Graphical abstract
Keywords: BDNF, TrkB, Dorsal root ganglia, Spinal cord injury, Pain, Nociceptors
Highlights
-
•
Central and peripheral mechanisms mediate both inflammatory and neuropathic pain.
-
•
DRGs represent an important peripheral site of plasticity driving neuropathic pain.
-
•
Changes in ion channel/receptor function are critical to nociceptor hyperexcitability.
-
•
Peripheral BDNF-TrkB signaling contributes to neuropathic pain after SCI.
-
•
Understanding peripheral mechanisms may reveal relevant clinical targets for pain.
Abstract
Pain is a sensory state resulting from complex integration of peripheral nociceptive inputs and central processing. Pain consists of adaptive pain that is acute and beneficial for healing and maladaptive pain that is often persistent and pathological. Pain is indeed heterogeneous, and can be expressed as nociceptive, inflammatory, or neuropathic in nature. Neuropathic pain is an example of maladaptive pain that occurs after spinal cord injury (SCI), which triggers a wide range of neural plasticity. The nociceptive processing that underlies pain hypersensitivity is well-studied in the spinal cord. However, recent investigations show maladaptive plasticity that leads to pain, including neuropathic pain after SCI, also exists at peripheral sites, such as the dorsal root ganglia (DRG), which contains the cell bodies of sensory neurons. This review discusses the important role DRGs play in nociceptive processing that underlies inflammatory and neuropathic pain. Specifically, it highlights nociceptor hyperexcitability as critical to increased pain states. Furthermore, it reviews prior literature on glutamate and glutamate receptors, voltage-gated sodium channels (VGSC), and brain-derived neurotrophic factor (BDNF) signaling in the DRG as important contributors to inflammatory and neuropathic pain. We previously reviewed BDNF’s role as a bidirectional neuromodulator of spinal plasticity. Here, we shift focus to the periphery and discuss BDNF-TrkB expression on nociceptors, non-nociceptor sensory neurons, and non-neuronal cells in the periphery as a potential contributor to induction and persistence of pain after SCI. Overall, this review presents a comprehensive evaluation of large bodies of work that individually focus on pain, DRG, BDNF, and SCI, to understand their interaction in nociceptive processing.
1. Introduction
Pain is a physiological response to injury that has been defined by the IASP as ‘an unpleasant sensory and emotional experience associated with, or resembling that associated with, actual or potential tissue damage’ (Pain, 2017). Pain can be categorized as adaptive or maladaptive. Adaptive pain includes inflammatory pain, which is an increase in sensitivity due to an inflammatory response resulting from tissue damage, and nociceptive pain which is caused by the activation of primary nociceptors located in the peripheral nervous system in somatic (skin, muscle, or bone) or visceral (body organs) tissue. Maladaptive pain typically involves abnormal functioning of the nervous system and is neither protective nor informative. Neuropathic pain, which results from injury to the somatosensory nervous system, is an example of maladaptive pain. Nociceptive processing underlying adaptive and maladaptive pain involves both peripheral and central mechanisms. In the periphery, post-translational modification results in peripheral sensitization, a process where there is an increased sensitivity of the peripheral terminals of primary nociceptors (Lewin et al., 1993, Lewin et al., 1994). Meanwhile, central mechanisms involve central sensitization (Woolf, 1983), which is an increased excitability of spinal neurons triggered by peripheral noxious input thereby creating a state where the response to normal input is greatly enhanced.
The dorsal root ganglion (DRG) houses the cell bodies of primary sensory neurons and projects axons to both the peripheral site of injury and the dorsal horn of the spinal cord. Hence, it is conceivable that the DRG is a major site of nociceptive processing in both adaptive and maladaptive pain states. Various membrane proteins implicated in the pain pathways are found in DRGs and expressed in nociceptors, including voltage gated ion channels, glutamate receptors and transporters, and G-coupled receptors. DRG neurons also express numerous cytokines and chemokines and their receptors, highlighting the important role DRGs play in nociception after tissue injury. While much focus has been given to the spinal cord dorsal horn (SCDH), an important central site for the integration of incoming sensory and descending input, less attention has been given to the DRG and peripheral processes that lead to pain hypersensitivity, particularly neuropathic pain.
Spinal cord injury (SCI) leads to significant sensorimotor dysfunction, including chronic neuropathic pain. A wealth of studies has focused on changes in the spinal cord that potentially contribute to development and maintenance of neuropathic pain. One of the most promising targets for adaptive plasticity and functional recovery after SCI is brain-derived neurotrophic factor (BDNF) because of its role in neuronal growth and development (Barde et al., 1982, Park and Poo, 2013). BDNF acting through its high affinity receptor, tropomyosin receptor kinase (Trk) B, has been shown to promote adaptive plasticity in both uninjured and injured spinal cord. However, BDNF has also been implicated in pain modulation (Merighi et al., 2008, Pezet et al., 2002a) and in central sensitization (Alles et al., 2021, Biggs et al., 2010, Sikandar et al., 2018). While the expression of BDNF in nociceptors has long been recognized, more recent studies have shown that BDNF is expressed in non-nociceptors in the DRG (Rutlin et al., 2014) as well as non-neuronal cells in the periphery (Hahn et al., 2006, Hahn et al., 2005, Wang et al., 2015). Therefore, any exploration into the peripheral processes mediating neuropathic pain and the role of the DRG must also include BDNF signaling, as BDNF is very likely modulating the activity and function of ion channels, glutamate receptors, and cytokines in the DRG.
Given the limited number of studies that have methodically examined DRG plasticity and peripheral BDNF signaling in the development of neuropathic pain after SCI, we take this opportunity to write a review evaluating what is currently known. We recognize a need to bring together the large bodies of work that independently focus on pain, nociceptors and DRG, BDNF, and SCI, and to understand how all the substrates implicated in the individual components work together (Fig. 1, Table 1). Although similar acknowledgments can be made of other ganglia systems such as the trigeminal, this review focuses on the critical role of the DRG to the development and expression of chronic pain. The present review begins with a brief overview of adaptive and maladaptive pain. Next, we discuss the DRG as an important peripheral site for nociceptor hyperexcitability and nociceptive processing that leads to pain, including neuropathic pain after SCI. BDNF-TrkB signaling in pain after SCI is also reviewed; however, because detailed reviews of BDNF-TrkB signaling in pain and plasticity after SCI were recently published by our laboratory (Garraway, 2023, Garraway and Huie, 2016), the present review focuses on cellular and molecular changes in the DRGs and the primary afferent sensory neurons after peripheral inflammatory insults and SCI to explore how the altered mechanisms may underlie development and maintenance of neuropathic pain.
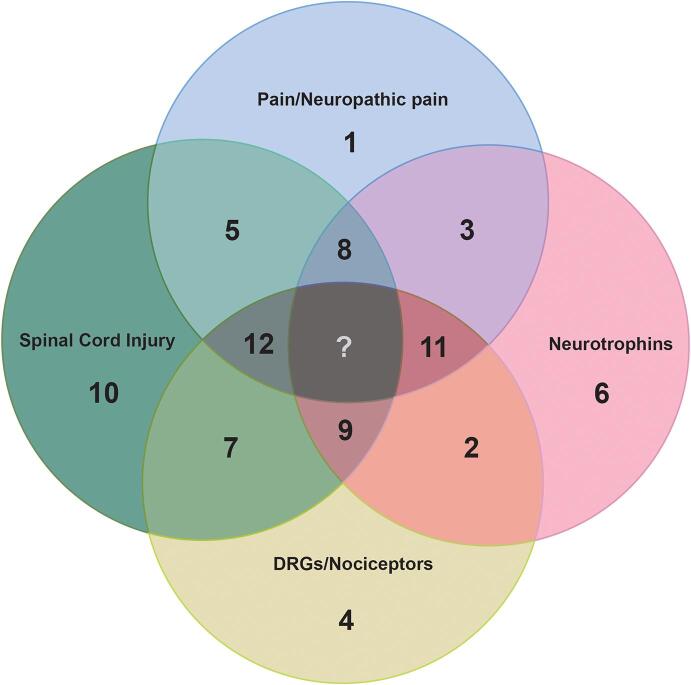
Fig. 1.
Examples of some review literature on pain, SCI, neurotrophins, and nociceptors through the past 30 years. This figure shows 12 recent review articles related to the field. Each number in the diagram can be linked to an article listed in Table 1. Although not demonstrative of the full scope of each topic, these reviews i) show most recent developments in the field or ii) are highly cited in other work, which implies their impact on driving the direction of other research. It should be noted that while several articles focus on 2 (article #2, 3, 5 and 7) or 3 (article # 8, 9, 11 and 12) topics, none of the articles examines all 4 topics (center space designated by ‘?’). This demonstrates a lack of reviews that discuss all the topics together to shed light on central as well as peripheral mechanisms including DRG and nociceptor plasticity in pain hypersensitivity, including neuropathic pain after SCI. The gap in perspective shows potential future research opportunities and development of new research questions for the field.
Table 1.
Examples of 12 representative review literatures on pain, SCI, neurotrophins, and/or nociceptors through the past 30 years. Each article can be located as a corresponding number (designated by # column) in Fig. 1.
| # | Reference | Conclusions/summary | Topic | |
|---|---|---|---|---|
| 1 | Millan (1999) | The induction of pain: an integrative review | Origin and pathophysiological significance of pain from evolutionary perspective | Pain |
| 2 | Mendell (2003) | Peripheral neurotrophic factors and pain | Mechanisms underlying sensitization, specifically the substances released and availability of the receptors that contribute to hyperalgesia | Neurotrophic factors Periphery/nociceptors |
| 3 | Pezet and McMahon (2006) | Neurotrophins: mediators and modulators of pain | Evidence for the contribution of neurotrophins (NGF, BDNF), the range of conditions that trigger their actions, and the mechanism of action in relation to pain | Neurotrophic factors Pain |
| 4 | Woolf and Ma (2007) | Nociceptors: noxious stimulus detectors | Nociceptor components, function, regulation of ion channels/receptors after injury | Nociceptors |
| 5 | Yezierski (2009) | SCI pain: Spinal and supraspinal mechanisms | Review of experimental studies focused on the spinal and supraspinal mechanisms with at- and below-level pain after SCI | Pain SCI |
| 6 | Numakawa et al. (2010) | BDNF function and intracellular signaling in neurons |
Broad overview of the current knowledge concerning BDNF action and associated intracellular signaling in neuronal protection, synaptic function, and morphological change, and understanding the secretion and intracellular dynamics of BDNF | Neurotrophins |
| 7 | Walters (2012) | Nociceptors as chronic drivers of pain and hyperreflexia after SCI: an adaptive-maladaptive hyperfunctional state hypothesis | Proposes SCI as trigger for persistent hyperfunctional state in nociceptors that originally evolved as an adaptive response. Focus on uninjured nociceptors altered by SCI and how they contribute to behavioral hypersensitivity. | Nociceptors SCI |
| 8 |
Garraway and Huie. (2016) |
Spinal Plasticity and Behavior: BDNF-Induced Neuromodulation in Uninjured and Injured Spinal Cord | Review of diverse actions of BDNF from recent literatures and comparison of BDNF-induced nociceptive plasticity in naïve and SCI conditions | SCI Pain Neurotrophins |
| 9 | Keefe et al. (2017) | Targeting Neurotrophins to Specific Populations of Neurons: NGF, BDNF, and NT-3 and Their Relevance for Treatment of Spinal Cord Injury | Review of neurotrophins NGF, BDNF, and NT-3 and their effects on specific populations of neurons, including nociceptors, after SCI | SCI Neurotrophins Nociceptors |
| 10 | Alizadeh et al. (2019) | Traumatic SCI: An overview of pathophysiology, models, and acute injury mechanism | Comprehensive overview of pathophysiology of SCI, neurological outcomes of human SCI, and available experimental model systems that have been used to identify SCI mechanisms | SCI |
| 11 | Cao et al. (2020) | Function and Mechanisms of truncated BDNF receptor TrkB.T1 in Neuropathic pain | Review of studies on truncated TrkB.T1 isoform, and its potential contribution to hyperpathic pain through interaction with neurotrophins and change in intracellular calcium levels. | Neuropathic pain Neurotrophins Nociceptors |
| 12 | Garraway (2023) | BDNF-Induced plasticity of spinal circuits underlying pain and learning | Review of literature on various types of plasticity that occur in the spinal cord and discussion of BDNF contribution in mediating cellular plasticity that underlies pain processing and spinal learning. | Pain SCI Neurotrophins |
2. Overview of pain
Pain is a complex sensory state that integrates a variety of external pain-causing, or noxious, inputs through peripheral and central nervous system (CNS) processing. Pain can be broadly defined as adaptive or maladaptive and is composed of a perceptive, reflexive component as well as an affective and emotional component (Melzack and Casey, 1968, Raja et al., 2020). Normal pain is acute and occurs to minimize contact with the noxious mechanical, thermal, or chemical stimuli that is high-threshold and intense (Basbaum et al., 2009), demanding immediate attention and activating a motor withdrawal reflex to prevent further damage (Millan, 1999). In response to injury or tissue damage, specialized peripheral sensory neurons called nociceptors detect nociceptive stimuli and transmit pain information to the initial pain processing site of the CNS, SCDH. Nociceptors release neuropeptides and other substances to trigger an immune response that promotes wound healing and protection against potential infections (Millan, 1999). Activation of the immune system serves to lower the pain-sensing threshold, so that the nociceptors can now detect stimuli with a wider range of intensities, which prevents further damage. The decreased activation threshold results in a phenomenon known as pain hypersensitivity (Woolf, 2010).
Some pain outlasts the initial inflammatory, tissue or nerve injury and is not elicited by a particular external stimulus. This maladaptive pain becomes chronic and debilitating and can be expressed as hyperalgesia (i.e., painful stimulus is perceived as more painful) and allodynia (i.e., non-painful stimulus now perceived as painful). Maladaptive pain is neither protective nor adaptive and can occur spontaneously. Moreover, it can result in potential cognitive effects that are comparable to depressive-like states (Millan, 1999, Peirs and Seal, 2016). Neuropathic pain, which is pain caused by injury to the nervous system (Costigan et al., 2009, Woolf and Mannion, 1999, Woolf and Costigan, 1999) is an example of maladaptive pain. Fig. 2 illustrates the fundamental difference between nociceptive and neuropathic pain.
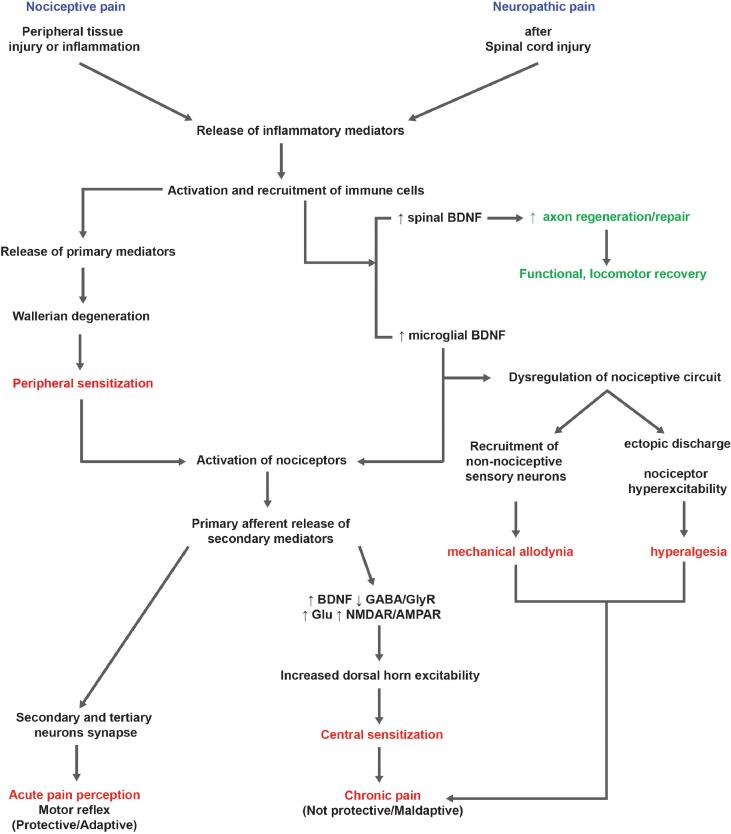
Fig. 2.
Comparison of nociceptive and neuropathic pain. Diagram illustrates an overview of critical mechanisms that lead to development of nociceptive and neuropathic pain after peripheral or central (e.g., SCI) injuries. Some mechanisms overlap, but distinct pathways and modulators involved are noted. Highlighted text indicates negative (red) or positive (green) outcomes of neural plasticity. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
2.1. Neuropathic pain after SCI
SCI is a mechanical damage to the spinal cord that leads to motor deficits below the site of the lesion, autonomic dysfunction, and chronic pain. In the U.S., there are approximately 302,000 individuals living with SCI, with an estimated 18,000 new cases occurring each year (NSCISC, 2023). After SCI, spontaneous functional recovery can take place to regenerate and restore the connectivity of the damaged axons. Studies have shown that while neuroplasticity is necessary for recovery, the same mechanisms may also be maladaptive. Nociceptive pain is a very common effect of SCI that occurs shortly after the primary injury, typically due to inflammation of the lesion area (Finnerup, 2013). However, within months after the injury, a more clinically relevant neuropathic pain develops in 40–50 % of individuals, often described as burning, stabbing, and/or shock-like (Finnerup et al., 2001, Siddall et al., 2003). Although its underlying neural mechanisms are not fully elucidated, neuropathic pain after SCI involves central and peripheral mechanisms (Bedi et al., 2010, Carlton et al., 2009, Christensen and Hulsebosch, 1997, Crown et al., 2006, Garraway et al., 2014, Hulsebosch et al., 2009, Yezierski et al., 2004), with many neural substrates implicated in these processes. Because the mechanisms of neuropathic pain are complex, therapeutic interventions aimed at the alleviation of neuropathic pain are often limited. For instance, despite development of diverse treatment strategies, from neurosurgery (Jug et al., 2015, Rath and Balain, 2017) to behavioral (Ilha et al., 2019, Sandrow-Feinberg and Houle, 2015) and pharmacological (Baroncini et al., 2021, Bracken et al., 1997) therapies, chronic neuropathic pain remains refractory (Baastrup & Finnerup, 2008), an effect that is directly linked to the diverse neural processes and substrates that are implicated and the heterogeneity of SCI and chronic pain etiologies.
Neuropathic pain after SCI can be localized or widespread in that the symptoms can occur at the level of the injury (“at-level”) or below the injury site (“below-level”) throughout the entire body. A variety of factors change after SCI that can ultimately contribute to neuropathic pain, which makes studying the underlying mechanism particularly difficult (Finnerup, 2013). One major cellular change is central sensitization, which is a potentiation of pain by CNS mechanisms. Central sensitization occurs because neurons in the nociceptive pathway exhibit increased membrane excitability and synaptic efficacy (Woolf, 1983), although it is now thought to involve spinal mechanisms that are independent of primary afferent input (Harte et al., 2018). Nonetheless, the onset of central sensitization drives nociceptive neurons to become hyperexcitable and consequently leads to pain (Brown & Weaver, 2012).
3. Overview of the dorsal root ganglia (DRG)
Pain is transmitted by primary afferent sensory fibers known as nociceptors. The cell bodies of nociceptors, along with other primary sensory neurons, are in the DRG. DRGs are highly complex structures situated on either side of the spinal cord, spanning the length of the spinal column. Each DRG rises from the SCDH as an enlargement of the dorsal root (Krames, 2014). The structure and function of the DRGs have been studied primarily in rodents (Abraira and Ginty, 2013, Koltzenburg et al., 1997, Li et al., 2016, Lynn and Carpenter, 1982, Reinhold et al., 2015, Usoskin et al., 2015), providing insight into signaling pathways, network connectivity, and molecular and functional involvement in transduction of pain (Mogil, 2009). Rodent models have been useful as DRGs are almost impossible to access in living humans and even very difficult to obtain postmortem (Rostock et al., 2018). Recent advancements have been made in investigation of human DRGs using human-derived DRGs (Davidson et al., 2014, Rostock et al., 2018) or human embryonic stem cells that can be differentiated into human sensory neuron-like cells (Blanchard et al., 2015, Chambers et al., 2012). With genetic tools, such as RNA sequencing (RNA-seq) and proteomics, human DRGs have been better characterized and compared to previously established features of rodent DRGs, which is important for development of targeted treatments in the clinic. Ray et al. (2018) found that human DRG transcriptome highly corresponds to that of mouse DRGs, and that the majority of DRG-related genes found in the commonly used C57BL/6 mouse strain are strongly associated with human DRGs, suggesting that the mouse is indeed a valid translational model for studies of sensory neurons and pain research. Furthermore, proteomic studies comparing human and mouse DRGs have also found a large overlap. Schwaid et al. (2018) found shared proteins for extracellular trafficking and myelin sheath, which are both integral for signal transmission, as well as ion channels (such as voltage-gated sodium channel, Nav 1.7) that are important for nociception. However, while the general proteomic and transcriptome profiles of human and mouse DRGs are similar, specific expression levels and functions can be distinct. For example, human DRGs express Nav 1.7 in higher subtype proportions and lower Nav 1.8 expression, while the mouse DRGs show the opposite (Chang et al., 2018).
Because most previous molecular and functional analyses of nociceptive neurons have used rodent models, especially for studies of peripheral nerve injury (Dowdall et al., 2005), SCI, and inflammatory and neuropathic pain (Jang et al., 2017, Nirogi et al., 2012), the current review will discuss rodent DRGs unless specified (see (Haberberger et al., 2019)) for a thorough review of human DRGs).
DRGs are typically circular to oval (Esposito et al., 2019, Lee et al., 1986). They are pseudounipolar neurons with an offshoot cell body connected by a single axon that branches into two different directions. The DRG cell bodies do not synapse onto each other (Krames, 2014) as they are separated by layers of intermittent satellite glial cells (SGCs) (Pannese, 2010) and Schwann cells. The sheaths allow penetration of neurotransmitters and other molecules into the neuron, rendering the DRG somas susceptible to inflammatory mediators and drugs in the system (Crawford and Caterina, 2020, Esposito et al., 2019). DRG cell bodies can also filter information received from the peripheral branch at the T-junction (Du et al., 2017, Hao et al., 2023).
3.1. DRG modulation of sensory information
DRGs have a critical role in the modulation of peripheral and central sensory processing, such as inflammation and development of neuropathic pain. The offshoot cell body allows continuity of information transmission between the peripheral (somatosensory) end organ (e.g., skin, viscera) and the CNS (spinal cord) through two axons, which display distinct electrophysiological, structural, and molecular properties. The peripheral process behaves similarly to a dendrite. It generates the action potential at the terminal that propagates towards the stem and the T-junction. The central process receives the signal from the bifurcation and sends it to the CNS. Some factors that may contribute to pain have been observed in the peripheral axon after inflammation or injury. These include extracellular-signal-regulated kinase (ERK), a kinase that is usually found downstream of many receptors’ activation (Perlson et al., 2005), and Transient receptor potential vanilloid (TRPV) −1, a membrane ion channel that is linked to noxious heat stimuli in pain-sensing neurons. TRPV1 was one of the first receptors associated with sensory transduction (Caterina et al., 1997) and is expressed in peripheral and central terminals, and at the cell bodies of sensory neurons (Clark et al., 2018). TRPV1 was shown to be selectively transported to the peripheral axonal branch after inflammation (Ji et al., 2002), which implicates its role in the development of pain. The central branch of the DRG enters the spinal cord and terminates in the SCDH (Basbaum et al., 2009, Millan, 1999).
3.2. Sensory neurons – nociceptors
Primary sensory neurons are known as the first-order neurons because they receive external sensory stimuli and initiate the transmission process. Upon activation, the first-order neurons conduct action potentials to the SCDH, where information can be carried via second-order neurons through the dorsal column (e.g., mechanosensory, proprioception) or the spinothalamic pathway (e.g., temperature, pain) (Purves D, 2001). DRGs contain the largest proportion of sensory neurons, but other ganglia, such as trigeminal (Bhuiyan et al., 2023, Edvinsson et al., 2022, Goto et al., 2016, Lopes et al., 2017) and vagal (Herrity et al., 2015, Ichikawa et al., 2007, Lázár et al., 2018) ganglia are also enriched with sensory neurons, though outside of the scope of the current review. Traditionally, sensory neurons are morphologically and functionally classified as Aβ-, Aδ-, or C-fibers, based on the degree of myelination, sensory modality, and action potential conduction velocity (Gardner, 2010, Horch et al., 1977). Recent single-cell RNA-seq (Li et al., 2016, Usoskin et al., 2015, Wang et al., 2021) and spatial transcriptomic studies (Jung et al., 2023, Tavares-Ferreira et al., 2022) have found more diversity in subclasses of DRGs and describe a more complex and nuanced understanding of the molecular heterogeneity within the DRG neurons. High-coverage single-cell RNA-seq was performed to find 10 types and 14 subtypes of DRG neurons with distinct marker genes, such as galanin, natriuretic peptide B, tyrosine hydroxylase (TH), and MAS-related GPR family member D (Li et al., 2016). Electrophysiological analysis and deep sequencing have identified specific ion channels mediate subtype-specific stimulus modalities (Zheng et al., 2019) that correspond to the subtypes established by transcriptome profiles. Though not fully demonstrative of the heterogeneity of the sensory neurons, this review uses the traditional, albeit simpler, morphology- and function-based classification system, as it appropriately demonstrates the diversity of morphologies and molecular properties among the sensory neurons, especially in the context of how different sensory neurons can selectively fire action potentials with stimulus intensities that reach distinct thresholds (Basbaum et al., 2009). The sensory neurons process a variety of sensory modalities such as temperature (thermoreceptors), pain (nociceptors), pressure (mechanoreceptors), and orientation (proprioceptors). The nociceptors are the smallest of the primary afferents innervating the skin and deep visceral tissues, and are activated by high threshold, intense stimuli. They can detect thermal, mechanical, and combination of different sensory stimuli (Basbaum et al., 2009, Dubin and Patapoutian, 2010, Mendell et al., 1999). Nociceptors are broadly classified into two types: Aδ-fibers and C-fibers. Aδ-fibers are small-diameter, thinly myelinated neurons that produce fast onset pain (Djouhri & Lawson, 2004) described as sharp and pricking (Hladnik et al., 2015). Aδ-fibers can be type I, which is activated by mechanical stimuli and high threshold heat (>50 °C), or type II, which has a high mechanical threshold but lower temperature threshold and is responsible for the initial acute pain response (Hladnik et al., 2015). Most nociceptors are small-diameter unmyelinated C-fiber neurons (Lumpkin & Caterina, 2007), which are polymodal and can detect mechanical, thermal, and chemical stimuli. The activation of C-fibers produces diffused dull, burning pain that is poorly tolerated (Hladnik et al., 2015).
