Abstract
Despite the prominence of the weathering hypothesis as a mechanism underlying racialized inequities in morbidity and mortality, the life course social and economic determinants of Black–White disparities in biological aging remain inadequately understood. This study uses data from the Health and Retirement Study (), multivariable regression, and Kitagawa–Blinder–Oaxaca decomposition to assess Black–White disparities across three measures of biological aging: PhenoAge, Klemera–Doubal biological age, and homeostatic dysregulation. It also examines the contributions of racial differences in life course socioeconomic and stress exposures and vulnerability to those exposures to Black–White disparities in biological aging. Across the outcomes, Black individuals exhibited accelerated biological aging relative to White individuals. Decomposition analyses showed that racial differences in life course socioeconomic exposures accounted for roughly 27% to 55% of the racial disparities across the biological aging measures, and racial disparities in psychosocial stress exposure explained 7% to 11%. We found less evidence that heterogeneity in the associations between social exposures and biological aging by race contributed substantially to Black–White disparities in biological aging. Our findings offer new evidence of the role of life course social exposures in generating disparities in biological aging, with implications for understanding age patterns of morbidity and mortality risks.
Keywords: Biological aging, Weathering, Racialized disparities, Life course, Aging
Introduction
Relative to White people, Black people in the United States live shorter, sicker lives (Hayward et al. 2000; Hummer and Gutin 2018; Levine et al. 2001; Williams et al. 2010). Scholars describe Black health patterns as “first and worst”: Black people experience earlier onset of health decline, greater severity of disease, and poorer survival rates than Whites (Williams et al. 2010). Racism patterns exposure to many damaging social exposures linked to accelerated aging, including exposure to socioeconomic deprivation (Boen 2016; Phelan and Link 2015) and psychosocial stress (Boen 2020; Goosby et al. 2018; Umberson et al. 2017). The weathering hypothesis proposes that racial disparities in these social exposures generate racially divergent biological processes of aging, ultimately causing inequities in morbidity and mortality (Geronimus 1992; Geronimus et al. 2006). Analyses of molecular and physiological data reflecting racialized differences in aging support the weathering hypothesis; by multiple metrics, Black Americans are, on average, biologically older than White Americans of the same chronological age (Forde et al. 2019; Forrester et al. 2019; Geronimus et al. 2010; Graf, Crowe et al. 2022; Levine and Crimmins 2014).
Despite the prominence of the weathering hypothesis in research on racial inequities in health and longevity, the life course social and economic determinants of racial disparities in biological aging remain inadequately understood. In particular, research is needed on (1) the roles of multiple life course social exposures in generating racial disparities in biological aging and (2) heterogeneity in the impacts of social exposures on biological aging by race. Studies of racial disparities in biological aging have typically included single cross-sectional measures of adult socioeconomic status (SES) or stress exposure, and few have simultaneously integrated multiple life course measures of early-life or long-term SES or stress exposure into models of race gaps in biological age acceleration. Insights from cumulative inequality theory and its empirical applications (Ferraro and Shippee 2009) suggest that the racial gaps in morbidity and mortality risk arise from disparities in the accumulation of socioeconomic and stress exposures across the life span (Boen 2016; Brown and Hargrove 2018; Graetz et al. 2022; Shuey and Willson 2008; Simons et al. 2019). Hence, studies on racial disparities in biological aging should integrate multiple life course measures of socioeconomic and psychosocial stress exposures to more comprehensively capture the role of social and environmental factors in patterning race gaps in aging (Raffington and Belsky 2022). Further, a mounting body of evidence documents heterogeneity of the impacts of social exposures on health risks by race, with Black and White individuals having differential vulnerability to social exposures, including socioeconomic and stress exposures (Assari 2018; Boen 2016; Boen et al. 2020; Brown, Mitchell, and Ailshire 2020; Brown et al. 2023; Colen et al. 2018; Ferraro and Morton 2020; Gaydosh et al. 2018; Hargrove et al. 2022). Still, few studies of racial disparities in biological aging have assessed whether socioeconomic or stress gradients operate differently by race, accentuating the need for better understanding the role of these life course social exposures in generating racial disparities in biological aging.
Using data from the Health and Retirement Study (HRS), this study advances understanding of racial disparities in biological aging in three key ways. First, we assess racial disparities across three blood chemistry–based measures of biological aging. These measures quantify the progress of age-related deterioration across multiple bodily systems, and previous studies have validated that they predict aging-related morbidity and mortality in a large-scale representative study of aging (Cohen et al. 2013; Graf, Crowe et al. 2022; Hastings et al. 2019; Kwon and Belsky 2021; Levine 2013; Liu et al. 2018; Parker et al. 2020). Second, we integrate measures of life course SES and psychosocial stress in our models of racial disparities in biological aging and use regression and decomposition techniques to examine how the life course accumulation of socioeconomic and stress exposures contribute to later-life racial disparities in biological aging. Finally, we test racial differences in biological vulnerability to socioeconomic and stress exposures to improve understanding of their relative contributions to the racial patterning of biological aging and age-related biophysiological deterioration. Our study’s findings clarify the roles of life course social and economic exposures in generating Black–White disparities in biological aging, providing new evidence of how racism—a system of social stratification, domination, and oppression—“gets under the skin” to shape racialized inequalities in morbidity and mortality risks.
Background
Biological Aging
From a biological perspective, aging is the progressive loss of system integrity over time that drives disease and mortality risks (Harman 1981; Kaeberlein 2013). Population health researchers often measure chronological age and include it as a covariate in empirical work, partly to proxy biological aging and account for the increased risks of morbidity and mortality associated with aging across the life span. Still, although all humans age, the pace of aging is not universal or fixed. Across the life course, individuals experience an array of environments and exposures that can slow or accelerate aging (Crimmins 2020; George and Ferraro 2016). Chronic and repeated exposure to harmful social and environmental conditions and circumstances can manifest in the body as an accumulation of senescence and oxidative damage that accelerates biological aging (Danese and McEwen 2012; Vineis et al. 2016). Aging also reflects resilience. Material and psychosocial resources can buffer against the physiological impacts of damaging exposures, with inequalities in financial, network, and other resources also producing heterogeneity in physiological deterioration and biological aging (Brown and Hargrove 2018; Ferraro and Shippee 2009; Yang et al. 2016). As articulated through cumulative inequality theory, the accumulation of social and environmental exposures resulting from structural inequalities across the life span can produce divergent trajectories in physiological deterioration and the pace of aging within cohorts over time (Ferraro and Shippee 2009).
Still, there is no gold standard measure of biological aging (Ferrucci et al. 2020). The current state of the art in measurement are algorithms that combine information from multiple molecular or physiological measurements to estimate the state or pace of the underlying process of aging (Rutledge et al. 2022). Of these measures, composites of blood chemistry and other clinical measurements tend to be among the most predictive of morbidity and mortality (Belsky et al. 2018; Graf, Crowe et al. 2022; Li et al. 2020). These measures combine information across indicators of the body’s organ systems to quantify a latent process of aging-related deterioration in system integrity and resulting vulnerability to disease and death (Belsky et al. 2018; Kwon and Belsky 2021).
Biological aging measures offer two key advantages for analyses of population health disparities and their causes. Empirically, relative to measures of chronic disease, biological aging measures are more sensitive to the biological embedding of racialized living conditions. These measures are also sensitive to variations in aging processes that emerge beginning in young adulthood, well before chronic disease onset (Belsky et al. 2015). Conceptually, biological aging measures are better aligned with theoretical models of how living in a racialized society becomes biologically embedded, given that they integrate information across body systems and capture the diffuse effects of chronic exposure to the many physical and social toxins associated with occupying a marginalized position in a racialized social system (Bailey et al. 2017; Williams and Mohammed 2013).
In this study, we use three algorithms based on different models of aging-related decline in system integrity. The PhenoAge method (“PhenoAge”) is based on modeling differences in mortality risk (Levine et al. 2018). PhenoAge values reflect the age at which a person’s physiology-predicted mortality risk would be approximately “normal” in a reference sample. The Klemera–Doubal method biological age (“KDM biological age”) is based on modeling physiological differences between older and younger people (Klemera and Doubal 2006). KDM biological age values reflect the age at which a person’s physiology would be approximately “normal” in a reference sample. The homeostatic dysregulation method (“HD”) is based on modeling physiological deviation from a healthy reference (Cohen et al. 2013). HD values reflect how different a person’s physiology is from the reference norm. By conducting parallel analyses of these three measures, we increase confidence that findings reflect a true aging process rather than artifacts of a specific method of modeling aging (Belsky et al. 2018; Lawlor et al. 2016).
Racism, Biological Age Acceleration, and Racial Inequities in Morbidity and Mortality
Racial disparities in age patterns of physiological deterioration, biological aging, and morbidity and mortality risk stem from racism (Roberts 2012). Rather than reflecting a natural division among humans, race is a sociopolitical construct that is historically and contextually fluid and contingent and serves to justify and maintain racial domination (Dennis et al. 2021). Racism contributes to population health inequality partly by differentially exposing those racialized as White and those racialized as Black to disparate material, psychosocial, and environmental opportunities and risks across the life span (Ferraro and Shippee 2009; Gee et al. 2012; Geronimus et al. 2010; Graetz et al. 2022; Hayward et al. 2000), generating divergent age patterns of physiological dysregulation and biological aging and contributing to the racial patterning of morbidity and mortality risk (Graf, Crowe et al. 2022; Liu et al. 2018).
