Abstract
Introduction:
Acute myocardial infarction (AMI) remains a leading cause of death in the United States. The limited regenerative capacity of cardiomyocytes and the restricted contractility of scar tissue after AMI are not addressed by current pharmacologic interventions. Mesenchymal stem/stromal cells (MSCs) have emerged as a promising therapeutic approach due to their low antigenicity, ease of harvesting, and efficacy and safety in preclinical and clinical studies, despite their low survival and engraftment rates. Other stem cell types, such as induced pluripotent stem cells also show promise and optimizing cardiac repair requires integrating these emerging technologies and strategies.
Areas covered:
This review offers insights into advancing cell-based therapies for AMI, emphasizing meticulously planned trials with a standardized definition of AMI, for a bench-to-bedside approach. We critically evaluate fundamental studies and clinical trials to provide a comprehensive overview of the advances, limitations and prospects for stem cell therapy in AMI.
Expert opinion:
MSCs show undeniable promise for treating AMI, but addressing their low survival and engraftment rates is crucial for clinical success. Integrating emerging technologies and well-designed trials will harness MSC therapy’s full potential in AMI management. Collaborative efforts are vital to developing effective stem cell therapies for AMI patients.
Keywords: Acute myocardial infarction, Mesenchymal stem cells, induced pluripotent stem cells, Cell-based therapy, Cardiac repair, Precision medicine, Exosomes
1. Introduction
Acute myocardial infarction (AMI) prominently figures as one of the principal drivers of death in the United States. Despite efforts involving interventional and pharmacological strategies, AMI still accounts for more than one hundred thousand deaths annually [1]. Every 40 seconds on average, an American will suffer a myocardial infarction (MI) [1]. The resulting heart failure accounts for almost 30% of the mortality in patients aged 65 and older suffering from this condition [1].
Thus, there is an unmet and urgent need for novel strategies to contain the injury to the cardiac cells following an AMI. Current management guidelines aim to contain progressive cardiac necrosis [2,3]. In cases of extensive damage, when the severity reaches a certain threshold, the only option remaining is a heart transplant, which holds significant challenges, such as host-recipient immune compatibility and the short supply of heart donors [4]. And even if reperfusion is achieved in a timely manner, myocardial damage and dysfunction of the microcirculation are still unavoidable complications [5]. Thus, strategies to repair/regenerate the myocardium and improve the surrounding ischemic environment are vital for achieving optimal cardiac functional recovery following an AMI. Stem cells have been extensively explored in rescuing the damaged micro-vessels and reinforcing myocardial healing mechanisms due to their limited immunogenic properties and differentiation.
Mesenchymal stem/stromal cells (MSCs) show anti-apoptotic, vasculogenic, and anti-inflammatory benefits and improve myocardial structure and function [6]. Despite findings on the safety and positive impact of stem cells in improving left ventricular ejection fraction (LVEF) [7], challenges arise from the limited survival and inadequate engraftment of the transplanted cells. induced pluripotent stem cells (iPSCs) are novel candidates with encouraging prospects, iPSCs represent great potential, but their long-term expansion and manufacturing costs hamper their clinical translation. This review will center on the pivotal mechanisms employed by MSCs in cardiac repair and provide an overview of recent investigations into cell therapy as a potential treatment approach for AMI.
2. Acute myocardial infarction: a loosely defined concept
The conventional definition of MI encompasses identification of acute myocardial injury, typically characterized by atypical heart biomarkers, and evidence of acute myocardial ischemia (i.e., ischemic chest symptoms and changes in electrocardiography pattern) [8]. An MI is triggered by the erosion or rupture of an underlying atheroma, resulting in thrombosis, and reduced coronary lumen diameter [9]. The ischemic myocardium activates molecular and cellular signaling, intense inflammatory responses, dysregulation of angiogenic pathways and cardiomyocyte necrosis in the hypoperfused area. The subsequent healing processes aims to resolve the inflammation and re-establish the integrity of the injured area [10] by developing a collagen scar to replace the necrotic tissue. This healing process ultimately leads to adverse structural and mechanical changes known as ventricular remodeling to adapt to the changes in the myocardial mechanical strength of the left ventricle (LV). Cardiac remodeling yields thinned LV wall with less muscle mass, dilated ventricle and compromised cardiac contractility [11].
A myocardial injury is considered acute only when accompanied by changes in bio-markers of myocardial necrosis values, namely cardiac troponin I (cTnI) and cardiac troponin T (cTnT) [8]. After an MI, cardiac troponins reach a maximum concentration in the bloodstream at 10 to 20 hours after onset of acute ischemia in patients receiving reperfusion interventions or at 24 to 50 hours in non-treated patients. The concentrations of cTnI and cTnT directly correlate with the injury area in those patients without reperfusion therapy [12].
Criteria for diagnosing MI also include alterations in the ST segment observed by electrocardiography. Broadly, MI can be subclassified into two main subtypes: ST-elevation myocardial infarction (STEMI) and non-ST-elevation myocardial infarction (NSTEMI) [13]. MI classification is also based on histologic observation of characteristic cells at each phase of the repair process. The entire process of achieving a healed phase takes ∼5 to 6 weeks [14]. A less precise definition categorizes MI based on distinct phases of histologic features, namely acute (occurring within 6 hours to 7 days), healing (occurring between 7 and 28 days), and healed (occurring 29 days or more) from the onset of the initial injury [8]. Based on AMI interventional strategies, patients seen within 12 hours or between 12 to 48 after symptom onset are early or late presenters, respectively. According to the International Statistical Classification of Diseases and Related Health Problems 10th Revision, acute myocardial infarction is classified when it occurs within a period of 4 weeks from the onset [15]. The clinical and electrocardiographic timing of acute infarction onset may not necessarily match with histologic characteristics. Thus, the definition of AMI varies depending on the study approach.
The inherent ability of the human heart to self-renew is limited, and the rate of cardiomyocyte turnover decreases progressively through the lifespan. Only ∼60% of the cardiomyocytes present at birth will survive to the age of 50 [16]. The search for new approaches to reverse the loss of cardiac tissue has motivated the development of cardiovascular regenerative medicine (CRM) [10]. CRM represents a cutting-edge approach within the medical field, aiming to repair damaged heart tissue through strategies such as tissue engineering, cell and gene therapy. The Transnational Alliance for Regenerative Therapies in Cardiovascular Syndromes is an international consortium dedicated to advancing CRM by fostering global collaboration, setting unified standards, and translating scientific research into effective clinical applications for treating cardiovascular diseases [17].
3. The search for the best cell
The search for the optimal cell type in cell-based regenerative medicine for acute MI is a critical area of investigation to identify the cell type that offers the most significant regenerative potential and therapeutic benefits. Numerous cell types have been explored. Each cell type possesses distinct characteristics and mechanisms of action, making it crucial to identify the optimal cell source for effective cardiac repair and regeneration. Cell types used in treating AMI both clinically and pre-clinically include skeletal myoblasts [18], bone marrow mononuclear cells (BMMNCs) [19], cardiac progenitor cells [20,21], cardiosphere-derived cells [22], MSCs, embryonic stem cells (ESCs) [23], and iPSCs [24]. These studies have exhibited considerable variation in efficacy and while it is unclear which cell(s) will ultimately prove the most efficacious, here we will focus primarily on MSCs and iPSCs while emphasizing the necessity for additional research in this domain.
Skeletal myoblasts were used in pioneer preclinical and clinical trials in CRM. Skeletal muscle contains an intrinsic reserve of tissue-committed cells able to proliferate, differentiate and merge with existing myocytes to regenerate the muscle when recruited to the injured tissue. The initial excitement toward these cells was motivated by their high culture scalability, immune safety, and strong resistance under ischemic conditions. However, skeletal myoblasts are also characterized by lineage restriction, which limits their potential to differentiate into new cardiomyocytes [25]. Early studies of these cells also reported an increased risk of arrhythmias attributed to the limited cell electrical synchronization activity. with the native myocardial cells [26]. Findings from the Myoblast Autologous Grafting in Ischemic Cardiomyopathy (MAGIC) trial demonstrated the safety of skeletal myoblasts. Interestingly, the MAGIC trial provided evidence supporting the concept that transferred skeletal myoblasts convey a therapeutic paracrine significance in the short term rather than any long-term structural value [27].
Cell-based therapy for heart regeneration moved forward to explore BMMNCs, a heterogenous cell subset consisting mainly of mature hematopoietic lineage cells but also encompassing hematopoietic stem cells (HSCs), endothelial progenitor cells (EPCs) and MSCs. HSCs are remarkable capable to derive into all cell types withing the blood lineage [28]; but their application is limited by their limited supply requiring further ex vivo strategies for their expansion. EPCs constitute a pro-angiogenic subset of HSCs that share various CD surface markers, including CD133 and CD34 [29]. Their potential clinical significance arises from their capacity to stimulate angiogenesis in ischemic tissues, albeit with poor cardiac function improvement [30].
Most data on stem cell therapy in AMI has been collected from studies performed using BMMNCs. BMMNCs as a treatment for AMI in the first human patient [31] set the standard for the following protocols demonstrating long-term cardiac functional enhancement in STEMI patients [32]. The Reinfusion of Enriched Progenitor cells And Infarct Remodeling in Acute Myocardial Infarction (REPAIR-AMI) Phase II trial conducted in 2006 indicated that intracoronary administration of BMMNCs resulted in enhanced LV function and reduced major adverse cardiovascular events, such as mortality and rehospitalization during long-term follow up after AMI, supporting the potential of stem cells in promoting myocardial regeneration [19]. Further studies attempted to confirm these results but lacked the power necessary to demonstrate a clear benefit [33–35]. The largest trial investigating autologous BMMNCs in patients with acute myocardial infarction, the BAMI Phase III clinical trial, focused on long-term clinical outcomes over a period of 2 years in individuals with acute ST-elevation myocardial infarction treated with BMMNC [36]. Despite its extensive scope, the BAMI Phase III clinical trial failed to assess the efficacy of autologous BMMNC treatment in improving survival rates or reducing major adverse cardiovascular events, providing valuable insights into the durability and overall impact of stem cell therapy. The BAMI trial was limited by a markedly low patient recruitment and a low all-cause mortality rate; perhaps reflecting the successful implementation of timely interventional strategies, rather than the efficacy of stem cell transplantation [37].
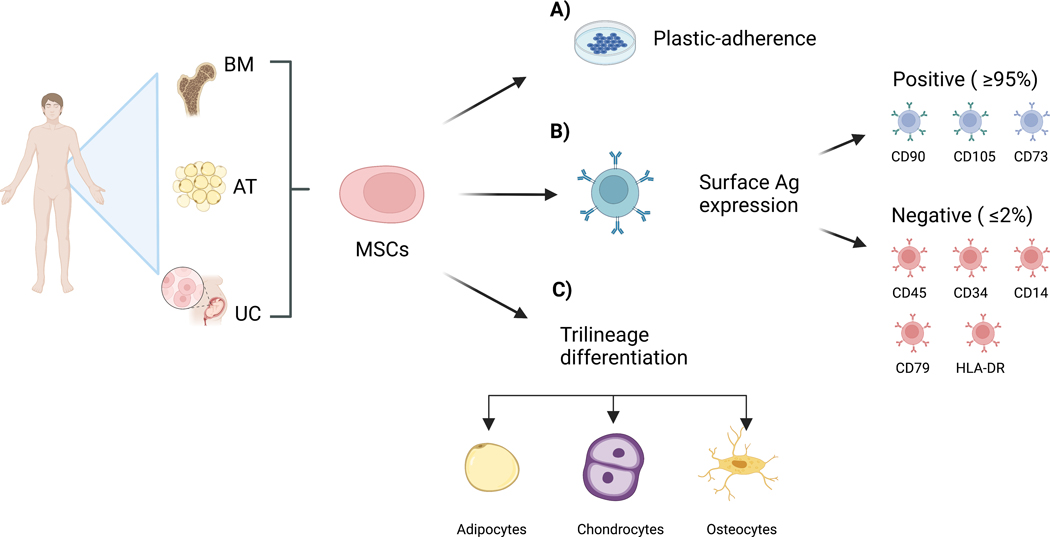
While the therapeutic potential of BMMNC was extensively studied, the following research shifted its focus towards the promising capabilities of MSCs, offering a broader scope of applicability in regenerative medicine. MSCs are a population of self-renewing and multipotent cells that are found in virtually all types of tissues, and were initially discovered from the bone marrow of guinea pigs [38]. In humans, they are primarily expanded from adipose tissue [39], bone marrow [40] and umbilical cord, Wharton’s jelly [41]. The International Society for Cellular Therapy (ISCT) has defined MSCs (1) as plastic adherent, (2) specific expression of surface antigen (Ag) and (3) the potential for trilineage differentiation under in vitro conditions (Figure 1) [42].
Figure 1.
MSCs can be harvested from a wide variety of tissues, including bone marrow (BM), adipose tissue (AT) and umbilical cord (UC). They are, a) plastic-adherent, b) express specific surface antigens, c) can differentiate into adipocytes, chondrocytes, and osteocytes. Ag (Antigen), CD (Cluster of differentiation). Created with BioRender.com
MSCs retrieved from diverse sources may vary in their differentiation potential, cell surface markers and paracrine signaling. For example, adipose stem cell lineage-specific surface markers. like CD34, vary during cell division, engendering different subsets of cells [39]. ASCs are readily obtained from the stromal-vascular fraction under local anesthesia in large quantities from lipoaspirates by enzymatic or non-enzymatic dissociation [43]. These cells can differentiate into the main cardiac cell lineages: cardiomyocytes (CMs), ECs, and smooth muscle vascular cells [44]. Bone marrow derived stem cells (BM-MSCs) represent a heterogenous subgroup found in the medullary stroma of bone marrow. Their high-grade immunosuppressive activity [45] makes them appealing prospects for cytotherapy. Adding to the burgeoning body of research investigating the safety and efficacy of cellular therapies in cardiac care, an early landmark double-blind, placebo-controlled study, the Prochymal study [46]. It reported comparable adverse event rates between hMSC- and placebo-treated cohorts and improved global symptom scores and ejection fraction, particularly among those with anterior MI, compared to their placebo-treated counterparts, supporting the concept that intravenous administration of allogeneic hMSCs is a safe and potentially efficacious therapeutic strategy for patients suffering from MI [46].