Nociceptors are composed of four functional components: i) peripheral terminal transduces the stimuli and initiates action potentials if the stimuli cross the threshold, ii) the axon propagates the action potentials towards the central terminal and becomes the presynaptic terminal of the first synapse in the CNS sensory pathway (Woolf & Ma, 2007), iii) the cell body responds to changes in the environment by altering transcription of neuropeptides, growth factors, and expression of ion channels and receptors, and iv) the central terminal. Noxious stimuli are received through the free unencapsulated nerve endings innervating the wall of arterioles, connective tissue, and skin (Zylka et al., 2005). Under normal, physiological circumstances, nociceptors respond exclusively to painful or potentially painful stimuli but are otherwise electrically silent (Dubin and Patapoutian, 2010, Woolf and Ma, 2007), requiring stimuli to cross the threshold at sufficient amplitude and duration to depolarize peripheral terminals. The nociceptors transduce the high intensity stimuli, conduct action potentials, and transmit signals to CNS neurons through activation of different receptors and channels on the membrane (Snider & McMahon, 1998). Some of the ion channels, receptors, and intracellular signaling proteins include voltage-gated sodium channels (VGSCs), specifically, Nav 1.7, 1.8 and 1.9 (Bennett et al., 2019, Wang et al., 2011), TRP receptors (Caterina et al., 1999), neurofilament peripherin (Bae et al., 2015), and isolectin B4 (IB4; Bogen et al., 2005). The depolarizing currents generated by the receptors, if sufficient, turn into action potentials, which are propagated to the CNS. VGSCs and potassium channels determine neuronal excitability by transferring the electrical input from the peripheral nerve terminals to the central neurons in the spinal cord (Woolf and Costigan, 1999, Woolf and Mannion, 1999).
The action potential that is generated in the first order neuron is conducted to specific laminae in the SCDH where nociceptor terminals are topographically organized. The distinct organization allows activation of second order projection neurons that underlie acute pain and emotional, affective, and reflexive responses (Woolf and Costigan, 1999, Woolf and Mannion, 1999). When nociceptors synapse with dorsal horn neurons, they release glutamate and peptides, such as substance P (SP), calcitonin gene-related peptide (CGRP), and somatostatin, to alter synaptic and efferent signaling (Dubin & Patapoutian, 2010). Glutamate acts primarily on the ionotropic glutamatergic receptors, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) (Yoshimura & Jessell, 1990) and N-methyl-D-aspartate (NMDA) (King et al., 1988, Woolf and Salter, 2000), to mediate fast excitatory postsynaptic potentials (EPSPs) produced by the AMPARs, followed by NMDAR-mediated slow EPSPs. Glutamate, along with the neuromodulators, act on metabotropic receptors that can induce the activation of several second messenger systems and downstream protein kinases. The growth factor, BDNF is also released from nociceptors and shown to mediate actions akin to central sensitization (Thompson et al., 1999). A more detailed description of the role of VGSCs, glutamate, and BDNF in pain sensation is provided in the following sections.
3.3. VGSCs in DRG and sensory neuron are critical in pain
While there is a large body of work evaluating other channels, this review focuses on VGSCs because sodium channels are critical to the action potential initiation and can be regulated by various receptors, including receptor tyrosine kinases (D'Arcangelo et al., 1993, Hilborn et al., 1998), such as TrkB. In fact, some subtypes of VGSCs that are exclusively expressed on sensory neurons have been reported to be in association with TrkB for normal function (Blum et al., 2002) and also contribute to neuropathic pain (Sun et al., 2022).
3.3.1. Types of VGSCs
VGSCs are generally expressed throughout the body as Nav 1.1–1.9 (Goldin et al., 2000) and are responsible for generation of the rising phase of the action potential (Hille, 1970, Hille, 2022). Thus, they are critical in neuronal excitability (Bennett et al., 2019). VGSCs are classified by their response to the channel blocker tetrodotoxin (TTX), as either TTX-sensitive (TTX-s; Nav 1.1–1.7) or -resistant (TTX-r; Nav 1.8 and 1.9) channels (Bossu and Feltz, 1984, Catterall, 1992, Elliott and Elliott, 1993). TTX-s VGSCs tend to activate more rapidly and display faster kinetics (Blair & Bean, 2002). TTX-r VGSCs recover faster from inactivated state than TTX-s currents almost by tenfold (Cummins and Waxman, 1997, Elliott and Elliott, 1993). At least 5 subtypes of VGSC (Nav 1.1, 1.6, 1.7, 1.8 and 1.9) are highly expressed in DRG neurons, which enable them to produce both fast-inactivating TTX-s and slow-inactivating TTX-r sodium currents (Rush et al., 1998). Specifically, TTX-r VGSCs are preferentially expressed in C and Aδ sensory neurons (Akopian et al., 1996, Djouhri et al., 2003), but other DRG neurons also express a combination of TTX-s and TTX-r channels, which influence their contribution to electrogenic properties under normal or pathological conditions. These subtypes include TTX-s Nav 1.7 (Dib-Hajj et al., 2013), and TTX-r Nav 1.8 (Hameed, 2019, Sangameswaran et al., 1996, Watanabe et al., 2014) and 1.9 (Dib-Hajj et al., 2015, Tate et al., 1998). These three VGSC subtypes are thought to be closely associated with nociceptor function, due to their identification in humans with abnormal sensitivity to pain (Dib-Hajj et al., 2005, Faber et al., 2012, Huang et al., 2015). Nav 1.7 is expressed predominantly in small-diameter DRG neurons (Black et al., 2004) and is also expressed in sympathetic neurons (Black et al., 1996, Toledo-Aral et al., 1997). Nav 1.8 and 1.9 are preferentially expressed in DRG neurons (Djouhri et al., 2003, Fukuoka et al., 2008), but Nav 1.9 is also expressed in myenteric neurons and can also be found in free nerve terminals and central terminals in the spinal cord (Dib-Hajj et al., 1998). Nav 1.9 is only expressed on C fibers, while Nav 1.8 is found in both C and A fibers (Amaya et al., 2000, Decosterd et al., 2002).
Nav 1.7 responds to small depolarizing stimuli close to the neuronal resting membrane potential (Cummins et al., 1998), amplifying accumulated subthreshold signals deployed at nociceptor nerve terminals (Toledo-Aral et al., 1997). Thus, Nav 1.7 determines the gain of nociceptor activation (Rush et al., 2007). Nav 1.7 also contributes to synaptic transmission by impacting neurotransmitter release (Alexandrou et al., 2016). In addition, Nav 1.7 hyperfunction has been implicated in driving evoked and spontaneous pain symptoms by increasing excitability and spontaneous activity (Dib-Hajj et al., 2005). A large portion of nociceptors are dependent on Nav 1.8 and 1.9 for axonal propagation (Klein et al., 2017). Nav 1.8 channels carry most of the sodium current (Blair & Bean, 2002) and recover more quickly from inactivation (Dib-Hajj et al., 1999), thereby contributing to repetitive firing and regulating neuronal excitability. Nav 1.9 is activated near resting membrane potential and produces a large persistent current due to its slow activation and inactivation kinetics (Cummins et al., 1999). Hence, like Nav 1.7, Nav 1.9 is thought to act as a threshold channel that determines neuronal excitability and prolongs the depolarizing response to stimuli rather than contributing to the amplitude of action potentials (Dib-Hajj et al., 2015, Herzog et al., 2001, Ostman et al., 2008).
3.3.2. VGSCs play a role in mediating injury-induced pain
VGSC expression and the development of neuropathic pain have been linked in other studies (Black et al., 1999, Gold et al., 2003, Lai et al., 2002). Nav 1.7, 1.8 and 1.9 have been targeted to relieve symptoms of neuropathic pain. It was reported that moderate loss of function of Nav 1.8 is associated with reduced pain sensitivity in humans and causes decreased excitability of mouse DRG neurons (Duan et al., 2016). The development of ectopic activity in nociceptors and other sensory neurons (Boucher et al., 2000, Liu et al., 2000, Wall and Gutnick, 1974, Wu et al., 2001) contribute to maintaining peripheral neuropathic pain (Devor, 2006, Haroutounian et al., 2014). Intravenous TTX inhibited ectopic activity in damaged rat DRG and SCDH neurons (Omana-Zapata et al., 1997) and reduced neuropathic pain behaviors (Lyu et al., 2000). After injury or inflammation, primary afferent hyperexcitability has been correlated with upregulation of both TTX-s and TTX-r VGSCs on SCDH neurons (Hains et al., 2003, Hains et al., 2005). Furthermore, studies have shown that the mRNA and/or protein levels of Nav 1.7, 1.8, and 1.9 in DRG neurons are increased after peripheral inflammation (Black et al., 2004, Coggeshall et al., 2004, Okuse et al., 1997, Strickland et al., 2008). Treating DRG neurons with inflammatory mediators increased the number of active Nav 1.9 channels and increased neuronal excitability (Binshtok et al., 2008, Maingret et al., 2008). This finding implicates Nav 1.9 in lowering the threshold and increasing the number of action potentials to drive DRG neuron hyperexcitability associated with inflammatory pain. Nav 1.8 expression was reported to increase in patients with neuropathic pain (Joshi et al., 2006), and Nav 1.9 gain-of-function mutations have also been identified in patients with painful peripheral neuropathy (Huang et al., 2014).
However, specific changes in VGSC expression after peripheral nerve injury or in experimental neuropathic pain models are less clear. For example, in animal models of neuropathic pain, Nav 1.7, 1.8 and 1.9 were reduced in the DRGs (Berta et al., 2008, Cummins and Waxman, 1997, Dib-Hajj et al., 1998, Gold et al., 2003, Kim et al., 2002). Likewise, there was a reduction in Nav 1.8 and Nav 1.9 in the DRGs following peripheral axotomy (Coward et al., 2000) and after lumbar (L) 5 sciatic nerve ligation in the nearby uninjured L4 DRG (Dong et al., 2007). Decosterd et al., 2002 reported near complete depletion of Nav 1.8 and 1.9 in L5 DRG after lesion of the L5 spinal segmental nerve, and the expression in neighboring uninjured DRGs was unchanged. Meanwhile, Nav 1.8 and 1.9 levels in L4 DRG were not changed following a central axonal injury of L4. Contrastingly, increased immunoreactivity for Nav 1.8 was observed in tissue samples from animal pain models (Novakovic et al., 1998), likely in uninjured C-fibers (Gold et al., 2003), and patients of persistent pain conditions (Shembalkar et al., 2001). In rodent neuropathic pain models, Nav 1.8-specific inhibitor reversed mechanical allodynia (Jarvis et al., 2007), but selective deletion of Nav 1.8 (Akopian et al., 1999, Kerr et al., 2001) did not prevent development of neuropathic pain. These different outcomes indicate the tremendous amount of injury-related plasticity that occurs within the DRG and furthermore show that VGSCs are differentially involved in pain modulation, an effect that might be dependent on both species’ differences and the type of injury.
3.4. Overview of glutamate and glutamate receptors
Glutamate is one of the most abundant excitatory and nociceptive neurotransmitters in the periphery (Keast & Stephensen, 2000). Glutamate binds to and activates ionotropic AMPA/kainate and NMDA glutamatergic receptors, and several metabotropic receptors. Glutamate receptors, such as NMDARs, are found throughout the nervous system (Monaghan and Cotman, 1985, Monyer et al., 1994) and primary sensory neurons. Receptors that engage with glutamate are synthesized in the DRG cell body and transported to the nerve terminals in the peripheral end organs, such as the skin, muscles, and joints (Carlton et al., 1995, Ma and Hargreaves, 2000). Primary sensory neurons express and release glutamate from central and peripheral terminals (Bae et al., 2000, deGroot et al., 2000) as well as from their cell bodies (Kung et al., 2013). Likewise, glutamatergic receptors and glutamate transporters have been observed in the cell bodies and terminals of the primary sensory neurons.
All four types of AMPAR subunits (GluA1-4) are expressed on sensory ganglia and central terminals. GluA1 is ubiquitously distributed in both myelinated and unmyelinated peptidergic nociceptors (Sato et al., 1993, Tachibana et al., 1994), while the GluA4 subunit is expressed predominantly on non-peptidergic neurons that are strongly associated with neuropathic pain (Willcockson & Valtschanoff, 2008). AMPARs are also expressed on the central terminals of the primary sensory neurons and in the SCDH, generally in laminae I-II, though the expression varies between different subtypes (Larsson & Broman, 2011). AMPARs are located in pre-, post-, and extra-synaptic membranes (Bredt and Nicoll, 2003, Malinow and Malenka, 2002). Synaptic AMPARs have been shown to contribute to nociceptive inputs (Hartmann et al., 2004) and changes to synaptic trafficking and Ca2 + -permeability have been implicated in excitotoxicity and persistent inflammatory pain (Choi et al., 2010, Ferguson et al., 2008, Hartmann et al., 2004, Tao, 2010). Extrasynaptic AMPARs move rapidly between the membrane and the intracellular compartments (Bredt and Nicoll, 2003, Carroll et al., 1999, Petrini et al., 2009) and have been implicated in the maintenance of persistent inflammatory pain (Kopach et al., 2011) and nerve injury-induced pain (Napier et al., 2012).
The NMDA receptor is a ligand-gated ion channel that mediates a major component of excitatory neurotransmission in the central nervous system. NMDA channel kinetics are much slower than that of AMPARs, due to the requirement for membrane depolarization to release the Mg2+ block (Paoletti, 2011). NMDARs consist of combinations of GluN1, GluN2, and GluN3 subunits (Paoletti et al., 2013). Functional receptors comprise of two GluN1 subunits, with either 2 GluN2 subunits or a combination of GluN2 and GluN3 subunits. Of the subunits, GluN2 is also expressed as isoforms GluN2A, 2B, 2C, 2D, and the differential composition and expression of these subunits determine NMDAR function (Salter et al., 2009). NMDARs are found on the cell bodies of both small and large diameter sensory neurons (Liu et al., 1994, Marvizon et al., 2002, Sato et al., 1993) and almost half of peripheral axons (Li et al., 2004). NMDARs and their receptor composition are also differentially distributed in the cell soma and pre-, post-, and extra-synaptic terminals. The differential distribution of NMDAR subunits demonstrates the complexity of NMDAR function and overall contribution in pain. Studies show synaptic inputs into superficial regions of the SCDH, where nociceptive afferents are integrated with interneurons and descending input from the brain (Todd, 2010) are mainly mediated by GluN2A and GluN2B subunits (Bardoni et al., 2004, Shiokawa et al., 2010).
Glutamate also engages metabotropic receptors, which are family C G-protein-coupled receptors. To date, there are eight subtypes of metabotropic glutamate receptors (mGluRs; R1–R8), which are classified in three groups based on sequence similarities. Group I, containing mGluR1 and 5 is excitatory, while Groups II and III are inhibitory (Conn and Pin, 1997, Schoepp et al., 1999). All three groups of mGluRs are also expressed in DRGs, mostly mGluR2/3 in 40–52 % of small-diameter cells (Carlton and Hargett, 2007, Carlton et al., 2001). These receptors may negatively regulate glutamate release to modulate nociceptive input (Carlton et al., 2001, Palazzo et al., 2014).
3.4.1. Glutamate and glutamate receptors in pain hypersensitivity
While both ionotropic and metabotropic glutamate receptors are implicated in persistent pain states, the role of the ionotropic receptors, primarily the NMDAR, is more thoroughly studied and will be emphasized in this section. NMDARs (Liu et al., 1994, Lu et al., 2003) and AMPARs (Carlton et al., 1995, Coggeshall and Carlton, 1998) are both transported from the cell bodies to the central terminals where they are activated by endogenously released glutamate that ultimately leads to sensitization of spinal cord neurons (Coderre and Melzack, 1992, Dickenson and Sullivan, 1987). Extensive studies have been done on postsynaptic NMDARs in the afferent terminals and their contributions to neuropathic pain, especially in central sensitization (Ji et al., 2003, Woolf and Salter, 2000, Zhang et al., 2016). GluN2B-containing NMDARs have been shown to be involved in neuropathic pain development following injury (Qu et al., 2009, Suzuki et al., 2001). Consistently, the GluN2B subunit is preferentially localized to the unmyelinated axons that synapse in the superficial regions of the SCDH (Temi et al., 2021). The GluN1 subunit is also implicated in pain. Targeted deletion of GluN1 in the SCDH of adult rats and/or mice attenuated both inflammatory (Garraway et al., 2009) and injury-induced pain (South et al., 2003).
In pathological conditions, such as following peripheral nerve injury, glutamate expression is increased in the DRG neurons (Kung et al., 2013), and after injury or inflammation, release of endogenous glutamate activates NMDARs on the primary afferent peripheral terminals, resulting in development of pain behaviors (Omote et al., 1998). Gong et al. (2014) showed that following chronic constriction injury of the sciatic nerve, glutamate-induced inward currents were increased in small diameter neurons, as well as neurons that were responsive to NMDA and AMPA. Also, peripheral inflammation increased the number of peripheral axons that express glutamate receptors (Du et al., 2006, Du et al., 2003). Exogenous administration of glutamate, NMDA, or AMPA caused an induction of mechanical hyperalgesia in the rat hind paws (Ferreira and Lorenzetti, 1994, Parada et al., 2003), and glutamate triggers action potentials in an NMDAR dependent manner (Laursen et al., 2014). On the other hand, blocking NMDA (Christoph et al., 2005) and AMPA/kainate (Lee et al., 2001) receptors resulted in an attenuation of pain behavior and blockade of nociceptor activity in inflammatory and neuropathic models (Jang et al., 2004). Similarly, in rodent neuropathic pain models, NMDAR antagonists, such as memantine, MK801, and ketamine led to a decrease in mechanical hyperalgesia (Burton et al., 1999) by the reduction of spinal cord sensitization.
3.4.2. Presynaptic NMDA receptors contribute to pain
Studies show NMDARs are also found at presynaptic nerve terminals in the SCDH (Bardoni et al., 2004, Krebs et al., 1991, Liu et al., 1994, Lu et al., 2003). Almost a third of SCDH NMDARs are presynaptic, which are translated in DRG neurons and transported to the synaptic terminal, adjacent to the vesicle release site (Liu et al., 1994). Unlike postsynaptic NMDARs, presynaptic NMDARs can spontaneously release neurotransmitters, such as SP, without neuronal depolarization and removal of the Mg2+ block (Dore et al., 2017, Kavalali, 2015). A distinct process involving metabotropic signaling through c-Jun N-terminal kinase (JNK) (Abrahamsson et al., 2017) can also trigger neurotransmitter release. Src family kinases, which phosphorylate NMDARs to activation, have also been implicated in primary afferent presynaptic NMDAR regulation (Marvizón et al., 1997) by increasing presynaptic glutamate release (Madara & Levine, 2008), which in turn can promote more persistent overall increase of glutamate receptor activity. Presynaptic NMDAR activation increases the frequency of miniature excitatory postsynaptic currents (EPSCs) associated with pain hypersensitivity in rodent models of neuropathic pain (Chen et al., 2014a, Chen et al., 2014b). Xie et al. (2016) found increased mEPSC frequency that was driven by presynaptic NMDARs in rats with paclitaxel-induced neuropathic pain that was through phosphorylation of GluN2A subunits instead of typical GluN2B. These results suggest that presynaptic NMDARs are recruited in conditions of neuropathic pain to facilitate glutamate release that increases SCDH neuronal excitability (Yan et al., 2013).
NMDARs are also found extrasynaptically and can be activated by glutamate in extracellular compartment (Meur et al., 2007), astrocytes (Carmignoto & Fellin, 2006), and glia (Nie et al., 2010), and accordingly, distributed in clusters in very specific cell contact areas to act as a point of contact with these processes (Kharazia and Weinberg, 1999, Papouin and Oliet, 2014, Petralia et al., 2010). Very little is known about the physiological function of extrasynaptic NMDARs, especially in the context of sensory neurons and nociception. Expression of various excitatory and inhibitory glutamate receptors show just a small fraction of variables that could change pain signaling after injury or inflammation. Added to this complex pool of receptors and channels is yet another diverse group of neuromodulators and inflammatory mediators, which we will discuss in more detail below.
4. Brain-derived neurotrophic factor modulates nociceptive and neuropathic pain
During nociceptive signal transduction, one of many different neuromodulators that is released from nociceptor terminals is the neurotrophin, BDNF. BDNF, which was first identified by Barde et al. (1982) has been well studied for its role in neuronal development and plasticity in the CNS (Ferrini and De Koninck, 2013, Merighi et al., 2008, Nijs et al., 2015), as well as in the development of sensory and motor neurons (Jones et al., 1994, Liu et al., 1995). Along with BDNF, other neurotrophins, including nerve growth factor (NGF), neurotrophin (NT) 3, and 4/5, are responsible for the development of the nervous system and are implicated in the survival and differentiation of neurons (Huang and Reichardt, 2001, Mitre et al., 2017, Numakawa et al., 2010). The neurotrophins mediate their actions by cell surface Trk receptors. NGF and NT3 preferentially bind TrkA and TrkC receptors, respectively, while BDNF and NT4/5 activate the TrkB receptor (Kaplan & Miller, 2000). Among the neurotrophins, BDNF is the most abundant and widely distributed in the CNS (Pezet, Malcangio, & McMahon, 2002). It is encoded by the bdnf gene (Leibrock et al., 1989, Timmusk et al., 1995, Timmusk et al., 1993) and is synthesized and released by sensory neurons, motor neurons, and peripheral and central immune cells (see reviews by Nijs et al. (2015) and Brigadski and Leßmann (2020)). BDNF exerts most of its cellular actions by engaging its high affinity TrkB receptor and the low affinity non-specific p75 neurotrophin receptor, but this review will focus on its actions through activation of TrkB.
4.1. TrkB receptor
TrkB is a transmembrane receptor with an extracellular ligand binding domain and a catalytically active intracellular domain. BDNF binds to TrkB, leading to the dimerization of the TrkB heteromer and auto-phosphorylation of its tyrosine residues. This activates downstream intracellular transduction pathways such as mitogen-activated protein kinase (MAPK) and phosphoinositide 3-kinase (PI3K) pathways (Huang and Reichardt, 2001, Numakawa et al., 2010, Pezet et al., 2002b). BDNF signaling through TrkB is essential for normal function in the brain such as neuronal survival, differentiation, and regulation of neuronal structure and function (Bonhoeffer, 1996, Numakawa et al., 2010). BDNF regulates neuronal excitability and synaptic plasticity in the CNS (Levine et al., 1995) by promoting neurotransmitter release (Takei et al., 1997), phosphorylating glutamatergic receptors (Levine et al., 1995), and inducing structural and functional changes in neurons (Abidin et al., 2008, Marty et al., 1997). In the CNS, BDNF itself can induce synaptic plasticity and long-term potentiation-like synaptic facilitation similarly to that observed in the hippocampus (Gottschalk et al., 1998, Korte et al., 1998, Novkovic et al., 2015).
4.2. BDNF’s expression and function in sensory neurons and spinal cord
BDNF is endogenously and constitutively synthesized in primary sensory neurons in the DRG, based on previous observations of mRNA (Wetmore & Olson, 1995) and protein (Barakat-Walter, 1996, Wetmore and Olson, 1995, Yan et al., 1997) expression. Once synthesized, BDNF is anterogradely transported from the DRG somas to the primary afferent terminals (Michael et al., 1997, Zhou and Rush, 1996) and is released into the SCDH (Lin et al., 2011, Matayoshi et al., 2005). BDNF is also expressed in Schwann cells (Apfel et al., 1996, Cho et al., 1997, Michael et al., 1997) and epithelial cells (Hahn et al., 2006, Hahn et al., 2005, Wang et al., 2015). The subpopulation of DRG neurons that express BDNF are small-to-medium sized peptidergic cells, presumably nociceptors (Luo et al., 2001, Michael et al., 1997). BDNF is stored in large dense core vesicles of these small cells along with SP and CGRP (Michael et al., 1997, Pezet et al., 2002a), which are released in the laminae I and II of the spinal cord in an activity-dependent fashion (Kerr et al., 1999, Lever et al., 2001, Mannion et al., 1999, Qiao et al., 2016, Zhou and Rush, 1996), where SP-containing C-fibers terminate (Michael et al., 1997).
Previous studies have implicated both spinal and peripheral BDNF in pro-nociceptive actions (Ferrini and De Koninck, 2013, Nijs et al., 2015) that can potentially contribute to the development of central sensitization leading to pain (see reviews by Thompson et al., 1999, Garraway and Huie, 2016, and Cappoli et al. (2020)). Short bursts of high frequency electrical stimulation of C-fibers and/or NGF treatment (i.e., inflammatory condition) (Lever et al., 2001) or sciatic nerve transection (Lever et al., 2001, Walker et al., 2001) induced the release of BDNF in the dorsal horn. Further, BDNF facilitated C-fiber evoked synaptic responses lamina II of the spinal cord, which required post-synaptic NMDARs (Garraway et al., 2003). BDNF is also linked to dysregulation of inhibitory neurotransmission, where reduction of BDNF release after spinal nerve ligation resulted in decreased levels of GABA to impair the GABAergic signaling in the SCDH contributing to hyperexcitability and central sensitization (Lever et al., 2003). Together, these observations show the importance of BDNF released from sensory neurons in synaptic and nociceptive plasticity in the spinal cord.