The social factors contributing to racial inequities in morbidity and mortality are complex and multifaceted, but studies point to two interrelated pathways linking racism to racialized morbidity and mortality risks (Brown et al. 2023). First, racism patterns Black–White health and mortality gaps through the unequal distribution of socioeconomic risks, resources, and opportunities across the life span (Boen 2016; Graetz et al. 2022; Hayward et al. 2000; Phelan and Link 2015). Socioeconomic conditions are fundamental determinants of health that shape health risks through biophysiological, behavioral, social, and environmental mechanisms (Link and Phelan 1995). Relative to Whites, Black Americans have markedly fewer socioeconomic resources, including substantially less wealth (Darity and Mullen 2020; Pfeffer and Killewald 2019), and experience more and longer spells of socioeconomic deprivation across the life span (Boen 2016; Shuey and Willson 2008). Black–White SES disparities result from historical legacies of racism, including the seizure of Black lands and assets through White violence beginning in the earliest days of chattel slavery, the failure of the Freedmen’s Bureau to provide “forty acres and a mule” to formerly enslaved individuals during Reconstruction, the exclusion of Black World War II veterans from receiving benefits under the GI Bill, and the de jure and de facto segregation of schools during and following Jim Crow (Darity and Mullen 2020). Contemporary forms of racism and discrimination across educational, housing, lending, carceral, and labor institutions and markets maintain and further exacerbate racial inequities in SES (Darity and Mullen 2020; Sewell 2016). The racial stratification of life course socioeconomic exposures is thought to be a primary pathway underlying racial disparities in health (Boen 2016; Brown et al. 2012; Graetz et al. 2022; Phelan and Link 2015) and biological aging (Simons et al. 2016; Simons et al. 2019).
Racial differences in psychosocial stress also contribute to Black–White health and mortality gaps (Boen 2020; Cheadle et al. 2020; Colen et al. 2018; Goosby et al. 2017; Sternthal et al. 2011). Much research has linked stress exposure to health and biophysiological risk. In response to stress, the hypothalamic–pituitary–adrenal axis and sympathetic nervous system secrete hormones to upregulate physiological functioning across body systems. Repeated activation of stress response systems in response to chronic stress can reduce the efficiency and effectiveness of these systems, thereby increasing inflammation and oxidative stress, allostatic load, and physiological dysregulation. These processes can accelerate cellular and biological aging and contribute to the development and progression of multiple pathologies over time (Epel et al. 2004; Goosby et al. 2018; McEwen 1998, 2007, 2012). Importantly, racism patterns social inequalities in stress exposure (Pearlin 1989; Sternthal et al. 2011; Williams 2018; Williams et al. 2010). Research has linked racial discrimination to racialized inequities in health and biophysiological functioning (Carter et al. 2019; Cheadle et al. 2020; Cuevas and Boen 2021; Goosby et al. 2018; Goosby et al. 2017). Racism also unequally burdens Black Americans with high levels of chronic, financial, and caregiving stress, as well as more stressful life events and traumas, contributing to racialized gaps in health and aging (Boen 2020; Goosby et al. 2018; Simons et al. 2021; Umberson et al. 2017).
Aims of the Present Study
This study has three key aims. First, we describe differences in biological age acceleration among older adults across three blood-based chemistry markers of biological aging. We use three measures because each makes different assumptions about the aging process and has different limitations.
Second, we quantify the relative contributions of life course socioeconomic and psychosocial stress exposures in producing Black–White gaps across these outcomes. We integrate markers of life course SES and stress exposure, using data from multiple life stages, to more fully capture how racial inequalities in the accumulation of social exposures shape patterns of biological aging.
Third, we assess the role of racial differences in vulnerability to socioeconomic and stress exposures in shaping racial disparities in biological aging. Evidence supporting the “diminishing returns hypothesis” indicates that Black individuals experience fewer health protections from increases in SES than Whites (Assari 2018; Boen 2016; Brown et al. 2023; Colen et al. 2018; Gaydosh et al. 2018; Graetz et al. 2022; Hargrove et al. 2022). Racial discrimination and exposure to high levels of contextual deprivation are some of the factors thought to restrict higher SES Black individuals, in particular, from converting socioeconomic gains into health improvements in the same ways as White individuals (Colen et al. 2018; Hargrove et al. 2022). Evidence also suggests that the health consequences of stress exposure may vary by race (Brown et al. 2023; Brown, Mitchell et al. 2020). Although a large body of research has documented higher stress levels among Black than White individuals, studies have also found lower levels of stress-related psychopathology among Black than White individuals (Brown et al. 2023; Mezuk et al. 2013). We therefore consider whether socioeconomic and stress gradients in biological aging vary by race.
Data and Methods
Data
Data for this study come from the Health and Retirement Study, a nationally representative, longitudinal study of U.S. adults aged 50 and older. Started in 1992, the HRS collects information about the social, economic, physical, and psychological well-being of older adults selected using a multistage area probability design (Heeringa and Connor 1995). Data for our outcome measures come from the 2016 Venous Blood Study. Although the HRS has collected blood-based biomarkers from finger sticks, dried blood spots, and salivary DNA since 2006, it first collected venous blood from respondents in 2016. All HRS panel respondents who completed a 2016 interview—except for proxy respondents and nursing home residents—were asked to consent to a venous blood draw by a trained phlebotomist (78.5% consented).1 We linked Venous Blood Study data with data from the RAND HRS (1992–2016) data file and the HRS Leave-Behind Questionnaire (2006–2012).
The analytic sample includes U.S.-born non-Hispanic Black and non-Hispanic White (hereafter, “Black” and “White”) HRS respondents who participated in the Venous Blood Study with complete data on the biomarkers used in the biological aging measures. We used multiple imputation with chained equations procedures to impute missing data on the stress measures; missing data on the other covariates were minimal (<5% of the sample had missing data). Our final sample includes 6,782 respondents.
Measures
Biological Aging
We measured biological aging using three published methods: the PhenoAge method (Levine et al. 2018), the Klemera–Doubal method biological age (Klemera and Doubal 2006), and the homeostatic dysregulation method (Cohen et al. 2013). Our discussion of results focuses on the PhenoAge algorithm, which shows strong and consistent associations with aging-related morbidity and mortality (Graf, Crowe et al. 2022; Liu et al. 2018); replications using the KDM biological age and homeostatic dysregulation measures appear in the tables in the online appendix.
An individual’s PhenoAge prediction corresponds to the chronological age at which their mortality risk would be approximately “normal” in a reference population. A PhenoAge older than chronological age indicates an advanced state of biological aging and increased risk for disease, disability, and mortality. A PhenoAge younger than chronological age indicates delayed biological aging and reduced risk for disease, disability, and mortality. The PhenoAge algorithm is derived from a multivariate analysis of mortality hazards (Levine and Crimmins 2018; Liu et al. 2018). The original PhenoAge algorithm was constructed from an elastic-net Gompertz regression of mortality on 42 biomarkers in the National Health and Nutrition Examination Survey III (NHANES III; Levine et al. 2018). That analysis selected nine biomarkers and chronological age as a parsimonious model used to compute a mortality prediction score. The mortality prediction score is then converted into a biological age value by matching the elastic-net model predicted score with mortality scores from a univariate Gompertz regression including only chronological age as a predictor. The chronological age at which the univariate model prediction matches the elastic-net model prediction is assigned as the biological age:
where represents the linear combination of biomarkers from the fitted model. is an ancillary parameter to be estimated from the data, and denotes time (here, in months). Thus, denotes the probability that the th individual will die within the next 120 months. The mortality score is then converted to a PhenoAge value based on a univariate Gompertz regression of the mortality hazard including only chronological age:
This regression estimates the probability that the individual will die within the next 120 months, where is the chronological age of the individual.
An individual’s KDM biological age prediction corresponds to the chronological age at which their physiology would be approximately considered “normal.” KDM biological age older than chronological age indicates an advanced state of biological aging and increased risk for disease, disability, and mortality. KDM biological age younger than chronological age indicates delayed biological aging and reduced risk for disease, disability, and mortality.
The KDM biological age algorithm is derived from a series of regressions of individual biomarkers on chronological age in a reference population. The equation takes information from number of regression lines of chronological age regressed on biomarkers:
where is the value of biomarker measured for an individual. For each biomarker , the parameters , and —the regression intercept, slope, and root-mean-square error, respectively—are estimated from a regression of chronological age on the biomarker in the reference sample. is a scaling factor equal to the square root of the variance in chronological age explained by the biomarker set in the reference sample. is chronological age. We formed the reference sample to compute the KDM biological age algorithm from nonpregnant NHANES III participants aged 30–75 years. Algorithm parameters are estimated separately for men and women.
An individual’s HD value corresponds to how different their physiology is from a healthy reference. Higher HD values indicate an advanced state of biological aging and increased risk for disease, disability, and mortality. Lower HD values indicate delayed biological aging and reduced risk for disease, disability, and mortality. HD is computed as the Mahalanobis distance (Mahalanobis 1936) for a set of biomarkers relative to a reference sample:
where is a multivariate observation (all biomarker values for an individual), and is the equivalent-length vector of reference sample means for each variable. is the reference sample variance–covariance matrix for the variables. If all variables are uncorrelated, then this calculation is equivalent to scaling each biomarker by its variance and summing the squared deviance for an observation:
where is the number of biomarkers, and is the variance in the ith biomarker. We specified the reference sample to be nonpregnant NHANES III participants aged 20–30 years for whom all user-selected biomarkers fall within the clinically normal range. On the basis of this reference sample, all biomarkers were standardized to have a mean of 0 and a standard deviation of 1 separately for men and women. Thus, we computed HD relative to a young, healthy sample, following the approach used in other published research (Belsky et al. 2018; Hastings et al. 2019; Parker et al. 2020).
We computed all three biological aging measures using eight of the nine biomarkers identified in Levine’s PhenoAge analysis (Levine et al. 2018): albumin, alkaline phosphatase, creatinine, C-reactive protein, lymphocyte %, mean cell volume, red cell distribution width, and white blood cell count. We substituted HbA1C for fasting glucose. Algorithm parameters were trained using data from NHANES III and projected onto the 2016 Venous Blood Study data using the BioAge R package (Kwon and Belsky 2021).
We calculated biological age advancement values for the PhenoAge and KDM biological age values by subtracting participants’ chronological ages from their biological age values. We log-transformed HD values for analysis.