Pluripotent stem cells include ESCs and iPSCs. ESCs were initially generated from murine [47] and later human blastocysts [48]. ESCs can self-renew and commit towards cell types deriving from all the germ layers, including CMs [49]. Promising pre-clinical studies of human embryonic stem cell-derived cardiomyocytes (hESC-CMs) transplantation showed their potential for cardiac regeneration, by reducing scar size and restoring cardiac function [50]. However, clinical applications of hESC-CMs are limited by their genetic instability, immunogenic features, tumorigenic potential, and ethical issues [51]. Graft-related arrhythmia is a common adverse effect reported in most of the studies following stem cells transplantation and is attributed to unresolved cell heterogenicity. Liu et al. provided significant insights into understanding the underlying mechanisms of arrhythmogenesis, suggesting that graft-induced arrhythmias are likely due to an ectopic impulse generation rather than abnormal conduction, as was initially hypothesized [50].
A breakthrough in regenerative medicine took place in 2006 when iPSCs were successfully generated in a murine model, in which specific somatic cells were reprogrammed back into a pluripotent stage through retroviral-mediated transduction of a well-defined set of the pluripotency factors, Oct3/4, Sox2, Klf4, and c-Myc [52]. Soon after, iPSCs were successfully obtained from human cells and, subsequently, patient cells were reprogrammed using this novel approach [53]. Similar to ESCs, iPSCs exhibit a broad differentiation plasticity [54] and are considered a promising source for autologous therapy [53]. A breakthrough in regenerative medicine took place in 2006 when iPSC were successfully generated in a mouse model, in which specific somatic cells were reprogrammed back to a pluripotent stage through retroviral-mediated transduction of a well-defined set of the pluripotency factors Oct3/4, Sox2, Klf4, and c-Myc [52]. A year later, researchers successfully obtained iPSCs from human cells and, subsequently, patient cells were reprogrammed using this novel approach [53]. Similar to ESCs, these iPSCs exhibit a broad differentiation plasticity [54] and are considered a promising source for autologous therapy [53].
The therapeutic potential of iPSCs to treat AMI was initially demonstrated using murine fibroblasts reprogrammed using the human stemness factors Oct3/4, Sox2, Klf4, and c-Myc. Compared to the parental fibroblasts, the resulting reprogrammed iPSC clones displayed spontaneous engraftment and efficient repair of damaged myocardium following intramyocardial administration into both immunodeficient and immunocompetent recipients. However, teratomas were found in the immunodeficient animals, an early indication that the risk associated with iPSC-based interventions significantly depends on the precise state of lineage differentiation and its intricate interaction with the host’s surrounding environment [55]. iPSCs can differentiate into functional CMs [56], ECs [57], and smooth muscle cells [58], paving the way for promising therapeutic applications in cardiac regeneration. Some studies showed that iPSCs could differentiate and exhibit a cardiac phenotype when delivered to the damaged heart [59]. However, structural, molecular, metabolic, and functional analyses of iPSC-derived cardiomyocytes showed an immature phenotype resembling cells at an embryonic stage [60], although attempts to prompt these cells to differentiate towards a more mature adult-like phenotype are ongoing [61–63].
Patient-specific iPSCs offer significant advantages over ESCs, bypassing ethical concerns and the same genetic makeup as the patient, offering opportunities to develop patient-specific treatments. In addition, patient-specific iPSCs were assumed to be immunologically safe but they can still trigger an immune response in vivo [64], [65], while the risk of developing teratomas have raised significant safety concerns [55,66].
4. Mesenchymal stem cells: mechanisms of action
The initial predicted role of stem cells in promoting cardiac repair and regeneration was by replacing damaged CMs with new functional cells [67]. While stem cells can be generated to develop into CM in vitro and in vivo by cultivating them under particular conditions, such as chemicals, growth factors, applied mechanical load, and co-culturing them with different cell populations [68,69], studies have failed to produce stem cells with a mechanical and electrophysiological phenotype suitable to fuse with the native tissue and fully perform cardiac functions [70]. Furthermore, a very low percentage of MSCs engraft and differentiate [71,72], prompting controversy as to the extent to which this mechanism influences cardiac repair.
The current consensus is that MSCs exert their beneficial properties through a paracrine effect mediated by secreted biomolecules, termed the secretome [73,74]. The secretome consists of factors that are “free-floating” or packed into small vesicles that are released into the surrounding microenvironment. The soluble fraction, contained within Conditioned Medium in vitro, is predominantly constituted by cytokines, chemokines, gene products, ECM proteases, and proliferating factors. Intramyocardial injection of BM-MSC conditioned medium overexpressing protein kinase B (Akt) produced improvement of LV function without any evidence of de novo cardiomyogenesis when administered during the early stage of MI, in small [75] and large animal models [76].
The vesicular fraction of the secretome consists of extracellular vesicles (EVs). EVs are nano-sized, lipid-membrane vesicles classified into exosomes (Exos; 40 to 100 nm), microvesicles (100 to 1000 nm), and apoptotic bodies (1 to 5 μm) [77,78]. Most cell types secrete Exos, and their cargo contains a combination of proteins and nucleotides [79]. The exosomes are developed along with the internal growth of the endosomal membrane and mature gradually until they are released extracellularly within a cellular structure identified as Multi-Vesicular Bodies (MVBs) [80,81]. Exos are essential intercellular messengers involved in transmitting biological signals. Intercellular message delivery is orchestrated through the controlled release of Exo cargo into the surrounding environment [82].
5. The role of MSCs in cardiac repair
The combined effects of stem cells’ immunomodulatory, angiogenic, and anti-fibrotic properties create a multifaceted approach to restoring cardiac function after AMI. By harnessing these mechanisms, stem cell therapy has shown promise in preclinical and clinical studies, with evidence of improved cardiac function, reduced scar formation, and improved neo-angiogenesis in the infarcted myocardium. Further understanding of the stem cells’ intricate interactions within the cardiac milieu will facilitate the development of more effective and directed therapies for AMI.
5.1. Immunosuppression
The role of MSCs in myocardial regeneration involves immunomodulation, anti-fibrotic features, and the ability to restore the damaged capillary network. At the onset of MI, an inadequate circulation supply induces massive death of cardiomyocytes and other cells crucial to cardiac function, eliciting an inflammatory response [83]. The interaction between Toll-like receptors and damage-associated molecular patterns that perform as “danger signals” during ischemia sets off the activation of the immune response [84]. The response of the immune system in the host impacts the prognosis of the ischemic heart. Progressive impairment of cardiac function and intensified cardiac remodeling are associated with excessive and persistent inflammation [85].
MSCs are considered immuno-privileged. Due to their lack of human leukocyte antigens (HLA) class II surface marker expression these cells bypass detection and clearance by the host immunity, opening the opportunity of considering MSCs suitable for allogenic therapy. The POSEIDON clinical trial proved that allogenic (allo) BM-derived human mesenchymal stem cells (hMSCs) were safe 12 months after transplantation in the context of non-ischemic dilated cardiomyopathy [86–88].
Human iPSC-derived MSCs exhibit superior immune privilege than BM-derived MSCs, being insensitive to the expression of interferon (IFN)-γ-induced HLA class II [89]. However, when iPSC-CMs are administered via intra-myocardial transplantation into non-human primates, the animals required concomitant immunosuppression with tacrolimus and methylprednisolone for cell survival [90].
MSCs are synchronized, potent immune modulators and immune suppressors able to exert their effects through multiple local and systemic pathways [91,92]. Paracrine signaling is the primary mechanism of immune modulation. MSC transplantation induces the downregulation of interleukin 1 (IL)-1, IL-6, and tumor necrosis factor (TNF)-α, resulting in reduced apoptosis of myocardial cells and substantial enhancement of heart function in murine models of myocardial infarction [93]. Remarkably, these properties are not considered to be an intrinsic feature of MSCs but rather a response to the adjacent milieu, particularly the severity and specific cell mediators involved in the inflammatory stimuli [94], i.e., the surrounding microenvironment influences MSC immune-regulatory properties by inducing them to assume an immune-suppressive phenotype when exposed to increased pro-inflammatory cytokine secretion [95].
Regulating the inflammatory response can be accomplished by modulating the activity of numerous cells participating in the process, including macrophages, T-lymphocytes (T-cells), and natural killer (NK) cells [96]. Within hours after acute ischemia of the myocardium, neutrophils are recruited in the injured cardiac area and exert many biological functions, i.e., they interact with apoptotic and necrotic cells propagating the inflammation. Next, monocytes relocate to the affected area where they differentiate into macrophages. In the ischemic myocardium, there are two types of macrophages, classified upon macrophage polarization. In the early stages of a myocardial infarction, M1 macrophages clear the debris, release pro-inflammatory cytokines, and initiate the immune response. M2 macrophages develop days after the onset of an AMI, exhibiting inflammation-suppressing characteristics and mitigating inflammation-promoting cytokines [97]. In addition, they promote cardiac regeneration by inducing angiogenesis and cell proliferation during scar formation [98]. Macrophages can be educated to regulate their polarization. In the presence of prostaglandin E2, MSCs can promote macrophage polarization toward an M2 phenotype that has greater scavenging/phagocytic activity and more active interactions with NK cells, i.e., suppressive effects on the adaptive and innate immune response [99].
Among all the immunomodulatory mechanisms effected by MSCs, it is worth noting their interaction with three primary lymphocyte types: cytotoxic T cells (CD8+), T helper cells (CD4+), and NK cells, as MSCs exert inhibitory effects on T-cell growth and activation while inducing apoptosis of T-helper and cytotoxic-T cells. T-cell suppression may be achieved directly by both intercellular interactions and by paracrine-mediated release of soluble mediators [100] such as Kynurenine (Kyn), the major metabolite of the amino acid tryptophan (Trp), which plays a pro-inflammatory role in the ischemic heart. Kyn metabolites increase oxidative damage and apoptosis in smooth muscle and endothelial cells. Indoleamine 2, 3-dioxygenase (IDO), is a rate-limiting enzyme that catalyzes the breakdown of Trp into Kyn. IDO inhibits T-cell proliferation and stimulates T-cell death [101]. MSCs co-cultured with T-cells, exhibit increased IDO expression, ultimately producing suppression of T-cell proliferation [102]. Due to their capacity to limit T-cell growth, MSCs were clinically implemented for treating therapy-resistant graft-versus-host disease (GvHD) [103]. Inflammatory-suppressing cytokines, such as IL-10 and transforming growth factor (TGF)-β, stimulate generation of regulatory T-cells (Treg). Cardiac Tregs are a protective subgroup of T-cells with acquired innate immune privilege and are able to shorten the pro-inflammatory phase, enhancing the shift from the initial inflammation-mediated stage to the regenerative phase at the injury site [104]. The function of NK cells is relevant in the ischemic myocardium, originating an intense inhibitory cytolytic function through multiple cytokines including Prostaglandin E2 (PGE2), TGF-β1, and IFN-γ. Adult stem cells can suppress the upregulation of NK cell-activating receptors NKp30, NKp44, and NKG2D by releasing PEG2 and IDO [105]. When hiPSC-MSCs are systemically transplanted intramyocardially prior to the induction of an MI in a murine model, the number of NK cells decreases, promoting the viability of the hiPSC-CMs, attenuating LV remodeling [106].
5.2. Angiogenesis
The expansion of the infarct border zone (BZ) is defined by the density of the capillary system that surrounds it. As infarction progresses, the perivascular fibrosis of the coronary circulation limits nutrient and oxygen to the heart [107]. The capillaries are then unable to sustain the cardiac overload. An impaired cardiac circulation is associated with decreased coronary flow reserve and poor clinical prognosis [108]. Thus, re-establishing the capillary system is essential for an optimal myocardial recovery. Therapeutic neovascularization comprises coronary angiogenesis and vasculogenesis, i.e., the generation of new vascular networks from existing ECs [109] or de novo formation from ECs, EPCs or alternative stem cells populations, respectively [110].
MSCs have previously been extensively explored in vitro and in vivo. They can engraft into the native myocardium and promote neovascularization by trans-differentiation into endothelial and smooth muscle cells [71,72]. Stem cells obtained from AT exhibit stronger proliferation potency and differentiate more rapidly than BM-MSCs [111]. ASCs improve capillary density in the BZ when transplanted into rats [112], improve cardiac function in pigs [113] and coronary perfusion in clinical trials [114].
Although stem cells can differentiate into ECs upon transplantation into the site of injury, enhancement of neovascularization is due mainly to the activation of paracrine secretion of promoting angiogenic factors, and matrix remodeling enzymes, like matrix metalloproteases (MMPs) [115,116]. MSCs exhibit extensive therapeutic potential primarily driven by their potential to secrete paracrine factors, such as stromal-derived factor (SDF)-1α, and suppress inflammatory markers, notably TNF-α. The SDF-1α, secreted by MSCs, supports endothelial function through the SDF1/CXCR4 pathway, enhancing chemotaxis and angiogenesis, which are fundamental to the restoration and regeneration of damaged tissues. This role of SDF-1α has been observed in the context of dilated cardiomyopathy (DCM), where MSCs have shown to enhance endothelial performance, with allogeneic MSCs demonstrating a more significant enhancement than autologous MSCs 3 months post-injection. Interestingly, this difference in efficacy is associated with the higher SDF-1α secretion of autologous MSCs compared to their allogeneic counterparts, which align with a decrease in serum TNFα concentrations in patients suffering of DCM treated with MSCs. These findings bear significant implications for the role of MSCs in treating acute MI, suggesting that allogeneic MSCs could be more effective in improving endothelial function. Thus, therapeutic strategies targeting endothelial function, potentially influenced by SDF-1α secretion, could hold significant value for the management of patients with cardiovascular diseases [117].