4.3. BDNF’s contribution to injury-induced pain
BDNF is also implicated in injury-induced pathophysiology, including neuropathic pain. Peripherally, Schwann cell-derived BDNF is increased after peripheral nerve axotomy (Michael et al., 1997, Pezet et al., 2002a). BDNF synthesis and anterograde transport from the DRG somas to the terminals is enhanced by peripheral injury (Mannion et al., 1999, Qiao et al., 2016, Zhou and Rush, 1996), and increase in BDNF in the DRGs correlated with development of allodynia in rats with spinal nerve injury (Zhou et al., 2000). Injury can also cause a phenotypic switch in BDNF expressing neurons in the DRG. After spinal nerve ligation, de novo synthesis and expression of BDNF is increased in the uninjured small neurons (Mannion et al., 1999) and in injured medium and large neurons, which was also observed after axotomy or peripheral nerve injury (Michael et al., 1999, Zhou et al., 1999). Similarly, after sciatic nerve crush, not only is the intensity of BDNF immunoreactivity increased in the small-diameter DRG neurons, but the number of medium- and large-diameter DRG neurons that express BDNF are also increased (Cho et al., 1998). Antisense and antibody against TrkB decreased mechanical hypersensitivity from repeated tactile stimulation (Mannion et al., 1999), acute heat pain, and thermal hyperalgesia (Groth & Aanonsen, 2002). Beyond the sensory neurons, microglial BDNF has been shown to downregulate the expression of the chloride transporter, K+-Cl− cotransporter (KCC2), to disrupt GABAergic-glycinergic-mediated inhibition and produce an overall increase in excitation. Altogether, these studies indicate the involvement of BDNF-TrkB signaling in the DRG and sensory neurons, in inflammatory and injury-induced neuropathic pain.
4.4. Peripheral BDNF-TrkB signaling in mechanotransduction and pain
The aforementioned studies support the pro-nociceptive role of BDNF-TrkB signaling in the nociceptive pathways after peripheral and central injury. Because BDNF’s functions in the CNS are well established, it is conceivable that BDNF will also mediate adaptive or maladaptive plasticity at peripheral sites. BDNF may regulate pro-nociceptive effects through regulation of synaptic transmission, afferent plasticity, and long-term modification in pain pathways to alter nociceptive processing both peripherally and centrally. For example, it was recently shown that BDNF in sensory neurons is necessary for the transition from acute to chronic inflammatory pain and some neuropathic states (Sikandar et al., 2018).
Previous studies have shown TrkB expression in different subpopulations of sensory neurons (Ernfors et al., 1993, Foster et al., 1994, Kashiba et al., 1995, Wright and Snider, 1995) and sensory nerve endings innervated by or connected to primary afferent sensory neurons (García-Piqueras et al., 2019, Montaño et al., 2010, Zimmerman et al., 2014). Importantly, BDNF and/or TrkB signaling has been shown to be required for normal mechanosensation (Carroll et al., 1998, Li et al., 2011, Rutlin et al., 2014). Specifically, BDNF-TrkB expression and signaling in hairy skin is required for development and normal functioning of a subpopulation of cutaneous afferents known as the Aδ-Low-Threshold Mechanoreceptors (LTMRs) (Li et al., 2011, Rutlin et al., 2014), previously classified as D hair cells (Abraira and Ginty, 2013, Koltzenburg et al., 1997). The thinly myelinated Aδ-LTMRs express TrkB and transduce directional touch in a BDNF dependent manner. BDNF is also expressed in a population of myelinated primary afferents, although according to Dembo et al. (2018), its expression in these primary afferents makes limited contributions to pain or itch. BDNF can be released in the periphery from non-neuronal cells, including Merkel cells (Reed-Geaghan et al., 2016) epithelial cells of the skin (Cefis et al., 2020, Rutlin et al., 2014), and peripheral immune cells (see references in Brigadski and Leßmann (2020)). Clearly, the widespread nature of BDNF’s expression suggests it can also modify nociceptor and sensory neuron function by actions in the periphery.
5. Other events that contribute to inflammatory or injury-induced plasticity of DRG
Inflammation and tissue or neural injury can lead to functional, chemical, or structural modification of all the components of the nociceptive pathway. When injury or inflammation takes place, primary nociceptors, mainly C- and Aδ-fibers, display lowered firing thresholds (hypersensitivity) and increased spontaneous firing (hyperexcitability), a phenomenon known as peripheral sensitization. During peripheral sensitization, neuropeptides are released from the peripheral branch of the nociceptor at the injured site or inflamed environment to attract leukocytes and activate receptors that are expressed on the terminals and/or cell bodies of nociceptors (Basbaum et al., 2009, Gold and Gebhart, 2010). Leukocytes, once recruited, are activated to macrophages for removal and regeneration of degenerating axons (Beuche and Friede, 1984, Brown et al., 1991, Hu and McLachlan, 2002, Perry and Brown, 1992). Extending to the CNS, glial activation, characterized by upregulated microglial (e.g., IBA1) and astroglial (e.g., GFAP; glial fibrillary acid protein) markers, and morphological changes can also occur that have been implicated in pathogenesis of chronic pain.
5.1. Effect of peripheral inflammation on DRG plasticity
Inflammatory responses, such as microglial proliferation and macrophage recruitment, have been observed in DRGs with peripheral nerve injury (Eckert et al., 1999, Hu and McLachlan, 2002, Lu and Richardson, 1993) or DRG compression (Zhang et al., 1999). SGCs around the DRG soma proliferate (Barron et al., 1990, Gehrmann et al., 1991, Lu and Richardson, 1991) and contribute to generation of ectopic discharges and oscillatory activity (Devor, 2006). Somal ectopic firing is pathological and produces sensory signals even in the absence of pain stimuli (Woolf & Ma, 2007). SGCs also release cytokines that contribute to development and persistence of nerve injury-induced mechanical hypersensitivity (Ji and Strichartz, 2004, Schafers et al., 2003, Svensson et al., 2005, Zelenka et al., 2005). Localized immune activation has been shown to induce prolonged inflammatory responses and elevated cytokine production, including interleukins (IL) −1β, −6, −18, monocyte chemoattractant protein (MCP)-1, and growth-related oncogene CXCL1 by 17 folds (Xie et al., 2006). Upregulation of the prototypical cytokines IL-1β and tumor necrosis factor (TNF) -α is one of the earliest indications of sensory inflammation and increased nociception (Miller et al., 2009). The release of cytokines from immune and other non-neuronal cells (e.g. keratinocytes) can directly interact with the sensory neurons or affect further downstream mediators, including other cytokines, chemokines, prostanoids, neurotrophins, and ATP (Binshtok et al., 2008, Chiu et al., 2012, Gold and Gebhart, 2010, Julius and Basbaum, 2001, Levin et al., 2008, Liu and Ji, 2013, Pezet and McMahon, 2006, White et al., 2005). Several cytokines such as IL-1β, IL-6 and TNF-α have been linked to DRG neuron excitability (Gadient and Otten, 1996, Gardiner et al., 2002, Inoue et al., 1999, Lee et al., 2004, Li et al., 2005, Nilsson et al., 2005) by sensitizing the TRP channels, which stimulate CGRP release (Obreja et al., 2005, Obreja et al., 2002, Opree and Kress, 2000), or by enhancing TTX-r Na + currents (Jin & Gereau, 2006). Consistent with this observation, TNF-α antibodies attenuate the development of thermal hyperalgesia and mechanical allodynia in models of neuropathic pain (Cunha et al., 2007, Sasaki et al., 2007, Zanella et al., 2008). Increased TRPV1 activation produces increasing inward current with repeated exposure to heat through peripherally released NGF (Zhang et al., 2005), which maintains peripheral sensitization. These changes lead to an increase in substrate for other receptors and amplify and prolong peripheral sensitization, leading to an increase in nociceptor activity.
5.2. Effect of SCI on pain and DRG plasticity
Immediately after the initial damage caused by SCI, the local area undergoes ischemia due to damaged blood vessels and cellular membranes, and inflammation. The insult leads to calcium and glutamate excitotoxicity, ionic imbalance, reactive oxygen species (ROS) production, and further inflammation and apoptosis, which progressively damage the surrounding spinal cord tissue. Microglia are activated to release cytokines and recruit more inflammatory cells like macrophages, which infiltrate the damaged spinal cord and remain activated for several weeks (Popovich et al., 1997, Sroga et al., 2003). The primary injury triggers systemic, cellular, and molecular changes that spread the damage to adjacent white and gray matter (secondary injury). Glutamate levels also increase that drive NMDA and AMPA receptors to be hyperactive. Hence, glutamate-induced excitotoxicity becomes a leading cause of several secondary mechanisms that further the damage. Amplification of pain responses and spread of pain sensitivity to uninjured regions results in secondary hyperalgesia (Ji et al., 2003).
Nociceptor-specific changes after SCI have yet to be investigated fully, but a few studies have shown that SCI can alter nociceptor activity typically by causing increased activity. Due to the injury, sensory signals are strengthened to transform nociceptors to be activated with just low-threshold, usually innocuous stimuli, which leads to neuronal hyperactivity and hyperexcitability. If C-fiber activity that does not cross the normal threshold is induced for prolonged periods, the heightened synaptic transmission results in persistent sensitization (Ji et al., 2003). CGRP-expressing peptidergic nociceptors (Ackery et al., 2007, Christensen and Hulsebosch, 1997, Helgren and Goldberger, 1993) and small- and medium-sized dissociated DRG neurons (Bedi et al., 2012) sprout new branches after SCI, indicating aberrant axonal growth and increased activity. Non-peptidergic C-fibers also undergo aberrant sprouting in the SCDH after SCI, consistent with injury-induced allodynia (Detloff et al., 2014). SCI can recruit typically non-nociceptive primary afferent neurons to the pain pathway (Torebjork et al., 1992), causing phenotypic changes in myelinated fibers, like Aβ-fibers (Woolf & Salter, 2000), which do not normally drive nociceptive output (Latremoliere & Woolf, 2009). Such functional changes in the primary afferent sensory neurons appear as allodynia.
Previous studies in DRG neurons have demonstrated that nociceptors become chronically hyperexcitable and display increased spontaneous activity (Bedi et al., 2010, Yang et al., 2014), which is neuronal firing not driven by any sensory input. While spontaneous activity occurring under physiological conditions aids in development of and rehabilitation of the spinal cord (e.g., motor neuron pathfinding, maturation of synapses, axon regrowth), increase in the incidence of spontaneous activity in the pain-transducing neurons after injury could lead to hyperalgesic priming (Reichling & Levine, 2009), so that the nociceptors are now more predisposed to enter a stable hypersensitive state (Walters, 2012). Because primary afferent somas are located distantly from their receptive fields, somal hyperexcitability may emerge to compensate for damage or loss of peripheral sensory branches after injury (Walters, 2012). Nociceptors also display increased afterdischarge (Eller et al., 2022). These aforementioned changes are consistent with the fact that sensory neurons can exhibit lowered thresholds to thermal and mechanical stimuli (Bishop et al., 2010) that indicate their involvement in the generation of pain.
Transcriptional changes occur in the DRG after SCI that are critically associated with the generation of pain (Costigan et al., 2002, Cuevas-Diaz Duran et al., 2023, Perkins et al., 2014, Wang et al., 2002). For example, changes have been reported in synaptogenesis signaling pathways and networks responsible for inflammatory signaling mechanisms (Yasko et al., 2019). Along these lines, we recently showed that pERK levels are robustly elevated in the adjacent trunk skin after SCI in adult mice that exhibited mechanical pain (Martin et al., 2022, Noble et al., 2022). While increased pERK levels might indicate plasticity of sensory neurons, we did not characterize the specific cell types that express pERK. However, a previous study by Dai et al. (2002) showed pERK is elevated in peripheral nerve terminals following noxious stimulation.
5.3. Peripheral changes after SCI that contribute to pain
While the neural mechanisms that underlie the emergence of nociceptive and neuropathic pain after SCI have been studied extensively in the spinal cord, the functional, morphological, and molecular changes in the periphery still need investigation. Studies suggest peripheral changes play a role in the induction and maintenance of pain, specifically after SCI. Also, sympathetic, and sensory neurons show increased interaction after injury through increased sympathetic fiber density and sprouting into the DRGs (García-Poblete et al., 2003, Kinnman and Levine, 1995b, Shinder et al., 1999, Xie et al., 2007). Many neurotransmitters and neuromodulators such as SP and BDNF are synthesized, stored, and released from the central terminals of the sensory neurons in the SCDH, in response to the injury. Previous studies also showed that intense activation of nociceptors shortly after SCI worsens hind paw mechanical hypersensitivity (Garraway et al., 2014, Martin et al., 2019), suggesting that peripheral nociceptor hyperexcitability can exacerbate pain responses after SCI. Although less understood, recent evidence suggests that peripheral TrkB signaling may also underlie pain after SCI. Specifically, we found that pharmacogenetic inhibition of TrkB systemically delayed the onset of mechanical allodynia after SCI for up to 4 weeks, and reversibly attenuated established pain (Martin et al., 2022, Noble et al., 2022). This observation is supported by a previous study that reported maladaptive TrkB signaling contributes to neuropathic pain after SCI (Wu et al., 2002). Altogether, these observations indicate that peripheral processes, including nociceptor hyperactivity, critically underlie pain after SCI.
5.4. Other SCI-induced changes: non-nociceptors such as C-LTMRs contribute to pain
Even though nociceptors are essential drivers of pain, non-nociceptors can also undergo plasticity that contributes to pain hypersensitivity. C-low threshold mechanoreceptors (C-LTMRs) are small diameter, unmyelinated afferents that innervate the trunk hairy skin and terminate in lamina II of the dorsal horn (Li et al., 2011). In the spinal cord, C-LTMRs form a synaptic glomeruli, demonstrating C-LTMR signals are integrated at the first synapse like other primary afferents (Larsson & Nagi, 2022). They are identified by TH expression (Li et al., 2011, Lou et al., 2013) and vesicular glutamate transporter (VGLUT) 3 expression (Gras et al., 2002, Seal et al., 2009). C-LTMRs normally encode gentle, pleasant touch contributing to social interactions (Bessou et al., 1971, Iggo, 1960, Li et al., 2011, Liljencrantz and Olausson, 2014, Löken et al., 2009, Morrison et al., 2010, Olausson et al., 2002, Zimmerman et al., 2014) and modulation of heat pain (Habig et al., 2017). However, C-LTMRs represent a sub-population of cutaneous afferents that may indeed convey pain under pathological conditions (Mahns and Nagi, 2013, Seal et al., 2009), including SCI. VGLUT3-knockout mice showed impaired acute mechanical allodynia in inflammatory and neuropathic pain models (Seal et al., 2009), demonstrating for the first time, that C-LTMRs are involved not only in low-threshold mechanosensation but may also be required for generation of pain response to innocuous mechanical stimuli. Similarly, ablation of Nav 1.8-expressing nociceptors (Abrahamsen et al., 2008) or knocking out T-type calcium channels (Cav3.2) in nociceptors and C-LTMRs also resulted in attenuation of mechanical allodynia (François et al., 2015), suggesting a possible mechanistic overlap between C-LTMRs and nociceptors. Previous studies undertaken in human subjects found that individuals with SCI respond with hyperesthesia and allodynia to activation of C-tactile fibers (i.e., human equivalent of C-LTMRs (Löken et al., 2009)) with a gentle brush stroke (Finnerup et al., 2003). Recently, we reported further evidence that C-LTMR afferent plasticity produces pain after SCI. Using a conditioned place-aversion paradigm, which investigates the affective component of pain, we showed that adult mice with SCI avoided the chamber associated with C-LTMR stimulation (i.e., mechanical, and optical stimulation) (Noble et al., 2022, Martin et al., 2022). Importantly, these changes took place in parallel to the establishment of hind paw mechanical hypersensitivity, suggesting that C-LTMR afferent plasticity promotes at-level affective pain following SCI, concurrent with below level pain. We also observed that stimulation of C-LTMRs increased pERK levels in TH+ DRG neurons (presumed C-LTMRs) only after SCI (Noble et al., 2022, Martin et al., 2022). C-LTMRs may excite nociceptive lamina I projection neurons through PKCγ-expressing neurons (Artola et al., 2020), which are also required for injury-associated mechanical hypersensitivity (Malmberg et al., 1997). Electrophysiological studies have shown sympathetic neurons directly excite C-LTMRs and increase their sensitivity to mechanical stimuli (Barasi and Lynn, 1986, Roberts and Elardo, 1985, Roberts and Levitt, 1982), and increased sympathetic activity is associated with injury-induced pain (Jänig et al., 1996, Kinnman and Levine, 1995a, Kinnman and Levine, 1995b, Ramer et al., 1999). Hence, it can be suggested that C-LTMR interaction with sympathetic neurons may take place after injury that consequently produces maladaptive changes. Despite these observations implicating C-LTMRs in pain, further investigation focusing on the neural mechanisms that enable the functional switch from touch-to-pain encoding is needed.
5.5. Sympathetic efferents and sensory neurons interact to produce pain
The organization of the sympathetic nervous system enables it to transmit information from the CNS to target tissue/organ. Sympathetic efferent fibers leave the spinal cord in the ventral roots to make their first synaptic connections with neurons in prevertebral sympathetic ganglia located in the abdomen. Apart from the C-LTMRs-sympathetic interaction mentioned in the preceding section, it has long been shown that the sympathetic nervous system contributes to pain in both animal models of neuropathic pain (Kim and Chung, 1991, Levine et al., 1986) and in human studies (McDonnell et al., 2011). Minett et al. (2012) showed that ablation of Nav 1.7 in sensory and sympathetic neurons abolished pain sensation following a nerve ligation model of neuropathic pain in mouse, thereby supporting sympathetic and sensory neurons interaction in distinct types of pain sensation. Specifically, the exact mechanism underlying the interaction remains unclear, but previous studies have investigated interactions at nerve terminals, cell bodies, and central terminals (Jänig & Häbler, 2000). Importantly, a prior study by Ren et al. (2005), showed that sympathetic efferent is necessary for C- and Aδ-pain fiber sensitization by capsaicin, also demonstrating that pain afferents are directly modulated by sympathetic activity (also see Lin et al. (2003)). Sensory neurons and sympathetic nerves are normally only associated with blood vessels, but several pain models have shown increased sympathetic fiber density and sprouting of sympathetic nerves into the DRGs as “basket” structures known as Dogiel’s arborizations (McLachlan et al., 1993). Dogiel’s arborizations were observed by Ramón y Cajal (García-Poblete et al., 2003), in human neuropathic pain patients (Shinder et al., 1999), axotomized cells (Ma & Bisby, 1999), and locally inflamed (Xie et al., 2006) or compressed (Chien et al., 2005) DRGs though in small numbers. The link between basket cells and behavioral studies have been conflicting (Xie et al., 2010), but electrophysiological data demonstrate basket cells to be the main source of spontaneous activity (Xie et al., 2011). Basket cells also showed increased nociceptive markers, such as TrkA, CGRP and SP, demonstrating that sprouting sympathetic fibers are probably closely apposed to nociceptive cells.
Sympathetic activity can indirectly interact with sensory pain systems through the neuro-endocrine and immune systems. Notably, sympathetic activity can impact immune functions by producing inflammation, which can then modify sensory systems such as nociceptor activity (see review by Jänig (2014)). Similarly, spontaneously active DRG cells can cause sympathetic sprouting, presumably by the release of neurotrophic factors or cytokines from SGC or glial cells (Xie et al., 2007, Zhang and Strong, 2008). Receptors for norepinephrine and ATP are also expressed on SGCs, which may be activated to release inflammatory neuromodulators after injury or inflammation that recruit sympathetic fibers (Hanani, 2005, Maruo et al., 2006, Tan et al., 2011). Here, we provide a brief overview of C-LTMRs and sympathetic contribution to pain, although noting that these interactions are quite complex and beyond the scope of this review.
5.6. Central sensitization
While this review aims to shed much needed light on the role DRG and sensory neuronal plasticity play in pain, it is important to briefly discuss cellular plasticity that occurs centrally, particularly the SCDH where nociceptors terminate. At the central axon terminal of the DRG, the release of neurotransmitters and neuromodulators triggers activation of postsynaptic glutamatergic receptors (i.e., NMDARs, AMPARs), which initiate recruitment of subthreshold synaptic inputs to nociceptive neurons to generate a state of potentiation known as the central sensitization (Woolf, 1983). Central sensitization is a C-fiber-mediated enhancement of synaptic efficacy in the spinal dorsal horn neurons following peripheral noxious stimuli, tissue injury, or nerve damage (Ji et al., 2003). Increased synaptic transmission decreases pain threshold, increases membrane excitability, and spreads pain sensitivity to non-injured areas by decreasing overall inhibitory tone (Ji et al., 2003). After the onset of central sensitization, sensory neurons respond to normally subthreshold innocuous stimuli. This response of the sensory neuron can occur independently of intense, noxious stimuli. For example, SCI can cause neuroinflammation and central sensitization that leads to chronic pain without peripheral insult (Hains & Waxman, 2006), by potentially inducing hyperexcitability of primary sensory neurons (Yang et al., 2014), or triggering the release of inflammatory mediators that can regulate DRG gene expression. Similarly, pain after SCI is worsened by intense activation of nociceptors, requiring mechanisms that overlap with central sensitization (Ferguson et al., 2006, Garraway et al., 2014, Grau, 2017). Thus, central and peripheral sensitization may be involved in bi-directional interactions (Ji et al., 2018) to mediate pain after inflammation or tissue injury.
6. Conclusion
Significant advances have been made in our understanding of the neurobiology of pain. Adaptive pain, such as nociceptive pain, is understood to involve peripheral and central mechanisms. Though more complex and less understood, neuropathic pain may also require plasticity at central and peripheral sites. Similarly, our understanding of functional plasticity after SCI has greatly improved with clinical and experimental evidence. Initially focused on neuronal survival, regeneration, and functional recovery of the damaged spinal cord, more recent investigations demonstrate that both adaptive and maladaptive plasticity can extend beyond the spinal cord to peripheral sites.
The DRGs are uniquely positioned at the intersection of the periphery and SCDH and are anatomically accessible, making them important targets for treatment and therapies of neuropathic pain. Additionally, the DRGs house the cell bodies of nociceptors, which are the drivers of pain under normal and pathological conditions. Thus, in this review, we highlighted critical features of the DRG, commonly expressed pain substrates, and DRG plasticity in inflammatory pain, as well as neuropathic pain after SCI.
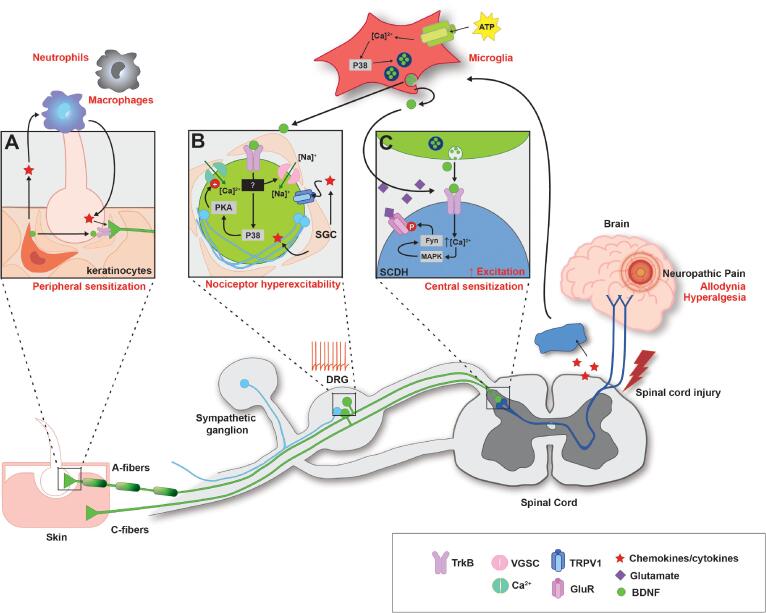
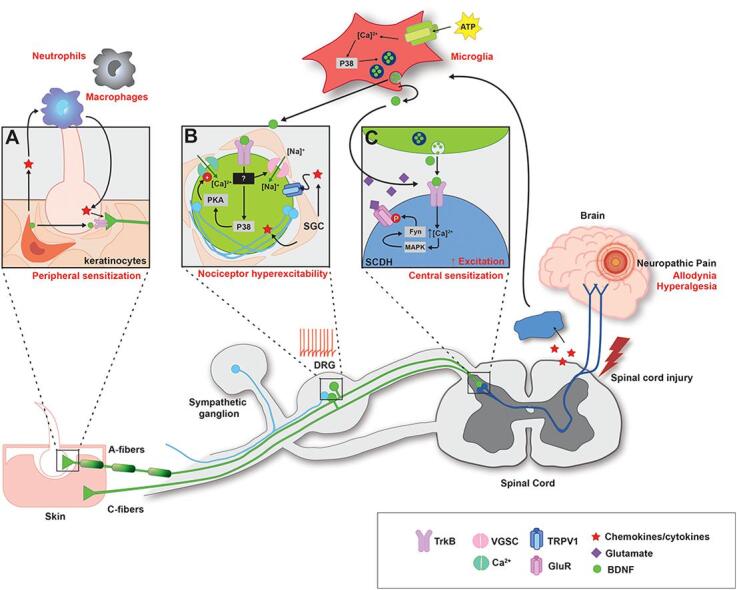
Also, despite the remarkable progress made in identifying the neuroanatomy and molecular mechanisms of peripheral pain transduction and diversity of sensory neurons involved, understanding individual components of the nociceptive circuits and how they interact to contribute to pain under pathological conditions remains to be studied. For example, while sensory neuron hyperexcitability is thought to underlie chronic pain after SCI, the exact mechanisms that lead to neuronal hyperexcitability is unknown. In examining the wealth of changes that occur after SCI, specifically those that may contribute to pain, our laboratory has focused on BDNF and recently reviewed BDNF as a bidirectional neuromodulator of spinal plasticity (Garraway & Huie, 2016), resulting in multifaceted effects on the spinal cord after SCI, and the role of BDNF-induced plasticity in spinal learning circuits (Garraway, 2023, Garraway and Huie, 2016). In the current review, we examine the role DRGs play in inflammatory and neuropathic pain states by focusing on the changes in nociceptor cell soma, terminals, and adjacent non-neuronal cells, such as microglia or skin cells (see Fig. 3 for summary of the peripheral processes potentially involved in injury-induced pain). We also discuss peripherally expressed BDNF and TrkB as an example of many potential contributors to induction and persistence of pain after SCI.