Other Measures
The racial disparity in the outcomes is indicated by a dummy variable for race (1 = Black). A composite factor score reflects three dimensions of life course SES: (1) early-life SES (parental education, father’s occupation, perceived SES in childhood, and an index of poverty-related events in childhood); (2) respondent education; and (3) long-term household wealth, which indicates inflation-adjusted, inverse hyperbolic sine (IHS)–transformed average household wealth across all observed HRS waves (1992–2016). We used an IHS-transformed wealth measure to reduce the extreme right skew while retaining negative wealth values. To generate the composite SES measure, we used principal components factor analysis (PCA). The SES components loaded onto a single factor with strong factor loadings (eigenvalue = 1.62; factor loadings = .69–.80). As in Boen (2020), cumulative stress burden is a composite factor score that includes measures of everyday discrimination, major life discrimination, chronic strain, financial strain, stressful life events, and life-time traumas. To create the composite indicator of stress burden, we first generated measures of average stress exposure for each stress measure across the 2006–2012 HRS waves.2 Respondents did not answer questions about stress exposure at every wave. For individuals with valid stress exposure data in multiple waves, we averaged scores for each stressor measure across waves. We then used factor analysis to generate the composite stress burden factor score. Our stress measure therefore reflects cumulative stress burden across domains of stress across multiple years. We generated the composite stress burden measure using PCA, and all stress measures loaded onto a single factor (eigenvalue = 2.16) with relatively high factor loadings. Supplementary analyses with alternative operationalizations of the SES and stress measures (e.g., including the individual SES and stress measures separately) yielded substantively similar results. We also included interaction terms for Black × SES and Black × stress. Other measures include age (years), age2, sex (1 = female), HRS birth cohort (1 = AHEAD; 2 = CODA; 3 = HRS; 4 = war babies; 5 = early baby boomers; 6 = mid–baby boomers), and region of birth (1 = born in the South). We included measures of Black × age and female × age to allow age patterns to vary by race and sex. Supplementary analyses included measures of Black × age2 and female × age2, but these measures were not related to the outcomes and were excluded from the final models. We also conducted supplemental sex-stratified analyses (described later).
Analytic Strategy
To examine the patterns and determinants of racial disparities in the markers of biological aging, we began with descriptive statistics, paying particular attention to Black–White differences in the outcomes and exposures. We also conducted preliminary linear regression analyses that assessed racial disparities in (1) biological aging, (2) socioeconomic gradients in biological age acceleration, and (3) the association between stress burden and biological age acceleration. These analyses provide preliminary evidence of racial, socioeconomic, and stress differences in biological aging and offer insights into whether the associations among SES, stress, and biological age vary by race.
We then estimated associations of socioeconomic and stress exposures with biological age measures using multivariable linear regression models. We built models stepwise. For each outcome, Model 1 is the basic adjusted model of the Black–White disparity with sociodemographic controls (including age, age2, Black × age, sex, female × age, birth cohort, and region of birth) but no adjustment for SES or stress. Model 2 builds on Model 1 by including the composite SES measure, and Model 3 builds on Model 2 by including Black × SES. Model 4 builds on Model 1 by adjusting for stress, and Model 5 builds on Model 4 by including Black × stress. Model 6 is the fully adjusted model. These stepwise models provide preliminary evidence of the contributions of racial differences in SES and stress—as well as racial variation in the associations of SES and stress with the outcomes—to racial disparities in biological age acceleration. All models include clustered standard errors at the household level. Finally, we used Kitagawa–Blinder–Oaxaca decomposition (Jann 2008; Kitagawa 1955; Oaxaca 1973) to quantify the contribution of racial differences in the covariates to race gaps in biological age acceleration, focusing on the roles of the SES and stress measures. We used a threefold decomposition (Jann 2008) that divides the racial disparities in the outcomes into (1) endowment effects, indicating the proportion of the disparity due to group differences in the distributions of the predictors or covariates; (2) coefficient effects, reflecting the contribution of differences in the associations between the predictors or covariates and the outcome to the disparity; and (3) interaction effects, indicating the contribution of interaction terms that account for group differences in endowments and coefficients that exist simultaneously. This method involves first estimating two group-specific regression models of the outcomes and then performing the decomposition of the mean group difference in each outcome. The decomposition is a counterfactual approach, replacing the levels of predictor variables and the coefficients of one group with corresponding values for the reference group. The expected change in a group’s mean outcome is obtained when this group is assigned the predictor values and regression coefficients of the reference (Jann 2008; Rahimi and Hashemi Nazari 2021). Using the threefold decomposition, we estimated how much of the Black–White gap in biological age acceleration can be linked to racial differences in the distributions of the socioeconomic and stress burden measures (i.e., endowment effects), as well as racial differences in the associations between the SES and stress measures and the markers of biological age acceleration (i.e., coefficient effects). This approach allows us to consider simultaneously how racial differences in the distribution of multiple social exposures and vulnerability to them contribute to racialized inequities in biological aging.
Although the multivariable and decomposition analyses adjust for sex, race and sex might intersect to shape biological aging patterns. Drawing on insights from intersectional studies of health and mortality (e.g., Brown et al. 2016; Warner and Brown 2011), we conducted supplemental sex-stratified analyses to assess how patterns of biological aging vary jointly by race and sex.
Results
Descriptive Analyses
Descriptive statistics are shown in Table 1. Across all three markers of biological aging, Black respondents show accelerated biological aging relative to White respondents. For example, Black individuals exhibit more advanced PhenoAge acceleration than Whites (2.730 and 0.113 years, respectively). Using the KDM biological age acceleration measure, we find that Black individuals are more than 10 years older than Whites (10.637 vs. −0.767, respectively). HD is also higher for Black than White individuals. Results in Table 1 further show racial disparities in life course socioeconomic and stress exposures: Black respondents have lower SES and higher stress burdens than White respondents (p < .001).
Table 1.
Descriptive statistics
| Full Sample | White | Black | p Valuea | |
|---|---|---|---|---|
|
| ||||
| Mean/Prop. (N = 6,782) | Mean/Prop. (n = 5,429) | Mean/Prop. (n = 1,353) | ||
|
| ||||
| Outcomes | ||||
| PhenoAge biological age acceleration | 0.635 | 0.113 | 2.730 | <.001 |
| KDM biological age acceleration | 1.508 | −0.767 | 10.637 | <.001 |
| Homeostatic dysregulation (log) | 3.969 | 3.887 | 4.298 | <.001 |
| Covariates | ||||
| Race (1 = Black) | .199 | |||
| Age (years) | 70.910 | 71.786 | 67.392 | <.001 |
| Sex (1 = female) | .582 | .568 | .636 | <.001 |
| Birth cohort | ||||
| AHEAD | .010 | .012 | .002 | |
| CODA | .060 | .069 | .024 | |
| HRS | .304 | .330 | .200 | <.001 |
| War babies | .165 | .178 | .117 | |
| Early baby boomers | .222 | .200 | .309 | |
| Mid-baby boomers | .238 | .211 | .348 | |
| Region of birth (1 = South) | .354 | .277 | .664 | <.001 |
| SES (factor score) | .128 | .304 | −.581 | <.001 |
| Stress burden (factor score) | .024 | −.064 | .377 | <.001 |
p value of the Black-White difference in mean/proportion, two-tailed test.
Figure 1 plots the unadjusted Black–White disparity in PhenoAge acceleration by age (panel a), the socioeconomic gradient in PhenoAge acceleration by race (panel b), and the stress gradient in PhenoAge by race (panel c). Panel a illustrates accelerated PhenoAge for Black relative to White individuals across midlife to late life, and the racial gap in PhenoAge acceleration converges slightly at later ages, possibly owing to mortality selection.
Fig. 1.
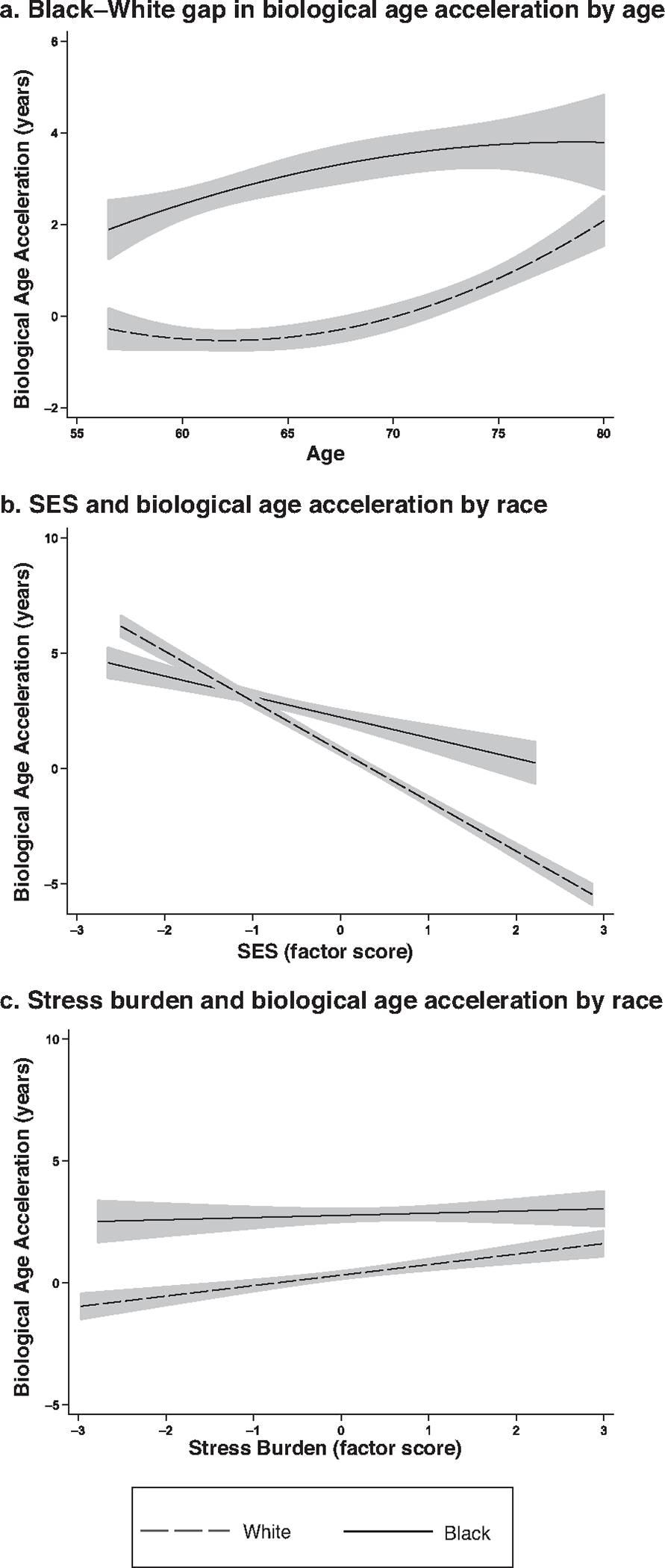
Associations of age, SES, and stress burden with biological age acceleration (PhenoAge algorithm) by race: Unadjusted Black–White disparity in biological age acceleration by age (panel a), unadjusted association between SES and biological age acceleration by race (panel b), and unadjusted association between stress burden and biological age acceleration by race (panel c). n = 6,782.