Potent pro-angiogenic cytokines include insulin-like growth factor (IGF)-1, basic fibroblast growth factor (bFGF), hepatocyte growth factor (HGF) and vascular endothelial growth factor (VEGF) [118]. Among these growth factors, the VEGF superfamily is a crucial mediator in upregulating angiogenesis via the phosphoinositide 3-kinase (PI3K)/AKT and rat sarcoma virus (RAS)/rapidly accelerated fibrosarcoma (RAF)/mitogen-activated protein kinase (MEK)/extracellular signal-regulated kinase (ERK) [119,120] signaling pathways. Following intramyocardial administration of BM-MSCs, VEGF upregulation of endogenous angiogenic factors, resulting in increased capillary density in the infarcted tissue [121].
Stem cells from different sources exhibit variable pro-angiogenic features. For example, ASCs secrete significantly higher levels of VEGF and HGF in vitro than BM-MSCs [112]. The capacity of hiPSCs to facilitate neovascularization and angiogenesis is of key interest in treating MI. iPSC-ECs secrete pro-angiogenic factors, including bFGF and VEGF, at comparable levels to ECs [122].
Interactions between ECs and the ECM are necessary to produce pro-angiogenic factors. MMPs critically participates in angiogenesis by modulating the capillary diameter and stabilizing emerging vessels [123]. MSCs derived from AT exert pro-angiogenic effects on ECs through the distinctive plasminogen activator/plasmin axis, which serves as the principal mechanism for vessel invasion and elongation within fibrin matrices [124].
iPSCs also promote angiogenesis, and recent studies have shed light on the regenerative capabilities of iPSC-derived fetal liver kinase-1 (Flk-1) progenitor cells for enhancing cardiac performance. Flk-1, also known as vascular endothelial growth factor receptor 2 (VEGFR-2), functions as a cell surface molecule associated with early cardiovascular progenitor cells. iPSC-Flk-1 progenitor cells, can differentiate into functional cardiovascular lineages, including CMs, ECs, and smooth myocytes, in vitro and in a murine model of AMI in vivo. This process stimulated neovascularization, enhanced blood flow to the injured region, and promoted tissue repair, preventing adverse remodeling, reduced infarct size and improved LV wall thickness, ultimately improving cardiac function [125]. Overall, iPSC-derived Flk-1 progenitor cells represent an appealing strategy to promote cardiac repair and enhance patient outcomes in AMI [125].
In addition to Flk-1, ETV2 deserves attention in the context of angiogenesis. ETV2, a member of the ETS transcription factor family, plays an important role in regulating the process of angiogenesis and has the remarkable ability to reprogram somatic cells into functional endothelial cells. A recent study suggests that iPSCs can be reprogrammed into ECs in vitro by briefly expressing ETV2, without VEGF expression [126].
5.3. Cell survival and apoptosis
Oxidative stress, arises from an uneven equilibrium between reactive oxygen species and antioxidants, is a common phenomenon observed during AMI that contributes to massive cell death and cardiac tissue damage. Recent studies show that transplanted MSCs and iPSCs can mitigate oxidative stress by activating specific cell survival pathways [127].
Diverse types of cellular demise have been elucidated in response to the ischemic injury, including apoptosis, necrosis, and pyroptosis [128]. The PI3K/Akt signaling cascade is critical for mediating cellular responses to ischemic injury, promoting cell survival, and reducing cell death by activating anti-apoptotic and anti-inflammatory pathways. Akt-modified MSCs promoted cardiomyocyte survival while reducing apoptosis under hypoxic conditions [129]. Similarly, transplanted human iPSCs could activate the Akt pathway in the ischemic myocardium, promoting cell survival and reducing tissue injury [130]. Co-expression of Akt1 and Wnt11, another relevant cellular pathway involved in tissue regeneration and cell migration, promotes stem cell proliferation and cardiac differentiation [131].
5.4. Fibrosis
Necrotic cardiomyocytes are replaced with fibroblasts, the main cellular component involved in MI remodeling and scar formation. Myocardial fibrosis causes contractility dysfunction by stiffening the myocardium [132]. Removal of debris and its replacement by connective tissue is observed approximately 5 weeks after AMI, and collagen deposition is complete approximately three months after AMI [133].
Stem cells have emerged as potential anti-fibrotic agents. Administration of HGF has shown remarkable anti-fibrotic effects, when transplanted into the BZ, MSCs promote secretion of HGF through cell-to-cell crosstalk. Promoting CM proliferation inhibits the fibrotic response after injury and reduces scar size. The Prospective Randomized Study of Mesenchymal Stem Cell Therapy in Patients Undergoing Cardiac Surgery (PROMETHEUS), is a landmark clinical trial investigating the efficacy of MSCs administered via trans-epicardial route in patients diagnosed with akinetic yet non-revascularized segments of the heart, resulting in decreased fibrosis, enhanced cardiac contractile function, and improved blood perfusion [134].
5.5. Cell cycle
The enhanced understanding of the complex signaling pathways governing the cell cycle of cardiomyocytes (CMs) is opening new avenues for creating novel approaches in the discipline of heart regeneration and repair. The cell cycle progression is governed by the regulation of cyclins and cyclin-dependent kinases (CDKs). The cyclin-D (CCND) family, comprising cyclin D1, D2, and D3 (CCND 1/2/3), is the most promising to induce CM proliferation. Following myocardial injury, CCND1 and CCND3 are found in the cytosol, and only CCND2 was retained within the nucleus, leading to enhanced synthesis of DNA and substantial myocardial regeneration [135].
The G1-S restriction point is mediated by CDK type 2 (CDK2) and type 4 and their co-factors, together with the CCND family members [136]. It is proposed that expression of cyclin D2 (CCND2) in CMs helps preserve heart performance by restoring some of the lost myocardium [137]. To address this concept, hiPSC-CMs with CCND2 driven by an α-myosin heavy chain (α-MHC) promoter were introduced into the LV myocardium of ischemic rodents. The transplantation of these CCND2-overexpressing Cardiomyocytes (hiPSC-CCND2OECMs) resulted in extensive areas of engraftment, a substantial reduction the size of the infarct, and improved left ventricular efficiency. Moreover, hiPSC-CCND2OECM–treated hearts also exhibited increased activation of paracrine mechanisms, promoting angiogenesis [137].
Further studies conducted in swine model of MI confirmed the regenerative potentiality of hiPSC-CCND2OECM as demonstrated by significant improvements in cardiac performance and reduced fibrosis. Furthermore, the hiPSC-CCND2OECMs proliferated from the first week throughout the fourth week after transplantation into the myocardium and promoted the proliferation of the host CMs, ECs, and SMCs. Interestingly, there were no reported instances of ventricular arrhythmias related to the treatment after 4-week follow-up, suggesting that CMs with enhanced proliferation capacity represent an effective and safe approach for myocardial repair [138].
Earlier research has shown that miR-302b-3p and miR-373–3p control the growth of human iPSC-derived cardiomyocytes through the HIPPO signaling pathway. Both miRNAs inhibit the expression of key components of the HIPPO pathway that leads to in enhanced hiPSC-CM proliferation. This research pinpointed the porcine LATS2 mRNA as an immediate target of hsa-miR-302b-3p, a microRNA assumes a pivotal function in regulating stem cell activities.in regulating stem cell growth and differentiation. The binding of hsa-miR-302b-3p to the 3’ untranslated region of LATS2 mRNA results in decreased LATS2 expression, a pivotal controller of the HIPPO signal transduction pathway [138]. Together these findings contribute significantly to alternative strategies for promoting hiPSC-CM differentiation and proliferation in cardiac regeneration.
6. Strategies for effective cell delivery
Optimum delivery technologies in CRM aim to provide a sufficient cellular dosage necessary to effect beneficial changes in the targeted site, achieving maximal retention while presenting a minimum risk for patients. Despite decades of research and various delivery modalities for regenerative products showcasing different degrees of ease of use, safety, clinical usefulness, and affordability [17], myocardial cell delivery remains constrained due to the heart’s anatomical complexity and difficult accessibility, coupled with the high risk of complications associated with surgical interventions. Thus, approaches that are minimally invasive and highly effective represent a challenge (Figure 2). A comprehensive meta-analysis of preclinical and clinical trials has compellingly demonstrated that the delivery route significantly influences the therapeutic efficacy of MSC in treating acute MI [139].
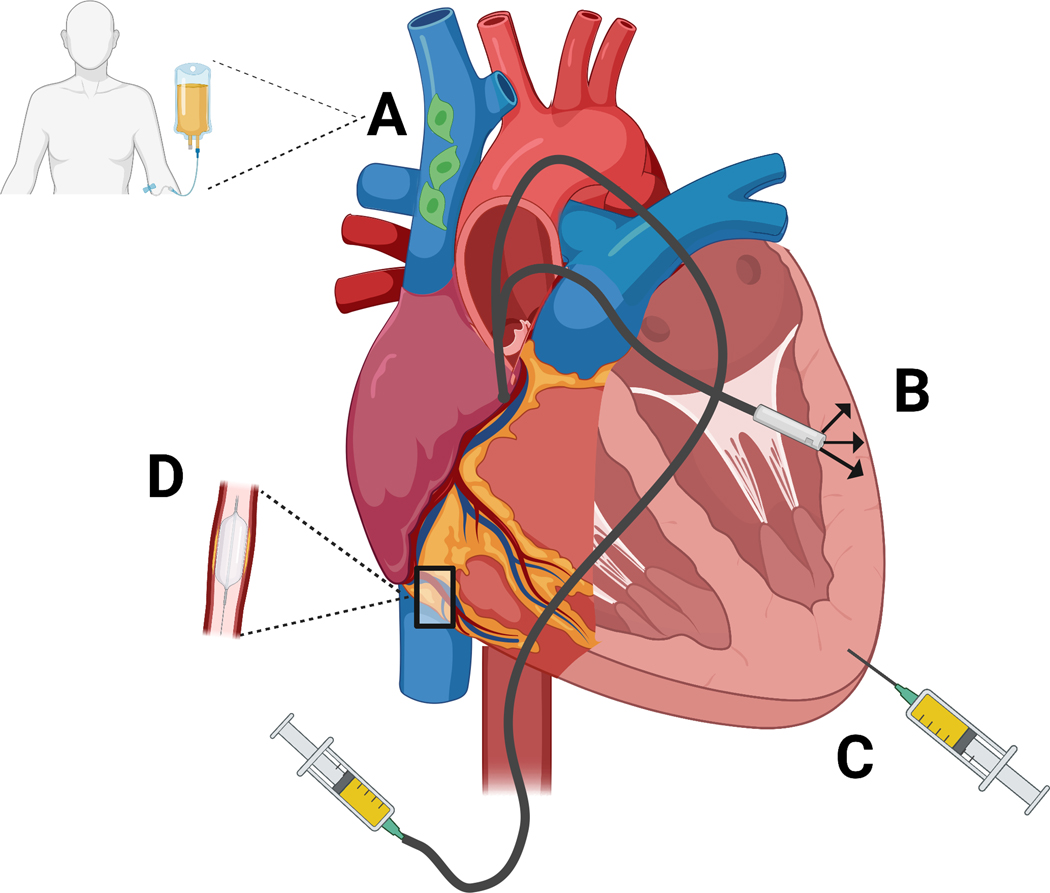
Figure 2.
Stem cell delivery approaches for AMI. A: Intravenous delivery (peripheral veins not shown). B: Transendocardial stem cell injection (TESI) via catheter. C: Epicardial injection. D: Catheter-based intracoronary infusion. Adapted from [86]. Created with BioRender.com
Traditionally, a systemic (IV) approach is the most common and accessible cell delivery route, in which cells are transferred directly to the bloodstream. Stem cells encounter physical barriers upon delivery, resulting in non-specific biodistribution [140,141]. For instance, MSCs administered intravenously become physically trapped within the lung microcirculation [142,143]. Early studies hypothesized that the phenomenon of pulmonary first-pass effect could result in fewer cells successfully reaching the arterial circulation and their desired cellular endpoint, which was assumed to reduce therapeutic efficacy [144–146]. In contrast, studies in a murine MI model report that cells entrapped within the pulmonary circulation could produce therapeutic effects remotely through secreted factors [147]. The premise of cells acting via paracrine signaling, along with a debate on whether a single cell dose is sufficient to deliver a satisfactory long-lasting beneficial effect, prompted reconsideration of intravenous delivery as this method allows multiple cell dose interventions due to its minimal invasiveness [148].
The intracoronary (IC) artery delivery method, which can be performed with or without stop-flow conditions, allows for the injection of cells directly into one of the main coronary arteries, improving cell homing to the myocardial infarcted zone while circumventing the adverse effects associated with direct myocardial injection. The stop-flow technique uses percutaneous intracoronary intervention, a minimally invasive procedure in which an inflated balloon is used to dilate occluded vessels, allowing cells to be delivered into the distal coronary bed close to the infarcted zone. Although the stop-flow technique might seem appealing in theory, manipulating a non-stented coronary artery with an intraluminal balloon carries considerable risks, including coronary dissection, arterial perforation, and vascular rupture [149]. Moreover, excessive cell injection can cause coronary artery occlusion, and reinfarction [150,151]. Comparisons of continuous-flow and stop-flow conditions in a porcine model showed no significant differences in the distribution or quantity of c-kit positive (c-kit+) human cardiac stem cells (hCSCs) at 24 hours. Approximately 4–5% of the infused hCSCs remained in the heart 24 hours after intracoronary delivery, regardless of the infusion technique [152].