Fig. 3.
Summary of various components in the periphery implicated for dysregulation of nociceptive circuit after SCI with BDNF-TrkB system as an example. A) Keratinocytes release growth factors (including BDNF) and cytokines to recruit macrophages and neutrophils, which further amplify inflammatory response by secreting more pro-inflammatory cytokines and chemokines (e.g., IL-1β, TNF-α). TrkB receptors are expressed on non-nociceptor sensory neurons (e.g., Aδ-LTMRs). During pathological conditions, BDNF derived from immune, epithelial, and Schwann cell can presumably interact with peripherally situated TrkB receptors to functionally alter the nociceptive circuit. B) BDNF acting through TrkB may participate in nociceptor hyperactivity by subsequent activation of downstream signaling cascades, such as PI3K and MAPK (p38). Studies implicate p38-dependent PKA signaling that stimulates T-type calcium Cav3.2 to regulate T-currents that may contribute to nociceptor hyperfunction. Certain subtype of VGSCs (TTX-R Nav 1.9) have been observed to underlie BDNF-TrkB-evoked excitation. Interaction between TrkB and VGSCs has not been clarified, but it may alter influx of sodium to change nociceptor excitability. DRGs also express TRPV1, which is sensitized by cytokines such as TNF-α. Proliferating SGCs surrounding DRGs release cytokines to further activate immune cells and trigger release of microglial BDNF. Sympathetic neurons sprout into the DRGs to form Dogiel’s arborization, which have been observed in spontaneously firing DRG neurons. Complex interactions between these components lead to changes in nociceptor threshold and behavior, leading to hyperexcitability. C) Synaptic interactions between primary afferent terminals and dorsal horn neurons lead to central sensitization. Primary afferent terminals release neurotransmitters and modulators (e.g., glutamate and BDNF) that activate respective receptors on SCDH neurons. Sensitized C-fibers release glutamate and BDNF. BDNF binds to TrkB receptors, which engage downstream intracellular signaling cascades including PLC, PKC, and Fyn to increase intracellular Ca2+. Consequently, increased Ca2+ increases phosphorylation of GluN2B subunit of NMDAR to facilitate glutamatergic currents. Released glutamate activates NMDA/AMPA receptors to activate post-synaptic interneurons.
The present review brings together many studies and reviews on mechanisms of pain, SCI, neurotrophins, and nociceptors, and their interactions, while highlighting the contribution of the DRGs underlying the individual components. Whereas significant progress has been made in our understanding of nociceptive processing in the SCDH, the initial CNS processing site, there remains a need for continued research emphasizing the critical roles peripheral plasticity and DRGs play in nociception.
CRediT authorship contribution statement
Kyeongran Jang: Investigation, Writing – original draft, Writing – review & editing. Sandra M. Garraway: .
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors thank Shangrila Parvin (Emory University) for critically reading and providing input on this review. Work presented in this article was supported in part by funds from Craig H. Neilsen Foundation grant #351718 and NINDS grants #R01NS102850 and #1R21NS116665 to SMG. Additional financial support was provided by Emory University Discretionary Funds.
Contributor Information
Kyeongran Jang, Email: kyeong.ran.jang@emory.edu.
Sandra M. Garraway, Email: smgarraway@emory.edu.
Data availability
No data was used for the research described in the article.
References
- Abidin I., Eysel U.T., Lessmann V., Mittmann T. Impaired GABAergic inhibition in the visual cortex of brain-derived neurotrophic factor heterozygous knockout mice. J. Physiol. 2008;586(7):1885–1901. doi: 10.1113/jphysiol.2007.148627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrahamsen B., Zhao J., Asante C.O., Cendan C.M., Marsh S., Martinez-Barbera J.P., Nassar M.A., Dickenson A.H., Wood J.N. The cell and molecular basis of mechanical, cold, and inflammatory pain. Science. 2008;321(5889):702–705. doi: 10.1126/science.1156916. [DOI] [PubMed] [Google Scholar]
- Abrahamsson T., Chou C.Y.C., Li S.Y., Mancino A., Costa R.P., Brock J.A., Nuro E., Buchanan K.A., Elgar D., Blackman A.V., Tudor-Jones A., Oyrer J., Farmer W.T., Murai K.K., Sjöström P.J. Differential regulation of evoked and spontaneous release by presynaptic NMDA receptors. Neuron. 2017;96(4):839–855.e835. doi: 10.1016/j.neuron.2017.09.030. [DOI] [PubMed] [Google Scholar]
- Abraira V.E., Ginty D.D. The Sensory Neurons of Touch. Neuron. 2013;79(4):618–639. doi: 10.1016/j.neuron.2013.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ackery A.D., Norenberg M.D., Krassioukov A. Calcitonin gene-related peptide immunoreactivity in chronic human spinal cord injury. Spinal Cord. 2007;45(10):678–686. doi: 10.1038/sj.sc.3102020. [DOI] [PubMed] [Google Scholar]
- Akopian A.N., Sivilotti L., Wood J.N. A tetrodotoxin-resistant voltage-gated sodium channel expressed by sensory neurons. Nature. 1996;379(6562):257–262. doi: 10.1038/379257a0. [DOI] [PubMed] [Google Scholar]
- Akopian A.N., Souslova V., England S., Okuse K., Ogata N., Ure J., Smith A., Kerr B.J., McMahon S.B., Boyce S., Hill R., Stanfa L.C., Dickenson A.H., Wood J.N. The tetrodotoxin-resistant sodium channel SNS has a specialized function in pain pathways. Nat. Neurosci. 1999;2(6):541–548. doi: 10.1038/9195. [DOI] [PubMed] [Google Scholar]
- Alexandrou A.J., Brown A.R., Chapman M.L., Estacion M., Turner J., Mis M.A., Wilbrey A., Payne E.C., Gutteridge A., Cox P.J., Doyle R., Printzenhoff D., Lin Z., Marron B.E., West C., Swain N.A., Storer R.I., Stupple P.A., Castle N.A., Stevens E.B. Subtype-selective small molecule inhibitors reveal a fundamental role for nav1.7 in nociceptor electrogenesis, axonal conduction and presynaptic release. PLoS One. 2016;11(4):e0152405. doi: 10.1371/journal.pone.0152405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alles S.R.A., Odem M.A., Lu V.B., Cassidy R.M., Smith P.A. Chronic BDNF simultaneously inhibits and unmasks superficial dorsal horn neuronal activity. Sci. Rep. 2021;11(1):2249. doi: 10.1038/s41598-021-81269-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaya F., Decosterd I., Samad T.A., Plumpton C., Tate S., Mannion R.J., Costigan M., Woolf C.J. Diversity of expression of the sensory neuron-specific TTX-resistant voltage-gated sodium ion channels SNS and SNS2. Mol. Cell. Neurosci. 2000;15(4):331–342. doi: 10.1006/mcne.1999.0828. [DOI] [PubMed] [Google Scholar]
- Apfel S.C., Wright D.E., Wiideman A.M., Dormia C., Snider W.D., Kessler J.A. Nerve growth factor regulates the expression of brain-derived neurotrophic factor mRNA in the peripheral nervous system. Mol. Cell. Neurosci. 1996;7(2):134–142. doi: 10.1006/mcne.1996.0010. [DOI] [PubMed] [Google Scholar]
- Artola A., Voisin D., Dallel R. PKCγ interneurons, a gateway to pathological pain in the dorsal horn. J. Neural Transm. 2020;127(4):527–540. doi: 10.1007/s00702-020-02162-6. [DOI] [PubMed] [Google Scholar]
- Baastrup C., Finnerup N.B. Pharmacological management of neuropathic pain following spinal cord injury. CNS Drugs. 2008;22(6):455–475. doi: 10.2165/00023210-200822060-00002. [DOI] [PubMed] [Google Scholar]
- Bae Y.C., Ihn H.J., Park M.J., Ottersen O.P., Moritani M., Yoshida A., Shigenaga Y. Identification of signal substances in synapses made between primary afferents and their associated axon terminals in the rat trigeminal sensory nuclei. J Comp Neurol. 2000;418(3):299–309. https://www.ncbi.nlm.nih.gov/pubmed/10701828 [PubMed] [Google Scholar]
- Bae J.Y., Kim J.H., Cho Y.S., Mah W., Bae Y.C. Quantitative analysis of afferents expressing substance P, calcitonin gene-related peptide, isolectin B4, neurofilament 200, and Peripherin in the sensory root of the rat trigeminal ganglion. J. Comp. Neurol. 2015;523(1):126–138. doi: 10.1002/cne.23672. [DOI] [PubMed] [Google Scholar]
- Barakat-Walter I. Brain-derived neurotrophic factor-like immunoreactivity is localized mainly in small sensory neurons of rat dorsal root ganglia. J. Neurosci. Methods. 1996;68(2):281–288. doi: 10.1016/0165-0270(96)00093-3. [DOI] [PubMed] [Google Scholar]
- Barasi S., Lynn B. Effects of sympathetic stimulation on mechanoreceptive and nociceptive afferent units from the rabbit pinna. Brain Res. 1986;378(1):21–27. doi: 10.1016/0006-8993(86)90282-9. [DOI] [PubMed] [Google Scholar]
- Barde Y.A., Edgar D., Thoenen H. Purification of a new neurotrophic factor from mammalian brain. EMBO J. 1982;1(5):549–553. doi: 10.1002/j.1460-2075.1982.tb01207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardoni R., Torsney C., Tong C.K., Prandini M., MacDermott A.B. Presynaptic NMDA receptors modulate glutamate release from primary sensory neurons in rat spinal cord dorsal horn. J. Neurosci. 2004;24(11):2774–2781. doi: 10.1523/jneurosci.4637-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baroncini A., Maffulli N., Eschweiler J., Tingart M., Migliorini F. Pharmacological management of secondary spinal cord injury. Expert Opin. Pharmacother. 2021;22(13):1793–1800. doi: 10.1080/14656566.2021.1918674. [DOI] [PubMed] [Google Scholar]
- Barron K.D., Marciano F.F., Amundson R., Mankes R. Perineuronal glial responses after axotomy of central and peripheral axons. A Comparison. Brain Res. 1990;523(2):219–229. doi: 10.1016/0006-8993(90)91490-8. [DOI] [PubMed] [Google Scholar]
- Basbaum A.I., Bautista D.M., Scherrer G., Julius D. Cellular and molecular mechanisms of pain. Cell. 2009;139(2):267–284. doi: 10.1016/j.cell.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedi S.S., Yang Q., Crook R.J., Du J., Wu Z., Fishman H.M., Grill R.J., Carlton S.M., Walters E.T. Chronic spontaneous activity generated in the somata of primary nociceptors is associated with pain-related behavior after spinal cord injury. J. Neurosci. 2010;30(44):14870–14882. doi: 10.1523/JNEUROSCI.2428-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedi S.S., Lago M.T., Masha L.I., Crook R.J., Grill R.J., Walters E.T. Spinal cord injury triggers an intrinsic growth-promoting state in nociceptors. J. Neurotrauma. 2012;29(5):925–935. doi: 10.1089/neu.2011.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett D.L., Clark A.J., Huang J., Waxman S.G., Dib-Hajj S.D. The Role of Voltage-Gated Sodium Channels in Pain Signaling. Physiol. Rev. 2019;99(2):1079–1151. doi: 10.1152/physrev.00052.2017. [DOI] [PubMed] [Google Scholar]
- Berta T., Poirot O., Pertin M., Ji R.R., Kellenberger S., Decosterd I. Transcriptional and functional profiles of voltage-gated Na(+) channels in injured and non-injured DRG neurons in the SNI model of neuropathic pain. Mol. Cell. Neurosci. 2008;37(2):196–208. doi: 10.1016/j.mcn.2007.09.007. [DOI] [PubMed] [Google Scholar]
- Bessou P., Burgess P.R., Perl E.R., Taylor C.B. Dynamic properties of mechanoreceptors with unmyelinated (C) fibers. J. Neurophysiol. 1971;34(1):116–131. doi: 10.1152/jn.1971.34.1.116. [DOI] [PubMed] [Google Scholar]
- Beuche W., Friede R.L. The role of non-resident cells in Wallerian degeneration. J. Neurocytol. 1984;13(5):767–796. doi: 10.1007/BF01148493. [DOI] [PubMed] [Google Scholar]
- Bhuiyan, S. A., Xu, M., Yang, L., Semizoglou, E., Bhatia, P., Pantaleo, K. I., Tochitsky, I., Jain, A., Erdogan, B., Blair, S., Cat, V., Mwirigi, J. M., Sankaranarayanan, I., Tavares-Ferreira, D., Green, U., McIlvried, L. A., Copits, B. A., Bertels, Z., Del Rosario, J. S., . . . Renthal, W. (2023). Harmonized cross-species cell atlases of trigeminal and dorsal root ganglia. bioRxiv. https://doi.org/10.1101/2023.07.04.547740.
- Biggs J.E., Lu V.B., Stebbing M.J., Balasubramanyan S., Smith P.A. Is BDNF sufficient for information transfer between microglia and dorsal horn neurons during the onset of central sensitization? Mol. Pain. 2010;6:44. doi: 10.1186/1744-8069-6-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binshtok A.M., Wang H., Zimmermann K., Amaya F., Vardeh D., Shi L., Brenner G.J., Ji R.R., Bean B.P., Woolf C.J., Samad T.A. Nociceptors are interleukin-1beta sensors. J. Neurosci. 2008;28(52):14062–14073. doi: 10.1523/JNEUROSCI.3795-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop T., Marchand F., Young A.R., Lewin G.R., McMahon S.B. Ultraviolet-B-induced mechanical hyperalgesia: a role for peripheral sensitisation. Pain. 2010;150(1):141–152. doi: 10.1016/j.pain.2010.04.018. [DOI] [PubMed] [Google Scholar]
- Black J.A., Dib-Hajj S., McNabola K., Jeste S., Rizzo M.A., Kocsis J.D., Waxman S.G. Spinal sensory neurons express multiple sodium channel alpha-subunit mRNAs. Brain Res. Mol. Brain Res. 1996;43(1–2):117–131. doi: 10.1016/s0169-328x(96)00163-5. [DOI] [PubMed] [Google Scholar]
- Black J.A., Cummins T.R., Plumpton C., Chen Y.H., Hormuzdiar W., Clare J.J., Waxman S.G. Upregulation of a silent sodium channel after peripheral, but not central, nerve injury in DRG neurons. J. Neurophysiol. 1999;82(5):2776–2785. doi: 10.1152/jn.1999.82.5.2776. [DOI] [PubMed] [Google Scholar]
- Black J.A., Liu S., Tanaka M., Cummins T.R., Waxman S.G. Changes in the expression of tetrodotoxin-sensitive sodium channels within dorsal root ganglia neurons in inflammatory pain. Pain. 2004;108(3):237–247. doi: 10.1016/j.pain.2003.12.035. [DOI] [PubMed] [Google Scholar]
- Blair N.T., Bean B.P. Roles of tetrodotoxin (TTX)-sensitive Na+ current, TTX-resistant Na+ current, and Ca2+ current in the action potentials of nociceptive sensory neurons. J. Neurosci. 2002;22(23):10277–10290. doi: 10.1523/JNEUROSCI.22-23-10277.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard J.W., Eade K.T., Szűcs A., Lo Sardo V., Tsunemoto R.K., Williams D., Sanna P.P., Baldwin K.K. Selective conversion of fibroblasts into peripheral sensory neurons. Nat. Neurosci. 2015;18(1):25–35. doi: 10.1038/nn.3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum R., Kafitz K.W., Konnerth A. Neurotrophin-evoked depolarization requires the sodium channel Na(V)1.9. Nature. 2002;419(6908):687–693. doi: 10.1038/nature01085. [DOI] [PubMed] [Google Scholar]
- Bogen O., Dreger M., Gillen C., Schroder W., Hucho F. Identification of versican as an isolectin B4-binding glycoprotein from mammalian spinal cord tissue. FEBS J. 2005;272(5):1090–1102. doi: 10.1111/j.1742-4658.2005.04543.x. [DOI] [PubMed] [Google Scholar]
- Bonhoeffer T. Neurotrophins and activity-dependent development of the neocortex. Curr. Opin. Neurobiol. 1996;6(1):119–126. doi: 10.1016/s0959-4388(96)80017-1. [DOI] [PubMed] [Google Scholar]
- Bossu J.L., Feltz A. Patch-clamp study of the tetrodotoxin-resistant sodium current in group C sensory neurones. Neurosci. Lett. 1984;51(2):241–246. doi: 10.1016/0304-3940(84)90558-5. [DOI] [PubMed] [Google Scholar]
- Boucher T.J., Okuse K., Bennett D.L.H., Munson J.B., Wood J.N., McMahon S.B. Potent Analgesic Effects of GDNF in Neuropathic Pain States. Science. 2000;290(5489):124–127. doi: 10.1126/science.290.5489.124. [DOI] [PubMed] [Google Scholar]
- Bracken M.B., Shepard M.J., Holford T.R., Leo-Summers L., Aldrich E.F., Fazl M., Fehlings M., Herr D.L., Hitchon P.W., Marshall L.F., Nockels R.P., Pascale V., Perot P.L., Jr., Piepmeier J., Sonntag V.K., Wagner F., Wilberger J.E., Winn H.R., Young W. Administration of methylprednisolone for 24 or 48 hours or tirilazad mesylate for 48 hours in the treatment of acute spinal cord injury. Results of the Third National Acute Spinal Cord Injury Randomized Controlled Trial. National Acute Spinal Cord Injury Study. J. Am. Med. Assoc. 1997;277(20):1597–1604. https://www.ncbi.nlm.nih.gov/pubmed/9168289 [PubMed] [Google Scholar]
- Bredt D.S., Nicoll R.A. AMPA receptor trafficking at excitatory synapses. Neuron. 2003;40(2):361–379. doi: 10.1016/s0896-6273(03)00640-8. [DOI] [PubMed] [Google Scholar]
- Brigadski T., Leßmann V. The physiology of regulated BDNF release. Cell Tissue Res. 2020;382(1):15–45. doi: 10.1007/s00441-020-03253-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M.C., Perry V.H., Lunn E.R., Gordon S., Heumann R. Macrophage dependence of peripheral sensory nerve regeneration: possible involvement of nerve growth factor. Neuron. 1991;6(3):359–370. doi: 10.1016/0896-6273(91)90245-u. [DOI] [PubMed] [Google Scholar]
- Brown A., Weaver L.C. The dark side of neuroplasticity. Exp. Neurol. 2012;235(1):133–141. doi: 10.1016/j.expneurol.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton A.W., Lee D.H., Saab C., Chung J.M. Preemptive intrathecal ketamine injection produces a long-lasting decrease in neuropathic pain behaviors in a rat model. Reg. Anesth. Pain Med. 1999;24(3):208–213. doi: 10.1016/s1098-7339(99)90129-3. [DOI] [PubMed] [Google Scholar]
- Cappoli N., Tabolacci E., Aceto P., Dello Russo C. The emerging role of the BDNF-TrkB signaling pathway in the modulation of pain perception. J. Neuroimmunol. 2020;349 doi: 10.1016/j.jneuroim.2020.577406. [DOI] [PubMed] [Google Scholar]
- Carlton S.M., Hargett G.L., Coggeshall R.E. Localization and activation of glutamate receptors in unmyelinated axons of rat glabrous skin. Neurosci. Lett. 1995;197(1):25–28. doi: 10.1016/0304-3940(95)11889-5. [DOI] [PubMed] [Google Scholar]
- Carlton S.M., Hargett G.L., Coggeshall R.E. Localization of metabotropic glutamate receptors 2/3 on primary afferent axons in the rat. Neuroscience. 2001;105(4):957–969. doi: 10.1016/s0306-4522(01)00238-x. [DOI] [PubMed] [Google Scholar]
- Carlton S.M., Du J., Tan H.Y., Nesic O., Hargett G.L., Bopp A.C., Yamani A., Lin Q., Willis W.D., Hulsebosch C.E. Peripheral and central sensitization in remote spinal cord regions contribute to central neuropathic pain after spinal cord injury. Pain. 2009;147(1–3):265–276. doi: 10.1016/j.pain.2009.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlton S.M., Hargett G.L. Colocalization of metabotropic glutamate receptors in rat dorsal root ganglion cells. J Comp Neurol. 2007;501(5):780–789. doi: 10.1002/cne.21285. [DOI] [PubMed] [Google Scholar]
- Carmignoto G., Fellin T. Glutamate release from astrocytes as a non-synaptic mechanism for neuronal synchronization in the hippocampus. Journal of Physiology-Paris. 2006;99(2):98–102. doi: 10.1016/j.jphysparis.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Carroll P., Lewin G.R., Koltzenburg M., Toyka K.V., Thoenen H. A role for BDNF in mechanosensation. Nat. Neurosci. 1998;1(1):42–46. doi: 10.1038/242. [DOI] [PubMed] [Google Scholar]
- Carroll R.C., Lissin D.V., von Zastrow M., Nicoll R.A., Malenka R.C. Rapid redistribution of glutamate receptors contributes to long-term depression in hippocampal cultures. Nat. Neurosci. 1999;2(5):454–460. doi: 10.1038/8123. [DOI] [PubMed] [Google Scholar]
- Caterina M.J., Schumacher M.A., Tominaga M., Rosen T.A., Levine J.D., Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389(6653):816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- Caterina M.J., Rosen T.A., Tominaga M., Brake A.J., Julius D. A capsaicin-receptor homologue with a high threshold for noxious heat. Nature. 1999;398(6726):436–441. doi: 10.1038/18906. [DOI] [PubMed] [Google Scholar]
- Catterall W.A. Cellular and molecular biology of voltage-gated sodium channels. Physiol. Rev. 1992;72(4 Suppl):S15–S48. doi: 10.1152/physrev.1992.72.suppl_4.S15. [DOI] [PubMed] [Google Scholar]
- Cefis M., Quirié A., Pernet N., Marie C., Garnier P., Prigent-Tessier A. Brain-derived neurotrophic factor is a full endothelium-derived factor in rats. Vasc.Pharmacol. 2020;128–129 doi: 10.1016/j.vph.2020.106674. [DOI] [PubMed] [Google Scholar]
- Chambers S.M., Qi Y., Mica Y., Lee G., Zhang X.J., Niu L., Bilsland J., Cao L., Stevens E., Whiting P., Shi S.H., Studer L. Combined small-molecule inhibition accelerates developmental timing and converts human pluripotent stem cells into nociceptors. Nat. Biotechnol. 2012;30(7):715–720. doi: 10.1038/nbt.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang W., Berta T., Kim Y.H., Lee S., Lee S.Y., Ji R.R. Expression and Role of Voltage-Gated Sodium Channels in Human Dorsal Root Ganglion Neurons with Special Focus on Nav1.7, Species Differences, and Regulation by Paclitaxel. Neurosci. Bull. 2018;34(1):4–12. doi: 10.1007/s12264-017-0132-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S.R., Hu Y.M., Chen H., Pan H.L. Calcineurin inhibitor induces pain hypersensitivity by potentiating pre- and postsynaptic NMDA receptor activity in spinal cords. J. Physiol. 2014;592(1):215–227. doi: 10.1113/jphysiol.2013.263814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W., Walwyn W., Ennes H.S., Kim H., McRoberts J.A., Marvizon J.C. BDNF released during neuropathic pain potentiates NMDA receptors in primary afferent terminals. Eur. J. Neurosci. 2014;39(9):1439–1454. doi: 10.1111/ejn.12516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien S.Q., Li C., Li H., Xie W., Pablo C.S., Zhang J.M. Sympathetic Fiber Sprouting in Chronically Compressed Dorsal Root Ganglia Without Peripheral Axotomy. J Neuropathic Pain Symptom Palliation. 2005;1(1):19–23. doi: 10.1300/J426v01n01_05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu I.M., von Hehn C.A., Woolf C.J. Neurogenic inflammation and the peripheral nervous system in host defense and immunopathology. Nat. Neurosci. 2012;15(8):1063–1067. doi: 10.1038/nn.3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho H.J., Kim J.K., Zhou X.F., Rush R.A. Increased brain-derived neurotrophic factor immunoreactivity in rat dorsal root ganglia and spinal cord following peripheral inflammation. Brain Res. 1997;764(1–2):269–272. doi: 10.1016/s0006-8993(97)00597-0. [DOI] [PubMed] [Google Scholar]
- Cho H.J., Kim J.K., Park H.C., Kim J.K., Kim D.S., Ha S.O., Hong H.S. Changes in brain-derived neurotrophic factor immunoreactivity in rat dorsal root ganglia, spinal cord, and gracile nuclei following cut or crush injuries. Exp. Neurol. 1998;154(1):224–230. doi: 10.1006/exnr.1998.6936. [DOI] [PubMed] [Google Scholar]
- Choi J.I., Svensson C.I., Koehrn F.J., Bhuskute A., Sorkin L.S. Peripheral inflammation induces tumor necrosis factor dependent AMPA receptor trafficking and Akt phosphorylation in spinal cord in addition to pain behavior. Pain. 2010;149(2):243–253. doi: 10.1016/j.pain.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen M.D., Hulsebosch C.E. Spinal cord injury and anti-NGF treatment results in changes in CGRP density and distribution in the dorsal horn in the rat. Exp. Neurol. 1997;147(2):463–475. doi: 10.1006/exnr.1997.6608. [DOI] [PubMed] [Google Scholar]