Panel b of Figure 1 displays the bivariate associations between the SES factor score and PhenoAge acceleration by race, showing smoothed PhenoAge acceleration scores over SES by race. SES and biological age acceleration are strongly and negatively associated, especially among Whites, for whom increases in SES are associated with slowed biological aging. The SES gradient in biological age acceleration is flatter for Black than White individuals, and the racial gap in biological age acceleration is widest among higher SES respondents, suggesting that socioeconomic gradients may vary by race. Panel c of Figure 1 shows the unadjusted association between stress burden and PhenoAge acceleration by race. At all stress levels, biological age acceleration is more advanced for Black relative to White individuals. The association between stress and biological age acceleration is positive for White individuals: increases in stress correspond to more advanced biological age acceleration. The stress gradient in biological age acceleration is flatter for Black than White individuals, suggesting that the associations between stress and biological aging may also vary by race.
Multivariable Regression Models
Results of the multivariate biological age models using the PhenoAge measure are shown in Table 2; model results for the KDM biological age and HD measures are shown in Tables A1 and A2, respectively (in the online appendix, along with other tables designated with an “A”). Although the parameter estimates vary across the three outcomes, the substantive interpretations of the findings are largely consistent, speaking to the robustness of the results.
Table 2.
Multivariable models of Black-White disparity in biological age acceleration (PhenoAge algorithm): HRS
| Model 1: | Model 2: | Model 3: | Model 4: | Model 5: | Model 6: | |
|---|---|---|---|---|---|---|
|
|
||||||
| Basic Adjusted | Model 1 + SES | Model 2 + Race × SES | Model 1 + Stress | Model 4 + Race × Stress | Fully Adjusted | |
|
| ||||||
| Racial Disparity (1 = Black) | 8.716*** (2.263) |
7.429*** (2.251) |
7.477*** (2.244) |
8.245*** (2.268) |
10.735*** (2.385) |
9.159*** (2.371) |
| Age | 0.048 (0.194) |
0.166 (0.192) |
0.180 (0.192) |
0.106 (0.194) |
0.118 (0.194) |
0.217 (0.192) |
| Black × Age | −0.084* (0.033) |
−0.085* (0.033) |
−0.081* (0.033) |
−0.081* (0.033) |
−0.113** (0.035) |
−0.105** (0.035) |
| Sex (1 = female) | 4.181** (1.555) |
4.051** (1.536) |
4.090** (1.534) |
4.227** (1.553) |
4.136** (1.552) |
4.050** (1.533) |
| Female × Age | −0.093*** (0.022) |
−0.094*** (0.022) |
−0.095*** (0.022) |
−0.093*** (0.022) |
−0.091*** (0.022) |
−0.093*** (0.022) |
| Age2 | 0.001 (0.001) |
−0.000 (0.001) |
−0.001 (0.001) |
0.000 (0.001) |
0.000 (0.001) |
−0.001 (0.001) |
| Birth Cohort | −0.455 (0.329) |
−0.557† (0.324) |
−0.533 (0.324) |
−0.467 (0.329) |
−0.474 (0.328) |
−0.544 (0.324) |
| Region of Birth (1 = South) | 0.824*** (0.235) |
0.470* (0.232) |
0.478* (0.232) |
0.853*** (0.235) |
0.833*** (0.235) |
0.501* (0.231) |
| SES (factor score) | −1.651*** (0.113) |
−1.763*** (0.120) |
−1.644*** (0.123) |
|||
| Black × SES | 0.671* (0.336) |
0.544 (0.340) |
||||
| Stress Burden (factor score) | 0.705*** (0.125) |
0.985*** (0.139) |
0.677*** (0.141) |
|||
| Black × Stress Burden | −0.985*** (0.294) |
−0.761** (0.294) |
||||
| Intercept | −2.982 (9.040) |
−5.340 (8.928) |
−6.003 (8.918) |
−5.690 (9.011) |
−6.271 (9.006) |
−7.958 (8.904) |
Notes: Results of ordinary least-squares regression models are presented. Standard errors, shown in parentheses, are clustered at the household level. n = 6,782.
p < .10;
p < .05;
p < .01;
p < .001
Results from Model 1 across Tables 2, A1, and A2 indicate that, on average, Black individuals experience accelerated biological aging relative to White individuals. Model 1 for the PhenoAge measure (Table 2) estimates that Black respondents are biologically roughly 8.7 years older than White respondents of the same chronological age (β = 8.716, p < .001). For both the PhenoAge acceleration (Table 2) and HD (Table A2) measures, the racial disparity in biological age acceleration declines with advanced chronological age, as indicated by the parameter estimates for Black × age; by contrast, the racial disparity in the measure of KMD biological age acceleration remains stable with age (Table A1).
For each outcome, Model 2 builds on Model 1 by adjusting for SES. Results from Model 2 in Table 2 reveal that SES is negatively associated with biological age acceleration using the PhenoAge measure (β = −1.651, p < .001). We also estimate negative associations between SES and the KDM biological age acceleration and HD measures. Across the outcomes, adjusting for SES in Model 2 reduces the magnitude of the racial disparity in biological age acceleration over Model 1, providing early suggestive evidence that racial disparities in life course SES may contribute to racial disparities in biological aging.
Model 3 builds on Model 2 by including the interaction for Black × SES to assess whether the SES gradients in biological age acceleration vary by race. Using the PhenoAge measure, results from Model 3 in Table 2 reveal a positive parameter estimate for Black × SES: although SES is negatively associated with biological age acceleration, the SES gradient in biological age acceleration is steeper for White than Black adults. This finding is consistent with the preliminary results in panel b of Figure 1. We also find evidence of the diminishing returns hypothesis using the HD measure (Table A2), although the parameter estimate for Black × SES is only marginally statistically significant (p < .10).
Model 4 builds on Model 1 by adjusting for stress burden. In the PhenoAge models (Table 2), stress burden is positively associated with biological age acceleration (β = 0.705, p < .001). Stress burden is also positively associated with KDM biological age acceleration and HD. After we adjust for stress burden, the racial disparity in the outcomes is attenuated over Model 1. Still, results from Model 5 in Tables 2, A1, and A2 suggest that the links between stress burden and biological aging vary by race. Across all three outcomes, the parameter estimates for Black × stress are negative, indicating that White individuals experience more rapid biological age acceleration than Black individuals with increases in stress exposure.
Finally, Model 6 is the fully adjusted model. Across all outcomes, the racial disparity in biological age acceleration is still large and statistically significant: Black individuals have substantially older biological age profiles than White individuals. In Model 6 of Table 2, Black respondents are biologically more than 9 years older than White individuals of the same chronological age (β = 9.159, p < .001). In the fully adjusted models in Tables 2, A1, and A2, the summary SES score maintains a negative association with the outcomes, but the coefficient estimates for Black × SES are not statistically significant. In Model 6, stress burden maintains a positive association with all three outcomes, and the coefficient estimates for Black × stress are negative, again suggesting more rapid biological age acceleration with increases in stress burden among White than Black individuals.
Kitagawa–Blinder–Oaxaca Decomposition
Figure 2 shows abbreviated Kitagawa–Blinder–Oaxaca decomposition results using the PhenoAge measure. In the interest of space, we focus on the SES and stress “endowment” and “coefficient” estimates in this description of the decomposition results. Full decomposition results for all three outcomes are shown in Table 3.
Fig. 2.
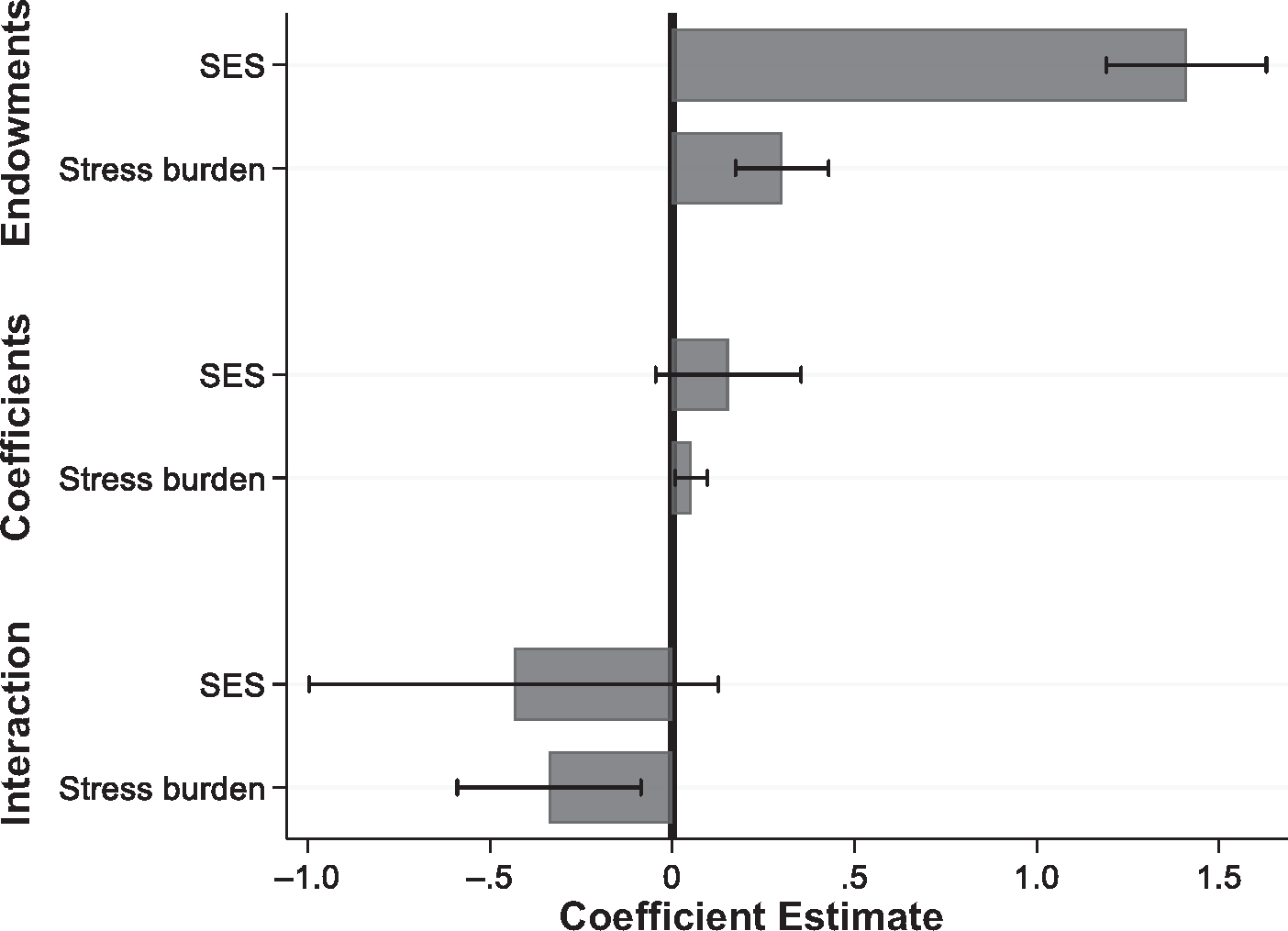
Contributions of life course SES and stress burden to Black–White disparities in biological age acceleration (PhenoAge algorithm). Results of Kitagawa–Blinder–Oaxaca decomposition of racial disparities in the PhenoAge measure of biological age acceleration. Analyses included the full set of covariates (age, sex, cohort, region of birth, SES, and stress burden). In the interest of space, only estimates for SES and stress burden are displayed. Full decomposition results are shown in Table 3. Whiskers represent 95% confidence intervals.