To circumvent the need to administer a significant quantity of cells in a single-dose cell treatment either during the acute or subacute phase following MI, studies have explored the potential advantages of repeated dosing of stem cells. In a rat MI model, repeated IC delivery of skeletal myoblasts [153] revealed that recurrent cell administration led to a significantly larger engrafted area and improved LV contractility compared to single-dose transplantation. In clinical trials, repetitive cell therapy has shown promise in promoting myocardial recovery. A notable example is the re-administration of autologous BMMNCs in patients with AMI three months after the first transfer. The findings indicated that this approach is safe and can further improve LVEF while reducing left ventricular remodeling, suggesting that repeated IC delivery could potentially be more effective for these patients, considering the greater improvements observed at 12-month follow-up [153]. The limited clinical data, despite being promising, indicates that rigorous and well-designed clinical trials will be essential to fully assess the utility and optimal timing for repeated dosing in cardiac repair.
Intramyocardial delivery approaches, including trans-endocardial and trans-epicardial techniques, have been widely used [154], allowing for cell delivery directly onto the infarcted area. In addition to the potential invasiveness of intramyocardial delivery techniques, there is the challenge of retaining transplanted cells locally within the myocardium. The therapeutic advantage of one technique over the others is controversial. A comprehensive meta-analysis that included both preclinical and clinical studies, concluded that trans-endocardial stem-cell injection led to significant improvements in LVEF and ischemic area, whereas a lack of improvement occurred with IC cell delivery. This finding highlights the potential promise of trans-endocardial stem-cell injection as a valuable therapeutic approach for enhancing cardiac repair and addressing ischemic myocardial injury, supporting further investigation and consideration for clinical applications in CRM [139].
Aside from the type of stem cell, the dose of transplanted cells also impacts the therapeutic outcomes. Studies performed in a murine ischemic myocardial infarction model, comparing three different doses reported enhanced effectiveness in higher doses of allo MSCs [155]. Further investigations have proposed that administering repeated doses could yield more substantial benefits compared to a single dose. This strategy supports the IV route, the least invasive method. This progression of research, which considers both dosage volume and frequency, accentuates the promising role of high, repeated administrations of allo MSCs as a potent strategy in managing ischemic myocardial infarctions [156].
Nanoparticles (NPs) portray an excellent drug delivery system engineered as carriers enabling regulated therapeutic release directly at the injury site. Gold nanoparticles (AuNPs), which are easily engineered, show low immunogenicity, and are highly stable [157] have been used as drug delivery system resulting in enhanced local circulatory perfusion [158]. In vivo research reported that miRNA combined with a polymeric nanoparticle (miNP) enhances endothelial stem-cell-derived CMs proliferation, leading to regeneration of the myocardium and reduction of the scar size [159].
The time between AMI and cell delivery also appears to be an important parameter. The Late-TIME randomized trial assessed the optimal timing of BM-MSC administration and reported significant improvements in LVEF when stem cells were transplanted 7 to 10 days after AMI [33]. The TIME trial was the first study with sufficient power to determine if cell delivery influenced LV function recovery in STEMI patients [160]. This trial measured LV function at the initial stage, 6 months, 1 and 2 years, reporting a lack of benefit regardless of therapy time application BMMNCs administration.
Exploring the regenerative characteristics of CMs has offered valuable insights for optimal post-myocardial infarction treatment. Research on Cardiac Myocytes indicates their growth potential remains active for approximately 7 days, inferring that the ideal period for treating a myocardial infarction would be within the first week of post-reperfusion therapy [63]. Cardiac tissue engineering offers a potential solution by creating a cell-seeded bioengineered cardiac patch (BCP) that is placed onto the epicardial surface of the myocardium to enhance retention and engraftment.
In a murine model of AMI in mice overexpressing adenylyl cyclase 6 (AC6), a therapeutic tricell patch (Tri-P) comprised of peritoneum seeded with iPSC-CMs, endothelial cells, and mouse embryonic fibroblasts was affixed over the ischemic zone 7 days following MI. The mouse embryonic fibroblasts produce fibrosis-related molecules while expression of AC6 reduces collagen deposition. The reduced collagen deposition correlated with enhanced progenitor cell migration and engraftment and the restoration of LV function [161].
Various stem cell-based therapies have shown promise for cardiac repair and regeneration. However, their limited efficiency due to poor cell survival and engraftment has prompted new strategies. Decellularized placenta (DP) stands out for its availability, highly vascularized tissue structure and rich ECM among the investigated natural scaffolds. A DP-derived BCP demonstrated organized mechanical contraction and synchronized electrical propagation, supporting its potential for myocardial repair and expressed various growth and angiogenic factors, such as VEGF, PDGF, IGF-1, bFGF, angiogenin, and angiopoietin-2, which may play pivotal roles in cardiac repair and regeneration [162].
hiPSC-CMs were seeded into this DP-derived BCP in a rat MI model. RNA sequencing analysis showed enhanced maturation of hiPSC-CMs on the DP-derived BCP, as evidenced by significant upregulation of representative genes associated with cardiac function. Transmission electron microscopy revealed a more mature and organized sarcomeric structure of hiPSC-CMs on the DP-derived BCP compared to monolayer cultures in vitro indicating that DP-derived BCP actively promotes hiPSC-CM maturation and function, thus solidifying its potential as a promising scaffold material for myocardial repair. In vivo evaluations further substantiated the efficacy of the BCP, as evidenced by significant improvements in left ventricular function, reduced infarct size, increased cell retention, and enhanced neovascularization when compared to non-treated MI group, DP-derived BCP or hiPSC-CM transplantation. These compelling findings underscore the potential of decellularized placenta as a natural scaffold material for creating an effective bioengineered cardiac patch [162].
7. Relevant studies in the field
Preclinical development in CRM heavily relies on employing animal models that closely mirror human cardiovascular diseases, providing valuable insights into the mechanisms underpinning these novel therapeutic strategies. While the mechanisms of CRM have been largely elucidated through research on small animals, the practical and translational significance of these findings can be constrained due to notable anatomical and functional differences between small animals and humans. Consequently, the use of large mammals, such as pigs, sheep, and monkeys, is increasingly emphasized to acquire a holistic comprehension of CRM and improve the translational relevance of the research. Large animal models have been particularly instrumental in the study of acute MI, contributing to our understanding of the potential and challenges of CRM in treating this serious condition [17].
Over the past few years, clinical trials aimed at investigating stem cell therapies’ potential in treating acute MI have significantly increased. These trials have aimed to evaluate the efficacy and safety of various stem cell types, administration routes, and patient populations. A selected list of ongoing clinical trials evaluating MSC-based treating AMI registered on the ClinicalTrials.com website [163–167], is listed in Table 1.
Table 1.
Selected Ongoing and Future Clinical Trials using diverse MSCs in the setting of AMI
| Cell Type | Phase | Acronym | ClinicalTrials.gov NCTID | Reference |
|---|---|---|---|---|
| Hearticellgram-AMI (Autologous bone marrow derived mesenchymal stem cells) | Phase III | N/A | NCT01652209 | [163] |
| MiSaver® Stem Cell | Phase I | N/A | NCT04050163 | [164] |
| UC-MSC | Phase I | N/A | NCT03902067 | [165] |
| Umbilical Cord-Derived WJ-MSCs | Phase III | PREVENT-TAHA | NCT05043610 | [166] |
| ProtheraCytes® (CD34+) | Phase II | EXCELLENT | NCT02669810 | [167] |
Source: ClinicalTrials.gov website. Hearticellgram®-AMI are bone marrow-derived mesenchymal stem cells, MiSaver® are Stem Cells and Plasma-Lyte is placebo. Abbreviations: NCT: National Clinical Trial, MiSaver®: Myocardial Infarction Saver, eNOS: endothelial Nitric Oxide Synthase, UC: Umbilical Cord, MSC: Mesenchymal Stem Cell, WJ-MSCs: Wharton’s Jelly Mesenchymal Stem Cells.
To highlight some of the most relevant clinical trials that have significantly contributed to our comprehension of cytotherapy for AMI are discussed. Table 2 provides an overview of clinical trials investigating the use of various cell types beyond MSCs for the treatment of cardiac conditions, specifically focusing on MI and heart failure [22,168,169]. These trials represent a significant advancement in regenerative therapies for cardiovascular diseases, offering promising insights into the potential of cytotherapy as a viable treatment option. By exploring different cell types, such as cardiac progenitor cells, cardiosphere-derived cells, EPCs, and iPSCs, these studies aim to improve our understanding of cellular therapies and their impact on cardiac repair and function. The outcomes of these ongoing trials will contribute valuable information towards the development of innovative and effective treatments for patients suffering from these debilitating conditions.
Table 2.
Some relevant clinical trials for AMI not involving MSCs.
| Trial | Trial | Cell Type | Clinical Outcome Measures | Limitations | Reference |
|---|---|---|---|---|---|
| ENACT-AMI (Enhanced Angiogenic Cell Therapy - Acute Myocardial Infarction) | Randomized, double-blinded, placebo-controlled Phase 3 trial | Endothelial Progenitor Cells (EPCs) | LV function and clinical events | Open-label design, relatively small sample size. | [168] |
| CAREMI (Cardiac Stem Cells in Patients with Acute Myocardial Infarction) | Phase I/II, randomized, double-blind, placebo-controlled | Allogeneic cardiac stem cells (AlloCSC-01) | Safety and efficacy in STEMI patients | Small sample size (n=49), only valid conclusions regarding safety, no definitive evaluation of efficacy | [169] |
| CADUCEUS (CArdiosphere-Derived aUtologous stem CElls to reverse ventricUlar dySfunction) | Phase I/II, double-blinded, placebo-controlled clinical trial | Autologous cardiosphere-derived cells (CDCs) | Scar size reduction, LV function improvement | The study population was limited to reperfused patients, the results may not be generalizable to other patient populations. The study did not include a placebo group. It did not assess the potential for adverse immune reactions to CDC therapy. | [22] |
Despite the promising potential of CRM, its clinical implementation faces several significant obstacles that must be carefully addressed. Firstly, our limited understanding of the complex mechanisms at the molecular, cellular, and organ levels that regulate cardiovascular functions. repair processes complicate the design of pragmatic clinical trials. A future emphasis on identifying particular causes at the molecular or cellular level of cardiovascular diseases will enhance trial success rates. Secondly, the inconsistent results of clinical studies frequently stem from the lack of standardized trial designs. For example, different definitions of AMI have been generally used in trials as an inclusion criterion or as endpoints. Implications of loose definitions impact sample demographics and outcomes, impeding an accurate comparison among trials [8]. Lastly, there is a need for increased multidisciplinary and multinational collaborations to address these limitations, augment our understanding of regenerative treatments will support the execution of extensive preclinical and clinical trials [17].
8. From tailored therapies to off-the-shelf strategies
The Human Genome Project taught us that the human DNA sequence is 99.9% similar. The remaining 0.1%, along with other epigenetic and molecular interactions, accounts for the wide phenotypic variations across people, including disease susceptibility and drug response [170].
The traditional one-therapy-fits-all practice is being replaced by individualized approaches based on patient characteristics to improve outcomes [171]. Precision medicine refers to a medical model that applies clinical and molecular research-based knowledge to identify novel, accurate and efficient theragnostic strategies considering the variability across patients’ genetic information [172], phenotype, biomarkers, specific pathological conditions, lifestyle, and environmental factors [173]. The importance of a precision medicine approach for stem cell therapy was highlighted by Rieger et al. in patients with idiopathic dilated cardiomyopathy participating in the POSEIDON-DCM clinical trial. This study showed that patients identified through genetic sequence analysis as negative for specific pathological variants responded more effectively to MSC treatment compared to population identified as “positive for pathological variants” or those with “uncertain significance” [174].
Research on stem cells within precision medicine is vital to understanding AMI models and defining the mechanisms underlying the great variability in clinical presentations observed in patients in LV function-recovery after myocardial infarction. Some studies have explored stem cell-therapy responses based on patients’ individual characteristics, suggesting a strong association between patient characteristics and outcomes [175]. As an illustration, patients experiencing an AMI frequently exhibit multivessel disease and undetected recurrent ischemic incidents that may directly affect the outcome of any regenerative treatment strategy. In the REPAIR-AMI trial, it was identified that patients with higher body weight and severe cardiac function loss at baseline, exhibited an improved therapeutic reaction to intracoronary administration of BMMNC treating AMI [108].
The revolution of iPSC therapies provided patient-specific autologous therapies reflecting the variability of interventional responses among patients and providing accurate patient stratification. However, the costs of manufacturing and the development processes in compliance with safety regulations exceed the many advantages that human PSC-based therapies may offer to personalize medicine. Moreover, due to time restrictions, manufacturing human iPSC-CMs for autologous therapies is unsustainable in clinical settings, such as acute MI treatment. The affordability of human iPSCs for allogenic treatments would likely improve with the engineering of universal donor iPSC lines for off-the-shelf cell products.
9. Beyond cells: the promising frontier of cell-free therapies
We have discussed strategies to remuscularize the injured heart by replacing damaged cells. In recent years, CRM has shifted toward cell-derived products since cell-based therapy appears to be more beneficial in treating HF rather than regenerating the bio-architecture of the ischemic myocardium in acute clinical settings [176]. The existing research on using MSC-derived products in the context of AMI treatment remains restricted. As mentioned above, the secretome comprises EVs and other factors (growth factors, RNA, peptides), but this section particularly emphasizing the use of Exos due to their crucial role in intercellular communication [177,178] among cells relevant to cardiac structure and performance, including CMs and ECs. Exos stimulate regeneration of the capillary network and damaged cardiac tissue in the infarct zone. The release of exosome cargo is regulated by cellular stress and cues from the surrounding environment, such as hypoxia or ischemia [179]. Exos derived from MSCs are potent therapy effectors after stem cell transplantation [80]. Unlike cell-based therapy, Exos tumorigenicity and risk for triggering adverse immune reactions are minimal, being either recognized and endocytosed or cleared by the recipient cell [180,181].
Like adult stem cells, Exos secreted by various stem cells have been studied for their promising capability in treating AMI, including MSCs [182], ASCs [183], ESCs [184], and iPSCs [185]. Among all types of cell-derived exosomes explored, iPSCs-derived Exos are considered encouraging and can be robustly expanded in vitro [186].