- Christoph T., Reissmuller E., Schiene K., Englberger W., Chizh B.A. Antiallodynic effects of NMDA glycine(B) antagonists in neuropathic pain: possible peripheral mechanisms. Brain Res. 2005;1048(1–2):218–227. doi: 10.1016/j.brainres.2005.04.081. [DOI] [PubMed] [Google Scholar]
- Clark A.J., Menendez G., AlQatari M., Patel N., Arstad E., Schiavo G., Koltzenburg M. Functional imaging in microfluidic chambers reveals sensory neuron sensitivity is differentially regulated between neuronal regions. Pain. 2018;159(7):1413–1425. doi: 10.1097/j.pain.0000000000001145. [DOI] [PubMed] [Google Scholar]
- Coderre T.J., Melzack R. The role of NMDA receptor-operated calcium channels in persistent nociception after formalin-induced tissue injury. J. Neurosci. 1992;12(9):3671–3675. doi: 10.1523/JNEUROSCI.12-09-03671.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coggeshall R.E., Carlton S.M. Ultrastructural analysis of NMDA, AMPA, and kainate receptors on unmyelinated and myelinated axons in the periphery. J Comp Neurol. 1998;391(1):78–86. doi: 10.1002/(sici)1096-9861(19980202)391:1<78::aid-cne7>3.3.co;2-8. [DOI] [PubMed] [Google Scholar]
- Coggeshall R.E., Tate S., Carlton S.M. Differential expression of tetrodotoxin-resistant sodium channels Nav1.8 and Nav1.9 in normal and inflamed rats. Neurosci. Lett. 2004;355(1–2):45–48. doi: 10.1016/j.neulet.2003.10.023. [DOI] [PubMed] [Google Scholar]
- Conn, P.J., Pin, J.P., 1997. Pharmacology and functions of metabotropic glutamate receptors. Annu. Rev. Pharmacol. Toxicol. 37, 205–237. doi:10.1146/annurev.pharmtox.37.1.205. Copyright ©, 2009. [DOI] [PubMed]
- Costigan M., Befort K., Karchewski L., Griffin R.S., D'Urso D., Allchorne A., Sitarski J., Mannion J.W., Pratt R.E., Woolf C.J. Replicate high-density rat genome oligonucleotide microarrays reveal hundreds of regulated genes in the dorsal root ganglion after peripheral nerve injury. BMC Neurosci. 2002;3(1):16. doi: 10.1186/1471-2202-3-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costigan M., Scholz J., Woolf C.J. Neuropathic pain: a maladaptive response of the nervous system to damage. Annu. Rev. Neurosci. 2009;32:1–32. doi: 10.1146/annurev.neuro.051508.135531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coward K., Plumpton C., Facer P., Birch R., Carlstedt T., Tate S., Bountra C., Anand P. Immunolocalization of SNS/PN3 and NaN/SNS2 sodium channels in human pain states. Pain. 2000;85(1):41–50. doi: 10.1016/s0304-3959(99)00251-1. [DOI] [PubMed] [Google Scholar]
- Crawford L.K., Caterina M.J. Functional Anatomy of the Sensory Nervous System: Updates From the Neuroscience Bench. Toxicol. Pathol. 2020;48(1):174–189. doi: 10.1177/0192623319869011. [DOI] [PubMed] [Google Scholar]
- Crown E.D., Ye Z., Johnson K.M., Xu G.-Y., McAdoo D.J., Hulsebosch C.E. Increases in the activated forms of ERK 1/2, p38 MAPK, and CREB are correlated with the expression of at-level mechanical allodynia following spinal cord injury. Exp. Neurol. 2006;199(2):397–407. doi: 10.1016/j.expneurol.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Cuevas-Diaz Duran R., Li Y., Garza Carbajal A., You Y., Dessauer C.W., Wu J., Walters E.T. Major Differences in Transcriptional Alterations in Dorsal Root Ganglia Between Spinal Cord Injury and Peripheral Neuropathic Pain Models. J. Neurotrauma. 2023;40(9–10):883–900. doi: 10.1089/neu.2022.0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummins T.R., Howe J.R., Waxman S.G. Slow closed-state inactivation: a novel mechanism underlying ramp currents in cells expressing the hNE/PN1 sodium channel. J. Neurosci. 1998;18(23):9607–9619. doi: 10.1523/JNEUROSCI.18-23-09607.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummins T.R., Waxman S.G. Downregulation of tetrodotoxin-resistant sodium currents and upregulation of a rapidly repriming tetrodotoxin-sensitive sodium current in small spinal sensory neurons after nerve injury. J. Neurosci. 1997;17(10):3503–3514. doi: 10.1523/JNEUROSCI.17-10-03503.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummins T.R., Dib-Hajj S.D., Black J.A., Akopian A.N., Wood J.N., Waxman S.G. A novel persistent tetrodotoxin-resistant sodium current in SNS-null and wild-type small primary sensory neurons. J. Neurosci. 1999;19(24):Rc43. doi: 10.1523/JNEUROSCI.19-24-j0001.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha T.M., Verri W.A., Jr., Fukada S.Y., Guerrero A.T., Santodomingo-Garzon T., Poole S., Parada C.A., Ferreira S.H., Cunha F.Q. TNF-alpha and IL-1beta mediate inflammatory hypernociception in mice triggered by B1 but not B2 kinin receptor. Eur. J. Pharmacol. 2007;573(1–3):221–229. doi: 10.1016/j.ejphar.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Dai Y., Iwata K., Fukuoka T., Kondo E., Tokunaga A., Yamanaka H., Tachibana T., Liu Y., Noguchi K. Phosphorylation of extracellular signal-regulated kinase in primary afferent neurons by noxious stimuli and its involvement in peripheral sensitization. J. Neurosci. 2002;22(17):7737–7745. doi: 10.1523/jneurosci.22-17-07737.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Arcangelo G., Paradiso K., Shepherd D., Brehm P., Halegoua S., Mandel G. Neuronal growth factor regulation of two different sodium channel types through distinct signal transduction pathways. J. Cell Biol. 1993;122(4):915–921. doi: 10.1083/jcb.122.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson S., Copits B.A., Zhang J., Page G., Ghetti A., Gereau R.W., t. Human sensory neurons: Membrane properties and sensitization by inflammatory mediators. Pain. 2014;155(9):1861–1870. doi: 10.1016/j.pain.2014.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decosterd I., Ji R.R., Abdi S., Tate S., Woolf C.J. The pattern of expression of the voltage-gated sodium channels Na(v)1.8 and Na(v)1.9 does not change in uninjured primary sensory neurons in experimental neuropathic pain models. Pain. 2002;96(3):269–277. doi: 10.1016/s0304-3959(01)00456-0. [DOI] [PubMed] [Google Scholar]
- deGroot J., Zhou S., Carlton S.M. Peripheral glutamate release in the hindpaw following low and high intensity sciatic stimulation. Neuroreport. 2000;11(3):497–502. doi: 10.1097/00001756-200002280-00014. [DOI] [PubMed] [Google Scholar]
- Dembo T., Braz J.M., Hamel K.A., Kuhn J.A., Basbaum A.I. Primary Afferent-Derived BDNF Contributes Minimally to the Processing of Pain and Itch. eNeuro. 2018;5(6) doi: 10.1523/ENEURO.0402-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detloff M.R., Smith E.J., Quiros Molina D., Ganzer P.D., Houlé J.D. Acute exercise prevents the development of neuropathic pain and the sprouting of non-peptidergic (GDNF- and artemin-responsive) c-fibers after spinal cord injury. Exp. Neurol. 2014;255:38–48. doi: 10.1016/j.expneurol.2014.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devor M. Sodium channels and mechanisms of neuropathic pain. J. Pain. 2006;7(1 Suppl 1):S3–S12. doi: 10.1016/j.jpain.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Dib-Hajj S.D., Tyrrell L., Black J.A., Waxman S.G. NaN, a novel voltage-gated Na channel, is expressed preferentially in peripheral sensory neurons and down-regulated after axotomy. PNAS. 1998;95(15):8963–8968. doi: 10.1073/pnas.95.15.8963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dib-Hajj S.D., Fjell J., Cummins T.R., Zheng Z., Fried K., LaMotte R., Black J.A., Waxman S.G. Plasticity of sodium channel expression in DRG neurons in the chronic constriction injury model of neuropathic pain. Pain. 1999;83(3):591–600. doi: 10.1016/s0304-3959(99)00169-4. [DOI] [PubMed] [Google Scholar]
- Dib-Hajj S.D., Rush A.M., Cummins T.R., Hisama F.M., Novella S., Tyrrell L., Marshall L., Waxman S.G. Gain-of-function mutation in Nav1.7 in familial erythromelalgia induces bursting of sensory neurons. Brain. 2005;128(Pt 8):1847–1854. doi: 10.1093/brain/awh514. [DOI] [PubMed] [Google Scholar]
- Dib-Hajj S.D., Yang Y., Black J.A., Waxman S.G. The Na(V)1.7 sodium channel: from molecule to man. Nat. Rev. Neurosci. 2013;14(1):49–62. doi: 10.1038/nrn3404. [DOI] [PubMed] [Google Scholar]
- Dib-Hajj S.D., Black J.A., Waxman S.G. NaV1.9: a sodium channel linked to human pain. Nat. Rev. Neurosci. 2015;16(9):511–519. doi: 10.1038/nrn3977. [DOI] [PubMed] [Google Scholar]
- Dickenson A.H., Sullivan A.F. Evidence for a role of the NMDA receptor in the frequency dependent potentiation of deep rat dorsal horn nociceptive neurones following C fibre stimulation. Neuropharmacology. 1987;26(8):1235–1238. doi: 10.1016/0028-3908(87)90275-9. [DOI] [PubMed] [Google Scholar]
- Djouhri L., Lawson S.N. Abeta-fiber nociceptive primary afferent neurons: a review of incidence and properties in relation to other afferent A-fiber neurons in mammals. Brain Res. Brain Res. Rev. 2004;46(2):131–145. doi: 10.1016/j.brainresrev.2004.07.015. [DOI] [PubMed] [Google Scholar]
- Djouhri L., Fang X., Okuse K., Wood J.N., Berry C.M., Lawson S.N. The TTX-resistant sodium channel Nav1.8 (SNS/PN3): expression and correlation with membrane properties in rat nociceptive primary afferent neurons. J. Physiol. 2003;550(Pt 3):739–752. doi: 10.1113/jphysiol.2003.042127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong X.W., Goregoaker S., Engler H., Zhou X., Mark L., Crona J., Terry R., Hunter J., Priestley T. Small interfering RNA-mediated selective knockdown of Na(V)1.8 tetrodotoxin-resistant sodium channel reverses mechanical allodynia in neuropathic rats. Neuroscience. 2007;146(2):812–821. doi: 10.1016/j.neuroscience.2007.01.054. [DOI] [PubMed] [Google Scholar]
- Dore K., Stein I.S., Brock J.A., Castillo P.E., Zito K., Sjöström P.J. Unconventional NMDA Receptor Signaling. J. Neurosci. 2017;37(45):10800–10807. doi: 10.1523/jneurosci.1825-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowdall T., Robinson I., Meert T.F. Comparison of five different rat models of peripheral nerve injury. Pharmacol. Biochem. Behav. 2005;80(1):93–108. doi: 10.1016/j.pbb.2004.10.016. [DOI] [PubMed] [Google Scholar]
- Du X., Hao H., Yang Y., Huang S., Wang C., Gigout S., Ramli R., Li X., Jaworska E., Edwards I., Deuchars J., Yanagawa Y., Qi J., Guan B., Jaffe D.B., Zhang H., Gamper N. Local GABAergic signaling within sensory ganglia controls peripheral nociceptive transmission. J. Clin. Invest. 2017;127(5):1741–1756. doi: 10.1172/JCI86812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J., Zhou S., Coggeshall R.E., Carlton S.M. N-methyl-D-aspartate-induced excitation and sensitization of normal and inflamed nociceptors. Neuroscience. 2003;118(2):547–562. doi: 10.1016/s0306-4522(03)00009-5. [DOI] [PubMed] [Google Scholar]
- Du J., Zhou S., Carlton S.M. Kainate-induced excitation and sensitization of nociceptors in normal and inflamed rat glabrous skin. Neuroscience. 2006;137(3):999–1013. doi: 10.1016/j.neuroscience.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Duan G., Han C., Wang Q., Guo S., Zhang Y., Ying Y., Huang P., Zhang L., Macala L., Shah P., Zhang M., Li N., Dib-Hajj S.D., Waxman S.G., Zhang X. A SCN10A SNP biases human pain sensitivity. Mol. Pain. 2016;12 doi: 10.1177/1744806916666083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubin A.E., Patapoutian A. Nociceptors: the sensors of the pain pathway. J. Clin. Invest. 2010;120(11):3760–3772. doi: 10.1172/JCI42843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert A., Segond von Banchet G., Sopper S., Petersen M. Spatio-temporal pattern of induction of bradykinin receptors and inflammation in rat dorsal root ganglia after unilateral nerve ligation. Pain. 1999;83(3):487–497. doi: 10.1016/S0304-3959(99)00152-9. [DOI] [PubMed] [Google Scholar]
- Edvinsson L., Edvinsson J.C.A., Haanes K.A. Identifying molecular targets in trigeminal nociception. Nat. Rev. Neurol. 2022;18(7):385–386. doi: 10.1038/s41582-022-00671-4. [DOI] [PubMed] [Google Scholar]
- Eller O.C., Stair R.N., Neal C., Rowe P.S.N., Nelson-Brantley J., Young E.E., Baumbauer K.M. Comprehensive phenotyping of cutaneous afferents reveals early-onset alterations in nociceptor response properties, release of CGRP, and hindpaw edema following spinal cord injury. Neurobiol Pain. 2022;12 doi: 10.1016/j.ynpai.2022.100097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott A.A., Elliott J.R. Characterization of TTX-sensitive and TTX-resistant sodium currents in small cells from adult rat dorsal root ganglia. J. Physiol. 1993;463:39–56. doi: 10.1113/jphysiol.1993.sp019583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernfors P., Rosario C.M., Merlio J.P., Grant G., Aldskogius H., Persson H. Expression of mRNAs for neurotrophin receptors in the dorsal root ganglion and spinal cord during development and following peripheral or central axotomy. Brain Res. Mol. Brain Res. 1993;17(3–4):217–226. doi: 10.1016/0169-328x(93)90005-a. [DOI] [PubMed] [Google Scholar]
- Esposito M.F., Malayil R., Hanes M., Deer T. Unique Characteristics of the Dorsal Root Ganglion as a Target for Neuromodulation. Pain Med. 2019;20(Suppl 1):S23–S30. doi: 10.1093/pm/pnz012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber C.G., Lauria G., Merkies I.S., Cheng X., Han C., Ahn H.S., Persson A.K., Hoeijmakers J.G., Gerrits M.M., Pierro T., Lombardi R., Kapetis D., Dib-Hajj S.D., Waxman S.G. Gain-of-function Nav1.8 mutations in painful neuropathy. PNAS. 2012;109(47):19444–19449. doi: 10.1073/pnas.1216080109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson A.R., Crown E.D., Grau J.W. Nociceptive plasticity inhibits adaptive learning in the spinal cord. Neuroscience. 2006;141(1):421–431. doi: 10.1016/j.neuroscience.2006.03.029. [DOI] [PubMed] [Google Scholar]
- Ferguson A.R., Christensen R.N., Gensel J.C., Miller B.A., Sun F., Beattie E.C., Bresnahan J.C., Beattie M.S. Cell death after spinal cord injury is exacerbated by rapid TNF alpha-induced trafficking of GluR2-lacking AMPARs to the plasma membrane. J. Neurosci. 2008;28(44):11391–11400. doi: 10.1523/jneurosci.3708-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira S.H., Lorenzetti B.B. Glutamate spinal retrograde sensitization of primary sensory neurons associated with nociception. Neuropharmacology. 1994;33(11):1479–1485. doi: 10.1016/0028-3908(94)90052-3. [DOI] [PubMed] [Google Scholar]
- Ferrini F., De Koninck Y. Microglia control neuronal network excitability via BDNF signalling. Neural Plast. 2013;2013 doi: 10.1155/2013/429815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnerup N.B. Pain in patients with spinal cord injury. Pain. 2013;154(Suppl 1):S71–S76. doi: 10.1016/j.pain.2012.12.007. [DOI] [PubMed] [Google Scholar]
- Finnerup N.B., Johannesen I.L., Sindrup S.H., Bach F.W., Jensen T.S. Pain and dysesthesia in patients with spinal cord injury: A postal survey. Spinal Cord. 2001;39(5):256–262. doi: 10.1038/sj.sc.3101161. [DOI] [PubMed] [Google Scholar]
- Finnerup N.B., Johannesen I.L., Fuglsang-Frederiksen A., Bach F.W., Jensen T.S. Sensory function in spinal cord injury patients with and without central pain. Brain. 2003;126(Pt 1):57–70. doi: 10.1093/brain/awg007. [DOI] [PubMed] [Google Scholar]
- Foster E., Robertson B., Fried K. trkB-like immunoreactivity in rat dorsal root ganglia following sciatic nerve injury. Brain Res. 1994;659(1–2):267–271. doi: 10.1016/0006-8993(94)90891-5. [DOI] [PubMed] [Google Scholar]
- François A., Schüetter N., Laffray S., Sanguesa J., Pizzoccaro A., Dubel S., Mantilleri A., Nargeot J., Noël J., Wood J.N., Moqrich A., Pongs O., Bourinet E. The Low-Threshold Calcium Channel Cav3.2 Determines Low-Threshold Mechanoreceptor Function. Cell Rep. 2015;10(3):370–382. doi: 10.1016/j.celrep.2014.12.042. [DOI] [PubMed] [Google Scholar]
- Fukuoka T., Kobayashi K., Yamanaka H., Obata K., Dai Y., Noguchi K. Comparative study of the distribution of the alpha-subunits of voltage-gated sodium channels in normal and axotomized rat dorsal root ganglion neurons. J Comp Neurol. 2008;510(2):188–206. doi: 10.1002/cne.21786. [DOI] [PubMed] [Google Scholar]
- Gadient R.A., Otten U. Postnatal expression of interleukin-6 (IL-6) and IL-6 receptor (IL-6R) mRNAs in rat sympathetic and sensory ganglia. Brain Res. 1996;724(1):41–46. doi: 10.1016/0006-8993(96)00264-8. [DOI] [PubMed] [Google Scholar]
- García-Piqueras J., García-Mesa Y., Cárcaba L., Feito J., Torres-Parejo I., Martín-Biedma B., Cobo J., García-Suárez O., Vega J.A. Ageing of the somatosensory system at the periphery: age-related changes in cutaneous mechanoreceptors. J. Anat. 2019;234(6):839–852. doi: 10.1111/joa.12983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Poblete E., Fernández-García H., Moro-Rodríguez E., Catalá-Rodríguez M., Rico-Morales M.L., García-Gómez-de-las-Heras S., Palomar-Gallego M.A. Sympathetic sprouting in dorsal root ganglia (DRG): a recent histological finding? Histol. Histopathol. 2003;18(2):575–586. doi: 10.14670/hh-18.575. [DOI] [PubMed] [Google Scholar]
- Gardiner N.J., Cafferty W.B., Slack S.E., Thompson S.W. Expression of gp130 and leukaemia inhibitory factor receptor subunits in adult rat sensory neurones: regulation by nerve injury. J. Neurochem. 2002;83(1):100–109. doi: 10.1046/j.1471-4159.2002.01101.x. [DOI] [PubMed] [Google Scholar]
- Gardner, Esther P., 2010. Touch. In: Encyclopedia of Life Sciences (ELS). John Wiley & Sons, Ltd: Chichester. DOI: 10.1002/9780470015902.a0000219.pub.
- Garraway S.M., Huie J.R. Spinal Plasticity and Behavior: BDNF-Induced Neuromodulation in Uninjured and Injured Spinal Cord. Neural Plast. 2016;2016:9857201. doi: 10.1155/2016/9857201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garraway S.M., Petruska J.C., Mendell L.M. BDNF sensitizes the response of lamina II neurons to high threshold primary afferent inputs. Eur. J. Neurosci. 2003;18(9):2467–2476. doi: 10.1046/j.1460-9568.2003.02982.x. [DOI] [PubMed] [Google Scholar]
- Garraway S.M., Xu Q., Inturrisi C.E. siRNA-mediated knockdown of the NR1 subunit gene of the NMDA receptor attenuates formalin-induced pain behaviors in adult rats. J. Pain. 2009;10(4):380–390. doi: 10.1016/j.jpain.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garraway S.M., Woller S.A., Huie J.R., Hartman J.J., Hook M.A., Miranda R.C., Huang Y.J., Ferguson A.R., Grau J.W. Peripheral noxious stimulation reduces withdrawal threshold to mechanical stimuli after spinal cord injury: role of tumor necrosis factor alpha and apoptosis. Pain. 2014;155(11):2344–2359. doi: 10.1016/j.pain.2014.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garraway, S. M. (2023). BDNF-Induced Plasticity of Spinal Circuits Underlying Pain and Learning. In: Oxford University Press.
- Gehrmann J., Monaco S., Kreutzberg G.W. Spinal cord microglial cells and DRG satellite cells rapidly respond to transection of the rat sciatic nerve. Restor. Neurol. Neurosci. 1991;2(4):181–198. doi: 10.3233/RNN-1991-245605. [DOI] [PubMed] [Google Scholar]
- Gold M.S., Gebhart G.F. Nociceptor sensitization in pain pathogenesis. Nat. Med. 2010;16(11):1248–1257. doi: 10.1038/nm.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold M.S., Weinreich D., Kim C.S., Wang R., Treanor J., Porreca F., Lai J. Redistribution of Na(V)1.8 in uninjured axons enables neuropathic pain. J. Neurosci. 2003;23(1):158–166. doi: 10.1523/JNEUROSCI.23-01-00158.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin A.L., Barchi R.L., Caldwell J.H., Hofmann F., Howe J.R., Hunter J.C., Kallen R.G., Mandel G., Meisler M.H., Netter Y.B., Noda M., Tamkun M.M., Waxman S.G., Wood J.N., Catterall W.A. Nomenclature of voltage-gated sodium channels. Neuron. 2000;28(2):365–368. doi: 10.1016/s0896-6273(00)00116-1. [DOI] [PubMed] [Google Scholar]
- Gong K., Kung L.H., Magni G., Bhargava A., Jasmin L. Increased response to glutamate in small diameter dorsal root ganglion neurons after sciatic nerve injury. PLoS One. 2014;9(4):e95491. doi: 10.1371/journal.pone.0095491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto T., Oh S.B., Takeda M., Shinoda M., Sato T., Gunjikake K.K., Iwata K. Recent advances in basic research on the trigeminal ganglion. J. Physiol. Sci. 2016;66(5):381–386. doi: 10.1007/s12576-016-0448-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottschalk W., Pozzo-Miller L.D., Figurov A., Lu B. Presynaptic modulation of synaptic transmission and plasticity by brain-derived neurotrophic factor in the developing hippocampus. J. Neurosci. 1998;18(17):6830–6839. doi: 10.1523/JNEUROSCI.18-17-06830.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gras C., Herzog E., Bellenchi G.C., Bernard V., Ravassard P., Pohl M., Gasnier B., Giros B., El Mestikawy S. A third vesicular glutamate transporter expressed by cholinergic and serotoninergic neurons. J. Neurosci. 2002;22(13):5442–5451. doi: 10.1523/jneurosci.22-13-05442.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grau J.W. When Pain Hurts: Nociceptive Stimulation Induces a State of Maladaptive Plasticity and Impairs Recovery after Spinal Cord Injury. J. Neurotrauma. 2017;34(10):1873–1890. doi: 10.1089/neu.2016.4626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groth R., Aanonsen L. Spinal brain-derived neurotrophic factor (BDNF) produces hyperalgesia in normal mice while antisense directed against either BDNF or trkB, prevent inflammation-induced hyperalgesia. Pain. 2002;100(1–2):171–181. doi: 10.1016/s0304-3959(02)00264-6. [DOI] [PubMed] [Google Scholar]
- Haberberger R.V., Barry C., Dominguez N., Matusica D. Human Dorsal Root Ganglia [Review] Front. Cell. Neurosci. 2019;13 doi: 10.3389/fncel.2019.00271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habig K., Schanzer A., Schirner W., Lautenschlager G., Dassinger B., Olausson H., Birklein F., Gizewski E.R., Kramer H.H. Low threshold unmyelinated mechanoafferents can modulate pain. BMC Neurol. 2017;17(1):184. doi: 10.1186/s12883-017-0963-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn, C., Schuhmann, B., Mkhlof, S., Pirayesh, A., Renz, H., & Nockher, W. A. (2005). Epithelial NGF and BDNF production induce prolonged survival of eosinophils during allergic airway inflammation. Journal of Allergy and Clinical Immunology, 115(2, Supplement), S194. https://doi.org/https://doi.org/10.1016/j.jaci.2004.12.789.