Table 3.
Kitagawa-Oaxaca-Blinder decomposition of racial disparities in biological age acceleration
| PhenoAge |
KDM Biological Age |
Homeostatic Dysregulation |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Coeff. (SE) | p Value | % | Coeff. (SE) | p Value | % | Coeff. (SE) | p Value | % | |
|
| |||||||||
| Overall | |||||||||
| Black-White disparity in outcome | 2.622 (0.282) |
*** | — | 11.422 (0.884) |
*** | — | 0.412 (0.027) |
*** | — |
| Endowments | 1.229 (0.170) |
*** | 46.89 | 5.045 (0.516) |
*** | 44.17 | 0.058 (0.018) |
** | 14.20 |
| Coefficients | 2.347 (0.485) |
*** | 89.49 | 9.851 (1.554) |
*** | 86.24 | 0.417 (0.043) |
*** | 101.37 |
| Interaction | −0.954 (0.437) |
* | −36.37 | −3.474 (1.344) |
* | −30.41 | −0.064 (0.039) |
−15.57 | |
| Endowments | |||||||||
| SES | 1.436 (0.116) |
*** | 54.77 | 4.077 (0.358) |
*** | 35.69 | 0.111 (0.012) |
*** | 26.85 |
| Stress burden | 0.301 (0.067) |
*** | 11.46 | 0.856 (0.201) |
*** | 7.49 | 0.027 (0.007) |
*** | 6.61 |
| Age | −0.189 (0.197) |
−7.22 | 0.305 (0.617) |
2.67 | −0.102 (0.021) |
*** | −24.72 | ||
| Sex | −0.185 (0.043) |
*** | −7.04 | −0.549 (0.129) |
*** | −4.81 | −0.012 (0.003) |
*** | −2.85 |
| Cohort | −0.469 (0.205) |
* | −17.87 | −0.474 (0.634) |
−4.15 | −0.006 (0.022) |
−1.45 | ||
| Region of birth (1 = South) | 0.335 (0.097) |
** | 12.79 | 0.830 (0.305) |
** | 7.27 | 0.040 (0.010) |
*** | 9.76 |
| Coefficients | |||||||||
| SES | 0.129 (0.103) |
4.91 | 0.209 (0.313) |
1.83 | 0.011 (0.009) |
2.77 | |||
| Stress burden | 0.051 (0.022) |
* | 1.96 | 0.141 (0.068) |
* | 1.23 | 0.005 (0.002) |
* | 1.16 |
| Age | −0.372 (9.103) |
−14.18 | −4.453 (29.328) |
−38.99 | −0.437 (0.831) |
−106.08 | |||
| Sex | 0.488 (0.331) |
18.62 | −0.340 (1.084) |
−2.98 | 0.023 (0.031) |
5.67 | |||
| Cohort | 2.547 (3.561) |
97.14 | 2.509 (11.634) |
21.97 | 0.133 (0.333) |
32.19 | |||
| Region of birth (1 = South) | −0.500 (0.173) |
** | −19.05 | −1.791 (0.545) |
** | −15.68 | −0.034 (0.016) |
* | −8.23 |
| Interaction | |||||||||
| SES | −0.374 (0.299) |
−14.27 | −0.607 (0.910) |
−5.32 | −0.033 (0.028) |
−8.05 | |||
| Stress burden | −0.356 (0.133) |
** | −13.59 | −0.984 (0.432) |
* | −8.62 | −0.033 (0.013) |
* | −8.11 |
| Age | 0.023 (0.551) |
0.86 | 0.270 (1.776) |
2.36 | 0.026 (0.050) |
6.42 | |||
| Sex | 0.058 (0.041) |
2.22 | −0.041 (0.129) |
−0.35 | 0.003 (0.004) |
0.68 | |||
| Cohort | 0.393 (0.550) |
14.99 | 0.387 (1.796) |
3.39 | 0.020 (0.051) |
4.97 | |||
| Region of birth (1 = South) | −0.697 (0.243) |
** | −26.58 | −2.498 (0.763) |
** | −21.87 | −0.047 (0.023) |
* | −11.48 |
Notes: Results of threefold decomposition that decomposes Black-White disparity. n = 6,782.
p<.05;
p<.01;
p<.001
The decomposition analyses support the hypothesis that Black–White differences in socioeconomic and stress exposures (or race differences in SES and stress “endowments”) account for substantial proportions of the racial gaps, with racial differences in life course SES playing a particularly critical role. Specifically, the racial disparity in life course SES accounts for more than half (54.77%) of the Black–White gap in PhenoAge acceleration (p < .001). Race differences in SES endowments account for comparatively less of the race gaps in the KMD biological age acceleration and HD measures (35.69% and 26.85%, respectively), although race differences in SES are still prominent contributors to disparities in these outcomes. Black–White differences in the distribution of stress burden also contribute substantially to race gaps in the outcomes (PhenoAge measure: 11.46% of the race gap, p < .001; KDM measure: 7.49%, p < .001; HD measure: 6.61%, p < .001).
The decomposition results support the hypothesis that racial differences in vulner-bility to stress contributed to racial disparities in the outcomes, although the magnitude of these contributions is substantially smaller than the endowment contributions. Racial differences in the associations between stress burden and PhenoAge account for 1.96% of the racial disparity in the outcome (p = .021), and estimates for the KDM biological age and HD measures are similar. We find no evidence that Black–White differences in returns to SES are significant determinants of racial disparities in any of the three outcomes.
Sex-Stratified Analyses
Table A3 displays the results of sex-stratified multivariable ordinary least-squares models of the PhenoAge acceleration measure. For both sexes, results for Models 1–6 suggest weathering among Black adults. The magnitude of the race differences in PhenoAge acceleration are similar for males and females. Further, supplementary analyses using the full sample and including a Black × female interaction showed that race differences in PhenoAge acceleration did not vary significantly by sex. Consistent with the results in Table 2, both SES and stress burden are associated with PhenoAge acceleration in the sex-stratified models.
In Model 4 for males, the coefficient estimate for Black × SES (β = 1.739, p < .01) indicates that the SES gradient in biological aging is steeper for White than Black males. The coefficient estimate for Black × SES remains positive and statistically significant for males in Model 6. By contrast, for females, the coefficient estimate for Black × SES is not statistically significant, indicating no evidence of differential returns among females. Still, supplementary analyses (not shown here) using a three-way interaction (sex × Black × SES) revealed that this sex difference in the association of SES by race was not statistically significant; for this reason, we interpret these coefficient differences between males and females as suggestive in nature.
The coefficient estimate for Black × stress burden is only marginally significant for males in Model 5 but is not statistically significant in Model 6, the fully adjusted model. Results of Models 5 and 6 suggest more rapid biological age acceleration with increases in stress burden for White than Black females. Supplementary analyses using a three-way interaction (sex × Black × stress burden) indicated that this sex difference was not statistically significant. Thus, we interpret these sex differences cautiously.
Table A4 presents the results of the sex-stratified decomposition analyses for all three outcomes. For both males and females, racial differences in SES account for the greatest proportion of Black–White gaps across the three biological aging measures. Qualitatively, though, race differences in SES generally account for more of the racialized gaps in PhenoAge acceleration and KDM biological age for males than females. Estimates of the contribution of race differences in stress burden to the Black–White gaps in the outcomes are similar for males and females. Again, the evidence suggests sex differences in the associations between the SES and stress measures with the outcomes by race, but we interpret these sex differences cautiously, given the lack of statistical significance.
Discussion
Black people in the United States experience higher and earlier risks of morbidity and mortality than White Americans for almost all leading causes of death (Williams et al. 2010). The weathering hypothesis (Geronimus 1992; Geronimus et al. 2006) contends that racialized differences in biological aging may underlie these inequities in life course morbidity and mortality risks. The accumulation of oxidative damage and senescence resulting from repeated adverse social and economic exposures across the life span is hypothesized to accelerate the biological aging process for Black people, in particular (Geronimus et al. 2006; Geronimus et al. 2010). Many previous empirical tests of this hypothesis used singular biomarkers with rather limited abilities to capture the complex biological process of aging. In contrast, this study provided a new, rigorous test by examining Black–White disparities across three comprehensive measures of biological aging linked to morbidity and mortality. In addition to documenting racialized inequities in biological aging, we used a combination of multivariable regression and decomposition analyses to further assess the contributions of social and economic exposures to Black–White disparities in biological aging. We considered the roles of racial differences in both life course exposures and vulnerability to those exposures in patterning disparities in biological aging. We also considered how race and sex potentially intersect to shape biological aging patterns. Our study offers three contributions to understanding the patterns and life course determinants of racialized disparities in biological age acceleration across midlife to late life.