Emerging studies show that the cardioprotective potential of Exos containing heart-specific microRNAs (endogenous, single-stranded, non-coding RNAs, crucial in regulating gene expression post-transcriptionally) is attributed to their ability to exert multiple mechanisms crucial for cardiac repair, including apoptosis suppression and cell proliferation [187]. Although sequence of miRNA showed that stem cells and MSC-derived Exos possess comparable characteristic expression profiles, MSC-derived exosomes may exhibit a superior cardioprotective effect over MSCs in treating MI [188]. These analyses also found that certain miRNA such as miR-371–373, known for their capacity to enhance cell proliferation and/or inhibit apoptosis [189] and miR-302–367 cluster [190], were significantly overexpressed, whereas The miRNAs implicated in inhibiting cell growth and facilitating programmed cell death or apoptosis, like miR-143–3p [191] and miR-506–3p [192], exhibited downregulation.
While certain studies have focused on the impact of specific miRNAs, it is evident that exploring the broader scope of these regulatory molecules may uncover additional layers of complexity and potential therapeutic targets in numerous physiological and pathological contexts. For example, through a comprehensive approach combining systems biology and tissue engineering, MicroRNA-21–5p (miR-21–5p) has been identified to be a cardioactive Exo miR in paracrine signaling of restorative hMSCs. Bioinformatics prediction and experimental data from human-engineered cardiac tissues (hECT) show that exosomal miR-21–5p effectively facilitates hMSCs paracrine influences on hECT contractile performance. Mirroring the human MSC Exo-treatment’s effects on hECT ability to contract, the delivery of miR-21–5p boosted contractility, while the knockdown of miR-21–5p in human MSCs attenuated the exosome-mediated improvement in contractility.
In exploring the mechanistic underpinnings, miR-21–5p was found to augment cardiac calcium management, which in turn enhances contractility, probably through the PI3K pathway. Support for these ramifications of miR-21–5p on calcium handling and contractile function was affirmed at protein and mRNA levels. A mathematical simulation of the process of excitation-contraction coupling was also modified to predict miR-21–5p therapy’s potential to reestablish proper calcium regulation in ischemic hCMs. The observations made in this study highlight the central role of exosomal miR-21–5p in refining subsequent cell-derived cardiovascular therapies, emphasizing its value in treating heart disease [193].
A study on MSCs modified by cardiac transcription factors GATA-4, showed that Exos derived from GATA4-overexpressing MSCs increased CM survival and reduced apoptosis under hypoxia stress contrasted to Exos released by non-modified MSCs [194].
MSC-derived Exos can also heal an I/R injury by promoting angiogenesis [195]. Human iPSC-CMs-derived exosomes promote in vitro angiogenesis upregulating growth factors like VEGFR-2 type A, platelet derived growth factor subunit A (PDGFA), and fibroblast growth factor type 2 (FGF2) in endothelial cells [196].
10. Expert opinion
Cardiac repair following an AMI involves multiple interconnected processes, including tissue regeneration, inflammation control, fibrosis resolution, and restoration of the circulatory system. Successful heart regeneration not only requires morphological restoration but also the establishment of electrical and mechanical coupling. The prospects for using stem cells for the treatment of AMI are compelling, although several challenges persist. Given the advances in the field, an analysis of potential developments and their implications on patient outcomes, including diagnosis and treatment protocols, is paramount.
A better understanding of stem cell behavior and their role in cardiac repair could lead to the development of predictive models, which could aid clinicians in pinpointing the ideal time window for stem cell intervention post-AMI. Timing remains a critical challenge due to the complex nature of the inflammatory response, but most important is limiting myocardial damage. Off-the-shelf products such as allogeneic cells and/or exosomes are well suited for rapid administration. Furthermore, standardizing therapeutic strategies could revolutionize treatment guidelines, optimizing effectiveness and potentially decreasing mortality rates.
In an economic context, identifying more effective and readily available stem cell sources could alleviate the financial burden associated with AMI considerably. This is particularly crucial given that heart disease is one of the leading causes of mortality worldwide and comes with significant healthcare costs. However, the large-scale implementation of such therapies in clinical practice necessitates transformative changes in infrastructure, clinician training, and regulatory oversight.
Notwithstanding the potential benefits, the field faces a plethora of both biological and technical hurdles that require concerted attention. A primary concern is the relatively low survival of transplanted stem cells, which can limit the overall effectiveness of therapy. Enhancing post-transplantation cell survival would extend the availability of secretome products, and potentially, fostering better integration with host cardiac tissue. Technological advancements, particularly in cell imaging and monitoring, may significantly aid these endeavors.
Another critical issue is the restricted accessibility and large-scale production of hCMs, which are vital to cardiac repair. Emerging technologies like iPSCs and advancements in bioreactor designs and cell culture conditions promise to surmount these limitations and could facilitate the large-scale production of hCMs, making them more accessible for research and clinical applications.
Pre-clinical studies are needed to develop safe, effective, and accessible stem cell therapies for post-AMI cardiac repair. This goal comprises several sub-objectives, including creating individualized therapies based on patient-specific factors, enriching our understanding of the stem cell repair process and standardizing methodologies for better inter-study comparisons.
While the road ahead for stem cell research in the context of AMI is promising, it is critical to acknowledge that this is not the only path forward. Tissue engineering and gene therapy are all promising fields that could offer complementary or alternative solutions. These areas represent parallel tracks for research that can contribute to the comprehensive goal of efficient cardiac repair.
We anticipate that a precision medicine approach will foster considerable progress in the field of regenerative medicine in the short and medium term with standard procedures being adapted toward a more personalized approach. Simultaneously, bioengineered cardiac patches, which can provide a more optimal environment for stem cell survival and integration, might prove clinically beneficial. However, these projected advancements hinge on resolving the biological and technical challenges inherent in the field and, more importantly, demonstrating long-term safety and efficacy in large-scale clinical trials. Beyond the scientific and technical aspects, ethical considerations, regulatory compliance, and fostering a multidisciplinary collaboration will all be influential in shaping the future of this field. Therefore, while the path forward is challenging, it is equally promising, offering hope for improved AMI management and patient outcomes.
Article highlights.
AMI ranks as a primary contributor to mortality in the United States. With ischemia, injured myocardium prompts an inflammatory response that is followed by tissue repair and cardiac remodeling processes leading to heart failure.
The therapeutic actions of MSCs are primarily due to paracrine-mediated effects rather than by their capacity to engraft and structurally repair the myocardium.
MSCs are immune evasive, pro-angiogenic and anti-fibrotic, ideal characteristics for cardiovascular regenerative medicine.
To achieve efficient cell-based therapies, promoting cell survival and engraftment at the ischemic area is crucial to contain cardiac remodeling and to preserve cardiac hemodynamic performance.
iPSCs can be reprogrammed from somatic cells into a pluripotent state, and can differentiate into any cell type, including all cardiac lineages.
In preclinical trials, iPSC-derived cardiomyocytes improve cardiac function and reduce infarct size. Further studies are needed to optimize their safety and efficacy.
The MSC secretome has significant potential for translation into cell-free biotherapies as off-the-shelf-products.
Combining cell-based regenerative therapy with precision medicine approaches will maximize efficacy, minimize the adverse effects, and reduce costs by avoiding ineffective therapies.
Funding
This paper was funded by National Heart, Lung, and Blood Institute (grant 1R01HL134558-01) and U.S. Department of Defense (grant W81XWH-19-PRMRP-CTA).
Footnotes
Declaration of interest
JM Hare reported having a patent for cardiac cell-based therapy. He holds equity in Vestion Inc. and maintains a professional relationship with Vestion Inc. as a consultant and member of the Board of Directors and Scientific Advisory Board. JM Hare is the Chief Scientific Officer, a compensated consultant and advisory board member for Longeveron, and holds equity in Longeveron. He is also the co-inventor of intellectual property licensed to Longeveron. Longeveron LLC and Vestion Inc. did not participate in funding this work. JM Hare’s relationships are disclosed to the University of Miami, and a management plan is in place. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
- 1.Tsao CW, Aday AW, Almarzooq ZI, et al. Heart Disease and Stroke Statistics-2022 Update: A Report From the American Heart Association. Circulation. 2022. Feb 22;145(8):e153–e639. [DOI] [PubMed] [Google Scholar]
- 2.Collet JP, Thiele H, Barbato E, et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. 2021. Apr 7;42(14):1289–1367. [DOI] [PubMed] [Google Scholar]
- 3.Ibanez B, James S, Agewall S, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). European Heart Journal. 2017;39(2):119–177. [DOI] [PubMed] [Google Scholar]
- 4.Alraies MC, Eckman P. Adult heart transplant: indications and outcomes. J Thorac Dis. 2014. Aug;6(8):1120–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Niccoli G, Burzotta F, Galiuto L, et al. Myocardial No-Reflow in Humans. Journal of the American College of Cardiology. 2009 2009/July/21/;54(4):281–292. [DOI] [PubMed] [Google Scholar]
- 6.Przybyt E, Harmsen MC. Mesenchymal stem cells: promising for myocardial regeneration? Curr Stem Cell Res Ther. 2013. Jul;8(4):270–7. [DOI] [PubMed] [Google Scholar]
- 7.Lalu MM, Mazzarello S, Zlepnig J, et al. Safety and Efficacy of Adult Stem Cell Therapy for Acute Myocardial Infarction and Ischemic Heart Failure (SafeCell Heart): A Systematic Review and Meta-Analysis. Stem Cells Transl Med. 2018. Dec;7(12):857–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thygesen K, Alpert JS, Jaffe AS, et al. Fourth Universal Definition of Myocardial Infarction (2018). Circulation. 2018;138(20):e618–e651. [DOI] [PubMed] [Google Scholar]
- 9.Anderson JL, Morrow DA. Acute Myocardial Infarction. N Engl J Med. 2017. May 25;376(21):2053–2064. [DOI] [PubMed] [Google Scholar]
- 10.Broughton KM, Wang BJ, Firouzi F, et al. Mechanisms of Cardiac Repair and Regeneration. Circulation Research. 2018;122(8):1151–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prabhu SD, Frangogiannis NG. The Biological Basis for Cardiac Repair After Myocardial Infarction: From Inflammation to Fibrosis. Circ Res. 2016. Jun 24;119(1):91–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Katrukha IA, Katrukha AG. Myocardial Injury and the Release of Troponins I and T in the Blood of Patients. Clinical Chemistry. 2020;67(1):124–130. [DOI] [PubMed] [Google Scholar]
- 13.Arora S, Stouffer GA, Kucharska-Newton A, et al. Fifteen-Year Trends in Management and Outcomes of Non-ST-Segment-Elevation Myocardial Infarction Among Black and White Patients: The ARIC Community Surveillance Study, 2000–2014. J Am Heart Assoc. 2018. Oct 2;7(19):e010203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Romero ME, Fernandez-Jimenez R, Ladich E, et al. PATHOLOGY OF MYOCARDIAL INFARCTION AND SUDDEN DEATH. In: Fuster V, Harrington RA, Narula J, et al., editors. Hurst’s The Heart, 14e. New York, NY: McGraw-Hill Education; 2017. [Google Scholar]
- 15.DeFilippis AP, Hall ME. Impact of New ICD Codes on Acute MI Characteristics and Outcomes. Journal of the American College of Cardiology. 2021;78(12):1254–1256. [DOI] [PubMed] [Google Scholar]
- 16.Bergmann O, Bhardwaj RD, Bernard S, et al. Evidence for cardiomyocyte renewal in humans. Science. 2009. Apr 3;324(5923):98–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fernández-Avilés F, Sanz-Ruiz R, Climent AM, et al. Global position paper on cardiovascular regenerative medicine. Eur Heart J. 2017. Sep 1;38(33):2532–2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iseoka H, Miyagawa S, Saito A, et al. Role and therapeutic effects of skeletal muscle-derived non-myogenic cells in a rat myocardial infarction model. Stem Cell Research & Therapy. 2020 2020/February/18;11(1):69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schächinger V, Erbs S, Elsässer A, et al. Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction. N Engl J Med. 2006. Sep 21;355(12):1210–21. [DOI] [PubMed] [Google Scholar]
- 20.Prat-Vidal C, Crisóstomo V, Moscoso I, et al. Intracoronary Delivery of Porcine Cardiac Progenitor Cells Overexpressing IGF-1 and HGF in a Pig Model of Sub-Acute Myocardial Infarction. Cells. 2021;10(10):2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tokunaga M, Liu M-L, Nagai T, et al. Implantation of cardiac progenitor cells using self-assembling peptide improves cardiac function after myocardial infarction. Journal of Molecular and Cellular Cardiology. 2010 2010/December/01/;49(6):972–983. [DOI] [PubMed] [Google Scholar]
- 22.Malliaras K, Makkar RR, Smith RR, et al. Intracoronary Cardiosphere-Derived Cells After Myocardial Infarction: Evidence of Therapeutic Regeneration in the Final 1-Year Results of the CADUCEUS Trial (CArdiosphere-Derived aUtologous stem CElls to reverse ventricUlar dySfunction). Journal of the American College of Cardiology. 2014 2014/January/21/;63(2):110–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chong JJ, Yang X, Don CW, et al. Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature. 2014. Jun 12;510(7504):273–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thavapalachandran S, Le TYL, Romanazzo S, et al. Pluripotent stem cell-derived mesenchymal stromal cells improve cardiac function and vascularity after myocardial infarction. Cytotherapy. 2021. Dec;23(12):1074–1084. [DOI] [PubMed] [Google Scholar]