- Hahn C., Islamian A.P., Renz H., Nockher W.A. Airway epithelial cells produce neurotrophins and promote the survival of eosinophils during allergic airway inflammation. J. Allergy Clin. Immunol. 2006;117(4):787–794. doi: 10.1016/j.jaci.2005.12.1339. [DOI] [PubMed] [Google Scholar]
- Hains B.C., Waxman S.G. Activated microglia contribute to the maintenance of chronic pain after spinal cord injury. J. Neurosci. 2006;26(16):4308–4317. doi: 10.1523/JNEUROSCI.0003-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hains B.C., Klein J.P., Saab C.Y., Craner M.J., Black J.A., Waxman S.G. Upregulation of sodium channel Nav1.3 and functional involvement in neuronal hyperexcitability associated with central neuropathic pain after spinal cord injury. J. Neurosci. 2003;23(26):8881–8892. doi: 10.1523/JNEUROSCI.23-26-08881.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hains B.C., Saab C.Y., Waxman S.G. Changes in electrophysiological properties and sodium channel Nav1.3 expression in thalamic neurons after spinal cord injury. Brain. 2005;128(Pt 10):2359–2371. doi: 10.1093/brain/awh623. [DOI] [PubMed] [Google Scholar]
- Hameed S. Na(v)1.7 and Na(v)1.8: Role in the pathophysiology of pain. Mol. Pain. 2019;15 doi: 10.1177/1744806919858801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanani M. Satellite glial cells in sensory ganglia: from form to function. Brain Res. Brain Res. Rev. 2005;48(3):457–476. doi: 10.1016/j.brainresrev.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Hao H., Ramli R., Wang C., Liu C., Shah S., Mullen P., Lall V., Jones F., Shao J., Zhang H., Jaffe D.B., Gamper N., Du X. Dorsal root ganglia control nociceptive input to the central nervous system. PLoS Biol. 2023;21(1):e3001958. doi: 10.1371/journal.pbio.3001958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haroutounian S., Nikolajsen L., Bendtsen T.F., Finnerup N.B., Kristensen A.D., Hasselstrøm J.B., Jensen T.S. Primary afferent input critical for maintaining spontaneous pain in peripheral neuropathy. Pain. 2014;155(7):1272–1279. doi: 10.1016/j.pain.2014.03.022. [DOI] [PubMed] [Google Scholar]
- Harte S.E., Harris R.E., Clauw D.J. The neurobiology of central sensitization. J. Appl. Biobehav. Res. 2018;23(2) doi: 10.1111/jabr.12137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann B., Ahmadi S., Heppenstall P.A., Lewin G.R., Schott C., Borchardt T., Seeburg P.H., Zeilhofer H.U., Sprengel R., Kuner R. The AMPA receptor subunits GluR-A and GluR-B reciprocally modulate spinal synaptic plasticity and inflammatory pain. Neuron. 2004;44(4):637–650. doi: 10.1016/j.neuron.2004.10.029. [DOI] [PubMed] [Google Scholar]
- Helgren M.E., Goldberger M.E. The recovery of postural reflexes and locomotion following low thoracic hemisection in adult cats involves compensation by undamaged primary afferent pathways. Exp. Neurol. 1993;123(1):17–34. doi: 10.1006/exnr.1993.1137. [DOI] [PubMed] [Google Scholar]
- Herrity A.N., Petruska J.C., Stirling D.P., Rau K.K., Hubscher C.H. The effect of spinal cord injury on the neurochemical properties of vagal sensory neurons. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015;308(12):R1021–R1033. doi: 10.1152/ajpregu.00445.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog R.I., Cummins T.R., Waxman S.G. Persistent TTX-resistant Na+ current affects resting potential and response to depolarization in simulated spinal sensory neurons. J. Neurophysiol. 2001;86(3):1351–1364. doi: 10.1152/jn.2001.86.3.1351. [DOI] [PubMed] [Google Scholar]
- Hilborn M.D., Vaillancourt R.R., Rane S.G. Growth factor receptor tyrosine kinases acutely regulate neuronal sodium channels through the src signaling pathway. J. Neurosci. 1998;18(2):590–600. doi: 10.1523/jneurosci.18-02-00590.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. Ionic channels in nerve membranes. Prog. Biophys. Mol. Biol. 1970;21:1–32. doi: 10.1016/0079-6107(70)90022-2. [DOI] [PubMed] [Google Scholar]
- Hille B. Ionic channels in nerve membranes, 50 years on. Prog. Biophys. Mol. Biol. 2022;169–170:12–20. doi: 10.1016/j.pbiomolbio.2021.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hladnik A., Bicanic I.A.E., Petanjek Z. Functional neuroanatomy of nociception and pain. Period. Biol. 2015;117:195–204. [Google Scholar]
- Horch K.W., Tuckett R.P., Burgess P.R. A key to the classification of cutaneous mechanoreceptors. J, Invest. Dermatol. 1977;69(1):75–82. doi: 10.1111/1523-1747.ep12497887. [DOI] [PubMed] [Google Scholar]
- Hu P., McLachlan E.M. Macrophage and lymphocyte invasion of dorsal root ganglia after peripheral nerve lesions in the rat. Neuroscience. 2002;112(1):23–38. doi: 10.1016/s0306-4522(02)00065-9. [DOI] [PubMed] [Google Scholar]
- Huang J., Han C., Estacion M., Vasylyev D., Hoeijmakers J.G., Gerrits M.M., Tyrrell L., Lauria G., Faber C.G., Dib-Hajj S.D., Merkies I.S., Waxman S.G. Gain-of-function mutations in sodium channel Na(v)1.9 in painful neuropathy. Brain. 2014;137(Pt 6):1627–1642. doi: 10.1093/brain/awu079. [DOI] [PubMed] [Google Scholar]
- Huang E.J., Reichardt L.F. Neurotrophins: roles in neuronal development and function. Annu. Rev. Neurosci. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y., Zhou X., Tang C., Zhang Y., Tao H., Chen P., Liu Z. Molecular basis of the inhibition of the fast inactivation of voltage-gated sodium channel Nav1.5 by tarantula toxin Jingzhaotoxin-II. Peptides. 2015;68:175–182. doi: 10.1016/j.peptides.2015.03.012. [DOI] [PubMed] [Google Scholar]
- Hulsebosch C.E., Hains B.C., Crown E.D., Carlton S.M. Mechanisms of chronic central neuropathic pain after spinal cord injury. Brain Res. Rev. 2009;60(1):202–213. doi: 10.1016/j.brainresrev.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichikawa H., Terayama R., Yamaai T., Yan Z., Sugimoto T. Brain-derived neurotrophic factor-immunoreactive neurons in the rat vagal and glossopharyngeal sensory ganglia; co-expression with other neurochemical substances. Brain Res. 2007;1155:93–99. doi: 10.1016/j.brainres.2006.11.024. [DOI] [PubMed] [Google Scholar]
- Iggo A. Cutaneous mechanoreceptors with afferent C fibres. J. Physiol. 1960;152(2):337–353. doi: 10.1113/jphysiol.1960.sp006491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- J. Ilha A. Meireles G.R. de Freitas do Espirito Santo, C. C., Machado-Pereira, N., Swarowsky, A., & Santos, A. R. S. Overground gait training promotes functional recovery and cortical neuroplasticity in an incomplete spinal cord injury model Life Sci 232 2019 116627 10.1016/j.lfs.2019.116627. [DOI] [PubMed]
- Inoue A., Ikoma K., Morioka N., Kumagai K., Hashimoto T., Hide I., Nakata Y. Interleukin-1beta induces substance P release from primary afferent neurons through the cyclooxygenase-2 system. J. Neurochem. 1999;73(5):2206–2213. https://www.ncbi.nlm.nih.gov/pubmed/10537081 [PubMed] [Google Scholar]
- Jang I.J., Davies A.J., Akimoto N., Back S.K., Lee P.R., Na H.S., Furue H., Jung S.J., Kim Y.H., Oh S.B. Acute inflammation reveals GABA(A) receptor-mediated nociception in mouse dorsal root ganglion neurons via PGE(2) receptor 4 signaling. Physiol. Rep. 2017;5(8) doi: 10.14814/phy2.13178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang J.H., Kim D.W., Sang Nam T., Se Paik K., Leem J.W. Peripheral glutamate receptors contribute to mechanical hyperalgesia in a neuropathic pain model of the rat. Neuroscience. 2004;128(1):169–176. doi: 10.1016/j.neuroscience.2004.06.040. [DOI] [PubMed] [Google Scholar]
- Jänig W. Sympathetic nervous system and inflammation: a conceptual view. Auton. Neurosci. 2014;182:4–14. doi: 10.1016/j.autneu.2014.01.004. [DOI] [PubMed] [Google Scholar]
- Jänig W., Häbler H.J. Sympathetic nervous system: contribution to chronic pain. Prog. Brain Res. 2000;129:451–468. [PubMed] [Google Scholar]
- Jänig W., Levine J.D., Michaelis M. In: Kumazawa T., Kruger L., Mizumura K., editors. Vol. 113. Elsevier; 1996. Chapter 10. Interactions of sympathetic and primary afferent neurons following nerve injury and tissue trauma; pp. 161–184. (Progress in Brain Research). [DOI] [PubMed] [Google Scholar]
- Jarvis M.F., Honore P., Shieh C.C., Chapman M., Joshi S., Zhang X.F., Kort M., Carroll W., Marron B., Atkinson R., Thomas J., Liu D., Krambis M., Liu Y., McGaraughty S., Chu K., Roeloffs R., Zhong C., Mikusa J.P., Krafte D.S. A-803467, a potent and selective Nav1.8 sodium channel blocker, attenuates neuropathic and inflammatory pain in the rat. PNAS. 2007;104(20):8520–8525. doi: 10.1073/pnas.0611364104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji R.R., Samad T.A., Jin S.X., Schmoll R., Woolf C.J. p38 MAPK activation by NGF in primary sensory neurons after inflammation increases TRPV1 levels and maintains heat hyperalgesia. Neuron. 2002;36(1):57–68. doi: 10.1016/s0896-6273(02)00908-x. [DOI] [PubMed] [Google Scholar]
- Ji R.R., Kohno T., Moore K.A., Woolf C.J. Central sensitization and LTP: do pain and memory share similar mechanisms? Trends Neurosci. 2003;26(12):696–705. doi: 10.1016/j.tins.2003.09.017. [DOI] [PubMed] [Google Scholar]
- Ji R.R., Strichartz G. Cell signaling and the genesis of neuropathic pain. Sci. STKE. 2004;2004(252):reE14. doi: 10.1126/stke.2522004re14. [DOI] [PubMed] [Google Scholar]
- Ji R.R., Nackley A., Huh Y., Terrando N., Maixner W. Neuroinflammation and Central Sensitization in Chronic and Widespread Pain. Anesthesiology. 2018;129(2):343–366. doi: 10.1097/ALN.0000000000002130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin X., Gereau R.W., t. Acute p38-mediated modulation of tetrodotoxin-resistant sodium channels in mouse sensory neurons by tumor necrosis factor-alpha. J. Neurosci. 2006;26(1):246–255. doi: 10.1523/JNEUROSCI.3858-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones K.R., Fariñas I., Backus C., Reichardt L.F. Targeted disruption of the BDNF gene perturbs brain and sensory neuron development but not motor neuron development. Cell. 1994;76(6):989–999. doi: 10.1016/0092-8674(94)90377-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi S.K., Mikusa J.P., Hernandez G., Baker S., Shieh C.C., Neelands T., Zhang X.F., Niforatos W., Kage K., Han P., Krafte D., Faltynek C., Sullivan J.P., Jarvis M.F., Honore P. Involvement of the TTX-resistant sodium channel Nav 1.8 in inflammatory and neuropathic, but not post-operative, pain states. Pain. 2006;123(1–2):75–82. doi: 10.1016/j.pain.2006.02.011. [DOI] [PubMed] [Google Scholar]
- Jug M., Kejzar N., Vesel M., Al Mawed S., Dobravec M., Herman S., Bajrovic F.F. Neurological Recovery after Traumatic Cervical Spinal Cord Injury Is Superior if Surgical Decompression and Instrumented Fusion Are Performed within 8 Hours versus 8 to 24 Hours after Injury: A Single Center Experience. J. Neurotrauma. 2015;32(18):1385–1392. doi: 10.1089/neu.2014.3767. [DOI] [PubMed] [Google Scholar]
- Julius D., Basbaum A.I. Molecular mechanisms of nociception. Nature. 2001;413(6852):203–210. doi: 10.1038/35093019. [DOI] [PubMed] [Google Scholar]
- Jung M., Dourado M., Maksymetz J., Jacobson A., Laufer B.I., Baca M., Foreman O., Hackos D.H., Riol-Blanco L., Kaminker J.S. Cross-species transcriptomic atlas of dorsal root ganglia reveals species-specific programs for sensory function. Nat. Commun. 2023;14(1):366. doi: 10.1038/s41467-023-36014-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan D.R., Miller F.D. Neurotrophin signal transduction in the nervous system. Curr. Opin. Neurobiol. 2000;10(3):381–391. doi: 10.1016/s0959-4388(00)00092-1. [DOI] [PubMed] [Google Scholar]
- Kashiba H., Noguchi K., Ueda Y., Senba E. Coexpression of trk family members and low-affinity neurotrophin receptors in rat dorsal root ganglion neurons. Brain Res. Mol. Brain Res. 1995;30(1):158–164. doi: 10.1016/0169-328x(94)00249-e. [DOI] [PubMed] [Google Scholar]
- Kavalali E.T. The mechanisms and functions of spontaneous neurotransmitter release. Nat. Rev. Neurosci. 2015;16(1):5–16. doi: 10.1038/nrn3875. [DOI] [PubMed] [Google Scholar]
- Keast J.R., Stephensen T.M. Glutamate and aspartate immunoreactivity in dorsal root ganglion cells supplying visceral and somatic targets and evidence for peripheral axonal transport. J Comp Neurol. 2000;424(4):577–587. https://www.ncbi.nlm.nih.gov/pubmed/10931482 [PubMed] [Google Scholar]
- Kerr B.J., Bradbury E.J., Bennett D.L., Trivedi P.M., Dassan P., French J., Shelton D.B., McMahon S.B., Thompson S.W. Brain-derived neurotrophic factor modulates nociceptive sensory inputs and NMDA-evoked responses in the rat spinal cord. J. Neurosci. 1999;19(12):5138–5148. doi: 10.1523/JNEUROSCI.19-12-05138.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr B.J., Souslova V., McMahon S.B., Wood J.N. A role for the TTX-resistant sodium channel Nav 1.8 in NGF-induced hyperalgesia, but not neuropathic pain. Neuroreport. 2001;12(14):3077–3080. doi: 10.1097/00001756-200110080-00019. [DOI] [PubMed] [Google Scholar]
- Kharazia V.N., Weinberg R.J. Immunogold localization of AMPA and NMDA receptors in somatic sensory cortex of albino rat. J Comp Neurol. 1999;412(2):292–302. doi: 10.1002/(sici)1096-9861(19990920)412:2<292::aid-cne8>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Kim S.H., Chung J.M. Sympathectomy alleviates mechanical allodynia in an experimental animal model for neuropathy in the rat. Neurosci. Lett. 1991;134(1):131–134. doi: 10.1016/0304-3940(91)90524-w. [DOI] [PubMed] [Google Scholar]
- Kim C.H., Oh Y., Chung J.M., Chung K. Changes in three subtypes of tetrodotoxin sensitive sodium channel expression in the axotomized dorsal root ganglion in the rat. Neurosci. Lett. 2002;323(2):125–128. doi: 10.1016/s0304-3940(02)00127-1. [DOI] [PubMed] [Google Scholar]
- King A.E., Thompson S.W., Urban L., Woolf C.J. An intracellular analysis of amino acid induced excitations of deep dorsal horn neurones in the rat spinal cord slice. Neurosci. Lett. 1988;89(3):286–292. doi: 10.1016/0304-3940(88)90541-1. [DOI] [PubMed] [Google Scholar]
- Kinnman E., Levine J.D. Involvement of the sympathetic postganglionic neuron in capsaicin-induced secondary hyperalgesia in the rat. Neuroscience. 1995;65(1):283–291. doi: 10.1016/0306-4522(94)00474-j. [DOI] [PubMed] [Google Scholar]
- Kinnman E., Levine J.D. Sensory and sympathetic contributions to nerve injury-induced sensory abnormalities in the rat. Neuroscience. 1995;64(3):751–767. doi: 10.1016/0306-4522(94)00435-8. [DOI] [PubMed] [Google Scholar]
- Klein A.H., Vyshnevska A., Hartke T.V., De Col R., Mankowski J.L., Turnquist B., Bosmans F., Reeh P.W., Schmelz M., Carr R.W., Ringkamp M. Sodium Channel Na(v)1.8 Underlies TTX-Resistant Axonal Action Potential Conduction in Somatosensory C-Fibers of Distal Cutaneous Nerves. J. Neurosci. 2017;37(20):5204–5214. doi: 10.1523/jneurosci.3799-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koltzenburg M., Stucky C.L., Lewin G.R. Receptive properties of mouse sensory neurons innervating hairy skin. J. Neurophysiol. 1997;78(4):1841–1850. doi: 10.1152/jn.1997.78.4.1841. [DOI] [PubMed] [Google Scholar]
- Kopach O., Kao S.C., Petralia R.S., Belan P., Tao Y.X., Voitenko N. Inflammation alters trafficking of extrasynaptic AMPA receptors in tonically firing lamina II neurons of the rat spinal dorsal horn. Pain. 2011;152(4):912–923. doi: 10.1016/j.pain.2011.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korte M., Kang H., Bonhoeffer T., Schuman E. A role for BDNF in the late-phase of hippocampal long-term potentiation. Neuropharmacology. 1998;37(4–5):553–559. doi: 10.1016/s0028-3908(98)00035-5. [DOI] [PubMed] [Google Scholar]
- Krames E.S. The role of the dorsal root ganglion in the development of neuropathic pain. Pain Med. 2014;15(10):1669–1685. doi: 10.1111/pme.12413. [DOI] [PubMed] [Google Scholar]
- Krebs M.O., Desce J.M., Kemel M.L., Gauchy C., Godeheu G., Cheramy A., Glowinski J. Glutamatergic control of dopamine release in the rat striatum: evidence for presynaptic N-methyl-D-aspartate receptors on dopaminergic nerve terminals. J. Neurochem. 1991;56(1):81–85. doi: 10.1111/j.1471-4159.1991.tb02565.x. [DOI] [PubMed] [Google Scholar]
- Kung L.H., Gong K., Adedoyin M., Ng J., Bhargava A., Ohara P.T., Jasmin L. Evidence for glutamate as a neuroglial transmitter within sensory ganglia. PLoS One. 2013;8(7):e68312. doi: 10.1371/journal.pone.0068312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai J., Gold M.S., Kim C.S., Bian D., Ossipov M.H., Hunter J.C., Porreca F. Inhibition of neuropathic pain by decreased expression of the tetrodotoxin-resistant sodium channel, NaV1.8. Pain. 2002;95(1–2):143–152. doi: 10.1016/s0304-3959(01)00391-8. [DOI] [PubMed] [Google Scholar]
- Larsson M., Broman J. Synaptic plasticity and pain: role of ionotropic glutamate receptors. Neuroscientist. 2011;17(3):256–273. doi: 10.1177/1073858409349913. [DOI] [PubMed] [Google Scholar]
- Larsson M., Nagi S.S. Role of C-tactile fibers in pain modulation: animal and human perspectives. Curr. Opin. Behav. Sci. 2022;43:138–144. doi: 10.1016/j.cobeha.2021.09.005. [DOI] [Google Scholar]
- Latremoliere A., Woolf C.J. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J. Pain. 2009;10(9):895–926. doi: 10.1016/j.jpain.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laursen J.C., Cairns B.E., Dong X.D., Kumar U., Somvanshi R.K., Arendt-Nielsen L., Gazerani P. Glutamate dysregulation in the trigeminal ganglion: a novel mechanism for peripheral sensitization of the craniofacial region. Neuroscience. 2014;256:23–35. doi: 10.1016/j.neuroscience.2013.10.009. [DOI] [PubMed] [Google Scholar]
- Lázár B.A., Jancsó G., Oszlács O., Nagy I., Sántha P. The Insulin Receptor Is Colocalized With the TRPV1 Nociceptive Ion Channel and Neuropeptides in Pancreatic Spinal and Vagal Primary Sensory Neurons. Pancreas. 2018;47(1):110–115. doi: 10.1097/mpa.0000000000000959. [DOI] [PubMed] [Google Scholar]
- Lee K.H., Chung K., Chung J.M., Coggeshall R.E. Correlation of cell body size, axon size, and signal conduction velocity for individually labelled dorsal root ganglion cells in the cat. J Comp Neurol. 1986;243(3):335–346. doi: 10.1002/cne.902430305. [DOI] [PubMed] [Google Scholar]
- Lee C.J., Kong H., Manzini M.C., Albuquerque C., Chao M.V., MacDermott A.B. Kainate receptors expressed by a subpopulation of developing nociceptors rapidly switch from high to low Ca2+ permeability. J. Neurosci. 2001;21(13):4572–4581. doi: 10.1523/JNEUROSCI.21-13-04572.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H.L., Lee K.M., Son S.J., Hwang S.H., Cho H.J. Temporal expression of cytokines and their receptors mRNAs in a neuropathic pain model. Neuroreport. 2004;15(18):2807–2811. https://www.ncbi.nlm.nih.gov/pubmed/15597059 [PubMed] [Google Scholar]
- Leibrock J., Lottspeich F., Hohn A., Hofer M., Hengerer B., Masiakowski P., Thoenen H., Barde Y.A. Molecular cloning and expression of brain-derived neurotrophic factor. Nature. 1989;341(6238):149–152. doi: 10.1038/341149a0. [DOI] [PubMed] [Google Scholar]
- Lever I.J., Bradbury E.J., Cunningham J.R., Adelson D.W., Jones M.G., McMahon S.B., Marvizon J.C., Malcangio M. Brain-derived neurotrophic factor is released in the dorsal horn by distinctive patterns of afferent fiber stimulation. J. Neurosci. 2001;21(12):4469–4477. doi: 10.1523/JNEUROSCI.21-12-04469.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lever I., Cunningham J., Grist J., Yip P.K., Malcangio M. Release of BDNF and GABA in the dorsal horn of neuropathic rats. Eur. J. Neurosci. 2003;18(5):1169–1174. doi: 10.1046/j.1460-9568.2003.02848.x. [DOI] [PubMed] [Google Scholar]
- Levin M.E., Jin J.G., Ji R.R., Tong J., Pomonis J.D., Lavery D.J., Miller S.W., Chiang L.W. Complement activation in the peripheral nervous system following the spinal nerve ligation model of neuropathic pain. Pain. 2008;137(1):182–201. doi: 10.1016/j.pain.2007.11.005. [DOI] [PubMed] [Google Scholar]
