu E, Oancea C, Turcu-Stiolică A. Process optimization using quality by design (QBD) approach of a gentamicin loaded PLGA biocomposite. J Sci Arts. 2021;4(57):1069–1080. [Google Scholar]