First, our findings indicate that using chronological age as a proxy for age-related physiological deterioration and dysregulation in population health research may mask substantial heterogeneity in biological aging by race. Consistent with the weathering hypothesis (Geronimus 1992; Geronimus et al. 2006) and in accordance with previous analyses (Forrester et al. 2019; Graf, Crowe et al. 2022; Levine and Crimmins 2018), our results showed strong evidence of Black–White disparities in biological age acceleration across three outcomes. For example, PhenoAge models showed accelerated biological aging among Black relative to White individuals (Table 2); in Model 1, Black individuals were biologically nearly 9 years older than Whites of the same chronological age. We also documented significant racial gaps in KDM biological age acceleration and HD. The similarity in results across the biological age acceleration measures suggests that a consistent latent construct underlies the aging process, boosting confidence that future studies adopting any of these measures can capture the essence of biological senescence. Racial disparities in biological age acceleration may therefore represent an important biological mechanism underlying Black–White morbidity and mortality risks in adulthood (Graf, Crowe et al. 2022; Levine and Crimmins 2014).
Second, our results indicate that racialized disparities in life course social and economic exposures play a central role in patterning Black–White inequities in biological age acceleration. Descriptive statistics in Table 1 indicate striking Black–White inequities in the socioeconomic and stress exposure measures. Results in Tables 2, A1, and A2 showed that life course SES and stress burden measures were strongly associated with the markers of biological age acceleration. Decomposition analyses in Figure 2 and Table 3 further revealed that Black–White differences in the distributions of life course SES and stress burden explained a substantial portion of the racial disparities in biological age acceleration: Black–White disparities in SES accounted for roughly 27% to 55%, and Black–White disparities in stress burden accounted for 7% to 11% of the racial disparities across the outcomes.
Our results help clarify how racism accelerates biological aging among Black relative to White people. For one, racism produces highly racialized distributions of SES across the life span in ways that pattern biological aging. Centuries of legally sanctioned and de facto racial segregation, discrimination, and violence have excluded Black individuals and families from many processes and instruments of wealth accumulation, financial security, and social mobility while generating tremendous socioeconomic resources and opportunities for White people (Darity and Mullen 2020). These racialized SES inequities are central contributors to Black–White gaps in biological aging. Consistent with other work (Boen 2020; Cheadle et al. 2020; Goosby et al. 2018; Umberson et al. 2017), we also showed that racism unequally patterns exposure to stressors across the life span—including discrimination, trauma, and grief—in ways that generate biological aging inequities. Importantly, although we included SES and stress as separate measures in our study, there are numerous inter-connected pathways through which SES and stress jointly pattern biological risks (Schnittker and McLeod 2005).
A central principle of the life course perspective is that aging is a lifelong process that begins at (or before) birth (Pavalko and Willson 2011), with social and economic conditions across the life span influencing life course trajectories of health and aging (Belsky et al. 2017; Boen 2016; Ferraro and Shippee 2009; Hayward et al. 2000; Yang et al. 2020). Still, research in this area has largely relied on cross-sectional indicators of SES or stress. In this study, we integrated measures of life course SES, including markers of early-life socioeconomic conditions, educational attainment, and long-term wealth. We also examined cumulative stress burden, accounting for exposure to chronic and acute stressors and strains across the life span. We thereby more comprehensively accounted for the variety of life course social and economic processes undergirding aging patterns in later life. Our results showed that racial inequities in the life course accumulation of social and economic exposures are central drivers of racial disparities in aging (Ferraro and Shippee 2009; Gee et al. 2012; Geronimus 1992; Geronimus et al. 2006).
A third contribution of this study is that results showed that although SES and stress gradients in biological aging may operate differently for Black and White individuals, racial differences in biological vulnerability to the SES and stress measures accounted for much less of the racial disparities in biological age acceleration than racial differences in social and economic exposures. A growing body of research has found that the associations between SES and health (Boen 2016; Colen et al. 2018; Gaydosh et al. 2018; Graetz et al. 2022) and stress and health (Brown, Abrams et al. 2020; Brown and Hargrove 2018) may vary by race. Results from our descriptive analyses lend preliminary support to these differential vulnerability hypotheses.
Still, the unique insights from decomposition analyses are that these heterogeneous associations played a substantially smaller role in explaining Black–White gaps in biological aging than racial disparities in the exposures. Notably, whereas racialized differences in SES exposures explained roughly 27% to 55% of the racial disparities across the outcomes, race differences in the associations between SES and the outcomes did not significantly explain Black–White gaps in the outcomes. Similarly, racial disparities in stress exposures explained approximately 7% to 11% of the race gaps in the outcomes, whereas race differences in the associations between stress burden and the outcomes explained less than 2%. In fact, our findings showed that White individuals are more biologically vulnerable to psychosocial stress than Black individuals. This finding adds to a growing body of research showing that despite having greater stress burdens, Black individuals may have better access to psychosocial resources, social supports, and coping strategies than Whites, leaving them better equipped to manage chronic and acute stressors and strains (Brown, Abrams et al. 2020; Brown, Mitchell et al. 2020; Brown et al. 2023; Mezuk et al. 2013). Nevertheless, our results point to the prominence of racialized inequities in life course social and economic exposures, rather than race differences in vulnerability to those exposures, in generating disparities in biological aging.
Our findings have critical implications for policy and intervention aimed at reducing racialized inequities in biological aging and, consequently, morbidity and mortality risks. Foremost, our results highlight the urgency of closing Black–White inequities in SES, which might substantially reduce the patterns of weathering documented here and in past research. Socioeconomic exposures accumulate in the body as people age (Ferraro and Shippee 2009), highlighting the need for efforts targeting racialized socioeconomic gaps from infancy through late life. The root causes of such gaps are structural, rooted in racist historical and contemporary policies and institutional practices that unequally distribute socioeconomic resources and rewards along racial lines. Therefore, racial economic equity will require systemic change to improve the economic, educational, and financial well-being of American households while targeting racial disparities in poverty, educational attainment, wealth, and other socioeconomic resources. Results of our supplementary analyses using the component measures of the composite SES measure showed that among the SES measures, racial differences in long-term wealth played the most substantial role in generating Black–White gaps in biological aging. This finding is consistent with other work (Graf, Zhang et al. 2022; Himmelstein et al. 2022) showing that efforts to reduce wealth inequality can greatly reduce population inequities in health and aging. Our results also highlight the central role of racism in patterning psychosocial stress across domains of stress and throughout the life span in ways that shape racialized risks of weathering. Reducing Black–White inequities in biological aging therefore requires attention to how racism differentially burdens Black people, in particular, with a host of chronic and acute traumas and strains that pattern morbidity and mortality risks.
Drawing on intersectional frameworks emphasizing how various dimensions of social stratification can jointly pattern health and aging (Brown et al. 2016; Dill and Zambrana 2009; Warner and Brown 2011), we also analyzed how racialized patterns of biological aging may vary by sex. For both males and females, we documented consistent evidence of weathering and strong, consistent evidence of SES and stress gradients in biological aging. We also found indications of possible heterogeneity in the associations among SES, stress, and the outcomes at the intersection of race and sex that warrant attention in future research. Still, we urge caution in interpreting these results because the sex and race differences were not statistically significant.
This study is not without limitations. First, the biological aging measures are available in only one HRS wave, preventing us from examining longitudinal trajectories of biological aging. Black individuals may experience more rapid rates of biological aging earlier in the life course in ways that our data cannot reveal. Second, although our measures of SES and stress burden predate the biological aging measures, concerns about reverse causality linger because we had only one wave of biological aging data. Third, our results may be subject to concerns about mortality selection. Given that the most structurally vulnerable individuals may have died before inclusion in these data, our estimates of the magnitude of the Black–White gaps in the outcomes and social exposures may be conservative. Fourth, although our analyses included multiple measures of life course SES and stress, other exposures not included here (e.g., exposure to vicarious discrimination and neighborhood stressors) might shape racialized inequities in biological aging. Fifth, because of small samples, our analyses excluded foreign-born and Hispanic HRS respondents. Future research should investigate how the patterns and drivers of biological aging vary by immigrant status and among Latinx/Hispanic people. Sixth, our supplementary sex-stratified models may have been underpowered to detect sex differences. Future research using larger samples could further assess how the patterns documented here might vary by sex and gender. Finally, although we used individual- and household-level measures of socioeconomic and stress exposures in our analysis, we encourage future research that more directly captures the structural and institutional processes generating inequities in these more proximate measures.
This study shows that Black people in the United States age more quickly than Whites because of their cumulative exposure to toxic social and economic exposures across the life span. Given documented links between our biological aging measures and morbidity and mortality, our results indicate that racism may shift the age curve of morbidity and mortality risks, putting Black people at greater risk of disease and death at earlier ages than White people. Because racial disparities in biological aging result from racialized differences in socioeconomic and stress exposures, efforts to reduce staggering and persistent Black–White gaps in morbidity and mortality should target policies and institutional practices that generate these racialized inequities in social and economic environments, risks, and opportunities. ■
Supplementary Material
Acknowledgments
This study used data from the Health and Retirement Study (HRS). The HRS is sponsored by the National Institute on Aging (NIA U01AG009740) and is conducted by the University of Michigan. The authors are grateful to the Population Studies Center at the University of Pennsylvania (National Institutes of Health’s Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), NIH grant R24HD044964), the Carolina Population Center (NICHD, NIH grant R21HD095448), and the Axilrod Faculty Fellowship program at the University of Pennsylvania for general support. Y.C.Y. acknowledges funding support from the National Institutes of Aging (NIH grant NIA R01AG057800).
Footnotes
For more information about the Venous Blood Study, see Crimmins et al. (2017).