- Levine J., Dardick S., Roizen M., Helms C., Basbaum A. Contribution of sensory afferents and sympathetic efferents to joint injury in experimental arthritis. J. Neurosci. 1986;6(12):3423–3429. doi: 10.1523/jneurosci.06-12-03423.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine E.S., Dreyfus C.F., Black I.B., Plummer M.R. Brain-derived neurotrophic factor rapidly enhances synaptic transmission in hippocampal neurons via postsynaptic tyrosine kinase receptors. PNAS. 1995;92(17):8074–8077. doi: 10.1073/pnas.92.17.8074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewin G.R., Ritter A.M., Mendell L.M. Nerve growth factor-induced hyperalgesia in the neonatal and adult rat. J. Neurosci. 1993;13(5):2136–2148. doi: 10.1523/JNEUROSCI.13-05-02136.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewin G.R., Rueff A., Mendell L.M. Peripheral and central mechanisms of NGF-induced hyperalgesia. Eur. J. Neurosci. 1994;6(12):1903–1912. doi: 10.1111/j.1460-9568.1994.tb00581.x. [DOI] [PubMed] [Google Scholar]
- Li C.L., Li K.C., Wu D., Chen Y., Luo H., Zhao J.R., Wang S.S., Sun M.M., Lu Y.J., Zhong Y.Q., Hu X.Y., Hou R., Zhou B.B., Bao L., Xiao H.S., Zhang X. Somatosensory neuron types identified by high-coverage single-cell RNA-sequencing and functional heterogeneity. Cell Res. 2016;26(8):967. doi: 10.1038/cr.2016.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., McRoberts J.A., Nie J., Ennes H.S., Mayer E.A. Electrophysiological characterization of N-methyl-D-aspartate receptors in rat dorsal root ganglia neurons. Pain. 2004;109(3):443–452. doi: 10.1016/j.pain.2004.02.021. [DOI] [PubMed] [Google Scholar]
- Li L., Rutlin M., Abraira V.E., Cassidy C., Kus L., Gong S., Jankowski M.P., Luo W., Heintz N., Koerber H.R., Woodbury C.J., Ginty D.D. The functional organization of cutaneous low-threshold mechanosensory neurons. Cell. 2011;147(7):1615–1627. doi: 10.1016/j.cell.2011.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M., Shi J., Tang J.R., Chen D., Ai B., Chen J., Wang L.N., Cao F.Y., Li L.L., Lin C.Y., Guan X.M. Effects of complete Freund's adjuvant on immunohistochemical distribution of IL-1beta and IL-1R I in neurons and glia cells of dorsal root ganglion. Acta Pharmacol. Sin. 2005;26(2):192–198. doi: 10.1111/j.1745-7254.2005.00522.x. [DOI] [PubMed] [Google Scholar]
- Liljencrantz J., Olausson H. Tactile C fibers and their contributions to pleasant sensations and to tactile allodynia. Front. Behav. Neurosci. 2014;8:37. doi: 10.3389/fnbeh.2014.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y.T., Ro L.S., Wang H.L., Chen J.C. Up-regulation of dorsal root ganglia BDNF and trkB receptor in inflammatory pain: an in vivo and in vitro study. J. Neuroinflammation. 2011;8:126. doi: 10.1186/1742-2094-8-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Q., Zou X., Fang L., Willis W.D. Sympathetic modulation of acute cutaneous flare induced by intradermal injection of capsaicin in anesthetized rats. J. Neurophysiol. 2003;89(2):853–861. doi: 10.1152/jn.00568.2002. [DOI] [PubMed] [Google Scholar]
- Liu X., Ernfors P., Wu H., Jaenisch R. Sensory but not motor neuron deficits in mice lacking NT4 and BDNF. Nature. 1995;375(6528):238–241. doi: 10.1038/375238a0. [DOI] [PubMed] [Google Scholar]
- Liu T., Ji R.R. New insights into the mechanisms of itch: are pain and itch controlled by distinct mechanisms? Pflugers Arch. 2013;465(12):1671–1685. doi: 10.1007/s00424-013-1284-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C.N., Wall P.D., Ben-Dor E., Michaelis M., Amir R., Devor M. Tactile allodynia in the absence of C-fiber activation: altered firing properties of DRG neurons following spinal nerve injury. Pain. 2000;85(3):503–521. doi: 10.1016/S0304-3959(00)00251-7. [DOI] [PubMed] [Google Scholar]
- Liu H., Wang H., Sheng M., Jan L.Y., Jan Y.N., Basbaum A.I. Evidence for presynaptic N-methyl-D-aspartate autoreceptors in the spinal cord dorsal horn. PNAS. 1994;91(18):8383–8387. doi: 10.1073/pnas.91.18.8383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löken L.S., Wessberg J., Morrison I., McGlone F., Olausson H. Coding of pleasant touch by unmyelinated afferents in humans. Nat. Neurosci. 2009;12(5):547–548. doi: 10.1038/nn.2312. [DOI] [PubMed] [Google Scholar]
- Lopes D.M., Denk F., McMahon S.B. The Molecular Fingerprint of Dorsal Root and Trigeminal Ganglion Neurons. Front. Mol. Neurosci. 2017;10:304. doi: 10.3389/fnmol.2017.00304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou S., Duan B., Vong L., Lowell B.B., Ma Q. Runx1 controls terminal morphology and mechanosensitivity of VGLUT3-expressing C-mechanoreceptors. J. Neurosci. 2013;33(3):870–882. doi: 10.1523/jneurosci.3942-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C.R., Hwang S.J., Phend K.D., Rustioni A., Valtschanoff J.G. Primary afferent terminals that express presynaptic NR1 in rats are mainly from myelinated, mechanosensitive fibers. J Comp Neurol. 2003;460(2):191–202. doi: 10.1002/cne.10632. [DOI] [PubMed] [Google Scholar]
- Lu X., Richardson P.M. Inflammation near the nerve cell body enhances axonal regeneration. J. Neurosci. 1991;11(4):972–978. doi: 10.1523/JNEUROSCI.11-04-00972.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X., Richardson P.M. Responses of macrophages in rat dorsal root ganglia following peripheral nerve injury. J. Neurocytol. 1993;22(5):334–341. doi: 10.1007/BF01195557. [DOI] [PubMed] [Google Scholar]
- Lumpkin E.A., Caterina M.J. Mechanisms of sensory transduction in the skin. Nature. 2007;445(7130):858–865. doi: 10.1038/nature05662. [DOI] [PubMed] [Google Scholar]
- Luo X.G., Rush R.A., Zhou X.F. Ultrastructural localization of brain-derived neurotrophic factor in rat primary sensory neurons. Neurosci. Res. 2001;39(4):377–384. doi: 10.1016/s0168-0102(00)00238-8. [DOI] [PubMed] [Google Scholar]
- Lynn B., Carpenter S.E. Primary afferent units from the hairy skin of the rat hind limb. Brain Res. 1982;238(1):29–43. doi: 10.1016/0006-8993(82)90768-5. [DOI] [PubMed] [Google Scholar]
- Lyu Y.S., Park S.K., Chung K., Chung J.M. Low dose of tetrodotoxin reduces neuropathic pain behaviors in an animal model. Brain Res. 2000;871(1):98–103. doi: 10.1016/s0006-8993(00)02451-3. [DOI] [PubMed] [Google Scholar]
- Ma W., Bisby M.A. Partial sciatic nerve transection induced tyrosine hydroxidase immunoreactive axon sprouting around both injured and spared dorsal root ganglion neurons which project to the gracile nucleus in middle-aged rats. Neurosci. Lett. 1999;275(2):117–120. doi: 10.1016/s0304-3940(99)00746-6. [DOI] [PubMed] [Google Scholar]
- Ma Q.P., Hargreaves R.J. Localization of N-methyl-D-aspartate NR2B subunits on primary sensory neurons that give rise to small-caliber sciatic nerve fibers in rats. Neuroscience. 2000;101(3):699–707. doi: 10.1016/s0306-4522(00)00419-x. [DOI] [PubMed] [Google Scholar]
- Madara J.C., Levine E.S. Presynaptic and postsynaptic NMDA receptors mediate distinct effects of brain-derived neurotrophic factor on synaptic transmission. J. Neurophysiol. 2008;100(6):3175–3184. doi: 10.1152/jn.90880.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahns D.A., Nagi S.S. An investigation into the peripheral substrates involved in the tactile modulation of cutaneous pain with emphasis on the C-tactile fibres. Exp. Brain Res. 2013;227(4):457–465. doi: 10.1007/s00221-013-3521-5. [DOI] [PubMed] [Google Scholar]
- Maingret F., Coste B., Padilla F., Clerc N., Crest M., Korogod S.M., Delmas P. Inflammatory mediators increase Nav1.9 current and excitability in nociceptors through a coincident detection mechanism. J. Gen. Physiol. 2008;131(3):211–225. doi: 10.1085/jgp.200709935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinow R., Malenka R.C. AMPA receptor trafficking and synaptic plasticity. Annu. Rev. Neurosci. 2002;25:103–126. doi: 10.1146/annurev.neuro.25.112701.142758. [DOI] [PubMed] [Google Scholar]
- Malmberg A.B., Chen C., Tonegawa S., Basbaum A.I. Preserved acute pain and reduced neuropathic pain in mice lacking PKCgamma. Science. 1997;278(5336):279–283. doi: 10.1126/science.278.5336.279. [DOI] [PubMed] [Google Scholar]
- Mannion R.J., Costigan M., Decosterd I., Amaya F., Ma Q.P., Holstege J.C., Ji R.R., Acheson A., Lindsay R.M., Wilkinson G.A., Woolf C.J. Neurotrophins: peripherally and centrally acting modulators of tactile stimulus-induced inflammatory pain hypersensitivity. PNAS. 1999;96(16):9385–9390. doi: 10.1073/pnas.96.16.9385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin K.K., Parvin S., Garraway S.M. Peripheral Inflammation Accelerates the Onset of Mechanical Hypersensitivity after Spinal Cord Injury and Engages Tumor Necrosis Factor α Signaling Mechanisms. J. Neurotrauma. 2019;36(12):2000–2010. doi: 10.1089/neu.2018.5953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin K.K., Noble D.J., Parvin S., Jang K., Garraway S.M. Pharmacogenetic inhibition of TrkB signaling in adult mice attenuates mechanical hypersensitivity and improves locomotor function after spinal cord injury. Front. Cell. Neurosci. 2022;16 doi: 10.3389/fncel.2022.987236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marty S., Berzaghi Mda P., Berninger B. Neurotrophins and activity-dependent plasticity of cortical interneurons. Trends Neurosci. 1997;20(5):198–202. doi: 10.1016/s0166-2236(96)01026-0. [DOI] [PubMed] [Google Scholar]
- Maruo K., Yamamoto H., Yamamoto S., Nagata T., Fujikawa H., Kanno T., Yaguchi T., Maruo S., Yoshiya S., Nishizaki T. Modulation of P2X receptors via adrenergic pathways in rat dorsal root ganglion neurons after sciatic nerve injury. Pain. 2006;120(1–2):106–112. doi: 10.1016/j.pain.2005.10.016. [DOI] [PubMed] [Google Scholar]
- Marvizón J.C., Martínez V., Grady E.F., Bunnett N.W., Mayer E.A. Neurokinin 1 receptor internalization in spinal cord slices induced by dorsal root stimulation is mediated by NMDA receptors. J. Neurosci. 1997;17(21):8129–8136. doi: 10.1523/jneurosci.17-21-08129.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marvizon J.C., McRoberts J.A., Ennes H.S., Song B., Wang X., Jinton L., Corneliussen B., Mayer E.A. Two N-methyl-D-aspartate receptors in rat dorsal root ganglia with different subunit composition and localization. J Comp Neurol. 2002;446(4):325–341. doi: 10.1002/cne.10202. [DOI] [PubMed] [Google Scholar]
- Matayoshi S., Jiang N., Katafuchi T., Koga K., Furue H., Yasaka T., Nakatsuka T., Zhou X.F., Kawasaki Y., Tanaka N., Yoshimura M. Actions of brain-derived neurotrophic factor on spinal nociceptive transmission during inflammation in the rat. J. Physiol. 2005;569(Pt 2):685–695. doi: 10.1113/jphysiol.2005.095331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonnell J.G., Finnerty O., Laffey J.G. Stellate ganglion blockade for analgesia following upper limb surgery. Anaesthesia. 2011;66(7):611–614. doi: 10.1111/j.1365-2044.2011.06626.x. [DOI] [PubMed] [Google Scholar]
- McLachlan E.M., Janig W., Devor M., Michaelis M. Peripheral nerve injury triggers noradrenergic sprouting within dorsal root ganglia. Nature. 1993;363(6429):543–546. doi: 10.1038/363543a0. [DOI] [PubMed] [Google Scholar]
- Melzack R., Casey K. Sensory, Motivational, and Central Control Determinants of Pain. In. 1968:423–439. [Google Scholar]
- Mendell L.M., Albers K.M., Davis B.M. Neurotrophins, nociceptors, and pain. Microsc. Res. Tech. 1999;45(4–5):252–261. doi: 10.1002/(SICI)1097-0029(19990515/01)45:4/5<252::AID-JEMT9>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Merighi A., Salio C., Ghirri A., Lossi L., Ferrini F., Betelli C., Bardoni R. BDNF as a pain modulator. Prog. Neurobiol. 2008;85(3):297–317. doi: 10.1016/j.pneurobio.2008.04.004. [DOI] [PubMed] [Google Scholar]
- Meur K.L., Galante M., Angulo M.C., Audinat E. Tonic activation of NMDA receptors by ambient glutamate of non-synaptic origin in the rat hippocampus. J. Physiol. 2007;580(2):373–383. doi: 10.1113/jphysiol.2006.123570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael G.J., Averill S., Nitkunan A., Rattray M., Bennett D.L., Yan Q., Priestley J.V. Nerve growth factor treatment increases brain-derived neurotrophic factor selectively in TrkA-expressing dorsal root ganglion cells and in their central terminations within the spinal cord. J. Neurosci. 1997;17(21):8476–8490. doi: 10.1523/JNEUROSCI.17-21-08476.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael G.J., Averill S., Shortland P.J., Yan Q., Priestley J.V. Axotomy results in major changes in BDNF expression by dorsal root ganglion cells: BDNF expression in large trkB and trkC cells, in pericellular baskets, and in projections to deep dorsal horn and dorsal column nuclei. Eur. J. Neurosci. 1999;11(10):3539–3551. doi: 10.1046/j.1460-9568.1999.00767.x. [DOI] [PubMed] [Google Scholar]
- Millan M.J. The induction of pain: an integrative review. Prog. Neurobiol. 1999;57(1):1–164. doi: 10.1016/s0301-0082(98)00048-3. [DOI] [PubMed] [Google Scholar]
- Miller R.J., Jung H., Bhangoo S.K., White F.A. Cytokine and chemokine regulation of sensory neuron function. Handb Exp. 2009;Pharmacol(194):417–449. doi: 10.1007/978-3-540-79090-7_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minett M.S., Nassar M.A., Clark A.K., Passmore G., Dickenson A.H., Wang F., Malcangio M., Wood J.N. Distinct Nav1.7-dependent pain sensations require different sets of sensory and sympathetic neurons. Nat. Commun. 2012;3:791. doi: 10.1038/ncomms1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitre M., Mariga A., Chao M.V. Neurotrophin signalling: novel insights into mechanisms and pathophysiology. Clin. Sci. (Lond.) 2017;131(1):13–23. doi: 10.1042/CS20160044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogil J.S. Animal models of pain: progress and challenges. Nat. Rev. Neurosci. 2009;10(4):283–294. doi: 10.1038/nrn2606. [DOI] [PubMed] [Google Scholar]
- Monaghan D.T., Cotman C.W. Distribution of N-methyl-D-aspartate-sensitive L-[3H]glutamate-binding sites in rat brain. J. Neurosci. 1985;5(11):2909–2919. doi: 10.1523/JNEUROSCI.05-11-02909.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montaño J.A., Pérez-Piñera P., García-Suárez O., Cobo J., Vega J.A. Development and neuronal dependence of cutaneous sensory nerve formations: Lessons from neurotrophins. Microsc. Res. Tech. 2010;73(5):513–529. doi: 10.1002/jemt.20790. [DOI] [PubMed] [Google Scholar]
- Monyer H., Burnashev N., Laurie D.J., Sakmann B., Seeburg P.H. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron. 1994;12(3):529–540. doi: 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- Morrison I., Löken L., Olausson H. The skin as a social organ. Exp. Brain Res. 2010;204(3):305–314. doi: 10.1007/s00221-009-2007-y. [DOI] [PubMed] [Google Scholar]
- Napier I.A., Mohammadi S.A., Christie M.J. Glutamate transporter dysfunction associated with nerve injury-induced pain in mice. J. Neurophysiol. 2012;107(2):649–657. doi: 10.1152/jn.00763.2011. [DOI] [PubMed] [Google Scholar]
- Nie H., Zhang H., Weng H.-R. Bidirectional Neuron-Glia Interactions Triggered by Deficiency of Glutamate Uptake at Spinal Sensory Synapses. J. Neurophysiol. 2010;104(2):713–725. doi: 10.1152/jn.00282.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nijs J., Meeus M., Versijpt J., Moens M., Bos I., Knaepen K., Meeusen R. Brain-derived neurotrophic factor as a driving force behind neuroplasticity in neuropathic and central sensitization pain: a new therapeutic target? Expert Opin. Ther. Targets. 2015;19(4):565–576. doi: 10.1517/14728222.2014.994506. [DOI] [PubMed] [Google Scholar]
- Nilsson A., Moller K., Dahlin L., Lundborg G., Kanje M. Early changes in gene expression in the dorsal root ganglia after transection of the sciatic nerve; effects of amphiregulin and PAI-1 on regeneration. Brain Res. Mol. Brain Res. 2005;136(1–2):65–74. doi: 10.1016/j.molbrainres.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Nirogi R., Goura V., Shanmuganathan D., Jayarajan P., Abraham R. Comparison of manual and automated filaments for evaluation of neuropathic pain behavior in rats. J. Pharmacol. Toxicol. Methods. 2012;66(1):8–13. doi: 10.1016/j.vascn.2012.04.006. [DOI] [PubMed] [Google Scholar]
- Noble D.J., Dongmo R., Parvin S., Martin K.K., Garraway S.M. C-low threshold mechanoreceptor activation becomes sufficient to trigger affective pain in spinal cord-injured mice in association with increased respiratory rates. Front. Integr. Neurosci. 2022;16:1081172. doi: 10.3389/fnint.2022.1081172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novakovic S.D., Tzoumaka E., McGivern J.G., Haraguchi M., Sangameswaran L., Gogas K.R., Eglen R.M., Hunter J.C. Distribution of the tetrodotoxin-resistant sodium channel PN3 in rat sensory neurons in normal and neuropathic conditions. J. Neurosci. 1998;18(6):2174–2187. doi: 10.1523/jneurosci.18-06-02174.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novkovic T., Mittmann T., Manahan-Vaughan D. BDNF contributes to the facilitation of hippocampal synaptic plasticity and learning enabled by environmental enrichment. Hippocampus. 2015;25(1):1–15. doi: 10.1002/hipo.22342. [DOI] [PubMed] [Google Scholar]
- Nscisc . Univeristy of Alabama at Birmingham; 2023. Traumatic Spinal Cord Injury Facts and Figures at a Glance. [Google Scholar]
- Numakawa T., Suzuki S., Kumamaru E., Adachi N., Richards M., Kunugi H. BDNF function and intracellular signaling in neurons. Histol. Histopathol. 2010;25(2):237–258. doi: 10.14670/HH-25.237. [DOI] [PubMed] [Google Scholar]
- Obreja O., Rathee P.K., Lips K.S., Distler C., Kress M. IL-1 beta potentiates heat-activated currents in rat sensory neurons: involvement of IL-1RI, tyrosine kinase, and protein kinase C. FASEB J. 2002;16(12):1497–1503. doi: 10.1096/fj.02-0101com. [DOI] [PubMed] [Google Scholar]
- Obreja O., Biasio W., Andratsch M., Lips K.S., Rathee P.K., Ludwig A., Rose-John S., Kress M. Fast modulation of heat-activated ionic current by proinflammatory interleukin 6 in rat sensory neurons. Brain. 2005;128(Pt 7):1634–1641. doi: 10.1093/brain/awh490. [DOI] [PubMed] [Google Scholar]
- Okuse K., Chaplan S.R., McMahon S.B., Luo Z.D., Calcutt N.A., Scott B.P., Akopian A.N., Wood J.N. Regulation of expression of the sensory neuron-specific sodium channel SNS in inflammatory and neuropathic pain. Mol. Cell. Neurosci. 1997;10(3–4):196–207. doi: 10.1006/mcne.1997.0657. [DOI] [PubMed] [Google Scholar]
- Olausson H., Lamarre Y., Backlund H., Morin C., Wallin B.G., Starck G., Ekholm S., Strigo I., Worsley K., Vallbo A.B., Bushnell M.C. Unmyelinated tactile afferents signal touch and project to insular cortex. Nat. Neurosci. 2002;5(9):900–904. doi: 10.1038/nn896. [DOI] [PubMed] [Google Scholar]
- Omana-Zapata I., Khabbaz M.A., Hunter J.C., Clarke D.E., Bley K.R. Tetrodotoxin inhibits neuropathic ectopic activity in neuromas, dorsal root ganglia and dorsal horn neurons. Pain. 1997;72(1–2):41–49. doi: 10.1016/s0304-3959(97)00012-2. [DOI] [PubMed] [Google Scholar]
- Omote K., Kawamata T., Kawamata M., Namiki A. Formalin-induced release of excitatory amino acids in the skin of the rat hindpaw. Brain Res. 1998;787(1):161–164. doi: 10.1016/s0006-8993(97)01568-0. [DOI] [PubMed] [Google Scholar]
- Opree A., Kress M. Involvement of the proinflammatory cytokines tumor necrosis factor-alpha, IL-1 beta, and IL-6 but not IL-8 in the development of heat hyperalgesia: effects on heat-evoked calcitonin gene-related peptide release from rat skin. J. Neurosci. 2000;20(16):6289–6293. doi: 10.1523/JNEUROSCI.20-16-06289.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostman J.A., Nassar M.A., Wood J.N., Baker M.D. GTP up-regulated persistent Na+ current and enhanced nociceptor excitability require NaV1.9. J. Physiol. 2008;586(4):1077–1087. doi: 10.1113/jphysiol.2007.147942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pain, I. A. f. t. S. o. (2017). IASP terminology. Pain Terms, 19(6), 2019.