- 25.Menasché P. Skeletal myoblasts and cardiac repair. Journal of Molecular and Cellular Cardiology. 2008 2008/October/01/;45(4):545–553. [DOI] [PubMed] [Google Scholar]
- 26.Scorsin M, Hagège A, Vilquin JT, et al. Comparison of the effects of fetal cardiomyocyte and skeletal myoblast transplantation on postinfarction left ventricular function. J Thorac Cardiovasc Surg. 2000. Jun;119(6):1169–75. [DOI] [PubMed] [Google Scholar]
- 27.Menasché P, Alfieri O, Janssens S, et al. The Myoblast Autologous Grafting in Ischemic Cardiomyopathy (MAGIC) trial: first randomized placebo-controlled study of myoblast transplantation. Circulation. 2008. Mar 4;117(9):1189–200. [DOI] [PubMed] [Google Scholar]
- 28.Eaves CJ. Hematopoietic stem cells: concepts, definitions, and the new reality. Blood. 2015. Apr 23;125(17):2605–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Asahara T, Kawamoto A, Masuda H. Concise review: Circulating endothelial progenitor cells for vascular medicine. Stem Cells. 2011. Nov;29(11):1650–5. [DOI] [PubMed] [Google Scholar]
- 30.Steinhoff G, Nesteruk J, Wolfien M, et al. Cardiac Function Improvement and Bone Marrow Response -: Outcome Analysis of the Randomized PERFECT Phase III Clinical Trial of Intramyocardial CD133(+) Application After Myocardial Infarction. EBioMedicine. 2017. Aug;22:208–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Strauer BE, Brehm M, Zeus T, et al. Repair of infarcted myocardium by autologous intracoronary mononuclear bone marrow cell transplantation in humans. Circulation. 2002. Oct 8;106(15):1913–8. [DOI] [PubMed] [Google Scholar]
- 32.Cao F, Sun D, Li C, et al. Long-term myocardial functional improvement after autologous bone marrow mononuclear cells transplantation in patients with ST-segment elevation myocardial infarction: 4 years follow-up. Eur Heart J. 2009. Aug;30(16):1986–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Traverse JH, Henry TD, Ellis SG, et al. Effect of intracoronary delivery of autologous bone marrow mononuclear cells 2 to 3 weeks following acute myocardial infarction on left ventricular function: the LateTIME randomized trial. JAMA. 2011. Nov 16;306(19):2110–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Traverse JH, Henry TD, Pepine CJ, et al. Effect of the use and timing of bone marrow mononuclear cell delivery on left ventricular function after acute myocardial infarction: the TIME randomized trial. Jama. 2012. Dec 12;308(22):2380–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Choudry F, Hamshere S, Saunders N, et al. A randomized double-blind control study of early intra-coronary autologous bone marrow cell infusion in acute myocardial infarction: the REGENERATE-AMI clinical trial†. Eur Heart J. 2016. Jan 14;37(3):256–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mathur A, Fernández-Avilés F, Bartunek J, et al. The effect of intracoronary infusion of bone marrow-derived mononuclear cells on all-cause mortality in acute myocardial infarction: the BAMI trial. European Heart Journal. 2020;41(38):3702–3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bolli R, Solankhi M, Tang XL, et al. Cell therapy in patients with heart failure: a comprehensive review and emerging concepts. Cardiovasc Res. 2022. Mar 16;118(4):951–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Friedenstein AJ, Chailakhjan RK, Lalykina KS. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell and tissue kinetics. 1970. Oct;3(4):393–403. [DOI] [PubMed] [Google Scholar]
- 39.Zuk PA, Zhu M, Ashjian P, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002. Dec;13(12):4279–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Haynesworth SE, Goshima J, Goldberg VM, et al. Characterization of cells with osteogenic potential from human marrow. Bone. 1992;13(1):81–8. [DOI] [PubMed] [Google Scholar]
- 41.Girdlestone J, Limbani VA, Cutler AJ, et al. Efficient expansion of mesenchymal stromal cells from umbilical cord under low serum conditions. Cytotherapy. 2009;11(6):738–48. [DOI] [PubMed] [Google Scholar]
- 42.Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–317. [DOI] [PubMed] [Google Scholar]
- 43.Multilineage Cells from Human Adipose Tissue: Implications for Cell-Based Therapies. Tissue Engineering. 2001;7(2):211–228. [DOI] [PubMed] [Google Scholar]
- 44.Planat-Benard V, Silvestre JS, Cousin B, et al. Plasticity of human adipose lineage cells toward endothelial cells: physiological and therapeutic perspectives. Circulation. 2004. Feb 10;109(5):656–63. [DOI] [PubMed] [Google Scholar]
- 45.Karaöz E, Çetinalp Demircan P, Erman G, et al. Comparative Analyses of Immunosuppressive Characteristics of Bone-Marrow, Wharton’s Jelly, and Adipose Tissue-Derived Human Mesenchymal Stem Cells. Turk J Haematol. 2017. Aug 2;34(3):213–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hare JM, Traverse JH, Henry TD, et al. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J Am Coll Cardiol. 2009. Dec 8;54(24):2277–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci U S A. 1981. Dec;78(12):7634–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thomson JA, Itskovitz-Eldor J, Shapiro SS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998. Nov 6;282(5391):1145–7. [DOI] [PubMed] [Google Scholar]
- 49.Hartman ME, Dai DF, Laflamme MA. Human pluripotent stem cells: Prospects and challenges as a source of cardiomyocytes for in vitro modeling and cell-based cardiac repair. Adv Drug Deliv Rev. 2016. Jan 15;96:3–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu YW, Chen B, Yang X, et al. Human embryonic stem cell-derived cardiomyocytes restore function in infarcted hearts of non-human primates. Nat Biotechnol. 2018. Aug;36(7):597–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Robertson JA. Human embryonic stem cell research: ethical and legal issues. Nature Reviews Genetics. 2001 2001/January/01;2(1):74–78. [DOI] [PubMed] [Google Scholar]
- 52.Yamanaka S. Strategies and new developments in the generation of patient-specific pluripotent stem cells. Cell Stem Cell. 2007. Jun 7;1(1):39–49. [DOI] [PubMed] [Google Scholar]
- 53.Takahashi K, Tanabe K, Ohnuki M, et al. Induction of Pluripotent Stem Cells from Adult Human Fibroblasts by Defined Factors. Cell. 2007;131(5):861–872. [DOI] [PubMed] [Google Scholar]
- 54.Lee J-H, Lee JB, Shapovalova Z, et al. Somatic transcriptome priming gates lineage-specific differentiation potential of human-induced pluripotent stem cell states. Nature Communications. 2014 2014/December/03;5(1):5605. [DOI] [PubMed] [Google Scholar]
- 55.Nelson TJ, Martinez-Fernandez A, Yamada S, et al. Repair of Acute Myocardial Infarction by Human Stemness Factors Induced Pluripotent Stem Cells. Circulation. 2009;120(5):408–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Batalov I, Feinberg AW. Differentiation of Cardiomyocytes from Human Pluripotent Stem Cells Using Monolayer Culture. Biomark Insights. 2015;10(Suppl 1):71–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Orlova VV, van den Hil FE, Petrus-Reurer S, et al. Generation, expansion and functional analysis of endothelial cells and pericytes derived from human pluripotent stem cells. Nat Protoc. 2014;9(6):1514–31. [DOI] [PubMed] [Google Scholar]
- 58.Xie CQ, Huang H, Wei S, et al. A comparison of murine smooth muscle cells generated from embryonic versus induced pluripotent stem cells. Stem Cells Dev. 2009. Jun;18(5):741–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mauritz C, Schwanke K, Reppel M, et al. Generation of functional murine cardiac myocytes from induced pluripotent stem cells. Circulation. 2008. Jul 29;118(5):507–17. [DOI] [PubMed] [Google Scholar]
- 60.Barbuti A, Benzoni P, Campostrini G, et al. Human derived cardiomyocytes: A decade of knowledge after the discovery of induced pluripotent stem cells. Dev Dyn. 2016. Dec;245(12):1145–1158. [DOI] [PubMed] [Google Scholar]
- 61.Karbassi E, Fenix A, Marchiano S, et al. Cardiomyocyte maturation: advances in knowledge and implications for regenerative medicine. Nature reviews Cardiology. 2020. Jun;17(6):341–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Machiraju P, Greenway SC. Current methods for the maturation of induced pluripotent stem cell-derived cardiomyocytes. World J Stem Cells. 2019. Jan 26;11(1):33–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Scuderi GJ, Butcher J. Naturally Engineered Maturation of Cardiomyocytes. Front Cell Dev Biol. 2017;5:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kaneko S, Yamanaka S. To be immunogenic, or not to be: that’s the iPSC question. Cell Stem Cell. 2013. Apr 4;12(4):385–6. [DOI] [PubMed] [Google Scholar]
- 65.Qiao Y, Agboola OS, Hu X, et al. Tumorigenic and Immunogenic Properties of Induced Pluripotent Stem Cells: a Promising Cancer Vaccine. Stem Cell Rev Rep. 2020. Dec;16(6):1049–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tu C, Zoldan J. Moving iPSC-Derived Cardiomyocytes Forward to Treat Myocardial Infarction. Cell Stem Cell. 2018. Sep 6;23(3):322–323. [DOI] [PubMed] [Google Scholar]
- 67.Mazo M, Araña M, Pelacho B, et al. Mesenchymal Stem Cells and Cardiovascular Disease: A Bench to Bedside Roadmap. Stem Cells International. 2012 2012/January/22;2012:175979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hafez P, Jose S, Chowdhury SR, et al. Cardiomyogenic differentiation of human sternal bone marrow mesenchymal stem cells using a combination of basic fibroblast growth factor and hydrocortisone. Cell Biology International. 2016;40(1):55–64. [DOI] [PubMed] [Google Scholar]
- 69.Raman N, Imran SAM, Ahmad Amin Noordin KB, et al. Mechanotransduction of mesenchymal stem cells (MSCs) during cardiomyocytes differentiation. Heliyon. 2022 2022/November/01/;8(11):e11624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Martin-Rendon E, Sweeney D, Lu F, et al. 5-Azacytidine-treated human mesenchymal stem/progenitor cells derived from umbilical cord, cord blood and bone marrow do not generate cardiomyocytes in vitro at high frequencies. Vox Sang. 2008. Aug;95(2):137–48. [DOI] [PubMed] [Google Scholar]
- 71.Hatzistergos KE, Saur D, Seidler B, et al. Stimulatory Effects of Mesenchymal Stem Cells on cKit+ Cardiac Stem Cells Are Mediated by SDF1/CXCR4 and SCF/cKit Signaling Pathways. Circulation research. 2016. Sep 30;119(8):921–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Quevedo HC, Hatzistergos KE, Oskouei BN, et al. Allogeneic mesenchymal stem cells restore cardiac function in chronic ischemic cardiomyopathy via trilineage differentiating capacity. Proc Natl Acad Sci U S A. 2009;106(33):14022–14027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Leri A, Rota M, Hosoda T, et al. Cardiac stem cell niches. Stem Cell Res. 2014. Nov;13(3 Pt B):631–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pokrovskaya LA, Zubareva EV, Nadezhdin SV, et al. Biological activity of mesenchymal stem cells secretome as a basis for cell-free therapeutic approach. Research Results in Pharmacology. 2020;6(1). [Google Scholar]
- 75.Gnecchi M, He H, Noiseux N, et al. Evidence supporting paracrine hypothesis for Akt-modified mesenchymal stem cell-mediated cardiac protection and functional improvement. Faseb j. 2006. Apr;20(6):661–9. [DOI] [PubMed] [Google Scholar]
- 76.Timmers L, Lim SK, Hoefer IE, et al. Human mesenchymal stem cell-conditioned medium improves cardiac function following myocardial infarction. Stem Cell Res. 2011. May;6(3):206–14. [DOI] [PubMed] [Google Scholar]
- 77.Gallina C, Turinetto V, Giachino C. A New Paradigm in Cardiac Regeneration: The Mesenchymal Stem Cell Secretome. Stem Cells Int. 2015;2015:765846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Teixeira FG, Salgado AJ. Mesenchymal stem cells secretome: current trends and future challenges. Neural Regen Res. 2020. Jan;15(1):75–77. *Of interest. Paper highlights MSCs secretome’s therapeutic potential - growth factors, cytokines, and exosomes for regenerative medicine breakthrough. Ready-to-use drug delivery system, addressing transplantation challenges.
- 79.Kishore R, Garikipati VNS, Gumpert A. Tiny Shuttles for Information Transfer: Exosomes in Cardiac Health and Disease. J Cardiovasc Transl Res. 2016. Jun;9(3):169–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Taheri B, Soleimani M, Fekri Aval S, et al. Induced pluripotent stem cell-derived extracellular vesicles: A novel approach for cell-free regenerative medicine. J Cell Physiol. 2019. Jun;234(6):8455–8464. [DOI] [PubMed] [Google Scholar]
- 81.M HR, Bayraktar E, G KH, et al. Exosomes: From Garbage Bins to Promising Therapeutic Targets. Int J Mol Sci. 2017. Mar 2;18(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rana S, Yue S, Stadel D, et al. Toward tailored exosomes: the exosomal tetraspanin web contributes to target cell selection. Int J Biochem Cell Biol. 2012. Sep;44(9):1574–84. [DOI] [PubMed] [Google Scholar]
- 83.Gentek R, Hoeffel G. The Innate Immune Response in Myocardial Infarction, Repair, and Regeneration. Adv Exp Med Biol. 2017;1003:251–272. [DOI] [PubMed] [Google Scholar]
- 84.van den Akker F, de Jager SCA, Sluijter JPG. Mesenchymal Stem Cell Therapy for Cardiac Inflammation: Immunomodulatory Properties and the Influence of Toll-Like Receptors. Mediators of Inflammation. 2013 2013/December/10;2013:181020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ruparelia N, Chai JT, Fisher EA, et al. Inflammatory processes in cardiovascular disease: a route to targeted therapies. Nat Rev Cardiol. 2017. Mar;14(3):133–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Golpanian S, Wolf A, Hatzistergos KE, et al. Rebuilding the Damaged Heart: Mesenchymal Stem Cells, Cell-Based Therapy, and Engineered Heart Tissue. Physiol Rev. 2016. Jul;96(3):1127–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ankrum JA, Ong JF, Karp JM. Mesenchymal stem cells: immune evasive, not immune privileged. Nature Biotechnology. 2014 2014/March/01;32(3):252–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Williams AR, Hare JM. Mesenchymal stem cells: biology, pathophysiology, translational findings, and therapeutic implications for cardiac disease. Circ Res. 2011. Sep 30;109(8):923–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sun YQ, Zhang Y, Li X, et al. Insensitivity of Human iPS Cells-Derived Mesenchymal Stem Cells to Interferon-γ-induced HLA Expression Potentiates Repair Efficiency of Hind Limb Ischemia in Immune Humanized NOD Scid Gamma Mice. Stem Cells. 2015. Dec;33(12):3452–67. [DOI] [PubMed] [Google Scholar]
- 90. Haworth R, Sharpe M. Accept or Reject: The Role of Immune Tolerance in the Development of Stem Cell Therapies and Possible Future Approaches. Toxicologic Pathology. 2021;49(7):1308–1316. *Of interest. The paper examines safety concerns in stem cell-derived treatments, emphasizing immune tolerance’s role. Authors foresee improved immunogenic epitopes and delivery techniques for sustained clinical successes.