Stress measures were assessed consistently beginning in 2006. In addition, using stress measures through the 2012 wave allowed us to include two waves of data on stress exposure for Venous Blood Study sample respondents without using data from the 2016 wave, which could raise concerns about reverse causality.
ELECTRONIC SUPPLEMENTARY MATERIAL The online version of this article (https://doi.org/10.1215/00703370-11057546) contains supplementary material.
Contributor Information
Courtney E. Boen, Department of Sociology and Population Studies Center, University of Pennsylvania, Philadelphia, PA, USA
Y. Claire Yang, Department of Sociology and Carolina Population Center, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA
Allison E. Aiello, Robert N. Butler Columbia Aging Center, Columbia University, New York, NY, USA
Alexis C. Dennis, Department of Sociology, McGill University, Montreal, Quebec, Canada
Kathleen Mullan Harris, Department of Sociology and Carolina Population Center, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA.
Dayoon Kwon, Fielding School of Public Health, University of California at Los Angeles, Los Angeles, CA, USA.
Daniel W. Belsky, Columbia Mailman School of Public Health and Robert N. Butler Columbia Aging Center, Columbia University, New York, NY, USA
References
- Assari S (2018). Unequal gain of equal resources across racial groups. International Journal of Health Policy and Management, 7, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey ZD, Krieger N, Agénor M, Graves J, Linos N, & Bassett MT (2017). Structural racism and health inequities in the USA: Evidence and interventions. Lancet, 389, 1453–1463. [DOI] [PubMed] [Google Scholar]
- Belsky DW, Caspi A, Cohen HJ, Kraus WE, Ramrakha S, Poulton R, & Moffitt TE (2017). Impact of early personal-history characteristics on the pace of aging: Implications for clinical trials of therapies to slow aging and extend healthspan. Aging Cell, 16, 644–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky DW, Caspi A, Houts R, Cohen HJ, Corcoran DL, Danese A, … Moffitt TE. (2015). Quantification of biological aging in young adults. Proceedings of the National Academy of Sciences, 112, E4104–E4110. 10.1073/pnas.1506264112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky DW, Moffitt TE, Cohen AA, Corcoran DL, Levine ME, Prinz JA, … Caspi A. (2018). Eleven telomere, epigenetic clock, and biomarker-composite quantifications of biological aging: Do they measure the same thing? American Journal of Epidemiology, 187, 1220–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boen C (2016). The role of socioeconomic factors in Black–White health inequities across the life course: Point-in-time measures, long-term exposures, and differential health returns. Social Science & Medicine, 170, 63–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boen C (2020). Death by a thousand cuts: Stress exposure and Black–White disparities in physiological functioning in late life. Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 75, 1937–1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boen C, Keister L, & Aronson B (2020). Beyond net worth: Racial differences in wealth portfolios and Black–White health inequality across the life course. Journal of Health and Social Behavior, 61, 153–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown LL, Abrams LR, Mitchell UA, & Ailshire JA (2020). Measuring more than exposure: Does stress appraisal matter for Black–White differences in anxiety and depressive symptoms among older adults? Innovation in Aging, 4, igaa040. 10.1093/geroni/igaa040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown LL, Mitchell UA, & Ailshire JA (2020). Disentangling the stress process: Race/ethnic differences in the exposure and appraisal of chronic stressors among older adults. Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 75, 650–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TH, & Hargrove TW (2018). Psychosocial mechanisms underlying older Black men’s health.Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 73, 188–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TH, Hargrove TW, Homan P, & Adkins DE (2023). Racialized health inequities: Quantifying socioeconomic and stress pathways using moderated mediation. Demography, 60, 675–705. 10.1215/00703370-10740718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TH, O’Rand AM, & Adkins DE (2012). Race-ethnicity and health trajectories: Tests of three hypotheses across multiple groups and health outcomes. Journal of Health and Social Behavior, 53, 359–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TH, Richardson LJ, Hargrove TW, & Thomas CS (2016). Using multiple-hierarchy stratification and life course approaches to understand health inequalities: The intersecting consequences of race, gender, SES, and age. Journal of Health and Social Behavior, 57, 200–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter SE, Ong ML, Simons RL, Gibbons FX, Lei MK, & Beach SRH (2019). The effect of early discrimination on accelerated aging among African Americans. Health Psychology, 38, 1010–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheadle JE, Goosby BJ, Jochman JC, Tomaso CC, Kozikowski Yancey CB, & Nelson TD (2020). Race and ethnic variation in college students’ allostatic regulation of racism-related stress. Proceedings of the National Academy of Sciences, 117, 31053–31062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AA, Milot E, Yong J, Seplaki CL, Fülöp T, Bandeen-Roche K, & Fried LP (2013). A novel statistical approach shows evidence for multi-system physiological dysregulation during aging. Mechanisms of Ageing and Development, 134, 110–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colen CG, Krueger PM, & Boettner BL (2018). Do rising tides lift all boats? Racial disparities in health across the lifecourse among middle-class African Americans and Whites. SSM—Population Health, 6, 125–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crimmins E, Faul J, Thyagarajan B, & Weir D (2017). Venous blood collection and assay protocol in the 2016 Health and Retirement Study: 2016 Venous Blood Study (VBS) (HRS documentation report). Retrieved from https://hrs.isr.umich.edu/sites/default/files/biblio/2016%20VBS%20data%20ver%207_1.pdf [Google Scholar]
- Crimmins EM (2020). Social hallmarks of aging: Suggestions for geroscience research. Ageing Research Reviews, 63, 101136. 10.1016/j.arr.2020.101136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuevas AG, & Boen C (2021). Tip of the iceberg: Measuring racial discrimination in studies of health. Stress and Health, 37, 1043–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danese A, & McEwen BS (2012). Adverse childhood experiences, allostasis, allostatic load, and age-related disease. Physiology & Behavior, 106, 29–39. [DOI] [PubMed] [Google Scholar]
- Darity WA Jr., & Mullen AK (2020). From here to equality: Reparations for Black Americans in the twenty-first century. Chapel Hill: University of North Carolina Press. [Google Scholar]
- Dennis AC, Chung EO, Lodge EK, Martinez RA, & Wilbur RE (2021). Looking back to leap forward: A framework for operationalizing the structural racism construct in minority and immigrant health research. Ethnicity & Disease, 31(Suppl.), 301–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dill BT, & Zambrana RE (Eds.). (2009). Emerging intersections: Race, class, and gender in theory, policy, and practice. New Brunswick, NJ: Rutgers University Press. [Google Scholar]
- Epel ES, Blackburn EH, Lin J, Dhabhar FS, Adler NE, Morrow JD, & Cawthon RM (2004). Accelerated telomere shortening in response to life stress. Proceedings of the National Academy of Sciences, 101, 17312–17315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraro K, & Morton P (2020). Do mediators linking childhood conditions to late-life chronic inflammation vary by race? Innovation in Aging, 4(Suppl. 1), 461–462. [Google Scholar]
- Ferraro KF, & Shippee TP (2009). Aging and cumulative inequality: How does inequality get under the skin? Gerontologist, 49, 333–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrucci L, Gonzalez-Freire M, Fabbri E, Simonsick E, Tanaka T, Moore Z, … Cabo R. (2020). Measuring biological aging in humans: A quest. Aging Cell, 19, e13080. 10.1111/acel.13080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forde AT, Crookes DM, Suglia SF, & Demmer RT (2019). The weathering hypothesis as an explanation for racial disparities in health: A systematic review. Annals of Epidemiology, 33, 1–18.e3. 10.1016/j.annepidem.2019.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrester S, Jacobs D, Zmora R, Schreiner P, Roger V, & Kiefe CI (2019). Racial differences in weathering and its associations with psychosocial stress: The CARDIA study. SSM—Population Health, 7, 100319. 10.1016/j.ssmph.2018.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaydosh L, Schorpp KM, Chen E, Miller GE, & Mullan Harris K (2018). College completion predicts lower depression but higher metabolic syndrome among disadvantaged minorities in young adulthood. Proceedings of the National Academy of Sciences, 115, 109–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee GC, Walsemann KM, & Brondolo E (2012). A life course perspective on how racism may be related to health inequities. American Journal of Public Health, 102, 967–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George LK, & Ferraro KF (2016). Aging and the social sciences. In George LK & Ferraro KF (Eds.), Handbook of aging and the social sciences (8th ed., pp. 3–22). San Diego, CA: Elsevier. [Google Scholar]
- Geronimus AT (1992). The weathering hypothesis and the health of African American women and infants: Evidence and speculations. Ethnicity & Disease, 2, 207–221. [PubMed] [Google Scholar]
- Geronimus AT, Hicken M, Keene D, & Bound J (2006). “Weathering” and age patterns of allostatic load scores among Blacks and Whites in the United States. American Journal of Public Health, 96, 826–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geronimus AT, Hicken MT, Pearson JA, Seashols SJ, Brown KL, & Dawson Cruz T (2010). Do U.S. Black women experience stress-related accelerated biological aging? A novel theory and first population-based test of Black–White differences in telomere length. Human Nature, 21, 19–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goosby BJ, Cheadle JE, & Mitchell C (2018). Stress-related biosocial mechanisms of discrimination and African American health inequities. Annual Review of Sociology, 44, 319–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goosby BJ, Straley E, & Cheadle JE (2017). Discrimination, sleep, and stress reactivity: Pathways to African American–White cardiometabolic risk inequities. Population Research and Policy Review, 36, 699–716. [Google Scholar]