- Palazzo E., de Novellis V., Rossi F., Maione S. Supraspinal metabotropic glutamate receptor subtype 8: a switch to turn off pain. Amino Acids. 2014;46(6):1441–1448. doi: 10.1007/s00726-014-1703-5. [DOI] [PubMed] [Google Scholar]
- Pannese E. The structure of the perineuronal sheath of satellite glial cells (SGCs) in sensory ganglia. Neuron Glia Biol. 2010;6(1):3–10. doi: 10.1017/S1740925X10000037. [DOI] [PubMed] [Google Scholar]
- Paoletti P. Molecular basis of NMDA receptor functional diversity. Eur. J. Neurosci. 2011;33(8):1351–1365. doi: 10.1111/j.1460-9568.2011.07628.x. [DOI] [PubMed] [Google Scholar]
- Paoletti P., Bellone C., Zhou Q. NMDA receptor subunit diversity: impact on receptor properties, synaptic plasticity and disease. Nat. Rev. Neurosci. 2013;14(6):383–400. doi: 10.1038/nrn3504. [DOI] [PubMed] [Google Scholar]
- Papouin T., Oliet S.H. Organization, control and function of extrasynaptic NMDA receptors. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014;369(1654):20130601. doi: 10.1098/rstb.2013.0601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parada C.A., Vivancos G.G., Tambeli C.H., Cunha F.Q., Ferreira S.H. Activation of presynaptic NMDA receptors coupled to NaV1.8-resistant sodium channel C-fibers causes retrograde mechanical nociceptor sensitization. PNAS. 2003;100(5):2923–2928. doi: 10.1073/pnas.252777799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H., Poo M.M. Neurotrophin regulation of neural circuit development and function. Nat. Rev. Neurosci. 2013;14(1):7–23. doi: 10.1038/nrn3379. [DOI] [PubMed] [Google Scholar]
- Peirs C., Seal R.P. Neural circuits for pain: Recent advances and current views. Science. 2016;354(6312):578–584. doi: 10.1126/science.aaf8933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins J.R., Antunes-Martins A., Calvo M., Grist J., Rust W., Schmid R., Hildebrandt T., Kohl M., Orengo C., McMahon S.B., Bennett D.L. A Comparison of RNA-Seq and Exon Arrays for Whole Genome Transcription Profiling of the L5 Spinal Nerve Transection Model of Neuropathic Pain in the Rat. Mol. Pain. 2014;10 doi: 10.1186/1744-8069-10-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlson E., Hanz S., Ben-Yaakov K., Segal-Ruder Y., Seger R., Fainzilber M. Vimentin-dependent spatial translocation of an activated MAP kinase in injured nerve. Neuron. 2005;45(5):715–726. doi: 10.1016/j.neuron.2005.01.023. [DOI] [PubMed] [Google Scholar]
- Perry V.H., Brown M.C. Role of macrophages in peripheral nerve degeneration and repair. Bioessays. 1992;14(6):401–406. doi: 10.1002/bies.950140610. [DOI] [PubMed] [Google Scholar]
- Petralia R.S., Wang Y.X., Hua F., Yi Z., Zhou A., Ge L., Stephenson F.A., Wenthold R.J. Organization of NMDA receptors at extrasynaptic locations. Neuroscience. 2010;167(1):68–87. doi: 10.1016/j.neuroscience.2010.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrini E.M., Lu J., Cognet L., Lounis B., Ehlers M.D., Choquet D. Endocytic trafficking and recycling maintain a pool of mobile surface AMPA receptors required for synaptic potentiation. Neuron. 2009;63(1):92–105. doi: 10.1016/j.neuron.2009.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezet S., Malcangio M., Lever I.J., Perkinton M.S., Thompson S.W., Williams R.J., McMahon S.B. Noxious stimulation induces Trk receptor and downstream ERK phosphorylation in spinal dorsal horn. Mol. Cell. Neurosci. 2002;21(4):684–695. doi: 10.1006/mcne.2002.1205. [DOI] [PubMed] [Google Scholar]
- Pezet S., McMahon S.B. Neurotrophins: mediators and modulators of pain. Annu. Rev. Neurosci. 2006;29:507–538. doi: 10.1146/annurev.neuro.29.051605.112929. [DOI] [PubMed] [Google Scholar]
- Pezet S., Malcangio M., McMahon S.B. BDNF: a neuromodulator in nociceptive pathways? Brain Res. Brain Res. Rev. 2002;40(1–3):240–249. doi: 10.1016/s0165-0173(02)00206-0. [DOI] [PubMed] [Google Scholar]
- Popovich P.G., Wei P., Stokes B.T. Cellular inflammatory response after spinal cord injury in Sprague-Dawley and Lewis rats. J Comp Neurol. 1997;377(3):443–464. doi: 10.1002/(sici)1096-9861(19970120)377:3<443::aid-cne10>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Purves D., A., G.,, Fitzpatrick, D,, et al. 2nd edition. Sinauer Associates; 2001. The Major Afferent Pathway for Mechanosensory Information: The Dorsal Column-Medial Lemniscus System. In Neuroscience. [Google Scholar]
- Qiao L.Y., Shen S., Liu M., Xia C., Kay J.C., Zhang Q.L. Inflammation and activity augment brain-derived neurotrophic factor peripheral release. Neuroscience. 2016;318:114–121. doi: 10.1016/j.neuroscience.2016.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu X.X., Cai J., Li M.J., Chi Y.N., Liao F.F., Liu F.Y., Wan Y., Han J.S., Xing G.G. Role of the spinal cord NR2B-containing NMDA receptors in the development of neuropathic pain. Exp. Neurol. 2009;215(2):298–307. doi: 10.1016/j.expneurol.2008.10.018. [DOI] [PubMed] [Google Scholar]
- Raja S.N., Carr D.B., Cohen M., Finnerup N.B., Flor H., Gibson S., Keefe F.J., Mogil J.S., Ringkamp M., Sluka K.A., Song X.J., Stevens B., Sullivan M.D., Tutelman P.R., Ushida T., Vader K. The revised International Association for the Study of Pain definition of pain: concepts, challenges, and compromises. Pain. 2020;161(9):1976–1982. doi: 10.1097/j.pain.0000000000001939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramer M.S., Thompson S.W.N., McMahon S.B. Causes and consequences of sympathetic basket formation in dorsal root ganglia. Pain, Suppl. 1999;6:S111–S120. doi: 10.1016/s0304-3959(99)00144-x. [DOI] [PubMed] [Google Scholar]
- Rath N., Balain B. Spinal cord injury-The role of surgical treatment for neurological improvement. J Clin Orthop Trauma. 2017;8(2):99–102. doi: 10.1016/j.jcot.2017.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray P., Torck A., Quigley L., Wangzhou A., Neiman M., Rao C., Lam T., Kim J.Y., Kim T.H., Zhang M.Q., Dussor G., Price T.J. Comparative transcriptome profiling of the human and mouse dorsal root ganglia: an RNA-seq-based resource for pain and sensory neuroscience research. Pain. 2018;159(7):1325–1345. doi: 10.1097/j.pain.0000000000001217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed-Geaghan E.G., Wright M.C., See L.A., Adelman P.C., Lee K.H., Koerber H.R., Maricich S.M. Merkel Cell-Driven BDNF Signaling Specifies SAI Neuron Molecular and Electrophysiological Phenotypes. J. Neurosci. 2016;36(15):4362–4376. doi: 10.1523/jneurosci.3781-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichling D.B., Levine J.D. Critical role of nociceptor plasticity in chronic pain. Trends Neurosci. 2009;32(12):611–618. doi: 10.1016/j.tins.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhold A.K., Batti L., Bilbao D., Buness A., Rittner H.L., Heppenstall P.A. Differential Transcriptional Profiling of Damaged and Intact Adjacent Dorsal Root Ganglia Neurons in Neuropathic Pain. PLoS One. 2015;10(4):e0123342. doi: 10.1371/journal.pone.0123342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Y., Zou X., Fang L., Lin Q. Sympathetic Modulation of Activity in Aδ- and C-Primary Nociceptive Afferents After Intradermal Injection of Capsaicin in Rats. J. Neurophysiol. 2005;93(1):365–377. doi: 10.1152/jn.00804.2004. [DOI] [PubMed] [Google Scholar]
- Roberts W.J., Elardo S.M. Sympathetic activation of unmyelinated mechanoreceptors in cat skin. Brain Res. 1985;339(1):123–125. doi: 10.1016/0006-8993(85)90629-8. [DOI] [PubMed] [Google Scholar]
- Roberts W.J., Levitt G.R. Histochemical evidence for sympathetic innervation of hair receptor afferents in cat skin. J Comp Neurol. 1982;210(2):204–209. doi: 10.1002/cne.902100211. [DOI] [PubMed] [Google Scholar]
- Rostock C., Schrenk-Siemens K., Pohle J., Siemens J. Human vs. Mouse Nociceptors - Similarities and Differences. Neuroscience. 2018;387:13–27. doi: 10.1016/j.neuroscience.2017.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush A.M., Bräu M.E., Elliott A.A., Elliott J.R. Electrophysiological properties of sodium current subtypes in small cells from adult rat dorsal root ganglia [Article] J. Physiol. 1998;511(3):771–789. doi: 10.1111/j.1469-7793.1998.771bg.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush A.M., Cummins T.R., Waxman S.G. Multiple sodium channels and their roles in electrogenesis within dorsal root ganglion neurons. J. Physiol. 2007;579(Pt 1):1–14. doi: 10.1113/jphysiol.2006.121483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutlin M., Ho C.Y., Abraira V.E., Cassidy C., Bai L., Woodbury C.J., Ginty D.D. The cellular and molecular basis of direction selectivity of Adelta-LTMRs. Cell. 2014;159(7):1640–1651. doi: 10.1016/j.cell.2014.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salter M.W., Dong Y., Kalia L.V., Liu X.J., Pitcher G. In: Biology of the NMDA Receptor. Van Dongen A.M., editor. CRC Press/Taylor & Francis; 2009. Regulation of NMDA Receptors by Kinases and Phosphatases. [PubMed] [Google Scholar]
- Sandrow-Feinberg H.R., Houle J.D. Exercise after spinal cord injury as an agent for neuroprotection, regeneration and rehabilitation. Brain Res. 2015;1619:12–21. doi: 10.1016/j.brainres.2015.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangameswaran L., Delgado S.G., Fish L.M., Koch B.D., Jakeman L.B., Stewart G.R., Sze P., Hunter J.C., Eglen R.M., Herman R.C. Structure and function of a novel voltage-gated, tetrodotoxin-resistant sodium channel specific to sensory neurons. J. Biol. Chem. 1996;271(11):5953–5956. doi: 10.1074/jbc.271.11.5953. [DOI] [PubMed] [Google Scholar]
- Sasaki, N., Kikuchi, S., Konno, S., Sekiguchi, M., & Watanabe, K. (2007). Anti-TNF-alpha antibody reduces pain-behavioral changes induced by epidural application of nucleus pulposus in a rat model depending on the timing of administration. Spine (Phila Pa 1976), 32(4), 413-416. https://doi.org/10.1097/01.brs.0000255097.18246.bc. [DOI] [PubMed]
- Sato K., Kiyama H., Park H.T., Tohyama M. AMPA, KA and NMDA receptors are expressed in the rat DRG neurones. Neuroreport. 1993;4(11):1263–1265. doi: 10.1097/00001756-199309000-00013. [DOI] [PubMed] [Google Scholar]
- Schafers M., Svensson C.I., Sommer C., Sorkin L.S. Tumor necrosis factor-alpha induces mechanical allodynia after spinal nerve ligation by activation of p38 MAPK in primary sensory neurons. J. Neurosci. 2003;23(7):2517–2521. doi: 10.1523/JNEUROSCI.23-07-02517.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoepp D.D., Jane D.E., Monn J.A. Pharmacological agents acting at subtypes of metabotropic glutamate receptors. Neuropharmacology. 1999;38(10):1431–1476. doi: 10.1016/s0028-3908(99)00092-1. [DOI] [PubMed] [Google Scholar]
- Schwaid A.G., Krasowka-Zoladek A., Chi A., Cornella-Taracido I. Comparison of the Rat and Human Dorsal Root Ganglion Proteome. Sci. Rep. 2018;8(1):13469. doi: 10.1038/s41598-018-31189-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seal R.P., Wang X., Guan Y., Raja S.N., Woodbury C.J., Basbaum A.I., Edwards R.H. Injury-induced mechanical hypersensitivity requires C-low threshold mechanoreceptors. Nature. 2009;462(7273):651–655. doi: 10.1038/nature08505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shembalkar P.K., Till S., Boettger M.K., Terenghi G., Tate S., Bountra C., Anand P. Increased sodium channel SNS/PN3 immunoreactivity in a causalgic finger. Eur. J. Pain. 2001;5(3):319–323. doi: 10.1053/eujp.2001.0251. [DOI] [PubMed] [Google Scholar]
- Shinder V., Govrin-Lippmann R., Cohen S., Belenky M., Ilin P., Fried K., Wilkinson H.A., Devor M. Structural basis of sympathetic-sensory coupling in rat and human dorsal root ganglia following peripheral nerve injury. J. Neurocytol. 1999;28(9):743–761. doi: 10.1023/a:1007090105840. [DOI] [PubMed] [Google Scholar]
- Shiokawa H., Kaftan E.J., MacDermott A.B., Tong C.K. NR2 subunits and NMDA receptors on lamina II inhibitory and excitatory interneurons of the mouse dorsal horn. Mol. Pain. 2010;6:26. doi: 10.1186/1744-8069-6-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddall P.J., McClelland J.M., Rutkowski S.B., Cousins M.J. A longitudinal study of the prevalence and characteristics of pain in the first 5 years following spinal cord injury. Pain. 2003;103(3):249–257. doi: 10.1016/S0304-3959(02)00452-9. [DOI] [PubMed] [Google Scholar]
- Sikandar S., Minett M.S., Millet Q., Santana-Varela S., Lau J., Wood J.N., Zhao J. Brain-derived neurotrophic factor derived from sensory neurons plays a critical role in chronic pain. Brain. 2018;141(4):1028–1039. doi: 10.1093/brain/awy009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider W.D., McMahon S.B. Tackling pain at the source: new ideas about nociceptors. Neuron. 1998;20(4):629–632. doi: 10.1016/s0896-6273(00)81003-x. [DOI] [PubMed] [Google Scholar]
- South S.M., Kohno T., Kaspar B.K., Hegarty D., Vissel B., Drake C.T., Ohata M., Jenab S., Sailer A.W., Malkmus S., Masuyama T., Horner P., Bogulavsky J., Gage F.H., Yaksh T.L., Woolf C.J., Heinemann S.F., Inturrisi C.E. A conditional deletion of the NR1 subunit of the NMDA receptor in adult spinal cord dorsal horn reduces NMDA currents and injury-induced pain. J. Neurosci. 2003;23(12):5031–5040. doi: 10.1523/jneurosci.23-12-05031.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sroga J.M., Jones T.B., Kigerl K.A., McGaughy V.M., Popovich P.G. Rats and mice exhibit distinct inflammatory reactions after spinal cord injury. J Comp Neurol. 2003;462(2):223–240. doi: 10.1002/cne.10736. [DOI] [PubMed] [Google Scholar]
- Strickland I.T., Martindale J.C., Woodhams P.L., Reeve A.J., Chessell I.P., McQueen D.S. Changes in the expression of NaV1.7, NaV1.8 and NaV1.9 in a distinct population of dorsal root ganglia innervating the rat knee joint in a model of chronic inflammatory joint pain. Eur. J. Pain. 2008;12(5):564–572. doi: 10.1016/j.ejpain.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Sun, L., Xian, H., Shi, Y., Yang, T., Shuai, H., Xia, R., Wen, T., Xia, W., Qian, R., Zhu, F., Liu, Y., Tian, Z., Li, L., Cong, R., Luo, C., Wu, S., Shen, X., Yu, X., Xie, R.-G., & Peng, C. (2022). Nav1.7 and Nav1.8 form supramolecular active clusters with TRKB in mouse and human DRG neurons during development of neuropathic pain. medRxiv, 2022.2011.2005.22281929. https://doi.org/10.1101/2022.11.05.22281929.
- Suzuki R., Matthews E.A., Dickenson A.H. Comparison of the effects of MK-801, ketamine and memantine on responses of spinal dorsal horn neurones in a rat model of mononeuropathy. Pain. 2001;91(1–2):101–109. doi: 10.1016/s0304-3959(00)00423-1. [DOI] [PubMed] [Google Scholar]
- Svensson C.I., Schafers M., Jones T.L., Powell H., Sorkin L.S. Spinal blockade of TNF blocks spinal nerve ligation-induced increases in spinal P-p38. Neurosci. Lett. 2005;379(3):209–213. doi: 10.1016/j.neulet.2004.12.064. [DOI] [PubMed] [Google Scholar]
- Tachibana M., Wenthold R.J., Morioka H., Petralia R.S. Light and electron microscopic immunocytochemical localization of AMPA-selective glutamate receptors in the rat spinal cord. J Comp Neurol. 1994;344(3):431–454. doi: 10.1002/cne.903440307. [DOI] [PubMed] [Google Scholar]
- Takei N., Sasaoka K., Inoue K., Takahashi M., Endo Y., Hatanaka H. Brain-derived neurotrophic factor increases the stimulation-evoked release of glutamate and the levels of exocytosis-associated proteins in cultured cortical neurons from embryonic rats. J. Neurochem. 1997;68(1):370–375. doi: 10.1046/j.1471-4159.1997.68010370.x. [DOI] [PubMed] [Google Scholar]
- Tan Y., Sun L., Zhang Q. Noradrenaline enhances ATP P2X3 receptor expression in dorsal root ganglion neurons of rats. Neuroscience. 2011;176:32–38. doi: 10.1016/j.neuroscience.2010.12.048. [DOI] [PubMed] [Google Scholar]
- Tao Y.X. Dorsal horn alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor trafficking in inflammatory pain. Anesthesiology. 2010;112(5):1259–1265. doi: 10.1097/ALN.0b013e3181d3e1ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tate S., Benn S., Hick C., Trezise D., John V., Mannion R.J., Costigan M., Plumpton C., Grose D., Gladwell Z., Kendall G., Dale K., Bountra C., Woolf C.J. Two sodium channels contribute to the TTX-R sodium current in primary sensory neurons. Nat. Neurosci. 1998;1(8):653–655. doi: 10.1038/3652. [DOI] [PubMed] [Google Scholar]
- Tavares-Ferreira D., Shiers S., Ray P.R., Wangzhou A., Jeevakumar V., Sankaranarayanan I., Cervantes A.M., Reese J.C., Chamessian A., Copits B.A., Dougherty P.M., Gereau R.W., Burton M.D., Dussor G., Price T.J. Spatial transcriptomics of dorsal root ganglia identifies molecular signatures of human nociceptors. Sci. Transl. Med. 2022;14(632):eabj8186. doi: 10.1126/scitranslmed.abj8186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temi S., Rudyk C., Armstrong J., Landrigan J.A., Dedek C., Salmaso N., Hildebrand M.E. Differential expression of GluN2 NMDA receptor subunits in the dorsal horn of male and female rats. Channels (Austin) 2021;15(1):179–192. doi: 10.1080/19336950.2020.1871205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson S.W., Bennett D.L., Kerr B.J., Bradbury E.J., McMahon S.B. Brain-derived neurotrophic factor is an endogenous modulator of nociceptive responses in the spinal cord. PNAS. 1999;96(14):7714–7718. doi: 10.1073/pnas.96.14.7714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmusk T., Palm K., Metsis M., Reintam T., Paalme V., Saarma M., Persson H. Multiple promoters direct tissue-specific expression of the rat BDNF gene. Neuron. 1993;10(3):475–489. doi: 10.1016/0896-6273(93)90335-o. [DOI] [PubMed] [Google Scholar]
- Timmusk T., Lendahl U., Funakoshi H., Arenas E., Persson H., Metsis M. Identification of brain-derived neurotrophic factor promoter regions mediating tissue-specific, axotomy-, and neuronal activity-induced expression in transgenic mice. J. Cell Biol. 1995;128(1–2):185–199. doi: 10.1083/jcb.128.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd A.J. Neuronal circuitry for pain processing in the dorsal horn. Nat. Rev. Neurosci. 2010;11(12):823–836. doi: 10.1038/nrn2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledo-Aral J.J., Moss B.L., He Z.J., Koszowski A.G., Whisenand T., Levinson S.R., Wolf J.J., Silos-Santiago I., Halegoua S., Mandel G. Identification of PN1, a predominant voltage-dependent sodium channel expressed principally in peripheral neurons. PNAS. 1997;94(4):1527–1532. doi: 10.1073/pnas.94.4.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torebjork H.E., Lundberg L.E., LaMotte R.H. Central changes in processing of mechanoreceptive input in capsaicin-induced secondary hyperalgesia in humans. J. Physiol. 1992;448:765–780. doi: 10.1113/jphysiol.1992.sp019069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usoskin D., Furlan A., Islam S., Abdo H., Lönnerberg P., Lou D., Hjerling-Leffler J., Haeggström J., Kharchenko O., Kharchenko P.V., Linnarsson S., Ernfors P. Unbiased classification of sensory neuron types by large-scale single-cell RNA sequencing. Nat. Neurosci. 2015;18(1):145–153. doi: 10.1038/nn.3881. [DOI] [PubMed] [Google Scholar]
- Walker S.M., Mitchell V.A., White D.M., Rush R.A., Duggan A.W. Release of immunoreactive brain-derived neurotrophic factor in the spinal cord of the rat following sciatic nerve transection. Brain Res. 2001;899(1–2):240–247. doi: 10.1016/s0006-8993(01)02259-4. [DOI] [PubMed] [Google Scholar]
- Wall P.D., Gutnick M. Properties of afferent nerve impulses originating from a neuroma. Nature. 1974;248(5451):740–743. doi: 10.1038/248740a0. [DOI] [PubMed] [Google Scholar]
- Walters E.T. Nociceptors as chronic drivers of pain and hyperreflexia after spinal cord injury: an adaptive-maladaptive hyperfunctional state hypothesis. Front. Physiol. 2012;3:309. doi: 10.3389/fphys.2012.00309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P., Chen F.X., Du C., Li C.Q., Yu Y.B., Zuo X.L., Li Y.Q. Increased production of BDNF in colonic epithelial cells induced by fecal supernatants from diarrheic IBS patients. Sci. Rep. 2015;5:10121. doi: 10.1038/srep10121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Gu J., Li Y.Q., Tao Y.X. Are voltage-gated sodium channels on the dorsal root ganglion involved in the development of neuropathic pain? Mol. Pain. 2011;7:16. doi: 10.1186/1744-8069-7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Sun H., Della Penna K., Benz R.J., Xu J., Gerhold D.L., Holder D.J., Koblan K.S. Chronic neuropathic pain is accompanied by global changes in gene expression and shares pathobiology with neurodegenerative diseases. Neuroscience. 2002;114(3):529–546. doi: 10.1016/s0306-4522(02)00341-x. [DOI] [PubMed] [Google Scholar]
- Wang K., Wang S., Chen Y., Wu D., Hu X., Lu Y., Wang L., Bao L., Li C., Zhang X. Single-cell transcriptomic analysis of somatosensory neurons uncovers temporal development of neuropathic pain. Cell Res. 2021;31(8):904–918. doi: 10.1038/s41422-021-00479-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe K., Larsson K., Rydevik B., Konno S., Nordborg C., Olmarker K. Increase of sodium channels (nav 1.8 and nav 1.9) in rat dorsal root ganglion neurons exposed to autologous nucleus pulposus. Open Orthop. J. 2014;8:69–73. doi: 10.2174/1874325001408010069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetmore C., Olson L. Neuronal and nonneuronal expression of neurotrophins and their receptors in sensory and sympathetic ganglia suggest new intercellular trophic interactions. J Comp Neurol. 1995;353(1):143–159. doi: 10.1002/cne.903530113. [DOI] [PubMed] [Google Scholar]
- White F.A., Bhangoo S.K., Miller R.J. Chemokines: integrators of pain and inflammation. Nat. Rev. Drug Discov. 2005;4(10):834–844. doi: 10.1038/nrd1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willcockson H., Valtschanoff J. AMPA and NMDA glutamate receptors are found in both peptidergic and non-peptidergic primary afferent neurons in the rat. Cell Tissue Res. 2008;334(1):17–23. doi: 10.1007/s00441-008-0662-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf C.J. Evidence for a central component of post-injury pain hypersensitivity. Nature. 1983;306(5944):686–688. doi: 10.1038/306686a0. [DOI] [PubMed] [Google Scholar]
- Woolf C.J. What is this thing called pain? J. Clin. Invest. 2010;120(11):3742–3744. doi: 10.1172/JCI45178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf C.J., Costigan M. Transcriptional and posttranslational plasticity and the generation of inflammatory pain. PNAS. 1999;96(14):7723–7730. doi: 10.1073/pnas.96.14.7723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf C.J., Ma Q. Nociceptors–noxious stimulus detectors. Neuron. 2007;55(3):353–364. doi: 10.1016/j.neuron.2007.07.016. [DOI] [PubMed] [Google Scholar]
- Woolf C.J., Mannion R.J. Neuropathic pain: aetiology, symptoms, mechanisms, and management. Lancet. 1999;353(9168):1959–1964. doi: 10.1016/s0140-6736(99)01307-0. [DOI] [PubMed] [Google Scholar]
- Woolf C.J., Salter M.W. Neuronal plasticity: increasing the gain in pain. Science. 2000;288(5472):1765–1769. doi: 10.1126/science.288.5472.1765. [DOI] [PubMed] [Google Scholar]
- Wright D.E., Snider W.D. Neurotrophin receptor mRNA expression defines distinct populations of neurons in rat dorsal root ganglia. J Comp Neurol. 1995;351(3):329–338. doi: 10.1002/cne.903510302. [DOI] [PubMed] [Google Scholar]
- Wu G., Ringkamp M., Hartke T.V., Murinson B.B., Campbell J.N., Griffin J.W., Meyer R.A. Early Onset of Spontaneous Activity in Uninjured C-Fiber Nociceptors after Injury to Neighboring Nerve Fibers. J. Neurosci. 2001;21(8) doi: 10.1523/JNEUROSCI.21-08-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G., Ringkamp M., Murinson B.B., Pogatzki E.M., Hartke T.V., Weerahandi H.M., Campbell J.N., Griffin J.W., Meyer R.A. Degeneration of myelinated efferent fibers induces spontaneous activity in uninjured C-fiber afferents. J. Neurosci. 2002;22(17):7746–7753. doi: 10.1523/jneurosci.22-17-07746.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie J.D., Chen S.R., Chen H., Zeng W.A., Pan H.L. Presynaptic N-Methyl-d-aspartate (NMDA) Receptor Activity Is Increased Through Protein Kinase C in Paclitaxel-induced Neuropathic Pain. J. Biol. Chem. 2016;291(37):19364–19373. doi: 10.1074/jbc.M116.732347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie W.R., Deng H., Li H., Bowen T.L., Strong J.A., Zhang J.M. Robust increase of cutaneous sensitivity, cytokine production and sympathetic sprouting in rats with localized inflammatory irritation of the spinal ganglia. Neuroscience. 2006;142(3):809–822. doi: 10.1016/j.neuroscience.2006.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie W., Strong J.A., Li H., Zhang J.M. Sympathetic sprouting near sensory neurons after nerve injury occurs preferentially on spontaneously active cells and is reduced by early nerve block. J. Neurophysiol. 2007;97(1):492–502. doi: 10.1152/jn.00899.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie W., Strong J.A., Zhang J.M. Increased excitability and spontaneous activity of rat sensory neurons following in vitro stimulation of sympathetic fiber sprouts in the isolated dorsal root ganglion. Pain. 2010;151(2):447–459. doi: 10.1016/j.pain.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie W., Strong J.A., Mao J., Zhang J.M. Highly localized interactions between sensory neurons and sprouting sympathetic fibers observed in a transgenic tyrosine hydroxylase reporter mouse. Mol. Pain. 2011;7:53. doi: 10.1186/1744-8069-7-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan X., Jiang E., Gao M., Weng H.R. Endogenous activation of presynaptic NMDA receptors enhances glutamate release from the primary afferents in the spinal dorsal horn in a rat model of neuropathic pain. J. Physiol. 2013;591(7):2001–2019. doi: 10.1113/jphysiol.2012.250522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Q., Rosenfeld R.D., Matheson C.R., Hawkins N., Lopez O.T., Bennett L., Welcher A.A. Expression of brain-derived neurotrophic factor protein in the adult rat central nervous system. Neuroscience. 1997;78(2):431–448. doi: 10.1016/s0306-4522(96)00613-6. [DOI] [PubMed] [Google Scholar]
- Yang Q., Wu Z., Hadden J.K., Odem M.A., Zuo Y., Crook R.J., Frost J.A., Walters E.T. Persistent pain after spinal cord injury is maintained by primary afferent activity. J. Neurosci. 2014;34(32):10765–10769. doi: 10.1523/JNEUROSCI.5316-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasko J.R., Moss I.L., Mains R.E. Transcriptional Profiling of Non-injured Nociceptors After Spinal Cord Injury Reveals Diverse Molecular Changes. Front. Mol. Neurosci. 2019;12:284. doi: 10.3389/fnmol.2019.00284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yezierski R.P., Yu C.G., Mantyh P.W., Vierck C.J., Lappi D.A. Spinal neurons involved in the generation of at-level pain following spinal injury in the rat. Neurosci. Lett. 2004;361(1–3):232–236. doi: 10.1016/j.neulet.2003.12.035. [DOI] [PubMed] [Google Scholar]
- Yoshimura M., Jessell T. Amino acid-mediated EPSPs at primary afferent synapses with substantia gelatinosa neurones in the rat spinal cord. J. Physiol. 1990;430:315–335. doi: 10.1113/jphysiol.1990.sp018293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanella, J. M., Burright, E. N., Hildebrand, K., Hobot, C., Cox, M., Christoferson, L., & McKay, W. F. (2008). Effect of etanercept, a tumor necrosis factor-alpha inhibitor, on neuropathic pain in the rat chronic constriction injury model. Spine (Phila Pa 1976), 33(3), 227-234. https://doi.org/10.1097/BRS.0b013e318162340a. [DOI] [PubMed]
- Zelenka M., Schafers M., Sommer C. Intraneural injection of interleukin-1beta and tumor necrosis factor-alpha into rat sciatic nerve at physiological doses induces signs of neuropathic pain. Pain. 2005;116(3):257–263. doi: 10.1016/j.pain.2005.04.018. [DOI] [PubMed] [Google Scholar]
- Zhang X., Huang J., McNaughton P.A. NGF rapidly increases membrane expression of TRPV1 heat-gated ion channels. EMBO J. 2005;24(24):4211–4223. doi: 10.1038/sj.emboj.7600893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W.B., Ross P.J., Tu Y., Wang Y., Beggs S., Sengar A.S., Ellis J., Salter M.W. Fyn Kinase regulates GluN2B subunit-dominant NMDA receptors in human induced pluripotent stem cell-derived neurons. Sci. Rep. 2016;6:23837. doi: 10.1038/srep23837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J.M., Strong J.A. Recent evidence for activity-dependent initiation of sympathetic sprouting and neuropathic pain. Sheng Li Xue Bao. 2008;60(5):617–627. [PubMed] [Google Scholar]
- Zhang J.M., Song X.J., LaMotte R.H. Enhanced excitability of sensory neurons in rats with cutaneous hyperalgesia produced by chronic compression of the dorsal root ganglion. J. Neurophysiol. 1999;82(6):3359–3366. doi: 10.1152/jn.1999.82.6.3359. [DOI] [PubMed] [Google Scholar]
- Zheng Y., Liu P., Bai L., Trimmer J.S., Bean B.P., Ginty D.D. Deep Sequencing of Somatosensory Neurons Reveals Molecular Determinants of Intrinsic Physiological Properties. Neuron. 2019;103(4):598–616.e597. doi: 10.1016/j.neuron.2019.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X.F., Rush R.A. Endogenous brain-derived neurotrophic factor is anterogradely transported in primary sensory neurons. Neuroscience. 1996;74(4):945–953. doi: 10.1016/0306-4522(96)00237-0. [DOI] [PubMed] [Google Scholar]
- Zhou X.F., Chie E.T., Deng Y.S., Zhong J.H., Xue Q., Rush R.A., Xian C.J. Injured primary sensory neurons switch phenotype for brain-derived neurotrophic factor in the rat. Neuroscience. 1999;92(3):841–853. doi: 10.1016/s0306-4522(99)00027-5. [DOI] [PubMed] [Google Scholar]
- Zhou X.F., Deng Y.S., Xian C.J., Zhong J.H. Neurotrophins from dorsal root ganglia trigger allodynia after spinal nerve injury in rats. Eur. J. Neurosci. 2000;12(1):100–105. doi: 10.1046/j.1460-9568.2000.00884.x. [DOI] [PubMed] [Google Scholar]
- Zimmerman A., Bai L., Ginty D.D. The gentle touch receptors of mammalian skin. Science. 2014;346(6212):950–954. doi: 10.1126/science.1254229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zylka M.J., Rice F.L., Anderson D.J. Topographically distinct epidermal nociceptive circuits revealed by axonal tracers targeted to Mrgprd. Neuron. 2005;45(1):17–25. doi: 10.1016/j.neuron.2004.12.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.