- 91.Li N, Hua J. Interactions between mesenchymal stem cells and the immune system. Cell Mol Life Sci. 2017. Jul;74(13):2345–2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dittrich A, Lauridsen H. Myocardial infarction and the immune response - Scarring or regeneration? A comparative look at mammals and popular regenerating animal models. Journal of Immunology and Regenerative Medicine. 2019 2019/June/01/;4:100016. [Google Scholar]
- 93.Guo J, Lin GS, Bao CY, et al. Anti-inflammation role for mesenchymal stem cells transplantation in myocardial infarction. Inflammation. 2007. Aug;30(3–4):97–104. [DOI] [PubMed] [Google Scholar]
- 94.Wang Y, Chen X, Cao W, et al. Plasticity of mesenchymal stem cells in immunomodulation: pathological and therapeutic implications. Nat Immunol. 2014. Nov;15(11):1009–16. [DOI] [PubMed] [Google Scholar]
- 95.Ren G, Zhang L, Zhao X, et al. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell. 2008. Feb 7;2(2):141–50. [DOI] [PubMed] [Google Scholar]
- 96.Yan X, Anzai A, Katsumata Y, et al. Temporal dynamics of cardiac immune cell accumulation following acute myocardial infarction. J Mol Cell Cardiol. 2013. Sep;62:24–35. [DOI] [PubMed] [Google Scholar]
- 97.Duncan SE, Gao S, Sarhene M, et al. Macrophage Activities in Myocardial Infarction and Heart Failure. Cardiol Res Pract. 2020;2020:4375127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Najar M, Raicevic G, Fayyad-Kazan H, et al. Mesenchymal stromal cells and immunomodulation: A gathering of regulatory immune cells. Cytotherapy. 2016. Feb;18(2):160–71. [DOI] [PubMed] [Google Scholar]
- 99.Chiossone L, Conte R, Spaggiari GM, et al. Mesenchymal Stromal Cells Induce Peculiar Alternatively Activated Macrophages Capable of Dampening Both Innate and Adaptive Immune Responses. Stem Cells. 2016. Jul;34(7):1909–21. [DOI] [PubMed] [Google Scholar]
- 100.Davies LC, Heldring N, Kadri N, et al. Mesenchymal Stromal Cell Secretion of Programmed Death-1 Ligands Regulates T Cell Mediated Immunosuppression. Stem Cells. 2017. Mar;35(3):766–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ala M, Eftekhar SP. The Footprint of Kynurenine Pathway in Cardiovascular Diseases. Int J Tryptophan Res. 2022;15:11786469221096643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Laing AG, Fanelli G, Ramirez-Valdez A, et al. Mesenchymal stem cells inhibit T-cell function through conserved induction of cellular stress. PLoS One. 2019;14(3):e0213170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ringdén O, Uzunel M, Rasmusson I, et al. Mesenchymal stem cells for treatment of therapy-resistant graft-versus-host disease. Transplantation. 2006. May 27;81(10):1390–7. [DOI] [PubMed] [Google Scholar]
- 104.Weiβ E, Ramos GC, Delgobo M. Myocardial-Treg Crosstalk: How to Tame a Wolf. Front Immunol. 2022;13:914033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Spaggiari GM, Capobianco A, Abdelrazik H, et al. Mesenchymal stem cells inhibit natural killer-cell proliferation, cytotoxicity, and cytokine production: role of indoleamine 2,3-dioxygenase and prostaglandin E2. Blood. 2008. Feb 1;111(3):1327–33. [DOI] [PubMed] [Google Scholar]
- 106.Sun SJ, Lai WH, Jiang Y, et al. Immunomodulation by systemic administration of human-induced pluripotent stem cell-derived mesenchymal stromal cells to enhance the therapeutic efficacy of cell-based therapy for treatment of myocardial infarction. Theranostics. 2021;11(4):1641–1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Dai Z, Aoki T, Fukumoto Y, et al. Coronary perivascular fibrosis is associated with impairment of coronary blood flow in patients with non-ischemic heart failure. J Cardiol. 2012. Nov;60(5):416–21. [DOI] [PubMed] [Google Scholar]
- 108.Mills JS, Rao SV. REPAIR-AMI: stem cells for acute myocardial infarction. Future Cardiol. 2007. Mar;3(2):137–40. [DOI] [PubMed] [Google Scholar]
- 109.Cochain C, Channon KM, Silvestre JS. Angiogenesis in the infarcted myocardium. Antioxid Redox Signal. 2013. Mar 20;18(9):1100–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tang J, Wang J, Yang J, et al. Mesenchymal stem cells over-expressing SDF-1 promote angiogenesis and improve heart function in experimental myocardial infarction in rats. Eur J Cardiothorac Surg. 2009. Oct;36(4):644–50. [DOI] [PubMed] [Google Scholar]
- 111.Hutchings G, Janowicz K, Moncrieff L, et al. The Proliferation and Differentiation of Adipose-Derived Stem Cells in Neovascularization and Angiogenesis. Int J Mol Sci. 2020. May 27;21(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wang L, Deng J, Tian W, et al. Adipose-derived stem cells are an effective cell candidate for treatment of heart failure: an MR imaging study of rat hearts. American Journal of Physiology-Heart and Circulatory Physiology. 2009;297(3):H1020–H1031. [DOI] [PubMed] [Google Scholar]
- 113.Valina C, Pinkernell K, Song YH, et al. Intracoronary administration of autologous adipose tissue-derived stem cells improves left ventricular function, perfusion, and remodelling after acute myocardial infarction. Eur Heart J. 2007. Nov;28(21):2667–77. [DOI] [PubMed] [Google Scholar]
- 114.Houtgraaf JH, Dekker WKd, Dalen BMv, et al. First Experience in Humans Using Adipose Tissue–Derived Regenerative Cells in the Treatment of Patients With ST-Segment Elevation Myocardial Infarction. Journal of the American College of Cardiology. 2012;59(5):539–540. [DOI] [PubMed] [Google Scholar]
- 115.Gupta S, Sharma A, S A, et al. Mesenchymal Stem Cells for Cardiac Regeneration: from Differentiation to Cell Delivery. Stem Cell Rev Rep. 2021. Oct;17(5):1666–1694. [DOI] [PubMed] [Google Scholar]
- 116.Oskowitz A, McFerrin H, Gutschow M, et al. Serum-deprived human multipotent mesenchymal stromal cells (MSCs) are highly angiogenic. Stem Cell Res. 2011. May;6(3):215–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Premer C, Wanschel A, Porras V, et al. Mesenchymal Stem Cell Secretion of SDF-1α Modulates Endothelial Function in Dilated Cardiomyopathy. Front Physiol. 2019;10:1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kwon HM, Hur S-M, Park K-Y, et al. Multiple paracrine factors secreted by mesenchymal stem cells contribute to angiogenesis. Vascular Pharmacology. 2014 2014/October/01/;63(1):19–28. [DOI] [PubMed] [Google Scholar]
- 119.Gerber HP, McMurtrey A, Kowalski J, et al. Vascular endothelial growth factor regulates endothelial cell survival through the phosphatidylinositol 3’-kinase/Akt signal transduction pathway. Requirement for Flk-1/KDR activation. J Biol Chem. 1998. Nov 13;273(46):30336–43. [DOI] [PubMed] [Google Scholar]
- 120.Meadows KN, Bryant P, Pumiglia K. Vascular endothelial growth factor induction of the angiogenic phenotype requires Ras activation. J Biol Chem. 2001. Dec 28;276(52):49289–98. [DOI] [PubMed] [Google Scholar]
- 121.Liang X, Ding Y, Zhang Y, et al. Paracrine mechanisms of mesenchymal stem cell-based therapy: current status and perspectives. Cell Transplant. 2014;23(9):1045–59. [DOI] [PubMed] [Google Scholar]
- 122.Rufaihah AJ, Huang NF, Jamé S, et al. Endothelial cells derived from human iPSCS increase capillary density and improve perfusion in a mouse model of peripheral arterial disease. Arterioscler Thromb Vasc Biol. 2011. Nov;31(11):e72–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Assis-Ribas T, Forni MF, Winnischofer SMB, et al. Extracellular matrix dynamics during mesenchymal stem cells differentiation. Dev Biol. 2018. May 15;437(2):63–74. [DOI] [PubMed] [Google Scholar]
- 124.Kachgal S, Putnam AJ. Mesenchymal stem cells from adipose and bone marrow promote angiogenesis via distinct cytokine and protease expression mechanisms. Angiogenesis. 2011. Mar;14(1):47–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Mauritz C, Martens A, Rojas SV, et al. Induced pluripotent stem cell (iPSC)-derived Flk-1 progenitor cells engraft, differentiate, and improve heart function in a mouse model of acute myocardial infarction. European Heart Journal. 2011;32(21):2634–2641. [DOI] [PubMed] [Google Scholar]
- 126. Zhang H, Yamaguchi T, Kokubu Y, et al. Transient ETV2 Expression Promotes the Generation of Mature Endothelial Cells from Human Pluripotent Stem Cells. Biol Pharm Bull. 2022;45(4):483–490. *Of interest. The paper shows that ETV2 overexpression in DPSCs boosts endothelial cell differentiation, holding promise for neovascularization in tissue engineering. Assays and proteomic analysis support their potential as a cell source.
- 127.Kurian GA, Rajagopal R, Vedantham S, et al. The Role of Oxidative Stress in Myocardial Ischemia and Reperfusion Injury and Remodeling: Revisited. Oxid Med Cell Longev. 2016;2016:1656450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lodrini AM, Goumans M-J. Cardiomyocytes Cellular Phenotypes After Myocardial Infarction [Review]. Frontiers in Cardiovascular Medicine. 2021 2021-November-08;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Walkowski B, Kleibert M, Majka M, et al. Insight into the Role of the PI3K/Akt Pathway in Ischemic Injury and Post-Infarct Left Ventricular Remodeling in Normal and Diabetic Heart. Cells. 2022. May 5;11(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Yan B, Singla DK. Transplanted induced pluripotent stem cells mitigate oxidative stress and improve cardiac function through the Akt cell survival pathway in diabetic cardiomyopathy. Mol Pharm. 2013. Sep 3;10(9):3425–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Chen B, Chen X, Liu C, et al. Co-expression of Akt1 and Wnt11 promotes the proliferation and cardiac differentiation of mesenchymal stem cells and attenuates hypoxia/reoxygenation-induced cardiomyocyte apoptosis. Biomed Pharmacother. 2018. Dec;108:508–514. [DOI] [PubMed] [Google Scholar]
- 132.White HD, Norris RM, Brown MA, et al. Left ventricular end-systolic volume as the major determinant of survival after recovery from myocardial infarction. Circulation. 1987. Jul;76(1):44–51. [DOI] [PubMed] [Google Scholar]
- 133.Arai AE. Healing After Myocardial Infarction: A Loosely Defined Process. JACC Cardiovasc Imaging. 2015. Jun;8(6):680–3. [DOI] [PubMed] [Google Scholar]
- 134.Karantalis V, DiFede DL, Gerstenblith G, et al. Autologous mesenchymal stem cells produce concordant improvements in regional function, tissue perfusion, and fibrotic burden when administered to patients undergoing coronary artery bypass grafting: The Prospective Randomized Study of Mesenchymal Stem Cell Therapy in Patients Undergoing Cardiac Surgery (PROMETHEUS) trial. Circ Res. 2014. Apr 11;114(8):1302–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hassink RJ, Pasumarthi KB, Nakajima H, et al. Cardiomyocyte cell cycle activation improves cardiac function after myocardial infarction. Cardiovasc Res. 2008. Apr 1;78(1):18–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Schang LM. The cell cycle, cyclin-dependent kinases, and viral infections: new horizons and unexpected connections. Prog Cell Cycle Res. 2003;5:103–24. [PubMed] [Google Scholar]
- 137. Zhu W, Zhao M, Mattapally S, et al. CCND2 Overexpression Enhances the Regenerative Potency of Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes: Remuscularization of Injured Ventricle. Circ Res. 2018. Jan 5;122(1):88–96. *Of interest. The paper finds that cyclin D2 overexpression improves human induced pluripotent stem cell-derived cardiomyocytes’ efficacy for myocardial repair in a swine model. CCND2 overexpression enhances therapeutic potential for myocardial infarction treatment.