- Graetz N, Boen CE, & Esposito MH (2022). Structural racism and quantitative causal inference: A life course mediation framework for decomposing racial health disparities. Journal of Health and Social Behavior, 63, 232–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf GH, Crowe CL, Kothari M, Kwon D, Manly JJ, Turney IC, … Belsky DW. (2022). Testing Black–White disparities in biological aging in older adults in the United States: Analysis of DNA-methylation and blood-chemistry methods. American Journal of Epidemiology, 191, 613–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf GH-J, Zhang Y, Domingue BW, Mullan Harris K, Kothari M, Kwon D, … Belsky DW. (2022). Social mobility and biological aging among older adults in the United States. PNAS Nexus, 1, pgac029. 10.1093/pnasnexus/pgac029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargrove TW, Gaydosh L, & Dennis AC (2022). Contextualizing educational disparities in health: Variations by race/ethnicity, nativity, and county-level characteristics. Demography, 59, 267–292. 10.1215/00703370-9664206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harman D (1981). The aging process. Proceedings of the National Academy of Sciences, 78, 7124–7128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings WJ., Shalev I, & Belsky DW. (2019). Comparability of biological aging measures in the National Health and Nutrition Examination Study, 1999–2002. Psychoneuroendocrinology, 106, 171–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward MD, Miles TP, Crimmins EM, & Yang Y (2000). The significance of socioeconomic status in explaining the racial gap in chronic health conditions. American Sociological Review, 65, 910–930. [Google Scholar]
- Heeringa SG, & Connor JH (1995). Technical description of the Health and Retirement Survey sample design (Report). Ann Arbor: Institute for Social Research, University of Michigan. Retrieved from https://hrs.isr.umich.edu/publications/biblio/5310 [Google Scholar]
- Himmelstein KEW, Lawrence JA, Jahn JL, Ceasar JN, Morse M, Bassett MT, … Venkataramani AS. (2022). Association between racial wealth inequities and racial disparities in longevity among U.S. adults and role of reparations payments, 1992 to 2018. JAMA Network Open, 5, e2240519. 10.1001/jamanetworkopen.2022.40519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummer RA, & Gutin I (2018). Racial/ethnic and nativity disparities in the health of older U.S. men and women. In Hayward MD & Majmundar MK (Eds.), Future directions for the demography of aging: Proceedings of a workshop (pp. 31–66). Washington, DC: National Academies Press. [PubMed] [Google Scholar]
- Jann B (2008). The Blinder–Oaxaca decomposition for linear regression models. Stata Journal, 8, 453–479. [Google Scholar]
- Kaeberlein M (2013). Longevity and aging. F1000Prime Reports, 5, 5. 10.12703/P5-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitagawa EM (1955). Components of a difference between two rates. Journal of the American Statistical Association, 50(272), 1168–1194. 10.1080/01621459.1955.10501299 [DOI] [Google Scholar]
- Klemera P, & Doubal S (2006). A new approach to the concept and computation of biological age. Mechanisms of Ageing and Development, 127, 240–248. [DOI] [PubMed] [Google Scholar]
- Kwon D, & Belsky D (2021). A toolkit for quantification of biological age from blood chemistry and organ function test data: BioAge. GeroScience, 43, 2795–2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawlor DA, Tilling K, & Davey Smith G (2016). Triangulation in aetiological epidemiology. International Journal of Epidemiology, 45, 1866–1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine ME (2013). Modeling the rate of senescence: Can estimated biological age predict mortality more accurately than chronological age? Journals of Gerontology, Series A: Biological Sciences and Medical Sciences, 68, 667–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine ME, & Crimmins EM (2014). Evidence of accelerated aging among African Americans and its implications for mortality. Social Science & Medicine, 118, 27–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine ME, & Crimmins EM (2018). Is 60 the new 50? Examining changes in biological age over the past two decades. Demography, 55, 387–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine ME, Lu AT, Quach A, Chen BH, Assimes TL, Bandinelli S, … Horvath S. (2018). An epigenetic biomarker of aging for lifespan and healthspan. Aging, 10, 573–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine RS, Foster JE, Fullilove RE, Fullilove MT, Briggs NC, Hull PC, … Hennekens CH. (2001). Black–White inequalities in mortality and life expectancy, 1933–1999: Implications for Healthy People 2010. Public Health Reports, 116, 474–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Ploner A, Wang Y, Magnusson PKE, Reynolds C, Finkel D, … Hägg S. (2020). Longitudinal trajectories, correlations and mortality associations of nine biological ages across 20-years follow-up. eLife, 9, e51507. 10.7554/eLife.51507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Link BG, & Phelan J (1995). Social conditions as fundamental causes of disease. Journal of Health and Social Behavior, 35(Extra issue), 80–94. [PubMed] [Google Scholar]
- Liu Z, Kuo P-L, Horvath S, Crimmins E, Ferrucci L, & Levine M (2018). A new aging measure captures morbidity and mortality risk across diverse subpopulations from NHANES IV: A cohort study. PLoS Medicine, 15, e1002718. 10.1371/journal.pmed.1002718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahalanobis PC (1936). On the generalized distance in statistics. Proceedings of the National Institute of Science of India, 2, 49–55. [Google Scholar]
- McEwen BS (1998). Stress, adaptation, and disease: Allostasis and allostatic load. Annals of the New York Academy of Sciences, 840, 33–44. [DOI] [PubMed] [Google Scholar]
- McEwen BS (2007). Physiology and neurobiology of stress and adaptation: Central role of the brain. Physiological Reviews, 87, 873–904. [DOI] [PubMed] [Google Scholar]
- McEwen BS (2012). Brain on stress: How the social environment gets under the skin. Proceedings of the National Academy of Sciences, 109(Suppl. 2), 17180–17185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezuk B, Abdou CM, Hudson D, Kershaw KN, Rafferty JA, Lee H, & Jackson JS (2013). “White box” epidemiology and the social neuroscience of health behaviors: The environmental affordances model. Society and Mental Health, 3, 79–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oaxaca R (1973). Male–female wage differentials in urban labor markets. International Economic Review, 14(3), 693. 10.2307/2525981 [DOI] [Google Scholar]
- Parker DC, Bartlett BN, Cohen HJ, Fillenbaum G, Huebner JL, Kraus VB, … Belsky DW. (2020). Association of blood chemistry quantifications of biological aging with disability and mortality in older adults. Journals of Gerontology, Series A: Biological Sciences and Medical Sciences, 75, 1671–1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavalko EK, & Willson AE (2011). Life course approaches to health, illness and healing. In Pescosolido BA, Martin JK, McLeod JD, & Rogers A (Eds.), Handbook of the sociology of health, illness, and healing: A blueprint for the 21st century (pp. 449–464). New York, NY: Springer Science+Business Media. [Google Scholar]
- Pearlin LI (1989). The sociological study of stress. Journal of Health and Social Behavior, 30, 241–256. [PubMed] [Google Scholar]
- Pfeffer FT, & Killewald A (2019). Intergenerational wealth mobility and racial inequality. Socius, 5. 10.1177/2378023119831799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelan JC, & Link BG (2015). Is racism a fundamental cause of inequalities in health? Annual Review of Sociology, 41, 311–330. [Google Scholar]
- Raffington L, & Belsky DW (2022). Integrating DNA methylation measures of biological aging into social determinants of health research. Current Environmental Health Reports, 9, 196–210. [DOI] [PubMed] [Google Scholar]
- Rahimi E, & Hashemi Nazari SS (2021). A detailed explanation and graphical representation of the Blinder–Oaxaca decomposition method with its application in health inequalities. Emerging Themes in Epidemiology, 18, 12. 10.1186/s12982-021-00100-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts D (2012). Fatal invention: How science, politics, and big business re-create race in the twenty-first century. New York, NY: The New Press. [Google Scholar]
- Rutledge J, Oh H, & Wyss-Coray T (2022). Measuring biological age using omics data. Nature Reviews Genetics, 23, 715–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnittker J, & McLeod JD (2005). The social psychology of health disparities. Annual Review of Sociology, 31, 75–103. [Google Scholar]
- Sewell AA (2016). The racism–race reification process: A mesolevel political economic framework for understanding racial health disparities. Sociology of Race and Ethnicity, 2, 402–432. [Google Scholar]
- Shuey KM, & Willson AE (2008). Cumulative disadvantage and Black–White disparities in lifecourse health trajectories. Research on Aging, 30, 200–225. [Google Scholar]
- Simons RL, Lei MK, Beach SRH, Philibert RA, Cutrona CE, Gibbons FX, & Barr A (2016). Economic hardship and biological weathering: The epigenetics of aging in a U.S. sample of Black women. Social Science & Medicine, 150, 192–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons RL, Lei M-K, Beach SRH, Simons LG, Barr AB, Gibbons FZ, & Philibert RA (2019). Testing life course models whereby juvenile and adult adversity combine to influence speed of biological aging. Journal of Health and Social Behavior, 60, 291–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons RL, Lei M-K, Klopack E, Beach SRH, Gibbons FX, & Philibert RA (2021). The effects of social adversity, discrimination, and health risk behaviors on the accelerated aging of African Americans: Further support for the weathering hypothesis. Social Science & Medicine, 282, 113169. 10.1016/j.socscimed.2020.113169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternthal MJ, Slopen N, & Williams DR (2011). Racial disparities in health: How much does stress really matter? Du Bois Review, 8, 95–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umberson D, Olson JS, Crosnoe R, Liu H, Pudrovska T, & Donnelly R (2017). Death of family members as an overlooked source of racial disadvantage in the United States. Proceedings of the National Academy of Sciences, 114, 915–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vineis P, Kelly-Irving M, Rappaport S, & Stringhini S (2016). The biological embedding of social differences in ageing trajectories. Journal of Epidemiology and Community Health, 70, 111–113. [DOI] [PubMed] [Google Scholar]
- Warner DF, & Brown TH (2011). Understanding how race/ethnicity and gender define age-trajectories of disability: An intersectionality approach. Social Science & Medicine, 72, 1236–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DR (2018). Stress and the mental health of populations of color: Advancing our understanding of race-related stressors. Journal of Health and Social Behavior, 59, 466–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DR, & Mohammed SA (2013). Racism and health I: Pathways and scientific evidence. American Behavioral Scientist, 57, 1152–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DR, Mohammed SA, Leavell J, & Collins C (2010). Race, socioeconomic status, and health: Complexities, ongoing challenges, and research opportunities. Annals of the New York Academy of Sciences, 1186, 69–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YC, Boen C, Gerken K, Li T, Schorpp K, & Mullan Harris K (2016). Social relationships and physiological determinants of longevity across the human life span. Proceedings of the National Academy of Sciences, 113, 578–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YC, Schorpp K, Boen C, Johnson M, & Mullan Harris K (2020). Socioeconomic status and biological risks for health and illness across the life course. Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 75, 613–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