- 138.Zhao M, Nakada Y, Wei Y, et al. Cyclin D2 Overexpression Enhances the Efficacy of Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes for Myocardial Repair in a Swine Model of Myocardial Infarction. Circulation. 2021. Jul 20;144(3):210–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kanelidis AJ, Premer C, Lopez J, et al. Route of Delivery Modulates the Efficacy of Mesenchymal Stem Cell Therapy for Myocardial Infarction: A Meta-Analysis of Preclinical Studies and Clinical Trials. Circ Res. 2017. Mar 31;120(7):1139–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Fakoya AO. New Delivery Systems of Stem Cells for Vascular Regeneration in Ischemia. Front Cardiovasc Med. 2017;4:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Bagno LL, Salerno AG, Balkan W, et al. Mechanism of Action of Mesenchymal Stem Cells (MSCs): impact of delivery method. Expert Opin Biol Ther. 2022. Apr;22(4):449–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Masterson CH, Tabuchi A, Hogan G, et al. Intra-vital imaging of mesenchymal stromal cell kinetics in the pulmonary vasculature during infection. Scientific Reports. 2021 2021/March/04;11(1):5265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Pulmonary Passage is a Major Obstacle for Intravenous Stem Cell Delivery: The Pulmonary First-Pass Effect. Stem Cells and Development. 2009;18(5):683–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Schrepfer S, Deuse T, Reichenspurner H, et al. Stem cell transplantation: the lung barrier. Transplant Proc. 2007. Mar;39(2):573–6. [DOI] [PubMed] [Google Scholar]
- 145.De Becker A, Riet IV. Homing and migration of mesenchymal stromal cells: How to improve the efficacy of cell therapy? World J Stem Cells. 2016. Mar 26;8(3):73–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Karp JM, Leng Teo GS. Mesenchymal stem cell homing: the devil is in the details. Cell Stem Cell. 2009. Mar 6;4(3):206–16. [DOI] [PubMed] [Google Scholar]
- 147.Lee RH, Pulin AA, Seo MJ, et al. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell. 2009. Jul 2;5(1):54–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zhang J, Bolli R, Garry DJ, et al. Basic and Translational Research in Cardiac Repair and Regeneration: JACC State-of-the-Art Review. Journal of the American College of Cardiology. 2021. Nov 23;78(21):2092–2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Levine GN, Bates ER, Blankenship JC, et al. 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation. 2011. Dec 6;124(23):e574–651. [DOI] [PubMed] [Google Scholar]
- 150.de Jong R, Houtgraaf JH, Samiei S, et al. Intracoronary stem cell infusion after acute myocardial infarction: a meta-analysis and update on clinical trials. Circ Cardiovasc Interv. 2014. Apr;7(2):156–67. [DOI] [PubMed] [Google Scholar]
- 151.Watanabe M, Yavagal DR. Intra-arterial delivery of mesenchymal stem cells. Brain Circ. 2016. Jul-Sep;2(3):114–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Keith MCL, Tokita Y, Tang X-L, et al. Effect of the stop-flow technique on cardiac retention of c-kit positive human cardiac stem cells after intracoronary infusion in a porcine model of chronic ischemic cardiomyopathy. Basic Research in Cardiology. 2015 2015/July/07;110(5):46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Premaratne GU, Tambara K, Fujita M, et al. Repeated implantation is a more effective cell delivery method in skeletal myoblast transplantation for rat myocardial infarction. Circ J. 2006. Sep;70(9):1184–9. [DOI] [PubMed] [Google Scholar]
- 154.Yigman Z, Ozdemir ED, Turan NN, et al. Umbilical cord mesenchymal stromal cells engraft and transdifferentiate into cardiomyocyte-like cells following acute myocardial ischemia⋆. Acta Histochem. 2020. Sep;122(6):151578. [DOI] [PubMed] [Google Scholar]
- 155.Bagno L, Hatzistergos KE, Balkan W, et al. Mesenchymal Stem Cell-Based Therapy for Cardiovascular Disease: Progress and Challenges. Mol Ther. 2018. Jul 5;26(7):1610–1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Wysoczynski M, Khan A, Bolli R. New Paradigms in Cell Therapy: Repeated Dosing, Intravenous Delivery, Immunomodulatory Actions, and New Cell Types. Circ Res. 2018. Jul 6;123(2):138–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ravichandran R, Sridhar R, Venugopal JR, et al. Gold Nanoparticle Loaded Hybrid Nanofibers for Cardiogenic Differentiation of Stem Cells for Infarcted Myocardium Regeneration. Macromolecular Bioscience. 2014;14(4):515–525. [DOI] [PubMed] [Google Scholar]
- 158.Pala R, Anju VT, Dyavaiah M, et al. Nanoparticle-Mediated Drug Delivery for the Treatment of Cardiovascular Diseases. Int J Nanomedicine. 2020;15:3741–3769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Yang H, Qin X, Wang H, et al. An in Vivo miRNA Delivery System for Restoring Infarcted Myocardium. ACS Nano. 2019. Sep 24;13(9):9880–9894. *Of interest. Paper presents effective miRNA delivery system: polymeric nanoparticles and hydrogel post-MI treatment. Smaller scar, improved cardiac function, and angiogenesis. Promising future miRNA therapy for MI.
- 160.Traverse JH, Henry TD, Pepine CJ, et al. TIME Trial: Effect of Timing of Stem Cell Delivery Following ST-Elevation Myocardial Infarction on the Recovery of Global and Regional Left Ventricular Function: Final 2-Year Analysis. Circ Res. 2018. Feb 2;122(3):479–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Dai B, Huang W, Xu M, et al. Reduced Collagen Deposition in Infarcted Myocardium Facilitates Induced Pluripotent Stem Cell Engraftment and Angiomyogenesis for Improvement of Left Ventricular Function. Journal of the American College of Cardiology. 2011 2011/November/08/;58(20):2118–2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Jiang Y, Sun S-J, Zhen Z, et al. Myocardial repair of bioengineered cardiac patches with decellularized placental scaffold and human-induced pluripotent stem cells in a rat model of myocardial infarction. Stem Cell Research & Therapy. 2021 2021/January/07;12(1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Pharmicell Co. L. A Randomized, Open Labeled, Multicenter Trial for Safety and Efficacy of Intracoronary Adult Human Mesenchymal Stem Cells Acute Myocardial Infarction 2013. Available from: https://classic.clinicaltrials.gov/ct2/show/NCT01652209
- 164.Inc HM. MiSaver® Stem Cell Treatment for Heart Attack (Acute Myocardial Infarction) 2019. Available from: https://classic.clinicaltrials.gov/ct2/show/record/NCT04050163
- 165.Technology SLS. UC-MSC Transplantation for Left Ventricular Dysfunction After AMI 2023. Available from: https://classic.clinicaltrials.gov/ct2/show/NCT03902067
- 166.Sciences SUoM. MSCs for Prevention of MI-induced HF (PREVENT-TAHA) 2021. Available from: https://classic.clinicaltrials.gov/ct2/show/NCT05043610
- 167.CellProthera. EXCELLENT (EXpanded CELL ENdocardiac Transplantation) 2024. Available from: https://classic.clinicaltrials.gov/ct2/show/NCT02669810
- 168.Taljaard M, Ward MR, Kutryk MJB, et al. Rationale and design of Enhanced Angiogenic Cell Therapy in Acute Myocardial Infarction (ENACT-AMI): The first randomized placebo-controlled trial of enhanced progenitor cell therapy for acute myocardial infarction. American Heart Journal. 2010 2010/March/01/;159(3):354–360. [DOI] [PubMed] [Google Scholar]
- 169.Fernández-Avilés F, Sanz-Ruiz R, Bogaert J, et al. Safety and Efficacy of Intracoronary Infusion of Allogeneic Human Cardiac Stem Cells in Patients With ST-Segment Elevation Myocardial Infarction and Left Ventricular Dysfunction. Circ Res. 2018. Aug 17;123(5):579–589. [DOI] [PubMed] [Google Scholar]
- 170.Collins FS, Mansoura MK. The Human Genome Project. Revealing the shared inheritance of all humankind. Cancer. 2001. Jan 1;91(1 Suppl):221–5. [DOI] [PubMed] [Google Scholar]
- 171.Litman T. Personalized medicine—concepts, technologies, and applications in inflammatory skin diseases. APMIS. 2019;127(5):386–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Lu YF, Goldstein DB, Angrist M, et al. Personalized medicine and human genetic diversity. Cold Spring Harb Perspect Med. 2014. Jul 24;4(9):a008581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Katsnelson A. Momentum grows to make ‘personalized’ medicine more ‘precise’. Nat Med. 2013. Mar;19(3):249. [DOI] [PubMed] [Google Scholar]
- 174.Rieger AC, Myerburg RJ, Florea V, et al. Genetic determinants of responsiveness to mesenchymal stem cell injections in non-ischemic dilated cardiomyopathy. EBioMedicine. 2019. Oct;48:377–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Delewi R, Hirsch A, Tijssen JG, et al. Impact of intracoronary bone marrow cell therapy on left ventricular function in the setting of ST-segment elevation myocardial infarction: a collaborative meta-analysis. Eur Heart J. 2014. Apr;35(15):989–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Loffredo FS, Steinhauser ML, Gannon J, et al. Bone marrow-derived cell therapy stimulates endogenous cardiomyocyte progenitors and promotes cardiac repair. Cell Stem Cell. 2011. Apr 8;8(4):389–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Ye M, Ni Q, Qi H, et al. Exosomes Derived from Human Induced Pluripotent Stem Cells-Endothelia Cells Promotes Postnatal Angiogenesis in Mice Bearing Ischemic Limbs. Int J Biol Sci. 2019;15(1):158–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Moghaddam AS, Afshari JT, Esmaeili SA, et al. Cardioprotective microRNAs: Lessons from stem cell-derived exosomal microRNAs to treat cardiovascular disease. Atherosclerosis. 2019. Jun;285:1–9. [DOI] [PubMed] [Google Scholar]
- 179.Carotenuto F, Teodori L, Maccari AM, et al. Turning regenerative technologies into treatment to repair myocardial injuries. Journal of Cellular and Molecular Medicine. 2020;24(5):2704–2716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Lai CP, Kim EY, Badr CE, et al. Visualization and tracking of tumour extracellular vesicle delivery and RNA translation using multiplexed reporters. Nat Commun. 2015. May 13;6:7029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Bradley JA, Bolton EM, Pedersen RA. Stem cell medicine encounters the immune system. Nat Rev Immunol. 2002. Nov;2(11):859–71. [DOI] [PubMed] [Google Scholar]
- 182.Lai RC, Arslan F, Lee MM, et al. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 2010. May;4(3):214–22. [DOI] [PubMed] [Google Scholar]
- 183.Xu MY, Ye ZS, Song XT, et al. Differences in the cargos and functions of exosomes derived from six cardiac cell types: a systematic review. Stem Cell Res Ther. 2019. Jun 27;10(1):194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Khan M, Nickoloff E, Abramova T, et al. Embryonic stem cell-derived exosomes promote endogenous repair mechanisms and enhance cardiac function following myocardial infarction. Circ Res. 2015. Jun 19;117(1):52–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Adamiak M, Cheng G, Bobis-Wozowicz S, et al. Induced Pluripotent Stem Cell (iPSC)-Derived Extracellular Vesicles Are Safer and More Effective for Cardiac Repair Than iPSCs. Circ Res. 2018. Jan 19;122(2):296–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Maleki B, Alani B, Tamehri Zadeh SS, et al. MicroRNAs and exosomes: Cardiac stem cells in heart diseases. Pathology - Research and Practice. 2022 2022/January/01/;229:153701. [DOI] [PubMed] [Google Scholar]
- 187.Çakmak HA, Demir M. MicroRNA and Cardiovascular Diseases. Balkan Med J. 2020. Feb 28;37(2):60–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Shao L, Zhang Y, Lan B, et al. MiRNA-Sequence Indicates That Mesenchymal Stem Cells and Exosomes Have Similar Mechanism to Enhance Cardiac Repair. Biomed Res Int. 2017;2017:4150705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Torrini C, Cubero RJ, Dirkx E, et al. Common Regulatory Pathways Mediate Activity of MicroRNAs Inducing Cardiomyocyte Proliferation. Cell Rep. 2019. May 28;27(9):2759–2771.e5. *Of interest. The paper reveals intriguing findings on pro-regenerative miRNAs in acute myocardial infarction. miR-199a-3p activates YAP and enhances cardiomyocyte proliferation, offering potential therapeutic insights for stem cell-based approaches.
- 190.Tian Y, Liu Y, Wang T, et al. A microRNA-Hippo pathway that promotes cardiomyocyte proliferation and cardiac regeneration in mice. Sci Transl Med. 2015. Mar 18;7(279):279ra38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Sun X, Dai G, Yu L, et al. miR-143–3p inhibits the proliferation, migration and invasion in osteosarcoma by targeting FOSL2. Scientific Reports. 2018 2018/January/12;8(1):606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Wen SY, Lin Y, Yu YQ, et al. miR-506 acts as a tumor suppressor by directly targeting the hedgehog pathway transcription factor Gli3 in human cervical cancer. Oncogene. 2015. Feb 5;34(6):717–25. [DOI] [PubMed] [Google Scholar]
- 193.Mayourian J, Ceholski DK, Gorski PA, et al. Exosomal microRNA-21–5p Mediates Mesenchymal Stem Cell Paracrine Effects on Human Cardiac Tissue Contractility. Circulation research. 2018. Mar 30;122(7):933–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Yu B, Kim HW, Gong M, et al. Exosomes secreted from GATA-4 overexpressing mesenchymal stem cells serve as a reservoir of anti-apoptotic microRNAs for cardioprotection. Int J Cardiol. 2015. Mar 1;182:349–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Liu L, Jin X, Hu CF, et al. Exosomes Derived from Mesenchymal Stem Cells Rescue Myocardial Ischaemia/Reperfusion Injury by Inducing Cardiomyocyte Autophagy Via AMPK and Akt Pathways. Cellular Physiology and Biochemistry. 2017;43(1):52–68. [DOI] [PubMed] [Google Scholar]
- 196.Dougherty JA, Kumar N, Noor M, et al. Extracellular Vesicles Released by Human Induced-Pluripotent Stem Cell-Derived Cardiomyocytes Promote Angiogenesis. Front Physiol. 2018;9:1794. [DOI] [PMC free article] [PubMed] [Google Scholar]